User login
Surge in pediatric ADHD med errors prompts call for prevention
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
according to results of a study published in the journal Pediatrics.
The dramatic jump is likely attributable to an increase in the prescribing of ADHD medications for children. According to the study authors, in 2019, nearly 10% of children in the United States had been diagnosed with ADHD, and some 3.3 million – or about 5% of all children in the country – had received a prescription for an ADHD medication.
“Because therapeutic errors are preventable, more attention should be given to patient and caregiver education and development of improved child-resistant medication dispensing and tracking systems,” the authors commented.
The investigators analyzed data from the National Poison Data System from 2000 through 2021 for therapeutic errors associated with ADHD medication among patients younger than 20 years.
“As medicine changes, it’s nice to look back at some of these things and see how some of these problems have changed,” said Natalie I. Rine, PharmD, a coauthor of the study and director of the Central Ohio Poison Center at Nationwide Children’s Hospital in Columbus.
The researchers identified 124,383 such errors reported to U.S. poison centers during the study period. The frequency increased by 299%.
Two-thirds (66.6%) of the exposures involved children aged 6-12 years, three-fourths (76.4%) were among males, and half (50.5%) involved amphetamines and related compounds. Most (79.7%) therapeutic errors were linked to exposure to a single substance. Nearly 83% of patients did not receive treatment at a health care facility; however, 2.3% were admitted to the hospital, and 4.2% had a “serious medical outcome,” the researchers found.
The most common scenarios were “inadvertently took or given medication twice” (53.9%), followed by “inadvertently took or given someone else’s medication” (13.4%) and “wrong medication taken or given” (12.9%), according to the researchers. Two percent involved mistakes by a pharmacist or nurse.
Easily preventable
Dr. Rine attributed the errors to simple mistakes and said they were likely the product of busy households and distracted caregivers. She added that the errors are easily avoided by storing the medication properly, keeping a sheet with the medication to document what was taken and when, and using a pillbox or one of many apps that can assist in documenting the dispensing of medications.
“I think the biggest thing is that a lot of these errors are preventable, more than anything else,” Dr. Rine said.
The increase in ADHD diagnoses among children and the subsequent prescribing of medications are reasons for the nearly 300% increase in poison control calls. A 2018 study showed that the estimated prevalence of ADHD diagnoses among U.S. children and adolescents increased from 6.1% in 1997-1998 to 10.2% in 2015-2016. The Centers for Disease Control and Prevention states that 6 million children and adolescents aged 3-17 years have been diagnosed with ADHD, and 62% have received ADHD medication.
Colleen Kraft, MD, a pediatrician at Children’s Hospital Los Angeles, said she was not surprised by the reported increase in errors. In addition to the simple uptick in ADHD diagnoses and prescriptions in the past 2 decades, Dr. Kraft said the growing variety of ADHD medication is a cause for more errors.
“Because we have so many more different types of these medications, it’s easy to confuse them, and it’s easy to make an error when you give this to a child,” she said in an interview.
Dr. Kraft also hypothesized that because ADHD can have a genetic component, some parents with undiagnosed and untreated ADHD are responsible for their child’s medication, a scenario ripe for mistakes.
Potential dangers
Not all ADHD medicinal overdosing is created equal, Dr. Kraft pointed out. Doubling up on a stimulant such as methylphenidate (Ritalin) or the combination of amphetamine and dextroamphetamine (Adderall) may cause headaches, suppress appetite, and cause an upset stomach, although those symptoms usually clear up in a few hours.
However, she noted, the use of alpha-1 adrenergic blockers is more concerning. Also used to treat high blood pressure, medications such as guanfacine and clonidine cause sedation. A double dose can cause blood pressure to decrease to dangerous levels.
The study’s primary limitation was bias in self-reporting, which may have led to underreporting of incidences, according to the researchers. Not every case in which an error occurs that involves a child’s taking ADHD medication gets reported to poison control, because some will take a wait-and-see approach and may not call if their child is asymptomatic.
“Our data is only as good as what the callers report to us,” Dr. Rine said.
A version of this article appeared on Medscape.com.
FROM PEDIATRICS
Sotatercept tied to disease modification in pulmonary arterial hypertension
MILAN – Sotatercept, a first-in-class activin signaling inhibitor, is currently under scrutiny as a potential game-changer in the treatment of pulmonary arterial hypertension (PAH). Data unveiled at the annual congress of the European Respiratory Society, held in Milan, suggest that sotatercept treatment has the capacity to deliver significant clinical benefits and could reshape the trajectory of this challenging disease. Experts are cautiously optimistic that this drug may soon find a place within the PAH treatment algorithm.
The STELLAR trial: A milestone in PAH research
PAH is intricately linked to the dysregulation of members within the TGF-beta superfamily, including activin receptor type IIA (ActRIIA) and its ligands activin A and activin B. This signaling pathway is believed to be a driving force behind the pulmonary vascular remodeling observed in PAH patients. Sotatercept, a fusion protein acting as a ligand trap for selected TGF-beta superfamily members, has been proposed to recalibrate pulmonary vascular homeostasis by promoting growth-inhibiting and pro-apoptotic signaling.
Sotatercept was tested first in a phase 2 trial (PULSAR) and later in a phase 3 trial (STELLAR). The STELLAR clinical trial, funded by Acceleron Pharma (now a subsidiary of Merck), was the subject of two presentations given by Marius M. Hoeper, MD, director of the department of respiratory medicine at Hannover Medical School, Hannover, Germany.
Dr. Hoeper commented on results published in the New England Journal of Medicine during a session titled, “Disease modification in pulmonary arterial hypertension.” Later, during the “From the Editor’s Desk” session, he presented new results recently published in the European Respiratory Journal about the effects of sotatercept on hemodynamics and right heart function.
Disease modification in PAH
In his initial address, Dr. Hoeper expounded on the concept of reverse remodeling as a therapeutic avenue for PAH. “PAH is not a disease of pulmonary vasoconstriction,” he clarified, “but a disease of proliferation. Endothelial cells and pulmonary vascular muscle cells proliferate and obliterate the lumen. It has been hypothesized that when we target this system successfully, we may not only stop disease progression, but we may have a chance to have at least some reverse remodeling, because, if these cells go into apoptosis, there may be a partial reopening of the vessels.”
“Sotatercept is probably going to be a game changer in our field,” Dr. Hoeper continued. “Is sotatercept a disease-modifying agent? It certainly induces disease improvement; in a few patients, although not in the majority, we see a normalization of hemodynamics. We target the underlying pathophysiology; this is clearly distinct from symptomatic treatment.” Dr. Hoeper went through the list of characteristics that a disease-modifying agent should have.
“To be able to say that a drug endures sustained clinical benefit, according to the FDA, you need to withdraw the drug, and this is something we do not know. We know that we can interrupt the treatment once or twice, but long-term I do not believe that,” he said, while acknowledging the need for more extended-term safety and efficacy data.
Unmasking hemodynamic impact
Dr. Hoeper’s second presentation focused on a post hoc analysis of the STELLAR trial never presented before. He analyzed right heart catheterization (RHC) and echocardiography (ECHO) data. With sotatercept treatment at week 24, the researchers observed:
- A small increase in systemic blood pressure and systemic vascular resistance.
- No changes in systolic and diastolic volumes of the left ventricle (lv).
- A small but significant reduction in lv ejection fraction.
- A great reduction in the mean pulmonary artery pressure (mPAP).
- No change in cardiac output.
- An improvement in pulmonary artery compliance.
- A reduction in the right ventricle work and in right atrial pressure.
- An improvement of echocardiographic parameters, including a significant decrease in tricuspid regurgitation.
“A drop of roughly 14 mm Hg in mPAP is something that we have never seen in PAH with any other add-on medication. This was entirely driven by improvement in the sotatercept group, not by deterioration in the placebo group,” Dr. Hoeper pointed out. Of note, change in mPAP correlated with changes in NT-proNBP and with changes in 6-minute walk distance (6MWD), the primary endpoint of the STELLAR trial. “We effectively unload the right ventricle by lowing the artery pressure. What we observe is exactly what we want to achieve in patients with PAH, because the heart is what really matters,” he concluded.
A new course in PAH treatment?
Olivier Sitbon, MD, PhD, professor of respiratory medicine at Université Paris-Saclay and consultant at the French Referral Center for Pulmonary Hypertension, echoed Dr. Hoeper’s enthusiasm. ,” he told this news organization.
Dr. Sitbon highlighted ongoing studies with sotatercept, including the ZENITH trial, focused on high-risk PAH patients, and the HYPERION trial, aimed at patients diagnosed within the first year of their PAH journey. He acknowledged that experts currently lack consensus on the ideal position for sotatercept within the PAH treatment algorithm. However, he anticipates a lively debate and expects sotatercept to find its place as a second-line treatment for intermediate low-risk or intermediate high-risk patients, with potential consideration for high-risk patients.
“There are two more studies ongoing with sotatercept: the ZENITH trial, dedicated to PAH patients at high risk, whose primary endpoint is mortality/need for lung transplant, and the HYPERION trial, dedicated to patients diagnosed less than 1 year (not really newly diagnosed but quite incident, while patients included in previous trial were very prevalent), whose primary endpoint is time to clinical worsening,” Dr. Sitbon noted, pointing out that there is currently no consensus among the experts about where to place sotatercept in the PAH treatment algorithm.
Further insights into sotatercept
The ERS Congress also unveiled two additional studies that provided fresh perspectives on sotatercept’s potential. Ioana R. Preston, MD, from Tufts Medical Center in Boston, presented the first interim analysis of SOTERIA, a long-term follow-up study involving 409 patients with a median exposure duration of 462 days to sotatercept. Treatment-emergent adverse events (TEAEs) were reported by 80% of patients, with 20% reporting a serious TEAE. Overall, four serious TEAEs (1% of patients) led to death, but only five patients (1.2%) discontinued sotatercept because of TEAE.
Notably, improvements in clinical efficacy measures persisted after 1 year. During SOTERIA, roughly 3% of patients on any prostacyclin discontinued it. “Results of SOTERIA support the long-term durable clinical benefit and safety of sotatercept for the treatment of PAH. Of note, patients were offered home self-administration therapy, so they do not need to come back to the office,” Dr. Preston said.
A second late-breaking abstract presented by Vallerie McLaughlin, MD, University of Michigan, Ann Arbor, described the possible long-term impact of sotatercept on morbidity and mortality. STELLAR trial data were analyzed to see how the risk profile of patients changed in the 24 weeks of study. Real-world registry data from the COMPERA registry were then used to extrapolate mortality and transplant need over 30 years based on risk transition. According to the simulation model, adding sotatercept to background therapy is expected to increase life expectancy by threefold, while avoiding nearly 700 hospitalizations and four lung/heart-lung transplantations per 1,000 patients. “Real-world data are needed to confirm these findings,” cautioned Dr. McLaughlin.
Dr. Hoeper disclosed speaking and consulting fees from Acceleron, Actelion, Altavant, AOP Health, Bayer, Ferrer, Janssen, Keros, and MSD. Dr. Sitbon disclosed speaking and consulting fees from Acceleron Pharmaceuticals, Altavant Sciences, AOP Orphan, Bayer, Ferrer, Gossamer Bio, Janssen, MSD, and United Therapeutics, and grant/research support from Acceleron Pharmaceuticals, AOP Orphan, Bayer, Janssen, and MSD. Dr. Preston disclosed speaking and consulting fees from Janssen and United Therapeutics, and grant/research support from Janssen and Respira Therapeutics. She has participated in scientific advisory boards for Aereovate, Altavant, and Gossamer Bio, and was in the Steering Committee of Acceleron, Liquidia, and United Therapeutics. Dr. McLaughlin has received speaking and consulting fees from Aerami, Aereovate, Caremark, Corvista, Enzyvant, Gossamer Bio, Janssen, Merck, United Therapeutics, and Vertex, and grant/research support from Aerovate, Enzyvant, Gossamer Bio, Janssen, Merck, and Sonovia. She is a member of the Board of Directors of Clene.
A version of this article first appeared on Medscape.com.
