User login
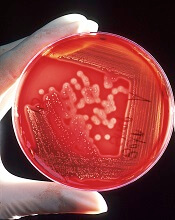
Researchers propose new acute leukemia subtypes
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization classification for acute leukemia.
Each of these subtypes share genomic characteristics with other acute leukemias, which suggests they might respond to treatments that are already in use.
This research also has shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said study author Charles G. Mullighan, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn.
In the current study, published in Nature, Dr. Mullighan and his colleagues used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL – B/myeloid and T/myeloid – and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than that of patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL.
In addition, the analysis showed that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3.
WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include: ZNF384r acute leukemia (either B-ALL or MPAL), WT1-mutant T/myeloid MPAL, and Ph-like B/myeloid MPAL.
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations. The researchers reported having no competing interests.
SOURCE: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
FROM NATURE
Key clinical point:
Major finding: In total, 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL.
Study details: Whole-genome, -exome, and RNA sequencing of 115 samples from pediatric patients with MPAL.
Disclosures: This research was supported by the National Cancer Institute and other organizations. The researchers reported having no competing interests.
Source: Alexander TB et al. Nature. 2018 Sep 12. doi: 10.1038/s41586-018-0436-0.
Pruritus linked to wide variety of cancers
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
A wide variety of hematologic, dermatologic, and solid organ malignancies are associated with pruritus, a large, single-center, retrospective study suggests.
Blacks with pruritus had a higher odds ratio of hematologic malignancies, among others, while whites had higher likelihood of liver, gastrointestinal, respiratory and gynecologic cancers, results of the study show.
The results by race help address a gap in the literature, according to Shawn G. Kwatra, MD, of Johns Hopkins University, Baltimore, and his coinvestigators.
“Little is known about the association between pruritus and malignancy among different ethnic groups,” Dr. Kwatra and his coauthors wrote in the Journal of the American Academy of Dermatology.
The study shows a stronger association with more types of malignancies than has been reported previously, according to the investigators.
“The main difference is that prior studies focused on diagnosis of malignancy after the onset of pruritus, while our study includes malignancies diagnosed on or after pruritus onset,” they wrote.
Retrospective data for the study, which came from the Johns Hopkins Health System, included 16,925 patients aged 18 years or older who presented with itching or pruritus between April 4, 2013 and Dec. 31, 2017.
Of those 16,925 patients, 2,903 were also diagnosed with a concomitant malignancy during that time period. Compared with patients with no itching diagnosis during that time period, the pruritus patients more likely to have a concomitant malignancy, with an OR of 5.76 (95% confidence interval, 5.53-6.00), Dr. Kwatra and his colleagues found.
Malignancies most strongly associated with pruritus included those of the skin, liver, gallbladder and biliary tract, and hematopoietic system.
Among hematologic malignancies, pruritus was most strongly linked to myeloid leukemia and primary cutaneous lymphoma, while among skin cancers, squamous cell carcinoma was most strongly linked.
Whites had higher odds of any malignancy versus blacks, according to investigators, with ORs of 6.12 (95% CI, 5.81-6.46) and 5.61 (95% CI, 5.21-6.04), respectively.
Blacks with pruritus had higher ORs for hematologic and soft tissue malignancies including those of the muscle, fat, and peripheral nerve, investigators said, while whites had higher ORs for skin and liver malignancies.
The investigators also looked at the prevalence of skin eruptions in patients with pruritus and malignancy. “Eruption is variable by malignancy type and points to differing underlying mechanisms of pruritus,” they reported.
The highest rates of skin eruption were in patients with myeloid leukemia at 66%, followed by bone cancers at 58%, lymphocytic leukemia at 57%, multiple myeloma at 53%, and bronchus at 53%. The lowest rates of skin eruption were in patients with gallbladder and biliary tract, colon, pancreas, and liver malignancies.
Dr. Kwatra reported that he is an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
SOURCE: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point:
Major finding: Blacks with pruritus had higher odds ratios for hematologic and soft tissue malignancies, while whites had higher ORs for skin and liver malignancies.
Study details: A retrospective study of 16,925 adults with itching or pruritus seen at a tertiary care center.
Disclosures: Dr. Kwatra reported serving as an advisory board member for Menlo Therapeutics and Trevi Therapeutics.
Source: Kwatra SG et al. J Am Acad Dermatol. 2018 Sep 11. doi: 10.1016/j.jaad.2018.08.044.
Insights could change treatment, classification of MPAL
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
An extensive analysis of mixed phenotype acute leukemia (MPAL) has led to new insights that may have implications for disease classification and treatment.
Researchers believe they have identified new subtypes of MPAL that should be included in the World Health Organization (WHO) classification for acute leukemia.
Each of these subtypes shares genomic characteristics with other acute leukemias, which suggests the new subtypes might respond to treatments that are already in use.
This research has also shed light on how MPAL evolves and appears to provide an explanation for why MPAL displays characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
“ALL and AML have very different treatments, but MPAL has features of both, so the question of how best to treat patients with MPAL has been challenging the leukemia community worldwide, and long-term survival of patients has been poor,” said Charles Mullighan, MBBS, MD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
With these issues in mind, Dr. Mullighan and his colleagues conducted their study of MPAL and described their findings in Nature.
New classifications
The researchers used whole-genome, whole-exome, and RNA sequencing to analyze 115 samples from pediatric patients with MPAL.
The analysis revealed mutations that define the two most common subtypes of MPAL—B/myeloid and T/myeloid—and suggested these subtypes share similarities with other leukemia subtypes.
The researchers found that 48% of B/myeloid MPAL cases carried rearrangements in ZNF384, a characteristic that is also found in cases of B-cell ALL. In fact, the team said the gene expression profiles of ZNF384r B-ALL and ZNF384r MPAL were indistinguishable.
“That is biologically and clinically important,” Dr. Mullighan said. “The findings suggest the ZNF384 rearrangement defines a distinct leukemia subtype, and the alteration should be used to guide treatment.”
The researchers noted that patients with ZNF384r exhibited higher FLT3 expression than patients with other types of B/myeloid or T/myeloid MPAL, so patients with ZNF384r MPAL might respond well to treatment with a FLT3 inhibitor.
This study also showed that cases of B/myeloid MPAL without ZNF384r shared genomic features with other B-ALL subtypes, such as Ph-like B-ALL, which may have implications for treatment.
Another of the researchers’ discoveries was that T/myeloid MPAL and early T-cell precursor ALL have similar gene expression profiles.
The team identified several genes that were mutated at similar frequencies in T/myeloid MPAL and early T-cell precursor ALL, including WT1, ETV6, EZH2, and FLT3. WT1 was the most frequently mutated transcription factor gene in T/myeloid MPAL.
Based on these findings, the researchers said the WHO classification of acute leukemia should be updated to include:
- ZNF384r acute leukemia (either B-ALL or MPAL)
- WT1-mutant T/myeloid MPAL
- Ph-like B/myeloid MPAL.
Evolution of MPAL
The researchers’ analyses also revealed leukemia-initiating genetic alterations in early hematopoietic progenitors.
The team said this and other findings—including the common genomic features of ZNF384r MPAL and B-ALL—suggest the ambiguous phenotype of MPAL results from alterations in immature hematopoietic progenitors.
“These findings suggest that the founding mutation occurs early in blood cell development, in some cases in hematopoietic stem cells, and results in an acute leukemia with features of both myeloid and lymphoid cells,” said study author Thomas Alexander, MD, of the University of North Carolina at Chapel Hill.
