User login
Proton pump inhibitors linked to increased dementia risk
TOPLINE:
and was highest among those diagnosed before age 70 years regardless of when PPI treatment was initiated.
METHODOLOGY:
- Researchers used four Danish registries to collect data on dementia diagnoses and prescription PPI use among 1,983,785 individuals aged 60-75 years between 2000 and 2018.
- The median follow-up time was 10.3 years.
TAKEAWAY:
- There were 99,384 (5.0%) cases of all-cause dementia during follow-up, with a median age of diagnosis of 79 years.
- Twenty-one-point-two percent of dementia cases and 18.9% of controls reported a history of PPI use.
- Risk for all-cause dementia before age 90 years was 36% higher with PPI use in people aged 60-69 years at baseline (adjusted incidence rate ratio, 1.36; 95% confidence interval, 1.29-1.43) and 6% higher in those who were age 80-89 years at baseline (aIRR, 1.06; 95% CI, 1.03-1.09).
- Investigators found significant increased dementia risk before age 90 years with PPI use regardless of when PPI treatment began and found no link between PPI use and dementia diagnoses after age 90 years.
IN PRACTICE:
“The association between PPI use and dementia was unambiguously largest among the youngest cases of dementia, potentially suggestive of a critical window of exposure where midlife PPI use affects dementia risk to a larger degree compared to late-life use,” the authors wrote. “Further, the finding could signify a declining impact of individual risk factors with advancing age owing to lengthy ongoing neuropathological processes.”
SOURCE:
Lead author of the study was Nelsan Pourhadi, MD, Danish Dementia Research Centre, department of neurology, Copenhagen University Hospital–Rigshospitalet. It was published online in Alzheimer’s and Dementia.
LIMITATIONS:
The study did not include data on PPI prescriptions before 1995, over-the-counter PPI use, and in-hospital intravenous use of PPI during the study period.
DISCLOSURES:
The study was funded by the Danish Ministry of Health. The authors reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
and was highest among those diagnosed before age 70 years regardless of when PPI treatment was initiated.
METHODOLOGY:
- Researchers used four Danish registries to collect data on dementia diagnoses and prescription PPI use among 1,983,785 individuals aged 60-75 years between 2000 and 2018.
- The median follow-up time was 10.3 years.
TAKEAWAY:
- There were 99,384 (5.0%) cases of all-cause dementia during follow-up, with a median age of diagnosis of 79 years.
- Twenty-one-point-two percent of dementia cases and 18.9% of controls reported a history of PPI use.
- Risk for all-cause dementia before age 90 years was 36% higher with PPI use in people aged 60-69 years at baseline (adjusted incidence rate ratio, 1.36; 95% confidence interval, 1.29-1.43) and 6% higher in those who were age 80-89 years at baseline (aIRR, 1.06; 95% CI, 1.03-1.09).
- Investigators found significant increased dementia risk before age 90 years with PPI use regardless of when PPI treatment began and found no link between PPI use and dementia diagnoses after age 90 years.
IN PRACTICE:
“The association between PPI use and dementia was unambiguously largest among the youngest cases of dementia, potentially suggestive of a critical window of exposure where midlife PPI use affects dementia risk to a larger degree compared to late-life use,” the authors wrote. “Further, the finding could signify a declining impact of individual risk factors with advancing age owing to lengthy ongoing neuropathological processes.”
SOURCE:
Lead author of the study was Nelsan Pourhadi, MD, Danish Dementia Research Centre, department of neurology, Copenhagen University Hospital–Rigshospitalet. It was published online in Alzheimer’s and Dementia.
LIMITATIONS:
The study did not include data on PPI prescriptions before 1995, over-the-counter PPI use, and in-hospital intravenous use of PPI during the study period.
DISCLOSURES:
The study was funded by the Danish Ministry of Health. The authors reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
and was highest among those diagnosed before age 70 years regardless of when PPI treatment was initiated.
METHODOLOGY:
- Researchers used four Danish registries to collect data on dementia diagnoses and prescription PPI use among 1,983,785 individuals aged 60-75 years between 2000 and 2018.
- The median follow-up time was 10.3 years.
TAKEAWAY:
- There were 99,384 (5.0%) cases of all-cause dementia during follow-up, with a median age of diagnosis of 79 years.
- Twenty-one-point-two percent of dementia cases and 18.9% of controls reported a history of PPI use.
- Risk for all-cause dementia before age 90 years was 36% higher with PPI use in people aged 60-69 years at baseline (adjusted incidence rate ratio, 1.36; 95% confidence interval, 1.29-1.43) and 6% higher in those who were age 80-89 years at baseline (aIRR, 1.06; 95% CI, 1.03-1.09).
- Investigators found significant increased dementia risk before age 90 years with PPI use regardless of when PPI treatment began and found no link between PPI use and dementia diagnoses after age 90 years.
IN PRACTICE:
“The association between PPI use and dementia was unambiguously largest among the youngest cases of dementia, potentially suggestive of a critical window of exposure where midlife PPI use affects dementia risk to a larger degree compared to late-life use,” the authors wrote. “Further, the finding could signify a declining impact of individual risk factors with advancing age owing to lengthy ongoing neuropathological processes.”
SOURCE:
Lead author of the study was Nelsan Pourhadi, MD, Danish Dementia Research Centre, department of neurology, Copenhagen University Hospital–Rigshospitalet. It was published online in Alzheimer’s and Dementia.
LIMITATIONS:
The study did not include data on PPI prescriptions before 1995, over-the-counter PPI use, and in-hospital intravenous use of PPI during the study period.
DISCLOSURES:
The study was funded by the Danish Ministry of Health. The authors reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
Tirzepatide with insulin glargine improves type 2 diabetes
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
AT EASD 2023
FDA approves first tocilizumab biosimilar
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
The Food and Drug Administration has approved the biosimilar tocilizumab-bavi (Tofidence), Biogen, the drug’s manufacturer, announced on Sept. 29.
It is the first tocilizumab biosimilar approved by the FDA. The reference product, Actemra (Genentech), was first approved by the agency in 2010.
“The approval of Tofidence in the U.S. marks another positive step toward helping more people with chronic autoimmune conditions gain access to leading therapies,” Ian Henshaw, global head of biosimilars at Biogen, said in a statement. “With the increasing numbers of approved biosimilars, we expect increased savings and sustainability for health care systems and an increase in physician choice and patient access to biologics.”
Biogen’s pricing for tocilizumab-bavi will be available closer to the product’s launch date, which has yet to be determined, a company spokesman said. The U.S. average monthly cost of Actemra for rheumatoid arthritis, administered intravenously, is $2,134-$4,268 depending on dosage, according to a Genentech spokesperson.
