User login
Does hormone therapy increase breast cancer risk in BRCA1 mutation carriers?
EXPERT COMMENTARY
Prophylactic bilateral oophorectomy (BO) reduces the risk of future ovarian cancer in women who have BRCA1 gene mutations. Women in this high-risk population may be reluctant, however, to use menopausal hormone therapy (HT) to mitigate the symptoms of surgical menopause because of concerns that it might elevate their risk of breast cancer.
To determine the relationship between HT use and BRCA1-associated breast cancer, Kotsopoulos and colleagues conducted a multicenter international cohort study. They prospectively followed women with BRCA1 mutations who had undergone BO and had intact breasts and no history of breast cancer.
Details of the study
The study included women who had a BRCA1 mutation and considered HT use following BO. Women were excluded from the analysis if they had a prior diagnosis of breast cancer or had BO prior to study enrollment. Study participants completed a questionnaire at baseline and a follow-up questionnaire every 2 years thereafter. The primary end point was invasive breast cancer.
Among 872 participating BRCA1 carriers, 43% (n = 377) used HT following BO. Mean duration of HT use following BO was 3.9 years, with 69% of users taking estrogen therapy alone (ET) and 19% using estrogen plus progestogen therapy (EPT). Those who used HT were younger at the time of BO compared with women who never used HT (mean age, 43.0 vs 48.4 years).
During follow-up (mean, 7.6 years; range, 0.4–22.1), invasive breast cancer was diagnosed in similar proportions of HT users and nonusers—10.3% and 10.7%, respectively (P = .86). The hazard ratio was 0.97 (95% confidence interval, 0.62–1.52; P = .89) for ever use of any type of hormone therapy versus no use.
When the type of HT used was examined, the 10-year actuarial risk of breast cancer was significantly lower with ET than with EPT (12% vs 22%, respectively; P = .04); this difference was more marked for women who underwent BO prior to age 45 (9% vs 24%; P = .009).
Study strengths and weaknesses
This investigation had several strengths, including the large number of BRCA1 mutation carriers studied, the relatively long follow-up, and the detailed exposure data obtained.
The use of self-administered questionnaires for collecting information on lifetime HT use and breast cancer diagnoses may be a limitation. In addition, the HT route, regimen, and dose were not considered in the analysis, and the effect of intrauterine devices as progestational endometrial protection was not evaluated. Finally, the relationship between HT and breast cancer risk in women with intact ovaries was not evaluated.
Because women with BRCA1 mutations have an elevated risk of ovarian cancer, risk-reducing gynecologic surgery is recommended for these women who have completed childbearing. In young women, BO without HT is associated with severe vasomotor symptoms, osteoporosis, cardiovascular disease, and cognitive decline. The clear reduction in breast cancer risk associated with ET (vs EPT) following BO suggests that in BRCA1 carriers who have completed childbearing, hysterectomy (which precludes the need for progestogen therapy) should be considered as part of risk-reducing gynecologic surgery. Further, the findings of this prospective study in high-risk women parallels the findings of the large randomized Women's Health Initiative trial (performed in the general population of menopausal women), which found that ET (conjugated equine estrogen) reduces the risk.1
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
EXPERT COMMENTARY
Prophylactic bilateral oophorectomy (BO) reduces the risk of future ovarian cancer in women who have BRCA1 gene mutations. Women in this high-risk population may be reluctant, however, to use menopausal hormone therapy (HT) to mitigate the symptoms of surgical menopause because of concerns that it might elevate their risk of breast cancer.
To determine the relationship between HT use and BRCA1-associated breast cancer, Kotsopoulos and colleagues conducted a multicenter international cohort study. They prospectively followed women with BRCA1 mutations who had undergone BO and had intact breasts and no history of breast cancer.
Details of the study
The study included women who had a BRCA1 mutation and considered HT use following BO. Women were excluded from the analysis if they had a prior diagnosis of breast cancer or had BO prior to study enrollment. Study participants completed a questionnaire at baseline and a follow-up questionnaire every 2 years thereafter. The primary end point was invasive breast cancer.
Among 872 participating BRCA1 carriers, 43% (n = 377) used HT following BO. Mean duration of HT use following BO was 3.9 years, with 69% of users taking estrogen therapy alone (ET) and 19% using estrogen plus progestogen therapy (EPT). Those who used HT were younger at the time of BO compared with women who never used HT (mean age, 43.0 vs 48.4 years).
During follow-up (mean, 7.6 years; range, 0.4–22.1), invasive breast cancer was diagnosed in similar proportions of HT users and nonusers—10.3% and 10.7%, respectively (P = .86). The hazard ratio was 0.97 (95% confidence interval, 0.62–1.52; P = .89) for ever use of any type of hormone therapy versus no use.
When the type of HT used was examined, the 10-year actuarial risk of breast cancer was significantly lower with ET than with EPT (12% vs 22%, respectively; P = .04); this difference was more marked for women who underwent BO prior to age 45 (9% vs 24%; P = .009).
Study strengths and weaknesses
This investigation had several strengths, including the large number of BRCA1 mutation carriers studied, the relatively long follow-up, and the detailed exposure data obtained.
The use of self-administered questionnaires for collecting information on lifetime HT use and breast cancer diagnoses may be a limitation. In addition, the HT route, regimen, and dose were not considered in the analysis, and the effect of intrauterine devices as progestational endometrial protection was not evaluated. Finally, the relationship between HT and breast cancer risk in women with intact ovaries was not evaluated.
Because women with BRCA1 mutations have an elevated risk of ovarian cancer, risk-reducing gynecologic surgery is recommended for these women who have completed childbearing. In young women, BO without HT is associated with severe vasomotor symptoms, osteoporosis, cardiovascular disease, and cognitive decline. The clear reduction in breast cancer risk associated with ET (vs EPT) following BO suggests that in BRCA1 carriers who have completed childbearing, hysterectomy (which precludes the need for progestogen therapy) should be considered as part of risk-reducing gynecologic surgery. Further, the findings of this prospective study in high-risk women parallels the findings of the large randomized Women's Health Initiative trial (performed in the general population of menopausal women), which found that ET (conjugated equine estrogen) reduces the risk.1
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Prophylactic bilateral oophorectomy (BO) reduces the risk of future ovarian cancer in women who have BRCA1 gene mutations. Women in this high-risk population may be reluctant, however, to use menopausal hormone therapy (HT) to mitigate the symptoms of surgical menopause because of concerns that it might elevate their risk of breast cancer.
To determine the relationship between HT use and BRCA1-associated breast cancer, Kotsopoulos and colleagues conducted a multicenter international cohort study. They prospectively followed women with BRCA1 mutations who had undergone BO and had intact breasts and no history of breast cancer.
Details of the study
The study included women who had a BRCA1 mutation and considered HT use following BO. Women were excluded from the analysis if they had a prior diagnosis of breast cancer or had BO prior to study enrollment. Study participants completed a questionnaire at baseline and a follow-up questionnaire every 2 years thereafter. The primary end point was invasive breast cancer.
Among 872 participating BRCA1 carriers, 43% (n = 377) used HT following BO. Mean duration of HT use following BO was 3.9 years, with 69% of users taking estrogen therapy alone (ET) and 19% using estrogen plus progestogen therapy (EPT). Those who used HT were younger at the time of BO compared with women who never used HT (mean age, 43.0 vs 48.4 years).
During follow-up (mean, 7.6 years; range, 0.4–22.1), invasive breast cancer was diagnosed in similar proportions of HT users and nonusers—10.3% and 10.7%, respectively (P = .86). The hazard ratio was 0.97 (95% confidence interval, 0.62–1.52; P = .89) for ever use of any type of hormone therapy versus no use.
When the type of HT used was examined, the 10-year actuarial risk of breast cancer was significantly lower with ET than with EPT (12% vs 22%, respectively; P = .04); this difference was more marked for women who underwent BO prior to age 45 (9% vs 24%; P = .009).
Study strengths and weaknesses
This investigation had several strengths, including the large number of BRCA1 mutation carriers studied, the relatively long follow-up, and the detailed exposure data obtained.
The use of self-administered questionnaires for collecting information on lifetime HT use and breast cancer diagnoses may be a limitation. In addition, the HT route, regimen, and dose were not considered in the analysis, and the effect of intrauterine devices as progestational endometrial protection was not evaluated. Finally, the relationship between HT and breast cancer risk in women with intact ovaries was not evaluated.
Because women with BRCA1 mutations have an elevated risk of ovarian cancer, risk-reducing gynecologic surgery is recommended for these women who have completed childbearing. In young women, BO without HT is associated with severe vasomotor symptoms, osteoporosis, cardiovascular disease, and cognitive decline. The clear reduction in breast cancer risk associated with ET (vs EPT) following BO suggests that in BRCA1 carriers who have completed childbearing, hysterectomy (which precludes the need for progestogen therapy) should be considered as part of risk-reducing gynecologic surgery. Further, the findings of this prospective study in high-risk women parallels the findings of the large randomized Women's Health Initiative trial (performed in the general population of menopausal women), which found that ET (conjugated equine estrogen) reduces the risk.1
-- Andrew M. Kaunitz, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
Higher BMI tied to lower breast cancer risk in women before menopause
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
Although obesity increases the risk of breast cancer in postmenopausal women, a large multicenter analysis has confirmed the opposite effect in premenopausal women.
