User login
Recurrence Rates of Mohs Micrographic Surgery vs Radiation Therapy for Basal Cell Carcinoma of the Ear
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
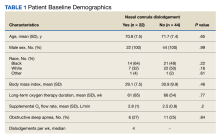
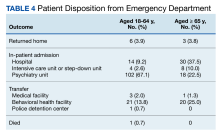
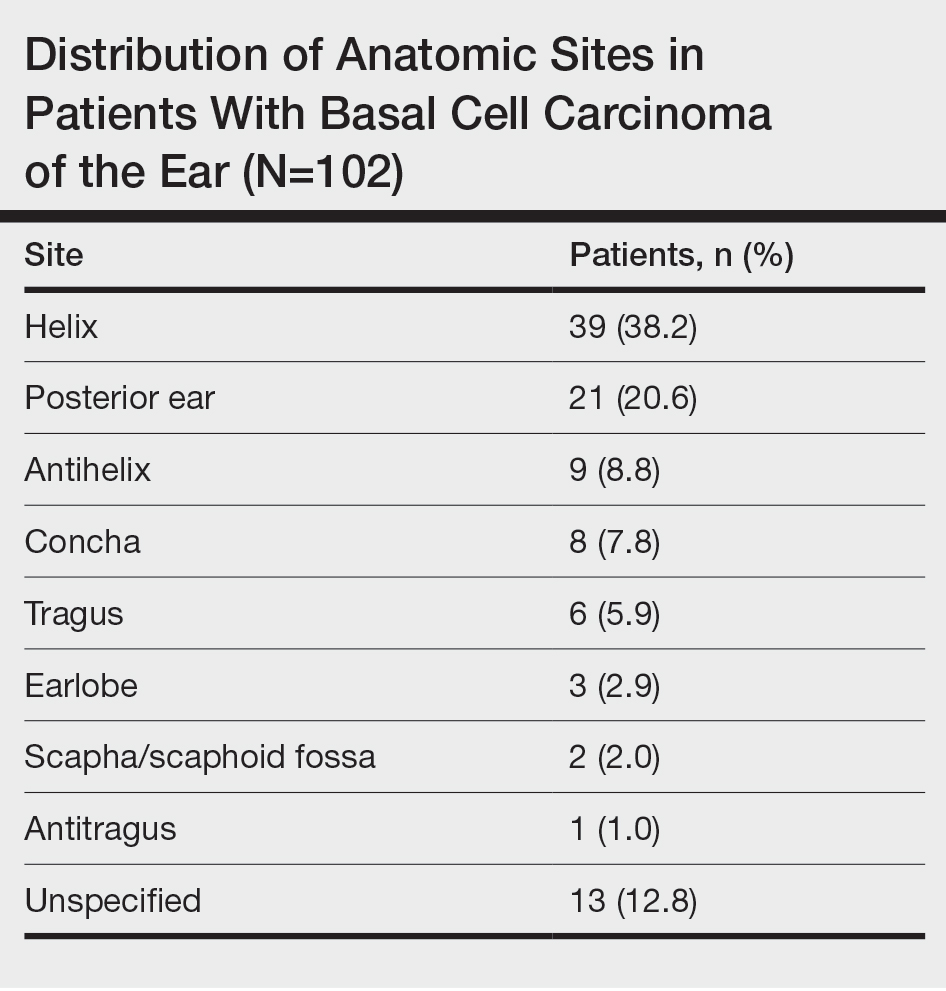
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.
Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. In this retrospective analysis, we evaluated recurrence rates of BCC of the ear in 102 patients who underwent treatment with Mohs micrographic surgery (MMS) or radiation therapy (RT) at a single institution between January 2017 and December 2019. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. Recurrence rates were assessed over a mean follow-up time of 2.8 years. Although MMS is the gold standard for treatment of BCC of the ear, RT may be a suitable alternative for nonsurgical candidates.
Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations. Given that these aggressive histologic subtypes—defined as morpheaform, basosquamous, sclerosing, infiltrative, or micronodular in any portion of the tumor—have been reported as independent predictors of recurrence,1,2 BCC of the ear may be more likely to recur.
Mohs micrographic surgery (MMS) is the gold standard for the treatment of BCC of the ear. For nonsurgical candidates—those with high bleeding risk, low life expectancy, or other medical or social factors—definitive radiation therapy (RT) may be an option. Our study sought to examine recurrence rates in patients with BCC of the ear treated with MMS vs RT.
Methods
A retrospective review of patients undergoing treatment of BCC of the ear at Bighorn Mohs Surgery and Dermatology Center (San Diego, California) between January 2017 and December 2019 was conducted. A total of 507 medical records were reviewed, and 102 patients were included in the study. Inclusion criteria consisted of biopsy-confirmed BCC of the ear that was treated with MMS, RT, or both. Data on patient demographics, tumor characteristics, treatment modality, and recurrence rates were collected from medical records. This retrospective review of medical records was exempt from institutional review board approval, as it did not involve direct human research subjects, solely entailing a retrospective examination of existing data.
Results
Of the 102 patients included, 82 were male and 20 were female, with an average age of 71 years. All patients were White with the exception of 1 patient whose race was unknown. Two patients were immunocompromised. The helix was identified as the most frequently involved site on the ear (Table). Most of the tumors (56/102) exhibited aggressive histologic subtypes; 36 tumors had nonaggressive histology, and 10 had no subtype listed. Two of the BCCs demonstrated perineural invasion on biopsy. Mohs micrographic surgery was used to treat 96 BCCs, definitive RT was used to treat 5 BCCs (all of which occurred in nonsurgical candidates), and MMS and adjuvant RT were used in 1 patient given multifocal perineural involvement. All 5 patients treated with definitive RT received electron beam radiation therapy; the total dose ranged from 5100 to 6000 cGy divided into 17 to 24 fractions. The final MMS defects ranged from 6 to 55 mm in size. The average follow-up time was 2.8 years. One of the BCCs on the helix that was treated with MMS recurred after 1.3 years. The overall recurrence rate was 0.98%. None of the patients treated with definitive RT experienced recurrence after the mean follow-up time of 2.8 years.

Comment
Basal cell carcinoma is the most commonly diagnosed cancer in the United States, with approximately 2 million new cases each year.1 Treatment modalities for localized BCC include MMS, surgical excision, electrodesiccation and curettage, topical and intralesional medications, laser therapy, and RT. For high-risk BCCs, MMS is associated with the lowest recurrence rates4 and remains the gold standard for treatment. For patients with contraindications to surgery, definitive RT is an alternative treatment for high-risk BCC.1
Definitive RT can be employed for patients who are poor surgical candidates or when surgery would result in substantial morbidity, impaired function, and/or poor cosmesis.3 Radiation therapy for skin cancers of the ear commonly is administered using high-energy electrons that produce double-strand breaks in the DNA of malignant cells, leading to cell death.4 Disadvantages of RT compared to MMS include a longer treatment course (3 to 6 weeks), possible minimal long-term cosmetic sequelae (eg, color or texture mismatch), lack of pathologic confirmation of margin control, and small risk for secondary malignancy in the treatment field over 2 to 3 decades. For patients with incurable or metastatic disease, palliative RT can provide local control and/or symptomatic relief to improve quality of life.4 Adjuvant RT may be indicated if there is substantial perineural involvement or positive margins after MMS when margins are unable to be achieved or in patients who may not tolerate prolonged or extensive surgical procedures.3
Basal cell carcinoma of the ear is considered a high-risk anatomic location independent of other prognostic factors. Basal cell carcinomas of the ear have a higher propensity for more aggressive histologic subtypes and subclinical spread.5 Our study demonstrated a higher proportion of aggressive histologic subtypes (56/102 [54.9%]) compared with nonaggressive subtypes (36/102 [35.3%]). There was 1 recurrence of a nodular, sclerosing, and infiltrative BCC on the helix treated with MMS after 1.3 years.
Limitations of our study include that it was conducted at a single institution with a homogenous study population and with relatively short follow-up.
Conclusion
Our study further validates the well-known utility of MMS for the treatment of BCC of the ears. Definitive RT is a suitable alternative for patients who are not surgical candidates. Adjuvant RT may be considered for substantial perineural involvement or positive margins after MMS.3
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
- Lee CT, Lehrer EJ, Aphale A, et al. Surgical excision, Mohs micrographic surgery, external-beam radiotherapy, or brachytherapy for indolent skin cancer: an international meta-analysis of 58 studies with 21,000 patients. Cancer. 2019;125:3582-3594.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part II: when is radiation therapy indicated? J Am Acad Dermatol. 2021;85:551-562.
- Wilmas KM, Garner WB, Ballo MT, et al. The role of radiation therapy in the management of cutaneous malignancies. part I: diagnostic modalities and applications. J Am Acad Dermatol. 2021;85:539-548.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:574-597.
PRACTICE POINTS
- Basal cell carcinoma (BCC) of the ear may have aggressive histologic subtypes and a greater propensity for subclinical spread than BCC in other anatomic locations, highlighting the importance of careful management and follow-up.
- Although Mohs micrographic surgery remains the gold standard for treating BCC of the ear, radiation therapy can be considered as a suitable alternative for nonsurgical candidates.
Risk for COVID-19 Infection in Patients With Vitiligo
To the Editor:
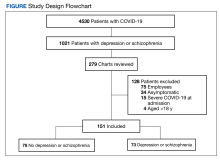
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
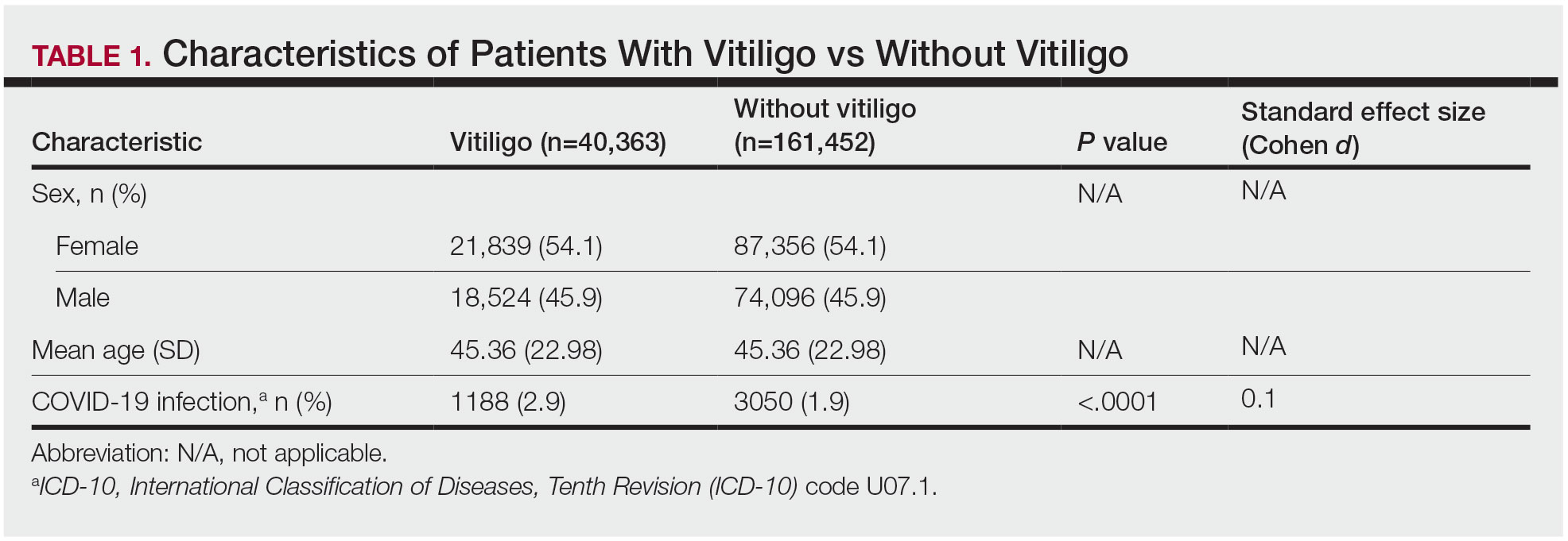
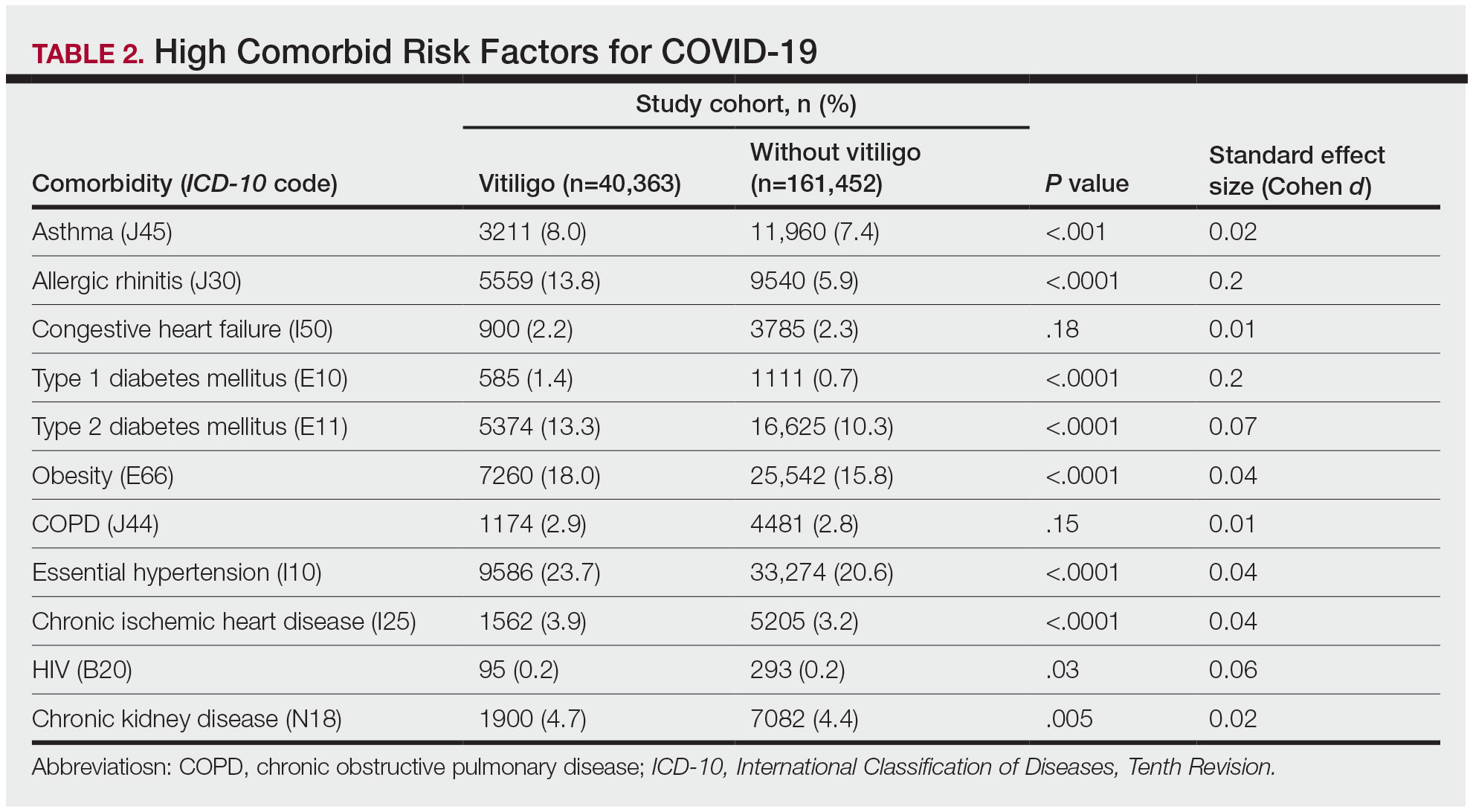
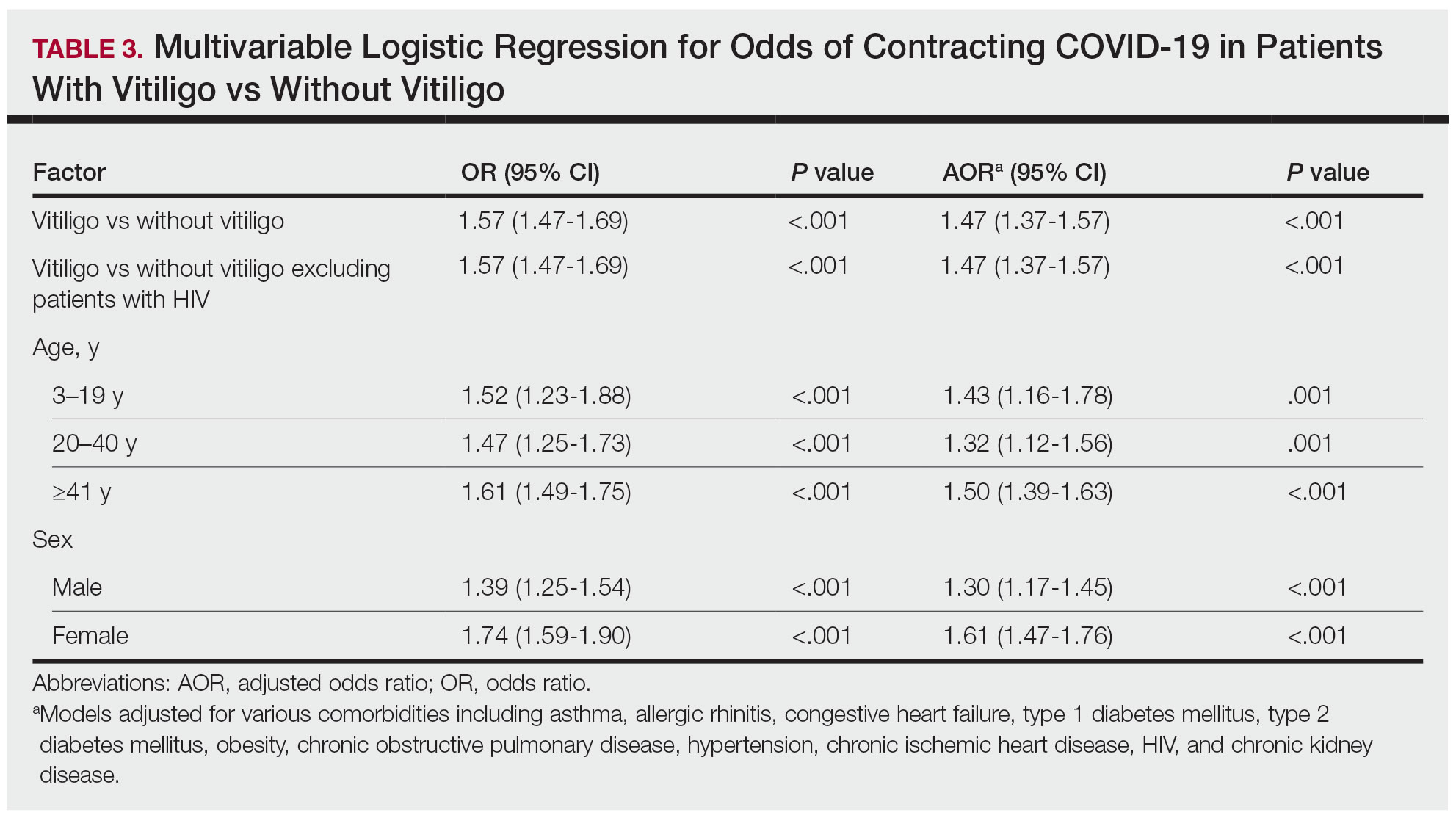
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).
Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.
Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

To the Editor:
Vitiligo is a depigmentation disorder that results from the loss of melanocytes in the epidermis.1 The most widely accepted pathophysiology for melanocyte destruction in vitiligo is an autoimmune process involving dysregulated cytokine production and autoreactive T-cell activation.1 Individuals with cutaneous autoinflammatory conditions currently are vital patient populations warranting research, as their susceptibility to COVID-19 infection may differ from the general population. We previously found a small increased risk for COVID-19 infection in patients with psoriasis,2 which suggests that other dermatologic conditions also may impact COVID-19 risk. The risk for COVID-19 infection in patients with vitiligo remains largely unknown. In this retrospective cohort study, we investigated the risk for COVID-19 infection in patients with vitiligo compared with those without vitiligo utilizing claims data from the COVID-19 Research Database (https://covid19researchdatabase.org/).
Claims were evaluated for patients aged 3 years and older with a vitiligo diagnosis (International Classification of Diseases, Tenth Revision [ICD-10] code L80) that was made between January 1, 2016, and January 1, 2020. Individuals without a vitiligo diagnosis during the same period were placed (4:1 ratio) in the control group and were matched with study group patients for age and sex. All comorbidity variables and vitiligo diagnoses were extracted from ICD-10 codes that were given prior to a diagnosis of COVID-19. We then constructed multivariable logistic regression models adjusting for measured confounders to evaluate if vitiligo was associated with higher risk for COVID-19 infection after January 1, 2020.
The vitiligo and nonvitiligo cohorts included 40,363 and 161,452 patients, respectively (Table 1). Logistic regression analysis with adjustment for confounding variables, including high comorbid risk factors (Table 2) revealed that patients with a diagnosis of vitiligo had significantly increased odds of COVID-19 infection compared with patients without vitiligo (adjusted odds ratio [AOR], 1.47; 95% CI, 1.37-1.57; P<.001)(Table 3). Additionally, subgroup logistic analyses for sex, age, and exclusion of patients who were HIV positive revealed that females with vitiligo had higher odds of contracting COVID-19 than males with vitiligo (Table 3).

Our results showed that patients with vitiligo had a higher relative risk for contracting COVID-19 than individuals without vitiligo. It has been reported that the prevalence of COVID-19 is higher among patients with autoimmune diseases compared to the general population.3 Additionally, a handful of vitiligo patients are managed with immunosuppressive agents that may further weaken their immune response.1 Moreover, survey results from dermatologists managing vitiligo patients revealed that physicians were fairly comfortable prescribing immunosuppressants and encouraging in-office phototherapy during the COVID-19 pandemic.4 As a result, more patients may have been attending in-office visits for their phototherapy, which may have increased their risk for COVID-19. Although these factors play a role in COVID-19 infection rates, the underlying immune dysregulation in vitiligo in relation to COVID-19 remains unknown and should be further explored.

Our findings are limited by the use of ICD-10 codes, the inability to control for all potential confounding variables, the lack of data regarding the stage of vitiligo, and the absence of data for undiagnosed COVID-19 infections. In addition, patients with vitiligo may be more likely to seek care, potentially increasing their rates of COVID-19 testing. The inability to identify the stage of vitiligo during enrollment in the database may have altered our results, as individuals with active disease have increased levels of IFN-γ. Increased secretion of IFN-γ also potentially helps in the clearance of COVID-19 infection.1 Future studies should investigate this relationship via planned COVID-19 testing, identification of vitiligo stage, and controlling for other associated comorbidities.

- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Wu JJ, Liu J, Thatiparthi A, et al. The risk of COVID-19 in patients with psoriasis—a retrospective cohort study [published online September 20, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.040
- Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2:E557-E564. doi:10.1016/S2665-9913(20)30227-7
- Chatterjee M, Das A. Management of vitiligo amidst the COVID-19 pandemic: a survey and resulting consensus. Indian J Dermatol. 2021;66:479-483. doi:10.4103/ijd.ijd_859_20
Practice Points
- The underlying autoimmune process in vitiligo can result in various changes to the immune system.
- A diagnosis of vitiligo may alter the body’s immune response to COVID-19 infection.
Association of Atrial Fibrillation and/or Flutter With Adverse Cardiac Outcomes and Mortality in Patients With Wolff-Parkinson-White Syndrome
Wolff-Parkinson-White (WPW) syndrome is characterized by the presence of ≥ 1 accessory pathways and the development of both recurrent paroxysmal atrial fibrillation (AF) and supraventricular tachycardia that can lead to further malignant arrhythmias resulting in sudden cardiac death (SCD).1-7 Historically, incidental, ventricular pre-excitation on electrocardiogram has conferred a relatively low SCD risk in adults; however, newer WPW syndrome data suggest the endpoint may not be as benign as previously thought.7 The current literature has defined atrioventricular reentrant tachycardia triggering AF, rather than symptoms, as an independent risk factor for malignant arrhythmias. Still, long-term data detailing the association of AF with serious cardiac events and death in patients with WPW syndrome are still limited.1-7
While previous guidelines for the treatment of WPW syndrome only recommended routine electrophysiology testing (EPT) with liberal catheter ablation for symptomatic individuals, the 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines now suggest its potential benefit for risk stratification in the asymptomatic population.8-12 Given the limited existing data, more long-term studies are needed to corroborate the latest EPT recommendations before routinely applying them in practice. Furthermore, since concomitant AF can lead to adverse cardiac outcomes in patients with WPW syndrome, additional data evaluating this association are also necessary. In this study, we aimed to determine the impact of atrial fibrillation and/or flutter (AF/AFL) on adverse cardiac outcomes and mortality in patients with WPW syndrome.
METHODS
This study used data from the Military Health System (MHS) Database Repository. The MHS is one of the largest health care systems in the country and includes information on about 10 million active duty and retired military service members and their families (51% male; 49% female).13,14 Data were fully anonymized and complied in accordance with federal and state laws, including the Health Insurance Portability and Accountability Act of 1996. The Naval Medical Center Portsmouth Institutional Review Board approved this study.
Study Design
This retrospective, observational cohort study identified MHS patients with WPW syndrome from January 1, 2014, to December 31, 2019. Patients were included if they had ≥ 2 International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes for WPW syndrome (ICD-9, 426.7; ICD-10, I45.6) on separate dates; were aged ≥ 18 years at index date; and had ≥ 1 year of continuous eligibility prior to the index date (enrollment gaps ≤ 30 days were considered continuous). Patients were then divided into 2 subgroups by the presence or absence of AF/AFL using diagnostic codes. Patients were excluded if they had evidence of an implantable cardioverter-defibrillator, permanent pacemaker or were missing age or sex data. Patients were followed from index date until the first occurrence of the outcome of interest, MHS disenrollment, or the end of the study period.
Cardiac composite outcomes comprised of sudden cardiac arrest (SCA), ventricular fibrillation (VF), ventricular tachycardia and death, as well as death specifically, were the outcomes of interest and assessed after index date using ICD-9 and ICD-10 codes. Death was defined as all-cause mortality. Time to event was calculated based on the date of the initial component from the composite outcome and date of death specifically for mortality. Those not experiencing an outcome were followed until MHS disenrollment or the end of the study period.
Various patient characteristics were assessed at index including age, sex, military sponsor (the patient’s active or retired duty member through which their dependent receives TRICARE benefits) rank and branch, geographic region, type of US Department of Defense beneficiary, and index year. Clinical characteristics were assessed over a 1-year baseline period prior to index date and included the number of cardiologist and clinical visits for WPW syndrome, Charlson Comorbidity Index (CCI) scores calculated from diagnostic codes outlined in the Quan coding method, and preindex time.15 Comorbidities were assessed at baseline and defined as having ≥ 1 ICD-9 or ICD-10 code for a corresponding condition within 1 year prior to index.