Tocilizumab-bavi is an intravenous formulation (20 mg/mL) indicated for treatment of moderately to severely active RA, polyarticular juvenile idiopathic arthritis (PJIA), and systemic juvenile idiopathic arthritis (SJIA). The medication is administered every 4 weeks in RA and PJIA and every 8 weeks in SJIA as a single intravenous drip infusion over 1 hour.
The European Commission approved its first tocilizumab biosimilar, Tyenne (Fresenius Kabi), earlier in 2023 in both subcutaneous and intravenous formulations. Biogen did not comment on whether the company is working on a subcutaneous formulation for tocilizumab-bavi.
A version of this article appeared on Medscape.com.
Impact of Liraglutide to Semaglutide Conversion on Glycemic Control and Cost Savings at a Veterans Affairs Medical Center
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
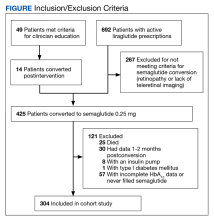
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
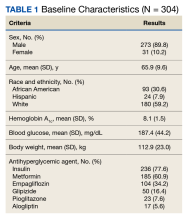
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
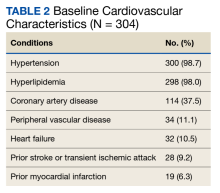
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
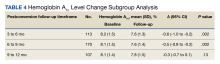
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
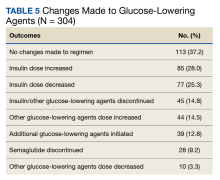
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
Semaglutide and liraglutide are glucagon-like peptide 1 receptor agonists (GLP-1 RAs) that are approved by the US Food and Drug Administration as subcutaneous injections for patients with type 2 diabetes mellitus (T2DM). Both are recommended by the American Diabetes Association (ADA) as first-line options for patients with concomitant atherosclerotic cardiovascular (CV) disease and exert therapeutic effect via incretin-like mechanisms.1 These agents lower blood glucose levels by stimulating insulin release, increasing the body’s sensitivity to insulin, and inhibiting inappropriate glucagon secretion.2,3 They also slow gastric emptying, resulting in decreased appetite and potential weight loss.4
The SUSTAIN (1-7) trials concluded that semaglutide presented an equivalent safety profile and greater efficacy compared with other GLP-1 RAs, including exenatide and dulaglutide.2 The SUSTAIN-10 open-label, head-to-head trial evaluating 1 mg semaglutide once weekly vs 1.2 mg liraglutide daily concluded that semaglutide was superior in hemoglobin A1c (HbA1c) and body weight reduction compared with liraglutide, with slightly increased gastrointestinal (GI) adverse effects (AEs).5 Similar to the LEADER trial assessing liraglutide, SUSTAIN-6 evaluated semaglutide in patients at increased CV risk and found that compared with placebo, semaglutide decreased rates of serious CV events, such as CV death, myocardial infarction, and stroke and were similar to the CV outcomes in the LEADER trial.2,6 Although initial results of the SUSTAIN-6 trial were thought to be nearly equivalent to the LEADER trial, analyses later published comparing both trials noted that semaglutide had more potent HbA1c lowering and weight loss benefit when compared with liraglutide.2,6 The cardioprotective outcomes of SUSTAIN-6 qualified semaglutide for inclusion in the current ADA Standards of Medical Care recommendations for CV risk reduction.6,7 However, despite the CV safety profile and efficacy associated with semaglutide, the SUSTAIN-6 trial noted an increased risk of diabetic retinopathy (DR) complications in 50 of 1648 patients (3%) treated with semaglutide compared with 29 of 1649 (1.8%) who received placebo (P = .02; hazard ratio, 1.76; 95% CI, 1.11-2.78).6 Of the 79 total patients who experienced retinopathy complications, 66 had retinopathy at baseline (42 of 50 [84%]) in the semaglutide group; 24 of 29 [83%] in the placebo group).6 Worsening of DR became one of the most notable AEs of semaglutide evaluated in clinical trials. This further deemed the effect as a warning in the semaglutide package insert to assist clinicians with treatment decisions.
As part of a US Department of Veterans Affairs (VA) National Lost Opportunity Cost Savings Initiative, which encompasses administrative efforts to promote more cost-effective yet safe and efficacious therapy options for veterans, the Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas, converted a portion of patients with T2DM established on liraglutide to semaglutide. The 30-day supply cost of the 2-pack liraglutide 6 mg/mL (3 mL) injection pens for the MEDVAMC was $197.64. The 30-day supply cost for the singular multidose semaglutide 0.5 mg/0.375 mL (1.5 mL) injection pen was $115.15. Cost savings for the MEDVAMC facility were initially estimated to reach $642,522.
The subset of patients converted had to have undergone teleretinal imaging and not have a diagnosis of nonproliferative DR (NPDR), proliferative DR (PDR), or PDR with or without
In the fall of 2021, there was also a standing list of patients on liraglutide who were not converted due to a lack of teleretinal imaging. As a result, there was potential for a quality improvement (QI) intervention to target this patient population, which could result in further cost savings for MEDVAMC and improved glycemic control because of increased conversion from liraglutide to semaglutide. The purpose of this project was to perform a QI assessment on this subset of patients both initially converted from liraglutide to semaglutide, and those who were yet to be converted due to a lack of teleretinal imaging to determine the impact on glycemic control and cost savings.
Methods
This QI project was a single-center, prospective cohort study with a retrospective chart review of veterans with T2DM converted from liraglutide to semaglutide at the MEDVAMC. Patient data were collected from the Computerized Patient Record System (CPRS) between March 1, 2021, and November 30, 2021. An initial subset of patients was converted to semaglutide in March and April 2021. Patients initially excluded underwent a second chart review to determine whether they truly met exclusion criteria. Patients who did not have a definitive diagnosis of NPDR or PDR, those due for updated teleretinal imaging, as well as those with updated teleretinal imaging that excluded NPDR or PDR were targeted for clinician education interventions.
Following this intervention, a subset of patients with negative DR findings were converted from liraglutide to semaglutide. Primary care and endocrinology clinicians were notified that patients who met the criteria should be referred for teleretinal imaging if no updated results were present or that patients were eligible for semaglutide conversion based on negative findings. Both patients who were initially converted as well as those converted following education were included for data collection/analysis of glycemic control via HbA1c and blood glucose levels.
Cost savings were evaluated using outpatient pharmacy procurement pricing data. This project was approved by the MEDVAMC Quality Assurance and Regulatory Affairs Office.
