User login
Complex Ankle and Hindfoot Arthrodesis Using Circular External Fixation
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
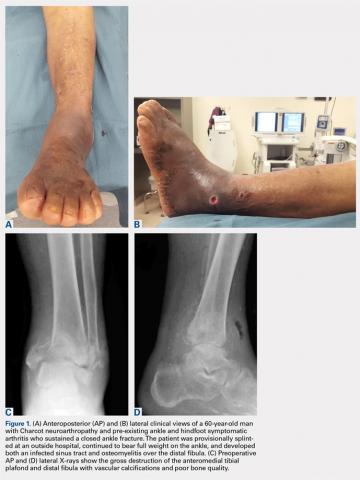
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
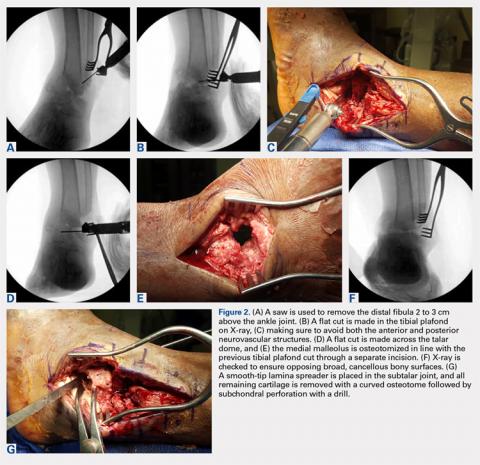
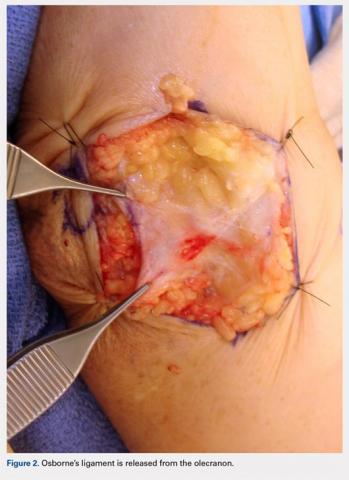
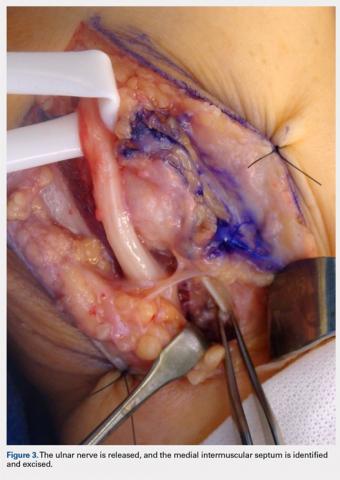
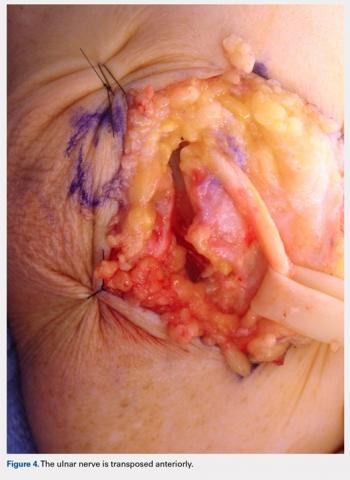
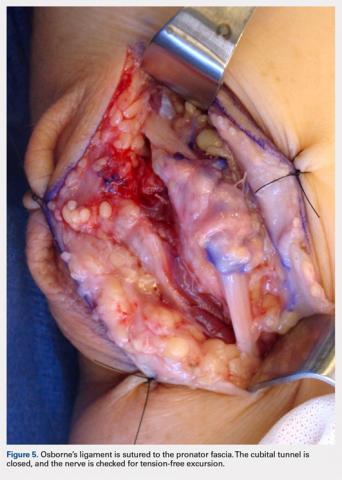
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
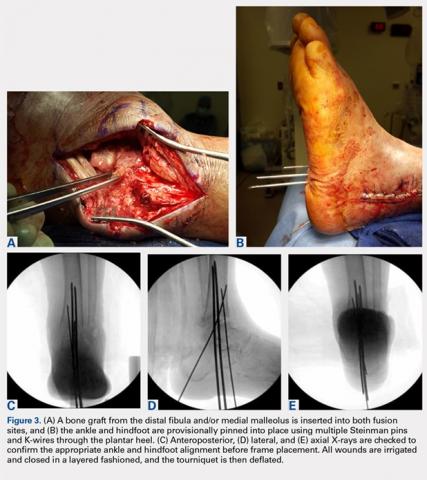
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
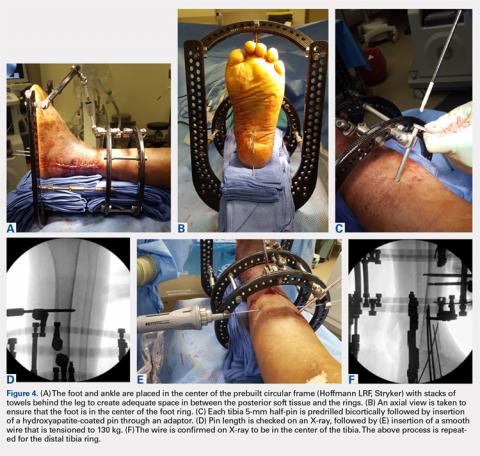
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
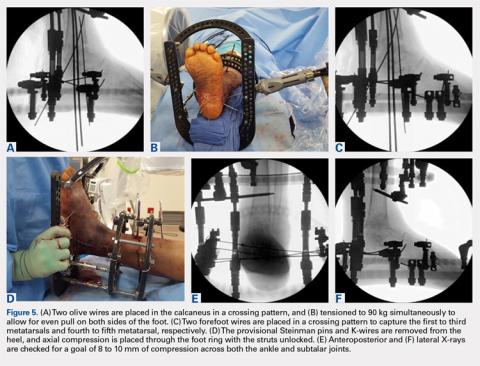
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
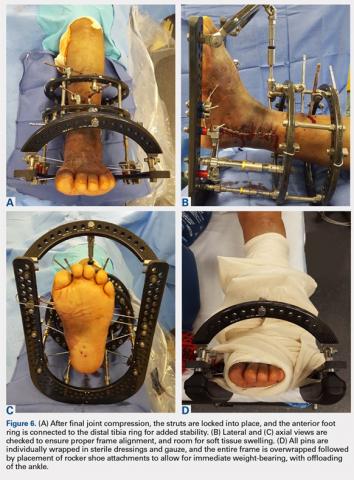
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
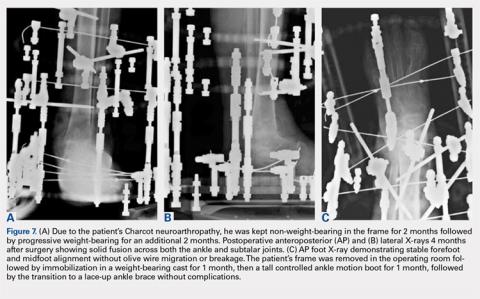
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
ABSTRACT
Surgical reconstruction of the ankle and hindfoot in patients with diabetes, Charcot neuroarthropathy, osteomyelitis, deformity, and/or bone loss can be challenging and often results in amputation. In these patients, conventional internal fixation with plates, screws, and intramedullary nails is often not feasible because of ongoing infection or poor bone stock and soft tissue quality. The Ilizarov method of ankle and hindfoot arthrodesis is a well-established technique for limb reconstruction that uses circular external fixation to achieve solid bony fusion, optimal leg length, and eradication of infection in cases of complex pathology. This article discusses indications, contraindications, pearls, and pitfalls of performing ankle and hindfoot arthrodesis using the Ilizarov technique.
Continue to: Patients with complex ankle and hindfoot deformity...
Patients with complex ankle and hindfoot deformity present a unique challenge to both nonoperative management and surgical reconstruction. Nonoperative management focuses on wound care, bracing, and immobilization using ankle-foot orthoses, total contact casts, and Charcot restraint orthotic walker boots for external stabilization. Fusion using the Ilizarov technique with circular fixation is a salvage limb-preservation procedure that has shown good results in select patient populations.1-5 Indications include post-traumatic, degenerative, and rheumatoid arthritis, osteomyelitis, tumors, neuromuscular conditions, and salvage of failed ankle and hindfoot procedures.6-9 Relative contraindications include wet gangrene, severe limb ischemia, and soft tissue compromise requiring urgent amputation. In addition, circular frames are not recommended in patients who are unable to comply with postoperative restrictions, and pin and wire care for the duration of frame placement because of personal, psychological, or socioeconomic reasons.
The Ilizarov technique of ring fixation provides dynamic, modular, and rigid fixation in multiple planes to control shear, bending, and rotational forces, and allows for early weight-bearing and postoperative adjustments as needed.10,11 Percutaneously placed half-pins and wires allow for solid fixation in the setting of both poor bone and soft tissue quality, and fusion can be achieved in the presence of active infection in a 1-stage procedure. The goal of ankle and hindfoot fusion using the Ilizarov technique is to achieve an infection-free, stable, plantigrade foot with neutral ankle alignment to allow for patient ambulation and return to activities of daily living.
Nonunion rates with circular fixation are reported to be as high as 16% to 54%, due to medical comorbidities, such as smoking, peripheral vascular disease, and Charcot neuroarthropathy.1Charcot, in particular, is a risk factor for nonunion as patients lack protective sensation, and have a higher rate of wound dehiscence, noncompliance with weight-bearing precautions, pin site infections, and frame breakage. In these patients, tibiotalocalcaneal (TTC) arthrodesis is preferred over the isolated ankle, or subtalar fusion to both provide a stable platform for ambulation and reduce the incidence of adjacent joint breakdown. Common complications of the Ilizarov technique include pin site infections, wire breakage, talar necrosis, and tibial stress fractures after frame removal.1,2,6,11-13 Circular frames are typically maintained for 3 to 8 months, until solid fusion is achieved radiographically. Frames are removed in the operating room with the concurrent examination of the fusion sites under anesthesia followed by a period of protected weight-bearing in a cast or tall controlled ankle motion (CAM) boot.
This article reviews several technical details, tips, and tricks that can help improve the intraoperative and postoperative outcomes of combined ankle and hindfoot arthrodesis using the Ilizarov technique with circular external fixation.
Continue to: SURGICAL TECHNIQUE...
SURGICAL TECHNIQUE
SETUP AND APPROACH
Patients are positioned supine with padding under the operative extremity to achieve neutral leg rotation (Figures 1A-1D). A thigh tourniquet is placed with the foot positioned at the end of the bed and on top of the radiolucent padding to avoid interference of the contralateral leg during lateral X-rays. After sterile prepping and draping, the extremity is exsanguinated above the level of an active infection, and the tourniquet inflated.
For isolated ankle arthrodesis, an anterior or lateral approach can be used, while for TTC arthrodesis, a lateral approach is required to access both the ankle and subtalar joints. A 10-cm longitudinal incision is made along the distal fibula, curving slightly and anteriorly along the distal extent of the incision. Dissection is continued down to bone using full thickness flaps, and the distal fibula is removed 2 to 3 cm above the ankle joint using a saw and osteotome (Figures 2A-2G). The distal fibula can be used subsequently as bone grafts depending on the quality of bone. The peroneal tendons are retracted posteriorly, and dissection is then continued to the posterior facet of the subtalar joint.
JOINT PREPARATION AND ALIGNMENT
Both the anterior and posterior neurovascular bundles are protected along the distal tibia with Hohmann retractors while a saw is used to create flat cuts across the tibial plafond and talus to allow apposition of flat, broad cancellous bony surfaces. Flat cuts followed by later joint compression will often shorten the limb by 2 to 3 cm. This leg length discrepancy can later be accommodated using a shoe lift, as needed. All retained hardware and/or infected and necrotic tissues in the ankle and hindfoot are removed using a rongeur and a pituitary rongeur.
The medial malleolus is osteotomized vertically using a direct medial incision and approach with full thickness flaps, and in line with the previous tibial plafond, is both cut and removed. The medial malleolus can also be used for bone grafts in fusion sites. A smooth-tip lamina spreader is placed in the subtalar joint for distraction and a curved osteotome, curettes, and a small rongeur are used to remove all remaining cartilage from the subtalar joint. Flat cuts in the subtalar joint can remove excessive bone, particularly from the inferior aspect of the talus. The subchondral bone is perforated using a 2.5- to 3.0-mm drill bit and a curved osteotome.
A bone graft from the distal fibula and medial malleolus, with or without the addition of allograft adjuvants, is placed evenly across the ankle and subtalar joints (Figures 3A-3E). At this point, the ankle and subtalar joints can be manipulated in multiple planes to achieve neutral coronal, sagittal, and axial alignment. With both the ankle and hindfoot held in a neutral position, multiple Steinman pins and K-wires in different orientations are inserted through the plantar aspect of the heel to hold the ankle and subtalar joints in place temporarily. Wires are cut short to prevent interference with subsequent foot olive wire placement through the frame.
Continue to: X-rays should be carefully checked...
X-rays should be carefully checked to ensure proper alignment. Wounds are gently irrigated, and vancomycin powder (2 g) can be placed within wounds for local antibiotic delivery. Lateral tissues are sharply debulked to allow for decreased tension on the incision, and small ulcers can be excised in their entirety. Wounds are closed in a layered fashion using 0-polydioxanone (PDS, Ethicon) suture for deep tissue, 2-0 PDS for subcutaneous tissue, and 2-0 nylon for skin closure. The tourniquet is deflated for the remainder of the case to reduce limb ischemia during frame placement.
CIRCULAR FRAME CONCEPTS AND PLACEMENT
The majority of circular frames for both ankle and hindfoot fusion have multiple ring sizes available in aluminum and radiolucent carbon fiber reinforced polymer (Hoffmann LRF, Stryker). Rings are available in full, open, segment, and both short- and long-foot options. Frames can be sterilized in a prebuilt 3 to 4 ring construct with 4 static or dynamic (telescopic) struts (100-277 mm). The most commonly used tibia and foot ring sizes are 155 cm, 180 cm, and 210 cm. Ring size should be able to accommodate posterior soft tissue swelling and avoid circumferential soft tissue abrasion against the rings. Anterior foot arches are used for increased construct stability and can be locked to the distal tibia ring for weight-bearing support. Wire and half-pin bolts, adaptors, and nuts are used to join each ring of the frame to the patient’s bone.
For TTC arthrodesis, 2 rings are typically used in the tibia, and 1 ring is used in the foot. For isolated ankle arthrodesis, an additional ring can be added with olive wires in the talus to permit compression only across the ankle joint. Multiple points of fixation are used in each ring in different planes to achieve both maximal stability and rotational control. If a single wire or half-pin becomes infected and requires removal, there are still multiple other points of fixation in the ring to maintain stability. Fixation within each ring should be off axis compared with the adjacent ring to both avoid stress risers and increase construct rigidity.
The prebuilt frame is checked on the back table to ensure proper orientation and component alignment. The frame is then placed over both the foot and ankle, and multiple stacks of towels are placed behind the heel, ankle, and calf to center the foot and ankle in the frame (Figures 4A-4F). At least 4 to 6 cm of space is needed in between the posterior soft tissues and each ring to accommodate postoperative swelling. On the lateral view, the foot ring should be in the mid-portion of the calcaneus. If there is a concern, particularly in Charcot patients, regarding early weight-bearing noncompliance, the foot ring can be placed flush with the plantar aspect of the foot, and olive wires can be inserted using longer adaptors. The frame should be checked from multiple viewpoints to ensure that both the foot and ankle are centered and in neutral rotation.
Continue to: TIBIA RING FIXATION...
TIBIA RING FIXATION
Tibia rings can be fixed using 2 to 3 half-pins (4-6 mm) alone or 2 half-pins in combination with a smooth wire. A small incision is made over the area of planned half-pin insertion, and the periosteum is cleared away using a hemostat. An adaptor sleeve is used, and the bone is drilled bicortically, followed by insertion of the half-pin. Hydroxyapatite-coated pins are used to improve the strength of the bone-pin interface and reduce the incidence of pin tract infections. Pins are inserted along both the anterior and medial aspects of the tibia, avoiding the thick lateral musculature. Care is taken to protect the medial neurovascular structures during pin placement following established Ilizarov safe zones.
After each pin is placed in the bone, the pin is secured to the adaptor that is then tightened to the ring. This process is repeated for both the proximal and distal tibia rings. Pins should be placed above and below each ring to avoid creating stress risers. During smooth wire placement, each wire is pushed by hand through the soft tissues and then drilled into the bone while the exposed segment is held with a damp sponge to reduce the incidence of thermal bone necrosis. Once the wire is drilled bicortically, a mallet is used to tap the wire through the remaining soft tissues to avoid wrapping them up in the wire. Each wire should be parallel to the ring to get an even line of compression.
