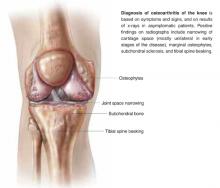
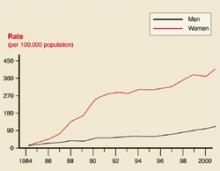
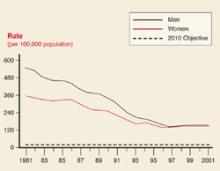
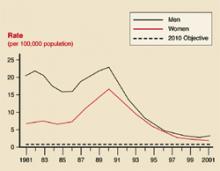
User login
HIV prevention enters a new era
The Centers for Disease Control and Prevention (CDC) estimates 40,000 new HIV infections occur annually in the US, and this may be increasing. Close to 1 million people in this country are living with HIV, and an estimated one quarter of them do not know they are infected.1 Thus, the infection often is detected late; 40% of those infected find out about it <1 year before AIDS develops. The result of delayed detection, or failed detection, is a large group of infected persons who unknowingly expose others to the disease for a prolonged period.2
Alarming trends
Recent epidemiologic trends indicate our preventive efforts are inadequate. Risky behavior is increasing among certain subpopulations of men who have sex with men.3,4 In the US, 300 babies a year are born with HIV infection, despite effective interventions to prevent mother-to-baby transmission, largely because infection in the mother is not detected during pregnancy.1 Needle exchange for IV drug users, a proven effective intervention, remains underused because of political objections.5
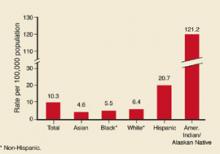
While the HIV epidemic in the US remains driven by infections in men who have sex with men and those who use illicit intravenous drugs, the number of heterosexually transmitted infections has increased each year and was estimated at 9183 new infections in 2002; 3234 among men and 5949 among women. In addition, the disease has become a major cause of health disparity in this country. Comparative AIDS rates per 100,000 in 2002 were 5.9 for whites, 8.5 for American Indians/Native Americans, 19.2 for Hispanics and 58.7 for African Americans.6
Effective treatments warrant more effective detection
On the other hand, the use of highly active anti-retroviral therapy has been very successful in altering the course of the disease in those infected, lowering death rates dramatically. The result has been an increasing number of people living with HIV/AIDS. While treatment lowers viral loads and presumably makes one less infectious, the overall community effect of an increasing number of infected persons still able to transmit the virus to others could be negative unless education is effective in reducing behavior that places others at risk.
Change is needed
All of these trends have created a need to reexamine HIV prevention efforts. The main HIV prevention interventions used in the US for 2 decades have included screening donated blood; screening pregnant women and administering antiretroviral agents to HIV-positive mothers during pregnancy and to their newborns; needle exchange programs (in a few locations); community-wide and risk-group specific education; and confidential or anonymous HIV counseling and testing programs.
Counseling and testing programs have used extensive pretest and posttest counseling sessions in an attempt to keep those who are HIV negative from contracting the disease. However, studies have shown that counseling and testing do not significantly alter sexual behavior among those who are HIV negative; they are effective for those who are HIV positive.7-11 Counseling of those who are HIV negative can be more effective if patient centered methods are used.12
CDC’S new initiative
The CDC has recently reviewed its HIV prevention efforts and initiated a new campaign called Advancing HIV Prevention (AHP). This initiative has 4 components:
- Make HIV testing a routine part of medical care whenever and wherever patients go for care.
- Use new models for diagnosing HIV infections outside traditional medical settings.
- Prevent new infections by working with people diagnosed with HIV and their partners.
- Decrease mother-to-child HIV transmission.
Potential benefits of increased testing include earlier detection and entry of infected persons into treatment, earlier notification and testing of contacts, shorter periods during which infected persons unknowingly transmit the infection to others, and reduced stigma of testing as it becomes routine. However, this strategy will be effective only if those who are HIV positive can receive medical care and social support and be convinced to avoid exposing others. Fortunately, the evidence is good that intensive counseling and case management can achieve these goals.8-11 Another potential benefit is earlier notification of contacts, either by the patient or the public health department, depending on local public health practice.
Testing during pregnancy is well accepted and widely used but is still not universally implemented. Voluntary testing is more acceptable if implemented as a routine test with a choice to opt out—ie, informing women that the HIV test is being offered as part of routine testing and that they have the option of refusing it. Selective testing based on perceived risk misses cases and contributes to stigmatization of those tested.13 The CDC recommends that women who refuse testing should be counseled on the potential benefits of HIV testing to them and to their babies; and that providers should recommend the test while, preserving the mother’s right to refuse should she decide the test is not in her best interest.14
Family physician involvement
Family physicians can contribute to the country’s HIV prevention efforts by implementing the steps listed in the Table. This new approach places more emphasis on finding those infected with HIV and initiating actions beneficial to them and their partners while reducing risk of transmission.
Explore acceptability of needle exchange programs. Another intervention proven effective, but more controversial, is needle exchange programs for illicit drug users. The evidence to date is that needle exchange programs prevent transmission of HIV and other blood borne pathogens and do not encourage use of illicit drugs.5 Because these programs have proven as controversial as they are effective, they have not been widely adopted. If the community political climate is receptive, family physicians could also advocate for these programs.
Implement routine testing. As family physicians move to make HIV testing routine, several issues must be considered. Though HIV testing methods are quite accurate, an initial positive test in a person with a very low pretest probability is more likely to be a false positive than a true positive. Risks, however, are not always apparent or admitted to by patients. Positive tests should be repeated and confirmed. Newly approved rapid HIV tests allow for results within a half-hour, but positive test results must be confirmed by western blot or immunofluorescence assay.15
Report cases promptly. In 31 states, HIV infection is a reportable disease. This may cause concern among patients and lead physicians to under report. This practice is discouraged for several reasons. Accurate tracking of the HIV epidemic is critical to measure the effectiveness of preventive interventions and to enable quick implementation of needed changes in public health practice. Federal funds to support treatment for those with HIV/AIDS depend on the number of persons with documented HIV infection; under-reporting causes the community to lose treatment funds. Finally, public health departments have a long established record of maintaining confidentiality of infectious disease reports and, in most jurisdictions, have more confidentiality legal protections than do physician offices.
HIV remains a significant public health problem in the US. As the epidemic evolves, new public health efforts will be needed. Full control of the epidemic might not be achieved until a more effective intervention, such as a vaccine, is available. However, interventions have proven effective and more widespread use would reduce the community burden of the disease.
TABLE
Practice based initiatives that could contribute to HIV reduction in the community
| Make HIV testing a routine part of general medical care. |
| Make HIV testing a routine part of pregnancy care. Test as early as possible in pregnancy and retest those at high risk in the third trimester. |
| Refer those who are HIV-positive to the local public health department for case management. |
| Work collaboratively with the public health department to insure that people with HIV infection receive medical care and social services. |
| Reinforce the message to those infected about how to avoid transmitting the infection to others. |
| Counsel uninfected patients who practice high-risk behaviors about how to reduce their risks of infection, using patient centered methods. |
| Promptly diagnose and treat other sexually transmitted infections. |
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). National Center for HIV, STD, and TB Prevention. Advancing HIV prevention: the science behind the new initiative. Available at: www.cdc.gov/hiv/partners/ahp_science.htm.
2. Hays RB, Paul J, Ekstrand M, Kegeles SM, et al. Actual versus perceived HIV status, sexual behaviors and predictors of unprotected sex among young gay and bisexual men who identify as HIV-negative, HIV-positive and untested. AIDS 1997;11:1495-1502.
3. CDC. Primary and secondary syphilis among men who have sex with men – New York City, 2001. MMWR Morb Mortal Wkly Rep 2002;51:853-856.
4. CDC. Resurgent bacterial sexually transmitted disease among men who have sex with men—King County, Washington, 1997–1999. MMWR Morb Mortal Wkly Rep 1999;48:773-777.
5. Institute of Medicine No Time to Lose: Getting More from HIV Prevention. Committee on HIV Prevention Strategies in the United States, Division of Health Promotion and Disease Prevention. Washington, DC: National Academies Press; 2001.
6. CDC. Cases of HIV infection and AIDS in the United States, 2002. HIV/AIDS Surveillance Report 2002;14:1-58.Available at: www.cdc.gov/hiv/stats/hasr1402.htm.
7. Kamb ML, Fishbein M, Douglas JM, et al. efficacy of risk reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. JAMA 1998;280:1161-1167.
8. Wenger NS, Kussling FS, Beck K, Shapiro MF. Sexual behavior of individuals infected with the human immunodeficiency virus: The need for intervention. Arch Int Med 1994;154:1849-1854.
9. Kilmarx PH, Hamers FF, Peterman TA. Living with HIV. Experiences and perspectives of HIV-infected sexually transmitted disease clinic patients after posttest counseling. Sex Transm Dis 1998;25:28-37.
10. Higgins DL, Galavotti C, O’Reilly KE, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA 1991;266:2419-2429
11. Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health 1999;89:1397-1405.
12. Kamb ML, Fishbein M, Douglas JM, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases. JAMA 1998;280:1161-1167.
13. Walensky RP, Losina E, Steger-Craven KA, Freedberg KA. Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary inpatient testing. Arch Intern Med 2002;162:887-892.
14. CDC. Revised recommendations for HIV screening of pregnant women. MMWR Recomm Rep 2001;50(RR-19):59-86.
15. CDC. Notice to readers: Approval of a new rapid test for HIV antibody. MMWR Morn Mortal Wkly Rep 2002;51:1051-1052.
The Centers for Disease Control and Prevention (CDC) estimates 40,000 new HIV infections occur annually in the US, and this may be increasing. Close to 1 million people in this country are living with HIV, and an estimated one quarter of them do not know they are infected.1 Thus, the infection often is detected late; 40% of those infected find out about it <1 year before AIDS develops. The result of delayed detection, or failed detection, is a large group of infected persons who unknowingly expose others to the disease for a prolonged period.2
Alarming trends
Recent epidemiologic trends indicate our preventive efforts are inadequate. Risky behavior is increasing among certain subpopulations of men who have sex with men.3,4 In the US, 300 babies a year are born with HIV infection, despite effective interventions to prevent mother-to-baby transmission, largely because infection in the mother is not detected during pregnancy.1 Needle exchange for IV drug users, a proven effective intervention, remains underused because of political objections.5
While the HIV epidemic in the US remains driven by infections in men who have sex with men and those who use illicit intravenous drugs, the number of heterosexually transmitted infections has increased each year and was estimated at 9183 new infections in 2002; 3234 among men and 5949 among women. In addition, the disease has become a major cause of health disparity in this country. Comparative AIDS rates per 100,000 in 2002 were 5.9 for whites, 8.5 for American Indians/Native Americans, 19.2 for Hispanics and 58.7 for African Americans.6
Effective treatments warrant more effective detection
On the other hand, the use of highly active anti-retroviral therapy has been very successful in altering the course of the disease in those infected, lowering death rates dramatically. The result has been an increasing number of people living with HIV/AIDS. While treatment lowers viral loads and presumably makes one less infectious, the overall community effect of an increasing number of infected persons still able to transmit the virus to others could be negative unless education is effective in reducing behavior that places others at risk.
Change is needed
All of these trends have created a need to reexamine HIV prevention efforts. The main HIV prevention interventions used in the US for 2 decades have included screening donated blood; screening pregnant women and administering antiretroviral agents to HIV-positive mothers during pregnancy and to their newborns; needle exchange programs (in a few locations); community-wide and risk-group specific education; and confidential or anonymous HIV counseling and testing programs.
Counseling and testing programs have used extensive pretest and posttest counseling sessions in an attempt to keep those who are HIV negative from contracting the disease. However, studies have shown that counseling and testing do not significantly alter sexual behavior among those who are HIV negative; they are effective for those who are HIV positive.7-11 Counseling of those who are HIV negative can be more effective if patient centered methods are used.12
CDC’S new initiative
The CDC has recently reviewed its HIV prevention efforts and initiated a new campaign called Advancing HIV Prevention (AHP). This initiative has 4 components:
- Make HIV testing a routine part of medical care whenever and wherever patients go for care.
- Use new models for diagnosing HIV infections outside traditional medical settings.
- Prevent new infections by working with people diagnosed with HIV and their partners.
- Decrease mother-to-child HIV transmission.
Potential benefits of increased testing include earlier detection and entry of infected persons into treatment, earlier notification and testing of contacts, shorter periods during which infected persons unknowingly transmit the infection to others, and reduced stigma of testing as it becomes routine. However, this strategy will be effective only if those who are HIV positive can receive medical care and social support and be convinced to avoid exposing others. Fortunately, the evidence is good that intensive counseling and case management can achieve these goals.8-11 Another potential benefit is earlier notification of contacts, either by the patient or the public health department, depending on local public health practice.
Testing during pregnancy is well accepted and widely used but is still not universally implemented. Voluntary testing is more acceptable if implemented as a routine test with a choice to opt out—ie, informing women that the HIV test is being offered as part of routine testing and that they have the option of refusing it. Selective testing based on perceived risk misses cases and contributes to stigmatization of those tested.13 The CDC recommends that women who refuse testing should be counseled on the potential benefits of HIV testing to them and to their babies; and that providers should recommend the test while, preserving the mother’s right to refuse should she decide the test is not in her best interest.14
Family physician involvement
Family physicians can contribute to the country’s HIV prevention efforts by implementing the steps listed in the Table. This new approach places more emphasis on finding those infected with HIV and initiating actions beneficial to them and their partners while reducing risk of transmission.
Explore acceptability of needle exchange programs. Another intervention proven effective, but more controversial, is needle exchange programs for illicit drug users. The evidence to date is that needle exchange programs prevent transmission of HIV and other blood borne pathogens and do not encourage use of illicit drugs.5 Because these programs have proven as controversial as they are effective, they have not been widely adopted. If the community political climate is receptive, family physicians could also advocate for these programs.
Implement routine testing. As family physicians move to make HIV testing routine, several issues must be considered. Though HIV testing methods are quite accurate, an initial positive test in a person with a very low pretest probability is more likely to be a false positive than a true positive. Risks, however, are not always apparent or admitted to by patients. Positive tests should be repeated and confirmed. Newly approved rapid HIV tests allow for results within a half-hour, but positive test results must be confirmed by western blot or immunofluorescence assay.15
Report cases promptly. In 31 states, HIV infection is a reportable disease. This may cause concern among patients and lead physicians to under report. This practice is discouraged for several reasons. Accurate tracking of the HIV epidemic is critical to measure the effectiveness of preventive interventions and to enable quick implementation of needed changes in public health practice. Federal funds to support treatment for those with HIV/AIDS depend on the number of persons with documented HIV infection; under-reporting causes the community to lose treatment funds. Finally, public health departments have a long established record of maintaining confidentiality of infectious disease reports and, in most jurisdictions, have more confidentiality legal protections than do physician offices.
HIV remains a significant public health problem in the US. As the epidemic evolves, new public health efforts will be needed. Full control of the epidemic might not be achieved until a more effective intervention, such as a vaccine, is available. However, interventions have proven effective and more widespread use would reduce the community burden of the disease.
TABLE
Practice based initiatives that could contribute to HIV reduction in the community
| Make HIV testing a routine part of general medical care. |
| Make HIV testing a routine part of pregnancy care. Test as early as possible in pregnancy and retest those at high risk in the third trimester. |
| Refer those who are HIV-positive to the local public health department for case management. |
| Work collaboratively with the public health department to insure that people with HIV infection receive medical care and social services. |
| Reinforce the message to those infected about how to avoid transmitting the infection to others. |
| Counsel uninfected patients who practice high-risk behaviors about how to reduce their risks of infection, using patient centered methods. |
| Promptly diagnose and treat other sexually transmitted infections. |
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
The Centers for Disease Control and Prevention (CDC) estimates 40,000 new HIV infections occur annually in the US, and this may be increasing. Close to 1 million people in this country are living with HIV, and an estimated one quarter of them do not know they are infected.1 Thus, the infection often is detected late; 40% of those infected find out about it <1 year before AIDS develops. The result of delayed detection, or failed detection, is a large group of infected persons who unknowingly expose others to the disease for a prolonged period.2
Alarming trends
Recent epidemiologic trends indicate our preventive efforts are inadequate. Risky behavior is increasing among certain subpopulations of men who have sex with men.3,4 In the US, 300 babies a year are born with HIV infection, despite effective interventions to prevent mother-to-baby transmission, largely because infection in the mother is not detected during pregnancy.1 Needle exchange for IV drug users, a proven effective intervention, remains underused because of political objections.5
While the HIV epidemic in the US remains driven by infections in men who have sex with men and those who use illicit intravenous drugs, the number of heterosexually transmitted infections has increased each year and was estimated at 9183 new infections in 2002; 3234 among men and 5949 among women. In addition, the disease has become a major cause of health disparity in this country. Comparative AIDS rates per 100,000 in 2002 were 5.9 for whites, 8.5 for American Indians/Native Americans, 19.2 for Hispanics and 58.7 for African Americans.6
Effective treatments warrant more effective detection
On the other hand, the use of highly active anti-retroviral therapy has been very successful in altering the course of the disease in those infected, lowering death rates dramatically. The result has been an increasing number of people living with HIV/AIDS. While treatment lowers viral loads and presumably makes one less infectious, the overall community effect of an increasing number of infected persons still able to transmit the virus to others could be negative unless education is effective in reducing behavior that places others at risk.
Change is needed
All of these trends have created a need to reexamine HIV prevention efforts. The main HIV prevention interventions used in the US for 2 decades have included screening donated blood; screening pregnant women and administering antiretroviral agents to HIV-positive mothers during pregnancy and to their newborns; needle exchange programs (in a few locations); community-wide and risk-group specific education; and confidential or anonymous HIV counseling and testing programs.
Counseling and testing programs have used extensive pretest and posttest counseling sessions in an attempt to keep those who are HIV negative from contracting the disease. However, studies have shown that counseling and testing do not significantly alter sexual behavior among those who are HIV negative; they are effective for those who are HIV positive.7-11 Counseling of those who are HIV negative can be more effective if patient centered methods are used.12
CDC’S new initiative
The CDC has recently reviewed its HIV prevention efforts and initiated a new campaign called Advancing HIV Prevention (AHP). This initiative has 4 components:
- Make HIV testing a routine part of medical care whenever and wherever patients go for care.
- Use new models for diagnosing HIV infections outside traditional medical settings.
- Prevent new infections by working with people diagnosed with HIV and their partners.
- Decrease mother-to-child HIV transmission.
Potential benefits of increased testing include earlier detection and entry of infected persons into treatment, earlier notification and testing of contacts, shorter periods during which infected persons unknowingly transmit the infection to others, and reduced stigma of testing as it becomes routine. However, this strategy will be effective only if those who are HIV positive can receive medical care and social support and be convinced to avoid exposing others. Fortunately, the evidence is good that intensive counseling and case management can achieve these goals.8-11 Another potential benefit is earlier notification of contacts, either by the patient or the public health department, depending on local public health practice.
Testing during pregnancy is well accepted and widely used but is still not universally implemented. Voluntary testing is more acceptable if implemented as a routine test with a choice to opt out—ie, informing women that the HIV test is being offered as part of routine testing and that they have the option of refusing it. Selective testing based on perceived risk misses cases and contributes to stigmatization of those tested.13 The CDC recommends that women who refuse testing should be counseled on the potential benefits of HIV testing to them and to their babies; and that providers should recommend the test while, preserving the mother’s right to refuse should she decide the test is not in her best interest.14
Family physician involvement
Family physicians can contribute to the country’s HIV prevention efforts by implementing the steps listed in the Table. This new approach places more emphasis on finding those infected with HIV and initiating actions beneficial to them and their partners while reducing risk of transmission.
Explore acceptability of needle exchange programs. Another intervention proven effective, but more controversial, is needle exchange programs for illicit drug users. The evidence to date is that needle exchange programs prevent transmission of HIV and other blood borne pathogens and do not encourage use of illicit drugs.5 Because these programs have proven as controversial as they are effective, they have not been widely adopted. If the community political climate is receptive, family physicians could also advocate for these programs.
Implement routine testing. As family physicians move to make HIV testing routine, several issues must be considered. Though HIV testing methods are quite accurate, an initial positive test in a person with a very low pretest probability is more likely to be a false positive than a true positive. Risks, however, are not always apparent or admitted to by patients. Positive tests should be repeated and confirmed. Newly approved rapid HIV tests allow for results within a half-hour, but positive test results must be confirmed by western blot or immunofluorescence assay.15
Report cases promptly. In 31 states, HIV infection is a reportable disease. This may cause concern among patients and lead physicians to under report. This practice is discouraged for several reasons. Accurate tracking of the HIV epidemic is critical to measure the effectiveness of preventive interventions and to enable quick implementation of needed changes in public health practice. Federal funds to support treatment for those with HIV/AIDS depend on the number of persons with documented HIV infection; under-reporting causes the community to lose treatment funds. Finally, public health departments have a long established record of maintaining confidentiality of infectious disease reports and, in most jurisdictions, have more confidentiality legal protections than do physician offices.
HIV remains a significant public health problem in the US. As the epidemic evolves, new public health efforts will be needed. Full control of the epidemic might not be achieved until a more effective intervention, such as a vaccine, is available. However, interventions have proven effective and more widespread use would reduce the community burden of the disease.
TABLE
Practice based initiatives that could contribute to HIV reduction in the community
| Make HIV testing a routine part of general medical care. |
| Make HIV testing a routine part of pregnancy care. Test as early as possible in pregnancy and retest those at high risk in the third trimester. |
| Refer those who are HIV-positive to the local public health department for case management. |
| Work collaboratively with the public health department to insure that people with HIV infection receive medical care and social services. |
| Reinforce the message to those infected about how to avoid transmitting the infection to others. |
| Counsel uninfected patients who practice high-risk behaviors about how to reduce their risks of infection, using patient centered methods. |
| Promptly diagnose and treat other sexually transmitted infections. |
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). National Center for HIV, STD, and TB Prevention. Advancing HIV prevention: the science behind the new initiative. Available at: www.cdc.gov/hiv/partners/ahp_science.htm.
2. Hays RB, Paul J, Ekstrand M, Kegeles SM, et al. Actual versus perceived HIV status, sexual behaviors and predictors of unprotected sex among young gay and bisexual men who identify as HIV-negative, HIV-positive and untested. AIDS 1997;11:1495-1502.
3. CDC. Primary and secondary syphilis among men who have sex with men – New York City, 2001. MMWR Morb Mortal Wkly Rep 2002;51:853-856.
4. CDC. Resurgent bacterial sexually transmitted disease among men who have sex with men—King County, Washington, 1997–1999. MMWR Morb Mortal Wkly Rep 1999;48:773-777.
5. Institute of Medicine No Time to Lose: Getting More from HIV Prevention. Committee on HIV Prevention Strategies in the United States, Division of Health Promotion and Disease Prevention. Washington, DC: National Academies Press; 2001.
6. CDC. Cases of HIV infection and AIDS in the United States, 2002. HIV/AIDS Surveillance Report 2002;14:1-58.Available at: www.cdc.gov/hiv/stats/hasr1402.htm.
7. Kamb ML, Fishbein M, Douglas JM, et al. efficacy of risk reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. JAMA 1998;280:1161-1167.
8. Wenger NS, Kussling FS, Beck K, Shapiro MF. Sexual behavior of individuals infected with the human immunodeficiency virus: The need for intervention. Arch Int Med 1994;154:1849-1854.
9. Kilmarx PH, Hamers FF, Peterman TA. Living with HIV. Experiences and perspectives of HIV-infected sexually transmitted disease clinic patients after posttest counseling. Sex Transm Dis 1998;25:28-37.
10. Higgins DL, Galavotti C, O’Reilly KE, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA 1991;266:2419-2429
11. Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health 1999;89:1397-1405.
12. Kamb ML, Fishbein M, Douglas JM, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases. JAMA 1998;280:1161-1167.
13. Walensky RP, Losina E, Steger-Craven KA, Freedberg KA. Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary inpatient testing. Arch Intern Med 2002;162:887-892.
14. CDC. Revised recommendations for HIV screening of pregnant women. MMWR Recomm Rep 2001;50(RR-19):59-86.
15. CDC. Notice to readers: Approval of a new rapid test for HIV antibody. MMWR Morn Mortal Wkly Rep 2002;51:1051-1052.
1. Centers for Disease Control and Prevention (CDC). National Center for HIV, STD, and TB Prevention. Advancing HIV prevention: the science behind the new initiative. Available at: www.cdc.gov/hiv/partners/ahp_science.htm.
2. Hays RB, Paul J, Ekstrand M, Kegeles SM, et al. Actual versus perceived HIV status, sexual behaviors and predictors of unprotected sex among young gay and bisexual men who identify as HIV-negative, HIV-positive and untested. AIDS 1997;11:1495-1502.
3. CDC. Primary and secondary syphilis among men who have sex with men – New York City, 2001. MMWR Morb Mortal Wkly Rep 2002;51:853-856.
4. CDC. Resurgent bacterial sexually transmitted disease among men who have sex with men—King County, Washington, 1997–1999. MMWR Morb Mortal Wkly Rep 1999;48:773-777.
5. Institute of Medicine No Time to Lose: Getting More from HIV Prevention. Committee on HIV Prevention Strategies in the United States, Division of Health Promotion and Disease Prevention. Washington, DC: National Academies Press; 2001.
6. CDC. Cases of HIV infection and AIDS in the United States, 2002. HIV/AIDS Surveillance Report 2002;14:1-58.Available at: www.cdc.gov/hiv/stats/hasr1402.htm.
7. Kamb ML, Fishbein M, Douglas JM, et al. efficacy of risk reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: A randomized controlled trial. JAMA 1998;280:1161-1167.
8. Wenger NS, Kussling FS, Beck K, Shapiro MF. Sexual behavior of individuals infected with the human immunodeficiency virus: The need for intervention. Arch Int Med 1994;154:1849-1854.
9. Kilmarx PH, Hamers FF, Peterman TA. Living with HIV. Experiences and perspectives of HIV-infected sexually transmitted disease clinic patients after posttest counseling. Sex Transm Dis 1998;25:28-37.
10. Higgins DL, Galavotti C, O’Reilly KE, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA 1991;266:2419-2429
11. Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health 1999;89:1397-1405.
12. Kamb ML, Fishbein M, Douglas JM, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases. JAMA 1998;280:1161-1167.
13. Walensky RP, Losina E, Steger-Craven KA, Freedberg KA. Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary inpatient testing. Arch Intern Med 2002;162:887-892.
14. CDC. Revised recommendations for HIV screening of pregnant women. MMWR Recomm Rep 2001;50(RR-19):59-86.
15. CDC. Notice to readers: Approval of a new rapid test for HIV antibody. MMWR Morn Mortal Wkly Rep 2002;51:1051-1052.
Infection control in the outpatient setting
Microbial antibiotic resistance, emergence of infectious diseases against which there are no treatments or vaccines, and the persisting possibility of intentional release of infectious agents have made the prevention of infectious disease transmission a top public health concern.
It is easy to overlook the potential for spreading infectious diseases in the outpatient setting. Relying on healthcare workers to practice good hygiene is unlikely to be enough. This public health battle must employ a comprehensive plan for clinic design, involving staff in setting and enforcing policies, and repeatedly emphasizing the importance of good hygiene.
Reasons for concern
Outpatient clinical settings are a prime location for the spread of infectious diseases, to staff and patients. In the past, when measles was common, physician offices were the source of infection in a significant proportion of all cases. Last year, hospitals were a principal focus for the spread of serious acute respiratory syndrome (SARS). This year’s influenza season was complicated by doubts about vaccine effectiveness and the possibility of a more virulent strain, making prevention of disease transmission a primary concern.
As the Centers for Disease Control and Prevention (CDC) and state and local health departments struggle to contain these and other infectious diseases, physicians should insure that their facilities and systems are designed to prevent disease spread and, in so doing, set an example for the community. Physicians and office managers can take 4 basic steps:
- Institute and enforce policies on respiratory hygiene
- Institute and enforce policies on hand hygiene
- Immunize all office staff
- Establish triage policies that separate potentially contagious patients from the rest.
Respiratory hygiene
The concept of respiratory hygiene is not new, but it has not been a visible part of American culture. This is changing. The CDC has published recommendations on respiratory hygiene and cough etiquette for healthcare settings (Table).1 As the public becomes aware of the dangers of respiratory diseases, these recommendations may become the norm of common courtesy.
TABLE
Respiratory hygiene in healthcare settings Visual alerts
| Visual alerts |
| Signs at outpatient entrances asking patients and companions to inform office staff if they have symptoms of a respiratory infection. |
| Signs describing expectations regarding respiratory hygiene. |
| Respiratory hygiene expectations |
| Covering mouth and nose when coughing or sneezing. |
| Using tissues to contain respiratory secretions and disposing them in a receptacle. |
| Performing hand hygiene after contact with respiratory secretions. |
| Respiratory hygiene measures |
| Offering procedure masks (with ear loops) or surgical masks (with ties) to those who are coughing. |
| Requesting those with respiratory symptoms to sit in a separate location. |
| Providing tissues and no-touch receptacles for tissue disposal. |
| Providing dispensers of alcohol-based hand rub, or sinks with soap and disposable towels for hand washing. |
| Advising healthcare personnel to wear a surgical or procedure mask when examining a patient with symptoms of a respiratory infection. |
Hand hygiene
Hand hygiene has also recently received more attention, and a variety of new products can assist healthcare professionals with this task. The CDC recommends physicians should use an alcohol-based hand rub or an antimicrobial soap for routinely decontaminating hands.2 If hands are visibly dirty or soiled they should be washed with soap (either antimicrobial or non-antimicrobial) and water. Situations in which hands should be decontaminated:
- Before direct patient contact
- After contact with a patient’s skin
- After gloved contact with body fluids, mucous membranes, and dressings
- After contact with contaminated medical equipment
- Before and after gloving for procedures.
Alcohol-based products are preferred for decontamination; antimicrobial soaps are acceptable, but neither works well against bacterial spores. In the rare instance when exposure to anthrax spores is suspected from a suspicious powder, hand washing with soap and water is recommended. Consult CDC guidelines for hand hygiene for a description of antiseptic agents and their activity against different infectious agents.2
Immunization
Immunization of physicians and others on the healthcare team is important for the protection of staff and patients. The CDC strongly recommends3 that all healthcare workers, except with contraindications, receive the following:
- Influenza vaccine annually
- Measles, mumps, and rubella vaccine or proof of immunity, including birth before 1957, lab evidence of immunity, or proof of immunization with 2 doses of live measles separated by at least 28 days, 1 dose of live rubella, and 1 dose of live mumps vaccine, all on or after the first birthday
- Varicella vaccine, or proof of immunity including a reliable history of varicella infection, laboratory evidence of immunity, or proof of immunization. Adequate immunization is 1 dose of varicella vaccine, if administered before age 13, or 2 doses at least a month apart if administered after age 13.
In addition, CDC recommends hepatitis B vaccine for all healthcare workers who have contact with or exposure to blood and body fluids. Two months after the completion of a 3-dose series, those with risks of injury from needle sticks or sharp instruments should be tested for antibody to hepatitis B surface antigen. If they have not developed any antibody, they should be checked for hepatitis B surface antigen; if results are negative, they should receive a second 3-dose series.
Guidelines should also be in place for management of healthcare workers exposed to certain infectious diseases at work, including tuberculosis, blood-borne pathogens, varicella, and others.
Triage policies
Physicians and clinic managers should consider implementing triage policies to separate infectious patients from others when they arrive at the facility. If respiratory hygiene measures are followed, asking those with common respiratory infections sit in common waiting areas and using common exam rooms is acceptable practice.
Patients with rash and fever present a different problem. Diseases with this presentation include measles, rubella, and varicella—all highly contagious and with potentially serious effects, especially in the immune compromised. Smallpox and monkeypox can also present with these symptoms, although the likelihood is remote. Consider triaging rash and fever patients immediately to a “rash room” and keeping them confined there until the clinical diagnosis is clarified. The best practice is to have a separate entrance and exit location for such patients.
Should one of the more serious diseases remain a possibility after evaluation, further measures might be necessary including avoidance of use of the rash room until disinfected and consultation with the local health department about recommendations for post exposure measures for staff and patients.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected]
1. CDC. Respiratory hygiene/cough etiquette in healthcare settings. Available at: www.cdc.gov/flu/professionals/pdf/resphygiene.pdf. Accessed on May 12, 2004.
2. CDC. Guideline for hand hygiene in healthcare settings. MMWR Recomm Rep 2002; 51(RR-16). Available at www.cdc.gov/mmwr/PDF/RR/RR5116.pdf. Accessed on May 12, 2004.
3. CDC. Immunization of healthcare workers: recommendations of the advisory committee on immunization practices and the hospital infection control practices advisory committee. MMWR Recomm Rep 1997: 46 (RR-18). Available at www.cdc.gov/mmwr/PDF/RR/RR4618.pdf. Accessed on May 12, 2004.
