User login
Benzene Detected in Benzoyl Peroxide Products: Debate On Implications Continues
Nine days after the independent laboratory, it published another report in Environmental Health Perspectives (EHP), on March 14, also warning about BP acne products.
The bottom line was the same: The laboratory, based in New Haven, Connecticut, said its analyses raise substantial concerns about the safety of BP-containing acne products currently on the market.
The lab’s results showed that the products can form over 800 times the conditionally restricted FDA concentration limit of 2 parts per million (ppm) of benzene, with both prescription and over-the-counter products affected.
“This is a problem of degradation, not contamination,” David Light, CEO and founder of Valisure, said in a telephone interview. BP can decompose into benzene, and exposure to benzene has been linked with a higher risk for leukemia and other blood cancers, according to the American Cancer Society.
In the wake of the findings, however, debate has erupted over the method and approach used by Valisure to test these products, with critics and companies contending that more “real-world” use data are needed. And the US Pharmacopeia (USP) is asking for full transparency about the testing methods.
In a March 8 statement, USP said the petition indicated that modified USP methods were used in the study, noting that “if changes are made to a USP method, complete validation data is necessary to demonstrate that a product meets USP standards.”
However, Valisure contended that drug products need to demonstrate stability over the entire life cycle, from shipment to continued use, emphasizing that constitutes the best “real-world” approach. It also defended the methodology it used.
The reports have led to a state of uncertainty about the use of BP products.
“Right now, we have more unknowns than anything else,” John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said in a video posted on X and YouTube, summarizing the findings released by Valisure on March 6 and 14. He was not involved in the Valisure research.
In a telephone interview, Dr. Barbieri said the report “needs to be taken seriously,” but he also believed the Valisure report is lacking information about testing under “real-world” conditions. He is calling for more information and more transparency about the data. What’s clear, Dr. Barbieri told this news organization, is that the findings about high benzene levels are not a manufacturing error. “It’s something to do with the molecule itself.”
Valisure’s Analyses
Valisure performed an initial analysis, using a method called gas chromatography-mass spectrometry, which is the FDA-preferred method for detecting benzene, Mr. Light said. It tested 175 acne products, 99 containing BP and 76 with other ingredients, such as salicylic acid. All the products without BP had no detectable benzene or values below 2 ppm, the FDA concentration limit for benzene.
Of the 99 BP products, 94 contained benzene without any elevated temperature incubation, according to Valisure. Using 50 °C (122 °F, the accepted pharmaceutical stability testing temperature) on 66 products, Valisure detected over 1500 ppm of benzene in two products, over 100 ppm in 17 products, and over 10 ppm in 42 products over an 18-day period.
The analysis confirmed, Valisure said in a press release and the petition, that a substantial amount of benzene can form in a BP product and leak outside the packaging into surrounding air.
The EHP paper, which includes authors from Valisure, reported that researchers took single lots of seven branded BP products, namely, Equate Beauty 2.5% BP cleansers, Neutrogena 10% BP cleanser, CVS Health 10% BP face wash, Walgreens 10% BP cream, Clean & Clear 10% cleanser, Equate Beauty 10% BP acne wash, and Proactiv 2.5% BP cleanser.
Using testing that involved gas chromatography-mass spectrometry, benzene was detected in all the BP products samples tested, and levels increased during incubation at body and shelf-life performance temperatures to more than 2 ppm. The authors concluded that the study “raises substantial concerns” about the safety of BP products currently on the market.
Methodology Debates
Two days after Valisure released its analysis on March 6, the USP reviewed the citizen’s petition filed by Valisure and called for more transparency around the testing methods.
“The petition referenced USP and indicated that modified USP methods and procedures were used in the study. The presence of unsafe levels of benzene should be taken seriously,” the statement said. The USP statement also noted that the Valisure analysis used modified USP methods and said that “if changes are made to a USP method, complete validation data is necessary to demonstrate that a product meets USP standards.”
In its statement, USP took issue with a practice known as accelerated thermal degradation, which it said Valisure used. USP said the approach involves raising the storage temperature of a product to higher than the temperature indicated on the label for the purpose of simulating degradation over a longer period. While the approach may be acceptable, USP said, the temperatures chosen may not be what is expected to happen to the products.
In response, Mr. Light of Valisure referenced guidance issued in August, 2020, from the FDA, stating that the method it used in the BP analysis can be used to detect impurities in hand sanitizers, including benzene. (In 2021, Valisure detected high levels of benzene in some hand sanitizers and asked the FDA to take action.)
Company Response
Among the companies that took issue with the report was Reckitt, which makes Clearasil, which contains BP. In a statement, the company said, in part: “The products and their ingredients are stable over the storage conditions described on their packaging which represent all reasonable and foreseeable conditions.” It said the findings presented by Valisure reflect “unrealistic scenarios rather than real-world conditions.”
The Personal Care Products Council, a national trade association that represents cosmetic and personal care product manufacturers, also took issue with the findings and the approach used to evaluate the products.
FDA and the Citizen’s Petition
The FDA accepted the petition, Mr. Light said, and gave it a docket number. “We’ll hopefully hear more soon” because the FDA is required to respond to a citizen’s petition within 180 days, he said.
“We generally don’t comment on pending citizens’ petitions,” an FDA spokesperson said in an email. “When we respond, we will respond directly to the petitioner and post the response in the designated agency public docket.”
Valisure’s Patent Application
Mr. Light and others have applied for a patent on methods of producing shelf-stable formulations to prevent degradation of BP to benzene.
“We saw the problem long before we had any sort of application,” Mr. Light said. The issue has been “known for decades.”
Role of BP Products for Acne
In the midst of uncertainty, “the first discussion is, do we want to use it?” Dr. Barbieri said in the interview. Some patients may want to avoid it altogether, until more data are available, including more verification of the findings, while others may be comfortable accepting the potential risk, he said.
“Benzoyl peroxide is one of our foundational acne treatments,” Dr. Barbieri said. In the American Academy of Dermatology updated guidelines on acne, published in January, 2024, strong recommendations were made for BP products, as well as topical retinoids, topical antibiotics, and oral doxycycline.
“When you take away BP, there’s no substitute for it,” Dr. Barbieri said. And if patients don’t get improvement with topicals, oral medications might be needed, and “these all have their own risks.”
In the Interim
Until more information is available, Dr. Barbieri is advising patients not to store the products at high temperatures or for a long time. Don’t keep the products past their expiration date, and perhaps keep products for a shorter time, “something like a month,” he said.
Those living in a hot climate might consider storing the products in the refrigerator, he said.
“We need more data from Valisure, from other groups that confirm their findings, and we need to hear from the FDA,” Dr. Barbieri said. “There’s a lot of uncertainty right now. But it’s important not to overreact.”
Dr. Barbieri had no relevant disclosures.
A version of this article appeared on Medscape.com.
Nine days after the independent laboratory, it published another report in Environmental Health Perspectives (EHP), on March 14, also warning about BP acne products.
The bottom line was the same: The laboratory, based in New Haven, Connecticut, said its analyses raise substantial concerns about the safety of BP-containing acne products currently on the market.
The lab’s results showed that the products can form over 800 times the conditionally restricted FDA concentration limit of 2 parts per million (ppm) of benzene, with both prescription and over-the-counter products affected.
“This is a problem of degradation, not contamination,” David Light, CEO and founder of Valisure, said in a telephone interview. BP can decompose into benzene, and exposure to benzene has been linked with a higher risk for leukemia and other blood cancers, according to the American Cancer Society.
In the wake of the findings, however, debate has erupted over the method and approach used by Valisure to test these products, with critics and companies contending that more “real-world” use data are needed. And the US Pharmacopeia (USP) is asking for full transparency about the testing methods.
In a March 8 statement, USP said the petition indicated that modified USP methods were used in the study, noting that “if changes are made to a USP method, complete validation data is necessary to demonstrate that a product meets USP standards.”
However, Valisure contended that drug products need to demonstrate stability over the entire life cycle, from shipment to continued use, emphasizing that constitutes the best “real-world” approach. It also defended the methodology it used.
The reports have led to a state of uncertainty about the use of BP products.
“Right now, we have more unknowns than anything else,” John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said in a video posted on X and YouTube, summarizing the findings released by Valisure on March 6 and 14. He was not involved in the Valisure research.
In a telephone interview, Dr. Barbieri said the report “needs to be taken seriously,” but he also believed the Valisure report is lacking information about testing under “real-world” conditions. He is calling for more information and more transparency about the data. What’s clear, Dr. Barbieri told this news organization, is that the findings about high benzene levels are not a manufacturing error. “It’s something to do with the molecule itself.”
Valisure’s Analyses
Valisure performed an initial analysis, using a method called gas chromatography-mass spectrometry, which is the FDA-preferred method for detecting benzene, Mr. Light said. It tested 175 acne products, 99 containing BP and 76 with other ingredients, such as salicylic acid. All the products without BP had no detectable benzene or values below 2 ppm, the FDA concentration limit for benzene.
Of the 99 BP products, 94 contained benzene without any elevated temperature incubation, according to Valisure. Using 50 °C (122 °F, the accepted pharmaceutical stability testing temperature) on 66 products, Valisure detected over 1500 ppm of benzene in two products, over 100 ppm in 17 products, and over 10 ppm in 42 products over an 18-day period.
The analysis confirmed, Valisure said in a press release and the petition, that a substantial amount of benzene can form in a BP product and leak outside the packaging into surrounding air.
The EHP paper, which includes authors from Valisure, reported that researchers took single lots of seven branded BP products, namely, Equate Beauty 2.5% BP cleansers, Neutrogena 10% BP cleanser, CVS Health 10% BP face wash, Walgreens 10% BP cream, Clean & Clear 10% cleanser, Equate Beauty 10% BP acne wash, and Proactiv 2.5% BP cleanser.
Using testing that involved gas chromatography-mass spectrometry, benzene was detected in all the BP products samples tested, and levels increased during incubation at body and shelf-life performance temperatures to more than 2 ppm. The authors concluded that the study “raises substantial concerns” about the safety of BP products currently on the market.
Methodology Debates
Two days after Valisure released its analysis on March 6, the USP reviewed the citizen’s petition filed by Valisure and called for more transparency around the testing methods.
“The petition referenced USP and indicated that modified USP methods and procedures were used in the study. The presence of unsafe levels of benzene should be taken seriously,” the statement said. The USP statement also noted that the Valisure analysis used modified USP methods and said that “if changes are made to a USP method, complete validation data is necessary to demonstrate that a product meets USP standards.”
In its statement, USP took issue with a practice known as accelerated thermal degradation, which it said Valisure used. USP said the approach involves raising the storage temperature of a product to higher than the temperature indicated on the label for the purpose of simulating degradation over a longer period. While the approach may be acceptable, USP said, the temperatures chosen may not be what is expected to happen to the products.