The association had “a greater magnitude than previously shown and across the entire distribution of body mass index,” wrote Minouk J. Schoemaker, PhD, of the Institute of Cancer Research in London, with his associates, on behalf of the Premenopausal Breast Cancer Collaborative Group. The protective effect of adiposity was strongest during young adulthood (ages 18-24 years), when it spanned breast cancer subtypes. “Understanding the biological mechanisms underlying these associations could have important preventive potential,” they wrote in JAMA Oncology.
Prior studies have linked greater body fat with reduced risk of breast cancer in younger women, but the effect has not been well characterized. For this analysis, the investigators pooled data from 19 cohort studies that included a total of 758,592 premenopausal women; median age was 40.6 years (interquartile range, 35.2-45.5 years).
For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years. There was no BMI threshold for risk reduction: the inverse correlation existed even when women were not overweight. Risk also did vary significantly among subgroups stratified by other risk factors for breast cancer. Adiposity was more protective against estrogen receptor-positive and progesterone-receptor positive breast cancers and less protective against hormone receptor–negative breast cancers, which “implies a hormonal mechanism,” the investigators said. “Body mass index at ages 25-54 years was not consistently associated with triple-negative or hormone receptor–negative breast cancer overall.”
Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
SOURCE: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
FROM JAMA ONCOLOGY
Key clinical point: In premenopausal women, adiposity inversely correlated with risk of breast cancer, and showed a stronger protective effect than previously documented.
Major finding: For each 5-unit increase in BMI, the estimated reduction in risk of breast cancer was 23% among women aged 18-24 years (hazard ratio, 0.77; 95% confidence interval, 0.73-0.80), 15% in women aged 25-34 years, 13% in women aged 35-44 years, and 12% in women aged 45-54 years.
Study details: Multicenter analysis of 19 cohort studies.
Disclosures: Funders included Breast Cancer Now, the Institute of Cancer Research, the National Institutes of Health, and many others. The researchers reported having no relevant conflicts of interest.
Source: Schoemaker MJ et al. JAMA Oncol. 2018; Jun 21. doi: 10.1001/jamaoncol.2018.1771.
ASCO 2018: Less is more as ‘tailoring’ takes on new meaning
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
A record-setting 40,000-plus oncology professionals attended this year’s annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. The outstanding education and scientific program, with the theme of Delivering Discoveries: Expanding the Reach of Precision Medicine, was planned and led by ASCO President Dr Bruce Johnson, professor and director of Thoracic Oncology at the Dana Farber Cancer Institute in Boston, and chaired by Sarah Cannon’s Dr David Spigel and Harvard’s Dr Ann Partridge. A recurring finding throughout the meeting was that “less is more” in several key areas of cancer therapy. From small molecules targeting driver mutations across various tumors to the application of immunotherapy in subsets of common cancers, it is clear that more patients are experiencing dramatic results from novel approaches.
A featured plenary session trial was TAILORx, a study of 10,273 women with hormone-receptor–positive, surgically resected breast cancer that had not spread to the lymph nodes, was less than 5 cm, and was not positive for the HER2 gene amplification. This clinical trial was sponsored by the NCI and initiated in 2006. It used the OncotypeDX genetic test to stratify patients into groups of low, intermediate, or high risk for recurrence. The low-risk patients received only hormonal therapy, and the high-risk patients were treated with hormonal therapy plus chemotherapy.
Dr Joseph Sparano, professor of Medicine and Women’s Health at the Albert Einstein College of Medicine in New York, presented the results from the group of 6,700 intermediate risk women who were randomized to receive hormonal therapy alone or in combination with chemotherapy. After 9 years of follow-up, 83.3% of the volunteers, as Dr Sparano appropriately referred to them, who were treated with hormonal therapy were still cancer free, compared with 84.3% of those who also received chemotherapy, demonstrating no statistical benefit for the addition of chemotherapy. Of note, breast cancer experts discussing the trial, including Dr Lisa Carey, professor of Breast Cancer Research at the UNC Lineberger Cancer Institute in Chapel Hill, urged that younger women, under the age of 50, with recurrence scores (RS) toward the higher end of the intermediate risk group (RS, 16-25) should still discuss and consider chemotherapy with their physicians. In summary, all patients fitting the study criteria with low (
These landmark and practice changing results mean that each year about 60,000 women in the United States will be spared the side effects of toxic drugs. These 10,273 study volunteers are true heroes to the women who will be diagnosed with breast cancer in coming years.
In the field of lung cancer, many new trial results using immunotherapy were presented, with the most talked about being single-agent pembrolizumab, a PD1 inhibitor, improving survival over traditional chemotherapy in patients with PD-L1 positive tumors, which comprise the majority of squamous cell and adenocarcinomas of the lung. Also in the plenary, Dr Gilberto Lopes of the Sylvester Cancer Center at the University of Miami, presented these results from the KEYNOTE-042 study. In patients with PD-L1 tumor proportion score (TPS) of >1%, the benefit in overall survival (OS) of pembrolizumab compared with chemotherapy was 16.7 versus 12.1 months, respectively (HR, 0.81). In those patients with a TPS of >20%, the OS benefit was 17.7 versus 1.0 months (HR, 0.77), and in the group with a TPS of >50%, the benefit was 20.0 versus 12.2 months (HR, 0.69). Overall, the quality of life and the occurrence of side effects were substantially better for those patients receiving immunotherapy alone. Other findings presented at the meeting demonstrated the benefit of adding immunotherapy to chemotherapy and of treating with combination immunotherapy (PD-1 and CTLA-4 inhibitors). Many options now exist, much work remains to be done, and accrual to clinical trials is more important than ever.
Another plenary session trial evaluated the benefit of performing a nephrectomy in patients with advanced or metastatic renal cell carcinoma (RCC), a long-held and practiced standard of care. Dr Arnaud Mejean of Paris Descartes University presented findings from the CARMENA trial, which randomized 450 patients with metastatic clear cell RCC to receive cytoreductive nephrectomy followed by sunitinib, or sunitinib alone. The OS results of 18.4 versus 13.9 months, respectively (HR, 0.89) favored sunitinib alone in this noninferiority analysis. Other endpoints lined up in favor of not removing the cancerous kidney, and the presenter and discussants were united in their opinion of the results and the resulting change in doing less surgery in these patients.
In a step away from less therapy, the European Pediatric Soft Tissue Sarcoma Study showed that adding 6 months of low-dose maintenance chemotherapy after standard intensive therapy improves survival in children with high-risk rhabdomyosarcoma. The addition of a vinorelbine and cyclophosphamide low-dose regimen improved 5-year disease-free survival from 69.8% to 77.6% (HR, 0.68) and OS from 73.7% to 86.5% (HR, 0.52) as presented by Dr Gianni Bisogno, University of Padovani, Italy. The maintenance regimen showed no increase in toxicity and actually fewer infections were noted.
In the area of molecular profiling, multiple studies at the meeting demonstrated the importance of assessing cancers for mutations as outstanding results were seen with therapies for NTRK, RET, ROS, and MSI-high driven tumors. In a debate on the role of molecular profiling, I had the opportunity to declare and support our position at Sarah Cannon that all patients with relapsed or metastatic cancers should have this testing performed. It will be through better understanding of the biology of these cancers that we will advance the field for all patients while sometimes finding a target or mutation that will dramatically change the life of a patient.
In keeping with the meeting’s theme, Delivering Discoveries: Expanding the Reach of Precision Medicine, the presentations and the discussions clearly demonstrated that through the use of precision medicine techniques such as prognostic gene assays and molecular profiling, patients can receive the best therapy, even “tailored” therapy, which may often actually be less therapy. It is an exciting time in cancer research, and I have never been more optimistic about the future of cancer treatment for our patients.
The long-term effects of posttreatment exercise on pain in young women with breast cancer
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
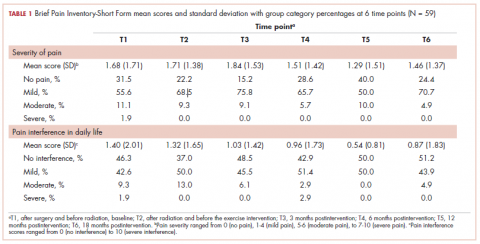
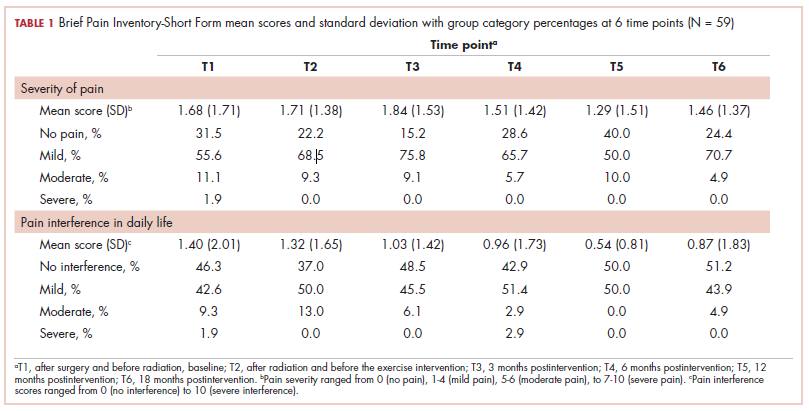
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
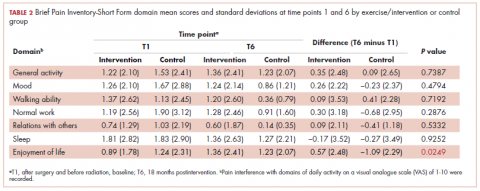
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
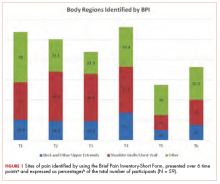
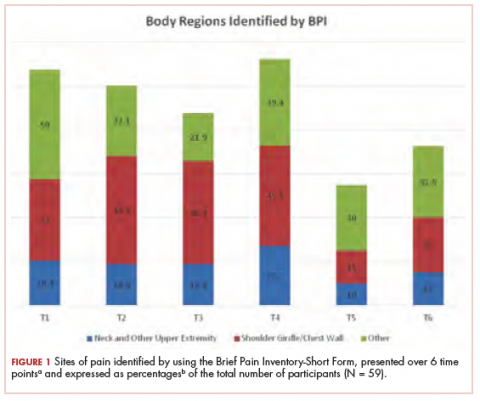
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
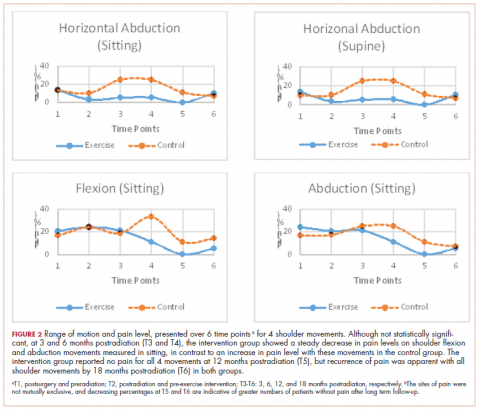
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
Rural cancer patients report faster care than urban counterparts
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
in a survey of 6,826 Medicare beneficiaries.