“One previous theory was that the reason you have two different cancer types within the same patient is that they acquire different mutations that drive them to become AML or ALL, with genomically distinct tumors within the same patient. That doesn’t seem to be the case from our data. Our proposed model is that the mutations occur earlier in development in cells that retain the potential to acquire myeloid or lymphoid features.”
This research was supported by the National Cancer Institute, the National Institutes of Health, Cookies for Kids’ Cancer, and other organizations.
Prophylaxis reduces bacteremia in some kids
In a phase 3 study, levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias who received intensive chemotherapy.
However, the risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children who underwent hematopoietic stem cell transplant (HSCT).
Sarah Alexander, MD, of the Hospital for Sick Children in Toronto, Ontario, Canada, and her colleagues reported these findings in JAMA.
This multicenter, randomized trial (ACCL0934) enrolled patients aged 6 months to 21 years.
There were 200 patients with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were set to receive chemotherapy and 424 patients who were to receive a myeloablative autologous or allogeneic HSCT.
The acute leukemia patients were randomized to receive no prophylaxis (n=100) or levofloxacin prophylaxis (n=100) for two consecutive cycles of chemotherapy.
The HSCT recipients were randomized to receive no prophylaxis (n=214) or levofloxacin prophylaxis (n=210) during one HSCT procedure.
Results
In the primary analysis of the acute leukemia group (n=195), the incidence of bacteremia was 21.9% for those randomized to levofloxacin and 43.4% for those who did not receive prophylaxis (P=0.001).
In the primary analysis of the HSCT group (n=418), the incidence of bacteremia was 11.0% in the levofloxacin arm and 17.3% in the control arm (P=0.06).
However, a post hoc analysis accounting for time at risk showed a significant difference in favor of prophylaxis in both the acute leukemia and HSCT groups and a similar effect size between groups.
For the acute leukemia group, the rate of bacteremic episodes in the post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.008).
In the HSCT group, the rate of bacteremic episodes was 5.3 versus 10.0 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.02).
The researchers said it is possible that the effect of prophylaxis was similar between the HSCT and acute leukemia groups, but there was reduced power to detect a significant difference because of fewer events among HSCT recipients.
However, the differences between the HSCT and acute leukemia groups in the primary analysis might also be explained by differences in supportive care measures or infections with pathogens that had differential sensitivity to levofloxacin.
The researchers noted that levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk.
Dr. Alexander and her colleagues also said further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits.
In the current study, there were 23 serious adverse events reported in 8 patients. Twelve of these events, occurring in two patients, may have been related to levofloxacin.
This research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
In a phase 3 study, levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias who received intensive chemotherapy.
However, the risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children who underwent hematopoietic stem cell transplant (HSCT).
Sarah Alexander, MD, of the Hospital for Sick Children in Toronto, Ontario, Canada, and her colleagues reported these findings in JAMA.
This multicenter, randomized trial (ACCL0934) enrolled patients aged 6 months to 21 years.
There were 200 patients with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were set to receive chemotherapy and 424 patients who were to receive a myeloablative autologous or allogeneic HSCT.
The acute leukemia patients were randomized to receive no prophylaxis (n=100) or levofloxacin prophylaxis (n=100) for two consecutive cycles of chemotherapy.
The HSCT recipients were randomized to receive no prophylaxis (n=214) or levofloxacin prophylaxis (n=210) during one HSCT procedure.
Results
In the primary analysis of the acute leukemia group (n=195), the incidence of bacteremia was 21.9% for those randomized to levofloxacin and 43.4% for those who did not receive prophylaxis (P=0.001).
In the primary analysis of the HSCT group (n=418), the incidence of bacteremia was 11.0% in the levofloxacin arm and 17.3% in the control arm (P=0.06).
However, a post hoc analysis accounting for time at risk showed a significant difference in favor of prophylaxis in both the acute leukemia and HSCT groups and a similar effect size between groups.
For the acute leukemia group, the rate of bacteremic episodes in the post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.008).
In the HSCT group, the rate of bacteremic episodes was 5.3 versus 10.0 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.02).
The researchers said it is possible that the effect of prophylaxis was similar between the HSCT and acute leukemia groups, but there was reduced power to detect a significant difference because of fewer events among HSCT recipients.
However, the differences between the HSCT and acute leukemia groups in the primary analysis might also be explained by differences in supportive care measures or infections with pathogens that had differential sensitivity to levofloxacin.
The researchers noted that levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk.
Dr. Alexander and her colleagues also said further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits.
In the current study, there were 23 serious adverse events reported in 8 patients. Twelve of these events, occurring in two patients, may have been related to levofloxacin.
This research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
In a phase 3 study, levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias who received intensive chemotherapy.
However, the risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children who underwent hematopoietic stem cell transplant (HSCT).
Sarah Alexander, MD, of the Hospital for Sick Children in Toronto, Ontario, Canada, and her colleagues reported these findings in JAMA.
This multicenter, randomized trial (ACCL0934) enrolled patients aged 6 months to 21 years.
There were 200 patients with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were set to receive chemotherapy and 424 patients who were to receive a myeloablative autologous or allogeneic HSCT.
The acute leukemia patients were randomized to receive no prophylaxis (n=100) or levofloxacin prophylaxis (n=100) for two consecutive cycles of chemotherapy.
The HSCT recipients were randomized to receive no prophylaxis (n=214) or levofloxacin prophylaxis (n=210) during one HSCT procedure.
Results
In the primary analysis of the acute leukemia group (n=195), the incidence of bacteremia was 21.9% for those randomized to levofloxacin and 43.4% for those who did not receive prophylaxis (P=0.001).
In the primary analysis of the HSCT group (n=418), the incidence of bacteremia was 11.0% in the levofloxacin arm and 17.3% in the control arm (P=0.06).
However, a post hoc analysis accounting for time at risk showed a significant difference in favor of prophylaxis in both the acute leukemia and HSCT groups and a similar effect size between groups.
For the acute leukemia group, the rate of bacteremic episodes in the post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.008).
In the HSCT group, the rate of bacteremic episodes was 5.3 versus 10.0 per 1,000 patient-days in the prophylaxis and control arms, respectively (P=0.02).
The researchers said it is possible that the effect of prophylaxis was similar between the HSCT and acute leukemia groups, but there was reduced power to detect a significant difference because of fewer events among HSCT recipients.
However, the differences between the HSCT and acute leukemia groups in the primary analysis might also be explained by differences in supportive care measures or infections with pathogens that had differential sensitivity to levofloxacin.
The researchers noted that levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk.
Dr. Alexander and her colleagues also said further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits.
In the current study, there were 23 serious adverse events reported in 8 patients. Twelve of these events, occurring in two patients, may have been related to levofloxacin.
This research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
Cell population appears to drive relapse in AML
Researchers believe they have identified cells that are responsible for relapse of acute myeloid leukemia (AML).
These “leukemic-regenerating cells” (LRCs), which are distinct from leukemic stem cells (LSCs), seem to arise in response to chemotherapy.
Experiments in mouse models of AML suggested that targeting LRCs could reduce the risk of relapse, and analyses of AML patient samples suggested LRCs might be used to predict relapse.
Allison Boyd, PhD, of McMaster University in Hamilton, Ont., and her colleagues reported these findings in Cancer Cell.
The researchers evaluated the leukemic populations that persist after chemotherapy by analyzing AML patient samples and xenograft AML models. The team found that LSCs were depleted by chemotherapy, and a different cell population, LRCs, appeared to arise in response to treatment.
LRCs are “molecularly distinct from therapy-naive LSCs,” the researchers said. In fact, the team identified 19 genes that are preferentially expressed by LRCs and could be treated with drugs.
