User login
It’s Time to Use an Age-based Approach to D-dimer
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
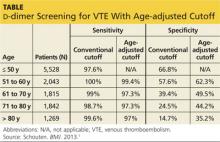
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
PRACTICE CHANGER
Use an age-adjusted d-dimer cutoff (patient age in years × 10 μg/L) for patients older than 50 when evaluating for venous thromboembolism (VTE); it reduces false-positives without substantially increasing false-negatives.1
STRENGTH OF RECOMMENDATION
A: Based on consistent and good-quality patient-centered evidence from a meta-analysis of cohort studies.1
ILLUSTRATIVE CASE
A 78-year-old woman with no significant medical history or recent immobility comes to your clinic complaining of left lower extremity pain and swelling. Her d-dimer is 650 μg/L. What is your next step?
Although d-dimer is recognized as a reasonable screening tool for VTE, the specificity of d-dimer testing using a conventional cutoff value of 500 μg/L is particularly poor in patients older than 50. In low-risk patients older than 80, the specificity is 14.7%.2-5 As a result, conventional d-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1,000 in younger patients to 8:1,000 in older patients,4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and CT pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
Continued on next page >>
STUDY SUMMARY
Using age-adjusted d-dimer cutoffs significantly reduced false-positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had d-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N = 12,497; 6,969 patients older than 50). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and d-dimer sensitivity and specificity for patients ages ≤ 50, 51 to 60, 61 to 70, 71 to 80, and > 80.
The specificity of the conventional d-dimer cutoff value for VTE decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 μg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (see table). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1,000 patients older than 80, compared with 124/1,000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1,000 patients) is comparable to the false-negative rate in those ages 50 and younger (3 per 1,000). The advantages are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
Continued on next page >>
WHAT’S NEW?
We can now use d-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive d-dimer test was less useful for older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients older than 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted d-dimer cutoff improves the diagnostic accuracy of d-dimer screening in older adults.
CAVEATS
Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of d-dimer results. Not all patients included in this meta-analysis whose d-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of the age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION
You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is its identification of a simple calculation that can directly improve patient care. Clinicians can easily apply an age-adjusted d-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 μg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted d-dimer calculation was provided with the lab results.
REFERENCES
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:
765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. http://bit.ly/1gPkLoE. Accessed March 3, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Accessed March 3, 2014.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(3):155-156, 158.
Prolotherapy: A nontraditional approach to knee osteoarthritis
Recommend prolotherapy for patients with knee osteoarthritis (OA) that does not respond to conventional therapies.1
Strength of recommendation
B: Based on a 3-arm, blinded, randomized controlled trial (RCT).
Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
Illustrative case
A 59-year-old woman with OA comes to your office with chronic knee pain. She has tried acetaminophen, ibuprofen, intra-articular corticosteroid injections, and physical therapy without significant improvement in pain or functioning. She wants to avoid daily medications or surgery and wonders if there are any interventions that will not lead to prolonged time away from work. What would you consider?
Additional options needed for knee OA
More than 25% of adults ages 55 years and older suffer from knee pain, and OA is an increasingly common cause.2 Knee pain is a major source of morbidity in the United States; it limits patients’ activities and increases comorbidities such as depression and obesity.
Conventional outpatient treatments for knee pain range from acetaminophen, nonsteroidal anti-inflammatory drugs, glucosamine, chondroitin, and opiates to topical capsaicin therapy, intra-articular hyaluronic acid, and corticosteroid injections. Cost, efficacy, and safety limit these therapies.3
Prolotherapy is another option used to treat musculoskeletal pain. It involves repeatedly injecting a sclerosing solution (usually dextrose) into the sites of chronic musculoskeletal pain.4 The mechanism of action is thought to be the result of local tissue irritation stimulating inflammatory pathways, which leads to the release of growth factors and subsequent healing.4,5 Previous studies evaluating the usefulness of prolotherapy have lacked methodological rigor, have not been randomized adequately, or have lacked a placebo comparison.6-9
STUDY SUMMARY: Prolotherapy reduces pain more than exercise or placebo
Rabago et al1 randomized 90 participants to dextrose prolotherapy, placebo saline injections, or at-home exercise. Participants had a ≥3 month history of painful knee OA based on a self-reported pain scale, radiographic evidence of knee OA within the past 5 years, and tenderness of ≥1 or more anterior knee structures on exam.
Sixty-six percent of participants were female. The mean age was 56.7 years and 74% were overweight (body mass index [BMI], 25-29.9) or obese (BMI ≥30). Participants chose to have one or both knees treated; 43 knees were injected in the dextrose group, 41 received saline injections, and 47 were assessed in the exercise group. There were no significant differences among groups at baseline.
Participants in the prolotherapy and saline groups received injections at 1, 5, and 9 weeks, plus optional injections at 13 and 17 weeks per physician and participant preference. Injections were administered both extra- and intra-articularly. Intra-articular injections were delivered using a 25-gauge needle with a mixture of 25% dextrose, 1% saline, and 1% lidocaine for a total volume of 6 mL. Extra-articular injections were delivered with a peppering technique with a maximum of 15 punctures over painful ligaments and tendons around the knee. The extra-articular solution was similar to the intra-articular except 15% dextrose was used, with a total maximum volume of 22.5 mL.
The placebo injection group received injections in the same pattern and technique, but the solution was the same quantity of 1% lidocaine plus 1% saline to achieve the same volume. The injector, outcome assessor, primary investigator, and participants were blinded to injection group.
In the exercise group, a study coordinator taught participants knee exercises and gave them a pamphlet with 10 exercises to perform at home. Adherence to at-home exercises was assessed with monthly logs that participants mailed in for the first 20 weeks of the study. Seventy-seven percent of participants reported doing their at-home exercises.
The primary outcome measure was change in composite score on the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), a validated questionnaire used to evaluate knee-related quality of life that features subscales for pain, stiffness, and function.10 The minimal clinically important difference in change in score on this 100-point instrument is 12 points; higher scores indicate better quality of life.11 The secondary outcome was change in score on the Knee Pain Scale (KPS), a validated questionnaire that uses a 4-point scale to measure pain frequency and a 5-point scale to measure pain severity; higher scores indicate worse symptoms.12
Improvements seen in both scores
Using an intention-to-treat analysis for all groups, WOMAC composite scores improved at 9 weeks and remained improved through 52 weeks. At 9 weeks, the dextrose group increased 13.91 points, compared with 6.75 (P=.020) in the saline group and 2.51 (P=.001) points in the exercise group.
At 52 weeks, the dextrose group showed an improvement of 15.32 points compared with 7.59 (P=.022) in the saline group and 8.24 (P=.034) in the exercise group. Fifty percent (15/30) of participants in the dextrose group had clinically meaningful improvement as measured by an increase of ≥12 points on the WOMAC, compared with 34% (10/29) and 26% (8/31) in the saline and exercise groups, respectively. At 52 weeks, the dextrose group had significantly decreased KPS knee pain frequency scores compared with the saline group (mean difference [MD], -1.20 vs. -0.60; P<.05) and exercise group (MD, -1.20 vs. -0.40; P<.05). Knee pain severity scores also decreased in the dextrose group compared to the saline (MD, -0.92 vs. -0.32, P<.05) and exercise groups (MD, -0.92 vs. -0.11; P<.05). There were no significant differences in KPS score decreases between the saline and exercise groups.
What about patient satisfaction?
At week 52, all participants were asked, “Would you recommend the therapy you received in this study to others with knee OA like yours?” Ninety-one percent of the dextrose group, 82% of the saline group, and 89% of the exercise group answered “Yes.”
All participants who received injections reported mild to moderate post-injection pain. Five participants in the saline group and 3 in the dextrose group experienced bruising. No other side effects or adverse events were documented. According to daily logs of medication use in the 7 days after injection, 74% of patients in the dextrose group used acetaminophen and 47% used oxycodone, compared with 63% and 43%, respectively, in the saline group. The study authors did not comment on the significance of these differences.
WHAT'S NEW: A randomized study provides support for prolotherapy
This study is the first to adequately demonstrate improvement in knee-related quality of life with prolotherapy compared with placebo (saline) or exercise. Family physicians can now add this therapy to their “toolbox” for patient complaints of OA pain.
CAVEATS: Efficacy is unknown in patients with certain comorbidities
Efficacy is unknown in patients with certain comorbidities Of 894 people screened, only 118 met initial eligibility criteria. This study did not include patients who were taking daily opioids, had diabetes, or had a BMI >40, so its results may not be generalizable to such patients.
Also, while the study demonstrated no side effects or adverse events other than bruising in 8 patients, the sample size may have been too small to detect less common adverse events. However, prior studies of prolotherapy have not revealed any substantial adverse effects.7
Strong evidence for some conditions… not for others. The strongest data support the efficacy of prolotherapy for focal tendinopathy (lateral epicondylosis) and knee OA. Evidence supporting prolotherapy for multimodal conditions, such as chronic low back pain, is less robust.4
CHALLENGES TO IMPLEMENTATION: Finding a prolotherapist near you may not be easy
The main challenge to implementation is finding a certified prolotherapist, or obtaining training in the technique. The prolotherapy knee protocol can be performed in an outpatient setting in less than 15 minutes, but the technique requires training. Prolotherapy training is available from multiple organizations, including the American Association of Orthopaedic Medicine, which requires 100 course hours for prolotherapy certification.4 No formal survey on the number of prolotherapists in the United States has been conducted since 1993,13 but Rabago et al1 estimated that the number is in the hundreds.
Insurance coverage frequently is a challenge. Most third-party payers do not cover prolotherapy, and currently most patients pay out-of-pocket. Rabago et al1 indicated that at their institution, the cost is $218 per injection session. Another study published in 2010 put the average total cost of 4 to 6 prolotherapy sessions at $1800.14
And from the patient’s perspective … The multiple needle sticks involved in prolotherapy can be painful.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center of Research Resources or the National Institutes of Health.
1. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
2. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91-97.
3. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
4. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
5. Hackett GS, Hemwall GA, Montgomery GA. Ligament and Tendon Relaxation Treated by Prolotherapy. 5th ed. Oak Park, IL: Institute in Basic Life Principles; 1991.
6. Schultz LW. A treatment for subluxation of the temporomandibular joint. JAMA. 1937;109:1032-1035.
7. Rabago D, Best TM, Beamsley M, et al. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15:376-380.
8. Reeves KD, Hassanein KM. Long-term effects of dextrose prolotherapy for anterior cruciate ligament laxity. Altern Ther Health Med. 2003;9:58-62.
9. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68-74,77-80.
10. Roos EM, Klässbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28:210-215.
11. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
12. Rejeski WJ, Ettinger WH Jr, Shumaker S, et al. The evaluation of pain in patients with knee osteoarthritis: the knee pain scale. J Rheumatol. 1995;22:1124-1129.
13. Dorman TA. Prolotherapy: A survey. J Orthop Med. 1993;15:28-32.
14. Hauser RA, Hauser MA, Baird NM, et al. Prolotherapy as an alternative to surgery: A prospective pilot study of 34 patients from a private medical practice. J Prolotherapy. 2010;2:272-281.
Recommend prolotherapy for patients with knee osteoarthritis (OA) that does not respond to conventional therapies.1
Strength of recommendation
B: Based on a 3-arm, blinded, randomized controlled trial (RCT).
Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
Illustrative case
A 59-year-old woman with OA comes to your office with chronic knee pain. She has tried acetaminophen, ibuprofen, intra-articular corticosteroid injections, and physical therapy without significant improvement in pain or functioning. She wants to avoid daily medications or surgery and wonders if there are any interventions that will not lead to prolonged time away from work. What would you consider?
Additional options needed for knee OA
More than 25% of adults ages 55 years and older suffer from knee pain, and OA is an increasingly common cause.2 Knee pain is a major source of morbidity in the United States; it limits patients’ activities and increases comorbidities such as depression and obesity.
Conventional outpatient treatments for knee pain range from acetaminophen, nonsteroidal anti-inflammatory drugs, glucosamine, chondroitin, and opiates to topical capsaicin therapy, intra-articular hyaluronic acid, and corticosteroid injections. Cost, efficacy, and safety limit these therapies.3
Prolotherapy is another option used to treat musculoskeletal pain. It involves repeatedly injecting a sclerosing solution (usually dextrose) into the sites of chronic musculoskeletal pain.4 The mechanism of action is thought to be the result of local tissue irritation stimulating inflammatory pathways, which leads to the release of growth factors and subsequent healing.4,5 Previous studies evaluating the usefulness of prolotherapy have lacked methodological rigor, have not been randomized adequately, or have lacked a placebo comparison.6-9
STUDY SUMMARY: Prolotherapy reduces pain more than exercise or placebo
Rabago et al1 randomized 90 participants to dextrose prolotherapy, placebo saline injections, or at-home exercise. Participants had a ≥3 month history of painful knee OA based on a self-reported pain scale, radiographic evidence of knee OA within the past 5 years, and tenderness of ≥1 or more anterior knee structures on exam.
Sixty-six percent of participants were female. The mean age was 56.7 years and 74% were overweight (body mass index [BMI], 25-29.9) or obese (BMI ≥30). Participants chose to have one or both knees treated; 43 knees were injected in the dextrose group, 41 received saline injections, and 47 were assessed in the exercise group. There were no significant differences among groups at baseline.
Participants in the prolotherapy and saline groups received injections at 1, 5, and 9 weeks, plus optional injections at 13 and 17 weeks per physician and participant preference. Injections were administered both extra- and intra-articularly. Intra-articular injections were delivered using a 25-gauge needle with a mixture of 25% dextrose, 1% saline, and 1% lidocaine for a total volume of 6 mL. Extra-articular injections were delivered with a peppering technique with a maximum of 15 punctures over painful ligaments and tendons around the knee. The extra-articular solution was similar to the intra-articular except 15% dextrose was used, with a total maximum volume of 22.5 mL.
The placebo injection group received injections in the same pattern and technique, but the solution was the same quantity of 1% lidocaine plus 1% saline to achieve the same volume. The injector, outcome assessor, primary investigator, and participants were blinded to injection group.
In the exercise group, a study coordinator taught participants knee exercises and gave them a pamphlet with 10 exercises to perform at home. Adherence to at-home exercises was assessed with monthly logs that participants mailed in for the first 20 weeks of the study. Seventy-seven percent of participants reported doing their at-home exercises.
The primary outcome measure was change in composite score on the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), a validated questionnaire used to evaluate knee-related quality of life that features subscales for pain, stiffness, and function.10 The minimal clinically important difference in change in score on this 100-point instrument is 12 points; higher scores indicate better quality of life.11 The secondary outcome was change in score on the Knee Pain Scale (KPS), a validated questionnaire that uses a 4-point scale to measure pain frequency and a 5-point scale to measure pain severity; higher scores indicate worse symptoms.12
Improvements seen in both scores
Using an intention-to-treat analysis for all groups, WOMAC composite scores improved at 9 weeks and remained improved through 52 weeks. At 9 weeks, the dextrose group increased 13.91 points, compared with 6.75 (P=.020) in the saline group and 2.51 (P=.001) points in the exercise group.
At 52 weeks, the dextrose group showed an improvement of 15.32 points compared with 7.59 (P=.022) in the saline group and 8.24 (P=.034) in the exercise group. Fifty percent (15/30) of participants in the dextrose group had clinically meaningful improvement as measured by an increase of ≥12 points on the WOMAC, compared with 34% (10/29) and 26% (8/31) in the saline and exercise groups, respectively. At 52 weeks, the dextrose group had significantly decreased KPS knee pain frequency scores compared with the saline group (mean difference [MD], -1.20 vs. -0.60; P<.05) and exercise group (MD, -1.20 vs. -0.40; P<.05). Knee pain severity scores also decreased in the dextrose group compared to the saline (MD, -0.92 vs. -0.32, P<.05) and exercise groups (MD, -0.92 vs. -0.11; P<.05). There were no significant differences in KPS score decreases between the saline and exercise groups.
What about patient satisfaction?
At week 52, all participants were asked, “Would you recommend the therapy you received in this study to others with knee OA like yours?” Ninety-one percent of the dextrose group, 82% of the saline group, and 89% of the exercise group answered “Yes.”
All participants who received injections reported mild to moderate post-injection pain. Five participants in the saline group and 3 in the dextrose group experienced bruising. No other side effects or adverse events were documented. According to daily logs of medication use in the 7 days after injection, 74% of patients in the dextrose group used acetaminophen and 47% used oxycodone, compared with 63% and 43%, respectively, in the saline group. The study authors did not comment on the significance of these differences.
WHAT'S NEW: A randomized study provides support for prolotherapy
This study is the first to adequately demonstrate improvement in knee-related quality of life with prolotherapy compared with placebo (saline) or exercise. Family physicians can now add this therapy to their “toolbox” for patient complaints of OA pain.
CAVEATS: Efficacy is unknown in patients with certain comorbidities
Efficacy is unknown in patients with certain comorbidities Of 894 people screened, only 118 met initial eligibility criteria. This study did not include patients who were taking daily opioids, had diabetes, or had a BMI >40, so its results may not be generalizable to such patients.
Also, while the study demonstrated no side effects or adverse events other than bruising in 8 patients, the sample size may have been too small to detect less common adverse events. However, prior studies of prolotherapy have not revealed any substantial adverse effects.7
Strong evidence for some conditions… not for others. The strongest data support the efficacy of prolotherapy for focal tendinopathy (lateral epicondylosis) and knee OA. Evidence supporting prolotherapy for multimodal conditions, such as chronic low back pain, is less robust.4
CHALLENGES TO IMPLEMENTATION: Finding a prolotherapist near you may not be easy
The main challenge to implementation is finding a certified prolotherapist, or obtaining training in the technique. The prolotherapy knee protocol can be performed in an outpatient setting in less than 15 minutes, but the technique requires training. Prolotherapy training is available from multiple organizations, including the American Association of Orthopaedic Medicine, which requires 100 course hours for prolotherapy certification.4 No formal survey on the number of prolotherapists in the United States has been conducted since 1993,13 but Rabago et al1 estimated that the number is in the hundreds.
Insurance coverage frequently is a challenge. Most third-party payers do not cover prolotherapy, and currently most patients pay out-of-pocket. Rabago et al1 indicated that at their institution, the cost is $218 per injection session. Another study published in 2010 put the average total cost of 4 to 6 prolotherapy sessions at $1800.14
And from the patient’s perspective … The multiple needle sticks involved in prolotherapy can be painful.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center of Research Resources or the National Institutes of Health.
