User login
Survival similar with bioprosthetic and mechanical valves
Among younger patients who underwent mitral valve replacement, 15-year survival was not significantly different between those who received bioprosthetic valves and those who received mechanical devices, based on data from a retrospective study published online April 14 in JAMA.
However, “there is a tradeoff between the incremental risk of reoperation associated with bioprosthetic valves and the greater long-term risk of stroke and major bleeding with mechanical prosthetic valves,” said Dr. Joanna Chikwe of Mount Sinai Hospital, New York, and her associates.
“Even though [our] findings suggest bioprosthetic valve replacement may be a reasonable alternative to mechanical prosthetic valve replacement in patients aged 50-69 years, the 15-year follow-up was insufficient to fully assess lifetime risks, particularly of reoperation,” the researchers noted (JAMA 2015;313:1435-42 [doi:10.1001/jama.2015.3164]).
The choice between bioprosthetic and mechanical mitral valves is controversial in patients younger than 70 years. Bioprosthetics are much more likely to degenerate over time and require reoperation, but mechanical valves put recipients at increased risk of thromboembolism and hemorrhage and require lifelong anticoagulation. In this age group, the use of bioprosthetic devices has steadily and markedly increased in the past decade, from a small fraction of patients to the majority of patients. Yet until now, no large-scale studies have compared long-term survival and other outcomes between the two valves in this patient population.
Dr. Chikwe and her associates reviewed data from a New York state database of all inpatient hospitalizations. They included 3,433 patients aged 50-69 years at baseline who underwent mitral valve replacement with bioprosthetic (23.2%) or mechanical (76.8%) devices from 1997 through 2007. They also assessed a subset of 664 patient pairs who were propensity matched.
After a median follow-up of 8.2 years (maximum, 16.8 years), there was no significant difference in long-term survival between the two groups. Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical devices, the investigators said.
This lack of survival difference “refocuses the emphasis” onto major complications and quality of life, the researchers noted. Regarding these secondary outcomes, the incidence of stroke was significantly higher with mechanical mitral valves (14.0% vs 6.8%) and carried a high mortality (8.5%). The incidence of serious bleeding events also was significantly higher with mechanical valves (14.9% vs 9.0%), and also carried a high (7.4%) mortality. Such risks should “be a major consideration in any discussion of prosthesis choice,” Dr. Chikwe and her associates noted.
Conversely, the incidence of mitral valve reoperation was significantly lower with mechanical devices (5.0% vs 11.1%), and related mortality was 5.3%.
These findings differ from those of recent single-center retrospective series, which reported a long-term survival benefit with mechanical valves in younger patients. Those studies, however, were much smaller and had methodological flaws such as failure to control for competing causes of death, the investigators noted.
This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
Among younger patients who underwent mitral valve replacement, 15-year survival was not significantly different between those who received bioprosthetic valves and those who received mechanical devices, based on data from a retrospective study published online April 14 in JAMA.
However, “there is a tradeoff between the incremental risk of reoperation associated with bioprosthetic valves and the greater long-term risk of stroke and major bleeding with mechanical prosthetic valves,” said Dr. Joanna Chikwe of Mount Sinai Hospital, New York, and her associates.
“Even though [our] findings suggest bioprosthetic valve replacement may be a reasonable alternative to mechanical prosthetic valve replacement in patients aged 50-69 years, the 15-year follow-up was insufficient to fully assess lifetime risks, particularly of reoperation,” the researchers noted (JAMA 2015;313:1435-42 [doi:10.1001/jama.2015.3164]).
The choice between bioprosthetic and mechanical mitral valves is controversial in patients younger than 70 years. Bioprosthetics are much more likely to degenerate over time and require reoperation, but mechanical valves put recipients at increased risk of thromboembolism and hemorrhage and require lifelong anticoagulation. In this age group, the use of bioprosthetic devices has steadily and markedly increased in the past decade, from a small fraction of patients to the majority of patients. Yet until now, no large-scale studies have compared long-term survival and other outcomes between the two valves in this patient population.
Dr. Chikwe and her associates reviewed data from a New York state database of all inpatient hospitalizations. They included 3,433 patients aged 50-69 years at baseline who underwent mitral valve replacement with bioprosthetic (23.2%) or mechanical (76.8%) devices from 1997 through 2007. They also assessed a subset of 664 patient pairs who were propensity matched.
After a median follow-up of 8.2 years (maximum, 16.8 years), there was no significant difference in long-term survival between the two groups. Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical devices, the investigators said.
This lack of survival difference “refocuses the emphasis” onto major complications and quality of life, the researchers noted. Regarding these secondary outcomes, the incidence of stroke was significantly higher with mechanical mitral valves (14.0% vs 6.8%) and carried a high mortality (8.5%). The incidence of serious bleeding events also was significantly higher with mechanical valves (14.9% vs 9.0%), and also carried a high (7.4%) mortality. Such risks should “be a major consideration in any discussion of prosthesis choice,” Dr. Chikwe and her associates noted.
Conversely, the incidence of mitral valve reoperation was significantly lower with mechanical devices (5.0% vs 11.1%), and related mortality was 5.3%.
These findings differ from those of recent single-center retrospective series, which reported a long-term survival benefit with mechanical valves in younger patients. Those studies, however, were much smaller and had methodological flaws such as failure to control for competing causes of death, the investigators noted.
This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
Among younger patients who underwent mitral valve replacement, 15-year survival was not significantly different between those who received bioprosthetic valves and those who received mechanical devices, based on data from a retrospective study published online April 14 in JAMA.
However, “there is a tradeoff between the incremental risk of reoperation associated with bioprosthetic valves and the greater long-term risk of stroke and major bleeding with mechanical prosthetic valves,” said Dr. Joanna Chikwe of Mount Sinai Hospital, New York, and her associates.
“Even though [our] findings suggest bioprosthetic valve replacement may be a reasonable alternative to mechanical prosthetic valve replacement in patients aged 50-69 years, the 15-year follow-up was insufficient to fully assess lifetime risks, particularly of reoperation,” the researchers noted (JAMA 2015;313:1435-42 [doi:10.1001/jama.2015.3164]).
The choice between bioprosthetic and mechanical mitral valves is controversial in patients younger than 70 years. Bioprosthetics are much more likely to degenerate over time and require reoperation, but mechanical valves put recipients at increased risk of thromboembolism and hemorrhage and require lifelong anticoagulation. In this age group, the use of bioprosthetic devices has steadily and markedly increased in the past decade, from a small fraction of patients to the majority of patients. Yet until now, no large-scale studies have compared long-term survival and other outcomes between the two valves in this patient population.
Dr. Chikwe and her associates reviewed data from a New York state database of all inpatient hospitalizations. They included 3,433 patients aged 50-69 years at baseline who underwent mitral valve replacement with bioprosthetic (23.2%) or mechanical (76.8%) devices from 1997 through 2007. They also assessed a subset of 664 patient pairs who were propensity matched.
After a median follow-up of 8.2 years (maximum, 16.8 years), there was no significant difference in long-term survival between the two groups. Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical devices, the investigators said.
This lack of survival difference “refocuses the emphasis” onto major complications and quality of life, the researchers noted. Regarding these secondary outcomes, the incidence of stroke was significantly higher with mechanical mitral valves (14.0% vs 6.8%) and carried a high mortality (8.5%). The incidence of serious bleeding events also was significantly higher with mechanical valves (14.9% vs 9.0%), and also carried a high (7.4%) mortality. Such risks should “be a major consideration in any discussion of prosthesis choice,” Dr. Chikwe and her associates noted.
Conversely, the incidence of mitral valve reoperation was significantly lower with mechanical devices (5.0% vs 11.1%), and related mortality was 5.3%.
These findings differ from those of recent single-center retrospective series, which reported a long-term survival benefit with mechanical valves in younger patients. Those studies, however, were much smaller and had methodological flaws such as failure to control for competing causes of death, the investigators noted.
This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
FROM JAMA
Key clinical point: 15-year survival was not significantly different between younger patients who received a mechanical mitral valve and those who received a bioprosthetic valve.
Major finding: Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical mitral valves.
Data source: A retrospective cohort study comparing long-term outcomes after mitral valve replacement in 3,433 patients aged 50-69 years living in New York.
Disclosures: This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
Evidence builds for complete revascularization in STEMI
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
AT ACC/CRFi2
Key clinical point: Complete revascularization of multivessel disease in patients with STEMI improved long-term outcomes, but may not be the optimal strategy for all.
Major finding: Complete revascularization reduced the risk of the primary endpoint from 22% with infarct-only PCI to 13%.
Data source: Randomized trial in 627 STEMI patients with multivessel disease.
Disclosures: The presenter had no financial disclosures.