Microbial antibiotic resistance, emergence of infectious diseases against which there are no treatments or vaccines, and the persisting possibility of intentional release of infectious agents have made the prevention of infectious disease transmission a top public health concern.
It is easy to overlook the potential for spreading infectious diseases in the outpatient setting. Relying on healthcare workers to practice good hygiene is unlikely to be enough. This public health battle must employ a comprehensive plan for clinic design, involving staff in setting and enforcing policies, and repeatedly emphasizing the importance of good hygiene.
Reasons for concern
Outpatient clinical settings are a prime location for the spread of infectious diseases, to staff and patients. In the past, when measles was common, physician offices were the source of infection in a significant proportion of all cases. Last year, hospitals were a principal focus for the spread of serious acute respiratory syndrome (SARS). This year’s influenza season was complicated by doubts about vaccine effectiveness and the possibility of a more virulent strain, making prevention of disease transmission a primary concern.
As the Centers for Disease Control and Prevention (CDC) and state and local health departments struggle to contain these and other infectious diseases, physicians should insure that their facilities and systems are designed to prevent disease spread and, in so doing, set an example for the community. Physicians and office managers can take 4 basic steps:
- Institute and enforce policies on respiratory hygiene
- Institute and enforce policies on hand hygiene
- Immunize all office staff
- Establish triage policies that separate potentially contagious patients from the rest.
Respiratory hygiene
The concept of respiratory hygiene is not new, but it has not been a visible part of American culture. This is changing. The CDC has published recommendations on respiratory hygiene and cough etiquette for healthcare settings (Table).1 As the public becomes aware of the dangers of respiratory diseases, these recommendations may become the norm of common courtesy.
TABLE
Respiratory hygiene in healthcare settings Visual alerts
| Visual alerts |
| Signs at outpatient entrances asking patients and companions to inform office staff if they have symptoms of a respiratory infection. |
| Signs describing expectations regarding respiratory hygiene. |
| Respiratory hygiene expectations |
| Covering mouth and nose when coughing or sneezing. |
| Using tissues to contain respiratory secretions and disposing them in a receptacle. |
| Performing hand hygiene after contact with respiratory secretions. |
| Respiratory hygiene measures |
| Offering procedure masks (with ear loops) or surgical masks (with ties) to those who are coughing. |
| Requesting those with respiratory symptoms to sit in a separate location. |
| Providing tissues and no-touch receptacles for tissue disposal. |
| Providing dispensers of alcohol-based hand rub, or sinks with soap and disposable towels for hand washing. |
| Advising healthcare personnel to wear a surgical or procedure mask when examining a patient with symptoms of a respiratory infection. |
Hand hygiene
Hand hygiene has also recently received more attention, and a variety of new products can assist healthcare professionals with this task. The CDC recommends physicians should use an alcohol-based hand rub or an antimicrobial soap for routinely decontaminating hands.2 If hands are visibly dirty or soiled they should be washed with soap (either antimicrobial or non-antimicrobial) and water. Situations in which hands should be decontaminated:
- Before direct patient contact
- After contact with a patient’s skin
- After gloved contact with body fluids, mucous membranes, and dressings
- After contact with contaminated medical equipment
- Before and after gloving for procedures.
Alcohol-based products are preferred for decontamination; antimicrobial soaps are acceptable, but neither works well against bacterial spores. In the rare instance when exposure to anthrax spores is suspected from a suspicious powder, hand washing with soap and water is recommended. Consult CDC guidelines for hand hygiene for a description of antiseptic agents and their activity against different infectious agents.2
Immunization
Immunization of physicians and others on the healthcare team is important for the protection of staff and patients. The CDC strongly recommends3 that all healthcare workers, except with contraindications, receive the following:
- Influenza vaccine annually
- Measles, mumps, and rubella vaccine or proof of immunity, including birth before 1957, lab evidence of immunity, or proof of immunization with 2 doses of live measles separated by at least 28 days, 1 dose of live rubella, and 1 dose of live mumps vaccine, all on or after the first birthday
- Varicella vaccine, or proof of immunity including a reliable history of varicella infection, laboratory evidence of immunity, or proof of immunization. Adequate immunization is 1 dose of varicella vaccine, if administered before age 13, or 2 doses at least a month apart if administered after age 13.
In addition, CDC recommends hepatitis B vaccine for all healthcare workers who have contact with or exposure to blood and body fluids. Two months after the completion of a 3-dose series, those with risks of injury from needle sticks or sharp instruments should be tested for antibody to hepatitis B surface antigen. If they have not developed any antibody, they should be checked for hepatitis B surface antigen; if results are negative, they should receive a second 3-dose series.
Guidelines should also be in place for management of healthcare workers exposed to certain infectious diseases at work, including tuberculosis, blood-borne pathogens, varicella, and others.
Triage policies
Physicians and clinic managers should consider implementing triage policies to separate infectious patients from others when they arrive at the facility. If respiratory hygiene measures are followed, asking those with common respiratory infections sit in common waiting areas and using common exam rooms is acceptable practice.
Patients with rash and fever present a different problem. Diseases with this presentation include measles, rubella, and varicella—all highly contagious and with potentially serious effects, especially in the immune compromised. Smallpox and monkeypox can also present with these symptoms, although the likelihood is remote. Consider triaging rash and fever patients immediately to a “rash room” and keeping them confined there until the clinical diagnosis is clarified. The best practice is to have a separate entrance and exit location for such patients.
Should one of the more serious diseases remain a possibility after evaluation, further measures might be necessary including avoidance of use of the rash room until disinfected and consultation with the local health department about recommendations for post exposure measures for staff and patients.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected]
Microbial antibiotic resistance, emergence of infectious diseases against which there are no treatments or vaccines, and the persisting possibility of intentional release of infectious agents have made the prevention of infectious disease transmission a top public health concern.
It is easy to overlook the potential for spreading infectious diseases in the outpatient setting. Relying on healthcare workers to practice good hygiene is unlikely to be enough. This public health battle must employ a comprehensive plan for clinic design, involving staff in setting and enforcing policies, and repeatedly emphasizing the importance of good hygiene.
Reasons for concern
Outpatient clinical settings are a prime location for the spread of infectious diseases, to staff and patients. In the past, when measles was common, physician offices were the source of infection in a significant proportion of all cases. Last year, hospitals were a principal focus for the spread of serious acute respiratory syndrome (SARS). This year’s influenza season was complicated by doubts about vaccine effectiveness and the possibility of a more virulent strain, making prevention of disease transmission a primary concern.
As the Centers for Disease Control and Prevention (CDC) and state and local health departments struggle to contain these and other infectious diseases, physicians should insure that their facilities and systems are designed to prevent disease spread and, in so doing, set an example for the community. Physicians and office managers can take 4 basic steps:
- Institute and enforce policies on respiratory hygiene
- Institute and enforce policies on hand hygiene
- Immunize all office staff
- Establish triage policies that separate potentially contagious patients from the rest.
Respiratory hygiene
The concept of respiratory hygiene is not new, but it has not been a visible part of American culture. This is changing. The CDC has published recommendations on respiratory hygiene and cough etiquette for healthcare settings (Table).1 As the public becomes aware of the dangers of respiratory diseases, these recommendations may become the norm of common courtesy.
TABLE
Respiratory hygiene in healthcare settings Visual alerts
| Visual alerts |
| Signs at outpatient entrances asking patients and companions to inform office staff if they have symptoms of a respiratory infection. |
| Signs describing expectations regarding respiratory hygiene. |
| Respiratory hygiene expectations |
| Covering mouth and nose when coughing or sneezing. |
| Using tissues to contain respiratory secretions and disposing them in a receptacle. |
| Performing hand hygiene after contact with respiratory secretions. |
| Respiratory hygiene measures |
| Offering procedure masks (with ear loops) or surgical masks (with ties) to those who are coughing. |
| Requesting those with respiratory symptoms to sit in a separate location. |
| Providing tissues and no-touch receptacles for tissue disposal. |
| Providing dispensers of alcohol-based hand rub, or sinks with soap and disposable towels for hand washing. |
| Advising healthcare personnel to wear a surgical or procedure mask when examining a patient with symptoms of a respiratory infection. |
Hand hygiene
Hand hygiene has also recently received more attention, and a variety of new products can assist healthcare professionals with this task. The CDC recommends physicians should use an alcohol-based hand rub or an antimicrobial soap for routinely decontaminating hands.2 If hands are visibly dirty or soiled they should be washed with soap (either antimicrobial or non-antimicrobial) and water. Situations in which hands should be decontaminated:
- Before direct patient contact
- After contact with a patient’s skin
- After gloved contact with body fluids, mucous membranes, and dressings
- After contact with contaminated medical equipment
- Before and after gloving for procedures.
Alcohol-based products are preferred for decontamination; antimicrobial soaps are acceptable, but neither works well against bacterial spores. In the rare instance when exposure to anthrax spores is suspected from a suspicious powder, hand washing with soap and water is recommended. Consult CDC guidelines for hand hygiene for a description of antiseptic agents and their activity against different infectious agents.2
Immunization
Immunization of physicians and others on the healthcare team is important for the protection of staff and patients. The CDC strongly recommends3 that all healthcare workers, except with contraindications, receive the following:
- Influenza vaccine annually
- Measles, mumps, and rubella vaccine or proof of immunity, including birth before 1957, lab evidence of immunity, or proof of immunization with 2 doses of live measles separated by at least 28 days, 1 dose of live rubella, and 1 dose of live mumps vaccine, all on or after the first birthday
- Varicella vaccine, or proof of immunity including a reliable history of varicella infection, laboratory evidence of immunity, or proof of immunization. Adequate immunization is 1 dose of varicella vaccine, if administered before age 13, or 2 doses at least a month apart if administered after age 13.
In addition, CDC recommends hepatitis B vaccine for all healthcare workers who have contact with or exposure to blood and body fluids. Two months after the completion of a 3-dose series, those with risks of injury from needle sticks or sharp instruments should be tested for antibody to hepatitis B surface antigen. If they have not developed any antibody, they should be checked for hepatitis B surface antigen; if results are negative, they should receive a second 3-dose series.
Guidelines should also be in place for management of healthcare workers exposed to certain infectious diseases at work, including tuberculosis, blood-borne pathogens, varicella, and others.
Triage policies
Physicians and clinic managers should consider implementing triage policies to separate infectious patients from others when they arrive at the facility. If respiratory hygiene measures are followed, asking those with common respiratory infections sit in common waiting areas and using common exam rooms is acceptable practice.
Patients with rash and fever present a different problem. Diseases with this presentation include measles, rubella, and varicella—all highly contagious and with potentially serious effects, especially in the immune compromised. Smallpox and monkeypox can also present with these symptoms, although the likelihood is remote. Consider triaging rash and fever patients immediately to a “rash room” and keeping them confined there until the clinical diagnosis is clarified. The best practice is to have a separate entrance and exit location for such patients.
Should one of the more serious diseases remain a possibility after evaluation, further measures might be necessary including avoidance of use of the rash room until disinfected and consultation with the local health department about recommendations for post exposure measures for staff and patients.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected]
1. CDC. Respiratory hygiene/cough etiquette in healthcare settings. Available at: www.cdc.gov/flu/professionals/pdf/resphygiene.pdf. Accessed on May 12, 2004.
2. CDC. Guideline for hand hygiene in healthcare settings. MMWR Recomm Rep 2002; 51(RR-16). Available at www.cdc.gov/mmwr/PDF/RR/RR5116.pdf. Accessed on May 12, 2004.
3. CDC. Immunization of healthcare workers: recommendations of the advisory committee on immunization practices and the hospital infection control practices advisory committee. MMWR Recomm Rep 1997: 46 (RR-18). Available at www.cdc.gov/mmwr/PDF/RR/RR4618.pdf. Accessed on May 12, 2004.
1. CDC. Respiratory hygiene/cough etiquette in healthcare settings. Available at: www.cdc.gov/flu/professionals/pdf/resphygiene.pdf. Accessed on May 12, 2004.
2. CDC. Guideline for hand hygiene in healthcare settings. MMWR Recomm Rep 2002; 51(RR-16). Available at www.cdc.gov/mmwr/PDF/RR/RR5116.pdf. Accessed on May 12, 2004.
3. CDC. Immunization of healthcare workers: recommendations of the advisory committee on immunization practices and the hospital infection control practices advisory committee. MMWR Recomm Rep 1997: 46 (RR-18). Available at www.cdc.gov/mmwr/PDF/RR/RR4618.pdf. Accessed on May 12, 2004.
How should we evaluate a solitary pulmonary nodule found on chest x-ray?
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When is a CT scan indicated to examine a solitary pulmonary nodule found on a chest x-ray film?
- Is there an indication for positron-emission tomography scanning?
- When should a biopsy be performed?
- What is the best biopsy method?
In January 2003 the American College of Chest Physicians Expert Panel on Lung Cancer Guidelines released its guideline on evaluating a solitary pulmonary nodule (SPN), an intraparenchymal lung lesion <3 cm in diameter unassociated with atelectasis or adenopathy. The objectives of this guideline were to define appropriate evidence-based practices for imaging and diagnostic tests, as well as indications for obtaining a tissue evaluation for the patient with a SPN. This expert panel included physicians from nuclear medicine, oncology, pulmonary medicine, radiology, and thoracic surgery. The major recommendations were summarized in the National Guideline Clearinghouse (available at www.guideline.gov).
The evidence categories for this guideline are diagnosis and management. Outcomes considered were sensitivity and specificity of diagnostic tests and diagnostic yield. No cost analysis was performed.
The committee used a complex recommendation rating scheme (A, B, C, D, I) after comparing levels of evidence (good, fair, or poor) compared with net benefits (substantial, moderate, small/weak, or none). The scheme was then revised to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine.
Guideline relevance and limitations
Solitary pulmonary nodules are discovered in 150,000 patients per year, and a delay in performing diagnostic studies can have dire consequences for those whose nodule proves malignant.
The guideline is weakened by the lack of a cost-effectiveness analysis.
A lengthy bibliography accompanies the guideline, but the support document does not provide evidence tables.
Guideline development and evidence review
Computerized bibliographic databases including Medline, Cancerlit, CINAHL, HealthStar, the Cochrane Collaboration Database of Abstracts of Reviews of Effectiveness, the National Guideline Clearinghouse, and the National Cancer Institute Physician Data Query database were searched for existing evidence. Priority was given to secondary sources including guidelines, systematic reviews, and meta-analyses. Search terms were lung neoplasms or bronchial neoplasms. Reference lists of review articles were also studied for additional evidence. There were 55 references.
Source for this guideline
Tan BB, Flaherty KR, Kazerooni EA, Iannettoni MD. The solitary pulmonary nodule. Chest 2003; 123(1 suppl):89S–96S.
Other guidelines on solitary pulmonary nodules
ACR Appropriateness Criteria™ for work-up of the solitary pulmonary nodule (SPN). 1995 (revised 2000). This guideline is one in a series of guidelines developed by the American College of Radiology. It ranks the utility of various diagnostic testing modalities based on evidence. This guideline is complex, because there are several “variants” based on the size of the lesion (≥1 cm or ≤1 cm) and the clinical suspicion of cancer (low, moderate to high). The clinical utility for primary care physicians is limited.
Source: Henschke CI, Yankelevitz D, Westcott J, et al. Work-up of the solitary pulmonary nodule. American College of Radiology. ACR Appropriateness Criteria. Radiology 2000; 215(Suppl):607–609. (19 references)
Diagnosis
- A solitary pulmonary nodule (SPN) with benign central calcification does not require further diagnostic testing (A).
- Spiral chest computed tomography (CT) scan with contrast should be performed for new SPNs (B).
- Review all previous chest x-rays when a SPN is found (C).
- Magnetic resonance imaging (MRI) is not indicated (D).
- Positron-emission tomography (PET) scan is not recommended for SPN <1 cm in size (D).
Management and follow-up evaluations
- Lymph node dissection should be performed for all pulmonary resections (A).
- If a wedge resection is not possible, a diagnostic lobectomy is an acceptable alternative (A).
- SPN that does not change on chest x-ray after 2 years of follow-up requires no further evaluation (B).
- PET scan of the chest with 18-fluorodeoxyglucose, might be considered preoperatively for SPN patients who are surgical candidates and have a negative mediastinal chest CT (B).
- Chest x-ray and chest CT scanning at 3, 6, 12, and 24 months should be performed for patients who are not good surgical candidates (B).
- An alternative to surgical intervention is percutaneous transthoracic needle aspiration (TTNA) or transbronchial needle biopsy for patients who refuse surgery (B).
- High surgical risk patients may be candidates for TTNA (B).
- Wedge resection followed by lobectomy is appropriate for pathology positive for cancer (B).
- Wedge resection or segmentectomy may be appropriate for marginal surgical candidates (B).
- Without a definitive tissue diagnosis, follow-up for 2 years is recommended with chest x-ray and chest CT (at 3, 6, 12, and 24 months) (C).
- Marginal surgical candidates who have a negative PET scan should have a CT scan at least in 3 months (C).
- For patients who are surgical candidates, TTNA is not indicated (D).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
What the new Medicare prescription drug bill may mean for providers and patients
In November 2003, President Bush signed the Medicare prescription-drug bill, which will usher in the largest change in the Medicare program in terms of money and number of people affected since the program’s creation in 1965. The final version of the bill was controversial, passing by a small margin in both the House and Senate.
Conservatives criticized the bill for not giving a large enough role to the private sector as an alternative to the traditional Medicare program, for spending too much money, and for risking even larger budget deficits than already predicted.
Liberals criticized it for providing an inadequate drug benefit, for allowing the prescription program to be run by private industry, and for creating an experimental private-sector program that will compete with traditional Medicare.
In the end, passage was ensured with support from the American Association of Retired Persons (AARP), drug companies, private health insurers, and national medical groups—and with the usual political maneuvering.
Public support among seniors and other groups remains unclear. For example, the American Academy of Family Physicians supported the bill, but negative reaction by members led President Michael Fleming to write a letter explaining the reasons for the decision (www.aafp.org/medicareletter.xml). In addition, Republican concerns about the overall cost of the legislation seem borne out by the administration’s recent announcement projecting costs of $530 billion over 10 years, about one third more than the price tag used to convince Congress to pass the legislation about 2 months before.
This article reviews the bill and some of its health policy implications.
Not all details clear; more than drug benefits affected
Several generalizations about Federal legislation hold true with this bill.
First, while the bill establishes the intent of Congress, a number of details will not be made clear until it is implemented by the executive branch—the administration and the responsible cabinet departments such as the Center for Medicare and Medicaid Services. The importance of these implementation details is most relevant to the prescription drug benefit section of the bill.
Second, the bill changes or adds programs in a number of health areas besides prescription drugs (see Supplementary changes with the Medicare prescription drug bill). These additions partly reflected the need of proponents to satisfy diverse special interests (private insurers, hospitals and physicians, rural areas) and thereby gain their support for other parts of the bill that were more controversial, principally the drug benefit and private competition for Medicare. Thus, there is funding to increase Medicare payments to physicians and rural hospitals and to hospitals serving large numbers of low-income patients.
- Medicare payments to rural hospitals and doctors increase by $25 billion over 10 years.
- Payments to hospitals serving large numbers of low-income patients would increase.
- Hospitals can avoid some future cuts in Medicare payments by submitting quality of care data to the government.
- Doctors would receive increases of 1.5% per year in Medicare payments for 2004 and 2005 rather than the cuts currently planned.
- Medicare would cover an initial physical for new beneficiaries and screening for diabetes and cardiovascular disease.
- Support for development of health savings accounts that allow people with high-deductible health insurance to shelter income from taxes and obtain tax deductions if the money is used for health expenses.
- Home health agencies would see cuts in payments, but patient co-pays would not be required.
- Medicare Part B premiums (for physician and outpatient services) would be greater for those with incomes over $80,000.
Third, the changes also reflect genuine goals of improving health by expanding Medicare coverage of preventive services and requiring participating hospitals to submit quality-of-care data.
Prescription drug coverage under the new bill
Although many seniors have drug coverage through retirement health plans or Medigap policies purchased privately, about one quarter of beneficiaries (some 10 million people) do not have such coverage. Even those with drug coverage may have difficulty affording recommended medications since the median income for a senior is little more than $23,000. Many physicians have seen the ill effects of seniors not filling their prescriptions or skipping doses of prescribed medications.
Until the benefit takes effect. The actual prescription drug benefit will not begin until 2006. Until then, Medicare recipients will be given the option of purchasing a drug-discount card for $30 per year starting this spring. It is estimated these cards may save 10% to 15% of prescription costs. In addition, low-income seniors will receive $600 per year toward drug purchases.
After it takes effect. The drug benefit starting in 2006 will be funded through a complex arrangement of patient and government payments (Figure).
- Premium: A premium estimated to begin at $35 per month.
- Deductible: An annual deductible starting at $250 and indexed to increase to $445 in 2013.
- Co-pay: After paying the deductible, enrollees will pay 25% of additional drug costs up to $2250, at which point a $2850 gap in cover-age—the so-called “doughut hole”—leaves the onus of payment with the patient until $5100 is reached.
- Catastrophic coverage: After $5100, patients will pay 5% of any additional annual drug costs.
In 2006, catastrophic coverage will begin after $3600 in out-of-pocket costs ($250 deductible + $500 in co-pays to $2250 + the $2850 doughnut gap), not counting the premium. Indexing provisions are projected to raise this out-of-pocket cost requirement to $6400 in 2013. These indexing features have received less attention in the media, but may become increasingly important to seniors. For lower-income individuals, as determined by specific yearly income and total assets guidelines, a small per-prescription fee will replace the premium, deductible, and doughnut hole gap payments.
Coverage will vary. As the yearly cost of drugs changes, so will the relative contributions made by the patient and the government (Table). The new bill provides substantial benefit to those with catastrophic drug costs and to very low-income seniors. The idea of linking payments to income (for the drug benefit and the Part B premium) is a change in the Medicare program, as it has traditionally provided the same benefit to all beneficiaries, regardless of income.
Expected effects of privatization. The manner in which the drug benefit will be administered was controversial in Congress. The legislation, written primarily by Republicans, provides that beneficiaries can obtain coverage by participating with an HMO or PPO or by purchasing standalone coverage through a private prescription drug insurance program. Patient enrollment is voluntary. Managed care plans would be encouraged to participate in the prescription benefit program through eligibility for government subsidies. In turn, more beneficiaries would be encouraged to choose managed care plans, thus decreasing the number of patients covered by traditional Medicare. Furthermore, current private Medigap supplemental plans will be barred from offering drug benefits.
With the HMO/PPO and stand-alone programs, it is likely that any reduction in drug costs will result from private pharmacy benefit managers negotiating discounts from drug companies as they do now for many employer-sponsored plans. Presumably, formularies will vary from plan to plan, and it may be difficult for patients to know ahead of time whether the plan they join will cover their current medications. The legislation prohibits the government from using its vast purchasing power to negotiate substantial discounts from drug companies as it does now for the Medicaid program. There are provisions to increase availability of generic drugs, but importation of drugs from Canada is prohibited unless FDA approval is given (so far, the FDA has opposed this). Many Democrats who opposed the bill argued that it allowed too large a role for the private sector and constrained the ability of the government to control drug costs.
A final controversial measure in the bill provided for conducting an experiment in 6 cities beginning in 2010, in which at least 1 private insurance plan would be funded to compete directly with the traditional Medicare program. Many Republicans believe this type of competition is necessary to decrease the rate of cost increases in the Medicare program, while many Democrats believe the private market is a big reason for increasing problems with the quality and cost of the entire health care system.
FIGURE
Out-of-pocket spending under new legislation
Out-of-pocket drug spending in 2006 for Medicare beneficiaries under new Medicare legislation. Note: Benefit levels are indexed to growth in per capita expenditures for covered Part D drugs. As a result, the Part D deductible in projected to increase from $250 in 2006 to $445 in 2013; the catastrophic threshold is projected to increase from $5100 in 2006 to $9066 in 2013. From the Kaiser Family Foundation website(www.kff.org/medicare/medicarebenefitataglance.ctm).
Looming questions
The new Medicare legislation is vast in scope, cost, and controversy. In the coming months, a number of organizations—AARP, the Department of Health and Human Services, and various foundations—will attempt to explain its provisions to the public, most likely in different ways.
TABLE
Deciphering the 2006 drug benefit
The chart above shows what portion of yearly drug costs would be paid by the Medicare recipient and what portion would be paid by Medicare beginning in 2006. It does not include the $420 yearly premium.Family physicians may be asked by patients to explain provisions of the program and to offer advice in making decisions about their participation.
In addition, preoccupation with explaining and implementing the Medicare bill may keep Congress and the President from addressing other pressing health issues such as the growing number of uninsured.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Pear R. Bush’s aides put higher price tag on Medicare law. New York Times, January 30, 2004.
2. Altman D. The new Medicare prescription-drug legislation. N Engl J Med 2004;350:9-10
3. American Academy of Family Physicians. Medicare Prescription Drug, Improvement and Modernization Act. Available at: www.aafp.org/x25558.xml. Accessed on April 2, 2004.
4. National Association of Chain Drug Stores. Medicare Prescription Drug Benefit and Discount Card Program Q & A. Available at: www.nacds.org/user-assets/PDF_files/MedicareRx_Q&A.pdf. Accessed on April 2, 2004.
5. New Medicare law/key provisions. Christian Science Monitor, December 4, 2003
In November 2003, President Bush signed the Medicare prescription-drug bill, which will usher in the largest change in the Medicare program in terms of money and number of people affected since the program’s creation in 1965. The final version of the bill was controversial, passing by a small margin in both the House and Senate.
Conservatives criticized the bill for not giving a large enough role to the private sector as an alternative to the traditional Medicare program, for spending too much money, and for risking even larger budget deficits than already predicted.
Liberals criticized it for providing an inadequate drug benefit, for allowing the prescription program to be run by private industry, and for creating an experimental private-sector program that will compete with traditional Medicare.
In the end, passage was ensured with support from the American Association of Retired Persons (AARP), drug companies, private health insurers, and national medical groups—and with the usual political maneuvering.
Public support among seniors and other groups remains unclear. For example, the American Academy of Family Physicians supported the bill, but negative reaction by members led President Michael Fleming to write a letter explaining the reasons for the decision (www.aafp.org/medicareletter.xml). In addition, Republican concerns about the overall cost of the legislation seem borne out by the administration’s recent announcement projecting costs of $530 billion over 10 years, about one third more than the price tag used to convince Congress to pass the legislation about 2 months before.
This article reviews the bill and some of its health policy implications.
Not all details clear; more than drug benefits affected
Several generalizations about Federal legislation hold true with this bill.
First, while the bill establishes the intent of Congress, a number of details will not be made clear until it is implemented by the executive branch—the administration and the responsible cabinet departments such as the Center for Medicare and Medicaid Services. The importance of these implementation details is most relevant to the prescription drug benefit section of the bill.
Second, the bill changes or adds programs in a number of health areas besides prescription drugs (see Supplementary changes with the Medicare prescription drug bill). These additions partly reflected the need of proponents to satisfy diverse special interests (private insurers, hospitals and physicians, rural areas) and thereby gain their support for other parts of the bill that were more controversial, principally the drug benefit and private competition for Medicare. Thus, there is funding to increase Medicare payments to physicians and rural hospitals and to hospitals serving large numbers of low-income patients.
- Medicare payments to rural hospitals and doctors increase by $25 billion over 10 years.
- Payments to hospitals serving large numbers of low-income patients would increase.
- Hospitals can avoid some future cuts in Medicare payments by submitting quality of care data to the government.
- Doctors would receive increases of 1.5% per year in Medicare payments for 2004 and 2005 rather than the cuts currently planned.
- Medicare would cover an initial physical for new beneficiaries and screening for diabetes and cardiovascular disease.
- Support for development of health savings accounts that allow people with high-deductible health insurance to shelter income from taxes and obtain tax deductions if the money is used for health expenses.
- Home health agencies would see cuts in payments, but patient co-pays would not be required.
- Medicare Part B premiums (for physician and outpatient services) would be greater for those with incomes over $80,000.
Third, the changes also reflect genuine goals of improving health by expanding Medicare coverage of preventive services and requiring participating hospitals to submit quality-of-care data.
Prescription drug coverage under the new bill
Although many seniors have drug coverage through retirement health plans or Medigap policies purchased privately, about one quarter of beneficiaries (some 10 million people) do not have such coverage. Even those with drug coverage may have difficulty affording recommended medications since the median income for a senior is little more than $23,000. Many physicians have seen the ill effects of seniors not filling their prescriptions or skipping doses of prescribed medications.
Until the benefit takes effect. The actual prescription drug benefit will not begin until 2006. Until then, Medicare recipients will be given the option of purchasing a drug-discount card for $30 per year starting this spring. It is estimated these cards may save 10% to 15% of prescription costs. In addition, low-income seniors will receive $600 per year toward drug purchases.
After it takes effect. The drug benefit starting in 2006 will be funded through a complex arrangement of patient and government payments (Figure).
- Premium: A premium estimated to begin at $35 per month.
- Deductible: An annual deductible starting at $250 and indexed to increase to $445 in 2013.
- Co-pay: After paying the deductible, enrollees will pay 25% of additional drug costs up to $2250, at which point a $2850 gap in cover-age—the so-called “doughut hole”—leaves the onus of payment with the patient until $5100 is reached.
- Catastrophic coverage: After $5100, patients will pay 5% of any additional annual drug costs.
In 2006, catastrophic coverage will begin after $3600 in out-of-pocket costs ($250 deductible + $500 in co-pays to $2250 + the $2850 doughnut gap), not counting the premium. Indexing provisions are projected to raise this out-of-pocket cost requirement to $6400 in 2013. These indexing features have received less attention in the media, but may become increasingly important to seniors. For lower-income individuals, as determined by specific yearly income and total assets guidelines, a small per-prescription fee will replace the premium, deductible, and doughnut hole gap payments.
Coverage will vary. As the yearly cost of drugs changes, so will the relative contributions made by the patient and the government (Table). The new bill provides substantial benefit to those with catastrophic drug costs and to very low-income seniors. The idea of linking payments to income (for the drug benefit and the Part B premium) is a change in the Medicare program, as it has traditionally provided the same benefit to all beneficiaries, regardless of income.
Expected effects of privatization. The manner in which the drug benefit will be administered was controversial in Congress. The legislation, written primarily by Republicans, provides that beneficiaries can obtain coverage by participating with an HMO or PPO or by purchasing standalone coverage through a private prescription drug insurance program. Patient enrollment is voluntary. Managed care plans would be encouraged to participate in the prescription benefit program through eligibility for government subsidies. In turn, more beneficiaries would be encouraged to choose managed care plans, thus decreasing the number of patients covered by traditional Medicare. Furthermore, current private Medigap supplemental plans will be barred from offering drug benefits.
With the HMO/PPO and stand-alone programs, it is likely that any reduction in drug costs will result from private pharmacy benefit managers negotiating discounts from drug companies as they do now for many employer-sponsored plans. Presumably, formularies will vary from plan to plan, and it may be difficult for patients to know ahead of time whether the plan they join will cover their current medications. The legislation prohibits the government from using its vast purchasing power to negotiate substantial discounts from drug companies as it does now for the Medicaid program. There are provisions to increase availability of generic drugs, but importation of drugs from Canada is prohibited unless FDA approval is given (so far, the FDA has opposed this). Many Democrats who opposed the bill argued that it allowed too large a role for the private sector and constrained the ability of the government to control drug costs.
A final controversial measure in the bill provided for conducting an experiment in 6 cities beginning in 2010, in which at least 1 private insurance plan would be funded to compete directly with the traditional Medicare program. Many Republicans believe this type of competition is necessary to decrease the rate of cost increases in the Medicare program, while many Democrats believe the private market is a big reason for increasing problems with the quality and cost of the entire health care system.
FIGURE
Out-of-pocket spending under new legislation
Out-of-pocket drug spending in 2006 for Medicare beneficiaries under new Medicare legislation. Note: Benefit levels are indexed to growth in per capita expenditures for covered Part D drugs. As a result, the Part D deductible in projected to increase from $250 in 2006 to $445 in 2013; the catastrophic threshold is projected to increase from $5100 in 2006 to $9066 in 2013. From the Kaiser Family Foundation website(www.kff.org/medicare/medicarebenefitataglance.ctm).
Looming questions
The new Medicare legislation is vast in scope, cost, and controversy. In the coming months, a number of organizations—AARP, the Department of Health and Human Services, and various foundations—will attempt to explain its provisions to the public, most likely in different ways.
TABLE
Deciphering the 2006 drug benefit
The chart above shows what portion of yearly drug costs would be paid by the Medicare recipient and what portion would be paid by Medicare beginning in 2006. It does not include the $420 yearly premium.Family physicians may be asked by patients to explain provisions of the program and to offer advice in making decisions about their participation.