MILAN – Sotatercept, a first-in-class activin signaling inhibitor, is currently under scrutiny as a potential game-changer in the treatment of pulmonary arterial hypertension (PAH). Data unveiled at the annual congress of the European Respiratory Society, held in Milan, suggest that sotatercept treatment has the capacity to deliver significant clinical benefits and could reshape the trajectory of this challenging disease. Experts are cautiously optimistic that this drug may soon find a place within the PAH treatment algorithm.
The STELLAR trial: A milestone in PAH research
PAH is intricately linked to the dysregulation of members within the TGF-beta superfamily, including activin receptor type IIA (ActRIIA) and its ligands activin A and activin B. This signaling pathway is believed to be a driving force behind the pulmonary vascular remodeling observed in PAH patients. Sotatercept, a fusion protein acting as a ligand trap for selected TGF-beta superfamily members, has been proposed to recalibrate pulmonary vascular homeostasis by promoting growth-inhibiting and pro-apoptotic signaling.
Sotatercept was tested first in a phase 2 trial (PULSAR) and later in a phase 3 trial (STELLAR). The STELLAR clinical trial, funded by Acceleron Pharma (now a subsidiary of Merck), was the subject of two presentations given by Marius M. Hoeper, MD, director of the department of respiratory medicine at Hannover Medical School, Hannover, Germany.
Dr. Hoeper commented on results published in the New England Journal of Medicine during a session titled, “Disease modification in pulmonary arterial hypertension.” Later, during the “From the Editor’s Desk” session, he presented new results recently published in the European Respiratory Journal about the effects of sotatercept on hemodynamics and right heart function.
Disease modification in PAH
In his initial address, Dr. Hoeper expounded on the concept of reverse remodeling as a therapeutic avenue for PAH. “PAH is not a disease of pulmonary vasoconstriction,” he clarified, “but a disease of proliferation. Endothelial cells and pulmonary vascular muscle cells proliferate and obliterate the lumen. It has been hypothesized that when we target this system successfully, we may not only stop disease progression, but we may have a chance to have at least some reverse remodeling, because, if these cells go into apoptosis, there may be a partial reopening of the vessels.”
“Sotatercept is probably going to be a game changer in our field,” Dr. Hoeper continued. “Is sotatercept a disease-modifying agent? It certainly induces disease improvement; in a few patients, although not in the majority, we see a normalization of hemodynamics. We target the underlying pathophysiology; this is clearly distinct from symptomatic treatment.” Dr. Hoeper went through the list of characteristics that a disease-modifying agent should have.
“To be able to say that a drug endures sustained clinical benefit, according to the FDA, you need to withdraw the drug, and this is something we do not know. We know that we can interrupt the treatment once or twice, but long-term I do not believe that,” he said, while acknowledging the need for more extended-term safety and efficacy data.
Unmasking hemodynamic impact
Dr. Hoeper’s second presentation focused on a post hoc analysis of the STELLAR trial never presented before. He analyzed right heart catheterization (RHC) and echocardiography (ECHO) data. With sotatercept treatment at week 24, the researchers observed:
- A small increase in systemic blood pressure and systemic vascular resistance.
- No changes in systolic and diastolic volumes of the left ventricle (lv).
- A small but significant reduction in lv ejection fraction.
- A great reduction in the mean pulmonary artery pressure (mPAP).
- No change in cardiac output.
- An improvement in pulmonary artery compliance.
- A reduction in the right ventricle work and in right atrial pressure.
- An improvement of echocardiographic parameters, including a significant decrease in tricuspid regurgitation.
“A drop of roughly 14 mm Hg in mPAP is something that we have never seen in PAH with any other add-on medication. This was entirely driven by improvement in the sotatercept group, not by deterioration in the placebo group,” Dr. Hoeper pointed out. Of note, change in mPAP correlated with changes in NT-proNBP and with changes in 6-minute walk distance (6MWD), the primary endpoint of the STELLAR trial. “We effectively unload the right ventricle by lowing the artery pressure. What we observe is exactly what we want to achieve in patients with PAH, because the heart is what really matters,” he concluded.
A new course in PAH treatment?
Olivier Sitbon, MD, PhD, professor of respiratory medicine at Université Paris-Saclay and consultant at the French Referral Center for Pulmonary Hypertension, echoed Dr. Hoeper’s enthusiasm. ,” he told this news organization.
Dr. Sitbon highlighted ongoing studies with sotatercept, including the ZENITH trial, focused on high-risk PAH patients, and the HYPERION trial, aimed at patients diagnosed within the first year of their PAH journey. He acknowledged that experts currently lack consensus on the ideal position for sotatercept within the PAH treatment algorithm. However, he anticipates a lively debate and expects sotatercept to find its place as a second-line treatment for intermediate low-risk or intermediate high-risk patients, with potential consideration for high-risk patients.
“There are two more studies ongoing with sotatercept: the ZENITH trial, dedicated to PAH patients at high risk, whose primary endpoint is mortality/need for lung transplant, and the HYPERION trial, dedicated to patients diagnosed less than 1 year (not really newly diagnosed but quite incident, while patients included in previous trial were very prevalent), whose primary endpoint is time to clinical worsening,” Dr. Sitbon noted, pointing out that there is currently no consensus among the experts about where to place sotatercept in the PAH treatment algorithm.
Further insights into sotatercept
The ERS Congress also unveiled two additional studies that provided fresh perspectives on sotatercept’s potential. Ioana R. Preston, MD, from Tufts Medical Center in Boston, presented the first interim analysis of SOTERIA, a long-term follow-up study involving 409 patients with a median exposure duration of 462 days to sotatercept. Treatment-emergent adverse events (TEAEs) were reported by 80% of patients, with 20% reporting a serious TEAE. Overall, four serious TEAEs (1% of patients) led to death, but only five patients (1.2%) discontinued sotatercept because of TEAE.
Notably, improvements in clinical efficacy measures persisted after 1 year. During SOTERIA, roughly 3% of patients on any prostacyclin discontinued it. “Results of SOTERIA support the long-term durable clinical benefit and safety of sotatercept for the treatment of PAH. Of note, patients were offered home self-administration therapy, so they do not need to come back to the office,” Dr. Preston said.
A second late-breaking abstract presented by Vallerie McLaughlin, MD, University of Michigan, Ann Arbor, described the possible long-term impact of sotatercept on morbidity and mortality. STELLAR trial data were analyzed to see how the risk profile of patients changed in the 24 weeks of study. Real-world registry data from the COMPERA registry were then used to extrapolate mortality and transplant need over 30 years based on risk transition. According to the simulation model, adding sotatercept to background therapy is expected to increase life expectancy by threefold, while avoiding nearly 700 hospitalizations and four lung/heart-lung transplantations per 1,000 patients. “Real-world data are needed to confirm these findings,” cautioned Dr. McLaughlin.
Dr. Hoeper disclosed speaking and consulting fees from Acceleron, Actelion, Altavant, AOP Health, Bayer, Ferrer, Janssen, Keros, and MSD. Dr. Sitbon disclosed speaking and consulting fees from Acceleron Pharmaceuticals, Altavant Sciences, AOP Orphan, Bayer, Ferrer, Gossamer Bio, Janssen, MSD, and United Therapeutics, and grant/research support from Acceleron Pharmaceuticals, AOP Orphan, Bayer, Janssen, and MSD. Dr. Preston disclosed speaking and consulting fees from Janssen and United Therapeutics, and grant/research support from Janssen and Respira Therapeutics. She has participated in scientific advisory boards for Aereovate, Altavant, and Gossamer Bio, and was in the Steering Committee of Acceleron, Liquidia, and United Therapeutics. Dr. McLaughlin has received speaking and consulting fees from Aerami, Aereovate, Caremark, Corvista, Enzyvant, Gossamer Bio, Janssen, Merck, United Therapeutics, and Vertex, and grant/research support from Aerovate, Enzyvant, Gossamer Bio, Janssen, Merck, and Sonovia. She is a member of the Board of Directors of Clene.
A version of this article first appeared on Medscape.com.
MILAN – Sotatercept, a first-in-class activin signaling inhibitor, is currently under scrutiny as a potential game-changer in the treatment of pulmonary arterial hypertension (PAH). Data unveiled at the annual congress of the European Respiratory Society, held in Milan, suggest that sotatercept treatment has the capacity to deliver significant clinical benefits and could reshape the trajectory of this challenging disease. Experts are cautiously optimistic that this drug may soon find a place within the PAH treatment algorithm.
The STELLAR trial: A milestone in PAH research
PAH is intricately linked to the dysregulation of members within the TGF-beta superfamily, including activin receptor type IIA (ActRIIA) and its ligands activin A and activin B. This signaling pathway is believed to be a driving force behind the pulmonary vascular remodeling observed in PAH patients. Sotatercept, a fusion protein acting as a ligand trap for selected TGF-beta superfamily members, has been proposed to recalibrate pulmonary vascular homeostasis by promoting growth-inhibiting and pro-apoptotic signaling.
Sotatercept was tested first in a phase 2 trial (PULSAR) and later in a phase 3 trial (STELLAR). The STELLAR clinical trial, funded by Acceleron Pharma (now a subsidiary of Merck), was the subject of two presentations given by Marius M. Hoeper, MD, director of the department of respiratory medicine at Hannover Medical School, Hannover, Germany.
Dr. Hoeper commented on results published in the New England Journal of Medicine during a session titled, “Disease modification in pulmonary arterial hypertension.” Later, during the “From the Editor’s Desk” session, he presented new results recently published in the European Respiratory Journal about the effects of sotatercept on hemodynamics and right heart function.
Disease modification in PAH
In his initial address, Dr. Hoeper expounded on the concept of reverse remodeling as a therapeutic avenue for PAH. “PAH is not a disease of pulmonary vasoconstriction,” he clarified, “but a disease of proliferation. Endothelial cells and pulmonary vascular muscle cells proliferate and obliterate the lumen. It has been hypothesized that when we target this system successfully, we may not only stop disease progression, but we may have a chance to have at least some reverse remodeling, because, if these cells go into apoptosis, there may be a partial reopening of the vessels.”
“Sotatercept is probably going to be a game changer in our field,” Dr. Hoeper continued. “Is sotatercept a disease-modifying agent? It certainly induces disease improvement; in a few patients, although not in the majority, we see a normalization of hemodynamics. We target the underlying pathophysiology; this is clearly distinct from symptomatic treatment.” Dr. Hoeper went through the list of characteristics that a disease-modifying agent should have.
“To be able to say that a drug endures sustained clinical benefit, according to the FDA, you need to withdraw the drug, and this is something we do not know. We know that we can interrupt the treatment once or twice, but long-term I do not believe that,” he said, while acknowledging the need for more extended-term safety and efficacy data.
Unmasking hemodynamic impact
Dr. Hoeper’s second presentation focused on a post hoc analysis of the STELLAR trial never presented before. He analyzed right heart catheterization (RHC) and echocardiography (ECHO) data. With sotatercept treatment at week 24, the researchers observed:
- A small increase in systemic blood pressure and systemic vascular resistance.
- No changes in systolic and diastolic volumes of the left ventricle (lv).
- A small but significant reduction in lv ejection fraction.
- A great reduction in the mean pulmonary artery pressure (mPAP).
- No change in cardiac output.
- An improvement in pulmonary artery compliance.
- A reduction in the right ventricle work and in right atrial pressure.
- An improvement of echocardiographic parameters, including a significant decrease in tricuspid regurgitation.
“A drop of roughly 14 mm Hg in mPAP is something that we have never seen in PAH with any other add-on medication. This was entirely driven by improvement in the sotatercept group, not by deterioration in the placebo group,” Dr. Hoeper pointed out. Of note, change in mPAP correlated with changes in NT-proNBP and with changes in 6-minute walk distance (6MWD), the primary endpoint of the STELLAR trial. “We effectively unload the right ventricle by lowing the artery pressure. What we observe is exactly what we want to achieve in patients with PAH, because the heart is what really matters,” he concluded.
A new course in PAH treatment?
Olivier Sitbon, MD, PhD, professor of respiratory medicine at Université Paris-Saclay and consultant at the French Referral Center for Pulmonary Hypertension, echoed Dr. Hoeper’s enthusiasm. ,” he told this news organization.
