User login
FDA authorizes first direct-to-consumer BRCA1/2 test
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
The Food and Drug Administration has authorized the first direct-to-consumer (DTC) test to report on three specific BRCA1/BRCA2 breast cancer gene mutations.
Personal Genome Service Genetic Health Risk (GHR) Report for BRCA1/BRCA2 (Selected Variants) does not identify the most common BRCA1/2 mutations but rather the three most common in people of Ashkenazi (Eastern European) Jewish descent, the FDA said in a press statement.
The test, marketed by 23andMe, analyzes DNA from a self-collected saliva sample.
The three mutations identified by the test are present in about 2% of Ashkenazi Jewish women, but rarely in other ethnic populations. Any individual who takes the test may have other mutations in BRCA1 or BRCA2 genes, or other cancer-related gene mutations that are not detected by this test.
“This test provides information to certain individuals who may be at increased breast, ovarian, or prostate cancer risk and who might not otherwise get genetic screening and is a step forward in the availability of DTC genetic tests. But it has a lot of caveats,” Donald St. Pierre, acting director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, said in the press statement. “While the detection of a BRCA mutation on this test does indicate an increased risk, only a small percentage of Americans carry one of these three mutations and most BRCA mutations that increase an individual’s risk are not detected by this test. The test should not be used as a substitute for seeing your doctor for cancer screenings or counseling on genetic and lifestyle factors that can increase or decrease cancer risk.”
The authorization was based on data provided by the company to indicate the test correctly identifies the three genetic variants in saliva samples and is reproducible. In addition, the company submitted data to demonstrate that the instructions are comprehensible and easy to follow.
The FDA cautions that consumers and health care professionals “should not use the test results to determine any treatments, including antihormone therapies and prophylactic removal of the breasts or ovaries.” Decisions should be made only after confirmatory testing and genetic counseling, they said.
FDA approves abemaciclib plus aromatase inhibitor as initial therapy
Abemaciclib (Verzenio) in combination with an aromatase inhibitor has been approved as initial endocrine-based therapy for postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, the US Food and Drug Administration announced in a press release.
Approval was based on the results of the MONARCH 3 study, a randomized, double-blind, placebo-controlled, multicenter clinical trial in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer. A total of 493 patients were randomized to receive either abemaciclib 150 mg or placebo orally twice daily, plus the treating physician’s choice of letrozole or anastrozole. The estimated median progression-free survival (PFS) (RECIST 1.1) was 28.2 months (95% CI: 23.5, not reached) for patients receiving abemaciclib and 14.8 months (95% CI: 11.2, 19.2) for those receiving placebo (HR 0.540; 95% CI: 0.418, 0.698; p<0.0001).
The most common adverse reactions that were seen in at least 20% of patients receiving abemaciclib in MONARCH 3 and were reported at a rate more than 2% higher than the rates seen in the placebo arm were diarrhea, neutropenia, fatigue, infections, nausea, abdominal pain, anemia, vomiting, alopecia, decreased appetite, and leukopenia.
The recommended starting dose of abemaciclib in combination with an aromatase inhibitor is 150 mg twice daily orally with or without food.
Abemaciclib (Verzenio) is manufactured by Eli Lilly.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf.
Abemaciclib (Verzenio) in combination with an aromatase inhibitor has been approved as initial endocrine-based therapy for postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, the US Food and Drug Administration announced in a press release.
Approval was based on the results of the MONARCH 3 study, a randomized, double-blind, placebo-controlled, multicenter clinical trial in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer. A total of 493 patients were randomized to receive either abemaciclib 150 mg or placebo orally twice daily, plus the treating physician’s choice of letrozole or anastrozole. The estimated median progression-free survival (PFS) (RECIST 1.1) was 28.2 months (95% CI: 23.5, not reached) for patients receiving abemaciclib and 14.8 months (95% CI: 11.2, 19.2) for those receiving placebo (HR 0.540; 95% CI: 0.418, 0.698; p<0.0001).
The most common adverse reactions that were seen in at least 20% of patients receiving abemaciclib in MONARCH 3 and were reported at a rate more than 2% higher than the rates seen in the placebo arm were diarrhea, neutropenia, fatigue, infections, nausea, abdominal pain, anemia, vomiting, alopecia, decreased appetite, and leukopenia.
The recommended starting dose of abemaciclib in combination with an aromatase inhibitor is 150 mg twice daily orally with or without food.
Abemaciclib (Verzenio) is manufactured by Eli Lilly.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf.
Abemaciclib (Verzenio) in combination with an aromatase inhibitor has been approved as initial endocrine-based therapy for postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, the US Food and Drug Administration announced in a press release.
Approval was based on the results of the MONARCH 3 study, a randomized, double-blind, placebo-controlled, multicenter clinical trial in postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer. A total of 493 patients were randomized to receive either abemaciclib 150 mg or placebo orally twice daily, plus the treating physician’s choice of letrozole or anastrozole. The estimated median progression-free survival (PFS) (RECIST 1.1) was 28.2 months (95% CI: 23.5, not reached) for patients receiving abemaciclib and 14.8 months (95% CI: 11.2, 19.2) for those receiving placebo (HR 0.540; 95% CI: 0.418, 0.698; p<0.0001).
The most common adverse reactions that were seen in at least 20% of patients receiving abemaciclib in MONARCH 3 and were reported at a rate more than 2% higher than the rates seen in the placebo arm were diarrhea, neutropenia, fatigue, infections, nausea, abdominal pain, anemia, vomiting, alopecia, decreased appetite, and leukopenia.
The recommended starting dose of abemaciclib in combination with an aromatase inhibitor is 150 mg twice daily orally with or without food.
Abemaciclib (Verzenio) is manufactured by Eli Lilly.
Full prescribing information is available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208855s000lbl.pdf.
Breast cancer deaths projected for 2018
Female breast cancer mortality is expected to be about 25.3 per 100,000 women in 2018, with the highest rate in the District of Columbia and the lowest in Utah.
Approximately 40,920 deaths from invasive female breast cancer are predicted in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, which is based on analysis of 2001-2015 data from the National Center for Health Statistics. The death rate has declined 39% since its peak in 1989, and over the last 10 years, the annual decline has been 1.8% for white women and 1.5% for black women per year, the ACS said.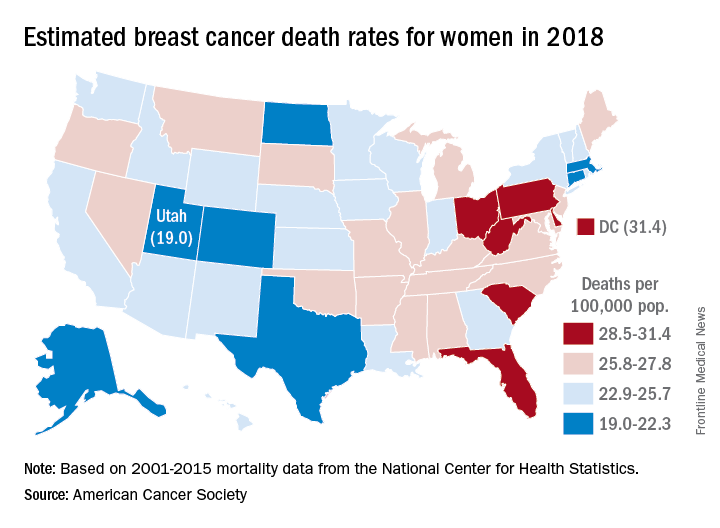
Breast cancer is the most common cancer in women, as it is expected to account for 30% of the almost 880,000 new cancer cases in 2018, compared with 13% for lung cancer, which is second. Lung cancer, however, is projected to cause more deaths among women – 70,500 – than any other cancer, the ACS reported.
Female breast cancer mortality is expected to be about 25.3 per 100,000 women in 2018, with the highest rate in the District of Columbia and the lowest in Utah.
Approximately 40,920 deaths from invasive female breast cancer are predicted in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, which is based on analysis of 2001-2015 data from the National Center for Health Statistics. The death rate has declined 39% since its peak in 1989, and over the last 10 years, the annual decline has been 1.8% for white women and 1.5% for black women per year, the ACS said.
Breast cancer is the most common cancer in women, as it is expected to account for 30% of the almost 880,000 new cancer cases in 2018, compared with 13% for lung cancer, which is second. Lung cancer, however, is projected to cause more deaths among women – 70,500 – than any other cancer, the ACS reported.
