User login
HER2-Positive Breast Cancer: Current Management
Introduction
Breast cancer is the second leading cause of cancer deaths among women in the United States, according to the SEER database. It is estimated that 1 in 8 women will be diagnosed with breast cancer at some point during their lifetime (12.4% lifetime risk).1,2 Because breast tumors are clinically and histopathologically heterogeneous, different diagnostic and therapeutic approaches are required for each subtype. Among the subtypes, tumors that are positive for human epidermal growth factor receptor 2 (HER2) account for approximately 15% to 20% of all newly diagnosed localized and metastatic invasive breast tumors.3,4 Historically, this subset of tumors has been considered the most aggressive due to a higher propensity to relapse and metastasize, translating into poorer prognosis compared with other subtypes.5–7 However, with the advent of HER2-targeted therapy in the late 1990s, prognosis has significantly improved for both early- and late-stage HER2-positive tumors.8
Pathogenesis
The HER2 proto-oncogene belongs to a family of human epidermal growth factor receptors that includes 4 transmembrane tyrosine kinase receptors: HER1 (also commonly known as epidermal growth factor receptor, EGFR), HER2, HER3, and HER4. Another commonly used nomenclature for this family of receptors is ERBB1 to ERBB4. Each of the receptors has a similar structure consisting of a growth factor–binding extracellular domain, a single transmembrane segment, an intracellular protein-tyrosine kinase catalytic domain, and a tyrosine-containing cytoplasmic tail. Activation of the extracellular domain leads to conformational changes that initiate a cascade of reactions resulting in protein kinase activation. ERBB tyrosine receptor kinases subsequently activate several intracellular pathways that are critical for cellular function and survival, including the PI3K-AKT, RAS-MAPK, and mTOR pathways. Hyperactivation or overexpression of these receptors leads to uncontrolled cell growth and proliferation, and eventually cancerogenesis.9,10
HER2 gene amplification can cause activation of the receptor’s extramembranous domain by way of either dimerization of two HER2 receptors or heterodimerization with other ERBB family receptors, leading to ligand-independent activation of cell signaling (ie, activation in the absence of external growth factors). Besides breast cancer, HER2 protein is overexpressed in several other tumor types, including esophageal and gastric adenocarcinomas, colon and gynecological malignancies, and to a lesser extent in other malignancies.
Biomarker Testing
All patients with newly diagnosed breast cancer should have their tumor tissue submitted for biomarker testing for estrogen receptors (ER), progesterone receptors (PR), and HER2 overexpression, as the result this testing dictates therapy choices. The purpose of HER2 testing is to investigate whether the HER2 gene, located on chromosome 17, is overexpressed or amplified. HER2 status provides the basis for treatment selection, which impacts long-term outcome measures such as recurrence and survival. Routine testing of carcinoma in situ for HER2 expression/amplification is not recommended and has no implication on choice of therapy at this time.
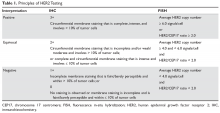
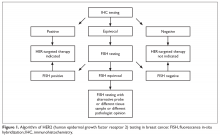
In 2013, the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) updated their clinical guideline recommendations for HER2 testing in breast cancer to improve its accuracy and its utility as a predictive marker.11 There are currently 2 approved modalities for HER2 testing: detection of HER2 protein overexpression by i
Fluorescence in-situ hybridization (FISH) testing assesses for HER2 amplification by determining the number of HER2 signals and
Neoadjuvant and Adjuvant Therapy for Locoregional Disease
Case Patient 1
A 56-year-old woman undergoes ultrasound-guided biopsy of a self-palpated breast lump. Pathology shows invasive ductal carcinoma that is ER-positive, PR-negative, and HER2 equivocal by IHC (2+ staining). Follow-up FISH testing shows a HER2/CEP17 ratio of 2.5. The tumor is estimated to be 2 cm in diameter by imaging and exam with no clinically palpable axillary lymphadenopathy. The patient exhibits no constitutional or localized symptoms concerning for metastases.
- What is the recommended management approach for this patient?
According to the ASCO/CAP guidelines, this patient’s tumor qualifies as HER2-positive based upon testing results showing amplification of the gene. This result has important implications for management since nearly all patients with macroscopically invasive HER2-positive tumors should be considered for adjuvant chemotherapy in combination with anti-HER2 therapy. The patient should proceed with upfront tumor resection and sentinel lymph node biopsy. Systemic staging imaging (ie, computed tomography [CT] or bone scan) is not indicated in early stage breast cancer.12,13 Systemic staging scans are indicated when (1) any anatomical stage III disease is suspected (eg, with involvement of the skin or chest wall, the presence of enlarged matted or fixed axillary lymph nodes, and involvement of nodal stations other than in the axilla), and (2) when symptoms or abnormal laboratory values raise suspicion for distant metastases (eg, unexplained bone pain, unintentional weight loss, elevated serum alkaline phosphatase, and transaminitis).
Case 1 Continued
The patient presents to discuss treatment options after undergoing a lumpectomy and sentinel node biopsy procedure. The pathology report notes a single focus of carcinoma measuring 2 cm with negative sentinel lymph nodes.
- What agents are used for adjuvant therapy in HER2-postive breast cancer?
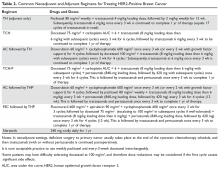
Nearly all patients with macroscopically invasive (> 1 mm) breast carcinoma should be considered for adjuvant therapy using a regimen that contains a taxane and trastuzumab. However, the benefit may be small for patients with tumors ≤ 5 mm (T1a, N0), so it is important to carefully weigh the risk against the benefit. Among the agents that targeting HER2, only trastuzumab has been shown to improve overall survival (OS) in the adjuvant setting; long-term follow-up data are awaited for other agents.8 A trastuzumab biosimilar, trastuzumab-dkst, was recently approved by the US Food and Drug Administration (FDA) for the same indications as trastuzumab.14 The regimens most commonly used in the adjuvant and neoadjuvant settings for nonmetastatic breast cancer are summarized in Table 2.
Patients with small (≤ 3 cm), node-negative tumors can generally be considered for a reduced-intensity regimen that includes weekly paclitaxel plus trastuzumab. This combination proved efficacious in a single-group, multicenter study that enrolled 406 patients.15 Paclitaxel and trastuzumab were given once weekly for 12 weeks, followed by trastuzumab, either weekly or every 3 weeks, to complete 1 year of therapy.After a median follow-up of more than 6 years, the rates of distant and locoregional recurrence were 1% and 1.2%, respectively.16
A combination of docetaxel, carboplatin, and trastuzumab is a nonanthracycline regimen that is also appropriate in this setting, based on the results of the Breast Cancer International Research Group 006 (BCIRG-006) trial.17 This phase 3 randomized trial enrolled 3222 women with HER2-positive, invasive, high-risk adenocarcinoma. Eligible patients had a T1–3 tumor and either lymph node–negative or –positive disease and were randomly assigned to receive 1 of 3 regimens: group 1 received doxorubicin and cyclophosphamide every 3 weeks for 4 cycles followed by docetaxel every 3 weeks for 4 cycles (AC-T); group 2 received the AC-T regimen in combination with trastuzumab; and group 3 received docetaxel, carboplatin, and trastuzumab once every 3 weeks for 6 cycles (TCH). Groups 2 and 3 also received trastuzumab for an additional 34 weeks to complete 1 year of therapy. Trastuzumab-containing regimens were found to offer superior disease-free survival (DFS) and OS. When comparing the 2 trastuzumab arms after more than 10 years of follow-up, no statistically significant advantage of an anthracycline regimen over a nonanthracycline regimen was found.18 Furthermore, the anthracycline arm had a fivefold higher incidence of symptomatic congestive heart failure (grades 3 and 4), and the nonanthracycline regimen was associated with a lower incidence of treatment-related leukemia, a clinically significant finding despite not reaching statistical significance due to low overall numbers.
BCIRG-006, NSABP B-31, NCCTG N9831, and HERA are all large randomized trials with consistent results confirming trastuzumab’s role in reducing recurrence and improving survival in HER2-positive breast cancer in the adjuvant settings. The estimated overall benefit from addition of this agent was a 34% to 41% improvement in survival and a 33% to 52% improvement in DFS.8,17–20
Dual anti-HER2 therapy containing both trastuzumab and pertuzumab should be strongly considered for patients with macroscopic lymph node involvement based on the results of the APHINITY trial.21 In this study, the addition of pertuzumab to standard trastuzumab-based therapy led to a significant improvement in invasive-disease-free survival at 3 years. In subgroup analysis, the benefit was restricted to the node-positive group (3-year invasive-disease-free survival rates of 92% in the pertuzumab group versus 90.2% in the placebo group, P = 0.02). Patients with hormone receptor–negative disease derived greater benefit from the addition of pertuzumab. Regimens used in the APHINITY trial included the anti-HER2 agents trastuzumab and pertuzumab in combination with 1 of the following chemotherapy regimens: sequential cyclophosphamide plus either doxorubicin or epirubicin, followed by either 4 cycles of docetaxel or 12 weekly doses of paclitaxel; sequential fluorouracil plus either epirubicin or doxorubicin plus cyclophosphamide (3 or 4 cycles), followed by 3 or 4 cycles of docetaxel or 12 weekly cycles of paclitaxel; or 6 cycles of concurrent docetaxel plus carboplatin.
One-year therapy with neratinib, an oral tyrosine kinase inhibitor of HER2, is now approved by the FDA after completion of trastuzumab in the adjuvant setting, based on the results of the ExteNET trial.22 In this study, patients who had completed trastuzumab within the preceding 12 months, without evidence of recurrence, were randomly assigned to receive either oral neratinib or placebo daily for 1 year. The 2-year DFS rate was 93.9% and 91.6% for the neratinib and placebo groups, respectively. The most common adverse effect of neratinib was diarrhea, with approximately 40% of patients experiencing grade 3 diarrhea. In subgroup analyses, hormone receptor–positive patients derived the most benefit, while hormone receptor–negative patients derived no or marginal benefit.22 OS benefit has not yet been established.23
Trastuzumab therapy (with pertuzumab if indicated) should be offered for an optimal duration of 12 months (17 cycles, including those given with chemotherapy backbone). A shorter duration of therapy, 6 months, has been shown to be inferior,24 while a longer duration, 24 months, has been shown to provide no additional benefit.25
Finally, sequential addition of anti-estrogen endocrine therapy is indicated for hormone-positive tumors. Endocrine therapy is usually added after completion of the chemotherapy backbone of the regimen, but may be given concurrently with anti-HER2 therapy. If radiation is being administered, endocrine therapy can be given concurrently or started after radiation therapy is completed.
Case 1 Conclusion
The patient can be offered 1 of 2 adjuvant treatment regimens, either TH or TCH (Table 2). Since the patient had lumpectomy, she is an appropriate candidate for adjuvant radiation, which would be started after completion of the chemotherapy backbone (taxane/platinum). Endocrine therapy for at least 5 years should be offered sequentially or concurrently with radiation. Her long-term prognosis is very favorable.
Case Patient 2
A 43-year-old woman presents with a 4-cm breast mass, a separate skin nodule, and palpable matted axillary lymphadenopathy. Biopsies of the breast mass and subcutaneous nodule reveal invasive ductal carcinoma that is ER-negative, PR-negative, and HER2-positive by IHC (3+ staining). Based on clinical findings, the patient is staged as T4b (separate tumor nodule), N2 (matted axillary lymph nodes). Systemic staging with CT scan of the chest, abdomen, and pelvis shows no evidence of distant metastases.
- What is the recommended approach to management for this patient?
Recommendations for neoadjuvant therapy, given before definitive surgery, follow the same path as with other subtypes of breast cancer. Patients with suspected anatomical stage III disease are strongly encouraged to undergo upfront (neoadjuvant) chemotherapy in combination with HER2-targeted agents. In addition, all HER2-positive patients with clinically node-positive disease can be offered neoadjuvant therapy using chemotherapy plus dual anti-HER2 therapy (trastuzumab and pertuzumab), with complete pathological response expected in more than 60% of patients.26,27 Because this patient has locally advanced disease, especially skin involvement and matted axillary nodes, she should undergo neoadjuvant therapy. Preferred regimens contain both trastuzumab and pertuzumab in combination with cytotoxic chemotherapy. The latter may be given concurrently (nonanthracycline regimens, such as docetaxel plus carboplatin) or sequentially (anthracycline-based regimens), as outlined in Table 2. Administration of anthracyclines and trastuzumab simultaneously is contraindicated due to increased risk of cardiomyopathy.28
Endocrine therapy is not indicated for this patient per the current standard of care because the tumor was ER- and PR-negative. Had the tumor been hormone receptor–positive, endocrine therapy for a minimum of 5 years would have been indicated. Likewise, in the case of hormone receptor–positive disease, 12 months of neratinib therapy after completion of trastuzumab may add further benefit, as shown in the ExteNET trial.22,23 Neratinib seems to have a propensity to prevent or delay trastuzumab-induced overexpression of estrogen receptors. This is mainly due to hormone receptor/HER2 crosstalk, a potential mechanism of resistance to trastuzumab.29,30
In addition to the medical therapy options discussed here, this patient would be expected to benefit from adjuvant radiation to the breast and regional lymph nodes, given the presence of T4 disease and bulky adenopathy in the axilla.31
Case 2 Conclusion
The patient undergoes neoadjuvant treatment (docetaxel, carboplatin, trastuzumab, and pertuzumab every 21 days for a total of 6 cycles), followed by surgical resection (modified radical mastectomy) that reveals complete pathological response (no residual invasive carcinoma). Subsequently, she receives radiation therapy to the primary tumor site and regional lymph nodes while continuing trastuzumab and pertuzumab for 11 more cycles (17 total). Despite presenting with locally advanced disease, the patient has a favorable overall prognosis due to an excellent pathological response.
- What is the approach to follow-up after completion of primary therapy?
Patients may follow up every 3 to 6 months for clinical evaluation in the first 5 years after completing primary adjuvant therapy. An annual screening mammogram is recommended as well. Body imaging can be done if dictated by symptoms. However, routine CT, positron emission tomography, or bone scans are not recommended as part of follow-up in the absence of symptoms, mainly because of a lack of evidence that such surveillance improves survival.32
Metastatic HER2-Positive Breast Cancer
Metastatic breast cancer most commonly presents as a distant recurrence of previously treated local disease. However, 6% to 18% of patients have no prior history of breast cancer and present with de novo metastatic disease.33,34 The most commonly involved distant organs are the skeletal bones, liver, lung, distant lymph node stations, and brain. Compared to other subtypes, HER2-positive tumors have an increased tendency to involve the central nervous system.35–38 Although metastatic HER2-positive breast cancer is not considered curable, significant improvement in survival has been achieved, and patients with metastatic disease have median survival approaching 5 years.39
Case Presentation 3
A 69-year-old woman with a history of breast cancer 4 years ago presents with new-onset back pain and unintentional weight loss. On exam, she is found to have palpable axillary adenopathy on the same side as her previous cancer. Her initial disease was stage IIB ER-positive and HER2-positive and was treated with chemotherapy, mastectomy, and anastrozole, which the patient is still taking. She undergoes CT scan of the chest, abdomen, and pelvis and radionucleotide bone scan, which show multiple liver and bony lesions suspicious for metastatic disease. Axillary lymph node biopsy confirms recurrent invasive carcinoma that is ER-positive and HER2-positive by IHC (3+).
- What is the approach to management of a patient who presents with symptoms of recurrent HER2-positive disease?
This patient likely has metastatic breast cancer based on the imaging findings. In such cases, a biopsy of the recurrent disease should always be considered, if feasible, to confirm the diagnosis and rule out other etiologies such as different malignances and benign conditions. Hormone-receptor and HER2 testing should also be performed on recurrent disease, since a change in HER2 status can be seen in 15% to 33% of cases.40–42
Based on data from the phase 3 CLEOPATRA trial, first-line systemic regimens for patients with metastatic breast cancer that is positive for HER2 should consist of a combination of docetaxel, trastuzumab, and pertuzumab. Compared to placebo, adding pertuzumab yielded superior progression-free survival of 18.4 months (versus 12.4 months for placebo) and an unprecedented OS of 56.5 months (versus 40.8 for placebo).39 Weekly paclitaxel can replace docetaxel with comparable efficacy (Table 3).43
Patients can develop significant neuropathy as well as skin and nail changes after multiple cycles of taxane-based chemotherapy. Therefore, the taxane backbone may be dropped after 6 to 8 cycles, while patients continue the trastuzumab and pertuzumab combination until disease progression or unacceptable toxicity. Some patients may enjoy remarkable long-term survival on “maintenance” anti-HER2 therapy.44 Despite lack of high-level evidence, such as from large randomized trials, some experts recommend the addition of a hormone blocker after discontinuation of the taxane in ER-positive tumors.45
Premenopausal and perimenopausal women with hormone receptor–positive metastatic disease should be considered for simultaneous ovarian suppression. Ovarian suppression can be accomplished medically using a gonadotropin-releasing hormone agonist (goserelin) or surgically via salpingo-oophorectomy.46–48
Case 3 Conclusion
The patient receives 6 cycles of docetaxel, trastuzumab, and pertuzumab, after which the docetaxel is discontinued due to neuropathy while she continues the other 2 agents. After 26 months of disease control, the patient is found to have new liver metastatic lesions, indicating progression of disease.
- What therapeutic options are available for this patient?
Patients whose disease progresses after receiving taxane- and trastuzumab-containing regimens are candidates to receive the novel antibody-drug conjugate ado-trastuzumab emtansine (T-DM1). Early progressors (ie, patients with early stage disease who have progression of disease while receiving adjuvant trastuzumab or within 6 months of completion of adjuvant trastuzumab) are also candidates for T-DM1. Treatment usually fits in the second line or beyond based on data from the EMILIA trial, which randomly assigned patients to receive either capecitabine plus lapatinib or T-DM1.49,50 Progression-free survival in the T-DM1 group was 9.6 months versus 6.4 months for the comparator. Improvement of 4 months in OS persisted with longer follow-up despite a crossover rate of 27%. Furthermore, a significantly higher objective response rate and fewer adverse effects were reported in the T-DM1 patients. Most patients included in the EMILIA trial were pertuzumab-naive. However, the benefit of T-DM1 appears to persist, albeit to a lesser extent, for pertuzumab-pretreated patients.51,52
Patients in whom treatment fails with 2 or more lines of therapy containing taxane-trastuzumab (with or without pertuzumab) and T-DM1 are candidates to receive a combination of capecitabine and lapatinib, a TKI, in the third line and beyond. Similarly, the combination of capecitabine with trastuzumab in the same settings appears to have equal efficacy.53,54 Trastuzumab may be continued beyond progression while changing the single-agent chemotherapy drug for subsequent lines of therapy, per ASCO guidelines,55 although improvement in OS has not been demonstrated beyond the third line in a large randomized trial (Table 3).
