User login
Donor EBV status affects recipient graft-vs-host disease risk
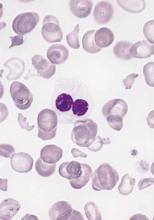
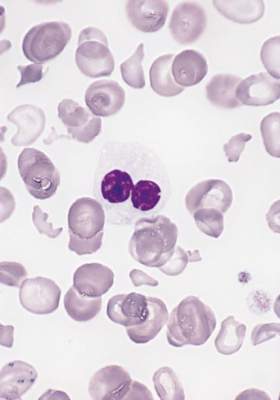
In allogeneic hematopoietic stem-cell transplantation, the donor’s status regarding Epstein-Barr virus affects the recipient’s risk of developing graft-vs-host disease – a “completely new and striking” finding, according to a report published online April 18 in the Journal of Clinical Oncology.
Approximately 80% of the general population has been infected with EBV and carries persistent virus in memory B cells. When viral material is transmitted to stem-cell recipients, it is known to cause posttransplantation lymphoproliferative disorder. Until now, however, no data were available to examine EBV serology’s effect on other posttransplantation outcomes, said Dr. Jan Styczynski of the department of pediatric hematology and oncology at Nicolaus Copernicus University, Bydgoszcz, Poland, and his associates.
They analyzed information in the European Society of Blood and Marrow Transplantation database for 11,364 patients with acute lymphoblastic leukemia or acute myeloblastic leukemia who underwent stem-cell transplantation between 1997 and 2012 and who were followed for approximately 5 years. Most of the donors (82%) were seropositive for EBV. Acute graft-vs-host disease (GVHD) developed in 32% and chronic GVHD developed in 40% of these stem cell–transplant recipients.
The incidence of chronic GVHD was significantly higher when the donor was EBV-seropositive (41%) than when the donor was EBV-seronegative (31%). Similarly, the incidence of acute GVHD was significantly higher when the donor was EBV-seropositive (32% vs 30%), but the magnitude of the difference between the two groups was smaller. The risk for GVHD increased even though patients receiving transplants from EBV-seropositive donors underwent more intensive GVHD prophylaxis than did those who had seronegative donors, the investigators said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.64.2405).
In contrast, the transplant recipients’ EBV status did not affect their risk of developing GVHD.
“Despite the effect of donor EBV serostatus on GVHD, we did not observe a corresponding GVHD-related death rate, and as a result, there was no effect on overall survival, relapse-free survival, relapse incidence, and nonrelapse mortality. However, it should be kept in mind that many other pre- and posttransplantation factors play a role in contributing to final transplantation outcomes,” Dr. Styczynski and his associates noted.
The current recommendation to monitor transplantation recipients for EBV and to give them “preemptive” rituximab to stave off the development of posttransplantation lymphoproliferative disorder might prove useful in also preventing GVHD, they added.
The findings of Dr. Styczynski and his associates raise the possibility that we may be able to prevent or treat GVHD in transplant recipients by controlling EBV infection.
Selecting only EBV-negative donors would be one way to accomplish this, but that would be impractical given the high seroprevalence of EBV in the general population. Depleting memory B cells, the reservoir of EBV infection, using monoclonal antibodies may prove helpful, and these agents might provide additional therapeutic effects. And novel antivirals such as retroviral integrase inhibitors may be more specific at targeting EBV than acyclovir and related agents, which have limited activity against latently infected B cells. These novel drugs, however, are not without risks and adverse effects.
A promising alternative might be to boost immunity to EBV using vaccination or adoptive transfer of ex vivo expanded EBV-specific cytotoxic T cells.
Dr. Katayoun Rezvani and Dr. Richard E. Champlin are with the University of Texas MD Andersen Cancer Center, Houston. Their financial disclosures are available at www.jco.org. They made these remarks in an editorial accompanying Dr. Styczynski’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2016.66.6099).
The findings of Dr. Styczynski and his associates raise the possibility that we may be able to prevent or treat GVHD in transplant recipients by controlling EBV infection.
Selecting only EBV-negative donors would be one way to accomplish this, but that would be impractical given the high seroprevalence of EBV in the general population. Depleting memory B cells, the reservoir of EBV infection, using monoclonal antibodies may prove helpful, and these agents might provide additional therapeutic effects. And novel antivirals such as retroviral integrase inhibitors may be more specific at targeting EBV than acyclovir and related agents, which have limited activity against latently infected B cells. These novel drugs, however, are not without risks and adverse effects.
A promising alternative might be to boost immunity to EBV using vaccination or adoptive transfer of ex vivo expanded EBV-specific cytotoxic T cells.
Dr. Katayoun Rezvani and Dr. Richard E. Champlin are with the University of Texas MD Andersen Cancer Center, Houston. Their financial disclosures are available at www.jco.org. They made these remarks in an editorial accompanying Dr. Styczynski’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2016.66.6099).
The findings of Dr. Styczynski and his associates raise the possibility that we may be able to prevent or treat GVHD in transplant recipients by controlling EBV infection.
Selecting only EBV-negative donors would be one way to accomplish this, but that would be impractical given the high seroprevalence of EBV in the general population. Depleting memory B cells, the reservoir of EBV infection, using monoclonal antibodies may prove helpful, and these agents might provide additional therapeutic effects. And novel antivirals such as retroviral integrase inhibitors may be more specific at targeting EBV than acyclovir and related agents, which have limited activity against latently infected B cells. These novel drugs, however, are not without risks and adverse effects.
A promising alternative might be to boost immunity to EBV using vaccination or adoptive transfer of ex vivo expanded EBV-specific cytotoxic T cells.
Dr. Katayoun Rezvani and Dr. Richard E. Champlin are with the University of Texas MD Andersen Cancer Center, Houston. Their financial disclosures are available at www.jco.org. They made these remarks in an editorial accompanying Dr. Styczynski’s report (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2016.66.6099).
In allogeneic hematopoietic stem-cell transplantation, the donor’s status regarding Epstein-Barr virus affects the recipient’s risk of developing graft-vs-host disease – a “completely new and striking” finding, according to a report published online April 18 in the Journal of Clinical Oncology.
Approximately 80% of the general population has been infected with EBV and carries persistent virus in memory B cells. When viral material is transmitted to stem-cell recipients, it is known to cause posttransplantation lymphoproliferative disorder. Until now, however, no data were available to examine EBV serology’s effect on other posttransplantation outcomes, said Dr. Jan Styczynski of the department of pediatric hematology and oncology at Nicolaus Copernicus University, Bydgoszcz, Poland, and his associates.
They analyzed information in the European Society of Blood and Marrow Transplantation database for 11,364 patients with acute lymphoblastic leukemia or acute myeloblastic leukemia who underwent stem-cell transplantation between 1997 and 2012 and who were followed for approximately 5 years. Most of the donors (82%) were seropositive for EBV. Acute graft-vs-host disease (GVHD) developed in 32% and chronic GVHD developed in 40% of these stem cell–transplant recipients.
The incidence of chronic GVHD was significantly higher when the donor was EBV-seropositive (41%) than when the donor was EBV-seronegative (31%). Similarly, the incidence of acute GVHD was significantly higher when the donor was EBV-seropositive (32% vs 30%), but the magnitude of the difference between the two groups was smaller. The risk for GVHD increased even though patients receiving transplants from EBV-seropositive donors underwent more intensive GVHD prophylaxis than did those who had seronegative donors, the investigators said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.64.2405).
In contrast, the transplant recipients’ EBV status did not affect their risk of developing GVHD.
“Despite the effect of donor EBV serostatus on GVHD, we did not observe a corresponding GVHD-related death rate, and as a result, there was no effect on overall survival, relapse-free survival, relapse incidence, and nonrelapse mortality. However, it should be kept in mind that many other pre- and posttransplantation factors play a role in contributing to final transplantation outcomes,” Dr. Styczynski and his associates noted.
