User login
Device closure of PFO doesn’t drop combined major adverse event risk
NASHVILLE, TENN. – Device closure of a patent foramen ovale didn’t reduce the composite risk of death, stroke, or transient ischemic attack when compared against medical therapy alone in a pooled analysis of three trials.
Device intervention, did, however, reduce the individual risk of recurrent stroke, although the absolute reduction seemed modest, Dr. David M. Kent said at the International Stroke Conference, sponsored by the American Heart Association.
Overall device closure conferred about a 30% annual risk reduction of recurrent stroke upon those with patent foramen ovale (PFO) who had experienced a prior cryptogenic stroke. About 30 patients would have to be treated to prevent one stroke over 5 years, said Dr. Kent of Tufts University, Boston.
“It’s not a huge benefit – it’s not like thrombectomy. But it’s comparable to the benefit of high-dose statins seen in the SPARCL [Stroke Prevention by Aggressive Reduction in Cholesterol Levels] trial.”
Dr. Kent and his colleagues’ analysis used three trials: the CLOSURE trial, which used the STARflex device, and two trials that tested the Amplatzer device, the PC TRIAL and RESPECT.
Altogether, the pooled analysis comprised about 2,300 patients. Of these, 440 were lost to 5-year follow-up; their outcomes were imputed into an intent-to-treat analysis based on last-known clinical status.
Dr. Kent and his colleagues also conducted a subanalysis of only the two Amplatzer device trials, which comprised 1,400 patients.
The primary outcome for each analysis was a composite of recurrent stroke, transient ischemic attack (TIA), and early death. The secondary outcome was recurrent stroke alone.
The patients were a mean of 45 years old. About a third had dyslipidemia, and a third had hypertension. The PFO was considered large in 60%.
During the 5-year follow-up period, there were 58 strokes and 54 TIAs. Four patients died during the trial – two in the device arms and two in the medical therapy arms.
When all three trials were analyzed together, PFO closure was not significantly better than medical therapy in the composite endpoint (1.5% vs. 2.3%). The difference in recurrent stroke rate alone was significant (0.7% vs. 1.3%), although Dr. Kent did note that the event rate was very low in both groups.
In the two Amplatzer trials, the device also did not significantly reduce the risk of the composite endpoint. It did confer significant benefit on the stroke-only outcome (hazard ratio, 0.41; 0.4% vs. 1.1%). Again, Dr. Kent said, the event rate was very low for both intervention groups.
There was no significantly increased risk of bleeding with PFO closure in the analysis of all three trials or in the two Amplatzer device trials alone, although both did find a significantly increased risk of atrial fibrillation. In the three-trial analysis, rates of atrial fibrillation were 1.5% vs. 0.48%; in the Amplatzer-only analysis, rates were 0.87% vs. 0.47%. The investigators weren’t able to identify any factors that might predispose to a safety event.
The analyses were sponsored by the National Institutes of Health. Dr. Kent had no financial disclosures, although several coauthors had ties with multiple pharmaceutical companies, or were investigators on the original trials, which were sponsored by the device manufacturers.
On Twitter @alz_gal
NASHVILLE, TENN. – Device closure of a patent foramen ovale didn’t reduce the composite risk of death, stroke, or transient ischemic attack when compared against medical therapy alone in a pooled analysis of three trials.
Device intervention, did, however, reduce the individual risk of recurrent stroke, although the absolute reduction seemed modest, Dr. David M. Kent said at the International Stroke Conference, sponsored by the American Heart Association.
Overall device closure conferred about a 30% annual risk reduction of recurrent stroke upon those with patent foramen ovale (PFO) who had experienced a prior cryptogenic stroke. About 30 patients would have to be treated to prevent one stroke over 5 years, said Dr. Kent of Tufts University, Boston.
“It’s not a huge benefit – it’s not like thrombectomy. But it’s comparable to the benefit of high-dose statins seen in the SPARCL [Stroke Prevention by Aggressive Reduction in Cholesterol Levels] trial.”
Dr. Kent and his colleagues’ analysis used three trials: the CLOSURE trial, which used the STARflex device, and two trials that tested the Amplatzer device, the PC TRIAL and RESPECT.
Altogether, the pooled analysis comprised about 2,300 patients. Of these, 440 were lost to 5-year follow-up; their outcomes were imputed into an intent-to-treat analysis based on last-known clinical status.
Dr. Kent and his colleagues also conducted a subanalysis of only the two Amplatzer device trials, which comprised 1,400 patients.
The primary outcome for each analysis was a composite of recurrent stroke, transient ischemic attack (TIA), and early death. The secondary outcome was recurrent stroke alone.
The patients were a mean of 45 years old. About a third had dyslipidemia, and a third had hypertension. The PFO was considered large in 60%.
During the 5-year follow-up period, there were 58 strokes and 54 TIAs. Four patients died during the trial – two in the device arms and two in the medical therapy arms.
When all three trials were analyzed together, PFO closure was not significantly better than medical therapy in the composite endpoint (1.5% vs. 2.3%). The difference in recurrent stroke rate alone was significant (0.7% vs. 1.3%), although Dr. Kent did note that the event rate was very low in both groups.
In the two Amplatzer trials, the device also did not significantly reduce the risk of the composite endpoint. It did confer significant benefit on the stroke-only outcome (hazard ratio, 0.41; 0.4% vs. 1.1%). Again, Dr. Kent said, the event rate was very low for both intervention groups.
There was no significantly increased risk of bleeding with PFO closure in the analysis of all three trials or in the two Amplatzer device trials alone, although both did find a significantly increased risk of atrial fibrillation. In the three-trial analysis, rates of atrial fibrillation were 1.5% vs. 0.48%; in the Amplatzer-only analysis, rates were 0.87% vs. 0.47%. The investigators weren’t able to identify any factors that might predispose to a safety event.
The analyses were sponsored by the National Institutes of Health. Dr. Kent had no financial disclosures, although several coauthors had ties with multiple pharmaceutical companies, or were investigators on the original trials, which were sponsored by the device manufacturers.
On Twitter @alz_gal
NASHVILLE, TENN. – Device closure of a patent foramen ovale didn’t reduce the composite risk of death, stroke, or transient ischemic attack when compared against medical therapy alone in a pooled analysis of three trials.
Device intervention, did, however, reduce the individual risk of recurrent stroke, although the absolute reduction seemed modest, Dr. David M. Kent said at the International Stroke Conference, sponsored by the American Heart Association.
Overall device closure conferred about a 30% annual risk reduction of recurrent stroke upon those with patent foramen ovale (PFO) who had experienced a prior cryptogenic stroke. About 30 patients would have to be treated to prevent one stroke over 5 years, said Dr. Kent of Tufts University, Boston.
“It’s not a huge benefit – it’s not like thrombectomy. But it’s comparable to the benefit of high-dose statins seen in the SPARCL [Stroke Prevention by Aggressive Reduction in Cholesterol Levels] trial.”
Dr. Kent and his colleagues’ analysis used three trials: the CLOSURE trial, which used the STARflex device, and two trials that tested the Amplatzer device, the PC TRIAL and RESPECT.
Altogether, the pooled analysis comprised about 2,300 patients. Of these, 440 were lost to 5-year follow-up; their outcomes were imputed into an intent-to-treat analysis based on last-known clinical status.
Dr. Kent and his colleagues also conducted a subanalysis of only the two Amplatzer device trials, which comprised 1,400 patients.
The primary outcome for each analysis was a composite of recurrent stroke, transient ischemic attack (TIA), and early death. The secondary outcome was recurrent stroke alone.
The patients were a mean of 45 years old. About a third had dyslipidemia, and a third had hypertension. The PFO was considered large in 60%.
During the 5-year follow-up period, there were 58 strokes and 54 TIAs. Four patients died during the trial – two in the device arms and two in the medical therapy arms.
When all three trials were analyzed together, PFO closure was not significantly better than medical therapy in the composite endpoint (1.5% vs. 2.3%). The difference in recurrent stroke rate alone was significant (0.7% vs. 1.3%), although Dr. Kent did note that the event rate was very low in both groups.
In the two Amplatzer trials, the device also did not significantly reduce the risk of the composite endpoint. It did confer significant benefit on the stroke-only outcome (hazard ratio, 0.41; 0.4% vs. 1.1%). Again, Dr. Kent said, the event rate was very low for both intervention groups.
There was no significantly increased risk of bleeding with PFO closure in the analysis of all three trials or in the two Amplatzer device trials alone, although both did find a significantly increased risk of atrial fibrillation. In the three-trial analysis, rates of atrial fibrillation were 1.5% vs. 0.48%; in the Amplatzer-only analysis, rates were 0.87% vs. 0.47%. The investigators weren’t able to identify any factors that might predispose to a safety event.
The analyses were sponsored by the National Institutes of Health. Dr. Kent had no financial disclosures, although several coauthors had ties with multiple pharmaceutical companies, or were investigators on the original trials, which were sponsored by the device manufacturers.
