User login
Prevention and treatment of influenza
With this flu season, there are new indications for the traditional inactivated (killed) vaccine, a new intranasal vaccine, lab tests for rapid identification of influenza, and a need to review the role of antiviral treatments.
New prevention measures
Inactivated influenza vaccine is the best preventive measure against both type A and B strains of the virus. The vaccine’s effectiveness depends somewhat on how well it matches to circulating virus antigens. Table 1 lists the benefits of vaccination in various populations.
Table 2 identifies the usual target populations for vaccine coverage. In the past 5 years, research has yielded several findings: the very young are at excess risk of influenza-related hospitalizations; adults aged 50 to 64 years have more high-risk conditions than previously thought; and cost-benefit analyses show a large economic toll of flu outbreaks manifested mainly as work and school absence. Consequently, the Centers for Disease Control and Prevention (CDC) now recommends routine vaccination of persons older than 50 years, and encourages vaccination of children between 6 and 24 months.Children under 9 years being immunized for the first time must receive 2 vaccines at least a month apart to gain optimal protection. This requirement will make it challenging to immunize children aged <24 months, since they are already receiving a number of other vaccinations.
This year enough vaccine has been produced to allow both targeted and nontargeted groups to receive inactivated vaccine as soon as it is available.
TABLE 1
Effectiveness of inactivated influenza vaccine
| In the patient group… | …the vaccine prevents a potential… |
|---|---|
| Healthy adults <65 years | 70%–90% of influenza illness |
| Children 1–15 years | 77%–91% of influenza respiratory illness;no evidence that it prevents otitis media7 |
| Adults >65 years | 58% of influenza respiratory illness |
| 30%–70% of hospitalizations for pneumonia and flu | |
| Adults >65 years in nursing homes | 30%–40% of influenza illness |
| 50%–60% of hospitalizations | |
| 80% death rate |
TABLE 2
Persons who should receive inactivated influenza vaccine
| Recommendations to date |
|---|
|
| New recommendations |
|
FluMist
The US Food and Drug Administration (FDA) recently approved FluMist, an intranasal vaccine with live, attenuated influenza virus, effective against both type A and B strains. Indications for its use are healthy people from 5 to 49 years. In this group, FluMist is an alternative to the traditional inactivated vaccine, but it is more expensive at $46 a dose (compared with $6 to $10 for inactivated vaccine). Unvaccinated children 5 to 8 years of age should receive 2 doses 6 to 10 weeks apart.4
People with chronic conditions such as asthma, cardiovascular disease, diabetes, and known or suspected immunodeficiency should not receive this vaccine until additional data are acquired about its effectiveness in these situations. In addition, because FluMist contains live influenza viruses, there is a potential for transmission from the vaccinated person to other persons. Therefore, clinicians should be cautious in its use when a patient requiring vaccination lives with immunosuppressed persons.
The rate of serious side effects with FluMist has been <1%, although mild side effects such as runny nose, fever, and headache occur slightly more often among vaccine than placebo recipients.
Improved diagnostic tests
The development of new outpatient treatments for influenza has increased the desirability of making an accurate diagnosis. Clinical symptoms, particularly fever and cough, are somewhat helpful (in adults, sensitivity is 63%–78% and specificity is 55%–71%). Diagnostic accuracy is enhanced by awareness of active flu cases in your community. This information is available from local or state health departments and the CDC, and it is based on active surveillance through networks of sentinel physician practices and emergency rooms. This is a good example of a reliable surveillance system helping physicians provide better clinical care.
Since 1989, a concerted public health effort has increased flu vaccine usage in adults older than 65 from 33% to 66% in 1999.Like many successful population health programs, this improvement has resulted from a focus on the core functions of public health:
- Assessment—regular surveys of vaccine coverage and local influenza outbreaks, continual identification of high-risk groups, and studies of vaccine efficacy and cost-effectiveness.
- Assurance—media campaigns to heighten awareness among consumers and providers of the benefits of vaccination and increased access to vaccine through physician offices, health departments, other health care worksites, and non-traditional sites such as malls and drug stores.
- Policy development — Medicare coverage of vaccine costs since 1993, Healthy People 2000 and 2010 national goals, and marketing campaigns to increase vaccine coverage supported by the Public Health Service in partnership with private organizations.
In the past several years, the FDA has approved an array of rapid diagnostic tests that may improve medical decision-making. Approved tests are now available for Clinical Laboratory Improvement Act (CLIA)-waived (QuickVue Influenza A/B; ZstatFlu) and nonwaived labs (BD Directigen Flu A+B; BD Directigen Flu A).
Nasal washes or swabs, not throat swabs, are the best method for obtaining specimens.
Compared with viral culture (the gold standard), reported sensitivities for these tests are 62%–73%, and specificities are 80%–99%.5
A study conducted in a private practice reported sensitivities of 72%–95% and specificities of 76%–84%.3 In this mainly pediatric population, the prevalence of influenza was about 50% by culture, and the positive predictive value (the likelihood that a positive test indicates true disease) ranged from 80%–86%, with a negative predictive value (the likelihood that a negative test indicates absence of disease) of 77%–90%.
In both studies, QuickVue was the best performing CLIA-waived test; the ZstatFlu test did not perform as well as the others. The tests generally give results in under 15 minutes, except ZstatFlu, which was more cumbersome to use. Since the prevalence of a condition in the population influences the predictive value of tests, all the tests perform better at finding true disease during active flu outbreaks than at the beginning or end of outbreaks when patients are less likely to have influenza.
The tests range in price from $15 to $25.
Antiviral treatments
Amantadine and rimantadine can reduce the duration of uncomplicated influenza A by about 1 day when started within 2 days of the onset of illness.
The newer drugs, zanamivir and oseltamivir, can reduce the duration of uncomplicated influenza A and B by about 1 day compared with placebo. Data are limited regarding the benefits of these drugs for patients at high risk of serious complications, or for children, although 1 study has shown a decrease in the incidence of otitis media among children taking oseltamivir.
Zanamivir is approved for adults and for children older than 7 years. It is administered via inhalation twice a day and costs $48 for the standard 5-day treatment.
Oseltamivir is approved for adults and for children older than 1 year. It is taken orally twice daily, with dose based on age and weight. It costs $60 for a 5-day treatment.
Prophylaxis. Amantadine and rimantadine are approved for prophylaxis against influenza A, and prevent 70%–90% of cases. Oseltamivir is approved for prophylaxis in adults and children older than 13 years. When used prophylactically, these drugs must be taken daily for the duration of influenza activity in the community. This can mean taking medication for weeks, which would be quite expensive in the case of oseltamivir.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Advisory Committee on Immunization Practices. Prevention and control of influenza; recommendations of the Advisory Committee on immunization Practices. MMWR Recomm Rep 2003;52(RR08):1-34.
2. Montalto N. An office-based approach to influenza: clinical diagnosis and laboratory testing. Am Fam Physician 2003;67:111-118.
3. Rodriguez W, Schwartz R, Thorne M. Evaluation of diagnostic tests for influenza in a pediatric practice. Pediatr Infect Dis J 2002;21:193-196.
4. Harper SA, Fukuda K, Cox NJ, Bridges CB. Using live, attenuated influenza vaccine for prevention and control of influenza; supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2003;52(RR13):1-8.
5. US Food and Drug Administration. Performance and caution in using rapid influenza virus diagnostic tests. Available at www.fda.gov/cdrh/oivd/laboratory.html#tip2. Accessed on October 6, 2003.
6. Colgan R, Michocki R, Greisman L, Moore TA. Antiviral drugs in the immunocompetent host: part II. Treatment of influenza and respiratory syncytial virus infections. Am Fam Physician 2003;67:763-766.
7. Hoberman A, Greenberg DP, Paradise JL, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290:1608-1616.
With this flu season, there are new indications for the traditional inactivated (killed) vaccine, a new intranasal vaccine, lab tests for rapid identification of influenza, and a need to review the role of antiviral treatments.
New prevention measures
Inactivated influenza vaccine is the best preventive measure against both type A and B strains of the virus. The vaccine’s effectiveness depends somewhat on how well it matches to circulating virus antigens. Table 1 lists the benefits of vaccination in various populations.
Table 2 identifies the usual target populations for vaccine coverage. In the past 5 years, research has yielded several findings: the very young are at excess risk of influenza-related hospitalizations; adults aged 50 to 64 years have more high-risk conditions than previously thought; and cost-benefit analyses show a large economic toll of flu outbreaks manifested mainly as work and school absence. Consequently, the Centers for Disease Control and Prevention (CDC) now recommends routine vaccination of persons older than 50 years, and encourages vaccination of children between 6 and 24 months.Children under 9 years being immunized for the first time must receive 2 vaccines at least a month apart to gain optimal protection. This requirement will make it challenging to immunize children aged <24 months, since they are already receiving a number of other vaccinations.
This year enough vaccine has been produced to allow both targeted and nontargeted groups to receive inactivated vaccine as soon as it is available.
TABLE 1
Effectiveness of inactivated influenza vaccine
| In the patient group… | …the vaccine prevents a potential… |
|---|---|
| Healthy adults <65 years | 70%–90% of influenza illness |
| Children 1–15 years | 77%–91% of influenza respiratory illness;no evidence that it prevents otitis media7 |
| Adults >65 years | 58% of influenza respiratory illness |
| 30%–70% of hospitalizations for pneumonia and flu | |
| Adults >65 years in nursing homes | 30%–40% of influenza illness |
| 50%–60% of hospitalizations | |
| 80% death rate |
TABLE 2
Persons who should receive inactivated influenza vaccine
| Recommendations to date |
|---|
|
| New recommendations |
|
FluMist
The US Food and Drug Administration (FDA) recently approved FluMist, an intranasal vaccine with live, attenuated influenza virus, effective against both type A and B strains. Indications for its use are healthy people from 5 to 49 years. In this group, FluMist is an alternative to the traditional inactivated vaccine, but it is more expensive at $46 a dose (compared with $6 to $10 for inactivated vaccine). Unvaccinated children 5 to 8 years of age should receive 2 doses 6 to 10 weeks apart.4
People with chronic conditions such as asthma, cardiovascular disease, diabetes, and known or suspected immunodeficiency should not receive this vaccine until additional data are acquired about its effectiveness in these situations. In addition, because FluMist contains live influenza viruses, there is a potential for transmission from the vaccinated person to other persons. Therefore, clinicians should be cautious in its use when a patient requiring vaccination lives with immunosuppressed persons.
The rate of serious side effects with FluMist has been <1%, although mild side effects such as runny nose, fever, and headache occur slightly more often among vaccine than placebo recipients.
Improved diagnostic tests
The development of new outpatient treatments for influenza has increased the desirability of making an accurate diagnosis. Clinical symptoms, particularly fever and cough, are somewhat helpful (in adults, sensitivity is 63%–78% and specificity is 55%–71%). Diagnostic accuracy is enhanced by awareness of active flu cases in your community. This information is available from local or state health departments and the CDC, and it is based on active surveillance through networks of sentinel physician practices and emergency rooms. This is a good example of a reliable surveillance system helping physicians provide better clinical care.
Since 1989, a concerted public health effort has increased flu vaccine usage in adults older than 65 from 33% to 66% in 1999.Like many successful population health programs, this improvement has resulted from a focus on the core functions of public health:
- Assessment—regular surveys of vaccine coverage and local influenza outbreaks, continual identification of high-risk groups, and studies of vaccine efficacy and cost-effectiveness.
- Assurance—media campaigns to heighten awareness among consumers and providers of the benefits of vaccination and increased access to vaccine through physician offices, health departments, other health care worksites, and non-traditional sites such as malls and drug stores.
- Policy development — Medicare coverage of vaccine costs since 1993, Healthy People 2000 and 2010 national goals, and marketing campaigns to increase vaccine coverage supported by the Public Health Service in partnership with private organizations.
In the past several years, the FDA has approved an array of rapid diagnostic tests that may improve medical decision-making. Approved tests are now available for Clinical Laboratory Improvement Act (CLIA)-waived (QuickVue Influenza A/B; ZstatFlu) and nonwaived labs (BD Directigen Flu A+B; BD Directigen Flu A).
Nasal washes or swabs, not throat swabs, are the best method for obtaining specimens.
Compared with viral culture (the gold standard), reported sensitivities for these tests are 62%–73%, and specificities are 80%–99%.5
A study conducted in a private practice reported sensitivities of 72%–95% and specificities of 76%–84%.3 In this mainly pediatric population, the prevalence of influenza was about 50% by culture, and the positive predictive value (the likelihood that a positive test indicates true disease) ranged from 80%–86%, with a negative predictive value (the likelihood that a negative test indicates absence of disease) of 77%–90%.
In both studies, QuickVue was the best performing CLIA-waived test; the ZstatFlu test did not perform as well as the others. The tests generally give results in under 15 minutes, except ZstatFlu, which was more cumbersome to use. Since the prevalence of a condition in the population influences the predictive value of tests, all the tests perform better at finding true disease during active flu outbreaks than at the beginning or end of outbreaks when patients are less likely to have influenza.
The tests range in price from $15 to $25.
Antiviral treatments
Amantadine and rimantadine can reduce the duration of uncomplicated influenza A by about 1 day when started within 2 days of the onset of illness.
The newer drugs, zanamivir and oseltamivir, can reduce the duration of uncomplicated influenza A and B by about 1 day compared with placebo. Data are limited regarding the benefits of these drugs for patients at high risk of serious complications, or for children, although 1 study has shown a decrease in the incidence of otitis media among children taking oseltamivir.
Zanamivir is approved for adults and for children older than 7 years. It is administered via inhalation twice a day and costs $48 for the standard 5-day treatment.
Oseltamivir is approved for adults and for children older than 1 year. It is taken orally twice daily, with dose based on age and weight. It costs $60 for a 5-day treatment.
Prophylaxis. Amantadine and rimantadine are approved for prophylaxis against influenza A, and prevent 70%–90% of cases. Oseltamivir is approved for prophylaxis in adults and children older than 13 years. When used prophylactically, these drugs must be taken daily for the duration of influenza activity in the community. This can mean taking medication for weeks, which would be quite expensive in the case of oseltamivir.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
With this flu season, there are new indications for the traditional inactivated (killed) vaccine, a new intranasal vaccine, lab tests for rapid identification of influenza, and a need to review the role of antiviral treatments.
New prevention measures
Inactivated influenza vaccine is the best preventive measure against both type A and B strains of the virus. The vaccine’s effectiveness depends somewhat on how well it matches to circulating virus antigens. Table 1 lists the benefits of vaccination in various populations.
Table 2 identifies the usual target populations for vaccine coverage. In the past 5 years, research has yielded several findings: the very young are at excess risk of influenza-related hospitalizations; adults aged 50 to 64 years have more high-risk conditions than previously thought; and cost-benefit analyses show a large economic toll of flu outbreaks manifested mainly as work and school absence. Consequently, the Centers for Disease Control and Prevention (CDC) now recommends routine vaccination of persons older than 50 years, and encourages vaccination of children between 6 and 24 months.Children under 9 years being immunized for the first time must receive 2 vaccines at least a month apart to gain optimal protection. This requirement will make it challenging to immunize children aged <24 months, since they are already receiving a number of other vaccinations.
This year enough vaccine has been produced to allow both targeted and nontargeted groups to receive inactivated vaccine as soon as it is available.
TABLE 1
Effectiveness of inactivated influenza vaccine
| In the patient group… | …the vaccine prevents a potential… |
|---|---|
| Healthy adults <65 years | 70%–90% of influenza illness |
| Children 1–15 years | 77%–91% of influenza respiratory illness;no evidence that it prevents otitis media7 |
| Adults >65 years | 58% of influenza respiratory illness |
| 30%–70% of hospitalizations for pneumonia and flu | |
| Adults >65 years in nursing homes | 30%–40% of influenza illness |
| 50%–60% of hospitalizations | |
| 80% death rate |
TABLE 2
Persons who should receive inactivated influenza vaccine
| Recommendations to date |
|---|
|
| New recommendations |
|
FluMist
The US Food and Drug Administration (FDA) recently approved FluMist, an intranasal vaccine with live, attenuated influenza virus, effective against both type A and B strains. Indications for its use are healthy people from 5 to 49 years. In this group, FluMist is an alternative to the traditional inactivated vaccine, but it is more expensive at $46 a dose (compared with $6 to $10 for inactivated vaccine). Unvaccinated children 5 to 8 years of age should receive 2 doses 6 to 10 weeks apart.4
People with chronic conditions such as asthma, cardiovascular disease, diabetes, and known or suspected immunodeficiency should not receive this vaccine until additional data are acquired about its effectiveness in these situations. In addition, because FluMist contains live influenza viruses, there is a potential for transmission from the vaccinated person to other persons. Therefore, clinicians should be cautious in its use when a patient requiring vaccination lives with immunosuppressed persons.
The rate of serious side effects with FluMist has been <1%, although mild side effects such as runny nose, fever, and headache occur slightly more often among vaccine than placebo recipients.
Improved diagnostic tests
The development of new outpatient treatments for influenza has increased the desirability of making an accurate diagnosis. Clinical symptoms, particularly fever and cough, are somewhat helpful (in adults, sensitivity is 63%–78% and specificity is 55%–71%). Diagnostic accuracy is enhanced by awareness of active flu cases in your community. This information is available from local or state health departments and the CDC, and it is based on active surveillance through networks of sentinel physician practices and emergency rooms. This is a good example of a reliable surveillance system helping physicians provide better clinical care.
Since 1989, a concerted public health effort has increased flu vaccine usage in adults older than 65 from 33% to 66% in 1999.Like many successful population health programs, this improvement has resulted from a focus on the core functions of public health:
- Assessment—regular surveys of vaccine coverage and local influenza outbreaks, continual identification of high-risk groups, and studies of vaccine efficacy and cost-effectiveness.
- Assurance—media campaigns to heighten awareness among consumers and providers of the benefits of vaccination and increased access to vaccine through physician offices, health departments, other health care worksites, and non-traditional sites such as malls and drug stores.
- Policy development — Medicare coverage of vaccine costs since 1993, Healthy People 2000 and 2010 national goals, and marketing campaigns to increase vaccine coverage supported by the Public Health Service in partnership with private organizations.
In the past several years, the FDA has approved an array of rapid diagnostic tests that may improve medical decision-making. Approved tests are now available for Clinical Laboratory Improvement Act (CLIA)-waived (QuickVue Influenza A/B; ZstatFlu) and nonwaived labs (BD Directigen Flu A+B; BD Directigen Flu A).
Nasal washes or swabs, not throat swabs, are the best method for obtaining specimens.
Compared with viral culture (the gold standard), reported sensitivities for these tests are 62%–73%, and specificities are 80%–99%.5
A study conducted in a private practice reported sensitivities of 72%–95% and specificities of 76%–84%.3 In this mainly pediatric population, the prevalence of influenza was about 50% by culture, and the positive predictive value (the likelihood that a positive test indicates true disease) ranged from 80%–86%, with a negative predictive value (the likelihood that a negative test indicates absence of disease) of 77%–90%.
In both studies, QuickVue was the best performing CLIA-waived test; the ZstatFlu test did not perform as well as the others. The tests generally give results in under 15 minutes, except ZstatFlu, which was more cumbersome to use. Since the prevalence of a condition in the population influences the predictive value of tests, all the tests perform better at finding true disease during active flu outbreaks than at the beginning or end of outbreaks when patients are less likely to have influenza.
The tests range in price from $15 to $25.
Antiviral treatments
Amantadine and rimantadine can reduce the duration of uncomplicated influenza A by about 1 day when started within 2 days of the onset of illness.
The newer drugs, zanamivir and oseltamivir, can reduce the duration of uncomplicated influenza A and B by about 1 day compared with placebo. Data are limited regarding the benefits of these drugs for patients at high risk of serious complications, or for children, although 1 study has shown a decrease in the incidence of otitis media among children taking oseltamivir.
Zanamivir is approved for adults and for children older than 7 years. It is administered via inhalation twice a day and costs $48 for the standard 5-day treatment.
Oseltamivir is approved for adults and for children older than 1 year. It is taken orally twice daily, with dose based on age and weight. It costs $60 for a 5-day treatment.
Prophylaxis. Amantadine and rimantadine are approved for prophylaxis against influenza A, and prevent 70%–90% of cases. Oseltamivir is approved for prophylaxis in adults and children older than 13 years. When used prophylactically, these drugs must be taken daily for the duration of influenza activity in the community. This can mean taking medication for weeks, which would be quite expensive in the case of oseltamivir.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Advisory Committee on Immunization Practices. Prevention and control of influenza; recommendations of the Advisory Committee on immunization Practices. MMWR Recomm Rep 2003;52(RR08):1-34.
2. Montalto N. An office-based approach to influenza: clinical diagnosis and laboratory testing. Am Fam Physician 2003;67:111-118.
3. Rodriguez W, Schwartz R, Thorne M. Evaluation of diagnostic tests for influenza in a pediatric practice. Pediatr Infect Dis J 2002;21:193-196.
4. Harper SA, Fukuda K, Cox NJ, Bridges CB. Using live, attenuated influenza vaccine for prevention and control of influenza; supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2003;52(RR13):1-8.
5. US Food and Drug Administration. Performance and caution in using rapid influenza virus diagnostic tests. Available at www.fda.gov/cdrh/oivd/laboratory.html#tip2. Accessed on October 6, 2003.
6. Colgan R, Michocki R, Greisman L, Moore TA. Antiviral drugs in the immunocompetent host: part II. Treatment of influenza and respiratory syncytial virus infections. Am Fam Physician 2003;67:763-766.
7. Hoberman A, Greenberg DP, Paradise JL, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290:1608-1616.
1. Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Advisory Committee on Immunization Practices. Prevention and control of influenza; recommendations of the Advisory Committee on immunization Practices. MMWR Recomm Rep 2003;52(RR08):1-34.
2. Montalto N. An office-based approach to influenza: clinical diagnosis and laboratory testing. Am Fam Physician 2003;67:111-118.
3. Rodriguez W, Schwartz R, Thorne M. Evaluation of diagnostic tests for influenza in a pediatric practice. Pediatr Infect Dis J 2002;21:193-196.
4. Harper SA, Fukuda K, Cox NJ, Bridges CB. Using live, attenuated influenza vaccine for prevention and control of influenza; supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2003;52(RR13):1-8.
5. US Food and Drug Administration. Performance and caution in using rapid influenza virus diagnostic tests. Available at www.fda.gov/cdrh/oivd/laboratory.html#tip2. Accessed on October 6, 2003.
6. Colgan R, Michocki R, Greisman L, Moore TA. Antiviral drugs in the immunocompetent host: part II. Treatment of influenza and respiratory syncytial virus infections. Am Fam Physician 2003;67:763-766.
7. Hoberman A, Greenberg DP, Paradise JL, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003;290:1608-1616.
Tuberculosis : Old problem, new concerns
- For the initial evaluation of any adult who requires routine evaluation for TB exposure, administer a second TB skin test within 1 to 3 weeks if the first test result is negative.
- The workup for active TB consists of a chest x-ray film, a human immunodeficiency virus (HIV) test, and possibly sputum collection. HIV-negative persons with a normal chest x-ray result are unlikely to have pulmonary TB, and sputum collection is unnecessary.
- Contrary to common belief, there is no age cutoff for treating latent TB.
- Treat active TB with isoniazid, rifampin, and pyrazinamide. Add ethambutol if a patient is a current or former resident of an area in which bacterial resistance to isoniazid is greater than 5%.
- Refer all suspected cases of TB to your local public health department for the purpose of tracking contacts.
With the decline of tuberculosis (TB), physician familiarity with this disease has substantially diminished. Yet TB remains common in immigrants, individuals with HIV infection, and other high-risk populations. How do we remain vigilant for TB? How do we screen? How should the physician interpret TB test results? What are the preferred options for treatment? What are the public health implications of TB? This article offers an update on this recalcitrant public health problem.
Whom, and how, to screen
Two factors are key in remaining vigilant for tuberculosis: knowing who in your patient population is most at risk for exposure, and knowing who is most likely to develop active disease if infected.
Persons at risk for exposure to TB (Table 1) or at risk for developing active disease if infected (Table 2) should receive a TB skin test regularly, although the optimal frequency has not been determined. Routine testing is not indicated for others.
Proper technique. The TB skin test should be administered with intermediate-strength purified protein derivative (PPD), 0.1 mL injected intradermally, resulting in a raised bleb. The test should be read 48 to 72 hours later and the area of induration, not erythema, measured and recorded in millimeters. With no induration, the result should be recorded as 0 mm, not as “negative.”
Interpreting test results. Interpretation of the test results depends on a person’s risk factors and age. For those listed in Table 3, a 5-mm induration is considered positive; for those listed in Tables 1 and 2 who are not in Table 3 , a 10-mm induration is positive. For everyone else, 15 mm is positive.
