User login
A Cross-sectional Analysis of Regional Trends in Medicare Reimbursement for Phototherapy Services From 2010 to 2023
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
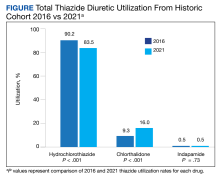
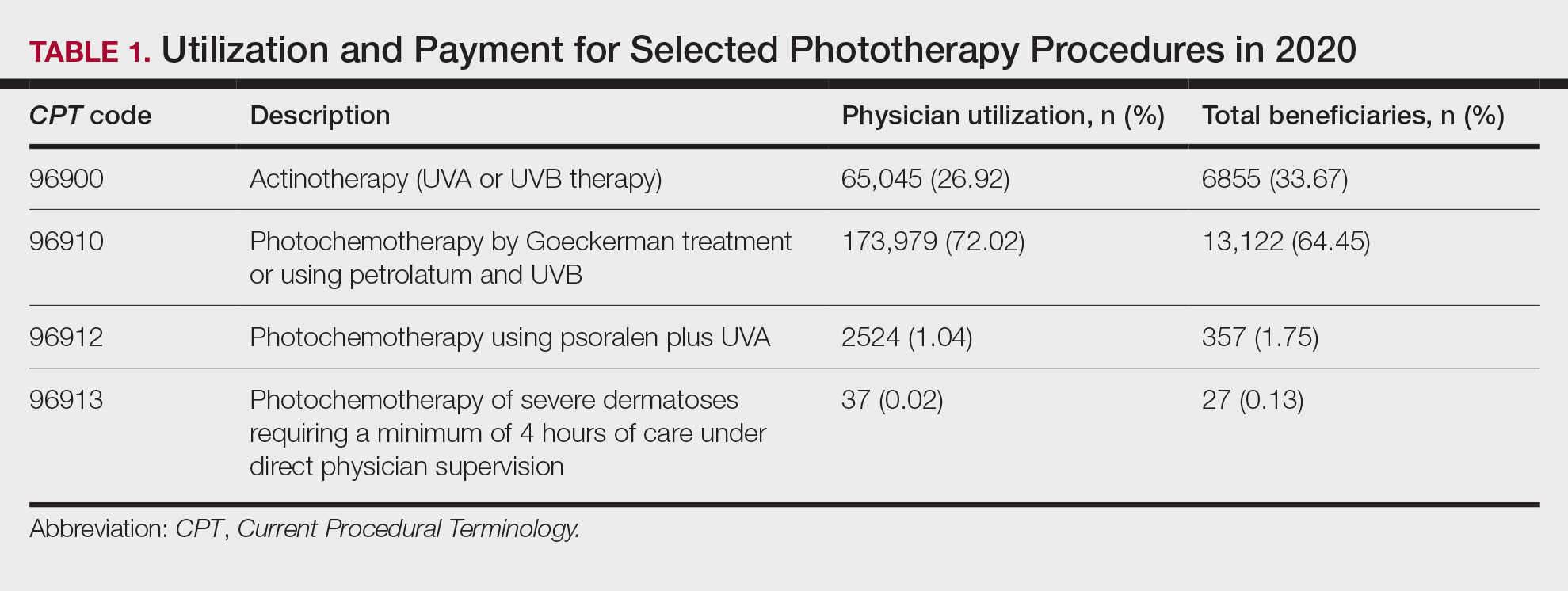
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.
On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).
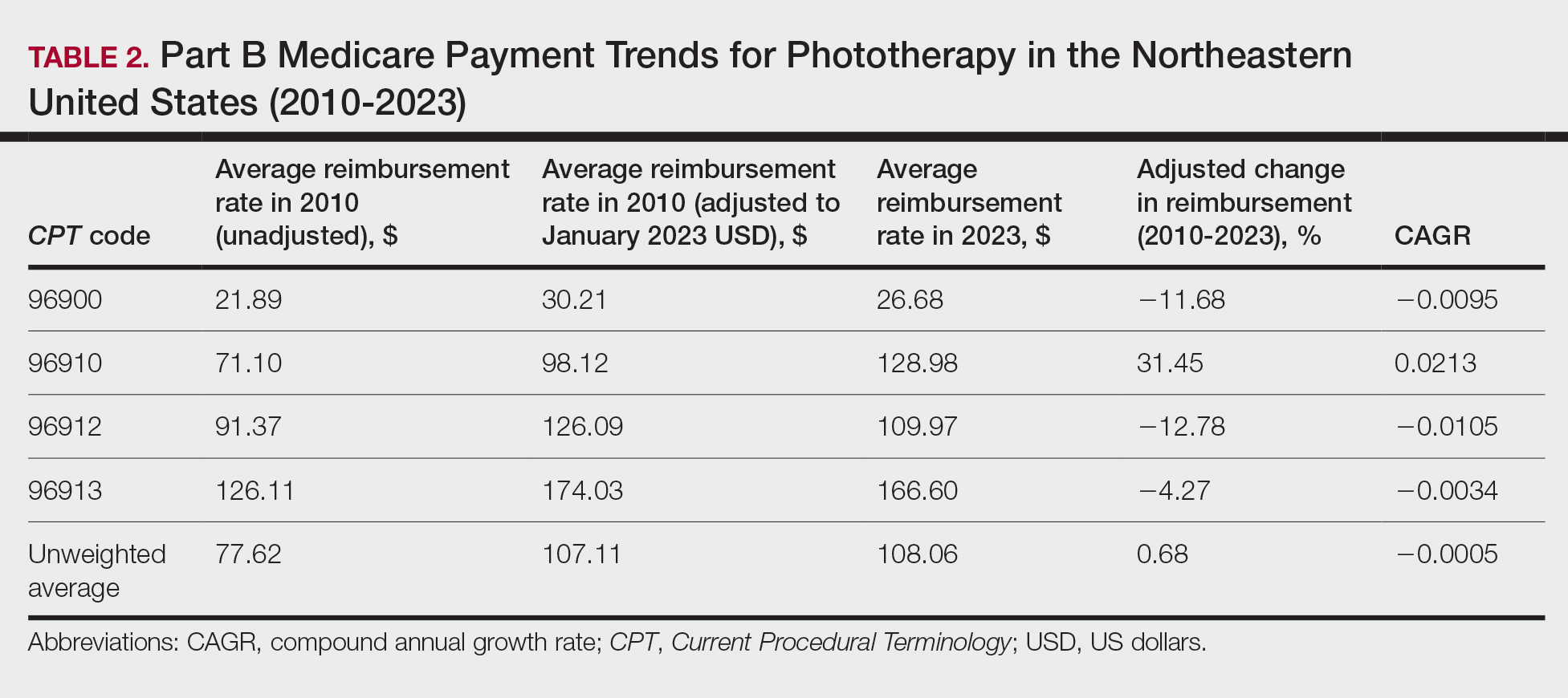
On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).
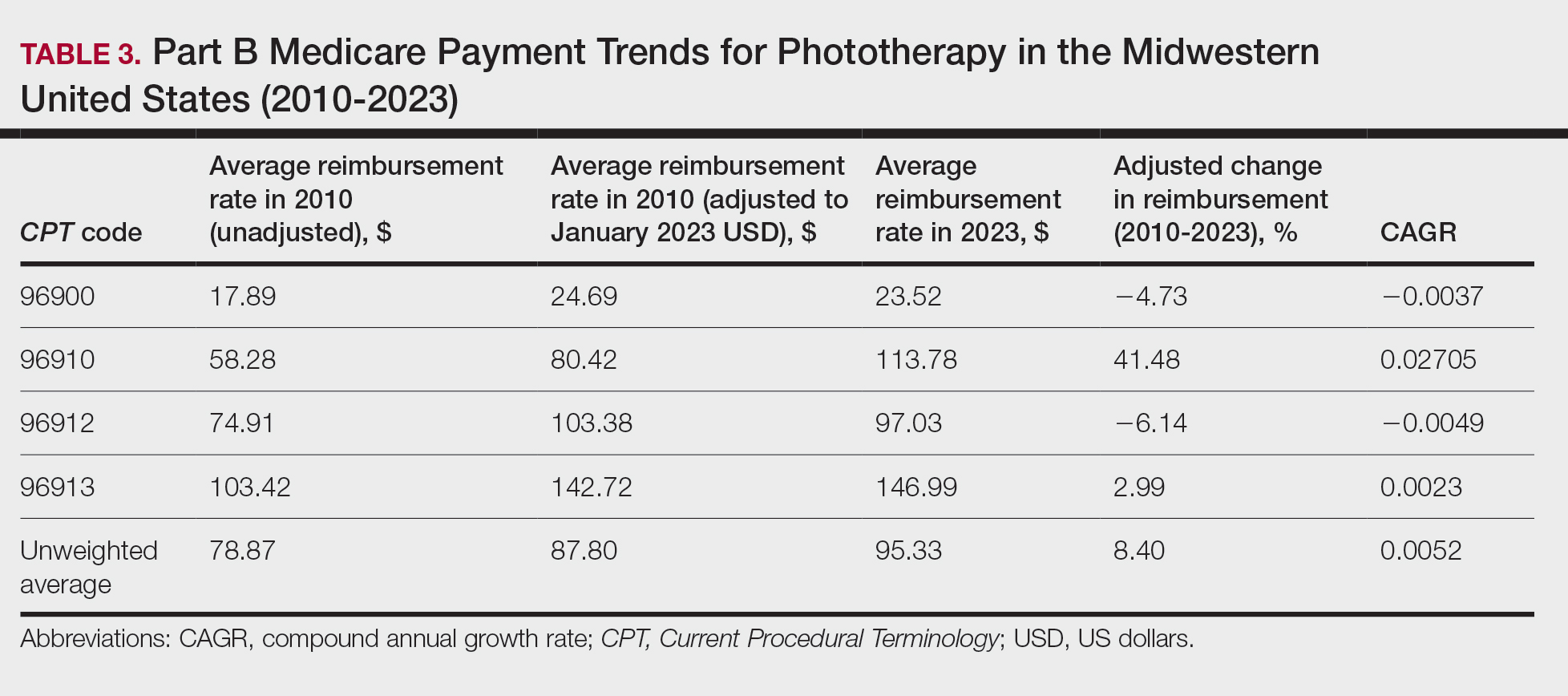
On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).
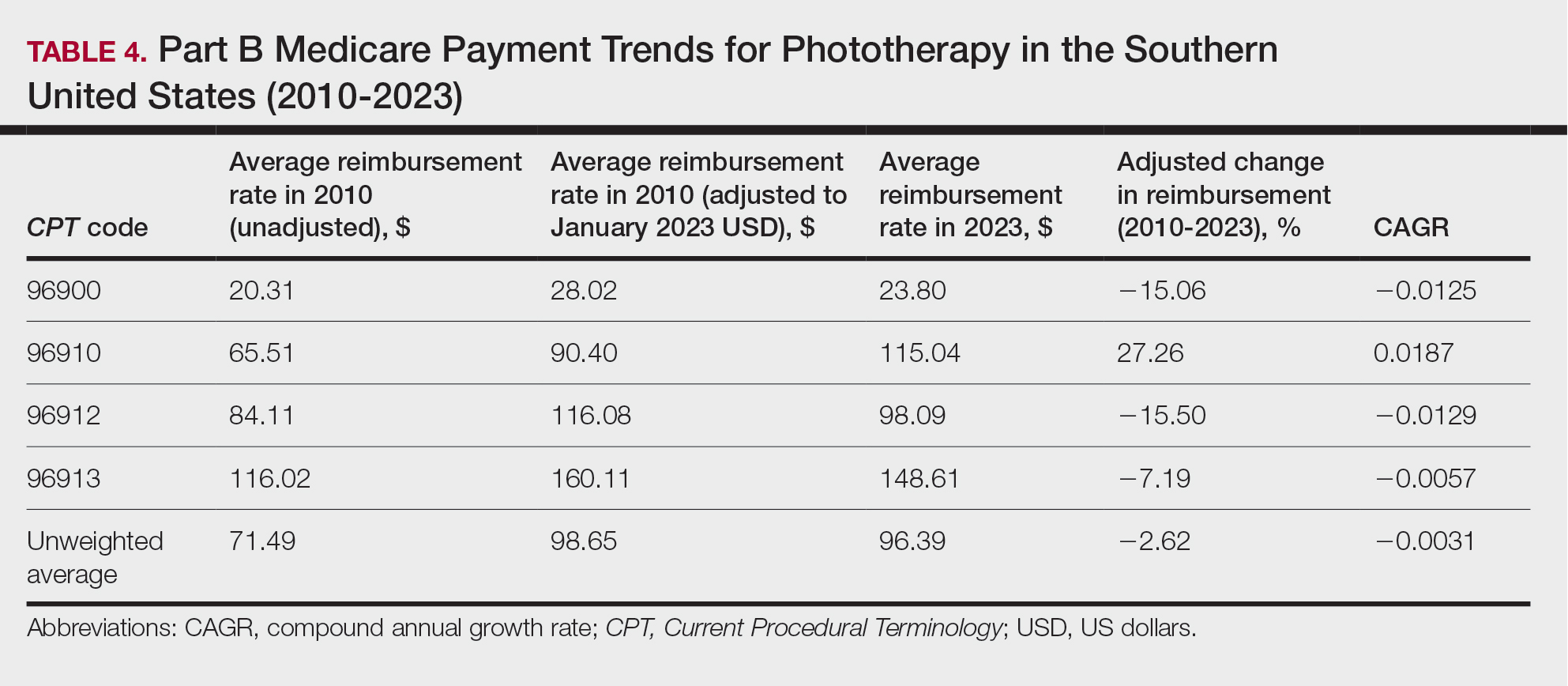
On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).
In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
Practice Points
- After weighting for procedure utilization, mean reimbursement for phototherapy increased across all US regions from 2010 to 2023 (mean change, +28.62%), yet with marked regional diversity.
- The southern United States reported the least growth in weighted mean reimbursement (+15.41%), and the western United States reported the greatest growth in weighted mean reimbursement (+51.16%).
- Region- and procedure-specific payment changes are especially valuable to dermatologists and policymakers alike, potentially reinvigorating payment reform discussions.