In addition, preoccupation with explaining and implementing the Medicare bill may keep Congress and the President from addressing other pressing health issues such as the growing number of uninsured.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
In November 2003, President Bush signed the Medicare prescription-drug bill, which will usher in the largest change in the Medicare program in terms of money and number of people affected since the program’s creation in 1965. The final version of the bill was controversial, passing by a small margin in both the House and Senate.
Conservatives criticized the bill for not giving a large enough role to the private sector as an alternative to the traditional Medicare program, for spending too much money, and for risking even larger budget deficits than already predicted.
Liberals criticized it for providing an inadequate drug benefit, for allowing the prescription program to be run by private industry, and for creating an experimental private-sector program that will compete with traditional Medicare.
In the end, passage was ensured with support from the American Association of Retired Persons (AARP), drug companies, private health insurers, and national medical groups—and with the usual political maneuvering.
Public support among seniors and other groups remains unclear. For example, the American Academy of Family Physicians supported the bill, but negative reaction by members led President Michael Fleming to write a letter explaining the reasons for the decision (www.aafp.org/medicareletter.xml). In addition, Republican concerns about the overall cost of the legislation seem borne out by the administration’s recent announcement projecting costs of $530 billion over 10 years, about one third more than the price tag used to convince Congress to pass the legislation about 2 months before.
This article reviews the bill and some of its health policy implications.
Not all details clear; more than drug benefits affected
Several generalizations about Federal legislation hold true with this bill.
First, while the bill establishes the intent of Congress, a number of details will not be made clear until it is implemented by the executive branch—the administration and the responsible cabinet departments such as the Center for Medicare and Medicaid Services. The importance of these implementation details is most relevant to the prescription drug benefit section of the bill.
Second, the bill changes or adds programs in a number of health areas besides prescription drugs (see Supplementary changes with the Medicare prescription drug bill). These additions partly reflected the need of proponents to satisfy diverse special interests (private insurers, hospitals and physicians, rural areas) and thereby gain their support for other parts of the bill that were more controversial, principally the drug benefit and private competition for Medicare. Thus, there is funding to increase Medicare payments to physicians and rural hospitals and to hospitals serving large numbers of low-income patients.
- Medicare payments to rural hospitals and doctors increase by $25 billion over 10 years.
- Payments to hospitals serving large numbers of low-income patients would increase.
- Hospitals can avoid some future cuts in Medicare payments by submitting quality of care data to the government.
- Doctors would receive increases of 1.5% per year in Medicare payments for 2004 and 2005 rather than the cuts currently planned.
- Medicare would cover an initial physical for new beneficiaries and screening for diabetes and cardiovascular disease.
- Support for development of health savings accounts that allow people with high-deductible health insurance to shelter income from taxes and obtain tax deductions if the money is used for health expenses.
- Home health agencies would see cuts in payments, but patient co-pays would not be required.
- Medicare Part B premiums (for physician and outpatient services) would be greater for those with incomes over $80,000.
Third, the changes also reflect genuine goals of improving health by expanding Medicare coverage of preventive services and requiring participating hospitals to submit quality-of-care data.
Prescription drug coverage under the new bill
Although many seniors have drug coverage through retirement health plans or Medigap policies purchased privately, about one quarter of beneficiaries (some 10 million people) do not have such coverage. Even those with drug coverage may have difficulty affording recommended medications since the median income for a senior is little more than $23,000. Many physicians have seen the ill effects of seniors not filling their prescriptions or skipping doses of prescribed medications.
Until the benefit takes effect. The actual prescription drug benefit will not begin until 2006. Until then, Medicare recipients will be given the option of purchasing a drug-discount card for $30 per year starting this spring. It is estimated these cards may save 10% to 15% of prescription costs. In addition, low-income seniors will receive $600 per year toward drug purchases.
After it takes effect. The drug benefit starting in 2006 will be funded through a complex arrangement of patient and government payments (Figure).
- Premium: A premium estimated to begin at $35 per month.
- Deductible: An annual deductible starting at $250 and indexed to increase to $445 in 2013.
- Co-pay: After paying the deductible, enrollees will pay 25% of additional drug costs up to $2250, at which point a $2850 gap in cover-age—the so-called “doughut hole”—leaves the onus of payment with the patient until $5100 is reached.
- Catastrophic coverage: After $5100, patients will pay 5% of any additional annual drug costs.
In 2006, catastrophic coverage will begin after $3600 in out-of-pocket costs ($250 deductible + $500 in co-pays to $2250 + the $2850 doughnut gap), not counting the premium. Indexing provisions are projected to raise this out-of-pocket cost requirement to $6400 in 2013. These indexing features have received less attention in the media, but may become increasingly important to seniors. For lower-income individuals, as determined by specific yearly income and total assets guidelines, a small per-prescription fee will replace the premium, deductible, and doughnut hole gap payments.
Coverage will vary. As the yearly cost of drugs changes, so will the relative contributions made by the patient and the government (Table). The new bill provides substantial benefit to those with catastrophic drug costs and to very low-income seniors. The idea of linking payments to income (for the drug benefit and the Part B premium) is a change in the Medicare program, as it has traditionally provided the same benefit to all beneficiaries, regardless of income.
Expected effects of privatization. The manner in which the drug benefit will be administered was controversial in Congress. The legislation, written primarily by Republicans, provides that beneficiaries can obtain coverage by participating with an HMO or PPO or by purchasing standalone coverage through a private prescription drug insurance program. Patient enrollment is voluntary. Managed care plans would be encouraged to participate in the prescription benefit program through eligibility for government subsidies. In turn, more beneficiaries would be encouraged to choose managed care plans, thus decreasing the number of patients covered by traditional Medicare. Furthermore, current private Medigap supplemental plans will be barred from offering drug benefits.
With the HMO/PPO and stand-alone programs, it is likely that any reduction in drug costs will result from private pharmacy benefit managers negotiating discounts from drug companies as they do now for many employer-sponsored plans. Presumably, formularies will vary from plan to plan, and it may be difficult for patients to know ahead of time whether the plan they join will cover their current medications. The legislation prohibits the government from using its vast purchasing power to negotiate substantial discounts from drug companies as it does now for the Medicaid program. There are provisions to increase availability of generic drugs, but importation of drugs from Canada is prohibited unless FDA approval is given (so far, the FDA has opposed this). Many Democrats who opposed the bill argued that it allowed too large a role for the private sector and constrained the ability of the government to control drug costs.
A final controversial measure in the bill provided for conducting an experiment in 6 cities beginning in 2010, in which at least 1 private insurance plan would be funded to compete directly with the traditional Medicare program. Many Republicans believe this type of competition is necessary to decrease the rate of cost increases in the Medicare program, while many Democrats believe the private market is a big reason for increasing problems with the quality and cost of the entire health care system.
FIGURE
Out-of-pocket spending under new legislation
Out-of-pocket drug spending in 2006 for Medicare beneficiaries under new Medicare legislation. Note: Benefit levels are indexed to growth in per capita expenditures for covered Part D drugs. As a result, the Part D deductible in projected to increase from $250 in 2006 to $445 in 2013; the catastrophic threshold is projected to increase from $5100 in 2006 to $9066 in 2013. From the Kaiser Family Foundation website(www.kff.org/medicare/medicarebenefitataglance.ctm).
Looming questions
The new Medicare legislation is vast in scope, cost, and controversy. In the coming months, a number of organizations—AARP, the Department of Health and Human Services, and various foundations—will attempt to explain its provisions to the public, most likely in different ways.
TABLE
Deciphering the 2006 drug benefit
The chart above shows what portion of yearly drug costs would be paid by the Medicare recipient and what portion would be paid by Medicare beginning in 2006. It does not include the $420 yearly premium.Family physicians may be asked by patients to explain provisions of the program and to offer advice in making decisions about their participation.
In addition, preoccupation with explaining and implementing the Medicare bill may keep Congress and the President from addressing other pressing health issues such as the growing number of uninsured.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Pear R. Bush’s aides put higher price tag on Medicare law. New York Times, January 30, 2004.
2. Altman D. The new Medicare prescription-drug legislation. N Engl J Med 2004;350:9-10
3. American Academy of Family Physicians. Medicare Prescription Drug, Improvement and Modernization Act. Available at: www.aafp.org/x25558.xml. Accessed on April 2, 2004.
4. National Association of Chain Drug Stores. Medicare Prescription Drug Benefit and Discount Card Program Q & A. Available at: www.nacds.org/user-assets/PDF_files/MedicareRx_Q&A.pdf. Accessed on April 2, 2004.
5. New Medicare law/key provisions. Christian Science Monitor, December 4, 2003
1. Pear R. Bush’s aides put higher price tag on Medicare law. New York Times, January 30, 2004.
2. Altman D. The new Medicare prescription-drug legislation. N Engl J Med 2004;350:9-10
3. American Academy of Family Physicians. Medicare Prescription Drug, Improvement and Modernization Act. Available at: www.aafp.org/x25558.xml. Accessed on April 2, 2004.
4. National Association of Chain Drug Stores. Medicare Prescription Drug Benefit and Discount Card Program Q & A. Available at: www.nacds.org/user-assets/PDF_files/MedicareRx_Q&A.pdf. Accessed on April 2, 2004.
5. New Medicare law/key provisions. Christian Science Monitor, December 4, 2003
Hepatitis A: Matching preventive resources to needs
A recent outbreak of Hepatitis A—linked to imported green onions served at a restaurant in Pennsylvania—sickened close to 600 patrons and restaurant staff and killed 3.1 Large-scale hepatitis outbreaks are uncommon. But this well-publicized event drew attention to Hepatitis A virus (HAV) infections and the issue of food safety, and it provides an example of the importance of accurate diagnosis, prompt reporting to public health authorities, and implementation of the preventive measures discussed here to lessen the community impact of this infectious disease.
Those at risk of infection
Hepatitis A virus is passed fecal-orally, and infection usually results from person-to-person transmission within a household or between sexual partners. Young children with hepatitis A are often the source of infection for adults. Day care centers are an important source of infection of children, staff, and parents of children.
Disease incidence varies considerably by race/ethnicity and geography. American-Indians have the highest rates; Hispanics have the second-highest (Figure 1). Rates are higher in the western US (Figure 2).
Persons at highest risk for HAV infection include travelers to developing countries, men who have sex with men, users of illicit drugs, those with clotting factor disorders, and persons who work with nonhuman primates. Those who have chronic liver disease are at higher risk of fatal, fulminate infection.
Surprisingly, workers in day care centers, health care institutions, schools, and sewage facilities appear not to be at higher than average risk for the community.
FIGURE 1
Hepatitis A by race/ethnicity
Rates of reported hepatitis A by race/ethnicity—United States, 1994. Source: CDC, National Notifiable Diseases Surveillance System.
FIGURE 2
Average reported cases of hepatitis A per 100,000 population, 1987–1997
Approximately the national average of reported cases during 1987–1997, by county. Source: CDC.
Clinical onset is sudden
Symptoms of illness appear suddenly and include fever, malaise, anorexia, nausea, abdominal pain, dark urine, and jaundice. By the time symptoms appear, the infection has been incubating from 2 weeks to 2 months (average, 28 days).
Mild symptoms without jaundice are much more likely in children (70%) than in adults (30%). Symptoms usually last less than 2 months but can occasionally persist or relapse for up to 6 months.
Confirm with IgM testing. Diagnosis is confirmed by serologic testing for anti-HAV immunoglobulin M (IgM), which is present before symptoms appear and persists for 6 months. Anti-HAV IgG also occurs early in infection but persists lifelong and cannot be used to distinguish old from current infection. Infectivity is highest in the 2 weeks before jaundice appears.
Matching prevention measures to patient needs
Two forms of HAV prevention are available: immune globulin (IG) and Hepatitis A vaccine.2
Immune globulin
For those traveling to high-risk areas, pre-exposure prophylaxis IG at a dose of 0.02 mL/kg intramuscularly offers up to 3 months of protection; a dose of 0.06 mL/kg intramuscularly provides protection up to 5 months.
Post-exposure prophylaxis is recommended at a dose of 0.02 mL/kg and, if given within 2 weeks of exposure, is 85% effective in preventing disease. If it can be provided within 2 weeks of the last exposure, IG is recommended for all unvaccinated household and sexual contacts of those with laboratory confirmed hepatitis A, and for those who have shared illicit drugs with an infected person.
Caveats. Immune globulin can interfere with the effectiveness of both mumps, measles, and rubella vaccine (MMR) and varicella vaccine. MMR administration should be delayed for 3 months and varicella vaccine for 5 months following IG administration. Immune globulin should not be given for at least 2 weeks after MMR vaccination or three weeks after varicella vaccination. Some IG preparations contain thimerosal; these should not be used in infants or pregnant women.
Hepatitis A vaccine
Two single-antigen hepatitis A vaccines are marketed, both of which are prepared from inactivated virus. Neither product is approved for use in children younger than 2 years. Both vaccines must be given in 2 doses, 6 months apart, for full protection; although in over 90 % of adults, protective antibody levels develop within 4 weeks of the first dose. The Table lists the dose and schedule for each hepatitis A single-antigen vaccine.
Candidates for hepatitis A vaccine:
- Children who live in states, counties, or communities with average annual hepatitis A rates of 20/100,000 or greater
- Travelers or those working in countries with high or intermediate rates of HAV infection (Figure 3)
- Men who have sex with men
- Users of illicit drugs
- Those who work with HAV in laboratories or with non-human primates
- Those with clotting factor disorders
- Those with chronic liver disease
Vaccines are safe. Hepatitis A vaccine does not interfere with other vaccines, appears safe in pregnancy, and causes only minor reactions such as pain and redness at the injection site, headache, or malaise. No credible evidence of serious complications from the vaccine exists. Since it takes 4 weeks for a full immune response to the vaccine to develop, those who need protection sooner should receive IG or both IG and vaccine administered at different sites.
Combination vaccine. There is also a combined hepatitis A and B vaccine (Twinrix) that is approved for those over age 18 years. This vaccine product contains trace amounts of thimerosal, neomycin, formalin, and yeast protein.3
TABLE
Recommended dosages and schedules of hepatitis A vaccines
| Vaccine | Age group | Dose | Volume | # Doses | Schedule |
|---|---|---|---|---|---|
| Havrix | 2–18 years | 720 ELU | 0.5 mL | 2 | 0, 6–12 mos |
| 19 years and older | 1440 ELU | 1.0 mL | 2 | 0, 6–12 mos | |
| Vaqta | 2–18 years | 25 U | 0.5 mL | 2 | 0, 6–18 mos |
| 19 years and older | 50 U | 1.0 mL | 2 | 0, 6–12 mos | |
| ELU, enzyme-linked immunosorbent assay (ELISA) units; U=units | |||||
FIGURE 3
Endemicity patterns of hepatitis A virus infection worldwide
This map generalizes currently available data, and patterns might vary within countries. Source: CDC.
Public health measures
Hepatitis A virus infection is reportable in every state. Prompt reporting by physicians is important, especially if the infected person is a food handler; serious, widespread, common-source outbreaks can be prevented by such actions. The role of the public health department is to verify cases, investigate outbreaks, institute outbreak control measures and make recommendations regarding routine vaccination. Some local public health departments may offer postexposure IG prophylaxis.
Use of immune globulin. Outbreak control measures will vary with the circumstances. Immune globulin for children and staff has proven effective in limiting outbreaks at daycare centers and should also be considered for parents of children at day care centers with a documented outbreak. A daycare center outbreak is defined as 1 or more cases in children or staff at a center or in 2 or more households of center attendees. Immune globulin is usually not effective in common source outbreaks, such as the recent Pennsylvania case, because the 2-week time period for protection has usually passed before the source of the outbreak is discovered.
While deaths from HAV are not common— approximately 100 per year—the individual and community morbidity caused by the virus can be considerable. Infection leads to hospitalization in 11% to 22% of cases and large economic losses with adults suffering 27 days of lost work and each case totaling over $2000 of direct and indirect costs.2 Local public health departments can also expend considerable resources trying to control outbreaks of this disease.
Indications for vaccination. The Centers for Disease Control and Prevention recommends routine vaccination for children who live in communities, counties, or states with HAV rates equal to or greater than 20/100,000, and it recommends considering routine vaccination when rates are equal to or greater than 10/100,000.
Following through. To lower community morbidity from HAV, be aware of the incidence and epidemiology of HAV in your community, suspect and accurately diagnose HAV illness using serological confirmation, promptly report all HAV cases to the local or state public health department, vaccinate patients who are in high-risk groups, offer prompt post-exposure prophylaxis to household and sexual contacts of those with acute HAV infection, and adhere to local recommendations regarding routine vaccination of children.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). Hepatitis A outbreak associated with green onions at a restaurant –Monaca, Pennsylvania, 2003. MMWR Morb Mortal Wkly Rep 2003;53:1-3.
2. Prevention of hepatitis A through active or passive immunization: recommendations of the advisory committee on immunization practices. MMWR Rapid Res 1990;48(RR-12):1-37.
3. CDC. FDA approval for a combined hepatitis A and B vaccine. MMWR Morb Mortal Wkly Rep 2001;50:806-807.
A recent outbreak of Hepatitis A—linked to imported green onions served at a restaurant in Pennsylvania—sickened close to 600 patrons and restaurant staff and killed 3.1 Large-scale hepatitis outbreaks are uncommon. But this well-publicized event drew attention to Hepatitis A virus (HAV) infections and the issue of food safety, and it provides an example of the importance of accurate diagnosis, prompt reporting to public health authorities, and implementation of the preventive measures discussed here to lessen the community impact of this infectious disease.
Those at risk of infection
Hepatitis A virus is passed fecal-orally, and infection usually results from person-to-person transmission within a household or between sexual partners. Young children with hepatitis A are often the source of infection for adults. Day care centers are an important source of infection of children, staff, and parents of children.
Disease incidence varies considerably by race/ethnicity and geography. American-Indians have the highest rates; Hispanics have the second-highest (Figure 1). Rates are higher in the western US (Figure 2).
Persons at highest risk for HAV infection include travelers to developing countries, men who have sex with men, users of illicit drugs, those with clotting factor disorders, and persons who work with nonhuman primates. Those who have chronic liver disease are at higher risk of fatal, fulminate infection.
Surprisingly, workers in day care centers, health care institutions, schools, and sewage facilities appear not to be at higher than average risk for the community.
FIGURE 1
Hepatitis A by race/ethnicity
Rates of reported hepatitis A by race/ethnicity—United States, 1994. Source: CDC, National Notifiable Diseases Surveillance System.
FIGURE 2
Average reported cases of hepatitis A per 100,000 population, 1987–1997
Approximately the national average of reported cases during 1987–1997, by county. Source: CDC.
Clinical onset is sudden
Symptoms of illness appear suddenly and include fever, malaise, anorexia, nausea, abdominal pain, dark urine, and jaundice. By the time symptoms appear, the infection has been incubating from 2 weeks to 2 months (average, 28 days).
Mild symptoms without jaundice are much more likely in children (70%) than in adults (30%). Symptoms usually last less than 2 months but can occasionally persist or relapse for up to 6 months.
Confirm with IgM testing. Diagnosis is confirmed by serologic testing for anti-HAV immunoglobulin M (IgM), which is present before symptoms appear and persists for 6 months. Anti-HAV IgG also occurs early in infection but persists lifelong and cannot be used to distinguish old from current infection. Infectivity is highest in the 2 weeks before jaundice appears.
Matching prevention measures to patient needs
Two forms of HAV prevention are available: immune globulin (IG) and Hepatitis A vaccine.2
Immune globulin
For those traveling to high-risk areas, pre-exposure prophylaxis IG at a dose of 0.02 mL/kg intramuscularly offers up to 3 months of protection; a dose of 0.06 mL/kg intramuscularly provides protection up to 5 months.
Post-exposure prophylaxis is recommended at a dose of 0.02 mL/kg and, if given within 2 weeks of exposure, is 85% effective in preventing disease. If it can be provided within 2 weeks of the last exposure, IG is recommended for all unvaccinated household and sexual contacts of those with laboratory confirmed hepatitis A, and for those who have shared illicit drugs with an infected person.
Caveats. Immune globulin can interfere with the effectiveness of both mumps, measles, and rubella vaccine (MMR) and varicella vaccine. MMR administration should be delayed for 3 months and varicella vaccine for 5 months following IG administration. Immune globulin should not be given for at least 2 weeks after MMR vaccination or three weeks after varicella vaccination. Some IG preparations contain thimerosal; these should not be used in infants or pregnant women.
Hepatitis A vaccine
Two single-antigen hepatitis A vaccines are marketed, both of which are prepared from inactivated virus. Neither product is approved for use in children younger than 2 years. Both vaccines must be given in 2 doses, 6 months apart, for full protection; although in over 90 % of adults, protective antibody levels develop within 4 weeks of the first dose. The Table lists the dose and schedule for each hepatitis A single-antigen vaccine.
Candidates for hepatitis A vaccine:
- Children who live in states, counties, or communities with average annual hepatitis A rates of 20/100,000 or greater
- Travelers or those working in countries with high or intermediate rates of HAV infection (Figure 3)
- Men who have sex with men
- Users of illicit drugs
- Those who work with HAV in laboratories or with non-human primates
- Those with clotting factor disorders
- Those with chronic liver disease
Vaccines are safe. Hepatitis A vaccine does not interfere with other vaccines, appears safe in pregnancy, and causes only minor reactions such as pain and redness at the injection site, headache, or malaise. No credible evidence of serious complications from the vaccine exists. Since it takes 4 weeks for a full immune response to the vaccine to develop, those who need protection sooner should receive IG or both IG and vaccine administered at different sites.
Combination vaccine. There is also a combined hepatitis A and B vaccine (Twinrix) that is approved for those over age 18 years. This vaccine product contains trace amounts of thimerosal, neomycin, formalin, and yeast protein.3
TABLE
Recommended dosages and schedules of hepatitis A vaccines
| Vaccine | Age group | Dose | Volume | # Doses | Schedule |
|---|---|---|---|---|---|
| Havrix | 2–18 years | 720 ELU | 0.5 mL | 2 | 0, 6–12 mos |
| 19 years and older | 1440 ELU | 1.0 mL | 2 | 0, 6–12 mos | |
| Vaqta | 2–18 years | 25 U | 0.5 mL | 2 | 0, 6–18 mos |
| 19 years and older | 50 U | 1.0 mL | 2 | 0, 6–12 mos | |
| ELU, enzyme-linked immunosorbent assay (ELISA) units; U=units | |||||
FIGURE 3
Endemicity patterns of hepatitis A virus infection worldwide
This map generalizes currently available data, and patterns might vary within countries. Source: CDC.
Public health measures
Hepatitis A virus infection is reportable in every state. Prompt reporting by physicians is important, especially if the infected person is a food handler; serious, widespread, common-source outbreaks can be prevented by such actions. The role of the public health department is to verify cases, investigate outbreaks, institute outbreak control measures and make recommendations regarding routine vaccination. Some local public health departments may offer postexposure IG prophylaxis.
Use of immune globulin. Outbreak control measures will vary with the circumstances. Immune globulin for children and staff has proven effective in limiting outbreaks at daycare centers and should also be considered for parents of children at day care centers with a documented outbreak. A daycare center outbreak is defined as 1 or more cases in children or staff at a center or in 2 or more households of center attendees. Immune globulin is usually not effective in common source outbreaks, such as the recent Pennsylvania case, because the 2-week time period for protection has usually passed before the source of the outbreak is discovered.
While deaths from HAV are not common— approximately 100 per year—the individual and community morbidity caused by the virus can be considerable. Infection leads to hospitalization in 11% to 22% of cases and large economic losses with adults suffering 27 days of lost work and each case totaling over $2000 of direct and indirect costs.2 Local public health departments can also expend considerable resources trying to control outbreaks of this disease.
Indications for vaccination. The Centers for Disease Control and Prevention recommends routine vaccination for children who live in communities, counties, or states with HAV rates equal to or greater than 20/100,000, and it recommends considering routine vaccination when rates are equal to or greater than 10/100,000.
Following through. To lower community morbidity from HAV, be aware of the incidence and epidemiology of HAV in your community, suspect and accurately diagnose HAV illness using serological confirmation, promptly report all HAV cases to the local or state public health department, vaccinate patients who are in high-risk groups, offer prompt post-exposure prophylaxis to household and sexual contacts of those with acute HAV infection, and adhere to local recommendations regarding routine vaccination of children.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
A recent outbreak of Hepatitis A—linked to imported green onions served at a restaurant in Pennsylvania—sickened close to 600 patrons and restaurant staff and killed 3.1 Large-scale hepatitis outbreaks are uncommon. But this well-publicized event drew attention to Hepatitis A virus (HAV) infections and the issue of food safety, and it provides an example of the importance of accurate diagnosis, prompt reporting to public health authorities, and implementation of the preventive measures discussed here to lessen the community impact of this infectious disease.
Those at risk of infection
Hepatitis A virus is passed fecal-orally, and infection usually results from person-to-person transmission within a household or between sexual partners. Young children with hepatitis A are often the source of infection for adults. Day care centers are an important source of infection of children, staff, and parents of children.
Disease incidence varies considerably by race/ethnicity and geography. American-Indians have the highest rates; Hispanics have the second-highest (Figure 1). Rates are higher in the western US (Figure 2).
Persons at highest risk for HAV infection include travelers to developing countries, men who have sex with men, users of illicit drugs, those with clotting factor disorders, and persons who work with nonhuman primates. Those who have chronic liver disease are at higher risk of fatal, fulminate infection.
Surprisingly, workers in day care centers, health care institutions, schools, and sewage facilities appear not to be at higher than average risk for the community.
FIGURE 1
Hepatitis A by race/ethnicity
Rates of reported hepatitis A by race/ethnicity—United States, 1994. Source: CDC, National Notifiable Diseases Surveillance System.
FIGURE 2
Average reported cases of hepatitis A per 100,000 population, 1987–1997
Approximately the national average of reported cases during 1987–1997, by county. Source: CDC.
Clinical onset is sudden
Symptoms of illness appear suddenly and include fever, malaise, anorexia, nausea, abdominal pain, dark urine, and jaundice. By the time symptoms appear, the infection has been incubating from 2 weeks to 2 months (average, 28 days).
Mild symptoms without jaundice are much more likely in children (70%) than in adults (30%). Symptoms usually last less than 2 months but can occasionally persist or relapse for up to 6 months.
Confirm with IgM testing. Diagnosis is confirmed by serologic testing for anti-HAV immunoglobulin M (IgM), which is present before symptoms appear and persists for 6 months. Anti-HAV IgG also occurs early in infection but persists lifelong and cannot be used to distinguish old from current infection. Infectivity is highest in the 2 weeks before jaundice appears.
Matching prevention measures to patient needs
Two forms of HAV prevention are available: immune globulin (IG) and Hepatitis A vaccine.2
Immune globulin
For those traveling to high-risk areas, pre-exposure prophylaxis IG at a dose of 0.02 mL/kg intramuscularly offers up to 3 months of protection; a dose of 0.06 mL/kg intramuscularly provides protection up to 5 months.
Post-exposure prophylaxis is recommended at a dose of 0.02 mL/kg and, if given within 2 weeks of exposure, is 85% effective in preventing disease. If it can be provided within 2 weeks of the last exposure, IG is recommended for all unvaccinated household and sexual contacts of those with laboratory confirmed hepatitis A, and for those who have shared illicit drugs with an infected person.
Caveats. Immune globulin can interfere with the effectiveness of both mumps, measles, and rubella vaccine (MMR) and varicella vaccine. MMR administration should be delayed for 3 months and varicella vaccine for 5 months following IG administration. Immune globulin should not be given for at least 2 weeks after MMR vaccination or three weeks after varicella vaccination. Some IG preparations contain thimerosal; these should not be used in infants or pregnant women.
Hepatitis A vaccine
Two single-antigen hepatitis A vaccines are marketed, both of which are prepared from inactivated virus. Neither product is approved for use in children younger than 2 years. Both vaccines must be given in 2 doses, 6 months apart, for full protection; although in over 90 % of adults, protective antibody levels develop within 4 weeks of the first dose. The Table lists the dose and schedule for each hepatitis A single-antigen vaccine.
Candidates for hepatitis A vaccine:
- Children who live in states, counties, or communities with average annual hepatitis A rates of 20/100,000 or greater
- Travelers or those working in countries with high or intermediate rates of HAV infection (Figure 3)
- Men who have sex with men
- Users of illicit drugs
- Those who work with HAV in laboratories or with non-human primates
- Those with clotting factor disorders
- Those with chronic liver disease
Vaccines are safe. Hepatitis A vaccine does not interfere with other vaccines, appears safe in pregnancy, and causes only minor reactions such as pain and redness at the injection site, headache, or malaise. No credible evidence of serious complications from the vaccine exists. Since it takes 4 weeks for a full immune response to the vaccine to develop, those who need protection sooner should receive IG or both IG and vaccine administered at different sites.
Combination vaccine. There is also a combined hepatitis A and B vaccine (Twinrix) that is approved for those over age 18 years. This vaccine product contains trace amounts of thimerosal, neomycin, formalin, and yeast protein.3
TABLE
Recommended dosages and schedules of hepatitis A vaccines
| Vaccine | Age group | Dose | Volume | # Doses | Schedule |
|---|---|---|---|---|---|
| Havrix | 2–18 years | 720 ELU | 0.5 mL | 2 | 0, 6–12 mos |
| 19 years and older | 1440 ELU | 1.0 mL | 2 | 0, 6–12 mos | |
| Vaqta | 2–18 years | 25 U | 0.5 mL | 2 | 0, 6–18 mos |
| 19 years and older | 50 U | 1.0 mL | 2 | 0, 6–12 mos | |
| ELU, enzyme-linked immunosorbent assay (ELISA) units; U=units | |||||
FIGURE 3
Endemicity patterns of hepatitis A virus infection worldwide
This map generalizes currently available data, and patterns might vary within countries. Source: CDC.
Public health measures
Hepatitis A virus infection is reportable in every state. Prompt reporting by physicians is important, especially if the infected person is a food handler; serious, widespread, common-source outbreaks can be prevented by such actions. The role of the public health department is to verify cases, investigate outbreaks, institute outbreak control measures and make recommendations regarding routine vaccination. Some local public health departments may offer postexposure IG prophylaxis.
Use of immune globulin. Outbreak control measures will vary with the circumstances. Immune globulin for children and staff has proven effective in limiting outbreaks at daycare centers and should also be considered for parents of children at day care centers with a documented outbreak. A daycare center outbreak is defined as 1 or more cases in children or staff at a center or in 2 or more households of center attendees. Immune globulin is usually not effective in common source outbreaks, such as the recent Pennsylvania case, because the 2-week time period for protection has usually passed before the source of the outbreak is discovered.
While deaths from HAV are not common— approximately 100 per year—the individual and community morbidity caused by the virus can be considerable. Infection leads to hospitalization in 11% to 22% of cases and large economic losses with adults suffering 27 days of lost work and each case totaling over $2000 of direct and indirect costs.2 Local public health departments can also expend considerable resources trying to control outbreaks of this disease.
Indications for vaccination. The Centers for Disease Control and Prevention recommends routine vaccination for children who live in communities, counties, or states with HAV rates equal to or greater than 20/100,000, and it recommends considering routine vaccination when rates are equal to or greater than 10/100,000.
Following through. To lower community morbidity from HAV, be aware of the incidence and epidemiology of HAV in your community, suspect and accurately diagnose HAV illness using serological confirmation, promptly report all HAV cases to the local or state public health department, vaccinate patients who are in high-risk groups, offer prompt post-exposure prophylaxis to household and sexual contacts of those with acute HAV infection, and adhere to local recommendations regarding routine vaccination of children.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). Hepatitis A outbreak associated with green onions at a restaurant –Monaca, Pennsylvania, 2003. MMWR Morb Mortal Wkly Rep 2003;53:1-3.
2. Prevention of hepatitis A through active or passive immunization: recommendations of the advisory committee on immunization practices. MMWR Rapid Res 1990;48(RR-12):1-37.
3. CDC. FDA approval for a combined hepatitis A and B vaccine. MMWR Morb Mortal Wkly Rep 2001;50:806-807.
1. Centers for Disease Control and Prevention (CDC). Hepatitis A outbreak associated with green onions at a restaurant –Monaca, Pennsylvania, 2003. MMWR Morb Mortal Wkly Rep 2003;53:1-3.
2. Prevention of hepatitis A through active or passive immunization: recommendations of the advisory committee on immunization practices. MMWR Rapid Res 1990;48(RR-12):1-37.
3. CDC. FDA approval for a combined hepatitis A and B vaccine. MMWR Morb Mortal Wkly Rep 2001;50:806-807.
Type 2 diabetes: The role of basal insulin therapy
- Patients should be screened for diabetes at age 45 years—earlier if they are overweight and have at least 1 other risk factor.