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Device closure of a patent foramen ovale seems to reduce the risk of recurrent stroke – but not a composite of death, stroke, or TIA – better than medical therapy.
Major finding: Device closure of PFO conferred about a 30% decline in the risk of recurrent stroke over a 5-year follow-up.
Data source: The analysis pooled results for 2,300 patients who participated in three trials.
Disclosures: The analyses were sponsored by the National Institutes of Health. Dr. Kent had no financial disclosures, although several coauthors had ties with multiple pharmaceutical companies, or were investigators on the original trials, which were sponsored by the device manufacturers.
Venous thromboembolism common after heart transplant
For every 1,000 patients who underwent heart transplantation, about 45 had an episode of venous thromboembolism within a year after surgery, according to a retrospective study reported in the February issue of the Journal of Heart and Lung Transplantation.
Furthermore, patients who had a single VTE episode after transplant had a “high” risk of recurrent VTE, said Dr. Rolando Alvarez, a cardiologist at Complejo Hospitalario Universitario A Coruna in A Coruna, Spain, and his associates.
“Our opinion is that long-term oral anticoagulation should be maintained in these patients, especially if other risk factors are present and provided that the bleeding risk is not excessive,” said the researchers.
Venous thromboembolism is a common complication of lung, kidney, and liver transplantation, but less is known about VTE after heart transplant. The researchers found that “classic” risk factors for VTE, such as being older, obese, or having renal dysfunction, also increased the risk of VTE after heart transplant (J. Heart Lung Transplant. 2015;34:167-74).
The study included data from 635 consecutive patients who underwent heart transplantation at a single hospital between 1991 and 2013. During a median of 8.4 years of follow-up, the cumulative incidence of VTE was 8.5%, for an annual incidence rate of 12.7 episodes per year for every 1,000 patients, the researchers reported. The risk of VTE was far higher during the first year after transplant (45.1 episodes per 1,000 patients), but even after excluding these episodes, VTE was six times more common among heart transplant recipients than among the general population. Furthermore, VTE recurred an estimated 30.5 times/1,000 patient-years, and 50.8 times/1,000 patients-years among patients who had stopped anticoagulants.
The cumulative incidence rate of DVT and PE were 8.4 and 8.7 episodes per 1,000 patient-years.
In the multivariate analysis, significant risk factors for VTE at less than 1 year after transplantation included age, obesity, chronic kidney disease, and emergency transplantation, the investigators said. More than a year after transplantation, only use of the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus significantly increased VTE risk.
“The evidence that supports a potential association between mTOR inhibitors and an increased risk of VTE events is still weak, and might be confounded by a high prevalence of comorbid conditions such as chronic renal failure, dyslipidemia, or malignancy in patients taking these kinds of drugs,” the investigators cautioned.
The authors suggested that in view of the high recurrence rate, long-term anticoagulation should be considered in heart transplant patients after their first VTE episode.
The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
For every 1,000 patients who underwent heart transplantation, about 45 had an episode of venous thromboembolism within a year after surgery, according to a retrospective study reported in the February issue of the Journal of Heart and Lung Transplantation.
Furthermore, patients who had a single VTE episode after transplant had a “high” risk of recurrent VTE, said Dr. Rolando Alvarez, a cardiologist at Complejo Hospitalario Universitario A Coruna in A Coruna, Spain, and his associates.
“Our opinion is that long-term oral anticoagulation should be maintained in these patients, especially if other risk factors are present and provided that the bleeding risk is not excessive,” said the researchers.
Venous thromboembolism is a common complication of lung, kidney, and liver transplantation, but less is known about VTE after heart transplant. The researchers found that “classic” risk factors for VTE, such as being older, obese, or having renal dysfunction, also increased the risk of VTE after heart transplant (J. Heart Lung Transplant. 2015;34:167-74).
The study included data from 635 consecutive patients who underwent heart transplantation at a single hospital between 1991 and 2013. During a median of 8.4 years of follow-up, the cumulative incidence of VTE was 8.5%, for an annual incidence rate of 12.7 episodes per year for every 1,000 patients, the researchers reported. The risk of VTE was far higher during the first year after transplant (45.1 episodes per 1,000 patients), but even after excluding these episodes, VTE was six times more common among heart transplant recipients than among the general population. Furthermore, VTE recurred an estimated 30.5 times/1,000 patient-years, and 50.8 times/1,000 patients-years among patients who had stopped anticoagulants.
The cumulative incidence rate of DVT and PE were 8.4 and 8.7 episodes per 1,000 patient-years.
In the multivariate analysis, significant risk factors for VTE at less than 1 year after transplantation included age, obesity, chronic kidney disease, and emergency transplantation, the investigators said. More than a year after transplantation, only use of the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus significantly increased VTE risk.
“The evidence that supports a potential association between mTOR inhibitors and an increased risk of VTE events is still weak, and might be confounded by a high prevalence of comorbid conditions such as chronic renal failure, dyslipidemia, or malignancy in patients taking these kinds of drugs,” the investigators cautioned.
The authors suggested that in view of the high recurrence rate, long-term anticoagulation should be considered in heart transplant patients after their first VTE episode.
The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
For every 1,000 patients who underwent heart transplantation, about 45 had an episode of venous thromboembolism within a year after surgery, according to a retrospective study reported in the February issue of the Journal of Heart and Lung Transplantation.
Furthermore, patients who had a single VTE episode after transplant had a “high” risk of recurrent VTE, said Dr. Rolando Alvarez, a cardiologist at Complejo Hospitalario Universitario A Coruna in A Coruna, Spain, and his associates.
“Our opinion is that long-term oral anticoagulation should be maintained in these patients, especially if other risk factors are present and provided that the bleeding risk is not excessive,” said the researchers.
Venous thromboembolism is a common complication of lung, kidney, and liver transplantation, but less is known about VTE after heart transplant. The researchers found that “classic” risk factors for VTE, such as being older, obese, or having renal dysfunction, also increased the risk of VTE after heart transplant (J. Heart Lung Transplant. 2015;34:167-74).
The study included data from 635 consecutive patients who underwent heart transplantation at a single hospital between 1991 and 2013. During a median of 8.4 years of follow-up, the cumulative incidence of VTE was 8.5%, for an annual incidence rate of 12.7 episodes per year for every 1,000 patients, the researchers reported. The risk of VTE was far higher during the first year after transplant (45.1 episodes per 1,000 patients), but even after excluding these episodes, VTE was six times more common among heart transplant recipients than among the general population. Furthermore, VTE recurred an estimated 30.5 times/1,000 patient-years, and 50.8 times/1,000 patients-years among patients who had stopped anticoagulants.
The cumulative incidence rate of DVT and PE were 8.4 and 8.7 episodes per 1,000 patient-years.
In the multivariate analysis, significant risk factors for VTE at less than 1 year after transplantation included age, obesity, chronic kidney disease, and emergency transplantation, the investigators said. More than a year after transplantation, only use of the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus significantly increased VTE risk.
“The evidence that supports a potential association between mTOR inhibitors and an increased risk of VTE events is still weak, and might be confounded by a high prevalence of comorbid conditions such as chronic renal failure, dyslipidemia, or malignancy in patients taking these kinds of drugs,” the investigators cautioned.
The authors suggested that in view of the high recurrence rate, long-term anticoagulation should be considered in heart transplant patients after their first VTE episode.
The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
Key clinical point: Venous thromboembolism (VTE) was common after heart transplant, especially when patients had relevant risk factors and were not on anticoagulants.
Major finding: Cumulative incidence of VTE was 8.5% during eight years of follow-up and was much higher during the first year after transplant.
Data source: Single-center retrospective cohort study of 635 heart transplant recipients.
Disclosures: The Fundacion BBVA-Carolina funded the study. Four coauthors reported receiving travel support from Novartis Pharma and Astellas Pharma. The other authors reported no relevant conflicts of interest.
Ranolazine plus beta-blockers might prevent postop AF
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
PHOENIX – Twice-daily ranolazine following adult cardiac surgery seemed to protect against atrial fibrillation, based on a retrospective cohort study at the University of Florida Jacksonville Medical Center.
Ranolazine (Ranexa) was dosed orally at 1,000 mg the morning of surgery, and then resumed after extubation, generally the night of surgery. The goal was 1,000 mg orally twice a day, for a maximum of 7 hospital days; patients usually went home before then, so they received an average of nine doses. The drug was discontinued at discharge.
Six (10.5%) of 57 patients in the ranolazine group developed postoperative atrial fibrillation (POAF) versus 26 (45.6%) of 57 matched controls (P < .0001). The first case came at postop day 3 in the ranolazine group, but within 24 hours in the control group. One person in the ranolazine group and one in the control group had a history of AF.
There was no statistical difference in ICU length of stay, 30-day readmission for cardiac causes, or 30-day cardiovascular mortality; the one cardiovascular death was in the control group.