Recommend prolotherapy for patients with knee osteoarthritis (OA) that does not respond to conventional therapies.1
Strength of recommendation
B: Based on a 3-arm, blinded, randomized controlled trial (RCT).
Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
Illustrative case
A 59-year-old woman with OA comes to your office with chronic knee pain. She has tried acetaminophen, ibuprofen, intra-articular corticosteroid injections, and physical therapy without significant improvement in pain or functioning. She wants to avoid daily medications or surgery and wonders if there are any interventions that will not lead to prolonged time away from work. What would you consider?
Additional options needed for knee OA
More than 25% of adults ages 55 years and older suffer from knee pain, and OA is an increasingly common cause.2 Knee pain is a major source of morbidity in the United States; it limits patients’ activities and increases comorbidities such as depression and obesity.
Conventional outpatient treatments for knee pain range from acetaminophen, nonsteroidal anti-inflammatory drugs, glucosamine, chondroitin, and opiates to topical capsaicin therapy, intra-articular hyaluronic acid, and corticosteroid injections. Cost, efficacy, and safety limit these therapies.3
Prolotherapy is another option used to treat musculoskeletal pain. It involves repeatedly injecting a sclerosing solution (usually dextrose) into the sites of chronic musculoskeletal pain.4 The mechanism of action is thought to be the result of local tissue irritation stimulating inflammatory pathways, which leads to the release of growth factors and subsequent healing.4,5 Previous studies evaluating the usefulness of prolotherapy have lacked methodological rigor, have not been randomized adequately, or have lacked a placebo comparison.6-9
STUDY SUMMARY: Prolotherapy reduces pain more than exercise or placebo
Rabago et al1 randomized 90 participants to dextrose prolotherapy, placebo saline injections, or at-home exercise. Participants had a ≥3 month history of painful knee OA based on a self-reported pain scale, radiographic evidence of knee OA within the past 5 years, and tenderness of ≥1 or more anterior knee structures on exam.
Sixty-six percent of participants were female. The mean age was 56.7 years and 74% were overweight (body mass index [BMI], 25-29.9) or obese (BMI ≥30). Participants chose to have one or both knees treated; 43 knees were injected in the dextrose group, 41 received saline injections, and 47 were assessed in the exercise group. There were no significant differences among groups at baseline.
Participants in the prolotherapy and saline groups received injections at 1, 5, and 9 weeks, plus optional injections at 13 and 17 weeks per physician and participant preference. Injections were administered both extra- and intra-articularly. Intra-articular injections were delivered using a 25-gauge needle with a mixture of 25% dextrose, 1% saline, and 1% lidocaine for a total volume of 6 mL. Extra-articular injections were delivered with a peppering technique with a maximum of 15 punctures over painful ligaments and tendons around the knee. The extra-articular solution was similar to the intra-articular except 15% dextrose was used, with a total maximum volume of 22.5 mL.
The placebo injection group received injections in the same pattern and technique, but the solution was the same quantity of 1% lidocaine plus 1% saline to achieve the same volume. The injector, outcome assessor, primary investigator, and participants were blinded to injection group.
In the exercise group, a study coordinator taught participants knee exercises and gave them a pamphlet with 10 exercises to perform at home. Adherence to at-home exercises was assessed with monthly logs that participants mailed in for the first 20 weeks of the study. Seventy-seven percent of participants reported doing their at-home exercises.
The primary outcome measure was change in composite score on the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC), a validated questionnaire used to evaluate knee-related quality of life that features subscales for pain, stiffness, and function.10 The minimal clinically important difference in change in score on this 100-point instrument is 12 points; higher scores indicate better quality of life.11 The secondary outcome was change in score on the Knee Pain Scale (KPS), a validated questionnaire that uses a 4-point scale to measure pain frequency and a 5-point scale to measure pain severity; higher scores indicate worse symptoms.12
Improvements seen in both scores
Using an intention-to-treat analysis for all groups, WOMAC composite scores improved at 9 weeks and remained improved through 52 weeks. At 9 weeks, the dextrose group increased 13.91 points, compared with 6.75 (P=.020) in the saline group and 2.51 (P=.001) points in the exercise group.
At 52 weeks, the dextrose group showed an improvement of 15.32 points compared with 7.59 (P=.022) in the saline group and 8.24 (P=.034) in the exercise group. Fifty percent (15/30) of participants in the dextrose group had clinically meaningful improvement as measured by an increase of ≥12 points on the WOMAC, compared with 34% (10/29) and 26% (8/31) in the saline and exercise groups, respectively. At 52 weeks, the dextrose group had significantly decreased KPS knee pain frequency scores compared with the saline group (mean difference [MD], -1.20 vs. -0.60; P<.05) and exercise group (MD, -1.20 vs. -0.40; P<.05). Knee pain severity scores also decreased in the dextrose group compared to the saline (MD, -0.92 vs. -0.32, P<.05) and exercise groups (MD, -0.92 vs. -0.11; P<.05). There were no significant differences in KPS score decreases between the saline and exercise groups.
What about patient satisfaction?
At week 52, all participants were asked, “Would you recommend the therapy you received in this study to others with knee OA like yours?” Ninety-one percent of the dextrose group, 82% of the saline group, and 89% of the exercise group answered “Yes.”
All participants who received injections reported mild to moderate post-injection pain. Five participants in the saline group and 3 in the dextrose group experienced bruising. No other side effects or adverse events were documented. According to daily logs of medication use in the 7 days after injection, 74% of patients in the dextrose group used acetaminophen and 47% used oxycodone, compared with 63% and 43%, respectively, in the saline group. The study authors did not comment on the significance of these differences.
WHAT'S NEW: A randomized study provides support for prolotherapy
This study is the first to adequately demonstrate improvement in knee-related quality of life with prolotherapy compared with placebo (saline) or exercise. Family physicians can now add this therapy to their “toolbox” for patient complaints of OA pain.
CAVEATS: Efficacy is unknown in patients with certain comorbidities
Efficacy is unknown in patients with certain comorbidities Of 894 people screened, only 118 met initial eligibility criteria. This study did not include patients who were taking daily opioids, had diabetes, or had a BMI >40, so its results may not be generalizable to such patients.
Also, while the study demonstrated no side effects or adverse events other than bruising in 8 patients, the sample size may have been too small to detect less common adverse events. However, prior studies of prolotherapy have not revealed any substantial adverse effects.7
Strong evidence for some conditions… not for others. The strongest data support the efficacy of prolotherapy for focal tendinopathy (lateral epicondylosis) and knee OA. Evidence supporting prolotherapy for multimodal conditions, such as chronic low back pain, is less robust.4
CHALLENGES TO IMPLEMENTATION: Finding a prolotherapist near you may not be easy
The main challenge to implementation is finding a certified prolotherapist, or obtaining training in the technique. The prolotherapy knee protocol can be performed in an outpatient setting in less than 15 minutes, but the technique requires training. Prolotherapy training is available from multiple organizations, including the American Association of Orthopaedic Medicine, which requires 100 course hours for prolotherapy certification.4 No formal survey on the number of prolotherapists in the United States has been conducted since 1993,13 but Rabago et al1 estimated that the number is in the hundreds.
Insurance coverage frequently is a challenge. Most third-party payers do not cover prolotherapy, and currently most patients pay out-of-pocket. Rabago et al1 indicated that at their institution, the cost is $218 per injection session. Another study published in 2010 put the average total cost of 4 to 6 prolotherapy sessions at $1800.14
And from the patient’s perspective … The multiple needle sticks involved in prolotherapy can be painful.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center of Research Resources or the National Institutes of Health.
1. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
2. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91-97.
3. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
4. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
5. Hackett GS, Hemwall GA, Montgomery GA. Ligament and Tendon Relaxation Treated by Prolotherapy. 5th ed. Oak Park, IL: Institute in Basic Life Principles; 1991.
6. Schultz LW. A treatment for subluxation of the temporomandibular joint. JAMA. 1937;109:1032-1035.
7. Rabago D, Best TM, Beamsley M, et al. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15:376-380.
8. Reeves KD, Hassanein KM. Long-term effects of dextrose prolotherapy for anterior cruciate ligament laxity. Altern Ther Health Med. 2003;9:58-62.
9. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68-74,77-80.
10. Roos EM, Klässbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28:210-215.
11. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
12. Rejeski WJ, Ettinger WH Jr, Shumaker S, et al. The evaluation of pain in patients with knee osteoarthritis: the knee pain scale. J Rheumatol. 1995;22:1124-1129.
13. Dorman TA. Prolotherapy: A survey. J Orthop Med. 1993;15:28-32.
14. Hauser RA, Hauser MA, Baird NM, et al. Prolotherapy as an alternative to surgery: A prospective pilot study of 34 patients from a private medical practice. J Prolotherapy. 2010;2:272-281.
1. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
2. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91-97.
3. Lawrence RC, Felson DT, Helmick CG, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35.
4. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
5. Hackett GS, Hemwall GA, Montgomery GA. Ligament and Tendon Relaxation Treated by Prolotherapy. 5th ed. Oak Park, IL: Institute in Basic Life Principles; 1991.
6. Schultz LW. A treatment for subluxation of the temporomandibular joint. JAMA. 1937;109:1032-1035.
7. Rabago D, Best TM, Beamsley M, et al. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15:376-380.
8. Reeves KD, Hassanein KM. Long-term effects of dextrose prolotherapy for anterior cruciate ligament laxity. Altern Ther Health Med. 2003;9:58-62.
9. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68-74,77-80.
10. Roos EM, Klässbo M, Lohmander LS. WOMAC osteoarthritis index. Reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis. Western Ontario and MacMaster Universities. Scand J Rheumatol. 1999;28:210-215.
11. Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27:2635-2641.
12. Rejeski WJ, Ettinger WH Jr, Shumaker S, et al. The evaluation of pain in patients with knee osteoarthritis: the knee pain scale. J Rheumatol. 1995;22:1124-1129.
13. Dorman TA. Prolotherapy: A survey. J Orthop Med. 1993;15:28-32.
14. Hauser RA, Hauser MA, Baird NM, et al. Prolotherapy as an alternative to surgery: A prospective pilot study of 34 patients from a private medical practice. J Prolotherapy. 2010;2:272-281.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
It’s time to use an age-based approach to D-dimer
Use an age-adjusted D-dimer cutoff (patient’s age in years × 10 mcg/L) for patients over age 50 years when evaluating for venous thromboembolism (VTE); it reduces false positives without substantially increasing false negatives.1
Strength of recommendation
A: Based on consistent and good quality patient-centered evidence from a meta-analysis of cohort studies.
Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and metaanalysis. BMJ. 2013;346:f2492.
Illustrative case
A 78-year-old woman with no significant past medical history or recent immobility comes into your clinic complaining of left lower extremity pain and swelling. Her D-dimer is 650 mcg/L. What is your next step?
Although D-dimer is recognized as a reasonable screening tool for VTE, the specificity of D-dimer testing using a conventional cutoff value of 500 mcg/L is particularly poor in patients over 50 years. In low-risk patients over 80 years old, the specificity is 14.7% (95% confidence interval, 11.3%-18.6%).2-5 As a result, conventional D-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1000 in younger patients to 8:1000 in older patients4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and computed tomography pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
STUDY SUMMARY: Using age-adjusted D-dimer cutoffs significantly reduced false positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had D-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies published before June 21, 2012 that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N=12,497; 6969 patients >50 years). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and D-dimer sensitivity and specificity for patients ages ≤50, 51 to 60, 61 to 70, 71 to 80, and >80 years.
The specificity of using the conventional D-dimer cutoff value for VTE (500 mcg/L) decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 mcg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (TABLE). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1000 patients >80 years, compared with 124/1000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than age 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1000 patients) is comparable to the false negative rate in those age, ≤50 (3 per 1000). The advantages of an age-adjusted cutoff are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
WHAT'S NEW?: We can now make use of the D-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive D-dimer test was less useful for our older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients over age 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted D-dimer cutoff improves the diagnostic accuracy of D-dimer screening in older adults.
CAVEATS: Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of D-dimer results. Not all patients included in this meta-analysis whose D-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of using an age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION: You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is it identifies a simple calculation that can directly improve patient care. Physicians can easily apply an age-adjusted D-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 mcg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted D-dimer calculation was provided with the lab results.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013; 346:f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. Available at: http://bit.ly/1vStJtm. Updated January 30, 2014. Accessed February 13, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Updated January 2013. Accessed October 23, 2013.
Use an age-adjusted D-dimer cutoff (patient’s age in years × 10 mcg/L) for patients over age 50 years when evaluating for venous thromboembolism (VTE); it reduces false positives without substantially increasing false negatives.1
Strength of recommendation
A: Based on consistent and good quality patient-centered evidence from a meta-analysis of cohort studies.
Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and metaanalysis. BMJ. 2013;346:f2492.
Illustrative case
A 78-year-old woman with no significant past medical history or recent immobility comes into your clinic complaining of left lower extremity pain and swelling. Her D-dimer is 650 mcg/L. What is your next step?
Although D-dimer is recognized as a reasonable screening tool for VTE, the specificity of D-dimer testing using a conventional cutoff value of 500 mcg/L is particularly poor in patients over 50 years. In low-risk patients over 80 years old, the specificity is 14.7% (95% confidence interval, 11.3%-18.6%).2-5 As a result, conventional D-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1000 in younger patients to 8:1000 in older patients4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and computed tomography pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
STUDY SUMMARY: Using age-adjusted D-dimer cutoffs significantly reduced false positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had D-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies published before June 21, 2012 that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N=12,497; 6969 patients >50 years). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and D-dimer sensitivity and specificity for patients ages ≤50, 51 to 60, 61 to 70, 71 to 80, and >80 years.
The specificity of using the conventional D-dimer cutoff value for VTE (500 mcg/L) decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 mcg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (TABLE). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1000 patients >80 years, compared with 124/1000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than age 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1000 patients) is comparable to the false negative rate in those age, ≤50 (3 per 1000). The advantages of an age-adjusted cutoff are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
WHAT'S NEW?: We can now make use of the D-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive D-dimer test was less useful for our older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients over age 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted D-dimer cutoff improves the diagnostic accuracy of D-dimer screening in older adults.
CAVEATS: Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of D-dimer results. Not all patients included in this meta-analysis whose D-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of using an age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION: You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is it identifies a simple calculation that can directly improve patient care. Physicians can easily apply an age-adjusted D-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 mcg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted D-dimer calculation was provided with the lab results.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Use an age-adjusted D-dimer cutoff (patient’s age in years × 10 mcg/L) for patients over age 50 years when evaluating for venous thromboembolism (VTE); it reduces false positives without substantially increasing false negatives.1
Strength of recommendation
A: Based on consistent and good quality patient-centered evidence from a meta-analysis of cohort studies.
Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and metaanalysis. BMJ. 2013;346:f2492.
Illustrative case
A 78-year-old woman with no significant past medical history or recent immobility comes into your clinic complaining of left lower extremity pain and swelling. Her D-dimer is 650 mcg/L. What is your next step?
Although D-dimer is recognized as a reasonable screening tool for VTE, the specificity of D-dimer testing using a conventional cutoff value of 500 mcg/L is particularly poor in patients over 50 years. In low-risk patients over 80 years old, the specificity is 14.7% (95% confidence interval, 11.3%-18.6%).2-5 As a result, conventional D-dimer testing is not very helpful for ruling out VTE in older patients.2-5
Improved testing is needed for a population at heightened risk
In the United States, there are more than 600,000 cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) each year.2 The incidence of PE increases from 1:1000 in younger patients to 8:1000 in older patients4 and the mortality rate can reach 30%.6 The gold standards of venography and pulmonary angiography have been replaced by less burdensome tests, primarily lower extremity duplex ultrasound and computed tomography pulmonary angiogram. However, even these tests are expensive and often present logistical challenges in elderly patients. For these reasons, it is helpful to have a simple, less-expensive tool to rule out VTE in older patients who have signs or symptoms.
STUDY SUMMARY: Using age-adjusted D-dimer cutoffs significantly reduced false positives
Schouten et al1 performed a systematic review and meta-analysis of studies of older patients with suspected VTE who had D-dimer testing using both conventional and age-adjusted cutoff values. The authors searched Medline and Embase for studies published before June 21, 2012 that were performed in outpatient, inpatient, or emergency department settings. They excluded studies of high-risk patients, specifically perioperative patients and those who’d had VTE, cancer, or a coagulation disorder.
Five high-quality studies of 13 cohorts were included in this analysis (N=12,497; 6969 patients >50 years). Each of these studies was a retrospective analysis of patients with a low clinical probability of VTE, as determined by Geneva or Wells scoring. The authors calculated the VTE prevalence and D-dimer sensitivity and specificity for patients ages ≤50, 51 to 60, 61 to 70, 71 to 80, and >80 years.
The specificity of using the conventional D-dimer cutoff value for VTE (500 mcg/L) decreased with age from 57.6% in those ages 51 to 60 to 14.7% in those older than 80. When age-adjusted cutoffs were used (age in years × 10 mcg/L), specificities improved in all age categories, particularly for older patients. For example, using age-adjusted cutoff values improved specificity to 62.3% in patients ages 51 to 60 and to 35.2% in those older than 80 (TABLE). Using a hypothetical model, Schouten et al1 calculated that applying age-adjusted cutoff values would exclude VTE in 303/1000 patients >80 years, compared with 124/1000 when using the conventional cutoff.
The benefit of using an age-adjusted cutoff is the ability to exclude VTE in more patients (1 out of 3 in those older than age 80) while not significantly increasing the number of missed VTE. In fact, the number of missed cases in the older population using the age-adjusted cutoff (approximately 1 to 4 per 1000 patients) is comparable to the false negative rate in those age, ≤50 (3 per 1000). The advantages of an age-adjusted cutoff are most notable with the use of enzyme linked fluorescent assays because these assays have a higher sensitivity and a trend toward lower specificity compared with other assays.
WHAT'S NEW?: We can now make use of the D-dimer in older patients
Up until now, it was acknowledged that the simple and less expensive D-dimer test was less useful for our older patients. In fact, in their 2007 clinical practice guideline on the diagnosis of VTE in primary care, the American Academy of Family Physicians and the American College of Physicians commented on the poor performance of the test in older patients.2 A more recent guideline—released by the Institute for Clinical Systems Improvement in January 2013—provided no specific guidance for patients over age 50.7 The meta-analysis reported on here, however, provides that guidance: Using an age-adjusted D-dimer cutoff improves the diagnostic accuracy of D-dimer screening in older adults.