- Management of type 2 diabetes requires a multifactorial approach that includes not only glycemic control but also addresses such risk factors as hypertension, dyslipidemia, renal impairment, and obesity.
- Tight glucose control (A1C <7%) may require intensive therapy with more than one antiglycemic agent. Early addition of basal insulin may be an efficient way to achieve A1C targets in some patients.
According to the latest estimates by the American Diabetes Association (ADA), more than 12.1 million persons in the United States have been diagnosed with diabetes and about 6 million remain undiagnosed.1 Type 2 diabetes, which comprises the majority (up to 95%) of all diabetes cases,2 has a profound impact on patient health and quality of life and places significant burdens on the health care system. For example, it confers a risk for myocardial infarction (MI) and cardiovascular mortality that is comparable to that for patients who have previously had an MI.3
Economically, the costs of diabetes in the United States are overwhelming: direct and indirect medical expenditures related to diabetes totaled $132 billion in 2002 and are expected to increase to $192 billion annually by 2020.1 Demographically adjusted, per-capita expenses for persons diagnosed with diabetes are more than double those of persons without diabetes.1
Clinical challenge
Type 2 diabetes poses a major challenge to primary care physicians, who are the main providers of care for about 80% of patients with this disease.4 Patients may present with a variety of complications, including neurologic, peripheral vascular, cardiovascular (eg, MI, stroke), renal, and ophthalmic disorders.1 Often, both microvascular and macrovascular complications precede the initial diagnosis of diabetes.5
Results of randomized clinical trials and population-based studies confirm that secondary preventive measures (ie, ameliorating risk factors in patients with known diabetes) and improved glycemic control can help reduce diabetes complications.3,6 A multifactorial approach that both achieves glycemic control and addresses other risk factors, such as dyslipidemia, hypertension, and microalbuminuria, has been demonstrated to be important, particularly for reducing macrovascular complications.7-9 Treatment goals for patients with type 2 diabetes include: an A1C value of <7.0%, a low-density lipoprotein cholesterol level of <100 mg/dL, and a blood pressure level of <130/80 mm Hg.10-12
The United Kingdom Prospective Diabetes Study (UKPDS 35)—in a posthoc observational substudy—suggested that there is “no threshold” for A1C lowering for any type of diabetes complication, indicating that treatment probably should aim to bring the A1C level as close to normal as possible.6 Thus, the American College of Endocrinology (ACE), for example, has set an even more aggressive A1C goal of ≤6.5%.13 Organizations such as the ADA and ACE believe that lower A1C targets not only are beneficial but that they also are achievable with currently available therapeutic agents. Ultimately, an individual patient’s comorbid conditions, life expectancy, and preferences must be considered when determining goals.14
Despite compelling outcomes data, the prevalence of diabetes remains high and clinical control remains suboptimal. The Health Plan Employer Data and Information Set, a widely used tool for measuring quality of health care, currently considers an A1C of >9.5% as “poorly controlled” disease.15 According to data from the Third National Health and Nutrition Examination Survey, 18% of patients with diabetes have an A1C of >9.5%.16 In areas where obesity is highly prevalent, such as New Orleans, the percentages are even higher, with up to 43% of obese patients treated at urban teaching hospitals in that area having A1C values of >9.5%.17 The Diabetes Quality Improvement Project, which governs 20 public and private health care organizations, found that 35% of its patients had a mean A1C of >9.5%.18 Considering that 9.5% is well above the ADA goal of <7.0%, data such as these underscore the need for more widespread diabetes screening and earlier intervention.
Identifying diabetes
The ADA recommends that screening with a fasting plasma glucose test be considered in all patients aged 45 years and older, particularly those with a body mass index of 25 kg/m2 or more.19 If the results are normal, screening should be repeated every 3 years. However, patients who are thought to be at increased risk should be considered for more frequent screening. The ADA recommendations have been endorsed by the American Academy of Pediatrics. The American Academy of Family Physicians follows the recommendations established by the US Preventive Services Task Force: screening is suggested in adults with hypertension or dyslipidemia.20
Routine screening for type 1 diabetes among healthy children is not expected to yield many cases and is not recommended. However, because the incidence of type 2 diabetes is increasing among children, it has been suggested that those who are overweight (defined as weight for height >85th percentile or weight >120% of ideal for height) with 2 or more additional risk factors should be screened every 2 years, beginning at age 10 years or at the onset of puberty.19
The ADA criteria for diagnosis of diabetes are listed in Table 1 . Whichever criterion is used, diagnostic test results must be confirmed on a subsequent day.19
Patients who do not meet the diagnostic criteria but have either impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) are considered to have prediabetes (see Table 1 ).19,21 Based on recent estimates, more than 12 million adults in the United States have prediabetes.22 Of great importance is regular monitoring of patients with IGT or IFG because in addition to diabetes, they are at a heightened risk of MI or stroke compared with normoglycemic persons.
TABLE 1
Diagnostic criteria for prediabetes and diabetes
| Prediabetes | Diabetes |
|---|---|
| One or both of the following: | One or more of the following: |
|
|
| *Oral glucose tolerance test should use glucose load containing equivalent of 75 g anhydrous glucose dissolved in water. | |
| Adapted from American Diabetes Association.19 | |
Intensive management
The UKPDS showed that intensive glycemic control in type 2 diabetes significantly reduced the risk of microvascular complications.23,24 In UKPDS 33, patients with newly diagnosed type 2 diabetes whose disease remained uncontrolled after completing 3 months of dietary management were initially randomized to intensive treatment (goal: FPG <108 mg/dL) with sulfonylurea or insulin monotherapy or to conventional treatment (goal: FPG <270 mg/dL) that began with diet. After 10 years, the median A1C was 7.0% in the intensive group and 7.9% in the conventional group.23 The reduction in A1C was associated with a 25% reduction of microvascular endpoints, mainly a reduced need for retinal photocoagulation (relative risk [RR] for intensive therapy, 0.75; 95% CI, 0.60–0.93; P<.01). While there was a 16% reduction in the risk of MI, this difference was not statistically significant (RR, 0.84; 95% CI, 0.71–1.00; P=.052). The UKPDS 35, which was an epidemiologic analysis of data (ie, not a direct comparison of the 2 treatment arms), demonstrated that each 1% reduction in mean A1C was associated with a 21% decrease in risk for any diabetes-related endpoint (95% CI, 17%–24%; P<.0001), a 21% decrease in diabetes-related deaths (95% CI, 15%–27%; P<.0001), a 37% decrease in the risk for microvascular complications (95% CI, 33%–41%; P<.0001), and a 14% decrease in the risk of MI (95% CI, 8%–21%; P<.0001) ( Figure 1 ).6
Another study that supports intensive management of type 2 diabetes is the Steno-2 study, a randomized controlled trial of 160 patients with type 2 diabetes and microalbuminuria.8 After 7.8 years of follow-up, 54% of conventionally treated and 57% of intensively treated patients were receiving insulin. Decreases in risk factors (eg, A1C, blood pressure, lipids, and albumin excretion) were significantly greater in patients receiving intensive therapy ( Figure 2 ). Significant reductions also were seen in the risk of cardiovascular disease (RR, 0.47; 95% CI, 0.24–0.73), nephropathy (RR, 0.39; 95% CI, 0.17–0.87), retinopathy (RR, 0.42; 95% CI, 0.21–0.86), and autonomic neuropathy (RR, 0.37; 95% CI, 0.18–0.79). Overall, intensive therapy in the Steno-2 study reduced risk of cardiovascular and microvascular events by about 50%.
FIGURE 1
UKPDS 35: Risk reductions associated with each 1% decrease in updated mean A1C
FIGURE 2 Percentage of patients in Steno-2 who reached treatment goals at mean follow-up of 7.8 years
Role of insulin in achieving targets
Another finding of the UKPDS was that type 2 diabetes is routinely progressive and that treatment with a single agent is unlikely to be successful for more than 5 years. Such observations mirror the physiologic progression from insulin resistance to absolute insulin deficiency. Thus, lifestyle modification often needs to be supplemented with oral antihyperglycemic therapy. In some cases, early treatment with insulin is preferable to relieve symptoms of hyperglycemia (eg, blurred vision, frequent thirst), which are suggestive of glucotoxicity and deteriorating β-cell function. As absolute insulin deficiency occurs, multiple oral agents, with or without insulin, may be required for optimal control of the disease.4,25
In UKPDS 57, 826 patients with type 2 diabetes were randomized to diet (n=242), ultralente insulin, a long-acting basal insulin (n=245), or sulfonylurea with the addition of ultralente insulin if FPG remained >108 mg/dL at maximal sulfonylurea dosage (n=339). Over 6 years, 53% of those started on sulfonylurea required addition of insulin.26 Patients treated with insulin monotherapy achieved a median A1C of 7.1%, while those on insulin plus sulfonylurea had a median A1C of 6.6% (P<.01). Episodes of major hypoglycemia occurred at a rate of 1.6% per year in the combined therapy group, compared with 3.2% a year in the insulin monotherapy group (P=0.17). In view of the observed progression of β-cell dysfunction and the failure of oral agents to maintain glycemic control, early addition of insulin to existing oral therapy may be a successful strategy in patients with type 2 diabetes.
Surmounting barriers
Insulin therapy may be delayed for a variety of reasons, including concerns about weight gain and hypo-glycemia as well as misconceptions that it increases the risk of cardiovascular disease.27 UKPDS 33 noted that relatively low proportions of patients treated with insulin experienced major (needing third-party help or medical intervention) hypoglycemic events (1.8% per year; intent-to-treat analysis).23 While both hypoglycemia and weight gain may occur with any form of insulin therapy or use of oral secretagogues, the benefits of effective glycemic control should be considered; moreover, tactics that may reduce these problems are available.28 For example, combined therapy with metformin and insulin can reduce or abolish the weight gain that otherwise may occur when insulin monotherapy is started or intensified.29 Treatment-associated hypoglycemia is generally mild to moderate in type 2 diabetes, and can be addressed with self–blood-glucose monitoring and patient education regarding recognition of symptoms and self-treatment. Also, the new long-acting insulin analogue, insulin glargine, causes fewer episodes of hypoglycemia than does neutral protamine Hagedorn (NPH) insulin.30-32
It is sometimes thought that insulin therapy may be associated with increased insulin resistance; however, studies examining peripheral insulin sensitivity have demonstrated that restoration of glycemic control with insulin improved insulin sensitivity.27,33 Also, the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction trial showed that intensive glycemic control with insulin therapy after an MI reduced the risk of adverse cardiovascular outcomes.6,34
Patient barriers to insulin therapy also include fear of needles and injections or a belief that insulin therapy represents failure, punishment, or a worsened prognosis.35 The notion that starting insulin therapy is a penalty for failure may be reinforced by physicians’ misconceptions about the role of insulin in type 2 diabetes.35 Setting the appropriate expectation that type 2 diabetes is a progressive disease that often eventually requires insulin can help to prevent such negative attitudes.
Finally, both patients and physicians may perceive insulin therapy as a complex and time-consuming treatment.27 Lack of office personnel and time to provide appropriate patient education for insulin therapy may contribute to such a perception. Concerns over complexity may be ameliorated by use of devices such as insulin pens and jet injectors that provide a precise dose and simplify self-administration. Premixed insulins are given twice daily and provide a measure of convenience, although flexibility of meal timing is limited in order to prevent hypoglycemia. Newer treatment regimens may also reduce patient apprehension concerning complexity and increase compliance.4 Notably, a simple and effective approach is to add once-daily basal insulin to established oral therapy.
Basal insulin studies
The efficiency and efficacy of adding basal insulin to existing oral antidiabetic agents was demonstrated in the randomized, open-label Treat-to-Target Trial.32 Overweight patients (N=756) who had been inadequately controlled (A1C >7.5%) on 1 or 2 oral agents (sulfonylurea, metformin, or thiazolidinedione) received insulin glargine or NPH insulin. The insulin dosage, taken at bedtime, was systematically titrated, based on before-breakfast glucose tests performed daily by the patients. Mean A1C levels fell from 8.6% to 6.9% after 24 weeks of therapy ( Figure 3 ). Both insulins performed similarly in terms of glycemic control, with 57% of patients in each group achieving an A1C of ≥7.0%. However, there was a significant difference in the incidence of hypoglycemia. Complete treatment success, defined as reaching target A1C without a single episode of nocturnal hypoglycemia (≤72 mg/dL), was achieved in 33% of the glargine patients and in 27% of the NPH group (P<.05). Overall rates of hypoglycemia, expressed as events per patient per year, were as follows: 14 symptomatic events in glargine patients vs 18 in NPH patients (P<.02), 9 confirmed events of ≤72 mg/dL in glargine patients vs 13 in NPH patients (P<.005), and 3 confirmed events of ≤56 mg/dL in glargine patients vs 5 in NPH patients (P<.003).
In another recent multicenter trial, 695 patients were randomized to 24 weeks of treatment with glimepiride plus 1 of 3 insulin regimens: morning insulin glargine, bedtime insulin glargine, or bedtime NPH insulin.36 Mean A1C levels decreased by 1.24% with morning glargine, 0.96% with bedtime glargine, and 0.84% with bedtime NPH. The percentage of patients achieving an A1C of ≤7.5% was 43% with morning glargine, 33% with bedtime glargine (P=.021 vs morning glargine), and 32% with bedtime NPH (P=.017 vs morning glargine). The percentage of patients who experienced nocturnal hypoglycemia was 17% with morning glargine, 23% with bedtime glargine, and 38% with bedtime NPH (P=.001 vs both glargine regimens). Finally, the percentage of patients who experienced symptomatic hypoglycemia was 56% with morning glargine (P=.004 vs bedtime glargine), 43% with bedtime glargine, and 58% with bedtime NPH (P=.001 vs bedtime glargine).
FIGURE 3 Treat-to-Target Trial: Rapid achievement of A1C goal
Practical aspects
Most commonly, insulin (Table 2) is introduced when single- or multipleagent oral therapy has failed to maintain glycemic control. Insulin may be added to existing oral therapy or, less typically, used as monotherapy. Of the 3 available insulins that have been used for basal therapy (NPH insulin, insulin glargine, and ultralente [insulin zinc extended]), ultralente may not be ideal since considerable variation has been noted in its pharmacokinetic and pharmacodynamic effects.37 Either NPH or glargine can be started at a dose of 10 U, at bedtime, while oral agents are continued at previous dosages. To provide 24-hour coverage, NPH may be needed twice daily whereas a single dose of glargine usually provides 24-hour coverage when administered at the same time each day.38 Patients usually need a total daily basal insulin dose of 0.5 to 0.6 U/kg (typically about 45 U daily for a 100-kg person).39 The dose may be adjusted weekly according to the patient’s FPG level.
Cost is a consideration in insulin therapy. Although more expensive than NPH, basal glargine is associated with 25% fewer episodes of nocturnal hypoglycemia, improved postdinner control, and slightly less weight gain.31,40 A retrospective review of medical and pharmacy claims demonstrated that considerable direct costs result from treatment of hypoglycemia associated with antidiabetic therapy, suggesting that therapies with less potential for inducing hypoglycemia would likely reduce these costs.41
The intensive insulin titration schedule used in the Treat-to-Target Trial is shown in Table 3. In clinical practice, a somewhat less intensive approach may work well. For example, the dose may be increased weekly by 4 U if the FPG level is >140 mg/dL on 3 consecutive occasions, or by 2 U if the FPG level is 120 to 140 mg/dL on 3 consecutive occasions.
Insulin glargine should not be mixed in the same syringe with other insulins as the pharmacokinetic profile may be altered. In the case of NPH, careful rolling or turning of the vial prior to injection to fully resuspend the crystals is advisable to minimize the variability of effect that may otherwise occur. Glargine, as with the regular and rapid-acting insulin analogues lispro and aspart, is a clear solution that does not need to be resuspended before administration. The addition of rapid-acting insulin analogues prandially to a regimen of basal insulin or basal-bolus therapy can be used to more closely mimic physiologic insulin secretion and may be needed when basal insulin replacement alone is insufficient to reach target levels of glycemic control.4
TABLE 2
Insulin formulations
| Insulin* | Onset | Peak | Effective duration (h) |
|---|---|---|---|
| Rapid-acting | |||
| Lispro | 5–15 min | 30–90 min | 5 |
| Aspart | 5–15 min | 30–90 min | 5 |
| Short-acting | |||
| Regular U100 | 30–60 min | 2–3 h | 5–8 |
| Regular U500 | 30–60 min | 2–3 h | 5–8 |
| Intermediate-acting | |||
| Isophane insulin (NPH) | 2–4 h | 4–10 h | 10–16 |
| Insulin zinc | 2–4 h | 4–12 h | 12–18 |
| Long-acting | |||
| Insulin zinc extended | 6–10 h | 10–16 h | 18–24 |
| Insulin glargine | 2–4 h† | No pronounced peak | 20–24 |
| Premixed | |||
| 70% NPH/30% regular | 30–60 min | Dual | 10–16 |
| 50% NPH/50% regular | 30–60 min | Dual | 10–16 |
| 75% NPL/25% lispro | 5–15 min | Dual | 10–16 |
| 70% NP/30% aspart | 5–15 min | Dual | 10–16 |
| *Assuming 0.1–0.2 U/kg per injection; onset and duration may vary by injection site (except for insulin glargine). | |||
| †Time to steady state. | |||
| NPH = neutral protamine Hagedorn; NPL = neutral protamine lispro; NP = neutral protamine. | |||
| Adapted from DeWitt and Hirsch.4 | |||
TABLE 3
Basal insulin: Initiation and dosage adjustment
| Forced titration schedule | |||
|---|---|---|---|
| |||
| Self-monitored FPG (mg/dL) | Dosage increase (IU/day) in Treat-to-Target Trial | ||
| ≥180 | 8 | ||
| ≥140 – <180 | 6 | ||
| ≥120 – <140 | 4 | ||
| >100 – <120 | 2 | ||
| *No increase if plasma glucose is <72 mg/dL in the preceding week; decrease dosage (2–4 IU/day) if plasma glucose is <56 mg/dL or severe hypoglycemia (requiring assistance) occurred within the preceding week. | |||
| FPG = fasting plasma glucose. | |||
| Adapted from Riddle et al.32 | |||
Summary
New evidence and methods continue to alter management of patients with type 2 diabetes. Appropriate screening and earlier intervention may help reduce the incidence and progression of microvascular and macrovascular complications. A multifactorial approach that addresses such risk factors as blood pressure, lipids, and glycemia has demonstrated reduced morbidity and mortality. While glycemic targets are getting lower, they can be efficiently attained with combined basal insulin and oral antidiabetic therapy. Insulin, too often considered a therapy of last resort, is an important intervention that can be used safely and effectively earlier in the course of type 2 diabetes.
1. American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917-932.
2. National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics fact sheet: general information and national estimates on diabetes in the United States, 2000. Bethesda, Md: U.S. Department of Health and Human Services, National Institutes of Health, 2002. National Institute of Diabetes and Digestive and Kidney Diseases, 2002.
3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
4. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
5. Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802-1817.
6. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405-412.
7. Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno-2 randomised study. Lancet. 1999;353:617-622.
8. Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
9. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998;317:703-713.
10. American Diabetes Association. Clinical practice recommendations 2003. Diabetes Care. 2003;26(suppl 1):S1-S156.
11. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
12. National Institutes of Health, National Heart Lung and Blood Institute. JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md: US Dept of Health and Human Services; May 2003. NIH publication 03-5233. Available at: http://www.nhlbi.nih.gov/guidelines/hypertension/express. Accessed November 14, 2003.
13. American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management: 2000 update. Endocr Pract. 2000;8(suppl 1):40-82.
14. Woolf SH, Davidson MB, Greenfield S, et al. Controlling blood glucose levels in patients with type 2 diabetes mellitus: an evidence-based policy statement by the American Academy of Family Physicians and American Diabetes Association. J Fam Pract. 2000;49:453-460.
15. National Committee for Quality Assurance. The State of Health Care Quality: 2003. Washington, DC: NCQA; 2003;1-34.
16. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KMV. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
17. Suwattee P, Lynch JC, Pendergrass ML. Quality of care for diabetic patients in a large urban public hospital. Diabetes Care. 2003;26:563-568.
18. Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24:1815-1820.
19. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27:S11-S14.
20. U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med. 2003;138:212-214.
21. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167.
22. Centers for Disease Control and Prevention. Prevalence of diabetes and impaired fasting glucose in adults: United States, 1999-2000. Morb Mortal Wkly Rep. 2003;52:833-837.
23. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
24. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
25. Feld S. AACE diabetes guidelines. Endocr Pract. 2002;8(suppl 1):41-65.
26. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
27. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18:S42-S49.
28. Rosenstock J. Insulin therapy: optimizing control in type 1 and type 2 diabetes. Clin Cornerstone. 2001;4:50-64.
29. Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182-188.
30. Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639-643.
31. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
32. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
33. Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353-363.
34. Malmberg K. and the DIGAMI (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction) Study Group Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. Br Med J. 1997;314:1512-1515.
35. Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctance. Diabetes Care. 1997;20:292-298.
36. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
37. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
38. Full U.S. Prescribing Information for Lantus. Available at: http://www.lantus.com/professional/home.jsp .Accessed March 5, 2003.
39. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
40. Rosenstock J, Schwartz SL, Clark CM, Jr.,, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
41. Raut M, Sung JCY, Law AW. Cost of treating hypoglycemia among patients with diabetes [abstract]. Diabetes Care. 2003;26(suppl 2):A1152.-
Disclosures: Dr LeRoith serves as a consultant to Aventis Pharmaceuticals, Pfizer Inc, and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals, Pfizer Inc, and Eli Lilly and Co. Dr Levetan serves as a consultant to Eli Lilly and Co. and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals and Novo Nordisk Pharmaceuticals, Inc. Dr Hirsch serves as a consultant to Eli Lilly and Co., Novo Nordisk Pharmaceuticals, Inc., Aventis Pharmaceuticals, and Medtronic MiniMed. Dr Riddle has received grant/research support from Amylin Pharmaceuticals, Aventis Pharmaceuticals, and Pfizer Inc. He serves as a consultant to and is on the speakers’ bureaus of Amylin Pharmaceuticals, Aventis Pharmaceuticals, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, Inc. Corresponding author: Derek Le Roith, MD, PhD, Room 8D12, Bldg 10, National Institutes of Health, MSC 1758, Bethesda MD 20892-1758. E-mail: [email protected].
- Patients should be screened for diabetes at age 45 years—earlier if they are overweight and have at least 1 other risk factor.
- Management of type 2 diabetes requires a multifactorial approach that includes not only glycemic control but also addresses such risk factors as hypertension, dyslipidemia, renal impairment, and obesity.
- Tight glucose control (A1C <7%) may require intensive therapy with more than one antiglycemic agent. Early addition of basal insulin may be an efficient way to achieve A1C targets in some patients.
According to the latest estimates by the American Diabetes Association (ADA), more than 12.1 million persons in the United States have been diagnosed with diabetes and about 6 million remain undiagnosed.1 Type 2 diabetes, which comprises the majority (up to 95%) of all diabetes cases,2 has a profound impact on patient health and quality of life and places significant burdens on the health care system. For example, it confers a risk for myocardial infarction (MI) and cardiovascular mortality that is comparable to that for patients who have previously had an MI.3
Economically, the costs of diabetes in the United States are overwhelming: direct and indirect medical expenditures related to diabetes totaled $132 billion in 2002 and are expected to increase to $192 billion annually by 2020.1 Demographically adjusted, per-capita expenses for persons diagnosed with diabetes are more than double those of persons without diabetes.1
Clinical challenge
Type 2 diabetes poses a major challenge to primary care physicians, who are the main providers of care for about 80% of patients with this disease.4 Patients may present with a variety of complications, including neurologic, peripheral vascular, cardiovascular (eg, MI, stroke), renal, and ophthalmic disorders.1 Often, both microvascular and macrovascular complications precede the initial diagnosis of diabetes.5
Results of randomized clinical trials and population-based studies confirm that secondary preventive measures (ie, ameliorating risk factors in patients with known diabetes) and improved glycemic control can help reduce diabetes complications.3,6 A multifactorial approach that both achieves glycemic control and addresses other risk factors, such as dyslipidemia, hypertension, and microalbuminuria, has been demonstrated to be important, particularly for reducing macrovascular complications.7-9 Treatment goals for patients with type 2 diabetes include: an A1C value of <7.0%, a low-density lipoprotein cholesterol level of <100 mg/dL, and a blood pressure level of <130/80 mm Hg.10-12
The United Kingdom Prospective Diabetes Study (UKPDS 35)—in a posthoc observational substudy—suggested that there is “no threshold” for A1C lowering for any type of diabetes complication, indicating that treatment probably should aim to bring the A1C level as close to normal as possible.6 Thus, the American College of Endocrinology (ACE), for example, has set an even more aggressive A1C goal of ≤6.5%.13 Organizations such as the ADA and ACE believe that lower A1C targets not only are beneficial but that they also are achievable with currently available therapeutic agents. Ultimately, an individual patient’s comorbid conditions, life expectancy, and preferences must be considered when determining goals.14
Despite compelling outcomes data, the prevalence of diabetes remains high and clinical control remains suboptimal. The Health Plan Employer Data and Information Set, a widely used tool for measuring quality of health care, currently considers an A1C of >9.5% as “poorly controlled” disease.15 According to data from the Third National Health and Nutrition Examination Survey, 18% of patients with diabetes have an A1C of >9.5%.16 In areas where obesity is highly prevalent, such as New Orleans, the percentages are even higher, with up to 43% of obese patients treated at urban teaching hospitals in that area having A1C values of >9.5%.17 The Diabetes Quality Improvement Project, which governs 20 public and private health care organizations, found that 35% of its patients had a mean A1C of >9.5%.18 Considering that 9.5% is well above the ADA goal of <7.0%, data such as these underscore the need for more widespread diabetes screening and earlier intervention.
Identifying diabetes
The ADA recommends that screening with a fasting plasma glucose test be considered in all patients aged 45 years and older, particularly those with a body mass index of 25 kg/m2 or more.19 If the results are normal, screening should be repeated every 3 years. However, patients who are thought to be at increased risk should be considered for more frequent screening. The ADA recommendations have been endorsed by the American Academy of Pediatrics. The American Academy of Family Physicians follows the recommendations established by the US Preventive Services Task Force: screening is suggested in adults with hypertension or dyslipidemia.20
Routine screening for type 1 diabetes among healthy children is not expected to yield many cases and is not recommended. However, because the incidence of type 2 diabetes is increasing among children, it has been suggested that those who are overweight (defined as weight for height >85th percentile or weight >120% of ideal for height) with 2 or more additional risk factors should be screened every 2 years, beginning at age 10 years or at the onset of puberty.19
The ADA criteria for diagnosis of diabetes are listed in Table 1 . Whichever criterion is used, diagnostic test results must be confirmed on a subsequent day.19
Patients who do not meet the diagnostic criteria but have either impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) are considered to have prediabetes (see Table 1 ).19,21 Based on recent estimates, more than 12 million adults in the United States have prediabetes.22 Of great importance is regular monitoring of patients with IGT or IFG because in addition to diabetes, they are at a heightened risk of MI or stroke compared with normoglycemic persons.
TABLE 1
Diagnostic criteria for prediabetes and diabetes
| Prediabetes | Diabetes |
|---|---|
| One or both of the following: | One or more of the following: |
|
|
| *Oral glucose tolerance test should use glucose load containing equivalent of 75 g anhydrous glucose dissolved in water. | |
| Adapted from American Diabetes Association.19 | |
Intensive management
The UKPDS showed that intensive glycemic control in type 2 diabetes significantly reduced the risk of microvascular complications.23,24 In UKPDS 33, patients with newly diagnosed type 2 diabetes whose disease remained uncontrolled after completing 3 months of dietary management were initially randomized to intensive treatment (goal: FPG <108 mg/dL) with sulfonylurea or insulin monotherapy or to conventional treatment (goal: FPG <270 mg/dL) that began with diet. After 10 years, the median A1C was 7.0% in the intensive group and 7.9% in the conventional group.23 The reduction in A1C was associated with a 25% reduction of microvascular endpoints, mainly a reduced need for retinal photocoagulation (relative risk [RR] for intensive therapy, 0.75; 95% CI, 0.60–0.93; P<.01). While there was a 16% reduction in the risk of MI, this difference was not statistically significant (RR, 0.84; 95% CI, 0.71–1.00; P=.052). The UKPDS 35, which was an epidemiologic analysis of data (ie, not a direct comparison of the 2 treatment arms), demonstrated that each 1% reduction in mean A1C was associated with a 21% decrease in risk for any diabetes-related endpoint (95% CI, 17%–24%; P<.0001), a 21% decrease in diabetes-related deaths (95% CI, 15%–27%; P<.0001), a 37% decrease in the risk for microvascular complications (95% CI, 33%–41%; P<.0001), and a 14% decrease in the risk of MI (95% CI, 8%–21%; P<.0001) ( Figure 1 ).6
Another study that supports intensive management of type 2 diabetes is the Steno-2 study, a randomized controlled trial of 160 patients with type 2 diabetes and microalbuminuria.8 After 7.8 years of follow-up, 54% of conventionally treated and 57% of intensively treated patients were receiving insulin. Decreases in risk factors (eg, A1C, blood pressure, lipids, and albumin excretion) were significantly greater in patients receiving intensive therapy ( Figure 2 ). Significant reductions also were seen in the risk of cardiovascular disease (RR, 0.47; 95% CI, 0.24–0.73), nephropathy (RR, 0.39; 95% CI, 0.17–0.87), retinopathy (RR, 0.42; 95% CI, 0.21–0.86), and autonomic neuropathy (RR, 0.37; 95% CI, 0.18–0.79). Overall, intensive therapy in the Steno-2 study reduced risk of cardiovascular and microvascular events by about 50%.
FIGURE 1
UKPDS 35: Risk reductions associated with each 1% decrease in updated mean A1C
FIGURE 2 Percentage of patients in Steno-2 who reached treatment goals at mean follow-up of 7.8 years
Role of insulin in achieving targets
Another finding of the UKPDS was that type 2 diabetes is routinely progressive and that treatment with a single agent is unlikely to be successful for more than 5 years. Such observations mirror the physiologic progression from insulin resistance to absolute insulin deficiency. Thus, lifestyle modification often needs to be supplemented with oral antihyperglycemic therapy. In some cases, early treatment with insulin is preferable to relieve symptoms of hyperglycemia (eg, blurred vision, frequent thirst), which are suggestive of glucotoxicity and deteriorating β-cell function. As absolute insulin deficiency occurs, multiple oral agents, with or without insulin, may be required for optimal control of the disease.4,25
In UKPDS 57, 826 patients with type 2 diabetes were randomized to diet (n=242), ultralente insulin, a long-acting basal insulin (n=245), or sulfonylurea with the addition of ultralente insulin if FPG remained >108 mg/dL at maximal sulfonylurea dosage (n=339). Over 6 years, 53% of those started on sulfonylurea required addition of insulin.26 Patients treated with insulin monotherapy achieved a median A1C of 7.1%, while those on insulin plus sulfonylurea had a median A1C of 6.6% (P<.01). Episodes of major hypoglycemia occurred at a rate of 1.6% per year in the combined therapy group, compared with 3.2% a year in the insulin monotherapy group (P=0.17). In view of the observed progression of β-cell dysfunction and the failure of oral agents to maintain glycemic control, early addition of insulin to existing oral therapy may be a successful strategy in patients with type 2 diabetes.
Surmounting barriers
Insulin therapy may be delayed for a variety of reasons, including concerns about weight gain and hypo-glycemia as well as misconceptions that it increases the risk of cardiovascular disease.27 UKPDS 33 noted that relatively low proportions of patients treated with insulin experienced major (needing third-party help or medical intervention) hypoglycemic events (1.8% per year; intent-to-treat analysis).23 While both hypoglycemia and weight gain may occur with any form of insulin therapy or use of oral secretagogues, the benefits of effective glycemic control should be considered; moreover, tactics that may reduce these problems are available.28 For example, combined therapy with metformin and insulin can reduce or abolish the weight gain that otherwise may occur when insulin monotherapy is started or intensified.29 Treatment-associated hypoglycemia is generally mild to moderate in type 2 diabetes, and can be addressed with self–blood-glucose monitoring and patient education regarding recognition of symptoms and self-treatment. Also, the new long-acting insulin analogue, insulin glargine, causes fewer episodes of hypoglycemia than does neutral protamine Hagedorn (NPH) insulin.30-32
It is sometimes thought that insulin therapy may be associated with increased insulin resistance; however, studies examining peripheral insulin sensitivity have demonstrated that restoration of glycemic control with insulin improved insulin sensitivity.27,33 Also, the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction trial showed that intensive glycemic control with insulin therapy after an MI reduced the risk of adverse cardiovascular outcomes.6,34
Patient barriers to insulin therapy also include fear of needles and injections or a belief that insulin therapy represents failure, punishment, or a worsened prognosis.35 The notion that starting insulin therapy is a penalty for failure may be reinforced by physicians’ misconceptions about the role of insulin in type 2 diabetes.35 Setting the appropriate expectation that type 2 diabetes is a progressive disease that often eventually requires insulin can help to prevent such negative attitudes.
