User login
Topical POLG nanoemulsion improves dryness and itchiness in atopic dermatitis
Key clinical point: Daily continuous use of 10% phytosteryl/octyldodecyl lauroyl glutamate (POLG) nanoemulsion improved several dry skin symptoms and itchiness in patients with atopic dermatitis (AD).
Major finding: Significant improvement was observed with POLG nanoemulsion in itchiness as early as at 3 weeks (P < .05) and dryness at 6 weeks (P < .01). By 6 weeks, scaliness and smoothness of the skin were also significantly improved (P < .01).
Study details: Findings are from a clinical study including patients with AD who received 10% POLG nanoemulsion for 6 weeks.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Takada M et al. A nano-emulsion containing ceramide-like lipo-amino acid cholesteryl derivatives improves skin symptoms in patients with atopic dermatitis by ameliorating the water-holding function. Int J Mol Sci. 2022;23(21):13362 (Nov 1). Doi: 10.3390/ijms232113362
Key clinical point: Daily continuous use of 10% phytosteryl/octyldodecyl lauroyl glutamate (POLG) nanoemulsion improved several dry skin symptoms and itchiness in patients with atopic dermatitis (AD).
Major finding: Significant improvement was observed with POLG nanoemulsion in itchiness as early as at 3 weeks (P < .05) and dryness at 6 weeks (P < .01). By 6 weeks, scaliness and smoothness of the skin were also significantly improved (P < .01).
Study details: Findings are from a clinical study including patients with AD who received 10% POLG nanoemulsion for 6 weeks.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Takada M et al. A nano-emulsion containing ceramide-like lipo-amino acid cholesteryl derivatives improves skin symptoms in patients with atopic dermatitis by ameliorating the water-holding function. Int J Mol Sci. 2022;23(21):13362 (Nov 1). Doi: 10.3390/ijms232113362
Key clinical point: Daily continuous use of 10% phytosteryl/octyldodecyl lauroyl glutamate (POLG) nanoemulsion improved several dry skin symptoms and itchiness in patients with atopic dermatitis (AD).
Major finding: Significant improvement was observed with POLG nanoemulsion in itchiness as early as at 3 weeks (P < .05) and dryness at 6 weeks (P < .01). By 6 weeks, scaliness and smoothness of the skin were also significantly improved (P < .01).
Study details: Findings are from a clinical study including patients with AD who received 10% POLG nanoemulsion for 6 weeks.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Takada M et al. A nano-emulsion containing ceramide-like lipo-amino acid cholesteryl derivatives improves skin symptoms in patients with atopic dermatitis by ameliorating the water-holding function. Int J Mol Sci. 2022;23(21):13362 (Nov 1). Doi: 10.3390/ijms232113362
Emollient use in the first year of life does not protect against atopic dermatitis in the long term
Key clinical point: Daily application of emollients during the first year of life did not prevent the development of atopic dermatitis (AD) in the long term.
Major finding: A similar proportion of children in the emollient and standard skincare groups were clinically diagnosed with AD between 12 and 60 months (31% and 28%, respectively; adjusted relative risk 1.10; 95% CI 0.93-1.30).
Study details: Findings are from the multicenter, parallel, Barrier Enhancement for Eczema Prevention trial including 1394 infants at high risk of developing AD who were randomly assigned to receive emollient for the first year plus standard skincare or only standard skincare.
Disclosures: This study was funded by the UK National Institute for Health and Care Research Health Technology Assessment. Some authors declared receiving personal fees, grants, or research funding from or serving as an investigator or director for several sources.
Source: Bradshaw LE et al. Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy. 2022 (Oct 19). Doi: 10.1111/all.15555
Key clinical point: Daily application of emollients during the first year of life did not prevent the development of atopic dermatitis (AD) in the long term.
Major finding: A similar proportion of children in the emollient and standard skincare groups were clinically diagnosed with AD between 12 and 60 months (31% and 28%, respectively; adjusted relative risk 1.10; 95% CI 0.93-1.30).
Study details: Findings are from the multicenter, parallel, Barrier Enhancement for Eczema Prevention trial including 1394 infants at high risk of developing AD who were randomly assigned to receive emollient for the first year plus standard skincare or only standard skincare.
Disclosures: This study was funded by the UK National Institute for Health and Care Research Health Technology Assessment. Some authors declared receiving personal fees, grants, or research funding from or serving as an investigator or director for several sources.
Source: Bradshaw LE et al. Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy. 2022 (Oct 19). Doi: 10.1111/all.15555
Key clinical point: Daily application of emollients during the first year of life did not prevent the development of atopic dermatitis (AD) in the long term.
