User login
Effect of Multidisciplinary Transitional Pain Service on Health Care Use and Costs Following Orthopedic Surgery
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
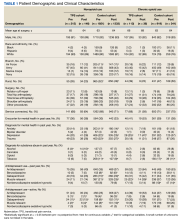
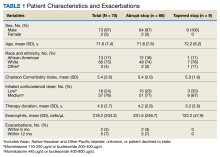
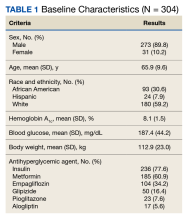
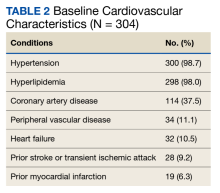
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
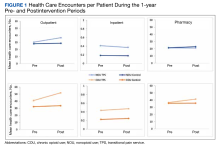
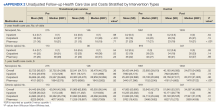
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
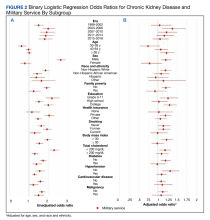
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
Opioid use disorder (OUD) is a significant cause of morbidity, mortality, and health care costs in the US.1,2 Surgery can be the inciting cause for exposure to an opioid; as many as 23% of patients develop chronic OUD following surgery.3,4 Patients with a history of substance use, mood disorders, anxiety, or previous chronic opioid use (COU) are at risk for relapse, dose escalation, and poor pain control after high-risk surgery, such as orthopedic joint procedures.5 Recently focus has been on identifying high-risk patients before orthopedic joint surgery and implementing evidence-based strategies that reduce the postoperative incidence of COU.
A transitional pain service (TPS) has been shown to reduce COU for high-risk surgical patients in different health care settings.6-9 The TPS model bundles multiple interventions that can be applied to patients at high risk for COU within a health care system. This includes individually tailored programs for preoperative education or pain management planning, use of multimodal analgesia (including regional or neuraxial techniques or nonopioid systemic medications), application of nonpharmacologic modalities (such as cognitive-based intervention), and a coordinated approach to postdischarge instructions and transitions of care. These interventions are coordinated by a multidisciplinary clinical service consisting of anesthesiologists and advanced practice clinicians with specialization in acute pain management and opioid tapering, nurse care coordinators, and psychologists with expertise in cognitive behavioral therapy.
TPS has been shown to reduce the incidence of COU for patients undergoing orthopedic joint surgery, but its impact on health care use and costs is unknown.6-9 The TPS intervention is resource intensive and increases the use of health care for preoperative education or pain management, which may increase the burden of costs. However, reducing long-term COU may reduce the use of health care for COU- and OUD-related complications, leading to cost savings. This study evaluated whether the TPS intervention influenced health care use and cost for inpatient, outpatient, or pharmacy services during the year following orthopedic joint surgery compared with that of the standard pain management care for procedures that place patients at high risk for COU. We used a difference-in-differences (DID) analysis to estimate this intervention effect, using multivariable regression models that controlled for unobserved time trends and cohort characteristics.
METHODS
This was a quasi-experimental study of patients who underwent orthopedic joint surgery and associated procedures at high risk for COU at the Veterans Affairs Salt Lake City Healthcare System (VASLCHS) between January 2016 through April 2020. The pre-TPS period between January 2016 through December 2017 was compared with the post-TPS period between January 2018 to September 2019. The control patient cohort was selected from 5 geographically diverse VA health care systems throughout the US: Eastern Colorado, Central Plains (Nebraska), White River Junction (Vermont), North Florida/South Georgia, and Portland (Oregon). By sampling health care costs from VA medical centers (VAMCs) across these different regions, our control group was generalizable to veterans receiving orthopedic joint surgery across the US. This study used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a repository of nearly all clinical and administrative data found in electronic health records for VA-provided care and fee-basis care paid for by the VA.10 All data were hosted and analyzed in the VA Informatics and Computing Infrastructure (VINCI) workspace. The University of Utah Institutional Review Board and the VASLCHS Office of Research and Development approved the protocol for this study.
TPS Intervention
The VASLCHS TPS has already been described in detail elsewhere.6,7 Briefly, patients at high risk for COU at the VASLCHS were enrolled in the TPS program before surgery for total knee, hip, or shoulder arthroplasty or rotator cuff procedures. The TPS service consists of an anesthesiologist and advanced practice clinician with specialization in acute pain management and opioid tapering, a psychologist with expertise in cognitive behavioral therapy, and 3 nurse care coordinators. These TPS practitioners work together to provide preoperative education, including setting expectations regarding postoperative pain, recommending nonopioid pain management strategies, and providing guidance regarding the appropriate use of opioids for surgical pain. Individual pain plans were developed and implemented for the perioperative period. After surgery, the TPS provided recommendations and support for nonopioid pain therapies and opioid tapers. Patients were followed by the TPS team for at least 12 months after surgery. At a minimum, the goals set by TPS included cessation of all opioid use for prior nonopioid users (NOU) by 90 days after surgery and the return to baseline opioid use or lower for prior COU patients by 90 days after surgery. The TPS also encouraged and supported opioid tapering among COU patients to reduce or completely stop opioid use after surgery.
Patient Cohorts
Veterans having primary or revision total knee, hip, or shoulder arthroplasty or rotator cuff repair between January 1, 2016, and September 30, 2019, at the aforementioned VAMCs were included in the study. Patients who had any hospitalization within 90 days pre- or postindex surgery or who died within 8 months after surgery were excluded from analysis. Patients who had multiple surgeries during the index inpatient visit or within 90 days after the index surgery also were excluded. Comorbid conditions for mental health and substance use were identified using the International Classification of Diseases, 10th revision Clinical Modification (ICD-10) codes or 9th revision equivalent grouped by Clinical Classifications Software Refined (CCS-R).11 Preoperative exposure to clinically relevant pharmacotherapy (ie, agents associated with prolonged opioid use and nonopioid adjuvants) was captured using VA outpatient prescription records (eAppendix 1).
Outcome Variables
Outcome variables included health care use and costs during 1-year pre- and postperiods from the date of surgery. VA health care costs for outpatient, inpatient, and pharmacy services for direct patient care were collected from the Managerial Cost Accounting System, an activity-based cost allocation system that generates estimates of the cost of individual VA hospital stays, health care encounters, and medications. Health care use was defined as the number of encounters for each visit type in the Managerial Cost Accounting System. All costs were adjusted to 2019 US dollars, using the Personal Consumption Expenditures price index for health care services.15
A set of sociodemographic variables including sex, age at surgery, race and ethnicity, rurality, military branch (Army, Air Force, Marine Corps, Navy, and other), and service connectivity were included as covariates in our regression models.
Statistical Analyses
Descriptive analyses were used to evaluate differences in baseline patient sociodemographic and clinical characteristics between pre- and postperiods for TPS intervention and control cohorts using 2-sample t tests for continuous variables and χ2 tests for categorical variables. We summarized unadjusted health care use and costs for outpatient, inpatient, and pharmacy visits and compared the pre- and postintervention periods using the Mann-Whitney test. Both mean (SD) and median (IQR) were considered, reflecting the skewed distribution of the outcome variables.
We used a DID approach to assess the intervention effect while minimizing confounding from the nonrandom sample. The DID approach controls for unobserved differences between VAMCs that are related to both the intervention and outcomes while controlling for trends over time that could affect outcomes across clinics. To implement the DID approach, we included 3 key independent variables in our regression models: (1) an indicator for whether the observation occurred in the postintervention period; (2) an indicator for whether the patient was exposed to the TPS intervention; and (3) the interaction between these 2 variables.
For cost outcomes, we used multivariable generalized linear models with a log link and a Poisson or Υ family. We analyzed inpatient costs using a 2-part generalized linear model because only 17% to 20% of patients had ≥ 1 inpatient visit. We used multivariable negative binomial regression for health care use outcomes. Demographic and clinical covariates described earlier were included in the regression models to control for differences in the composition of patient groups and clinics that could lead to confounding bias.
RESULTS
Of the 4954 patients included in our study cohort, 3545 (71.6%) were in the NOU group and 1409 (28.4%) were in the COU group. Among the NOU cohort, 361 patients were in the intervention group and 3184 in the control group. Among the COU cohort, 149 patients were in the intervention group and 1260 in the control group (Table 1). Most patients were male, White race, with a mean (SD) age of 64 (11) years. The most common orthopedic procedure was total knee arthroplasty, followed by total hip arthroplasty. Among both NOU and COU cohorts, patients’ characteristics were similar between the pre- and postintervention period among either TPS or control cohort.
Figures 1 and 2 and eAppendix 2 depict unadjusted per-person average outpatient, inpatient, and pharmacy visits and costs incurred during the 1-year pre- and postintervention periods for the NOU and COU cohorts. Average total health care follow-up costs ranged from $40,000 to $53,000 for NOU and from $47,000 to $82,000 for COU cohort. Cost for outpatient visits accounted for about 70% of the average total costs, followed by costs for inpatient visits of about 20%, and costs for pharmacy for the remaining.
For the NOU cohort, the number of health care encounters remained fairly stable between periods except for the outpatient visits among the TPS group. The TPS group experienced an increase in mean outpatient visits in the postperiod: 30 vs 37 visits (23%) (
Table 2 summarizes the results from the multivariable DID analyses for the outpatient, inpatient, and pharmacy visit and cost outcomes. Here, the estimated effect of the TPS intervention is the coefficient from the interaction between the postintervention and TPS exposure indicator variables. This coefficient was calculated as the difference in the outcome before and after the TPS intervention among the TPS group minus the difference in the outcome before and after the TPS intervention among the control group. For the NOU cohort, TPS was associated with an increase in the use of outpatient health care (mean [SD] increase of 6.9 [2] visits; P < .001) after the surgery with no statistically significant effect on outpatient costs (mean [SD] increase of $2787 [$3749]; P = .55). There was no statistically significant effect of TPS on the use of inpatient visits or pharmacy, but a decrease in costs for inpatient visits among those who had at least 1 inpatient visit (mean [SD] decrease of $12,170 [$6100]; P = .02). For the COU cohort, TPS had no statistically significant impact on the use of outpatient, inpatient, or pharmacy or the corresponding costs.
DISCUSSION
TPS is a multidisciplinary approach to perioperative pain management that has been shown to reduce both the quantity and duration of opioid use among orthopedic surgery patients.6,7 Although the cost burden of providing TPS services to prevent COU is borne by the individual health care system, it is unclear whether this expense is offset by lower long-term medical costs and health care use for COU- and OUD-related complications. In this study focused on a veteran population undergoing orthopedic joint procedures, a DID analysis of cost and health care use showed that TPS, which has been shown to reduce COU for high-risk surgical patients, can be implemented without increasing the overall costs to the VA health care system during the 1 year following surgery, even with increased outpatient visits. For NOU patients, there was no difference in outpatient visit costs or pharmacy costs over 12 months after surgery, although there was a significant reduction in subsequent inpatient costs over the same period. Further, there was no difference in outpatient, inpatient, or pharmacy costs after surgery for COU patients. These findings suggest that TPS can be a cost-effective approach to reduce opioid use among patients undergoing orthopedic joint surgery in VAMCs.
The costs of managing COU after surgery are substantial. Prior reports have shown that adjusted total health care costs are 1.6 to 2.5 times higher for previously NOU patients with new COU after major surgery than those for such patients without persistent use.16 The 1-year costs associated with new COU in this prior study ranged between $7944 and $17,702 after inpatient surgery and between $5598 and $12,834 after outpatient index surgery, depending on the payer, which are in line with the cost differences found in our current study. Another report among patients with COU following orthopedic joint replacement showed that they had higher use of inpatient, emergency department, and ambulance/paramedic services in the 12 months following their surgery than did those without persistent use.17 Although these results highlight the impact that COU plays in driving increased costs after major surgery, there have been limited studies focused on interventions that can neutralize the costs associated with opioid misuse after surgery. To our knowledge, our study is the first analysis to show the impact of using an intervention such as TPS to reduce postoperative opioid use on health care use and cost.
Although a rigorous and comprehensive return on investment analysis was beyond the scope of this analysis, these results may have several implications for other health care systems and hospitals that wish to invest in a multidisciplinary perioperative pain management program such as TPS but may be reluctant due to the upfront investment. First, the increased number of patient follow-up visits needed during TPS seems to be more than offset by the reduction in opioid use and associated complications that may occur after surgery. Second, TPS did not seem to be associated with an increase in overall health care costs during the 1-year follow-up period. Together, these results indicate that the return on investment for a TPS approach to perioperative pain management in which optimal patient-centered outcomes are achieved without increasing long-term costs to a health care system may be positive.
Limitations
This study has several limitations. First, this was a quasi-experimental observational study, and the associations we identified between intervention and outcomes should not be assumed to demonstrate causality. Although our DID analysis controlled for an array of demographic and clinical characteristics, differences in medical costs and health care use between the 2 cohorts might be driven by unobserved confounding variables.
Our study also was limited to veterans who received medical care at the VA, and results may not be generalizable to other non-VA health care systems or to veterans with Medicare insurance who have dual benefits. While our finding on health care use and costs may be incomplete because of the uncaptured health care use outside the VA, our DID analysis helped reduce unobserved bias because the absence of data outside of VA care applies to both TPS and control groups. Further, the total costs of operating a TPS program at any given institution will depend on the size of the hospital and volume of surgical patients who meet criteria for enrollment. However, the relative differences in health care use and costs may be extrapolated to patients undergoing orthopedic surgery in other types of academic and community-based health care systems.
Furthermore, this analysis focused primarily on COU and NOU patients undergoing orthopedic joint surgery. While this represents a high-risk population for OUD, the costs and health care use associated with delivering the TPS intervention to other types of surgical procedures may be significantly different. All costs in this analysis were based on 2019 estimates and do not account for the potential inflation over the past several years. Nonmonetary costs to the patient and per-person average total intervention costs were not included in the study. However, we assumed that costs associated with TPS and standard of care would have increased to an equivalent degree over the same period. Further, the average cost of TPS per patient (approximately $900) is relatively small compared with the average annual costs during 1-year pre- and postoperative periods and was not expected to have a significant effect on the analysis.
Conclusions
We found that the significant reduction in COU seen in previous studies following the implementation of TPS was not accompanied by increased health care costs.6,7 When considering the other costs of long-term opioid use, such as abuse potential, overdose, death, and increased disability, implementation of a TPS service has the potential to improve patient quality of life while reducing other health-related costs. Health care systems should consider the implementation of similar multidisciplinary approaches to perioperative pain management to improve outcomes after orthopedic joint surgery and other high-risk procedures.
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
1. Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452. doi:10.15585/mmwr.mm655051e1
2. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906. doi:10.1097/MLR.0000000000000625
3. Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722-727. doi:10.1097/SLA.0000000000001780
4. Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947-957, e3. doi:10.1016/j.jhsa.2016.07.113
5. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. doi:10.1001/jamasurg.2017.0504
6. Buys MJ, Bayless K, Romesser J, et al. Multidisciplinary transitional pain service for the veteran population. Fed Pract. 2020;37(10):472-478. doi:10.12788/fp.0053
7. Buys MJ, Bayless K, Romesser J, et al. Opioid use among veterans undergoing major joint surgery managed by a multidisciplinary transitional pain service. Reg Anesth Pain Med. 2020;45(11):847-852. doi:10.1136/rapm-2020-101797
8. Huang A, Katz J, Clarke H. Ensuring safe prescribing of controlled substances for pain following surgery by developing a transitional pain service. Pain Manag. 2015;5(2):97-105. doi:10.2217/pmt.15.7
9. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695-702. doi:10.2147/JPR.S91924
10. Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
11. Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR). Updated December 9, 2022. Accessed October 30, 2023. www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
12. Mosher HJ, Richardson KK, Lund BC. The 1-year treatment course of new opioid recipients in Veterans Health Administration. Pain Med. 2016;17(7):1282-1291. doi:10.1093/pm/pnw058
13. Hadlandsmyth K, Mosher HJ, Vander Weg MW, O’Shea AM, McCoy KD, Lund BC. Utility of accumulated opioid supply days and individual patient factors in predicting probability of transitioning to long-term opioid use: an observational study in the Veterans Health Administration. Pharmacol Res Perspect. 2020;8(2):e00571. doi:10.1002/prp2.571
14. Pagé MG, Kudrina I, Zomahoun HTV, et al. Relative frequency and risk factors for long-term opioid therapy following surgery and trauma among adults: a systematic review protocol. Syst Rev. 2018;7(1):97. doi:10.1186/s13643-018-0760-3
15. US. Bureau of Economic Analysis. Price indexes for personal consumption expenditures by major type of product. Accessed October 30, 2023. https://apps.bea.gov/iTable/?reqid=19&step=3&isuri=1&nipa_table_list=64&categories=survey
16. Brummett CM, Evans-Shields J, England C, et al. Increased health care costs associated with new persistent opioid use after major surgery in opioid-naive patients. J Manag Care Spec Pharm. 2021;27(6):760-771. doi:10.18553/jmcp.2021.20507
17. Gold LS, Strassels SA, Hansen RN. Health care costs and utilization in patients receiving prescriptions for long-acting opioids for acute postsurgical pain. Clin J Pain. 2016;32(9):747-754. doi:10.1097/ajp.0000000000000322
Increasing Local Productivity Through a Regional Antimicrobial Stewardship Collaborative
The importance of formalized antimicrobial stewardship programs (ASPs) has gained recognition over the past 2 decades. The increasing requirements for ASP programs from national entities often outpace the staffing, technology, and analytic support needed to meet these demands.1,2 A multimodal approach to stewardship that includes education initiatives, audit-and-feedback methodology, and system support is effective in producing sustained change.3 However, this approach is resource intensive, and many ASPs must look outward for additional support.
Centralized ASP collaboratives and stewardship networks have been effective in positively impacting initiatives and outcomes through resource sharing.3-5 These collaboratives can take on multiple forms ranging from centralized education distribution to individual sites coming together to set goals and develop strategies to address common issues.5-8 Collaboratives can provide enhanced data analysis through data pooling, which may lead to shared dashboards or antibiotic use (AU) reports, allowing for robust benchmarking.5-7 Productivity at individual centers is often measured by AU and antimicrobial resistance (AMR) rates, but these measures alone do not fully capture the benefits of collaborative participation.
The US Department of Veterans Affairs (VA), similar to other large health care systems, is uniquely positioned to promote the development of ASP collaboratives due to the use of the same electronic health record system and infrastructure for data. This centralized data lends itself more readily to data dashboards and interfacility comparison. In turn, the identification of facilities that have outlying data for specific measures can lead to a collaborative effort to identify aberrant processes or facility-specific problems and identify, implement, and track the progress of appropriate solutions with less effort and resources.7 The VA has a national stewardship group, the Antimicrobial Stewardship Task Force (ASTF), that identifies and disseminates best practices and advocates for stewardship resources.
VA facilities are heterogeneous with regard to patient population, services, availability of specialists, and antibiotic resistance patterns.9 Therefore, clinical practice and needs vary. The ASTF has spearheaded the development of regional collaboratives, recognizing the potential benefit of smaller groups with shared leadership. The Veterans Integrated Services Networks (VISNs) are geographically demarcated regions that lend themselves well to coordination among member facilities due to similar populations, challenges, and opportunities. The Veterans Affairs Midsouth Healthcare Network (VISN 9) includes 5 facilities across Tennessee, Kentucky, Mississippi, Arkansas, Georgia, Virginia, and Indiana and serves about 293,000 veterans, ranging from 35,000 to 105,000 per facility.