The current recommendation to monitor transplantation recipients for EBV and to give them “preemptive” rituximab to stave off the development of posttransplantation lymphoproliferative disorder might prove useful in also preventing GVHD, they added.
In allogeneic hematopoietic stem-cell transplantation, the donor’s status regarding Epstein-Barr virus affects the recipient’s risk of developing graft-vs-host disease – a “completely new and striking” finding, according to a report published online April 18 in the Journal of Clinical Oncology.
Approximately 80% of the general population has been infected with EBV and carries persistent virus in memory B cells. When viral material is transmitted to stem-cell recipients, it is known to cause posttransplantation lymphoproliferative disorder. Until now, however, no data were available to examine EBV serology’s effect on other posttransplantation outcomes, said Dr. Jan Styczynski of the department of pediatric hematology and oncology at Nicolaus Copernicus University, Bydgoszcz, Poland, and his associates.
They analyzed information in the European Society of Blood and Marrow Transplantation database for 11,364 patients with acute lymphoblastic leukemia or acute myeloblastic leukemia who underwent stem-cell transplantation between 1997 and 2012 and who were followed for approximately 5 years. Most of the donors (82%) were seropositive for EBV. Acute graft-vs-host disease (GVHD) developed in 32% and chronic GVHD developed in 40% of these stem cell–transplant recipients.
The incidence of chronic GVHD was significantly higher when the donor was EBV-seropositive (41%) than when the donor was EBV-seronegative (31%). Similarly, the incidence of acute GVHD was significantly higher when the donor was EBV-seropositive (32% vs 30%), but the magnitude of the difference between the two groups was smaller. The risk for GVHD increased even though patients receiving transplants from EBV-seropositive donors underwent more intensive GVHD prophylaxis than did those who had seronegative donors, the investigators said (J Clin Oncol. 2016 Apr 18. doi: 10.1200/JCO.2015.64.2405).
In contrast, the transplant recipients’ EBV status did not affect their risk of developing GVHD.
“Despite the effect of donor EBV serostatus on GVHD, we did not observe a corresponding GVHD-related death rate, and as a result, there was no effect on overall survival, relapse-free survival, relapse incidence, and nonrelapse mortality. However, it should be kept in mind that many other pre- and posttransplantation factors play a role in contributing to final transplantation outcomes,” Dr. Styczynski and his associates noted.
The current recommendation to monitor transplantation recipients for EBV and to give them “preemptive” rituximab to stave off the development of posttransplantation lymphoproliferative disorder might prove useful in also preventing GVHD, they added.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: In allogeneic hematopoietic stem-cell transplantation, the donor’s EBV status affects the recipient’s risk of developing GVHD.
Major finding: Chronic GVHD was significantly more likely to develop when the donor was EBV-seropositive (41%) than EBV-seronegative (31%).
Data source: A retrospective analysis of data regarding 11,364 European patients with acute leukemia who underwent stem-cell transplantation and were followed for 5 years.
Disclosures: No study sponsor was identified. Dr. Styczynski reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
In myelodysplastic syndrome, improved tool for predicting death after HCT
A new risk-stratification tool goes one better than the standard tools used to predict survival in those undergoing allogeneic hematopoietic cell transplantation (allo HCT) for myelodysplastic syndrome, based on a study published online April 4 in the Journal of Clinical Oncology.
The concordance index for the new risk-stratification tool was modestly better at 0.575, compared with 0.538 for the standard International Prognostic Scoring System (IPSS) and 0.554 for the revised IPSS (IPSS-R), according to Dr. Brian C. Shaffer of Memorial Sloan Kettering Cancer Center, New York, and his colleagues who participate in the Center for International Blood and Marrow Transplant Research (CIBMTR) network.
“The proposed system generally agrees with the IPSS-R in the very high–risk subcategory; however, a significant portion of patients in high- and very high–risk IPSS-R groups were represented in the low- and intermediate-risk proposed scoring subcategories. The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system,” the researchers wrote.
Further, the “scoring system uses readily available clinical data and can be calculated quickly, facilitating patient consultation with respect to allo HCT, and may also be used to identify high-risk populations where interventions such as post–allo HCT maintenance therapies may be of benefit,” they wrote (J Clin Oncol. 2016 April 4. doi: 10.1200/JCO.2015.65.0515).
The data were obtained from the CIBMTR, a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
The researchers applied the prognostic tool to 2,133 patients with MDS undergoing HLA-matched (n = 1,728) or -mismatched (n = 405) allo HCT. Factors prognostic of mortality were identified in a training subset (n = 1,151) of the HLA-matched cohort. A weighted score using these factors was then assigned to the validation cohort of 577 remaining patients undergoing HLA-matched allo HCT as well as to patients undergoing HLA-mismatched allo HCT. The training data set was used to develop a prognostic scoring system, and the validation data set was used to assess the prognostic ability of the scoring system, the researchers noted.
In the scoring system, 1 point was assigned for the following factors: Blood blasts greater than 3%, platelet levels of 50 × 109/L or less at transplantation, Karnofsky performance status less than 90%, comprehensive cytogenetic risk score of poor or very poor, and age 30-49 years. Two points were assigned for monosomal karyotype and age 50 years or older.
Based on the scoring system, 3-year overall survival after transplantation was 71% in patients with scores of 1 point, 49% with scores of 2-3, 41% with scores of 4-5, and 25% with scores of 6 or more. Increasing score was predictive of increased relapse and treatment-related mortality in the HLA-matched set and of relapse in the HLA-mismatched cohort.
To develop the scoring system, the researchers used a model that weighed patient age; sex; and Karnofsky performance status; disease stage at transplantation; comprehensive cytogenetic risk status; bone marrow and peripheral blood blast percentages; hemoglobin, neutrophil, and platelet counts at diagnosis and pretransplantation; lactate dehydrogenase at transplantation; pretransplantation therapy (hypomethylating agents, chemotherapy, neither, or both); time from diagnosis to transplantation; year of transplantation; conditioning regimen and regimen intensity (myeloablative v reduced intensity); donor–recipient sex match or mismatch; graft-versus-host disease prophylaxis; graft type (bone marrow vs. peripheral blood); presence of secondary myelodysplastic syndrome; and unrelated donor vs. related donor.
There were no significant differences in overall survival at 1, 3, and 5 years or in the 3-year incidences of relapse and treatment-related mortality in the training subset and the validation cohort.
Data on somatic mutations have become relevant in myelodysplastic syndrome prognostication and were missing from this analysis, the researchers wrote. “The next generation of prognostic tools will need to account for this information.”
Dr. Shaffer had no relevant financial disclosures.
On Twitter @maryjodales
A new risk-stratification tool goes one better than the standard tools used to predict survival in those undergoing allogeneic hematopoietic cell transplantation (allo HCT) for myelodysplastic syndrome, based on a study published online April 4 in the Journal of Clinical Oncology.
The concordance index for the new risk-stratification tool was modestly better at 0.575, compared with 0.538 for the standard International Prognostic Scoring System (IPSS) and 0.554 for the revised IPSS (IPSS-R), according to Dr. Brian C. Shaffer of Memorial Sloan Kettering Cancer Center, New York, and his colleagues who participate in the Center for International Blood and Marrow Transplant Research (CIBMTR) network.
“The proposed system generally agrees with the IPSS-R in the very high–risk subcategory; however, a significant portion of patients in high- and very high–risk IPSS-R groups were represented in the low- and intermediate-risk proposed scoring subcategories. The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system,” the researchers wrote.
Further, the “scoring system uses readily available clinical data and can be calculated quickly, facilitating patient consultation with respect to allo HCT, and may also be used to identify high-risk populations where interventions such as post–allo HCT maintenance therapies may be of benefit,” they wrote (J Clin Oncol. 2016 April 4. doi: 10.1200/JCO.2015.65.0515).