Each wire is secured on 1 end and then tensioned to 130 kg using a hand tensioner. An additional tool can be placed in the wire adaptor to prevent the wire from bending during tensioning. If the wire is passing above or below the ring, longer wire adaptors should be used to build to the wire. The wire should never be bent toward the ring as this can increase the likelihood of improper pin tensioning and breakage. Wire placement should be avoided posteriorly as this can make it difficult to secure and/or tension wires, and also increases the risk of damage to posterior structures.
Ring fixation in the distal tibia near the plafond may require 1 half-pin and 2 wires to avoid damage to the tibialis anterior and posterior tibial tendons. In this case, smooth wires should be placed in a crossing pattern and tensioned simultaneously to avoid pulling the ankle away from the center of the frame. Wires should be bent and curved over each ring and then cut to facilitate subsequent removal.
FOOT RING FIXATION
In the foot, olive wires are used to increase fixation against bone. For each olive wire, a small incision is made to accommodate the diameter of the olive through the soft tissue. Similar to the distal tibia, 2 olive wires should be placed above and below the foot ring in a crossing pattern through the calcaneus (Figures 5A-5F). The axial view of the frame should be checked to ensure proper wire orientation. When using olive wires, it is essential to tension both at the same time to 90 kg, as the foot can be pulled medially or laterally in the frame if 1 wire is tensioned before the other.
Forefoot olive wires should also be placed in a crossing pattern, with 1 wire fixed through the first, second, and third metatarsals, and 1 wire through the fourth and fifth metatarsals. Additional forefoot olive wires can be placed if compression is needed across the midfoot or Chopart joints for fusion. Multiple X-rays should be checked to ensure that the calcaneus and forefoot olive wires are firmly fixed both in and against bone.
Continue to: JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS...
JOINT COMPRESSION AND FINAL FRAME ADJUSTMENTS
Once all rings are secured to the bone with half-pins and wires, the previously placed Steinman pins, and K-wires through the heel are removed. Both ankle and subtalar joint alignments are rechecked, and then axial compression is placed through the foot ring with the knee extended and the struts unlocked. Static or telescopic struts are used to achieve 8 to 10 mm of bony compression. X-rays are taken before and after to analyze final joint compression and alignment. Struts should be sequentially tightened (1/2 turn of a static strut) 1 at a time as final tightening of 1 strut alone can bind and interfere with both the compression and tightening of the remaining struts.
Once final compression is achieved, the struts are locked, and the front foot arch is closed anteriorly and connected to the distal tibia ring for increased stability (Figures 6A-6D). Each pin and wire is covered in a sterile dressing followed by gauze to allow for soft tissue padding. The entire frame is then overwrapped in bias stockinette rolls or ace wraps.
Walking attachments can be added immediately to the frame that allows for early weight-bearing. Rocker shoe attachments with a 15° anterior and posterior slope and rubber soles can help offload the ankle and subtalar joints, decrease pressure on heel strike, and reduce ankle motion during ambulation (Hoffmann LRF, Stryker).
POSTOPERATIVE PROTOCOL
Depending on individual characteristics, patients can be immediately weight-bearing in the circular frame. Patients with Charcot neuroarthropathy are recommended to remain non-weight-bearing for the first 2 months to reduce the likelihood of pin, wire, and frame breakage along with nonunion. Pin and wire site care and maintenance are initiated the day after surgery and continue on a daily basis for the duration of frame placement. Sutures are removed 4 to 5 weeks after surgery to ensure adequate wound healing. Serial X-rays are taken monthly to analyze fusion sites.
If pins or wires become infected, patients are placed on oral antibiotics, and both pins and wires can be removed or exchanged in the operating room. Once fusion is achieved in 3 to 8 months (Figures 7A-7C), the frame is removed in the operating room, and fusion sites are examined under dynamic fluoroscopy. If fusion is confirmed, patients are made weight-bearing as tolerated in a short-leg cast or tall CAM boot for 6 to 8 weeks, and then transitioned to an ankle brace in an accommodative shoe.
Continue to: DISCUSSION...
DISCUSSION
A key aspect of recovery after ankle and hindfoot fusion using the Ilizarov technique is balancing pin care, soft tissue swelling, and weight-bearing status. The average time patients will spend in the frame is approximately 25 to 28 weeks, but can range from 12 to 84 weeks.1,2Given the considerable variability in both soft tissue healing and bony union, patients should be extensively counseled before surgery to set expectations correctly and ensure that they have the necessary help and support to care for the frame during the treatment period. Patients should be followed closely during the first 6 weeks to ensure that pins and wires do not become infected or break, as both of these issues require immediate intervention.
In a review of 11 patients who underwent tibiocalcaneal arthrodesis using an Ilizarov external fixator for infected talar nonunions or extrusions, Rochman and colleagues8 reported an 81% rate of successful fusion with a final mean American Orthopaedic Foot and Ankle Society score of 65 (out of a maximum 86). Similar results were reported by Saltzman9 in a series of 8 patients with diffuse ankle osteomyelitis treated with resection of all infected tissue and hybrid-frame compression arthrodesis. All patients received 6 weeks of intravenous antibiotics, and frames were removed at 3 months, and walking casts were applied for 1 to 2 additional months. Ankle sepsis was eradicated in all patients, and 7/8 (87.5%) ankles successfully fused at an average of 13.5 weeks (range, 10-16 weeks). One limb required below-knee amputation at 5 weeks due to non-reconstructible vascular insufficiency. At an average of 3.4-year follow-up, none of the 7 fused ankles required further surgery.
Fragomen and colleagues1 retrospectively reviewed 101 patients who underwent complex ankle fusion using the Ilizarov technique and found that 76/91 (83.5%) patients achieved fusion at an average of 25 weeks (range, 10-65 weeks). Smoking was associated with a 54% rate of nonunion and 15/19 (79%) patients with Charcot neuroarthropathy achieved ankle fusion, but had a subsequent subtalar joint failure, thus highlighting the need for TTC arthrodesis in Charcot patients. Salem and colleagues2 reviewed 21 Ilizarov ankle fusions and reported that all patients achieved fusion at an average of 28 weeks (range, 12-84 weeks). Complications occurred in 11 patients, including 2 nonunions that healed after revision frame application and 4 pin tract infections.
CONCLUSION
Overall, the Ilizarov technique using circular external fixation is a powerful tool that can be used to treat a variety of disorders including complex foot and ankle deformity and infection. While case series generally show favorable outcomes, patients must be informed that this technique is a salvage procedure for limb preservation that requires meticulous operative technique, diligent postoperative care, and tight control of medical comorbidities, such as blood sugar levels in individuals with diabetes to achieve a successful outcome.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
1. Fragomen AT, Borst E, Schachter L, Lyman S, Rozbruch SR. Complex ankle arthrodesis using the Ilizarov method yields high rate of fusion. Clin Orthop Relat Res. 2012;470(10):2864-2873. doi:10.1007/s11999-012-2470-9.
2. Salem KH, Kinzl L, Schmelz A. Ankle arthrodesis using Ilizarov ring fixators: a review of 22 cases. Foot Ankle Int. 2006;27(10):764-770. doi:10.1177/107110070602701002.
3. Cierny G 3rd, Cook WG, Mader JT. Ankle arthrodesis in the presence of ongoing sepsis. Indications, methods, and results. Orthop Clin North Am. 1989;20(4):709-721.
4. Dalla Paola L, Brocco E, Ceccacci T, et al. Limb salvage in Charcot foot and ankle osteomyelitis: combined use single stage/double stage of arthrodesis and external fixation. Foot Ankle Int. 2009;30(11):1065-1070. doi:10.3113/FAI.2009.1065.
5. Eylon S, Porat S, Bor N, Leibner ED. Outcome of Ilizarov ankle arthrodesis. Foot Ankle Int. 2007;28(8):873-879. doi:10.3113/FAI.2007.0873.
6. Kalish S, Fleming J, Weinstein R. External fixators for elective rearfoot and ankle arthrodesis. Techniques and indications. Clin Podiatr Med Surg. 2003;20(1):65-96, vi.
7. Kollig E, Esenwein SA, Muhr G, Kutscha-Lissberg F. Fusion of the septic ankle: experience with 15 cases using hybrid external fixation. J Trauma. 2003;55(4):685-691. doi:10.1097/01.TA.0000051933.83342.E4.
8. Rochman R, Jackson Hutson J, Alade O. Tibiocalcaneal arthrodesis using the Ilizarov technique in the presence of bone loss and infection of the talus. Foot Ankle Int. 2008;29(10):1001-1008. doi:10.3113/FAI.2008.1001.
9. Saltzman CL. Salvage of diffuse ankle osteomyelitis by single-stage resection and circumferential frame compression arthrodesis. Iowa Orthop J. 2005;2547-52.
10. Fragomen AT, Rozbruch SR. The mechanics of external fixation. HSS J. 2007;3(1):13-29. doi:10.1007/s11420-006-9025-0.
11. Hawkins BJ, Langerman RJ, Anger DM, Calhoun JH. The Ilizarov technique in ankle fusion. Clin Orthop Relat Res. 1994;(303):217-225.
12. Jones CP, Youngblood CS, Waldrop N, Davis WH, Pinzur MS. Tibial Stress Fracture Secondary to Half-Pins in Circular Ring External Fixation for Charcot Foot. Foot Ankle Int. 2014;35(6):572-577. doi:10.1177/1071100714531229.
13. Kazmers NH, Fragomen AT, Rozbruch SR. Prevention of pin site infection in external fixation: a review of the literature. Strategies Trauma Limb Reconstr. 2016;11(2):75-85. doi:10.1007/s11751-016-0256-4.
TAKE-HOME POINTS
- Ankle and hindfoot fusion using circular external fixation is a useful surgical technique in patients with diabetes, Charcot, osteomyelitis, deformity, and/or bone and soft tissue compromise in order to obtain solid bony fusion, stable limb alignment, and eradication of infection in cases of complex pathology.
- Deformity correction with osteotomies and meticulous joint preparation is required in order to obtain broad, cancellous bony surfaces for fusion with neutral alignment. Autograft from the distal fibula and/or medial malleolus can be combined with bone allograft to assist with joint fusion.
- The ankle and hindfoot are provisionally pinned into neutral coronal and sagittal alignment through the plantar surface of the foot using large K-wires prior to placement of the lower leg in the center of a circular 3-ring compression frame. Typically, 2 to 3 points of fixation are used per ring with a combination of half-pins and smooth wires.
- Ring attachments are built up or down to the level of the half-pins and wires in order to prevent pins and wires from bending, breaking, or causing iatrogenic deformity during tensioning. Crossing olive wires are used in the midfoot and calcaneus with dual tensioning devices to ensure an even pull on both sides of the foot.
- Dynamic or static compression struts are used to obtain 8 to 10 mm of compression across the ankle and hindfoot, followed by addition of an anterior foot ring to increase construct rigidity. Daily pin care is started 3 to 4 days after surgery and patients are kept non-weight-bearing for approximately 2 months in the frame with a total frame period of 3 to 8 months depending on bony healing on X-ray.
The Flint Lock: A Novel Technique in Total Knee Arthroplasty Closure
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.
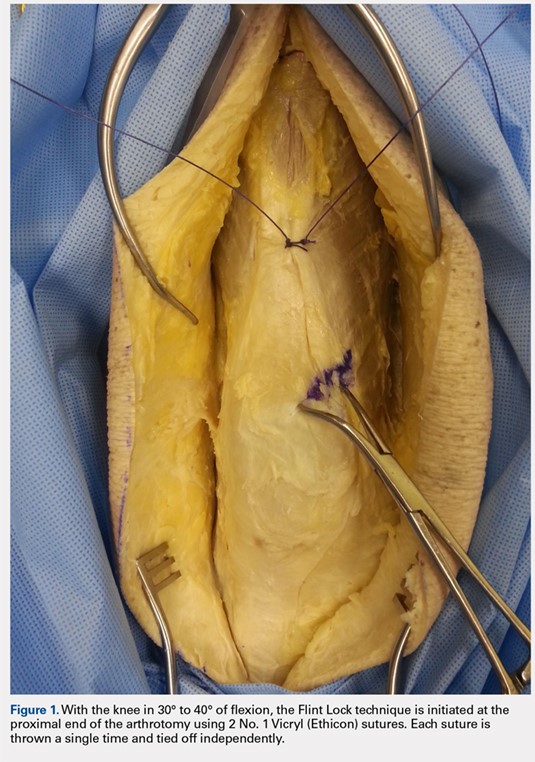
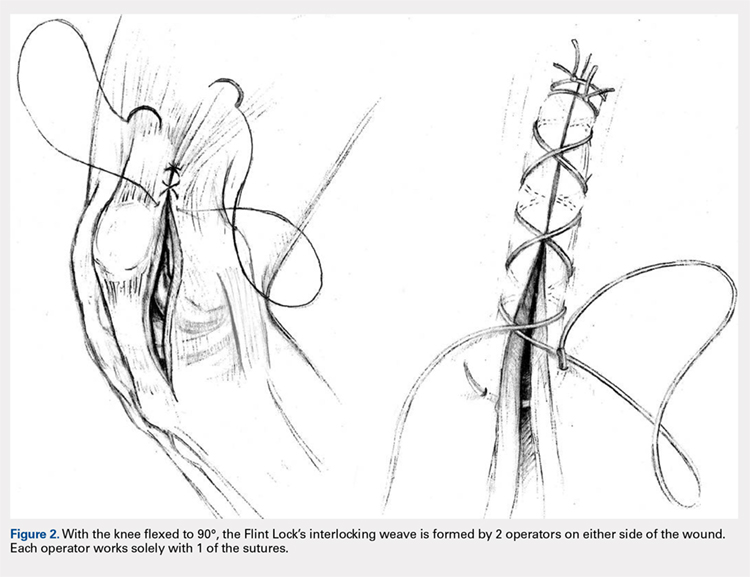
The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.
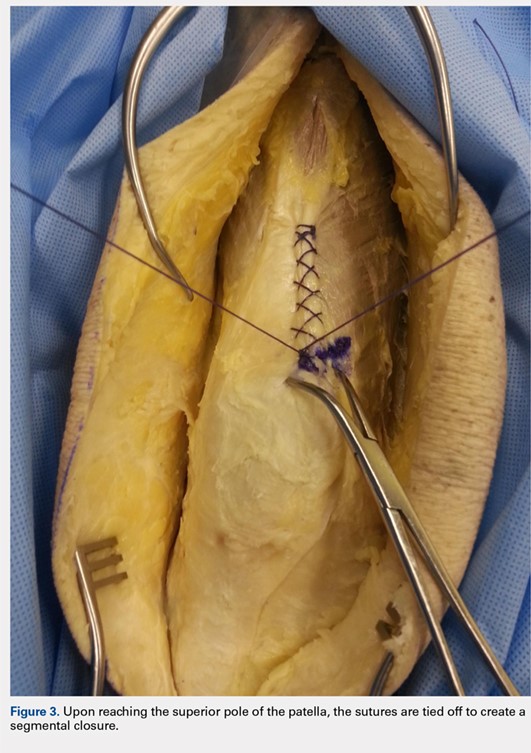
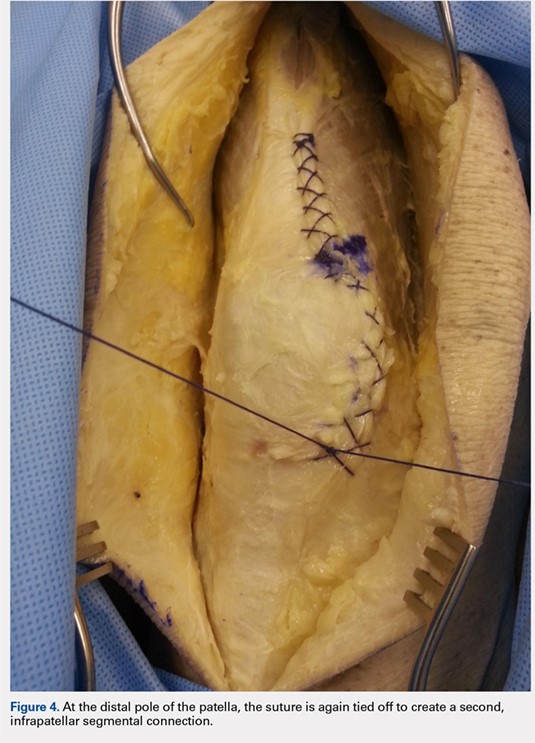
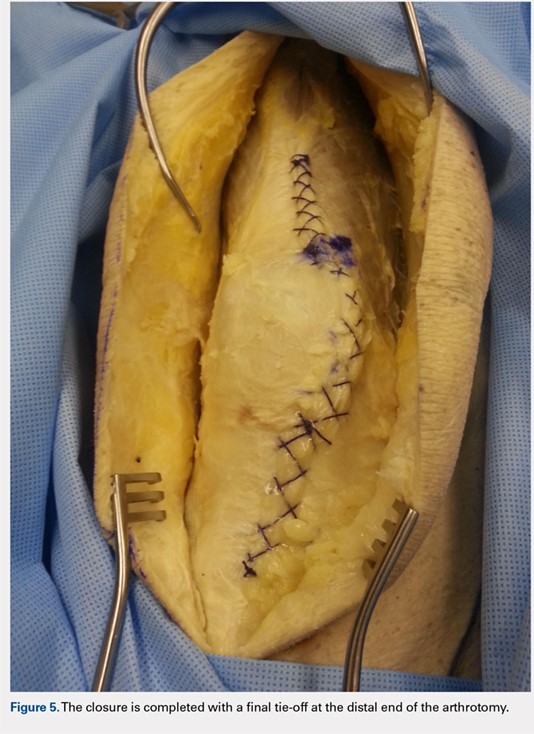
When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).
Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.

