User login
Children’s cancer survival steadily increasing
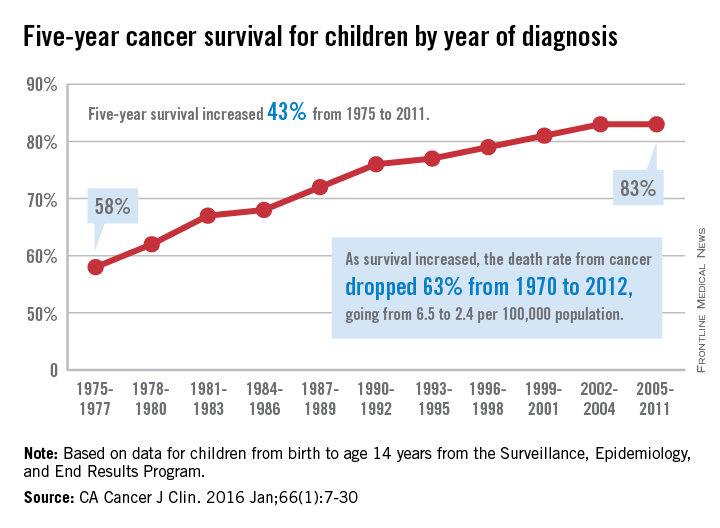
The 5-year cancer survival rate for children younger than 15 years old is up by 43% since 1975, according to investigators from the American Cancer Society.
The 5-year survival rate for all cancers showed a statistically significant rise from 58% in 1975 to 83% in 2011, said Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).
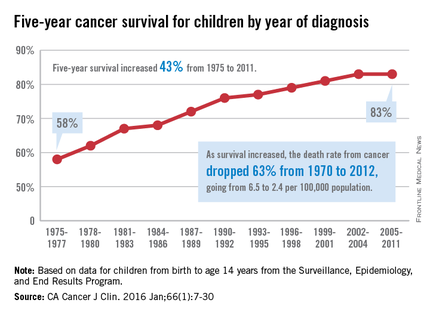
“The substantial progress for all of the major childhood cancers reflects both improvements in treatment and high levels of participation in clinical trials,” they wrote.
Survival for cancers of the brain and nervous system – now the leading cause of cancer death for those younger than 20 years old – increased from 57% in 1975 to 74% in 2011. The next-most-common cause of cancer death in children and adolescents is leukemia, and 5-year survival for acute myeloid leukemia went from 19% in 1975 to 67% in 2011, while 5-year survival for acute lymphocytic leukemia rose from 57% to 91% over that time period, the investigators reported.
The authors reported no conflicts of interest.
The 5-year cancer survival rate for children younger than 15 years old is up by 43% since 1975, according to investigators from the American Cancer Society.
The 5-year survival rate for all cancers showed a statistically significant rise from 58% in 1975 to 83% in 2011, said Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

“The substantial progress for all of the major childhood cancers reflects both improvements in treatment and high levels of participation in clinical trials,” they wrote.
Survival for cancers of the brain and nervous system – now the leading cause of cancer death for those younger than 20 years old – increased from 57% in 1975 to 74% in 2011. The next-most-common cause of cancer death in children and adolescents is leukemia, and 5-year survival for acute myeloid leukemia went from 19% in 1975 to 67% in 2011, while 5-year survival for acute lymphocytic leukemia rose from 57% to 91% over that time period, the investigators reported.
The authors reported no conflicts of interest.
The 5-year cancer survival rate for children younger than 15 years old is up by 43% since 1975, according to investigators from the American Cancer Society.
The 5-year survival rate for all cancers showed a statistically significant rise from 58% in 1975 to 83% in 2011, said Rebecca L. Siegel and her associates at the ACS (CA Cancer J Clin. 2016 Jan;66[1]:7-30).

“The substantial progress for all of the major childhood cancers reflects both improvements in treatment and high levels of participation in clinical trials,” they wrote.
Survival for cancers of the brain and nervous system – now the leading cause of cancer death for those younger than 20 years old – increased from 57% in 1975 to 74% in 2011. The next-most-common cause of cancer death in children and adolescents is leukemia, and 5-year survival for acute myeloid leukemia went from 19% in 1975 to 67% in 2011, while 5-year survival for acute lymphocytic leukemia rose from 57% to 91% over that time period, the investigators reported.
The authors reported no conflicts of interest.