Statistical Analysis
Baseline characteristics were assessed and descriptive statistics for categorical and continuous variables were presented accordingly. To assess bivariate association with exposure, χ2 tests were used to compare categorical variables, while t tests were used to compare continuous variables by exposure status. Incidence proportions and rates were reported for each outcome of interest. Kaplan-Meier curves were constructed to assess the bivariate association between exposure and study outcomes. Cox proportional hazard modeling was performed to estimate the association between AF/AFL and time to each of the outcomes. Multiple models were designed to assess cardiac and metabolic covariates, in addition to baseline characteristics. This included a base model adjusted for age, sex, military sponsor rank and branch, geographic region, and duty status.
Additional models adjusted for cardiac and metabolic confounders and CCI score. A comprehensive model included the base, cardiac, and metabolic covariates. Multicollinearity between covariates was assessed. Variables with a variance inflation factor > 4 or a tolerance level < 0.1 were added to the models. Cox proportional hazard models were used to estimate the unadjusted and adjusted hazard ratios (HRs) and 95% CIs of the association between AF/AFL and the study outcomes. Data were analyzed using SAS, version 9.4 for Windows.
RESULTS
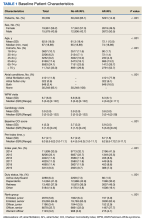
From 2014 through 2019, 35,539 patients with WPW syndrome were identified in the MHS, 5291 had AF/AFL (14.9%); 19,961 were female (56.2%), the mean (SD) age was 62.9 (18.0) years, and 11,742 were aged ≥ 75 years (33.0%) (Table 1).
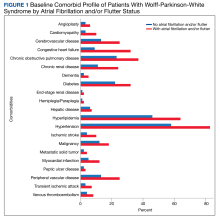
There were 4121 (11.6%), 322 (0.9%), and 848 (2.4%) patients with AF, AFL, and both arrhythmias, respectively. The mean (SD) number of cardiology visits was 3.9 (3.0). The mean (SD) baseline CCI score for the AF/AFL subgroup was 5.9 (3.5) vs 3.7 (2.2) for the non-AF/AFL subgroup (P < .001). The most prevalent comorbid conditions were hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and diabetes (P < .001) (Figure 1).
Composite Outcomes
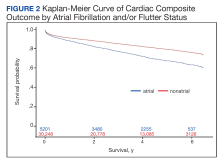
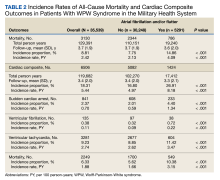
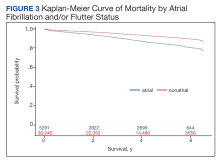
In the overall cohort, during a mean (SD) follow-up time of 3.4 (2.0) years comprising 119,682 total person-years, the components of the composite outcome occurred 6506 times with an incidence rate of 5.44 per 100 person-years. Ventricular tachycardia was the most common event, occurring 3281 times with an incidence rate of 2.74 per 100 person-years. SCA and VF occurred 841 and 135 times with incidence rates of 0.70 and 0.11 per 100 person-years, respectively. Death was the initial event 2249 times with an incidence rate of 1.88 per 100 person-years. Figure 2 shows the Kaplan-Meier curve of cardiac composite outcome by AF/AFL status.
The subgroup with AF/AFL comprised 17,412 total person-years and 1424 cardiac composite incidences compared with 102,270 person years and 5082 incidences in the no AF/AFL group (Table 2). Comparing AF/AFL vs no AF/AFL incidence rates were 8.18 vs 4.97 per 100 person-years, respectively (P < .001). SCA and VF occurred 233 and 38 times and respectively had incidence rates of 1.34 and 0.22 per 100 person-years in the AF/AFL group vs 0.59 and 0.09 per 100 person-years in the no AF/AFL group (P < .001). There were 549 deaths and a 3.15 per 100 person-years incidence rate in the AF/AFL group vs 1700 deaths and a 1.66 incidence rate in the no AF/AFL group (P < .001).
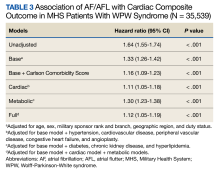
The HR for the composite outcome in the base model was 1.33 (95% CI, 1.26-1.42, P < .001) (Table 3). The association between AF/AFL and the composite outcome remained significant after adjusting for additional metabolic and cardiac covariates. The HRs for the metabolic and cardiac models were 1.30 (95% CI, 1.23-1.38, P < .001) and 1.11 (95% CI, 1.05-1.18, P < .001), respectively. After adjusting for the full model, the HR was 1.12 (95% CI, 1.05-1.19, P < .001).
Mortality
Over the 6-year study period, there was a lower survival probability for patients with AF/AFL. In the overall cohort, during a mean (SD) follow-up time of 3.7 (1.9) years comprising 129,391 total person-years, there were 3130 (8.8%) deaths and an incidence rate of 2.42 per 100 person-years. Death occurred 786 times with a 4.09 incidence rate per 100 person-years in the AF/AFL vs 2344 deaths and a 2.13 incidence rate per 100 person-years in the no AF/AFL group (P < .001). In the non-AF/AFL subgroup, death occurred 2344 times during a mean (SD) follow-up of 3.7 (1.9) years comprising 110,151 total person-years. Figure 3 shows the Kaplan-Meier curve of mortality by AF/AFL status.
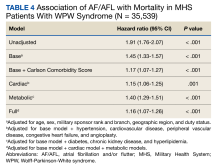
After adjusting for the base, metabolic and cardiac covariates, the HRs for mortality were 1.45 (95% CI, 1.33-1.57, P < .001), 1.40 (95% CI, 1.29-1.51, P < .001) and 1.15 (95% CI, 1.06-1.25, P = .001), respectively (Table 4). The HR after adjusting for the full model was 1.16 (95% CI, 1.07-1.26, P < .001).
DISCUSSION
In this large retrospective cohort study, patients with WPW syndrome and comorbid AF/AFL had a significantly higher association with the cardiac composite outcome and death during a 3-year follow-up period when compared with patients without AF/AFL. After adjusting for confounding variables, the AF/AFL subgroup maintained a 12% and 16% higher association with the composite outcome and mortality, respectively. There was minimal difference in confounding effects between demographic data and metabolic profiles, suggesting one may serve as a proxy for the other.
To our knowledge, this is the largest WPW syndrome cohort study evaluating cardiac outcomes and mortality to date. Although previous research has shown the relatively low and mostly anecdotal SCD incidence within this population, our results demonstrate a higher association of adverse cardiac outcomes and death in an AF/AFL subgroup.16-18 Notably, in this study the AF/AFL cohort was older and had higher CCI scores than their counterparts (P < .001), thus inferring an inherently greater degree of morbidity and 10-year mortality risk. Our study is also unique in that the mean patient age was significantly older than previously reported (63 vs 27 years), which may suggest a longer living history of both ventricular pre-excitation and the comorbidities outlined in Figure 1.19 Given these age discrepancies, it is possible that our overall study population was still relatively low risk and that not all reported deaths were necessarily related to WPW syndrome. Despite these assumptions, when comparing the WPW syndrome subgroups, we still found the AF/AFL cohort maintained a statistically significant higher association with the 2 study outcomes, even after adjusting for the greater presence of comorbidities. This suggests that the presence of AF/AFL may still portend a worse prognosis in patients with WPW syndrome.
Although the association of AF and development of VF in patients with WPW syndrome—due to rapid conduction over the accessory pathway(s)—was first reported > 40 years ago, there has still been few large, long-term data studies exploring mortality in this cohort.19-25 Furthermore, even though the current literature attributes the development of AF with the electrophysiologic properties of the accessory pathway, as well as intrinsic atrial architecture and muscle vulnerability, there is still equivocal consensus regarding EPT screening and ablation indications for asymptomatic patients with WPW syndrome.26-28 Notably, Pappone and colleagues demonstrated the potential benefit of liberal ablation indications for asymptomatic patients, arguing that the intrinsic electrophysiologic properties of the accessory pathway—ie, short accessory-pathway antegrade effective refractory period, inducibility of atrioventricular reentrant tachycardia triggering AF, and multiple accessory pathway—rather than symptoms, are independent predictors of developing malignant arrhythmia.1-5
These findings contradict those reported by Obeyesekere and colleagues, who concluded that the low SCD incidence rates in patients with WPW syndrome precluded routine invasive screening.19,28 They argued that Pappone and colleagues used malignant arrhythmia as a surrogate marker for death, and that the positive predictive value of a short accessory-pathway antegrade effective refractory period for developing malignant arrhythmia was lower than reported (15% vs 82%, respectively) and that its negative predictive value was 100%.1,19,28 Given these conflicting recommendations, we hope our data elucidates the higher association of adverse outcomes and support considerations for more intensive EPT indications in patients with WPW syndrome.
While our study does not report SCD incidence, it does provide robust and reliable mortality data that suggests a greater association of death within an AF/AFL subgroup. Our findings would support more liberal EPT recommendations in patients with WPW syndrome.1-5,8,9 In this study, the SCA incidence rate was more than double the rate in the AF/AFL cohort (P < .001) and is commonly the initial presenting event in WPW syndrome.9 Even though the reported SCD incidence rate is low in WPW syndrome, our data demonstrated an increased association of death within the AF/AFL cohort. Physicians should consider early risk stratification and ablation to prevent potential recurrent malignant arrhythmia leading to death.1-5,8,9,12,19,20
Limitations
As a retrospective study and without access to the National Death Index, we were unable to determine the exact cause or events leading to death and instead utilized all-cause mortality data. Subsequently, our observations may only demonstrate association, rather than causality, between AF/AFL and death in patients with WPW syndrome. Additionally, we could not distinguish between AF and AFL as the arrhythmia leading to death. However, since overall survivability was the outcome of interest, our adjusted HR models were still able to demonstrate the increased association of the composite outcome and death within an AF/AFL cohort.
Although a large cohort was analyzed, due to the constraints of utilizing diagnostic codes to determine study outcomes, we could not distinguish between symptomatic and asymptomatic patients, nor how they were managed prior to the outcome event. However, as recent literature demonstrates, updated predictors of malignant arrhythmia and decisions for early EPT are similar for both symptomatic and asymptomatic patients and should be driven by the intrinsic electrophysiologic properties of the accessory pathway, rather than symptomatology; thus, our inability to discern this should have negligible consequence in determining when to perform risk stratification and ablation.1
MHS eligible patients have direct access to care; the generalizability of our data may not necessarily correspond to a community population with lower socioeconomic status (we did adjust for military sponsor rank which has been used as a proxy), reduced access to care, or uninsured individuals. However, the prevalence of WPW syndrome within our cohort was comparable to the general population, 0.4% vs 0.1%-0.3%, respectively.13,14,19 Similarly, the incidence of AF within our population was comparable to the general population, 15% vs 16%-26%, respectively.23 These similar data points suggest our results may apply beyond MHS patients.
CONCLUSIONS
Patients with WPW syndrome and AF/AFL have a higher association with adverse cardiac outcomes and death. Despite previously reported low SCD incidence rates in this population, our study demonstrates the increased association of mortality in an AF/AFL cohort. The limitations of utilizing all-cause mortality data necessitate further investigation into the etiology behind the deaths in our study population. Since ventricular pre-excitation can predispose patients to AF and potentially lead to malignant arrhythmia and SCD, understanding the cause of mortality will allow physicians to determine the appropriate monitoring and intervention strategies to improve outcomes in this population. Our results suggest consideration for more aggressive EPT screening and ablation recommendations in patients with WPW syndrome may be warranted.
1. Pappone C, Vicedomini G, Manguso F, et al. The natural history of WPW syndrome. Eur Heart J Suppl. 2015; 17 (Supplement A):A8-A11.doi:10.1093/eurheartj/suv004
2. Pappone C, Vicedomini G, Manguso F, et al. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125(5):661-668. doi:10.1161/CIRCULATIONAHA.111.065722
3. Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003;41(2):239-244. doi:10.1016/s0735-1097(02)02706-7
4. Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130(10):811-819. doi:10.1161/CIRCULATIONAHA.114.011154
5. Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003;349(19):1803-1811. doi:10.1056/NEJMoa035345
6. Santinelli V, Radinovic A, Manguso F, et al. Asymptomatic ventricular preexcitation: a long-term prospective follow-up study of 293 adult patients. Circ Arrhythm Electrophysiol. 2009;2(2):102-107. doi:10.1161/CIRCEP.108.827550
7. Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280. doi:10.1016/j.jacc.2008.09.037
8. Al-Khatib SM, Arshad A, Balk EM, et al. Risk Stratification for Arrhythmic Events in Patients With Asymptomatic Pre-Excitation: A Systematic Review for the 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):1624-1638. doi:10.1016/j.jacc.2015.09.018
9. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108(15):1871-1909.doi:10.1161/01.CIR.0000091380.04100.84
10. Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012;9(6):1006-1024. doi:10.1016/j.hrthm.2012.03.050
11. Cohen M, Triedman J. Guidelines for management of asymptomatic ventricular pre-excitation: brave new world or Pandora’s box?. Circ Arrhythm Electrophysiol. 2014;7(2):187-189. doi:10.1161/CIRCEP.114.001528
12. Svendsen JH, Dagres N, Dobreanu D, et al. Current strategy for treatment of patients with Wolff-Parkinson-White syndrome and asymptomatic preexcitation in Europe: European Heart Rhythm Association survey. Europace. 2013;15(5):750-753. doi:10.1093/europace/eut094
13. Gimbel RW, Pangaro L, Barbour G. America’s “undiscovered” laboratory for health services research. Med Care. 2010;48(8):751-756. doi:10.1097/MLR.0b013e3181e35be8
14. Dorrance KA, Ramchandani S, Neil N, Fisher H. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142-145. doi:10.7205/milmed-d-12-00168
15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi:10.1097/01.mlr.0000182534.19832.83
16. Finocchiaro G, Papadakis M, Behr ER, Sharma S, Sheppard M. Sudden Cardiac Death in Pre-Excitation and Wolff-Parkinson-White: Demographic and Clinical Features. J Am Coll Cardiol. 2017;69(12):1644-1645. doi:10.1016/j.jacc.2017.01.023
17. Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993;87(3):866-873. doi:10.1161/01.cir.87.3.866
18. Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142(3):530-536. doi:10.1067/mhj.2001.117779
19. Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125(19):2308-2315. doi:10.1161/CIRCULATIONAHA.111.055350
20. Waspe LE, Brodman R, Kim SG, Fisher JD. Susceptibility to atrial fibrillation and ventricular tachyarrhythmia in the Wolff-Parkinson-White syndrome: role of the accessory pathway. Am Heart J. 1986;112(6):1141-1152. doi:10.1016/0002-8703(86)90342-x
21. Pietersen AH, Andersen ED, Sandøe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70(5):38A-43A. doi:10.1016/0002-9149(92)91076-g
22. Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17(6):1352-1356. doi:10.1016/s0735-1097(10)80146-9
23. Fujimura O, Klein GJ, Yee R, Sharma AD. Mode of onset of atrial fibrillation in the Wolff-Parkinson-White syndrome: how important is the accessory pathway?. J Am Coll Cardiol. 1990;15(5):1082-1086. doi:10.1016/0735-1097(90)90244-j
24. Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12(2):144-150. doi:10.1093/oxfordjournals.eurheartj.a059860
25. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301(20):1080-1085. doi:10.1056/NEJM197911153012003
26. Centurion OA. Atrial Fibrillation in the Wolff-Parkinson-White Syndrome. J Atr Fibrillation. 2011;4(1):287. Published 2011 May 4. doi:10.4022/jafib.287
27. Song C, Guo Y, Zheng X, et al. Prognostic Significance and Risk of Atrial Fibrillation of Wolff-Parkinson-White Syndrome in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2018;122(9):1546-1550. doi:10.1016/j.amjcard.2018.07.021
28. Obeyesekere M, Gula LJ, Skanes AC, Leong-Sit P, Klein GJ. Risk of sudden death in Wolff-Parkinson-White syndrome: how high is the risk?. Circulation. 2012;125(5):659-660. doi:10.1161/CIRCULATIONAHA.111.085159
Wolff-Parkinson-White (WPW) syndrome is characterized by the presence of ≥ 1 accessory pathways and the development of both recurrent paroxysmal atrial fibrillation (AF) and supraventricular tachycardia that can lead to further malignant arrhythmias resulting in sudden cardiac death (SCD).1-7 Historically, incidental, ventricular pre-excitation on electrocardiogram has conferred a relatively low SCD risk in adults; however, newer WPW syndrome data suggest the endpoint may not be as benign as previously thought.7 The current literature has defined atrioventricular reentrant tachycardia triggering AF, rather than symptoms, as an independent risk factor for malignant arrhythmias. Still, long-term data detailing the association of AF with serious cardiac events and death in patients with WPW syndrome are still limited.1-7
While previous guidelines for the treatment of WPW syndrome only recommended routine electrophysiology testing (EPT) with liberal catheter ablation for symptomatic individuals, the 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines now suggest its potential benefit for risk stratification in the asymptomatic population.8-12 Given the limited existing data, more long-term studies are needed to corroborate the latest EPT recommendations before routinely applying them in practice. Furthermore, since concomitant AF can lead to adverse cardiac outcomes in patients with WPW syndrome, additional data evaluating this association are also necessary. In this study, we aimed to determine the impact of atrial fibrillation and/or flutter (AF/AFL) on adverse cardiac outcomes and mortality in patients with WPW syndrome.
METHODS
This study used data from the Military Health System (MHS) Database Repository. The MHS is one of the largest health care systems in the country and includes information on about 10 million active duty and retired military service members and their families (51% male; 49% female).13,14 Data were fully anonymized and complied in accordance with federal and state laws, including the Health Insurance Portability and Accountability Act of 1996. The Naval Medical Center Portsmouth Institutional Review Board approved this study.
Study Design
This retrospective, observational cohort study identified MHS patients with WPW syndrome from January 1, 2014, to December 31, 2019. Patients were included if they had ≥ 2 International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes for WPW syndrome (ICD-9, 426.7; ICD-10, I45.6) on separate dates; were aged ≥ 18 years at index date; and had ≥ 1 year of continuous eligibility prior to the index date (enrollment gaps ≤ 30 days were considered continuous). Patients were then divided into 2 subgroups by the presence or absence of AF/AFL using diagnostic codes. Patients were excluded if they had evidence of an implantable cardioverter-defibrillator, permanent pacemaker or were missing age or sex data. Patients were followed from index date until the first occurrence of the outcome of interest, MHS disenrollment, or the end of the study period.
Cardiac composite outcomes comprised of sudden cardiac arrest (SCA), ventricular fibrillation (VF), ventricular tachycardia and death, as well as death specifically, were the outcomes of interest and assessed after index date using ICD-9 and ICD-10 codes. Death was defined as all-cause mortality. Time to event was calculated based on the date of the initial component from the composite outcome and date of death specifically for mortality. Those not experiencing an outcome were followed until MHS disenrollment or the end of the study period.
Various patient characteristics were assessed at index including age, sex, military sponsor (the patient’s active or retired duty member through which their dependent receives TRICARE benefits) rank and branch, geographic region, type of US Department of Defense beneficiary, and index year. Clinical characteristics were assessed over a 1-year baseline period prior to index date and included the number of cardiologist and clinical visits for WPW syndrome, Charlson Comorbidity Index (CCI) scores calculated from diagnostic codes outlined in the Quan coding method, and preindex time.15 Comorbidities were assessed at baseline and defined as having ≥ 1 ICD-9 or ICD-10 code for a corresponding condition within 1 year prior to index.
Statistical Analysis
Baseline characteristics were assessed and descriptive statistics for categorical and continuous variables were presented accordingly. To assess bivariate association with exposure, χ2 tests were used to compare categorical variables, while t tests were used to compare continuous variables by exposure status. Incidence proportions and rates were reported for each outcome of interest. Kaplan-Meier curves were constructed to assess the bivariate association between exposure and study outcomes. Cox proportional hazard modeling was performed to estimate the association between AF/AFL and time to each of the outcomes. Multiple models were designed to assess cardiac and metabolic covariates, in addition to baseline characteristics. This included a base model adjusted for age, sex, military sponsor rank and branch, geographic region, and duty status.
Additional models adjusted for cardiac and metabolic confounders and CCI score. A comprehensive model included the base, cardiac, and metabolic covariates. Multicollinearity between covariates was assessed. Variables with a variance inflation factor > 4 or a tolerance level < 0.1 were added to the models. Cox proportional hazard models were used to estimate the unadjusted and adjusted hazard ratios (HRs) and 95% CIs of the association between AF/AFL and the study outcomes. Data were analyzed using SAS, version 9.4 for Windows.
RESULTS
From 2014 through 2019, 35,539 patients with WPW syndrome were identified in the MHS, 5291 had AF/AFL (14.9%); 19,961 were female (56.2%), the mean (SD) age was 62.9 (18.0) years, and 11,742 were aged ≥ 75 years (33.0%) (Table 1).
There were 4121 (11.6%), 322 (0.9%), and 848 (2.4%) patients with AF, AFL, and both arrhythmias, respectively. The mean (SD) number of cardiology visits was 3.9 (3.0). The mean (SD) baseline CCI score for the AF/AFL subgroup was 5.9 (3.5) vs 3.7 (2.2) for the non-AF/AFL subgroup (P < .001). The most prevalent comorbid conditions were hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and diabetes (P < .001) (Figure 1).
Composite Outcomes
In the overall cohort, during a mean (SD) follow-up time of 3.4 (2.0) years comprising 119,682 total person-years, the components of the composite outcome occurred 6506 times with an incidence rate of 5.44 per 100 person-years. Ventricular tachycardia was the most common event, occurring 3281 times with an incidence rate of 2.74 per 100 person-years. SCA and VF occurred 841 and 135 times with incidence rates of 0.70 and 0.11 per 100 person-years, respectively. Death was the initial event 2249 times with an incidence rate of 1.88 per 100 person-years. Figure 2 shows the Kaplan-Meier curve of cardiac composite outcome by AF/AFL status.
The subgroup with AF/AFL comprised 17,412 total person-years and 1424 cardiac composite incidences compared with 102,270 person years and 5082 incidences in the no AF/AFL group (Table 2). Comparing AF/AFL vs no AF/AFL incidence rates were 8.18 vs 4.97 per 100 person-years, respectively (P < .001). SCA and VF occurred 233 and 38 times and respectively had incidence rates of 1.34 and 0.22 per 100 person-years in the AF/AFL group vs 0.59 and 0.09 per 100 person-years in the no AF/AFL group (P < .001). There were 549 deaths and a 3.15 per 100 person-years incidence rate in the AF/AFL group vs 1700 deaths and a 1.66 incidence rate in the no AF/AFL group (P < .001).
The HR for the composite outcome in the base model was 1.33 (95% CI, 1.26-1.42, P < .001) (Table 3). The association between AF/AFL and the composite outcome remained significant after adjusting for additional metabolic and cardiac covariates. The HRs for the metabolic and cardiac models were 1.30 (95% CI, 1.23-1.38, P < .001) and 1.11 (95% CI, 1.05-1.18, P < .001), respectively. After adjusting for the full model, the HR was 1.12 (95% CI, 1.05-1.19, P < .001).
Mortality
Over the 6-year study period, there was a lower survival probability for patients with AF/AFL. In the overall cohort, during a mean (SD) follow-up time of 3.7 (1.9) years comprising 129,391 total person-years, there were 3130 (8.8%) deaths and an incidence rate of 2.42 per 100 person-years. Death occurred 786 times with a 4.09 incidence rate per 100 person-years in the AF/AFL vs 2344 deaths and a 2.13 incidence rate per 100 person-years in the no AF/AFL group (P < .001). In the non-AF/AFL subgroup, death occurred 2344 times during a mean (SD) follow-up of 3.7 (1.9) years comprising 110,151 total person-years. Figure 3 shows the Kaplan-Meier curve of mortality by AF/AFL status.
After adjusting for the base, metabolic and cardiac covariates, the HRs for mortality were 1.45 (95% CI, 1.33-1.57, P < .001), 1.40 (95% CI, 1.29-1.51, P < .001) and 1.15 (95% CI, 1.06-1.25, P = .001), respectively (Table 4). The HR after adjusting for the full model was 1.16 (95% CI, 1.07-1.26, P < .001).
DISCUSSION
In this large retrospective cohort study, patients with WPW syndrome and comorbid AF/AFL had a significantly higher association with the cardiac composite outcome and death during a 3-year follow-up period when compared with patients without AF/AFL. After adjusting for confounding variables, the AF/AFL subgroup maintained a 12% and 16% higher association with the composite outcome and mortality, respectively. There was minimal difference in confounding effects between demographic data and metabolic profiles, suggesting one may serve as a proxy for the other.
To our knowledge, this is the largest WPW syndrome cohort study evaluating cardiac outcomes and mortality to date. Although previous research has shown the relatively low and mostly anecdotal SCD incidence within this population, our results demonstrate a higher association of adverse cardiac outcomes and death in an AF/AFL subgroup.16-18 Notably, in this study the AF/AFL cohort was older and had higher CCI scores than their counterparts (P < .001), thus inferring an inherently greater degree of morbidity and 10-year mortality risk. Our study is also unique in that the mean patient age was significantly older than previously reported (63 vs 27 years), which may suggest a longer living history of both ventricular pre-excitation and the comorbidities outlined in Figure 1.19 Given these age discrepancies, it is possible that our overall study population was still relatively low risk and that not all reported deaths were necessarily related to WPW syndrome. Despite these assumptions, when comparing the WPW syndrome subgroups, we still found the AF/AFL cohort maintained a statistically significant higher association with the 2 study outcomes, even after adjusting for the greater presence of comorbidities. This suggests that the presence of AF/AFL may still portend a worse prognosis in patients with WPW syndrome.
Although the association of AF and development of VF in patients with WPW syndrome—due to rapid conduction over the accessory pathway(s)—was first reported > 40 years ago, there has still been few large, long-term data studies exploring mortality in this cohort.19-25 Furthermore, even though the current literature attributes the development of AF with the electrophysiologic properties of the accessory pathway, as well as intrinsic atrial architecture and muscle vulnerability, there is still equivocal consensus regarding EPT screening and ablation indications for asymptomatic patients with WPW syndrome.26-28 Notably, Pappone and colleagues demonstrated the potential benefit of liberal ablation indications for asymptomatic patients, arguing that the intrinsic electrophysiologic properties of the accessory pathway—ie, short accessory-pathway antegrade effective refractory period, inducibility of atrioventricular reentrant tachycardia triggering AF, and multiple accessory pathway—rather than symptoms, are independent predictors of developing malignant arrhythmia.1-5
These findings contradict those reported by Obeyesekere and colleagues, who concluded that the low SCD incidence rates in patients with WPW syndrome precluded routine invasive screening.19,28 They argued that Pappone and colleagues used malignant arrhythmia as a surrogate marker for death, and that the positive predictive value of a short accessory-pathway antegrade effective refractory period for developing malignant arrhythmia was lower than reported (15% vs 82%, respectively) and that its negative predictive value was 100%.1,19,28 Given these conflicting recommendations, we hope our data elucidates the higher association of adverse outcomes and support considerations for more intensive EPT indications in patients with WPW syndrome.