A VISN 9 stewardship collaborative (as described by Buckel and colleagues in 2022) was established to enhance member facility ASPs through shared goal setting.6 Initially, the collaborative met quarterly; however, with increased participation and the onset of COVID-19, the collaborative evolved to meet burgeoning ASP needs. While intrafacility multidisciplinary ASP collaboration has been previously published, few publications on interfacility collaborations exist.3-6 To our knowledge, no previous publications have reported the impact of a VA ASP collaborative on the productivity and effectiveness of participating ASP facilities and the region. We aim to share the structure and processes of this ASP collaborative, demonstrate its impact through quantification of productivity, and aid others in developing similar collaboratives to further ASPs’ impact.
Methods
The regional VISN 9 ASP collaborative was formed in January 2020 to address common issues across facilities and optimize human capital and resources. The initial collaborative included ASP pharmacists but quickly expanded to include physicians and nurse practitioners. The collaborative is co-led by 2 members from different facilities that rotate.
In April 2021, clinical guidance and research/quality improvement (QI) subcommittees were created. The monthly research/QI subcommittee discusses current initiatives and barriers to ongoing research, adapt and disseminate successful interventions to other facilities, and develop new collaborative initiatives. The clinical guidance subcommittee creates and disseminates clinical expert recommendations regarding common issues or emerging needs.
Data Plan and Collection
To measure success and growth, we evaluated annual facility reports that convey the state of each facility’s ASP, outline its current initiatives and progress, highlight areas of need, and set a programmatic goal and strategy for the upcoming year. These reports, required by a VA directive, are submitted annually by each facility to local and VISN leadership and must address the following 7 areas: (1) ASP structure and fulfillment of national VA policy for ASP; (2) fulfillment of the Joint Commission ASP standards; (3) ASP metrics; (4) ASP activities and interventions; (5) ASP QI and research initiatives; (6) education; and (7) goals and priorities.
To standardize evaluation and accurately reflect ASP effort across heterogeneous reports, 4 core areas were identified from areas 1, 3, 4 and 5 listed previously. Area 2 was excluded for its similarity among all facilities, and areas 6 and 7 were excluded for significant differences in definitions and reporting across facilities.
The project team consisted of 5 members from the collaborative who initially discussed definitions and annual report review methodology. A subgroup was assigned to area 1 and another to areas 3, 4, and 5 for initial review and data extraction. Results were later reviewed to address discrepancies and finalize collation and presentation.
The impact of the collaborative on individual facilities was measured by both quantitative and qualitative measures. Quantitative measures included: (1) designated ASP pharmacy, physician, or advanced practice provider (APP) full-time equivalents (FTE) at each facility compared with the recommended FTE for facility size; (2) the number of inpatient and outpatient ASP AU metrics for each facility and the VISN total; (3) reported improvement in annual ASP metrics calculated as frequency of improved metrics for each facility and the VISN; (4) the number of QI or research initiatives for each facility and the VISN, which included clinical pathways and order sets; and (5) the number of initiatives published as either abstract or manuscript.10 Additionally, the number of collaborative efforts involving more than 1 facility was tracked. Qualitative data included categories of metrics and QI and research initiatives. Data were collected by year and facility. Facilities are labeled A to E throughout this article.
Along with facility annual ASP reports, facility and VISN AU trends for fiscal years (FY) 2019-2022 were collected from existing VA dashboards tracking AU in both acute respiratory infections (ARI) and in patients with COVID-19. Quantitative data included facility and VISN quarterly AU rates for ARI, extracted from the national VA dashboard. Facility and VISN AU rates in patients with COVID-19 were extracted from a dashboard developed by the VISN 9 ASP collaborative. The VISN 9 Institutional Review Board deemed this work QI and approval was waived.
Results
In 2019, only 2 sites (A and C) reported dedicated FTE compared with recommended minimum staffing; neither met minimum requirements. In 2020, 1 facility (B) met the physician FTE recommendation, and 2 facilities met the pharmacy minimum FTE (D and E). In 2021 and 2022, 2 of 5 facilities (B and E) met the physician minimum FTE, and 2 of 5 (D and E) met the minimum pharmacy FTE recommendations. For the study years 2019 to 2022, 1 facility (E) met both pharmacy and physician FTE recommendations in 2021 and 2022, and 2 facilities (A and C) never met minimum FTE recommendations.
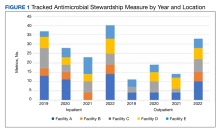
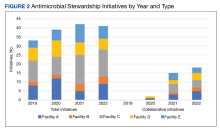
Regarding ASP metrics, all facilities tracked and reported inpatient AU; however, facility A did not document inpatient metrics for FY 2021. The number of individual inpatient metrics varied annually; however, FY 2022 saw the highest reported for the VISN (n = 40), with a more even distribution across facilities (Figure 1). Common metrics in 2022 included total AU, broad-spectrum gram-negative AU, anti–methicillin-resistant Staphylococcus aureus (MRSA) agent use, antibiotics with high risk for Clostridioides difficile infection (CDI), and AU in patients with COVID-19. The percentage of improved metrics for VISN 9 was consistent, ranging from 26.5% to 34.8%, throughout the study period.
From 2019 to 2022, facilities reporting outpatient AU increased from 3 to 5 and included fluoroquinolone use and AU in ARI. VISN 9 outpatient metrics increased every year except in 2021 with improved distribution across facilities. The number of total metrics with reported improvement in the outpatient setting overall increased from 3 of 11 (27%) in 2019 to 20 of 33 (60%) in 2022.
Antimicrobial Stewardship Initiatives
Quantitative and qualitative data regarding initiatives are reported in Figure 2 and the eAppendix respectively. Since the formation of the collaborative, total initiatives increased from 33 in 2019 to 41 in 2022. In 2019, before the collaborative, individual facilities were working on similar projects in parallel, which included MRSA decolonization (A and C), surgical prophylaxis (A and E), asymptomatic bacteriuria (A and C), and CDI (B, C, D, and E). The development of clinical pathways and order sets remained consistent, ranging from 15 to 19 throughout the study period except for 2020, when 33 clinical pathways and/or order sets were developed. Collaboration between sites also remained consistent, with 1 shared clinical pathway and/or order menu between at least 1 site reported yearly for 2020, 2021, and 2022. The number of publications from VISN 9 grew from 2 in 2019 to 17 in 2022. In 2019, there were no collaborative research or QI publications, but in 2022 there were 2 joint publications, 1 between 2 facilities (A and C) and 1 including all facilities.
ARI and COVID-19 were identified by the collaborative as VISN priorities, leading to shared metrics and benchmarking across facilities. From 2019 to 2022, increased collaboration on these initiatives was noted at all facilities. The ARI goal was established to reduce inappropriate prescribing for ARI/bronchitis to under 20% across VISN 9. Rates dropped from 50.3% (range, 35.4%-77.6%) in FY 2019 quarter (Q) 1 to 15% (range, 8%-18.3%) in FY 2022 Q4. The clinical guidance subcommittee developed a guideline for AU in patients with COVID-19 that was approved by the VISN 9 Pharmacy & Therapeutics Committee. A VISN 9 dashboard was developed to track inpatient and outpatient AU for COVID-19. Antibiotic prescribing in the first 4 days of hospitalization decreased from 62.2% at the start of the COVID-19 pandemic to 48.7% after dissemination of COVID-19 guidance.
Discussion
This study demonstrates the benefit of participating in a regional ASP collaborative for individual facilities and the region. Some products from the collaborative include the development of regionwide guidance for the use of antimicrobials in COVID-19, interfacility collaborative initiatives, a COVID-19 dashboard, improvement in metrics, and several publications. Importantly, this expansion occurred during the COVID-19 pandemic when many ASP members were spread thin. Moreover, despite 4 sites not meeting VA-recommended ASP staffing requirements for both pharmacists and physicians, productivity increased within the VISN as facilities worked together sharing local challenges and successful paths in removing ASP barriers. The collaborative shared QI strategies, advocated for technological support (ie, Theradoc and dashboards) to maximize available ASP human capital, standardized metric reporting, and made continued efforts sustainable.
Previous reports in the literature have found ASP collaboratives to be an effective model for long-term program growth.3 Two collaboratives found improved adherence to the Centers for Disease Control and Prevention core elements for ASP.4,5
Our findings highlight that ASP collaboratives can help answer the recent call to action from McGregor, Fitzpatrick, and Suda who advocated for ASPs to take the next steps in stewardship, which include standardization of evaluating metrics and the use of robust QI frameworks.11 Moving forward, an area for research could include a comparison of ASP collaborative infrastructures and productivity to identify optimal fit dependent on facility structure and setting. Parallel to our experience, other reports cite heterogeneous ASP metrics and a lack of benchmarking, spotlighting the need for standardization.8,11,12
Limitations
Using annual reports was a limitation for analyzing and reporting the full impact of the collaborative. Local facility-level discretion of content inclusion led to many facilities only reporting on the forefront of new initiatives that they had developed and may have led to the omission of other ongoing work. Further, time invested into the ASP regional collaborative was not captured within annual reports; therefore, the opportunity cost cannot be determined.
Conclusions
The VA has an advantage that many private health care facilities do not: the ability to work across systems to ease the burden of duplicative work and more readily disseminate effective strategies. The regional ASP collaborative bred innovation and the tearing down of silos. The implementation of the collaborative aided in robust QI infrastructure, standardization of reporting and metrics, and greater support through facility alignments with regional guidance. ASP interfacility collaboratives provide a sustainable solution in a resource-limited landscape.
Acknowledgments
This work was made possible by the resources provided through the Antimicrobial Stewardship Programs in the Veterans Integrated Services Network (VISN) 9.
1. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103-108. doi:10.1016/j.ijid.2021.10.001
2. Emberger J, Tassone D, Stevens MP, Markley JD. The current state of antimicrobial stewardship: challenges, successes, and future directions. Curr Infect Dis Rep. 2018;20(9):31. doi:10.1007/s11908-018-0637-6
3. Moehring RW, Yarrington ME, Davis AE, et al. Effects of a collaborative, community hospital network for antimicrobial stewardship program implementation. Clin Infect Dis. 2021;73(9):1656-1663. doi:10.1093/cid/ciab356
4. Logan AY, Williamson JE, Reinke EK, Jarrett SW, Boger MS, Davidson LE. Establishing an antimicrobial stewardship collaborative across a large, diverse health care system. Jt Comm J Qual Patient Saf. 2019;45(9):591-599. doi:10.1016/j.jcjq.2019.03.002
5. Dukhovny D, Buus-Frank ME, Edwards EM, et al. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144(6):e20190589. doi:10.1542/peds.2019-0589
6. Buckel WR, Stenehjem EA, Hersh AL, Hyun DY, Zetts RM. Harnessing the power of health systems and networks for antimicrobial stewardship. Clin Infect Dis. 2022;75(11):2038-2044. doi:10.1093/cid/ciac515
7. Graber CJ, Jones MM, Goetz MB, et al. Decreases in antimicrobial use associated with multihospital implementation of electronic antimicrobial stewardship tools. Clin Infect Dis. 2020;71(5):1168-1176. doi:10.1093/cid/ciz941
8. Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38(5):513-520. doi:10.1017/ice.2016.328
9. US Department of Veterans Affairs. About VHA. 2022. Updated September 7, 2023. Accessed November 7, 2023. https://www.va.gov/health/aboutVHA.asp
10. Echevarria K, Groppi J, Kelly AA, Morreale AP, Neuhauser MM, Roselle GA. Development and application of an objective staffing calculator for antimicrobial stewardship programs in the Veterans Health Administration. Am J Health Syst Pharm. 2017;74(21):1785-1790. doi:10.2146/ajhp160825
11. McGregor JC, Fitzpatrick MA, Suda KJ. Expanding antimicrobial stewardship through quality improvement. JAMA Netw Open. 2021;4(2):e211072. doi:10.1001/jamanetworkopen.2021.1072
12. Newland JG, Gerber JS, Kronman MP, et al. Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS): a quality improvement collaborative. J Pediatr Infect Dis Soc. 2018;7(2):124-128. doi:10.1093/jpids/pix020
The importance of formalized antimicrobial stewardship programs (ASPs) has gained recognition over the past 2 decades. The increasing requirements for ASP programs from national entities often outpace the staffing, technology, and analytic support needed to meet these demands.1,2 A multimodal approach to stewardship that includes education initiatives, audit-and-feedback methodology, and system support is effective in producing sustained change.3 However, this approach is resource intensive, and many ASPs must look outward for additional support.
Centralized ASP collaboratives and stewardship networks have been effective in positively impacting initiatives and outcomes through resource sharing.3-5 These collaboratives can take on multiple forms ranging from centralized education distribution to individual sites coming together to set goals and develop strategies to address common issues.5-8 Collaboratives can provide enhanced data analysis through data pooling, which may lead to shared dashboards or antibiotic use (AU) reports, allowing for robust benchmarking.5-7 Productivity at individual centers is often measured by AU and antimicrobial resistance (AMR) rates, but these measures alone do not fully capture the benefits of collaborative participation.
The US Department of Veterans Affairs (VA), similar to other large health care systems, is uniquely positioned to promote the development of ASP collaboratives due to the use of the same electronic health record system and infrastructure for data. This centralized data lends itself more readily to data dashboards and interfacility comparison. In turn, the identification of facilities that have outlying data for specific measures can lead to a collaborative effort to identify aberrant processes or facility-specific problems and identify, implement, and track the progress of appropriate solutions with less effort and resources.7 The VA has a national stewardship group, the Antimicrobial Stewardship Task Force (ASTF), that identifies and disseminates best practices and advocates for stewardship resources.
VA facilities are heterogeneous with regard to patient population, services, availability of specialists, and antibiotic resistance patterns.9 Therefore, clinical practice and needs vary. The ASTF has spearheaded the development of regional collaboratives, recognizing the potential benefit of smaller groups with shared leadership. The Veterans Integrated Services Networks (VISNs) are geographically demarcated regions that lend themselves well to coordination among member facilities due to similar populations, challenges, and opportunities. The Veterans Affairs Midsouth Healthcare Network (VISN 9) includes 5 facilities across Tennessee, Kentucky, Mississippi, Arkansas, Georgia, Virginia, and Indiana and serves about 293,000 veterans, ranging from 35,000 to 105,000 per facility.
A VISN 9 stewardship collaborative (as described by Buckel and colleagues in 2022) was established to enhance member facility ASPs through shared goal setting.6 Initially, the collaborative met quarterly; however, with increased participation and the onset of COVID-19, the collaborative evolved to meet burgeoning ASP needs. While intrafacility multidisciplinary ASP collaboration has been previously published, few publications on interfacility collaborations exist.3-6 To our knowledge, no previous publications have reported the impact of a VA ASP collaborative on the productivity and effectiveness of participating ASP facilities and the region. We aim to share the structure and processes of this ASP collaborative, demonstrate its impact through quantification of productivity, and aid others in developing similar collaboratives to further ASPs’ impact.
Methods
The regional VISN 9 ASP collaborative was formed in January 2020 to address common issues across facilities and optimize human capital and resources. The initial collaborative included ASP pharmacists but quickly expanded to include physicians and nurse practitioners. The collaborative is co-led by 2 members from different facilities that rotate.
In April 2021, clinical guidance and research/quality improvement (QI) subcommittees were created. The monthly research/QI subcommittee discusses current initiatives and barriers to ongoing research, adapt and disseminate successful interventions to other facilities, and develop new collaborative initiatives. The clinical guidance subcommittee creates and disseminates clinical expert recommendations regarding common issues or emerging needs.
Data Plan and Collection
To measure success and growth, we evaluated annual facility reports that convey the state of each facility’s ASP, outline its current initiatives and progress, highlight areas of need, and set a programmatic goal and strategy for the upcoming year. These reports, required by a VA directive, are submitted annually by each facility to local and VISN leadership and must address the following 7 areas: (1) ASP structure and fulfillment of national VA policy for ASP; (2) fulfillment of the Joint Commission ASP standards; (3) ASP metrics; (4) ASP activities and interventions; (5) ASP QI and research initiatives; (6) education; and (7) goals and priorities.
To standardize evaluation and accurately reflect ASP effort across heterogeneous reports, 4 core areas were identified from areas 1, 3, 4 and 5 listed previously. Area 2 was excluded for its similarity among all facilities, and areas 6 and 7 were excluded for significant differences in definitions and reporting across facilities.
The project team consisted of 5 members from the collaborative who initially discussed definitions and annual report review methodology. A subgroup was assigned to area 1 and another to areas 3, 4, and 5 for initial review and data extraction. Results were later reviewed to address discrepancies and finalize collation and presentation.
The impact of the collaborative on individual facilities was measured by both quantitative and qualitative measures. Quantitative measures included: (1) designated ASP pharmacy, physician, or advanced practice provider (APP) full-time equivalents (FTE) at each facility compared with the recommended FTE for facility size; (2) the number of inpatient and outpatient ASP AU metrics for each facility and the VISN total; (3) reported improvement in annual ASP metrics calculated as frequency of improved metrics for each facility and the VISN; (4) the number of QI or research initiatives for each facility and the VISN, which included clinical pathways and order sets; and (5) the number of initiatives published as either abstract or manuscript.10 Additionally, the number of collaborative efforts involving more than 1 facility was tracked. Qualitative data included categories of metrics and QI and research initiatives. Data were collected by year and facility. Facilities are labeled A to E throughout this article.
Along with facility annual ASP reports, facility and VISN AU trends for fiscal years (FY) 2019-2022 were collected from existing VA dashboards tracking AU in both acute respiratory infections (ARI) and in patients with COVID-19. Quantitative data included facility and VISN quarterly AU rates for ARI, extracted from the national VA dashboard. Facility and VISN AU rates in patients with COVID-19 were extracted from a dashboard developed by the VISN 9 ASP collaborative. The VISN 9 Institutional Review Board deemed this work QI and approval was waived.
Results
In 2019, only 2 sites (A and C) reported dedicated FTE compared with recommended minimum staffing; neither met minimum requirements. In 2020, 1 facility (B) met the physician FTE recommendation, and 2 facilities met the pharmacy minimum FTE (D and E). In 2021 and 2022, 2 of 5 facilities (B and E) met the physician minimum FTE, and 2 of 5 (D and E) met the minimum pharmacy FTE recommendations. For the study years 2019 to 2022, 1 facility (E) met both pharmacy and physician FTE recommendations in 2021 and 2022, and 2 facilities (A and C) never met minimum FTE recommendations.
Regarding ASP metrics, all facilities tracked and reported inpatient AU; however, facility A did not document inpatient metrics for FY 2021. The number of individual inpatient metrics varied annually; however, FY 2022 saw the highest reported for the VISN (n = 40), with a more even distribution across facilities (Figure 1). Common metrics in 2022 included total AU, broad-spectrum gram-negative AU, anti–methicillin-resistant Staphylococcus aureus (MRSA) agent use, antibiotics with high risk for Clostridioides difficile infection (CDI), and AU in patients with COVID-19. The percentage of improved metrics for VISN 9 was consistent, ranging from 26.5% to 34.8%, throughout the study period.