Participants
Patients included in the study were adults aged ≥ 18 years with T2DM, converted from liraglutide 0.6 and 1.2 mg daily to semaglutide 0.25 mg weekly (titrated to 0.5 mg weekly after 4 weeks), and had an active prescription for semaglutide, with or without insulin or other oral antihyperglycemics. Patients with NPDR or PDR, type 1 DM, no HbA1c data, no filled semaglutide prescriptions, insulin pumps, and those without teleretinal imaging within the postintervention period or who died during the study period were excluded.
Patient baseline characteristics collected included demographic data, CV comorbidities, antihyperglycemic medications, and changes in insulin doses. Parameters analyzed at baseline and 3 to 12 months postconversion included body weight, HbA1c, and blood glucose levels.
Outcomes
The primary objectives of this QI project were to assess glycemic control (via changes in HbA1c levels) and cost savings following patient conversion from liraglutide to semaglutide. A second objective was to educate clinicians for referral of T2DM patients without teleretinal imaging in the past 2 years.
The purpose of the latter objective was to encourage conversion from liraglutide to semaglutide in the absence of DR. We predicted that 50% of patients with clinician education would be converted. Secondary objectives included assessing body weight differences, evaluating modifications in diabetes regimen, and documenting AEs. We predicted that glycemic control would either remain stable or improve with conversion to semaglutide.
Statistical Analysis
Patient demographic data were analyzed using descriptive statistics. Quantitative data (HbA1c, blood glucose, and body weight differences as continuous variables) were analyzed using a paired Student t test, and categorical variables were analyzed using the χ2 test.
Results
During the study period, 692 patients were identified with active liraglutide prescriptions (Figure). Of these, 49 patients who were initially excluded due to outdated teleretinal imaging or negative findings met the criteria for clinician education, and 14 of those 49 patients (28.6%) were converted from liraglutide to semaglutide. Thirty-three patients (67.3%) did not schedule teleretinal imaging or did not convert to semaglutide following negative teleretinal findings. Two patients (4.1%) either scheduled or proceeded with teleretinal imaging, without any further action from the clinician.
Including the 14 patients converted posteducational intervention, 425 patients were converted to semaglutide. Excluded from analysis were 121 patients: 57 for incomplete HbA1c data or no filled semaglutide prescription; 30 for HbA1c and weight data outside of the study timeframe; 25 died of causes unrelated to the project; 8 had insulin pumps; and 1 was diagnosed with late-onset type 1 DM. The final sample was 304 patients who underwent analysis.
Two hundred seventy-three patients (89.8%) were male, and 180 (59.2%) were White (Table 1). The mean (SD) age of patients was 65.9 (9.6) years, and 236 (77.6%) were established on insulin therapy (either basal, bolus, or a combination). The 3 most common antihyperglycemic agents (other than insulin) that patients used included 185 metformin (60.9%), 104 empagliflozin (34.2%), and 50 glipizide (16.4%) prescriptions.
Most patients had CV disease. Three hundred patients (98.7%) had comorbid hypertension, 298 (98.0%) had hyperlipidemia, and 114 (37.5%) had coronary artery disease (Table 2). Other diseases that patients were concomitantly diagnosed with included peripheral vascular disease, heart failure, history of stroke or transient ischemic attack, and history of myocardial infarction.
Documented AEs included 83 patients (27.3%) with hypoglycemia at any point within 3 to 12 months of conversion and 25 patients (8.2%) with mainly GI-related events, including nausea, vomiting, diarrhea, decreased appetite, and abdominal pain. Six patients (2.0%) had a new diagnosis of DR 3 to 12 months postconversion.
Glycemic Control and Weight Changes
At baseline, mean (SD) HbA1c was 8.1% (1.5), blood glucose was 187.4 (44.2) mg/dL, and body weight was 112.9 (23.0) kg (Table 3). In the timeframe evaluated (3 to 12 months postconversion), patients’ mean (SD) HbA1c was found to have significantly decreased to 7.6% (1.4) (P < .001; 95% CI, -0.7 to -0.3), blood glucose decreased to 172.6 (39.0) mg/dL (P < .001; 95% CI, -19.3 to -10.2), and body weight decreased to 105.2 (32.3) kg (P < .001; 95% CI, -10.6 to -4.8). All parameters evaluated were deemed statistically significant.
Further analyses evaluating specific changes in HbA1c observed postconversion are as follows: 199 patients (65.5%) experienced a decrease, 92 (30.3%) experienced an increase, and 13 (4.3%) experienced no change in their HbA1c.
As the timeframe was fairly broad to assess HbA1c changes, a prespecified subgroup analysis was conducted to determine specific changes in HbA1c within 3 to 6, 6 to 9, and 9 to 12 months postconversion (Table 4). At 3 to 6 months postconversion, patient mean (SD) HbA1c levels significantly decreased from 8.2% (1.5) at baseline to 7.6% (1.3) postconversion (P = .002; 95% CI, -1.0 to -0.2). At 6 to 9 months postconversion, the mean (SD) HbA1c significantly decreased from 8.1% (1.5) at baseline to 7.6% (1.4) postconversion (P = .002; 95% CI, -0.8 to -0.2).
Glucose-Lowering Agent Adjustments
One hundred thirteen patients (37.2%) required no changes to their antihyperglycemic regimen with the conversion, 85 (28.0%) required increased insulin doses, and 77 (25.3%) required decreased insulin doses (Table 5). Forty-five (14.8%) patients underwent discontinuation of either insulin or other antihyperglycemic agents; 44 (14.5%) had other antihyperglycemic agents dose increased, 39 (12.8%) required adding other glucose-lowering agents, 28 (9.2%) discontinued semaglutide, and 10 (3.3%) had other glucose-lowering medication doses decreased.
Cost Savings
Cost savings were evaluated using the MEDVAMC outpatient pharmacy procurement service. The total cost savings per patient per month was $82.49. For the 411 preclinician education patients converted to semaglutide, this resulted in a prospective annual cost savings of $406,840.68. An additional $13,858.32 was saved due to the intervention/clinician education for 14 patients converted to semaglutide. The total annual cost savings was $420,699.00.
Discussion
Overall, glycemic control significantly improved with veterans’ conversion from liraglutide to semaglutide. Not only were significant changes noted with HbA1c levels and weight, but consistencies were noted with mean HbA1c decrease and weight loss expected of GLP-1 RAs noted in clinical trials. The typical range for HbA1c changes expected is -1% to -2% and weight loss of 1 to 6 kg.4,7 Data from the LEAD-5 and SUSTAIN-4 trials, evaluating glycemic control in liraglutide and semaglutide, respectively, have noted comparable yet slightly more potent HbA1c decreases (-1.33% for liraglutide 1.8 mg daily vs -1.2% and -1.6% for semaglutide 0.5 mg and 1 mg weekly, respectively).8,9 However, more robust weight loss has been noted with semaglutide vs liraglutide (-4.62 kg for semaglutide 0.5 mg weekly and -6.33 kg for semaglutide 1 mg weekly vs -3.43 kg for liraglutide 1.8 mg daily).8,9 Results from the SUSTAIN-10 trial also noted mean changes in HbA1c of -1.7% for semaglutide 1 mg weekly vs -1.0% for liraglutide 1.2 mg daily; mean body weight differences were -5.8 kg for semaglutide and -1.9 kg for liraglutide at their respective doses.5 The mean weight loss noted with this QI project is consistent with prior trials of semaglutide.