FROM CA: A CANCER JOURNAL FOR CLINICIANS
Lysolipid antigens prominent in MGUS and myeloma
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
Clonal immunoglobulin reactive to lyso-glucosylceramide (LGL1), which is elevated in Gaucher disease, was found in patients with Gaucher disease as well as in one third of patients with sporadic monoclonal gammopathies.
Antigen-specific immunoblots showed that 17 of 20 patients with Gaucher disease had clonal immunoglobulin against LGL1, researchers reported (N Engl J Med. 2016 Feb 10).
Analysis of immunoglobulin gene mutations has provided evidence for antigen-driven clonal expansion of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS), but the underlying antigens remained unknown. Myeloma risk is increased with lipid disorders such as Gaucher disease and obesity, suggesting lipid involvement in pathogenesis.
“These studies set the stage for newer approaches to lower the levels of these lipids in patients with Gaucher disease and others with precursors for myeloma. Potentially, this could be achieved with drugs or lifestyle changes to reduce the levels of lipids to lower the risk of cancer,” senior author Dr. Madhav Dhodapkar, chief of hematology at Yale Cancer Center, New Haven, Conn., said in a press release.
All six mouse models with Gaucher-like disease had clonal immunoglobulin against LGL1. Gaucher disease–associated gammopathy in mouse models can be targeted by the reduction of the underlying antigen. Feeding eliglustat to the mice with clonal immunoglobulins reduced anti-LGL1 antibodies detected by immunoblot and reduced clonal immunoglobulin in vivo.
Dysregulated lipids are sometimes present in sporadic myeloma, and M spikes on LGL1-specific blotting were LGL1-reactive in 22 of 66 patients (33%). These clonal immunoglobulins also were cross reactive to lysophosphatidylcholine. Patients with polyclonal gammopathies not associated with Gaucher disease did not react to the lysolipids.
“Understanding the antigenic reactivity of clonal immunoglobulin not only has direct implications for antigenic origins of myeloma but also may lead to new strategies to prevent or treat clinical cancer by targeting the underlying antigen,” wrote Shiny Nair, Ph.D., of Yale University and colleagues.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: In patients with Gaucher disease and in nearly one-third of patients with MGUS or myeloma, clonal immunoglobulins reacted with lysolipid antigens.
Major finding: Clonal immunoglobulin from 17 of 20 patients with Gaucher disease and from 22 of 66 patients with sporadic myeloma were reactive to lyso-glucosylceramide.
Data source: Peripheral blood or bone marrow samples from 25 healthy donors, 20 patients with Gaucher disease, and 66 patients with sporadic monoclonal gammopathy.
Disclosures: Dr. Nair reported having no disclosures. One of her coauthors reported ties to industry.
Danish study finds increased glioma risk in a rosacea population
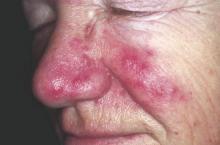
An increased focus on neurologic symptoms in patients with rosacea may be warranted, according to Danish researchers, who found a significantly increased risk of glioma associated with rosacea, in a nationwide study of Danish citizens.
The observational study followed 5,484,910 Danish adults from January 1997 through December 2011; 68,372 were diagnosed with rosacea, and the remaining 5,416,538 were the reference group. The incidence rate of glioma per 10,000 person-years (adjusted for age, sex, and socioeconomic status) was 3.34 in the reference population, but was 4.99 among those with rosacea, reported Dr. Alexander Egeberg of the department of dermatoallergology, Herlev and Gentofte University Hospital, University of Copenhagen, Hellerup, and his coauthors (JAMA Dermatol. 2016 Jan 27. doi: 10.1001/jamadermatol.2015.5549).
The adjusted incidence rate ratio (IRR) of glioma in patients with rosacea was 1.36 (P less than .001). When the researchers limited the analysis to patients who had been diagnosed by a hospital dermatologist, the adjusted IRR was 1.82. The results remained significant after sensitivity analyses and after adjustment for potential confounders.
Among the patients with rosacea, men had an increased risk of glioma, compared with women (an incidence rate per 10,000 person-years of 6.45 vs. 4.30), although “gliomas and rosacea were generally more common among women,” the authors reported.
The association might be partially mediated by mechanisms dependent on matrix metalloproteinases (MMPs), the authors said, referring to studies indicating that MMPs, in particular MMP-9, “play a pivotal role in rosacea and regulation of the invasiveness of malignant glioma cells.” While speculative, “mechanisms dependent on MMPs may contribute to the link between rosacea and the risk for glioma,” the investigators added.
