User login
Enjoy Las Vegas and HM17
Welcome to HM17 and Las Vegas! We invite you to network and get to know more than 4,000 of your closest colleagues over the next 3 days. Please have fun taking advantage of the many unique learning opportunities we have developed for this year’s meeting. We hope you will be pleased with the offerings that our Annual Meeting Committee has produced on your behalf. You will see committee members wearing buttons that identify them as members of the Annual Meeting Committee. Please take the time to give them your feedback about the meeting and, if you feel so inclined, thank them for the time and energy they committed to create this year’s meeting.
We think you will find this meeting and the precourses have something (many things!) for everyone. Whether you are a community or an academic hospitalist, a newly minted hospitalist or a seasoned veteran, a clinician who takes care of the young or the old (and everyone in between), an advanced practice clinician or hospital medicine administrator, a researcher or educator or clinician (or any combination of the three), we had you in mind as we developed the content for HM17.
We have added medical education and health policy tracks and are bringing back favorites like the young-hospitalist, academic, pediatric, practice management, and quality tracks. Don’t forget to attend our interactive workshops. More than 150 workshop ideas were submitted, and we are proud to feature 18 of the best.
Finally, your HM17 experience will not be complete until you attend the much-anticipated Updates in Hospital Medicine talks and plenary sessions; network with your colleagues at the Research, Innovations, and Clinical Vignettes (RIV) Poster Competition; roam the Exhibit Hall; and join in a Special Interest Forum.
This meeting would not be possible without the tireless effort of the SHM staff and leadership, conference faculty, and committee members. Most importantly, we sincerely thank all of you for attending HM17. We have created this meeting for you, and we hope it is your most valuable educational and networking opportunity of 2017.
Enjoy Las Vegas and HM17, and we will see you in 2018 in Orlando!
Dr. Feldman is a hospitalist at Johns Hopkins in Baltimore and course director for HM17.
Welcome to HM17 and Las Vegas! We invite you to network and get to know more than 4,000 of your closest colleagues over the next 3 days. Please have fun taking advantage of the many unique learning opportunities we have developed for this year’s meeting. We hope you will be pleased with the offerings that our Annual Meeting Committee has produced on your behalf. You will see committee members wearing buttons that identify them as members of the Annual Meeting Committee. Please take the time to give them your feedback about the meeting and, if you feel so inclined, thank them for the time and energy they committed to create this year’s meeting.
We think you will find this meeting and the precourses have something (many things!) for everyone. Whether you are a community or an academic hospitalist, a newly minted hospitalist or a seasoned veteran, a clinician who takes care of the young or the old (and everyone in between), an advanced practice clinician or hospital medicine administrator, a researcher or educator or clinician (or any combination of the three), we had you in mind as we developed the content for HM17.
We have added medical education and health policy tracks and are bringing back favorites like the young-hospitalist, academic, pediatric, practice management, and quality tracks. Don’t forget to attend our interactive workshops. More than 150 workshop ideas were submitted, and we are proud to feature 18 of the best.
Finally, your HM17 experience will not be complete until you attend the much-anticipated Updates in Hospital Medicine talks and plenary sessions; network with your colleagues at the Research, Innovations, and Clinical Vignettes (RIV) Poster Competition; roam the Exhibit Hall; and join in a Special Interest Forum.
This meeting would not be possible without the tireless effort of the SHM staff and leadership, conference faculty, and committee members. Most importantly, we sincerely thank all of you for attending HM17. We have created this meeting for you, and we hope it is your most valuable educational and networking opportunity of 2017.
Enjoy Las Vegas and HM17, and we will see you in 2018 in Orlando!
Dr. Feldman is a hospitalist at Johns Hopkins in Baltimore and course director for HM17.
Welcome to HM17 and Las Vegas! We invite you to network and get to know more than 4,000 of your closest colleagues over the next 3 days. Please have fun taking advantage of the many unique learning opportunities we have developed for this year’s meeting. We hope you will be pleased with the offerings that our Annual Meeting Committee has produced on your behalf. You will see committee members wearing buttons that identify them as members of the Annual Meeting Committee. Please take the time to give them your feedback about the meeting and, if you feel so inclined, thank them for the time and energy they committed to create this year’s meeting.
We think you will find this meeting and the precourses have something (many things!) for everyone. Whether you are a community or an academic hospitalist, a newly minted hospitalist or a seasoned veteran, a clinician who takes care of the young or the old (and everyone in between), an advanced practice clinician or hospital medicine administrator, a researcher or educator or clinician (or any combination of the three), we had you in mind as we developed the content for HM17.
We have added medical education and health policy tracks and are bringing back favorites like the young-hospitalist, academic, pediatric, practice management, and quality tracks. Don’t forget to attend our interactive workshops. More than 150 workshop ideas were submitted, and we are proud to feature 18 of the best.
Finally, your HM17 experience will not be complete until you attend the much-anticipated Updates in Hospital Medicine talks and plenary sessions; network with your colleagues at the Research, Innovations, and Clinical Vignettes (RIV) Poster Competition; roam the Exhibit Hall; and join in a Special Interest Forum.
This meeting would not be possible without the tireless effort of the SHM staff and leadership, conference faculty, and committee members. Most importantly, we sincerely thank all of you for attending HM17. We have created this meeting for you, and we hope it is your most valuable educational and networking opportunity of 2017.
Enjoy Las Vegas and HM17, and we will see you in 2018 in Orlando!
Dr. Feldman is a hospitalist at Johns Hopkins in Baltimore and course director for HM17.
Hospital infections top WHO’s list of priority pathogens
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
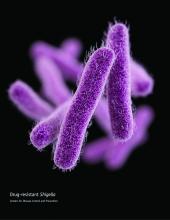
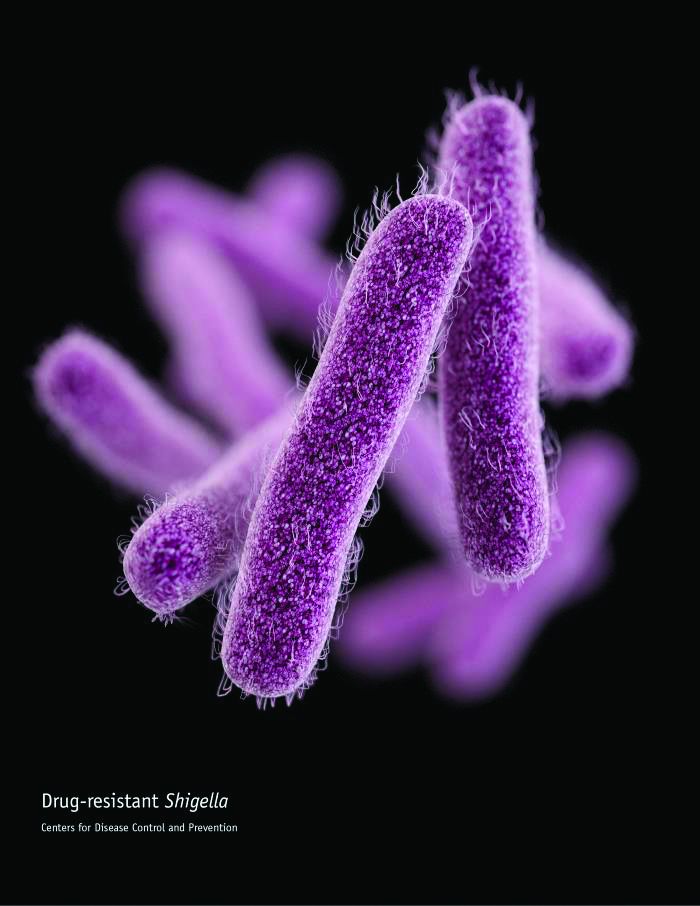
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
The World Health Organization is urging governments to focus antibiotic research efforts on a list of urgent bacterial threats, topped by several increasingly powerful superbugs that cause hospital-based infections and other potentially deadly conditions.
The WHO listed the top 20 bacteria that it believes are most harmful to human health, other than mycobacteria such as Mycobacterium tuberculosis, which causes tuberculosis. The germ was not included in the list because it’s generally accepted to be the most urgent priority for new antibiotic research and development, Marie-Paule Kieny, PhD, a WHO assistant director, said at a press conference.
The priority list is needed because the antibiotic pipeline is “practically dry,” thanks to scientific research challenges and a lack of financial incentives, according to Dr. Kieny. “Antibiotics are generally used for the short term, unlike therapies for chronic diseases, which bring in much higher returns on investment,” she said. The list “is intended to signal to the scientific community and the pharmaceutical industry the areas they should focus on to address urgent public health threats.”
The WHO list begins with Priority 1/“Critical” pathogens that it believes most urgently need to be targeted through antibiotic research and development: Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; and Enterobacteriaceae (including Klebsiella pneumonia, Escherichia coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp., and Morganella spp.), carbapenem-resistant, extended-spectrum beta-lactamase–producing.
“These bacteria are responsible for severe infections and high mortality rates, mostly in hospitalized patients, transplant recipients, those receiving chemotherapy, or patients in intensive care units,” Dr. Kieny said. “While these bacteria are not widespread and do not generally affect healthy individuals, the burden for patients and society is now alarming – and new, effective therapies are imperative.”
Priority 2/”High” pathogens are Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and resistant; Helicobacter pylori, clarithromycin-resistant; Campylobacter, fluoroquinolone-resistant; Salmonella spp., fluoroquinolone-resistant; Neisseria gonorrhoeae, third-generation cephalosporin-resistant and fluoroquinolone-resistant.
Pathogens in this category can infect healthy individuals, Dr. Kieny noted. “These infections, although not associated with significant mortality, have a dramatic health and economic impact on communities and, in particular, in low-income countries.”
Priority 3/”Medium” pathogens are Streptococcus pneumoniae, penicillin–non-susceptible; Haemophilus influenzae, ampicillin-resistant; and Shigella spp., fluoroquinolone-resistant.
These pathogens “represent a threat because of increasing resistance but still have some effective antibiotic options available,” Dr. Kieny said.
According to a statement provided by the WHO, the priority list doesn’t include streptococcus A and B or chlamydia, because resistance hasn’t reached the level of a public health threat.
One goal of the list is to focus attention on the development of small-market, gram-negative drugs that combat hospital-based infections, explained Nicola Magrini, MD, a WHO scientist who also spoke at the press conference.
Over the last decade, he said, the pipeline has instead focused more on gram-positive agents – mostly linked to beta-lactamase – that have wider market potential and generate less resistance.
“From a clinical point of view, these multidrug-resistant gram-negative clinical trials are very difficult and expensive to do, more than for gram-positive,” noted Evelina Tacconelli, MD, PhD, a contributor to the WHO report. “Because when we talk about gram-negative, we need to cover multiple pathogens and not just one or two, as in the case of gram-positive.”