CAVEATS: Results are not generalizable to patients at higher risk
These findings are not generalizable to all patients, particularly those at higher clinical risk who would undergo imaging regardless of D-dimer results. Not all patients included in this meta-analysis whose D-dimer was negative received imaging to confirm that they did not have VTE. As a result, the diagnostic accuracy of using an age-adjusted cutoff could have been overestimated, although this is likely not clinically important because these cases would have remained symptomatic within the 45-day to 3-month follow-up period.
CHALLENGES TO IMPLEMENTATION: You, not the lab, will need to do the calculation
One of the more valuable aspects of this study is it identifies a simple calculation that can directly improve patient care. Physicians can easily apply an age-adjusted D-dimer cutoff as they interpret lab results by multiplying the patient’s age in years × 10 mcg/L. While this does not require institutional changes by the lab, hospital, or clinic, it would be helpful if the age-adjusted D-dimer calculation was provided with the lab results.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013; 346:f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. Available at: http://bit.ly/1vStJtm. Updated January 30, 2014. Accessed February 13, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Updated January 2013. Accessed October 23, 2013.
1. Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013; 346:f2492.
2. Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: a clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Fam Med. 2007;5:57-62.
3. Vossen JA, Albrektson J, Sensarma A, et al. Clinical usefulness of adjusted D-dimer cutoff values to exclude pulmonary embolism in a community hospital emergency department patient population. Acta Radiol. 2012;53:765-768.
4. van Es J, Mos I, Douma R, et al. The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded. Thromb Haemost. 2012;107:167-171.
5. Deep vein thrombosis (DVT). DynaMed Web site. Available at: http://bit.ly/1vStJtm. Updated January 30, 2014. Accessed February 13, 2014.
6. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711–1717.
7. Dupras D, Bluhm J, Felty C, et al. Venous thromboembolism diagnosis and treatment. Institute for Clinical Systems Improvement Web site. Available at: https://www.icsi.org/_asset/sw0pgp/VTE.pdf. Updated January 2013. Accessed October 23, 2013.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Treating Migraine: The Case for Aspirin
PRACTICE CHANGER
Recommend aspirin 975 mg (three adult tablets) as a viable firstline treatment for acute migraine. Consider prescribing metoclopramide 10 mg to be taken with aspirin to markedly decrease associated nausea and help achieve maximum symptom relief.1
STRENGTH OF RECOMMENDATION
B: Based on a Cochrane meta-analysis of 13 good-quality, randomized controlled trials (RCTs).1
ILLUSTRATIVE CASE
During a routine physical, a 37-year-old patient asks you what she should take for occasional migraine. She describes a unilateral headache with associated nausea, vomiting, phonophobia, and photophobia. What medication should you recommend?
Migraine headache affects more than 37 million Americans.2 Women are three times more likely than men to experience migraine, with the highest prevalence among those ages 30 to 50.3,4 More than 50% of patients report that episodes cause severe impairment, resulting in an average loss of four to six workdays each year due to migraine.5,6
Do you recommend this low-cost option?
Although many patients try OTC headache remedies for migraine, when they do seek medical care for this condition, most (67%) turn to their primary care provider.7 But despite a 2010 Cochrane review showing aspirin’s efficacy for acute migraine,8 our experience—based on discussions with physicians at numerous residency programs—suggests that family practice providers are not likely to recommend it.
Further evidence of the underuse of aspirin for migraine comes from a 2013 review of national surveillance studies,5 which found that in 2009, triptans accounted for nearly 80% of antimigraine analgesics prescribed during office visits.5 Thus, when the Cochrane reviewers issued this update of the earlier meta-analysis, we welcomed the opportunity to feature a practice changer that might not be getting the “traction” it deserves.
Continue reading for the study summary...
STUDY SUMMARY
Multiple RCTs highlight aspirin’s efficacy
The 2013 Cochrane reviewers used the same 13 good-quality, double-blind RCTs involving 4,222 participants as the earlier review; no new studies that warranted inclusion were found. A total of 5,261 episodes of moderate-to-severe migraine were treated with either aspirin alone or aspirin plus the antiemetic metoclopramide.1
Five studies had placebo controls, four had active controls (eg, sumatriptan, zolmitriptan, ibuprofen, acetaminophen plus codeine, and ergotamine plus caffeine), and four had both active and placebo controls. Primary outcomes were painfree status at two hours and headache relief (defined as a reduction in pain from moderate/severe to none/mild without the use of rescue medication) at two hours. Sustained headache relief at 24 hours was a secondary outcome.
Patients self-assessed their headache pain, using either a four-point categorical scale (none, mild, moderate, or severe) or a 100-mm visual analog scale. On the analog scale, less than 30 mm was considered mild or no pain; 30 mm or more was considered moderate or severe.
Study participants were ages 18 to 65 (mean age range, 37 to 44), and their symptoms met International Headache Society criteria for migraine with or without aura.9 All participants had migraine symptoms for at least 12 months, with one to six attacks of moderate to severe intensity per month prior to the study period.
In six studies (n = 2,027), investigators compared either 900- or 1,000-mg aspirin alone with placebo. For both primary outcomes, aspirin alone was superior to placebo, with a number needed to treat (NNT) of 8.1 for two-hour painfree status and 4.9 for two-hour headache relief. In three studies (n = 1,142), aspirin was superior to placebo for 24-hour headache relief, with an NNT of 6.6.
Aspirin plus metoclopramide was also better than placebo for primary and secondary outcomes, with an NNT of 8.8 for two-hour painfree status, 3.3 for two-hour headache relief, and 6.2 for 24-hour headache relief. Based on subgroup analysis, aspirin plus metoclopramide was more effective than aspirin alone for two-hour headache relief but equivalent for two-hour painfree status and 24-hour headache relief. The addition of metoclopramide to aspirin significantly reduced nausea and vomiting.
In two studies (n = 726), aspirin alone was equivalent to sumatriptan 50 mg for reaching painfree and headache relief status at two hours. Two additional studies (n = 523) compared aspirin plus metoclopramide with sumatriptan 100 mg and found them to be equal for two-hour headache relief, but the aspirin combination was inferior to the triptan for painfree status at two hours (n = 528). Data were insufficient to compare the efficacy of aspirin with zolmitriptan, ibuprofen, or acetaminophen plus codeine.
There were no reports of gastrointestinal bleeding or other serious adverse events attributable to aspirin therapy. Most adverse effects were mild or moderate disturbances of the digestive and nervous systems, with a number needed to harm of 34 for aspirin (with or without metoclopramide) versus placebo.
WHAT’S NEW?
A reminder of aspirin’s efficacy in treating migraine
The update of this meta-analysis confirms that high-dose aspirin (900 to 1,000 mg) is an effective treatment for migraine headache in adults ages 18 to 65. The addition of metoclopramide reduces nausea and vomiting but offers little if any benefit for headache/pain relief.
Continue reading for the caveats and challenges to implementation...
CAVEATS
Lack of comparison with other treatments
Data were insufficient to compare the efficacy of aspirin with zolmitriptan, other NSAIDs alone, or acetaminophen plus codeine. Aspirin should be used with caution in patients who have chronic renal disease and/or a history of peptic ulcer disease.
CHALLENGES TO IMPLEMENTATION
Patients want a prescription
Patients often expect a prescription when they present with complaints of migraine headache and may feel shortchanged if they’re told to take an aspirin. Providing a prescription for the antiemetic metoclopramide, as well as a brief explanation of the evidence indicating that aspirin is effective for migraine, may adequately address such expectations.
Continue reading for the references...
REFERENCES
1. Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
2. National Headache Foundation. Migraine. www.headaches.org/education/Headache_Topic_Sheets/Migraine. Accessed February 14, 2014.
3. Lipton RB, Stewart WF, Diamond S, et. al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657.
4. Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: a life-span study. Cephalagia. 2010; 9:1065-1072.
5. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
6. Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999; 159:813-818.
7. Gibbs TS, Fleischer AB Jr, Feldman SR, et al. Health care utilization in patients with migraine: demographics and patterns of care in the ambulatory setting. Headache. 2003;43:330-335.
8. Kirthi V, Derry S, Moore RA, et al. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(4):CD008041.
9. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004; 24 (suppl 1):S9-S160.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(2):94-96.
PRACTICE CHANGER
Recommend aspirin 975 mg (three adult tablets) as a viable firstline treatment for acute migraine. Consider prescribing metoclopramide 10 mg to be taken with aspirin to markedly decrease associated nausea and help achieve maximum symptom relief.1
STRENGTH OF RECOMMENDATION
B: Based on a Cochrane meta-analysis of 13 good-quality, randomized controlled trials (RCTs).1
ILLUSTRATIVE CASE
During a routine physical, a 37-year-old patient asks you what she should take for occasional migraine. She describes a unilateral headache with associated nausea, vomiting, phonophobia, and photophobia. What medication should you recommend?
Migraine headache affects more than 37 million Americans.2 Women are three times more likely than men to experience migraine, with the highest prevalence among those ages 30 to 50.3,4 More than 50% of patients report that episodes cause severe impairment, resulting in an average loss of four to six workdays each year due to migraine.5,6
Do you recommend this low-cost option?
Although many patients try OTC headache remedies for migraine, when they do seek medical care for this condition, most (67%) turn to their primary care provider.7 But despite a 2010 Cochrane review showing aspirin’s efficacy for acute migraine,8 our experience—based on discussions with physicians at numerous residency programs—suggests that family practice providers are not likely to recommend it.
Further evidence of the underuse of aspirin for migraine comes from a 2013 review of national surveillance studies,5 which found that in 2009, triptans accounted for nearly 80% of antimigraine analgesics prescribed during office visits.5 Thus, when the Cochrane reviewers issued this update of the earlier meta-analysis, we welcomed the opportunity to feature a practice changer that might not be getting the “traction” it deserves.
Continue reading for the study summary...
STUDY SUMMARY
Multiple RCTs highlight aspirin’s efficacy
The 2013 Cochrane reviewers used the same 13 good-quality, double-blind RCTs involving 4,222 participants as the earlier review; no new studies that warranted inclusion were found. A total of 5,261 episodes of moderate-to-severe migraine were treated with either aspirin alone or aspirin plus the antiemetic metoclopramide.1
Five studies had placebo controls, four had active controls (eg, sumatriptan, zolmitriptan, ibuprofen, acetaminophen plus codeine, and ergotamine plus caffeine), and four had both active and placebo controls. Primary outcomes were painfree status at two hours and headache relief (defined as a reduction in pain from moderate/severe to none/mild without the use of rescue medication) at two hours. Sustained headache relief at 24 hours was a secondary outcome.
Patients self-assessed their headache pain, using either a four-point categorical scale (none, mild, moderate, or severe) or a 100-mm visual analog scale. On the analog scale, less than 30 mm was considered mild or no pain; 30 mm or more was considered moderate or severe.
Study participants were ages 18 to 65 (mean age range, 37 to 44), and their symptoms met International Headache Society criteria for migraine with or without aura.9 All participants had migraine symptoms for at least 12 months, with one to six attacks of moderate to severe intensity per month prior to the study period.
In six studies (n = 2,027), investigators compared either 900- or 1,000-mg aspirin alone with placebo. For both primary outcomes, aspirin alone was superior to placebo, with a number needed to treat (NNT) of 8.1 for two-hour painfree status and 4.9 for two-hour headache relief. In three studies (n = 1,142), aspirin was superior to placebo for 24-hour headache relief, with an NNT of 6.6.
Aspirin plus metoclopramide was also better than placebo for primary and secondary outcomes, with an NNT of 8.8 for two-hour painfree status, 3.3 for two-hour headache relief, and 6.2 for 24-hour headache relief. Based on subgroup analysis, aspirin plus metoclopramide was more effective than aspirin alone for two-hour headache relief but equivalent for two-hour painfree status and 24-hour headache relief. The addition of metoclopramide to aspirin significantly reduced nausea and vomiting.
In two studies (n = 726), aspirin alone was equivalent to sumatriptan 50 mg for reaching painfree and headache relief status at two hours. Two additional studies (n = 523) compared aspirin plus metoclopramide with sumatriptan 100 mg and found them to be equal for two-hour headache relief, but the aspirin combination was inferior to the triptan for painfree status at two hours (n = 528). Data were insufficient to compare the efficacy of aspirin with zolmitriptan, ibuprofen, or acetaminophen plus codeine.
There were no reports of gastrointestinal bleeding or other serious adverse events attributable to aspirin therapy. Most adverse effects were mild or moderate disturbances of the digestive and nervous systems, with a number needed to harm of 34 for aspirin (with or without metoclopramide) versus placebo.
WHAT’S NEW?
A reminder of aspirin’s efficacy in treating migraine
The update of this meta-analysis confirms that high-dose aspirin (900 to 1,000 mg) is an effective treatment for migraine headache in adults ages 18 to 65. The addition of metoclopramide reduces nausea and vomiting but offers little if any benefit for headache/pain relief.
Continue reading for the caveats and challenges to implementation...
CAVEATS
Lack of comparison with other treatments
Data were insufficient to compare the efficacy of aspirin with zolmitriptan, other NSAIDs alone, or acetaminophen plus codeine. Aspirin should be used with caution in patients who have chronic renal disease and/or a history of peptic ulcer disease.
CHALLENGES TO IMPLEMENTATION
Patients want a prescription
Patients often expect a prescription when they present with complaints of migraine headache and may feel shortchanged if they’re told to take an aspirin. Providing a prescription for the antiemetic metoclopramide, as well as a brief explanation of the evidence indicating that aspirin is effective for migraine, may adequately address such expectations.
Continue reading for the references...
REFERENCES
1. Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
2. National Headache Foundation. Migraine. www.headaches.org/education/Headache_Topic_Sheets/Migraine. Accessed February 14, 2014.
3. Lipton RB, Stewart WF, Diamond S, et. al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657.
4. Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: a life-span study. Cephalagia. 2010; 9:1065-1072.
5. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
6. Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999; 159:813-818.
7. Gibbs TS, Fleischer AB Jr, Feldman SR, et al. Health care utilization in patients with migraine: demographics and patterns of care in the ambulatory setting. Headache. 2003;43:330-335.
8. Kirthi V, Derry S, Moore RA, et al. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(4):CD008041.
9. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004; 24 (suppl 1):S9-S160.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(2):94-96.
PRACTICE CHANGER
Recommend aspirin 975 mg (three adult tablets) as a viable firstline treatment for acute migraine. Consider prescribing metoclopramide 10 mg to be taken with aspirin to markedly decrease associated nausea and help achieve maximum symptom relief.1
STRENGTH OF RECOMMENDATION
B: Based on a Cochrane meta-analysis of 13 good-quality, randomized controlled trials (RCTs).1
ILLUSTRATIVE CASE
During a routine physical, a 37-year-old patient asks you what she should take for occasional migraine. She describes a unilateral headache with associated nausea, vomiting, phonophobia, and photophobia. What medication should you recommend?
Migraine headache affects more than 37 million Americans.2 Women are three times more likely than men to experience migraine, with the highest prevalence among those ages 30 to 50.3,4 More than 50% of patients report that episodes cause severe impairment, resulting in an average loss of four to six workdays each year due to migraine.5,6
Do you recommend this low-cost option?
Although many patients try OTC headache remedies for migraine, when they do seek medical care for this condition, most (67%) turn to their primary care provider.7 But despite a 2010 Cochrane review showing aspirin’s efficacy for acute migraine,8 our experience—based on discussions with physicians at numerous residency programs—suggests that family practice providers are not likely to recommend it.
Further evidence of the underuse of aspirin for migraine comes from a 2013 review of national surveillance studies,5 which found that in 2009, triptans accounted for nearly 80% of antimigraine analgesics prescribed during office visits.5 Thus, when the Cochrane reviewers issued this update of the earlier meta-analysis, we welcomed the opportunity to feature a practice changer that might not be getting the “traction” it deserves.
Continue reading for the study summary...
STUDY SUMMARY
Multiple RCTs highlight aspirin’s efficacy
The 2013 Cochrane reviewers used the same 13 good-quality, double-blind RCTs involving 4,222 participants as the earlier review; no new studies that warranted inclusion were found. A total of 5,261 episodes of moderate-to-severe migraine were treated with either aspirin alone or aspirin plus the antiemetic metoclopramide.1
Five studies had placebo controls, four had active controls (eg, sumatriptan, zolmitriptan, ibuprofen, acetaminophen plus codeine, and ergotamine plus caffeine), and four had both active and placebo controls. Primary outcomes were painfree status at two hours and headache relief (defined as a reduction in pain from moderate/severe to none/mild without the use of rescue medication) at two hours. Sustained headache relief at 24 hours was a secondary outcome.
Patients self-assessed their headache pain, using either a four-point categorical scale (none, mild, moderate, or severe) or a 100-mm visual analog scale. On the analog scale, less than 30 mm was considered mild or no pain; 30 mm or more was considered moderate or severe.
Study participants were ages 18 to 65 (mean age range, 37 to 44), and their symptoms met International Headache Society criteria for migraine with or without aura.9 All participants had migraine symptoms for at least 12 months, with one to six attacks of moderate to severe intensity per month prior to the study period.
In six studies (n = 2,027), investigators compared either 900- or 1,000-mg aspirin alone with placebo. For both primary outcomes, aspirin alone was superior to placebo, with a number needed to treat (NNT) of 8.1 for two-hour painfree status and 4.9 for two-hour headache relief. In three studies (n = 1,142), aspirin was superior to placebo for 24-hour headache relief, with an NNT of 6.6.
Aspirin plus metoclopramide was also better than placebo for primary and secondary outcomes, with an NNT of 8.8 for two-hour painfree status, 3.3 for two-hour headache relief, and 6.2 for 24-hour headache relief. Based on subgroup analysis, aspirin plus metoclopramide was more effective than aspirin alone for two-hour headache relief but equivalent for two-hour painfree status and 24-hour headache relief. The addition of metoclopramide to aspirin significantly reduced nausea and vomiting.