Major finding: A similar proportion of children in the emollient and standard skincare groups were clinically diagnosed with AD between 12 and 60 months (31% and 28%, respectively; adjusted relative risk 1.10; 95% CI 0.93-1.30).
Study details: Findings are from the multicenter, parallel, Barrier Enhancement for Eczema Prevention trial including 1394 infants at high risk of developing AD who were randomly assigned to receive emollient for the first year plus standard skincare or only standard skincare.
Disclosures: This study was funded by the UK National Institute for Health and Care Research Health Technology Assessment. Some authors declared receiving personal fees, grants, or research funding from or serving as an investigator or director for several sources.
Source: Bradshaw LE et al. Emollients for prevention of atopic dermatitis: 5-year findings from the BEEP randomized trial. Allergy. 2022 (Oct 19). Doi: 10.1111/all.15555
Topical prebiotics and postbiotics effective and well tolerated in mild-to-moderate atopic dermatitis
Key clinical point: A topical formulation containing a mixture of prebiotics and postbiotics was effective and well tolerated in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: After 15 weeks, the SCORing AD index (−59.2%; P < .001) and the PRURISCORE (−64.1%; P < .001) reduced significantly, with 68.0% of patients reporting the tolerability of the drug as “very good” or “excellent.”
Study details: Findings are from a study including 396 patients with mild or moderate AD who received a topical formulation containing a mixture of prebiotics and postbiotics.
Disclosures: This work was supported by the Istituto Ganassini di Ricerche Biochimiche, Italy. The authors declared no conflicts of interest.
Source: Gelmetti C et al. Topical prebiotics/postbiotics and PRURISCORE validation in atopic dermatitis. International study of 396 patients. J Dermatolog Treat. 2022 (Oct 17). Doi: 10.1080/09546634.2022.2131703
Key clinical point: A topical formulation containing a mixture of prebiotics and postbiotics was effective and well tolerated in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: After 15 weeks, the SCORing AD index (−59.2%; P < .001) and the PRURISCORE (−64.1%; P < .001) reduced significantly, with 68.0% of patients reporting the tolerability of the drug as “very good” or “excellent.”
Study details: Findings are from a study including 396 patients with mild or moderate AD who received a topical formulation containing a mixture of prebiotics and postbiotics.
Disclosures: This work was supported by the Istituto Ganassini di Ricerche Biochimiche, Italy. The authors declared no conflicts of interest.
Source: Gelmetti C et al. Topical prebiotics/postbiotics and PRURISCORE validation in atopic dermatitis. International study of 396 patients. J Dermatolog Treat. 2022 (Oct 17). Doi: 10.1080/09546634.2022.2131703
Key clinical point: A topical formulation containing a mixture of prebiotics and postbiotics was effective and well tolerated in patients with mild-to-moderate atopic dermatitis (AD).
Major finding: After 15 weeks, the SCORing AD index (−59.2%; P < .001) and the PRURISCORE (−64.1%; P < .001) reduced significantly, with 68.0% of patients reporting the tolerability of the drug as “very good” or “excellent.”
Study details: Findings are from a study including 396 patients with mild or moderate AD who received a topical formulation containing a mixture of prebiotics and postbiotics.
Disclosures: This work was supported by the Istituto Ganassini di Ricerche Biochimiche, Italy. The authors declared no conflicts of interest.
Source: Gelmetti C et al. Topical prebiotics/postbiotics and PRURISCORE validation in atopic dermatitis. International study of 396 patients. J Dermatolog Treat. 2022 (Oct 17). Doi: 10.1080/09546634.2022.2131703
Atopic dermatitis patients with good clinical response or conjunctivitis may opt for longer dupilumab dosing interval
Key clinical point: A longer dupilumab dosing interval might be a good treatment option for patients with atopic dermatitis (AD) who have achieved good clinical response (GCR) or report treatment-related conjunctivitis with a previous dupilumab treatment (600 mg followed by 300 mg every 2 weeks).
Major finding: In the GCR group, the mean Eczema Area and Severity Index (EASI) score was 28.22, which reduced significantly to 0.44 among patients receiving dupilumab once every 3 weeks (Q3W) and to 0.19 among patients receiving dupilumab once every 4 weeks (Q4W) after >60 weeks (both P < .0001). EASI improved after 18 weeks in the treatment-resistant conjunctivitis group (P < .0001).
Study details: Findings are retrospectively collected data of 59 adult patients with AD who implemented Q3W (84.75%) or Q4W (15.25%) dupilumab dosing interval due to GCR or conjunctivitis.