Dr. Sitbon highlighted ongoing studies with sotatercept, including the ZENITH trial, focused on high-risk PAH patients, and the HYPERION trial, aimed at patients diagnosed within the first year of their PAH journey. He acknowledged that experts currently lack consensus on the ideal position for sotatercept within the PAH treatment algorithm. However, he anticipates a lively debate and expects sotatercept to find its place as a second-line treatment for intermediate low-risk or intermediate high-risk patients, with potential consideration for high-risk patients.
“There are two more studies ongoing with sotatercept: the ZENITH trial, dedicated to PAH patients at high risk, whose primary endpoint is mortality/need for lung transplant, and the HYPERION trial, dedicated to patients diagnosed less than 1 year (not really newly diagnosed but quite incident, while patients included in previous trial were very prevalent), whose primary endpoint is time to clinical worsening,” Dr. Sitbon noted, pointing out that there is currently no consensus among the experts about where to place sotatercept in the PAH treatment algorithm.
Further insights into sotatercept
The ERS Congress also unveiled two additional studies that provided fresh perspectives on sotatercept’s potential. Ioana R. Preston, MD, from Tufts Medical Center in Boston, presented the first interim analysis of SOTERIA, a long-term follow-up study involving 409 patients with a median exposure duration of 462 days to sotatercept. Treatment-emergent adverse events (TEAEs) were reported by 80% of patients, with 20% reporting a serious TEAE. Overall, four serious TEAEs (1% of patients) led to death, but only five patients (1.2%) discontinued sotatercept because of TEAE.
Notably, improvements in clinical efficacy measures persisted after 1 year. During SOTERIA, roughly 3% of patients on any prostacyclin discontinued it. “Results of SOTERIA support the long-term durable clinical benefit and safety of sotatercept for the treatment of PAH. Of note, patients were offered home self-administration therapy, so they do not need to come back to the office,” Dr. Preston said.
A second late-breaking abstract presented by Vallerie McLaughlin, MD, University of Michigan, Ann Arbor, described the possible long-term impact of sotatercept on morbidity and mortality. STELLAR trial data were analyzed to see how the risk profile of patients changed in the 24 weeks of study. Real-world registry data from the COMPERA registry were then used to extrapolate mortality and transplant need over 30 years based on risk transition. According to the simulation model, adding sotatercept to background therapy is expected to increase life expectancy by threefold, while avoiding nearly 700 hospitalizations and four lung/heart-lung transplantations per 1,000 patients. “Real-world data are needed to confirm these findings,” cautioned Dr. McLaughlin.
Dr. Hoeper disclosed speaking and consulting fees from Acceleron, Actelion, Altavant, AOP Health, Bayer, Ferrer, Janssen, Keros, and MSD. Dr. Sitbon disclosed speaking and consulting fees from Acceleron Pharmaceuticals, Altavant Sciences, AOP Orphan, Bayer, Ferrer, Gossamer Bio, Janssen, MSD, and United Therapeutics, and grant/research support from Acceleron Pharmaceuticals, AOP Orphan, Bayer, Janssen, and MSD. Dr. Preston disclosed speaking and consulting fees from Janssen and United Therapeutics, and grant/research support from Janssen and Respira Therapeutics. She has participated in scientific advisory boards for Aereovate, Altavant, and Gossamer Bio, and was in the Steering Committee of Acceleron, Liquidia, and United Therapeutics. Dr. McLaughlin has received speaking and consulting fees from Aerami, Aereovate, Caremark, Corvista, Enzyvant, Gossamer Bio, Janssen, Merck, United Therapeutics, and Vertex, and grant/research support from Aerovate, Enzyvant, Gossamer Bio, Janssen, Merck, and Sonovia. She is a member of the Board of Directors of Clene.
A version of this article first appeared on Medscape.com.
AT ERS 2023
Nivolumab/Ipillimumab combo demonstrates long-term efficacy in NSCLC
Long-term follow-up from the CheckMate 227 study has revealed lasting benefit from the combination of the CTLA-4 inhibitor ipilimumab (IPI) and the PD-1 inhibitor nivolumab (NIVO) in non-small cell lung cancer. , according to the latest analysis from the study.
“Patients treated with NIVO-IPI versus chemotherapy continue to derive long term durable efficacy benefit in CheckMate 227, regardless of PD-L1 expression. This represents the longest ever reported follow-up across phase three studies of frontline immunotherapy in patients with metastatic non–small cell lung cancer, and this further highlights the clinical benefit of frontline NIVO-IPI as a treatment in these patients with metastatic non–small cell lung cancer, regardless of the PD-L1 expression,” said Solange Peters, MD, PhD, during a presentation of the latest analysis at the annual World Conference on Lung Cancer. Dr. Peters is a professor of oncology at Lausanne (Switzerland) University Hospital.
The combination of nivolumab and ipilimumab has shown long-term survival benefit in other cancer types, including advanced melanoma, advanced renal cell carcinoma, and unresectable pleural mesothelioma.
The same session featured other studies demonstrating positive outcomes of immunotherapy in NSCLC. Serving as a discussant, Ferdinandos Skoulidis MD, PhD, commented, “I would argue that we are now at an inflection point where we can claim that we are altering the natural history of the disease for a subset of patients.” Dr. Skoulidis is an associate professor of thoracic oncology at the University of Texas MD Anderson Cancer Center.
Updated results
CheckMate 227 enrolled patients with metastatic or recurrent NSCLC, excluding those with EGFR/ALK alterations. Patients with PD-L1 expression greater than or equal to 1% (PD-L1 positive, n = 1,189) were randomized to NIVO-IPI, NIVO, or chemotherapy. Patients with PD-L1 expression less than 1% (n = 550, PD-L1 negative) were randomized to NIVO-IPI, NIVO plus chemotherapy, or chemotherapy alone. The 5-year landmark analysis, which was published by the National Center for Biotechnology Information, showed overall survival rate of 24% among PD-L1 greater than or equal to 1% patients (PD-L1 positive) and 19% in PD-L1 less than 1% (PD-L1 negative) patients who received IPI-NIVO therapy, compared with 14% and 7%, respectively, in the chemotherapy only groups.
At WCLC, Dr. Peters presented data extending to 6 years of follow-up, as well as exploratory analyses. At 6 years of follow-up, in PD-L1 positive patients, 22% of the NIVO-IPI group remained alive, versus 13% of the chemotherapy group (hazard ratio, 0.78; 95% confidence interval, 0.67-0.91), while there was no significant improvement in OS for nivolumab alone, compared with chemotherapy. In the PD-L1 negative group, 16% were alive at 6 years in the IPI-NIVO group (HR, 0.65; 95% CI, 0.52-0.81), as were 10% in NIVO plus chemotherapy (HR, 0.79; 95% CI, 0.64-0.98) group, versus 5% in the chemotherapy group. The benefit of NIVO-IPI was significant in both squamous and non-squamous tumors for both PDL1-positive and PD-L1 negative patients.
At 6 years follow-up, 27% of PD-L1 positive patients who responded to NIVO-IPI remained in response, versus 22% in the NIVO group and 4% in the chemotherapy only group. Among PD-L1 negative patients, 25% of combination therapy responders remained in response at 6 years, while there were 10% still in response among the NIVO group, and none in the chemotherapy only group.
Exploratory analyses
Dr. Peters presented a slide showing tumor burden reductions occurring in responders. “What has to be concluded from this very interesting graph is that there are more, deeper responses in the NIVO-IPI versus chemotherapy. Very importantly, too, this is strongly correlated with survival. In both treatment arms, a high magnitude of tumor burden reduction is correlated with an improved survival,” said Dr. Peters. Specifically, among PD-L1 positive patients with more than 80% tumor reduction, survival was 59% at 6 years (95% CI, 44-71%). The figure was 68% in the NIVO only arm (95% CI, 47-82%), and 42% in the chemotherapy only arm (95% CI, 15-66%).
Among PD-L1 negative patients, “there are more, deeper responses in NIVO-IPI versus chemotherapy. That is very clear. And probably differently from the positive PD-L1 arm, the tumor burden reduction is correlated with survival but really only strongly observed in the NIVO-IPI arm,” said Dr. Peters. The figure was 20% in the nivolumab arm (95% CI, 3-48%) and 0% in the chemotherapy only arm (95% CI, not available). “So really something is correlating the tumor burden reduction with the outcome and specifically correlating it in the negative PD-L1 with the treatment of NIVO-IPI,” said Dr. Peters.
The researchers also noted longer progression-free survival and overall response rate in the NIVO-IPI group than the chemotherapy group in both PD-L1 positive and PD-L1 negative patients.
With respect to health-related quality of life, the researchers found a correlation between higher scores at baseline on the EQ-5D-3L scale and overall survival in the chemotherapy group (HR, 0.61; 95% CI, 0.51-0.74) and a trend in the NIVO-IPI group (HR, 0.83; 95% CI, 0.69-1.01). “So this baseline history, the quality of life, is correlated with the outcome regardless of the treatment you deliver,” said Dr. Peters.
Personalizing immunotherapy in NSCLC
In his comments, Dr. Skoulidis highlighted the length of responses. “Most importantly, approximately 50% of these patients that are alive at six years are also disease free, suggesting that we are indeed making a dent on the natural history of the disease for these patients,” he said.
He also made a case for personalizing immunotherapy and suggested that CheckMate 227 could provide some guidance. “Ipilimumab/nivolumab – the CheckMate 227 regimen – appears to be particularly active in terms of inducing long-term, long-lasting responses and overall survival in patients harboring tumors that are negative for PD-L1,” he said.
Dr. Skoulidis also highlighted the 16% six-year overall survival among PD-L1 negative patients who received NIVO-IPI, calling it “impressive.” Of those who responded, 25% continued to respond at 6 years. “This is particularly notable in the subset of patients with squamous histology and lack of PD-L1 expression, where the six year overall survival rate with NIVO-IPI versus chemo was 18% versus 4%. So perhaps in patients with squamous histology and lack of PD-L1 expression, NIVO-IPI might represent a favorable regimen to improve long term outcomes,” said Dr. Skoulidis.
CheckMate 227 was funded by Bristol Myers Sqiubb. Dr. Peters has financial relationships with a wide range of pharmaceutical companies, including Bristol Myers Squibb. Dr. Skoulidis has financial relationships with Moderna, BioNTech, Amgen, Intellisphere, Navire, BeiGene, Medscape, Calithera Biosciences, Tango Therapeutics, Guardant Health, Novartis, AIMM Therapeutics, Mirati Therapeutics, Boehringer Ingelheim, Merck, and Pfizer.
Long-term follow-up from the CheckMate 227 study has revealed lasting benefit from the combination of the CTLA-4 inhibitor ipilimumab (IPI) and the PD-1 inhibitor nivolumab (NIVO) in non-small cell lung cancer. , according to the latest analysis from the study.
“Patients treated with NIVO-IPI versus chemotherapy continue to derive long term durable efficacy benefit in CheckMate 227, regardless of PD-L1 expression. This represents the longest ever reported follow-up across phase three studies of frontline immunotherapy in patients with metastatic non–small cell lung cancer, and this further highlights the clinical benefit of frontline NIVO-IPI as a treatment in these patients with metastatic non–small cell lung cancer, regardless of the PD-L1 expression,” said Solange Peters, MD, PhD, during a presentation of the latest analysis at the annual World Conference on Lung Cancer. Dr. Peters is a professor of oncology at Lausanne (Switzerland) University Hospital.
The combination of nivolumab and ipilimumab has shown long-term survival benefit in other cancer types, including advanced melanoma, advanced renal cell carcinoma, and unresectable pleural mesothelioma.
The same session featured other studies demonstrating positive outcomes of immunotherapy in NSCLC. Serving as a discussant, Ferdinandos Skoulidis MD, PhD, commented, “I would argue that we are now at an inflection point where we can claim that we are altering the natural history of the disease for a subset of patients.” Dr. Skoulidis is an associate professor of thoracic oncology at the University of Texas MD Anderson Cancer Center.