In two studies (n = 726), aspirin alone was equivalent to sumatriptan 50 mg for reaching painfree and headache relief status at two hours. Two additional studies (n = 523) compared aspirin plus metoclopramide with sumatriptan 100 mg and found them to be equal for two-hour headache relief, but the aspirin combination was inferior to the triptan for painfree status at two hours (n = 528). Data were insufficient to compare the efficacy of aspirin with zolmitriptan, ibuprofen, or acetaminophen plus codeine.
There were no reports of gastrointestinal bleeding or other serious adverse events attributable to aspirin therapy. Most adverse effects were mild or moderate disturbances of the digestive and nervous systems, with a number needed to harm of 34 for aspirin (with or without metoclopramide) versus placebo.
WHAT’S NEW?
A reminder of aspirin’s efficacy in treating migraine
The update of this meta-analysis confirms that high-dose aspirin (900 to 1,000 mg) is an effective treatment for migraine headache in adults ages 18 to 65. The addition of metoclopramide reduces nausea and vomiting but offers little if any benefit for headache/pain relief.
Continue reading for the caveats and challenges to implementation...
CAVEATS
Lack of comparison with other treatments
Data were insufficient to compare the efficacy of aspirin with zolmitriptan, other NSAIDs alone, or acetaminophen plus codeine. Aspirin should be used with caution in patients who have chronic renal disease and/or a history of peptic ulcer disease.
CHALLENGES TO IMPLEMENTATION
Patients want a prescription
Patients often expect a prescription when they present with complaints of migraine headache and may feel shortchanged if they’re told to take an aspirin. Providing a prescription for the antiemetic metoclopramide, as well as a brief explanation of the evidence indicating that aspirin is effective for migraine, may adequately address such expectations.
Continue reading for the references...
REFERENCES
1. Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
2. National Headache Foundation. Migraine. www.headaches.org/education/Headache_Topic_Sheets/Migraine. Accessed February 14, 2014.
3. Lipton RB, Stewart WF, Diamond S, et. al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657.
4. Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: a life-span study. Cephalagia. 2010; 9:1065-1072.
5. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
6. Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999; 159:813-818.
7. Gibbs TS, Fleischer AB Jr, Feldman SR, et al. Health care utilization in patients with migraine: demographics and patterns of care in the ambulatory setting. Headache. 2003;43:330-335.
8. Kirthi V, Derry S, Moore RA, et al. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(4):CD008041.
9. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004; 24 (suppl 1):S9-S160.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(2):94-96.
Steroids for Acute COPD—But for How Long?
PRACTICE CHANGER
Prescribe a five-day regimen of glucocorticoid therapy for acute exacerbations of chronic obstructive pulmonary disease (COPD); the shorter course of treatment appears to be as effective as a 14-day regimen.1
Strength of recommendation
B: Based on a single well-designed randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
A 55-year-old man with COPD presents to the emergency department (ED) with progressive shortness of breath, cough, and sputum production in the past four days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk for early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids versus a longer (eight-week) duration come from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to 14-day regimen (30 to 40 mg/d) but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (three to seven days) with a longer regimen (10 to 15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing five-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
Continue reading for the study summary...
STUDY SUMMARY
Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a five- and a 14-day course of prednisone 40 mg/d to treat patients with COPD exacerbations. A patient was considered to have an exacerbation if he or she had a change from baseline in two or more of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who presented to the EDs of five Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 or older and have at least 20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (FEV1/FVC > 70%), pneumonia, an estimated survival of less than six months, pregnancy, and lactation.
All the participants (N = 311) received 40 mg methylprednisolone intravenously on day 1, followed by prednisone 40 mg orally on days 2 through 5. The researchers then randomly divided participants into two groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional nine days. Participants in both groups also received antibiotics for seven days, twice-daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the five-day steroid regimen versus 57 days for those on the
14-day regimen in the intention-to-treat analysis (HR, 0.95). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took more than 400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg, respectively).
WHAT’S NEW?
Now we know: five days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for more than five days.3 This trial clearly demonstrated that 40-mg prednisone for five days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS
Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION
Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a five-day regimen may be appropriate. For those in whom prior attempts at short-course treatment have failed, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which patients previously treated with a longer regimen will find the shorter course of treatment unsuccessful.
Continue for references...
REFERENCES
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.org. Accessed January 9, 2014.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(1):29-30, 32.
PRACTICE CHANGER
Prescribe a five-day regimen of glucocorticoid therapy for acute exacerbations of chronic obstructive pulmonary disease (COPD); the shorter course of treatment appears to be as effective as a 14-day regimen.1
Strength of recommendation
B: Based on a single well-designed randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
A 55-year-old man with COPD presents to the emergency department (ED) with progressive shortness of breath, cough, and sputum production in the past four days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk for early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids versus a longer (eight-week) duration come from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to 14-day regimen (30 to 40 mg/d) but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (three to seven days) with a longer regimen (10 to 15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing five-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
Continue reading for the study summary...
STUDY SUMMARY
Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a five- and a 14-day course of prednisone 40 mg/d to treat patients with COPD exacerbations. A patient was considered to have an exacerbation if he or she had a change from baseline in two or more of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who presented to the EDs of five Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 or older and have at least 20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (FEV1/FVC > 70%), pneumonia, an estimated survival of less than six months, pregnancy, and lactation.
All the participants (N = 311) received 40 mg methylprednisolone intravenously on day 1, followed by prednisone 40 mg orally on days 2 through 5. The researchers then randomly divided participants into two groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional nine days. Participants in both groups also received antibiotics for seven days, twice-daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the five-day steroid regimen versus 57 days for those on the
14-day regimen in the intention-to-treat analysis (HR, 0.95). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took more than 400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg, respectively).
WHAT’S NEW?
Now we know: five days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for more than five days.3 This trial clearly demonstrated that 40-mg prednisone for five days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS
Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION
Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a five-day regimen may be appropriate. For those in whom prior attempts at short-course treatment have failed, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which patients previously treated with a longer regimen will find the shorter course of treatment unsuccessful.
Continue for references...
REFERENCES
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.org. Accessed January 9, 2014.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(1):29-30, 32.
PRACTICE CHANGER
Prescribe a five-day regimen of glucocorticoid therapy for acute exacerbations of chronic obstructive pulmonary disease (COPD); the shorter course of treatment appears to be as effective as a 14-day regimen.1
Strength of recommendation
B: Based on a single well-designed randomized controlled trial (RCT).1
ILLUSTRATIVE CASE
A 55-year-old man with COPD presents to the emergency department (ED) with progressive shortness of breath, cough, and sputum production in the past four days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk for early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids versus a longer (eight-week) duration come from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to 14-day regimen (30 to 40 mg/d) but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (three to seven days) with a longer regimen (10 to 15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing five-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
Continue reading for the study summary...
STUDY SUMMARY
Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a five- and a 14-day course of prednisone 40 mg/d to treat patients with COPD exacerbations. A patient was considered to have an exacerbation if he or she had a change from baseline in two or more of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who presented to the EDs of five Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 or older and have at least 20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (FEV1/FVC > 70%), pneumonia, an estimated survival of less than six months, pregnancy, and lactation.
All the participants (N = 311) received 40 mg methylprednisolone intravenously on day 1, followed by prednisone 40 mg orally on days 2 through 5. The researchers then randomly divided participants into two groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional nine days. Participants in both groups also received antibiotics for seven days, twice-daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the five-day steroid regimen versus 57 days for those on the
14-day regimen in the intention-to-treat analysis (HR, 0.95). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took more than 400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg, respectively).
WHAT’S NEW?
Now we know: five days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for more than five days.3 This trial clearly demonstrated that 40-mg prednisone for five days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS
Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION
Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a five-day regimen may be appropriate. For those in whom prior attempts at short-course treatment have failed, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which patients previously treated with a longer regimen will find the shorter course of treatment unsuccessful.
Continue for references...
REFERENCES
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. www.goldcopd.org. Accessed January 9, 2014.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2014;63(1):29-30, 32.
Treating migraine: The case for aspirin
Recommend aspirin 975 mg (3 adult tablets) as a viable first-line treatment for acute migraine. Consider prescribing metoclopramide 10 mg to be taken with aspirin to markedly decrease associated nausea and help achieve maximum symptom relief.1
Strength of recommendation
A: Based on a Cochrane meta-analysis of 13 good quality, randomized controlled trials (RCTs).
Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
Illustrative case
During a routine physical, a 37-year-old patient asks you what she should take for her occasional migraines. She describes a unilateral headache with associated nausea, vomiting, phonophobia, and photophobia. What medication should you recommend?
Migraine headache affects more than 37 million Americans.2 Women are 3 times more likely than men to suffer from migraine, with the highest prevalence among those between the ages of 30 and 50 years.3,4 More than 50% of patients report that episodes cause severe impairment, resulting in an average loss of 4 to 6 workdays each year due to migraine.5,6
Do you recommend this low-cost option?
Although many patients try over-the-counter headache remedies for migraine, when they do seek medical care for this condition, most (67%) turn to their primary care providers.7 But despite a 2010 Cochrane review showing aspirin’s efficacy for acute migraine,8 our experience—based on discussions with physicians at numerous residency programs—suggests that family physicians are not likely to recommend it.
Further evidence of the underuse of aspirin for migraine comes from a 2013 review of national surveillance studies,5 which found that in 2009, triptans accounted for nearly 80% of antimigraine analgesics prescribed during office visits.5 Thus, when the Cochrane reviewers issued this update of the earlier meta-analysis, we welcomed the opportunity to feature a practice changer that might not be getting the “traction” it deserves.
STUDY SUMMARY: Multiple RCTs highlight aspirin's efficacy
The 2013 Cochrane reviewers used the same 13 good quality, double-blind RCTs involving 4222 participants as the earlier review; no new studies that warranted inclusion were found. A total of 5261 episodes of migraine of moderate to severe intensity were treated with either aspirin alone or aspirin plus the antiemetic metoclopramide.1
Five studies had placebo controls, 4 had active controls (sumatriptan, zolmitriptan, ibuprofen, acetaminophen plus codeine, and ergotamine plus caffeine among them), and 4 had both active and placebo controls. Primary outcomes were pain-free status at 2 hours and headache relief (defined as a reduction in pain from moderate or severe to none or mild without the use of rescue medication) at 2 hours. Sustained headache relief at 24 hours was a secondary outcome.
Patients self-assessed their headache pain, using either a 4-point categorical scale (none, mild, moderate, or severe) or a 100 mm visual analog scale. On the analog scale, <30 mm was considered mild or no pain; ≥30 mm was considered moderate or severe.
Study participants were 18 to 65 years of age (the mean age range was 37-44), and their symptoms met International Headache Society criteria for migraine with or without aura.9 All participants had migraine symptoms for ≥12 months, with between one and 6 attacks of moderate to severe intensity per month prior to the study period.
In 6 studies (n=2027), investigators compared either 900 or 1000 mg aspirin alone with placebo. For both primary outcomes, aspirin alone was superior to placebo, with a number needed to treat (NNT) of 8.1 for 2-hour pain-free status and 4.9 for 2-hour headache relief. In 3 studies (n=1142), aspirin was superior to placebo for 24-hour headache relief, with an NNT of 6.6. Aspirin plus metoclopramide was also better than placebo for primary and secondary outcomes, with an NNT of 8.8 for 2-hour pain-free status, 3.3 for 2-hour headache relief, and 6.2 for 24-hour headache relief. Based on subgroup analysis, aspirin plus metoclopramide was more effective than aspirin alone for 2-hour headache relief (P=.0131), but equivalent for 2-hour pain-free status and 24-hour headache relief. The addition of metoclopramide to aspirin significantly reduced nausea (P<.00006) and vomiting (P=.002).
In 2 studies (n=726), aspirin alone was equivalent to sumatriptan 50 mg for reaching pain-free and headache relief status at 2 hours. Two additional studies (n=523) compared aspirin plus metoclopramide with sumatriptan 100 mg and found them to be equal for 2-hour headache relief, but the aspirin combination was inferior to the triptan for pain-free status at 2 hours (n=528). Data were insufficient to compare the efficacy of aspirin with zolmitriptan, ibuprofen, or acetaminophen plus codeine.
There were no reports of gastrointestinal bleed or other serious adverse events attributable to aspirin therapy. Most adverse effects were mild or moderate disturbances of the digestive and nervous systems, with a number needed to harm of 34 (95% confidence interval, 18-340) for aspirin (with or without metoclopramide) vs placebo.
WHAT'S NEW?: A reminder of aspirin's efficacy in treating migraine
The update of this meta-analysis confirms that high-dose aspirin (900-1000 mg) is an effective treatment for migraine headache in adults between the ages of 18 and 65 years. The addition of metoclopramide reduces nausea and vomiting, but offers little if any benefit for headache/pain relief.
CAVEATS: Lack of comparison with other treatments
Data were insufficient to compare the efficacy of aspirin with zolmitriptan, other nonsteroidal anti-inflammatory drugs alone, or acetaminophen plus codeine. Aspirin should be used with caution in patients with chronic renal disease and/or a history of peptic ulcer disease.
CHALLENGES TO IMPLEMENTATION: Patients want a prescription
Patients often expect a prescription when they visit a physician with complaints of migraine headache and may feel shortchanged if they’re told to take an aspirin. Providing a prescription for the antiemetic metoclopramide, as well as a brief explanation of the evidence indicating that aspirin is effective for migraine, may adequately address such expectations.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
2. Migraine. National Headache Foundation Web site. Available at: http://www.headaches.org/education/Headache_Topic_Sheets/Migraine. Accessed January 10, 2014.
3. Lipton RB, Stewart WF, Diamond S, et. al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657.
4. Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;9:1065-1072.
5. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
6. Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Intern Med.1999;159:813-818.
7. Gibbs TS, Fleischer AB Jr, Feldman SR, et al. Health care utilization in patients with migraine: demographics and patterns of care in the ambulatory setting. Headache. 2003;43:330-335.
8. Kirthi V, Derry S, Moore RA, et al. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(4):CD008041.
9. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004;24(suppl 1):S9-S160.
Recommend aspirin 975 mg (3 adult tablets) as a viable first-line treatment for acute migraine. Consider prescribing metoclopramide 10 mg to be taken with aspirin to markedly decrease associated nausea and help achieve maximum symptom relief.1
Strength of recommendation
A: Based on a Cochrane meta-analysis of 13 good quality, randomized controlled trials (RCTs).
Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
Illustrative case
During a routine physical, a 37-year-old patient asks you what she should take for her occasional migraines. She describes a unilateral headache with associated nausea, vomiting, phonophobia, and photophobia. What medication should you recommend?
Migraine headache affects more than 37 million Americans.2 Women are 3 times more likely than men to suffer from migraine, with the highest prevalence among those between the ages of 30 and 50 years.3,4 More than 50% of patients report that episodes cause severe impairment, resulting in an average loss of 4 to 6 workdays each year due to migraine.5,6
Do you recommend this low-cost option?
Although many patients try over-the-counter headache remedies for migraine, when they do seek medical care for this condition, most (67%) turn to their primary care providers.7 But despite a 2010 Cochrane review showing aspirin’s efficacy for acute migraine,8 our experience—based on discussions with physicians at numerous residency programs—suggests that family physicians are not likely to recommend it.
Further evidence of the underuse of aspirin for migraine comes from a 2013 review of national surveillance studies,5 which found that in 2009, triptans accounted for nearly 80% of antimigraine analgesics prescribed during office visits.5 Thus, when the Cochrane reviewers issued this update of the earlier meta-analysis, we welcomed the opportunity to feature a practice changer that might not be getting the “traction” it deserves.
STUDY SUMMARY: Multiple RCTs highlight aspirin's efficacy
The 2013 Cochrane reviewers used the same 13 good quality, double-blind RCTs involving 4222 participants as the earlier review; no new studies that warranted inclusion were found. A total of 5261 episodes of migraine of moderate to severe intensity were treated with either aspirin alone or aspirin plus the antiemetic metoclopramide.1
Five studies had placebo controls, 4 had active controls (sumatriptan, zolmitriptan, ibuprofen, acetaminophen plus codeine, and ergotamine plus caffeine among them), and 4 had both active and placebo controls. Primary outcomes were pain-free status at 2 hours and headache relief (defined as a reduction in pain from moderate or severe to none or mild without the use of rescue medication) at 2 hours. Sustained headache relief at 24 hours was a secondary outcome.
Patients self-assessed their headache pain, using either a 4-point categorical scale (none, mild, moderate, or severe) or a 100 mm visual analog scale. On the analog scale, <30 mm was considered mild or no pain; ≥30 mm was considered moderate or severe.
Study participants were 18 to 65 years of age (the mean age range was 37-44), and their symptoms met International Headache Society criteria for migraine with or without aura.9 All participants had migraine symptoms for ≥12 months, with between one and 6 attacks of moderate to severe intensity per month prior to the study period.
In 6 studies (n=2027), investigators compared either 900 or 1000 mg aspirin alone with placebo. For both primary outcomes, aspirin alone was superior to placebo, with a number needed to treat (NNT) of 8.1 for 2-hour pain-free status and 4.9 for 2-hour headache relief. In 3 studies (n=1142), aspirin was superior to placebo for 24-hour headache relief, with an NNT of 6.6. Aspirin plus metoclopramide was also better than placebo for primary and secondary outcomes, with an NNT of 8.8 for 2-hour pain-free status, 3.3 for 2-hour headache relief, and 6.2 for 24-hour headache relief. Based on subgroup analysis, aspirin plus metoclopramide was more effective than aspirin alone for 2-hour headache relief (P=.0131), but equivalent for 2-hour pain-free status and 24-hour headache relief. The addition of metoclopramide to aspirin significantly reduced nausea (P<.00006) and vomiting (P=.002).
In 2 studies (n=726), aspirin alone was equivalent to sumatriptan 50 mg for reaching pain-free and headache relief status at 2 hours. Two additional studies (n=523) compared aspirin plus metoclopramide with sumatriptan 100 mg and found them to be equal for 2-hour headache relief, but the aspirin combination was inferior to the triptan for pain-free status at 2 hours (n=528). Data were insufficient to compare the efficacy of aspirin with zolmitriptan, ibuprofen, or acetaminophen plus codeine.
There were no reports of gastrointestinal bleed or other serious adverse events attributable to aspirin therapy. Most adverse effects were mild or moderate disturbances of the digestive and nervous systems, with a number needed to harm of 34 (95% confidence interval, 18-340) for aspirin (with or without metoclopramide) vs placebo.