Of note, 44 patients (14.5%) required the dosage increase of either one or multiple additional glucose-lowering agents at any time point within the 3- to 12-month period. Of those patients, 38 (86.4%) underwent further semaglutide dose titration to 1 mg weekly. Common reasons for a further dose increase to 1 mg weekly were an indication for more robust HbA1c lowering, a desire to decrease patients’ either basal or bolus insulin requirements, or a treatment goal of completely titrating patients off insulin.
It is uncertain why 30.3% of patients experienced an increase in HbA1c and 4.3% experienced no change. However, possibilities for the divergence in HbA1c outcomes in these subsets of patients may include suboptimal adherence to semaglutide or other antihyperglycemic agents as indicated by clinicians or nonadherence to dietary and lifestyle modifications.
Most patients (65.5%) experienced a decrease in HbA1c because of conversion to semaglutide, and
At the MEDVAMC, liraglutide is a nonformulary agent and semaglutide is now the formulary-preferred option. For patients with uncontrolled T2DM, if a GLP-1 RA is desired for therapy, clinicians are to place a prior authorization drug request (PADR) consultation for semaglutide for further evaluation and review of VA Criteria for Use (CFU) by clinical pharmacist practitioners. Liraglutide is the alternative option if patients do not meet the CFU for semaglutide (ie, have a diagnosis of DR among other exclusions). However, the semaglutide CFU was updated in April 2022 to exclude those specifically diagnosed with PDR, severe NPDR, and macular edema unless an ophthalmologist deems semaglutide acceptable. This indicates that patients with mild-to-moderate NPDR (who were originally excluded from this QI project) are now eligible to receive semaglutide. The incidence of new DR diagnoses (2%) observed in this study could indicate an unclear relationship between semaglutide and increased rates of DR; however, no definitive correlation can be established due to the retrospective nature of this project. The implications of the results of this QI project in relation to the updated CFU remain undetermined.
Due to the comparable improvements in HbA1c and more robust weight loss noted with semaglutide vs liraglutide, we deem it appropriate to select semaglutide as the more cost-efficient GLP-1 RA and formulary preferred option. The data of this QI project supports the overall safety and treatment utility of this option. Although significant cost savings were achieved (> $400,000), the long-term benefit of the liraglutide to semaglutide conversion remains unknown.
Strengths and Limitations
Strengths of this project include the large sample size, its setting in a large VA medical center, and the evaluation of multiple outcomes beyond HbA1c for assessment of glycemic control (ie, mean blood glucose, insulin titration, and dose adjustment of other glucose-lowering agents).
Limitations of this study include the retrospective chart review used for data collection, limited accuracy of objective data due to the COVID-19 pandemic, and inconsistencies with documentation in patients’ electronic health records. As a protective measure in the height of the pandemic between March 2021 and November 2021, the VA promoted using telephone and virtual-visit clinics to minimize exposure for patients with nonurgent follow-up needs. Patient hesitance to present to the clinic in person due to COVID-19 was also a significant factor in obtaining objective follow-up data. As a result, less accurate and timely baseline and postconversion weight and HbA1c data resulted, leading to our decision to extend the timeframe evaluated postconversion to 3 to 12 months. We also noted inconsistencies with documentation in CPRS. Unless veterans were closely followed by clinical pharmacist practitioners or endocrine consultation service clinicians, it was more difficult to follow and document trends of insulin titration to assess the impact of semaglutide conversion. The number of AEs, including hypoglycemia and GI intolerance, were also not consistently documented within the CPRS, and the frequency of AEs may be underestimated.
Another possible limitation regarding the interpretation of the results includes the portion of patients titrated up to semaglutide 1 mg weekly. As the focal point of this project was to review changes in glycemic control in the conversion to semaglutide 0.5 mg, this population of patients converted to 1 mg could potentially overestimate the HbA1c and weight changes described, as it is consistent with the SUSTAIN trials that show more robust decreases in those parameters described earlier.
Conclusions
A subset of patients with T2DM converted from liraglutide to semaglutide experienced significant changes in glycemic control and body weight. Significant differences were noted for a decreased HbA1c, decreased mean blood glucose, and weight loss. A fair portion of patients’ antihyperglycemic regimens required no changes on conversion to semaglutide. Although the semaglutide discontinuation rate neared 10%, AEs that may have contributed to this discontinuation rate included hypoglycemia and GI intolerance. Clinician education resulted in a substantial number of patients undergoing teleretinal imaging and further conversion to semaglutide; however, due to the low conversion response rate, a more effective method of educating clinicians is warranted. Although the semaglutide cost savings initiative at MEDVAMC resulted in significant savings, a full cost-effective analysis is needed to assess more comprehensive institution savings.
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
1. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
2. Aroda VR, Ahmann A, Cariou B, et al. Comparative efficacy, safety, and cardiovascular outcome with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019;45(5):409-418. doi:10.1016/j.diabet.2018.12.001
3. Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2021;12:2042018821997320. Published 2021 Mar 9. doi:10.1177/2042018821997320
4. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. doi:10.1016/j.cmet.2018.03.001
5. Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020;46(2):100-109. doi:10.1016/j.diabet.2019.101117
6. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi:10.1056/NEJMoa1607141
7. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190. doi:10.2337/dc23-S010
8. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046-2055. doi:10.1007/s00125-009-1472-y
9. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(5):355-366. doi:10.1016/S2213-8587(17)30085-2
FDA gives semaglutide two drug safety–related label changes
The FDA added a warning to the drug-interaction section of the Ozempiclabel that reiterates a warning that is already in place in other label sections, reinforcing the message that the glucagon-like peptide-1 (GLP-1) receptor agonist Ozempiccan potentially interact with the action of certain other agents to increase a person’s risk for hypoglycemia.
The added text says: “Ozempic stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving Ozempic in combination with an insulin secretagogue (for instance, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.”
This text was already included in both the “Warning and Precautions” and the “Adverse Reactions” sections of the label. The warning also advises, “The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.”
Reports of ileus episodes after approval
The second addition concerns a new adverse reaction that was identified during the postmarketing experience.
