User login
Top research findings of 2018-2019 for clinical practice
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
In Part 1 of this article, published in

1. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
In light of the association of major depressive disorder (MDD) with an increased risk of aging-related diseases, Han et al2 examined whether MDD was associated with higher epigenetic aging in blood as measured by DNA methylation patterns. They also studied whether clinical characteristics of MDD had a further impact on these patterns, and whether the findings replicated in brain tissue. Many differentially methylated regions of our DNA tend to change as we age. Han et al2 used these age-sensitive differentially methylated regions to estimate chronological age, using DNA extracted from various tissues, including blood and brain.
Study design
- As a part of the Netherlands Study of Depression and Anxiety (NESDA), this study included 811 patients with MDD and 319 control participants with no lifetime psychiatric disorders and low depressive symptoms (Inventory of Depressive Symptomatology score <14).
- Diagnosis of MDD and clinical characteristics were assessed by questionnaires and psychiatric interviews. Childhood trauma was assessed using the NEMESIS childhood trauma interview, which included a structured inventory of trauma exposure during childhood.
- DNA methylation age was estimated using all methylation sites in the blood of 811 patients with MDD and 319 control participants. The residuals of the DNA methylation age estimates regressed on chronological age were calculated to indicate epigenetic aging.
- Analyses were adjusted for sociodemographic characteristics, lifestyle, and health status.
- Postmortem brain samples of 74 patients with MDD and 64 control participants were used for replication.
Outcomes
- Significantly higher epigenetic aging was observed in patients with MDD compared with control participants (Cohen’s d = 0.18), which suggests that patients with MDD are biologically older than their corresponding chronological age. There was a significant dose effect with increasing symptom severity in the overall sample.
- In the MDD group, epigenetic aging was positively and significantly associated with childhood trauma.
- The case-control difference was replicated in an independent analysis of postmortem brain samples.
Conclusion
- These findings suggest that patients with MDD and people with a history of childhood trauma may biologically age relatively faster than those without MDD or childhood trauma. These findings may represent a biomarker of aging and might help identify patients who may benefit from early and intensive interventions to reduce the physical comorbidities of MDD.
- This study raises the possibility that MDD may be causally related to epigenetic age acceleration. However, it only points out the associations; there are other possible explanations for this correlation, including the possibility that a shared risk factor accounts for the observed association.
2. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
Delirium is common and often goes underdiagnosed. It is particularly prevalent among hospitalized geriatric patients. Several medications have been suggested to have a role in treating or preventing delirium. However, it remains uncertain which medications provide the best response rate, the lowest rate of delirium occurrence, and the best tolerability. In an attempt to find answers to these questions, Wu et al3 reviewed studies that evaluated the use of various medications used for delirium.
Study design
- Researchers conducted a systematic review and network meta-analysis of randomized controlled trials (RCTs) that investigated various pharmacologic agents used to treat or prevent delirium.
- Fifty-eight RCTs were included in the analyses. Of these, 20 RCTs with a total of 1,435 participants compared the outcomes of treatments of delirium, and 38 RCTs with a total of 8,168 participants examined prevention.
- A network meta-analysis was performed to determine if an agent or combinations of agents were superior to placebo or widely used medications.
Continue to: Outcomes
Outcomes
- Haloperidol plus lorazepam provided the best response rate for treating delirium compared with placebo/control.
- For delirium prevention, patients who received ramelteon, olanzapine, risperidone, or dexmedetomidine had significantly lower delirium occurrence rates than those receiving placebo/control.
- None of the pharmacologic treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusion
- Haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacologic interventions for treatment or prophylaxis increased all-cause mortality.
- However, network meta-analyses involve extrapolating treatment comparisons that are not made directly. As Blazer8 pointed out, both findings in this study (that haloperidol plus lorazepam is a unique intervention among the treatment trials and ramelteon is a unique intervention for prevention) seemed to be driven by 2 of the 58 studies that Wu et al3 examined.Wu et al3 also cautioned that both of these interventions needed to be further researched for efficacy.
3. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
While some evidence suggests that elevated brain noradrenergic activity is involved in the initiation and maintenance of alcohol use disorder,9 current medications used to treat alcohol use disorder do not target brain noradrenergic pathways. In an RCT, Simpson et al4 tested prazosin, an alpha-1 adrenergic receptor antagonist, for the treatment of alcohol use disorder.
Study design
- In this 12-week double-blind study, 92 participants with alcohol use disorder were randomly assigned to receive prazosin or placebo. Individuals with posttraumatic stress disorder were excluded.
- Prazosin was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of Week 2. The behavioral platform was medical management. Participants provided daily data on their alcohol consumption.
- Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Outcomes
- Among the 80 participants who completed the titration period and were included in the primary analyses, prazosin was associated with self-reported fewer heavy drinking days, and fewer drinks per week (Palatino LT Std−8 vs Palatino LT Std−1.5 with placebo). Drinking days per week and craving showed no group differences.
- The rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants receiving prazosin compared with those receiving placebo.
Continue to: Conclusion
Conclusion
- These findings of moderate reductions in heavy drinking days and drinks per week with prazosin suggest that prazosin may be a promising harm-reduction treatment for alcohol use disorder.
4. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
Postpartum depression is among the most common complications of childbirth. It can result in considerable suffering for mothers, children, and families. Gamma-aminobutyric acid (GABA) signaling has previously been reported to be involved in the pathophysiology of postpartum depression. Meltzer-Brody et al5 conducted 2 double-blind, randomized, placebo-controlled, phase 3 trials comparing brexanolone with placebo in women with postpartum depression at 30 clinical research centers and specialized psychiatric units in the United States.
Study design
- Participants were women age 18 to 45, Palatino LT Std≤6 months postpartum at screening, with postpartum depression as indicated by a qualifying 17-item Hamilton Depression Rating Scale (HAM-D) score of ≥26 for Study 1 or 20 to 25 for Study 2.
- Of the 375 women who were screened simultaneously across both studies, 138 were randomly assigned (1:1:1) to receive a single IV injection of brexanolone, 90 μg/kg per hour (BRX90) (n = 45), brexanolone, 60 μg/kg per hour (BRX60) (n = 47), or placebo (n = 46) for 60 hours in Study 1, and 108 were randomly assigned (1:1) to receive BRX90 (n = 54) or placebo (n = 54) for 60 hours in Study 2.
- The primary efficacy endpoint was change in total score on the HAM-D from baseline to 60 hours. Patients were followed until Day 30.
Outcomes
- In Study 1, at 60 hours, the least-squares (LS) mean reduction in HAM-D total score from baseline was 19.5 points (standard error [SE] 1.2) in the BRX60 group and 17.7 points (SE 1.2) in the BRX90 group, compared with 14.0 points (SE 1.1) in the placebo group.
- In Study 2, at 60 hours, the LS mean reduction in HAM-D total score from baseline was 14.6 points (SE 0.8) in the BRX90 group compared with 12.1 points (SE 0.8) for the placebo group.
- In Study 1, one patient in the BRX60 group had 2 serious adverse events (suicidal ideation and intentional overdose attempt during follow-up). In Study 2, one patient in the BRX90 group had 2 serious adverse events (altered state of consciousness and syncope), which were considered treatment-related.
Conclusion
- Administration of brexanolone injection for postpartum depression resulted in significant, clinically meaningful reductions in HAM-D total score at 60 hours compared with placebo, with a rapid onset of action and durable treatment response during the study period. These results suggest that brexanolone injection has the potential to improve treatment options for women with this disorder.
Continue to: #5
5. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
In clinical practice, the use of multiple antipsychotic agents for the maintenance treatment of schizophrenia is common but generally not recommended. The effectiveness of antipsychotic polypharmacy in preventing relapse of schizophrenia has not been established, and whether specific antipsychotic combinations are superior to monotherapies for maintenance treatment of schizophrenia is unknown. Tiihonen et al6 investigated the association of specific antipsychotic combinations with psychiatric rehospitalization, which was used as a marker for relapse.
Study design
- This study included 62,250 patients with schizophrenia, treated between January 1, 1996 and December 31, 2015, in a comprehensive, nationwide cohort in Finland. Overall, 31,257 individuals (50.2%) were men, and the median age was 45.6 (interquartile range, 34.6 to 57.9).
- Patients were receiving 29 different antipsychotic monotherapy or polypharmacy regimens.
- Researchers analyzed data from April 24 to June 15, 2018 using psychiatric rehospitalization as a marker for relapse. To minimize selection bias, rehospitalization risks were investigated using within-individual analyses.
- The main outcome was the hazard ratio (HR) for psychiatric rehospitalization during use of polypharmacy vs monotherapy by the same patient.
Outcomes
- Clozapine plus aripiprazole was associated with the lowest risk of psychiatric rehospitalization, with a difference of 14% (HR, .86; CI, .79 to .94) compared with clozapine monotherapy in the analysis that included all polypharmacy periods, and 18% (HR, .82; CI, .75 to .89) in the conservatively defined polypharmacy analysis that excluded periods <90 days.
- Among patients experiencing their first episode of schizophrenia, the differences between clozapine plus aripiprazole vs clozapine monotherapy were greater, with a difference of 22% in the analysis that included all polypharmacy periods, and 23% in the conservatively defined polypharmacy analysis.
- At the aggregate level, any antipsychotic polypharmacy was associated with a 7% to 13% lower risk of psychiatric rehospitalization compared with any monotherapy.
- Clozapine was the only monotherapy among the 10 best treatments.
- Results on all-cause and somatic hospitalization, mortality, and other sensitivity analyses were in line with the primary outcomes.
Conclusion
- This study suggests that certain types of antipsychotic polypharmacy may reduce the risk of rehospitalization in patients with schizophrenia. Current treatment guidelines state that clinicians should prefer antipsychotic monotherapy and avoid polypharmacy. Tiihonen et al6 raise the question whether current treatment guidelines should continue to discourage antipsychotic polypharmacy in the maintenance treatment of schizophrenia.
- Despite the large administrative databases and sophisticated statistical methods used in this study, this approach has important limitations. As Goff10 points out, despite efforts to minimize bias, these results should be considered preliminary until confirmed by RCTs.
6. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
In routine clinical practice, patients with schizophrenia are often treated with combinations of antipsychotics and other psychotropic medications. However, there is little evidence about the comparative effectiveness of these adjunctive treatment strategies. Stroup et al7 investigated the comparative real-world effectiveness of adjunctive psychotropic treatments for patients with schizophrenia.
Continue to: Study design
Study design
- This comparative effectiveness study used US Medicaid data from January 1, 2001, to December 31, 2010. Data analysis was performed from January 1, 2017, to June 30, 2018.
- The study cohort included 81,921 adult outpatients diagnosed with schizophrenia with a mean age of 40.7 (range: 18 to 64), including 37,515 women (45.8%). All patients were stably treated with a single antipsychotic and then started on an adjunctive antidepressant (n = 31,117), benzodiazepine (n = 11,941), mood stabilizer (n = 12,849), or another antipsychotic (n = 26,014).
- Researchers used multinomial logistic regression models to estimate propensity scores to balance covariates across the 4 medication groups. Weighted Cox proportional hazards regression models were used to compare treatment outcomes during 365 days on an intention-to-treat basis.