From 2019 to 2022, facilities reporting outpatient AU increased from 3 to 5 and included fluoroquinolone use and AU in ARI. VISN 9 outpatient metrics increased every year except in 2021 with improved distribution across facilities. The number of total metrics with reported improvement in the outpatient setting overall increased from 3 of 11 (27%) in 2019 to 20 of 33 (60%) in 2022.
Antimicrobial Stewardship Initiatives
Quantitative and qualitative data regarding initiatives are reported in Figure 2 and the eAppendix respectively. Since the formation of the collaborative, total initiatives increased from 33 in 2019 to 41 in 2022. In 2019, before the collaborative, individual facilities were working on similar projects in parallel, which included MRSA decolonization (A and C), surgical prophylaxis (A and E), asymptomatic bacteriuria (A and C), and CDI (B, C, D, and E). The development of clinical pathways and order sets remained consistent, ranging from 15 to 19 throughout the study period except for 2020, when 33 clinical pathways and/or order sets were developed. Collaboration between sites also remained consistent, with 1 shared clinical pathway and/or order menu between at least 1 site reported yearly for 2020, 2021, and 2022. The number of publications from VISN 9 grew from 2 in 2019 to 17 in 2022. In 2019, there were no collaborative research or QI publications, but in 2022 there were 2 joint publications, 1 between 2 facilities (A and C) and 1 including all facilities.
ARI and COVID-19 were identified by the collaborative as VISN priorities, leading to shared metrics and benchmarking across facilities. From 2019 to 2022, increased collaboration on these initiatives was noted at all facilities. The ARI goal was established to reduce inappropriate prescribing for ARI/bronchitis to under 20% across VISN 9. Rates dropped from 50.3% (range, 35.4%-77.6%) in FY 2019 quarter (Q) 1 to 15% (range, 8%-18.3%) in FY 2022 Q4. The clinical guidance subcommittee developed a guideline for AU in patients with COVID-19 that was approved by the VISN 9 Pharmacy & Therapeutics Committee. A VISN 9 dashboard was developed to track inpatient and outpatient AU for COVID-19. Antibiotic prescribing in the first 4 days of hospitalization decreased from 62.2% at the start of the COVID-19 pandemic to 48.7% after dissemination of COVID-19 guidance.
Discussion
This study demonstrates the benefit of participating in a regional ASP collaborative for individual facilities and the region. Some products from the collaborative include the development of regionwide guidance for the use of antimicrobials in COVID-19, interfacility collaborative initiatives, a COVID-19 dashboard, improvement in metrics, and several publications. Importantly, this expansion occurred during the COVID-19 pandemic when many ASP members were spread thin. Moreover, despite 4 sites not meeting VA-recommended ASP staffing requirements for both pharmacists and physicians, productivity increased within the VISN as facilities worked together sharing local challenges and successful paths in removing ASP barriers. The collaborative shared QI strategies, advocated for technological support (ie, Theradoc and dashboards) to maximize available ASP human capital, standardized metric reporting, and made continued efforts sustainable.
Previous reports in the literature have found ASP collaboratives to be an effective model for long-term program growth.3 Two collaboratives found improved adherence to the Centers for Disease Control and Prevention core elements for ASP.4,5
Our findings highlight that ASP collaboratives can help answer the recent call to action from McGregor, Fitzpatrick, and Suda who advocated for ASPs to take the next steps in stewardship, which include standardization of evaluating metrics and the use of robust QI frameworks.11 Moving forward, an area for research could include a comparison of ASP collaborative infrastructures and productivity to identify optimal fit dependent on facility structure and setting. Parallel to our experience, other reports cite heterogeneous ASP metrics and a lack of benchmarking, spotlighting the need for standardization.8,11,12
Limitations
Using annual reports was a limitation for analyzing and reporting the full impact of the collaborative. Local facility-level discretion of content inclusion led to many facilities only reporting on the forefront of new initiatives that they had developed and may have led to the omission of other ongoing work. Further, time invested into the ASP regional collaborative was not captured within annual reports; therefore, the opportunity cost cannot be determined.
Conclusions
The VA has an advantage that many private health care facilities do not: the ability to work across systems to ease the burden of duplicative work and more readily disseminate effective strategies. The regional ASP collaborative bred innovation and the tearing down of silos. The implementation of the collaborative aided in robust QI infrastructure, standardization of reporting and metrics, and greater support through facility alignments with regional guidance. ASP interfacility collaboratives provide a sustainable solution in a resource-limited landscape.
Acknowledgments
This work was made possible by the resources provided through the Antimicrobial Stewardship Programs in the Veterans Integrated Services Network (VISN) 9.
The importance of formalized antimicrobial stewardship programs (ASPs) has gained recognition over the past 2 decades. The increasing requirements for ASP programs from national entities often outpace the staffing, technology, and analytic support needed to meet these demands.1,2 A multimodal approach to stewardship that includes education initiatives, audit-and-feedback methodology, and system support is effective in producing sustained change.3 However, this approach is resource intensive, and many ASPs must look outward for additional support.
Centralized ASP collaboratives and stewardship networks have been effective in positively impacting initiatives and outcomes through resource sharing.3-5 These collaboratives can take on multiple forms ranging from centralized education distribution to individual sites coming together to set goals and develop strategies to address common issues.5-8 Collaboratives can provide enhanced data analysis through data pooling, which may lead to shared dashboards or antibiotic use (AU) reports, allowing for robust benchmarking.5-7 Productivity at individual centers is often measured by AU and antimicrobial resistance (AMR) rates, but these measures alone do not fully capture the benefits of collaborative participation.
The US Department of Veterans Affairs (VA), similar to other large health care systems, is uniquely positioned to promote the development of ASP collaboratives due to the use of the same electronic health record system and infrastructure for data. This centralized data lends itself more readily to data dashboards and interfacility comparison. In turn, the identification of facilities that have outlying data for specific measures can lead to a collaborative effort to identify aberrant processes or facility-specific problems and identify, implement, and track the progress of appropriate solutions with less effort and resources.7 The VA has a national stewardship group, the Antimicrobial Stewardship Task Force (ASTF), that identifies and disseminates best practices and advocates for stewardship resources.
VA facilities are heterogeneous with regard to patient population, services, availability of specialists, and antibiotic resistance patterns.9 Therefore, clinical practice and needs vary. The ASTF has spearheaded the development of regional collaboratives, recognizing the potential benefit of smaller groups with shared leadership. The Veterans Integrated Services Networks (VISNs) are geographically demarcated regions that lend themselves well to coordination among member facilities due to similar populations, challenges, and opportunities. The Veterans Affairs Midsouth Healthcare Network (VISN 9) includes 5 facilities across Tennessee, Kentucky, Mississippi, Arkansas, Georgia, Virginia, and Indiana and serves about 293,000 veterans, ranging from 35,000 to 105,000 per facility.
A VISN 9 stewardship collaborative (as described by Buckel and colleagues in 2022) was established to enhance member facility ASPs through shared goal setting.6 Initially, the collaborative met quarterly; however, with increased participation and the onset of COVID-19, the collaborative evolved to meet burgeoning ASP needs. While intrafacility multidisciplinary ASP collaboration has been previously published, few publications on interfacility collaborations exist.3-6 To our knowledge, no previous publications have reported the impact of a VA ASP collaborative on the productivity and effectiveness of participating ASP facilities and the region. We aim to share the structure and processes of this ASP collaborative, demonstrate its impact through quantification of productivity, and aid others in developing similar collaboratives to further ASPs’ impact.
Methods
The regional VISN 9 ASP collaborative was formed in January 2020 to address common issues across facilities and optimize human capital and resources. The initial collaborative included ASP pharmacists but quickly expanded to include physicians and nurse practitioners. The collaborative is co-led by 2 members from different facilities that rotate.
In April 2021, clinical guidance and research/quality improvement (QI) subcommittees were created. The monthly research/QI subcommittee discusses current initiatives and barriers to ongoing research, adapt and disseminate successful interventions to other facilities, and develop new collaborative initiatives. The clinical guidance subcommittee creates and disseminates clinical expert recommendations regarding common issues or emerging needs.
Data Plan and Collection
To measure success and growth, we evaluated annual facility reports that convey the state of each facility’s ASP, outline its current initiatives and progress, highlight areas of need, and set a programmatic goal and strategy for the upcoming year. These reports, required by a VA directive, are submitted annually by each facility to local and VISN leadership and must address the following 7 areas: (1) ASP structure and fulfillment of national VA policy for ASP; (2) fulfillment of the Joint Commission ASP standards; (3) ASP metrics; (4) ASP activities and interventions; (5) ASP QI and research initiatives; (6) education; and (7) goals and priorities.
To standardize evaluation and accurately reflect ASP effort across heterogeneous reports, 4 core areas were identified from areas 1, 3, 4 and 5 listed previously. Area 2 was excluded for its similarity among all facilities, and areas 6 and 7 were excluded for significant differences in definitions and reporting across facilities.
The project team consisted of 5 members from the collaborative who initially discussed definitions and annual report review methodology. A subgroup was assigned to area 1 and another to areas 3, 4, and 5 for initial review and data extraction. Results were later reviewed to address discrepancies and finalize collation and presentation.
The impact of the collaborative on individual facilities was measured by both quantitative and qualitative measures. Quantitative measures included: (1) designated ASP pharmacy, physician, or advanced practice provider (APP) full-time equivalents (FTE) at each facility compared with the recommended FTE for facility size; (2) the number of inpatient and outpatient ASP AU metrics for each facility and the VISN total; (3) reported improvement in annual ASP metrics calculated as frequency of improved metrics for each facility and the VISN; (4) the number of QI or research initiatives for each facility and the VISN, which included clinical pathways and order sets; and (5) the number of initiatives published as either abstract or manuscript.10 Additionally, the number of collaborative efforts involving more than 1 facility was tracked. Qualitative data included categories of metrics and QI and research initiatives. Data were collected by year and facility. Facilities are labeled A to E throughout this article.
Along with facility annual ASP reports, facility and VISN AU trends for fiscal years (FY) 2019-2022 were collected from existing VA dashboards tracking AU in both acute respiratory infections (ARI) and in patients with COVID-19. Quantitative data included facility and VISN quarterly AU rates for ARI, extracted from the national VA dashboard. Facility and VISN AU rates in patients with COVID-19 were extracted from a dashboard developed by the VISN 9 ASP collaborative. The VISN 9 Institutional Review Board deemed this work QI and approval was waived.
Results
In 2019, only 2 sites (A and C) reported dedicated FTE compared with recommended minimum staffing; neither met minimum requirements. In 2020, 1 facility (B) met the physician FTE recommendation, and 2 facilities met the pharmacy minimum FTE (D and E). In 2021 and 2022, 2 of 5 facilities (B and E) met the physician minimum FTE, and 2 of 5 (D and E) met the minimum pharmacy FTE recommendations. For the study years 2019 to 2022, 1 facility (E) met both pharmacy and physician FTE recommendations in 2021 and 2022, and 2 facilities (A and C) never met minimum FTE recommendations.
Regarding ASP metrics, all facilities tracked and reported inpatient AU; however, facility A did not document inpatient metrics for FY 2021. The number of individual inpatient metrics varied annually; however, FY 2022 saw the highest reported for the VISN (n = 40), with a more even distribution across facilities (Figure 1). Common metrics in 2022 included total AU, broad-spectrum gram-negative AU, anti–methicillin-resistant Staphylococcus aureus (MRSA) agent use, antibiotics with high risk for Clostridioides difficile infection (CDI), and AU in patients with COVID-19. The percentage of improved metrics for VISN 9 was consistent, ranging from 26.5% to 34.8%, throughout the study period.
From 2019 to 2022, facilities reporting outpatient AU increased from 3 to 5 and included fluoroquinolone use and AU in ARI. VISN 9 outpatient metrics increased every year except in 2021 with improved distribution across facilities. The number of total metrics with reported improvement in the outpatient setting overall increased from 3 of 11 (27%) in 2019 to 20 of 33 (60%) in 2022.
Antimicrobial Stewardship Initiatives
Quantitative and qualitative data regarding initiatives are reported in Figure 2 and the eAppendix respectively. Since the formation of the collaborative, total initiatives increased from 33 in 2019 to 41 in 2022. In 2019, before the collaborative, individual facilities were working on similar projects in parallel, which included MRSA decolonization (A and C), surgical prophylaxis (A and E), asymptomatic bacteriuria (A and C), and CDI (B, C, D, and E). The development of clinical pathways and order sets remained consistent, ranging from 15 to 19 throughout the study period except for 2020, when 33 clinical pathways and/or order sets were developed. Collaboration between sites also remained consistent, with 1 shared clinical pathway and/or order menu between at least 1 site reported yearly for 2020, 2021, and 2022. The number of publications from VISN 9 grew from 2 in 2019 to 17 in 2022. In 2019, there were no collaborative research or QI publications, but in 2022 there were 2 joint publications, 1 between 2 facilities (A and C) and 1 including all facilities.
ARI and COVID-19 were identified by the collaborative as VISN priorities, leading to shared metrics and benchmarking across facilities. From 2019 to 2022, increased collaboration on these initiatives was noted at all facilities. The ARI goal was established to reduce inappropriate prescribing for ARI/bronchitis to under 20% across VISN 9. Rates dropped from 50.3% (range, 35.4%-77.6%) in FY 2019 quarter (Q) 1 to 15% (range, 8%-18.3%) in FY 2022 Q4. The clinical guidance subcommittee developed a guideline for AU in patients with COVID-19 that was approved by the VISN 9 Pharmacy & Therapeutics Committee. A VISN 9 dashboard was developed to track inpatient and outpatient AU for COVID-19. Antibiotic prescribing in the first 4 days of hospitalization decreased from 62.2% at the start of the COVID-19 pandemic to 48.7% after dissemination of COVID-19 guidance.
Discussion
This study demonstrates the benefit of participating in a regional ASP collaborative for individual facilities and the region. Some products from the collaborative include the development of regionwide guidance for the use of antimicrobials in COVID-19, interfacility collaborative initiatives, a COVID-19 dashboard, improvement in metrics, and several publications. Importantly, this expansion occurred during the COVID-19 pandemic when many ASP members were spread thin. Moreover, despite 4 sites not meeting VA-recommended ASP staffing requirements for both pharmacists and physicians, productivity increased within the VISN as facilities worked together sharing local challenges and successful paths in removing ASP barriers. The collaborative shared QI strategies, advocated for technological support (ie, Theradoc and dashboards) to maximize available ASP human capital, standardized metric reporting, and made continued efforts sustainable.
Previous reports in the literature have found ASP collaboratives to be an effective model for long-term program growth.3 Two collaboratives found improved adherence to the Centers for Disease Control and Prevention core elements for ASP.4,5
Our findings highlight that ASP collaboratives can help answer the recent call to action from McGregor, Fitzpatrick, and Suda who advocated for ASPs to take the next steps in stewardship, which include standardization of evaluating metrics and the use of robust QI frameworks.11 Moving forward, an area for research could include a comparison of ASP collaborative infrastructures and productivity to identify optimal fit dependent on facility structure and setting. Parallel to our experience, other reports cite heterogeneous ASP metrics and a lack of benchmarking, spotlighting the need for standardization.8,11,12
Limitations
Using annual reports was a limitation for analyzing and reporting the full impact of the collaborative. Local facility-level discretion of content inclusion led to many facilities only reporting on the forefront of new initiatives that they had developed and may have led to the omission of other ongoing work. Further, time invested into the ASP regional collaborative was not captured within annual reports; therefore, the opportunity cost cannot be determined.
Conclusions
The VA has an advantage that many private health care facilities do not: the ability to work across systems to ease the burden of duplicative work and more readily disseminate effective strategies. The regional ASP collaborative bred innovation and the tearing down of silos. The implementation of the collaborative aided in robust QI infrastructure, standardization of reporting and metrics, and greater support through facility alignments with regional guidance. ASP interfacility collaboratives provide a sustainable solution in a resource-limited landscape.
Acknowledgments
This work was made possible by the resources provided through the Antimicrobial Stewardship Programs in the Veterans Integrated Services Network (VISN) 9.
1. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103-108. doi:10.1016/j.ijid.2021.10.001
2. Emberger J, Tassone D, Stevens MP, Markley JD. The current state of antimicrobial stewardship: challenges, successes, and future directions. Curr Infect Dis Rep. 2018;20(9):31. doi:10.1007/s11908-018-0637-6
3. Moehring RW, Yarrington ME, Davis AE, et al. Effects of a collaborative, community hospital network for antimicrobial stewardship program implementation. Clin Infect Dis. 2021;73(9):1656-1663. doi:10.1093/cid/ciab356
4. Logan AY, Williamson JE, Reinke EK, Jarrett SW, Boger MS, Davidson LE. Establishing an antimicrobial stewardship collaborative across a large, diverse health care system. Jt Comm J Qual Patient Saf. 2019;45(9):591-599. doi:10.1016/j.jcjq.2019.03.002
5. Dukhovny D, Buus-Frank ME, Edwards EM, et al. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144(6):e20190589. doi:10.1542/peds.2019-0589
6. Buckel WR, Stenehjem EA, Hersh AL, Hyun DY, Zetts RM. Harnessing the power of health systems and networks for antimicrobial stewardship. Clin Infect Dis. 2022;75(11):2038-2044. doi:10.1093/cid/ciac515
7. Graber CJ, Jones MM, Goetz MB, et al. Decreases in antimicrobial use associated with multihospital implementation of electronic antimicrobial stewardship tools. Clin Infect Dis. 2020;71(5):1168-1176. doi:10.1093/cid/ciz941
8. Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38(5):513-520. doi:10.1017/ice.2016.328
9. US Department of Veterans Affairs. About VHA. 2022. Updated September 7, 2023. Accessed November 7, 2023. https://www.va.gov/health/aboutVHA.asp
10. Echevarria K, Groppi J, Kelly AA, Morreale AP, Neuhauser MM, Roselle GA. Development and application of an objective staffing calculator for antimicrobial stewardship programs in the Veterans Health Administration. Am J Health Syst Pharm. 2017;74(21):1785-1790. doi:10.2146/ajhp160825
11. McGregor JC, Fitzpatrick MA, Suda KJ. Expanding antimicrobial stewardship through quality improvement. JAMA Netw Open. 2021;4(2):e211072. doi:10.1001/jamanetworkopen.2021.1072
12. Newland JG, Gerber JS, Kronman MP, et al. Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS): a quality improvement collaborative. J Pediatr Infect Dis Soc. 2018;7(2):124-128. doi:10.1093/jpids/pix020
1. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis. 2021;113:103-108. doi:10.1016/j.ijid.2021.10.001
2. Emberger J, Tassone D, Stevens MP, Markley JD. The current state of antimicrobial stewardship: challenges, successes, and future directions. Curr Infect Dis Rep. 2018;20(9):31. doi:10.1007/s11908-018-0637-6
3. Moehring RW, Yarrington ME, Davis AE, et al. Effects of a collaborative, community hospital network for antimicrobial stewardship program implementation. Clin Infect Dis. 2021;73(9):1656-1663. doi:10.1093/cid/ciab356
4. Logan AY, Williamson JE, Reinke EK, Jarrett SW, Boger MS, Davidson LE. Establishing an antimicrobial stewardship collaborative across a large, diverse health care system. Jt Comm J Qual Patient Saf. 2019;45(9):591-599. doi:10.1016/j.jcjq.2019.03.002
5. Dukhovny D, Buus-Frank ME, Edwards EM, et al. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144(6):e20190589. doi:10.1542/peds.2019-0589
6. Buckel WR, Stenehjem EA, Hersh AL, Hyun DY, Zetts RM. Harnessing the power of health systems and networks for antimicrobial stewardship. Clin Infect Dis. 2022;75(11):2038-2044. doi:10.1093/cid/ciac515
7. Graber CJ, Jones MM, Goetz MB, et al. Decreases in antimicrobial use associated with multihospital implementation of electronic antimicrobial stewardship tools. Clin Infect Dis. 2020;71(5):1168-1176. doi:10.1093/cid/ciz941
8. Kelly AA, Jones MM, Echevarria KL, et al. A report of the efforts of the Veterans Health Administration national antimicrobial stewardship initiative. Infect Control Hosp Epidemiol. 2017;38(5):513-520. doi:10.1017/ice.2016.328
9. US Department of Veterans Affairs. About VHA. 2022. Updated September 7, 2023. Accessed November 7, 2023. https://www.va.gov/health/aboutVHA.asp
10. Echevarria K, Groppi J, Kelly AA, Morreale AP, Neuhauser MM, Roselle GA. Development and application of an objective staffing calculator for antimicrobial stewardship programs in the Veterans Health Administration. Am J Health Syst Pharm. 2017;74(21):1785-1790. doi:10.2146/ajhp160825
11. McGregor JC, Fitzpatrick MA, Suda KJ. Expanding antimicrobial stewardship through quality improvement. JAMA Netw Open. 2021;4(2):e211072. doi:10.1001/jamanetworkopen.2021.1072
12. Newland JG, Gerber JS, Kronman MP, et al. Sharing Antimicrobial Reports for Pediatric Stewardship (SHARPS): a quality improvement collaborative. J Pediatr Infect Dis Soc. 2018;7(2):124-128. doi:10.1093/jpids/pix020
Chronic Kidney Disease and Military Service in US Adults, 1999-2018
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
Chronic kidney disease (CKD) affects nearly 37 million people (11%) in the US and is a leading cause of death and morbidity. Due to their older age and higher prevalence of comorbid conditions, the prevalence of CKD among veterans is approximately 34% higher than in the general population and the fourth most common chronic disease diagnosed among US veterans.1,2 US veterans and those with prior military service (MS) may be at a particularly high risk for CKD and associated health care outcomes including increased hospitalization and death. The observed excess burden of CKD is not mirrored in the general population, and it is unclear whether prior MS confers a unique risk profile for CKD.