Taken as a whole, a similar quality of care was reported between the two groups, but the picture changed when racial/ethnic subgroups were considered. Non-Hispanic black and Hispanic patients in rural locations reported inferior care to their urban counterparts, investigators wrote in Cancer.
“Cancer patients living in rural areas are vulnerable and have unique health care needs,” wrote lead author Michelle A. Mollica, PhD, of the National Cancer Institute, and her colleagues. “To our knowledge, this is the first study to explore the patient’s perception of the timeliness of care in such a large, multiregion sample of cancer patients.”
In 2003, the National Academy of Medicine concluded that living in a rural environment was associated with poorer health. Existing research surrounding cancer has echoed this concern, showing that rural patients have higher rates of cancer and mortality, longer delays in diagnosis, and limited access to care.
The current, retrospective study involved 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. Consumer Assessment of Healthcare Providers and Systems surveys were conducted between 1998 and 2013, then linked with data from the Surveillance, Epidemiology, and End Results registry program.
Surveys were conducted within 12 months of diagnosis, during which time patients were asked about their access to care as defined by two composites: “Getting Needed Care” and “Getting Care Quickly.” Getting Needed Care included ease of making appointments and receiving treatments and Getting Care Quickly questions asked about appointment delays and time spent waiting at the doctor’s office. Answers were converted to a numerical score from 0 to 100, with 0 being the worst and 100 being the best.
For both composites, mean scores for urban and rural locations were greater than 85 out of 100.
In contrast to previous studies, urban patients reported longer delays in care, scoring Getting Care Quickly 2.27 points lower than rural patients (P = .02). Pacific Islanders and non-Hispanic Asian patients from rural places reported even faster care, ranking about 8 points higher than urban patients of the same race/ethnicity.
Locality did not have a significant impact on Getting Needed Care unless race/ethnicity was also considered (P = .04). Non-Hispanic white patients from rural locations scored Getting Needed Care about 2 points higher than urban white patients, while Hispanic and non-Hispanic black patients had an opposite trend, with this rural cohort ranking Getting Needed Care lower than urban patients of the same race/ethnicity.
“Geographic residence is but one important factor in cancer care delivery,” the authors noted. “There is a need for fine-grained research looking at specific barriers for urban residents, experiences of racial/ethnic minority survivors residing in rural areas, and rural-urban differences in the clinic settings in which medical care is delivered.”
The authors had no disclosures to report.
SOURCE: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
FROM CANCER
Key clinical point: Cancer patients living in rural areas reported more timely care than urban patients.
Major finding: In a Consumer Assessment of Healthcare Providers and Systems (CAHPS) survey, urban patients rated “Getting Care Quickly” 2.27 points lower than rural patients (P = .02).
Study details: A retrospective study of 6,140 urban and 686 rural Medicare beneficiaries who were aged at least 65 years when diagnosed with either breast, lung, colorectal, or prostate cancer. CAHPS patient experience surveys were conducted between 1998 and 2013, then linked with Surveillance, Epidemiology, and End Results data.
Disclosures: The authors had no disclosures to report.
Source: Mollica MA et al. Cancer. 2018 Jun 7. doi: 10.1002/cncr.31541.
Predicting Platinum Efficacy
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Platinum-based chemotherapy is effective in metastatic triple negative breast cancer (mTNBC), but predictive biomarkers would help identify the best candidates for the treatment. Two sets of parameters—neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)—have already demonstrated their prognostic prowess in many malignancies, but how well will they do in platinum-treated mTNBC patients? Researchers from Fondazione IRCCS Istituto Nazionale dei Tumori, in Milan, Italy conducted a retrospective, single-center study to evaluate the association between baseline NLR or PLR and progression-free survival (PFS) in 57 mTNBC patients treated with carboplatin-paclitaxel or carboplatin-gemcitabine between 2007 and 2017, compared with 148 patients with hormone receptor-positive HER2-negative metastatic breast cancer.
Response was assessed every 3 chemotherapy cycles. Among platinum-treated patients, high NLR and PLR were associated with significantly lower PFS. Median PFS was 304 days in patients with NLR < 2.5, and 158 days in those with NLR ≥ 2.5. Progression-free survival was longer in patients with baseline PLR < 200, compared with PLR ≥ 200. The researchers found no significant association between NLR or PLR and the PFS of control patients.
When the same parameters were evaluated before the administration of the third treatment cycle, NLR < 2.5 was still associated with reduced risk of disease progression, although PLR < 200 was not.
In patients with mTNBC, median overall survival was significantly longer in patients with NLR < 2.5 compared with NLR ≥ 2.5. Platelet-to-lymphocyte ratio values were not associated with overall survival. The ratios also appeared to have a generally prognostic role independently from tumor biology.
The hormone receptors for NLR and PLR in multivariable analysis for PFS were similar, and the parameters correlated with each other, the researchers say, suggesting that both NLR and PLR “well reflect the inflammatory/immune contexture in mTNBC, and may be redundant as predictive biomarkers.”
Source:
Vernieri C, Mennitto A, Prisciandaro M, et al. Sci Rep. 2018;8(1):8703.
Everolimus/exemestane improves PFS of ER+/HER2– breast cancer vs. everolimus alone
CHICAGO – For women with estrogen receptor–positive breast cancer resistant to endocrine therapy, the combination of everolimus and exemestane had better efficacy than did everolimus alone, but single-agent capecitabine appeared to offer benefit comparable to that of the combination therapy, results of the BOLERO-6 trial suggest.
Among 309 postmenopausal women with ER-positive, HER2-negative advanced breast cancer, the combination of everolimus (Afinitor) and exemestane (Aromasin and generics) was associated with a 26% improvement in progression-free survival (PFS) compared with everolimus alone, reported Guy Jerusalem, MD, PhD, of Liege University, Belgium.
There was also, however, a numerical but not statistically significant difference in PFS favoring capecitabine (Xeloda and generics) “which may be attributed to various baseline characteristics favoring capecitabine, and potential informative censoring,” he said at the annual meeting of the American Society of Clinical Oncology.
“We have noted in BOLERO-6 a better-than-expected outcome in median progression-free survival of capecitabine compared with the previously reported 4.1 to 7.9 months median progression-free survival,” he said.
BOLERO-6, results of which were published online June 3 in JAMA Oncology, was a postmarketing study by the sponsors to fulfill commitments to both the Food and Drug Administration and the European Medicines Agency to estimate the treatment benefit with combined everolimus and exemestane vs. monotherapy with everolimus or capecitabine in patients with ER-positive, HER2-negative breast cancer that progressed during nonsteroidal aromatase inhibitor therapy.
Patients from 83 centers in 18 countries were enrolled in the open label, phase 2 study and randomly assigned to receive oral everolimus 10 mg daily with oral exemestane 25 mg daily, everolimus at the same dose alone, or oral capecitabine 1,250 mg/m2 twice daily for 2 weeks on, 1 week off.
The trial was not powered for statistical comparisons between arms, but was instead designed with the primary objective of estimated investigator-assessed PFS for the combination vs. everolimus alone.
At baseline, more patients assigned to capecitabine vs. everolimus-containing regimens were younger than 65, white, had an Eastern Cooperative Oncology Group status of 0 (fully active), and had bone-only metastases. In addition, fewer patients in the capecitabine arm had three or more metastatic sites, Dr. Jerusalem noted,
For the primary analysis, the median PFS with everolimus/exemestane was 8.4 months, compared with 6.8 months for everolimus alone. The estimated hazard ratio (HR) for PFS with everolimus/exemestane vs. everolimus alone was 0.74 (90% confidence interval [CI], 0.57-0.97)
In contrast, median PFS with capecitabine was 9.6 months, with a nonsignificant hazard ratio of 1.26 for the combination (90% CI, 0.96-1.66).