Caveat. Multiple puncture tests, though easier to administer, do not inject a standardized amount of tuberculin into the skin; results are more difficult to interpret and, if judged reactive, must be confirmed with a PPD test. This option is not recommended for testing.
Prior receipt of the bacillus Calmette-Guerin (BCG) vaccine does not affect the interpretation of the TB skin test, nor should it affect decisions to treat latent TB. The effectiveness of BCG vaccine in preventing TB infection in highly questionable, and the reaction to PPD caused by BCG wanes after a few years.
Two-step testing. Two-step testing means administering a second TB skin test within 1 to 3 weeks if the first test result is negative. This procedure should be used for the initial evaluation of adults who require routine testing. If the second skin test result is positive, it indicates the person was infected with TB before, and that immunity has waned and was “boosted” by the first test.
Without the 2-step process, a positive result on repeat testing would suggest recent infection rather than prior exposure. This could have implications for the decision to accept or not accept treatment for latent TB.
TABLE 1
Conditions associated with high risk of exposure to tuberculosis
| Foreign-born in area with high tuberculosis rates |
| Use of illicit drugs |
Institutionalization or work in
|
| Work in health care facilities with high-risk clients |
| Socioeconomic disadvantage |
| Children of those at high risk |
TABLE 2
Conditions associated with high risk of developing active tuberculosis
| HIV infection |
| Recent infection with Mycobacterium tuberculosis |
| Chest x-ray indicating old tuberculosis |
Immunosuppression
|
| Leukemia and other cancers of blood, lymph, and bone marrow |
| Cancer of the head and neck |
| Gastrectomy or intestinal bypass |
| Chronic malabsorption |
| End-stage renal disease |
| Diabetes |
| Silicosis |
| Illicit drug use |
TABLE 3
How to interpret a tuberculosis skin test
| 5 mm considered positive in those with: |
|
| 10 mm is considered positive in: |
Work-up for suspected tuberculosis
For those suspected of having TB because of chronic cough, night sweats, fever, and weight loss, or because of a positive TB skin test result, the workup consists of a chest x-ray film, an HIV test, and possibly sputum collection for microscopic evaluation and culture.
Those with a normal chest x-ray result who are HIV-negative are unlikely to have pulmonary TB, and sputum collection is unnecessary. For those with suspicious chest x-ray films and for those who are HIV positive with TB symptoms, sputum samples are needed for microscopic evaluation and culture (3 samples, preferably on 3 consecutive days).
Acid-fast organisms seen under the microscope may be Mycobacterium tuberculosis or mycobacteria other than tuberculosis (commonly referred to as MOTT), and the final determination must await culture confirmation, which now takes about 4 weeks. Preliminary confirmation using polymerase chain reaction can be accomplished in a few days. Treatment for active TB should be initiated, however, and the suspicion reported to the local health department as soon as TB is suspected.
Treating latent and active disease
Latent tuberculosis. Treatment for latent TB (positive TB skin test result, negative chest x-ray film, HIV-negative) should not be initiated until active TB is ruled out. This may require waiting 3 to 4 weeks for sputum culture results.
Contrary to common belief, there is no age cutoff for initiation of treatment for latent TB. Treatment requires 6 to 9 months of isoniazid or 4 months of rifampin. A shorter course with pyrazinamide and rifampin is also possible, although questions about the safety of this regimen have been raised. Patient compliance for the duration of therapy is difficult to achieve; but if accomplished, the risk of active disease decreases from 10% to less than 1%. At highest risk of developing active disease are children, and those who are HIV-positive, recently infected with TB, or whose chest films indicate old disease.
Since isoniazid is hepatotoxic, patients should be asked about symptoms of liver inflammation (abdominal pain, decline in appetite, dark urine, light-colored stools), and liver function tests should be ordered if symptoms are present. Routine monthly testing of liver function tests is not necessary and is recommended only for those who have chronic liver disease, an alcohol abuse disorder, or are pregnant.
Active tuberculosis. Always initiate treatment with at least 3 drugs (isoniazid, rifampin, and pyrazinamide). Ethambutol should be added to the regimen if patients are current or former residents of an area where resistance to isoniazid is more than 5%. If the organism proves sensitive to isoniazid and rifampin, ethambutol can be discontinued. Pyrazinamide should be continued for a full 2 months, and isoniazid and rifampin for a full 6 months. A variety of protocols for dosing and administration frequency are available to enhance convenience and patient compliance. Drug regimens may have to be adjusted based on culture results and success of therapy.
The standard of care for active TB is directly observed therapy. This means watching the patient swallow the pills. Although such care is labor intensive and implies a lack of trust in the patient, with its implementation, the successful completion of therapy rises from 50% to close to 100%. Widespread use of directly observed therapy since the late 1980s has resulted in a marked reduction of TB rates and rates of bacterial resistance.
To prevent bacterial resistance to drugs, treatment for active disease must be administered according to guidelines and be completed. One cardinal rule in the prevention of bacterial resistance is never to add just 1 drug to a failing regimen.
Any deviation from standard therapy because of bacterial resistance, patient nonadherence, or adverse drug effects is reason to consult the local public health department or state public health department TB program.
Working with the public health department
Three complementary activities are needed to control TB in the community:
- Finding and treating those with active TB
- Investigating close contacts of persons with active disease and offering them treatment of latent TB
- Screening persons at high risk, to find and treat latent TB.
The detection and management of tuberculosis offers an excellent opportunity for family physicians and health departments to work collaboratively to improve the community’s health.
Family physicians can make several contributions to reducing TB’s impact on the community:
- Screen appropriately
- Correctly apply and interpret TB skin tests
- Accurately make the diagnosis of latent and active TB
- Treat active TB according to recommended guidelines
- Encourage treatment of latent TB among those at highest risk of activation
- Promptly report to the local health department those suspected of having TB
- Collaborate with the public health department to reduce the spread of disease.
Local or state public health departments assist in several ways:
- Communicate with and investigating family and other close contacts of those with active, contagious TB, to find anyone with latent or active TB
- Offer consultation on how to diagnose and treat latent and active TB
- Assist with directly observed therapy
- Use public health authority to isolate and quarantine patients who are infectious and pose a risk to the community through non-adherence to treatment and infection control guidelines.
Many public health departments have TB programs wherein patients with active or latent TB can receive free care and medication.
State and local public health departments have the responsibility of monitoring TB care provided by physicians in the community, to insure it is applied according to guidelines and that it is completed. The intrusiveness of this can be minimized with regular communication and appreciation of the roles and responsibilities of each party.
Excellent review of the basics of TB screening, diagnosis, and treatment: Centers for Disease Control and Prevention (CDC). Core Curriculum on Tuberculosis. 4th ed. Atlanta, Ga: CDC; 2000.
Self-study modules on TB: CDC. National Center for HIV, STD, and TB Prevention, Divisionof Tuberculosis Elimination. Available at: www.phppo.cdc.gov/PHTN/tbmodules.(Accessed on September 8, 2003.)
Training modules and statistics on TB diagnosis, treatment, and epidemiology, including state-specific analyses: CDC. National Center for HIV, STD, and TB Prevention. Division of Tuberculosis Elimination. Available at: www.cdc.gov/nchstp/tb/default.htm. (Accessedon September 8, 2003.)
The most recent, comprehensive description of TB treatment recommendations: Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003; 167:603–662.
Prospects and trends
More reliable testing. The TB skin test is an imperfect screening tool. The development of a blood test for TB antibodies has progressed and will be evaluated and standardized. It will likely be a useful clinical and epidemiological tool in the near future.
Tuberculosis secondary to HIV. By some indicators, the rate of HIV infections has recently increased. This factor, combined with the increased life span of those with HIV, has the potential for reversing some of the recent progress in slowing TB rates. The continued development of anti-HIV medications and availability of the medications through AIDS/HIV treatment programs could help make those who are HIV infected less susceptible to TB infection and disease activation.
Tuberculosis and immigration. It is likely that as endemic TB declines in the US, a higher proportion of TB infections will occur in those who are foreign born and move to this country (see “Trends reversing in tuberculosis”). This underscores the global nature of public health and the importance of international collaboration in the control of contagious diseases and other public health threats.
At the turn of the 20th century, TB was the leading cause of death in the US and much of the world. Public health efforts and improved living conditions resulted in a steady decline in TB morbidity and mortality until the mid-1980s.
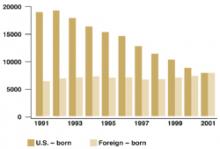
At that time a combination of events—the HIV epidemic, increased immigration from countries with high TB rates, and a decline in funding for TB control programs—resulted in a reversal of this downward trend and for several years there were increases in US TB rates. Of equal concern was an increase in multidrug resistance. These worrisome trends were reversed in the early 1990s and since that time TB rates have again been declining. In 2001 there were 15,989 new cases of TB in the US for a rate of 5.6/100,000. An increasing proportion of cases have been occurring among those born in countries with high TB rates; in 2001 49% of all those with TB in the US were foreign-born.
Increasing proportion of foreign-born TB cases
Number of tuberculosis cases in US-born vs foreign-born persons, United States, 1991–2001. Adapted from the Centers for Disease Control and Prevention, Atlanta, Ga.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
- For the initial evaluation of any adult who requires routine evaluation for TB exposure, administer a second TB skin test within 1 to 3 weeks if the first test result is negative.
- The workup for active TB consists of a chest x-ray film, a human immunodeficiency virus (HIV) test, and possibly sputum collection. HIV-negative persons with a normal chest x-ray result are unlikely to have pulmonary TB, and sputum collection is unnecessary.
- Contrary to common belief, there is no age cutoff for treating latent TB.
- Treat active TB with isoniazid, rifampin, and pyrazinamide. Add ethambutol if a patient is a current or former resident of an area in which bacterial resistance to isoniazid is greater than 5%.
- Refer all suspected cases of TB to your local public health department for the purpose of tracking contacts.
With the decline of tuberculosis (TB), physician familiarity with this disease has substantially diminished. Yet TB remains common in immigrants, individuals with HIV infection, and other high-risk populations. How do we remain vigilant for TB? How do we screen? How should the physician interpret TB test results? What are the preferred options for treatment? What are the public health implications of TB? This article offers an update on this recalcitrant public health problem.
Whom, and how, to screen
Two factors are key in remaining vigilant for tuberculosis: knowing who in your patient population is most at risk for exposure, and knowing who is most likely to develop active disease if infected.
Persons at risk for exposure to TB (Table 1) or at risk for developing active disease if infected (Table 2) should receive a TB skin test regularly, although the optimal frequency has not been determined. Routine testing is not indicated for others.
Proper technique. The TB skin test should be administered with intermediate-strength purified protein derivative (PPD), 0.1 mL injected intradermally, resulting in a raised bleb. The test should be read 48 to 72 hours later and the area of induration, not erythema, measured and recorded in millimeters. With no induration, the result should be recorded as 0 mm, not as “negative.”
Interpreting test results. Interpretation of the test results depends on a person’s risk factors and age. For those listed in Table 3, a 5-mm induration is considered positive; for those listed in Tables 1 and 2 who are not in Table 3 , a 10-mm induration is positive. For everyone else, 15 mm is positive.
Caveat. Multiple puncture tests, though easier to administer, do not inject a standardized amount of tuberculin into the skin; results are more difficult to interpret and, if judged reactive, must be confirmed with a PPD test. This option is not recommended for testing.
Prior receipt of the bacillus Calmette-Guerin (BCG) vaccine does not affect the interpretation of the TB skin test, nor should it affect decisions to treat latent TB. The effectiveness of BCG vaccine in preventing TB infection in highly questionable, and the reaction to PPD caused by BCG wanes after a few years.
Two-step testing. Two-step testing means administering a second TB skin test within 1 to 3 weeks if the first test result is negative. This procedure should be used for the initial evaluation of adults who require routine testing. If the second skin test result is positive, it indicates the person was infected with TB before, and that immunity has waned and was “boosted” by the first test.
Without the 2-step process, a positive result on repeat testing would suggest recent infection rather than prior exposure. This could have implications for the decision to accept or not accept treatment for latent TB.
TABLE 1
Conditions associated with high risk of exposure to tuberculosis
| Foreign-born in area with high tuberculosis rates |
| Use of illicit drugs |
Institutionalization or work in
|
| Work in health care facilities with high-risk clients |
| Socioeconomic disadvantage |
| Children of those at high risk |
TABLE 2
Conditions associated with high risk of developing active tuberculosis
| HIV infection |
| Recent infection with Mycobacterium tuberculosis |
| Chest x-ray indicating old tuberculosis |
Immunosuppression
|
| Leukemia and other cancers of blood, lymph, and bone marrow |
| Cancer of the head and neck |
| Gastrectomy or intestinal bypass |
| Chronic malabsorption |
| End-stage renal disease |
| Diabetes |
| Silicosis |
| Illicit drug use |
TABLE 3
How to interpret a tuberculosis skin test
| 5 mm considered positive in those with: |
|
| 10 mm is considered positive in: |
Work-up for suspected tuberculosis
For those suspected of having TB because of chronic cough, night sweats, fever, and weight loss, or because of a positive TB skin test result, the workup consists of a chest x-ray film, an HIV test, and possibly sputum collection for microscopic evaluation and culture.
Those with a normal chest x-ray result who are HIV-negative are unlikely to have pulmonary TB, and sputum collection is unnecessary. For those with suspicious chest x-ray films and for those who are HIV positive with TB symptoms, sputum samples are needed for microscopic evaluation and culture (3 samples, preferably on 3 consecutive days).
Acid-fast organisms seen under the microscope may be Mycobacterium tuberculosis or mycobacteria other than tuberculosis (commonly referred to as MOTT), and the final determination must await culture confirmation, which now takes about 4 weeks. Preliminary confirmation using polymerase chain reaction can be accomplished in a few days. Treatment for active TB should be initiated, however, and the suspicion reported to the local health department as soon as TB is suspected.
Treating latent and active disease
Latent tuberculosis. Treatment for latent TB (positive TB skin test result, negative chest x-ray film, HIV-negative) should not be initiated until active TB is ruled out. This may require waiting 3 to 4 weeks for sputum culture results.
Contrary to common belief, there is no age cutoff for initiation of treatment for latent TB. Treatment requires 6 to 9 months of isoniazid or 4 months of rifampin. A shorter course with pyrazinamide and rifampin is also possible, although questions about the safety of this regimen have been raised. Patient compliance for the duration of therapy is difficult to achieve; but if accomplished, the risk of active disease decreases from 10% to less than 1%. At highest risk of developing active disease are children, and those who are HIV-positive, recently infected with TB, or whose chest films indicate old disease.
Since isoniazid is hepatotoxic, patients should be asked about symptoms of liver inflammation (abdominal pain, decline in appetite, dark urine, light-colored stools), and liver function tests should be ordered if symptoms are present. Routine monthly testing of liver function tests is not necessary and is recommended only for those who have chronic liver disease, an alcohol abuse disorder, or are pregnant.
Active tuberculosis. Always initiate treatment with at least 3 drugs (isoniazid, rifampin, and pyrazinamide). Ethambutol should be added to the regimen if patients are current or former residents of an area where resistance to isoniazid is more than 5%. If the organism proves sensitive to isoniazid and rifampin, ethambutol can be discontinued. Pyrazinamide should be continued for a full 2 months, and isoniazid and rifampin for a full 6 months. A variety of protocols for dosing and administration frequency are available to enhance convenience and patient compliance. Drug regimens may have to be adjusted based on culture results and success of therapy.
The standard of care for active TB is directly observed therapy. This means watching the patient swallow the pills. Although such care is labor intensive and implies a lack of trust in the patient, with its implementation, the successful completion of therapy rises from 50% to close to 100%. Widespread use of directly observed therapy since the late 1980s has resulted in a marked reduction of TB rates and rates of bacterial resistance.
To prevent bacterial resistance to drugs, treatment for active disease must be administered according to guidelines and be completed. One cardinal rule in the prevention of bacterial resistance is never to add just 1 drug to a failing regimen.
Any deviation from standard therapy because of bacterial resistance, patient nonadherence, or adverse drug effects is reason to consult the local public health department or state public health department TB program.
Working with the public health department
Three complementary activities are needed to control TB in the community:
- Finding and treating those with active TB
- Investigating close contacts of persons with active disease and offering them treatment of latent TB
- Screening persons at high risk, to find and treat latent TB.
The detection and management of tuberculosis offers an excellent opportunity for family physicians and health departments to work collaboratively to improve the community’s health.
Family physicians can make several contributions to reducing TB’s impact on the community:
- Screen appropriately
- Correctly apply and interpret TB skin tests
- Accurately make the diagnosis of latent and active TB
- Treat active TB according to recommended guidelines
- Encourage treatment of latent TB among those at highest risk of activation
- Promptly report to the local health department those suspected of having TB
- Collaborate with the public health department to reduce the spread of disease.
Local or state public health departments assist in several ways:
- Communicate with and investigating family and other close contacts of those with active, contagious TB, to find anyone with latent or active TB
- Offer consultation on how to diagnose and treat latent and active TB
- Assist with directly observed therapy
- Use public health authority to isolate and quarantine patients who are infectious and pose a risk to the community through non-adherence to treatment and infection control guidelines.
Many public health departments have TB programs wherein patients with active or latent TB can receive free care and medication.
State and local public health departments have the responsibility of monitoring TB care provided by physicians in the community, to insure it is applied according to guidelines and that it is completed. The intrusiveness of this can be minimized with regular communication and appreciation of the roles and responsibilities of each party.
Excellent review of the basics of TB screening, diagnosis, and treatment: Centers for Disease Control and Prevention (CDC). Core Curriculum on Tuberculosis. 4th ed. Atlanta, Ga: CDC; 2000.
Self-study modules on TB: CDC. National Center for HIV, STD, and TB Prevention, Divisionof Tuberculosis Elimination. Available at: www.phppo.cdc.gov/PHTN/tbmodules.(Accessed on September 8, 2003.)
Training modules and statistics on TB diagnosis, treatment, and epidemiology, including state-specific analyses: CDC. National Center for HIV, STD, and TB Prevention. Division of Tuberculosis Elimination. Available at: www.cdc.gov/nchstp/tb/default.htm. (Accessedon September 8, 2003.)
The most recent, comprehensive description of TB treatment recommendations: Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003; 167:603–662.
Prospects and trends
More reliable testing. The TB skin test is an imperfect screening tool. The development of a blood test for TB antibodies has progressed and will be evaluated and standardized. It will likely be a useful clinical and epidemiological tool in the near future.
Tuberculosis secondary to HIV. By some indicators, the rate of HIV infections has recently increased. This factor, combined with the increased life span of those with HIV, has the potential for reversing some of the recent progress in slowing TB rates. The continued development of anti-HIV medications and availability of the medications through AIDS/HIV treatment programs could help make those who are HIV infected less susceptible to TB infection and disease activation.
Tuberculosis and immigration. It is likely that as endemic TB declines in the US, a higher proportion of TB infections will occur in those who are foreign born and move to this country (see “Trends reversing in tuberculosis”). This underscores the global nature of public health and the importance of international collaboration in the control of contagious diseases and other public health threats.
At the turn of the 20th century, TB was the leading cause of death in the US and much of the world. Public health efforts and improved living conditions resulted in a steady decline in TB morbidity and mortality until the mid-1980s.
At that time a combination of events—the HIV epidemic, increased immigration from countries with high TB rates, and a decline in funding for TB control programs—resulted in a reversal of this downward trend and for several years there were increases in US TB rates. Of equal concern was an increase in multidrug resistance. These worrisome trends were reversed in the early 1990s and since that time TB rates have again been declining. In 2001 there were 15,989 new cases of TB in the US for a rate of 5.6/100,000. An increasing proportion of cases have been occurring among those born in countries with high TB rates; in 2001 49% of all those with TB in the US were foreign-born.
Increasing proportion of foreign-born TB cases
Number of tuberculosis cases in US-born vs foreign-born persons, United States, 1991–2001. Adapted from the Centers for Disease Control and Prevention, Atlanta, Ga.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
- For the initial evaluation of any adult who requires routine evaluation for TB exposure, administer a second TB skin test within 1 to 3 weeks if the first test result is negative.
- The workup for active TB consists of a chest x-ray film, a human immunodeficiency virus (HIV) test, and possibly sputum collection. HIV-negative persons with a normal chest x-ray result are unlikely to have pulmonary TB, and sputum collection is unnecessary.
- Contrary to common belief, there is no age cutoff for treating latent TB.
- Treat active TB with isoniazid, rifampin, and pyrazinamide. Add ethambutol if a patient is a current or former resident of an area in which bacterial resistance to isoniazid is greater than 5%.
- Refer all suspected cases of TB to your local public health department for the purpose of tracking contacts.
With the decline of tuberculosis (TB), physician familiarity with this disease has substantially diminished. Yet TB remains common in immigrants, individuals with HIV infection, and other high-risk populations. How do we remain vigilant for TB? How do we screen? How should the physician interpret TB test results? What are the preferred options for treatment? What are the public health implications of TB? This article offers an update on this recalcitrant public health problem.
Whom, and how, to screen
Two factors are key in remaining vigilant for tuberculosis: knowing who in your patient population is most at risk for exposure, and knowing who is most likely to develop active disease if infected.
Persons at risk for exposure to TB (Table 1) or at risk for developing active disease if infected (Table 2) should receive a TB skin test regularly, although the optimal frequency has not been determined. Routine testing is not indicated for others.
Proper technique. The TB skin test should be administered with intermediate-strength purified protein derivative (PPD), 0.1 mL injected intradermally, resulting in a raised bleb. The test should be read 48 to 72 hours later and the area of induration, not erythema, measured and recorded in millimeters. With no induration, the result should be recorded as 0 mm, not as “negative.”
Interpreting test results. Interpretation of the test results depends on a person’s risk factors and age. For those listed in Table 3, a 5-mm induration is considered positive; for those listed in Tables 1 and 2 who are not in Table 3 , a 10-mm induration is positive. For everyone else, 15 mm is positive.
Caveat. Multiple puncture tests, though easier to administer, do not inject a standardized amount of tuberculin into the skin; results are more difficult to interpret and, if judged reactive, must be confirmed with a PPD test. This option is not recommended for testing.
Prior receipt of the bacillus Calmette-Guerin (BCG) vaccine does not affect the interpretation of the TB skin test, nor should it affect decisions to treat latent TB. The effectiveness of BCG vaccine in preventing TB infection in highly questionable, and the reaction to PPD caused by BCG wanes after a few years.
Two-step testing. Two-step testing means administering a second TB skin test within 1 to 3 weeks if the first test result is negative. This procedure should be used for the initial evaluation of adults who require routine testing. If the second skin test result is positive, it indicates the person was infected with TB before, and that immunity has waned and was “boosted” by the first test.
Without the 2-step process, a positive result on repeat testing would suggest recent infection rather than prior exposure. This could have implications for the decision to accept or not accept treatment for latent TB.
TABLE 1
Conditions associated with high risk of exposure to tuberculosis
| Foreign-born in area with high tuberculosis rates |
| Use of illicit drugs |
Institutionalization or work in
|
| Work in health care facilities with high-risk clients |
| Socioeconomic disadvantage |
| Children of those at high risk |
TABLE 2
Conditions associated with high risk of developing active tuberculosis
| HIV infection |
| Recent infection with Mycobacterium tuberculosis |
| Chest x-ray indicating old tuberculosis |
Immunosuppression
|
| Leukemia and other cancers of blood, lymph, and bone marrow |
| Cancer of the head and neck |
| Gastrectomy or intestinal bypass |
| Chronic malabsorption |
| End-stage renal disease |
| Diabetes |
| Silicosis |
| Illicit drug use |
TABLE 3
How to interpret a tuberculosis skin test
| 5 mm considered positive in those with: |
|
| 10 mm is considered positive in: |
Work-up for suspected tuberculosis
For those suspected of having TB because of chronic cough, night sweats, fever, and weight loss, or because of a positive TB skin test result, the workup consists of a chest x-ray film, an HIV test, and possibly sputum collection for microscopic evaluation and culture.
Those with a normal chest x-ray result who are HIV-negative are unlikely to have pulmonary TB, and sputum collection is unnecessary. For those with suspicious chest x-ray films and for those who are HIV positive with TB symptoms, sputum samples are needed for microscopic evaluation and culture (3 samples, preferably on 3 consecutive days).
Acid-fast organisms seen under the microscope may be Mycobacterium tuberculosis or mycobacteria other than tuberculosis (commonly referred to as MOTT), and the final determination must await culture confirmation, which now takes about 4 weeks. Preliminary confirmation using polymerase chain reaction can be accomplished in a few days. Treatment for active TB should be initiated, however, and the suspicion reported to the local health department as soon as TB is suspected.