Approved HER2-Targeted Drugs
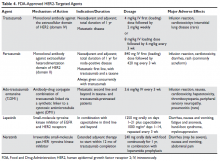
HER2-directed therapy is implemented in the management of nearly all stages of HER2-positive invasive breast cancer, including early and late stages (Table 4).
Trastuzumab
Trastuzumab was the first anti-HER2 agent to be approved by the FDA in 1998. It is a humanized monoclonal antibody directed against the extracellular domain of the HER2 receptor (domain IV). Trastuzumab functions by interrupting HER2 signal transduction and by flagging tumor cells for immune destruction.56 Cardiotoxicity, usually manifested as left ventricular systolic dysfunction, is the most noteworthy adverse effect of trastuzumab. The most prominent risk factors for cardiomyopathy in patients receiving trastuzumab are low baseline ejection fraction (< 55%), age > 50 years, co-administration and higher cumulative dose of anthracyclines, and increased body mass index and obesity.57–59 Whether patients receive therapy in the neoadjuvant, adjuvant, or metastatic settings, baseline cardiac function assessment with echocardiogram or multiple-gated acquisition scan is required. While well-designed randomized trials validating the value and frequency of monitoring are lacking, repeated cardiac testing every 3 months is generally recommended for patients undergoing adjuvant therapy. Patients with metastatic disease who are receiving treatment with palliative intent may be monitored less frequently.60,61
An asymptomatic drop in ejection fraction is the most common manifestation of cardiac toxicity. Other cardiac manifestations have also been reported with much less frequency, including arrhythmias, severe congestive heart failure, ventricular thrombus formation, and even cardiac death. Until monitoring and dose-adjustment guidelines are issued, the guidance provided in the FDA-approved prescribing information should be followed, which recommends holding trastuzumab when there is ≥ 16% absolute reduction in left ventricular ejection fraction (LVEF) from the baseline value; or if the LVEF value is below the institutional lower limit of normal and the drop is ≥ 10%. After holding the drug, cardiac function can be re-evaluated every 4 weeks. In most patients, trastuzumab-induced cardiotoxicity can be reversed by withholding trastuzumab and initiating cardioprotective therapy, although the latter remains controversial. Re-challenging after recovery of ejection fraction is possible and toxicity does not appear to be proportional to cumulative dose. Cardiomyopathy due to trastuzumab therapy is potentially reversible within 6 months in more than 80% of cases.28,57,60–63
Other notable adverse effects of trastuzumab include pulmonary toxicity (such as interstitial lung disease) and infusion reactions (usually during or within 24 hours of first dose).
Pertuzumab
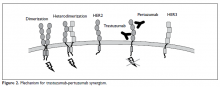
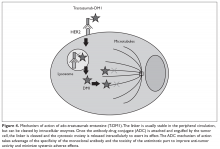
Pertuzumab is another humanized monoclonal antibody directed to a different extracellular domain of the HER2 receptor, the dimerization domain (domain II), which is responsible for heterodimerization of HER2 with other HER receptors, especially HER3. This agent should always be co-administered with trastuzumab as the 2 drugs produce synergistic anti-tumor effect, without competition for the receptor. Activation of HER3, via dimerization with HER2, produces an alternative mechanism of downstream signaling, even in the presence of trastuzumab and in the absence of growth factors (Figure 2).
Ado-Trastuzumab Emtansine
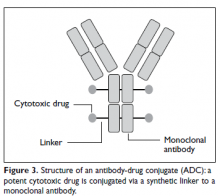
Ado-trastuzumab emtansine (T-DM1) is an antibody-drug conjugate that combines the monoclonal antibody trastuzumab with the cytotoxic agent DM1 (emtansine), a potent microtubule inhibitor and a derivative of maytansine, in a single structure (Figure 3).
Lapatinib
Lapatinib is an oral small-molecule tyrosine kinase inhibitor of EGFR (HER1) and HER2 receptors. It is approved in combination with capecitabine for patients with HER2-expressing metastatic breast cancer who previously received trastuzumab, an anthracycline, and a taxane chemotherapy or T-DM1. Lapatinib is also approved in combination with letrozole in postmenopausal women with HER2-positive, hormone receptor–positive metastatic disease, although it is unclear where this regimen would fit in the current schema. It may be considered for patients with hormone receptor–positive disease who are not candidates for therapy with taxane-trastuzumab and T-DM1 or who decline this therapy. Diarrhea is seen in most patients treated with lapatinib and may be severe in 20% of cases when lapatinib is combined with capecitabine. Decreases in LVEF have been reported and cardiac function monitoring at baseline and periodically may be considered.69,70 Lapatinib is not approved for use in adjuvant settings.
Neratinib
Neratinib is an oral small-molecule irreversible tyrosine kinase inhibitor of HER1, HER2, and HER4. It is currently approved only for extended adjuvant therapy after completion of 1 year of standard trastuzumab therapy. It is given orally every day for 1 year. The main side effect, expected in nearly all patients, is diarrhea, which can be severe in up to 40% of patients and may lead to dehydration and electrolyte imbalance. Diarrhea usually starts early in the course of therapy and can be most intense during the first cycle. Therefore, prophylactic antidiarrheal therapy is recommended to reduce the intensity of diarrhea. Loperamide prophylaxis may be initiated simultaneously for all patients using a tapering schedule. Drug interruption or dose reduction may be required if diarrhea is severe or refractory.21,71 Neratinib is not FDA-approved in the metastatic settings.
Conclusion
HER2-positive tumors represent a distinct subset(s) of breast tumors with unique pathological and clinical characteristics. Treatment with a combination of cytotoxic chemotherapy and HER2-targeted agents has led to a dramatic improvement in survival for patients with locoregional and advanced disease. Trastuzumab is an integral part of adjuvant therapy for HER2-positive invasive disease. Pertuzumab should be added to trastuzumab in node-positive disease. Neratinib may be considered after completion of trastuzumab therapy in patients with hormone receptor–positive disease. For metastatic HER2-positive breast cancer, a regimen consisting of docetaxel plus trastuzumab and pertuzumab is the standard first-line therapy. Ado-trastuzumab is an ideal next line option for patients whose disease progresses on trastuzumab and taxanes.
1. Yedjou CG, Tchounwou PB, Payton M, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health 2017;14(5).
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89.
3. Huang HJ, Neven P, Drijkoningen M, et al. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol 2005;58:611–6.
4. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41.
5. Cronin KA, Harlan LC, Dodd KW, et al. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest 2010;28:963–-8.
6. Huang HJ, Neven P, Drijkoningen M, et al. Hormone receptors do not predict the HER2/neu status in all age groups of women with an operable breast cancer. Ann Oncol 2005;16:1755–61.
7. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502.
8. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744–52.
9. Brennan PJ, Kumagai T, Berezov A, et al. HER2/neu: mechanisms of dimerization/oligomerization. Oncogene 2000;19:6093–101.
10. Roskoski R Jr. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun 2004;319:1–11.
11. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013.
12. Ravaioli A, Pasini G, Polselli A, et al. Staging of breast cancer: new recommended standard procedure. Breast Cancer Res Treat 2002;72:53–60.
13. Puglisi F, Follador A, Minisini AM, et al. Baseline staging tests after a new diagnosis of breast cancer: further evidence of their limited indications. Ann Oncol 2005;16:263–6.
14. FDA approves trastuzumab biosimilar. Cancer Discov 2018;8:130.
15. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134–41.
16. Tolaney SM, Barry WT, Guo H, Dillon D, et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC) [ASCO abstract]. J Clin Oncol. 2017;35(suppl):511.
17. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83.
18. Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC -> T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC -> TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+early breast cancer [SABC abstract]. Cancer Res 2016;76(4 supplement):S5-04.
19. Jahanzeb M. Adjuvant trastuzumab therapy for HER2-positive breast cancer. Clin Breast Cancer 2008;8:324–33.
20. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195–205.
21. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122–31.
22. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:367–77.
23. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1688–700.
24. Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 2013;14:741–8.
25. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021–8.
26. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–84.
27. Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer 2018;89:27–35
28. de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol 2014;32:2159–65.
29. Dowsett M, Harper-Wynne C, Boeddinghaus I, et al. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res 2001;61:8452–8.
30. Nahta R, O’Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat 2012;135:39–48.
31. Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Pract Radiat Oncol 2016;6:e219-e34.
32. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 2016;34:611–35.
33. Zeichner SB, Herna S, Mani A, et al. Survival of patients with de-novo metastatic breast cancer: analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Cancer Res Treat 2015;153:617–24.
34. Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 2010;21:2169–74.
35. Savci-Heijink CD, Halfwerk H, Hooijer GK, et al. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat 2015;150:547–57.
36. Kimbung S, Loman N, Hedenfalk I. Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol 2015;35:85–95.
37. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972–7.
38. Burstein HJ, Lieberman G, Slamon DJ, et al. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol 2005;16:1772–7.
39. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724–34.
40. Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 2012;30:2601–8.
41. Guarneri V, Giovannelli S, Ficarra G, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist 2008;13:838–44.
42. Salkeni MA, Hall SJ. Metastatic breast cancer: Endocrine therapy landscape reshaped. Avicenna J Med 2017;7:144–52.
43. Dang C, Iyengar N, Datko F, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015;33:442–7.
44. Cantini L, Pistelli M, Savini A, et al. Long-responders to anti-HER2 therapies: A case report and review of the literature. Mol Clin Oncol 2018;8:147–52.
45. Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer 2016;69:216–22.
46. Falkson G, Holcroft C, Gelman RS, et al. Ten-year follow-up study of premenopausal women with metastatic breast cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 1995;13:1453–8.
47. Boccardo F, Rubagotti A, Perrotta A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol 1994;5:337–42.
48 Taylor CW, Green S, Dalton WS, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol 1998;16:994–9.
49. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91.
50. Dieras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732–42.
51. Dzimitrowicz H, Berger M, Vargo C, et al. T-DM1 Activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol 2016;34:3511–7.
52. Fabi A, Giannarelli D, Moscetti L, et al. Ado-trastuzumab emtansine (T-DM1) in HER2+ advanced breast cancer patients: does pretreatment with pertuzumab matter? Future Oncol 2017;13:2791–7.
53. Madden R, Kosari S, Peterson GM, et al. Lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer: A systematic review. Int J Clin Pharmacol Ther 2018;56:72–80.
54. Pivot X, Manikhas A, Zurawski B, et al. CEREBEL (EGF111438): A phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015;33:1564–73.
55. Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:2078–99.
56. Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39–51.
57. Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416–21.
58. Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf 2008;31:459–67.
59. Guenancia C, Lefebvre A, Cardinale D, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol 2016;34:3157–65.
60. Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol 2016;34:1030–3.
61. Brann AM, Cobleigh MA, Okwuosa TM. Cardiovascular monitoring with trastuzumab therapy: how frequent is too frequent? JAMA Oncol 2016;2:1123–4.
62. Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859–65.
63. Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010;28:3422–8.
64. Yamashita-Kashima Y, Shu S, Yorozu K, et al. Mode of action of pertuzumab in combination with trastuzumab plus docetaxel therapy in a HER2-positive breast cancer xenograft model. Oncol Lett 2017;14:4197–205.
65. Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer 2017;117:1736–42.
66. Girish S, Gupta M, Wang B, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 2012;69:1229–40.
67. Uppal H, Doudement E, Mahapatra K, et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin Cancer Res 2015;21:123–33.
68. Yan H, Endo Y, Shen Y, et al. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther 2016;15:480–90.
69. Spector NL, Xia W, Burris H 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 2005;23:2502–12.
70. Johnston S, Pippen J Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27:5538–46.
71. Neratinib (Nerlynx) for HER2-positive breast cancer. Med Lett Drugs Ther 2018;60(1539):23.
Introduction
Breast cancer is the second leading cause of cancer deaths among women in the United States, according to the SEER database. It is estimated that 1 in 8 women will be diagnosed with breast cancer at some point during their lifetime (12.4% lifetime risk).1,2 Because breast tumors are clinically and histopathologically heterogeneous, different diagnostic and therapeutic approaches are required for each subtype. Among the subtypes, tumors that are positive for human epidermal growth factor receptor 2 (HER2) account for approximately 15% to 20% of all newly diagnosed localized and metastatic invasive breast tumors.3,4 Historically, this subset of tumors has been considered the most aggressive due to a higher propensity to relapse and metastasize, translating into poorer prognosis compared with other subtypes.5–7 However, with the advent of HER2-targeted therapy in the late 1990s, prognosis has significantly improved for both early- and late-stage HER2-positive tumors.8
Pathogenesis
The HER2 proto-oncogene belongs to a family of human epidermal growth factor receptors that includes 4 transmembrane tyrosine kinase receptors: HER1 (also commonly known as epidermal growth factor receptor, EGFR), HER2, HER3, and HER4. Another commonly used nomenclature for this family of receptors is ERBB1 to ERBB4. Each of the receptors has a similar structure consisting of a growth factor–binding extracellular domain, a single transmembrane segment, an intracellular protein-tyrosine kinase catalytic domain, and a tyrosine-containing cytoplasmic tail. Activation of the extracellular domain leads to conformational changes that initiate a cascade of reactions resulting in protein kinase activation. ERBB tyrosine receptor kinases subsequently activate several intracellular pathways that are critical for cellular function and survival, including the PI3K-AKT, RAS-MAPK, and mTOR pathways. Hyperactivation or overexpression of these receptors leads to uncontrolled cell growth and proliferation, and eventually cancerogenesis.9,10
HER2 gene amplification can cause activation of the receptor’s extramembranous domain by way of either dimerization of two HER2 receptors or heterodimerization with other ERBB family receptors, leading to ligand-independent activation of cell signaling (ie, activation in the absence of external growth factors). Besides breast cancer, HER2 protein is overexpressed in several other tumor types, including esophageal and gastric adenocarcinomas, colon and gynecological malignancies, and to a lesser extent in other malignancies.
Biomarker Testing
All patients with newly diagnosed breast cancer should have their tumor tissue submitted for biomarker testing for estrogen receptors (ER), progesterone receptors (PR), and HER2 overexpression, as the result this testing dictates therapy choices. The purpose of HER2 testing is to investigate whether the HER2 gene, located on chromosome 17, is overexpressed or amplified. HER2 status provides the basis for treatment selection, which impacts long-term outcome measures such as recurrence and survival. Routine testing of carcinoma in situ for HER2 expression/amplification is not recommended and has no implication on choice of therapy at this time.
In 2013, the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) updated their clinical guideline recommendations for HER2 testing in breast cancer to improve its accuracy and its utility as a predictive marker.11 There are currently 2 approved modalities for HER2 testing: detection of HER2 protein overexpression by i
Fluorescence in-situ hybridization (FISH) testing assesses for HER2 amplification by determining the number of HER2 signals and
Neoadjuvant and Adjuvant Therapy for Locoregional Disease
Case Patient 1
A 56-year-old woman undergoes ultrasound-guided biopsy of a self-palpated breast lump. Pathology shows invasive ductal carcinoma that is ER-positive, PR-negative, and HER2 equivocal by IHC (2+ staining). Follow-up FISH testing shows a HER2/CEP17 ratio of 2.5. The tumor is estimated to be 2 cm in diameter by imaging and exam with no clinically palpable axillary lymphadenopathy. The patient exhibits no constitutional or localized symptoms concerning for metastases.
- What is the recommended management approach for this patient?
According to the ASCO/CAP guidelines, this patient’s tumor qualifies as HER2-positive based upon testing results showing amplification of the gene. This result has important implications for management since nearly all patients with macroscopically invasive HER2-positive tumors should be considered for adjuvant chemotherapy in combination with anti-HER2 therapy. The patient should proceed with upfront tumor resection and sentinel lymph node biopsy. Systemic staging imaging (ie, computed tomography [CT] or bone scan) is not indicated in early stage breast cancer.12,13 Systemic staging scans are indicated when (1) any anatomical stage III disease is suspected (eg, with involvement of the skin or chest wall, the presence of enlarged matted or fixed axillary lymph nodes, and involvement of nodal stations other than in the axilla), and (2) when symptoms or abnormal laboratory values raise suspicion for distant metastases (eg, unexplained bone pain, unintentional weight loss, elevated serum alkaline phosphatase, and transaminitis).
Case 1 Continued
The patient presents to discuss treatment options after undergoing a lumpectomy and sentinel node biopsy procedure. The pathology report notes a single focus of carcinoma measuring 2 cm with negative sentinel lymph nodes.
- What agents are used for adjuvant therapy in HER2-postive breast cancer?
Nearly all patients with macroscopically invasive (> 1 mm) breast carcinoma should be considered for adjuvant therapy using a regimen that contains a taxane and trastuzumab. However, the benefit may be small for patients with tumors ≤ 5 mm (T1a, N0), so it is important to carefully weigh the risk against the benefit. Among the agents that targeting HER2, only trastuzumab has been shown to improve overall survival (OS) in the adjuvant setting; long-term follow-up data are awaited for other agents.8 A trastuzumab biosimilar, trastuzumab-dkst, was recently approved by the US Food and Drug Administration (FDA) for the same indications as trastuzumab.14 The regimens most commonly used in the adjuvant and neoadjuvant settings for nonmetastatic breast cancer are summarized in Table 2.
