User login
Debunking Atopic Dermatitis Myths: Does Eczema Limit Patients' Daily Activities?
Myth: Eczema has a minimal impact on patients’ daily activities
Although eczema may be considered a relatively mild skin condition, the effects of the disease can be debilitating for many patients. Low quality of life (QoL) due to eczema flares and trigger avoidance can lead to decreased productivity in this population, and patients often report interference of the disease with participation in daily life, including activities at work and school.
In a series of patient satisfaction surveys administered by the National Eczema Foundation, 75% of adults with eczema said their disease interferes with their job and household chores. A study of 380 adult atopic dermatitis (AD) patients assessing the impact of the disease on QoL showed similar results, as 39.0% of participants said AD impacted shopping, home, and garden activities a lot or very much and 36.8% said it impacted these activities a little. Additionally, reports from caregivers of children with AD indicated that nearly 50% of children miss at least 1 day of the school year due to their disease, and 17% miss 5 or more days. Over time, these limitations can have a serious psychological impact in eczema patients of any age.
In the National Eczema Foundation survey, 71% of respondents said their disease also gets in the way of participating in sports or hobbies. Eczema patients may miss out on the physical and mental health benefits of activities associated with increased body temperature or prolonged contact with sports equipment, which can exacerbate symptoms. In general, outdoor activities in all seasons can be particularly troublesome in this population, as eczema flares can be triggered by cold or hot temperatures, humidity, wind, dry air, sun exposure, pollution, and contact with allergens like pollen or mold.
The impact of eczema treatments on patients’ daily activities also should be considered when evaluating QoL in this population, as it frequently takes considerable time out of a patient’s day to manage his/her disease. Control of symptoms often requires a multistep daily regimen involving medication, bathing, moisturizing, applying wet compresses, ridding the house of allergens, and/or cleaning sheets and clothing. According to the National Eczema Foundation survey, 1 in 3 respondents said it takes 1 or more hours per day to treat their disease. To encourage adherence and ensure optimal outcomes, physicians should work with patients to develop an eczema treatment plan that is both effective and manageable in terms of their daily routines.
Ultimately, the disease burden in eczema patients is multidimensional, extending beyond only cutaneous symptoms; therefore, it is important for clinicians to consider the impact on QoL when choosing treatments for patients with eczema and to initiate appropriate therapy at the onset of disease presentation to mitigate the effects of the condition on patients’ daily lives. Eczema management strategies should include QoL screening to ensure the disease has a minimal impact on patients' daily lives and preferred activities.
Atopic dermatitis affects all ages. https://www.aad.org/media/news-releases/adult-atopic-dermatitis. American Academy of Dermatology website. Posted July 27, 2017. Accessed March 12, 2018.
Impacts of eczema on exercise, social life and hobbies. HealthTalkOnline website. http://www.healthtalk.org/young-peoples-experiences/eczema/impacts-eczema-exercise-social-life-and-hobbies. Accessed March 12, 2018.
In your words. National Eczema Foundation website. https://nationaleczema.org/in-your-words-survey-series. Accessed March 11, 2018.
Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults [published online January 14, 2016]. J Am Acad Dermatol. 2016;74:491-498.
Myth: Eczema has a minimal impact on patients’ daily activities
Although eczema may be considered a relatively mild skin condition, the effects of the disease can be debilitating for many patients. Low quality of life (QoL) due to eczema flares and trigger avoidance can lead to decreased productivity in this population, and patients often report interference of the disease with participation in daily life, including activities at work and school.
In a series of patient satisfaction surveys administered by the National Eczema Foundation, 75% of adults with eczema said their disease interferes with their job and household chores. A study of 380 adult atopic dermatitis (AD) patients assessing the impact of the disease on QoL showed similar results, as 39.0% of participants said AD impacted shopping, home, and garden activities a lot or very much and 36.8% said it impacted these activities a little. Additionally, reports from caregivers of children with AD indicated that nearly 50% of children miss at least 1 day of the school year due to their disease, and 17% miss 5 or more days. Over time, these limitations can have a serious psychological impact in eczema patients of any age.
In the National Eczema Foundation survey, 71% of respondents said their disease also gets in the way of participating in sports or hobbies. Eczema patients may miss out on the physical and mental health benefits of activities associated with increased body temperature or prolonged contact with sports equipment, which can exacerbate symptoms. In general, outdoor activities in all seasons can be particularly troublesome in this population, as eczema flares can be triggered by cold or hot temperatures, humidity, wind, dry air, sun exposure, pollution, and contact with allergens like pollen or mold.
The impact of eczema treatments on patients’ daily activities also should be considered when evaluating QoL in this population, as it frequently takes considerable time out of a patient’s day to manage his/her disease. Control of symptoms often requires a multistep daily regimen involving medication, bathing, moisturizing, applying wet compresses, ridding the house of allergens, and/or cleaning sheets and clothing. According to the National Eczema Foundation survey, 1 in 3 respondents said it takes 1 or more hours per day to treat their disease. To encourage adherence and ensure optimal outcomes, physicians should work with patients to develop an eczema treatment plan that is both effective and manageable in terms of their daily routines.
Ultimately, the disease burden in eczema patients is multidimensional, extending beyond only cutaneous symptoms; therefore, it is important for clinicians to consider the impact on QoL when choosing treatments for patients with eczema and to initiate appropriate therapy at the onset of disease presentation to mitigate the effects of the condition on patients’ daily lives. Eczema management strategies should include QoL screening to ensure the disease has a minimal impact on patients' daily lives and preferred activities.
Myth: Eczema has a minimal impact on patients’ daily activities
Although eczema may be considered a relatively mild skin condition, the effects of the disease can be debilitating for many patients. Low quality of life (QoL) due to eczema flares and trigger avoidance can lead to decreased productivity in this population, and patients often report interference of the disease with participation in daily life, including activities at work and school.
In a series of patient satisfaction surveys administered by the National Eczema Foundation, 75% of adults with eczema said their disease interferes with their job and household chores. A study of 380 adult atopic dermatitis (AD) patients assessing the impact of the disease on QoL showed similar results, as 39.0% of participants said AD impacted shopping, home, and garden activities a lot or very much and 36.8% said it impacted these activities a little. Additionally, reports from caregivers of children with AD indicated that nearly 50% of children miss at least 1 day of the school year due to their disease, and 17% miss 5 or more days. Over time, these limitations can have a serious psychological impact in eczema patients of any age.
In the National Eczema Foundation survey, 71% of respondents said their disease also gets in the way of participating in sports or hobbies. Eczema patients may miss out on the physical and mental health benefits of activities associated with increased body temperature or prolonged contact with sports equipment, which can exacerbate symptoms. In general, outdoor activities in all seasons can be particularly troublesome in this population, as eczema flares can be triggered by cold or hot temperatures, humidity, wind, dry air, sun exposure, pollution, and contact with allergens like pollen or mold.
The impact of eczema treatments on patients’ daily activities also should be considered when evaluating QoL in this population, as it frequently takes considerable time out of a patient’s day to manage his/her disease. Control of symptoms often requires a multistep daily regimen involving medication, bathing, moisturizing, applying wet compresses, ridding the house of allergens, and/or cleaning sheets and clothing. According to the National Eczema Foundation survey, 1 in 3 respondents said it takes 1 or more hours per day to treat their disease. To encourage adherence and ensure optimal outcomes, physicians should work with patients to develop an eczema treatment plan that is both effective and manageable in terms of their daily routines.
Ultimately, the disease burden in eczema patients is multidimensional, extending beyond only cutaneous symptoms; therefore, it is important for clinicians to consider the impact on QoL when choosing treatments for patients with eczema and to initiate appropriate therapy at the onset of disease presentation to mitigate the effects of the condition on patients’ daily lives. Eczema management strategies should include QoL screening to ensure the disease has a minimal impact on patients' daily lives and preferred activities.
Atopic dermatitis affects all ages. https://www.aad.org/media/news-releases/adult-atopic-dermatitis. American Academy of Dermatology website. Posted July 27, 2017. Accessed March 12, 2018.
Impacts of eczema on exercise, social life and hobbies. HealthTalkOnline website. http://www.healthtalk.org/young-peoples-experiences/eczema/impacts-eczema-exercise-social-life-and-hobbies. Accessed March 12, 2018.
In your words. National Eczema Foundation website. https://nationaleczema.org/in-your-words-survey-series. Accessed March 11, 2018.
Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults [published online January 14, 2016]. J Am Acad Dermatol. 2016;74:491-498.
Atopic dermatitis affects all ages. https://www.aad.org/media/news-releases/adult-atopic-dermatitis. American Academy of Dermatology website. Posted July 27, 2017. Accessed March 12, 2018.
Impacts of eczema on exercise, social life and hobbies. HealthTalkOnline website. http://www.healthtalk.org/young-peoples-experiences/eczema/impacts-eczema-exercise-social-life-and-hobbies. Accessed March 12, 2018.
In your words. National Eczema Foundation website. https://nationaleczema.org/in-your-words-survey-series. Accessed March 11, 2018.
Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): Insights from a phase 2b clinical trial of dupilumab in adults [published online January 14, 2016]. J Am Acad Dermatol. 2016;74:491-498.
Debunking Acne Myths: Does Wearing Makeup Cause Acne?
Myth: Wearing makeup causes acne breakouts
Acne breakouts caused by makeup and other skin care products, known as acne cosmetica, typically resolve when patients stop using pore-clogging products; however, the overall impact of cosmetics on the development of acne lesions is considered to be negligible. Many cosmetics are not inherently comedogenic and can be used safely by patients in combination with proper skin care techniques.
Although dermatologists may be inclined to discourage makeup use during acne treatment or breakouts due to its potential to aggravate the patient’s condition, research has shown that treatment results and quality of life (QoL) scores associated with makeup use in acne patients may improve when patients receive instruction on how to use skin care products and cosmetics effectively. In one study of 50 female acne patients, 25 participants were instructed on how to use skin care products and cosmetics, and the other 25 participants received no specific instructions from dermatologists. After 4 weeks of treatment with conventional topical and/or oral acne medications, the investigators concluded that use of skin care products did not negatively impact acne treatment, and the group that received application instructions showed more notable improvements in QoL scores versus those who did not. In another study, the overall number of acne eruptions decreased over a 2- to 4-week period in female acne patients who were trained by a makeup artist to apply cosmetics while undergoing acne treatment. These results suggest that acne patients who wear makeup may benefit from a conversation with their dermatologist about what products and skin care techniques they can use to minimize exacerbation of or even improve their condition.
When choosing makeup that will not cause or exacerbate acne breakouts, patients should look for packaging that indicates the product will not clog pores and is oil-free, noncomedogenic, and/or nonacnegenic. Some makeup products are specifically formulated to help camouflage redness and pimples, which can help improve quality of life and self-esteem in acne patients who otherwise may be self-conscious about their appearance. Mineral-based cosmetics containing powdered formulas of silica, titanium dioxide, and zinc oxide can be used to absorb oil, camouflage redness, and prevent irritation. Anti-inflammatory ingredients and antioxidants also are used in some makeup products to reduce skin irritation and promote barrier repair. Additional cosmetic ingredients that can affect the mechanisms of acne pathogenesis and may contribute to a decrease in acne lesions include nicotinamide, lactic acid, triethyl acetate/ethyllineolate, and prebiotic plant extracts.
Makeup should be applied gently to avoid irritating the skin. It also is important to remind patients not to share their makeup brushes and applicators and to clean them weekly to ensure that bacteria, dead skin cells, and oil are not spread to the skin, which can lead to new breakouts. Although patients may be compelled to scrub the skin to remove makeup, a mild cleanser should be gently applied using the fingertips and rinsed off with lukewarm water to minimize skin irritation. Any makeup remaining on the skin after washing should be gently removed with an oil-free makeup remover.
Hayashi N, Imori M, Yanagisawa M, et al. Make-up improves the quality of life of acne patients without aggravating acne eruptions during treatments. Eur J Dermatol. 2005;15:284-287.
I have acne! is it okay to wear makeup? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/makeup-with-acne. Accessed February 13, 2018.
Korting HC, Borelli C, Schöllmann C. Acne vulgaris. role of cosmetics [in German]. 2010;61:126-131.
Matsuoka Y, Yoneda K, Sadahira C, et al. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol. 2006;33:745-752.
Proper skin care lays the foundation for successful acne and rosacea treatment. American Academy of Dermatology website. https://www.aad.org/media/news-releases/proper-skin-care-lays-the-foundation-for-successful-acne-and-rosacea-treatment Published July 31, 2013. Accessed February 13, 2018.
Myth: Wearing makeup causes acne breakouts
Acne breakouts caused by makeup and other skin care products, known as acne cosmetica, typically resolve when patients stop using pore-clogging products; however, the overall impact of cosmetics on the development of acne lesions is considered to be negligible. Many cosmetics are not inherently comedogenic and can be used safely by patients in combination with proper skin care techniques.