Heart surgeons get serious about RCTs
SAN DIEGO – The days when cardiologists could look down their noses at cardiac surgeons as primitive when it comes to conducting high-quality clinical research have come and gone.
“Cardiologists have been much more sophisticated than we have in doing randomized controlled trials. But cardiac surgeons are finally making strong progress in conducting randomized clinical trials, an area we’re not typically known for,” Dr. Vinod H. Thourani asserted at the annual meeting of the American College of Cardiology.
Much of this progress can be credited to the relatively recent creation of the Cardiothoracic Surgical Trials Network (CSTN), funded by the U.S. National Institutions of Health and the Canadian Institutes of Health Research. This surgical network is carrying out cutting-edge RCTs that will change the practice of cardiology as well as heart surgery, said Dr. Thourani, professor of surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
To illustrate the breadth of current research in cardiothoracic surgery, he presented thumbnail sketches of RCTs due to report findings as early as this spring and no later than the latter part of next year or early 2017. The trials he selected, some conducted by the CSTN and others with industry sponsorship, address the aortic valve, the mitral valve, postoperative atrial fibrillation, revascularization in patients with unprotected left main coronary artery disease, and advanced heart failure.
• Heart failure: Outcomes of the ENDURANCE destination trial are due to be presented this spring at the annual meeting of the International Society for Heart and Lung Transplantation in Nice, France. This study will compare 12-month outcomes of continuous-flow ventricular assist devices as destination therapy in advanced heart failure. In this HeartWare-sponsored study, 310 patients received the investigational HeartWare ventricular assist system and 155 controls were implanted with the FDA-approved HeartMate II device marketed by Thoratec.
• Atrial fibrillation: The Rate Control Versus Rhythm Control for Postoperative Atrial Fibrillation trial compares the two management strategies in patients with new-onset AF following cardiac surgery. This CSTN-conducted study, to be presented next year, looks at which treatment approach results in fewer days in the hospital, as well as heart rhythm at discharge and through 60 days of follow-up, economic costs, and the incidence of postoperative clinical events.
• Coronary artery disease: The EXCEL trial has randomized 2,600 patients with unprotected left main coronary artery disease to coronary artery bypass surgery or percutaneous intervention with a XIENCE everolimus-eluting stent. The primary outcome is the composite of all-cause mortality, acute MI, or stroke. First results of this Abbott Vascular–sponsored trial are due to be reported next year.“This is a study that will clearly be impactful for you,” Dr. Thourani observed.
• Aortic valve disease: Two major RCTs are looking at the impact of extending transcatheter aortic valve replacement (TAVR) to an intermediate-surgical-risk population of patients with symptomatic severe aortic stenosis. The PARTNER II trial compares transfemoral or transapical/transaortic TAVR with a SAPIEN XT valve to surgical aortic valve replacement in patients with a Society of Thoracic Surgeons mortality risk score of 4%-8%. The primary endpoint is all-cause mortality and disabling stroke at 2 years. Results of this Edwards Lifesciences–sponsored trial will be presented by early 2016.
The Medtronic-sponsored SURTAVI trial compares TAVR using the company’s CoreValve alone or with PCI if revascularization is indicated versus surgical aortic valve replacement alone or with coronary artery bypass grafting if revascularization is indicated. This is a randomized trial involving 2,500 intermediate-risk patients. Of note, the SURTAVI investigators have revised the study protocol to open the trial to patients who are age 75 years or older or have an STS score of 2%-10%, which really redefines the concept of intermediate risk, Dr. Thourani said. Results will be presented by early 2017.
• Mitral valve disease: The Evaluation of Outcomes Following Mitral Valve Repair/Replacement in Severe Chronic Ischemic Mitral Regurgitation trial, carried out by the CSTN, will present 2-year outcome data later this year or in early 2016. The 1-year results caused major consternation in the surgical world. The eye opener was that 33% of patients in the repair group had moderate or severe mitral regurgitation at 12 months, compared with just 2% in the replacement group (N. Engl. J. Med. 2014;370:23-32). Mitral valve repair has traditionally been by far the more popular strategy. If the 2-year results show a growing disparity in terms of rates of severe mitral regurgitation, that may change.
Another CSTN study, the Surgical Intervention in Moderate Ischemic Mitral Regurgitation trial, found no demonstrable clinical benefit in adding a mitral valve repair operation to CABG surgery at 1 year of follow-up. The incidence of moderate or severe mitral regurgitation was lower at 1 year with concomitant valve repair and CABG, but this was offset by more neurologic events, longer ICU and total hospital stays, and no differences in the degree of reverse remodeling, mortality, or quality of life (N. Engl. J. Med. 2014;371:2178-88).
“Two-year follow-up is ongoing. It really becomes very important that later this year or next year we’re going to have the results available for you to determine if the lower incidence of moderate or severe mitral regurgitation at 1 year translates into a net clinical benefit for patients undergoing CABG and mitral repair. This study has big implications for the practice of thoracic surgery and for how cardiologists refer patients,” according to Dr. Thourani.
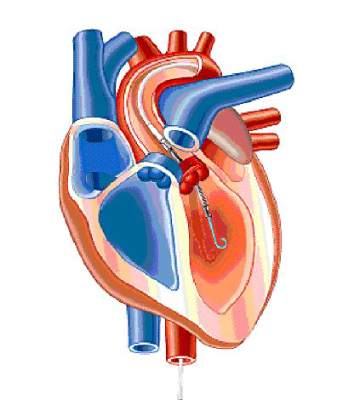
The COAPT trial is randomizing patients with symptomatic functional mitral regurgitation and very high surgical risk to percutaneous catheter-based treatment with the MitraClip or to a standard-care control group. One-year outcomes will be presented in 2016, and follow-up out to 5 years is planned.
“You can see now that in cardiac surgery there’s a lot going on,” Dr. Thourani concluded. “It’s not only good for us, but it’s good for you. As a cardiovascular community, we need to work together more.”
He reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott Medical, Boston Scientific, Medtronic, and Sorin.
SAN DIEGO – The days when cardiologists could look down their noses at cardiac surgeons as primitive when it comes to conducting high-quality clinical research have come and gone.
“Cardiologists have been much more sophisticated than we have in doing randomized controlled trials. But cardiac surgeons are finally making strong progress in conducting randomized clinical trials, an area we’re not typically known for,” Dr. Vinod H. Thourani asserted at the annual meeting of the American College of Cardiology.
Much of this progress can be credited to the relatively recent creation of the Cardiothoracic Surgical Trials Network (CSTN), funded by the U.S. National Institutions of Health and the Canadian Institutes of Health Research. This surgical network is carrying out cutting-edge RCTs that will change the practice of cardiology as well as heart surgery, said Dr. Thourani, professor of surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
To illustrate the breadth of current research in cardiothoracic surgery, he presented thumbnail sketches of RCTs due to report findings as early as this spring and no later than the latter part of next year or early 2017. The trials he selected, some conducted by the CSTN and others with industry sponsorship, address the aortic valve, the mitral valve, postoperative atrial fibrillation, revascularization in patients with unprotected left main coronary artery disease, and advanced heart failure.
• Heart failure: Outcomes of the ENDURANCE destination trial are due to be presented this spring at the annual meeting of the International Society for Heart and Lung Transplantation in Nice, France. This study will compare 12-month outcomes of continuous-flow ventricular assist devices as destination therapy in advanced heart failure. In this HeartWare-sponsored study, 310 patients received the investigational HeartWare ventricular assist system and 155 controls were implanted with the FDA-approved HeartMate II device marketed by Thoratec.
• Atrial fibrillation: The Rate Control Versus Rhythm Control for Postoperative Atrial Fibrillation trial compares the two management strategies in patients with new-onset AF following cardiac surgery. This CSTN-conducted study, to be presented next year, looks at which treatment approach results in fewer days in the hospital, as well as heart rhythm at discharge and through 60 days of follow-up, economic costs, and the incidence of postoperative clinical events.
• Coronary artery disease: The EXCEL trial has randomized 2,600 patients with unprotected left main coronary artery disease to coronary artery bypass surgery or percutaneous intervention with a XIENCE everolimus-eluting stent. The primary outcome is the composite of all-cause mortality, acute MI, or stroke. First results of this Abbott Vascular–sponsored trial are due to be reported next year.“This is a study that will clearly be impactful for you,” Dr. Thourani observed.
• Aortic valve disease: Two major RCTs are looking at the impact of extending transcatheter aortic valve replacement (TAVR) to an intermediate-surgical-risk population of patients with symptomatic severe aortic stenosis. The PARTNER II trial compares transfemoral or transapical/transaortic TAVR with a SAPIEN XT valve to surgical aortic valve replacement in patients with a Society of Thoracic Surgeons mortality risk score of 4%-8%. The primary endpoint is all-cause mortality and disabling stroke at 2 years. Results of this Edwards Lifesciences–sponsored trial will be presented by early 2016.