Two-thirds of the patients had coronary artery bypass grafts, and the rest had either valve surgery or a combination of both surgeries. Patients were 60 years old on average, and two-thirds were men, Drayton Hammond, Pharm.D., said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
Ranolazine is indicated for chronic angina, not POAF prevention, but some previous investigations have suggested a possible benefit. A randomized, controlled clinical trial is currently looking into the matter.
At least retrospectively, the drug was “beneficial, definitely. There is about a 35% absolute-risk reduction,” said Dr. Hammond, who conducted the study while at the Jacksonville hospital.
Doctors there continue to use ranolazine for postop AF prophylaxis, as they see fit, said Dr. Hammond, now an assistant professor of pharmacy practice at the University of Arkansas for Medical Sciences, in Little Rock.
Gilead, the maker of the Ranexa, is also working on a ranolazine-dronedarone combination for paroxysmal AF.
More than half of the ranolazine patients in the study developed symptomatic hypotension within 72 hours of surgery, versus about a third in the control group (P = .0004). The drug was discontinued in one ranolazine patient because of hypotension. The problem resolved after 72 hours.
“We don’t have a good explanation” for the side effect. Perhaps there were differences in myocardial stunning or vasopressor use between the groups, but “we had the same three surgeons” for all the cases, Dr. Hammond said.
Ranolazine labeling notes the risk of hypotension and orthostatic hypotension. Labeling also warns of QT interval prolongation and renal failure in susceptible patients. The investigators found no between-group differences in bradycardia, new renal failure, or neurological events.
Overall, 53 (93%) patients in the ranolazine group were on postoperative beta-blockers, and 54 (94.7%) on postop statins; 48 (84.2%) in the control group were on beta-blockers postop and 47 (82.5%) on statins. Beta-blockers are first-line treatment to prevent postop AF; patients on any other antiarrhythmic were excluded from the trial, as were those who died during surgery.
AT THE CRITICAL CARE CONGRESS
Key clinical point: Ranolazine’s protective effect seems to come at the cost of symptomatic hypotension in the first 3 days after surgery.
Major finding: Postop atrial fibrillation occurred in 6 (10.5%) of 57 patients in the ranolazine group and 26 (45.6%) of 57 matched controls (P < .0001).
Data source: Retrospective cohort study of postop follow-up in 114 adults who had cardiac surgery.
Disclosures: There was no outside funding for the work. The investigators said they have no financial relationship with Gilead, maker of ranolazine (Ranexa).
CHADS2 predicts postop atrial fibrillation
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
PHOENIX – For every unit increase in baseline CHADS2 score, the risk of postop atrial fibrillation increases by 17%, according to a retrospective chart review of 1,550 adults who had major vascular or thoracic surgery at the Mayo Clinic in Rochester, Minn.
On multivariate analysis, postop day 1 Sequential Organ Failure Assessment score (HR 1.08, 95% CI 1.03-1.12, per unit increase) and cumulative fluid balance (HR 1.03, 95% CI 1.01-1.06, per 1,000 mL) also correlated with the risk for new-onset atrial fibrillation (AF).
Baseline calcium channel blockers protected against new-onset AF (HR 0.52, 95% CI 0.37-0.73), but, paradoxically, the risk increased with baseline (HR 1.78, 95% CI 1.24-2.56) and postop (HR 1.44, 95% CI 1.05-1.99) beta-blocker use.
The relationship of CHADS2 to new-onset AF (HR 1.17, 95% CI 1.04-1.31) could prove handy in the surgical ICU because “everyone is familiar with it, and it’s easy to calculate.” CHADS2 (heart failure, hypertension, age, diabetes, prior stroke) has also recently been shown to predict AF after cardiac surgery, said lead investigator Kirstin Kooda, Pharm.D., a critical care pharmacist at Mayo.
The beta-blocker finding was a surprise, since beta-blockers are a standard AF treatment, Dr. Kooda said at the Critical Care Congress, sponsored by the Society for Critical Care Medicine. About 80% (175) of new-onset AF patients were on baseline beta-blockers, versus about 68% (892) who did not develop AF. Patients using beta-blockers received them the morning of surgery, and resumed them a median of 7 hours afterward. There were no significant differences in heart rates during surgery.
The team excluded patients with any history of AF and censored patients if they developed it, so the drugs’ use probably wasn’t related to a concern about the condition. Just under 70% of patients in both groups had baseline hypertension, another indication for the drugs.
Even so, the finding is probably real given the number of patients in the study. Most likely, the drugs were markers for additional risk factors not captured in the study, Dr. Kooda said.
Overall, 112 (20.7%) of the 540 thoracic patients and 107 (11%) of the 1,010 vascular patients developed new-onset AF a median of 55 hours after surgery. The incidence difference and timing are in line with previous reports.
The mean age in the AF group was 70 years, and in the non-AF group it was 66 years. In both, 65% were men, 5% had heart failure, 30% had diabetes, and 10% had prior strokes. Patients with pacemakers and recent myocardial infarctions – also possible settings for beta-blockers – were excluded from the trial.
The majority of the vascular cases were open aortic aneurysms, aortic bypasses, and thrombectomies or endarterectomies of central arteries. Most of the thoracic surgeries were lobectomies, pneumonectomies, and wedge or chest wall resections.
AT THE CRITICAL CARE CONGRESS
Key clinical point: Postop atrial fibrillation is more likely if patients go into surgery with an elevated CHADS 2 score.
Major finding: For every unit increase in baseline CHADS2 score, there is a 17% increase in the risk of new-onset AF following major vascular or thoracic surgery (HR 1.17, 95% CI 1.04-1.31).
Data source: Retrospective chart review of 1,550 adult patients.
Disclosures: The investigators said they had no disclosures. No outside funding was reported for the work.
Scoring system identifies mortality risks in esophagectomy patients
SAN DIEGO – Predictive factors such as Zubrod score, previous cardiothoracic surgery, history of smoking, and hypertension can give health care providers a powerful tool for predicting the likelihood of patient morbidity resulting from esophagectomy, thus allowing accurate stratification of such patients and thereby mitigating morbidity rates, a study showed.
Patients who had previously undergone cardiothoracic surgery, had Zubrod scores of at least 2, had diabetes mellitus requiring insulin therapy, were currently smoking, had hypertension, were female, and had a forced expiratory volume in 1 second (FEV1) score of less than 60% were the most highly predisposed to mortality after undergoing esophagectomy, said Dr. J. Matthew Reinersman of the Mayo Clinic in Rochester, Minn., who presented the findings at the annual meeting of the Society of Thoracic Surgeons.
Dr. Reinersman led a team of investigators in a retrospective analysis of 343 consecutive patients in the STS General Thoracic Surgery Database who underwent esophagectomies for malignancies between August 2009 and December 2012. “Our primary outcome variables were operative mortality, both in-hospital and 30-day mortality, as well as major morbidity, which we defined as anastomotic leak, myocardial infarction, pulmonary embolism, pneumonia, reintubation for respiratory failure, empyema, chylothorax, and any reoperation,” he explained.
Univariate and multivariate analyses, using a chi-square test or Fisher’s exact analyses, were performed to look for predictors within the data set, and were subsequently used to craft the risk-based model that assigned each patient a score for how likely he or she would be to experience morbidity or mortality after undergoing an esophagectomy. Each patient was then assigned to one of four groups based on this score – group A (86 subjects), group B (138 subjects), group C (81 subjects), or group D (38 subjects) – in ascending order of score.
Dr. Reinersman and his coauthors found that 17 subjects (19.8%) in group A had either morbidity or mortality, as did 45 subjects (32.6%) in group B, 61 subjects (75.3%) in group C, and 36 (94.7%) in group D, indicating that the model and scoring system developed by the investigators was successful in predicting likely morbidity and mortality outcomes based on patients’ medical histories.
The mean patient age was 63.2 years, and the majority of subjects were male. Smokers were prevalent in the study population: Roughly 56% were former smokers, and 12% were current smokers. Endocarcinoma was the predominant tissue type observed by the authors, and the most common tumor location was the gastroesophageal junction or lower third of the esophagus. Approximately, 75% of patients received neoadjuvant therapy as well.
“This risk-assessment tool, which uses only seven factors and an easy-to-remember scoring system dividing patients into four risk categories, can be easily integrated into everyday clinical practice,” said Dr. Reinersman. “It can help inform patient selection and education, [and] also identifies smoking as an important but modifiable preoperative risk factor.”
Dr. Reinersman reported no relevant financial conflicts.
SAN DIEGO – Predictive factors such as Zubrod score, previous cardiothoracic surgery, history of smoking, and hypertension can give health care providers a powerful tool for predicting the likelihood of patient morbidity resulting from esophagectomy, thus allowing accurate stratification of such patients and thereby mitigating morbidity rates, a study showed.
