User login
Bronchitis the leader at putting children in the hospital
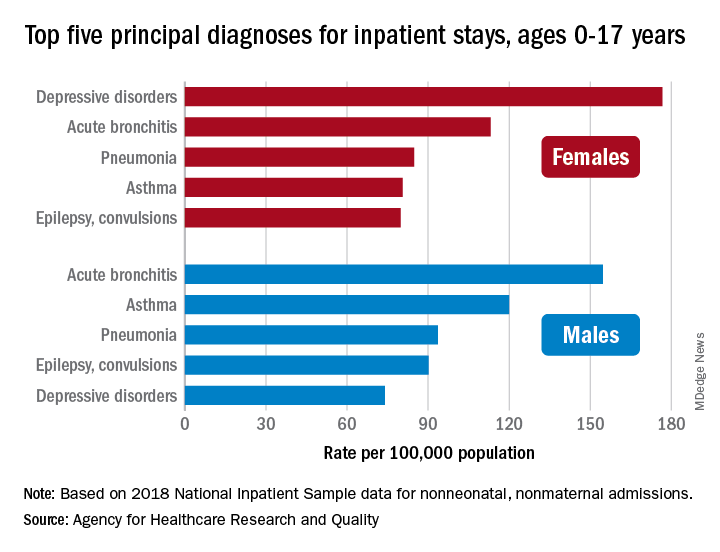
About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.
No link between childhood vaccinations and allergies or asthma
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A meta-analysis by Australian researchers found no link between childhood vaccinations and an increase in allergies and asthma. In fact, children who received the BCG vaccine actually had a lesser incidence of eczema than other children, but there was no difference shown in any of the allergies or asthma.
The researchers, in a report published in the journal Allergy, write, “We found no evidence that childhood vaccination with commonly administered vaccines was associated with increased risk of later allergic disease.”
“Allergies have increased worldwide in the last 50 years, and in developed countries, earlier,” said study author Caroline J. Lodge, PhD, principal research fellow at the University of Melbourne, in an interview. “In developing countries, it is still a crisis.” No one knows why, she said. That was the reason for the recent study.
Allergic diseases such as allergic rhinitis (hay fever) and food allergies have a serious influence on quality of life, and the incidence is growing. According to the Global Asthma Network, there are 334 million people living with asthma. Between 2%-10% of adults have atopic eczema, and more than a 250,000 people have food allergies. This coincides temporally with an increase in mass vaccination of children.
Unlike the controversy surrounding vaccinations and autism, which has long been debunked as baseless, a hygiene hypothesis postulates that when children acquire immunity from many diseases, they become vulnerable to allergic reactions. Thanks to vaccinations, children in the developed world now are routinely immune to dozens of diseases.
That immunity leads to suppression of a major antibody response, increasing sensitivity to allergens and allergic disease. Suspicion of a link with childhood vaccinations has been used by opponents of vaccines in lobbying campaigns jeopardizing the sustainability of vaccine programs. In recent days, for example, the state of Tennessee has halted a program to encourage vaccination for COVID-19 as well as all other vaccinations, the result of pressure on the state by anti-vaccination lobbying.
But the Melbourne researchers reported that the meta-analysis of 42 published research studies doesn’t support the vaccine–allergy hypothesis. Using PubMed and EMBASE records between January 1946 and January 2018, researchers selected studies to be included in the analysis, looking for allergic outcomes in children given BCG or vaccines for measles or pertussis. Thirty-five publications reported cohort studies, and seven were based on randomized controlled trials.
The Australian study is not the only one showing the same lack of linkage between vaccination and allergy. The International Study of Asthma and Allergies in Childhood (ISAAC) found no association between mass vaccination and atopic disease. A 1998 Swedish study of 669 children found no differences in the incidence of allergic diseases between those who received pertussis vaccine and those who did not.
“The bottom line is that vaccines prevent infectious diseases,” said Matthew B. Laurens, associate professor of pediatrics at the University of Maryland, Baltimore, in an interview. Dr. Laurens was not part of the Australian study.
“Large-scale epidemiological studies do not support the theory that vaccines are associated with an increased risk of allergy or asthma,” he stressed. “Parents should not be deterred from vaccinating their children because of fears that this would increase risks of allergy and/or asthma.”
Dr. Lodge and Dr. Laurens have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dupilumab safe, effective in kids 6-11 with moderate-to-severe asthma
Dupilumab (Dupixent, Sanofi and Regeneron) significantly reduced exacerbations compared with placebo in children ages 6-11 years who had moderate-to-severe asthma in a phase 3 trial.
A fully human monoclonal antibody, dupilumab also improved lung function versus placebo by week 12, an improvement that lasted the length of the 52-week trial.
Dupilumab previously had been shown to be safe and effective in adolescents and adults with moderate-to-severe asthma, patients 6 years and older with moderate-to-severe atopic dermatitis, and adults with chronic rhinosinusitis with nasal polyposis, but its safety and effectiveness for moderate-to-severe asthma in the 6-11 years age group was not known.
Results from the randomized, double-blind VOYAGE study conducted across several countries were presented Saturday, July 10, at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2021.
Leonard B. Bacharier, MD, professor of pediatrics, allergy/immunology/pulmonary medicine at Vanderbilt University Medical Center in Nashville, Tennessee, presented the results from the trial, which was funded by Sanofi/Regeneron.
Researchers enrolled 408 children ages 6-11 years with uncontrolled moderate-to-severe asthma. Children on high-dose inhaled corticosteroids (ICS) alone or medium-to-high–dose ICS with a second controller were randomly assigned either to add-on subcutaneous dupilumab 100 mg or 200 mg, based on body weight at study start, or to placebo every 2 weeks for 52 weeks.
Analyses were done in two populations: 350 patients with markers of type 2 inflammation (baseline blood eosinophils ≥150 cells/μl or fractional exhaled nitric oxide [FeNO] ≥20 ppb) and 259 patients with baseline blood eosinophils ≥300 cells/µl.
“The primary endpoint was the annualized rate of severe asthma exacerbations,” Dr. Bacharier said. “The key secondary endpoint was change in percent predicted prebronchodilator FEV1 [forced expiratory volume at 1 second] from baseline to week 12.”
At week 12, the annualized severe asthma exacerbation rate was reduced by 59% (P < .0001) in children with blood eosinophils ≥300 cells/µL and results were similar in those with the type 2 inflammatory phenotype compared with placebo.
Results also indicate a favorable safety profile for dupilumab.
James M. Tracy, DO, an expert with the American College of Allergy, Asthma, and Immunology, told this news organization that adding the dupilumab option for children in the 6-11 age group is “huge.”
Dr. Tracy, who was not involved with the study, said although omalizumab (Xolair, Genentech) is also available for these children, dupilumab stands out because of the range of comorbidities it can treat.
“[Children] don’t have the same rhinosinusitis and polyposis that adults would have, but a lot of them have eczema, and this drug with multiple prongs is incredibly useful and addresses a broad array of allergic conditions,” Dr. Tracy said.
More than 90% of children in the study had at least one concurrent type 2 inflammatory condition, including atopic dermatitis and eosinophilic esophagitis. Dupilumab blocks the shared receptor for interleukin (IL)-4/IL-13, which are key drivers of type 2 inflammation in multiple diseases.