The Medtronic-sponsored SURTAVI trial compares TAVR using the company’s CoreValve alone or with PCI if revascularization is indicated versus surgical aortic valve replacement alone or with coronary artery bypass grafting if revascularization is indicated. This is a randomized trial involving 2,500 intermediate-risk patients. Of note, the SURTAVI investigators have revised the study protocol to open the trial to patients who are age 75 years or older or have an STS score of 2%-10%, which really redefines the concept of intermediate risk, Dr. Thourani said. Results will be presented by early 2017.
• Mitral valve disease: The Evaluation of Outcomes Following Mitral Valve Repair/Replacement in Severe Chronic Ischemic Mitral Regurgitation trial, carried out by the CSTN, will present 2-year outcome data later this year or in early 2016. The 1-year results caused major consternation in the surgical world. The eye opener was that 33% of patients in the repair group had moderate or severe mitral regurgitation at 12 months, compared with just 2% in the replacement group (N. Engl. J. Med. 2014;370:23-32). Mitral valve repair has traditionally been by far the more popular strategy. If the 2-year results show a growing disparity in terms of rates of severe mitral regurgitation, that may change.
Another CSTN study, the Surgical Intervention in Moderate Ischemic Mitral Regurgitation trial, found no demonstrable clinical benefit in adding a mitral valve repair operation to CABG surgery at 1 year of follow-up. The incidence of moderate or severe mitral regurgitation was lower at 1 year with concomitant valve repair and CABG, but this was offset by more neurologic events, longer ICU and total hospital stays, and no differences in the degree of reverse remodeling, mortality, or quality of life (N. Engl. J. Med. 2014;371:2178-88).
“Two-year follow-up is ongoing. It really becomes very important that later this year or next year we’re going to have the results available for you to determine if the lower incidence of moderate or severe mitral regurgitation at 1 year translates into a net clinical benefit for patients undergoing CABG and mitral repair. This study has big implications for the practice of thoracic surgery and for how cardiologists refer patients,” according to Dr. Thourani.
The COAPT trial is randomizing patients with symptomatic functional mitral regurgitation and very high surgical risk to percutaneous catheter-based treatment with the MitraClip or to a standard-care control group. One-year outcomes will be presented in 2016, and follow-up out to 5 years is planned.
“You can see now that in cardiac surgery there’s a lot going on,” Dr. Thourani concluded. “It’s not only good for us, but it’s good for you. As a cardiovascular community, we need to work together more.”
He reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott Medical, Boston Scientific, Medtronic, and Sorin.
SAN DIEGO – The days when cardiologists could look down their noses at cardiac surgeons as primitive when it comes to conducting high-quality clinical research have come and gone.
“Cardiologists have been much more sophisticated than we have in doing randomized controlled trials. But cardiac surgeons are finally making strong progress in conducting randomized clinical trials, an area we’re not typically known for,” Dr. Vinod H. Thourani asserted at the annual meeting of the American College of Cardiology.
Much of this progress can be credited to the relatively recent creation of the Cardiothoracic Surgical Trials Network (CSTN), funded by the U.S. National Institutions of Health and the Canadian Institutes of Health Research. This surgical network is carrying out cutting-edge RCTs that will change the practice of cardiology as well as heart surgery, said Dr. Thourani, professor of surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
To illustrate the breadth of current research in cardiothoracic surgery, he presented thumbnail sketches of RCTs due to report findings as early as this spring and no later than the latter part of next year or early 2017. The trials he selected, some conducted by the CSTN and others with industry sponsorship, address the aortic valve, the mitral valve, postoperative atrial fibrillation, revascularization in patients with unprotected left main coronary artery disease, and advanced heart failure.
• Heart failure: Outcomes of the ENDURANCE destination trial are due to be presented this spring at the annual meeting of the International Society for Heart and Lung Transplantation in Nice, France. This study will compare 12-month outcomes of continuous-flow ventricular assist devices as destination therapy in advanced heart failure. In this HeartWare-sponsored study, 310 patients received the investigational HeartWare ventricular assist system and 155 controls were implanted with the FDA-approved HeartMate II device marketed by Thoratec.
• Atrial fibrillation: The Rate Control Versus Rhythm Control for Postoperative Atrial Fibrillation trial compares the two management strategies in patients with new-onset AF following cardiac surgery. This CSTN-conducted study, to be presented next year, looks at which treatment approach results in fewer days in the hospital, as well as heart rhythm at discharge and through 60 days of follow-up, economic costs, and the incidence of postoperative clinical events.
• Coronary artery disease: The EXCEL trial has randomized 2,600 patients with unprotected left main coronary artery disease to coronary artery bypass surgery or percutaneous intervention with a XIENCE everolimus-eluting stent. The primary outcome is the composite of all-cause mortality, acute MI, or stroke. First results of this Abbott Vascular–sponsored trial are due to be reported next year.“This is a study that will clearly be impactful for you,” Dr. Thourani observed.
• Aortic valve disease: Two major RCTs are looking at the impact of extending transcatheter aortic valve replacement (TAVR) to an intermediate-surgical-risk population of patients with symptomatic severe aortic stenosis. The PARTNER II trial compares transfemoral or transapical/transaortic TAVR with a SAPIEN XT valve to surgical aortic valve replacement in patients with a Society of Thoracic Surgeons mortality risk score of 4%-8%. The primary endpoint is all-cause mortality and disabling stroke at 2 years. Results of this Edwards Lifesciences–sponsored trial will be presented by early 2016.
The Medtronic-sponsored SURTAVI trial compares TAVR using the company’s CoreValve alone or with PCI if revascularization is indicated versus surgical aortic valve replacement alone or with coronary artery bypass grafting if revascularization is indicated. This is a randomized trial involving 2,500 intermediate-risk patients. Of note, the SURTAVI investigators have revised the study protocol to open the trial to patients who are age 75 years or older or have an STS score of 2%-10%, which really redefines the concept of intermediate risk, Dr. Thourani said. Results will be presented by early 2017.
• Mitral valve disease: The Evaluation of Outcomes Following Mitral Valve Repair/Replacement in Severe Chronic Ischemic Mitral Regurgitation trial, carried out by the CSTN, will present 2-year outcome data later this year or in early 2016. The 1-year results caused major consternation in the surgical world. The eye opener was that 33% of patients in the repair group had moderate or severe mitral regurgitation at 12 months, compared with just 2% in the replacement group (N. Engl. J. Med. 2014;370:23-32). Mitral valve repair has traditionally been by far the more popular strategy. If the 2-year results show a growing disparity in terms of rates of severe mitral regurgitation, that may change.
Another CSTN study, the Surgical Intervention in Moderate Ischemic Mitral Regurgitation trial, found no demonstrable clinical benefit in adding a mitral valve repair operation to CABG surgery at 1 year of follow-up. The incidence of moderate or severe mitral regurgitation was lower at 1 year with concomitant valve repair and CABG, but this was offset by more neurologic events, longer ICU and total hospital stays, and no differences in the degree of reverse remodeling, mortality, or quality of life (N. Engl. J. Med. 2014;371:2178-88).
“Two-year follow-up is ongoing. It really becomes very important that later this year or next year we’re going to have the results available for you to determine if the lower incidence of moderate or severe mitral regurgitation at 1 year translates into a net clinical benefit for patients undergoing CABG and mitral repair. This study has big implications for the practice of thoracic surgery and for how cardiologists refer patients,” according to Dr. Thourani.
The COAPT trial is randomizing patients with symptomatic functional mitral regurgitation and very high surgical risk to percutaneous catheter-based treatment with the MitraClip or to a standard-care control group. One-year outcomes will be presented in 2016, and follow-up out to 5 years is planned.
“You can see now that in cardiac surgery there’s a lot going on,” Dr. Thourani concluded. “It’s not only good for us, but it’s good for you. As a cardiovascular community, we need to work together more.”
He reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott Medical, Boston Scientific, Medtronic, and Sorin.
EXPERT ANALYSIS FROM ACC 15
Avoid voriconazole in transplant patients at risk for skin cancer
SAN FRANCISCO – Voriconazole increased the risk of squamous cell carcinoma by 73% in a review of 455 lung transplant patients at the University of California, San Francisco.
The increase was for any exposure to the drug after transplant (adjusted hazard ratio, 1.73; P = .03). The investigators also found that each additional 30-day exposure at 200 mg of voriconazole twice daily increased the risk of squamous cell carcinoma (SCC) by 3.0% (HR, 1.03; P < .001). The results were adjusted for age at transplant, sex, and race. Overall, SCC risk was highest among white men aged 50 years or older at the time of transplant.