Female breast cancer mortality is expected to be about 25.3 per 100,000 women in 2018, with the highest rate in the District of Columbia and the lowest in Utah.
Approximately 40,920 deaths from invasive female breast cancer are predicted in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, which is based on analysis of 2001-2015 data from the National Center for Health Statistics. The death rate has declined 39% since its peak in 1989, and over the last 10 years, the annual decline has been 1.8% for white women and 1.5% for black women per year, the ACS said.
Breast cancer is the most common cancer in women, as it is expected to account for 30% of the almost 880,000 new cancer cases in 2018, compared with 13% for lung cancer, which is second. Lung cancer, however, is projected to cause more deaths among women – 70,500 – than any other cancer, the ACS reported.
Measurement of physical activity and sedentary behavior in breast cancer survivors
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
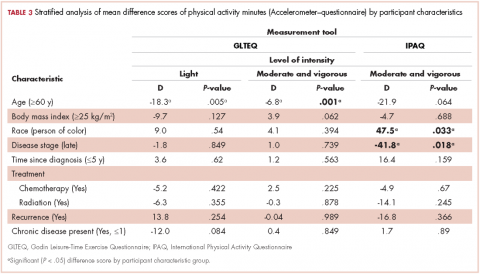
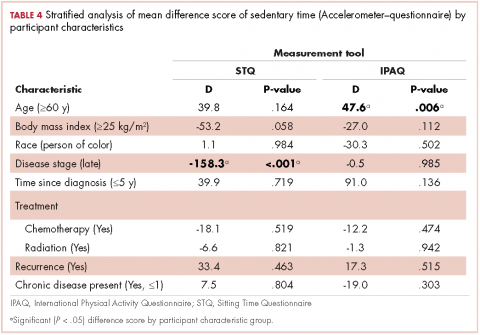
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
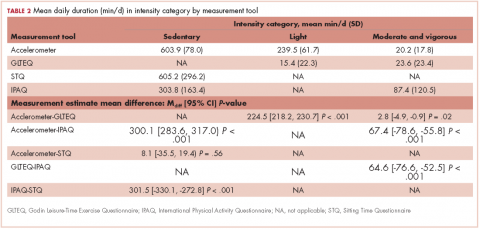
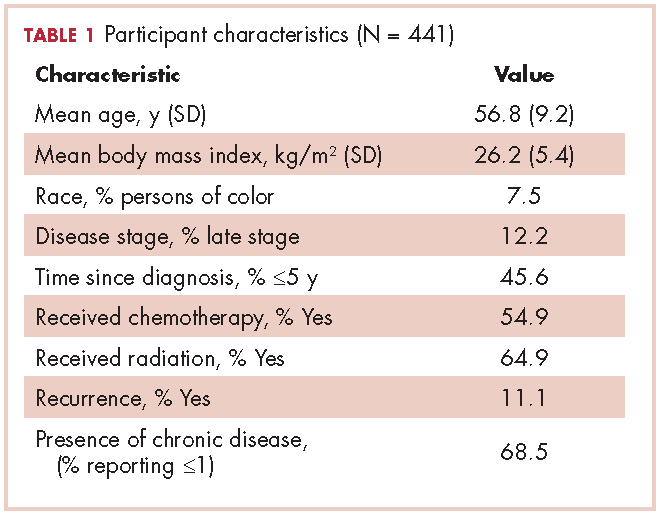
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
Abemaciclib becomes first CDK inhibitor to clinch single-agent approval for breast cancer
The fall 2017 approval by the US Food and Drug Administration (FDA) of abemaciclib made it the third cyclin-dependent kinase (CDK) inhibitor approved for the treatment of hormone receptor (HR)-positive breast cancer, and the first to receive an approved indication as monotherapy in that setting. Abemaciclib is a small-molecule inhibitor of the CDK4 and CDK6 proteins, which are key gatekeepers of the cell cycle and frequently dysregulated in HR-positive breast cancer. On the basis of the randomized, placebo-controlled, multicenter phase 3 MONARCH-2 trial, it was approved in combination with fulvestrant for the treatment of women with HR-positive, HER2-negative advanced or metastatic breast cancer who had progressed during endocrine therapy.1
A total of 669 women aged 18 years and older, with any menopausal status, an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) or nonmeasurable bone-only disease, were enrolled. Patients had progressed during neoadjuvant or adjuvant endocrine therapy, within 12 months of adjuvant endocrine therapy, or during frontline endocrine treatment for metastatic disease.
Those who had received more than 1 endocrine therapy or any prior chemotherapy for metastatic breast cancer or prior treatment with everolimus or CDK4/6 inhibitors, as well as those with the presence of visceral crisis or evidence or history of central nervous system (CNS) metastases, were excluded from the study.
Patients were randomized 2:1 to receive 150 mg abemaciclib or placebo, both in combination with 500 mg fulvestrant. The initial dose of abemaciclib was 200 mg, but this was amended to 150mg after enrollment of the first 178 patients to alleviate diarrhea-related toxicity concerns. Randomization was stratified according to metastatic site (visceral, bone only, or other) and endocrine therapy resistance (primary or secondary).
Tumors were measured by computed tomography (CT) and magnetic-resonance imaging (MRI) according to RECIST-1.1 within 28 days before random assignment, every 8 weeks for the first year, every 12 weeks thereafter, and then within 2 weeks of clinical progression. Bone scintigraphy was also performed at baseline and then every 6th cycle starting with cycle 7. Hematologic and blood chemistry laboratory tests were performed centrally on days 1 and 15 of the first cycle and day 1 of all remaining cycles.
The primary endpoint was progression-free survival (PFS); median PFS was 16.4 months in the abemaciclib arm, compared with 9.3 months in the placebo arm in the intent-to-treat population (hazard ratio [HR], 0.553;P < .0000001), translating to a 45% reduction in the risk of disease progression or death with the combination. Objective response rate in the 2 groups among patients with measurable disease was 48.1% and 21.3%, respectively, which included a complete response rate of 3.5% in the abemaciclib arm. The median duration of response was not yet reached in the study group, compared with 25.6 months for placebo. Overall survival data were not yet mature.
The agency also approved abemaciclib as monotherapy for women and men with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting. That approval was based on data from the single-arm MONARCH-1 trial of 132 patients who received 200 mg abemaciclib twice daily on a continuous schedule.2
Patients had adequate organ function, measurable disease per RECIST-1.1, and an ECOG performance status of 0 or 1. Patients must have progressed on or after previous endocrine therapy and have received prior treatment with at least 2 chemotherapy regimens, at least 1 of them, but no more than 2, having been administered in the metastatic setting. Exclusion criteria included prior receipt of a CDK inhibitor, major surgery within 14 days of the start of the study, and CNS metastases.
Tumor assessments were performed by CT or MRI according to RECIST-1.1 within the 4 weeks prior to the first dose of study drug and then subsequently at every other cycle. Responses were confirmed at least 4 weeks after the initial observation. The overall response rate was 19.7%, made up completely of partial responses. Median duration of response was 8.6 months, median PFS was 6 months and median OS was 17.7 months.
Adverse events
The most common adverse events experienced with the combination of abemaciclib and fulvestrant were neutropenia (23.6%) and diarrhea (13.4%). The rate of grade 4 neutropenia was higher in the combination arm (2.9% vs 0.4%) and there were 3 deaths with the combination that were linked to treatment-related AEs. In the monotherapy trial, abemaciclib treatment most commonly caused diarrhea (90.2%), fatigue (65.2%), nausea (64.4%), decreased appetite (45.5%), and abdominal pain (38.6%). Grade 3 diarrhea and fatigue occurred in 19.7% and 12.9% of patients, respectively. Serious AEs occurred in 24.2% of patients and AEs led to treatment discontinuation in 7.6% of patients.
Warnings and precautions
Abemaciclib is marketed as Verzenio by Eli Lilly and Company. Warnings and precautions relating to diarrhea, neutropenia, hepatotoxicity, venous thromboembolism (VTE), and embryofetal toxicity are detailed in the prescribing information. In the event of diarrhea, patients should be treated with antidiarrheal therapy and should increase oral fluids and notify their health care provider. Treatment should be interrupted for grade 3 or 4 diarrhea and then resumed at a lower dose upon return to grade 1.
To guard against neutropenia, complete blood counts should be performed prior to starting therapy, every 2 weeks for the first 2 months, monthly for the subsequent 2 months, and then as clinically indicated. Treatment should be interrupted or delayed or the dose reduced for grade 3 or 4 neutropenia and patients should report episodes of fever.