Patients who had previously undergone cardiothoracic surgery, had Zubrod scores of at least 2, had diabetes mellitus requiring insulin therapy, were currently smoking, had hypertension, were female, and had a forced expiratory volume in 1 second (FEV1) score of less than 60% were the most highly predisposed to mortality after undergoing esophagectomy, said Dr. J. Matthew Reinersman of the Mayo Clinic in Rochester, Minn., who presented the findings at the annual meeting of the Society of Thoracic Surgeons.
Dr. Reinersman led a team of investigators in a retrospective analysis of 343 consecutive patients in the STS General Thoracic Surgery Database who underwent esophagectomies for malignancies between August 2009 and December 2012. “Our primary outcome variables were operative mortality, both in-hospital and 30-day mortality, as well as major morbidity, which we defined as anastomotic leak, myocardial infarction, pulmonary embolism, pneumonia, reintubation for respiratory failure, empyema, chylothorax, and any reoperation,” he explained.
Univariate and multivariate analyses, using a chi-square test or Fisher’s exact analyses, were performed to look for predictors within the data set, and were subsequently used to craft the risk-based model that assigned each patient a score for how likely he or she would be to experience morbidity or mortality after undergoing an esophagectomy. Each patient was then assigned to one of four groups based on this score – group A (86 subjects), group B (138 subjects), group C (81 subjects), or group D (38 subjects) – in ascending order of score.
Dr. Reinersman and his coauthors found that 17 subjects (19.8%) in group A had either morbidity or mortality, as did 45 subjects (32.6%) in group B, 61 subjects (75.3%) in group C, and 36 (94.7%) in group D, indicating that the model and scoring system developed by the investigators was successful in predicting likely morbidity and mortality outcomes based on patients’ medical histories.
The mean patient age was 63.2 years, and the majority of subjects were male. Smokers were prevalent in the study population: Roughly 56% were former smokers, and 12% were current smokers. Endocarcinoma was the predominant tissue type observed by the authors, and the most common tumor location was the gastroesophageal junction or lower third of the esophagus. Approximately, 75% of patients received neoadjuvant therapy as well.
“This risk-assessment tool, which uses only seven factors and an easy-to-remember scoring system dividing patients into four risk categories, can be easily integrated into everyday clinical practice,” said Dr. Reinersman. “It can help inform patient selection and education, [and] also identifies smoking as an important but modifiable preoperative risk factor.”
Dr. Reinersman reported no relevant financial conflicts.
SAN DIEGO – Predictive factors such as Zubrod score, previous cardiothoracic surgery, history of smoking, and hypertension can give health care providers a powerful tool for predicting the likelihood of patient morbidity resulting from esophagectomy, thus allowing accurate stratification of such patients and thereby mitigating morbidity rates, a study showed.
Patients who had previously undergone cardiothoracic surgery, had Zubrod scores of at least 2, had diabetes mellitus requiring insulin therapy, were currently smoking, had hypertension, were female, and had a forced expiratory volume in 1 second (FEV1) score of less than 60% were the most highly predisposed to mortality after undergoing esophagectomy, said Dr. J. Matthew Reinersman of the Mayo Clinic in Rochester, Minn., who presented the findings at the annual meeting of the Society of Thoracic Surgeons.
Dr. Reinersman led a team of investigators in a retrospective analysis of 343 consecutive patients in the STS General Thoracic Surgery Database who underwent esophagectomies for malignancies between August 2009 and December 2012. “Our primary outcome variables were operative mortality, both in-hospital and 30-day mortality, as well as major morbidity, which we defined as anastomotic leak, myocardial infarction, pulmonary embolism, pneumonia, reintubation for respiratory failure, empyema, chylothorax, and any reoperation,” he explained.
Univariate and multivariate analyses, using a chi-square test or Fisher’s exact analyses, were performed to look for predictors within the data set, and were subsequently used to craft the risk-based model that assigned each patient a score for how likely he or she would be to experience morbidity or mortality after undergoing an esophagectomy. Each patient was then assigned to one of four groups based on this score – group A (86 subjects), group B (138 subjects), group C (81 subjects), or group D (38 subjects) – in ascending order of score.
Dr. Reinersman and his coauthors found that 17 subjects (19.8%) in group A had either morbidity or mortality, as did 45 subjects (32.6%) in group B, 61 subjects (75.3%) in group C, and 36 (94.7%) in group D, indicating that the model and scoring system developed by the investigators was successful in predicting likely morbidity and mortality outcomes based on patients’ medical histories.
The mean patient age was 63.2 years, and the majority of subjects were male. Smokers were prevalent in the study population: Roughly 56% were former smokers, and 12% were current smokers. Endocarcinoma was the predominant tissue type observed by the authors, and the most common tumor location was the gastroesophageal junction or lower third of the esophagus. Approximately, 75% of patients received neoadjuvant therapy as well.
“This risk-assessment tool, which uses only seven factors and an easy-to-remember scoring system dividing patients into four risk categories, can be easily integrated into everyday clinical practice,” said Dr. Reinersman. “It can help inform patient selection and education, [and] also identifies smoking as an important but modifiable preoperative risk factor.”
Dr. Reinersman reported no relevant financial conflicts.
AT THE STS ANNUAL MEETING
Key clinical point: Certain predictive indicators can help stratify esophagectomy patients based on preoperative variables and risk factors so that morbidity rates are mitigated.
Major finding: Of 343 patients studied, morbidity occurred in 159 (45.8%); the combined 30-day and in-hospital mortality in subjects was 12 (3.5%); the most reliable predictors were prior cardiothoracic surgery, Zubrod score ≥ 2, diabetes mellitus requiring insulin therapy, current smoking, hypertension, female gender, and an FEV1 score less than 60% of predicted.
Data source: A retrospective analysis of 343 patients with esophageal cancer who underwent esophagectomy from August 2009 to December 2012.
Disclosures: Dr. Reinersman reported no relevant financial conflicts.
Hybrid revascularization remains a rare bird
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.
He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
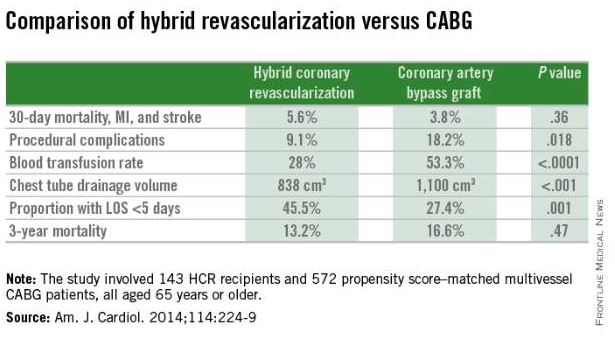
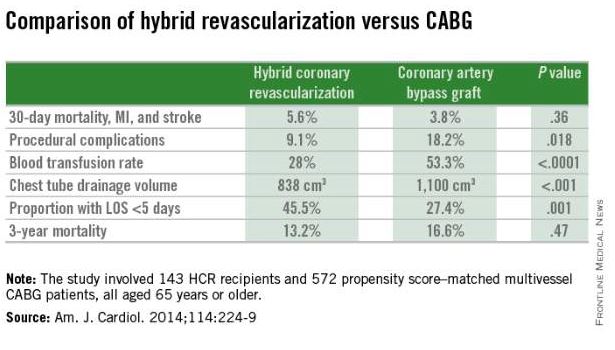
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.

He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
SNOWMASS, COLO. – For all the talk of embracing the heart team approach to coronary revascularization as the new standard, emphasizing interdisciplinary collaboration between cardiologists and surgeons, hybrid coronary revascularization remains a rarely employed strategy.
Hybrid coronary revascularization (HCR) is performed at one-third of U.S. centers providing coronary artery bypass graft (CABG) surgery, but that’s a misleading statistic. A mere handful of the centers have extensive experience with this strategy, according to the first nationwide assessment of HCR in patients with multivessel coronary artery disease.
“Only a very few centers – maybe 5 or 10 – do hybrid procedures as 8%-10% of their volume,” Dr. Vinod H. Thourani* said at the Annual Cardiovascular Conference at Snowmass.

He was a coinvestigator in the analysis of nearly 200,000 CABG procedures in the Society of Thoracic Surgeons (STS) national database for 2011-2013. Hybrid coronary revascularization – a combination of surgical and percutaneous techniques coupling minimally invasive bypass of the left anterior descending (LAD) coronary artery using an internal mammary artery graft along with drug-eluting stents for non-LAD lesions – accounted for just 0.48% of total CABG volume. And no trend for growth in the HCR approach was evident during the study period.
Moreover, even in the one-third of U.S. CABG centers that performed hybrid coronary revascularization, the strategy was used on average in less than 1% of all CABG procedures (Circulation 2014;130:872-9), noted Dr. Thourani, professor of cardiothoracic surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
In contrast, he and his Emory colleagues have fully embraced HCR. At Emory, even as the annual number of primary isolated CABG procedures with sternotomy fell by 44% from 2005 to 2012, there was a compensatory increase in the number of HCRs.