Dr. Magrini said he couldn’t provide estimates about how many people worldwide are affected by the listed pathogens. However, he said a full report with numbers will be released by June.
It does appear that patients with severe infection from antibiotic-resistant germs face a mortality rate of up to 60%, while extended-spectrum beta-lactamase–positive E. coli accounts for up to 70% of urinary tract infections in many countries, explained Dr. Tacconelli, head of the division of infectious diseases at the University of Tübingen, Germany.
“Even if we don’t know exactly how many,” she said, “we are talking about millions of people affected.”
VHA warns of a ‘second epidemic’ of carbapenem-resistant E. cloacae complex
Veterans Health Administration monitoring of carbapenem-resistant Enterobacteriaceae (CRE) trends from 2006 to 2015 shows a rise in resistance rates of E. cloacae complex nationwide.
The first major CRE outbreak, Klebsiella pneumoniae, occurred in the eastern United States in the early 2000s and has since spread across the country. K. pneumoniae has recently shown a decrease in resistance rates in the region including New York, both in the current VHA-based study and in a 2016 study of three New York City hospitals.
“CRE trends during 2006-2015 in the VHA recapitulate the epidemic of carbapenem-resistant K. pneumoniae in the United States and indicate that a ‘second epidemic’ of carbapenem-resistant E. cloacae complex appears to be unfolding,” wrote Brigid M. Wilson, PhD, of Louis Stokes Cleveland Department of Veterans Affairs Medical Center, and her coauthors.
The researchers used VHA network data to identify 128,431 K. pneumoniae and 38,219 E. cloacae complex (which refers to the species E. cloacae, E. asburiae, E. kobei, E. hormaechei, and E. xiafangensis) isolates from patients hospitalized in 140 facilities in 40 states, the District of Columbia, and Puerto Rico from 2006 to 2015. These isolates, paired with their carbapenem susceptibility test results, show the rise and geographic concentration of the CRE cases over the decade.
The increased E. cloacae complex resistance in 2014-2015 was centered around the Pacific Coast and Southwest regions. The researchers noted that E. cloacae complex has a less well defined genetic makeup, compared with K. pneumoniae.
“We hypothesize that E. cloacae complex contains genotypes with epidemic potential associated with increasing rates of carbapenem resistance observed in the VHA,” they wrote, concluding that “the VHA may serve as a vantage point for detecting nationwide trends in antimicrobial drug resistance” (Emerg Infect Dis. 2017 Mar. doi: 10.3201/eid2305.162034).
Veterans Health Administration monitoring of carbapenem-resistant Enterobacteriaceae (CRE) trends from 2006 to 2015 shows a rise in resistance rates of E. cloacae complex nationwide.
The first major CRE outbreak, Klebsiella pneumoniae, occurred in the eastern United States in the early 2000s and has since spread across the country. K. pneumoniae has recently shown a decrease in resistance rates in the region including New York, both in the current VHA-based study and in a 2016 study of three New York City hospitals.
“CRE trends during 2006-2015 in the VHA recapitulate the epidemic of carbapenem-resistant K. pneumoniae in the United States and indicate that a ‘second epidemic’ of carbapenem-resistant E. cloacae complex appears to be unfolding,” wrote Brigid M. Wilson, PhD, of Louis Stokes Cleveland Department of Veterans Affairs Medical Center, and her coauthors.
The researchers used VHA network data to identify 128,431 K. pneumoniae and 38,219 E. cloacae complex (which refers to the species E. cloacae, E. asburiae, E. kobei, E. hormaechei, and E. xiafangensis) isolates from patients hospitalized in 140 facilities in 40 states, the District of Columbia, and Puerto Rico from 2006 to 2015. These isolates, paired with their carbapenem susceptibility test results, show the rise and geographic concentration of the CRE cases over the decade.
The increased E. cloacae complex resistance in 2014-2015 was centered around the Pacific Coast and Southwest regions. The researchers noted that E. cloacae complex has a less well defined genetic makeup, compared with K. pneumoniae.
“We hypothesize that E. cloacae complex contains genotypes with epidemic potential associated with increasing rates of carbapenem resistance observed in the VHA,” they wrote, concluding that “the VHA may serve as a vantage point for detecting nationwide trends in antimicrobial drug resistance” (Emerg Infect Dis. 2017 Mar. doi: 10.3201/eid2305.162034).
Veterans Health Administration monitoring of carbapenem-resistant Enterobacteriaceae (CRE) trends from 2006 to 2015 shows a rise in resistance rates of E. cloacae complex nationwide.
The first major CRE outbreak, Klebsiella pneumoniae, occurred in the eastern United States in the early 2000s and has since spread across the country. K. pneumoniae has recently shown a decrease in resistance rates in the region including New York, both in the current VHA-based study and in a 2016 study of three New York City hospitals.
“CRE trends during 2006-2015 in the VHA recapitulate the epidemic of carbapenem-resistant K. pneumoniae in the United States and indicate that a ‘second epidemic’ of carbapenem-resistant E. cloacae complex appears to be unfolding,” wrote Brigid M. Wilson, PhD, of Louis Stokes Cleveland Department of Veterans Affairs Medical Center, and her coauthors.
The researchers used VHA network data to identify 128,431 K. pneumoniae and 38,219 E. cloacae complex (which refers to the species E. cloacae, E. asburiae, E. kobei, E. hormaechei, and E. xiafangensis) isolates from patients hospitalized in 140 facilities in 40 states, the District of Columbia, and Puerto Rico from 2006 to 2015. These isolates, paired with their carbapenem susceptibility test results, show the rise and geographic concentration of the CRE cases over the decade.
The increased E. cloacae complex resistance in 2014-2015 was centered around the Pacific Coast and Southwest regions. The researchers noted that E. cloacae complex has a less well defined genetic makeup, compared with K. pneumoniae.
“We hypothesize that E. cloacae complex contains genotypes with epidemic potential associated with increasing rates of carbapenem resistance observed in the VHA,” they wrote, concluding that “the VHA may serve as a vantage point for detecting nationwide trends in antimicrobial drug resistance” (Emerg Infect Dis. 2017 Mar. doi: 10.3201/eid2305.162034).
Monotherapy as good as combo for antibiotic-resistant infections in low-risk patients
VIENNA – A single, well-targeted antibiotic may be enough to effectively combat serous bloodstream infections in patients who have a low baseline mortality risk.
Among these patients, overall mortality was similar among those receiving a single antibiotic and those getting multiple antibiotics (35% vs. 41%). Patients with a high baseline mortality risk, however, did experience a significant 44% survival benefit when treated with a combination regimen, Jesus Rodríguez-Baño, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The finding is important when considering the ever-increasing imperative of antibiotic stewardship, Dr. Rodríguez-Baño said in an interview.
“In areas where these pathogens are common, particularly in intensive care units, where they can become epidemic and infect many patients, the overuse of combination therapy will be fueling the problem,” said Dr. Rodríguez-Baño, head of infectious diseases and clinical microbiology at the University Hospital Virgin Macarena, Seville, Spain. “This is a way to avoid the overuse of some broad-spectrum antibiotics. Selecting the patients who should not receive combination therapy may significantly reduce the total consumption” on a unit.
The retrospective study, dubbed INCREMENT, was conducted at 37 hospitals in 10 countries. It enrolled patients with bloodstream infections caused by extended-spectrum beta-lactamase- or carbapenemase-producing Enterobacteriaceae. Dr. Rodríguez-Baño reported results for 437 patients whose infections were caused by the carbapenemase-producing strain.
It was simultaneously published in Lancet Infectious Diseases (Lancet Inf Dis 2017; DOI: http://dx.doi.org/10.1016/S1473-3099(17)30228-1).
These patients were a mean of 66 years old; most (60%) were male. The primary infective agent was Klebsiella pneumonia (86%); most infections were nosocomial. The origin of infections varied, but most (80%) arose from places other than the urinary or biliary tract. Sources were vascular catheters, pneumonia, intraabdominal, and skin and soft tissue. About half of the patients were in severe sepsis or septic shock when treated.
The group was first divided into those who received appropriate or inappropriate therapy (78% vs. 22%). Appropriate therapy was considered to be the early administration of a drug that could effectively target the infective organism. Next, those who got appropriate therapy were parsed by whether they received mono- or combination therapy (61%, 39%). Finally, these patients were stratified by a specially designed mortality risk score, the INCREMENT Carbapenemase-Producing Enterobacteriaceae (CPE) Mortality Score (Mayo Clinic Proceedings. doi.org/10.1016/j.mayocp.2016.06.024).
- Severe sepsis or shock at presentation (5 points)
- Pitt score of 6 or more (4 points)
- Charlson comorbidity index of 2 or more (3 points)
- Source of bloodstream infection other than urinary or biliary tract (3 points)
- Inappropriate empirical therapy and inappropriate early targeted therapy (2 points)
Patients were considered low risk if they had a score of 0-7, and high of they had a score of 8 or more.
The risk assessment took is quick, easy to figure, and extremely important, Dr. Rodríguez-Baño noted. “This is a very easy-to-use tool that can help us make many patient management decisions. All of the information is already available in the patient’s chart, so it doesn’t require any additional assessments. It’s a very good way to individualize treatment.”
In the initial analysis, all-cause mortality at 30 days was 22% lower among patients who received appropriate early therapy than those who did not (38.5% vs. 60.6%). This translated to a 55% decrease in the risk of death (HR 0.45 in the fully adjusted model).
The investigators next turned their attention toward the group that received appropriate therapy. All-cause 30-day mortality was 41% in those who got monotherapy and 34.8% among those who got combination therapy..
Finally, this group was stratified according to the INCREMENT-CPE mortality risk score.
In the low-risk category, combination therapy did not confer a survival advantage over monotherapy. Death occurred in 20% of those getting monotherapy and 24% receiving combination treatment – not a significant difference (HR 1.21).
Combination therapy did, however, confer a significant survival benefit in the high-risk group. Death occurred in 62% of those receiving monotherapy and 48% of those receiving combination therapy – a 44% risk reduction (HR 0.56).
As long as they were appropriately targeted against the infective organism, all drugs used in the high-mortality risk group were similarly effective at reducing the risk of death. Compared to colistin monotherapy, a combination that included tigecycline reduced the risk of death by 55% (HR 0.45); combination with aminoglycosides by 58% (HR 0.42); and combination with carbapenems by 44% (HR 0.56).
A secondary analysis of this group determined that time was a critical factor in survival. Each day delay after day 2 significantly increased the risk of death, Dr. Rodríguez-Baño said. This 48-hour period gives clinicians a chance to wait for the culture and antibiogram to return, and then choose and initiate the best treatment. Before the results come back, empiric antibiotic therapy is appropriate, but changes should be made immediately after the results come back.