The FDA has received more than 8,500 reports of gastrointestinal issues among patients prescribed glucagon-like peptide-1 (GLP-1) receptor agonists. Ileus is mentioned in 33 cases, including two deaths, associated with semaglutide. The FDA stopped short of saying there is a direct link between the drug and intestinal blockages.
“Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the FDA stated in its approval of the label update.
The same warning for the risk of intestinal blockages is already listed on the labels for tirzepatide (Mounjaro, Lilly) and semaglutide injection 2.4 mg (Wegovy, Novo Nordisk).
The label change comes after a Louisiana woman filed a lawsuit in August that claims she was “severely injured” after using Mounjaro and Ozempic. She claimed the drug makers failed to disclose risks of vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
*Correction, 10/3/23: An earlier version of this article misstated the semaglutide formulation that received the updates.
A version of this article first appeared on Medscape.com.
The FDA added a warning to the drug-interaction section of the Ozempiclabel that reiterates a warning that is already in place in other label sections, reinforcing the message that the glucagon-like peptide-1 (GLP-1) receptor agonist Ozempiccan potentially interact with the action of certain other agents to increase a person’s risk for hypoglycemia.
The added text says: “Ozempic stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving Ozempic in combination with an insulin secretagogue (for instance, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.”
This text was already included in both the “Warning and Precautions” and the “Adverse Reactions” sections of the label. The warning also advises, “The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.”
Reports of ileus episodes after approval
The second addition concerns a new adverse reaction that was identified during the postmarketing experience.
The FDA has received more than 8,500 reports of gastrointestinal issues among patients prescribed glucagon-like peptide-1 (GLP-1) receptor agonists. Ileus is mentioned in 33 cases, including two deaths, associated with semaglutide. The FDA stopped short of saying there is a direct link between the drug and intestinal blockages.
“Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the FDA stated in its approval of the label update.
The same warning for the risk of intestinal blockages is already listed on the labels for tirzepatide (Mounjaro, Lilly) and semaglutide injection 2.4 mg (Wegovy, Novo Nordisk).
The label change comes after a Louisiana woman filed a lawsuit in August that claims she was “severely injured” after using Mounjaro and Ozempic. She claimed the drug makers failed to disclose risks of vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
*Correction, 10/3/23: An earlier version of this article misstated the semaglutide formulation that received the updates.
A version of this article first appeared on Medscape.com.
The FDA added a warning to the drug-interaction section of the Ozempiclabel that reiterates a warning that is already in place in other label sections, reinforcing the message that the glucagon-like peptide-1 (GLP-1) receptor agonist Ozempiccan potentially interact with the action of certain other agents to increase a person’s risk for hypoglycemia.
The added text says: “Ozempic stimulates insulin release in the presence of elevated blood glucose concentrations. Patients receiving Ozempic in combination with an insulin secretagogue (for instance, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia.”
This text was already included in both the “Warning and Precautions” and the “Adverse Reactions” sections of the label. The warning also advises, “The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.”
Reports of ileus episodes after approval
The second addition concerns a new adverse reaction that was identified during the postmarketing experience.
The FDA has received more than 8,500 reports of gastrointestinal issues among patients prescribed glucagon-like peptide-1 (GLP-1) receptor agonists. Ileus is mentioned in 33 cases, including two deaths, associated with semaglutide. The FDA stopped short of saying there is a direct link between the drug and intestinal blockages.
“Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure,” the FDA stated in its approval of the label update.
The same warning for the risk of intestinal blockages is already listed on the labels for tirzepatide (Mounjaro, Lilly) and semaglutide injection 2.4 mg (Wegovy, Novo Nordisk).
The label change comes after a Louisiana woman filed a lawsuit in August that claims she was “severely injured” after using Mounjaro and Ozempic. She claimed the drug makers failed to disclose risks of vomiting and diarrhea due to inflammation of the stomach lining, as well as the risk of gastroparesis.
*Correction, 10/3/23: An earlier version of this article misstated the semaglutide formulation that received the updates.
A version of this article first appeared on Medscape.com.
Study: Antiviral med linked to COVID mutations that can spread
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
published in the online journal Nature.
There’s no evidence that molnupiravir, sold under the brand name Lagevrio, has caused the creation of more transmissible or severe variants of COVID, the study says, but researchers called for more scrutiny of the drug.
Researchers looked at 15 million COVID genomes and discovered that hallmark mutations linked to molnupiravir increased in 2022, especially in places where the drug was widely used, such as the United States and the United Kingdom. Levels of the mutations were also found in populations where the drug was heavily prescribed, such as seniors.
Molnupiravir is an antiviral given to people after they show signs of having COVID-19. It interferes with the COVID-19 virus’s ability to make copies of itself, thus stopping the spread of the virus throughout the body and keeping the virus level low.
The study found the virus can sometimes survive molnupiravir, resulting in mutations that have spread to other people.
Theo Sanderson, PhD, the lead author on the study and a postdoctoral researcher at the Francis Crick Institute in London, told The Guardian that the implications of the mutations were unclear.
“The signature is very clear, but there aren’t any widely circulating variants that have the signature. At the moment there’s nothing that’s transmitted very widely that’s due to molnupiravir,” he said.
The study doesn’t say people should not use molnupiravir but calls for public health officials to scrutinize it.
“The observation that molnupiravir treatment has left a visible trace in global sequencing databases, including onwards transmission of molnupiravir-derived sequences, will be an important consideration for assessing the effects and evolutionary safety of this drug,” the researchers concluded.
When reached for comment, Merck questioned the evidence.
“The authors assume these mutations were associated with viral spread from molnupiravir-treated patients without documented evidence of that transmission. Instead, the authors rely on circumstantial associations between the region from which the sequence was identified and time frame of sequence collection in countries where molnupiravir is available to draw their conclusions,” the company said.
The Food and Drug Administration authorized the use of molnupiravir for the treatment of mild to moderate COVID-19 in adults in December 2021. The FDA has also authorized the use of nirmatrelvir/ritonavir (Paxlovid), an antiviral made by Pfizer.
A version of this article appeared on WebMD.com.
FROM NATURE
Paxlovid weaker against current COVID-19 variants
But when looking at death alone, the antiviral was still highly effective.
Paxlovid was about 37% effective at preventing death or hospitalization in high-risk patients, compared with no treatment. The study also looked at the antiviral Lagevrio, made by Merck, and found it was about 41% effective. In preventing death alone, Paxlovid was about 84% effective, compared with no treatment, and Lagevrio was about 77% effective.
The investigators, of the University of North Carolina at Chapel Hill and the Cleveland Clinic, examined electronic health records of 68,867 patients at hospitals in Cleveland and Florida who were diagnosed with COVID from April 1, 2022, to Feb. 20, 2023.
For Paxlovid, the effectiveness against death and hospitalization was lower than the effectiveness rate of about 86% found in clinical trials in 2021, according to Bloomberg.