The Impact of a Paracentesis Clinic on Internal Medicine Resident Procedural Competency
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
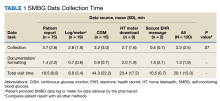
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
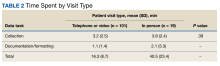
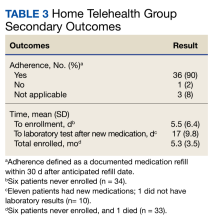
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
Piperacillin/Tazobactam Use vs Cefepime May Be Associated With Acute Decompensated Heart Failure
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
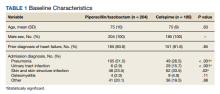
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
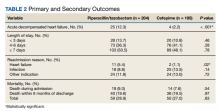
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
Association Between LDL-C and Androgenetic Alopecia Among Female Patients in a Specialty Alopecia Clinic
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).
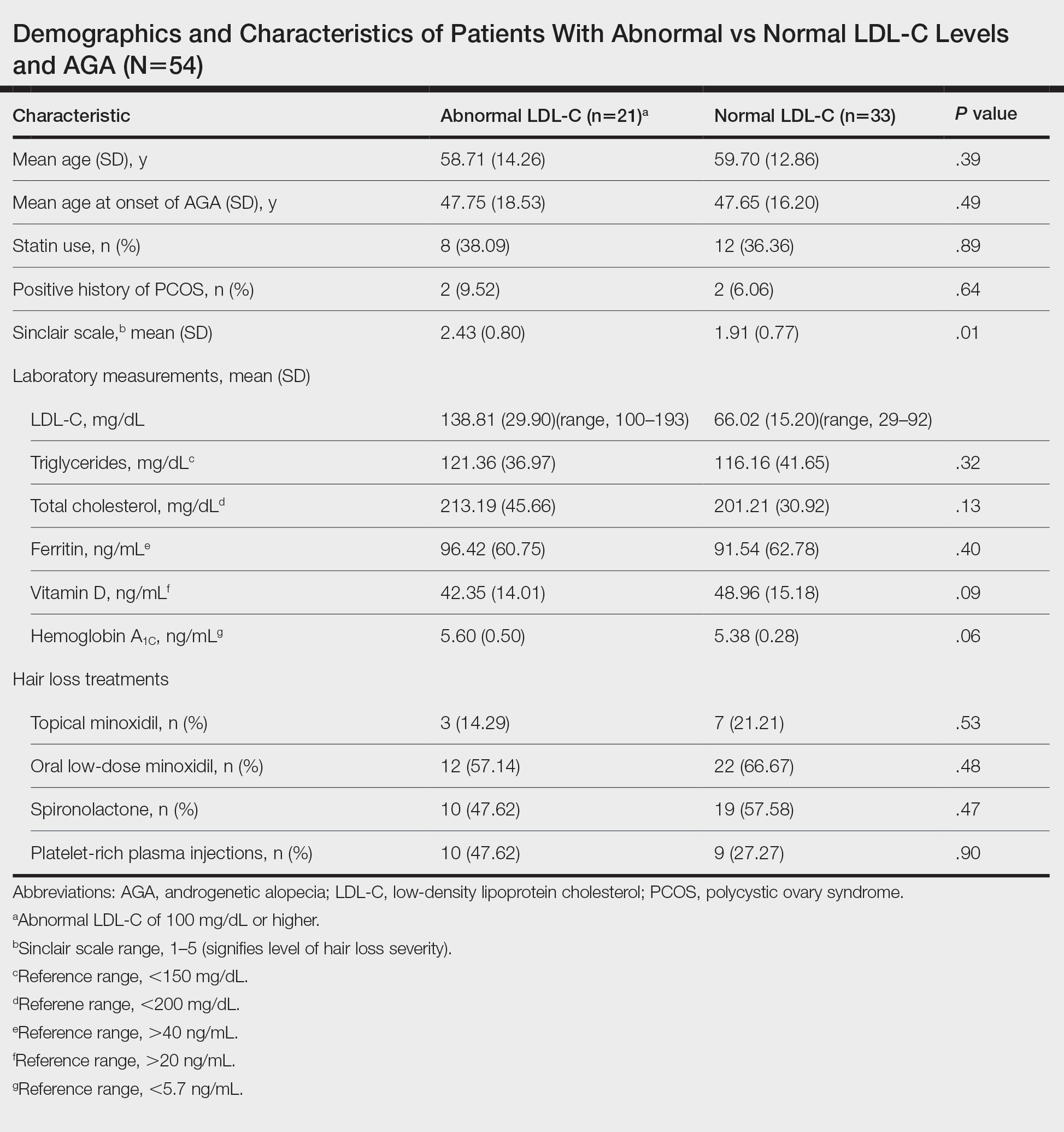
We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
Practice Points
- Associations have been shown between hair loss and markers of bad health such as insulin resistance and high cholesterol. Research has not yet shown the relationship between hair loss severity and these markers, particularly cholesterol.
Analysis of Nail Excision Practice Patterns in the Medicare Provider Utilization and Payment Database 2012-2017
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.
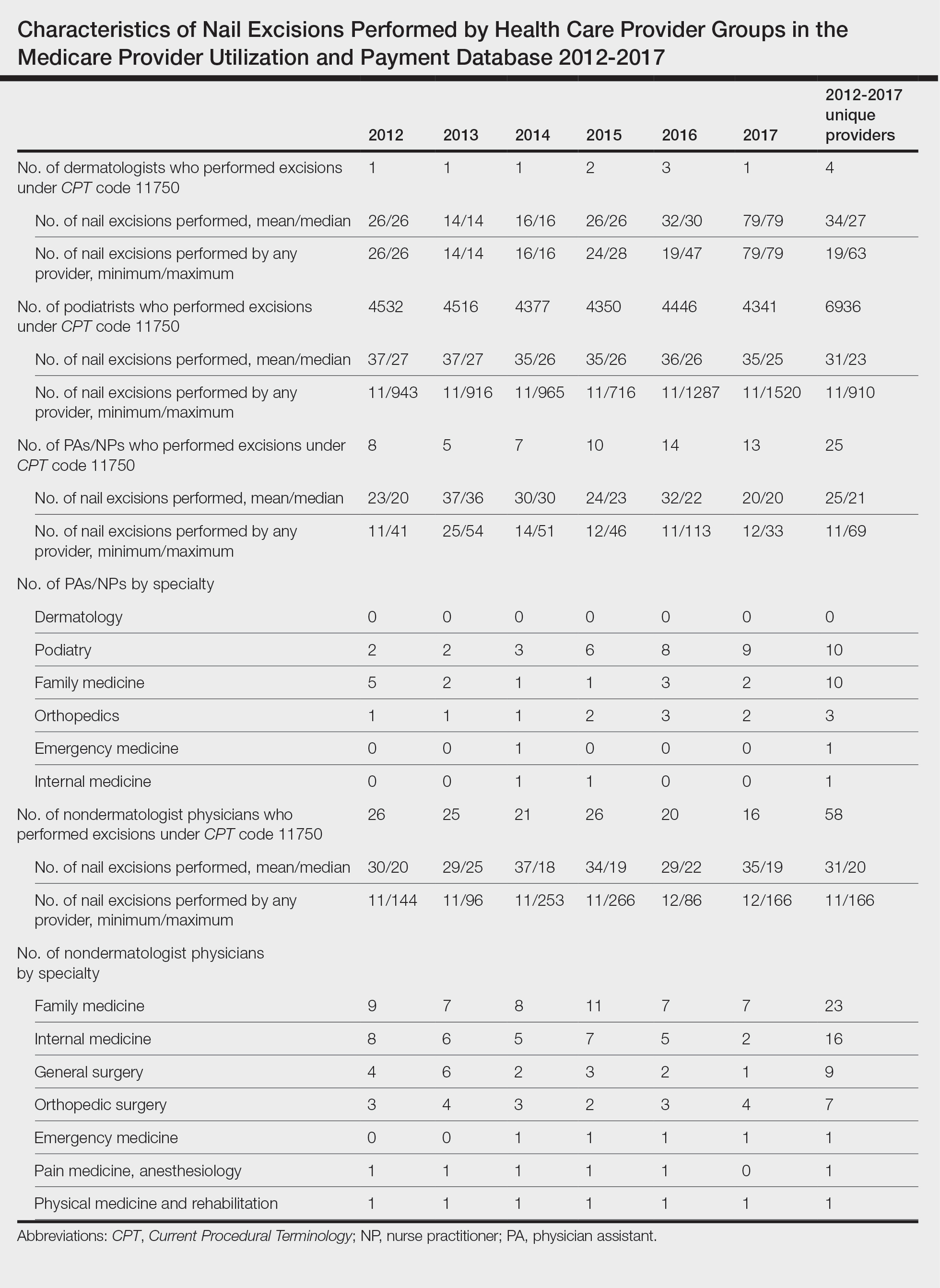
A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).
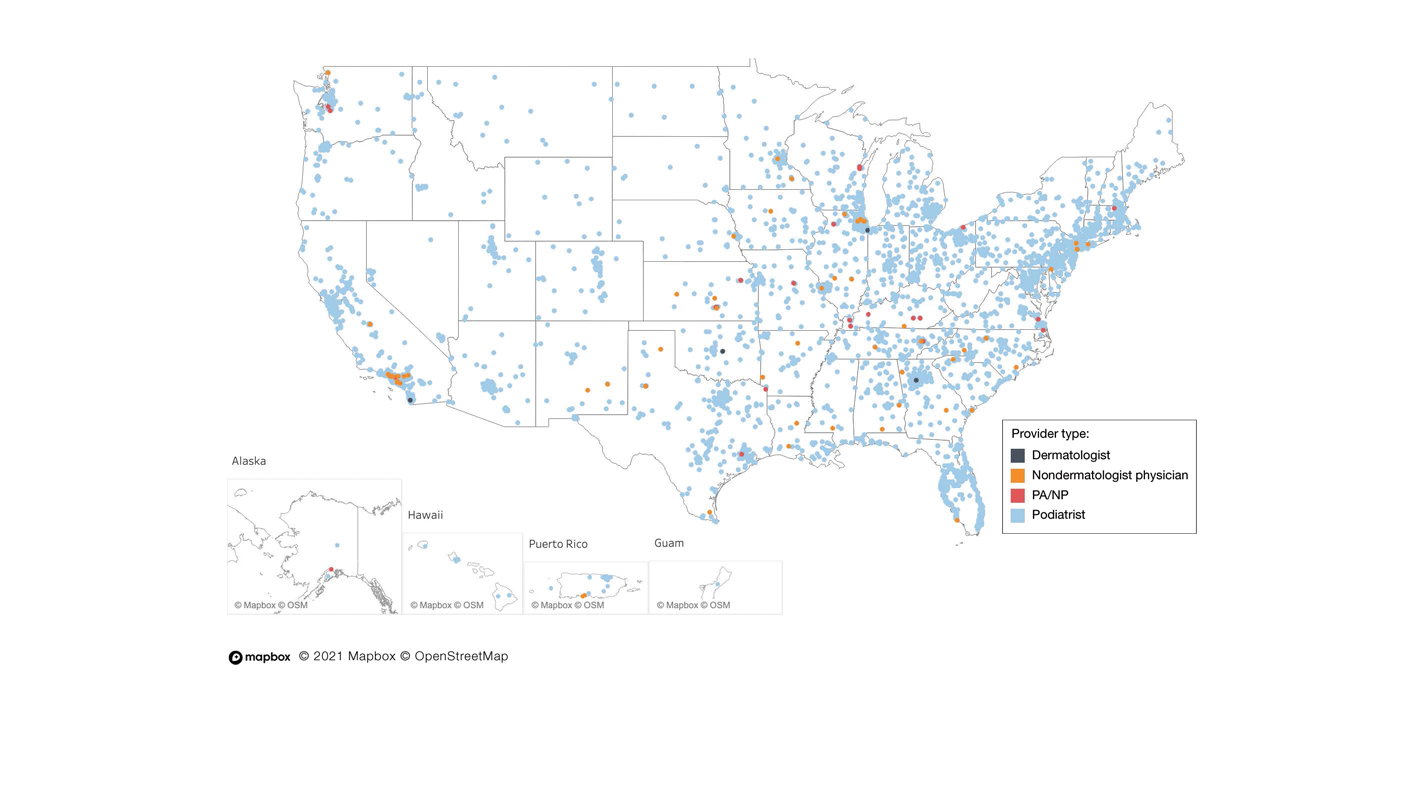
Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
Practice Points
- Dermatologists are considered nail experts but perform nail excisions less frequently than their podiatric counterparts and physicians in other specialties.
- Aspects of podiatric surgical training should be incorporated into dermatology residency to increase competency and comfort of dermatologists in nail excision procedures.
- Dermatologists may not be perceived as nail experts by the public, indicating a need for increased community education on the role of dermatologists in treating nail disease.
PRAME Expression in Melanocytic Proliferations in Special Sites
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.
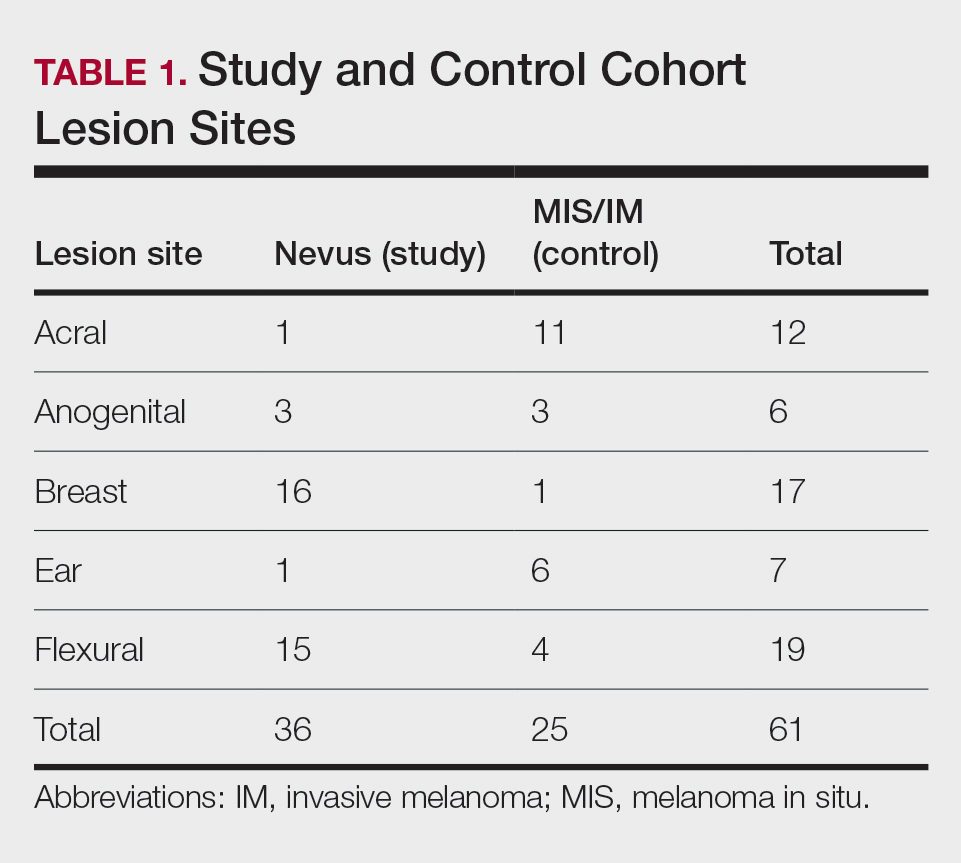
Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).