Liver function tests should be performed before starting abemaciclib, every 2 weeks for the first 2 months, monthly for the next 2 months, and then as
Patients should be monitored for signs and symptoms of VTE and pulmonary embolism, and treated appropriately. Pregnant women should be advised of the potential risk to a fetus, and those of reproductive potential should be counselled on the importance of using effective contraception during treatment and for at least 3 weeks after the last dose.3
1. Sledge Jr GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884.
2. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res. http://clincancerres.aacrjournals.org/content/early/2017/05/20/1078-0432.CCR-17-0754. Published online first on May 22, 2017. Accessed January 19, 2018.
3. Verzenio (abemaciclib) tablets, for oral use. Prescribing information. Eli Lilly and Co. http://uspl.lilly.com/verzenio/verzenio.html#pi. September 2017. Accessed November 20, 2017.
The fall 2017 approval by the US Food and Drug Administration (FDA) of abemaciclib made it the third cyclin-dependent kinase (CDK) inhibitor approved for the treatment of hormone receptor (HR)-positive breast cancer, and the first to receive an approved indication as monotherapy in that setting. Abemaciclib is a small-molecule inhibitor of the CDK4 and CDK6 proteins, which are key gatekeepers of the cell cycle and frequently dysregulated in HR-positive breast cancer. On the basis of the randomized, placebo-controlled, multicenter phase 3 MONARCH-2 trial, it was approved in combination with fulvestrant for the treatment of women with HR-positive, HER2-negative advanced or metastatic breast cancer who had progressed during endocrine therapy.1
A total of 669 women aged 18 years and older, with any menopausal status, an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) or nonmeasurable bone-only disease, were enrolled. Patients had progressed during neoadjuvant or adjuvant endocrine therapy, within 12 months of adjuvant endocrine therapy, or during frontline endocrine treatment for metastatic disease.
Those who had received more than 1 endocrine therapy or any prior chemotherapy for metastatic breast cancer or prior treatment with everolimus or CDK4/6 inhibitors, as well as those with the presence of visceral crisis or evidence or history of central nervous system (CNS) metastases, were excluded from the study.
Patients were randomized 2:1 to receive 150 mg abemaciclib or placebo, both in combination with 500 mg fulvestrant. The initial dose of abemaciclib was 200 mg, but this was amended to 150mg after enrollment of the first 178 patients to alleviate diarrhea-related toxicity concerns. Randomization was stratified according to metastatic site (visceral, bone only, or other) and endocrine therapy resistance (primary or secondary).
Tumors were measured by computed tomography (CT) and magnetic-resonance imaging (MRI) according to RECIST-1.1 within 28 days before random assignment, every 8 weeks for the first year, every 12 weeks thereafter, and then within 2 weeks of clinical progression. Bone scintigraphy was also performed at baseline and then every 6th cycle starting with cycle 7. Hematologic and blood chemistry laboratory tests were performed centrally on days 1 and 15 of the first cycle and day 1 of all remaining cycles.
The primary endpoint was progression-free survival (PFS); median PFS was 16.4 months in the abemaciclib arm, compared with 9.3 months in the placebo arm in the intent-to-treat population (hazard ratio [HR], 0.553;P < .0000001), translating to a 45% reduction in the risk of disease progression or death with the combination. Objective response rate in the 2 groups among patients with measurable disease was 48.1% and 21.3%, respectively, which included a complete response rate of 3.5% in the abemaciclib arm. The median duration of response was not yet reached in the study group, compared with 25.6 months for placebo. Overall survival data were not yet mature.
The agency also approved abemaciclib as monotherapy for women and men with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting. That approval was based on data from the single-arm MONARCH-1 trial of 132 patients who received 200 mg abemaciclib twice daily on a continuous schedule.2
Patients had adequate organ function, measurable disease per RECIST-1.1, and an ECOG performance status of 0 or 1. Patients must have progressed on or after previous endocrine therapy and have received prior treatment with at least 2 chemotherapy regimens, at least 1 of them, but no more than 2, having been administered in the metastatic setting. Exclusion criteria included prior receipt of a CDK inhibitor, major surgery within 14 days of the start of the study, and CNS metastases.
Tumor assessments were performed by CT or MRI according to RECIST-1.1 within the 4 weeks prior to the first dose of study drug and then subsequently at every other cycle. Responses were confirmed at least 4 weeks after the initial observation. The overall response rate was 19.7%, made up completely of partial responses. Median duration of response was 8.6 months, median PFS was 6 months and median OS was 17.7 months.
Adverse events
The most common adverse events experienced with the combination of abemaciclib and fulvestrant were neutropenia (23.6%) and diarrhea (13.4%). The rate of grade 4 neutropenia was higher in the combination arm (2.9% vs 0.4%) and there were 3 deaths with the combination that were linked to treatment-related AEs. In the monotherapy trial, abemaciclib treatment most commonly caused diarrhea (90.2%), fatigue (65.2%), nausea (64.4%), decreased appetite (45.5%), and abdominal pain (38.6%). Grade 3 diarrhea and fatigue occurred in 19.7% and 12.9% of patients, respectively. Serious AEs occurred in 24.2% of patients and AEs led to treatment discontinuation in 7.6% of patients.
Warnings and precautions
Abemaciclib is marketed as Verzenio by Eli Lilly and Company. Warnings and precautions relating to diarrhea, neutropenia, hepatotoxicity, venous thromboembolism (VTE), and embryofetal toxicity are detailed in the prescribing information. In the event of diarrhea, patients should be treated with antidiarrheal therapy and should increase oral fluids and notify their health care provider. Treatment should be interrupted for grade 3 or 4 diarrhea and then resumed at a lower dose upon return to grade 1.
To guard against neutropenia, complete blood counts should be performed prior to starting therapy, every 2 weeks for the first 2 months, monthly for the subsequent 2 months, and then as clinically indicated. Treatment should be interrupted or delayed or the dose reduced for grade 3 or 4 neutropenia and patients should report episodes of fever.
Liver function tests should be performed before starting abemaciclib, every 2 weeks for the first 2 months, monthly for the next 2 months, and then as
Patients should be monitored for signs and symptoms of VTE and pulmonary embolism, and treated appropriately. Pregnant women should be advised of the potential risk to a fetus, and those of reproductive potential should be counselled on the importance of using effective contraception during treatment and for at least 3 weeks after the last dose.3
The fall 2017 approval by the US Food and Drug Administration (FDA) of abemaciclib made it the third cyclin-dependent kinase (CDK) inhibitor approved for the treatment of hormone receptor (HR)-positive breast cancer, and the first to receive an approved indication as monotherapy in that setting. Abemaciclib is a small-molecule inhibitor of the CDK4 and CDK6 proteins, which are key gatekeepers of the cell cycle and frequently dysregulated in HR-positive breast cancer. On the basis of the randomized, placebo-controlled, multicenter phase 3 MONARCH-2 trial, it was approved in combination with fulvestrant for the treatment of women with HR-positive, HER2-negative advanced or metastatic breast cancer who had progressed during endocrine therapy.1
A total of 669 women aged 18 years and older, with any menopausal status, an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) or nonmeasurable bone-only disease, were enrolled. Patients had progressed during neoadjuvant or adjuvant endocrine therapy, within 12 months of adjuvant endocrine therapy, or during frontline endocrine treatment for metastatic disease.
Those who had received more than 1 endocrine therapy or any prior chemotherapy for metastatic breast cancer or prior treatment with everolimus or CDK4/6 inhibitors, as well as those with the presence of visceral crisis or evidence or history of central nervous system (CNS) metastases, were excluded from the study.
Patients were randomized 2:1 to receive 150 mg abemaciclib or placebo, both in combination with 500 mg fulvestrant. The initial dose of abemaciclib was 200 mg, but this was amended to 150mg after enrollment of the first 178 patients to alleviate diarrhea-related toxicity concerns. Randomization was stratified according to metastatic site (visceral, bone only, or other) and endocrine therapy resistance (primary or secondary).
Tumors were measured by computed tomography (CT) and magnetic-resonance imaging (MRI) according to RECIST-1.1 within 28 days before random assignment, every 8 weeks for the first year, every 12 weeks thereafter, and then within 2 weeks of clinical progression. Bone scintigraphy was also performed at baseline and then every 6th cycle starting with cycle 7. Hematologic and blood chemistry laboratory tests were performed centrally on days 1 and 15 of the first cycle and day 1 of all remaining cycles.