Patients with small (≤ 3 cm), node-negative tumors can generally be considered for a reduced-intensity regimen that includes weekly paclitaxel plus trastuzumab. This combination proved efficacious in a single-group, multicenter study that enrolled 406 patients.15 Paclitaxel and trastuzumab were given once weekly for 12 weeks, followed by trastuzumab, either weekly or every 3 weeks, to complete 1 year of therapy.After a median follow-up of more than 6 years, the rates of distant and locoregional recurrence were 1% and 1.2%, respectively.16
A combination of docetaxel, carboplatin, and trastuzumab is a nonanthracycline regimen that is also appropriate in this setting, based on the results of the Breast Cancer International Research Group 006 (BCIRG-006) trial.17 This phase 3 randomized trial enrolled 3222 women with HER2-positive, invasive, high-risk adenocarcinoma. Eligible patients had a T1–3 tumor and either lymph node–negative or –positive disease and were randomly assigned to receive 1 of 3 regimens: group 1 received doxorubicin and cyclophosphamide every 3 weeks for 4 cycles followed by docetaxel every 3 weeks for 4 cycles (AC-T); group 2 received the AC-T regimen in combination with trastuzumab; and group 3 received docetaxel, carboplatin, and trastuzumab once every 3 weeks for 6 cycles (TCH). Groups 2 and 3 also received trastuzumab for an additional 34 weeks to complete 1 year of therapy. Trastuzumab-containing regimens were found to offer superior disease-free survival (DFS) and OS. When comparing the 2 trastuzumab arms after more than 10 years of follow-up, no statistically significant advantage of an anthracycline regimen over a nonanthracycline regimen was found.18 Furthermore, the anthracycline arm had a fivefold higher incidence of symptomatic congestive heart failure (grades 3 and 4), and the nonanthracycline regimen was associated with a lower incidence of treatment-related leukemia, a clinically significant finding despite not reaching statistical significance due to low overall numbers.
BCIRG-006, NSABP B-31, NCCTG N9831, and HERA are all large randomized trials with consistent results confirming trastuzumab’s role in reducing recurrence and improving survival in HER2-positive breast cancer in the adjuvant settings. The estimated overall benefit from addition of this agent was a 34% to 41% improvement in survival and a 33% to 52% improvement in DFS.8,17–20
Dual anti-HER2 therapy containing both trastuzumab and pertuzumab should be strongly considered for patients with macroscopic lymph node involvement based on the results of the APHINITY trial.21 In this study, the addition of pertuzumab to standard trastuzumab-based therapy led to a significant improvement in invasive-disease-free survival at 3 years. In subgroup analysis, the benefit was restricted to the node-positive group (3-year invasive-disease-free survival rates of 92% in the pertuzumab group versus 90.2% in the placebo group, P = 0.02). Patients with hormone receptor–negative disease derived greater benefit from the addition of pertuzumab. Regimens used in the APHINITY trial included the anti-HER2 agents trastuzumab and pertuzumab in combination with 1 of the following chemotherapy regimens: sequential cyclophosphamide plus either doxorubicin or epirubicin, followed by either 4 cycles of docetaxel or 12 weekly doses of paclitaxel; sequential fluorouracil plus either epirubicin or doxorubicin plus cyclophosphamide (3 or 4 cycles), followed by 3 or 4 cycles of docetaxel or 12 weekly cycles of paclitaxel; or 6 cycles of concurrent docetaxel plus carboplatin.
One-year therapy with neratinib, an oral tyrosine kinase inhibitor of HER2, is now approved by the FDA after completion of trastuzumab in the adjuvant setting, based on the results of the ExteNET trial.22 In this study, patients who had completed trastuzumab within the preceding 12 months, without evidence of recurrence, were randomly assigned to receive either oral neratinib or placebo daily for 1 year. The 2-year DFS rate was 93.9% and 91.6% for the neratinib and placebo groups, respectively. The most common adverse effect of neratinib was diarrhea, with approximately 40% of patients experiencing grade 3 diarrhea. In subgroup analyses, hormone receptor–positive patients derived the most benefit, while hormone receptor–negative patients derived no or marginal benefit.22 OS benefit has not yet been established.23
Trastuzumab therapy (with pertuzumab if indicated) should be offered for an optimal duration of 12 months (17 cycles, including those given with chemotherapy backbone). A shorter duration of therapy, 6 months, has been shown to be inferior,24 while a longer duration, 24 months, has been shown to provide no additional benefit.25
Finally, sequential addition of anti-estrogen endocrine therapy is indicated for hormone-positive tumors. Endocrine therapy is usually added after completion of the chemotherapy backbone of the regimen, but may be given concurrently with anti-HER2 therapy. If radiation is being administered, endocrine therapy can be given concurrently or started after radiation therapy is completed.
Case 1 Conclusion
The patient can be offered 1 of 2 adjuvant treatment regimens, either TH or TCH (Table 2). Since the patient had lumpectomy, she is an appropriate candidate for adjuvant radiation, which would be started after completion of the chemotherapy backbone (taxane/platinum). Endocrine therapy for at least 5 years should be offered sequentially or concurrently with radiation. Her long-term prognosis is very favorable.
Case Patient 2
A 43-year-old woman presents with a 4-cm breast mass, a separate skin nodule, and palpable matted axillary lymphadenopathy. Biopsies of the breast mass and subcutaneous nodule reveal invasive ductal carcinoma that is ER-negative, PR-negative, and HER2-positive by IHC (3+ staining). Based on clinical findings, the patient is staged as T4b (separate tumor nodule), N2 (matted axillary lymph nodes). Systemic staging with CT scan of the chest, abdomen, and pelvis shows no evidence of distant metastases.
- What is the recommended approach to management for this patient?
Recommendations for neoadjuvant therapy, given before definitive surgery, follow the same path as with other subtypes of breast cancer. Patients with suspected anatomical stage III disease are strongly encouraged to undergo upfront (neoadjuvant) chemotherapy in combination with HER2-targeted agents. In addition, all HER2-positive patients with clinically node-positive disease can be offered neoadjuvant therapy using chemotherapy plus dual anti-HER2 therapy (trastuzumab and pertuzumab), with complete pathological response expected in more than 60% of patients.26,27 Because this patient has locally advanced disease, especially skin involvement and matted axillary nodes, she should undergo neoadjuvant therapy. Preferred regimens contain both trastuzumab and pertuzumab in combination with cytotoxic chemotherapy. The latter may be given concurrently (nonanthracycline regimens, such as docetaxel plus carboplatin) or sequentially (anthracycline-based regimens), as outlined in Table 2. Administration of anthracyclines and trastuzumab simultaneously is contraindicated due to increased risk of cardiomyopathy.28
Endocrine therapy is not indicated for this patient per the current standard of care because the tumor was ER- and PR-negative. Had the tumor been hormone receptor–positive, endocrine therapy for a minimum of 5 years would have been indicated. Likewise, in the case of hormone receptor–positive disease, 12 months of neratinib therapy after completion of trastuzumab may add further benefit, as shown in the ExteNET trial.22,23 Neratinib seems to have a propensity to prevent or delay trastuzumab-induced overexpression of estrogen receptors. This is mainly due to hormone receptor/HER2 crosstalk, a potential mechanism of resistance to trastuzumab.29,30
In addition to the medical therapy options discussed here, this patient would be expected to benefit from adjuvant radiation to the breast and regional lymph nodes, given the presence of T4 disease and bulky adenopathy in the axilla.31
Case 2 Conclusion
The patient undergoes neoadjuvant treatment (docetaxel, carboplatin, trastuzumab, and pertuzumab every 21 days for a total of 6 cycles), followed by surgical resection (modified radical mastectomy) that reveals complete pathological response (no residual invasive carcinoma). Subsequently, she receives radiation therapy to the primary tumor site and regional lymph nodes while continuing trastuzumab and pertuzumab for 11 more cycles (17 total). Despite presenting with locally advanced disease, the patient has a favorable overall prognosis due to an excellent pathological response.
- What is the approach to follow-up after completion of primary therapy?
Patients may follow up every 3 to 6 months for clinical evaluation in the first 5 years after completing primary adjuvant therapy. An annual screening mammogram is recommended as well. Body imaging can be done if dictated by symptoms. However, routine CT, positron emission tomography, or bone scans are not recommended as part of follow-up in the absence of symptoms, mainly because of a lack of evidence that such surveillance improves survival.32
Metastatic HER2-Positive Breast Cancer
Metastatic breast cancer most commonly presents as a distant recurrence of previously treated local disease. However, 6% to 18% of patients have no prior history of breast cancer and present with de novo metastatic disease.33,34 The most commonly involved distant organs are the skeletal bones, liver, lung, distant lymph node stations, and brain. Compared to other subtypes, HER2-positive tumors have an increased tendency to involve the central nervous system.35–38 Although metastatic HER2-positive breast cancer is not considered curable, significant improvement in survival has been achieved, and patients with metastatic disease have median survival approaching 5 years.39
Case Presentation 3
A 69-year-old woman with a history of breast cancer 4 years ago presents with new-onset back pain and unintentional weight loss. On exam, she is found to have palpable axillary adenopathy on the same side as her previous cancer. Her initial disease was stage IIB ER-positive and HER2-positive and was treated with chemotherapy, mastectomy, and anastrozole, which the patient is still taking. She undergoes CT scan of the chest, abdomen, and pelvis and radionucleotide bone scan, which show multiple liver and bony lesions suspicious for metastatic disease. Axillary lymph node biopsy confirms recurrent invasive carcinoma that is ER-positive and HER2-positive by IHC (3+).
- What is the approach to management of a patient who presents with symptoms of recurrent HER2-positive disease?
This patient likely has metastatic breast cancer based on the imaging findings. In such cases, a biopsy of the recurrent disease should always be considered, if feasible, to confirm the diagnosis and rule out other etiologies such as different malignances and benign conditions. Hormone-receptor and HER2 testing should also be performed on recurrent disease, since a change in HER2 status can be seen in 15% to 33% of cases.40–42
Based on data from the phase 3 CLEOPATRA trial, first-line systemic regimens for patients with metastatic breast cancer that is positive for HER2 should consist of a combination of docetaxel, trastuzumab, and pertuzumab. Compared to placebo, adding pertuzumab yielded superior progression-free survival of 18.4 months (versus 12.4 months for placebo) and an unprecedented OS of 56.5 months (versus 40.8 for placebo).39 Weekly paclitaxel can replace docetaxel with comparable efficacy (Table 3).43
Patients can develop significant neuropathy as well as skin and nail changes after multiple cycles of taxane-based chemotherapy. Therefore, the taxane backbone may be dropped after 6 to 8 cycles, while patients continue the trastuzumab and pertuzumab combination until disease progression or unacceptable toxicity. Some patients may enjoy remarkable long-term survival on “maintenance” anti-HER2 therapy.44 Despite lack of high-level evidence, such as from large randomized trials, some experts recommend the addition of a hormone blocker after discontinuation of the taxane in ER-positive tumors.45
Premenopausal and perimenopausal women with hormone receptor–positive metastatic disease should be considered for simultaneous ovarian suppression. Ovarian suppression can be accomplished medically using a gonadotropin-releasing hormone agonist (goserelin) or surgically via salpingo-oophorectomy.46–48
Case 3 Conclusion
The patient receives 6 cycles of docetaxel, trastuzumab, and pertuzumab, after which the docetaxel is discontinued due to neuropathy while she continues the other 2 agents. After 26 months of disease control, the patient is found to have new liver metastatic lesions, indicating progression of disease.
- What therapeutic options are available for this patient?
Patients whose disease progresses after receiving taxane- and trastuzumab-containing regimens are candidates to receive the novel antibody-drug conjugate ado-trastuzumab emtansine (T-DM1). Early progressors (ie, patients with early stage disease who have progression of disease while receiving adjuvant trastuzumab or within 6 months of completion of adjuvant trastuzumab) are also candidates for T-DM1. Treatment usually fits in the second line or beyond based on data from the EMILIA trial, which randomly assigned patients to receive either capecitabine plus lapatinib or T-DM1.49,50 Progression-free survival in the T-DM1 group was 9.6 months versus 6.4 months for the comparator. Improvement of 4 months in OS persisted with longer follow-up despite a crossover rate of 27%. Furthermore, a significantly higher objective response rate and fewer adverse effects were reported in the T-DM1 patients. Most patients included in the EMILIA trial were pertuzumab-naive. However, the benefit of T-DM1 appears to persist, albeit to a lesser extent, for pertuzumab-pretreated patients.51,52
Patients in whom treatment fails with 2 or more lines of therapy containing taxane-trastuzumab (with or without pertuzumab) and T-DM1 are candidates to receive a combination of capecitabine and lapatinib, a TKI, in the third line and beyond. Similarly, the combination of capecitabine with trastuzumab in the same settings appears to have equal efficacy.53,54 Trastuzumab may be continued beyond progression while changing the single-agent chemotherapy drug for subsequent lines of therapy, per ASCO guidelines,55 although improvement in OS has not been demonstrated beyond the third line in a large randomized trial (Table 3).
Approved HER2-Targeted Drugs
HER2-directed therapy is implemented in the management of nearly all stages of HER2-positive invasive breast cancer, including early and late stages (Table 4).
Trastuzumab
Trastuzumab was the first anti-HER2 agent to be approved by the FDA in 1998. It is a humanized monoclonal antibody directed against the extracellular domain of the HER2 receptor (domain IV). Trastuzumab functions by interrupting HER2 signal transduction and by flagging tumor cells for immune destruction.56 Cardiotoxicity, usually manifested as left ventricular systolic dysfunction, is the most noteworthy adverse effect of trastuzumab. The most prominent risk factors for cardiomyopathy in patients receiving trastuzumab are low baseline ejection fraction (< 55%), age > 50 years, co-administration and higher cumulative dose of anthracyclines, and increased body mass index and obesity.57–59 Whether patients receive therapy in the neoadjuvant, adjuvant, or metastatic settings, baseline cardiac function assessment with echocardiogram or multiple-gated acquisition scan is required. While well-designed randomized trials validating the value and frequency of monitoring are lacking, repeated cardiac testing every 3 months is generally recommended for patients undergoing adjuvant therapy. Patients with metastatic disease who are receiving treatment with palliative intent may be monitored less frequently.60,61
An asymptomatic drop in ejection fraction is the most common manifestation of cardiac toxicity. Other cardiac manifestations have also been reported with much less frequency, including arrhythmias, severe congestive heart failure, ventricular thrombus formation, and even cardiac death. Until monitoring and dose-adjustment guidelines are issued, the guidance provided in the FDA-approved prescribing information should be followed, which recommends holding trastuzumab when there is ≥ 16% absolute reduction in left ventricular ejection fraction (LVEF) from the baseline value; or if the LVEF value is below the institutional lower limit of normal and the drop is ≥ 10%. After holding the drug, cardiac function can be re-evaluated every 4 weeks. In most patients, trastuzumab-induced cardiotoxicity can be reversed by withholding trastuzumab and initiating cardioprotective therapy, although the latter remains controversial. Re-challenging after recovery of ejection fraction is possible and toxicity does not appear to be proportional to cumulative dose. Cardiomyopathy due to trastuzumab therapy is potentially reversible within 6 months in more than 80% of cases.28,57,60–63
Other notable adverse effects of trastuzumab include pulmonary toxicity (such as interstitial lung disease) and infusion reactions (usually during or within 24 hours of first dose).
Pertuzumab
Pertuzumab is another humanized monoclonal antibody directed to a different extracellular domain of the HER2 receptor, the dimerization domain (domain II), which is responsible for heterodimerization of HER2 with other HER receptors, especially HER3. This agent should always be co-administered with trastuzumab as the 2 drugs produce synergistic anti-tumor effect, without competition for the receptor. Activation of HER3, via dimerization with HER2, produces an alternative mechanism of downstream signaling, even in the presence of trastuzumab and in the absence of growth factors (Figure 2).
Ado-Trastuzumab Emtansine
Ado-trastuzumab emtansine (T-DM1) is an antibody-drug conjugate that combines the monoclonal antibody trastuzumab with the cytotoxic agent DM1 (emtansine), a potent microtubule inhibitor and a derivative of maytansine, in a single structure (Figure 3).
Lapatinib
Lapatinib is an oral small-molecule tyrosine kinase inhibitor of EGFR (HER1) and HER2 receptors. It is approved in combination with capecitabine for patients with HER2-expressing metastatic breast cancer who previously received trastuzumab, an anthracycline, and a taxane chemotherapy or T-DM1. Lapatinib is also approved in combination with letrozole in postmenopausal women with HER2-positive, hormone receptor–positive metastatic disease, although it is unclear where this regimen would fit in the current schema. It may be considered for patients with hormone receptor–positive disease who are not candidates for therapy with taxane-trastuzumab and T-DM1 or who decline this therapy. Diarrhea is seen in most patients treated with lapatinib and may be severe in 20% of cases when lapatinib is combined with capecitabine. Decreases in LVEF have been reported and cardiac function monitoring at baseline and periodically may be considered.69,70 Lapatinib is not approved for use in adjuvant settings.
Neratinib
Neratinib is an oral small-molecule irreversible tyrosine kinase inhibitor of HER1, HER2, and HER4. It is currently approved only for extended adjuvant therapy after completion of 1 year of standard trastuzumab therapy. It is given orally every day for 1 year. The main side effect, expected in nearly all patients, is diarrhea, which can be severe in up to 40% of patients and may lead to dehydration and electrolyte imbalance. Diarrhea usually starts early in the course of therapy and can be most intense during the first cycle. Therefore, prophylactic antidiarrheal therapy is recommended to reduce the intensity of diarrhea. Loperamide prophylaxis may be initiated simultaneously for all patients using a tapering schedule. Drug interruption or dose reduction may be required if diarrhea is severe or refractory.21,71 Neratinib is not FDA-approved in the metastatic settings.
Conclusion
HER2-positive tumors represent a distinct subset(s) of breast tumors with unique pathological and clinical characteristics. Treatment with a combination of cytotoxic chemotherapy and HER2-targeted agents has led to a dramatic improvement in survival for patients with locoregional and advanced disease. Trastuzumab is an integral part of adjuvant therapy for HER2-positive invasive disease. Pertuzumab should be added to trastuzumab in node-positive disease. Neratinib may be considered after completion of trastuzumab therapy in patients with hormone receptor–positive disease. For metastatic HER2-positive breast cancer, a regimen consisting of docetaxel plus trastuzumab and pertuzumab is the standard first-line therapy. Ado-trastuzumab is an ideal next line option for patients whose disease progresses on trastuzumab and taxanes.