The data were obtained from the CIBMTR, a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
The researchers applied the prognostic tool to 2,133 patients with MDS undergoing HLA-matched (n = 1,728) or -mismatched (n = 405) allo HCT. Factors prognostic of mortality were identified in a training subset (n = 1,151) of the HLA-matched cohort. A weighted score using these factors was then assigned to the validation cohort of 577 remaining patients undergoing HLA-matched allo HCT as well as to patients undergoing HLA-mismatched allo HCT. The training data set was used to develop a prognostic scoring system, and the validation data set was used to assess the prognostic ability of the scoring system, the researchers noted.
In the scoring system, 1 point was assigned for the following factors: Blood blasts greater than 3%, platelet levels of 50 × 109/L or less at transplantation, Karnofsky performance status less than 90%, comprehensive cytogenetic risk score of poor or very poor, and age 30-49 years. Two points were assigned for monosomal karyotype and age 50 years or older.
Based on the scoring system, 3-year overall survival after transplantation was 71% in patients with scores of 1 point, 49% with scores of 2-3, 41% with scores of 4-5, and 25% with scores of 6 or more. Increasing score was predictive of increased relapse and treatment-related mortality in the HLA-matched set and of relapse in the HLA-mismatched cohort.
To develop the scoring system, the researchers used a model that weighed patient age; sex; and Karnofsky performance status; disease stage at transplantation; comprehensive cytogenetic risk status; bone marrow and peripheral blood blast percentages; hemoglobin, neutrophil, and platelet counts at diagnosis and pretransplantation; lactate dehydrogenase at transplantation; pretransplantation therapy (hypomethylating agents, chemotherapy, neither, or both); time from diagnosis to transplantation; year of transplantation; conditioning regimen and regimen intensity (myeloablative v reduced intensity); donor–recipient sex match or mismatch; graft-versus-host disease prophylaxis; graft type (bone marrow vs. peripheral blood); presence of secondary myelodysplastic syndrome; and unrelated donor vs. related donor.
There were no significant differences in overall survival at 1, 3, and 5 years or in the 3-year incidences of relapse and treatment-related mortality in the training subset and the validation cohort.
Data on somatic mutations have become relevant in myelodysplastic syndrome prognostication and were missing from this analysis, the researchers wrote. “The next generation of prognostic tools will need to account for this information.”
Dr. Shaffer had no relevant financial disclosures.
On Twitter @maryjodales
A new risk-stratification tool goes one better than the standard tools used to predict survival in those undergoing allogeneic hematopoietic cell transplantation (allo HCT) for myelodysplastic syndrome, based on a study published online April 4 in the Journal of Clinical Oncology.
The concordance index for the new risk-stratification tool was modestly better at 0.575, compared with 0.538 for the standard International Prognostic Scoring System (IPSS) and 0.554 for the revised IPSS (IPSS-R), according to Dr. Brian C. Shaffer of Memorial Sloan Kettering Cancer Center, New York, and his colleagues who participate in the Center for International Blood and Marrow Transplant Research (CIBMTR) network.
“The proposed system generally agrees with the IPSS-R in the very high–risk subcategory; however, a significant portion of patients in high- and very high–risk IPSS-R groups were represented in the low- and intermediate-risk proposed scoring subcategories. The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system,” the researchers wrote.
Further, the “scoring system uses readily available clinical data and can be calculated quickly, facilitating patient consultation with respect to allo HCT, and may also be used to identify high-risk populations where interventions such as post–allo HCT maintenance therapies may be of benefit,” they wrote (J Clin Oncol. 2016 April 4. doi: 10.1200/JCO.2015.65.0515).
The data were obtained from the CIBMTR, a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
The researchers applied the prognostic tool to 2,133 patients with MDS undergoing HLA-matched (n = 1,728) or -mismatched (n = 405) allo HCT. Factors prognostic of mortality were identified in a training subset (n = 1,151) of the HLA-matched cohort. A weighted score using these factors was then assigned to the validation cohort of 577 remaining patients undergoing HLA-matched allo HCT as well as to patients undergoing HLA-mismatched allo HCT. The training data set was used to develop a prognostic scoring system, and the validation data set was used to assess the prognostic ability of the scoring system, the researchers noted.
In the scoring system, 1 point was assigned for the following factors: Blood blasts greater than 3%, platelet levels of 50 × 109/L or less at transplantation, Karnofsky performance status less than 90%, comprehensive cytogenetic risk score of poor or very poor, and age 30-49 years. Two points were assigned for monosomal karyotype and age 50 years or older.
Based on the scoring system, 3-year overall survival after transplantation was 71% in patients with scores of 1 point, 49% with scores of 2-3, 41% with scores of 4-5, and 25% with scores of 6 or more. Increasing score was predictive of increased relapse and treatment-related mortality in the HLA-matched set and of relapse in the HLA-mismatched cohort.
To develop the scoring system, the researchers used a model that weighed patient age; sex; and Karnofsky performance status; disease stage at transplantation; comprehensive cytogenetic risk status; bone marrow and peripheral blood blast percentages; hemoglobin, neutrophil, and platelet counts at diagnosis and pretransplantation; lactate dehydrogenase at transplantation; pretransplantation therapy (hypomethylating agents, chemotherapy, neither, or both); time from diagnosis to transplantation; year of transplantation; conditioning regimen and regimen intensity (myeloablative v reduced intensity); donor–recipient sex match or mismatch; graft-versus-host disease prophylaxis; graft type (bone marrow vs. peripheral blood); presence of secondary myelodysplastic syndrome; and unrelated donor vs. related donor.
There were no significant differences in overall survival at 1, 3, and 5 years or in the 3-year incidences of relapse and treatment-related mortality in the training subset and the validation cohort.
Data on somatic mutations have become relevant in myelodysplastic syndrome prognostication and were missing from this analysis, the researchers wrote. “The next generation of prognostic tools will need to account for this information.”
Dr. Shaffer had no relevant financial disclosures.
On Twitter @maryjodales
FROM JCO
Key clinical point: A portion of patients with myelodysplastic syndrome in high- and very high–risk groups of the revised International Prognostic Scoring System (IPSS-R) were represented in the low- and intermediate-risk groups of the proposed scoring subcategories.
Major finding: The 3-year survival in patients classified as high risk with the IPSS-R was 75%; it was 57% in those classified as low or intermediate risk with the proposed system.
Data source: The Center for International Blood and Marrow Transplant Research (CIBMTR), a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute data on consecutive allo and autologous HCTs to a centralized statistical center.
Disclosures: Dr. Shaffer had no relevant financial disclosures.
Cyclophosphamide nets low rate of chronic GVHD after mobilized blood cell transplantation
High-dose cyclophosphamide is safe and effective when given as prophylaxis for chronic graft-versus-host disease (GVHD) to patients who have undergone transplantation of mobilized blood cells, finds a phase 2 trial reported in Blood.
Investigators led by Dr. Marco Mielcarek, medical director of the Adult Blood and Marrow Transplant Program and an oncologist at the Fred Hutchinson Cancer Research Center in Seattle, Washington, enrolled in the trial 43 patients with high-risk hematologic malignancies.
The patients underwent myeloablative conditioning followed by transplantation with growth factor–mobilized blood cells from related or unrelated donors, and were given high-dose cyclophosphamide on two early posttransplantation days.
Main results showed that the cumulative 1-year incidence of chronic GVHD was 16%, less than half of the roughly 35% seen historically with conventional immunosuppression.
Moreover, cyclophosphamide did not appear to compromise engraftment or control of the underlying malignancy. Only a single patient, one with an HLA-mismatched donor, had failure of primary engraftment; after amendment of the protocol to require HLA matching, there were no additional cases. Just 17% of patients experienced a recurrence of their malignancy by 2 years.
Taken together, the findings suggest that high-dose cyclophosphamide—as combined with two myeloablative conditioning options (to accommodate different malignancies) and with posttransplantation cyclosporine (to reduce the risk of acute GVHD)—may eliminate most of the drawbacks to using mobilized blood cells for transplantation, according to the investigators.