Although dermatologists may be inclined to discourage makeup use during acne treatment or breakouts due to its potential to aggravate the patient’s condition, research has shown that treatment results and quality of life (QoL) scores associated with makeup use in acne patients may improve when patients receive instruction on how to use skin care products and cosmetics effectively. In one study of 50 female acne patients, 25 participants were instructed on how to use skin care products and cosmetics, and the other 25 participants received no specific instructions from dermatologists. After 4 weeks of treatment with conventional topical and/or oral acne medications, the investigators concluded that use of skin care products did not negatively impact acne treatment, and the group that received application instructions showed more notable improvements in QoL scores versus those who did not. In another study, the overall number of acne eruptions decreased over a 2- to 4-week period in female acne patients who were trained by a makeup artist to apply cosmetics while undergoing acne treatment. These results suggest that acne patients who wear makeup may benefit from a conversation with their dermatologist about what products and skin care techniques they can use to minimize exacerbation of or even improve their condition.
When choosing makeup that will not cause or exacerbate acne breakouts, patients should look for packaging that indicates the product will not clog pores and is oil-free, noncomedogenic, and/or nonacnegenic. Some makeup products are specifically formulated to help camouflage redness and pimples, which can help improve quality of life and self-esteem in acne patients who otherwise may be self-conscious about their appearance. Mineral-based cosmetics containing powdered formulas of silica, titanium dioxide, and zinc oxide can be used to absorb oil, camouflage redness, and prevent irritation. Anti-inflammatory ingredients and antioxidants also are used in some makeup products to reduce skin irritation and promote barrier repair. Additional cosmetic ingredients that can affect the mechanisms of acne pathogenesis and may contribute to a decrease in acne lesions include nicotinamide, lactic acid, triethyl acetate/ethyllineolate, and prebiotic plant extracts.
Makeup should be applied gently to avoid irritating the skin. It also is important to remind patients not to share their makeup brushes and applicators and to clean them weekly to ensure that bacteria, dead skin cells, and oil are not spread to the skin, which can lead to new breakouts. Although patients may be compelled to scrub the skin to remove makeup, a mild cleanser should be gently applied using the fingertips and rinsed off with lukewarm water to minimize skin irritation. Any makeup remaining on the skin after washing should be gently removed with an oil-free makeup remover.
Myth: Wearing makeup causes acne breakouts
Acne breakouts caused by makeup and other skin care products, known as acne cosmetica, typically resolve when patients stop using pore-clogging products; however, the overall impact of cosmetics on the development of acne lesions is considered to be negligible. Many cosmetics are not inherently comedogenic and can be used safely by patients in combination with proper skin care techniques.
Although dermatologists may be inclined to discourage makeup use during acne treatment or breakouts due to its potential to aggravate the patient’s condition, research has shown that treatment results and quality of life (QoL) scores associated with makeup use in acne patients may improve when patients receive instruction on how to use skin care products and cosmetics effectively. In one study of 50 female acne patients, 25 participants were instructed on how to use skin care products and cosmetics, and the other 25 participants received no specific instructions from dermatologists. After 4 weeks of treatment with conventional topical and/or oral acne medications, the investigators concluded that use of skin care products did not negatively impact acne treatment, and the group that received application instructions showed more notable improvements in QoL scores versus those who did not. In another study, the overall number of acne eruptions decreased over a 2- to 4-week period in female acne patients who were trained by a makeup artist to apply cosmetics while undergoing acne treatment. These results suggest that acne patients who wear makeup may benefit from a conversation with their dermatologist about what products and skin care techniques they can use to minimize exacerbation of or even improve their condition.
When choosing makeup that will not cause or exacerbate acne breakouts, patients should look for packaging that indicates the product will not clog pores and is oil-free, noncomedogenic, and/or nonacnegenic. Some makeup products are specifically formulated to help camouflage redness and pimples, which can help improve quality of life and self-esteem in acne patients who otherwise may be self-conscious about their appearance. Mineral-based cosmetics containing powdered formulas of silica, titanium dioxide, and zinc oxide can be used to absorb oil, camouflage redness, and prevent irritation. Anti-inflammatory ingredients and antioxidants also are used in some makeup products to reduce skin irritation and promote barrier repair. Additional cosmetic ingredients that can affect the mechanisms of acne pathogenesis and may contribute to a decrease in acne lesions include nicotinamide, lactic acid, triethyl acetate/ethyllineolate, and prebiotic plant extracts.
Makeup should be applied gently to avoid irritating the skin. It also is important to remind patients not to share their makeup brushes and applicators and to clean them weekly to ensure that bacteria, dead skin cells, and oil are not spread to the skin, which can lead to new breakouts. Although patients may be compelled to scrub the skin to remove makeup, a mild cleanser should be gently applied using the fingertips and rinsed off with lukewarm water to minimize skin irritation. Any makeup remaining on the skin after washing should be gently removed with an oil-free makeup remover.
Hayashi N, Imori M, Yanagisawa M, et al. Make-up improves the quality of life of acne patients without aggravating acne eruptions during treatments. Eur J Dermatol. 2005;15:284-287.
I have acne! is it okay to wear makeup? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/makeup-with-acne. Accessed February 13, 2018.
Korting HC, Borelli C, Schöllmann C. Acne vulgaris. role of cosmetics [in German]. 2010;61:126-131.
Matsuoka Y, Yoneda K, Sadahira C, et al. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol. 2006;33:745-752.
Proper skin care lays the foundation for successful acne and rosacea treatment. American Academy of Dermatology website. https://www.aad.org/media/news-releases/proper-skin-care-lays-the-foundation-for-successful-acne-and-rosacea-treatment Published July 31, 2013. Accessed February 13, 2018.
Hayashi N, Imori M, Yanagisawa M, et al. Make-up improves the quality of life of acne patients without aggravating acne eruptions during treatments. Eur J Dermatol. 2005;15:284-287.
I have acne! is it okay to wear makeup? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/makeup-with-acne. Accessed February 13, 2018.
Korting HC, Borelli C, Schöllmann C. Acne vulgaris. role of cosmetics [in German]. 2010;61:126-131.
Matsuoka Y, Yoneda K, Sadahira C, et al. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol. 2006;33:745-752.
Proper skin care lays the foundation for successful acne and rosacea treatment. American Academy of Dermatology website. https://www.aad.org/media/news-releases/proper-skin-care-lays-the-foundation-for-successful-acne-and-rosacea-treatment Published July 31, 2013. Accessed February 13, 2018.
Debunking Psoriasis Myths: How Long Do Patients Have to Wait to See Results With Biologics?
Myth: Biologics Work Slowly
Biologics have demonstrated efficacy in psoriasis and often are used in psoriasis patients who have not achieved desired results with other treatments, patients who have had intolerable side effects from other treatments, and patients with concurrent diseases that preclude the use of systemic therapies. Because of the quality-of-life impact of psoriasis, patients look for quick clearance of their symptoms, but can biologics deliver fast results or do they work slowly?
Biologics such as etanercept and adalimumab block tumor necrosis factor α signaling, while ustekinumab targets IL-12 and IL-23 and others target IL-17. Some patients may begin to see improvement in skin lesions within 1 month of initiating biologic therapies because they target specific proinflammatory pathways that are critical to the pathogenesis of psoriasis, but response time varies among patients and specific therapy used.
The psoriasis area and severity index (PASI) measures psoriasis treatment success. Based on the American Academy of Dermatology’s guidelines of care for the management of psoriasis and psoriatic arthritis published in 2008, short-term response was achieved in 10 to 14 weeks for the following biologics:
- Adalimumab: 80% of patients achieved PASI 75 at week 12
- Etanercept: 49% of patients given 50 mg twice weekly achieved PASI 75 at 12 weeks; 34% of patients given 25 mg twice weekly achieved PASI 75 at 12 weeks
- Infliximab: 80% of patients achieved PASI 75 at week 10
Of the newer biologics, Premier Research recently noted that PASI 75 was achieved after 12 weeks with the following biologics:
- Brodalumab: 83% after 12 weeks
- Ixekizumab: 90% after 12 weeks
- Secukinumab: 80% after 12 weeks
- Ustekinumab: 70% after 12 weeks
There are a variety of factors to consider when determining which biologic to use for a psoriasis patient. These data may help in the decision process. However, dermatologists must educate psoriasis patients with a high body mass index that their disease may take longer to respond and may need combination therapy for optimal clearance.
Expert Commentary
All of the biologics, especially the IL-17 inhibitors, work very quickly to clear psoriasis. The only way they work “slowly” is that it may take time (usually a few days) for the payers to approve biologic prescriptions.
—Jashin J. Wu, MD (Los Angeles, California)
Biologics. DermNet New Zealand website. https://www.dermnetnz.org/topics/biologics/. Accessed February 6, 2018.
Biologics in psoriasis treatment. Premier Research website. https://premier-research.com/perspectives-biologics-psoriasis-treatment/. Published May 9, 2017. Accessed February 6, 2018.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
Vilarrasa E, Notario J, Bordas X, et al. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74:1066-1072.
Myth: Biologics Work Slowly
Biologics have demonstrated efficacy in psoriasis and often are used in psoriasis patients who have not achieved desired results with other treatments, patients who have had intolerable side effects from other treatments, and patients with concurrent diseases that preclude the use of systemic therapies. Because of the quality-of-life impact of psoriasis, patients look for quick clearance of their symptoms, but can biologics deliver fast results or do they work slowly?
Biologics such as etanercept and adalimumab block tumor necrosis factor α signaling, while ustekinumab targets IL-12 and IL-23 and others target IL-17. Some patients may begin to see improvement in skin lesions within 1 month of initiating biologic therapies because they target specific proinflammatory pathways that are critical to the pathogenesis of psoriasis, but response time varies among patients and specific therapy used.
The psoriasis area and severity index (PASI) measures psoriasis treatment success. Based on the American Academy of Dermatology’s guidelines of care for the management of psoriasis and psoriatic arthritis published in 2008, short-term response was achieved in 10 to 14 weeks for the following biologics:
- Adalimumab: 80% of patients achieved PASI 75 at week 12
- Etanercept: 49% of patients given 50 mg twice weekly achieved PASI 75 at 12 weeks; 34% of patients given 25 mg twice weekly achieved PASI 75 at 12 weeks
- Infliximab: 80% of patients achieved PASI 75 at week 10
Of the newer biologics, Premier Research recently noted that PASI 75 was achieved after 12 weeks with the following biologics:
- Brodalumab: 83% after 12 weeks
- Ixekizumab: 90% after 12 weeks
- Secukinumab: 80% after 12 weeks
- Ustekinumab: 70% after 12 weeks
There are a variety of factors to consider when determining which biologic to use for a psoriasis patient. These data may help in the decision process. However, dermatologists must educate psoriasis patients with a high body mass index that their disease may take longer to respond and may need combination therapy for optimal clearance.
Expert Commentary
All of the biologics, especially the IL-17 inhibitors, work very quickly to clear psoriasis. The only way they work “slowly” is that it may take time (usually a few days) for the payers to approve biologic prescriptions.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Biologics Work Slowly
Biologics have demonstrated efficacy in psoriasis and often are used in psoriasis patients who have not achieved desired results with other treatments, patients who have had intolerable side effects from other treatments, and patients with concurrent diseases that preclude the use of systemic therapies. Because of the quality-of-life impact of psoriasis, patients look for quick clearance of their symptoms, but can biologics deliver fast results or do they work slowly?
Biologics such as etanercept and adalimumab block tumor necrosis factor α signaling, while ustekinumab targets IL-12 and IL-23 and others target IL-17. Some patients may begin to see improvement in skin lesions within 1 month of initiating biologic therapies because they target specific proinflammatory pathways that are critical to the pathogenesis of psoriasis, but response time varies among patients and specific therapy used.
The psoriasis area and severity index (PASI) measures psoriasis treatment success. Based on the American Academy of Dermatology’s guidelines of care for the management of psoriasis and psoriatic arthritis published in 2008, short-term response was achieved in 10 to 14 weeks for the following biologics:
- Adalimumab: 80% of patients achieved PASI 75 at week 12
- Etanercept: 49% of patients given 50 mg twice weekly achieved PASI 75 at 12 weeks; 34% of patients given 25 mg twice weekly achieved PASI 75 at 12 weeks
- Infliximab: 80% of patients achieved PASI 75 at week 10
Of the newer biologics, Premier Research recently noted that PASI 75 was achieved after 12 weeks with the following biologics:
- Brodalumab: 83% after 12 weeks
- Ixekizumab: 90% after 12 weeks
- Secukinumab: 80% after 12 weeks
- Ustekinumab: 70% after 12 weeks
There are a variety of factors to consider when determining which biologic to use for a psoriasis patient. These data may help in the decision process. However, dermatologists must educate psoriasis patients with a high body mass index that their disease may take longer to respond and may need combination therapy for optimal clearance.
Expert Commentary
All of the biologics, especially the IL-17 inhibitors, work very quickly to clear psoriasis. The only way they work “slowly” is that it may take time (usually a few days) for the payers to approve biologic prescriptions.
—Jashin J. Wu, MD (Los Angeles, California)
Biologics. DermNet New Zealand website. https://www.dermnetnz.org/topics/biologics/. Accessed February 6, 2018.
Biologics in psoriasis treatment. Premier Research website. https://premier-research.com/perspectives-biologics-psoriasis-treatment/. Published May 9, 2017. Accessed February 6, 2018.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
Vilarrasa E, Notario J, Bordas X, et al. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74:1066-1072.
Biologics. DermNet New Zealand website. https://www.dermnetnz.org/topics/biologics/. Accessed February 6, 2018.
Biologics in psoriasis treatment. Premier Research website. https://premier-research.com/perspectives-biologics-psoriasis-treatment/. Published May 9, 2017. Accessed February 6, 2018.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
Vilarrasa E, Notario J, Bordas X, et al. ORBIT (Outcome and Retention Rate of Biologic Treatments for Psoriasis): a retrospective observational study on biologic drug survival in daily practice. J Am Acad Dermatol. 2016;74:1066-1072.
Total Shoulder Arthroplasty Using a Bone-Sparing, Precision Multiplanar Humeral Prosthesis
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).
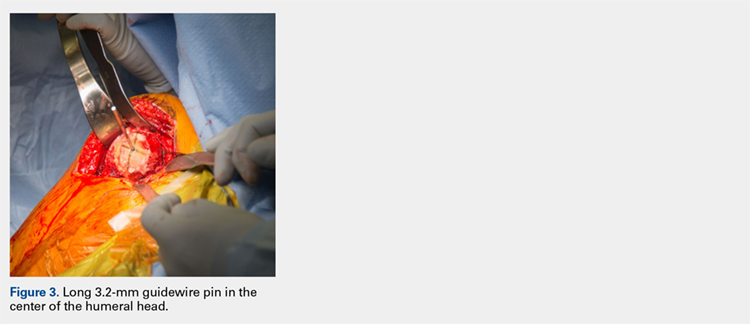
Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

