User login
MACRA Monday: BMI screening and follow-up
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
MACRA Monday: Osteoarthritis assessment
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #109: Osteoarthritis Function and Pain Assessment
This measure is aimed at capturing the percentage of visits that included an assessment of function and pain for patients with a diagnosis of osteoarthritis (OA) who are aged 21 years or older.
What you need to do: Perform an assessment of symptoms and functional status for patients with OA and document it in the medical record. Validated scales and questionnaires may be used but are not required.
Eligible cases include patients aged 21 years and older with a diagnosis of OA and a patient encounter during the performance period. Applicable codes include (CPT): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1006F indicates that OA symptoms and functional status were assessed. Add the 8P modifier to CPT II 1006F if the assessment was not performed and the reason is not otherwise specified.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #109: Osteoarthritis Function and Pain Assessment
This measure is aimed at capturing the percentage of visits that included an assessment of function and pain for patients with a diagnosis of osteoarthritis (OA) who are aged 21 years or older.
What you need to do: Perform an assessment of symptoms and functional status for patients with OA and document it in the medical record. Validated scales and questionnaires may be used but are not required.
Eligible cases include patients aged 21 years and older with a diagnosis of OA and a patient encounter during the performance period. Applicable codes include (CPT): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1006F indicates that OA symptoms and functional status were assessed. Add the 8P modifier to CPT II 1006F if the assessment was not performed and the reason is not otherwise specified.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
Measure #109: Osteoarthritis Function and Pain Assessment
This measure is aimed at capturing the percentage of visits that included an assessment of function and pain for patients with a diagnosis of osteoarthritis (OA) who are aged 21 years or older.
What you need to do: Perform an assessment of symptoms and functional status for patients with OA and document it in the medical record. Validated scales and questionnaires may be used but are not required.
Eligible cases include patients aged 21 years and older with a diagnosis of OA and a patient encounter during the performance period. Applicable codes include (CPT): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 1006F indicates that OA symptoms and functional status were assessed. Add the 8P modifier to CPT II 1006F if the assessment was not performed and the reason is not otherwise specified.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dietary restrictions with MAOIs
VIDEO: Canakinumab’s cardiovascular benefits linked with hsCRP cuts
ANAHEIM, CALIF. – Targeting the anti-inflammatory drug canakinumab to cardiovascular disease patients with a robust response to a single dose may be an effective way to enhance the cost benefit of this novel treatment that is effective but also expensive.
A post hoc analysis of data collected in the ground-breaking CANTOS trial showed that among patients with a robust anti-inflammatory response to their first dose of canakinumab, the 4-year rate of major adverse cardiovascular events fell by 25% compared with patients who received placebo, a much higher relative risk reduction compared with all canakinumab recipients in the study. In this responsive subgroup, which constituted 55% of all patients assessed after their first dose, canakinumab also cut both cardiovascular death and all-cause death by 31% each relative to placebo, statistically significant reductions that had not been seen in the trial’s primary analysis that included all drug recipients, Paul M. Ridker, MD, said at the American Heart Association scientific sessions.
“The current analysis suggests that the magnitude of hsCRP [high sensitivity C-reactive protein] reduction following a single dose of canakinumab may provide a simple clinical method to identify individuals most likely to accrue the largest cardiovascular and cancer benefits from continued treatment.” The findings have importance “for patient selection, cost-effectiveness, and personalized medicine” as researchers and clinicians begin using anti-inflammatory drugs such as canakinumab to treat cardiovascular disease patients, said Dr. Ridker, professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
“The magnitude of the hsCRP reduction” after the first dose “is telling us who benefits most,” Dr. Ridker said in a video interview.
The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) enrolled patients with a history of a MI and a “residual” inflammatory risk based on having an hsCRP level above 2 mg/L at baseline despite receiving optimized standard treatments and having a median LDL cholesterol level of 82 mg/dL. Dr. Ridker and his associates reported the study’s primary outcome results in August at the European Society of Cardiology meeting and in a simultaneously-published article (N Engl J Med. 2017 Sept 21; 377[12]:1119-31).
For the current analyses they defined a robust anti-inflammatory response as CANTOS patients who maintained an hsCRP level below 2 mg/L when measured 3 months after their first canakinumab dose. When the researchers divided all canakinumab recipients into tertiles based on their achieved hsCRP level after one dose, those with the lowest level, less than 1.2 mg/L, also had the best outcome, with a 4-year rate of major adverse cardiovascular events that was 29% lower than the placebo patients. Patients in the middle tertile of achieved hsCRP, from 1.2 up to but not including 2.6 mg/L, showed a smaller but still statistically significant relative 17% reduction in events, while patients with the tertile with the highest hsCRP levels following one canakinumab dose had essentially no difference in their outcomes compared with placebo-treated control patients.