- The main outcomes and measures included risk of hospitalization for a mental disorder (primary), emergency department (ED) visits for a mental disorder, and all-cause mortality.
Outcomes
- Compared with starting another antipsychotic, initiating use of an antidepressant was associated with a lower risk of psychiatric hospitalization, and initiating use of a benzodiazepine was associated with a higher risk. Initiating use of a mood stabilizer was not significantly different from initiating use of another antipsychotic.
- A similar pattern of associations was observed in psychiatric ED visits for initiating use of an antidepressant, benzodiazepine, or mood stabilizer.
- Initiating use of a mood stabilizer was associated with an increased risk of mortality.
Conclusion
- Compared with the addition of a second antipsychotic, adding an antidepressant was associated with substantially reduced rates of hospitalization, whereas adding a benzodiazepine was associated with a modest increase in the risk of hospitalization. While the addition of a mood stabilizer was not associated with a significant difference in the risk of hospitalization, it was associated with higher mortality.
- Despite the limitations associated with this study, the associations of benzodiazepines and mood stabilizers with poorer outcomes warrant clinical caution and further investigation.
Bottom Line
Significantly higher epigenetic aging has been observed in patients with major depressive disorder. Haloperidol plus lorazepam might be an effective treatment for delirium; and ramelteon may be effective for preventing delirium. Prazosin reduces heavy drinking in patients with alcohol use disorder. A 60-hour infusion of brexanolone can help alleviate postpartum depression. Clozapine plus aripiprazole reduces the risk of rehospitalization among patients with schizophrenia. Adding an antidepressant to an antipsychotic also can reduce the risk of rehospitalization among patients with schizophrenia.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Aripiprazole • Abilify
Brexanolone • Zulresso
Clozapine • Clozaril
Dexmedetomidine • Precedex
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Prazosin • Minipress
Ramelteon • Rozerem
Risperidone • Risperdal
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
1. Saeed SA, Stanley JB. Top research findings of 2018-2019. First of 2 parts. Current Psychiatry. 2020;19(1):13-18.
2. Han LKM, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774-782.
3. Wu YC, Tseng PT, Tu YK, et al. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatry. 2019;76(5):526-535.
4. Simpson TL, Saxon AJ, Stappenbeck C, et al. Double-blind randomized clinical trial of prazosin for alcohol use disorder. Am J Psychiatry. 2018;175(12):1216-1224.
5. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
6. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499-507.
7. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry. 2019;76(5):508-515.
8. Blazer DG. Pharmacologic intervention for the treatment and prevention of delirium: looking beneath the modeling. JAMA Psychiatry. 2019;76(5):472-473.
9. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61-75.
10. Goff DC. Can adjunctive pharmacotherapy reduce hospitalization in schizophrenia? Insights from administrative databases. JAMA Psychiatry. 2019;76(5):468-469.
Lumateperone for schizophrenia
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
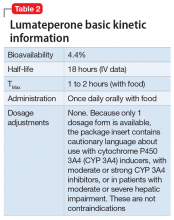
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
Antipsychotic nonadherence is a known contributor to relapse risk among patients with schizophrenia.1 Because relapse episodes may be associated with antipsychotic treatment resistance, this must be avoided as much as possible by appropriate medication selection.2 Adverse effect burden is an important factor leading to oral antipsychotic nonadherence, with patient-derived data indicating that extrapyramidal symptoms (EPS) (odds ratio [OR] 0.57, P = .0007), sedation/cognitive adverse effects (OR 0.70, P = .033), prolactin/endocrine effects (OR 0.69, P = .0342), and metabolic adverse effects (OR 0.64, P = .0079) are all significantly related to lower rates of adherence.3 With this in mind, successive generations of antipsychotics have been released, with fewer tolerability issues present than seen with earlier compounds.1,4 Although these newer second-generation antipsychotics (SGAs) have not proven more effective for schizophrenia than those first marketed in the 1990s, they generally possess lower rates of EPS, hyperprolactinemia, anticholinergic and antihistaminic properties, metabolic adverse effects, and orthostasis.5 This improved adverse effect profile will hopefully increase the chances of antipsychotic acceptance in patients with schizophrenia, and thereby promote improved adherence.
Lumateperone (Caplyta) is a novel oral antipsychotic approved for the treatment of adult patients with schizophrenia (Table 1). It possesses some properties seen with other SGAs, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM) and lower affinity for dopamine D2 receptors (Ki 32 nM), along with low affinity for alpha1-adrenergic receptors (Ki 73 nM), and muscarinic and histaminergic receptors (Ki > 100 nM).6,7 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that of other SGAs such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18)8; at steady state, the D2 occupancy remains <40% (Figure) and the corresponding rates of EPS/akathisia were only 6.7% for lumateperone vs 6.3% for placebo in short-term clinical trials.7,9

How it works
A unique aspect of lumateperone’s pharmacology may relate to differential actions at presynaptic and postsynaptic dopamine D2 receptors. Other antipsychotics possess comparable antagonist (or partial agonist) properties at postsynaptic D2 receptors (the D2 long isoform) and the presynaptic autoreceptor (the D2 short isoform). By blocking the presynaptic autoreceptor, feedback inhibition on dopamine release is removed; therefore, the required higher levels of postsynaptic D2 receptor occupancy needed for effective antipsychotic action (eg, 60% to 80% for antagonists, and 83% to 100% for partial agonists) may be a product of the need to oppose this increased presynaptic release of dopamine. In vitro assays show that lumateperone does not increase presynaptic dopamine release, indicating that it possesses agonist properties at the presynaptic D2 short receptor.10 That property may explain how lumateperone functions as an antipsychotic despite low levels of D2 receptor occupancy.10
Another hypothesis is based on our understanding of pimavanserin’s pharmacology. Pimavanserin is a selective 5HT2A antagonist FDA-approved for the treatment of Parkinson’s disease psychosis (PDP), with extremely high receptor affinity (Ki 0.087 nM) and no appreciable binding at dopamine receptors.5 Pimavanserin not only treats PDP, but is being evaluated in clinical trials for dementia-related psychosis, and has positive data for augmenting antipsychotics when there is a low level of D2 blockade.11,12 In a controlled trial, pimavanserin added to risperidone, 2 mg/d, was as effective as risperidone, 6 mg/d, illustrating the point that near-saturation of the 5HT2A receptor can increase antipsychotic efficacy when dopamine blockade is relatively low. For risperidone, 2 mg/d, the expected D2 occupancy is only 60%.13
Lumateperone also has moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM). Serotonin transporter occupancy at the dose approved for schizophrenia (42 mg/d) is approximately 30%,14 below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitor (SSRI) antidepressants; nevertheless, there is evidence for antidepressant effects seen in preclinical assays, schizophrenia studies, and phase III trials for bipolar depression.8,15,16 It is hypothesized that near-saturation of the 5HT2A receptor might act synergistically with the modest extent of 5HT reuptake inhibition to promote downstream effects associated with effective antidepressant treatments.8 In vivo data also showed phosphorylation of N-methyl-
Clinical implications
Nonadherence with oral antipsychotics among patients with schizophrenia is often related to adverse effects.17 The SGAs marketed since 2000 generally have lower rates of sedation and metabolic and/or endocrine adverse events than earlier compounds, yet each has limitations:
- asenapine: sedation and weight gain
- the partial agonists (aripiprazole, brexpiprazole, cariprazine): akathisia
- lurasidone: dose-dependent EPS and akathisia
- iloperidone: orthostasis.18
Ziprasidone is an exception, because it had low rates of most adverse effects in schizophrenia trials, but the need to take it twice daily with a 500 kcal meal hampers its use. A meta-analysis of 32 oral antipsychotics, including first-generation agents, noted that the efficacy differences between medications are slight for patients without treatment-resistant schizophrenia, but “differences in side-effects are more marked.”18
Continue to: Until novel mechanisms are discovered...
Until novel mechanisms are discovered that increase schizophrenia response rates, the availability of newer antipsychotics with more favorable tolerability profiles presents clinicians and patients with added options when adverse effects interfere with prior treatment. In all phases of the adult schizophrenia trial program for lumateperone, 811 patients received short-term (4- to 6-week) exposure (dose range: 14 to 84 mg/d), while 329 had ≥6 months exposure and 108 had ≥1 year of exposure to the 42-mg/d dose. In these studies, there was no single adverse reaction leading to discontinuation that occurred at a rate >2%. The only adverse events that occurred at rates ≥5% and more than twice the rate of placebo were somnolence/sedation (lumateperone 24%, placebo 10%), and dry mouth (lumateperone 6%, placebo 2%). Nausea was present in 9% of the lumateperone group compared with 5% for placebo.7 In the short-term studies, the combined rate of EPS and akathisia was 6.7% for lumateperone and 6.3% for placebo.7 This difference translates to a number needed to harm of 250 for these neurologic adverse effects. The functional impact of lumateperone’s glutamatergic mechanisms is not well characterized within the schizophrenia population, but the antidepressant potential has been studied for patients with bipolar depression, with 1 positive phase III trial.19
Efficacy in adults with schizophrenia. The efficacy of lumateperone has been established in 2 pivotal, double-blind, placebo-controlled trials. The first was a 4-week, phase II trial (Study 005) in which 335 adults age 18 to 55 with an acute exacerbation of schizophrenia were randomized in a 1:1:1:1 manner to lumateperone, 42 mg/d (60 mg of lumateperone tosylate), lumateperone, 84 mg/d (120 mg of lumateperone tosylate), risperidone, 4 mg/d, or placebo, all taken once daily.20 For the 4 treatment arms, the least squares mean changes from baseline to the Day 28 endpoint on the primary outcome measure, Positive and Negative Syndrome Scale (PANSS) total score, were: lumateperone, 42 mg/d: −13.2 points; lumateperone, 84 mg/d: −8.3 points; risperidone, 4 mg/d: −13.4 points; and placebo: −7.4 points. Both lumateperone, 42 mg/d, and risperidone, 4 mg/d, were significantly different than placebo, and with identical moderate effect sizes of 0.4.20 Lumateperone, 84 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that a similar proportion of patients (40%) randomized to lumateperone, 42 mg/d, or risperidone, 4 mg/d, improved by ≥30% on PANSS total score.
The second pivotal trial (Study 301) was a phase III, double-blind, placebo-controlled trial of 450 adults, age 18 to 60, with an acute exacerbation of schizophrenia who were randomized in 1:1:1 manner to receive lumateperone, 42 mg/d (lumateperone tosylate 60 mg), lumateperone, 28 mg/d (lumateperone tosylate 40 mg), or placebo once daily for 4 weeks.21 For the 3 treatment arms, the least squares mean changes on PANSS total score from baseline to the Day 28 endpoint were: lumateperone, 42 mg/d: −14.5 points; lumateperone, 28 mg/d: −12.9 points; and placebo: −10.3 points. Lumateperone, 28 mg/d, did not separate from placebo on the primary outcome. The responder analysis also indicated that 36.5% of those receiving lumateperone, 42 mg/d, and 36.3% of those receiving lumateperone, 28 mg/d, improved by ≥30% on PANSS total score, compared with 25.5% of patients treated with placebo.