A stratified multivariate Cox regression model controlling for baseline difference and known prognostic factor yielded an HR for PFS of 1.15 (90% CI, 0.86-1.52) for the combination.
Censoring of patients was more frequent in the capecitabine arm (33% vs. 23% in the combination arm), which included 20% of patients on capecitabine who were censored for starting on a new antineoplastic therapy vs. 9% of patients on everolimus/exemestane.
The median time to treatment failure was 5.8 months with the combination, vs. 4.2 months with everolimus alone (HR, 0.66, 90% CI, 0.52-0.4), and 6.2 months with capecitabine alone (HR, 1.03, 90% CI, 0.81-1.31).
Median overall survival was 23.1 months in the combination arm, 29.3 months in the everolimus arm, and 25.6 months in the capecitabine arm. There were no statistically significant differences in overall survival among the groups.
Grade 3 or greater adverse events were more frequent in the combination vs. everolimus arms, and comparable between the combination and capecitabine arms, Dr. Jerusalem said.
Serious adverse events of any grade were more frequent in the combination arm than in the other two arms, but there were no significant differences in discontinuations due to adverse events
“The results of the present study suggest that mTOR inhibitor and endocrine therapy combinations remain important for aromatase inhibitor–refractory disease. Safety and PFS with everolimus plus exemestane in this study were consistent with BOLERO-2 and are now supported by real-world evidence,” the investigators wrote.
“The take home from the BOLERO-6 trial is that the progression-free survival for the combination of everolimus and exemestane is superior to everolimus alone, and is in line with data from the BOLERO-2 trial, and also the PrE0102 study, demonstrating the consistent activity of mTOR inhibition in combination with endocrine therapy in the aromatase inhibitor resistance setting, and this supports our use of the combination in the endocrine resistant patients,” said Cynthia X. Ma, MD, PhD, of Washington University, St. Louis, the invited discussant.
Novartis funded the study. Dr Jerusalem received research funding from Novartis and Roche; honoraria from Novartis, Roche, Pfizer, Lilly, Celgene, Amgen, BMS, and Puma Technology; and nonfinancial support from Novartis, Roche, Pfizer, Lilly, Amgen, and BMS. Dr. Ma reported consulting/advising, travel expenses, and institutional research funding from Novartis and others.
SOURCE: Jerusalem G et al. ASCO 2018 Abstract 1005
CHICAGO – For women with estrogen receptor–positive breast cancer resistant to endocrine therapy, the combination of everolimus and exemestane had better efficacy than did everolimus alone, but single-agent capecitabine appeared to offer benefit comparable to that of the combination therapy, results of the BOLERO-6 trial suggest.
Among 309 postmenopausal women with ER-positive, HER2-negative advanced breast cancer, the combination of everolimus (Afinitor) and exemestane (Aromasin and generics) was associated with a 26% improvement in progression-free survival (PFS) compared with everolimus alone, reported Guy Jerusalem, MD, PhD, of Liege University, Belgium.
There was also, however, a numerical but not statistically significant difference in PFS favoring capecitabine (Xeloda and generics) “which may be attributed to various baseline characteristics favoring capecitabine, and potential informative censoring,” he said at the annual meeting of the American Society of Clinical Oncology.
“We have noted in BOLERO-6 a better-than-expected outcome in median progression-free survival of capecitabine compared with the previously reported 4.1 to 7.9 months median progression-free survival,” he said.
BOLERO-6, results of which were published online June 3 in JAMA Oncology, was a postmarketing study by the sponsors to fulfill commitments to both the Food and Drug Administration and the European Medicines Agency to estimate the treatment benefit with combined everolimus and exemestane vs. monotherapy with everolimus or capecitabine in patients with ER-positive, HER2-negative breast cancer that progressed during nonsteroidal aromatase inhibitor therapy.
Patients from 83 centers in 18 countries were enrolled in the open label, phase 2 study and randomly assigned to receive oral everolimus 10 mg daily with oral exemestane 25 mg daily, everolimus at the same dose alone, or oral capecitabine 1,250 mg/m2 twice daily for 2 weeks on, 1 week off.
The trial was not powered for statistical comparisons between arms, but was instead designed with the primary objective of estimated investigator-assessed PFS for the combination vs. everolimus alone.
At baseline, more patients assigned to capecitabine vs. everolimus-containing regimens were younger than 65, white, had an Eastern Cooperative Oncology Group status of 0 (fully active), and had bone-only metastases. In addition, fewer patients in the capecitabine arm had three or more metastatic sites, Dr. Jerusalem noted,
For the primary analysis, the median PFS with everolimus/exemestane was 8.4 months, compared with 6.8 months for everolimus alone. The estimated hazard ratio (HR) for PFS with everolimus/exemestane vs. everolimus alone was 0.74 (90% confidence interval [CI], 0.57-0.97)
In contrast, median PFS with capecitabine was 9.6 months, with a nonsignificant hazard ratio of 1.26 for the combination (90% CI, 0.96-1.66).
A stratified multivariate Cox regression model controlling for baseline difference and known prognostic factor yielded an HR for PFS of 1.15 (90% CI, 0.86-1.52) for the combination.
Censoring of patients was more frequent in the capecitabine arm (33% vs. 23% in the combination arm), which included 20% of patients on capecitabine who were censored for starting on a new antineoplastic therapy vs. 9% of patients on everolimus/exemestane.
The median time to treatment failure was 5.8 months with the combination, vs. 4.2 months with everolimus alone (HR, 0.66, 90% CI, 0.52-0.4), and 6.2 months with capecitabine alone (HR, 1.03, 90% CI, 0.81-1.31).
Median overall survival was 23.1 months in the combination arm, 29.3 months in the everolimus arm, and 25.6 months in the capecitabine arm. There were no statistically significant differences in overall survival among the groups.
Grade 3 or greater adverse events were more frequent in the combination vs. everolimus arms, and comparable between the combination and capecitabine arms, Dr. Jerusalem said.
Serious adverse events of any grade were more frequent in the combination arm than in the other two arms, but there were no significant differences in discontinuations due to adverse events
“The results of the present study suggest that mTOR inhibitor and endocrine therapy combinations remain important for aromatase inhibitor–refractory disease. Safety and PFS with everolimus plus exemestane in this study were consistent with BOLERO-2 and are now supported by real-world evidence,” the investigators wrote.
“The take home from the BOLERO-6 trial is that the progression-free survival for the combination of everolimus and exemestane is superior to everolimus alone, and is in line with data from the BOLERO-2 trial, and also the PrE0102 study, demonstrating the consistent activity of mTOR inhibition in combination with endocrine therapy in the aromatase inhibitor resistance setting, and this supports our use of the combination in the endocrine resistant patients,” said Cynthia X. Ma, MD, PhD, of Washington University, St. Louis, the invited discussant.
Novartis funded the study. Dr Jerusalem received research funding from Novartis and Roche; honoraria from Novartis, Roche, Pfizer, Lilly, Celgene, Amgen, BMS, and Puma Technology; and nonfinancial support from Novartis, Roche, Pfizer, Lilly, Amgen, and BMS. Dr. Ma reported consulting/advising, travel expenses, and institutional research funding from Novartis and others.
SOURCE: Jerusalem G et al. ASCO 2018 Abstract 1005
CHICAGO – For women with estrogen receptor–positive breast cancer resistant to endocrine therapy, the combination of everolimus and exemestane had better efficacy than did everolimus alone, but single-agent capecitabine appeared to offer benefit comparable to that of the combination therapy, results of the BOLERO-6 trial suggest.
Among 309 postmenopausal women with ER-positive, HER2-negative advanced breast cancer, the combination of everolimus (Afinitor) and exemestane (Aromasin and generics) was associated with a 26% improvement in progression-free survival (PFS) compared with everolimus alone, reported Guy Jerusalem, MD, PhD, of Liege University, Belgium.
There was also, however, a numerical but not statistically significant difference in PFS favoring capecitabine (Xeloda and generics) “which may be attributed to various baseline characteristics favoring capecitabine, and potential informative censoring,” he said at the annual meeting of the American Society of Clinical Oncology.
“We have noted in BOLERO-6 a better-than-expected outcome in median progression-free survival of capecitabine compared with the previously reported 4.1 to 7.9 months median progression-free survival,” he said.
BOLERO-6, results of which were published online June 3 in JAMA Oncology, was a postmarketing study by the sponsors to fulfill commitments to both the Food and Drug Administration and the European Medicines Agency to estimate the treatment benefit with combined everolimus and exemestane vs. monotherapy with everolimus or capecitabine in patients with ER-positive, HER2-negative breast cancer that progressed during nonsteroidal aromatase inhibitor therapy.
Patients from 83 centers in 18 countries were enrolled in the open label, phase 2 study and randomly assigned to receive oral everolimus 10 mg daily with oral exemestane 25 mg daily, everolimus at the same dose alone, or oral capecitabine 1,250 mg/m2 twice daily for 2 weeks on, 1 week off.
The trial was not powered for statistical comparisons between arms, but was instead designed with the primary objective of estimated investigator-assessed PFS for the combination vs. everolimus alone.