The primary endpoint was progression-free survival (PFS); median PFS was 16.4 months in the abemaciclib arm, compared with 9.3 months in the placebo arm in the intent-to-treat population (hazard ratio [HR], 0.553;P < .0000001), translating to a 45% reduction in the risk of disease progression or death with the combination. Objective response rate in the 2 groups among patients with measurable disease was 48.1% and 21.3%, respectively, which included a complete response rate of 3.5% in the abemaciclib arm. The median duration of response was not yet reached in the study group, compared with 25.6 months for placebo. Overall survival data were not yet mature.
The agency also approved abemaciclib as monotherapy for women and men with HR-positive, HER2-negative advanced or metastatic breast cancer with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting. That approval was based on data from the single-arm MONARCH-1 trial of 132 patients who received 200 mg abemaciclib twice daily on a continuous schedule.2
Patients had adequate organ function, measurable disease per RECIST-1.1, and an ECOG performance status of 0 or 1. Patients must have progressed on or after previous endocrine therapy and have received prior treatment with at least 2 chemotherapy regimens, at least 1 of them, but no more than 2, having been administered in the metastatic setting. Exclusion criteria included prior receipt of a CDK inhibitor, major surgery within 14 days of the start of the study, and CNS metastases.
Tumor assessments were performed by CT or MRI according to RECIST-1.1 within the 4 weeks prior to the first dose of study drug and then subsequently at every other cycle. Responses were confirmed at least 4 weeks after the initial observation. The overall response rate was 19.7%, made up completely of partial responses. Median duration of response was 8.6 months, median PFS was 6 months and median OS was 17.7 months.
Adverse events
The most common adverse events experienced with the combination of abemaciclib and fulvestrant were neutropenia (23.6%) and diarrhea (13.4%). The rate of grade 4 neutropenia was higher in the combination arm (2.9% vs 0.4%) and there were 3 deaths with the combination that were linked to treatment-related AEs. In the monotherapy trial, abemaciclib treatment most commonly caused diarrhea (90.2%), fatigue (65.2%), nausea (64.4%), decreased appetite (45.5%), and abdominal pain (38.6%). Grade 3 diarrhea and fatigue occurred in 19.7% and 12.9% of patients, respectively. Serious AEs occurred in 24.2% of patients and AEs led to treatment discontinuation in 7.6% of patients.
Warnings and precautions
Abemaciclib is marketed as Verzenio by Eli Lilly and Company. Warnings and precautions relating to diarrhea, neutropenia, hepatotoxicity, venous thromboembolism (VTE), and embryofetal toxicity are detailed in the prescribing information. In the event of diarrhea, patients should be treated with antidiarrheal therapy and should increase oral fluids and notify their health care provider. Treatment should be interrupted for grade 3 or 4 diarrhea and then resumed at a lower dose upon return to grade 1.
To guard against neutropenia, complete blood counts should be performed prior to starting therapy, every 2 weeks for the first 2 months, monthly for the subsequent 2 months, and then as clinically indicated. Treatment should be interrupted or delayed or the dose reduced for grade 3 or 4 neutropenia and patients should report episodes of fever.
Liver function tests should be performed before starting abemaciclib, every 2 weeks for the first 2 months, monthly for the next 2 months, and then as
Patients should be monitored for signs and symptoms of VTE and pulmonary embolism, and treated appropriately. Pregnant women should be advised of the potential risk to a fetus, and those of reproductive potential should be counselled on the importance of using effective contraception during treatment and for at least 3 weeks after the last dose.3
1. Sledge Jr GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884.
2. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res. http://clincancerres.aacrjournals.org/content/early/2017/05/20/1078-0432.CCR-17-0754. Published online first on May 22, 2017. Accessed January 19, 2018.
3. Verzenio (abemaciclib) tablets, for oral use. Prescribing information. Eli Lilly and Co. http://uspl.lilly.com/verzenio/verzenio.html#pi. September 2017. Accessed November 20, 2017.
1. Sledge Jr GW, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884.
2. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res. http://clincancerres.aacrjournals.org/content/early/2017/05/20/1078-0432.CCR-17-0754. Published online first on May 22, 2017. Accessed January 19, 2018.
3. Verzenio (abemaciclib) tablets, for oral use. Prescribing information. Eli Lilly and Co. http://uspl.lilly.com/verzenio/verzenio.html#pi. September 2017. Accessed November 20, 2017.
Exercise during chemotherapy may yield long-term physical benefits
ORLANDO – Physical exercise during adjuvant chemotherapy may lead to improved physical activity and decreased fatigue years after the treatment is completed, results of a recent analysis suggest.
Four years after participating in an exercise program that took place during cancer treatment, patients reported more moderate-to-vigorous activity and less fatigue, compared with patients who did not participate in the program, according to long-term follow-up results presented at the Cancer Survivorship Symposium.
“We think that offering exercise during cancer treatment, including chemotherapy, is recommended and has beneficial short- and long-term effects on health,” said Anne M. May, PhD, of University Medical Center, Utrecht, the Netherlands.
Speaking in a press conference at the symposium, Dr. May described results of the analysis, which included 128 patients who had previously participated in PACT, a 237-patient randomized controlled trial evaluating a supervised exercise program versus usual care in patients undergoing adjuvant treatment for breast or colon cancer.
The 18-week exercise program included moderate- to high-intensity aerobic and strength training under physical therapist supervision for 60 minutes twice weekly, plus home-based physical activity for 30 minutes three times weekly.
At 4 years’ follow-up, patients in the exercise group reported on average 90 minutes of moderate-to-vigorous exercise per week, compared to an average of 70 minutes per week in the usual care group (P less than .05), Dr. May reported at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
There was also a trend toward decreased fatigue reported in the exercise vs. usual care group, though the finding did not reach statistical significance, she said.
It is “encouraging” to see that this exercise program had a long-term impact on patients’ physical activity levels, said ASCO expert Timothy Gilligan, MD, MSc.
“I think the public sometimes gets jaded because the nutritional recommendations seem to change every year, but if you look at the research on exercise in health … it’s interesting how consistent the data is that exercise really is good for us – if we can only get people to do it,” said Dr. Gilligan, who moderated the press conference.
Dr. May said she had no disclosures to report for the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
SOURCE: May AM et al. Cancer Survivorship Symposium, Abstract 99.
ORLANDO – Physical exercise during adjuvant chemotherapy may lead to improved physical activity and decreased fatigue years after the treatment is completed, results of a recent analysis suggest.
Four years after participating in an exercise program that took place during cancer treatment, patients reported more moderate-to-vigorous activity and less fatigue, compared with patients who did not participate in the program, according to long-term follow-up results presented at the Cancer Survivorship Symposium.
“We think that offering exercise during cancer treatment, including chemotherapy, is recommended and has beneficial short- and long-term effects on health,” said Anne M. May, PhD, of University Medical Center, Utrecht, the Netherlands.
Speaking in a press conference at the symposium, Dr. May described results of the analysis, which included 128 patients who had previously participated in PACT, a 237-patient randomized controlled trial evaluating a supervised exercise program versus usual care in patients undergoing adjuvant treatment for breast or colon cancer.
The 18-week exercise program included moderate- to high-intensity aerobic and strength training under physical therapist supervision for 60 minutes twice weekly, plus home-based physical activity for 30 minutes three times weekly.
At 4 years’ follow-up, patients in the exercise group reported on average 90 minutes of moderate-to-vigorous exercise per week, compared to an average of 70 minutes per week in the usual care group (P less than .05), Dr. May reported at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
There was also a trend toward decreased fatigue reported in the exercise vs. usual care group, though the finding did not reach statistical significance, she said.
It is “encouraging” to see that this exercise program had a long-term impact on patients’ physical activity levels, said ASCO expert Timothy Gilligan, MD, MSc.
“I think the public sometimes gets jaded because the nutritional recommendations seem to change every year, but if you look at the research on exercise in health … it’s interesting how consistent the data is that exercise really is good for us – if we can only get people to do it,” said Dr. Gilligan, who moderated the press conference.
Dr. May said she had no disclosures to report for the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
SOURCE: May AM et al. Cancer Survivorship Symposium, Abstract 99.
ORLANDO – Physical exercise during adjuvant chemotherapy may lead to improved physical activity and decreased fatigue years after the treatment is completed, results of a recent analysis suggest.
Four years after participating in an exercise program that took place during cancer treatment, patients reported more moderate-to-vigorous activity and less fatigue, compared with patients who did not participate in the program, according to long-term follow-up results presented at the Cancer Survivorship Symposium.