Finally, both patients and physicians may perceive insulin therapy as a complex and time-consuming treatment.27 Lack of office personnel and time to provide appropriate patient education for insulin therapy may contribute to such a perception. Concerns over complexity may be ameliorated by use of devices such as insulin pens and jet injectors that provide a precise dose and simplify self-administration. Premixed insulins are given twice daily and provide a measure of convenience, although flexibility of meal timing is limited in order to prevent hypoglycemia. Newer treatment regimens may also reduce patient apprehension concerning complexity and increase compliance.4 Notably, a simple and effective approach is to add once-daily basal insulin to established oral therapy.
Basal insulin studies
The efficiency and efficacy of adding basal insulin to existing oral antidiabetic agents was demonstrated in the randomized, open-label Treat-to-Target Trial.32 Overweight patients (N=756) who had been inadequately controlled (A1C >7.5%) on 1 or 2 oral agents (sulfonylurea, metformin, or thiazolidinedione) received insulin glargine or NPH insulin. The insulin dosage, taken at bedtime, was systematically titrated, based on before-breakfast glucose tests performed daily by the patients. Mean A1C levels fell from 8.6% to 6.9% after 24 weeks of therapy ( Figure 3 ). Both insulins performed similarly in terms of glycemic control, with 57% of patients in each group achieving an A1C of ≥7.0%. However, there was a significant difference in the incidence of hypoglycemia. Complete treatment success, defined as reaching target A1C without a single episode of nocturnal hypoglycemia (≤72 mg/dL), was achieved in 33% of the glargine patients and in 27% of the NPH group (P<.05). Overall rates of hypoglycemia, expressed as events per patient per year, were as follows: 14 symptomatic events in glargine patients vs 18 in NPH patients (P<.02), 9 confirmed events of ≤72 mg/dL in glargine patients vs 13 in NPH patients (P<.005), and 3 confirmed events of ≤56 mg/dL in glargine patients vs 5 in NPH patients (P<.003).
In another recent multicenter trial, 695 patients were randomized to 24 weeks of treatment with glimepiride plus 1 of 3 insulin regimens: morning insulin glargine, bedtime insulin glargine, or bedtime NPH insulin.36 Mean A1C levels decreased by 1.24% with morning glargine, 0.96% with bedtime glargine, and 0.84% with bedtime NPH. The percentage of patients achieving an A1C of ≤7.5% was 43% with morning glargine, 33% with bedtime glargine (P=.021 vs morning glargine), and 32% with bedtime NPH (P=.017 vs morning glargine). The percentage of patients who experienced nocturnal hypoglycemia was 17% with morning glargine, 23% with bedtime glargine, and 38% with bedtime NPH (P=.001 vs both glargine regimens). Finally, the percentage of patients who experienced symptomatic hypoglycemia was 56% with morning glargine (P=.004 vs bedtime glargine), 43% with bedtime glargine, and 58% with bedtime NPH (P=.001 vs bedtime glargine).
FIGURE 3 Treat-to-Target Trial: Rapid achievement of A1C goal
Practical aspects
Most commonly, insulin (Table 2) is introduced when single- or multipleagent oral therapy has failed to maintain glycemic control. Insulin may be added to existing oral therapy or, less typically, used as monotherapy. Of the 3 available insulins that have been used for basal therapy (NPH insulin, insulin glargine, and ultralente [insulin zinc extended]), ultralente may not be ideal since considerable variation has been noted in its pharmacokinetic and pharmacodynamic effects.37 Either NPH or glargine can be started at a dose of 10 U, at bedtime, while oral agents are continued at previous dosages. To provide 24-hour coverage, NPH may be needed twice daily whereas a single dose of glargine usually provides 24-hour coverage when administered at the same time each day.38 Patients usually need a total daily basal insulin dose of 0.5 to 0.6 U/kg (typically about 45 U daily for a 100-kg person).39 The dose may be adjusted weekly according to the patient’s FPG level.
Cost is a consideration in insulin therapy. Although more expensive than NPH, basal glargine is associated with 25% fewer episodes of nocturnal hypoglycemia, improved postdinner control, and slightly less weight gain.31,40 A retrospective review of medical and pharmacy claims demonstrated that considerable direct costs result from treatment of hypoglycemia associated with antidiabetic therapy, suggesting that therapies with less potential for inducing hypoglycemia would likely reduce these costs.41
The intensive insulin titration schedule used in the Treat-to-Target Trial is shown in Table 3. In clinical practice, a somewhat less intensive approach may work well. For example, the dose may be increased weekly by 4 U if the FPG level is >140 mg/dL on 3 consecutive occasions, or by 2 U if the FPG level is 120 to 140 mg/dL on 3 consecutive occasions.
Insulin glargine should not be mixed in the same syringe with other insulins as the pharmacokinetic profile may be altered. In the case of NPH, careful rolling or turning of the vial prior to injection to fully resuspend the crystals is advisable to minimize the variability of effect that may otherwise occur. Glargine, as with the regular and rapid-acting insulin analogues lispro and aspart, is a clear solution that does not need to be resuspended before administration. The addition of rapid-acting insulin analogues prandially to a regimen of basal insulin or basal-bolus therapy can be used to more closely mimic physiologic insulin secretion and may be needed when basal insulin replacement alone is insufficient to reach target levels of glycemic control.4
TABLE 2
Insulin formulations
| Insulin* | Onset | Peak | Effective duration (h) |
|---|---|---|---|
| Rapid-acting | |||
| Lispro | 5–15 min | 30–90 min | 5 |
| Aspart | 5–15 min | 30–90 min | 5 |
| Short-acting | |||
| Regular U100 | 30–60 min | 2–3 h | 5–8 |
| Regular U500 | 30–60 min | 2–3 h | 5–8 |
| Intermediate-acting | |||
| Isophane insulin (NPH) | 2–4 h | 4–10 h | 10–16 |
| Insulin zinc | 2–4 h | 4–12 h | 12–18 |
| Long-acting | |||
| Insulin zinc extended | 6–10 h | 10–16 h | 18–24 |
| Insulin glargine | 2–4 h† | No pronounced peak | 20–24 |
| Premixed | |||
| 70% NPH/30% regular | 30–60 min | Dual | 10–16 |
| 50% NPH/50% regular | 30–60 min | Dual | 10–16 |
| 75% NPL/25% lispro | 5–15 min | Dual | 10–16 |
| 70% NP/30% aspart | 5–15 min | Dual | 10–16 |
| *Assuming 0.1–0.2 U/kg per injection; onset and duration may vary by injection site (except for insulin glargine). | |||
| †Time to steady state. | |||
| NPH = neutral protamine Hagedorn; NPL = neutral protamine lispro; NP = neutral protamine. | |||
| Adapted from DeWitt and Hirsch.4 | |||
TABLE 3
Basal insulin: Initiation and dosage adjustment
| Forced titration schedule | |||
|---|---|---|---|
| |||
| Self-monitored FPG (mg/dL) | Dosage increase (IU/day) in Treat-to-Target Trial | ||
| ≥180 | 8 | ||
| ≥140 – <180 | 6 | ||
| ≥120 – <140 | 4 | ||
| >100 – <120 | 2 | ||
| *No increase if plasma glucose is <72 mg/dL in the preceding week; decrease dosage (2–4 IU/day) if plasma glucose is <56 mg/dL or severe hypoglycemia (requiring assistance) occurred within the preceding week. | |||
| FPG = fasting plasma glucose. | |||
| Adapted from Riddle et al.32 | |||
Summary
New evidence and methods continue to alter management of patients with type 2 diabetes. Appropriate screening and earlier intervention may help reduce the incidence and progression of microvascular and macrovascular complications. A multifactorial approach that addresses such risk factors as blood pressure, lipids, and glycemia has demonstrated reduced morbidity and mortality. While glycemic targets are getting lower, they can be efficiently attained with combined basal insulin and oral antidiabetic therapy. Insulin, too often considered a therapy of last resort, is an important intervention that can be used safely and effectively earlier in the course of type 2 diabetes.
- Patients should be screened for diabetes at age 45 years—earlier if they are overweight and have at least 1 other risk factor.
- Management of type 2 diabetes requires a multifactorial approach that includes not only glycemic control but also addresses such risk factors as hypertension, dyslipidemia, renal impairment, and obesity.
- Tight glucose control (A1C <7%) may require intensive therapy with more than one antiglycemic agent. Early addition of basal insulin may be an efficient way to achieve A1C targets in some patients.
According to the latest estimates by the American Diabetes Association (ADA), more than 12.1 million persons in the United States have been diagnosed with diabetes and about 6 million remain undiagnosed.1 Type 2 diabetes, which comprises the majority (up to 95%) of all diabetes cases,2 has a profound impact on patient health and quality of life and places significant burdens on the health care system. For example, it confers a risk for myocardial infarction (MI) and cardiovascular mortality that is comparable to that for patients who have previously had an MI.3
Economically, the costs of diabetes in the United States are overwhelming: direct and indirect medical expenditures related to diabetes totaled $132 billion in 2002 and are expected to increase to $192 billion annually by 2020.1 Demographically adjusted, per-capita expenses for persons diagnosed with diabetes are more than double those of persons without diabetes.1
Clinical challenge
Type 2 diabetes poses a major challenge to primary care physicians, who are the main providers of care for about 80% of patients with this disease.4 Patients may present with a variety of complications, including neurologic, peripheral vascular, cardiovascular (eg, MI, stroke), renal, and ophthalmic disorders.1 Often, both microvascular and macrovascular complications precede the initial diagnosis of diabetes.5
Results of randomized clinical trials and population-based studies confirm that secondary preventive measures (ie, ameliorating risk factors in patients with known diabetes) and improved glycemic control can help reduce diabetes complications.3,6 A multifactorial approach that both achieves glycemic control and addresses other risk factors, such as dyslipidemia, hypertension, and microalbuminuria, has been demonstrated to be important, particularly for reducing macrovascular complications.7-9 Treatment goals for patients with type 2 diabetes include: an A1C value of <7.0%, a low-density lipoprotein cholesterol level of <100 mg/dL, and a blood pressure level of <130/80 mm Hg.10-12
The United Kingdom Prospective Diabetes Study (UKPDS 35)—in a posthoc observational substudy—suggested that there is “no threshold” for A1C lowering for any type of diabetes complication, indicating that treatment probably should aim to bring the A1C level as close to normal as possible.6 Thus, the American College of Endocrinology (ACE), for example, has set an even more aggressive A1C goal of ≤6.5%.13 Organizations such as the ADA and ACE believe that lower A1C targets not only are beneficial but that they also are achievable with currently available therapeutic agents. Ultimately, an individual patient’s comorbid conditions, life expectancy, and preferences must be considered when determining goals.14
Despite compelling outcomes data, the prevalence of diabetes remains high and clinical control remains suboptimal. The Health Plan Employer Data and Information Set, a widely used tool for measuring quality of health care, currently considers an A1C of >9.5% as “poorly controlled” disease.15 According to data from the Third National Health and Nutrition Examination Survey, 18% of patients with diabetes have an A1C of >9.5%.16 In areas where obesity is highly prevalent, such as New Orleans, the percentages are even higher, with up to 43% of obese patients treated at urban teaching hospitals in that area having A1C values of >9.5%.17 The Diabetes Quality Improvement Project, which governs 20 public and private health care organizations, found that 35% of its patients had a mean A1C of >9.5%.18 Considering that 9.5% is well above the ADA goal of <7.0%, data such as these underscore the need for more widespread diabetes screening and earlier intervention.
Identifying diabetes
The ADA recommends that screening with a fasting plasma glucose test be considered in all patients aged 45 years and older, particularly those with a body mass index of 25 kg/m2 or more.19 If the results are normal, screening should be repeated every 3 years. However, patients who are thought to be at increased risk should be considered for more frequent screening. The ADA recommendations have been endorsed by the American Academy of Pediatrics. The American Academy of Family Physicians follows the recommendations established by the US Preventive Services Task Force: screening is suggested in adults with hypertension or dyslipidemia.20
Routine screening for type 1 diabetes among healthy children is not expected to yield many cases and is not recommended. However, because the incidence of type 2 diabetes is increasing among children, it has been suggested that those who are overweight (defined as weight for height >85th percentile or weight >120% of ideal for height) with 2 or more additional risk factors should be screened every 2 years, beginning at age 10 years or at the onset of puberty.19
The ADA criteria for diagnosis of diabetes are listed in Table 1 . Whichever criterion is used, diagnostic test results must be confirmed on a subsequent day.19
Patients who do not meet the diagnostic criteria but have either impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) are considered to have prediabetes (see Table 1 ).19,21 Based on recent estimates, more than 12 million adults in the United States have prediabetes.22 Of great importance is regular monitoring of patients with IGT or IFG because in addition to diabetes, they are at a heightened risk of MI or stroke compared with normoglycemic persons.
TABLE 1
Diagnostic criteria for prediabetes and diabetes
| Prediabetes | Diabetes |
|---|---|
| One or both of the following: | One or more of the following: |
|
|
| *Oral glucose tolerance test should use glucose load containing equivalent of 75 g anhydrous glucose dissolved in water. | |
| Adapted from American Diabetes Association.19 | |
Intensive management
The UKPDS showed that intensive glycemic control in type 2 diabetes significantly reduced the risk of microvascular complications.23,24 In UKPDS 33, patients with newly diagnosed type 2 diabetes whose disease remained uncontrolled after completing 3 months of dietary management were initially randomized to intensive treatment (goal: FPG <108 mg/dL) with sulfonylurea or insulin monotherapy or to conventional treatment (goal: FPG <270 mg/dL) that began with diet. After 10 years, the median A1C was 7.0% in the intensive group and 7.9% in the conventional group.23 The reduction in A1C was associated with a 25% reduction of microvascular endpoints, mainly a reduced need for retinal photocoagulation (relative risk [RR] for intensive therapy, 0.75; 95% CI, 0.60–0.93; P<.01). While there was a 16% reduction in the risk of MI, this difference was not statistically significant (RR, 0.84; 95% CI, 0.71–1.00; P=.052). The UKPDS 35, which was an epidemiologic analysis of data (ie, not a direct comparison of the 2 treatment arms), demonstrated that each 1% reduction in mean A1C was associated with a 21% decrease in risk for any diabetes-related endpoint (95% CI, 17%–24%; P<.0001), a 21% decrease in diabetes-related deaths (95% CI, 15%–27%; P<.0001), a 37% decrease in the risk for microvascular complications (95% CI, 33%–41%; P<.0001), and a 14% decrease in the risk of MI (95% CI, 8%–21%; P<.0001) ( Figure 1 ).6
Another study that supports intensive management of type 2 diabetes is the Steno-2 study, a randomized controlled trial of 160 patients with type 2 diabetes and microalbuminuria.8 After 7.8 years of follow-up, 54% of conventionally treated and 57% of intensively treated patients were receiving insulin. Decreases in risk factors (eg, A1C, blood pressure, lipids, and albumin excretion) were significantly greater in patients receiving intensive therapy ( Figure 2 ). Significant reductions also were seen in the risk of cardiovascular disease (RR, 0.47; 95% CI, 0.24–0.73), nephropathy (RR, 0.39; 95% CI, 0.17–0.87), retinopathy (RR, 0.42; 95% CI, 0.21–0.86), and autonomic neuropathy (RR, 0.37; 95% CI, 0.18–0.79). Overall, intensive therapy in the Steno-2 study reduced risk of cardiovascular and microvascular events by about 50%.
FIGURE 1
UKPDS 35: Risk reductions associated with each 1% decrease in updated mean A1C
FIGURE 2 Percentage of patients in Steno-2 who reached treatment goals at mean follow-up of 7.8 years
Role of insulin in achieving targets
Another finding of the UKPDS was that type 2 diabetes is routinely progressive and that treatment with a single agent is unlikely to be successful for more than 5 years. Such observations mirror the physiologic progression from insulin resistance to absolute insulin deficiency. Thus, lifestyle modification often needs to be supplemented with oral antihyperglycemic therapy. In some cases, early treatment with insulin is preferable to relieve symptoms of hyperglycemia (eg, blurred vision, frequent thirst), which are suggestive of glucotoxicity and deteriorating β-cell function. As absolute insulin deficiency occurs, multiple oral agents, with or without insulin, may be required for optimal control of the disease.4,25
In UKPDS 57, 826 patients with type 2 diabetes were randomized to diet (n=242), ultralente insulin, a long-acting basal insulin (n=245), or sulfonylurea with the addition of ultralente insulin if FPG remained >108 mg/dL at maximal sulfonylurea dosage (n=339). Over 6 years, 53% of those started on sulfonylurea required addition of insulin.26 Patients treated with insulin monotherapy achieved a median A1C of 7.1%, while those on insulin plus sulfonylurea had a median A1C of 6.6% (P<.01). Episodes of major hypoglycemia occurred at a rate of 1.6% per year in the combined therapy group, compared with 3.2% a year in the insulin monotherapy group (P=0.17). In view of the observed progression of β-cell dysfunction and the failure of oral agents to maintain glycemic control, early addition of insulin to existing oral therapy may be a successful strategy in patients with type 2 diabetes.
Surmounting barriers
Insulin therapy may be delayed for a variety of reasons, including concerns about weight gain and hypo-glycemia as well as misconceptions that it increases the risk of cardiovascular disease.27 UKPDS 33 noted that relatively low proportions of patients treated with insulin experienced major (needing third-party help or medical intervention) hypoglycemic events (1.8% per year; intent-to-treat analysis).23 While both hypoglycemia and weight gain may occur with any form of insulin therapy or use of oral secretagogues, the benefits of effective glycemic control should be considered; moreover, tactics that may reduce these problems are available.28 For example, combined therapy with metformin and insulin can reduce or abolish the weight gain that otherwise may occur when insulin monotherapy is started or intensified.29 Treatment-associated hypoglycemia is generally mild to moderate in type 2 diabetes, and can be addressed with self–blood-glucose monitoring and patient education regarding recognition of symptoms and self-treatment. Also, the new long-acting insulin analogue, insulin glargine, causes fewer episodes of hypoglycemia than does neutral protamine Hagedorn (NPH) insulin.30-32
It is sometimes thought that insulin therapy may be associated with increased insulin resistance; however, studies examining peripheral insulin sensitivity have demonstrated that restoration of glycemic control with insulin improved insulin sensitivity.27,33 Also, the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction trial showed that intensive glycemic control with insulin therapy after an MI reduced the risk of adverse cardiovascular outcomes.6,34
Patient barriers to insulin therapy also include fear of needles and injections or a belief that insulin therapy represents failure, punishment, or a worsened prognosis.35 The notion that starting insulin therapy is a penalty for failure may be reinforced by physicians’ misconceptions about the role of insulin in type 2 diabetes.35 Setting the appropriate expectation that type 2 diabetes is a progressive disease that often eventually requires insulin can help to prevent such negative attitudes.
Finally, both patients and physicians may perceive insulin therapy as a complex and time-consuming treatment.27 Lack of office personnel and time to provide appropriate patient education for insulin therapy may contribute to such a perception. Concerns over complexity may be ameliorated by use of devices such as insulin pens and jet injectors that provide a precise dose and simplify self-administration. Premixed insulins are given twice daily and provide a measure of convenience, although flexibility of meal timing is limited in order to prevent hypoglycemia. Newer treatment regimens may also reduce patient apprehension concerning complexity and increase compliance.4 Notably, a simple and effective approach is to add once-daily basal insulin to established oral therapy.
Basal insulin studies
The efficiency and efficacy of adding basal insulin to existing oral antidiabetic agents was demonstrated in the randomized, open-label Treat-to-Target Trial.32 Overweight patients (N=756) who had been inadequately controlled (A1C >7.5%) on 1 or 2 oral agents (sulfonylurea, metformin, or thiazolidinedione) received insulin glargine or NPH insulin. The insulin dosage, taken at bedtime, was systematically titrated, based on before-breakfast glucose tests performed daily by the patients. Mean A1C levels fell from 8.6% to 6.9% after 24 weeks of therapy ( Figure 3 ). Both insulins performed similarly in terms of glycemic control, with 57% of patients in each group achieving an A1C of ≥7.0%. However, there was a significant difference in the incidence of hypoglycemia. Complete treatment success, defined as reaching target A1C without a single episode of nocturnal hypoglycemia (≤72 mg/dL), was achieved in 33% of the glargine patients and in 27% of the NPH group (P<.05). Overall rates of hypoglycemia, expressed as events per patient per year, were as follows: 14 symptomatic events in glargine patients vs 18 in NPH patients (P<.02), 9 confirmed events of ≤72 mg/dL in glargine patients vs 13 in NPH patients (P<.005), and 3 confirmed events of ≤56 mg/dL in glargine patients vs 5 in NPH patients (P<.003).
In another recent multicenter trial, 695 patients were randomized to 24 weeks of treatment with glimepiride plus 1 of 3 insulin regimens: morning insulin glargine, bedtime insulin glargine, or bedtime NPH insulin.36 Mean A1C levels decreased by 1.24% with morning glargine, 0.96% with bedtime glargine, and 0.84% with bedtime NPH. The percentage of patients achieving an A1C of ≤7.5% was 43% with morning glargine, 33% with bedtime glargine (P=.021 vs morning glargine), and 32% with bedtime NPH (P=.017 vs morning glargine). The percentage of patients who experienced nocturnal hypoglycemia was 17% with morning glargine, 23% with bedtime glargine, and 38% with bedtime NPH (P=.001 vs both glargine regimens). Finally, the percentage of patients who experienced symptomatic hypoglycemia was 56% with morning glargine (P=.004 vs bedtime glargine), 43% with bedtime glargine, and 58% with bedtime NPH (P=.001 vs bedtime glargine).
FIGURE 3 Treat-to-Target Trial: Rapid achievement of A1C goal
Practical aspects
Most commonly, insulin (Table 2) is introduced when single- or multipleagent oral therapy has failed to maintain glycemic control. Insulin may be added to existing oral therapy or, less typically, used as monotherapy. Of the 3 available insulins that have been used for basal therapy (NPH insulin, insulin glargine, and ultralente [insulin zinc extended]), ultralente may not be ideal since considerable variation has been noted in its pharmacokinetic and pharmacodynamic effects.37 Either NPH or glargine can be started at a dose of 10 U, at bedtime, while oral agents are continued at previous dosages. To provide 24-hour coverage, NPH may be needed twice daily whereas a single dose of glargine usually provides 24-hour coverage when administered at the same time each day.38 Patients usually need a total daily basal insulin dose of 0.5 to 0.6 U/kg (typically about 45 U daily for a 100-kg person).39 The dose may be adjusted weekly according to the patient’s FPG level.
Cost is a consideration in insulin therapy. Although more expensive than NPH, basal glargine is associated with 25% fewer episodes of nocturnal hypoglycemia, improved postdinner control, and slightly less weight gain.31,40 A retrospective review of medical and pharmacy claims demonstrated that considerable direct costs result from treatment of hypoglycemia associated with antidiabetic therapy, suggesting that therapies with less potential for inducing hypoglycemia would likely reduce these costs.41
The intensive insulin titration schedule used in the Treat-to-Target Trial is shown in Table 3. In clinical practice, a somewhat less intensive approach may work well. For example, the dose may be increased weekly by 4 U if the FPG level is >140 mg/dL on 3 consecutive occasions, or by 2 U if the FPG level is 120 to 140 mg/dL on 3 consecutive occasions.
Insulin glargine should not be mixed in the same syringe with other insulins as the pharmacokinetic profile may be altered. In the case of NPH, careful rolling or turning of the vial prior to injection to fully resuspend the crystals is advisable to minimize the variability of effect that may otherwise occur. Glargine, as with the regular and rapid-acting insulin analogues lispro and aspart, is a clear solution that does not need to be resuspended before administration. The addition of rapid-acting insulin analogues prandially to a regimen of basal insulin or basal-bolus therapy can be used to more closely mimic physiologic insulin secretion and may be needed when basal insulin replacement alone is insufficient to reach target levels of glycemic control.4
TABLE 2
Insulin formulations
| Insulin* | Onset | Peak | Effective duration (h) |
|---|---|---|---|
| Rapid-acting | |||
| Lispro | 5–15 min | 30–90 min | 5 |
| Aspart | 5–15 min | 30–90 min | 5 |
| Short-acting | |||
| Regular U100 | 30–60 min | 2–3 h | 5–8 |
| Regular U500 | 30–60 min | 2–3 h | 5–8 |
| Intermediate-acting | |||
| Isophane insulin (NPH) | 2–4 h | 4–10 h | 10–16 |
| Insulin zinc | 2–4 h | 4–12 h | 12–18 |
| Long-acting | |||
| Insulin zinc extended | 6–10 h | 10–16 h | 18–24 |
| Insulin glargine | 2–4 h† | No pronounced peak | 20–24 |
| Premixed | |||
| 70% NPH/30% regular | 30–60 min | Dual | 10–16 |
| 50% NPH/50% regular | 30–60 min | Dual | 10–16 |
| 75% NPL/25% lispro | 5–15 min | Dual | 10–16 |
| 70% NP/30% aspart | 5–15 min | Dual | 10–16 |
| *Assuming 0.1–0.2 U/kg per injection; onset and duration may vary by injection site (except for insulin glargine). | |||
| †Time to steady state. | |||
| NPH = neutral protamine Hagedorn; NPL = neutral protamine lispro; NP = neutral protamine. | |||
| Adapted from DeWitt and Hirsch.4 | |||
TABLE 3
Basal insulin: Initiation and dosage adjustment
| Forced titration schedule | |||
|---|---|---|---|
| |||
| Self-monitored FPG (mg/dL) | Dosage increase (IU/day) in Treat-to-Target Trial | ||
| ≥180 | 8 | ||
| ≥140 – <180 | 6 | ||
| ≥120 – <140 | 4 | ||
| >100 – <120 | 2 | ||
| *No increase if plasma glucose is <72 mg/dL in the preceding week; decrease dosage (2–4 IU/day) if plasma glucose is <56 mg/dL or severe hypoglycemia (requiring assistance) occurred within the preceding week. | |||
| FPG = fasting plasma glucose. | |||
| Adapted from Riddle et al.32 | |||
Summary
New evidence and methods continue to alter management of patients with type 2 diabetes. Appropriate screening and earlier intervention may help reduce the incidence and progression of microvascular and macrovascular complications. A multifactorial approach that addresses such risk factors as blood pressure, lipids, and glycemia has demonstrated reduced morbidity and mortality. While glycemic targets are getting lower, they can be efficiently attained with combined basal insulin and oral antidiabetic therapy. Insulin, too often considered a therapy of last resort, is an important intervention that can be used safely and effectively earlier in the course of type 2 diabetes.
1. American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917-932.
2. National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics fact sheet: general information and national estimates on diabetes in the United States, 2000. Bethesda, Md: U.S. Department of Health and Human Services, National Institutes of Health, 2002. National Institute of Diabetes and Digestive and Kidney Diseases, 2002.
3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
4. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
5. Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802-1817.
6. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405-412.
7. Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno-2 randomised study. Lancet. 1999;353:617-622.
8. Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
9. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998;317:703-713.
10. American Diabetes Association. Clinical practice recommendations 2003. Diabetes Care. 2003;26(suppl 1):S1-S156.
11. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
12. National Institutes of Health, National Heart Lung and Blood Institute. JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md: US Dept of Health and Human Services; May 2003. NIH publication 03-5233. Available at: http://www.nhlbi.nih.gov/guidelines/hypertension/express. Accessed November 14, 2003.
13. American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management: 2000 update. Endocr Pract. 2000;8(suppl 1):40-82.
14. Woolf SH, Davidson MB, Greenfield S, et al. Controlling blood glucose levels in patients with type 2 diabetes mellitus: an evidence-based policy statement by the American Academy of Family Physicians and American Diabetes Association. J Fam Pract. 2000;49:453-460.
15. National Committee for Quality Assurance. The State of Health Care Quality: 2003. Washington, DC: NCQA; 2003;1-34.
16. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KMV. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
17. Suwattee P, Lynch JC, Pendergrass ML. Quality of care for diabetic patients in a large urban public hospital. Diabetes Care. 2003;26:563-568.
18. Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24:1815-1820.
19. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27:S11-S14.
20. U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med. 2003;138:212-214.
21. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167.
22. Centers for Disease Control and Prevention. Prevalence of diabetes and impaired fasting glucose in adults: United States, 1999-2000. Morb Mortal Wkly Rep. 2003;52:833-837.
23. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
24. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
25. Feld S. AACE diabetes guidelines. Endocr Pract. 2002;8(suppl 1):41-65.
26. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
27. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18:S42-S49.
28. Rosenstock J. Insulin therapy: optimizing control in type 1 and type 2 diabetes. Clin Cornerstone. 2001;4:50-64.
29. Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182-188.
30. Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639-643.
31. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
32. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
33. Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353-363.
34. Malmberg K. and the DIGAMI (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction) Study Group Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. Br Med J. 1997;314:1512-1515.
35. Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctance. Diabetes Care. 1997;20:292-298.
36. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
37. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
38. Full U.S. Prescribing Information for Lantus. Available at: http://www.lantus.com/professional/home.jsp .Accessed March 5, 2003.
39. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
40. Rosenstock J, Schwartz SL, Clark CM, Jr.,, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
41. Raut M, Sung JCY, Law AW. Cost of treating hypoglycemia among patients with diabetes [abstract]. Diabetes Care. 2003;26(suppl 2):A1152.-
Disclosures: Dr LeRoith serves as a consultant to Aventis Pharmaceuticals, Pfizer Inc, and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals, Pfizer Inc, and Eli Lilly and Co. Dr Levetan serves as a consultant to Eli Lilly and Co. and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals and Novo Nordisk Pharmaceuticals, Inc. Dr Hirsch serves as a consultant to Eli Lilly and Co., Novo Nordisk Pharmaceuticals, Inc., Aventis Pharmaceuticals, and Medtronic MiniMed. Dr Riddle has received grant/research support from Amylin Pharmaceuticals, Aventis Pharmaceuticals, and Pfizer Inc. He serves as a consultant to and is on the speakers’ bureaus of Amylin Pharmaceuticals, Aventis Pharmaceuticals, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, Inc. Corresponding author: Derek Le Roith, MD, PhD, Room 8D12, Bldg 10, National Institutes of Health, MSC 1758, Bethesda MD 20892-1758. E-mail: [email protected].
1. American Diabetes Association. Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917-932.
2. National Institute of Diabetes and Digestive and Kidney Diseases. National diabetes statistics fact sheet: general information and national estimates on diabetes in the United States, 2000. Bethesda, Md: U.S. Department of Health and Human Services, National Institutes of Health, 2002. National Institute of Diabetes and Digestive and Kidney Diseases, 2002.
3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
4. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264.
5. Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802-1817.
6. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321:405-412.
7. Gaede P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno-2 randomised study. Lancet. 1999;353:617-622.
8. Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383-393.
9. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J. 1998;317:703-713.
10. American Diabetes Association. Clinical practice recommendations 2003. Diabetes Care. 2003;26(suppl 1):S1-S156.
11. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
12. National Institutes of Health, National Heart Lung and Blood Institute. JNC 7 Express: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, Md: US Dept of Health and Human Services; May 2003. NIH publication 03-5233. Available at: http://www.nhlbi.nih.gov/guidelines/hypertension/express. Accessed November 14, 2003.
13. American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management: 2000 update. Endocr Pract. 2000;8(suppl 1):40-82.
14. Woolf SH, Davidson MB, Greenfield S, et al. Controlling blood glucose levels in patients with type 2 diabetes mellitus: an evidence-based policy statement by the American Academy of Family Physicians and American Diabetes Association. J Fam Pract. 2000;49:453-460.
15. National Committee for Quality Assurance. The State of Health Care Quality: 2003. Washington, DC: NCQA; 2003;1-34.
16. Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Venkat Narayan KMV. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136:565-574.
17. Suwattee P, Lynch JC, Pendergrass ML. Quality of care for diabetic patients in a large urban public hospital. Diabetes Care. 2003;26:563-568.
18. Fleming BB, Greenfield S, Engelgau MM, Pogach LM, Clauser SB, Parrott MA. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24:1815-1820.
19. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004;27:S11-S14.
20. U.S. Preventive Services Task Force. Screening for type 2 diabetes mellitus in adults: recommendations and rationale. Ann Intern Med. 2003;138:212-214.
21. Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160-3167.
22. Centers for Disease Control and Prevention. Prevalence of diabetes and impaired fasting glucose in adults: United States, 1999-2000. Morb Mortal Wkly Rep. 2003;52:833-837.
23. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853.
24. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854-865.
25. Feld S. AACE diabetes guidelines. Endocr Pract. 2002;8(suppl 1):41-65.
26. Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002;25:330-336.
27. Riddle MC. The underuse of insulin therapy in North America. Diabetes Metab Res Rev. 2002;18:S42-S49.
28. Rosenstock J. Insulin therapy: optimizing control in type 1 and type 2 diabetes. Clin Cornerstone. 2001;4:50-64.
29. Avilés-Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;131:182-188.
30. Ratner RE, Hirsch IB, Neifing JL, Garg SK, Mecca TE, Wilson CA. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. U.S. Study Group of Insulin Glargine in Type 1 Diabetes. Diabetes Care. 2000;23:639-643.
31. Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136.
32. Riddle MC, Rosenstock J, Gerich J. The Treat-to-Target Trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080-3086.
33. Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353-363.
34. Malmberg K. and the DIGAMI (Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction) Study Group Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. Br Med J. 1997;314:1512-1515.
35. Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients’ fears and hopes about insulin therapy: the basis of patient reluctance. Diabetes Care. 1997;20:292-298.
36. Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2003;138:952-959.
37. Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142-2148.
38. Full U.S. Prescribing Information for Lantus. Available at: http://www.lantus.com/professional/home.jsp .Accessed March 5, 2003.
39. DeWitt DE, Dugdale DC. Using new insulin strategies in the outpatient treatment of diabetes: clinical applications. JAMA. 2003;289:2265-2269.
40. Rosenstock J, Schwartz SL, Clark CM, Jr.,, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636.
41. Raut M, Sung JCY, Law AW. Cost of treating hypoglycemia among patients with diabetes [abstract]. Diabetes Care. 2003;26(suppl 2):A1152.-
Disclosures: Dr LeRoith serves as a consultant to Aventis Pharmaceuticals, Pfizer Inc, and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals, Pfizer Inc, and Eli Lilly and Co. Dr Levetan serves as a consultant to Eli Lilly and Co. and Novo Nordisk Pharmaceuticals, Inc. and on the speakers’ bureaus of Aventis Pharmaceuticals and Novo Nordisk Pharmaceuticals, Inc. Dr Hirsch serves as a consultant to Eli Lilly and Co., Novo Nordisk Pharmaceuticals, Inc., Aventis Pharmaceuticals, and Medtronic MiniMed. Dr Riddle has received grant/research support from Amylin Pharmaceuticals, Aventis Pharmaceuticals, and Pfizer Inc. He serves as a consultant to and is on the speakers’ bureaus of Amylin Pharmaceuticals, Aventis Pharmaceuticals, GlaxoSmithKline, and Novo Nordisk Pharmaceuticals, Inc. Corresponding author: Derek Le Roith, MD, PhD, Room 8D12, Bldg 10, National Institutes of Health, MSC 1758, Bethesda MD 20892-1758. E-mail: [email protected].
10 steps for avoiding health disparities in your practice
We hope the answer to the question above is no. However, the evidence regarding differences in the care of patients based on race, ethnicity, gender, and socioeconomic status suggests that if this patient is a woman or African American or from a lower socioeconomic class, resultant morbidity or mortality will be higher.
Differences are seen in the provision of cardiovascular care, cancer diagnosis and treament, and HIV care. African Americans, Latino Americans, Asian Americans, and Native Americans have higher morbidity and mortality than Caucasian chemical dependency, diabetes, heart disease, infant Americans for multiple problems including cancer, mortality, and unintentional and intentional injuries.1
This article explores possible explanations for health care disparities and offers 10 practical strategies for tackling this challenging issue.
Examples of health disparities
The United States has dramatically improved the health status of its citizens—increasing longevity, reducing infant mortality and teenage pregnancies, and increasing the number of children being immunized. Despite these improvements, though, there remain persistent and disproportionate burdens of disease and illness borne by subgroups of the population (Table 1). 2,3
The Institute of Medicine in its recent report, “Unequal Treatment,” approaches the issue from another perspective: they define these disparities as “racial or ethnic differences in the quality of healthcare that are not due to access-related factors or clinical needs, preferences and appropriateness of intervention.”4
TABLE 1
Examples of health disparities that could be changed
| Disparity in mortality |
| Infant mortality |
| Infant mortality is higher for infants of African American, Native Hawaiian, and Native American mothers (13.8, 10.0, and 9.3 deaths per 1000 live births, respectively) than for infants of other race groups. Infant mortality decreases as the mother’s level of education increases. |
| Disparity in morbidity |
| Cancer (males) |
| The incidence of cancer among black males exceeds that of white males for prostate cancer (60%), lung and bronchial cancer (58% ), and colon and rectum cancers (14%). |
| Disparity in health behaviors |
| Cigarette smoking |
| Smoking among persons aged 25 years and over ranges from 11% among college graduates to 32% for those without a high school diploma; 19% of adolescents in the most rural counties smoke compared to 11% in central counties. |
| Disparity in preventive health care |
| Mammography |
| Poor women are 27% less likely to have had a recent mammogram than are women with family incomes above the poverty level. |
| Disparity in access to care |
| Health insurance coverage |
| 13% of children under aged <18 years have no health insurance coverage; 28% of children with family incomes of 1 to 1.5 times the poverty level are without coverage, compared with 5% of those with family incomes at least twice the poverty level. |
| Source: Adapted from Health, United States, 2001. |
| Hyattsville, Md: National Center for Health Statistics; 2001. |
Correcting health disparity begins with understanding its causes
A number of factors account for disparities in health and health care.
Population-influenced factors
Leading candidates are some population groups’ lower socioeconomic status (eg, income, occupation, education) and increased exposure to unhealthy environments. Individuals may also exhibit preferences for or against treatment (when appropriate treatment recommendations are offered) that mirror group preferences.
For example, African American patients’ distrust of the healthcare system may be based in part on their experience of discrimination as research subjects in the Tuskegee syphilis study and Los Angeles measles immunization study. Research has shown that while these issues are relevant, they do not fully account for observed disparities.
System factors
Problems with access to care are common: inadequate insurance, transportation difficulties, geographic barriers to needed services (rural/urban), and language barriers. Again, research has shown that access to care matters, but not necessarily more than other factors.
Individual factors
At the individual level, a clinical encounter may be adversely affected by physician-patient racial/ethnic discordance, patient health literacy, and physician cultural competence. Also, there is the high prevalence of risky behavior such as smoking.
Finally, provider-specific issues may be operative: bias (prejudice) against certain groups of patients, clinical uncertainty when dealing with patients, and stereotypes held by providers about the behavior or health of different groups of patients according to race, ethnicity, or culture.
Addressing disparities in practice
Clearly, improving the socioeconomic status and access to care for all people are among the most important ways to eliminate health disparities. Physicians can influence these areas through individual participation in political activities, in nonprofit organizations, and in their professional organizations.
Steps can also be taken in your own practice (Table 2).
TABLE 2
Ten practical measures for avoiding health disparity in your practice
| Use evidence-based clinical guidelines as much as possible. |
| Consider the health literacy level of your patients when planning care and treatment, when explaining medical recommendations, and when handing out written material. |
| Ensure that front desk staff are sensitive to patient backgrounds and cultures. |
| Provide culturally sensitive patient education materials (eg, brochures in Spanish). |
| Keep a “black book” with the names and numbers of community health resources. |
| Volunteer with a nonprofit community-based agency in your area. |
| Ask your local health department or managed care plans if they have a community health improvement plan. Get involved in creating or implementing the plan. |
| Create a special program for one or more of the populations you care for (eg, a school-based program to help reduce teenage pregnancy). |
| Develop a plan for translation services. |
| Browse through the Institute of Medicine report, “Unequal Treatment” (available at www.iom.edu/report.asp?id=4475). |
Use evidence-based guidelines
To minimize the effect of possible bias and stereotyping in caring for patients of different races, ethnicities, and cultures, an important foundation is to standardize care for all patients by using evidence-based practice guidelines when appropriate. Clinical guidelines such as those published by the US Preventive Services Task Force and those available on the Internet through the National Guideline Clearinghouse provide well-researched and substantiated recommendations (available at www.ngc.gov).
Using guidelines is consistent with national recommendations to incorporate more evidence-based practices in clinical care.
Make your office patient-friendly
Create an office environment that is sensitive to the needs of all patients. Addressing language issues, having front desk staff who are sensitive and unbiased, and providing culturally relevant patient education material (eg, posters, magazines) are important components of a supportive office environment.1
Advocate patient education
Strategies to improve patient health literacy and physician cultural competence may be of benefit. The literacy issue can be helped considerably by enabling patients to increase their understanding of health terminology, and there are national efforts to address patient health literacy. Physicians can also help by explaining options and care plans simply, carefully, and without medical jargon. The American Medical Association has a national campaign in support of health literacy (www.amaassn.org/ama/pub/category/8115.html).
Increase cross-cultural communication skills
The Institute of Medicine and academicians have increasingly recommended training healthcare professionals to be more culturally competent. Experts have agreed that the “essence of cultural competence is not the mastery of ‘facts’ about different ethnic groups, but rather a patient-centered approach that incorporates fundamental skills and attitudes that may be applicable across ethnic boundaries.”6
A recent national survey supported this idea by showing that racial differences in patient satisfaction disappeared after adjustment for the quality of physician behaviors (eg, showing respect for patients, spending adequate time with patients). The fact that these positive physician behaviors were reported more frequently by white than non-white patients points to the need for continued effort at improving physicians’ interpersonal skills.
Eliminating health disparities is one of the top 2 goals of Healthy People 2010, the document that guides the nation’s health promotion and disease prevention agenda. Healthy People 2010 (www.health.gov/healthypeople) is a compilation of important prevention objectives for the Nation identified by the US Public Health Service that helps to focus health care system and community efforts. The vision for Healthy People 2010 is “Healthy People in Healthy Communities,” a theme emphasizing that the health of the individual is closely linked with the health of the community.
The Leading Health Indicators are a subset of the Healthy People 2010 objectives and were chosen for emphasis because they account for more than 50% of the leading preventable causes of morbidity and premature morality in the US. 5 Data on these 10 objectives also point to disparities in health status and health outcomes among population groups in the US. Most states and many local communities have used the Healthy People 2010/Leading Health Indicators to develop and implement state and local “Healthy People” plans.
Physicians have an important role in efforts to meet these goals because many of them can only be met by utilizing multicomponent intervention strategies that include actions at the clinic, health care system and community level.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Tucker C, Herman K, Pedersen T, Higley B, Montrichard M, Ivery P. Cultural sensitivity in physician-patient relationships: perspectives of an ethnically diverse sample of low-income primary care patients. Med Care 2003;41:859-870.
2. Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial and ethnic disparities in health care. JAMA 2000;283:2579-2584.
3. Navarro V. Race or class versus race and class: mortality differentials in the United States. Lancet 1990;336:1238-1240.
4. Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academy Press; 2002. Available at: www.iom.edu/report.asp?id=4475. Accessed on February 13, 2004.
5. McGinnis JM, Foege W. Actual causes of death in the Unites States. JAMA 1993;270:2207-2212.
6. Saha S, Arbelaez JJ, Cooper LA. Patient-Physician relationships and racial disparities in the quality of health care. Am J Public Health 2003;93:1713-1719.
We hope the answer to the question above is no. However, the evidence regarding differences in the care of patients based on race, ethnicity, gender, and socioeconomic status suggests that if this patient is a woman or African American or from a lower socioeconomic class, resultant morbidity or mortality will be higher.
Differences are seen in the provision of cardiovascular care, cancer diagnosis and treament, and HIV care. African Americans, Latino Americans, Asian Americans, and Native Americans have higher morbidity and mortality than Caucasian chemical dependency, diabetes, heart disease, infant Americans for multiple problems including cancer, mortality, and unintentional and intentional injuries.1
This article explores possible explanations for health care disparities and offers 10 practical strategies for tackling this challenging issue.
Examples of health disparities
The United States has dramatically improved the health status of its citizens—increasing longevity, reducing infant mortality and teenage pregnancies, and increasing the number of children being immunized. Despite these improvements, though, there remain persistent and disproportionate burdens of disease and illness borne by subgroups of the population (Table 1). 2,3
The Institute of Medicine in its recent report, “Unequal Treatment,” approaches the issue from another perspective: they define these disparities as “racial or ethnic differences in the quality of healthcare that are not due to access-related factors or clinical needs, preferences and appropriateness of intervention.”4
TABLE 1
Examples of health disparities that could be changed
| Disparity in mortality |
| Infant mortality |
| Infant mortality is higher for infants of African American, Native Hawaiian, and Native American mothers (13.8, 10.0, and 9.3 deaths per 1000 live births, respectively) than for infants of other race groups. Infant mortality decreases as the mother’s level of education increases. |
| Disparity in morbidity |
| Cancer (males) |
| The incidence of cancer among black males exceeds that of white males for prostate cancer (60%), lung and bronchial cancer (58% ), and colon and rectum cancers (14%). |
| Disparity in health behaviors |
| Cigarette smoking |
| Smoking among persons aged 25 years and over ranges from 11% among college graduates to 32% for those without a high school diploma; 19% of adolescents in the most rural counties smoke compared to 11% in central counties. |
| Disparity in preventive health care |
| Mammography |
| Poor women are 27% less likely to have had a recent mammogram than are women with family incomes above the poverty level. |
| Disparity in access to care |
| Health insurance coverage |
| 13% of children under aged <18 years have no health insurance coverage; 28% of children with family incomes of 1 to 1.5 times the poverty level are without coverage, compared with 5% of those with family incomes at least twice the poverty level. |
| Source: Adapted from Health, United States, 2001. |
| Hyattsville, Md: National Center for Health Statistics; 2001. |
Correcting health disparity begins with understanding its causes
A number of factors account for disparities in health and health care.
Population-influenced factors
Leading candidates are some population groups’ lower socioeconomic status (eg, income, occupation, education) and increased exposure to unhealthy environments. Individuals may also exhibit preferences for or against treatment (when appropriate treatment recommendations are offered) that mirror group preferences.
For example, African American patients’ distrust of the healthcare system may be based in part on their experience of discrimination as research subjects in the Tuskegee syphilis study and Los Angeles measles immunization study. Research has shown that while these issues are relevant, they do not fully account for observed disparities.
System factors
Problems with access to care are common: inadequate insurance, transportation difficulties, geographic barriers to needed services (rural/urban), and language barriers. Again, research has shown that access to care matters, but not necessarily more than other factors.
Individual factors
At the individual level, a clinical encounter may be adversely affected by physician-patient racial/ethnic discordance, patient health literacy, and physician cultural competence. Also, there is the high prevalence of risky behavior such as smoking.
Finally, provider-specific issues may be operative: bias (prejudice) against certain groups of patients, clinical uncertainty when dealing with patients, and stereotypes held by providers about the behavior or health of different groups of patients according to race, ethnicity, or culture.
Addressing disparities in practice
Clearly, improving the socioeconomic status and access to care for all people are among the most important ways to eliminate health disparities. Physicians can influence these areas through individual participation in political activities, in nonprofit organizations, and in their professional organizations.
Steps can also be taken in your own practice (Table 2).
TABLE 2
Ten practical measures for avoiding health disparity in your practice
| Use evidence-based clinical guidelines as much as possible. |
| Consider the health literacy level of your patients when planning care and treatment, when explaining medical recommendations, and when handing out written material. |
| Ensure that front desk staff are sensitive to patient backgrounds and cultures. |
| Provide culturally sensitive patient education materials (eg, brochures in Spanish). |
| Keep a “black book” with the names and numbers of community health resources. |
| Volunteer with a nonprofit community-based agency in your area. |
| Ask your local health department or managed care plans if they have a community health improvement plan. Get involved in creating or implementing the plan. |
| Create a special program for one or more of the populations you care for (eg, a school-based program to help reduce teenage pregnancy). |
| Develop a plan for translation services. |
| Browse through the Institute of Medicine report, “Unequal Treatment” (available at www.iom.edu/report.asp?id=4475). |
Use evidence-based guidelines
To minimize the effect of possible bias and stereotyping in caring for patients of different races, ethnicities, and cultures, an important foundation is to standardize care for all patients by using evidence-based practice guidelines when appropriate. Clinical guidelines such as those published by the US Preventive Services Task Force and those available on the Internet through the National Guideline Clearinghouse provide well-researched and substantiated recommendations (available at www.ngc.gov).
Using guidelines is consistent with national recommendations to incorporate more evidence-based practices in clinical care.
Make your office patient-friendly
Create an office environment that is sensitive to the needs of all patients. Addressing language issues, having front desk staff who are sensitive and unbiased, and providing culturally relevant patient education material (eg, posters, magazines) are important components of a supportive office environment.1
Advocate patient education
Strategies to improve patient health literacy and physician cultural competence may be of benefit. The literacy issue can be helped considerably by enabling patients to increase their understanding of health terminology, and there are national efforts to address patient health literacy. Physicians can also help by explaining options and care plans simply, carefully, and without medical jargon. The American Medical Association has a national campaign in support of health literacy (www.amaassn.org/ama/pub/category/8115.html).
Increase cross-cultural communication skills
The Institute of Medicine and academicians have increasingly recommended training healthcare professionals to be more culturally competent. Experts have agreed that the “essence of cultural competence is not the mastery of ‘facts’ about different ethnic groups, but rather a patient-centered approach that incorporates fundamental skills and attitudes that may be applicable across ethnic boundaries.”6
A recent national survey supported this idea by showing that racial differences in patient satisfaction disappeared after adjustment for the quality of physician behaviors (eg, showing respect for patients, spending adequate time with patients). The fact that these positive physician behaviors were reported more frequently by white than non-white patients points to the need for continued effort at improving physicians’ interpersonal skills.
Eliminating health disparities is one of the top 2 goals of Healthy People 2010, the document that guides the nation’s health promotion and disease prevention agenda. Healthy People 2010 (www.health.gov/healthypeople) is a compilation of important prevention objectives for the Nation identified by the US Public Health Service that helps to focus health care system and community efforts. The vision for Healthy People 2010 is “Healthy People in Healthy Communities,” a theme emphasizing that the health of the individual is closely linked with the health of the community.
The Leading Health Indicators are a subset of the Healthy People 2010 objectives and were chosen for emphasis because they account for more than 50% of the leading preventable causes of morbidity and premature morality in the US. 5 Data on these 10 objectives also point to disparities in health status and health outcomes among population groups in the US. Most states and many local communities have used the Healthy People 2010/Leading Health Indicators to develop and implement state and local “Healthy People” plans.
Physicians have an important role in efforts to meet these goals because many of them can only be met by utilizing multicomponent intervention strategies that include actions at the clinic, health care system and community level.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
We hope the answer to the question above is no. However, the evidence regarding differences in the care of patients based on race, ethnicity, gender, and socioeconomic status suggests that if this patient is a woman or African American or from a lower socioeconomic class, resultant morbidity or mortality will be higher.
Differences are seen in the provision of cardiovascular care, cancer diagnosis and treament, and HIV care. African Americans, Latino Americans, Asian Americans, and Native Americans have higher morbidity and mortality than Caucasian chemical dependency, diabetes, heart disease, infant Americans for multiple problems including cancer, mortality, and unintentional and intentional injuries.1
This article explores possible explanations for health care disparities and offers 10 practical strategies for tackling this challenging issue.
Examples of health disparities
The United States has dramatically improved the health status of its citizens—increasing longevity, reducing infant mortality and teenage pregnancies, and increasing the number of children being immunized. Despite these improvements, though, there remain persistent and disproportionate burdens of disease and illness borne by subgroups of the population (Table 1). 2,3
The Institute of Medicine in its recent report, “Unequal Treatment,” approaches the issue from another perspective: they define these disparities as “racial or ethnic differences in the quality of healthcare that are not due to access-related factors or clinical needs, preferences and appropriateness of intervention.”4
TABLE 1
Examples of health disparities that could be changed
| Disparity in mortality |
| Infant mortality |
| Infant mortality is higher for infants of African American, Native Hawaiian, and Native American mothers (13.8, 10.0, and 9.3 deaths per 1000 live births, respectively) than for infants of other race groups. Infant mortality decreases as the mother’s level of education increases. |
| Disparity in morbidity |
| Cancer (males) |
| The incidence of cancer among black males exceeds that of white males for prostate cancer (60%), lung and bronchial cancer (58% ), and colon and rectum cancers (14%). |
| Disparity in health behaviors |
| Cigarette smoking |
| Smoking among persons aged 25 years and over ranges from 11% among college graduates to 32% for those without a high school diploma; 19% of adolescents in the most rural counties smoke compared to 11% in central counties. |
| Disparity in preventive health care |
| Mammography |
| Poor women are 27% less likely to have had a recent mammogram than are women with family incomes above the poverty level. |
| Disparity in access to care |
| Health insurance coverage |
| 13% of children under aged <18 years have no health insurance coverage; 28% of children with family incomes of 1 to 1.5 times the poverty level are without coverage, compared with 5% of those with family incomes at least twice the poverty level. |
| Source: Adapted from Health, United States, 2001. |
| Hyattsville, Md: National Center for Health Statistics; 2001. |
Correcting health disparity begins with understanding its causes
A number of factors account for disparities in health and health care.
Population-influenced factors
Leading candidates are some population groups’ lower socioeconomic status (eg, income, occupation, education) and increased exposure to unhealthy environments. Individuals may also exhibit preferences for or against treatment (when appropriate treatment recommendations are offered) that mirror group preferences.
For example, African American patients’ distrust of the healthcare system may be based in part on their experience of discrimination as research subjects in the Tuskegee syphilis study and Los Angeles measles immunization study. Research has shown that while these issues are relevant, they do not fully account for observed disparities.
System factors
Problems with access to care are common: inadequate insurance, transportation difficulties, geographic barriers to needed services (rural/urban), and language barriers. Again, research has shown that access to care matters, but not necessarily more than other factors.
Individual factors
At the individual level, a clinical encounter may be adversely affected by physician-patient racial/ethnic discordance, patient health literacy, and physician cultural competence. Also, there is the high prevalence of risky behavior such as smoking.
Finally, provider-specific issues may be operative: bias (prejudice) against certain groups of patients, clinical uncertainty when dealing with patients, and stereotypes held by providers about the behavior or health of different groups of patients according to race, ethnicity, or culture.
Addressing disparities in practice
Clearly, improving the socioeconomic status and access to care for all people are among the most important ways to eliminate health disparities. Physicians can influence these areas through individual participation in political activities, in nonprofit organizations, and in their professional organizations.
Steps can also be taken in your own practice (Table 2).
TABLE 2
Ten practical measures for avoiding health disparity in your practice
| Use evidence-based clinical guidelines as much as possible. |
| Consider the health literacy level of your patients when planning care and treatment, when explaining medical recommendations, and when handing out written material. |
| Ensure that front desk staff are sensitive to patient backgrounds and cultures. |
| Provide culturally sensitive patient education materials (eg, brochures in Spanish). |
| Keep a “black book” with the names and numbers of community health resources. |
| Volunteer with a nonprofit community-based agency in your area. |
| Ask your local health department or managed care plans if they have a community health improvement plan. Get involved in creating or implementing the plan. |
| Create a special program for one or more of the populations you care for (eg, a school-based program to help reduce teenage pregnancy). |
| Develop a plan for translation services. |
| Browse through the Institute of Medicine report, “Unequal Treatment” (available at www.iom.edu/report.asp?id=4475). |
Use evidence-based guidelines
To minimize the effect of possible bias and stereotyping in caring for patients of different races, ethnicities, and cultures, an important foundation is to standardize care for all patients by using evidence-based practice guidelines when appropriate. Clinical guidelines such as those published by the US Preventive Services Task Force and those available on the Internet through the National Guideline Clearinghouse provide well-researched and substantiated recommendations (available at www.ngc.gov).
Using guidelines is consistent with national recommendations to incorporate more evidence-based practices in clinical care.
Make your office patient-friendly
Create an office environment that is sensitive to the needs of all patients. Addressing language issues, having front desk staff who are sensitive and unbiased, and providing culturally relevant patient education material (eg, posters, magazines) are important components of a supportive office environment.1
Advocate patient education
Strategies to improve patient health literacy and physician cultural competence may be of benefit. The literacy issue can be helped considerably by enabling patients to increase their understanding of health terminology, and there are national efforts to address patient health literacy. Physicians can also help by explaining options and care plans simply, carefully, and without medical jargon. The American Medical Association has a national campaign in support of health literacy (www.amaassn.org/ama/pub/category/8115.html).
Increase cross-cultural communication skills
The Institute of Medicine and academicians have increasingly recommended training healthcare professionals to be more culturally competent. Experts have agreed that the “essence of cultural competence is not the mastery of ‘facts’ about different ethnic groups, but rather a patient-centered approach that incorporates fundamental skills and attitudes that may be applicable across ethnic boundaries.”6
A recent national survey supported this idea by showing that racial differences in patient satisfaction disappeared after adjustment for the quality of physician behaviors (eg, showing respect for patients, spending adequate time with patients). The fact that these positive physician behaviors were reported more frequently by white than non-white patients points to the need for continued effort at improving physicians’ interpersonal skills.
Eliminating health disparities is one of the top 2 goals of Healthy People 2010, the document that guides the nation’s health promotion and disease prevention agenda. Healthy People 2010 (www.health.gov/healthypeople) is a compilation of important prevention objectives for the Nation identified by the US Public Health Service that helps to focus health care system and community efforts. The vision for Healthy People 2010 is “Healthy People in Healthy Communities,” a theme emphasizing that the health of the individual is closely linked with the health of the community.
The Leading Health Indicators are a subset of the Healthy People 2010 objectives and were chosen for emphasis because they account for more than 50% of the leading preventable causes of morbidity and premature morality in the US. 5 Data on these 10 objectives also point to disparities in health status and health outcomes among population groups in the US. Most states and many local communities have used the Healthy People 2010/Leading Health Indicators to develop and implement state and local “Healthy People” plans.
Physicians have an important role in efforts to meet these goals because many of them can only be met by utilizing multicomponent intervention strategies that include actions at the clinic, health care system and community level.
Corresponding author
Eric Henley, MD, MPH, Co-Editor, Practice Alert, 1601 Parkview Avenue, Rockford, IL 61107. E-mail: [email protected].
1. Tucker C, Herman K, Pedersen T, Higley B, Montrichard M, Ivery P. Cultural sensitivity in physician-patient relationships: perspectives of an ethnically diverse sample of low-income primary care patients. Med Care 2003;41:859-870.
2. Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial and ethnic disparities in health care. JAMA 2000;283:2579-2584.
3. Navarro V. Race or class versus race and class: mortality differentials in the United States. Lancet 1990;336:1238-1240.
4. Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academy Press; 2002. Available at: www.iom.edu/report.asp?id=4475. Accessed on February 13, 2004.
5. McGinnis JM, Foege W. Actual causes of death in the Unites States. JAMA 1993;270:2207-2212.
6. Saha S, Arbelaez JJ, Cooper LA. Patient-Physician relationships and racial disparities in the quality of health care. Am J Public Health 2003;93:1713-1719.
1. Tucker C, Herman K, Pedersen T, Higley B, Montrichard M, Ivery P. Cultural sensitivity in physician-patient relationships: perspectives of an ethnically diverse sample of low-income primary care patients. Med Care 2003;41:859-870.
2. Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial and ethnic disparities in health care. JAMA 2000;283:2579-2584.
3. Navarro V. Race or class versus race and class: mortality differentials in the United States. Lancet 1990;336:1238-1240.
4. Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academy Press; 2002. Available at: www.iom.edu/report.asp?id=4475. Accessed on February 13, 2004.
5. McGinnis JM, Foege W. Actual causes of death in the Unites States. JAMA 1993;270:2207-2212.
6. Saha S, Arbelaez JJ, Cooper LA. Patient-Physician relationships and racial disparities in the quality of health care. Am J Public Health 2003;93:1713-1719.
How should we diagnose and treat osteoarthritis of the knee?
- When are x-ray films indicated for a patient with knee pain?
- When should we prescribe selective cyclo-oxygenase-2 (COX-2) inhibitors, instead of nonsteroidal anti-inflammatory drugs (NSAIDs)?
- How often can intraarticular steroids be used?
- What is the role of viscosupplementation?
- When is total knee replacement appropriate?
Answers to these and other questions can be found in a guideline revised within the year by the Evidence-Based Practice Committee of the American Academy of Orthopedic Surgeons. The guideline—revised from a version developed and released in 1996—is divided into 2 phases: care provided by the first-contact primary care physician (the focus of this review), and recommendations for specialists (not addressed in this review).
The major recommendations summarized in the National Guideline Clearinghouse (www.ngc.gov) did not include the excellent care algorithm. For this update, therefore, the source document was accessed. It summarizes the following recommendations for referral to a musculoskeletal specialist (orthopedist, physiatrist, or rheumatologist)—poor response to 12 weeks of treatment, suspected infection, or hemarthrosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Targeted patients were adults with longstanding knee pain. Outcomes measured were symptomatic pain relief, improved range of motion, better physical functioning, and complications associated with treatment.
The committee used a recommendation rating scheme of A to D, based on a review of the evidence. Ratings were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (As explained on pages 111 to 120 of this issue, The journal of family practice and many other family-medicine publications will be using an evidence-rating system ranging from A to C. For this review, however, the scheme of A to D originally used by the guideline’s authors has been left intact.)
Limitations of guideline usefulness
Although this guideline was just published, the evidence is complete only through 2000. The bibliography is lengthy, but the support document does not provide evidence tables. The established outcomes set forth were not used to design the algorithm, which also lacks grades of evidence. The guideline is further weakened by the lack of cost-effectiveness analysis.
Guideline development and evidence review
The 1996 guideline was developed by a multidisciplinary group of American Academy of Orthopedic Surgeons, the American Association of Neurological Surgeons, the American College of Physical Medicine and Rehabilitation, and the American College of Rheumatology. The 2003 revision group performed a new literature search for 1990–2000 for human subjects aged 19 years and older. In all, 128 articles were reviewed, 114 references were cited, the evidence was graded, and the original guideline was revised based on the evidence.
Sources for this guideline
American Academy of Orthopaedic Surgeons. AAOS clinical practice guideline on osteoarthritis of the knee. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2003.
Source document available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_04.pdf. Algorithm available at: www.aaos.org/word-html/pdfs_r/guidelin/chart_oakn.pdf. Accessed on December 30, 2003.
Grade A Recommendations
- Initial treatment with NSAIDs or acetaminophen. acetaminophen is as effective as NSAIDs
- Physical therapy, including conditioning, quadriceps strengthening, and range of motion exercises should be considered for patients with osteoarthritis (confirmed by radiographs) after 4 to 6 weeks of conservative therapy.
Grade B Recommendations
- COX-2 Inhibitors should be used only for patients at risk of adverse renal and gastrointestinal effects from NSAIDs.
- A tangential view of the patellofemoral joint and a standing posterior-anterior view of the knee flexed 20° should be obtained for patients who do not respond to treatment in 1 to 4 weeks or whose pain returns. Positive findings are narrowing of cartilage space, marginal osteophytes, subchondral sclerosis, and tibial spine beaking.
- If the patient is unresponsive to 1 NSAID, changing to another NSAID is an option.
- Use durable medical equipment assistive devices such as canes, fitted footwear, and braces.
- Educate patients regarding weight loss, support groups, and avoidance of activities that worsen knee pain.
Grade C Recommendations
- Viscosupplementation may be effective during the first 12 weeks of symptoms.
Grade D Recommendations
- Knee x-ray for patients with persistent pain (1–4 weeks) or return of pain after a symptom-free interval.
- With long-term NSAID use, monitor complete blood count, renal functions, liver functions, and stool guiac every 6 months.
- Arthrocentesis and intra-articular steroid injection are options for persistent pain (1–4 weeks).
- Chondroitin and glucosamine have not been studied adequately to make recommendations.
FIGURE
Osteoarthritis of the knee
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000; 43:1905–1915. Available at: www.rheumatology.org/publications/guide-lines/oa-knee/oa-knee.asp. Accessed on December 30, 2003.
- Knee pain or swelling: acute or chronic. University of Michigan Health System. Ann Arbor, Mich: University of Michigan Health System; 2002 Aug. Available at: cme.med.umich. edu/pdf/guideline/knee.pdf.
- Diagnosis and treatment of adult degenerative joint disease (DJD) of the knee. Institute for Clinical Systems Improvement (ICSI). Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002 May. Available at: www.icsi.org/knowledge/browse_ category.asp?catID=29.
- Pain in osteoarthritis, rheumatoid arthritis, and juvenile chronic arthritis. Simon LS, Lipman AG, Jacox AK, et al. 2nd ed. Glenview, Ill: American Pain Society (APS); 2002. Not available on-line.
- Physical activity in the prevention, treatment, and rehabilitation of diseases. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 May 7. Available at: www.ebm-guidelines.com/home.html (fee for access).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When are x-ray films indicated for a patient with knee pain?