“If these findings are confirmed in future studies, HLA-matched mobilized blood cell transplantation may gain even greater acceptance and further replace marrow as a source of stem cells for most indications,” they maintain.
The patients studied had a median age of 43 years, and slightly more than half were in remission without minimal residual disease.
Blood cells were mobilized with granulocyte colony-stimulating factor (G-CSF). Overall, 28% of patients received grafts from related donors, while 72% received grafts from unrelated donors.
For pretransplant conditioning, patients received fludarabine and targeted busulfan, or total body irradiation with use of a minimum dose of 12 Gy.
The patients were given cyclophosphamide at 50 mg/kg per day on days 3 and 4 after transplantation. This was followed by cyclosporine starting on day 5.
The cumulative 1-year incidence of chronic GVHD as defined by National Institutes of Health criteria (i.e., that requiring systemic immunosuppressive therapy)—the trial’s primary endpoint—was 16%, which fell just short of the goal of 15% the investigators were aiming for (Blood. 2016;127:1502-8). Analyses failed to identify any predictors of this outcome.
Although the estimated cumulative incidence of grade 2 acute GVHD was high, at 77%, none of the patients developed grade 3 or 4 acute GVHD, according to the investigators, who disclosed that they had no competing financial interests.
The single patient who experienced failure of primary engraftment had familial myelodysplastic syndrome and had received a graft from an HLA A-antigen–mismatched unrelated donor.
The 2-year cumulative incidence of nonrelapse mortality was 14%, and the 2-year cumulative incidence of recurrent malignancy was 17%. Projected overall survival was 70%.
Among the 42 patients having at least a year of follow-up, 50% were alive and free of relapse without any systemic immunosuppression at 1 year after transplantation.
High-dose cyclophosphamide is safe and effective when given as prophylaxis for chronic graft-versus-host disease (GVHD) to patients who have undergone transplantation of mobilized blood cells, finds a phase 2 trial reported in Blood.
Investigators led by Dr. Marco Mielcarek, medical director of the Adult Blood and Marrow Transplant Program and an oncologist at the Fred Hutchinson Cancer Research Center in Seattle, Washington, enrolled in the trial 43 patients with high-risk hematologic malignancies.
The patients underwent myeloablative conditioning followed by transplantation with growth factor–mobilized blood cells from related or unrelated donors, and were given high-dose cyclophosphamide on two early posttransplantation days.
Main results showed that the cumulative 1-year incidence of chronic GVHD was 16%, less than half of the roughly 35% seen historically with conventional immunosuppression.
Moreover, cyclophosphamide did not appear to compromise engraftment or control of the underlying malignancy. Only a single patient, one with an HLA-mismatched donor, had failure of primary engraftment; after amendment of the protocol to require HLA matching, there were no additional cases. Just 17% of patients experienced a recurrence of their malignancy by 2 years.
Taken together, the findings suggest that high-dose cyclophosphamide—as combined with two myeloablative conditioning options (to accommodate different malignancies) and with posttransplantation cyclosporine (to reduce the risk of acute GVHD)—may eliminate most of the drawbacks to using mobilized blood cells for transplantation, according to the investigators.
“If these findings are confirmed in future studies, HLA-matched mobilized blood cell transplantation may gain even greater acceptance and further replace marrow as a source of stem cells for most indications,” they maintain.
The patients studied had a median age of 43 years, and slightly more than half were in remission without minimal residual disease.
Blood cells were mobilized with granulocyte colony-stimulating factor (G-CSF). Overall, 28% of patients received grafts from related donors, while 72% received grafts from unrelated donors.
For pretransplant conditioning, patients received fludarabine and targeted busulfan, or total body irradiation with use of a minimum dose of 12 Gy.
The patients were given cyclophosphamide at 50 mg/kg per day on days 3 and 4 after transplantation. This was followed by cyclosporine starting on day 5.
The cumulative 1-year incidence of chronic GVHD as defined by National Institutes of Health criteria (i.e., that requiring systemic immunosuppressive therapy)—the trial’s primary endpoint—was 16%, which fell just short of the goal of 15% the investigators were aiming for (Blood. 2016;127:1502-8). Analyses failed to identify any predictors of this outcome.
Although the estimated cumulative incidence of grade 2 acute GVHD was high, at 77%, none of the patients developed grade 3 or 4 acute GVHD, according to the investigators, who disclosed that they had no competing financial interests.
The single patient who experienced failure of primary engraftment had familial myelodysplastic syndrome and had received a graft from an HLA A-antigen–mismatched unrelated donor.
The 2-year cumulative incidence of nonrelapse mortality was 14%, and the 2-year cumulative incidence of recurrent malignancy was 17%. Projected overall survival was 70%.
Among the 42 patients having at least a year of follow-up, 50% were alive and free of relapse without any systemic immunosuppression at 1 year after transplantation.
High-dose cyclophosphamide is safe and effective when given as prophylaxis for chronic graft-versus-host disease (GVHD) to patients who have undergone transplantation of mobilized blood cells, finds a phase 2 trial reported in Blood.
Investigators led by Dr. Marco Mielcarek, medical director of the Adult Blood and Marrow Transplant Program and an oncologist at the Fred Hutchinson Cancer Research Center in Seattle, Washington, enrolled in the trial 43 patients with high-risk hematologic malignancies.
The patients underwent myeloablative conditioning followed by transplantation with growth factor–mobilized blood cells from related or unrelated donors, and were given high-dose cyclophosphamide on two early posttransplantation days.
Main results showed that the cumulative 1-year incidence of chronic GVHD was 16%, less than half of the roughly 35% seen historically with conventional immunosuppression.
Moreover, cyclophosphamide did not appear to compromise engraftment or control of the underlying malignancy. Only a single patient, one with an HLA-mismatched donor, had failure of primary engraftment; after amendment of the protocol to require HLA matching, there were no additional cases. Just 17% of patients experienced a recurrence of their malignancy by 2 years.
Taken together, the findings suggest that high-dose cyclophosphamide—as combined with two myeloablative conditioning options (to accommodate different malignancies) and with posttransplantation cyclosporine (to reduce the risk of acute GVHD)—may eliminate most of the drawbacks to using mobilized blood cells for transplantation, according to the investigators.
“If these findings are confirmed in future studies, HLA-matched mobilized blood cell transplantation may gain even greater acceptance and further replace marrow as a source of stem cells for most indications,” they maintain.
The patients studied had a median age of 43 years, and slightly more than half were in remission without minimal residual disease.
Blood cells were mobilized with granulocyte colony-stimulating factor (G-CSF). Overall, 28% of patients received grafts from related donors, while 72% received grafts from unrelated donors.
For pretransplant conditioning, patients received fludarabine and targeted busulfan, or total body irradiation with use of a minimum dose of 12 Gy.
The patients were given cyclophosphamide at 50 mg/kg per day on days 3 and 4 after transplantation. This was followed by cyclosporine starting on day 5.
The cumulative 1-year incidence of chronic GVHD as defined by National Institutes of Health criteria (i.e., that requiring systemic immunosuppressive therapy)—the trial’s primary endpoint—was 16%, which fell just short of the goal of 15% the investigators were aiming for (Blood. 2016;127:1502-8). Analyses failed to identify any predictors of this outcome.
Although the estimated cumulative incidence of grade 2 acute GVHD was high, at 77%, none of the patients developed grade 3 or 4 acute GVHD, according to the investigators, who disclosed that they had no competing financial interests.
The single patient who experienced failure of primary engraftment had familial myelodysplastic syndrome and had received a graft from an HLA A-antigen–mismatched unrelated donor.
The 2-year cumulative incidence of nonrelapse mortality was 14%, and the 2-year cumulative incidence of recurrent malignancy was 17%. Projected overall survival was 70%.
Among the 42 patients having at least a year of follow-up, 50% were alive and free of relapse without any systemic immunosuppression at 1 year after transplantation.