Dr. Tracy said that while dupilumab is not the only drug available to treat children 6-11 years with moderate-to-severe asthma, it is “a significant and unique addition to the armamentarium of the individual practitioner taking care of these very severe asthmatics in the 6-11 age group.”
Dupilumab also led to rapid and sustained improvement in lung function. At 12 weeks, children assigned dupilumab improved their lung function as measured by FEV1 by 5.21% (P = .0009), and that continued through the 52-week study period.
“What we know is the [improved lung function] effect is sustained. What we don’t know is how long you have to keep on the drug for a more permanent effect, which is an issue for all these biologics,” Tracy said.
Dr. Bacharier reported speaker fees and research support from Sanofi/Regeneron. Dr. Tracy has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dupilumab (Dupixent, Sanofi and Regeneron) significantly reduced exacerbations compared with placebo in children ages 6-11 years who had moderate-to-severe asthma in a phase 3 trial.
A fully human monoclonal antibody, dupilumab also improved lung function versus placebo by week 12, an improvement that lasted the length of the 52-week trial.
Dupilumab previously had been shown to be safe and effective in adolescents and adults with moderate-to-severe asthma, patients 6 years and older with moderate-to-severe atopic dermatitis, and adults with chronic rhinosinusitis with nasal polyposis, but its safety and effectiveness for moderate-to-severe asthma in the 6-11 years age group was not known.
Results from the randomized, double-blind VOYAGE study conducted across several countries were presented Saturday, July 10, at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2021.
Leonard B. Bacharier, MD, professor of pediatrics, allergy/immunology/pulmonary medicine at Vanderbilt University Medical Center in Nashville, Tennessee, presented the results from the trial, which was funded by Sanofi/Regeneron.
Researchers enrolled 408 children ages 6-11 years with uncontrolled moderate-to-severe asthma. Children on high-dose inhaled corticosteroids (ICS) alone or medium-to-high–dose ICS with a second controller were randomly assigned either to add-on subcutaneous dupilumab 100 mg or 200 mg, based on body weight at study start, or to placebo every 2 weeks for 52 weeks.
Analyses were done in two populations: 350 patients with markers of type 2 inflammation (baseline blood eosinophils ≥150 cells/μl or fractional exhaled nitric oxide [FeNO] ≥20 ppb) and 259 patients with baseline blood eosinophils ≥300 cells/µl.
“The primary endpoint was the annualized rate of severe asthma exacerbations,” Dr. Bacharier said. “The key secondary endpoint was change in percent predicted prebronchodilator FEV1 [forced expiratory volume at 1 second] from baseline to week 12.”
At week 12, the annualized severe asthma exacerbation rate was reduced by 59% (P < .0001) in children with blood eosinophils ≥300 cells/µL and results were similar in those with the type 2 inflammatory phenotype compared with placebo.
Results also indicate a favorable safety profile for dupilumab.
James M. Tracy, DO, an expert with the American College of Allergy, Asthma, and Immunology, told this news organization that adding the dupilumab option for children in the 6-11 age group is “huge.”
Dr. Tracy, who was not involved with the study, said although omalizumab (Xolair, Genentech) is also available for these children, dupilumab stands out because of the range of comorbidities it can treat.
“[Children] don’t have the same rhinosinusitis and polyposis that adults would have, but a lot of them have eczema, and this drug with multiple prongs is incredibly useful and addresses a broad array of allergic conditions,” Dr. Tracy said.
More than 90% of children in the study had at least one concurrent type 2 inflammatory condition, including atopic dermatitis and eosinophilic esophagitis. Dupilumab blocks the shared receptor for interleukin (IL)-4/IL-13, which are key drivers of type 2 inflammation in multiple diseases.
Dr. Tracy said that while dupilumab is not the only drug available to treat children 6-11 years with moderate-to-severe asthma, it is “a significant and unique addition to the armamentarium of the individual practitioner taking care of these very severe asthmatics in the 6-11 age group.”
Dupilumab also led to rapid and sustained improvement in lung function. At 12 weeks, children assigned dupilumab improved their lung function as measured by FEV1 by 5.21% (P = .0009), and that continued through the 52-week study period.
“What we know is the [improved lung function] effect is sustained. What we don’t know is how long you have to keep on the drug for a more permanent effect, which is an issue for all these biologics,” Tracy said.
Dr. Bacharier reported speaker fees and research support from Sanofi/Regeneron. Dr. Tracy has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dupilumab (Dupixent, Sanofi and Regeneron) significantly reduced exacerbations compared with placebo in children ages 6-11 years who had moderate-to-severe asthma in a phase 3 trial.
A fully human monoclonal antibody, dupilumab also improved lung function versus placebo by week 12, an improvement that lasted the length of the 52-week trial.
Dupilumab previously had been shown to be safe and effective in adolescents and adults with moderate-to-severe asthma, patients 6 years and older with moderate-to-severe atopic dermatitis, and adults with chronic rhinosinusitis with nasal polyposis, but its safety and effectiveness for moderate-to-severe asthma in the 6-11 years age group was not known.
Results from the randomized, double-blind VOYAGE study conducted across several countries were presented Saturday, July 10, at the European Academy of Allergy and Clinical Immunology (EAACI) Hybrid Congress 2021.
Leonard B. Bacharier, MD, professor of pediatrics, allergy/immunology/pulmonary medicine at Vanderbilt University Medical Center in Nashville, Tennessee, presented the results from the trial, which was funded by Sanofi/Regeneron.
Researchers enrolled 408 children ages 6-11 years with uncontrolled moderate-to-severe asthma. Children on high-dose inhaled corticosteroids (ICS) alone or medium-to-high–dose ICS with a second controller were randomly assigned either to add-on subcutaneous dupilumab 100 mg or 200 mg, based on body weight at study start, or to placebo every 2 weeks for 52 weeks.
Analyses were done in two populations: 350 patients with markers of type 2 inflammation (baseline blood eosinophils ≥150 cells/μl or fractional exhaled nitric oxide [FeNO] ≥20 ppb) and 259 patients with baseline blood eosinophils ≥300 cells/µl.
“The primary endpoint was the annualized rate of severe asthma exacerbations,” Dr. Bacharier said. “The key secondary endpoint was change in percent predicted prebronchodilator FEV1 [forced expiratory volume at 1 second] from baseline to week 12.”
At week 12, the annualized severe asthma exacerbation rate was reduced by 59% (P < .0001) in children with blood eosinophils ≥300 cells/µL and results were similar in those with the type 2 inflammatory phenotype compared with placebo.
Results also indicate a favorable safety profile for dupilumab.
James M. Tracy, DO, an expert with the American College of Allergy, Asthma, and Immunology, told this news organization that adding the dupilumab option for children in the 6-11 age group is “huge.”
Dr. Tracy, who was not involved with the study, said although omalizumab (Xolair, Genentech) is also available for these children, dupilumab stands out because of the range of comorbidities it can treat.
“[Children] don’t have the same rhinosinusitis and polyposis that adults would have, but a lot of them have eczema, and this drug with multiple prongs is incredibly useful and addresses a broad array of allergic conditions,” Dr. Tracy said.