The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.

When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).



Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
ABSTRACT
Conventional interrupted sutures are traditionally used in extensor mechanism closure during total knee arthroplasty (TKA). In recent years, barbed suture has been introduced with the proposed benefits of decreased closure time and a watertight seal that is superior to interrupted sutures. Complication rates using barbed sutures and conventional interrupted sutures are similar. We propose a novel closure technique known as the Flint Lock, which is a double continuous interlocking stitch. The Flint Lock provides a quick and efficient closure to the extensor mechanism in TKA. In addition, similar to barbed suture, the Flint Lock should provide a superior watertight seal. It utilizes relatively inexpensive and readily available materials.
Continue to: In 2003, more than 400,000 total knee replacements...
In 2003, more than 400,000 total knee replacements were performed in the United States. This number is expected to increase in the coming decades to 3 million by the year 2030.1 The surgical approach to knee arthroplasty always involves a capsular incision that needs to be repaired after implantation of the components. The capsular incision repair should be strong enough to allow for immediate range of motion.
Traditionally, repair of the arthrotomy is performed using interrupted sutures. Recently, a running technique using barbed suture has been demonstrated to enable faster closure times.2-6 In addition, a running suture technique using barbed suture provides a superior watertight closure compared with an interrupted suture.7 It has been reported that the barbed suture has the same safety profile as that of interrupted sutures,2,3,4 although extensor mechanism repair failure8 and wound complications9,10 have been reported.
This study proposes a novel technique for arthrotomy closure in total knee arthroplasty (TKA). It is a double continuous interlocking stitch, termed the “Flint Lock.” Based on our clinical experience using this method, this technique has been found to be safe and effective.
TECHNIQUE
The Flint Lock was developed for closure in TKA, which was performed through a standard medial parapatellar approach. Before creating the arthrotomy, a horizontal line is drawn along the medial side of the patella to ensure anatomic alignment of the extensor mechanism during closure of the capsule.

The Flint Lock is performed by 2 people working simultaneously. Closure begins at the proximal end of the arthrotomy using 2 No. 1 Vicryl (Ethicon) sutures. Each suture is thrown a single time at the most proximal extent of the arthrotomy with the knee in 30° to 40° of flexion. These sutures are tied off independently from each other (Figure 1). At this point, the knee is flexed to 90° and the sutures are thrown alternately, with the first operator passing medial to lateral through the capsule and the second operator passing lateral to medial. While 1 operator is passing a suture, the other operator holds the other suture tight to maintain tension on the closure. The alternating throws create an interlocking weave as the pattern is repeated and progressively moves distally (Figure 2). This technique results in 2 continuous sutures running in opposing directions. Each No. 1 Vicryl suture is specific to each operator. Therefore, each operator uses the same suture for the entirety of the closure.

When the superior pole of the patella is reached, the 2 sutures are tied together, thus creating a segmental closure (Figure 3). Following this tie off, the closure is continued in a similar manner until the inferior pole of the patella is reached. The sutures are then tied off to each other again, creating another segmental closure (Figure 4). The remainder of the arthrotomy is closed continuing the Flint Lock technique, and the 2 sutures are tied off to each other at the distal end of the arthrotomy and cut (Figure 5).



