User login
Obesity treatment in mental illness: Is semaglutide a game changer?
It’s probably fair to say that most people would like to be thinner. More than 42% of Americans have obesity and another 30% are classified as being overweight, according to the latest statistics from the CDC.
Excess body weight is associated with many illnesses and plays a role in mental health; being heavy can take a toll on self-esteem. Many people worry that carrying excess weight makes them less attractive to potential romantic partners, and both physicians and employers treat those with obesity differently. Furthermore, in psychiatry, many of the medications we prescribe lead to weight gain.
In my clinical practice, I have listened as patients blamed themselves for their body habitus; many won’t consider biological treatments as they feel that would be “cheating” or taking an easy way out. They often point to periods in their life when they did lose weight and believe that they should be able to do it again, even if the weight loss took tremendous effort, was not sustained, and occurred decades ago.
That said, we psychiatrists often find ourselves in the position of managing obesity in our patients. I have been known to give patients who gain weight on antipsychotics either stimulants or metformin, or to add naltrexone to their Wellbutrin (bupropion) to effectively mimic a weight-loss medicine called Contrave.
Obesity a treatable medical condition
It wasn’t until 2013 that the American Medical Association recognized obesity as a medical condition.
In a New Yorker article that same year, “Diet Drugs Work: Why Won’t Doctors Prescribe Them?” Suzanne Koven wrote: “Several obesity experts told me they’ve encountered doctors who confide that they just didn’t like fat people and don’t enjoy taking care of them. Even doctors who treat obese patients feel stigmatized: ‘diet doctor’ is not a flattering term.”
Eat less, exercise more – with a blame-the-patient attitude – is still what people see as the “right” way to lose weight.
On June 4, 2021, the FDA approved semaglutide, a glucagonlike peptide–1 receptor agonist, previously used for the treatment of diabetes, for use as a weight loss agent for patients with obesity, or for those with a body mass index over 27 kg/m2 if they also have a weight-related comorbidity.
Semaglutide has three trade names, all manufactured by Novo Nordisk. The pill version is called Rybelsus and comes in 7-mg and 14-mg tablets. Ozempic is available in 0.5-mg and 1.0-mg doses and is administered weekly by subcutaneous injection for diabetes. The new, higher-dose preparation for weight loss, Wegovy, 2.4 mg, also comes as a weekly subcutaneous dose and is now available for the hefty price of $1,400 per month.
In STEP 1 trials, the higher-dose Wegovy was associated with an average 14.9% weight loss (15.3 kg) over 68 weeks, more than any other single-agent weight loss medication on the market.
GLP-1 receptor agonists work in the brain to decrease appetite, slow gastric emptying, increase insulin secretion, and stimulate brown adipose tissue thermogenesis.
Psych drugs lead to weight gain
Elaine Weiner, MD, is the medical director in the outpatient research program of the Maryland Psychiatric Research Center in Catonsville, where she treats patients with schizophrenia.
“Nearly all of our patients gain 20 pounds or more on the combinations of medications we use, mostly atypical antipsychotics,” she said. “Weight management is difficult for people who don’t have problems with motivation, but in our patients, lack of motivation is a core part of their illness, so asking them to adhere to diet and exercise regimens is of limited utility.
“Then, add to that the fact that they sometimes don’t have primary care doctors, and these issues of weight gain and metabolic syndrome come back to the psychiatrist. It is a really bad problem and we need more treatments.”
Fatima Cody Stanford, MD, MPH, MPA, is a fellowship-trained obesity medicine physician-scientist at the Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston. She has treated thousands of patients with obesity, speaks internationally on the topic of weight loss medicine, and has published over 100 peer-reviewed articles on obesity.
We spoke at length about recent changes in the field of obesity medicine and the introduction of the new GLP-1 receptor agonists.
“We as physicians have learned so little,” Dr. Stanford said. “This mantra of ‘calories in, calories out’ is not working; this is inaccurate and our focus on this has led to a rise in obesity. All calories are not created the same, and I think we are finally starting to see obesity medicine take off.”
Dr. Stanford is quick to note that obesity is a complex problem. Several different hormones are involved in regulating both appetite and satiety, processed foods promote weight gain, sleep is crucial to weight loss, and exercise helps maintain weight loss but is not usually effective in promoting it. “There are many contributors to energy storage,” she said.
The stimulant phentermine was approved in 1959. Addiction was a concern, and then in the 1990s, it was used in combination with fenfluramine to promote weight loss, a combination known as phen-fen. Fenfluramine was pulled from the market in 1997 when it was found to be associated with pulmonary hypertension and then heart valve abnormalities.
“This frightened quite a few physicians,” Dr. Stanford noted. Phentermine is still used for weight loss, either alone or together with topiramate, as a combination medication called Qsymia, nicknamed phen-top.
“Phen-top is the next best thing we have to semaglutide, and there is an average weight loss of 8%-9% of body weight. Semaglutide is going to be really significant for those people who are responders, and this has been quite well tolerated, the most common side effect being nausea,” she said.
However, she is quick to note that not everyone responds to every medication. “I use each patient’s clinical profile to determine what strategies and which medications to use.”
Cardiologists getting in the game
Michael Miller, MD, is a cardiologist at the University of Maryland, Baltimore, and author of “Heal Your Heart” (Emmaus, Pa.: Rodale, 2014). He is very enthusiastic about the approval of semaglutide.
“We are so excited because you finally can use these medicines without having to be diabetic,” Dr. Miller said. “We’re waiting on the results of the SELECT [Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity] trials looking at people who are not diabetic or who are prediabetic, to see the 5-year outcomes with regard to cardiac events.
“Usually endocrinologists prescribe these medications, but cardiologists have started to get into the game since GLP-1 receptor agonists reduce cardiovascular events.” Dr. Miller is hopeful that this medication may neutralize the weight gain caused by psychotropic medications.
Wegovy is administered via weekly injection and, like insulin, is a subcutaneous medication that patients self-administer. Will patients be amenable to injecting a medication for weight loss? Dr. Stanford said that roughly 20%-30% of her patients are hesitant when she suggests that they use liraglutide, another GLP-1 receptor agonist that is approved for weight loss, and some are very fearful of needles.
However, she also noted that during the COVID-19 pandemic, many more patients have sought treatment from obesity medicine physicians because of the association between obesity and mortality from COVID-19. Patients have been willing to consider treatments that they were not previously open to pursuing.
So if people are willing to take Wegovy and doctors are willing to prescribe it, will insurers pay for it? As of this writing, the medication is not yet available, but Ozempic, the lower-dose agent for diabetes, costs $850-$900 for a 4-week supply, according to the GoodRx website.
Liraglutide (Saxenda), the GLP-1 receptor agonist that is currently available for weight loss as a daily injectable, costs $1,300-$1,400 per month.
These medications are not covered by Medicare or Medicaid, and Dr. Stanford, who is well versed as to exactly which private insurers in Massachusetts will and will not reimburse specific medications, said her patients with insurance coverage have been known to delay retirement so that they can remain on the more expensive medications.
“For the past 8 years,” she said, “the Treat and Reduce Obesity Act has had bipartisan support in Congress but has not passed. We are still hopeful that insurers will be required to cover medical and behavioral treatments for obesity.”
As our society struggles to destigmatize so many disorders, obesity remains a highly stigmatized condition, one that our patients cannot hide and one that leads to so many other comorbid illnesses. As new treatments are approved, there will be more for physicians to offer. Semaglutide, if it becomes available to those who need it most, could be a game changer. For patients who have not had success with traditional weight-loss methods, it’s encouraging to have another option available, one that may be reasonable to try before resorting to bariatric surgery.
For decades, psychiatrists have been comfortable prescribing treatments that lead to weight gain. Now, maybe it’s time they also prescribe those that prevent it.
A version of this article first appeared on Medscape.com.
It’s probably fair to say that most people would like to be thinner. More than 42% of Americans have obesity and another 30% are classified as being overweight, according to the latest statistics from the CDC.
Excess body weight is associated with many illnesses and plays a role in mental health; being heavy can take a toll on self-esteem. Many people worry that carrying excess weight makes them less attractive to potential romantic partners, and both physicians and employers treat those with obesity differently. Furthermore, in psychiatry, many of the medications we prescribe lead to weight gain.
In my clinical practice, I have listened as patients blamed themselves for their body habitus; many won’t consider biological treatments as they feel that would be “cheating” or taking an easy way out. They often point to periods in their life when they did lose weight and believe that they should be able to do it again, even if the weight loss took tremendous effort, was not sustained, and occurred decades ago.
That said, we psychiatrists often find ourselves in the position of managing obesity in our patients. I have been known to give patients who gain weight on antipsychotics either stimulants or metformin, or to add naltrexone to their Wellbutrin (bupropion) to effectively mimic a weight-loss medicine called Contrave.
Obesity a treatable medical condition
It wasn’t until 2013 that the American Medical Association recognized obesity as a medical condition.
In a New Yorker article that same year, “Diet Drugs Work: Why Won’t Doctors Prescribe Them?” Suzanne Koven wrote: “Several obesity experts told me they’ve encountered doctors who confide that they just didn’t like fat people and don’t enjoy taking care of them. Even doctors who treat obese patients feel stigmatized: ‘diet doctor’ is not a flattering term.”
Eat less, exercise more – with a blame-the-patient attitude – is still what people see as the “right” way to lose weight.
On June 4, 2021, the FDA approved semaglutide, a glucagonlike peptide–1 receptor agonist, previously used for the treatment of diabetes, for use as a weight loss agent for patients with obesity, or for those with a body mass index over 27 kg/m2 if they also have a weight-related comorbidity.
Semaglutide has three trade names, all manufactured by Novo Nordisk. The pill version is called Rybelsus and comes in 7-mg and 14-mg tablets. Ozempic is available in 0.5-mg and 1.0-mg doses and is administered weekly by subcutaneous injection for diabetes. The new, higher-dose preparation for weight loss, Wegovy, 2.4 mg, also comes as a weekly subcutaneous dose and is now available for the hefty price of $1,400 per month.
In STEP 1 trials, the higher-dose Wegovy was associated with an average 14.9% weight loss (15.3 kg) over 68 weeks, more than any other single-agent weight loss medication on the market.
GLP-1 receptor agonists work in the brain to decrease appetite, slow gastric emptying, increase insulin secretion, and stimulate brown adipose tissue thermogenesis.
Psych drugs lead to weight gain
Elaine Weiner, MD, is the medical director in the outpatient research program of the Maryland Psychiatric Research Center in Catonsville, where she treats patients with schizophrenia.
“Nearly all of our patients gain 20 pounds or more on the combinations of medications we use, mostly atypical antipsychotics,” she said. “Weight management is difficult for people who don’t have problems with motivation, but in our patients, lack of motivation is a core part of their illness, so asking them to adhere to diet and exercise regimens is of limited utility.
“Then, add to that the fact that they sometimes don’t have primary care doctors, and these issues of weight gain and metabolic syndrome come back to the psychiatrist. It is a really bad problem and we need more treatments.”
Fatima Cody Stanford, MD, MPH, MPA, is a fellowship-trained obesity medicine physician-scientist at the Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston. She has treated thousands of patients with obesity, speaks internationally on the topic of weight loss medicine, and has published over 100 peer-reviewed articles on obesity.
We spoke at length about recent changes in the field of obesity medicine and the introduction of the new GLP-1 receptor agonists.
“We as physicians have learned so little,” Dr. Stanford said. “This mantra of ‘calories in, calories out’ is not working; this is inaccurate and our focus on this has led to a rise in obesity. All calories are not created the same, and I think we are finally starting to see obesity medicine take off.”
Dr. Stanford is quick to note that obesity is a complex problem. Several different hormones are involved in regulating both appetite and satiety, processed foods promote weight gain, sleep is crucial to weight loss, and exercise helps maintain weight loss but is not usually effective in promoting it. “There are many contributors to energy storage,” she said.
The stimulant phentermine was approved in 1959. Addiction was a concern, and then in the 1990s, it was used in combination with fenfluramine to promote weight loss, a combination known as phen-fen. Fenfluramine was pulled from the market in 1997 when it was found to be associated with pulmonary hypertension and then heart valve abnormalities.
“This frightened quite a few physicians,” Dr. Stanford noted. Phentermine is still used for weight loss, either alone or together with topiramate, as a combination medication called Qsymia, nicknamed phen-top.
“Phen-top is the next best thing we have to semaglutide, and there is an average weight loss of 8%-9% of body weight. Semaglutide is going to be really significant for those people who are responders, and this has been quite well tolerated, the most common side effect being nausea,” she said.
However, she is quick to note that not everyone responds to every medication. “I use each patient’s clinical profile to determine what strategies and which medications to use.”
Cardiologists getting in the game
Michael Miller, MD, is a cardiologist at the University of Maryland, Baltimore, and author of “Heal Your Heart” (Emmaus, Pa.: Rodale, 2014). He is very enthusiastic about the approval of semaglutide.
“We are so excited because you finally can use these medicines without having to be diabetic,” Dr. Miller said. “We’re waiting on the results of the SELECT [Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity] trials looking at people who are not diabetic or who are prediabetic, to see the 5-year outcomes with regard to cardiac events.
“Usually endocrinologists prescribe these medications, but cardiologists have started to get into the game since GLP-1 receptor agonists reduce cardiovascular events.” Dr. Miller is hopeful that this medication may neutralize the weight gain caused by psychotropic medications.
Wegovy is administered via weekly injection and, like insulin, is a subcutaneous medication that patients self-administer. Will patients be amenable to injecting a medication for weight loss? Dr. Stanford said that roughly 20%-30% of her patients are hesitant when she suggests that they use liraglutide, another GLP-1 receptor agonist that is approved for weight loss, and some are very fearful of needles.
However, she also noted that during the COVID-19 pandemic, many more patients have sought treatment from obesity medicine physicians because of the association between obesity and mortality from COVID-19. Patients have been willing to consider treatments that they were not previously open to pursuing.
So if people are willing to take Wegovy and doctors are willing to prescribe it, will insurers pay for it? As of this writing, the medication is not yet available, but Ozempic, the lower-dose agent for diabetes, costs $850-$900 for a 4-week supply, according to the GoodRx website.
Liraglutide (Saxenda), the GLP-1 receptor agonist that is currently available for weight loss as a daily injectable, costs $1,300-$1,400 per month.
These medications are not covered by Medicare or Medicaid, and Dr. Stanford, who is well versed as to exactly which private insurers in Massachusetts will and will not reimburse specific medications, said her patients with insurance coverage have been known to delay retirement so that they can remain on the more expensive medications.
“For the past 8 years,” she said, “the Treat and Reduce Obesity Act has had bipartisan support in Congress but has not passed. We are still hopeful that insurers will be required to cover medical and behavioral treatments for obesity.”
As our society struggles to destigmatize so many disorders, obesity remains a highly stigmatized condition, one that our patients cannot hide and one that leads to so many other comorbid illnesses. As new treatments are approved, there will be more for physicians to offer. Semaglutide, if it becomes available to those who need it most, could be a game changer. For patients who have not had success with traditional weight-loss methods, it’s encouraging to have another option available, one that may be reasonable to try before resorting to bariatric surgery.
For decades, psychiatrists have been comfortable prescribing treatments that lead to weight gain. Now, maybe it’s time they also prescribe those that prevent it.
A version of this article first appeared on Medscape.com.
It’s probably fair to say that most people would like to be thinner. More than 42% of Americans have obesity and another 30% are classified as being overweight, according to the latest statistics from the CDC.
Excess body weight is associated with many illnesses and plays a role in mental health; being heavy can take a toll on self-esteem. Many people worry that carrying excess weight makes them less attractive to potential romantic partners, and both physicians and employers treat those with obesity differently. Furthermore, in psychiatry, many of the medications we prescribe lead to weight gain.
In my clinical practice, I have listened as patients blamed themselves for their body habitus; many won’t consider biological treatments as they feel that would be “cheating” or taking an easy way out. They often point to periods in their life when they did lose weight and believe that they should be able to do it again, even if the weight loss took tremendous effort, was not sustained, and occurred decades ago.
That said, we psychiatrists often find ourselves in the position of managing obesity in our patients. I have been known to give patients who gain weight on antipsychotics either stimulants or metformin, or to add naltrexone to their Wellbutrin (bupropion) to effectively mimic a weight-loss medicine called Contrave.
Obesity a treatable medical condition
It wasn’t until 2013 that the American Medical Association recognized obesity as a medical condition.
In a New Yorker article that same year, “Diet Drugs Work: Why Won’t Doctors Prescribe Them?” Suzanne Koven wrote: “Several obesity experts told me they’ve encountered doctors who confide that they just didn’t like fat people and don’t enjoy taking care of them. Even doctors who treat obese patients feel stigmatized: ‘diet doctor’ is not a flattering term.”
Eat less, exercise more – with a blame-the-patient attitude – is still what people see as the “right” way to lose weight.
On June 4, 2021, the FDA approved semaglutide, a glucagonlike peptide–1 receptor agonist, previously used for the treatment of diabetes, for use as a weight loss agent for patients with obesity, or for those with a body mass index over 27 kg/m2 if they also have a weight-related comorbidity.
Semaglutide has three trade names, all manufactured by Novo Nordisk. The pill version is called Rybelsus and comes in 7-mg and 14-mg tablets. Ozempic is available in 0.5-mg and 1.0-mg doses and is administered weekly by subcutaneous injection for diabetes. The new, higher-dose preparation for weight loss, Wegovy, 2.4 mg, also comes as a weekly subcutaneous dose and is now available for the hefty price of $1,400 per month.
In STEP 1 trials, the higher-dose Wegovy was associated with an average 14.9% weight loss (15.3 kg) over 68 weeks, more than any other single-agent weight loss medication on the market.
GLP-1 receptor agonists work in the brain to decrease appetite, slow gastric emptying, increase insulin secretion, and stimulate brown adipose tissue thermogenesis.
Psych drugs lead to weight gain
Elaine Weiner, MD, is the medical director in the outpatient research program of the Maryland Psychiatric Research Center in Catonsville, where she treats patients with schizophrenia.
“Nearly all of our patients gain 20 pounds or more on the combinations of medications we use, mostly atypical antipsychotics,” she said. “Weight management is difficult for people who don’t have problems with motivation, but in our patients, lack of motivation is a core part of their illness, so asking them to adhere to diet and exercise regimens is of limited utility.
“Then, add to that the fact that they sometimes don’t have primary care doctors, and these issues of weight gain and metabolic syndrome come back to the psychiatrist. It is a really bad problem and we need more treatments.”
Fatima Cody Stanford, MD, MPH, MPA, is a fellowship-trained obesity medicine physician-scientist at the Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston. She has treated thousands of patients with obesity, speaks internationally on the topic of weight loss medicine, and has published over 100 peer-reviewed articles on obesity.