While our study does not report SCD incidence, it does provide robust and reliable mortality data that suggests a greater association of death within an AF/AFL subgroup. Our findings would support more liberal EPT recommendations in patients with WPW syndrome.1-5,8,9 In this study, the SCA incidence rate was more than double the rate in the AF/AFL cohort (P < .001) and is commonly the initial presenting event in WPW syndrome.9 Even though the reported SCD incidence rate is low in WPW syndrome, our data demonstrated an increased association of death within the AF/AFL cohort. Physicians should consider early risk stratification and ablation to prevent potential recurrent malignant arrhythmia leading to death.1-5,8,9,12,19,20
Limitations
As a retrospective study and without access to the National Death Index, we were unable to determine the exact cause or events leading to death and instead utilized all-cause mortality data. Subsequently, our observations may only demonstrate association, rather than causality, between AF/AFL and death in patients with WPW syndrome. Additionally, we could not distinguish between AF and AFL as the arrhythmia leading to death. However, since overall survivability was the outcome of interest, our adjusted HR models were still able to demonstrate the increased association of the composite outcome and death within an AF/AFL cohort.
Although a large cohort was analyzed, due to the constraints of utilizing diagnostic codes to determine study outcomes, we could not distinguish between symptomatic and asymptomatic patients, nor how they were managed prior to the outcome event. However, as recent literature demonstrates, updated predictors of malignant arrhythmia and decisions for early EPT are similar for both symptomatic and asymptomatic patients and should be driven by the intrinsic electrophysiologic properties of the accessory pathway, rather than symptomatology; thus, our inability to discern this should have negligible consequence in determining when to perform risk stratification and ablation.1
MHS eligible patients have direct access to care; the generalizability of our data may not necessarily correspond to a community population with lower socioeconomic status (we did adjust for military sponsor rank which has been used as a proxy), reduced access to care, or uninsured individuals. However, the prevalence of WPW syndrome within our cohort was comparable to the general population, 0.4% vs 0.1%-0.3%, respectively.13,14,19 Similarly, the incidence of AF within our population was comparable to the general population, 15% vs 16%-26%, respectively.23 These similar data points suggest our results may apply beyond MHS patients.
CONCLUSIONS
Patients with WPW syndrome and AF/AFL have a higher association with adverse cardiac outcomes and death. Despite previously reported low SCD incidence rates in this population, our study demonstrates the increased association of mortality in an AF/AFL cohort. The limitations of utilizing all-cause mortality data necessitate further investigation into the etiology behind the deaths in our study population. Since ventricular pre-excitation can predispose patients to AF and potentially lead to malignant arrhythmia and SCD, understanding the cause of mortality will allow physicians to determine the appropriate monitoring and intervention strategies to improve outcomes in this population. Our results suggest consideration for more aggressive EPT screening and ablation recommendations in patients with WPW syndrome may be warranted.
Wolff-Parkinson-White (WPW) syndrome is characterized by the presence of ≥ 1 accessory pathways and the development of both recurrent paroxysmal atrial fibrillation (AF) and supraventricular tachycardia that can lead to further malignant arrhythmias resulting in sudden cardiac death (SCD).1-7 Historically, incidental, ventricular pre-excitation on electrocardiogram has conferred a relatively low SCD risk in adults; however, newer WPW syndrome data suggest the endpoint may not be as benign as previously thought.7 The current literature has defined atrioventricular reentrant tachycardia triggering AF, rather than symptoms, as an independent risk factor for malignant arrhythmias. Still, long-term data detailing the association of AF with serious cardiac events and death in patients with WPW syndrome are still limited.1-7
While previous guidelines for the treatment of WPW syndrome only recommended routine electrophysiology testing (EPT) with liberal catheter ablation for symptomatic individuals, the 2015 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines now suggest its potential benefit for risk stratification in the asymptomatic population.8-12 Given the limited existing data, more long-term studies are needed to corroborate the latest EPT recommendations before routinely applying them in practice. Furthermore, since concomitant AF can lead to adverse cardiac outcomes in patients with WPW syndrome, additional data evaluating this association are also necessary. In this study, we aimed to determine the impact of atrial fibrillation and/or flutter (AF/AFL) on adverse cardiac outcomes and mortality in patients with WPW syndrome.
METHODS
This study used data from the Military Health System (MHS) Database Repository. The MHS is one of the largest health care systems in the country and includes information on about 10 million active duty and retired military service members and their families (51% male; 49% female).13,14 Data were fully anonymized and complied in accordance with federal and state laws, including the Health Insurance Portability and Accountability Act of 1996. The Naval Medical Center Portsmouth Institutional Review Board approved this study.
Study Design
This retrospective, observational cohort study identified MHS patients with WPW syndrome from January 1, 2014, to December 31, 2019. Patients were included if they had ≥ 2 International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes for WPW syndrome (ICD-9, 426.7; ICD-10, I45.6) on separate dates; were aged ≥ 18 years at index date; and had ≥ 1 year of continuous eligibility prior to the index date (enrollment gaps ≤ 30 days were considered continuous). Patients were then divided into 2 subgroups by the presence or absence of AF/AFL using diagnostic codes. Patients were excluded if they had evidence of an implantable cardioverter-defibrillator, permanent pacemaker or were missing age or sex data. Patients were followed from index date until the first occurrence of the outcome of interest, MHS disenrollment, or the end of the study period.
Cardiac composite outcomes comprised of sudden cardiac arrest (SCA), ventricular fibrillation (VF), ventricular tachycardia and death, as well as death specifically, were the outcomes of interest and assessed after index date using ICD-9 and ICD-10 codes. Death was defined as all-cause mortality. Time to event was calculated based on the date of the initial component from the composite outcome and date of death specifically for mortality. Those not experiencing an outcome were followed until MHS disenrollment or the end of the study period.
Various patient characteristics were assessed at index including age, sex, military sponsor (the patient’s active or retired duty member through which their dependent receives TRICARE benefits) rank and branch, geographic region, type of US Department of Defense beneficiary, and index year. Clinical characteristics were assessed over a 1-year baseline period prior to index date and included the number of cardiologist and clinical visits for WPW syndrome, Charlson Comorbidity Index (CCI) scores calculated from diagnostic codes outlined in the Quan coding method, and preindex time.15 Comorbidities were assessed at baseline and defined as having ≥ 1 ICD-9 or ICD-10 code for a corresponding condition within 1 year prior to index.
Statistical Analysis
Baseline characteristics were assessed and descriptive statistics for categorical and continuous variables were presented accordingly. To assess bivariate association with exposure, χ2 tests were used to compare categorical variables, while t tests were used to compare continuous variables by exposure status. Incidence proportions and rates were reported for each outcome of interest. Kaplan-Meier curves were constructed to assess the bivariate association between exposure and study outcomes. Cox proportional hazard modeling was performed to estimate the association between AF/AFL and time to each of the outcomes. Multiple models were designed to assess cardiac and metabolic covariates, in addition to baseline characteristics. This included a base model adjusted for age, sex, military sponsor rank and branch, geographic region, and duty status.
Additional models adjusted for cardiac and metabolic confounders and CCI score. A comprehensive model included the base, cardiac, and metabolic covariates. Multicollinearity between covariates was assessed. Variables with a variance inflation factor > 4 or a tolerance level < 0.1 were added to the models. Cox proportional hazard models were used to estimate the unadjusted and adjusted hazard ratios (HRs) and 95% CIs of the association between AF/AFL and the study outcomes. Data were analyzed using SAS, version 9.4 for Windows.
RESULTS
From 2014 through 2019, 35,539 patients with WPW syndrome were identified in the MHS, 5291 had AF/AFL (14.9%); 19,961 were female (56.2%), the mean (SD) age was 62.9 (18.0) years, and 11,742 were aged ≥ 75 years (33.0%) (Table 1).
There were 4121 (11.6%), 322 (0.9%), and 848 (2.4%) patients with AF, AFL, and both arrhythmias, respectively. The mean (SD) number of cardiology visits was 3.9 (3.0). The mean (SD) baseline CCI score for the AF/AFL subgroup was 5.9 (3.5) vs 3.7 (2.2) for the non-AF/AFL subgroup (P < .001). The most prevalent comorbid conditions were hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and diabetes (P < .001) (Figure 1).
Composite Outcomes
In the overall cohort, during a mean (SD) follow-up time of 3.4 (2.0) years comprising 119,682 total person-years, the components of the composite outcome occurred 6506 times with an incidence rate of 5.44 per 100 person-years. Ventricular tachycardia was the most common event, occurring 3281 times with an incidence rate of 2.74 per 100 person-years. SCA and VF occurred 841 and 135 times with incidence rates of 0.70 and 0.11 per 100 person-years, respectively. Death was the initial event 2249 times with an incidence rate of 1.88 per 100 person-years. Figure 2 shows the Kaplan-Meier curve of cardiac composite outcome by AF/AFL status.
The subgroup with AF/AFL comprised 17,412 total person-years and 1424 cardiac composite incidences compared with 102,270 person years and 5082 incidences in the no AF/AFL group (Table 2). Comparing AF/AFL vs no AF/AFL incidence rates were 8.18 vs 4.97 per 100 person-years, respectively (P < .001). SCA and VF occurred 233 and 38 times and respectively had incidence rates of 1.34 and 0.22 per 100 person-years in the AF/AFL group vs 0.59 and 0.09 per 100 person-years in the no AF/AFL group (P < .001). There were 549 deaths and a 3.15 per 100 person-years incidence rate in the AF/AFL group vs 1700 deaths and a 1.66 incidence rate in the no AF/AFL group (P < .001).
The HR for the composite outcome in the base model was 1.33 (95% CI, 1.26-1.42, P < .001) (Table 3). The association between AF/AFL and the composite outcome remained significant after adjusting for additional metabolic and cardiac covariates. The HRs for the metabolic and cardiac models were 1.30 (95% CI, 1.23-1.38, P < .001) and 1.11 (95% CI, 1.05-1.18, P < .001), respectively. After adjusting for the full model, the HR was 1.12 (95% CI, 1.05-1.19, P < .001).
Mortality
Over the 6-year study period, there was a lower survival probability for patients with AF/AFL. In the overall cohort, during a mean (SD) follow-up time of 3.7 (1.9) years comprising 129,391 total person-years, there were 3130 (8.8%) deaths and an incidence rate of 2.42 per 100 person-years. Death occurred 786 times with a 4.09 incidence rate per 100 person-years in the AF/AFL vs 2344 deaths and a 2.13 incidence rate per 100 person-years in the no AF/AFL group (P < .001). In the non-AF/AFL subgroup, death occurred 2344 times during a mean (SD) follow-up of 3.7 (1.9) years comprising 110,151 total person-years. Figure 3 shows the Kaplan-Meier curve of mortality by AF/AFL status.
After adjusting for the base, metabolic and cardiac covariates, the HRs for mortality were 1.45 (95% CI, 1.33-1.57, P < .001), 1.40 (95% CI, 1.29-1.51, P < .001) and 1.15 (95% CI, 1.06-1.25, P = .001), respectively (Table 4). The HR after adjusting for the full model was 1.16 (95% CI, 1.07-1.26, P < .001).
DISCUSSION
In this large retrospective cohort study, patients with WPW syndrome and comorbid AF/AFL had a significantly higher association with the cardiac composite outcome and death during a 3-year follow-up period when compared with patients without AF/AFL. After adjusting for confounding variables, the AF/AFL subgroup maintained a 12% and 16% higher association with the composite outcome and mortality, respectively. There was minimal difference in confounding effects between demographic data and metabolic profiles, suggesting one may serve as a proxy for the other.
To our knowledge, this is the largest WPW syndrome cohort study evaluating cardiac outcomes and mortality to date. Although previous research has shown the relatively low and mostly anecdotal SCD incidence within this population, our results demonstrate a higher association of adverse cardiac outcomes and death in an AF/AFL subgroup.16-18 Notably, in this study the AF/AFL cohort was older and had higher CCI scores than their counterparts (P < .001), thus inferring an inherently greater degree of morbidity and 10-year mortality risk. Our study is also unique in that the mean patient age was significantly older than previously reported (63 vs 27 years), which may suggest a longer living history of both ventricular pre-excitation and the comorbidities outlined in Figure 1.19 Given these age discrepancies, it is possible that our overall study population was still relatively low risk and that not all reported deaths were necessarily related to WPW syndrome. Despite these assumptions, when comparing the WPW syndrome subgroups, we still found the AF/AFL cohort maintained a statistically significant higher association with the 2 study outcomes, even after adjusting for the greater presence of comorbidities. This suggests that the presence of AF/AFL may still portend a worse prognosis in patients with WPW syndrome.
Although the association of AF and development of VF in patients with WPW syndrome—due to rapid conduction over the accessory pathway(s)—was first reported > 40 years ago, there has still been few large, long-term data studies exploring mortality in this cohort.19-25 Furthermore, even though the current literature attributes the development of AF with the electrophysiologic properties of the accessory pathway, as well as intrinsic atrial architecture and muscle vulnerability, there is still equivocal consensus regarding EPT screening and ablation indications for asymptomatic patients with WPW syndrome.26-28 Notably, Pappone and colleagues demonstrated the potential benefit of liberal ablation indications for asymptomatic patients, arguing that the intrinsic electrophysiologic properties of the accessory pathway—ie, short accessory-pathway antegrade effective refractory period, inducibility of atrioventricular reentrant tachycardia triggering AF, and multiple accessory pathway—rather than symptoms, are independent predictors of developing malignant arrhythmia.1-5
These findings contradict those reported by Obeyesekere and colleagues, who concluded that the low SCD incidence rates in patients with WPW syndrome precluded routine invasive screening.19,28 They argued that Pappone and colleagues used malignant arrhythmia as a surrogate marker for death, and that the positive predictive value of a short accessory-pathway antegrade effective refractory period for developing malignant arrhythmia was lower than reported (15% vs 82%, respectively) and that its negative predictive value was 100%.1,19,28 Given these conflicting recommendations, we hope our data elucidates the higher association of adverse outcomes and support considerations for more intensive EPT indications in patients with WPW syndrome.
While our study does not report SCD incidence, it does provide robust and reliable mortality data that suggests a greater association of death within an AF/AFL subgroup. Our findings would support more liberal EPT recommendations in patients with WPW syndrome.1-5,8,9 In this study, the SCA incidence rate was more than double the rate in the AF/AFL cohort (P < .001) and is commonly the initial presenting event in WPW syndrome.9 Even though the reported SCD incidence rate is low in WPW syndrome, our data demonstrated an increased association of death within the AF/AFL cohort. Physicians should consider early risk stratification and ablation to prevent potential recurrent malignant arrhythmia leading to death.1-5,8,9,12,19,20
Limitations
As a retrospective study and without access to the National Death Index, we were unable to determine the exact cause or events leading to death and instead utilized all-cause mortality data. Subsequently, our observations may only demonstrate association, rather than causality, between AF/AFL and death in patients with WPW syndrome. Additionally, we could not distinguish between AF and AFL as the arrhythmia leading to death. However, since overall survivability was the outcome of interest, our adjusted HR models were still able to demonstrate the increased association of the composite outcome and death within an AF/AFL cohort.
Although a large cohort was analyzed, due to the constraints of utilizing diagnostic codes to determine study outcomes, we could not distinguish between symptomatic and asymptomatic patients, nor how they were managed prior to the outcome event. However, as recent literature demonstrates, updated predictors of malignant arrhythmia and decisions for early EPT are similar for both symptomatic and asymptomatic patients and should be driven by the intrinsic electrophysiologic properties of the accessory pathway, rather than symptomatology; thus, our inability to discern this should have negligible consequence in determining when to perform risk stratification and ablation.1
MHS eligible patients have direct access to care; the generalizability of our data may not necessarily correspond to a community population with lower socioeconomic status (we did adjust for military sponsor rank which has been used as a proxy), reduced access to care, or uninsured individuals. However, the prevalence of WPW syndrome within our cohort was comparable to the general population, 0.4% vs 0.1%-0.3%, respectively.13,14,19 Similarly, the incidence of AF within our population was comparable to the general population, 15% vs 16%-26%, respectively.23 These similar data points suggest our results may apply beyond MHS patients.
CONCLUSIONS
Patients with WPW syndrome and AF/AFL have a higher association with adverse cardiac outcomes and death. Despite previously reported low SCD incidence rates in this population, our study demonstrates the increased association of mortality in an AF/AFL cohort. The limitations of utilizing all-cause mortality data necessitate further investigation into the etiology behind the deaths in our study population. Since ventricular pre-excitation can predispose patients to AF and potentially lead to malignant arrhythmia and SCD, understanding the cause of mortality will allow physicians to determine the appropriate monitoring and intervention strategies to improve outcomes in this population. Our results suggest consideration for more aggressive EPT screening and ablation recommendations in patients with WPW syndrome may be warranted.
1. Pappone C, Vicedomini G, Manguso F, et al. The natural history of WPW syndrome. Eur Heart J Suppl. 2015; 17 (Supplement A):A8-A11.doi:10.1093/eurheartj/suv004
2. Pappone C, Vicedomini G, Manguso F, et al. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125(5):661-668. doi:10.1161/CIRCULATIONAHA.111.065722
3. Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003;41(2):239-244. doi:10.1016/s0735-1097(02)02706-7
4. Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130(10):811-819. doi:10.1161/CIRCULATIONAHA.114.011154
5. Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003;349(19):1803-1811. doi:10.1056/NEJMoa035345
6. Santinelli V, Radinovic A, Manguso F, et al. Asymptomatic ventricular preexcitation: a long-term prospective follow-up study of 293 adult patients. Circ Arrhythm Electrophysiol. 2009;2(2):102-107. doi:10.1161/CIRCEP.108.827550
7. Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280. doi:10.1016/j.jacc.2008.09.037
8. Al-Khatib SM, Arshad A, Balk EM, et al. Risk Stratification for Arrhythmic Events in Patients With Asymptomatic Pre-Excitation: A Systematic Review for the 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):1624-1638. doi:10.1016/j.jacc.2015.09.018
9. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108(15):1871-1909.doi:10.1161/01.CIR.0000091380.04100.84
10. Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012;9(6):1006-1024. doi:10.1016/j.hrthm.2012.03.050
11. Cohen M, Triedman J. Guidelines for management of asymptomatic ventricular pre-excitation: brave new world or Pandora’s box?. Circ Arrhythm Electrophysiol. 2014;7(2):187-189. doi:10.1161/CIRCEP.114.001528
12. Svendsen JH, Dagres N, Dobreanu D, et al. Current strategy for treatment of patients with Wolff-Parkinson-White syndrome and asymptomatic preexcitation in Europe: European Heart Rhythm Association survey. Europace. 2013;15(5):750-753. doi:10.1093/europace/eut094
13. Gimbel RW, Pangaro L, Barbour G. America’s “undiscovered” laboratory for health services research. Med Care. 2010;48(8):751-756. doi:10.1097/MLR.0b013e3181e35be8
14. Dorrance KA, Ramchandani S, Neil N, Fisher H. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142-145. doi:10.7205/milmed-d-12-00168
15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi:10.1097/01.mlr.0000182534.19832.83
16. Finocchiaro G, Papadakis M, Behr ER, Sharma S, Sheppard M. Sudden Cardiac Death in Pre-Excitation and Wolff-Parkinson-White: Demographic and Clinical Features. J Am Coll Cardiol. 2017;69(12):1644-1645. doi:10.1016/j.jacc.2017.01.023
17. Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993;87(3):866-873. doi:10.1161/01.cir.87.3.866
18. Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142(3):530-536. doi:10.1067/mhj.2001.117779
19. Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125(19):2308-2315. doi:10.1161/CIRCULATIONAHA.111.055350
20. Waspe LE, Brodman R, Kim SG, Fisher JD. Susceptibility to atrial fibrillation and ventricular tachyarrhythmia in the Wolff-Parkinson-White syndrome: role of the accessory pathway. Am Heart J. 1986;112(6):1141-1152. doi:10.1016/0002-8703(86)90342-x
21. Pietersen AH, Andersen ED, Sandøe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70(5):38A-43A. doi:10.1016/0002-9149(92)91076-g
22. Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17(6):1352-1356. doi:10.1016/s0735-1097(10)80146-9
23. Fujimura O, Klein GJ, Yee R, Sharma AD. Mode of onset of atrial fibrillation in the Wolff-Parkinson-White syndrome: how important is the accessory pathway?. J Am Coll Cardiol. 1990;15(5):1082-1086. doi:10.1016/0735-1097(90)90244-j
24. Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12(2):144-150. doi:10.1093/oxfordjournals.eurheartj.a059860
25. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301(20):1080-1085. doi:10.1056/NEJM197911153012003
26. Centurion OA. Atrial Fibrillation in the Wolff-Parkinson-White Syndrome. J Atr Fibrillation. 2011;4(1):287. Published 2011 May 4. doi:10.4022/jafib.287
27. Song C, Guo Y, Zheng X, et al. Prognostic Significance and Risk of Atrial Fibrillation of Wolff-Parkinson-White Syndrome in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2018;122(9):1546-1550. doi:10.1016/j.amjcard.2018.07.021
28. Obeyesekere M, Gula LJ, Skanes AC, Leong-Sit P, Klein GJ. Risk of sudden death in Wolff-Parkinson-White syndrome: how high is the risk?. Circulation. 2012;125(5):659-660. doi:10.1161/CIRCULATIONAHA.111.085159
1. Pappone C, Vicedomini G, Manguso F, et al. The natural history of WPW syndrome. Eur Heart J Suppl. 2015; 17 (Supplement A):A8-A11.doi:10.1093/eurheartj/suv004
2. Pappone C, Vicedomini G, Manguso F, et al. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125(5):661-668. doi:10.1161/CIRCULATIONAHA.111.065722
3. Pappone C, Santinelli V, Rosanio S, et al. Usefulness of invasive electrophysiologic testing to stratify the risk of arrhythmic events in asymptomatic patients with Wolff-Parkinson-White pattern: results from a large prospective long-term follow-up study. J Am Coll Cardiol. 2003;41(2):239-244. doi:10.1016/s0735-1097(02)02706-7
4. Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130(10):811-819. doi:10.1161/CIRCULATIONAHA.114.011154
5. Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-Parkinson-White syndrome. N Engl J Med. 2003;349(19):1803-1811. doi:10.1056/NEJMoa035345
6. Santinelli V, Radinovic A, Manguso F, et al. Asymptomatic ventricular preexcitation: a long-term prospective follow-up study of 293 adult patients. Circ Arrhythm Electrophysiol. 2009;2(2):102-107. doi:10.1161/CIRCEP.108.827550
7. Santinelli V, Radinovic A, Manguso F, et al. The natural history of asymptomatic ventricular pre-excitation a long-term prospective follow-up study of 184 asymptomatic children. J Am Coll Cardiol. 2009;53(3):275-280. doi:10.1016/j.jacc.2008.09.037
8. Al-Khatib SM, Arshad A, Balk EM, et al. Risk Stratification for Arrhythmic Events in Patients With Asymptomatic Pre-Excitation: A Systematic Review for the 2015 ACC/AHA/HRS Guideline for the Management of Adult Patients With Supraventricular Tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016;67(13):1624-1638. doi:10.1016/j.jacc.2015.09.018
9. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias). Circulation. 2003;108(15):1871-1909.doi:10.1161/01.CIR.0000091380.04100.84
10. Pediatric and Congenital Electrophysiology Society (PACES); Heart Rhythm Society (HRS); American College of Cardiology Foundation (ACCF); PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS). Heart Rhythm. 2012;9(6):1006-1024. doi:10.1016/j.hrthm.2012.03.050
11. Cohen M, Triedman J. Guidelines for management of asymptomatic ventricular pre-excitation: brave new world or Pandora’s box?. Circ Arrhythm Electrophysiol. 2014;7(2):187-189. doi:10.1161/CIRCEP.114.001528
12. Svendsen JH, Dagres N, Dobreanu D, et al. Current strategy for treatment of patients with Wolff-Parkinson-White syndrome and asymptomatic preexcitation in Europe: European Heart Rhythm Association survey. Europace. 2013;15(5):750-753. doi:10.1093/europace/eut094
13. Gimbel RW, Pangaro L, Barbour G. America’s “undiscovered” laboratory for health services research. Med Care. 2010;48(8):751-756. doi:10.1097/MLR.0b013e3181e35be8
14. Dorrance KA, Ramchandani S, Neil N, Fisher H. Leveraging the military health system as a laboratory for health care reform. Mil Med. 2013;178(2):142-145. doi:10.7205/milmed-d-12-00168
15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi:10.1097/01.mlr.0000182534.19832.83
16. Finocchiaro G, Papadakis M, Behr ER, Sharma S, Sheppard M. Sudden Cardiac Death in Pre-Excitation and Wolff-Parkinson-White: Demographic and Clinical Features. J Am Coll Cardiol. 2017;69(12):1644-1645. doi:10.1016/j.jacc.2017.01.023
17. Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993;87(3):866-873. doi:10.1161/01.cir.87.3.866
18. Fitzsimmons PJ, McWhirter PD, Peterson DW, Kruyer WB. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142(3):530-536. doi:10.1067/mhj.2001.117779
19. Obeyesekere MN, Leong-Sit P, Massel D, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125(19):2308-2315. doi:10.1161/CIRCULATIONAHA.111.055350
20. Waspe LE, Brodman R, Kim SG, Fisher JD. Susceptibility to atrial fibrillation and ventricular tachyarrhythmia in the Wolff-Parkinson-White syndrome: role of the accessory pathway. Am Heart J. 1986;112(6):1141-1152. doi:10.1016/0002-8703(86)90342-x
21. Pietersen AH, Andersen ED, Sandøe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70(5):38A-43A. doi:10.1016/0002-9149(92)91076-g
22. Della Bella P, Brugada P, Talajic M, et al. Atrial fibrillation in patients with an accessory pathway: importance of the conduction properties of the accessory pathway. J Am Coll Cardiol. 1991;17(6):1352-1356. doi:10.1016/s0735-1097(10)80146-9
23. Fujimura O, Klein GJ, Yee R, Sharma AD. Mode of onset of atrial fibrillation in the Wolff-Parkinson-White syndrome: how important is the accessory pathway?. J Am Coll Cardiol. 1990;15(5):1082-1086. doi:10.1016/0735-1097(90)90244-j
24. Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J. 1991;12(2):144-150. doi:10.1093/oxfordjournals.eurheartj.a059860
25. Klein GJ, Bashore TM, Sellers TD, Pritchett EL, Smith WM, Gallagher JJ. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med. 1979;301(20):1080-1085. doi:10.1056/NEJM197911153012003
26. Centurion OA. Atrial Fibrillation in the Wolff-Parkinson-White Syndrome. J Atr Fibrillation. 2011;4(1):287. Published 2011 May 4. doi:10.4022/jafib.287
27. Song C, Guo Y, Zheng X, et al. Prognostic Significance and Risk of Atrial Fibrillation of Wolff-Parkinson-White Syndrome in Patients With Hypertrophic Cardiomyopathy. Am J Cardiol. 2018;122(9):1546-1550. doi:10.1016/j.amjcard.2018.07.021
28. Obeyesekere M, Gula LJ, Skanes AC, Leong-Sit P, Klein GJ. Risk of sudden death in Wolff-Parkinson-White syndrome: how high is the risk?. Circulation. 2012;125(5):659-660. doi:10.1161/CIRCULATIONAHA.111.085159
Nasal Cannula Dislodgement During Sleep in Veterans Receiving Long-term Oxygen Therapy for Hypoxemic Chronic Respiratory Failure
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
The prevalence of chronic obstructive pulmonary disease (COPD) among male US veterans is higher than in the general population.1 Veterans with COPD have higher rates of comorbidities and increased respiratory-related and all-cause health care use, including the use of long-term oxygen therapy (LTOT).2-5 It has been well established that LTOT reduces all-cause mortality in patients with COPD and
Delivery of domiciliary LTOT entails placing a nasal cannula into both nostrils and loosely securing it around both ears throughout the wake-sleep cycle. Several veterans with hypoxemic CRF due to COPD at the Jesse Brown Veterans Affairs Medical Center (JBVAMC) in Chicago, Illinois, who were receiving LTOT reported nasal cannula dislodgement (NCD) while they slept. However, the clinical significance and impact of these repeated episodes on respiratory-related health care utilization, such as frequent COPD exacerbations with hospitalization, were not recognized.