Continue to: The superficial layers are closed at the surgeon’s discretion...
The superficial layers are closed at the surgeon’s discretion. The authors prefer interrupted 2-0 Vicryl sutures followed by a running 3-0 Monocryl (Ethicon) suture in the subcutaneous layer. Dermabond (Ethicon) skin glue and an Aquacel Ag (ConvaTec) dressing are applied, followed by a compressive bandage.
DISCUSSION
The importance of a strong, tight closure of the arthrotomy in TKA is critical to the success of the procedure. Nevertheless, there are multiple methods to achieve closure. The Flint Lock technique is a novel method that employs basic concepts of surgical technique in an original manner. The continuous nature of the closure should provide a tighter seal, leading to less wound drainage. Persistent wound drainage has been associated with deep wound infections following total joint arthroplasty.11,12 In addition, the double suture provides a safeguard to a single suture rupture, while the segmental quality protects against complete arthrotomy failure.
A potential downside of this technique is that it requires 2 individuals operating 2 needles simultaneously. This presents a potential for a sharp injury to the operators; however, this has not occurred in our experience. A comparable risk with interrupted sutures is probably present because there are often multiple sutures utilized during closure via the interrupted technique.
In 2015, the cost of a single No. 1 barbed suture was $13.14 at our institution, whereas the cost of 2 No. 1 Vicryl sutures was $3.66. Although pricing differs across hospitals, the Vicryl sutures are probably less costly compared with the barbed sutures.
Our experience with the Flint Lock technique has been favorable thus far, with no incidences of postoperative drainage, infection, or extensor mechanism failure. Our current use has been in closure of the knee, but it could be considered in closure of long incisions about the hip as well. A more in-depth analysis of relevant factors, such as time for closure, mechanical strength, cost savings, and clinical outcomes, is needed to further evaluate this method of closure. In addition, biomechanical analysis of the technique would aid in its evaluation. Future studies are needed to analyze these factors to verify the benefits and viability of the Flint Lock technique.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi:10.2106/JBJS.F.00222.
2. Eickmann T, Quane E. Total knee arthroplasty closure with barbed sutures. J Knee Surg. 2010;23(3):163-167. doi:10.1055/s-0030-1268692.
3. Gililland JM, Anderson LA, Sun G, Erickson JA, Peters CL. Perioperative closure-related complication rates and cost analysis of barbed suture for closure in TKA. Clin Orthop Relat Res. 2012;470(1):125-129. doi:10.1007/s11999-011-2104-7.
4. Ting NT, Moric MM, Della Valle CJ, Levine BR. Use of knotless suture for closure of total hip and knee arthroplasties: a prospective, randomized clinical trial. J Arthroplasty. 2012;27(10):1783-1788. doi:10.1016/j.arth.2012.05.022.
5. Stephens S, Politi J, Taylor BC. Evaluation of primary total knee arthroplasty incision closure with use of continuous bidirectional barbed suture. Surg Technol Int. 2011;21:199-203.
6. Levine BR, Ting N, Della Valle CJ. Use of a barbed suture in the closure of hip and knee arthroplasty wounds. Orthopedics. 2011;34(9):e473-e475. doi:10.3928/01477447-20110714-35.
7. Nett M, Avelar R, Sheehan M, Cushner F. Water-tight knee arthrotomy closure: comparison of a novel single bidirectional barbed self-retaining running suture versus conventional interrupted sutures. J Knee Surg. 2011;24(1):55-59. doi:10.1055/s-0031-1275400.
8. Wright RC, Gillis CT, Yacoubian SV, Raven RB 3rd, Falkinstein Y, Yacoubian SV. Extensor mechanism repair failure with use of birectional barbed suture in total knee arthroplasty. J Arthroplasty. 2012;27(7):1413.e1-e4. doi:10.1016/j.arth.2011.08.013.
9. Campbell AL, Patrick DA Jr, Liabaud B, Geller JA. Superficial wound closure complications with barbed sutures following knee arthroplasty. J Arthroplasty. 2014;29(5):966-969. doi:10.1016/j.arth.2013.09.045.
10. Smith EL, DiSegna ST, Shukla PY, Matzkin EG. Barbed versus traditional sutures: closure time, cost, and wound related outcomes in total joint arthroplasty. J Arthroplasty. 2014;29(2):283-287. doi:10.1016/j.arth.2013.05.031.
11. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515. doi:10.1016/S0736-0266(01)00153-X.
12. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289. doi:10.1016/S0883-5403(06)80091-4.
TAKE-HOME POINTS
- The Flint Lock is a novel technique in TKA closure.
- Its continuous nature provides a tight seal with extensor mechanism closure.
- The utilization of a segmental closure with double suture provides a safeguard for suture failure.
- The suture used in the technique is less expensive than barbed suture.
- Future investigation is warranted to further validate the use of the Flint Lock.
Subcutaneous Ulnar Nerve Transposition Using Osborne’s Ligament as a Ligamentodermal or Ligamentofascial Sling
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The ulnar nerve is most commonly compressed at the elbow in the cubital tunnel. Conservative and operative treatments have been applied for cubital tunnel syndrome. Surgical management options include decompression, medial epicondylectomy, and various anterior transposition techniques. We describe a novel technique of anterior transposition of the ulnar nerve by using Osborne’s ligament as a sling to avoid subluxation. Osborne’s ligament is incised posteriorly and medially on the olecranon to create a sling with 2 to 3 cm width. The sling is tailored to wrap around the ulnar nerve and attached to the flexor-pronator fascia or dermis to create a smooth gliding surface without causing compression. Ten patients with cubital tunnel syndrome, established by physical examination findings and electromyography/nerve conduction studies underwent ulnar nerve transposition using this technique and were able to participate in a phone survey. The average follow-up was 15.6 months (range, 4-28 months). The average time to become subjectively “better” after surgery was 4.2 weeks. The pain intensity was reduced from an average of 7.5 preoperatively to <1, on a 10-point scale, at the time of the survey. All patients had symptomatic relief without any complication. The proposed technique using Osborne’s ligament as a ligamentofascial or ligamentodermal sling offers a unique way of creating a non-compressive sling with the component of the cubital tunnel itself and has an additional benefit of creating a smooth gliding surface for early return of function.
Continue to: Ulnar nerve compression at the elbow...
Ulnar nerve compression at the elbow is a common nerve compression syndrome in the upper extremity. There are multiple sites of compression of the ulnar nerve distal to the axilla. The most common site of ulnar nerve compression is at the cubital tunnel.1 When ulnar nerve compression is clinically suspected, electromyography (EMG) and nerve conduction velocity studies (NCS) may be performed to help support the diagnosis. However, a false negative rate in excess of 10% is found in patients with clinical signs and symptoms of cubital tunnel syndrome.2 Treatment of cubital tunnel syndrome involves nonsurgical treatments, including activity modification, use of nonsteroidal anti-inflammatory drugs, splinting, and physical therapy or surgical treatment.3-5
Surgical management of cubital tunnel syndrome is indicated after a failed nonsurgical management or a presentation with motor weakness. The most common surgical treatments include in situ decompression, subcutaneous transposition, intramuscular transposition, submuscular transposition, and medial epicondylectomy, or their combination.6 However, optimal surgical management of cubital tunnel syndrome remains controversial.2,7 The overall goal of surgery is to eliminate all sites of compression and obtain a tension-free nerve that glides smoothly.
After the initial concept of subcutaneous anterior ulnar nerve transposition was developed by Curtis8 in 1898, many different techniques have been derived including epineurial suture, fasciodermal sling, and subcutaneous to fascia suture.8-10 Common complications of subcutaneous ulnar nerve transposition include nerve fibrosis, recurrent subluxation, and inadequate division of the intermuscular septum.9 Additionally, thin patients often have repeated trauma to their ulnar nerves after subcutaneous transposition.3
The anatomy of the cubital tunnel is well described, but it has multiple names and descriptions throughout the literature. Osborne11 originally described a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel. O’Driscoll and colleagues5 conducted a cadaver study and proposed calling Osborne’s band as the cubital tunnel retinaculum. They described 4 different variations of anatomy and the retinaculum as a 4-mm wide band of tissue located proximally in the cubital tunnel that is distinct from the arcuate ligament and the fascia between the 2 heads of the flexor carpi ulnaris.5 Green and Rayan12 studied cubital tunnel anatomy and referred to the ligament that spans the medial epicondyle and the olecranon as the arcuate ligament, which is also distinct from the flexor carpi ulnaris aponeurosis. These variations in named anatomy make describing procedures around the cubital tunnel challenging. In this study, the fascial band between the 2 heads of the flexor carpi ulnaris, as originally described by Osborne,11 will be referred to as Osborne’s ligament.
We describe a novel technique of anterior subcutaneous ulnar nerve transposition, where Osborne’s ligament is used as a sling to prevent ulnar nerve subluxation over the medial epicondyle. We also describe the results of our initial subset of patients who were treated with this technique.
Continue to: MATERIALS AND METHODS...
MATERIALS AND METHODS
We performed a chart review of all patients operated on between January 2010 and March 2012 by the same surgeon. We recruited 15 consecutive patients who were diagnosed with ulnar nerve transposition for moderate to severe cubital tunnel syndrome through EMG/NCS and physical examination during this time frame. Operative reports were then reviewed. In 14 of these 15 cases, Osborne’s ligament was used as a ligamentofascial or ligamentodermal sling. In the fifteenth patient, preoperative subluxation of the ulnar nerve was identified with movement of elbow, and Osborne’s ligament was found to not be large enough to provide an appropriate sling. Three patients were unreachable, and 1 patient chose to not participate in the study. Of the initial 15 patients, 10 were given a telephone survey (Appendix A), which was prepared based on the recommendation of Novak and colleagues13 and incorporated with questions regarding preoperative symptoms, satisfaction, smoking history, and employment status. This study was Institutional Review Board approved at our institution, and appropriate consent was obtained from the participants.
Appendix A. Ulnar Nerve Telephone Survey
SURGICAL TECHNIQUE
A 10 to 12 cm incision centered over the cubital tunnel is made. The medial antebrachial cutaneous nerve is identified and protected. After dissection through superficial fascia, Osborne’s ligament is identified. The ligament is then released posteriorly from the olecranon and is assessed. The ulnar nerve is then freed in a proximal to distal manner to preserve vascular structures that supply the epineurium. The medial intermuscular septum is examined and excised as a site of compression. The ulnar nerve is then mobilized. Once mobilized, the ulnar nerve is transposed anterior to the medial epicondyle and checked to ensure that no sharp curves are made and nothing is impinging on the nerve while passively flexing and extending the elbow. The Osborne’s ligament is then passed over the top of the previously transposed ulnar nerve to create a sling that is ligamentofascial if sutured to the flexor/pronator fascia or ligamentodermal if sutured to dermis. Importantly, the flexor/pronator fascia is not incised. The remaining soft tissue and fascia of the cubital tunnel are then closed with 2-0 vicryl suture. The free end of the Osborne’s ligament is sutured to flexor/pronator fascia or to dermis, anterior to the medial epicondyle with No. 0 vicryl suture. This process is conducted in a tension-free manner to prevent creating a new site of compression. The nerve is then rechecked for appropriate, tension-free gliding followed by closure of the wound in layers after irrigation (additional details are shown in Figures 1-5).
RESULTS
Ten of the 15 patients were available for telephone review. The results of the telephone survey are as follows. The average time to telephone survey was 15.6 months (range, 4-28 months). The average time to become subjectively “better” was 4.2 weeks (range, 2-6 weeks). The average time back to work was 1.6 weeks (range, 1 day to 3 weeks). Three patients were retired and did not go back to work. All patients stated they were subjectively “better” after surgery, and when asked, all patients stated that they would choose surgery again. The average pain prior to surgery was 7.5 (range, 5.5-9.5) on a 10-point scale. The average pain after surgery at final phone interview was 0.1 on a 10-point scale (range, 0-1). All patients stated that their sensation was subjectively better after the surgery. One patient said that his strength worsened, another patient said that his strength was the same, and the remaining patients said that their strength was better. One patient was a smoker, and no patients had acute traumatic injuries that caused their ulnar nerve symptoms.
Continue to: DISCUSSION...
DISCUSSION
Subcutaneous ulnar nerve transposition is an effective way to treat ulnar nerve compression at the cubital tunnel in appropriate patients. Many techniques have been described, including epineurial suture, fasciodermal sling, and using the medial intermuscular septum as a sling for the ulnar nerve.9,10,14,15 Eaton and colleagues14 described the creation of a 1 cm × 1 cm flap based on antebrachial fascial connected to the medial epicondyle. This flap is reflected medially and acts as a fasciodermal sling posterior to the transposed nerve at the medial epicondyle. This sling also acts like a septum to prevent posterior subluxation. Only subcutaneous fat is superficial to the nerve, in contrast to previous attempts at subcutaneous transposition. At an average of 18 months of follow-up, 14 patients showed improvement in their symptoms.14 Pribyl and Robinson,9 in 1998, described a procedure where a portion of the intermuscular septum is divided from a distance of 3 to 4 cm proximal to its insertion on the medial epicondyle; the portion is used as a sling and sutured to the fascia of the flexor/pronator mass or alternatively to the subcutaneous tissues. Tan and colleagues15 modified Pribyl and Robinson’s technique by creating a “V” sling with the intermuscular septum; this technique led to complete resolution of symptoms in 17 of 20 patients and improved the symptoms in the 3 remaining patients. Richmond and Southmayd10 reported excellent results in 83% of patients who had epineurium sutured to the fascia during subcutaneous transposition. However, each aforementioned technique has its own unique theoretical set of problems. The shortcoming of Eaton and colleagues’14 fasciodermal sling is the creation of a raw bed while creating the sling over the flexor-pronator fascia, which is prone to scarring. Moreover, given that the flexor-pronator fascia is incised, theoretically, the healing period is prolonged and the grip strength in the initial postoperative period decreases. Utilizing the medial intermuscular septum as a sling can create a narrow band, which creates sharp angles that limit nerve gliding. Suturing the epineurium to the fascia by using the technique of Richmond and Southmayd10 creates a construct that is resistant to tension-free gliding.
In this study, Osborne’s ligament was successfully used as a ligamentofascial or ligamentodermal sling in our subset of patients. We believe this is partially due to the large smooth gliding surface of Osborne’s ligament that helps to minimize sharp curves and allows for the ulnar nerve to glide tension free. This could be seen with other techniques as described previously. Furthermore, our technique is different because the flexor pronator fascia is not incised, which results in less soft tissue trauma and less pain generation; we suspect that the patients were able to have an early return to work and did not complain of decreased strength because the flexor pronator fascia was not disturbed. Our surveyed patients essentially had complete cessation of pain and were able to return to work in about 10 to 11 days. The patients reported that they felt subjectively “better” in approximately 4 weeks and reported no complications. Sensation was also subjectively “better” in all of the patients surveyed.
This study presents several limitations. The study was retrospective in nature and did not include randomization or a control group. In addition, there is a possibility of significant recall bias in the telephone survey that relies on patient recollection. Finally, the telephone survey is an invalidated outcome measure, and no formal statistical analysis was performed.
CONCLUSION
Subcutaneous ulnar nerve transposition using Osborne’s ligament as a ligamentofascial or ligamentodermal sling is a novel technique that creates a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve and showed success in our subset of patients.
This paper will be judged for the Resident Writer’s Award.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
1. Chiou HJ, Chou YH, Cheng SP, et al. Cubital tunnel syndrome: diagnosis by high-resolution ultrasonography. J Ultrasound Med. 1998;17(10):643-648. doi:10.7863/jum.1998.17.10.643.
2. Palmer BA, Hughes TB. Cubital tunnel syndrome. J Hand Surg. 2010;35(1):153-163. doi:10.1016/j.jhsa.2009.11.004.
3. Elhassan B, Steinmann SP. Entrapment neuropathy of the ulnar nerve. J Am Acad Orthop Surg. 2007;15(11):672-681. doi:10.5435/00124635-200711000-00006.
4. Robertson C, Saratsiotis J. A review of compressive ulnar neuropathy at the elbow. J Manip Physiol Ther. 2005;28(5):345. doi:10.1016/j.jmpt.2005.04.005.
5. O'Driscoll SW, Horii E, Carmichael SW, Morrey BF. The cubital tunnel and ulnar neuropathy. Bone Joint Surg Br. 1991;73(4):613-617. doi:10.1302/0301-620X.73B4.2071645.
6. Svernlöv B, Larsson M, Rehn K, Adolfsson L. Conservative treatment of the cubital tunnel syndrome. J Hand Surg Eur Vol. 2009;34(2):201-207. doi:10.1177/1753193408098480.
7. Mowlavi A, Andrews K, Lille S, Verhulst S, Zook EG, Milner S. The management of cubital tunnel syndrome: A meta-analysis of clinical studies. Plast Reconstr Surg. 2000;106(2):327-334. doi:10.1097/00006534-200008000-00014.
8. Curtis. Traumatic ulnar neuritis: transplantation of the nerve. J Nerv Ment Dis. 1898;25(480):169.
9. Pribyl CR, Robinson B. Use of the medial intermuscular septum as a fascial sling during anterior transposition of the ulnar nerve. J Hand Surg. 1998;23(3):500-504. doi:10.1016/S0363-5023(05)80468-X.
10. Richmond JC, Southmayd WW. Superficial anterior transposition of the ulnar nerve at the elbow for ulnar neuritis. Clin Orthop Relat Res. 1982;164(164):42-44. doi:10.1097/00003086-198204000-00010.
11. Osborne G. Compression neuritis of the ulnar nerve at the elbow. Hand. 1970;2(1):10-13. doi:10.1016/0072-968X(70)90027-6.
12. Green JR Jr, Rayan GM. The cubital tunnel: anatomic, histologic, and biomechanical study. J Shoulder Elbow Surg. 1999;8(5):466-470.
13. Novak CB, Mackinnon SE, Stuebe AM. Patient self-reported outcome After ulnar nerve transposition. Ann Plast Surg. 2002;48(3):274-280. doi:10.1097/00000637-200203000-00008.
14. Eaton RG, Crowe JF, Parkes JC. Anterior transposition of the ulnar nerve using a non-compressing fasciodermal sling. J Bone Joint Surg Am. 1980;62(5):820-825. doi:10.2106/00004623-198062050-00019.
15. Tan V, Pope J, Daluiski A, Capo JT, Weiland AJ. The V-sling: a modified medial intermuscular septal sling for anterior transposition of the ulnar nerve. J Hand Surg. 2004;29(2):325-327. doi:10.1016/j.jhsa.2003.11.011.
TAKE-HOME POINTS
- Optimal management of cubital tunnel syndrome is controversial.
- There are many different techniques for ulnar nerve transposition, each with their own set of pitfalls.
- Goal of any surgery for ulnar nerve compression is to eliminate all sites of compression and create a tension-free nerve that glides freely.
- Osborne’s ligament is a transverse fibrous band as the fascial connection between the 2 heads of the flexor carpi ulnaris that forms the roof of the cubital tunnel.
- Osborne’s ligament can be used in ulnar nerve transposition to create a broad based, smooth-gliding sling for tension-free excursion of the ulnar nerve.
A Three-View Radiographic Approach to Femoroacetabular Impingement
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40° may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140° may indicate coxa valga
<130° may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.
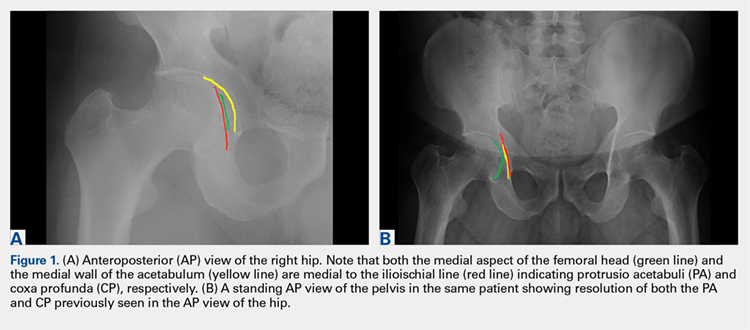
A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40° may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140° may indicate coxa valga
<130° may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
ABSTRACT
Femoroacetabular impingement (FAI) is an abnormality of the hip joint that is increasingly being recognized as a cause of athletic disability and early degenerative hip disease. Despite significant advances in the knowledge of FAI, it remains a frequently unrecognized cause of hip pain in adolescents and young adults among orthopedic providers. The purpose of this article is to present a simple 3-view radiographic approach to young adults with hip pain. The radiographs include a standing anteroposterior view of the pelvis, a cross-table lateral view, and a false profile view. Good quality radiographs showing the common sites of potential impingement combined with a basic understanding of certain radiographic parameters may allow faster diagnosis, eliminate unnecessary studies, and allow earlier referral and management.
Continue to: The prevalence of femoroacetabular impingement...
The prevalence of femoroacetabular impingement (FAI) in the general population is estimated at 23.1%.1 While FAI is often bilateral,2 patients usually present with unilateral symptoms.3 Young, highly active individuals are most commonly affected.3 Despite significant improvement in our understanding of FAI in recent years, it remains a poorly recognized cause of hip pain among orthopedic providers. Clohisy and colleagues3 found that the average time to diagnosis was 3.1 years (range, 3-15 years) and the average number of providers seen before correct diagnosis was 4.2 (range, 1-16) with nearly half those providers being orthopedic specialists. This is likely attributed to limited training and lack of appropriate imaging. Multiple comprehensive radiographic approaches have been described, including plain films, computed tomography, and magnetic resonance imaging.2,4 The objective of this article is to present a simple 3-view plain film approach for young adults with hip pain. While history and physical examination remain key to FAI diagnosis, a basic knowledge of the common sites of impingement with appropriate radiographic views to visualize these sites may help eliminate unnecessary imaging and delayed diagnosis.
STANDING ANTEROPOSTERIOR VIEW OF THE PELVIS
An anteroposterior (AP) view of the pelvis, as opposed to an AP view of the hip, is an important first radiograph in the evaluation of young patients presenting with hip pain. Not only does it permit visualization of the contralateral hip for comparison, but it also allows more accurate measurements of several radiographic parameters (Table). An AP view of the hip often gives the false impression of global over coverage, such as coxa profunda2 and protrusio acetabuli (Figures 1A, 1B), and may overestimate the amount of acetabular anteversion.2
Table. Summary of Common Radiographic Parameters When Assessing Young Adults with Hip Pain2,4
Sign | Best Radiographic View | Measurement | Quoted Normal Valuesa | Clinical Relevance of Abnormal Values |
Acetabular depth | AP pelvis | Medial wall of the acetabulum (MWA) relative to the ilioischial line (IIL) | MWA is lateral to IIL | Global overcoverage (ie, coxa profunda) |
Femoral depth | AP pelvis | Medial surface of the femoral head (MFH) relative to the IIL | MFH is lateral and within 10 mm of the IIL | >10 mm may indicate undercoverage (ie, dysplasia)
MFH medial to IIL may indicate overcoverage (ie, protrusio acetabuli) |
Tonnis angle | AP pelvis | Angle between the weight-bearing surface of the acetabulum and a line parallel to the horizontal axis of the pelvis (eg, inter-teardrop line) | 0°-10° | >10° may indicate undercoverage (ie, dysplasia)
<0° may indicate overcoverage (ie, pincer-type FAI) |
Lateral center edge angle | AP pelvis | Angle between a line perpendicular to the horizontal axis of the pelvis through the center of the femoral head and a line connecting the center of the femoral head to the lateral most edge of the acetabular weight-bearing surface | 25°-40° | >40° may indicate overcoverage (ie, pincer-type FAI)
<25° may indicate undercoverage (ie, dysplasia) |
Crossover sign | AP pelvis | Intersection between the anterior and posterior rims of the acetabulum | Crossover occurs at the lateral most aspect of the acetabular weight-bearing surface | Crossover occurring distal to the lateral most aspect of the acetabular weight-bearing surface may indicate acetabular retroversion |
Femoral neck-shaft angle | AP pelvis | Angle between the femoral shaft and the longitudinal axis of the neck | 135° ± 5° | >140° may indicate coxa valga
<130° may indicate coxa vara |
Alpha angle | Cross-table lateral | Angle between a line connecting the center of the femoral neck to the center of the femoral head and a line connecting the center of the head to a point on the anterolateral aspect of the head-neck junction where the head sphericity ends | >55° | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset | Cross-table lateral | Distance between 2 lines parallel to the longitudinal axis of the femoral neck: 1 line tangent to the anterior most aspect of the neck and 1 line tangent to the anterior surface of the femoral head | >10 mm | Decreased head-neck offset (ie, cam-type impingement) |
Anterior head-neck offset ratio | Cross-table lateral | Anterior head-neck offset divided by the diameter of the femoral head | >0.14 | Decreased head-neck offset (ie, cam-type impingement) |
Femoral version | Cross-table lateral | Angle between the longitudinal axis of the femoral neck and the longitudinal axis of the femoral shaft | 15° ± 5° | Developmental disorders (eg, dysplasia, slipped capital femoral epiphysis) |
Anterior center edge angle | False profile view | Angle between a vertical line through the center of the femoral head and a line connecting the center of the femoral head to the anterior most edge of the acetabular weight-bearing surface | >20° | Undercoverage (ie, dysplasia) |
aNormal values are provided for reference only and should not be solely relied on for diagnosis.
Abbreviations: AP, anteroposterior; FAI, femoroacetabular impingement.