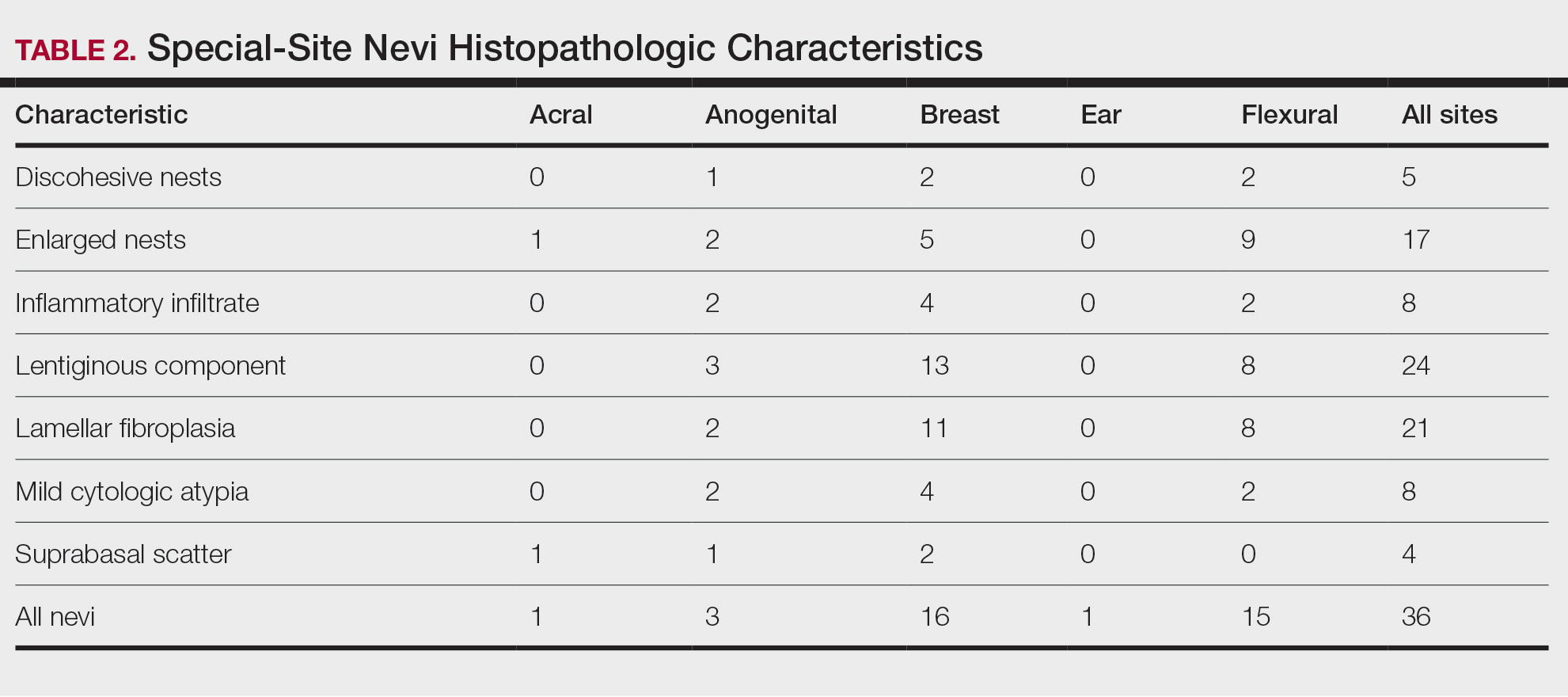
Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.
Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.
Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.
Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.
Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
Practice Points
- Special-site nevi are benign melanocytic proliferations at special anatomic sites. Although cytologic atypia and architectural distortion may be present, they are centrally located and should not be present at the borders of the lesion.
- Strong expression of the preferentially expressed antigen in melanoma (PRAME) via immunohistochemistry provides a reliable indicator for benignity in differentiating a special-site nevus from a malignant melanoma occurring at a special site.
Association Between Atopic Dermatitis and Chronic Obstructive Pulmonary Disease Among US Adults in the 1999-2006 NHANES Survey
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).
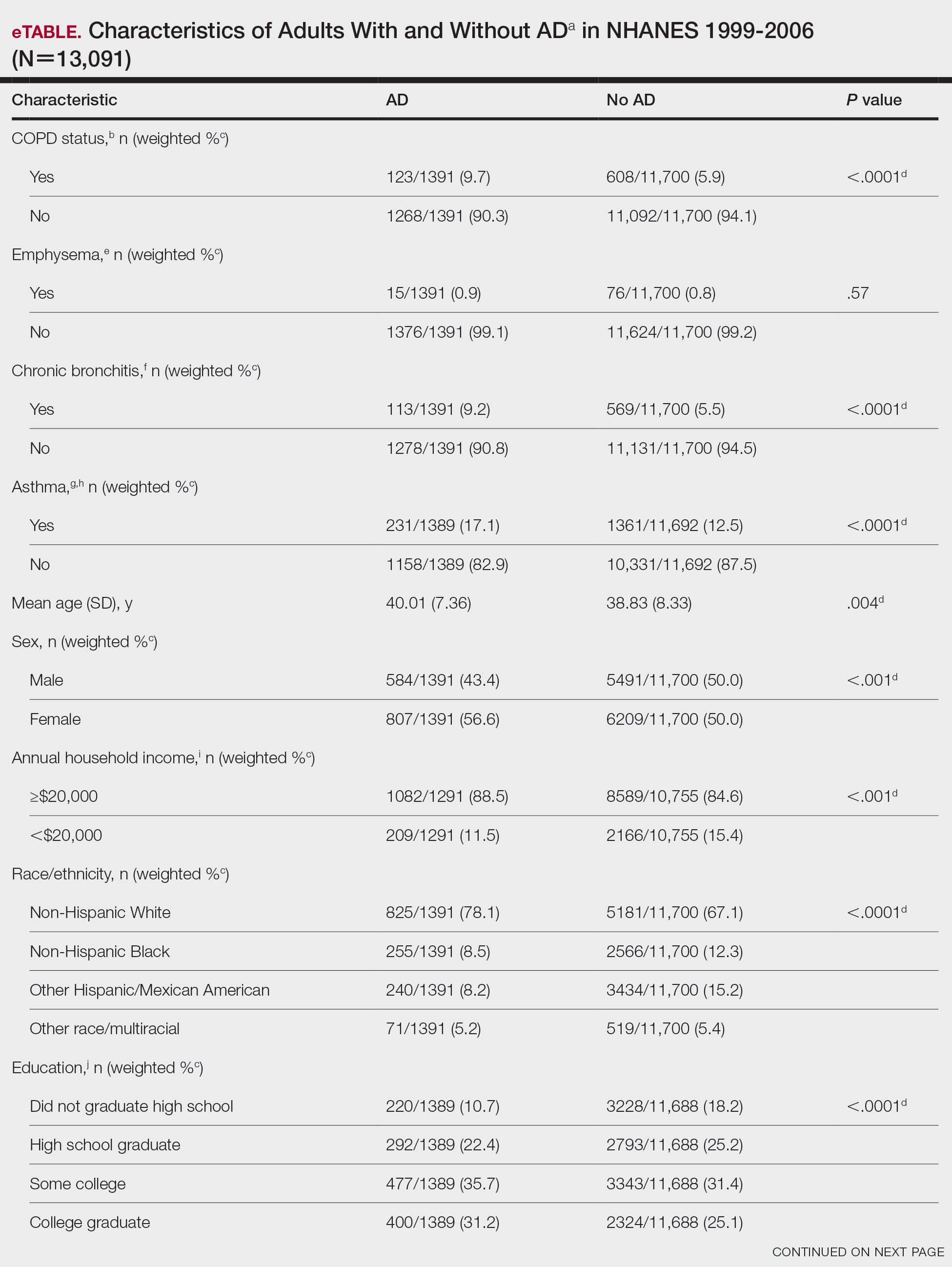
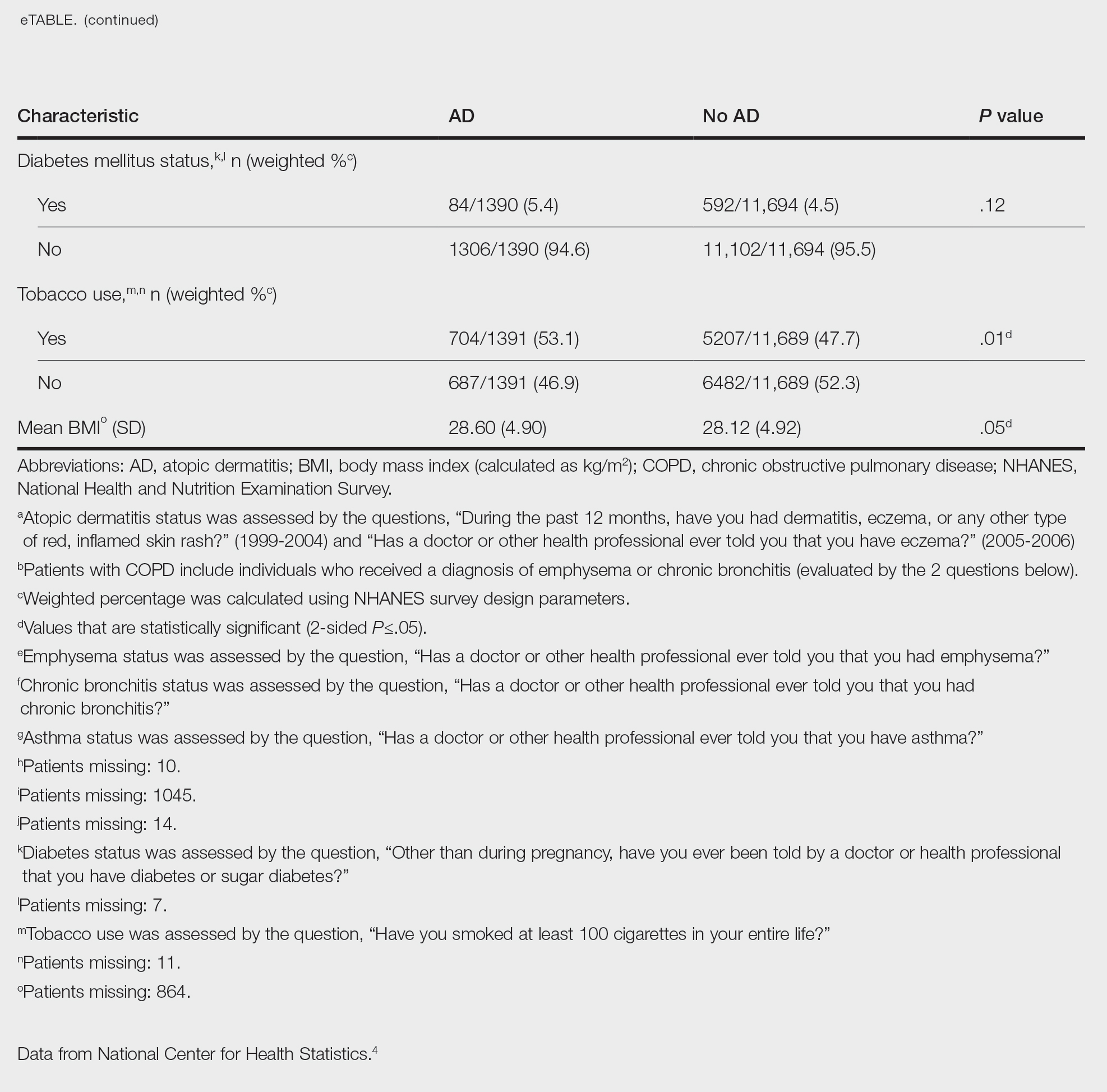
Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).