Updated results
CheckMate 227 enrolled patients with metastatic or recurrent NSCLC, excluding those with EGFR/ALK alterations. Patients with PD-L1 expression greater than or equal to 1% (PD-L1 positive, n = 1,189) were randomized to NIVO-IPI, NIVO, or chemotherapy. Patients with PD-L1 expression less than 1% (n = 550, PD-L1 negative) were randomized to NIVO-IPI, NIVO plus chemotherapy, or chemotherapy alone. The 5-year landmark analysis, which was published by the National Center for Biotechnology Information, showed overall survival rate of 24% among PD-L1 greater than or equal to 1% patients (PD-L1 positive) and 19% in PD-L1 less than 1% (PD-L1 negative) patients who received IPI-NIVO therapy, compared with 14% and 7%, respectively, in the chemotherapy only groups.
At WCLC, Dr. Peters presented data extending to 6 years of follow-up, as well as exploratory analyses. At 6 years of follow-up, in PD-L1 positive patients, 22% of the NIVO-IPI group remained alive, versus 13% of the chemotherapy group (hazard ratio, 0.78; 95% confidence interval, 0.67-0.91), while there was no significant improvement in OS for nivolumab alone, compared with chemotherapy. In the PD-L1 negative group, 16% were alive at 6 years in the IPI-NIVO group (HR, 0.65; 95% CI, 0.52-0.81), as were 10% in NIVO plus chemotherapy (HR, 0.79; 95% CI, 0.64-0.98) group, versus 5% in the chemotherapy group. The benefit of NIVO-IPI was significant in both squamous and non-squamous tumors for both PDL1-positive and PD-L1 negative patients.
At 6 years follow-up, 27% of PD-L1 positive patients who responded to NIVO-IPI remained in response, versus 22% in the NIVO group and 4% in the chemotherapy only group. Among PD-L1 negative patients, 25% of combination therapy responders remained in response at 6 years, while there were 10% still in response among the NIVO group, and none in the chemotherapy only group.
Exploratory analyses
Dr. Peters presented a slide showing tumor burden reductions occurring in responders. “What has to be concluded from this very interesting graph is that there are more, deeper responses in the NIVO-IPI versus chemotherapy. Very importantly, too, this is strongly correlated with survival. In both treatment arms, a high magnitude of tumor burden reduction is correlated with an improved survival,” said Dr. Peters. Specifically, among PD-L1 positive patients with more than 80% tumor reduction, survival was 59% at 6 years (95% CI, 44-71%). The figure was 68% in the NIVO only arm (95% CI, 47-82%), and 42% in the chemotherapy only arm (95% CI, 15-66%).
Among PD-L1 negative patients, “there are more, deeper responses in NIVO-IPI versus chemotherapy. That is very clear. And probably differently from the positive PD-L1 arm, the tumor burden reduction is correlated with survival but really only strongly observed in the NIVO-IPI arm,” said Dr. Peters. The figure was 20% in the nivolumab arm (95% CI, 3-48%) and 0% in the chemotherapy only arm (95% CI, not available). “So really something is correlating the tumor burden reduction with the outcome and specifically correlating it in the negative PD-L1 with the treatment of NIVO-IPI,” said Dr. Peters.
The researchers also noted longer progression-free survival and overall response rate in the NIVO-IPI group than the chemotherapy group in both PD-L1 positive and PD-L1 negative patients.
With respect to health-related quality of life, the researchers found a correlation between higher scores at baseline on the EQ-5D-3L scale and overall survival in the chemotherapy group (HR, 0.61; 95% CI, 0.51-0.74) and a trend in the NIVO-IPI group (HR, 0.83; 95% CI, 0.69-1.01). “So this baseline history, the quality of life, is correlated with the outcome regardless of the treatment you deliver,” said Dr. Peters.
Personalizing immunotherapy in NSCLC
In his comments, Dr. Skoulidis highlighted the length of responses. “Most importantly, approximately 50% of these patients that are alive at six years are also disease free, suggesting that we are indeed making a dent on the natural history of the disease for these patients,” he said.
He also made a case for personalizing immunotherapy and suggested that CheckMate 227 could provide some guidance. “Ipilimumab/nivolumab – the CheckMate 227 regimen – appears to be particularly active in terms of inducing long-term, long-lasting responses and overall survival in patients harboring tumors that are negative for PD-L1,” he said.
Dr. Skoulidis also highlighted the 16% six-year overall survival among PD-L1 negative patients who received NIVO-IPI, calling it “impressive.” Of those who responded, 25% continued to respond at 6 years. “This is particularly notable in the subset of patients with squamous histology and lack of PD-L1 expression, where the six year overall survival rate with NIVO-IPI versus chemo was 18% versus 4%. So perhaps in patients with squamous histology and lack of PD-L1 expression, NIVO-IPI might represent a favorable regimen to improve long term outcomes,” said Dr. Skoulidis.
CheckMate 227 was funded by Bristol Myers Sqiubb. Dr. Peters has financial relationships with a wide range of pharmaceutical companies, including Bristol Myers Squibb. Dr. Skoulidis has financial relationships with Moderna, BioNTech, Amgen, Intellisphere, Navire, BeiGene, Medscape, Calithera Biosciences, Tango Therapeutics, Guardant Health, Novartis, AIMM Therapeutics, Mirati Therapeutics, Boehringer Ingelheim, Merck, and Pfizer.
Long-term follow-up from the CheckMate 227 study has revealed lasting benefit from the combination of the CTLA-4 inhibitor ipilimumab (IPI) and the PD-1 inhibitor nivolumab (NIVO) in non-small cell lung cancer. , according to the latest analysis from the study.
“Patients treated with NIVO-IPI versus chemotherapy continue to derive long term durable efficacy benefit in CheckMate 227, regardless of PD-L1 expression. This represents the longest ever reported follow-up across phase three studies of frontline immunotherapy in patients with metastatic non–small cell lung cancer, and this further highlights the clinical benefit of frontline NIVO-IPI as a treatment in these patients with metastatic non–small cell lung cancer, regardless of the PD-L1 expression,” said Solange Peters, MD, PhD, during a presentation of the latest analysis at the annual World Conference on Lung Cancer. Dr. Peters is a professor of oncology at Lausanne (Switzerland) University Hospital.
The combination of nivolumab and ipilimumab has shown long-term survival benefit in other cancer types, including advanced melanoma, advanced renal cell carcinoma, and unresectable pleural mesothelioma.
The same session featured other studies demonstrating positive outcomes of immunotherapy in NSCLC. Serving as a discussant, Ferdinandos Skoulidis MD, PhD, commented, “I would argue that we are now at an inflection point where we can claim that we are altering the natural history of the disease for a subset of patients.” Dr. Skoulidis is an associate professor of thoracic oncology at the University of Texas MD Anderson Cancer Center.
Updated results
CheckMate 227 enrolled patients with metastatic or recurrent NSCLC, excluding those with EGFR/ALK alterations. Patients with PD-L1 expression greater than or equal to 1% (PD-L1 positive, n = 1,189) were randomized to NIVO-IPI, NIVO, or chemotherapy. Patients with PD-L1 expression less than 1% (n = 550, PD-L1 negative) were randomized to NIVO-IPI, NIVO plus chemotherapy, or chemotherapy alone. The 5-year landmark analysis, which was published by the National Center for Biotechnology Information, showed overall survival rate of 24% among PD-L1 greater than or equal to 1% patients (PD-L1 positive) and 19% in PD-L1 less than 1% (PD-L1 negative) patients who received IPI-NIVO therapy, compared with 14% and 7%, respectively, in the chemotherapy only groups.
At WCLC, Dr. Peters presented data extending to 6 years of follow-up, as well as exploratory analyses. At 6 years of follow-up, in PD-L1 positive patients, 22% of the NIVO-IPI group remained alive, versus 13% of the chemotherapy group (hazard ratio, 0.78; 95% confidence interval, 0.67-0.91), while there was no significant improvement in OS for nivolumab alone, compared with chemotherapy. In the PD-L1 negative group, 16% were alive at 6 years in the IPI-NIVO group (HR, 0.65; 95% CI, 0.52-0.81), as were 10% in NIVO plus chemotherapy (HR, 0.79; 95% CI, 0.64-0.98) group, versus 5% in the chemotherapy group. The benefit of NIVO-IPI was significant in both squamous and non-squamous tumors for both PDL1-positive and PD-L1 negative patients.
At 6 years follow-up, 27% of PD-L1 positive patients who responded to NIVO-IPI remained in response, versus 22% in the NIVO group and 4% in the chemotherapy only group. Among PD-L1 negative patients, 25% of combination therapy responders remained in response at 6 years, while there were 10% still in response among the NIVO group, and none in the chemotherapy only group.
Exploratory analyses
Dr. Peters presented a slide showing tumor burden reductions occurring in responders. “What has to be concluded from this very interesting graph is that there are more, deeper responses in the NIVO-IPI versus chemotherapy. Very importantly, too, this is strongly correlated with survival. In both treatment arms, a high magnitude of tumor burden reduction is correlated with an improved survival,” said Dr. Peters. Specifically, among PD-L1 positive patients with more than 80% tumor reduction, survival was 59% at 6 years (95% CI, 44-71%). The figure was 68% in the NIVO only arm (95% CI, 47-82%), and 42% in the chemotherapy only arm (95% CI, 15-66%).
Among PD-L1 negative patients, “there are more, deeper responses in NIVO-IPI versus chemotherapy. That is very clear. And probably differently from the positive PD-L1 arm, the tumor burden reduction is correlated with survival but really only strongly observed in the NIVO-IPI arm,” said Dr. Peters. The figure was 20% in the nivolumab arm (95% CI, 3-48%) and 0% in the chemotherapy only arm (95% CI, not available). “So really something is correlating the tumor burden reduction with the outcome and specifically correlating it in the negative PD-L1 with the treatment of NIVO-IPI,” said Dr. Peters.
The researchers also noted longer progression-free survival and overall response rate in the NIVO-IPI group than the chemotherapy group in both PD-L1 positive and PD-L1 negative patients.
With respect to health-related quality of life, the researchers found a correlation between higher scores at baseline on the EQ-5D-3L scale and overall survival in the chemotherapy group (HR, 0.61; 95% CI, 0.51-0.74) and a trend in the NIVO-IPI group (HR, 0.83; 95% CI, 0.69-1.01). “So this baseline history, the quality of life, is correlated with the outcome regardless of the treatment you deliver,” said Dr. Peters.
Personalizing immunotherapy in NSCLC
In his comments, Dr. Skoulidis highlighted the length of responses. “Most importantly, approximately 50% of these patients that are alive at six years are also disease free, suggesting that we are indeed making a dent on the natural history of the disease for these patients,” he said.
He also made a case for personalizing immunotherapy and suggested that CheckMate 227 could provide some guidance. “Ipilimumab/nivolumab – the CheckMate 227 regimen – appears to be particularly active in terms of inducing long-term, long-lasting responses and overall survival in patients harboring tumors that are negative for PD-L1,” he said.
Dr. Skoulidis also highlighted the 16% six-year overall survival among PD-L1 negative patients who received NIVO-IPI, calling it “impressive.” Of those who responded, 25% continued to respond at 6 years. “This is particularly notable in the subset of patients with squamous histology and lack of PD-L1 expression, where the six year overall survival rate with NIVO-IPI versus chemo was 18% versus 4%. So perhaps in patients with squamous histology and lack of PD-L1 expression, NIVO-IPI might represent a favorable regimen to improve long term outcomes,” said Dr. Skoulidis.
CheckMate 227 was funded by Bristol Myers Sqiubb. Dr. Peters has financial relationships with a wide range of pharmaceutical companies, including Bristol Myers Squibb. Dr. Skoulidis has financial relationships with Moderna, BioNTech, Amgen, Intellisphere, Navire, BeiGene, Medscape, Calithera Biosciences, Tango Therapeutics, Guardant Health, Novartis, AIMM Therapeutics, Mirati Therapeutics, Boehringer Ingelheim, Merck, and Pfizer.
FROM WCLC 2023
FDA approves JAK inhibitor momelotinib for myelofibrosis with anemia
Momelotinib is the fourth JAK inhibitor to be approved by the agency for myelofibrosis but the only one indicated for patients with hemoglobin levels below 10 g/dL.