More than 90% of children in the study had at least one concurrent type 2 inflammatory condition, including atopic dermatitis and eosinophilic esophagitis. Dupilumab blocks the shared receptor for interleukin (IL)-4/IL-13, which are key drivers of type 2 inflammation in multiple diseases.
Dr. Tracy said that while dupilumab is not the only drug available to treat children 6-11 years with moderate-to-severe asthma, it is “a significant and unique addition to the armamentarium of the individual practitioner taking care of these very severe asthmatics in the 6-11 age group.”
Dupilumab also led to rapid and sustained improvement in lung function. At 12 weeks, children assigned dupilumab improved their lung function as measured by FEV1 by 5.21% (P = .0009), and that continued through the 52-week study period.
“What we know is the [improved lung function] effect is sustained. What we don’t know is how long you have to keep on the drug for a more permanent effect, which is an issue for all these biologics,” Tracy said.
Dr. Bacharier reported speaker fees and research support from Sanofi/Regeneron. Dr. Tracy has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Optimizing Severe Asthma Treatment: Challenges and Approaches to Care
Asthma is a complex, heterogeneous disease with unmet treatment needs. Patients with severe asthma generally continue to have severe disease despite being on controller therapies.
Uncontrolled asthma can result in unnecessary suffering and interfere with daily activities. It also increases the risk for exacerbations and places substantial burden on the healthcare system.
In this ReCAP, Drs Sandhya Khurana and Steve N. Georas, from the Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care in Rochester, New York, discuss current challenges in the management of severe asthma, and how advances in the understanding of phenotypes and therapeutic options guide their approaches to asthma management.
They review key indicators of severe asthma, tools to assess asthma control, approaches to phenotyping patients, treatment options for type 2 and non–type 2 asthma, and emerging agents.
--
Sandhya Khurana, MD is a Professor, Department of Medicine, University of Rochester, Director, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Sandhya Khurana, MD, has disclosed the following relevant financial relationships: Received research grant from: GlaxoSmithKline.
Steve N. Georas, MD is a Professor, Department of Medicine, University of Rochester; Walter & Carmina Mary Parkes Family Endowed Professor; Director, Pulmonary Function Labs, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Steve N. Georas, MD, has disclosed no relevant financial relationships.
Asthma is a complex, heterogeneous disease with unmet treatment needs. Patients with severe asthma generally continue to have severe disease despite being on controller therapies.
Uncontrolled asthma can result in unnecessary suffering and interfere with daily activities. It also increases the risk for exacerbations and places substantial burden on the healthcare system.
In this ReCAP, Drs Sandhya Khurana and Steve N. Georas, from the Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care in Rochester, New York, discuss current challenges in the management of severe asthma, and how advances in the understanding of phenotypes and therapeutic options guide their approaches to asthma management.
They review key indicators of severe asthma, tools to assess asthma control, approaches to phenotyping patients, treatment options for type 2 and non–type 2 asthma, and emerging agents.
--
Sandhya Khurana, MD is a Professor, Department of Medicine, University of Rochester, Director, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Sandhya Khurana, MD, has disclosed the following relevant financial relationships: Received research grant from: GlaxoSmithKline.
Steve N. Georas, MD is a Professor, Department of Medicine, University of Rochester; Walter & Carmina Mary Parkes Family Endowed Professor; Director, Pulmonary Function Labs, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Steve N. Georas, MD, has disclosed no relevant financial relationships.
Asthma is a complex, heterogeneous disease with unmet treatment needs. Patients with severe asthma generally continue to have severe disease despite being on controller therapies.
Uncontrolled asthma can result in unnecessary suffering and interfere with daily activities. It also increases the risk for exacerbations and places substantial burden on the healthcare system.
In this ReCAP, Drs Sandhya Khurana and Steve N. Georas, from the Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care in Rochester, New York, discuss current challenges in the management of severe asthma, and how advances in the understanding of phenotypes and therapeutic options guide their approaches to asthma management.
They review key indicators of severe asthma, tools to assess asthma control, approaches to phenotyping patients, treatment options for type 2 and non–type 2 asthma, and emerging agents.
--
Sandhya Khurana, MD is a Professor, Department of Medicine, University of Rochester, Director, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Sandhya Khurana, MD, has disclosed the following relevant financial relationships: Received research grant from: GlaxoSmithKline.
Steve N. Georas, MD is a Professor, Department of Medicine, University of Rochester; Walter & Carmina Mary Parkes Family Endowed Professor; Director, Pulmonary Function Labs, Mary H. Parkes Center for Asthma, Allergy, and Pulmonary Care, Rochester, New York.
Steve N. Georas, MD, has disclosed no relevant financial relationships.

Respiratory infection– and asthma-prone children
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).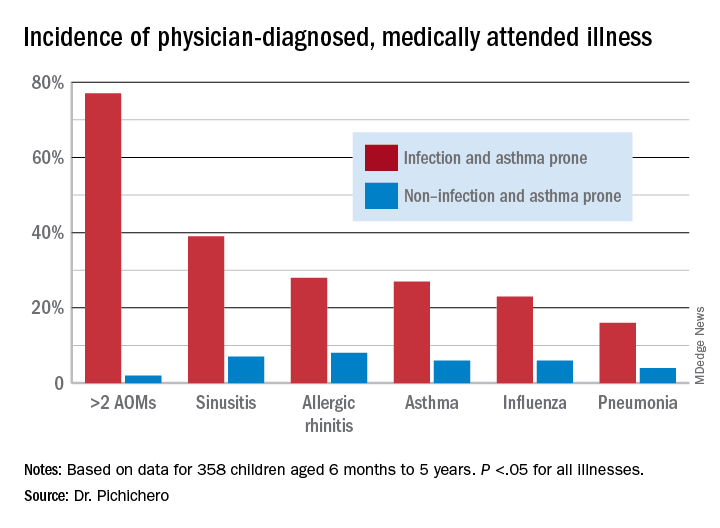
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Sublingual immunotherapy: Where does it stand?
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Sublingual immunotherapy (SLIT) emerged over a century ago as a gentler alternative to allergy shots. It uses the same antigens found in allergy shots, delivering them through tablets or drops under the tongue rather than by injecting them into the skin.
Yet injection immunotherapy has been the mainstay of allergy treatment in the United States. Allergy shots are “the bread and butter, keeping the lights on at allergy practices,” said allergist Sakina Bajowala, MD, of Kaneland Allergy and Asthma Center, in the Chicago area. So even “when environmental SLIT showed quite clearly that it had efficacy, people were so slow to adapt.”
SLIT – a daily treatment that builds protection from allergens gradually over years with few side effects – is popular around the globe, particularly for environmental allergies. But only a handful of clinics offer food SLIT. Even though recent trials in peanut-allergic children show that SLIT is far safer than oral immunotherapy and about as effective as the Food and Drug Administration–approved peanut-allergy product and has lasting benefits for toddlers, many allergists lack experience with customized immunotherapies and hesitate to offer an unregulated treatment for which the evidence base is still emerging.
Why hasn’t food allergy SLIT caught on?
One issue is that there is scant evidence from randomized, controlled trials. The treatments that clinics offer often hinge on insurance coverage, and increasingly, insurers only cover FDA-approved products. FDA approval requires thousands of patients being enrolled in long, expensive studies to prove the treatment’s merit. In a similar vein, doctors are trained to question methods that lack a strong publication base, for good reason.