One of these genes is DRD2, and the researchers found they could target LRCs using a small-molecule antagonist of DRD2.
Targeting LRCs
Dr. Boyd and her colleagues compared the effects of treatment with a DRD2 antagonist in AML xenografts populated with therapy-naive LSCs and AML xenografts that harbored LRCs following exposure to cytarabine.
The researchers said DRD2 antagonist therapy “moderately” affected AML progenitors in the LSC model but “had profound effects on regenerating LRCs.”
Treatment with the DRD2 antagonist also improved the efficacy of chemotherapy.
In xenografts derived from one AML patient, treatment with cytarabine alone left 50% of mice with residual disease. However, the addition of the DRD2 antagonist enabled 100% of the mice to achieve disease-free status.
In xenografts derived from a patient with more aggressive AML, all recipient mice had residual disease after receiving cytarabine. Treatment with the DRD2 antagonist slowed leukemic regrowth and nearly doubled the time to relapse.
Targeting LRCs also reduced disease regeneration potential in samples from other AML patients.
“This is a major clinical opportunity because this type of leukemia is very diverse and responds differently across patients,” Dr. Boyd said. “It has been a challenge in a clinical setting to find a commonality for therapeutic targeting across the wide array of patients, and these regenerative cells provide that similarity.”
Predicting relapse
Dr. Boyd and her colleagues also analyzed bone marrow samples collected from AML patients approximately 3 weeks after they completed standard induction chemotherapy.
The team found that progenitor activity was enriched among residual leukemic cells. However, patient cells lacked gene expression signatures related to therapy-naive LSCs.
“Instead, these highly regenerative AML cells preferentially expressed our LRC signature,” the researchers said.
The team also found evidence to suggest that LRC molecular profiles arise temporarily after chemotherapy. The LRC signature was not observed at diagnosis or once AML was reestablished at relapse.
“We think there are opportunities here because now we have a window where we can kick the cancer while it’s down,” Dr. Boyd said.
She and her colleagues also found the LRC signature might be useful for predicting relapse in AML patients.
The team assessed expression of SLC2A2, an LRC marker that has overlapping expression with DRD2, in seven patients who were in remission after induction.
Chemotherapy increased expression of SLC2A2 only in the four patients who had residual disease – not in the three patients who remained in disease-free remission for at least 5 years. “These results suggest that LRC populations represent reservoirs of residual disease, and LRC marker expression levels can be linked to clinical outcomes of AML relapse,” the researchers said.
This study was supported by the Canadian Cancer Society, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other organizations.
SOURCE: Boyd AL et al. Cancer Cell. 2018 Sep 10. doi: 10.1016/j.ccell.2018.08.007.
Researchers believe they have identified cells that are responsible for relapse of acute myeloid leukemia (AML).
These “leukemic-regenerating cells” (LRCs), which are distinct from leukemic stem cells (LSCs), seem to arise in response to chemotherapy.
Experiments in mouse models of AML suggested that targeting LRCs could reduce the risk of relapse, and analyses of AML patient samples suggested LRCs might be used to predict relapse.
Allison Boyd, PhD, of McMaster University in Hamilton, Ont., and her colleagues reported these findings in Cancer Cell.
The researchers evaluated the leukemic populations that persist after chemotherapy by analyzing AML patient samples and xenograft AML models. The team found that LSCs were depleted by chemotherapy, and a different cell population, LRCs, appeared to arise in response to treatment.
LRCs are “molecularly distinct from therapy-naive LSCs,” the researchers said. In fact, the team identified 19 genes that are preferentially expressed by LRCs and could be treated with drugs.
One of these genes is DRD2, and the researchers found they could target LRCs using a small-molecule antagonist of DRD2.
Targeting LRCs
Dr. Boyd and her colleagues compared the effects of treatment with a DRD2 antagonist in AML xenografts populated with therapy-naive LSCs and AML xenografts that harbored LRCs following exposure to cytarabine.
The researchers said DRD2 antagonist therapy “moderately” affected AML progenitors in the LSC model but “had profound effects on regenerating LRCs.”
Treatment with the DRD2 antagonist also improved the efficacy of chemotherapy.
In xenografts derived from one AML patient, treatment with cytarabine alone left 50% of mice with residual disease. However, the addition of the DRD2 antagonist enabled 100% of the mice to achieve disease-free status.
In xenografts derived from a patient with more aggressive AML, all recipient mice had residual disease after receiving cytarabine. Treatment with the DRD2 antagonist slowed leukemic regrowth and nearly doubled the time to relapse.
Targeting LRCs also reduced disease regeneration potential in samples from other AML patients.
“This is a major clinical opportunity because this type of leukemia is very diverse and responds differently across patients,” Dr. Boyd said. “It has been a challenge in a clinical setting to find a commonality for therapeutic targeting across the wide array of patients, and these regenerative cells provide that similarity.”
Predicting relapse
Dr. Boyd and her colleagues also analyzed bone marrow samples collected from AML patients approximately 3 weeks after they completed standard induction chemotherapy.
The team found that progenitor activity was enriched among residual leukemic cells. However, patient cells lacked gene expression signatures related to therapy-naive LSCs.
“Instead, these highly regenerative AML cells preferentially expressed our LRC signature,” the researchers said.
The team also found evidence to suggest that LRC molecular profiles arise temporarily after chemotherapy. The LRC signature was not observed at diagnosis or once AML was reestablished at relapse.
“We think there are opportunities here because now we have a window where we can kick the cancer while it’s down,” Dr. Boyd said.
She and her colleagues also found the LRC signature might be useful for predicting relapse in AML patients.
The team assessed expression of SLC2A2, an LRC marker that has overlapping expression with DRD2, in seven patients who were in remission after induction.
Chemotherapy increased expression of SLC2A2 only in the four patients who had residual disease – not in the three patients who remained in disease-free remission for at least 5 years. “These results suggest that LRC populations represent reservoirs of residual disease, and LRC marker expression levels can be linked to clinical outcomes of AML relapse,” the researchers said.
This study was supported by the Canadian Cancer Society, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other organizations.
SOURCE: Boyd AL et al. Cancer Cell. 2018 Sep 10. doi: 10.1016/j.ccell.2018.08.007.
Researchers believe they have identified cells that are responsible for relapse of acute myeloid leukemia (AML).
These “leukemic-regenerating cells” (LRCs), which are distinct from leukemic stem cells (LSCs), seem to arise in response to chemotherapy.
Experiments in mouse models of AML suggested that targeting LRCs could reduce the risk of relapse, and analyses of AML patient samples suggested LRCs might be used to predict relapse.
Allison Boyd, PhD, of McMaster University in Hamilton, Ont., and her colleagues reported these findings in Cancer Cell.
The researchers evaluated the leukemic populations that persist after chemotherapy by analyzing AML patient samples and xenograft AML models. The team found that LSCs were depleted by chemotherapy, and a different cell population, LRCs, appeared to arise in response to treatment.
LRCs are “molecularly distinct from therapy-naive LSCs,” the researchers said. In fact, the team identified 19 genes that are preferentially expressed by LRCs and could be treated with drugs.
One of these genes is DRD2, and the researchers found they could target LRCs using a small-molecule antagonist of DRD2.
Targeting LRCs
Dr. Boyd and her colleagues compared the effects of treatment with a DRD2 antagonist in AML xenografts populated with therapy-naive LSCs and AML xenografts that harbored LRCs following exposure to cytarabine.
The researchers said DRD2 antagonist therapy “moderately” affected AML progenitors in the LSC model but “had profound effects on regenerating LRCs.”
Treatment with the DRD2 antagonist also improved the efficacy of chemotherapy.