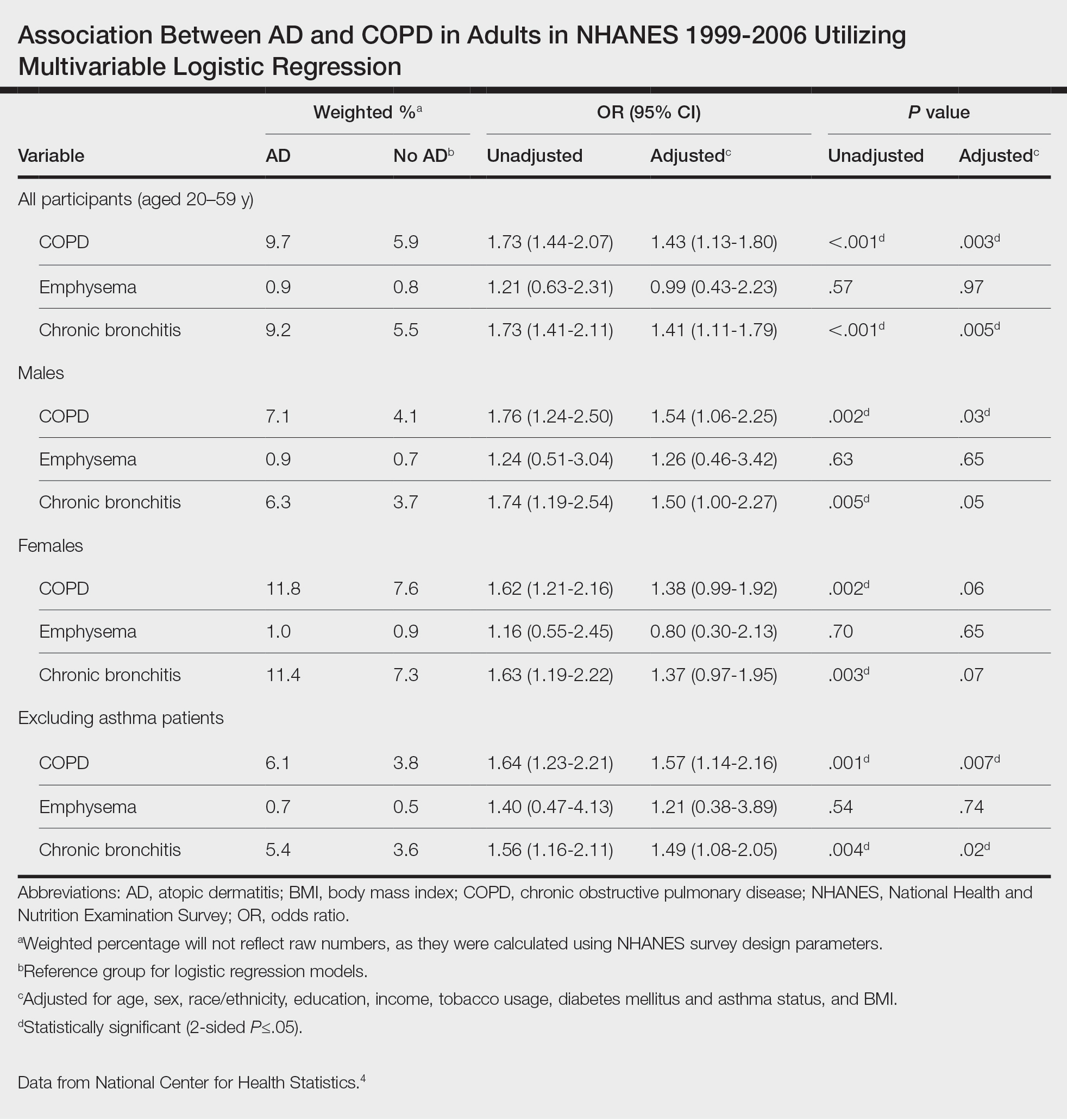
Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is an inflammatory skin condition that affects approximately 16.5 million adults in the United States.1 Atopic dermatitis is associated with skin barrier dysfunction and the activation of type 2 inflammatory cytokines. Multiorgan involvement of AD has been demonstrated, as patients with AD are more prone to asthma, allergic rhinitis, and other systemic diseases.2 In 2020, Smirnova et al3 reported a significant association (adjusted odds ratio [AOR], 1.58; 95% CI, 1.30-1.92) between AD and chronic obstructive pulmonary disease (COPD) in a large Swedish population. Currently, there is a lack of research evaluating the association between AD and COPD in a population of US adults. Therefore, we explored the association between AD and COPD (chronic bronchitis or emphysema) in a population of US adults utilizing the 1999-2006 National Health and Nutrition Examination Survey (NHANES), as these were the latest data for AD available in NHANES.4
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 1999-2006 NHANES database. Three outcome variables—emphysema, chronic bronchitis, and COPD—and numerous confounding variables for each participant were extracted from the NHANES database. The original cohort consisted of 13,134 participants, and 43 patients were excluded from our analysis owing to the lack of response to survey questions regarding AD and COPD status. The relationship between AD and COPD was evaluated by multivariable logistic regression analyses utilizing Stata/MP 17 (StataCorp LLC). In our logistic regression models, we controlled for age, sex, race/ethnicity, education, income, tobacco usage, diabetes mellitus and asthma status, and body mass index (eTable).


Our study consisted of 13,091 participants. Multivariable logistic regressions were utilized to examine the association between AD and COPD (Table). Approximately 12.5% (weighted) of the patients in our analysis had AD. Additionally, 9.7% (weighted) of patients with AD had received a diagnosis of COPD; conversely, 5.9% (weighted) of patients without AD had received a diagnosis of COPD. More patients with AD reported a diagnosis of chronic bronchitis (9.2%) rather than emphysema (0.9%). Our analysis revealed a significant association between AD and COPD among adults aged 20 to 59 years (AOR, 1.43; 95% CI, 1.13-1.80; P=.003) after controlling for potential confounding variables. Subsequently, we performed subgroup analyses, including exclusion of patients with an asthma diagnosis, to further explore the association between AD and COPD. After excluding participants with asthma, there was still a significant association between AD and COPD (AOR, 1.57; 95% CI, 1.14-2.16; P=.007). Moreover, the odds of receiving a COPD diagnosis were significantly higher among male patients with AD (AOR, 1.54; 95% CI, 1.06-2.25; P=.03).

Our results support the association between AD and COPD, more specifically chronic bronchitis. This finding may be due to similar pathogenic mechanisms in both conditions, including overlapping cytokine production and immune pathways.5 Additionally, Harazin et al6 discussed the role of a novel gene, collagen 29A1 (COL29A1), in the pathogenesis of AD, COPD, and asthma. Variations in this gene may predispose patients to not only atopic diseases but also COPD.6
Limitations of our study include self-reported diagnoses and lack of patients older than 59 years. Self-reported diagnoses could have resulted in some misclassification of COPD, as some individuals may have reported a diagnosis of COPD rather than their true diagnosis of asthma. We mitigated this limitation by constructing a subpopulation model with exclusion of individuals with asthma. Further studies with spirometry-diagnosed COPD are needed to explore this relationship and the potential contributory pathophysiologic mechanisms. Understanding this association may increase awareness of potential comorbidities and assist clinicians with adequate management of patients with AD.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32:409-413. doi:10.1016/j.clindermatol.2013.11.007
- Smirnova J, Montgomery S, Lindberg M, et al. Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 2020;20:23. doi:10.1186/s12895-020-00117-8
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- Kawayama T, Okamoto M, Imaoka H, et al. Interleukin-18 in pulmonary inflammatory diseases. J Interferon Cytokine Res. 2012;32:443-449. doi:10.1089/jir.2012.0029
- Harazin M, Parwez Q, Petrasch-Parwez E, et al. Variation in the COL29A1 gene in German patients with atopic dermatitis, asthma and chronic obstructive pulmonary disease. J Dermatol. 2010;37:740-742. doi:10.1111/j.1346-8138.2010.00923.x
Practice Points
- Various comorbidities are associated with atopic dermatitis (AD). Currently, research exploring the association between AD and chronic obstructive pulmonary disease is limited.
- Understanding the systemic diseases associated with inflammatory skin diseases can assist with adequate patient management.
Thiazide Diuretic Utilization Within the VA
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
Hypertension is one of the most common cardiovascular disease (CVD) states, affecting nearly half of all adults in the United States.1 Numerous classes of antihypertensives are available for blood pressure (BP) management, including thiazide diuretics, which contain both thiazide and thiazide-like agents. Thiazide diuretics available in the US include hydrochlorothiazide (HCTZ), chlorthalidone, metolazone, and indapamide. These agents are commonly used and recommended as first-line treatment in the current 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline for the prevention, detection, evaluation, and management of high BP in adults.2
The ACC/AHA guideline recommends chlorthalidone as the preferred thiazide diuretic.2 This recommendation is based on its prolonged half-life compared with other thiazide agents, as well as the reduction of CVD seen with chlorthalidone in previous trials. The main evidence supporting chlorthalidone use comes from the ALLHAT trial, which compared chlorthalidone, amlodipine, and lisinopril in patients with hypertension. The primary composite outcome of fatal coronary artery disease or nonfatal myocardial infarction was not significantly different between groups. However, when looking at the incidence of heart failure, chlorthalidone was superior to both amlodipine and lisinopril.3 In the TOMHS trial, chlorthalidone was more effective in reducing left ventricular hypertrophy than amlodipine, enalapril, doxazosin, or acebutolol.4 Furthermore, both a systematic review and a retrospective cohort analysis suggested that chlorthalidone may be associated with improved CVD outcomes compared with HCTZ.5,6 However, prospective randomized trial data is needed to confirm the superiority of chlorthalidone over other thiazide diuretics.
HCTZ has historically been the most common thiazide diuretic.7 However, with the available evidence and 2017 ACC/AHA BP guideline recommendations, it is unclear whether this trend continues and what impact it may have on CVD outcomes. It is unclear which thiazide diuretic is most commonly used in the US Department of Veterans Affairs (VA) health care system. The purpose of this project was to evaluate current thiazide diuretic utilization within the VA.
Methods
This retrospective, observational study evaluated the prescribing pattern of thiazide diuretics from all VA health care systems from January 1, 2016, to January 21, 2022. Thiazide diuretic agents included in this study were HCTZ, chlorthalidone, indapamide, and any combination antihypertensive products that included these 3 thiazide diuretics. Metolazone was excluded as it is commonly used in the setting of diuretic resistance with heart failure. Data was obtained from the VA Corporate Data Warehouse (CDW) and divided into 2 cohorts: the active and historic cohorts. The active cohort was of primary interest and included any active VA thiazide diuretic prescriptions on January 21, 2022. The historic cohort included thiazide prescriptions assessed at yearly intervals from January 1, 2016, to December 31, 2021. This date range was selected to assess what impact the 2017 ACC/AHA BP guideline had on clinician preferences and thiazide diuretic prescribing rates.
Within the active cohort, demographic data, vital information, and concomitant potassium or magnesium supplementation were collected. Baseline characteristics included were age, sex, race and ethnicity, and BP. Patients with > 1 race or ethnicity reported were categorized as other. The first BP reading documented after the active thiazide diuretic initiation date was included for analysis to capture on-therapy BPs while limiting confounding factors due to other potential antihypertensive changes. This project was ruled exempt from institutional review board review by the West Palm Beach VA Healthcare System Research and Development Committee.
The primary outcome was the evaluation of utilization rates of each thiazide in the active cohort, reported as a proportion of overall thiazide class utilization within the VA. Secondary outcomes in the active thiazide cohort included concomitant potassium or magnesium supplement utilization rates in each of the thiazide groups, BP values, and BP control rates. BP control was defined as a systolic BP < 130 mm Hg and a diastolic BP < 80 mm Hg. Finally, the change in thiazide diuretic utilization patterns from January 1, 2016, to December 31, 2021, was evaluated in the historic cohort.
Statistical Analysis
Data collection and analysis were completed using the CDW analyzed with Microsoft SQL Server Management Studio 18 and Microsoft Excel. All exported data to Microsoft Excel was kept in a secure network drive that was only accessible to the authors. Protected health information remained confidential per VA policy and the Health Insurance Portability and Accountability Act.
Baseline demographics were evaluated across thiazide arms using descriptive statistics. The primary outcome was assessed and a χ2 test with a single comparison α level of 0.05 with Bonferroni correction to adjust for multiple comparisons when appropriate. For the secondary outcomes, analysis of continuous data was assessed using analysis of variance (ANOVA), and nominal data were assessed with a χ2 test with a single comparison α level of 0.05 and Bonferroni correction to adjust for multiple comparisons where appropriate. When comparing all 3 thiazide groups, after the Bonferroni correction, P < .01667 was considered statistically significant to avoid a type 1 error in a family of statistical tests.
Results
As of January 21, 2022, the active thiazide cohort yielded 628,994 thiazide prescriptions within the VA nationwide. Most patients were male, with female patients representing 8.4%, 6.6%, and 5.6% of the HCTZ, chlorthalidone, and indapamide arms, respectively (Table 1). Utilization rates were significantly different between thiazide groups (P < .001). HCTZ was the most prescribed thiazide diuretic (84.6%) followed by chlorthalidone (14.9%) and indapamide (0.5%) (Table 2).
BP values documented after prescription initiation date were available for few individuals in the HCTZ, chlorthalidone, and indapamide groups (0.3%, 0.2%, and 0.5%, respectively). Overall, the mean BP values were similar among thiazide groups: 135/79 mm Hg for HCTZ, 137/78 mm Hg for chlorthalidone, and 133/79 mm Hg for indapamide (P = .32). BP control was also similar with control rates of 26.0%, 27.1%, and 33.3% for those on HCTZ, chlorthalidone, and indapamide, respectively (P = .75). The use of concomitant potassium or magnesium supplementation was significantly different between thiazide groups with rates of 12.4%, 22.6%, and 27.1% for HCTZ, chlorthalidone, and indapamide, respectively (P < .001). When comparing chlorthalidone to HCTZ, there was a significantly higher rate of concomitant supplementation with chlorthalidone (P < .001) (Table 3).
In the historic cohort, HCTZ utilization decreased from 90.2% to 83.5% (P < .001) and chlorthalidone utilization increased significantly from 9.3% to 16.0% (P < .001) (Figure). There was no significant change in the use of indapamide during this period (P = .73). Yearly trends from 2016 to 2021 are listed in Table 4.
Discussion
The findings of our evaluation demonstrate that despite the 2017 ACC/AHA BP guideline recommendations for using chlorthalidone, HCTZ predominates as the most prescribed thiazide diuretic within the VA. However, since the publication of this guideline, there has been an increase in chlorthalidone prescribing and a decrease in HCTZ prescribing within the VA.
A 2010 study by Ernst and colleagues revealed a similar trend to what was seen in our study. At that time, HCTZ was the most prescribed thiazide encompassing 95% of total thiazide utilization; however, chlorthalidone utilization increased from 1.1% in 2003 to 2.4% in 2008.8 In comparing our chlorthalidone utilization rates with these results, 9.3% in 2016 and 16.0% in 2021, the change in chlorthalidone prescribing from 2003 to 2016 represents a more than linear increase. This trend continued in our study from 2016 to 2021; the expected chlorthalidone utilization would be 21.2% in 2021 if it followed the 2003 to 2016 rate of change. Thus the trend in increasing chlorthalidone use predated the 2017 guideline recommendation. Nonetheless, this change in the thiazide prescribing pattern represents a positive shift in practice.
Our evaluation found a significantly higher rate of concomitant potassium or magnesium supplementation with chlorthalidone and indapamide compared with HCTZ in the active cohort. Electrolyte abnormalities are well documented adverse effects associated with thiazide diuretic use.9 A cross-sectional analysis by Ravioli and colleagues revealed thiazide diuretic use was an independent predictor of both hyponatremia (22.1% incidence) and hypokalemia (19% incidence) and that chlorthalidone was associated with the highest risk of electrolyte abnormalities whereas HCTZ was associated with the lowest risk. Their study also found these electrolyte abnormalities to have a dose-dependent relationship with the thiazide diuretic prescribed.10
While Ravioli and colleagues did not address the incidence of hypomagnesemia with thiazide diuretic use, a cross-sectional analysis by Kieboom and colleagues reported a significant increase in hypomagnesemia in patients prescribed thiazide diuretics.11 Although rates of electrolyte abnormalities are reported in the literature, the rates of concomitant supplementation are unclear, especially when compared across thiazide agents. Our study provides insight into the use of concomitant potassium and magnesium supplementation compared between HCTZ, chlorthalidone, and indapamide. In our active cohort, potassium was more commonly prescribed than magnesium. Interestingly, magnesium supplementation accounted for 25.9% of the total supplement use for HCTZ compared with rates of 22.4% and 21.0% for chlorthalidone and indapamide, respectively. It is unclear if this trend highlights a greater incidence of hypomagnesemia with HCTZ or greater clinician awareness to monitor this agent, but this finding may warrant further investigation. In addition, when considering the overall lower rate of supplementation seen with HCTZ in our study, the use of potassium-sparing diuretics should be considered. These agents, including triamterene, amiloride, eplerenone, and spironolactone, can be supplement-sparing and are available in combination products only with HCTZ.