In response, Mr. Light of Valisure referenced guidance issued in August, 2020, from the FDA, stating that the method it used in the BP analysis can be used to detect impurities in hand sanitizers, including benzene. (In 2021, Valisure detected high levels of benzene in some hand sanitizers and asked the FDA to take action.)
Company Response
Among the companies that took issue with the report was Reckitt, which makes Clearasil, which contains BP. In a statement, the company said, in part: “The products and their ingredients are stable over the storage conditions described on their packaging which represent all reasonable and foreseeable conditions.” It said the findings presented by Valisure reflect “unrealistic scenarios rather than real-world conditions.”
The Personal Care Products Council, a national trade association that represents cosmetic and personal care product manufacturers, also took issue with the findings and the approach used to evaluate the products.
FDA and the Citizen’s Petition
The FDA accepted the petition, Mr. Light said, and gave it a docket number. “We’ll hopefully hear more soon” because the FDA is required to respond to a citizen’s petition within 180 days, he said.
“We generally don’t comment on pending citizens’ petitions,” an FDA spokesperson said in an email. “When we respond, we will respond directly to the petitioner and post the response in the designated agency public docket.”
Valisure’s Patent Application
Mr. Light and others have applied for a patent on methods of producing shelf-stable formulations to prevent degradation of BP to benzene.
“We saw the problem long before we had any sort of application,” Mr. Light said. The issue has been “known for decades.”
Role of BP Products for Acne
In the midst of uncertainty, “the first discussion is, do we want to use it?” Dr. Barbieri said in the interview. Some patients may want to avoid it altogether, until more data are available, including more verification of the findings, while others may be comfortable accepting the potential risk, he said.
“Benzoyl peroxide is one of our foundational acne treatments,” Dr. Barbieri said. In the American Academy of Dermatology updated guidelines on acne, published in January, 2024, strong recommendations were made for BP products, as well as topical retinoids, topical antibiotics, and oral doxycycline.
“When you take away BP, there’s no substitute for it,” Dr. Barbieri said. And if patients don’t get improvement with topicals, oral medications might be needed, and “these all have their own risks.”
In the Interim
Until more information is available, Dr. Barbieri is advising patients not to store the products at high temperatures or for a long time. Don’t keep the products past their expiration date, and perhaps keep products for a shorter time, “something like a month,” he said.
Those living in a hot climate might consider storing the products in the refrigerator, he said.
“We need more data from Valisure, from other groups that confirm their findings, and we need to hear from the FDA,” Dr. Barbieri said. “There’s a lot of uncertainty right now. But it’s important not to overreact.”
Dr. Barbieri had no relevant disclosures.
A version of this article appeared on Medscape.com.
Nine days after the independent laboratory, it published another report in Environmental Health Perspectives (EHP), on March 14, also warning about BP acne products.
The bottom line was the same: The laboratory, based in New Haven, Connecticut, said its analyses raise substantial concerns about the safety of BP-containing acne products currently on the market.
The lab’s results showed that the products can form over 800 times the conditionally restricted FDA concentration limit of 2 parts per million (ppm) of benzene, with both prescription and over-the-counter products affected.
“This is a problem of degradation, not contamination,” David Light, CEO and founder of Valisure, said in a telephone interview. BP can decompose into benzene, and exposure to benzene has been linked with a higher risk for leukemia and other blood cancers, according to the American Cancer Society.
In the wake of the findings, however, debate has erupted over the method and approach used by Valisure to test these products, with critics and companies contending that more “real-world” use data are needed. And the US Pharmacopeia (USP) is asking for full transparency about the testing methods.
In a March 8 statement, USP said the petition indicated that modified USP methods were used in the study, noting that “if changes are made to a USP method, complete validation data is necessary to demonstrate that a product meets USP standards.”
However, Valisure contended that drug products need to demonstrate stability over the entire life cycle, from shipment to continued use, emphasizing that constitutes the best “real-world” approach. It also defended the methodology it used.
The reports have led to a state of uncertainty about the use of BP products.
“Right now, we have more unknowns than anything else,” John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, said in a video posted on X and YouTube, summarizing the findings released by Valisure on March 6 and 14. He was not involved in the Valisure research.
In a telephone interview, Dr. Barbieri said the report “needs to be taken seriously,” but he also believed the Valisure report is lacking information about testing under “real-world” conditions. He is calling for more information and more transparency about the data. What’s clear, Dr. Barbieri told this news organization, is that the findings about high benzene levels are not a manufacturing error. “It’s something to do with the molecule itself.”
Valisure’s Analyses
Valisure performed an initial analysis, using a method called gas chromatography-mass spectrometry, which is the FDA-preferred method for detecting benzene, Mr. Light said. It tested 175 acne products, 99 containing BP and 76 with other ingredients, such as salicylic acid. All the products without BP had no detectable benzene or values below 2 ppm, the FDA concentration limit for benzene.
Of the 99 BP products, 94 contained benzene without any elevated temperature incubation, according to Valisure. Using 50 °C (122 °F, the accepted pharmaceutical stability testing temperature) on 66 products, Valisure detected over 1500 ppm of benzene in two products, over 100 ppm in 17 products, and over 10 ppm in 42 products over an 18-day period.
The analysis confirmed, Valisure said in a press release and the petition, that a substantial amount of benzene can form in a BP product and leak outside the packaging into surrounding air.
The EHP paper, which includes authors from Valisure, reported that researchers took single lots of seven branded BP products, namely, Equate Beauty 2.5% BP cleansers, Neutrogena 10% BP cleanser, CVS Health 10% BP face wash, Walgreens 10% BP cream, Clean & Clear 10% cleanser, Equate Beauty 10% BP acne wash, and Proactiv 2.5% BP cleanser.
Using testing that involved gas chromatography-mass spectrometry, benzene was detected in all the BP products samples tested, and levels increased during incubation at body and shelf-life performance temperatures to more than 2 ppm. The authors concluded that the study “raises substantial concerns” about the safety of BP products currently on the market.
Methodology Debates
Two days after Valisure released its analysis on March 6, the USP reviewed the citizen’s petition filed by Valisure and called for more transparency around the testing methods.
“The petition referenced USP and indicated that modified USP methods and procedures were used in the study. The presence of unsafe levels of benzene should be taken seriously,” the statement said. The USP statement also noted that the Valisure analysis used modified USP methods and said that “if changes are made to a USP method, complete validation data is necessary to demonstrate that a product meets USP standards.”
In its statement, USP took issue with a practice known as accelerated thermal degradation, which it said Valisure used. USP said the approach involves raising the storage temperature of a product to higher than the temperature indicated on the label for the purpose of simulating degradation over a longer period. While the approach may be acceptable, USP said, the temperatures chosen may not be what is expected to happen to the products.
In response, Mr. Light of Valisure referenced guidance issued in August, 2020, from the FDA, stating that the method it used in the BP analysis can be used to detect impurities in hand sanitizers, including benzene. (In 2021, Valisure detected high levels of benzene in some hand sanitizers and asked the FDA to take action.)
Company Response
Among the companies that took issue with the report was Reckitt, which makes Clearasil, which contains BP. In a statement, the company said, in part: “The products and their ingredients are stable over the storage conditions described on their packaging which represent all reasonable and foreseeable conditions.” It said the findings presented by Valisure reflect “unrealistic scenarios rather than real-world conditions.”
The Personal Care Products Council, a national trade association that represents cosmetic and personal care product manufacturers, also took issue with the findings and the approach used to evaluate the products.
FDA and the Citizen’s Petition
The FDA accepted the petition, Mr. Light said, and gave it a docket number. “We’ll hopefully hear more soon” because the FDA is required to respond to a citizen’s petition within 180 days, he said.
“We generally don’t comment on pending citizens’ petitions,” an FDA spokesperson said in an email. “When we respond, we will respond directly to the petitioner and post the response in the designated agency public docket.”
Valisure’s Patent Application
Mr. Light and others have applied for a patent on methods of producing shelf-stable formulations to prevent degradation of BP to benzene.
“We saw the problem long before we had any sort of application,” Mr. Light said. The issue has been “known for decades.”
Role of BP Products for Acne
In the midst of uncertainty, “the first discussion is, do we want to use it?” Dr. Barbieri said in the interview. Some patients may want to avoid it altogether, until more data are available, including more verification of the findings, while others may be comfortable accepting the potential risk, he said.
“Benzoyl peroxide is one of our foundational acne treatments,” Dr. Barbieri said. In the American Academy of Dermatology updated guidelines on acne, published in January, 2024, strong recommendations were made for BP products, as well as topical retinoids, topical antibiotics, and oral doxycycline.
“When you take away BP, there’s no substitute for it,” Dr. Barbieri said. And if patients don’t get improvement with topicals, oral medications might be needed, and “these all have their own risks.”
In the Interim
Until more information is available, Dr. Barbieri is advising patients not to store the products at high temperatures or for a long time. Don’t keep the products past their expiration date, and perhaps keep products for a shorter time, “something like a month,” he said.
Those living in a hot climate might consider storing the products in the refrigerator, he said.
“We need more data from Valisure, from other groups that confirm their findings, and we need to hear from the FDA,” Dr. Barbieri said. “There’s a lot of uncertainty right now. But it’s important not to overreact.”
Dr. Barbieri had no relevant disclosures.
A version of this article appeared on Medscape.com.
Acne Risk With Progestin-Only Long-Acting Reversible Contraceptives Evaluated
TOPLINE:
Despite the .
METHODOLOGY:
- Progestin-only LARC may increase the risk for acne, but this has not been well studied in adolescents and young adults.
- In the study, researchers evaluated the incidence of acne, acne as a reason for removal, and strategies used to manage acne after insertion of a progestin-only intrauterine device (IUD) or contraceptive implant in 1319 adolescents and young adults across four Adolescent Medicine LARC Collaborative study sites from January 2017 to June 2021.The mean age at insertion was 18.6 years.
- Overall, 24% of participants had acne at the time of LARC insertion.
- Worsening acne was defined as new patient reports of concern about acne, observations of acne, or addition of an acne medication after insertion; increased severity noted on an exam during follow-up or at the time of LARC removal; or acne reported as a side effect and/or reason for LARC removal.
TAKEAWAY:
- During the study period, 376 participants (28.5%) experienced worsening acne after LARC insertion, and 17% reported acne as a new concern, with no differences between those who received an IUD or an implant.
- Only 44 of the 376 participants (11.7%) who reported worsening acne were being treated with an oral agent at follow-up.
- Of the 542 individuals (41% of the total) who had the LARC device removed, 40 (7.4%) cited concerns about acne for removing the device, although just 5 (0.92%) said that acne was the only reason for removal. Of the 40 with concerns about acne when the device was removed, 18 (45%) had documented acne at the time of insertion.