The purpose of this study was to determine whether veterans with hypoxemic CRF due to COPD and receiving 24-hour LTOT at JBVAMC were experiencing NCD during sleep and, if so, its impact on
METHODS
We reviewed electronic health records (EHRs) of veterans with hypoxemic CRF from COPD who received 24-hour LTOT administered through nasal cannula and were followed
Pertinent patient demographics, clinical and physiologic variables, and hospitalizations with length of JBVAMC stay for each physician-diagnosed COPD exacerbation in the preceding year from the date last seen in the clinic were abstracted from EHRs. Overall hospital cost, defined as a veteran overnight stay in either the medical intensive care unit (MICU) or a general acute medicine bed in a US Department of Veterans Affairs (VA) facility, was calculated for each hospitalization for physician-diagnosed COPD exacerbation using VA Managerial Cost Accounting System National Cost Extracts for inpatient encounters.15 We then contacted each veteran by telephone and asked whether they had experienced NCD and, if so, its weekly frequency ranging from once to nightly.
Data Analysis
Data were reported as mean (SD) where appropriate. The t test and Fisher exact test were used as indicated. P < .05 was considered statistically significant. The study protocol
RESULTS
During the study period,
Of the 75 patients, 66 (88%) responded to the telephone survey and 22 patients (33%) reported weekly episodes of NCD while they slept (median, 4 dislodgments per week). (Table 1). Eight patients (36%) reported nightly NCDs (Figure). All 66 respondents were male and 14 of 22 in the NCD group as well as 21 of 44 in the no NCD group were Black veterans. The mean age was similar in both groups: 71 years in the NCD group and 72 years in the no NCD group. There were no statistically significant differences in demographics, including prevalence of obstructive sleep apnea (OSA), supplemental oxygen flow rate, and duration of LTOT, or in pulmonary function test results between patients who did and did not experience NCD while sleeping (Table 2).
Ten of 22 patients (45%) with NCD and 9 of 44 patients (20%) without NCD were hospitalized at the JBVAMC for ≥ 1 COPD exacerbation in the preceding year that was diagnosed by a physician (P = .045). Three of 22 patients (14%) with NCD and no patients in the no NCD group were admitted to the MICU. No patients required intubation and mechanical ventilation during hospitalization, and no patients died. Overall hospital costs were 25% ($64,342) higher in NCD group compared with the no NCD group and were attributed to the MICU admissions in the NCD group (Table 3). Nine veterans did not respond to repeated telephone calls. One physician-diagnosed COPD exacerbation requiring hospitalization was documented in the nonresponder group; the patient was hospitalized for 2 days. One veteran died before being contacted.
DISCUSSION
There are 3 new findings in this study.
Nocturnal arterial oxygen desaturation in patients with COPD without evidence of OSA may contribute to the frequency of exacerbations.16 Although the mechanism(s) underlying this phenomenon is uncertain, we posit that prolonged nocturnal airway wall hypoxia could amplify underlying chronic inflammation through local generation of reactive oxygen species, thereby predisposing patients to exacerbations. Frequent COPD exacerbations promote disease progression and health status decline and are associated with increased mortality.11,13 Moreover, hospitalization of patients with COPD is the largest contributor to the annual direct cost of COPD per patient.10,12 The higher hospitalization rate observed in the NCD group in our study suggests that interruption of supplemental oxygen delivery while asleep may be a risk factor for COPD exacerbation. Alternatively, an independent factor or factors may have contributed to both NCD during sleep and COPD exacerbation in these patients or an impending exacerbation resulted in sleep disturbances that led to NCD. Additional research is warranted on veterans with hypoxemic CRF from COPD who are receiving LTOT and report frequent NCD during sleep that may support or refute these hypotheses.
To the best of our knowledge, NCD during sleep has not been previously reported in patients
Limitations
This was a small, single-site study, comprised entirely of male patients who are predominantly Black veterans. The telephone interviews with veterans self-reporting NCD during their sleep are prone to recall bias. In addition, the validity and reproducibility of NCD during sleep were not addressed in this study. Missing data from 9 nonresponders may have introduced a nonresponse bias in data analysis and interpretation. The overall hospital cost for a COPD exacerbation at JBVAMC was derived from VA data; US Centers for Medicare & Medicaid Services or commercial carrier data may be different.15,21 Lastly, access to LTOT for veterans with hypoxemic CRF from COPD is regulated and supervised at VA medical facilities.14 This process may be different for patients outside the VA. Taken together, it is difficult to generalize our initial observations to non-VA patients with hypoxemic CRF from COPD who are receiving LTOT. We suggest a large, prospective study of veterans be conducted to determine the prevalence of NCD during sleep and its relationship with COPD exacerbations in veterans receiving LTOT with hypoxemic CRF due to COPD.
CONCLUSIONS
Acknowledgments
We thank Yolanda Davis, RRT, and George Adam for their assistance with this project.
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
1. Boersma P, Cohen RA, Zelaya CE, Moy E. Multiple chronic conditions among veterans and nonveterans: United States, 2015-2018. Natl Health Stat Report. 2021;(153):1-13. doi:10.15620/cdc:101659
2. Sharafkhaneh A, Petersen NJ, Yu H-J, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132. doi:10.2147/copd.s8047
3. LaBedz SL, Krishnan JA, Chung Y-C, et al. Chronic obstructive pulmonary disease outcomes at Veterans Affairs versus non-Veterans Affairs hospitals. Chronic Obstr Pulm Dis. 2021;8(3):306-313. doi:10.15326/jcopdf.2021.0201
4. Darnell K, Dwivedi AK, Weng Z, Panos RJ. Disproportionate utilization of healthcare resources among veterans with COPD: a retrospective analysis of factors associated with COPD healthcare cost. Cost Eff Resour Alloc. 2013;11:13. doi:10.1186/1478-7547-11-13
5. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US Veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
6. Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):CD001744. doi:10.1002/14651858.CD001744.pub2
7. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254-1264. doi:10.1164/rccm.201802-0382CI
8. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202(10):e121-e141. doi:10.1164/rccm.202009-3608ST
9. AARC. AARC clinical practice guideline. Oxygen therapy in the home or alternate site health care facility--2007 revision & update. Respir Care. 2007;52(8):1063-1068.
10. Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618
11. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464-471. doi:10.1164/rccm.201710-2029OC
12. Stanford RH, Engel-Nitz NM, Bancroft T, Essoi B. The identification and cost of acute chronic obstructive pulmonary disease exacerbations in a United States population healthcare claims database. COPD. 2020;17(5):499-508. doi:10.1080/15412555.2020.1817357
13. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
14. US Department of Veterans Affairs, Veterans Health Administration. Home oxygen program. VHA Directive 1173.13(1). Published August 5, 2020. Accessed February 28, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8947
15. Phibbs CS, Barnett PG, Fan A, Harden C, King SS, Scott JY. Research guide to decision support system national cost extracts. Health Economics Resource Center of Health Service R&D Services, US Department of Veterans Affairs. September 2010. Accessed February 14, 2024. https://www.herc.research.va.gov/files/book_621.pdf
16. Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183-194. doi:10.1183/09059180.00004311
17. Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174(4):373-378. doi:10.1164/rccm.200507-1161WS
18. Melani AS, Sestini P, Rottoli P. Home oxygen therapy: re-thinking the role of devices. Expert Rev Clin Pharmacol. 2018;11(3):279-289. doi:10.1080/17512433.2018.1421457
19. Sculley JA, Corbridge SJ, Prieto-Centurion V, et al. Home oxygen therapy for patients with COPD: time for a reboot. Respir Care. 2019;64(12):1574-1585. doi:10.4187/respcare.07135
20. Jacobs SS, Lindell KO, Collins EG, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24-32. doi:10.1513/AnnalsATS.201703-209OC
21. US Centers for Medicare & Medicaid Services. Home use of oxygen. Publication number 100-3. January 3, 2023. Accessed February 14, 2024. https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=169
Recurrent Aphthous Stomatitis: Clinical Experience From a University Hospital in Brazil
To the Editor:
Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,1,2 round ulcerations of variable sizes with a tendency for recurrence, most commonly located in nonkeratinized areas of the oral mucosa. Pathergy commonly is observed.3 Although many authors consider the terms RAS andaphtha to be synonymous,4,5 differentiating the clinical lesion (aphthous ulceration) from the disease (aphtha or RAS) can be useful, as several other diseases can at times manifest with similar ulcers (called aphthoid lesions), such as pemphigus vulgaris, mucous membrane pemphigoid, and erythema multiforme.6
It is estimated that approximately 20% of individuals worldwide have at least one episode of aphtha during their lifetime,7 and it is considered the most common disease of the oral mucosa.8,9 However, only patients presenting with severe acute outbreaks or frequent relapses typically seek medical treatment. Clinically, aphthous ulcers are classified as aphtha minor (small number of small lesions), aphtha major (large deep lesions that also can affect the minor salivary glands with intense necrosis, difficulty in healing, and mucosal scarring), and aphtha herpetiformis (innumerous tiny lesions that reappear in recurring outbreaks).1-3 The term complex aphthosis was introduced in 198510 and is defined as recurrent oral and genital aphthous ulcerations or recurring multiple oral aphthous ulcers in the absence of systemic manifestations or Behçet disease11,12; however, complex aphthosis also has been reported as frequent episodes of ulcerations that may be associated with systemic diseases including Behçet disease.13,14
Currently, RAS is considered an immunologically mediated alteration in cutaneous mucosal reactivity with a multifactorial systemic cause. Underlying conditions such as Behçet disease, inflammatory bowel disease (IBD), iatrogenic immunosuppression (eg, following solid organ transplantation), AIDS, and cyclic neutropenia may or may not be detected.11-13
Our retrospective study explored the systemic nature of RAS. We reviewed patient records to evaluate underlying systemic conditions associated with the diagnosis of RAS and the use of oral medications in managing the disease. Medical records from the Department of Dermatology of the University of São Paulo, Brazil, from 2003 to 2017 were reviewed to identify patients with a diagnosis of RAS. Clinical classification of RAS—minor, major, or herpetiform—as well as the presence of aphthous lesions in other locations and the presence of other associated inflammatory cutaneous manifestations also were noted. Associated systemic diseases and treatments for RAS were recorded. Patients for whom the diagnosis of RAS was changed during follow-up were excluded. Because this was a retrospective analysis of medical records and without any patient risk, informed consent was not needed.
Medical records for 125 patients were reviewed; 63 were male (50.4%), and 62 were female (49.6%). The age at onset of symptoms, which ranged from a few months after birth to 74 years, was reported in only 92 (73.6%) patient medical records. Of these, 30 (32.6%) reported onset before 20 years of age, 39 (42.4%) between 20 and 39 years, 17 (18.5%) between 40 and 59 years, and 6 (6.5%) at 60 years or older. Morphologically, 72 (57.6%) had minor, 42 (33.6%) had major, and 11 (8.8%) had herpetiform aphthous ulcers. None of the patients presented with sporadic lesions; the disease was long-standing and persistent in all cases (complex aphthosis).
Regarding the location of the ulcers, 92 (73.6%) patients had lesions on the oral mucosa only. Some patients had lesions in more than one site in addition to the oral mucosa: 32 (25.6%) had aphthae in the genital/groin region and 4 (3.2%) presented with perianal/anal aphthae. Nineteen patients (19.2%) presented other cutaneous manifestations in addition to aphthae: 11 (45.8%) had folliculitis/pseudofolliculitis, and 8 (33.3%) had erythema nodosum (EN). Eight patients (33.3%) presented with uveitis, and 6 (25%) presented with concomitant arthralgia/arthritis. Fifty-four patients (43.2%) had confirmed or suspected associated disease: Behçet disease (21 [38.9%]), IBD (10 [18.5%]), solid organ transplantation (7 [13.0%])(kidney, 4 [57.1%]; heart, 2 [28.6%]; liver, 1 [14.3%]), HIV infection (6 [11.1%]), lymphoma (1 [1.9%]), aplastic anemia (1 [1.9%]), or myelodysplastic syndrome (1 [1.9%]). Ten patients (18.5%) presented with other diseases under investigation (eg, unidentified rheumatologic disease, unexplained neutropenia, undiagnosed immunodeficiencies, autoinflammatory syndromes, possible cyclic neutropenia).
Biopsies of the oral mucosa were performed in 31 patients. Histopathologic findings will be discussed in a future publication (unpublished data).
Five patients (4.0%) were lost to follow-up and did not receive treatment; 10 (8.0%) received only topical treatment (analgesics and/or corticosteroids). All 9 (7.2%) patients undergoing intralesional corticosteroid injections also were on a systemic treatment. One hundred ten (88.0%) patients were treated systemically—with colchicine (84/110 [76.4%]), thalidomide (43/110 [39.1%]), small pulses of oral corticosteroids (26 [23.6%]), dapsone (12/110 [10.9%]), or pentoxifylline (3 [2.7%]). Furthermore, in patients with associated diseases, treatment of the underlying condition was conducted when available, and follow-up was carried out in conjunction with the appropriate specialists. For treatment of the associated disease, patients received other medications such as methotrexate, azathioprine, cyclophosphamide, intravenous corticosteroid pulse, and immunobiologics.
The prevalence of RAS between sexes in our study population was similar (50.4% male; 49.6% female). Results from prior studies have been mixed; some reported a higher prevalence in females,15-18 while others found no predilection for sex among patients diagnosed with RAS.19,20 In our analysis, 75% of patients experienced symptoms of RAS before 40 years of age; in prior studies, up to 56% of patients experienced symptoms between the ages of 20 and 40 years.21,22
In our study, 26.4% of patients had extraoral aphthae. Genital lesions have been described as infrequent,23 and lesions manifesting in other mucous membranes or on the skin are rare.24 A study reported genital involvement in 8% to 13% of patients with oral aphtha.25 We observed genital involvement in 25.6% of patients. Likewise, this higher value may be due to our study population of patients referred to our university hospital. In our study, 19.2% of patients presented with other inflammatory manifestations in addition to aphthous ulcerations (eg, folliculitis, EN, uveitis, arthritis). As dermatologists in a tertiary reference hospital, we actively look for such associations in every aphtha patient, which may not be the case in many nondermatologic oral care services.
In our study population, 43.2% of patients were diagnosed with or were under investigation for systemic diseases known to be associated with RAS. We found associations with Behçet disease most frequently, followed by IBD,26 solid organ transplantation, and HIV. In this group of patients, the respective systemic disease was active or poorly controlled. In transplant recipients, aphtha major was the most common type, similar to other studies.27 We observed no notable difference in the clinical picture of the oral ulcers in patients with a well-established systemic disease vs those without.
Most of our cases did not present findings other than aphtha, indicating that the intrinsic defect that predisposes to RAS is always systemic. Even mild and sporadic cases may be attributable to a systemic disorder of cutaneous-mucosal reactivity. The predisposition to RAS never originates in the oral cavity, hence the confusion caused and the uselessness of studies that relate aphthae to factors such as local food allergies, pH changes, or local infection with microorganisms.5,28 The disease course (reducing the frequency of lesion appearance and accelerating the healing of extensive lesions) is only modified with systemic treatment, with local measures proving to be only moderately useful to relieve pain. We believe that RAS can in many ways be compared to EN and pyoderma gangrenosum (PG): some systemic conditions that predispose patients to EN and PG also may predispose them to RAS (eg, IBD, hematologic disorders). Similar to RAS, many cases of EN and PG are idiopathic. In addition, pathergy also occurs in PG.11,13
We were unable to observe or establish any predictive clinical element that could indicate a better or worse response to the prescribed treatments, which also has been noted by other authors.3,4 Treatment of RAS is empiric, generally starting with drugs that are easier to prescribe and with fewer adverse effects, then progressing to more complex drugs when a good response is not obtained. Colchicine was the most commonly prescribed medication (76.4% [84/110]). It has been proposed by several authors3,4 as a first-line systemic medication for the treatment of recurrent aphthae, as it has been shown to be effective and safe. The dosage ranged from 0.5 mg twice daily to 0.5 mg 4 times daily. Dapsone is an established drug for aphtha29,30 and was used in 12 of our patients. The dosage used in our patients ranged from 50 to 100 mg/d. Adverse effects such as hemolytic anemia frequently are seen, and one of the patients in our study developed DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome in response to dapsone. In 7 cases, colchicine and dapsone were used together, which is believed to potentiate the therapeutic effects. This combination may be useful in patients for whom thalidomide cannot be used or those who have not improved with monotherapy.29 Thalidomide is considered one of the most effective drugs for RAS.30,31 Forty-three patients in our analysis were treated with thalidomide, usually as a first choice. The dosage ranged from 100 to 200 mg/d. It was mainly chosen in disabling pediatric cases, adult men with aphthous major, and women with no risk for pregnancy. Due to its potential adverse effects, thalidomide has been recommended when there is no response with other medications that are dose dependent; severe adverse effects such as thromboembolism and peripheral neuropathy are rare.31 Oral corticosteroids were used in 26 patients, aiming at rapid improvement in very symptomatic cases; however, due to the potential for long-term adverse effects, in all cases they were prescribed in combination with another medication that was maintained after the corticosteroid was discontinued.
We highlight the systemic nature of RAS as well as its frequent association with systemic diseases and other correlated manifestations (pustules, EN, arthralgia). We also emphasize the importance of using oral medications to adequately control the disease and do not recommend topical medications aimed at treating local causes. Dermatologists should be consulted in managing severe cases of RAS.
- Buño IJ, Huff JC, Weston WL, et al. Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch Dermatol. 1998;134:827-831.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728- 732.
- Natah SS, Konttinen YT, Enattah NS, et al. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221-234.
- Zunt SL. Recurrent aphthous stomatitis. Dermatol Clin. 2003;21:33-39.
- Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. number VI. recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21.
- Chams-Davatchi C, Shizarpour M, Davatchi F, et al. Comparison of oral aphthae in Behçet’s disease and idiopathic recurrent aphthous stomatitis. Adv Exp Med Biol. 2003;528:317-320.
- Schemel-Suárez M, López-López J, Chimenos-Küstner E. Oral ulcers: differential diagnosis and treatment [in Spanish]. Med Clin (Barc). 2015;145:499-503.
- S´lebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz). 2014;62:205-215.
- Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10:26-36.
- Jorizzo JL, Taylor RS, Schmalstieg FC, et al. Complex aphthosis: a forme fruste of Behçet’s syndrome? J Am Acad Dermatol. 1985;13:80-84.
- McCarty MA, Garton RA, Jorizzo JL. Complex aphthosis and Behçet’s disease. Dermatol Clin. 2003;21:41-48.
- Bulur I, Melrem O. Behçet disease: new aspects. Clin Dermatol. 2017;35:421-434.
- Cui RZ, Rogers RS 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34:475-481.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728-732.
- Ship II. Epidemiologic aspects of recurrent aphthous ulcerations. Oral Surg Oral Med Oral Pathol. 1972;33:400-406.
- Ship JA. Recurrent aphthous stomatitis. an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:141-147.
- Wilhelmsen NS, Weber R, Monteiro F, et al. Correlation between histocompatibility antigens and recurrent aphthous stomatitis in the Brazilian population. Braz J Otorhinolaryngol. 2009;75:426-431.
- S´lebioda Z, Dorocka-Bobkowska B. Systemic and environmental risk factors for recurrent aphthous stomatitis in a Polish cohort of patients. Postepy Dermatol Alergol. 2019;36:196-201.
- Ship JA, Chavez EM, Doerr PA, et al. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95-112.
- Brocklehurst P, Tickle M, Glenny AM, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database Syst Rev. 2012;12:CD005411.
- Belenguer-Guallar I, Jiménez-Soriano Y, Ariadna Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis. a literature review. J Clin Exp Dent. 2014;6:E168-E174.
- Bagán JV, Sanchis JM, Milián MA, et al. Recurrent aphthous stomatitis. a study of the clinical characteristics of lesions in 93 cases. J Oral Pathol Med. 1991;20:395-397.
- Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19:195-204.
- Scully C, Porter S. Recurrent aphthous stomatitis: current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18:21-27
- Chapel TA. Origins of penile ulcerations. Arch Androl. 1979; 3: 351-357.
- Lourenço SV, Hussein TP, Bologna SB, et al. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204-207.
- Nico MM, Brito AE, Martins LE, et al. Oral ulcers in an immunosuppressed 5-year-old boy. Clin Exp Dermatol. 2008;33:367-368.
- Trakji B, Baroudi K, Kharma Y. The effect of dietary habits on the development of the recurrent aphthous stomatitis. Niger Med J. 2012;53:9-11.
- Lynde CB, Bruce AJ, Rogers RS 3rd. Successful treatment of complex aphthosis with colchicine and dapsone. Arch Dermatol. 2009;145:273-276.
- Letsinger JA, McCarty MA, Jorizzo JL. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J Am Acad Dermatol. 2005(3 pt 1);52:500-508.
- Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. Medicine (Baltimore). 2010;89:176-182.
To the Editor:
Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,1,2 round ulcerations of variable sizes with a tendency for recurrence, most commonly located in nonkeratinized areas of the oral mucosa. Pathergy commonly is observed.3 Although many authors consider the terms RAS andaphtha to be synonymous,4,5 differentiating the clinical lesion (aphthous ulceration) from the disease (aphtha or RAS) can be useful, as several other diseases can at times manifest with similar ulcers (called aphthoid lesions), such as pemphigus vulgaris, mucous membrane pemphigoid, and erythema multiforme.6
It is estimated that approximately 20% of individuals worldwide have at least one episode of aphtha during their lifetime,7 and it is considered the most common disease of the oral mucosa.8,9 However, only patients presenting with severe acute outbreaks or frequent relapses typically seek medical treatment. Clinically, aphthous ulcers are classified as aphtha minor (small number of small lesions), aphtha major (large deep lesions that also can affect the minor salivary glands with intense necrosis, difficulty in healing, and mucosal scarring), and aphtha herpetiformis (innumerous tiny lesions that reappear in recurring outbreaks).1-3 The term complex aphthosis was introduced in 198510 and is defined as recurrent oral and genital aphthous ulcerations or recurring multiple oral aphthous ulcers in the absence of systemic manifestations or Behçet disease11,12; however, complex aphthosis also has been reported as frequent episodes of ulcerations that may be associated with systemic diseases including Behçet disease.13,14
Currently, RAS is considered an immunologically mediated alteration in cutaneous mucosal reactivity with a multifactorial systemic cause. Underlying conditions such as Behçet disease, inflammatory bowel disease (IBD), iatrogenic immunosuppression (eg, following solid organ transplantation), AIDS, and cyclic neutropenia may or may not be detected.11-13
Our retrospective study explored the systemic nature of RAS. We reviewed patient records to evaluate underlying systemic conditions associated with the diagnosis of RAS and the use of oral medications in managing the disease. Medical records from the Department of Dermatology of the University of São Paulo, Brazil, from 2003 to 2017 were reviewed to identify patients with a diagnosis of RAS. Clinical classification of RAS—minor, major, or herpetiform—as well as the presence of aphthous lesions in other locations and the presence of other associated inflammatory cutaneous manifestations also were noted. Associated systemic diseases and treatments for RAS were recorded. Patients for whom the diagnosis of RAS was changed during follow-up were excluded. Because this was a retrospective analysis of medical records and without any patient risk, informed consent was not needed.
Medical records for 125 patients were reviewed; 63 were male (50.4%), and 62 were female (49.6%). The age at onset of symptoms, which ranged from a few months after birth to 74 years, was reported in only 92 (73.6%) patient medical records. Of these, 30 (32.6%) reported onset before 20 years of age, 39 (42.4%) between 20 and 39 years, 17 (18.5%) between 40 and 59 years, and 6 (6.5%) at 60 years or older. Morphologically, 72 (57.6%) had minor, 42 (33.6%) had major, and 11 (8.8%) had herpetiform aphthous ulcers. None of the patients presented with sporadic lesions; the disease was long-standing and persistent in all cases (complex aphthosis).
Regarding the location of the ulcers, 92 (73.6%) patients had lesions on the oral mucosa only. Some patients had lesions in more than one site in addition to the oral mucosa: 32 (25.6%) had aphthae in the genital/groin region and 4 (3.2%) presented with perianal/anal aphthae. Nineteen patients (19.2%) presented other cutaneous manifestations in addition to aphthae: 11 (45.8%) had folliculitis/pseudofolliculitis, and 8 (33.3%) had erythema nodosum (EN). Eight patients (33.3%) presented with uveitis, and 6 (25%) presented with concomitant arthralgia/arthritis. Fifty-four patients (43.2%) had confirmed or suspected associated disease: Behçet disease (21 [38.9%]), IBD (10 [18.5%]), solid organ transplantation (7 [13.0%])(kidney, 4 [57.1%]; heart, 2 [28.6%]; liver, 1 [14.3%]), HIV infection (6 [11.1%]), lymphoma (1 [1.9%]), aplastic anemia (1 [1.9%]), or myelodysplastic syndrome (1 [1.9%]). Ten patients (18.5%) presented with other diseases under investigation (eg, unidentified rheumatologic disease, unexplained neutropenia, undiagnosed immunodeficiencies, autoinflammatory syndromes, possible cyclic neutropenia).
Biopsies of the oral mucosa were performed in 31 patients. Histopathologic findings will be discussed in a future publication (unpublished data).
Five patients (4.0%) were lost to follow-up and did not receive treatment; 10 (8.0%) received only topical treatment (analgesics and/or corticosteroids). All 9 (7.2%) patients undergoing intralesional corticosteroid injections also were on a systemic treatment. One hundred ten (88.0%) patients were treated systemically—with colchicine (84/110 [76.4%]), thalidomide (43/110 [39.1%]), small pulses of oral corticosteroids (26 [23.6%]), dapsone (12/110 [10.9%]), or pentoxifylline (3 [2.7%]). Furthermore, in patients with associated diseases, treatment of the underlying condition was conducted when available, and follow-up was carried out in conjunction with the appropriate specialists. For treatment of the associated disease, patients received other medications such as methotrexate, azathioprine, cyclophosphamide, intravenous corticosteroid pulse, and immunobiologics.