Unlike the 2 positive trials in which placebo change in total PANSS scores were −7.4 and −10.3 points, respectively, in a phase III trial (Study 302) with 696 participants, placebo showed a 15.1-point decrease from baseline PANSS total score.19 Among the 3 treatment arms of this study (lumateperone, 14 mg/d, lumateperone, 42 mg/d, and risperidone, 4 mg/d), only risperidone was superior to placebo.
Adverse events
In the phase II pivotal study, completion rates among the 4 arms were comparable: lumateperone, 42 mg/d: 71%; lumateperone, 84 mg/d: 76%; risperidone, 4 mg/d: 77%; and placebo: 72%.20 There were no serious adverse events (SAEs) associated with lumateperone; the 2 SAEs that occurred involved worsening of schizophrenia/psychotic disorder for risperidone (n = 1) and for placebo (n = 1). Five participants discontinued the study due to an adverse event: 2 who were receiving lumateperone (1 due to dry mouth, and 1 due to worsening of schizophrenia) and 3 who were receiving risperidone (2 due to akathisia, and 1 due to blood creatine phosphokinase increase).20 The most frequent adverse event was somnolence/sedation (placebo: 13%; lumateperone, 42 mg/d: 17%; risperidone, 4 mg/d: 21%; and lumateperone, 84 mg/d: 32.5%). Neither dose of lumateperone was associated with increased rates of EPS. Median weight gain to Day 28 was 1 kg for placebo and for each dose of lumateperone, and 2.5 kg for risperidone. Compared with risperidone, lumateperone showed statistically significantly lower prolactin levels (lumateperone, 42 mg/d and 84 mg/d: P < .001), and metabolic parameters, including fasting glucose (lumateperone 42 mg/d: P = .007; lumateperone, 84 mg/d: P = .023), total cholesterol (lumateperone, 42 mg/d: P = .012; lumateperone, 84 mg/d: P = .004), and triglycerides (lumateperone, 42 mg/d: P = .074; lumateperone, 84 mg/d: P = .002).20 There was no significant impact on the corrected QT interval.
Continue to: In the phase III trial...
In the phase III trial, completion rates among the 3 arms were lumateperone, 42 mg/d: 85%; lumateperone, 28 mg/d: 80%; and placebo: 74%. There was 1 SAE in a patient receiving lumateperone, 28 mg/d. This individual had preexisting risk factors and a history of seizures, and experienced a seizure during the study. Adverse events that occurred in either lumateperone group at a rate ≥5% and more than twice the rate of placebo were somnolence (lumateperone, 42 mg/d: 17.3%; lumateperone, 28 mg/d: 11.3%; and placebo: 4.0%); sedation (lumateperone, 42 mg/d: 12.7%; lumateperone, 28 mg/d: 9.3%; and placebo: 5.4%); fatigue (lumateperone, 42 mg/d: 5.3%; lumateperone, 28 mg/d: 4.7%; and placebo: 1.3%); and constipation (lumateperone, 42 mg/d: 6.7%; lumateperone, 28 mg/d: 4.0%; and placebo: 2.7%).21 No EPS-related adverse events occurred in ≥5% patients in any treatment arm. Median change in weight from baseline to Day 28 was 0.9 kg for lumateperone, 42 mg/d, 0.6 kg for lumateperone, 28 mg/d, and 0.7 kg for placebo. There were no significant mean changes in metabolic parameters for any treatment arm, and none of the patients had a corrected QT interval (QTc) >500 ms or a change in QTc >60 ms from baseline.21
Pharmacologic profile
Lumateperone’s in vitro binding profile includes high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), lower affinity for dopamine D2 receptors (Ki 32 nM), moderate binding affinity for SERT (Ki 33 nM), and lower affinity for alpha 1-adrenergic receptors (Ki 73 nM) and muscarinic and histaminergic receptors (Ki >100 nM).6,7 As noted above, this 60-fold ratio of 5HT2A to D2 affinity is extremely high; moreover, imaging data reveal low D2 receptor occupancy, consistent with the lack of clinically significant EPS seen in the trials. In vitro assays also reveal impact on glutamate pathways, and pathways associated with antidepressant response.8 The clinical benefits of the glutamatergic properties remain theoretical, but the antidepressant benefit has been seen in a positive phase III trial for bipolar depression.19
Clinical considerations
Effect sizes in the 2 positive pivotal trials were 0.3 and 0.4, comparable with those for other antipsychotics approved within the last decade: brexpiprazole, 0.26; cariprazine, 0.34; and lurasidone, 0.36.21 The absence of clinically significant EPS, lack of impact on metabolic or endocrine parameters, and lack of titration are all appealing properties. That only 42 mg/d proved effective may reflect the fact that the other doses studied to date in randomized, fixed-dose studies were 14 mg/d (Study 302) and 84 mg/d (Study 005), evaluated in one study each. While those 2 doses might indeed be outside the therapeutic window, given the heterogeneity of schizophrenia, future studies might help further refine the therapeutic range for schizophrenia, especially for doses closer to 42 mg/d (eg, 28 mg/d, 63 mg/d). Should 42 mg/d not prove effective, there is no data for now to suggest whether a dose increase may be helpful. As there is only 1 marketed dose of lumateperone (42-mg capsules), and no easy way to modify this dose, lumateperone’s package insert includes cautionary language regarding situations where there will be less-than-expected drug exposure (use of cytochrome P450 [CYP] 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant EPS and metabolic or endocrine adverse effects. In vitro data indicate glutamatergic effects, and human data indicate antidepressant effects in bipolar patients. Despite the absence of significant histamine H1 or muscarinic affinity, the rate of somnolence/sedation was twice that of placebo (lumateperone 24%, placebo 10%).7
Why Rx? Reasons to prescribe lumateperone for adult patients with schizophrenia include:
- Favorable tolerability profile, including no significant signal for EPS or endocrine or metabolic adverse effects, and no QT prolongation
- No need for titration.
Dosing. There is only 1 dose available for lumateperone, 42-mg capsules (Table 2). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less-than-expected drug exposure (use of CYP 3A4 inducers), greater-than-expected drug exposure (moderate or strong CYP 3A4 inhibitors), or use in patients with moderate or severe hepatic impairment as defined by Child-Pugh criteria (Child-Pugh B or C). These are not contraindications.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Continue to: Bottom Line
Bottom Line
Lumateperone is a novel oral antipsychotic indicated for treating adults with schizophrenia. Its unique properties include the lack of presynaptic dopamine D2 antagonism, low D2 receptor occupancy, and the absence of significant extrapyramidal symptoms and metabolic or endocrine adverse effects. In clinical trials, the most frequent adverse event was somnolence/sedation.
Related Resource
- Fulton D. FDA approves Caplyta to treat schizophrenenia in adults. https://www.mdedge.com/psychiatry/article/214733/schizophrenia-other-psychotic-disorders/fda-approves-caplyta-treat.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Iloperidone • Fanapt
Lumateperone • Caplyta
Lurasidone • Latuda
Olanzapine • Zyprexa
Pimavanserin • Nuplazid
Risperidone • Risperdal
Ziprasidone • Geodon
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
1. Dufort A, Zipursky RB. Understanding and managing treatment adherence in schizophrenia [published online January 3, 2019]. Clin Schizophr Relat Psychoses. 2019. doi: 10.3371/CSRP.ADRZ.121218.
2. Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? Antipsychotic response in first- vs. second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042.
3. Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
4. Kurokawa S, Kishimoto T, Su K-P, et al. Psychiatrists’ perceptions of medication adherence among patients with schizophrenia: an international survey. Schizophr Res. 2019;211:105-107.
5. Meyer JM. Pharmacotherapy of psychosis and mania. In: Brunton LL, Hilal-Dandan R, Knollmann BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. Chicago, Illinois: McGraw-Hill; 2018:279-302.
6. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
7. Caplyta [package Insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2019.
8. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
9. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
10. Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018. doi: 10.19185/matters.201712000006.
11. Meltzer HY, Elkis H, Vanover K, et al. Pimavanserin, a selective serotonin (5-HT)2A-inverse agonist, enhances the efficacy and safety of risperidone, 2mg/day, but does not enhance efficacy of haloperidol, 2mg/day: comparison with reference dose risperidone, 6mg/day. Schizophr Res. 2012;141(2-3):144-152.
12. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
13. Remington G, Mamo D, Labelle A, et al. A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry. 2006;163(3):396-401.
14. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-2872.
15. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
16. Vanover K, Glass S, Kozauer S, et al. 30 lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectr. 2019;24(1):190-191.
17. Young SL, Taylor M, Lawrie SM. “First do no harm.” A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. 2015;29(4):353-362.
18. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951.
19. Vyas P, Hwang BJ, Brašic ´ JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2019;1-7.
20. Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961.
21. Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia [published online January 8, 2020]. JAMA Psychiatry. 2020;E1-E10.
MAPS.EDU, GAS POPS, and AEIOU: Acronyms to guide your assessments
Mnemonics and acronyms are part of our daily lives, helping us to memorize and retain clinical information. They play an invaluable role in medical school because they can help students recall vast amounts of information in a moment’s notice, such as psychiatric conditions to consider during a “review of systems.”
Most medical students are trained to conduct a review of systems as a standard approach when a thorough medical history is indicated. Clinicians need to assess all patients for an extremely broad range of syndromes. Because of the extensive comorbidity of many psychiatric disorders, it is important to review the most common conditions before establishing a diagnosis and formulating a treatment plan.1
For example, a patient presenting with a chief complaint consistent with a depressive disorder may have unipolar depression, bipolar depression, or substance-induced depression (after general medical comorbidity has been excluded). In this scenario, it would be equally important to identify co-occurring conditions, such as an anxiety disorder or psychotic symptoms, because these can have a major impact on treatment and prognosis.
In our work as clinical educators, we have noticed that many students struggle with a review of psychiatric systems during their evaluation of a new patient. Acronyms could serve as a map to guide them during assessments. While these may be most valuable to medical students, they are also helpful for clinicians on the frontline of medical practice (primary care, family practice, OB-GYN) as well as for early-career psychiatrists.
MAPS.EDU
The acronym MAPS.EDU covers several common psychiatric conditions seen in routine practice: Mood disorders, Anxiety disorders, Personality disorders, Schizophrenia and related disorders, Eating disorders, Developmental disorders (eg, attention-deficit/hyperactivity disorder), and substance Use disorders. While not comprehensive, MAPS.EDU can be a quick method to help psychiatrists remember these common conditions.
GAS POPS
Anxiety is a core symptom of several psychiatric disorders. The mnemonic GAS POPS can help clinicians recall disorders to consider when screening patients who report anxiety: Generalized anxiety disorder, Agoraphobia, Social anxiety disorder, Panic disorder, Obsessive-compulsive disorder, Posttraumatic stress disorder (PTSD), and Specific phobias.
AEIOU for PTSD
The diagnostic criteria of PTSD can be memorized by using the acronym AEIOU: Avoidance (of triggers), Exposure (to trauma), Intrusions (reliving phenomena), Outbursts (or other manifestations of hyperarousal), and Unhappiness (negative alterations in mood and cognition).
1. Rush J, Zimmerman M, Wisniewski S, et al. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43-55.
Mnemonics and acronyms are part of our daily lives, helping us to memorize and retain clinical information. They play an invaluable role in medical school because they can help students recall vast amounts of information in a moment’s notice, such as psychiatric conditions to consider during a “review of systems.”