These findings suggest that for hsCRP, “lower is better,” Dr. Ridker noted. “It’s similar to where we were in 1994” when trial results showed how LDL levels related to cardiovascular disease events and the potential that statin treatment held had for reducing event rates.
The new analyses included three additional notable findings:
• The main serious adverse effect from canakinumab treatment, fatal infections, which occurred at a small but significantly higher rate than in control patients, had a similar incidence of about one episode/300 patient years regardless of whether the achieved hsCRP level fell below 2 mg/L or remained above that level. The placebo rate of fatal infections was about one for every 500 patient years.
• The substantially reduced incidence of lung cancer seen in the primary CANTOS results also tracked with achieved hsCRP. Patients with an achieved hsCRP that was below the median for all treated patients had a 71% cut in new-onset lung cancer compared with controls, while patients with hsCRP levels that fell above the median showed no significant difference compared with control patients.
• All three doses of canakinumab tested in CANTOS – 50 mg, 150 mg, and 300 mg administered by subcutaneous injection once every 3 months – produced a drop in hsCRP to below 2 mg/L in some patients, but the rate at which patients reached this level differed depending on dose: 44% among patients who received the lowest dose, 55% of those who received a 150-mg dose, and 65% among those on the highest dose.
But this “responder analysis” of patients who received canakinumab received a strong cautionary caveat from Robert M. Califf, MD, professor of medicine and vice chancellor for health data sciences at Duke University in Durham, N.C.
“Beware of responder analyses,” Dr. Califf said during a talk at the meeting in which he commented on the new CANTOS findings. “There is a long history in cardiology of being misled” by responder analyses, he said.
Dr. Ridker added that the mechanism that determines whether patients have a robust or modest response to canakinumab remains unclear, although it likely depends on genetic factors. He also noted that research being done by himself and others is investigating the efficacy of other anti-inflammatory drugs that could potentially cut cardiovascular disease rates, including methotrexate and colchicine.
Canakinumab (Ilaris) had Food and Drug Administration marketing approval to treat systemic juvenile idiopathic arthritis and pediatric fever syndromes. Concurrently with the meeting report, the results appeared in an article online (Lancet 2017 Nov 13. doi: 10.1016/S0140-6736[17]32814-3).
CANTOS was sponsored by Novartis, the company that markets canakinumab. Dr. Ridker has been a consultant to Novartis and was lead investigator of CANTOS. He also holds patents on using hsCRP to assess cardiovascular disease risk. Dr. Califf is an adviser to Verily Health Sciences.
[email protected]
On Twitter @mitchelzoler
CANTOS is a landmark trial for showing the importance of inflammation in causing cardiovascular disease events. The new analyses from CANTOS strengthen the biological premise of the study and better address some concerns about the magnitude of benefit raised following the first report of the CANTOS findings. We now see an increased effect on major cardiovascular disease events and, for the first time, an impact from treatment on cardiovascular death and all-cause death in the subgroup of patients with a robust anti-inflammatory response to the drug. This shows the possibility of an enhanced benefit-to-risk balance by focusing canakinumab treatment on responsive patients.
New anti-inflammatory agents now in development offer the prospect for oral drugs that could have similar benefits, and by targeting such treatments to selected patients we can envision using an anti-inflammatory strategy for primary prevention of cardiovascular disease events as well as for secondary prevention. We can also anticipate studies that will show how to better integrate anti-inflammatory interventions with lipid-lowering drugs, as well as selected drugs used to treat diabetes.
The data from CANTOS has opened a new world of possibilities related to anti-inflammatory treatments for preventing and treating cardiovascular diseases.
Ira Tabas, MD , is professor of medicine, cell biology, and physiology at Columbia University in New York. He had no disclosures. He made these comments as designated discussant for Dr. Ridker’s report.
CANTOS is a landmark trial for showing the importance of inflammation in causing cardiovascular disease events. The new analyses from CANTOS strengthen the biological premise of the study and better address some concerns about the magnitude of benefit raised following the first report of the CANTOS findings. We now see an increased effect on major cardiovascular disease events and, for the first time, an impact from treatment on cardiovascular death and all-cause death in the subgroup of patients with a robust anti-inflammatory response to the drug. This shows the possibility of an enhanced benefit-to-risk balance by focusing canakinumab treatment on responsive patients.