In xenografts derived from one AML patient, treatment with cytarabine alone left 50% of mice with residual disease. However, the addition of the DRD2 antagonist enabled 100% of the mice to achieve disease-free status.
In xenografts derived from a patient with more aggressive AML, all recipient mice had residual disease after receiving cytarabine. Treatment with the DRD2 antagonist slowed leukemic regrowth and nearly doubled the time to relapse.
Targeting LRCs also reduced disease regeneration potential in samples from other AML patients.
“This is a major clinical opportunity because this type of leukemia is very diverse and responds differently across patients,” Dr. Boyd said. “It has been a challenge in a clinical setting to find a commonality for therapeutic targeting across the wide array of patients, and these regenerative cells provide that similarity.”
Predicting relapse
Dr. Boyd and her colleagues also analyzed bone marrow samples collected from AML patients approximately 3 weeks after they completed standard induction chemotherapy.
The team found that progenitor activity was enriched among residual leukemic cells. However, patient cells lacked gene expression signatures related to therapy-naive LSCs.
“Instead, these highly regenerative AML cells preferentially expressed our LRC signature,” the researchers said.
The team also found evidence to suggest that LRC molecular profiles arise temporarily after chemotherapy. The LRC signature was not observed at diagnosis or once AML was reestablished at relapse.
“We think there are opportunities here because now we have a window where we can kick the cancer while it’s down,” Dr. Boyd said.
She and her colleagues also found the LRC signature might be useful for predicting relapse in AML patients.
The team assessed expression of SLC2A2, an LRC marker that has overlapping expression with DRD2, in seven patients who were in remission after induction.
Chemotherapy increased expression of SLC2A2 only in the four patients who had residual disease – not in the three patients who remained in disease-free remission for at least 5 years. “These results suggest that LRC populations represent reservoirs of residual disease, and LRC marker expression levels can be linked to clinical outcomes of AML relapse,” the researchers said.
This study was supported by the Canadian Cancer Society, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other organizations.
SOURCE: Boyd AL et al. Cancer Cell. 2018 Sep 10. doi: 10.1016/j.ccell.2018.08.007.
FROM CANCER CELL
Key clinical point: “Leukemic-regenerating cells” (LRCs) may drive relapse in acute myeloid leukemia (AML).
Major finding: LRCs could be useful for predicting relapse, and targeting LRCs might reduce relapse risk.
Study details: Research in AML patient samples and xenograft AML models.
Disclosures: This study was supported by the Canadian Cancer Society and other organizations.
Source: Boyd AL et al. Cancer Cell. 2018 Sep 10. doi: 10.1016/j.ccell.2018.08.007.
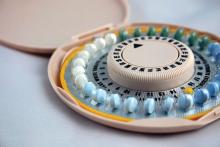
Hormonal contraceptives tied to leukemia in progeny
A nationwide cohort study suggests an association between a woman’s use of hormonal contraceptives and leukemia in her offspring.
Children of mothers who used hormonal contraception, either during pregnancy or in the 3 months beforehand, had a 1.5-fold greater risk of leukemia, when compared to children of mothers who had never used hormonal contraception.
This increased risk translated to one additional case of leukemia per about 50,000 children exposed to hormonal contraceptives.
The increased risk appeared limited to non-lymphoid leukemia.
Marie Hargreave, PhD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, and her colleagues reported these findings in The Lancet Oncology.
The study included 1,185,157 children born between 1996 and 2014 and followed for a median of 9.3 years. Data on these children were collected from the Danish Medical Birth Registry and the Danish Cancer Registry.
The researchers looked at redeemed prescriptions from the Danish National Prescription Registry to determine the mothers’ contraceptive use and divided the women into three categories:
- Mothers who had never used hormonal contraceptives
- Those with previous hormonal contraceptive use, defined as greater than 3 months before the start of pregnancy
- Mothers with recent contraceptive use, defined as during or within 3 months of pregnancy.
Results
There were 606 children diagnosed with leukemia in the study cohort—465 with lymphoid leukemia and 141 with non-lymphoid leukemia.
Overall, children born to mothers with previous or recent use of hormonal contraceptives had a significantly increased risk of developing any leukemia. The hazard ratios (HRs) were as follows:
- Previous use of hormonal contraceptives—HR=1.25 (P=0.039)
- Recent use—HR=1.46 (P=0.011)
- Use within 3 months of pregnancy—HR=1.42 (P=0.025)
- Use during pregnancy—HR=1.78 (P=0.070).
The risk of lymphoid leukemia did not increase significantly with maternal use of hormonal contraceptives. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.23 (P=0.089)
- Recent use—HR=1.27 (P=0.167)
- Use within 3 months of pregnancy—HR=1.28 (P=0.173)
- Use during pregnancy—HR=1.22 (P=0.635).
However, the risk of non-lymphoid leukemia was significantly increased in children born to mothers with recent hormonal contraceptive use. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.33 (P=0.232)
- Recent use—HR=2.17 (P=0.008)
- Use within 3 months of pregnancy—HR=1.95 (P=0.033)
- Use during pregnancy—HR=3.87 (P=0.006).
The association between recent contraceptive use and any leukemia was strongest in children ages 6 to 10 years. The researchers said this was not surprising because the incidence of non-lymphoid leukemia increases after the age of 6.
The researchers estimated that a mother’s recent use of hormonal contraceptives would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia over the study period.
This low risk of leukemia “is not a major concern with regard to the safety of hormonal contraceptives,” the researchers said.
However, the findings do suggest the intrauterine hormonal environment affects leukemia development in children, and this should be explored in future research.
This study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations, and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
A nationwide cohort study suggests an association between a woman’s use of hormonal contraceptives and leukemia in her offspring.
Children of mothers who used hormonal contraception, either during pregnancy or in the 3 months beforehand, had a 1.5-fold greater risk of leukemia, when compared to children of mothers who had never used hormonal contraception.
This increased risk translated to one additional case of leukemia per about 50,000 children exposed to hormonal contraceptives.
The increased risk appeared limited to non-lymphoid leukemia.
Marie Hargreave, PhD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, and her colleagues reported these findings in The Lancet Oncology.
The study included 1,185,157 children born between 1996 and 2014 and followed for a median of 9.3 years. Data on these children were collected from the Danish Medical Birth Registry and the Danish Cancer Registry.
The researchers looked at redeemed prescriptions from the Danish National Prescription Registry to determine the mothers’ contraceptive use and divided the women into three categories:
- Mothers who had never used hormonal contraceptives
- Those with previous hormonal contraceptive use, defined as greater than 3 months before the start of pregnancy
- Mothers with recent contraceptive use, defined as during or within 3 months of pregnancy.
Results
There were 606 children diagnosed with leukemia in the study cohort—465 with lymphoid leukemia and 141 with non-lymphoid leukemia.
Overall, children born to mothers with previous or recent use of hormonal contraceptives had a significantly increased risk of developing any leukemia. The hazard ratios (HRs) were as follows:
- Previous use of hormonal contraceptives—HR=1.25 (P=0.039)
- Recent use—HR=1.46 (P=0.011)
- Use within 3 months of pregnancy—HR=1.42 (P=0.025)
- Use during pregnancy—HR=1.78 (P=0.070).
The risk of lymphoid leukemia did not increase significantly with maternal use of hormonal contraceptives. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.23 (P=0.089)
- Recent use—HR=1.27 (P=0.167)
- Use within 3 months of pregnancy—HR=1.28 (P=0.173)
- Use during pregnancy—HR=1.22 (P=0.635).