At baseline, more patients assigned to capecitabine vs. everolimus-containing regimens were younger than 65, white, had an Eastern Cooperative Oncology Group status of 0 (fully active), and had bone-only metastases. In addition, fewer patients in the capecitabine arm had three or more metastatic sites, Dr. Jerusalem noted,
For the primary analysis, the median PFS with everolimus/exemestane was 8.4 months, compared with 6.8 months for everolimus alone. The estimated hazard ratio (HR) for PFS with everolimus/exemestane vs. everolimus alone was 0.74 (90% confidence interval [CI], 0.57-0.97)
In contrast, median PFS with capecitabine was 9.6 months, with a nonsignificant hazard ratio of 1.26 for the combination (90% CI, 0.96-1.66).
A stratified multivariate Cox regression model controlling for baseline difference and known prognostic factor yielded an HR for PFS of 1.15 (90% CI, 0.86-1.52) for the combination.
Censoring of patients was more frequent in the capecitabine arm (33% vs. 23% in the combination arm), which included 20% of patients on capecitabine who were censored for starting on a new antineoplastic therapy vs. 9% of patients on everolimus/exemestane.
The median time to treatment failure was 5.8 months with the combination, vs. 4.2 months with everolimus alone (HR, 0.66, 90% CI, 0.52-0.4), and 6.2 months with capecitabine alone (HR, 1.03, 90% CI, 0.81-1.31).
Median overall survival was 23.1 months in the combination arm, 29.3 months in the everolimus arm, and 25.6 months in the capecitabine arm. There were no statistically significant differences in overall survival among the groups.
Grade 3 or greater adverse events were more frequent in the combination vs. everolimus arms, and comparable between the combination and capecitabine arms, Dr. Jerusalem said.
Serious adverse events of any grade were more frequent in the combination arm than in the other two arms, but there were no significant differences in discontinuations due to adverse events
“The results of the present study suggest that mTOR inhibitor and endocrine therapy combinations remain important for aromatase inhibitor–refractory disease. Safety and PFS with everolimus plus exemestane in this study were consistent with BOLERO-2 and are now supported by real-world evidence,” the investigators wrote.
“The take home from the BOLERO-6 trial is that the progression-free survival for the combination of everolimus and exemestane is superior to everolimus alone, and is in line with data from the BOLERO-2 trial, and also the PrE0102 study, demonstrating the consistent activity of mTOR inhibition in combination with endocrine therapy in the aromatase inhibitor resistance setting, and this supports our use of the combination in the endocrine resistant patients,” said Cynthia X. Ma, MD, PhD, of Washington University, St. Louis, the invited discussant.
Novartis funded the study. Dr Jerusalem received research funding from Novartis and Roche; honoraria from Novartis, Roche, Pfizer, Lilly, Celgene, Amgen, BMS, and Puma Technology; and nonfinancial support from Novartis, Roche, Pfizer, Lilly, Amgen, and BMS. Dr. Ma reported consulting/advising, travel expenses, and institutional research funding from Novartis and others.
SOURCE: Jerusalem G et al. ASCO 2018 Abstract 1005
REPORTING FROM ASCO 2018
Key clinical point: The combination of everolimus and exemestane had better efficacy than did everolimus alone in women with ER+/HER2– breast cancer resistant to endocrine therapy.
Major finding: Median PFS with everolimus/exemestane was 8.4 months vs 6.8 months for everolimus.
Study details: Randomized, open label, phase 2 trial of 309 women with ER-positive, HER2-negative breast cancer that progressed during nonsteroidal aromatase inhibitor therapy.
Disclosures: Novartis funded the study. Dr Jerusalem received research funding from Novartis and Roche; honoraria from Novartis, Roche, Pfizer, Lilly, Celgene, Amgen, BMS, and Puma Technology; and nonfinancial support from Novartis, Roche, Pfizer, Lilly, Amgen, and BMS. Dr. Ma reported consulting/advising, travel expenses, and institutional research funding from Novartis and others.
Source: Jerusalem G et al. ASCO 2018, Abstract 1005.
TAILORx marks major advance for precision medicine in breast cancer
CHICAGO – , sparing them adverse effects and preventing overtreatment, TAILORx trial results show.
The findings, which were reported in the plenary session at the annual meeting of the American Society of Clinical Oncology and simultaneously published in the New England Journal of Medicine, mark a major advance in precision medicine.
The recurrence score is known to be prognostic and to be predictive of benefit from adding chemotherapy to endocrine therapy, Dr. Sparano said. “But there was a major gap: There was uncertain benefit for patients who had a midrange score, about two-thirds of all patients who are treated.”
The phase 3 TAILORx trial registered 10,273 women with hormone receptor–positive, HER2-negative, node-negative early-stage breast cancer, making it the largest adjuvant breast cancer trial to date. Analyses focused on the 6,711 evaluable women with a midrange recurrence score (defined as 11 through 25 in the trial), who were randomized to receive endocrine therapy alone or adjuvant chemotherapy plus endocrine therapy, with a noninferiority design. Of note, contemporary drugs and regimens were used.
Results at a median follow-up of 7.5 years showed that the trial met its primary endpoint: The risk of invasive disease-free survival events (invasive disease recurrence, second primary cancer, or death) was not inferior for women given endocrine therapy alone compared with counterparts given chemotherapy plus endocrine therapy (hazard ratio, 1.08; P = .26), Dr. Sparano reported.
The groups were also on par, with absolute differences of no more than 1% between rates, with respect to a variety of other efficacy outcomes: freedom from distant recurrence and any recurrence, and overall survival.
Findings were similar across most subgroups. But analyses suggested that women aged 50 years and younger having a recurrence score of 16-25 did fare better when they received chemotherapy. “Though exploratory from a statistical perspective, this is a highly clinically relevant observation,” he maintained. “It suggests ... that chemotherapy should be spared with caution in this subgroup, after a careful discussion of potential benefits and risks in a shared decision process.”
In other findings, analyses of the trial’s nonrandomized groups confirmed excellent outcomes among women with a low recurrence score (defined as 0-10) given endocrine therapy alone, and at the other end of the spectrum, need for a more aggressive approach, including chemotherapy, among women with a high recurrence score (defined as 26-100).
Ultimately, application of the recurrence score allowed 69% of the entire trial population to skip chemotherapy: all of those women with a score of 0-10 (16% of the trial population), those older than 50 years with a score of 11-25 (45%), and those aged 50 years or younger with a score of 11-15 (8%).
“Although this trial was designed in 2003, it was designed with the goal of addressing one of the themes at this 2018 meeting, expanding the reach of precision medicine,” Dr. Sparano pointed out. “It also embodies the core values of ASCO: By providing the highest level of evidence, it can have a direct and immediate impact on the care of our patients.”
An ongoing companion phase 3 trial, RxPONDER, is assessing the benefit of applying the recurrence score in women who are similar but instead have node-positive disease.
Tailoring treatment: ‘not too much and not too little’
“These are very important data because this is the most common form of breast cancer in the United States and other developed countries, and the most challenging decision we make with these patients is whether or not to recommend adjuvant chemotherapy with all of its side effects and with its potential benefits,” said ASCO Expert Harold Burstein, MD, PhD, FASCO. “The data provided here today from this massive NCI-sponsored trial show that the vast majority of women who have this test performed on their tumor can be told that they don’t need chemotherapy, and that can be said with tremendous confidence and reassurance.”
“This is not so much about de-escalation ... The goal of this study was not to just use less treatment, the goal was to tailor treatment – they chose the title very aptly, with the idea of saying some women are going to need more of one kind of therapy and less of another, and others will get a different treatment based on the biology of their tumor,” said Dr. Burstein, a medical oncologist at the Dana-Farber Cancer Institute and associate professor of medicine, Harvard Medical School, Boston.
“This is extraordinary data for breast cancer doctors and women who have breast cancer. It allows you to individualize treatment based on extraordinary science, which now has tremendous prospective validation,” he said. Overall, “women with breast cancer who are getting modern therapy are doing extraordinarily well, and this test shows us how to tailor that management so they get exactly the right amount of treatment – not too much and not too little.”
Study details
All of the women with hormone receptor–positive, HER2-negative, node-negative early-stage breast cancer enrolled in TAILORx met National Comprehensive Cancer Network guidelines for receiving adjuvant chemotherapy.
Roughly 69% had an intermediate recurrence score (11-25) and were randomized. All of the 17% having a low recurrence score (0-10) were given only endocrine therapy, and all of the 14% with a high recurrence score (26-100) were given both adjuvant chemotherapy and endocrine therapy.
Of note, the recurrence scores used to define midrange were adjusted downward from those conventionally used to account for exclusion of patients with higher-risk HER2-positive disease and to minimize potential for undertreatment, Dr. Sparano explained. “I think you will see changes in the near future as to how Genomic Health reports their results.”
Among the women with midrange scores who were randomized, the hazard ratio for invasive disease-free survival with endocrine therapy alone compared with chemotherapy plus endocrine therapy (1.08) fell well within the predefined hazard ratio for noninferiority (1.322). The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy and 84.3% with chemotherapy plus endocrine therapy.
The groups had similar rates of freedom from distant recurrence (94.5% vs. 95.0%; hazard ratio, 1.10; P = .48) and distant or locoregional recurrence (92.2% vs. 92.9%; hazard ratio, 1.11; P = .33), and similar overall survival (93.9% vs. 93.8%; hazard ratio for death, 0.99; P = .89).
In exploratory analyses, there was an interaction of age and recurrence score (P = .004) whereby women aged 50 or younger derived some benefit from chemotherapy if they had a recurrence score of 16-20 (9% fewer invasive disease–free survival events, including 2% fewer distant recurrences) or a recurrence score 21-25 (6% fewer invasive disease–free survival events, mainly distant recurrences). “This is information that could drive some younger women who have a recurrence score in this range to accept chemotherapy,” Dr. Sparano said.