IN PRACTICE:
The authors recommend that clinicians prescribing progestin-only LARC should counsel patients that acne may be a side effect, reassuring them that if they develop acne, “it typically is not problematic enough to warrant discontinuation,” and concluded that “concerns about the development or worsening of acne should not be cause to avoid these forms of contraception.”
SOURCE:
The study, led by Markus D. Boos, MD, PhD, of the division of dermatology in the Department of Pediatrics, University of Washington in Seattle and Seattle Children’s Hospital, was published in Pediatric Dermatology.
LIMITATIONS:
Individuals without documented acne were assumed to be acne-free, creating potential bias. Acne evaluation and treatment were not standardized and were not performed by dermatologists; acne severity was not recorded for many participants, possibly underestimating severity, and excluding LARC insertions without follow-up or with removal within 8 weeks may have underestimated the percentage of participants who developed new or worsening acne.
DISCLOSURES:
The study was supported by Investigator-Initiated Studies Program of Organon and by the Health Resources and Services Administration of the US Department of Health and Human Services. Many authors received grants for this work. The authors did not disclose any other competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
Despite the .
METHODOLOGY:
- Progestin-only LARC may increase the risk for acne, but this has not been well studied in adolescents and young adults.
- In the study, researchers evaluated the incidence of acne, acne as a reason for removal, and strategies used to manage acne after insertion of a progestin-only intrauterine device (IUD) or contraceptive implant in 1319 adolescents and young adults across four Adolescent Medicine LARC Collaborative study sites from January 2017 to June 2021.The mean age at insertion was 18.6 years.
- Overall, 24% of participants had acne at the time of LARC insertion.
- Worsening acne was defined as new patient reports of concern about acne, observations of acne, or addition of an acne medication after insertion; increased severity noted on an exam during follow-up or at the time of LARC removal; or acne reported as a side effect and/or reason for LARC removal.
TAKEAWAY:
- During the study period, 376 participants (28.5%) experienced worsening acne after LARC insertion, and 17% reported acne as a new concern, with no differences between those who received an IUD or an implant.
- Only 44 of the 376 participants (11.7%) who reported worsening acne were being treated with an oral agent at follow-up.
- Of the 542 individuals (41% of the total) who had the LARC device removed, 40 (7.4%) cited concerns about acne for removing the device, although just 5 (0.92%) said that acne was the only reason for removal. Of the 40 with concerns about acne when the device was removed, 18 (45%) had documented acne at the time of insertion.
IN PRACTICE:
The authors recommend that clinicians prescribing progestin-only LARC should counsel patients that acne may be a side effect, reassuring them that if they develop acne, “it typically is not problematic enough to warrant discontinuation,” and concluded that “concerns about the development or worsening of acne should not be cause to avoid these forms of contraception.”
SOURCE:
The study, led by Markus D. Boos, MD, PhD, of the division of dermatology in the Department of Pediatrics, University of Washington in Seattle and Seattle Children’s Hospital, was published in Pediatric Dermatology.
LIMITATIONS:
Individuals without documented acne were assumed to be acne-free, creating potential bias. Acne evaluation and treatment were not standardized and were not performed by dermatologists; acne severity was not recorded for many participants, possibly underestimating severity, and excluding LARC insertions without follow-up or with removal within 8 weeks may have underestimated the percentage of participants who developed new or worsening acne.
DISCLOSURES:
The study was supported by Investigator-Initiated Studies Program of Organon and by the Health Resources and Services Administration of the US Department of Health and Human Services. Many authors received grants for this work. The authors did not disclose any other competing interests.
A version of this article appeared on Medscape.com.
TOPLINE:
Despite the .
METHODOLOGY:
- Progestin-only LARC may increase the risk for acne, but this has not been well studied in adolescents and young adults.
- In the study, researchers evaluated the incidence of acne, acne as a reason for removal, and strategies used to manage acne after insertion of a progestin-only intrauterine device (IUD) or contraceptive implant in 1319 adolescents and young adults across four Adolescent Medicine LARC Collaborative study sites from January 2017 to June 2021.The mean age at insertion was 18.6 years.
- Overall, 24% of participants had acne at the time of LARC insertion.
- Worsening acne was defined as new patient reports of concern about acne, observations of acne, or addition of an acne medication after insertion; increased severity noted on an exam during follow-up or at the time of LARC removal; or acne reported as a side effect and/or reason for LARC removal.
TAKEAWAY:
- During the study period, 376 participants (28.5%) experienced worsening acne after LARC insertion, and 17% reported acne as a new concern, with no differences between those who received an IUD or an implant.
- Only 44 of the 376 participants (11.7%) who reported worsening acne were being treated with an oral agent at follow-up.
- Of the 542 individuals (41% of the total) who had the LARC device removed, 40 (7.4%) cited concerns about acne for removing the device, although just 5 (0.92%) said that acne was the only reason for removal. Of the 40 with concerns about acne when the device was removed, 18 (45%) had documented acne at the time of insertion.
IN PRACTICE:
The authors recommend that clinicians prescribing progestin-only LARC should counsel patients that acne may be a side effect, reassuring them that if they develop acne, “it typically is not problematic enough to warrant discontinuation,” and concluded that “concerns about the development or worsening of acne should not be cause to avoid these forms of contraception.”
SOURCE:
The study, led by Markus D. Boos, MD, PhD, of the division of dermatology in the Department of Pediatrics, University of Washington in Seattle and Seattle Children’s Hospital, was published in Pediatric Dermatology.
LIMITATIONS:
Individuals without documented acne were assumed to be acne-free, creating potential bias. Acne evaluation and treatment were not standardized and were not performed by dermatologists; acne severity was not recorded for many participants, possibly underestimating severity, and excluding LARC insertions without follow-up or with removal within 8 weeks may have underestimated the percentage of participants who developed new or worsening acne.
DISCLOSURES:
The study was supported by Investigator-Initiated Studies Program of Organon and by the Health Resources and Services Administration of the US Department of Health and Human Services. Many authors received grants for this work. The authors did not disclose any other competing interests.
A version of this article appeared on Medscape.com.
Paid Parental Leave: Impact on Maternal Mental Health and Child Wellbeing
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Maternal mental health has a profound impact on the health and wellbeing of the child. Since the onset of the pandemic, rates of postpartum depression have increased, affecting an estimated 1 in 5 women.1 Numerous studies show the impact of postpartum depression on the newborn child across multiple domains, from bonding to healthy weight gain to meeting developmental milestones.
While new medications are being studied and approved to specifically target postpartum depression, these treatments are inaccessible to many because of high costs and long wait lists. Beyond medication, structural changes such as paid parental leave have been shown to have a substantial impact on maternal mental health, thus impacting the health of children as well.
Implications for Mothers and Children
Psychiatric diagnoses such as postpartum depression are on the rise.1,2 This is likely attributable to a combination of factors, including increased isolation since the start of the pandemic, worsening health inequities across race and socioeconomic status, and difficulty accessing mental health care.3-5 The effect that postpartum depression has on the family is significant for the newborn as well as other children in the home.
Data suggest that postpartum depression impacts both the physical and mental health of the child. Infants of mothers with postpartum depression may experience challenges with weight gain, decreased breastfeeding, sleep disruptions, and delays in achieving developmental milestones.6-9 They may also show decreased maternal infant bonding, challenges with cognitive development including language and IQ, and increased risk of behavioral disturbances.10,11 These effects are likely attributable to a combination of factors, including decreased maternal responsiveness to infant cues.7,12 Many of these effects are mediated by the chronicity and severity of depressive symptoms, suggesting the importance of screening and treatment of postpartum depression.10,11 However, treatment for postpartum depression can be difficult to access, particularly given the increased level of need.
It is therefore critical to consider what structural interventions and policy changes can decrease the risk of developing postpartum depression. Data consistently show that access to paid parental leave improves maternal mental health outcomes. Among patients with access to parental leave, research shows that paid leave of longer duration, at least 2-3 months, is the most protective.13 Studies have identified decreased depressive symptoms, decreased stress, decreased use of mental health services, and decreased hospital admissions among women with longer parental leave.13 The positive effects of paid parental leave on maternal mental health can extend beyond the postpartum period, solidifying its impact on the long-term health outcomes of both mother and child.13
Advocacy Is Imperative
In 2024, the United States is the only high-income country, and one of only seven countries in the world, that does not guarantee access to paid parental leave. The Family Medical Leave Act is a 31-year-old federal law that requires some employers to provide unpaid leave to eligible employees. It is narrow in scope, and it excludes many low-wage workers and LGBTQ+ families. Thirteen states — California, Colorado, Connecticut, Delaware, Maine, Massachusetts, Maryland, Minnesota, New Jersey, New York, Oregon, Rhode Island, and Washington — as well as the District of Columbia, have enacted their own paid leave policies. However, there are no federal laws requiring access to paid parental leave. As of 2023, fewer than 30% of workers in the United States have access to paid parental leave, and only 16% of employees in the service industry have access to paid parental leave.14 This disproportionately affects families from lower income backgrounds, and further exacerbates socioeconomic, racial, and gender inequities. From a health systems lens, this increases risk of adverse maternal mental health outcomes among those who already have decreased access to mental health services, worsening health disparities.
Paid parental leave has strong public support across party lines, with polls showing the majority of Americans support comprehensive paid family and medical leave.15 Despite this, the United States has failed to enact legislation on this issue since 1993. Multiple attempts at expanding leave have not come to fruition. In the past year, both the house and the senate have announced bipartisan efforts to expand access to paid parental leave. However, legislative frameworks are still in early stages.
As physicians, it is crucial that we advocate for expanded access to paid parental leave. We must use our expertise to speak to the impact that paid parental leave can have on the mental and physical health of parents, children, and families. By advocating for paid parental leave, we can help create a more just and equitable healthcare system.
Dr. Shannon is a second-year psychiatry resident at University of California, Los Angeles. She attended Stanford University for her undergraduate degree and Dartmouth Geisel School of Medicine for medical school. Her interests include perinatal psychiatry, health systems research, and mental health policy advocacy. Dr. Richards is assistant clinical professor in the department of psychiatry and biobehavioral sciences; program director of the child and adolescent psychiatry fellowship; and associate medical director of the perinatal program at the UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles.
References
1. Wang Z et al. Mapping Global Prevalence of Depression Among Postpartum Women. Transl Psychiatry. 2021 Oct 20. doi: 10.1038/s41398-021-01663-6.
2. Iyengar U et al. One Year Into the Pandemic: A Systematic Review of Perinatal Mental Health Outcomes During COVID-19. Front Psychiatry. 2021 Jun 24. doi: 10.3389/fpsyt.2021.674194.
3. World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2022 Mar 2. www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1.
4. Masters GA et al. Impact of the COVID-19 Pandemic on Mental Health, Access to Care, and Health Disparities in the Perinatal Period. J Psychiatr Res. 2021 May. doi: 10.1016/j.jpsychires.2021.02.056.