A good quality radiograph is important for accurate assessment. The X-ray beam should be perpendicular to the coronal plane of the pelvis. Neutral rotation of the pelvis is a prerequisite and can be confirmed by the presence of symmetric obturator foramina, iliac wings, and coccyx vertically in line with the pubic symphysis. Deviations from this configuration can significantly affect the ability to accurately assess the acetabular version. This is because the rotational profile of the acetabulum is sensitive to pelvic rotation.5,6
While the AP view of the pelvis can be obtained in either supine or standing positions, the standing position is recommended. A supine view tends to increase the likelihood of finding a crossover sign that often disappears in the standing position (Figures 2A, 2B). This is attributed to the posterior tilt of the pelvis in the sagittal plane with standing, which functionally increases acetabular anteversion, eliminating the crossover sign.5,6 In contrast, a crossover sign that persists in the standing position combined with other abnormal radiographic parameters, such as a negative Tonnis angle and/or increased lateral center edge angle, are concerning for pincer-type FAI (Figures 3A, 3B). An isolated crossover sign may be a normal variant in young asymptomatic patients7 and is not a reliable indicator of acetabular retroversion.5
In addition to assessing the acetabular coverage and version (Figures 1A, 1B, 3A, 3B, and 4A, 4B), the AP view of the pelvis can provide valuable information regarding the proximal femur. One should pay attention to the sphericity of the head (pistol grip cam lesions are most obvious on this view), congruency between the femoral head and the acetabulum, femoral offset, and neck-shaft angle. While we tend to traditionally classify FAI into cam and pincer osseous bumps, alterations in hip dynamics (i.e., coxa vara and coxa breva) can result in functional impingement even in the absence of the osseous bumps.
Continue to: CROSS-TABLE LATERAL...
CROSS-TABLE LATERAL
A cross-table lateral of the affected hip is another important radiographic adjunct in the evaluation of hip pain in young patients. This view provides AP axial visualization of the hip joint identifying potential pathologies such as anterior cam lesions that may not be apparent on frog-leg lateral radiographs (Figures 5A, 5B and 6A, 6B). The cross-table lateral view can also show posterior impingement and/or joint space narrowing from countercoup lesions associated with pincer-type FAI (Figures 3A, 3B). In addition, the rotational profile of the proximal femur is best assessed in this view (Figure 4B). The challenge with a cross-table lateral, however, is that it is operator-dependent. In circumstances where a good quality cross-table lateral cannot be obtained, we default to a frog-leg lateral to avoid excess radiation exposure.
FALSE PROFILE VIEW
A false profile view provides a good visualization of the anterosuperior aspect of the acetabulum. It can show anterior acetabular over or under coverage. It may also show sub-spine impingement (Figures 7A, 7B). Sub-spine impingement is characterized by a prominent anterior inferior iliac spine (AIIS) that extends to the level of the anterosuperior acetabular rim. The prominent AIIS can impinge on the femoral head-neck junction during hip flexion. A prominent AIIS has also been shown to give the false impression of a crossover sign.8
CONCLUSION
Even to the trained eye, radiographic findings of FAI can be quite subtle and easily missed. A systematic approach when interpreting plain radiographs is important. Radiographic assessment starts with good quality X-rays with the pelvis in neutral rotation. Because of the young age of most patients, radiation exposure should be minimized. An understanding of the potential sites of impingement and the specific radiographs to visualize these sites minimizes radiation exposure and other unnecessary imaging. In our experience, the 3-view radiographic approach presented combined with supportive history and physical examination findings are highly sensitive to identify cases of FAI. Advanced imaging is reserved for patients who have failed conservative management or considering surgical intervention.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
1. Fernquest S, Arnold C, Palmer A, et al. Osseous impingement occurs early in flexion in cam-type femoroacetabular impingement: a 4D CT model. Bone Joint J. 2017;99-B(4 Supple B):41-48. doi:10.1302/0301-620X.99B4.BJJ-2016-1274.R1.
2. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552. doi:10.2214/AJR.06.0921.
3. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644. doi:10.1007/s11999-008-0680-y.
4. Clohisy JC, Carlisle JC, Beaule PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am. 2008;90 Suppl 4:47-66. doi:10.2106/JBJS.H.00756.
5. Dandachli W, Islam SU, Liu M, Richards R, Hall-Craggs M, Witt J. Three-dimensional CT analysis to determine acetabular retroversion and the implications for the management of femoro-acetabular impingement. J Bone Joint Surg Br. 2009;91(8):1031-1036. doi:10.1302/0301-620X.91B8.22389.
6. Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90(11):1428-1434. doi:10.1302/0301-620X.90B11.20073.
7. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254. doi:10.1007/s11999-014-4055-2.
8. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470. doi:10.1007/s11999-012-2689-5.
TAKE-HOME POINTS
- FAI is a frequently unrecognized cause of hip pain in adolescents and young adults.
- Understanding the potential sites of impingement and the specific radiographs to visualize these sites can help avoid unnecessary imaging and delayed diagnosis.
- A simple radiographic approach consisting of a standing AP view of the pelvis, a cross-table lateral view, and a false profile view is often a sufficient screening tool.
- While we tend to classify FAI into cam and pincer osseous bumps, alterations in hip dynamics can result in functional impingement even in the absence of the osseous bumps.
- Advanced imaging is reserved for patients who have failed conservative management or are considering surgical intervention.
5 Points on Meniscal Allograft Transplantation
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.
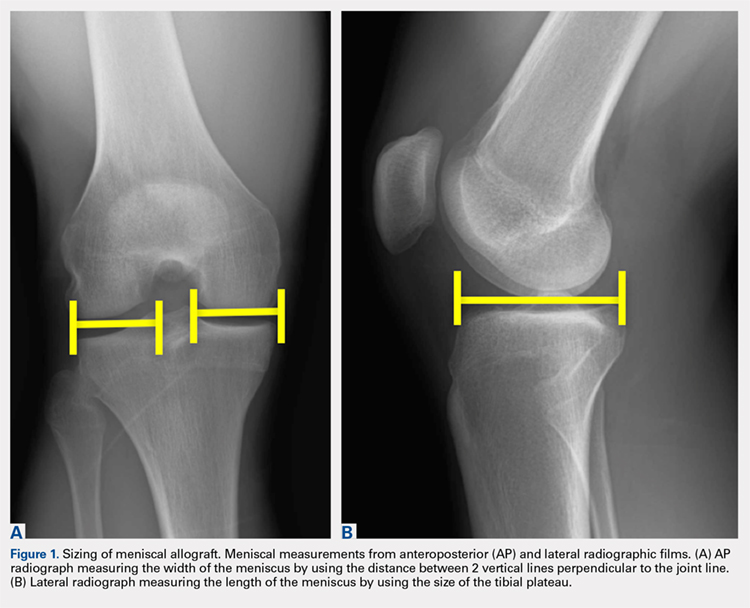
Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.
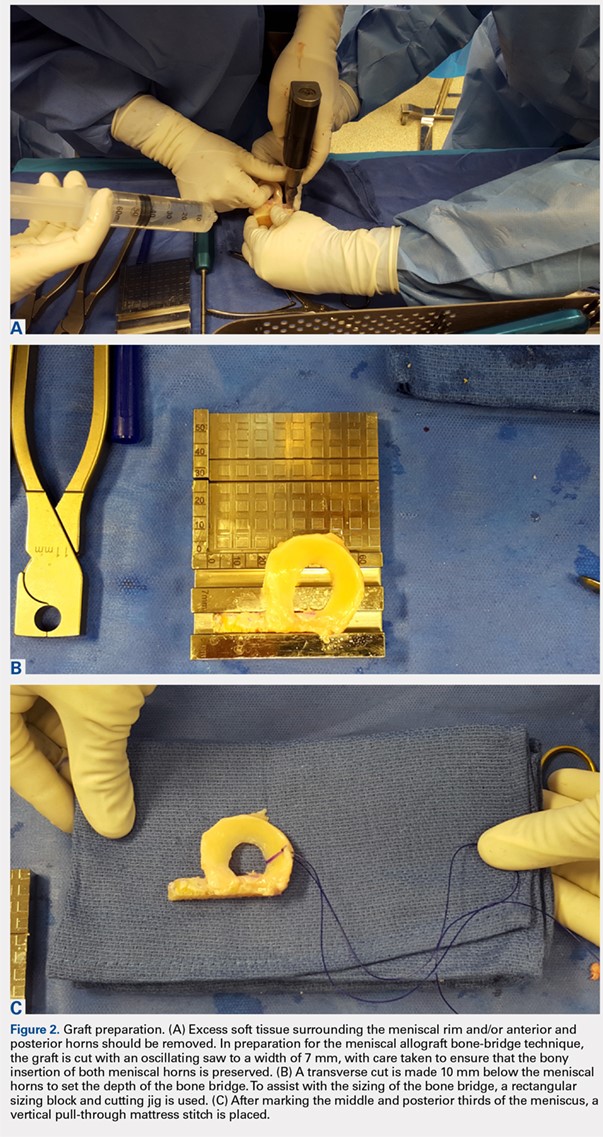
Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
ABSTRACT
Meniscus allograft transplantation (MAT) has yielded excellent long-term functional outcomes when performed in properly indicated patients. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency. Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure. Once an ideal candidate is identified, graft selection and preparation are critical steps to ensure a proper fit and long-term viability of the meniscus. When selecting the graft, accurate measurements must be taken, and this is most commonly performed using plain radiographs for this. Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Continue to: Meniscus tears are common in the young, athletic patient population...
Meniscus tears are common in the young, athletic patient population. In the United States alone, approximately 700,000 meniscectomies are performed annually.1 Given discouraging long-term clinical results following subtotal meniscectomy in young patients, meniscal repair is preferred whenever possible.2 Despite short-term symptom relief if subtotal meniscectomy is required, some patients often go on to develop localized pain in the affected compartment, effusions, and eventual development of osteoarthritis. In such patients with symptomatic meniscal deficiency, meniscal allograft transplantation (MAT) has yielded excellent long-term functional outcomes.3-5 Three recently published systematic reviews describe the outcomes of MAT in thousands of patients, noting positive outcomes in regard to pain and function for the majority of patients.6-8 Specifically, in a review conducted by Elattar and colleagues7 consisting of 44 studies comprising 1136 grafts in 1068 patients, the authors reported clinical improvement in Lysholm Knee Scoring Scale score (44 to 77), visual analog scale (48 mm to 17 mm), and International Knee Documentation Committee (84% normal/nearly normal, 89% satisfaction), among other outcomes measures. Additionally, the complication (21.3%) and failure rates (10.6%) were considered acceptable by all authors. The purpose of this article is to review indications, operative preparation, critical aspects of surgical technique, and additional concomitant procedures commonly performed alongside MAT.
1. PATIENT SELECTION
When used with the proper indications, MAT offers improved functional outcomes and reduced pain for patients with symptomatic meniscal deficiency. When evaluating a patient for potential MAT, it is imperative to evaluate past medical history and past surgical procedures. The ideal MAT candidate is a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency who does not have (1) evidence of diffuse osteoarthritis (Outerbridge grade <2), including the absence of significant bony flattening or osteophytes in the involved compartment; (2) inflammatory arthritis; (3) active or previous joint infection; (4) mechanical axis malalignment; or (5) morbid obesity (Table). Long-leg weight-bearing anterior-posterior alignment radiographs are important in the work-up of any patient being considered for MAT, and consideration for concomitant or staged realignment high tibial osteotomy (HTO) or distal femoral osteotomy (DFO) should be given for patients in excessive varus or valgus, respectively. Although the decision to perform a realignment osteotomy is made on a patient-specific basis, if the weight-bearing line passes medial to the medial tibial spine or lateral to the lateral tibial spine, HTO or DFO, respectively, should be considered. Importantly, MAT is not typically recommended in the asymptomatic patient.9 Although some recent evidence suggests MAT may have chondroprotective effects on articular cartilage following meniscectomy, there is insufficient long-term outcome data to support the use of MAT as a prophylactic measure, especially given the fact that graft deterioration inevitably occurs at 7 to 10 years, with patients having to consider avoiding meniscus-dependent activities following transplant to protect their graft from traumatic failure.10,11
Table. Summary of Indications and Contraindications for Meniscal Allograft Transplant (MAT)
Indications | Contraindicationsa |
Patients younger than 50 years old with a chief complaint of pain limiting their desired activities | Diffuse femoral and/or tibial articular cartilage wear |
Body mass index <35 kg/m2 | Radiographic evidence of arthritis |
Previous meniscectomy (or non-viable meniscus state) with pain localized to the affected compartment | Inflammatory arthritis conditions |
Normal or correctable coronal and sagittal alignment | MAT performed as a prophylactic measure in the absence of appropriate symptoms is highly controversial |
Normal or correctable ligamentous stability |
|
Normal or correctable articular cartilage |
|
Willingness to comply with rehabilitation protocol |
|
Realistic post-surgical activity expectations |
|
aContraindications for MAT are controversial, as the available literature discussing contraindications is very limited. This list is based on the experience of the senior author.
Long-term prospective studies have shown high graft survival and predominantly positive functional results after MAT. Age indications have expanded, with 1 recent study reporting 6% reoperation rate and zero failures in a cohort of 37 adolescent MAT patients.12 High survival rates hold even among an athletic population, where rates of return to play after MAT have been reported to be >75% for those competing at a high school level or higher.13 In an active military population, <2% of patients progressed to revision MAT or total knee arthroplasty at minimum 2-year follow-up, but 22% of patients were unable to return to military duty owing to residual knee limitations.14 In this series, tobacco use correlated with failure, whereas MAT by high-volume, fellowship-trained orthopedic surgeons decreased rates of failure.
2. GRAFT SELECTION
In preparation for MAT, accurate measurements must be taken for appropriate size matching. Several measurement techniques have been described, including using plain radiographs, 3D computed tomography (CT), and magnetic resonance imaging (MRI).15-18 There is limited data regarding the consequences of an improperly sized donor meniscus; however, an oversized lateral meniscus has been shown to increase the contact forces across the articular cartilage.19 Additionally, an undersized allograft may result in normal forces across the articular cartilage but greater forces across the meniscus.19
When sizing the recipient knee for MAT, accurate width and length measurements are critical. The most common technique used today includes measurements using anteroposterior and lateral radiographic images as described by Pollard and colleagues.15 The width of the meniscus is determined by the distance between 2 vertical lines perpendicular to the joint line, 1 of them tangential to the margin of the tibia metaphysis and the other between the medial and lateral tibial eminence in both knees (Figures 1A,1B). The length of the meniscus is measured on a lateral radiograph. A line is drawn at the level of the articular line between the anterior surface of the tibia above the tuberosity and a parallel line that is tangential to the posterior margin of the tibial plateau. Percent corrections are performed for these dimensions as described in previous publications.

Other techniques have been described to obtain accurate measurements of the recipient knee. For example, obtaining an MRI of the contralateral knee may provide a reproducible method of measuring both the width and length of the medial and lateral menisci.20 CT has been used to measure the lateral meniscus independently, and it has been shown to exhibit less error in the measure of the tibial plateau when compared with X-rays.18 Both CT and MRI are more expensive than simple radiographs, and CT exposes the patient to an increased amount of radiation. Current evidence does not support standard use of these advanced imaging modalities for meniscal sizing.
Continue to: GRAFT PREPARATION AND PLACEMENT...
3. GRAFT PREPARATION AND PLACEMENT
At the time of surgery, the meniscus allograft is thawed in sterile saline and prepared on the back table. This can be done before or after the diagnostic arthroscopy and bone-slot preparation. Excess soft tissue surrounding the meniscal rim and/or anterior and posterior horns should be removed. Several techniques for MAT have been described, but we generally prefer a bridge-in-slot technique for both medial and lateral MAT.21 To prepare the meniscus allograft for a bridge-in-slot technique, the graft is cut with an oscillating saw to a width of 7 mm, with care taken to ensure that the bony insertions of both meniscal horns are preserved. Next, a transverse cut is made 10 mm below the meniscal horns to set the depth of the bone bridge. To assist with the sizing of the bone bridge, a rectangular sizing block and cutting jig is used (Figures 2A-2C). After marking the middle and posterior thirds of the meniscus, a No. 2 non-absorbable suture is placed at the junction of the posterior and middle thirds of the meniscus. This completes preparation of the allograft prior to implantation.