Introduction
Breast cancer is the second leading cause of cancer deaths among women in the United States, according to the SEER database. It is estimated that 1 in 8 women will be diagnosed with breast cancer at some point during their lifetime (12.4% lifetime risk).1,2 Because breast tumors are clinically and histopathologically heterogeneous, different diagnostic and therapeutic approaches are required for each subtype. Among the subtypes, tumors that are positive for human epidermal growth factor receptor 2 (HER2) account for approximately 15% to 20% of all newly diagnosed localized and metastatic invasive breast tumors.3,4 Historically, this subset of tumors has been considered the most aggressive due to a higher propensity to relapse and metastasize, translating into poorer prognosis compared with other subtypes.5–7 However, with the advent of HER2-targeted therapy in the late 1990s, prognosis has significantly improved for both early- and late-stage HER2-positive tumors.8
Pathogenesis
The HER2 proto-oncogene belongs to a family of human epidermal growth factor receptors that includes 4 transmembrane tyrosine kinase receptors: HER1 (also commonly known as epidermal growth factor receptor, EGFR), HER2, HER3, and HER4. Another commonly used nomenclature for this family of receptors is ERBB1 to ERBB4. Each of the receptors has a similar structure consisting of a growth factor–binding extracellular domain, a single transmembrane segment, an intracellular protein-tyrosine kinase catalytic domain, and a tyrosine-containing cytoplasmic tail. Activation of the extracellular domain leads to conformational changes that initiate a cascade of reactions resulting in protein kinase activation. ERBB tyrosine receptor kinases subsequently activate several intracellular pathways that are critical for cellular function and survival, including the PI3K-AKT, RAS-MAPK, and mTOR pathways. Hyperactivation or overexpression of these receptors leads to uncontrolled cell growth and proliferation, and eventually cancerogenesis.9,10
HER2 gene amplification can cause activation of the receptor’s extramembranous domain by way of either dimerization of two HER2 receptors or heterodimerization with other ERBB family receptors, leading to ligand-independent activation of cell signaling (ie, activation in the absence of external growth factors). Besides breast cancer, HER2 protein is overexpressed in several other tumor types, including esophageal and gastric adenocarcinomas, colon and gynecological malignancies, and to a lesser extent in other malignancies.
Biomarker Testing
All patients with newly diagnosed breast cancer should have their tumor tissue submitted for biomarker testing for estrogen receptors (ER), progesterone receptors (PR), and HER2 overexpression, as the result this testing dictates therapy choices. The purpose of HER2 testing is to investigate whether the HER2 gene, located on chromosome 17, is overexpressed or amplified. HER2 status provides the basis for treatment selection, which impacts long-term outcome measures such as recurrence and survival. Routine testing of carcinoma in situ for HER2 expression/amplification is not recommended and has no implication on choice of therapy at this time.
In 2013, the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) updated their clinical guideline recommendations for HER2 testing in breast cancer to improve its accuracy and its utility as a predictive marker.11 There are currently 2 approved modalities for HER2 testing: detection of HER2 protein overexpression by i
Fluorescence in-situ hybridization (FISH) testing assesses for HER2 amplification by determining the number of HER2 signals and
Neoadjuvant and Adjuvant Therapy for Locoregional Disease
Case Patient 1
A 56-year-old woman undergoes ultrasound-guided biopsy of a self-palpated breast lump. Pathology shows invasive ductal carcinoma that is ER-positive, PR-negative, and HER2 equivocal by IHC (2+ staining). Follow-up FISH testing shows a HER2/CEP17 ratio of 2.5. The tumor is estimated to be 2 cm in diameter by imaging and exam with no clinically palpable axillary lymphadenopathy. The patient exhibits no constitutional or localized symptoms concerning for metastases.
- What is the recommended management approach for this patient?
According to the ASCO/CAP guidelines, this patient’s tumor qualifies as HER2-positive based upon testing results showing amplification of the gene. This result has important implications for management since nearly all patients with macroscopically invasive HER2-positive tumors should be considered for adjuvant chemotherapy in combination with anti-HER2 therapy. The patient should proceed with upfront tumor resection and sentinel lymph node biopsy. Systemic staging imaging (ie, computed tomography [CT] or bone scan) is not indicated in early stage breast cancer.12,13 Systemic staging scans are indicated when (1) any anatomical stage III disease is suspected (eg, with involvement of the skin or chest wall, the presence of enlarged matted or fixed axillary lymph nodes, and involvement of nodal stations other than in the axilla), and (2) when symptoms or abnormal laboratory values raise suspicion for distant metastases (eg, unexplained bone pain, unintentional weight loss, elevated serum alkaline phosphatase, and transaminitis).
Case 1 Continued
The patient presents to discuss treatment options after undergoing a lumpectomy and sentinel node biopsy procedure. The pathology report notes a single focus of carcinoma measuring 2 cm with negative sentinel lymph nodes.
- What agents are used for adjuvant therapy in HER2-postive breast cancer?
Nearly all patients with macroscopically invasive (> 1 mm) breast carcinoma should be considered for adjuvant therapy using a regimen that contains a taxane and trastuzumab. However, the benefit may be small for patients with tumors ≤ 5 mm (T1a, N0), so it is important to carefully weigh the risk against the benefit. Among the agents that targeting HER2, only trastuzumab has been shown to improve overall survival (OS) in the adjuvant setting; long-term follow-up data are awaited for other agents.8 A trastuzumab biosimilar, trastuzumab-dkst, was recently approved by the US Food and Drug Administration (FDA) for the same indications as trastuzumab.14 The regimens most commonly used in the adjuvant and neoadjuvant settings for nonmetastatic breast cancer are summarized in Table 2.
Patients with small (≤ 3 cm), node-negative tumors can generally be considered for a reduced-intensity regimen that includes weekly paclitaxel plus trastuzumab. This combination proved efficacious in a single-group, multicenter study that enrolled 406 patients.15 Paclitaxel and trastuzumab were given once weekly for 12 weeks, followed by trastuzumab, either weekly or every 3 weeks, to complete 1 year of therapy.After a median follow-up of more than 6 years, the rates of distant and locoregional recurrence were 1% and 1.2%, respectively.16
A combination of docetaxel, carboplatin, and trastuzumab is a nonanthracycline regimen that is also appropriate in this setting, based on the results of the Breast Cancer International Research Group 006 (BCIRG-006) trial.17 This phase 3 randomized trial enrolled 3222 women with HER2-positive, invasive, high-risk adenocarcinoma. Eligible patients had a T1–3 tumor and either lymph node–negative or –positive disease and were randomly assigned to receive 1 of 3 regimens: group 1 received doxorubicin and cyclophosphamide every 3 weeks for 4 cycles followed by docetaxel every 3 weeks for 4 cycles (AC-T); group 2 received the AC-T regimen in combination with trastuzumab; and group 3 received docetaxel, carboplatin, and trastuzumab once every 3 weeks for 6 cycles (TCH). Groups 2 and 3 also received trastuzumab for an additional 34 weeks to complete 1 year of therapy. Trastuzumab-containing regimens were found to offer superior disease-free survival (DFS) and OS. When comparing the 2 trastuzumab arms after more than 10 years of follow-up, no statistically significant advantage of an anthracycline regimen over a nonanthracycline regimen was found.18 Furthermore, the anthracycline arm had a fivefold higher incidence of symptomatic congestive heart failure (grades 3 and 4), and the nonanthracycline regimen was associated with a lower incidence of treatment-related leukemia, a clinically significant finding despite not reaching statistical significance due to low overall numbers.
BCIRG-006, NSABP B-31, NCCTG N9831, and HERA are all large randomized trials with consistent results confirming trastuzumab’s role in reducing recurrence and improving survival in HER2-positive breast cancer in the adjuvant settings. The estimated overall benefit from addition of this agent was a 34% to 41% improvement in survival and a 33% to 52% improvement in DFS.8,17–20
Dual anti-HER2 therapy containing both trastuzumab and pertuzumab should be strongly considered for patients with macroscopic lymph node involvement based on the results of the APHINITY trial.21 In this study, the addition of pertuzumab to standard trastuzumab-based therapy led to a significant improvement in invasive-disease-free survival at 3 years. In subgroup analysis, the benefit was restricted to the node-positive group (3-year invasive-disease-free survival rates of 92% in the pertuzumab group versus 90.2% in the placebo group, P = 0.02). Patients with hormone receptor–negative disease derived greater benefit from the addition of pertuzumab. Regimens used in the APHINITY trial included the anti-HER2 agents trastuzumab and pertuzumab in combination with 1 of the following chemotherapy regimens: sequential cyclophosphamide plus either doxorubicin or epirubicin, followed by either 4 cycles of docetaxel or 12 weekly doses of paclitaxel; sequential fluorouracil plus either epirubicin or doxorubicin plus cyclophosphamide (3 or 4 cycles), followed by 3 or 4 cycles of docetaxel or 12 weekly cycles of paclitaxel; or 6 cycles of concurrent docetaxel plus carboplatin.
One-year therapy with neratinib, an oral tyrosine kinase inhibitor of HER2, is now approved by the FDA after completion of trastuzumab in the adjuvant setting, based on the results of the ExteNET trial.22 In this study, patients who had completed trastuzumab within the preceding 12 months, without evidence of recurrence, were randomly assigned to receive either oral neratinib or placebo daily for 1 year. The 2-year DFS rate was 93.9% and 91.6% for the neratinib and placebo groups, respectively. The most common adverse effect of neratinib was diarrhea, with approximately 40% of patients experiencing grade 3 diarrhea. In subgroup analyses, hormone receptor–positive patients derived the most benefit, while hormone receptor–negative patients derived no or marginal benefit.22 OS benefit has not yet been established.23
Trastuzumab therapy (with pertuzumab if indicated) should be offered for an optimal duration of 12 months (17 cycles, including those given with chemotherapy backbone). A shorter duration of therapy, 6 months, has been shown to be inferior,24 while a longer duration, 24 months, has been shown to provide no additional benefit.25
Finally, sequential addition of anti-estrogen endocrine therapy is indicated for hormone-positive tumors. Endocrine therapy is usually added after completion of the chemotherapy backbone of the regimen, but may be given concurrently with anti-HER2 therapy. If radiation is being administered, endocrine therapy can be given concurrently or started after radiation therapy is completed.
Case 1 Conclusion
The patient can be offered 1 of 2 adjuvant treatment regimens, either TH or TCH (Table 2). Since the patient had lumpectomy, she is an appropriate candidate for adjuvant radiation, which would be started after completion of the chemotherapy backbone (taxane/platinum). Endocrine therapy for at least 5 years should be offered sequentially or concurrently with radiation. Her long-term prognosis is very favorable.
Case Patient 2
A 43-year-old woman presents with a 4-cm breast mass, a separate skin nodule, and palpable matted axillary lymphadenopathy. Biopsies of the breast mass and subcutaneous nodule reveal invasive ductal carcinoma that is ER-negative, PR-negative, and HER2-positive by IHC (3+ staining). Based on clinical findings, the patient is staged as T4b (separate tumor nodule), N2 (matted axillary lymph nodes). Systemic staging with CT scan of the chest, abdomen, and pelvis shows no evidence of distant metastases.
- What is the recommended approach to management for this patient?
Recommendations for neoadjuvant therapy, given before definitive surgery, follow the same path as with other subtypes of breast cancer. Patients with suspected anatomical stage III disease are strongly encouraged to undergo upfront (neoadjuvant) chemotherapy in combination with HER2-targeted agents. In addition, all HER2-positive patients with clinically node-positive disease can be offered neoadjuvant therapy using chemotherapy plus dual anti-HER2 therapy (trastuzumab and pertuzumab), with complete pathological response expected in more than 60% of patients.26,27 Because this patient has locally advanced disease, especially skin involvement and matted axillary nodes, she should undergo neoadjuvant therapy. Preferred regimens contain both trastuzumab and pertuzumab in combination with cytotoxic chemotherapy. The latter may be given concurrently (nonanthracycline regimens, such as docetaxel plus carboplatin) or sequentially (anthracycline-based regimens), as outlined in Table 2. Administration of anthracyclines and trastuzumab simultaneously is contraindicated due to increased risk of cardiomyopathy.28
Endocrine therapy is not indicated for this patient per the current standard of care because the tumor was ER- and PR-negative. Had the tumor been hormone receptor–positive, endocrine therapy for a minimum of 5 years would have been indicated. Likewise, in the case of hormone receptor–positive disease, 12 months of neratinib therapy after completion of trastuzumab may add further benefit, as shown in the ExteNET trial.22,23 Neratinib seems to have a propensity to prevent or delay trastuzumab-induced overexpression of estrogen receptors. This is mainly due to hormone receptor/HER2 crosstalk, a potential mechanism of resistance to trastuzumab.29,30
In addition to the medical therapy options discussed here, this patient would be expected to benefit from adjuvant radiation to the breast and regional lymph nodes, given the presence of T4 disease and bulky adenopathy in the axilla.31
Case 2 Conclusion
The patient undergoes neoadjuvant treatment (docetaxel, carboplatin, trastuzumab, and pertuzumab every 21 days for a total of 6 cycles), followed by surgical resection (modified radical mastectomy) that reveals complete pathological response (no residual invasive carcinoma). Subsequently, she receives radiation therapy to the primary tumor site and regional lymph nodes while continuing trastuzumab and pertuzumab for 11 more cycles (17 total). Despite presenting with locally advanced disease, the patient has a favorable overall prognosis due to an excellent pathological response.
- What is the approach to follow-up after completion of primary therapy?
Patients may follow up every 3 to 6 months for clinical evaluation in the first 5 years after completing primary adjuvant therapy. An annual screening mammogram is recommended as well. Body imaging can be done if dictated by symptoms. However, routine CT, positron emission tomography, or bone scans are not recommended as part of follow-up in the absence of symptoms, mainly because of a lack of evidence that such surveillance improves survival.32
Metastatic HER2-Positive Breast Cancer
Metastatic breast cancer most commonly presents as a distant recurrence of previously treated local disease. However, 6% to 18% of patients have no prior history of breast cancer and present with de novo metastatic disease.33,34 The most commonly involved distant organs are the skeletal bones, liver, lung, distant lymph node stations, and brain. Compared to other subtypes, HER2-positive tumors have an increased tendency to involve the central nervous system.35–38 Although metastatic HER2-positive breast cancer is not considered curable, significant improvement in survival has been achieved, and patients with metastatic disease have median survival approaching 5 years.39
Case Presentation 3
A 69-year-old woman with a history of breast cancer 4 years ago presents with new-onset back pain and unintentional weight loss. On exam, she is found to have palpable axillary adenopathy on the same side as her previous cancer. Her initial disease was stage IIB ER-positive and HER2-positive and was treated with chemotherapy, mastectomy, and anastrozole, which the patient is still taking. She undergoes CT scan of the chest, abdomen, and pelvis and radionucleotide bone scan, which show multiple liver and bony lesions suspicious for metastatic disease. Axillary lymph node biopsy confirms recurrent invasive carcinoma that is ER-positive and HER2-positive by IHC (3+).
- What is the approach to management of a patient who presents with symptoms of recurrent HER2-positive disease?
This patient likely has metastatic breast cancer based on the imaging findings. In such cases, a biopsy of the recurrent disease should always be considered, if feasible, to confirm the diagnosis and rule out other etiologies such as different malignances and benign conditions. Hormone-receptor and HER2 testing should also be performed on recurrent disease, since a change in HER2 status can be seen in 15% to 33% of cases.40–42
Based on data from the phase 3 CLEOPATRA trial, first-line systemic regimens for patients with metastatic breast cancer that is positive for HER2 should consist of a combination of docetaxel, trastuzumab, and pertuzumab. Compared to placebo, adding pertuzumab yielded superior progression-free survival of 18.4 months (versus 12.4 months for placebo) and an unprecedented OS of 56.5 months (versus 40.8 for placebo).39 Weekly paclitaxel can replace docetaxel with comparable efficacy (Table 3).43
Patients can develop significant neuropathy as well as skin and nail changes after multiple cycles of taxane-based chemotherapy. Therefore, the taxane backbone may be dropped after 6 to 8 cycles, while patients continue the trastuzumab and pertuzumab combination until disease progression or unacceptable toxicity. Some patients may enjoy remarkable long-term survival on “maintenance” anti-HER2 therapy.44 Despite lack of high-level evidence, such as from large randomized trials, some experts recommend the addition of a hormone blocker after discontinuation of the taxane in ER-positive tumors.45
Premenopausal and perimenopausal women with hormone receptor–positive metastatic disease should be considered for simultaneous ovarian suppression. Ovarian suppression can be accomplished medically using a gonadotropin-releasing hormone agonist (goserelin) or surgically via salpingo-oophorectomy.46–48
Case 3 Conclusion
The patient receives 6 cycles of docetaxel, trastuzumab, and pertuzumab, after which the docetaxel is discontinued due to neuropathy while she continues the other 2 agents. After 26 months of disease control, the patient is found to have new liver metastatic lesions, indicating progression of disease.
- What therapeutic options are available for this patient?
Patients whose disease progresses after receiving taxane- and trastuzumab-containing regimens are candidates to receive the novel antibody-drug conjugate ado-trastuzumab emtansine (T-DM1). Early progressors (ie, patients with early stage disease who have progression of disease while receiving adjuvant trastuzumab or within 6 months of completion of adjuvant trastuzumab) are also candidates for T-DM1. Treatment usually fits in the second line or beyond based on data from the EMILIA trial, which randomly assigned patients to receive either capecitabine plus lapatinib or T-DM1.49,50 Progression-free survival in the T-DM1 group was 9.6 months versus 6.4 months for the comparator. Improvement of 4 months in OS persisted with longer follow-up despite a crossover rate of 27%. Furthermore, a significantly higher objective response rate and fewer adverse effects were reported in the T-DM1 patients. Most patients included in the EMILIA trial were pertuzumab-naive. However, the benefit of T-DM1 appears to persist, albeit to a lesser extent, for pertuzumab-pretreated patients.51,52
Patients in whom treatment fails with 2 or more lines of therapy containing taxane-trastuzumab (with or without pertuzumab) and T-DM1 are candidates to receive a combination of capecitabine and lapatinib, a TKI, in the third line and beyond. Similarly, the combination of capecitabine with trastuzumab in the same settings appears to have equal efficacy.53,54 Trastuzumab may be continued beyond progression while changing the single-agent chemotherapy drug for subsequent lines of therapy, per ASCO guidelines,55 although improvement in OS has not been demonstrated beyond the third line in a large randomized trial (Table 3).
Approved HER2-Targeted Drugs
HER2-directed therapy is implemented in the management of nearly all stages of HER2-positive invasive breast cancer, including early and late stages (Table 4).