5. Shuffrey LC et al. Improving Perinatal Maternal Mental Health Starts With Addressing Structural Inequities. JAMA Psychiatry. 2022 May 1. doi: 10.1001/jamapsychiatry.2022.0097.
6. Lubotzky-Gete S et al. Postpartum Depression and Infant Development Up to 24 months: A Nationwide Population-Based Study. J Affect Disord. 2021 Apr 15. doi: 10.1016/j.jad.2021.02.042.
7. Saharoy R et al. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023 Jul 4. doi: 10.7759/cureus.41381..
8. Gress-Smith JL et al. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Matern Child Health J. 2012 May. doi: 10.1007/s10995-011-0812-y.
9. Kim S et al. The Impact of Antepartum Depression and Postpartum Depression on Exclusive Breastfeeding: A Systematic Review and Meta-Analysis. Clin Nurs Res. 2022 Jun. doi: 10.1177/10547738211053507.
10. Mirhosseini H et al. Cognitive Behavioral Development in Children Following Maternal Postpartum Depression: A Review Article. Electron Physician. 2015 Dec 20. doi: 10.19082/1673.
11. Grace SL et al. The Effect of Postpartum Depression on Child Cognitive Development and Behavior: A Review and Critical Analysis of the Literature. Arch Womens Ment Health. 2003 Nov. doi: 10.1007/s00737-003-0024-6.
12. Milgrom J et al. The Mediating Role of Maternal Responsiveness in Some Longer Term Effects of Postnatal Depression on Infant Development. Infant Behavior and Development. 2004 Sep 11. doi.org/10.1016/j.infbeh.2004.03.003.
13. Heshmati A et al. The Effect of Parental Leave on Parents’ Mental Health: A Systematic Review. Lancet Public Health. 2023 Jan. doi: 10.1016/S2468-2667(22)00311-5.
14. U.S. Bureau of Labor Statistics, What Data Does the BLS Publish on Family Leave? 2023 Sept 21. www.bls.gov/ebs/factsheets/family-leave-benefits-fact-sheet.htm.
15. Horowitz JM et al. Americans Widely Support Paid Family and Medical Leave, But Differ Over Specific Policies. Pew Research Center’s Social & Demographic Trends Project, Pew Research Center. 2017 Mar 23. www.pewresearch.org/social-trends/2017/03/23/americans-widely-support-paid-family-and-medical-leave-but-differ-over-specific-policies/.
Timing the New Meningitis Shots Serogroup Top 5’s
The first pentavalent vaccine approved against all five major serogroups of meningococcal disease has clinicians evaluating the optimal timing for vaccination, according to a new analysis.
Vaccines have helped greatly reduce the rate of invasive meningococcal disease among adolescents over the past 20 years, and the new formulation that covers all main types of the bacteria could help improve vaccination coverage and drive infection rates even lower, reported the research led by senior author Gregory Zimet from the department of pediatrics at the Indiana University School of Medicine in Indianapolis, Indiana.
The five main serogroups — labeled A, B, C, W, and Y — cause most of the disease set off by the bacteria Neisseria meningitidis. It is a rare but serious illness that mostly affects adolescents and young adults.
Meningitis often presents with nonspecific symptoms and can progress to serious illness and even death within hours.
“Clinical features of invasive meningococcal disease, coupled with its unpredictable epidemiology, suggest that vaccination is the best strategy for preventing associated adverse outcomes,” the researchers reported.
Before the introduction of vaccines in 2005, the incidence of disease in the United States ranged from 0.5 to 1.1 cases per 100,000 people, with ≥ 10% of cases being fatal.
The Quadrivalent Vaccine
In 2005, the first quadrivalent meningococcal vaccine, covering serogroups A, C, W, and Y, was approved in the United States and recommended for routine use in 11- and 12-year-olds, followed by a 2010 booster recommendation at age 16 years.
Between 2006 and 2017, the estimated incidence among 11- to 15-year-olds dropped by > 26% each year.
For those aged 16-22 years, the incidence dropped even further by > 35% per year between 2011 and 2017 after the booster was introduced.
Rates also fell in other groups that had not been vaccinated, such as in infants and adults, suggesting possible herd protection after the vaccines.
With Serogroup B
By 2015, a vaccine covering serogroup B was also approved. However, it was not added to the routine vaccination schedule and was subject to shared clinical decision-making between clinicians and patients.
The B vaccine has been less successful, reported the researchers, who said this is likely because uptake was much lower due to it not being part of the routine schedule.
Today, serogroup B makes up a greater proportion of meningitis cases. Before the vaccines were introduced, it accounted for about one third of cases, and now it is the cause of about half of all cases.
Two Doses With a Boost?
In October, the US Food and Drug Administration approved the first pentavalent vaccine against all five major serogroups, which the authors of the analysis said, “may help optimize the existing US adolescent meningococcal vaccination platform”.
A modeling study suggested that the current vaccination schedule of two doses each of the vaccines would prevent 165 cases of meningitis over 10 years. However, a two-dose pentavalent vaccine at age 11 years plus a booster at age 16 years would not only simplify the process and reduce the number of injections required but would also increase the number of cases prevented to 256.
“Use of pentavalent vaccines yields the potential to build on the success of the incumbent program, raising B vaccination coverage by simplifying existing recommendations and decreasing the number of injections required,” the researchers reported, thus “…reducing the clinical and economic burden of meningococcal disease.”
A version of this article appeared on Medscape.com.
The first pentavalent vaccine approved against all five major serogroups of meningococcal disease has clinicians evaluating the optimal timing for vaccination, according to a new analysis.
Vaccines have helped greatly reduce the rate of invasive meningococcal disease among adolescents over the past 20 years, and the new formulation that covers all main types of the bacteria could help improve vaccination coverage and drive infection rates even lower, reported the research led by senior author Gregory Zimet from the department of pediatrics at the Indiana University School of Medicine in Indianapolis, Indiana.
The five main serogroups — labeled A, B, C, W, and Y — cause most of the disease set off by the bacteria Neisseria meningitidis. It is a rare but serious illness that mostly affects adolescents and young adults.
Meningitis often presents with nonspecific symptoms and can progress to serious illness and even death within hours.
“Clinical features of invasive meningococcal disease, coupled with its unpredictable epidemiology, suggest that vaccination is the best strategy for preventing associated adverse outcomes,” the researchers reported.
Before the introduction of vaccines in 2005, the incidence of disease in the United States ranged from 0.5 to 1.1 cases per 100,000 people, with ≥ 10% of cases being fatal.
The Quadrivalent Vaccine
In 2005, the first quadrivalent meningococcal vaccine, covering serogroups A, C, W, and Y, was approved in the United States and recommended for routine use in 11- and 12-year-olds, followed by a 2010 booster recommendation at age 16 years.
Between 2006 and 2017, the estimated incidence among 11- to 15-year-olds dropped by > 26% each year.
For those aged 16-22 years, the incidence dropped even further by > 35% per year between 2011 and 2017 after the booster was introduced.
Rates also fell in other groups that had not been vaccinated, such as in infants and adults, suggesting possible herd protection after the vaccines.
With Serogroup B
By 2015, a vaccine covering serogroup B was also approved. However, it was not added to the routine vaccination schedule and was subject to shared clinical decision-making between clinicians and patients.
The B vaccine has been less successful, reported the researchers, who said this is likely because uptake was much lower due to it not being part of the routine schedule.
Today, serogroup B makes up a greater proportion of meningitis cases. Before the vaccines were introduced, it accounted for about one third of cases, and now it is the cause of about half of all cases.
Two Doses With a Boost?
In October, the US Food and Drug Administration approved the first pentavalent vaccine against all five major serogroups, which the authors of the analysis said, “may help optimize the existing US adolescent meningococcal vaccination platform”.
A modeling study suggested that the current vaccination schedule of two doses each of the vaccines would prevent 165 cases of meningitis over 10 years. However, a two-dose pentavalent vaccine at age 11 years plus a booster at age 16 years would not only simplify the process and reduce the number of injections required but would also increase the number of cases prevented to 256.
“Use of pentavalent vaccines yields the potential to build on the success of the incumbent program, raising B vaccination coverage by simplifying existing recommendations and decreasing the number of injections required,” the researchers reported, thus “…reducing the clinical and economic burden of meningococcal disease.”
A version of this article appeared on Medscape.com.
The first pentavalent vaccine approved against all five major serogroups of meningococcal disease has clinicians evaluating the optimal timing for vaccination, according to a new analysis.
Vaccines have helped greatly reduce the rate of invasive meningococcal disease among adolescents over the past 20 years, and the new formulation that covers all main types of the bacteria could help improve vaccination coverage and drive infection rates even lower, reported the research led by senior author Gregory Zimet from the department of pediatrics at the Indiana University School of Medicine in Indianapolis, Indiana.
The five main serogroups — labeled A, B, C, W, and Y — cause most of the disease set off by the bacteria Neisseria meningitidis. It is a rare but serious illness that mostly affects adolescents and young adults.
Meningitis often presents with nonspecific symptoms and can progress to serious illness and even death within hours.
“Clinical features of invasive meningococcal disease, coupled with its unpredictable epidemiology, suggest that vaccination is the best strategy for preventing associated adverse outcomes,” the researchers reported.
Before the introduction of vaccines in 2005, the incidence of disease in the United States ranged from 0.5 to 1.1 cases per 100,000 people, with ≥ 10% of cases being fatal.
The Quadrivalent Vaccine
In 2005, the first quadrivalent meningococcal vaccine, covering serogroups A, C, W, and Y, was approved in the United States and recommended for routine use in 11- and 12-year-olds, followed by a 2010 booster recommendation at age 16 years.
Between 2006 and 2017, the estimated incidence among 11- to 15-year-olds dropped by > 26% each year.
For those aged 16-22 years, the incidence dropped even further by > 35% per year between 2011 and 2017 after the booster was introduced.
Rates also fell in other groups that had not been vaccinated, such as in infants and adults, suggesting possible herd protection after the vaccines.
With Serogroup B
By 2015, a vaccine covering serogroup B was also approved. However, it was not added to the routine vaccination schedule and was subject to shared clinical decision-making between clinicians and patients.
The B vaccine has been less successful, reported the researchers, who said this is likely because uptake was much lower due to it not being part of the routine schedule.
Today, serogroup B makes up a greater proportion of meningitis cases. Before the vaccines were introduced, it accounted for about one third of cases, and now it is the cause of about half of all cases.
Two Doses With a Boost?
In October, the US Food and Drug Administration approved the first pentavalent vaccine against all five major serogroups, which the authors of the analysis said, “may help optimize the existing US adolescent meningococcal vaccination platform”.
A modeling study suggested that the current vaccination schedule of two doses each of the vaccines would prevent 165 cases of meningitis over 10 years. However, a two-dose pentavalent vaccine at age 11 years plus a booster at age 16 years would not only simplify the process and reduce the number of injections required but would also increase the number of cases prevented to 256.