The prevalence of RAS between sexes in our study population was similar (50.4% male; 49.6% female). Results from prior studies have been mixed; some reported a higher prevalence in females,15-18 while others found no predilection for sex among patients diagnosed with RAS.19,20 In our analysis, 75% of patients experienced symptoms of RAS before 40 years of age; in prior studies, up to 56% of patients experienced symptoms between the ages of 20 and 40 years.21,22
In our study, 26.4% of patients had extraoral aphthae. Genital lesions have been described as infrequent,23 and lesions manifesting in other mucous membranes or on the skin are rare.24 A study reported genital involvement in 8% to 13% of patients with oral aphtha.25 We observed genital involvement in 25.6% of patients. Likewise, this higher value may be due to our study population of patients referred to our university hospital. In our study, 19.2% of patients presented with other inflammatory manifestations in addition to aphthous ulcerations (eg, folliculitis, EN, uveitis, arthritis). As dermatologists in a tertiary reference hospital, we actively look for such associations in every aphtha patient, which may not be the case in many nondermatologic oral care services.
In our study population, 43.2% of patients were diagnosed with or were under investigation for systemic diseases known to be associated with RAS. We found associations with Behçet disease most frequently, followed by IBD,26 solid organ transplantation, and HIV. In this group of patients, the respective systemic disease was active or poorly controlled. In transplant recipients, aphtha major was the most common type, similar to other studies.27 We observed no notable difference in the clinical picture of the oral ulcers in patients with a well-established systemic disease vs those without.
Most of our cases did not present findings other than aphtha, indicating that the intrinsic defect that predisposes to RAS is always systemic. Even mild and sporadic cases may be attributable to a systemic disorder of cutaneous-mucosal reactivity. The predisposition to RAS never originates in the oral cavity, hence the confusion caused and the uselessness of studies that relate aphthae to factors such as local food allergies, pH changes, or local infection with microorganisms.5,28 The disease course (reducing the frequency of lesion appearance and accelerating the healing of extensive lesions) is only modified with systemic treatment, with local measures proving to be only moderately useful to relieve pain. We believe that RAS can in many ways be compared to EN and pyoderma gangrenosum (PG): some systemic conditions that predispose patients to EN and PG also may predispose them to RAS (eg, IBD, hematologic disorders). Similar to RAS, many cases of EN and PG are idiopathic. In addition, pathergy also occurs in PG.11,13
We were unable to observe or establish any predictive clinical element that could indicate a better or worse response to the prescribed treatments, which also has been noted by other authors.3,4 Treatment of RAS is empiric, generally starting with drugs that are easier to prescribe and with fewer adverse effects, then progressing to more complex drugs when a good response is not obtained. Colchicine was the most commonly prescribed medication (76.4% [84/110]). It has been proposed by several authors3,4 as a first-line systemic medication for the treatment of recurrent aphthae, as it has been shown to be effective and safe. The dosage ranged from 0.5 mg twice daily to 0.5 mg 4 times daily. Dapsone is an established drug for aphtha29,30 and was used in 12 of our patients. The dosage used in our patients ranged from 50 to 100 mg/d. Adverse effects such as hemolytic anemia frequently are seen, and one of the patients in our study developed DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome in response to dapsone. In 7 cases, colchicine and dapsone were used together, which is believed to potentiate the therapeutic effects. This combination may be useful in patients for whom thalidomide cannot be used or those who have not improved with monotherapy.29 Thalidomide is considered one of the most effective drugs for RAS.30,31 Forty-three patients in our analysis were treated with thalidomide, usually as a first choice. The dosage ranged from 100 to 200 mg/d. It was mainly chosen in disabling pediatric cases, adult men with aphthous major, and women with no risk for pregnancy. Due to its potential adverse effects, thalidomide has been recommended when there is no response with other medications that are dose dependent; severe adverse effects such as thromboembolism and peripheral neuropathy are rare.31 Oral corticosteroids were used in 26 patients, aiming at rapid improvement in very symptomatic cases; however, due to the potential for long-term adverse effects, in all cases they were prescribed in combination with another medication that was maintained after the corticosteroid was discontinued.
We highlight the systemic nature of RAS as well as its frequent association with systemic diseases and other correlated manifestations (pustules, EN, arthralgia). We also emphasize the importance of using oral medications to adequately control the disease and do not recommend topical medications aimed at treating local causes. Dermatologists should be consulted in managing severe cases of RAS.
To the Editor:
Recurrent aphthous stomatitis (RAS) is a mucocutaneous condition characterized by single or multiple, painful,1,2 round ulcerations of variable sizes with a tendency for recurrence, most commonly located in nonkeratinized areas of the oral mucosa. Pathergy commonly is observed.3 Although many authors consider the terms RAS andaphtha to be synonymous,4,5 differentiating the clinical lesion (aphthous ulceration) from the disease (aphtha or RAS) can be useful, as several other diseases can at times manifest with similar ulcers (called aphthoid lesions), such as pemphigus vulgaris, mucous membrane pemphigoid, and erythema multiforme.6
It is estimated that approximately 20% of individuals worldwide have at least one episode of aphtha during their lifetime,7 and it is considered the most common disease of the oral mucosa.8,9 However, only patients presenting with severe acute outbreaks or frequent relapses typically seek medical treatment. Clinically, aphthous ulcers are classified as aphtha minor (small number of small lesions), aphtha major (large deep lesions that also can affect the minor salivary glands with intense necrosis, difficulty in healing, and mucosal scarring), and aphtha herpetiformis (innumerous tiny lesions that reappear in recurring outbreaks).1-3 The term complex aphthosis was introduced in 198510 and is defined as recurrent oral and genital aphthous ulcerations or recurring multiple oral aphthous ulcers in the absence of systemic manifestations or Behçet disease11,12; however, complex aphthosis also has been reported as frequent episodes of ulcerations that may be associated with systemic diseases including Behçet disease.13,14
Currently, RAS is considered an immunologically mediated alteration in cutaneous mucosal reactivity with a multifactorial systemic cause. Underlying conditions such as Behçet disease, inflammatory bowel disease (IBD), iatrogenic immunosuppression (eg, following solid organ transplantation), AIDS, and cyclic neutropenia may or may not be detected.11-13
Our retrospective study explored the systemic nature of RAS. We reviewed patient records to evaluate underlying systemic conditions associated with the diagnosis of RAS and the use of oral medications in managing the disease. Medical records from the Department of Dermatology of the University of São Paulo, Brazil, from 2003 to 2017 were reviewed to identify patients with a diagnosis of RAS. Clinical classification of RAS—minor, major, or herpetiform—as well as the presence of aphthous lesions in other locations and the presence of other associated inflammatory cutaneous manifestations also were noted. Associated systemic diseases and treatments for RAS were recorded. Patients for whom the diagnosis of RAS was changed during follow-up were excluded. Because this was a retrospective analysis of medical records and without any patient risk, informed consent was not needed.
Medical records for 125 patients were reviewed; 63 were male (50.4%), and 62 were female (49.6%). The age at onset of symptoms, which ranged from a few months after birth to 74 years, was reported in only 92 (73.6%) patient medical records. Of these, 30 (32.6%) reported onset before 20 years of age, 39 (42.4%) between 20 and 39 years, 17 (18.5%) between 40 and 59 years, and 6 (6.5%) at 60 years or older. Morphologically, 72 (57.6%) had minor, 42 (33.6%) had major, and 11 (8.8%) had herpetiform aphthous ulcers. None of the patients presented with sporadic lesions; the disease was long-standing and persistent in all cases (complex aphthosis).
Regarding the location of the ulcers, 92 (73.6%) patients had lesions on the oral mucosa only. Some patients had lesions in more than one site in addition to the oral mucosa: 32 (25.6%) had aphthae in the genital/groin region and 4 (3.2%) presented with perianal/anal aphthae. Nineteen patients (19.2%) presented other cutaneous manifestations in addition to aphthae: 11 (45.8%) had folliculitis/pseudofolliculitis, and 8 (33.3%) had erythema nodosum (EN). Eight patients (33.3%) presented with uveitis, and 6 (25%) presented with concomitant arthralgia/arthritis. Fifty-four patients (43.2%) had confirmed or suspected associated disease: Behçet disease (21 [38.9%]), IBD (10 [18.5%]), solid organ transplantation (7 [13.0%])(kidney, 4 [57.1%]; heart, 2 [28.6%]; liver, 1 [14.3%]), HIV infection (6 [11.1%]), lymphoma (1 [1.9%]), aplastic anemia (1 [1.9%]), or myelodysplastic syndrome (1 [1.9%]). Ten patients (18.5%) presented with other diseases under investigation (eg, unidentified rheumatologic disease, unexplained neutropenia, undiagnosed immunodeficiencies, autoinflammatory syndromes, possible cyclic neutropenia).
Biopsies of the oral mucosa were performed in 31 patients. Histopathologic findings will be discussed in a future publication (unpublished data).
Five patients (4.0%) were lost to follow-up and did not receive treatment; 10 (8.0%) received only topical treatment (analgesics and/or corticosteroids). All 9 (7.2%) patients undergoing intralesional corticosteroid injections also were on a systemic treatment. One hundred ten (88.0%) patients were treated systemically—with colchicine (84/110 [76.4%]), thalidomide (43/110 [39.1%]), small pulses of oral corticosteroids (26 [23.6%]), dapsone (12/110 [10.9%]), or pentoxifylline (3 [2.7%]). Furthermore, in patients with associated diseases, treatment of the underlying condition was conducted when available, and follow-up was carried out in conjunction with the appropriate specialists. For treatment of the associated disease, patients received other medications such as methotrexate, azathioprine, cyclophosphamide, intravenous corticosteroid pulse, and immunobiologics.
The prevalence of RAS between sexes in our study population was similar (50.4% male; 49.6% female). Results from prior studies have been mixed; some reported a higher prevalence in females,15-18 while others found no predilection for sex among patients diagnosed with RAS.19,20 In our analysis, 75% of patients experienced symptoms of RAS before 40 years of age; in prior studies, up to 56% of patients experienced symptoms between the ages of 20 and 40 years.21,22
In our study, 26.4% of patients had extraoral aphthae. Genital lesions have been described as infrequent,23 and lesions manifesting in other mucous membranes or on the skin are rare.24 A study reported genital involvement in 8% to 13% of patients with oral aphtha.25 We observed genital involvement in 25.6% of patients. Likewise, this higher value may be due to our study population of patients referred to our university hospital. In our study, 19.2% of patients presented with other inflammatory manifestations in addition to aphthous ulcerations (eg, folliculitis, EN, uveitis, arthritis). As dermatologists in a tertiary reference hospital, we actively look for such associations in every aphtha patient, which may not be the case in many nondermatologic oral care services.
In our study population, 43.2% of patients were diagnosed with or were under investigation for systemic diseases known to be associated with RAS. We found associations with Behçet disease most frequently, followed by IBD,26 solid organ transplantation, and HIV. In this group of patients, the respective systemic disease was active or poorly controlled. In transplant recipients, aphtha major was the most common type, similar to other studies.27 We observed no notable difference in the clinical picture of the oral ulcers in patients with a well-established systemic disease vs those without.
Most of our cases did not present findings other than aphtha, indicating that the intrinsic defect that predisposes to RAS is always systemic. Even mild and sporadic cases may be attributable to a systemic disorder of cutaneous-mucosal reactivity. The predisposition to RAS never originates in the oral cavity, hence the confusion caused and the uselessness of studies that relate aphthae to factors such as local food allergies, pH changes, or local infection with microorganisms.5,28 The disease course (reducing the frequency of lesion appearance and accelerating the healing of extensive lesions) is only modified with systemic treatment, with local measures proving to be only moderately useful to relieve pain. We believe that RAS can in many ways be compared to EN and pyoderma gangrenosum (PG): some systemic conditions that predispose patients to EN and PG also may predispose them to RAS (eg, IBD, hematologic disorders). Similar to RAS, many cases of EN and PG are idiopathic. In addition, pathergy also occurs in PG.11,13
We were unable to observe or establish any predictive clinical element that could indicate a better or worse response to the prescribed treatments, which also has been noted by other authors.3,4 Treatment of RAS is empiric, generally starting with drugs that are easier to prescribe and with fewer adverse effects, then progressing to more complex drugs when a good response is not obtained. Colchicine was the most commonly prescribed medication (76.4% [84/110]). It has been proposed by several authors3,4 as a first-line systemic medication for the treatment of recurrent aphthae, as it has been shown to be effective and safe. The dosage ranged from 0.5 mg twice daily to 0.5 mg 4 times daily. Dapsone is an established drug for aphtha29,30 and was used in 12 of our patients. The dosage used in our patients ranged from 50 to 100 mg/d. Adverse effects such as hemolytic anemia frequently are seen, and one of the patients in our study developed DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome in response to dapsone. In 7 cases, colchicine and dapsone were used together, which is believed to potentiate the therapeutic effects. This combination may be useful in patients for whom thalidomide cannot be used or those who have not improved with monotherapy.29 Thalidomide is considered one of the most effective drugs for RAS.30,31 Forty-three patients in our analysis were treated with thalidomide, usually as a first choice. The dosage ranged from 100 to 200 mg/d. It was mainly chosen in disabling pediatric cases, adult men with aphthous major, and women with no risk for pregnancy. Due to its potential adverse effects, thalidomide has been recommended when there is no response with other medications that are dose dependent; severe adverse effects such as thromboembolism and peripheral neuropathy are rare.31 Oral corticosteroids were used in 26 patients, aiming at rapid improvement in very symptomatic cases; however, due to the potential for long-term adverse effects, in all cases they were prescribed in combination with another medication that was maintained after the corticosteroid was discontinued.
We highlight the systemic nature of RAS as well as its frequent association with systemic diseases and other correlated manifestations (pustules, EN, arthralgia). We also emphasize the importance of using oral medications to adequately control the disease and do not recommend topical medications aimed at treating local causes. Dermatologists should be consulted in managing severe cases of RAS.
- Buño IJ, Huff JC, Weston WL, et al. Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch Dermatol. 1998;134:827-831.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728- 732.
- Natah SS, Konttinen YT, Enattah NS, et al. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221-234.
- Zunt SL. Recurrent aphthous stomatitis. Dermatol Clin. 2003;21:33-39.
- Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. number VI. recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21.
- Chams-Davatchi C, Shizarpour M, Davatchi F, et al. Comparison of oral aphthae in Behçet’s disease and idiopathic recurrent aphthous stomatitis. Adv Exp Med Biol. 2003;528:317-320.
- Schemel-Suárez M, López-López J, Chimenos-Küstner E. Oral ulcers: differential diagnosis and treatment [in Spanish]. Med Clin (Barc). 2015;145:499-503.
- S´lebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz). 2014;62:205-215.
- Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10:26-36.
- Jorizzo JL, Taylor RS, Schmalstieg FC, et al. Complex aphthosis: a forme fruste of Behçet’s syndrome? J Am Acad Dermatol. 1985;13:80-84.
- McCarty MA, Garton RA, Jorizzo JL. Complex aphthosis and Behçet’s disease. Dermatol Clin. 2003;21:41-48.
- Bulur I, Melrem O. Behçet disease: new aspects. Clin Dermatol. 2017;35:421-434.
- Cui RZ, Rogers RS 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34:475-481.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728-732.
- Ship II. Epidemiologic aspects of recurrent aphthous ulcerations. Oral Surg Oral Med Oral Pathol. 1972;33:400-406.
- Ship JA. Recurrent aphthous stomatitis. an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:141-147.
- Wilhelmsen NS, Weber R, Monteiro F, et al. Correlation between histocompatibility antigens and recurrent aphthous stomatitis in the Brazilian population. Braz J Otorhinolaryngol. 2009;75:426-431.
- S´lebioda Z, Dorocka-Bobkowska B. Systemic and environmental risk factors for recurrent aphthous stomatitis in a Polish cohort of patients. Postepy Dermatol Alergol. 2019;36:196-201.
- Ship JA, Chavez EM, Doerr PA, et al. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95-112.
- Brocklehurst P, Tickle M, Glenny AM, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database Syst Rev. 2012;12:CD005411.
- Belenguer-Guallar I, Jiménez-Soriano Y, Ariadna Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis. a literature review. J Clin Exp Dent. 2014;6:E168-E174.
- Bagán JV, Sanchis JM, Milián MA, et al. Recurrent aphthous stomatitis. a study of the clinical characteristics of lesions in 93 cases. J Oral Pathol Med. 1991;20:395-397.
- Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19:195-204.
- Scully C, Porter S. Recurrent aphthous stomatitis: current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18:21-27
- Chapel TA. Origins of penile ulcerations. Arch Androl. 1979; 3: 351-357.
- Lourenço SV, Hussein TP, Bologna SB, et al. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204-207.
- Nico MM, Brito AE, Martins LE, et al. Oral ulcers in an immunosuppressed 5-year-old boy. Clin Exp Dermatol. 2008;33:367-368.
- Trakji B, Baroudi K, Kharma Y. The effect of dietary habits on the development of the recurrent aphthous stomatitis. Niger Med J. 2012;53:9-11.
- Lynde CB, Bruce AJ, Rogers RS 3rd. Successful treatment of complex aphthosis with colchicine and dapsone. Arch Dermatol. 2009;145:273-276.
- Letsinger JA, McCarty MA, Jorizzo JL. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J Am Acad Dermatol. 2005(3 pt 1);52:500-508.
- Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. Medicine (Baltimore). 2010;89:176-182.
- Buño IJ, Huff JC, Weston WL, et al. Elevated levels of interferon gamma, tumor necrosis factor alpha, interleukins 2, 4, and 5, but not interleukin 10, are present in recurrent aphthous stomatitis. Arch Dermatol. 1998;134:827-831.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728- 732.
- Natah SS, Konttinen YT, Enattah NS, et al. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg. 2004;33:221-234.
- Zunt SL. Recurrent aphthous stomatitis. Dermatol Clin. 2003;21:33-39.
- Jurge S, Kuffer R, Scully C, et al. Mucosal disease series. number VI. recurrent aphthous stomatitis. Oral Dis. 2006;12:1-21.
- Chams-Davatchi C, Shizarpour M, Davatchi F, et al. Comparison of oral aphthae in Behçet’s disease and idiopathic recurrent aphthous stomatitis. Adv Exp Med Biol. 2003;528:317-320.
- Schemel-Suárez M, López-López J, Chimenos-Küstner E. Oral ulcers: differential diagnosis and treatment [in Spanish]. Med Clin (Barc). 2015;145:499-503.
- S´lebioda Z, Szponar E, Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: literature review. Arch Immunol Ther Exp (Warsz). 2014;62:205-215.
- Edgar NR, Saleh D, Miller RA. Recurrent aphthous stomatitis: a review. J Clin Aesthet Dermatol. 2017;10:26-36.
- Jorizzo JL, Taylor RS, Schmalstieg FC, et al. Complex aphthosis: a forme fruste of Behçet’s syndrome? J Am Acad Dermatol. 1985;13:80-84.
- McCarty MA, Garton RA, Jorizzo JL. Complex aphthosis and Behçet’s disease. Dermatol Clin. 2003;21:41-48.
- Bulur I, Melrem O. Behçet disease: new aspects. Clin Dermatol. 2017;35:421-434.
- Cui RZ, Rogers RS 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016;34:475-481.
- Femiano F, Lanza A, Buonaiuto C, et al. Guidelines for diagnosis and management of aphthous stomatitis. Pediatr Infect Dis J. 2007;26:728-732.
- Ship II. Epidemiologic aspects of recurrent aphthous ulcerations. Oral Surg Oral Med Oral Pathol. 1972;33:400-406.
- Ship JA. Recurrent aphthous stomatitis. an update. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:141-147.
- Wilhelmsen NS, Weber R, Monteiro F, et al. Correlation between histocompatibility antigens and recurrent aphthous stomatitis in the Brazilian population. Braz J Otorhinolaryngol. 2009;75:426-431.
- S´lebioda Z, Dorocka-Bobkowska B. Systemic and environmental risk factors for recurrent aphthous stomatitis in a Polish cohort of patients. Postepy Dermatol Alergol. 2019;36:196-201.
- Ship JA, Chavez EM, Doerr PA, et al. Recurrent aphthous stomatitis. Quintessence Int. 2000;31:95-112.
- Brocklehurst P, Tickle M, Glenny AM, et al. Systemic interventions for recurrent aphthous stomatitis (mouth ulcers). Cochrane Database Syst Rev. 2012;12:CD005411.
- Belenguer-Guallar I, Jiménez-Soriano Y, Ariadna Claramunt-Lozano A. Treatment of recurrent aphthous stomatitis. a literature review. J Clin Exp Dent. 2014;6:E168-E174.
- Bagán JV, Sanchis JM, Milián MA, et al. Recurrent aphthous stomatitis. a study of the clinical characteristics of lesions in 93 cases. J Oral Pathol Med. 1991;20:395-397.
- Huppert JS, Gerber MA, Deitch HR, et al. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol. 2006;19:195-204.
- Scully C, Porter S. Recurrent aphthous stomatitis: current concepts of etiology, pathogenesis and management. J Oral Pathol Med. 1989;18:21-27
- Chapel TA. Origins of penile ulcerations. Arch Androl. 1979; 3: 351-357.
- Lourenço SV, Hussein TP, Bologna SB, et al. Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol. 2010;24:204-207.
- Nico MM, Brito AE, Martins LE, et al. Oral ulcers in an immunosuppressed 5-year-old boy. Clin Exp Dermatol. 2008;33:367-368.
- Trakji B, Baroudi K, Kharma Y. The effect of dietary habits on the development of the recurrent aphthous stomatitis. Niger Med J. 2012;53:9-11.
- Lynde CB, Bruce AJ, Rogers RS 3rd. Successful treatment of complex aphthosis with colchicine and dapsone. Arch Dermatol. 2009;145:273-276.
- Letsinger JA, McCarty MA, Jorizzo JL. Complex aphthosis: a large case series with evaluation algorithm and therapeutic ladder from topicals to thalidomide. J Am Acad Dermatol. 2005(3 pt 1);52:500-508.
- Hello M, Barbarot S, Bastuji-Garin S, et al. Use of thalidomide for severe recurrent aphthous stomatitis: a multicenter cohort analysis. Medicine (Baltimore). 2010;89:176-182.
Practice Points
- The process that leads to the formation of aphthous ulcerations is always systemic, not local, even in the absence of a diagnosable systemic disease.
- Relapsing cases of aphthae should be treated with systemic medication.
The Impact of Primary Tumor Site on Survival in Mycosis Fungoides
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.
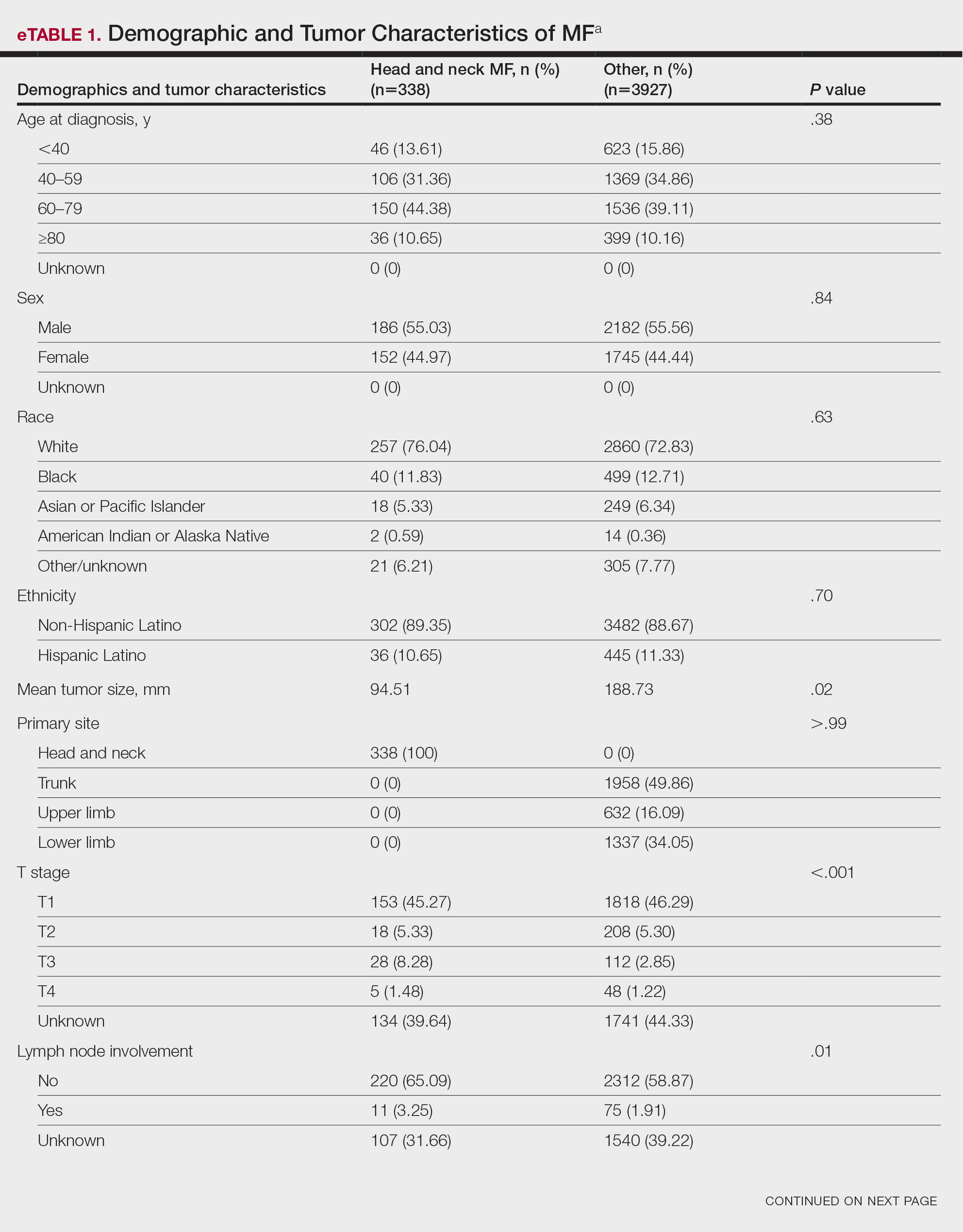
Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).
For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).