It’s an important development because, while JAK inhibitors are standard treatment for myelofibrosis, those previously approved for the uncommon blood cancer can cause cytopenia, particularly anemia, which, ironically, is also a hallmark of myelofibrosis itself.
This issue makes using JAK inhibitors for myelofibrosis challenging, according to Anthony Hunter, MD, a myeloid malignancies specialist at Emory University, Atlanta, who spoke on the topic recently at the annual meeting of the Society of Hematologic Oncology in Houston. “Momelotinib is an important emerging agent for these more anemic patients.” Momelotinib has a spleen response comparable with ruxolitinib – the first JAK inhibitor approved for myelofibrosis in the United States – and significantly higher rates of transfusion independence, although lower rates of symptom control, he said.
In GSK’s press release, hematologist/oncologist Ruben Mesa, MD, executive director of Atrium Health Wake Forest Baptist Comprehensive Cancer Center, Winston-Salem, N.C., said that, “with momelotinib, we have the potential to establish a new standard of care for myelofibrosis patients with anemia.”
Momelotinib’s specific indication is for “the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis or secondary myelofibrosis (post–polycythemia vera and post–essential thrombocythemia), in adults with anemia.”
The once-daily oral medication was approved based on two trials. One trial, MOMENTUM, showed statistically significant response with respect to constitutional symptoms, splenic response, and transfusion independence in anemic patients treated with momelotinib versus danazol.
An anemic subset of the SIMPLIFY-1 trial showed comparable spleen volume reduction versus ruxolitinib but a numerically lower symptom response rate.
The most common momelotinib adverse reactions in trials were thrombocytopenia, hemorrhage, bacterial infection, fatigue, dizziness, diarrhea, and nausea.
A version of this article appeared on Medscape.com.
Momelotinib is the fourth JAK inhibitor to be approved by the agency for myelofibrosis but the only one indicated for patients with hemoglobin levels below 10 g/dL.
It’s an important development because, while JAK inhibitors are standard treatment for myelofibrosis, those previously approved for the uncommon blood cancer can cause cytopenia, particularly anemia, which, ironically, is also a hallmark of myelofibrosis itself.
This issue makes using JAK inhibitors for myelofibrosis challenging, according to Anthony Hunter, MD, a myeloid malignancies specialist at Emory University, Atlanta, who spoke on the topic recently at the annual meeting of the Society of Hematologic Oncology in Houston. “Momelotinib is an important emerging agent for these more anemic patients.” Momelotinib has a spleen response comparable with ruxolitinib – the first JAK inhibitor approved for myelofibrosis in the United States – and significantly higher rates of transfusion independence, although lower rates of symptom control, he said.
In GSK’s press release, hematologist/oncologist Ruben Mesa, MD, executive director of Atrium Health Wake Forest Baptist Comprehensive Cancer Center, Winston-Salem, N.C., said that, “with momelotinib, we have the potential to establish a new standard of care for myelofibrosis patients with anemia.”
Momelotinib’s specific indication is for “the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis or secondary myelofibrosis (post–polycythemia vera and post–essential thrombocythemia), in adults with anemia.”
The once-daily oral medication was approved based on two trials. One trial, MOMENTUM, showed statistically significant response with respect to constitutional symptoms, splenic response, and transfusion independence in anemic patients treated with momelotinib versus danazol.
An anemic subset of the SIMPLIFY-1 trial showed comparable spleen volume reduction versus ruxolitinib but a numerically lower symptom response rate.
The most common momelotinib adverse reactions in trials were thrombocytopenia, hemorrhage, bacterial infection, fatigue, dizziness, diarrhea, and nausea.
A version of this article appeared on Medscape.com.
Momelotinib is the fourth JAK inhibitor to be approved by the agency for myelofibrosis but the only one indicated for patients with hemoglobin levels below 10 g/dL.
It’s an important development because, while JAK inhibitors are standard treatment for myelofibrosis, those previously approved for the uncommon blood cancer can cause cytopenia, particularly anemia, which, ironically, is also a hallmark of myelofibrosis itself.
This issue makes using JAK inhibitors for myelofibrosis challenging, according to Anthony Hunter, MD, a myeloid malignancies specialist at Emory University, Atlanta, who spoke on the topic recently at the annual meeting of the Society of Hematologic Oncology in Houston. “Momelotinib is an important emerging agent for these more anemic patients.” Momelotinib has a spleen response comparable with ruxolitinib – the first JAK inhibitor approved for myelofibrosis in the United States – and significantly higher rates of transfusion independence, although lower rates of symptom control, he said.
In GSK’s press release, hematologist/oncologist Ruben Mesa, MD, executive director of Atrium Health Wake Forest Baptist Comprehensive Cancer Center, Winston-Salem, N.C., said that, “with momelotinib, we have the potential to establish a new standard of care for myelofibrosis patients with anemia.”
Momelotinib’s specific indication is for “the treatment of intermediate or high-risk myelofibrosis, including primary myelofibrosis or secondary myelofibrosis (post–polycythemia vera and post–essential thrombocythemia), in adults with anemia.”
The once-daily oral medication was approved based on two trials. One trial, MOMENTUM, showed statistically significant response with respect to constitutional symptoms, splenic response, and transfusion independence in anemic patients treated with momelotinib versus danazol.
An anemic subset of the SIMPLIFY-1 trial showed comparable spleen volume reduction versus ruxolitinib but a numerically lower symptom response rate.
The most common momelotinib adverse reactions in trials were thrombocytopenia, hemorrhage, bacterial infection, fatigue, dizziness, diarrhea, and nausea.
A version of this article appeared on Medscape.com.
Supplements Are Not a Synonym for Safe: Suspected Liver Injury From Ashwagandha
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
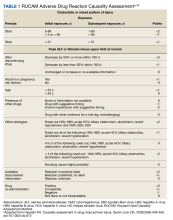
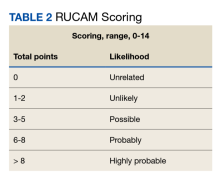
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
Many patients take herbals as alternative supplements to boost energy and mood. There are increasing reports of unintended adverse effects related to these supplements, particularly to the liver.1-3 A study by the Drug-Induced Liver Injury Network found that liver injury caused by herbals and dietary supplements has increased from 7% in 2004 to 20% in 2013.4
The supplement ashwagandha has become increasingly popular. Ashwagandha is extracted from the root of Withania somnifera (
To date, the factors defining the population at risk for ashwagandha toxicity are unclear, and an understanding of how to diagnose drug-induced liver injury is still immature in clinical practice. The regulation and study of the herbal and dietary supplement industry remain challenging. While many so-called natural substances are well tolerated, others can have unanticipated and harmful adverse effects and drug interactions. Future research should not only identify potentially harmful substances, but also which patients may be at greatest risk.
Case Presentation
A 48-year-old man with a history of severe alcohol use disorder (AUD) complicated by fatty liver and withdrawal seizures and delirium tremens, hypertension, depression, and anxiety presented to the emergency department (ED) after 4 days of having jaundice, epigastric abdominal pain, dark urine, and pale stools. In the preceding months, he had increased his alcohol use to as many as 12 drinks daily due to depression. After experiencing a blackout, he stopped drinking 7 days before presenting to the ED. He felt withdrawal symptoms, including tremors, diaphoresis, abdominal pain, nausea, and vomiting. On the third day of withdrawals, he reported that he had started taking an over-the-counter testosterone-boosting supplement to increase his energy, which he referred to as TestBoost—a mix of 8 ingredients, including ashwagandha, eleuthero root, Hawthorn berry, longjack, ginseng root, mushroom extract, bindii, and horny goat weed. After taking the supplement for 2 days, he noticed that his urine darkened, his stools became paler, his abdominal pain worsened, and he became jaundiced. After 2 additional days without improvement, and still taking the supplement, he presented to the ED. He reported having no fever, chills, recent illness, chest pain, shortness of breath, melena, lower extremity swelling, recent travel, or any changes in medications.
The patient had a 100.1 °F temperature, 102 beats per minute pulse; 129/94 mm Hg blood pressure, 18 beats per minute respiratory rate, and 97% oxygen saturation on room air on admission. He was in no acute distress, though his examination was notable for generalized jaundice and scleral icterus. He was mildly tender to palpation in the epigastric and right upper quadrant region. He was alert and oriented without confusion. He did not have any asterixis or spider angiomas, though he had scattered bruises on his left flank and left calf. His laboratory results were notable for mildly elevated aspartate aminotransferase (AST), 58 U/L (reference range, 13-35); alanine transaminase (ALT), 49 U/L (reference range, 7-45); and alkaline phosphatase (ALP), 98 U/L (reference range 33-94); total bilirubin, 13.6 mg/dL (reference range, 0.2-1.0); direct bilirubin, 8.4 mg/dL (reference range, 0.2-1); and international normalized ratio (INR), 1.11 (reference range, 2-3). His white blood cell and platelet counts were not remarkable at 9790/μL (reference range, 4500-11,000) and 337,000/μL (reference range, 150,000-440,000), respectively. Abdominal ultrasound and computed tomography (CT) revealed fatty liver with contracted gallbladder and no biliary dilatation. Urine ethanol levels were negative. The gastrointestinal (GI) service was consulted and agreed that his cholestatic injury was nonobstructive and likely related to the ashwagandha component of his supplement. The recommendation was cessation with close outpatient follow-up.
The patient was not prescribed any additional medications, such as steroids or ursodiol. He ceased supplement use following hospitalization; but relapsed into alcohol use 1 month after his discharge. Within 3 weeks, his total bilirubin had improved to 2.87 mg/dL, though AST, ALT, and ALP worsened to 127 U/L, 152 U/L, and 140 U/L, respectively. According to the notes of his psychiatrist who saw him at the time the laboratory tests were drawn, he had remained sober since discharge. His acute hepatitis panel drawn on admission was negative, and he demonstrated immunity to hepatitis A and B. Urine toxicology was negative. Antinuclear antibody (ANA) test was negative 1 year prior to discharge. Epstein-Barr virus (EBV), cytomegalovirus (CMV), ANA, antismooth muscle antibody, and immunoglobulins were not checked as suspicion for these etiologies was low. The Roussel Uclaf Causality Assessment Method (RUCAM) score was calculated as 6 (+1 for timing, +2 for drop in total bilirubin, +1 for ethanol risk factor, 0 for no other drugs, 0 for rule out of other diseases, +2 for known hepatotoxicity, 0 no repeat administration) for this patient indicating probable adverse drug reaction liver injury (Tables 1 and 2). However, we acknowledge that CMV, EBV, and herpes simplex virus status were not tested.
The 8 ingredients contained in TestBoost aside from ashwagandha did not have any major known liver adverse effects per a major database of medications. The other ingredients include eleuthero root, Hawthorn berry (crataegus laevigata), longjack (eurycoma longifolla) root, American ginseng root (American panax ginseng—panax quinquefolius), and Cordyceps mycelium (mushroom) extract, bindii (Tribulus terrestris), and epimedium grandiflorum (horny goat weed).6 No assays were performed to confirm purity of the ingredients in the patient’s supplement container.
Alcoholic hepatitis is an important consideration in this patient with AUD, though the timing of symptoms with supplement use and the cholestatic injury pattern with normal INR seems more consistent with drug-induced injury. Viral, infectious, and obstructive etiologies also were investigated. Acute viral hepatitis was ruled out based on bloodwork. The normal hepatobiliary tree on both ultrasound and CT effectively ruled out acute cholecystitis, cholangitis, and choledocholithiasis and there was no further indication for magnetic resonance cholangiopancreatography. There was no hepatic vein clot suggestive of Budd-Chiari syndrome. Autoimmune hepatitis was thought to be unlikely given that the etiology of injury seemed cholestatic in nature. Given the timing of the liver injury relative to supplement use it is likely that ashwagandha was a causative factor of this patient’s liver injury overlaid on an already strained liver from increased alcohol abuse.