However, the risk of non-lymphoid leukemia was significantly increased in children born to mothers with recent hormonal contraceptive use. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.33 (P=0.232)
- Recent use—HR=2.17 (P=0.008)
- Use within 3 months of pregnancy—HR=1.95 (P=0.033)
- Use during pregnancy—HR=3.87 (P=0.006).
The association between recent contraceptive use and any leukemia was strongest in children ages 6 to 10 years. The researchers said this was not surprising because the incidence of non-lymphoid leukemia increases after the age of 6.
The researchers estimated that a mother’s recent use of hormonal contraceptives would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia over the study period.
This low risk of leukemia “is not a major concern with regard to the safety of hormonal contraceptives,” the researchers said.
However, the findings do suggest the intrauterine hormonal environment affects leukemia development in children, and this should be explored in future research.
This study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations, and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
A nationwide cohort study suggests an association between a woman’s use of hormonal contraceptives and leukemia in her offspring.
Children of mothers who used hormonal contraception, either during pregnancy or in the 3 months beforehand, had a 1.5-fold greater risk of leukemia, when compared to children of mothers who had never used hormonal contraception.
This increased risk translated to one additional case of leukemia per about 50,000 children exposed to hormonal contraceptives.
The increased risk appeared limited to non-lymphoid leukemia.
Marie Hargreave, PhD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, and her colleagues reported these findings in The Lancet Oncology.
The study included 1,185,157 children born between 1996 and 2014 and followed for a median of 9.3 years. Data on these children were collected from the Danish Medical Birth Registry and the Danish Cancer Registry.
The researchers looked at redeemed prescriptions from the Danish National Prescription Registry to determine the mothers’ contraceptive use and divided the women into three categories:
- Mothers who had never used hormonal contraceptives
- Those with previous hormonal contraceptive use, defined as greater than 3 months before the start of pregnancy
- Mothers with recent contraceptive use, defined as during or within 3 months of pregnancy.
Results
There were 606 children diagnosed with leukemia in the study cohort—465 with lymphoid leukemia and 141 with non-lymphoid leukemia.
Overall, children born to mothers with previous or recent use of hormonal contraceptives had a significantly increased risk of developing any leukemia. The hazard ratios (HRs) were as follows:
- Previous use of hormonal contraceptives—HR=1.25 (P=0.039)
- Recent use—HR=1.46 (P=0.011)
- Use within 3 months of pregnancy—HR=1.42 (P=0.025)
- Use during pregnancy—HR=1.78 (P=0.070).
The risk of lymphoid leukemia did not increase significantly with maternal use of hormonal contraceptives. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.23 (P=0.089)
- Recent use—HR=1.27 (P=0.167)
- Use within 3 months of pregnancy—HR=1.28 (P=0.173)
- Use during pregnancy—HR=1.22 (P=0.635).
However, the risk of non-lymphoid leukemia was significantly increased in children born to mothers with recent hormonal contraceptive use. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.33 (P=0.232)
- Recent use—HR=2.17 (P=0.008)
- Use within 3 months of pregnancy—HR=1.95 (P=0.033)
- Use during pregnancy—HR=3.87 (P=0.006).
The association between recent contraceptive use and any leukemia was strongest in children ages 6 to 10 years. The researchers said this was not surprising because the incidence of non-lymphoid leukemia increases after the age of 6.
The researchers estimated that a mother’s recent use of hormonal contraceptives would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia over the study period.
This low risk of leukemia “is not a major concern with regard to the safety of hormonal contraceptives,” the researchers said.
However, the findings do suggest the intrauterine hormonal environment affects leukemia development in children, and this should be explored in future research.
This study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations, and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Levofloxacin prophylaxis cuts bacteremia in pediatric acute leukemias
according to results of a multicenter, randomized phase 3 trial.
Risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children undergoing hematopoietic stem cell transplantation (HSCT), although a post hoc analysis accounting for time at risk did show a significant difference, according to results of this Children’s Oncology Group (COG) trial.
The reduction in risk for children with acute leukemias was similar to findings of adult studies showing the benefit of prophylactic antibiotics in patients with cancer-related neutropenia, said Sarah Alexander, MD, of the division of hematology/oncology, the Hospital for Sick Children, Toronto, and her coinvestigators.
Before this COG study, data on prophylactic antibiotics in children with cancer were limited to several small, single-group observational studies, Dr. Alexander and her coauthors wrote in JAMA.
Bacteremia was the primary outcome of the COG study, according to the investigators, because of its link to sepsis, increased health care utilization, and infection-related mortality. “Consequently, this outcome is meaningful to both clinicians and patients,” the investigators noted.
The multicenter, randomized, open-label phase 3 trial (ACCL0934) enrolled patients aged 6 months to 21 years, including 200 with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were to receive at least two intensive chemotherapy cycles, and 424 who were to receive a myeloablative autologous or allogeneic HSCT.
In the final analysis of the acute leukemias group, which included 195 patients, likelihood of bacteremia was 21.9% for those randomized to levofloxacin prophylaxis, versus 43.4% for no prophylaxis (P = 0.001).
In the final analysis of the HSCT group, which included 418 patients, likelihood of bacteremia was not significantly different, at 11.0% for levofloxacin prophylaxis, versus 17.3% for no prophylaxis (P = 0.06).
“Levofloxacin prophylaxis was effective at reducing the risk of bacteremia among patients with acute leukemia, but not among patients undergoing HSCT,” Dr. Armstrong and her coauthors said.
A post hoc analysis accounting for time at risk, however, showed a significant difference in favor of prophylaxis in both groups and a similar effect size between groups, according to investigators.
For the acute leukemias group, the rate of bacteremic episodes in that post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and no prophylaxis arms, respectively (P = 0.008). In the HSCT group, the rate was 5.3 versus 10.0 bacteremias per 1,000 patient-days in the prophylaxis and no prophylaxis arms (P = .02).
The similar effect size suggests that in the primary analysis, there was reduced power to detect a significant difference in the HSCT group because of fewer events, driven partly by a shorter duration of neutropenia in that group, Dr. Armstrong and her associates said.
“However, it is also plausible that the leukemia and HSCT groups had different supportive care measures or were infected with pathogens that had differential sensitivity to levofloxacin resulting in different efficacy of levofloxacin in the 2 groups,” they added.
Levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several Gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests that other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk, the investigators said.
Further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits, according to the investigators, who reported 23 serious adverse events in 8 patients, 11 of which were considered unrelated or unlikely to be related to levofloxacin.
“The adoption of antibacterial prophylaxis is tempered by potential negative consequences including Clostridium difficile-associated diarrhea, bacterial resistance, and musculoskeletal toxicities,” Dr. Armstrong and her colleagues noted.
The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
SOURCE: Alexander S, et al . JAMA. 2018;320(10):995-1004.
according to results of a multicenter, randomized phase 3 trial.
Risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children undergoing hematopoietic stem cell transplantation (HSCT), although a post hoc analysis accounting for time at risk did show a significant difference, according to results of this Children’s Oncology Group (COG) trial.
The reduction in risk for children with acute leukemias was similar to findings of adult studies showing the benefit of prophylactic antibiotics in patients with cancer-related neutropenia, said Sarah Alexander, MD, of the division of hematology/oncology, the Hospital for Sick Children, Toronto, and her coinvestigators.
Before this COG study, data on prophylactic antibiotics in children with cancer were limited to several small, single-group observational studies, Dr. Alexander and her coauthors wrote in JAMA.
Bacteremia was the primary outcome of the COG study, according to the investigators, because of its link to sepsis, increased health care utilization, and infection-related mortality. “Consequently, this outcome is meaningful to both clinicians and patients,” the investigators noted.