The difference in effectiveness in the real-world and clinical studies may have occurred because the early studies were conducted with unvaccinated people. Also, the virus has evolved since those first studies, Bloomberg reported.
The researchers said Paxlovid and Lagevrio are recommended for use because they reduce hospitalization and death among high-risk patients who get COVID, even taking recent Omicron subvariants into account.
“These findings suggest that the use of either nirmatrelvir (Paxlovid) or molnupiravir (Lagevrio) is associated with reductions in mortality and hospitalization in patients infected with Omicron, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions,” the researchers wrote. “Both drugs can, therefore, be used to treat nonhospitalized patients who are at high risk of progressing to severe COVID-19.”
Both drugs should be taken within 5 days of the onset of COVID symptoms.
The study was supported by the National Institutes of Health. Three coauthors reported conflicts of interest with various companies and organizations.
A version of this article first appeared on WebMD.com.
But when looking at death alone, the antiviral was still highly effective.
Paxlovid was about 37% effective at preventing death or hospitalization in high-risk patients, compared with no treatment. The study also looked at the antiviral Lagevrio, made by Merck, and found it was about 41% effective. In preventing death alone, Paxlovid was about 84% effective, compared with no treatment, and Lagevrio was about 77% effective.
The investigators, of the University of North Carolina at Chapel Hill and the Cleveland Clinic, examined electronic health records of 68,867 patients at hospitals in Cleveland and Florida who were diagnosed with COVID from April 1, 2022, to Feb. 20, 2023.
For Paxlovid, the effectiveness against death and hospitalization was lower than the effectiveness rate of about 86% found in clinical trials in 2021, according to Bloomberg.
The difference in effectiveness in the real-world and clinical studies may have occurred because the early studies were conducted with unvaccinated people. Also, the virus has evolved since those first studies, Bloomberg reported.
The researchers said Paxlovid and Lagevrio are recommended for use because they reduce hospitalization and death among high-risk patients who get COVID, even taking recent Omicron subvariants into account.
“These findings suggest that the use of either nirmatrelvir (Paxlovid) or molnupiravir (Lagevrio) is associated with reductions in mortality and hospitalization in patients infected with Omicron, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions,” the researchers wrote. “Both drugs can, therefore, be used to treat nonhospitalized patients who are at high risk of progressing to severe COVID-19.”
Both drugs should be taken within 5 days of the onset of COVID symptoms.
The study was supported by the National Institutes of Health. Three coauthors reported conflicts of interest with various companies and organizations.
A version of this article first appeared on WebMD.com.
But when looking at death alone, the antiviral was still highly effective.
Paxlovid was about 37% effective at preventing death or hospitalization in high-risk patients, compared with no treatment. The study also looked at the antiviral Lagevrio, made by Merck, and found it was about 41% effective. In preventing death alone, Paxlovid was about 84% effective, compared with no treatment, and Lagevrio was about 77% effective.
The investigators, of the University of North Carolina at Chapel Hill and the Cleveland Clinic, examined electronic health records of 68,867 patients at hospitals in Cleveland and Florida who were diagnosed with COVID from April 1, 2022, to Feb. 20, 2023.
For Paxlovid, the effectiveness against death and hospitalization was lower than the effectiveness rate of about 86% found in clinical trials in 2021, according to Bloomberg.
The difference in effectiveness in the real-world and clinical studies may have occurred because the early studies were conducted with unvaccinated people. Also, the virus has evolved since those first studies, Bloomberg reported.
The researchers said Paxlovid and Lagevrio are recommended for use because they reduce hospitalization and death among high-risk patients who get COVID, even taking recent Omicron subvariants into account.
“These findings suggest that the use of either nirmatrelvir (Paxlovid) or molnupiravir (Lagevrio) is associated with reductions in mortality and hospitalization in patients infected with Omicron, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions,” the researchers wrote. “Both drugs can, therefore, be used to treat nonhospitalized patients who are at high risk of progressing to severe COVID-19.”
Both drugs should be taken within 5 days of the onset of COVID symptoms.
The study was supported by the National Institutes of Health. Three coauthors reported conflicts of interest with various companies and organizations.
A version of this article first appeared on WebMD.com.
FROM JAMA NETWORK OPEN
Tirzepatide superior to semaglutide for A1c control, weight loss
, results from a meta-analysis of 22 randomized controlled trials show.
“The results indicate tirzepatide’s superior performance over subcutaneous semaglutide in managing blood sugar and achieving weight loss, making it a promising option in the pharmaceutical management of type 2 diabetes,” first author Thomas Karagiannis, MD, PhD, Aristotle University of Thessaloniki, Greece, said in an interview.
“In clinical context, the most potent doses of each drug revealed a clear difference regarding weight loss, with tirzepatide resulting in an average weight reduction that exceeded that of semaglutide by 5.7 kg (12.6 pounds),” he said.
The study is scheduled to be presented at the annual meeting of the European Association for the Study of Diabetes (EASD) in early October.
While a multitude of studies have been conducted for tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist, and semaglutide, a selective GLP-1 agonist, studies comparing the two drugs directly are lacking.
For a more comprehensive understanding of how the drugs compare, Dr. Karagiannis and colleagues conducted the meta-analysis of 22 trials, including two direct comparisons, the SURPASS-2 trial and a smaller trial, and 20 other studies comparing either semaglutide or tirzepatide with a common comparator, such as placebo, basal insulin, or other GLP-RA-1 drugs.
Overall, 18,472 participants were included in the studies.
All included studies had assessed a maintenance dose of tirzepatide of either 5, 10, or 15 mg once weekly or semaglutide at doses of 0.5, 1.0, or 2.0 mg once weekly for at least 12 weeks. All comparisons were for subcutaneous injection formulations (semaglutide can also be taken orally).
Blood glucose reduction
Tirzepatide at 15 mg was found to have the highest efficacy in the reduction of A1c compared with placebo, with a mean difference of –2.00%, followed by tirzepatide 10 mg (–1.86%) and semaglutide 2.0 mg (–1.62%).
All three of the tirzepatide doses had greater reductions in A1c compared with the respective low, medium, and high doses of semaglutide.
Dr. Karagiannis noted that the differences are significant: “An A1c reduction even by 0.5% is often deemed clinically important,” he said.
Body weight reduction comparisons
The reductions in body weight across the three drug doses were greater with tirzepatide (–10.96 kg [24.2 pounds], –8.75 kg [19.3 pounds], and –6.16 kg [13.6 pounds] for 15, 10, and 5 mg, respectively) compared with semaglutide (–5.24 kg [11.6 pounds], –4.44 kg [9.8 pounds], and –2.72 kg [6 pounds] for semaglutide 2.0, 1.0, and 0.5 mg, respectively).