Current estimates of CKD burden among veterans or those with prior MS are widely variable and have been limited by unique regions, specific exposure profiles, or to single health care systems. As such, there remains a paucity of data examining CKD burden more broadly. We performed a study in the adult population of the US to quantify associations with the extent of CKD, enumerate temporal trends of CKD among those with prior MS, describe risk within subgroups, and compare heterogeneity of risk factors for CKD by MS.
Methods
The National Health and Nutrition Examination Survey (NHANES) is a suite of nationally representative, cross-sectional surveys of the noninstitutionalized US population. It is conducted by the National Center for Health Statistics and uses a stratified, clustered probability design, with surveys carried out without interruption, collated, and made accessible to the public at 2-year intervals.3 The survey consists of a questionnaire, physical examination, and laboratory data.
The inclusion criteria for our study were age ≥ 20 years along with serum creatinine and urinary albumin-creatinine measurements. The following definitions were used for the study:
• CKD: Estimated glomerular filtration rate < 60 mL/min/1.73 m2 calibrated to isotope dilution mass spectrometry (IDMS).
• Traceable: Creatinine-based CKD Epidemiology Collaboration formula or urinary albumin-creatine ratio ≥ 30 mg/g.
• MS: Positive response to the questions “Did you ever serve in the Armed Forces of the United States?” (1999 to 2010) or “Have you ever served on active duty in the US Armed Forces, military Reserves, or National Guard?” (2011 to 2018).
• Diabetes: Self-reported history, medication for diabetes, or glycated hemoglobin ≥ 7%.
• Hypertension: Blood pressure ≥ 140/90 or ≥ 130/40 mm Hg in the presence of diabetes, medication for hypertension, cardiovascular disease, or CKD, myocardial infarction, cardiac failure, or cerebrovascular disease by self-report.2,3
Analysis
Primary sampling unit, stratum, and weight variables were employed throughout to generate parameter estimates that are generalizable to the US population.4,5 The χ2 test and logistic regression, respectively, were employed for comparison of proportions and estimation of odds ratios. R Version 4.1.2 was employed for data analysis.
Results
In the overall sample, the frequencies (95% standard error [SE]) of CKD and prior MS were 15.2% (0.3) and 11.5% (0.3) (Table 1). The proportion (SE) with CKD was significantly higher among those with prior MS vs the overall population: 22.7% (0.7) vs 15.2% (0.3) (P < .001). Significant associations with CKD were observed (P < .05) by age, sex, race and ethnicity, family poverty, school education, health insurance, smoking, body mass index, diabetes, hypertension, cardiovascular disease, and malignancy. Within those reporting prior MS, the proportion (SE) with CKD differed by era: 1999 to 2002, 18.9% (1.1); 2003 to 2006, 24.9% (1.5); 2007 to 2010, 22.3% (1.5); 2011 to 2014, 24.3% (1.7); and 2015 to 2018, 24.0% (1.8) (P = .02) (Figure 1).
Without covariate adjustment, prior MS was significantly associated with an increased risk of CKD (unadjusted odds ratio [OR], 1.78; 95% CI, 1.64-1.93; P < .05) (Table 2). Prior MS was significantly associated with CKD in the following subgroups: 2003 to 2006, 2011 to 2014, 2015 to 2018, age groups of 40 to 64 years and ≥ 65 years, male sex, non-Hispanic White and Hispanic ethnicity, school education of grade 0 to 11, and private or other health insurance. Additional comorbidities strongly associated with CKD included hypertension (OR, 6.37; 95% CI, 5.37-7.55), diabetes (OR, 4.16; 95% CI, 3.45-5.03), and cardiovascular disease (OR, 4.20; 95% CI, 3.57-4.95).
In the population reporting prior MS, the unadjusted OR of CKD vs 1999 to 2002 was greater for all other examined eras; with the greatest likelihood observed for the 2003 to 2006 era. Unadjusted ORs of CKD differed in groups with and without prior MS (P value for interaction < .05) for 2003 to 2006, those aged 40 to 64 years and ≥ 65 years, female sex, non-Hispanic African American and Hispanic race and ethnicity, family poverty, high school education, private health insurance, any smoking history, diabetes, hypertension, and cardiovascular disease (Figure 2A).
Following adjustment for age, sex, and race and ethnicity, MS was associated with a 17% higher likelihood of CKD (adjusted odds ratio [AOR], 1.17; 95% CI, 1.06-1.28; P < .01) (Table 3). Prior MS was significantly associated (P < .05) with CKD in the subgroups: age groups 40 to 64 years and ≥ 65 years, non-Hispanic African American, and body mass index ≥ 30. Among those with prior MS, comorbidities strongly associated with CKD in adjusted models included hypertension (AOR, 3.86; 95% CI, 3.18-4.69), diabetes (AOR, 3.05; 95% CI, 2.44-3.82), and cardiovascular disease (AOR, 2.51; 95% CI, 2.09-3.01). In the population with prior MS, the adjusted likelihood of CKD vs 1999 to 2002 was similar across all eras. Adjusted associations of CKD differed in groups with and without prior MS for age groups 40 to 64 years and ≥ 65 years, female sex, and family poverty (P < .05) (Figure 2B).
Discussion
We observed that prior MS was associated with CKD, all eras were associated with CKD in the subgroup with MS, and risk factors for CKD differed among many subgroups both with and without MS history, a finding that remained present in adjusted models. In addition, the finding of CKD was relatively common among those with prior MS (approximately 15%) and was most strongly associated with increasing age and comorbidities frequently associated with CKD.
Although many studies have demonstrated associations of US veteran status with various comorbidities, including hypertension, obesity, and diabetes, these studies often are limited to those both qualifying and receiving care within the US Department of Veterans Affairs (VA) health care system.6-9 The crude proportion of individuals reporting multiple chronic conditions, which included hypertension, diabetes, and weak or failing kidneys, was 49.7% for US veterans compared with 24.1% for nonveterans.2 Large-scale, nationally representative cohorts for use in this context have been limited by the heterogeneity of definitions of CKD applied with limited timeframes yielding variable estimates.1,10 Moreover, few studies have examined the clinical epidemiology of CKD more broadly in the US among those with prior MS. For example, a PubMed search on March 3, 2022, with the terms “epidemiology”, “military service”, and “chronic kidney disease” produced only 9 citations, one of which examined trends among a non-US cohort and quantifying disease burden another among adolescents.
Whether or not prior MS confers a unique risk profile for CKD is unknown. While our findings of an increased CKD burden among those reporting MS may partially reflect observed increases in baseline comorbidities, the observed excess CKD among those with MS remained across multiple categories even after adjustment for baseline demography. As several studies have demonstrated, enlistment into MS may select for a more diverse population; however those enlisted personnel may be of lower socioeconomic status and possibly at higher risk of CKD.11,12 Our findings of important differences in baseline determinants of health mirror this. The proportion of MS respondents with CKD vs CKD alone reporting a high school education or lower was higher (36.0% vs 21.8%) as well as among those with a history of family poverty (21.1% vs 18.0%).
Limitations
Our study has several limitations, including its cross-sectional study design, a lack of longitudinal data within individuals, and exclusion of institutionalized individuals. Limitations notwithstanding this study has several important aspects. As prior MS is highly variable, we were limited in our inability to stratify by service type or length of service. For example, veteran status is conferred to a “Reservist or member of the National Guard called to federal active duty or disabled from a disease or injury incurred or aggravated in line of duty or while in training status also qualify as a veteran” (13 CFR § 125.11). For the purposes of our study, prior MS would include all active-duty service (veterans) as well as reservists and National Guard members who have not been activated. This may be more representative of the overall effect of MS, as limitation to those receiving care within the VA may select for an older, more multimorbid population of patients, limiting generalizability.
In addition, more detailed information regarding service-related exposures and other service-connected conditions would allow for a more granular risk assessment by service type, era, and military conflict. Our finding of excess CKD burden among those with prior MS compared with the overall population is timely given the recent passage of the Promise to Address Comprehensive Toxics (PACT) Act. Exposure to and injury from Agent Orange—a known service-connected exposure associated with incident hypertension and diabetes—may be a significant contributor to CKD that may have a significant era effect. In addition, water contamination among those stationed in Camp Lejeune in North Carolina has notable genitourinary associations. Finally, burn pit exposures in more recent military conflicts may also have important associations with chronic disease, possibly including CKD. While similar attempts at the creation of large-scale US veteran cohorts have been limited by incomplete capture of creatinine, the large proportion of missing race data, and limited inclusion of additional markers of kidney disease, our use of a well-described, nationally representative survey along with standardized capture of clinical and laboratory elements mitigate the use of various societal or other codified definitions.1
Conclusions
Prior MS is associated with an increased risk of CKD overall and across several important subgroups. This finding was observed in various unadjusted and adjusted models and may constitute a unique risk profile of risk.
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
1. Ozieh MN, Gebregziabher M, Ward RC, Taber DJ, Egede LE. Creating a 13-year National Longitudinal Cohort of veterans with chronic kidney disease. BMC Nephrol. 2019;20(1):241. doi:10.1186/s12882-019-1430-y
2. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13.
3. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Survey. 2022. Accessed October 31, 2023. www.cdc.gov/nchs/nhanes/index.htm
4. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi:10.7326/0003-4819-150-9-200905050-00006
5. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50(6):918-926. doi:10.1053/j.ajkd.2007.08.020
6. Smoley BA, Smith NL, Runkle GP. Hypertension in a population of active duty service members. J Am Board Fam Med. 2008;21(6):504-511. doi:10.3122/jabfm.2008.06.070182
7. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139. doi:10.1056/NEJMoa0808431
8. Smith TJ, Marriott BP, Dotson L, et al. Overweight and obesity in military personnel: sociodemographic predictors. Obesity (Silver Spring). 2012;20(7):1534-1538. doi:10.1038/oby.2012.25
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi:10.1001/archinte.160.21.3252
10. Saran R, Pearson A, Tilea A, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA Renal Information System (VA-REINS). Am J Kidney Dis. 2021;77(3):397-405. doi:10.1053/j.ajkd.2020.07.013
11. Wang L, Elder GH, Jr., Spence NJ. Status configurations, military service and higher education. Soc Forces. 2012;91(2):397-422. doi:10.1093/sf/sos174
12. Zeng X, Liu J, Tao S, Hong HG, Li Y, Fu P. Associations between socioeconomic status and chronic kidney disease: a meta-analysis. J Epidemiol Community Health. 2018;72(4):270-279. doi:10.1136/jech-2017-209815
Discontinuation Schedule of Inhaled Corticosteroids in Patients With Chronic Obstructive Pulmonary Disease
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
Impact of the COVID-19 Pandemic on Care for Patients With Atopic Dermatitis
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.
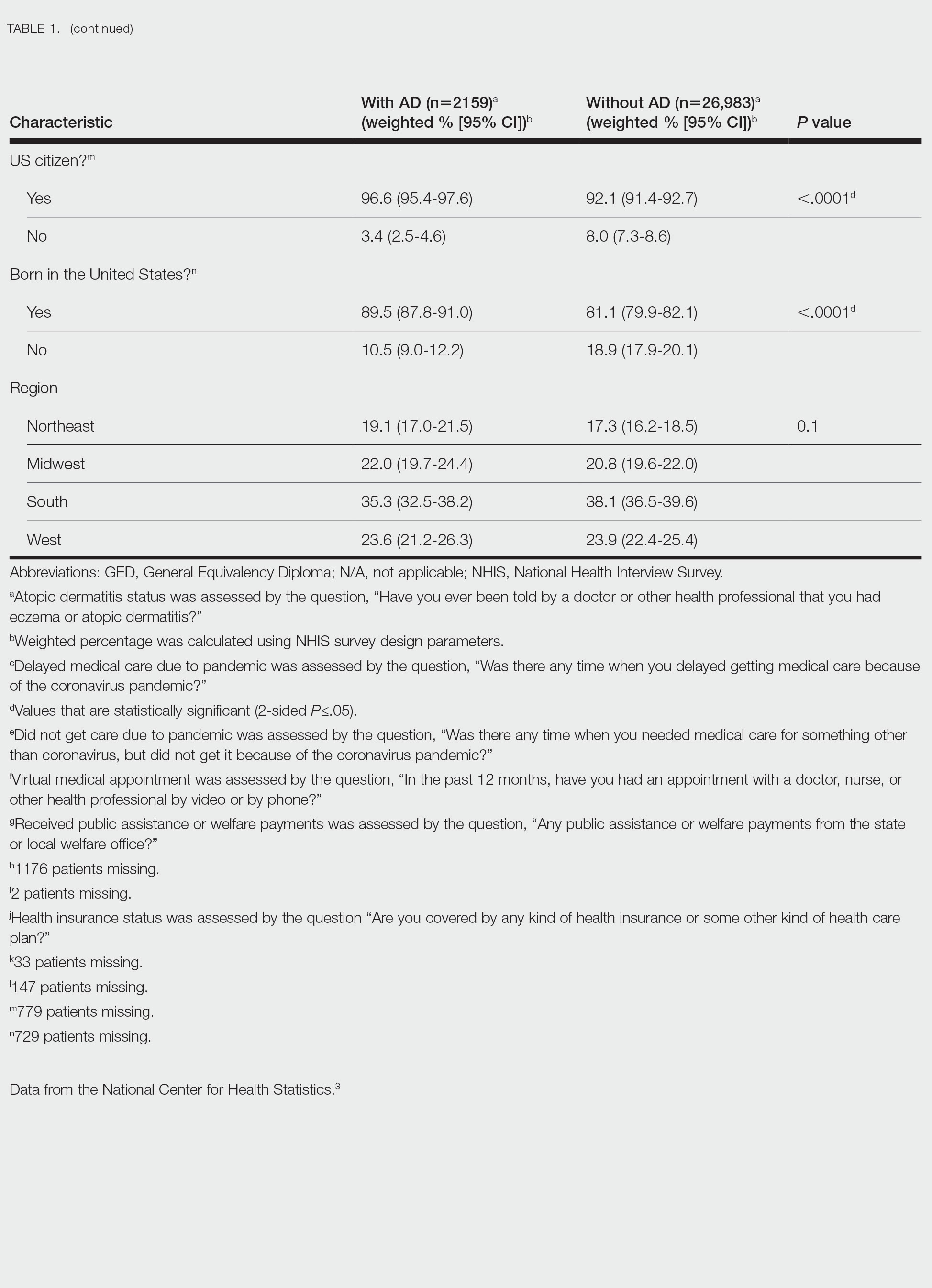
There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.
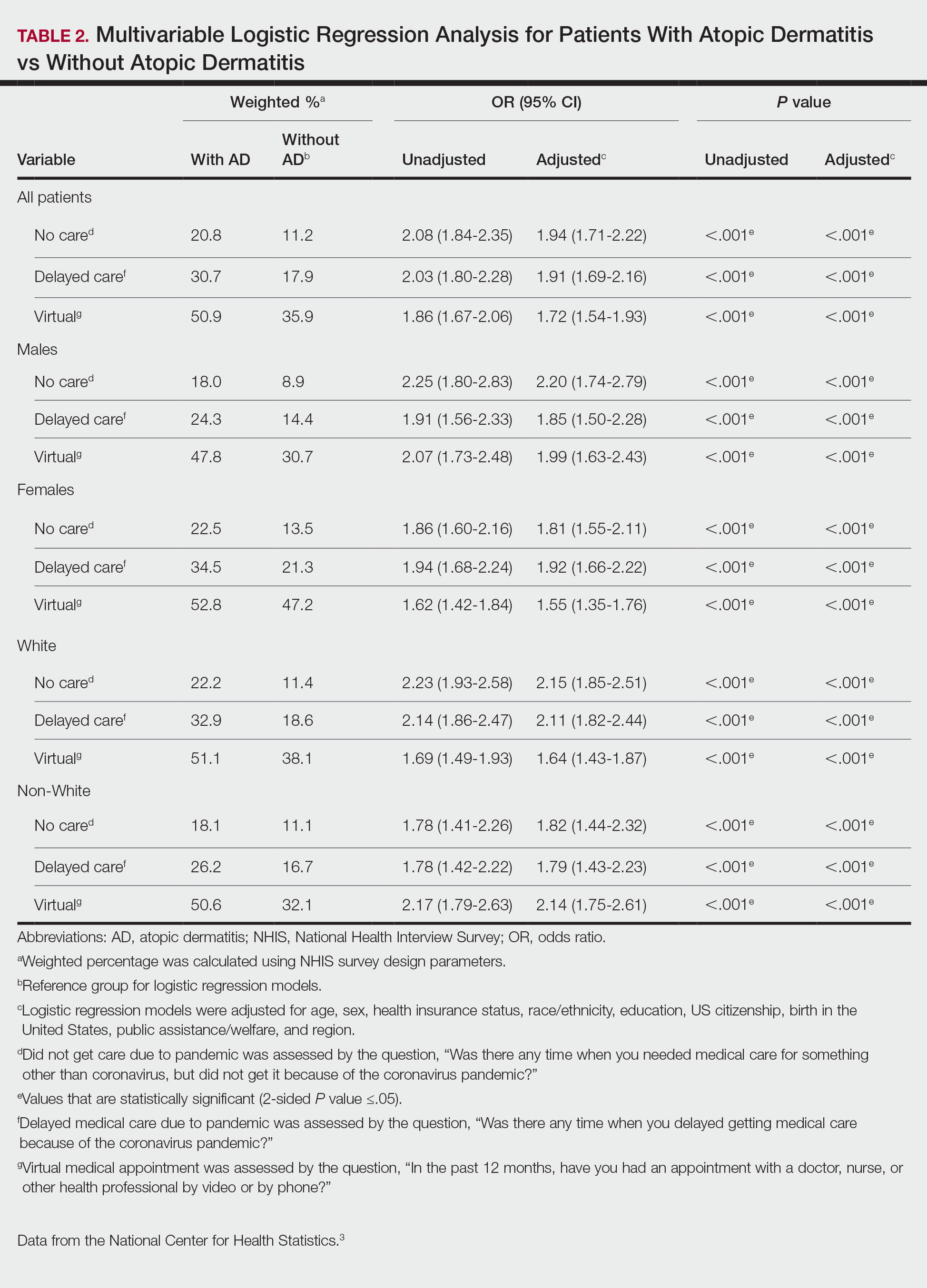
Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
To the Editor:
Atopic dermatitis (AD) is a widely prevalent dermatologic condition that can severely impact a patient’s quality of life.1 Individuals with AD have been substantially affected during the COVID-19 pandemic due to the increased use of irritants, decreased access to care, and rise in psychological stress.1,2 These factors have resulted in lower quality of life and worsening dermatologic symptoms for many AD patients over the last few years.1 One major potential contributory component of these findings is decreased accessibility to in-office care during the pandemic, with a shift to telemedicine instead. Accessibility to care during the COVID-19 pandemic for AD patients compared to those without AD remains unknown. Therefore, we explored the impact of the COVID-19 pandemic on care for patients with AD in a large US population.