“We’ve actually maintained our coronary bypass volume based on introducing HCR, unlike a lot of programs,” according to the surgeon.
The appeal of HCR is that it combines the durability of the internal mammary artery-to-LAD graft – long recognized as the main source of the survival advantage CABG holds over percutaneous interventions – with drug-eluting stents as a less invasive alternative to often-unreliable vein grafts in non-LAD target vessels.
The current American College of Cardiology/American Heart Association guidelines on coronary revascularization give HCR a favorable class IIa recommendation. However, since there are few data on clinical outcomes with HCR versus conventional CABG, Dr. Thourani and his coinvestigators compared in-hospital outcomes in the STS database. They found that after adjustment for the higher cardiovascular risk profiles present in patients undergoing HCR, the rates of in-hospital mortality and major morbidity in the two groups were similar, suggesting HCR may offer an equally safe alternative to CABG in selected patients.
Patients want less invasive alternatives to CABG with sternotomy. The three most common minimally invasive, sternal-sparing approaches are minimally invasive direct coronary artery bypass, or MIDCAB; robotic-assisted totally endoscopic coronary artery bypass, or TECAB; and robotic-assisted coronary artery bypass, a favored approach at Emory. In robotic-assisted coronary artery bypass, the harvest of the left internal mammary artery, pericardiotomy, and targeting of the LAD are accomplished with robotic assistance, but the anastomosis is hand sewn under direct vision, off pump, via a non–rib-spreading 3- to 4-cm minithoracotomy.
In a report on 307 consecutive patients who underwent robotic-assisted CABG at the university, including 159 who had HCR for multivessel disease, short-term clinical and angiographic outcomes were excellent (J. Thorac. Cardiovasc. Surg. 2014;147:179-85). Particularly striking, in Dr. Thourani’s view, was that only one postoperative stroke occurred, for a 0.3% incidence. That’s a far lower rate than typical with CABG with vein grafts.
In a more recent, as-yet-unpublished update including 477 consecutive HCR patients at Emory, the stroke rate remained extraordinarily low at 0.2%, he said.
“You can almost eliminate stroke with this procedure,” according to Dr. Thourani.
Thirty-day mortality was 0.8%, with an MI rate of 1.0%, a 3.8% incidence of conversion to sternotomy, and a 1.9% repeat revascularization rate. The rate of Fitzgibbon A patency was 96.2%, he noted.
“Return to work is really at the patient’s discretion. We tell them, ‘when you don’t hurt, you can go back.’ Some people go back 4-5 days after surgery. And there are really zero limitations for the patient – none whatsoever,” the surgeon said.
The HCR approach to multivessel revascularization appears to be particularly advantageous in diabetic patients and in older individuals. In a retrospective, nonrandomized single-center comparison of 143 HCR recipients who were propensity score matched to 572 patients who underwent multivessel CABG (Am. J. Cardiol. 2014;114:224-9), the HCR recipients had significantly fewer procedural complications, faster recovery, and similar 3-year mortality (see graphic).
Dr. Thourani said that because of the dirth of randomized trials data, it must be conceded that there is equipoise at present regarding the most effective treatment strategy in patients with multivessel coronary disease. The options are CABG with left internal mammary artery and vein grafts, HCR, medical management, multivessel percutaneous coronary intervention (PCI), and multiarterial CABG. But he added that the available evidence, while not definitive, does point the way forward.
“As we move into 2015 and the future, I think we should be doing less of the left intermal mammary artery with vein grafts, potentially less of the medical therapy, and I think we need to do a lot more multiarterial grafts if we think patients need to be opened up with a sternotomy. And if we don’t think they need to be opened up, we need to think very strongly about hybrid revascularization instead of multivessel PCI, especially in patients who are diabetic or old,” according to the surgeon.
Dr. Thourani reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott, Boston Scientific, Medtronic, and Sorin.
*Clarification, 4/20/2015: Dr. Vinod H. Thourani wishes to clarify that, as he stated in his presentation, the current work he reported on from Emory University was performed by Dr. Michael Halkos, who is the leader of hybrid revascularization at Emory, and that Dr. Halkos was lead author and researcher in several of the other studies reported upon.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Hybrid carotid stents eyed with cautious optimism
CHICAGO – The next generation of hybrid carotid stents is slowly breathing life into the stagnant field of carotid artery stenting.
The new hybrid stents combine the flexibility of a traditional open-cell, nitinol stent with the stabilization typically offered by a closed-cell stent design. The initial clinical experience is limited, but shows promising results against embolization, Dr. Claudio Schönholz said at a symposium on vascular surgery sponsored by Northwestern University.
Last year, Dr. Schönholz and his colleagues at the Medical University of South Carolina in Charleston reported the first-in-man use of the investigational Gore Carotid Stent (W.L. Gore & Associates) (J. Endovasc. Thera. 2014 Aug;21:601-4).
As part of the Gore Carotid Stent Clinical Study for the Treatment of Carotid Artery Stenosis in Patients at Increased Risk for Adverse Events from Carotid Endarterectomy (SCAFFOLD) study, the team has successfully treated another four patients with no evidence of peri- or postprocedural neurological events. This included a case with such severe high-grade stenosis and slow flow that the external carotid artery was not even visible on imaging before the stent was placed, Dr. Schönholz said.
The Food and Drug Administration recently reviewed unreleased data for the first 100 patients enrolled in SCAFFOLD and given the green light for the multicenter, 312-patient study to resume with the start of the new year, he said.
Medtronic’s Cristallo Ideale hybrid stent is already approved outside the United States and was associated with no major neurological events and two cases of transient ischemic attack in 124 patients treated at four expert centers in Italy and Germany in the Cristallo study (J. Endovasc. Ther. 2008;15:186-92).
A more recent retrospective study revealed only one minor stroke in the perioperative period and during the first 30 days in 68 patients with symptomatic carotid stenosis treated by Turkish surgeons with the Cristallo Ideale stent and a proximal protection device (MO.MA, Invatec s.r.l., Medtronic, Italy) (Int. Angiol. 2014 Nov. 14. [Epub ahead of print]).
Better patient selection, increased operator experience, and use of embolic protection devices has reduced neurological events associated with carotid artery stenting, but embolization still occurs after protection devices are removed due to plaque protrusion through the stent struts, Dr. Schönholz said.
The unique design of the hybrid stents “may prevent plaque protrusion, eliminating peri- and postprocedural events,” he said.
The Cristallo Ideale hybrid stent is a nitinol-based stent that has a closed-cell portion at its center and an open-cell configuration on the distal and proximal sections.
In contrast, the Gore Carotid Stent has a closed-cell component throughout the entire device length that is created by placing an expanded polytetrafluoroethylene lattice with 500-micron pores over an open-cell frame. Once combined, both the stent frame and lattice are coated on all surfaces with Carmeda Bioactive Surface (CBAS) heparin. It’s action is limited only to the device surface and has no systemic anticoagulation effects, said Dr. Schönholz, who disclosed serving on Gore’s scientific advisory board.
The open-cell frame allows a high degree of flexibility and conformity to the native anatomy, while the stent lattice provides a high degree of plaque scaffolding that can reduce plaque prolapse, he said. The lattice also reduces the amount of emboli released during and after stent deployment and stabilizes the stent frame by resisting elongation as well as “fish-scaling,” or the misalignment of stent struts that protrude into the vessel wall, particularly when stents are deployed in tortuous anatomy.
When asked during a discussion whether the same results couldn’t be achieved by simply putting in another covered stent like a Viabahn, Dr. Schönholz replied that the Gore Stent isn’t a true covered stent because the 500-micron pores allow perfusion to be maintained to the external carotid artery. “It was intended to prevent plaque profusion, but at the same time allowing perfusion of the external carotid,” he said.
So far, the investigators have not incorporated intravascular ultrasound during stent placement, as it was not part of the SCAFFOLD protocol, but this will likely be used once the device is approved, he added.
Course director Dr. Mark K. Eskandari, chief of vascular surgery at Northwestern University in Chicago, said the results show that “carotid stenting isn’t dead yet and we can persevere. Advances in technology, both in regards to mechanical embolic protection devices and stent design systems, continue to improve the already great results of carotid artery stenting.”
CHICAGO – The next generation of hybrid carotid stents is slowly breathing life into the stagnant field of carotid artery stenting.
The new hybrid stents combine the flexibility of a traditional open-cell, nitinol stent with the stabilization typically offered by a closed-cell stent design. The initial clinical experience is limited, but shows promising results against embolization, Dr. Claudio Schönholz said at a symposium on vascular surgery sponsored by Northwestern University.
Last year, Dr. Schönholz and his colleagues at the Medical University of South Carolina in Charleston reported the first-in-man use of the investigational Gore Carotid Stent (W.L. Gore & Associates) (J. Endovasc. Thera. 2014 Aug;21:601-4).