In terms of drug-to-drug comparisons, tirzepatide 15 mg had a mean of 5.72 kg (12.6 pounds) greater reduction in body weight vs. semaglutide 2.0 mg; tirzepatide 10 mg had a mean of 3.52 kg (7.8 pounds) reduction vs. semaglutide 2.0 mg; and tirzepatide 5 mg had a mean of a 1.72 kg (3.8 pounds) greater reduction vs. semaglutide 1.0 mg.
Adverse events: Increased GI events with highest tirzepatide dose
Regarding the gastrointestinal adverse events associated with the drugs, tirzepatide 15 mg had the highest rate of the two drugs at their various doses, with a risk ratio (RR) of 3.57 compared with placebo for nausea, an RR of 4.35 for vomiting, and 2.04 for diarrhea.
There were no significant differences between the two drugs for the gastrointestinal events, with the exception of the highest dose of tirzepatide, 15 mg, which had a higher risk of vomiting vs. semaglutide 1.0 (RR 1.39) and semaglutide 0.5 mg (RR 1.85).
In addition, tirzepatide 15 mg had a higher risk vs. semaglutide 0.5 mg for nausea (RR 1.45).
There were no significant differences between the two drugs and placebo in the risk of serious adverse events.
Real-world applications, comparisons
Dr. Karagiannis noted that the results indicate that benefits of the efficacy of the higher tirzepatide dose need to be balanced with those potential side effects.
“Although the efficacy of the high tirzepatide dose might make it a favorable choice, its real-world application can be affected on an individual’s ability to tolerate these side effects in case they occur,” he explained.
Ultimately, “some patients may prioritize tolerability over enhanced efficacy,” he added.
Furthermore, while all three maintenance doses of tirzepatide analyzed have received marketing authorization, “to get a clearer picture of the real-world tolerance to these doses outside the context of randomized controlled trials, well-designed observational studies would be necessary,” Dr. Karagiannis said.
Among other issues of comparison with the two drugs is cost.
In a recent analysis, the cost per 1% of body weight reduction was reported to be $1,197 for high-dose tirzepatide (15 mg) vs. $1,511 for semaglutide 2.4 mg, with an overall cost of 72 weeks of therapy with tirzepatide at $17,527 compared with $22,878 for semaglutide.
Overall, patients and clinicians should consider the full range of differences and similarities between the medications, “from [their] efficacy and side effects to cost-effectiveness, long-term safety, and cardiovascular profile,” Dr. Karagiannis said.
Semaglutide is currently approved by the Food and Drug Administration for treatment of type 2 diabetes and obesity/weight loss management.
Tirzepatide has also received approval for the treatment of type 2 diabetes and its manufacturers have submitted applications for its approval for obesity/weight loss management.
Dr. Karagiannis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, results from a meta-analysis of 22 randomized controlled trials show.
“The results indicate tirzepatide’s superior performance over subcutaneous semaglutide in managing blood sugar and achieving weight loss, making it a promising option in the pharmaceutical management of type 2 diabetes,” first author Thomas Karagiannis, MD, PhD, Aristotle University of Thessaloniki, Greece, said in an interview.
“In clinical context, the most potent doses of each drug revealed a clear difference regarding weight loss, with tirzepatide resulting in an average weight reduction that exceeded that of semaglutide by 5.7 kg (12.6 pounds),” he said.
The study is scheduled to be presented at the annual meeting of the European Association for the Study of Diabetes (EASD) in early October.
While a multitude of studies have been conducted for tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist, and semaglutide, a selective GLP-1 agonist, studies comparing the two drugs directly are lacking.
For a more comprehensive understanding of how the drugs compare, Dr. Karagiannis and colleagues conducted the meta-analysis of 22 trials, including two direct comparisons, the SURPASS-2 trial and a smaller trial, and 20 other studies comparing either semaglutide or tirzepatide with a common comparator, such as placebo, basal insulin, or other GLP-RA-1 drugs.
Overall, 18,472 participants were included in the studies.
All included studies had assessed a maintenance dose of tirzepatide of either 5, 10, or 15 mg once weekly or semaglutide at doses of 0.5, 1.0, or 2.0 mg once weekly for at least 12 weeks. All comparisons were for subcutaneous injection formulations (semaglutide can also be taken orally).
Blood glucose reduction
Tirzepatide at 15 mg was found to have the highest efficacy in the reduction of A1c compared with placebo, with a mean difference of –2.00%, followed by tirzepatide 10 mg (–1.86%) and semaglutide 2.0 mg (–1.62%).
All three of the tirzepatide doses had greater reductions in A1c compared with the respective low, medium, and high doses of semaglutide.
Dr. Karagiannis noted that the differences are significant: “An A1c reduction even by 0.5% is often deemed clinically important,” he said.
Body weight reduction comparisons
The reductions in body weight across the three drug doses were greater with tirzepatide (–10.96 kg [24.2 pounds], –8.75 kg [19.3 pounds], and –6.16 kg [13.6 pounds] for 15, 10, and 5 mg, respectively) compared with semaglutide (–5.24 kg [11.6 pounds], –4.44 kg [9.8 pounds], and –2.72 kg [6 pounds] for semaglutide 2.0, 1.0, and 0.5 mg, respectively).
In terms of drug-to-drug comparisons, tirzepatide 15 mg had a mean of 5.72 kg (12.6 pounds) greater reduction in body weight vs. semaglutide 2.0 mg; tirzepatide 10 mg had a mean of 3.52 kg (7.8 pounds) reduction vs. semaglutide 2.0 mg; and tirzepatide 5 mg had a mean of a 1.72 kg (3.8 pounds) greater reduction vs. semaglutide 1.0 mg.
Adverse events: Increased GI events with highest tirzepatide dose
Regarding the gastrointestinal adverse events associated with the drugs, tirzepatide 15 mg had the highest rate of the two drugs at their various doses, with a risk ratio (RR) of 3.57 compared with placebo for nausea, an RR of 4.35 for vomiting, and 2.04 for diarrhea.
There were no significant differences between the two drugs for the gastrointestinal events, with the exception of the highest dose of tirzepatide, 15 mg, which had a higher risk of vomiting vs. semaglutide 1.0 (RR 1.39) and semaglutide 0.5 mg (RR 1.85).
In addition, tirzepatide 15 mg had a higher risk vs. semaglutide 0.5 mg for nausea (RR 1.45).
There were no significant differences between the two drugs and placebo in the risk of serious adverse events.
Real-world applications, comparisons
Dr. Karagiannis noted that the results indicate that benefits of the efficacy of the higher tirzepatide dose need to be balanced with those potential side effects.