Although voriconazole did protect against posttransplant Aspergillus colonization (aHR, 0.50; P < .001), it did not reduce the risk of invasive aspergillosis. The drug reduced all-cause mortality only among colonized subjects (aHR, 0.34; P = .03), and offered no mortality benefit among those who were not colonized.
There was no difference in all-cause mortality between patients who had any exposure to voriconazole and those who did not, “but we actually found a 2% increased risk of death for each 1 month on the medication. Patients who weren’t colonized were the ones contributing to this increased risk of death,” said lead investigator Matthew Mansh, now a medical student at Stanford (Calif.) University.
There was no increased risk of SCC with alternative antifungals, including inhaled amphotericin and posaconazole. These alternatives should be considered instead of voriconazole in people at higher risk for skin cancer after lung transplants, according to the study, Mr. Mansh noted.
Voriconazole, which is widely used for antifungal prophylaxis after solid organ transplants, has been linked to skin cancer. The reason for the carcinogenic effect is not known; researchers are working to unravel the molecular mechanisms.
“Physicians should be cautious when using voriconazole in the care of transplant recipients. If you see a patient who is developing phototoxicity” with voriconazole, “and if they don’t have evidence of Aspergillus colonization, you may want to limit exposure to high doses of this drug or suggest an alternative,” Mr. Mansh said.
“We have now demonstrated that the alternatives “don’t carry this increased risk of cutaneous SCC,” Mr. Mansh said at the American Academy of Dermatology annual meeting.
The mean age of the study patients at transplant was 52 years, and the majority of patients were white; slightly more than half were men. Most had bilateral lung transplants, with pulmonary fibrosis at the leading indication.
Voriconazole was used in 85% of the patients for an average of 10 months. A quarter of voriconazole patients developed SCC within 5 years of transplant, and 43% within 10 years. Among patients who did not receive the drug, 15% developed SCC within 5 years of transplant, and 28% developed SCC within 10 years of transplant.
“The benefit of voriconazole in terms of death was limited to patients with evidence of Aspergillus colonization, and it wasn’t dose dependent. Patients who had a higher cumulative exposure did not get more benefit,” Mr. Mansh said.
Mr. Mansh had no relevant disclosures.

|
Dr. Paul T. Nghiem |
This is a carefully done study with a practical message: voriconazole patients are at a prolonged increased risk for squamous cell carcinoma. If patients develop phototoxicity or are fair-skinned, have sun damage, a history of squamous cell carcinoma or other risk factors, I think it’s highly appropriate to suggest an alternative. The alternatives are not at all associated with phototoxicity or squamous cell carcinoma.
Dr. Paul T. Nghiem moderated the late-breaker presentation in which the study was presented and is a professor of dermatology at the University of Washington, Seattle. Dr. Nghiem had no disclosures related to the study.

|
Dr. Paul T. Nghiem |
This is a carefully done study with a practical message: voriconazole patients are at a prolonged increased risk for squamous cell carcinoma. If patients develop phototoxicity or are fair-skinned, have sun damage, a history of squamous cell carcinoma or other risk factors, I think it’s highly appropriate to suggest an alternative. The alternatives are not at all associated with phototoxicity or squamous cell carcinoma.
Dr. Paul T. Nghiem moderated the late-breaker presentation in which the study was presented and is a professor of dermatology at the University of Washington, Seattle. Dr. Nghiem had no disclosures related to the study.

|
Dr. Paul T. Nghiem |
This is a carefully done study with a practical message: voriconazole patients are at a prolonged increased risk for squamous cell carcinoma. If patients develop phototoxicity or are fair-skinned, have sun damage, a history of squamous cell carcinoma or other risk factors, I think it’s highly appropriate to suggest an alternative. The alternatives are not at all associated with phototoxicity or squamous cell carcinoma.
Dr. Paul T. Nghiem moderated the late-breaker presentation in which the study was presented and is a professor of dermatology at the University of Washington, Seattle. Dr. Nghiem had no disclosures related to the study.
SAN FRANCISCO – Voriconazole increased the risk of squamous cell carcinoma by 73% in a review of 455 lung transplant patients at the University of California, San Francisco.
The increase was for any exposure to the drug after transplant (adjusted hazard ratio, 1.73; P = .03). The investigators also found that each additional 30-day exposure at 200 mg of voriconazole twice daily increased the risk of squamous cell carcinoma (SCC) by 3.0% (HR, 1.03; P < .001). The results were adjusted for age at transplant, sex, and race. Overall, SCC risk was highest among white men aged 50 years or older at the time of transplant.
Although voriconazole did protect against posttransplant Aspergillus colonization (aHR, 0.50; P < .001), it did not reduce the risk of invasive aspergillosis. The drug reduced all-cause mortality only among colonized subjects (aHR, 0.34; P = .03), and offered no mortality benefit among those who were not colonized.
There was no difference in all-cause mortality between patients who had any exposure to voriconazole and those who did not, “but we actually found a 2% increased risk of death for each 1 month on the medication. Patients who weren’t colonized were the ones contributing to this increased risk of death,” said lead investigator Matthew Mansh, now a medical student at Stanford (Calif.) University.
There was no increased risk of SCC with alternative antifungals, including inhaled amphotericin and posaconazole. These alternatives should be considered instead of voriconazole in people at higher risk for skin cancer after lung transplants, according to the study, Mr. Mansh noted.
Voriconazole, which is widely used for antifungal prophylaxis after solid organ transplants, has been linked to skin cancer. The reason for the carcinogenic effect is not known; researchers are working to unravel the molecular mechanisms.
“Physicians should be cautious when using voriconazole in the care of transplant recipients. If you see a patient who is developing phototoxicity” with voriconazole, “and if they don’t have evidence of Aspergillus colonization, you may want to limit exposure to high doses of this drug or suggest an alternative,” Mr. Mansh said.
“We have now demonstrated that the alternatives “don’t carry this increased risk of cutaneous SCC,” Mr. Mansh said at the American Academy of Dermatology annual meeting.
The mean age of the study patients at transplant was 52 years, and the majority of patients were white; slightly more than half were men. Most had bilateral lung transplants, with pulmonary fibrosis at the leading indication.
Voriconazole was used in 85% of the patients for an average of 10 months. A quarter of voriconazole patients developed SCC within 5 years of transplant, and 43% within 10 years. Among patients who did not receive the drug, 15% developed SCC within 5 years of transplant, and 28% developed SCC within 10 years of transplant.
“The benefit of voriconazole in terms of death was limited to patients with evidence of Aspergillus colonization, and it wasn’t dose dependent. Patients who had a higher cumulative exposure did not get more benefit,” Mr. Mansh said.
Mr. Mansh had no relevant disclosures.
SAN FRANCISCO – Voriconazole increased the risk of squamous cell carcinoma by 73% in a review of 455 lung transplant patients at the University of California, San Francisco.
The increase was for any exposure to the drug after transplant (adjusted hazard ratio, 1.73; P = .03). The investigators also found that each additional 30-day exposure at 200 mg of voriconazole twice daily increased the risk of squamous cell carcinoma (SCC) by 3.0% (HR, 1.03; P < .001). The results were adjusted for age at transplant, sex, and race. Overall, SCC risk was highest among white men aged 50 years or older at the time of transplant.
Although voriconazole did protect against posttransplant Aspergillus colonization (aHR, 0.50; P < .001), it did not reduce the risk of invasive aspergillosis. The drug reduced all-cause mortality only among colonized subjects (aHR, 0.34; P = .03), and offered no mortality benefit among those who were not colonized.
There was no difference in all-cause mortality between patients who had any exposure to voriconazole and those who did not, “but we actually found a 2% increased risk of death for each 1 month on the medication. Patients who weren’t colonized were the ones contributing to this increased risk of death,” said lead investigator Matthew Mansh, now a medical student at Stanford (Calif.) University.
There was no increased risk of SCC with alternative antifungals, including inhaled amphotericin and posaconazole. These alternatives should be considered instead of voriconazole in people at higher risk for skin cancer after lung transplants, according to the study, Mr. Mansh noted.
Voriconazole, which is widely used for antifungal prophylaxis after solid organ transplants, has been linked to skin cancer. The reason for the carcinogenic effect is not known; researchers are working to unravel the molecular mechanisms.
“Physicians should be cautious when using voriconazole in the care of transplant recipients. If you see a patient who is developing phototoxicity” with voriconazole, “and if they don’t have evidence of Aspergillus colonization, you may want to limit exposure to high doses of this drug or suggest an alternative,” Mr. Mansh said.
“We have now demonstrated that the alternatives “don’t carry this increased risk of cutaneous SCC,” Mr. Mansh said at the American Academy of Dermatology annual meeting.