“Use of pentavalent vaccines yields the potential to build on the success of the incumbent program, raising B vaccination coverage by simplifying existing recommendations and decreasing the number of injections required,” the researchers reported, thus “…reducing the clinical and economic burden of meningococcal disease.”
A version of this article appeared on Medscape.com.
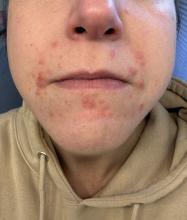
A 35-year-old female presented with a 1-day history of eroded papules and vesicles distributed periorally
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
.1 While it predominantly affects children, it is important to note that it can also affect adults. Although it is not a life threatening infection, it can cause a painful rash and is highly contagious. The infection is easily spread in multiple ways, including via respiratory droplets, contact with vesicular or nasal secretions, or through fecal-oral transmission. Most cases occur during the summer and fall seasons but individuals can be infected at any time of the year.
HFMD typically starts with a few days of non-specific viral symptoms, such as fever, cough, sore throat, and fatigue. It is then followed by an eruption of intraoral macules and vesicles and a widespread distribution of oval shaped macules that predominantly involve the hands and feet.1 Both children and adults can present atypically. Atypical presentations include vesicles and bullae on extensor surfaces such as the forearms, as well as eruptions on the face or buttocks.2 Other atypical morphologies include eczema herpeticum-like, Gianotti-Crosti-like, and purpuric/petechiae.3 Atypical hand, food, and mouth disease cases are often caused by coxsackievirus A6, however other strains of coxsackievirus can also cause atypical symptoms.2,3
Our 35-year-old female patient presented with eroded papules and vesicles around the mouth. A diagnosis of atypical HFMD was made clinically in the following days when the patient developed the more classic intraoral and acral macules and vesicles.
Similar to our case, HFMD is most often diagnosed clinically. PCR testing from an active vesicle or nasopharyngeal swab can be obtained. Treatment for HFMD is supportive and symptoms generally resolve over 7-10 days. Over-the-counter analgesics, such as ibuprofen and acetaminophen, as well as oral analgesics that contain lidocaine or diphenhydramine are often helpful3. In this case, our patient improved over the course of seven days without needing therapy.
This case and the photos were submitted by Vanessa Ortega, BS, University of California, San Diego; Brooke Resh Sateesh, MD, and Justin Gordon, MD, San Diego Family Dermatology. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Centers for Disease Control and Prevention. (2023, June 20). Symptoms of hand, foot, and mouth disease.
2. Drago F et al. J Am Acad Dermatol. 2017 Aug;77(2):e51-6. doi: 10.1016/j.jaad.2017.03.046.
3. Starkey SY et al. Pediatr Dermatol. 2024 Jan-Feb;41(1):23-7. doi: 10.1111/pde.15461.
Treating Pediatric Vitiligo: Consensus Statement Provides Recommendations
TOPLINE:
METHODOLOGY:
- While half of all vitiligo cases manifest within the initial two decades of life, no guidelines specifically address the management of vitiligo in children, adolescents, and young adults with vitiligo.
- A protocol was established to formulate consensus recommendations addressing questions related to pediatric vitiligo.
- Overall, 50 articles on topical corticosteroids and/or topical calcineurin inhibitors, five on topical Janus kinase inhibitors, and two each on pseudocatalase and microdermabrasion were included.
- The participants recorded their agreement levels with the formulated statements, using a 5-point Likert scale.
TAKEAWAY:
- TCIs, TCSs, JAK inhibitors, and phototherapy, specifically narrowband ultraviolet (UV)-B light therapy, are mainstay treatments; the combination of UV-B light and topical therapy may enhance initial repigmentation.
- Long-term monitoring for skin cancers is advised, and short outdoor UV exposure is suggested for pediatric patients.
- TCIs, such as tacrolimus and pimecrolimus, are recommended as first-line therapy, particularly on the face, applied twice daily for ≥ 3 months; continued use for 6-12 additional months is recommended if repigmentation is observed.
- The choice of TCS class depends on the site and planned usage duration. Short-term use or overlap with TCIs is recommended because of the risk for atrophy with long-term TCS use. Class 5-6 agents are another option.
- For areas with thin skin, TCSs can be considered second-line treatments.
- Topical JAK inhibitors, specifically topical 1.5% ruxolitinib cream, are recommended for patients aged ≥ 12 years, as first- or second-line therapy. Limitation to 10% body surface area is recommended to minimize systemic absorption. Limited evidence exists for children aged < 12 years.
IN PRACTICE:
“Effective therapy requires a focus on long-term therapeutic interventions to maximize the local gain and retention of pigmentation with a trial period of twice-weekly application. Counseling should include discussion of the chronicity of vitiligo and the need for long-term care,” the authors wrote.
LIMITATIONS:
Some of the recommendations were opinion-based because of the scarcity of evidence-based literature.
SOURCE:
The consensus statement was published on March 13 in JAMA Dermatology.
DISCLOSURES:
This work was supported by grants from Vitiligo Research Foundation and Incyte Pharmaceuticals. The majority of authors disclosed financial relationships outside this work; several reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- While half of all vitiligo cases manifest within the initial two decades of life, no guidelines specifically address the management of vitiligo in children, adolescents, and young adults with vitiligo.
- A protocol was established to formulate consensus recommendations addressing questions related to pediatric vitiligo.
- Overall, 50 articles on topical corticosteroids and/or topical calcineurin inhibitors, five on topical Janus kinase inhibitors, and two each on pseudocatalase and microdermabrasion were included.
- The participants recorded their agreement levels with the formulated statements, using a 5-point Likert scale.
TAKEAWAY:
- TCIs, TCSs, JAK inhibitors, and phototherapy, specifically narrowband ultraviolet (UV)-B light therapy, are mainstay treatments; the combination of UV-B light and topical therapy may enhance initial repigmentation.
- Long-term monitoring for skin cancers is advised, and short outdoor UV exposure is suggested for pediatric patients.
- TCIs, such as tacrolimus and pimecrolimus, are recommended as first-line therapy, particularly on the face, applied twice daily for ≥ 3 months; continued use for 6-12 additional months is recommended if repigmentation is observed.
- The choice of TCS class depends on the site and planned usage duration. Short-term use or overlap with TCIs is recommended because of the risk for atrophy with long-term TCS use. Class 5-6 agents are another option.
- For areas with thin skin, TCSs can be considered second-line treatments.
- Topical JAK inhibitors, specifically topical 1.5% ruxolitinib cream, are recommended for patients aged ≥ 12 years, as first- or second-line therapy. Limitation to 10% body surface area is recommended to minimize systemic absorption. Limited evidence exists for children aged < 12 years.
IN PRACTICE:
“Effective therapy requires a focus on long-term therapeutic interventions to maximize the local gain and retention of pigmentation with a trial period of twice-weekly application. Counseling should include discussion of the chronicity of vitiligo and the need for long-term care,” the authors wrote.
LIMITATIONS:
Some of the recommendations were opinion-based because of the scarcity of evidence-based literature.
SOURCE:
The consensus statement was published on March 13 in JAMA Dermatology.
DISCLOSURES:
This work was supported by grants from Vitiligo Research Foundation and Incyte Pharmaceuticals. The majority of authors disclosed financial relationships outside this work; several reported no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- While half of all vitiligo cases manifest within the initial two decades of life, no guidelines specifically address the management of vitiligo in children, adolescents, and young adults with vitiligo.
- A protocol was established to formulate consensus recommendations addressing questions related to pediatric vitiligo.
- Overall, 50 articles on topical corticosteroids and/or topical calcineurin inhibitors, five on topical Janus kinase inhibitors, and two each on pseudocatalase and microdermabrasion were included.
- The participants recorded their agreement levels with the formulated statements, using a 5-point Likert scale.
TAKEAWAY:
- TCIs, TCSs, JAK inhibitors, and phototherapy, specifically narrowband ultraviolet (UV)-B light therapy, are mainstay treatments; the combination of UV-B light and topical therapy may enhance initial repigmentation.
- Long-term monitoring for skin cancers is advised, and short outdoor UV exposure is suggested for pediatric patients.
- TCIs, such as tacrolimus and pimecrolimus, are recommended as first-line therapy, particularly on the face, applied twice daily for ≥ 3 months; continued use for 6-12 additional months is recommended if repigmentation is observed.
- The choice of TCS class depends on the site and planned usage duration. Short-term use or overlap with TCIs is recommended because of the risk for atrophy with long-term TCS use. Class 5-6 agents are another option.
- For areas with thin skin, TCSs can be considered second-line treatments.
- Topical JAK inhibitors, specifically topical 1.5% ruxolitinib cream, are recommended for patients aged ≥ 12 years, as first- or second-line therapy. Limitation to 10% body surface area is recommended to minimize systemic absorption. Limited evidence exists for children aged < 12 years.
IN PRACTICE:
“Effective therapy requires a focus on long-term therapeutic interventions to maximize the local gain and retention of pigmentation with a trial period of twice-weekly application. Counseling should include discussion of the chronicity of vitiligo and the need for long-term care,” the authors wrote.
LIMITATIONS:
Some of the recommendations were opinion-based because of the scarcity of evidence-based literature.
SOURCE:
The consensus statement was published on March 13 in JAMA Dermatology.
DISCLOSURES:
This work was supported by grants from Vitiligo Research Foundation and Incyte Pharmaceuticals. The majority of authors disclosed financial relationships outside this work; several reported no disclosures.
A version of this article appeared on Medscape.com.
Inside the 2024 AAD Acne Guidelines: New Therapies Join Old Standbys
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
SAN DIEGO — Just weeks after the American Academy of Dermatology (AAD) published its updated acne management guidelines, a dermatologist who helped write the recommendations provided colleagues with insight into recently approved topical therapies, the importance of multimodal therapy, and a controversial report linking benzoyl peroxide (BP) to the carcinogen benzene.
In regard to topical treatments, the guidelines make a “strong” recommendation for topical retinoids based on “moderate” evidence, Andrea L. Zaenglein, MD, professor of dermatology and pediatrics, Penn State University, Hershey, Pennsylvania, said at the annual meeting of the American Academy of Dermatology. The recommendation was based on a pooled analysis of four randomized controlled trials that found patients with acne who used the medications were more likely to have improvement via the Investigator Global Assessment (IGA) scale at 12 weeks than were those treated with a vehicle (risk ratio [RR], 1.57; 1.21-2.04).
The updated guidelines were published on January 30 in the Journal of the American Academy of Dermatology. The previous guidelines were issued in 2016.
“We have four current retinoids that we use: adapalene, tretinoin, tazarotene, and trifarotene,” Dr. Zaenglein said. “Typically, when we think about retinoids, we think of adapalene as being more tolerable and tazarotene as being more effective. But we also know that they can work to prevent and treat scarring, and they work against comedonal lesions and inflammatory lesions.”