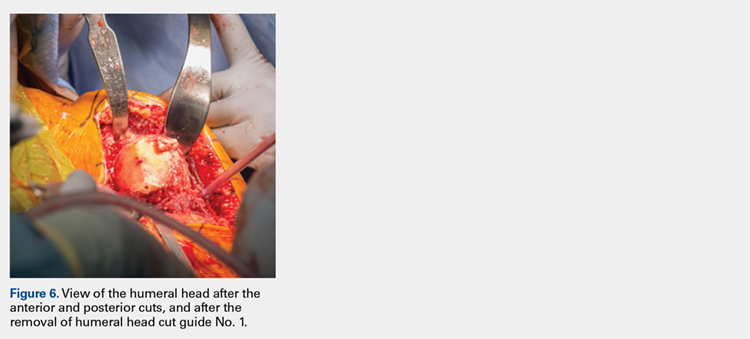
The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).
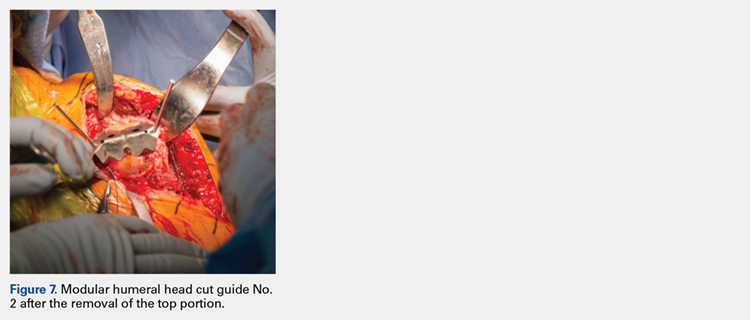
The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).
The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
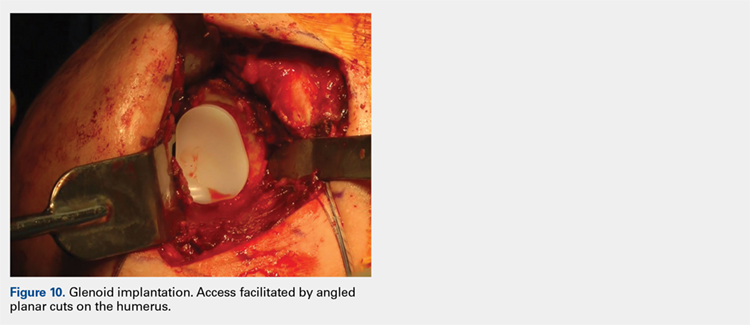
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