We spoke at length about recent changes in the field of obesity medicine and the introduction of the new GLP-1 receptor agonists.
“We as physicians have learned so little,” Dr. Stanford said. “This mantra of ‘calories in, calories out’ is not working; this is inaccurate and our focus on this has led to a rise in obesity. All calories are not created the same, and I think we are finally starting to see obesity medicine take off.”
Dr. Stanford is quick to note that obesity is a complex problem. Several different hormones are involved in regulating both appetite and satiety, processed foods promote weight gain, sleep is crucial to weight loss, and exercise helps maintain weight loss but is not usually effective in promoting it. “There are many contributors to energy storage,” she said.
The stimulant phentermine was approved in 1959. Addiction was a concern, and then in the 1990s, it was used in combination with fenfluramine to promote weight loss, a combination known as phen-fen. Fenfluramine was pulled from the market in 1997 when it was found to be associated with pulmonary hypertension and then heart valve abnormalities.
“This frightened quite a few physicians,” Dr. Stanford noted. Phentermine is still used for weight loss, either alone or together with topiramate, as a combination medication called Qsymia, nicknamed phen-top.
“Phen-top is the next best thing we have to semaglutide, and there is an average weight loss of 8%-9% of body weight. Semaglutide is going to be really significant for those people who are responders, and this has been quite well tolerated, the most common side effect being nausea,” she said.
However, she is quick to note that not everyone responds to every medication. “I use each patient’s clinical profile to determine what strategies and which medications to use.”
Cardiologists getting in the game
Michael Miller, MD, is a cardiologist at the University of Maryland, Baltimore, and author of “Heal Your Heart” (Emmaus, Pa.: Rodale, 2014). He is very enthusiastic about the approval of semaglutide.
“We are so excited because you finally can use these medicines without having to be diabetic,” Dr. Miller said. “We’re waiting on the results of the SELECT [Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity] trials looking at people who are not diabetic or who are prediabetic, to see the 5-year outcomes with regard to cardiac events.
“Usually endocrinologists prescribe these medications, but cardiologists have started to get into the game since GLP-1 receptor agonists reduce cardiovascular events.” Dr. Miller is hopeful that this medication may neutralize the weight gain caused by psychotropic medications.
Wegovy is administered via weekly injection and, like insulin, is a subcutaneous medication that patients self-administer. Will patients be amenable to injecting a medication for weight loss? Dr. Stanford said that roughly 20%-30% of her patients are hesitant when she suggests that they use liraglutide, another GLP-1 receptor agonist that is approved for weight loss, and some are very fearful of needles.
However, she also noted that during the COVID-19 pandemic, many more patients have sought treatment from obesity medicine physicians because of the association between obesity and mortality from COVID-19. Patients have been willing to consider treatments that they were not previously open to pursuing.
So if people are willing to take Wegovy and doctors are willing to prescribe it, will insurers pay for it? As of this writing, the medication is not yet available, but Ozempic, the lower-dose agent for diabetes, costs $850-$900 for a 4-week supply, according to the GoodRx website.
Liraglutide (Saxenda), the GLP-1 receptor agonist that is currently available for weight loss as a daily injectable, costs $1,300-$1,400 per month.
These medications are not covered by Medicare or Medicaid, and Dr. Stanford, who is well versed as to exactly which private insurers in Massachusetts will and will not reimburse specific medications, said her patients with insurance coverage have been known to delay retirement so that they can remain on the more expensive medications.
“For the past 8 years,” she said, “the Treat and Reduce Obesity Act has had bipartisan support in Congress but has not passed. We are still hopeful that insurers will be required to cover medical and behavioral treatments for obesity.”
As our society struggles to destigmatize so many disorders, obesity remains a highly stigmatized condition, one that our patients cannot hide and one that leads to so many other comorbid illnesses. As new treatments are approved, there will be more for physicians to offer. Semaglutide, if it becomes available to those who need it most, could be a game changer. For patients who have not had success with traditional weight-loss methods, it’s encouraging to have another option available, one that may be reasonable to try before resorting to bariatric surgery.
For decades, psychiatrists have been comfortable prescribing treatments that lead to weight gain. Now, maybe it’s time they also prescribe those that prevent it.
A version of this article first appeared on Medscape.com.
Let’s talk about race
“I feel like my aggression is being racialized.” “Of course I wouldn’t call the cops if I felt like hurting myself. I’m Black.”
Those statements represent the heightened trauma our Black and Brown patients with mental health issues have been experiencing. In the wake of increasingly publicized police brutality against Black and Brown communities, the role race plays in mental health decompensation is evident. At this moment in time, we must continue to improve our understanding of the role race plays in psychiatric disorders. We must also ask ourselves: At times, does psychiatry worsen the traumas of the communities we serve?
Some psychiatrists are afraid to speak about race. They may believe it to be too “political.” But avoiding these necessary conversations perpetuates the trauma of those we treat. It suggests that physicians are ignorant of an issue at the forefront of patients’ mental health. Psychiatry, today, is primarily focused on the biological aspects of disease. We must not forget that psychiatry is biopsychosocial. It is imperative that psychiatrists have conversations about race – and its significance to our patients and their care.
Only 10.4% of psychiatrists in the United States comprise those considered underrepresented in medicine (URM). Yet, those very groups make up 32.6% of the U.S. population and are overrepresented in psychiatric hospitals.1 Many studies have shown that concordant racial backgrounds between patient and physician lead to a more positive patient experience2 and arguably, the subsequent potential for better health outcomes. Our efforts in addressing this disparity often fall short. URM applicants may be hesitant to join an institution where diversity is lacking or where they may be the only minority.3 While there is no simple solution, I propose that psychiatrists promote the importance of mental health to Black and Brown students of all ages by collaborating with schools and community leaders.
It is important to acknowledge that the lack of diversity within psychiatry is reflective of that among all physicians. This in part stems from the barriers to medical education that Black and Brown communities face. Those who start off with more resources or have parents who are physicians are at an advantage when trying to get into medical school. In fact, one in five medical students have a parent who is a physician4 and about three-fourths of students come from families whose income falls among the top two quintiles.5 Impoverished communities, which have a disproportionate share of Black and Brown people, cannot afford to take MCAT preparatory classes or to accept unpaid “resume building” opportunities. Many medical schools continue to place more weight on test scores and research/medical experiences, despite a shift to a more holistic review process. Institutions that have tried a different approach and accepted students from more diverse backgrounds may often overlook the challenges that URM students face while in medical school and fail to provide appropriate support resources.
The result is a failure to retain such students. A study conducted at Stony Brook (N.Y.) University showed that those underrepresented in medicine were six times more likely to get dismissed from medical school, and three times more likely to both withdraw or graduate beyond 4 years, compared with their White counterparts.6 This is a serious issue that needs to change on a structural and systemic level.
Any discussion of race and psychiatry must acknowledge psychiatry’s history of racism against Black and Brown communities to engage in racially informed discussions with our patients. Only then can we play a better role advocating against racism within the field in the future. Dating back to the 18th century, psychiatry has promoted ideologies that promote racism. Benjamin Rush, considered the “father of American Psychiatry,” believed that Black skin was a disease derived from leprosy called “negritude.” In the late 19th century, this twisted ideology continued with the invention of the term “drapetomania,” which was used to describe enslaved people who ran away as having a mental disorder.7 Black prisoners were subjected to experimental treatment with substances such as LSD and bulbocapnine to subdue them.8 This idea that minorities were dangerous and needed to be subdued translated into a higher number of schizophrenia diagnoses, particularly among Black men, as it was used as a tool to vilify them in the 1970s. Although schizophrenia is equally prevalent among Whites and non-Whites, Black people are four times more likely to be diagnosed, compared with their White counterparts, while Hispanics are three times more likely. Studies have shown that Black and Brown men are also more likely to receive higher doses of antipsychotics.9
Given this history, it is not surprising that Black and Brown representation within the field is lacking and that patients may be hesitant to share their feelings about race with us. While we can’t change history, we can take a stance condemning the harmful behavior of the past. The American Psychiatric Association issued an apology earlier this year to Black, Indigenous, and People of Color for its support in structural racism.10 This is a step in the right direction, but we need more than statements or performative actions. We need to amplify the voices of Black and Brown psychiatrists and patients, as well as highlight their current and past contributions to the field. While my educational experiences focused on the work of prominent White scholars, medical curricula should showcase the work of people like Solomon Carter Fuller, MD, a Black psychiatrist who was essential to understanding Alzheimer’s, or Joseph White, PhD, sometimes referred to as the “godfather of Black psychology.”11
At times, I have found myself witness to situations where colleagues make statements that not only do not condemn racism, but in fact encourage it. I have unfortunately heard some use the all-too-familiar rhetoric of reverse racism, such as: “They just assume I am racist because I am a White male” or “They’re being racist against me” or statements like “Don’t you think it is far-fetched to believe she was just sitting on a college campus doing nothing when the police were called?” Rhetoric such as this is problematic to the field of psychiatry and medicine as a whole – and only serves to further invalidate the feelings of our Black and Brown patients. We must increase exposure and education regarding racism to address this, especially the meaning of microaggressions, a concept many fail to understand.
Attention to the subject of racism has increased within medical schools and residency training programs in the wake of George Floyd’s death. However, most programs often make these lectures optional or only have one to two limited sessions. Furthermore, many do not make it mandatory for faculty to attend; they are arguably the most in need of this training given that they set the precedent of how to practice psychiatry. Some institutions have incorporated comprehensive antiracist curriculums into medical training. One model that has been successful is the Social Justice and Health Equity program within Yale University’s psychiatry residency. The curriculum has four tracks:
- Structural competency, which focuses on the mental health impact of extraclinical structures, for example a patient’s neighborhood and associated barriers of access.
- Human experience, which explores the interaction of patients and providers and how biases play a role.
- Advocacy, which teaches residents the written and oral skills to lobby for patient interests on a community and legislative level.
- History of psychiatry, which focuses on understanding psychiatry’s prior role in racism.
In each track, there are group discussions, cases led by faculty, and meetings with community leaders. Through this curriculum, residents learn about power, privilege, and how to interact with and advocate for patients in a way that promotes equity, rather than racial disparity.12,13 This is a model that other psychiatric residency programs can promote, emulate, and benefit from.
Educating ourselves will hopefully lead to a deeper introspection of how we interact with patients and if we are promoting antiracism through our attitude and actions. Reflecting on my own personal practice, I have noted that the interplay of race, mental health, and provider decision-making becomes particularly complex when dealing with situations in which a patient exhibits increased aggression or agitation. As a second-year psychiatric resident immersed in the inpatient world, I have become familiar with patients at higher risk and greater need. The first attempt toward de-escalation involves verbal cues without any other more intrusive measures. If that fails, intramuscular (IM) medications are typically considered. If a patient has a history of aggressive behavior, the threshold to use IM medications can decrease dramatically. This is mainly to protect ourselves and our nursing staff and to prioritize safety. While I understand this rationale, I wonder about the patient’s experience. What constitutes “aggressive” behavior? For patients who have had violence used against them because of their race or who have suffered from police brutality, having police present or threatening IM medications will increasingly trigger them and escalate the situation. The aftermath can deepen the distrust of psychiatry by Black and Brown people.
How do we then handle such situations in a way that both protects our staff from physical harm and protects our patients from racial trauma? While I don’t have a great answer, I think we can benefit from standardizing what we consider aggressive behavior and have specific criteria that patients need to exhibit before administering an IM medication. In addition, discussions with the team, including residents, nurses, and attending physicians, about how to address an emergent situation before it actually happens are essential. Specifically discussing the patient’s history and race and how it may affect the situation is not something to be shied away from. Lastly, in the event that an IM medication is administered and police are present, debriefing with the patient afterward is necessary. The patient may not be willing or able to listen to you or trust you, but taking accountability and acknowledging what happened, justified or not, is a part of the process of rebuilding rapport.
Both in the purview of the individual psychiatrist and the field of psychiatry as a whole, we need to examine our behavior and not be afraid to make changes for the betterment of our patients. We must learn to talk about race with our patients and in the process, advocate for more representation of Black and Brown psychiatrists, understanding the barriers faced by these communities when pursuing the medical field. We must educate ourselves on psychiatry’s history, and equip ourselves with knowledge and tools to promote antiracism and shape psychiatry’s future. We can then apply these very tools to challenging situations we may encounter daily with the ultimate goal of improving the mental health of our patients. This is the only way we will progress and ensure that psychiatry is an equitable, antiracist field. As Ibram X. Kendi, PhD, has written, “The heartbeat of antiracism is self-reflection, recognition, admission, and fundamentally self-critique.”
Dr. Malik is a second-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
References
1. Wyse R et al. Acad Psychiatry. 2020 Oct;44(5):523-30.
2. Cooper LA et al. Ann Intern Med. 2003;139:907-15.
3. Pierre JM et al. Acad Psychiatry. 2017;41:226-32.
4. Hartocollis A. “Getting into med school without hard sciences.” New York Times. 2010 Jul 29.
5. AAMC. An updated look at the economic diversity of U.S. medical students. Analysis in Brief. 2018 Oct;18(5).
6. Rainey ML. How do we retain minority health professions students. In: Smedley BD et al. The right thing to do, the smart thing to do: Enhancing diversity in the health professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, M.D. Institute of Medicine. National Academies Press. 2001.
7. Geller J. “Structural racism in American psychiatry and APA: Part 1.” Psychiatric News. 2020 Jun 23.
8. Mohr CL and Gordon JE. Tulane: The emergence of a modern university, 1945-1980. Louisiana State University Press, Baton Rouge. 2001.
9. Metzl JM. The protest psychosis: How schizophrenia became a Black disease. Beacon Press. 2010.
10. APA’s apology to Black, indigenous and people of color for its support of structural racism in psychiatry. American Psychiatric Association. 2021 Jan 18.
11. Black pioneers in mental health. Mental Health America. 2021.
12. Belli B. For Yale’s emerging psychiatrists, confronting racism is in the curriculum. Yale News. 2020 Jul 30.
13. Jordan A and Jackson D. Social justice and health equity curriculum. Yale School of Medicine. 2019 Sep 24.
“I feel like my aggression is being racialized.” “Of course I wouldn’t call the cops if I felt like hurting myself. I’m Black.”
Those statements represent the heightened trauma our Black and Brown patients with mental health issues have been experiencing. In the wake of increasingly publicized police brutality against Black and Brown communities, the role race plays in mental health decompensation is evident. At this moment in time, we must continue to improve our understanding of the role race plays in psychiatric disorders. We must also ask ourselves: At times, does psychiatry worsen the traumas of the communities we serve?
Some psychiatrists are afraid to speak about race. They may believe it to be too “political.” But avoiding these necessary conversations perpetuates the trauma of those we treat. It suggests that physicians are ignorant of an issue at the forefront of patients’ mental health. Psychiatry, today, is primarily focused on the biological aspects of disease. We must not forget that psychiatry is biopsychosocial. It is imperative that psychiatrists have conversations about race – and its significance to our patients and their care.
Only 10.4% of psychiatrists in the United States comprise those considered underrepresented in medicine (URM). Yet, those very groups make up 32.6% of the U.S. population and are overrepresented in psychiatric hospitals.1 Many studies have shown that concordant racial backgrounds between patient and physician lead to a more positive patient experience2 and arguably, the subsequent potential for better health outcomes. Our efforts in addressing this disparity often fall short. URM applicants may be hesitant to join an institution where diversity is lacking or where they may be the only minority.3 While there is no simple solution, I propose that psychiatrists promote the importance of mental health to Black and Brown students of all ages by collaborating with schools and community leaders.
It is important to acknowledge that the lack of diversity within psychiatry is reflective of that among all physicians. This in part stems from the barriers to medical education that Black and Brown communities face. Those who start off with more resources or have parents who are physicians are at an advantage when trying to get into medical school. In fact, one in five medical students have a parent who is a physician4 and about three-fourths of students come from families whose income falls among the top two quintiles.5 Impoverished communities, which have a disproportionate share of Black and Brown people, cannot afford to take MCAT preparatory classes or to accept unpaid “resume building” opportunities. Many medical schools continue to place more weight on test scores and research/medical experiences, despite a shift to a more holistic review process. Institutions that have tried a different approach and accepted students from more diverse backgrounds may often overlook the challenges that URM students face while in medical school and fail to provide appropriate support resources.
The result is a failure to retain such students. A study conducted at Stony Brook (N.Y.) University showed that those underrepresented in medicine were six times more likely to get dismissed from medical school, and three times more likely to both withdraw or graduate beyond 4 years, compared with their White counterparts.6 This is a serious issue that needs to change on a structural and systemic level.
Any discussion of race and psychiatry must acknowledge psychiatry’s history of racism against Black and Brown communities to engage in racially informed discussions with our patients. Only then can we play a better role advocating against racism within the field in the future. Dating back to the 18th century, psychiatry has promoted ideologies that promote racism. Benjamin Rush, considered the “father of American Psychiatry,” believed that Black skin was a disease derived from leprosy called “negritude.” In the late 19th century, this twisted ideology continued with the invention of the term “drapetomania,” which was used to describe enslaved people who ran away as having a mental disorder.7 Black prisoners were subjected to experimental treatment with substances such as LSD and bulbocapnine to subdue them.8 This idea that minorities were dangerous and needed to be subdued translated into a higher number of schizophrenia diagnoses, particularly among Black men, as it was used as a tool to vilify them in the 1970s. Although schizophrenia is equally prevalent among Whites and non-Whites, Black people are four times more likely to be diagnosed, compared with their White counterparts, while Hispanics are three times more likely. Studies have shown that Black and Brown men are also more likely to receive higher doses of antipsychotics.9
Given this history, it is not surprising that Black and Brown representation within the field is lacking and that patients may be hesitant to share their feelings about race with us. While we can’t change history, we can take a stance condemning the harmful behavior of the past. The American Psychiatric Association issued an apology earlier this year to Black, Indigenous, and People of Color for its support in structural racism.10 This is a step in the right direction, but we need more than statements or performative actions. We need to amplify the voices of Black and Brown psychiatrists and patients, as well as highlight their current and past contributions to the field. While my educational experiences focused on the work of prominent White scholars, medical curricula should showcase the work of people like Solomon Carter Fuller, MD, a Black psychiatrist who was essential to understanding Alzheimer’s, or Joseph White, PhD, sometimes referred to as the “godfather of Black psychology.”11
At times, I have found myself witness to situations where colleagues make statements that not only do not condemn racism, but in fact encourage it. I have unfortunately heard some use the all-too-familiar rhetoric of reverse racism, such as: “They just assume I am racist because I am a White male” or “They’re being racist against me” or statements like “Don’t you think it is far-fetched to believe she was just sitting on a college campus doing nothing when the police were called?” Rhetoric such as this is problematic to the field of psychiatry and medicine as a whole – and only serves to further invalidate the feelings of our Black and Brown patients. We must increase exposure and education regarding racism to address this, especially the meaning of microaggressions, a concept many fail to understand.