Newer concentrations include tretinoin 0.05% lotion, tazarotene 0.045% lotion, and trifarotene 0.005% cream. She noted that this trifarotene concentration can be helpful for moderate truncal acne and also referred to evidence that whey protein appears to exacerbate that condition. “I always ask teenage kids about that: Are they using those protein powders?”
Recommendations for ‘Multimodal Therapy,’ Especially With Antibiotics
Dr. Zaenglein highlighted a “good practice statement” in the new guidelines that says, “when managing acne with topical medications, we recommend multimodal therapy combining multiple mechanisms of action.”
Topical antibiotics are effective treatments on their own and include erythromycin, clindamycin, and minocycline (Minocin), she said. But the guidelines, which refer to evidence supporting them as “moderate,” do not recommend them as monotherapy because of the risk for antibiotic resistance.
The oral retinoid isotretinoin may be appropriate in conjunction with topical medications, she said, “and we also recommend fixed combination products because they’re associated with increased adherence.”
Dermatologists are familiar with several of these products because “we’ve been using them for years and years,” she said. The guidelines note that “compared to vehicle at 12 weeks, a greater proportion of patients treated with combined BP and topical retinoid achieved IGA success in three RCTs (RR, 2.19; 1.77-2.72).”
Dr. Zaenglein noted that the guidelines recommend that patients taking antibiotics also use benzoyl peroxide, which has “moderate” evidence regarding preventing the development of antibiotic resistance. “Lower strengths tend to be less irritating, and over-the-counter formulations are readily available,” she said, adding that colleagues should make sure to warn patients about the risk of bleaching clothes and towels with BP.
Now, there’s a newly approved treatment, the first fixed-dose triple combination therapy for acne, she said. It combines 1.2% clindamycin, 3.1% benzoyl peroxide, and 0.15% adapalene (Cabtreo) and is Food and Drug Administration (FDA)-approved for treating acne in patients ages 12 and up.
The new AAD guidelines note that “potential adverse effect profiles of the fixed-dose combinations generally reflect those of the individual agents in summation. Some fixed-dose combination products may be less expensive than prescribing their individual components separately.” The evidence supporting fixed-dose combinations in conjunction with benzoyl peroxide is considered “moderate.”
Dapsone gel, 7.5% (Aczone) is another option for acne. “It’s a topical so you don’t need to do G6PD [glucose-6-phosphate dehydrogenase] testing,” Dr. Zaenglein said. “It’s well tolerated, and mean total lesions fell by 48.9% vs 43.2% for vehicle,” in a 2018 study, which she said also found that females benefited more than males from this treatment.
Clascoterone 1% cream (Winlevi), approved in 2020, is appropriate for males and females aged 12 and up, Dr. Zaenglein said. She noted that it’s the only topical anti-androgen that can be used in males. However, while it has a “high” level of evidence because of phase 3 clinical trials showing benefits in moderate to severe acne, the AAD guidelines only conditionally recommend this option because the high price of clascoterone “may impact equitable acne treatment access.” The price listed on the website GoodRx (accessed on March 12) lists drugstore prices for a single 60-gram tube as ranging from $590 to $671.
“One of the harder things is trying to figure out where clascoterone fits in our kind of standard combination therapy,” she said. “Much like other hormonal therapies, it works better over the long term.”
Two more topical options per the AAD guidelines are salicylic acid, based on one randomized controlled trial, and azelaic acid (Azelex, Finacea), based on three randomized controlled trials. Both of these recommendations are conditional because of limited evidence: Evidence is considered “low” for salicylic acid and “moderate” for azelaic acid, the guidelines say, and azelaic acid “may be particularly helpful for patients with sensitive skin or darker skin types due to its lightening effect on dyspigmentation.”
As for risk for topical treatments during pregnancy/lactation, the guidelines note that topical therapies other than topical retinoids are “preferred” during pregnancy. Tazarotene is contraindicated during pregnancy, and salicylic acid should be used only in limited areas of exposure. There are no data for dapsone and clascoterone during pregnancy/lactation, and minocycline is “not recommended.”
The guideline authors noted that “available evidence is insufficient to develop a recommendation on the use of topical glycolic acid, sulfur, sodium sulfacetamide, and resorcinol for acne treatment or to make recommendations that compare topical BP, retinoids, antibiotics, and their combinations directly against each other.”
Could BP Post a Risk From Benzene?
Dr. Zaenglein highlighted a recently released report by Valisure, an independent laboratory, which reported finding high levels of the cancer-causing chemical benzene in several acne treatments, including brands such as Clearasil. “They didn’t release all of the ones that they evaluated, but there were a lot ... that we commonly recommend for our patients,” she said.
On March 6, CBS News reported that Valisure “ran tests at various temperatures over 18 days and found some products ‘can form over 800 times the conditionally restricted FDA concentration limit of two parts per million (ppm) for benzene’ in 2 weeks at 50° C (122° F),” but that benzene levels “at room temperature were more modest, ranging from about one to 24 parts per million.”
Dr. Zaenglein said she’s not ready to urge patients to discontinue BP, although in light of the findings, “I will tell them to store it at room temperature or lower.”
For now, it’s important to wait for independent verification of the results, she said. “And then it’s up to the manufacturers to reevaluate the stability of their benzoyl peroxide products with heat.”
Dr. Zaenglein disclosed relationships with AbbVie, Arcutis, Biofrontera, Galderma, and Incyte (grants/research funding), Church & Dwight (consulting fees), and UCB (consulting honoraria).
A version of this article appeared on Medscape.com.
FROM AAD 2024
Attrition in Youth Sports
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
.
Seventy-five years ago news of this dramatic decline in participation would have received a quizzical shrug because organized youth sports was in its infancy. It consisted primarily of Little League Baseball and for the most part excluded girls. Prior to middle school and high school, children were self-organizing their sports activities – picking their own teams, demarcating their own fields, and making up the rules to fit the conditions. Soccer Dads and Hockey Moms hadn’t been invented. To what extent this attrition from youth sports is contributing to the fact that more than 75% of this country’s adolescents fail to meet even the most lenient activity requirements is unclear. But, it certainly isn’t helping the situation.
Parsing out the contributors to this decline in organized sport should be high on our priority list. In the recent AAP Clinical Report published in Pediatrics (same reference as above) the authors claim “Burnout represents one of the primary reasons for attrition in youth sports.” This statement doesn’t quite agree with my experiences. So, I decided to chase down their reference. It turns out their assertion comes from an article coming out of Australia by eight authors who “brainstormed” 83 unique statements of 61 stakeholders regarding “athlete participation in the high-performance pathway” and concluded “Athlete health was considered the most important athlete retention to address.” While injury and overuse may explain why some elite youth participants drop out and represent a topic for the AAP to address, I’m not sure this paper’s anecdotal conclusion helps us understand the overall decline in youth sports. A broader and deeper discussion can be found in a 2019 AAP Clinical Report that addresses the advantages and pitfalls of youth sports as organized in this country.
How we arrived at this point in which, as the AAP report observes, “Youth sport participation represents the primary route to physical activity” is unclear. One obvious cause is the blossoming of the sedentary entertainment alternatives that has easily overpowered the attraction of self-organized outdoor games. The first attack in this war that we are losing came with affordable color television. Here we must blame ourselves both as parents and pediatricians for not acknowledging the risks and creating some limits. Sadly, the AAP’s initial focus was on content and not on time watched. And, of course, by the time handheld electronic devices arrived the cat was out of the bag.
We also must accept some blame for allowing physical activity to disappear as a meaningful part of the school day. Recesses have been curtailed, leaving free play and all its benefits as an endangered species. Physical education classes have been pared down tragically just as teenagers are making their own choices about how they will spend their time.
We must not underestimate the role that parental anxiety has played in the popularity of organized sport. I’m not sure of the origins of this change, but folks in my cohort recall that our parents let us roam free. As long as we showed up for meals without a policeman in tow, our parents were happy. For some reason parents seem more concerned about risks of their children being outside unmonitored, even in what are clearly safe neighborhoods.
Into this void created by sedentary amusements, limited in-school opportunities for physical activity, and parental anxiety, adult (often parent) organized sports have flourished. Unfortunately, they have too often been over-organized and allowed to morph into a model that mimics professional sports. The myth that to succeed a child must start early, narrow his/her focus and practice, practice, practice has created a situation that is a major contributor to the decline in youth sports participation. This philosophy also contributes to burnout and overuse injuries, but this is primarily among the few and the more elite.
When the child who is already involved in an organized sport sees and believes that he or she hasn’t a chance against the “early bloomers,” that child will quit. Without an appealing alternative, he/she will become sedentary. Further damage is done when children themselves and their parents have witnessed other families heavily invested in professionalized youth programs and decide it doesn’t make sense to even sign up.
In full disclosure, I must say that I have children and grandchildren who have participated in travel teams. Luckily they have not been tempted to seek even more elite programs. They have played at least two or three sports each year and still remain physically active as adults.
I don’t think the answer to the decline in youth sports is to eliminate travel and super-elite teams. The drive to succeed is too strong in some individuals. The answers lie in setting limits on sedentary alternatives, continuing to loudly question the myth of early specialization, and to work harder at offering the broadest range of opportunities that can appeal to children of all skill levels.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Sulfites Selected as ACDS Allergen of the Year
by the American Contact Dermatitis Society (ACDS).
Sulfites are currently not found in most screening patch test series, so may be missed as a relevant contact allergen, Donald V. Belsito, MD, emeritus professor in the Department of Dermatology at Columbia University, New York City, said in his presentation on the Allergen of the Year on March 7 at the annual meeting of the American Contact Dermatitis Society in San Diego. Sulfites, he noted, are distinct from sulfates, and the groups do not cross-react with each other.
Sodium disulfite, an inorganic compound, belongs to a group of sulfiting agents, which contain the sulfite ion SO32− and include ammonium sulfite, potassium sulfite, and sodium sulfite, Dr. Belsito said. Sulfites function as antioxidants and preservatives in a range of products including food and beverages, personal care products, and pharmaceuticals.
The type of sulfite allergy diagnosed by patch testing is type IV hypersensitivity or delayed-type hypersensitivity, where patients present with pruritic, red, scaling macules, papulovesicles, and patches, Dr. Belsito told this news organization. “It is not the type I, immediate hypersensitivity that causes hives and, in some cases, anaphylaxis,” he said. Sulfites also can cause these side effects, so correct labeling of food and beverages is important, he noted.
Some common nonoccupational sulfite sources include hair coloring and bleach products, hairspray, tanning lotions, makeup, sunscreens, and deodorants, Dr. Belsito said in his presentation. Medications including topical antifungals, topical corticosteroids, and nasal solutions can be culprits, as can water in swimming pools, he noted.