New anti-inflammatory agents now in development offer the prospect for oral drugs that could have similar benefits, and by targeting such treatments to selected patients we can envision using an anti-inflammatory strategy for primary prevention of cardiovascular disease events as well as for secondary prevention. We can also anticipate studies that will show how to better integrate anti-inflammatory interventions with lipid-lowering drugs, as well as selected drugs used to treat diabetes.
The data from CANTOS has opened a new world of possibilities related to anti-inflammatory treatments for preventing and treating cardiovascular diseases.
Ira Tabas, MD , is professor of medicine, cell biology, and physiology at Columbia University in New York. He had no disclosures. He made these comments as designated discussant for Dr. Ridker’s report.
CANTOS is a landmark trial for showing the importance of inflammation in causing cardiovascular disease events. The new analyses from CANTOS strengthen the biological premise of the study and better address some concerns about the magnitude of benefit raised following the first report of the CANTOS findings. We now see an increased effect on major cardiovascular disease events and, for the first time, an impact from treatment on cardiovascular death and all-cause death in the subgroup of patients with a robust anti-inflammatory response to the drug. This shows the possibility of an enhanced benefit-to-risk balance by focusing canakinumab treatment on responsive patients.
New anti-inflammatory agents now in development offer the prospect for oral drugs that could have similar benefits, and by targeting such treatments to selected patients we can envision using an anti-inflammatory strategy for primary prevention of cardiovascular disease events as well as for secondary prevention. We can also anticipate studies that will show how to better integrate anti-inflammatory interventions with lipid-lowering drugs, as well as selected drugs used to treat diabetes.
The data from CANTOS has opened a new world of possibilities related to anti-inflammatory treatments for preventing and treating cardiovascular diseases.
Ira Tabas, MD , is professor of medicine, cell biology, and physiology at Columbia University in New York. He had no disclosures. He made these comments as designated discussant for Dr. Ridker’s report.
ANAHEIM, CALIF. – Targeting the anti-inflammatory drug canakinumab to cardiovascular disease patients with a robust response to a single dose may be an effective way to enhance the cost benefit of this novel treatment that is effective but also expensive.
A post hoc analysis of data collected in the ground-breaking CANTOS trial showed that among patients with a robust anti-inflammatory response to their first dose of canakinumab, the 4-year rate of major adverse cardiovascular events fell by 25% compared with patients who received placebo, a much higher relative risk reduction compared with all canakinumab recipients in the study. In this responsive subgroup, which constituted 55% of all patients assessed after their first dose, canakinumab also cut both cardiovascular death and all-cause death by 31% each relative to placebo, statistically significant reductions that had not been seen in the trial’s primary analysis that included all drug recipients, Paul M. Ridker, MD, said at the American Heart Association scientific sessions.
“The current analysis suggests that the magnitude of hsCRP [high sensitivity C-reactive protein] reduction following a single dose of canakinumab may provide a simple clinical method to identify individuals most likely to accrue the largest cardiovascular and cancer benefits from continued treatment.” The findings have importance “for patient selection, cost-effectiveness, and personalized medicine” as researchers and clinicians begin using anti-inflammatory drugs such as canakinumab to treat cardiovascular disease patients, said Dr. Ridker, professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
“The magnitude of the hsCRP reduction” after the first dose “is telling us who benefits most,” Dr. Ridker said in a video interview.
The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) enrolled patients with a history of a MI and a “residual” inflammatory risk based on having an hsCRP level above 2 mg/L at baseline despite receiving optimized standard treatments and having a median LDL cholesterol level of 82 mg/dL. Dr. Ridker and his associates reported the study’s primary outcome results in August at the European Society of Cardiology meeting and in a simultaneously-published article (N Engl J Med. 2017 Sept 21; 377[12]:1119-31).
For the current analyses they defined a robust anti-inflammatory response as CANTOS patients who maintained an hsCRP level below 2 mg/L when measured 3 months after their first canakinumab dose. When the researchers divided all canakinumab recipients into tertiles based on their achieved hsCRP level after one dose, those with the lowest level, less than 1.2 mg/L, also had the best outcome, with a 4-year rate of major adverse cardiovascular events that was 29% lower than the placebo patients. Patients in the middle tertile of achieved hsCRP, from 1.2 up to but not including 2.6 mg/L, showed a smaller but still statistically significant relative 17% reduction in events, while patients with the tertile with the highest hsCRP levels following one canakinumab dose had essentially no difference in their outcomes compared with placebo-treated control patients.