An increased focus on neurologic symptoms such as headaches, memory loss, visual symptoms, cognitive decline, and personality changes in patients with rosacea “and timely referral to relevant specialists may be warranted,” they concluded.
Limitations of the study included the observational design, which cannot establish causation, the authors noted. Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
An increased focus on neurologic symptoms in patients with rosacea may be warranted, according to Danish researchers, who found a significantly increased risk of glioma associated with rosacea, in a nationwide study of Danish citizens.
The observational study followed 5,484,910 Danish adults from January 1997 through December 2011; 68,372 were diagnosed with rosacea, and the remaining 5,416,538 were the reference group. The incidence rate of glioma per 10,000 person-years (adjusted for age, sex, and socioeconomic status) was 3.34 in the reference population, but was 4.99 among those with rosacea, reported Dr. Alexander Egeberg of the department of dermatoallergology, Herlev and Gentofte University Hospital, University of Copenhagen, Hellerup, and his coauthors (JAMA Dermatol. 2016 Jan 27. doi: 10.1001/jamadermatol.2015.5549).
The adjusted incidence rate ratio (IRR) of glioma in patients with rosacea was 1.36 (P less than .001). When the researchers limited the analysis to patients who had been diagnosed by a hospital dermatologist, the adjusted IRR was 1.82. The results remained significant after sensitivity analyses and after adjustment for potential confounders.
Among the patients with rosacea, men had an increased risk of glioma, compared with women (an incidence rate per 10,000 person-years of 6.45 vs. 4.30), although “gliomas and rosacea were generally more common among women,” the authors reported.
The association might be partially mediated by mechanisms dependent on matrix metalloproteinases (MMPs), the authors said, referring to studies indicating that MMPs, in particular MMP-9, “play a pivotal role in rosacea and regulation of the invasiveness of malignant glioma cells.” While speculative, “mechanisms dependent on MMPs may contribute to the link between rosacea and the risk for glioma,” the investigators added.
An increased focus on neurologic symptoms such as headaches, memory loss, visual symptoms, cognitive decline, and personality changes in patients with rosacea “and timely referral to relevant specialists may be warranted,” they concluded.
Limitations of the study included the observational design, which cannot establish causation, the authors noted. Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
An increased focus on neurologic symptoms in patients with rosacea may be warranted, according to Danish researchers, who found a significantly increased risk of glioma associated with rosacea, in a nationwide study of Danish citizens.
The observational study followed 5,484,910 Danish adults from January 1997 through December 2011; 68,372 were diagnosed with rosacea, and the remaining 5,416,538 were the reference group. The incidence rate of glioma per 10,000 person-years (adjusted for age, sex, and socioeconomic status) was 3.34 in the reference population, but was 4.99 among those with rosacea, reported Dr. Alexander Egeberg of the department of dermatoallergology, Herlev and Gentofte University Hospital, University of Copenhagen, Hellerup, and his coauthors (JAMA Dermatol. 2016 Jan 27. doi: 10.1001/jamadermatol.2015.5549).
The adjusted incidence rate ratio (IRR) of glioma in patients with rosacea was 1.36 (P less than .001). When the researchers limited the analysis to patients who had been diagnosed by a hospital dermatologist, the adjusted IRR was 1.82. The results remained significant after sensitivity analyses and after adjustment for potential confounders.
Among the patients with rosacea, men had an increased risk of glioma, compared with women (an incidence rate per 10,000 person-years of 6.45 vs. 4.30), although “gliomas and rosacea were generally more common among women,” the authors reported.
The association might be partially mediated by mechanisms dependent on matrix metalloproteinases (MMPs), the authors said, referring to studies indicating that MMPs, in particular MMP-9, “play a pivotal role in rosacea and regulation of the invasiveness of malignant glioma cells.” While speculative, “mechanisms dependent on MMPs may contribute to the link between rosacea and the risk for glioma,” the investigators added.
An increased focus on neurologic symptoms such as headaches, memory loss, visual symptoms, cognitive decline, and personality changes in patients with rosacea “and timely referral to relevant specialists may be warranted,” they concluded.
Limitations of the study included the observational design, which cannot establish causation, the authors noted. Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
FROM JAMA DERMATOLOGY
Key clinical point: Clinicians should be mindful of neurologic symptoms in patients with rosacea because of a significant association between glioma and rosacea.