Attention to the subject of racism has increased within medical schools and residency training programs in the wake of George Floyd’s death. However, most programs often make these lectures optional or only have one to two limited sessions. Furthermore, many do not make it mandatory for faculty to attend; they are arguably the most in need of this training given that they set the precedent of how to practice psychiatry. Some institutions have incorporated comprehensive antiracist curriculums into medical training. One model that has been successful is the Social Justice and Health Equity program within Yale University’s psychiatry residency. The curriculum has four tracks:
- Structural competency, which focuses on the mental health impact of extraclinical structures, for example a patient’s neighborhood and associated barriers of access.
- Human experience, which explores the interaction of patients and providers and how biases play a role.
- Advocacy, which teaches residents the written and oral skills to lobby for patient interests on a community and legislative level.
- History of psychiatry, which focuses on understanding psychiatry’s prior role in racism.
In each track, there are group discussions, cases led by faculty, and meetings with community leaders. Through this curriculum, residents learn about power, privilege, and how to interact with and advocate for patients in a way that promotes equity, rather than racial disparity.12,13 This is a model that other psychiatric residency programs can promote, emulate, and benefit from.
Educating ourselves will hopefully lead to a deeper introspection of how we interact with patients and if we are promoting antiracism through our attitude and actions. Reflecting on my own personal practice, I have noted that the interplay of race, mental health, and provider decision-making becomes particularly complex when dealing with situations in which a patient exhibits increased aggression or agitation. As a second-year psychiatric resident immersed in the inpatient world, I have become familiar with patients at higher risk and greater need. The first attempt toward de-escalation involves verbal cues without any other more intrusive measures. If that fails, intramuscular (IM) medications are typically considered. If a patient has a history of aggressive behavior, the threshold to use IM medications can decrease dramatically. This is mainly to protect ourselves and our nursing staff and to prioritize safety. While I understand this rationale, I wonder about the patient’s experience. What constitutes “aggressive” behavior? For patients who have had violence used against them because of their race or who have suffered from police brutality, having police present or threatening IM medications will increasingly trigger them and escalate the situation. The aftermath can deepen the distrust of psychiatry by Black and Brown people.
How do we then handle such situations in a way that both protects our staff from physical harm and protects our patients from racial trauma? While I don’t have a great answer, I think we can benefit from standardizing what we consider aggressive behavior and have specific criteria that patients need to exhibit before administering an IM medication. In addition, discussions with the team, including residents, nurses, and attending physicians, about how to address an emergent situation before it actually happens are essential. Specifically discussing the patient’s history and race and how it may affect the situation is not something to be shied away from. Lastly, in the event that an IM medication is administered and police are present, debriefing with the patient afterward is necessary. The patient may not be willing or able to listen to you or trust you, but taking accountability and acknowledging what happened, justified or not, is a part of the process of rebuilding rapport.
Both in the purview of the individual psychiatrist and the field of psychiatry as a whole, we need to examine our behavior and not be afraid to make changes for the betterment of our patients. We must learn to talk about race with our patients and in the process, advocate for more representation of Black and Brown psychiatrists, understanding the barriers faced by these communities when pursuing the medical field. We must educate ourselves on psychiatry’s history, and equip ourselves with knowledge and tools to promote antiracism and shape psychiatry’s future. We can then apply these very tools to challenging situations we may encounter daily with the ultimate goal of improving the mental health of our patients. This is the only way we will progress and ensure that psychiatry is an equitable, antiracist field. As Ibram X. Kendi, PhD, has written, “The heartbeat of antiracism is self-reflection, recognition, admission, and fundamentally self-critique.”
Dr. Malik is a second-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
References
1. Wyse R et al. Acad Psychiatry. 2020 Oct;44(5):523-30.
2. Cooper LA et al. Ann Intern Med. 2003;139:907-15.
3. Pierre JM et al. Acad Psychiatry. 2017;41:226-32.
4. Hartocollis A. “Getting into med school without hard sciences.” New York Times. 2010 Jul 29.
5. AAMC. An updated look at the economic diversity of U.S. medical students. Analysis in Brief. 2018 Oct;18(5).
6. Rainey ML. How do we retain minority health professions students. In: Smedley BD et al. The right thing to do, the smart thing to do: Enhancing diversity in the health professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, M.D. Institute of Medicine. National Academies Press. 2001.
7. Geller J. “Structural racism in American psychiatry and APA: Part 1.” Psychiatric News. 2020 Jun 23.
8. Mohr CL and Gordon JE. Tulane: The emergence of a modern university, 1945-1980. Louisiana State University Press, Baton Rouge. 2001.
9. Metzl JM. The protest psychosis: How schizophrenia became a Black disease. Beacon Press. 2010.
10. APA’s apology to Black, indigenous and people of color for its support of structural racism in psychiatry. American Psychiatric Association. 2021 Jan 18.
11. Black pioneers in mental health. Mental Health America. 2021.
12. Belli B. For Yale’s emerging psychiatrists, confronting racism is in the curriculum. Yale News. 2020 Jul 30.
13. Jordan A and Jackson D. Social justice and health equity curriculum. Yale School of Medicine. 2019 Sep 24.
“I feel like my aggression is being racialized.” “Of course I wouldn’t call the cops if I felt like hurting myself. I’m Black.”
Those statements represent the heightened trauma our Black and Brown patients with mental health issues have been experiencing. In the wake of increasingly publicized police brutality against Black and Brown communities, the role race plays in mental health decompensation is evident. At this moment in time, we must continue to improve our understanding of the role race plays in psychiatric disorders. We must also ask ourselves: At times, does psychiatry worsen the traumas of the communities we serve?
Some psychiatrists are afraid to speak about race. They may believe it to be too “political.” But avoiding these necessary conversations perpetuates the trauma of those we treat. It suggests that physicians are ignorant of an issue at the forefront of patients’ mental health. Psychiatry, today, is primarily focused on the biological aspects of disease. We must not forget that psychiatry is biopsychosocial. It is imperative that psychiatrists have conversations about race – and its significance to our patients and their care.
Only 10.4% of psychiatrists in the United States comprise those considered underrepresented in medicine (URM). Yet, those very groups make up 32.6% of the U.S. population and are overrepresented in psychiatric hospitals.1 Many studies have shown that concordant racial backgrounds between patient and physician lead to a more positive patient experience2 and arguably, the subsequent potential for better health outcomes. Our efforts in addressing this disparity often fall short. URM applicants may be hesitant to join an institution where diversity is lacking or where they may be the only minority.3 While there is no simple solution, I propose that psychiatrists promote the importance of mental health to Black and Brown students of all ages by collaborating with schools and community leaders.
It is important to acknowledge that the lack of diversity within psychiatry is reflective of that among all physicians. This in part stems from the barriers to medical education that Black and Brown communities face. Those who start off with more resources or have parents who are physicians are at an advantage when trying to get into medical school. In fact, one in five medical students have a parent who is a physician4 and about three-fourths of students come from families whose income falls among the top two quintiles.5 Impoverished communities, which have a disproportionate share of Black and Brown people, cannot afford to take MCAT preparatory classes or to accept unpaid “resume building” opportunities. Many medical schools continue to place more weight on test scores and research/medical experiences, despite a shift to a more holistic review process. Institutions that have tried a different approach and accepted students from more diverse backgrounds may often overlook the challenges that URM students face while in medical school and fail to provide appropriate support resources.
The result is a failure to retain such students. A study conducted at Stony Brook (N.Y.) University showed that those underrepresented in medicine were six times more likely to get dismissed from medical school, and three times more likely to both withdraw or graduate beyond 4 years, compared with their White counterparts.6 This is a serious issue that needs to change on a structural and systemic level.
Any discussion of race and psychiatry must acknowledge psychiatry’s history of racism against Black and Brown communities to engage in racially informed discussions with our patients. Only then can we play a better role advocating against racism within the field in the future. Dating back to the 18th century, psychiatry has promoted ideologies that promote racism. Benjamin Rush, considered the “father of American Psychiatry,” believed that Black skin was a disease derived from leprosy called “negritude.” In the late 19th century, this twisted ideology continued with the invention of the term “drapetomania,” which was used to describe enslaved people who ran away as having a mental disorder.7 Black prisoners were subjected to experimental treatment with substances such as LSD and bulbocapnine to subdue them.8 This idea that minorities were dangerous and needed to be subdued translated into a higher number of schizophrenia diagnoses, particularly among Black men, as it was used as a tool to vilify them in the 1970s. Although schizophrenia is equally prevalent among Whites and non-Whites, Black people are four times more likely to be diagnosed, compared with their White counterparts, while Hispanics are three times more likely. Studies have shown that Black and Brown men are also more likely to receive higher doses of antipsychotics.9
Given this history, it is not surprising that Black and Brown representation within the field is lacking and that patients may be hesitant to share their feelings about race with us. While we can’t change history, we can take a stance condemning the harmful behavior of the past. The American Psychiatric Association issued an apology earlier this year to Black, Indigenous, and People of Color for its support in structural racism.10 This is a step in the right direction, but we need more than statements or performative actions. We need to amplify the voices of Black and Brown psychiatrists and patients, as well as highlight their current and past contributions to the field. While my educational experiences focused on the work of prominent White scholars, medical curricula should showcase the work of people like Solomon Carter Fuller, MD, a Black psychiatrist who was essential to understanding Alzheimer’s, or Joseph White, PhD, sometimes referred to as the “godfather of Black psychology.”11
At times, I have found myself witness to situations where colleagues make statements that not only do not condemn racism, but in fact encourage it. I have unfortunately heard some use the all-too-familiar rhetoric of reverse racism, such as: “They just assume I am racist because I am a White male” or “They’re being racist against me” or statements like “Don’t you think it is far-fetched to believe she was just sitting on a college campus doing nothing when the police were called?” Rhetoric such as this is problematic to the field of psychiatry and medicine as a whole – and only serves to further invalidate the feelings of our Black and Brown patients. We must increase exposure and education regarding racism to address this, especially the meaning of microaggressions, a concept many fail to understand.
Attention to the subject of racism has increased within medical schools and residency training programs in the wake of George Floyd’s death. However, most programs often make these lectures optional or only have one to two limited sessions. Furthermore, many do not make it mandatory for faculty to attend; they are arguably the most in need of this training given that they set the precedent of how to practice psychiatry. Some institutions have incorporated comprehensive antiracist curriculums into medical training. One model that has been successful is the Social Justice and Health Equity program within Yale University’s psychiatry residency. The curriculum has four tracks:
- Structural competency, which focuses on the mental health impact of extraclinical structures, for example a patient’s neighborhood and associated barriers of access.
- Human experience, which explores the interaction of patients and providers and how biases play a role.
- Advocacy, which teaches residents the written and oral skills to lobby for patient interests on a community and legislative level.
- History of psychiatry, which focuses on understanding psychiatry’s prior role in racism.
In each track, there are group discussions, cases led by faculty, and meetings with community leaders. Through this curriculum, residents learn about power, privilege, and how to interact with and advocate for patients in a way that promotes equity, rather than racial disparity.12,13 This is a model that other psychiatric residency programs can promote, emulate, and benefit from.
Educating ourselves will hopefully lead to a deeper introspection of how we interact with patients and if we are promoting antiracism through our attitude and actions. Reflecting on my own personal practice, I have noted that the interplay of race, mental health, and provider decision-making becomes particularly complex when dealing with situations in which a patient exhibits increased aggression or agitation. As a second-year psychiatric resident immersed in the inpatient world, I have become familiar with patients at higher risk and greater need. The first attempt toward de-escalation involves verbal cues without any other more intrusive measures. If that fails, intramuscular (IM) medications are typically considered. If a patient has a history of aggressive behavior, the threshold to use IM medications can decrease dramatically. This is mainly to protect ourselves and our nursing staff and to prioritize safety. While I understand this rationale, I wonder about the patient’s experience. What constitutes “aggressive” behavior? For patients who have had violence used against them because of their race or who have suffered from police brutality, having police present or threatening IM medications will increasingly trigger them and escalate the situation. The aftermath can deepen the distrust of psychiatry by Black and Brown people.
How do we then handle such situations in a way that both protects our staff from physical harm and protects our patients from racial trauma? While I don’t have a great answer, I think we can benefit from standardizing what we consider aggressive behavior and have specific criteria that patients need to exhibit before administering an IM medication. In addition, discussions with the team, including residents, nurses, and attending physicians, about how to address an emergent situation before it actually happens are essential. Specifically discussing the patient’s history and race and how it may affect the situation is not something to be shied away from. Lastly, in the event that an IM medication is administered and police are present, debriefing with the patient afterward is necessary. The patient may not be willing or able to listen to you or trust you, but taking accountability and acknowledging what happened, justified or not, is a part of the process of rebuilding rapport.
Both in the purview of the individual psychiatrist and the field of psychiatry as a whole, we need to examine our behavior and not be afraid to make changes for the betterment of our patients. We must learn to talk about race with our patients and in the process, advocate for more representation of Black and Brown psychiatrists, understanding the barriers faced by these communities when pursuing the medical field. We must educate ourselves on psychiatry’s history, and equip ourselves with knowledge and tools to promote antiracism and shape psychiatry’s future. We can then apply these very tools to challenging situations we may encounter daily with the ultimate goal of improving the mental health of our patients. This is the only way we will progress and ensure that psychiatry is an equitable, antiracist field. As Ibram X. Kendi, PhD, has written, “The heartbeat of antiracism is self-reflection, recognition, admission, and fundamentally self-critique.”
Dr. Malik is a second-year psychiatry resident at the University of California, San Diego. She has a background in policy and grassroots organizing through her time working at the National Coalition for the Homeless and the Women’s Law Project. Dr. Malik has no disclosures.
References
1. Wyse R et al. Acad Psychiatry. 2020 Oct;44(5):523-30.
2. Cooper LA et al. Ann Intern Med. 2003;139:907-15.
3. Pierre JM et al. Acad Psychiatry. 2017;41:226-32.
4. Hartocollis A. “Getting into med school without hard sciences.” New York Times. 2010 Jul 29.
5. AAMC. An updated look at the economic diversity of U.S. medical students. Analysis in Brief. 2018 Oct;18(5).
6. Rainey ML. How do we retain minority health professions students. In: Smedley BD et al. The right thing to do, the smart thing to do: Enhancing diversity in the health professions: Summary of the Symposium on Diversity in Health Professions in Honor of Herbert W. Nickens, M.D. Institute of Medicine. National Academies Press. 2001.
7. Geller J. “Structural racism in American psychiatry and APA: Part 1.” Psychiatric News. 2020 Jun 23.
8. Mohr CL and Gordon JE. Tulane: The emergence of a modern university, 1945-1980. Louisiana State University Press, Baton Rouge. 2001.
9. Metzl JM. The protest psychosis: How schizophrenia became a Black disease. Beacon Press. 2010.
10. APA’s apology to Black, indigenous and people of color for its support of structural racism in psychiatry. American Psychiatric Association. 2021 Jan 18.
11. Black pioneers in mental health. Mental Health America. 2021.
12. Belli B. For Yale’s emerging psychiatrists, confronting racism is in the curriculum. Yale News. 2020 Jul 30.
13. Jordan A and Jackson D. Social justice and health equity curriculum. Yale School of Medicine. 2019 Sep 24.
Ketamine and psychosis risk: New data
Ketamine used to treat severe depression in patients with a history of psychosis does not exacerbate psychosis risk, new research suggests.
A meta-analysis of nine studies, encompassing 41 patients with TRD and a history of psychosis, suggests ketamine is safe and effective and did not exacerbate psychotic symptoms in this patient population.
“We believe our findings could encourage clinicians and researchers to examine a broadened indication for ketamine treatment in individual patients with high levels of treatment resistance, carefully monitoring both clinical response and side effects, specifically looking at possible increases in psychotic symptoms,” study investigator Jolien K. E. Veraart, MD, University of Groningen, University Medical Center Groningen, the Netherlands, told this news organization.
The study was published online July 13 in the Journal of Clinical Psychiatry.
Rapid, robust effects
Ketamine has shown “rapid and robust antidepressant effects” in clinical studies. However, this research has not included patients with past or current psychosis, based on the assumption that psychosis will increase with ketamine administration, since side effects of ketamine can include transient “schizophrenia-like” psychotomimetic phenomena, including perceptual disorders and hallucinations in healthy individuals, the investigators note.
Dr. Veraart said psychotic symptoms are “common in people with severe depression,” and these patients have poorer outcomes with pharmacotherapy, psychotherapy, and electroconvulsive therapy.
Additionally, up to 60% of patients with schizophrenia experience negative symptomatology, including loss of motivation, affective blunting, and anhedonia, which “has a clear phenomenological overlap with depression,” the authors write. They also note anti-anhedonic effects of subanesthetic ketamine doses have been reported, without adversely impacting long-term psychotic symptoms in patients with schizophrenia.
“Positive results from carefully monitored trials with ketamine treatment in these patients have motivated us to summarize the currently available knowledge to inform our colleagues,” she said.
To investigate, the researchers conducted a literature search and selected 9 articles (N = 41 patients) that reported on ketamine treatment in patients with a history of psychosis or current psychotic symptoms.
All studies were either case reports or pilot studies, the authors report. Types of patients included those with bipolar or unipolar depression, or depression in schizoaffective disorder , or patients with schizophrenia and concurrent depression. Depressive symptomatology was the treatment target in eight studies, and one study targeted negative symptoms in patients with schizophrenia.
Dosing, frequency, and types of administration (ketamine IV, esketamine IV, or esketamine subcutaneous) varied from study to study.
In seven studies, ketamine was found to improve depressive symptoms, and in two studies, improvement in psychotic symptoms was also shown. Two studies revealed improvement in symptoms of suicidality. Results of the study that measured negative symptoms showed “significant improvement” in five of six patients, with a -37.3% decrease in mean Brief Negative Symptoms Scale (BNSS) from the baseline to the end of four infusions.
“Ketamine showed good antidepressant effects, and, in some cases, the comorbid symptoms even improved or disappeared after ketamine treatment,” Dr. Veraart summarized. However, the effect size of ketamine might be lower in those with a history of psychosis, she added.
She also noted that
She pointed to one study limitation, which is that only small, uncontrolled trials were included and that there is a risk for publication bias.
Larger trials needed
Commenting on the study, Dan Iosifescu, MD, MSc, associate professor of psychiatry, New York University School of Medicine, said that if the finding “were based on a larger study it would be very important, as a theoretical risk of psychosis is preventing such patients from access to an otherwise beneficial treatment.”