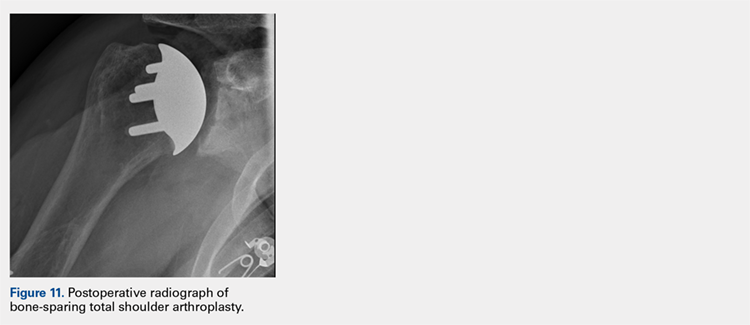
After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.
DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12
Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
ABSTRACT
Proper reconstruction of proximal humeral anatomy is of primary importance to maximize patient outcomes after total shoulder arthroplasty. This article describes a new arthroplasty technique, where a fixed multiplanar bone resection is made and a novel implant, which is designed to precisely match the bone resection, is inserted.
Continue to: The success of total shoulder arthroplasty...
The success of total shoulder arthroplasty (TSA) is largely dependent on how accurate the proximal humeral anatomy is reconstructed and the glenohumeral relationships are restored.1-4 Numerous studies have demonstrated a relationship of worse clinical outcomes and implant failure with nonanatomic implant placement.5-8 The majority of arthroplasty systems rely on surgeon-dependent decision-making to determine the location of the border of the articular surface and, ultimately, the amount and location of bone to be resected. Even in experienced hands, the ability to reproducibly restore the joint line is inconsistent.3
In contrast, the majority of total knee arthroplasty (TKA) systems have been designed with instrumentation that guides the surgeon precisely regarding where and how much femoral bone must be resected, and the corresponding implant is designed with the same thickness to preserve the location of the joint line. Cutting block instrumentation rather than freehand cuts enables reproducibility of TKA while being performed for an estimated 700,000 times annually in the US.9
To achieve similar high levels of reproducibility in shoulder arthroplasty, a new technique was developed based on the principle of providing instrumentation to assist the surgeon in accurately restoring the proximal humeral joint line. This technical article describes the technique of using a multiplanar instrumented cutting system and matching implants to perform TSA. The technique shown was previously studied and was found to allow surgeons to recreate the original anatomy of the humerus with very high precision.10

The humeral prosthesis described in this article has an articular surface that is slightly elliptical to more closely match the actual shape of the humerus bone.11 Biomechanical studies have demonstrated that implants designed with a nonspherical shape have more similar motion and kinematics to those of the native humeral head.

This provides rotation stability, and the implant rests on the strong subchondral bone of the proximal humerus proximal to the anatomic neck rather than relying on metaphyseal bone or canal fixation, as recommended by Aldoiusti.13 It also allows optimal implant placement with complete freedom with respect to inclination, version, and medial/posterior offset from the humeral canal.
Continue to: The implant respects the relationship...
The implant respects the relationship of the rotator cuff insertion and has a recessed superior margin to keep both the implant and the saw blade 3 mm to 5 mm away from the supraspinatus fibers to protect the rotator cuff from iatrogenic injury.
TECHNIQUE
The technique described in this article uses the Catalyst CSR Total Shoulder System (Catalyst OrthoScience), which was cleared to treat arthritis of the shoulder by the US Food and Drug Administration in May 2016.
A standard deltopectoral incision is made, and the surgeon dissects the interval between the pectoralis major medially and the deltoid laterally. The subscapularis can be incised by tenotomy; alternatively, the surgeon can perform a subscapularis peel or a lesser tuberosity osteotomy using this technique.
Once the glenohumeral joint is exposed, the surgeon delivers the humeral head anteriorly. A preferred method is to place a Darrach retractor between the humeral head and the glenoid, and a cobra or a second Darrach retractor behind the superolateral humeral head superficial to the supraspinatus tendon. By simultaneously pressing on both retractors and externally rotating the patient’s arm, the humeral head is delivered anteriorly. Osteophytes on the anterior and inferior edge of the humeral head are generously removed at this time using a rongeur.
Using a pin guide, the long 3.2-mm guidewire pin is drilled under power into the center of the articular surface. The pin guide is then removed, leaving the pin in the center of the humerus (Figure 3).

Continue to: Next, the surgeon...
Next, the surgeon slides the cannulated reamer over the long guidewire pin and under power removes a small portion of the humeral head subchondral bone until the surgeon feels and observes that the reamer is no longer removing bone (Figure 4). The patent-pending reamer design prevents the surgeon from removing more than a few millimeters of bone, after which point the reamer spins on the surface of the bone without resecting further.

The surgeon is aware that the reamer has achieved its desired depth when it is no longer creating new bone shavings, and the surgeon can hear and feel that the reamer is spinning and no longer cutting. Then the surgeon removes the reamer.

The surgeon places the first humeral cut guide over the long guidewire pin, oriented superiorly-inferiorly and secures the guide using 4 short pins, and the long pin is removed. The surgeon uses an oscillating saw to cut the anterior and posterior plane cuts through the saw captures in the cut guide (Figure 5). The humeral cut guide and short pins are removed (Figure 6).

The surgeon then applies the second humeral cut guide to the proximal humerus and secures it using 2 short pins. The surgeon then uses the 6-mm drill to drill the 4 holes for the pegs of the implant. The top portion of the guide is removed, and the surgeon makes the superior and inferior cuts along the top and bottom surfaces of the guide using an oscillating saw (Figure 7).

The surgeon then uses a rongeur to slightly round the edges of the 4 corners at the periphery of the humerus. The second humeral cut guide and short pins are removed (Figure 8).

Continue to: Next, the surgeon trials...
Next, the surgeon trials humeral implants to determine the correct implant size (Figure 9). Once the proper humeral size is chosen, the trial is removed and the humeral cover is placed over the prepared humeral head. The surgeon then proceeds to glenoid preparation (Figure 10), which is easily accessible and facilitated by angled planar cuts on the humeral head. Glenoid technique will be discussed in a subsequent article.

After glenoid preparation and insertion, the humerus is delivered anteriorly. The proximal humerus is washed and dried, and cement is applied to the peg holes in the humerus bone and the underside of the humeral implant. The implant is then inserted using the humeral impactor to apply pressure and assure that the implant is fully seated. Once the humeral cement is hardened, the glenohumeral joint is irrigated and closure begins. Postoperative radiograph is shown in Figure 11.