“We tend to think we must give the very best antibiotic at the very first moment that we see a patient with a serious infection,” he said. “But what we found is that it’s not critical to give the perfect antibiotic on the first day. It is critical, however, to give the correct one once you know which bacteria is causing the infection. Since it takes 48 hours for those results to come back, this is perfect timing.”
INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
[email protected]
On Twitter @Alz_gal
VIENNA – A single, well-targeted antibiotic may be enough to effectively combat serous bloodstream infections in patients who have a low baseline mortality risk.
Among these patients, overall mortality was similar among those receiving a single antibiotic and those getting multiple antibiotics (35% vs. 41%). Patients with a high baseline mortality risk, however, did experience a significant 44% survival benefit when treated with a combination regimen, Jesus Rodríguez-Baño, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The finding is important when considering the ever-increasing imperative of antibiotic stewardship, Dr. Rodríguez-Baño said in an interview.
“In areas where these pathogens are common, particularly in intensive care units, where they can become epidemic and infect many patients, the overuse of combination therapy will be fueling the problem,” said Dr. Rodríguez-Baño, head of infectious diseases and clinical microbiology at the University Hospital Virgin Macarena, Seville, Spain. “This is a way to avoid the overuse of some broad-spectrum antibiotics. Selecting the patients who should not receive combination therapy may significantly reduce the total consumption” on a unit.
The retrospective study, dubbed INCREMENT, was conducted at 37 hospitals in 10 countries. It enrolled patients with bloodstream infections caused by extended-spectrum beta-lactamase- or carbapenemase-producing Enterobacteriaceae. Dr. Rodríguez-Baño reported results for 437 patients whose infections were caused by the carbapenemase-producing strain.
It was simultaneously published in Lancet Infectious Diseases (Lancet Inf Dis 2017; DOI: http://dx.doi.org/10.1016/S1473-3099(17)30228-1).
These patients were a mean of 66 years old; most (60%) were male. The primary infective agent was Klebsiella pneumonia (86%); most infections were nosocomial. The origin of infections varied, but most (80%) arose from places other than the urinary or biliary tract. Sources were vascular catheters, pneumonia, intraabdominal, and skin and soft tissue. About half of the patients were in severe sepsis or septic shock when treated.
The group was first divided into those who received appropriate or inappropriate therapy (78% vs. 22%). Appropriate therapy was considered to be the early administration of a drug that could effectively target the infective organism. Next, those who got appropriate therapy were parsed by whether they received mono- or combination therapy (61%, 39%). Finally, these patients were stratified by a specially designed mortality risk score, the INCREMENT Carbapenemase-Producing Enterobacteriaceae (CPE) Mortality Score (Mayo Clinic Proceedings. doi.org/10.1016/j.mayocp.2016.06.024).
- Severe sepsis or shock at presentation (5 points)
- Pitt score of 6 or more (4 points)
- Charlson comorbidity index of 2 or more (3 points)
- Source of bloodstream infection other than urinary or biliary tract (3 points)
- Inappropriate empirical therapy and inappropriate early targeted therapy (2 points)
Patients were considered low risk if they had a score of 0-7, and high of they had a score of 8 or more.
The risk assessment took is quick, easy to figure, and extremely important, Dr. Rodríguez-Baño noted. “This is a very easy-to-use tool that can help us make many patient management decisions. All of the information is already available in the patient’s chart, so it doesn’t require any additional assessments. It’s a very good way to individualize treatment.”
In the initial analysis, all-cause mortality at 30 days was 22% lower among patients who received appropriate early therapy than those who did not (38.5% vs. 60.6%). This translated to a 55% decrease in the risk of death (HR 0.45 in the fully adjusted model).
The investigators next turned their attention toward the group that received appropriate therapy. All-cause 30-day mortality was 41% in those who got monotherapy and 34.8% among those who got combination therapy..
Finally, this group was stratified according to the INCREMENT-CPE mortality risk score.
In the low-risk category, combination therapy did not confer a survival advantage over monotherapy. Death occurred in 20% of those getting monotherapy and 24% receiving combination treatment – not a significant difference (HR 1.21).
Combination therapy did, however, confer a significant survival benefit in the high-risk group. Death occurred in 62% of those receiving monotherapy and 48% of those receiving combination therapy – a 44% risk reduction (HR 0.56).
As long as they were appropriately targeted against the infective organism, all drugs used in the high-mortality risk group were similarly effective at reducing the risk of death. Compared to colistin monotherapy, a combination that included tigecycline reduced the risk of death by 55% (HR 0.45); combination with aminoglycosides by 58% (HR 0.42); and combination with carbapenems by 44% (HR 0.56).
A secondary analysis of this group determined that time was a critical factor in survival. Each day delay after day 2 significantly increased the risk of death, Dr. Rodríguez-Baño said. This 48-hour period gives clinicians a chance to wait for the culture and antibiogram to return, and then choose and initiate the best treatment. Before the results come back, empiric antibiotic therapy is appropriate, but changes should be made immediately after the results come back.
“We tend to think we must give the very best antibiotic at the very first moment that we see a patient with a serious infection,” he said. “But what we found is that it’s not critical to give the perfect antibiotic on the first day. It is critical, however, to give the correct one once you know which bacteria is causing the infection. Since it takes 48 hours for those results to come back, this is perfect timing.”
INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
[email protected]
On Twitter @Alz_gal
VIENNA – A single, well-targeted antibiotic may be enough to effectively combat serous bloodstream infections in patients who have a low baseline mortality risk.
Among these patients, overall mortality was similar among those receiving a single antibiotic and those getting multiple antibiotics (35% vs. 41%). Patients with a high baseline mortality risk, however, did experience a significant 44% survival benefit when treated with a combination regimen, Jesus Rodríguez-Baño, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The finding is important when considering the ever-increasing imperative of antibiotic stewardship, Dr. Rodríguez-Baño said in an interview.
“In areas where these pathogens are common, particularly in intensive care units, where they can become epidemic and infect many patients, the overuse of combination therapy will be fueling the problem,” said Dr. Rodríguez-Baño, head of infectious diseases and clinical microbiology at the University Hospital Virgin Macarena, Seville, Spain. “This is a way to avoid the overuse of some broad-spectrum antibiotics. Selecting the patients who should not receive combination therapy may significantly reduce the total consumption” on a unit.
The retrospective study, dubbed INCREMENT, was conducted at 37 hospitals in 10 countries. It enrolled patients with bloodstream infections caused by extended-spectrum beta-lactamase- or carbapenemase-producing Enterobacteriaceae. Dr. Rodríguez-Baño reported results for 437 patients whose infections were caused by the carbapenemase-producing strain.
It was simultaneously published in Lancet Infectious Diseases (Lancet Inf Dis 2017; DOI: http://dx.doi.org/10.1016/S1473-3099(17)30228-1).
These patients were a mean of 66 years old; most (60%) were male. The primary infective agent was Klebsiella pneumonia (86%); most infections were nosocomial. The origin of infections varied, but most (80%) arose from places other than the urinary or biliary tract. Sources were vascular catheters, pneumonia, intraabdominal, and skin and soft tissue. About half of the patients were in severe sepsis or septic shock when treated.
The group was first divided into those who received appropriate or inappropriate therapy (78% vs. 22%). Appropriate therapy was considered to be the early administration of a drug that could effectively target the infective organism. Next, those who got appropriate therapy were parsed by whether they received mono- or combination therapy (61%, 39%). Finally, these patients were stratified by a specially designed mortality risk score, the INCREMENT Carbapenemase-Producing Enterobacteriaceae (CPE) Mortality Score (Mayo Clinic Proceedings. doi.org/10.1016/j.mayocp.2016.06.024).
- Severe sepsis or shock at presentation (5 points)
- Pitt score of 6 or more (4 points)
- Charlson comorbidity index of 2 or more (3 points)
- Source of bloodstream infection other than urinary or biliary tract (3 points)
- Inappropriate empirical therapy and inappropriate early targeted therapy (2 points)
Patients were considered low risk if they had a score of 0-7, and high of they had a score of 8 or more.
The risk assessment took is quick, easy to figure, and extremely important, Dr. Rodríguez-Baño noted. “This is a very easy-to-use tool that can help us make many patient management decisions. All of the information is already available in the patient’s chart, so it doesn’t require any additional assessments. It’s a very good way to individualize treatment.”
In the initial analysis, all-cause mortality at 30 days was 22% lower among patients who received appropriate early therapy than those who did not (38.5% vs. 60.6%). This translated to a 55% decrease in the risk of death (HR 0.45 in the fully adjusted model).
The investigators next turned their attention toward the group that received appropriate therapy. All-cause 30-day mortality was 41% in those who got monotherapy and 34.8% among those who got combination therapy..
Finally, this group was stratified according to the INCREMENT-CPE mortality risk score.
In the low-risk category, combination therapy did not confer a survival advantage over monotherapy. Death occurred in 20% of those getting monotherapy and 24% receiving combination treatment – not a significant difference (HR 1.21).
Combination therapy did, however, confer a significant survival benefit in the high-risk group. Death occurred in 62% of those receiving monotherapy and 48% of those receiving combination therapy – a 44% risk reduction (HR 0.56).
As long as they were appropriately targeted against the infective organism, all drugs used in the high-mortality risk group were similarly effective at reducing the risk of death. Compared to colistin monotherapy, a combination that included tigecycline reduced the risk of death by 55% (HR 0.45); combination with aminoglycosides by 58% (HR 0.42); and combination with carbapenems by 44% (HR 0.56).
A secondary analysis of this group determined that time was a critical factor in survival. Each day delay after day 2 significantly increased the risk of death, Dr. Rodríguez-Baño said. This 48-hour period gives clinicians a chance to wait for the culture and antibiogram to return, and then choose and initiate the best treatment. Before the results come back, empiric antibiotic therapy is appropriate, but changes should be made immediately after the results come back.
“We tend to think we must give the very best antibiotic at the very first moment that we see a patient with a serious infection,” he said. “But what we found is that it’s not critical to give the perfect antibiotic on the first day. It is critical, however, to give the correct one once you know which bacteria is causing the infection. Since it takes 48 hours for those results to come back, this is perfect timing.”
INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
[email protected]
On Twitter @Alz_gal
Key clinical point:
Major finding: Among these patients, correctly targeted monotherapy decreased the risk of death by 44% – just about the same risk reduction as conferred by combination therapy.
Data source: The INCREMENT retrospective study comprised 437 patients.
Disclosures: INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
Plazomicin beats gold standard antibiotics in complex, gram-negative bacterial infections
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
CDC: Some Shigella strains show reduced ciprofloxacin susceptibility
The Centers for Disease Control and Prevention has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
• Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
• Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
• Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
• Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible – with special attention given to the MIC for fluoroquinolone antibiotics.
• Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
• Consult local or state health departments for guidance regarding when patients may return to child care, school, or work.
• Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition; all cases should be reported to the local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
The Centers for Disease Control and Prevention has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
• Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
• Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
• Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
• Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible – with special attention given to the MIC for fluoroquinolone antibiotics.
• Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
• Consult local or state health departments for guidance regarding when patients may return to child care, school, or work.
• Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition; all cases should be reported to the local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
The Centers for Disease Control and Prevention has identified an increase in Shigella isolates with reduced susceptibility to ciprofloxacin, and has released an official health advisory outlining new recommendations for clinical diagnosis, management, and reporting, as well as for laboratories and public health officials.
The Shigella isolates of concern in the United States have minimum inhibitory concentration (MIC) values of 0.12-1 mcg/mL for ciprofloxacin, which is within the range considered susceptible. These strains, however, “often have a quinolone resistance gene that may lead to clinically significant reduced susceptibility to fluoroquinolone antibiotics,” such as ciprofloxacin, according to the CDC advisory.
It is possible that strains with MIC in the 0.12-1 mcg/mL range may have worse clinical outcome or increased risk of transmission, so the CDC made the following recommendations to clinicians:
• Order a stool culture to obtain isolates for antimicrobial susceptibility testing in suspected cases.
• Order antimicrobial susceptibility testing when ordering a stool culture for Shigella.
• Avoid routine prescribing of antibiotic therapy for Shigella infection, instead reserving antibiotics for patients with a clinical indication or when advised by public health officials in an outbreak setting.
• Tailor antibiotic choice (when antibiotics are indicated) to susceptibility results as soon as possible – with special attention given to the MIC for fluoroquinolone antibiotics.
• Obtain follow-up stool cultures in shigellosis patients who have continued or worsening symptoms despite antibiotic therapy.
• Consult local or state health departments for guidance regarding when patients may return to child care, school, or work.
• Counsel patients with active diarrhea on how they can prevent spreading the infection to others, regardless of whether antibiotic treatment is prescribed.
Additionally, the CDC noted that shigellosis is a nationally notifiable condition; all cases should be reported to the local health department. If a patient with shigellosis and a ciprofloxacin MIC of 0.12-1 mcg/mL is identified, this information should be included in the report to facilitate further testing of the isolate.
The CDC reported that it is working with state and local public health departments and clinical partners to determine if outcomes are indeed worse for patients treated with ciprofloxacin for Shigella strains harboring a quinolone resistance gene, and it will continue to monitor trends in susceptibility of Shigella isolates and to perform genetic testing on select strains to confirm the presence and type of resistance genes.
Sneak Peak: The Hospital Leader Blog “The Impact of Hospital Design on Health – for Patients AND Providers”
I was rounding on the inpatient general medicine teaching service last weekend and offered to meet my team of students and residents in the “resident library” on Saturday morning. (Although it holds the name “library,” there were no books or periodicals to be seen.) I had not been in the library for many months and was struck by a few things as I entered.
It is a dimly lit space, lined on three of the four walls with rickety desks and desktop computers all facing the walls. The walls are painted an off-white color with innumerable dings and nicks, presumably accumulated over the course of years. There was a string of garland in the shape of a Christmas tree pinned to the wall (P.S. It is March), the entire left side of which was sagging and misshapen. There were various tattered and coffee-stained papers scattered haphazardly throughout the room, including what appeared to be progress notes and test results printed from the EHR; a few worn ECGs; a telemetry strip; even a few (REALLY old, no doubt) chest x-ray films. Lining the fourth wall was a large foldable table, topped with crumbs and food scraps, a half-eaten chocolate Bundt cake, and scattered napkins and utensils, some of which appeared to be used. The one exterior-facing wall had a row of windows with crinkled blinds, some completely closed, others opened at awkward angles and seemingly stuck in place. There was a cadre of chairs in the room, none matching, all in various stages of disrepair, with one completely missing an armrest and another tucked in the corner, probably needing the addition of a handwritten sign “BRokEn.”
This library is a place where the students, interns, and residents go for a bit of a safe haven. They can take their coats off, sit down, have their own computer space, answer pages, and complain about their woes. They can bounce questions off each other, vent frustrations, find the humor in a situation, and just be themselves. So,But what struck me about their sanctuary is that it is totally and utterly depressing. And it was as if they didn’t even notice the chaos and filth laying everywhere around them. I find it impossible to believe that it does not have an effect on their mood and outlook. Although we are all social animals, and we have a real need to congregate and connect with one another, is this really the best environment to do that?
Read the full text of this blog post at hospitalleader.org.
Dr. Scheurer is a clinical hospitalist and the medical director of quality and safety at the Medical University of South Carolina in Charleston.
Also on The Hospital Leader…
- Should Overuse Be Considered an Adverse Event By Chris Moriates, MD
- Pulling the Welcome Mat Out from Under Our Colleagues By Brett Hendel-Paterson, MD
- The Upside of Anger By Tracy Cardin, ACNP-BC, SFHM
- Does Your Onboarding Process Really Get Folks on Board? By Leslie Flores, MH, SFHM
- A GIF Is Worth 3000 Words: Introducing #Visual Abstract for #JHMChat By Charlie Wray, DO
I was rounding on the inpatient general medicine teaching service last weekend and offered to meet my team of students and residents in the “resident library” on Saturday morning. (Although it holds the name “library,” there were no books or periodicals to be seen.) I had not been in the library for many months and was struck by a few things as I entered.
It is a dimly lit space, lined on three of the four walls with rickety desks and desktop computers all facing the walls. The walls are painted an off-white color with innumerable dings and nicks, presumably accumulated over the course of years. There was a string of garland in the shape of a Christmas tree pinned to the wall (P.S. It is March), the entire left side of which was sagging and misshapen. There were various tattered and coffee-stained papers scattered haphazardly throughout the room, including what appeared to be progress notes and test results printed from the EHR; a few worn ECGs; a telemetry strip; even a few (REALLY old, no doubt) chest x-ray films. Lining the fourth wall was a large foldable table, topped with crumbs and food scraps, a half-eaten chocolate Bundt cake, and scattered napkins and utensils, some of which appeared to be used. The one exterior-facing wall had a row of windows with crinkled blinds, some completely closed, others opened at awkward angles and seemingly stuck in place. There was a cadre of chairs in the room, none matching, all in various stages of disrepair, with one completely missing an armrest and another tucked in the corner, probably needing the addition of a handwritten sign “BRokEn.”
This library is a place where the students, interns, and residents go for a bit of a safe haven. They can take their coats off, sit down, have their own computer space, answer pages, and complain about their woes. They can bounce questions off each other, vent frustrations, find the humor in a situation, and just be themselves. So,But what struck me about their sanctuary is that it is totally and utterly depressing. And it was as if they didn’t even notice the chaos and filth laying everywhere around them. I find it impossible to believe that it does not have an effect on their mood and outlook. Although we are all social animals, and we have a real need to congregate and connect with one another, is this really the best environment to do that?
Read the full text of this blog post at hospitalleader.org.
Dr. Scheurer is a clinical hospitalist and the medical director of quality and safety at the Medical University of South Carolina in Charleston.
Also on The Hospital Leader…
- Should Overuse Be Considered an Adverse Event By Chris Moriates, MD
- Pulling the Welcome Mat Out from Under Our Colleagues By Brett Hendel-Paterson, MD
- The Upside of Anger By Tracy Cardin, ACNP-BC, SFHM
- Does Your Onboarding Process Really Get Folks on Board? By Leslie Flores, MH, SFHM
- A GIF Is Worth 3000 Words: Introducing #Visual Abstract for #JHMChat By Charlie Wray, DO
I was rounding on the inpatient general medicine teaching service last weekend and offered to meet my team of students and residents in the “resident library” on Saturday morning. (Although it holds the name “library,” there were no books or periodicals to be seen.) I had not been in the library for many months and was struck by a few things as I entered.
It is a dimly lit space, lined on three of the four walls with rickety desks and desktop computers all facing the walls. The walls are painted an off-white color with innumerable dings and nicks, presumably accumulated over the course of years. There was a string of garland in the shape of a Christmas tree pinned to the wall (P.S. It is March), the entire left side of which was sagging and misshapen. There were various tattered and coffee-stained papers scattered haphazardly throughout the room, including what appeared to be progress notes and test results printed from the EHR; a few worn ECGs; a telemetry strip; even a few (REALLY old, no doubt) chest x-ray films. Lining the fourth wall was a large foldable table, topped with crumbs and food scraps, a half-eaten chocolate Bundt cake, and scattered napkins and utensils, some of which appeared to be used. The one exterior-facing wall had a row of windows with crinkled blinds, some completely closed, others opened at awkward angles and seemingly stuck in place. There was a cadre of chairs in the room, none matching, all in various stages of disrepair, with one completely missing an armrest and another tucked in the corner, probably needing the addition of a handwritten sign “BRokEn.”
This library is a place where the students, interns, and residents go for a bit of a safe haven. They can take their coats off, sit down, have their own computer space, answer pages, and complain about their woes. They can bounce questions off each other, vent frustrations, find the humor in a situation, and just be themselves. So,But what struck me about their sanctuary is that it is totally and utterly depressing. And it was as if they didn’t even notice the chaos and filth laying everywhere around them. I find it impossible to believe that it does not have an effect on their mood and outlook. Although we are all social animals, and we have a real need to congregate and connect with one another, is this really the best environment to do that?
Read the full text of this blog post at hospitalleader.org.
Dr. Scheurer is a clinical hospitalist and the medical director of quality and safety at the Medical University of South Carolina in Charleston.
Also on The Hospital Leader…
- Should Overuse Be Considered an Adverse Event By Chris Moriates, MD
- Pulling the Welcome Mat Out from Under Our Colleagues By Brett Hendel-Paterson, MD
- The Upside of Anger By Tracy Cardin, ACNP-BC, SFHM
- Does Your Onboarding Process Really Get Folks on Board? By Leslie Flores, MH, SFHM
- A GIF Is Worth 3000 Words: Introducing #Visual Abstract for #JHMChat By Charlie Wray, DO
Procalcitonin guidance improves antibiotic stewardship
The case
A 72-year-old male with COPD presents to the emergency department with increased dyspnea and cough. He is afebrile, and oxygen saturation is 87% on room air. WBC count is 9.5 with a normal differential, and chest x-ray is read by the radiologist as atelectasis versus early consolidation in the left lower lobe. Should antibiotics be initiated?