Treating latent and active disease
Latent tuberculosis. Treatment for latent TB (positive TB skin test result, negative chest x-ray film, HIV-negative) should not be initiated until active TB is ruled out. This may require waiting 3 to 4 weeks for sputum culture results.
Contrary to common belief, there is no age cutoff for initiation of treatment for latent TB. Treatment requires 6 to 9 months of isoniazid or 4 months of rifampin. A shorter course with pyrazinamide and rifampin is also possible, although questions about the safety of this regimen have been raised. Patient compliance for the duration of therapy is difficult to achieve; but if accomplished, the risk of active disease decreases from 10% to less than 1%. At highest risk of developing active disease are children, and those who are HIV-positive, recently infected with TB, or whose chest films indicate old disease.
Since isoniazid is hepatotoxic, patients should be asked about symptoms of liver inflammation (abdominal pain, decline in appetite, dark urine, light-colored stools), and liver function tests should be ordered if symptoms are present. Routine monthly testing of liver function tests is not necessary and is recommended only for those who have chronic liver disease, an alcohol abuse disorder, or are pregnant.
Active tuberculosis. Always initiate treatment with at least 3 drugs (isoniazid, rifampin, and pyrazinamide). Ethambutol should be added to the regimen if patients are current or former residents of an area where resistance to isoniazid is more than 5%. If the organism proves sensitive to isoniazid and rifampin, ethambutol can be discontinued. Pyrazinamide should be continued for a full 2 months, and isoniazid and rifampin for a full 6 months. A variety of protocols for dosing and administration frequency are available to enhance convenience and patient compliance. Drug regimens may have to be adjusted based on culture results and success of therapy.
The standard of care for active TB is directly observed therapy. This means watching the patient swallow the pills. Although such care is labor intensive and implies a lack of trust in the patient, with its implementation, the successful completion of therapy rises from 50% to close to 100%. Widespread use of directly observed therapy since the late 1980s has resulted in a marked reduction of TB rates and rates of bacterial resistance.
To prevent bacterial resistance to drugs, treatment for active disease must be administered according to guidelines and be completed. One cardinal rule in the prevention of bacterial resistance is never to add just 1 drug to a failing regimen.
Any deviation from standard therapy because of bacterial resistance, patient nonadherence, or adverse drug effects is reason to consult the local public health department or state public health department TB program.
Working with the public health department
Three complementary activities are needed to control TB in the community:
- Finding and treating those with active TB
- Investigating close contacts of persons with active disease and offering them treatment of latent TB
- Screening persons at high risk, to find and treat latent TB.
The detection and management of tuberculosis offers an excellent opportunity for family physicians and health departments to work collaboratively to improve the community’s health.
Family physicians can make several contributions to reducing TB’s impact on the community:
- Screen appropriately
- Correctly apply and interpret TB skin tests
- Accurately make the diagnosis of latent and active TB
- Treat active TB according to recommended guidelines
- Encourage treatment of latent TB among those at highest risk of activation
- Promptly report to the local health department those suspected of having TB
- Collaborate with the public health department to reduce the spread of disease.
Local or state public health departments assist in several ways:
- Communicate with and investigating family and other close contacts of those with active, contagious TB, to find anyone with latent or active TB
- Offer consultation on how to diagnose and treat latent and active TB
- Assist with directly observed therapy
- Use public health authority to isolate and quarantine patients who are infectious and pose a risk to the community through non-adherence to treatment and infection control guidelines.
Many public health departments have TB programs wherein patients with active or latent TB can receive free care and medication.
State and local public health departments have the responsibility of monitoring TB care provided by physicians in the community, to insure it is applied according to guidelines and that it is completed. The intrusiveness of this can be minimized with regular communication and appreciation of the roles and responsibilities of each party.
Excellent review of the basics of TB screening, diagnosis, and treatment: Centers for Disease Control and Prevention (CDC). Core Curriculum on Tuberculosis. 4th ed. Atlanta, Ga: CDC; 2000.
Self-study modules on TB: CDC. National Center for HIV, STD, and TB Prevention, Divisionof Tuberculosis Elimination. Available at: www.phppo.cdc.gov/PHTN/tbmodules.(Accessed on September 8, 2003.)
Training modules and statistics on TB diagnosis, treatment, and epidemiology, including state-specific analyses: CDC. National Center for HIV, STD, and TB Prevention. Division of Tuberculosis Elimination. Available at: www.cdc.gov/nchstp/tb/default.htm. (Accessedon September 8, 2003.)
The most recent, comprehensive description of TB treatment recommendations: Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003; 167:603–662.
Prospects and trends
More reliable testing. The TB skin test is an imperfect screening tool. The development of a blood test for TB antibodies has progressed and will be evaluated and standardized. It will likely be a useful clinical and epidemiological tool in the near future.
Tuberculosis secondary to HIV. By some indicators, the rate of HIV infections has recently increased. This factor, combined with the increased life span of those with HIV, has the potential for reversing some of the recent progress in slowing TB rates. The continued development of anti-HIV medications and availability of the medications through AIDS/HIV treatment programs could help make those who are HIV infected less susceptible to TB infection and disease activation.
Tuberculosis and immigration. It is likely that as endemic TB declines in the US, a higher proportion of TB infections will occur in those who are foreign born and move to this country (see “Trends reversing in tuberculosis”). This underscores the global nature of public health and the importance of international collaboration in the control of contagious diseases and other public health threats.
At the turn of the 20th century, TB was the leading cause of death in the US and much of the world. Public health efforts and improved living conditions resulted in a steady decline in TB morbidity and mortality until the mid-1980s.
At that time a combination of events—the HIV epidemic, increased immigration from countries with high TB rates, and a decline in funding for TB control programs—resulted in a reversal of this downward trend and for several years there were increases in US TB rates. Of equal concern was an increase in multidrug resistance. These worrisome trends were reversed in the early 1990s and since that time TB rates have again been declining. In 2001 there were 15,989 new cases of TB in the US for a rate of 5.6/100,000. An increasing proportion of cases have been occurring among those born in countries with high TB rates; in 2001 49% of all those with TB in the US were foreign-born.
Increasing proportion of foreign-born TB cases
Number of tuberculosis cases in US-born vs foreign-born persons, United States, 1991–2001. Adapted from the Centers for Disease Control and Prevention, Atlanta, Ga.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
How should we manage an acute exacerbation of COPD?
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
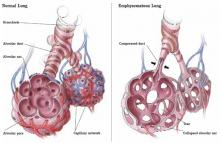
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
Diagnosis
- Chest radiography is useful (B).
- Spirometry should not be used to diagnose an exacerbation or to assess its severity (A).
- An arterial blood gas reading is helpful in gauging the severity of an exacerbation (A).
- There is little evidence regarding the contribution of additional laboratory testing, the predictive value of physical examination findings, or the usefulness of electrocardiography or echocardiography.
Treatment
- Inhaled short-acting beta-2 agonists and anticholinergic bronchodilators have positive effects. Since inhaled anti-cholinergic bronchodilators have fewer side effects, use them first. If improvement is slow with the initial bronchodilator, even at maximum dose, add a second bronchodilator (A).
- Parenteral agents (methyxanthines and sympathomimetics) are not as effective and have potential adverse effects (B).
- Mucolytic medications and chest physiotherapy are not effective (C).
- Systemic corticosteroids improve respiration and reduce relapse rate (A).
- Noninvasive positive-pressure ventilation decreases risk for invasive mechanical ventilation (A).
- Oxygen is beneficial for hypoxemic patients (B).
- Antibiotics are beneficial. Narrow-spectrum antibiotics (eg, amoxicillin, trimethoprimsulfamethoxazole, or tetracycline) are recommended as first-line agents. The more severe the episode, the more beneficial are antibiotics (A). There is no data regarding the optimal length of antibiotic treatment.
- Little evidence is available regarding the empiric use of diuretics.
Prognosis
- No methods reliably predict readmission to the hospital within 14 days after discharge (B).
- No methods reliably predict inpatient mortality (B).
Would you order a chest film to evaluate an acute exacerbation of chronic obstructive pulmonary disease (COPD)? Which medication would you first prescribe—a short-acting inhaled beta-2 agonist or an anticholinergic bronchodilator?
These are important questions for family physicians who commonly manage acute exacerbations of COPD.
The guideline summarized here was developed by a joint expert panel of the American College of Physicians–American Society of Internal Medicine and the American College of Chest Physicians. Three outcomes were considered: treatment efficacy, 6-month mortality, and relapse, as defined by return visit to the emergency department within 14 days of initial presentation. Systematic reviews with evidence tables were used to analyze data. The rationale for each recommendation is clear and well documented.
We added strength-of-recommendation ratings, which are not in the original guideline.
Limitations of the Guideline and Additional Evidence
Several weaknesses underlie this guideline. The authors found that, despite the importance of COPD, it has been the subject of very few high-quality studies. The highest-quality studies were few in number and had enrolled a small number of participants. The authors did not grade the strength of each recommendation in the summary document or in the detailed manuscripts, making it difficult to rapidly review.
Different diagnostic criteria are used in the source studies, making the context of treatment recommendations difficult to fully understand. Outcome endpoints also varied among studies. Goals for oxygen therapy were not addressed. Antibiotic treatment was based on studies before the emergence of multidrug-resistant organisms, particularly Streptococcus pneumoniae. It did not address tobacco use or smoking cessation, vaccine administration, outpatient management, management of stable COPD, or stratification of patients by severity.
Guideline Development and Evidence Review
Literature searches were performed using MEDLINE (1966–2000), EMBASE (1966– 2000), Health Star (1966–2000), and the Cochrane Controlled Trials Register (2000, Issue 1).
Search strategies included the index terms and text words chronic obstructive pulmonary disease and acute exacerbation and specific terms relating to interventions and outcomes. Variations on several search strategies were tested to locate the greatest number of relevant articles. Reference lists of retrieved articles were also examined. In all, 770 source articles were found.
Two other Guidelines for COPD
- Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, Md: Global Initiative for Chronic Obstructive Lung Disease, World Health Organization/National Heart, Lung, and Blood Institute; 2001. Various pagings. (Web access at: www.goldcopd.com.)
- Veterans Health Administration (VHA). Clinical practice guideline for the management of chronic obstructive pulmonary disease. Version 1.1a. Washington, DC: Department of Veterans Affairs (US), Veterans Health Administration; 1999 Aug. 116 p. (Web access at: www.oqp.med.va.gov/cpg/COPD/ COPD_base.htm).
FIGURE
Emphysematous dysfunction in COPD
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
GUIDELINE SOURCES
Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians–American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: A summary and appraisal of published evidence. Ann Intern Med 2001; 134:600-620. (Available at: www.annals.org/issues/ v134n7/full/200104030-00016.html. Accessed on September 5, 2003.)
McCrory DC, Brown C, Gelfand SE, Bach PB. Management of acute exacerbations of COPD: a summary and appraisal of published evidence. Chest 2001; 119:1190-1209.
Snow V, Lascher S, Mottur-Pilson C; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001; 134: 595-599. (Available at: www.annals.org/issues/v134n7/full/20010403000015.html. Accessed on September 5, 2003.)
What FPs need to know about West Nile virus disease
The Centers for Disease Control and Prevention (CDC) reports that West Nile virus infection in humans or animals has occurred in most states, and mosquito bite is now known to be just 1 of several means of virus transmission. Since many infected persons are asymptomatic, the challenge of controlling the spread is made even more difficult. At the time this issue went to press, August 19, a total of 599 human cases and 11 deaths had been reported to the CDC by state and local health authorities. Last year, a total of 638 cases and 31 deaths had been reported by August 30, and for the entire year, 4156 lab-positive human cases and 284 deaths.
This article describes transmission, diagnosis, treatment, and prevention of West Nile virus infection.
How the virus spreads
The virus, an RNA virus from the Flaviviridae family, is maintained in a bird-mosquito-bird cycle that begins in the spring when mosquitoes emerge and ends in early fall when they become dormant. By mid to late summer, the virus population has sufficiently amplified in both these hosts. At this point, other mosquitoes act as "bridge vectors" that bite both humans and birds, thus initiating West Nile virus infection in humans.
“Advice from your doctor: How to prevent West Nile virus infection,” may be photocopied for distribution to patients.
Avian mortality is documented in 162 North American species. It approaches 100% in laboratory-infected crows (making crows an important marker for the spread of West Nile virus in a specific community). House sparrows may develop high-level viremia for several days without dying, making them important amplifying hosts. Viremia in humans is low-grade and short-lived.
Transmission to humans: new routes discovered
Most cases of West Nile virus infections in humans result from mosquito bites, but other mechanisms have been discovered: needle sticks in lab workers, possibly breast milk (1 case), and possible transplacental transmission to the fetus (1 case).
More importantly, there is evidence of transmission through contaminated blood. Since many people infected with West Nile virus are asymptomatic, screening donors by clinical history is inadequate. In June 2003, blood-testing centers began screening the blood supply for West Nile virus using an experimental kit approved by the FDA.
What you can do to prevent West Nile virus infection
Here are practical steps you can take to avoid being infected with West Nile virus, and to help stop the spread of disease.
To prevent mosquito bites
- Use an insect repellant that contains DEET at concentrations less than 50% for adults and less than 10% for children.
- Wear long sleeves and socks when outdoors.
- Be particularly careful during evening and early morning hours, when mosquitoes are most active.
Mosquito-proof your home
- Drain standing water regularly to minimize mosquito breeding grounds.
- Install or repair screens on windows and doors.
Report dead birds to local health authorities so they can better monitor the activity of West Nile virus transmission.
Clinical course
Like many other viral infections, West Nile virus infection manifests in several ways.
Asymptomatic infection. Infection is not clinically apparent in most people.
Mild infection. About 20% of persons infected exhibit West Nile fever, a mild illness that follows an incubation period of 3 to 14 days.
The syndrome lasts 3 to 6 days, and is characterized by a sudden febrile illness often with an array of nonspecific signs and symptoms such as malaise, anorexia, nausea and vomiting, eye pain, headache, myalgia, rash, and lymphadenopathy.
Severe infection. About 1 in 150 infected persons develops severe neurological disease (encephalitis or meningitis are most common).
Being older than 50 years is the most significant risk factor for neurologic disease. In hospitalized patients, the most common symptoms accompanying neurologic disease are fever, weakness, gastrointestinal symptoms, and mental status changes. A smaller number of patients have a maculopapular or morbilliform rash of the trunk or extremities.
Outcomes. Advanced age is the most important risk factor for death, with mortality reaching 20% among patients older than 70 years. Unfortunately, there appears to be substantial neurologic morbidity for those surviving hospitalization.
The US case-fatality rate in 2002 was 9% in patients with meningoencephalitis.
Diagnosis
Diagnosis of symptomatic West Nile virus disease is based on clinical findings, a suggestive epidemiologic context, and specific laboratory test results.
Clinical and epidemiologic clues. Unexplained encephalitis or meningitis, particularly in a patient older than 50 years, during the summer or early fall, should trigger suspicion. Evidence of locally active disease in birds or humans, or recent travel to an area of known active disease, increases suspicion.
MAC-ELISA test and PanBio assay. The best test for diagnosis is detection of immunoglobulin M (IgM) antibody to West Nile virus in serum or cerebrospinal fluid within 8 days of illness onset (MAC-ELISA test, available through local and state health departments).
The FDA recently approved a commercial product, the PanBio West Nile virus IgM assay, which correctly identified the antibody in 90%–99% of cases. IgM antibody does not cross the blood-brain barrier, so its detection in cerebrospinal fluid is presumptive evidence of central nervous system infection.
Other laboratory findings include:
- normal or increased white blood cell counts, sometimes with lymphopenia or anemia
- occasional hyponatremia, especially in patients with encephalitis
- cerebrospinal fluid pleocytosis (usually lymphocytic), increased protein and normal glucose
- normal computed tomography brain scans
- abnormal magnetic resonance images in one third of patients.
Treatment is supportive
Treatment of severe disease is supportive. No evidence indicates efficacy of ribavirin, interferon, steroids, or other agents.
How to use public health resources
Prevention of West Nile virus disease will require both clinical and public health efforts. A good surveillance system is vital, providing clinicians and the community with knowledge about disease activity in birds and humans.
- Local or state health departments must coordinate, investigate, and track reports of dead birds by community members.
- Clinicians must notify the health department about suspected infections in humans.
- By publicizing the results of an active surveillance program, the health department assists clinicians in identifying cases more quickly and helps motivate the community to take appropriate preventive measures.
In late August 1999, an infectious disease specialist reported 2 patients with encephalitis at 1 hospital in Queens to the New York City Department of Health. An ensuing investigation revealed 6 additional cases at nearby hospitals. The illnesses were characterized by fever, severe muscle weakness (7 of 8 persons), and flaccid paralysis (4 of 8). Cerebrospinal fluid test results suggested viral infection.
So began the saga of human West Nile virus in the United States.
The virus was first isolated from a patient in Uganda, and is now distributed throughout Africa, the Middle East, parts of Europe, southwestern Asia, and Australia. Disease outbreaks in other parts of the world were infrequent until 1996.
West Nile virus is thought to have come to North America from Israel, but it is not clear how. Since 1999, the virus has spread rapidly throughout the US. Interestingly, the number of human cases reported annually was low (20–60) until 2002, when more than 4000 cases were reported. Only 9 continental states had avoided human cases of West Nile virus, and only 4 had reported no human or animal cases.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected]
SOURCES
Centers for Disease Control and Prevention. West Nile virus Web page. Available at: www.cdc.gov/ncidod/dvbid/ westnile/index.htm. Accessed on August 19, 2003.
Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 2001; 344:1807-1814.
Petersen L, Marfin A, and Gubler D. West Nile virus. JAMA 2003; 290:524-528.
US Food and Drug Administration. West Nile virus Web page. Available at: www.fda.gov/oc/opacom/hottopics/westnile.html. Accessed on August 11, 2003.
The Centers for Disease Control and Prevention (CDC) reports that West Nile virus infection in humans or animals has occurred in most states, and mosquito bite is now known to be just 1 of several means of virus transmission. Since many infected persons are asymptomatic, the challenge of controlling the spread is made even more difficult. At the time this issue went to press, August 19, a total of 599 human cases and 11 deaths had been reported to the CDC by state and local health authorities. Last year, a total of 638 cases and 31 deaths had been reported by August 30, and for the entire year, 4156 lab-positive human cases and 284 deaths.
This article describes transmission, diagnosis, treatment, and prevention of West Nile virus infection.
How the virus spreads
The virus, an RNA virus from the Flaviviridae family, is maintained in a bird-mosquito-bird cycle that begins in the spring when mosquitoes emerge and ends in early fall when they become dormant. By mid to late summer, the virus population has sufficiently amplified in both these hosts. At this point, other mosquitoes act as "bridge vectors" that bite both humans and birds, thus initiating West Nile virus infection in humans.
“Advice from your doctor: How to prevent West Nile virus infection,” may be photocopied for distribution to patients.
Avian mortality is documented in 162 North American species. It approaches 100% in laboratory-infected crows (making crows an important marker for the spread of West Nile virus in a specific community). House sparrows may develop high-level viremia for several days without dying, making them important amplifying hosts. Viremia in humans is low-grade and short-lived.
Transmission to humans: new routes discovered
Most cases of West Nile virus infections in humans result from mosquito bites, but other mechanisms have been discovered: needle sticks in lab workers, possibly breast milk (1 case), and possible transplacental transmission to the fetus (1 case).
More importantly, there is evidence of transmission through contaminated blood. Since many people infected with West Nile virus are asymptomatic, screening donors by clinical history is inadequate. In June 2003, blood-testing centers began screening the blood supply for West Nile virus using an experimental kit approved by the FDA.
What you can do to prevent West Nile virus infection
Here are practical steps you can take to avoid being infected with West Nile virus, and to help stop the spread of disease.
To prevent mosquito bites
- Use an insect repellant that contains DEET at concentrations less than 50% for adults and less than 10% for children.
- Wear long sleeves and socks when outdoors.
- Be particularly careful during evening and early morning hours, when mosquitoes are most active.
Mosquito-proof your home
- Drain standing water regularly to minimize mosquito breeding grounds.
- Install or repair screens on windows and doors.
Report dead birds to local health authorities so they can better monitor the activity of West Nile virus transmission.
Clinical course
Like many other viral infections, West Nile virus infection manifests in several ways.
Asymptomatic infection. Infection is not clinically apparent in most people.
Mild infection. About 20% of persons infected exhibit West Nile fever, a mild illness that follows an incubation period of 3 to 14 days.
The syndrome lasts 3 to 6 days, and is characterized by a sudden febrile illness often with an array of nonspecific signs and symptoms such as malaise, anorexia, nausea and vomiting, eye pain, headache, myalgia, rash, and lymphadenopathy.
Severe infection. About 1 in 150 infected persons develops severe neurological disease (encephalitis or meningitis are most common).
Being older than 50 years is the most significant risk factor for neurologic disease. In hospitalized patients, the most common symptoms accompanying neurologic disease are fever, weakness, gastrointestinal symptoms, and mental status changes. A smaller number of patients have a maculopapular or morbilliform rash of the trunk or extremities.
Outcomes. Advanced age is the most important risk factor for death, with mortality reaching 20% among patients older than 70 years. Unfortunately, there appears to be substantial neurologic morbidity for those surviving hospitalization.
The US case-fatality rate in 2002 was 9% in patients with meningoencephalitis.
Diagnosis
Diagnosis of symptomatic West Nile virus disease is based on clinical findings, a suggestive epidemiologic context, and specific laboratory test results.
Clinical and epidemiologic clues. Unexplained encephalitis or meningitis, particularly in a patient older than 50 years, during the summer or early fall, should trigger suspicion. Evidence of locally active disease in birds or humans, or recent travel to an area of known active disease, increases suspicion.
MAC-ELISA test and PanBio assay. The best test for diagnosis is detection of immunoglobulin M (IgM) antibody to West Nile virus in serum or cerebrospinal fluid within 8 days of illness onset (MAC-ELISA test, available through local and state health departments).
The FDA recently approved a commercial product, the PanBio West Nile virus IgM assay, which correctly identified the antibody in 90%–99% of cases. IgM antibody does not cross the blood-brain barrier, so its detection in cerebrospinal fluid is presumptive evidence of central nervous system infection.
Other laboratory findings include:
- normal or increased white blood cell counts, sometimes with lymphopenia or anemia
- occasional hyponatremia, especially in patients with encephalitis
- cerebrospinal fluid pleocytosis (usually lymphocytic), increased protein and normal glucose
- normal computed tomography brain scans
- abnormal magnetic resonance images in one third of patients.
Treatment is supportive
Treatment of severe disease is supportive. No evidence indicates efficacy of ribavirin, interferon, steroids, or other agents.
How to use public health resources
Prevention of West Nile virus disease will require both clinical and public health efforts. A good surveillance system is vital, providing clinicians and the community with knowledge about disease activity in birds and humans.
- Local or state health departments must coordinate, investigate, and track reports of dead birds by community members.
- Clinicians must notify the health department about suspected infections in humans.
- By publicizing the results of an active surveillance program, the health department assists clinicians in identifying cases more quickly and helps motivate the community to take appropriate preventive measures.
In late August 1999, an infectious disease specialist reported 2 patients with encephalitis at 1 hospital in Queens to the New York City Department of Health. An ensuing investigation revealed 6 additional cases at nearby hospitals. The illnesses were characterized by fever, severe muscle weakness (7 of 8 persons), and flaccid paralysis (4 of 8). Cerebrospinal fluid test results suggested viral infection.
So began the saga of human West Nile virus in the United States.
The virus was first isolated from a patient in Uganda, and is now distributed throughout Africa, the Middle East, parts of Europe, southwestern Asia, and Australia. Disease outbreaks in other parts of the world were infrequent until 1996.
West Nile virus is thought to have come to North America from Israel, but it is not clear how. Since 1999, the virus has spread rapidly throughout the US. Interestingly, the number of human cases reported annually was low (20–60) until 2002, when more than 4000 cases were reported. Only 9 continental states had avoided human cases of West Nile virus, and only 4 had reported no human or animal cases.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected]
The Centers for Disease Control and Prevention (CDC) reports that West Nile virus infection in humans or animals has occurred in most states, and mosquito bite is now known to be just 1 of several means of virus transmission. Since many infected persons are asymptomatic, the challenge of controlling the spread is made even more difficult. At the time this issue went to press, August 19, a total of 599 human cases and 11 deaths had been reported to the CDC by state and local health authorities. Last year, a total of 638 cases and 31 deaths had been reported by August 30, and for the entire year, 4156 lab-positive human cases and 284 deaths.
This article describes transmission, diagnosis, treatment, and prevention of West Nile virus infection.