The mean age of the study patients at transplant was 52 years, and the majority of patients were white; slightly more than half were men. Most had bilateral lung transplants, with pulmonary fibrosis at the leading indication.
Voriconazole was used in 85% of the patients for an average of 10 months. A quarter of voriconazole patients developed SCC within 5 years of transplant, and 43% within 10 years. Among patients who did not receive the drug, 15% developed SCC within 5 years of transplant, and 28% developed SCC within 10 years of transplant.
“The benefit of voriconazole in terms of death was limited to patients with evidence of Aspergillus colonization, and it wasn’t dose dependent. Patients who had a higher cumulative exposure did not get more benefit,” Mr. Mansh said.
Mr. Mansh had no relevant disclosures.
AT AAD 2015
Key clinical point: Use an alternative antifungal after lung transplant in white men aged 50 years and older.
Major finding: Exposure to voriconazole increased the risk of squamous cell carcinoma by 73% after lung transplant (aHR, 1.73; P = .03); each additional 30‐day exposure at 200 mg twice daily increased the risk by 3.0% (HR 1.03; P < .001).
Data source: Retrospective cohort study of 455 lung transplant patients
Disclosures: The lead investigator had no relevant disclosures.
Disruptive medicine: 3-D printing revolution
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
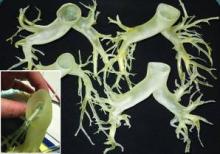
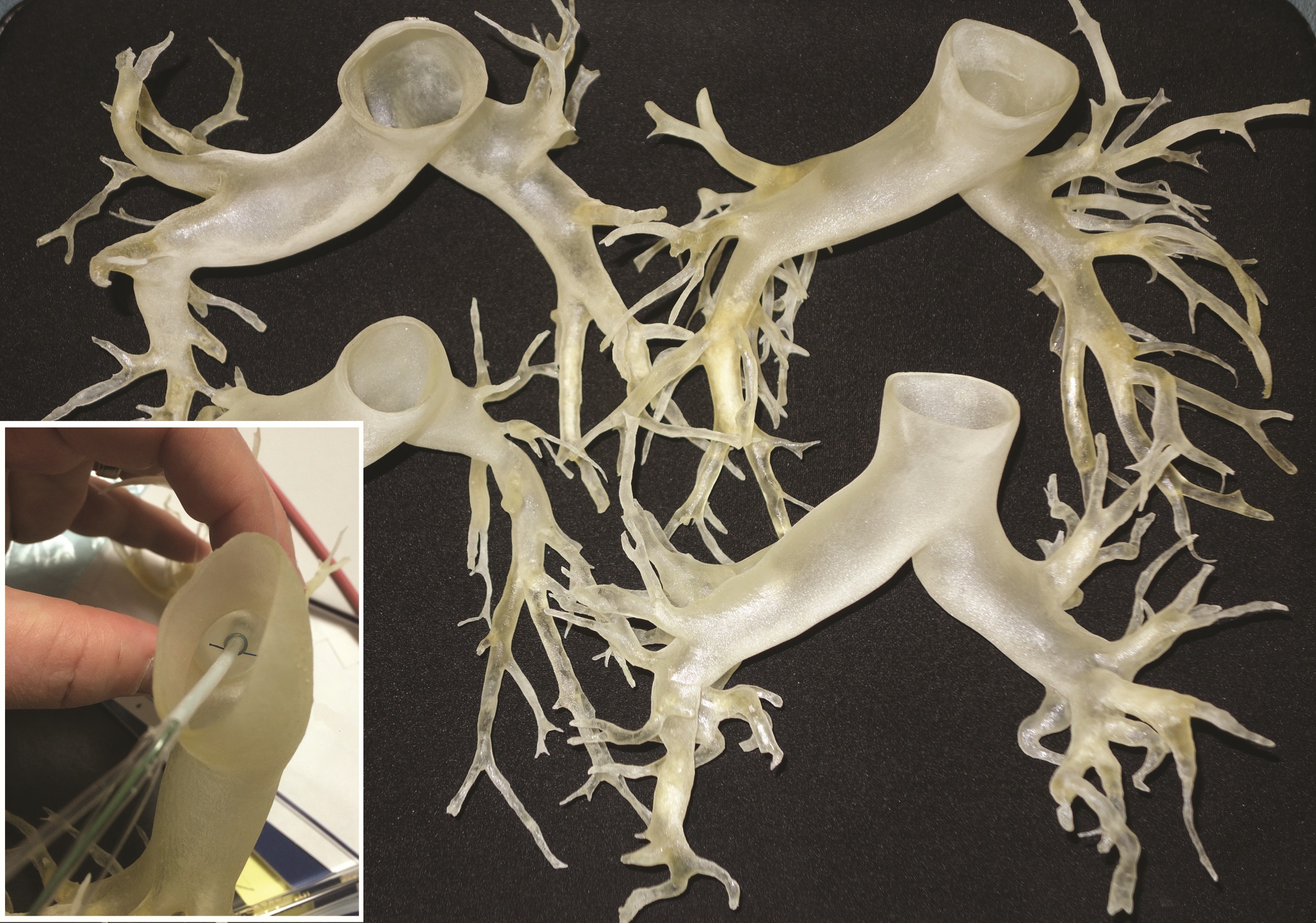
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
FDA approves miniature heart pump for use during high risk PCI
A miniature heart pump has been approved by the Food and Drug Administration to “help certain patients maintain stable heart function and circulation during certain high-risk percutaneous coronary intervention (HRPCI) procedures,” the agency has announced.
The Impella 2.5 System, manufactured by Abiomed, is “intended for temporary use by patients with severe symptomatic CAD [coronary artery disease] and diminished (but stable) heart function who are undergoing HRPCI but are not candidates for surgical coronary bypass treatment,” according to the FDA’s statement.
“Use of the Impella 2.5 System is intended to prevent episodes of unstable heart function, including unstable blood pressure and poor circulation, in patients who are at high risk for its occurrence,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the statement.
Approval was based on the PROTECT II study and observational data from the USpella Registry.
“The overall data provided evidence that, for patients with severe CAD and diminished heart function, the temporary circulatory support provided by the Impella 2.5 System during an HRPCI procedure may allow a longer and more thorough procedure by preventing episodes of hemodynamic instability ... due to temporary abnormalities in heart function,” the FDA statement said. In addition, “fewer later adverse events” such as the need for repeat HRPCI procedures, “may occur in patients undergoing HRPCI with the pump compared to patients undergoing HRPCI with an intra-aortic balloon pump,” according to the FDA.
The FDA statement also noted that the system can be used as an alternative to the intra-aortic balloon pump “without significantly increasing the safety risks of the HRPCI procedure.”
As a postmarketing requirement, the manufacturer will conduct a single arm study of the device in high-risk PCI patients, according to the company’s statement announcing approval.
The wording of the approved indication is as follows, according to Abiomed: “The Impella 2.5 is a temporary (less than or equal to 6 hours) ventricular support device indicated for use during high-risk PCI performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high-risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 in these patients may prevent hemodynamic instability that may occur during planned temporary coronary occlusions and may reduce peri- and postprocedural adverse events.”
A miniature heart pump has been approved by the Food and Drug Administration to “help certain patients maintain stable heart function and circulation during certain high-risk percutaneous coronary intervention (HRPCI) procedures,” the agency has announced.
The Impella 2.5 System, manufactured by Abiomed, is “intended for temporary use by patients with severe symptomatic CAD [coronary artery disease] and diminished (but stable) heart function who are undergoing HRPCI but are not candidates for surgical coronary bypass treatment,” according to the FDA’s statement.
“Use of the Impella 2.5 System is intended to prevent episodes of unstable heart function, including unstable blood pressure and poor circulation, in patients who are at high risk for its occurrence,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the statement.
Approval was based on the PROTECT II study and observational data from the USpella Registry.
“The overall data provided evidence that, for patients with severe CAD and diminished heart function, the temporary circulatory support provided by the Impella 2.5 System during an HRPCI procedure may allow a longer and more thorough procedure by preventing episodes of hemodynamic instability ... due to temporary abnormalities in heart function,” the FDA statement said. In addition, “fewer later adverse events” such as the need for repeat HRPCI procedures, “may occur in patients undergoing HRPCI with the pump compared to patients undergoing HRPCI with an intra-aortic balloon pump,” according to the FDA.
The FDA statement also noted that the system can be used as an alternative to the intra-aortic balloon pump “without significantly increasing the safety risks of the HRPCI procedure.”
As a postmarketing requirement, the manufacturer will conduct a single arm study of the device in high-risk PCI patients, according to the company’s statement announcing approval.