Background
The problem: Antibiotic overuse
With the increasing prevalence of antibiotic resistance in our nation’s hospitals, the need for robust antibiotic stewardship programs has continued to rise in importance. In 2016, the CDC reported a fatal case of septic shock due to a carbapenem-resistant strain of Klebsiella resistant to all tested antibiotics.1 This case received much media coverage; moreover, this patient represented only one of the approximately 23,000 patients infected with antibiotic-resistant bacteria in the United States who die each year. Although various approaches to curbing antibiotic resistance are being pursued, judicious antibiotic use is central to success. Current evidence suggests that up to 30% of antibiotics are not optimally prescribed,2 leaving a significant opportunity for improvement.
Lower respiratory infections account for a substantial proportion of antibiotic utilization in the United States. In a recent study, acute respiratory conditions generated 221 antibiotic prescriptions per 1,000 population, but only half of these were deemed appropriate.2 The inability to reliably discern viral from bacterial etiology is a driver of excess antibiotic use.
The procalcitonin assay has been touted as a possible solution to this problem. Multiple studies have evaluated its utility as a tool to help discriminate between bacterial infection and viral or noninfectious etiologies.
What is procalcitonin?
Thyroidal c-cells convert the prohormone procalcitonin to calcitonin, which is stored in secretory granules for release in response to fluctuations in calcium levels via a classical neuroendocrine feedback loop. Alternatively, procalcitonin can be synthesized in nonthyroidal parenchymal cells, and high levels of proinflammatory mediators secreted in response to bacterial endotoxin drive increased procalcitonin production. Interestingly, interferon gamma, up-regulated in viral infections, reduces procalcitonin production. Nonthyroidal parenchymal cells lack mechanisms for efficient conversion of procalcitonin to calcitonin and do not contain secretory granules to facilitate its regulated release. Hence bacterial infections correlate with higher serum procalcitonin levels.3
Evidence
Can procalcitonin guide antibiotic therapy in patients with acute respiratory illness while reducing antibiotic utilization?
The ability of procalcitonin to selectively identify bacterial infection makes it a potentially promising tool to advance the antibiotic stewardship agenda. Multiple randomized controlled trials have explored the use of procalcitonin-guided antibiotic therapy for treatment of lower respiratory tract infections such as acute bronchitis, exacerbations of COPD, and pneumonia. Each study discussed below was done in Switzerland, involved the same key investigator (Mirjam Christ-Crain, MD, PhD), and shared a similar design in which a threshold for low procalcitonin values (less than 0.1 mcg/L) and high procalcitonin values (greater than 0.25 mcg/L) was prespecified. Antibiotic therapy was strongly discouraged for patients with low procalcitonin and encouraged for those with high procalcitonin; antibiotics were not recommended for patients with intermediate values, but the treating physician was allowed ultimate discretion (Figure 1). All studies compared a procalcitonin-guided treatment group to a standard care group, in which antibiotics were prescribed by the treating physician based on established clinical guidelines.
Figure 1. Procalcitonin treatment algorithm
Procalcitonin Level (mcg/L) | Likelihood of bacterial infection | Antibiotic treatment |
less than 0.1 | Absent | Strongly discouraged |
0.1-0.25 | Unlikely | Discouraged |
0.25-0.5 | Possible | Encouraged |
greater than 0.5 | Present | Strongly encouraged |
Figure 1. Procalcitonin treatment algorithm
In a study focusing on outpatients presenting to their primary care physicians with acute respiratory tract infection, 53 primary care physicians in Switzerland recruited 458 patients. There was no significant difference in time to symptom resolution, as determined by patient report during an interview 14 days after initial presentation; however, 97% of patients in the standard-care group received antibiotics, compared with 25% in the procalcitonin-guided group. Equal numbers of patients (30% in each group) reported persistent symptoms at 28-day follow-up. Among the cohort of patients with upper respiratory infections or acute bronchitis, procalcitonin guidance reduced antibiotic prescriptions by 80%.4
In a blinded, single-center, randomized, controlled trial of 226 patients presenting to a university hospital with a COPD exacerbation severe enough to require a change in the baseline medication regimen, procalcitonin-guided therapy allowed for an absolute reduction of antibiotic use by 32% without an impact on outcomes. Rates of clinical improvement, ICU utilization, recurrent exacerbations, hospital length of stay, and mortality did not differ between the groups.5
Limitations include the possible impact of the Hawthorne effect, as physicians knew their antibiotic usage patterns were being monitored, which may impact generalizability of the findings to a real-world setting. Similarly, it is difficult to control for a spillover effect as providers exposed to the procalcitonin-guided algorithm became more comfortable with a restrictive prescribing approach. The costs of the additional procalcitonin assay must be weighed against the benefits. Incidence and cost of other adverse effects of antibiotic use (rates of Clostridium difficile, renal insufficiency, urticarial drug eruptions, etc.) were not addressed. The rapid assay currently has limited availability in the United States, though that is changing. Finally, recent additional studies (unrelated to procalcitonin) have suggested shorter antibiotic treatment durations for patients with pneumonia.8
Is there evidence for using procalcitonin to guide treatment in the broader population of ICU patients?
While there is good evidence for using procalcitonin to guide antibiotic use in patients with acute respiratory illness, the evidence for using procalcitonin in the broader cohort of critically-ill patients with sepsis is less well established.
The most promising results were reported by the Stop Antibiotics on Procalcitonin guidance Study (SAPS). Published in July 2016, this was a prospective, multicenter, randomized, controlled, open-label study of patients admitted to the ICU (not limited to respiratory illness) in the Netherlands. A total of 1,575 patients were assigned to the procalcitonin-guided group or the standard-of-care group. In the procalcitonin-guided group, procalcitonin levels were checked daily, and physicians were given nonbinding advice to discontinue antibiotics if procalcitonin levels decreased by greater than 80% from peak levels or to below 0.5 mcg/L.
Patients received an average of 7.5 daily defined antibiotic doses in the procalcitonin-guided group versus 9.3 daily defined doses in the standard-of-care group (P less than .0001). The median duration of antibiotic treatment in the procalcitonin arm was 5 days versus 7 days in the control group (P less than .0001). Mortality at 28 days was 20% in the procalcitonin group and 25% in the control group (P = .0122). At 1 year, mortality was 36% in the procalcitonin group and 43% in the control group (P = .0188). The authors hypothesized that the unexpected decrease in mortality in the procalcitonin group may have been due to earlier consideration of alternate illness etiologies in patients with a low procalcitonin level or decreased antibiotic side effects.9While the SAPS trial supports decreased antibiotic usage in ICU patients with the use of the procalcitonin assay, there are some important limitations. First, the trial was done in the Netherlands, where baseline antibiotic usage was comparatively low. Second, daily procalcitonin level monitoring was not continued for patients transferred out of the ICU while still on antibiotics. Further, guidelines for antibiotic discontinuation were nonbinding, and in many cases physicians did not stop antibiotics based on procalcitonin guidelines suggested by the study authors.
Earlier trials regarding the procalcitonin assay in the critical care setting similarly showed some promise but also concerns. One trial reported a 25% reduction in antibiotic exposure and noninferiority of 28-day mortality, but there was a nonsignificant 3.8% absolute increase in mortality at 60 days.10 Another trial reported similar survival in the procalcitonin group but more side effects and longer ICU stays.11Ultimately, while the SAPS trial supports the potential use of procalcitonin in critically-ill patients, these patients likely have complex sepsis physiology that requires clinicians to consider a number of clinical factors when making antibiotic decisions.
Back to the case
The case illustrates a common emergency department presentation where clinical and radiographic features are not convincing for bacterial infection. This patient has an acute respiratory illness, but is afebrile and lacks leukocytosis with left shift, and x-rays are indeterminate for pneumonia. The differential diagnosis also includes COPD exacerbation, viral infection, or noninfectious triggers of dyspnea.
In this scenario, obtaining procalcitonin levels is useful in the decision to initiate or withhold antibiotic treatment. An elevated procalcitonin level suggests a bacterial infection and would favor initiation of antibiotics for pneumonia. A low procalcitonin level makes a bacterial infection less likely, and a clinician may consider withholding antibiotics and consider alternate etiologies for the patient’s presentation.
Bottom line
Procalcitonin can be safely used to guide the decision to initiate antibiotics in patients presenting with acute respiratory illness. Use of the procalcitonin assay has been shown to reduce antibiotic utilization without an increase in adverse outcomes. There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
Bryan J. Huang, MD, FHM, and Gregory B. Seymann, MD, SFHM, are in the division of hospital medicine, University of California, San Diego.
- Key Points
- Elevated procalcitonin levels suggest the presence of bacterial infection.
- In patients presenting with acute respiratory illness, procalcitonin levels can be used to guide the decision to initiate or withhold antibiotics, improving antibiotic stewardship.
- Sequential monitoring of procalcitonin levels may help guide duration of antibiotic therapy.
- There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
References
1. Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae – Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(1):33.
2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315(17):1864-73.
3. Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31-32):451-60.
4. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008; 168(18): 2000-7.
5. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131(1): 9-19.
6. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93.
7. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10): 1059-66.
8. Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-65.
9. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
10. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463-74.
11. Jensen J-U, Lundgren B, Hein L, et al. The procalcitonin and survival study (PASS) – a randomised multicenter investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and proactive diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. BMC infectious diseases. 2008;8:91-100.
1. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059-66.
2. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
3. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
The case
A 72-year-old male with COPD presents to the emergency department with increased dyspnea and cough. He is afebrile, and oxygen saturation is 87% on room air. WBC count is 9.5 with a normal differential, and chest x-ray is read by the radiologist as atelectasis versus early consolidation in the left lower lobe. Should antibiotics be initiated?
Background
The problem: Antibiotic overuse
With the increasing prevalence of antibiotic resistance in our nation’s hospitals, the need for robust antibiotic stewardship programs has continued to rise in importance. In 2016, the CDC reported a fatal case of septic shock due to a carbapenem-resistant strain of Klebsiella resistant to all tested antibiotics.1 This case received much media coverage; moreover, this patient represented only one of the approximately 23,000 patients infected with antibiotic-resistant bacteria in the United States who die each year. Although various approaches to curbing antibiotic resistance are being pursued, judicious antibiotic use is central to success. Current evidence suggests that up to 30% of antibiotics are not optimally prescribed,2 leaving a significant opportunity for improvement.
Lower respiratory infections account for a substantial proportion of antibiotic utilization in the United States. In a recent study, acute respiratory conditions generated 221 antibiotic prescriptions per 1,000 population, but only half of these were deemed appropriate.2 The inability to reliably discern viral from bacterial etiology is a driver of excess antibiotic use.
The procalcitonin assay has been touted as a possible solution to this problem. Multiple studies have evaluated its utility as a tool to help discriminate between bacterial infection and viral or noninfectious etiologies.
What is procalcitonin?