However, “since the review is based on a small sample, a low risk of psychosis exacerbation after IV ketamine is still possible,” said Dr. Iosifescu, who is also the director of clinical research at the Kline Institute for Psychiatric Research in Orangeburg, New York, and was not involved with the study.
Dr. Veraart agreed, adding that the “efficacy, safety, and tolerability of ketamine in depressed patients with a vulnerability to psychosis should be investigated in well-designed randomized controlled trials before application on a large scale is promoted.”
The study had no specific funding. Dr. Veraart has received speaker honoraria from Janssen outside of the submitted work. The other authors’ disclosures are listed in the original article. Dr. Iosifescu has been a consultant to the Centers of Psychiatric Excellence, advising clinics on the best methods of providing treatment with IV ketamine.
A version of this article first appeared on Medscape.com.
Ketamine used to treat severe depression in patients with a history of psychosis does not exacerbate psychosis risk, new research suggests.
A meta-analysis of nine studies, encompassing 41 patients with TRD and a history of psychosis, suggests ketamine is safe and effective and did not exacerbate psychotic symptoms in this patient population.
“We believe our findings could encourage clinicians and researchers to examine a broadened indication for ketamine treatment in individual patients with high levels of treatment resistance, carefully monitoring both clinical response and side effects, specifically looking at possible increases in psychotic symptoms,” study investigator Jolien K. E. Veraart, MD, University of Groningen, University Medical Center Groningen, the Netherlands, told this news organization.
The study was published online July 13 in the Journal of Clinical Psychiatry.
Rapid, robust effects
Ketamine has shown “rapid and robust antidepressant effects” in clinical studies. However, this research has not included patients with past or current psychosis, based on the assumption that psychosis will increase with ketamine administration, since side effects of ketamine can include transient “schizophrenia-like” psychotomimetic phenomena, including perceptual disorders and hallucinations in healthy individuals, the investigators note.
Dr. Veraart said psychotic symptoms are “common in people with severe depression,” and these patients have poorer outcomes with pharmacotherapy, psychotherapy, and electroconvulsive therapy.
Additionally, up to 60% of patients with schizophrenia experience negative symptomatology, including loss of motivation, affective blunting, and anhedonia, which “has a clear phenomenological overlap with depression,” the authors write. They also note anti-anhedonic effects of subanesthetic ketamine doses have been reported, without adversely impacting long-term psychotic symptoms in patients with schizophrenia.
“Positive results from carefully monitored trials with ketamine treatment in these patients have motivated us to summarize the currently available knowledge to inform our colleagues,” she said.
To investigate, the researchers conducted a literature search and selected 9 articles (N = 41 patients) that reported on ketamine treatment in patients with a history of psychosis or current psychotic symptoms.
All studies were either case reports or pilot studies, the authors report. Types of patients included those with bipolar or unipolar depression, or depression in schizoaffective disorder , or patients with schizophrenia and concurrent depression. Depressive symptomatology was the treatment target in eight studies, and one study targeted negative symptoms in patients with schizophrenia.
Dosing, frequency, and types of administration (ketamine IV, esketamine IV, or esketamine subcutaneous) varied from study to study.
In seven studies, ketamine was found to improve depressive symptoms, and in two studies, improvement in psychotic symptoms was also shown. Two studies revealed improvement in symptoms of suicidality. Results of the study that measured negative symptoms showed “significant improvement” in five of six patients, with a -37.3% decrease in mean Brief Negative Symptoms Scale (BNSS) from the baseline to the end of four infusions.
“Ketamine showed good antidepressant effects, and, in some cases, the comorbid symptoms even improved or disappeared after ketamine treatment,” Dr. Veraart summarized. However, the effect size of ketamine might be lower in those with a history of psychosis, she added.
She also noted that
She pointed to one study limitation, which is that only small, uncontrolled trials were included and that there is a risk for publication bias.
Larger trials needed
Commenting on the study, Dan Iosifescu, MD, MSc, associate professor of psychiatry, New York University School of Medicine, said that if the finding “were based on a larger study it would be very important, as a theoretical risk of psychosis is preventing such patients from access to an otherwise beneficial treatment.”
However, “since the review is based on a small sample, a low risk of psychosis exacerbation after IV ketamine is still possible,” said Dr. Iosifescu, who is also the director of clinical research at the Kline Institute for Psychiatric Research in Orangeburg, New York, and was not involved with the study.
Dr. Veraart agreed, adding that the “efficacy, safety, and tolerability of ketamine in depressed patients with a vulnerability to psychosis should be investigated in well-designed randomized controlled trials before application on a large scale is promoted.”
The study had no specific funding. Dr. Veraart has received speaker honoraria from Janssen outside of the submitted work. The other authors’ disclosures are listed in the original article. Dr. Iosifescu has been a consultant to the Centers of Psychiatric Excellence, advising clinics on the best methods of providing treatment with IV ketamine.
A version of this article first appeared on Medscape.com.
Ketamine used to treat severe depression in patients with a history of psychosis does not exacerbate psychosis risk, new research suggests.
A meta-analysis of nine studies, encompassing 41 patients with TRD and a history of psychosis, suggests ketamine is safe and effective and did not exacerbate psychotic symptoms in this patient population.
“We believe our findings could encourage clinicians and researchers to examine a broadened indication for ketamine treatment in individual patients with high levels of treatment resistance, carefully monitoring both clinical response and side effects, specifically looking at possible increases in psychotic symptoms,” study investigator Jolien K. E. Veraart, MD, University of Groningen, University Medical Center Groningen, the Netherlands, told this news organization.
The study was published online July 13 in the Journal of Clinical Psychiatry.
Rapid, robust effects
Ketamine has shown “rapid and robust antidepressant effects” in clinical studies. However, this research has not included patients with past or current psychosis, based on the assumption that psychosis will increase with ketamine administration, since side effects of ketamine can include transient “schizophrenia-like” psychotomimetic phenomena, including perceptual disorders and hallucinations in healthy individuals, the investigators note.
Dr. Veraart said psychotic symptoms are “common in people with severe depression,” and these patients have poorer outcomes with pharmacotherapy, psychotherapy, and electroconvulsive therapy.
Additionally, up to 60% of patients with schizophrenia experience negative symptomatology, including loss of motivation, affective blunting, and anhedonia, which “has a clear phenomenological overlap with depression,” the authors write. They also note anti-anhedonic effects of subanesthetic ketamine doses have been reported, without adversely impacting long-term psychotic symptoms in patients with schizophrenia.
“Positive results from carefully monitored trials with ketamine treatment in these patients have motivated us to summarize the currently available knowledge to inform our colleagues,” she said.
To investigate, the researchers conducted a literature search and selected 9 articles (N = 41 patients) that reported on ketamine treatment in patients with a history of psychosis or current psychotic symptoms.
All studies were either case reports or pilot studies, the authors report. Types of patients included those with bipolar or unipolar depression, or depression in schizoaffective disorder , or patients with schizophrenia and concurrent depression. Depressive symptomatology was the treatment target in eight studies, and one study targeted negative symptoms in patients with schizophrenia.
Dosing, frequency, and types of administration (ketamine IV, esketamine IV, or esketamine subcutaneous) varied from study to study.
In seven studies, ketamine was found to improve depressive symptoms, and in two studies, improvement in psychotic symptoms was also shown. Two studies revealed improvement in symptoms of suicidality. Results of the study that measured negative symptoms showed “significant improvement” in five of six patients, with a -37.3% decrease in mean Brief Negative Symptoms Scale (BNSS) from the baseline to the end of four infusions.
“Ketamine showed good antidepressant effects, and, in some cases, the comorbid symptoms even improved or disappeared after ketamine treatment,” Dr. Veraart summarized. However, the effect size of ketamine might be lower in those with a history of psychosis, she added.
She also noted that
She pointed to one study limitation, which is that only small, uncontrolled trials were included and that there is a risk for publication bias.
Larger trials needed
Commenting on the study, Dan Iosifescu, MD, MSc, associate professor of psychiatry, New York University School of Medicine, said that if the finding “were based on a larger study it would be very important, as a theoretical risk of psychosis is preventing such patients from access to an otherwise beneficial treatment.”
However, “since the review is based on a small sample, a low risk of psychosis exacerbation after IV ketamine is still possible,” said Dr. Iosifescu, who is also the director of clinical research at the Kline Institute for Psychiatric Research in Orangeburg, New York, and was not involved with the study.
Dr. Veraart agreed, adding that the “efficacy, safety, and tolerability of ketamine in depressed patients with a vulnerability to psychosis should be investigated in well-designed randomized controlled trials before application on a large scale is promoted.”
The study had no specific funding. Dr. Veraart has received speaker honoraria from Janssen outside of the submitted work. The other authors’ disclosures are listed in the original article. Dr. Iosifescu has been a consultant to the Centers of Psychiatric Excellence, advising clinics on the best methods of providing treatment with IV ketamine.
A version of this article first appeared on Medscape.com.
Psychiatric genomics has a diversity problem
In combing the genome, scientists can use genetic clues to determine a person’s risk for psychiatric disease and even identify new drug targets. But the benefits of these discoveries will be limited to people of European descent.
Nearly 90% of participants in genome-wide association studies (GWASs), which search for gene variants linked to disease, are of European ancestry. This Eurocentric focus threatens to widen existing disparities in racial and ethnic mental health.
“If you develop certain interventions based on only a single population profile, then you’ll be leaving out the rest of the populations in the world,” says Solomon Teferra, MD, PhD, associate professor of psychiatry at Addis Ababa University, Ethiopia. In a growing trend, psychiatric researchers are diverging from the field’s European bias and are working to correct the imbalance in DNA databases.
The significant downsides of genomics’ one-track mind
One obstacle hindering therapeutic advances in psychiatry is a shallow understanding of the mechanisms of disorders. “The biggest problem in terms of advancing research for mental health conditions is that we don’t understand the underlying biology,” says Laramie Duncan, PhD, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “Genetics is one of the best ways to systematically look for new clues about the underlying biology.”
At the advent of genomic research, scientists thought it best to study DNA from people of a single ancestry from one continent. “Researchers for a long time held the idea that it was going to be too complicated to include multiple ancestries in the first rounds of genetic analyses,” says Dr. Duncan.
Studying DNA from someone with ancestors from multiple parts of the world wasn’t compatible with methods used in the early days of GWASs. “Individual parts of a person’s DNA can be linked back to one region of the world or another, and most of our methods essentially assume that all of a person’s DNA came from one region of the world,” says Dr. Duncan.
Because many genes are usually involved in psychiatric disorders, scientists need large numbers of participants to detect uncommon, influential variants. Early research was concentrated in North America and Europe so that scientists could readily collect samples from people of European ancestry.
“It then went out of hand because it became routine practice to use only this one group, essentially White, European ancestry people,” says Karoline Kuchenbaecker, PhD, associate professor of psychiatry at University College London.
Yet findings from one population won’t necessarily translate to others. “And that’s exactly what has been shown,” says Dr. Teferra. Polygenic risk scores developed for schizophrenia from European samples, for example, perform poorly among people of African ancestry, although among Europeans, they are strongly effective at differentiating European individuals with and those without schizophrenia. Moreover, drugs that target a gene identified from studies in European populations may be harmful to other groups.
Studies drawn from a diverse pool of participants would benefit a wider swath of humanity. They would also allow scientists to discover small areas of overlap in genomes of different populations, which would help them close in on the true biology of diseases and ensure that “we’re all benefiting from more diverse data in genetics and psychiatric genetics,” says Dr. Kuchenbaecker.
New efforts aim at filling the gaps
, not African, Latin American, or Indigenous ancestry.
Efforts to increase representation of persons of African ancestry have largely focused on African Americans; fewer efforts have extended to the African continent, home to the most genetically diverse populations. Even fewer have focused on mental health. “The little that was being done was on a very small scale,” says Karestan Koenen, PhD, a professor at Harvard School of Public Health, Boston.
With this in mind, researchers from institutions in Kenya, Uganda, South Africa, and Ethiopia partnered with researchers at the Broad Institute of the Massachusetts Institute of Technology and Harvard to conduct the largest GWAS of psychiatric disorders in Africa. Dr. Koenen leads the project, Neuropsychiatric Genetics of African Populations–Psychosis (NeuroGAP-Psychosis), which will analyze DNA from over 35,000 people of African ancestry in each of these four countries. Investigators will compare the half of participants who have no history of psychosis with the half with schizophrenia or bipolar disorder in the hopes of identifying the genetic determinants of psychosis.
“Then any potential intervention or therapeutics that will be developed will also be useful for Africans,” says Dr. Teferra, a NeuroGAP principal investigator. Because of the tremendous degree of genetic diversity among people on the continent, however, findings still might not translate to all African populations.
But correcting equity problems in genomics isn’t as simple as recruiting people with non-European backgrounds, especially if those people are unfamiliar with research or have been subject to scientific exploitation. “Special care needs to be taken to, first of all, provide information that’s appropriate [to participants], but also motivate people to take part and then find ways to keep these communities involved and understand what they’re interested in,” says Dr. Kuchenbaecker, who is not involved with NeuroGAP.
For NeuroGAP, the team needed to work with ethical committees at all of the institutions involved, ensure research materials were appropriate for each community’s cultural context, and gain the trust of local communities.
“One of the biggest criticisms within the scientific world is that people from more endowed countries just fly in, bully everyone, collect the data, and leave, with no credit to the local scientists or communities,” says NeuroGAP principal investigator Lukoye Atwoli, MMed, PhD, professor of psychiatry and dean of the Medical College, East Africa, at the Aga Khan University, Nairobi, Kenya. “That is one of the biggest pitfalls we had to grapple with.”
To address that concern, NeuroGAP is training local researchers and is providing them with requested resources so they can carry out similar studies in the future. “We will be looking to address a real need in the academic community and in clinical service delivery,” says Dr. Atwoli.
Dr. Kuchenbaecker says that NeuroGAP demonstrates features necessary for projects seeking to improve equity in psychiatric genomics. “What they’re doing right is recruiting really large numbers, recruiting from different African countries, and involving African investigators,” she says.
In the Americas, Janitza Montalvo-Ortiz, PhD, assistant professor in the Division of Genetics, department of psychiatry, Yale University, New Haven, Conn., and her colleagues are expanding psychiatric genomics projects in Latin America. She co-founded the Latin American Genomics Consortium in 2019, a network of scientists supporting psychiatric genomic research in the region. The consortium also involves the Neuropsychiatric Genetics in Mexican Populations project, which is similar to NeuroGAP and is also led by Dr. Koenan.
The study of Latin American populations is complicated, because genes in these populations reflect Indigenous American, European, and African ancestries. Even when investigators sampled DNA from Latin American individuals, that data often went unused. “Now with new methods emerging to allow us to properly analyze admixed populations in GWAS studies, we’re making efforts to compile different datasets scattered across different large-scale cohorts,” says Dr. Montalvo-Ortiz. “Our ultimate goal is to conduct the first large-scale LatinX GWAS of psychiatry,” she says.
With these projects, researchers hope that new psychiatric research will produce clinical advances for people historically left on the sidelines of genomic studies. By involving their communities in genomic research, “whatever is going to be developed will also benefit our community,” says Dr. Teferra. “We will not be left out.”
A version of this article first appeared on Medscape.com.
In combing the genome, scientists can use genetic clues to determine a person’s risk for psychiatric disease and even identify new drug targets. But the benefits of these discoveries will be limited to people of European descent.
Nearly 90% of participants in genome-wide association studies (GWASs), which search for gene variants linked to disease, are of European ancestry. This Eurocentric focus threatens to widen existing disparities in racial and ethnic mental health.
“If you develop certain interventions based on only a single population profile, then you’ll be leaving out the rest of the populations in the world,” says Solomon Teferra, MD, PhD, associate professor of psychiatry at Addis Ababa University, Ethiopia. In a growing trend, psychiatric researchers are diverging from the field’s European bias and are working to correct the imbalance in DNA databases.
The significant downsides of genomics’ one-track mind
One obstacle hindering therapeutic advances in psychiatry is a shallow understanding of the mechanisms of disorders. “The biggest problem in terms of advancing research for mental health conditions is that we don’t understand the underlying biology,” says Laramie Duncan, PhD, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “Genetics is one of the best ways to systematically look for new clues about the underlying biology.”
At the advent of genomic research, scientists thought it best to study DNA from people of a single ancestry from one continent. “Researchers for a long time held the idea that it was going to be too complicated to include multiple ancestries in the first rounds of genetic analyses,” says Dr. Duncan.
Studying DNA from someone with ancestors from multiple parts of the world wasn’t compatible with methods used in the early days of GWASs. “Individual parts of a person’s DNA can be linked back to one region of the world or another, and most of our methods essentially assume that all of a person’s DNA came from one region of the world,” says Dr. Duncan.
Because many genes are usually involved in psychiatric disorders, scientists need large numbers of participants to detect uncommon, influential variants. Early research was concentrated in North America and Europe so that scientists could readily collect samples from people of European ancestry.
“It then went out of hand because it became routine practice to use only this one group, essentially White, European ancestry people,” says Karoline Kuchenbaecker, PhD, associate professor of psychiatry at University College London.
Yet findings from one population won’t necessarily translate to others. “And that’s exactly what has been shown,” says Dr. Teferra. Polygenic risk scores developed for schizophrenia from European samples, for example, perform poorly among people of African ancestry, although among Europeans, they are strongly effective at differentiating European individuals with and those without schizophrenia. Moreover, drugs that target a gene identified from studies in European populations may be harmful to other groups.
Studies drawn from a diverse pool of participants would benefit a wider swath of humanity. They would also allow scientists to discover small areas of overlap in genomes of different populations, which would help them close in on the true biology of diseases and ensure that “we’re all benefiting from more diverse data in genetics and psychiatric genetics,” says Dr. Kuchenbaecker.
New efforts aim at filling the gaps
, not African, Latin American, or Indigenous ancestry.
Efforts to increase representation of persons of African ancestry have largely focused on African Americans; fewer efforts have extended to the African continent, home to the most genetically diverse populations. Even fewer have focused on mental health. “The little that was being done was on a very small scale,” says Karestan Koenen, PhD, a professor at Harvard School of Public Health, Boston.
With this in mind, researchers from institutions in Kenya, Uganda, South Africa, and Ethiopia partnered with researchers at the Broad Institute of the Massachusetts Institute of Technology and Harvard to conduct the largest GWAS of psychiatric disorders in Africa. Dr. Koenen leads the project, Neuropsychiatric Genetics of African Populations–Psychosis (NeuroGAP-Psychosis), which will analyze DNA from over 35,000 people of African ancestry in each of these four countries. Investigators will compare the half of participants who have no history of psychosis with the half with schizophrenia or bipolar disorder in the hopes of identifying the genetic determinants of psychosis.