As part of the Gore Carotid Stent Clinical Study for the Treatment of Carotid Artery Stenosis in Patients at Increased Risk for Adverse Events from Carotid Endarterectomy (SCAFFOLD) study, the team has successfully treated another four patients with no evidence of peri- or postprocedural neurological events. This included a case with such severe high-grade stenosis and slow flow that the external carotid artery was not even visible on imaging before the stent was placed, Dr. Schönholz said.
The Food and Drug Administration recently reviewed unreleased data for the first 100 patients enrolled in SCAFFOLD and given the green light for the multicenter, 312-patient study to resume with the start of the new year, he said.
Medtronic’s Cristallo Ideale hybrid stent is already approved outside the United States and was associated with no major neurological events and two cases of transient ischemic attack in 124 patients treated at four expert centers in Italy and Germany in the Cristallo study (J. Endovasc. Ther. 2008;15:186-92).
A more recent retrospective study revealed only one minor stroke in the perioperative period and during the first 30 days in 68 patients with symptomatic carotid stenosis treated by Turkish surgeons with the Cristallo Ideale stent and a proximal protection device (MO.MA, Invatec s.r.l., Medtronic, Italy) (Int. Angiol. 2014 Nov. 14. [Epub ahead of print]).
Better patient selection, increased operator experience, and use of embolic protection devices has reduced neurological events associated with carotid artery stenting, but embolization still occurs after protection devices are removed due to plaque protrusion through the stent struts, Dr. Schönholz said.
The unique design of the hybrid stents “may prevent plaque protrusion, eliminating peri- and postprocedural events,” he said.
The Cristallo Ideale hybrid stent is a nitinol-based stent that has a closed-cell portion at its center and an open-cell configuration on the distal and proximal sections.
In contrast, the Gore Carotid Stent has a closed-cell component throughout the entire device length that is created by placing an expanded polytetrafluoroethylene lattice with 500-micron pores over an open-cell frame. Once combined, both the stent frame and lattice are coated on all surfaces with Carmeda Bioactive Surface (CBAS) heparin. It’s action is limited only to the device surface and has no systemic anticoagulation effects, said Dr. Schönholz, who disclosed serving on Gore’s scientific advisory board.
The open-cell frame allows a high degree of flexibility and conformity to the native anatomy, while the stent lattice provides a high degree of plaque scaffolding that can reduce plaque prolapse, he said. The lattice also reduces the amount of emboli released during and after stent deployment and stabilizes the stent frame by resisting elongation as well as “fish-scaling,” or the misalignment of stent struts that protrude into the vessel wall, particularly when stents are deployed in tortuous anatomy.
When asked during a discussion whether the same results couldn’t be achieved by simply putting in another covered stent like a Viabahn, Dr. Schönholz replied that the Gore Stent isn’t a true covered stent because the 500-micron pores allow perfusion to be maintained to the external carotid artery. “It was intended to prevent plaque profusion, but at the same time allowing perfusion of the external carotid,” he said.
So far, the investigators have not incorporated intravascular ultrasound during stent placement, as it was not part of the SCAFFOLD protocol, but this will likely be used once the device is approved, he added.
Course director Dr. Mark K. Eskandari, chief of vascular surgery at Northwestern University in Chicago, said the results show that “carotid stenting isn’t dead yet and we can persevere. Advances in technology, both in regards to mechanical embolic protection devices and stent design systems, continue to improve the already great results of carotid artery stenting.”
CHICAGO – The next generation of hybrid carotid stents is slowly breathing life into the stagnant field of carotid artery stenting.
The new hybrid stents combine the flexibility of a traditional open-cell, nitinol stent with the stabilization typically offered by a closed-cell stent design. The initial clinical experience is limited, but shows promising results against embolization, Dr. Claudio Schönholz said at a symposium on vascular surgery sponsored by Northwestern University.
Last year, Dr. Schönholz and his colleagues at the Medical University of South Carolina in Charleston reported the first-in-man use of the investigational Gore Carotid Stent (W.L. Gore & Associates) (J. Endovasc. Thera. 2014 Aug;21:601-4).
As part of the Gore Carotid Stent Clinical Study for the Treatment of Carotid Artery Stenosis in Patients at Increased Risk for Adverse Events from Carotid Endarterectomy (SCAFFOLD) study, the team has successfully treated another four patients with no evidence of peri- or postprocedural neurological events. This included a case with such severe high-grade stenosis and slow flow that the external carotid artery was not even visible on imaging before the stent was placed, Dr. Schönholz said.
The Food and Drug Administration recently reviewed unreleased data for the first 100 patients enrolled in SCAFFOLD and given the green light for the multicenter, 312-patient study to resume with the start of the new year, he said.
Medtronic’s Cristallo Ideale hybrid stent is already approved outside the United States and was associated with no major neurological events and two cases of transient ischemic attack in 124 patients treated at four expert centers in Italy and Germany in the Cristallo study (J. Endovasc. Ther. 2008;15:186-92).
A more recent retrospective study revealed only one minor stroke in the perioperative period and during the first 30 days in 68 patients with symptomatic carotid stenosis treated by Turkish surgeons with the Cristallo Ideale stent and a proximal protection device (MO.MA, Invatec s.r.l., Medtronic, Italy) (Int. Angiol. 2014 Nov. 14. [Epub ahead of print]).
Better patient selection, increased operator experience, and use of embolic protection devices has reduced neurological events associated with carotid artery stenting, but embolization still occurs after protection devices are removed due to plaque protrusion through the stent struts, Dr. Schönholz said.
The unique design of the hybrid stents “may prevent plaque protrusion, eliminating peri- and postprocedural events,” he said.
The Cristallo Ideale hybrid stent is a nitinol-based stent that has a closed-cell portion at its center and an open-cell configuration on the distal and proximal sections.
In contrast, the Gore Carotid Stent has a closed-cell component throughout the entire device length that is created by placing an expanded polytetrafluoroethylene lattice with 500-micron pores over an open-cell frame. Once combined, both the stent frame and lattice are coated on all surfaces with Carmeda Bioactive Surface (CBAS) heparin. It’s action is limited only to the device surface and has no systemic anticoagulation effects, said Dr. Schönholz, who disclosed serving on Gore’s scientific advisory board.
The open-cell frame allows a high degree of flexibility and conformity to the native anatomy, while the stent lattice provides a high degree of plaque scaffolding that can reduce plaque prolapse, he said. The lattice also reduces the amount of emboli released during and after stent deployment and stabilizes the stent frame by resisting elongation as well as “fish-scaling,” or the misalignment of stent struts that protrude into the vessel wall, particularly when stents are deployed in tortuous anatomy.
When asked during a discussion whether the same results couldn’t be achieved by simply putting in another covered stent like a Viabahn, Dr. Schönholz replied that the Gore Stent isn’t a true covered stent because the 500-micron pores allow perfusion to be maintained to the external carotid artery. “It was intended to prevent plaque profusion, but at the same time allowing perfusion of the external carotid,” he said.
So far, the investigators have not incorporated intravascular ultrasound during stent placement, as it was not part of the SCAFFOLD protocol, but this will likely be used once the device is approved, he added.
Course director Dr. Mark K. Eskandari, chief of vascular surgery at Northwestern University in Chicago, said the results show that “carotid stenting isn’t dead yet and we can persevere. Advances in technology, both in regards to mechanical embolic protection devices and stent design systems, continue to improve the already great results of carotid artery stenting.”
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
STS releases public surgical outcomes report
The Society of Thoracic Surgeons has released a public online report of national surgical outcomes from its Congenital Heart Surgery Database, the association has announced.
The STS report focuses on pediatric and congenital cardiac malformations, and includes 4-year observed, expected, and risk-adjusted mortality rates, the society said in a statement.
Twenty-five sites participated in the first round of STS public online reporting, similar to the observed participation in the first round of public reporting in the STS Adult Cardiac Surgery Database, which started in 2010.
“Reporting hospital surgical outcomes using risk-adjusted analysis is extremely important because it allows for a fair assessment, on a level playing field, of outcomes across hospitals that treat different populations of patients,” Dr. Marshall L. Jacobs, STS CHSD Task Force chair, said in the statement.
For more information, visit http://www.sts.org/quality-research-patient-safety/sts-public-reporting-online.
The Society of Thoracic Surgeons has released a public online report of national surgical outcomes from its Congenital Heart Surgery Database, the association has announced.
The STS report focuses on pediatric and congenital cardiac malformations, and includes 4-year observed, expected, and risk-adjusted mortality rates, the society said in a statement.
Twenty-five sites participated in the first round of STS public online reporting, similar to the observed participation in the first round of public reporting in the STS Adult Cardiac Surgery Database, which started in 2010.
“Reporting hospital surgical outcomes using risk-adjusted analysis is extremely important because it allows for a fair assessment, on a level playing field, of outcomes across hospitals that treat different populations of patients,” Dr. Marshall L. Jacobs, STS CHSD Task Force chair, said in the statement.
For more information, visit http://www.sts.org/quality-research-patient-safety/sts-public-reporting-online.