DISCUSSION
Numerous authors have demonstrated that accurate implant placement is crucial for restoring normal glenoid kinematics and motion,1-4 while some authors have reported worsening clinical outcomes and higher rates of pain and implant loosening when the implants were not placed anatomically.5-8 This is such an important concept that it essentially was the primary inspiration for creating this TSA system. In addition, the system utilizes a nonspherical, elliptical humeral head that more closely matches the anatomy of the proximal humerus,14,15 and this type of shape has shown improved biomechanics in laboratory testing.12

Good results have been demonstrated in restoring the normal anatomy using stemmed devices on the radiographic analysis of cadavers.16 The creation of stemmed implants with variable inclination and offset has improved computer models17 compared with previous studies,18 with the exception of scenarios with extreme offset.
In theory, resurfacing implants and implants without a canal stem should have a better implant placement than that with stemmed implants; however, the ability to restore the center of rotation was even worse for resurfacing prostheses, with 65% of all implants being measured as outliers postoperatively in one study.19 Most of the resurfacing implants and their instrumentation techniques offer little to help the surgeon control for implant height. The depth of the reaming is variable, not calibrated, and not correlated with the implant size, frequently leading to overstuffing after surgery. Second, the use of spherical prostheses forces the surgeon to choose between matching the superior-inferior humeral size, leading to overhang of the implant, or matching the anteroposterior, leading to frequent undersizing in the coronal plane. The nonspherical, elliptical head shape can potentially simplify implant selection.
In summary, new techniques have been developed in an attempt to achieve increased consistency and precision in TSA. By more accurately reproducing the proximal humeral anatomy, it is proposed that clinical outcomes in terms of the range of motion and patient satisfaction may also be improved through newer techniques. Cadaver studies have validated the anatomic precision of this technique.10 Clinical data comprising of patient-reported outcome measures and radiographic outcome studies are currently underway for this arthroplasty system.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
1. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409.
2. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction. An in vitro study. J Bone Joint Surg Am. 2004;86-A(3):575-580.
3. Iannotti JP, Spencer EE, Winter U, Deffenbaugh D, Williams G. Prosthetic positioning in total shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14(1 Supple S):111S-121S.
4. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(8):1184-1190.
5. Denard PJ, Raiss P, Sowa B, Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22(7):894-900.
6. Figgie HE 3rd, Inglis AE, Goldberg VM, Ranawat CS, Figgie MP, Wile JM. An analysis of factors affecting the long-term results of total shoulder arthroplasty in inflammatory arthritis. J Arthroplasty. 1988;3(2):123-130.
7. Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA 3rd. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16(5):555-562.
8. Flurin PH, Roche CP, Wright TW, Zuckerman JD. Correlation between clinical outcomes and anatomic reconstruction with anatomic total shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S92-S98.
9. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Goldberg SS, Akyuz E, Murthi AM, Blaine T. Accuracy of humeral articular surface restoration in a novel anatomic shoulder arthroplasty technique and design: a cadaveric study. Journal of Shoulder and Elbow Arthroplasty. 2018;2:2471549217750791.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25(7):1084-1093.
13. Alidousti H, Giles JW, Emery RJH, Jeffers J. Spatial mapping of humeral head bone density. J Shoulder Elbow Surg. 2017;26(9):1653-1661.
14. Harrold F, Wigderowitz C. Humeral head arthroplasty and its ability to restore original humeral head geometry. J Shoulder Elbow Surg. 2013;22(1):115-121.
15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338.
16. Wirth MA, Ondrla J, Southworth C, Kaar K, Anderson BC, Rockwood CA 3rd. Replicating proximal humeral articular geometry with a third-generation implant: a radiographic study in cadaveric shoulders. J Shoulder Elbow Surg. 2007;16(3 Suppl):S111-S116.
17. Pearl ML, Kurutz S, Postacchini R. Geometric variables in anatomic replacement of the proximal humerus: How much prosthetic geometry is necessary? J Shoulder Elbow Surg. 2009;18(3):366-370.
18. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326.
19. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1740-1746.
TAKE-HOME POINTS
- Bone-preserving shoulder arthroplasty is now available and rapidly growing in the US.
- The calibrated, multiplanar instruments and prosthesis shown here allow surgeons to recreate the normal humerus shape with high precision.
- The elliptical, non-spherical design of the humerus prosthesis has shown improved shoulder kinematics compared to standard spherical prostheses.
- The implant rests on dense bone proximal to the anatomic neck where bone support is strong.
- Glenoid implant insertion is routinely performed using this technique and access is facilitated by the angled bone resections.
Knotless Tape Suture Fixation of Quadriceps Tendon Rupture: A Novel Technique
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
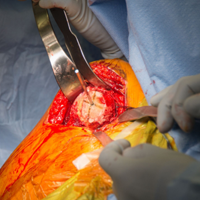
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
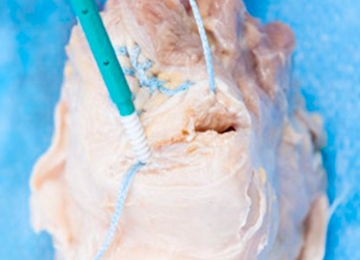
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).
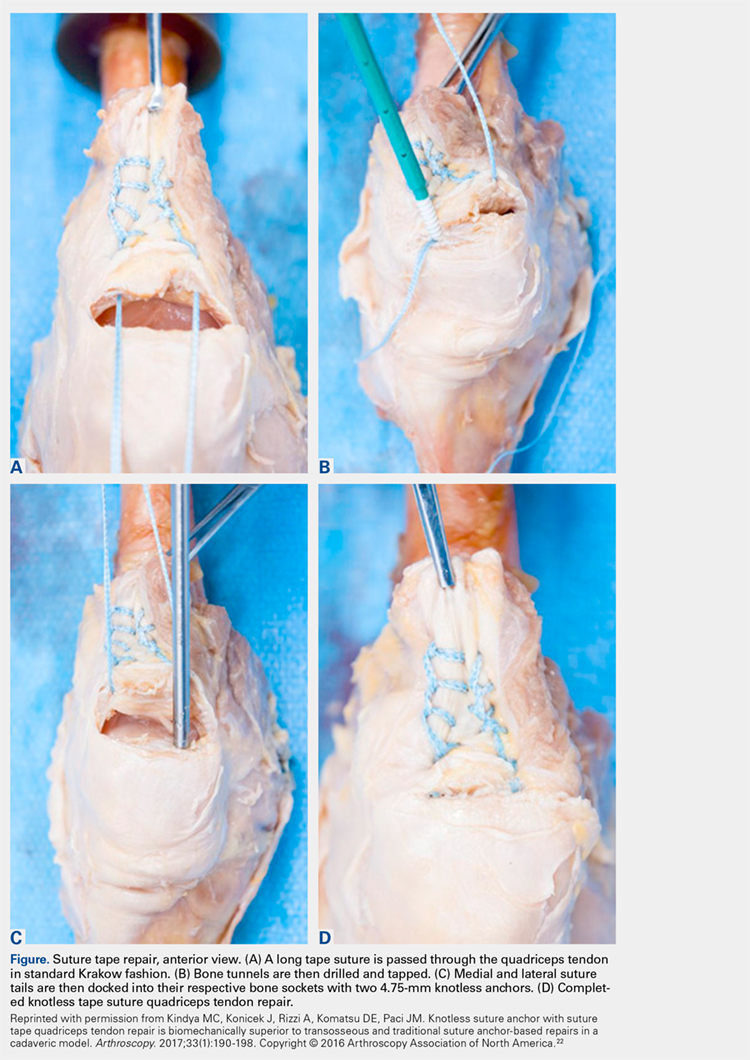
After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).

After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
ABSTRACT
Quadriceps tendon ruptures disrupt the extensor mechanism of the knee and require urgent surgical management. Traditional repair techniques have had mixed biomechanical and clinical results risking weakness and extensor lag. We describe a novel technique using tape suture and knotless anchors, which has performed superiorly during biomechanical testing and yielded terrific early clinical results.
Continue to: Quadriceps tendon rupture...
Quadriceps tendon rupture is an uncommon yet potentially devastating knee injury with an estimated incidence of 1.37 in 100,000.1 It most often occurs in male, middle-aged or older patients with degenerative tendon changes and serious systemic diseases, such as chronic renal failure, diabetes mellitus, rheumatoid arthritis, and disorders requiring long-term steroid use (tissue quality is often compromised by patient age and comorbidities).2-10 Whereas partial tears with an intact extensor mechanism may be managed nonoperatively, prompt operative intervention is indicated in cases of complete tear or an incompetent extensor mechanism to facilitate early range of motion (ROM) and return of knee function.2-4,8,9
The standard of care is repair with a nonabsorbable suture passed through transosseous patellar tunnels, often with several weeks of postoperative immobilization to protect the repair.3,4,7,10-12 Reported complications of this method include significant extension lag, decreased strength, and ROM compared with the contralateral knee, chronic pain, and iatrogenic patellar fracture.8,13-18 Repair techniques using suture anchors have been proposed as viable alternatives, but biomechanical studies comparing them with standard transosseous repair have reported mixed results.7,10-12,18-20 Two studies found improved biomechanical characteristics with suture anchors,10,21 but 2 others found the characteristics of suture anchor fixation equal to11 or worse than12 those of transosseous fixation. In light of the controversy regarding strength and clinical outcomes of suture anchor repair compared with transosseous repair, new and potentially superior surgical interventions should be considered.
We recently completed a cadaveric study comparing the biomechanical properties of a novel quadriceps tendon repair technique using 4.75-mm biocomposite knotless suture anchors with suture tape and the properties of conventional techniques using either transosseous or suture anchor repair alone.22 In the cadaveric model, compared with transosseous and fully threaded suture anchor techniques, repair of quadriceps tendon ruptures with this knotless suture anchor with suture tape technique was biomechanically superior in cyclic displacement, construct stiffness, and ultimate load to failure.22 Additionally, this method allows for less extensive dissection, shorter operative times, and the potential for earlier and more aggressive rehabilitation protocols.22 We propose this technique, presented in this article, as a superior alternative to traditional quadriceps tendon repair techniques.
TECHNIQUE
The patient is placed in supine position with a tourniquet placed on the proximal thigh. A midline incision is made from the proximal pole of the patella, proximally by 5 cm. A combination of sharp and blunt dissection is performed through skin and subcutaneous tissues down to the extensor mechanism, exposing the proximal pole of the patella and the torn quadriceps tendon.
The distal aspect of the quadriceps tendon is then débrided of any devitalized tissue and secured with an Allis clamp. A long tape suture (FiberTape; Arthrex) is then used to place a locking Krackow stitch in a distal-to-proximal and then proximal-to-distal direction for 5 throws in each direction within the quadriceps tendon, with the tails exiting distally at the tear site. Care is taken with each pass to ensure that there is no slack within the system.
Continue to: The proximal pole of the patella...
The proximal pole of the patella is then prepared by débriding any remaining soft tissue back to an area of exposed subcortical bone, which is débrided to a bleeding bony bed. Holes are drilled in the medial and lateral thirds of the patella at the proximal pole using the drill for 4.75-mm biocomposite knotless suture anchors (SwiveLock; Arthrex). The tap for the 4.75-mm anchors is then passed at each guide hole. In hard bone, double-tapping is recommended.
Next, the medial strand of tape suture is loaded within a 4.75-mm biocomposite knotless suture anchor eyelet and reduced to the patella. The medial anchor is malleted and screwed into place, while tension is kept on the lateral strand with the knee in full extension. The lateral strand is then placed into its 4.75-mm biocomposite knotless suture anchor, reduced to the patella, and then malleted and screwed into place in the lateral hole, thereby completing the core portion of the repair (Figures A-D). The core strands from the 4.75-mm biocomposite knotless suture anchors are then back-passed in mattress fashion and tied, and medial and lateral retinacular repairs are then performed using supersuture tape (SutureTape or FiberWire; Arthrex).