Trastuzumab
Trastuzumab was the first anti-HER2 agent to be approved by the FDA in 1998. It is a humanized monoclonal antibody directed against the extracellular domain of the HER2 receptor (domain IV). Trastuzumab functions by interrupting HER2 signal transduction and by flagging tumor cells for immune destruction.56 Cardiotoxicity, usually manifested as left ventricular systolic dysfunction, is the most noteworthy adverse effect of trastuzumab. The most prominent risk factors for cardiomyopathy in patients receiving trastuzumab are low baseline ejection fraction (< 55%), age > 50 years, co-administration and higher cumulative dose of anthracyclines, and increased body mass index and obesity.57–59 Whether patients receive therapy in the neoadjuvant, adjuvant, or metastatic settings, baseline cardiac function assessment with echocardiogram or multiple-gated acquisition scan is required. While well-designed randomized trials validating the value and frequency of monitoring are lacking, repeated cardiac testing every 3 months is generally recommended for patients undergoing adjuvant therapy. Patients with metastatic disease who are receiving treatment with palliative intent may be monitored less frequently.60,61
An asymptomatic drop in ejection fraction is the most common manifestation of cardiac toxicity. Other cardiac manifestations have also been reported with much less frequency, including arrhythmias, severe congestive heart failure, ventricular thrombus formation, and even cardiac death. Until monitoring and dose-adjustment guidelines are issued, the guidance provided in the FDA-approved prescribing information should be followed, which recommends holding trastuzumab when there is ≥ 16% absolute reduction in left ventricular ejection fraction (LVEF) from the baseline value; or if the LVEF value is below the institutional lower limit of normal and the drop is ≥ 10%. After holding the drug, cardiac function can be re-evaluated every 4 weeks. In most patients, trastuzumab-induced cardiotoxicity can be reversed by withholding trastuzumab and initiating cardioprotective therapy, although the latter remains controversial. Re-challenging after recovery of ejection fraction is possible and toxicity does not appear to be proportional to cumulative dose. Cardiomyopathy due to trastuzumab therapy is potentially reversible within 6 months in more than 80% of cases.28,57,60–63
Other notable adverse effects of trastuzumab include pulmonary toxicity (such as interstitial lung disease) and infusion reactions (usually during or within 24 hours of first dose).
Pertuzumab
Pertuzumab is another humanized monoclonal antibody directed to a different extracellular domain of the HER2 receptor, the dimerization domain (domain II), which is responsible for heterodimerization of HER2 with other HER receptors, especially HER3. This agent should always be co-administered with trastuzumab as the 2 drugs produce synergistic anti-tumor effect, without competition for the receptor. Activation of HER3, via dimerization with HER2, produces an alternative mechanism of downstream signaling, even in the presence of trastuzumab and in the absence of growth factors (Figure 2).
Ado-Trastuzumab Emtansine
Ado-trastuzumab emtansine (T-DM1) is an antibody-drug conjugate that combines the monoclonal antibody trastuzumab with the cytotoxic agent DM1 (emtansine), a potent microtubule inhibitor and a derivative of maytansine, in a single structure (Figure 3).
Lapatinib
Lapatinib is an oral small-molecule tyrosine kinase inhibitor of EGFR (HER1) and HER2 receptors. It is approved in combination with capecitabine for patients with HER2-expressing metastatic breast cancer who previously received trastuzumab, an anthracycline, and a taxane chemotherapy or T-DM1. Lapatinib is also approved in combination with letrozole in postmenopausal women with HER2-positive, hormone receptor–positive metastatic disease, although it is unclear where this regimen would fit in the current schema. It may be considered for patients with hormone receptor–positive disease who are not candidates for therapy with taxane-trastuzumab and T-DM1 or who decline this therapy. Diarrhea is seen in most patients treated with lapatinib and may be severe in 20% of cases when lapatinib is combined with capecitabine. Decreases in LVEF have been reported and cardiac function monitoring at baseline and periodically may be considered.69,70 Lapatinib is not approved for use in adjuvant settings.
Neratinib
Neratinib is an oral small-molecule irreversible tyrosine kinase inhibitor of HER1, HER2, and HER4. It is currently approved only for extended adjuvant therapy after completion of 1 year of standard trastuzumab therapy. It is given orally every day for 1 year. The main side effect, expected in nearly all patients, is diarrhea, which can be severe in up to 40% of patients and may lead to dehydration and electrolyte imbalance. Diarrhea usually starts early in the course of therapy and can be most intense during the first cycle. Therefore, prophylactic antidiarrheal therapy is recommended to reduce the intensity of diarrhea. Loperamide prophylaxis may be initiated simultaneously for all patients using a tapering schedule. Drug interruption or dose reduction may be required if diarrhea is severe or refractory.21,71 Neratinib is not FDA-approved in the metastatic settings.
Conclusion
HER2-positive tumors represent a distinct subset(s) of breast tumors with unique pathological and clinical characteristics. Treatment with a combination of cytotoxic chemotherapy and HER2-targeted agents has led to a dramatic improvement in survival for patients with locoregional and advanced disease. Trastuzumab is an integral part of adjuvant therapy for HER2-positive invasive disease. Pertuzumab should be added to trastuzumab in node-positive disease. Neratinib may be considered after completion of trastuzumab therapy in patients with hormone receptor–positive disease. For metastatic HER2-positive breast cancer, a regimen consisting of docetaxel plus trastuzumab and pertuzumab is the standard first-line therapy. Ado-trastuzumab is an ideal next line option for patients whose disease progresses on trastuzumab and taxanes.
1. Yedjou CG, Tchounwou PB, Payton M, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health 2017;14(5).
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89.
3. Huang HJ, Neven P, Drijkoningen M, et al. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol 2005;58:611–6.
4. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41.
5. Cronin KA, Harlan LC, Dodd KW, et al. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest 2010;28:963–-8.
6. Huang HJ, Neven P, Drijkoningen M, et al. Hormone receptors do not predict the HER2/neu status in all age groups of women with an operable breast cancer. Ann Oncol 2005;16:1755–61.
7. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502.
8. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744–52.
9. Brennan PJ, Kumagai T, Berezov A, et al. HER2/neu: mechanisms of dimerization/oligomerization. Oncogene 2000;19:6093–101.
10. Roskoski R Jr. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun 2004;319:1–11.
11. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013.
12. Ravaioli A, Pasini G, Polselli A, et al. Staging of breast cancer: new recommended standard procedure. Breast Cancer Res Treat 2002;72:53–60.
13. Puglisi F, Follador A, Minisini AM, et al. Baseline staging tests after a new diagnosis of breast cancer: further evidence of their limited indications. Ann Oncol 2005;16:263–6.
14. FDA approves trastuzumab biosimilar. Cancer Discov 2018;8:130.
15. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134–41.
16. Tolaney SM, Barry WT, Guo H, Dillon D, et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC) [ASCO abstract]. J Clin Oncol. 2017;35(suppl):511.
17. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83.
18. Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC -> T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC -> TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+early breast cancer [SABC abstract]. Cancer Res 2016;76(4 supplement):S5-04.
19. Jahanzeb M. Adjuvant trastuzumab therapy for HER2-positive breast cancer. Clin Breast Cancer 2008;8:324–33.
20. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195–205.
21. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122–31.
22. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:367–77.
23. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1688–700.
24. Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 2013;14:741–8.
25. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021–8.
26. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–84.
27. Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer 2018;89:27–35
28. de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol 2014;32:2159–65.
29. Dowsett M, Harper-Wynne C, Boeddinghaus I, et al. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res 2001;61:8452–8.
30. Nahta R, O’Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat 2012;135:39–48.
31. Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Pract Radiat Oncol 2016;6:e219-e34.
32. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 2016;34:611–35.
33. Zeichner SB, Herna S, Mani A, et al. Survival of patients with de-novo metastatic breast cancer: analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Cancer Res Treat 2015;153:617–24.
34. Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 2010;21:2169–74.
35. Savci-Heijink CD, Halfwerk H, Hooijer GK, et al. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat 2015;150:547–57.
36. Kimbung S, Loman N, Hedenfalk I. Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol 2015;35:85–95.
37. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972–7.
38. Burstein HJ, Lieberman G, Slamon DJ, et al. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol 2005;16:1772–7.
39. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724–34.
40. Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 2012;30:2601–8.
41. Guarneri V, Giovannelli S, Ficarra G, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist 2008;13:838–44.
42. Salkeni MA, Hall SJ. Metastatic breast cancer: Endocrine therapy landscape reshaped. Avicenna J Med 2017;7:144–52.
43. Dang C, Iyengar N, Datko F, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015;33:442–7.
44. Cantini L, Pistelli M, Savini A, et al. Long-responders to anti-HER2 therapies: A case report and review of the literature. Mol Clin Oncol 2018;8:147–52.
45. Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer 2016;69:216–22.
46. Falkson G, Holcroft C, Gelman RS, et al. Ten-year follow-up study of premenopausal women with metastatic breast cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 1995;13:1453–8.
47. Boccardo F, Rubagotti A, Perrotta A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol 1994;5:337–42.
48 Taylor CW, Green S, Dalton WS, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol 1998;16:994–9.
49. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91.
50. Dieras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732–42.
51. Dzimitrowicz H, Berger M, Vargo C, et al. T-DM1 Activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol 2016;34:3511–7.
52. Fabi A, Giannarelli D, Moscetti L, et al. Ado-trastuzumab emtansine (T-DM1) in HER2+ advanced breast cancer patients: does pretreatment with pertuzumab matter? Future Oncol 2017;13:2791–7.
53. Madden R, Kosari S, Peterson GM, et al. Lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer: A systematic review. Int J Clin Pharmacol Ther 2018;56:72–80.
54. Pivot X, Manikhas A, Zurawski B, et al. CEREBEL (EGF111438): A phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015;33:1564–73.
55. Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:2078–99.
56. Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39–51.
57. Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416–21.
58. Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf 2008;31:459–67.
59. Guenancia C, Lefebvre A, Cardinale D, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol 2016;34:3157–65.
60. Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol 2016;34:1030–3.
61. Brann AM, Cobleigh MA, Okwuosa TM. Cardiovascular monitoring with trastuzumab therapy: how frequent is too frequent? JAMA Oncol 2016;2:1123–4.
62. Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859–65.
63. Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010;28:3422–8.
64. Yamashita-Kashima Y, Shu S, Yorozu K, et al. Mode of action of pertuzumab in combination with trastuzumab plus docetaxel therapy in a HER2-positive breast cancer xenograft model. Oncol Lett 2017;14:4197–205.
65. Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer 2017;117:1736–42.
66. Girish S, Gupta M, Wang B, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 2012;69:1229–40.
67. Uppal H, Doudement E, Mahapatra K, et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin Cancer Res 2015;21:123–33.
68. Yan H, Endo Y, Shen Y, et al. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther 2016;15:480–90.
69. Spector NL, Xia W, Burris H 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 2005;23:2502–12.
70. Johnston S, Pippen J Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27:5538–46.
71. Neratinib (Nerlynx) for HER2-positive breast cancer. Med Lett Drugs Ther 2018;60(1539):23.
1. Yedjou CG, Tchounwou PB, Payton M, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health 2017;14(5).
2. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271–89.
3. Huang HJ, Neven P, Drijkoningen M, et al. Association between tumour characteristics and HER-2/neu by immunohistochemistry in 1362 women with primary operable breast cancer. J Clin Pathol 2005;58:611–6.
4. Noone AM, Cronin KA, Altekruse SF, et al. Cancer incidence and survival trends by subtype using data from the Surveillance Epidemiology and End Results Program, 1992-2013. Cancer Epidemiol Biomarkers Prev 2017;26:632–41.
5. Cronin KA, Harlan LC, Dodd KW, et al. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US. Cancer Invest 2010;28:963–-8.
6. Huang HJ, Neven P, Drijkoningen M, et al. Hormone receptors do not predict the HER2/neu status in all age groups of women with an operable breast cancer. Ann Oncol 2005;16:1755–61.
7. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502.
8. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014;32:3744–52.
9. Brennan PJ, Kumagai T, Berezov A, et al. HER2/neu: mechanisms of dimerization/oligomerization. Oncogene 2000;19:6093–101.
10. Roskoski R Jr. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun 2004;319:1–11.
11. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013.
12. Ravaioli A, Pasini G, Polselli A, et al. Staging of breast cancer: new recommended standard procedure. Breast Cancer Res Treat 2002;72:53–60.
13. Puglisi F, Follador A, Minisini AM, et al. Baseline staging tests after a new diagnosis of breast cancer: further evidence of their limited indications. Ann Oncol 2005;16:263–6.
14. FDA approves trastuzumab biosimilar. Cancer Discov 2018;8:130.
15. Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134–41.
16. Tolaney SM, Barry WT, Guo H, Dillon D, et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC) [ASCO abstract]. J Clin Oncol. 2017;35(suppl):511.
17. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273–83.
18. Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC -> T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC -> TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+early breast cancer [SABC abstract]. Cancer Res 2016;76(4 supplement):S5-04.
19. Jahanzeb M. Adjuvant trastuzumab therapy for HER2-positive breast cancer. Clin Breast Cancer 2008;8:324–33.
20. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017;389:1195–205.
21. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122–31.
22. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:367–77.
23. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1688–700.
24. Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 2013;14:741–8.
25. Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 2013;382:1021–8.
26. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–84.
27. Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer 2018;89:27–35
28. de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol 2014;32:2159–65.
29. Dowsett M, Harper-Wynne C, Boeddinghaus I, et al. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res 2001;61:8452–8.
30. Nahta R, O’Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat 2012;135:39–48.
31. Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. Pract Radiat Oncol 2016;6:e219-e34.
32. Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 2016;34:611–35.
33. Zeichner SB, Herna S, Mani A, et al. Survival of patients with de-novo metastatic breast cancer: analysis of data from a large breast cancer-specific private practice, a university-based cancer center and review of the literature. Breast Cancer Res Treat 2015;153:617–24.
34. Dawood S, Broglio K, Ensor J, et al. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 2010;21:2169–74.
35. Savci-Heijink CD, Halfwerk H, Hooijer GK, et al. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat 2015;150:547–57.
36. Kimbung S, Loman N, Hedenfalk I. Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol 2015;35:85–95.
37. Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972–7.
38. Burstein HJ, Lieberman G, Slamon DJ, et al. Isolated central nervous system metastases in patients with HER2-overexpressing advanced breast cancer treated with first-line trastuzumab-based therapy. Ann Oncol 2005;16:1772–7.
39. Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724–34.
40. Lindstrom LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol 2012;30:2601–8.
41. Guarneri V, Giovannelli S, Ficarra G, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: impact on patient management. Oncologist 2008;13:838–44.
42. Salkeni MA, Hall SJ. Metastatic breast cancer: Endocrine therapy landscape reshaped. Avicenna J Med 2017;7:144–52.
43. Dang C, Iyengar N, Datko F, et al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015;33:442–7.
44. Cantini L, Pistelli M, Savini A, et al. Long-responders to anti-HER2 therapies: A case report and review of the literature. Mol Clin Oncol 2018;8:147–52.
45. Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer 2016;69:216–22.
46. Falkson G, Holcroft C, Gelman RS, et al. Ten-year follow-up study of premenopausal women with metastatic breast cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 1995;13:1453–8.
47. Boccardo F, Rubagotti A, Perrotta A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol 1994;5:337–42.
48 Taylor CW, Green S, Dalton WS, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol 1998;16:994–9.
49. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91.
50. Dieras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:732–42.
51. Dzimitrowicz H, Berger M, Vargo C, et al. T-DM1 Activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol 2016;34:3511–7.
52. Fabi A, Giannarelli D, Moscetti L, et al. Ado-trastuzumab emtansine (T-DM1) in HER2+ advanced breast cancer patients: does pretreatment with pertuzumab matter? Future Oncol 2017;13:2791–7.
53. Madden R, Kosari S, Peterson GM, et al. Lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer: A systematic review. Int J Clin Pharmacol Ther 2018;56:72–80.
54. Pivot X, Manikhas A, Zurawski B, et al. CEREBEL (EGF111438): A phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2015;33:1564–73.
55. Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:2078–99.
56. Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39–51.
57. Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 2010;28:3416–21.
58. Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf 2008;31:459–67.
59. Guenancia C, Lefebvre A, Cardinale D, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol 2016;34:3157–65.
60. Dang CT, Yu AF, Jones LW, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol 2016;34:1030–3.
61. Brann AM, Cobleigh MA, Okwuosa TM. Cardiovascular monitoring with trastuzumab therapy: how frequent is too frequent? JAMA Oncol 2016;2:1123–4.
62. Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007;25:3859–65.
63. Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010;28:3422–8.
64. Yamashita-Kashima Y, Shu S, Yorozu K, et al. Mode of action of pertuzumab in combination with trastuzumab plus docetaxel therapy in a HER2-positive breast cancer xenograft model. Oncol Lett 2017;14:4197–205.
65. Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer 2017;117:1736–42.
66. Girish S, Gupta M, Wang B, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol 2012;69:1229–40.
67. Uppal H, Doudement E, Mahapatra K, et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin Cancer Res 2015;21:123–33.
68. Yan H, Endo Y, Shen Y, et al. Ado-trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther 2016;15:480–90.
69. Spector NL, Xia W, Burris H 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 2005;23:2502–12.
70. Johnston S, Pippen J Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 2009;27:5538–46.
71. Neratinib (Nerlynx) for HER2-positive breast cancer. Med Lett Drugs Ther 2018;60(1539):23.
PI3K inhibitor/fulvestrant has modest benefit, serious toxicity in breast cancer
CHICAGO – Is two months of progression-free survival worth it if those months mean living with serious side effects?
For women with advanced estrogen receptor-positive, HER2-negative breast cancer, the combination of the PI3K inhibitor taselisib and the selective estrogen receptor modifier fulvestrant (Faslodex) bought two additional months of PFS, compared with fulvestrant alone, but at the cost of serious toxicities in half the patients treated with the combination, results of the SANDPIPER trial show.
“These results are positive, but I think we all agree they are modest,” lead investigator José Baselga, MD, PhD from Memorial Sloan Kettering Cancer Center in New York, said at a briefing at the annual meeting of the American Society of Clinical Oncology.
The “challenging tolerability” of the combination led to frequent treatment discontinuations, and may have limited the clinical benefit of the combination, he said, but added that the study serves as proof of principle that PI3K may be a bona fide target in advanced breast cancer.
ASCO expert Harold Burstein, MD, from the Dana-Farber Cancer Institute in Boston agreed that PI3K “is a very appealing target. It’s a mutation that probably is the most common in breast cancer when you do genomic sequencing, and it arises in other tumors as well.”
He likened the study findings, however, to a key opening a locked door, only to find that there is a chain latch on the other side preventing entry.