FROM BLOOD
Key clinical point: High-dose posttransplant cyclophosphamide is safe and effective for reducing the risk of chronic GVHD after mobilized blood cell transplantation.
Major finding: The cumulative 1-year incidence of chronic GVHD requiring immunosuppressive therapy was 16%.
Data source: A single-arm trial among 43 patients with high-risk hematologic malignancies undergoing growth factor–mobilized blood cell transplantation.
Disclosures: The authors disclosed that they have no competing financial interests.
Drug for conditioning AML patients for transplant gets orphan drug designation
A radioimmunotherapeutic drug for conditioning relapsed and refractory acute myeloid leukemia (AML) patients for a hematopoietic stem cell transplant has been granted orphan drug designation by the Food and Drug Administration.
Iomab-B is a radioimmunoconjugate consisting of the murine monoclonal antibody BC8 and an iodine-131 radioisotope. The Fred Hutchinson Cancer Research Center developed BC8 to target CD45, a panleukocytic antigen widely expressed on white blood cells. “When labeled with radioactive isotopes, BC8 carries radioactivity directly to the site of cancerous growth and bone marrow while avoiding effects of radiation on most healthy tissues,” says a statement from Actinium Pharmaceuticals, which would market the drug.
Iomab-B will be tested in a multicenter trial that will include 150 patients over age 55 with refractory and relapsed AML. “There has not been a new drug approved for relapsed and refractory AML patients over the age of 55 in decades and with Iomab-B being the only therapy of its kind, we are pleased to have achieved this important milestone,” Sandesh Seth, executive chairman of Actinium, said in the statement.
A radioimmunotherapeutic drug for conditioning relapsed and refractory acute myeloid leukemia (AML) patients for a hematopoietic stem cell transplant has been granted orphan drug designation by the Food and Drug Administration.
Iomab-B is a radioimmunoconjugate consisting of the murine monoclonal antibody BC8 and an iodine-131 radioisotope. The Fred Hutchinson Cancer Research Center developed BC8 to target CD45, a panleukocytic antigen widely expressed on white blood cells. “When labeled with radioactive isotopes, BC8 carries radioactivity directly to the site of cancerous growth and bone marrow while avoiding effects of radiation on most healthy tissues,” says a statement from Actinium Pharmaceuticals, which would market the drug.
Iomab-B will be tested in a multicenter trial that will include 150 patients over age 55 with refractory and relapsed AML. “There has not been a new drug approved for relapsed and refractory AML patients over the age of 55 in decades and with Iomab-B being the only therapy of its kind, we are pleased to have achieved this important milestone,” Sandesh Seth, executive chairman of Actinium, said in the statement.
A radioimmunotherapeutic drug for conditioning relapsed and refractory acute myeloid leukemia (AML) patients for a hematopoietic stem cell transplant has been granted orphan drug designation by the Food and Drug Administration.
Iomab-B is a radioimmunoconjugate consisting of the murine monoclonal antibody BC8 and an iodine-131 radioisotope. The Fred Hutchinson Cancer Research Center developed BC8 to target CD45, a panleukocytic antigen widely expressed on white blood cells. “When labeled with radioactive isotopes, BC8 carries radioactivity directly to the site of cancerous growth and bone marrow while avoiding effects of radiation on most healthy tissues,” says a statement from Actinium Pharmaceuticals, which would market the drug.
Iomab-B will be tested in a multicenter trial that will include 150 patients over age 55 with refractory and relapsed AML. “There has not been a new drug approved for relapsed and refractory AML patients over the age of 55 in decades and with Iomab-B being the only therapy of its kind, we are pleased to have achieved this important milestone,” Sandesh Seth, executive chairman of Actinium, said in the statement.
New drug approved for hepatic veno-occlusive disease
Defibrotide sodium has been approved to treat hepatic veno-occlusive disease (VOD) in patients with renal or pulmonary dysfunction following a hematopoietic stem cell transplantation, the Food and Drug Administration has announced.
The drug, which will be marketed as Defitelio by Jazz Pharmaceuticals, was tested in two prospective clinical trials and an expanded access study that included a total of 528 patients with hepatic VOD and multiorgan dysfunction following a transplantation. All patients received 6.25 mg/kg doses of the drug intravenously, every 6 hours until resolution of VOD. The percentages of patients surviving more than 100 days after receiving a stem cell transplantation in each of the studies were 38%, 44%, and 45%, respectively, according to a statement from the FDA.
Contraindications for taking the drug are concurrent use of anticoagulants or fibrinolytic therapies.
Hypotension, diarrhea, vomiting, nausea, and epistaxis are the most common adverse reactions to the drug.
Full prescribing information is available at the FDA website.
Defibrotide sodium has been approved to treat hepatic veno-occlusive disease (VOD) in patients with renal or pulmonary dysfunction following a hematopoietic stem cell transplantation, the Food and Drug Administration has announced.
The drug, which will be marketed as Defitelio by Jazz Pharmaceuticals, was tested in two prospective clinical trials and an expanded access study that included a total of 528 patients with hepatic VOD and multiorgan dysfunction following a transplantation. All patients received 6.25 mg/kg doses of the drug intravenously, every 6 hours until resolution of VOD. The percentages of patients surviving more than 100 days after receiving a stem cell transplantation in each of the studies were 38%, 44%, and 45%, respectively, according to a statement from the FDA.
Contraindications for taking the drug are concurrent use of anticoagulants or fibrinolytic therapies.
Hypotension, diarrhea, vomiting, nausea, and epistaxis are the most common adverse reactions to the drug.
Full prescribing information is available at the FDA website.
Defibrotide sodium has been approved to treat hepatic veno-occlusive disease (VOD) in patients with renal or pulmonary dysfunction following a hematopoietic stem cell transplantation, the Food and Drug Administration has announced.
The drug, which will be marketed as Defitelio by Jazz Pharmaceuticals, was tested in two prospective clinical trials and an expanded access study that included a total of 528 patients with hepatic VOD and multiorgan dysfunction following a transplantation. All patients received 6.25 mg/kg doses of the drug intravenously, every 6 hours until resolution of VOD. The percentages of patients surviving more than 100 days after receiving a stem cell transplantation in each of the studies were 38%, 44%, and 45%, respectively, according to a statement from the FDA.
Contraindications for taking the drug are concurrent use of anticoagulants or fibrinolytic therapies.
Hypotension, diarrhea, vomiting, nausea, and epistaxis are the most common adverse reactions to the drug.
Full prescribing information is available at the FDA website.
Children who have stem cell transplants need skin exams, sun protection
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
WASHINGTON – Children who have had a hematopoietic stem cell transplant (HSCT) have an increased risk of benign and atypical nevi, Dr. Johanna Sheu reported at the annual meeting of the American Academy of Dermatology.
These patients need to have routine skin exams and be educated about sun protection needs, she said. Based on her study, these needs are not routinely met.
At least 1 year after undergoing HSCT at Boston Children’s Hospital, 85 posttransplant patients had significantly more nevi and more atypical nevi than did 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type. In addition, 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Moreover, “sun protection … and dermatology follow-up was poor” among the transplant recipients, said Dr. Sheu, of MassGeneral Hospital for Children, Boston. About 40% of the transplant recipients reported having a sunburn since their transplant, only 15% reported daily use of sunscreen, and 53% said they did not recall being told that sunburn could trigger graft-versus-host disease (GVHD).
About one-third of the patients had never seen a dermatologist; of those who had, two-thirds had only seen the dermatologist once, Dr. Sheu reported.
Late skin effects of HSCT are not as well described in children as they are in adults, she said. In adults, late skin effects include vitiligo, psoriasis, nonmelanoma skin cancers, and an increased nevi count.
The children in the study had undergone an HSCT between 1998 and 2013, at a median age of about 7 years (range was 1 month to 19 years). At the time of their skin exams, their mean age was 14 years, and they had been followed for a median of almost 4 years. Nevi were counted on the forearms, backs, legs, palms, and soles.