“Then any potential intervention or therapeutics that will be developed will also be useful for Africans,” says Dr. Teferra, a NeuroGAP principal investigator. Because of the tremendous degree of genetic diversity among people on the continent, however, findings still might not translate to all African populations.
But correcting equity problems in genomics isn’t as simple as recruiting people with non-European backgrounds, especially if those people are unfamiliar with research or have been subject to scientific exploitation. “Special care needs to be taken to, first of all, provide information that’s appropriate [to participants], but also motivate people to take part and then find ways to keep these communities involved and understand what they’re interested in,” says Dr. Kuchenbaecker, who is not involved with NeuroGAP.
For NeuroGAP, the team needed to work with ethical committees at all of the institutions involved, ensure research materials were appropriate for each community’s cultural context, and gain the trust of local communities.
“One of the biggest criticisms within the scientific world is that people from more endowed countries just fly in, bully everyone, collect the data, and leave, with no credit to the local scientists or communities,” says NeuroGAP principal investigator Lukoye Atwoli, MMed, PhD, professor of psychiatry and dean of the Medical College, East Africa, at the Aga Khan University, Nairobi, Kenya. “That is one of the biggest pitfalls we had to grapple with.”
To address that concern, NeuroGAP is training local researchers and is providing them with requested resources so they can carry out similar studies in the future. “We will be looking to address a real need in the academic community and in clinical service delivery,” says Dr. Atwoli.
Dr. Kuchenbaecker says that NeuroGAP demonstrates features necessary for projects seeking to improve equity in psychiatric genomics. “What they’re doing right is recruiting really large numbers, recruiting from different African countries, and involving African investigators,” she says.
In the Americas, Janitza Montalvo-Ortiz, PhD, assistant professor in the Division of Genetics, department of psychiatry, Yale University, New Haven, Conn., and her colleagues are expanding psychiatric genomics projects in Latin America. She co-founded the Latin American Genomics Consortium in 2019, a network of scientists supporting psychiatric genomic research in the region. The consortium also involves the Neuropsychiatric Genetics in Mexican Populations project, which is similar to NeuroGAP and is also led by Dr. Koenan.
The study of Latin American populations is complicated, because genes in these populations reflect Indigenous American, European, and African ancestries. Even when investigators sampled DNA from Latin American individuals, that data often went unused. “Now with new methods emerging to allow us to properly analyze admixed populations in GWAS studies, we’re making efforts to compile different datasets scattered across different large-scale cohorts,” says Dr. Montalvo-Ortiz. “Our ultimate goal is to conduct the first large-scale LatinX GWAS of psychiatry,” she says.
With these projects, researchers hope that new psychiatric research will produce clinical advances for people historically left on the sidelines of genomic studies. By involving their communities in genomic research, “whatever is going to be developed will also benefit our community,” says Dr. Teferra. “We will not be left out.”
A version of this article first appeared on Medscape.com.
In combing the genome, scientists can use genetic clues to determine a person’s risk for psychiatric disease and even identify new drug targets. But the benefits of these discoveries will be limited to people of European descent.
Nearly 90% of participants in genome-wide association studies (GWASs), which search for gene variants linked to disease, are of European ancestry. This Eurocentric focus threatens to widen existing disparities in racial and ethnic mental health.
“If you develop certain interventions based on only a single population profile, then you’ll be leaving out the rest of the populations in the world,” says Solomon Teferra, MD, PhD, associate professor of psychiatry at Addis Ababa University, Ethiopia. In a growing trend, psychiatric researchers are diverging from the field’s European bias and are working to correct the imbalance in DNA databases.
The significant downsides of genomics’ one-track mind
One obstacle hindering therapeutic advances in psychiatry is a shallow understanding of the mechanisms of disorders. “The biggest problem in terms of advancing research for mental health conditions is that we don’t understand the underlying biology,” says Laramie Duncan, PhD, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University. “Genetics is one of the best ways to systematically look for new clues about the underlying biology.”
At the advent of genomic research, scientists thought it best to study DNA from people of a single ancestry from one continent. “Researchers for a long time held the idea that it was going to be too complicated to include multiple ancestries in the first rounds of genetic analyses,” says Dr. Duncan.
Studying DNA from someone with ancestors from multiple parts of the world wasn’t compatible with methods used in the early days of GWASs. “Individual parts of a person’s DNA can be linked back to one region of the world or another, and most of our methods essentially assume that all of a person’s DNA came from one region of the world,” says Dr. Duncan.
Because many genes are usually involved in psychiatric disorders, scientists need large numbers of participants to detect uncommon, influential variants. Early research was concentrated in North America and Europe so that scientists could readily collect samples from people of European ancestry.
“It then went out of hand because it became routine practice to use only this one group, essentially White, European ancestry people,” says Karoline Kuchenbaecker, PhD, associate professor of psychiatry at University College London.
Yet findings from one population won’t necessarily translate to others. “And that’s exactly what has been shown,” says Dr. Teferra. Polygenic risk scores developed for schizophrenia from European samples, for example, perform poorly among people of African ancestry, although among Europeans, they are strongly effective at differentiating European individuals with and those without schizophrenia. Moreover, drugs that target a gene identified from studies in European populations may be harmful to other groups.
Studies drawn from a diverse pool of participants would benefit a wider swath of humanity. They would also allow scientists to discover small areas of overlap in genomes of different populations, which would help them close in on the true biology of diseases and ensure that “we’re all benefiting from more diverse data in genetics and psychiatric genetics,” says Dr. Kuchenbaecker.
New efforts aim at filling the gaps
, not African, Latin American, or Indigenous ancestry.
Efforts to increase representation of persons of African ancestry have largely focused on African Americans; fewer efforts have extended to the African continent, home to the most genetically diverse populations. Even fewer have focused on mental health. “The little that was being done was on a very small scale,” says Karestan Koenen, PhD, a professor at Harvard School of Public Health, Boston.
With this in mind, researchers from institutions in Kenya, Uganda, South Africa, and Ethiopia partnered with researchers at the Broad Institute of the Massachusetts Institute of Technology and Harvard to conduct the largest GWAS of psychiatric disorders in Africa. Dr. Koenen leads the project, Neuropsychiatric Genetics of African Populations–Psychosis (NeuroGAP-Psychosis), which will analyze DNA from over 35,000 people of African ancestry in each of these four countries. Investigators will compare the half of participants who have no history of psychosis with the half with schizophrenia or bipolar disorder in the hopes of identifying the genetic determinants of psychosis.
“Then any potential intervention or therapeutics that will be developed will also be useful for Africans,” says Dr. Teferra, a NeuroGAP principal investigator. Because of the tremendous degree of genetic diversity among people on the continent, however, findings still might not translate to all African populations.
But correcting equity problems in genomics isn’t as simple as recruiting people with non-European backgrounds, especially if those people are unfamiliar with research or have been subject to scientific exploitation. “Special care needs to be taken to, first of all, provide information that’s appropriate [to participants], but also motivate people to take part and then find ways to keep these communities involved and understand what they’re interested in,” says Dr. Kuchenbaecker, who is not involved with NeuroGAP.
For NeuroGAP, the team needed to work with ethical committees at all of the institutions involved, ensure research materials were appropriate for each community’s cultural context, and gain the trust of local communities.
“One of the biggest criticisms within the scientific world is that people from more endowed countries just fly in, bully everyone, collect the data, and leave, with no credit to the local scientists or communities,” says NeuroGAP principal investigator Lukoye Atwoli, MMed, PhD, professor of psychiatry and dean of the Medical College, East Africa, at the Aga Khan University, Nairobi, Kenya. “That is one of the biggest pitfalls we had to grapple with.”
To address that concern, NeuroGAP is training local researchers and is providing them with requested resources so they can carry out similar studies in the future. “We will be looking to address a real need in the academic community and in clinical service delivery,” says Dr. Atwoli.
Dr. Kuchenbaecker says that NeuroGAP demonstrates features necessary for projects seeking to improve equity in psychiatric genomics. “What they’re doing right is recruiting really large numbers, recruiting from different African countries, and involving African investigators,” she says.
In the Americas, Janitza Montalvo-Ortiz, PhD, assistant professor in the Division of Genetics, department of psychiatry, Yale University, New Haven, Conn., and her colleagues are expanding psychiatric genomics projects in Latin America. She co-founded the Latin American Genomics Consortium in 2019, a network of scientists supporting psychiatric genomic research in the region. The consortium also involves the Neuropsychiatric Genetics in Mexican Populations project, which is similar to NeuroGAP and is also led by Dr. Koenan.
The study of Latin American populations is complicated, because genes in these populations reflect Indigenous American, European, and African ancestries. Even when investigators sampled DNA from Latin American individuals, that data often went unused. “Now with new methods emerging to allow us to properly analyze admixed populations in GWAS studies, we’re making efforts to compile different datasets scattered across different large-scale cohorts,” says Dr. Montalvo-Ortiz. “Our ultimate goal is to conduct the first large-scale LatinX GWAS of psychiatry,” she says.
With these projects, researchers hope that new psychiatric research will produce clinical advances for people historically left on the sidelines of genomic studies. By involving their communities in genomic research, “whatever is going to be developed will also benefit our community,” says Dr. Teferra. “We will not be left out.”
A version of this article first appeared on Medscape.com.
Britney Spears and her 13-year conservatorship: An abuse of involuntary care?
The public has watched the ongoing drama unfold in the media for the past 13 years. In 2008, pop star Britney Spears was placed on conservatorship – a court process that gave decision-making powers over her personal, legal, and financial decisions to another person.
On June 23, 2021, Ms. Spears announced in open court that she is traumatized by being conserved and wants her rights back, but we know little about what behaviors left her family and a judge to determine that she was not capable of managing her own affairs, and why this would remain the case for so many years.
Adam Nelson, MD, practices psychiatry in Marin County, Calif. He explained in an interview that there are different types of conservatorships. “Probate conservatorship is the traditional path for conservatorship of person, estate, and/or finances based on evidence of incapacity due to any medical condition. This is the type of conservatorship that Britney Spears has had and which she is now contesting.”
Ms. Spears was placed on conservatorship after two involuntary hospitalizations for psychiatric illness and/or substance abuse. Her father, Jamie Spears, was appointed by the court to be her conservator.
In a New York Times article, reporters Liz Day, Samantha Stark, and Joe Coscarelli recently wrote: “But now, confidential court records obtained by the New York Times reveal that Ms. Spears, 39, expressed serious opposition to the conservatorship earlier and more often than had previously been known, and said that it restricted everything from whom she dated to the color of her kitchen cabinets.” The article goes on to say: “The newly obtained court records show that Ms. Spears questioned [her father’s] fitness for the role. As early as 2014, in a hearing closed to the public, Ms. Spears’s court-appointed lawyer, Samuel D. Ingham III, said she wanted to explore removing her father as conservator, citing his drinking, among other objections on a ‘shopping list’ of grievances.
“As the fight drags on, the bills are piling up – and, in a quirk of the conservatorship system, Ms. Spears has to pay for lawyers on both sides, including those arguing against her wishes in court. A recent $890,000 bill from one set of Mr. Spears’s lawyers, covering about 4 months of work, included media strategizing for defending the conservatorship.”
The case heated up at the June 23 hearing, when Ms. Spears had a telephone hearing with Los Angeles probate Judge Brenda Penney. The call was transcribed and published in Variety. The purpose of the hearing was for Ms. Spears to request an expedited release from her conservatorship without a psychiatric evaluation.
Ms. Spears began her 23-minute testimony to the judge by discussing her work and how she felt compelled to perform. “My management said, if I don’t do this tour, I will have to find an attorney, and by contract my own management could sue me if I didn’t follow through with the tour. ... So out of fear, I went ahead and I did the tour.”
She then discussed concerns by her manager that she was not complying with her medication regimen.
“Three days later, after I said no to Vegas,” Ms. Spears continued, “my therapist sat me down in a room and said he had a million phone calls about how I was not cooperating in rehearsals, and I haven’t been taking my medication. All this was false. He immediately, the next day, put me on lithium out of nowhere. He took me off my normal meds I’ve been on for 5 years. ... There were six different nurses in my home and they wouldn’t let me get in my car to go anywhere for a month.”
She spoke about entering rehab at the insistence of the conservatorship, and relayed her distress about this experience. She talked poignantly about her frustration of feeling she was not being heard by the court the last time she spoke and about the financial conflicts of interest created by her conservatorship. Ms. Spears, who has appeared on national television, recorded albums, and gone on performance tours during this period, has a net worth estimated at $60 million.
“It’s been a long time since I’ve owned my money. And it’s my wish and my dream for all of this to end without being tested,” she told the judge. “Again, it makes no sense whatsoever for the state of California to sit back and literally watch me with their own two eyes, make a living for so many people, and pay so many people trucks and buses on the road with me and be told, I’m not good enough. But I’m great at what I do. And I allow these people to control what I do, ma’am. And it’s enough. It makes no sense at all.”
Finally, Ms. Spears expressed a heart-wrenching desire to have another child and she asserted that the conservatorship will not allow her to see a doctor to have her IUD removed. She talked about being required to go to therapy three times a week and contended that she is traumatized by all that has transpired.
Ms. Spears has not filed the necessary paperwork to have her conservatorship ended. In an interview with Vice, attorney Scott Rahn noted that the process to end conservatorship can be a lengthy and difficult path. To do so, she might first need to petition the court to be allowed to hire her own attorney. If uncontested, the conservatorship could possibly be ended within months, but otherwise this could entail a lengthy trial over the course of years. While ending conservatorship may entail discovery, depositions, and hearings over years, a scathing story in the New Yorker detailed how Ms. Spears was placed into this conservatorship in a matter of days, without being present to give her own testimony. In the usual circumstances, California law requires that the person being conserved must be given 5 days’ notice before a conservatorship takes place, but Ms. Spears was deemed to be at risk of substantial harm and the judge allowed for an immediate conservatorship. The article notes that even axe murderers are allowed to hire lawyers, while those placed in conservatorships are not.
Reasoning behind such actions
Often, people are appointed guardians, conservators, or payees because of concerns that their psychiatric or substance use disorders, dementia, or impaired intellectual states lead them to poor decisions that endanger their financial stability. Usually the money they may lose is from a government disability benefit, an inheritance, or former accrued wealth. In this unusual celebrity case, Britney Spears has been conserved while she maintained a rigorous work schedule and actively earned the money she is being protected from spending.
Dr. Nelson talked about how people come to be conserved. “The law regarding conservatorship is a state law, but conservatorships in California are done at the county level, and the counties don’t have a vested interest in protecting people from themselves unless a third party or a family member comes forward. I imagine there is another side to this story, I have never seen it used like this for someone who is working. Questions remain about why this conservatorship has gone on for 13 years.”
Dr. Nelson believes that the current California laws leave room for abuse. “If the children of a wealthy parent observes the parent spending their inheritance in a way they don’t approve of, they can claim the parent is impaired and needs to be conserved. Usually it doesn’t work, but it’s possible there are times when the courts are swayed.”
Why does it matter and why should psychiatrists be concerned? The issue of involuntary treatment is a contentious one, and the stakeholders on all sides are vocal when it comes to our country’s sickest and most vulnerable individuals. Any story with a whiff of abuse, or of someone who is not severely impaired being denied basic civil rights – including the right to refuse treatment – dilutes and stains the efforts of those who are trying to protect people who suffer from chronic psychotic disorders. And when society reaches further to say that an individual is not entitled to make their own basic life decisions, this further stigmatizes those with psychiatric illnesses. And people with both mental illnesses and substance use disorders often get better, so why would conservatorships be permanent?
Does our society want the courts to protect people from their own poor judgment? Should there be judges at every casino entrance? Where are the conservators for those who live in the streets? Again, this is a half-told story, one where the potential for abuse of the conserved remains a high risk, and the long-term message about involuntary care is one of taking a way a person’s rights unnecessarily.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. She has no disclosures.
The public has watched the ongoing drama unfold in the media for the past 13 years. In 2008, pop star Britney Spears was placed on conservatorship – a court process that gave decision-making powers over her personal, legal, and financial decisions to another person.
On June 23, 2021, Ms. Spears announced in open court that she is traumatized by being conserved and wants her rights back, but we know little about what behaviors left her family and a judge to determine that she was not capable of managing her own affairs, and why this would remain the case for so many years.
Adam Nelson, MD, practices psychiatry in Marin County, Calif. He explained in an interview that there are different types of conservatorships. “Probate conservatorship is the traditional path for conservatorship of person, estate, and/or finances based on evidence of incapacity due to any medical condition. This is the type of conservatorship that Britney Spears has had and which she is now contesting.”
Ms. Spears was placed on conservatorship after two involuntary hospitalizations for psychiatric illness and/or substance abuse. Her father, Jamie Spears, was appointed by the court to be her conservator.
In a New York Times article, reporters Liz Day, Samantha Stark, and Joe Coscarelli recently wrote: “But now, confidential court records obtained by the New York Times reveal that Ms. Spears, 39, expressed serious opposition to the conservatorship earlier and more often than had previously been known, and said that it restricted everything from whom she dated to the color of her kitchen cabinets.” The article goes on to say: “The newly obtained court records show that Ms. Spears questioned [her father’s] fitness for the role. As early as 2014, in a hearing closed to the public, Ms. Spears’s court-appointed lawyer, Samuel D. Ingham III, said she wanted to explore removing her father as conservator, citing his drinking, among other objections on a ‘shopping list’ of grievances.
“As the fight drags on, the bills are piling up – and, in a quirk of the conservatorship system, Ms. Spears has to pay for lawyers on both sides, including those arguing against her wishes in court. A recent $890,000 bill from one set of Mr. Spears’s lawyers, covering about 4 months of work, included media strategizing for defending the conservatorship.”
The case heated up at the June 23 hearing, when Ms. Spears had a telephone hearing with Los Angeles probate Judge Brenda Penney. The call was transcribed and published in Variety. The purpose of the hearing was for Ms. Spears to request an expedited release from her conservatorship without a psychiatric evaluation.
Ms. Spears began her 23-minute testimony to the judge by discussing her work and how she felt compelled to perform. “My management said, if I don’t do this tour, I will have to find an attorney, and by contract my own management could sue me if I didn’t follow through with the tour. ... So out of fear, I went ahead and I did the tour.”
She then discussed concerns by her manager that she was not complying with her medication regimen.
“Three days later, after I said no to Vegas,” Ms. Spears continued, “my therapist sat me down in a room and said he had a million phone calls about how I was not cooperating in rehearsals, and I haven’t been taking my medication. All this was false. He immediately, the next day, put me on lithium out of nowhere. He took me off my normal meds I’ve been on for 5 years. ... There were six different nurses in my home and they wouldn’t let me get in my car to go anywhere for a month.”