The patient did not follow up with the GI service as an outpatient. There are no reports that the patient continued using the testosterone booster. His bilirubin improved dramatically within 1.5 months while his liver enzymes peaked 3 weeks later, with ALT ≥ AST. During his next admission 3 months later, he had relapsed, and his liver enzymes had the classic 2:1 AST to ALT ratio.
Discussion
Generally, ashwagandha has been thought to be well tolerated and possibly hepatoprotective.7-10 However, recent studies suggest potential for hepatotoxicity, though without clear guidance about which patients are most at risk.5,11,12 A study by Inagaki and colleagues suggests the potential for dose-dependent mechanism of liver injury, and this is supported by in vitro CYP450 inhibition with high doses of W Somnifera extract.11,13 We hypothesize that there may be a multihit process that makes some patients more susceptible to supplement harm, particularly those with repeated exposures and with ongoing exposure to hepatic toxins, such as AUD.14 Supplements should be used with more caution in these individuals.
Additionally, although there are no validated guidelines to confirm the diagnosis of drug-induced liver injury (DILI) from a manufactured medication or herbal remedy, the Council for International Organizations of Medical Sciences (CIOMS) developed RUCAM, a set of diagnostic criteria for DILI, which can be used to determine the probability of DILI based on pattern of injury.15 Although not widely used in clinical practice, RUCAM can help identify the possibility of DILI outside of expert consensus.16 It seems to have better discriminative ability than the Maria and Victorino scale, also used to identify DILI.16,17 While there is no replacement for clinical judgment, these scales may aid in identifying potential causes of DILI. The National Institutes of Health also has a LiverTox online tool that can assist health care professionals in identifying potentially hepatotoxic substances.6
Conclusions
We present a patient with AUD who developed cholestatic liver injury after ashwagandha use. Crucial to the diagnostic process is quantifying the amount ingested before presentation and the presence of contaminants, which is currently difficult to quantify given the lack of mechanisms to test supplements expediently in this manner in the clinical setting, which also requires the patient to bring in the supplements directly. There is also a lack of regulation and uniformity in these products. A clinician may be inclined to measure ashwagandha serum levels; however, such a test is not available to our knowledge. Nonetheless, using clinical tools such as RUCAM and utilizing databases, such as LiverTox, may help clinicians identify and remove potentially unsafe supplements. While there are many possible synergies between current medical practice and herbal remedies, practitioners must take care to first do no harm, as outlined in our Hippocratic Oath.
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
1. Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29(4):373-382. doi:10.1055/s-0029-1240006
2. Suk KT, Kim DJ, Kim CH, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380-1387. doi:10.1038/ajg.2012.138
3. Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology. 2019;156(8):2230-2241.e11. doi:10.1053/j.gastro.2019.02.002
4. Navarro VJ, Barnhart H, Bonkovsky HL, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399-1408. doi:10.1002/hep.27317
5. Björnsson HK, Björnsson, Avula B, et al. (2020). Ashwagandha‐induced liver injury: a case series from Iceland and the US Drug‐Induced Liver Injury Network. Liver Int. 2020;40(4):825-829. doi:10.1111/liv.14393
6. National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: clinical and research information on drug-induced liver injury [internet]. Ashwagandha. Updated May 2, 2019. Accessed August 7, 2023. https://www.ncbi.nlm.nih.gov/books/NBK548536
7. Kumar G, Srivastava A, Sharma SK, Rao TD, Gupta YK. Efficacy and safety evaluation of Ayurvedic treatment (ashwagandha powder & Sidh Makardhwaj) in rheumatoid arthritis patients: a pilot prospective study. Indian J Med Res. 2015;141(1):100-106. doi:10.4103/0971-5916.154510
8. Kumar G, Srivastava A, Sharma SK, Gupta YK. Safety and efficacy evaluation of Ayurvedic treatment (arjuna powder and Arogyavardhini Vati) in dyslipidemia patients: a pilot prospective cohort clinical study. 2012;33(2):197-201. doi:10.4103/0974-8520.105238
9. Sultana N, Shimmi S, Parash MT, Akhtar J. Effects of ashwagandha (Withania somnifera) root extract on some serum liver marker enzymes (AST, ALT) in gentamicin intoxicated rats. J Bangladesh Soc Physiologist. 2012;7(1): 1-7. doi:10.3329/JBSP.V7I1.11152
10. Patel DP, Yan T, Kim D, et al. Withaferin A improves nonalcoholic steatohepatitis in mice. J Pharmacol Exp Ther. 2019;371(2):360-374. doi:10.1124/jpet.119.256792
11. Inagaki K, Mori N, Honda Y, Takaki S, Tsuji K, Chayama K. A case of drug-induced liver injury with prolonged severe intrahepatic cholestasis induced by ashwagandha. Kanzo. 2017;58(8):448-454. doi:10.2957/kanzo.58.448
12. Alali F, Hermez K, Ullah N. Acute hepatitis induced by a unique combination of herbal supplements. Am J Gastroenterol. 2018;113:S1661.
13. Sava J, Varghese A, Pandita N. Lack of the cytochrome P450 3A interaction of methanolic extract of Withania somnifera, Withaferin A, Withanolide A and Withanoside IV. J Pharm Negative Results. 2013;4(1):26.
14. Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474-485. doi:10.1056/NEJMra021844.
15. Danan G, Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of International Consensus Meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1333. doi:10.1016/0895-4356(93)90101-6
16. Hayashi PH. Causality assessment in drug-induced liver injury. Semin Liver Dis. 2009;29(4):348-356. doi.10.1002/cld.615
17. Lucena MI, Camargo R, Andrade RJ, Perez-Sanchez CJ, Sanchez De La Cuesta F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology. 2001;33(1):123-130. doi:10.1053/jhep.2001.20645
Olfactory Hallucinations Following COVID-19 Vaccination
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
The rapid development of multiple vaccines for COVID-19 significantly contributed to reducing the morbidity and mortality associated with COVID-19 infection.1 The vaccination campaign against COVID-19 started in December 2020 within the US Department of Veterans Affairs (VA) health care system with the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines followed by the Johnson & Johnson (J&J) vaccine in March 2021.2,3
Because of the importance of maintaining a safe vaccination campaign, surveillance reports documenting cases of malignant or benign adverse effects (AEs) are fundamental to generate awareness and accurate knowledge on these newly developed vaccines. Here we report the case of a veteran who developed olfactory hallucinations following the administration of the J&J COVID-19 vaccine.
Case Presentation
A 39-year-old veteran with a history of tension-type headaches presented to the neurology clinic with concern of a burning smell sensation in the absence of an identifiable source. He first noticed this symptom approximately 3 weeks after he received the J&J COVID-19 vaccine about 4 months prior. At the symptom’s first occurrence, he underwent a nasal swab antigen COVID-19 test, which was negative. Initially, symptoms would occur daily lasting about 1 hour. Thereafter, they started to decrease in duration, frequency, and intensity, and about 11 months postvaccination, milder episodes were occurring 1 to 2 times weekly. These episodes lasted nearly 2 years (21 months postvaccination). They happened randomly during the day and were not associated with any other symptoms. Specifically, there were no headaches, loss of consciousness, abnormal movements, nausea, vomiting, photophobia or phonophobia, or alteration of consciousness, such as confusion or drowsiness during or after the events. Additionally, there were no clear triggers the veteran could identify. The veteran did not sustain any head injuries or exposure to toxic odors before the onset of symptoms.
At the time of his presentation to the clinic, both his general and neurological examinations were unremarkable.
Discussion
It has been previously observed that infection with COVID-19 can lead to the loss of taste and smell, but only less commonly olfactory hallucination.4 The pathophysiology of olfactory hallucinations following COVID-19 infection is unknown, but several mechanisms have been proposed. These include obstruction of the olfactory cleft; infection of the sustentacular supporting cells, which express angiotensin‐converting enzyme 2 (ACE‐2); injury to olfactory sensory cells via neuropilin‐1 receptors (NRP1); and injury to the olfactory bulb.5
The case we present represents the only report of phantosmia following a J&J COVID-19 vaccination. Phantosmia, featured by a burning or smoke odor, has been reported prior in a case of a 57-year-old woman following the administration of the Pfizer-BioNTech mRNA vaccine.6 Similar to our case, symptoms were not associated with a concurrent COVID-19 infection ruled out via a COVID-19 polymerase chain reaction test. For the Pfizer-BioNTech phantosmia case, a 3 Tesla (T) brain MRI showed left greater than right olfactory bulb and tract gadolinium enhancement on T1-weighted postcontrast images. On axial T2-weighted fluid-attenuated inversion recovery images, hyperintensity along the left olfactory bulb and bilateral olfactory tracts was noted and interpreted as edema. On sagittal thin sections of T2-weighted images, the olfactory nerve filia were thickened and clumped.6 On the contrary, in the case we present, a brain MRI obtained with a 1.5 T magnet showed no abnormalities. It is possible that a high-resolution scan targeting the olfactory bulb could have disclosed pathological changes. At the time when the veteran presented to the neurology clinic, symptoms were already improving, and repeat MRI was deferred as it would not have changed the clinical management.
Konstantinidis and colleagues reported hyposmia in 2 patients following Pfizer-BioNTech COVID-19 vaccination.5 Both patients, 42- and 39-year-old women, experienced hyposmia following their second dose of the vaccine with symptom onset 3 and 5 days after vaccination, respectively. The first patient reported improvement of symptoms after 1 week, while the second patient participated in olfactory training and experienced only partial recovery after 1 month. Multiple studies have reported cranial nerve involvement secondary to other COVID-19 vaccines, including olfactory dysfunction, optic neuritis, acute abducens nerve palsy, Bell palsy, tinnitus, and cochleopathy.7
There are no previous reports of phantosmia following the J&J COVID-19 vaccine. In our case, reported symptoms were mild, although they persisted for nearly 2 years following vaccination.
In the evaluation of this veteran, although the timing between symptom onset and vaccination was indicative of a possible link between the 2, other etiologies of phantosmia were ruled out. Isolated olfactory hallucination is most associated with temporal lobe epilepsy, which is the most common form of epilepsy to present in adulthood. However, given the absence of other symptoms suggestive of epilepsy and the duration of the episodes (approximately 1 hour), the clinical suspicion was low. This was reinforced by the EEG that showed no abnormalities in the temporal region. Notwithstanding these considerations, one must keep in mind that no episodes of phantosmia occurred during the EEG recording, the correlates of which are the gold standard to rule out a diagnosis of epilepsy.
A normal brain MRI argued against possible structural abnormalities leading to these symptoms. Thus, the origin of these symptoms remains unknown.
Conclusions
The emergency approval and use of vaccines against COVID-19 was a major victory for public health in 2021. However, given the rapid rollout of these vaccines, the medical community is responsible for reporting adverse effects as they are observed. The authors believe that the clinical events featuring the J&J COVID-19 vaccine in this veteran should not discourage the use of the COVID-19 vaccine. However, sharing the clinical outcome of this veteran is relevant to inform the community regarding this rare and benign possible adverse effect of the J&J COVID-19 vaccine.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Tennessee Valley Veteran Healthcare System (Nashville). The authors thank Dr. Martin Gallagher (Tennessee Valley Veteran Healthcare System) for providing clinical expertise with electroencephalogram interpretation.