“We think that offering exercise during cancer treatment, including chemotherapy, is recommended and has beneficial short- and long-term effects on health,” said Anne M. May, PhD, of University Medical Center, Utrecht, the Netherlands.
Speaking in a press conference at the symposium, Dr. May described results of the analysis, which included 128 patients who had previously participated in PACT, a 237-patient randomized controlled trial evaluating a supervised exercise program versus usual care in patients undergoing adjuvant treatment for breast or colon cancer.
The 18-week exercise program included moderate- to high-intensity aerobic and strength training under physical therapist supervision for 60 minutes twice weekly, plus home-based physical activity for 30 minutes three times weekly.
At 4 years’ follow-up, patients in the exercise group reported on average 90 minutes of moderate-to-vigorous exercise per week, compared to an average of 70 minutes per week in the usual care group (P less than .05), Dr. May reported at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
There was also a trend toward decreased fatigue reported in the exercise vs. usual care group, though the finding did not reach statistical significance, she said.
It is “encouraging” to see that this exercise program had a long-term impact on patients’ physical activity levels, said ASCO expert Timothy Gilligan, MD, MSc.
“I think the public sometimes gets jaded because the nutritional recommendations seem to change every year, but if you look at the research on exercise in health … it’s interesting how consistent the data is that exercise really is good for us – if we can only get people to do it,” said Dr. Gilligan, who moderated the press conference.
Dr. May said she had no disclosures to report for the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
SOURCE: May AM et al. Cancer Survivorship Symposium, Abstract 99.
REPORTING FROM CSC 2018
Key clinical point: Exercise during adjuvant chemotherapy may lead to lower levels of fatigue and higher levels of physical activity years after treatment is completed.
Major finding: Four years after participating in an 18-week exercise program during cancer treatment, patients reported an average of 90 minutes of moderate-to-vigorous physical activity per day, compared with 70 minutes for patients who did not participate (P less than .05).
Study details: Long-term follow-up of 128 patients who had participated in the PACT study, a randomized controlled trial of a supervised exercise program versus usual care in breast and colon cancer patients undergoing adjuvant treatment including chemotherapy.
Disclosures: The authors reported no disclosures relevant to the study, which was supported by grants from the Dutch Cancer Society, the Dutch Pink Ribbon Foundation, and the Netherlands Organization for Health Research.
Source: May AM et al. Cancer Survivorship Symposium, Abstract 99.
Cyclophosphamide extends PFS in elderly HER2+ breast cancer patients
by 7 months – with acceptable toxicity – vs. dual HER2 blockade alone in a randomized, open-label, phase 2 trial.
After a median follow-up of 20.7 months, the median progression-free survival (PFS) among 41 women who received trastuzumab and pertuzumab plus metronomic oral cyclophosphamide was 12.7 months, compared with 5.6 months among 39 women who received only trastuzumab and pertuzumab. Estimated PFS at 6 months was 73.4% and 46.2% in the groups, respectively (hazard ratio, 0.65), Hans Wildiers, MD, PhD, of University Hospitals Leuven, Belgium, and his colleagues reported in Lancet Oncology.
The most frequent grade 3-4 adverse events occurring in each group, respectively, were hypertension (12% and 15%), diarrhea (12% and 10%), dyspnea (10% and 5%), fatigue (5% and 8%), and pain (5% in each group). Thromboembolic events occurred in four patients (10%) receiving cyclophosphamide, while none occurred in the dual HER2 blockade-only group. Severe cardiac toxicities were occasionally observed in both groups.
Study subjects were women aged at least 70 years, or at least 60 years with confirmed functional restrictions. All had confirmed HER2-positive metastatic breast cancer and no prior chemotherapy for metastatic disease. They received either intravenous trastuzumab at a loading dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks, and intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks (median of 6 cycles), or those same doses of trastuzumab and pertuzumab plus metronomic oral cyclophosphamide at a dose of 50 mg daily (median of 13 cycles). Subsequent treatment with trastuzumab emtansine in 29 patients who progressed during the study was active and well tolerated; the overall PFS at 6 months in this group of patients was 49.5% and median PFS was 5 months after starting trastuzumab emtansine.
“The results of this study indicate that the benefit of avoiding the side effects of chemotherapy with the use of dual anti–HER2 blockade only does not compensate for an important loss of activity in the metastatic setting,” the investigators wrote, concluding that “trastuzumab and pertuzumab plus metronomic oral cyclophosphamide, potentially followed by trastuzumab emtansine after progression, might delay the need for, or supersede, the use of taxane-based chemotherapy in this population.”
Further evaluation of this combination in a randomized phase 3 study is warranted, as the phase 2 findings do not provide “robust justification for a change in practice.” They do, however, provide a scientific framework for supporting more specific trials in older patients, who compromise nearly a third of breast cancer patients worldwide, and up to 50% in high-income countries, they said.
This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, Astra Zeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
SOURCE: Wildiers H et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
The findings by Wildiers et al. regarding the benefits of adding oral cyclophosphamide to dual HER2 blockade should be evaluated further, according to Charles E. Geyer Jr., MD, who applauded the investigators’ demonstration of a framework for much-needed clinical trials in frail elderly patients with breast cancer.
In an editorial, Dr. Geyer wrote that the trial results provide sufficient evidence for consideration of trastuzumab and pertuzumab plus oral cyclophosphamide in frail elderly patients at increased risk of adverse events from taxane-based therapies, but he also encouraged additional study (Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30084-6).
An important consideration in future studies will be the choice of a comparator group; he suggested a version of the CLEOPATRA study to look at trastuzumab plus metronomic oral cyclophosphamide with and without pertuzumab. A prior small study suggested that the problematic risk of diarrhea seen in patients in the current study did not occur in patients pretreated with trastuzumab who showed activity with trastuzumab and metronomic oral cyclophosphamide chemotherapy, he noted.
Dr. Geyer is with the Massey Cancer Center at Virginia Commonwealth University, Richmond. He reported receiving personal fees from Myriad and Heron Therapeutics for advisory board participation, and travel support from AstraZeneca, Genentech, and Macrogenics, outside the submitted work.
The findings by Wildiers et al. regarding the benefits of adding oral cyclophosphamide to dual HER2 blockade should be evaluated further, according to Charles E. Geyer Jr., MD, who applauded the investigators’ demonstration of a framework for much-needed clinical trials in frail elderly patients with breast cancer.
In an editorial, Dr. Geyer wrote that the trial results provide sufficient evidence for consideration of trastuzumab and pertuzumab plus oral cyclophosphamide in frail elderly patients at increased risk of adverse events from taxane-based therapies, but he also encouraged additional study (Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30084-6).
An important consideration in future studies will be the choice of a comparator group; he suggested a version of the CLEOPATRA study to look at trastuzumab plus metronomic oral cyclophosphamide with and without pertuzumab. A prior small study suggested that the problematic risk of diarrhea seen in patients in the current study did not occur in patients pretreated with trastuzumab who showed activity with trastuzumab and metronomic oral cyclophosphamide chemotherapy, he noted.
Dr. Geyer is with the Massey Cancer Center at Virginia Commonwealth University, Richmond. He reported receiving personal fees from Myriad and Heron Therapeutics for advisory board participation, and travel support from AstraZeneca, Genentech, and Macrogenics, outside the submitted work.
The findings by Wildiers et al. regarding the benefits of adding oral cyclophosphamide to dual HER2 blockade should be evaluated further, according to Charles E. Geyer Jr., MD, who applauded the investigators’ demonstration of a framework for much-needed clinical trials in frail elderly patients with breast cancer.
In an editorial, Dr. Geyer wrote that the trial results provide sufficient evidence for consideration of trastuzumab and pertuzumab plus oral cyclophosphamide in frail elderly patients at increased risk of adverse events from taxane-based therapies, but he also encouraged additional study (Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30084-6).
An important consideration in future studies will be the choice of a comparator group; he suggested a version of the CLEOPATRA study to look at trastuzumab plus metronomic oral cyclophosphamide with and without pertuzumab. A prior small study suggested that the problematic risk of diarrhea seen in patients in the current study did not occur in patients pretreated with trastuzumab who showed activity with trastuzumab and metronomic oral cyclophosphamide chemotherapy, he noted.