The multicenter, randomized, open-label phase 3 trial (ACCL0934) enrolled patients aged 6 months to 21 years, including 200 with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were to receive at least two intensive chemotherapy cycles, and 424 who were to receive a myeloablative autologous or allogeneic HSCT.
In the final analysis of the acute leukemias group, which included 195 patients, likelihood of bacteremia was 21.9% for those randomized to levofloxacin prophylaxis, versus 43.4% for no prophylaxis (P = 0.001).
In the final analysis of the HSCT group, which included 418 patients, likelihood of bacteremia was not significantly different, at 11.0% for levofloxacin prophylaxis, versus 17.3% for no prophylaxis (P = 0.06).
“Levofloxacin prophylaxis was effective at reducing the risk of bacteremia among patients with acute leukemia, but not among patients undergoing HSCT,” Dr. Armstrong and her coauthors said.
A post hoc analysis accounting for time at risk, however, showed a significant difference in favor of prophylaxis in both groups and a similar effect size between groups, according to investigators.
For the acute leukemias group, the rate of bacteremic episodes in that post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and no prophylaxis arms, respectively (P = 0.008). In the HSCT group, the rate was 5.3 versus 10.0 bacteremias per 1,000 patient-days in the prophylaxis and no prophylaxis arms (P = .02).
The similar effect size suggests that in the primary analysis, there was reduced power to detect a significant difference in the HSCT group because of fewer events, driven partly by a shorter duration of neutropenia in that group, Dr. Armstrong and her associates said.
“However, it is also plausible that the leukemia and HSCT groups had different supportive care measures or were infected with pathogens that had differential sensitivity to levofloxacin resulting in different efficacy of levofloxacin in the 2 groups,” they added.
Levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several Gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests that other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk, the investigators said.
Further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits, according to the investigators, who reported 23 serious adverse events in 8 patients, 11 of which were considered unrelated or unlikely to be related to levofloxacin.
“The adoption of antibacterial prophylaxis is tempered by potential negative consequences including Clostridium difficile-associated diarrhea, bacterial resistance, and musculoskeletal toxicities,” Dr. Armstrong and her colleagues noted.
The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
SOURCE: Alexander S, et al . JAMA. 2018;320(10):995-1004.
according to results of a multicenter, randomized phase 3 trial.
Risk of bacteremia was not significantly reduced with levofloxacin in another cohort of children undergoing hematopoietic stem cell transplantation (HSCT), although a post hoc analysis accounting for time at risk did show a significant difference, according to results of this Children’s Oncology Group (COG) trial.
The reduction in risk for children with acute leukemias was similar to findings of adult studies showing the benefit of prophylactic antibiotics in patients with cancer-related neutropenia, said Sarah Alexander, MD, of the division of hematology/oncology, the Hospital for Sick Children, Toronto, and her coinvestigators.
Before this COG study, data on prophylactic antibiotics in children with cancer were limited to several small, single-group observational studies, Dr. Alexander and her coauthors wrote in JAMA.
Bacteremia was the primary outcome of the COG study, according to the investigators, because of its link to sepsis, increased health care utilization, and infection-related mortality. “Consequently, this outcome is meaningful to both clinicians and patients,” the investigators noted.
The multicenter, randomized, open-label phase 3 trial (ACCL0934) enrolled patients aged 6 months to 21 years, including 200 with acute leukemias (acute myeloid leukemia or relapsed acute lymphoblastic leukemia) who were to receive at least two intensive chemotherapy cycles, and 424 who were to receive a myeloablative autologous or allogeneic HSCT.
In the final analysis of the acute leukemias group, which included 195 patients, likelihood of bacteremia was 21.9% for those randomized to levofloxacin prophylaxis, versus 43.4% for no prophylaxis (P = 0.001).
In the final analysis of the HSCT group, which included 418 patients, likelihood of bacteremia was not significantly different, at 11.0% for levofloxacin prophylaxis, versus 17.3% for no prophylaxis (P = 0.06).
“Levofloxacin prophylaxis was effective at reducing the risk of bacteremia among patients with acute leukemia, but not among patients undergoing HSCT,” Dr. Armstrong and her coauthors said.
A post hoc analysis accounting for time at risk, however, showed a significant difference in favor of prophylaxis in both groups and a similar effect size between groups, according to investigators.
For the acute leukemias group, the rate of bacteremic episodes in that post hoc analysis was 4.9 versus 9.4 per 1,000 patient-days in the prophylaxis and no prophylaxis arms, respectively (P = 0.008). In the HSCT group, the rate was 5.3 versus 10.0 bacteremias per 1,000 patient-days in the prophylaxis and no prophylaxis arms (P = .02).
The similar effect size suggests that in the primary analysis, there was reduced power to detect a significant difference in the HSCT group because of fewer events, driven partly by a shorter duration of neutropenia in that group, Dr. Armstrong and her associates said.
“However, it is also plausible that the leukemia and HSCT groups had different supportive care measures or were infected with pathogens that had differential sensitivity to levofloxacin resulting in different efficacy of levofloxacin in the 2 groups,” they added.
Levofloxacin-resistant pathogens, such as viridans group streptococcal isolates and several Gram-negative isolates, often were detected in patients who had bacteremia events despite prophylaxis. This suggests that other interventions in combination with levofloxacin prophylaxis are probably needed to further decrease risk, the investigators said.
Further randomized studies are needed to better understand the risks of levofloxacin in relation to its benefits, according to the investigators, who reported 23 serious adverse events in 8 patients, 11 of which were considered unrelated or unlikely to be related to levofloxacin.
“The adoption of antibacterial prophylaxis is tempered by potential negative consequences including Clostridium difficile-associated diarrhea, bacterial resistance, and musculoskeletal toxicities,” Dr. Armstrong and her colleagues noted.
The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Dr. Alexander reported no disclosures. Coauthors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
SOURCE: Alexander S, et al . JAMA. 2018;320(10):995-1004.
FROM JAMA
Key clinical point: Levofloxacin prophylaxis significantly reduced bacteremia in children with acute leukemias undergoing intensive chemotherapy, but not in children undergoing hematopoietic stem cell transplantation (HSCT).
Major finding: Bacteremia likelihood was 21.9% versus 43.4% for prophylaxis and no prophylaxis, respectively, in the acute leukemias group (P = 0.001), and 11.0% versus 17.3% in the HSCT group (P = 0.06).
Study details: A randomized phase 3 clinical trial, including 200 patients with acute leukemias and 424 patients undergoing HSCT.
Disclosures: The research was supported by grants from the Community Clinical Oncology Program and National Cancer Institute. Study authors reported disclosures related to Bristol-Myers Squibb, Chimerix, Jazz Pharmaceuticals, and the Children’s Oncology Group.
Source: Alexander S, et al. JAMA. 2018;320(10):995-1004.
Hormonal contraceptive use linked to leukemia risk in offspring
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: Recent maternal hormonal contraceptive use was linked to one additional case of leukemia per 47,170 children.
Study details: Danish nationwide cohort study in 1,185,157 children.
Disclosures: The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Source: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Cell population appears to drive relapse in AML
Researchers believe they have identified cells that are responsible for relapse of acute myeloid leukemia (AML).
These “leukemic-regenerating cells” (LRCs), which are distinct from leukemic stem cells (LSCs), seem to arise in response to chemotherapy.
Experiments in mouse models of AML suggested that targeting LRCs could reduce the risk of relapse, and analyses of AML patient samples suggested LRCs might be used to predict relapse.
Allison Boyd, PhD, of McMaster University in Hamilton, Ontario, Canada, and her colleagues reported these findings in Cancer Cell.