Disclosures: This study did not receive any funding. Some authors declared serving as speakers, investigators, consultants, or advisory board members, or receiving personal fees from several sources.
Source: Patruno C et al. Dupilumab dose spacing after initial successful treatment or adverse events in adult patients with atopic dermatitis: A retrospective analysis. Dermatol Ther. 2022 (Oct 13). Doi: 10.1111/dth.15933
Key clinical point: A longer dupilumab dosing interval might be a good treatment option for patients with atopic dermatitis (AD) who have achieved good clinical response (GCR) or report treatment-related conjunctivitis with a previous dupilumab treatment (600 mg followed by 300 mg every 2 weeks).
Major finding: In the GCR group, the mean Eczema Area and Severity Index (EASI) score was 28.22, which reduced significantly to 0.44 among patients receiving dupilumab once every 3 weeks (Q3W) and to 0.19 among patients receiving dupilumab once every 4 weeks (Q4W) after >60 weeks (both P < .0001). EASI improved after 18 weeks in the treatment-resistant conjunctivitis group (P < .0001).
Study details: Findings are retrospectively collected data of 59 adult patients with AD who implemented Q3W (84.75%) or Q4W (15.25%) dupilumab dosing interval due to GCR or conjunctivitis.
Disclosures: This study did not receive any funding. Some authors declared serving as speakers, investigators, consultants, or advisory board members, or receiving personal fees from several sources.
Source: Patruno C et al. Dupilumab dose spacing after initial successful treatment or adverse events in adult patients with atopic dermatitis: A retrospective analysis. Dermatol Ther. 2022 (Oct 13). Doi: 10.1111/dth.15933
Key clinical point: A longer dupilumab dosing interval might be a good treatment option for patients with atopic dermatitis (AD) who have achieved good clinical response (GCR) or report treatment-related conjunctivitis with a previous dupilumab treatment (600 mg followed by 300 mg every 2 weeks).
Major finding: In the GCR group, the mean Eczema Area and Severity Index (EASI) score was 28.22, which reduced significantly to 0.44 among patients receiving dupilumab once every 3 weeks (Q3W) and to 0.19 among patients receiving dupilumab once every 4 weeks (Q4W) after >60 weeks (both P < .0001). EASI improved after 18 weeks in the treatment-resistant conjunctivitis group (P < .0001).
Study details: Findings are retrospectively collected data of 59 adult patients with AD who implemented Q3W (84.75%) or Q4W (15.25%) dupilumab dosing interval due to GCR or conjunctivitis.
Disclosures: This study did not receive any funding. Some authors declared serving as speakers, investigators, consultants, or advisory board members, or receiving personal fees from several sources.
Source: Patruno C et al. Dupilumab dose spacing after initial successful treatment or adverse events in adult patients with atopic dermatitis: A retrospective analysis. Dermatol Ther. 2022 (Oct 13). Doi: 10.1111/dth.15933
Long-term efficacy of baricitinib in moderate-to-severe atopic dermatitis
Key clinical point: Baricitinib demonstrated long-term (52 weeks) efficacy in reducing disease severity in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 52, >45% of patients achieved ≥75% improvement in the Eczema Area and Severity Index (EASI), with a mean improvement of 56.8 points in the total EASI score.
Study details: Findings are from the phase 3, BREEZE-AD5 study including 146 patients with moderate-to-severe AD who were assigned to receive 2 mg baricitinib, of which 98 patients participated in the open-label extension, BREEZE-AD6 study.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lily.
Source: Simpson E et al. Baricitinib 2 mg for the treatment of atopic dermatitis in North America: Long-term efficacy and patient-reported outcomes. Dermatol Ther. 2022 (Oct 21). Doi: 10.1111/dth.15954
Key clinical point: Baricitinib demonstrated long-term (52 weeks) efficacy in reducing disease severity in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 52, >45% of patients achieved ≥75% improvement in the Eczema Area and Severity Index (EASI), with a mean improvement of 56.8 points in the total EASI score.
Study details: Findings are from the phase 3, BREEZE-AD5 study including 146 patients with moderate-to-severe AD who were assigned to receive 2 mg baricitinib, of which 98 patients participated in the open-label extension, BREEZE-AD6 study.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lily.
Source: Simpson E et al. Baricitinib 2 mg for the treatment of atopic dermatitis in North America: Long-term efficacy and patient-reported outcomes. Dermatol Ther. 2022 (Oct 21). Doi: 10.1111/dth.15954
Key clinical point: Baricitinib demonstrated long-term (52 weeks) efficacy in reducing disease severity in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 52, >45% of patients achieved ≥75% improvement in the Eczema Area and Severity Index (EASI), with a mean improvement of 56.8 points in the total EASI score.