The Society of Thoracic Surgeons has released a public online report of national surgical outcomes from its Congenital Heart Surgery Database, the association has announced.
The STS report focuses on pediatric and congenital cardiac malformations, and includes 4-year observed, expected, and risk-adjusted mortality rates, the society said in a statement.
Twenty-five sites participated in the first round of STS public online reporting, similar to the observed participation in the first round of public reporting in the STS Adult Cardiac Surgery Database, which started in 2010.
“Reporting hospital surgical outcomes using risk-adjusted analysis is extremely important because it allows for a fair assessment, on a level playing field, of outcomes across hospitals that treat different populations of patients,” Dr. Marshall L. Jacobs, STS CHSD Task Force chair, said in the statement.
For more information, visit http://www.sts.org/quality-research-patient-safety/sts-public-reporting-online.
A percutaneous modification of RVAD placement shows promise
The use of a modified percutaneous placement technique for right ventricular assist device (RVAD) implantation showed the potential to lessen complications and improve outcomes, according to the results of a retrospective review of 21 patients with right ventricular failure (RVF) implanted with RVADs using this technique.
“This study shows the feasibility and safety of the proposed RVAD implantation technique for various forms of perioperative RVF. A satisfactory outcome can be achieved with a minimal rate of complications,” according to Dr. Diyar Saeed and colleagues at the Clinic for Cardiovascular Surgery, Heinrich-Heine University of Dusseldorf (Germany). Their report appears in the March issue of The Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j/jtcvs.2014.10.104].
Although this technique has been previously described anecdotally in a few case reports, this study is the largest series reported to date, according to the authors.
A total of 63 left ventricular assist devices (LVADs) were placed during the study period (January 2010 to February 2014); 17 (27%) of these patients subsequently received RVADs. An additional four patients were included who received RVADs after developing postcardiotomy RVF. The mean patient age of the RVAD patients 58 years, and 71% were men, The primary diagnosis was ischemic cardiomyopathy in 57% of the patients, dilative cardiomyopathy in 24%, and acute myocardial infarction in 19% .
The initial operation for RVAD implantation required a median sternotomy and was performed via cardiopulmonary bypass in 14 patients, LVAD support in 3 patients, ECMO support in 3 patients, and off-pump in 1 patient. A dacron graft was attached to the pulmonary artery and passed through a subxiphoid exit, where the RVAD outflow cannula was inserted. The inflow cannula was percutaneously cannulated in the femoral vein, and the sternum was primarily closed.
The median duration of RVAD support was 9 days (range 2-88 days). Explantation of the RVAD was performed by pulling and ligating the outflow graft followed by closure of the insertion site. The RVAD inflow cannula was removed and direct pressure applied.
The overall outcomes were that 52% of patients were successfully weaned from the RVAD; 38% of patients died; and 10% of patients received cardiac transplants, giving a survival rate to discharge or heart transplantation of 62%. The overall 1-year survival rate was 52%, but this comprised an improved survival rate of the 4 postcardiotomy patients (75%) compared with the 17 LVAD patients who received an RVAD (47%). Because of the small population size, these latter differences were not significant.
The main drawback of the technique is the limited mobility of the patients owing to the presence of the inflow cannula in the groin, according to the authors, with the majority of the patients remaining bed or chair bound.
“Early extubation, extended support duration, and reduction of resternotomy risks may be the main advantage of this technique. The RVAD removal can be reproducibly performed under minimal anesthesia and without the need for resternotomy. [However], the survival rate remains limited in patients requiring RVAD support after LVAD implantation,” the researchers concluded.
The authors reported having no financial disclosures.
What makes this series unique is the fact that Dr. Saeed and his colleagues placed the RVAD inflow and outflow cannulae percutaneously to avoid the need for resternotomy for removal, according to the invited editorial commentary by Dr. Robert L. Kormos [doi:10.1016/j/jtcvs.2014.11.031].
“Although this is an expanded observational study, it should make us consider further trials to define the real benefits of this approach,” he stated. “The lingering question for all of us, however, is: How does the clinical community define the need for these systems? The balance, of course, will be between utilizing a lower clinical threshold for implantation of LVADs, thus reducing the inherent RV dysfunction, versus ease of insertion and management of percutaneous RVADs, which could lead to earlier RV support decisions, obviating the syndrome of multiorgan failure that accompanies RV failure, and promoting the potential for RV recovery.”
Dr. Kormos is is a professor of surgery at the Presbyterian University Hospital, University of Pittsburgh.
What makes this series unique is the fact that Dr. Saeed and his colleagues placed the RVAD inflow and outflow cannulae percutaneously to avoid the need for resternotomy for removal, according to the invited editorial commentary by Dr. Robert L. Kormos [doi:10.1016/j/jtcvs.2014.11.031].
“Although this is an expanded observational study, it should make us consider further trials to define the real benefits of this approach,” he stated. “The lingering question for all of us, however, is: How does the clinical community define the need for these systems? The balance, of course, will be between utilizing a lower clinical threshold for implantation of LVADs, thus reducing the inherent RV dysfunction, versus ease of insertion and management of percutaneous RVADs, which could lead to earlier RV support decisions, obviating the syndrome of multiorgan failure that accompanies RV failure, and promoting the potential for RV recovery.”
Dr. Kormos is is a professor of surgery at the Presbyterian University Hospital, University of Pittsburgh.
What makes this series unique is the fact that Dr. Saeed and his colleagues placed the RVAD inflow and outflow cannulae percutaneously to avoid the need for resternotomy for removal, according to the invited editorial commentary by Dr. Robert L. Kormos [doi:10.1016/j/jtcvs.2014.11.031].
“Although this is an expanded observational study, it should make us consider further trials to define the real benefits of this approach,” he stated. “The lingering question for all of us, however, is: How does the clinical community define the need for these systems? The balance, of course, will be between utilizing a lower clinical threshold for implantation of LVADs, thus reducing the inherent RV dysfunction, versus ease of insertion and management of percutaneous RVADs, which could lead to earlier RV support decisions, obviating the syndrome of multiorgan failure that accompanies RV failure, and promoting the potential for RV recovery.”
Dr. Kormos is is a professor of surgery at the Presbyterian University Hospital, University of Pittsburgh.
The use of a modified percutaneous placement technique for right ventricular assist device (RVAD) implantation showed the potential to lessen complications and improve outcomes, according to the results of a retrospective review of 21 patients with right ventricular failure (RVF) implanted with RVADs using this technique.
“This study shows the feasibility and safety of the proposed RVAD implantation technique for various forms of perioperative RVF. A satisfactory outcome can be achieved with a minimal rate of complications,” according to Dr. Diyar Saeed and colleagues at the Clinic for Cardiovascular Surgery, Heinrich-Heine University of Dusseldorf (Germany). Their report appears in the March issue of The Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j/jtcvs.2014.10.104].
Although this technique has been previously described anecdotally in a few case reports, this study is the largest series reported to date, according to the authors.
A total of 63 left ventricular assist devices (LVADs) were placed during the study period (January 2010 to February 2014); 17 (27%) of these patients subsequently received RVADs. An additional four patients were included who received RVADs after developing postcardiotomy RVF. The mean patient age of the RVAD patients 58 years, and 71% were men, The primary diagnosis was ischemic cardiomyopathy in 57% of the patients, dilative cardiomyopathy in 24%, and acute myocardial infarction in 19% .
The initial operation for RVAD implantation required a median sternotomy and was performed via cardiopulmonary bypass in 14 patients, LVAD support in 3 patients, ECMO support in 3 patients, and off-pump in 1 patient. A dacron graft was attached to the pulmonary artery and passed through a subxiphoid exit, where the RVAD outflow cannula was inserted. The inflow cannula was percutaneously cannulated in the femoral vein, and the sternum was primarily closed.
The median duration of RVAD support was 9 days (range 2-88 days). Explantation of the RVAD was performed by pulling and ligating the outflow graft followed by closure of the insertion site. The RVAD inflow cannula was removed and direct pressure applied.
The overall outcomes were that 52% of patients were successfully weaned from the RVAD; 38% of patients died; and 10% of patients received cardiac transplants, giving a survival rate to discharge or heart transplantation of 62%. The overall 1-year survival rate was 52%, but this comprised an improved survival rate of the 4 postcardiotomy patients (75%) compared with the 17 LVAD patients who received an RVAD (47%). Because of the small population size, these latter differences were not significant.
The main drawback of the technique is the limited mobility of the patients owing to the presence of the inflow cannula in the groin, according to the authors, with the majority of the patients remaining bed or chair bound.
“Early extubation, extended support duration, and reduction of resternotomy risks may be the main advantage of this technique. The RVAD removal can be reproducibly performed under minimal anesthesia and without the need for resternotomy. [However], the survival rate remains limited in patients requiring RVAD support after LVAD implantation,” the researchers concluded.
The authors reported having no financial disclosures.