Using anonymous survey data from the 2021 National Health Interview Survey,3 we conducted a population-based, cross-sectional study to evaluate access to care during the COVID-19 pandemic for patients with AD compared to those without AD. We assigned the following 3 survey questions as outcome variables to assess access to care: delayed medical care due to COVID-19 pandemic (yes/no), did not get care due to COVID-19 pandemic (yes/no), and virtual medical appointment in the last 12 months (yes/no). In Table 1, numerous categorical survey variables, including sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region, were analyzed using χ2 testing to evaluate for differences among individuals with and without AD. Multivariable logistic regression models evaluating the relationship between AD and access to care were constructed using Stata/MP 17 (StataCorp LLC). In our analysis we controlled for age, sex, health insurance status, race/ethnicity, education, US citizenship, birth in the United States, public assistance/welfare, and region.


There were 29,142 adult patients (aged ≥18 years) included in our analysis. Approximately 7.4% (weighted) of individuals had AD (Table 1). After adjusting for confounding variables, patients with AD had a higher odds of delaying medical care due to the COVID-19 pandemic (adjusted odds ratio [AOR], 1.91; 95% CI, 1.69-2.16; P<.001), not receiving care due to the COVID-19 pandemic (AOR, 1.94; 95% CI, 1.71-2.22; P<.001), and having a virtual medical visit in the last 12 months (AOR, 1.72; 95% CI, 1.54-1.93; P<.001)(Table 2) compared with patients without AD.

Our findings support the association between AD and decreased access to in-person care due to the COVID-19 pandemic. Moreover, telemedicine was utilized more among individuals with AD, possibly due to the accessibility of diagnostic tools for dermatologic diagnoses, such as high-quality photographs.4 According to Trinidad et al,4 telemedicine became an invaluable tool for dermatology hospitalists during the COVID-19 pandemic, as many physicians were able to comfortably diagnose patients with cutaneous diseases without an in-person visit. Utilizing telemedicine for patient care can help reduce the risk for COVID-19 transmission while also providing quality care for individuals living in rural areas.5 Chiricozzi et al6 discussed the importance of telemedicine in Italy during the pandemic, as many AD patients were able to maintain control of their disease while on systemic treatments.
Limitations of this study include self-reported measures; inability to compare patients with AD to individuals with other cutaneous diseases; and additional potential confounders, such as chronic comorbidities. Future studies should evaluate the use of telemedicine and access to care among individuals with other common skin diseases and help determine why such discrepancies exist. Understanding the difficulties in access to care and the viable alternatives in place may increase awareness and assist clinicians with adequate management of patients with AD.
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
1. Sieniawska J, Lesiak A, Cia˛z˙yn´ski K, et al. Impact of the COVID-19 pandemic on atopic dermatitis patients. Int J Environ Res Public Health. 2022;19:1734. doi:10.3390/ijerph19031734
2. Pourani MR, Ganji R, Dashti T, et al. Impact of COVID-19 pandemic on patients with atopic dermatitis [in Spanish]. Actas Dermosifiliogr. 2022;113:T286-T293. doi:10.1016/j.ad.2021.08.004
3. National Center for Health Statistics. NHIS Data, Questionnaires and Related Documentation. Centers for Disease Control and Prevention website. Accessed February 1, 2023. https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm
4. Trinidad J, Gabel CK, Han JJ, et al. Telemedicine and dermatology hospital consultations during the COVID-19 pandemic: a multi-centre observational study on resource utilization and conversion to in-person consultations during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2022;36:E323-E325. doi:10.1111/jdv.17898
5. Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511. doi:10.3390/jcm11061511
6. Chiricozzi A, Talamonti M, De Simone C, et al. Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: data from the DA-COVID-19 registry. Allergy. 2021;76:1813-1824. doi:10.1111/all.14767
Practice Points
- The landscape of dermatology has seen major changes due to the COVID-19 pandemic, as many patients now utilize telemedicine to receive care.
- Understanding accessibility to in-person care for patients with atopic dermatitis during the COVID-19 pandemic can assist with the development of methods to enhance management.
Monitoring Thyrotropin in Veterans With Thyroid Nodules
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
When thyroid nodules are found clinically or incidentally on imaging, the patient’s thyrotropin level should be measured.1 Ultrasound is the first-line imaging recommended to assess thyroid nodules.1,2 Nodules can then be evaluated by a fine-needle aspiration (FNA) biopsy, which provides cytological information to determine whether the nodule is benign or malignant.1,3,4 Most thyroid nodules pose a low risk of malignancy.1
The American Thyroid Association guidelines on thyroid nodule management do not specify any recommendations for follow-up thyrotropin testing in patients who do not have any history that is known to affect thyroid function.1 Therefore, clinicians have to make decisions regarding follow-up testing in these patients without any evidence-based guidelines. There is a lack of data in the literature on whether thyrotropin levels change over time in this patient population. If thyrotropin levels do not become abnormal over time, then patients would not need thyrotropin monitoring or treatment for hypo- or hyperthyroidism.
The aim of this study was to determine whether thyrotropin levels change over time in patients with thyroid nodules and determine whether repeat thyrotropin testing was required after initial testing. The authors hypothesized that thyrotropin values do not change substantially over time in patients with thyroid nodules, except in patients with a history of hot nodules, autoimmune thyroid disease, thyroid or pituitary surgery, radioactive iodine ablation, neck radiation, or use of medications affecting thyroid function. This study may be able to contribute to the clinical guidelines for thyrotropin testing in patients with thyroid nodules so that clinicians can make evidence-based decisions.
METHODS
This retrospective chart review was conducted using the Computerized Patient Record System at the Veterans Affairs Dayton Healthcare System (VADHS) in Ohio. Patients aged ≥ 18 years who were diagnosed with ≥ 1 thyroid nodule from January 2010 to December 2016 and had a normal thyrotropin level at the time of diagnosis were included in the study. Patients who were found to have thyroid nodules multiple times were included only once from the time of the initial diagnosis. Patients were excluded if they had a medical history known to affect thyroid function. Exclusion criteria included a history of hot thyroid nodules; autoimmune thyroid disease on imaging or blood work; history of thyroid surgery, including pituitary surgery; history of radioactive iodine treatment; history of neck radiation; use of thyroxine before nodule diagnosis; use of amiodarone, programmed cell death-1 inhibitors, programmed cell death ligand-1 inhibitors, or cytotoxic T-lymphocyte-associated protein-4 inhibitors; or 3 consecutive months of steroid use.
Age at nodule diagnosis, sex, race, thyrotropin values at and after the time of nodule diagnosis, and duration from nodule diagnosis to most recent thyrotropin value were retrospectively collected until 100 patients met inclusion criteria for the study. Of note, from 2010 to 2016, the assays used at the VADHS to measure thyrotropin values changed over time, as did the normal reference ranges and the type of sample used for the assays. Normal thyrotropin range at time of diagnosis based on serum or plasma samples and for repeat thyrotropin levels are provided in Table 1, also based on serum or plasma samples. All collected data in the study was de-identified for analysis.
Statistical Analysis
Patients were divided into 2 groups: those who had an abnormal most recent thyrotropin value and those who did not. Mean (SD) of both groups was calculated for continuous variables of age at diagnosis, initial thyrotropin value and most recent thyrotropin value, and time from diagnosis to most recent thyrotropin value. Percentages for both groups were calculated for categorical variables of sex, race, and whether initial and most recent thyrotropin values were based on serum or plasma samples and old or new reference ranges. A 95% CI was determined for the true population rate of patients with an abnormal thyrotropin value at most recent testing. Independent sample t tests were used to compare the continuous variables between the abnormal and normal most recent thyrotropin groups. Categorical variables between the 2 groups were compared using χ2 tests. P < .05 was considered statistically significant. Statistical analyses were completed using IBM SPSS Statistics 27. This study was approved by the Wright State University Institutional Review Board and the VADHS Research and Development Committee.
RESULTS
Of 557 patient charts studied, 100 patients were included; the mean (SD) age at nodule diagnosis was 62.4 (11.1) years, and the mean (SD) initial thyrotropin level at nodule diagnosis was 1.51 (0.87) μIU/mL. The mean (SD) most recent thyrotropin level was 1.60 (1.03) μIU/mL after a mean duration of 5.7 (2.5) years postnodule diagnosis (Table 2).
Six patients (6%; 95% CI, 2.5%-12.7%) who had a normal thyrotropin level at nodule diagnosis developed an abnormal thyrotropin level in a mean (SD) of 6.9 (3.1) years. These 6 patients had a mean age at nodule diagnosis of 69.2 (11.4) years. Five of the 6 were male, and all were White patients. One patient’s thyrotropin level rose from an initial thyrotropin of 3.38 μIU/mL at nodule diagnosis to a high of 7.76 μIU/mL after 8.5 years. This patient was diagnosed with subclinical hypothyroidism and did not require treatment.
Five patients’ thyrotropin levels dropped below normal in a mean 7 years, with levels ranging from 0.25 to 0.52 μIU/mL. Of these patients, 2 became symptomatic from the nodules, experiencing dysphagia or hoarseness, with 1 diagnosed with hyperthyroidism. This patient was treated with methimazole and radioactive iodine ablation 9 years after diagnosis. The other 3 patients who developed low thyrotropin had no nodule symptoms or treatment. Ninety-four patients maintained thyrotropin values in the normal range for a mean (SD) of 5.7 (2.5) years and had a mean (SD) age at nodule diagnosis of 61.9 (11.0) years.
Both thyrotropin groups were compared. For categorical variables, there were no significant differences for sex (
Of note, the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma and had 3 different normal reference ranges during the 2010 to 2016 period studied. The thyrotropin values fell into 4 categories: serum sample with normal range 0.4 to 5.5 μIU/mL, serum sample with normal range 0.4 to 4.0 μIU/mL, plasma sample with normal range 0.4 to 4.0 μIU/mL, and plasma sample with normal range 0.6 to 4.8 μIU/mL. There were no significant differences between the abnormal and normal most recent thyrotropin groups in sample type for initial or most recent thyrotropin (P = .44 and P = .99, respectively) or in normal range for initial or most recent thyrotropin level (P = .99 and P = .09, respectively).
DISCUSSION
We found no statistically significant change in blood thyrotropin levels over time among patients with thyroid nodules with no history of medical conditions or medications known to affect thyroid hormone levels. Six of 100 patients developed abnormal thyrotropin, but only 2 eventually were treated for thyroid dysfunction: 1 for hypothyroidism and 1 for hyperthyroidism. The other 4 patients who did not receive treatment developed low thyrotropin but had no official diagnosis of hyperthyroidism in their health records, seemingly due to lack of multiple, consistently low thyrotropin values or due to lack of follow-up. Based on these data, monitoring thyrotropin over time may not be necessary in patients without any medical history known to affect thyroid function. The results provide support for the original hypothesis.
Although only thyrotropin values at the time of nodule diagnosis and most recent thyrotropin values were analyzed, thyrotropin trends over time were considered. Some patients did have transient abnormal thyrotropin values; however, a search of the patients’ records showed that these transient abnormalities did not lead to any initiation of hypothyroidism or hyperthyroidism treatment.
Another consideration is that changes in the sample type processed and in the normal thyrotropin ranges over time could have been confounding variables. However, statistical analyses showed that the abnormal and normal most recent thyrotropin groups did not show any significant differences in sample type or reference range for either the initial or most recent thyrotropin values. Hospitals change the laboratory assays they use for clinical tests over time, but these changes likely did not affect the results of this study.
The data from this study showed similar results to previously reported research. This study found that 6% of patients developed abnormal thyrotropin levels over time. A study of 157 patients with nonfunctioning benign thyroid nodules found that 8.3% of patients developed thyroid dysfunction.5 In another follow-up study on patients with thyroid nodules who were otherwise euthyroid, 2 of 118 patients eventually received treatment for hyperthyroidism.6 In the current study, we report that just 1 of 100 included patients had to begin treatment for hyperthyroidism.
The literature also includes research on using thyrotropin and age to predict malignancy in patients with thyroid nodules. One study suggested that a thyrotropin cutoff point of ≥ 2.1 mU/I and an age cutoff point of ≥ 47 years were significantly associated with a diagnosis of malignancy.7 Although the current study did not study malignancy, the results showed that the mean age at nodule diagnosis was higher in patients who had abnormal vs normal most recent thyrotropin levels: 69 vs 62 years, respectively. Future studies could determine whether a certain initial thyrotropin value or age could be used as a cutoff for requiring further thyrotropin monitoring to check for development of hyperthyroidism or hypothyroidism.
Limitations
This study was limited by its small size of 100 subjects. Most patients had to be excluded to focus on the aim of determining whether thyrotropin monitoring is needed in the specific group of patients without medical history that would be expected to affect thyroid function. Another limitation was that 83% of the patients included in the study were male, which does not reflect the general population. Future studies should include a greater number of patients and aim for a balance of 50% male and 50% female patients.
Additionally, it is important to note that the changing definition of the normal thyrotropin range was a limitation. It is possible that some patients who were considered normal at the time of a particular thyrotropin measurement may have had an abnormal reading if measured at a different time. Another consideration is that the VADHS changed the type of blood sample used to generate thyrotropin values from serum to plasma during the time that analyzed thyrotropin values were measured. This could have led to different thyrotropin values and, therefore, different results of this study compared with if the sample type had stayed the same. However, a previous study showed very similar thyrotropin values generated from serum and plasma samples in 17 patients.8 Therefore, possibly the change in sample type in the current study only minimally affected the results.
CONCLUSIONS
Current American Thyroid Association guidelines do not specify recommendations for follow-up thyrotropin testing in patients with thyroid nodules who do not have a history of conditions or medications known to affect thyroid hormone levels.1 This study suggests that repeat thyrotropin monitoring may not be necessary for this group of patients. Individuals who had an abnormal most recent thyrotropin had an older age at thyroid nodule diagnosis compared with patients who had a normal most recent thyrotropin, so it is possible that thyrotropin monitoring may be recommended for people with nodules who are above a certain age. The results of this study as well as future studies could help create new clinical recommendations for thyrotropin monitoring in patients with thyroid nodules that clinicians can use to make evidence-based clinical decisions. There would also be a decreased financial, physical, and time burden on the patients if guidelines specify that they are not required to get continued blood thyrotropin testing.
Acknowledgments
The authors acknowledge Ronald J. Markert, PhD, formerly of Wright State University Boonshoft School of Medicine, for his contributions to the statistical analysis of this research.
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi:10.1089/thy.2015.0020
2. Chambara N, Liu SYW, Lo X, Ying M. Diagnostic performance evaluation of different TI-RADS using ultrasound computer-aided diagnosis of thyroid nodules: an experience with adjusted settings. PLoS One. 2021;16(1):e0245617. doi:10.1371/journal.pone.0245617
3. Livhits MJ, Zhu CY, Kuo EJ, et al. Effectiveness of molecular testing techniques for diagnosis of indeterminate thyroid nodules: a randomized clinical trial. JAMA Oncol. 2021;7(1):70-77. doi:10.1001/jamaoncol.2020.5935
4. Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95-102. doi:10.1210/jc.2018-01674
5. Memon R, Salgado Nunez Del Prado SR, Lamos EM, et al. Biochemical follow-up of nonfunctioning benign thyroid nodules. Clin Endocrinol (Oxf). 2021;94(2):322-329. doi:10.1111/cen.14303
6. Bajuk Studen K, Gaberscek S, Pirnat E, Zaletel K. Five-year follow-up and clinical outcome in euthyroid patients with thyroid nodules. Radiol Oncol. 2021;55(3):317-322. Published 2021 May 31. doi:10.2478/raon-2021-0025
7. Fernández-Trujillo C, Pérez-Zaballos J, Rodríguez-Pérez CA, et al. TSH level and risk of malignancy in patients with Bethesda category IV thyroid nodules. Horm Cancer. 2020;11(3-4):200-204. doi:10.1007/s12672-020-00384-4
8. Villanger GD, Learner E, Longnecker MP, et al. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology. 2017;28(3):365-369. doi:10.1097/EDE.0000000000000606
Elective Hand Surgery and Antithrombotic Use in Veterans
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
Patients planning plastic surgery traditionally were instructed to stop anticoagulants and antiplatelet medications during the perioperative period to avoid bleeding, which could result in flap loss, pain, skin necrosis, and blood transfusions. In the veteran patient population, anticoagulants are prescribed for the prevention of limb- and life-threatening embolic and thrombotic events.1-3 As of June 2021, > 332,000 veterans were prescribed direct oral anticoagulants.1
In 2015, the Malcom Randall Veterans Affairs Medical Center (MRVAMC) in Gainesville, Florida, Plastic Surgery Service began instructing patients planning elective hand surgery to continue their prescription anticoagulants and antiplatelets during the perioperative period. This decision was prompted by a patient who needed carpal tunnel release surgery and was prescribed coumadin for repeated thrombosis of his dialysis grafts. Hand surgery literature at the time suggested allowing patients to continue their anticoagulants and antiplatelets through the perioperative period to avoid life- and limb-threatening events and wide fluctuations in blood anticoagulant levels.4-6 The MRVAMC Plastic Surgery Service chose to accept the risk of perioperative bleeding after shared decision making with the patients rather than risk a cardiac stent obstruction, pulmonary embolism, or embolic stroke in the at-risk patients.
The objective of this study was to determine the postoperative bleeding complication rate over a 7.5-year period in the veteran patients who did not interrupt their prescription blood thinners. This would assist the MRVAMC Plastic Surgery Service with providing data-driven informed consent and determine whether this protocol should continue.