The wording of the approved indication is as follows, according to Abiomed: “The Impella 2.5 is a temporary (less than or equal to 6 hours) ventricular support device indicated for use during high-risk PCI performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high-risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 in these patients may prevent hemodynamic instability that may occur during planned temporary coronary occlusions and may reduce peri- and postprocedural adverse events.”
A miniature heart pump has been approved by the Food and Drug Administration to “help certain patients maintain stable heart function and circulation during certain high-risk percutaneous coronary intervention (HRPCI) procedures,” the agency has announced.
The Impella 2.5 System, manufactured by Abiomed, is “intended for temporary use by patients with severe symptomatic CAD [coronary artery disease] and diminished (but stable) heart function who are undergoing HRPCI but are not candidates for surgical coronary bypass treatment,” according to the FDA’s statement.
“Use of the Impella 2.5 System is intended to prevent episodes of unstable heart function, including unstable blood pressure and poor circulation, in patients who are at high risk for its occurrence,” Dr. William Maisel, acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, said in the statement.
Approval was based on the PROTECT II study and observational data from the USpella Registry.
“The overall data provided evidence that, for patients with severe CAD and diminished heart function, the temporary circulatory support provided by the Impella 2.5 System during an HRPCI procedure may allow a longer and more thorough procedure by preventing episodes of hemodynamic instability ... due to temporary abnormalities in heart function,” the FDA statement said. In addition, “fewer later adverse events” such as the need for repeat HRPCI procedures, “may occur in patients undergoing HRPCI with the pump compared to patients undergoing HRPCI with an intra-aortic balloon pump,” according to the FDA.
The FDA statement also noted that the system can be used as an alternative to the intra-aortic balloon pump “without significantly increasing the safety risks of the HRPCI procedure.”
As a postmarketing requirement, the manufacturer will conduct a single arm study of the device in high-risk PCI patients, according to the company’s statement announcing approval.
The wording of the approved indication is as follows, according to Abiomed: “The Impella 2.5 is a temporary (less than or equal to 6 hours) ventricular support device indicated for use during high-risk PCI performed in elective or urgent hemodynamically stable patients with severe coronary artery disease and depressed left ventricular ejection fraction, when a heart team, including a cardiac surgeon, has determined high-risk PCI is the appropriate therapeutic option. Use of the Impella 2.5 in these patients may prevent hemodynamic instability that may occur during planned temporary coronary occlusions and may reduce peri- and postprocedural adverse events.”
VIDEO: Long-term PARTNER 1 data tip scales toward TAVR
SAN DIEGO – The 5-year results of the PARTNER 1 trial in presented at the annual meeting of the American College of Cardiology were reassuring for clinicians treating patients with severe aortic stenosis at high risk for surgery, said to Dr. Jeffrey J. Popma of Beth Israel DeaconessMedical Center, Boston, in a video interview. The data showed comparable mortality between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), as well as long-term durability of the SAPIEN transcatheter valve, he said.
With these similar outcome results, Dr. Popma asks, can the less-invasive TAVR procedure be considered the preferred treatment over SAVR in these very-sick patients?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – The 5-year results of the PARTNER 1 trial in presented at the annual meeting of the American College of Cardiology were reassuring for clinicians treating patients with severe aortic stenosis at high risk for surgery, said to Dr. Jeffrey J. Popma of Beth Israel DeaconessMedical Center, Boston, in a video interview. The data showed comparable mortality between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), as well as long-term durability of the SAPIEN transcatheter valve, he said.
With these similar outcome results, Dr. Popma asks, can the less-invasive TAVR procedure be considered the preferred treatment over SAVR in these very-sick patients?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – The 5-year results of the PARTNER 1 trial in presented at the annual meeting of the American College of Cardiology were reassuring for clinicians treating patients with severe aortic stenosis at high risk for surgery, said to Dr. Jeffrey J. Popma of Beth Israel DeaconessMedical Center, Boston, in a video interview. The data showed comparable mortality between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR), as well as long-term durability of the SAPIEN transcatheter valve, he said.
With these similar outcome results, Dr. Popma asks, can the less-invasive TAVR procedure be considered the preferred treatment over SAVR in these very-sick patients?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 15
Cost comparison favors minimally invasive over conventional AVR
Outcomes were similar, but hospital costs improved with use of mini-aortic valve replacement, compared with conventional AVR, according to the results of a Society of Thoracic Surgeons (STS) database study of 1,341 patients who underwent primary AVR at 17 hospitals.
A propensity match cohort analysis was done to compare patients who had conventional (67%) vs. mini-AVR (33%) performed using either partial sternotomy or right thoracotomy.
Mortality, stroke, renal failure, atrial fibrillation, reoperation for bleeding, and respiratory insufficiency were not statistically significantly different between the two groups. There was also no significant difference in ICU or hospital length of stay between the two groups. However, mini-AVR was associated with both significantly decreased ventilator time (5 vs. 6 hours) and blood product transfusion (25% vs. 32%), according to the report, which was published online and scheduled for the April issue of the Journal of Thoracic and Cardiovascular Surgery (doi:10.1016/j/jtcvs.2015.01.014).
Total hospital cost was significantly lower in the mini-AVR group ($36,348) vs. the conventional repair group ($38,239, P = .02), wrote Dr. Ravi Kiran Ghanta of the University of Virginia, Charlottesville, and his colleagues.
The authors discussed the previously raised issue of longer cross-clamp and bypass times seen in earlier studies of mini-AVR. In their current study, such was not the case, with mini-AVR appearing equivalent with conventional operations. The authors suggested that surgeons have now adopted techniques to reduce bypass and cross-clamp times with mini-AVR.
Data were limited to in-hospital costs. Other costs, such as those of rehabilitation and lost productivity, were not included in the analysis. “Including these health-care costs may have increased overall savings with mini-AVR compared to conventional AVR,” the authors noted.
“Mini-AVR is associated with decreased ventilator time, blood product utilization, early discharge, and reduced total hospital cost. In contemporary clinical practice, mini-AVR is safe and cost-effective,” the researchers concluded.
The authors reported that they had no conflicts.
“Measurement of cost and outcome, the determinant of ‘value’ in health care, is assuming increasing importance in the evaluation of all medial interventions, especially those surgical procedures done frequently and at higher cost,” wrote Dr. Verdi J. DiSesa in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.049]).
Although applauding the timeliness and importance of analyzing the costs and outcomes in propensity-matched patients undergoing aortic valve replacement via full sternotomy of mini-AVR using a partial sternotomy or limited thoracotomy incision, he questioned some of the methodology. The cost-to-charge ratio used, which provides an estimate of the cost of services, is not uniform throughout departments in a single hospital, and differs from hospital to hospital, although the latter was accounted for. He suggested that “activity-based costing” would have been a better approach, although more labor intensive. In this approach, every encounter with a caregiver is assigned a unit cost and the cost of a patient encounter is calculated by estimating the number of units of service and multiplying by the unit cost for service.
“This method is demanding of resources but has the potential to become the most accurate and therefore the preferred way to determine the costs of medical services,” he wrote. In addition, the authors included but did not separately analyze patients having AVR via minithoracotomy.
“What might be the conclusions of this research were these patients excluded or analyzed separately?” he asked.
In terms of the propensity model, the matching procedure included only three clinical factors – STS Predicted Rate of Mortality (PROM) score, the operative year, and the surgeon who performed the procedure – resulting in 35% of patients remaining unmatched, which may have biased the results.
This analysis, Dr. DiSesa concluded, is “perhaps an early version of the kind of analyses, which increasingly will be required for the evaluation of the services provided by all health care providers, not only cardiac surgeons.”
Dr. DiSesa is professor of surgery at Temple University, Philadelphia.
“Measurement of cost and outcome, the determinant of ‘value’ in health care, is assuming increasing importance in the evaluation of all medial interventions, especially those surgical procedures done frequently and at higher cost,” wrote Dr. Verdi J. DiSesa in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.049]).
Although applauding the timeliness and importance of analyzing the costs and outcomes in propensity-matched patients undergoing aortic valve replacement via full sternotomy of mini-AVR using a partial sternotomy or limited thoracotomy incision, he questioned some of the methodology. The cost-to-charge ratio used, which provides an estimate of the cost of services, is not uniform throughout departments in a single hospital, and differs from hospital to hospital, although the latter was accounted for. He suggested that “activity-based costing” would have been a better approach, although more labor intensive. In this approach, every encounter with a caregiver is assigned a unit cost and the cost of a patient encounter is calculated by estimating the number of units of service and multiplying by the unit cost for service.
“This method is demanding of resources but has the potential to become the most accurate and therefore the preferred way to determine the costs of medical services,” he wrote. In addition, the authors included but did not separately analyze patients having AVR via minithoracotomy.
“What might be the conclusions of this research were these patients excluded or analyzed separately?” he asked.