She spoke about entering rehab at the insistence of the conservatorship, and relayed her distress about this experience. She talked poignantly about her frustration of feeling she was not being heard by the court the last time she spoke and about the financial conflicts of interest created by her conservatorship. Ms. Spears, who has appeared on national television, recorded albums, and gone on performance tours during this period, has a net worth estimated at $60 million.
“It’s been a long time since I’ve owned my money. And it’s my wish and my dream for all of this to end without being tested,” she told the judge. “Again, it makes no sense whatsoever for the state of California to sit back and literally watch me with their own two eyes, make a living for so many people, and pay so many people trucks and buses on the road with me and be told, I’m not good enough. But I’m great at what I do. And I allow these people to control what I do, ma’am. And it’s enough. It makes no sense at all.”
Finally, Ms. Spears expressed a heart-wrenching desire to have another child and she asserted that the conservatorship will not allow her to see a doctor to have her IUD removed. She talked about being required to go to therapy three times a week and contended that she is traumatized by all that has transpired.
Ms. Spears has not filed the necessary paperwork to have her conservatorship ended. In an interview with Vice, attorney Scott Rahn noted that the process to end conservatorship can be a lengthy and difficult path. To do so, she might first need to petition the court to be allowed to hire her own attorney. If uncontested, the conservatorship could possibly be ended within months, but otherwise this could entail a lengthy trial over the course of years. While ending conservatorship may entail discovery, depositions, and hearings over years, a scathing story in the New Yorker detailed how Ms. Spears was placed into this conservatorship in a matter of days, without being present to give her own testimony. In the usual circumstances, California law requires that the person being conserved must be given 5 days’ notice before a conservatorship takes place, but Ms. Spears was deemed to be at risk of substantial harm and the judge allowed for an immediate conservatorship. The article notes that even axe murderers are allowed to hire lawyers, while those placed in conservatorships are not.
Reasoning behind such actions
Often, people are appointed guardians, conservators, or payees because of concerns that their psychiatric or substance use disorders, dementia, or impaired intellectual states lead them to poor decisions that endanger their financial stability. Usually the money they may lose is from a government disability benefit, an inheritance, or former accrued wealth. In this unusual celebrity case, Britney Spears has been conserved while she maintained a rigorous work schedule and actively earned the money she is being protected from spending.
Dr. Nelson talked about how people come to be conserved. “The law regarding conservatorship is a state law, but conservatorships in California are done at the county level, and the counties don’t have a vested interest in protecting people from themselves unless a third party or a family member comes forward. I imagine there is another side to this story, I have never seen it used like this for someone who is working. Questions remain about why this conservatorship has gone on for 13 years.”
Dr. Nelson believes that the current California laws leave room for abuse. “If the children of a wealthy parent observes the parent spending their inheritance in a way they don’t approve of, they can claim the parent is impaired and needs to be conserved. Usually it doesn’t work, but it’s possible there are times when the courts are swayed.”
Why does it matter and why should psychiatrists be concerned? The issue of involuntary treatment is a contentious one, and the stakeholders on all sides are vocal when it comes to our country’s sickest and most vulnerable individuals. Any story with a whiff of abuse, or of someone who is not severely impaired being denied basic civil rights – including the right to refuse treatment – dilutes and stains the efforts of those who are trying to protect people who suffer from chronic psychotic disorders. And when society reaches further to say that an individual is not entitled to make their own basic life decisions, this further stigmatizes those with psychiatric illnesses. And people with both mental illnesses and substance use disorders often get better, so why would conservatorships be permanent?
Does our society want the courts to protect people from their own poor judgment? Should there be judges at every casino entrance? Where are the conservators for those who live in the streets? Again, this is a half-told story, one where the potential for abuse of the conserved remains a high risk, and the long-term message about involuntary care is one of taking a way a person’s rights unnecessarily.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. She has no disclosures.
The public has watched the ongoing drama unfold in the media for the past 13 years. In 2008, pop star Britney Spears was placed on conservatorship – a court process that gave decision-making powers over her personal, legal, and financial decisions to another person.
On June 23, 2021, Ms. Spears announced in open court that she is traumatized by being conserved and wants her rights back, but we know little about what behaviors left her family and a judge to determine that she was not capable of managing her own affairs, and why this would remain the case for so many years.
Adam Nelson, MD, practices psychiatry in Marin County, Calif. He explained in an interview that there are different types of conservatorships. “Probate conservatorship is the traditional path for conservatorship of person, estate, and/or finances based on evidence of incapacity due to any medical condition. This is the type of conservatorship that Britney Spears has had and which she is now contesting.”
Ms. Spears was placed on conservatorship after two involuntary hospitalizations for psychiatric illness and/or substance abuse. Her father, Jamie Spears, was appointed by the court to be her conservator.
In a New York Times article, reporters Liz Day, Samantha Stark, and Joe Coscarelli recently wrote: “But now, confidential court records obtained by the New York Times reveal that Ms. Spears, 39, expressed serious opposition to the conservatorship earlier and more often than had previously been known, and said that it restricted everything from whom she dated to the color of her kitchen cabinets.” The article goes on to say: “The newly obtained court records show that Ms. Spears questioned [her father’s] fitness for the role. As early as 2014, in a hearing closed to the public, Ms. Spears’s court-appointed lawyer, Samuel D. Ingham III, said she wanted to explore removing her father as conservator, citing his drinking, among other objections on a ‘shopping list’ of grievances.
“As the fight drags on, the bills are piling up – and, in a quirk of the conservatorship system, Ms. Spears has to pay for lawyers on both sides, including those arguing against her wishes in court. A recent $890,000 bill from one set of Mr. Spears’s lawyers, covering about 4 months of work, included media strategizing for defending the conservatorship.”
The case heated up at the June 23 hearing, when Ms. Spears had a telephone hearing with Los Angeles probate Judge Brenda Penney. The call was transcribed and published in Variety. The purpose of the hearing was for Ms. Spears to request an expedited release from her conservatorship without a psychiatric evaluation.
Ms. Spears began her 23-minute testimony to the judge by discussing her work and how she felt compelled to perform. “My management said, if I don’t do this tour, I will have to find an attorney, and by contract my own management could sue me if I didn’t follow through with the tour. ... So out of fear, I went ahead and I did the tour.”
She then discussed concerns by her manager that she was not complying with her medication regimen.
“Three days later, after I said no to Vegas,” Ms. Spears continued, “my therapist sat me down in a room and said he had a million phone calls about how I was not cooperating in rehearsals, and I haven’t been taking my medication. All this was false. He immediately, the next day, put me on lithium out of nowhere. He took me off my normal meds I’ve been on for 5 years. ... There were six different nurses in my home and they wouldn’t let me get in my car to go anywhere for a month.”
She spoke about entering rehab at the insistence of the conservatorship, and relayed her distress about this experience. She talked poignantly about her frustration of feeling she was not being heard by the court the last time she spoke and about the financial conflicts of interest created by her conservatorship. Ms. Spears, who has appeared on national television, recorded albums, and gone on performance tours during this period, has a net worth estimated at $60 million.
“It’s been a long time since I’ve owned my money. And it’s my wish and my dream for all of this to end without being tested,” she told the judge. “Again, it makes no sense whatsoever for the state of California to sit back and literally watch me with their own two eyes, make a living for so many people, and pay so many people trucks and buses on the road with me and be told, I’m not good enough. But I’m great at what I do. And I allow these people to control what I do, ma’am. And it’s enough. It makes no sense at all.”
Finally, Ms. Spears expressed a heart-wrenching desire to have another child and she asserted that the conservatorship will not allow her to see a doctor to have her IUD removed. She talked about being required to go to therapy three times a week and contended that she is traumatized by all that has transpired.
Ms. Spears has not filed the necessary paperwork to have her conservatorship ended. In an interview with Vice, attorney Scott Rahn noted that the process to end conservatorship can be a lengthy and difficult path. To do so, she might first need to petition the court to be allowed to hire her own attorney. If uncontested, the conservatorship could possibly be ended within months, but otherwise this could entail a lengthy trial over the course of years. While ending conservatorship may entail discovery, depositions, and hearings over years, a scathing story in the New Yorker detailed how Ms. Spears was placed into this conservatorship in a matter of days, without being present to give her own testimony. In the usual circumstances, California law requires that the person being conserved must be given 5 days’ notice before a conservatorship takes place, but Ms. Spears was deemed to be at risk of substantial harm and the judge allowed for an immediate conservatorship. The article notes that even axe murderers are allowed to hire lawyers, while those placed in conservatorships are not.
Reasoning behind such actions
Often, people are appointed guardians, conservators, or payees because of concerns that their psychiatric or substance use disorders, dementia, or impaired intellectual states lead them to poor decisions that endanger their financial stability. Usually the money they may lose is from a government disability benefit, an inheritance, or former accrued wealth. In this unusual celebrity case, Britney Spears has been conserved while she maintained a rigorous work schedule and actively earned the money she is being protected from spending.
Dr. Nelson talked about how people come to be conserved. “The law regarding conservatorship is a state law, but conservatorships in California are done at the county level, and the counties don’t have a vested interest in protecting people from themselves unless a third party or a family member comes forward. I imagine there is another side to this story, I have never seen it used like this for someone who is working. Questions remain about why this conservatorship has gone on for 13 years.”
Dr. Nelson believes that the current California laws leave room for abuse. “If the children of a wealthy parent observes the parent spending their inheritance in a way they don’t approve of, they can claim the parent is impaired and needs to be conserved. Usually it doesn’t work, but it’s possible there are times when the courts are swayed.”
Why does it matter and why should psychiatrists be concerned? The issue of involuntary treatment is a contentious one, and the stakeholders on all sides are vocal when it comes to our country’s sickest and most vulnerable individuals. Any story with a whiff of abuse, or of someone who is not severely impaired being denied basic civil rights – including the right to refuse treatment – dilutes and stains the efforts of those who are trying to protect people who suffer from chronic psychotic disorders. And when society reaches further to say that an individual is not entitled to make their own basic life decisions, this further stigmatizes those with psychiatric illnesses. And people with both mental illnesses and substance use disorders often get better, so why would conservatorships be permanent?
Does our society want the courts to protect people from their own poor judgment? Should there be judges at every casino entrance? Where are the conservators for those who live in the streets? Again, this is a half-told story, one where the potential for abuse of the conserved remains a high risk, and the long-term message about involuntary care is one of taking a way a person’s rights unnecessarily.
Dr. Miller is coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore. She has no disclosures.
Mood stabilizers: Balancing tolerability, serum levels, and dosage
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.
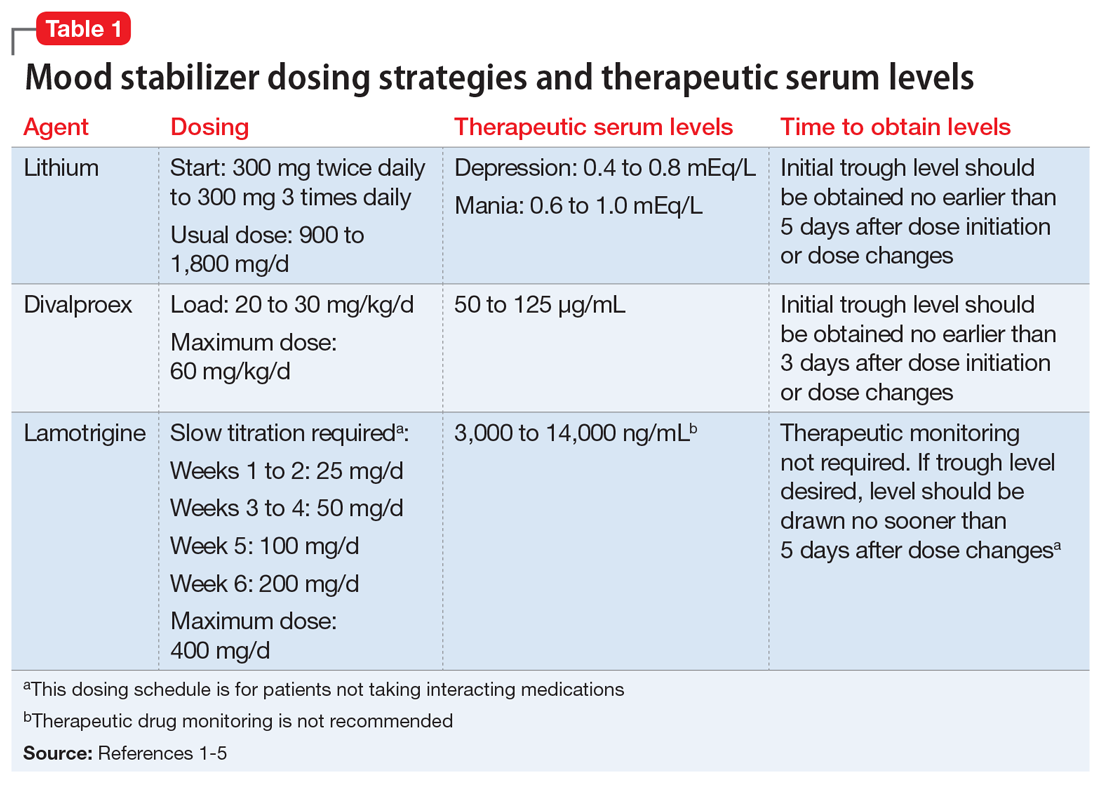
Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.

Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.
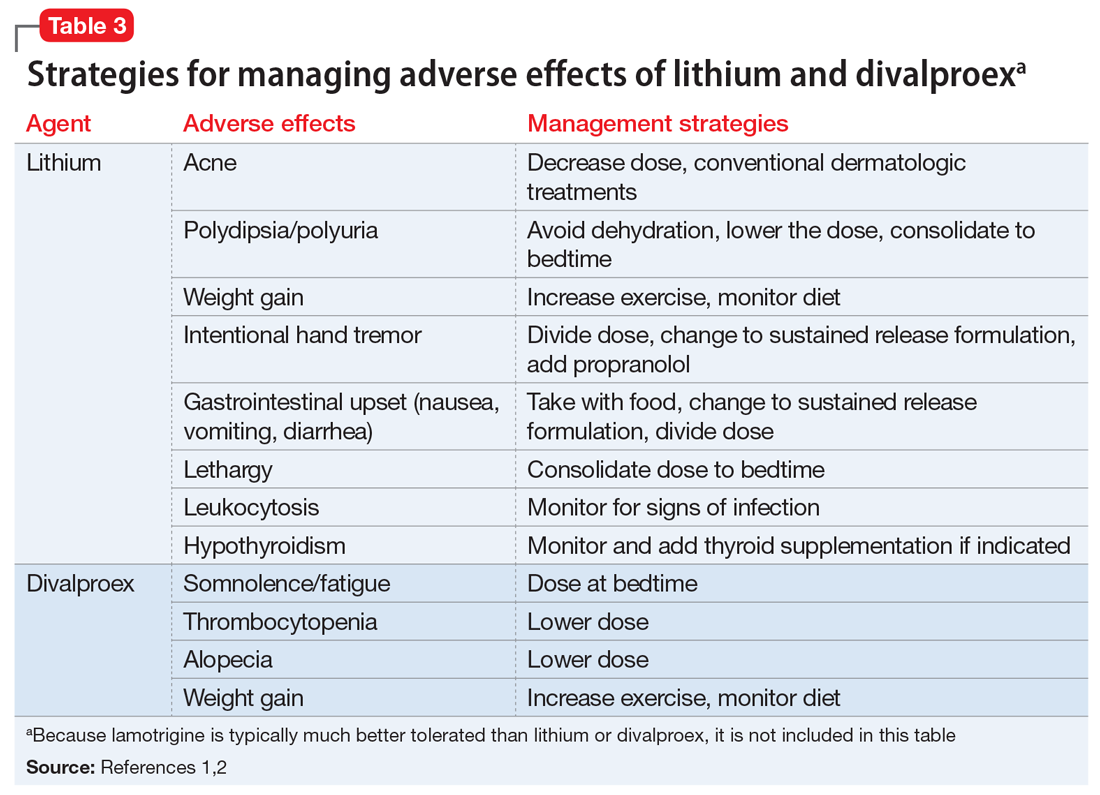
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.

Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.

Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.

Mr. B, age 32, was diagnosed with bipolar disorder 10 years ago after experiencing a manic episode that resulted in his first psychiatric hospitalization. He was prescribed quetiapine, 400 mg/d, and remained stable for the next several years. Unfortunately, Mr. B developed significant metabolic adverse effects, including diabetes and a 30-pound weight gain, so he was switched from quetiapine to lithium. Mr. B was unable to tolerate the sedation and cognitive effects of lithium, and the dose could not be titrated to within the therapeutic window. As a result, Mr. B experienced a moderate depressive episode. His current clinician would like to initiate lamotrigine at a starting dose of 25 mg/d. Mr. B has not had a manic episode since the index hospitalization, and this is his first depressive episode.
The term “mood stabilizer” has come to refer to medications that treat a depressive and/or manic episode without inducing the other. In conventional terms, it refers to non-antipsychotic medications such as lithium, divalproex, and lamotrigine. Except for lithium, mood stabilizers are also antiepileptic drugs (AEDs). The role of AEDs for treating psychiatric conditions was discovered after they were originally FDA-approved for treating seizures. Following this discovery, the recommended doses and therapeutic ranges for these agents when applied to psychiatric treatment fell into a gray area.
Every patient is different and requires an individualized treatment plan, but this often leaves the clinician wondering, “How high is too high for this mood stabilizer?” or “My patient is responding well, but could a higher dose be even more effective?” In the case of Mr. B, who has trialed 2 medications with poor tolerability, how high can the lamotrigine dose be titrated to achieve a therapeutic response without adverse effects? The literature on this topic does not provide an exact answer, but does shed some light on key considerations for such decisions.
Which mood stabilizers are recommended?
One of the most recently updated guidelines for the treatment of bipolar disorder was released in 2018 by the Canadian Network for Mood and Anxiety Treatments (CANMAT).1 Lithium, divalproex, and lamotrigine were each recommended as a first-line option for treating bipolar disorder. For lithium and divalproex, the CANMAT guidelines recommend serum level monitoring for efficacy and tolerability; however, they do not recommend serum level monitoring for lamotrigine. Lithium and divalproex each have safety and tolerability concerns, particularly when selected for maintenance therapy, whereas lamotrigine is typically much better tolerated.1 Divalproex and lithium can cause weight gain, gastrointestinal adverse effects (nausea, vomiting, diarrhea), and tremor. Additional tolerability concerns with lithium include renal toxicity, electrocardiogram abnormalities, hypothyroidism, cognitive impairment, and dermatologic reactions. Divalproex can produce greater levels of sedation and may impact reproductive function (oligomenorrhea or hyperandrogenism). One of the most common adverse effects of lamotrigine is a non-serious rash; however, slow dose titration is necessary to decrease the risk of a serious, life-threatening rash such as Stevens-Johnson syndrome.