The median nevi count was 44 nevi, significantly more than the level seen in control subjects. Transplant recipients also had significantly more nevi in sun-exposed areas of the body, as well as on the palms and soles. Transplant recipients were more likely to have atypical nevi and to have nevi greater than 5 mm in diameter.
In addition to fair skin, factors associated with an increase in the overall nevi count included being older than age 10 at the time of the transplant and having total body irradiation, pretransplant chemotherapy, and myeloablative conditioning. Having had a sunburn since the transplant, reported by 40%, was also a risk factor.
Chronic GVHD and chronic GVHD of the skin were associated with the presence of atypical nevi; acute GVHD, the duration of immune suppression, and the use of topical steroids or calcineurin inhibitors were not associated with increased risk of atypical nevi.
She and her coinvestigators are currently analyzing the pathogenesis of these late effects in this population, and autoimmune skin conditions – vitiligo and alopecia – in 25% of the transplant recipients in the study.
In 2013, 1,100 children under aged 16 years in the United States underwent a bone marrow transplant, she noted.
Dr. Sheu had no disclosures.
AT AAD 16
Key clinical point: Children who have had a hematopoietic stem cell transplant need to have routine skin exams and be educated about sun protection needs.
Major finding: 41% of the transplant recipients had at least one actinic keratosis, a basal or squamous cell carcinoma, or a solar lentigo; 11% had at least one nevus spilus.
Data source: A single-center study of 85 posttransplant patients and 85 healthy controls who were matched by age, gender, and Fitzpatrick skin type.
Disclosures: The study was not sponsored and Dr. Sheu had no disclosures.
New drug comparable to voriconazole for aspergillosis
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
FROM THE LANCET
Key clinical point: Isavuconazole is an appropriate alternative for primary treatment of suspected invasive aspergillosis.
Major finding: All-cause mortality at 6 weeks in the intention-to-treat group of 516 patients was 19% with isavuconazole and 20% with voriconazole. Fewer drug-related adverse events were reported with isavuconazole, however (42% vs. 60% of patients).
Data source: A phase III randomized, double-blind noninferiority trial – the SECURE trial – comparing the safety and efficacy of intravenous and oral formulations of isavuconazole and voriconazole for the primary treatment of invasive aspergillosis and disease caused by other molds.
Disclosures: Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International. Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees, and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer, during the study.
Resources limit availability of bone marrow transplants
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Peripheral blood is a major stem cell source for hematopoietic stem cell transplantation in many parts of the world – even though bone marrow is preferred – likely due to costs, the need for patient hospitalization, and a lack of trained physicians and necessary equipment for performing bone marrow transplant, Dr. Ayami Yoshimi, and colleagues reported for the Worldwide Network of Blood and Marrow Transplantation.
Peripheral blood stem cells are readily available at transplant centers, as they are collected routinely for other indications, and they offer short-term cost savings due to rapid engraftment, the network reported in a research letter (JAMA. 2016;315[2]:198-200. doi:10.1001/jama.2015.13706).
In patients with nonmalignant disorders, use of peripheral blood stem cells in HSCT has a higher rate of graft-vs.-host disease and lower survival rates.
In the network’s retrospective survey, which they estimate covered 90% of transplants performed in 2009 and 2010, 114,217 HSCTs were reported by 1,482 transplant teams and 3,282 allogeneic HSCTs were performed for bone marrow failure. Use of peripheral blood stem cells in HSCT varied from 80% in countries with low and middle incomes to 50% in those with high-middle incomes to 36% in those with high incomes (P less than .001). For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Use of unrelated donors was highest in Europe (515/1107; 47%); use of matched sibling donors was highest in the Eastern Mediterranean region and Africa (249/274; 91%).
Bone marrow was used most commonly in the Americas (631/843; 75%) and in Europe (632/1057; 60%), but not in the Eastern Mediterranean region and Africa (123/266; 46%) and in the Asia Pacific region (380/936; 41%; excluding Japan, 19%).
“National and international transplant organizations and authorities should foster regional accredited bone marrow harvest centers for patients with nonmalignant disorders and provide resources to establish such infrastructures. Unrelated donor registries should provide information on the necessity of bone marrow donation for patients with bone marrow failure,” wrote Dr. Yoshimi of the University of Freiburg, Germany, and colleagues.
Funding for the study was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
FROM JAMA
Key clinical point: In regions with limited resources, peripheral blood stem cells were used more frequently than bone marrow in hematopoietic stem cell transplantation for bone marrow failure.
Major finding: For the 3,282 allogeneic HSCTs performed for bone marrow failure worldwide, stem cell sources included bone marrow (1,766, 54%), peripheral blood stem cells (1,336, 41%), and cord blood (180, 5%).
Data source: Data on 3,282 allogeneic HSCTs for bone marrow failure performed in 2009 and 2010 were collected by retrospective surveys by the Worldwide Network for Blood and Marrow Transplantation, and by direct contact with transplant centers in countries without registries.
Disclosures: Funding was indirectly provided by the Worldwide Network of Blood and Marrow Transplantation. Dr. Yoshimi reported having no disclosures.
Medicare to cover HSCT in approved clinical trials for myeloma, myelofibrosis, sickle cell disease
Medicare will cover allogeneic hematopoietic stem cell transplantation (HSCT) for beneficiaries with multiple myeloma, myelofibrosis, or sickle cell disease in the context of approved, prospective clinical trials, the Centers for Medicare & Medicaid Services announced in a final decision memo Jan. 27.
Approvable studies must examine whether Medicare beneficiaries who receive allogeneic HSCT have improved outcomes, compared with patients who do not receive allogeneic HSCT as measured by graft vs. host disease, other transplant-related adverse events, overall survival, and, optionally, quality of life measures.
In multiple myeloma, allogeneic HSCT will be covered only for Medicare beneficiaries who have Durie-Salmon Stage II or III multiple myeloma, or International Staging System (ISS) Stage II or Stage III multiple myeloma, and are participating in an approved prospective clinical study. Such studies must control for selection bias and potential confounding by age, duration of diagnosis, disease classification, International Myeloma Working Group (IMWG) classification, ISS staging, Durie-Salmon staging, comorbid conditions, type of preparative/conditioning regimen, graft vs. host disease (GVHD) prophylaxis, donor type, and cell source, the CMS said in its memo.
In myelofibrosis, allogeneic HSCT will be covered by Medicare in an approved prospective study only for beneficiaries with Dynamic International Prognostic Scoring System (DIPSS plus) intermediate-2 or high primary or secondary myelofibrosis. Studies must be controlled for selection bias and potential confounding by age, duration of diagnosis, disease classification, DIPSS plus score, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
In sickle cell disease, allogeneic HSCT will be covered by Medicare only for beneficiaries who have severe, symptomatic disease. Approvable studies must control for selection bias and potential confounding by age, duration of diagnosis, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
On Twitter @maryjodales
Dr. Navneet Majhail comments: I welcome the decision by CMS [the Centers for Medicare & Medicaid Services] to cover allogeneic hematopoietic stem cell transplantation (HSCT) for multiple myeloma, myelofibrosis, and sickle cell disease under the coverage with evidence development (CED) mechanism. This action will allow us to provide transplant as a treatment option for older patients with myeloma and myelofibrosis and for Medicare beneficiaries with sickle cell disease: Lack of coverage is a real challenge at present for this population and prevents us from offering a potentially curative treatment option to these high-risk patients.

|
Dr. Navneet Majhail |
The decision is the result of collective advocacy efforts of our transplant community, patients, and patient advocacy organizations, Be the Match, and the American Society for Blood and Marrow Transplantation.
The CED asks for a prospective clinical trial that mandates the presence of a control arm of comparable patients who do not receive allogeneic transplantation. I completely support provision of transplantation on a clinical trial for CED purposes; however, I believe it would have been better to allow hematology and transplant experts to determine the appropriate study design in consultation with CMS to fulfill the CED requirements. For example, on the basis of available evidence, it will be challenging to enroll patients with high-risk myelofibrosis to a nontransplant arm. Irrespective, this is a big win for patients with these life-threatening diseases and for physicians who treat them.