In terms of the propensity model, the matching procedure included only three clinical factors – STS Predicted Rate of Mortality (PROM) score, the operative year, and the surgeon who performed the procedure – resulting in 35% of patients remaining unmatched, which may have biased the results.
This analysis, Dr. DiSesa concluded, is “perhaps an early version of the kind of analyses, which increasingly will be required for the evaluation of the services provided by all health care providers, not only cardiac surgeons.”
Dr. DiSesa is professor of surgery at Temple University, Philadelphia.
“Measurement of cost and outcome, the determinant of ‘value’ in health care, is assuming increasing importance in the evaluation of all medial interventions, especially those surgical procedures done frequently and at higher cost,” wrote Dr. Verdi J. DiSesa in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.049]).
Although applauding the timeliness and importance of analyzing the costs and outcomes in propensity-matched patients undergoing aortic valve replacement via full sternotomy of mini-AVR using a partial sternotomy or limited thoracotomy incision, he questioned some of the methodology. The cost-to-charge ratio used, which provides an estimate of the cost of services, is not uniform throughout departments in a single hospital, and differs from hospital to hospital, although the latter was accounted for. He suggested that “activity-based costing” would have been a better approach, although more labor intensive. In this approach, every encounter with a caregiver is assigned a unit cost and the cost of a patient encounter is calculated by estimating the number of units of service and multiplying by the unit cost for service.
“This method is demanding of resources but has the potential to become the most accurate and therefore the preferred way to determine the costs of medical services,” he wrote. In addition, the authors included but did not separately analyze patients having AVR via minithoracotomy.
“What might be the conclusions of this research were these patients excluded or analyzed separately?” he asked.
In terms of the propensity model, the matching procedure included only three clinical factors – STS Predicted Rate of Mortality (PROM) score, the operative year, and the surgeon who performed the procedure – resulting in 35% of patients remaining unmatched, which may have biased the results.
This analysis, Dr. DiSesa concluded, is “perhaps an early version of the kind of analyses, which increasingly will be required for the evaluation of the services provided by all health care providers, not only cardiac surgeons.”
Dr. DiSesa is professor of surgery at Temple University, Philadelphia.
Outcomes were similar, but hospital costs improved with use of mini-aortic valve replacement, compared with conventional AVR, according to the results of a Society of Thoracic Surgeons (STS) database study of 1,341 patients who underwent primary AVR at 17 hospitals.
A propensity match cohort analysis was done to compare patients who had conventional (67%) vs. mini-AVR (33%) performed using either partial sternotomy or right thoracotomy.
Mortality, stroke, renal failure, atrial fibrillation, reoperation for bleeding, and respiratory insufficiency were not statistically significantly different between the two groups. There was also no significant difference in ICU or hospital length of stay between the two groups. However, mini-AVR was associated with both significantly decreased ventilator time (5 vs. 6 hours) and blood product transfusion (25% vs. 32%), according to the report, which was published online and scheduled for the April issue of the Journal of Thoracic and Cardiovascular Surgery (doi:10.1016/j/jtcvs.2015.01.014).
Total hospital cost was significantly lower in the mini-AVR group ($36,348) vs. the conventional repair group ($38,239, P = .02), wrote Dr. Ravi Kiran Ghanta of the University of Virginia, Charlottesville, and his colleagues.
The authors discussed the previously raised issue of longer cross-clamp and bypass times seen in earlier studies of mini-AVR. In their current study, such was not the case, with mini-AVR appearing equivalent with conventional operations. The authors suggested that surgeons have now adopted techniques to reduce bypass and cross-clamp times with mini-AVR.
Data were limited to in-hospital costs. Other costs, such as those of rehabilitation and lost productivity, were not included in the analysis. “Including these health-care costs may have increased overall savings with mini-AVR compared to conventional AVR,” the authors noted.
“Mini-AVR is associated with decreased ventilator time, blood product utilization, early discharge, and reduced total hospital cost. In contemporary clinical practice, mini-AVR is safe and cost-effective,” the researchers concluded.
The authors reported that they had no conflicts.
Outcomes were similar, but hospital costs improved with use of mini-aortic valve replacement, compared with conventional AVR, according to the results of a Society of Thoracic Surgeons (STS) database study of 1,341 patients who underwent primary AVR at 17 hospitals.
A propensity match cohort analysis was done to compare patients who had conventional (67%) vs. mini-AVR (33%) performed using either partial sternotomy or right thoracotomy.
Mortality, stroke, renal failure, atrial fibrillation, reoperation for bleeding, and respiratory insufficiency were not statistically significantly different between the two groups. There was also no significant difference in ICU or hospital length of stay between the two groups. However, mini-AVR was associated with both significantly decreased ventilator time (5 vs. 6 hours) and blood product transfusion (25% vs. 32%), according to the report, which was published online and scheduled for the April issue of the Journal of Thoracic and Cardiovascular Surgery (doi:10.1016/j/jtcvs.2015.01.014).
Total hospital cost was significantly lower in the mini-AVR group ($36,348) vs. the conventional repair group ($38,239, P = .02), wrote Dr. Ravi Kiran Ghanta of the University of Virginia, Charlottesville, and his colleagues.
The authors discussed the previously raised issue of longer cross-clamp and bypass times seen in earlier studies of mini-AVR. In their current study, such was not the case, with mini-AVR appearing equivalent with conventional operations. The authors suggested that surgeons have now adopted techniques to reduce bypass and cross-clamp times with mini-AVR.
Data were limited to in-hospital costs. Other costs, such as those of rehabilitation and lost productivity, were not included in the analysis. “Including these health-care costs may have increased overall savings with mini-AVR compared to conventional AVR,” the authors noted.
“Mini-AVR is associated with decreased ventilator time, blood product utilization, early discharge, and reduced total hospital cost. In contemporary clinical practice, mini-AVR is safe and cost-effective,” the researchers concluded.
The authors reported that they had no conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Mortality, stroke, renal failure, and other complications were not significantly different between mini-aortic valve replacement and conventional replacement.
Major finding: Total hospital costs were significantly lower with mini-AVR, compared with conventional replacement ($36,348 vs. $38,239).
Data source: A retrospective data analysis of patient records for isolated AVR repair, extracted from a regional, multi-institutional Society of Thoracic Surgeons database.
Disclosures: The authors reported that they had no relevant conflicts of interest.
Greater surgeon experience linked to better long-term survival in NSCLC
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:Insufficient lymph-node sampling by less-experienced surgeons may be a reason why patients with stage I NSCLC had better long-term survival if operated on by moderate- and high-experience surgeons.
Major finding: Short-term outcomes in pathologic stage I NSCLC were not affected by surgeon experience, but 5-year survival was significantly lower for the low-experience compared to the moderate-experience surgeons (76.9% vs. 67.5%).
Data source: An institutional database analysis was conducted of 800 operations on stage I NSCLC patients performed from 2000 to 2012.
Disclosures: The authors reported having no conflicts of interest.
Low-volume centers using ECMO have poorest survival rates
SAN DIEGO – Lung transplantation centers that are considered low volume tend to have lower rates of survival than do those of their medium- and high-volume counterparts when patients are bridged via extracorporeal membrane oxygenation (ECMO), according to researchers.
Even so, there is a point at which survival outcomes begin to improve for low-volume centers, they added.
“Increasingly, [ECMO] is used as a bridge to lung transplantation; indeed, the use of ECMO has tripled over the past 15 years and survival has increased by the same magnitude,” Dr. Jeremiah A. Hayanga said at the annual meeting of the Society of Thoracic Surgeons.
“An entire body of literature has linked high-volume [centers] to improved outcomes in the context of complex surgical procedures. Lung transplantation [LTx] falls within the same domain, and has been considered subject to the same inverse volume-outcome paradigm,” said Dr. Hayanga of Michigan State University, Grand Rapids.
He and his coinvestigators conducted a retrospective analysis of 16,603 LTx recipients in the International Registry for Heart and Lung Transplantation (ISHLT) who underwent ECMO as their bridging strategy between 2005 and 2010. Centers were stratified into categories of low, medium, and high based on the volume of LTx procedures performed over the study interval: Low was defined as fewer than 25, medium as 25-50, and more than 50 as high volume.
Overall, 85 of the 16,603 transplant recipients in the study population were bridged via ECMO: 20 (23.5%) of them in low-volume centers, 30 (35.3%) in medium-volume centers, and 35 (41.2%) in high-volume centers. The researchers used Cox proportional hazard modeling to identify predictors of both 1- and 5-year survival rates, which were found to be significantly lower in low-volume centers – 13.61% at 5 years post LTx.