Methods
The North Florida/South Georgia Veterans Health System Research Committee and the University of Florida Institutional Review Board approved a retrospective chart review of elective hand cases performed by the MRVAMC Plastic Surgery Service from January 1, 2015, through June 30, 2022. Elective hand cases were identified based on the operation description and included nerve decompressions, tendon releases, trapeziectomy, small-joint fusion, neurectomy, elective amputations, and benign neoplasm removals (Table). Hand surgery included cubital tunnel releases (decompression of the ulnar nerve at the level of the elbow) because hand surgery fellowships, hand surgery training, and hand surgery practices traditionally include a high volume of cubital tunnel releases. We wanted this study to have real-world applications.
Patients’ histories and physicals were reviewed for prescription antithrombotics and for instructions not to interrupt these medications. Postoperative notes were reviewed for 30 days for evidence of postoperative bleeding complications.
The following prescription anticoagulants were included in the study: dabigatran, rivaroxaban, warfarin, edoxaban, and apixaban. In addition, the following prescription antiplatelets were included in the study:
Results
One hundred seventy-eight patients were identified for maintaining prescription blood thinners during their elective hand surgery. There was 1 major complication (0.6%) and 4 minor bleeding complications (2.2%). The major complication occurred when a patient had to return to surgery from the recovery room for emergent control of bleeding. The surgery was for an in situ cubital tunnel release. The patient, aged 48 years, was taking clopidogrel and aspirin and had a personal and family history of cardiovascular disease. The bleeding was controlled with bipolar cautery and Floseal, a topical haemostatic matrix made of bovine gelatin and human thrombin. The minor bleeding complications were treated in the clinic with compression, wound care, or expedited follow-up for reassurance. These included an in situ cubital tunnel release for a patient taking warfarin and aspirin, a digital inclusion cyst for a patient taking apixaban, an endoscopic carpal tunnel for a patient taking aspirin and clopidogrel, and an open carpal tunnel and ulnar tunnel release for a patient taking aspirin and clopidogrel. There were no thrombotic events during the study.
Discussion
Higher utilization of anticoagulation has been evidenced by a 30% increase in Medicare claims and a 277% increase in Medicaid anticoagulation claims between 2014 and 2019, driven by more prescriptions for direct oral anticoagulants such as apixaban and rivaroxaban.7 The MRVAMC Plastic Surgery Service began a protocol for managing perioperative anticoagulation in 2015 to avoid the risk of perioperative thrombotic events in veteran patients. Patients who choose elective hand surgery were instructed to continue their prescription blood thinners. Exceptions to this protocol were patients scheduled for a partial fasciectomy (for Dupuytren contracture) or cubital tunnel release with anterior ulnar nerve transposition. A hematoma would increase the risk for skin necrosis in the patients receiving a fasciectomy, resulting from the thin skin flaps and meticulous dissection to identify and protect the digital nerves. Worsening nerve dysfunction could result from hematoma compression and scarring in the ulnar nerve cases. If the risk of holding the blood thinner was felt to be unreasonably high, based on recommendations from the patients’ cardiologist or primary care doctor, we offered an in situ cubital tunnel release for the ulnar nerve patients.
Concerns regarding interrupting chronic anticoagulation involve the increased risk of thromboembolism and the theoretical risk of a rebound hypercoagulable effect.8 Patients prescribed warfarin have been found to unintentionally discontinue this medication after outpatient surgery at more than 1.5 times the rate of the general population.9
A systematic review of 9 published studies looking specifically at elective hand and wrist surgeries demonstrated no significant increase in perioperative bleeding risk with the continuation of anticoagulation and antiplatelet medications.10 Sardenberg and colleagues reviewed 7 studies in which 410 hand and wrist surgeries were performed in patients prescribed warfarin or aspirin and clopidogrel. These patients had a 0.7% serious complication rate, requiring surgical treatment only in patients having complex wrist surgeries (wrist arthrodesis with tenosynovectomy, resection of the distal ulna with tenosynovectomy and tendon transfer, and proximal row carpectomy).11 Bogunovic and colleagues compared 50 hand and wrist patients who were on uninterrupted warfarin with those who were not. They required patients to have an
These and our study are consistent with other disciplines, such as facial plastic surgery, dermatology, and ophthalmology, which do not support routine suspension of anticoagulants.13-16 A review of 30 cutaneous surgery studies involving > 14,000 patients recommended meticulous hemostasis over cessation of blood thinners.15 The University of Massachusetts Dermatology Clinic found a 40 times higher rate of bleeding complications in patients on clopidogrel and warfarin but still recommended continuation of these medications to avoid thrombotic events.16
Limitations
This study is a retrospective chart review and limited by what is already documented in the electronic health record. We can verify that the patients were given instructions to continue their medications up to the day of surgery but cannot be certain whether the instructions were followed. No control group was told to hold their anticoagulants for the same surgery. Once we decided on a protocol, we applied it to all patients. The study approval was for the specific time frame when the protocol was in place.
Our study was designed for elective hand cases because those surgeries can be anticipated, predicted, and patients can be given instructions during the preoperative appointments. We did incidentally find several nonelective hand cases (traumas, infections, and cancers) during the review of patients taking prescription blood thinners that had to be expedited to the operating room. Based on morbidity data during that time period, there were no additional postoperative hand surgery bleeding complications that had to return to the operating room. Future studies are indicated, but we believe our protocol can be applied to urgent and emergent hand surgeries as well as elective cases.
Conclusions
Our study supports continuing prescription anticoagulant and antiplatelet medications during the perioperative period for elective hand surgery. We found this is a safe practice in our veteran population with an acceptably low local bleeding complication rate.
Acknowledgments
This manuscript is the result of work supported with the resources and the use of facilities at the North Florida/South Georgia Veterans Health System in Gainesville, Florida.
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
1. Allen AL, Lucas J, Parra D, et al. Shifting the paradigm: a population health approach to the management of direct oral anticoagulants. J Am Heart Assoc. 2021;10(24):e022758. doi:10.1161/JAHA.121.022758
2. Buck J, Kaboli P, Gage BF, Cram P, Vaughan Sarrazin MS. Trends in antithrombotic therapy for atrial fibrillation: data from the Veterans Health Administration health system. Am Heart J. 2016;179:186-191. doi:10.1016/j.ahj.2016.03.029
3. Kinlay S, Young MM, Sherrod R, Gagnon DR. Long-term outcomes and duration of dual antiplatelet therapy after coronary intervention with second-generation drug-eluting stents: the Veterans Affairs Extended DAPT Study. J Am Heart Assoc. 2023;12(2):e027055.
4. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of antiplatelet medication on hand and wrist surgery. J Hand Surg Am. 2013;38(6):1063-1070. doi:10.1016/j.jhsa.2013.03.034
5. Wallace DL, Latimer MD, Belcher HJ. Stopping warfarin therapy is unnecessary for hand surgery. J Hand Surg Br. 2004;29(3):203-205. doi:10.1016/j.jhsb.2003.12.008
6. Edmunds I, Avakian Z. Hand surgery on anticoagulated patients: a prospective study of 121 operations. Hand Surg. 2010;15(2):109-113. doi:10.1142/S021881041000468
7. Duvalyan A, Pandey A, Vaduganathan M, et al. Trends in anticoagulation prescription spending among Medicare Part D and Medicaid beneficiaries between 2014 and 2019. J Am Heart Assoc. 2021;10(24):e022644. doi:10.1161/JAHA.121.022644
8. Thakur NA, Czerwein JK, Butera JN, Palumbo MA. Perioperative management of chronic anticoagulation in orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(12):729-738. doi:10.5435/00124635-201012000-00003
9. Bell C, Bajca J, Bierman A, Li P, Mamdani M, Urbach D. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Int Med. 2003;166(22):2525-2531.
10. Stone MJ, Wilks DJ, Wade RG. Hand and wrist surgery on anticoagulants and antiplatelets: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2020;73(8):1413-1423.
11. Sardenberg T, Deienno FS, Miranda RF, et al. Hand and wrist surgery without suspending warfarin or oral antiplatelet - systematic review. Rev Bras Ortop. 2017;52(4):390-395. doi:10.1016/j.rboe.2017.07.001
12. Bogunovic L, Gelberman RH, Goldfarb CA, Boyer MI, Calfee RP. The impact of uninterrupted warfarin on hand and wrist surgery. J Hand Surg Am. 2015;40(11):2133-2140. doi:10.1016/j.jhsa.2015.07.037
13. Kraft CT, Bellile E, Baker SR, Kim JC, Moyer JS. Anticoagulant complications in facial plastic and reconstructive surgery. JAMA Facial Plast Surg. 2015;17(2):103-107. doi:10.1001/jamafacial.2014.1147
14. He X, Chen AF, Nirwan RS, Sridhar J, Kuriyan AE. Perioperative management of anticoagulants in ocular surgeries. Int Ophthalmol Clin. 2020;60(3):3-15. doi:10.1097/IIO.0000000000000316
15. Isted A, Cooper L, Colville RJ. Bleeding on the cutting edge: a systematic review of anticoagulant and antiplatelet continuation in minor cutaneous surgery. J Plast Reconstr Aesthet Surg. 2018;71(4):455-467. doi:10.1016/j.bjps.2017.11.024
16. Bordeaux JS, Martires KJ, Goldberg D, Pattee SF, Fu P, Maloney ME. Prospective evaluation of dermatologic surgery complications including patients on multiple antiplatelet and anticoagulant medications. J Am Acad Dermatol. 2011;65(3):576-583. doi:10.1016/j.jaad.2011.02.012
Are Text Pages an Effective Nudge to Increase Attendance at Internal Medicine Morning Report Conferences? A Cluster Randomized Controlled Trial
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
Community Nursing Home Program Oversight: Can the VA Meet Increased Demand for Community-Based Care?
The US Department of Veterans Affairs (VA) Community Nursing Home (CNH) program provides 24-hour skilled nursing care for eligible veterans in public or private community-based facilities that have established a contract to care for veterans. Veteran eligibility is based on service-connected status and level of disability, covering the cost of care for veterans who need long-term care because of their service-connected disability or for veterans with disabilities rated at ≥ 70%.1 Between 2014 and 2018, the average daily census of veterans in CNHs increased by 26% and the percentage of funds obligated to this program increased by 49%.2 The VA projects that the number of veterans receiving care in a CNH program will increase by 80% between 2017 and 2037, corresponding to a 149% increase in CNH expenditures.2
CNH program oversight teams are mandated at each VA medical center (VAMC) to monitor care coordination within the CNH program. These teams include nurses and social workers (SWs) who perform regular on-site assessments to monitor the clinical, functional, and psychosocial needs of veterans. These assessments include a review of the electronic health record (EHR) and face-to-face contact with veterans and CNH staff, regardless of the purchasing authority (hospice, long-term care, short-term rehabilitation, respite care).3 These teams represent key stakeholders impacted by CNH program expansion.
While the CNH program has focused primarily on the provision of long-term care, the VA is now expanding to include short-term rehabilitation through Veteran Care Agreements.4 These agreements are authorized under the MISSION Act, designed to improve care for veterans.5 Veteran Care Agreements are expected to be less burdensome to execute than traditional contracts and will permit the VA to partner with more CNHs, as noted in a Congressional Research Service report regarding long-term care services for veterans.6 However, increasing the number of CNHs increases demands on oversight teams, particularly if the coordinators are compelled to perform monthly on-site visits to facilities required under current guidelines.3
The objective of this study was to describe the experiences of VA and CNH staff involved in care coordination and the oversight of veterans receiving CNH care amid Veteran Care Agreement implementation and in anticipation of CNH program expansion. The results are intended to inform expansion efforts within the CNH program.
METHODS
This study was a component of a larger research project examining VA-purchased CNH care; recruitment methods are available in previous publications describing this work.7 Participants provided written or verbal consent before video and phone interviews, respectively. This study was approved by the Colorado Multiple Institutional Review Board (Protocol #18-1186).
Video and phone interviews were conducted by 3 team members from October 2018 to March 2020 with CNH staff and VA CNH program oversight team members. Participant recruitment was paused from May to October 2020 as a result of the COVID-19 pandemic and ambiguity about VA NH care purchasing policies following the passage of the VA MISSION Act.5 We used semistructured interview guides (eAppendix 1 for VA staff and eAppendix 2 for NH staff, available online at doi:10.12788/fp.0421). Recorded and transcribed interviews ranged from 15 to 90 minutes.
Two members of the research team analyzed transcripts using both deductive and inductive content analysis.8 The interview guide informed an a priori codebook, and in vivo codes were included as they emerged. We jointly coded 6 transcripts to reach a consensus on coding approaches and analyzed the remaining transcripts independently with frequent meetings to develop themes with a qualitative methodologist. All qualitative data were analyzed using ATLAS.ti software.
This was a retrospective observational study of veterans who received VA-paid care in CNHs during the 2019 fiscal year (10/1/2018-9/30/2019) using data from the enrollment, inpatient and outpatient encounters, and other care paid for by the VA in the VA Corporate Data Warehouse. We linked Centers for Medicare and Medicaid monthly Nursing Home Compare reports and the Brown University Long Term Care: Facts on Care in the US (LTC FoCUS) annual files to identify facility addresses.9
Descriptive analyses of quantitative data were conducted in parallel with the qualitative findings.8 Distance from the contracting VAMC to CNH was calculated using the greater-circle formula to find the linear distance between geographic coordinates. Quantitative and qualitative data were collected concurrently, analyzed independently, and integrated into the interpretation of results.10
RESULTS
We conducted 36 interviews with VA and NH staff who were affiliated with 6 VAMCs and 17 CNHs. Four themes emerged concerning CNH oversight: (1) benefits of VA CNH team engagement/visits; (2) burden of VA CNH oversight; (3) burden of oversight limited the ability to contract with additional NHs; and (4) factors that ease the burden and facilitate successful oversight.
Benefits of Engagement/Visits
VA SWs and nurses visit each veteran every 30 to 45 days to review their health records, meet with them, and check in with NH staff. In addition, VA SWs and nurses coordinate each veteran’s care by working as liaisons between the VA and the NH to help NH staff problem solve veteran-related issues through care conferences. VA SWs and nurses act as extra advocates for veterans to make sure their needs are met. “This program definitely helps ensure that veterans are receiving higher quality care because if we see that they aren’t, then we do something about it,” a VA NH coordinator reported in an interview.
NH staff noted benefits to monthly VA staff visits, including having an additional person coordinating care and built-in VA liaisons. “It’s nice to have that extra set of eyes, people that you can care plan with,” an NH administrator shared. “It’s definitely a true partnership, and we have open and honest conversations so we can really provide a good service for our veterans.”
Distance & High Veteran Census Burdens
VA participants described oversight components as burdensome. Specifically, several VA participants mentioned that the charting they completed in the facility during each visit proved time consuming and onerous, particularly for distant NHs. To accommodate veterans’ preferences to receive care in a facility close to their homes and families, VAMCs contract with NHs that are geographically spread out. “We’re just all spread out… staff have issues driving 2 and a half hours just to review charts all day,” a VA CNH coordinator explained. In 2019, the mean distance between VAMC and NH was 48 miles, with half located > 32 miles from the VAMC. One-quarter of NHs were > 70 miles and 44% were located > 50 miles from the VAMC (Figure 1).
Participants highlighted how regular oversight visits were particularly time consuming at CNHs with a large contracted population. VA nurses and SWs spend multiple days and up to a week conducting oversight visits at facilities with large numbers of veterans. Another VA nurse highlighted how charting requirements resulted in several days of documentation outside of the NH visit for facilities with many contracted veteran residents. Multiple VA participants noted that having many veterans at an NH exacerbated the oversight burdens. In 2019, 252 (28%) of VA CNHs had > 10 contracted veterans and 1 facility had 34 veterans (Figure 2). VA participants perceived having too many veterans concentrated at 1 facility as potentially challenging for CNHs due to the complex care needs of veterans and the added need for care coordination with the VA. One VA NH coordinator noted that while some facilities were “adept at being able to handle higher numbers” of veterans, others were “overwhelmed.” Too many veterans at an NH, an SW explained, might lead the “facility to fail because we are such a cumbersome system.”
Oversight & Staffing Burden
While several participants described wanting to contract with more NHs to avoid overwhelming existing CNHs and to increase choice for veterans, they expressed concerns about their ability to provide oversight at more facilities due to limited staffing and oversight requirements. Across VAMCs, the median number of VA CNHs varied substantially (Figure 3). One VA participant with about 35 CNHs explained that while adding more NHs could create “more opportunities and options” for veterans, it needs to be balanced with the required oversight responsibilities. One VA nurse insisted that more staff were needed to meet current and future oversight needs. “We’re all getting stretched pretty thin, and just so we don’t drop the ball on things… I would like to see a little more staff if we’re gonna have a lot more nursing homes.”
Participants had concerns related to the VA MISSION Act and the possibility of more VA-paid NHs for rehabilitation or short-term care. Participants underscored the necessity for additional staff to account for the increased oversight burden or a reduction in oversight requirements. One SW felt that increasing the number of CNHs would increase the required oversight and the need for collaboration with NH staff, which would limit her ability to establish close and trusting working relationships with NH staff. Participants also described the challenges of meeting their current oversight requirements, which limited extra visits for acute issues and care conferences. This was attributed to a lack of adequate staffing in the VA CNH program, given the time-intensive nature of VA oversight requirements.
Easing Burden & Facilitating Oversight
Participants noted how obtaining remote access to veterans’ EHRs allowed them to conduct chart reviews before oversight visits. This permitted more time for interaction with veterans and CNH staff as well as coordinating care. While providing access to the VA EHR would not change the chart review component of VA oversight, some participants felt it might improve care coordination between VA and NH staff during monthly visits.
Participants felt they were able to build strong working relationships with facilities with more veterans due to frequent communication and collaboration. VA participants also noted that CNHs with larger veteran censuses were more likely to respond to VA concerns about care to maintain the business relationship and contract. To optimize strong working relationships and decrease the challenges of having too many veterans at a facility, some VA participants suggested that CNH programs create a local policy to recommend the number of veterans placed in a CNH.
Discussion
Participants interviewed for this study echoed findings from previous work that identified the importance of developing trusted working relationships with CNHs to care for veterans.11,12 However, interorganizational care coordination, a shortage of health care professionals, and resource demands associated with caring for veterans reported in other community care settings were also noted in our findings.12,13
Building upon prior recommendations related to community care of veterans, our analysis identified key areas that could improve CNH program oversight efficiency, including: (1) improving the interoperability of EHRs to facilitate coordination of care and oversight; (2) addressing inefficiencies associated with traveling to geographically dispersed CNHs; and (3) “right-sizing” the number of veterans residing in each CNH.