These findings suggest that for hsCRP, “lower is better,” Dr. Ridker noted. “It’s similar to where we were in 1994” when trial results showed how LDL levels related to cardiovascular disease events and the potential that statin treatment held had for reducing event rates.
The new analyses included three additional notable findings:
• The main serious adverse effect from canakinumab treatment, fatal infections, which occurred at a small but significantly higher rate than in control patients, had a similar incidence of about one episode/300 patient years regardless of whether the achieved hsCRP level fell below 2 mg/L or remained above that level. The placebo rate of fatal infections was about one for every 500 patient years.
• The substantially reduced incidence of lung cancer seen in the primary CANTOS results also tracked with achieved hsCRP. Patients with an achieved hsCRP that was below the median for all treated patients had a 71% cut in new-onset lung cancer compared with controls, while patients with hsCRP levels that fell above the median showed no significant difference compared with control patients.
• All three doses of canakinumab tested in CANTOS – 50 mg, 150 mg, and 300 mg administered by subcutaneous injection once every 3 months – produced a drop in hsCRP to below 2 mg/L in some patients, but the rate at which patients reached this level differed depending on dose: 44% among patients who received the lowest dose, 55% of those who received a 150-mg dose, and 65% among those on the highest dose.
But this “responder analysis” of patients who received canakinumab received a strong cautionary caveat from Robert M. Califf, MD, professor of medicine and vice chancellor for health data sciences at Duke University in Durham, N.C.
“Beware of responder analyses,” Dr. Califf said during a talk at the meeting in which he commented on the new CANTOS findings. “There is a long history in cardiology of being misled” by responder analyses, he said.
Dr. Ridker added that the mechanism that determines whether patients have a robust or modest response to canakinumab remains unclear, although it likely depends on genetic factors. He also noted that research being done by himself and others is investigating the efficacy of other anti-inflammatory drugs that could potentially cut cardiovascular disease rates, including methotrexate and colchicine.
Canakinumab (Ilaris) had Food and Drug Administration marketing approval to treat systemic juvenile idiopathic arthritis and pediatric fever syndromes. Concurrently with the meeting report, the results appeared in an article online (Lancet 2017 Nov 13. doi: 10.1016/S0140-6736[17]32814-3).
CANTOS was sponsored by Novartis, the company that markets canakinumab. Dr. Ridker has been a consultant to Novartis and was lead investigator of CANTOS. He also holds patents on using hsCRP to assess cardiovascular disease risk. Dr. Califf is an adviser to Verily Health Sciences.
[email protected]
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Targeting the anti-inflammatory drug canakinumab to cardiovascular disease patients with a robust response to a single dose may be an effective way to enhance the cost benefit of this novel treatment that is effective but also expensive.
A post hoc analysis of data collected in the ground-breaking CANTOS trial showed that among patients with a robust anti-inflammatory response to their first dose of canakinumab, the 4-year rate of major adverse cardiovascular events fell by 25% compared with patients who received placebo, a much higher relative risk reduction compared with all canakinumab recipients in the study. In this responsive subgroup, which constituted 55% of all patients assessed after their first dose, canakinumab also cut both cardiovascular death and all-cause death by 31% each relative to placebo, statistically significant reductions that had not been seen in the trial’s primary analysis that included all drug recipients, Paul M. Ridker, MD, said at the American Heart Association scientific sessions.
“The current analysis suggests that the magnitude of hsCRP [high sensitivity C-reactive protein] reduction following a single dose of canakinumab may provide a simple clinical method to identify individuals most likely to accrue the largest cardiovascular and cancer benefits from continued treatment.” The findings have importance “for patient selection, cost-effectiveness, and personalized medicine” as researchers and clinicians begin using anti-inflammatory drugs such as canakinumab to treat cardiovascular disease patients, said Dr. Ridker, professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
“The magnitude of the hsCRP reduction” after the first dose “is telling us who benefits most,” Dr. Ridker said in a video interview.
The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) enrolled patients with a history of a MI and a “residual” inflammatory risk based on having an hsCRP level above 2 mg/L at baseline despite receiving optimized standard treatments and having a median LDL cholesterol level of 82 mg/dL. Dr. Ridker and his associates reported the study’s primary outcome results in August at the European Society of Cardiology meeting and in a simultaneously-published article (N Engl J Med. 2017 Sept 21; 377[12]:1119-31).