How the virus spreads
The virus, an RNA virus from the Flaviviridae family, is maintained in a bird-mosquito-bird cycle that begins in the spring when mosquitoes emerge and ends in early fall when they become dormant. By mid to late summer, the virus population has sufficiently amplified in both these hosts. At this point, other mosquitoes act as "bridge vectors" that bite both humans and birds, thus initiating West Nile virus infection in humans.
“Advice from your doctor: How to prevent West Nile virus infection,” may be photocopied for distribution to patients.
Avian mortality is documented in 162 North American species. It approaches 100% in laboratory-infected crows (making crows an important marker for the spread of West Nile virus in a specific community). House sparrows may develop high-level viremia for several days without dying, making them important amplifying hosts. Viremia in humans is low-grade and short-lived.
Transmission to humans: new routes discovered
Most cases of West Nile virus infections in humans result from mosquito bites, but other mechanisms have been discovered: needle sticks in lab workers, possibly breast milk (1 case), and possible transplacental transmission to the fetus (1 case).
More importantly, there is evidence of transmission through contaminated blood. Since many people infected with West Nile virus are asymptomatic, screening donors by clinical history is inadequate. In June 2003, blood-testing centers began screening the blood supply for West Nile virus using an experimental kit approved by the FDA.
What you can do to prevent West Nile virus infection
Here are practical steps you can take to avoid being infected with West Nile virus, and to help stop the spread of disease.
To prevent mosquito bites
- Use an insect repellant that contains DEET at concentrations less than 50% for adults and less than 10% for children.
- Wear long sleeves and socks when outdoors.
- Be particularly careful during evening and early morning hours, when mosquitoes are most active.
Mosquito-proof your home
- Drain standing water regularly to minimize mosquito breeding grounds.
- Install or repair screens on windows and doors.
Report dead birds to local health authorities so they can better monitor the activity of West Nile virus transmission.
Clinical course
Like many other viral infections, West Nile virus infection manifests in several ways.
Asymptomatic infection. Infection is not clinically apparent in most people.
Mild infection. About 20% of persons infected exhibit West Nile fever, a mild illness that follows an incubation period of 3 to 14 days.
The syndrome lasts 3 to 6 days, and is characterized by a sudden febrile illness often with an array of nonspecific signs and symptoms such as malaise, anorexia, nausea and vomiting, eye pain, headache, myalgia, rash, and lymphadenopathy.
Severe infection. About 1 in 150 infected persons develops severe neurological disease (encephalitis or meningitis are most common).
Being older than 50 years is the most significant risk factor for neurologic disease. In hospitalized patients, the most common symptoms accompanying neurologic disease are fever, weakness, gastrointestinal symptoms, and mental status changes. A smaller number of patients have a maculopapular or morbilliform rash of the trunk or extremities.
Outcomes. Advanced age is the most important risk factor for death, with mortality reaching 20% among patients older than 70 years. Unfortunately, there appears to be substantial neurologic morbidity for those surviving hospitalization.
The US case-fatality rate in 2002 was 9% in patients with meningoencephalitis.
Diagnosis
Diagnosis of symptomatic West Nile virus disease is based on clinical findings, a suggestive epidemiologic context, and specific laboratory test results.
Clinical and epidemiologic clues. Unexplained encephalitis or meningitis, particularly in a patient older than 50 years, during the summer or early fall, should trigger suspicion. Evidence of locally active disease in birds or humans, or recent travel to an area of known active disease, increases suspicion.
MAC-ELISA test and PanBio assay. The best test for diagnosis is detection of immunoglobulin M (IgM) antibody to West Nile virus in serum or cerebrospinal fluid within 8 days of illness onset (MAC-ELISA test, available through local and state health departments).
The FDA recently approved a commercial product, the PanBio West Nile virus IgM assay, which correctly identified the antibody in 90%–99% of cases. IgM antibody does not cross the blood-brain barrier, so its detection in cerebrospinal fluid is presumptive evidence of central nervous system infection.
Other laboratory findings include:
- normal or increased white blood cell counts, sometimes with lymphopenia or anemia
- occasional hyponatremia, especially in patients with encephalitis
- cerebrospinal fluid pleocytosis (usually lymphocytic), increased protein and normal glucose
- normal computed tomography brain scans
- abnormal magnetic resonance images in one third of patients.
Treatment is supportive
Treatment of severe disease is supportive. No evidence indicates efficacy of ribavirin, interferon, steroids, or other agents.
How to use public health resources
Prevention of West Nile virus disease will require both clinical and public health efforts. A good surveillance system is vital, providing clinicians and the community with knowledge about disease activity in birds and humans.
- Local or state health departments must coordinate, investigate, and track reports of dead birds by community members.
- Clinicians must notify the health department about suspected infections in humans.
- By publicizing the results of an active surveillance program, the health department assists clinicians in identifying cases more quickly and helps motivate the community to take appropriate preventive measures.
In late August 1999, an infectious disease specialist reported 2 patients with encephalitis at 1 hospital in Queens to the New York City Department of Health. An ensuing investigation revealed 6 additional cases at nearby hospitals. The illnesses were characterized by fever, severe muscle weakness (7 of 8 persons), and flaccid paralysis (4 of 8). Cerebrospinal fluid test results suggested viral infection.
So began the saga of human West Nile virus in the United States.
The virus was first isolated from a patient in Uganda, and is now distributed throughout Africa, the Middle East, parts of Europe, southwestern Asia, and Australia. Disease outbreaks in other parts of the world were infrequent until 1996.
West Nile virus is thought to have come to North America from Israel, but it is not clear how. Since 1999, the virus has spread rapidly throughout the US. Interestingly, the number of human cases reported annually was low (20–60) until 2002, when more than 4000 cases were reported. Only 9 continental states had avoided human cases of West Nile virus, and only 4 had reported no human or animal cases.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected]
SOURCES
Centers for Disease Control and Prevention. West Nile virus Web page. Available at: www.cdc.gov/ncidod/dvbid/ westnile/index.htm. Accessed on August 19, 2003.
Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 2001; 344:1807-1814.
Petersen L, Marfin A, and Gubler D. West Nile virus. JAMA 2003; 290:524-528.
US Food and Drug Administration. West Nile virus Web page. Available at: www.fda.gov/oc/opacom/hottopics/westnile.html. Accessed on August 11, 2003.
SOURCES
Centers for Disease Control and Prevention. West Nile virus Web page. Available at: www.cdc.gov/ncidod/dvbid/ westnile/index.htm. Accessed on August 19, 2003.
Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 2001; 344:1807-1814.
Petersen L, Marfin A, and Gubler D. West Nile virus. JAMA 2003; 290:524-528.
US Food and Drug Administration. West Nile virus Web page. Available at: www.fda.gov/oc/opacom/hottopics/westnile.html. Accessed on August 11, 2003.
Diagnosing coronary heart disease: When to use stress imaging studies
- Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting electrocardiogram (ECG) and without contraindications to exercise.
- Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test.
- Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to target level are candidates for nuclear or echocardiographic pharmacologic stress testing.
- Patients with suspected coronary heart disease and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Most men and women experience symptoms before myocardial infarction (MI).1 Early recognition of these symptoms and prompt treatment are essential for prevention of death and disability related to coronary heart disease (CHD).
Patients with multiple risk factors, chest pain typically suggestive of CHD, or a history of CHD are usually easy to identify and triage. However, many patients do not have obvious risks for CHD but experience occasional symptoms of cardiac ischemia.
Patients can be stratified into low-, intermediate-, and high-risk categories that will help determine appropriate work-up. Those at intermediate risk can be difficult to assess, and may particularly benefit from stress-imaging studies.
The algorithm (Figure 1) is based on current guidelines,2 and indicates how patients with chest pain/symptoms may be identified and treated according to an initial estimate of the probability of obstructive coronary artery disease. The choice of noninvasive diagnostic tests for individuals with stable chest pain and a lower risk for CHD is then outlined.
Case studies
Patient 1
A 64-year-old, nonsmoking, obese woman with degenerative osteoarthritis of the knees occasionally experiences chest discomfort that lasts for a few minutes, sometimes radiating to her back. The discomfort, which started 4 weeks ago, occasionally becomes worse after a brief walk, but is not usually related to exertion, or associated with nausea or diaphoresis. She sometimes becomes short of breath climbing stairs.
Physical examination: In no acute distress; body-mass index 31.2, waist circumference 42 inches, heart rate 70 beats/min, blood pressure 142/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: resting electrocardiogram (ECG)—sinus rhythm otherwise normal; creatinine 1.2 mg/dL; fasting glucose 122 mg/dL; glycosylated hemoglobin 6.4%. Lipids: total cholesterol 232 mg/dL; triglycerides 230 mg/dL; high-density lipoprotein (HDL) cholesterol 28 mg/dL; low-density lipoprotein (LDL) cholesterol 158 mg/dL.
Patient 2
A 58-year-old, nonsmoking, otherwise healthy man experiences tightness in the chest, usually at night. The pain began 4 to 8 weeks ago; it lasts as long as 1 to 2 hours but is “very mild.” It does not radiate to the arm or jaw and is unrelated to exertion. There is no diaphoresis or nausea. He sometimes feels a bit winded, which “might be due to anxiety.”
Physical examination: slightly overweight man in no acute distress; body-mass index 27.0, waist circumference 36 inches, heart rate 74 beats/min, blood pressure 138/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: ECG— sinus rhythm, otherwise normal; creatinine 1.0 mg/dL; fasting glucose 98 mg/dL. Lipids: total cholesterol 215 mg/dL; triglycerides 150 mg/dL; HDL 40 mg/dL; LDL 145 mg/dL.
Diagnostic approaches
Standard diagnostic techniques include history, physical examination, laboratory testing as indicated, resting ECG, and assessment of risk factors for CHD.
Evaluation of chest pain
A careful history and physical examination can often quickly exclude many noncardiac causes of chest discomfort or pain. Table 1 contrasts the characteristics of atypical (noncardiac) symptoms with those of typical (cardiac) symptoms. Its quality, location, and the factors that relieve or provoke it, duration, and any associated symptoms should be evaluated. If high-risk or unstable signs or symptoms are present that suggest acute coronary syndrome (unstable angina or MI), evaluation in the emergency department should be performed.
Patients exhibiting stable or atypical (noncardiac) symptoms with some, but not all, of the features of angina described above have a lower probability of coronary artery disease, and should be considered for diagnostic evaluation under the guidance of the primary care physician.3
TABLE 1
Characteristics of atypical (noncardiac) vs typical cardiac symptoms
| Characteristic | Atypical/noncardiac | Typical/cardiac |
|---|---|---|
| Quality | Sharp, stabbing, positional | Squeezing, ache, pressure, fullness, burning, heavy, suffocating, “discomfort” |
| Location | Highly localized, below the epigastrium, above the mandible | Diffuse area—substernal, chest, jaw, back, arms |
| Provoked by | “Nothing,” body movement, cough, deep inspiration, chest palpation | Exertion, emotional stress, cold air |
| Relieved by | “Nothing,” position change, analgesics, heat, antacids | Rest; nitroglycerin |
| Duration | “Seconds” (fleeting), or hours, days | 30 seconds to 5 minutes |
| Associated symptoms | Reflux/heartburn | Dyspnea, diaphoresis, nausea, fatigue |
Evaluating women
In women aged <55 years, noncardiac chest pain is common, but since the prevalence of CHD is increasing among younger women, their symptoms should not be dismissed as “noncardiac” without full evaluation.
Women are also more likely than men to report dyspnea or pain in the jaw or back instead of, or in addition to, chest symptoms. Further, since women are often older and less active when they develop CHD, they may not exhibit typical exertional symptoms. Diagnosis in women is also hampered by lower accuracy of standard stress ECG testing compared with men. False-positive and false-negative tests may occur more frequently in women due to hormonal effects on the ECG, and more frequent comorbidities that limit maximal exercise capacity.
Physical examination
Palpation and auscultation of the chest may detect the presence of a friction rub or significant murmur, and thus identify a nonischemic cause for the chest symptoms. Carotid bruits or reduced pedal pulses indicate the presence of other vascular diseases. Patients with xanthomas, hypertension, or signs of congestive heart failure are more likely to have CHD, while those whose pain can be reproduced by body movement or by palpating the chest are less likely to have CHD.
Risk factor assessment. The assessment of risk factors for CHD allows the identification of many patients at high risk for CHD and can be helpful in guiding the choice of additional tests. As evident in the Framingham Heart Study,4 independent risk factors such as cigarette smoking, hypertension, diabetes mellitus, and hyperlipidemia are direct causes of CHD.
Laboratory tests
In the patient at low risk of CHD, blood testing for cardiac markers is not indicated. A lipid profile and blood glucose level help to establish the risk level associated with hyperlipidemia and diabetes. A complete blood count (eg, for anemia), thyroid hormone studies (eg, for hyperthyroidism), arterial blood gases (eg, PCO2 for chronic obstructive pulmonary disease), and other tests may help in diagnosing contributory conditions.
Resting electrocardiogram
A routine resting 12-lead ECG is an inexpensive but critical test that can provide important diagnostic and prognostic information. Evidence of infarction, ischemia, hypertrophy arrhythmias, and conduction disturbances can be detected and, if present, substantially increase the likelihood of a cardiac cause of symptoms.
Even the presence of mild or nonspecific ST-T wave changes, while not diagnostic, can aid the clinician by suggesting a higher probability of a nondiagnostic stress ECG and the need for an imaging stress test.3 An abnormal resting ECG with ST-T wave changes associated with digoxin use, left bundle branch block, left ventricular hypertrophy, and so on, limit interpretation of an exercise ECG, and points to a need for exercise testing with imaging.
It is important to note that a normal ECG obtained when the patient is asymptomatic does not exclude CHD, and additional risk stratification with noninvasive diagnostic stress testing may be indicated.5
Chest x-ray
A chest x-ray is often appropriate for patients with cardiac or pulmonary signs/symptoms. It may show cardiac enlargement, ventricular aneurysm, or evidence of heart failure, which may support the diagnosis of CHD and help to assess the extent of cardiopulmonary involvement.
Noninvasive stress testing
Considering our 2 patients with occasional episodes of unexplained chest discomfort: Based on the ECG and clinical findings, their risk for CHD is considered low to intermediate.
Test selection
Diagnostic tests should be selected based on the clinician’s estimate of probability of CHD.2
Low probability. If the likelihood of CHD is low, stress testing is generally not indicated, as its specificity is extremely low, and test results do not improve diagnostic accuracy over the clinical impression alone.
Intermediate probability. If the patient is able to exercise to capacity, the choice is exercise testing. Patients who can exercise and have an interpretable ECG, with no evidence of left ventricular dysfunction and no prior revascularization procedure, should usually undergo standard stress ECG testing. If the ECG is not interpretable, (due to repolarization abnormalities, left bundle branch block, left ventricular hypertrophy, digoxin use, etc) an exercise test with imaging (nuclear or echocardiographic) is indicated.
For patients unable to exercise, pharmacologic stress testing with imaging is indicated.
High probability. If the probability of CHD is high, it is reasonable to proceed directly to coronary angiography.
Exercise stress test
Exercise testing is a cardiovascular stress that uses treadmill or bicycle exercise with ECG and blood pressure monitoring. Such testing is widely available and relatively inexpensive.2 It allows assessment of exercise capacity and correlation of symptoms with ECG changes typical of myocardial ischemia.
Exercise testing provides the highest level of incremental diagnostic and prognostic information for patients with an intermediate probability of CHD.2 An important objective of stress testing is to identify individuals with a high risk for severe (left main or 3-vessel) CHD. More invasive procedures, such as percutaneous cutaneous angioplasty (PCTA), are recommended for these high-risk individuals to improve their survival.
Candidates for exercise treadmill testing include patients with stable symptoms who can be expected to exercise to an adequate workload. Patients with repolarization abnormalities on the resting ECG, such as left bundle branch block, left ventricular hypertrophy, or digoxin use, frequently have noninterpretable stress ECGs and may benefit from imaging techniques.
Limitations. Some patients referred for exercise treadmill testing are unable to achieve either adequate exercise levels or the target heart rate due to comorbid conditions,6 such as degenerative joint disease, obesity, pulmonary disease, peripheral vascular disease, central nervous system disorders, physical deconditioning, chronotropic incompetence, and medications such as beta blockers. More subtle factors, such as an unwillingness to exercise, may also affect a patient’s suitability for stress testing. These patients should be considered for pharmacologic stress testing.
Additionally, stress-induced ST-T wave changes do not accurately localize the site of myocardial ischemia and provide no direct information on left ventricular function and other clinically important variables. The sensitivity and specificity of exercise ECG testing ranges from approximately 67% to 72%, which is below that of stress imaging techniques, whose average sensitivity ranges from 80% to 85%.7-9
Stress imaging modalities
For a patient with an abnormal resting ECG, evidence of left ventricular dysfunction, or a prior coronary revascularization, stress imaging with either echocardiography or nuclear perfusion scanning is appropriate. Both techniques show higher specificity than the stress ECG alone.
Nuclear imaging. Nuclear imaging uses radiotracers (thallium-201, technetium-99m tetrofosmin [Myoview], or technetium-99m sestamibi [Cardiolite]) to evaluate myocardial perfusion and function, and has greatly advanced the ability to detect and assess the extent of CHD. Stress myocardial perfusion imaging has a sensitivity of >90% for detecting patients at risk of cardiac death or MI.6
To detect ischemia or infarction, a radioisotope is injected at rest and after stress to produce images of myocardial regional uptake, which is proportional to regional blood flow. Normally, with maximal exercise or pharmacologic stress, myocardial blood flow is greatly increased above the resting condition. If a fixed coronary stenosis is present, myocardial perfusion in the territory supplied by the stenosis cannot be increased, which will create a flow differential and uneven distribution of the tracer.
As illustrated in Figure 2, a normal myocardial perfusion image shows homogenous accumulation of radiotracer on both the stress and rest images. A perfusion defect appears as an area of reduced tracer uptake.
Nuclear perfusion studies can also provide a measure of left ventricular function and wall motion utilizing a bolus injection of radiotracer. While images can be obtained in most patients utilizing current techniques, artifacts due to breast and diaphragmatic tissue attenuation can lead to false-positive interpretation, particularly when examining women and when using thallium.
Echocardiography. Echocardiography visualizes the heart directly in real time using ultrasound, providing convenient assessment of the cardiac chambers, myocardium, valves, pericardium, and great vessels. The test can also identify mechanical complications of acute myocardial infarction, differentiate causes of reduced cardiac output and blood pressure, and help guide therapy. Stress echocardiography (exercise or pharmacologic stress) can be used to detect the presence, location, and severity of inducible myocardial ischemia as well as for risk stratification and prognosis.
During stress-induced ischemia, decrements in contractile function are directly related to decreases in regional subendocardial blood flow. Wall-motion changes precede ischemic ECG changes, accounting for the increased sensitivity of echocardiography versus ECG stress testing.
Interpretation of stress echocardiograms is based on analysis of segmental wall motion before and soon after stress. Normally, with exercise, or dobutamine infusion, left ventricular wall motion becomes hyperdynamic. The hallmark of ischemia is the development with stress of new, or the worsening of preexisting, wall motion abnormalities. The lack of improvement with stress in an already hypokinetic segment indicates infarction. Stress-induced left ventricular cavity enlargement, systolic dysfunction, or mitral regurgitation may also suggest CHD. Accuracy of stress echocardiography is similar to that of nuclear stress testing.
Considerable expertise in echocardiography is needed to rapidly acquire diagnostic images, so that its selection is limited by the skill of the technician. Image quality can be compromised by obesity and other factors, but the widespread use of intravenous contrast agents has significantly reduced the proportion of patients with uninterpretable images.
FIGURE 2
Accumulation of radiotracer in nuclear imaging (stress and rest images)
Patients who are not expected to achieve an adequate exercise capacity (as in our patient with osteoarthritis) should undergo pharmacologic stress testing with adenosine, dipyridamole, or dobutamine. Atrial pacing utilizing a swallowed esophageal electrode is also used in some cases. These agents, combined with echocardiographic or nuclear imaging, are particularly useful in patients who are unable to exercise adequately.
Pharmacologic stress agents are sometimes combined with low-level exercise protocols which may reduce the noncardiac side effects and improve image quality.10
Adenosine is the pharmacologic agent used most commonly in nuclear perfusion stress testing. An intravenous infusion of adenosine produces coronary vasodilation which is quickly attenuated when the infusion is terminated. Side effects, which are short-lived, include flushing, palpitations, and chest pain.
Dipyridamole is used less commonly due to its prolonged side effects and reports of lower specificity.11 Dobutamine, a beta-adrenergic agonist, increases heart rate and contractility in a dose-related fashion when infused intravenously. This agent is most commonly used in echocardiographic imaging. It can also be utilized with nuclear imaging when adenosine is contraindicated due to severe pulmonary or cerebrovascular disease. Side effects include transient arrhythmias, hypertension or hypotension, tremor, and chest pain.
Referral to a cardiologist
Referral to a cardiologist should be considered when the suspicion for cardiac disease is high, there is substantial diagnostic uncertainty after initial evaluation, or if symptoms persist, despite treatment of a noncardiac cause. Further evaluation and treatment often includes coronary angiography.
Coronary angiography
Most outpatients, such as the 2 presented, can be diagnosed with clinical and noninvasive measures. Coronary angiography is most commonly used to determine the presence and extent of obstructive CHD, and to guide decisions about revascularization in high-risk patients, or in patients with an abnormal stress test.
Cardiac catheterization presents a small but real risk to the patient, involves discomfort and substantial cost, and can challenge effective resource utilization. Risks and benefits to individual patients should be discussed between primary care physician and cardiologist.
Summary
Findings in our 2 patients are summarized below. Diagnostic decisions reflect the algorithm in Figure 1and are based on current guidelines.2
Patient 1. After initial assessment, our 64-year-old asymptomatic woman still falls into “intermediate probability of CHD” due to her multiple CHD risks. Stress testing was therefore indicated. Due to her inability to exercise because of an orthopedic limitation, she underwent pharmacologic stress testing with an adenosine sestamibi study. A small inferior reversible defect was identified, suggestive of myocardial ischemia.
Aggressive medical therapy aimed at minimizing symptoms and reducing risk was selected: aspirin, a beta-blocker for ischemia and hypertension, and a statin for hyperlipidemia. Longacting nitrates or calcium-channel blockers would have been reasonable alternatives. Consideration of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker is also indicated in light of her new CHD diagnosis and glucose intolerance. She was advised to initiate a low-fat, low-carbohydrate diet and to exercise (swim) regularly to lower risk. She will be seen in 6 weeks to reevaluate her symptoms, blood pressure, and lipid and glycemic control.
Patient 2. Our male patient also warranted stress testing. He was referred for a standard stress ECG due to his normal resting ECG, and the expectation that he would be able to exercise adequately. He satisfactorily completed 10.5 minutes (10 METS) of a Bruce protocol on a treadmill exercise stress test, which was entirely normal.
This admittedly anxious individual was reassured that his chest symptoms are not due to heart disease. An empiric trial with a proton pump inhibitor could be initiated if gastro-esophageal reflux is suspected.
Conclusions
Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting ECG and without contraindications to exercise, as in our male patient. Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test. Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to the target level are candidates for nuclear or echocardiographic pharmacologic stress testing, as was indicated for our female patient. Patients with suspected CHD and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Finally, when selecting a specific stress imaging technique, physicians should consider the local expertise with the various techniques available, together with their strengths and limitations in the individual patient.19
1. Spertus JA, Radford MJ, Every NR, et al. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction. Summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation 2003;107:1681-1691.
2. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). Circulation 2002;106:1883-1892.
3. Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999;33:2092-2197.
4. Grundy SM, Balady GJ, Criqui MH, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the American Heart Association Task Force on Risk Reduction. Circulation 1998;97:1876-1887.
5. Zanger DR, Solomon AJ, Gersh BJ. Contemporary management of angina: part I: risk assessment. Am Fam Physician 1999;60:2543-2552.
6. Bar Harbor Invitation Meeting 2000. Panel 9: Nuclear imaging in coronary artery disease. J Nucl Cardiol 2001;8:305-316.
7. Kotler TS, Diamond GA. Exercise thallium-201 scintigraphy in the diagnosis and prognosis of coronary artery disease. Ann Intern Med 1990;113:684-702.
8. Gibbons RJ. Rest and exercise radionuclide angiography for diagnosis in chronic ischemic heart disease. Circulation 1991;84 (3 Suppl):I93-I99.
9. Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc 1995;70:5-15.
10. Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol 2000;7:439-446.
11. Li T, Ahlberg A, Hachamovitch R, et al. Comparison of adenosine and dipyridamole in detecting coronary artery disease using Tc-99m sestamibi single-photon emission computed tomography imaging: a randomized, prospective clinical study. J Am Coll Cardiol 2003;41(suppl A):461A. Abstract 858-3.