WHAT'S NEW?: A reminder of aspirin's efficacy in treating migraine
The update of this meta-analysis confirms that high-dose aspirin (900-1000 mg) is an effective treatment for migraine headache in adults between the ages of 18 and 65 years. The addition of metoclopramide reduces nausea and vomiting, but offers little if any benefit for headache/pain relief.
CAVEATS: Lack of comparison with other treatments
Data were insufficient to compare the efficacy of aspirin with zolmitriptan, other nonsteroidal anti-inflammatory drugs alone, or acetaminophen plus codeine. Aspirin should be used with caution in patients with chronic renal disease and/or a history of peptic ulcer disease.
CHALLENGES TO IMPLEMENTATION: Patients want a prescription
Patients often expect a prescription when they visit a physician with complaints of migraine headache and may feel shortchanged if they’re told to take an aspirin. Providing a prescription for the antiemetic metoclopramide, as well as a brief explanation of the evidence indicating that aspirin is effective for migraine, may adequately address such expectations.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Recommend aspirin 975 mg (3 adult tablets) as a viable first-line treatment for acute migraine. Consider prescribing metoclopramide 10 mg to be taken with aspirin to markedly decrease associated nausea and help achieve maximum symptom relief.1
Strength of recommendation
A: Based on a Cochrane meta-analysis of 13 good quality, randomized controlled trials (RCTs).
Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
Illustrative case
During a routine physical, a 37-year-old patient asks you what she should take for her occasional migraines. She describes a unilateral headache with associated nausea, vomiting, phonophobia, and photophobia. What medication should you recommend?
Migraine headache affects more than 37 million Americans.2 Women are 3 times more likely than men to suffer from migraine, with the highest prevalence among those between the ages of 30 and 50 years.3,4 More than 50% of patients report that episodes cause severe impairment, resulting in an average loss of 4 to 6 workdays each year due to migraine.5,6
Do you recommend this low-cost option?
Although many patients try over-the-counter headache remedies for migraine, when they do seek medical care for this condition, most (67%) turn to their primary care providers.7 But despite a 2010 Cochrane review showing aspirin’s efficacy for acute migraine,8 our experience—based on discussions with physicians at numerous residency programs—suggests that family physicians are not likely to recommend it.
Further evidence of the underuse of aspirin for migraine comes from a 2013 review of national surveillance studies,5 which found that in 2009, triptans accounted for nearly 80% of antimigraine analgesics prescribed during office visits.5 Thus, when the Cochrane reviewers issued this update of the earlier meta-analysis, we welcomed the opportunity to feature a practice changer that might not be getting the “traction” it deserves.
STUDY SUMMARY: Multiple RCTs highlight aspirin's efficacy
The 2013 Cochrane reviewers used the same 13 good quality, double-blind RCTs involving 4222 participants as the earlier review; no new studies that warranted inclusion were found. A total of 5261 episodes of migraine of moderate to severe intensity were treated with either aspirin alone or aspirin plus the antiemetic metoclopramide.1
Five studies had placebo controls, 4 had active controls (sumatriptan, zolmitriptan, ibuprofen, acetaminophen plus codeine, and ergotamine plus caffeine among them), and 4 had both active and placebo controls. Primary outcomes were pain-free status at 2 hours and headache relief (defined as a reduction in pain from moderate or severe to none or mild without the use of rescue medication) at 2 hours. Sustained headache relief at 24 hours was a secondary outcome.
Patients self-assessed their headache pain, using either a 4-point categorical scale (none, mild, moderate, or severe) or a 100 mm visual analog scale. On the analog scale, <30 mm was considered mild or no pain; ≥30 mm was considered moderate or severe.
Study participants were 18 to 65 years of age (the mean age range was 37-44), and their symptoms met International Headache Society criteria for migraine with or without aura.9 All participants had migraine symptoms for ≥12 months, with between one and 6 attacks of moderate to severe intensity per month prior to the study period.
In 6 studies (n=2027), investigators compared either 900 or 1000 mg aspirin alone with placebo. For both primary outcomes, aspirin alone was superior to placebo, with a number needed to treat (NNT) of 8.1 for 2-hour pain-free status and 4.9 for 2-hour headache relief. In 3 studies (n=1142), aspirin was superior to placebo for 24-hour headache relief, with an NNT of 6.6. Aspirin plus metoclopramide was also better than placebo for primary and secondary outcomes, with an NNT of 8.8 for 2-hour pain-free status, 3.3 for 2-hour headache relief, and 6.2 for 24-hour headache relief. Based on subgroup analysis, aspirin plus metoclopramide was more effective than aspirin alone for 2-hour headache relief (P=.0131), but equivalent for 2-hour pain-free status and 24-hour headache relief. The addition of metoclopramide to aspirin significantly reduced nausea (P<.00006) and vomiting (P=.002).
In 2 studies (n=726), aspirin alone was equivalent to sumatriptan 50 mg for reaching pain-free and headache relief status at 2 hours. Two additional studies (n=523) compared aspirin plus metoclopramide with sumatriptan 100 mg and found them to be equal for 2-hour headache relief, but the aspirin combination was inferior to the triptan for pain-free status at 2 hours (n=528). Data were insufficient to compare the efficacy of aspirin with zolmitriptan, ibuprofen, or acetaminophen plus codeine.
There were no reports of gastrointestinal bleed or other serious adverse events attributable to aspirin therapy. Most adverse effects were mild or moderate disturbances of the digestive and nervous systems, with a number needed to harm of 34 (95% confidence interval, 18-340) for aspirin (with or without metoclopramide) vs placebo.
WHAT'S NEW?: A reminder of aspirin's efficacy in treating migraine
The update of this meta-analysis confirms that high-dose aspirin (900-1000 mg) is an effective treatment for migraine headache in adults between the ages of 18 and 65 years. The addition of metoclopramide reduces nausea and vomiting, but offers little if any benefit for headache/pain relief.
CAVEATS: Lack of comparison with other treatments
Data were insufficient to compare the efficacy of aspirin with zolmitriptan, other nonsteroidal anti-inflammatory drugs alone, or acetaminophen plus codeine. Aspirin should be used with caution in patients with chronic renal disease and/or a history of peptic ulcer disease.
CHALLENGES TO IMPLEMENTATION: Patients want a prescription
Patients often expect a prescription when they visit a physician with complaints of migraine headache and may feel shortchanged if they’re told to take an aspirin. Providing a prescription for the antiemetic metoclopramide, as well as a brief explanation of the evidence indicating that aspirin is effective for migraine, may adequately address such expectations.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
2. Migraine. National Headache Foundation Web site. Available at: http://www.headaches.org/education/Headache_Topic_Sheets/Migraine. Accessed January 10, 2014.
3. Lipton RB, Stewart WF, Diamond S, et. al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657.
4. Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;9:1065-1072.
5. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
6. Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Intern Med.1999;159:813-818.
7. Gibbs TS, Fleischer AB Jr, Feldman SR, et al. Health care utilization in patients with migraine: demographics and patterns of care in the ambulatory setting. Headache. 2003;43:330-335.
8. Kirthi V, Derry S, Moore RA, et al. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(4):CD008041.
9. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004;24(suppl 1):S9-S160.
1. Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041.
2. Migraine. National Headache Foundation Web site. Available at: http://www.headaches.org/education/Headache_Topic_Sheets/Migraine. Accessed January 10, 2014.
3. Lipton RB, Stewart WF, Diamond S, et. al. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646-657.
4. Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;9:1065-1072.
5. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
6. Hu XH, Markson LE, Lipton RB, et al. Burden of migraine in the United States: disability and economic costs. Arch Intern Med.1999;159:813-818.
7. Gibbs TS, Fleischer AB Jr, Feldman SR, et al. Health care utilization in patients with migraine: demographics and patterns of care in the ambulatory setting. Headache. 2003;43:330-335.
8. Kirthi V, Derry S, Moore RA, et al. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2010;(4):CD008041.
9. The international classification of headache disorders. 2nd ed. Cephalalgia. 2004;24(suppl 1):S9-S160.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Steroids for acute COPD—but for how long?
Prescribe a 5-day regimen of glucocorticoid therapy for acute chronic obstructive pulmonary disease (COPD) exacerbations; the shorter course of treatment appears to be as effective as a 14-day regiment.1
Strength of recommendation
B: Based on a single well-design randomized controlled trial (RCT).
Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
Illustrative case
A 55-year-old man with COPD presents to the emergency department (ED) because of progressive shortness of breath, cough, and sputum production over the past 4 days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk of early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids vs a longer (8-week) duration comes from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to-14-day regimen (30-40 mg/d), but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (3-7 days) with a longer regimen (10-15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing 5-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
STUDY SUMMARY: Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a 5- vs a 14-day course of prednisone (40 mg/d) to treat patients with COPD exacerbations. A patient was considered to have a COPD exacerbation if he or she had a change from baseline in ≥2 of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who came to the EDs of 5 Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 years or older and have ≥20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (forced expiratory volume in one second/forced vital capacity >70%), pneumonia, an estimated survival <6 months, pregnancy, and lactation.
All the participants (N=311) received 40 mg methylprednisolone intravenously on Day 1, followed by prednisone 40 mg orally on Days 2 through 5. The researchers then randomly divided participants into 2 groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional 9 days. Participants in both groups also received antibiotics for 7 days, twice daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the 5-day steroid regimen vs 57 days for those on the 14-day regimen in the intention to treat analysis (HR=0.95; 90% confidence interval, 0.70-1.29; P=.006). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took >400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg; P<.001).
WHAT'S NEW?: Now we know: 5 days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for >5 days.3 This trial clearly demonstrated that 40 mg prednisone for 5 days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS: Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION: Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a 5-day regimen may be appropriate. For those who have failed prior attempts at short-course treatment, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which of those who were previously treated with a longer regimen may fail on the shorter course of treatment.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosismanagement.html. Accessed December 13, 2013.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C,et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
Prescribe a 5-day regimen of glucocorticoid therapy for acute chronic obstructive pulmonary disease (COPD) exacerbations; the shorter course of treatment appears to be as effective as a 14-day regiment.1
Strength of recommendation
B: Based on a single well-design randomized controlled trial (RCT).
Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
Illustrative case
A 55-year-old man with COPD presents to the emergency department (ED) because of progressive shortness of breath, cough, and sputum production over the past 4 days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk of early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids vs a longer (8-week) duration comes from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to-14-day regimen (30-40 mg/d), but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (3-7 days) with a longer regimen (10-15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing 5-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
STUDY SUMMARY: Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a 5- vs a 14-day course of prednisone (40 mg/d) to treat patients with COPD exacerbations. A patient was considered to have a COPD exacerbation if he or she had a change from baseline in ≥2 of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who came to the EDs of 5 Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 years or older and have ≥20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (forced expiratory volume in one second/forced vital capacity >70%), pneumonia, an estimated survival <6 months, pregnancy, and lactation.
All the participants (N=311) received 40 mg methylprednisolone intravenously on Day 1, followed by prednisone 40 mg orally on Days 2 through 5. The researchers then randomly divided participants into 2 groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional 9 days. Participants in both groups also received antibiotics for 7 days, twice daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the 5-day steroid regimen vs 57 days for those on the 14-day regimen in the intention to treat analysis (HR=0.95; 90% confidence interval, 0.70-1.29; P=.006). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took >400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg; P<.001).
WHAT'S NEW?: Now we know: 5 days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for >5 days.3 This trial clearly demonstrated that 40 mg prednisone for 5 days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS: Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION: Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a 5-day regimen may be appropriate. For those who have failed prior attempts at short-course treatment, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which of those who were previously treated with a longer regimen may fail on the shorter course of treatment.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Prescribe a 5-day regimen of glucocorticoid therapy for acute chronic obstructive pulmonary disease (COPD) exacerbations; the shorter course of treatment appears to be as effective as a 14-day regiment.1
Strength of recommendation
B: Based on a single well-design randomized controlled trial (RCT).
Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
Illustrative case
A 55-year-old man with COPD presents to the emergency department (ED) because of progressive shortness of breath, cough, and sputum production over the past 4 days. He is diagnosed with a COPD exacerbation, treated with corticosteroids, and admitted to the hospital. His inpatient treatment includes antibiotics, inhaled albuterol and ipratropium, supplemental oxygen, and oral corticosteroids.
How many days should he take oral steroids?
Severe exacerbations of COPD are independently associated with mortality,2 regardless of baseline severity. Guidelines and systematic reviews highlight the importance of using oral glucocorticoids in the management of acute COPD exacerbations, as the drugs have been found to shorten recovery time and length of hospital stay, improve lung function, and reduce the risk of early relapse and treatment failure.3-5 What is not clear is how long the course of oral steroids should be.
What we know (and don’t know) about duration
Data supporting a 14-day course of steroids vs a longer (8-week) duration comes from the Systemic Corticosteroids in COPD Exacerbations trial.6 Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria suggest a 10- to-14-day regimen (30-40 mg/d), but acknowledge that there is a lack of data from clinical and observational studies to support this recommendation.3 A recent Cochrane review compared a short course of treatment (3-7 days) with a longer regimen (10-15 days) and found that the evidence to support a clinical practice change was inconclusive.5
The study detailed in this PURL—a double-blind RCT comparing 5-day with 14-day oral steroid treatment in patients hospitalized for acute COPD exacerbation—had more definitive results.1
STUDY SUMMARY: Shorter and longer regimens produce equal results
Leuppi et al1 used noninferiority methodology to compare a 5- vs a 14-day course of prednisone (40 mg/d) to treat patients with COPD exacerbations. A patient was considered to have a COPD exacerbation if he or she had a change from baseline in ≥2 of the following: dyspnea, cough, sputum quantity, or purulence.
Participants were patients who came to the EDs of 5 Swiss teaching hospitals between March 2006 and February 2011. To be eligible, individuals had to be 40 years or older and have ≥20 pack-years of smoking. Exclusion criteria included asthma, mild obstruction (forced expiratory volume in one second/forced vital capacity >70%), pneumonia, an estimated survival <6 months, pregnancy, and lactation.
All the participants (N=311) received 40 mg methylprednisolone intravenously on Day 1, followed by prednisone 40 mg orally on Days 2 through 5. The researchers then randomly divided participants into 2 groups: One group continued to take prednisone 40 mg/d and the other group received a matching placebo for an additional 9 days. Participants in both groups also received antibiotics for 7 days, twice daily inhaled steroids, daily tiotropium, and nebulized albuterol, as needed; additional oral glucocorticoids could be administered, as well, at the discretion of the treating physicians.
The primary outcome was the time to the next COPD exacerbation, up to 180 days. Noninferiority between the groups was defined as no more than a 15% absolute increase in exacerbations. The dropout rate was 5.7%, evenly divided between groups. Intention to treat and per-protocol analyses were conducted, and hazard ratios (HRs) were calculated using the Kaplan-Meier method and Cox proportional hazards models.
The time to next COPD exacerbation did not differ between the study groups: 56 days for those on the 5-day steroid regimen vs 57 days for those on the 14-day regimen in the intention to treat analysis (HR=0.95; 90% confidence interval, 0.70-1.29; P=.006). Sensitivity analyses adjusting for baseline characteristics provided similar results, as did the per-protocol analysis.
Secondary outcomes (overall survival; need for mechanical ventilation; need for additional corticosteroids; and clinical performance measures, such as dyspnea score and quality of life) also did not differ between groups. Nor were there differences in hyperglycemia, worsening hypertension, infection, or other adverse effects typically associated with glucocorticoid use. The active treatment group took >400 mg more prednisone than the placebo group (mean, 793 mg vs 379 mg; P<.001).
WHAT'S NEW?: Now we know: 5 days is enough
While randomized trials have found that glucocorticoids improve COPD symptoms, the optimal treatment dose and duration were not known. Indeed, current guidelines recommend treatment for >5 days.3 This trial clearly demonstrated that 40 mg prednisone for 5 days is at least as good as a 14-day treatment course. Furthermore, it is unnecessary to taper the short-course therapy, which simplifies the regimen.
CAVEATS: Will the results apply to those less severely ill?
More than 80% of patients with acute COPD exacerbations can be managed in an outpatient setting.3 However, participants in this trial were hospitalized for a median of 8.5 days, and most had severe or very severe COPD—and thus, were not fully representative of COPD patients typically seen in an outpatient practice. Yet patients with less severe disease should be at least as likely to respond to short-course steroids as those whose COPD is more severe.
It is important to note that participants in this study all received optimal guideline-based therapies during hospitalization, which may be difficult to achieve for some patients treated in an outpatient setting. Finally, treatment adherence observed during the hospitalization period in this trial is unlikely to be replicated in the outpatient setting.
CHALLENGES TO IMPLEMENTATION: Identifying patients who need steroids for a longer duration
For patients with new COPD exacerbations or those successfully treated using short-course therapy in the past, a 5-day regimen may be appropriate. For those who have failed prior attempts at short-course treatment, however, a 14-day course of treatment may be more advisable. That said, no guidelines are available to help us determine which of those who were previously treated with a longer regimen may fail on the shorter course of treatment.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosismanagement.html. Accessed December 13, 2013.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C,et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
1. Leuppi JD, Schuetz P, Bingisser R, et al. Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013;309:2223-2231.
2. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925-931.
3. Global Initiative for Chronic Obstructive Lung Disease, Inc. The global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosismanagement.html. Accessed December 13, 2013.
4. Quon BS, Gan WQ, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
5. Walters JA, Wang W, Morley C,et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD006897.
6. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941-1947.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Low-dose penicillin for recurrent cellulitis?
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe low-dose penicillin to patients with recurrent leg cellulitis to decrease the frequency of recurrent episodes.1
Strength of recommendation
B: Based on a single blinded randomized controlled trial (RCT).
Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
Illustrative case
An obese 50-year-old man presents with cellulitis of his right lower leg. He has a history of chronic venous insufficiency and has had 2 previous episodes of leg cellulitis in the past year. Should you initiate prophylactic antibiotics?
The incidence of cellulitis is 24.6 in 1000 person-years, according to a US population-based study of insurance claims for the years 1997 to 2002—the most recent data available.2 The lower extremities are most commonly affected, accounting for 70% to 80% of cases.3 Risk factors associated with recurrence include venous insufficiency, lymphedema, overweight, skin breakdown, and leg edema, which are often difficult to modify.4,5
Keeping well hydrated to avoid skin breakdown, elevating affected extremities to decrease edema, wearing compression stockings, and treating tinea pedis can help reduce the incidence of recurrent cellulitis.6,7 Prophylactic antibiotics can help, as well.