Lithium
Lithium continues to be regarded as a gold-standard therapy for bipolar disorder. The exact serum levels corresponding to efficacy and tolerability vary. The Lithiumeter: Version 2.0 is a schematic that incorporates the various levels recommended by different clinical guidelines.2 The recommended serum levels range from 0.6 to 1.0 mEq/L for mania and 0.4 to 0.8 mEq/L for depression.2 One of the main issues with lithium dosing is balancing a therapeutic level with tolerability and toxicity. Toxicity may begin when lithium levels exceed 1.2 mEq/L, and levels >2.0 mEq/L can be lethal. Signs of acute toxicity include tremor, headache, arrhythmia, nausea, vomiting, diarrhea, polyuria, and polydipsia. Conversely, chronic lithium use may lead to chronic toxicity as patients age and their physical health changes. Signs of chronic toxicity include ataxia, confusion, renal dysfunction, and tremor. There is no “one size fits all” when it comes to lithium dosing. Individualized dosing is necessary to balance efficacy and tolerability.
Divalproex
Divalproex was initially studied for use as an AED, and its therapeutic levels as an AED are not the same as those indicated for bipolar disorder. Generally, patients with bipolar disorder require a divalproex serum level >50 µg/mL. Ranges closer to 100 µg/mL have been found to be most effective for treating acute mania.3 A loading dose of 20 to 30 mg/kg/d can be administered to help achieve mood stabilization. Again, efficacy must be balanced against toxicity. The maximum dose of divalproex is 60 mg/kg/d, which is rarely seen in psychiatric practice. Early studies of divalproex found adverse effects greatest in individuals with plasma levels >100 µg/mL. Reported adverse effects included alopecia, weight gain, tremor, and mental status changes.4
Lamotrigine
Unlike lithium and divalproex, lamotrigine therapeutic drug monitoring is not common. The accepted therapeutic reference range (TRR) for lamotrigine as an AED is 3,000 to 14,000 ng/mL. Unholzer et al5 evaluated the dose and TRR for individuals with bipolar disorder treated with lamotrigine. No statistically significant difference in lamotrigine serum levels was found in responders vs nonresponders.5 Most patients were prescribed ≤200 mg/d; however, some were prescribed higher doses. The maximum dose recommended when lamotrigine is used as an AED is 400 mg/d; however, this study furthered the evidence that lower doses tend to be effective in bipolar disorder.
Continue to: CASE
CASE CONTINUED
It has been 3 months since Mr. B was initiated on lamotrigine, and he has since been titrated to his current, stable dose of 100 mg/d. Mr. B is no longer experiencing the sedation he had with lithium and has the energy to commit to an exercise routine. This has allowed him to lose 15 pounds so far and greatly improve control of his diabetes.

Dosage summary
Most available evidence supports dosing lithium and divalproex to effect, typically seen between 0.6 to 1.0 mEq/L and 50 to 125 µg/mL, respectively. Higher plasma levels tend to correspond to more adverse effects and toxicity. Lamotrigine does not have such a narrow therapeutic window. Lamotrigine for psychiatric treatment yields greatest efficacy at approximately 200 mg/d, but doses can be increased if warranted, which could be the case in Mr. B.

Table 11-5 outlines dosing strategies and therapeutic serum levels for lithium, divalproex, and lamotrigine. Table 22 lists signs and symptoms of lithium toxicity, and Table 31,2 describes strategies for managing adverse effects of lithium and divalproex.

1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
1. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170.
2. Malhi GS, Gershon S, Outhred T. Lithiumeter: version 2.0. Bipolar Disord. 2016;18(8):631-641.
3. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
4. Turnbull DM, Rawlins MD, Weightman D, et al. Plasma concentrations of sodium valproate: their clinical value. Ann Neurol. 1983;14(1):38-42.
5. Unholzer S, Haen E. Retrospective analysis of therapeutic drug monitoring data for treatment of bipolar disorder with lamotrigine. Pharmacopsychiatry. 2015;48(7):296.
Stuck in a rut with the wrong diagnosis
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.
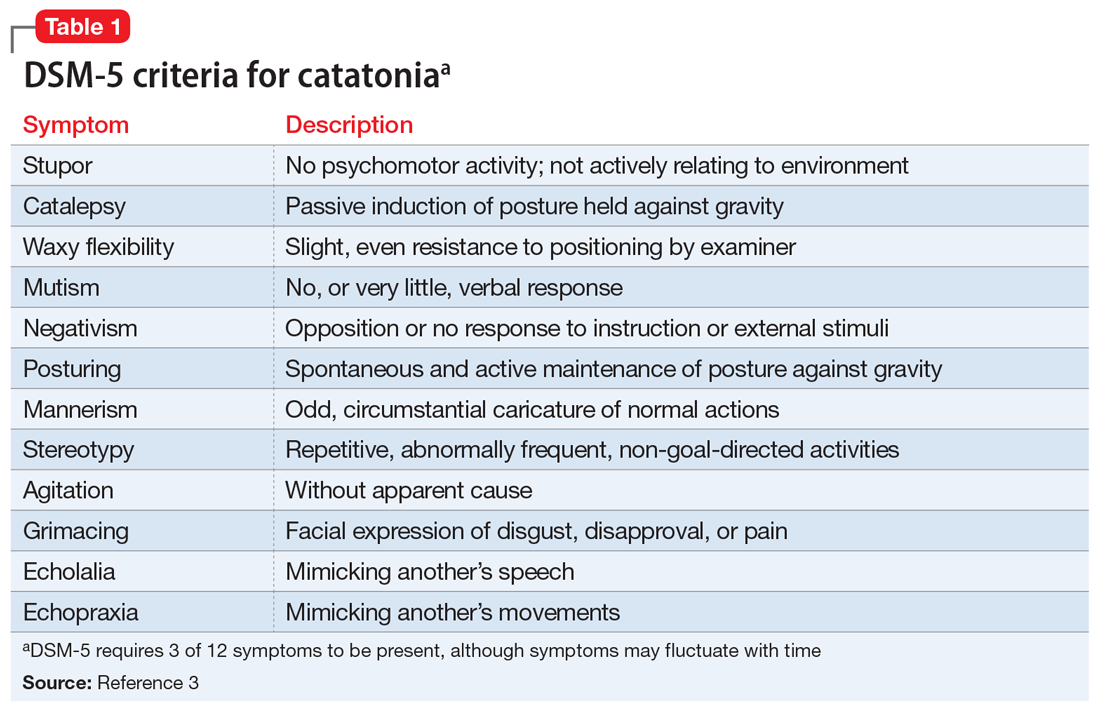
A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.
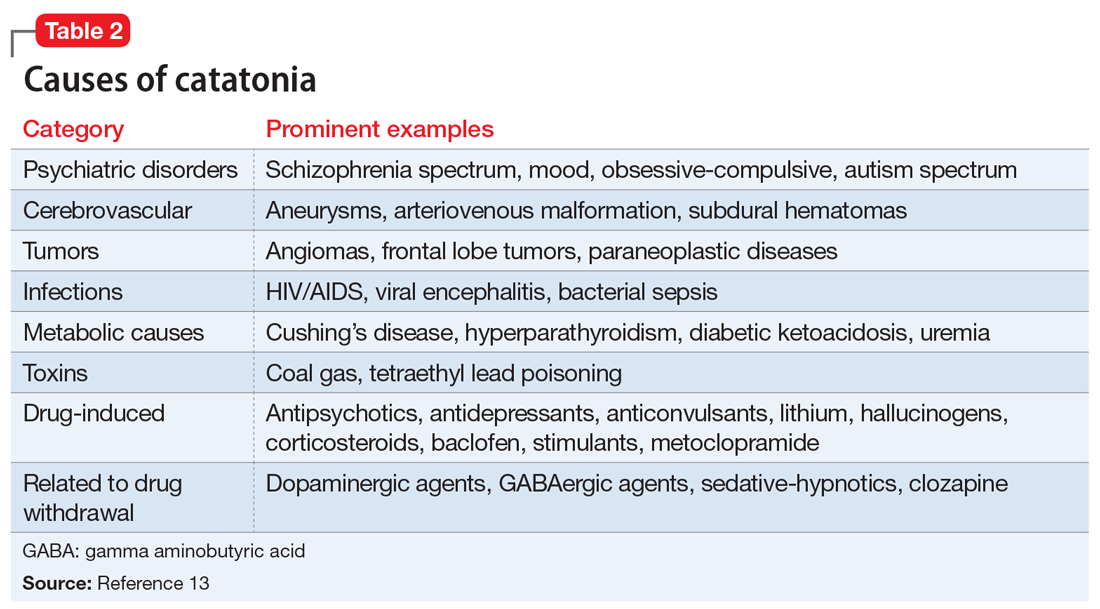
Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-
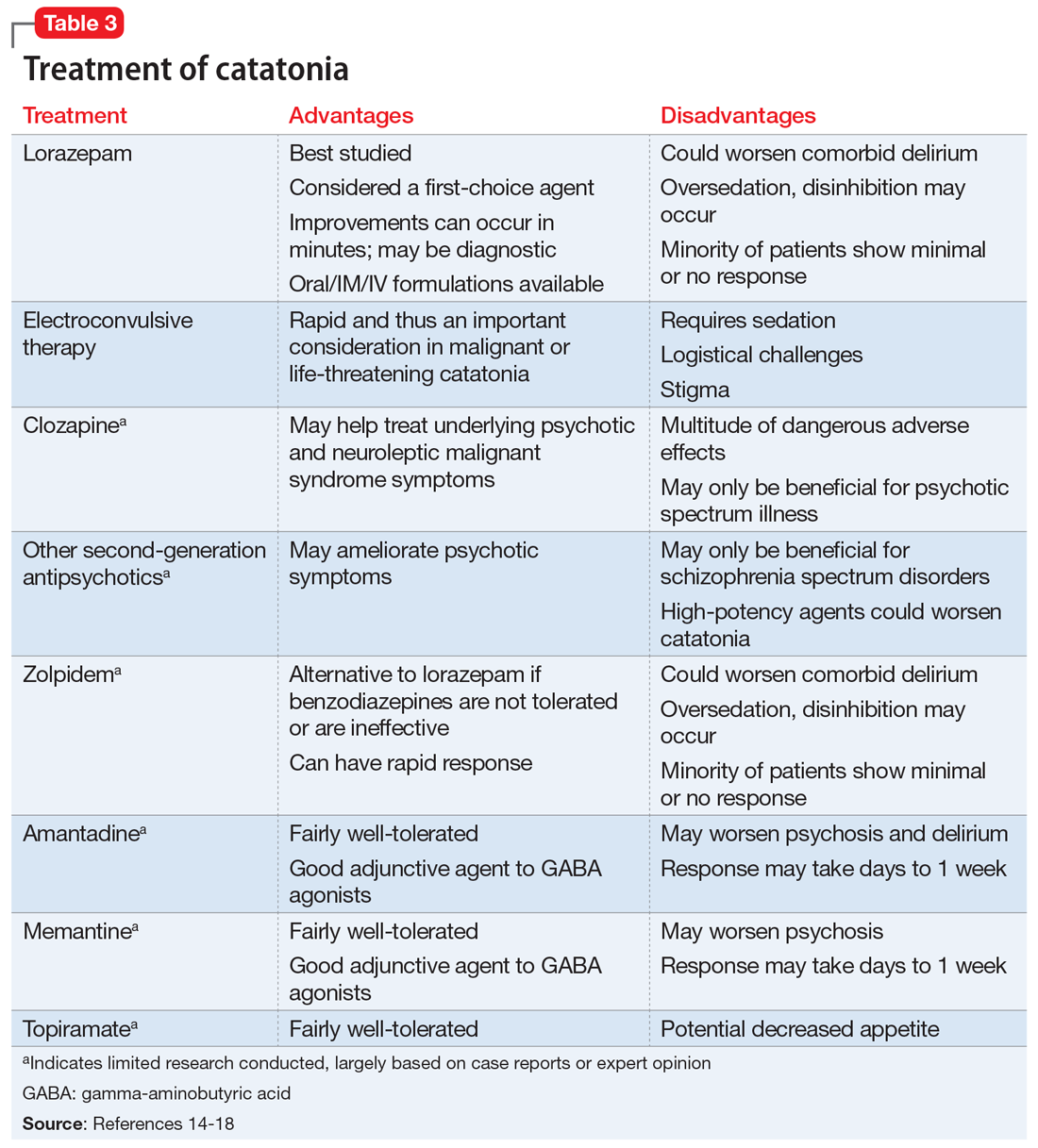
Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.

A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.

Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-

Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
CASE Aggressive behaviors, psychosis
Ms. N, age 58, has a long history of bipolar disorder with psychotic features. She presents to our emergency department (ED) after an acute fall and frequent violent behaviors at her nursing home, where she had resided since being diagnosed with an unspecified neurocognitive disorder. For several weeks before her fall, she was physically aggressive, throwing objects at nursing home staff, and was unable to have her behavior redirected.
While in the ED, Ms. N rambles and appears to be responding to internal stimuli. Suddenly, she stops responding and begins to stare.
HISTORY Severe, chronic psychosis and hospitalization
Ms. N is well-known at our inpatient psychiatry and electroconvulsive therapy (ECT) services. During the last 10 years, she has had worsening manic, psychotic, and catatonic (both excited and stuporous subtype) episodes. Three years ago, she had experienced a period of severe, chronic psychosis and excited catatonia that required extended inpatient treatment. While hospitalized, Ms. N had marginal responses to clozapine and benzodiazepines, but improved dramatically with ECT. After Ms. N left the hospital, she went to live with her boyfriend. She remained stable on monthly maintenance ECT treatments (bifrontal) before she was lost to follow-up 14 months prior to the current presentation. Ms. N’s family reports that she needed a cardiac clearance before continuing ECT treatment; however, she was hospitalized at another hospital with pneumonia and subsequent complications that interrupted the maintenance ECT treatments.
Approximately 3 months after medical issues requiring hospitalization began, Ms. N received a diagnosis of neurocognitive disorder due to difficulty with activities of daily living and cognitive decline. She was transferred to a nursing home by the outside hospital. When Ms. N’s symptoms of psychosis returned and she required inpatient psychiatric care, she was transferred to a nearby facility that did not have ECT available or knowledge of her history of catatonia resistant to pharmacologic management. Ms. N had a documented history of catatonia that spanned 10 years. During the last 4 years, Ms. N often required ECT treatment. Her current medication regimen prescribed by an outpatient psychiatrist includes clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily, both for bipolar disorder.
EVALUATION An unusual mix of symptoms
In the ED, Ms. N undergoes a CT of the head, which is found to be nonacute. Laboratory results show that her white blood cell count is 14.3 K/µL, which is mildly elevated. Results from a urinalysis and electrocardiogram (ECG) are unremarkable.
After Ms. N punches a radiology technician, she is administered IV lorazepam, 2 mg once, for her agitation. Twenty minutes after receiving IV lorazepam, she is calm and cooperative. However, approximately 4 hours later, Ms. N is yelling, tearful, and expressing delusions of grandeur—she believes she is God.
After she is admitted to the medical floor, Ms. N is seen by our consultation and liaison psychiatry service. She exhibits several signs of catatonia, including grasp reflex, gegenhalten (oppositional paratonia), waxy flexibility, and echolalia. Ms. N also has an episode of urinary incontinence. At some parts of the day, she is alert and oriented to self and location; at other times, she is somnolent and disoriented. The treatment team continues Ms. N’s previous medication regimen of clozapine, 300 mg twice daily, and clonazepam, 0.5 mg twice daily. Unfortunately, at times Ms. N spits out and hides her administered oral medications, which leads to the decision to discontinue clozapine. Once medically cleared, Ms. N is transferred to the psychiatric floor.
[polldaddy:10869949]
Continue to: TREATMENT
TREATMENT Bifrontal ECT initiated
On hospital Day 3 Ms. N is administered a trial of IM lorazepam, titrated up to 6 mg/d (maximum tolerated dose) while the treatment team initiates the legal process to conduct ECT because she is unable to give consent. Once Ms. N begins tolerating oral medications, amantadine, 100 mg twice daily, is added to treat her catatonia. As in prior hospitalizations, Ms. N is unresponsive to pharmacotherapy alone for her catatonic symptoms. On hospital Day 8, forced ECT is granted, which is 5 days after the process of filing paperwork was started. Bifrontal ECT is utilized with the following settings: frequency 70 Hz, pulse width 1.5 ms, 100% energy dose, 504 mC. Ms. N does not experience a significant improvement until she receives 10 ECT treatments as part of a 3-times-per-week acute series protocol. The Bush-Francis Catatonia Rating Scale (BFCRS) and the KANNER scale are used to monitor her progress. Her initial BFCRS score is 17 and initial KANNER scale, part 2 score is 26.
Ms. N spends a total of 61 days in the hospital, which is significantly longer than her previous hospital admissions on our psychiatric unit; these previous admissions were for treatment of both stuporous and excited subtypes of catatonia. This increased length of stay coincides with a significantly longer duration of untreated catatonia. Knowledge of her history of both the stuporous and excited subtypes of catatonia would have allowed for faster diagnosis and treatment.1
The authors’ observations
Originally conceptualized as a separate syndrome by Karl Kahlbaum, catatonia was considered only as a specifier for neuropsychiatric conditions (primarily schizophrenia) as recently as DSM-IV-TR.2 DSM-5 describes catatonia as a marked psychomotor disturbance and acknowledges its connection to schizophrenia by keeping it in the same chapter.3 DSM-5 includes separate diagnoses for catatonia, catatonia due to a general medical condition, and unspecified catatonia (for catatonia without a known underlying disorder).3 A recent meta-analysis found the prevalence of catatonia is higher in patients with medical/neurologic illness, bipolar disorder, and autism than in those with schizophrenia.4
Table 13 highlights the DSM-5 criteria for catatonia. DSM-5 requires 3 of 12 symptoms to be present, although symptoms may fluctuate with time.3 If a clinician is not specifically looking for catatonia, it can be a difficult syndrome to diagnose. Does rigidity indicate catatonia, or excessive dopamine blockade from an antipsychotic? How can seemingly contradictory symptoms be part of the same syndrome? Many clinicians associate catatonia with the stuporous subtype (immobility, posturing, catalepsy), which is more prevalent, but the excited subtype, which may involve severe agitation, autonomic dysfunction, and impaired consciousness, can be lethal.2 The diversity in presentation of catatonia is not unlike the challenging variety of symptoms of heart attacks.