The 9-year rate of distant recurrence averaged 5% among the women with midrange scores overall. It was just 3% among the women with a low recurrence score given endocrine therapy alone, but it was still 13% among the women with a high recurrence score despite receiving both endocrine therapy and chemotherapy. The last finding may “indicate the need to explore potentially more effective therapies in this setting,” he proposed.
Dr. Sparano disclosed that he has a consulting or advisory role with Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack; has stock or other ownership interests with Metastat; and receives research funding (institutional) from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. This study received funding primarily from the National Cancer Institute, National Institutes of Health. Additional support was provided by the Breast Cancer Research Foundation, Komen Foundation, and U.S. Postal Service Breast Cancer Stamp.
SOURCE: Sparano et al. ASCO 2018 Abstract LBA1
CHICAGO – , sparing them adverse effects and preventing overtreatment, TAILORx trial results show.
The findings, which were reported in the plenary session at the annual meeting of the American Society of Clinical Oncology and simultaneously published in the New England Journal of Medicine, mark a major advance in precision medicine.
The recurrence score is known to be prognostic and to be predictive of benefit from adding chemotherapy to endocrine therapy, Dr. Sparano said. “But there was a major gap: There was uncertain benefit for patients who had a midrange score, about two-thirds of all patients who are treated.”
The phase 3 TAILORx trial registered 10,273 women with hormone receptor–positive, HER2-negative, node-negative early-stage breast cancer, making it the largest adjuvant breast cancer trial to date. Analyses focused on the 6,711 evaluable women with a midrange recurrence score (defined as 11 through 25 in the trial), who were randomized to receive endocrine therapy alone or adjuvant chemotherapy plus endocrine therapy, with a noninferiority design. Of note, contemporary drugs and regimens were used.
Results at a median follow-up of 7.5 years showed that the trial met its primary endpoint: The risk of invasive disease-free survival events (invasive disease recurrence, second primary cancer, or death) was not inferior for women given endocrine therapy alone compared with counterparts given chemotherapy plus endocrine therapy (hazard ratio, 1.08; P = .26), Dr. Sparano reported.
The groups were also on par, with absolute differences of no more than 1% between rates, with respect to a variety of other efficacy outcomes: freedom from distant recurrence and any recurrence, and overall survival.
Findings were similar across most subgroups. But analyses suggested that women aged 50 years and younger having a recurrence score of 16-25 did fare better when they received chemotherapy. “Though exploratory from a statistical perspective, this is a highly clinically relevant observation,” he maintained. “It suggests ... that chemotherapy should be spared with caution in this subgroup, after a careful discussion of potential benefits and risks in a shared decision process.”
In other findings, analyses of the trial’s nonrandomized groups confirmed excellent outcomes among women with a low recurrence score (defined as 0-10) given endocrine therapy alone, and at the other end of the spectrum, need for a more aggressive approach, including chemotherapy, among women with a high recurrence score (defined as 26-100).
Ultimately, application of the recurrence score allowed 69% of the entire trial population to skip chemotherapy: all of those women with a score of 0-10 (16% of the trial population), those older than 50 years with a score of 11-25 (45%), and those aged 50 years or younger with a score of 11-15 (8%).
“Although this trial was designed in 2003, it was designed with the goal of addressing one of the themes at this 2018 meeting, expanding the reach of precision medicine,” Dr. Sparano pointed out. “It also embodies the core values of ASCO: By providing the highest level of evidence, it can have a direct and immediate impact on the care of our patients.”
An ongoing companion phase 3 trial, RxPONDER, is assessing the benefit of applying the recurrence score in women who are similar but instead have node-positive disease.
Tailoring treatment: ‘not too much and not too little’
“These are very important data because this is the most common form of breast cancer in the United States and other developed countries, and the most challenging decision we make with these patients is whether or not to recommend adjuvant chemotherapy with all of its side effects and with its potential benefits,” said ASCO Expert Harold Burstein, MD, PhD, FASCO. “The data provided here today from this massive NCI-sponsored trial show that the vast majority of women who have this test performed on their tumor can be told that they don’t need chemotherapy, and that can be said with tremendous confidence and reassurance.”
“This is not so much about de-escalation ... The goal of this study was not to just use less treatment, the goal was to tailor treatment – they chose the title very aptly, with the idea of saying some women are going to need more of one kind of therapy and less of another, and others will get a different treatment based on the biology of their tumor,” said Dr. Burstein, a medical oncologist at the Dana-Farber Cancer Institute and associate professor of medicine, Harvard Medical School, Boston.
“This is extraordinary data for breast cancer doctors and women who have breast cancer. It allows you to individualize treatment based on extraordinary science, which now has tremendous prospective validation,” he said. Overall, “women with breast cancer who are getting modern therapy are doing extraordinarily well, and this test shows us how to tailor that management so they get exactly the right amount of treatment – not too much and not too little.”
Study details
All of the women with hormone receptor–positive, HER2-negative, node-negative early-stage breast cancer enrolled in TAILORx met National Comprehensive Cancer Network guidelines for receiving adjuvant chemotherapy.
Roughly 69% had an intermediate recurrence score (11-25) and were randomized. All of the 17% having a low recurrence score (0-10) were given only endocrine therapy, and all of the 14% with a high recurrence score (26-100) were given both adjuvant chemotherapy and endocrine therapy.
Of note, the recurrence scores used to define midrange were adjusted downward from those conventionally used to account for exclusion of patients with higher-risk HER2-positive disease and to minimize potential for undertreatment, Dr. Sparano explained. “I think you will see changes in the near future as to how Genomic Health reports their results.”
Among the women with midrange scores who were randomized, the hazard ratio for invasive disease-free survival with endocrine therapy alone compared with chemotherapy plus endocrine therapy (1.08) fell well within the predefined hazard ratio for noninferiority (1.322). The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy and 84.3% with chemotherapy plus endocrine therapy.
The groups had similar rates of freedom from distant recurrence (94.5% vs. 95.0%; hazard ratio, 1.10; P = .48) and distant or locoregional recurrence (92.2% vs. 92.9%; hazard ratio, 1.11; P = .33), and similar overall survival (93.9% vs. 93.8%; hazard ratio for death, 0.99; P = .89).
In exploratory analyses, there was an interaction of age and recurrence score (P = .004) whereby women aged 50 or younger derived some benefit from chemotherapy if they had a recurrence score of 16-20 (9% fewer invasive disease–free survival events, including 2% fewer distant recurrences) or a recurrence score 21-25 (6% fewer invasive disease–free survival events, mainly distant recurrences). “This is information that could drive some younger women who have a recurrence score in this range to accept chemotherapy,” Dr. Sparano said.
The 9-year rate of distant recurrence averaged 5% among the women with midrange scores overall. It was just 3% among the women with a low recurrence score given endocrine therapy alone, but it was still 13% among the women with a high recurrence score despite receiving both endocrine therapy and chemotherapy. The last finding may “indicate the need to explore potentially more effective therapies in this setting,” he proposed.
Dr. Sparano disclosed that he has a consulting or advisory role with Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack; has stock or other ownership interests with Metastat; and receives research funding (institutional) from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. This study received funding primarily from the National Cancer Institute, National Institutes of Health. Additional support was provided by the Breast Cancer Research Foundation, Komen Foundation, and U.S. Postal Service Breast Cancer Stamp.
SOURCE: Sparano et al. ASCO 2018 Abstract LBA1
CHICAGO – , sparing them adverse effects and preventing overtreatment, TAILORx trial results show.
The findings, which were reported in the plenary session at the annual meeting of the American Society of Clinical Oncology and simultaneously published in the New England Journal of Medicine, mark a major advance in precision medicine.
The recurrence score is known to be prognostic and to be predictive of benefit from adding chemotherapy to endocrine therapy, Dr. Sparano said. “But there was a major gap: There was uncertain benefit for patients who had a midrange score, about two-thirds of all patients who are treated.”
The phase 3 TAILORx trial registered 10,273 women with hormone receptor–positive, HER2-negative, node-negative early-stage breast cancer, making it the largest adjuvant breast cancer trial to date. Analyses focused on the 6,711 evaluable women with a midrange recurrence score (defined as 11 through 25 in the trial), who were randomized to receive endocrine therapy alone or adjuvant chemotherapy plus endocrine therapy, with a noninferiority design. Of note, contemporary drugs and regimens were used.
Results at a median follow-up of 7.5 years showed that the trial met its primary endpoint: The risk of invasive disease-free survival events (invasive disease recurrence, second primary cancer, or death) was not inferior for women given endocrine therapy alone compared with counterparts given chemotherapy plus endocrine therapy (hazard ratio, 1.08; P = .26), Dr. Sparano reported.
The groups were also on par, with absolute differences of no more than 1% between rates, with respect to a variety of other efficacy outcomes: freedom from distant recurrence and any recurrence, and overall survival.
Findings were similar across most subgroups. But analyses suggested that women aged 50 years and younger having a recurrence score of 16-25 did fare better when they received chemotherapy. “Though exploratory from a statistical perspective, this is a highly clinically relevant observation,” he maintained. “It suggests ... that chemotherapy should be spared with caution in this subgroup, after a careful discussion of potential benefits and risks in a shared decision process.”