For the current analyses they defined a robust anti-inflammatory response as CANTOS patients who maintained an hsCRP level below 2 mg/L when measured 3 months after their first canakinumab dose. When the researchers divided all canakinumab recipients into tertiles based on their achieved hsCRP level after one dose, those with the lowest level, less than 1.2 mg/L, also had the best outcome, with a 4-year rate of major adverse cardiovascular events that was 29% lower than the placebo patients. Patients in the middle tertile of achieved hsCRP, from 1.2 up to but not including 2.6 mg/L, showed a smaller but still statistically significant relative 17% reduction in events, while patients with the tertile with the highest hsCRP levels following one canakinumab dose had essentially no difference in their outcomes compared with placebo-treated control patients.
These findings suggest that for hsCRP, “lower is better,” Dr. Ridker noted. “It’s similar to where we were in 1994” when trial results showed how LDL levels related to cardiovascular disease events and the potential that statin treatment held had for reducing event rates.
The new analyses included three additional notable findings:
• The main serious adverse effect from canakinumab treatment, fatal infections, which occurred at a small but significantly higher rate than in control patients, had a similar incidence of about one episode/300 patient years regardless of whether the achieved hsCRP level fell below 2 mg/L or remained above that level. The placebo rate of fatal infections was about one for every 500 patient years.
• The substantially reduced incidence of lung cancer seen in the primary CANTOS results also tracked with achieved hsCRP. Patients with an achieved hsCRP that was below the median for all treated patients had a 71% cut in new-onset lung cancer compared with controls, while patients with hsCRP levels that fell above the median showed no significant difference compared with control patients.
• All three doses of canakinumab tested in CANTOS – 50 mg, 150 mg, and 300 mg administered by subcutaneous injection once every 3 months – produced a drop in hsCRP to below 2 mg/L in some patients, but the rate at which patients reached this level differed depending on dose: 44% among patients who received the lowest dose, 55% of those who received a 150-mg dose, and 65% among those on the highest dose.
But this “responder analysis” of patients who received canakinumab received a strong cautionary caveat from Robert M. Califf, MD, professor of medicine and vice chancellor for health data sciences at Duke University in Durham, N.C.
“Beware of responder analyses,” Dr. Califf said during a talk at the meeting in which he commented on the new CANTOS findings. “There is a long history in cardiology of being misled” by responder analyses, he said.
Dr. Ridker added that the mechanism that determines whether patients have a robust or modest response to canakinumab remains unclear, although it likely depends on genetic factors. He also noted that research being done by himself and others is investigating the efficacy of other anti-inflammatory drugs that could potentially cut cardiovascular disease rates, including methotrexate and colchicine.
Canakinumab (Ilaris) had Food and Drug Administration marketing approval to treat systemic juvenile idiopathic arthritis and pediatric fever syndromes. Concurrently with the meeting report, the results appeared in an article online (Lancet 2017 Nov 13. doi: 10.1016/S0140-6736[17]32814-3).
CANTOS was sponsored by Novartis, the company that markets canakinumab. Dr. Ridker has been a consultant to Novartis and was lead investigator of CANTOS. He also holds patents on using hsCRP to assess cardiovascular disease risk. Dr. Califf is an adviser to Verily Health Sciences.
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Patients with a strong hsCRP canakinumab response had a 25% drop in cardiovascular disease events compared with controls.
Data source: Post hoc analyses of data collected in CANTOS, a multicenter trial with 10,061 patients.
Disclosures: CANTOS was sponsored by Novartis, the company that markets canakinumab (Ilaris). Dr. Ridker has been a consultant to Novartis and was lead investigator of CANTOS. He also holds patents on using hsCRP to assess cardiovascular disease risk. Dr. Califf is an adviser to Verily Health Sciences.
VIDEO: Study supports close follow-up of patients with high-risk adenomas plus serrated polyps
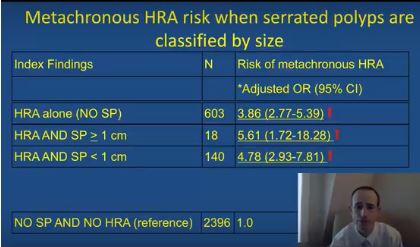
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
The simultaneous colonoscopic presence of serrated polyps and high-risk adenomas led to a fivefold increase in the odds of metachronous high-risk adenomas in a large population-based registry study reported in Gastroenterology (2017. doi: 10.1053/j.gastro.2017.09.011).
The data “support the recommendation that individuals with large and high-risk serrated lesions require closer surveillance,” said Joseph C. Anderson, MD, of White River Junction Department of Veterans Affairs Medical Center, Vt., with his associates. When discounting size and histology, the presence of serrated polyps alone was not associated with an increased risk for metachronous high-risk adenoma, they also reported. Although serrated polyps are important precursors of colorectal cancer, relevant longitudinal surveillance data are sparse. Therefore, the investigators studied 5,433 adults who underwent index and follow-up colonoscopies a median of 4.9 years apart and were tracked in the population-based New Hampshire Colonoscopy Registry. The cohort had a median age of 61 years and half of individuals were male.