Yet SLIT caught the attention of pioneering physicians who were intrigued by this “low-and-slow” immune-modifying approach, despite limited published evidence, and they sought real-world experience.
The late physician David Morris, MD, came across SLIT in the 1960s while searching for alternative ways to help mold-allergic farmers who were suffering terrible side effects from allergy shots. Dr. Morris attended conferences, learned more about sublingual techniques, got board certified in allergy, and opened Allergy Associates of La Crosse (Wis.), in 1970 to offer SLIT as a treatment for food and environmental allergies.
Dr. Morris and colleagues developed a protocol to create custom SLIT drops tailored to individual patients’ clinical histories and allergy test results. The method has been used to treat more than 200,000 patients. It has been used by allergist Nikhila Schroeder, MD, MEng, who learned SLIT methods while treating nearly 1,000 patients at Allergy Associates. In 2018, she opened her own direct-care SLIT practice, Allergenuity Health, in the Charlotte metropolitan area of North Carolina (see part 2 of this series).
Dr. Bajowala’s clinic offers SLIT in addition to oral immunotherapy (OIT). She was encouraged by the recent toddler SLIT data but wondered whether it would translate to a real-world setting. According to her calculations, the published protocol – according to which participants receive up to 4 mg/d over 6 months and continue receiving a daily maintenance dose of 4 mg for 3 years – would cost $10,000 per patient.
With this dosing regimen, the intervention is unaffordable, Dr. Bajowala said. And “there’s no way to make it cheaper because that’s the raw materials cost. It does not include labor or bottles or profit at all. That’s just $10,000 in peanut extract.”
Owing to cost, Dr. Bajowala’s clinic generally uses SLIT as a bridge to OIT. Her food allergy patients receive up to 1 mg/d and remain at that dose for a month or so before transitioning to OIT, “for which the supplies are orders of magnitude cheaper,” she said.
Dr. Schroeder said there is evidence for efficacy at microgram and even nanogram dosing – much lower than used in the recent food SLIT trials. Maintenance doses range from 50 ng/d to 25 mcg/d for environmental SLIT and 4-37 mcg/d for food SLIT, she said. The La Crosse method uses even lower dose ranges.
However, dosing information is not readily available, Dr. Schroeder noted. She has spent years scrutinizing articles and compiling information from allergen extract suppliers – all the while treating hundreds of SLIT patients. “I have had to expend a lot of time and effort,” said Dr. Schroeder. “It’s really hard to explain quickly.”
In the published literature, SLIT dosing recommendations vary widely. According to a 2007 analysis, environmental allergy symptoms improved with doses over a 1,000-fold range. What’s more, success did not scale with increased dosing and seemed to depend more on frequency and duration of treatment.
There are fewer studies regarding food SLIT. The most promising data come from recent trials of peanut-allergic children led by Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill. Still, “I am nervous to tell people to go do this based on 150 kids at one site,” Dr. Kim said. “We need to have a gigantic study across multiple sites that actually confirms what we have found in our single center.”
Because there are few published trials of food SLIT, confusion about which doses are optimal, how early to start, and how long the benefits last will be a barrier for many clinicians, said Douglas Mack, MD, FRCPC, assistant clinical professor in pediatrics at McMaster University, Hamilton, Ont.
Much could be learned from Allergy Associates of La Crosse, Allergenuity Health, and other clinics with SLIT experience involving thousands of patients. But that real-world data are messy and difficult to publish. Plus, it is hard for private allergists to find time to review charts, analyze data, and draft papers alongside seeing patients and running a clinic – especially without students and interns, who typically assist with academic research, Dr. Schroeder said.
Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern University, Chicago, and colleagues worked with a La Crosse team 6 or 7 years ago to try to analyze and publish SLIT outcomes for 121 peanut-allergic children who were treated for food and environmental allergies at the Wisconsin clinic. The researchers had hoped to publish an article describing caregiver-reported and clinical outcomes.
Among 73 caregivers who responded to a survey, more than half reported improved eczema, asthma, and environmental allergy symptoms, and virtually all families said SLIT calmed anxieties and minimized fear of allergic reactions. However, the clinical outcomes – skinprick test results, immune changes, and oral food challenges – were not as robust. And the data were incomplete. Some patients had traveled to La Crosse for SLIT drops but underwent skin and blood testing with their local allergist. Compiling records is “so much harder when you’re not doing a prospective clinical trial,” Dr. Gupta said.
The caregiver-reported outcomes were presented as a poster at the 2015 annual meeting of the American College of Allergy, Asthma, and Immunology and the 2016 annual meeting of the Pediatric Academic Society, said Jeff Kessler, MBA, FACHE, who is practice executive at La Crosse. However, with only self-reported data and no convincing lab metrics, the findings were never submitted for publication.
Others are eager to see clearer proof that SLIT works at doses lower than those published in the most recent trials. “If we can get efficacy with lower doses, that means we can increase accessibility, because we can lower the cost,” Dr. Bajowala said.
Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins University, Baltimore, has a pending grant proposal for a multifood trial of SLIT. “It’s a big missing piece,” he said.
Dr. Mack said that in Canada there was “almost an instant change in group think” when the Canadian Society of Allergy and Clinical Immunology published guidelines in support of OIT. With the new guidelines, “people are less concerned about liability. Once they start getting into OIT, I think you’re going to see SLIT coming right along for the ride.”
The shift will be slower in the United States, which has 20 times as many practicing allergists as Canada. Nevertheless, “I totally think SLIT has a place at the table,” Dr. Mack said. “I hope we start to see more high-quality data and people start to use it and experiment with it a bit and see how it works.”
A version of this article first appeared on Medscape.com. This is part three of a three-part series. Part one is here. Part two is here.
Direct-care allergy clinic specializes in sublingual immunotherapy
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
With degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Nikhila Schroeder, MD, MEng, brings a problem-solving mindset to medicine.
Being a doctor means having to “figure out all aspects of [a patient’s] situation and do my best to come up with an answer,” said Dr. Schroeder, who founded Allergenuity Health, a solo allergy practice in Huntersville, N.C., with her husband James, who serves as practice executive. It’s “being a medical detective for your patient.”
Yet, during her training, Dr. Schroeder found that market-driven health care makes it hard to practice medicine with a patient’s best interest foremost. Procedures for diagnosing and treating disease cater to insurance companies’ reimbursement policies. “You wind up having to tailor your care to whatever insurance will cover,” she said.
Insurers, in turn, look for evidence from large, peer-reviewed studies to prove that a treatment works. Many physicians hesitate to offer therapies that aren’t covered by insurance, for both liability and financial reasons. So treatment tends to be limited to those options that were rigorously vetted in long, costly, multisite trials that are difficult to conduct without a corporate sponsor.
This is why there is still only one licensed treatment for people with food allergies – a set of standardized peanut powder capsules (Palforzia) that was approved by the Food and Drug Administration in early 2020 for peanut-allergic children aged 4-17 years. A small but growing number of allergists offer unapproved oral immunotherapy (OIT) using commercial food products to treat allergies to peanuts and other foods.