Most medical students are trained to conduct a review of systems as a standard approach when a thorough medical history is indicated. Clinicians need to assess all patients for an extremely broad range of syndromes. Because of the extensive comorbidity of many psychiatric disorders, it is important to review the most common conditions before establishing a diagnosis and formulating a treatment plan.1
For example, a patient presenting with a chief complaint consistent with a depressive disorder may have unipolar depression, bipolar depression, or substance-induced depression (after general medical comorbidity has been excluded). In this scenario, it would be equally important to identify co-occurring conditions, such as an anxiety disorder or psychotic symptoms, because these can have a major impact on treatment and prognosis.
In our work as clinical educators, we have noticed that many students struggle with a review of psychiatric systems during their evaluation of a new patient. Acronyms could serve as a map to guide them during assessments. While these may be most valuable to medical students, they are also helpful for clinicians on the frontline of medical practice (primary care, family practice, OB-GYN) as well as for early-career psychiatrists.
MAPS.EDU
The acronym MAPS.EDU covers several common psychiatric conditions seen in routine practice: Mood disorders, Anxiety disorders, Personality disorders, Schizophrenia and related disorders, Eating disorders, Developmental disorders (eg, attention-deficit/hyperactivity disorder), and substance Use disorders. While not comprehensive, MAPS.EDU can be a quick method to help psychiatrists remember these common conditions.
GAS POPS
Anxiety is a core symptom of several psychiatric disorders. The mnemonic GAS POPS can help clinicians recall disorders to consider when screening patients who report anxiety: Generalized anxiety disorder, Agoraphobia, Social anxiety disorder, Panic disorder, Obsessive-compulsive disorder, Posttraumatic stress disorder (PTSD), and Specific phobias.
AEIOU for PTSD
The diagnostic criteria of PTSD can be memorized by using the acronym AEIOU: Avoidance (of triggers), Exposure (to trauma), Intrusions (reliving phenomena), Outbursts (or other manifestations of hyperarousal), and Unhappiness (negative alterations in mood and cognition).
Mnemonics and acronyms are part of our daily lives, helping us to memorize and retain clinical information. They play an invaluable role in medical school because they can help students recall vast amounts of information in a moment’s notice, such as psychiatric conditions to consider during a “review of systems.”
Most medical students are trained to conduct a review of systems as a standard approach when a thorough medical history is indicated. Clinicians need to assess all patients for an extremely broad range of syndromes. Because of the extensive comorbidity of many psychiatric disorders, it is important to review the most common conditions before establishing a diagnosis and formulating a treatment plan.1
For example, a patient presenting with a chief complaint consistent with a depressive disorder may have unipolar depression, bipolar depression, or substance-induced depression (after general medical comorbidity has been excluded). In this scenario, it would be equally important to identify co-occurring conditions, such as an anxiety disorder or psychotic symptoms, because these can have a major impact on treatment and prognosis.
In our work as clinical educators, we have noticed that many students struggle with a review of psychiatric systems during their evaluation of a new patient. Acronyms could serve as a map to guide them during assessments. While these may be most valuable to medical students, they are also helpful for clinicians on the frontline of medical practice (primary care, family practice, OB-GYN) as well as for early-career psychiatrists.
MAPS.EDU
The acronym MAPS.EDU covers several common psychiatric conditions seen in routine practice: Mood disorders, Anxiety disorders, Personality disorders, Schizophrenia and related disorders, Eating disorders, Developmental disorders (eg, attention-deficit/hyperactivity disorder), and substance Use disorders. While not comprehensive, MAPS.EDU can be a quick method to help psychiatrists remember these common conditions.
GAS POPS
Anxiety is a core symptom of several psychiatric disorders. The mnemonic GAS POPS can help clinicians recall disorders to consider when screening patients who report anxiety: Generalized anxiety disorder, Agoraphobia, Social anxiety disorder, Panic disorder, Obsessive-compulsive disorder, Posttraumatic stress disorder (PTSD), and Specific phobias.
AEIOU for PTSD
The diagnostic criteria of PTSD can be memorized by using the acronym AEIOU: Avoidance (of triggers), Exposure (to trauma), Intrusions (reliving phenomena), Outbursts (or other manifestations of hyperarousal), and Unhappiness (negative alterations in mood and cognition).
1. Rush J, Zimmerman M, Wisniewski S, et al. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43-55.
1. Rush J, Zimmerman M, Wisniewski S, et al. Comorbid psychiatric disorders in depressed outpatients: demographic and clinical features. J Affect Disord. 2005;87(1):43-55.
Called to court? Tips for testifying
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
As a psychiatrist, you could be called to court to testify as a fact witness in a hearing or trial. Your role as a fact witness would differ from that of an expert witness in that you would likely testify about the information that you have gathered through direct observation of patients or others. Fact witnesses are generally not asked to give expert opinions regarding forensic issues, and treating psychiatrists should not do so about their patients. As a fact witness, depending on the form of litigation, you might be in one of the following 4 roles1:
- Observer. As the term implies, you have observed an event. For example, you are asked to testify about a fight that you witnessed between another clinician’s patient and a nurse while you were making your rounds on an inpatient unit.
- Non-defendant treater. You are the treating psychiatrist for a patient who is involved in litigation to recover damages for injuries sustained from a third party. For example, you are asked to testify about your patient’s premorbid functioning before a claimed injury that spurred the lawsuit.
- Plaintiff. You are suing someone else and may be claiming your own damages. For example, in your attempt to claim damages as a plaintiff, you use your clinical knowledge to testify about your own mental health symptoms and the adverse impact these have had on you.
- Defendant treater. You are being sued by one of your patients. For example, a patient brings a malpractice case against you for allegations of not meeting the standard of care. You testify about your direct observations of the patient, the diagnoses you provided, and your rationale for the implemented treatment plan.
Preparing yourself as a fact witness
For many psychiatrists, testifying can be an intimidating process. Although there are similarities between testifying in a courtroom and giving a deposition, there are also significant differences. For guidelines on providing depositions, see Knoll and Resnick’s “Deposition dos and don’ts: How to answer 8 tricky questions” (
Don’t panic. Although your first reaction may be to panic upon receiving a subpoena or court order, you should “keep your cool” and remember that the observations you made or treatment provided have already taken place.1 Your role as a fact witness is to inform the judge and jury about what you saw and did.1
Continue to: Refresh your memory and practice
Refresh your memory and practice. Gather all required information (eg, medical records, your notes, etc.) and review it before testifying. This will help you to recall the facts more accurately when you are asked a question. Consider practicing your testimony with the attorney who requested you to get feedback on how you present yourself.1 However, do not try to memorize what you are going to say because this could make your testimony sound rehearsed and unconvincing.
Plan ahead, and have a pretrial conference. Because court proceedings are unpredictable, you should clear your schedule to allow enough time to appear in court. Before your court appearance, meet with the attorney who requested you to discuss any new facts or issues as well as learn what the attorney aims to accomplish with your testimony.1
Speak clearly in your own words, and avoid jargon. Courtroom officials are unlikely to understand psychiatric jargon. Therefore, you should explain psychiatric terms in language that laypeople would comprehend. Because the court stenographer will require you to use actual words for the court transcripts, you should answer clearly and verbally or respond with a definitive “yes” or “no” (and not by nodding or shaking your head).
Testimony is also not a time for guessing. If you don’t know the answer, you should say “I don’t know.”
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
1. Gutheil TG. The psychiatrist in court: a survival guide. Washington, DC: American Psychiatric Press, Inc.; 1998.
2. Knoll JL, Resnick PJ. Deposition dos and don’ts: how to answer 8 tricky questions. Current Psychiatry. 2008;7(3):25-28,36,39-40.
The evolution of manic and hypomanic symptoms
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
Lofexidine: An option for treating opioid withdrawal
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
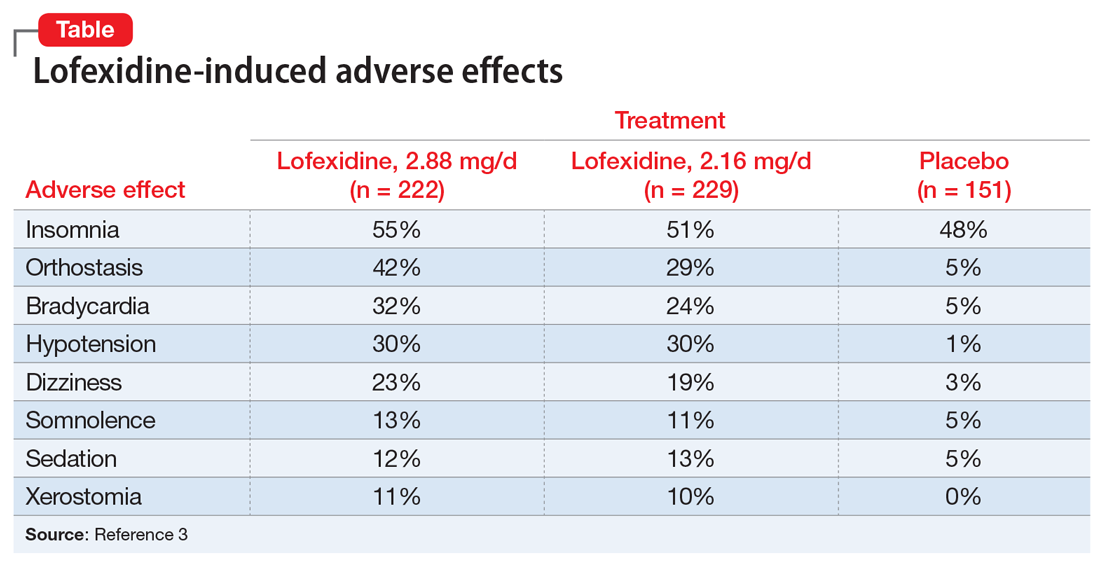
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).

Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Antipsychotics, dopamine, and pain
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.
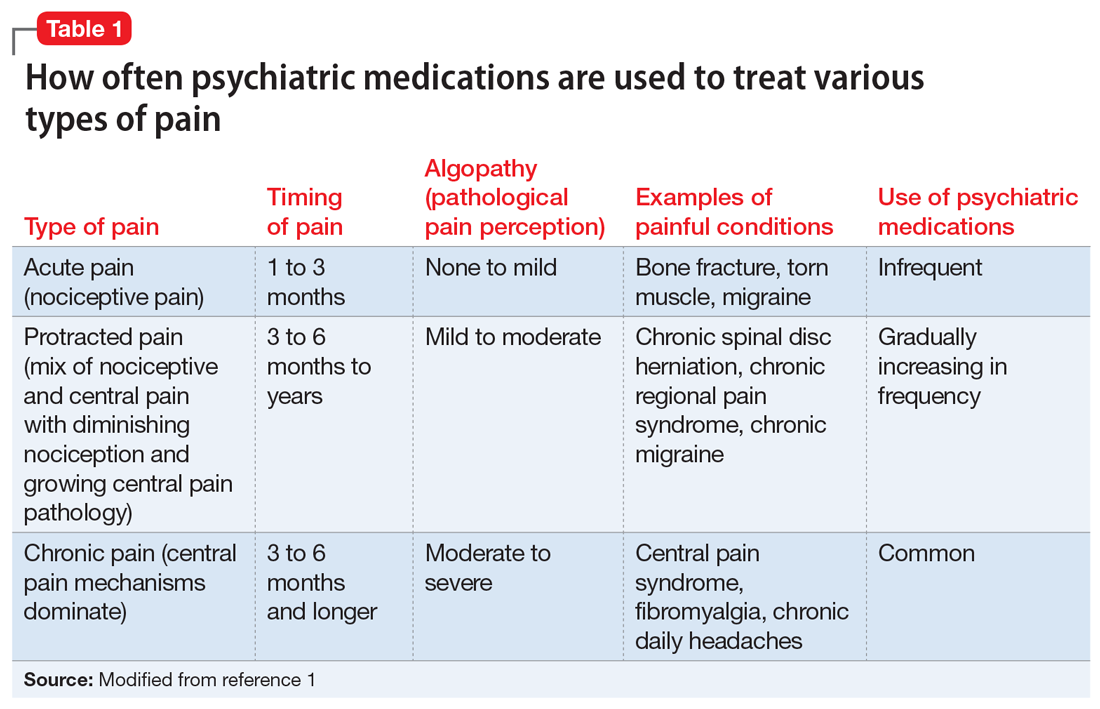
In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.