The use of a modified percutaneous placement technique for right ventricular assist device (RVAD) implantation showed the potential to lessen complications and improve outcomes, according to the results of a retrospective review of 21 patients with right ventricular failure (RVF) implanted with RVADs using this technique.
“This study shows the feasibility and safety of the proposed RVAD implantation technique for various forms of perioperative RVF. A satisfactory outcome can be achieved with a minimal rate of complications,” according to Dr. Diyar Saeed and colleagues at the Clinic for Cardiovascular Surgery, Heinrich-Heine University of Dusseldorf (Germany). Their report appears in the March issue of The Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j/jtcvs.2014.10.104].
Although this technique has been previously described anecdotally in a few case reports, this study is the largest series reported to date, according to the authors.
A total of 63 left ventricular assist devices (LVADs) were placed during the study period (January 2010 to February 2014); 17 (27%) of these patients subsequently received RVADs. An additional four patients were included who received RVADs after developing postcardiotomy RVF. The mean patient age of the RVAD patients 58 years, and 71% were men, The primary diagnosis was ischemic cardiomyopathy in 57% of the patients, dilative cardiomyopathy in 24%, and acute myocardial infarction in 19% .
The initial operation for RVAD implantation required a median sternotomy and was performed via cardiopulmonary bypass in 14 patients, LVAD support in 3 patients, ECMO support in 3 patients, and off-pump in 1 patient. A dacron graft was attached to the pulmonary artery and passed through a subxiphoid exit, where the RVAD outflow cannula was inserted. The inflow cannula was percutaneously cannulated in the femoral vein, and the sternum was primarily closed.
The median duration of RVAD support was 9 days (range 2-88 days). Explantation of the RVAD was performed by pulling and ligating the outflow graft followed by closure of the insertion site. The RVAD inflow cannula was removed and direct pressure applied.
The overall outcomes were that 52% of patients were successfully weaned from the RVAD; 38% of patients died; and 10% of patients received cardiac transplants, giving a survival rate to discharge or heart transplantation of 62%. The overall 1-year survival rate was 52%, but this comprised an improved survival rate of the 4 postcardiotomy patients (75%) compared with the 17 LVAD patients who received an RVAD (47%). Because of the small population size, these latter differences were not significant.
The main drawback of the technique is the limited mobility of the patients owing to the presence of the inflow cannula in the groin, according to the authors, with the majority of the patients remaining bed or chair bound.
“Early extubation, extended support duration, and reduction of resternotomy risks may be the main advantage of this technique. The RVAD removal can be reproducibly performed under minimal anesthesia and without the need for resternotomy. [However], the survival rate remains limited in patients requiring RVAD support after LVAD implantation,” the researchers concluded.
The authors reported having no financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Use of a percutaneous modification of right ventricular assist device (RVAD) implantation appears to provide extended support duration and reduces the resternotomy risks, compared with conventional RVAD implantation.
Major finding: The survival rates to discharge or heart transplantation, and to 1 year, were 62% and 52%, respectively.
Data source: A retrospective review of 21 patients with RVF implanted with RVADs via the modified percutaneous placement technique.
Disclosures: The authors reported having no financial disclosures.
More donated hearts rejected, even as wait list grows
Only about one in three available hearts was accepted for transplant in the United States in 2010, down from 44% 2 decades ago, researchers reported online Feb. 9 in the American Journal of Transplantation.
The decline stems in part from transplant centers rejecting “marginal” donor hearts, belying a growing need for heart transplants, longer waiting times, and multiple campaigns to expand the use of organs donated for transplantation, said Dr. Kiran Khush of Stanford (Calif.) University and her associates.
The researchers analyzed data on 82,053 potential donor hearts from the Organ Procurement and Transplantation Network. In 1995, 44% of available hearts were accepted for transplant, compared with only 29% in 2006 and 32% in 2010, they found. Meanwhile, rejection rates for donor hearts rose from 37% in 1995 to 52% in 2010, they reported (Am. J. Transplant. 2015 Feb. 10 [doi:10.1111/ajt.13055]).
Several factors might explain the trends, the investigators said. Potential heart donors tended to be older and more often had hypertension and diabetes by the final years of the study period, and transplant centers were less likely to accept hearts from such individuals. Also, mechanical circulatory devices were more commonly used, and centers might hesitate to transplant “marginal” hearts into “stable” recipients of such devices, Dr. Khush and her associates said. Furthermore, government scrutiny of post-transplant outcomes might make centers more conservative when evaluating potential donors, they added.
The study also uncovered regional variations in acceptance rates for donor hearts, with the lowest – about 25%-28% – found primarily in the southeastern United States. “Unfortunately, there are no standard guidelines for donor heart evaluation and acceptance, resulting in considerable inconsistencies in the types of donor hearts that are accepted by different transplant centers, and likely resulting in nonrecovery of potentially useful organs,” the investigators said. The findings “lend support to research and policy efforts aimed at establishing evidence-based criteria for donor heart evaluation and acceptance,” they added.
The work was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.
Only about one in three available hearts was accepted for transplant in the United States in 2010, down from 44% 2 decades ago, researchers reported online Feb. 9 in the American Journal of Transplantation.
The decline stems in part from transplant centers rejecting “marginal” donor hearts, belying a growing need for heart transplants, longer waiting times, and multiple campaigns to expand the use of organs donated for transplantation, said Dr. Kiran Khush of Stanford (Calif.) University and her associates.
The researchers analyzed data on 82,053 potential donor hearts from the Organ Procurement and Transplantation Network. In 1995, 44% of available hearts were accepted for transplant, compared with only 29% in 2006 and 32% in 2010, they found. Meanwhile, rejection rates for donor hearts rose from 37% in 1995 to 52% in 2010, they reported (Am. J. Transplant. 2015 Feb. 10 [doi:10.1111/ajt.13055]).
Several factors might explain the trends, the investigators said. Potential heart donors tended to be older and more often had hypertension and diabetes by the final years of the study period, and transplant centers were less likely to accept hearts from such individuals. Also, mechanical circulatory devices were more commonly used, and centers might hesitate to transplant “marginal” hearts into “stable” recipients of such devices, Dr. Khush and her associates said. Furthermore, government scrutiny of post-transplant outcomes might make centers more conservative when evaluating potential donors, they added.
The study also uncovered regional variations in acceptance rates for donor hearts, with the lowest – about 25%-28% – found primarily in the southeastern United States. “Unfortunately, there are no standard guidelines for donor heart evaluation and acceptance, resulting in considerable inconsistencies in the types of donor hearts that are accepted by different transplant centers, and likely resulting in nonrecovery of potentially useful organs,” the investigators said. The findings “lend support to research and policy efforts aimed at establishing evidence-based criteria for donor heart evaluation and acceptance,” they added.
The work was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.
Only about one in three available hearts was accepted for transplant in the United States in 2010, down from 44% 2 decades ago, researchers reported online Feb. 9 in the American Journal of Transplantation.
The decline stems in part from transplant centers rejecting “marginal” donor hearts, belying a growing need for heart transplants, longer waiting times, and multiple campaigns to expand the use of organs donated for transplantation, said Dr. Kiran Khush of Stanford (Calif.) University and her associates.
The researchers analyzed data on 82,053 potential donor hearts from the Organ Procurement and Transplantation Network. In 1995, 44% of available hearts were accepted for transplant, compared with only 29% in 2006 and 32% in 2010, they found. Meanwhile, rejection rates for donor hearts rose from 37% in 1995 to 52% in 2010, they reported (Am. J. Transplant. 2015 Feb. 10 [doi:10.1111/ajt.13055]).
Several factors might explain the trends, the investigators said. Potential heart donors tended to be older and more often had hypertension and diabetes by the final years of the study period, and transplant centers were less likely to accept hearts from such individuals. Also, mechanical circulatory devices were more commonly used, and centers might hesitate to transplant “marginal” hearts into “stable” recipients of such devices, Dr. Khush and her associates said. Furthermore, government scrutiny of post-transplant outcomes might make centers more conservative when evaluating potential donors, they added.
The study also uncovered regional variations in acceptance rates for donor hearts, with the lowest – about 25%-28% – found primarily in the southeastern United States. “Unfortunately, there are no standard guidelines for donor heart evaluation and acceptance, resulting in considerable inconsistencies in the types of donor hearts that are accepted by different transplant centers, and likely resulting in nonrecovery of potentially useful organs,” the investigators said. The findings “lend support to research and policy efforts aimed at establishing evidence-based criteria for donor heart evaluation and acceptance,” they added.
The work was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.
FROM THE AMERICAN JOURNAL OF TRANSPLANTATION
Key clinical point: Acceptance rates of hearts donated for transplantation have declined substantially in the United States.
Major finding: Only 32% of donated hearts were accepted for transplant in 2010, compared with 44% in 1995.
Data source: Analysis of 82,053 potential donor hearts from the Organ Procurement and Transplantation Network.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; and the Health Resources and Services Administration. The authors reported having no conflicts of interest.