Even fewer allergists treat food allergy patients with another immune-modifying treatment, sublingual immunotherapy (SLIT), which delivers allergens through liquid drops held for several minutes under the tongue. Since 2018, Allergenuity Health, which offers SLIT to treat food and environmental allergies, has provided care to more than 400 patients. More than a third have come from out of state.
The clinic uses a direct-care approach. Rather than taking insurance, the clinic offers a monthly billing program that includes tests, SLIT bottles, and access to Dr. Schroeder via phone, email, or text. “I’m only contracted with the patient, and my only focus is the patient,” Dr. Schroeder said in an interview.
Unforgettable day
Allergy was not on Dr. Schroeder’s radar in medical school. She wanted to be a surgeon. But she loved working with children, so she did a pediatrics residency at the University of Virginia Children’s Hospital in Charlottesville. There Dr. Schroeder started seeing kids with eczema and allergies. While covering a friend’s clinic shift in 2010, she was thrust into an emergency. A family who didn’t speak English had just brought in their screaming 6-month-old baby, red and puffy with hives. “We didn’t know what was going on with this child,” Dr. Schroeder said. “Somehow I was elected to go in there.”
All of a sudden, things got quiet. Yet the baby was still screaming, mouth wide open. Dr. Schroeder had learned about anaphylaxis but had never witnessed it – until that day. The baby›s airways swelled so much that the crying became hoarse and soft. After working with a nurse to administer epinephrine, Dr. Schroeder saw something equally unforgettable: The baby’s heart rate soared, but within minutes the hives and swelling subsided and smiles returned. “It was incredible how quickly things changed,” Dr. Schroeder said. The baby had a reaction to rice, an uncommon allergen.
Dr. Schroeder stayed at UVA 2 more years to complete an allergy and immunology fellowship. She learned to diagnose food allergies but became frustrated having to tell patients they had little recourse but to avoid the food and to check in every year or 2. “I was, like, aren’t we specialists? Shouldn’t we have a little more expertise and maybe see if there are ways we could change this?” Dr. Schroeder said.
During those years, allergy shots were the only form of immunotherapy being taught to fellows. At clinic, Dr. Schroeder served as backup to the nurses when someone reacted to shots. She was troubled that some patients needed epinephrine to stop asthma attacks caused by injections they had received as treatment. The idea of injecting substances under the skin seemed akin to vaccination – where “you want to aggravate the immune system, you want it to get revved up, you want to build it up to fight,” she said. “But that’s not what you want for allergy. You want to tone it down. It didn’t really, to be honest, make a lot of sense to me.”
Dr. Schroeder started digging and asking questions. How does the immune system decide what is safe? Which cells and molecules communicate these decisions? She thought about babies and how they “learn” by putting stuff into their mouths. “If we don’t tolerate most of what we take in there, we wouldn’t survive,” Dr. Schroeder said. “It makes a lot of sense that a lot of tolerance begins with cells of the mouth.”
Dr. Schroeder discussed these concepts with her attendings. “They were all, like, no, there’s really no good evidence for that,” she said. But at some point, someone mentioned sublingual immunotherapy, and Schroeder came across Allergy Associates of La Crosse (Wis.).
The clinic’s late founder, David Morris, MD, learned about SLIT in the 1960s as an alternative option for farmers who suffered terrible side effects from injection immunotherapy they received to treat their mold allergies. Dr. Morris attended conferences, learned more about sublingual techniques – at times seeking advice from European allergists who offered SLIT – and became board certified in allergy before opening the La Crosse clinic in 1970. According to the clinic, more than 200,000 patients with environmental and food allergies have been treated with its SLIT protocol.
Dr. Schroeder was shocked to discover that this clinic had existed for 40 years, yet “I, as an allergist, had heard nothing about them,” she said.
Toward the end of her fellowship, OIT was becoming more well known. But she felt its risks were often downplayed. After years of talking with food allergy patients, Schroeder realized that most didn’t actually care about eating peanut butter sandwiches or sesame or walnuts. “Often I would hear, through tears: ‘I just want my child to be able to sit with their friends at lunch, to not be put at this other table, to not feel so isolated,’ ” she said. What mattered most to many families was gaining enough protection to not feel anxious about participating in social activities involving food.
Dr. Schroeder had a growing sense that SLIT – given its ease, safety, and sensible route of allergen delivery – seemed more useful. She wanted to learn more.
Her mentors urged her to stay in academia instead. “They were, like, you have a good academic reputation. You’re a solid thinker. You’re great at what you do. Do the traditional stuff,” Dr. Schroeder said.
Despite these admonitions, Dr. Schroeder left academia and took a job at La Crosse after completing her allergy fellowship. Determined to see whether SLIT could be effective, “I decided in the end, you know what, I have to go do this,” she said. “I need to know, and the only way I’m going to know is to do it, because no one was giving me good information.”
Before treating anyone with SLIT, Dr. Schroeder tried it herself – as a La Crosse patient. Growing up with severe eczema, eye swelling, and chronic nasal congestion leading to sinus infections, “I myself was a severely allergic person,” she said. Within several months, Dr. Schroeder saw dramatic improvement in her symptoms – “a night and day difference.” She experienced some mouth tingling, one of SLIT’s most common side effects, but found it “very tolerable, very mild.”
Allergenuity Health doesn’t aim to promote SLIT as the best treatment, said Dr. Schroeder, who has helped some families use avoidance or OIT as a better option. “An initial evaluation is always about proper diagnosis and education about all the treatment options available. Really, the point is education – be a detective for them and figure out what’s going on, be honest about what we know and what we don’t know, and give them the tools to figure out how to proceed.”
A version of this article first appeared on Medscape.com. This is part two of a three-part series. Part one is here. Part three is here.
There’s a much safer food allergy immunotherapy – why don’t more doctors offer it?
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
For the 32 million people in the United States with food allergies, those who seek relief beyond constant vigilance and EpiPens face a confusing treatment landscape. In January 2020, the Food and Drug Administration approved an oral immunotherapy product (Palforzia) for peanut-allergic children. Yet the product’s ill-timed release during a pandemic and its black-box warning about the risk for anaphylaxis has slowed uptake.
A small number of allergists offer home-grown oral immunotherapy (OIT), which builds protection by exposing patients to increasing daily doses of commercial food products over months. However, as with Palforzia, allergic reactions are common during treatment, and the hard-earned protection can fade if not maintained with regular dosing.
An alternate approach, sublingual immunotherapy (SLIT), delivers food proteins through liquid drops held in the mouth – a site rich in tolerance-inducing immune cells. In a 2019 study of peanut-allergic children aged 1-11 years, SLIT offered a level of protection on par with Palforzia while causing considerably fewer adverse events. And at the 2021 annual meeting of the American Academy of Allergy, Asthma and Immunology, researchers reported that SLIT produced stronger, more durable benefits in toddlers aged 1-4.
Sublingual immunotherapy is “a bunch of drops you put under your tongue, you hold it for a couple minutes, and then you’re done for the day,” said Edwin Kim, MD, director of the UNC Food Allergy Initiative, University of North Carolina at Chapel Hill, who led the two recent studies. For protecting against accidental ingestions, SLIT “is pushing pretty close to what OIT is able to provide but seemingly with a superior ease of administration and safety profile.”