In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
Our understanding of pain mechanisms continues to evolve and, accordingly, so do our treatment strategies. The fundamental differences between acute and chronic pain were only recently recognized; this lack of recognition led to the application of acute pain treatments to chronic pain, contributing to the opioid epidemic in the United States.
With the diminishing emphasis on opioid medications, researchers are exploring other pharmacologic modalities for treating pain. Many nonopioid psychiatric medications are used off-label for the treatment of pain. Psychiatric medications play a larger role in the management of pain as pain becomes more chronic (Table 11). For simplicity, acute pain may be seen as nociception colored by emotions, and chronic pain as emotions colored by nociception. Protracted pain connects those extremes with a diminishing role of nociception and an increasing role of emotion,1 which may increase the potential role of psychiatric medications, including antipsychotics.

In this article, I discuss the potential role of dopamine in the perception of pain, and review the potential use of first- and second-generation antipsychotics for treating various pain syndromes.
Role of dopamine in pain
There is increasing interest in exploring antipsychotics to treat chronic pain2 because dopamine dysfunction is part of pathological pain perception. Excess dopamine is associated with headaches (dopamine hypersensitivity hypothesis3,4) and dopamine dysfunction is a part of posttraumatic stress disorder (PTSD),5 dissociation,6 paranoia,7 and catastrophizing.8 Somatic psychosis, like any psychosis, can be based on dopamine pathology. Dopaminergic neurons affect nociceptive function in the spinal dorsal horn,9 and dopamine receptors are altered in atypical facial pain,10 burning mouth syndrome,11 and fibromyalgia.12
In normal circumstances, dopamine is fundamentally a protective neurotransmitter. In acute pain, dopamine is powerfully released, making the pain bearable. A patient may describe acute pain as seeming “like it was not happening to me” or “it was like a dream”; both are examples of dopamine-caused dissociation and a possible prediction of subsequent chronification. In chronic pain, pathological mechanisms settle in and take root; therefore, keeping protective dopamine levels high becomes a priority. This is especially common in patients who have experienced abuse or PTSD. The only natural way to keep dopamine up for prolonged periods of time is to decrease pain and stress thresholds. Both phenomena are readily observed in patients with pain. In extreme cases, self-mutilation and involvement in conflicts become pathologically gratifying.
The dopaminergic system is essential for pain control with a tissue injury.13 It becomes pathologically stimulated and increasingly dysfunctional as algopathy (a pathological pain perception) develops. At the same time, a flood or drought of any neurotransmitter is equally bad and may produce similar clinical pictures. Both a lack of and excess of dopamine are associated with pain.14 This is why opposite treatments may be beneficial in different patients with chronic pain. As an example, the use of stimulants15 and bupropion16 has been reported in the treatment of abdominal pain. And, reversely, antipsychotics, especially first-generation agents, may be associated with chronic (tardive) pain, including orofacial and genital pain.17
First-generation antipsychotics
First-generation antipsychotics (FGAs) have been used to treat various nonpsychiatric conditions (Table 2). Although they are powerful D2 receptor inhibitors, FGAs lack the intrinsic ability to counteract the unwanted adverse effects of strong inhibition. As a result, movement disorders and prolactinemia are commonly induced by FGAs. The most dangerous consequence of treatment with these agents is neuroleptic malignant syndrome (NMS).
Continue to: Haloperidol
Haloperidol is prescribed widely by nonpsychiatrists, primarily to treat agitation. Intravenous haloperidol has been used for the abortive treatment of headaches.18 Paradoxically, IV haloperidol is less likely to induce extrapyramidal symptoms (EPS) than the oral formulation because of a more pronounced anticholinergic action in IV use. Haloperidol can help relieve gastroparesis and nausea, especially in IV administration,19 but prolonged oral administration is associated with unwanted movement problems and should be avoided.20
Chlorpromazine is more anticholinergic than haloperidol. It can be used in the abortive treatment of headaches (preferably via IV and IM administration), nausea, hiccups, porphyria, and serotonin syndrome, but it is very sedating and frequently produces hypotension, dangerous QT prolongation, and sensations of thought-blocking.21
Pimozide is reported to help with skin picking, trichotillomania, and somatic hallucinations.22
Droperidol, promethazine, and prochlorperazine are used off-label to treat nausea and headaches. Primary care clinicians may not be aware that these commonly used medications are antipsychotics. Similar to other FGAs, these 3 agents may produce NMS and tardive dyskinesia (TD). The same applies to the prokinetic drug metoclopramide.
Second-generation antipsychotics
Second-generation antipsychotics (SGAs) work with various serotonin receptors, offsetting and enhancing the antipsychotic function of dopamine blockade. This diminishes but does not eliminate EPS and the risk of TD. Fortunately, the risk of NMS is lower with SGAs than with FGAs. Many SGAs are FDA-approved for treating schizophrenia and other psychiatric disorders, and some have relevance for pain management (Table 3). Many SGAs help with depressive symptoms and are powerful mood stabilizers. As such, they may diminish central over-firing of dopaminergic and serotonergic neurons involved in the pain cascade, which in turn decreases pain transmission and perception. The downside is that in general, SGAs increase the risk of diabetes and hyperlipidemia.
Continue to: Risperidone
Risperidone was the second FDA-approved SGA. Pain practitioners primarily prescribe it for treatmeant-resistant headaches, but patients with fibromyalgia and those with phantom and thalamic pain also may respond. Because risperidone’s properties are similar to that of many FGAs, it may potently cause EPS, TD, and prolactinemia. Neuroleptic malignant syndrome also has been reported.23
Ziprasidone is frequently overlooked by clinicians who treat pain. Although ziprasidone may be sedating, it is powerful as both a preventive and abortive (in an IM formulation) agent for treatment-resistant headaches. This might be attributed to its effects on the 5HT9 receptor. It is approved for treating bipolar depression and has been prescribed to effectively treat anxiety. For patients receiving ziprasidone, QT prolongation needs to be monitored closely.24
Olanzapine was modeled after clozapine and is effective as a mood stabilizer and an antianxiety, antipsychotic, and sleep-promoting medication. It has a useful “mellowing” effect and helps with central pain syndrome management. Patients with fibromyalgia respond well; in some cases, patients with phantom and thalamic pain also respond. Among SGAs prescribed to treat chronic pain, olanzapine has the most published studies. However, the downside is the risk of severe weight gain and diabetes. Usually, if a patient is already overweight, they gain less, but these patients typically are concerned about any additional weight gain.25
Aripiprazole is a partial dopamine agonist. It increases dopamine function in the prefrontal cortex, and by doing so it possibly improves cognition, mental acuity, goal-oriented activity, and attention. At the same time, it decreases dopamine activity in the basal ganglia and limbic system, improving catastrophizing, paranoia, abnormal pain perception, and multiple homeostasis functions. This combination of effects can be invaluable for some patients, but depending on individual susceptibility, aripiprazole might be too activating (causing agitation and akathisia) or too sedating.26
Brexpiprazole is a relative of aripiprazole, but for some patients it is better tolerated, and compliance with this medication usually is good. It partially antagonizes the D2 and 5HT1A receptors while antagonizing the 5HT2A receptors (which decreases the dopamine release in the striatum) and mimics the mechanism of action of an antidepressant. Through alpha-1-adrenergic receptor antagonism, it reduces EPS. All these effects are also part of the mechanisms of action of quetiapine, clozapine, and iloperidone, but brexpiprazole is considered to be the most alpha-1 antagonistic, which is a mechanism of action of other potential pain-controlling medications such as clonidine and tizanidine. In patients with pain who have an overactive noradrenergic system, this property may be beneficial. Its major problem stems from cytochrome P450 2D6 (CYP2D6) enzyme-dependent metabolism, which causes an approximately 5-fold increase in brexpiprazole blood level in poor CYP2D6 metabolizers. Therefore, combining brexpiprazole with CYP2D6 inhibitors such as fluoxetine, paroxetine, and duloxetine would be unwise. Aripiprazole and brexpiprazole are less associated with diabetes and sexual adverse effects than many other SGAs.27
Continue to: Asenapine
Asenapine is an underutilized antipsychotic. Its mechanism of action spans multiple receptors and is less specific in individual receptor activity than other dopamine blockers. It is administered under the tongue due to poor absorption when swallowed, and its molecule has an anesthetic property that causes mouth and tongue numbness/paresthesia. This function may help patients with orofacial pain. Significant somnolence and weight gain (although less than with olanzapine) limit its use. Some patients cannot tolerate the taste.28
Quetiapine is prescribed rather frequently due to its significant antianxiety effect. It is also reported to be beneficial in pain control.29 Weight gain may be severe. In doses smaller than typically administered to patients with bipolar disorder or schizophrenia, quetiapine is widely prescribed off-label for sleep. In lower doses, it acts primarily as an antihistamine (hence the sedation), but at an increased dose it activates the adrenergic system, which offsets sedation. Quetiapine antagonizes H1 histamine and 5HT2
Cariprazine is typically well tolerated because of its benign metabolic profile. It does not increase the QT interval and is not sedating. Cariprazine is a D2 and D3 partial receptor agonist. This allows the medication to inhibit overstimulated dopamine receptors (a desirable effect in pain management) and induces them when the endogenous dopamine level is low (helping with cognition, volition, and attention). Pro-cognitive effects are always beneficial for patients with pain. Cariprazine produces less EPS due to more ventral striatum vs dorsal striatum activity. Mood improvement caused by this medication is attributed to its 5HT2A, 5HT2B, and 5HT2C inverse agonism, which modulates the serotonergic system. Cariprazine will likely have a positive future in pain management because it has shown efficacy in the chronic stress model.33
A complex condition
No single medication or group of medications may be exclusively relied on for treating patients with chronic pain. Identifying alternatives to opioids for treating pain brings more attention to centrally-acting medications that may aid in the stabilization of the nervous system, which can decrease pathological pain perception and help patients cope with chronic painful conditions.
Bottom Line
Antipsychotics may be a valuable asset in the treatment of chronic pain, offering a potential alternative to prescribing opioids for pain. More research is needed to identify specific ways of using dopamine blockade or dopamine enhancement to help patients with chronic pain.