Study details: Findings are from the phase 3, BREEZE-AD5 study including 146 patients with moderate-to-severe AD who were assigned to receive 2 mg baricitinib, of which 98 patients participated in the open-label extension, BREEZE-AD6 study.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lily.
Source: Simpson E et al. Baricitinib 2 mg for the treatment of atopic dermatitis in North America: Long-term efficacy and patient-reported outcomes. Dermatol Ther. 2022 (Oct 21). Doi: 10.1111/dth.15954
Long-term efficacy of baricitinib in moderate-to-severe atopic dermatitis
Key clinical point: Baricitinib demonstrated long-term (52 weeks) efficacy in reducing disease severity in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 52, >45% of patients achieved ≥75% improvement in the Eczema Area and Severity Index (EASI), with a mean improvement of 56.8 points in the total EASI score.
Study details: Findings are from the phase 3, BREEZE-AD5 study including 146 patients with moderate-to-severe AD who were assigned to receive 2 mg baricitinib, of which 98 patients participated in the open-label extension, BREEZE-AD6 study.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lily.
Source: Simpson E et al. Baricitinib 2 mg for the treatment of atopic dermatitis in North America: Long-term efficacy and patient-reported outcomes. Dermatol Ther. 2022 (Oct 21). Doi: 10.1111/dth.15954
Key clinical point: Baricitinib demonstrated long-term (52 weeks) efficacy in reducing disease severity in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 52, >45% of patients achieved ≥75% improvement in the Eczema Area and Severity Index (EASI), with a mean improvement of 56.8 points in the total EASI score.
Study details: Findings are from the phase 3, BREEZE-AD5 study including 146 patients with moderate-to-severe AD who were assigned to receive 2 mg baricitinib, of which 98 patients participated in the open-label extension, BREEZE-AD6 study.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lily.
Source: Simpson E et al. Baricitinib 2 mg for the treatment of atopic dermatitis in North America: Long-term efficacy and patient-reported outcomes. Dermatol Ther. 2022 (Oct 21). Doi: 10.1111/dth.15954
Key clinical point: Baricitinib demonstrated long-term (52 weeks) efficacy in reducing disease severity in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: At week 52, >45% of patients achieved ≥75% improvement in the Eczema Area and Severity Index (EASI), with a mean improvement of 56.8 points in the total EASI score.
Study details: Findings are from the phase 3, BREEZE-AD5 study including 146 patients with moderate-to-severe AD who were assigned to receive 2 mg baricitinib, of which 98 patients participated in the open-label extension, BREEZE-AD6 study.
Disclosures: This study was funded by Eli Lilly and Company, under license from Incyte Corporation. Five authors declared being employees and shareholders of Eli Lilly, and the other authors reported ties with several sources, including Eli Lily.
Source: Simpson E et al. Baricitinib 2 mg for the treatment of atopic dermatitis in North America: Long-term efficacy and patient-reported outcomes. Dermatol Ther. 2022 (Oct 21). Doi: 10.1111/dth.15954
Moderate-to-severe atopic dermatitis: No increased infection risk with long-term dupilumab use
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous long-term dupilumab treatment was not associated with an increased risk for overall systemic/cutaneous infections.
Major finding: At 4 years, the overall infection rate was 71.27 number of patients with ≥1 event per 100 patient-years (nP/100 PY), with most infections being mild to moderate in severity, and only a very small number of infections resulted in treatment discontinuation (0.34 nP/100 PY). The rate of total skin infections decreased from 28.10 to 11.48 nP/100 PY from week 16 to year 4.
Study details: Findings are from the analysis of the LIBERTY AD OLE study including 2677 patients with moderate-to-severe AD who received dupilumab, of which 13.1% completed treatment up to week 204.
Disclosures: This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Four authors declared being employees and shareholders of Regeneron Pharmaceuticals. Three authors declared being employees or holding stock options in Sanofi. The other authors reported ties with several sources, including Regeneron and Sanofi.
Source: Blauvelt A et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2022 (Nov 1). Doi: 10.1007/s12325-022-02322-y
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous long-term dupilumab treatment was not associated with an increased risk for overall systemic/cutaneous infections.
Major finding: At 4 years, the overall infection rate was 71.27 number of patients with ≥1 event per 100 patient-years (nP/100 PY), with most infections being mild to moderate in severity, and only a very small number of infections resulted in treatment discontinuation (0.34 nP/100 PY). The rate of total skin infections decreased from 28.10 to 11.48 nP/100 PY from week 16 to year 4.