After surgery, the patient is placed in a knee brace locked in full extension and allowed to weight-bear as tolerated using crutches. During the first week, knee ROM is allowed up to 30°. During weeks 2 to 6 passive ROM is gradually increased to 90°, and use of crutches is tapered. At week 6 the brace is unlocked for ambulation; it may be discontinued after 7 to 8 weeks or when determined safe. Light activity is permitted from month 4 to month 6. A patient who achieves satisfactory strength, is clinically examined, and progresses through rehabilitation is allowed to return to fully unrestricted sport.
DISCUSSION
Quadriceps tendon rupture is an uncommon clinical entity that requires early surgical management.1-5,12,17,19 The standard of care is passage of nonabsorbable sutures through transosseous patellar bone tunnels, but repair with suture anchors has been studied as an alternative that allows for less tissue trauma, decreased operative time, safe early initiation of rehabilitation protocols, and reduced risk of patella fracture or damage.3,7,10-12,18-20,21,23 Despite these potential advantages, biomechanical studies have yielded inconsistent results regarding the superiority of suture anchor repair over repair with transosseous tunnels.7,10-12,18-20 We propose quadriceps tendon repair using the 4.75-mm biocomposite knotless suture anchor with tape suture technique as a biomechanically superior alternative to either transosseous tunnels or suture anchor repair alone, with significant advantages both in and out of the operating room.
Results of biomechanical studies comparing transosseous tunnel repair and standard suture anchor repair have been mixed, though the heterogeneity of their study methods and endpoints makes direct comparisons difficult.7,10-12,18-20 Petri and colleagues10 and Sherman and colleagues21 reported statistically significant higher load to failure10 and reduced gapping during cyclic loading10,21 with suture anchor repair relative to transosseous repair. However, Hart and colleagues12 found that repair with suture anchors had lower ultimate tensile load, and they concluded that transosseous repair is superior. Lighthart and colleagues11 found no significant difference in displacement between the 2 repairs.
Continue to: In our cadaveric biomechanical study...
In our cadaveric biomechanical study, a novel 4.75-mm biocomposite knotless suture anchor with suture tape repair was compared with traditional 3-tunnel transosseous repair and with standard 2-anchor suture anchor repair.22 Statistically significant superiority was found across multiple parameters, including initial tendon displacement, stiffness, and ultimate load to failure (vs 5.5-mm biocomposite fully threaded suture anchor repair), as well as initial and late tendon displacement, stiffness, and ultimate load to failure (vs transosseous repair).22 Although definitive conclusions are difficult to draw on the basis of prior cadaveric studies comparing standard suture anchor repair and transosseous repair, our results decidedly favor the biomechanical characteristics of this 4.75-mm biocomposite knotless suture anchor with suture tape repair and make it a potentially superior repair technique based on biomechanics alone.22
Similarly to standard repair with suture anchors, repair using a 4.75-mm biocomposite knotless suture anchor with tape suture eliminates the need to expose the distal pole of the patella.7,10-12,21 This allows for a smaller surgical incision, less extensive dissection, and prevents possible interference with the patellar tendon.7,10-12,21 Additionally, it eliminates the risk of iatrogenic patellar fracture and damage to the articular surface from drilling the transpatellar tunnels.17,18 Both our own review of cases repaired with our 4.75-mm biocomposite knotless suture anchor with suture tape technique as well as studies of suture anchor repair have consistently found operative times of <1 hour.21 Shorter operative times and smaller surgical wounds are advantageous given that many of these patients have medical comorbidities that predispose them to intraoperative and wound-healing complications.12,19-22
Optimal rehabilitation protocols for quadriceps tendon repair are a matter of controversy. Multiple studies of repair with transosseous patellar tunnels describe immobilization for 6 weeks after surgery, but there has been a recent push toward early motion.7,13,23,24 Reported complications of extended immobilization include limited flexion, pain, weakness, decreased patellar mobility, and patella baja.14 Studies have suggested that, while excessive loading can cause gap formation and weaken the repair, some controlled motion is necessary to heal the tendon23,25 and reduce the risks of stiffness and atrophy.14 The improved biomechanical characteristics of the 4.75-mm biocomposite knotless suture anchor with tape suture technique allow for safe early initiation of ROM exercises and accelerated rehabilitation protocols.
In our early experience with this technique, functional outcomes have been excellent. A formal 2-year outcome study of patients who have undergone quadriceps tendon repair with this 4.75-mm biocomposite knotless suture anchor with tape suture technique is under way.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
1. Clayton RA, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338-1344.
2. Rasul AT Jr, Fischer DA. Primary repair of quadriceps tendon ruptures. Clin Orthop Relat Res. 1993;(289):205-207.
3. Ilan DI, Tejwani N, Keschner M, Leibman M. Quadriceps tendon rupture. J Am Acad Orthop Surg. 2003;11(3):192-200.
4. Ramseier LE, Werner CM, Heinzelmann M. Quadriceps and patellar tendon rupture. Injury. 2006;37(6):516-519.
5. Ciriello V, Gudipati S, Tosounidis T, Soucacos PN, Giannoudis PV. Clinical outcomes after repair of quadriceps tendon rupture: a systematic review. Injury. 2012;43(11):1931-1938.
6. O’Shea K, Kenny P, Donovan J, Condon F, McElwain JP. Outcomes following quadriceps tendon ruptures. Injury. 2002;33(3):257-260.
7. Richards DP, Barber FA. Repair of quadriceps tendon ruptures using suture anchors. Arthroscopy. 2002;18(5):556-559.
8. Wenzl ME, Kirchner R, Seide K, Strametz S, Jürgens C. Quadriceps tendon ruptures—is there a complete functional restitution? Injury. 2004;35(9):922-926.
9. Boudissa M, Roudet A, Rubens-Duval B, Chaussard C, Saragaglia D. Acute quadriceps tendon ruptures: a series of 50 knees with an average follow-up of more than 6 years. Orthop Traumatol Surg Res. 2014;100(2):213-216.
10. Petri M, Dratzidis A, Brand S, et al. Suture anchor repair yields better biomechanical properties than transosseous sutures in ruptured quadriceps tendons. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1039-1045.
11. Lighthart WC, Cohen DA, Levine RG, Parks BG, Boucher HR. Suture anchor versus suture through tunnel fixation for quadriceps tendon rupture: a biomechanical study. Orthopedics. 2008;31(5):441.
12. Hart ND, Wallace MK, Scovell JF, Krupp RJ, Cook C, Wyland DJ. Quadriceps tendon rupture: a biomechanical comparison of transosseous equivalent double-row suture anchor versus transosseous tunnel repair. J Knee Surg. 2012;25(4):335-339.
13. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19(6):509-514.
14. West JL, Keene JS, Kaplan LD. Early motion after quadriceps and patellar tendon repairs: outcomes with single-suture augmentation. Am J Sports Med. 2008;36(2):316-323.
15. De Baere T, Geulette B, Manche E, Barras L. Functional results after surgical repair of quadriceps tendon rupture. Acta Orthop Belg. 2002;68(2):146-149.
16. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12(4):273-279.
17. Gregory JM, Sherman SL, Mather R, Bach BR Jr. Patellar stress fracture after transosseous extensor mechanism repair: report of 3 cases. Am J Sports Med. 2012;40(7):1668-1672.
18. Bushnell BD, Whitener GB, Rubright JH, Creighton RA, Logel KJ, Wood ML. The use of suture anchors to repair the ruptured quadriceps tendon. J Orthop Trauma. 2007;21(6):407-413.
19. Harris JD, Abrams GD, Yanke AB, Hellman MD, Erickson BJ, Bach BR Jr. Suture anchor repair of quadriceps tendon rupture. Orthopedics. 2014;37(3):183-186.
20. Maniscalco P, Bertone C, Rivera F, Bocchi L. A new method of repair for quadriceps tendon ruptures. A case report. Panminerva Med. 2000;42(3):223-225.
21. Sherman SL, Copeland ME, Milles JL, Flood DA, Pfeiffer FM. Biomechanical evaluation of suture anchor versus transosseous tunnel quadriceps tendon repair techniques. Arthroscopy. 2016;32(6):1117-1124.
22. Kindya MC, Konicek J, Rizzi A, Komatsu DE, Paci JM. Knotless suture anchor with suture tape quadriceps tendon repair is biomechanically superior to transosseous and traditional suture anchor-based repairs in a cadaveric model. Arthroscopy. 2017;33(1):190-198.
23. Brossard P, Le Roux G, Vasse B; Orthopedics, Traumatology Society of Western France (SOO). Acute quadriceps tendon rupture repaired by suture anchors: outcomes at 7 years’ follow-up in 25 cases. Orthop Traumatol Surg Res. 2017;103(4):597-601.
24. Langenhan R, Baumann M, Ricart P, et al. Postoperative functional rehabilitation after repair of quadriceps tendon ruptures: a comparison of two different protocols. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2275-2278.
25. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228-237.
TAKE-HOME POINTS
- Knotless tape suture fixation of the quadriceps tendon is biomechanically superior to traditional fixation techniques.
- When passing locking Krackow stitches, be sure to take all slack out with each pass.
- Consider double tapping the patella pilot holes prior to placing anchors, as the bone is very hard.
- Palpate the articular surface of the patella when drilling pilot holes for safe placement.
- Perform an adequate retinacular repair to complete the repair.
Debunking Atopic Dermatitis Myths: Should Patients Avoid Products With Parabens?
Myth: Parabens are dangerous
Some atopic dermatitis (AD) patients may be misinformed by reports that parabens have estrogenic and antiandrogenic effects and may be involved in carcinogenesis via endocrine modulation. Although in Europe some parabens have been banned or restricted, in the United States there are no regulations against the use of parabens in cosmetics. Dermatologists must acknowledge that their AD patients may have concerns about cosmetic products and they must be prepared to dispel any myths.
Parabens such as methylparaben, propylparaben, butylparaben, and ethylparaben are common in cosmetics such as moisturizers. Parabens have protective properties to prevent the growth of harmful bacteria and mold. According to the US Food and Drug Administration, “scientists continue to review published studies on the safety of parabens. At this time, we do not have information showing that parabens as they are used in cosmetics have an effect on human health. . . . If we determine that a health hazard exists, we will advise the industry and the public.”
Here are some important facts to note for patients, based on a research article published in Cosmetics & Toiletries in June 2017:
- Parabens are not toxic at the concentrations used in personal care products
- Parabens are not genotoxic or carcinogenic
- Parabens are readily excreted in urine and do not accumulate in tissues
In patients with chronic dermatitis, the Cosmetic Ingredient Review Expert Panel reported that parabens generally induce sensitization in less than 4% of patients. The panel concluded that they can support the safety of cosmetic products in which parabens are used as preservatives.
In fact, one study published in the Journal of the American Academy of Dermatology found that AD patients were not predisposed to allergies to parabens, formaldehyde, or diazolidinyl urea, but they were more likely to have allergic reactions to formaldehyde releasers. As a result, AD patients should choose moisturizers containing parabens and should have no fears about using them.
Expert Commentary
In general I recommend paraben-containing cleansers and emollients on a daily basis in practice. However, patient concerns exist due to negative online content easily accessed and fear can prevent usage of agents. Therefore, I am also open to offering options lacking in parabens, such as coconut oil.
—Nanette B. Silverberg, MD (New York, New York)
Doyle K. Some skin creams bad news for eczema. Reuters. December 12, 2013. https://www.reuters.com/article/us-skin-creams-eczema/some-skin-creams-bad-news-for-eczema-idUSBRE9BB14720131212. Accessed January 12, 2018.
Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. June 1, 2017. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the-Trade-425784294.html. Accessed January 12, 2018.
Parabens in cosmetics. US Food and Drug Administration website. https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128042.html. Accessed January 12, 2018.
Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
Shaughnessy CN, Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis: reactivity to topical preservatives. J Am Acad Dermatol. 2014;70:102-107.
Myth: Parabens are dangerous
Some atopic dermatitis (AD) patients may be misinformed by reports that parabens have estrogenic and antiandrogenic effects and may be involved in carcinogenesis via endocrine modulation. Although in Europe some parabens have been banned or restricted, in the United States there are no regulations against the use of parabens in cosmetics. Dermatologists must acknowledge that their AD patients may have concerns about cosmetic products and they must be prepared to dispel any myths.
Parabens such as methylparaben, propylparaben, butylparaben, and ethylparaben are common in cosmetics such as moisturizers. Parabens have protective properties to prevent the growth of harmful bacteria and mold. According to the US Food and Drug Administration, “scientists continue to review published studies on the safety of parabens. At this time, we do not have information showing that parabens as they are used in cosmetics have an effect on human health. . . . If we determine that a health hazard exists, we will advise the industry and the public.”
Here are some important facts to note for patients, based on a research article published in Cosmetics & Toiletries in June 2017:
- Parabens are not toxic at the concentrations used in personal care products
- Parabens are not genotoxic or carcinogenic
- Parabens are readily excreted in urine and do not accumulate in tissues
In patients with chronic dermatitis, the Cosmetic Ingredient Review Expert Panel reported that parabens generally induce sensitization in less than 4% of patients. The panel concluded that they can support the safety of cosmetic products in which parabens are used as preservatives.
In fact, one study published in the Journal of the American Academy of Dermatology found that AD patients were not predisposed to allergies to parabens, formaldehyde, or diazolidinyl urea, but they were more likely to have allergic reactions to formaldehyde releasers. As a result, AD patients should choose moisturizers containing parabens and should have no fears about using them.
Expert Commentary
In general I recommend paraben-containing cleansers and emollients on a daily basis in practice. However, patient concerns exist due to negative online content easily accessed and fear can prevent usage of agents. Therefore, I am also open to offering options lacking in parabens, such as coconut oil.
—Nanette B. Silverberg, MD (New York, New York)
Myth: Parabens are dangerous
Some atopic dermatitis (AD) patients may be misinformed by reports that parabens have estrogenic and antiandrogenic effects and may be involved in carcinogenesis via endocrine modulation. Although in Europe some parabens have been banned or restricted, in the United States there are no regulations against the use of parabens in cosmetics. Dermatologists must acknowledge that their AD patients may have concerns about cosmetic products and they must be prepared to dispel any myths.
Parabens such as methylparaben, propylparaben, butylparaben, and ethylparaben are common in cosmetics such as moisturizers. Parabens have protective properties to prevent the growth of harmful bacteria and mold. According to the US Food and Drug Administration, “scientists continue to review published studies on the safety of parabens. At this time, we do not have information showing that parabens as they are used in cosmetics have an effect on human health. . . . If we determine that a health hazard exists, we will advise the industry and the public.”
Here are some important facts to note for patients, based on a research article published in Cosmetics & Toiletries in June 2017:
- Parabens are not toxic at the concentrations used in personal care products
- Parabens are not genotoxic or carcinogenic
- Parabens are readily excreted in urine and do not accumulate in tissues
In patients with chronic dermatitis, the Cosmetic Ingredient Review Expert Panel reported that parabens generally induce sensitization in less than 4% of patients. The panel concluded that they can support the safety of cosmetic products in which parabens are used as preservatives.
In fact, one study published in the Journal of the American Academy of Dermatology found that AD patients were not predisposed to allergies to parabens, formaldehyde, or diazolidinyl urea, but they were more likely to have allergic reactions to formaldehyde releasers. As a result, AD patients should choose moisturizers containing parabens and should have no fears about using them.
Expert Commentary
In general I recommend paraben-containing cleansers and emollients on a daily basis in practice. However, patient concerns exist due to negative online content easily accessed and fear can prevent usage of agents. Therefore, I am also open to offering options lacking in parabens, such as coconut oil.
—Nanette B. Silverberg, MD (New York, New York)
Doyle K. Some skin creams bad news for eczema. Reuters. December 12, 2013. https://www.reuters.com/article/us-skin-creams-eczema/some-skin-creams-bad-news-for-eczema-idUSBRE9BB14720131212. Accessed January 12, 2018.
Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. June 1, 2017. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the-Trade-425784294.html. Accessed January 12, 2018.
Parabens in cosmetics. US Food and Drug Administration website. https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128042.html. Accessed January 12, 2018.
Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
Shaughnessy CN, Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis: reactivity to topical preservatives. J Am Acad Dermatol. 2014;70:102-107.
Doyle K. Some skin creams bad news for eczema. Reuters. December 12, 2013. https://www.reuters.com/article/us-skin-creams-eczema/some-skin-creams-bad-news-for-eczema-idUSBRE9BB14720131212. Accessed January 12, 2018.
Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. June 1, 2017. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the-Trade-425784294.html. Accessed January 12, 2018.
Parabens in cosmetics. US Food and Drug Administration website. https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128042.html. Accessed January 12, 2018.
Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
Shaughnessy CN, Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis: reactivity to topical preservatives. J Am Acad Dermatol. 2014;70:102-107.
Debunking Acne Myths: Do Patients Need to Worry About Acne After Adolescence?
Myth: Acne only occurs in teenagers
Acne typically is associated with teenagers and puberty, and many adult patients may not be aware that acne can persist beyond adolescence or even develop for the first time in adulthood. As the prevalence of adults with acne increases, it is important to educate this population about factors associated with postadolescent acne development and let them know that effective treatments are available.
There are 2 types of adult acne: persistent acne, which refers to adolescent acne that continues beyond 25 years of age, and late-onset acne, which develops for the first time after 25 years of age. Adult acne generally is mild to moderate in severity and may be refractory to treatment. Unlike adolescent acne, which is more prominent in adolescent boys and manifests as the more severe forms of the disease, adult acne primarily affects women and is more inflammatory in nature, making these patients more susceptible to scarring. In one study, acne prevalence among 1055 adult participants (age range, 20–60 years) was estimated at 61.5%; however, only 36.8% were aware of their condition and only 25% sought treatment. The most commonly affected area was the malar region, which differs from acne seen in teenagers. In addition to the cheeks, adult acne generally is more prominent on the lower chin, jawline, and neck, and lesions more commonly present as closed comedones.