Dr. Navneet Majhail is the director of the Cleveland Clinic’s Blood & Marrow Transplant Program. He serves as a staff physician at the Taussig Cancer Institute and is a professor of medicine with the Cleveland Clinic Lerner College of Medicine.
Dr. Navneet Majhail comments: I welcome the decision by CMS [the Centers for Medicare & Medicaid Services] to cover allogeneic hematopoietic stem cell transplantation (HSCT) for multiple myeloma, myelofibrosis, and sickle cell disease under the coverage with evidence development (CED) mechanism. This action will allow us to provide transplant as a treatment option for older patients with myeloma and myelofibrosis and for Medicare beneficiaries with sickle cell disease: Lack of coverage is a real challenge at present for this population and prevents us from offering a potentially curative treatment option to these high-risk patients.

|
Dr. Navneet Majhail |
The decision is the result of collective advocacy efforts of our transplant community, patients, and patient advocacy organizations, Be the Match, and the American Society for Blood and Marrow Transplantation.
The CED asks for a prospective clinical trial that mandates the presence of a control arm of comparable patients who do not receive allogeneic transplantation. I completely support provision of transplantation on a clinical trial for CED purposes; however, I believe it would have been better to allow hematology and transplant experts to determine the appropriate study design in consultation with CMS to fulfill the CED requirements. For example, on the basis of available evidence, it will be challenging to enroll patients with high-risk myelofibrosis to a nontransplant arm. Irrespective, this is a big win for patients with these life-threatening diseases and for physicians who treat them.
Dr. Navneet Majhail is the director of the Cleveland Clinic’s Blood & Marrow Transplant Program. He serves as a staff physician at the Taussig Cancer Institute and is a professor of medicine with the Cleveland Clinic Lerner College of Medicine.
Dr. Navneet Majhail comments: I welcome the decision by CMS [the Centers for Medicare & Medicaid Services] to cover allogeneic hematopoietic stem cell transplantation (HSCT) for multiple myeloma, myelofibrosis, and sickle cell disease under the coverage with evidence development (CED) mechanism. This action will allow us to provide transplant as a treatment option for older patients with myeloma and myelofibrosis and for Medicare beneficiaries with sickle cell disease: Lack of coverage is a real challenge at present for this population and prevents us from offering a potentially curative treatment option to these high-risk patients.

|
Dr. Navneet Majhail |
The decision is the result of collective advocacy efforts of our transplant community, patients, and patient advocacy organizations, Be the Match, and the American Society for Blood and Marrow Transplantation.
The CED asks for a prospective clinical trial that mandates the presence of a control arm of comparable patients who do not receive allogeneic transplantation. I completely support provision of transplantation on a clinical trial for CED purposes; however, I believe it would have been better to allow hematology and transplant experts to determine the appropriate study design in consultation with CMS to fulfill the CED requirements. For example, on the basis of available evidence, it will be challenging to enroll patients with high-risk myelofibrosis to a nontransplant arm. Irrespective, this is a big win for patients with these life-threatening diseases and for physicians who treat them.
Dr. Navneet Majhail is the director of the Cleveland Clinic’s Blood & Marrow Transplant Program. He serves as a staff physician at the Taussig Cancer Institute and is a professor of medicine with the Cleveland Clinic Lerner College of Medicine.
Medicare will cover allogeneic hematopoietic stem cell transplantation (HSCT) for beneficiaries with multiple myeloma, myelofibrosis, or sickle cell disease in the context of approved, prospective clinical trials, the Centers for Medicare & Medicaid Services announced in a final decision memo Jan. 27.
Approvable studies must examine whether Medicare beneficiaries who receive allogeneic HSCT have improved outcomes, compared with patients who do not receive allogeneic HSCT as measured by graft vs. host disease, other transplant-related adverse events, overall survival, and, optionally, quality of life measures.
In multiple myeloma, allogeneic HSCT will be covered only for Medicare beneficiaries who have Durie-Salmon Stage II or III multiple myeloma, or International Staging System (ISS) Stage II or Stage III multiple myeloma, and are participating in an approved prospective clinical study. Such studies must control for selection bias and potential confounding by age, duration of diagnosis, disease classification, International Myeloma Working Group (IMWG) classification, ISS staging, Durie-Salmon staging, comorbid conditions, type of preparative/conditioning regimen, graft vs. host disease (GVHD) prophylaxis, donor type, and cell source, the CMS said in its memo.
In myelofibrosis, allogeneic HSCT will be covered by Medicare in an approved prospective study only for beneficiaries with Dynamic International Prognostic Scoring System (DIPSS plus) intermediate-2 or high primary or secondary myelofibrosis. Studies must be controlled for selection bias and potential confounding by age, duration of diagnosis, disease classification, DIPSS plus score, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
In sickle cell disease, allogeneic HSCT will be covered by Medicare only for beneficiaries who have severe, symptomatic disease. Approvable studies must control for selection bias and potential confounding by age, duration of diagnosis, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
On Twitter @maryjodales
Medicare will cover allogeneic hematopoietic stem cell transplantation (HSCT) for beneficiaries with multiple myeloma, myelofibrosis, or sickle cell disease in the context of approved, prospective clinical trials, the Centers for Medicare & Medicaid Services announced in a final decision memo Jan. 27.
Approvable studies must examine whether Medicare beneficiaries who receive allogeneic HSCT have improved outcomes, compared with patients who do not receive allogeneic HSCT as measured by graft vs. host disease, other transplant-related adverse events, overall survival, and, optionally, quality of life measures.
In multiple myeloma, allogeneic HSCT will be covered only for Medicare beneficiaries who have Durie-Salmon Stage II or III multiple myeloma, or International Staging System (ISS) Stage II or Stage III multiple myeloma, and are participating in an approved prospective clinical study. Such studies must control for selection bias and potential confounding by age, duration of diagnosis, disease classification, International Myeloma Working Group (IMWG) classification, ISS staging, Durie-Salmon staging, comorbid conditions, type of preparative/conditioning regimen, graft vs. host disease (GVHD) prophylaxis, donor type, and cell source, the CMS said in its memo.
In myelofibrosis, allogeneic HSCT will be covered by Medicare in an approved prospective study only for beneficiaries with Dynamic International Prognostic Scoring System (DIPSS plus) intermediate-2 or high primary or secondary myelofibrosis. Studies must be controlled for selection bias and potential confounding by age, duration of diagnosis, disease classification, DIPSS plus score, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
In sickle cell disease, allogeneic HSCT will be covered by Medicare only for beneficiaries who have severe, symptomatic disease. Approvable studies must control for selection bias and potential confounding by age, duration of diagnosis, comorbid conditions, type of preparative/conditioning regimen, GVHD prophylaxis, donor type, and cell source.
On Twitter @maryjodales
CAR T-cells induce remissions, avoid GVHD in relapsed B-cell malignancies
In patients with B-cell malignancies who did not obtain remissions after allogeneic hematopoietic stem-cell transplantation (alloHSCT), infusion of anti-CD19 chimeric antigen receptor (CAR19) T-cells induced remissions without causing graft-versus-host disease (GVHD).
Of 20 patients treated, 6 obtained complete remission and 2 obtained partial remissions, while none of the patients experienced new-onset acute GVHD.
“The increased antimalignancy potency of CAR19 T-cells allowed small doses of T-cells to eradicate malignancy without causing GVHD; therefore, this work demonstrates a solution to the central problem of alloHSCT, the separation of [graft-versus-malignancy] from GVHD,” wrote Dr. Jennifer Brudno of the National Cancer Institute, Bethesda, Md., and colleagues (J Clin Onc. 2016 Jan. 25. doi: 10.1200/JCO.2015.64.5929).