Study details: Findings are from the analysis of the LIBERTY AD OLE study including 2677 patients with moderate-to-severe AD who received dupilumab, of which 13.1% completed treatment up to week 204.
Disclosures: This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Four authors declared being employees and shareholders of Regeneron Pharmaceuticals. Three authors declared being employees or holding stock options in Sanofi. The other authors reported ties with several sources, including Regeneron and Sanofi.
Source: Blauvelt A et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2022 (Nov 1). Doi: 10.1007/s12325-022-02322-y
Key clinical point: In patients with moderate-to-severe atopic dermatitis (AD), continuous long-term dupilumab treatment was not associated with an increased risk for overall systemic/cutaneous infections.
Major finding: At 4 years, the overall infection rate was 71.27 number of patients with ≥1 event per 100 patient-years (nP/100 PY), with most infections being mild to moderate in severity, and only a very small number of infections resulted in treatment discontinuation (0.34 nP/100 PY). The rate of total skin infections decreased from 28.10 to 11.48 nP/100 PY from week 16 to year 4.
Study details: Findings are from the analysis of the LIBERTY AD OLE study including 2677 patients with moderate-to-severe AD who received dupilumab, of which 13.1% completed treatment up to week 204.
Disclosures: This research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Four authors declared being employees and shareholders of Regeneron Pharmaceuticals. Three authors declared being employees or holding stock options in Sanofi. The other authors reported ties with several sources, including Regeneron and Sanofi.
Source: Blauvelt A et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2022 (Nov 1). Doi: 10.1007/s12325-022-02322-y
Exposure to wildfire air pollution increases atopic dermatitis risk in older adults
Key clinical point: Air pollution due to a wildfire increased the rate of clinic visits for atopic dermatitis (AD), especially at a 0-week lag, in adults aged ≥65 years.
Major finding: In adults aged ≥65 years, the adjusted rate of clinic visits for AD during a week with a wildfire was 1.4 (95% CI 1.1-1.9) times the rate during weeks without wildfire and every 1-unit increase in the mean weekly smoke plume density score increased the rate of clinic visits for AD by 1.3 (95% CI 1.1-1.6) times.
Study details: This study analyzed the data of outpatient dermatology visits for AD (5529 visits) and itch (1319 visits).
Disclosures: This study did not report the source of funding. Dr. Grimes declared receiving grants from the University of California, San Francisco.
Source: Fadadu RP et al. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw Open. 2022;5(10):e2238594 (Oct 26). Doi: 10.1001/jamanetworkopen.2022.38594
Key clinical point: Air pollution due to a wildfire increased the rate of clinic visits for atopic dermatitis (AD), especially at a 0-week lag, in adults aged ≥65 years.
Major finding: In adults aged ≥65 years, the adjusted rate of clinic visits for AD during a week with a wildfire was 1.4 (95% CI 1.1-1.9) times the rate during weeks without wildfire and every 1-unit increase in the mean weekly smoke plume density score increased the rate of clinic visits for AD by 1.3 (95% CI 1.1-1.6) times.
Study details: This study analyzed the data of outpatient dermatology visits for AD (5529 visits) and itch (1319 visits).
Disclosures: This study did not report the source of funding. Dr. Grimes declared receiving grants from the University of California, San Francisco.
Source: Fadadu RP et al. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw Open. 2022;5(10):e2238594 (Oct 26). Doi: 10.1001/jamanetworkopen.2022.38594
Key clinical point: Air pollution due to a wildfire increased the rate of clinic visits for atopic dermatitis (AD), especially at a 0-week lag, in adults aged ≥65 years.
Major finding: In adults aged ≥65 years, the adjusted rate of clinic visits for AD during a week with a wildfire was 1.4 (95% CI 1.1-1.9) times the rate during weeks without wildfire and every 1-unit increase in the mean weekly smoke plume density score increased the rate of clinic visits for AD by 1.3 (95% CI 1.1-1.6) times.
Study details: This study analyzed the data of outpatient dermatology visits for AD (5529 visits) and itch (1319 visits).
Disclosures: This study did not report the source of funding. Dr. Grimes declared receiving grants from the University of California, San Francisco.
Source: Fadadu RP et al. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw Open. 2022;5(10):e2238594 (Oct 26). Doi: 10.1001/jamanetworkopen.2022.38594
Atopic dermatitis: Dupilumab serum levels not associated with treatment response or adverse effects
Key clinical point: In patients with atopic dermatitis (AD), serum dupilumab levels at week 16 were not associated with treatment response or adverse effects due to dupilumab during the first year of treatment.