A retrospective study of all adults admitted to a hospital found that only 41% of patients who met criteria for catatonia received this diagnosis.5 Further complicating the diagnosis, delirium and catatonia can co-exist; one study found this was the case in 1 of 3 critically ill patients.6 DSM-5 criteria for catatonia due to another medical condition exclude the diagnosis if delirium is present, but this study and others suggest this needs to be reconsidered.3
Continue to: A standardized evaluation is key
A standardized evaluation is key
Just as a patient who presents with chest pain requires a standardized evaluation, including a pertinent history, laboratory workup, and ECG, psychiatrists may also use standardized diagnostic instruments to aid in the diagnosis of catatonia. One study of hospitalized patients with schizophrenia found that using a standardized diagnostic procedure for catatonia resulted in a 7-fold increase in the diagnosis.7 The BFCRS is the most common standardized instrument for catatonia, likely due to its high inter-rater reliability.8 Other scales include the KANNER scale and Northoff Catatonia Scale, which emphasize different aspects of the disease or for certain clinical populations (eg, the KANNER scale adjusts for patients who are nonverbal at baseline). One study suggested that BFCRS has lower reliability for less-severe illness.9 These differences emphasize that psychiatry does not have a thorough understanding of the intricacies of catatonia. However, using validated screening tools can lead to more consistent diagnoses and continue important research on this often-misunderstood illness.
Dangers of untreated catatonia
Rapid treatment of catatonia is necessary to prevent mortality. A study of patients in Kentucky’s state psychiatric hospitals found that untreated catatonia with resultant death from pulmonary embolism was the leading cause of preventable death.10 A 17-year retrospective study of patients with schizophrenia admitted to 1 hospital found that those with catatonia were >4 times as likely to die during hospitalization than those without catatonia.11 The significant morbidity and mortality from untreated catatonia are typically attributed to the consequences of poorly controlled movements, immobility, autonomic instability, and poor/no oral intake. Reduced oral intake can result in malnutrition, dehydration, arrhythmias, and increased risk of infections. Furthermore, chronic catatonic episodes are more difficult to treat.12 In addition to the aggressive management of neuropsychiatric symptoms, it is vital to evaluate relevant medical etiologies that may be contributing to the syndrome (Table 213). Tracking vital signs and laboratory values, such as creatine kinase, electrolytes, and complete blood count, is required to ensure the medical condition does not become life-threatening.

Treatment options
Studies and expert opinion suggest that benzodiazepines (specifically lorazepam, because it is the most studied agent) are the first-line treatment for catatonia. A lorazepam challenge test—providing 1 or 2 mg of IV lorazepam—is considered diagnostic and therapeutic given the high rate of response within 10 minutes.14 Patients with limited response to lorazepam or who are medically compromised should undergo ECT. Electroconvulsive therapy is considered the gold-standard treatment for catatonia; estimated response rates range from 59% to 100%, even in patients who fail to respond to pharmacotherapy.15 Although highly effective, ECT is often hindered by the time required to initiate treatment, stigma, lack of access, and other logistical challenges.
Table 314-18 highlights the advantages and disadvantages of treatment options for catatonia. Some researchers have suggested a zolpidem challenge test could augment lorazepam because some patients respond only to zolpidem.14 The efficacy of these medications along with some evidence of anti-N-methyl-

Ms. N was ultimately diagnosed with bipolar disorder, current episode mixed, with psychotic and catatonic features. Ms. N had symptoms of mania including grandiosity, periods of lack of sleep, delusions as well as depressive symptoms of tearfulness and low mood. The treatment team had considered that Ms. N had delirious mania because she had fluctuating sensorium, which included varying degrees of orientation and ability to answer questioning. However, the literature supporting the differentiation between delirious mania and excited catatonia is unclear, and both conditions may respond to ECT.18 A diagnosis of catatonia allowed the team to use rating scales to track Ms. N’s progress by monitoring for specific signs, such as grasp reflex and waxy flexibility.
Continue to: OUTCOME
OUTCOME Return to baseline
Before discharge, Ms. N’s BFCRS score decreases from the initial score of 17 to 0, and her KANNER scale score decreases from the initial score of 26 to 4, which correlates with vast improvement in clinical presentation. Once Ms. N completes the acute ECT treatment, she returns to her baseline level of functioning, and is discharged to live with her boyfriend. She is advised to continue weekly ECT for the first several months to ensure clinical stability. This regimen is later transitioned to biweekly and then monthly. Electroconvulsive therapy protocols from previous research were utilized in Ms. N’s case, but ultimately the lowest number of ECT treatments needed to maintain stability is determined clinically over many years.19 Ms. N is discharged on aripiprazole, 15 mg/d; bupropion ER, 300 mg/d (added after depressive symptoms emerge while catatonia symptoms improve midway through her lengthy hospitalization); and memantine, 10 mg/d. Ideally, clozapine would have been continued; however, due to her history of nonadherence and frequent restarting of the medication at a low dose, clozapine was discontinued and aripiprazole initiated.
More than 1 year later, Ms. N remains stable and continues to receive monthly ECT maintenance treatments.
Bottom Line
Catatonia should always be considered in a patient who presents with acute neuropsychiatric symptoms. Rapid diagnosis with standardized screening instruments and aggressive treatment are vital to prevent morbidity and mortality.
Related Resource
- Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing textbook of psychosomatic medicine and consultation-liaison psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Baclofen • Ozobax
Bupropion ER • Wellbutrin XL
Clonazepam • Klonopin
Clozapine • Clozaril
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Metoclopramide • Reglan
Memantine • Namenda
Topiramate • Topamax
Zolpidem • Ambien
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
1. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums. 2000;5(7):26-33.
2. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6(4):391‐398.
3. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013. 119-121.
4. Solmi M, Pigato GG, Roiter B, et al. Prevalence of catatonia and its moderators in clinical samples: results from a meta-analysis and meta-regression analysis. Schizophrenia Bulletin. 2017;44(5):1133-1150.
5. Llesuy JR, Medina M, Jacobson KC, et al. Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci. 2018;30(2):145-151.
6. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients. Crit Care Med. 2017;45(11):1837-1844.
7. Heijden FVD, Tuinier S, Arts N, et al. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38(1):3-8.
8. Sarkar S, Sakey S, Mathan K, et al. Assessing catatonia using four different instruments: inter-rater reliability and prevalence in inpatient clinical population. Asian J Psychiatr. 2016;23:27-31.
9. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
10. Puentes R, Brenzel A, Leon JD. Pulmonary embolism during stuporous episodes of catatonia was found to be the most frequent cause of preventable death according to a state mortality review: 6 deaths in 15 years. Clin Schizophr Relat Psychoses. 2017; doi:10.3371/csrp.rpab.071317
11. Funayama M, Takata T, Koreki A, et al. Catatonic stupor in schizophrenic disorders and subsequent medical complications and mortality. Psychosomatic Medicine. 2018:80(4):370-376.
12. Perugi G, Medda P, Toni C, et al. The role of electroconvulsive therapy (ECT) in bipolar disorder: effectiveness in 522 patients with bipolar depression, mixed-state, mania and catatonic features. Curr Neuropharmacol. 2017;15(3):359-371.
13. Freudenreich O, Francis A, Fricchione GL. Chapter 9. Psychosis, mania, and catatonia. In: Levenson, James L, ed. The American Psychiatric Association Publishing Textbook of Psychosomatic medicine and Consultation-Liaison Psychiatry. 3rd ed. American Psychiatric Association Publishing; 2019.
14. Sienaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
15. Pelzer A, Heijden FVD, Boer ED. Systematic review of catatonia treatment. Neuropsychiatr Dis Treat. 2018;14:317-326.
16. Carroll BT, Goforth HW, Thomas C, et al. Review of adjunctive glutamate antagonist therapy in the treatment of catatonic syndromes. J Neuropsychiatry and Clin Neurosci. 2007;19(4):406-412.
17. Fink M. Rediscovering catatonia: the biography of a treatable syndrome. Acta Psychiatr Scand Suppl. 2013;(441):1-47.
18. Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge University Press; 2006.
19. Petrides G, Tobias KG, Kellner CH, et al. Continuation and maintenance electroconvulsive therapy for mood disorders: review of the literature. Neuropsychobiology. 2011;64(3):129-140.
How a community-based program for SMI pivoted during the pandemic
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
‘Remarkable’ response to diabetes drug in resistant bipolar depression
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treating insulin resistance may improve treatment-resistant bipolar depression, early research suggests.
In a randomized, placebo-controlled trial, treatment with the diabetes drug metformin reversed insulin resistance in 50% of patients, and this reversal was associated with significant improvement of depressive symptoms. One patient randomly assigned to placebo also achieved a reversal of insulin resistance and improved depressive symptoms.
“The study needs replication, but this early clinical trial suggests that the mitigation of insulin resistance by metformin significantly improves depressive symptoms in a significant percentage of treatment resistant bipolar patients,” presenting author Jessica M. Gannon, MD, University of Pittsburgh Medical Center (UPMC), said in an interview.
“It looks like in treatment-resistant bipolar depression, treating insulin resistance is a way to get people well again, to get out of their depression,” principal investigator Cynthia Calkin, MD, Dalhousie University, Halifax, N.S., added.
The findings were presented at the virtual American Society of Clinical Psychopharmacology 2021 Annual Meeting.
Chronic inflammation
The study was a joint effort by UPMC and Dalhousie University and was sponsored by the Stanley Medical Research Institute.
Patients with bipolar disorder (BD) who are obese tend to have more serious illness, with a more chronic course, more rapid cycling, and more morbidity. These patients also fail to respond to lithium, Dr. Calkin said.
“Untreated hyperinsulinemia could be contributing to a state of chronic inflammation and be involved in disease progression. So the question for me was, if we treat this insulin resistance, would patients get better?” she said.
Dr. Calkin said investigators used metformin because it is already used by psychiatrists for weight management in patients on antipsychotics.
“I wanted to test the drug that would work to reverse insulin resistance and that psychiatrists would be comfortable prescribing,” she said.
The 26-week study randomly assigned 20 patients to receive metformin and 25 patients to placebo.
All participants were 18 years and older, had a diagnosis of BD I or II, and had nonremitting BD defined by moderate depressive symptoms as measured on the Montgomery-Asberg Depression Rating Scale (MADRS) score of 15 or greater, despite being on optimal, guideline-compatible treatment.
All patients were stable, were on optimal doses of mood-stabilizing medications for at least 4 weeks prior to study entry, and had insulin resistance as defined by a Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) ≥1.8.
Characteristics were similar between the two groups, including baseline MADRS scores, body mass index, fasting glucose and insulin serum levels.
Patients were titrated up to 2,000 mg of metformin, which was the full dose, over 2 weeks and then maintained on treatment for a further 24 weeks.
Highly resistant population
The study’s primary outcome measure was change in MADRS score, with a response defined as a 30% reduction in MADRS from baseline.
By week 14, 10 metformin-treated patients (50%) and one patient in the placebo group (4%) no longer met insulin resistance criteria.
“It was a bit of a surprise to me that 50% of patients converted to being insulin sensitive again. When you use metformin to treat diabetes, people respond to it at more than a 50% rate, so I was expecting more people to respond,” Dr. Calkin said.
Nevertheless, the 11 patients who did respond and reversed insulin resistance achieved greater reduction in MADRS scores compared with nonconverters.
“Those who reversed their insulin resistance showed a remarkable resolution in their depressive symptoms. The reduction in MADRS scores began at week six, and were maintained through to the end of the study, and the Cohen’s d effect size for MADRS depression scores for converters was 0.52 at week 14 and 0.55 at week 26,” Dr. Calkin said.
“They were moderately to severely depressed going in, and at the end of the study, they had mild residual depressive symptoms, or they were completely well. These were very treatment-resistant patients.”
“All had failed, on average, eight or nine trials in their lifetime. When they came to us, nothing else would work. That’s one of the remarkable things about our results, just how well they responded when they had not responded to any other psychotropic medications. This approach may be very helpful for some patients,” Dr. Calkin said.
A holistic approach
Commenting on the study, Michael E. Thase, MD, professor of psychiatry, University of Pennsylvania, Philadelphia, said the findings need to be replicated but provide further support for the broader strategy of taking a holistic approach to the care of patients with difficult-to-treat mood disorders.
“Approximately one-half of people with treatment-resistant bipolar depression showed evidence of glucose resistance, and that adjunctive treatment with metformin, a medication that enhances insulin sensitivity, was moderately effective in normalizing glucose metabolism, with about a 50% response rate. Among those who experienced improved glucose regulation, there was a significant reduction in depressive symptoms,” he noted.
The study was funded by the Stanley Medical Research Institute (SMRI). Dr. Calkin and Dr. Thase have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA okays new drug option for schizophrenia, bipolar I disorder
The U.S. Food and Drug Administration has approved a once-daily oral medication, which is a combination of olanzapine and samidorphan (Lybalvi, Alkermes), for the treatment of schizophrenia and bipolar I disorder.
The drug is approved for the treatment of adults with schizophrenia and for adults with bipolar I disorder as a maintenance monotherapy or to treat acute manic or mixed episodes, as either monotherapy or an adjunct to lithium or valproate.
An atypical antipsychotic, the drug is a combination of olanzapine, an established antipsychotic medication, and samidorphan, a new chemical entity.
“Schizophrenia and bipolar I disorder are complex, chronic diseases, and there remains a persistent need for new medications with proven efficacy and safety. Olanzapine, a highly efficacious atypical antipsychotic, is associated with significant side effects, including weight gain that may impact patients’ treatment experiences and limit its use. With the efficacy of olanzapine and evidence of less weight gain in patients with schizophrenia, Lybalvi brings a welcome new addition to our medication arsenal,” René S. Kahn, MD, PhD, Esther and Joseph Klingenstein professor & chair, department of psychiatry and Behavioral Health System at the Icahn School of Medicine at Mount Sinai, New York, said in a company press release.
In a clinical development program, the drug demonstrated antipsychotic efficacy, safety, and tolerability, including significantly less weight gain than olanzapine in patients with schizophrenia in the ENLIGHTEN-2 study.
The FDA approved Lybalvi under the 505(b)(2) regulatory pathway based on data from 27 clinical studies, including 18 studies evaluating Lybalvi and nine studies evaluating samidorphan alone and the FDA’s findings of the safety and effectiveness of olanzapine in the treatment of bipolar I disorder and schizophrenia. Data suggest that olanzapine-associated weight gain is disease independent, the company reports.
“People living with schizophrenia or bipolar I disorder must evaluate both efficacy and tolerability when making treatment decisions,” Paul Gionfriddo, president and CEO of Mental Health America, said in the same company press release. “We are grateful that companies like Alkermes are driven to continue developing new treatment options in psychiatry that seek to address unmet needs of our community, and we applaud the FDA for considering the experiences of individuals living with these conditions.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a once-daily oral medication, which is a combination of olanzapine and samidorphan (Lybalvi, Alkermes), for the treatment of schizophrenia and bipolar I disorder.
The drug is approved for the treatment of adults with schizophrenia and for adults with bipolar I disorder as a maintenance monotherapy or to treat acute manic or mixed episodes, as either monotherapy or an adjunct to lithium or valproate.
An atypical antipsychotic, the drug is a combination of olanzapine, an established antipsychotic medication, and samidorphan, a new chemical entity.
“Schizophrenia and bipolar I disorder are complex, chronic diseases, and there remains a persistent need for new medications with proven efficacy and safety. Olanzapine, a highly efficacious atypical antipsychotic, is associated with significant side effects, including weight gain that may impact patients’ treatment experiences and limit its use. With the efficacy of olanzapine and evidence of less weight gain in patients with schizophrenia, Lybalvi brings a welcome new addition to our medication arsenal,” René S. Kahn, MD, PhD, Esther and Joseph Klingenstein professor & chair, department of psychiatry and Behavioral Health System at the Icahn School of Medicine at Mount Sinai, New York, said in a company press release.
In a clinical development program, the drug demonstrated antipsychotic efficacy, safety, and tolerability, including significantly less weight gain than olanzapine in patients with schizophrenia in the ENLIGHTEN-2 study.
The FDA approved Lybalvi under the 505(b)(2) regulatory pathway based on data from 27 clinical studies, including 18 studies evaluating Lybalvi and nine studies evaluating samidorphan alone and the FDA’s findings of the safety and effectiveness of olanzapine in the treatment of bipolar I disorder and schizophrenia. Data suggest that olanzapine-associated weight gain is disease independent, the company reports.
“People living with schizophrenia or bipolar I disorder must evaluate both efficacy and tolerability when making treatment decisions,” Paul Gionfriddo, president and CEO of Mental Health America, said in the same company press release. “We are grateful that companies like Alkermes are driven to continue developing new treatment options in psychiatry that seek to address unmet needs of our community, and we applaud the FDA for considering the experiences of individuals living with these conditions.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved a once-daily oral medication, which is a combination of olanzapine and samidorphan (Lybalvi, Alkermes), for the treatment of schizophrenia and bipolar I disorder.
The drug is approved for the treatment of adults with schizophrenia and for adults with bipolar I disorder as a maintenance monotherapy or to treat acute manic or mixed episodes, as either monotherapy or an adjunct to lithium or valproate.
An atypical antipsychotic, the drug is a combination of olanzapine, an established antipsychotic medication, and samidorphan, a new chemical entity.
“Schizophrenia and bipolar I disorder are complex, chronic diseases, and there remains a persistent need for new medications with proven efficacy and safety. Olanzapine, a highly efficacious atypical antipsychotic, is associated with significant side effects, including weight gain that may impact patients’ treatment experiences and limit its use. With the efficacy of olanzapine and evidence of less weight gain in patients with schizophrenia, Lybalvi brings a welcome new addition to our medication arsenal,” René S. Kahn, MD, PhD, Esther and Joseph Klingenstein professor & chair, department of psychiatry and Behavioral Health System at the Icahn School of Medicine at Mount Sinai, New York, said in a company press release.
In a clinical development program, the drug demonstrated antipsychotic efficacy, safety, and tolerability, including significantly less weight gain than olanzapine in patients with schizophrenia in the ENLIGHTEN-2 study.
The FDA approved Lybalvi under the 505(b)(2) regulatory pathway based on data from 27 clinical studies, including 18 studies evaluating Lybalvi and nine studies evaluating samidorphan alone and the FDA’s findings of the safety and effectiveness of olanzapine in the treatment of bipolar I disorder and schizophrenia. Data suggest that olanzapine-associated weight gain is disease independent, the company reports.
“People living with schizophrenia or bipolar I disorder must evaluate both efficacy and tolerability when making treatment decisions,” Paul Gionfriddo, president and CEO of Mental Health America, said in the same company press release. “We are grateful that companies like Alkermes are driven to continue developing new treatment options in psychiatry that seek to address unmet needs of our community, and we applaud the FDA for considering the experiences of individuals living with these conditions.”
A version of this article first appeared on Medscape.com.