Which drug? What dose and duration?
These questions aren’t easily answered, as recommendations vary among specialty groups. The Infectious Diseases Society of America recommends benzathine penicillin (1.2 million units/month IM), erythromycin 250 mg PO BID, penicillin V 1 g PO BID, or nasal mupirocin BID for 5 days per month for “frequent” cellulitis, but “frequent” is not clearly defined.6,8-11 The British Lymphology Society (BLS)’s first-line recommendation for patients with ≥2 episodes of cellulitis per year is penicillin V 250 mg BID (or penicillin V 500 mg BID for patients with a BMI ≥33) for one year, then penicillin V 250 mg daily for an additional year. The BLS suggests lifelong antibiotic prophylaxis if cellulitis recurs after 2 years of prophylaxis.6
Consensus is lacking as to the optimal duration of prophylactic antibiotics. Kremer et al11 found that prophylactic erythromycin for 18 months reduced recurrent cellulitis; Thomas et al12 showed an insignificant reduction in cellulitis recurrence with 6 months of low-dose penicillin in patients who’d had one prior episode. The study detailed in this PURL provides additional evidence about the use of prophylactic antibiotics for recurrent lower extremity cellulitis and tests the efficacy of a particular dose and duration.
STUDY SUMMARY: Low-dose penicillin reduces recurrence rate
This double-blind RCT compared penicillin with placebo for the prevention of recurrent leg cellulitis. To be included in the study, patients had to have had at least 2 episodes of cellulitis within the previous 3 years, one of which occurred in the preceding 6 months. Participants (N=274) were recruited at hospitals in the United Kingdom and Ireland. Baseline characteristics included obesity (mean BMI, 35), mean age late 50s, and 3 to 4 prior episodes of cellulitis. About 25% of the participants had a history of venous insufficiency, as well.
The study had 2 phases—one for prophylaxis, the other for follow-up. During the prophylaxis phase, which lasted 12 months, patients received either penicillin 250 mg PO BID or placebo. Participants were followed for up to 3 years. They received phone calls every 3 months during the prophylaxis phase and every 6 months during the follow-up phase to assess adverse events, use of health care services, and recurrence of cellulitis.
Protection diminishes after prophylaxis ends
The primary outcome was the time from randomization to recurrence of cellulitis: Median times to recurrent cellulitis were 626 days for the penicillin group and 532 days for patients on placebo. Recurrence rates were 45% lower in those who received penicillin (hazard ratio=0.55; 95% confidence interval, 0.35-0.86; number needed to treat=5; P=.01) during the prophylaxis phase, but there was no difference in incidence in the follow-up phase.
Secondary outcomes measured were the proportions of patients with recurrent cellulitis in both the prophylaxis and follow-up phases, new leg edema or ulceration, duration of hospital admission for cellulitis, cost-effectiveness, and adverse drug effects/events of interest. The penicillin group had fewer episodes of recurrent cellulitis (119 vs 164; P=.02). The percentage of patients with new edema or ulceration between the 2 groups (40% penicillin vs 48% placebo; P=.46) and difference in cost-effectiveness between the 2 groups were not significant. Mean duration of hospitalization was 10 days (penicillin) and 9.2 days (placebo). There was no significant difference in the number of participants who experienced one or more adverse events (37 for those taking penicillin vs 48 for the placebo group; P=.50), including nausea, diarrhea, vulvovaginitis/thrush, rash, and death. There were 8 deaths in the penicillin group and 3 in the placebo group, although none was considered study related.
WHAT'S NEW?: Evidence that low-dose penicillin is effective
This trial provides strong evidence that a lower dose of penicillin than is currently recommended by the IDSA (250 mg vs 1 g BID) is effective in reducing leg cellulitis recurrence. It also shows that 12 months of prophylaxis significantly reduces the risk of recurrent leg cellulitis, but that the effect may diminish when the penicillin is stopped.
CAVEATS: Questions about dose and duration remain
Participant characteristics predictive of prophylaxis failure in this study included BMI≥33 and ≥3 previous episodes of cellulitis. It could be that patients with higher BMIs need a higher dose of penicillin. And we still don’t know whether prophylactic treatment for longer than 12 months would provide continued benefit, what the optimal time period for prophylactic antibiotics should be, and whether the higher recommended dose of penicillin would be more effective than the low dose that was used in this study. Antibiotic resistance associated with long-term penicillin use is a concern, as well.
challenges to implementation
Even when we know that patients are likely to benefit, we are often hesitant to prescribe long-term antibiotics because of reasonable fears of resistance and adverse effects.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
1. Thomas KS, Crook AM, Nunn AJ, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med. 2013; 368:1695-1703.
2. Ellis Simonsen SM, Van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect 2006;134:293-299.
3. Hirschmann Raugi GJ. Lower limb cellulitis and its mimics. Part I: Lower Limb Cellulitis. J Am Acad Dermatol. 2012;67:163e1-163e12.
4. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg(cellulitis): case-control study. BMJ 1999;18:1591-1594.
5. McNamara DR, Tleyjah IM, Berbari EF, et al. A predictive model of recurrent lower extremity cellulitis in a population-based cohort. Arch Int Med. 2007;167:709-715.
6. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373-1406.
7. Consensus document on the management of cellulitis in lymphoedema. British Lymphology Society, 2013. Available at: http://www.lymphoedema.org/Menu3/Revised%20Cellulitis%20Consensus%202013.pdf. Accessed January 2, 2014.
8. Babb RR, Spittell JA Jr, Martin WJ, et al. Prophylaxis of recurrent lymphangitis complicating lymphedema. JAMA. 1966;195:871-873.
9. Sjoblom AC, Eriksson B, Jorup-Ronstrom C, et al. Antibiotic prophylaxis in recurrent erysipelas. Infection 1993;21:390-393.
10. Wang JH, Liu YC, Cheng DL, et al. Role of benzathine penicillin G in prophylaxis for recurrent streptococcal cellulitis of the the lower legs. Clin Infect Dis. 1997;25:685-689.
11. Kremer M, Zuckerman R, Avraham Z, et al. Long-term antimicrobial therapy in the prevention of recurrent soft-tissue infections. J Infect. 1991;22:37-40.
12. Thomas K, Crook A, Foster K, et al. Prophylactic antibiotics for the prevention of cellulitis (erysipelas) of the leg: results of the UK Dermatology Clinical Trials Network’s PATCH II trial team. Br J Dermatol. 2012;166:169-178.
Copyright © 2014. The Family Physicians Inquiries Network. All rights reserved.
Mediterranean Diet: Higher Fat But Lower Risk
PRACTICE CHANGER
Counsel patients at high risk for cardiovascular disease and stroke to follow a Mediterranean diet, which is associated with a 30% risk reduction.1
STRENGTH OF RECOMMENDATION
B: Based on one well-designed randomized controlled trial.1
ILLUSTRATIVE CASE
A 62-year-old patient with diabetes, obesity, and a family history of early-onset coronary artery disease is motivated to make significant lifestyle changes. You recommend moderate aerobic exercise (30 min five d/wk) but wonder whether a low-fat or a Mediterranean diet would be more effective in reducing her risk.
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of mortality in the United States. CVD accounts for one in every three deaths,2 and stroke is a leading cause of long-term disability.2 The direct cost of treating CVD is estimated at $312.6 billion annually.2
Many modifiable risk factors contribute to CVD, including smoking, sedentary lifestyle, obesity, alcohol consumption, and poorly controlled chronic disease, as well as an unhealthy diet. A recent report from the American Heart Association suggests that 13% of deaths from CVD can be attributed to poor diet.2
Focus counseling on at-risk patients
Primary care providers (PCPs) often struggle to effectively counsel patients on behavioral change strategies, facing many barriers. Chief among them are the lack of time, training, and confidence in their counseling techniques, as well as a lack of patient motivation and readiness to change.3 In recognition of these barriers, the US Preventive Services Task Force recently recommended that PCPs focus behavioral counseling efforts on patients at high risk for heart disease.4
Large observational studies have found an association between trans fat and increased risk for CVD, as well as decreased risk for CVD in patients adhering to a Mediterranean diet.5-11 This type of diet typically includes a high intake of olive oil, fruit, nuts, vegetables, and cereals; moderate intake of fish and poultry; and low intake of dairy products, red meat, processed meats, and sweets. It also includes wine in moderation, consumed with meals.
Data on the physiologic properties of olive oil, including its antioxidant, vasodilating, and antiplatelet effects—as well as its effects on LDL cholesterol that may inhibit atherogenesis—support the link between a Mediterranean diet and a decreased risk for CVD found in the observational studies.12,13 Until recently, however, no randomized controlled trial (RCT) had compared the effect of a Mediterranean diet with that of a low-fat diet for primary prevention of CVD.
STUDY SUMMARY
Mediterranean diet significantly lowers risk
Prevencion con Dieta Mediterranea (PREDIMED) was a large RCT (N = 7,447) comparing two variations of a Mediterranean diet with a low-fat diet for primary prevention of CVD. This Spanish study enrolled men ages 55 to 80 and women ages 60 to 80 who were at high risk for CVD. The risk was based on either a diagnosis of type 2 diabetes or the presence of ≥ 3 major risk factors, including smoking, hypertension, elevated LDL cholesterol, low HDL cholesterol, overweight or obesity, and a family history of early heart disease.
Participants were randomly assigned to one of three dietary groups: One group followed a Mediterranean diet supplemented with ≥ 4 Tb of extra virgin olive oil per day; a second group was put on a Mediterranean diet supplemented by 30 g (about 1/3 cup) of mixed nuts daily; a third group (the controls) was advised to follow a low-fat diet. The majority of baseline characteristics and medications taken throughout the study were similar among all three groups.
Those in both Mediterranean diet groups were followed for a median of 4.8 years, during which they received quarterly dietary classes and individual and group counseling. The controls received baseline training, plus a leaflet about low-fat diets annually. In year 3, however, the researchers began giving the control group the same level of counseling as those in the Mediterranean diet groups to avoid confounding results.
Adherence to the diets was determined by a self-reported 14-item dietary screening questionnaire, plus urinary hydroxytyrosol and serum alpha-linoleic acid levels to assess for olive oil and mixed nut compliance. Self-reporting5 and biometric data indicated good compliance with the Mediterranean diets, and there was no difference found in levels of exercise among the groups.
After five years, those in the Mediterranean diet groups had consumed significantly more olive oil, nuts, vegetables, fruits, wine, legumes, seafood, and sofrito sauce (a popular tomato-based sauce) than the control group. Participants in the low-fat diet group had decreased their fat intake by 2%, while those in the Mediterranean groups had increased fat intake (by 2.03% for the olive oil group and 2.1% for the nut group). Overall, 37% of energy intake by those in the low-fat diet group came from fat (exceeding the < 30% of calories derived from fat intake that defines a low-fat diet), compared with 39% fat intake for those in both Mediterranean diet groups.
The primary outcome was a composite of MI, stroke, and death from cardiovascular causes, and there were clinically meaningful and statistically significant differences between the Mediterranean diet groups and the controls. The primary outcome rate for the supplemental olive oil group was 3.8%; 3.4% for the extra nuts group; and 4.4% for the controls. This represents a 30% reduction in risk for combined stroke, MI, and death due to cardiovascular causes for the Mediterranean diet groups (hazard ratio [HR], 0.7 and number needed to treat [NNT], 148 for the olive oil group; HR, 0.7 and NNT, 100 for the group consuming extra nuts). Similar benefits were found in the multivariable adjusted analyses. The results correspond to three fewer events (stroke, MI, or cardiovascular death) per 1,000 person-years for this high-risk population.
The only individual outcome that showed a significant decrease was stroke, with an NNT of 125 in both Mediterranean diet groups. Outcomes for the controls were similar before and after they began receiving quarterly counseling.
WHAT’S NEW?
Mediterranean diet is better than a lower-fat regimen
This study indicates that a Mediterranean diet, with increased intake of either olive oil or mixed nuts, is more protective against CVD than a recommended low-fat diet. It also shows that advising patients at high risk to follow a Mediterranean diet, providing dietary counseling, and monitoring them for adherence, rather than simply recommending a low-fat diet, can significantly decrease the risk for stroke.
Rates of CVD are higher in the US than in Spain, so implementing a Mediterranean diet on a large scale in this country has the potential to produce a greater response than that seen in this study.
CAVEATS
Would a true low-fat diet be a better comparison?
Although the control group’s diet was meant to be low fat, the participants did not achieve this, possibly due to the relatively low level of dietary education and personalized counseling at the start of the study. Their inability to reach the < 30% fat target could also reflect the difficulty patients have, in general, in decreasing fat content in their diet, which may mean the diet they maintained was a more realistic comparison.
This study used one brand of olive oil and a particular mixture of nuts (walnuts, hazelnuts, and almonds); it is possible that variations on either of these could affect the benefits of the diet.
CHALLENGES TO IMPLEMENTATION
Fitting a Mediterranean diet into an American lifestyle
The typical US diet is significantly different from that of most Spaniards. Americans may find it difficult to add either ≥ 4 Tb of olive oil or 30 g (1/3 cup) of nuts daily, for example, due to both cost and availability. Limited access to both individual and group counseling could be a barrier, as well.
On the other hand, this practice changer has the potential to simplify dietary counseling by allowing clinicians to focus on just one type of diet, for which there are many resources available both online and in print. We believe it makes sense to recommend a Mediterranean diet, while continuing to recommend increased exercise, smoking cessation, and improved control of chronic disease to lower patients’ risk for poor outcomes from CVD.
REFERENCES
1. Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368: 1279-1290.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
3. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546-552.
4. USPSTF. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults. www.uspreventiveservicestaskforce.org/uspstf/usp sphys.htm. Accessed December 18, 2013.
5. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491-1499.
6. Oomen C, Ocké MC, Feskens JM, et al. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746-751.
7. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785.
8. Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292: 1433-1439.
9. Kris-Etherton P, Eckel RH, Howard BV, et al; Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Education Program/AHA Step 1 Dietary Pattern on cardiovascular disease. Circulation. 2001;103:1823-1825.
10. Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. The association of Mediterranean diet with lower risk of acute coronary syndromes, in hypertensive subjects. Int J Cardiol. 2002; 82:141-147.
11. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. The role of traditional Mediterranean-type of diet and lifestyle, in the development of acute coronary syndromes: preliminary results from CARDIO 2000 study. Centr Eur J Public Health. 2002;10:11-15.
12. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
13. Vincent-Baudry S, Defoort C, Gerber M, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82: 964-971.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(12):745-746, 748.
PRACTICE CHANGER
Counsel patients at high risk for cardiovascular disease and stroke to follow a Mediterranean diet, which is associated with a 30% risk reduction.1
STRENGTH OF RECOMMENDATION
B: Based on one well-designed randomized controlled trial.1
ILLUSTRATIVE CASE
A 62-year-old patient with diabetes, obesity, and a family history of early-onset coronary artery disease is motivated to make significant lifestyle changes. You recommend moderate aerobic exercise (30 min five d/wk) but wonder whether a low-fat or a Mediterranean diet would be more effective in reducing her risk.
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of mortality in the United States. CVD accounts for one in every three deaths,2 and stroke is a leading cause of long-term disability.2 The direct cost of treating CVD is estimated at $312.6 billion annually.2
Many modifiable risk factors contribute to CVD, including smoking, sedentary lifestyle, obesity, alcohol consumption, and poorly controlled chronic disease, as well as an unhealthy diet. A recent report from the American Heart Association suggests that 13% of deaths from CVD can be attributed to poor diet.2
Focus counseling on at-risk patients
Primary care providers (PCPs) often struggle to effectively counsel patients on behavioral change strategies, facing many barriers. Chief among them are the lack of time, training, and confidence in their counseling techniques, as well as a lack of patient motivation and readiness to change.3 In recognition of these barriers, the US Preventive Services Task Force recently recommended that PCPs focus behavioral counseling efforts on patients at high risk for heart disease.4
Large observational studies have found an association between trans fat and increased risk for CVD, as well as decreased risk for CVD in patients adhering to a Mediterranean diet.5-11 This type of diet typically includes a high intake of olive oil, fruit, nuts, vegetables, and cereals; moderate intake of fish and poultry; and low intake of dairy products, red meat, processed meats, and sweets. It also includes wine in moderation, consumed with meals.
Data on the physiologic properties of olive oil, including its antioxidant, vasodilating, and antiplatelet effects—as well as its effects on LDL cholesterol that may inhibit atherogenesis—support the link between a Mediterranean diet and a decreased risk for CVD found in the observational studies.12,13 Until recently, however, no randomized controlled trial (RCT) had compared the effect of a Mediterranean diet with that of a low-fat diet for primary prevention of CVD.
STUDY SUMMARY
Mediterranean diet significantly lowers risk
Prevencion con Dieta Mediterranea (PREDIMED) was a large RCT (N = 7,447) comparing two variations of a Mediterranean diet with a low-fat diet for primary prevention of CVD. This Spanish study enrolled men ages 55 to 80 and women ages 60 to 80 who were at high risk for CVD. The risk was based on either a diagnosis of type 2 diabetes or the presence of ≥ 3 major risk factors, including smoking, hypertension, elevated LDL cholesterol, low HDL cholesterol, overweight or obesity, and a family history of early heart disease.
Participants were randomly assigned to one of three dietary groups: One group followed a Mediterranean diet supplemented with ≥ 4 Tb of extra virgin olive oil per day; a second group was put on a Mediterranean diet supplemented by 30 g (about 1/3 cup) of mixed nuts daily; a third group (the controls) was advised to follow a low-fat diet. The majority of baseline characteristics and medications taken throughout the study were similar among all three groups.
Those in both Mediterranean diet groups were followed for a median of 4.8 years, during which they received quarterly dietary classes and individual and group counseling. The controls received baseline training, plus a leaflet about low-fat diets annually. In year 3, however, the researchers began giving the control group the same level of counseling as those in the Mediterranean diet groups to avoid confounding results.
Adherence to the diets was determined by a self-reported 14-item dietary screening questionnaire, plus urinary hydroxytyrosol and serum alpha-linoleic acid levels to assess for olive oil and mixed nut compliance. Self-reporting5 and biometric data indicated good compliance with the Mediterranean diets, and there was no difference found in levels of exercise among the groups.