In occupational settings, sulfites may be present not only in food and drink products but also can be used in production of products, such as those used for sterilization during beer and wine fermentation, Dr. Belsito said. Other potential occupational sources of sulfite exposure include healthcare settings and textile, chemical, rubber, and pharmaceutical manufacturing.
High-sulfite food products (> 100 ppm) to be aware of include dried fruit (raisins and prunes are exceptions), bottled lemon or lime juice (but not frozen products), wine, molasses, grape juice (white, or white, pink, and red sparkling), and pickled cocktail onions, Dr. Belsito said.
“Like other contact allergens, the clinical presentation correlates with exposure,” he added. A study by the North American Contact Dermatitis Group (NACDG) found that 28.8% of patients positive for sulfite allergy on patch testing presented with facial dermatitis, which was not only related to cosmetics and medications used on the face but also from products, such as shampoo, used on the scalp that dripped onto the face. “The scalp is relatively resistant to the expression of contact allergy and may not be involved at all,” he said.
According to the NACDG study, the hands were the second most common site of dermatitis associated with sulfites (20.5%) followed by generalized distribution (13.6%). These sites are to be expected, given the sources of food and beverage, personal care products, and occupational materials, Dr. Belsito said.
“Eczematous dermatitis of the lips is also common in patients with ingested food sources of sulfites,” he said.
Systemic contact dermatitis to sulfites has been documented following oral, rectal, and parental exposure, Dr. Belsito told this news organization. “Systemic dermatitis may present as a scattered/generalized dermatitis, symmetrical drug-related intertriginous and flexural exanthema (also referred to as baboon syndrome), or erythroderma,” he said.
How to Spot Sulfite Allergies
The exclusion of sulfites from most patch test series means that sulfite allergy diagnoses are often missed, despite the wide range of potential exposures, Dr. Belsito said.
“Most cases of allergic contact dermatitis occur at the site of application of the allergen,” he noted. Depending on the location of the dermatitis, a detailed history of exposures that includes cosmetics and topical medications, work-related materials, and foods and beverages might suggest a sulfite allergy, he said.
Given the range of potential clinical presentations and the many and varied exposures to sulfites, Dr. Belsito’s best tip for clinicians is to routinely screen for them and evaluate the many avenues of exposure if a patch test is positive, he said.
For now, he said he does not think additional research is needed on sulfites as allergens; instead, sulfites, such as sodium metabisulfite/sodium disulfite, should be included in all clinicians’ baseline screening series, he said.
The Allergen of the Year was also recently announced in the journal Dermatitis. Authors Samuel F. Ekstein, MS, and Erin M. Warshaw, MD, from the Department of Dermatology, Park Nicollet Health Services, Minneapolis, Minnesota, noted that the ACDS hoped to raise awareness of sulfites as a “significant allergen” and called for their increased inclusion in screening patch test series.
Patients identified with sulfite allergies can find alternative products on the ACDS CAMP (Contact Allergen Management Program) website, Dr. Warshaw said in an interview.
She also highlighted some examples of sulfites as allergens in healthcare settings in particular. She described one patient who presented with dermatitis at the site of three previous hand orthopedic procedures.
“Although surgical cleansers were suspected, the patient reacted to sodium metabisulfite. Review of the operating room contactants confirmed sulfites as preservatives in an injectable anesthetic and antibiotic used for wound irrigation,” she said. Another patient who had been treated for recurrent otitis externa and seborrheic dermatitis was found to be allergic to sulfites in an otic antibiotic suspension as well as in a ketoconazole cream product, she added.
In the paper, Dr. Warshaw and Mr. Ekstein called for the addition of sulfites to the test series. Although the NACDG added sodium metabisulfite to the series in 2017, sulfites are not part of the American Contact Dermatitis Core Series, they wrote. Sodium metabisulfite, they said, was added to the European baseline standard series after review of the 2019-2020 patch test reactivity and clinical relevance data.
The ACDS meeting is held every year the day before the annual meeting of the American Academy of Dermatology.
Dr. Belsito and Dr. Warshaw had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
by the American Contact Dermatitis Society (ACDS).
Sulfites are currently not found in most screening patch test series, so may be missed as a relevant contact allergen, Donald V. Belsito, MD, emeritus professor in the Department of Dermatology at Columbia University, New York City, said in his presentation on the Allergen of the Year on March 7 at the annual meeting of the American Contact Dermatitis Society in San Diego. Sulfites, he noted, are distinct from sulfates, and the groups do not cross-react with each other.
Sodium disulfite, an inorganic compound, belongs to a group of sulfiting agents, which contain the sulfite ion SO32− and include ammonium sulfite, potassium sulfite, and sodium sulfite, Dr. Belsito said. Sulfites function as antioxidants and preservatives in a range of products including food and beverages, personal care products, and pharmaceuticals.
The type of sulfite allergy diagnosed by patch testing is type IV hypersensitivity or delayed-type hypersensitivity, where patients present with pruritic, red, scaling macules, papulovesicles, and patches, Dr. Belsito told this news organization. “It is not the type I, immediate hypersensitivity that causes hives and, in some cases, anaphylaxis,” he said. Sulfites also can cause these side effects, so correct labeling of food and beverages is important, he noted.
Some common nonoccupational sulfite sources include hair coloring and bleach products, hairspray, tanning lotions, makeup, sunscreens, and deodorants, Dr. Belsito said in his presentation. Medications including topical antifungals, topical corticosteroids, and nasal solutions can be culprits, as can water in swimming pools, he noted.
In occupational settings, sulfites may be present not only in food and drink products but also can be used in production of products, such as those used for sterilization during beer and wine fermentation, Dr. Belsito said. Other potential occupational sources of sulfite exposure include healthcare settings and textile, chemical, rubber, and pharmaceutical manufacturing.
High-sulfite food products (> 100 ppm) to be aware of include dried fruit (raisins and prunes are exceptions), bottled lemon or lime juice (but not frozen products), wine, molasses, grape juice (white, or white, pink, and red sparkling), and pickled cocktail onions, Dr. Belsito said.
“Like other contact allergens, the clinical presentation correlates with exposure,” he added. A study by the North American Contact Dermatitis Group (NACDG) found that 28.8% of patients positive for sulfite allergy on patch testing presented with facial dermatitis, which was not only related to cosmetics and medications used on the face but also from products, such as shampoo, used on the scalp that dripped onto the face. “The scalp is relatively resistant to the expression of contact allergy and may not be involved at all,” he said.
According to the NACDG study, the hands were the second most common site of dermatitis associated with sulfites (20.5%) followed by generalized distribution (13.6%). These sites are to be expected, given the sources of food and beverage, personal care products, and occupational materials, Dr. Belsito said.
“Eczematous dermatitis of the lips is also common in patients with ingested food sources of sulfites,” he said.
Systemic contact dermatitis to sulfites has been documented following oral, rectal, and parental exposure, Dr. Belsito told this news organization. “Systemic dermatitis may present as a scattered/generalized dermatitis, symmetrical drug-related intertriginous and flexural exanthema (also referred to as baboon syndrome), or erythroderma,” he said.
How to Spot Sulfite Allergies
The exclusion of sulfites from most patch test series means that sulfite allergy diagnoses are often missed, despite the wide range of potential exposures, Dr. Belsito said.
“Most cases of allergic contact dermatitis occur at the site of application of the allergen,” he noted. Depending on the location of the dermatitis, a detailed history of exposures that includes cosmetics and topical medications, work-related materials, and foods and beverages might suggest a sulfite allergy, he said.
Given the range of potential clinical presentations and the many and varied exposures to sulfites, Dr. Belsito’s best tip for clinicians is to routinely screen for them and evaluate the many avenues of exposure if a patch test is positive, he said.
For now, he said he does not think additional research is needed on sulfites as allergens; instead, sulfites, such as sodium metabisulfite/sodium disulfite, should be included in all clinicians’ baseline screening series, he said.
The Allergen of the Year was also recently announced in the journal Dermatitis. Authors Samuel F. Ekstein, MS, and Erin M. Warshaw, MD, from the Department of Dermatology, Park Nicollet Health Services, Minneapolis, Minnesota, noted that the ACDS hoped to raise awareness of sulfites as a “significant allergen” and called for their increased inclusion in screening patch test series.
Patients identified with sulfite allergies can find alternative products on the ACDS CAMP (Contact Allergen Management Program) website, Dr. Warshaw said in an interview.
She also highlighted some examples of sulfites as allergens in healthcare settings in particular. She described one patient who presented with dermatitis at the site of three previous hand orthopedic procedures.
“Although surgical cleansers were suspected, the patient reacted to sodium metabisulfite. Review of the operating room contactants confirmed sulfites as preservatives in an injectable anesthetic and antibiotic used for wound irrigation,” she said. Another patient who had been treated for recurrent otitis externa and seborrheic dermatitis was found to be allergic to sulfites in an otic antibiotic suspension as well as in a ketoconazole cream product, she added.
In the paper, Dr. Warshaw and Mr. Ekstein called for the addition of sulfites to the test series. Although the NACDG added sodium metabisulfite to the series in 2017, sulfites are not part of the American Contact Dermatitis Core Series, they wrote. Sodium metabisulfite, they said, was added to the European baseline standard series after review of the 2019-2020 patch test reactivity and clinical relevance data.
The ACDS meeting is held every year the day before the annual meeting of the American Academy of Dermatology.
Dr. Belsito and Dr. Warshaw had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
by the American Contact Dermatitis Society (ACDS).
Sulfites are currently not found in most screening patch test series, so may be missed as a relevant contact allergen, Donald V. Belsito, MD, emeritus professor in the Department of Dermatology at Columbia University, New York City, said in his presentation on the Allergen of the Year on March 7 at the annual meeting of the American Contact Dermatitis Society in San Diego. Sulfites, he noted, are distinct from sulfates, and the groups do not cross-react with each other.
Sodium disulfite, an inorganic compound, belongs to a group of sulfiting agents, which contain the sulfite ion SO32− and include ammonium sulfite, potassium sulfite, and sodium sulfite, Dr. Belsito said. Sulfites function as antioxidants and preservatives in a range of products including food and beverages, personal care products, and pharmaceuticals.
The type of sulfite allergy diagnosed by patch testing is type IV hypersensitivity or delayed-type hypersensitivity, where patients present with pruritic, red, scaling macules, papulovesicles, and patches, Dr. Belsito told this news organization. “It is not the type I, immediate hypersensitivity that causes hives and, in some cases, anaphylaxis,” he said. Sulfites also can cause these side effects, so correct labeling of food and beverages is important, he noted.
Some common nonoccupational sulfite sources include hair coloring and bleach products, hairspray, tanning lotions, makeup, sunscreens, and deodorants, Dr. Belsito said in his presentation. Medications including topical antifungals, topical corticosteroids, and nasal solutions can be culprits, as can water in swimming pools, he noted.