Major finding: Serum dupilumab levels at 16 weeks were not associated with the prediction of treatment response at 52 weeks (≥90% improvement in the Eczema Area and Severity Index; odds ratio [OR] 0.96; P = .34) or adverse events during the first year of treatment (OR 1.01; P = .83).
Study details: Findings are from a prospective clinical cohort study including 295 patients with AD who started dupilumab and had treatment week 16 serum samples available.
Disclosures: This study was funded by AbbVie, Eli Lilly, and other sources. The authors declared receiving consulting fees, speaking fees, investigator fees, or research funding from several sources.
Source: Spekhorst LS et al. Association of serum dupilumab levels at 16 weeks with treatment response and adverse effects in patients with atopic dermatitis: A prospective clinical cohort study from the BioDay registry. JAMA Dermatol. 2022 (Nov 2). Doi: 10.1001/jamadermatol.2022.4639
Key clinical point: In patients with atopic dermatitis (AD), serum dupilumab levels at week 16 were not associated with treatment response or adverse effects due to dupilumab during the first year of treatment.
Major finding: Serum dupilumab levels at 16 weeks were not associated with the prediction of treatment response at 52 weeks (≥90% improvement in the Eczema Area and Severity Index; odds ratio [OR] 0.96; P = .34) or adverse events during the first year of treatment (OR 1.01; P = .83).
Study details: Findings are from a prospective clinical cohort study including 295 patients with AD who started dupilumab and had treatment week 16 serum samples available.
Disclosures: This study was funded by AbbVie, Eli Lilly, and other sources. The authors declared receiving consulting fees, speaking fees, investigator fees, or research funding from several sources.
Source: Spekhorst LS et al. Association of serum dupilumab levels at 16 weeks with treatment response and adverse effects in patients with atopic dermatitis: A prospective clinical cohort study from the BioDay registry. JAMA Dermatol. 2022 (Nov 2). Doi: 10.1001/jamadermatol.2022.4639
Key clinical point: In patients with atopic dermatitis (AD), serum dupilumab levels at week 16 were not associated with treatment response or adverse effects due to dupilumab during the first year of treatment.
Major finding: Serum dupilumab levels at 16 weeks were not associated with the prediction of treatment response at 52 weeks (≥90% improvement in the Eczema Area and Severity Index; odds ratio [OR] 0.96; P = .34) or adverse events during the first year of treatment (OR 1.01; P = .83).
Study details: Findings are from a prospective clinical cohort study including 295 patients with AD who started dupilumab and had treatment week 16 serum samples available.
Disclosures: This study was funded by AbbVie, Eli Lilly, and other sources. The authors declared receiving consulting fees, speaking fees, investigator fees, or research funding from several sources.
Source: Spekhorst LS et al. Association of serum dupilumab levels at 16 weeks with treatment response and adverse effects in patients with atopic dermatitis: A prospective clinical cohort study from the BioDay registry. JAMA Dermatol. 2022 (Nov 2). Doi: 10.1001/jamadermatol.2022.4639
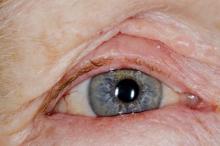
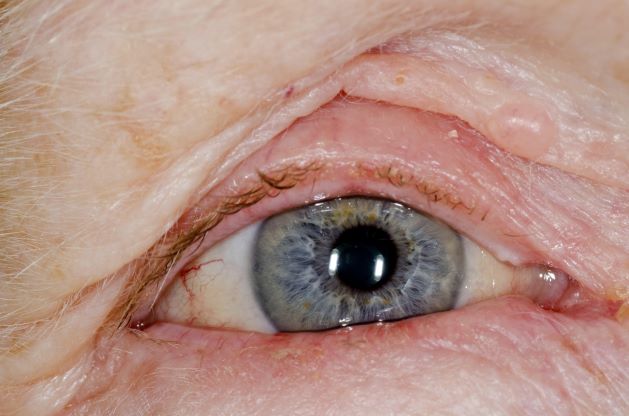
Red swollen eyelids
This patient's symptoms are consistent with a diagnosis of blepharitis.
Blepharitis is an inflammatory disorder of the eyelids that is frequently associated with bacterial colonization of the eyelid. Anatomically, it can be categorized as anterior blepharitis or posterior blepharitis. Anterior blepharitis refers to inflammation primarily positioned around the skin, eyelashes, and lash follicles and is usually further divided into staphylococcal and seborrheic variants. Posterior blepharitis involves the meibomian gland orifices, meibomian glands, tarsal plate, and blepharo-conjunctival junction.