In general, CAR19 T-cells did not persist longer than 4 weeks, the median time it takes for GVHD to develop after donor lymphocyte infusion, which may be a factor in avoiding GVHD. Smaller doses may be another contributing factor: CAR19 T-cell doses administered in the trial ranged from 106/kg to 107/kg, which is 10-fold smaller than typical donor lymphocyte infusions.
In contrast to previous CAR T-cell studies, patients did not receive chemotherapy before CAR19 T-cell infusion, so endogenous T-cells and natural killer cells were not depleted. Evidence indicates that lymphocyte depletion enhances antitumor activity of adoptively transferred T-cells, but the current results indicate that prior lymphocyte depletion is not an absolute requirement.
The CAR19 T-cell infusion was especially effective in acute lymphoblastic leukemia (ALL), with four of the five ALL patients obtaining complete remission. Patients with chronic lymphocyte leukemia and lymphoma also obtained remissions.
Toxicities observed were consistent with previous CAR T-cell studies and included fever, tachycardia, and hypotension, indicative of cytokine release syndrome.
Peak levels of CAR19 T-cells were significantly higher in patients with complete or partial responses, and patients who did not obtain a complete or partial response were more likely to have undetectable or very low levels. Increasing peak blood levels of CAR19 T-cells in vivo is an important goal for future research, according to investigators. Since endogenous CD19+ cells may promote proliferation of CAR19 T- cells, CD19+ cellular vaccines might enhance CAR19 T-cell proliferation in patients with low levels, they suggested.
Administration of programmed cell death protein-1 (PD-1) antagonists may also offer improvements. High levels of PD-1 expression on CAR19 T-cells were observed at the time of peak blood CAR19 T-cell levels.
Dr. Brudno reported having no disclosures. Dr. Goy and Dr. Rosenberg reported ties to industry, and Dr. Kochenderfer reported patents pending on CAR T-cell therapy.
In patients with B-cell malignancies who did not obtain remissions after allogeneic hematopoietic stem-cell transplantation (alloHSCT), infusion of anti-CD19 chimeric antigen receptor (CAR19) T-cells induced remissions without causing graft-versus-host disease (GVHD).
Of 20 patients treated, 6 obtained complete remission and 2 obtained partial remissions, while none of the patients experienced new-onset acute GVHD.
“The increased antimalignancy potency of CAR19 T-cells allowed small doses of T-cells to eradicate malignancy without causing GVHD; therefore, this work demonstrates a solution to the central problem of alloHSCT, the separation of [graft-versus-malignancy] from GVHD,” wrote Dr. Jennifer Brudno of the National Cancer Institute, Bethesda, Md., and colleagues (J Clin Onc. 2016 Jan. 25. doi: 10.1200/JCO.2015.64.5929).
In general, CAR19 T-cells did not persist longer than 4 weeks, the median time it takes for GVHD to develop after donor lymphocyte infusion, which may be a factor in avoiding GVHD. Smaller doses may be another contributing factor: CAR19 T-cell doses administered in the trial ranged from 106/kg to 107/kg, which is 10-fold smaller than typical donor lymphocyte infusions.
In contrast to previous CAR T-cell studies, patients did not receive chemotherapy before CAR19 T-cell infusion, so endogenous T-cells and natural killer cells were not depleted. Evidence indicates that lymphocyte depletion enhances antitumor activity of adoptively transferred T-cells, but the current results indicate that prior lymphocyte depletion is not an absolute requirement.
The CAR19 T-cell infusion was especially effective in acute lymphoblastic leukemia (ALL), with four of the five ALL patients obtaining complete remission. Patients with chronic lymphocyte leukemia and lymphoma also obtained remissions.
Toxicities observed were consistent with previous CAR T-cell studies and included fever, tachycardia, and hypotension, indicative of cytokine release syndrome.
Peak levels of CAR19 T-cells were significantly higher in patients with complete or partial responses, and patients who did not obtain a complete or partial response were more likely to have undetectable or very low levels. Increasing peak blood levels of CAR19 T-cells in vivo is an important goal for future research, according to investigators. Since endogenous CD19+ cells may promote proliferation of CAR19 T- cells, CD19+ cellular vaccines might enhance CAR19 T-cell proliferation in patients with low levels, they suggested.
Administration of programmed cell death protein-1 (PD-1) antagonists may also offer improvements. High levels of PD-1 expression on CAR19 T-cells were observed at the time of peak blood CAR19 T-cell levels.
Dr. Brudno reported having no disclosures. Dr. Goy and Dr. Rosenberg reported ties to industry, and Dr. Kochenderfer reported patents pending on CAR T-cell therapy.
In patients with B-cell malignancies who did not obtain remissions after allogeneic hematopoietic stem-cell transplantation (alloHSCT), infusion of anti-CD19 chimeric antigen receptor (CAR19) T-cells induced remissions without causing graft-versus-host disease (GVHD).
Of 20 patients treated, 6 obtained complete remission and 2 obtained partial remissions, while none of the patients experienced new-onset acute GVHD.
“The increased antimalignancy potency of CAR19 T-cells allowed small doses of T-cells to eradicate malignancy without causing GVHD; therefore, this work demonstrates a solution to the central problem of alloHSCT, the separation of [graft-versus-malignancy] from GVHD,” wrote Dr. Jennifer Brudno of the National Cancer Institute, Bethesda, Md., and colleagues (J Clin Onc. 2016 Jan. 25. doi: 10.1200/JCO.2015.64.5929).
In general, CAR19 T-cells did not persist longer than 4 weeks, the median time it takes for GVHD to develop after donor lymphocyte infusion, which may be a factor in avoiding GVHD. Smaller doses may be another contributing factor: CAR19 T-cell doses administered in the trial ranged from 106/kg to 107/kg, which is 10-fold smaller than typical donor lymphocyte infusions.
In contrast to previous CAR T-cell studies, patients did not receive chemotherapy before CAR19 T-cell infusion, so endogenous T-cells and natural killer cells were not depleted. Evidence indicates that lymphocyte depletion enhances antitumor activity of adoptively transferred T-cells, but the current results indicate that prior lymphocyte depletion is not an absolute requirement.
The CAR19 T-cell infusion was especially effective in acute lymphoblastic leukemia (ALL), with four of the five ALL patients obtaining complete remission. Patients with chronic lymphocyte leukemia and lymphoma also obtained remissions.
Toxicities observed were consistent with previous CAR T-cell studies and included fever, tachycardia, and hypotension, indicative of cytokine release syndrome.
Peak levels of CAR19 T-cells were significantly higher in patients with complete or partial responses, and patients who did not obtain a complete or partial response were more likely to have undetectable or very low levels. Increasing peak blood levels of CAR19 T-cells in vivo is an important goal for future research, according to investigators. Since endogenous CD19+ cells may promote proliferation of CAR19 T- cells, CD19+ cellular vaccines might enhance CAR19 T-cell proliferation in patients with low levels, they suggested.
Administration of programmed cell death protein-1 (PD-1) antagonists may also offer improvements. High levels of PD-1 expression on CAR19 T-cells were observed at the time of peak blood CAR19 T-cell levels.
Dr. Brudno reported having no disclosures. Dr. Goy and Dr. Rosenberg reported ties to industry, and Dr. Kochenderfer reported patents pending on CAR T-cell therapy.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: In patients who relapsed after allogeneic hematopoietic stem-cell transplantation, anti-CD19 chimeric antigen receptor (CAR) T-cell therapy induced remissions without causing graft-versus-host disease (GVHD).
Major finding: Of 20 patients treated, 6 obtained complete remission and 2 obtained partial remissions; none experienced new-onset acute GVHD.
Data source: Patients with B-cell malignancies who had progressed after allogeneic hematopoietic stem-cell transplantation.
Disclosures: Dr. Brudno reported having no disclosures. Dr. Goy and Dr. Rosenberg reported ties to industry, and Dr. Kochenderfer reported patents pending on CAR T-cell therapy.