Thyroidal c-cells convert the prohormone procalcitonin to calcitonin, which is stored in secretory granules for release in response to fluctuations in calcium levels via a classical neuroendocrine feedback loop. Alternatively, procalcitonin can be synthesized in nonthyroidal parenchymal cells, and high levels of proinflammatory mediators secreted in response to bacterial endotoxin drive increased procalcitonin production. Interestingly, interferon gamma, up-regulated in viral infections, reduces procalcitonin production. Nonthyroidal parenchymal cells lack mechanisms for efficient conversion of procalcitonin to calcitonin and do not contain secretory granules to facilitate its regulated release. Hence bacterial infections correlate with higher serum procalcitonin levels.3
Evidence
Can procalcitonin guide antibiotic therapy in patients with acute respiratory illness while reducing antibiotic utilization?
The ability of procalcitonin to selectively identify bacterial infection makes it a potentially promising tool to advance the antibiotic stewardship agenda. Multiple randomized controlled trials have explored the use of procalcitonin-guided antibiotic therapy for treatment of lower respiratory tract infections such as acute bronchitis, exacerbations of COPD, and pneumonia. Each study discussed below was done in Switzerland, involved the same key investigator (Mirjam Christ-Crain, MD, PhD), and shared a similar design in which a threshold for low procalcitonin values (less than 0.1 mcg/L) and high procalcitonin values (greater than 0.25 mcg/L) was prespecified. Antibiotic therapy was strongly discouraged for patients with low procalcitonin and encouraged for those with high procalcitonin; antibiotics were not recommended for patients with intermediate values, but the treating physician was allowed ultimate discretion (Figure 1). All studies compared a procalcitonin-guided treatment group to a standard care group, in which antibiotics were prescribed by the treating physician based on established clinical guidelines.
Figure 1. Procalcitonin treatment algorithm
Procalcitonin Level (mcg/L) | Likelihood of bacterial infection | Antibiotic treatment |
less than 0.1 | Absent | Strongly discouraged |
0.1-0.25 | Unlikely | Discouraged |
0.25-0.5 | Possible | Encouraged |
greater than 0.5 | Present | Strongly encouraged |
Figure 1. Procalcitonin treatment algorithm
In a study focusing on outpatients presenting to their primary care physicians with acute respiratory tract infection, 53 primary care physicians in Switzerland recruited 458 patients. There was no significant difference in time to symptom resolution, as determined by patient report during an interview 14 days after initial presentation; however, 97% of patients in the standard-care group received antibiotics, compared with 25% in the procalcitonin-guided group. Equal numbers of patients (30% in each group) reported persistent symptoms at 28-day follow-up. Among the cohort of patients with upper respiratory infections or acute bronchitis, procalcitonin guidance reduced antibiotic prescriptions by 80%.4
In a blinded, single-center, randomized, controlled trial of 226 patients presenting to a university hospital with a COPD exacerbation severe enough to require a change in the baseline medication regimen, procalcitonin-guided therapy allowed for an absolute reduction of antibiotic use by 32% without an impact on outcomes. Rates of clinical improvement, ICU utilization, recurrent exacerbations, hospital length of stay, and mortality did not differ between the groups.5
Limitations include the possible impact of the Hawthorne effect, as physicians knew their antibiotic usage patterns were being monitored, which may impact generalizability of the findings to a real-world setting. Similarly, it is difficult to control for a spillover effect as providers exposed to the procalcitonin-guided algorithm became more comfortable with a restrictive prescribing approach. The costs of the additional procalcitonin assay must be weighed against the benefits. Incidence and cost of other adverse effects of antibiotic use (rates of Clostridium difficile, renal insufficiency, urticarial drug eruptions, etc.) were not addressed. The rapid assay currently has limited availability in the United States, though that is changing. Finally, recent additional studies (unrelated to procalcitonin) have suggested shorter antibiotic treatment durations for patients with pneumonia.8
Is there evidence for using procalcitonin to guide treatment in the broader population of ICU patients?
While there is good evidence for using procalcitonin to guide antibiotic use in patients with acute respiratory illness, the evidence for using procalcitonin in the broader cohort of critically-ill patients with sepsis is less well established.
The most promising results were reported by the Stop Antibiotics on Procalcitonin guidance Study (SAPS). Published in July 2016, this was a prospective, multicenter, randomized, controlled, open-label study of patients admitted to the ICU (not limited to respiratory illness) in the Netherlands. A total of 1,575 patients were assigned to the procalcitonin-guided group or the standard-of-care group. In the procalcitonin-guided group, procalcitonin levels were checked daily, and physicians were given nonbinding advice to discontinue antibiotics if procalcitonin levels decreased by greater than 80% from peak levels or to below 0.5 mcg/L.
Patients received an average of 7.5 daily defined antibiotic doses in the procalcitonin-guided group versus 9.3 daily defined doses in the standard-of-care group (P less than .0001). The median duration of antibiotic treatment in the procalcitonin arm was 5 days versus 7 days in the control group (P less than .0001). Mortality at 28 days was 20% in the procalcitonin group and 25% in the control group (P = .0122). At 1 year, mortality was 36% in the procalcitonin group and 43% in the control group (P = .0188). The authors hypothesized that the unexpected decrease in mortality in the procalcitonin group may have been due to earlier consideration of alternate illness etiologies in patients with a low procalcitonin level or decreased antibiotic side effects.9While the SAPS trial supports decreased antibiotic usage in ICU patients with the use of the procalcitonin assay, there are some important limitations. First, the trial was done in the Netherlands, where baseline antibiotic usage was comparatively low. Second, daily procalcitonin level monitoring was not continued for patients transferred out of the ICU while still on antibiotics. Further, guidelines for antibiotic discontinuation were nonbinding, and in many cases physicians did not stop antibiotics based on procalcitonin guidelines suggested by the study authors.
Earlier trials regarding the procalcitonin assay in the critical care setting similarly showed some promise but also concerns. One trial reported a 25% reduction in antibiotic exposure and noninferiority of 28-day mortality, but there was a nonsignificant 3.8% absolute increase in mortality at 60 days.10 Another trial reported similar survival in the procalcitonin group but more side effects and longer ICU stays.11Ultimately, while the SAPS trial supports the potential use of procalcitonin in critically-ill patients, these patients likely have complex sepsis physiology that requires clinicians to consider a number of clinical factors when making antibiotic decisions.
Back to the case
The case illustrates a common emergency department presentation where clinical and radiographic features are not convincing for bacterial infection. This patient has an acute respiratory illness, but is afebrile and lacks leukocytosis with left shift, and x-rays are indeterminate for pneumonia. The differential diagnosis also includes COPD exacerbation, viral infection, or noninfectious triggers of dyspnea.
In this scenario, obtaining procalcitonin levels is useful in the decision to initiate or withhold antibiotic treatment. An elevated procalcitonin level suggests a bacterial infection and would favor initiation of antibiotics for pneumonia. A low procalcitonin level makes a bacterial infection less likely, and a clinician may consider withholding antibiotics and consider alternate etiologies for the patient’s presentation.
Bottom line
Procalcitonin can be safely used to guide the decision to initiate antibiotics in patients presenting with acute respiratory illness. Use of the procalcitonin assay has been shown to reduce antibiotic utilization without an increase in adverse outcomes. There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
Bryan J. Huang, MD, FHM, and Gregory B. Seymann, MD, SFHM, are in the division of hospital medicine, University of California, San Diego.
- Key Points
- Elevated procalcitonin levels suggest the presence of bacterial infection.
- In patients presenting with acute respiratory illness, procalcitonin levels can be used to guide the decision to initiate or withhold antibiotics, improving antibiotic stewardship.
- Sequential monitoring of procalcitonin levels may help guide duration of antibiotic therapy.
- There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
References
1. Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae – Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(1):33.
2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315(17):1864-73.
3. Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31-32):451-60.
4. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008; 168(18): 2000-7.
5. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131(1): 9-19.
6. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93.
7. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10): 1059-66.
8. Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-65.
9. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
10. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463-74.
11. Jensen J-U, Lundgren B, Hein L, et al. The procalcitonin and survival study (PASS) – a randomised multicenter investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and proactive diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. BMC infectious diseases. 2008;8:91-100.
1. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059-66.
2. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
3. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
The case
A 72-year-old male with COPD presents to the emergency department with increased dyspnea and cough. He is afebrile, and oxygen saturation is 87% on room air. WBC count is 9.5 with a normal differential, and chest x-ray is read by the radiologist as atelectasis versus early consolidation in the left lower lobe. Should antibiotics be initiated?
Background
The problem: Antibiotic overuse
With the increasing prevalence of antibiotic resistance in our nation’s hospitals, the need for robust antibiotic stewardship programs has continued to rise in importance. In 2016, the CDC reported a fatal case of septic shock due to a carbapenem-resistant strain of Klebsiella resistant to all tested antibiotics.1 This case received much media coverage; moreover, this patient represented only one of the approximately 23,000 patients infected with antibiotic-resistant bacteria in the United States who die each year. Although various approaches to curbing antibiotic resistance are being pursued, judicious antibiotic use is central to success. Current evidence suggests that up to 30% of antibiotics are not optimally prescribed,2 leaving a significant opportunity for improvement.
Lower respiratory infections account for a substantial proportion of antibiotic utilization in the United States. In a recent study, acute respiratory conditions generated 221 antibiotic prescriptions per 1,000 population, but only half of these were deemed appropriate.2 The inability to reliably discern viral from bacterial etiology is a driver of excess antibiotic use.
The procalcitonin assay has been touted as a possible solution to this problem. Multiple studies have evaluated its utility as a tool to help discriminate between bacterial infection and viral or noninfectious etiologies.
What is procalcitonin?
Thyroidal c-cells convert the prohormone procalcitonin to calcitonin, which is stored in secretory granules for release in response to fluctuations in calcium levels via a classical neuroendocrine feedback loop. Alternatively, procalcitonin can be synthesized in nonthyroidal parenchymal cells, and high levels of proinflammatory mediators secreted in response to bacterial endotoxin drive increased procalcitonin production. Interestingly, interferon gamma, up-regulated in viral infections, reduces procalcitonin production. Nonthyroidal parenchymal cells lack mechanisms for efficient conversion of procalcitonin to calcitonin and do not contain secretory granules to facilitate its regulated release. Hence bacterial infections correlate with higher serum procalcitonin levels.3
Evidence
Can procalcitonin guide antibiotic therapy in patients with acute respiratory illness while reducing antibiotic utilization?