Many parents don’t necessarily want their allergic kids to be able to eat a peanut butter sandwich – but do want them to be able to safely sit at the same lunch table and attend birthday parties with other kids. SLIT achieves this level of protection about as well as OIT, with fewer side effects.
Still, because of concerns about the treatment’s cost, unclear dosing regimens, and lack of FDA approval, very few U.S. allergists – likely less than 5% – offer sublingual immunotherapy to treat food allergies, making SLIT even less available than OIT.
Concerns about SLIT
One possible reason: Success is slower and less visible for SLIT. When patients undergo OIT, they build up to dosing with the actual food. “To a family who has a concern about their kid reacting, they can see them eating chunks of peanut in our office. That is really encouraging,” said Douglas Mack, MD, FRCPC, an allergist with Halton Pediatric Allergy and assistant clinical professor of pediatrics at McMaster University, Hamilton, Ont.
On the other hand, ingestion isn’t the focus for SLIT, so progress is harder to measure using metrics in published trials. After holding SLIT drops under the tongue, some patients spit them out. If they swallow the dose, it’s a vanishingly small amount. Immune changes that reflect increasing tolerance, such as a decrease in IgE antibodies, tend to be more gradual with SLIT than with OIT. And because SLIT is only offered in private clinics, such tests are not conducted as regularly as they would be for published trials.
But there may be a bigger factor: Some think earlier trials comparing the two immunotherapy regimens gave SLIT a bad rap. For example, in studies of milk- and peanut-allergic children conducted in 2011 and 2014, investigators concluded that SLIT was safer and that OIT appeared to be more effective. However, those trials compared SLIT with OIT using a much higher dose (2,000 mg) than is used in the licensed product (300 mg).
Over the years, endpoints for food allergy treatment trials have shifted from enabling patients to eat a full serving of their allergen to merely raising their threshold to guard against accidental exposures. So in those earlier articles, “we would probably write the discussion section differently now,” said Corinne Keet, MD, PhD, first author on the 2011 milk study and an associate professor of pediatrics at Johns Hopkins University, Baltimore.
Indeed, “when you compare [SLIT] to Palforzia or other studies of low-dose OIT (300 mg/d), they look equal in terms of their efficacy,” said senior author Robert Wood, MD, professor of pediatrics and director of pediatric allergy and immunology at Johns Hopkins. Yet, “I’m afraid we had a major [negative] impact on pharma’s interest in pursuing SLIT.”
Without corporate funding, it’s nearly impossible to conduct the large, multisite trials required for FDA approval of a treatment. And without approved products, many allergists are reluctant to offer the therapy, Dr. Wood said. It “makes your life a lot more complicated to be dabbling in things that are not approved,” he noted.
But at least one company is giving it a go. Applying the SLIT principle of delivering food allergens to tolerance-promoting immune cells in the mouth, New York–based Intrommune Therapeutics recently started enrolling peanut-allergic adults for a phase 1 trial of its experimental toothpaste.
Interest in food-allergy SLIT seems to be growing. “I definitely think that it could be an option for the future,” said Jaclyn Bjelac, MD, associate director of the Food Allergy Center of Excellence at the Cleveland Clinic. “Up until a few months ago, it really wasn’t on our radar.”
On conversations with Dr. Kim, philanthropists and drug developers said they found the recent data on SLIT promising, yet pointed out that food SLIT protocols and products are already in the public domain – they are described in published research using allergen extracts that are on the market. They “can’t see a commercial path forward,” Dr. Kim said in an interview. “And that’s kind of where many of my conversations end.”
Although there are no licensed SLIT products for food allergies, between 2014 and 2017, the FDA approved four sublingual immunotherapy tablets to treat environmental allergies – Stallergenes-Greer’s Oralair and ALK’s Grastek for grass pollens, ALK’s Odactra for dust mites, and ALK’s Ragwitek for short ragweed.
SLIT tablets work as well as allergy shots (subcutaneous immunotherapy) for controlling environmental allergy symptoms, they have a better safety profile, according to AAAAI guidelines, and they can be self-administered at home, which has made them a popular option globally. “Our European colleagues have used sublingual immunotherapy much more frequently than, for example, in the U.S.,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
Use of SLIT is also increasing in the United States, especially as FDA-approved products become available. In a 2019 survey, the percentage of U.S. allergists who said they were offering sublingual treatment for environmental allergies increased from 5.9% in 2007 to 73.5% in 2019. However, only 11.2% reported extensive SLIT use; the remainder reported some (50.5%) or little (38.3%) use.
As noted above, considerably fewer U.S. allergists use SLIT to treat food allergies. Similarly, a 2021 survey of allergists in Canada found that only 7% offered food sublingual immunotherapy; more than half reported offering OIT.
One practice, Allergy Associates of La Crosse (Wis.), has offered SLIT drops for food and environmental allergies for decades. Since the clinic opened in 1970, more than 200,000 people have been treated with its protocol. Every patient receives customized sublingual drops – “exactly what they’re allergic to, exactly how allergic they are, and then we build from there,” said Jeff Kessler, MBA, FACHE, practice executive at Allergy Associates of La Crosse. “Quite frankly, it’s the way immunotherapy should be done.
A version of this article first appeared on Medscape.com. This is part one of a three-part series. Part two is here. Part three is here.
Tezepelumab reduces serious exacerbations in severe asthma
Results from the NAVIGATOR study of tezepelumab showed that treatment of adults and adolescents with severe, uncontrolled asthma with the new biologic led to a large reduction in exacerbations requiring hospital stays and ED visits.
Tezepelumab, codeveloped by Amgen and AstraZeneca, has a novel mechanism of action. It blocks thymic stromal lymphopoietin (TSLP), which is a cytokine produced by epithelial cells. TSLP levels correlate with airway obstruction, severity of disease, and glucocorticoid resistance. TSLP is involved in T2 inflammation within the airway, but also plays a role in the interactions between airway cells and immune cells, which doesn’t rely only solely on T2 inflammation. That broad mechanism of action distinguishes tezepelumab from most other biologics for the treatment of asthma, which are more targeted.
“By working at the top of the cascade, tezepelumab helps stop inflammation at a key source. Clinical trials with tezepelumab showed a clinical benefit in patients irrespective of their baseline biomarker level, including patients with low eosinophil levels at baseline,” said Jean-Pierre Llanos-Ackert, MD, who is executive medical director and global medical affairs lead for tezepelumab at Amgen.
The primary endpoint data look robust, according to Praveen Akuthota, MD, who is an associate professor of medicine at the University of California, San Diego, and comoderated the session at the American Thoracic Society’s virtual international conference, where the research was presented. The study was also published on May 13, 2021, in the New England Journal of Medicine. The conference session included updated results.
The drug holds promise, but more study is needed. “The question really will be, how is this drug different from the existing biologics? How much better is this drug in patients who have borderline T2 biomarkers, or even low T2. The study does show some efficacy in patients whose T2 signals may not be as robust. We’ll have to see with ongoing longitudinal data, how this drug positions, compared to the other agents. It’s obviously exciting, though, to have another option, given that we know what our current armamentarium of agents there are still nonresponders,” said Dr. Akuthota in an interview.