SOURCE: AMERICAN GASTROENTEROLOGICAL ASSOCIATION
After adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies, individuals were at significantly increased risk of metachronous high-risk adenoma if their baseline colonoscopy showed high-risk adenoma and synchronous serrated polyps (odds ratio, 5.6; 95% confidence interval, 1.7-18.3), high-risk adenoma with synchronous sessile serrated adenomas (or polyps) or traditional serrated adenomas (OR, 16.0; 95% CI, 7.0-37.0), or high-risk adenoma alone (OR, 3.9; 95% CI, 2.8-5.4), vs. participants with no findings.
The researchers also found that the index presence of large (at least 1-cm) serrated polyps greatly increased the likelihood of finding large metachronous serrated polyps on subsequent colonoscopy (OR, 14.0; 95% CI, 5.0-40.9). “This has clinical relevance, since previous studies have demonstrated an increased risk for colorectal cancer in individuals with large serrated polyps,” the researchers wrote. “However, this increased risk may occur over a protracted time period of 10 years or more, and addressing variation in serrated polyp detection rates and completeness of resection may be more effective than a shorter surveillance interval at reducing risk in these individuals.”
The index presence of sessile serrated adenomas or polyps, or traditional serrated adenomas, also predicted the subsequent development of large serrated polyps (OR, 9.7; 95% CI, 3.6-25.9). The study did not examine polyp location or morphology (flat versus polypoid), but the association might be related to right-sided or flat lesions, which colonoscopists are more likely to miss or to incompletely excise than more defined polypoid lesions, the researchers commented. “Additional research is needed to further clarify the associations between index patient characteristics, polyp location, size, endoscopic appearance and histology, and the metachronous risk of advanced lesions and colorectal cancer in order to refine current surveillance recommendations for individuals undergoing colonoscopy,” they commented.
The study spanned January 2004 to June 2015, and awareness about the importance of serrated polyps rose during this period, they also noted.
The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: High-risk adenomas and the synchronous presence of serrated polyps significantly increased the risk of metachronous high-risk adenomas.
Major finding: Compared with individuals with unremarkable colonoscopies, the odds ratio was 5.6 after adjusting for age, sex, smoking status, body mass index, and median interval between colonoscopies.
Data source: Analyses of index and follow-up colonoscopies of 5,433 individuals from a population-based surveillance registry.
Disclosures: The National Cancer Institute and the Norris Cotton Cancer Center provided funding. The researchers reported having no conflicts of interest.
MACRA Monday: Pneumococcal vaccination
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #111: Pneumococcal Vaccination Status for Older Adults
This measure is aimed at capturing the percentage of patients aged 65 years and older who have ever received a pneumococcal vaccine.
What you need to do: Review the medical record to find out if the patient has ever received a pneumococcal vaccine and if not, administer the vaccine.
Eligible cases include patients who were aged 65 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 4040F indicates that the pneumococcal vaccine was administered or previously received. Use exclusion code G9707 to indicate that the patient was not eligible because they received hospice services at any time during the measurement period.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #111: Pneumococcal Vaccination Status for Older Adults
This measure is aimed at capturing the percentage of patients aged 65 years and older who have ever received a pneumococcal vaccine.
What you need to do: Review the medical record to find out if the patient has ever received a pneumococcal vaccine and if not, administer the vaccine.
Eligible cases include patients who were aged 65 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 4040F indicates that the pneumococcal vaccine was administered or previously received. Use exclusion code G9707 to indicate that the patient was not eligible because they received hospice services at any time during the measurement period.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #111: Pneumococcal Vaccination Status for Older Adults
This measure is aimed at capturing the percentage of patients aged 65 years and older who have ever received a pneumococcal vaccine.
What you need to do: Review the medical record to find out if the patient has ever received a pneumococcal vaccine and if not, administer the vaccine.
Eligible cases include patients who were aged 65 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 4040F indicates that the pneumococcal vaccine was administered or previously received. Use exclusion code G9707 to indicate that the patient was not eligible because they received hospice services at any time during the measurement period.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
• Those who enrolled in Medicare for the first time during a performance period.
• Those who have Medicare Part B allowed charges of $30,000 or less.
• Those who have 100 or fewer Medicare Part B patients.