12. American Heart Association. Heart disease and stroke statistics—2003 update. Dallas, Tex: American Heart Association; 2003.
This special section of The Journal of Family Practiceis provided by an unrestricted grant fromFujisawa Healthcare, Inc. Disclosures: Dr McBride has served on the speakers’ bureau for the following: Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Glaxo SmithKline, KOS Pharmaceuticals, Merck & Co Inc, Pfizer Inc, Reliant Pharmaceuticals, and Sankyo. He has served as consultant to KOS Pharmaceuticals, Merck & Co Inc, and Pfizer Inc. He has received grant/research support from Merck & Co Inc and Pfizer Inc. Dr Hayes had no commercial interests to report. Corresponding author: Sharonne N. Hayes, MD, Mayo Clinic Women’s Heart Clinic, Rochester MN, 55905. E-mail: [email protected].
- Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting electrocardiogram (ECG) and without contraindications to exercise.
- Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test.
- Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to target level are candidates for nuclear or echocardiographic pharmacologic stress testing.
- Patients with suspected coronary heart disease and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Most men and women experience symptoms before myocardial infarction (MI).1 Early recognition of these symptoms and prompt treatment are essential for prevention of death and disability related to coronary heart disease (CHD).
Patients with multiple risk factors, chest pain typically suggestive of CHD, or a history of CHD are usually easy to identify and triage. However, many patients do not have obvious risks for CHD but experience occasional symptoms of cardiac ischemia.
Patients can be stratified into low-, intermediate-, and high-risk categories that will help determine appropriate work-up. Those at intermediate risk can be difficult to assess, and may particularly benefit from stress-imaging studies.
The algorithm (Figure 1) is based on current guidelines,2 and indicates how patients with chest pain/symptoms may be identified and treated according to an initial estimate of the probability of obstructive coronary artery disease. The choice of noninvasive diagnostic tests for individuals with stable chest pain and a lower risk for CHD is then outlined.
Case studies
Patient 1
A 64-year-old, nonsmoking, obese woman with degenerative osteoarthritis of the knees occasionally experiences chest discomfort that lasts for a few minutes, sometimes radiating to her back. The discomfort, which started 4 weeks ago, occasionally becomes worse after a brief walk, but is not usually related to exertion, or associated with nausea or diaphoresis. She sometimes becomes short of breath climbing stairs.
Physical examination: In no acute distress; body-mass index 31.2, waist circumference 42 inches, heart rate 70 beats/min, blood pressure 142/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: resting electrocardiogram (ECG)—sinus rhythm otherwise normal; creatinine 1.2 mg/dL; fasting glucose 122 mg/dL; glycosylated hemoglobin 6.4%. Lipids: total cholesterol 232 mg/dL; triglycerides 230 mg/dL; high-density lipoprotein (HDL) cholesterol 28 mg/dL; low-density lipoprotein (LDL) cholesterol 158 mg/dL.
Patient 2
A 58-year-old, nonsmoking, otherwise healthy man experiences tightness in the chest, usually at night. The pain began 4 to 8 weeks ago; it lasts as long as 1 to 2 hours but is “very mild.” It does not radiate to the arm or jaw and is unrelated to exertion. There is no diaphoresis or nausea. He sometimes feels a bit winded, which “might be due to anxiety.”
Physical examination: slightly overweight man in no acute distress; body-mass index 27.0, waist circumference 36 inches, heart rate 74 beats/min, blood pressure 138/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: ECG— sinus rhythm, otherwise normal; creatinine 1.0 mg/dL; fasting glucose 98 mg/dL. Lipids: total cholesterol 215 mg/dL; triglycerides 150 mg/dL; HDL 40 mg/dL; LDL 145 mg/dL.
Diagnostic approaches
Standard diagnostic techniques include history, physical examination, laboratory testing as indicated, resting ECG, and assessment of risk factors for CHD.
Evaluation of chest pain
A careful history and physical examination can often quickly exclude many noncardiac causes of chest discomfort or pain. Table 1 contrasts the characteristics of atypical (noncardiac) symptoms with those of typical (cardiac) symptoms. Its quality, location, and the factors that relieve or provoke it, duration, and any associated symptoms should be evaluated. If high-risk or unstable signs or symptoms are present that suggest acute coronary syndrome (unstable angina or MI), evaluation in the emergency department should be performed.
Patients exhibiting stable or atypical (noncardiac) symptoms with some, but not all, of the features of angina described above have a lower probability of coronary artery disease, and should be considered for diagnostic evaluation under the guidance of the primary care physician.3
TABLE 1
Characteristics of atypical (noncardiac) vs typical cardiac symptoms
| Characteristic | Atypical/noncardiac | Typical/cardiac |
|---|---|---|
| Quality | Sharp, stabbing, positional | Squeezing, ache, pressure, fullness, burning, heavy, suffocating, “discomfort” |
| Location | Highly localized, below the epigastrium, above the mandible | Diffuse area—substernal, chest, jaw, back, arms |
| Provoked by | “Nothing,” body movement, cough, deep inspiration, chest palpation | Exertion, emotional stress, cold air |
| Relieved by | “Nothing,” position change, analgesics, heat, antacids | Rest; nitroglycerin |
| Duration | “Seconds” (fleeting), or hours, days | 30 seconds to 5 minutes |
| Associated symptoms | Reflux/heartburn | Dyspnea, diaphoresis, nausea, fatigue |
Evaluating women
In women aged <55 years, noncardiac chest pain is common, but since the prevalence of CHD is increasing among younger women, their symptoms should not be dismissed as “noncardiac” without full evaluation.
Women are also more likely than men to report dyspnea or pain in the jaw or back instead of, or in addition to, chest symptoms. Further, since women are often older and less active when they develop CHD, they may not exhibit typical exertional symptoms. Diagnosis in women is also hampered by lower accuracy of standard stress ECG testing compared with men. False-positive and false-negative tests may occur more frequently in women due to hormonal effects on the ECG, and more frequent comorbidities that limit maximal exercise capacity.
Physical examination
Palpation and auscultation of the chest may detect the presence of a friction rub or significant murmur, and thus identify a nonischemic cause for the chest symptoms. Carotid bruits or reduced pedal pulses indicate the presence of other vascular diseases. Patients with xanthomas, hypertension, or signs of congestive heart failure are more likely to have CHD, while those whose pain can be reproduced by body movement or by palpating the chest are less likely to have CHD.
Risk factor assessment. The assessment of risk factors for CHD allows the identification of many patients at high risk for CHD and can be helpful in guiding the choice of additional tests. As evident in the Framingham Heart Study,4 independent risk factors such as cigarette smoking, hypertension, diabetes mellitus, and hyperlipidemia are direct causes of CHD.
Laboratory tests
In the patient at low risk of CHD, blood testing for cardiac markers is not indicated. A lipid profile and blood glucose level help to establish the risk level associated with hyperlipidemia and diabetes. A complete blood count (eg, for anemia), thyroid hormone studies (eg, for hyperthyroidism), arterial blood gases (eg, PCO2 for chronic obstructive pulmonary disease), and other tests may help in diagnosing contributory conditions.
Resting electrocardiogram
A routine resting 12-lead ECG is an inexpensive but critical test that can provide important diagnostic and prognostic information. Evidence of infarction, ischemia, hypertrophy arrhythmias, and conduction disturbances can be detected and, if present, substantially increase the likelihood of a cardiac cause of symptoms.
Even the presence of mild or nonspecific ST-T wave changes, while not diagnostic, can aid the clinician by suggesting a higher probability of a nondiagnostic stress ECG and the need for an imaging stress test.3 An abnormal resting ECG with ST-T wave changes associated with digoxin use, left bundle branch block, left ventricular hypertrophy, and so on, limit interpretation of an exercise ECG, and points to a need for exercise testing with imaging.
It is important to note that a normal ECG obtained when the patient is asymptomatic does not exclude CHD, and additional risk stratification with noninvasive diagnostic stress testing may be indicated.5
Chest x-ray
A chest x-ray is often appropriate for patients with cardiac or pulmonary signs/symptoms. It may show cardiac enlargement, ventricular aneurysm, or evidence of heart failure, which may support the diagnosis of CHD and help to assess the extent of cardiopulmonary involvement.
Noninvasive stress testing
Considering our 2 patients with occasional episodes of unexplained chest discomfort: Based on the ECG and clinical findings, their risk for CHD is considered low to intermediate.
Test selection
Diagnostic tests should be selected based on the clinician’s estimate of probability of CHD.2
Low probability. If the likelihood of CHD is low, stress testing is generally not indicated, as its specificity is extremely low, and test results do not improve diagnostic accuracy over the clinical impression alone.
Intermediate probability. If the patient is able to exercise to capacity, the choice is exercise testing. Patients who can exercise and have an interpretable ECG, with no evidence of left ventricular dysfunction and no prior revascularization procedure, should usually undergo standard stress ECG testing. If the ECG is not interpretable, (due to repolarization abnormalities, left bundle branch block, left ventricular hypertrophy, digoxin use, etc) an exercise test with imaging (nuclear or echocardiographic) is indicated.
For patients unable to exercise, pharmacologic stress testing with imaging is indicated.
High probability. If the probability of CHD is high, it is reasonable to proceed directly to coronary angiography.
Exercise stress test
Exercise testing is a cardiovascular stress that uses treadmill or bicycle exercise with ECG and blood pressure monitoring. Such testing is widely available and relatively inexpensive.2 It allows assessment of exercise capacity and correlation of symptoms with ECG changes typical of myocardial ischemia.
Exercise testing provides the highest level of incremental diagnostic and prognostic information for patients with an intermediate probability of CHD.2 An important objective of stress testing is to identify individuals with a high risk for severe (left main or 3-vessel) CHD. More invasive procedures, such as percutaneous cutaneous angioplasty (PCTA), are recommended for these high-risk individuals to improve their survival.
Candidates for exercise treadmill testing include patients with stable symptoms who can be expected to exercise to an adequate workload. Patients with repolarization abnormalities on the resting ECG, such as left bundle branch block, left ventricular hypertrophy, or digoxin use, frequently have noninterpretable stress ECGs and may benefit from imaging techniques.
Limitations. Some patients referred for exercise treadmill testing are unable to achieve either adequate exercise levels or the target heart rate due to comorbid conditions,6 such as degenerative joint disease, obesity, pulmonary disease, peripheral vascular disease, central nervous system disorders, physical deconditioning, chronotropic incompetence, and medications such as beta blockers. More subtle factors, such as an unwillingness to exercise, may also affect a patient’s suitability for stress testing. These patients should be considered for pharmacologic stress testing.
Additionally, stress-induced ST-T wave changes do not accurately localize the site of myocardial ischemia and provide no direct information on left ventricular function and other clinically important variables. The sensitivity and specificity of exercise ECG testing ranges from approximately 67% to 72%, which is below that of stress imaging techniques, whose average sensitivity ranges from 80% to 85%.7-9
Stress imaging modalities
For a patient with an abnormal resting ECG, evidence of left ventricular dysfunction, or a prior coronary revascularization, stress imaging with either echocardiography or nuclear perfusion scanning is appropriate. Both techniques show higher specificity than the stress ECG alone.
Nuclear imaging. Nuclear imaging uses radiotracers (thallium-201, technetium-99m tetrofosmin [Myoview], or technetium-99m sestamibi [Cardiolite]) to evaluate myocardial perfusion and function, and has greatly advanced the ability to detect and assess the extent of CHD. Stress myocardial perfusion imaging has a sensitivity of >90% for detecting patients at risk of cardiac death or MI.6
To detect ischemia or infarction, a radioisotope is injected at rest and after stress to produce images of myocardial regional uptake, which is proportional to regional blood flow. Normally, with maximal exercise or pharmacologic stress, myocardial blood flow is greatly increased above the resting condition. If a fixed coronary stenosis is present, myocardial perfusion in the territory supplied by the stenosis cannot be increased, which will create a flow differential and uneven distribution of the tracer.
As illustrated in Figure 2, a normal myocardial perfusion image shows homogenous accumulation of radiotracer on both the stress and rest images. A perfusion defect appears as an area of reduced tracer uptake.
Nuclear perfusion studies can also provide a measure of left ventricular function and wall motion utilizing a bolus injection of radiotracer. While images can be obtained in most patients utilizing current techniques, artifacts due to breast and diaphragmatic tissue attenuation can lead to false-positive interpretation, particularly when examining women and when using thallium.
Echocardiography. Echocardiography visualizes the heart directly in real time using ultrasound, providing convenient assessment of the cardiac chambers, myocardium, valves, pericardium, and great vessels. The test can also identify mechanical complications of acute myocardial infarction, differentiate causes of reduced cardiac output and blood pressure, and help guide therapy. Stress echocardiography (exercise or pharmacologic stress) can be used to detect the presence, location, and severity of inducible myocardial ischemia as well as for risk stratification and prognosis.
During stress-induced ischemia, decrements in contractile function are directly related to decreases in regional subendocardial blood flow. Wall-motion changes precede ischemic ECG changes, accounting for the increased sensitivity of echocardiography versus ECG stress testing.
Interpretation of stress echocardiograms is based on analysis of segmental wall motion before and soon after stress. Normally, with exercise, or dobutamine infusion, left ventricular wall motion becomes hyperdynamic. The hallmark of ischemia is the development with stress of new, or the worsening of preexisting, wall motion abnormalities. The lack of improvement with stress in an already hypokinetic segment indicates infarction. Stress-induced left ventricular cavity enlargement, systolic dysfunction, or mitral regurgitation may also suggest CHD. Accuracy of stress echocardiography is similar to that of nuclear stress testing.
Considerable expertise in echocardiography is needed to rapidly acquire diagnostic images, so that its selection is limited by the skill of the technician. Image quality can be compromised by obesity and other factors, but the widespread use of intravenous contrast agents has significantly reduced the proportion of patients with uninterpretable images.
FIGURE 2
Accumulation of radiotracer in nuclear imaging (stress and rest images)
Patients who are not expected to achieve an adequate exercise capacity (as in our patient with osteoarthritis) should undergo pharmacologic stress testing with adenosine, dipyridamole, or dobutamine. Atrial pacing utilizing a swallowed esophageal electrode is also used in some cases. These agents, combined with echocardiographic or nuclear imaging, are particularly useful in patients who are unable to exercise adequately.
Pharmacologic stress agents are sometimes combined with low-level exercise protocols which may reduce the noncardiac side effects and improve image quality.10
Adenosine is the pharmacologic agent used most commonly in nuclear perfusion stress testing. An intravenous infusion of adenosine produces coronary vasodilation which is quickly attenuated when the infusion is terminated. Side effects, which are short-lived, include flushing, palpitations, and chest pain.
Dipyridamole is used less commonly due to its prolonged side effects and reports of lower specificity.11 Dobutamine, a beta-adrenergic agonist, increases heart rate and contractility in a dose-related fashion when infused intravenously. This agent is most commonly used in echocardiographic imaging. It can also be utilized with nuclear imaging when adenosine is contraindicated due to severe pulmonary or cerebrovascular disease. Side effects include transient arrhythmias, hypertension or hypotension, tremor, and chest pain.
Referral to a cardiologist
Referral to a cardiologist should be considered when the suspicion for cardiac disease is high, there is substantial diagnostic uncertainty after initial evaluation, or if symptoms persist, despite treatment of a noncardiac cause. Further evaluation and treatment often includes coronary angiography.
Coronary angiography
Most outpatients, such as the 2 presented, can be diagnosed with clinical and noninvasive measures. Coronary angiography is most commonly used to determine the presence and extent of obstructive CHD, and to guide decisions about revascularization in high-risk patients, or in patients with an abnormal stress test.
Cardiac catheterization presents a small but real risk to the patient, involves discomfort and substantial cost, and can challenge effective resource utilization. Risks and benefits to individual patients should be discussed between primary care physician and cardiologist.
Summary
Findings in our 2 patients are summarized below. Diagnostic decisions reflect the algorithm in Figure 1and are based on current guidelines.2
Patient 1. After initial assessment, our 64-year-old asymptomatic woman still falls into “intermediate probability of CHD” due to her multiple CHD risks. Stress testing was therefore indicated. Due to her inability to exercise because of an orthopedic limitation, she underwent pharmacologic stress testing with an adenosine sestamibi study. A small inferior reversible defect was identified, suggestive of myocardial ischemia.
Aggressive medical therapy aimed at minimizing symptoms and reducing risk was selected: aspirin, a beta-blocker for ischemia and hypertension, and a statin for hyperlipidemia. Longacting nitrates or calcium-channel blockers would have been reasonable alternatives. Consideration of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker is also indicated in light of her new CHD diagnosis and glucose intolerance. She was advised to initiate a low-fat, low-carbohydrate diet and to exercise (swim) regularly to lower risk. She will be seen in 6 weeks to reevaluate her symptoms, blood pressure, and lipid and glycemic control.
Patient 2. Our male patient also warranted stress testing. He was referred for a standard stress ECG due to his normal resting ECG, and the expectation that he would be able to exercise adequately. He satisfactorily completed 10.5 minutes (10 METS) of a Bruce protocol on a treadmill exercise stress test, which was entirely normal.
This admittedly anxious individual was reassured that his chest symptoms are not due to heart disease. An empiric trial with a proton pump inhibitor could be initiated if gastro-esophageal reflux is suspected.
Conclusions
Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting ECG and without contraindications to exercise, as in our male patient. Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test. Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to the target level are candidates for nuclear or echocardiographic pharmacologic stress testing, as was indicated for our female patient. Patients with suspected CHD and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Finally, when selecting a specific stress imaging technique, physicians should consider the local expertise with the various techniques available, together with their strengths and limitations in the individual patient.19
- Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting electrocardiogram (ECG) and without contraindications to exercise.
- Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test.
- Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to target level are candidates for nuclear or echocardiographic pharmacologic stress testing.
- Patients with suspected coronary heart disease and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Most men and women experience symptoms before myocardial infarction (MI).1 Early recognition of these symptoms and prompt treatment are essential for prevention of death and disability related to coronary heart disease (CHD).
Patients with multiple risk factors, chest pain typically suggestive of CHD, or a history of CHD are usually easy to identify and triage. However, many patients do not have obvious risks for CHD but experience occasional symptoms of cardiac ischemia.
Patients can be stratified into low-, intermediate-, and high-risk categories that will help determine appropriate work-up. Those at intermediate risk can be difficult to assess, and may particularly benefit from stress-imaging studies.
The algorithm (Figure 1) is based on current guidelines,2 and indicates how patients with chest pain/symptoms may be identified and treated according to an initial estimate of the probability of obstructive coronary artery disease. The choice of noninvasive diagnostic tests for individuals with stable chest pain and a lower risk for CHD is then outlined.
Case studies
Patient 1
A 64-year-old, nonsmoking, obese woman with degenerative osteoarthritis of the knees occasionally experiences chest discomfort that lasts for a few minutes, sometimes radiating to her back. The discomfort, which started 4 weeks ago, occasionally becomes worse after a brief walk, but is not usually related to exertion, or associated with nausea or diaphoresis. She sometimes becomes short of breath climbing stairs.
Physical examination: In no acute distress; body-mass index 31.2, waist circumference 42 inches, heart rate 70 beats/min, blood pressure 142/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: resting electrocardiogram (ECG)—sinus rhythm otherwise normal; creatinine 1.2 mg/dL; fasting glucose 122 mg/dL; glycosylated hemoglobin 6.4%. Lipids: total cholesterol 232 mg/dL; triglycerides 230 mg/dL; high-density lipoprotein (HDL) cholesterol 28 mg/dL; low-density lipoprotein (LDL) cholesterol 158 mg/dL.
Patient 2
A 58-year-old, nonsmoking, otherwise healthy man experiences tightness in the chest, usually at night. The pain began 4 to 8 weeks ago; it lasts as long as 1 to 2 hours but is “very mild.” It does not radiate to the arm or jaw and is unrelated to exertion. There is no diaphoresis or nausea. He sometimes feels a bit winded, which “might be due to anxiety.”
Physical examination: slightly overweight man in no acute distress; body-mass index 27.0, waist circumference 36 inches, heart rate 74 beats/min, blood pressure 138/88 mm Hg, cardiovascular examination unremarkable.
Laboratory evaluation: ECG— sinus rhythm, otherwise normal; creatinine 1.0 mg/dL; fasting glucose 98 mg/dL. Lipids: total cholesterol 215 mg/dL; triglycerides 150 mg/dL; HDL 40 mg/dL; LDL 145 mg/dL.
Diagnostic approaches
Standard diagnostic techniques include history, physical examination, laboratory testing as indicated, resting ECG, and assessment of risk factors for CHD.
Evaluation of chest pain
A careful history and physical examination can often quickly exclude many noncardiac causes of chest discomfort or pain. Table 1 contrasts the characteristics of atypical (noncardiac) symptoms with those of typical (cardiac) symptoms. Its quality, location, and the factors that relieve or provoke it, duration, and any associated symptoms should be evaluated. If high-risk or unstable signs or symptoms are present that suggest acute coronary syndrome (unstable angina or MI), evaluation in the emergency department should be performed.
Patients exhibiting stable or atypical (noncardiac) symptoms with some, but not all, of the features of angina described above have a lower probability of coronary artery disease, and should be considered for diagnostic evaluation under the guidance of the primary care physician.3
TABLE 1
Characteristics of atypical (noncardiac) vs typical cardiac symptoms
| Characteristic | Atypical/noncardiac | Typical/cardiac |
|---|---|---|
| Quality | Sharp, stabbing, positional | Squeezing, ache, pressure, fullness, burning, heavy, suffocating, “discomfort” |
| Location | Highly localized, below the epigastrium, above the mandible | Diffuse area—substernal, chest, jaw, back, arms |
| Provoked by | “Nothing,” body movement, cough, deep inspiration, chest palpation | Exertion, emotional stress, cold air |
| Relieved by | “Nothing,” position change, analgesics, heat, antacids | Rest; nitroglycerin |
| Duration | “Seconds” (fleeting), or hours, days | 30 seconds to 5 minutes |
| Associated symptoms | Reflux/heartburn | Dyspnea, diaphoresis, nausea, fatigue |
Evaluating women
In women aged <55 years, noncardiac chest pain is common, but since the prevalence of CHD is increasing among younger women, their symptoms should not be dismissed as “noncardiac” without full evaluation.
Women are also more likely than men to report dyspnea or pain in the jaw or back instead of, or in addition to, chest symptoms. Further, since women are often older and less active when they develop CHD, they may not exhibit typical exertional symptoms. Diagnosis in women is also hampered by lower accuracy of standard stress ECG testing compared with men. False-positive and false-negative tests may occur more frequently in women due to hormonal effects on the ECG, and more frequent comorbidities that limit maximal exercise capacity.
Physical examination
Palpation and auscultation of the chest may detect the presence of a friction rub or significant murmur, and thus identify a nonischemic cause for the chest symptoms. Carotid bruits or reduced pedal pulses indicate the presence of other vascular diseases. Patients with xanthomas, hypertension, or signs of congestive heart failure are more likely to have CHD, while those whose pain can be reproduced by body movement or by palpating the chest are less likely to have CHD.
Risk factor assessment. The assessment of risk factors for CHD allows the identification of many patients at high risk for CHD and can be helpful in guiding the choice of additional tests. As evident in the Framingham Heart Study,4 independent risk factors such as cigarette smoking, hypertension, diabetes mellitus, and hyperlipidemia are direct causes of CHD.
Laboratory tests
In the patient at low risk of CHD, blood testing for cardiac markers is not indicated. A lipid profile and blood glucose level help to establish the risk level associated with hyperlipidemia and diabetes. A complete blood count (eg, for anemia), thyroid hormone studies (eg, for hyperthyroidism), arterial blood gases (eg, PCO2 for chronic obstructive pulmonary disease), and other tests may help in diagnosing contributory conditions.
Resting electrocardiogram
A routine resting 12-lead ECG is an inexpensive but critical test that can provide important diagnostic and prognostic information. Evidence of infarction, ischemia, hypertrophy arrhythmias, and conduction disturbances can be detected and, if present, substantially increase the likelihood of a cardiac cause of symptoms.
Even the presence of mild or nonspecific ST-T wave changes, while not diagnostic, can aid the clinician by suggesting a higher probability of a nondiagnostic stress ECG and the need for an imaging stress test.3 An abnormal resting ECG with ST-T wave changes associated with digoxin use, left bundle branch block, left ventricular hypertrophy, and so on, limit interpretation of an exercise ECG, and points to a need for exercise testing with imaging.
It is important to note that a normal ECG obtained when the patient is asymptomatic does not exclude CHD, and additional risk stratification with noninvasive diagnostic stress testing may be indicated.5
Chest x-ray
A chest x-ray is often appropriate for patients with cardiac or pulmonary signs/symptoms. It may show cardiac enlargement, ventricular aneurysm, or evidence of heart failure, which may support the diagnosis of CHD and help to assess the extent of cardiopulmonary involvement.