The other comoderator in the session, Laura Crotty Alexander, MD, commented: “It seems like it might work possibly even better than some that are directly covering one pathway only. Hopefully, this agent will be efficacious in a broader population than some of the more targeted biologics.” Dr. Alexander is an associate professor of medicine at the University of California, San Diego, and section chief of pulmonary critical care at the Veterans Affairs San Diego Healthcare System.
She pointed out that physicians often think of asthma patients in broad brush terms, as high or low T2, or T2 high and Th1 or neutrophilic or obese, but many patients present a more complicated picture. “There is some overlap across those phenotypes, such that an agent that works really well for one group doesn’t mean that it won’t have an impact, especially clinically, on some of these other phenotypes,” said Dr. Alexander.
Dr. Akuthota agreed. “Having options for patients whose biomarkers are not maybe as clear is, I think, important.”
Promising results
The study included 1,059 patients aged 12-80 who received 210 mg tezepelumab or placebo. Over 52 weeks, the treatment group had a 79% reduction in exacerbations requiring hospitalization or an ED visit, compared with placebo (rate ratio, 0.21; 95% confidence interval, 0.12-0.37), and an 85% reduction in exacerbations requiring hospitalization (RR, 0.15; 95% CI, 0.07-0.33). The drug increased the time to first exacerbation requiring hospitalization that required hospitalization or an ED visit, reducing risk by 65% (hazard ratio, 0.35; 95% CI, 0.22-0.56).
Fewer patients in the treatment group than placebo used asthma-related health care resources, including: ED visits (32 vs. 94), unscheduled visit to a specialist (285 vs. 406), telephone calls to a health care provider (234 vs. 599), ambulance transport (5 vs. 22), and home visits from a health care provider (18 vs. 22). Fewer patients in the tezepelumab group had hospital stays (3.2% vs. 7.0%), and they had a lower total number of hospital days (108 vs. 497) and days in the ICU (0 vs. 31).
The study was funded by Amgen and AstraZeneca. Dr. Llanos-Ackert is an employee of Amgen. Dr. Alexander has no relevant financial disclosures. Dr. Akuthota has consulted for AstraZeneca and participated in their clinical trials.
Results from the NAVIGATOR study of tezepelumab showed that treatment of adults and adolescents with severe, uncontrolled asthma with the new biologic led to a large reduction in exacerbations requiring hospital stays and ED visits.
Tezepelumab, codeveloped by Amgen and AstraZeneca, has a novel mechanism of action. It blocks thymic stromal lymphopoietin (TSLP), which is a cytokine produced by epithelial cells. TSLP levels correlate with airway obstruction, severity of disease, and glucocorticoid resistance. TSLP is involved in T2 inflammation within the airway, but also plays a role in the interactions between airway cells and immune cells, which doesn’t rely only solely on T2 inflammation. That broad mechanism of action distinguishes tezepelumab from most other biologics for the treatment of asthma, which are more targeted.
“By working at the top of the cascade, tezepelumab helps stop inflammation at a key source. Clinical trials with tezepelumab showed a clinical benefit in patients irrespective of their baseline biomarker level, including patients with low eosinophil levels at baseline,” said Jean-Pierre Llanos-Ackert, MD, who is executive medical director and global medical affairs lead for tezepelumab at Amgen.
The primary endpoint data look robust, according to Praveen Akuthota, MD, who is an associate professor of medicine at the University of California, San Diego, and comoderated the session at the American Thoracic Society’s virtual international conference, where the research was presented. The study was also published on May 13, 2021, in the New England Journal of Medicine. The conference session included updated results.
The drug holds promise, but more study is needed. “The question really will be, how is this drug different from the existing biologics? How much better is this drug in patients who have borderline T2 biomarkers, or even low T2. The study does show some efficacy in patients whose T2 signals may not be as robust. We’ll have to see with ongoing longitudinal data, how this drug positions, compared to the other agents. It’s obviously exciting, though, to have another option, given that we know what our current armamentarium of agents there are still nonresponders,” said Dr. Akuthota in an interview.
The other comoderator in the session, Laura Crotty Alexander, MD, commented: “It seems like it might work possibly even better than some that are directly covering one pathway only. Hopefully, this agent will be efficacious in a broader population than some of the more targeted biologics.” Dr. Alexander is an associate professor of medicine at the University of California, San Diego, and section chief of pulmonary critical care at the Veterans Affairs San Diego Healthcare System.
She pointed out that physicians often think of asthma patients in broad brush terms, as high or low T2, or T2 high and Th1 or neutrophilic or obese, but many patients present a more complicated picture. “There is some overlap across those phenotypes, such that an agent that works really well for one group doesn’t mean that it won’t have an impact, especially clinically, on some of these other phenotypes,” said Dr. Alexander.
Dr. Akuthota agreed. “Having options for patients whose biomarkers are not maybe as clear is, I think, important.”
Promising results
The study included 1,059 patients aged 12-80 who received 210 mg tezepelumab or placebo. Over 52 weeks, the treatment group had a 79% reduction in exacerbations requiring hospitalization or an ED visit, compared with placebo (rate ratio, 0.21; 95% confidence interval, 0.12-0.37), and an 85% reduction in exacerbations requiring hospitalization (RR, 0.15; 95% CI, 0.07-0.33). The drug increased the time to first exacerbation requiring hospitalization that required hospitalization or an ED visit, reducing risk by 65% (hazard ratio, 0.35; 95% CI, 0.22-0.56).
Fewer patients in the treatment group than placebo used asthma-related health care resources, including: ED visits (32 vs. 94), unscheduled visit to a specialist (285 vs. 406), telephone calls to a health care provider (234 vs. 599), ambulance transport (5 vs. 22), and home visits from a health care provider (18 vs. 22). Fewer patients in the tezepelumab group had hospital stays (3.2% vs. 7.0%), and they had a lower total number of hospital days (108 vs. 497) and days in the ICU (0 vs. 31).
The study was funded by Amgen and AstraZeneca. Dr. Llanos-Ackert is an employee of Amgen. Dr. Alexander has no relevant financial disclosures. Dr. Akuthota has consulted for AstraZeneca and participated in their clinical trials.
Results from the NAVIGATOR study of tezepelumab showed that treatment of adults and adolescents with severe, uncontrolled asthma with the new biologic led to a large reduction in exacerbations requiring hospital stays and ED visits.
Tezepelumab, codeveloped by Amgen and AstraZeneca, has a novel mechanism of action. It blocks thymic stromal lymphopoietin (TSLP), which is a cytokine produced by epithelial cells. TSLP levels correlate with airway obstruction, severity of disease, and glucocorticoid resistance. TSLP is involved in T2 inflammation within the airway, but also plays a role in the interactions between airway cells and immune cells, which doesn’t rely only solely on T2 inflammation. That broad mechanism of action distinguishes tezepelumab from most other biologics for the treatment of asthma, which are more targeted.
“By working at the top of the cascade, tezepelumab helps stop inflammation at a key source. Clinical trials with tezepelumab showed a clinical benefit in patients irrespective of their baseline biomarker level, including patients with low eosinophil levels at baseline,” said Jean-Pierre Llanos-Ackert, MD, who is executive medical director and global medical affairs lead for tezepelumab at Amgen.