Attention is then turned to back the arthroscopy. A standard posteromedial (medial meniscus) or posterolateral (lateral meniscus) accessory incision is made, and a Henning retractor is carefully placed in order to receive the sutures that will be placed through the meniscus allograft via a standard inside-out repair technique. First, a zone-specific cannula is used to place a nitinol wire out the accessory incision. The looped end of the wire is pulled out of the anterior arthrotomy incision that will be used to shuttle the meniscus allograft into the joint. In order to pass the meniscal allograft into the joint, the passing suture previously placed through the meniscus is shuttled through the nitinol wire, and the wire is then pulled out the accessory incision, advancing the meniscus through the anteiror arthrotomy. As the meniscus is introduced, the traction suture is then gently tensioned to get the allograft completely into the joint. Next, the bone bridge is seated into the previously created bone slot, as the soft tissue component is manually pushed beneath the ipsilateral femoral condyle. Under direct visualization, the soft tissue component is reduced with a probe using firm, constant traction. To aid in reduction, it may be useful to apply compartment-specific varus or valgus stress and to cycle the knee once the meniscal complex is reduced.
4. GRAFT FIXATION
Once the graft has been passed completely into the joint, with the bone bridge seated into the bone slot, the long end of an Army-Navy retractor is placed firmly through the arthrotomy on the meniscal bone bridge, maintaining a downward force to allow the bridge to remain slotted. To lever down on the posterior aspect of the graft, a freer elevator is used from anterosuperior to posteroinferior. The bone bridge is then secured using a bioabsorbable interference screw, placed central to the bone bridge opposing the block to the ipsilateral compartment. The remainder of the meniscus is secured with an inside-out repair technique, working from posterior to anterior through a standard medial or lateral meniscal repair approach. In total, approximately 6 to 10 vertical mattress sutures are placed, and these can be placed both superiorly and inferiorly on the meniscus. Posteriorly, an all-inside suture repair device may be helpful. Finally, the anterior aspect of the meniscus is repaired to the capsule in an open fashion prior to closing the arthrotomy. Sutures are tied with the leg in extension. The meniscal repair incision is closed in a standard fashion using layers.
5. CONCOMITANT PATHOLOGY AND MAT
The presence of concomitant knee pathology in the context of meniscus deficiency is a challenging problem that requires careful attention to all aspects of the underlying condition of the knee. In cases where MAT is indicated, issues of malalignment, cartilage defects, and/or ligamentous instability may also need to be addressed either concomitantly or in staged fashion. For example, medial meniscal deficiency in the setting of varus alignment can be addressed with a concomitant HTO, whereas lateral meniscal deficiency in the setting of valgus malalignment can be addressed with a concomitant DFO. In both cases, the osteotomy corrects an abnormal mechanical axis, offloading the diseased compartment. This accomplishes 2 goals, namely to preserve the new MAT graft and to protect underlying articular cartilage.22-24 The osteotomy is an important contributor to additional pain relief by offloading the compartment, and clinical studies have demonstrated that failure to address malalignment in the setting of surgical intervention for cartilage and meniscal insufficiency leads to inferior clinical outcomes and poor survival of transplanted tissue.25-28
Continue to: In a meniscus-deficient patient with chondral lesions...
In a meniscus-deficient patient with chondral lesions (Outerbridge grade 3 or 4), concomitant MAT and cartilage restoration should be considered. Depending on the size and location of the chondral lesion, options include marrow stimulation, autologous chondrocyte implantation, osteochondral autograft transfer, as well as chondral and/or osteochondral allograft transplantation. In a systematic review of concomitant MAT and cartilage restoration procedures, Harris and colleagues25 found that failure rates of the combined surgery were similar to those of either surgery in isolation.
Young athletes sustaining anterior cruciate ligament (ACL) tears commonly also have meniscal pathology that must be addressed. Most cases are treated with meniscal repair or partial meniscectomy, but occasionally patients present with ACL tear and symptomatic meniscal deficiency. Specifically, MAT survival relies largely on a knee with ligamentous stability, whereas outcomes of ACL reconstruction are improved with intact and functional menisci.29 The surgical technique for MAT is modified slightly in the setting of performing a concomitant ACL reconstruction, with the ACL tibial tunnel drilled to avoid the meniscal bone slot if possible, followed by femoral tunnel creation. Femoral fixation of the ACL graft is accomplished after preparation of the meniscal slot. The meniscal graft is set into place (sutures are not yet tied), and tibial fixation of the ACL graft is performed next. We typically use an Achilles allograft for the ACL reconstruction, with the bone block used for femoral fixation to avoid bony impingement between the MAT bone bridge/block and the ACL graft. With the knee in full extension, the MAT sutures are tied at the conclusion of the surgical procedure. Concomitant MAT and ACL reconstruction has yielded positive long-term clinical outcomes, improved joint stability, and findings similar to historical results of ACL reconstruction or MAT performed in isolation.30,31
CONCLUSION
When used with the proper indications, MAT has demonstrated the ability to restore function and reduce pain. Successful meniscal transplant requires attention to the patient’s past medical and surgical history. Similarly, care must be taken to address any concomitant knee pathology, such as coronal realignment, ligament reconstruction, or cartilage restoration.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Rep. 2009;11(11):1-25.
2. Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
3. Saltzman BM, Bajaj S, Salata M, et al. Prospective long-term evaluation of meniscal allograft transplantation procedure: a minimum of 7-year follow-up. J Knee Surg. 2012;25(2):165-175. doi:10.1055/s-0032-1313738.
4. van der Wal RJ, Thomassen BJ, van Arkel ER. Long-term clinical outcome of open meniscal allograft transplantation. Am J Sports Med. 2009;37(11):2134-2139. doi:10.1177/0363546509336725.
5. Vundelinckx B, Vanlauwe J, Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. Am J Sports Med. 2014;42(7):1592-1599. doi:10.1177/0363546514530092.
6. Hergan D, Thut D, Sherman O, Day MS. Meniscal allograft transplantation. Arthroscopy. 2011;27(1):101-112. doi:10.1016/j.arthro.2010.05.019.
7. Elattar M, Dhollander A, Verdonk R, Almqvist KF, Verdonk P. Twenty-six years of meniscal allograft transplantation: is it still experimental? A meta-analysis of 44 trials. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):147-157. doi:10.1007/s00167-010-1351-6.
8. Verdonk R, Volpi P, Verdonk P, et al. Indications and limits of meniscal allografts. Injury. 2013;44(Suppl 1):S21-S27. doi:10.1016/S0020-1383(13)70006-8.
9. Frank RM, Yanke A, Verma NN, Cole BJ. Immediate versus delayed meniscus allograft transplantation: letter to the editor. Am J Sports Med. 2015;43(5):NP8-NP9. doi:10.1177/0363546515571065.
10. Aagaard H, Jørgensen U, Bojsen-Møller F. Immediate versus delayed meniscal allograft transplantation in sheep. Clin Orthop Relat Res. 2003;406(406):218-227. doi:10.1097/01.blo.0000030066.92399.7f.
11. Jiang D, Ao YF, Gong X, Wang YJ, Zheng ZZ, Yu JK. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am J Sports Med. 2014;42(10):2329-2337. doi:10.1177/0363546514541653.
12. Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA, Cole BJ. Meniscal allograft transplantation in the adolescent population. Arthroscopy. 2016;32(6):1133-1140.e1. doi:10.1016/j.arthro.2015.11.041.
13. Chalmers PN, Karas V, Sherman SL, Cole BJ. Return to high-level sport after meniscal allograft transplantation. Arthroscopy. 2013;29(3):539-544. doi:10.1016/j.arthro.2012.10.027.
14. Waterman BR, Rensing N, Cameron KL, Owens BD, Pallis M. Survivorship of meniscal allograft transplantation in an athletic patient population. Am J Sports Med. 2016;44(5):1237-1242. doi:10.1177/0363546515626184.
15. Pollard ME, Kang Q, Berg EE. Radiographic sizing for meniscal transplantation. Arthroscopy. 1995;11(6):684-687. doi:10.1016/0749-8063(95)90110-8.
16. Haut TL, Hull ML, Howell SM. Use of roentgenography and magnetic resonance imaging to predict meniscal geometry determined with a three-dimensional coordinate digitizing system. J Orthop Res. 2000;18(2):228-237. doi:10.1002/jor.1100180210.
17. Van Thiel GS, Verma N, Yanke A, Basu S, Farr J, Cole B. Meniscal allograft size can be predicted by height, weight, and gender. Arthroscopy. 2009;25(7):722-727. doi:10.1016/j.arthro.2009.01.004.
18. McConkey M, Lyon C, Bennett DL, et al. Radiographic sizing for meniscal transplantation using 3-D CT reconstruction. J Knee Surg. 2012;25(3):221-225. doi:10.1055/s-0031-1292651.
19. Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35(1):34-42. doi:10.1177/0363546506291404.
20. Yoon JR, Jeong HI, Seo MJ, et al. The use of contralateral knee magnetic resonance imaging to predict meniscal size during meniscal allograft transplantation. Arthroscopy. 2014;30(10):1287-1293. doi:10.1016/j.arthro.2014.05.009.
21. Lee AS, Kang RW, Kroin E, Verma NN, Cole BJ. Allograft meniscus transplantation. Sports Med Arthrosc. 2012;20(2):106-114. doi:10.1097/JSA.0b013e318246f005.
22. Agneskirchner JD, Hurschler C, Wrann CD, Lobenhoffer P. The effects of valgus medial opening wedge high tibial osteotomy on articular cartilage pressure of the knee: a biomechanical study. Arthroscopy. 2007;23(8):852-861. doi:10.1016/j.arthro.2007.05.018.
23. Loening AM, James IE, Levenston ME, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys. 2000;381(2):205-212. doi:10.1006/abbi.2000.1988.
24. Mina C, Garrett WE Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36(5):949-955. doi:10.1177/0363546508315471.
25. Harris JD, Cavo M, Brophy R, Siston R, Flanigan D. Biological knee reconstruction: a systematic review of combined meniscal allograft transplantation and cartilage repair or restoration. Arthroscopy: 2011;27(3):409-418. doi:10.1016/j.arthro.2010.08.007.
26. Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36(9):1770-1778. doi:10.1177/0363546508317122.
27. Kazi HA, Abdel-Rahman W, Brady PA, Cameron JC. Meniscal allograft with or without osteotomy: a 15-year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):303-309. doi:10.1007/s00167-014-3291-z.
28. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. doi:10.1007/s00167-005-0033-2.
29. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28(4):446-452. doi:10.1177/03635465000280040201.
30. Graf KW Jr, Sekiya JK, Wojtys EM; Department of Orthopaedic Surgery, University of Michigan Medical Center, Ann Arbor, Michigan, USA. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20(2):129-140. doi:10.1016/j.arthro.2003.11.032.
31. Binnet MS, Akan B, Kaya A. Lyophilised medial meniscus transplantations in ACL-deficient knees: a 19-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):109-113. doi:10.1007/s00167-011-1556-3.
TAKE-HOME POINTS
- Patient selection is critical for obtaining long-term functional outcome improvements and reduced pain, with the ideal MAT candidate being a chronologically and physiologically young patient (<50 years) with symptomatic meniscal deficiency.
- Existing pathology in the knee needs to be carefully considered and issues such as malalignment, cartilage defects, and/or ligamentous instability may require a staged or concomitant procedure.
- Accurate graft width and length measurements are vital, and the most common technique used today includes measuring the meniscus on anteroposterior and lateral radiographic images.
- When preparing the graft for the bone-bridge technique, the bone is fashioned to create a bone bridge 10 mm in depth by approximately 7 mm in width, incorporating the anterior and posterior horns of the meniscus.
- Graft fixation can be accomplished by placing vertical mattress sutures and tying those down with the knee in full extension.
Debunking Atopic Dermatitis Myths: Can Adults Develop Eczema?
Myth: Atopic Dermatitis Does Not Start in Adulthood
Atopic dermatitis (AD) typically first appears in childhood and tends to disappear before puberty begins; however, some patients experience AD that persists into adulthood or occurs de novo. Bannister and Freeman coined the term adult-onset atopic dermatitis after reviewing 2604 cases of AD and noting that 243 patients (9%) were first diagnosed with AD at 20 years of age or older. Adult-onset AD may be its own subset of AD or childhood AD that was simply not diagnosed until adulthood or was forgotten by the patient.
Characteristically, AD presents in adults as inflammatory eczema with areas of lichenification. It could occur after a change in residence to a cold dry climate or exposure to central heating, as patients who grew up in warm, sunny, humid climates might not have had diagnosable AD in childhood or adolescence. The more common forms of adult-onset AD are hand and neck dermatitis, hand eczema, nummular eczema, or prurigo, while childhood AD often manifests in a flexural distribution. Because it is difficult to detect, adult-onset AD is diagnosed after ruling out other diseases. Diagnostic procedures, such as patch tests, skin prick tests, biopsies, or blood screenings, usually are necessary to rule out other diseases or types of eczema. Contact eczema is the first diagnostic sign of AD in adults.
Maintaining AD in the differential diagnosis for patients with clinical symptoms of pruritus and eczema is essential due to the quality of life impact of the condition. Sleep disturbance is common in adults with severe AD and treatment may help to improve sleep quality.
Hanifin suggested the following when assessing adults for AD:
- Verify diagnosis (not allergic contact dermatitis or psoriasis)
- Determine patient's history of allergies (eg, food allergy) or childhood eczema
- Obtain family history of eczema/allergies
- Evaluate if patient's occupation may impact condition (eg, contact with irritants or known contact allergens)
- Inquire about patient's childhood residence (eg, tropical climate)
Adult-onset AD is a recalcitrant condition that can be difficult to treat, and appropriately labeling/diagnosing the condition will lead to better management.
Expert Commentary
Whereas once it was the rite of the pediatric AD patient to outgrow disease, it has now become clear that resolution is not as hard a stop in atopic disease as expected. Adult-onset and persistent disease in AD is clearly a significant problem, especially in developed nations and carries a host of comorbidites. Time and enhanced research will hopefully identify interventions to reverse the trend towards persistence into adulthood.
—Nanette B. Silverberg, MD (New York, New York)
Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225-228.
Hanifin JM. Adult-onset atopic dermatitis: fact or fancy? Dermatol Clin. 2017;35:299-302.
Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78-88.
Myth: Atopic Dermatitis Does Not Start in Adulthood
Atopic dermatitis (AD) typically first appears in childhood and tends to disappear before puberty begins; however, some patients experience AD that persists into adulthood or occurs de novo. Bannister and Freeman coined the term adult-onset atopic dermatitis after reviewing 2604 cases of AD and noting that 243 patients (9%) were first diagnosed with AD at 20 years of age or older. Adult-onset AD may be its own subset of AD or childhood AD that was simply not diagnosed until adulthood or was forgotten by the patient.
Characteristically, AD presents in adults as inflammatory eczema with areas of lichenification. It could occur after a change in residence to a cold dry climate or exposure to central heating, as patients who grew up in warm, sunny, humid climates might not have had diagnosable AD in childhood or adolescence. The more common forms of adult-onset AD are hand and neck dermatitis, hand eczema, nummular eczema, or prurigo, while childhood AD often manifests in a flexural distribution. Because it is difficult to detect, adult-onset AD is diagnosed after ruling out other diseases. Diagnostic procedures, such as patch tests, skin prick tests, biopsies, or blood screenings, usually are necessary to rule out other diseases or types of eczema. Contact eczema is the first diagnostic sign of AD in adults.
Maintaining AD in the differential diagnosis for patients with clinical symptoms of pruritus and eczema is essential due to the quality of life impact of the condition. Sleep disturbance is common in adults with severe AD and treatment may help to improve sleep quality.
Hanifin suggested the following when assessing adults for AD:
- Verify diagnosis (not allergic contact dermatitis or psoriasis)
- Determine patient's history of allergies (eg, food allergy) or childhood eczema
- Obtain family history of eczema/allergies
- Evaluate if patient's occupation may impact condition (eg, contact with irritants or known contact allergens)
- Inquire about patient's childhood residence (eg, tropical climate)
Adult-onset AD is a recalcitrant condition that can be difficult to treat, and appropriately labeling/diagnosing the condition will lead to better management.
Expert Commentary
Whereas once it was the rite of the pediatric AD patient to outgrow disease, it has now become clear that resolution is not as hard a stop in atopic disease as expected. Adult-onset and persistent disease in AD is clearly a significant problem, especially in developed nations and carries a host of comorbidites. Time and enhanced research will hopefully identify interventions to reverse the trend towards persistence into adulthood.
—Nanette B. Silverberg, MD (New York, New York)
Myth: Atopic Dermatitis Does Not Start in Adulthood
Atopic dermatitis (AD) typically first appears in childhood and tends to disappear before puberty begins; however, some patients experience AD that persists into adulthood or occurs de novo. Bannister and Freeman coined the term adult-onset atopic dermatitis after reviewing 2604 cases of AD and noting that 243 patients (9%) were first diagnosed with AD at 20 years of age or older. Adult-onset AD may be its own subset of AD or childhood AD that was simply not diagnosed until adulthood or was forgotten by the patient.
Characteristically, AD presents in adults as inflammatory eczema with areas of lichenification. It could occur after a change in residence to a cold dry climate or exposure to central heating, as patients who grew up in warm, sunny, humid climates might not have had diagnosable AD in childhood or adolescence. The more common forms of adult-onset AD are hand and neck dermatitis, hand eczema, nummular eczema, or prurigo, while childhood AD often manifests in a flexural distribution. Because it is difficult to detect, adult-onset AD is diagnosed after ruling out other diseases. Diagnostic procedures, such as patch tests, skin prick tests, biopsies, or blood screenings, usually are necessary to rule out other diseases or types of eczema. Contact eczema is the first diagnostic sign of AD in adults.
Maintaining AD in the differential diagnosis for patients with clinical symptoms of pruritus and eczema is essential due to the quality of life impact of the condition. Sleep disturbance is common in adults with severe AD and treatment may help to improve sleep quality.
Hanifin suggested the following when assessing adults for AD:
- Verify diagnosis (not allergic contact dermatitis or psoriasis)
- Determine patient's history of allergies (eg, food allergy) or childhood eczema
- Obtain family history of eczema/allergies
- Evaluate if patient's occupation may impact condition (eg, contact with irritants or known contact allergens)
- Inquire about patient's childhood residence (eg, tropical climate)
Adult-onset AD is a recalcitrant condition that can be difficult to treat, and appropriately labeling/diagnosing the condition will lead to better management.
Expert Commentary
Whereas once it was the rite of the pediatric AD patient to outgrow disease, it has now become clear that resolution is not as hard a stop in atopic disease as expected. Adult-onset and persistent disease in AD is clearly a significant problem, especially in developed nations and carries a host of comorbidites. Time and enhanced research will hopefully identify interventions to reverse the trend towards persistence into adulthood.
—Nanette B. Silverberg, MD (New York, New York)
Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225-228.
Hanifin JM. Adult-onset atopic dermatitis: fact or fancy? Dermatol Clin. 2017;35:299-302.
Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78-88.
Bannister MJ, Freeman S. Adult-onset atopic dermatitis. Australas J Dermatol. 2000;41:225-228.
Hanifin JM. Adult-onset atopic dermatitis: fact or fancy? Dermatol Clin. 2017;35:299-302.
Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27:78-88.
Debunking Atopic Dermatitis Myths: Should You Use Systemic Therapy?
Myth: Because atopic dermatitis is skin-deep, systemic therapy is unnecessary.
Although atopic dermatitis (AD) primarily is known as a skin condition, recent research has indicated that it may be the start of the “atopic march” leading to the development of 1 or more other atopic conditions with multiorgan involvement. In infancy AD can progress to asthma and allergic rhinitis. Adult AD can be accompanied by systemic diseases such as inflammatory bowel disease, nephritic syndrome, and others. There also is a link between impairment of epidermal barrier function and disturbed skin microbiome in patients with AD. Therefore, systemic therapy may be warranted; the question is when should you use systemic therapy?
Most AD patients have mild to moderate disease that responds well to emollients and avoidance of disease triggers and other skin irritants. However, many AD patients experience a more severe disease course that does not respond adequately to topical therapy. For these patients, systemic therapy is a viable treatment option to improve quality of life (QOL), prevent flares, and control skin inflammation and other AD symptoms.
In 2017 an expert panel of the International Eczema Council proposed an algorithm to be used to determine if systemic therapy is warranted in patients with AD. Dermatologists must consider disease severity, impact on QOL, and risks and benefits of systemic therapies. Before starting systemic therapy, the panel recommends the following:
- Consider alternate or concomitant diagnoses
- Avoid triggers
- Optimize topical therapy
- Ensure adequate patient/caregiver education
- Treat coexistent infection
- Assess QOL
- Consider phototherapy
The American Academy of Dermatology established Guidelines of Care for the Management of AD in 2014, which provide recommendations for the most efficacious systemic agents.
Armed with these guidelines, dermatologists can work with patients to determine the most appropriate treatment course for this condition that is more than skin-deep.
Expert Commentary
Atopic dermatitis is a skin barrier abnormality that causes inflammatory skin disease and an inflammatory disorder triggering abnormal barrier. Whether we choose the outside-in or inside-out approach, it is clear that there is a systemic inflammation associated with skin disease. It is true that children respond well to barrier repair and topical therapy in many settings, as do many adults. However, chronic skin inflammation is not in isolation, triggering mucosal barrier changes allowing for more sensitization to foods and respiratory allergens as well as systemic inflammation in adults. Despite the utility of systemic steroids, the side effects generally outweigh benefit. On the other hand, phototherapy and systemic agents can clear skin and induce remissions and improved QOL. The AAD guidelines were reported before US Food and Drug Administration approval of newer agents such as dupilumab, leaving it up to the dermatologist to find the niche for this first biologic agent for AD.
—Nanette B. Silverberg, MD
Suggested Readings
Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease [published online November 22, 2013]. Clin Dermatol. 2014;32:409-413.
Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents [published online May 9, 2014]. J Am Acad Dermatol. 2014;71:327-349.
Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? recommendations from an expert panel of the International Eczema Council [published online August 10, 2017]. J Am Acad Dermatol. 2017;77:623-633.
Thomas CL, Fernández-Peñas P. The microbiome and atopic eczema: more than skin deep [published online January 28, 2016]. Australas J Dermatol. 2017;58:18-24.
Myth: Because atopic dermatitis is skin-deep, systemic therapy is unnecessary.
Although atopic dermatitis (AD) primarily is known as a skin condition, recent research has indicated that it may be the start of the “atopic march” leading to the development of 1 or more other atopic conditions with multiorgan involvement. In infancy AD can progress to asthma and allergic rhinitis. Adult AD can be accompanied by systemic diseases such as inflammatory bowel disease, nephritic syndrome, and others. There also is a link between impairment of epidermal barrier function and disturbed skin microbiome in patients with AD. Therefore, systemic therapy may be warranted; the question is when should you use systemic therapy?
Most AD patients have mild to moderate disease that responds well to emollients and avoidance of disease triggers and other skin irritants. However, many AD patients experience a more severe disease course that does not respond adequately to topical therapy. For these patients, systemic therapy is a viable treatment option to improve quality of life (QOL), prevent flares, and control skin inflammation and other AD symptoms.
In 2017 an expert panel of the International Eczema Council proposed an algorithm to be used to determine if systemic therapy is warranted in patients with AD. Dermatologists must consider disease severity, impact on QOL, and risks and benefits of systemic therapies. Before starting systemic therapy, the panel recommends the following:
- Consider alternate or concomitant diagnoses
- Avoid triggers
- Optimize topical therapy
- Ensure adequate patient/caregiver education
- Treat coexistent infection
- Assess QOL
- Consider phototherapy
The American Academy of Dermatology established Guidelines of Care for the Management of AD in 2014, which provide recommendations for the most efficacious systemic agents.
Armed with these guidelines, dermatologists can work with patients to determine the most appropriate treatment course for this condition that is more than skin-deep.
Expert Commentary
Atopic dermatitis is a skin barrier abnormality that causes inflammatory skin disease and an inflammatory disorder triggering abnormal barrier. Whether we choose the outside-in or inside-out approach, it is clear that there is a systemic inflammation associated with skin disease. It is true that children respond well to barrier repair and topical therapy in many settings, as do many adults. However, chronic skin inflammation is not in isolation, triggering mucosal barrier changes allowing for more sensitization to foods and respiratory allergens as well as systemic inflammation in adults. Despite the utility of systemic steroids, the side effects generally outweigh benefit. On the other hand, phototherapy and systemic agents can clear skin and induce remissions and improved QOL. The AAD guidelines were reported before US Food and Drug Administration approval of newer agents such as dupilumab, leaving it up to the dermatologist to find the niche for this first biologic agent for AD.
—Nanette B. Silverberg, MD
Suggested Readings
Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease [published online November 22, 2013]. Clin Dermatol. 2014;32:409-413.
Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents [published online May 9, 2014]. J Am Acad Dermatol. 2014;71:327-349.
Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? recommendations from an expert panel of the International Eczema Council [published online August 10, 2017]. J Am Acad Dermatol. 2017;77:623-633.
Thomas CL, Fernández-Peñas P. The microbiome and atopic eczema: more than skin deep [published online January 28, 2016]. Australas J Dermatol. 2017;58:18-24.
Myth: Because atopic dermatitis is skin-deep, systemic therapy is unnecessary.
Although atopic dermatitis (AD) primarily is known as a skin condition, recent research has indicated that it may be the start of the “atopic march” leading to the development of 1 or more other atopic conditions with multiorgan involvement. In infancy AD can progress to asthma and allergic rhinitis. Adult AD can be accompanied by systemic diseases such as inflammatory bowel disease, nephritic syndrome, and others. There also is a link between impairment of epidermal barrier function and disturbed skin microbiome in patients with AD. Therefore, systemic therapy may be warranted; the question is when should you use systemic therapy?
Most AD patients have mild to moderate disease that responds well to emollients and avoidance of disease triggers and other skin irritants. However, many AD patients experience a more severe disease course that does not respond adequately to topical therapy. For these patients, systemic therapy is a viable treatment option to improve quality of life (QOL), prevent flares, and control skin inflammation and other AD symptoms.
In 2017 an expert panel of the International Eczema Council proposed an algorithm to be used to determine if systemic therapy is warranted in patients with AD. Dermatologists must consider disease severity, impact on QOL, and risks and benefits of systemic therapies. Before starting systemic therapy, the panel recommends the following:
- Consider alternate or concomitant diagnoses
- Avoid triggers
- Optimize topical therapy
- Ensure adequate patient/caregiver education
- Treat coexistent infection
- Assess QOL
- Consider phototherapy
The American Academy of Dermatology established Guidelines of Care for the Management of AD in 2014, which provide recommendations for the most efficacious systemic agents.
Armed with these guidelines, dermatologists can work with patients to determine the most appropriate treatment course for this condition that is more than skin-deep.
Expert Commentary
Atopic dermatitis is a skin barrier abnormality that causes inflammatory skin disease and an inflammatory disorder triggering abnormal barrier. Whether we choose the outside-in or inside-out approach, it is clear that there is a systemic inflammation associated with skin disease. It is true that children respond well to barrier repair and topical therapy in many settings, as do many adults. However, chronic skin inflammation is not in isolation, triggering mucosal barrier changes allowing for more sensitization to foods and respiratory allergens as well as systemic inflammation in adults. Despite the utility of systemic steroids, the side effects generally outweigh benefit. On the other hand, phototherapy and systemic agents can clear skin and induce remissions and improved QOL. The AAD guidelines were reported before US Food and Drug Administration approval of newer agents such as dupilumab, leaving it up to the dermatologist to find the niche for this first biologic agent for AD.
—Nanette B. Silverberg, MD
Suggested Readings
Darlenski R, Kazandjieva J, Hristakieva E, et al. Atopic dermatitis as a systemic disease [published online November 22, 2013]. Clin Dermatol. 2014;32:409-413.
Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents [published online May 9, 2014]. J Am Acad Dermatol. 2014;71:327-349.
Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? recommendations from an expert panel of the International Eczema Council [published online August 10, 2017]. J Am Acad Dermatol. 2017;77:623-633.
Thomas CL, Fernández-Peñas P. The microbiome and atopic eczema: more than skin deep [published online January 28, 2016]. Australas J Dermatol. 2017;58:18-24.
A Novel Technique for the Treatment of Jersey Fingers
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
TAKE-HOME POINTS
- Transosseous repair of FDP has been long utilized, tying the sutures over a polyethylene button at the nail plate, which is associated with significant complications.
- Avoiding use of a button decreases these complications, eliminating damage to the nailbed and eliminating external sutures, thus decreasing infection risk.
- Keith needles can be utilized to pass the sutures from volar to dorsal, and can be inserted using a wire drive; their position can be checked fluoroscopically prior to suture passage.
- This technique can be used in conjunction with skeletal fixation of associated fractures.
- This technique can be utilized in pediatric patients, placing the sutures distal to the physis.
Debunking Atopic Dermatitis Myths: Do Most Children Outgrow Atopic Dermatitis?
Myth: Children eventually outgrow atopic dermatitis and therefore do not need treatment
The negative impact of atopic dermatitis (AD) on quality of life in the pediatric population often prompts parents/guardians to inquire about whether a child with AD will ever outgrow their disease. If remission is expected as the child gets older, many may question if it is necessary to pursue treatment or just let the disease run its course. Although AD often is reported to resolve soon after the first decade of life, symptoms can persist well into the second decade and beyond, suggesting that AD may be a lifelong disease with periods of waxing and waning symptoms that require persistent treatment throughout the patient’s life.
A 2014 study included 7157 children with AD (mean age of disease onset, 1.7 years) who were enrolled in the Pediatric Eczema Elective Registry (PEER) program between the ages of 2 and 17 years with measurement of disease activity at regular 6-month intervals for up to 5 years. The study results indicated that more than 80% of patients at every age (age range, 2–26 years) had symptoms of AD and/or were using medication to treat their disease, and the majority (64%) of patients had never reported a 6-month period during which they achieved clearance of symptoms without medication. At the age of 20 years, 50% of patients reported at least 1 lifetime 6-month period during which they were both symptom and treatment free. In another study of adolescents with AD who also had AD in childhood (N=82), 48% of patients remained in the same AD severity grades and 13% deteriorated from childhood to adolescence; only 39% of patients showed improvement in disease severity from childhood to adolescence. The findings of these reports are contradictory to conventional clinical teaching, which indicates that AD generally resolves by age 12 in 50% to 70% of children.
Even though some children with AD may experience periods of disease clearance, these findings often do not persist and should not be confused with a permanent remission. Most patients require continued treatment with medications to achieve relief of symptoms. Therefore, physicians should not assure parents/guardians that a child can outgrow AD; rather, they should educate pediatric patients and their caregivers about the potentially lifelong disease course and encourage early intervention to mitigate symptoms and manage comorbidities as the patient ages.
Hon KL, Tsang YCK, Poon TCW, et al. Predicating eczema severity beyond childhood. World J Pediatr. 2016;12:44-48.
Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis [published online April 2, 2014]. JAMA Dermatol. 2014;150:593-600.
Myth: Children eventually outgrow atopic dermatitis and therefore do not need treatment
The negative impact of atopic dermatitis (AD) on quality of life in the pediatric population often prompts parents/guardians to inquire about whether a child with AD will ever outgrow their disease. If remission is expected as the child gets older, many may question if it is necessary to pursue treatment or just let the disease run its course. Although AD often is reported to resolve soon after the first decade of life, symptoms can persist well into the second decade and beyond, suggesting that AD may be a lifelong disease with periods of waxing and waning symptoms that require persistent treatment throughout the patient’s life.
A 2014 study included 7157 children with AD (mean age of disease onset, 1.7 years) who were enrolled in the Pediatric Eczema Elective Registry (PEER) program between the ages of 2 and 17 years with measurement of disease activity at regular 6-month intervals for up to 5 years. The study results indicated that more than 80% of patients at every age (age range, 2–26 years) had symptoms of AD and/or were using medication to treat their disease, and the majority (64%) of patients had never reported a 6-month period during which they achieved clearance of symptoms without medication. At the age of 20 years, 50% of patients reported at least 1 lifetime 6-month period during which they were both symptom and treatment free. In another study of adolescents with AD who also had AD in childhood (N=82), 48% of patients remained in the same AD severity grades and 13% deteriorated from childhood to adolescence; only 39% of patients showed improvement in disease severity from childhood to adolescence. The findings of these reports are contradictory to conventional clinical teaching, which indicates that AD generally resolves by age 12 in 50% to 70% of children.
Even though some children with AD may experience periods of disease clearance, these findings often do not persist and should not be confused with a permanent remission. Most patients require continued treatment with medications to achieve relief of symptoms. Therefore, physicians should not assure parents/guardians that a child can outgrow AD; rather, they should educate pediatric patients and their caregivers about the potentially lifelong disease course and encourage early intervention to mitigate symptoms and manage comorbidities as the patient ages.
Myth: Children eventually outgrow atopic dermatitis and therefore do not need treatment
The negative impact of atopic dermatitis (AD) on quality of life in the pediatric population often prompts parents/guardians to inquire about whether a child with AD will ever outgrow their disease. If remission is expected as the child gets older, many may question if it is necessary to pursue treatment or just let the disease run its course. Although AD often is reported to resolve soon after the first decade of life, symptoms can persist well into the second decade and beyond, suggesting that AD may be a lifelong disease with periods of waxing and waning symptoms that require persistent treatment throughout the patient’s life.
A 2014 study included 7157 children with AD (mean age of disease onset, 1.7 years) who were enrolled in the Pediatric Eczema Elective Registry (PEER) program between the ages of 2 and 17 years with measurement of disease activity at regular 6-month intervals for up to 5 years. The study results indicated that more than 80% of patients at every age (age range, 2–26 years) had symptoms of AD and/or were using medication to treat their disease, and the majority (64%) of patients had never reported a 6-month period during which they achieved clearance of symptoms without medication. At the age of 20 years, 50% of patients reported at least 1 lifetime 6-month period during which they were both symptom and treatment free. In another study of adolescents with AD who also had AD in childhood (N=82), 48% of patients remained in the same AD severity grades and 13% deteriorated from childhood to adolescence; only 39% of patients showed improvement in disease severity from childhood to adolescence. The findings of these reports are contradictory to conventional clinical teaching, which indicates that AD generally resolves by age 12 in 50% to 70% of children.
Even though some children with AD may experience periods of disease clearance, these findings often do not persist and should not be confused with a permanent remission. Most patients require continued treatment with medications to achieve relief of symptoms. Therefore, physicians should not assure parents/guardians that a child can outgrow AD; rather, they should educate pediatric patients and their caregivers about the potentially lifelong disease course and encourage early intervention to mitigate symptoms and manage comorbidities as the patient ages.
Hon KL, Tsang YCK, Poon TCW, et al. Predicating eczema severity beyond childhood. World J Pediatr. 2016;12:44-48.
Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis [published online April 2, 2014]. JAMA Dermatol. 2014;150:593-600.
Hon KL, Tsang YCK, Poon TCW, et al. Predicating eczema severity beyond childhood. World J Pediatr. 2016;12:44-48.
Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis [published online April 2, 2014]. JAMA Dermatol. 2014;150:593-600.
Glenoid Bone Loss in Reverse Shoulder Arthroplasty Treated with Bone Graft Techniques
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).
In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8
Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9
All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.
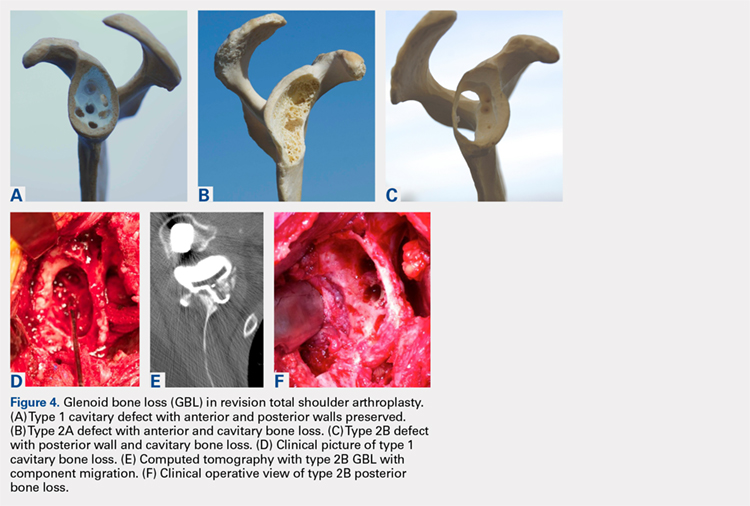
Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).
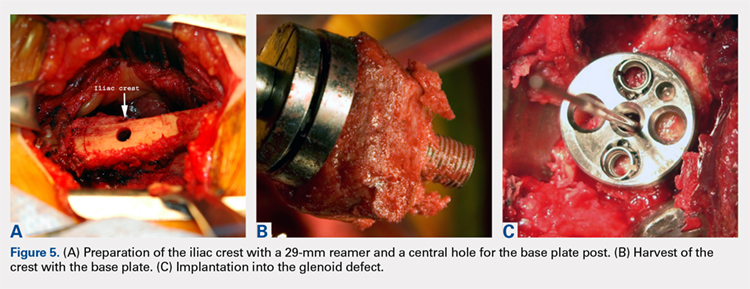
This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).
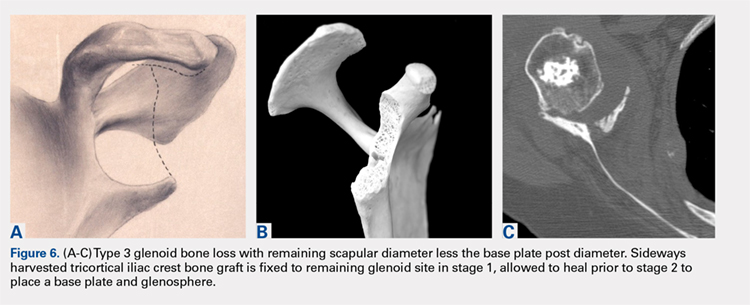
An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26
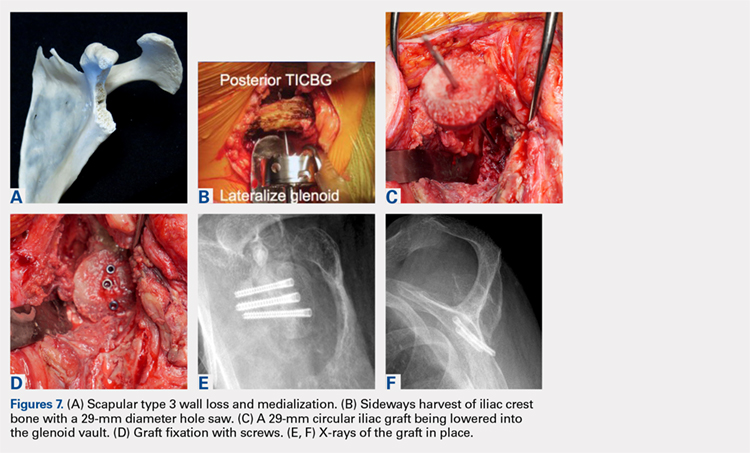
CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.
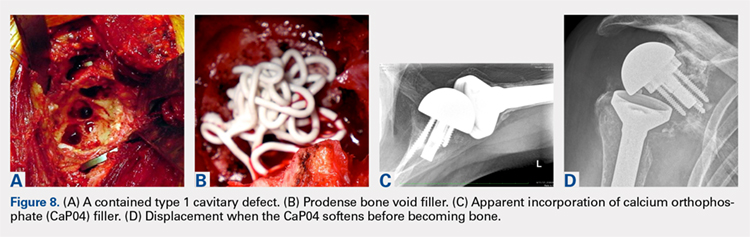
For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).