Blepharitis can be acute or chronic. It is frequently associated with systemic diseases, such as rosacea, atopy, and seborrheic dermatitis, as well as ocular diseases, such as dry eye syndromes, chalazion, trichiasis, ectropion and entropion, infectious or other inflammatory conjunctivitis, and keratitis. Moreover, high rates of blepharitis have been reported in patients treated with dupilumab for atopic dermatitis.
Eye irritation, itching, erythema of the lids, flaking of the lid margins, and/or changes in the eyelashes are common presenting symptoms in patients with blepharitis. Other symptoms may include:
• Burning
• Watering
• Foreign-body sensation
• Crusting and mattering of the lashes and medial canthus
• Red lids
• Red eyes
• Photophobia
• Pain
• Decreased vision
• Visual fluctuations
• Heat, cold, alcohol, and spicy-food intolerance
The differential diagnosis for blepharitis includes bacterial keratitis, which is a serious ocular disorder that can lead to vision loss if not properly treated. Bacterial keratitis progresses rapidly and can result in corneal destruction within 24-48 hours with some particularly virulent bacteria. Patients with bacterial keratitis typically report rapid onset of pain, photophobia, and decreased vision.
Ocular rosacea should also be considered in the differential diagnosis of blepharitis, and the two conditions can co-occur. Patients with ocular rosacea may experience facial symptoms (eg, recurrent flushing episodes, persistent and/or recurrent midfacial erythema, papular and pustular lesions) in addition to ocular symptoms, which can range from minor irritation, foreign-body sensation, and blurry vision to severe ocular surface disruption and inflammatory keratitis.
Bacterial conjunctivitis involves inflammation of the bulbar and/or palpebral conjunctiva, whereas blepharitis involves inflammation of the eyelids only. Other conditions to consider in the diagnosis of blepharitis can be found here.
Given the unprecedented efficacy seen in clinical trials, dupilumab is emerging as a first-line therapeutic for moderate to severe atopic dermatitis. However, clinicians should be alert to ocular complications among their patients with atopic dermatitis who are being treated with dupilumab. In some patients, this may be because of preexisting meibomian gland disease and ocular surface disease. After a diagnosis of ocular complications, the continued use of dupilumab should be jointly evaluated by the ophthalmologist and dermatologist or allergist on the basis of the ocular risk vs systemic benefit. Treatment for blepharitis typically includes strict eyelid hygiene and topical antibiotic ointment; oral antibiotics can be beneficial for refractory disease.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's symptoms are consistent with a diagnosis of blepharitis.
Blepharitis is an inflammatory disorder of the eyelids that is frequently associated with bacterial colonization of the eyelid. Anatomically, it can be categorized as anterior blepharitis or posterior blepharitis. Anterior blepharitis refers to inflammation primarily positioned around the skin, eyelashes, and lash follicles and is usually further divided into staphylococcal and seborrheic variants. Posterior blepharitis involves the meibomian gland orifices, meibomian glands, tarsal plate, and blepharo-conjunctival junction.
Blepharitis can be acute or chronic. It is frequently associated with systemic diseases, such as rosacea, atopy, and seborrheic dermatitis, as well as ocular diseases, such as dry eye syndromes, chalazion, trichiasis, ectropion and entropion, infectious or other inflammatory conjunctivitis, and keratitis. Moreover, high rates of blepharitis have been reported in patients treated with dupilumab for atopic dermatitis.
Eye irritation, itching, erythema of the lids, flaking of the lid margins, and/or changes in the eyelashes are common presenting symptoms in patients with blepharitis. Other symptoms may include:
• Burning
• Watering
• Foreign-body sensation
• Crusting and mattering of the lashes and medial canthus
• Red lids
• Red eyes
• Photophobia
• Pain
• Decreased vision
• Visual fluctuations
• Heat, cold, alcohol, and spicy-food intolerance
The differential diagnosis for blepharitis includes bacterial keratitis, which is a serious ocular disorder that can lead to vision loss if not properly treated. Bacterial keratitis progresses rapidly and can result in corneal destruction within 24-48 hours with some particularly virulent bacteria. Patients with bacterial keratitis typically report rapid onset of pain, photophobia, and decreased vision.
Ocular rosacea should also be considered in the differential diagnosis of blepharitis, and the two conditions can co-occur. Patients with ocular rosacea may experience facial symptoms (eg, recurrent flushing episodes, persistent and/or recurrent midfacial erythema, papular and pustular lesions) in addition to ocular symptoms, which can range from minor irritation, foreign-body sensation, and blurry vision to severe ocular surface disruption and inflammatory keratitis.