• Those who are significantly participating in an Advanced Alternative Payment Model (APM).
VIDEO: Laparoscopy is a safe approach throughout pregnancy, expert says
NATIONAL HARBOR, MD. – Laparoscopy offers advantages over laparotomy when performing nonobstetrical surgery on pregnant women, Yuval Kaufman, MD, said at the AAGL Global Congress.
“When we talk about advantages in referral to the pregnant patient, one of the most important things is early ambulation,” Dr. Kaufman, a gynecologic surgeon at Carmel Medical Center in Haifa, Israel, said in an interview. “These patients are in a hypercoagulable state; they are more likely to have DVT and PE. You need them up and running as soon as possible.”
Laparoscopy also tends to be better in terms of handling of the uterus, offering a field of view so that the uterus doesn’t need to be moved as much. In addition, laparoscopy is associated with a smaller, more easily healed scar, and usually requires fewer analgesics, which is better for the fetus, he said.
The Society of American Gastrointestinal and Endoscopic Surgeons recently issued guidelines for the use of laparoscopy during pregnancy, advising surgeons that these procedures can be safely performed during any trimester when the operation is indicated, he said.
“There was an older misconception that surgery has to be done in the second trimester only,” Dr. Kaufman said. “But they actually contradict that; they show that if you postpone surgery for this reason you might be doing much more damage to the mother and to the fetus.”
Dr. Kaufman reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
NATIONAL HARBOR, MD. – Laparoscopy offers advantages over laparotomy when performing nonobstetrical surgery on pregnant women, Yuval Kaufman, MD, said at the AAGL Global Congress.
“When we talk about advantages in referral to the pregnant patient, one of the most important things is early ambulation,” Dr. Kaufman, a gynecologic surgeon at Carmel Medical Center in Haifa, Israel, said in an interview. “These patients are in a hypercoagulable state; they are more likely to have DVT and PE. You need them up and running as soon as possible.”
Laparoscopy also tends to be better in terms of handling of the uterus, offering a field of view so that the uterus doesn’t need to be moved as much. In addition, laparoscopy is associated with a smaller, more easily healed scar, and usually requires fewer analgesics, which is better for the fetus, he said.
The Society of American Gastrointestinal and Endoscopic Surgeons recently issued guidelines for the use of laparoscopy during pregnancy, advising surgeons that these procedures can be safely performed during any trimester when the operation is indicated, he said.
“There was an older misconception that surgery has to be done in the second trimester only,” Dr. Kaufman said. “But they actually contradict that; they show that if you postpone surgery for this reason you might be doing much more damage to the mother and to the fetus.”
Dr. Kaufman reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
NATIONAL HARBOR, MD. – Laparoscopy offers advantages over laparotomy when performing nonobstetrical surgery on pregnant women, Yuval Kaufman, MD, said at the AAGL Global Congress.
“When we talk about advantages in referral to the pregnant patient, one of the most important things is early ambulation,” Dr. Kaufman, a gynecologic surgeon at Carmel Medical Center in Haifa, Israel, said in an interview. “These patients are in a hypercoagulable state; they are more likely to have DVT and PE. You need them up and running as soon as possible.”
Laparoscopy also tends to be better in terms of handling of the uterus, offering a field of view so that the uterus doesn’t need to be moved as much. In addition, laparoscopy is associated with a smaller, more easily healed scar, and usually requires fewer analgesics, which is better for the fetus, he said.
The Society of American Gastrointestinal and Endoscopic Surgeons recently issued guidelines for the use of laparoscopy during pregnancy, advising surgeons that these procedures can be safely performed during any trimester when the operation is indicated, he said.
“There was an older misconception that surgery has to be done in the second trimester only,” Dr. Kaufman said. “But they actually contradict that; they show that if you postpone surgery for this reason you might be doing much more damage to the mother and to the fetus.”
Dr. Kaufman reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @eaztweets
AT AAGL 2017
VIDEO: Dr. Charles E. Miller’s AAGL highlights
NATIONAL HARBOR, MD. – The biggest theme of the 2017 AAGL Global Congress was the importance of understanding anatomy, Charles E. Miller, MD, a minimally invasive gynecologic surgeon in Naperville, Ill., and past president of the AAGL, said at the meeting.
“It’s about doing surgery in the right place, in the right space,” he said.
One of the advantages of this year’s Congress is a greater emphasis on cadaveric dissections, Dr. Miller said during an interview. “Understanding how the nerves are placed, how the vessels are in place, the muscles and the different spaces, and how that all relates to our most complex dissections.”