Looking at just the high-volume and low-volume centers, the researchers noted “significant differences” in both 1-year and 5-year survival rates when ECMO was used for bridging. One-year survival probability was roughly 40% in low-volume centers and roughly 70% in high-volume centers, while 5-year survival probability was well under 25% for recipients from low-volume centers and around 50% for those from high-volume centers (P = .0006). No significant differences existed for non-ECMO patients, regardless of center volume.
“No differences existed in survival in medium- and high-volume centers,” said Dr. Hayanga. “Transplanting without ECMO as a bridge showed fewer survival differences for both 1-year and 5-year survival. However, when ECMO was used as a bridge, the low-volume center [survival rates] were dramatically lower at both 1 year and 5 years.”
When Dr. Hayanga and his colleagues examined procedural volume as a continuous variable, however, a single inflection point was determined as the point at which survival outcomes steadily improve – 19 procedures. Centers that performed at least 19 LTx procedures between 2005 and 2010 experienced an uptick in survival rates, even though centers that saw 19-25 procedures were still considered low volume, the researchers noted.
“The corresponding c-statistic, however, is just under 60%,” cautioned Dr. Hayanga. “The C-statistic is a measure of the explanatory power of a variable – in this case, [center] volume – in accounting for the variability in outcome, or survival in this case. To put that number into context, a C-statistic of 50% means ‘no explanatory power’ whatsoever.”
Dr. Hayanga explained that he and his coauthors compared transplant recipient and donor characteristics using analysis of variance (ANOVA) and chi-square tests to compare variables, cumulative survival using Kaplan-Meier curves, and significance using log-rank tests.
Dr. Hayanga reported no financial conflicts of interest.
SAN DIEGO – Lung transplantation centers that are considered low volume tend to have lower rates of survival than do those of their medium- and high-volume counterparts when patients are bridged via extracorporeal membrane oxygenation (ECMO), according to researchers.
Even so, there is a point at which survival outcomes begin to improve for low-volume centers, they added.
“Increasingly, [ECMO] is used as a bridge to lung transplantation; indeed, the use of ECMO has tripled over the past 15 years and survival has increased by the same magnitude,” Dr. Jeremiah A. Hayanga said at the annual meeting of the Society of Thoracic Surgeons.
“An entire body of literature has linked high-volume [centers] to improved outcomes in the context of complex surgical procedures. Lung transplantation [LTx] falls within the same domain, and has been considered subject to the same inverse volume-outcome paradigm,” said Dr. Hayanga of Michigan State University, Grand Rapids.
He and his coinvestigators conducted a retrospective analysis of 16,603 LTx recipients in the International Registry for Heart and Lung Transplantation (ISHLT) who underwent ECMO as their bridging strategy between 2005 and 2010. Centers were stratified into categories of low, medium, and high based on the volume of LTx procedures performed over the study interval: Low was defined as fewer than 25, medium as 25-50, and more than 50 as high volume.
Overall, 85 of the 16,603 transplant recipients in the study population were bridged via ECMO: 20 (23.5%) of them in low-volume centers, 30 (35.3%) in medium-volume centers, and 35 (41.2%) in high-volume centers. The researchers used Cox proportional hazard modeling to identify predictors of both 1- and 5-year survival rates, which were found to be significantly lower in low-volume centers – 13.61% at 5 years post LTx.
Looking at just the high-volume and low-volume centers, the researchers noted “significant differences” in both 1-year and 5-year survival rates when ECMO was used for bridging. One-year survival probability was roughly 40% in low-volume centers and roughly 70% in high-volume centers, while 5-year survival probability was well under 25% for recipients from low-volume centers and around 50% for those from high-volume centers (P = .0006). No significant differences existed for non-ECMO patients, regardless of center volume.
“No differences existed in survival in medium- and high-volume centers,” said Dr. Hayanga. “Transplanting without ECMO as a bridge showed fewer survival differences for both 1-year and 5-year survival. However, when ECMO was used as a bridge, the low-volume center [survival rates] were dramatically lower at both 1 year and 5 years.”
When Dr. Hayanga and his colleagues examined procedural volume as a continuous variable, however, a single inflection point was determined as the point at which survival outcomes steadily improve – 19 procedures. Centers that performed at least 19 LTx procedures between 2005 and 2010 experienced an uptick in survival rates, even though centers that saw 19-25 procedures were still considered low volume, the researchers noted.
“The corresponding c-statistic, however, is just under 60%,” cautioned Dr. Hayanga. “The C-statistic is a measure of the explanatory power of a variable – in this case, [center] volume – in accounting for the variability in outcome, or survival in this case. To put that number into context, a C-statistic of 50% means ‘no explanatory power’ whatsoever.”
Dr. Hayanga explained that he and his coauthors compared transplant recipient and donor characteristics using analysis of variance (ANOVA) and chi-square tests to compare variables, cumulative survival using Kaplan-Meier curves, and significance using log-rank tests.
Dr. Hayanga reported no financial conflicts of interest.
SAN DIEGO – Lung transplantation centers that are considered low volume tend to have lower rates of survival than do those of their medium- and high-volume counterparts when patients are bridged via extracorporeal membrane oxygenation (ECMO), according to researchers.
Even so, there is a point at which survival outcomes begin to improve for low-volume centers, they added.
“Increasingly, [ECMO] is used as a bridge to lung transplantation; indeed, the use of ECMO has tripled over the past 15 years and survival has increased by the same magnitude,” Dr. Jeremiah A. Hayanga said at the annual meeting of the Society of Thoracic Surgeons.
“An entire body of literature has linked high-volume [centers] to improved outcomes in the context of complex surgical procedures. Lung transplantation [LTx] falls within the same domain, and has been considered subject to the same inverse volume-outcome paradigm,” said Dr. Hayanga of Michigan State University, Grand Rapids.
He and his coinvestigators conducted a retrospective analysis of 16,603 LTx recipients in the International Registry for Heart and Lung Transplantation (ISHLT) who underwent ECMO as their bridging strategy between 2005 and 2010. Centers were stratified into categories of low, medium, and high based on the volume of LTx procedures performed over the study interval: Low was defined as fewer than 25, medium as 25-50, and more than 50 as high volume.
Overall, 85 of the 16,603 transplant recipients in the study population were bridged via ECMO: 20 (23.5%) of them in low-volume centers, 30 (35.3%) in medium-volume centers, and 35 (41.2%) in high-volume centers. The researchers used Cox proportional hazard modeling to identify predictors of both 1- and 5-year survival rates, which were found to be significantly lower in low-volume centers – 13.61% at 5 years post LTx.
Looking at just the high-volume and low-volume centers, the researchers noted “significant differences” in both 1-year and 5-year survival rates when ECMO was used for bridging. One-year survival probability was roughly 40% in low-volume centers and roughly 70% in high-volume centers, while 5-year survival probability was well under 25% for recipients from low-volume centers and around 50% for those from high-volume centers (P = .0006). No significant differences existed for non-ECMO patients, regardless of center volume.
“No differences existed in survival in medium- and high-volume centers,” said Dr. Hayanga. “Transplanting without ECMO as a bridge showed fewer survival differences for both 1-year and 5-year survival. However, when ECMO was used as a bridge, the low-volume center [survival rates] were dramatically lower at both 1 year and 5 years.”
When Dr. Hayanga and his colleagues examined procedural volume as a continuous variable, however, a single inflection point was determined as the point at which survival outcomes steadily improve – 19 procedures. Centers that performed at least 19 LTx procedures between 2005 and 2010 experienced an uptick in survival rates, even though centers that saw 19-25 procedures were still considered low volume, the researchers noted.
“The corresponding c-statistic, however, is just under 60%,” cautioned Dr. Hayanga. “The C-statistic is a measure of the explanatory power of a variable – in this case, [center] volume – in accounting for the variability in outcome, or survival in this case. To put that number into context, a C-statistic of 50% means ‘no explanatory power’ whatsoever.”
Dr. Hayanga explained that he and his coauthors compared transplant recipient and donor characteristics using analysis of variance (ANOVA) and chi-square tests to compare variables, cumulative survival using Kaplan-Meier curves, and significance using log-rank tests.
Dr. Hayanga reported no financial conflicts of interest.
AT THE STS ANNUAL MEETING
Key clinical point: Low-volume lung transplantation centers in the United States typically have the poorest survival rates compared to those with higher volumes when using ECMO.
Major finding: Of 85 LTx subjects bridged via ECMO, 20 (23.5%) of these were bridged in low, 30 (35.3%) in medium, and 35 (41.2%) in high-volume centers; in the ECMO cohort, the lowest 5-year survival rate (13.61%) was observed at low-volume centers.
Data source: Retrospective analysis of 16,603 adult LTx recipients in the International Registry for Heart and Lung Transplantation during 2005-2010.
Disclosures: Dr. Hayanga reported no financial conflicts of interest.