Continue to: Related Resource
Related Resource
- Tripathi A. Antipsychotics for migraines, cluster headaches, and nausea. Current Psychiatry. 2013;12(2):E1-E4.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Brexpiprazole • Rexulti
Bupropion • Wellbutrin, Zyban
Cariprazine • Vraylar
Chlorpromazine • Thorazine
Clonidine • Catapres
Clozapine • Clozaril
Droperidol • Inapsine
Duloxetine • Cymbalta
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Metoclopramide • Reglan
Olanzapine • Zyprexa
Paroxetine • Paxil
Pimozide • Orap
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Risperidone • Risperdal
Tizanidine • Zanaflex
Ziprasidone • Geodon
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
1. Arbuck D, Pergolizzi J. Algopathy—acknowledging the pathological process of pain chronification. Pract Pain Manag. 2017;17(4):4,26-32.
2. Shin SW, Lee JS, Abdi S, et al. Antipsychotics for patients with pain. Korean J Pain. 2019;32(1):3-11.
3. D’Andrea G, Leone M, Bussone G, et al. Abnormal tyrosine metabolism in chronic cluster headache. Cephalalgia. 2017;37(2):148-153.
4. D’Andrea G, Granella F, Perini F, et al. Platelet levels of dopamine are increased in migraine and cluster headache. Headache. 2006;46(4):585-591.
5. Wolf EJ, Mitchell KS, Logue MW, et al. The dopamine D3 receptor gene, and posttraumatic stress disorder. J Trauma Stress. 2014;27(4):379-387.
6. den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100.
7. Nour MM, Dahoun T, Schwartenbeck P, et al. Dopaminergic basis for signaling belief updates, but not surprise, and the link to paranoia. Proc Natl Acad Sci U S A. 2018;115(43):E10167-E10176.
8. Zhu H, Clemens S, Sawchuk M, et al. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885-897.
9. Wood PB, Schweinhardt P, Jaeger E, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576-3582.
10. Hagelberg N, Fossell H, Aalto S, et al. Altered dopamine D2 receptor binding in atypical facial pain. Pain. 2003;106(1-2):43-48.
11. Hagelberg N, Fossell H, Rinne JD, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101(1-2):149-154.
12. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11-36.
13. Siahposht-Khachaki A, Pourreza P, Ezzatpanah S, et al. Nucleus accumbens dopamine receptors mediate hypothalamus-induced antinociception in the rat formalin test. Eur J Pain. 2017;21(7):1285-1294.
14. Thompson T, Gallop K, Correll CU, et al. Pain perception in Parkinson’s disease: a systematic review and meta-analysis of experimental studies. Aging Res Rev. 2017;35:74-86.
15. Check JH. Chronic unremitting lower abdominal pain quickly abrogated following treatment with amphetamine. Clin Exp Obstet Gynecol. 2016;43(1):109-111.
16. Wilkes S. Bupropion. Drugs Today (Barc). 2006;42(10):671-681.
17. Frei K, Truong DD, Fahn S, et al. The nosology of tardive syndromes. J Neurol Sci. 2018;389:10-16.
18. Honkaniemi J, Liimatainen S, Rainesalo S, et al. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787.
19. Murray-Brown F, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database Syst Rev. 2015;(11):CD006271.
20. Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334.
21. Weinman D, Nicastro O, Akala O, et al. Parenteral treatment of episodic tension-type headache: a systematic review. Headache. 2014;54(2):260-268.
22. Arnold LM, Auchenbach MB, McElroy SL. Psychogenic excoriation. Clinical features, proposed diagnostic criteria, epidemiology, and approaches to treatment. CNS Drugs. 2001;15(5):351-359.
23. Khouzam HR. Psychopharmacology of chronic pain: a focus on antidepressants and atypical antipsychotics. Postgrad Med. 2016;128(3):323-330.
24. Landsness EC, Wang LH, Bucelli RC. Ziprasidone as a potential abortive therapy for status migrainosus. Neurohospitalist. 2016;6(4):151-156.
25. Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585-591.
26. Fei L, Abrardi L, Mediati RD. Unexpected effect of aripiprazole on nociceptive pain. Ther Adv Psychopharmacol. 2012;2(5):211-212.
27. Markovic M, Gallipani A, Patel KH, et al. Brexpiprazole. Ann Pharmacother. 2017;51(4):315-322.
28. Gerrits M, de Greef R, Peeters P. Effect of absorption site on the pharmacokinetics of sublingual asenapine in healthy male subjects. Biopharm Drug Dispos. 2010;31(5-6):351-357.
29. Heo MH, Kim JY, Hwang I, et al. Analgesic effect of quetiapine in a mouse model of cancer-induced bone pain. Korean J Intern Med. 2017;32(6):1069-1074.
30. Tamburello AC, Lieberman JA, Baum RM, et al. Successful removal of quetiapine from a correctional formulary. J Am Acad Psychiatry Law. 2012;40(4):502-508.
31. Fountoulakis KN, Iacovides A, Kaprinis SG, et al. Diffuse muscle pain with quetiapine. Br J Psychiatry. 2003;182:81.
32. Shintani F. Diminished pain perception in schizophrenia. Lancet. 2010;376(9735):87.
33. Duric V, Banasr M, Franklin T, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. 2017;20(10):788-796
Buspirone: A forgotten friend
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
In general, when a medication goes off patent, marketing for it significantly slows down or comes to a halt. Studies have shown that physicians’ prescribing habits are influenced by pharmaceutical representatives and companies.1 This phenomenon may have an unforeseen adverse effect: once an effective and inexpensive medication “goes generic,” its use may fall out of favor. Additionally, physicians may have concerns about prescribing generic medications, such as perceiving them as less effective and conferring more adverse effects compared with brand-name formulations.2 One such generic medication is buspirone, which originally was branded as BuSpar.
Anxiety disorders are the most common psychiatric diagnoses, and at times are the most challenging to treat.3 Anecdotally, we often see benzodiazepines prescribed as first-line monotherapy for acute and chronic anxiety, but because these agents can cause physical dependence and a withdrawal reaction, alternative anxiolytic medications should be strongly considered. Despite its age, buspirone still plays a role in the treatment of anxiety, and its off-label use can also be useful in certain populations and scenarios. In this article, we delve into buspirone’s mechanism of action, discuss its advantages and challenges, and what you need to know when prescribing it.
How buspirone works
Buspirone was originally described as an anxiolytic agent that was pharmacologically unrelated to traditional anxiety-reducing medications (ie, benzodiazepines and barbiturates).4
The antidepressants vortioxetine and vilazodone exhibit dual-action at both serotonin reuptake transporters and 5HT1A receptors; thus, they work like an SSRI and buspirone combined.6 Although some patients may find it more convenient to take a dual-action pill over 2 separate ones, some insurance companies do not cover these newer agents. Additionally, prescribing buspirone separately allows for more precise dosing, which may lower the risk of adverse effects.
Buspirone is a major substrate for cytochrome P450 (CYP) 3A4 and a minor for CYP2D6, so caution must be advised if considering buspirone for a patient receiving any CYP3A4 inducers and/or inhibitors,7 including grapefruit juice.8
Dose adjustments are not necessary for age and sex, which allows for highly consistent dosing.4 However, as with prescribing medications in any geriatric population, lower starting doses and slower titration of buspirone may be necessary to avoid potential adverse effects due to the alterations of pharmacodynamic and pharmacokinetic processes that occur as patients age.9
Advantages of buspirone
Works well as an add-on to other medications. While buspirone in adequate doses may be helpful as monotherapy in GAD, it can also be helpful in other, more complex psychiatric scenarios. Sumiyoshi et al10 observed improvement in scores on the Digit Symbol Substitution Test when buspirone was added to a second-generation antipsychotic (SGA), which suggests buspirone may help improve attention in patients with schizophrenia. It has been postulated that buspirone may also be helpful for cognitive dysfunction in patients with Alzheimer’s disease.11 Buspirone has been used to treat comorbid anxiety and alcohol use disorder, resulting in reduced anxiety, longer latency to relapse, and fewer drinking days during a 12-week treatment program.12 Buspirone has been more effective than placebo for treating post-stroke anxiety.13
Continue to: Patients who receive...
Patients who receive an SSRI, such as citalopram, but are not able to achieve a substantial improvement in their depressive and/or anxious symptoms may benefit from the addition of buspirone to their treatment regimen.14,15
A favorable adverse-effect profile. There are no absolute contraindications to buspirone except a history of hypersensitivity.4 Buspirone generally is well tolerated and carries a low risk of adverse effects. The most common adverse effects are dizziness and nausea.6 Buspirone is not sedating.
Potentially safe for patients who are pregnant. Unlike many other first-line agents for anxiety, such as SSRIs, buspirone has an FDA Category B classification, meaning animal studies have shown no adverse events during pregnancy.4 The FDA Pregnancy and Lactation Labeling Rule applies only to medications that entered the market on or after June 30, 2001; unfortunately, buspirone is excluded from this updated categorization.16 As with any medication being considered for pregnant or lactating women, the prescriber and patient must weigh the benefits vs the risks to determine if buspirone is appropriate for any individual patient.
No adverse events have been reported from abrupt discontinuation of buspirone.17
Inexpensive. Buspirone is generic and extremely inexpensive. According to GoodRx.com, a 30-day supply of 5-mg tablets for twice-daily dosing can cost $4.18 A maximum daily dose (prescribed as 2 pills, 15 mg twice daily) may cost approximately $18/month.18 Thus, buspirone is a good option for uninsured or underinsured patients, for whom this would be more affordable than other anxiolytic medications.
Continue to: May offset certain adverse effects
May offset certain adverse effects. Sexual dysfunction is a common adverse effect of SSRIs. One strategy to offset this phenomenon is to add bupropion. However, in a randomized controlled trial, Landén et al19 found that sexual adverse effects induced by SSRIs were greatly mitigated by adding buspirone, even within the first week of treatment. This improvement was more marked in women than in men, which is helpful because sexual dysfunction in women is generally resistant to other interventions.20 Unlike
Unlikely to cause extrapyramidal symptoms (EPS). Because of its central D2 antagonism, buspirone has a low potential (<1%) to produce EPS. Buspirone has even been shown to reverse
The Table4 highlights key points to bear in mind when prescribing buspirone.
Challenges with buspirone
Response is not immediate. Unlike benzodiazepines, buspirone does not have an immediate onset of action.22 With buspirone monotherapy, response may be seen in approximately 2 to 4 weeks.23 Therefore, patients transitioning from a quick-onset benzodiazepine to buspirone may not report a good response. However, as noted above, when using buspirone to treat SSRI-induced sexual dysfunction, response may emerge within 1 week.19 Buspirone also lacks the euphoric and sedative qualities of benzodiazepines that patients may prefer.
Not for patients with hepatic and renal impairment. Because plasma levels of buspirone are elevated in patients with hepatic and renal impairment, this medication is not ideal for use in these populations.4
Continue to: Contraindicated in patients receiving MAOIs
Contraindicated in patients receiving MAOIs. Buspirone should not be prescribed to patients with depression who are receiving treatment with a monoamine oxidase inhibitor (MAOI) because the combination may precipitate a hypertensive reaction.4 A minimum washout period of 14 days from the MAOI is necessary before initiating buspirone.9
Idiosyncratic adverse effects. As with all pharmaceuticals, buspirone may produce idiosyncratic adverse effects. Faber and Sansone24 reported a case of a woman who experienced hair loss 3 months into treatment with buspirone. After cessation, her alopecia resolved.