- When should we prescribe selective cyclo-oxygenase-2 (COX-2) inhibitors, instead of nonsteroidal anti-inflammatory drugs (NSAIDs)?
- How often can intraarticular steroids be used?
- What is the role of viscosupplementation?
- When is total knee replacement appropriate?
Answers to these and other questions can be found in a guideline revised within the year by the Evidence-Based Practice Committee of the American Academy of Orthopedic Surgeons. The guideline—revised from a version developed and released in 1996—is divided into 2 phases: care provided by the first-contact primary care physician (the focus of this review), and recommendations for specialists (not addressed in this review).
The major recommendations summarized in the National Guideline Clearinghouse (www.ngc.gov) did not include the excellent care algorithm. For this update, therefore, the source document was accessed. It summarizes the following recommendations for referral to a musculoskeletal specialist (orthopedist, physiatrist, or rheumatologist)—poor response to 12 weeks of treatment, suspected infection, or hemarthrosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Targeted patients were adults with longstanding knee pain. Outcomes measured were symptomatic pain relief, improved range of motion, better physical functioning, and complications associated with treatment.
The committee used a recommendation rating scheme of A to D, based on a review of the evidence. Ratings were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (As explained on pages 111 to 120 of this issue, The journal of family practice and many other family-medicine publications will be using an evidence-rating system ranging from A to C. For this review, however, the scheme of A to D originally used by the guideline’s authors has been left intact.)
Limitations of guideline usefulness
Although this guideline was just published, the evidence is complete only through 2000. The bibliography is lengthy, but the support document does not provide evidence tables. The established outcomes set forth were not used to design the algorithm, which also lacks grades of evidence. The guideline is further weakened by the lack of cost-effectiveness analysis.
Guideline development and evidence review
The 1996 guideline was developed by a multidisciplinary group of American Academy of Orthopedic Surgeons, the American Association of Neurological Surgeons, the American College of Physical Medicine and Rehabilitation, and the American College of Rheumatology. The 2003 revision group performed a new literature search for 1990–2000 for human subjects aged 19 years and older. In all, 128 articles were reviewed, 114 references were cited, the evidence was graded, and the original guideline was revised based on the evidence.
Sources for this guideline
American Academy of Orthopaedic Surgeons. AAOS clinical practice guideline on osteoarthritis of the knee. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2003.
Source document available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_04.pdf. Algorithm available at: www.aaos.org/word-html/pdfs_r/guidelin/chart_oakn.pdf. Accessed on December 30, 2003.
Grade A Recommendations
- Initial treatment with NSAIDs or acetaminophen. acetaminophen is as effective as NSAIDs
- Physical therapy, including conditioning, quadriceps strengthening, and range of motion exercises should be considered for patients with osteoarthritis (confirmed by radiographs) after 4 to 6 weeks of conservative therapy.
Grade B Recommendations
- COX-2 Inhibitors should be used only for patients at risk of adverse renal and gastrointestinal effects from NSAIDs.
- A tangential view of the patellofemoral joint and a standing posterior-anterior view of the knee flexed 20° should be obtained for patients who do not respond to treatment in 1 to 4 weeks or whose pain returns. Positive findings are narrowing of cartilage space, marginal osteophytes, subchondral sclerosis, and tibial spine beaking.
- If the patient is unresponsive to 1 NSAID, changing to another NSAID is an option.
- Use durable medical equipment assistive devices such as canes, fitted footwear, and braces.
- Educate patients regarding weight loss, support groups, and avoidance of activities that worsen knee pain.
Grade C Recommendations
- Viscosupplementation may be effective during the first 12 weeks of symptoms.
Grade D Recommendations
- Knee x-ray for patients with persistent pain (1–4 weeks) or return of pain after a symptom-free interval.
- With long-term NSAID use, monitor complete blood count, renal functions, liver functions, and stool guiac every 6 months.
- Arthrocentesis and intra-articular steroid injection are options for persistent pain (1–4 weeks).
- Chondroitin and glucosamine have not been studied adequately to make recommendations.
FIGURE
Osteoarthritis of the knee
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000; 43:1905–1915. Available at: www.rheumatology.org/publications/guide-lines/oa-knee/oa-knee.asp. Accessed on December 30, 2003.
- Knee pain or swelling: acute or chronic. University of Michigan Health System. Ann Arbor, Mich: University of Michigan Health System; 2002 Aug. Available at: cme.med.umich. edu/pdf/guideline/knee.pdf.
- Diagnosis and treatment of adult degenerative joint disease (DJD) of the knee. Institute for Clinical Systems Improvement (ICSI). Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002 May. Available at: www.icsi.org/knowledge/browse_ category.asp?catID=29.
- Pain in osteoarthritis, rheumatoid arthritis, and juvenile chronic arthritis. Simon LS, Lipman AG, Jacox AK, et al. 2nd ed. Glenview, Ill: American Pain Society (APS); 2002. Not available on-line.
- Physical activity in the prevention, treatment, and rehabilitation of diseases. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 May 7. Available at: www.ebm-guidelines.com/home.html (fee for access).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- When are x-ray films indicated for a patient with knee pain?
- When should we prescribe selective cyclo-oxygenase-2 (COX-2) inhibitors, instead of nonsteroidal anti-inflammatory drugs (NSAIDs)?
- How often can intraarticular steroids be used?
- What is the role of viscosupplementation?
- When is total knee replacement appropriate?
Answers to these and other questions can be found in a guideline revised within the year by the Evidence-Based Practice Committee of the American Academy of Orthopedic Surgeons. The guideline—revised from a version developed and released in 1996—is divided into 2 phases: care provided by the first-contact primary care physician (the focus of this review), and recommendations for specialists (not addressed in this review).
The major recommendations summarized in the National Guideline Clearinghouse (www.ngc.gov) did not include the excellent care algorithm. For this update, therefore, the source document was accessed. It summarizes the following recommendations for referral to a musculoskeletal specialist (orthopedist, physiatrist, or rheumatologist)—poor response to 12 weeks of treatment, suspected infection, or hemarthrosis.
The evidence categories for this guideline are diagnosis, evaluation, management, and treatment. Targeted patients were adults with longstanding knee pain. Outcomes measured were symptomatic pain relief, improved range of motion, better physical functioning, and complications associated with treatment.
The committee used a recommendation rating scheme of A to D, based on a review of the evidence. Ratings were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (As explained on pages 111 to 120 of this issue, The journal of family practice and many other family-medicine publications will be using an evidence-rating system ranging from A to C. For this review, however, the scheme of A to D originally used by the guideline’s authors has been left intact.)
Limitations of guideline usefulness
Although this guideline was just published, the evidence is complete only through 2000. The bibliography is lengthy, but the support document does not provide evidence tables. The established outcomes set forth were not used to design the algorithm, which also lacks grades of evidence. The guideline is further weakened by the lack of cost-effectiveness analysis.
Guideline development and evidence review
The 1996 guideline was developed by a multidisciplinary group of American Academy of Orthopedic Surgeons, the American Association of Neurological Surgeons, the American College of Physical Medicine and Rehabilitation, and the American College of Rheumatology. The 2003 revision group performed a new literature search for 1990–2000 for human subjects aged 19 years and older. In all, 128 articles were reviewed, 114 references were cited, the evidence was graded, and the original guideline was revised based on the evidence.
Sources for this guideline
American Academy of Orthopaedic Surgeons. AAOS clinical practice guideline on osteoarthritis of the knee. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 2003.
Source document available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_04.pdf. Algorithm available at: www.aaos.org/word-html/pdfs_r/guidelin/chart_oakn.pdf. Accessed on December 30, 2003.
Grade A Recommendations
- Initial treatment with NSAIDs or acetaminophen. acetaminophen is as effective as NSAIDs
- Physical therapy, including conditioning, quadriceps strengthening, and range of motion exercises should be considered for patients with osteoarthritis (confirmed by radiographs) after 4 to 6 weeks of conservative therapy.
Grade B Recommendations
- COX-2 Inhibitors should be used only for patients at risk of adverse renal and gastrointestinal effects from NSAIDs.
- A tangential view of the patellofemoral joint and a standing posterior-anterior view of the knee flexed 20° should be obtained for patients who do not respond to treatment in 1 to 4 weeks or whose pain returns. Positive findings are narrowing of cartilage space, marginal osteophytes, subchondral sclerosis, and tibial spine beaking.
- If the patient is unresponsive to 1 NSAID, changing to another NSAID is an option.
- Use durable medical equipment assistive devices such as canes, fitted footwear, and braces.
- Educate patients regarding weight loss, support groups, and avoidance of activities that worsen knee pain.
Grade C Recommendations
- Viscosupplementation may be effective during the first 12 weeks of symptoms.
Grade D Recommendations
- Knee x-ray for patients with persistent pain (1–4 weeks) or return of pain after a symptom-free interval.
- With long-term NSAID use, monitor complete blood count, renal functions, liver functions, and stool guiac every 6 months.
- Arthrocentesis and intra-articular steroid injection are options for persistent pain (1–4 weeks).
- Chondroitin and glucosamine have not been studied adequately to make recommendations.
FIGURE
Osteoarthritis of the knee
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000; 43:1905–1915. Available at: www.rheumatology.org/publications/guide-lines/oa-knee/oa-knee.asp. Accessed on December 30, 2003.
- Knee pain or swelling: acute or chronic. University of Michigan Health System. Ann Arbor, Mich: University of Michigan Health System; 2002 Aug. Available at: cme.med.umich. edu/pdf/guideline/knee.pdf.
- Diagnosis and treatment of adult degenerative joint disease (DJD) of the knee. Institute for Clinical Systems Improvement (ICSI). Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002 May. Available at: www.icsi.org/knowledge/browse_ category.asp?catID=29.
- Pain in osteoarthritis, rheumatoid arthritis, and juvenile chronic arthritis. Simon LS, Lipman AG, Jacox AK, et al. 2nd ed. Glenview, Ill: American Pain Society (APS); 2002. Not available on-line.
- Physical activity in the prevention, treatment, and rehabilitation of diseases. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Medical Publications Ltd.; 2002 May 7. Available at: www.ebm-guidelines.com/home.html (fee for access).
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
Preventing VTE in hospitalized patients
- How do we determine risk of venous thromboembolism (VTE) in patients scheduled for surgery?
- Do all surgical patients require VTE prevention?
- Is aspirin adequate to prevent VTE in low-risk hospitalized patients?
- Which anticoagulant is appropriate for a patient scheduled for total knee replacement?
These important questions are answered in a guideline developed by a committee of the American College of Chest Physicians, which considered the following prophylaxis recommendations: early ambulation, aspirin, graduated compression stockings, intermittent pneumatic compression, low-dose unfractionated heparin, low-molecular-weight heparin, or oral antithrombotic agents.
The committee categorized recommendations by type of surgical procedure and risk status. In this summary, the recommendations are reorganized by strength of recommendation.
Three outcomes were regarded:
- Efficacy of various prophylactic strategies
- Rates and relative risk of venous thromboembolism outcomes—ie, fatal pulmonary embolism, symptomatic deep vein thrombosis, pulmonary embolism, or asymptomatic proximal deep vein thrombosis
- Cost-effectiveness of prophylaxis.
The committee used a rating scheme that accounted for both the risk/benefit ratio (clear or unclear) and the strength of the supporting recommendation (A, B, C). The grades of evidence were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (For an explanaton of these grades.)
Relevant recommendations
This guideline is clinically relevant because of the high mortality associated with pulmonary embolus complicating VTE.
It offers a practical, tabulated guide, listed by surgical procedure performed. It is pertinent to hospitalized patients under the care of family physicians. The rationale for each recommendation is clear and well supported by the referenced literature. The objectives of the guideline were met and the outcome measures were appropriate.
The guideline is weakened by the lack of cost-effectiveness considerations.
Determining surgical risk
| Surgery + | Patient age (yr) + | Risk factors = | Level of risk |
|---|---|---|---|
| Minor | < 40 | No | Low |
| Minor | Any | Yes* | Moderate |
| 40–60 | No | ||
| Major | < 40 | No | |
| Minor | > 60 | No | High |
| > 60 | Yes* | ||
| Major | > 40 | No | |
| > 40 | Yes* | ||
| Major | > 40 | Prior VTE, cancer, hypercoagulable states, hip/knee arthoplasty, hip fracture, major trauma, spinal injury | Very high |
*Additional risk factors: immobility, stroke, paralysis, trauma, obesity, varicose veins, cardiac dysfunction, indwelling central venous catheter, inflammatory bowel disease, nephrotic syndrome, pregnancy, estrogen use, congenital thrombophilic abnormalities
- For all risk groups of patients, aspirin is not recommended for prophylaxis (strength of recommendation [SOR]: A)
- Every hospital should have an appropriate thromboembolic event prevention strategy, determined by proper risk assessment (SOR: D)
- Antithrombotics should be used with caution before invasive spinal or epidural procedures (SOR: C)
Grade A Recommendations
- Low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), graduated compression stockings (GCS), or intermittent pneumatic compression (IPC) for moderate-risk surgery patients
- LDUH, LMWH, or IPC for higher-risk general surgery
- Twice-daily LDUH for major gynecological surgery for benign disease
- Three-times-daily dose LDUH for gynecological surgery for malignancy
- LMWH or warfarin for 7–10 days for total hip or total knee replacement surgery; continue for longer periods in higher-risk patients. Adjusted-dose intravenous heparin is an acceptable alternative, but more difficult to manage
- Aspirin alone is not acceptable for hip fracture patients
- IPC with GCS for intracranial surgery; LDUH or postoperative LMWH are acceptable alternatives
- LMWH or intravenous heparin for the acute myocardial infarction patient (for the VTE prevention indication)
- LDUH or LMWH for immobilized stroke patient. GCS if anticoagula tion is contraindicated
- LDUH or LMWH for medical patients with cancer, bedrest, congestive heart failure, or severe lung disease
Grade B Recommendations
- LDUH, GCF, IPC, or LMWH for open urologic procedures
- IPC for total knee replacement
- LMWH or warfarin for hip fracture; an alternative is IPC
- LMWH for acute spinal cord injury. Alternative GCS or IPC in combination with LMWH or LDUH, if LMWH is contraindicated
Grade C Recommendations
- Early ambulation (with no antithrombotic agents) for low-risk surgery patients or uncomplicated gynecologic procedures
- LDUH, LMWH, or IPC for higher-risk surgery patients
- For very-high-risk surgery patients, LDUH or LMWH combined with GCS or IPC.Some patients may benefit from post-hospital LMWH or warfarin
- Daily LDUH or IPC for major gynecologic procedures for benign disease
- LDUH plus GCS or LMWH for gynecologic surgery for malignancy
- Early ambulation for low risk urologic and gynecologic procedures
- High-risk urologic procedures GCS plus with LDUH or LMWH
- GCS or IPC added to antithrombotic drugs for total hip replacement
Guideline development and evidence review
Literature searches were performed for each patient group. Criteria for inclusion included relevant patient group, sample size of at least 10 patients per group, verified deep vein thrombosis, and patients with adequate outcome assessments.
In considering baseline risk of thrombosis, only either prospective cohort studies or control groups of randomized trials were considered. For prophylaxis efficacy recommendations, only randomized trials were considered. The consensus group analyzed data from 630 sources before making these recommendations.
Sources for this guideline
Sixth ACCP Consensus Conference on Antithrombotic Therapy
The Consensus Conference guidelines can be found at:
Geerts WH, et al. Prevention of thromboembolism. Chest 2001; 119:132S–175S. Available at: www.chestjournal.org/content/vol119/1_suppl/index. shtml. Accessed on December 16, 2003.
Tables illustrating these guideline, organized by type of surgical procedure can be accessed at: chestnet.safeserver.com/guidelines/antithrombotic/p8.php
In the same issue of this journal, there were reports on the mechanism of action for oral anticoagulants, managing oral anticoagulant therapy, platelet active drugs, mechanisms of action of heparin and low molecular weight heparin, hemorrhagic complications of anticoagulation, use of antithrombotic medications during pregnancy, antithrombotic therapy for heart disease and peripheral vascular disease, use of these for stroke, and their role in treating children.
OTHER GUIDELINES ON PREVENTION OF VTE
- Deep venous thrombosis. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Publications Ltd; 2002. Available at: www.ngc.gov/guidelines/FTNGC-2610.html. Accessed on December 16, 2003.
- Practice paramenters for the prevention of venous thromboembolism. The Standards Task Force of the Society of Colon and Rectal Surgeons. Dis Colon Rectum 2000; 43:1037–47. [54 references.] Available at: www.fascrs.org/ascrspp-pvt.html. Accessed on December 16, 2003.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- How do we determine risk of venous thromboembolism (VTE) in patients scheduled for surgery?
- Do all surgical patients require VTE prevention?
- Is aspirin adequate to prevent VTE in low-risk hospitalized patients?
- Which anticoagulant is appropriate for a patient scheduled for total knee replacement?
These important questions are answered in a guideline developed by a committee of the American College of Chest Physicians, which considered the following prophylaxis recommendations: early ambulation, aspirin, graduated compression stockings, intermittent pneumatic compression, low-dose unfractionated heparin, low-molecular-weight heparin, or oral antithrombotic agents.
The committee categorized recommendations by type of surgical procedure and risk status. In this summary, the recommendations are reorganized by strength of recommendation.
Three outcomes were regarded:
- Efficacy of various prophylactic strategies
- Rates and relative risk of venous thromboembolism outcomes—ie, fatal pulmonary embolism, symptomatic deep vein thrombosis, pulmonary embolism, or asymptomatic proximal deep vein thrombosis
- Cost-effectiveness of prophylaxis.
The committee used a rating scheme that accounted for both the risk/benefit ratio (clear or unclear) and the strength of the supporting recommendation (A, B, C). The grades of evidence were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (For an explanaton of these grades.)
Relevant recommendations
This guideline is clinically relevant because of the high mortality associated with pulmonary embolus complicating VTE.
It offers a practical, tabulated guide, listed by surgical procedure performed. It is pertinent to hospitalized patients under the care of family physicians. The rationale for each recommendation is clear and well supported by the referenced literature. The objectives of the guideline were met and the outcome measures were appropriate.
The guideline is weakened by the lack of cost-effectiveness considerations.
Determining surgical risk
| Surgery + | Patient age (yr) + | Risk factors = | Level of risk |
|---|---|---|---|
| Minor | < 40 | No | Low |
| Minor | Any | Yes* | Moderate |
| 40–60 | No | ||
| Major | < 40 | No | |
| Minor | > 60 | No | High |
| > 60 | Yes* | ||
| Major | > 40 | No | |
| > 40 | Yes* | ||
| Major | > 40 | Prior VTE, cancer, hypercoagulable states, hip/knee arthoplasty, hip fracture, major trauma, spinal injury | Very high |
*Additional risk factors: immobility, stroke, paralysis, trauma, obesity, varicose veins, cardiac dysfunction, indwelling central venous catheter, inflammatory bowel disease, nephrotic syndrome, pregnancy, estrogen use, congenital thrombophilic abnormalities
- For all risk groups of patients, aspirin is not recommended for prophylaxis (strength of recommendation [SOR]: A)
- Every hospital should have an appropriate thromboembolic event prevention strategy, determined by proper risk assessment (SOR: D)
- Antithrombotics should be used with caution before invasive spinal or epidural procedures (SOR: C)
Grade A Recommendations
- Low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), graduated compression stockings (GCS), or intermittent pneumatic compression (IPC) for moderate-risk surgery patients
- LDUH, LMWH, or IPC for higher-risk general surgery
- Twice-daily LDUH for major gynecological surgery for benign disease
- Three-times-daily dose LDUH for gynecological surgery for malignancy
- LMWH or warfarin for 7–10 days for total hip or total knee replacement surgery; continue for longer periods in higher-risk patients. Adjusted-dose intravenous heparin is an acceptable alternative, but more difficult to manage
- Aspirin alone is not acceptable for hip fracture patients
- IPC with GCS for intracranial surgery; LDUH or postoperative LMWH are acceptable alternatives
- LMWH or intravenous heparin for the acute myocardial infarction patient (for the VTE prevention indication)
- LDUH or LMWH for immobilized stroke patient. GCS if anticoagula tion is contraindicated
- LDUH or LMWH for medical patients with cancer, bedrest, congestive heart failure, or severe lung disease
Grade B Recommendations
- LDUH, GCF, IPC, or LMWH for open urologic procedures
- IPC for total knee replacement
- LMWH or warfarin for hip fracture; an alternative is IPC
- LMWH for acute spinal cord injury. Alternative GCS or IPC in combination with LMWH or LDUH, if LMWH is contraindicated
Grade C Recommendations
- Early ambulation (with no antithrombotic agents) for low-risk surgery patients or uncomplicated gynecologic procedures
- LDUH, LMWH, or IPC for higher-risk surgery patients
- For very-high-risk surgery patients, LDUH or LMWH combined with GCS or IPC.Some patients may benefit from post-hospital LMWH or warfarin
- Daily LDUH or IPC for major gynecologic procedures for benign disease
- LDUH plus GCS or LMWH for gynecologic surgery for malignancy
- Early ambulation for low risk urologic and gynecologic procedures
- High-risk urologic procedures GCS plus with LDUH or LMWH
- GCS or IPC added to antithrombotic drugs for total hip replacement
Guideline development and evidence review
Literature searches were performed for each patient group. Criteria for inclusion included relevant patient group, sample size of at least 10 patients per group, verified deep vein thrombosis, and patients with adequate outcome assessments.
In considering baseline risk of thrombosis, only either prospective cohort studies or control groups of randomized trials were considered. For prophylaxis efficacy recommendations, only randomized trials were considered. The consensus group analyzed data from 630 sources before making these recommendations.
Sources for this guideline
Sixth ACCP Consensus Conference on Antithrombotic Therapy
The Consensus Conference guidelines can be found at:
Geerts WH, et al. Prevention of thromboembolism. Chest 2001; 119:132S–175S. Available at: www.chestjournal.org/content/vol119/1_suppl/index. shtml. Accessed on December 16, 2003.
Tables illustrating these guideline, organized by type of surgical procedure can be accessed at: chestnet.safeserver.com/guidelines/antithrombotic/p8.php
In the same issue of this journal, there were reports on the mechanism of action for oral anticoagulants, managing oral anticoagulant therapy, platelet active drugs, mechanisms of action of heparin and low molecular weight heparin, hemorrhagic complications of anticoagulation, use of antithrombotic medications during pregnancy, antithrombotic therapy for heart disease and peripheral vascular disease, use of these for stroke, and their role in treating children.
OTHER GUIDELINES ON PREVENTION OF VTE
- Deep venous thrombosis. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Publications Ltd; 2002. Available at: www.ngc.gov/guidelines/FTNGC-2610.html. Accessed on December 16, 2003.
- Practice paramenters for the prevention of venous thromboembolism. The Standards Task Force of the Society of Colon and Rectal Surgeons. Dis Colon Rectum 2000; 43:1037–47. [54 references.] Available at: www.fascrs.org/ascrspp-pvt.html. Accessed on December 16, 2003.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
- How do we determine risk of venous thromboembolism (VTE) in patients scheduled for surgery?
- Do all surgical patients require VTE prevention?
- Is aspirin adequate to prevent VTE in low-risk hospitalized patients?
- Which anticoagulant is appropriate for a patient scheduled for total knee replacement?
These important questions are answered in a guideline developed by a committee of the American College of Chest Physicians, which considered the following prophylaxis recommendations: early ambulation, aspirin, graduated compression stockings, intermittent pneumatic compression, low-dose unfractionated heparin, low-molecular-weight heparin, or oral antithrombotic agents.
The committee categorized recommendations by type of surgical procedure and risk status. In this summary, the recommendations are reorganized by strength of recommendation.
Three outcomes were regarded:
- Efficacy of various prophylactic strategies
- Rates and relative risk of venous thromboembolism outcomes—ie, fatal pulmonary embolism, symptomatic deep vein thrombosis, pulmonary embolism, or asymptomatic proximal deep vein thrombosis
- Cost-effectiveness of prophylaxis.
The committee used a rating scheme that accounted for both the risk/benefit ratio (clear or unclear) and the strength of the supporting recommendation (A, B, C). The grades of evidence were altered to correspond to the grades of recommendation of the Oxford Centre for Evidence-Based Medicine. (For an explanaton of these grades.)
Relevant recommendations
This guideline is clinically relevant because of the high mortality associated with pulmonary embolus complicating VTE.
It offers a practical, tabulated guide, listed by surgical procedure performed. It is pertinent to hospitalized patients under the care of family physicians. The rationale for each recommendation is clear and well supported by the referenced literature. The objectives of the guideline were met and the outcome measures were appropriate.
The guideline is weakened by the lack of cost-effectiveness considerations.
Determining surgical risk
| Surgery + | Patient age (yr) + | Risk factors = | Level of risk |
|---|---|---|---|
| Minor | < 40 | No | Low |
| Minor | Any | Yes* | Moderate |
| 40–60 | No | ||
| Major | < 40 | No | |
| Minor | > 60 | No | High |
| > 60 | Yes* | ||
| Major | > 40 | No | |
| > 40 | Yes* | ||
| Major | > 40 | Prior VTE, cancer, hypercoagulable states, hip/knee arthoplasty, hip fracture, major trauma, spinal injury | Very high |
*Additional risk factors: immobility, stroke, paralysis, trauma, obesity, varicose veins, cardiac dysfunction, indwelling central venous catheter, inflammatory bowel disease, nephrotic syndrome, pregnancy, estrogen use, congenital thrombophilic abnormalities
- For all risk groups of patients, aspirin is not recommended for prophylaxis (strength of recommendation [SOR]: A)
- Every hospital should have an appropriate thromboembolic event prevention strategy, determined by proper risk assessment (SOR: D)
- Antithrombotics should be used with caution before invasive spinal or epidural procedures (SOR: C)
Grade A Recommendations
- Low-dose unfractionated heparin (LDUH), low-molecular-weight heparin (LMWH), graduated compression stockings (GCS), or intermittent pneumatic compression (IPC) for moderate-risk surgery patients
- LDUH, LMWH, or IPC for higher-risk general surgery
- Twice-daily LDUH for major gynecological surgery for benign disease
- Three-times-daily dose LDUH for gynecological surgery for malignancy
- LMWH or warfarin for 7–10 days for total hip or total knee replacement surgery; continue for longer periods in higher-risk patients. Adjusted-dose intravenous heparin is an acceptable alternative, but more difficult to manage
- Aspirin alone is not acceptable for hip fracture patients
- IPC with GCS for intracranial surgery; LDUH or postoperative LMWH are acceptable alternatives
- LMWH or intravenous heparin for the acute myocardial infarction patient (for the VTE prevention indication)
- LDUH or LMWH for immobilized stroke patient. GCS if anticoagula tion is contraindicated
- LDUH or LMWH for medical patients with cancer, bedrest, congestive heart failure, or severe lung disease
Grade B Recommendations
- LDUH, GCF, IPC, or LMWH for open urologic procedures
- IPC for total knee replacement
- LMWH or warfarin for hip fracture; an alternative is IPC
- LMWH for acute spinal cord injury. Alternative GCS or IPC in combination with LMWH or LDUH, if LMWH is contraindicated
Grade C Recommendations
- Early ambulation (with no antithrombotic agents) for low-risk surgery patients or uncomplicated gynecologic procedures
- LDUH, LMWH, or IPC for higher-risk surgery patients
- For very-high-risk surgery patients, LDUH or LMWH combined with GCS or IPC.Some patients may benefit from post-hospital LMWH or warfarin
- Daily LDUH or IPC for major gynecologic procedures for benign disease
- LDUH plus GCS or LMWH for gynecologic surgery for malignancy
- Early ambulation for low risk urologic and gynecologic procedures
- High-risk urologic procedures GCS plus with LDUH or LMWH
- GCS or IPC added to antithrombotic drugs for total hip replacement
Guideline development and evidence review
Literature searches were performed for each patient group. Criteria for inclusion included relevant patient group, sample size of at least 10 patients per group, verified deep vein thrombosis, and patients with adequate outcome assessments.
In considering baseline risk of thrombosis, only either prospective cohort studies or control groups of randomized trials were considered. For prophylaxis efficacy recommendations, only randomized trials were considered. The consensus group analyzed data from 630 sources before making these recommendations.
Sources for this guideline
Sixth ACCP Consensus Conference on Antithrombotic Therapy
The Consensus Conference guidelines can be found at:
Geerts WH, et al. Prevention of thromboembolism. Chest 2001; 119:132S–175S. Available at: www.chestjournal.org/content/vol119/1_suppl/index. shtml. Accessed on December 16, 2003.
Tables illustrating these guideline, organized by type of surgical procedure can be accessed at: chestnet.safeserver.com/guidelines/antithrombotic/p8.php
In the same issue of this journal, there were reports on the mechanism of action for oral anticoagulants, managing oral anticoagulant therapy, platelet active drugs, mechanisms of action of heparin and low molecular weight heparin, hemorrhagic complications of anticoagulation, use of antithrombotic medications during pregnancy, antithrombotic therapy for heart disease and peripheral vascular disease, use of these for stroke, and their role in treating children.
OTHER GUIDELINES ON PREVENTION OF VTE
- Deep venous thrombosis. Finnish Medical Society Duodecim. Helsinki, Finland: Duodecim Publications Ltd; 2002. Available at: www.ngc.gov/guidelines/FTNGC-2610.html. Accessed on December 16, 2003.
- Practice paramenters for the prevention of venous thromboembolism. The Standards Task Force of the Society of Colon and Rectal Surgeons. Dis Colon Rectum 2000; 43:1037–47. [54 references.] Available at: www.fascrs.org/ascrspp-pvt.html. Accessed on December 16, 2003.
Correspondence
Keith B. Holten, MD, Clinton Memorial Hospital/University of Cincinnati Family Practice Residency, 825 W. Locust St., Wilmington, OH, 45177. E-mail: [email protected].
Sexually transmitted disease: Easier screening tests, single-dose therapies
Success in the United States in reducing morbidity caused by syphilis and gonorrhea has been offset by the rising morbidity seen with other sexually transmitted diseases (STDs), such as chlamydia and herpes. Also, recent increases in the prevalence of syphilis and human immunodeficiency virus (HIV) underscore the fact that sustaining public health successes depends on constant surveillance and a commitment to control efforts.
This Practice Alert focuses on 3 prevalent STDs: syphilis, gonorrhea, and chlamydia—those patients most likely to be infected, and specific developments in screening, diagnosis, and treatment.
Those most likely to be infected
In 2001, 783,000 cases of chlamydia, 362,000 cases of gonorrhea, and 31,600 cases of syphilis were reported to the Centers for Disease Control and Prevention (CDC).1 The true incidence of each disease is unknown. Historical trends in the number of cases reported are reflected in Figures 1 ,2, and 3. In 2001, there was a slight increase in the number of syphilis cases, reversing a 10-year downward trend. The number of chlamydia cases continued to rise, which may reflect improved screening and reporting, while the number of gonorrhea cases continued downward.
FIGURE 1
Chlamydia rates
Chlamydia rates by sex: United States, 1984–2001.
FIGURE 2
Gonorrhea rates
Gonorrhea rates by sex: United States 1981–2001, and the Healthy People year 2010 objective.
FIGURE 3
Syphilis rates
Primary and secondary syphilis rates by sex: United States 1881–2001, and the Healthy People year 2010 objective.
Chlamydia
Infection with Chlamydia trachomatisis reported 4 times as often in women as in men, reflecting better screening of women in family planning programs and during prenatal care.2 The highest rates of chlamydia in women occur in the age groups 15 to 19 years (25/1000) and 20 to 25 years (24/1000). Testing in family planning clinics has yielded chlamydia infection rates of 5.6% among these women.2
Gonorrhea and syphilis
The age groups at highest risk for gonorrhea are 15 to 19 years for women (703/100,000) and 20 to 24 for men (563/100,000).3 For syphilis, the highest risk for women is age 20 to 24 years (3.8/100,000 for primary and secondary syphilis) and 35 to 39 years for men (7.2/100,000).4 There are marked geographic variations in the rates of syphilis (Figure 4). The recent increase in syphilis has been among men, largely attributed to homosexual activity.
FIGURE 4
Syphilis rates by county
Counties with primary and secondary syphilis rates above and below the Healthy People year 2010 objective: United States, 2001.
When and whom to screen
Many STDs persist asymptomatically. These silent infections can cause long-term morbidity such as infertility, pelvic inflammatory disease, ectopic pregnancies, and chronic pelvic pain. Both the United States Preventive Services Task Force and the American Academy of Family Physicians recommend that screening for STDs be performed in specific circumstances (Table 1).9,10
These recommendations should probably be considered a minimum standard, with other groups and diseases included based on the local epidemiology. The local or state health department can be a useful source of information on local epidemiologic patterns and screening and treatment recommendations. Keep in mind that the accuracy of local disease statistics depends on screening and disease reporting by local physicians.