In other findings, analyses of the trial’s nonrandomized groups confirmed excellent outcomes among women with a low recurrence score (defined as 0-10) given endocrine therapy alone, and at the other end of the spectrum, need for a more aggressive approach, including chemotherapy, among women with a high recurrence score (defined as 26-100).
Ultimately, application of the recurrence score allowed 69% of the entire trial population to skip chemotherapy: all of those women with a score of 0-10 (16% of the trial population), those older than 50 years with a score of 11-25 (45%), and those aged 50 years or younger with a score of 11-15 (8%).
“Although this trial was designed in 2003, it was designed with the goal of addressing one of the themes at this 2018 meeting, expanding the reach of precision medicine,” Dr. Sparano pointed out. “It also embodies the core values of ASCO: By providing the highest level of evidence, it can have a direct and immediate impact on the care of our patients.”
An ongoing companion phase 3 trial, RxPONDER, is assessing the benefit of applying the recurrence score in women who are similar but instead have node-positive disease.
Tailoring treatment: ‘not too much and not too little’
“These are very important data because this is the most common form of breast cancer in the United States and other developed countries, and the most challenging decision we make with these patients is whether or not to recommend adjuvant chemotherapy with all of its side effects and with its potential benefits,” said ASCO Expert Harold Burstein, MD, PhD, FASCO. “The data provided here today from this massive NCI-sponsored trial show that the vast majority of women who have this test performed on their tumor can be told that they don’t need chemotherapy, and that can be said with tremendous confidence and reassurance.”
“This is not so much about de-escalation ... The goal of this study was not to just use less treatment, the goal was to tailor treatment – they chose the title very aptly, with the idea of saying some women are going to need more of one kind of therapy and less of another, and others will get a different treatment based on the biology of their tumor,” said Dr. Burstein, a medical oncologist at the Dana-Farber Cancer Institute and associate professor of medicine, Harvard Medical School, Boston.
“This is extraordinary data for breast cancer doctors and women who have breast cancer. It allows you to individualize treatment based on extraordinary science, which now has tremendous prospective validation,” he said. Overall, “women with breast cancer who are getting modern therapy are doing extraordinarily well, and this test shows us how to tailor that management so they get exactly the right amount of treatment – not too much and not too little.”
Study details
All of the women with hormone receptor–positive, HER2-negative, node-negative early-stage breast cancer enrolled in TAILORx met National Comprehensive Cancer Network guidelines for receiving adjuvant chemotherapy.
Roughly 69% had an intermediate recurrence score (11-25) and were randomized. All of the 17% having a low recurrence score (0-10) were given only endocrine therapy, and all of the 14% with a high recurrence score (26-100) were given both adjuvant chemotherapy and endocrine therapy.
Of note, the recurrence scores used to define midrange were adjusted downward from those conventionally used to account for exclusion of patients with higher-risk HER2-positive disease and to minimize potential for undertreatment, Dr. Sparano explained. “I think you will see changes in the near future as to how Genomic Health reports their results.”
Among the women with midrange scores who were randomized, the hazard ratio for invasive disease-free survival with endocrine therapy alone compared with chemotherapy plus endocrine therapy (1.08) fell well within the predefined hazard ratio for noninferiority (1.322). The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy and 84.3% with chemotherapy plus endocrine therapy.
The groups had similar rates of freedom from distant recurrence (94.5% vs. 95.0%; hazard ratio, 1.10; P = .48) and distant or locoregional recurrence (92.2% vs. 92.9%; hazard ratio, 1.11; P = .33), and similar overall survival (93.9% vs. 93.8%; hazard ratio for death, 0.99; P = .89).
In exploratory analyses, there was an interaction of age and recurrence score (P = .004) whereby women aged 50 or younger derived some benefit from chemotherapy if they had a recurrence score of 16-20 (9% fewer invasive disease–free survival events, including 2% fewer distant recurrences) or a recurrence score 21-25 (6% fewer invasive disease–free survival events, mainly distant recurrences). “This is information that could drive some younger women who have a recurrence score in this range to accept chemotherapy,” Dr. Sparano said.
The 9-year rate of distant recurrence averaged 5% among the women with midrange scores overall. It was just 3% among the women with a low recurrence score given endocrine therapy alone, but it was still 13% among the women with a high recurrence score despite receiving both endocrine therapy and chemotherapy. The last finding may “indicate the need to explore potentially more effective therapies in this setting,” he proposed.
Dr. Sparano disclosed that he has a consulting or advisory role with Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack; has stock or other ownership interests with Metastat; and receives research funding (institutional) from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. This study received funding primarily from the National Cancer Institute, National Institutes of Health. Additional support was provided by the Breast Cancer Research Foundation, Komen Foundation, and U.S. Postal Service Breast Cancer Stamp.
SOURCE: Sparano et al. ASCO 2018 Abstract LBA1
REPORTING FROM ASCO 2018
Key clinical point: The majority of women with HR-positive, HER2-negative, node-negative early-stage breast cancer who have an intermediate recurrence score can safely skip adjuvant chemotherapy.
Major finding: Among women with an Oncotype DX Recurrence Score in the midrange (11-25), invasive disease–free survival with endocrine therapy alone was not inferior to that with chemotherapy plus endocrine therapy (hazard ratio, 1.08; P = .26).
Study details: A phase 3 trial among 10,273 women with HR-positive, HER2-negative, node-negative early-stage breast cancer, with a noninferiority randomized component among the 6,711 women with a midrange recurrence score (TAILORx trial).
Disclosures: Dr. Sparano disclosed that he has a consulting or advisory role with Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack; has stock or other ownership interests with MetaStat; and receives research funding (institutional) from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. This study received funding primarily from the National Cancer Institute, National Institutes of Health. Additional support was provided by the Breast Cancer Research Foundation, Komen Foundation, and U.S. Postal Service Breast Cancer Stamp.
Source: Sparano et al. ASCO 2018 Abstract LBA1.
Dr. William J. Gradishar shares breast cancer take-aways from ASCO 2018
CHICAGO – William J. Gradishar, MD, discussed the clinical impact of breast cancer research presented at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, said TAILORx was a “big win” in that it has no doubt diminished the number of women with early-stage breast cancer who will require chemotherapy. However, although the trial has provided some clarity, it also has left some questions open, particularly for patients under 50 years of age, he said.
Dr. Gradishar also discussed the results of combination trials of targeted therapy with either endocrine therapy or chemotherapy. In discussing SANDPIPER, which evaluated whether a phosphoinositide 3-kinase inhibitor could enhance the effect of anti-hormonal therapy, he said that although it was a positive trial, “from a clinician’s standpoint, it’s probably not sufficient in my mind to get really excited about.”
CHICAGO – William J. Gradishar, MD, discussed the clinical impact of breast cancer research presented at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, said TAILORx was a “big win” in that it has no doubt diminished the number of women with early-stage breast cancer who will require chemotherapy. However, although the trial has provided some clarity, it also has left some questions open, particularly for patients under 50 years of age, he said.
Dr. Gradishar also discussed the results of combination trials of targeted therapy with either endocrine therapy or chemotherapy. In discussing SANDPIPER, which evaluated whether a phosphoinositide 3-kinase inhibitor could enhance the effect of anti-hormonal therapy, he said that although it was a positive trial, “from a clinician’s standpoint, it’s probably not sufficient in my mind to get really excited about.”
CHICAGO – William J. Gradishar, MD, discussed the clinical impact of breast cancer research presented at the annual meeting of the American Society of Clinical Oncology.
In a video interview, Dr. Gradishar, the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago, said TAILORx was a “big win” in that it has no doubt diminished the number of women with early-stage breast cancer who will require chemotherapy. However, although the trial has provided some clarity, it also has left some questions open, particularly for patients under 50 years of age, he said.
Dr. Gradishar also discussed the results of combination trials of targeted therapy with either endocrine therapy or chemotherapy. In discussing SANDPIPER, which evaluated whether a phosphoinositide 3-kinase inhibitor could enhance the effect of anti-hormonal therapy, he said that although it was a positive trial, “from a clinician’s standpoint, it’s probably not sufficient in my mind to get really excited about.”
REPORTING FROM ASCO 2018
IMPACT study: Matched targeted therapy improves survival in advanced cancer
CHICAGO – according to findings from a retrospective analysis of molecularly profiled patients.
Of 3,743 patients tested as part of IMPACT (Initiative for Molecular Profiling and Advanced Cancer Therapy), 1,307 (34.9%) had at least one targetable molecular alteration. Of those, 711 (54.4%) received either matched targeted therapy that was being tested in a clinical trial or – in a small number of cases – therapy with an approved treatment used off label, and 596 (45.6%) received nonmatched therapy, Apostolia-Maria Tsimberidou, MD, reported during a press briefing at the annual meeting of the American Society of Clinical Oncology.
The objective response rates in 697 evaluable matched therapy patients was 16.2% versus 5.4% in 571 evaluable nonmatched patients, and stable disease for at least 6 months occurred in 18.7% and 14.7% of patients, respectively, for an overall disease control rate of 34.9% versus 20.1%, said Dr. Tsimberidou, a professor at the University of Texas MD Anderson Cancer Center, Houston.
Median progression-free survival in those who received matched versus nonmatched therapy was 4.0 months and 2.8 months, respectively (hazard ratio, 0.67), and median overall survival was 9.3 and 7.3 months, respectively (HR, 0.72), she said.
The 3-year overall survival rate was 15% versus 7%, respectively, and 10-year survival was 6% and 1%, respectively.