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
1. Xu S, Huang R, Sy LS, et al. COVID-19 vaccination and non-COVID-19 mortality risk - seven integrated health care organizations, United States, December 14, 2020-July 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1520-1524. Published 2021 Oct 29. doi:10.15585/mmwr.mm7043e2
2. Der-Martirosian C, Steers WN, Northcraft H, Chu K, Dobalian A. Vaccinating veterans for COVID-19 at the U.S. Department of Veterans Affairs. Am J Prev Med. 2022;62(6):e317-e324. doi:10.1016/j.amepre.2021.12.016
3. Bagnato F, Wallin M. COVID-19 vaccine in veterans with multiple sclerosis: protect the vulnerable. Fed Pract. 2021;38(suppl 1):S28-S32. doi:10.12788/fp.0113
4. Işlek A, Balcı MK. Phantosmia with COVID-19 related olfactory dysfunction: report of nine cases. Indian J Otolaryngol Head Neck Surg. 2022;74(suppl 2):2891-2893. doi:10.1007/s12070-021-02505-z
5. Konstantinidis I, Tsakiropoulou E, Hähner A, de With K, Poulas K, Hummel T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int Forum Allergy Rhinol. 2021;11(9):1399-1401. doi:10.1002/alr.22809
6. Keir G, Maria NI, Kirsch CFE. Unique imaging findings of neurologic phantosmia following Pfizer-BioNtech COVID-19 vaccination: a case report. Top Magn Reson Imaging. 2021;30(3):133-137. doi:10.1097/RMR.0000000000000287
7. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43(1):3-40. doi:10.1007/s10072-021-05662-9
Chimeric Antigen Receptor T-Cell Therapy in the Veterans Affairs Network: the Tennessee Valley Healthcare System Experience
BACKGROUND
Chimeric antigen receptor T-cell (CAR-T) therapy is a novel treatment for hematologic malignancies, with 6 FDA agents approved for commercial use. The Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS) is currently the only VA facility accredited to administer these agents and we are reporting the TVHS experience thus far.
METHODS
TVHS became an authorized treatment center for CAR-T therapy in September 2019 and performed its first CAR-T infusion in December 2019. This is a retrospective electronic chart review of all CAR-T veterans referred to TVHS from the program’s inception, December 1, 2019 through July 31, 2022 to evaluate at least one year of post infusion data. The primary objective is to evaluate the outcomes of veterans who received CAR-T therapy at TVHS including overall response rates (ORR), progression free survival (PFS), and overall survival (OS). Secondary objectives include assessment of toxicities, including rates and maximum grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
RESULTS
A total of 41 veterans have received CAR-T infusion at TVHS to date. Twenty-nine of these veterans have at least one year post-CAR-T infusion data and are included in this analysis. The majority of veterans were White (72%), male (93%), and were treated for diffuse large B-cell lymphoma (86%). Twenty-eight percent of veterans were under-represented minorities. Average age was 61 years with 62% being 65 years and older and five (17%) veterans being over the age of 74. Day 30 ORR was 90% (45% complete response [CR]). One-year PFS was 55.2% and 1-year OS was 65.5%. Of the 19 veterans who achieved CR by day 100, 79% remain in CR to date. CRS toxicity was observed in 66% of veterans (0% Grade 3 or higher). ICANS was observed in 27.5% of veterans (24% Grade 3 or higher). Only 5 (26%) veterans required transfer to the intensive care unit for additional monitoring.
CONCLUSIONS
CAR-T therapy has become a wellestablished practice at TVHS and is a safe and effective treatment option for veterans with aggressive lymphoid malignancies. Our outcomes are similar to that seen nationally with better access to under-represented minorities in an aging population.
BACKGROUND
Chimeric antigen receptor T-cell (CAR-T) therapy is a novel treatment for hematologic malignancies, with 6 FDA agents approved for commercial use. The Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS) is currently the only VA facility accredited to administer these agents and we are reporting the TVHS experience thus far.
METHODS
TVHS became an authorized treatment center for CAR-T therapy in September 2019 and performed its first CAR-T infusion in December 2019. This is a retrospective electronic chart review of all CAR-T veterans referred to TVHS from the program’s inception, December 1, 2019 through July 31, 2022 to evaluate at least one year of post infusion data. The primary objective is to evaluate the outcomes of veterans who received CAR-T therapy at TVHS including overall response rates (ORR), progression free survival (PFS), and overall survival (OS). Secondary objectives include assessment of toxicities, including rates and maximum grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
RESULTS
A total of 41 veterans have received CAR-T infusion at TVHS to date. Twenty-nine of these veterans have at least one year post-CAR-T infusion data and are included in this analysis. The majority of veterans were White (72%), male (93%), and were treated for diffuse large B-cell lymphoma (86%). Twenty-eight percent of veterans were under-represented minorities. Average age was 61 years with 62% being 65 years and older and five (17%) veterans being over the age of 74. Day 30 ORR was 90% (45% complete response [CR]). One-year PFS was 55.2% and 1-year OS was 65.5%. Of the 19 veterans who achieved CR by day 100, 79% remain in CR to date. CRS toxicity was observed in 66% of veterans (0% Grade 3 or higher). ICANS was observed in 27.5% of veterans (24% Grade 3 or higher). Only 5 (26%) veterans required transfer to the intensive care unit for additional monitoring.
CONCLUSIONS
CAR-T therapy has become a wellestablished practice at TVHS and is a safe and effective treatment option for veterans with aggressive lymphoid malignancies. Our outcomes are similar to that seen nationally with better access to under-represented minorities in an aging population.
BACKGROUND
Chimeric antigen receptor T-cell (CAR-T) therapy is a novel treatment for hematologic malignancies, with 6 FDA agents approved for commercial use. The Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS) is currently the only VA facility accredited to administer these agents and we are reporting the TVHS experience thus far.
METHODS
TVHS became an authorized treatment center for CAR-T therapy in September 2019 and performed its first CAR-T infusion in December 2019. This is a retrospective electronic chart review of all CAR-T veterans referred to TVHS from the program’s inception, December 1, 2019 through July 31, 2022 to evaluate at least one year of post infusion data. The primary objective is to evaluate the outcomes of veterans who received CAR-T therapy at TVHS including overall response rates (ORR), progression free survival (PFS), and overall survival (OS). Secondary objectives include assessment of toxicities, including rates and maximum grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
RESULTS
A total of 41 veterans have received CAR-T infusion at TVHS to date. Twenty-nine of these veterans have at least one year post-CAR-T infusion data and are included in this analysis. The majority of veterans were White (72%), male (93%), and were treated for diffuse large B-cell lymphoma (86%). Twenty-eight percent of veterans were under-represented minorities. Average age was 61 years with 62% being 65 years and older and five (17%) veterans being over the age of 74. Day 30 ORR was 90% (45% complete response [CR]). One-year PFS was 55.2% and 1-year OS was 65.5%. Of the 19 veterans who achieved CR by day 100, 79% remain in CR to date. CRS toxicity was observed in 66% of veterans (0% Grade 3 or higher). ICANS was observed in 27.5% of veterans (24% Grade 3 or higher). Only 5 (26%) veterans required transfer to the intensive care unit for additional monitoring.
CONCLUSIONS
CAR-T therapy has become a wellestablished practice at TVHS and is a safe and effective treatment option for veterans with aggressive lymphoid malignancies. Our outcomes are similar to that seen nationally with better access to under-represented minorities in an aging population.
Real-World Evidence of Safety Trends Using Rituximab-PVVR in Clinic Infusions
BACKGROUND
The safety and efficacy of biosimilars are carefully reviewed by the Food and Drug Administration (FDA) to ensure the biosimilar meets the high standards for approval. However, safety concerns from infusion nursing staff prompted a review of rituximab-PVVR and rituximab for any new trends in National VA, primary literature, and facility adverse events.
METHODS
Utilizing the VA ADERS (Veteran’s Affairs Adverse Drug Event Reporting System), data was analyzed from 01/01/21 thru 04/01/23. No clear trends were identified to support an increased reaction rate for Rituximab-PVVR or Rituximab. A total of 104 Rituximab product (both parent and biosimilar products) adverse reactions were reported nationally. Of those reported, about half 56 ADEs (54%) were specifically to Rituximab-PVVR.
RESULTS
Reviewing our facility specific VA ADERS data, Birmingham VA reported 7 ADEs. Similarly other sites reported a range of 0 to 13 Rituximab product ADEs. The total number of unique patients to receive a rituximab product in the Birmingham VA since 2021 is 106, resulting in an overall incidence rate of 6.6%.
DISCUSSION
Based on the recent publication, Safety of switching between rituximab biosimilars in onco-hematology “adverse events were similar, in terms of seriousness and frequency, to those described in the literature, providing further support to the clinical safety of biosimilars.” This prospective clinical trial published in 2021, reported grade 1 rituximab related infusion events in 7.1% of patients (n=83) which correlates closely to the reported incidence at our facility referenced above (6.6%). Our current pre-medications include acetaminophen, an antihistamine, and steroid 30 minutes prior to infusion. Although our interdisciplinary team deemed this appropriate, to improve and minimize infusion reaction symptoms, the following interventions were instituted including changing ORAL Diphenhydramine to intravenous Diphenhydramine 25mg IV and providing education to infusion nursing staff on the safety and efficacy of the rituximab and biosimilar products.
CONCLUSIONS
Following the intervention (04/07/23), 36 total unique patients received rituximab products with zero incidents reported. Although the results are limited, the data may suggest IV diphenhydramine reduces the severity of ADEs which may alter reporting or show a potential “nocebo” effect could be a factor with any rituximab infusion needing further evaluation.
BACKGROUND
The safety and efficacy of biosimilars are carefully reviewed by the Food and Drug Administration (FDA) to ensure the biosimilar meets the high standards for approval. However, safety concerns from infusion nursing staff prompted a review of rituximab-PVVR and rituximab for any new trends in National VA, primary literature, and facility adverse events.
METHODS
Utilizing the VA ADERS (Veteran’s Affairs Adverse Drug Event Reporting System), data was analyzed from 01/01/21 thru 04/01/23. No clear trends were identified to support an increased reaction rate for Rituximab-PVVR or Rituximab. A total of 104 Rituximab product (both parent and biosimilar products) adverse reactions were reported nationally. Of those reported, about half 56 ADEs (54%) were specifically to Rituximab-PVVR.
RESULTS
Reviewing our facility specific VA ADERS data, Birmingham VA reported 7 ADEs. Similarly other sites reported a range of 0 to 13 Rituximab product ADEs. The total number of unique patients to receive a rituximab product in the Birmingham VA since 2021 is 106, resulting in an overall incidence rate of 6.6%.
DISCUSSION
Based on the recent publication, Safety of switching between rituximab biosimilars in onco-hematology “adverse events were similar, in terms of seriousness and frequency, to those described in the literature, providing further support to the clinical safety of biosimilars.” This prospective clinical trial published in 2021, reported grade 1 rituximab related infusion events in 7.1% of patients (n=83) which correlates closely to the reported incidence at our facility referenced above (6.6%). Our current pre-medications include acetaminophen, an antihistamine, and steroid 30 minutes prior to infusion. Although our interdisciplinary team deemed this appropriate, to improve and minimize infusion reaction symptoms, the following interventions were instituted including changing ORAL Diphenhydramine to intravenous Diphenhydramine 25mg IV and providing education to infusion nursing staff on the safety and efficacy of the rituximab and biosimilar products.
CONCLUSIONS
Following the intervention (04/07/23), 36 total unique patients received rituximab products with zero incidents reported. Although the results are limited, the data may suggest IV diphenhydramine reduces the severity of ADEs which may alter reporting or show a potential “nocebo” effect could be a factor with any rituximab infusion needing further evaluation.
BACKGROUND
The safety and efficacy of biosimilars are carefully reviewed by the Food and Drug Administration (FDA) to ensure the biosimilar meets the high standards for approval. However, safety concerns from infusion nursing staff prompted a review of rituximab-PVVR and rituximab for any new trends in National VA, primary literature, and facility adverse events.
METHODS
Utilizing the VA ADERS (Veteran’s Affairs Adverse Drug Event Reporting System), data was analyzed from 01/01/21 thru 04/01/23. No clear trends were identified to support an increased reaction rate for Rituximab-PVVR or Rituximab. A total of 104 Rituximab product (both parent and biosimilar products) adverse reactions were reported nationally. Of those reported, about half 56 ADEs (54%) were specifically to Rituximab-PVVR.
RESULTS
Reviewing our facility specific VA ADERS data, Birmingham VA reported 7 ADEs. Similarly other sites reported a range of 0 to 13 Rituximab product ADEs. The total number of unique patients to receive a rituximab product in the Birmingham VA since 2021 is 106, resulting in an overall incidence rate of 6.6%.