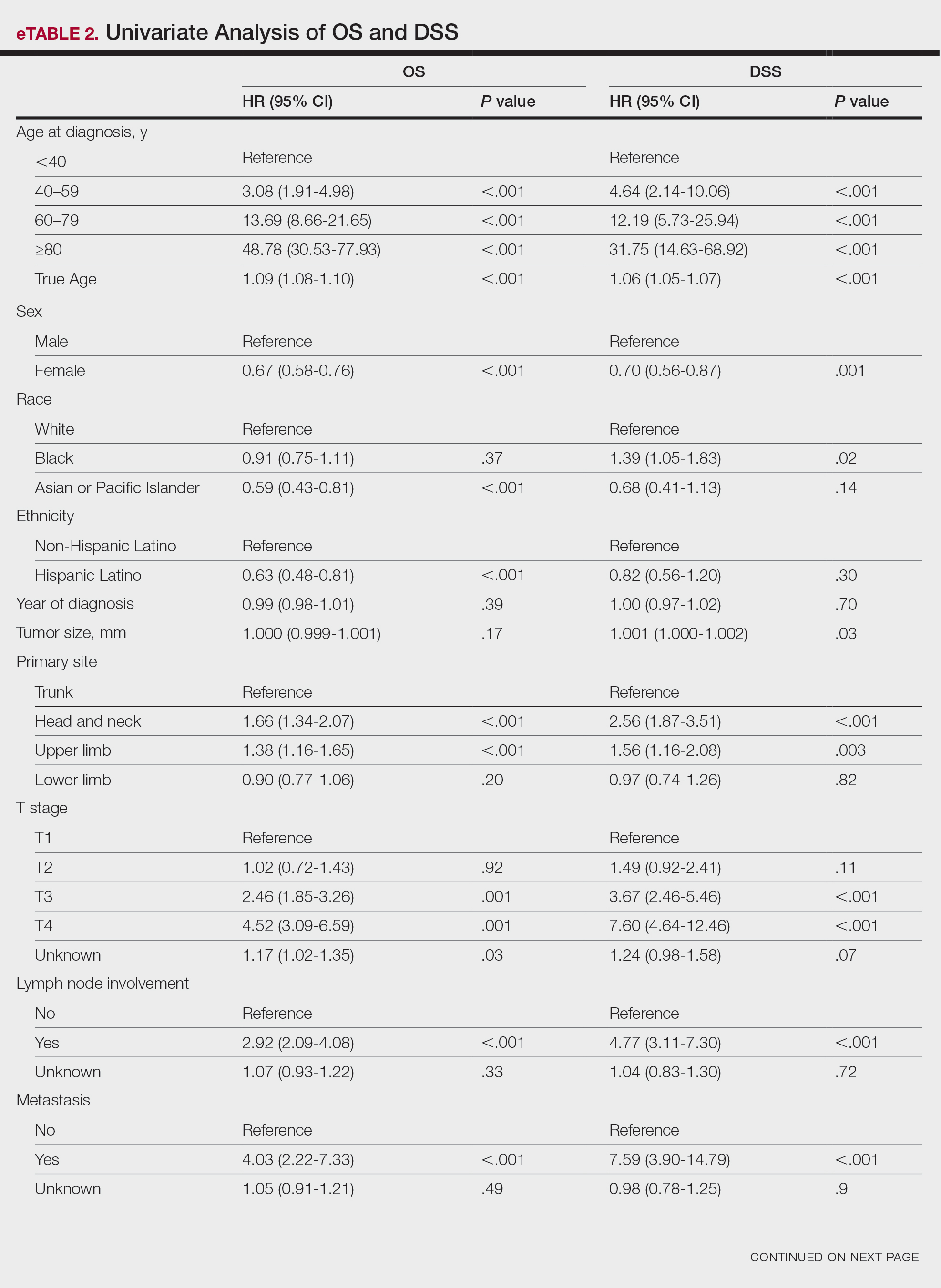
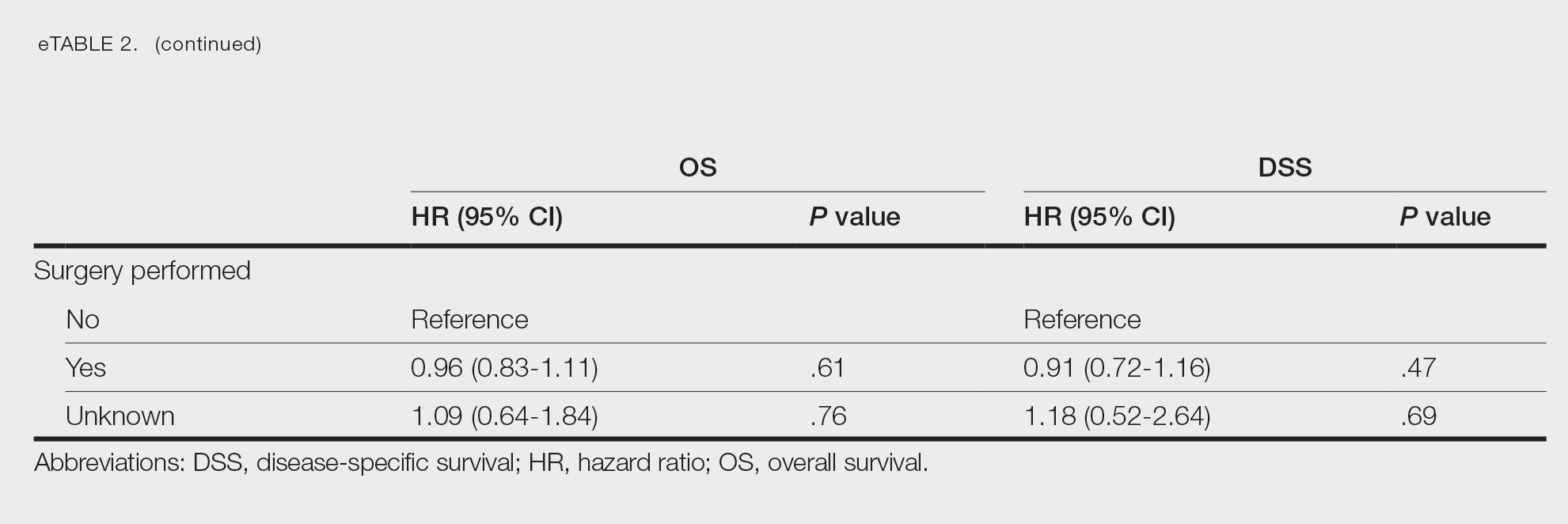
Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).
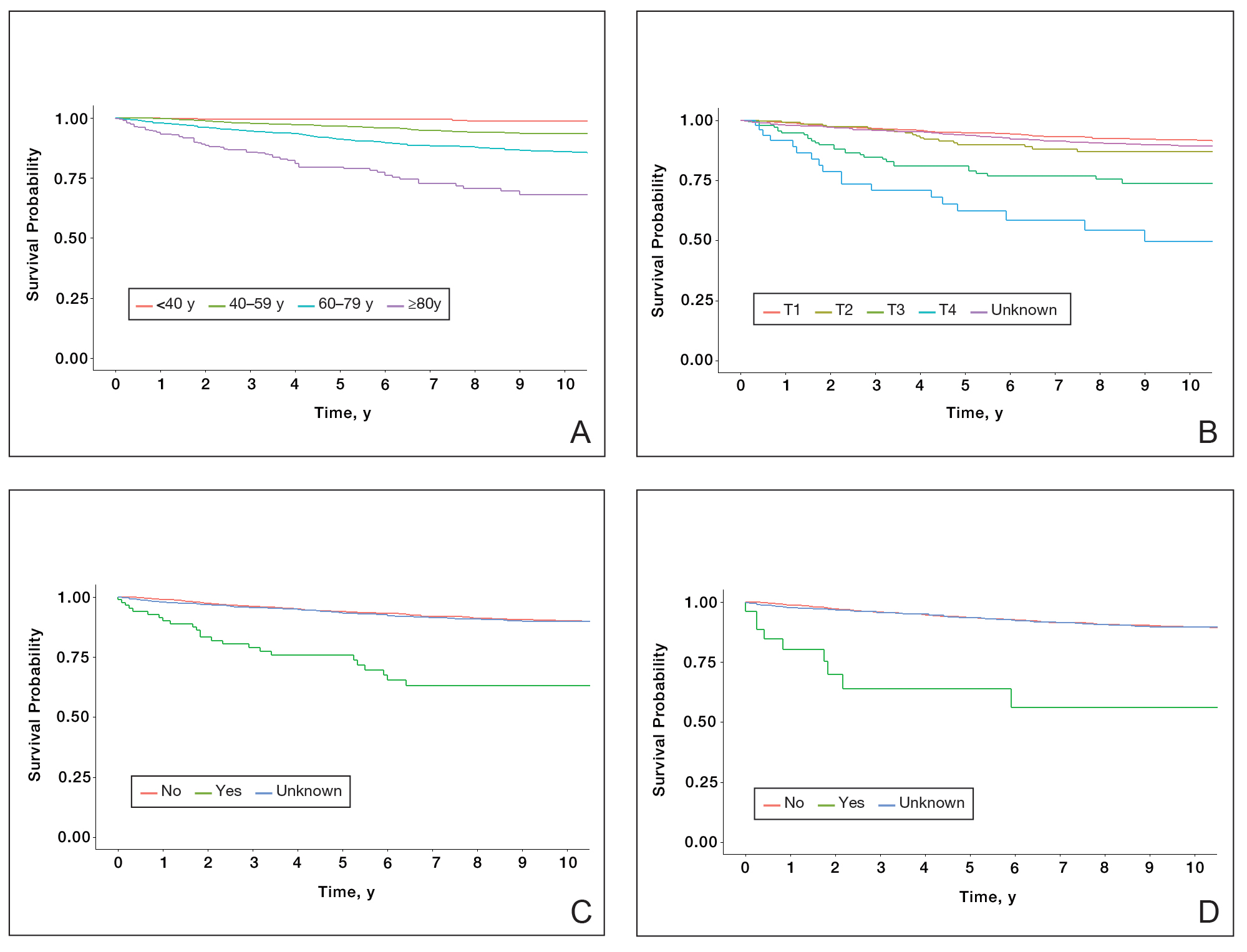
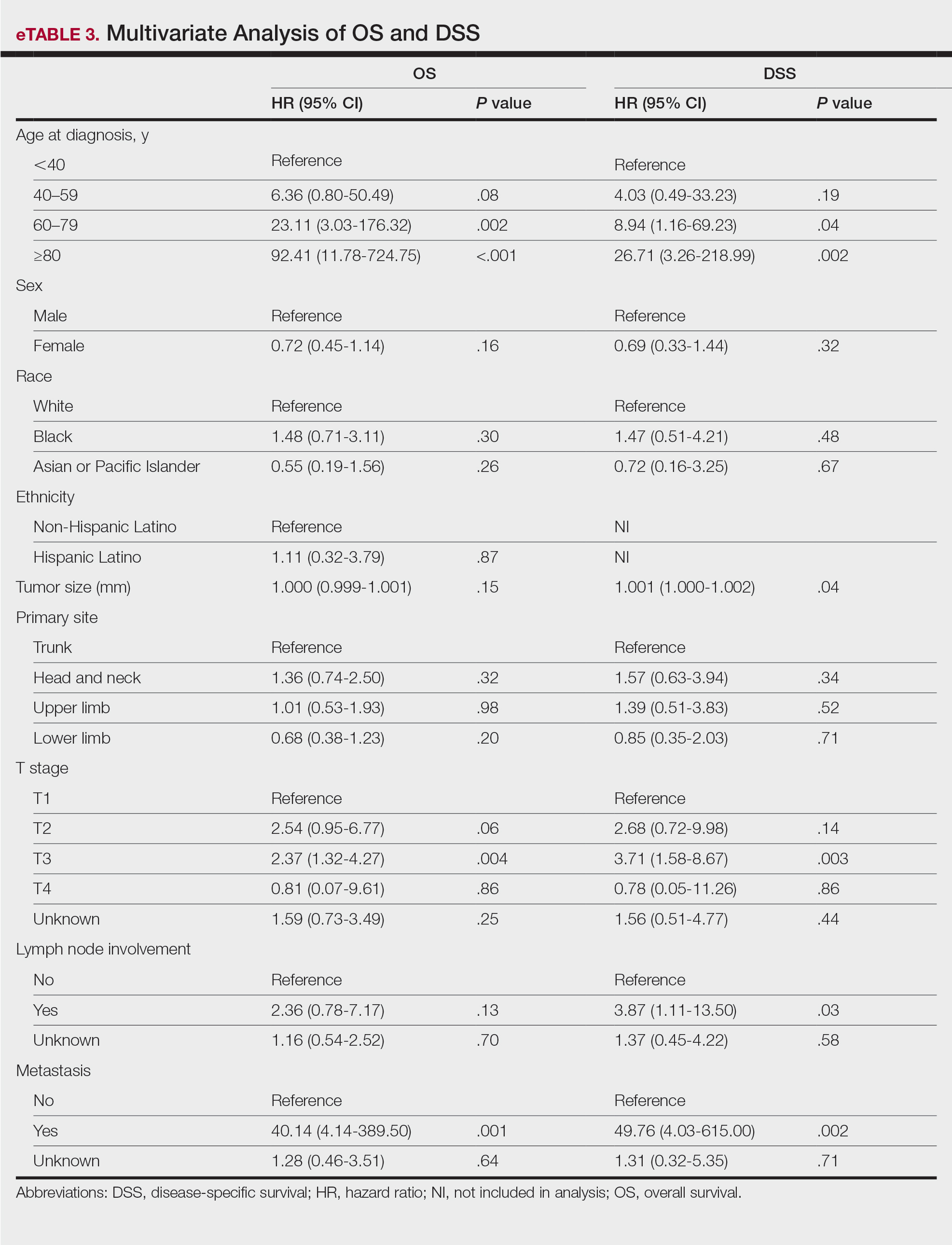
Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Practice Points
- Mycosis fungoides (MF) is the most common cutaneous T-cell lymphoma.
- Because MF is associated with diagnostic challenges due to its indolent course, data regarding primary tumor site as a prognostic factor are limited.
- Although MF originating from the head and neck region did not appear to influence survival, it was found that patients who were older or who had a larger tumor size at diagnosis, a higher T stage, lymph node involvement, or presence of metastasis had poorer survival overall and may benefit from additional counseling regarding their prognosis.
Evaluation of Anti-Agitation Medication Prescribing Patterns by Age in the Emergency Department
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
Each year, about 2.6% of emergency department (ED) visits involve agitation.1 ED clinicians are especially prone to workplace violence and assault, facing the challenge of caring for patients while maintaining safety. A 2013 prospective study found an average of 4.15 violent events per employee in 9 months; nurses and patient care assistants were most frequently affected.2 A 2022 survey from the American College of Emergency Physicians found 55% of respondents reported being physically assaulted in the ED and 79% of respondents reported witnessing another assault. Most of these assaults (98%) were committed by the patients.3 Appropriate management of patients experiencing acute agitation is critical for the safety of all parties involved.
The initial approach to acute agitation management involves nonpharmacologic measures in an attempt to avoid coercive actions, such as physical restraints. Reducing environmental stimulation and verbal de-escalation are effective and help the patients with agitation regain control over their behavior.4
When these measures fail, however, pharmacologic therapy is often administered to ensure safety. The goal of pharmacologic therapy is to calm the patient without causing sedation.5 This allows the patient to continue participating in their care and allows the care team to accurately assess them, which is critical in determining the underlying etiology of agitation. Historically, haloperidol has commonly been used to manage acute agitation. It is frequently administered with lorazepam and diphenhydramine to reduce the incidence of haloperidol’s extrapyramidal adverse effects. However, there are several potential concerns with this method, including oversedation, QTc prolongation, potential drug interactions, and polypharmacy.5,6
The American Association of Emergency Psychiatry Project BETA Psychopharmacology Workgroup published a Consensus Statement in 2012 regarding the psychopharmacology of agitation.5 When considering medication for agitation management, clinicians must first determine a provisional diagnosis outlining the most probable etiology of the patient’s behavior, such as delirium, intoxication, or a psychiatric disorder. Apart from alcohol intoxication, benzodiazepines (BZDs) or second-generation antipsychotics as monotherapy are generally preferred over haloperidol for acute agitation.5 Second-generation antipsychotics have demonstrated to be as effective as haloperidol but are thought to be safer options. Quetiapine is not recommended for use in the ED due to the risk of orthostatic hypotension, as patients are often volume depleted.5The Veterans Affairs Southern Nevada Healthcare System (VASNHS) serves veterans in the Las Vegas area. Among the nearly 220,000 veterans in Nevada, about 100,000 veterans are aged ≥ 65 years.7 The 2012 consensus statement on psychopharmacology for agitation offers no specific age-related guidance. However, there are safety concerns in older adults both with antipsychotics and BZDs, even with acute use. The US Food and Drug Administration (FDA) issued a boxed warning for all antipsychotics due to increased mortality in older adult patients with dementia-related psychosis.8 The 2023 American Geriatrics Society Beers Criteria provides guidance on pharmacological therapy for adults aged ≥ 65 years and recommends avoiding antipsychotics and BZDs.9 In addition to the FDA boxed warning, data suggest increased mortality with antipsychotic use independent of dementia. With BZDs, changes in pharmacodynamics make older adults more prone to adverse effects, including cognitive impairment, delirium, falls, and fractures. A retrospective chart review evaluated risperidone use in the ED and found that adults aged ≥ 65 years experienced higher rates of hypotension, even though this age group received about half the dose of risperidone compared with younger patients.10 For this patient population, the general approach in treating acute agitation has been to avoid the use of medications, but prescribe lower doses when necessary.11
With limited research on acute agitation management in older adults, the purpose of this study was to compare current prescribing practices of anti-agitation medications between adults aged 18 to 64 years and adults aged ≥ 65 years in the VASNHS ED. This study was also conducted to better understand the anti-agitation prescribing practices at VASNHS, as no order sets or protocols existed at the time of the study to guide medication selection in agitation management. To our knowledge, this is the first observational study evaluating pharmacologic acute agitation management in the ED based on age.
Methods
This study was a retrospective chart review of patients aged ≥ 18 years who presented to the VASNHS ED and received medication for acute agitation. Patients were identified through active orders for a formulary agitation medication from August 1, 2019, to July 31, 2022. Formulary medication options included intravenous, oral, and intramuscular routes for haloperidol, droperidol, lorazepam, olanzapine, or ziprasidone. Veterans were excluded if they presented with alcohol intoxication, alcohol or BZD withdrawal, if the medication administration was unrelated to agitation, or whether the medication was not administered. While alcohol and/or BZDs can contribute to acute agitation, these patients were excluded due to a clear indication for BZD therapy and the challenge in a retrospective chart review to determine whether patients received medication for agitation vs other withdrawal-related symptoms.
Endpoints
The primary endpoint was the medication selection between 2 age groups: 18 to 64 years and ≥ 65 years. The secondary endpoints included ordered medication dose by regimen, additional anti-agitation medication use within 3 hours of initial medication administration, and disposition. Safety outcomes included incidence of newly occurring oxygen desaturation < 95%, supplemental oxygen requirement, intubation, QTc prolongation, and hypotension with systolic blood pressure < 90 mm Hg within 1 hour of medication administration. Data collected included patient demographics, substance use, conditions contributing to altered mental status, active psychotropic medication prescriptions, medication adherence, agitation medication prescriber, and doses. Adherence to psychotropic medication in the past 6 months was defined as ≥ 80% of days covered with medication and based on fill history. This was only calculated for applicable patients and did not include patients with only as-needed medications, such as hydroxyzine for anxiety.
Statistical Analysis
Statistical analyses were performed using IBM SPSS. Baseline characteristics were analyzed using descriptive statistics. χ2 and Fisher exact tests were used to analyze categorical data. A student t test was used for continuous variables and a 2-sided P value of < .05 was considered statistically significant.
Results
During the study period, 2342 unique patient encounters with active anti-agitation medication orders in the ED were identified and 232 encounters met the inclusion criteria. Of those excluded, 605 encounters had alcohol involvement. The study included 152 patient encounters for 128 patients aged 18 to 64 years of whom 16 patients had > 1 encounter with a mean (SD) 2.5 (1.1) visits. The study included 80 patient encounters for 72 patients aged ≥ 65 years of whom 7 patients had > 1 encounter with a mean (SD) 2.1 (0.3) visits. The mean age was 45.5 years in the younger cohort and 72.2 years in the older cohort. For data analysis and characterization of the ED population, each patient encounter was treated as a unique patient.
Baseline characteristics significantly differed between the 2 groups (Table 1). When comparing patients aged 18 to 64 years and those aged ≥ 65 years, the younger cohort had higher rates of substance use disorder diagnosis (55.3% vs 27.5%, P < .001), positive urine drug screen (69.7% vs 22.5%, P < .001), and 72-hour legal hold (59.9% vs 32.5%, P < .001) and lower rates of cognitive impairment or dementia (0.7% vs 48.8%, P < .001), and altered mental status-related diagnosis (2.0% vs 18.8%, P < .001). Diagnoses in the younger cohort included 1 each for hyperglycemia, urinary tract infection, and hyponatremia. Diagnoses in the older cohort included 4 for urinary tract infections, 4 for sepsis, 2 for encephalopathy, 2, for hyperglycemia, 1 gastrointestinal bleed, 1 thyrotoxicosis, and 1 respiratory failure.
Endpoints
The primary outcome of anti-agitation medication selection significantly differed between the younger cohort and older cohort (P = .02). All medication combinations ordered are shown in the eAppendix based on patient age and the percentage of patients in the age cohort that received that medication combination. Lorazepam monotherapy was the most common anti-agitation medication regimen ordered: 43.4% in patients aged 18 to 64 years and 41.3% in patients aged ≥ 65 years. Second-generation antipsychotic use was low.
Only 10.5% of patients aged 18 to 64 years and 8.8% of patients aged ≥ 65 years received a medication combination including a second-generation antipsychotic. Intramuscular administration (41.4%) was most common followed by intravenous (37.5%), oral (19.8%), and oral disintegrating tablets (1.3%). The median (IQR) number of anti-agitation medications ordered by a prescriber was 6 (3-11) and 18 of 28 prescribers did not prescribe second-generation antipsychotics.
Medication doses ordered did not significantly differ except lorazepam monotherapy, as patients aged ≥ 65 received a lower dose (P = .007) (Table 2). Given the limited data within 1 hour, the first set of vital signs available after medication administration was used for analysis of safety outcomes. Vital signs were documented within 1 hour after medication administration for only 28.3% of patients aged 18 to 64 years and 42.5% of patients aged ≥ 65 years. The median (IQR) time to documentation for vital signs after medication administration was 96 minutes (56-177) for patients aged 18 to 64 years and 64 minutes (25-121) for patients aged ≥ 65 years. Electrocardiogram measurement after medication administration only occurred in 7.9% of patients aged 18 to 64 years and 5% of patients aged ≥ 65 years.
Fourteen patients (7.9%) aged 18 to 64 years and 17 patients (15.0%) aged ≥ 65 years experienced an adverse outcome (P = .09) (Table 3). Most patients who had an adverse safety outcome experienced new oxygen desaturation < 95%. Of those patients, only a small proportion required new supplemental oxygen or intubation. The 2 patients intubated had ongoing medical issues complicating their course in the ED. New QTc prolongation was only documented in haloperidol-containing regimens.
The proportion of patients requiring additional anti-agitation medication doses within 3 hours following initial administration was similar between the 2 groups. The mean (SD) amount of time to administration of subsequent dose was 55 minutes (30) in the younger cohort and 64 minutes (36) in the older cohort. Patient disposition from the ED, significantly differed based on age (P < .001) (Table 4). Patients aged 18 to 64 years were more frequently admitted to the psychiatry unit, while patients aged ≥ 65 years were primarily admitted to the hospital. One patient in the younger cohort died due to hyponatremia.
Discussion
The most likely causes of acute agitation significantly differed between patients aged 18 to 64 years and patients aged ≥ 65 years. Patients in the younger cohort were more likely to present with a history of substance use disorder or a positive urine drug screen for illicit substances. They were also more likely to have a 72-hour legal hold initiated, suggesting higher rates of suicidal and/or homicidal ideations. Patients in the older cohort were likely to present with a history of cognitive impairment or be diagnosed with a condition contributing to an altered mental status. To our knowledge, this is the first study that has assessed characteristics of patients experiencing acute agitation in the ED based on age and demonstrated significant differences in potential contributing factors to acute agitation. These findings may have important implications in helping guide the selection of empiric regimens, especially when the cause of agitation cannot immediately be elucidated.
Lorazepam monotherapy, haloperidol monotherapy, and a combination of haloperidol, lorazepam, and diphenhydramine were the 3 most frequently prescribed regimens for acute agitation. There was low second-generation antipsychotic use. Outside of the VASNHS formulary, there were no policies or restrictions that would have prevented clinicians from ordering a particular anti-agitation medication during the study period.
Since the end of the period assessed in this study, VASNHS clinicians have been educated on the guidelines for anti-agitation medication regimens to encourage higher use of second-generation antipsychotics when appropriate. Training has been developed to prevent unnecessary delays when using these products. Barriers to second-generation antipsychotic use at VASNHS have also been identified and addressed. Previously, second-generation antipsychotics and the sterile water required for medication reconstitution were not overridable in Pyxis machines, often resulting in delays in administering these medications to acutely agitated patients. As of February 2023, olanzapine, ziprasidone, and sterile water are overridable, making them more accessible in situations when medication is urgently needed. Clinicians also expressed concern regarding a lack of familiarity with reconstituting and administering intramuscular second-generation antipsychotics.
While the general guidance has been to use lower doses of anti-agitation medications in patients aged ≥ 65 years, no significant differences were seen in doses ordered other than for lorazepam. In our study, however, there were no significant differences in adverse safety outcomes, though a higher proportion of patients in the older cohort experienced new respiratory-related outcomes after medication administration. Given the retrospective nature of this study and limited documentation of vital signs after medication administration, we cannot conclude the adverse safety outcomes were directly related to the anti-agitation medications. Most patients in both groups did not require additional doses of anti-agitation medications. The results of this study have been used to guide the development of an order set for anti-agitation medications.
Limitations
As a retrospective chart review, this study is unable to prove any differences in prescribing patterns for anti-agitation medications based on age. As a single-center study, the prescribing patterns and baseline characteristics are unique to the facility and not generalizable to all patients with acute agitation in the ED. Future, higher-quality studies with adequate power in diverse patient populations are needed to further elucidate differences in acute agitation etiology and anti-agitation medications based on patient age.
The anti-agitation medication used may have been skewed for patients with multiple and/or previous ED encounters. If information was available on previous causes of agitation and/or previous efficacy of regimens, this may have influenced selection. Additionally, clinical pharmacy specialists began providing daytime coverage in the ED in April 2022. As a part of their role, these pharmacists provide recommendations for medication selection in the management of acute agitation and can order anti-agitation medications. While no pharmacist prescriptions were identified in the study, their recommendations may have influenced medication selection toward the end of the study period.
Given the retrospective nature of the study, it is unclear whether medication selection may have been guided by the patient’s presentation or comorbidities to avoid adverse effects. This may have influenced the safety outcomes observed. Another limitation to this data is vital signs documentation. Vital signs were rarely documented in the ED within 1 hour of medication administration, meaning the vital signs captured may not be related to the agitation medication. Among the patients with documented vital signs, 20 patients were documented within 10 minutes, likely prior to when the medication had taken full effect. This time variability further limits the ability to link safety outcomes to medications and demonstrates a need for additional research. Very few patients had electrocardiogram data after medication administration. If patients did have an electrocardiogram measured in the ED, this more commonly occurred prior to any medication administration, which may have also guided clinicians in initial medication selection.