TABLE 1
When and whom to screen for chlamydia, gonorrhea, and syphilis
| Chlamydia | Sexually active women aged 25 years should be routinely tested, and tested during pregnancy at the first prenatal care visit. |
| Other women at high risk* for chlamydia should be routinely tested, and tested during pregnancy at the first prenatal care visit. | |
| Gonorrhea | High-risk women* should be routinely tested, and tested during pregnancy at the first prenatal care visit |
| Syphilis | High-risk women should be routinely tested, and tested during pregnancy at the first prenatal care visit, again in the third trimester, and at delivery. |
| All pregnant women should be tested at the first prenatal care visit. | |
| * Definitions for high risk vary but generally include the following: those with multiple sex partners, other STDs, sexual contact to those with disease, or who exchange sex for money for drugs. | |
Urine screening tests
New nucleic acid amplification tests (NAAT) facilitate screening and diagnosis of chlamydia and gonorrhea with a urine sample. This offers the ease of urine collection in both men and women, with the added benefit of sensitivitiesand specificities equal to those obtained from urethral or endocervical samples. NAATs make possible urine screening in settings where urethral and cervical samples may not be possible because of logistics or patient nonacceptance.
NAATs do not require the presence of live organisms, and only a small number of organisms are needed for accurate test results.
One disadvantage of these tests is an inability to determine antibiotic sensitivities. Another is occasional false-positive results from dead organisms, which can occur if test of cures are performed too soon after treatment (less than 3 weeks).
NAATs can be used on urethral and endocervical swabs as well as urine samples. But they should not be used on oral or rectal samples. Some products test for both gonorrhea and chlamydia in a single specimen. A positive result could be due to either organism, however, requiring more specific testing.
The CDC believes that NAATs on urine samples are acceptable methods of screening for genital gonorrhea and chlamydia in both men and women, although for gonorrhea, cultures of urethral and endocervical swabs are preferred so that sensitivities can be obtained. Gonorrhea and chlamydia cultures are recommended for diagnosing oropharyngeal or anal infection.11
Treatment
Single-dose therapies
A variety of single-dose therapies for STDs are now available (Table 2). Single-dose therapies are convenient for patients, and they encourage quicker completion of therapy. If single-dose therapy is administered in the clinical setting, it is essentially directly observed therapy. Tests of cure can be avoided when common STDs are treated with recommended regimens and completion of treatment is assured.
A disadvantage often of single-dose therapy is its cost. However, when compared with the total costs of incomplete treatments—lower cure rates, return visits, increased infection of contacts—the price of a single-dose agent may seem more acceptable.
Many of the single-dose therapies for gonorrhea are quinolones, which should not be used to treat gonorrhea in (or acquired in) areas with high rates of quinolone resistance (Hawaii, parts of California). Consult your local or state health department to learn the local rates of resistance.
TABLE 2
Single-dose therapies available for common STDs
| Infection | Single-dose therapy |
|---|---|
| Chlamydia | Azithromycin 1 gm (oral) |
| Gonorrhea | Cefixime 400 mg (oral)*† |
| Ceftriaxone 125 mg (intramuscular) | |
| Ciprofloxacin 500 mg (oral) | |
| Ofloxacin 400 mg (oral)† | |
| Levofloxacin 250 mg (oral)† | |
| Nongonococcal urethritis | Azithromycin 1 gm (oral) |
| Syphilis (primary, secondary, early latent) | Benzathene penicillin 2.4 million units (intramuscular) |
| *Cefixime tablets are currently not being manufactured. | |
| †Not recommended for pharyngeal gonorrhea. | |
Management of sex partners
Treatment of patients with STDs is not complete until sex partners who have been exposed are also evaluated, tested, and treated. The common STDs discussed in this article are reportable to local and state health departments. However, in many jurisdictions, cost and staffing limitations prevent public health investigation and contact notification of gonorrhea or chlamydia infections. Family physicians in such locations need to advise their patients to notify sexual contacts of their exposure and recommend that they be examined and treated.
Confidentiality. When patients express concern about having their infections reported to the public health department, reassure them that these departments have a very good record of maintaining patient confidentiality and that public health information is usually afforded a greater degree of protection than information in an office medical record. When public health departments notify sexual contacts of their exposure, they do not reveal who exposed them, although in some instances the sexual contacts can figure this out.
Gonorrhea or chlamydia. The CDC recommends that when a patient is diagnosed with either gonorrhea or chlamydia, all their sex partners from the past 60 days should be evaluated and treated prophylactically.12 Patients and their sex partner(s) should avoid intercourse for 7 days after initiation of treatment and until symptoms resolve.
Syphilis. Syphilis is more complicated. Persons who were exposed to primary, secondary, and early latent syphilis within the 90 days prior to diagnosis should be treated prophylactically. Those exposed from 91 days to 6 months prior to diagnosis of secondary syphilis, or 91 days to 1 year prior to the diagnosis of early latent syphilis, should be treated prophylactically if serology testing is not available or if follow up is uncertain.
Other infections. Current sex partners of those with mucopurulent cervicitis or nongonococcal urethritis should also be evaluated and treated with the same regimen chosen for the index patient. Sex partners within the past 60 days of women with pelvic inflammatory disease should be evaluated and treated prophylactically for both gonorrhea and chlamydia while sex partners within the past 60 days of men with epididymitis should be evaluated but not necessarily treated prophylactically.
As of May 2001,169 million Americans were regular users of the Internet.5 Internet sites created for the purpose of facilitating sexual contact have proliferated and include those for heterosexuals, gay men, lesbians, swingers, and those interested in group sex.6
Use of the Internet to meet sex partners has generated concern in public health circles because of the potential for increased risk of STDs, including HIV/AIDS, from anonymous sex. One case report describes an outbreak of syphilis among gay men who were participants in an Internet chat room for sexual networking.7 Each man with syphilis who was located reported an average of 12 recent sex partners (range of 2–47); a mean of 6 partners (range of 2–15) were located and examined. Four out of the 7 with syphilis were also positive for HIV.
Another study of users of HIVcounseling and testing services found that 16% had sought sex partners on the Internet and 65% of these reported having sex with someone they met on the Internet.8 Internet users reported more previous STDs,more partners, more HIV-positive partners,and more sex with gay men than did non-Internet users.
While much remains to be learned about this topic,these studies indicate that Internet-initiated sex may involve higher risk than sex initiated through other means, although anonymous sex and having multiple sex partners should be considered high-risk activities however they are initiated. Warn patients about these risks.
Collaboration is key to control
The impact of STDs on a community can be moderated when family physicians and local public health departments collaborate. The department’s role includes providing information on the local epidemiology of STDs; assistance in screening, testing, and treating; and, depending on available resources, notifying sexual partners. Many larger public health departments operate a publicly funded STD clinic for patients and sexual contacts who lack financial resources.
Family physicians can screen high-risk persons, use recommended treatment regimens, report communicable diseases as required by state statute, and assist with partner notification by urging patients to cooperate with the local health agency and, if public health partner notification is not available, urging patients to notify their sexual partners.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). National Center for HIV, STD and TB Prevention. Division of Sexually Transmitted Diseases. 2001 National STD Surveillance Report. Table 1: Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 civilian population: United States, 1941–2001. Available at www.cdc.gov/std/stats/tables/table1.htm. Accessed on October 27, 2003.
2. CDC. Division of Sexually Transmitted Diseases. Chlamydia. Available at www.cdc.gov/std/stats/2001chlamydia.htm. Accessed on October 27, 2003.
3. CDC. Division of Sexually Transmitted Diseases. Gonorrhea. Available at www.cdc.gov/std/stats/2001gonorrhea.htm. Accessed on October 27, 2003.
4. CDC. Division of Sexually Transmitted Diseases. Syphilis. Available at www.cdc.gov/std/stats/2001syphilis.htm. Accessed on October 27, 2003.
5. Available at www.eurmktg.com/globstats.
6. Bull SS, McFarlane M. Soliciting sex on the internet. What are the risks for sexually transmitted diseases and HIV? Sex Transm Dis 2000;27:545-550.
7. Klausner JD, Wolf W, Fischer-Ponce L, Zolt I, Katz MH. Tracing a syphilis outbreak through cyberspace. JAMA 2000;284:447-449.
8. McFarlane M, Bull SS, Rietmeijer CA. The internet as a newly-emerging risk environment for sexually transmitted diseases. JAMA 2000;284:443-446.
9. Agency for Healthcare Research and Quality (AHRCQ). United States Preventive Services Task Force (USPSTF). Screening: chlamydia infection. Available at www.ahcpr.gov/clinic/uspstf/uspschlm.htm. Accessed on October 27, 2003.
10. American Academy of Family Physicians. Introduction to AAFP summary of policy recommendations for periodic health examinations. Available at www.aafp.org/x10601.xml. Accessed on October 27, 2003.
11. CDC. Screening tests to detect chlamydia trachomatis and Neisseria gonorrhea infections. MMWR Recomm Rep 2002;51(RR-15):1-38.
12. CDC. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR6):1-78.
Success in the United States in reducing morbidity caused by syphilis and gonorrhea has been offset by the rising morbidity seen with other sexually transmitted diseases (STDs), such as chlamydia and herpes. Also, recent increases in the prevalence of syphilis and human immunodeficiency virus (HIV) underscore the fact that sustaining public health successes depends on constant surveillance and a commitment to control efforts.
This Practice Alert focuses on 3 prevalent STDs: syphilis, gonorrhea, and chlamydia—those patients most likely to be infected, and specific developments in screening, diagnosis, and treatment.
Those most likely to be infected
In 2001, 783,000 cases of chlamydia, 362,000 cases of gonorrhea, and 31,600 cases of syphilis were reported to the Centers for Disease Control and Prevention (CDC).1 The true incidence of each disease is unknown. Historical trends in the number of cases reported are reflected in Figures 1 ,2, and 3. In 2001, there was a slight increase in the number of syphilis cases, reversing a 10-year downward trend. The number of chlamydia cases continued to rise, which may reflect improved screening and reporting, while the number of gonorrhea cases continued downward.
FIGURE 1
Chlamydia rates
Chlamydia rates by sex: United States, 1984–2001.
FIGURE 2
Gonorrhea rates
Gonorrhea rates by sex: United States 1981–2001, and the Healthy People year 2010 objective.
FIGURE 3
Syphilis rates
Primary and secondary syphilis rates by sex: United States 1881–2001, and the Healthy People year 2010 objective.
Chlamydia
Infection with Chlamydia trachomatisis reported 4 times as often in women as in men, reflecting better screening of women in family planning programs and during prenatal care.2 The highest rates of chlamydia in women occur in the age groups 15 to 19 years (25/1000) and 20 to 25 years (24/1000). Testing in family planning clinics has yielded chlamydia infection rates of 5.6% among these women.2
Gonorrhea and syphilis
The age groups at highest risk for gonorrhea are 15 to 19 years for women (703/100,000) and 20 to 24 for men (563/100,000).3 For syphilis, the highest risk for women is age 20 to 24 years (3.8/100,000 for primary and secondary syphilis) and 35 to 39 years for men (7.2/100,000).4 There are marked geographic variations in the rates of syphilis (Figure 4). The recent increase in syphilis has been among men, largely attributed to homosexual activity.
FIGURE 4
Syphilis rates by county
Counties with primary and secondary syphilis rates above and below the Healthy People year 2010 objective: United States, 2001.
When and whom to screen
Many STDs persist asymptomatically. These silent infections can cause long-term morbidity such as infertility, pelvic inflammatory disease, ectopic pregnancies, and chronic pelvic pain. Both the United States Preventive Services Task Force and the American Academy of Family Physicians recommend that screening for STDs be performed in specific circumstances (Table 1).9,10
These recommendations should probably be considered a minimum standard, with other groups and diseases included based on the local epidemiology. The local or state health department can be a useful source of information on local epidemiologic patterns and screening and treatment recommendations. Keep in mind that the accuracy of local disease statistics depends on screening and disease reporting by local physicians.
TABLE 1
When and whom to screen for chlamydia, gonorrhea, and syphilis
| Chlamydia | Sexually active women aged 25 years should be routinely tested, and tested during pregnancy at the first prenatal care visit. |
| Other women at high risk* for chlamydia should be routinely tested, and tested during pregnancy at the first prenatal care visit. | |
| Gonorrhea | High-risk women* should be routinely tested, and tested during pregnancy at the first prenatal care visit |
| Syphilis | High-risk women should be routinely tested, and tested during pregnancy at the first prenatal care visit, again in the third trimester, and at delivery. |
| All pregnant women should be tested at the first prenatal care visit. | |
| * Definitions for high risk vary but generally include the following: those with multiple sex partners, other STDs, sexual contact to those with disease, or who exchange sex for money for drugs. | |
Urine screening tests
New nucleic acid amplification tests (NAAT) facilitate screening and diagnosis of chlamydia and gonorrhea with a urine sample. This offers the ease of urine collection in both men and women, with the added benefit of sensitivitiesand specificities equal to those obtained from urethral or endocervical samples. NAATs make possible urine screening in settings where urethral and cervical samples may not be possible because of logistics or patient nonacceptance.
NAATs do not require the presence of live organisms, and only a small number of organisms are needed for accurate test results.
One disadvantage of these tests is an inability to determine antibiotic sensitivities. Another is occasional false-positive results from dead organisms, which can occur if test of cures are performed too soon after treatment (less than 3 weeks).
NAATs can be used on urethral and endocervical swabs as well as urine samples. But they should not be used on oral or rectal samples. Some products test for both gonorrhea and chlamydia in a single specimen. A positive result could be due to either organism, however, requiring more specific testing.
The CDC believes that NAATs on urine samples are acceptable methods of screening for genital gonorrhea and chlamydia in both men and women, although for gonorrhea, cultures of urethral and endocervical swabs are preferred so that sensitivities can be obtained. Gonorrhea and chlamydia cultures are recommended for diagnosing oropharyngeal or anal infection.11
Treatment
Single-dose therapies
A variety of single-dose therapies for STDs are now available (Table 2). Single-dose therapies are convenient for patients, and they encourage quicker completion of therapy. If single-dose therapy is administered in the clinical setting, it is essentially directly observed therapy. Tests of cure can be avoided when common STDs are treated with recommended regimens and completion of treatment is assured.
A disadvantage often of single-dose therapy is its cost. However, when compared with the total costs of incomplete treatments—lower cure rates, return visits, increased infection of contacts—the price of a single-dose agent may seem more acceptable.
Many of the single-dose therapies for gonorrhea are quinolones, which should not be used to treat gonorrhea in (or acquired in) areas with high rates of quinolone resistance (Hawaii, parts of California). Consult your local or state health department to learn the local rates of resistance.
TABLE 2
Single-dose therapies available for common STDs
| Infection | Single-dose therapy |
|---|---|
| Chlamydia | Azithromycin 1 gm (oral) |
| Gonorrhea | Cefixime 400 mg (oral)*† |
| Ceftriaxone 125 mg (intramuscular) | |
| Ciprofloxacin 500 mg (oral) | |
| Ofloxacin 400 mg (oral)† | |
| Levofloxacin 250 mg (oral)† | |
| Nongonococcal urethritis | Azithromycin 1 gm (oral) |
| Syphilis (primary, secondary, early latent) | Benzathene penicillin 2.4 million units (intramuscular) |
| *Cefixime tablets are currently not being manufactured. | |
| †Not recommended for pharyngeal gonorrhea. | |
Management of sex partners
Treatment of patients with STDs is not complete until sex partners who have been exposed are also evaluated, tested, and treated. The common STDs discussed in this article are reportable to local and state health departments. However, in many jurisdictions, cost and staffing limitations prevent public health investigation and contact notification of gonorrhea or chlamydia infections. Family physicians in such locations need to advise their patients to notify sexual contacts of their exposure and recommend that they be examined and treated.
Confidentiality. When patients express concern about having their infections reported to the public health department, reassure them that these departments have a very good record of maintaining patient confidentiality and that public health information is usually afforded a greater degree of protection than information in an office medical record. When public health departments notify sexual contacts of their exposure, they do not reveal who exposed them, although in some instances the sexual contacts can figure this out.
Gonorrhea or chlamydia. The CDC recommends that when a patient is diagnosed with either gonorrhea or chlamydia, all their sex partners from the past 60 days should be evaluated and treated prophylactically.12 Patients and their sex partner(s) should avoid intercourse for 7 days after initiation of treatment and until symptoms resolve.
Syphilis. Syphilis is more complicated. Persons who were exposed to primary, secondary, and early latent syphilis within the 90 days prior to diagnosis should be treated prophylactically. Those exposed from 91 days to 6 months prior to diagnosis of secondary syphilis, or 91 days to 1 year prior to the diagnosis of early latent syphilis, should be treated prophylactically if serology testing is not available or if follow up is uncertain.
Other infections. Current sex partners of those with mucopurulent cervicitis or nongonococcal urethritis should also be evaluated and treated with the same regimen chosen for the index patient. Sex partners within the past 60 days of women with pelvic inflammatory disease should be evaluated and treated prophylactically for both gonorrhea and chlamydia while sex partners within the past 60 days of men with epididymitis should be evaluated but not necessarily treated prophylactically.
As of May 2001,169 million Americans were regular users of the Internet.5 Internet sites created for the purpose of facilitating sexual contact have proliferated and include those for heterosexuals, gay men, lesbians, swingers, and those interested in group sex.6
Use of the Internet to meet sex partners has generated concern in public health circles because of the potential for increased risk of STDs, including HIV/AIDS, from anonymous sex. One case report describes an outbreak of syphilis among gay men who were participants in an Internet chat room for sexual networking.7 Each man with syphilis who was located reported an average of 12 recent sex partners (range of 2–47); a mean of 6 partners (range of 2–15) were located and examined. Four out of the 7 with syphilis were also positive for HIV.
Another study of users of HIVcounseling and testing services found that 16% had sought sex partners on the Internet and 65% of these reported having sex with someone they met on the Internet.8 Internet users reported more previous STDs,more partners, more HIV-positive partners,and more sex with gay men than did non-Internet users.
While much remains to be learned about this topic,these studies indicate that Internet-initiated sex may involve higher risk than sex initiated through other means, although anonymous sex and having multiple sex partners should be considered high-risk activities however they are initiated. Warn patients about these risks.
Collaboration is key to control
The impact of STDs on a community can be moderated when family physicians and local public health departments collaborate. The department’s role includes providing information on the local epidemiology of STDs; assistance in screening, testing, and treating; and, depending on available resources, notifying sexual partners. Many larger public health departments operate a publicly funded STD clinic for patients and sexual contacts who lack financial resources.
Family physicians can screen high-risk persons, use recommended treatment regimens, report communicable diseases as required by state statute, and assist with partner notification by urging patients to cooperate with the local health agency and, if public health partner notification is not available, urging patients to notify their sexual partners.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
Success in the United States in reducing morbidity caused by syphilis and gonorrhea has been offset by the rising morbidity seen with other sexually transmitted diseases (STDs), such as chlamydia and herpes. Also, recent increases in the prevalence of syphilis and human immunodeficiency virus (HIV) underscore the fact that sustaining public health successes depends on constant surveillance and a commitment to control efforts.
This Practice Alert focuses on 3 prevalent STDs: syphilis, gonorrhea, and chlamydia—those patients most likely to be infected, and specific developments in screening, diagnosis, and treatment.
Those most likely to be infected
In 2001, 783,000 cases of chlamydia, 362,000 cases of gonorrhea, and 31,600 cases of syphilis were reported to the Centers for Disease Control and Prevention (CDC).1 The true incidence of each disease is unknown. Historical trends in the number of cases reported are reflected in Figures 1 ,2, and 3. In 2001, there was a slight increase in the number of syphilis cases, reversing a 10-year downward trend. The number of chlamydia cases continued to rise, which may reflect improved screening and reporting, while the number of gonorrhea cases continued downward.
FIGURE 1
Chlamydia rates
Chlamydia rates by sex: United States, 1984–2001.
FIGURE 2
Gonorrhea rates
Gonorrhea rates by sex: United States 1981–2001, and the Healthy People year 2010 objective.
FIGURE 3
Syphilis rates
Primary and secondary syphilis rates by sex: United States 1881–2001, and the Healthy People year 2010 objective.
Chlamydia
Infection with Chlamydia trachomatisis reported 4 times as often in women as in men, reflecting better screening of women in family planning programs and during prenatal care.2 The highest rates of chlamydia in women occur in the age groups 15 to 19 years (25/1000) and 20 to 25 years (24/1000). Testing in family planning clinics has yielded chlamydia infection rates of 5.6% among these women.2
Gonorrhea and syphilis
The age groups at highest risk for gonorrhea are 15 to 19 years for women (703/100,000) and 20 to 24 for men (563/100,000).3 For syphilis, the highest risk for women is age 20 to 24 years (3.8/100,000 for primary and secondary syphilis) and 35 to 39 years for men (7.2/100,000).4 There are marked geographic variations in the rates of syphilis (Figure 4). The recent increase in syphilis has been among men, largely attributed to homosexual activity.
FIGURE 4
Syphilis rates by county
Counties with primary and secondary syphilis rates above and below the Healthy People year 2010 objective: United States, 2001.
When and whom to screen
Many STDs persist asymptomatically. These silent infections can cause long-term morbidity such as infertility, pelvic inflammatory disease, ectopic pregnancies, and chronic pelvic pain. Both the United States Preventive Services Task Force and the American Academy of Family Physicians recommend that screening for STDs be performed in specific circumstances (Table 1).9,10
These recommendations should probably be considered a minimum standard, with other groups and diseases included based on the local epidemiology. The local or state health department can be a useful source of information on local epidemiologic patterns and screening and treatment recommendations. Keep in mind that the accuracy of local disease statistics depends on screening and disease reporting by local physicians.
TABLE 1
When and whom to screen for chlamydia, gonorrhea, and syphilis
| Chlamydia | Sexually active women aged 25 years should be routinely tested, and tested during pregnancy at the first prenatal care visit. |
| Other women at high risk* for chlamydia should be routinely tested, and tested during pregnancy at the first prenatal care visit. | |
| Gonorrhea | High-risk women* should be routinely tested, and tested during pregnancy at the first prenatal care visit |
| Syphilis | High-risk women should be routinely tested, and tested during pregnancy at the first prenatal care visit, again in the third trimester, and at delivery. |
| All pregnant women should be tested at the first prenatal care visit. | |
| * Definitions for high risk vary but generally include the following: those with multiple sex partners, other STDs, sexual contact to those with disease, or who exchange sex for money for drugs. | |
Urine screening tests
New nucleic acid amplification tests (NAAT) facilitate screening and diagnosis of chlamydia and gonorrhea with a urine sample. This offers the ease of urine collection in both men and women, with the added benefit of sensitivitiesand specificities equal to those obtained from urethral or endocervical samples. NAATs make possible urine screening in settings where urethral and cervical samples may not be possible because of logistics or patient nonacceptance.
NAATs do not require the presence of live organisms, and only a small number of organisms are needed for accurate test results.
One disadvantage of these tests is an inability to determine antibiotic sensitivities. Another is occasional false-positive results from dead organisms, which can occur if test of cures are performed too soon after treatment (less than 3 weeks).
NAATs can be used on urethral and endocervical swabs as well as urine samples. But they should not be used on oral or rectal samples. Some products test for both gonorrhea and chlamydia in a single specimen. A positive result could be due to either organism, however, requiring more specific testing.
The CDC believes that NAATs on urine samples are acceptable methods of screening for genital gonorrhea and chlamydia in both men and women, although for gonorrhea, cultures of urethral and endocervical swabs are preferred so that sensitivities can be obtained. Gonorrhea and chlamydia cultures are recommended for diagnosing oropharyngeal or anal infection.11
Treatment
Single-dose therapies
A variety of single-dose therapies for STDs are now available (Table 2). Single-dose therapies are convenient for patients, and they encourage quicker completion of therapy. If single-dose therapy is administered in the clinical setting, it is essentially directly observed therapy. Tests of cure can be avoided when common STDs are treated with recommended regimens and completion of treatment is assured.
A disadvantage often of single-dose therapy is its cost. However, when compared with the total costs of incomplete treatments—lower cure rates, return visits, increased infection of contacts—the price of a single-dose agent may seem more acceptable.
Many of the single-dose therapies for gonorrhea are quinolones, which should not be used to treat gonorrhea in (or acquired in) areas with high rates of quinolone resistance (Hawaii, parts of California). Consult your local or state health department to learn the local rates of resistance.
TABLE 2
Single-dose therapies available for common STDs
| Infection | Single-dose therapy |
|---|---|
| Chlamydia | Azithromycin 1 gm (oral) |
| Gonorrhea | Cefixime 400 mg (oral)*† |
| Ceftriaxone 125 mg (intramuscular) | |
| Ciprofloxacin 500 mg (oral) | |
| Ofloxacin 400 mg (oral)† | |
| Levofloxacin 250 mg (oral)† | |
| Nongonococcal urethritis | Azithromycin 1 gm (oral) |
| Syphilis (primary, secondary, early latent) | Benzathene penicillin 2.4 million units (intramuscular) |
| *Cefixime tablets are currently not being manufactured. | |
| †Not recommended for pharyngeal gonorrhea. | |
Management of sex partners
Treatment of patients with STDs is not complete until sex partners who have been exposed are also evaluated, tested, and treated. The common STDs discussed in this article are reportable to local and state health departments. However, in many jurisdictions, cost and staffing limitations prevent public health investigation and contact notification of gonorrhea or chlamydia infections. Family physicians in such locations need to advise their patients to notify sexual contacts of their exposure and recommend that they be examined and treated.
Confidentiality. When patients express concern about having their infections reported to the public health department, reassure them that these departments have a very good record of maintaining patient confidentiality and that public health information is usually afforded a greater degree of protection than information in an office medical record. When public health departments notify sexual contacts of their exposure, they do not reveal who exposed them, although in some instances the sexual contacts can figure this out.
Gonorrhea or chlamydia. The CDC recommends that when a patient is diagnosed with either gonorrhea or chlamydia, all their sex partners from the past 60 days should be evaluated and treated prophylactically.12 Patients and their sex partner(s) should avoid intercourse for 7 days after initiation of treatment and until symptoms resolve.
Syphilis. Syphilis is more complicated. Persons who were exposed to primary, secondary, and early latent syphilis within the 90 days prior to diagnosis should be treated prophylactically. Those exposed from 91 days to 6 months prior to diagnosis of secondary syphilis, or 91 days to 1 year prior to the diagnosis of early latent syphilis, should be treated prophylactically if serology testing is not available or if follow up is uncertain.
Other infections. Current sex partners of those with mucopurulent cervicitis or nongonococcal urethritis should also be evaluated and treated with the same regimen chosen for the index patient. Sex partners within the past 60 days of women with pelvic inflammatory disease should be evaluated and treated prophylactically for both gonorrhea and chlamydia while sex partners within the past 60 days of men with epididymitis should be evaluated but not necessarily treated prophylactically.
As of May 2001,169 million Americans were regular users of the Internet.5 Internet sites created for the purpose of facilitating sexual contact have proliferated and include those for heterosexuals, gay men, lesbians, swingers, and those interested in group sex.6
Use of the Internet to meet sex partners has generated concern in public health circles because of the potential for increased risk of STDs, including HIV/AIDS, from anonymous sex. One case report describes an outbreak of syphilis among gay men who were participants in an Internet chat room for sexual networking.7 Each man with syphilis who was located reported an average of 12 recent sex partners (range of 2–47); a mean of 6 partners (range of 2–15) were located and examined. Four out of the 7 with syphilis were also positive for HIV.
Another study of users of HIVcounseling and testing services found that 16% had sought sex partners on the Internet and 65% of these reported having sex with someone they met on the Internet.8 Internet users reported more previous STDs,more partners, more HIV-positive partners,and more sex with gay men than did non-Internet users.
While much remains to be learned about this topic,these studies indicate that Internet-initiated sex may involve higher risk than sex initiated through other means, although anonymous sex and having multiple sex partners should be considered high-risk activities however they are initiated. Warn patients about these risks.
Collaboration is key to control
The impact of STDs on a community can be moderated when family physicians and local public health departments collaborate. The department’s role includes providing information on the local epidemiology of STDs; assistance in screening, testing, and treating; and, depending on available resources, notifying sexual partners. Many larger public health departments operate a publicly funded STD clinic for patients and sexual contacts who lack financial resources.
Family physicians can screen high-risk persons, use recommended treatment regimens, report communicable diseases as required by state statute, and assist with partner notification by urging patients to cooperate with the local health agency and, if public health partner notification is not available, urging patients to notify their sexual partners.
Correspondence
1825 E. Roosevelt, Phoenix, AZ 85006. E-mail: [email protected].
1. Centers for Disease Control and Prevention (CDC). National Center for HIV, STD and TB Prevention. Division of Sexually Transmitted Diseases. 2001 National STD Surveillance Report. Table 1: Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 civilian population: United States, 1941–2001. Available at www.cdc.gov/std/stats/tables/table1.htm. Accessed on October 27, 2003.
2. CDC. Division of Sexually Transmitted Diseases. Chlamydia. Available at www.cdc.gov/std/stats/2001chlamydia.htm. Accessed on October 27, 2003.
3. CDC. Division of Sexually Transmitted Diseases. Gonorrhea. Available at www.cdc.gov/std/stats/2001gonorrhea.htm. Accessed on October 27, 2003.
4. CDC. Division of Sexually Transmitted Diseases. Syphilis. Available at www.cdc.gov/std/stats/2001syphilis.htm. Accessed on October 27, 2003.
5. Available at www.eurmktg.com/globstats.
6. Bull SS, McFarlane M. Soliciting sex on the internet. What are the risks for sexually transmitted diseases and HIV? Sex Transm Dis 2000;27:545-550.
7. Klausner JD, Wolf W, Fischer-Ponce L, Zolt I, Katz MH. Tracing a syphilis outbreak through cyberspace. JAMA 2000;284:447-449.
8. McFarlane M, Bull SS, Rietmeijer CA. The internet as a newly-emerging risk environment for sexually transmitted diseases. JAMA 2000;284:443-446.
9. Agency for Healthcare Research and Quality (AHRCQ). United States Preventive Services Task Force (USPSTF). Screening: chlamydia infection. Available at www.ahcpr.gov/clinic/uspstf/uspschlm.htm. Accessed on October 27, 2003.
10. American Academy of Family Physicians. Introduction to AAFP summary of policy recommendations for periodic health examinations. Available at www.aafp.org/x10601.xml. Accessed on October 27, 2003.
11. CDC. Screening tests to detect chlamydia trachomatis and Neisseria gonorrhea infections. MMWR Recomm Rep 2002;51(RR-15):1-38.
12. CDC. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR6):1-78.
1. Centers for Disease Control and Prevention (CDC). National Center for HIV, STD and TB Prevention. Division of Sexually Transmitted Diseases. 2001 National STD Surveillance Report. Table 1: Cases of sexually transmitted diseases reported by state health departments and rates per 100,000 civilian population: United States, 1941–2001. Available at www.cdc.gov/std/stats/tables/table1.htm. Accessed on October 27, 2003.
2. CDC. Division of Sexually Transmitted Diseases. Chlamydia. Available at www.cdc.gov/std/stats/2001chlamydia.htm. Accessed on October 27, 2003.
3. CDC. Division of Sexually Transmitted Diseases. Gonorrhea. Available at www.cdc.gov/std/stats/2001gonorrhea.htm. Accessed on October 27, 2003.
4. CDC. Division of Sexually Transmitted Diseases. Syphilis. Available at www.cdc.gov/std/stats/2001syphilis.htm. Accessed on October 27, 2003.
5. Available at www.eurmktg.com/globstats.
6. Bull SS, McFarlane M. Soliciting sex on the internet. What are the risks for sexually transmitted diseases and HIV? Sex Transm Dis 2000;27:545-550.
7. Klausner JD, Wolf W, Fischer-Ponce L, Zolt I, Katz MH. Tracing a syphilis outbreak through cyberspace. JAMA 2000;284:447-449.
8. McFarlane M, Bull SS, Rietmeijer CA. The internet as a newly-emerging risk environment for sexually transmitted diseases. JAMA 2000;284:443-446.
9. Agency for Healthcare Research and Quality (AHRCQ). United States Preventive Services Task Force (USPSTF). Screening: chlamydia infection. Available at www.ahcpr.gov/clinic/uspstf/uspschlm.htm. Accessed on October 27, 2003.
10. American Academy of Family Physicians. Introduction to AAFP summary of policy recommendations for periodic health examinations. Available at www.aafp.org/x10601.xml. Accessed on October 27, 2003.
11. CDC. Screening tests to detect chlamydia trachomatis and Neisseria gonorrhea infections. MMWR Recomm Rep 2002;51(RR-15):1-38.
12. CDC. Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002;51(RR6):1-78.