Questionable efficacy for some anxiety subtypes. Buspirone has been studied as a treatment of other common psychiatric conditions, such as social phobia and anxiety in the setting of smoking cessation. However, it has not proven to be effective over placebo in treating these anxiety subtypes.25,26
Short half-life. Because of its relatively short half-life (2 to 3 hours), buspirone requires dosing 2 to 3 times a day, which could increase the risk of noncompliance.4 However, some patients might prefer multiple dosing throughout the day due to perceived better coverage of their anxiety symptoms.
Limited incentive for future research. Because buspirone is available only as a generic formulation, there is little financial incentive for pharmaceutical companies and other interested parties to study what may be valuable uses for buspirone. For example, there is no data available on comparative augmentation of buspirone and SGAs with antidepressants for depression and/or anxiety. There is also little data available about buspirone prescribing trends or why buspirone may be underutilized in clinical practice today.
Continue to: Unfortunately, historical and longitudinal...
Unfortunately, historical and longitudinal data on the prescribing practices of buspirone is limited because the original branded medication, BuSpar, is no longer on the market. However, this medication offers multiple advantages over other agents used to treat anxiety, and it should not be forgotten when formulating a treatment regimen for patients with anxiety and/or depression.
Bottom Line
Buspirone is a safe, low-cost, effective treatment option for patients with anxiety and may be helpful as an augmenting agent for depression. Because of its efficacy and high degree of tolerability, it should be prioritized higher in our treatment algorithms and be a part of our routine pharmacologic armamentarium.
Related Resources
- Howland RH. Buspirone: Back to the future. J Psychosoc Nurs Ment Health Serv. 2015;53(11):21-24.
- Strawn JR, Mills JA, Cornwall GJ, et al. Buspirone in children and adolescents with anxiety: a review and Bayesian analysis of abandoned randomized controlled trials. J Child Adolesc Psychopharmacol. 2018;28(1):2-9.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Citalopram • Celexa
Haloperidol • Haldol
Vilazodone • Viibryd
Vortioxetine • Trintellix
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
1. Fickweiler F, Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open. 2017;7(9):e016408. doi: 10.1136/bmjopen-2017-016408.
2. Haque M. Generic medicine and prescribing: a quick assessment. Adv Hum Biol. 2017;7(3):101-108.
3. National Alliance on Mental Illness. Anxiety disorders. https://www.nami.org/Learn-More/Mental-Health-Conditions/Anxiety-Disorders. Published December 2017. Accessed November 26, 2019.
4. Buspar [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2000.
5. Hjorth S, Carlsson A. Buspirone: effects on central monoaminergic transmission-possible relevance to animal experimental and clinical findings. Eur J Pharmacol. 1982:83;299-303.
6. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
7. Buspirone tablets [package insert]. East Brunswick, NJ: Strides Pharma Inc; 2017.
8. Lilja JJ, Kivistö KT, Backman, JT, et al. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655-660.
9. Stahl SM. Stahl’s essential psychopharmacology: prescriber’s guide, 6th ed. Cambridge, United Kingdom: Cambridge University Press; 2017.
10. Sumiyoshi T, Park S, Jayathilake K. Effect of buspirone, a serotonin1A partial agonist, on cognitive function in schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2007;95(1-3):158-168.
11. Schechter LE, Dawson LA, Harder JA. The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des. 2002;8(2):139-145.
12. Kranzler HR, Burleson JA, Del Boca FK. Buspirone treatment of anxious alcoholics: a placebo-controlled trial. Arch Gen Psychiatry. 1994;51(9):720-731.
13. Burton CA, Holmes J, Murray J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2011;12:1-25.
14. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001; 62(6):448-452.
15. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3rd edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Published May 2010. Accessed November 2019.
16. U.S. Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. https://www.fda.gov/drugs/labeling/pregnancy-and-lactation-labeling-drugs-final-rule. Published September 11, 2019. Accessed November 26, 2019.
17. Goa KL, Ward A. Buspirone. A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32(2):114-129.
18. GoodRx. Buspar prices, coupons, & savings tips in U.S. area code 08054. https://www.goodrx.com/buspar. Accessed June 6, 2019.
19. Landén M, Eriksson E, Agren H, et al. Effect of buspirone on sexual dysfunction in depressed patients treated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol. 1999;19(3):268-271.
20. Hensley PL, Nurnberg HG. SSRI sexual dysfunction: a female perspective. J Sex Marital Ther. 2002;28(suppl 1):143-153.
21. Haleem DJ, Samad N, Haleem MA. Reversal of haloperidol-induced extrapyramidal symptoms by buspirone: a time-related study. Behav Pharmacol. 2007;18(2):147-153.
22. Kaplan SS, Saddock BJ, Grebb JA. Synopsis of psychiatry. 11th ed. Philadelphia, PA: Wolters Kluwer; 2014.
23. National Alliance on Mental Health. Buspirone (BuSpar). https://www.nami.org/Learn-More/Treatment/Mental-Health-Medications/Types-of-Medication/Buspirone-(BuSpar). Published January 2019. Accessed November 26, 2019.
24. Faber J, Sansone RA. Buspirone: a possible cause of alopecia. Innov Clin Neurosci. 2013;10(1):13.
25. Van Vliet IM, Den Boer JA, Westenberg HGM, et al. Clinical effects of buspirone in social phobia, a double-blind placebo controlled study. J Clin Psychiatry. 1997;58(4):164-168.
26. Schneider NG, Olmstead RE, Steinberg C, et al. Efficacy of buspirone in smoking cessation: a placebo‐controlled trial. Clin Pharmacol Ther. 1996;60(5):568-575.
Top research findings of 2018-2019 for clinical practice
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of
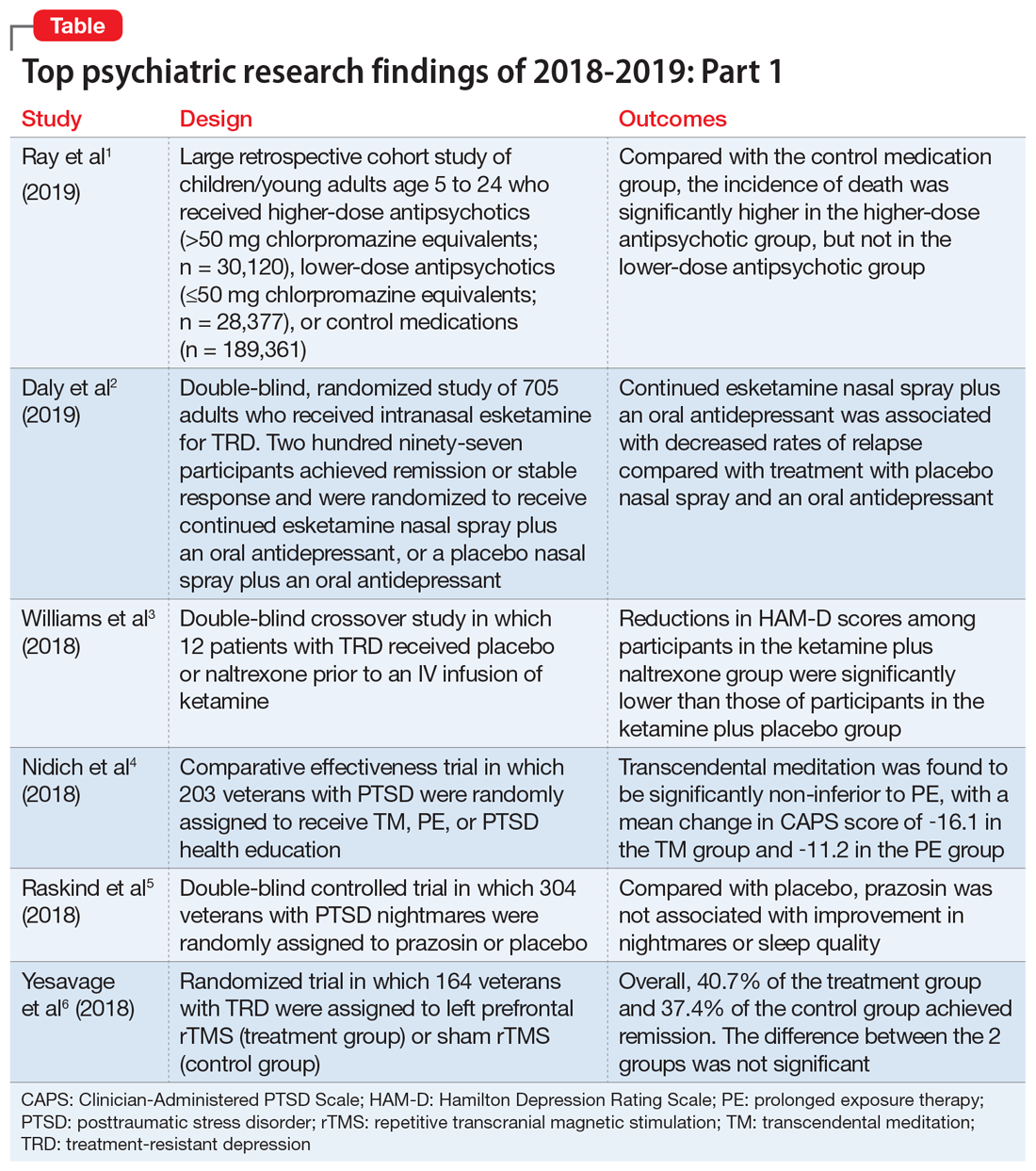
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of

1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
Medical knowledge is growing faster than ever, as is the challenge of keeping up with this ever-growing body of information. Clinicians need a system or method to help them sort and evaluate the quality of new information before they can apply it to clinical care. Without such a system, when facing an overload of information, most of us tend to take the first or the most easily accessed information, without considering the quality of such information. As a result, the use of poor-quality information affects the quality and outcome of care we provide, and costs billions of dollars annually in problems associated with underuse, overuse, and misuse of treatments.
In an effort to sort and evaluate recently published research that is ready for clinical use, the first author (SAS) used the following 3-step methodology:
1. Searched literature for research findings suggesting readiness for clinical utilization published between July 1, 2018 and June 30, 2019.
2. Surveyed members of the American Association of Chairs of Departments of Psychiatry, the American Association of Community Psychiatrists, the American Association of Psychiatric Administrators, the North Carolina Psychiatric Association, the Group for the Advancement of Psychiatry, and many other colleagues by asking them: “Among the articles published from July 1, 2018 to June 30, 2019, which ones in your opinion have (or are likely to have or should have) affected/changed the clinical practice of psychiatry?”
3. Looked for appraisals in post-publication reviews such as NEJM Journal Watch, F1000 Prime, Evidence-Based Mental Health, commentaries in peer-reviewed journals, and other sources (see Related Resources).