This study may have also overlooked risperidone use. Though risperidone is on the VASNHS formulary, it was not expected to be commonly used in the ED setting due to it only being available by mouth. However, oral medication use was higher than expected, and there were instances where clinicians initially ordered 1 of the included anti-agitation medications but patients ultimately received risperidone. Based on these findings, the current study may have overlooked this as an anti-agitation medication regimen. In addition, by excluding alcohol intoxication, alcohol withdrawal, and BZD withdrawal, this study did not fully capture the agitated population in our ED.
Conclusions
Anti-agitation medication prescribing patterns may differ between adults aged 18 to 64 years and those aged ≥ 65 years. The findings of this study also suggest that the most common agitation etiologies may differ based on patient age. Future studies should further explore anti-agitation medication use and agitation etiologies among older adults to guide medication prescribing.
Acknowledgments
We acknowledge Ted Turner, PharmD, BCPP, and Phong Ly, PharmD, BCPS, for their support and assistance on this project.
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
1. Miner JR, Klein LR, Cole JB, Driver BE, Moore JC, Ho JD. The characteristics and prevalence of agitation in an urban county emergency department. Ann Emerg Med. 2018;72(4):361-370. doi:10.1016/j.annemergmed.2018.06.001
2. Kowalenko T, Gates D, Gillespie GL, Succop P, Mentzel TK. Prospective study of violence against ED workers. Am J Emerg Med. 2013;31(1):197-205. doi:10.1016/j.ajem.2012.07.010
3. Marketing General Incorporated. ACEP emergency department violence poll results. American College of Emergency Physicians. August 2022. Accessed January 10, 2024. https://www.emergencyphysicians.org/siteassets/emphysicians/all-pdfs/acep-emergency-department-violence-report-2022-abridged.pdf
4. Richmond JS, Berlin JS, Fishkind AB, et al. Verbal de-escalation of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project BETA De-escalation Workgroup. West J Emerg Med. 2012;13(1):17-25. doi:10.5811/westjem.2011.9.6864
5. Wilson MP, Pepper D, Currier GW, Holloman GH Jr, Feifel D. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project BETA Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26-34. doi:10.5811/westjem.2011.9.6866
6. Pierre JM. Time to retire haloperidol? Current Psychiatry. 2020;19(5):18-28.
7. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Updated September 7, 2022. Accessed January 10, 2024. https://www.va.gov/vetdata/Veteran_Population.asp
8. Yan J. FDA extends black-box warning to all antipsychotics. Psychiatric News. 2008;43(14):1-27. doi:10.1176/pn.43.14.0001
9. 2023 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
10. Wilson MP, Nordstrom K, Hopper A, Porter A, Castillo EM, Vilke GM. Risperidone in the emergency setting is associated with more hypotension in elderly patients. J Emerg Med. 2017;53(5):735-739. doi:10.1016/j.jemermed.2017.06.026
11. Gottlieb M, Long B, Koyfman A. Approach to the agitated emergency department patient. J Emerg Med. 2018;54(4):447-457. doi:10.1016/j.jemermed.2017.12.049
Underlying Mental Illness and Risk of Severe Outcomes Associated With COVID-19
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
The Centers for Disease Control and Prevention (CDC) has identified factors that put patients at a higher risk of severe COVID-19 infection, which include advanced age, obesity, cardiovascular disease, diabetes, chronic kidney disease, lung disease, and immunocompromising conditions. The CDC also acknowledges that mood disorders, including depression and schizophrenia, contribute to the progression to severe COVID-19.1 Antiviral therapies, such as nirmatrelvir and ritonavir combination, remdesivir, and molnupiravir, and monoclonal antibody (mAb) therapies, have been used to prevent hospitalization and mortality from COVID-19 infection for individuals with mild-to-moderate COVID-19 who are at high risk of progressing to severe infection.2 Although antiviral and mAb therapies likely have mitigated many infections, poor prognoses are prevalent. It is important to identify all patients at risk of progressing to severe COVID-19 infection.
Although the CDC considers depression and schizophrenia to be risk factors for severe COVID-19 infection, the Captain James A. Lovell Federal Health Care Center (FHCC) in North Chicago, Illinois, does not, making these patients ineligible for antiviral or mAb therapies unless they have another risk factor. As a result, these patients could be at risk of severe COVID-19 infection, but might not be treated appropriately. Psychiatric diagnoses are common among veterans, with 19.7% experiencing a mental illness in 2020.3 It is imperative to determine whether depression or schizophrenia play a role in the progression of COVID-19 to expand access to individuals who are eligible for antiviral or mAb therapies.
Because COVID-19 is a novel virus, there are few studies of psychiatric disorders and COVID-19 prognosis. A 2020 case control study determined that those with a recent mental illness diagnosis were at higher risk of COVID-19 infection with worse outcomes compared with those without psychiatric diagnoses. This effect was most prevalent among individuals with depression and schizophrenia.4 However, these individuals also were found to have additional comorbidities that could have contributed to poorer outcomes. A meta-analysis determined that psychiatric disorders were associated with increased COVID-19-related mortality.5 A 2022 cohort study that included vaccinated US Department of Veterans Affairs (VA) patients determined that having a psychiatric diagnosis was associated with increased incidence of breakthrough infections.6 Individuals with psychiatric conditions are thought to be at higher risk of severe COVID-19 outcomes because of poor access to care and higher incidence of untreated underlying health conditions.7 Lifestyle factors also could play a role. Because there is minimal data on COVID-19 prognosis and mental illness, further research is warranted to determine whether psychiatric diagnoses could contribute to more severe COVID-19 infections.
Methods
This was a retrospective cohort chart review study at FHCC that compared COVID-19 outcomes in individuals with depression or schizophrenia with those without these diagnoses. FHCC patients with the International Classification of Diseases code for COVID-19 (U07.1) from fiscal years 2020 to 2022 were included. We then selected patients with a depression or schizophrenia diagnosis noted in the electronic health record (EHR). These 2 patient lists were consolidated to identify every individual with a COVID-19 diagnosis and a diagnosis of depression or schizophrenia.
Patients were included if they were aged ≥ 18 years with a positive COVID-19 infection confirmed via polymerase chain reaction or blood test. Patients also had to have mild-to-moderate COVID-19 with ≥ 1 symptom such as fever, cough, sore throat, malaise, headache, muscle pain, loss of taste and smell, or shortness of breath. Patients were excluded if they had an asymptomatic infection, presented with severe COVID-19 infection, or were an FHCC employee. Severe COVID-19 was defined as having oxygen saturation < 94%, a respiratory rate > 30 breaths per minute, or supplemental oxygen requirement.
Patient EHRs were reviewed and analyzed using the VA Computerized Patient Record System and Joint Legacy Viewer. Collected data included age, medical history, use of antiviral or mAb therapy, and admission or death within 30 days of a positive COVID-19 test. The primary outcome of this study was severe COVID-19 outcomes defined as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death within 30 days of infection. The primary outcome was analyzed with a student t test; P < .05 was considered statistically significant.
Results
More than 5000 individuals had a COVID-19 diagnosis during the study period. Among these patients, 4530 had no depression or schizophrenia diagnosis; 1021 individuals had COVID-19 and a preexisting diagnosis of depression or schizophrenia. Among these 1021 patients, 279 charts were reviewed due to time constraints; 128 patients met exclusion criteria and 151 patients were included in the study. Of the 151 patients with COVID-19, 78 had no depression or schizophrenia and 73 patients with COVID-19 had a preexisting depression or schizophrenia diagnosis (Figure).
The 2 groups were similar at baseline. The most common risk factors for severe COVID-19 included age > 60 years, obesity, and cardiovascular disease. However, more than half of the individuals analyzed had no risk factors (Table 1). Some patients with risk factors received antiviral or mAb therapy to prevent severe COVID-19 infection; combination nirmatrelvir and ritonavir was the most common agent (Table 2). Of the 73 individuals with a psychiatric diagnosis, 67 had depression (91.8%), and 6 had schizophrenia (8.2%).
Hospitalization or death within 30 days of COVID-19 infection between patients with depression or schizophrenia and patients without these psychiatric diagnoses was not statistically significant (P = .36). Sixteen individuals were hospitalized, 8 in each group. Three individuals died within 30 days; death only occurred in patients who had depression or schizophrenia (Table 3).
Discussion
This study found that hospitalization or death within 30 days of COVID-19 infection occurred more frequently among individuals with depression or schizophrenia compared with those without these psychiatric comorbidities. However, this difference was not statistically significant.
This study had several limitations. It was a retrospective, chart review study, which relied on accurate documentation. In addition, we reviewed COVID-19 cases from fiscal years 2020 to 2022 and as a result, several viral variants were analyzed. This made it difficult to draw conclusions, especially because the omicron variant is thought to be less deadly, which may have skewed the data. Vaccinations and COVID-19 treatments became available in late 2020, which likely affected the progression to severe disease. Our study did not assess vaccination status, therefore it is unclear whether COVID-19 vaccination played a role in mitigating infection. When the pandemic began, many individuals were afraid to come to the hospital and did not receive care until they progressed to severe COVID-19, which would have excluded them from the study. Many individuals had additional comorbidities that likely impacted their COVID-19 outcomes. It is not possible to conclude if the depression or schizophrenia diagnoses were responsible for hospitalization or death within 30 days of infection or if it was because of other known risk factors. Future research is needed to address these limitations.
Conclusions
More COVID-19 hospitalizations and deaths occurred within 30 days of infection among those with depression and schizophrenia compared with individuals without these comorbidities. However, this effect was not statistically significant. Many limitations could have contributed to this finding, which should be addressed in future studies. Because the sample size was small, further research with a larger patient population is warranted to explore the association between psychiatric comorbidities such as depression and schizophrenia and COVID-19 disease progression. Future studies also could include assessment of vaccination status and exclude individuals with other high-risk comorbidities for severe COVID-19 outcomes. These studies could determine if depression and schizophrenia are correlated with worse COVID-19 outcomes and ensure that all high-risk patients are identified and treated appropriately to prevent morbidity and mortality.
Acknowledgements
Thank you to the research committee at the Captain James A. Lovell Federal Health Care Center who assisted in the completion of this project, including Shaiza Khan, PharmD, BCPS; Yinka Alaka, PharmD; and Hong-Yen Vi, PharmD, BCPS, BCCCP.
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
1. Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated February 9, 2023. Accessed February 27, 2024. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
2. National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. Updated November 2, 2023. Accessed February 27, 2024. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults-therapeutic-management
3. National Alliance on Mental Illness. Mental health by the numbers. Updated April 2023. Accessed February 27, 2024. https://www.nami.org/mhstats
4. Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry . 2021;20(1):124-130. doi:10.1002/wps.20806
5. Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry . 2021;78(11):1208-1217. doi:10.1001/jamapsychiatry.2021.2274
6. Nishimi K, Neylan TC, Bertenthal D, Seal KH, O’Donovan A. Association of Psychiatric Disorders With Incidence of SARS-CoV-2 Breakthrough Infection Among Vaccinated Adults. JAMA Netw Open . 2022;5(4):e227287. Published 2022 Apr 1. doi:10.1001/jamanetworkopen.2022.7287
7. Koyama AK, Koumans EH, Sircar K, et al. Mental Health Conditions and Severe COVID-19 Outcomes after Hospitalization, United States. Emerg Infect Dis . 2022;28(7):1533-1536. doi:10.3201/eid2807.212208
Outcomes and Barriers Associated with Telehealth-Based Hepatitis C Treatment During Early Phases of the COVID-19 Pandemic
Although 2.4 million adults in the United States have been diagnosed with hepatitis C virus (HCV) infection, it remains underdiagnosed and undertreated, particularly among difficult to reach populations, such as persons who inject drugs, marginally housed individuals, correctional populations, and pregnant women.1 Though the US Preventive Services Task Force (USPSTF) broadened HCV screening recommendations to include individuals aged 18 to 79 years, rates of new HCV prescriptions sharply declined during the COVID-19 pandemic.2,3
During the pandemic, many health care systems adopted virtual health care modalities. Within the Veteran Health Administration (VHA), there was an 11-fold increase in virtual encounters. However, veterans aged > 45 years, homeless, and had other insurance were less likely to utilize virtual care.4,5 As health care delivery continues to evolve, health systems must adapt and test innovative models for the treatment of HCV.
There is limited understanding of HCV treatments when exclusively conducted virtually. The aim of this study was to evaluate the effects of the HCV treatment program at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) during the early phase of the COVID-19 pandemic, when telehealth modalities and mail-order prescriptions were used for HCV diagnosis and treatment. The secondary aim of this study was to understand patient factors associated with treatment initiation and discontinuation for patients using telehealth.
Methods
The VHA is the largest provider of HCV care in the US.6 At VAGLAHS, veterans with HCV are referred for evaluation to a viral hepatitis clinic staffed by gastroenterologists and infectious disease specialists. Veterans with detectable HCV on an HCV RNA test have an additional workup ordered if necessary and are referred to an HCV-specialist pharmacist or physician’s assistant to start treatment. In March 2020, all HCV evaluations and treatment initiation in the viral hepatitis clinic started being conducted exclusively via telehealth. This was the primary modality of HCV evaluations and treatment initiation until COVID-19 restrictions were lifted to permit in-person evaluations. Prescriptions were delivered by mail to patients following treatment initiation appointments.
We retrospectively reviewed electronic health records of veterans referred to start treatment March 1, 2020, through September 30, 2020. The endpoint of the reviewed records was set because during this specific time frame, VAGLAHS used an exclusively telehealth-based model for HCV evaluation and treatment. Patients were followed until June 15, 2021. Due to evolving COVID-19 restrictions at the time, and despite requests received, treatment initiations by the pharmacy team were suspended in March 2020 but HCV treatments resumed in May. Data collected included baseline demographics (age, sex, race, ethnicity, housing status, distance to VAGLAHS), comorbidities (cirrhosis, hepatitis B virus coinfection, HIV coinfection), psychiatric conditions (mood or psychotic disorder, alcohol use disorder [AUD], opioid use disorder), and treatment characteristics (HCV genotype, HCV treatment regimen, baseline viral load). Distance from the patient’s home to VAGLAHS was calculated using CDXZipStream software. Comorbidities and psychiatric conditions were identified by the presence of the appropriate diagnosis via International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in the health record and confirmed by review of clinician notes. Active AUD was defined as: (1) the presence of AUD diagnosis code; (2) AUD Identification Test-Consumption (AUDIT-C) score of high or severe risk based on established cutoffs; and (3) active alcohol use noted in the electronic health record. All patients had an AUDIT-C score completed within 1 year of initiating treatment. Opioid use disorder was defined by the presence of diagnostic codes for opioid dependence or opioid abuse.
The reasons for treatment noninitiation and discontinuation were each captured. We calculated descriptive statistics to analyze the frequency distributions of all variables. Independent t tests were used to analyze continuous data and Pearson χ2 test was used to analyze categorical data. Statistical significance was set as P < .05.
Results
From March 1, 2020, through September 30, 2020, 73 veterans were referred to the HCV clinical pharmacist for treatment (Figure). Forty-three veterans (59%) initiated HCV treatment and 34 (79%) completed the full treatment course (Table 1). Twenty-five patients (65%) had their sustained virologic response at 12 weeks (SVR12) testing and 22 patients achieved SVR12 (88%; 30% of total sample). One patient did not achieve SVR, and 2 patients died (variceal hemorrhage and progression of cerebral amyloidosis/function decline) before the completion of laboratory testing. From March 2020 to May 2020, HCV treatments requests were paused as new COVID-19 policies were being introduced; 33 patients were referred during this time and 21 initiated treatment.
Veterans that did not start HCV treatment had a significantly higher rate of active AUD when compared with those that initiated treatment: 30% vs 9% (P = .02). Of the patients who started and discontinued treatment, none had active AUD. Other baseline demographics, clinical characteristics, and treatment characteristics were similar between the groups. No patient demographic characteristics were significantly associated with HCV treatment discontinuation. We did not observe any major health disparities in initiation or discontinuation by sex, race, ethnicity, or geography. Eleven patients (37%) could not be contacted, which was the most common reason veterans did not initiate treatment (Table 2). Of the 9 patients that did not complete SVR12, 5 patients could not be contacted for follow-up, which was the most common reason veterans discontinued treatment.
Discussion
This study highlights the experience of treating patients with HCV with an exclusively telehealth model in the months following implementation of stay-at-home orders from March 19, 2020, to September 30, 2020, during the COVID-19 pandemic at VAGLAHS. We were able to successfully complete treatment for 34 veterans (47%) and achieved SVR rates of 88%. We found that AUD was associated with unsuccessful treatment initiation. There were no statistically significant patient characteristic findings for treatment discontinuation in our study (Table 3). Unhealthy alcohol use and AUD are highly prevalent among veterans with HCV and prior to the pandemic, studies have demonstrated AUD as a barrier to HCV treatment.7
Since worse hepatic outcomes have been observed in veterans with HCV and AUD and increased harmful patterns of drinking occurred during the pandemic, a renewed interest in treating AUD in these veterans during the era of telehealth is critical.8 While we were unable to ascertain whether alcohol misuse in our cohort increased during the pandemic or whether changes in drinking patterns affected HCV treatment outcomes before and after the pandemic, such an association should reinforce the need for clinicians to expeditiously link patients to substance use care. It should also stimulate further considerations of addressing social determinants of health not captured in this study.
During the pandemic, veterans with posttraumatic stress disorder, a history of serving in combat roles, and experiencing related financial stressors had higher risk of AUD.9,10 For veterans with AUD who initiated HCV treatment, none discontinued their therapy, aligning with other studies showed that patients with AUD were able to achieve high rates of SVR and emphasizing that veterans should be treated irrespective of an AUD diagnosis.11 However, more innovative engagement initiatives for veterans with AUD should be explored as we continue to adapt more telehealth-based care for HCV direct-acting antiviral treatments. A more in-depth understanding of how alcohol use relates to treatment noninitiation is warranted, as this may stem from behavioral patterns that could not be captured in the present study.
The inability to reach veterans by telephone was a major reason for noninitiation and discontinuation of treatment. While the expansion of telehealth services has been noted across the VHA, there is still room for improving methods of engaging veterans in health care postpandemic.12 Prior studies in veteran populations that were successful in increasing uptake of HCV treatment have employed telehealth strategies that further emphasizes its integral role in HCV elimination.13 Although our study did not show mental health comorbidities and housing status as statistically significant, it is important to note that 20% of patients referred for HCV treatment had an incomplete evaluation which can lead to potentially unobserved indicators not captured by our study such as quality of linkage to care. It is imperative to stress the best practices for HCV initiation by integrating a multidisciplinary team to address patients’ psychosocial comorbidities.14 Finally, we did not observe any major disparities in treating veterans with HCV during the pandemic. This observation is reassuring and consistent with other VHA data given the heightened recognition of health disparities seen in health care sectors across the country, especially evident during the COVID-19 pandemic and the current era of increased adaptation of telehealth.
Limitations
Limitations to this study include its retrospective nature, small sample size, and short study time frame as a proportion of veterans have yet to complete HCV treatment which can potentially explain how larger studies were able to find other statistically significant patient-related factors impacting treatment initiation compared to ours. Given the lack of universal standardized diagnostic criterion of AUD, this can limit how our study can be compared to others in similar populations. Additionally, this study was conducted at a single facility with a predominantly older male veteran population, which may not be generalizable to other populations.
Conclusions
Treating HCV during the COVID-19 pandemic with telehealth and mail-out medications was feasible and led to high SVR rates, but unhealthy alcohol use and an inability to contact veterans were predominant barriers to success. Future quality improvement efforts should focus on addressing these barriers and exploring the relationship between alcohol use and HCV treatment initiation.
1. Patel AA, Bui A, Prohl E, et al. Innovations in Hepatitis C Screening and Treatment. Hepatol Commun. 2020;5(3):371-386. Published 2020 Dec 7. doi:10.1002/hep4.1646
2. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(10):970-975. doi:10.1001/jama.2020.1123
3. Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med. 2021;61(3):369-376. doi:10.1016/j.amepre.2021.03.011
4. Rosen CS, Morland LA, Glassman LH, et al. Virtual mental health care in the Veterans Health Administration’s immediate response to coronavirus disease-19. Am Psychol. 2021;76(1):26-38. doi:10.1037/amp0000751
5. Balut MD, Wyte-Lake T, Steers WN, et al. Expansion of telemedicine during COVID-19 at a VA specialty clinic. Healthc (Amst). 2022;10(1):100599. doi:10.1016/j.hjdsi.2021.100599
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
7. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017;46(10):992-1000. doi:10.1111/apt.14328
8. Alavi M, Janjua NZ, Chong M, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68(3):393-401. doi:10.1016/j.jhep.2017.10.019
9. Pedersen ER, Davis JP, Fitzke RE, Lee DS, Saba S. American Veterans in the Era of COVID-19: Reactions to the Pandemic, Posttraumatic Stress Disorder, and Substance Use Behaviors. Int J Ment Health Addict. 2023;21(2):767-782. doi:10.1007/s11469-021-00620-0
10. Na PJ, Norman SB, Nichter B, et al. Prevalence, risk and protective factors of alcohol use disorder during the COVID-19 pandemic in U.S. military veterans. Drug Alcohol Depend. 2021;225:108818. doi:10.1016/j.drugalcdep.2021.108818
11. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-109. doi:10.1016/j.drugalcdep.2016.10.021
12. Baum A, Kaboli PJ, Schwartz MD. Reduced In-Person and Increased Telehealth Outpatient Visits During the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
13. Fleming BS, Ifeachor AP, Andres AM, et al. Improving Veteran Access to Treatment for Hepatitis C Virus Infection: Addressing social issues and treatment barriers significantly increases access to HCV care, and many veterans successfully start therapy with the help of additional support staff. Fed Pract. 2017;34(Suppl 4):S24-S28.
14. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
Although 2.4 million adults in the United States have been diagnosed with hepatitis C virus (HCV) infection, it remains underdiagnosed and undertreated, particularly among difficult to reach populations, such as persons who inject drugs, marginally housed individuals, correctional populations, and pregnant women.1 Though the US Preventive Services Task Force (USPSTF) broadened HCV screening recommendations to include individuals aged 18 to 79 years, rates of new HCV prescriptions sharply declined during the COVID-19 pandemic.2,3
During the pandemic, many health care systems adopted virtual health care modalities. Within the Veteran Health Administration (VHA), there was an 11-fold increase in virtual encounters. However, veterans aged > 45 years, homeless, and had other insurance were less likely to utilize virtual care.4,5 As health care delivery continues to evolve, health systems must adapt and test innovative models for the treatment of HCV.
There is limited understanding of HCV treatments when exclusively conducted virtually. The aim of this study was to evaluate the effects of the HCV treatment program at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) during the early phase of the COVID-19 pandemic, when telehealth modalities and mail-order prescriptions were used for HCV diagnosis and treatment. The secondary aim of this study was to understand patient factors associated with treatment initiation and discontinuation for patients using telehealth.
Methods
The VHA is the largest provider of HCV care in the US.6 At VAGLAHS, veterans with HCV are referred for evaluation to a viral hepatitis clinic staffed by gastroenterologists and infectious disease specialists. Veterans with detectable HCV on an HCV RNA test have an additional workup ordered if necessary and are referred to an HCV-specialist pharmacist or physician’s assistant to start treatment. In March 2020, all HCV evaluations and treatment initiation in the viral hepatitis clinic started being conducted exclusively via telehealth. This was the primary modality of HCV evaluations and treatment initiation until COVID-19 restrictions were lifted to permit in-person evaluations. Prescriptions were delivered by mail to patients following treatment initiation appointments.
We retrospectively reviewed electronic health records of veterans referred to start treatment March 1, 2020, through September 30, 2020. The endpoint of the reviewed records was set because during this specific time frame, VAGLAHS used an exclusively telehealth-based model for HCV evaluation and treatment. Patients were followed until June 15, 2021. Due to evolving COVID-19 restrictions at the time, and despite requests received, treatment initiations by the pharmacy team were suspended in March 2020 but HCV treatments resumed in May. Data collected included baseline demographics (age, sex, race, ethnicity, housing status, distance to VAGLAHS), comorbidities (cirrhosis, hepatitis B virus coinfection, HIV coinfection), psychiatric conditions (mood or psychotic disorder, alcohol use disorder [AUD], opioid use disorder), and treatment characteristics (HCV genotype, HCV treatment regimen, baseline viral load). Distance from the patient’s home to VAGLAHS was calculated using CDXZipStream software. Comorbidities and psychiatric conditions were identified by the presence of the appropriate diagnosis via International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in the health record and confirmed by review of clinician notes. Active AUD was defined as: (1) the presence of AUD diagnosis code; (2) AUD Identification Test-Consumption (AUDIT-C) score of high or severe risk based on established cutoffs; and (3) active alcohol use noted in the electronic health record. All patients had an AUDIT-C score completed within 1 year of initiating treatment. Opioid use disorder was defined by the presence of diagnostic codes for opioid dependence or opioid abuse.
The reasons for treatment noninitiation and discontinuation were each captured. We calculated descriptive statistics to analyze the frequency distributions of all variables. Independent t tests were used to analyze continuous data and Pearson χ2 test was used to analyze categorical data. Statistical significance was set as P < .05.
Results
From March 1, 2020, through September 30, 2020, 73 veterans were referred to the HCV clinical pharmacist for treatment (Figure). Forty-three veterans (59%) initiated HCV treatment and 34 (79%) completed the full treatment course (Table 1). Twenty-five patients (65%) had their sustained virologic response at 12 weeks (SVR12) testing and 22 patients achieved SVR12 (88%; 30% of total sample). One patient did not achieve SVR, and 2 patients died (variceal hemorrhage and progression of cerebral amyloidosis/function decline) before the completion of laboratory testing. From March 2020 to May 2020, HCV treatments requests were paused as new COVID-19 policies were being introduced; 33 patients were referred during this time and 21 initiated treatment.
Veterans that did not start HCV treatment had a significantly higher rate of active AUD when compared with those that initiated treatment: 30% vs 9% (P = .02). Of the patients who started and discontinued treatment, none had active AUD. Other baseline demographics, clinical characteristics, and treatment characteristics were similar between the groups. No patient demographic characteristics were significantly associated with HCV treatment discontinuation. We did not observe any major health disparities in initiation or discontinuation by sex, race, ethnicity, or geography. Eleven patients (37%) could not be contacted, which was the most common reason veterans did not initiate treatment (Table 2). Of the 9 patients that did not complete SVR12, 5 patients could not be contacted for follow-up, which was the most common reason veterans discontinued treatment.