After five years, those in the Mediterranean diet groups had consumed significantly more olive oil, nuts, vegetables, fruits, wine, legumes, seafood, and sofrito sauce (a popular tomato-based sauce) than the control group. Participants in the low-fat diet group had decreased their fat intake by 2%, while those in the Mediterranean groups had increased fat intake (by 2.03% for the olive oil group and 2.1% for the nut group). Overall, 37% of energy intake by those in the low-fat diet group came from fat (exceeding the < 30% of calories derived from fat intake that defines a low-fat diet), compared with 39% fat intake for those in both Mediterranean diet groups.
The primary outcome was a composite of MI, stroke, and death from cardiovascular causes, and there were clinically meaningful and statistically significant differences between the Mediterranean diet groups and the controls. The primary outcome rate for the supplemental olive oil group was 3.8%; 3.4% for the extra nuts group; and 4.4% for the controls. This represents a 30% reduction in risk for combined stroke, MI, and death due to cardiovascular causes for the Mediterranean diet groups (hazard ratio [HR], 0.7 and number needed to treat [NNT], 148 for the olive oil group; HR, 0.7 and NNT, 100 for the group consuming extra nuts). Similar benefits were found in the multivariable adjusted analyses. The results correspond to three fewer events (stroke, MI, or cardiovascular death) per 1,000 person-years for this high-risk population.
The only individual outcome that showed a significant decrease was stroke, with an NNT of 125 in both Mediterranean diet groups. Outcomes for the controls were similar before and after they began receiving quarterly counseling.
WHAT’S NEW?
Mediterranean diet is better than a lower-fat regimen
This study indicates that a Mediterranean diet, with increased intake of either olive oil or mixed nuts, is more protective against CVD than a recommended low-fat diet. It also shows that advising patients at high risk to follow a Mediterranean diet, providing dietary counseling, and monitoring them for adherence, rather than simply recommending a low-fat diet, can significantly decrease the risk for stroke.
Rates of CVD are higher in the US than in Spain, so implementing a Mediterranean diet on a large scale in this country has the potential to produce a greater response than that seen in this study.
CAVEATS
Would a true low-fat diet be a better comparison?
Although the control group’s diet was meant to be low fat, the participants did not achieve this, possibly due to the relatively low level of dietary education and personalized counseling at the start of the study. Their inability to reach the < 30% fat target could also reflect the difficulty patients have, in general, in decreasing fat content in their diet, which may mean the diet they maintained was a more realistic comparison.
This study used one brand of olive oil and a particular mixture of nuts (walnuts, hazelnuts, and almonds); it is possible that variations on either of these could affect the benefits of the diet.
CHALLENGES TO IMPLEMENTATION
Fitting a Mediterranean diet into an American lifestyle
The typical US diet is significantly different from that of most Spaniards. Americans may find it difficult to add either ≥ 4 Tb of olive oil or 30 g (1/3 cup) of nuts daily, for example, due to both cost and availability. Limited access to both individual and group counseling could be a barrier, as well.
On the other hand, this practice changer has the potential to simplify dietary counseling by allowing clinicians to focus on just one type of diet, for which there are many resources available both online and in print. We believe it makes sense to recommend a Mediterranean diet, while continuing to recommend increased exercise, smoking cessation, and improved control of chronic disease to lower patients’ risk for poor outcomes from CVD.
REFERENCES
1. Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368: 1279-1290.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
3. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546-552.
4. USPSTF. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults. www.uspreventiveservicestaskforce.org/uspstf/usp sphys.htm. Accessed December 18, 2013.
5. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491-1499.
6. Oomen C, Ocké MC, Feskens JM, et al. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746-751.
7. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785.
8. Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292: 1433-1439.
9. Kris-Etherton P, Eckel RH, Howard BV, et al; Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Education Program/AHA Step 1 Dietary Pattern on cardiovascular disease. Circulation. 2001;103:1823-1825.
10. Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. The association of Mediterranean diet with lower risk of acute coronary syndromes, in hypertensive subjects. Int J Cardiol. 2002; 82:141-147.
11. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. The role of traditional Mediterranean-type of diet and lifestyle, in the development of acute coronary syndromes: preliminary results from CARDIO 2000 study. Centr Eur J Public Health. 2002;10:11-15.
12. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
13. Vincent-Baudry S, Defoort C, Gerber M, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82: 964-971.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(12):745-746, 748.
PRACTICE CHANGER
Counsel patients at high risk for cardiovascular disease and stroke to follow a Mediterranean diet, which is associated with a 30% risk reduction.1
STRENGTH OF RECOMMENDATION
B: Based on one well-designed randomized controlled trial.1
ILLUSTRATIVE CASE
A 62-year-old patient with diabetes, obesity, and a family history of early-onset coronary artery disease is motivated to make significant lifestyle changes. You recommend moderate aerobic exercise (30 min five d/wk) but wonder whether a low-fat or a Mediterranean diet would be more effective in reducing her risk.
Cardiovascular disease (CVD), including heart disease and stroke, is the leading cause of mortality in the United States. CVD accounts for one in every three deaths,2 and stroke is a leading cause of long-term disability.2 The direct cost of treating CVD is estimated at $312.6 billion annually.2
Many modifiable risk factors contribute to CVD, including smoking, sedentary lifestyle, obesity, alcohol consumption, and poorly controlled chronic disease, as well as an unhealthy diet. A recent report from the American Heart Association suggests that 13% of deaths from CVD can be attributed to poor diet.2
Focus counseling on at-risk patients
Primary care providers (PCPs) often struggle to effectively counsel patients on behavioral change strategies, facing many barriers. Chief among them are the lack of time, training, and confidence in their counseling techniques, as well as a lack of patient motivation and readiness to change.3 In recognition of these barriers, the US Preventive Services Task Force recently recommended that PCPs focus behavioral counseling efforts on patients at high risk for heart disease.4
Large observational studies have found an association between trans fat and increased risk for CVD, as well as decreased risk for CVD in patients adhering to a Mediterranean diet.5-11 This type of diet typically includes a high intake of olive oil, fruit, nuts, vegetables, and cereals; moderate intake of fish and poultry; and low intake of dairy products, red meat, processed meats, and sweets. It also includes wine in moderation, consumed with meals.
Data on the physiologic properties of olive oil, including its antioxidant, vasodilating, and antiplatelet effects—as well as its effects on LDL cholesterol that may inhibit atherogenesis—support the link between a Mediterranean diet and a decreased risk for CVD found in the observational studies.12,13 Until recently, however, no randomized controlled trial (RCT) had compared the effect of a Mediterranean diet with that of a low-fat diet for primary prevention of CVD.
STUDY SUMMARY
Mediterranean diet significantly lowers risk
Prevencion con Dieta Mediterranea (PREDIMED) was a large RCT (N = 7,447) comparing two variations of a Mediterranean diet with a low-fat diet for primary prevention of CVD. This Spanish study enrolled men ages 55 to 80 and women ages 60 to 80 who were at high risk for CVD. The risk was based on either a diagnosis of type 2 diabetes or the presence of ≥ 3 major risk factors, including smoking, hypertension, elevated LDL cholesterol, low HDL cholesterol, overweight or obesity, and a family history of early heart disease.
Participants were randomly assigned to one of three dietary groups: One group followed a Mediterranean diet supplemented with ≥ 4 Tb of extra virgin olive oil per day; a second group was put on a Mediterranean diet supplemented by 30 g (about 1/3 cup) of mixed nuts daily; a third group (the controls) was advised to follow a low-fat diet. The majority of baseline characteristics and medications taken throughout the study were similar among all three groups.
Those in both Mediterranean diet groups were followed for a median of 4.8 years, during which they received quarterly dietary classes and individual and group counseling. The controls received baseline training, plus a leaflet about low-fat diets annually. In year 3, however, the researchers began giving the control group the same level of counseling as those in the Mediterranean diet groups to avoid confounding results.
Adherence to the diets was determined by a self-reported 14-item dietary screening questionnaire, plus urinary hydroxytyrosol and serum alpha-linoleic acid levels to assess for olive oil and mixed nut compliance. Self-reporting5 and biometric data indicated good compliance with the Mediterranean diets, and there was no difference found in levels of exercise among the groups.
After five years, those in the Mediterranean diet groups had consumed significantly more olive oil, nuts, vegetables, fruits, wine, legumes, seafood, and sofrito sauce (a popular tomato-based sauce) than the control group. Participants in the low-fat diet group had decreased their fat intake by 2%, while those in the Mediterranean groups had increased fat intake (by 2.03% for the olive oil group and 2.1% for the nut group). Overall, 37% of energy intake by those in the low-fat diet group came from fat (exceeding the < 30% of calories derived from fat intake that defines a low-fat diet), compared with 39% fat intake for those in both Mediterranean diet groups.
The primary outcome was a composite of MI, stroke, and death from cardiovascular causes, and there were clinically meaningful and statistically significant differences between the Mediterranean diet groups and the controls. The primary outcome rate for the supplemental olive oil group was 3.8%; 3.4% for the extra nuts group; and 4.4% for the controls. This represents a 30% reduction in risk for combined stroke, MI, and death due to cardiovascular causes for the Mediterranean diet groups (hazard ratio [HR], 0.7 and number needed to treat [NNT], 148 for the olive oil group; HR, 0.7 and NNT, 100 for the group consuming extra nuts). Similar benefits were found in the multivariable adjusted analyses. The results correspond to three fewer events (stroke, MI, or cardiovascular death) per 1,000 person-years for this high-risk population.
The only individual outcome that showed a significant decrease was stroke, with an NNT of 125 in both Mediterranean diet groups. Outcomes for the controls were similar before and after they began receiving quarterly counseling.
WHAT’S NEW?
Mediterranean diet is better than a lower-fat regimen
This study indicates that a Mediterranean diet, with increased intake of either olive oil or mixed nuts, is more protective against CVD than a recommended low-fat diet. It also shows that advising patients at high risk to follow a Mediterranean diet, providing dietary counseling, and monitoring them for adherence, rather than simply recommending a low-fat diet, can significantly decrease the risk for stroke.
Rates of CVD are higher in the US than in Spain, so implementing a Mediterranean diet on a large scale in this country has the potential to produce a greater response than that seen in this study.
CAVEATS
Would a true low-fat diet be a better comparison?
Although the control group’s diet was meant to be low fat, the participants did not achieve this, possibly due to the relatively low level of dietary education and personalized counseling at the start of the study. Their inability to reach the < 30% fat target could also reflect the difficulty patients have, in general, in decreasing fat content in their diet, which may mean the diet they maintained was a more realistic comparison.
This study used one brand of olive oil and a particular mixture of nuts (walnuts, hazelnuts, and almonds); it is possible that variations on either of these could affect the benefits of the diet.
CHALLENGES TO IMPLEMENTATION
Fitting a Mediterranean diet into an American lifestyle
The typical US diet is significantly different from that of most Spaniards. Americans may find it difficult to add either ≥ 4 Tb of olive oil or 30 g (1/3 cup) of nuts daily, for example, due to both cost and availability. Limited access to both individual and group counseling could be a barrier, as well.
On the other hand, this practice changer has the potential to simplify dietary counseling by allowing clinicians to focus on just one type of diet, for which there are many resources available both online and in print. We believe it makes sense to recommend a Mediterranean diet, while continuing to recommend increased exercise, smoking cessation, and improved control of chronic disease to lower patients’ risk for poor outcomes from CVD.
REFERENCES
1. Estruch R, Ros F, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368: 1279-1290.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
3. Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners. Prev Med. 1995;24:546-552.
4. USPSTF. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults. www.uspreventiveservicestaskforce.org/uspstf/usp sphys.htm. Accessed December 18, 2013.
5. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491-1499.
6. Oomen C, Ocké MC, Feskens JM, et al. Association between trans fatty acid intake and 10-year risk of coronary heart disease in the Zutphen Elderly Study: a prospective population-based study. Lancet. 2001;357:746-751.
7. de Lorgeril M, Salen P, Martin JL, et al. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779-785.
8. Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292: 1433-1439.
9. Kris-Etherton P, Eckel RH, Howard BV, et al; Nutrition Committee Population Science Committee and Clinical Science Committee of the American Heart Association. Lyon Diet Heart Study. Benefits of a Mediterranean-style, National Education Program/AHA Step 1 Dietary Pattern on cardiovascular disease. Circulation. 2001;103:1823-1825.
10. Panagiotakos DB, Chrysohoou C, Pitsavos C, et al. The association of Mediterranean diet with lower risk of acute coronary syndromes, in hypertensive subjects. Int J Cardiol. 2002; 82:141-147.
11. Panagiotakos DB, Pitsavos C, Chrysohoou C, et al. The role of traditional Mediterranean-type of diet and lifestyle, in the development of acute coronary syndromes: preliminary results from CARDIO 2000 study. Centr Eur J Public Health. 2002;10:11-15.
12. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
13. Vincent-Baudry S, Defoort C, Gerber M, et al. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82: 964-971.
Acknowledgement
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(12):745-746, 748.
Is Self-swabbing for STIs a Good Idea?
PRACTICE CHANGER
Ask women who are at risk for sexually transmitted infections (STIs) to self-swab for chlamydia and gonorrhea testing; self-collection of vulvovaginal swabs with nucleic acid amplification testing (NAAT) has excellent sensitivity in women with and without symptoms.1,2
STRENGTH OF RECOMMENDATION
B: Based on a prospective diagnostic cohort study.1,2
ILLUSTRATIVE CASE
An 18-year-old patient requests testing for STIs. She has no symptoms. What is the best way to collect samples for chlamydia and gonorrhea testing?
Despite public health efforts, chlamydia and gonorrhea remain significant health problems, with more than 1.4 million cases of chlamydia and 321,849 cases of gonorrhea reported in the United States in 2011.3 Both can have devastating effects on reproduction, even in women who are asymptomatic.
Annual testing recommended for at-risk women
According to the CDC, most reported cases of chlamydia (70%) and gonorrhea (62%) occur in men and women between the ages of 15 and 24.3 Both the CDC and the US Preventive Services Task Force recommend annual chlamydia screening for all sexually active women younger than 25 and for older women who have certain risk factors (eg, having multiple sex partners or living in communities with a high burden of disease).4,5 Annual gonorrhea screening is also recommended for sexually active women with risk factors.4,5
How best to test? A number of unknowns
NAAT is the most sensitive test for detection of chlamydia and gonorrhea, but other questions about how best to screen for STIs remain.1,6 It has not been clear whether self-collected vulvovaginal swabs are equivalent to clinician-collected urethral or endocervical swabs for the detection of gonorrhea, or whether NAAT testing of the self-collected swabs or culture of the clinician-collected swabs is a more sensitive test for gonorrhea.
While some studies have found self-collected vulvovaginal samples to be as sensitive as clinician-collected endocervical samples for the diagnosis of chlamydia and gonorrhea, samples are still often collected by clinicians.7,8 Collecting endocervical swabs is uncomfortable for patients and time consuming for clinicians, and evidence suggests that patients prefer noninvasive sampling.9
STUDY SUMMARY
Self-collected samples are highly sensitive
This study was designed to compare the sensitivity and specificity of self-collected vulvovaginal swabs with that of clinician-collected swabs for chlamydia and gonorrhea, both in asymptomatic women and women with symptoms of an STI. Test methods were also assessed for gonorrhea, comparing detection rates of self-swabs tested with NAAT and the culture of clinician-collected urethral and endocervical samples.
The researchers evaluated a total of 3,973 women, ages 16 to 59, who sought care at a single sexual health center in the United Kingdom. The average age was 25; 37% of the participants reported a prior STI, and 42% had at least one symptom suggestive of an STI. Women were excluded from the study if they had taken an antibiotic in the preceding 28 days or were unable/unwilling to take a vulvovaginal swab or undergo clinician examination and sample collection.
The women performed vulvovaginal swabs for NAAT prior to a speculum exam; endocervical swab for both NAAT and culture and a urethral swab for culture were collected by the clinician. All the swabs sent for NAAT were tested for chlamydia and gonorrhea, and cultures were performed to detect gonorrhea.
Chlamydia: Vulvovaginal swabs have higher detection rates
Of the 3,867 participants with complete results, 10.2% were infected with chlamydia. Self-collected vulvovaginal swabs were significantly more sensitive than endocervical swabs (97% vs 88%) and had equal specificity (99.9% vs 100%). In women with symptoms of an STI, the sensitivity was 97% vs 88%; in those with no symptoms, the sensitivity was 97% vs 89%.
Gonorrhea: Self-collection, NAAT yield better results
Gonorrhea was found in 2.5% of the 3,859 women with complete results for testing of this STI. Self-collected swabs and clinician-collected swabs analyzed by NAAT both had excellent sensitivity (99% and 96%, respectively). But self-collected samples that underwent NAAT were significantly more sensitive than clinician-collected urethral and endocervical samples that were cultured (99% vs 81%). The number needed to test by self-collection for NAAT (compared with clinician-collected culture) to detect one additional case of gonorrhea was 5.
In women with symptoms suggestive of infection, the NAAT assays—both clinician- and self-collected—were equivalent and were more sensitive than gonorrhea culture. In asymptomatic women, 1.8% of whom had gonorrhea, the vulvovaginal swab sent for NAAT was more sensitive than culture (98% vs 78%) and was equivalent to the endocervical swab for NAAT (90%).
The bottom line: Self-collected vulvovaginal swabs are the sample of choice for both chlamydia and gonorrhea testing in women, regardless of whether they have symptoms. When a clinical examination is needed, either the clinician or the patient can collect a vulvovaginal swab.
WHAT’S NEW
Endocervical samples, cultures have lower detection rates
In this study, endocervical samples collected by the clinician rather than self-collected vulvovaginal samples would have missed 9% (one in 11) of chlamydial infections in women with symptoms of an STI. Vulvovaginal swabs and endocervical swabs have equal sensitivity for the diagnosis of gonorrhea when NAAT is used, but culture would have missed one in five gonorrhea infections (in women with and without symptoms).
CAVEATS
NAAT is costly and does not test for drug sensitivity
Although NAAT has replaced cell culture methodology as the gold standard for gonorrhea and chlamydia diagnosis, it is potentially costly if not readily available in your practice setting. What’s more, NAAT does not allow testing for antibiotic sensitivity, which is particularly relevant with increasing resistance of gonorrhea to multiple antibiotics. In addition, it’s unclear whether these results would apply to all NAAT assays or just the one used in this study.