“Although the efficacy of the high tirzepatide dose might make it a favorable choice, its real-world application can be affected on an individual’s ability to tolerate these side effects in case they occur,” he explained.
Ultimately, “some patients may prioritize tolerability over enhanced efficacy,” he added.
Furthermore, while all three maintenance doses of tirzepatide analyzed have received marketing authorization, “to get a clearer picture of the real-world tolerance to these doses outside the context of randomized controlled trials, well-designed observational studies would be necessary,” Dr. Karagiannis said.
Among other issues of comparison with the two drugs is cost.
In a recent analysis, the cost per 1% of body weight reduction was reported to be $1,197 for high-dose tirzepatide (15 mg) vs. $1,511 for semaglutide 2.4 mg, with an overall cost of 72 weeks of therapy with tirzepatide at $17,527 compared with $22,878 for semaglutide.
Overall, patients and clinicians should consider the full range of differences and similarities between the medications, “from [their] efficacy and side effects to cost-effectiveness, long-term safety, and cardiovascular profile,” Dr. Karagiannis said.
Semaglutide is currently approved by the Food and Drug Administration for treatment of type 2 diabetes and obesity/weight loss management.
Tirzepatide has also received approval for the treatment of type 2 diabetes and its manufacturers have submitted applications for its approval for obesity/weight loss management.
Dr. Karagiannis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, results from a meta-analysis of 22 randomized controlled trials show.
“The results indicate tirzepatide’s superior performance over subcutaneous semaglutide in managing blood sugar and achieving weight loss, making it a promising option in the pharmaceutical management of type 2 diabetes,” first author Thomas Karagiannis, MD, PhD, Aristotle University of Thessaloniki, Greece, said in an interview.
“In clinical context, the most potent doses of each drug revealed a clear difference regarding weight loss, with tirzepatide resulting in an average weight reduction that exceeded that of semaglutide by 5.7 kg (12.6 pounds),” he said.
The study is scheduled to be presented at the annual meeting of the European Association for the Study of Diabetes (EASD) in early October.
While a multitude of studies have been conducted for tirzepatide, a dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1) receptor agonist, and semaglutide, a selective GLP-1 agonist, studies comparing the two drugs directly are lacking.
For a more comprehensive understanding of how the drugs compare, Dr. Karagiannis and colleagues conducted the meta-analysis of 22 trials, including two direct comparisons, the SURPASS-2 trial and a smaller trial, and 20 other studies comparing either semaglutide or tirzepatide with a common comparator, such as placebo, basal insulin, or other GLP-RA-1 drugs.
Overall, 18,472 participants were included in the studies.
All included studies had assessed a maintenance dose of tirzepatide of either 5, 10, or 15 mg once weekly or semaglutide at doses of 0.5, 1.0, or 2.0 mg once weekly for at least 12 weeks. All comparisons were for subcutaneous injection formulations (semaglutide can also be taken orally).
Blood glucose reduction
Tirzepatide at 15 mg was found to have the highest efficacy in the reduction of A1c compared with placebo, with a mean difference of –2.00%, followed by tirzepatide 10 mg (–1.86%) and semaglutide 2.0 mg (–1.62%).
All three of the tirzepatide doses had greater reductions in A1c compared with the respective low, medium, and high doses of semaglutide.
Dr. Karagiannis noted that the differences are significant: “An A1c reduction even by 0.5% is often deemed clinically important,” he said.
Body weight reduction comparisons
The reductions in body weight across the three drug doses were greater with tirzepatide (–10.96 kg [24.2 pounds], –8.75 kg [19.3 pounds], and –6.16 kg [13.6 pounds] for 15, 10, and 5 mg, respectively) compared with semaglutide (–5.24 kg [11.6 pounds], –4.44 kg [9.8 pounds], and –2.72 kg [6 pounds] for semaglutide 2.0, 1.0, and 0.5 mg, respectively).
In terms of drug-to-drug comparisons, tirzepatide 15 mg had a mean of 5.72 kg (12.6 pounds) greater reduction in body weight vs. semaglutide 2.0 mg; tirzepatide 10 mg had a mean of 3.52 kg (7.8 pounds) reduction vs. semaglutide 2.0 mg; and tirzepatide 5 mg had a mean of a 1.72 kg (3.8 pounds) greater reduction vs. semaglutide 1.0 mg.
Adverse events: Increased GI events with highest tirzepatide dose
Regarding the gastrointestinal adverse events associated with the drugs, tirzepatide 15 mg had the highest rate of the two drugs at their various doses, with a risk ratio (RR) of 3.57 compared with placebo for nausea, an RR of 4.35 for vomiting, and 2.04 for diarrhea.
There were no significant differences between the two drugs for the gastrointestinal events, with the exception of the highest dose of tirzepatide, 15 mg, which had a higher risk of vomiting vs. semaglutide 1.0 (RR 1.39) and semaglutide 0.5 mg (RR 1.85).
In addition, tirzepatide 15 mg had a higher risk vs. semaglutide 0.5 mg for nausea (RR 1.45).
There were no significant differences between the two drugs and placebo in the risk of serious adverse events.
Real-world applications, comparisons
Dr. Karagiannis noted that the results indicate that benefits of the efficacy of the higher tirzepatide dose need to be balanced with those potential side effects.
“Although the efficacy of the high tirzepatide dose might make it a favorable choice, its real-world application can be affected on an individual’s ability to tolerate these side effects in case they occur,” he explained.
Ultimately, “some patients may prioritize tolerability over enhanced efficacy,” he added.
Furthermore, while all three maintenance doses of tirzepatide analyzed have received marketing authorization, “to get a clearer picture of the real-world tolerance to these doses outside the context of randomized controlled trials, well-designed observational studies would be necessary,” Dr. Karagiannis said.
Among other issues of comparison with the two drugs is cost.
In a recent analysis, the cost per 1% of body weight reduction was reported to be $1,197 for high-dose tirzepatide (15 mg) vs. $1,511 for semaglutide 2.4 mg, with an overall cost of 72 weeks of therapy with tirzepatide at $17,527 compared with $22,878 for semaglutide.
Overall, patients and clinicians should consider the full range of differences and similarities between the medications, “from [their] efficacy and side effects to cost-effectiveness, long-term safety, and cardiovascular profile,” Dr. Karagiannis said.
Semaglutide is currently approved by the Food and Drug Administration for treatment of type 2 diabetes and obesity/weight loss management.
Tirzepatide has also received approval for the treatment of type 2 diabetes and its manufacturers have submitted applications for its approval for obesity/weight loss management.
Dr. Karagiannis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2023
MDMA effective in diverse patients with PTSD
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
New antibiotic could combat multidrug-resistant superbugs
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
FROM CELL