Low chlorthalidone utilization rates are concerning especially given the literature demonstrating CVD benefit with chlorthalidone and the lack of compelling outcomes data to support HCTZ as the preferred agent.3,4 There are several reasons why HCTZ use may be higher in practice. First is clinical inertia, which is defined as a lack of treatment intensification or lack of changing practice patterns, despite evidence-based goals of care.12 HCTZ has been the most widely prescribed thiazide diuretic for years.7 As a result, converting HCTZ to chlorthalidone for a patient with suboptimal BP control may not be considered and instead clinicians may add on another antihypertensive or titrate doses of current antihypertensives.
There is also a consideration for patient adherence. HCTZ has many more combination products available than chlorthalidone and indapamide. If switching a patient from an HCTZ-containing combination product to chlorthalidone, adherence and patient willingness to take another capsule or tablet must be considered. Finally, there may be clinical controversy and questions around switching patients from HCTZ to chlorthalidone. Although the guidelines do not explicitly recommend switching to chlorthalidone, it may be reasonable in most patients unless they have or are at significant risk of electrolyte or metabolic disturbances that may be exacerbated or triggered with conversion.
When converting from HCTZ to chlorthalidone, it is important to consider dosing. Previous studies have demonstrated that chlorthalidone is 1.5 to 2 times more potent than HCTZ.13,14 Therefore, the conversion from HCTZ to chlorthalidone is not 1:1, but instead 50 mg of HCTZ is approximately equal to25 to 37.5 mg of chlorthalidone.14
Limitations
This study was limited by its retrospective design, gaps in data, duplicate active prescription data, and the assessment of concomitant electrolyte supplementation. As with any retrospective study, there is a potential for confounding and a concern for information bias with missing information. This study relied on proper documentation of prescription and demographic information in the Veterans Health Information Systems and Technology Architecture (VistA), as the CDW compiles information from this electronic health record. Strengths of the VistA include ease in clinical functions, documentation, and the ability for records to be updated from any VA facility nationally. However, there is always the possibility of user error and information to be omitted.
In our study, the documentation of BP values and subsequent analysis of overall BP control were limited. For BP values to be included in this study, they had to be recorded after the active thiazide prescription was written and from an in-person encounter documented in VistA. The COVID-19 pandemic shifted the clinical landscape and many primary care appointments during the active cohort evaluation period were conducted virtually. Therefore, patients may not have had formal vitals recorded. There may also be an aspect of selection bias regarding the chlorthalidone group. Although rates of thiazide switching were not assessed, some patients may have been switched from HCTZ or indapamide to chlorthalidone to achieve additional BP control. Thus, patients receiving chlorthalidone may represent a more difficult-to-control hypertensive population, making a finding of similar BP control rates between HCTZ and chlorthalidone an actual positive finding regarding chlorthalidone. Finally, this study did not assess adherence to medications. As the intent of the study was to analyze prescribing patterns, it is impossible to know if the patient was actively taking the medication at the time of assessment. When considering the rates of BP control, there were limited BP values, a potential for selection bias, and neither adherence nor patient self-reported home BP values were assessed. Therefore, the interpretation of overall BP control must be done with caution.
Additionally, duplicate prescriptions were noted in the active cohort. Rates of duplication were 0.2%, 0.08%, and 0.09% for HCTZ, chlorthalidone, and indapamide, respectively. With these small percentages, we felt this would not have a significant impact on the overall thiazide use trends seen in our study. Patients can receive prescriptions from multiple VA facilities and may have > 1 active prescriptions. This has been mitigated in recent years with the introduction of the OneVA program, allowing pharmacists to access any prescription on file from any VA facility and refill if needed (except controlled substance prescriptions). However, there are certain instances in which duplicate prescriptions may be necessary. These include patients enrolled and receiving care at another VA facility (eg, traveling for part of a year) and patients hospitalized at a different facility and given medications on discharge.
With the overall low rate of duplication prescriptions seen in each thiazide group, we determined that this was not large enough to cause substantial variation in the results of this evaluation and was unlikely to alter the results. This study also does not inform on the incidence of switching between thiazide diuretics. If a patient was switched from HCTZ to chlorthalidone in 2017, for example, a prescription for HCTZ and chlorthalidone would have been reported in this study. We felt that the change in chlorthalidone prescribing from January 1, 2016, to December 31, 2021, would reflect overall utilization rates, which may include switching from HCTZ or indapamide to chlorthalidone in addition to new chlorthalidone prescriptions.
Finally, there are confounders and trends in concomitant potassium or magnesium supplementation that were not accounted for in our study. These include concomitant loop diuretics or other medications that may cause electrolyte abnormalities and the dose-dependent relationship between thiazide diuretics and electrolyte abnormalities.10 Actual laboratory values were not included in this analysis and thus we cannot assess whether supplementation or management of electrolyte disturbances was clinically appropriate.
Conclusions
Thiazide utilization patterns have shifted possibly due to the 2017 ACC/AHA BP guideline recommendations. However, HCTZ continues to be the most widely prescribed thiazide diuretic within the VA. There is a need for future projects and clinician education to increase the implementation of guideline-recommended therapy within the VA, particularly regarding chlorthalidone use.
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
1. Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Updated May 12, 2023. Accessed October 12, 2023. https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi:10.1161/HYP.0000000000000065
3. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. doi:10.1001/jama.288.23.2981
4. Liebson PR, Grandits GA, Dianzumba S, et al. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91(3):698-706. doi:10.1161/01.cir.91.3.698
5. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59(6):1110-1117. doi:10.1161/HYPERTENSIONAHA.112.191106
6. Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57(4):689-694. doi:10.1161/HYPERTENSIONAHA.110.161505
7. Vongpatanasin W. Hydrochlorothiazide is not the most useful nor versatile thiazide diuretic. Curr Opin Cardiol. 2015;30(4):361-365. doi:10.1097/HCO.0000000000000178
8. Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich). 2010;12(12):927-934. doi:10.1111/j.1751-7176.2010.00373.x
9. Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10-24. doi:10.1016/S0002-9629(15)40676-7
10. Ravioli S, Bahmad S, Funk GC, Schwarz C, Exadaktylos A, Lindner G. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: results of a cross-sectional study. Am J Med. 2021;134(9):1148-1154. doi:10.1016/j.amjmed.2021.04.007
11. Kieboom BCT, Zietse R, Ikram MA, Hoorn EJ, Stricker BH. Thiazide but not loop diuretics is associated with hypomagnesaemia in the general population. Pharmacoepidemiol Drug Saf. 2018;27(11):1166-1173. doi:10.1002/pds.4636
12. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, et al. Clinical Inertia and Outpatient Medical Errors. In: Henriksen K, Battles JB, Marks ES, et al, editors. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville (MD): Agency for Healthcare Research and Quality (US); 2005. https://www.ncbi.nlm.nih.gov/books/NBK20513/
13. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43(1):4-9. doi:10.1161/01.HYP.0000103632.19915.0E
14. Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21(11):2634-2642. doi:10.1111/jcmm.13205
Evaluating Pharmacists’ Time Collecting Self-Monitoring Blood Glucose Data
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting in person vs telephone or video SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2). The most common SMBG collection method for in-person/face-to-face visits was continuous glucose monitor (CGM) (n = 10), followed by meter download/home telehealth (n = 5), patient report (n = 3), and directly from log/meter (n = 1). For telephone or video visits, the most common collection method was patient report (n = 72), followed by directly from log/meter (n = 18), CGM (n = 5), meter download/home telehealth (n = 4), and secure message (n = 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPs could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of 23 patient encounters where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for 1 patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members, are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting in person vs telephone or video SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2). The most common SMBG collection method for in-person/face-to-face visits was continuous glucose monitor (CGM) (n = 10), followed by meter download/home telehealth (n = 5), patient report (n = 3), and directly from log/meter (n = 1). For telephone or video visits, the most common collection method was patient report (n = 72), followed by directly from log/meter (n = 18), CGM (n = 5), meter download/home telehealth (n = 4), and secure message (n = 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPs could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of 23 patient encounters where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for 1 patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members, are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting in person vs telephone or video SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2). The most common SMBG collection method for in-person/face-to-face visits was continuous glucose monitor (CGM) (n = 10), followed by meter download/home telehealth (n = 5), patient report (n = 3), and directly from log/meter (n = 1). For telephone or video visits, the most common collection method was patient report (n = 72), followed by directly from log/meter (n = 18), CGM (n = 5), meter download/home telehealth (n = 4), and secure message (n = 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPs could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of 23 patient encounters where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for 1 patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members, are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
VA Home Telehealth Program for Initiating and Optimizing Heart Failure Guideline-Directed Medical Therapy
Heart failure (HF) is a chronic, progressive condition that is characterized by the heart’s inability to effectively pump blood throughout the body. In 2018, approximately 6.2 million US adults had HF, and 13.4% of all death certificates noted HF as a precipitating factor.1 Patients not receiving appropriate guideline-directed medical therapy (GDMT) face a 29% excess mortality risk over a 2-year period.2 Each additional GDMT included in a patient’s regimen significantly reduces all-cause mortality.3
The Change the Management of Patients with Heart Failure (CHAMP) registry reports that only about 1% of patients with HF are prescribed 3 agents from contemporary GDMT at target doses, highlighting the need for optimizing clinicians’ approaches to GDMT.4 Similarly, The Get With The Guidelines Heart Failure Registry has noted that only 20.2% of patients with HF with reduced ejection fraction (HFrEF) are prescribed a sodium-glucose cotransporter 2 inhibitor (SGLT2i) following hospital discharge for HFrEF exacerbation.5 Overall, treatment rates with GDMT saw limited improvement between 2013 and 2019, with no significant difference between groups in mortality, indicating the need for optimized methods to encourage the initiation of GDMT.6
Remote monitoring and telecare are novel ways to improve GDMT rates in those with HFrEF. However, data are inconsistent regarding the impact of remote HF monitoring and improvements in GDMT or HF-related outcomes.6-10 The modalities of remote monitoring for GDMT vary among studies, but the potential for telehealth monitoring to improve GDMT, thereby potentially reducing HF-related hospitalizations, is clear.
Telemonitoring has demonstrated improved participant adherence with weight monitoring, although the withdrawal rate was high, and has the potential to reduce all-cause mortality and HF-related hospitalizations.11,12 Telemonitoring for GDMT optimization led to an increase in the proportion of patients who achieved optimal GDMT doses, a decrease in the time to dose optimization, and a reduction in the number of clinic visits.13 Remote GDMT titration was accomplished in the general patient population with HFrEF; however, in populations already followed by cardiologists or HF specialists, remote optimization strategies did not yield different proportions of GDMT use.14 The aim of this study was to assess the impact of the home telehealth (HT) monitoring program on the initiation and optimization of HF GDMT among veterans with HFrEF at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan.
Methods
This was a single-center retrospective study of Computerized Patient Record System (CPRS)data. Patients at the VAAAHS were evaluated if they were diagnosed with HFrEF and were eligible for enrollment in the HT monitoring program. Eligibility criteria included a diagnosis of stage C HF, irrespective of EF, and a history of any HF-related hospitalization. We focused on patients with HFrEF due to stronger guideline-based recommendations for certain pharmacotherapies as compared with HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). Initial patient data for HT enrolling were accessed using the Heart Failure Dashboard via the US Department of Veterans Affairs (VA) Academic Detailing Service. The target daily doses of typical agents used in HFrEF GDMT are listed in the Appendix.
The HT program is an embedded model in which HT nurses receive remote data from the patient and triage that with the VAAAHS cardiology team. Patients’ questions, concerns, and/or vital signs are recovered remotely. In this model, nurses are embedded in the cardiology team, working with the cardiologists, cardiology clinical pharmacist, and/or cardiology nurse practitioners to make medication interventions. Data are recorded with an HT device, including weight, blood pressure (BP), heart rate, and pulse oximetry. HT nurses are also available to the patient via phone or video. The program uses a 180-day disease management protocol for HF via remote device, enabling the patient to answer questions and receive education on their disease daily. Responses to questions and data are then reviewed by an HT nurse remotely during business hours and triaged as appropriate with the cardiology team. Data can be communicated to the cardiology team via the patient record, eliminating the need for the cardiology team to use the proprietary portal affiliated with the HT device.
Study Sample
Patient information was obtained from a list of 417 patients eligible for enrollment in the HT program; the list was sent to the HT program for review and enrollment. Patient data were extracted from the VAAAHS HF Dashboard and included all patients with HFrEF and available data on the platform. The sample for the retrospective chart review included 40 adults who had HFrEF, defined as a left ventricular EF (LVEF) of ≤ 40% as evidenced by a transthoracic echocardiogram or cardiac magnetic resonance imaging. These patients were contacted and agreed to enroll in the HT monitoring program. The HT program population was compared against a control group of 33 patients who were ineligible for the HT program. Patients were deemed ineligible for HT if they resided in a nursing home, lacked a VAAAHS primary care clinician, or declined participation in the HT program.
Procedures
Patients who declined participation in the HT program followed the standard of care, which was limited to visits with primary care clinicians and/or cardiologists as per the follow-up plan. Patient data were collected over 12 months. The study was approved by the VAAAHS Institutional Review Board (reference number, 1703034), Research and Development Committee, and Research Administration.
Primary and Secondary Goals
The primary goal of the study was to assess the impact of the HT program on drug interventions, specifically initiating and titrating HFrEF pharmacotherapies. Interventions were based on GDMT with known mortality- and morbidity-reducing properties when used at their maximum tolerated doses, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor-neprilysin inhibitor (ARNi), or angiotensin receptor blockers (ARB), with a preference for ARNi, β-blockers for HFrEF (metoprolol succinate, bisoprolol, or carvedilol), aldosterone antagonists, and SGLT2is.
Secondary goals included HF-related hospitalizations, medication adherence, time to enrollment in HT, time to laboratory analysis after the initiation or titration of an ACEi/ARB/ARNi or aldosterone antagonist, and time enrolled in the HT program. Patients were considered adherent if their drug refill history showed consistent fills of their medications. The χ2 test was used for total interventions made during the study period and Fisher exact test for all others.