The primary endpoint data look robust, according to Praveen Akuthota, MD, who is an associate professor of medicine at the University of California, San Diego, and comoderated the session at the American Thoracic Society’s virtual international conference, where the research was presented. The study was also published on May 13, 2021, in the New England Journal of Medicine. The conference session included updated results.
The drug holds promise, but more study is needed. “The question really will be, how is this drug different from the existing biologics? How much better is this drug in patients who have borderline T2 biomarkers, or even low T2. The study does show some efficacy in patients whose T2 signals may not be as robust. We’ll have to see with ongoing longitudinal data, how this drug positions, compared to the other agents. It’s obviously exciting, though, to have another option, given that we know what our current armamentarium of agents there are still nonresponders,” said Dr. Akuthota in an interview.
The other comoderator in the session, Laura Crotty Alexander, MD, commented: “It seems like it might work possibly even better than some that are directly covering one pathway only. Hopefully, this agent will be efficacious in a broader population than some of the more targeted biologics.” Dr. Alexander is an associate professor of medicine at the University of California, San Diego, and section chief of pulmonary critical care at the Veterans Affairs San Diego Healthcare System.
She pointed out that physicians often think of asthma patients in broad brush terms, as high or low T2, or T2 high and Th1 or neutrophilic or obese, but many patients present a more complicated picture. “There is some overlap across those phenotypes, such that an agent that works really well for one group doesn’t mean that it won’t have an impact, especially clinically, on some of these other phenotypes,” said Dr. Alexander.
Dr. Akuthota agreed. “Having options for patients whose biomarkers are not maybe as clear is, I think, important.”
Promising results
The study included 1,059 patients aged 12-80 who received 210 mg tezepelumab or placebo. Over 52 weeks, the treatment group had a 79% reduction in exacerbations requiring hospitalization or an ED visit, compared with placebo (rate ratio, 0.21; 95% confidence interval, 0.12-0.37), and an 85% reduction in exacerbations requiring hospitalization (RR, 0.15; 95% CI, 0.07-0.33). The drug increased the time to first exacerbation requiring hospitalization that required hospitalization or an ED visit, reducing risk by 65% (hazard ratio, 0.35; 95% CI, 0.22-0.56).
Fewer patients in the treatment group than placebo used asthma-related health care resources, including: ED visits (32 vs. 94), unscheduled visit to a specialist (285 vs. 406), telephone calls to a health care provider (234 vs. 599), ambulance transport (5 vs. 22), and home visits from a health care provider (18 vs. 22). Fewer patients in the tezepelumab group had hospital stays (3.2% vs. 7.0%), and they had a lower total number of hospital days (108 vs. 497) and days in the ICU (0 vs. 31).
The study was funded by Amgen and AstraZeneca. Dr. Llanos-Ackert is an employee of Amgen. Dr. Alexander has no relevant financial disclosures. Dr. Akuthota has consulted for AstraZeneca and participated in their clinical trials.
FROM ATS 2021
Combination Therapy for Severe Asthma
Patients with severe asthma often experience symptoms and exacerbations that can interfere with daily life and further compromise lung function.
These patients often need combination therapy to achieve optimal control. This typically includes a low-dose inhaled corticosteroid (ICS) plus a long-acting beta-agonist (LABA).
For some patients, however, adherence to these therapies will not result in optimal outcomes.
Dr Monica Kraft, of the University of Arizona Health Sciences Center, discusses additional therapeutic options for these patients, which include increasing the dose of ICS or adding an oral corticosteroid such as prednisone or methylprednisolone.
When treatment-adherent patients are still unable to maintain control of their asthma symptoms, it may be optimal to move to biologic therapy.
There are currently five available biologics that work against IgE, IL-4/IL-13, IL-5, and IL-5R. Biomarker testing for blood eosinophils, exhaled nitric oxide, and serum IgE can help determine which biologic is best suited to each individual patient.
--
Robert and Irene Flinn Professor, Department of Medicine, Banner University Medical Center, North Campus; Chair, Department of Medicine, University of Arizona Health Sciences Center, Tucson, Arizona.
Monica Kraft, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AstraZeneca; Genentech; Chiesi; Sanofi
Serve(d) as Chief Medical Officer for: RaeSedo, LLC
Received research grant from: National Institutes of Health; American Lung Association; Sanofi; AstraZeneca; Chiesi
received income in an amount equal to or greater than $250 from: AstraZeneca; Genentech; Chiesi; Sanof.
Patients with severe asthma often experience symptoms and exacerbations that can interfere with daily life and further compromise lung function.
These patients often need combination therapy to achieve optimal control. This typically includes a low-dose inhaled corticosteroid (ICS) plus a long-acting beta-agonist (LABA).
For some patients, however, adherence to these therapies will not result in optimal outcomes.
Dr Monica Kraft, of the University of Arizona Health Sciences Center, discusses additional therapeutic options for these patients, which include increasing the dose of ICS or adding an oral corticosteroid such as prednisone or methylprednisolone.
When treatment-adherent patients are still unable to maintain control of their asthma symptoms, it may be optimal to move to biologic therapy.
There are currently five available biologics that work against IgE, IL-4/IL-13, IL-5, and IL-5R. Biomarker testing for blood eosinophils, exhaled nitric oxide, and serum IgE can help determine which biologic is best suited to each individual patient.
--
Robert and Irene Flinn Professor, Department of Medicine, Banner University Medical Center, North Campus; Chair, Department of Medicine, University of Arizona Health Sciences Center, Tucson, Arizona.
Monica Kraft, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AstraZeneca; Genentech; Chiesi; Sanofi
Serve(d) as Chief Medical Officer for: RaeSedo, LLC
Received research grant from: National Institutes of Health; American Lung Association; Sanofi; AstraZeneca; Chiesi
received income in an amount equal to or greater than $250 from: AstraZeneca; Genentech; Chiesi; Sanof.
Patients with severe asthma often experience symptoms and exacerbations that can interfere with daily life and further compromise lung function.
These patients often need combination therapy to achieve optimal control. This typically includes a low-dose inhaled corticosteroid (ICS) plus a long-acting beta-agonist (LABA).
For some patients, however, adherence to these therapies will not result in optimal outcomes.
Dr Monica Kraft, of the University of Arizona Health Sciences Center, discusses additional therapeutic options for these patients, which include increasing the dose of ICS or adding an oral corticosteroid such as prednisone or methylprednisolone.
When treatment-adherent patients are still unable to maintain control of their asthma symptoms, it may be optimal to move to biologic therapy.
There are currently five available biologics that work against IgE, IL-4/IL-13, IL-5, and IL-5R. Biomarker testing for blood eosinophils, exhaled nitric oxide, and serum IgE can help determine which biologic is best suited to each individual patient.
--
Robert and Irene Flinn Professor, Department of Medicine, Banner University Medical Center, North Campus; Chair, Department of Medicine, University of Arizona Health Sciences Center, Tucson, Arizona.
Monica Kraft, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: AstraZeneca; Genentech; Chiesi; Sanofi
Serve(d) as Chief Medical Officer for: RaeSedo, LLC
Received research grant from: National Institutes of Health; American Lung Association; Sanofi; AstraZeneca; Chiesi
received income in an amount equal to or greater than $250 from: AstraZeneca; Genentech; Chiesi; Sanof.