We chose 12 articles based on their clinical relevance/applicability. Here in Part 1 we present brief descriptions of the 6 of top 12 papers chosen by this methodology; these studies are summarized in the Table.1-6 The order in which they appear in this article is arbitrary. The remaining 6 studies will be reviewed in Part 2 in the February 2020 issue of

1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
Children and young adults are increasingly being prescribed antipsychotic medications. Studies have suggested that when these medications are used in adults and older patients, they are associated with an increased risk of death.7-9 Whether or not these medications are associated with an increased risk of death in children and youth has been unknown. Ray et al1 compared the risk of unexpected death among children and youths who were beginning treatment with an antipsychotic or control medications.
Study design
- This retrospective cohort study evaluated children and young adults age 5 to 24 who were enrolled in Medicaid in Tennessee between 1999 and 2014.
- New antipsychotic use at both a higher dose (>50 mg chlorpromazine equivalents) and a lower dose (≤50 mg chlorpromazine equivalents) was compared with new use of a control medication, including attention-deficit/hyperactivity disorder medications, antidepressants, and mood stabilizers.
- There were 189,361 participants in the control group, 28,377 participants in the lower-dose antipsychotic group, and 30,120 participants in the higher-dose antipsychotic group.
Outcomes
- The primary outcome was death due to injury or suicide or unexpected death occurring during study follow-up.
- The incidence of death in the higher-dose antipsychotic group (146.2 per 100,000 person-years) was significantly higher (P < .001) than the incidence of death in the control medications group (54.5 per 100,000 person years).
- There was no similar significant difference between the lower-dose antipsychotic group and the control medications group.
Continue to: Conclusion
Conclusion
- Higher-dose antipsychotic use is associated with increased rates of unexpected deaths in children and young adults.
- As with all association studies, no direct line connected cause and effect. However, these results reinforce recommendations for careful prescribing and monitoring of antipsychotic regimens for children and youths, and the need for larger antipsychotic safety studies in this population.
- Examining risks associated with specific antipsychotics will require larger datasets, but will be critical for our understanding of the risks and benefits.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
Controlled studies have shown esketamine has efficacy for treatment-resistant depression (TRD), but these studies have been only short-term, and the long-term effects of esketamine for TRD have not been established. To fill that gap, Daly et al2 assessed the efficacy of esketamine nasal spray plus an oral antidepressant vs a placebo nasal spray plus an oral antidepressant in delaying relapse of depressive symptoms in patients with TRD. All patients were in stable remission after an optimization course of esketamine nasal spray plus an oral antidepressant.
Study design
- Between October 2015 and February 2018, researchers conducted a phase III, multicenter, double-blind, randomized withdrawal study to evaluate the effect of continuation of esketamine on rates of relapse in patients with TRD who had responded to initial treatment with esketamine.
- Initially, 705 adults were enrolled. Of these participants, 455 proceeded to the optimization phase, in which they were treated with esketamine nasal spray plus an oral antidepressant.
- After 16 weeks of optimization treatment, 297 participants achieved remission or stable response and were randomized to a treatment group, which received continued esketamine nasal spray plus an oral antidepressant, or to a control group, which received a placebo nasal spray plus an oral antidepressant.
Outcomes
- Treatment with esketamine nasal spray and an oral antidepressant was associated with decreased rates of relapse compared with treatment with placebo nasal spray and an oral antidepressant. This was the case among patients who had achieved remission as well as those who had achieved stable response.
- Continued treatment with esketamine decreased the risk of relapse by 51%, with 40 participants in the treatment group experiencing relapse compared with 73 participants in the placebo group.
Continue to: Conclusion
Conclusion
- In patients with TRD who responded to initial treatment with esketamine, continuing esketamine plus an oral antidepressant resulted in clinically meaningful superiority in preventing relapse compared with a placebo nasal spray plus an oral antidepressant.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
Many studies have documented the efficacy of ketamine as a rapid-onset antidepressant. Studies investigating the mechanism of this effect have focused on antagonism of N-methyl-
Study design
- This double-blind crossover study evaluated if opioid receptor activation is necessary for ketamine to have an antidepressant effect in patients with TRD.
- Twelve participants completed both sides of the study in a randomized order. Participants received placebo or naltrexone prior to an IV infusion of ketamine.
- Researchers measured patients’ scores on the Hamilton Depression Rating Scale (HAM-D) at baseline and 1 day after infusion. Response was defined as a ≥50% reduction in HAM-D score.
Outcomes
- Reductions in HAM-D scores among participants in the ketamine plus naltrexone group were significantly lower than those of participants in the ketamine plus placebo group.
- Dissociation related to ketamine use did not differ significantly between the naltrexone group and the placebo group.
Continue to: Conclusion
Conclusion
- This small study found a significant decrease in the antidepressant effect of ketamine infusion in patients with TRD when opioid receptors are blocked with naltrexone prior to infusion, which suggests opioid receptor activation is necessary for ketamine to be effective as an antidepressant.
- This appears to be consistent with observations of buprenorphine’s antidepressant effects. Caution is indicated until additional studies can further elucidate the mechanism of action of ketamine’s antidepressant effects (see "Ketamine/esketamine: Putative mechanism of action," page 32).
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomised controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
Posttraumatic stress disorder (PTSD) is a common and important public health problem. Evidence-based treatments for PTSD include trauma-focused therapies such as prolonged exposure therapy (PE). However, some patients may not respond to PE, drop out, or elect not to pursue it. Researchers continue to explore treatments that are non-trauma-focused, such as mindfulness meditation and interpersonal psychotherapy. In a 3-group comparative effectiveness trial, Nidich et al4 examined the efficacy of a non-trauma-focused intervention, transcendental meditation (TM), in reducing PTSD symptom severity and depression in veterans.
Study design
- Researchers recruited 203 veterans with PTSD from the Department of Veterans Affairs (VA) San Diego Healthcare System between June 2013 and October 2016.
- Participants were randomly assigned to 1 of 3 groups: 68 to TM, 68 to PE, and 67 to PTSD health education (HE).
- Each group received 12 sessions over 12 weeks. In addition to group and individual sessions, all participants received daily practice or assignments.
- The Clinician-Administered PTSD Scale (CAPS) was used to assess symptoms before and after treatment.
Outcomes
- The primary outcome assessed was change in PTSD symptom severity at the end of the study compared with baseline as measured by change in CAPS score.
- Transcendental meditation was found to be significantly non-inferior to PE, with a mean change in CAPS score of −16.1 in the TM group and −11.2 in the PE group.
- Both the TM and PE groups also had significant reductions in CAPS scores compared with the HE group, which had a mean change in CAPS score of −2.5.
Continue to: Conclusion
Conclusion
- Transcendental meditation is significantly not inferior to PE in the treatment of veterans with PTSD.
- The findings from this first comparative effectiveness trial comparing TM with an established psychotherapy for PTSD suggests the feasibility and efficacy of TM as an alternative therapy for veterans with PTSD.
- Because TM is self-administered after an initial expert training, it may offer an easy-to-implement approach that may be more accessible to veterans than other treatments.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
Several smaller randomized trials of prazosin involving a total of 283 active-duty service members, veterans, and civilian participants have shown efficacy of prazosin for PTSD-related nightmares, sleep disturbance, and overall clinical functioning. However, in a recent trial, Raskind et al5 failed to demonstrate such efficacy.
Study design
- Veterans with chronic PTSD nightmares were recruited from 13 VA medical centers to participate in a 26-week, double-blind, randomized controlled trial.
- A total of 304 participants were randomized to a prazosin treatment group (n = 152) or a placebo control group (n = 152).
- During the first 10 weeks, prazosin or placebo were administered in an escalating fashion up to a maximum dose.
- The CAPS, Pittsburgh Sleep Quality Index (PSQI), and Clinical Global Impressions of Change (CGIC) scores were measured at baseline, after 10 weeks, and after 26 weeks.
Outcomes
- Three primary outcomes measures were assessed: change in score from baseline to 10 weeks on CAPS item B2, the PSQI, and the CGIC.
- A secondary measure was change in score from baseline of the same measures at 26 weeks.
- There was no significant difference between the prazosin group and the placebo group in any of the primary or secondary measures.
Continue to: Conclusion
Conclusion
- Compared with placebo, prazosin was not associated with improvement in nightmares or sleep quality for veterans with chronic PTSD nightmares.
- Because psychosocial instability was an exclusion criterion, it is possible that a selection bias resulting from recruitment of patients who were mainly in clinically stable condition accounted for these negative results, since symptoms in such patients were less likely to be ameliorated with antiadrenergic treatment.
Treatment-resistant depression in veterans is a major clinical challenge because of these patients’ increased risk of suicide. Repetitive transcranial magnetic stimulation (rTMS) has shown promising results for TRD. In a randomized trial, Yesavage et al6 compared rTMS vs sham rTMS in veterans with TRD.
Study design
- Veterans with TRD were recruited from 9 VA medical centers throughout the United States between September 2012 and May 2016.
- Researchers randomized 164 participants into 1 of 2 groups in a double-blind fashion. The treatment group (n = 81) received left prefrontal rTMS, and the control group (n = 83) received sham rTMS.
Outcomes
- In an intention-to-treat analysis, remission rate (defined as a HAM-D score of ≤10) was assessed as the primary outcome measure.
- Remission was seen in both groups, with 40.7% of the treatment group achieving remission and 37.4% of the control group achieving remission. However, the difference between the 2 groups was not significant (P = .67), with an odds ratio of 1.16.
Continue to: Conclusion
Conclusion
- In this study, treatment with rTMS did not show a statistically significant difference in rates of remission from TRD in veterans compared with sham rTMS. This differs from previous rTMS trials in non-veteran patients.
- The findings of this study also differed from those of other rTMS research in terms of the high remission rates that were seen in both the active and sham groups.
Bottom Line
The risk of death might be increased in children and young adults who receive highdose antipsychotics. Continued treatment with intranasal esketamine may help prevent relapse in patients with treatment-resistant depression (TRD) who initially respond to esketamine. The antidepressant effects of ketamine might be associated with opioid receptor activation. Transcendental meditation may be helpful for patients with posttraumatic stress disorder (PTSD), while prazosin might not improve nightmares or sleep quality in patients with PTSD. Repetitive transcranial magnetic stimulation (rTMS) might not be any more effective than sham rTMS for veterans with TRD.
Related Resources
- NEJM Journal Watch. www.jwatch.org.
- F1000 Prime. https://f1000.com/prime/home.
- BMJ Journals Evidence-Based Mental Health. https://ebmh.bmj.com.
Drug Brand Names
Buprenorphine • Subutex
Chlorpromazine • Thorazine
Esketamine nasal spray • Spravato
Ketamine • Ketalar
Naltrexone • Narcan
Prazosin • Minipress
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.
1. Ray WA, Stein CM, Murray KT, et al. Association of antipsychotic treatment with risk of unexpected death among children and youths. JAMA Psychiatry. 2019;76(2):162-171.
2. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76(9):893-903.
3. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
4. Nidich S, Mills PJ, Rainforth M, et al. Non-trauma-focused meditation versus exposure therapy in veterans with post-traumatic stress disorder: a randomized controlled trial. Lancet Psychiatry. 2018;5(12):975-986.
5. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
6. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884-893.
7. Ray WA, Meredith S, Thapa PB, et al. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58(12):1161-1167.
8. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
9. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970.