Patients included in IMPACT had a mean age of 57 years, and 39% were men. They were heavily pretreated (mean number of prior therapies was 4); only 2.8% of patients had no prior treatment. Cancers included gastrointestinal (24.2%), gynecologic (19.4%), breast (13.5%), melanoma (11.9%) and lung (8.7%).
In this video interview, Dr. Tsimberidou describes the rationale, methodology, and findings of IMPACT, including the use of a prognostic scoring system developed as part of the study to predict overall survival based on baseline characteristics, such as baseline p13K/AKT/mTOR pathway molecular alterations, which were shown on multivariate analysis in IMPACT to predict shorter overall survival versus other alterations. Other predictors of shorter survival included liver metastases, elevated lactate dehydrogenase levels, poor functional status, low albumin levels, elevated platelet counts, and age of 60 years or older.
“We [also] wanted to see if adding the intervention ... would hold significance in this multivariate model, and we found that ... nonmatched therapy was associated with adverse survival; it was an independent factor associated with worse survival,” she said. “Therefore, matched targeted therapy is associated with longer survival.”
In the randomized, phase 2 trial IMPACT 2, progression-free survival will be compared in patients with and without matched targeted therapy, and the prognostic scoring system developed as part of IMPACT to predict overall survival based on baseline characteristics will be further evaluated, she said.
During a discussion of the findings during the press briefing, ASCO Expert Catherine M. Diefenbach, MD, said the type of precision medicine studied in IMPACT is “the wave of the future.
“Large scale efforts such as ASCO’s TAPUR or the NCI-MATCH trial will bring these efforts to many, many more patients, and hopefully usher in a new way of treating advanced cancer patients that will improve overall survival for many more patients,” said Dr. Diefenbach, of New York University.
Dr. Tsimberidou reported a consulting or advisory role with Roche, as well as research funding to her institution from EMD Serono, Baxter, Foundation Medicine, ONYX, Bayer, Boston Biomedical, Placon, IMMATICS, Karus Therapeutics, and StemCells.
SOURCE: Tsimberidou AM et al. ASCO 2018, Abstract LBA 2553.
CHICAGO – according to findings from a retrospective analysis of molecularly profiled patients.
Of 3,743 patients tested as part of IMPACT (Initiative for Molecular Profiling and Advanced Cancer Therapy), 1,307 (34.9%) had at least one targetable molecular alteration. Of those, 711 (54.4%) received either matched targeted therapy that was being tested in a clinical trial or – in a small number of cases – therapy with an approved treatment used off label, and 596 (45.6%) received nonmatched therapy, Apostolia-Maria Tsimberidou, MD, reported during a press briefing at the annual meeting of the American Society of Clinical Oncology.
The objective response rates in 697 evaluable matched therapy patients was 16.2% versus 5.4% in 571 evaluable nonmatched patients, and stable disease for at least 6 months occurred in 18.7% and 14.7% of patients, respectively, for an overall disease control rate of 34.9% versus 20.1%, said Dr. Tsimberidou, a professor at the University of Texas MD Anderson Cancer Center, Houston.
Median progression-free survival in those who received matched versus nonmatched therapy was 4.0 months and 2.8 months, respectively (hazard ratio, 0.67), and median overall survival was 9.3 and 7.3 months, respectively (HR, 0.72), she said.
The 3-year overall survival rate was 15% versus 7%, respectively, and 10-year survival was 6% and 1%, respectively.
Patients included in IMPACT had a mean age of 57 years, and 39% were men. They were heavily pretreated (mean number of prior therapies was 4); only 2.8% of patients had no prior treatment. Cancers included gastrointestinal (24.2%), gynecologic (19.4%), breast (13.5%), melanoma (11.9%) and lung (8.7%).
In this video interview, Dr. Tsimberidou describes the rationale, methodology, and findings of IMPACT, including the use of a prognostic scoring system developed as part of the study to predict overall survival based on baseline characteristics, such as baseline p13K/AKT/mTOR pathway molecular alterations, which were shown on multivariate analysis in IMPACT to predict shorter overall survival versus other alterations. Other predictors of shorter survival included liver metastases, elevated lactate dehydrogenase levels, poor functional status, low albumin levels, elevated platelet counts, and age of 60 years or older.
“We [also] wanted to see if adding the intervention ... would hold significance in this multivariate model, and we found that ... nonmatched therapy was associated with adverse survival; it was an independent factor associated with worse survival,” she said. “Therefore, matched targeted therapy is associated with longer survival.”
In the randomized, phase 2 trial IMPACT 2, progression-free survival will be compared in patients with and without matched targeted therapy, and the prognostic scoring system developed as part of IMPACT to predict overall survival based on baseline characteristics will be further evaluated, she said.
During a discussion of the findings during the press briefing, ASCO Expert Catherine M. Diefenbach, MD, said the type of precision medicine studied in IMPACT is “the wave of the future.
“Large scale efforts such as ASCO’s TAPUR or the NCI-MATCH trial will bring these efforts to many, many more patients, and hopefully usher in a new way of treating advanced cancer patients that will improve overall survival for many more patients,” said Dr. Diefenbach, of New York University.
Dr. Tsimberidou reported a consulting or advisory role with Roche, as well as research funding to her institution from EMD Serono, Baxter, Foundation Medicine, ONYX, Bayer, Boston Biomedical, Placon, IMMATICS, Karus Therapeutics, and StemCells.
SOURCE: Tsimberidou AM et al. ASCO 2018, Abstract LBA 2553.
CHICAGO – according to findings from a retrospective analysis of molecularly profiled patients.
Of 3,743 patients tested as part of IMPACT (Initiative for Molecular Profiling and Advanced Cancer Therapy), 1,307 (34.9%) had at least one targetable molecular alteration. Of those, 711 (54.4%) received either matched targeted therapy that was being tested in a clinical trial or – in a small number of cases – therapy with an approved treatment used off label, and 596 (45.6%) received nonmatched therapy, Apostolia-Maria Tsimberidou, MD, reported during a press briefing at the annual meeting of the American Society of Clinical Oncology.
The objective response rates in 697 evaluable matched therapy patients was 16.2% versus 5.4% in 571 evaluable nonmatched patients, and stable disease for at least 6 months occurred in 18.7% and 14.7% of patients, respectively, for an overall disease control rate of 34.9% versus 20.1%, said Dr. Tsimberidou, a professor at the University of Texas MD Anderson Cancer Center, Houston.
Median progression-free survival in those who received matched versus nonmatched therapy was 4.0 months and 2.8 months, respectively (hazard ratio, 0.67), and median overall survival was 9.3 and 7.3 months, respectively (HR, 0.72), she said.
The 3-year overall survival rate was 15% versus 7%, respectively, and 10-year survival was 6% and 1%, respectively.
Patients included in IMPACT had a mean age of 57 years, and 39% were men. They were heavily pretreated (mean number of prior therapies was 4); only 2.8% of patients had no prior treatment. Cancers included gastrointestinal (24.2%), gynecologic (19.4%), breast (13.5%), melanoma (11.9%) and lung (8.7%).
In this video interview, Dr. Tsimberidou describes the rationale, methodology, and findings of IMPACT, including the use of a prognostic scoring system developed as part of the study to predict overall survival based on baseline characteristics, such as baseline p13K/AKT/mTOR pathway molecular alterations, which were shown on multivariate analysis in IMPACT to predict shorter overall survival versus other alterations. Other predictors of shorter survival included liver metastases, elevated lactate dehydrogenase levels, poor functional status, low albumin levels, elevated platelet counts, and age of 60 years or older.
“We [also] wanted to see if adding the intervention ... would hold significance in this multivariate model, and we found that ... nonmatched therapy was associated with adverse survival; it was an independent factor associated with worse survival,” she said. “Therefore, matched targeted therapy is associated with longer survival.”
In the randomized, phase 2 trial IMPACT 2, progression-free survival will be compared in patients with and without matched targeted therapy, and the prognostic scoring system developed as part of IMPACT to predict overall survival based on baseline characteristics will be further evaluated, she said.
During a discussion of the findings during the press briefing, ASCO Expert Catherine M. Diefenbach, MD, said the type of precision medicine studied in IMPACT is “the wave of the future.
“Large scale efforts such as ASCO’s TAPUR or the NCI-MATCH trial will bring these efforts to many, many more patients, and hopefully usher in a new way of treating advanced cancer patients that will improve overall survival for many more patients,” said Dr. Diefenbach, of New York University.
Dr. Tsimberidou reported a consulting or advisory role with Roche, as well as research funding to her institution from EMD Serono, Baxter, Foundation Medicine, ONYX, Bayer, Boston Biomedical, Placon, IMMATICS, Karus Therapeutics, and StemCells.
SOURCE: Tsimberidou AM et al. ASCO 2018, Abstract LBA 2553.
REPORTING FROM ASCO 2018
Key clinical point: Matched targeted therapy improved survival in patients with advanced cancer.
Major finding: The 3-yearoverall survival rate with matched versus nonmatched therapy was 15% and 7%, respectively.
Study details: A retrospective analysis (IMPACT) of 3,743 molecularly profiled advanced cancer patients.
Disclosures: Dr. Tsimberidou reported a consulting or advisory role with Roche, as well as research funding to her institution from EMD Serono, Baxter, Foundation Medicine, ONYX Medical, Bayer, Boston Biomedical, Placon, IMMATICS, Karus Therapeutics, and StemCells.
Source: Tsimberidou AM et al. ASCO 2018, Abstract LBA 2553.