The interoperability of EHRs has been cited by multiple studies of VA community care programs as critical to reducing inefficiencies and allowing more in-person time with veterans and staff in care coordination, especially at rural locations.11-15 The Veterans Health Information Exchange Program is designed to optimize data sharing as veterans are increasingly referred to non-VA care through the MISSION Act. This program is organized around patient engagement, clinician adoption, partner engagement, and technological capabilities.16
Unfortunately, significant barriers exist for the VA CNH program within each of these information exchange domains. For example, patient engagement requires veteran consent for consumer-initiated exchange of medical information, which is not practical due to the high prevalence of cognitive impairment in NHs. Similarly, VA consent requirements prohibit EHR download and sharing with non-VA facilities like CNHs, limiting use. eHealth Exchange partnerships allow organizations caring for veterans to connect with the VA via networks that provide a common trust agreement and technical compliance testing. Unfortunately, in 2017, only 257 NHs in which veterans received care nationally were eHealth Exchange partners, which indicates that while NHs could partner in this information exchange, very few did.16
Finally, while the exchange is possible, it is not practical; most CNHs lack the staff that would be required to support data transfer on their technology platform into the appropriate translational gateways. Although remote access to EHRs in CNHs improved during the pandemic, the Veterans Health Information Exchange Program is not designed to facilitate interoperability of VA and CNH records and remains a significant barrier for this and many other VA community care programs.
The dispersal of veterans across CNHs that are > 50 miles from the nearest VAMC represents an additional area to improve program efficiency and meet growing demands for rural care. While recent field guidance to CNH oversight teams reduces the frequency of visits by VA CNH teams, the burden of driving to each facility is not likely to decrease as CNHs increasingly offer rehabilitation as a part of Veteran Care Agreements.17 Visits performed by telehealth or by trained local VA staff may represent alternatives.15
Finally, interview participants described the ideal range of the number of veterans in each CNH necessary to optimize efficiencies. Veterans who rely more heavily upon VA care tend to have more medical and mental health comorbidities than average Medicare beneficiaries.18,19 This was reflected in participants’ recommendation to have enough veterans to benefit from economies of scale but to also identify a limit when efficiencies are lost. Given that most CNHs cared for 8 to 15 veterans, facilities seem to have identified how best to match the resources available with veterans’ care needs. Based on these observations, new care networks that will be established because of the MISSION Act may benefit from establishing evidence-based policies that support flexibility in oversight frequency and either allow for remote oversight or consolidate the number of CNHs to improve efficiencies in care provision and oversight.20
Limitations
Limitations include the unique relationship between VA and CNH staff overseeing the quality of care provided to veterans in CNHs, which is not replicated in other models of care. Data collection was interrupted following the passage of the MISSION Act in 2018 until guidance on changes to practice resulting from the law were clarified in 2020. Interviews were also interrupted at the onset of the COVID-19 pandemic.
Conclusions
The current quality of the CNH care oversight structure will require adaptation as demand for CNH care increases. While the VA CNH program is one of the longest-standing programs collaborating with non-VA community care partners, it is now only one of many following the MISSION Act. The success of this and other programs will depend on matching available CNH resources to the complex medical and psychological needs of veterans. At a time when strategies to ease the burden on NHs and VA CNH coordinators are desperately needed, Veterans Health Information Exchange capabilities need to improve. Evidence is needed to guide the scaling of the program to meet the needs of the rapidly expanding veteran population who are eligible for CNH care.
Acknowledgments
The authors acknowledge Amy Mochel of the Providence Veterans Affairs Medical Center for project management support of this project.
1. Miller EA, Gadbois E, Gidmark S, Intrator O. Purchasing nursing home care within the Veterans Health Administration: lessons for nursing home recruitment, contracting, and oversight. J Health Admin Educ. 2015;32(2):165-197.
2. GAO. VA health care. Veterans’ use of long-term care is increasing, and VA faces challenges in meeting the demand. February 19, 2020. Accessed September 19, 2023. https://www.gao.gov/assets/gao-20-284.pdf
3. VHA Handbook 1143.2, VHA community nursing home oversight procedures. US Department of Veterans Affairs, Veterans Health Administration. June 2004. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=3740930&FileName=VA259-17-Q-0501-007.pdf
4. Community care: veteran care agreements. US Department of Veterans Affairs. 2022. Updated August 8, 2023. Accessed September 7, 2023. https://www.va.gov/COMMUNITYCARE/providers/Veterans_Care_Agreements.asp
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION Act and the future of veterans’ access to quality health care. JAMA. 2020;324(4):343-344. doi:10.1001/jama.2020.4505
6. Colello KJ, Panangala SV; Congressional Research Service. Long-term care services for veterans. February 14, 2017. Accessed September 7, 2023. https://crsreports.congress.gov/product/pdf/R/R44697
7. Magid KH, Galenbeck E, Haverhals LM, et al. Purchasing high-quality community nursing home care: a will to work with VHA diminished by contracting burdens. J Am Med Dir Assoc. 2022;23(11):1757-1764. doi:10.1016/j.jamda.2022.03.007
8. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398-405. doi:10.1111/nhs.12048
9. Brown University. LTC Focus. Accessed September 18, 2023. https://ltcfocus.org/about
10. Zhang W, Creswell J. The use of “mixing” procedure of mixed methods in health services research. Med Care. 2013;51(8):e51-e57. doi:10.1097/MLR.0b013e31824642fd
11. Haverhals LM, Magid KH, Blanchard KN, Levy CR. Veterans Health Administration staff perceptions of overseeing care in community nursing homes during COVID-19. Gerontol Geriatr Med. 2022;8:23337214221080307. Published 2022 Feb 15. doi:10.1177/23337214221080307
12. Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
13. Schlosser J, Kollisch D, Johnson D, Perkins T, Olson A. VA-community dual care: veteran and clinician perspectives. J Community Health. 2020;45(4):795-802. doi:10.1007/s10900-020-00795-y
14. Nevedal AL, Wong EP, Urech TH, Peppiatt JL, Sorie MR, Vashi AA. Veterans’ experiences with accessing community emergency care. Mil Med. 2023;188(1-2):e58-e64. doi:10.1093/milmed/usab196
15. Levenson SA. Smart case review: a model for successful remote medical direction and enhanced nursing home quality improvement. J Am Med Dir Assoc. 2021;22(10):2212-2215.e6. doi:10.1016/j.jamda.2021.05.043
16. Donahue M, Bouhaddou O, Hsing N, et al. Veterans Health Information Exchange: successes and challenges of nationwide interoperability. AMIA Annu Symp Proc. 2018;2018:385-394. Published 2018 Dec 5.
17. US Department of Veterans Affairs. VHA Notice 2023-07. Community Nursing Home Program. September 5, 2023:1-4.
18. Helmer DA, Dwibedi N, Rowneki M, et al. Mental health conditions and hospitalizations for ambulatory care sensitive conditions among veterans with diabetes. Am Health Drug Benefits. 2020;13(2):61-71.
19. Rosen AK, Wagner TH, Pettey WBP, et al. Differences in risk scores of veterans receiving community care purchased by the Veterans Health Administration. Health Serv Res. 2018;53(suppl 3):5438-5454. doi:10.1111/1475-6773.13051
20. Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
The US Department of Veterans Affairs (VA) Community Nursing Home (CNH) program provides 24-hour skilled nursing care for eligible veterans in public or private community-based facilities that have established a contract to care for veterans. Veteran eligibility is based on service-connected status and level of disability, covering the cost of care for veterans who need long-term care because of their service-connected disability or for veterans with disabilities rated at ≥ 70%.1 Between 2014 and 2018, the average daily census of veterans in CNHs increased by 26% and the percentage of funds obligated to this program increased by 49%.2 The VA projects that the number of veterans receiving care in a CNH program will increase by 80% between 2017 and 2037, corresponding to a 149% increase in CNH expenditures.2
CNH program oversight teams are mandated at each VA medical center (VAMC) to monitor care coordination within the CNH program. These teams include nurses and social workers (SWs) who perform regular on-site assessments to monitor the clinical, functional, and psychosocial needs of veterans. These assessments include a review of the electronic health record (EHR) and face-to-face contact with veterans and CNH staff, regardless of the purchasing authority (hospice, long-term care, short-term rehabilitation, respite care).3 These teams represent key stakeholders impacted by CNH program expansion.
While the CNH program has focused primarily on the provision of long-term care, the VA is now expanding to include short-term rehabilitation through Veteran Care Agreements.4 These agreements are authorized under the MISSION Act, designed to improve care for veterans.5 Veteran Care Agreements are expected to be less burdensome to execute than traditional contracts and will permit the VA to partner with more CNHs, as noted in a Congressional Research Service report regarding long-term care services for veterans.6 However, increasing the number of CNHs increases demands on oversight teams, particularly if the coordinators are compelled to perform monthly on-site visits to facilities required under current guidelines.3
The objective of this study was to describe the experiences of VA and CNH staff involved in care coordination and the oversight of veterans receiving CNH care amid Veteran Care Agreement implementation and in anticipation of CNH program expansion. The results are intended to inform expansion efforts within the CNH program.
METHODS
This study was a component of a larger research project examining VA-purchased CNH care; recruitment methods are available in previous publications describing this work.7 Participants provided written or verbal consent before video and phone interviews, respectively. This study was approved by the Colorado Multiple Institutional Review Board (Protocol #18-1186).
Video and phone interviews were conducted by 3 team members from October 2018 to March 2020 with CNH staff and VA CNH program oversight team members. Participant recruitment was paused from May to October 2020 as a result of the COVID-19 pandemic and ambiguity about VA NH care purchasing policies following the passage of the VA MISSION Act.5 We used semistructured interview guides (eAppendix 1 for VA staff and eAppendix 2 for NH staff, available online at doi:10.12788/fp.0421). Recorded and transcribed interviews ranged from 15 to 90 minutes.
Two members of the research team analyzed transcripts using both deductive and inductive content analysis.8 The interview guide informed an a priori codebook, and in vivo codes were included as they emerged. We jointly coded 6 transcripts to reach a consensus on coding approaches and analyzed the remaining transcripts independently with frequent meetings to develop themes with a qualitative methodologist. All qualitative data were analyzed using ATLAS.ti software.
This was a retrospective observational study of veterans who received VA-paid care in CNHs during the 2019 fiscal year (10/1/2018-9/30/2019) using data from the enrollment, inpatient and outpatient encounters, and other care paid for by the VA in the VA Corporate Data Warehouse. We linked Centers for Medicare and Medicaid monthly Nursing Home Compare reports and the Brown University Long Term Care: Facts on Care in the US (LTC FoCUS) annual files to identify facility addresses.9
Descriptive analyses of quantitative data were conducted in parallel with the qualitative findings.8 Distance from the contracting VAMC to CNH was calculated using the greater-circle formula to find the linear distance between geographic coordinates. Quantitative and qualitative data were collected concurrently, analyzed independently, and integrated into the interpretation of results.10
RESULTS
We conducted 36 interviews with VA and NH staff who were affiliated with 6 VAMCs and 17 CNHs. Four themes emerged concerning CNH oversight: (1) benefits of VA CNH team engagement/visits; (2) burden of VA CNH oversight; (3) burden of oversight limited the ability to contract with additional NHs; and (4) factors that ease the burden and facilitate successful oversight.
Benefits of Engagement/Visits
VA SWs and nurses visit each veteran every 30 to 45 days to review their health records, meet with them, and check in with NH staff. In addition, VA SWs and nurses coordinate each veteran’s care by working as liaisons between the VA and the NH to help NH staff problem solve veteran-related issues through care conferences. VA SWs and nurses act as extra advocates for veterans to make sure their needs are met. “This program definitely helps ensure that veterans are receiving higher quality care because if we see that they aren’t, then we do something about it,” a VA NH coordinator reported in an interview.
NH staff noted benefits to monthly VA staff visits, including having an additional person coordinating care and built-in VA liaisons. “It’s nice to have that extra set of eyes, people that you can care plan with,” an NH administrator shared. “It’s definitely a true partnership, and we have open and honest conversations so we can really provide a good service for our veterans.”
Distance & High Veteran Census Burdens
VA participants described oversight components as burdensome. Specifically, several VA participants mentioned that the charting they completed in the facility during each visit proved time consuming and onerous, particularly for distant NHs. To accommodate veterans’ preferences to receive care in a facility close to their homes and families, VAMCs contract with NHs that are geographically spread out. “We’re just all spread out… staff have issues driving 2 and a half hours just to review charts all day,” a VA CNH coordinator explained. In 2019, the mean distance between VAMC and NH was 48 miles, with half located > 32 miles from the VAMC. One-quarter of NHs were > 70 miles and 44% were located > 50 miles from the VAMC (Figure 1).
Participants highlighted how regular oversight visits were particularly time consuming at CNHs with a large contracted population. VA nurses and SWs spend multiple days and up to a week conducting oversight visits at facilities with large numbers of veterans. Another VA nurse highlighted how charting requirements resulted in several days of documentation outside of the NH visit for facilities with many contracted veteran residents. Multiple VA participants noted that having many veterans at an NH exacerbated the oversight burdens. In 2019, 252 (28%) of VA CNHs had > 10 contracted veterans and 1 facility had 34 veterans (Figure 2). VA participants perceived having too many veterans concentrated at 1 facility as potentially challenging for CNHs due to the complex care needs of veterans and the added need for care coordination with the VA. One VA NH coordinator noted that while some facilities were “adept at being able to handle higher numbers” of veterans, others were “overwhelmed.” Too many veterans at an NH, an SW explained, might lead the “facility to fail because we are such a cumbersome system.”
Oversight & Staffing Burden
While several participants described wanting to contract with more NHs to avoid overwhelming existing CNHs and to increase choice for veterans, they expressed concerns about their ability to provide oversight at more facilities due to limited staffing and oversight requirements. Across VAMCs, the median number of VA CNHs varied substantially (Figure 3). One VA participant with about 35 CNHs explained that while adding more NHs could create “more opportunities and options” for veterans, it needs to be balanced with the required oversight responsibilities. One VA nurse insisted that more staff were needed to meet current and future oversight needs. “We’re all getting stretched pretty thin, and just so we don’t drop the ball on things… I would like to see a little more staff if we’re gonna have a lot more nursing homes.”
Participants had concerns related to the VA MISSION Act and the possibility of more VA-paid NHs for rehabilitation or short-term care. Participants underscored the necessity for additional staff to account for the increased oversight burden or a reduction in oversight requirements. One SW felt that increasing the number of CNHs would increase the required oversight and the need for collaboration with NH staff, which would limit her ability to establish close and trusting working relationships with NH staff. Participants also described the challenges of meeting their current oversight requirements, which limited extra visits for acute issues and care conferences. This was attributed to a lack of adequate staffing in the VA CNH program, given the time-intensive nature of VA oversight requirements.
Easing Burden & Facilitating Oversight
Participants noted how obtaining remote access to veterans’ EHRs allowed them to conduct chart reviews before oversight visits. This permitted more time for interaction with veterans and CNH staff as well as coordinating care. While providing access to the VA EHR would not change the chart review component of VA oversight, some participants felt it might improve care coordination between VA and NH staff during monthly visits.
Participants felt they were able to build strong working relationships with facilities with more veterans due to frequent communication and collaboration. VA participants also noted that CNHs with larger veteran censuses were more likely to respond to VA concerns about care to maintain the business relationship and contract. To optimize strong working relationships and decrease the challenges of having too many veterans at a facility, some VA participants suggested that CNH programs create a local policy to recommend the number of veterans placed in a CNH.
Discussion
Participants interviewed for this study echoed findings from previous work that identified the importance of developing trusted working relationships with CNHs to care for veterans.11,12 However, interorganizational care coordination, a shortage of health care professionals, and resource demands associated with caring for veterans reported in other community care settings were also noted in our findings.12,13
Building upon prior recommendations related to community care of veterans, our analysis identified key areas that could improve CNH program oversight efficiency, including: (1) improving the interoperability of EHRs to facilitate coordination of care and oversight; (2) addressing inefficiencies associated with traveling to geographically dispersed CNHs; and (3) “right-sizing” the number of veterans residing in each CNH.
The interoperability of EHRs has been cited by multiple studies of VA community care programs as critical to reducing inefficiencies and allowing more in-person time with veterans and staff in care coordination, especially at rural locations.11-15 The Veterans Health Information Exchange Program is designed to optimize data sharing as veterans are increasingly referred to non-VA care through the MISSION Act. This program is organized around patient engagement, clinician adoption, partner engagement, and technological capabilities.16
Unfortunately, significant barriers exist for the VA CNH program within each of these information exchange domains. For example, patient engagement requires veteran consent for consumer-initiated exchange of medical information, which is not practical due to the high prevalence of cognitive impairment in NHs. Similarly, VA consent requirements prohibit EHR download and sharing with non-VA facilities like CNHs, limiting use. eHealth Exchange partnerships allow organizations caring for veterans to connect with the VA via networks that provide a common trust agreement and technical compliance testing. Unfortunately, in 2017, only 257 NHs in which veterans received care nationally were eHealth Exchange partners, which indicates that while NHs could partner in this information exchange, very few did.16
Finally, while the exchange is possible, it is not practical; most CNHs lack the staff that would be required to support data transfer on their technology platform into the appropriate translational gateways. Although remote access to EHRs in CNHs improved during the pandemic, the Veterans Health Information Exchange Program is not designed to facilitate interoperability of VA and CNH records and remains a significant barrier for this and many other VA community care programs.
The dispersal of veterans across CNHs that are > 50 miles from the nearest VAMC represents an additional area to improve program efficiency and meet growing demands for rural care. While recent field guidance to CNH oversight teams reduces the frequency of visits by VA CNH teams, the burden of driving to each facility is not likely to decrease as CNHs increasingly offer rehabilitation as a part of Veteran Care Agreements.17 Visits performed by telehealth or by trained local VA staff may represent alternatives.15
Finally, interview participants described the ideal range of the number of veterans in each CNH necessary to optimize efficiencies. Veterans who rely more heavily upon VA care tend to have more medical and mental health comorbidities than average Medicare beneficiaries.18,19 This was reflected in participants’ recommendation to have enough veterans to benefit from economies of scale but to also identify a limit when efficiencies are lost. Given that most CNHs cared for 8 to 15 veterans, facilities seem to have identified how best to match the resources available with veterans’ care needs. Based on these observations, new care networks that will be established because of the MISSION Act may benefit from establishing evidence-based policies that support flexibility in oversight frequency and either allow for remote oversight or consolidate the number of CNHs to improve efficiencies in care provision and oversight.20
Limitations
Limitations include the unique relationship between VA and CNH staff overseeing the quality of care provided to veterans in CNHs, which is not replicated in other models of care. Data collection was interrupted following the passage of the MISSION Act in 2018 until guidance on changes to practice resulting from the law were clarified in 2020. Interviews were also interrupted at the onset of the COVID-19 pandemic.
Conclusions
The current quality of the CNH care oversight structure will require adaptation as demand for CNH care increases. While the VA CNH program is one of the longest-standing programs collaborating with non-VA community care partners, it is now only one of many following the MISSION Act. The success of this and other programs will depend on matching available CNH resources to the complex medical and psychological needs of veterans. At a time when strategies to ease the burden on NHs and VA CNH coordinators are desperately needed, Veterans Health Information Exchange capabilities need to improve. Evidence is needed to guide the scaling of the program to meet the needs of the rapidly expanding veteran population who are eligible for CNH care.
Acknowledgments
The authors acknowledge Amy Mochel of the Providence Veterans Affairs Medical Center for project management support of this project.