Results
Patient data were collected between July 2022 and June 2023. All 73 patients were male, and the mean age in the HT group (n = 40) was 72.6 years and 75.2 years for the control group (n = 33). Overall, the baseline demographics were similar between the groups (Table 1). Of 40 patients screened for enrollment in the HT program, 33 were included in the analysis (Figure 1).
At baseline, the HT group included more individuals than the control group on ACEi/ARB/ARNi (24 vs 19, respectively), β-blocker (28 vs 24, respectively), SGLT2i (14 vs 11, respectively), and aldosterone antagonist (15 vs 9, respectively) (Figure 2). There were 20 interventions made in the HT group compared with 11 therapy changes in the control arm during the study (odds ratio, 1.43; P = .23) (Table 2). In the HT group, 1 patient achieved an ACEi target dose, 3 patients achieved a β-blocker target dose, and 7 achieved a target dose of spironolactone (titration is not required for SGLT2i therapy and is counted as target dose). In the HT group, 17 patients were on ≥ 3 recommended agents, while 9 patients were taking 4 agents. Seven of 20 HT group interventions resulted in titration to the target dose. In the control group, no patients achieved an ARNi target dose, 3 patients achieved a β-blocker target dose, and 2 patients achieved a spironolactone antagonist target dose. In the control arm, 7 patients were on ≥ 3 GDMTs, and 2 were taking 4 agents. No patient in either group achieved a target dose of 4 agents. Five of 11 control group interventions resulted in initiation or titration of GDMT to the target dose.
Of the 40 HT group patients, 7 were excluded from analysis (3 failed to schedule HT, 1 was at a long-term care facility, 1 was nonadherent, 1 declined participation, and 1 died) and 33 remained in the program for a mean (SD) 5.3 (3.5) months. Death rates were tracked during the study: 1 patient died in the HT group and 3 in the control group.
We analyzed the overall percentage of VAAAHS patients with HFrEF who were on appropriate GDMT. Given the ongoing drive to improve HF-related outcomes, HT interventions could not be compared to a static population, so the HT and control patients were compared with the rates of GDMT at VAAAHS, which was available in the Academic Detailing Service Heart Failure Dashboard (Figure 3). Titration and optimization rates were also compared (Figure 4). From July 2022 to June 2023, ARNi use increased by 16.6%, aldosterone antagonist by 6.8%, and β-blockers by 2.4%. Target doses of GDMTs were more difficult to achieve in the hospital system. There was an increase in aldosterone antagonist target dose achievement by 4.7%, but overall there were decreases in target doses in other GDMTs: ACEi/ARB/ARNi target dose use decreased by 3.2%, ARNi target dose use decreased by 2.7% and target β-blocker use decreased by 0.9%.
Discussion
Telehealth yielded clinically important interventions, with some titrations bringing patients to their target doses of medications for HFrEF. The 20 interventions made in the HT group can be largely attributed to the nurses’ efforts to alert clinicians to drug titrations or ACEi/ARB to ARNi transitions. Although the findings were not statistically significant, the difference in the number of drug therapy changes supports the use of the HT program for a GDMT optimization strategy. Patients may be difficult to titrate secondary to adverse effects that make medication initiation or titration inappropriate, such as hypotension and hyperkalemia, although this was not observed in this small sample size. Considering a mean HT enrollment of 5.3 months, many patients had adequate disease assessment and medication titration. Given that patients are discharged from the service once deemed appropriate, this decreases the burden on the patient and increases the utility and implementation of the HT program for other patients.
A surprising finding of this study was the lower rate of HF-related hospitalizations in the HT group. Although not the primary subject of interest in the study, it reinforced the importance of close health care professional follow-up for patients living with HF. Telehealth may improve communication and shared decision making over medication use. Because the finding was unanticipated, the rate of diuretic adjustments was not tracked.
Patients were reevaluated every 6 months for willingness to continue the program, adherence, and clinical needs. These results are similar to those of other trials that demonstrated improved rates of GDMT in the setting of pharmacist- or nurse-led HF treatment optimization.15,16 These positive results differ from other trials incorporating remote monitoring regarding patient continuation in HT programs. For example, in a study by Ding and colleagues, the withdrawal rate from their monitoring service was about 22%, while in our study only 1 patient withdrew from the HT program.11
The HT program resulted in fewer hospitalizations than the control arm. There were 6 HF-related hospitalizations in the control group, although 5 involved a single patient. Typically, such a patient would be encouraged to follow HT monitoring after just 1 HF-related hospitalization; however, the patient declined to participate.
Early optimization of GDMT in patients who were recently discharged from the hospital for an HF-related hospitalization yields a reduction in hospital rehospitalization.17 GDMT optimization has unequivocal benefits in HF outcomes. Unfortunately, the issues surrounding methodologies on how to best optimize GDMT are lacking. While HT has been found to be feasible in the aid of optimizing medical therapy, the TIM-HF trial concluded that remote monitoring services had no significant benefit in reducing mortality.7,8 On the other hand, the OptiLink HF Study showed that when clinicians respond to remote monitoring prompts from fluid index threshold crossing alerts, these interventions are associated with significantly improved clinical outcomes in patients with implantable cardioverter-defibrillators and advanced HF.9 In contrast to previous trials, the AMULET trial showed that remote monitoring compared with standard care significantly reduced the risk of HF hospitalization or cardiovascular death during the 12-month follow-up among patients with HF and LVEF ≤ 49% after an episode of acute exacerbation.10 Additionally, patients who received skilled home health services and participated in remote monitoring for their chronic HF experienced a reduction in all-cause 30-day readmission.18
Given the contrasting evidence regarding remote monitoring and variable modalities of implementing interventions, we investigated whether HT monitoring yields improvements in GDMT optimization. We found that HT nurses were able to nearly double the rate of interventions for patients with HFrEF. The HT program in providing expanded services will require adequate staffing responsibilities and support. The HT program is geared toward following a large, diverse patient population, such as those with chronic obstructive pulmonary disease, hypertension, and HF. We only evaluated services for patients with HFrEF, but the program also follows patients with HfmrEF and HfpEF. These patients were not included as GDMT optimization differs for patients with an LVEF > 40%.19,20
The lower rates of achieving target doses of GDMTs were likely obstructed by continuous use of initial drug doses and further limited by lack of follow-up. When compared with the rest of the VAAAHS, there was a greater effort to increase ARNi use in the HT group as 7 of 33 patients (21%) were started on ARNi compared with a background increase of ARNi use of 17%. There was a lower mortality rate observed in the HT group compared with the control group. One patient in each group died of unrelated causes, while 2 deaths in the control group were due to worsening HF. The difference in mortality is likely multifactorial, possibly related to the control group’s greater disease burden or higher mean age (75.2 years vs 72.6 years).
Limitations
This was an observational cohort design, which is subject to bias. Thus, the findings of this study are entirely hypothesis-generating and a randomized controlled trial would be necessary for clearer results. Second, low numbers of participants may have skewed the data set. Given the observational nature of the study, this nonetheless is a positive finding to support the HT program for assisting with HF monitoring and prompting drug interventions. Due to the low number of participants, a single patient may have skewed the results with 5 hospitalizations.
Conclusions
This pilot study demonstrates the applicability of HT monitoring to optimize veteran HFrEF GDMT. The HT program yielded numerically relevant interventions and fewer HF-related hospitalizations compared with the control arm. The study shows the feasibility of the program to safely optimize GDMT toward their target doses and may serve as an additional catalyst to further develop HT programs specifically targeted toward HF monitoring and management. Cost-savings analyses would likely need to demonstrate the cost utility of such a service.
We thank the home telehealth nursing staff for their assistance in data collection and enrollment of patients into the monitoring program.
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052
2. McCullough PA, Mehta HS, Barker CM, et al. Mortality and guideline-directed medical therapy in real-world heart failure patients with reduced ejection fraction. Clin Cardiol. 2021;44(9):1192-1198. doi:10.1002/clc.23664
3. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73-84. doi:10.1016/j.jchf.2021.09.004
4. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
5. Pierce JB, Vaduganathan M, Fonarow GC, et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: The Get With The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023;8(7):652-661. doi:10.1001/jamacardio.2023.1266
6. Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of quality of care for US veterans with recent-onset heart failure with reduced ejection fraction. JAMA Cardiol. 2022;7(2):130-139. doi:10.1001/jamacardio.2021.4585 7. Rahimi K, Nazarzadeh M, Pinho-Gomes AC, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106(20):1573-1578. doi:10.1136/heartjnl-2020-316773 8. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873-1880. doi:10.1161/CIRCULATIONAHA.111.018473
9. Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: results from the OptiLink HF study. Circ Arrhythm Electrophysiol. 2021;14(1):e008693. doi:10.1161/CIRCEP.120.008693
10. Krzesinski P, Jankowska EA, Siebert J, et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): a randomised controlled trial. Eur J Heart Fail. 2022;24(3):565-577. doi:10.1002/ejhf.2358
11. Ding H, Jayasena R, Chen SH, et al. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020;22(7):e17559. doi:10.2196/17559
12. Kitsiou S, Pare G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17(3):e63. doi:10.2196/jmir.4174
13. Artanian V, Ross HJ, Rac VE, O’Sullivan M, Brahmbhatt DH, Seto E. Impact of remote titration combined with telemonitoring on the optimization of guideline-directed medical therapy for patients with heart failure: internal pilot of a randomized controlled trial. JMIR Cardio. 2020;4(1):e21962. doi:10.2196/21962
14. Desai AS, Maclean T, Blood AJ, et al. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(12):1430-1434. doi:10.1001/jamacardio.2020.3757
15. Patil T, Ali S, Kaur A, et al. Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARM-HF). J Cardiovasc Transl Res. 2022;15(6):1424-1435. doi:10.1007/s12265-022-10262-9
16. Zheng J, Mednick T, Heidenreich PA, Sandhu AT. Pharmacist- and nurse-led medical optimization in heart failure: a systematic review and meta-analysis. J Card Fail. 2023;29(7):1000-1013. doi:10.1016/j.cardfail.2023.03.012
17. Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938-1952. doi:10.1016/S0140-6736(22)02076-1
18. O’Connor M, Asdornwised U, Dempsey ML, et al. Using telehealth to reduce all-cause 30-day hospital readmissions among heart failure patients receiving skilled home health services. Appl Clin Inform. 2016;7(2):238-47. doi:10.4338/ACI-2015-11-SOA-0157
19. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062
20. Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835-1878. doi:10.1016/j.jacc.2023.03.393
Heart failure (HF) is a chronic, progressive condition that is characterized by the heart’s inability to effectively pump blood throughout the body. In 2018, approximately 6.2 million US adults had HF, and 13.4% of all death certificates noted HF as a precipitating factor.1 Patients not receiving appropriate guideline-directed medical therapy (GDMT) face a 29% excess mortality risk over a 2-year period.2 Each additional GDMT included in a patient’s regimen significantly reduces all-cause mortality.3
The Change the Management of Patients with Heart Failure (CHAMP) registry reports that only about 1% of patients with HF are prescribed 3 agents from contemporary GDMT at target doses, highlighting the need for optimizing clinicians’ approaches to GDMT.4 Similarly, The Get With The Guidelines Heart Failure Registry has noted that only 20.2% of patients with HF with reduced ejection fraction (HFrEF) are prescribed a sodium-glucose cotransporter 2 inhibitor (SGLT2i) following hospital discharge for HFrEF exacerbation.5 Overall, treatment rates with GDMT saw limited improvement between 2013 and 2019, with no significant difference between groups in mortality, indicating the need for optimized methods to encourage the initiation of GDMT.6
Remote monitoring and telecare are novel ways to improve GDMT rates in those with HFrEF. However, data are inconsistent regarding the impact of remote HF monitoring and improvements in GDMT or HF-related outcomes.6-10 The modalities of remote monitoring for GDMT vary among studies, but the potential for telehealth monitoring to improve GDMT, thereby potentially reducing HF-related hospitalizations, is clear.
Telemonitoring has demonstrated improved participant adherence with weight monitoring, although the withdrawal rate was high, and has the potential to reduce all-cause mortality and HF-related hospitalizations.11,12 Telemonitoring for GDMT optimization led to an increase in the proportion of patients who achieved optimal GDMT doses, a decrease in the time to dose optimization, and a reduction in the number of clinic visits.13 Remote GDMT titration was accomplished in the general patient population with HFrEF; however, in populations already followed by cardiologists or HF specialists, remote optimization strategies did not yield different proportions of GDMT use.14 The aim of this study was to assess the impact of the home telehealth (HT) monitoring program on the initiation and optimization of HF GDMT among veterans with HFrEF at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan.
Methods
This was a single-center retrospective study of Computerized Patient Record System (CPRS)data. Patients at the VAAAHS were evaluated if they were diagnosed with HFrEF and were eligible for enrollment in the HT monitoring program. Eligibility criteria included a diagnosis of stage C HF, irrespective of EF, and a history of any HF-related hospitalization. We focused on patients with HFrEF due to stronger guideline-based recommendations for certain pharmacotherapies as compared with HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). Initial patient data for HT enrolling were accessed using the Heart Failure Dashboard via the US Department of Veterans Affairs (VA) Academic Detailing Service. The target daily doses of typical agents used in HFrEF GDMT are listed in the Appendix.
The HT program is an embedded model in which HT nurses receive remote data from the patient and triage that with the VAAAHS cardiology team. Patients’ questions, concerns, and/or vital signs are recovered remotely. In this model, nurses are embedded in the cardiology team, working with the cardiologists, cardiology clinical pharmacist, and/or cardiology nurse practitioners to make medication interventions. Data are recorded with an HT device, including weight, blood pressure (BP), heart rate, and pulse oximetry. HT nurses are also available to the patient via phone or video. The program uses a 180-day disease management protocol for HF via remote device, enabling the patient to answer questions and receive education on their disease daily. Responses to questions and data are then reviewed by an HT nurse remotely during business hours and triaged as appropriate with the cardiology team. Data can be communicated to the cardiology team via the patient record, eliminating the need for the cardiology team to use the proprietary portal affiliated with the HT device.