In an interview, Dr. Burstein said that despite the best efforts of Dr. Baselga and others to find a suitable approach to targeting the PI3K pathway, the evidence to date suggests that it may not be an important driver of breast cancer.
Taselisib is the first agent in its class to specifically block the PI3K alpha isoform that is found to be mutated in approximately 40% of advanced ER-positive breast cancer. The agent has been shown to offer clinical benefits in early trials for patients with head and neck and some gynecologic cancers.
In the phase 3 SANDPIPER trial, 516 women with locally advanced or metastatic ER-positive, HER2-negative breast cancer that had progressed or recurred following aromatase inhibitor therapy were enrolled and randomly assigned on a 2:1 basis to receive fulvestrant plus taselisib (340 patients) or fulvestrant plus a placebo (176 patients).
As noted, the median progression-free survival was 7.4 months for women who received the combination, compared with 5.4 months for controls. The stratified hazard ratio was 0.70 favoring taselisib (P = .0037).
But also as noted, the addition of taselisib “clearly led to toxicity,” Dr. Baselga said.
Serious adverse events occurred in 32% of patients in the fulvestrant/taselisib group, compared with 8.9% of controls. Grade 3 or greater side effects occurred in 49.5% vs. 16.4%, respectively, and side effects leading to discontinuation of taselisib occurred in 16.8% of patients, vs. 2.3% of those on placebo.
The primary toxicities were gastrointestinal effects, especially diarrhea, which occurred in 60.1% vs. 19.7% of patients (all grades). Hyperglycemia occurred in 40.4% of patients on taselisib, vs. 9.4% on placebo.
Dr. Baselga noted that the secondary endpoints of overall response rate, clinical benefit rate, and duration of response all favored taselisib.
Asked whether taselisib was the right agent in this setting, given the commercial availability of at least two other PI3K inhibitors – idelalisib (Zydelig) and copanlisib (Aliqopa) – Dr. Baselga agreed that another, more specific agent may offer similar or better efficacy with fewer off-target effects. He noted that taselisib is highly active against the alpha isoforms of PI3K, but also hits the delta and gamma isoforms.
“The side effects that we see that are limiting patients staying on [taselisib] are mostly delta and gamma. So I do think that in the case of breast cancer, what we need to do is to work on more specific alpha inhibitors that will be safer,” he said.
During the oral abstracts session where Dr. Baselga presented the SANDPIPER results, Cynthia X Ma, MD, PhD, from Washington University School of Medicine in St. Louis, the invited discussant, agreed that the trial provides proof of concept that PI3K inhibition may be an effective therapeutic strategy in breast cancer.
“However, the modest progression-free survival improvement and significant toxicity profile does not support its clinical application,” she said.
SOURCE: : Baselga J et al. ASCO 2018 Abstract LBA1006 .
CHICAGO – Is two months of progression-free survival worth it if those months mean living with serious side effects?
For women with advanced estrogen receptor-positive, HER2-negative breast cancer, the combination of the PI3K inhibitor taselisib and the selective estrogen receptor modifier fulvestrant (Faslodex) bought two additional months of PFS, compared with fulvestrant alone, but at the cost of serious toxicities in half the patients treated with the combination, results of the SANDPIPER trial show.
“These results are positive, but I think we all agree they are modest,” lead investigator José Baselga, MD, PhD from Memorial Sloan Kettering Cancer Center in New York, said at a briefing at the annual meeting of the American Society of Clinical Oncology.
The “challenging tolerability” of the combination led to frequent treatment discontinuations, and may have limited the clinical benefit of the combination, he said, but added that the study serves as proof of principle that PI3K may be a bona fide target in advanced breast cancer.
ASCO expert Harold Burstein, MD, from the Dana-Farber Cancer Institute in Boston agreed that PI3K “is a very appealing target. It’s a mutation that probably is the most common in breast cancer when you do genomic sequencing, and it arises in other tumors as well.”
He likened the study findings, however, to a key opening a locked door, only to find that there is a chain latch on the other side preventing entry.
In an interview, Dr. Burstein said that despite the best efforts of Dr. Baselga and others to find a suitable approach to targeting the PI3K pathway, the evidence to date suggests that it may not be an important driver of breast cancer.
Taselisib is the first agent in its class to specifically block the PI3K alpha isoform that is found to be mutated in approximately 40% of advanced ER-positive breast cancer. The agent has been shown to offer clinical benefits in early trials for patients with head and neck and some gynecologic cancers.
In the phase 3 SANDPIPER trial, 516 women with locally advanced or metastatic ER-positive, HER2-negative breast cancer that had progressed or recurred following aromatase inhibitor therapy were enrolled and randomly assigned on a 2:1 basis to receive fulvestrant plus taselisib (340 patients) or fulvestrant plus a placebo (176 patients).
As noted, the median progression-free survival was 7.4 months for women who received the combination, compared with 5.4 months for controls. The stratified hazard ratio was 0.70 favoring taselisib (P = .0037).
But also as noted, the addition of taselisib “clearly led to toxicity,” Dr. Baselga said.
Serious adverse events occurred in 32% of patients in the fulvestrant/taselisib group, compared with 8.9% of controls. Grade 3 or greater side effects occurred in 49.5% vs. 16.4%, respectively, and side effects leading to discontinuation of taselisib occurred in 16.8% of patients, vs. 2.3% of those on placebo.
The primary toxicities were gastrointestinal effects, especially diarrhea, which occurred in 60.1% vs. 19.7% of patients (all grades). Hyperglycemia occurred in 40.4% of patients on taselisib, vs. 9.4% on placebo.
Dr. Baselga noted that the secondary endpoints of overall response rate, clinical benefit rate, and duration of response all favored taselisib.
Asked whether taselisib was the right agent in this setting, given the commercial availability of at least two other PI3K inhibitors – idelalisib (Zydelig) and copanlisib (Aliqopa) – Dr. Baselga agreed that another, more specific agent may offer similar or better efficacy with fewer off-target effects. He noted that taselisib is highly active against the alpha isoforms of PI3K, but also hits the delta and gamma isoforms.
“The side effects that we see that are limiting patients staying on [taselisib] are mostly delta and gamma. So I do think that in the case of breast cancer, what we need to do is to work on more specific alpha inhibitors that will be safer,” he said.
During the oral abstracts session where Dr. Baselga presented the SANDPIPER results, Cynthia X Ma, MD, PhD, from Washington University School of Medicine in St. Louis, the invited discussant, agreed that the trial provides proof of concept that PI3K inhibition may be an effective therapeutic strategy in breast cancer.
“However, the modest progression-free survival improvement and significant toxicity profile does not support its clinical application,” she said.
SOURCE: : Baselga J et al. ASCO 2018 Abstract LBA1006 .
CHICAGO – Is two months of progression-free survival worth it if those months mean living with serious side effects?
For women with advanced estrogen receptor-positive, HER2-negative breast cancer, the combination of the PI3K inhibitor taselisib and the selective estrogen receptor modifier fulvestrant (Faslodex) bought two additional months of PFS, compared with fulvestrant alone, but at the cost of serious toxicities in half the patients treated with the combination, results of the SANDPIPER trial show.
“These results are positive, but I think we all agree they are modest,” lead investigator José Baselga, MD, PhD from Memorial Sloan Kettering Cancer Center in New York, said at a briefing at the annual meeting of the American Society of Clinical Oncology.
The “challenging tolerability” of the combination led to frequent treatment discontinuations, and may have limited the clinical benefit of the combination, he said, but added that the study serves as proof of principle that PI3K may be a bona fide target in advanced breast cancer.
ASCO expert Harold Burstein, MD, from the Dana-Farber Cancer Institute in Boston agreed that PI3K “is a very appealing target. It’s a mutation that probably is the most common in breast cancer when you do genomic sequencing, and it arises in other tumors as well.”
He likened the study findings, however, to a key opening a locked door, only to find that there is a chain latch on the other side preventing entry.
In an interview, Dr. Burstein said that despite the best efforts of Dr. Baselga and others to find a suitable approach to targeting the PI3K pathway, the evidence to date suggests that it may not be an important driver of breast cancer.
Taselisib is the first agent in its class to specifically block the PI3K alpha isoform that is found to be mutated in approximately 40% of advanced ER-positive breast cancer. The agent has been shown to offer clinical benefits in early trials for patients with head and neck and some gynecologic cancers.
In the phase 3 SANDPIPER trial, 516 women with locally advanced or metastatic ER-positive, HER2-negative breast cancer that had progressed or recurred following aromatase inhibitor therapy were enrolled and randomly assigned on a 2:1 basis to receive fulvestrant plus taselisib (340 patients) or fulvestrant plus a placebo (176 patients).
As noted, the median progression-free survival was 7.4 months for women who received the combination, compared with 5.4 months for controls. The stratified hazard ratio was 0.70 favoring taselisib (P = .0037).
But also as noted, the addition of taselisib “clearly led to toxicity,” Dr. Baselga said.
Serious adverse events occurred in 32% of patients in the fulvestrant/taselisib group, compared with 8.9% of controls. Grade 3 or greater side effects occurred in 49.5% vs. 16.4%, respectively, and side effects leading to discontinuation of taselisib occurred in 16.8% of patients, vs. 2.3% of those on placebo.
The primary toxicities were gastrointestinal effects, especially diarrhea, which occurred in 60.1% vs. 19.7% of patients (all grades). Hyperglycemia occurred in 40.4% of patients on taselisib, vs. 9.4% on placebo.
Dr. Baselga noted that the secondary endpoints of overall response rate, clinical benefit rate, and duration of response all favored taselisib.
Asked whether taselisib was the right agent in this setting, given the commercial availability of at least two other PI3K inhibitors – idelalisib (Zydelig) and copanlisib (Aliqopa) – Dr. Baselga agreed that another, more specific agent may offer similar or better efficacy with fewer off-target effects. He noted that taselisib is highly active against the alpha isoforms of PI3K, but also hits the delta and gamma isoforms.
“The side effects that we see that are limiting patients staying on [taselisib] are mostly delta and gamma. So I do think that in the case of breast cancer, what we need to do is to work on more specific alpha inhibitors that will be safer,” he said.
During the oral abstracts session where Dr. Baselga presented the SANDPIPER results, Cynthia X Ma, MD, PhD, from Washington University School of Medicine in St. Louis, the invited discussant, agreed that the trial provides proof of concept that PI3K inhibition may be an effective therapeutic strategy in breast cancer.
“However, the modest progression-free survival improvement and significant toxicity profile does not support its clinical application,” she said.
SOURCE: : Baselga J et al. ASCO 2018 Abstract LBA1006 .
REPORTING FROM ASCO 2018
Key clinical point:
Major finding: The combination of taselisib/fulvestrant extend median progression-free survival by two months.
Study details: Phase 3 randomized trial in 516 women with locally advanced or metastatic ER+/HER2- breast cancer.
Disclosures: The study was funded by F. Hoffman La-Roche. Dr. Baselga had disclosures related to GRAIL, Lilly, and Novartis, Infinity Pharmaceuticals, and Varian Medical Systems, PMV Pharma, and Juno Therapeutics. Dr. Burstein disclosed institutional research funding and speaker’s bureau activities for Novartis. Dr. Ma reported no relevant disclosures.
Source: Baselga et al. ASCO Abstract LBA1006.
TAILORx: Most women with intermediate risk ER+ breast cancer can safely skip chemo
CHICAGO – New data from the TAILORx trial are welcome news for women with HR-positive, HER2-negative, axillary node–negative early-stage breast cancer and their oncologists caught in the gray area surrounding the need for adjuvant chemotherapy.
Results of the noninferiority phase 3 trial—the largest adjuvant breast cancer treatment trial ever conducted—show that among the 6,711 women with an intermediate Oncotype DX Breast Recurrence Score (11-25), those who received only endocrine therapy and skipped adjuvant chemotherapy did not have worse invasive disease-free survival than counterparts who received both (hazard ratio, 1.08; P=.26).
The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy alone and 84.3% with both chemotherapy and endocrine therapy, and the pattern was essentially the same for freedom from any recurrence and distant recurrence, and overall survival.
The findings are practice changing, according to lead study author Joseph A. Sparano, MD, associate director for clinical research at the Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
In a video interview at the annual meeting of the American Society of Clinical Oncology, he discussed implications of the new data for decision making, results of interaction analyses showing that one size does not fit all and certain women with intermediate recurrence scores do derive benefit from adjuvant chemotherapy, as well as plans to use the tumor samples for future analyses on those that do recur.
CHICAGO – New data from the TAILORx trial are welcome news for women with HR-positive, HER2-negative, axillary node–negative early-stage breast cancer and their oncologists caught in the gray area surrounding the need for adjuvant chemotherapy.
Results of the noninferiority phase 3 trial—the largest adjuvant breast cancer treatment trial ever conducted—show that among the 6,711 women with an intermediate Oncotype DX Breast Recurrence Score (11-25), those who received only endocrine therapy and skipped adjuvant chemotherapy did not have worse invasive disease-free survival than counterparts who received both (hazard ratio, 1.08; P=.26).
The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy alone and 84.3% with both chemotherapy and endocrine therapy, and the pattern was essentially the same for freedom from any recurrence and distant recurrence, and overall survival.
The findings are practice changing, according to lead study author Joseph A. Sparano, MD, associate director for clinical research at the Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
In a video interview at the annual meeting of the American Society of Clinical Oncology, he discussed implications of the new data for decision making, results of interaction analyses showing that one size does not fit all and certain women with intermediate recurrence scores do derive benefit from adjuvant chemotherapy, as well as plans to use the tumor samples for future analyses on those that do recur.
CHICAGO – New data from the TAILORx trial are welcome news for women with HR-positive, HER2-negative, axillary node–negative early-stage breast cancer and their oncologists caught in the gray area surrounding the need for adjuvant chemotherapy.
Results of the noninferiority phase 3 trial—the largest adjuvant breast cancer treatment trial ever conducted—show that among the 6,711 women with an intermediate Oncotype DX Breast Recurrence Score (11-25), those who received only endocrine therapy and skipped adjuvant chemotherapy did not have worse invasive disease-free survival than counterparts who received both (hazard ratio, 1.08; P=.26).
The 9-year rate of invasive disease–free survival was 83.3% with endocrine therapy alone and 84.3% with both chemotherapy and endocrine therapy, and the pattern was essentially the same for freedom from any recurrence and distant recurrence, and overall survival.
The findings are practice changing, according to lead study author Joseph A. Sparano, MD, associate director for clinical research at the Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
In a video interview at the annual meeting of the American Society of Clinical Oncology, he discussed implications of the new data for decision making, results of interaction analyses showing that one size does not fit all and certain women with intermediate recurrence scores do derive benefit from adjuvant chemotherapy, as well as plans to use the tumor samples for future analyses on those that do recur.
REPORTING FROM ASCO 2018
Heading down the wrong pathway in advanced breast cancer?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – In the SANDPIPER trial, the combination of the phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib and the selective estrogen receptor modifier fulvestrant (Faslodex) was associated with a small but significant progression-free survival (PFS) benefit, compared with fulvestrant alone in women with advanced estrogen-receptor positive, HER2-negative breast cancer.
That 2-month PFS benefit, described as “modest” by investigators, came at the cost of significant toxicities, with nearly 50% of patients treated with the combination having grade 3 or greater toxicities, compared with 16% of patients treated with fulvestrant alone.
In this video interview from the annual meeting of the American Society of Clinical Oncology, ASCO expert Harold Burstein, MD, PhD, from the Dana-Farber Cancer Institute in Boston, questions whether the PI3K pathway, shown to be targetable in hematologic malignancies, is worth continuing to pursue in breast cancer.
Dr. Burstein had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – In the SANDPIPER trial, the combination of the phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib and the selective estrogen receptor modifier fulvestrant (Faslodex) was associated with a small but significant progression-free survival (PFS) benefit, compared with fulvestrant alone in women with advanced estrogen-receptor positive, HER2-negative breast cancer.
That 2-month PFS benefit, described as “modest” by investigators, came at the cost of significant toxicities, with nearly 50% of patients treated with the combination having grade 3 or greater toxicities, compared with 16% of patients treated with fulvestrant alone.
In this video interview from the annual meeting of the American Society of Clinical Oncology, ASCO expert Harold Burstein, MD, PhD, from the Dana-Farber Cancer Institute in Boston, questions whether the PI3K pathway, shown to be targetable in hematologic malignancies, is worth continuing to pursue in breast cancer.
Dr. Burstein had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – In the SANDPIPER trial, the combination of the phosphatidylinositol 3-kinase (PI3K) inhibitor taselisib and the selective estrogen receptor modifier fulvestrant (Faslodex) was associated with a small but significant progression-free survival (PFS) benefit, compared with fulvestrant alone in women with advanced estrogen-receptor positive, HER2-negative breast cancer.
That 2-month PFS benefit, described as “modest” by investigators, came at the cost of significant toxicities, with nearly 50% of patients treated with the combination having grade 3 or greater toxicities, compared with 16% of patients treated with fulvestrant alone.
In this video interview from the annual meeting of the American Society of Clinical Oncology, ASCO expert Harold Burstein, MD, PhD, from the Dana-Farber Cancer Institute in Boston, questions whether the PI3K pathway, shown to be targetable in hematologic malignancies, is worth continuing to pursue in breast cancer.
Dr. Burstein had no disclosures.
REPORTING FROM ASCO 2018
Who needs breast cancer genetics testing?
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13
Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13

Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Advances in cancer genetics are rapidly changing how clinicians assess an individual’s risk for breast cancer. ObGyns counsel many women with a personal or family history of the disease, many of whom can benefit from genetics counseling and testing. As patients with a hereditary predisposition to breast cancer are at higher risk and are younger at diagnosis, it is imperative to identify them early so they can benefit from enhanced surveillance, chemoprevention, and discussions regarding risk-reducing surgeries. ObGyns are uniquely poised to identify young women at risk for hereditary cancer syndromes, and they play a crucial role in screening and prevention over the life span.
CASE Patient with breast cancer history asks about screening for her daughters
A 52-year-old woman presents for her annual examination. She underwent breast cancer treatment 10 years earlier and has done well since then. When asked about family history of breast cancer and ethnicity, she reports her mother had breast cancer later in life, and her mother’s father was of Ashkenazi Jewish ancestry.In addition, a maternal uncle had metastatic prostate cancer. You recall that breast cancer diagnosed before age 50 years and Ashkenazi ancestry are “red flags” for a hereditary cancer syndrome. The patient wonders how her daughters should be screened. What do you do next?