Fluctuating hormone levels are a common cause of adult acne, particularly in women during menses or pregnancy, menopause, or perimenopause; women also may experience breakouts after starting or discontinuing birth control pills. Acne flare-ups in adults also have been linked to chronic stress, family history, hair and skin care products, medication side effects, undiagnosed medical conditions, steroid use, increased calorie intake, whole and fat-reduced milk consumption, and tobacco smoking. Adult acne also has been found to be associated with other dermatologic conditions including hirsutism, alopecia, and seborrhea.
Early diagnosis and treatment of adult acne is crucial to ensure good cosmetic outcomes and minimize disease burden. When treating adult acne, particularly in women, dermatologists should consider a variety of factors that set this condition apart from adolescent acne, including the predisposition of older skin to irritation, possible slow response to treatment, a high likelihood of good adherence to treatment, and the psychosocial impact of acne in the adult population. In adult women, it also is important to consider whether patients are of childbearing age when selecting a treatment. Patients also should be encouraged to read the labels on their personal care products to ensure they are noncomedogenic and will not clog pores.
Adult acne. American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/adult-acne. Accessed January 9, 2018.
Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm [published online January 10, 2013]. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
Khunger N, Kumar C. A clinic-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78:335-341.
Semedo D, Ladeiro F, Ruivo M, et al. Adult acne: prevalence and portrayal in primary healthcare patients, in the Great Porto Area, Portugal [published online September 30, 2016]. Acta Med Port. 2016;29:507-513.
Myth: Acne only occurs in teenagers
Acne typically is associated with teenagers and puberty, and many adult patients may not be aware that acne can persist beyond adolescence or even develop for the first time in adulthood. As the prevalence of adults with acne increases, it is important to educate this population about factors associated with postadolescent acne development and let them know that effective treatments are available.
There are 2 types of adult acne: persistent acne, which refers to adolescent acne that continues beyond 25 years of age, and late-onset acne, which develops for the first time after 25 years of age. Adult acne generally is mild to moderate in severity and may be refractory to treatment. Unlike adolescent acne, which is more prominent in adolescent boys and manifests as the more severe forms of the disease, adult acne primarily affects women and is more inflammatory in nature, making these patients more susceptible to scarring. In one study, acne prevalence among 1055 adult participants (age range, 20–60 years) was estimated at 61.5%; however, only 36.8% were aware of their condition and only 25% sought treatment. The most commonly affected area was the malar region, which differs from acne seen in teenagers. In addition to the cheeks, adult acne generally is more prominent on the lower chin, jawline, and neck, and lesions more commonly present as closed comedones.
Fluctuating hormone levels are a common cause of adult acne, particularly in women during menses or pregnancy, menopause, or perimenopause; women also may experience breakouts after starting or discontinuing birth control pills. Acne flare-ups in adults also have been linked to chronic stress, family history, hair and skin care products, medication side effects, undiagnosed medical conditions, steroid use, increased calorie intake, whole and fat-reduced milk consumption, and tobacco smoking. Adult acne also has been found to be associated with other dermatologic conditions including hirsutism, alopecia, and seborrhea.
Early diagnosis and treatment of adult acne is crucial to ensure good cosmetic outcomes and minimize disease burden. When treating adult acne, particularly in women, dermatologists should consider a variety of factors that set this condition apart from adolescent acne, including the predisposition of older skin to irritation, possible slow response to treatment, a high likelihood of good adherence to treatment, and the psychosocial impact of acne in the adult population. In adult women, it also is important to consider whether patients are of childbearing age when selecting a treatment. Patients also should be encouraged to read the labels on their personal care products to ensure they are noncomedogenic and will not clog pores.
Myth: Acne only occurs in teenagers
Acne typically is associated with teenagers and puberty, and many adult patients may not be aware that acne can persist beyond adolescence or even develop for the first time in adulthood. As the prevalence of adults with acne increases, it is important to educate this population about factors associated with postadolescent acne development and let them know that effective treatments are available.
There are 2 types of adult acne: persistent acne, which refers to adolescent acne that continues beyond 25 years of age, and late-onset acne, which develops for the first time after 25 years of age. Adult acne generally is mild to moderate in severity and may be refractory to treatment. Unlike adolescent acne, which is more prominent in adolescent boys and manifests as the more severe forms of the disease, adult acne primarily affects women and is more inflammatory in nature, making these patients more susceptible to scarring. In one study, acne prevalence among 1055 adult participants (age range, 20–60 years) was estimated at 61.5%; however, only 36.8% were aware of their condition and only 25% sought treatment. The most commonly affected area was the malar region, which differs from acne seen in teenagers. In addition to the cheeks, adult acne generally is more prominent on the lower chin, jawline, and neck, and lesions more commonly present as closed comedones.
Fluctuating hormone levels are a common cause of adult acne, particularly in women during menses or pregnancy, menopause, or perimenopause; women also may experience breakouts after starting or discontinuing birth control pills. Acne flare-ups in adults also have been linked to chronic stress, family history, hair and skin care products, medication side effects, undiagnosed medical conditions, steroid use, increased calorie intake, whole and fat-reduced milk consumption, and tobacco smoking. Adult acne also has been found to be associated with other dermatologic conditions including hirsutism, alopecia, and seborrhea.
Early diagnosis and treatment of adult acne is crucial to ensure good cosmetic outcomes and minimize disease burden. When treating adult acne, particularly in women, dermatologists should consider a variety of factors that set this condition apart from adolescent acne, including the predisposition of older skin to irritation, possible slow response to treatment, a high likelihood of good adherence to treatment, and the psychosocial impact of acne in the adult population. In adult women, it also is important to consider whether patients are of childbearing age when selecting a treatment. Patients also should be encouraged to read the labels on their personal care products to ensure they are noncomedogenic and will not clog pores.
Adult acne. American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/adult-acne. Accessed January 9, 2018.
Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm [published online January 10, 2013]. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
Khunger N, Kumar C. A clinic-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78:335-341.
Semedo D, Ladeiro F, Ruivo M, et al. Adult acne: prevalence and portrayal in primary healthcare patients, in the Great Porto Area, Portugal [published online September 30, 2016]. Acta Med Port. 2016;29:507-513.
Adult acne. American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/adult-acne. Accessed January 9, 2018.
Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm [published online January 10, 2013]. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
Khunger N, Kumar C. A clinic-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78:335-341.
Semedo D, Ladeiro F, Ruivo M, et al. Adult acne: prevalence and portrayal in primary healthcare patients, in the Great Porto Area, Portugal [published online September 30, 2016]. Acta Med Port. 2016;29:507-513.
Debunking Psoriasis Myths: How to Help Patients Who Are Afraid of Injections
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Intraoperative Use of External Fixator Attachments for Reduction of Lower Extremity Fractures and Dislocations
Take-Home Points
- External fixator attachments are fast and easy to assemble with existing external fixator equipment.
- They allow for multi-directional force application and use of extrinsic power grip.
- They limit radiation exposure and provides unobstructred line of sight to zone of injury.
- The attachments can then be removed once reduction is achieved.
External fixation has a long history both for initial open or closed management of fractures and for definitive management.1 After the introduction of internal fixation constructs using nails or plates, external fixation largely transitioned from a means of definitive management to a temporizing measure taken before definitive internal fixation.
The Delta Frame external fixator (DePuy Synthes), which is used for significantly swollen ankle and pilon fractures, features anteromedially placed tibial shaft pins and a transcalcaneal pin. For distal tibia fractures that are not amenable to urgent internal fixation because of the degree of swelling or soft-tissue injury, it provides ligamentotaxis and traction for reduction of fracture fragments and stabilization.2
Numerous other external fixator configurations, such as knee-spanning or tibia-spanning external fixators, can be used for similar purposes. These stabilization methods are all minimally invasive and thus cause little trauma to the zone of injury3 and give soft-tissue injuries time to heal before definitive internal fixation.
Several different external fixator configurations can be used for a variety of fracture patterns and locations, but we propose using the external fixator as a starting point and adding proximal and distal attachments. These attachments have the potential to create more reduction force, and they provide more control of proximal and distal fracture fragments, continue to be minimally invasive, offer extrinsic grip power, are easily assembled and disassembled for intraoperative fracture reduction, and reduce the surgeon’s radiation exposure.
Materials and Methods
Our institution employs an external fixator system that is often used for high-energy lower extremity pathology. This system facilitates assembly of a Sweet T–Cherry II configuration. For periarticular ankle injuries, a Delta Frame external fixator is applied as described in the AO (Arbeitsgemeinschaft für Osteosynthesefragen) surgical reference. Two diaphyseal Schanz pins are inserted into the tibia anterior to posterior based on the pin-placement guide and confirmed with fluoroscopy. These pins must be positioned close enough to the fracture site to provide stability, but not so close as to enter the zone of injury. A Denham pin is placed in the calcaneus medial to lateral. Care is taken to avoid the posterior tibial neurovascular bundle. Then, with use of pin-carbon fiber rod connectors, rods are attached so the Schanz pins connect with the Denham pin. In Sweet T–Cherry II assembly, a different rod configuration is used; rods are attached to the proximal-most Schanz pin and the Denham pin. In Sweet T assembly, a rod-rod connector is used to attach 2 carbon fiber rods to each other.
Next, in Cherry II assembly, 2 carbon fiber rods are attached to and locked to the Denham pin, one medial and the other lateral in an orientation orthogonal to the Denham pin extending distally. The Cherry II apparatus is completed with a third rod and is placed parallel to the Denham pin and orthogonal to the first 2 rods.
For knee-spanning external fixators, 2 Sweet T assemblies can be attached to the 2 Schanz pins. Furthermore, if 2 transverse pins are used for tibial external fixation, 2 Cherry II attachments can be used for multidirectional traction. An added benefit is extrinsic grip power, vs the intrinsic grip power provided with use of only the Schanz and Denham pins.
Results
The fully assembled apparatus provides a firm, well-fixed configuration for applying traction in multiple directions. Axial traction can be applied, as can anterior or posterior translation forces, which may be helpful in fracture reduction and joint dislocation. For difficult-to-reduce fractures or dislocations, the surgeon can apply multidirectional traction distally while the assistant applies countertraction proximally. Fracture reduction is confirmed with fluoroscopy, and the external fixator is locked in position to maintain reduction. After reduction is confirmed, Sweet T and Cherry II are easily removed. The end result is a reduced fracture or dislocation that has the typical appearance of the temporizing external fixator.
Discussion
The Sweet T–Cherry II configuration is assembled quickly and can aid the orthopedic surgeon and assistant in managing trauma cases involving difficult-to-reduce fractures. The principle is the same as in any other traction-countertraction model, though the materials required for assembly are already available to the surgeon, and additional equipment is not required. Furthermore, the large size of the attachments has the potential to offer more points of manipulation by the surgeon and assistant, when compared with Schanz and Denham pins alone. The configuration allows full extrinsic grip power as well (Figure 4), whereas only intrinsic hand power is allowed with traction applied through Schanz and Denham pins alone.
These attachments also have the potential to reduce surgeon and assistant radiation exposure. By positioning Sweet T and Cherry II proximally and distally, with the fluoroscopy machine over the zone of injury, the operators of the attachments increase their distance from the source of radiation. This strategic positioning decreases radiation exposure and reduces direct and scatter energy from the fluoroscopy machine and the patient.4 In addition, with the operators of the attachments farther from the zone of injury, the surgeon has a clear and direct view of the procedure, which may otherwise be obstructed with use of tensioning devices or conventional external fixator configurations. Sweet T and Cherry II attachments theoretically could be used for any configuration that uses proximal and distal fixation points. There is also the added benefit that these attachments are not restricted to applying traction axially but can also translate fracture fragments anteriorly, posteriorly, medially, or laterally, which has the potential to aid in reducing fractures and dislocations with varying degrees of translation, not only shortening. Although these attachments have mechanical counterparts, such as femoral distractors and other tensioning devices, the counterparts may take longer to assemble, apply, and activate. Sweet T and Cherry II attachments are included in the external fixator equipment and may generate more force and control while achieving reduction and stabilization similar to those achieved with other traction or tensioning devices. In addition, other tensioning devices may be cumbersome and unwieldy relative to Sweet T and Cherry II. For these attachments, research is needed on forces generated, time of assembly, and ease of use.
Conclusion
The Sweet T and Cherry II external fixator attachments have the potential to aid in managing difficult-to-reduce complex fractures and dislocations. These attachments are quickly assembled and may generate more force than does reduction with Schanz and Denham pins alone. The shape of these attachments may also provide a more comfortable and easier-to-manipulate base for application of traction in multiple directions. An added benefit is extrinsic grip power. Use of these attachments also has the potential to reduce operator radiation exposure and may provide an unobstructed view of the operative field, as the attachment operators are farther from the zone of injury. In addition, the materials used to assemble these attachments are included in almost all external fixation sets. More research comparing standard external fixator configurations with external fixator configurations using Sweet T and Cherry II attachments is needed.
1. Tejwani N, Polonet D, Wolinsky PR. External fixation of tibial fractures. J Am Acad Orthop Surg. 2015;23(2):126-130.
2. Patterson MJ, Cole JD. Two-staged delayed open reduction and internal fixation of severe pilon fractures. J Orthop Trauma. 1999;13(2):85-91.
3. Bible JE, Mir HR. External fixation: principles and applications. J Am Acad Orthop Surg. 2015;23(11):683-690.
4. Giordano BD, Ryder S, Baumhauer JF, DiGiovanni BF. Exposure to direct and scatter radiation with use of mini-c-arm fluoroscopy. J Bone Joint Surg Am. 2007;89(5):948-952.
Take-Home Points
- External fixator attachments are fast and easy to assemble with existing external fixator equipment.
- They allow for multi-directional force application and use of extrinsic power grip.
- They limit radiation exposure and provides unobstructred line of sight to zone of injury.
- The attachments can then be removed once reduction is achieved.
External fixation has a long history both for initial open or closed management of fractures and for definitive management.1 After the introduction of internal fixation constructs using nails or plates, external fixation largely transitioned from a means of definitive management to a temporizing measure taken before definitive internal fixation.
The Delta Frame external fixator (DePuy Synthes), which is used for significantly swollen ankle and pilon fractures, features anteromedially placed tibial shaft pins and a transcalcaneal pin. For distal tibia fractures that are not amenable to urgent internal fixation because of the degree of swelling or soft-tissue injury, it provides ligamentotaxis and traction for reduction of fracture fragments and stabilization.2
Numerous other external fixator configurations, such as knee-spanning or tibia-spanning external fixators, can be used for similar purposes. These stabilization methods are all minimally invasive and thus cause little trauma to the zone of injury3 and give soft-tissue injuries time to heal before definitive internal fixation.
Several different external fixator configurations can be used for a variety of fracture patterns and locations, but we propose using the external fixator as a starting point and adding proximal and distal attachments. These attachments have the potential to create more reduction force, and they provide more control of proximal and distal fracture fragments, continue to be minimally invasive, offer extrinsic grip power, are easily assembled and disassembled for intraoperative fracture reduction, and reduce the surgeon’s radiation exposure.
Materials and Methods
Our institution employs an external fixator system that is often used for high-energy lower extremity pathology. This system facilitates assembly of a Sweet T–Cherry II configuration. For periarticular ankle injuries, a Delta Frame external fixator is applied as described in the AO (Arbeitsgemeinschaft für Osteosynthesefragen) surgical reference. Two diaphyseal Schanz pins are inserted into the tibia anterior to posterior based on the pin-placement guide and confirmed with fluoroscopy. These pins must be positioned close enough to the fracture site to provide stability, but not so close as to enter the zone of injury. A Denham pin is placed in the calcaneus medial to lateral. Care is taken to avoid the posterior tibial neurovascular bundle. Then, with use of pin-carbon fiber rod connectors, rods are attached so the Schanz pins connect with the Denham pin. In Sweet T–Cherry II assembly, a different rod configuration is used; rods are attached to the proximal-most Schanz pin and the Denham pin. In Sweet T assembly, a rod-rod connector is used to attach 2 carbon fiber rods to each other.