Study Sample
Patient information was obtained from a list of 417 patients eligible for enrollment in the HT program; the list was sent to the HT program for review and enrollment. Patient data were extracted from the VAAAHS HF Dashboard and included all patients with HFrEF and available data on the platform. The sample for the retrospective chart review included 40 adults who had HFrEF, defined as a left ventricular EF (LVEF) of ≤ 40% as evidenced by a transthoracic echocardiogram or cardiac magnetic resonance imaging. These patients were contacted and agreed to enroll in the HT monitoring program. The HT program population was compared against a control group of 33 patients who were ineligible for the HT program. Patients were deemed ineligible for HT if they resided in a nursing home, lacked a VAAAHS primary care clinician, or declined participation in the HT program.
Procedures
Patients who declined participation in the HT program followed the standard of care, which was limited to visits with primary care clinicians and/or cardiologists as per the follow-up plan. Patient data were collected over 12 months. The study was approved by the VAAAHS Institutional Review Board (reference number, 1703034), Research and Development Committee, and Research Administration.
Primary and Secondary Goals
The primary goal of the study was to assess the impact of the HT program on drug interventions, specifically initiating and titrating HFrEF pharmacotherapies. Interventions were based on GDMT with known mortality- and morbidity-reducing properties when used at their maximum tolerated doses, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor-neprilysin inhibitor (ARNi), or angiotensin receptor blockers (ARB), with a preference for ARNi, β-blockers for HFrEF (metoprolol succinate, bisoprolol, or carvedilol), aldosterone antagonists, and SGLT2is.
Secondary goals included HF-related hospitalizations, medication adherence, time to enrollment in HT, time to laboratory analysis after the initiation or titration of an ACEi/ARB/ARNi or aldosterone antagonist, and time enrolled in the HT program. Patients were considered adherent if their drug refill history showed consistent fills of their medications. The χ2 test was used for total interventions made during the study period and Fisher exact test for all others.
Results
Patient data were collected between July 2022 and June 2023. All 73 patients were male, and the mean age in the HT group (n = 40) was 72.6 years and 75.2 years for the control group (n = 33). Overall, the baseline demographics were similar between the groups (Table 1). Of 40 patients screened for enrollment in the HT program, 33 were included in the analysis (Figure 1).
At baseline, the HT group included more individuals than the control group on ACEi/ARB/ARNi (24 vs 19, respectively), β-blocker (28 vs 24, respectively), SGLT2i (14 vs 11, respectively), and aldosterone antagonist (15 vs 9, respectively) (Figure 2). There were 20 interventions made in the HT group compared with 11 therapy changes in the control arm during the study (odds ratio, 1.43; P = .23) (Table 2). In the HT group, 1 patient achieved an ACEi target dose, 3 patients achieved a β-blocker target dose, and 7 achieved a target dose of spironolactone (titration is not required for SGLT2i therapy and is counted as target dose). In the HT group, 17 patients were on ≥ 3 recommended agents, while 9 patients were taking 4 agents. Seven of 20 HT group interventions resulted in titration to the target dose. In the control group, no patients achieved an ARNi target dose, 3 patients achieved a β-blocker target dose, and 2 patients achieved a spironolactone antagonist target dose. In the control arm, 7 patients were on ≥ 3 GDMTs, and 2 were taking 4 agents. No patient in either group achieved a target dose of 4 agents. Five of 11 control group interventions resulted in initiation or titration of GDMT to the target dose.
Of the 40 HT group patients, 7 were excluded from analysis (3 failed to schedule HT, 1 was at a long-term care facility, 1 was nonadherent, 1 declined participation, and 1 died) and 33 remained in the program for a mean (SD) 5.3 (3.5) months. Death rates were tracked during the study: 1 patient died in the HT group and 3 in the control group.
We analyzed the overall percentage of VAAAHS patients with HFrEF who were on appropriate GDMT. Given the ongoing drive to improve HF-related outcomes, HT interventions could not be compared to a static population, so the HT and control patients were compared with the rates of GDMT at VAAAHS, which was available in the Academic Detailing Service Heart Failure Dashboard (Figure 3). Titration and optimization rates were also compared (Figure 4). From July 2022 to June 2023, ARNi use increased by 16.6%, aldosterone antagonist by 6.8%, and β-blockers by 2.4%. Target doses of GDMTs were more difficult to achieve in the hospital system. There was an increase in aldosterone antagonist target dose achievement by 4.7%, but overall there were decreases in target doses in other GDMTs: ACEi/ARB/ARNi target dose use decreased by 3.2%, ARNi target dose use decreased by 2.7% and target β-blocker use decreased by 0.9%.
Discussion
Telehealth yielded clinically important interventions, with some titrations bringing patients to their target doses of medications for HFrEF. The 20 interventions made in the HT group can be largely attributed to the nurses’ efforts to alert clinicians to drug titrations or ACEi/ARB to ARNi transitions. Although the findings were not statistically significant, the difference in the number of drug therapy changes supports the use of the HT program for a GDMT optimization strategy. Patients may be difficult to titrate secondary to adverse effects that make medication initiation or titration inappropriate, such as hypotension and hyperkalemia, although this was not observed in this small sample size. Considering a mean HT enrollment of 5.3 months, many patients had adequate disease assessment and medication titration. Given that patients are discharged from the service once deemed appropriate, this decreases the burden on the patient and increases the utility and implementation of the HT program for other patients.
A surprising finding of this study was the lower rate of HF-related hospitalizations in the HT group. Although not the primary subject of interest in the study, it reinforced the importance of close health care professional follow-up for patients living with HF. Telehealth may improve communication and shared decision making over medication use. Because the finding was unanticipated, the rate of diuretic adjustments was not tracked.
Patients were reevaluated every 6 months for willingness to continue the program, adherence, and clinical needs. These results are similar to those of other trials that demonstrated improved rates of GDMT in the setting of pharmacist- or nurse-led HF treatment optimization.15,16 These positive results differ from other trials incorporating remote monitoring regarding patient continuation in HT programs. For example, in a study by Ding and colleagues, the withdrawal rate from their monitoring service was about 22%, while in our study only 1 patient withdrew from the HT program.11
The HT program resulted in fewer hospitalizations than the control arm. There were 6 HF-related hospitalizations in the control group, although 5 involved a single patient. Typically, such a patient would be encouraged to follow HT monitoring after just 1 HF-related hospitalization; however, the patient declined to participate.
Early optimization of GDMT in patients who were recently discharged from the hospital for an HF-related hospitalization yields a reduction in hospital rehospitalization.17 GDMT optimization has unequivocal benefits in HF outcomes. Unfortunately, the issues surrounding methodologies on how to best optimize GDMT are lacking. While HT has been found to be feasible in the aid of optimizing medical therapy, the TIM-HF trial concluded that remote monitoring services had no significant benefit in reducing mortality.7,8 On the other hand, the OptiLink HF Study showed that when clinicians respond to remote monitoring prompts from fluid index threshold crossing alerts, these interventions are associated with significantly improved clinical outcomes in patients with implantable cardioverter-defibrillators and advanced HF.9 In contrast to previous trials, the AMULET trial showed that remote monitoring compared with standard care significantly reduced the risk of HF hospitalization or cardiovascular death during the 12-month follow-up among patients with HF and LVEF ≤ 49% after an episode of acute exacerbation.10 Additionally, patients who received skilled home health services and participated in remote monitoring for their chronic HF experienced a reduction in all-cause 30-day readmission.18
Given the contrasting evidence regarding remote monitoring and variable modalities of implementing interventions, we investigated whether HT monitoring yields improvements in GDMT optimization. We found that HT nurses were able to nearly double the rate of interventions for patients with HFrEF. The HT program in providing expanded services will require adequate staffing responsibilities and support. The HT program is geared toward following a large, diverse patient population, such as those with chronic obstructive pulmonary disease, hypertension, and HF. We only evaluated services for patients with HFrEF, but the program also follows patients with HfmrEF and HfpEF. These patients were not included as GDMT optimization differs for patients with an LVEF > 40%.19,20
The lower rates of achieving target doses of GDMTs were likely obstructed by continuous use of initial drug doses and further limited by lack of follow-up. When compared with the rest of the VAAAHS, there was a greater effort to increase ARNi use in the HT group as 7 of 33 patients (21%) were started on ARNi compared with a background increase of ARNi use of 17%. There was a lower mortality rate observed in the HT group compared with the control group. One patient in each group died of unrelated causes, while 2 deaths in the control group were due to worsening HF. The difference in mortality is likely multifactorial, possibly related to the control group’s greater disease burden or higher mean age (75.2 years vs 72.6 years).
Limitations
This was an observational cohort design, which is subject to bias. Thus, the findings of this study are entirely hypothesis-generating and a randomized controlled trial would be necessary for clearer results. Second, low numbers of participants may have skewed the data set. Given the observational nature of the study, this nonetheless is a positive finding to support the HT program for assisting with HF monitoring and prompting drug interventions. Due to the low number of participants, a single patient may have skewed the results with 5 hospitalizations.
Conclusions
This pilot study demonstrates the applicability of HT monitoring to optimize veteran HFrEF GDMT. The HT program yielded numerically relevant interventions and fewer HF-related hospitalizations compared with the control arm. The study shows the feasibility of the program to safely optimize GDMT toward their target doses and may serve as an additional catalyst to further develop HT programs specifically targeted toward HF monitoring and management. Cost-savings analyses would likely need to demonstrate the cost utility of such a service.
We thank the home telehealth nursing staff for their assistance in data collection and enrollment of patients into the monitoring program.
Heart failure (HF) is a chronic, progressive condition that is characterized by the heart’s inability to effectively pump blood throughout the body. In 2018, approximately 6.2 million US adults had HF, and 13.4% of all death certificates noted HF as a precipitating factor.1 Patients not receiving appropriate guideline-directed medical therapy (GDMT) face a 29% excess mortality risk over a 2-year period.2 Each additional GDMT included in a patient’s regimen significantly reduces all-cause mortality.3
The Change the Management of Patients with Heart Failure (CHAMP) registry reports that only about 1% of patients with HF are prescribed 3 agents from contemporary GDMT at target doses, highlighting the need for optimizing clinicians’ approaches to GDMT.4 Similarly, The Get With The Guidelines Heart Failure Registry has noted that only 20.2% of patients with HF with reduced ejection fraction (HFrEF) are prescribed a sodium-glucose cotransporter 2 inhibitor (SGLT2i) following hospital discharge for HFrEF exacerbation.5 Overall, treatment rates with GDMT saw limited improvement between 2013 and 2019, with no significant difference between groups in mortality, indicating the need for optimized methods to encourage the initiation of GDMT.6
Remote monitoring and telecare are novel ways to improve GDMT rates in those with HFrEF. However, data are inconsistent regarding the impact of remote HF monitoring and improvements in GDMT or HF-related outcomes.6-10 The modalities of remote monitoring for GDMT vary among studies, but the potential for telehealth monitoring to improve GDMT, thereby potentially reducing HF-related hospitalizations, is clear.
Telemonitoring has demonstrated improved participant adherence with weight monitoring, although the withdrawal rate was high, and has the potential to reduce all-cause mortality and HF-related hospitalizations.11,12 Telemonitoring for GDMT optimization led to an increase in the proportion of patients who achieved optimal GDMT doses, a decrease in the time to dose optimization, and a reduction in the number of clinic visits.13 Remote GDMT titration was accomplished in the general patient population with HFrEF; however, in populations already followed by cardiologists or HF specialists, remote optimization strategies did not yield different proportions of GDMT use.14 The aim of this study was to assess the impact of the home telehealth (HT) monitoring program on the initiation and optimization of HF GDMT among veterans with HFrEF at the Veterans Affairs Ann Arbor Healthcare System (VAAAHS) in Michigan.
Methods
This was a single-center retrospective study of Computerized Patient Record System (CPRS)data. Patients at the VAAAHS were evaluated if they were diagnosed with HFrEF and were eligible for enrollment in the HT monitoring program. Eligibility criteria included a diagnosis of stage C HF, irrespective of EF, and a history of any HF-related hospitalization. We focused on patients with HFrEF due to stronger guideline-based recommendations for certain pharmacotherapies as compared with HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF). Initial patient data for HT enrolling were accessed using the Heart Failure Dashboard via the US Department of Veterans Affairs (VA) Academic Detailing Service. The target daily doses of typical agents used in HFrEF GDMT are listed in the Appendix.
The HT program is an embedded model in which HT nurses receive remote data from the patient and triage that with the VAAAHS cardiology team. Patients’ questions, concerns, and/or vital signs are recovered remotely. In this model, nurses are embedded in the cardiology team, working with the cardiologists, cardiology clinical pharmacist, and/or cardiology nurse practitioners to make medication interventions. Data are recorded with an HT device, including weight, blood pressure (BP), heart rate, and pulse oximetry. HT nurses are also available to the patient via phone or video. The program uses a 180-day disease management protocol for HF via remote device, enabling the patient to answer questions and receive education on their disease daily. Responses to questions and data are then reviewed by an HT nurse remotely during business hours and triaged as appropriate with the cardiology team. Data can be communicated to the cardiology team via the patient record, eliminating the need for the cardiology team to use the proprietary portal affiliated with the HT device.
Study Sample
Patient information was obtained from a list of 417 patients eligible for enrollment in the HT program; the list was sent to the HT program for review and enrollment. Patient data were extracted from the VAAAHS HF Dashboard and included all patients with HFrEF and available data on the platform. The sample for the retrospective chart review included 40 adults who had HFrEF, defined as a left ventricular EF (LVEF) of ≤ 40% as evidenced by a transthoracic echocardiogram or cardiac magnetic resonance imaging. These patients were contacted and agreed to enroll in the HT monitoring program. The HT program population was compared against a control group of 33 patients who were ineligible for the HT program. Patients were deemed ineligible for HT if they resided in a nursing home, lacked a VAAAHS primary care clinician, or declined participation in the HT program.
Procedures
Patients who declined participation in the HT program followed the standard of care, which was limited to visits with primary care clinicians and/or cardiologists as per the follow-up plan. Patient data were collected over 12 months. The study was approved by the VAAAHS Institutional Review Board (reference number, 1703034), Research and Development Committee, and Research Administration.
Primary and Secondary Goals
The primary goal of the study was to assess the impact of the HT program on drug interventions, specifically initiating and titrating HFrEF pharmacotherapies. Interventions were based on GDMT with known mortality- and morbidity-reducing properties when used at their maximum tolerated doses, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor-neprilysin inhibitor (ARNi), or angiotensin receptor blockers (ARB), with a preference for ARNi, β-blockers for HFrEF (metoprolol succinate, bisoprolol, or carvedilol), aldosterone antagonists, and SGLT2is.
Secondary goals included HF-related hospitalizations, medication adherence, time to enrollment in HT, time to laboratory analysis after the initiation or titration of an ACEi/ARB/ARNi or aldosterone antagonist, and time enrolled in the HT program. Patients were considered adherent if their drug refill history showed consistent fills of their medications. The χ2 test was used for total interventions made during the study period and Fisher exact test for all others.