DISCUSSION
Based on the recent publication, Safety of switching between rituximab biosimilars in onco-hematology “adverse events were similar, in terms of seriousness and frequency, to those described in the literature, providing further support to the clinical safety of biosimilars.” This prospective clinical trial published in 2021, reported grade 1 rituximab related infusion events in 7.1% of patients (n=83) which correlates closely to the reported incidence at our facility referenced above (6.6%). Our current pre-medications include acetaminophen, an antihistamine, and steroid 30 minutes prior to infusion. Although our interdisciplinary team deemed this appropriate, to improve and minimize infusion reaction symptoms, the following interventions were instituted including changing ORAL Diphenhydramine to intravenous Diphenhydramine 25mg IV and providing education to infusion nursing staff on the safety and efficacy of the rituximab and biosimilar products.
CONCLUSIONS
Following the intervention (04/07/23), 36 total unique patients received rituximab products with zero incidents reported. Although the results are limited, the data may suggest IV diphenhydramine reduces the severity of ADEs which may alter reporting or show a potential “nocebo” effect could be a factor with any rituximab infusion needing further evaluation.
Does Gemcitabine Have a Curative Role in Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia?
INTRODUCTION
Gemcitabine is a part of National Comprehensive Cancer Network (NCCN) guidelines as salvage therapy for relapsed/refractory B-cell lymphomas, but its role in chronic lymphocytic leukemia (CLL) remains unclear. We describe a case of relapsed CLL showing complete response while on gemcitabine for another primary malignancy, suggesting a potential curative role of gemcitabine for CLL.
CASE REPORT
A 78-year-old male with relapsed CD38+ CLL with del11q on ibrutinib with partial response, presented with gross hematuria for one week. Of note, he was diagnosed with BRCA-negative Stage Ib pancreatic adenocarcinoma within the previous year, treated with surgery and adjuvant capecitabine-gemcitabine. Physical examination was unremarkable and bloodwork showed a white cell count of 32,000 cells/ mm3 with 1.5% lymphocytes, hemoglobin 9.5 g/dL, and platelets 866,000 cells/mm3. Hematuria remained persistent despite frequent bladder irrigations but resolved within a week of stopping ibrutinib. Eight months later, his white cell count is 6,600 cells/mm3, with 16% lymphocytes, hemoglobin 10.2 g/dL, platelets 519,000/m3, and CT scans show no pathological lymphadenopathy. A recent flow cytometry done for academic purposes showed no clonal B cells.
DISCUSSION
Relapsed CLL has a poor prognosis with no curative treatment. Gemcitabine is a part of NCCN guidelines for relapse/refractory B-cell lymphomas but is not included in guidelines for CLL. A study by Jamie et al in 2001 suggested the pre-clinical effectiveness of gemcitabine for relapsed/refractory CLL and phase II trials conducted in 2005 and 2012 on combination chemotherapy including gemcitabine have shown overall CLL response rates of 50-65%. The resolution of B-cell clonality and improvement in biochemical markers after treatment with gemcitabine for an alternate primary malignancy suggested that gemcitabine played a potential curative role in our patient. Further prospective studies are needed to explore this avenue for the role of gemcitabine as a salvage as well as potentially curative therapy for relapsed CLL with variable cytogenetics and treatment histories.
CONCLUSIONS
Gemcitabine is not part of NCCN guidelines for CLL currently but it is a reasonable treatment option for relapsed/refractory CLL. Further studies are needed to explore its potential curative role for relapsed CLL, and update existing guidelines.
INTRODUCTION
Gemcitabine is a part of National Comprehensive Cancer Network (NCCN) guidelines as salvage therapy for relapsed/refractory B-cell lymphomas, but its role in chronic lymphocytic leukemia (CLL) remains unclear. We describe a case of relapsed CLL showing complete response while on gemcitabine for another primary malignancy, suggesting a potential curative role of gemcitabine for CLL.
CASE REPORT
A 78-year-old male with relapsed CD38+ CLL with del11q on ibrutinib with partial response, presented with gross hematuria for one week. Of note, he was diagnosed with BRCA-negative Stage Ib pancreatic adenocarcinoma within the previous year, treated with surgery and adjuvant capecitabine-gemcitabine. Physical examination was unremarkable and bloodwork showed a white cell count of 32,000 cells/ mm3 with 1.5% lymphocytes, hemoglobin 9.5 g/dL, and platelets 866,000 cells/mm3. Hematuria remained persistent despite frequent bladder irrigations but resolved within a week of stopping ibrutinib. Eight months later, his white cell count is 6,600 cells/mm3, with 16% lymphocytes, hemoglobin 10.2 g/dL, platelets 519,000/m3, and CT scans show no pathological lymphadenopathy. A recent flow cytometry done for academic purposes showed no clonal B cells.
DISCUSSION
Relapsed CLL has a poor prognosis with no curative treatment. Gemcitabine is a part of NCCN guidelines for relapse/refractory B-cell lymphomas but is not included in guidelines for CLL. A study by Jamie et al in 2001 suggested the pre-clinical effectiveness of gemcitabine for relapsed/refractory CLL and phase II trials conducted in 2005 and 2012 on combination chemotherapy including gemcitabine have shown overall CLL response rates of 50-65%. The resolution of B-cell clonality and improvement in biochemical markers after treatment with gemcitabine for an alternate primary malignancy suggested that gemcitabine played a potential curative role in our patient. Further prospective studies are needed to explore this avenue for the role of gemcitabine as a salvage as well as potentially curative therapy for relapsed CLL with variable cytogenetics and treatment histories.
CONCLUSIONS
Gemcitabine is not part of NCCN guidelines for CLL currently but it is a reasonable treatment option for relapsed/refractory CLL. Further studies are needed to explore its potential curative role for relapsed CLL, and update existing guidelines.
INTRODUCTION
Gemcitabine is a part of National Comprehensive Cancer Network (NCCN) guidelines as salvage therapy for relapsed/refractory B-cell lymphomas, but its role in chronic lymphocytic leukemia (CLL) remains unclear. We describe a case of relapsed CLL showing complete response while on gemcitabine for another primary malignancy, suggesting a potential curative role of gemcitabine for CLL.
CASE REPORT
A 78-year-old male with relapsed CD38+ CLL with del11q on ibrutinib with partial response, presented with gross hematuria for one week. Of note, he was diagnosed with BRCA-negative Stage Ib pancreatic adenocarcinoma within the previous year, treated with surgery and adjuvant capecitabine-gemcitabine. Physical examination was unremarkable and bloodwork showed a white cell count of 32,000 cells/ mm3 with 1.5% lymphocytes, hemoglobin 9.5 g/dL, and platelets 866,000 cells/mm3. Hematuria remained persistent despite frequent bladder irrigations but resolved within a week of stopping ibrutinib. Eight months later, his white cell count is 6,600 cells/mm3, with 16% lymphocytes, hemoglobin 10.2 g/dL, platelets 519,000/m3, and CT scans show no pathological lymphadenopathy. A recent flow cytometry done for academic purposes showed no clonal B cells.
DISCUSSION
Relapsed CLL has a poor prognosis with no curative treatment. Gemcitabine is a part of NCCN guidelines for relapse/refractory B-cell lymphomas but is not included in guidelines for CLL. A study by Jamie et al in 2001 suggested the pre-clinical effectiveness of gemcitabine for relapsed/refractory CLL and phase II trials conducted in 2005 and 2012 on combination chemotherapy including gemcitabine have shown overall CLL response rates of 50-65%. The resolution of B-cell clonality and improvement in biochemical markers after treatment with gemcitabine for an alternate primary malignancy suggested that gemcitabine played a potential curative role in our patient. Further prospective studies are needed to explore this avenue for the role of gemcitabine as a salvage as well as potentially curative therapy for relapsed CLL with variable cytogenetics and treatment histories.
CONCLUSIONS
Gemcitabine is not part of NCCN guidelines for CLL currently but it is a reasonable treatment option for relapsed/refractory CLL. Further studies are needed to explore its potential curative role for relapsed CLL, and update existing guidelines.
Implementation and Evaluation of a Clinical Pharmacist Practitioner-Led Pharmacogenomics Service in a Veterans Affairs Hematology and Oncology Clinic
BACKGROUND
The Pharmacogenomic Testing for Veterans (PHASER) program provides preemptive pharmacogenomic testing for Veterans nationally. Program implementation at the Madison VA began in the hematology and oncology (hem/onc) clinics. In these clinics, PHASER test results are reviewed by the hem/onc clinical pharmacist practitioner (CPP) who provides recommendations regarding therapy via an electronic health record note. The purpose of this retrospective chart review was to assess the impact of the CPP on medication management informed by pharmacogenomics.
METHODS
A retrospective chart review was completed for all Veterans enrolled in hem/onc services and offered PHASER testing between April 1, 2022 and November 1, 2022. The number and type of interventions recommended by the hem/onc CPP, acceptance of recommended interventions, and hem/onc CPP time spent were collected for all patients who accepted and completed PHASER testing. Interventions were categorized and descriptive statistics were used to summarize data.
RESULTS
Of the 98 patients reviewed by the CPP, 75 (77%) were prescribed a medication with potential pharmacogenomic implications. At least one actionable recommendation for medication therapy adjustment was identified for 40 (53%) of those patients based on their pharmacogenomic test results. The CPP spent an average of 12 minutes per patient review (range 5 to 30 minutes) and 100% of CPP recommendations were accepted.
CONCLUSIONS
The CPP efficiently reviewed pharmacogenomic test results and made meaningful recommendations for medication therapy adjustments. CPP recommendations were highly accepted in the hem/onc setting.
BACKGROUND
The Pharmacogenomic Testing for Veterans (PHASER) program provides preemptive pharmacogenomic testing for Veterans nationally. Program implementation at the Madison VA began in the hematology and oncology (hem/onc) clinics. In these clinics, PHASER test results are reviewed by the hem/onc clinical pharmacist practitioner (CPP) who provides recommendations regarding therapy via an electronic health record note. The purpose of this retrospective chart review was to assess the impact of the CPP on medication management informed by pharmacogenomics.
METHODS
A retrospective chart review was completed for all Veterans enrolled in hem/onc services and offered PHASER testing between April 1, 2022 and November 1, 2022. The number and type of interventions recommended by the hem/onc CPP, acceptance of recommended interventions, and hem/onc CPP time spent were collected for all patients who accepted and completed PHASER testing. Interventions were categorized and descriptive statistics were used to summarize data.
RESULTS
Of the 98 patients reviewed by the CPP, 75 (77%) were prescribed a medication with potential pharmacogenomic implications. At least one actionable recommendation for medication therapy adjustment was identified for 40 (53%) of those patients based on their pharmacogenomic test results. The CPP spent an average of 12 minutes per patient review (range 5 to 30 minutes) and 100% of CPP recommendations were accepted.
CONCLUSIONS
The CPP efficiently reviewed pharmacogenomic test results and made meaningful recommendations for medication therapy adjustments. CPP recommendations were highly accepted in the hem/onc setting.
BACKGROUND
The Pharmacogenomic Testing for Veterans (PHASER) program provides preemptive pharmacogenomic testing for Veterans nationally. Program implementation at the Madison VA began in the hematology and oncology (hem/onc) clinics. In these clinics, PHASER test results are reviewed by the hem/onc clinical pharmacist practitioner (CPP) who provides recommendations regarding therapy via an electronic health record note. The purpose of this retrospective chart review was to assess the impact of the CPP on medication management informed by pharmacogenomics.
METHODS
A retrospective chart review was completed for all Veterans enrolled in hem/onc services and offered PHASER testing between April 1, 2022 and November 1, 2022. The number and type of interventions recommended by the hem/onc CPP, acceptance of recommended interventions, and hem/onc CPP time spent were collected for all patients who accepted and completed PHASER testing. Interventions were categorized and descriptive statistics were used to summarize data.
RESULTS
Of the 98 patients reviewed by the CPP, 75 (77%) were prescribed a medication with potential pharmacogenomic implications. At least one actionable recommendation for medication therapy adjustment was identified for 40 (53%) of those patients based on their pharmacogenomic test results. The CPP spent an average of 12 minutes per patient review (range 5 to 30 minutes) and 100% of CPP recommendations were accepted.
CONCLUSIONS
The CPP efficiently reviewed pharmacogenomic test results and made meaningful recommendations for medication therapy adjustments. CPP recommendations were highly accepted in the hem/onc setting.