Bacterial conjunctivitis involves inflammation of the bulbar and/or palpebral conjunctiva, whereas blepharitis involves inflammation of the eyelids only. Other conditions to consider in the diagnosis of blepharitis can be found here.
Given the unprecedented efficacy seen in clinical trials, dupilumab is emerging as a first-line therapeutic for moderate to severe atopic dermatitis. However, clinicians should be alert to ocular complications among their patients with atopic dermatitis who are being treated with dupilumab. In some patients, this may be because of preexisting meibomian gland disease and ocular surface disease. After a diagnosis of ocular complications, the continued use of dupilumab should be jointly evaluated by the ophthalmologist and dermatologist or allergist on the basis of the ocular risk vs systemic benefit. Treatment for blepharitis typically includes strict eyelid hygiene and topical antibiotic ointment; oral antibiotics can be beneficial for refractory disease.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's symptoms are consistent with a diagnosis of blepharitis.
Blepharitis is an inflammatory disorder of the eyelids that is frequently associated with bacterial colonization of the eyelid. Anatomically, it can be categorized as anterior blepharitis or posterior blepharitis. Anterior blepharitis refers to inflammation primarily positioned around the skin, eyelashes, and lash follicles and is usually further divided into staphylococcal and seborrheic variants. Posterior blepharitis involves the meibomian gland orifices, meibomian glands, tarsal plate, and blepharo-conjunctival junction.
Blepharitis can be acute or chronic. It is frequently associated with systemic diseases, such as rosacea, atopy, and seborrheic dermatitis, as well as ocular diseases, such as dry eye syndromes, chalazion, trichiasis, ectropion and entropion, infectious or other inflammatory conjunctivitis, and keratitis. Moreover, high rates of blepharitis have been reported in patients treated with dupilumab for atopic dermatitis.
Eye irritation, itching, erythema of the lids, flaking of the lid margins, and/or changes in the eyelashes are common presenting symptoms in patients with blepharitis. Other symptoms may include:
• Burning
• Watering
• Foreign-body sensation
• Crusting and mattering of the lashes and medial canthus
• Red lids
• Red eyes
• Photophobia
• Pain
• Decreased vision
• Visual fluctuations
• Heat, cold, alcohol, and spicy-food intolerance
The differential diagnosis for blepharitis includes bacterial keratitis, which is a serious ocular disorder that can lead to vision loss if not properly treated. Bacterial keratitis progresses rapidly and can result in corneal destruction within 24-48 hours with some particularly virulent bacteria. Patients with bacterial keratitis typically report rapid onset of pain, photophobia, and decreased vision.
Ocular rosacea should also be considered in the differential diagnosis of blepharitis, and the two conditions can co-occur. Patients with ocular rosacea may experience facial symptoms (eg, recurrent flushing episodes, persistent and/or recurrent midfacial erythema, papular and pustular lesions) in addition to ocular symptoms, which can range from minor irritation, foreign-body sensation, and blurry vision to severe ocular surface disruption and inflammatory keratitis.
Bacterial conjunctivitis involves inflammation of the bulbar and/or palpebral conjunctiva, whereas blepharitis involves inflammation of the eyelids only. Other conditions to consider in the diagnosis of blepharitis can be found here.
Given the unprecedented efficacy seen in clinical trials, dupilumab is emerging as a first-line therapeutic for moderate to severe atopic dermatitis. However, clinicians should be alert to ocular complications among their patients with atopic dermatitis who are being treated with dupilumab. In some patients, this may be because of preexisting meibomian gland disease and ocular surface disease. After a diagnosis of ocular complications, the continued use of dupilumab should be jointly evaluated by the ophthalmologist and dermatologist or allergist on the basis of the ocular risk vs systemic benefit. Treatment for blepharitis typically includes strict eyelid hygiene and topical antibiotic ointment; oral antibiotics can be beneficial for refractory disease.
William D. James, MD, Professor, Department of Dermatology, University of Pennsylvania, Philadelphia.
Disclosure: William D. James, MD, has disclosed the following relevant financial relationships:
Received income in an amount equal to or greater than $250 from: Elsevier.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 71-year-old woman was referred for an ophthalmologic examination by her dermatologist. The patient reports recent onset of red, swollen eyelids; ocular itching; and a burning sensation. Prior medical history includes severe atopic dermatitis, type 2 diabetes, and osteoarthritis. Current medications include metformin 1000 mg/d, celecoxib 200 mg/d, and clobetasol propionate 0.05% cream twice daily. The patient began receiving subcutaneous dupilumab 300 mg/once every 2 weeks about 6 weeks earlier.