Dr. Geyer is with the Massey Cancer Center at Virginia Commonwealth University, Richmond. He reported receiving personal fees from Myriad and Heron Therapeutics for advisory board participation, and travel support from AstraZeneca, Genentech, and Macrogenics, outside the submitted work.
by 7 months – with acceptable toxicity – vs. dual HER2 blockade alone in a randomized, open-label, phase 2 trial.
After a median follow-up of 20.7 months, the median progression-free survival (PFS) among 41 women who received trastuzumab and pertuzumab plus metronomic oral cyclophosphamide was 12.7 months, compared with 5.6 months among 39 women who received only trastuzumab and pertuzumab. Estimated PFS at 6 months was 73.4% and 46.2% in the groups, respectively (hazard ratio, 0.65), Hans Wildiers, MD, PhD, of University Hospitals Leuven, Belgium, and his colleagues reported in Lancet Oncology.
The most frequent grade 3-4 adverse events occurring in each group, respectively, were hypertension (12% and 15%), diarrhea (12% and 10%), dyspnea (10% and 5%), fatigue (5% and 8%), and pain (5% in each group). Thromboembolic events occurred in four patients (10%) receiving cyclophosphamide, while none occurred in the dual HER2 blockade-only group. Severe cardiac toxicities were occasionally observed in both groups.
Study subjects were women aged at least 70 years, or at least 60 years with confirmed functional restrictions. All had confirmed HER2-positive metastatic breast cancer and no prior chemotherapy for metastatic disease. They received either intravenous trastuzumab at a loading dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks, and intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks (median of 6 cycles), or those same doses of trastuzumab and pertuzumab plus metronomic oral cyclophosphamide at a dose of 50 mg daily (median of 13 cycles). Subsequent treatment with trastuzumab emtansine in 29 patients who progressed during the study was active and well tolerated; the overall PFS at 6 months in this group of patients was 49.5% and median PFS was 5 months after starting trastuzumab emtansine.
“The results of this study indicate that the benefit of avoiding the side effects of chemotherapy with the use of dual anti–HER2 blockade only does not compensate for an important loss of activity in the metastatic setting,” the investigators wrote, concluding that “trastuzumab and pertuzumab plus metronomic oral cyclophosphamide, potentially followed by trastuzumab emtansine after progression, might delay the need for, or supersede, the use of taxane-based chemotherapy in this population.”
Further evaluation of this combination in a randomized phase 3 study is warranted, as the phase 2 findings do not provide “robust justification for a change in practice.” They do, however, provide a scientific framework for supporting more specific trials in older patients, who compromise nearly a third of breast cancer patients worldwide, and up to 50% in high-income countries, they said.
This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, Astra Zeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
SOURCE: Wildiers H et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
by 7 months – with acceptable toxicity – vs. dual HER2 blockade alone in a randomized, open-label, phase 2 trial.
After a median follow-up of 20.7 months, the median progression-free survival (PFS) among 41 women who received trastuzumab and pertuzumab plus metronomic oral cyclophosphamide was 12.7 months, compared with 5.6 months among 39 women who received only trastuzumab and pertuzumab. Estimated PFS at 6 months was 73.4% and 46.2% in the groups, respectively (hazard ratio, 0.65), Hans Wildiers, MD, PhD, of University Hospitals Leuven, Belgium, and his colleagues reported in Lancet Oncology.
The most frequent grade 3-4 adverse events occurring in each group, respectively, were hypertension (12% and 15%), diarrhea (12% and 10%), dyspnea (10% and 5%), fatigue (5% and 8%), and pain (5% in each group). Thromboembolic events occurred in four patients (10%) receiving cyclophosphamide, while none occurred in the dual HER2 blockade-only group. Severe cardiac toxicities were occasionally observed in both groups.
Study subjects were women aged at least 70 years, or at least 60 years with confirmed functional restrictions. All had confirmed HER2-positive metastatic breast cancer and no prior chemotherapy for metastatic disease. They received either intravenous trastuzumab at a loading dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks, and intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks (median of 6 cycles), or those same doses of trastuzumab and pertuzumab plus metronomic oral cyclophosphamide at a dose of 50 mg daily (median of 13 cycles). Subsequent treatment with trastuzumab emtansine in 29 patients who progressed during the study was active and well tolerated; the overall PFS at 6 months in this group of patients was 49.5% and median PFS was 5 months after starting trastuzumab emtansine.
“The results of this study indicate that the benefit of avoiding the side effects of chemotherapy with the use of dual anti–HER2 blockade only does not compensate for an important loss of activity in the metastatic setting,” the investigators wrote, concluding that “trastuzumab and pertuzumab plus metronomic oral cyclophosphamide, potentially followed by trastuzumab emtansine after progression, might delay the need for, or supersede, the use of taxane-based chemotherapy in this population.”
Further evaluation of this combination in a randomized phase 3 study is warranted, as the phase 2 findings do not provide “robust justification for a change in practice.” They do, however, provide a scientific framework for supporting more specific trials in older patients, who compromise nearly a third of breast cancer patients worldwide, and up to 50% in high-income countries, they said.
This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, Astra Zeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
SOURCE: Wildiers H et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
FROM THE LANCET ONCOLOGY
Key clinical point: Adding oral cyclophosphamide to trastuzumab and pertuzumab benefits frail elderly breast cancer patients.
Major finding: PFS was 12.7 months vs. 5.6 months with trastuzumab and pertuzumab plus metronomic oral cyclophosphamide vs. trastuzumab and pertuzumab alone.
Study details: A randomized phase 2 trial in 80 patients.
Disclosures: This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, MacroGenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
Source: Wildiers Hans et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
PAM50-based score identifies low-risk subset with node-positive early-stage breast cancer
The PAM50-based risk of recurrence (ROR) score enabled selection of a subset of patients with estrogen receptor (ER)–positive early-stage breast cancer with node-positive or node-negative disease with an estimated 10-year distant recurrence (DR) absolute risk of less than 5%, investigators reported.
This subset of patients with one to three positive lymph nodes presented a sufficiently low risk of disease recurrence and could safely avoid chemotherapy, reported Anne-Vibeke Laenkholm, MD, and her associates. The report was published in the Journal of Clinical Oncology.
The PAM50-based ROR score (Prosigna Score; Nanostring Technologies, Seattle) is a comparative gene expression profile of the tumor, relative to each of the four PAM50 molecular profiles (luminal A, luminal B, basal, and HER2 enriched) to determine the degree of similarity. The Prosigna assay currently has Food and Drug Administration clearance for prognostic use.
Postmenopausal women diagnosed with ER-positive/HER2-negative early-stage breast cancer in Denmark between 2000 and 2003, and who received adjuvant treatment with tamoxifen or an aromatase inhibitor without chemotherapy were eligible for this cohort study.
The primary endpoint was time to DR, defined as the interval from breast cancer surgery until DR or death as a result of breast cancer. Secondary endpoints included overall survival and time to any recurrence. “The median age of patients was 63 years (range, 50-89 years); 45.5% were node negative, and 54.5% had one to three positive nodes. Tumor characteristics including nodal status, tumor size, malignancy grade, and lymphovascular invasion were all associated with DR, whereas treatment adherence was not,” wrote Dr. Laenkholm of the department of surgical pathology, Zealand University Hospital, Denmark, and her colleagues.
“With a median follow-up of approximately 9 years, 26% of the patients with node-positive disease were categorized as having a low ROR score corresponding to a DR of 3.5% at 10 years compared with those with an intermediate ROR score with a DR of 11.5% and those with a high ROR score corresponding to a DR risk of 22%. In the node-negative group, those with a low ROR score had a 10-year DR risk of 5% and those with an intermediate ROR score had a 7.3% 10-year DR risk, compared with a DR of 17.8% in those with a high ROR score,” Dr. Laenkholm and her associates reported.
The data are entirely prognostic and do not address whether chemotherapy might offer some benefit in a node-positive population (i.e., one to three nodes), or whether adjuvant chemotherapy–associated adverse effects may offset the added benefit, if any, the investigators said.
Nonetheless, genomic assay–based scoring tools are an important aid in clinical decision making readily available to the physician at the patient’s bedside, they said.
Nanostring Technologies funded the study. Laboratory work flow was executed at the department of surgical pathology, Zealand University Hospital. Several authors reported ties with Roche and other pharmaceutical companies.
SOURCE: Laenkholm et al. J Clin Oncol. 2018 Jan 25. doi: 10.1200/JCO.2017.74.6586.
The study by Laenkholm et al. provides encouraging data for the clinical utility of molecular assays to aid decision making for node-positive disease.