In a presentation on neuropelveology, Michael Hibner, MD, and Mario Castellanos, MD, of St. Joseph’s Hospital and Medical Center, Phoenix, performed a live cadaveric dissection showing how to deal with a trapped pudendal nerve, working over the gluteus maximus and dissecting down.
In a video session, surgeons demonstrated a needleless robotic-assisted transabdominal cerclage. The nonneedle procedure used a unique, posterior placement of the cerclage knot, a technique which Dr. Miller said he plans to use in his own practice.
The incorporation of colleagues from around the country, and around the world, was another strength of this year’s Congress, said Dr. Miller, particularly a presentation from a Chinese ob.gyn. association on isthmoceles. “To be able to see that this transcends miles upon miles upon miles, but yet we’re seeing the same type of problems, is quite interesting,” he said.
On Twitter @eaztweets
NATIONAL HARBOR, MD. – The biggest theme of the 2017 AAGL Global Congress was the importance of understanding anatomy, Charles E. Miller, MD, a minimally invasive gynecologic surgeon in Naperville, Ill., and past president of the AAGL, said at the meeting.
“It’s about doing surgery in the right place, in the right space,” he said.
One of the advantages of this year’s Congress is a greater emphasis on cadaveric dissections, Dr. Miller said during an interview. “Understanding how the nerves are placed, how the vessels are in place, the muscles and the different spaces, and how that all relates to our most complex dissections.”
In a presentation on neuropelveology, Michael Hibner, MD, and Mario Castellanos, MD, of St. Joseph’s Hospital and Medical Center, Phoenix, performed a live cadaveric dissection showing how to deal with a trapped pudendal nerve, working over the gluteus maximus and dissecting down.
In a video session, surgeons demonstrated a needleless robotic-assisted transabdominal cerclage. The nonneedle procedure used a unique, posterior placement of the cerclage knot, a technique which Dr. Miller said he plans to use in his own practice.
The incorporation of colleagues from around the country, and around the world, was another strength of this year’s Congress, said Dr. Miller, particularly a presentation from a Chinese ob.gyn. association on isthmoceles. “To be able to see that this transcends miles upon miles upon miles, but yet we’re seeing the same type of problems, is quite interesting,” he said.
On Twitter @eaztweets
NATIONAL HARBOR, MD. – The biggest theme of the 2017 AAGL Global Congress was the importance of understanding anatomy, Charles E. Miller, MD, a minimally invasive gynecologic surgeon in Naperville, Ill., and past president of the AAGL, said at the meeting.
“It’s about doing surgery in the right place, in the right space,” he said.
One of the advantages of this year’s Congress is a greater emphasis on cadaveric dissections, Dr. Miller said during an interview. “Understanding how the nerves are placed, how the vessels are in place, the muscles and the different spaces, and how that all relates to our most complex dissections.”
In a presentation on neuropelveology, Michael Hibner, MD, and Mario Castellanos, MD, of St. Joseph’s Hospital and Medical Center, Phoenix, performed a live cadaveric dissection showing how to deal with a trapped pudendal nerve, working over the gluteus maximus and dissecting down.
In a video session, surgeons demonstrated a needleless robotic-assisted transabdominal cerclage. The nonneedle procedure used a unique, posterior placement of the cerclage knot, a technique which Dr. Miller said he plans to use in his own practice.
The incorporation of colleagues from around the country, and around the world, was another strength of this year’s Congress, said Dr. Miller, particularly a presentation from a Chinese ob.gyn. association on isthmoceles. “To be able to see that this transcends miles upon miles upon miles, but yet we’re seeing the same type of problems, is quite interesting,” he said.
On Twitter @eaztweets
AT AAGL 2017
VIDEO: Advanced alternative payment model for RA set to undergo testing
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”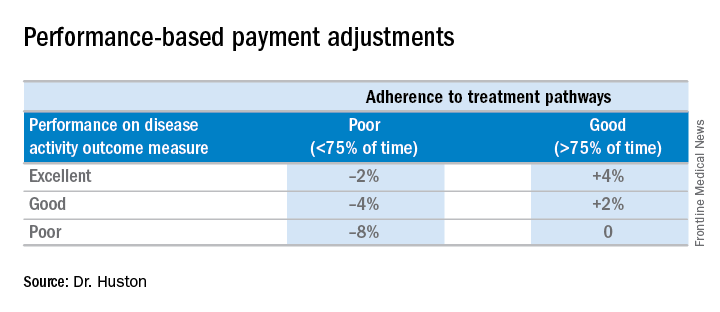
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
AT ACR 2017