The US Department of Veterans Affairs (VA) Community Nursing Home (CNH) program provides 24-hour skilled nursing care for eligible veterans in public or private community-based facilities that have established a contract to care for veterans. Veteran eligibility is based on service-connected status and level of disability, covering the cost of care for veterans who need long-term care because of their service-connected disability or for veterans with disabilities rated at ≥ 70%.1 Between 2014 and 2018, the average daily census of veterans in CNHs increased by 26% and the percentage of funds obligated to this program increased by 49%.2 The VA projects that the number of veterans receiving care in a CNH program will increase by 80% between 2017 and 2037, corresponding to a 149% increase in CNH expenditures.2
CNH program oversight teams are mandated at each VA medical center (VAMC) to monitor care coordination within the CNH program. These teams include nurses and social workers (SWs) who perform regular on-site assessments to monitor the clinical, functional, and psychosocial needs of veterans. These assessments include a review of the electronic health record (EHR) and face-to-face contact with veterans and CNH staff, regardless of the purchasing authority (hospice, long-term care, short-term rehabilitation, respite care).3 These teams represent key stakeholders impacted by CNH program expansion.
While the CNH program has focused primarily on the provision of long-term care, the VA is now expanding to include short-term rehabilitation through Veteran Care Agreements.4 These agreements are authorized under the MISSION Act, designed to improve care for veterans.5 Veteran Care Agreements are expected to be less burdensome to execute than traditional contracts and will permit the VA to partner with more CNHs, as noted in a Congressional Research Service report regarding long-term care services for veterans.6 However, increasing the number of CNHs increases demands on oversight teams, particularly if the coordinators are compelled to perform monthly on-site visits to facilities required under current guidelines.3
The objective of this study was to describe the experiences of VA and CNH staff involved in care coordination and the oversight of veterans receiving CNH care amid Veteran Care Agreement implementation and in anticipation of CNH program expansion. The results are intended to inform expansion efforts within the CNH program.
METHODS
This study was a component of a larger research project examining VA-purchased CNH care; recruitment methods are available in previous publications describing this work.7 Participants provided written or verbal consent before video and phone interviews, respectively. This study was approved by the Colorado Multiple Institutional Review Board (Protocol #18-1186).
Video and phone interviews were conducted by 3 team members from October 2018 to March 2020 with CNH staff and VA CNH program oversight team members. Participant recruitment was paused from May to October 2020 as a result of the COVID-19 pandemic and ambiguity about VA NH care purchasing policies following the passage of the VA MISSION Act.5 We used semistructured interview guides (eAppendix 1 for VA staff and eAppendix 2 for NH staff, available online at doi:10.12788/fp.0421). Recorded and transcribed interviews ranged from 15 to 90 minutes.
Two members of the research team analyzed transcripts using both deductive and inductive content analysis.8 The interview guide informed an a priori codebook, and in vivo codes were included as they emerged. We jointly coded 6 transcripts to reach a consensus on coding approaches and analyzed the remaining transcripts independently with frequent meetings to develop themes with a qualitative methodologist. All qualitative data were analyzed using ATLAS.ti software.
This was a retrospective observational study of veterans who received VA-paid care in CNHs during the 2019 fiscal year (10/1/2018-9/30/2019) using data from the enrollment, inpatient and outpatient encounters, and other care paid for by the VA in the VA Corporate Data Warehouse. We linked Centers for Medicare and Medicaid monthly Nursing Home Compare reports and the Brown University Long Term Care: Facts on Care in the US (LTC FoCUS) annual files to identify facility addresses.9
Descriptive analyses of quantitative data were conducted in parallel with the qualitative findings.8 Distance from the contracting VAMC to CNH was calculated using the greater-circle formula to find the linear distance between geographic coordinates. Quantitative and qualitative data were collected concurrently, analyzed independently, and integrated into the interpretation of results.10
RESULTS
We conducted 36 interviews with VA and NH staff who were affiliated with 6 VAMCs and 17 CNHs. Four themes emerged concerning CNH oversight: (1) benefits of VA CNH team engagement/visits; (2) burden of VA CNH oversight; (3) burden of oversight limited the ability to contract with additional NHs; and (4) factors that ease the burden and facilitate successful oversight.
Benefits of Engagement/Visits
VA SWs and nurses visit each veteran every 30 to 45 days to review their health records, meet with them, and check in with NH staff. In addition, VA SWs and nurses coordinate each veteran’s care by working as liaisons between the VA and the NH to help NH staff problem solve veteran-related issues through care conferences. VA SWs and nurses act as extra advocates for veterans to make sure their needs are met. “This program definitely helps ensure that veterans are receiving higher quality care because if we see that they aren’t, then we do something about it,” a VA NH coordinator reported in an interview.
NH staff noted benefits to monthly VA staff visits, including having an additional person coordinating care and built-in VA liaisons. “It’s nice to have that extra set of eyes, people that you can care plan with,” an NH administrator shared. “It’s definitely a true partnership, and we have open and honest conversations so we can really provide a good service for our veterans.”
Distance & High Veteran Census Burdens
VA participants described oversight components as burdensome. Specifically, several VA participants mentioned that the charting they completed in the facility during each visit proved time consuming and onerous, particularly for distant NHs. To accommodate veterans’ preferences to receive care in a facility close to their homes and families, VAMCs contract with NHs that are geographically spread out. “We’re just all spread out… staff have issues driving 2 and a half hours just to review charts all day,” a VA CNH coordinator explained. In 2019, the mean distance between VAMC and NH was 48 miles, with half located > 32 miles from the VAMC. One-quarter of NHs were > 70 miles and 44% were located > 50 miles from the VAMC (Figure 1).
Participants highlighted how regular oversight visits were particularly time consuming at CNHs with a large contracted population. VA nurses and SWs spend multiple days and up to a week conducting oversight visits at facilities with large numbers of veterans. Another VA nurse highlighted how charting requirements resulted in several days of documentation outside of the NH visit for facilities with many contracted veteran residents. Multiple VA participants noted that having many veterans at an NH exacerbated the oversight burdens. In 2019, 252 (28%) of VA CNHs had > 10 contracted veterans and 1 facility had 34 veterans (Figure 2). VA participants perceived having too many veterans concentrated at 1 facility as potentially challenging for CNHs due to the complex care needs of veterans and the added need for care coordination with the VA. One VA NH coordinator noted that while some facilities were “adept at being able to handle higher numbers” of veterans, others were “overwhelmed.” Too many veterans at an NH, an SW explained, might lead the “facility to fail because we are such a cumbersome system.”
Oversight & Staffing Burden
While several participants described wanting to contract with more NHs to avoid overwhelming existing CNHs and to increase choice for veterans, they expressed concerns about their ability to provide oversight at more facilities due to limited staffing and oversight requirements. Across VAMCs, the median number of VA CNHs varied substantially (Figure 3). One VA participant with about 35 CNHs explained that while adding more NHs could create “more opportunities and options” for veterans, it needs to be balanced with the required oversight responsibilities. One VA nurse insisted that more staff were needed to meet current and future oversight needs. “We’re all getting stretched pretty thin, and just so we don’t drop the ball on things… I would like to see a little more staff if we’re gonna have a lot more nursing homes.”
Participants had concerns related to the VA MISSION Act and the possibility of more VA-paid NHs for rehabilitation or short-term care. Participants underscored the necessity for additional staff to account for the increased oversight burden or a reduction in oversight requirements. One SW felt that increasing the number of CNHs would increase the required oversight and the need for collaboration with NH staff, which would limit her ability to establish close and trusting working relationships with NH staff. Participants also described the challenges of meeting their current oversight requirements, which limited extra visits for acute issues and care conferences. This was attributed to a lack of adequate staffing in the VA CNH program, given the time-intensive nature of VA oversight requirements.
Easing Burden & Facilitating Oversight
Participants noted how obtaining remote access to veterans’ EHRs allowed them to conduct chart reviews before oversight visits. This permitted more time for interaction with veterans and CNH staff as well as coordinating care. While providing access to the VA EHR would not change the chart review component of VA oversight, some participants felt it might improve care coordination between VA and NH staff during monthly visits.
Participants felt they were able to build strong working relationships with facilities with more veterans due to frequent communication and collaboration. VA participants also noted that CNHs with larger veteran censuses were more likely to respond to VA concerns about care to maintain the business relationship and contract. To optimize strong working relationships and decrease the challenges of having too many veterans at a facility, some VA participants suggested that CNH programs create a local policy to recommend the number of veterans placed in a CNH.
Discussion
Participants interviewed for this study echoed findings from previous work that identified the importance of developing trusted working relationships with CNHs to care for veterans.11,12 However, interorganizational care coordination, a shortage of health care professionals, and resource demands associated with caring for veterans reported in other community care settings were also noted in our findings.12,13
Building upon prior recommendations related to community care of veterans, our analysis identified key areas that could improve CNH program oversight efficiency, including: (1) improving the interoperability of EHRs to facilitate coordination of care and oversight; (2) addressing inefficiencies associated with traveling to geographically dispersed CNHs; and (3) “right-sizing” the number of veterans residing in each CNH.
The interoperability of EHRs has been cited by multiple studies of VA community care programs as critical to reducing inefficiencies and allowing more in-person time with veterans and staff in care coordination, especially at rural locations.11-15 The Veterans Health Information Exchange Program is designed to optimize data sharing as veterans are increasingly referred to non-VA care through the MISSION Act. This program is organized around patient engagement, clinician adoption, partner engagement, and technological capabilities.16
Unfortunately, significant barriers exist for the VA CNH program within each of these information exchange domains. For example, patient engagement requires veteran consent for consumer-initiated exchange of medical information, which is not practical due to the high prevalence of cognitive impairment in NHs. Similarly, VA consent requirements prohibit EHR download and sharing with non-VA facilities like CNHs, limiting use. eHealth Exchange partnerships allow organizations caring for veterans to connect with the VA via networks that provide a common trust agreement and technical compliance testing. Unfortunately, in 2017, only 257 NHs in which veterans received care nationally were eHealth Exchange partners, which indicates that while NHs could partner in this information exchange, very few did.16
Finally, while the exchange is possible, it is not practical; most CNHs lack the staff that would be required to support data transfer on their technology platform into the appropriate translational gateways. Although remote access to EHRs in CNHs improved during the pandemic, the Veterans Health Information Exchange Program is not designed to facilitate interoperability of VA and CNH records and remains a significant barrier for this and many other VA community care programs.
The dispersal of veterans across CNHs that are > 50 miles from the nearest VAMC represents an additional area to improve program efficiency and meet growing demands for rural care. While recent field guidance to CNH oversight teams reduces the frequency of visits by VA CNH teams, the burden of driving to each facility is not likely to decrease as CNHs increasingly offer rehabilitation as a part of Veteran Care Agreements.17 Visits performed by telehealth or by trained local VA staff may represent alternatives.15
Finally, interview participants described the ideal range of the number of veterans in each CNH necessary to optimize efficiencies. Veterans who rely more heavily upon VA care tend to have more medical and mental health comorbidities than average Medicare beneficiaries.18,19 This was reflected in participants’ recommendation to have enough veterans to benefit from economies of scale but to also identify a limit when efficiencies are lost. Given that most CNHs cared for 8 to 15 veterans, facilities seem to have identified how best to match the resources available with veterans’ care needs. Based on these observations, new care networks that will be established because of the MISSION Act may benefit from establishing evidence-based policies that support flexibility in oversight frequency and either allow for remote oversight or consolidate the number of CNHs to improve efficiencies in care provision and oversight.20
Limitations
Limitations include the unique relationship between VA and CNH staff overseeing the quality of care provided to veterans in CNHs, which is not replicated in other models of care. Data collection was interrupted following the passage of the MISSION Act in 2018 until guidance on changes to practice resulting from the law were clarified in 2020. Interviews were also interrupted at the onset of the COVID-19 pandemic.
Conclusions
The current quality of the CNH care oversight structure will require adaptation as demand for CNH care increases. While the VA CNH program is one of the longest-standing programs collaborating with non-VA community care partners, it is now only one of many following the MISSION Act. The success of this and other programs will depend on matching available CNH resources to the complex medical and psychological needs of veterans. At a time when strategies to ease the burden on NHs and VA CNH coordinators are desperately needed, Veterans Health Information Exchange capabilities need to improve. Evidence is needed to guide the scaling of the program to meet the needs of the rapidly expanding veteran population who are eligible for CNH care.
Acknowledgments
The authors acknowledge Amy Mochel of the Providence Veterans Affairs Medical Center for project management support of this project.
1. Miller EA, Gadbois E, Gidmark S, Intrator O. Purchasing nursing home care within the Veterans Health Administration: lessons for nursing home recruitment, contracting, and oversight. J Health Admin Educ. 2015;32(2):165-197.
2. GAO. VA health care. Veterans’ use of long-term care is increasing, and VA faces challenges in meeting the demand. February 19, 2020. Accessed September 19, 2023. https://www.gao.gov/assets/gao-20-284.pdf
3. VHA Handbook 1143.2, VHA community nursing home oversight procedures. US Department of Veterans Affairs, Veterans Health Administration. June 2004. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=3740930&FileName=VA259-17-Q-0501-007.pdf
4. Community care: veteran care agreements. US Department of Veterans Affairs. 2022. Updated August 8, 2023. Accessed September 7, 2023. https://www.va.gov/COMMUNITYCARE/providers/Veterans_Care_Agreements.asp
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION Act and the future of veterans’ access to quality health care. JAMA. 2020;324(4):343-344. doi:10.1001/jama.2020.4505
6. Colello KJ, Panangala SV; Congressional Research Service. Long-term care services for veterans. February 14, 2017. Accessed September 7, 2023. https://crsreports.congress.gov/product/pdf/R/R44697
7. Magid KH, Galenbeck E, Haverhals LM, et al. Purchasing high-quality community nursing home care: a will to work with VHA diminished by contracting burdens. J Am Med Dir Assoc. 2022;23(11):1757-1764. doi:10.1016/j.jamda.2022.03.007
8. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398-405. doi:10.1111/nhs.12048
9. Brown University. LTC Focus. Accessed September 18, 2023. https://ltcfocus.org/about
10. Zhang W, Creswell J. The use of “mixing” procedure of mixed methods in health services research. Med Care. 2013;51(8):e51-e57. doi:10.1097/MLR.0b013e31824642fd
11. Haverhals LM, Magid KH, Blanchard KN, Levy CR. Veterans Health Administration staff perceptions of overseeing care in community nursing homes during COVID-19. Gerontol Geriatr Med. 2022;8:23337214221080307. Published 2022 Feb 15. doi:10.1177/23337214221080307
12. Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
13. Schlosser J, Kollisch D, Johnson D, Perkins T, Olson A. VA-community dual care: veteran and clinician perspectives. J Community Health. 2020;45(4):795-802. doi:10.1007/s10900-020-00795-y
14. Nevedal AL, Wong EP, Urech TH, Peppiatt JL, Sorie MR, Vashi AA. Veterans’ experiences with accessing community emergency care. Mil Med. 2023;188(1-2):e58-e64. doi:10.1093/milmed/usab196
15. Levenson SA. Smart case review: a model for successful remote medical direction and enhanced nursing home quality improvement. J Am Med Dir Assoc. 2021;22(10):2212-2215.e6. doi:10.1016/j.jamda.2021.05.043
16. Donahue M, Bouhaddou O, Hsing N, et al. Veterans Health Information Exchange: successes and challenges of nationwide interoperability. AMIA Annu Symp Proc. 2018;2018:385-394. Published 2018 Dec 5.
17. US Department of Veterans Affairs. VHA Notice 2023-07. Community Nursing Home Program. September 5, 2023:1-4.
18. Helmer DA, Dwibedi N, Rowneki M, et al. Mental health conditions and hospitalizations for ambulatory care sensitive conditions among veterans with diabetes. Am Health Drug Benefits. 2020;13(2):61-71.
19. Rosen AK, Wagner TH, Pettey WBP, et al. Differences in risk scores of veterans receiving community care purchased by the Veterans Health Administration. Health Serv Res. 2018;53(suppl 3):5438-5454. doi:10.1111/1475-6773.13051
20. Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
1. Miller EA, Gadbois E, Gidmark S, Intrator O. Purchasing nursing home care within the Veterans Health Administration: lessons for nursing home recruitment, contracting, and oversight. J Health Admin Educ. 2015;32(2):165-197.
2. GAO. VA health care. Veterans’ use of long-term care is increasing, and VA faces challenges in meeting the demand. February 19, 2020. Accessed September 19, 2023. https://www.gao.gov/assets/gao-20-284.pdf
3. VHA Handbook 1143.2, VHA community nursing home oversight procedures. US Department of Veterans Affairs, Veterans Health Administration. June 2004. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=3740930&FileName=VA259-17-Q-0501-007.pdf
4. Community care: veteran care agreements. US Department of Veterans Affairs. 2022. Updated August 8, 2023. Accessed September 7, 2023. https://www.va.gov/COMMUNITYCARE/providers/Veterans_Care_Agreements.asp
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION Act and the future of veterans’ access to quality health care. JAMA. 2020;324(4):343-344. doi:10.1001/jama.2020.4505
6. Colello KJ, Panangala SV; Congressional Research Service. Long-term care services for veterans. February 14, 2017. Accessed September 7, 2023. https://crsreports.congress.gov/product/pdf/R/R44697
7. Magid KH, Galenbeck E, Haverhals LM, et al. Purchasing high-quality community nursing home care: a will to work with VHA diminished by contracting burdens. J Am Med Dir Assoc. 2022;23(11):1757-1764. doi:10.1016/j.jamda.2022.03.007
8. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398-405. doi:10.1111/nhs.12048
9. Brown University. LTC Focus. Accessed September 18, 2023. https://ltcfocus.org/about
10. Zhang W, Creswell J. The use of “mixing” procedure of mixed methods in health services research. Med Care. 2013;51(8):e51-e57. doi:10.1097/MLR.0b013e31824642fd
11. Haverhals LM, Magid KH, Blanchard KN, Levy CR. Veterans Health Administration staff perceptions of overseeing care in community nursing homes during COVID-19. Gerontol Geriatr Med. 2022;8:23337214221080307. Published 2022 Feb 15. doi:10.1177/23337214221080307
12. Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
13. Schlosser J, Kollisch D, Johnson D, Perkins T, Olson A. VA-community dual care: veteran and clinician perspectives. J Community Health. 2020;45(4):795-802. doi:10.1007/s10900-020-00795-y
14. Nevedal AL, Wong EP, Urech TH, Peppiatt JL, Sorie MR, Vashi AA. Veterans’ experiences with accessing community emergency care. Mil Med. 2023;188(1-2):e58-e64. doi:10.1093/milmed/usab196
15. Levenson SA. Smart case review: a model for successful remote medical direction and enhanced nursing home quality improvement. J Am Med Dir Assoc. 2021;22(10):2212-2215.e6. doi:10.1016/j.jamda.2021.05.043
16. Donahue M, Bouhaddou O, Hsing N, et al. Veterans Health Information Exchange: successes and challenges of nationwide interoperability. AMIA Annu Symp Proc. 2018;2018:385-394. Published 2018 Dec 5.
17. US Department of Veterans Affairs. VHA Notice 2023-07. Community Nursing Home Program. September 5, 2023:1-4.
18. Helmer DA, Dwibedi N, Rowneki M, et al. Mental health conditions and hospitalizations for ambulatory care sensitive conditions among veterans with diabetes. Am Health Drug Benefits. 2020;13(2):61-71.
19. Rosen AK, Wagner TH, Pettey WBP, et al. Differences in risk scores of veterans receiving community care purchased by the Veterans Health Administration. Health Serv Res. 2018;53(suppl 3):5438-5454. doi:10.1111/1475-6773.13051
20. Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
Impact of Liraglutide to Semaglutide Conversion on Glycemic Control and Cost Savings at a Veterans Affairs Medical Center
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2