These studies examine sensitivity and specificity of gonorrhea and chlamydia testing in a high-risk population: women who were seeking care in a sexual health center. Your patient population may be at lower risk, which will lower the prevalence of STIs and lower the positive predictive value of NAAT. A positive NAAT test for an STI should be followed by a confirmation NAAT in low-risk populations.
CHALLENGES TO IMPLEMENTATION
Reconsidering the way we practice
Most family practice providers are accustomed to performing a full examination on patients with a suspected STI, and changing the flow of the office visit may be difficult. And to implement this practice changer properly, it would be necessary to provide patient instruction in self-collection technique.
Also, making this change could be costly if you do not have this particular NAAT available. Once implemented, however, self-collection with NAAT will likely save time and be more comfortable for your patients. It will also provide a higher sensitivity in detecting chlamydia infections and equal sensitivity in detecting gonorrhea, compared with clinician-collected NAAT testing.
REFERENCES
1. Schoeman SA, Stewart CM, Booth RA, et al. Assessment of best single sample for finding chlamydia in women with and without symptoms: a diagnostic test study. BMJ. 2012;345:e8013.
2. Stewart CM, Schoeman SA, Booth RA, et al. Assessment of self taken swabs versus clinician taken swab for cultures for diagnosing gonorrhea in women: single centre, diagnostic accuracy study. BMJ. 2012;345: e8107.
3. CDC. Fact sheet: STD trends in the United States—2011 national data for chlamydia, gonorrhea, and syphilis (2012). www.cdc.gov/std/stats11/trends-2011.pdf. Accessed November 15, 2013.
4. CDC. Fact sheet: Incidence, prevalence, and cost of sexually transmitted infections in the United States (2013). www.cdc.gov/std/stats/STI-Estimates-Fact-Sheet-Feb-2013.pdf. Accessed November 15, 2013.
5. United States Preventive Services Task Force. USPSTF Recommendations for STI screening (2008). www.uspreventiveservicestaskforce.org/uspstf08/methods/stinfections.htm. Accessed November 15, 2013.
6. CDC. 2010 STD treatment guidelines. www.cdc.gov/std/treatment/2010/default.htm. Accessed November 15, 2013.
7. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: non-invasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 2005;142:914-925.
8. Moss S, Mallinson H. The contribution of APTIMA Combo 2 assay to the diagnosis of gonorrhoea in genitourinary medicine setting. Int J STD AIDS. 2007;18:551-554.
9. Chernesky MA, Hook EW 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhaea infections. Sex Transm Dis. 2005;32: 729-733.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(11):651-653.
PRACTICE CHANGER
Ask women who are at risk for sexually transmitted infections (STIs) to self-swab for chlamydia and gonorrhea testing; self-collection of vulvovaginal swabs with nucleic acid amplification testing (NAAT) has excellent sensitivity in women with and without symptoms.1,2
STRENGTH OF RECOMMENDATION
B: Based on a prospective diagnostic cohort study.1,2
ILLUSTRATIVE CASE
An 18-year-old patient requests testing for STIs. She has no symptoms. What is the best way to collect samples for chlamydia and gonorrhea testing?
Despite public health efforts, chlamydia and gonorrhea remain significant health problems, with more than 1.4 million cases of chlamydia and 321,849 cases of gonorrhea reported in the United States in 2011.3 Both can have devastating effects on reproduction, even in women who are asymptomatic.
Annual testing recommended for at-risk women
According to the CDC, most reported cases of chlamydia (70%) and gonorrhea (62%) occur in men and women between the ages of 15 and 24.3 Both the CDC and the US Preventive Services Task Force recommend annual chlamydia screening for all sexually active women younger than 25 and for older women who have certain risk factors (eg, having multiple sex partners or living in communities with a high burden of disease).4,5 Annual gonorrhea screening is also recommended for sexually active women with risk factors.4,5
How best to test? A number of unknowns
NAAT is the most sensitive test for detection of chlamydia and gonorrhea, but other questions about how best to screen for STIs remain.1,6 It has not been clear whether self-collected vulvovaginal swabs are equivalent to clinician-collected urethral or endocervical swabs for the detection of gonorrhea, or whether NAAT testing of the self-collected swabs or culture of the clinician-collected swabs is a more sensitive test for gonorrhea.
While some studies have found self-collected vulvovaginal samples to be as sensitive as clinician-collected endocervical samples for the diagnosis of chlamydia and gonorrhea, samples are still often collected by clinicians.7,8 Collecting endocervical swabs is uncomfortable for patients and time consuming for clinicians, and evidence suggests that patients prefer noninvasive sampling.9
STUDY SUMMARY
Self-collected samples are highly sensitive
This study was designed to compare the sensitivity and specificity of self-collected vulvovaginal swabs with that of clinician-collected swabs for chlamydia and gonorrhea, both in asymptomatic women and women with symptoms of an STI. Test methods were also assessed for gonorrhea, comparing detection rates of self-swabs tested with NAAT and the culture of clinician-collected urethral and endocervical samples.
The researchers evaluated a total of 3,973 women, ages 16 to 59, who sought care at a single sexual health center in the United Kingdom. The average age was 25; 37% of the participants reported a prior STI, and 42% had at least one symptom suggestive of an STI. Women were excluded from the study if they had taken an antibiotic in the preceding 28 days or were unable/unwilling to take a vulvovaginal swab or undergo clinician examination and sample collection.
The women performed vulvovaginal swabs for NAAT prior to a speculum exam; endocervical swab for both NAAT and culture and a urethral swab for culture were collected by the clinician. All the swabs sent for NAAT were tested for chlamydia and gonorrhea, and cultures were performed to detect gonorrhea.
Chlamydia: Vulvovaginal swabs have higher detection rates
Of the 3,867 participants with complete results, 10.2% were infected with chlamydia. Self-collected vulvovaginal swabs were significantly more sensitive than endocervical swabs (97% vs 88%) and had equal specificity (99.9% vs 100%). In women with symptoms of an STI, the sensitivity was 97% vs 88%; in those with no symptoms, the sensitivity was 97% vs 89%.
Gonorrhea: Self-collection, NAAT yield better results
Gonorrhea was found in 2.5% of the 3,859 women with complete results for testing of this STI. Self-collected swabs and clinician-collected swabs analyzed by NAAT both had excellent sensitivity (99% and 96%, respectively). But self-collected samples that underwent NAAT were significantly more sensitive than clinician-collected urethral and endocervical samples that were cultured (99% vs 81%). The number needed to test by self-collection for NAAT (compared with clinician-collected culture) to detect one additional case of gonorrhea was 5.
In women with symptoms suggestive of infection, the NAAT assays—both clinician- and self-collected—were equivalent and were more sensitive than gonorrhea culture. In asymptomatic women, 1.8% of whom had gonorrhea, the vulvovaginal swab sent for NAAT was more sensitive than culture (98% vs 78%) and was equivalent to the endocervical swab for NAAT (90%).
The bottom line: Self-collected vulvovaginal swabs are the sample of choice for both chlamydia and gonorrhea testing in women, regardless of whether they have symptoms. When a clinical examination is needed, either the clinician or the patient can collect a vulvovaginal swab.
WHAT’S NEW
Endocervical samples, cultures have lower detection rates
In this study, endocervical samples collected by the clinician rather than self-collected vulvovaginal samples would have missed 9% (one in 11) of chlamydial infections in women with symptoms of an STI. Vulvovaginal swabs and endocervical swabs have equal sensitivity for the diagnosis of gonorrhea when NAAT is used, but culture would have missed one in five gonorrhea infections (in women with and without symptoms).
CAVEATS
NAAT is costly and does not test for drug sensitivity
Although NAAT has replaced cell culture methodology as the gold standard for gonorrhea and chlamydia diagnosis, it is potentially costly if not readily available in your practice setting. What’s more, NAAT does not allow testing for antibiotic sensitivity, which is particularly relevant with increasing resistance of gonorrhea to multiple antibiotics. In addition, it’s unclear whether these results would apply to all NAAT assays or just the one used in this study.
These studies examine sensitivity and specificity of gonorrhea and chlamydia testing in a high-risk population: women who were seeking care in a sexual health center. Your patient population may be at lower risk, which will lower the prevalence of STIs and lower the positive predictive value of NAAT. A positive NAAT test for an STI should be followed by a confirmation NAAT in low-risk populations.
CHALLENGES TO IMPLEMENTATION
Reconsidering the way we practice
Most family practice providers are accustomed to performing a full examination on patients with a suspected STI, and changing the flow of the office visit may be difficult. And to implement this practice changer properly, it would be necessary to provide patient instruction in self-collection technique.
Also, making this change could be costly if you do not have this particular NAAT available. Once implemented, however, self-collection with NAAT will likely save time and be more comfortable for your patients. It will also provide a higher sensitivity in detecting chlamydia infections and equal sensitivity in detecting gonorrhea, compared with clinician-collected NAAT testing.
REFERENCES
1. Schoeman SA, Stewart CM, Booth RA, et al. Assessment of best single sample for finding chlamydia in women with and without symptoms: a diagnostic test study. BMJ. 2012;345:e8013.
2. Stewart CM, Schoeman SA, Booth RA, et al. Assessment of self taken swabs versus clinician taken swab for cultures for diagnosing gonorrhea in women: single centre, diagnostic accuracy study. BMJ. 2012;345: e8107.
3. CDC. Fact sheet: STD trends in the United States—2011 national data for chlamydia, gonorrhea, and syphilis (2012). www.cdc.gov/std/stats11/trends-2011.pdf. Accessed November 15, 2013.
4. CDC. Fact sheet: Incidence, prevalence, and cost of sexually transmitted infections in the United States (2013). www.cdc.gov/std/stats/STI-Estimates-Fact-Sheet-Feb-2013.pdf. Accessed November 15, 2013.
5. United States Preventive Services Task Force. USPSTF Recommendations for STI screening (2008). www.uspreventiveservicestaskforce.org/uspstf08/methods/stinfections.htm. Accessed November 15, 2013.
6. CDC. 2010 STD treatment guidelines. www.cdc.gov/std/treatment/2010/default.htm. Accessed November 15, 2013.
7. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: non-invasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 2005;142:914-925.
8. Moss S, Mallinson H. The contribution of APTIMA Combo 2 assay to the diagnosis of gonorrhoea in genitourinary medicine setting. Int J STD AIDS. 2007;18:551-554.
9. Chernesky MA, Hook EW 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhaea infections. Sex Transm Dis. 2005;32: 729-733.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(11):651-653.
PRACTICE CHANGER
Ask women who are at risk for sexually transmitted infections (STIs) to self-swab for chlamydia and gonorrhea testing; self-collection of vulvovaginal swabs with nucleic acid amplification testing (NAAT) has excellent sensitivity in women with and without symptoms.1,2
STRENGTH OF RECOMMENDATION
B: Based on a prospective diagnostic cohort study.1,2
ILLUSTRATIVE CASE
An 18-year-old patient requests testing for STIs. She has no symptoms. What is the best way to collect samples for chlamydia and gonorrhea testing?
Despite public health efforts, chlamydia and gonorrhea remain significant health problems, with more than 1.4 million cases of chlamydia and 321,849 cases of gonorrhea reported in the United States in 2011.3 Both can have devastating effects on reproduction, even in women who are asymptomatic.
Annual testing recommended for at-risk women
According to the CDC, most reported cases of chlamydia (70%) and gonorrhea (62%) occur in men and women between the ages of 15 and 24.3 Both the CDC and the US Preventive Services Task Force recommend annual chlamydia screening for all sexually active women younger than 25 and for older women who have certain risk factors (eg, having multiple sex partners or living in communities with a high burden of disease).4,5 Annual gonorrhea screening is also recommended for sexually active women with risk factors.4,5
How best to test? A number of unknowns
NAAT is the most sensitive test for detection of chlamydia and gonorrhea, but other questions about how best to screen for STIs remain.1,6 It has not been clear whether self-collected vulvovaginal swabs are equivalent to clinician-collected urethral or endocervical swabs for the detection of gonorrhea, or whether NAAT testing of the self-collected swabs or culture of the clinician-collected swabs is a more sensitive test for gonorrhea.
While some studies have found self-collected vulvovaginal samples to be as sensitive as clinician-collected endocervical samples for the diagnosis of chlamydia and gonorrhea, samples are still often collected by clinicians.7,8 Collecting endocervical swabs is uncomfortable for patients and time consuming for clinicians, and evidence suggests that patients prefer noninvasive sampling.9
STUDY SUMMARY
Self-collected samples are highly sensitive
This study was designed to compare the sensitivity and specificity of self-collected vulvovaginal swabs with that of clinician-collected swabs for chlamydia and gonorrhea, both in asymptomatic women and women with symptoms of an STI. Test methods were also assessed for gonorrhea, comparing detection rates of self-swabs tested with NAAT and the culture of clinician-collected urethral and endocervical samples.
The researchers evaluated a total of 3,973 women, ages 16 to 59, who sought care at a single sexual health center in the United Kingdom. The average age was 25; 37% of the participants reported a prior STI, and 42% had at least one symptom suggestive of an STI. Women were excluded from the study if they had taken an antibiotic in the preceding 28 days or were unable/unwilling to take a vulvovaginal swab or undergo clinician examination and sample collection.
The women performed vulvovaginal swabs for NAAT prior to a speculum exam; endocervical swab for both NAAT and culture and a urethral swab for culture were collected by the clinician. All the swabs sent for NAAT were tested for chlamydia and gonorrhea, and cultures were performed to detect gonorrhea.
Chlamydia: Vulvovaginal swabs have higher detection rates
Of the 3,867 participants with complete results, 10.2% were infected with chlamydia. Self-collected vulvovaginal swabs were significantly more sensitive than endocervical swabs (97% vs 88%) and had equal specificity (99.9% vs 100%). In women with symptoms of an STI, the sensitivity was 97% vs 88%; in those with no symptoms, the sensitivity was 97% vs 89%.
Gonorrhea: Self-collection, NAAT yield better results
Gonorrhea was found in 2.5% of the 3,859 women with complete results for testing of this STI. Self-collected swabs and clinician-collected swabs analyzed by NAAT both had excellent sensitivity (99% and 96%, respectively). But self-collected samples that underwent NAAT were significantly more sensitive than clinician-collected urethral and endocervical samples that were cultured (99% vs 81%). The number needed to test by self-collection for NAAT (compared with clinician-collected culture) to detect one additional case of gonorrhea was 5.
In women with symptoms suggestive of infection, the NAAT assays—both clinician- and self-collected—were equivalent and were more sensitive than gonorrhea culture. In asymptomatic women, 1.8% of whom had gonorrhea, the vulvovaginal swab sent for NAAT was more sensitive than culture (98% vs 78%) and was equivalent to the endocervical swab for NAAT (90%).
The bottom line: Self-collected vulvovaginal swabs are the sample of choice for both chlamydia and gonorrhea testing in women, regardless of whether they have symptoms. When a clinical examination is needed, either the clinician or the patient can collect a vulvovaginal swab.
WHAT’S NEW
Endocervical samples, cultures have lower detection rates
In this study, endocervical samples collected by the clinician rather than self-collected vulvovaginal samples would have missed 9% (one in 11) of chlamydial infections in women with symptoms of an STI. Vulvovaginal swabs and endocervical swabs have equal sensitivity for the diagnosis of gonorrhea when NAAT is used, but culture would have missed one in five gonorrhea infections (in women with and without symptoms).
CAVEATS
NAAT is costly and does not test for drug sensitivity
Although NAAT has replaced cell culture methodology as the gold standard for gonorrhea and chlamydia diagnosis, it is potentially costly if not readily available in your practice setting. What’s more, NAAT does not allow testing for antibiotic sensitivity, which is particularly relevant with increasing resistance of gonorrhea to multiple antibiotics. In addition, it’s unclear whether these results would apply to all NAAT assays or just the one used in this study.
These studies examine sensitivity and specificity of gonorrhea and chlamydia testing in a high-risk population: women who were seeking care in a sexual health center. Your patient population may be at lower risk, which will lower the prevalence of STIs and lower the positive predictive value of NAAT. A positive NAAT test for an STI should be followed by a confirmation NAAT in low-risk populations.
CHALLENGES TO IMPLEMENTATION
Reconsidering the way we practice
Most family practice providers are accustomed to performing a full examination on patients with a suspected STI, and changing the flow of the office visit may be difficult. And to implement this practice changer properly, it would be necessary to provide patient instruction in self-collection technique.
Also, making this change could be costly if you do not have this particular NAAT available. Once implemented, however, self-collection with NAAT will likely save time and be more comfortable for your patients. It will also provide a higher sensitivity in detecting chlamydia infections and equal sensitivity in detecting gonorrhea, compared with clinician-collected NAAT testing.
REFERENCES
1. Schoeman SA, Stewart CM, Booth RA, et al. Assessment of best single sample for finding chlamydia in women with and without symptoms: a diagnostic test study. BMJ. 2012;345:e8013.
2. Stewart CM, Schoeman SA, Booth RA, et al. Assessment of self taken swabs versus clinician taken swab for cultures for diagnosing gonorrhea in women: single centre, diagnostic accuracy study. BMJ. 2012;345: e8107.
3. CDC. Fact sheet: STD trends in the United States—2011 national data for chlamydia, gonorrhea, and syphilis (2012). www.cdc.gov/std/stats11/trends-2011.pdf. Accessed November 15, 2013.
4. CDC. Fact sheet: Incidence, prevalence, and cost of sexually transmitted infections in the United States (2013). www.cdc.gov/std/stats/STI-Estimates-Fact-Sheet-Feb-2013.pdf. Accessed November 15, 2013.
5. United States Preventive Services Task Force. USPSTF Recommendations for STI screening (2008). www.uspreventiveservicestaskforce.org/uspstf08/methods/stinfections.htm. Accessed November 15, 2013.
6. CDC. 2010 STD treatment guidelines. www.cdc.gov/std/treatment/2010/default.htm. Accessed November 15, 2013.
7. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: non-invasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae. Ann Intern Med. 2005;142:914-925.
8. Moss S, Mallinson H. The contribution of APTIMA Combo 2 assay to the diagnosis of gonorrhoea in genitourinary medicine setting. Int J STD AIDS. 2007;18:551-554.
9. Chernesky MA, Hook EW 3rd, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhaea infections. Sex Transm Dis. 2005;32: 729-733.
ACKNOWLEDGEMENT
The PURLs Surveillance System is supported in part by Grant Number UL 1RR 024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2013. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2013;62(11):651-653.