Noninvasive stress testing
Considering our 2 patients with occasional episodes of unexplained chest discomfort: Based on the ECG and clinical findings, their risk for CHD is considered low to intermediate.
Test selection
Diagnostic tests should be selected based on the clinician’s estimate of probability of CHD.2
Low probability. If the likelihood of CHD is low, stress testing is generally not indicated, as its specificity is extremely low, and test results do not improve diagnostic accuracy over the clinical impression alone.
Intermediate probability. If the patient is able to exercise to capacity, the choice is exercise testing. Patients who can exercise and have an interpretable ECG, with no evidence of left ventricular dysfunction and no prior revascularization procedure, should usually undergo standard stress ECG testing. If the ECG is not interpretable, (due to repolarization abnormalities, left bundle branch block, left ventricular hypertrophy, digoxin use, etc) an exercise test with imaging (nuclear or echocardiographic) is indicated.
For patients unable to exercise, pharmacologic stress testing with imaging is indicated.
High probability. If the probability of CHD is high, it is reasonable to proceed directly to coronary angiography.
Exercise stress test
Exercise testing is a cardiovascular stress that uses treadmill or bicycle exercise with ECG and blood pressure monitoring. Such testing is widely available and relatively inexpensive.2 It allows assessment of exercise capacity and correlation of symptoms with ECG changes typical of myocardial ischemia.
Exercise testing provides the highest level of incremental diagnostic and prognostic information for patients with an intermediate probability of CHD.2 An important objective of stress testing is to identify individuals with a high risk for severe (left main or 3-vessel) CHD. More invasive procedures, such as percutaneous cutaneous angioplasty (PCTA), are recommended for these high-risk individuals to improve their survival.
Candidates for exercise treadmill testing include patients with stable symptoms who can be expected to exercise to an adequate workload. Patients with repolarization abnormalities on the resting ECG, such as left bundle branch block, left ventricular hypertrophy, or digoxin use, frequently have noninterpretable stress ECGs and may benefit from imaging techniques.
Limitations. Some patients referred for exercise treadmill testing are unable to achieve either adequate exercise levels or the target heart rate due to comorbid conditions,6 such as degenerative joint disease, obesity, pulmonary disease, peripheral vascular disease, central nervous system disorders, physical deconditioning, chronotropic incompetence, and medications such as beta blockers. More subtle factors, such as an unwillingness to exercise, may also affect a patient’s suitability for stress testing. These patients should be considered for pharmacologic stress testing.
Additionally, stress-induced ST-T wave changes do not accurately localize the site of myocardial ischemia and provide no direct information on left ventricular function and other clinically important variables. The sensitivity and specificity of exercise ECG testing ranges from approximately 67% to 72%, which is below that of stress imaging techniques, whose average sensitivity ranges from 80% to 85%.7-9
Stress imaging modalities
For a patient with an abnormal resting ECG, evidence of left ventricular dysfunction, or a prior coronary revascularization, stress imaging with either echocardiography or nuclear perfusion scanning is appropriate. Both techniques show higher specificity than the stress ECG alone.
Nuclear imaging. Nuclear imaging uses radiotracers (thallium-201, technetium-99m tetrofosmin [Myoview], or technetium-99m sestamibi [Cardiolite]) to evaluate myocardial perfusion and function, and has greatly advanced the ability to detect and assess the extent of CHD. Stress myocardial perfusion imaging has a sensitivity of >90% for detecting patients at risk of cardiac death or MI.6
To detect ischemia or infarction, a radioisotope is injected at rest and after stress to produce images of myocardial regional uptake, which is proportional to regional blood flow. Normally, with maximal exercise or pharmacologic stress, myocardial blood flow is greatly increased above the resting condition. If a fixed coronary stenosis is present, myocardial perfusion in the territory supplied by the stenosis cannot be increased, which will create a flow differential and uneven distribution of the tracer.
As illustrated in Figure 2, a normal myocardial perfusion image shows homogenous accumulation of radiotracer on both the stress and rest images. A perfusion defect appears as an area of reduced tracer uptake.
Nuclear perfusion studies can also provide a measure of left ventricular function and wall motion utilizing a bolus injection of radiotracer. While images can be obtained in most patients utilizing current techniques, artifacts due to breast and diaphragmatic tissue attenuation can lead to false-positive interpretation, particularly when examining women and when using thallium.
Echocardiography. Echocardiography visualizes the heart directly in real time using ultrasound, providing convenient assessment of the cardiac chambers, myocardium, valves, pericardium, and great vessels. The test can also identify mechanical complications of acute myocardial infarction, differentiate causes of reduced cardiac output and blood pressure, and help guide therapy. Stress echocardiography (exercise or pharmacologic stress) can be used to detect the presence, location, and severity of inducible myocardial ischemia as well as for risk stratification and prognosis.
During stress-induced ischemia, decrements in contractile function are directly related to decreases in regional subendocardial blood flow. Wall-motion changes precede ischemic ECG changes, accounting for the increased sensitivity of echocardiography versus ECG stress testing.
Interpretation of stress echocardiograms is based on analysis of segmental wall motion before and soon after stress. Normally, with exercise, or dobutamine infusion, left ventricular wall motion becomes hyperdynamic. The hallmark of ischemia is the development with stress of new, or the worsening of preexisting, wall motion abnormalities. The lack of improvement with stress in an already hypokinetic segment indicates infarction. Stress-induced left ventricular cavity enlargement, systolic dysfunction, or mitral regurgitation may also suggest CHD. Accuracy of stress echocardiography is similar to that of nuclear stress testing.
Considerable expertise in echocardiography is needed to rapidly acquire diagnostic images, so that its selection is limited by the skill of the technician. Image quality can be compromised by obesity and other factors, but the widespread use of intravenous contrast agents has significantly reduced the proportion of patients with uninterpretable images.
FIGURE 2
Accumulation of radiotracer in nuclear imaging (stress and rest images)
Patients who are not expected to achieve an adequate exercise capacity (as in our patient with osteoarthritis) should undergo pharmacologic stress testing with adenosine, dipyridamole, or dobutamine. Atrial pacing utilizing a swallowed esophageal electrode is also used in some cases. These agents, combined with echocardiographic or nuclear imaging, are particularly useful in patients who are unable to exercise adequately.
Pharmacologic stress agents are sometimes combined with low-level exercise protocols which may reduce the noncardiac side effects and improve image quality.10
Adenosine is the pharmacologic agent used most commonly in nuclear perfusion stress testing. An intravenous infusion of adenosine produces coronary vasodilation which is quickly attenuated when the infusion is terminated. Side effects, which are short-lived, include flushing, palpitations, and chest pain.
Dipyridamole is used less commonly due to its prolonged side effects and reports of lower specificity.11 Dobutamine, a beta-adrenergic agonist, increases heart rate and contractility in a dose-related fashion when infused intravenously. This agent is most commonly used in echocardiographic imaging. It can also be utilized with nuclear imaging when adenosine is contraindicated due to severe pulmonary or cerebrovascular disease. Side effects include transient arrhythmias, hypertension or hypotension, tremor, and chest pain.
Referral to a cardiologist
Referral to a cardiologist should be considered when the suspicion for cardiac disease is high, there is substantial diagnostic uncertainty after initial evaluation, or if symptoms persist, despite treatment of a noncardiac cause. Further evaluation and treatment often includes coronary angiography.
Coronary angiography
Most outpatients, such as the 2 presented, can be diagnosed with clinical and noninvasive measures. Coronary angiography is most commonly used to determine the presence and extent of obstructive CHD, and to guide decisions about revascularization in high-risk patients, or in patients with an abnormal stress test.
Cardiac catheterization presents a small but real risk to the patient, involves discomfort and substantial cost, and can challenge effective resource utilization. Risks and benefits to individual patients should be discussed between primary care physician and cardiologist.
Summary
Findings in our 2 patients are summarized below. Diagnostic decisions reflect the algorithm in Figure 1and are based on current guidelines.2
Patient 1. After initial assessment, our 64-year-old asymptomatic woman still falls into “intermediate probability of CHD” due to her multiple CHD risks. Stress testing was therefore indicated. Due to her inability to exercise because of an orthopedic limitation, she underwent pharmacologic stress testing with an adenosine sestamibi study. A small inferior reversible defect was identified, suggestive of myocardial ischemia.
Aggressive medical therapy aimed at minimizing symptoms and reducing risk was selected: aspirin, a beta-blocker for ischemia and hypertension, and a statin for hyperlipidemia. Longacting nitrates or calcium-channel blockers would have been reasonable alternatives. Consideration of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker is also indicated in light of her new CHD diagnosis and glucose intolerance. She was advised to initiate a low-fat, low-carbohydrate diet and to exercise (swim) regularly to lower risk. She will be seen in 6 weeks to reevaluate her symptoms, blood pressure, and lipid and glycemic control.
Patient 2. Our male patient also warranted stress testing. He was referred for a standard stress ECG due to his normal resting ECG, and the expectation that he would be able to exercise adequately. He satisfactorily completed 10.5 minutes (10 METS) of a Bruce protocol on a treadmill exercise stress test, which was entirely normal.
This admittedly anxious individual was reassured that his chest symptoms are not due to heart disease. An empiric trial with a proton pump inhibitor could be initiated if gastro-esophageal reflux is suspected.
Conclusions
Standard treadmill exercise testing for diagnosis and risk stratification is suitable for patients with a normal resting ECG and without contraindications to exercise, as in our male patient. Those with an uninterpretable ECG should undergo either nuclear or echocardiographic imaging in concert with their exercise test. Patients in whom exercise is either contraindicated or who have a condition that interferes with exercising to the target level are candidates for nuclear or echocardiographic pharmacologic stress testing, as was indicated for our female patient. Patients with suspected CHD and for whom exercise or pharmacologic testing is contraindicated should be referred to a cardiologist for evaluation.
Finally, when selecting a specific stress imaging technique, physicians should consider the local expertise with the various techniques available, together with their strengths and limitations in the individual patient.19
1. Spertus JA, Radford MJ, Every NR, et al. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction. Summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation 2003;107:1681-1691.
2. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). Circulation 2002;106:1883-1892.
3. Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999;33:2092-2197.
4. Grundy SM, Balady GJ, Criqui MH, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the American Heart Association Task Force on Risk Reduction. Circulation 1998;97:1876-1887.
5. Zanger DR, Solomon AJ, Gersh BJ. Contemporary management of angina: part I: risk assessment. Am Fam Physician 1999;60:2543-2552.
6. Bar Harbor Invitation Meeting 2000. Panel 9: Nuclear imaging in coronary artery disease. J Nucl Cardiol 2001;8:305-316.
7. Kotler TS, Diamond GA. Exercise thallium-201 scintigraphy in the diagnosis and prognosis of coronary artery disease. Ann Intern Med 1990;113:684-702.
8. Gibbons RJ. Rest and exercise radionuclide angiography for diagnosis in chronic ischemic heart disease. Circulation 1991;84 (3 Suppl):I93-I99.
9. Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc 1995;70:5-15.
10. Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol 2000;7:439-446.
11. Li T, Ahlberg A, Hachamovitch R, et al. Comparison of adenosine and dipyridamole in detecting coronary artery disease using Tc-99m sestamibi single-photon emission computed tomography imaging: a randomized, prospective clinical study. J Am Coll Cardiol 2003;41(suppl A):461A. Abstract 858-3.
12. American Heart Association. Heart disease and stroke statistics—2003 update. Dallas, Tex: American Heart Association; 2003.
This special section of The Journal of Family Practiceis provided by an unrestricted grant fromFujisawa Healthcare, Inc. Disclosures: Dr McBride has served on the speakers’ bureau for the following: Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Glaxo SmithKline, KOS Pharmaceuticals, Merck & Co Inc, Pfizer Inc, Reliant Pharmaceuticals, and Sankyo. He has served as consultant to KOS Pharmaceuticals, Merck & Co Inc, and Pfizer Inc. He has received grant/research support from Merck & Co Inc and Pfizer Inc. Dr Hayes had no commercial interests to report. Corresponding author: Sharonne N. Hayes, MD, Mayo Clinic Women’s Heart Clinic, Rochester MN, 55905. E-mail: [email protected].
1. Spertus JA, Radford MJ, Every NR, et al. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction. Summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation 2003;107:1681-1691.
2. Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to update the 1997 exercise testing guidelines). Circulation 2002;106:1883-1892.
3. Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina). J Am Coll Cardiol 1999;33:2092-2197.
4. Grundy SM, Balady GJ, Criqui MH, et al. Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the American Heart Association Task Force on Risk Reduction. Circulation 1998;97:1876-1887.
5. Zanger DR, Solomon AJ, Gersh BJ. Contemporary management of angina: part I: risk assessment. Am Fam Physician 1999;60:2543-2552.
6. Bar Harbor Invitation Meeting 2000. Panel 9: Nuclear imaging in coronary artery disease. J Nucl Cardiol 2001;8:305-316.
7. Kotler TS, Diamond GA. Exercise thallium-201 scintigraphy in the diagnosis and prognosis of coronary artery disease. Ann Intern Med 1990;113:684-702.
8. Gibbons RJ. Rest and exercise radionuclide angiography for diagnosis in chronic ischemic heart disease. Circulation 1991;84 (3 Suppl):I93-I99.
9. Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc 1995;70:5-15.
10. Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol 2000;7:439-446.
11. Li T, Ahlberg A, Hachamovitch R, et al. Comparison of adenosine and dipyridamole in detecting coronary artery disease using Tc-99m sestamibi single-photon emission computed tomography imaging: a randomized, prospective clinical study. J Am Coll Cardiol 2003;41(suppl A):461A. Abstract 858-3.
12. American Heart Association. Heart disease and stroke statistics—2003 update. Dallas, Tex: American Heart Association; 2003.
This special section of The Journal of Family Practiceis provided by an unrestricted grant fromFujisawa Healthcare, Inc. Disclosures: Dr McBride has served on the speakers’ bureau for the following: Abbott Laboratories, Astra-Zeneca, Bristol-Myers Squibb, Glaxo SmithKline, KOS Pharmaceuticals, Merck & Co Inc, Pfizer Inc, Reliant Pharmaceuticals, and Sankyo. He has served as consultant to KOS Pharmaceuticals, Merck & Co Inc, and Pfizer Inc. He has received grant/research support from Merck & Co Inc and Pfizer Inc. Dr Hayes had no commercial interests to report. Corresponding author: Sharonne N. Hayes, MD, Mayo Clinic Women’s Heart Clinic, Rochester MN, 55905. E-mail: [email protected].
SARS: Lessons learned thus far
The speed with which public health agencies such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) have addressed the outbreak of severe acute respiratory distress syndrome, known as SARS, has been impressive. Working with academic epidemiologists and researchers, they appear to have identified a new virus as the likely causative agent, characterized some of the basic epidemiology and clinical course of the infection, and developed confirmatory lab tests.
Understanding the SARS story is important both for its medical implications and the public health principles it illustrates. This article summarizes key points about SARS, mainly using reference material from the CDC.1
Diagnosis
Infection with the SARS virus produces a range of clinical responses:
- Asymptomatic or mild respiratory illness
- Moderate illness: temperature <100.4°F (<38°C) and 1 or more clinical findings such as cough, shortness of breath, or hypoxia
- Severe illness: the above findings plus radiologic evidence or autopsy findings of pneumonia, respiratory distress syndrome, with or without an identifiable cause.
Suspect SARS when patients presenting with any of the above symptoms meet one of these epidemiologic criteria.
- having traveled to areas under CDC travel alerts or advisories
- having had close contact within 10 days of developing symptoms with a person known or suspected to have SARS.
When evaluating such patients, use careful hand hygiene and precautions against airborne transmission (N-95 respirator or standard face mask if this is not available) and direct contact (gloves, gowns).
Probable cases (clinical criteria of severe respiratory illness of unknown cause since February 1, 2003, epidemiological criteria, with or without lab criteria) and suspected cases (same criteria, but with moderate respiratory illness only) should be reported to local or state health departments.
Diagnostic testing
Diagnostic testing should include chest x-ray, pulse oximetry, blood cultures, sputum gram stain and culture, and testing for viral pathogens (influenza and respiratory syncytial virus).
Legionella and pneumococcal urine antigen testing can be considered. Acute and convalescent (21 days) serum should be saved for lab testing.
In May, the CDC announced the development of an enzyme-linked immunosorbent assay (ELISA) blood test to identify antibody to the presumed SARS virus. The test is now available to local and state health departments for acute and convalescent testing of patients’ serum. A more sensitive polymerase chain reaction test is under development.
Treat with supportive measures
No specific treatment exists for SARS. Treat patients as you would any community-acquired pneumonia of unknown origin and provide supportive therapy as necessary. Hospitalization should be based on the usual indications.
Traditional infection control methods can work
Most importantly, public health departments have demonstrated that traditional infection control measures such as surveillance and isolation/quarantine may be successful in limiting the spread of the infection. Physicians should be aware of these important concepts.
The incubation period for SARS is believed to be up to 10 days. During this time, people are not contagious. Transmission is believed to occur mainly during close face-to-face contact such as happens in households or patient-care settings. Aerosol or airborne transmission is also a possibility, although believed to be much less likely.
Surveillance is the system and process of monitoring for specific conditions. Infectious disease surveillance requires the cooperation of local, state, and federal health departments, private and public laboratories, and clinicians working in private and public settings. A definition of the condition being monitored and a method of identifying and reporting cases are necessary. To maximize surveillance, it helps to have a reporting requirement such as we have for diseases like tuberculosis or measles.
On February 11, 2003, the World Health Organization (WHO) was first informed by Chinese health authorities of 305 cases of acute respiratory syndrome in Guangdong province in southern China. As it turned out, these cases had started in November 2002, and the disease was characterized by transmission to health-care workers and household contacts.
People who visited China in this period and were exposed to SARS became unwitting carriers. Disease outbreaks occurred subsequently in Vietnam, Hong Kong, Singapore, Toronto, and Taiwan. In April, as international pressure increased, Chinese authorities began acknowledging the wider extent of the SARS outbreak, including hundreds of cases in Beijing and smaller numbers in other parts of the country.
As of June 16, 2003, the WHO had reported 8460 cases of SARS worldwide with a death rate of 9%.2 At that time, the CDC had reported 72 probable and 329 suspected cases in the US, almost all travel-related.1
Theoretical source: civet cats
In April, scientific teams from several countries identified a new coronavirus as the likely cause of SARS. This family of viruses had previously been identified as a cause of mild upper respiratory illnesses. In mid-April, CDC and others announced they had sequenced the genome of the specific coronavirus thought to cause SARS. In late May, researchers from Hong Kong and China announced they had discovered a virtually identical virus in a species of tree-dwelling cat, the civet, that is eaten as wild game in southern China, where SARS is believed to have started. One theory is that the virus may have lived in animals and passed to humans through a mutation or other mechanism.3
Both isolation of suspected cases and quarantine of contacts have been used to control SARS. In the US, quarantine is usually implemented voluntarily, but, for certain conditions such as SARS, people can be quarantined involuntarily. In the case of a communicable disease such as SARS for which there is no known treatment and which can spread readily under certain circumstances, the strategies of isolation and quarantine are even more important.
A need for better defenses
As of early June, countries most affected were mainland China, Hong Kong, and Taiwan. These countries were subject to a CDC travel advisory, which means people should travel there only if they had essential business. In addition, the CDC issued a travel alert for Singapore, and re-issued one for Toronto after the city failed to contain the initial outbreak. Alerts advise travelers that if they have visited a specific SARS-affected area, they should seek medical attention if they get sick within 10 days.
Strategies against SARS. While SARS appears to have been brought under control in certain areas (Hanoi and maybe Singapore), this has not happened in others. To date, the US has been spared a serious outbreak. Use of strategies such as travel alerts and advisories, screening airline passengers from affected countries, and heightened vigilance in following up suspected cases and exposures have all helped.
Another emerging infection: monkeypox
As SARS was being contained, an infectious disease new to the US erupted: monkeypox. On June 16, when the number of cases stood at 82 persons An ELISA blood test is now available to identify antibodies to the presumed SARS virus in 5 states, the federal government banned the sale and distribution of prairie dogs and all rodents from Africa, in an effort to control the spread. Monkeypox is believed to have spread from an African rat imported by a pet store and housed with prairie dogs for sale to the public.
Most infected persons had direct contact with diseased prairie dogs that had been purchased as pets. In some instances, however, direct contact with infected animals could not be documented; therefore, health officials cannot rule out the possibility of human-to-human transmission of the monkeypox virus.
Monkeypox was first identified in monkeys in 1959, but certain African rodents were later identified as its real host. Outbreaks in people occurred in the Congo in the 1990s.
The Centers for Disease Control and Prevention issued an interim case definition for human cases of monkeypox and a recommendation that certain individuals be offered smallpox vaccination for protection (available at http://www.cdc.gov/ncidod/monkeypox/ casedefinition.htm).
Our best defense
Continued emergence of infectious diseases and the dramatic spread of SARS internationally through airline travel and close contact in hospitals should prompt us to strengthen our public health systems. A well functioning surveillance system coupled with the infrastructure to apply traditional techniques such as case finding, tracking, isolation, quarantine—and bans, as in the case of monkeypox—may be our best defense against communicable disease epidemics.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Centers for Disease Control and Prevention. Severe acute respiratory syndrome (SARS). Available at: www.cdc.gov/od/oc/media/sars.htm. Accessed on June 17, 2003.
2. World Health Organization. Cumulative number of reported probably cases or SARS. Available at: www.who.int/csr/sars/country/2003_06_16/en. Accessed on June 17, 2003.
3. Bradsher K, Crampton T. Hong Kong travel advisory lifted; cat virus tied to SARS.New York Times May 23, 2003.
The speed with which public health agencies such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) have addressed the outbreak of severe acute respiratory distress syndrome, known as SARS, has been impressive. Working with academic epidemiologists and researchers, they appear to have identified a new virus as the likely causative agent, characterized some of the basic epidemiology and clinical course of the infection, and developed confirmatory lab tests.
Understanding the SARS story is important both for its medical implications and the public health principles it illustrates. This article summarizes key points about SARS, mainly using reference material from the CDC.1
Diagnosis
Infection with the SARS virus produces a range of clinical responses:
- Asymptomatic or mild respiratory illness
- Moderate illness: temperature <100.4°F (<38°C) and 1 or more clinical findings such as cough, shortness of breath, or hypoxia
- Severe illness: the above findings plus radiologic evidence or autopsy findings of pneumonia, respiratory distress syndrome, with or without an identifiable cause.
Suspect SARS when patients presenting with any of the above symptoms meet one of these epidemiologic criteria.
- having traveled to areas under CDC travel alerts or advisories
- having had close contact within 10 days of developing symptoms with a person known or suspected to have SARS.
When evaluating such patients, use careful hand hygiene and precautions against airborne transmission (N-95 respirator or standard face mask if this is not available) and direct contact (gloves, gowns).
Probable cases (clinical criteria of severe respiratory illness of unknown cause since February 1, 2003, epidemiological criteria, with or without lab criteria) and suspected cases (same criteria, but with moderate respiratory illness only) should be reported to local or state health departments.
Diagnostic testing
Diagnostic testing should include chest x-ray, pulse oximetry, blood cultures, sputum gram stain and culture, and testing for viral pathogens (influenza and respiratory syncytial virus).
Legionella and pneumococcal urine antigen testing can be considered. Acute and convalescent (21 days) serum should be saved for lab testing.
In May, the CDC announced the development of an enzyme-linked immunosorbent assay (ELISA) blood test to identify antibody to the presumed SARS virus. The test is now available to local and state health departments for acute and convalescent testing of patients’ serum. A more sensitive polymerase chain reaction test is under development.
Treat with supportive measures
No specific treatment exists for SARS. Treat patients as you would any community-acquired pneumonia of unknown origin and provide supportive therapy as necessary. Hospitalization should be based on the usual indications.
Traditional infection control methods can work
Most importantly, public health departments have demonstrated that traditional infection control measures such as surveillance and isolation/quarantine may be successful in limiting the spread of the infection. Physicians should be aware of these important concepts.
The incubation period for SARS is believed to be up to 10 days. During this time, people are not contagious. Transmission is believed to occur mainly during close face-to-face contact such as happens in households or patient-care settings. Aerosol or airborne transmission is also a possibility, although believed to be much less likely.
Surveillance is the system and process of monitoring for specific conditions. Infectious disease surveillance requires the cooperation of local, state, and federal health departments, private and public laboratories, and clinicians working in private and public settings. A definition of the condition being monitored and a method of identifying and reporting cases are necessary. To maximize surveillance, it helps to have a reporting requirement such as we have for diseases like tuberculosis or measles.