In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8

Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9

All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.

Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).

This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).

An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26

CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.

For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
ABSTRACT
The reverse shoulder arthroplasty facilitates surgical treatment of primary and revision shoulder with rotator cuff and bone deficiencies. Wear pattern classifications and a logical treatment approach for glenoid bone loss enable the surgeon to address a difficult series of problems in the reconstructions where the glenoid might not otherwise be able to support the implants. Bone grafting using the native humeral head in primary cases, and in revision cases, iliac crest are the most reliable sources for structural grafts for the worn or deficient glenoid vault.
Continue to: The reverse shoulder arthroplasty...
The reverse shoulder arthroplasty (RSA) technique was approved by the US Food and Drug Administration and introduced to the US market in 2004. It has been a successful addition to the treatment of shoulder pathologies with bone and rotator cuff loss. Its indications have expanded from treatment of very elderly patients with rotator cuff deficiencies to now include younger patients with humeral and glenoid bone loss, arthritis, soft-tissue losses, fractures, instability, and revision arthroplasty. Many of these conditions, when not adequately addressed with anatomic arthroplasty, now have viable treatment options for newer complex and successful reconstructions.
Glenoid bone deficiencies offer unique challenges for successful arthroplasty management. Basing treatment on bone loss classifications permits meaningful evaluation of these surgical options and whether they might be carried out in 1- or 2-stage reconstructions. An underlying premise is that restoration of the glenoid joint line and version assist in final stability, power, and functional results. For this purpose, bone graft options, or augmented implants are beneficial. This review covers the bone grafting options for autografts and allografts for deficient glenoids in reverse shoulder arthroplasty reconstructions.
OPERATIVE TECHNIQUES
For patients without prior arthroplasty, the humeral head is available for bone grafting the glenoid bone deficits. Favard and Hamada have described vertical glenoid classifications for uneven glenoid bone loss applicable to cuff tear arthropathy and inflammatory arthritis patients.1,2 The more severe E3 superior and medial bone loss is ideally addressed with the humeral head. An early example in 2004 confirmed that this was a good indication for glenoid bone grafting and using the reverse shoulder in these advanced cases (Figures 1A-1E).

In this case, it was noted that with bone grafts the base plate post did not engage the native scapula glenoid vault. Given that the on-growth central post was the strongest part of the fixation, it was fortunate that this healed. The need for a longer post with bone grafts was recognized. Laurent Comtat with the Wright Medical company accommodated the author’s request to develop the first 25- and 30-mm-long posts to allow better fixation and on-growth potential when used with bone grafts.
Gilles Walch’s classification addresses arthritic central and horizontal bone loss.3,4 Considerations relevant in RSA include the severe A2 central bone loss found in inflammatory arthritis and the B2, B3, and C patterns with posterior bone loss seen in osteoarthritis, rheumatoid arthritis, and congenital dysplasia5,6 as seen in Figures 2A, 2B. The 3-dimensional (3-D) computed tomography (CT) scan is considered the most accurate method of assessment when compared with axial radiographs.7 The glenoid vault model as a measurement of glenoid bone loss has great promise in designing prosthetic replacements and bone graft techniques.8

Continue to: Modern methods for determining glenoid version...
Modern methods for determining glenoid version, medialization, and eccentric bone wear include 3-D reconstruction and patient-specific instruments. For many years, version determination has been confirmed at surgery with subscapularis elevation, palpating the glenoid center point along Friedman’s line, and then inserting a Steinmann pin as a guide to restore version and the lateral joint line at the time of bone grafting. An example of this is demonstrated in Figures 3A-3E.9

All grafts are harvested with a hole saw from the humeral head. The inner diameter is 29 mm, the same as that of the base plate. Originally, the hole saw and mandrel were obtained from the hardware store, but Pascal Boileau upgraded the hole saw quality when he had industry develop a stainless-steel hole saw and published his results with the BIO-RSA (Wright Medical).10 In an unpublished study, Harmsen reviewed our 220 consecutive humeral head bone grafts for use of this technique with successful and reproducible results. In a separate evaluation, 29 shaped humeral head bone grafts for B2, B3, and C glenoid bone deficits showed 100% healing.11 This technique has good reproducibility when performed with an autogenous bone graft from a local donor source.
The more challenging cases involve glenoid bone loss from polyethylene osteolysis and, in some revision cases, concomitant sepsis.12 The humeral head is no longer available, and the distal clavicle or humeral metaphysis are often insufficient to restore the glenoid vault and joint line. Gunther and associates at the UC Berkeley biomaterials laboratory have made many contributions to our understanding of polyethylene wear and the factors leading to its loosening that result in massive glenoid bone loss.13
Antuna and colleagues14 classified these cases as having a central vault cavitary defect, or one combined with a peripheral glenoid wall bone loss of either the anterior or posterior glenoid. Newton and colleagues15 described the structural tricortical iliac crest bone graft as a 2-stage reconstruction. The second stage could be performed 4 to 6 months later after graft incorporation. With the excellent Association for Osteosynthesis (AO) type fixation using the base plate with compression and locking screws, it was reasonable to perform this in 1 stage, assuming that adequate fixation could be obtained with the iliac bone graft to the glenoid.16 This worked well with the cavitary glenoid defects and those in which either the anterior or posterior wall was absent.17-19
EXCEPTIONS TO THE 1-STAGE FIXATION TECHNIQUE
Fixation could still be obtained medially, but more severe cases were encountered with loss of both the anterior and posterior walls. In these more advanced cases, the vault was no longer present after removal of the polyethylene, cement, and rubbery osteolytic tissue that replaced the bone. To account for this, a simplified 3-stage classification was proposed.20 The cavitary vault defect is designated as type 1 bone loss. Type 2A includes the cavitary central defect plus loss of the anterior glenoid wall, and 2B is similar with loss of the posterior wall (Figures 4A-4F). Type 3 involves loss of the glenoid vault and both anterior and posterior walls with erosion down to the medial juncture of the base of the scapular spine, coracoid, and pillar of the scapula.

Continue to: The tricortical iliac crest bone graft...
The tricortical iliac crest bone graft (TICBG) offered a structural graft that worked well for these cases of bone loss. When the graft is performed in 1 stage, the glenoid is exposed, and the defect measured after removing the osteolytic, polyethylene-laden tissue from the glenoid. The iliac graft is harvested and placed with the long post base plate engaging the native scapula medially (Figures 5A-5C).

This technique worked well with the type 1 and 2 defects, but when attempted with the type 3 glenoid defect with global glenoid bone loss, adequate fixation for a single-stage reconstruction could not be predictably obtained with type 3 loss of the vault and both walls. In this situation, the base plate post is wider than the remaining medialized glenoid vault (Figures 6A-6C). The iliac crest provides better bone for this global loss when harvested sideways, fixed with screws, and after secure healing, the second-stage base plate is placed (Figures 7A-7F).

An alternative to the iliac crest as a bone graft donor site is the femoral neck allograft.21 It avoids the additional surgery and pain at the donor site, but healing is less assured. Scalise and Iannotti22 have had good clinical results but noted substantial graft resorption when revising a total shoulder to a humeral head arthroplasty. In a recent report by Ozgur and colleagues,23 64% of femoral neck allografts were still intact at 1-year follow-up. The technique involved harvesting the graft with a hole saw, shaping and affixing it to the deficient glenoid, and gaining central fixation with a threaded or solid post base plate and peripheral screws. Poor results were obtained with the use of the femoral shaft, as it is brittle. Angled peripheral screws caused the allograft shaft to fracture. Low-grade sepsis remained an unanswered problem in the patient group, which averaged 6 prior procedures, and often led to another revision. Less favorable results were found using the 1-piece threaded post base plate with grafts.24 It is assumed that the allograft has less healing potential, and micro motion plays a role when the long central screw has no on-growth healing potential in the native scapula. This graft choice is the author’s least favorite, but is available in desperate situations. Jones and colleagues25 report promising results with bulk allografts and autografts for large glenoid defects with good clinical results. The results in the graft cohort were inferior to those in a matched group not requiring grafts. Their complications were consistent with the revision setting for shoulders having multiple operations. It is well known that preoperative factors are strong predictors of postoperative outcomes.26

CONCLUSION
The author’s current technique is to use the native humeral head when available, or iliac crest for structural support to the base plate and glenosphere. Secure fixation to the native scapula is necessary if the operation is to be done in 1-stage. Incorporation with calcium orthophosphate bone substitution does not replace the need for structural support as shown in Figures 8A-8D.

For the type 2 vault and 1 wall glenoid bone loss defects, the TICBG is still the most useful option. For the type 3 global bone loss defects, a 2-stage approach is the safer option. Additional options that may replace some of these grafting techniques are the introduction of the metallic augmented ingrowth base plates to correct for superior, anterior, and posterior glenoid bone losses. The early unpublished experiences by Wright and colleagues are very promising. All of the above options should be available in the operating room for a busy arthroplasty surgeon.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
1. Hamada K, Fukuda H, Mikasa M, Kobay Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990;254:92-96.
2. Favard L, Alami G. The glenoid in the frontal plane: The Favard and Hamada radiographic classifications of cuff tear osteoarthritis. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:53-58.
3. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Walch G. The glenoid in the horizontal plane: Walch classification revisited humeral subluxation and glenoid retroversion. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:45-51.
4. Walch G, Badet R, Boulahia A, Khoury A. Morphological study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
5. Iannotti JP, Ricchetti E. Walch classification: adding two new glenoid types. Orthopaedic Insights Cleveland Clinic. 2017:6-7.
6. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse shoulder arthroplasty for primary glenohumeral osteoarthritis with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
7. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs verses computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
8. Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional compute tomography scans. [published online ahead of print January 22, 2008]. J Shoulder Elbow Surg. 2008;17(2):328-335.
9. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
10. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reverse shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558-2567.
11. Harmsen S, Casagrande D, Norris T: “Shaped” humeral head autograft reverse shoulder arthroplasty: Treatment for primary glenohumeral osteoarthritis with significant posterior glenoid bone loss (B2, B3, and C-type). Orthopade. 2017;46(12):1045-1054.
12. Norris TR, Phipatanakul WP. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15(1):84-87.
13. Farzana F, Lee T, Malito L, et al. Analysis of severely fractured glenoid components: clinical consequences of biomechanics, design, and materials selection on implant performance. J Shoulder Elbow Surg. 2016;25(7):1041-1050.
14. Antuña SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
15. Newton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid cortical cancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15(2):173-179.
16. Norris TR, Kelly JD, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Techniques Shoulder Elbow Surgery. 2007;8(1):37-46.
17. Kelly JD II, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525.
18. Norris TR. Reconstruction of glenoid bone loss in total shoulder arthroplasty. In: Boileau P, ed. Shoulder Concepts 2008-Arthroscopy and Arthroplasty. Paris, France: Sauramps Medical; 2008:397-404.
19. Humphrey CS, Kelly JD, Norris TR. Management of glenoid deficiency in reverse shoulder arthroplasty. In: Fealy S, Warren RF, Craig EV, Sperling JW, eds. Shoulder Arthroplasty. New York, NY: Thieme; 2006.
20. Norris TR, Abdus-Salaam S. Lessons learned from the Hylamer experience and technical salvage for glenoid reconstruction. In: Walch G, Boileau P, Favard ML, Lévigne C, Sirveaux F, eds. Shoulder Concepts 2010: The Glenoid. Paris, France: Sauramps Medical; 2010:265-278.
21. Bateman E, Donald SM. Reconstruction of massive uncontained glenoid defects using a combined autograft-allograft construct with reverse shoulder arthroplasty: preliminary results. J Shoulder Elbow Surg. 2012;21(7):925-934.
22. Scalise JJ, Iannotti JP. Bone grafting severe glenoid defects in revision shoulder arthroplasty. Clin Orthop. 2008;466(1):139-145.
23. Ozgur S, Sadeghpour R, Norris TR. Revision shoulder arthroplasty with a reverse shoulder prosthesis. Use of structural allograft for glenoid bone loss. Orthopade. 2017;46(12):1055-1062.
24. Sadeghpour R, Ozgur S, Norris TR. Threaded post baseplate failures in RSA. In: Hardy PH, Valenti PH, Scheibel M, eds. Shoulder Arthroplasty, Current Concepts. Paris International Shoulder Course 2017. 2017:148-157.
25. Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425-1432.
26. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg. 2003;85(2):251-258.
TAKE-HOME POINTS
- Glenoid deficiencies that occur from dysplasia, arthritis, or polyethylene osteolysis may be successfully addressed with bone grafting techniques and reverse shoulder arthroplasty.
- The intact humeral head in a primary case is ideal graft to be shaped to fit the glenoid deficits.
- The reverse shoulder with a long post base plate that is fixed securely to the native scapula is the author’s preferred technique.
- As the native humeral head is not available in revision cases, the tricortical iliac crest bone graft may be fixed as a structural graft in 1-stage.
- When the scapular walls are deficient and medial fixation is not secure, 2 stages 4 months to 6 months apart will be necessary before loading the construct.