Discussion
This study highlights the experience of treating patients with HCV with an exclusively telehealth model in the months following implementation of stay-at-home orders from March 19, 2020, to September 30, 2020, during the COVID-19 pandemic at VAGLAHS. We were able to successfully complete treatment for 34 veterans (47%) and achieved SVR rates of 88%. We found that AUD was associated with unsuccessful treatment initiation. There were no statistically significant patient characteristic findings for treatment discontinuation in our study (Table 3). Unhealthy alcohol use and AUD are highly prevalent among veterans with HCV and prior to the pandemic, studies have demonstrated AUD as a barrier to HCV treatment.7
Since worse hepatic outcomes have been observed in veterans with HCV and AUD and increased harmful patterns of drinking occurred during the pandemic, a renewed interest in treating AUD in these veterans during the era of telehealth is critical.8 While we were unable to ascertain whether alcohol misuse in our cohort increased during the pandemic or whether changes in drinking patterns affected HCV treatment outcomes before and after the pandemic, such an association should reinforce the need for clinicians to expeditiously link patients to substance use care. It should also stimulate further considerations of addressing social determinants of health not captured in this study.
During the pandemic, veterans with posttraumatic stress disorder, a history of serving in combat roles, and experiencing related financial stressors had higher risk of AUD.9,10 For veterans with AUD who initiated HCV treatment, none discontinued their therapy, aligning with other studies showed that patients with AUD were able to achieve high rates of SVR and emphasizing that veterans should be treated irrespective of an AUD diagnosis.11 However, more innovative engagement initiatives for veterans with AUD should be explored as we continue to adapt more telehealth-based care for HCV direct-acting antiviral treatments. A more in-depth understanding of how alcohol use relates to treatment noninitiation is warranted, as this may stem from behavioral patterns that could not be captured in the present study.
The inability to reach veterans by telephone was a major reason for noninitiation and discontinuation of treatment. While the expansion of telehealth services has been noted across the VHA, there is still room for improving methods of engaging veterans in health care postpandemic.12 Prior studies in veteran populations that were successful in increasing uptake of HCV treatment have employed telehealth strategies that further emphasizes its integral role in HCV elimination.13 Although our study did not show mental health comorbidities and housing status as statistically significant, it is important to note that 20% of patients referred for HCV treatment had an incomplete evaluation which can lead to potentially unobserved indicators not captured by our study such as quality of linkage to care. It is imperative to stress the best practices for HCV initiation by integrating a multidisciplinary team to address patients’ psychosocial comorbidities.14 Finally, we did not observe any major disparities in treating veterans with HCV during the pandemic. This observation is reassuring and consistent with other VHA data given the heightened recognition of health disparities seen in health care sectors across the country, especially evident during the COVID-19 pandemic and the current era of increased adaptation of telehealth.
Limitations
Limitations to this study include its retrospective nature, small sample size, and short study time frame as a proportion of veterans have yet to complete HCV treatment which can potentially explain how larger studies were able to find other statistically significant patient-related factors impacting treatment initiation compared to ours. Given the lack of universal standardized diagnostic criterion of AUD, this can limit how our study can be compared to others in similar populations. Additionally, this study was conducted at a single facility with a predominantly older male veteran population, which may not be generalizable to other populations.
Conclusions
Treating HCV during the COVID-19 pandemic with telehealth and mail-out medications was feasible and led to high SVR rates, but unhealthy alcohol use and an inability to contact veterans were predominant barriers to success. Future quality improvement efforts should focus on addressing these barriers and exploring the relationship between alcohol use and HCV treatment initiation.
Although 2.4 million adults in the United States have been diagnosed with hepatitis C virus (HCV) infection, it remains underdiagnosed and undertreated, particularly among difficult to reach populations, such as persons who inject drugs, marginally housed individuals, correctional populations, and pregnant women.1 Though the US Preventive Services Task Force (USPSTF) broadened HCV screening recommendations to include individuals aged 18 to 79 years, rates of new HCV prescriptions sharply declined during the COVID-19 pandemic.2,3
During the pandemic, many health care systems adopted virtual health care modalities. Within the Veteran Health Administration (VHA), there was an 11-fold increase in virtual encounters. However, veterans aged > 45 years, homeless, and had other insurance were less likely to utilize virtual care.4,5 As health care delivery continues to evolve, health systems must adapt and test innovative models for the treatment of HCV.
There is limited understanding of HCV treatments when exclusively conducted virtually. The aim of this study was to evaluate the effects of the HCV treatment program at the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) during the early phase of the COVID-19 pandemic, when telehealth modalities and mail-order prescriptions were used for HCV diagnosis and treatment. The secondary aim of this study was to understand patient factors associated with treatment initiation and discontinuation for patients using telehealth.
Methods
The VHA is the largest provider of HCV care in the US.6 At VAGLAHS, veterans with HCV are referred for evaluation to a viral hepatitis clinic staffed by gastroenterologists and infectious disease specialists. Veterans with detectable HCV on an HCV RNA test have an additional workup ordered if necessary and are referred to an HCV-specialist pharmacist or physician’s assistant to start treatment. In March 2020, all HCV evaluations and treatment initiation in the viral hepatitis clinic started being conducted exclusively via telehealth. This was the primary modality of HCV evaluations and treatment initiation until COVID-19 restrictions were lifted to permit in-person evaluations. Prescriptions were delivered by mail to patients following treatment initiation appointments.
We retrospectively reviewed electronic health records of veterans referred to start treatment March 1, 2020, through September 30, 2020. The endpoint of the reviewed records was set because during this specific time frame, VAGLAHS used an exclusively telehealth-based model for HCV evaluation and treatment. Patients were followed until June 15, 2021. Due to evolving COVID-19 restrictions at the time, and despite requests received, treatment initiations by the pharmacy team were suspended in March 2020 but HCV treatments resumed in May. Data collected included baseline demographics (age, sex, race, ethnicity, housing status, distance to VAGLAHS), comorbidities (cirrhosis, hepatitis B virus coinfection, HIV coinfection), psychiatric conditions (mood or psychotic disorder, alcohol use disorder [AUD], opioid use disorder), and treatment characteristics (HCV genotype, HCV treatment regimen, baseline viral load). Distance from the patient’s home to VAGLAHS was calculated using CDXZipStream software. Comorbidities and psychiatric conditions were identified by the presence of the appropriate diagnosis via International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes in the health record and confirmed by review of clinician notes. Active AUD was defined as: (1) the presence of AUD diagnosis code; (2) AUD Identification Test-Consumption (AUDIT-C) score of high or severe risk based on established cutoffs; and (3) active alcohol use noted in the electronic health record. All patients had an AUDIT-C score completed within 1 year of initiating treatment. Opioid use disorder was defined by the presence of diagnostic codes for opioid dependence or opioid abuse.
The reasons for treatment noninitiation and discontinuation were each captured. We calculated descriptive statistics to analyze the frequency distributions of all variables. Independent t tests were used to analyze continuous data and Pearson χ2 test was used to analyze categorical data. Statistical significance was set as P < .05.
Results
From March 1, 2020, through September 30, 2020, 73 veterans were referred to the HCV clinical pharmacist for treatment (Figure). Forty-three veterans (59%) initiated HCV treatment and 34 (79%) completed the full treatment course (Table 1). Twenty-five patients (65%) had their sustained virologic response at 12 weeks (SVR12) testing and 22 patients achieved SVR12 (88%; 30% of total sample). One patient did not achieve SVR, and 2 patients died (variceal hemorrhage and progression of cerebral amyloidosis/function decline) before the completion of laboratory testing. From March 2020 to May 2020, HCV treatments requests were paused as new COVID-19 policies were being introduced; 33 patients were referred during this time and 21 initiated treatment.
Veterans that did not start HCV treatment had a significantly higher rate of active AUD when compared with those that initiated treatment: 30% vs 9% (P = .02). Of the patients who started and discontinued treatment, none had active AUD. Other baseline demographics, clinical characteristics, and treatment characteristics were similar between the groups. No patient demographic characteristics were significantly associated with HCV treatment discontinuation. We did not observe any major health disparities in initiation or discontinuation by sex, race, ethnicity, or geography. Eleven patients (37%) could not be contacted, which was the most common reason veterans did not initiate treatment (Table 2). Of the 9 patients that did not complete SVR12, 5 patients could not be contacted for follow-up, which was the most common reason veterans discontinued treatment.
Discussion
This study highlights the experience of treating patients with HCV with an exclusively telehealth model in the months following implementation of stay-at-home orders from March 19, 2020, to September 30, 2020, during the COVID-19 pandemic at VAGLAHS. We were able to successfully complete treatment for 34 veterans (47%) and achieved SVR rates of 88%. We found that AUD was associated with unsuccessful treatment initiation. There were no statistically significant patient characteristic findings for treatment discontinuation in our study (Table 3). Unhealthy alcohol use and AUD are highly prevalent among veterans with HCV and prior to the pandemic, studies have demonstrated AUD as a barrier to HCV treatment.7
Since worse hepatic outcomes have been observed in veterans with HCV and AUD and increased harmful patterns of drinking occurred during the pandemic, a renewed interest in treating AUD in these veterans during the era of telehealth is critical.8 While we were unable to ascertain whether alcohol misuse in our cohort increased during the pandemic or whether changes in drinking patterns affected HCV treatment outcomes before and after the pandemic, such an association should reinforce the need for clinicians to expeditiously link patients to substance use care. It should also stimulate further considerations of addressing social determinants of health not captured in this study.
During the pandemic, veterans with posttraumatic stress disorder, a history of serving in combat roles, and experiencing related financial stressors had higher risk of AUD.9,10 For veterans with AUD who initiated HCV treatment, none discontinued their therapy, aligning with other studies showed that patients with AUD were able to achieve high rates of SVR and emphasizing that veterans should be treated irrespective of an AUD diagnosis.11 However, more innovative engagement initiatives for veterans with AUD should be explored as we continue to adapt more telehealth-based care for HCV direct-acting antiviral treatments. A more in-depth understanding of how alcohol use relates to treatment noninitiation is warranted, as this may stem from behavioral patterns that could not be captured in the present study.
The inability to reach veterans by telephone was a major reason for noninitiation and discontinuation of treatment. While the expansion of telehealth services has been noted across the VHA, there is still room for improving methods of engaging veterans in health care postpandemic.12 Prior studies in veteran populations that were successful in increasing uptake of HCV treatment have employed telehealth strategies that further emphasizes its integral role in HCV elimination.13 Although our study did not show mental health comorbidities and housing status as statistically significant, it is important to note that 20% of patients referred for HCV treatment had an incomplete evaluation which can lead to potentially unobserved indicators not captured by our study such as quality of linkage to care. It is imperative to stress the best practices for HCV initiation by integrating a multidisciplinary team to address patients’ psychosocial comorbidities.14 Finally, we did not observe any major disparities in treating veterans with HCV during the pandemic. This observation is reassuring and consistent with other VHA data given the heightened recognition of health disparities seen in health care sectors across the country, especially evident during the COVID-19 pandemic and the current era of increased adaptation of telehealth.
Limitations
Limitations to this study include its retrospective nature, small sample size, and short study time frame as a proportion of veterans have yet to complete HCV treatment which can potentially explain how larger studies were able to find other statistically significant patient-related factors impacting treatment initiation compared to ours. Given the lack of universal standardized diagnostic criterion of AUD, this can limit how our study can be compared to others in similar populations. Additionally, this study was conducted at a single facility with a predominantly older male veteran population, which may not be generalizable to other populations.
Conclusions
Treating HCV during the COVID-19 pandemic with telehealth and mail-out medications was feasible and led to high SVR rates, but unhealthy alcohol use and an inability to contact veterans were predominant barriers to success. Future quality improvement efforts should focus on addressing these barriers and exploring the relationship between alcohol use and HCV treatment initiation.
1. Patel AA, Bui A, Prohl E, et al. Innovations in Hepatitis C Screening and Treatment. Hepatol Commun. 2020;5(3):371-386. Published 2020 Dec 7. doi:10.1002/hep4.1646
2. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(10):970-975. doi:10.1001/jama.2020.1123
3. Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med. 2021;61(3):369-376. doi:10.1016/j.amepre.2021.03.011
4. Rosen CS, Morland LA, Glassman LH, et al. Virtual mental health care in the Veterans Health Administration’s immediate response to coronavirus disease-19. Am Psychol. 2021;76(1):26-38. doi:10.1037/amp0000751
5. Balut MD, Wyte-Lake T, Steers WN, et al. Expansion of telemedicine during COVID-19 at a VA specialty clinic. Healthc (Amst). 2022;10(1):100599. doi:10.1016/j.hjdsi.2021.100599
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
7. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017;46(10):992-1000. doi:10.1111/apt.14328
8. Alavi M, Janjua NZ, Chong M, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68(3):393-401. doi:10.1016/j.jhep.2017.10.019
9. Pedersen ER, Davis JP, Fitzke RE, Lee DS, Saba S. American Veterans in the Era of COVID-19: Reactions to the Pandemic, Posttraumatic Stress Disorder, and Substance Use Behaviors. Int J Ment Health Addict. 2023;21(2):767-782. doi:10.1007/s11469-021-00620-0
10. Na PJ, Norman SB, Nichter B, et al. Prevalence, risk and protective factors of alcohol use disorder during the COVID-19 pandemic in U.S. military veterans. Drug Alcohol Depend. 2021;225:108818. doi:10.1016/j.drugalcdep.2021.108818
11. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-109. doi:10.1016/j.drugalcdep.2016.10.021
12. Baum A, Kaboli PJ, Schwartz MD. Reduced In-Person and Increased Telehealth Outpatient Visits During the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
13. Fleming BS, Ifeachor AP, Andres AM, et al. Improving Veteran Access to Treatment for Hepatitis C Virus Infection: Addressing social issues and treatment barriers significantly increases access to HCV care, and many veterans successfully start therapy with the help of additional support staff. Fed Pract. 2017;34(Suppl 4):S24-S28.
14. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
1. Patel AA, Bui A, Prohl E, et al. Innovations in Hepatitis C Screening and Treatment. Hepatol Commun. 2020;5(3):371-386. Published 2020 Dec 7. doi:10.1002/hep4.1646
2. US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for Hepatitis C Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2020;323(10):970-975. doi:10.1001/jama.2020.1123
3. Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med. 2021;61(3):369-376. doi:10.1016/j.amepre.2021.03.011
4. Rosen CS, Morland LA, Glassman LH, et al. Virtual mental health care in the Veterans Health Administration’s immediate response to coronavirus disease-19. Am Psychol. 2021;76(1):26-38. doi:10.1037/amp0000751
5. Balut MD, Wyte-Lake T, Steers WN, et al. Expansion of telemedicine during COVID-19 at a VA specialty clinic. Healthc (Amst). 2022;10(1):100599. doi:10.1016/j.hjdsi.2021.100599
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
7. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct-acting anti-viral agents. Aliment Pharmacol Ther. 2017;46(10):992-1000. doi:10.1111/apt.14328
8. Alavi M, Janjua NZ, Chong M, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: An international study. J Hepatol. 2018;68(3):393-401. doi:10.1016/j.jhep.2017.10.019
9. Pedersen ER, Davis JP, Fitzke RE, Lee DS, Saba S. American Veterans in the Era of COVID-19: Reactions to the Pandemic, Posttraumatic Stress Disorder, and Substance Use Behaviors. Int J Ment Health Addict. 2023;21(2):767-782. doi:10.1007/s11469-021-00620-0
10. Na PJ, Norman SB, Nichter B, et al. Prevalence, risk and protective factors of alcohol use disorder during the COVID-19 pandemic in U.S. military veterans. Drug Alcohol Depend. 2021;225:108818. doi:10.1016/j.drugalcdep.2021.108818
11. Tsui JI, Williams EC, Green PK, Berry K, Su F, Ioannou GN. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101-109. doi:10.1016/j.drugalcdep.2016.10.021
12. Baum A, Kaboli PJ, Schwartz MD. Reduced In-Person and Increased Telehealth Outpatient Visits During the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
13. Fleming BS, Ifeachor AP, Andres AM, et al. Improving Veteran Access to Treatment for Hepatitis C Virus Infection: Addressing social issues and treatment barriers significantly increases access to HCV care, and many veterans successfully start therapy with the help of additional support staff. Fed Pract. 2017;34(Suppl 4):S24-S28.
14. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing Hepatitis C Virus Infection: Best Practices From the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504. doi:10.7326/M17-1073
Clinical Implications of a Formulary Conversion From Budesonide/formoterol to Fluticasone/salmeterol at a VA Medical Center
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder associated with slowly progressive systemic inflammation. It includes emphysema, chronic bronchitis, and small airway disease. Patients with COPD have an incomplete reversibility of airway obstruction, the key differentiating factor between it and asthma.1
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend a combination inhaler consisting of a long-acting β-2 agonist (LABA) and inhaled corticosteroid (ICS) for patients with a history of COPD exacerbations.2 Blood eosinophil count is another marker for the initiation of an ICS in patients with COPD. According to the 2023 GOLD Report, ICS therapy is appropriate for patients who experience frequent exacerbations and have a blood eosinophil count > 100 cells/μL, while on maximum tolerated inhaler therapy.3 A 2019 meta-analysis found an overall reduction in the risk of exacerbations in patients with blood eosinophil counts ≥ 100 cells/µL after initiating an ICS.4
Common ICS-LABA inhalers include the combination of budesonide/formoterol as well as fluticasone/salmeterol. Though these combinations are within the same therapeutic class, they have different delivery systems: budesonide/formoterol is a metered dose inhaler, while fluticasone/salmeterol is a dry powder inhaler. The PATHOS study compared the exacerbation rates for the 2 inhalers in primary care patients with COPD. Patients treated long-term with the budesonide/formoterol inhaler were significantly less likely to experience a COPD exacerbation than those treated with the fluticasone/salmeterol inhaler.5
In 2021, The Veteran Health Administration transitioned patients from budesonide/formoterol inhalers to fluticasone/salmeterol inhalers through a formulary conversion. The purpose of this study was to examine the outcomes for patients undergoing the transition.
Methods
A retrospective chart review was conducted on patients at the Hershel “Woody” Williams Veterans Affairs Medical Center in Huntington, West Virginia, with COPD and prescriptions for both budesonide/formoterol and fluticasone/salmeterol inhalers between February 1, 2021, and May 30, 2022. In 2018, the prevalence of COPD in West Virginia was 13.9%, highest in the US.6 Data was obtained through the US Department of Veteran Affairs (VA) Corporate Data Warehouse and stored on a VA Informatics and Computing Infrastructure server. Patients were randomly selected from this cohort and included if they were aged 18 to 89 years, prescribed both inhalers, and had a confirmed COPD diagnosis. Patients were excluded if they also had an asthma diagnosis, if they had an interstitial lung disease, or any tracheostomy tubes. The date of transition from a budesonide/formoterol inhaler to a fluticasone/salmeterol inhaler was collected to establish a timeline of 6 months before and 6 months after the transition.
The primary endpoint was to assess clinical outcomes such as the number of COPD exacerbations and hospitalizations within 6 months of the transition for patients affected by the formulary conversion. Secondary outcomes included the incidence of adverse effects (AEs), treatment failure, tobacco use, and systemic corticosteroid/antimicrobial utilization.
Statistical analyses were performed using STATA v.15. Numerical data was analyzed using a Wilcoxon signed rank test. Categorical data was analyzed by a logistic regression analysis.
Results
Of 1497 included patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers, 165 were randomly selected and 100 patients were included in this analysis. Of the 100 patients, 99 were male with a mean (SEM) age of 71 (0.69) years (range, 54-87) (Table).
The transition from budesonide/formoterol to fluticasone/salmeterol inhalers did not have a statistically significant impact on exacerbations (P = .56). Thirty patients had ≥ 1 exacerbation: 12 had an exacerbation before the transition, 10 had an exacerbation after the transition, and 8 had exacerbations before and after the transition. In the 6 months prior to the transition while on a budesonide/formoterol inhaler, there were 24 exacerbations among 20 patients. Five patients had > 1 exacerbation, accounting for 11 of the 24 exacerbations. There were 29 exacerbations among 19 patients while on a fluticasone/salmeterol inhaler in the 6 months after the transition. Four of these patients had > 1 exacerbation, accounting for 14 of 29 exacerbations (Figure).
Secondary endpoints showed 3 patients experienced an AE related to fluticasone/salmeterol, including thrush, coughing and throat irritation, and dyspnea. Eighteen fluticasone/salmeterol therapeutic failures were indicated by related prior authorization medication requests in the electronic health record. Twelve of 18 patients experienced no difference in exacerbations before vs after the transition to budesonide/formoterol. Twenty-three patients transitioned from fluticasone/salmeterol to a different ICS-LABA therapy; 20 of those 23 patients transitioned back to a budesonide/formoterol inhaler.
There were 48 documented active tobacco users in the study. There was no statistically significant correlation (P = .52) when comparing tobacco use at time of conversion and exacerbation frequency, although the coefficient showed a negative correlation of -0.387. In the 6 months prior to the transition, there were 17 prescriptions for systemic corticosteroids and 24 for antibiotics to treat COPD exacerbations. Following the transition, there were only 12 prescriptions for systemic corticosteroids and 23 for antibiotics. Fifty-two patients had an active prescription for a fluticasone/salmeterol inhaler at the time of the data review (November to December 2022); of the 48 patients who did not, 10 were no longer active due to patient death between the study period and data retrieval.
Discussion
Patients who transitioned from budesonide/formoterol to fluticasone/salmeterol inhalers did not show a significant difference in clinical COPD outcomes. While the total number of exacerbations increased after switching to the fluticasone/salmeterol inhaler, fewer patients had exacerbations during fluticasone/salmeterol therapy when compared with budesonide/fluticasone therapy. The number of patients receiving systemic corticosteroids and antibiotics to treat exacerbations before and after the transition were similar.
The frequency of treatment failures and AEs to the fluticasone/salmeterol inhaler could be due to the change of the inhaler delivery systems. Budesonide/formoterol is a metered dose inhaler (MDI). It is equipped with a pressurized canister that allows a spacer to be used to maximize benefit. Spacers can assist in preventing oral candidiasis by reducing the amount of medication that touches the back of the throat. Spacers are an option for patients, but not all use them for their MDIs, which can result in a less effective administered dose. Fluticasone/salmeterol is a dry powder inhaler, which requires a deep, fast breath to maximize the benefit, and spacers cannot be used with them. MDIs have been shown to be responsible for a negative impact on climate change, which can be reduced by switching to a dry powder inhaler.7
Tobacco cessation is very important in limiting the progression of COPD. As shown with the negative coefficient correlation, not being an active tobacco user at the time of transition correlated (although not significantly) with less frequent exacerbations. When comparing this study to similar research, such as the PATHOS study, several differences are observed.5 The PATHOS study compared long term treatment (> 1 year) of budesonide/formoterol or fluticasone/salmeterol, a longer period than this study. It regarded similar outcomes for the definition of an exacerbation, such as antibiotic/steroid use or hospital admission. While the current study showed no significant difference between the 2 inhalers and their effect on exacerbations, the PATHOS study found that those treated with a budesonide/formoterol inhaler were less likely to experience COPD-related exacerbations than those treated with the fluticasone/salmeterol inhaler. The PATHOS study had a larger mainly Scandinavian sample (N = 5500). This population could exhibit baseline differences from a study of US veterans.5 A similar Canadian matched cohort study of 2262 patients compared the 2 inhalers to assess their relative effectiveness. It found that COPD exacerbations did not differ between the 2 groups, but the budesonide/formoterol group was significantly less likely to have an emergency department visit compared to the fluticasone salmeterol group.8 Like the PATHOS study, the Canadian study had a larger sample size and longer timeframe than did our study.
Limitations
There are various limitations to this study. It was a retrospective, single-center study and the patient population was relatively homogenous, with only 1 female and a mean age of 71 years. As a study conducted in a veteran population in West Virginia, the findings may not be representative of the general population with COPD, which includes more women and more racial diversity.9 The American Lung Association discusses how environmental exposures to hazardous conditions increase the risks of pulmonary diseases for veterans.10 It has been reported that the prevalence of COPD is higher among veterans compared to the general population, but it is not different in terms of disease manifestation.10
Another limitation is the short time frame. Clinical guidelines, including the GOLD Report, typically track the number of exacerbations for 1 year to escalate therapy.3 Six months was a relatively short time frame, and it is possible that more exacerbations may have occurred beyond the study time frame. Ten patients in the sample died between the end of the study period and data retrieval, which might have been caught by a longer study period. An additional limitation was the inability to measure adherence. As this was a formulary conversion, many patients had been mailed a 30- or 90-day prescription of the budesonide/formoterol inhaler when transitioned to the fluticasone/salmeterol inhaler. There was no way to accurately determine when the patient made the switch to the fluticasone/salmeterol inhaler. This study also had a small sample group (a pre-post analysis of the same group), a limitation when evaluating the impact of this formulary change on a small percentage of the population transitioned.
This formulary conversion occurred during the COVID-19 pandemic, and some exacerbations could have been the result of a misdiagnosed COVID-19 infection. Respiratory infections, including COVID-19, are common causes of exacerbations. It is also possible that some patients elected not to receive medical care for symptoms of an exacerbation during the pandemic.11
Conclusions
Switching from the budesonide/formoterol inhaler to the fluticasone/salmeterol inhaler through formulary conversion did not have a significant impact on the clinical outcomes in patients with COPD. This study found that although the inhalers contain different active ingredients, products within the same therapeutic class yielded nonsignificant changes. When conducting formulary conversions, intolerances and treatment failures should be expected when switching from different inhaler delivery systems. This study further justifies the ability to be cost effective by making formulary conversions within the same therapeutic class within a veterans population.
Acknowledgments
The authors would like to acknowledge James Brown, PharmD, PhD.
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
1. US Department of Veterans Affairs. VA/DOD Clinical Practice Guideline. Management of Outpatient Chronic Obstructive Pulmonary Disease. 2021. Accessed January 22, 2024. https://www.healthquality.va.gov/guidelines/cd/copd/
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD Report. 2022. Accessed January 22, 2024. https://goldcopd.org/2022-gold-reports/
3. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis management, and prevention of chronic obstructive pulmonary disease 2023 report. Accessed January 26, 2024. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
4. Oshagbemi OA, Odiba JO, Daniel A, Yunusa I. Absolute blood eosinophil counts to guide inhaled corticosteroids therapy among patients with COPD: systematic review and meta-analysis. Curr Drug Targets. 2019;20(16):1670-1679. doi:10.2174/1389450120666190808141625
5. Larsson K, Janson C, Lisspers K, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584-594. doi:10.1111/joim.12067
6. West Virginia Department of Health and Human Resources, Division of Health Promotion and Chronic Disease. Statistics about the population of West Virginia. 2018. Accessed January 22, 2024. https://dhhr.wv.gov/hpcd/data_reports/ Pages/Fast-Facts.aspx
7. Fidler L, Green S, Wintemute K. Pressurized metered-dose inhalers and their impact on climate change. CMAJ. 2022;194(12):E460. doi:10.1503/cmaj.211747
8. Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): Effect on COPD-related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clin Ther. 2010;32(7):1320-1328. doi:10.1016/j.clinthera.2010.06.022
9. Wheaton AG, Cunningham TJ, Ford ES, Croft JB; Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease — United States, 2013. MMWR Morb Mortal Wkly Rep. 2015:64(11):289-295.
10. Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
11. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4