The investigators were able to select a subset of patients with node-positive disease with an estimated 10-year distant recurrence risk of less than or equal to 5%, based on the PAM50 risk of recurrence score. It could be argued that the data do not address the issue of whether chemotherapy would offer some benefit (predictive) in a node-positive population; however, a counterargument is that whatever small added benefit may be derived from adjuvant chemotherapy would be offset by adverse effects, resulting in a net gain of zero. The collective data from evaluation of other assays in this population suggest that patients with ER-positive disease, one to three positive nodes, and a low recurrence score can safely avoid adjuvant chemotherapy and receive endocrine therapy alone. This supports the current NCCN guidelines, which indicate the recurrence score assay can be considered in patients with one to three positive nodes. The more definitive answer on whether adjuvant chemotherapy adds benefit to endocrine therapy will be determined from the RxPONDER study, in which patients with ER+ disease, one to three positive nodes, and a recurrence score less than or equal to 25 will be randomly assigned to chemotherapy or not.
Ricardo L. B. Costa, MD, MS, is with the H. Lee Moffitt Cancer Center, Tampa. William J. Gradishar, MD, is with Northwestern University, Chicago. Dr. Costa disclosed ties with Bristol-Myers Squibb. Dr. Gradishar reported having no relevant financial disclosures. Their comments were adapted from an editorial (J Clin Oncol. 2018 Jan 25. doi: 10.1200/JCO.2017.76.9802).
The study by Laenkholm et al. provides encouraging data for the clinical utility of molecular assays to aid decision making for node-positive disease.
The investigators were able to select a subset of patients with node-positive disease with an estimated 10-year distant recurrence risk of less than or equal to 5%, based on the PAM50 risk of recurrence score. It could be argued that the data do not address the issue of whether chemotherapy would offer some benefit (predictive) in a node-positive population; however, a counterargument is that whatever small added benefit may be derived from adjuvant chemotherapy would be offset by adverse effects, resulting in a net gain of zero. The collective data from evaluation of other assays in this population suggest that patients with ER-positive disease, one to three positive nodes, and a low recurrence score can safely avoid adjuvant chemotherapy and receive endocrine therapy alone. This supports the current NCCN guidelines, which indicate the recurrence score assay can be considered in patients with one to three positive nodes. The more definitive answer on whether adjuvant chemotherapy adds benefit to endocrine therapy will be determined from the RxPONDER study, in which patients with ER+ disease, one to three positive nodes, and a recurrence score less than or equal to 25 will be randomly assigned to chemotherapy or not.
Ricardo L. B. Costa, MD, MS, is with the H. Lee Moffitt Cancer Center, Tampa. William J. Gradishar, MD, is with Northwestern University, Chicago. Dr. Costa disclosed ties with Bristol-Myers Squibb. Dr. Gradishar reported having no relevant financial disclosures. Their comments were adapted from an editorial (J Clin Oncol. 2018 Jan 25. doi: 10.1200/JCO.2017.76.9802).
The study by Laenkholm et al. provides encouraging data for the clinical utility of molecular assays to aid decision making for node-positive disease.
The investigators were able to select a subset of patients with node-positive disease with an estimated 10-year distant recurrence risk of less than or equal to 5%, based on the PAM50 risk of recurrence score. It could be argued that the data do not address the issue of whether chemotherapy would offer some benefit (predictive) in a node-positive population; however, a counterargument is that whatever small added benefit may be derived from adjuvant chemotherapy would be offset by adverse effects, resulting in a net gain of zero. The collective data from evaluation of other assays in this population suggest that patients with ER-positive disease, one to three positive nodes, and a low recurrence score can safely avoid adjuvant chemotherapy and receive endocrine therapy alone. This supports the current NCCN guidelines, which indicate the recurrence score assay can be considered in patients with one to three positive nodes. The more definitive answer on whether adjuvant chemotherapy adds benefit to endocrine therapy will be determined from the RxPONDER study, in which patients with ER+ disease, one to three positive nodes, and a recurrence score less than or equal to 25 will be randomly assigned to chemotherapy or not.
Ricardo L. B. Costa, MD, MS, is with the H. Lee Moffitt Cancer Center, Tampa. William J. Gradishar, MD, is with Northwestern University, Chicago. Dr. Costa disclosed ties with Bristol-Myers Squibb. Dr. Gradishar reported having no relevant financial disclosures. Their comments were adapted from an editorial (J Clin Oncol. 2018 Jan 25. doi: 10.1200/JCO.2017.76.9802).
The PAM50-based risk of recurrence (ROR) score enabled selection of a subset of patients with estrogen receptor (ER)–positive early-stage breast cancer with node-positive or node-negative disease with an estimated 10-year distant recurrence (DR) absolute risk of less than 5%, investigators reported.
This subset of patients with one to three positive lymph nodes presented a sufficiently low risk of disease recurrence and could safely avoid chemotherapy, reported Anne-Vibeke Laenkholm, MD, and her associates. The report was published in the Journal of Clinical Oncology.
The PAM50-based ROR score (Prosigna Score; Nanostring Technologies, Seattle) is a comparative gene expression profile of the tumor, relative to each of the four PAM50 molecular profiles (luminal A, luminal B, basal, and HER2 enriched) to determine the degree of similarity. The Prosigna assay currently has Food and Drug Administration clearance for prognostic use.
Postmenopausal women diagnosed with ER-positive/HER2-negative early-stage breast cancer in Denmark between 2000 and 2003, and who received adjuvant treatment with tamoxifen or an aromatase inhibitor without chemotherapy were eligible for this cohort study.
The primary endpoint was time to DR, defined as the interval from breast cancer surgery until DR or death as a result of breast cancer. Secondary endpoints included overall survival and time to any recurrence. “The median age of patients was 63 years (range, 50-89 years); 45.5% were node negative, and 54.5% had one to three positive nodes. Tumor characteristics including nodal status, tumor size, malignancy grade, and lymphovascular invasion were all associated with DR, whereas treatment adherence was not,” wrote Dr. Laenkholm of the department of surgical pathology, Zealand University Hospital, Denmark, and her colleagues.
“With a median follow-up of approximately 9 years, 26% of the patients with node-positive disease were categorized as having a low ROR score corresponding to a DR of 3.5% at 10 years compared with those with an intermediate ROR score with a DR of 11.5% and those with a high ROR score corresponding to a DR risk of 22%. In the node-negative group, those with a low ROR score had a 10-year DR risk of 5% and those with an intermediate ROR score had a 7.3% 10-year DR risk, compared with a DR of 17.8% in those with a high ROR score,” Dr. Laenkholm and her associates reported.
The data are entirely prognostic and do not address whether chemotherapy might offer some benefit in a node-positive population (i.e., one to three nodes), or whether adjuvant chemotherapy–associated adverse effects may offset the added benefit, if any, the investigators said.
Nonetheless, genomic assay–based scoring tools are an important aid in clinical decision making readily available to the physician at the patient’s bedside, they said.
Nanostring Technologies funded the study. Laboratory work flow was executed at the department of surgical pathology, Zealand University Hospital. Several authors reported ties with Roche and other pharmaceutical companies.
SOURCE: Laenkholm et al. J Clin Oncol. 2018 Jan 25. doi: 10.1200/JCO.2017.74.6586.
The PAM50-based risk of recurrence (ROR) score enabled selection of a subset of patients with estrogen receptor (ER)–positive early-stage breast cancer with node-positive or node-negative disease with an estimated 10-year distant recurrence (DR) absolute risk of less than 5%, investigators reported.
This subset of patients with one to three positive lymph nodes presented a sufficiently low risk of disease recurrence and could safely avoid chemotherapy, reported Anne-Vibeke Laenkholm, MD, and her associates. The report was published in the Journal of Clinical Oncology.
The PAM50-based ROR score (Prosigna Score; Nanostring Technologies, Seattle) is a comparative gene expression profile of the tumor, relative to each of the four PAM50 molecular profiles (luminal A, luminal B, basal, and HER2 enriched) to determine the degree of similarity. The Prosigna assay currently has Food and Drug Administration clearance for prognostic use.
Postmenopausal women diagnosed with ER-positive/HER2-negative early-stage breast cancer in Denmark between 2000 and 2003, and who received adjuvant treatment with tamoxifen or an aromatase inhibitor without chemotherapy were eligible for this cohort study.