On February 11, 2003, the World Health Organization (WHO) was first informed by Chinese health authorities of 305 cases of acute respiratory syndrome in Guangdong province in southern China. As it turned out, these cases had started in November 2002, and the disease was characterized by transmission to health-care workers and household contacts.
People who visited China in this period and were exposed to SARS became unwitting carriers. Disease outbreaks occurred subsequently in Vietnam, Hong Kong, Singapore, Toronto, and Taiwan. In April, as international pressure increased, Chinese authorities began acknowledging the wider extent of the SARS outbreak, including hundreds of cases in Beijing and smaller numbers in other parts of the country.
As of June 16, 2003, the WHO had reported 8460 cases of SARS worldwide with a death rate of 9%.2 At that time, the CDC had reported 72 probable and 329 suspected cases in the US, almost all travel-related.1
Theoretical source: civet cats
In April, scientific teams from several countries identified a new coronavirus as the likely cause of SARS. This family of viruses had previously been identified as a cause of mild upper respiratory illnesses. In mid-April, CDC and others announced they had sequenced the genome of the specific coronavirus thought to cause SARS. In late May, researchers from Hong Kong and China announced they had discovered a virtually identical virus in a species of tree-dwelling cat, the civet, that is eaten as wild game in southern China, where SARS is believed to have started. One theory is that the virus may have lived in animals and passed to humans through a mutation or other mechanism.3
Both isolation of suspected cases and quarantine of contacts have been used to control SARS. In the US, quarantine is usually implemented voluntarily, but, for certain conditions such as SARS, people can be quarantined involuntarily. In the case of a communicable disease such as SARS for which there is no known treatment and which can spread readily under certain circumstances, the strategies of isolation and quarantine are even more important.
A need for better defenses
As of early June, countries most affected were mainland China, Hong Kong, and Taiwan. These countries were subject to a CDC travel advisory, which means people should travel there only if they had essential business. In addition, the CDC issued a travel alert for Singapore, and re-issued one for Toronto after the city failed to contain the initial outbreak. Alerts advise travelers that if they have visited a specific SARS-affected area, they should seek medical attention if they get sick within 10 days.
Strategies against SARS. While SARS appears to have been brought under control in certain areas (Hanoi and maybe Singapore), this has not happened in others. To date, the US has been spared a serious outbreak. Use of strategies such as travel alerts and advisories, screening airline passengers from affected countries, and heightened vigilance in following up suspected cases and exposures have all helped.
Another emerging infection: monkeypox
As SARS was being contained, an infectious disease new to the US erupted: monkeypox. On June 16, when the number of cases stood at 82 persons An ELISA blood test is now available to identify antibodies to the presumed SARS virus in 5 states, the federal government banned the sale and distribution of prairie dogs and all rodents from Africa, in an effort to control the spread. Monkeypox is believed to have spread from an African rat imported by a pet store and housed with prairie dogs for sale to the public.
Most infected persons had direct contact with diseased prairie dogs that had been purchased as pets. In some instances, however, direct contact with infected animals could not be documented; therefore, health officials cannot rule out the possibility of human-to-human transmission of the monkeypox virus.
Monkeypox was first identified in monkeys in 1959, but certain African rodents were later identified as its real host. Outbreaks in people occurred in the Congo in the 1990s.
The Centers for Disease Control and Prevention issued an interim case definition for human cases of monkeypox and a recommendation that certain individuals be offered smallpox vaccination for protection (available at http://www.cdc.gov/ncidod/monkeypox/ casedefinition.htm).
Our best defense
Continued emergence of infectious diseases and the dramatic spread of SARS internationally through airline travel and close contact in hospitals should prompt us to strengthen our public health systems. A well functioning surveillance system coupled with the infrastructure to apply traditional techniques such as case finding, tracking, isolation, quarantine—and bans, as in the case of monkeypox—may be our best defense against communicable disease epidemics.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
The speed with which public health agencies such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) have addressed the outbreak of severe acute respiratory distress syndrome, known as SARS, has been impressive. Working with academic epidemiologists and researchers, they appear to have identified a new virus as the likely causative agent, characterized some of the basic epidemiology and clinical course of the infection, and developed confirmatory lab tests.
Understanding the SARS story is important both for its medical implications and the public health principles it illustrates. This article summarizes key points about SARS, mainly using reference material from the CDC.1
Diagnosis
Infection with the SARS virus produces a range of clinical responses:
- Asymptomatic or mild respiratory illness
- Moderate illness: temperature <100.4°F (<38°C) and 1 or more clinical findings such as cough, shortness of breath, or hypoxia
- Severe illness: the above findings plus radiologic evidence or autopsy findings of pneumonia, respiratory distress syndrome, with or without an identifiable cause.
Suspect SARS when patients presenting with any of the above symptoms meet one of these epidemiologic criteria.
- having traveled to areas under CDC travel alerts or advisories
- having had close contact within 10 days of developing symptoms with a person known or suspected to have SARS.
When evaluating such patients, use careful hand hygiene and precautions against airborne transmission (N-95 respirator or standard face mask if this is not available) and direct contact (gloves, gowns).
Probable cases (clinical criteria of severe respiratory illness of unknown cause since February 1, 2003, epidemiological criteria, with or without lab criteria) and suspected cases (same criteria, but with moderate respiratory illness only) should be reported to local or state health departments.
Diagnostic testing
Diagnostic testing should include chest x-ray, pulse oximetry, blood cultures, sputum gram stain and culture, and testing for viral pathogens (influenza and respiratory syncytial virus).
Legionella and pneumococcal urine antigen testing can be considered. Acute and convalescent (21 days) serum should be saved for lab testing.
In May, the CDC announced the development of an enzyme-linked immunosorbent assay (ELISA) blood test to identify antibody to the presumed SARS virus. The test is now available to local and state health departments for acute and convalescent testing of patients’ serum. A more sensitive polymerase chain reaction test is under development.
Treat with supportive measures
No specific treatment exists for SARS. Treat patients as you would any community-acquired pneumonia of unknown origin and provide supportive therapy as necessary. Hospitalization should be based on the usual indications.
Traditional infection control methods can work
Most importantly, public health departments have demonstrated that traditional infection control measures such as surveillance and isolation/quarantine may be successful in limiting the spread of the infection. Physicians should be aware of these important concepts.
The incubation period for SARS is believed to be up to 10 days. During this time, people are not contagious. Transmission is believed to occur mainly during close face-to-face contact such as happens in households or patient-care settings. Aerosol or airborne transmission is also a possibility, although believed to be much less likely.
Surveillance is the system and process of monitoring for specific conditions. Infectious disease surveillance requires the cooperation of local, state, and federal health departments, private and public laboratories, and clinicians working in private and public settings. A definition of the condition being monitored and a method of identifying and reporting cases are necessary. To maximize surveillance, it helps to have a reporting requirement such as we have for diseases like tuberculosis or measles.
On February 11, 2003, the World Health Organization (WHO) was first informed by Chinese health authorities of 305 cases of acute respiratory syndrome in Guangdong province in southern China. As it turned out, these cases had started in November 2002, and the disease was characterized by transmission to health-care workers and household contacts.
People who visited China in this period and were exposed to SARS became unwitting carriers. Disease outbreaks occurred subsequently in Vietnam, Hong Kong, Singapore, Toronto, and Taiwan. In April, as international pressure increased, Chinese authorities began acknowledging the wider extent of the SARS outbreak, including hundreds of cases in Beijing and smaller numbers in other parts of the country.
As of June 16, 2003, the WHO had reported 8460 cases of SARS worldwide with a death rate of 9%.2 At that time, the CDC had reported 72 probable and 329 suspected cases in the US, almost all travel-related.1
Theoretical source: civet cats
In April, scientific teams from several countries identified a new coronavirus as the likely cause of SARS. This family of viruses had previously been identified as a cause of mild upper respiratory illnesses. In mid-April, CDC and others announced they had sequenced the genome of the specific coronavirus thought to cause SARS. In late May, researchers from Hong Kong and China announced they had discovered a virtually identical virus in a species of tree-dwelling cat, the civet, that is eaten as wild game in southern China, where SARS is believed to have started. One theory is that the virus may have lived in animals and passed to humans through a mutation or other mechanism.3
Both isolation of suspected cases and quarantine of contacts have been used to control SARS. In the US, quarantine is usually implemented voluntarily, but, for certain conditions such as SARS, people can be quarantined involuntarily. In the case of a communicable disease such as SARS for which there is no known treatment and which can spread readily under certain circumstances, the strategies of isolation and quarantine are even more important.
A need for better defenses
As of early June, countries most affected were mainland China, Hong Kong, and Taiwan. These countries were subject to a CDC travel advisory, which means people should travel there only if they had essential business. In addition, the CDC issued a travel alert for Singapore, and re-issued one for Toronto after the city failed to contain the initial outbreak. Alerts advise travelers that if they have visited a specific SARS-affected area, they should seek medical attention if they get sick within 10 days.
Strategies against SARS. While SARS appears to have been brought under control in certain areas (Hanoi and maybe Singapore), this has not happened in others. To date, the US has been spared a serious outbreak. Use of strategies such as travel alerts and advisories, screening airline passengers from affected countries, and heightened vigilance in following up suspected cases and exposures have all helped.
Another emerging infection: monkeypox
As SARS was being contained, an infectious disease new to the US erupted: monkeypox. On June 16, when the number of cases stood at 82 persons An ELISA blood test is now available to identify antibodies to the presumed SARS virus in 5 states, the federal government banned the sale and distribution of prairie dogs and all rodents from Africa, in an effort to control the spread. Monkeypox is believed to have spread from an African rat imported by a pet store and housed with prairie dogs for sale to the public.
Most infected persons had direct contact with diseased prairie dogs that had been purchased as pets. In some instances, however, direct contact with infected animals could not be documented; therefore, health officials cannot rule out the possibility of human-to-human transmission of the monkeypox virus.
Monkeypox was first identified in monkeys in 1959, but certain African rodents were later identified as its real host. Outbreaks in people occurred in the Congo in the 1990s.
The Centers for Disease Control and Prevention issued an interim case definition for human cases of monkeypox and a recommendation that certain individuals be offered smallpox vaccination for protection (available at http://www.cdc.gov/ncidod/monkeypox/ casedefinition.htm).
Our best defense
Continued emergence of infectious diseases and the dramatic spread of SARS internationally through airline travel and close contact in hospitals should prompt us to strengthen our public health systems. A well functioning surveillance system coupled with the infrastructure to apply traditional techniques such as case finding, tracking, isolation, quarantine—and bans, as in the case of monkeypox—may be our best defense against communicable disease epidemics.
Correspondence
1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Centers for Disease Control and Prevention. Severe acute respiratory syndrome (SARS). Available at: www.cdc.gov/od/oc/media/sars.htm. Accessed on June 17, 2003.
2. World Health Organization. Cumulative number of reported probably cases or SARS. Available at: www.who.int/csr/sars/country/2003_06_16/en. Accessed on June 17, 2003.
3. Bradsher K, Crampton T. Hong Kong travel advisory lifted; cat virus tied to SARS.New York Times May 23, 2003.
1. Centers for Disease Control and Prevention. Severe acute respiratory syndrome (SARS). Available at: www.cdc.gov/od/oc/media/sars.htm. Accessed on June 17, 2003.
2. World Health Organization. Cumulative number of reported probably cases or SARS. Available at: www.who.int/csr/sars/country/2003_06_16/en. Accessed on June 17, 2003.
3. Bradsher K, Crampton T. Hong Kong travel advisory lifted; cat virus tied to SARS.New York Times May 23, 2003.
New JFP series on timely clinical issues
The 20th century offered hope that man could triumph over microbes. Public health interventions, such as massive vaccination campaigns, coupled with improved nutrition and living conditions have radically reduced the leading cause of death—infectious disease—at least in developed countries. Chronic diseases and injuries now cause most deaths in the US.
Yet influenza and pneumonia stubbornly persist; together, they are the 7th leading cause of death.1 New infectious diseases have emerged: legionella, hantavirus, and ebola hemorrhagic fever. AIDS took less than 2 decades to become the most common killer of African American men aged 35 to 44 and women aged 25 to 35.2 Existing diseases have migrated to new geographic areas (West Nile virus) or evolved new pathologic manifestations (severe acute respiratory syndrome, or SARS). And microbes continue to develop resistance against old and new antibiotics at an alarming rate.
We now face the very real threat of intentional production and release of microorganisms as a weapon of war and terror. Throughout history, mankind has tried, and at times succeeded, in weakening political adversaries by infecting them with microbes. Today, new technologies for DNA manipulation to enhance virulence and infectiousness considerably increase the potential for mischief and new global epidemics. Ironically, despite one of the greatest public health achievements of all time—eradication of history’s leading killer, smallpox—we find ourselves vulnerable to this organism’s reemergence due to worldwide cessation of mass immunization.
We know what to do
Although this picture is disturbing, we have the ability to hold our own. Vaccines, sanitation, potable water, and food safety technologies remain effective as long as we are willing to pay for and use them. Other measures include disease surveillance, laboratory analysis, public education, and, when needed, isolation and quarantine of individuals.
The public health infrastructure necessary to wield these tools effectively has eroded substantially over the past 40 years. It is now being strengthened through increased federal funding and national attention. However, implementation of public health measures remains largely a state and local responsibility.
New JFP series advises on crucial clinical issues
An unfortunate part of history is the separate development of medicine and public health, which has led to misunderstandings and lack of appreciation for the mutual benefits of cooperation. It is now apparent the 2 systems must work more closely.
This desirable interdependence is the basis for a new type of article in THE JOURNAL OF FAMILY PRACTICE that will focus on developments that are linking the practice of medicine with the practice of public health. The inaugural article, on page 528 of this issue, is on SARS.
We aim to advise family physicians on developments in medicine that apply so broadly that they can decisively affect the overall public health, such as
- effective office-based and community-based prevention interventions
- practical application of new recommendations, such as those on sexually transmitted disease diagnosis and treatment, immunization practices, and chronic disease control.
Articles in this series will assist family physicians improve the health of the nation as they
- stay informed of local and national disease trends
- use antibiotics judiciously
- remain alert for suspicious disease presentations and report contagious diseases and other reportable conditions
- participate in sentinel disease detection
- cooperate with and support local and state health departments
- help plan and participate in emergency preparedness systems
- serve on boards of health or as consultants or medical directors to local health departments.
The importance of these activities cannot be overestimated. When the Centers for Disease Control and Prevention examined outbreaks that might have involved intentional use of infectious agents, it cited reporting by front-line health professionals as the most critical component in the detection of suspicious events.3
Patient-by-patient, physician-by-physician
A strong public health infrastructure supported by public health–conscious physicians will not only strengthen protection against infectious diseases but also address other community-wide concerns, such as chronic and environmentally caused diseases, injuries, and substance abuse. Family medicine predoctoral and residency programs must be sure they are preparing trainees to fulfill these roles. Training requirements should include more than token mention of public health.
Appropriate roles for the American Academy of Family Physicians include leadership in testing and developing information systems that link local family physicians to state and local health departments and provide easy access to public health information, and training practicing physicians to be more involved in the public health system.
But nothing can substitute for the concern and involvement of the local physician caring for the individual patient, who sees beyond the individual patient encounter and works to ensure that their daily actions also contribute to better health for all.
1. Centers for Disease Control and Prevention. Deaths; preliminary data for 2000. National Vital Statistics Reports 2001;49(12):Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr49/ nvsr49_12.pdf. Accessed on June 17, 2003.
2. Centers for Disease Control and Prevention. Deaths: leading causes for 1999. National Vital Statistics Reports 2001;49(1):Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr49/ nvsr49_11.pdf. Accessed on June 17, 2003.
3. Ashford DA, Kaiser RM, Bales ME, et al. Planning against biological terrorism: lessons from outbreak investigations. Emerg Infect Dis 2003;9:515-519.
The 20th century offered hope that man could triumph over microbes. Public health interventions, such as massive vaccination campaigns, coupled with improved nutrition and living conditions have radically reduced the leading cause of death—infectious disease—at least in developed countries. Chronic diseases and injuries now cause most deaths in the US.
Yet influenza and pneumonia stubbornly persist; together, they are the 7th leading cause of death.1 New infectious diseases have emerged: legionella, hantavirus, and ebola hemorrhagic fever. AIDS took less than 2 decades to become the most common killer of African American men aged 35 to 44 and women aged 25 to 35.2 Existing diseases have migrated to new geographic areas (West Nile virus) or evolved new pathologic manifestations (severe acute respiratory syndrome, or SARS). And microbes continue to develop resistance against old and new antibiotics at an alarming rate.
We now face the very real threat of intentional production and release of microorganisms as a weapon of war and terror. Throughout history, mankind has tried, and at times succeeded, in weakening political adversaries by infecting them with microbes. Today, new technologies for DNA manipulation to enhance virulence and infectiousness considerably increase the potential for mischief and new global epidemics. Ironically, despite one of the greatest public health achievements of all time—eradication of history’s leading killer, smallpox—we find ourselves vulnerable to this organism’s reemergence due to worldwide cessation of mass immunization.
We know what to do
Although this picture is disturbing, we have the ability to hold our own. Vaccines, sanitation, potable water, and food safety technologies remain effective as long as we are willing to pay for and use them. Other measures include disease surveillance, laboratory analysis, public education, and, when needed, isolation and quarantine of individuals.
The public health infrastructure necessary to wield these tools effectively has eroded substantially over the past 40 years. It is now being strengthened through increased federal funding and national attention. However, implementation of public health measures remains largely a state and local responsibility.
New JFP series advises on crucial clinical issues
An unfortunate part of history is the separate development of medicine and public health, which has led to misunderstandings and lack of appreciation for the mutual benefits of cooperation. It is now apparent the 2 systems must work more closely.
This desirable interdependence is the basis for a new type of article in THE JOURNAL OF FAMILY PRACTICE that will focus on developments that are linking the practice of medicine with the practice of public health. The inaugural article, on page 528 of this issue, is on SARS.
We aim to advise family physicians on developments in medicine that apply so broadly that they can decisively affect the overall public health, such as
- effective office-based and community-based prevention interventions
- practical application of new recommendations, such as those on sexually transmitted disease diagnosis and treatment, immunization practices, and chronic disease control.
Articles in this series will assist family physicians improve the health of the nation as they
- stay informed of local and national disease trends
- use antibiotics judiciously
- remain alert for suspicious disease presentations and report contagious diseases and other reportable conditions
- participate in sentinel disease detection
- cooperate with and support local and state health departments
- help plan and participate in emergency preparedness systems
- serve on boards of health or as consultants or medical directors to local health departments.
The importance of these activities cannot be overestimated. When the Centers for Disease Control and Prevention examined outbreaks that might have involved intentional use of infectious agents, it cited reporting by front-line health professionals as the most critical component in the detection of suspicious events.3
Patient-by-patient, physician-by-physician
A strong public health infrastructure supported by public health–conscious physicians will not only strengthen protection against infectious diseases but also address other community-wide concerns, such as chronic and environmentally caused diseases, injuries, and substance abuse. Family medicine predoctoral and residency programs must be sure they are preparing trainees to fulfill these roles. Training requirements should include more than token mention of public health.
Appropriate roles for the American Academy of Family Physicians include leadership in testing and developing information systems that link local family physicians to state and local health departments and provide easy access to public health information, and training practicing physicians to be more involved in the public health system.
But nothing can substitute for the concern and involvement of the local physician caring for the individual patient, who sees beyond the individual patient encounter and works to ensure that their daily actions also contribute to better health for all.
The 20th century offered hope that man could triumph over microbes. Public health interventions, such as massive vaccination campaigns, coupled with improved nutrition and living conditions have radically reduced the leading cause of death—infectious disease—at least in developed countries. Chronic diseases and injuries now cause most deaths in the US.
Yet influenza and pneumonia stubbornly persist; together, they are the 7th leading cause of death.1 New infectious diseases have emerged: legionella, hantavirus, and ebola hemorrhagic fever. AIDS took less than 2 decades to become the most common killer of African American men aged 35 to 44 and women aged 25 to 35.2 Existing diseases have migrated to new geographic areas (West Nile virus) or evolved new pathologic manifestations (severe acute respiratory syndrome, or SARS). And microbes continue to develop resistance against old and new antibiotics at an alarming rate.
We now face the very real threat of intentional production and release of microorganisms as a weapon of war and terror. Throughout history, mankind has tried, and at times succeeded, in weakening political adversaries by infecting them with microbes. Today, new technologies for DNA manipulation to enhance virulence and infectiousness considerably increase the potential for mischief and new global epidemics. Ironically, despite one of the greatest public health achievements of all time—eradication of history’s leading killer, smallpox—we find ourselves vulnerable to this organism’s reemergence due to worldwide cessation of mass immunization.
We know what to do
Although this picture is disturbing, we have the ability to hold our own. Vaccines, sanitation, potable water, and food safety technologies remain effective as long as we are willing to pay for and use them. Other measures include disease surveillance, laboratory analysis, public education, and, when needed, isolation and quarantine of individuals.
The public health infrastructure necessary to wield these tools effectively has eroded substantially over the past 40 years. It is now being strengthened through increased federal funding and national attention. However, implementation of public health measures remains largely a state and local responsibility.
New JFP series advises on crucial clinical issues
An unfortunate part of history is the separate development of medicine and public health, which has led to misunderstandings and lack of appreciation for the mutual benefits of cooperation. It is now apparent the 2 systems must work more closely.
This desirable interdependence is the basis for a new type of article in THE JOURNAL OF FAMILY PRACTICE that will focus on developments that are linking the practice of medicine with the practice of public health. The inaugural article, on page 528 of this issue, is on SARS.
We aim to advise family physicians on developments in medicine that apply so broadly that they can decisively affect the overall public health, such as
- effective office-based and community-based prevention interventions
- practical application of new recommendations, such as those on sexually transmitted disease diagnosis and treatment, immunization practices, and chronic disease control.
Articles in this series will assist family physicians improve the health of the nation as they
- stay informed of local and national disease trends
- use antibiotics judiciously
- remain alert for suspicious disease presentations and report contagious diseases and other reportable conditions
- participate in sentinel disease detection
- cooperate with and support local and state health departments
- help plan and participate in emergency preparedness systems
- serve on boards of health or as consultants or medical directors to local health departments.
The importance of these activities cannot be overestimated. When the Centers for Disease Control and Prevention examined outbreaks that might have involved intentional use of infectious agents, it cited reporting by front-line health professionals as the most critical component in the detection of suspicious events.3
Patient-by-patient, physician-by-physician
A strong public health infrastructure supported by public health–conscious physicians will not only strengthen protection against infectious diseases but also address other community-wide concerns, such as chronic and environmentally caused diseases, injuries, and substance abuse. Family medicine predoctoral and residency programs must be sure they are preparing trainees to fulfill these roles. Training requirements should include more than token mention of public health.
Appropriate roles for the American Academy of Family Physicians include leadership in testing and developing information systems that link local family physicians to state and local health departments and provide easy access to public health information, and training practicing physicians to be more involved in the public health system.
But nothing can substitute for the concern and involvement of the local physician caring for the individual patient, who sees beyond the individual patient encounter and works to ensure that their daily actions also contribute to better health for all.
1. Centers for Disease Control and Prevention. Deaths; preliminary data for 2000. National Vital Statistics Reports 2001;49(12):Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr49/ nvsr49_12.pdf. Accessed on June 17, 2003.
2. Centers for Disease Control and Prevention. Deaths: leading causes for 1999. National Vital Statistics Reports 2001;49(1):Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr49/ nvsr49_11.pdf. Accessed on June 17, 2003.
3. Ashford DA, Kaiser RM, Bales ME, et al. Planning against biological terrorism: lessons from outbreak investigations. Emerg Infect Dis 2003;9:515-519.
1. Centers for Disease Control and Prevention. Deaths; preliminary data for 2000. National Vital Statistics Reports 2001;49(12):Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr49/ nvsr49_12.pdf. Accessed on June 17, 2003.
2. Centers for Disease Control and Prevention. Deaths: leading causes for 1999. National Vital Statistics Reports 2001;49(1):Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr49/ nvsr49_11.pdf. Accessed on June 17, 2003.
3. Ashford DA, Kaiser RM, Bales ME, et al. Planning against biological terrorism: lessons from outbreak investigations. Emerg Infect Dis 2003;9:515-519.
Evaluation and Treatment of Constipation
Constipation is an often-overlooked problem in primary care practice. It deserves careful evaluation, including consideration of the many possible causes and appropriate diagnostic testing. Fortunately, most patients respond well to conservative measures.
Constipation prompts a visit to a physician by 1.2% of the US population every year (although most persons with constipation do not seek the assistance of a physician).What You Should Know About Constipation,” is included with this article. (For your convenience, it may be freely duplicated and distributed.)