Having a risk assessment plan is crucial
Given increasing demands, limited time, and the abundance of information to be discussed with patients, primary care physicians may find it challenging to assess breast cancer risk, consider genetics testing for appropriate individuals, and counsel patients about risk management options. The process has become even more complex since the expansion in genetics knowledge and the advent of multigene panel testing. Not only is risk assessment crucial for this woman and her daughters, and for other patients, but a delay in diagnosing and treating breast cancer in patients with hereditary and familial cancer risks may represent a worrisome new trend in medical litigation.1,2 Clinicians must have a process in place for assessing risk in all patients and treating them appropriately.
The American Cancer Society (ACS) estimated that 252,710 cases of breast cancer would be diagnosed in 2017, leading to 40,610 deaths.3 Twelve percent to 14% of breast cancers are thought to be related to hereditary cancer predisposition syndromes.4–8 This means that, every year, almost 35,000 cases of breast cancer are attributable to hereditary risk. These cases can be detected early with enhanced surveillance, which carries the highest chance for cure, or prevented with risk-reducing surgery in identified genetic mutation carriers. Each child of a person with a genetic mutation predisposing to breast cancer has a 50% chance of inheriting the mutation and having a very high risk of cancer.
In this patient’s case, basic information is collected about her cancer-related personal and family history.
Asking a few key questions can help in stratifying risk:
- Have you or anyone in your family had cancer? What type, and at what age?
- If breast cancer, did it involve both breasts, or was it triple-negative?
- Is there a family history of ovarian cancer?
- Is there a family history of male breast cancer?
- Is there a family history of metastatic prostate cancer?
- Are you of Ashkenazi Jewish ethnicity?
- Have you or anyone in your family ever had genetics testing for cancer?
The hallmarks of hereditary cancer are multiple cancers in an individual or family; young age at diagnosis; and ovarian, pancreatic, or another rare cancer. Metastatic prostate cancer was added as a red flag for hereditary risk after a recent large series found that 11.8% of men with metastatic prostate cancer harbor germline mutations.9
CASE Continued
On further questioning, the patient reports she had triple-negative (estrogen receptor–, progesterone receptor–, and human epidermal growth factor receptor 2 [HER2]–negative) breast cancer, a feature of patients with germline BRCA1 (breast cancer susceptibility gene 1) mutations.10 In addition, her Ashkenazi ancestry is concerning, as there is a 1-in-40 chance of carrying 1 of the 3 Ashkenazi founder BRCA mutations.11 Is a genetics consultation needed?
Read about guidelines for referral and testing.
Guidelines for genetics referral and testing
According to the TABLE, which summarizes national guidelines for genetics referral, maternal and paternal family histories are equally important. Our patient was under age 50 at diagnosis, has a history of triple-negative breast cancer, is of Ashkenazi ancestry, and has a family history of metastatic prostate cancer. She meets the criteria for genetics testing, and screening for her daughters most certainly will depend on the findings of that testing. If she carries a BRCA1 mutation, as might be anticipated, each daughter would have a 50% chance of having inherited the mutation. If they carry the mutation as well, they would begin breast magnetic resonance imaging (MRI) screening at age 25.12 If they decide against genetics testing, they could still undergo MRI screening as untested first-degree relatives of a BRCA carrier, per ACS recommendations.13

Integrating evidence and experience
Over the past 10 to 20 years, other breast cancer susceptibility genes (eg, BRCA2, PALB2, CHEK2) have been identified. More recently, next-generation sequencing has become commercially available. Laboratories can use this newer method to sequence multiple genes rapidly and in parallel, and its cost is similar to that of single-syndrome testing.14 When more than 1 gene can explain an inherited cancer syndrome, multigene panel testing may be more efficient and cost-effective. Use of multigene panel testing is supported in guidelines issued by the National Comprehensive Cancer Network,12 the American College of Obstetricians and Gynecologists,15 and other medical societies.
For our patient, the most logical strategy would be to test for the 3 mutations most common in the Ashkenazi population and then, if no mutation is found, perform multigene panel testing.
Formal genetics counseling can be very helpful for a patient, particularly in the era of multigene panel testing.16,17 A detailed pedigree (family tree) is elicited, and a genetics specialist determines whether testing is indicated and which test is best for the patient. Possible test findings are explained. The patient may be found to have a pathogenic variant with associated increased cancer risk, a negative test result (informative or uninformative), or a variant of uncertain significance (VUS). VUS is a gene mutation identified with an unknown effect on protein function and an unclear association with cancer risk. A finding of VUS may make the patient anxious,18 create uncertainty in the treating physician,19 and lead to harmful overtreatment, excessive surveillance, or unnecessary use of a preventive measure.19–21 Genetics counseling allows the patient, even the patient with VUS, to make appropriate decisions.22 Counseling may also help a patient or family process emotional responses, such as fear and guilt. In addition, counselors are familiar with relevant laws and regulations, such as the Genetic Information Nondiscrimination Act of 2008 (GINA), which protects patients from insurance and employment discrimination. Many professional guidelines recommend providing genetics counseling in conjunction with genetics testing,12,23 and some insurance companies and some states require counseling for coverage of testing.
Cost of genetics counseling. If patients are concerned about the cost of genetics testing, they can be reassured with the following information24–26:
- The Patient Protection and Affordable Care Act (ACA) identifies BRCA testing as a preventive service
- Medicare provides coverage for affected patients with a qualifying personal history
- 97% of commercial insurers and most state Medicaid programs provide coverage for hereditary cancer testing
- Most commercial laboratories have affordability programs that may provide additional support.
If a BRCA mutation is found: Many patients question the value of knowing whether they have a BRCA mutation. What our patient, her daughters, and others may not realize is that, if a BRCA mutation is found, breast MRI screening can begin at age 25. Although contrast-enhanced MRI screening is highly sensitive in detecting breast cancer,27–29 it lacks specificity and commonly yields false positives.
Some patients also worry about overdiagnosis with this highly sensitive test. Many do not realize that preventively prescribed oral contraceptives can reduce the risk of ovarian cancer by 50%, and cosmetically acceptable risk-reducing breast surgeries can reduce the risk by 90%.
Many are unaware of the associated risks with ovarian, prostate, pancreatic, and other cancers; of risk management options; and of assisted reproduction options, such as preimplantation genetics diagnosis, which can prevent the passing of a genetic mutation to future generations. The guidelines on risk management options are increasingly clear and helpful,12,30–32 and women often turn to their ObGyns for advice about health and prevention.
ObGyns are often the first-line providers for women with a personal or family history of breast cancer. Identification of at-risk patients begins with taking a careful family history and becoming familiar with the rapidly evolving guidelines in this important field. Identification of appropriate candidates for breast cancer genetics testing is a key step toward prevention, value-based care, and avoidance of legal liability.
CASE Resolved
In this case, testing for the 3 common Ashkenazi BRCA founder mutations was negative, and multigene panel testing was also negative. Her husband is not of Ashkenazi Jewish descent and there is no significant family history of cancer on his side. The daughters are advised to begin high-risk screening at the age of 32, 10 years earlier than their mother was diagnosed, but no genetic testing is indicated for them.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
- Phillips RL Jr, Bartholomew LA, Dovey SM, Fryer GE Jr, Miyoshi TJ, Green LA. Learning from malpractice claims about negligent, adverse events in primary care in the United States. Qual Saf Health Care. 2004;13(2):121–126.
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- American Cancer Society. Breast Cancer Facts & Figures 2017-2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-factsand-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Published 2017. Accessed December 28, 2017.
- Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
- Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–1468.
- Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–2009.
- Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–2257.
- Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149(3):604–613.e20.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–453.
- Mavaddat N, Barrowdale D, Andrulis IL, et al; Consortium of Investigators of Modifiers of BRCA1/2. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev. 2012;21(1):134–147.
- Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336(20):1401–1408.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian. Version 1.2018. https://www.nccn.org. Accessed December 28, 2017.
- Saslow D, Boetes C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89.
- Heather JM, Chain B. The sequence of sequencers: the history of sequencing DNA. Genomics. 2016;107(1):1–8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130(3):e110–e126.
- Mester JL, Schreiber AH, Moran RT. Genetic counselors: your partners in clinical practice. Cleve Clin J Med. 2012;79(8):560–568.
- Smith M, Mester J, Eng C. How to spot heritable breast cancer: a primary care physician’s guide. Cleve Clin J Med. 2014;81(1):31–40.
- Welsh JL, Hoskin TL, Day CN, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 genes. Ann Surg Oncol. 2017;24(10):3067–3072.
- Kurian AW, Li Y, Hamilton AS, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–2239.
- Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. 2016;13(9):581–588.
- Yu PP, Vose JM, Hayes DF. Genetic cancer susceptibility testing: increased technology, increased complexity. J Clin Oncol. 2015;33(31):3533–3534.
- Pederson HJ, Gopalakrishnan D, Noss R, Yanda C, Eng C, Grobmyer SR. Impact of multigene panel testing on surgical decision making in breast cancer patients. J Am Coll Surg. 2018;226(4):560–565.
- Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33(31):3660–3667.
- Preventive care benefits for women: What Marketplace health insurance plans cover. HealthCare.gov. https://www.healthcare.gov/coverage/what-marketplace-plans-cover/. Accessed May 15, 2018.
- Centers for Medicare & Medicaid Services. The Center for Consumer Information & Insurance Oversight: Affordable Care Act Implementation FAQs – Set 12. https://www.cms.gov/CCIIO/Resources/Fact-Sheets-and-FAQs/aca_implementation_faqs12.html. Accessed May 15, 2018.
- US Preventive Services Task Force. Final Recommendation Statement: BRCA-Related Cancer: Risk Assessment, Genetic Counseling, and Genetic Testing. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing. Published December 2013. Accessed May 15, 2018.
- Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476.
- Lehman CD, Blume JD, Weatherall P, et al; International Breast MRI Consortium Working Group. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905.
- Kriege M, Brekelmans CT, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437.
- Pederson HJ, Padia SA, May M, Grobmyer S. Managing patients at genetic risk of breast cancer. Cleve Clin J Med. 2016;83(3):199–206.
- Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281.
- American Society of Breast Surgeons. Consensus Guideline on Hereditary Genetic Testing for Patients With and Without Breast Cancer. Columbia, MD: American Society of Breast Surgeons. https://www.breastsurgeons.org/new_layout/about/statements/PDF_Statements/BRCA_Testing.pdf. Published March 14, 2017. Accessed December 28, 2017.
Take-home points
- The best genetics test is a good family history, updated annually
- Each year, 35,000 breast cancers are attributable to hereditary risk
- It is crucial to identify families at risk for hereditary breast cancer early, as cancers may begin in a woman's 30s; screening begins at age 25
- Multigene panel testing is efficient and cost-effective
- For patients who have highly penetrant pathogenic variants and are of childbearing age, preimplantation genetics diagnosis is an option
Should breast cancer screening guidelines be tailored to a patient’s race and ethnicity?
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.
Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).
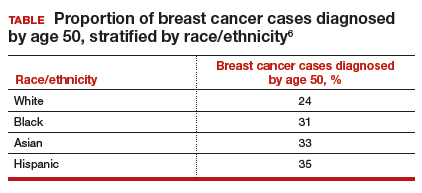
These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
Dr Jame Abraham's top ASCO selections in breast cancer
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Ketorolac may reduce breast cancer recurrence risk, particularly in overweight patients
Ketorolac administered during primary tumor surgery may cut risk of distant recurrences in patients with breast cancer, results of a retrospective study show.
Overweight patients appeared most likely to benefit from interoperative treatment with this nonsteroidal anti-inflammatory drug, study investigators reported.
“This approach could be extremely appealing for parts of the globe where obesity has been strongly increasing during the last decade and where resources for cancer treatment are scarce,” they wrote. The report was published in the Journal of the National Cancer Institute.
Ketorolac inhibits enzymes upregulated by leptin, a hormone abnormally secreted in the setting of overweight or obesity, which might explain the concentration of benefit in high–body mass index individuals, noted Christine Desmedt, PhD, of the Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Brussels, and her coauthors.
Indeed, the study also showed no benefit to intraoperative administration of another NSAID, diclofenac, which does not appear to have the same enzyme-inhibitory effects as ketorolac, the investigators said.
This recently published analysis by Dr. Desmedt and her colleagues was based on two retrospective series of patients: one evaluating intraoperative ketorolac in 529 patients versus 298 patients who received no ketorolac, and one evaluating intraoperative diclofenac in 787 patients, versus 220 who did not receive that NSAID.
The investigators found a significant association between ketorolac given during surgery and decreased incidence of distant metastasis (adjusted hazard ratio [aHR], 0.59, 95% confidence interval, 0.37-0.96, P = .03). Reduced recurrence was most evident in patients with high BMI (aHR, 0.55; 95% CI, 0.31-0.96; P = .04).
Further evaluation revealed that the benefit of ketorolac was “clearly associated” with a reduction in early metastases, both overall and in the high-BMI subgroup, the investigators said.
By contrast, intraoperative diclofenac was not associated with a decrease in distant recurrences, overall (adjusted HR, 1.04; 95% CI, 0.58-1.87, P = .88) or in BMI subgroup analysis, investigators said.
While some might be surprised that a single dose of ketorolac could reduce distant recurrence, it might be explained by the timing of NSAID delivery, they noted. In previous studies, primary tumor removal has been shown to disturb disease homeostasis, and thus might trigger early recurrences.
“Complex system dynamics are exquisitely sensitive on initial conditions, and, therefore, changes occurring in critical early times may be able to cause major changes in system evolution,” the investigators wrote in a discussion of the results.
The finding is also not without precedent. The authors cited one Scandinavian randomized trial in which a single course of perioperative cyclophosphamide significantly improved disease-free survival at more than 17 years of follow-up; by contrast, giving the treatment 2-4 weeks after mastectomy provided no such benefit.
In addition, ketorolac’s potential perioperative benefit has been shown in other tumor types, including improved disease-free survival in one institutional series of lung cancer patients, and reduced disease-specific mortality in a retrospective study of ovarian cancer patients.
The present breast cancer study is limited because it is retrospective, and does not address questions regarding optimal timing or duration of dose. However, “it suggests a potentially important repositioning of ketorolac in the intraoperative treatment of breast cancer patients with elevated BMI, and points to the need for a prospective confirmatory randomized trial,” the authors said.
Dr. Desmedt and her colleagues reported no conflicts of interest related to the study.
SOURCE: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.
Ketorolac administered during primary tumor surgery may cut risk of distant recurrences in patients with breast cancer, results of a retrospective study show.
Overweight patients appeared most likely to benefit from interoperative treatment with this nonsteroidal anti-inflammatory drug, study investigators reported.
“This approach could be extremely appealing for parts of the globe where obesity has been strongly increasing during the last decade and where resources for cancer treatment are scarce,” they wrote. The report was published in the Journal of the National Cancer Institute.
Ketorolac inhibits enzymes upregulated by leptin, a hormone abnormally secreted in the setting of overweight or obesity, which might explain the concentration of benefit in high–body mass index individuals, noted Christine Desmedt, PhD, of the Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Brussels, and her coauthors.
Indeed, the study also showed no benefit to intraoperative administration of another NSAID, diclofenac, which does not appear to have the same enzyme-inhibitory effects as ketorolac, the investigators said.
This recently published analysis by Dr. Desmedt and her colleagues was based on two retrospective series of patients: one evaluating intraoperative ketorolac in 529 patients versus 298 patients who received no ketorolac, and one evaluating intraoperative diclofenac in 787 patients, versus 220 who did not receive that NSAID.
The investigators found a significant association between ketorolac given during surgery and decreased incidence of distant metastasis (adjusted hazard ratio [aHR], 0.59, 95% confidence interval, 0.37-0.96, P = .03). Reduced recurrence was most evident in patients with high BMI (aHR, 0.55; 95% CI, 0.31-0.96; P = .04).
Further evaluation revealed that the benefit of ketorolac was “clearly associated” with a reduction in early metastases, both overall and in the high-BMI subgroup, the investigators said.
By contrast, intraoperative diclofenac was not associated with a decrease in distant recurrences, overall (adjusted HR, 1.04; 95% CI, 0.58-1.87, P = .88) or in BMI subgroup analysis, investigators said.
While some might be surprised that a single dose of ketorolac could reduce distant recurrence, it might be explained by the timing of NSAID delivery, they noted. In previous studies, primary tumor removal has been shown to disturb disease homeostasis, and thus might trigger early recurrences.
“Complex system dynamics are exquisitely sensitive on initial conditions, and, therefore, changes occurring in critical early times may be able to cause major changes in system evolution,” the investigators wrote in a discussion of the results.
The finding is also not without precedent. The authors cited one Scandinavian randomized trial in which a single course of perioperative cyclophosphamide significantly improved disease-free survival at more than 17 years of follow-up; by contrast, giving the treatment 2-4 weeks after mastectomy provided no such benefit.
In addition, ketorolac’s potential perioperative benefit has been shown in other tumor types, including improved disease-free survival in one institutional series of lung cancer patients, and reduced disease-specific mortality in a retrospective study of ovarian cancer patients.
The present breast cancer study is limited because it is retrospective, and does not address questions regarding optimal timing or duration of dose. However, “it suggests a potentially important repositioning of ketorolac in the intraoperative treatment of breast cancer patients with elevated BMI, and points to the need for a prospective confirmatory randomized trial,” the authors said.
Dr. Desmedt and her colleagues reported no conflicts of interest related to the study.
SOURCE: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.
Ketorolac administered during primary tumor surgery may cut risk of distant recurrences in patients with breast cancer, results of a retrospective study show.
Overweight patients appeared most likely to benefit from interoperative treatment with this nonsteroidal anti-inflammatory drug, study investigators reported.
“This approach could be extremely appealing for parts of the globe where obesity has been strongly increasing during the last decade and where resources for cancer treatment are scarce,” they wrote. The report was published in the Journal of the National Cancer Institute.
Ketorolac inhibits enzymes upregulated by leptin, a hormone abnormally secreted in the setting of overweight or obesity, which might explain the concentration of benefit in high–body mass index individuals, noted Christine Desmedt, PhD, of the Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Brussels, and her coauthors.
Indeed, the study also showed no benefit to intraoperative administration of another NSAID, diclofenac, which does not appear to have the same enzyme-inhibitory effects as ketorolac, the investigators said.