Results
Patient data were collected between July 2022 and June 2023. All 73 patients were male, and the mean age in the HT group (n = 40) was 72.6 years and 75.2 years for the control group (n = 33). Overall, the baseline demographics were similar between the groups (Table 1). Of 40 patients screened for enrollment in the HT program, 33 were included in the analysis (Figure 1).
At baseline, the HT group included more individuals than the control group on ACEi/ARB/ARNi (24 vs 19, respectively), β-blocker (28 vs 24, respectively), SGLT2i (14 vs 11, respectively), and aldosterone antagonist (15 vs 9, respectively) (Figure 2). There were 20 interventions made in the HT group compared with 11 therapy changes in the control arm during the study (odds ratio, 1.43; P = .23) (Table 2). In the HT group, 1 patient achieved an ACEi target dose, 3 patients achieved a β-blocker target dose, and 7 achieved a target dose of spironolactone (titration is not required for SGLT2i therapy and is counted as target dose). In the HT group, 17 patients were on ≥ 3 recommended agents, while 9 patients were taking 4 agents. Seven of 20 HT group interventions resulted in titration to the target dose. In the control group, no patients achieved an ARNi target dose, 3 patients achieved a β-blocker target dose, and 2 patients achieved a spironolactone antagonist target dose. In the control arm, 7 patients were on ≥ 3 GDMTs, and 2 were taking 4 agents. No patient in either group achieved a target dose of 4 agents. Five of 11 control group interventions resulted in initiation or titration of GDMT to the target dose.
Of the 40 HT group patients, 7 were excluded from analysis (3 failed to schedule HT, 1 was at a long-term care facility, 1 was nonadherent, 1 declined participation, and 1 died) and 33 remained in the program for a mean (SD) 5.3 (3.5) months. Death rates were tracked during the study: 1 patient died in the HT group and 3 in the control group.
We analyzed the overall percentage of VAAAHS patients with HFrEF who were on appropriate GDMT. Given the ongoing drive to improve HF-related outcomes, HT interventions could not be compared to a static population, so the HT and control patients were compared with the rates of GDMT at VAAAHS, which was available in the Academic Detailing Service Heart Failure Dashboard (Figure 3). Titration and optimization rates were also compared (Figure 4). From July 2022 to June 2023, ARNi use increased by 16.6%, aldosterone antagonist by 6.8%, and β-blockers by 2.4%. Target doses of GDMTs were more difficult to achieve in the hospital system. There was an increase in aldosterone antagonist target dose achievement by 4.7%, but overall there were decreases in target doses in other GDMTs: ACEi/ARB/ARNi target dose use decreased by 3.2%, ARNi target dose use decreased by 2.7% and target β-blocker use decreased by 0.9%.
Discussion
Telehealth yielded clinically important interventions, with some titrations bringing patients to their target doses of medications for HFrEF. The 20 interventions made in the HT group can be largely attributed to the nurses’ efforts to alert clinicians to drug titrations or ACEi/ARB to ARNi transitions. Although the findings were not statistically significant, the difference in the number of drug therapy changes supports the use of the HT program for a GDMT optimization strategy. Patients may be difficult to titrate secondary to adverse effects that make medication initiation or titration inappropriate, such as hypotension and hyperkalemia, although this was not observed in this small sample size. Considering a mean HT enrollment of 5.3 months, many patients had adequate disease assessment and medication titration. Given that patients are discharged from the service once deemed appropriate, this decreases the burden on the patient and increases the utility and implementation of the HT program for other patients.
A surprising finding of this study was the lower rate of HF-related hospitalizations in the HT group. Although not the primary subject of interest in the study, it reinforced the importance of close health care professional follow-up for patients living with HF. Telehealth may improve communication and shared decision making over medication use. Because the finding was unanticipated, the rate of diuretic adjustments was not tracked.
Patients were reevaluated every 6 months for willingness to continue the program, adherence, and clinical needs. These results are similar to those of other trials that demonstrated improved rates of GDMT in the setting of pharmacist- or nurse-led HF treatment optimization.15,16 These positive results differ from other trials incorporating remote monitoring regarding patient continuation in HT programs. For example, in a study by Ding and colleagues, the withdrawal rate from their monitoring service was about 22%, while in our study only 1 patient withdrew from the HT program.11
The HT program resulted in fewer hospitalizations than the control arm. There were 6 HF-related hospitalizations in the control group, although 5 involved a single patient. Typically, such a patient would be encouraged to follow HT monitoring after just 1 HF-related hospitalization; however, the patient declined to participate.
Early optimization of GDMT in patients who were recently discharged from the hospital for an HF-related hospitalization yields a reduction in hospital rehospitalization.17 GDMT optimization has unequivocal benefits in HF outcomes. Unfortunately, the issues surrounding methodologies on how to best optimize GDMT are lacking. While HT has been found to be feasible in the aid of optimizing medical therapy, the TIM-HF trial concluded that remote monitoring services had no significant benefit in reducing mortality.7,8 On the other hand, the OptiLink HF Study showed that when clinicians respond to remote monitoring prompts from fluid index threshold crossing alerts, these interventions are associated with significantly improved clinical outcomes in patients with implantable cardioverter-defibrillators and advanced HF.9 In contrast to previous trials, the AMULET trial showed that remote monitoring compared with standard care significantly reduced the risk of HF hospitalization or cardiovascular death during the 12-month follow-up among patients with HF and LVEF ≤ 49% after an episode of acute exacerbation.10 Additionally, patients who received skilled home health services and participated in remote monitoring for their chronic HF experienced a reduction in all-cause 30-day readmission.18
Given the contrasting evidence regarding remote monitoring and variable modalities of implementing interventions, we investigated whether HT monitoring yields improvements in GDMT optimization. We found that HT nurses were able to nearly double the rate of interventions for patients with HFrEF. The HT program in providing expanded services will require adequate staffing responsibilities and support. The HT program is geared toward following a large, diverse patient population, such as those with chronic obstructive pulmonary disease, hypertension, and HF. We only evaluated services for patients with HFrEF, but the program also follows patients with HfmrEF and HfpEF. These patients were not included as GDMT optimization differs for patients with an LVEF > 40%.19,20
The lower rates of achieving target doses of GDMTs were likely obstructed by continuous use of initial drug doses and further limited by lack of follow-up. When compared with the rest of the VAAAHS, there was a greater effort to increase ARNi use in the HT group as 7 of 33 patients (21%) were started on ARNi compared with a background increase of ARNi use of 17%. There was a lower mortality rate observed in the HT group compared with the control group. One patient in each group died of unrelated causes, while 2 deaths in the control group were due to worsening HF. The difference in mortality is likely multifactorial, possibly related to the control group’s greater disease burden or higher mean age (75.2 years vs 72.6 years).
Limitations
This was an observational cohort design, which is subject to bias. Thus, the findings of this study are entirely hypothesis-generating and a randomized controlled trial would be necessary for clearer results. Second, low numbers of participants may have skewed the data set. Given the observational nature of the study, this nonetheless is a positive finding to support the HT program for assisting with HF monitoring and prompting drug interventions. Due to the low number of participants, a single patient may have skewed the results with 5 hospitalizations.
Conclusions
This pilot study demonstrates the applicability of HT monitoring to optimize veteran HFrEF GDMT. The HT program yielded numerically relevant interventions and fewer HF-related hospitalizations compared with the control arm. The study shows the feasibility of the program to safely optimize GDMT toward their target doses and may serve as an additional catalyst to further develop HT programs specifically targeted toward HF monitoring and management. Cost-savings analyses would likely need to demonstrate the cost utility of such a service.
We thank the home telehealth nursing staff for their assistance in data collection and enrollment of patients into the monitoring program.
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052
2. McCullough PA, Mehta HS, Barker CM, et al. Mortality and guideline-directed medical therapy in real-world heart failure patients with reduced ejection fraction. Clin Cardiol. 2021;44(9):1192-1198. doi:10.1002/clc.23664
3. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73-84. doi:10.1016/j.jchf.2021.09.004
4. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
5. Pierce JB, Vaduganathan M, Fonarow GC, et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: The Get With The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023;8(7):652-661. doi:10.1001/jamacardio.2023.1266
6. Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of quality of care for US veterans with recent-onset heart failure with reduced ejection fraction. JAMA Cardiol. 2022;7(2):130-139. doi:10.1001/jamacardio.2021.4585 7. Rahimi K, Nazarzadeh M, Pinho-Gomes AC, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106(20):1573-1578. doi:10.1136/heartjnl-2020-316773 8. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873-1880. doi:10.1161/CIRCULATIONAHA.111.018473
9. Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: results from the OptiLink HF study. Circ Arrhythm Electrophysiol. 2021;14(1):e008693. doi:10.1161/CIRCEP.120.008693
10. Krzesinski P, Jankowska EA, Siebert J, et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): a randomised controlled trial. Eur J Heart Fail. 2022;24(3):565-577. doi:10.1002/ejhf.2358
11. Ding H, Jayasena R, Chen SH, et al. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020;22(7):e17559. doi:10.2196/17559
12. Kitsiou S, Pare G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17(3):e63. doi:10.2196/jmir.4174
13. Artanian V, Ross HJ, Rac VE, O’Sullivan M, Brahmbhatt DH, Seto E. Impact of remote titration combined with telemonitoring on the optimization of guideline-directed medical therapy for patients with heart failure: internal pilot of a randomized controlled trial. JMIR Cardio. 2020;4(1):e21962. doi:10.2196/21962
14. Desai AS, Maclean T, Blood AJ, et al. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(12):1430-1434. doi:10.1001/jamacardio.2020.3757
15. Patil T, Ali S, Kaur A, et al. Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARM-HF). J Cardiovasc Transl Res. 2022;15(6):1424-1435. doi:10.1007/s12265-022-10262-9
16. Zheng J, Mednick T, Heidenreich PA, Sandhu AT. Pharmacist- and nurse-led medical optimization in heart failure: a systematic review and meta-analysis. J Card Fail. 2023;29(7):1000-1013. doi:10.1016/j.cardfail.2023.03.012
17. Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938-1952. doi:10.1016/S0140-6736(22)02076-1
18. O’Connor M, Asdornwised U, Dempsey ML, et al. Using telehealth to reduce all-cause 30-day hospital readmissions among heart failure patients receiving skilled home health services. Appl Clin Inform. 2016;7(2):238-47. doi:10.4338/ACI-2015-11-SOA-0157
19. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062
20. Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835-1878. doi:10.1016/j.jacc.2023.03.393
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi:10.1161/CIR.0000000000001052
2. McCullough PA, Mehta HS, Barker CM, et al. Mortality and guideline-directed medical therapy in real-world heart failure patients with reduced ejection fraction. Clin Cardiol. 2021;44(9):1192-1198. doi:10.1002/clc.23664
3. Tromp J, Ouwerkerk W, van Veldhuisen DJ, et al. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10(2):73-84. doi:10.1016/j.jchf.2021.09.004
4. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol. 2018;72(4):351-366. doi:10.1016/j.jacc.2018.04.070
5. Pierce JB, Vaduganathan M, Fonarow GC, et al. Contemporary use of sodium-glucose cotransporter-2 inhibitor therapy among patients hospitalized for heart failure with reduced ejection fraction in the US: The Get With The Guidelines-Heart Failure Registry. JAMA Cardiol. 2023;8(7):652-661. doi:10.1001/jamacardio.2023.1266
6. Sandhu AT, Kohsaka S, Turakhia MP, Lewis EF, Heidenreich PA. Evaluation of quality of care for US veterans with recent-onset heart failure with reduced ejection fraction. JAMA Cardiol. 2022;7(2):130-139. doi:10.1001/jamacardio.2021.4585 7. Rahimi K, Nazarzadeh M, Pinho-Gomes AC, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. 2020;106(20):1573-1578. doi:10.1136/heartjnl-2020-316773 8. Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123(17):1873-1880. doi:10.1161/CIRCULATIONAHA.111.018473
9. Wintrich J, Pavlicek V, Brachmann J, et al. Remote monitoring with appropriate reaction to alerts was associated with improved outcomes in chronic heart failure: results from the OptiLink HF study. Circ Arrhythm Electrophysiol. 2021;14(1):e008693. doi:10.1161/CIRCEP.120.008693
10. Krzesinski P, Jankowska EA, Siebert J, et al. Effects of an outpatient intervention comprising nurse-led non-invasive assessments, telemedicine support and remote cardiologists’ decisions in patients with heart failure (AMULET study): a randomised controlled trial. Eur J Heart Fail. 2022;24(3):565-577. doi:10.1002/ejhf.2358
11. Ding H, Jayasena R, Chen SH, et al. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: randomized controlled trial. J Med Internet Res. 2020;22(7):e17559. doi:10.2196/17559
12. Kitsiou S, Pare G, Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. J Med Internet Res. 2015;17(3):e63. doi:10.2196/jmir.4174
13. Artanian V, Ross HJ, Rac VE, O’Sullivan M, Brahmbhatt DH, Seto E. Impact of remote titration combined with telemonitoring on the optimization of guideline-directed medical therapy for patients with heart failure: internal pilot of a randomized controlled trial. JMIR Cardio. 2020;4(1):e21962. doi:10.2196/21962
14. Desai AS, Maclean T, Blood AJ, et al. Remote optimization of guideline-directed medical therapy in patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5(12):1430-1434. doi:10.1001/jamacardio.2020.3757
15. Patil T, Ali S, Kaur A, et al. Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARM-HF). J Cardiovasc Transl Res. 2022;15(6):1424-1435. doi:10.1007/s12265-022-10262-9
16. Zheng J, Mednick T, Heidenreich PA, Sandhu AT. Pharmacist- and nurse-led medical optimization in heart failure: a systematic review and meta-analysis. J Card Fail. 2023;29(7):1000-1013. doi:10.1016/j.cardfail.2023.03.012
17. Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400(10367):1938-1952. doi:10.1016/S0140-6736(22)02076-1
18. O’Connor M, Asdornwised U, Dempsey ML, et al. Using telehealth to reduce all-cause 30-day hospital readmissions among heart failure patients receiving skilled home health services. Appl Clin Inform. 2016;7(2):238-47. doi:10.4338/ACI-2015-11-SOA-0157
19. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876-e894. doi:10.1161/CIR.0000000000001062
20. Kittleson MM, Panjrath GS, Amancherla K, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835-1878. doi:10.1016/j.jacc.2023.03.393