Suggested lifestyle changes include moderate physical activity, increased fluid intake, increased dietary fiber, and sitting on the toilet about 15–20 minutes after breakfast (taking advantage of the gastrocolic reflex). In selected patients, these changes may be useful, although specific benefits of moderate physical activity and increased fluid intake have not been conclusively proven.
Bulk laxatives
Wheat bran is one of the best and least expensive bulk laxatives. Methylcellulose (eg, Citrucel), psyllium (eg, Metamucil), and polycarbophil (eg, FiberCon) are bulk laxatives that are safe, more refined, and more concentrated than wheat bran, but they are also more expensive. Combined with diet and liquids, bulk laxatives are the most effective and “natural” long-term treatment for constipation. However, their slow onset of action (between 12 and 72 hours) limits their usefulness in acute management of constipation.
Saline laxatives
The saline laxatives include magnesium citrate (eg, Citroma) and magnesium hydroxide (eg, Milk of Magnesia). These agents decrease colonic transit time by stimulating cholecystokinin and draw fluid into the colon by their osmotic effect. Their rapid onset of action (between 30 minutes and 3 hours) makes saline laxatives an excellent choice for acute management of constipation. These laxatives commonly cause abdominal cramping and, in patients with renal failure, may cause magnesium toxicity. Nevertheless, saline laxatives are generally safe and effective.
Osmotic laxatives
Polyethylene glycol (eg, MiraLax) is an effective new osmotic laxative. Rapid onset of action (between 24 and 48 hours) makes an osmotic a good choice for patients who have chronic constipation that fails to respond to bulk and saline laxatives. Polyethylene glycol is equally effective, but better tolerated than the older osmotics, lactulose and sorbitol.16 Because it is not fermented, gas and cramps are minimal. Lactulose (eg, Chronulac) and sorbitol, which are poorly absorbed sugars, likewise have rapid onset of action, but flatulence and abdominal distention may limit tolerance. Sorbitol is generally less expensive than lactulose.
Stimulant laxatives
The oral stimulant laxatives include diphenylmethanes, the anthraquinones, and castor oil (eg, Emulsoil). They are more potent than bulk or osmotic laxatives, but long-term use is safe if limited to 3 days per week. Bisacodyl (eg, Dulcolax), a diphenylmethane, alters electrolyte transportation within intestinal mucosa and stimulates peristalsis. These actions may cause abdominal cramping and hypokalemia. Cascara (mildest), senna (eg, Senokot), and aloe (strongest) are anthraquinones, which are laxatives with actions and side effects similar to bisacodyl. These agents may cause a benign, reversible pigmentation of the colon (melanosis coli). It has been suggested that chronic use of these agents may damage the enteric nervous system, but a causal relationship has not been clearly established. The most prudent approach is to limit use of stimulant laxatives to constipation that is refractory to other laxatives.
Enemas and suppositories
Enemas and suppositories stimulate colonic contractions and soften stools. Water, saline, soap suds, hypertonic sodium phosphate, and mineral oil are used as enemas. Acute water intoxication can occur with water enemas, especially in infants, children, and the elderly, if they have difficulty evacuating the water. Phosphate enemas may cause hyperphosphatemia and hypocalcemic tetany in these patients and should therefore be used with caution in most patients and should not be used in children 3 years of age or younger. Glycerin and bisacodyl are stimulant suppositories that are clinically effective. Bisacodyl and soap suds enemas cause changes in the epithelium of the rectum, and the effect of glycerin on rectal mucosa is unclear. Therefore, these agents should only be used episodically. Mineral oil enemas are used to soften hardened stool in the rectal ampulla.
Other treatment options
More aggressive measures may be necessary for specific types of constipation. These include behavioral therapy and biofeedback for pelvic floor dysfunction, and surgery for slow-transit constipation or Hirschsprung’s disease.
Investigative pharmacologic treatments for constipation include agents that increase colonic contractions (prokinetic drugs) and prostaglandins. These agents have had limited efficacy and troublesome side effects. Therefore, at this time these drugs have limited usefulness in the treatment of constipation.
Fecal impaction
The management of fecal impaction begins with complete evacuation of the colon. Initially, patients with hard stool in the rectum may be given mineral oil retention enemas followed by manual disimpaction. Prior to further treatment, it is important to obtain an abdominal radiograph to rule out mechanical bowel obstruction. If there is no mechanical bowel obstruction, evacuation of the impaction can be accomplished with oral polyethylene glycol (eg, GoLytely) until clear (up to 8 liters or more may be required for complete evacuation).16 Administration of twice-daily enemas for 3 days or more is an acceptable alternative to oral polyethylene glycol. Lifestyle changes, bulk laxatives, saline, osmotic laxatives, and enemas should be used to maintain regular defecation after the colon has been cleansed. It is reasonable to attempt to withdraw laxatives after several months of regular bowel habits.
1. Sonnenberg A, Koch T. Physician visits in the United States for constipation. Dig Dis Sci 1989;34:606-11.
2. Sonnenberg A, Koch T. Epidemiology of constipation in the United States. Dis Colon Rectum 1989;32:1-8.
3. Johanson JF, Sonnenberg A, Koch T. Clinical epidemiology of chronic constipation. J Clin Gastroenterol 1989;11:525.-
4. Johanson JF. Geographic distribution of constipation in the United States. Am J Gastroenterol 1998;93:188-91.
5. Harari D, Gurwitz J, Avorn J, Bohn R, Minaker K. Bowel habit in relation to age and gender: findings from the National Health Interview Survey and clinical implications. Arch Intern Med 1996;156:315-20.
6. Nyam D, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997;40:273-7.
7. Koch A, Voderholzer W, Klauser A, Muller-Lissner SA. Symptoms in chronic constipation. Dis Colon Rectum 1997;40:902-6.
8. Ashraf W, Park F, Lof J, et al. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am J Gastroenterol 1996;91:26-32.
9. Kamal N, Chami T, Andersen A, et al. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology 1991;101:1320-4.
10. Garvey M, Noyes R, Jr, Yates W. Frequency of constipation in major depression: relationship to other clinical variables. Psychosomatics 1990;31:204-6.
11. Manning AP, Thompson WG, Heaton KW, Morris AF. Towards a positive diagnosis of the irritable bowel. BMJ 1978;2:653-4.
12. Locke GR, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology 2000;119:1766-78.
13. Tramonte SM, Brand MB, Mulrow CD, et al. The treatment of chronic constipation in adults. A systematic review. J Gen Intern Med 1997;12:15-24.
14. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-93.
15. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage 2000;19:130-6.
16. Tiongco F, Tsang T, Pollack J. Use of oral GoLytely solution in relief of refractory fecal impaction. Dig Dis Sci 1997;42:1454-7.
17. Anti M, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998;45:727-32.
18. Graham D, Moser S, Estes M. The effect of bran on bowel function in constipation. Gastroenterology 1982;77:599-603.
19. Marlett JA, Li BU, Patrow CJ, Bass P. Comparative laxation of psyllium with and without senna in an ambulatory constipated population. Am J Gastroenterol 1987;82:333-7.
20. Hamilton J, Wagner J, Burdick B, Bass P. Clinical evaluation of methylcellulose as a bulk laxative. Dig Dis Sci 1988;33:993-8.
21. Bass P, Clark C, DoPico GA. Comparison of the laxative efficacy and patient preference of calcium polycarbophil and psyllium suspension. Curr Ther Res Clin Exp 1988;43:770-4.
22. Attar A, Lemann M, Ferguson A, et al. Comparison of a low-dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999;44:226-30.
23. Lederle F, Busch D, Mattox K, West M, Aske D. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med 1990;89:597-601.
24. Sanders JF. Lactulose syrup assessed in a double-blind study of elderly constipated patients. J Am Geriatr Soc 1978;26:236-9.
25. Koustomanis D, Lennard-Jones J, Roy A, Kamm M. Controlled randomized trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut 1995;37:95-9.
26. Nyman DC, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997;40:273-9.
Constipation is an often-overlooked problem in primary care practice. It deserves careful evaluation, including consideration of the many possible causes and appropriate diagnostic testing. Fortunately, most patients respond well to conservative measures.
Constipation prompts a visit to a physician by 1.2% of the US population every year (although most persons with constipation do not seek the assistance of a physician).What You Should Know About Constipation,” is included with this article. (For your convenience, it may be freely duplicated and distributed.)
Suggested lifestyle changes include moderate physical activity, increased fluid intake, increased dietary fiber, and sitting on the toilet about 15–20 minutes after breakfast (taking advantage of the gastrocolic reflex). In selected patients, these changes may be useful, although specific benefits of moderate physical activity and increased fluid intake have not been conclusively proven.
Bulk laxatives
Wheat bran is one of the best and least expensive bulk laxatives. Methylcellulose (eg, Citrucel), psyllium (eg, Metamucil), and polycarbophil (eg, FiberCon) are bulk laxatives that are safe, more refined, and more concentrated than wheat bran, but they are also more expensive. Combined with diet and liquids, bulk laxatives are the most effective and “natural” long-term treatment for constipation. However, their slow onset of action (between 12 and 72 hours) limits their usefulness in acute management of constipation.
Saline laxatives
The saline laxatives include magnesium citrate (eg, Citroma) and magnesium hydroxide (eg, Milk of Magnesia). These agents decrease colonic transit time by stimulating cholecystokinin and draw fluid into the colon by their osmotic effect. Their rapid onset of action (between 30 minutes and 3 hours) makes saline laxatives an excellent choice for acute management of constipation. These laxatives commonly cause abdominal cramping and, in patients with renal failure, may cause magnesium toxicity. Nevertheless, saline laxatives are generally safe and effective.
Osmotic laxatives
Polyethylene glycol (eg, MiraLax) is an effective new osmotic laxative. Rapid onset of action (between 24 and 48 hours) makes an osmotic a good choice for patients who have chronic constipation that fails to respond to bulk and saline laxatives. Polyethylene glycol is equally effective, but better tolerated than the older osmotics, lactulose and sorbitol.16 Because it is not fermented, gas and cramps are minimal. Lactulose (eg, Chronulac) and sorbitol, which are poorly absorbed sugars, likewise have rapid onset of action, but flatulence and abdominal distention may limit tolerance. Sorbitol is generally less expensive than lactulose.
Stimulant laxatives
The oral stimulant laxatives include diphenylmethanes, the anthraquinones, and castor oil (eg, Emulsoil). They are more potent than bulk or osmotic laxatives, but long-term use is safe if limited to 3 days per week. Bisacodyl (eg, Dulcolax), a diphenylmethane, alters electrolyte transportation within intestinal mucosa and stimulates peristalsis. These actions may cause abdominal cramping and hypokalemia. Cascara (mildest), senna (eg, Senokot), and aloe (strongest) are anthraquinones, which are laxatives with actions and side effects similar to bisacodyl. These agents may cause a benign, reversible pigmentation of the colon (melanosis coli). It has been suggested that chronic use of these agents may damage the enteric nervous system, but a causal relationship has not been clearly established. The most prudent approach is to limit use of stimulant laxatives to constipation that is refractory to other laxatives.
Enemas and suppositories
Enemas and suppositories stimulate colonic contractions and soften stools. Water, saline, soap suds, hypertonic sodium phosphate, and mineral oil are used as enemas. Acute water intoxication can occur with water enemas, especially in infants, children, and the elderly, if they have difficulty evacuating the water. Phosphate enemas may cause hyperphosphatemia and hypocalcemic tetany in these patients and should therefore be used with caution in most patients and should not be used in children 3 years of age or younger. Glycerin and bisacodyl are stimulant suppositories that are clinically effective. Bisacodyl and soap suds enemas cause changes in the epithelium of the rectum, and the effect of glycerin on rectal mucosa is unclear. Therefore, these agents should only be used episodically. Mineral oil enemas are used to soften hardened stool in the rectal ampulla.
Other treatment options
More aggressive measures may be necessary for specific types of constipation. These include behavioral therapy and biofeedback for pelvic floor dysfunction, and surgery for slow-transit constipation or Hirschsprung’s disease.
Investigative pharmacologic treatments for constipation include agents that increase colonic contractions (prokinetic drugs) and prostaglandins. These agents have had limited efficacy and troublesome side effects. Therefore, at this time these drugs have limited usefulness in the treatment of constipation.
Fecal impaction
The management of fecal impaction begins with complete evacuation of the colon. Initially, patients with hard stool in the rectum may be given mineral oil retention enemas followed by manual disimpaction. Prior to further treatment, it is important to obtain an abdominal radiograph to rule out mechanical bowel obstruction. If there is no mechanical bowel obstruction, evacuation of the impaction can be accomplished with oral polyethylene glycol (eg, GoLytely) until clear (up to 8 liters or more may be required for complete evacuation).16 Administration of twice-daily enemas for 3 days or more is an acceptable alternative to oral polyethylene glycol. Lifestyle changes, bulk laxatives, saline, osmotic laxatives, and enemas should be used to maintain regular defecation after the colon has been cleansed. It is reasonable to attempt to withdraw laxatives after several months of regular bowel habits.
Constipation is an often-overlooked problem in primary care practice. It deserves careful evaluation, including consideration of the many possible causes and appropriate diagnostic testing. Fortunately, most patients respond well to conservative measures.
Constipation prompts a visit to a physician by 1.2% of the US population every year (although most persons with constipation do not seek the assistance of a physician).What You Should Know About Constipation,” is included with this article. (For your convenience, it may be freely duplicated and distributed.)
Suggested lifestyle changes include moderate physical activity, increased fluid intake, increased dietary fiber, and sitting on the toilet about 15–20 minutes after breakfast (taking advantage of the gastrocolic reflex). In selected patients, these changes may be useful, although specific benefits of moderate physical activity and increased fluid intake have not been conclusively proven.
Bulk laxatives
Wheat bran is one of the best and least expensive bulk laxatives. Methylcellulose (eg, Citrucel), psyllium (eg, Metamucil), and polycarbophil (eg, FiberCon) are bulk laxatives that are safe, more refined, and more concentrated than wheat bran, but they are also more expensive. Combined with diet and liquids, bulk laxatives are the most effective and “natural” long-term treatment for constipation. However, their slow onset of action (between 12 and 72 hours) limits their usefulness in acute management of constipation.
Saline laxatives
The saline laxatives include magnesium citrate (eg, Citroma) and magnesium hydroxide (eg, Milk of Magnesia). These agents decrease colonic transit time by stimulating cholecystokinin and draw fluid into the colon by their osmotic effect. Their rapid onset of action (between 30 minutes and 3 hours) makes saline laxatives an excellent choice for acute management of constipation. These laxatives commonly cause abdominal cramping and, in patients with renal failure, may cause magnesium toxicity. Nevertheless, saline laxatives are generally safe and effective.
Osmotic laxatives
Polyethylene glycol (eg, MiraLax) is an effective new osmotic laxative. Rapid onset of action (between 24 and 48 hours) makes an osmotic a good choice for patients who have chronic constipation that fails to respond to bulk and saline laxatives. Polyethylene glycol is equally effective, but better tolerated than the older osmotics, lactulose and sorbitol.16 Because it is not fermented, gas and cramps are minimal. Lactulose (eg, Chronulac) and sorbitol, which are poorly absorbed sugars, likewise have rapid onset of action, but flatulence and abdominal distention may limit tolerance. Sorbitol is generally less expensive than lactulose.
Stimulant laxatives
The oral stimulant laxatives include diphenylmethanes, the anthraquinones, and castor oil (eg, Emulsoil). They are more potent than bulk or osmotic laxatives, but long-term use is safe if limited to 3 days per week. Bisacodyl (eg, Dulcolax), a diphenylmethane, alters electrolyte transportation within intestinal mucosa and stimulates peristalsis. These actions may cause abdominal cramping and hypokalemia. Cascara (mildest), senna (eg, Senokot), and aloe (strongest) are anthraquinones, which are laxatives with actions and side effects similar to bisacodyl. These agents may cause a benign, reversible pigmentation of the colon (melanosis coli). It has been suggested that chronic use of these agents may damage the enteric nervous system, but a causal relationship has not been clearly established. The most prudent approach is to limit use of stimulant laxatives to constipation that is refractory to other laxatives.
Enemas and suppositories
Enemas and suppositories stimulate colonic contractions and soften stools. Water, saline, soap suds, hypertonic sodium phosphate, and mineral oil are used as enemas. Acute water intoxication can occur with water enemas, especially in infants, children, and the elderly, if they have difficulty evacuating the water. Phosphate enemas may cause hyperphosphatemia and hypocalcemic tetany in these patients and should therefore be used with caution in most patients and should not be used in children 3 years of age or younger. Glycerin and bisacodyl are stimulant suppositories that are clinically effective. Bisacodyl and soap suds enemas cause changes in the epithelium of the rectum, and the effect of glycerin on rectal mucosa is unclear. Therefore, these agents should only be used episodically. Mineral oil enemas are used to soften hardened stool in the rectal ampulla.
Other treatment options
More aggressive measures may be necessary for specific types of constipation. These include behavioral therapy and biofeedback for pelvic floor dysfunction, and surgery for slow-transit constipation or Hirschsprung’s disease.
Investigative pharmacologic treatments for constipation include agents that increase colonic contractions (prokinetic drugs) and prostaglandins. These agents have had limited efficacy and troublesome side effects. Therefore, at this time these drugs have limited usefulness in the treatment of constipation.
Fecal impaction
The management of fecal impaction begins with complete evacuation of the colon. Initially, patients with hard stool in the rectum may be given mineral oil retention enemas followed by manual disimpaction. Prior to further treatment, it is important to obtain an abdominal radiograph to rule out mechanical bowel obstruction. If there is no mechanical bowel obstruction, evacuation of the impaction can be accomplished with oral polyethylene glycol (eg, GoLytely) until clear (up to 8 liters or more may be required for complete evacuation).16 Administration of twice-daily enemas for 3 days or more is an acceptable alternative to oral polyethylene glycol. Lifestyle changes, bulk laxatives, saline, osmotic laxatives, and enemas should be used to maintain regular defecation after the colon has been cleansed. It is reasonable to attempt to withdraw laxatives after several months of regular bowel habits.
1. Sonnenberg A, Koch T. Physician visits in the United States for constipation. Dig Dis Sci 1989;34:606-11.
2. Sonnenberg A, Koch T. Epidemiology of constipation in the United States. Dis Colon Rectum 1989;32:1-8.
3. Johanson JF, Sonnenberg A, Koch T. Clinical epidemiology of chronic constipation. J Clin Gastroenterol 1989;11:525.-
4. Johanson JF. Geographic distribution of constipation in the United States. Am J Gastroenterol 1998;93:188-91.
5. Harari D, Gurwitz J, Avorn J, Bohn R, Minaker K. Bowel habit in relation to age and gender: findings from the National Health Interview Survey and clinical implications. Arch Intern Med 1996;156:315-20.
6. Nyam D, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997;40:273-7.
7. Koch A, Voderholzer W, Klauser A, Muller-Lissner SA. Symptoms in chronic constipation. Dis Colon Rectum 1997;40:902-6.
8. Ashraf W, Park F, Lof J, et al. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am J Gastroenterol 1996;91:26-32.
9. Kamal N, Chami T, Andersen A, et al. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology 1991;101:1320-4.
10. Garvey M, Noyes R, Jr, Yates W. Frequency of constipation in major depression: relationship to other clinical variables. Psychosomatics 1990;31:204-6.
11. Manning AP, Thompson WG, Heaton KW, Morris AF. Towards a positive diagnosis of the irritable bowel. BMJ 1978;2:653-4.
12. Locke GR, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology 2000;119:1766-78.
13. Tramonte SM, Brand MB, Mulrow CD, et al. The treatment of chronic constipation in adults. A systematic review. J Gen Intern Med 1997;12:15-24.
14. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-93.
15. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage 2000;19:130-6.
16. Tiongco F, Tsang T, Pollack J. Use of oral GoLytely solution in relief of refractory fecal impaction. Dig Dis Sci 1997;42:1454-7.
17. Anti M, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998;45:727-32.
18. Graham D, Moser S, Estes M. The effect of bran on bowel function in constipation. Gastroenterology 1982;77:599-603.
19. Marlett JA, Li BU, Patrow CJ, Bass P. Comparative laxation of psyllium with and without senna in an ambulatory constipated population. Am J Gastroenterol 1987;82:333-7.
20. Hamilton J, Wagner J, Burdick B, Bass P. Clinical evaluation of methylcellulose as a bulk laxative. Dig Dis Sci 1988;33:993-8.
21. Bass P, Clark C, DoPico GA. Comparison of the laxative efficacy and patient preference of calcium polycarbophil and psyllium suspension. Curr Ther Res Clin Exp 1988;43:770-4.
22. Attar A, Lemann M, Ferguson A, et al. Comparison of a low-dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999;44:226-30.
23. Lederle F, Busch D, Mattox K, West M, Aske D. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med 1990;89:597-601.
24. Sanders JF. Lactulose syrup assessed in a double-blind study of elderly constipated patients. J Am Geriatr Soc 1978;26:236-9.
25. Koustomanis D, Lennard-Jones J, Roy A, Kamm M. Controlled randomized trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut 1995;37:95-9.
26. Nyman DC, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997;40:273-9.
1. Sonnenberg A, Koch T. Physician visits in the United States for constipation. Dig Dis Sci 1989;34:606-11.
2. Sonnenberg A, Koch T. Epidemiology of constipation in the United States. Dis Colon Rectum 1989;32:1-8.
3. Johanson JF, Sonnenberg A, Koch T. Clinical epidemiology of chronic constipation. J Clin Gastroenterol 1989;11:525.-
4. Johanson JF. Geographic distribution of constipation in the United States. Am J Gastroenterol 1998;93:188-91.
5. Harari D, Gurwitz J, Avorn J, Bohn R, Minaker K. Bowel habit in relation to age and gender: findings from the National Health Interview Survey and clinical implications. Arch Intern Med 1996;156:315-20.
6. Nyam D, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997;40:273-7.
7. Koch A, Voderholzer W, Klauser A, Muller-Lissner SA. Symptoms in chronic constipation. Dis Colon Rectum 1997;40:902-6.
8. Ashraf W, Park F, Lof J, et al. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am J Gastroenterol 1996;91:26-32.
9. Kamal N, Chami T, Andersen A, et al. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology 1991;101:1320-4.
10. Garvey M, Noyes R, Jr, Yates W. Frequency of constipation in major depression: relationship to other clinical variables. Psychosomatics 1990;31:204-6.
11. Manning AP, Thompson WG, Heaton KW, Morris AF. Towards a positive diagnosis of the irritable bowel. BMJ 1978;2:653-4.
12. Locke GR, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology 2000;119:1766-78.
13. Tramonte SM, Brand MB, Mulrow CD, et al. The treatment of chronic constipation in adults. A systematic review. J Gen Intern Med 1997;12:15-24.
14. Petticrew M, Watt I, Brand M. What’s the “best buy” for treatment of constipation? Results of a systematic review of the efficacy and comparative efficacy of laxatives in the elderly. Br J Gen Pract 1999;49:387-93.
15. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage 2000;19:130-6.
16. Tiongco F, Tsang T, Pollack J. Use of oral GoLytely solution in relief of refractory fecal impaction. Dig Dis Sci 1997;42:1454-7.
17. Anti M, Pignataro G, Armuzzi A, et al. Water supplementation enhances the effect of high-fiber diet on stool frequency and laxative consumption in adult patients with functional constipation. Hepatogastroenterology 1998;45:727-32.
18. Graham D, Moser S, Estes M. The effect of bran on bowel function in constipation. Gastroenterology 1982;77:599-603.
19. Marlett JA, Li BU, Patrow CJ, Bass P. Comparative laxation of psyllium with and without senna in an ambulatory constipated population. Am J Gastroenterol 1987;82:333-7.
20. Hamilton J, Wagner J, Burdick B, Bass P. Clinical evaluation of methylcellulose as a bulk laxative. Dig Dis Sci 1988;33:993-8.
21. Bass P, Clark C, DoPico GA. Comparison of the laxative efficacy and patient preference of calcium polycarbophil and psyllium suspension. Curr Ther Res Clin Exp 1988;43:770-4.
22. Attar A, Lemann M, Ferguson A, et al. Comparison of a low-dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation. Gut 1999;44:226-30.
23. Lederle F, Busch D, Mattox K, West M, Aske D. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am J Med 1990;89:597-601.
24. Sanders JF. Lactulose syrup assessed in a double-blind study of elderly constipated patients. J Am Geriatr Soc 1978;26:236-9.
25. Koustomanis D, Lennard-Jones J, Roy A, Kamm M. Controlled randomized trial of visual biofeedback versus muscle training without a visual display for intractable constipation. Gut 1995;37:95-9.
26. Nyman DC, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997;40:273-9.