The ability of procalcitonin to selectively identify bacterial infection makes it a potentially promising tool to advance the antibiotic stewardship agenda. Multiple randomized controlled trials have explored the use of procalcitonin-guided antibiotic therapy for treatment of lower respiratory tract infections such as acute bronchitis, exacerbations of COPD, and pneumonia. Each study discussed below was done in Switzerland, involved the same key investigator (Mirjam Christ-Crain, MD, PhD), and shared a similar design in which a threshold for low procalcitonin values (less than 0.1 mcg/L) and high procalcitonin values (greater than 0.25 mcg/L) was prespecified. Antibiotic therapy was strongly discouraged for patients with low procalcitonin and encouraged for those with high procalcitonin; antibiotics were not recommended for patients with intermediate values, but the treating physician was allowed ultimate discretion (Figure 1). All studies compared a procalcitonin-guided treatment group to a standard care group, in which antibiotics were prescribed by the treating physician based on established clinical guidelines.
Figure 1. Procalcitonin treatment algorithm
Procalcitonin Level (mcg/L) | Likelihood of bacterial infection | Antibiotic treatment |
less than 0.1 | Absent | Strongly discouraged |
0.1-0.25 | Unlikely | Discouraged |
0.25-0.5 | Possible | Encouraged |
greater than 0.5 | Present | Strongly encouraged |
Figure 1. Procalcitonin treatment algorithm
In a study focusing on outpatients presenting to their primary care physicians with acute respiratory tract infection, 53 primary care physicians in Switzerland recruited 458 patients. There was no significant difference in time to symptom resolution, as determined by patient report during an interview 14 days after initial presentation; however, 97% of patients in the standard-care group received antibiotics, compared with 25% in the procalcitonin-guided group. Equal numbers of patients (30% in each group) reported persistent symptoms at 28-day follow-up. Among the cohort of patients with upper respiratory infections or acute bronchitis, procalcitonin guidance reduced antibiotic prescriptions by 80%.4
In a blinded, single-center, randomized, controlled trial of 226 patients presenting to a university hospital with a COPD exacerbation severe enough to require a change in the baseline medication regimen, procalcitonin-guided therapy allowed for an absolute reduction of antibiotic use by 32% without an impact on outcomes. Rates of clinical improvement, ICU utilization, recurrent exacerbations, hospital length of stay, and mortality did not differ between the groups.5
Limitations include the possible impact of the Hawthorne effect, as physicians knew their antibiotic usage patterns were being monitored, which may impact generalizability of the findings to a real-world setting. Similarly, it is difficult to control for a spillover effect as providers exposed to the procalcitonin-guided algorithm became more comfortable with a restrictive prescribing approach. The costs of the additional procalcitonin assay must be weighed against the benefits. Incidence and cost of other adverse effects of antibiotic use (rates of Clostridium difficile, renal insufficiency, urticarial drug eruptions, etc.) were not addressed. The rapid assay currently has limited availability in the United States, though that is changing. Finally, recent additional studies (unrelated to procalcitonin) have suggested shorter antibiotic treatment durations for patients with pneumonia.8
Is there evidence for using procalcitonin to guide treatment in the broader population of ICU patients?
While there is good evidence for using procalcitonin to guide antibiotic use in patients with acute respiratory illness, the evidence for using procalcitonin in the broader cohort of critically-ill patients with sepsis is less well established.
The most promising results were reported by the Stop Antibiotics on Procalcitonin guidance Study (SAPS). Published in July 2016, this was a prospective, multicenter, randomized, controlled, open-label study of patients admitted to the ICU (not limited to respiratory illness) in the Netherlands. A total of 1,575 patients were assigned to the procalcitonin-guided group or the standard-of-care group. In the procalcitonin-guided group, procalcitonin levels were checked daily, and physicians were given nonbinding advice to discontinue antibiotics if procalcitonin levels decreased by greater than 80% from peak levels or to below 0.5 mcg/L.
Patients received an average of 7.5 daily defined antibiotic doses in the procalcitonin-guided group versus 9.3 daily defined doses in the standard-of-care group (P less than .0001). The median duration of antibiotic treatment in the procalcitonin arm was 5 days versus 7 days in the control group (P less than .0001). Mortality at 28 days was 20% in the procalcitonin group and 25% in the control group (P = .0122). At 1 year, mortality was 36% in the procalcitonin group and 43% in the control group (P = .0188). The authors hypothesized that the unexpected decrease in mortality in the procalcitonin group may have been due to earlier consideration of alternate illness etiologies in patients with a low procalcitonin level or decreased antibiotic side effects.9While the SAPS trial supports decreased antibiotic usage in ICU patients with the use of the procalcitonin assay, there are some important limitations. First, the trial was done in the Netherlands, where baseline antibiotic usage was comparatively low. Second, daily procalcitonin level monitoring was not continued for patients transferred out of the ICU while still on antibiotics. Further, guidelines for antibiotic discontinuation were nonbinding, and in many cases physicians did not stop antibiotics based on procalcitonin guidelines suggested by the study authors.
Earlier trials regarding the procalcitonin assay in the critical care setting similarly showed some promise but also concerns. One trial reported a 25% reduction in antibiotic exposure and noninferiority of 28-day mortality, but there was a nonsignificant 3.8% absolute increase in mortality at 60 days.10 Another trial reported similar survival in the procalcitonin group but more side effects and longer ICU stays.11Ultimately, while the SAPS trial supports the potential use of procalcitonin in critically-ill patients, these patients likely have complex sepsis physiology that requires clinicians to consider a number of clinical factors when making antibiotic decisions.
Back to the case
The case illustrates a common emergency department presentation where clinical and radiographic features are not convincing for bacterial infection. This patient has an acute respiratory illness, but is afebrile and lacks leukocytosis with left shift, and x-rays are indeterminate for pneumonia. The differential diagnosis also includes COPD exacerbation, viral infection, or noninfectious triggers of dyspnea.
In this scenario, obtaining procalcitonin levels is useful in the decision to initiate or withhold antibiotic treatment. An elevated procalcitonin level suggests a bacterial infection and would favor initiation of antibiotics for pneumonia. A low procalcitonin level makes a bacterial infection less likely, and a clinician may consider withholding antibiotics and consider alternate etiologies for the patient’s presentation.
Bottom line
Procalcitonin can be safely used to guide the decision to initiate antibiotics in patients presenting with acute respiratory illness. Use of the procalcitonin assay has been shown to reduce antibiotic utilization without an increase in adverse outcomes. There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
Bryan J. Huang, MD, FHM, and Gregory B. Seymann, MD, SFHM, are in the division of hospital medicine, University of California, San Diego.
- Key Points
- Elevated procalcitonin levels suggest the presence of bacterial infection.
- In patients presenting with acute respiratory illness, procalcitonin levels can be used to guide the decision to initiate or withhold antibiotics, improving antibiotic stewardship.
- Sequential monitoring of procalcitonin levels may help guide duration of antibiotic therapy.
- There is potential but less conclusive evidence for procalcitonin usage in the broader population of ICU patients with sepsis.
References
1. Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae – Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(1):33.
2. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 2016;315(17):1864-73.
3. Christ-Crain M, Muller B. Procalcitonin in bacterial infections – hype, hope, more or less? Swiss Med Wkly. 2005;135(31-32):451-60.
4. Briel M, Schuetz P, Mueller B, et al. Procalcitonin-guided antibiotic use vs. a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008; 168(18): 2000-7.
5. Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest 2007;131(1): 9-19.
6. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93.
7. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302(10): 1059-66.
8. Uranga A, Espana PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-65.
9. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
10. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463-74.
11. Jensen J-U, Lundgren B, Hein L, et al. The procalcitonin and survival study (PASS) – a randomised multicenter investigator-initiated trial to investigate whether daily measurements biomarker procalcitonin and proactive diagnostic and therapeutic responses to abnormal procalcitonin levels, can improve survival in intensive care unit patients. BMC infectious diseases. 2008;8:91-100.
1. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059-66.
2. de Jong E, van Oers JA, Beishiozen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-27.
3. Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012;(9):CD007498.
Cutting back ICU antibiotics could significantly reduce MDRO transmissions
Cutting back on antibiotic courses in intensive care unit settings can significantly reduce the number of multidrug-resistant organism (MDRO) transmissions, according to the findings of a modeling study.
“Significant opportunities exist to optimize and reduce antibiotic usage, [but] the impact of reducing overall antibiotic usage on antibiotic resistance is not known and would be difficult to assess using traditional study designs,” wrote Sean L. Barnes, PhD, of the University of Maryland, College Park, and his colleagues. “Therefore, we applied mathematical modeling to estimate the effect of reducing antibiotic usage on antibiotic resistance.”
Using an agent-based model – which allows for a realistic prediction of interactions between patients and health care workers, while also allowing for heterogeneity in the characteristics of each distinct “person” – Dr. Barnes and his coinvestigators simulated the transmission of MDROs from health care workers to patients.
Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci were deemed “high-prevalence pathogens;” carbapenem-resistant Enterobacteriaceae, multidrug-resistant Acinetobacter baumannii, and multidrug-resistant Pseudomonas aeruginosa were deemed low-prevalence pathogens. These designations were based on transmission rates found in existing literature.
Patients on antibiotic courses were set at 75% (0.75) at baseline, which was then adjusted to determine its effect on overall MDRO transmission. The number of patients at baseline was 18, with nine nurses, two physicians, and six other health care workers. Mean length-of-stay was 3.5 days, hand hygiene rates were set at 80% for nurses and 50% for physicians, with a 0.83 (83%) efficacy rate when followed. The probability of worker-to-patient transmission was set at 0.025 (2.5%), and set at 0.075 (7.5%) for transmission going the other way.
“We simulated the transmission of the high- and low-prevalence MDROs for 1 year [and] performed 200 replications each for 33 parameter-based scenarios,” the authors said.
When the number of patients on an antibiotic course was dropped from 75% to 65% (a drop of 10%), the rate of high-prevalence MDRO transmission dropped by 11.2% (P < .001). When reduced from 75% to 50% (a drop of 25%), the high-prevalence MDRO transmission rate fell by 28.3% (P < .001), according to the model.
Low-prevalence MDROs also reduced by significant amounts when antibiotic regimens were cut back by the same percentages, with transmission rates falling by 14.3% (P < .001) and 29.8% (P < .001), respectively.
In terms of microbiome effects, the 10% reduction in antibiotics lowered high-prevalence rates by an effect of 1.5, and low-prevalence rates by 1.7; those numbers were 1.2 and 1.4, respectively, when antibiotics were dropped by 25%.
“These reductions are statistically significant and proportionally similar for both high- and low-prevalence MDROs,” the authors concluded, “and they can potentially decrease MDRO acquisition among patients who are receiving antibiotics, as well as among patients who are not receiving antibiotics.”
The National Institutes of Health and the Department of Veterans Affairs’ Health Services Research and Development Department funded the study. Dr. Barnes and his coauthors reported no relevant financial disclosures.