In occupational settings, sulfites may be present not only in food and drink products but also can be used in production of products, such as those used for sterilization during beer and wine fermentation, Dr. Belsito said. Other potential occupational sources of sulfite exposure include healthcare settings and textile, chemical, rubber, and pharmaceutical manufacturing.
High-sulfite food products (> 100 ppm) to be aware of include dried fruit (raisins and prunes are exceptions), bottled lemon or lime juice (but not frozen products), wine, molasses, grape juice (white, or white, pink, and red sparkling), and pickled cocktail onions, Dr. Belsito said.
“Like other contact allergens, the clinical presentation correlates with exposure,” he added. A study by the North American Contact Dermatitis Group (NACDG) found that 28.8% of patients positive for sulfite allergy on patch testing presented with facial dermatitis, which was not only related to cosmetics and medications used on the face but also from products, such as shampoo, used on the scalp that dripped onto the face. “The scalp is relatively resistant to the expression of contact allergy and may not be involved at all,” he said.
According to the NACDG study, the hands were the second most common site of dermatitis associated with sulfites (20.5%) followed by generalized distribution (13.6%). These sites are to be expected, given the sources of food and beverage, personal care products, and occupational materials, Dr. Belsito said.
“Eczematous dermatitis of the lips is also common in patients with ingested food sources of sulfites,” he said.
Systemic contact dermatitis to sulfites has been documented following oral, rectal, and parental exposure, Dr. Belsito told this news organization. “Systemic dermatitis may present as a scattered/generalized dermatitis, symmetrical drug-related intertriginous and flexural exanthema (also referred to as baboon syndrome), or erythroderma,” he said.
How to Spot Sulfite Allergies
The exclusion of sulfites from most patch test series means that sulfite allergy diagnoses are often missed, despite the wide range of potential exposures, Dr. Belsito said.
“Most cases of allergic contact dermatitis occur at the site of application of the allergen,” he noted. Depending on the location of the dermatitis, a detailed history of exposures that includes cosmetics and topical medications, work-related materials, and foods and beverages might suggest a sulfite allergy, he said.
Given the range of potential clinical presentations and the many and varied exposures to sulfites, Dr. Belsito’s best tip for clinicians is to routinely screen for them and evaluate the many avenues of exposure if a patch test is positive, he said.
For now, he said he does not think additional research is needed on sulfites as allergens; instead, sulfites, such as sodium metabisulfite/sodium disulfite, should be included in all clinicians’ baseline screening series, he said.
The Allergen of the Year was also recently announced in the journal Dermatitis. Authors Samuel F. Ekstein, MS, and Erin M. Warshaw, MD, from the Department of Dermatology, Park Nicollet Health Services, Minneapolis, Minnesota, noted that the ACDS hoped to raise awareness of sulfites as a “significant allergen” and called for their increased inclusion in screening patch test series.
Patients identified with sulfite allergies can find alternative products on the ACDS CAMP (Contact Allergen Management Program) website, Dr. Warshaw said in an interview.
She also highlighted some examples of sulfites as allergens in healthcare settings in particular. She described one patient who presented with dermatitis at the site of three previous hand orthopedic procedures.
“Although surgical cleansers were suspected, the patient reacted to sodium metabisulfite. Review of the operating room contactants confirmed sulfites as preservatives in an injectable anesthetic and antibiotic used for wound irrigation,” she said. Another patient who had been treated for recurrent otitis externa and seborrheic dermatitis was found to be allergic to sulfites in an otic antibiotic suspension as well as in a ketoconazole cream product, she added.
In the paper, Dr. Warshaw and Mr. Ekstein called for the addition of sulfites to the test series. Although the NACDG added sodium metabisulfite to the series in 2017, sulfites are not part of the American Contact Dermatitis Core Series, they wrote. Sodium metabisulfite, they said, was added to the European baseline standard series after review of the 2019-2020 patch test reactivity and clinical relevance data.
The ACDS meeting is held every year the day before the annual meeting of the American Academy of Dermatology.
Dr. Belsito and Dr. Warshaw had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
FROM ACDS 2024
Psilocybin Poison Control Calls Spike in Teens, Young Adults
Calls to US poison centers related to psilocybin more than tripled among teens and more than doubled in young adults between 2019 and 2022, new research suggests. Investigators say the increase may be linked to decriminalization efforts in US cities and states.
METHODOLOGY:
- Investigators used data from the National Poison Data System (NPDS) to identify calls involving psilocybin between January 2013 and December 2022.
- Researchers focused on calls about individuals between the ages of 13 and 25 years.
- Exposures to psilocybin were examined based on demographics, clinical effects, level of care, and medical outcome.
TAKEAWAY:
- During the entire 10-year study period, 4055 psilocybin-involved exposures were reported in the age groups studied, with 66% being single-substance exposures and close to three quarters receiving medical attention.
- Psilocybin’s most common effects were hallucinations or delusions (37% of calls), agitation (28%), tachycardia (20%), and confusion (16%).
- The number of psilocybin-related calls to poison control centers for youth were largely unchanged from 2013 to 2018 but more than tripled among adolescents (aged 13-19 years) from 2019 and 2022 and more than doubled among young adults (aged 20-25 years) between 2018 and 2022 (P < .0001).
IN PRACTICE:
The increase in poison center calls coincides with psilocybin decriminalization efforts in several states in 2019, the authors noted. However, because those efforts only legalized use in adults aged 21 years and older, the rise among younger people is concerning, they added. “As psilocybin may become more widely available, it is important for parents to be aware that psilocybin is also available in edible forms such as chocolate and gummies. And we learned from our experience with edible cannabis that young children can mistake edibles for candy,” lead author Rita Farah, PharmD, MPH, PhD, Blue Ridge Poison Center epidemiologist, said in a news release.
SOURCE:
Christopher Holstege, MD, director of UVA Health’s Blue Ridge Poison Center and chief of the Division of Medical Toxicology at the UVA School of Medicine was the senior and corresponding author of the study. It was published online on February 26 in the Journal of Adolescent Health.
LIMITATIONS:
NPDS data are not designed to assess potential risk factors leading to increases in psilocybin-related cases. Moreover, because reports to poison control centers are voluntary and don’t capture all exposures, NPDS data likely under-represent cases of hallucinogenic mushroom poisonings. Lastly, NPDS data are susceptible to reporting and misclassification biases.
DISCLOSURES:
Funding source was not disclosed. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Calls to US poison centers related to psilocybin more than tripled among teens and more than doubled in young adults between 2019 and 2022, new research suggests. Investigators say the increase may be linked to decriminalization efforts in US cities and states.
METHODOLOGY:
- Investigators used data from the National Poison Data System (NPDS) to identify calls involving psilocybin between January 2013 and December 2022.
- Researchers focused on calls about individuals between the ages of 13 and 25 years.
- Exposures to psilocybin were examined based on demographics, clinical effects, level of care, and medical outcome.
TAKEAWAY:
- During the entire 10-year study period, 4055 psilocybin-involved exposures were reported in the age groups studied, with 66% being single-substance exposures and close to three quarters receiving medical attention.
- Psilocybin’s most common effects were hallucinations or delusions (37% of calls), agitation (28%), tachycardia (20%), and confusion (16%).
- The number of psilocybin-related calls to poison control centers for youth were largely unchanged from 2013 to 2018 but more than tripled among adolescents (aged 13-19 years) from 2019 and 2022 and more than doubled among young adults (aged 20-25 years) between 2018 and 2022 (P < .0001).
IN PRACTICE:
The increase in poison center calls coincides with psilocybin decriminalization efforts in several states in 2019, the authors noted. However, because those efforts only legalized use in adults aged 21 years and older, the rise among younger people is concerning, they added. “As psilocybin may become more widely available, it is important for parents to be aware that psilocybin is also available in edible forms such as chocolate and gummies. And we learned from our experience with edible cannabis that young children can mistake edibles for candy,” lead author Rita Farah, PharmD, MPH, PhD, Blue Ridge Poison Center epidemiologist, said in a news release.
SOURCE:
Christopher Holstege, MD, director of UVA Health’s Blue Ridge Poison Center and chief of the Division of Medical Toxicology at the UVA School of Medicine was the senior and corresponding author of the study. It was published online on February 26 in the Journal of Adolescent Health.
LIMITATIONS:
NPDS data are not designed to assess potential risk factors leading to increases in psilocybin-related cases. Moreover, because reports to poison control centers are voluntary and don’t capture all exposures, NPDS data likely under-represent cases of hallucinogenic mushroom poisonings. Lastly, NPDS data are susceptible to reporting and misclassification biases.
DISCLOSURES:
Funding source was not disclosed. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Calls to US poison centers related to psilocybin more than tripled among teens and more than doubled in young adults between 2019 and 2022, new research suggests. Investigators say the increase may be linked to decriminalization efforts in US cities and states.
METHODOLOGY:
- Investigators used data from the National Poison Data System (NPDS) to identify calls involving psilocybin between January 2013 and December 2022.
- Researchers focused on calls about individuals between the ages of 13 and 25 years.
- Exposures to psilocybin were examined based on demographics, clinical effects, level of care, and medical outcome.
TAKEAWAY:
- During the entire 10-year study period, 4055 psilocybin-involved exposures were reported in the age groups studied, with 66% being single-substance exposures and close to three quarters receiving medical attention.
- Psilocybin’s most common effects were hallucinations or delusions (37% of calls), agitation (28%), tachycardia (20%), and confusion (16%).
- The number of psilocybin-related calls to poison control centers for youth were largely unchanged from 2013 to 2018 but more than tripled among adolescents (aged 13-19 years) from 2019 and 2022 and more than doubled among young adults (aged 20-25 years) between 2018 and 2022 (P < .0001).
IN PRACTICE:
The increase in poison center calls coincides with psilocybin decriminalization efforts in several states in 2019, the authors noted. However, because those efforts only legalized use in adults aged 21 years and older, the rise among younger people is concerning, they added. “As psilocybin may become more widely available, it is important for parents to be aware that psilocybin is also available in edible forms such as chocolate and gummies. And we learned from our experience with edible cannabis that young children can mistake edibles for candy,” lead author Rita Farah, PharmD, MPH, PhD, Blue Ridge Poison Center epidemiologist, said in a news release.
SOURCE:
Christopher Holstege, MD, director of UVA Health’s Blue Ridge Poison Center and chief of the Division of Medical Toxicology at the UVA School of Medicine was the senior and corresponding author of the study. It was published online on February 26 in the Journal of Adolescent Health.
LIMITATIONS:
NPDS data are not designed to assess potential risk factors leading to increases in psilocybin-related cases. Moreover, because reports to poison control centers are voluntary and don’t capture all exposures, NPDS data likely under-represent cases of hallucinogenic mushroom poisonings. Lastly, NPDS data are susceptible to reporting and misclassification biases.
DISCLOSURES:
Funding source was not disclosed. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.