The researchers evaluated the leukemic populations that persist after chemotherapy by analyzing AML patient samples and xenograft AML models. The team found that LSCs were depleted by chemotherapy, and a different cell population, LRCs, appeared to arise in response to treatment.
LRCs are “molecularly distinct from therapy-naïve LSCs,” the researchers said. In fact, the team identified 19 genes that are preferentially expressed by LRCs and could be druggable.
One of these genes is DRD2, and the researchers found they could target LRCs using a small-molecule antagonist of DRD2.
Targeting LRCs
Dr. Boyd and her colleagues compared the effects of treatment with a DRD2 antagonist in AML xenografts populated with therapy-naive LSCs and AML xenografts that harbored LRCs following exposure to cytarabine.
The researchers said DRD2 antagonist therapy “moderately” affected AML progenitors in the LSC model but “had profound effects on regenerating LRCs.”
Treatment with the DRD2 antagonist also improved the efficacy of chemotherapy.
In xenografts derived from one AML patient, treatment with cytarabine alone left 50% of mice with residual disease. However, the addition of the DRD2 antagonist enabled 100% of the mice to achieve disease-free status.
In xenografts derived from a patient with more aggressive AML, all recipient mice had residual disease after receiving cytarabine. Treatment with the DRD2 antagonist slowed leukemic re-growth and nearly doubled the time to relapse.
Targeting LRCs also reduced disease regeneration potential in samples from other AML patients.
“This is a major clinical opportunity because this type of leukemia is very diverse and responds differently across patients,” Dr. Boyd said. “It has been a challenge in a clinical setting to find a commonality for therapeutic targeting across the wide array of patients, and these regenerative cells provide that similarity.”
Predicting relapse
Dr. Boyd and her colleagues also analyzed bone marrow samples collected from AML patients approximately 3 weeks after they completed standard induction chemotherapy.
The team found that progenitor activity was enriched among residual leukemic cells. However, patient cells lacked gene expression signatures related to therapy-naive LSCs.
“Instead, these highly regenerative AML cells preferentially expressed our LRC signature,” the researchers said.
The team also found evidence to suggest that LRC molecular profiles arise temporarily after chemotherapy. The LRC signature was not observed at diagnosis or once AML was re-established at relapse.
“We think there are opportunities here because now we have a window where we can kick the cancer while it’s down,” Dr. Boyd said.
She and her colleagues also found the LRC signature might be useful for predicting relapse in AML patients.
The team assessed expression of SLC2A2, an LRC marker that has overlapping expression with DRD2, in 7 patients who were in clinical remission after induction.
The researchers found that chemotherapy increased expression of SLC2A2 only in the four patients who had residual disease—not in the three patients who remained in disease-free remission for at least 5 years.
“These results suggest that LRC populations represent reservoirs of residual disease, and LRC marker expression levels can be linked to clinical outcomes of AML relapse,” the researchers said.
This study was supported by the Canadian Cancer Society, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other organizations.
Researchers believe they have identified cells that are responsible for relapse of acute myeloid leukemia (AML).
These “leukemic-regenerating cells” (LRCs), which are distinct from leukemic stem cells (LSCs), seem to arise in response to chemotherapy.
Experiments in mouse models of AML suggested that targeting LRCs could reduce the risk of relapse, and analyses of AML patient samples suggested LRCs might be used to predict relapse.
Allison Boyd, PhD, of McMaster University in Hamilton, Ontario, Canada, and her colleagues reported these findings in Cancer Cell.
The researchers evaluated the leukemic populations that persist after chemotherapy by analyzing AML patient samples and xenograft AML models. The team found that LSCs were depleted by chemotherapy, and a different cell population, LRCs, appeared to arise in response to treatment.
LRCs are “molecularly distinct from therapy-naïve LSCs,” the researchers said. In fact, the team identified 19 genes that are preferentially expressed by LRCs and could be druggable.
One of these genes is DRD2, and the researchers found they could target LRCs using a small-molecule antagonist of DRD2.
Targeting LRCs
Dr. Boyd and her colleagues compared the effects of treatment with a DRD2 antagonist in AML xenografts populated with therapy-naive LSCs and AML xenografts that harbored LRCs following exposure to cytarabine.
The researchers said DRD2 antagonist therapy “moderately” affected AML progenitors in the LSC model but “had profound effects on regenerating LRCs.”
Treatment with the DRD2 antagonist also improved the efficacy of chemotherapy.
In xenografts derived from one AML patient, treatment with cytarabine alone left 50% of mice with residual disease. However, the addition of the DRD2 antagonist enabled 100% of the mice to achieve disease-free status.
In xenografts derived from a patient with more aggressive AML, all recipient mice had residual disease after receiving cytarabine. Treatment with the DRD2 antagonist slowed leukemic re-growth and nearly doubled the time to relapse.
Targeting LRCs also reduced disease regeneration potential in samples from other AML patients.
“This is a major clinical opportunity because this type of leukemia is very diverse and responds differently across patients,” Dr. Boyd said. “It has been a challenge in a clinical setting to find a commonality for therapeutic targeting across the wide array of patients, and these regenerative cells provide that similarity.”
Predicting relapse
Dr. Boyd and her colleagues also analyzed bone marrow samples collected from AML patients approximately 3 weeks after they completed standard induction chemotherapy.
The team found that progenitor activity was enriched among residual leukemic cells. However, patient cells lacked gene expression signatures related to therapy-naive LSCs.
“Instead, these highly regenerative AML cells preferentially expressed our LRC signature,” the researchers said.
The team also found evidence to suggest that LRC molecular profiles arise temporarily after chemotherapy. The LRC signature was not observed at diagnosis or once AML was re-established at relapse.
“We think there are opportunities here because now we have a window where we can kick the cancer while it’s down,” Dr. Boyd said.
She and her colleagues also found the LRC signature might be useful for predicting relapse in AML patients.
The team assessed expression of SLC2A2, an LRC marker that has overlapping expression with DRD2, in 7 patients who were in clinical remission after induction.
The researchers found that chemotherapy increased expression of SLC2A2 only in the four patients who had residual disease—not in the three patients who remained in disease-free remission for at least 5 years.
“These results suggest that LRC populations represent reservoirs of residual disease, and LRC marker expression levels can be linked to clinical outcomes of AML relapse,” the researchers said.
This study was supported by the Canadian Cancer Society, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other organizations.
Researchers believe they have identified cells that are responsible for relapse of acute myeloid leukemia (AML).
These “leukemic-regenerating cells” (LRCs), which are distinct from leukemic stem cells (LSCs), seem to arise in response to chemotherapy.
Experiments in mouse models of AML suggested that targeting LRCs could reduce the risk of relapse, and analyses of AML patient samples suggested LRCs might be used to predict relapse.
Allison Boyd, PhD, of McMaster University in Hamilton, Ontario, Canada, and her colleagues reported these findings in Cancer Cell.
The researchers evaluated the leukemic populations that persist after chemotherapy by analyzing AML patient samples and xenograft AML models. The team found that LSCs were depleted by chemotherapy, and a different cell population, LRCs, appeared to arise in response to treatment.
LRCs are “molecularly distinct from therapy-naïve LSCs,” the researchers said. In fact, the team identified 19 genes that are preferentially expressed by LRCs and could be druggable.
One of these genes is DRD2, and the researchers found they could target LRCs using a small-molecule antagonist of DRD2.
Targeting LRCs
Dr. Boyd and her colleagues compared the effects of treatment with a DRD2 antagonist in AML xenografts populated with therapy-naive LSCs and AML xenografts that harbored LRCs following exposure to cytarabine.