The primary endpoint was time to DR, defined as the interval from breast cancer surgery until DR or death as a result of breast cancer. Secondary endpoints included overall survival and time to any recurrence. “The median age of patients was 63 years (range, 50-89 years); 45.5% were node negative, and 54.5% had one to three positive nodes. Tumor characteristics including nodal status, tumor size, malignancy grade, and lymphovascular invasion were all associated with DR, whereas treatment adherence was not,” wrote Dr. Laenkholm of the department of surgical pathology, Zealand University Hospital, Denmark, and her colleagues.
“With a median follow-up of approximately 9 years, 26% of the patients with node-positive disease were categorized as having a low ROR score corresponding to a DR of 3.5% at 10 years compared with those with an intermediate ROR score with a DR of 11.5% and those with a high ROR score corresponding to a DR risk of 22%. In the node-negative group, those with a low ROR score had a 10-year DR risk of 5% and those with an intermediate ROR score had a 7.3% 10-year DR risk, compared with a DR of 17.8% in those with a high ROR score,” Dr. Laenkholm and her associates reported.
The data are entirely prognostic and do not address whether chemotherapy might offer some benefit in a node-positive population (i.e., one to three nodes), or whether adjuvant chemotherapy–associated adverse effects may offset the added benefit, if any, the investigators said.
Nonetheless, genomic assay–based scoring tools are an important aid in clinical decision making readily available to the physician at the patient’s bedside, they said.
Nanostring Technologies funded the study. Laboratory work flow was executed at the department of surgical pathology, Zealand University Hospital. Several authors reported ties with Roche and other pharmaceutical companies.
SOURCE: Laenkholm et al. J Clin Oncol. 2018 Jan 25. doi: 10.1200/JCO.2017.74.6586.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The score enabled selection of a subset of patients with node-positive or node-negative disease with an estimated 10-year DR absolute risk of less than 5%.
Major finding: About 37% of patients with a single positive lymph node and 15% of patients with two positive nodes were identified as low risk by Prosigna, with very favorable outcomes when treated with adjuvant endocrine therapy alone.
Study details: Danish Breast Cancer Cooperative Group database including postmenopausal patients in Denmark diagnosed with ER-positive invasive breast cancer from 2000 through 2003 and treated with endocrine therapy for 5 years.
Disclosures: Nanostring Technologies funded the study. Laboratory work flow was executed at the department of surgical pathology, Zealand University Hospital. Several authors reported ties with Roche and other pharmaceutical companies.
Source: Laenkholm et al. J Clin Oncol. 2018 Jan 25. doi: 10.1200/JCO.2017.74.6586.
AHA: Heart health helps optimize breast cancer outcomes
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
FROM CIRCULATION
Hodgkin lymphoma survivors are at an increased risk of subsequent ER-negative breast cancer
Young women with primary Hodgkin lymphoma had an increased relative risk of estrogen receptor–positive breast cancer if they received radiotherapy and, irrespective of the type of treatment they got, an elevated risk of ER-negative breast cancer, based on results of a study based on patient records from 12 U.S. National Cancer Institute Surveillance, Epidemiology, and End Results registries.
Of 7,355 women diagnosed with primary Hodgkin lymphoma during 1973-2009 and aged 10-39 years, 377 patients subsequently were diagnosed with breast cancer at a mean age of 45 years; 57% of the cancers were ER positive, 34% were ER negative, and 9% had unknown/borderline ER status, Diana R. Withrow, PhD, and her colleagues from the radiation epidemiology branch, division of cancer epidemiology and genetics, National Cancer Institute reported in JAMA Oncology.
Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8; 95% confidence interval, 4.8-6.9) than ER-positive breast cancer (SIR, 3.1; 95% CI, 2.7-3.5; P less than .001 for the difference), the researchers wrote.
For ER-positive disease, the increased SIR was observed only among women who had received radiotherapy for their Hodgkin lymphoma (SIR, 3.9; 95% CI, 3.4-4.5). In this group, the SIR for ER-positive disease was lower in the chemotherapy than in the no/unknown chemotherapy group (P = .04), said the researchers.
The authors acknowledged that lack of information on patient risk factors such as family history, reproductive factors, and hormone therapy, as well as detailed treatment information such as radiotherapy dose, fields, specific chemotherapeutic agents, and subsequent therapy is a limitation of the current study. Further research, including comprehensive treatment records, will lead to a better understanding of the association between treatment and breast cancer subtype in these patients, the researchers concluded.
None of the study authors reported any conflicts of interest.
SOURCE: Withrow D et al. doi: 10.1001/jamaoncol.2017.4887.
Young women with primary Hodgkin lymphoma had an increased relative risk of estrogen receptor–positive breast cancer if they received radiotherapy and, irrespective of the type of treatment they got, an elevated risk of ER-negative breast cancer, based on results of a study based on patient records from 12 U.S. National Cancer Institute Surveillance, Epidemiology, and End Results registries.
Of 7,355 women diagnosed with primary Hodgkin lymphoma during 1973-2009 and aged 10-39 years, 377 patients subsequently were diagnosed with breast cancer at a mean age of 45 years; 57% of the cancers were ER positive, 34% were ER negative, and 9% had unknown/borderline ER status, Diana R. Withrow, PhD, and her colleagues from the radiation epidemiology branch, division of cancer epidemiology and genetics, National Cancer Institute reported in JAMA Oncology.
Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8; 95% confidence interval, 4.8-6.9) than ER-positive breast cancer (SIR, 3.1; 95% CI, 2.7-3.5; P less than .001 for the difference), the researchers wrote.
For ER-positive disease, the increased SIR was observed only among women who had received radiotherapy for their Hodgkin lymphoma (SIR, 3.9; 95% CI, 3.4-4.5). In this group, the SIR for ER-positive disease was lower in the chemotherapy than in the no/unknown chemotherapy group (P = .04), said the researchers.
The authors acknowledged that lack of information on patient risk factors such as family history, reproductive factors, and hormone therapy, as well as detailed treatment information such as radiotherapy dose, fields, specific chemotherapeutic agents, and subsequent therapy is a limitation of the current study. Further research, including comprehensive treatment records, will lead to a better understanding of the association between treatment and breast cancer subtype in these patients, the researchers concluded.
None of the study authors reported any conflicts of interest.
SOURCE: Withrow D et al. doi: 10.1001/jamaoncol.2017.4887.
Young women with primary Hodgkin lymphoma had an increased relative risk of estrogen receptor–positive breast cancer if they received radiotherapy and, irrespective of the type of treatment they got, an elevated risk of ER-negative breast cancer, based on results of a study based on patient records from 12 U.S. National Cancer Institute Surveillance, Epidemiology, and End Results registries.
Of 7,355 women diagnosed with primary Hodgkin lymphoma during 1973-2009 and aged 10-39 years, 377 patients subsequently were diagnosed with breast cancer at a mean age of 45 years; 57% of the cancers were ER positive, 34% were ER negative, and 9% had unknown/borderline ER status, Diana R. Withrow, PhD, and her colleagues from the radiation epidemiology branch, division of cancer epidemiology and genetics, National Cancer Institute reported in JAMA Oncology.
Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8; 95% confidence interval, 4.8-6.9) than ER-positive breast cancer (SIR, 3.1; 95% CI, 2.7-3.5; P less than .001 for the difference), the researchers wrote.
For ER-positive disease, the increased SIR was observed only among women who had received radiotherapy for their Hodgkin lymphoma (SIR, 3.9; 95% CI, 3.4-4.5). In this group, the SIR for ER-positive disease was lower in the chemotherapy than in the no/unknown chemotherapy group (P = .04), said the researchers.
The authors acknowledged that lack of information on patient risk factors such as family history, reproductive factors, and hormone therapy, as well as detailed treatment information such as radiotherapy dose, fields, specific chemotherapeutic agents, and subsequent therapy is a limitation of the current study. Further research, including comprehensive treatment records, will lead to a better understanding of the association between treatment and breast cancer subtype in these patients, the researchers concluded.
None of the study authors reported any conflicts of interest.
SOURCE: Withrow D et al. doi: 10.1001/jamaoncol.2017.4887.
FROM JAMA ONCOLOGY
Key clinical point: .
Major finding: Survivors of Hodgkin lymphoma had a greater relative risk of ER-negative (standardized incidence ratio, 5.8) than ER-positive breast cancer (SIR, 3.1).
Study details: 7,355 women diagnosed with first primary Hodgkin lymphoma during 1973-2009, who were aged 10-39 years, and reported to 12 U.S. National Cancer Institute SEER registries.
Disclosures: None reported.
Source: Withrow D et al. doi: 10.1001/jamaoncolo.2017.4887.