Next, in Cherry II assembly, 2 carbon fiber rods are attached to and locked to the Denham pin, one medial and the other lateral in an orientation orthogonal to the Denham pin extending distally. The Cherry II apparatus is completed with a third rod and is placed parallel to the Denham pin and orthogonal to the first 2 rods.
For knee-spanning external fixators, 2 Sweet T assemblies can be attached to the 2 Schanz pins. Furthermore, if 2 transverse pins are used for tibial external fixation, 2 Cherry II attachments can be used for multidirectional traction. An added benefit is extrinsic grip power, vs the intrinsic grip power provided with use of only the Schanz and Denham pins.
Results
The fully assembled apparatus provides a firm, well-fixed configuration for applying traction in multiple directions. Axial traction can be applied, as can anterior or posterior translation forces, which may be helpful in fracture reduction and joint dislocation. For difficult-to-reduce fractures or dislocations, the surgeon can apply multidirectional traction distally while the assistant applies countertraction proximally. Fracture reduction is confirmed with fluoroscopy, and the external fixator is locked in position to maintain reduction. After reduction is confirmed, Sweet T and Cherry II are easily removed. The end result is a reduced fracture or dislocation that has the typical appearance of the temporizing external fixator.
Discussion
The Sweet T–Cherry II configuration is assembled quickly and can aid the orthopedic surgeon and assistant in managing trauma cases involving difficult-to-reduce fractures. The principle is the same as in any other traction-countertraction model, though the materials required for assembly are already available to the surgeon, and additional equipment is not required. Furthermore, the large size of the attachments has the potential to offer more points of manipulation by the surgeon and assistant, when compared with Schanz and Denham pins alone. The configuration allows full extrinsic grip power as well (Figure 4), whereas only intrinsic hand power is allowed with traction applied through Schanz and Denham pins alone.
These attachments also have the potential to reduce surgeon and assistant radiation exposure. By positioning Sweet T and Cherry II proximally and distally, with the fluoroscopy machine over the zone of injury, the operators of the attachments increase their distance from the source of radiation. This strategic positioning decreases radiation exposure and reduces direct and scatter energy from the fluoroscopy machine and the patient.4 In addition, with the operators of the attachments farther from the zone of injury, the surgeon has a clear and direct view of the procedure, which may otherwise be obstructed with use of tensioning devices or conventional external fixator configurations. Sweet T and Cherry II attachments theoretically could be used for any configuration that uses proximal and distal fixation points. There is also the added benefit that these attachments are not restricted to applying traction axially but can also translate fracture fragments anteriorly, posteriorly, medially, or laterally, which has the potential to aid in reducing fractures and dislocations with varying degrees of translation, not only shortening. Although these attachments have mechanical counterparts, such as femoral distractors and other tensioning devices, the counterparts may take longer to assemble, apply, and activate. Sweet T and Cherry II attachments are included in the external fixator equipment and may generate more force and control while achieving reduction and stabilization similar to those achieved with other traction or tensioning devices. In addition, other tensioning devices may be cumbersome and unwieldy relative to Sweet T and Cherry II. For these attachments, research is needed on forces generated, time of assembly, and ease of use.
Conclusion
The Sweet T and Cherry II external fixator attachments have the potential to aid in managing difficult-to-reduce complex fractures and dislocations. These attachments are quickly assembled and may generate more force than does reduction with Schanz and Denham pins alone. The shape of these attachments may also provide a more comfortable and easier-to-manipulate base for application of traction in multiple directions. An added benefit is extrinsic grip power. Use of these attachments also has the potential to reduce operator radiation exposure and may provide an unobstructed view of the operative field, as the attachment operators are farther from the zone of injury. In addition, the materials used to assemble these attachments are included in almost all external fixation sets. More research comparing standard external fixator configurations with external fixator configurations using Sweet T and Cherry II attachments is needed.
Take-Home Points
- External fixator attachments are fast and easy to assemble with existing external fixator equipment.
- They allow for multi-directional force application and use of extrinsic power grip.
- They limit radiation exposure and provides unobstructred line of sight to zone of injury.
- The attachments can then be removed once reduction is achieved.
External fixation has a long history both for initial open or closed management of fractures and for definitive management.1 After the introduction of internal fixation constructs using nails or plates, external fixation largely transitioned from a means of definitive management to a temporizing measure taken before definitive internal fixation.
The Delta Frame external fixator (DePuy Synthes), which is used for significantly swollen ankle and pilon fractures, features anteromedially placed tibial shaft pins and a transcalcaneal pin. For distal tibia fractures that are not amenable to urgent internal fixation because of the degree of swelling or soft-tissue injury, it provides ligamentotaxis and traction for reduction of fracture fragments and stabilization.2
Numerous other external fixator configurations, such as knee-spanning or tibia-spanning external fixators, can be used for similar purposes. These stabilization methods are all minimally invasive and thus cause little trauma to the zone of injury3 and give soft-tissue injuries time to heal before definitive internal fixation.
Several different external fixator configurations can be used for a variety of fracture patterns and locations, but we propose using the external fixator as a starting point and adding proximal and distal attachments. These attachments have the potential to create more reduction force, and they provide more control of proximal and distal fracture fragments, continue to be minimally invasive, offer extrinsic grip power, are easily assembled and disassembled for intraoperative fracture reduction, and reduce the surgeon’s radiation exposure.
Materials and Methods
Our institution employs an external fixator system that is often used for high-energy lower extremity pathology. This system facilitates assembly of a Sweet T–Cherry II configuration. For periarticular ankle injuries, a Delta Frame external fixator is applied as described in the AO (Arbeitsgemeinschaft für Osteosynthesefragen) surgical reference. Two diaphyseal Schanz pins are inserted into the tibia anterior to posterior based on the pin-placement guide and confirmed with fluoroscopy. These pins must be positioned close enough to the fracture site to provide stability, but not so close as to enter the zone of injury. A Denham pin is placed in the calcaneus medial to lateral. Care is taken to avoid the posterior tibial neurovascular bundle. Then, with use of pin-carbon fiber rod connectors, rods are attached so the Schanz pins connect with the Denham pin. In Sweet T–Cherry II assembly, a different rod configuration is used; rods are attached to the proximal-most Schanz pin and the Denham pin. In Sweet T assembly, a rod-rod connector is used to attach 2 carbon fiber rods to each other.
Next, in Cherry II assembly, 2 carbon fiber rods are attached to and locked to the Denham pin, one medial and the other lateral in an orientation orthogonal to the Denham pin extending distally. The Cherry II apparatus is completed with a third rod and is placed parallel to the Denham pin and orthogonal to the first 2 rods.
For knee-spanning external fixators, 2 Sweet T assemblies can be attached to the 2 Schanz pins. Furthermore, if 2 transverse pins are used for tibial external fixation, 2 Cherry II attachments can be used for multidirectional traction. An added benefit is extrinsic grip power, vs the intrinsic grip power provided with use of only the Schanz and Denham pins.
Results
The fully assembled apparatus provides a firm, well-fixed configuration for applying traction in multiple directions. Axial traction can be applied, as can anterior or posterior translation forces, which may be helpful in fracture reduction and joint dislocation. For difficult-to-reduce fractures or dislocations, the surgeon can apply multidirectional traction distally while the assistant applies countertraction proximally. Fracture reduction is confirmed with fluoroscopy, and the external fixator is locked in position to maintain reduction. After reduction is confirmed, Sweet T and Cherry II are easily removed. The end result is a reduced fracture or dislocation that has the typical appearance of the temporizing external fixator.
Discussion
The Sweet T–Cherry II configuration is assembled quickly and can aid the orthopedic surgeon and assistant in managing trauma cases involving difficult-to-reduce fractures. The principle is the same as in any other traction-countertraction model, though the materials required for assembly are already available to the surgeon, and additional equipment is not required. Furthermore, the large size of the attachments has the potential to offer more points of manipulation by the surgeon and assistant, when compared with Schanz and Denham pins alone. The configuration allows full extrinsic grip power as well (Figure 4), whereas only intrinsic hand power is allowed with traction applied through Schanz and Denham pins alone.
These attachments also have the potential to reduce surgeon and assistant radiation exposure. By positioning Sweet T and Cherry II proximally and distally, with the fluoroscopy machine over the zone of injury, the operators of the attachments increase their distance from the source of radiation. This strategic positioning decreases radiation exposure and reduces direct and scatter energy from the fluoroscopy machine and the patient.4 In addition, with the operators of the attachments farther from the zone of injury, the surgeon has a clear and direct view of the procedure, which may otherwise be obstructed with use of tensioning devices or conventional external fixator configurations. Sweet T and Cherry II attachments theoretically could be used for any configuration that uses proximal and distal fixation points. There is also the added benefit that these attachments are not restricted to applying traction axially but can also translate fracture fragments anteriorly, posteriorly, medially, or laterally, which has the potential to aid in reducing fractures and dislocations with varying degrees of translation, not only shortening. Although these attachments have mechanical counterparts, such as femoral distractors and other tensioning devices, the counterparts may take longer to assemble, apply, and activate. Sweet T and Cherry II attachments are included in the external fixator equipment and may generate more force and control while achieving reduction and stabilization similar to those achieved with other traction or tensioning devices. In addition, other tensioning devices may be cumbersome and unwieldy relative to Sweet T and Cherry II. For these attachments, research is needed on forces generated, time of assembly, and ease of use.
Conclusion
The Sweet T and Cherry II external fixator attachments have the potential to aid in managing difficult-to-reduce complex fractures and dislocations. These attachments are quickly assembled and may generate more force than does reduction with Schanz and Denham pins alone. The shape of these attachments may also provide a more comfortable and easier-to-manipulate base for application of traction in multiple directions. An added benefit is extrinsic grip power. Use of these attachments also has the potential to reduce operator radiation exposure and may provide an unobstructed view of the operative field, as the attachment operators are farther from the zone of injury. In addition, the materials used to assemble these attachments are included in almost all external fixation sets. More research comparing standard external fixator configurations with external fixator configurations using Sweet T and Cherry II attachments is needed.
1. Tejwani N, Polonet D, Wolinsky PR. External fixation of tibial fractures. J Am Acad Orthop Surg. 2015;23(2):126-130.
2. Patterson MJ, Cole JD. Two-staged delayed open reduction and internal fixation of severe pilon fractures. J Orthop Trauma. 1999;13(2):85-91.
3. Bible JE, Mir HR. External fixation: principles and applications. J Am Acad Orthop Surg. 2015;23(11):683-690.
4. Giordano BD, Ryder S, Baumhauer JF, DiGiovanni BF. Exposure to direct and scatter radiation with use of mini-c-arm fluoroscopy. J Bone Joint Surg Am. 2007;89(5):948-952.
1. Tejwani N, Polonet D, Wolinsky PR. External fixation of tibial fractures. J Am Acad Orthop Surg. 2015;23(2):126-130.
2. Patterson MJ, Cole JD. Two-staged delayed open reduction and internal fixation of severe pilon fractures. J Orthop Trauma. 1999;13(2):85-91.
3. Bible JE, Mir HR. External fixation: principles and applications. J Am Acad Orthop Surg. 2015;23(11):683-690.
4. Giordano BD, Ryder S, Baumhauer JF, DiGiovanni BF. Exposure to direct and scatter radiation with use of mini-c-arm fluoroscopy. J Bone Joint Surg Am. 2007;89(5):948-952.
Social Media Creates Anxiety for Teenagers With Acne
Adolescents with acne experience anxiety over using social media, according to a recent online survey of teenagers in the United States. The results of the survey, conducted by Harris Poll on behalf of Cutanea Life Sciences, Inc, demonstrate the negative impact of acne on body image and self-esteem.
Of 1010 teens surveyed (age range, 15–19 years), 86% said they have had acne, and a majority of respondents said that acne has a negative effect on their body image and attractiveness (71%) as well as their self-esteem (67%). Fifty-one percent of respondents who use social media said it makes having acne harder and 72% agreed most teenagers with acne are self-conscious about showing their acne on social media. As a result, 68% reported that most of their peers with acne edit or alter their photographs on social media, and 58% have offered to take a photograph to avoid being in a picture. Half of the respondents have taken at least 1 of the following actions to avoid displaying their acne on social media:
- Chose not to include a photograph on social media
- Deleted or untagged a photograph that showed their acne
- Asked someone else to take down a photograph because it showed their acne
- Altered, edited, retouched, or cropped a photograph to try and hide their acne
- Avoided having their picture taken with someone who had clearer skin
- Stayed off social media so they would not have to post or see photographs of themselves
Dermatologists should be aware of the psychosocial impact of acne in teenagers to provide effective management strategies. Although the majority of teens (61%) said they were doing everything possible to manage their acne, 1 in 3 respondents admitted to having difficulty managing their condition. To effectively treat acne, more than three-quarters said that it was at least very important to use a therapy that worked quickly (83%) and was affordable (80%) and easy to use (78%). Be sure to address the psychosocial impact of acne with your teenaged patients, especially pertaining to social media.
Adolescents with acne experience anxiety over using social media, according to a recent online survey of teenagers in the United States. The results of the survey, conducted by Harris Poll on behalf of Cutanea Life Sciences, Inc, demonstrate the negative impact of acne on body image and self-esteem.
Of 1010 teens surveyed (age range, 15–19 years), 86% said they have had acne, and a majority of respondents said that acne has a negative effect on their body image and attractiveness (71%) as well as their self-esteem (67%). Fifty-one percent of respondents who use social media said it makes having acne harder and 72% agreed most teenagers with acne are self-conscious about showing their acne on social media. As a result, 68% reported that most of their peers with acne edit or alter their photographs on social media, and 58% have offered to take a photograph to avoid being in a picture. Half of the respondents have taken at least 1 of the following actions to avoid displaying their acne on social media:
- Chose not to include a photograph on social media
- Deleted or untagged a photograph that showed their acne
- Asked someone else to take down a photograph because it showed their acne
- Altered, edited, retouched, or cropped a photograph to try and hide their acne
- Avoided having their picture taken with someone who had clearer skin
- Stayed off social media so they would not have to post or see photographs of themselves
Dermatologists should be aware of the psychosocial impact of acne in teenagers to provide effective management strategies. Although the majority of teens (61%) said they were doing everything possible to manage their acne, 1 in 3 respondents admitted to having difficulty managing their condition. To effectively treat acne, more than three-quarters said that it was at least very important to use a therapy that worked quickly (83%) and was affordable (80%) and easy to use (78%). Be sure to address the psychosocial impact of acne with your teenaged patients, especially pertaining to social media.
Adolescents with acne experience anxiety over using social media, according to a recent online survey of teenagers in the United States. The results of the survey, conducted by Harris Poll on behalf of Cutanea Life Sciences, Inc, demonstrate the negative impact of acne on body image and self-esteem.
Of 1010 teens surveyed (age range, 15–19 years), 86% said they have had acne, and a majority of respondents said that acne has a negative effect on their body image and attractiveness (71%) as well as their self-esteem (67%). Fifty-one percent of respondents who use social media said it makes having acne harder and 72% agreed most teenagers with acne are self-conscious about showing their acne on social media. As a result, 68% reported that most of their peers with acne edit or alter their photographs on social media, and 58% have offered to take a photograph to avoid being in a picture. Half of the respondents have taken at least 1 of the following actions to avoid displaying their acne on social media:
- Chose not to include a photograph on social media
- Deleted or untagged a photograph that showed their acne
- Asked someone else to take down a photograph because it showed their acne
- Altered, edited, retouched, or cropped a photograph to try and hide their acne
- Avoided having their picture taken with someone who had clearer skin
- Stayed off social media so they would not have to post or see photographs of themselves
Dermatologists should be aware of the psychosocial impact of acne in teenagers to provide effective management strategies. Although the majority of teens (61%) said they were doing everything possible to manage their acne, 1 in 3 respondents admitted to having difficulty managing their condition. To effectively treat acne, more than three-quarters said that it was at least very important to use a therapy that worked quickly (83%) and was affordable (80%) and easy to use (78%). Be sure to address the psychosocial impact of acne with your teenaged patients, especially pertaining to social media.