The researchers said DRD2 antagonist therapy “moderately” affected AML progenitors in the LSC model but “had profound effects on regenerating LRCs.”
Treatment with the DRD2 antagonist also improved the efficacy of chemotherapy.
In xenografts derived from one AML patient, treatment with cytarabine alone left 50% of mice with residual disease. However, the addition of the DRD2 antagonist enabled 100% of the mice to achieve disease-free status.
In xenografts derived from a patient with more aggressive AML, all recipient mice had residual disease after receiving cytarabine. Treatment with the DRD2 antagonist slowed leukemic re-growth and nearly doubled the time to relapse.
Targeting LRCs also reduced disease regeneration potential in samples from other AML patients.
“This is a major clinical opportunity because this type of leukemia is very diverse and responds differently across patients,” Dr. Boyd said. “It has been a challenge in a clinical setting to find a commonality for therapeutic targeting across the wide array of patients, and these regenerative cells provide that similarity.”
Predicting relapse
Dr. Boyd and her colleagues also analyzed bone marrow samples collected from AML patients approximately 3 weeks after they completed standard induction chemotherapy.
The team found that progenitor activity was enriched among residual leukemic cells. However, patient cells lacked gene expression signatures related to therapy-naive LSCs.
“Instead, these highly regenerative AML cells preferentially expressed our LRC signature,” the researchers said.
The team also found evidence to suggest that LRC molecular profiles arise temporarily after chemotherapy. The LRC signature was not observed at diagnosis or once AML was re-established at relapse.
“We think there are opportunities here because now we have a window where we can kick the cancer while it’s down,” Dr. Boyd said.
She and her colleagues also found the LRC signature might be useful for predicting relapse in AML patients.
The team assessed expression of SLC2A2, an LRC marker that has overlapping expression with DRD2, in 7 patients who were in clinical remission after induction.
The researchers found that chemotherapy increased expression of SLC2A2 only in the four patients who had residual disease—not in the three patients who remained in disease-free remission for at least 5 years.
“These results suggest that LRC populations represent reservoirs of residual disease, and LRC marker expression levels can be linked to clinical outcomes of AML relapse,” the researchers said.
This study was supported by the Canadian Cancer Society, the Canadian Institutes of Health Research, the Ontario Institute for Cancer Research, and other organizations.
Team identifies potential immunotherapy target for AML
New research could aid the development of immunotherapies tailored to patients with acute myeloid leukemia (AML) who are undergoing stem cell transplant (SCT).
Researchers found they could use genetic sequencing and computer software to identify minor histocompatibility antigens (mHAs) known to occur in AML.
The team used this method to predict novel graft-versus-leukemia (GVL) mHAs and demonstrated that one of these mHAs could be a “potentially useful” therapeutic target.
Ben Vincent, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, and his colleagues reported these findings in Blood Advances.
In their retrospective study, the researchers tested whether their software could predict antigenic targets in 101 SCT donor-recipient pairs.
The researchers found they could correctly identify 14 of 18 mHAs known to occur in AML, but they were also able to predict 102 new GVL mHAs.
The researchers then confirmed one of these GVL mHAs, called UNC-GRK4-V, as a potential target for immunotherapy. The team observed immune responses to UNC-GRK4-V in four of nine AML patients who had undergone SCT.
Looking ahead, the researchers want to optimize their software to predict the most common AML-associated mHAs present in the U.S. population and confirm these predicted antigens as valid immunotherapy targets.
The team believes they could potentially use their predictions to engineer donor immune cells to specifically target the cancer cell antigens while preventing graft-versus-host disease.
“We’ve developed a software package that predicts leukemia-specific immune targets in any leukemia patient undergoing a stem cell transplant based on DNA and RNA sequencing and demonstrated that these data can lead to actual targets expressed on leukemia cells,” Dr. Vincent said.
“The next step of our work is to use that information for patient-specific therapies to try to improve cure rates without making graft-versus-host disease worse.”
The current research was supported by a National Cancer Institute grant, an ASCO Young Investigator Award, the North Carolina University Cancer Research Fund, and the Scott Neil Schwirck Fellowship.
New research could aid the development of immunotherapies tailored to patients with acute myeloid leukemia (AML) who are undergoing stem cell transplant (SCT).
Researchers found they could use genetic sequencing and computer software to identify minor histocompatibility antigens (mHAs) known to occur in AML.
The team used this method to predict novel graft-versus-leukemia (GVL) mHAs and demonstrated that one of these mHAs could be a “potentially useful” therapeutic target.
Ben Vincent, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, and his colleagues reported these findings in Blood Advances.
In their retrospective study, the researchers tested whether their software could predict antigenic targets in 101 SCT donor-recipient pairs.
The researchers found they could correctly identify 14 of 18 mHAs known to occur in AML, but they were also able to predict 102 new GVL mHAs.
The researchers then confirmed one of these GVL mHAs, called UNC-GRK4-V, as a potential target for immunotherapy. The team observed immune responses to UNC-GRK4-V in four of nine AML patients who had undergone SCT.
Looking ahead, the researchers want to optimize their software to predict the most common AML-associated mHAs present in the U.S. population and confirm these predicted antigens as valid immunotherapy targets.
The team believes they could potentially use their predictions to engineer donor immune cells to specifically target the cancer cell antigens while preventing graft-versus-host disease.
“We’ve developed a software package that predicts leukemia-specific immune targets in any leukemia patient undergoing a stem cell transplant based on DNA and RNA sequencing and demonstrated that these data can lead to actual targets expressed on leukemia cells,” Dr. Vincent said.
“The next step of our work is to use that information for patient-specific therapies to try to improve cure rates without making graft-versus-host disease worse.”
The current research was supported by a National Cancer Institute grant, an ASCO Young Investigator Award, the North Carolina University Cancer Research Fund, and the Scott Neil Schwirck Fellowship.
New research could aid the development of immunotherapies tailored to patients with acute myeloid leukemia (AML) who are undergoing stem cell transplant (SCT).
Researchers found they could use genetic sequencing and computer software to identify minor histocompatibility antigens (mHAs) known to occur in AML.
The team used this method to predict novel graft-versus-leukemia (GVL) mHAs and demonstrated that one of these mHAs could be a “potentially useful” therapeutic target.
Ben Vincent, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, and his colleagues reported these findings in Blood Advances.
In their retrospective study, the researchers tested whether their software could predict antigenic targets in 101 SCT donor-recipient pairs.
The researchers found they could correctly identify 14 of 18 mHAs known to occur in AML, but they were also able to predict 102 new GVL mHAs.
The researchers then confirmed one of these GVL mHAs, called UNC-GRK4-V, as a potential target for immunotherapy. The team observed immune responses to UNC-GRK4-V in four of nine AML patients who had undergone SCT.
Looking ahead, the researchers want to optimize their software to predict the most common AML-associated mHAs present in the U.S. population and confirm these predicted antigens as valid immunotherapy targets.
The team believes they could potentially use their predictions to engineer donor immune cells to specifically target the cancer cell antigens while preventing graft-versus-host disease.
“We’ve developed a software package that predicts leukemia-specific immune targets in any leukemia patient undergoing a stem cell transplant based on DNA and RNA sequencing and demonstrated that these data can lead to actual targets expressed on leukemia cells,” Dr. Vincent said.
“The next step of our work is to use that information for patient-specific therapies to try to improve cure rates without making graft-versus-host disease worse.”
The current research was supported by a National Cancer Institute grant, an ASCO Young Investigator Award, the North Carolina University Cancer Research Fund, and the Scott Neil Schwirck Fellowship.