This recently published analysis by Dr. Desmedt and her colleagues was based on two retrospective series of patients: one evaluating intraoperative ketorolac in 529 patients versus 298 patients who received no ketorolac, and one evaluating intraoperative diclofenac in 787 patients, versus 220 who did not receive that NSAID.
The investigators found a significant association between ketorolac given during surgery and decreased incidence of distant metastasis (adjusted hazard ratio [aHR], 0.59, 95% confidence interval, 0.37-0.96, P = .03). Reduced recurrence was most evident in patients with high BMI (aHR, 0.55; 95% CI, 0.31-0.96; P = .04).
Further evaluation revealed that the benefit of ketorolac was “clearly associated” with a reduction in early metastases, both overall and in the high-BMI subgroup, the investigators said.
By contrast, intraoperative diclofenac was not associated with a decrease in distant recurrences, overall (adjusted HR, 1.04; 95% CI, 0.58-1.87, P = .88) or in BMI subgroup analysis, investigators said.
While some might be surprised that a single dose of ketorolac could reduce distant recurrence, it might be explained by the timing of NSAID delivery, they noted. In previous studies, primary tumor removal has been shown to disturb disease homeostasis, and thus might trigger early recurrences.
“Complex system dynamics are exquisitely sensitive on initial conditions, and, therefore, changes occurring in critical early times may be able to cause major changes in system evolution,” the investigators wrote in a discussion of the results.
The finding is also not without precedent. The authors cited one Scandinavian randomized trial in which a single course of perioperative cyclophosphamide significantly improved disease-free survival at more than 17 years of follow-up; by contrast, giving the treatment 2-4 weeks after mastectomy provided no such benefit.
In addition, ketorolac’s potential perioperative benefit has been shown in other tumor types, including improved disease-free survival in one institutional series of lung cancer patients, and reduced disease-specific mortality in a retrospective study of ovarian cancer patients.
The present breast cancer study is limited because it is retrospective, and does not address questions regarding optimal timing or duration of dose. However, “it suggests a potentially important repositioning of ketorolac in the intraoperative treatment of breast cancer patients with elevated BMI, and points to the need for a prospective confirmatory randomized trial,” the authors said.
Dr. Desmedt and her colleagues reported no conflicts of interest related to the study.
SOURCE: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Key clinical point: Administration of ketorolac during primary tumor surgery was associated with a reduction of distant recurrences, particularly in overweight patients.
Major finding: Reduced recurrence was most evident in patients with high BMI (adjusted hazard ratio, 0.55; 95% CI, 0.31-0.96; P = .04).
Study details: Analysis of two retrospective series, including a total of 1,834 patients with breast cancer, evaluating intraoperative administration of ketorolac or diclofenac.
Disclosures: The authors declared no conflicts of interest.
Source: Desmedt C et al. J Natl Cancer Inst. 2018 Apr 30. doi: 10.1093/jnci/djy042.
Screening for brain mets could improve quality of life for some with breast cancer
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
Despite having more extensive metastases at presentation, breast cancer patients had outcomes after brain-directed therapy similar to those of lung cancer patients, results of a retrospective, single-center study show.
The breast cancer patients had larger and more numerous brain metastases compared with the non-small-cell lung cancer (NSCLC) patients, according to study results published in JAMA Oncology.
However, median survival was not statistically different between groups, at 1.45 years for the breast cancer patients and 1.09 years for NSCLC patients (P = .06), wrote Daniel N. Cagney, MD, of Dana-Farber/Brigham and Women’s Cancer Center, Harvard Medical School, Boston, and his coauthors.
“This finding suggests that intracranial disease in patients with breast cancer was not more aggressive or resistant to treatment, but rather was diagnosed at a later stage,” noted Dr. Cagney and his colleagues.
They described a retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC, all treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Median metastasis diameter at presentation was 17 mm for the breast cancer patients, compared with 14 mm for the lung cancer patients (P less than .001). Breast cancer patients were significantly more likely to be symptomatic, have seizures, harbor brainstem involvement, and have leptomeningeal disease at the time of diagnosis, the researchers wrote.
“After initial brain-directed therapy, no significant differences in recurrence or treatment-based intracranial outcomes were found between the two groups,” they noted. However, neurological death was seen in 37.3% of the breast cancer group versus 19.9% of the lung cancer group (P less than .001).
Dr. Cagney and his coauthors said they conducted the study to identify the potential value of brain-directed MRI screening in breast cancer, which they said is “important given the impact of neurological compromise on quality of life.”
Brain metastases are common in some subsets of breast cancer patients, yet National Comprehensive Cancer Network guidelines do not recommend brain-directed screening in breast cancer, “a recommendation that is based only on expert consensus given the lack of definitive or prospective studies on this issue,” they wrote.
In light of their findings, the investigators suggest that brain-directed MRI screening is important for breast cancer patients who present with potential for intracranial involvement.
“Early identification of intracranial disease facilitates less invasive or less toxic approaches, such as stereotactic radiosurgery or careful use of promising systemic agents, rather than [whole brain radiation therapy] or neurosurgical resection.” they wrote.
In this study, whole brain radiation therapy was more common in the breast cancer group (59.9% versus 42.9% for the lung cancer group; P less than .001), the investigators noted.
Dr. Cagney and colleagues had no conflicts of interest to report.
SOURCE: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
FROM JAMA ONCOLOGY
Key clinical point: Breast cancer patients presented with larger and more numerous brain metastases compared with non–small-cell lung cancer patients, but after brain-directed therapy, there were no differences in outcomes between groups.
Major finding: Median survival was 1.45 years for breast cancer patients and 1.09 for NSCLC patients.
Study details: A retrospective analysis of 349 patients with breast cancer and 659 patients with NSCLC treated between 2000 and 2015 at Dana-Farber/Brigham and Women’s Cancer Center.
Disclosures: The authors reported no conflicts of interest.
Source: Cagney DN et al. JAMA Oncol. 2018 May 17. doi: 10.1001/jamaoncol.2018.0813.
CBT-I bests acupuncture for treating insomnia among cancer survivors
Cancer survivors who have trouble sleeping saw improvements with both cognitive-behavioral therapy designed specifically for insomnia (CBT-I) and acupuncture, according to results from the randomized, controlled CHOICE trial. But the former is more efficacious.
“Insomnia can have deleterious effects on quality of life and function, and occurs in up to 60% of cancer survivors,” lead study author Jun J. Mao, MD, chief of integrative medicine service at Memorial Sloan Kettering Cancer Center, New York, said in a press briefing held in advance of the annual meeting of the American Society of Clinical Oncology.
“CBT-I is a highly effective therapy and can be considered the gold standard of treatment,” he noted. However, this modality may be limited by poor adherence and nonresponse. Moreover, it is highly specialized and not currently available in many cancer centers or communities.
Functional imaging studies have shown that acupuncture can regulate brain regions involving cognition and emotion that are essential to sleep regulation, and clinical research has shown that it can improve pain- and hot flash–related sleep disturbances, according to Dr. Mao. About 73% of U.S. comprehensive cancer centers offer acupuncture for symptom management.
Main results of the CHOICE (Choosing Options for Insomnia in Cancer Effectively) trial showed that patients in both the CBT-I and acupuncture groups reduced their Insomnia Severity Index scores by more than one-half at the end of the 8-weeks treatment period, but the reduction was a statistically significant 2.6 points greater with CBT-I. Benefit of each treatment was still evident after 12 weeks.
Response rate was higher with CBT-I than with acupuncture only among patients having mild insomnia at baseline, and the two treatments yielded similar improvements in mental and physical quality of life.
“Among cancer patients with insomnia, we found that both acupuncture and CBT-I produced clinically meaningful and durable benefit, but overall, CBT-I is more effective in reducing insomnia severity,” Dr. Mao concluded. “Our hope is that by doing this type of research, we can help patients and clinicians pick the right kind of treatment and help them to manage their sleep. Our next step is to really examine for what type of patient treatment would be beneficial, and how to deliver this type of effective treatment to the broader community of cancer patients.”
Insomnia among cancer survivors is both prevalent and problematic, agreed ASCO President Bruce E. Johnson, MD, FASCO.
“The most common way we treat this is pharmacologically, with sleeping pills,” he noted. “This trial shows that two different methods using something other than medications can help people with sleep, and not only do they help people with sleep, but they improve their quality of life.
“We think this information will be helpful for clinicians who end up having to decide, and also, we would use this information to help decide about how the severity of the insomnia is going to influence the treatment,” maintained Dr. Johnson, who is also a professor of medicine at the Dana-Farber Cancer Institute in Boston, and a leader of the center’s lung cancer program.
Study details
The CHOICE trial did not have any restrictions on cancer type or stage; more than a half-dozen types were represented among the 160 patients enrolled, with breast cancer (31%) and prostate cancer (23%) accounting for the largest shares. The majority of patients were white (70%) and had moderate to severe insomnia (79%).
Patients were randomized to receive either acupuncture sessions (10 sessions, with points selected to treat insomnia plus comorbid symptoms such as fatigue and anxiety) or CBT-I (7 sessions), each over the course of 8 weeks.
Main results showed that at the end of treatment, the reduction in Insomnia Severity Index was 8.3 points with acupuncture and 10.9 points with CBT-I (P = .0007), Dr. Mao reported. Benefit of each treatment was sustained after 12 weeks.
In stratified analysis, the rate of response (defined as a greater than 8-point reduction) was higher with CBT-I than with acupuncture among patients with mild insomnia (Insomnia Severity Index of 8-14) (85% vs. 18%; P less than .0001), but not among patients with moderate or severe insomnia (Insomnia Severity Index of 15 or higher) (75% vs. 66%; P = .26).
The two treatments were similarly efficacious with respect to quality of life, assessed with the Patient-Reported Outcomes Measurement Information System over the entire course of the trial, for both the physical health component (P = .4) and the mental health component (P = .36).
Dr. Mao disclosed no relevant conflicts of interest. The study received funding from the Patient-Centered Outcomes Research Institute.
SOURCE: Mao JJ et al. ASCO 2018. Abstract 10001.
Cancer survivors who have trouble sleeping saw improvements with both cognitive-behavioral therapy designed specifically for insomnia (CBT-I) and acupuncture, according to results from the randomized, controlled CHOICE trial. But the former is more efficacious.
“Insomnia can have deleterious effects on quality of life and function, and occurs in up to 60% of cancer survivors,” lead study author Jun J. Mao, MD, chief of integrative medicine service at Memorial Sloan Kettering Cancer Center, New York, said in a press briefing held in advance of the annual meeting of the American Society of Clinical Oncology.
“CBT-I is a highly effective therapy and can be considered the gold standard of treatment,” he noted. However, this modality may be limited by poor adherence and nonresponse. Moreover, it is highly specialized and not currently available in many cancer centers or communities.
Functional imaging studies have shown that acupuncture can regulate brain regions involving cognition and emotion that are essential to sleep regulation, and clinical research has shown that it can improve pain- and hot flash–related sleep disturbances, according to Dr. Mao. About 73% of U.S. comprehensive cancer centers offer acupuncture for symptom management.
Main results of the CHOICE (Choosing Options for Insomnia in Cancer Effectively) trial showed that patients in both the CBT-I and acupuncture groups reduced their Insomnia Severity Index scores by more than one-half at the end of the 8-weeks treatment period, but the reduction was a statistically significant 2.6 points greater with CBT-I. Benefit of each treatment was still evident after 12 weeks.
Response rate was higher with CBT-I than with acupuncture only among patients having mild insomnia at baseline, and the two treatments yielded similar improvements in mental and physical quality of life.
“Among cancer patients with insomnia, we found that both acupuncture and CBT-I produced clinically meaningful and durable benefit, but overall, CBT-I is more effective in reducing insomnia severity,” Dr. Mao concluded. “Our hope is that by doing this type of research, we can help patients and clinicians pick the right kind of treatment and help them to manage their sleep. Our next step is to really examine for what type of patient treatment would be beneficial, and how to deliver this type of effective treatment to the broader community of cancer patients.”
Insomnia among cancer survivors is both prevalent and problematic, agreed ASCO President Bruce E. Johnson, MD, FASCO.
“The most common way we treat this is pharmacologically, with sleeping pills,” he noted. “This trial shows that two different methods using something other than medications can help people with sleep, and not only do they help people with sleep, but they improve their quality of life.
“We think this information will be helpful for clinicians who end up having to decide, and also, we would use this information to help decide about how the severity of the insomnia is going to influence the treatment,” maintained Dr. Johnson, who is also a professor of medicine at the Dana-Farber Cancer Institute in Boston, and a leader of the center’s lung cancer program.
Study details
The CHOICE trial did not have any restrictions on cancer type or stage; more than a half-dozen types were represented among the 160 patients enrolled, with breast cancer (31%) and prostate cancer (23%) accounting for the largest shares. The majority of patients were white (70%) and had moderate to severe insomnia (79%).
Patients were randomized to receive either acupuncture sessions (10 sessions, with points selected to treat insomnia plus comorbid symptoms such as fatigue and anxiety) or CBT-I (7 sessions), each over the course of 8 weeks.
Main results showed that at the end of treatment, the reduction in Insomnia Severity Index was 8.3 points with acupuncture and 10.9 points with CBT-I (P = .0007), Dr. Mao reported. Benefit of each treatment was sustained after 12 weeks.
In stratified analysis, the rate of response (defined as a greater than 8-point reduction) was higher with CBT-I than with acupuncture among patients with mild insomnia (Insomnia Severity Index of 8-14) (85% vs. 18%; P less than .0001), but not among patients with moderate or severe insomnia (Insomnia Severity Index of 15 or higher) (75% vs. 66%; P = .26).
The two treatments were similarly efficacious with respect to quality of life, assessed with the Patient-Reported Outcomes Measurement Information System over the entire course of the trial, for both the physical health component (P = .4) and the mental health component (P = .36).
Dr. Mao disclosed no relevant conflicts of interest. The study received funding from the Patient-Centered Outcomes Research Institute.
SOURCE: Mao JJ et al. ASCO 2018. Abstract 10001.
Cancer survivors who have trouble sleeping saw improvements with both cognitive-behavioral therapy designed specifically for insomnia (CBT-I) and acupuncture, according to results from the randomized, controlled CHOICE trial. But the former is more efficacious.
“Insomnia can have deleterious effects on quality of life and function, and occurs in up to 60% of cancer survivors,” lead study author Jun J. Mao, MD, chief of integrative medicine service at Memorial Sloan Kettering Cancer Center, New York, said in a press briefing held in advance of the annual meeting of the American Society of Clinical Oncology.
“CBT-I is a highly effective therapy and can be considered the gold standard of treatment,” he noted. However, this modality may be limited by poor adherence and nonresponse. Moreover, it is highly specialized and not currently available in many cancer centers or communities.
Functional imaging studies have shown that acupuncture can regulate brain regions involving cognition and emotion that are essential to sleep regulation, and clinical research has shown that it can improve pain- and hot flash–related sleep disturbances, according to Dr. Mao. About 73% of U.S. comprehensive cancer centers offer acupuncture for symptom management.
Main results of the CHOICE (Choosing Options for Insomnia in Cancer Effectively) trial showed that patients in both the CBT-I and acupuncture groups reduced their Insomnia Severity Index scores by more than one-half at the end of the 8-weeks treatment period, but the reduction was a statistically significant 2.6 points greater with CBT-I. Benefit of each treatment was still evident after 12 weeks.
Response rate was higher with CBT-I than with acupuncture only among patients having mild insomnia at baseline, and the two treatments yielded similar improvements in mental and physical quality of life.
“Among cancer patients with insomnia, we found that both acupuncture and CBT-I produced clinically meaningful and durable benefit, but overall, CBT-I is more effective in reducing insomnia severity,” Dr. Mao concluded. “Our hope is that by doing this type of research, we can help patients and clinicians pick the right kind of treatment and help them to manage their sleep. Our next step is to really examine for what type of patient treatment would be beneficial, and how to deliver this type of effective treatment to the broader community of cancer patients.”
Insomnia among cancer survivors is both prevalent and problematic, agreed ASCO President Bruce E. Johnson, MD, FASCO.
“The most common way we treat this is pharmacologically, with sleeping pills,” he noted. “This trial shows that two different methods using something other than medications can help people with sleep, and not only do they help people with sleep, but they improve their quality of life.
“We think this information will be helpful for clinicians who end up having to decide, and also, we would use this information to help decide about how the severity of the insomnia is going to influence the treatment,” maintained Dr. Johnson, who is also a professor of medicine at the Dana-Farber Cancer Institute in Boston, and a leader of the center’s lung cancer program.
Study details
The CHOICE trial did not have any restrictions on cancer type or stage; more than a half-dozen types were represented among the 160 patients enrolled, with breast cancer (31%) and prostate cancer (23%) accounting for the largest shares. The majority of patients were white (70%) and had moderate to severe insomnia (79%).
Patients were randomized to receive either acupuncture sessions (10 sessions, with points selected to treat insomnia plus comorbid symptoms such as fatigue and anxiety) or CBT-I (7 sessions), each over the course of 8 weeks.
Main results showed that at the end of treatment, the reduction in Insomnia Severity Index was 8.3 points with acupuncture and 10.9 points with CBT-I (P = .0007), Dr. Mao reported. Benefit of each treatment was sustained after 12 weeks.
In stratified analysis, the rate of response (defined as a greater than 8-point reduction) was higher with CBT-I than with acupuncture among patients with mild insomnia (Insomnia Severity Index of 8-14) (85% vs. 18%; P less than .0001), but not among patients with moderate or severe insomnia (Insomnia Severity Index of 15 or higher) (75% vs. 66%; P = .26).
The two treatments were similarly efficacious with respect to quality of life, assessed with the Patient-Reported Outcomes Measurement Information System over the entire course of the trial, for both the physical health component (P = .4) and the mental health component (P = .36).
Dr. Mao disclosed no relevant conflicts of interest. The study received funding from the Patient-Centered Outcomes Research Institute.
SOURCE: Mao JJ et al. ASCO 2018. Abstract 10001.
REPORTING FROM ASCO 2018
Key clinical point:
Major finding: After 8 weeks of treatment, the reduction in Insomnia Severity Index was 8.3 points with acupuncture and 10.9 points with CBT-I (P = .0007).
Study details: A randomized, controlled trial among 160 survivors of diverse cancers having any degree of insomnia.
Disclosures: Dr. Mao disclosed no relevant conflicts of interest. The study received funding from the Patient-Centered Outcomes Research Institute.
Source: Mao JJ et al. ASCO 2018. Abstract 10001.