Cutting back on antibiotic courses in intensive care unit settings can significantly reduce the number of multidrug-resistant organism (MDRO) transmissions, according to the findings of a modeling study.
“Significant opportunities exist to optimize and reduce antibiotic usage, [but] the impact of reducing overall antibiotic usage on antibiotic resistance is not known and would be difficult to assess using traditional study designs,” wrote Sean L. Barnes, PhD, of the University of Maryland, College Park, and his colleagues. “Therefore, we applied mathematical modeling to estimate the effect of reducing antibiotic usage on antibiotic resistance.”
Using an agent-based model – which allows for a realistic prediction of interactions between patients and health care workers, while also allowing for heterogeneity in the characteristics of each distinct “person” – Dr. Barnes and his coinvestigators simulated the transmission of MDROs from health care workers to patients.
Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci were deemed “high-prevalence pathogens;” carbapenem-resistant Enterobacteriaceae, multidrug-resistant Acinetobacter baumannii, and multidrug-resistant Pseudomonas aeruginosa were deemed low-prevalence pathogens. These designations were based on transmission rates found in existing literature.
Patients on antibiotic courses were set at 75% (0.75) at baseline, which was then adjusted to determine its effect on overall MDRO transmission. The number of patients at baseline was 18, with nine nurses, two physicians, and six other health care workers. Mean length-of-stay was 3.5 days, hand hygiene rates were set at 80% for nurses and 50% for physicians, with a 0.83 (83%) efficacy rate when followed. The probability of worker-to-patient transmission was set at 0.025 (2.5%), and set at 0.075 (7.5%) for transmission going the other way.
“We simulated the transmission of the high- and low-prevalence MDROs for 1 year [and] performed 200 replications each for 33 parameter-based scenarios,” the authors said.
When the number of patients on an antibiotic course was dropped from 75% to 65% (a drop of 10%), the rate of high-prevalence MDRO transmission dropped by 11.2% (P < .001). When reduced from 75% to 50% (a drop of 25%), the high-prevalence MDRO transmission rate fell by 28.3% (P < .001), according to the model.
Low-prevalence MDROs also reduced by significant amounts when antibiotic regimens were cut back by the same percentages, with transmission rates falling by 14.3% (P < .001) and 29.8% (P < .001), respectively.
In terms of microbiome effects, the 10% reduction in antibiotics lowered high-prevalence rates by an effect of 1.5, and low-prevalence rates by 1.7; those numbers were 1.2 and 1.4, respectively, when antibiotics were dropped by 25%.
“These reductions are statistically significant and proportionally similar for both high- and low-prevalence MDROs,” the authors concluded, “and they can potentially decrease MDRO acquisition among patients who are receiving antibiotics, as well as among patients who are not receiving antibiotics.”
The National Institutes of Health and the Department of Veterans Affairs’ Health Services Research and Development Department funded the study. Dr. Barnes and his coauthors reported no relevant financial disclosures.
Cutting back on antibiotic courses in intensive care unit settings can significantly reduce the number of multidrug-resistant organism (MDRO) transmissions, according to the findings of a modeling study.
“Significant opportunities exist to optimize and reduce antibiotic usage, [but] the impact of reducing overall antibiotic usage on antibiotic resistance is not known and would be difficult to assess using traditional study designs,” wrote Sean L. Barnes, PhD, of the University of Maryland, College Park, and his colleagues. “Therefore, we applied mathematical modeling to estimate the effect of reducing antibiotic usage on antibiotic resistance.”
Using an agent-based model – which allows for a realistic prediction of interactions between patients and health care workers, while also allowing for heterogeneity in the characteristics of each distinct “person” – Dr. Barnes and his coinvestigators simulated the transmission of MDROs from health care workers to patients.
Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci were deemed “high-prevalence pathogens;” carbapenem-resistant Enterobacteriaceae, multidrug-resistant Acinetobacter baumannii, and multidrug-resistant Pseudomonas aeruginosa were deemed low-prevalence pathogens. These designations were based on transmission rates found in existing literature.
Patients on antibiotic courses were set at 75% (0.75) at baseline, which was then adjusted to determine its effect on overall MDRO transmission. The number of patients at baseline was 18, with nine nurses, two physicians, and six other health care workers. Mean length-of-stay was 3.5 days, hand hygiene rates were set at 80% for nurses and 50% for physicians, with a 0.83 (83%) efficacy rate when followed. The probability of worker-to-patient transmission was set at 0.025 (2.5%), and set at 0.075 (7.5%) for transmission going the other way.
“We simulated the transmission of the high- and low-prevalence MDROs for 1 year [and] performed 200 replications each for 33 parameter-based scenarios,” the authors said.
When the number of patients on an antibiotic course was dropped from 75% to 65% (a drop of 10%), the rate of high-prevalence MDRO transmission dropped by 11.2% (P < .001). When reduced from 75% to 50% (a drop of 25%), the high-prevalence MDRO transmission rate fell by 28.3% (P < .001), according to the model.
Low-prevalence MDROs also reduced by significant amounts when antibiotic regimens were cut back by the same percentages, with transmission rates falling by 14.3% (P < .001) and 29.8% (P < .001), respectively.
In terms of microbiome effects, the 10% reduction in antibiotics lowered high-prevalence rates by an effect of 1.5, and low-prevalence rates by 1.7; those numbers were 1.2 and 1.4, respectively, when antibiotics were dropped by 25%.
“These reductions are statistically significant and proportionally similar for both high- and low-prevalence MDROs,” the authors concluded, “and they can potentially decrease MDRO acquisition among patients who are receiving antibiotics, as well as among patients who are not receiving antibiotics.”
The National Institutes of Health and the Department of Veterans Affairs’ Health Services Research and Development Department funded the study. Dr. Barnes and his coauthors reported no relevant financial disclosures.
FROM INFECTION CONTROL & HOSPITAL EPIDEMIOLOGY
Key clinical point:
Major finding: A 10% reduction in prescribed antibiotic courses saw high-prevalence MDRO transmission drop by 11.2%, and a 25% reduction caused a drop of 28.3%; low-prevalence MDROs dropped by 14.3% and 29.8%, respectively (P < .001 for all).
Data source: An agent-based model of a single ICU with 18 patients and 17 health care workers at baseline.
Disclosures: The National Institutes of Health and the Department of Veterans Affairs’ Health Services Research and Development Department funded the study. Dr. Barnes and his coauthors reported no relevant financial disclosures.
FDA clears procalcitonin test to hone antibiotic use in LRTI, sepsis
The Food and Drug Administration has cleared the expanded use of a procalcitonin test to help determine antibiotic use in patients with lower respiratory tract infections (LRTI) and sepsis.
The Vidas Brahms PCT Assay (bioMérieux) uses procalcitonin levels to determine whether a patient with a lower respiratory tract infection (LRTI) should begin or remain on antibiotics and when antibiotics should be withdrawn in a patient with sepsis.
The test will be used primarily in hospital settings and emergency departments, according to the FDA. Test levels that are high levels suggest bacterial infection and the need for antibiotics while low levels indicate viral or noninfectious processes. However, concerns exist regarding false-positive or false-negative test results, which can prompt clinicians to prematurely stop or unnecessarily continue an antibiotic regimen in certain patients.
“Health care providers should not rely solely on PCT test results when making treatment decisions but should interpret test results in the context of a patient’s clinical status and other laboratory results,” according to the FDA statement.
The expanded use of the test was approved based on promising data from clinical trials that was presented at an FDA advisory committee meeting in November 2016. The Vidas Brahms test was already approved by the FDA for use in determining a patient’s risk of dying from sepsis. The test was cleared via the FDA 510(k) regulatory pathway, which is meant for tests or devices for which there is already something similar on the market.
Support for the test’s expanded usage comes from published prospective, randomized clinical trials that compared PCT-guided therapy with standard therapy. In those studies, patients who had received PCT-guided therapy experienced significant decreases in antibiotic use without significant affects to their safety.
The Food and Drug Administration has cleared the expanded use of a procalcitonin test to help determine antibiotic use in patients with lower respiratory tract infections (LRTI) and sepsis.
The Vidas Brahms PCT Assay (bioMérieux) uses procalcitonin levels to determine whether a patient with a lower respiratory tract infection (LRTI) should begin or remain on antibiotics and when antibiotics should be withdrawn in a patient with sepsis.
The test will be used primarily in hospital settings and emergency departments, according to the FDA. Test levels that are high levels suggest bacterial infection and the need for antibiotics while low levels indicate viral or noninfectious processes. However, concerns exist regarding false-positive or false-negative test results, which can prompt clinicians to prematurely stop or unnecessarily continue an antibiotic regimen in certain patients.
“Health care providers should not rely solely on PCT test results when making treatment decisions but should interpret test results in the context of a patient’s clinical status and other laboratory results,” according to the FDA statement.
The expanded use of the test was approved based on promising data from clinical trials that was presented at an FDA advisory committee meeting in November 2016. The Vidas Brahms test was already approved by the FDA for use in determining a patient’s risk of dying from sepsis. The test was cleared via the FDA 510(k) regulatory pathway, which is meant for tests or devices for which there is already something similar on the market.
Support for the test’s expanded usage comes from published prospective, randomized clinical trials that compared PCT-guided therapy with standard therapy. In those studies, patients who had received PCT-guided therapy experienced significant decreases in antibiotic use without significant affects to their safety.
The Food and Drug Administration has cleared the expanded use of a procalcitonin test to help determine antibiotic use in patients with lower respiratory tract infections (LRTI) and sepsis.
The Vidas Brahms PCT Assay (bioMérieux) uses procalcitonin levels to determine whether a patient with a lower respiratory tract infection (LRTI) should begin or remain on antibiotics and when antibiotics should be withdrawn in a patient with sepsis.
The test will be used primarily in hospital settings and emergency departments, according to the FDA. Test levels that are high levels suggest bacterial infection and the need for antibiotics while low levels indicate viral or noninfectious processes. However, concerns exist regarding false-positive or false-negative test results, which can prompt clinicians to prematurely stop or unnecessarily continue an antibiotic regimen in certain patients.
“Health care providers should not rely solely on PCT test results when making treatment decisions but should interpret test results in the context of a patient’s clinical status and other laboratory results,” according to the FDA statement.
The expanded use of the test was approved based on promising data from clinical trials that was presented at an FDA advisory committee meeting in November 2016. The Vidas Brahms test was already approved by the FDA for use in determining a patient’s risk of dying from sepsis. The test was cleared via the FDA 510(k) regulatory pathway, which is meant for tests or devices for which there is already something similar on the market.
Support for the test’s expanded usage comes from published prospective, randomized clinical trials that compared PCT-guided therapy with standard therapy. In those studies, patients who had received PCT-guided therapy experienced significant decreases in antibiotic use without significant affects to their safety.