Major finding: The incidence rate ratio of glioma per 10,000 person-years was 3.34 in the reference population and 4.99 in patients with rosacea.
Data source: A nationwide cohort study that followed 5,484 910 Danish adults from 1997 through 2011.
Disclosures: Dr. Egeberg reported being a former employee of Pfizer; one coauthor reported receiving consultancy and/or speaker honoraria from Galderma. The study was supported by an unrestricted grant from the LEO Foundation and the Lundbeck Foundation and an unrestricted research scholarship from the Novo Nordisk Foundation.
Proton radiotherapy effective for childhood medulloblastoma
Treatment of childhood medulloblastoma with proton radiotherapy resulted in acceptable toxicity, with no observed cardiac, pulmonary, or gastrointestinal late effects, and achieved outcomes that were similar to those of photon (x-ray)-based therapy.
At a median follow up of 5 years, the cumulative incidence of grade 3 to 4 hearing loss was 16% (95% confidence interval, 6-29). The Full Scale Intelligence Quotient decreased significantly, particularly in children younger than 8 years, driven mostly by drops in processing speed and verbal comprehension. The cumulative incidence of any hormone deficit at 7 years was 63% (95% CI, 48-75).
For all patients, progression-free survival (PFS) at 5 years was 80% (95% CI, 67-88) and overall survival (OS) was 83% (95% CI, 70-90). For patients with standard-risk disease, PFS was 85% (95% CI, 69-93) and OS was 86% (95% CI, 70-94); for intermediate-risk disease, PFS was 67% (95% CI, 19-90) and OS was 67% (95% CI, 19-90); for high-risk disease, PFS was 71% (95% CI, 41-88) and OS was 79% (95% CI, 47-93). These rates are similar to previously published outcomes of 81%-83% for PFS and 85%-86% for OS (Lancet Onc. 2016 Jan 29. doi: 10.1016/S1470-2045(15)00167-9).
“Therefore, the similar disease control coupled with similar patterns of failure should quell concerns raised about the differences in relative biologic effectiveness of passively scattered proton radiotherapy,” wrote Dr. Torunn Yock, chief of pediatric radiation oncology at Massachusetts General Hospital, Boston, and colleagues.
“Although there remain some effects of treatment on hearing, endocrine, and neurocognitive outcomes – particularly in younger patients – other late effects common in photon-treated patients, such as cardiac, pulmonary, and gastrointestinal toxic effects, were absent,” they said.
Medulloblastoma survivors often have treatment-related adverse late effects, and proton radiotherapy is used to mitigate late effects by decreasing the volume of normal tissue irradiated.
The estimated mean loss per year of IQ points, at –1.5, was less than IQ differences reported in previous studies, which ranged from –1.9 to –5.8 depending on age, craniospinal irradiation dosing, boost volumes, and length of follow-up.
There were no observed cardiac effects, and no patients had restrictive lung disease, which can occur in 24%-50% of long-term survivors treated with craniospinal photon irradiation. In addition, there were no new cases of gastrointestinal toxic effects that have occurred in up to 44% of photon-treated patients.
The prospective, nonrandomized phase II study carried out at Massachusetts General Hospital, Boston, evaluated 59 patients aged 3-21 years (median age 6.6 years) who had medulloblastoma (39 standard risk, 6 intermediate risk, and 14 high risk). All patients received chemotherapy and 55 had a near or gross total resection.
The prospective study by Dr. Yock and colleagues sets a new benchmark for treatment of medulloblastoma in pediatric patients and alludes to the clinical benefits of advanced radiation treatment. It becomes increasingly important for radiation oncologists to incorporate new findings in genomics and molecular subtyping for diseases such as medulloblastoma in the design of prospective studies, and to implement strategies to prevent cognitive decline in pediatric patients. The investigators demonstrate benefits of low-dose sparing afforded by proton therapy, but further improvements are possible. With newer delivery techniques, such as spot scanning proton therapy for the craniospinal component of treatment, more improvements in hearing outcomes can be expected.
The rarity of the disease, combined with the compelling results of Dr. Yock and colleagues, make randomized trials of photons versus protons for medulloblastoma unlikely. Without randomized trial data, some states require that all pediatric patients be treated with photon therapy, a requirement that could result higher rates of cardiovascular disease and other adverse effects. Radiation oncologists understand the potential for severe adverse effects of treatment, and many embrace new technologies that mitigate effects of radiation therapy on patients’ quality of life, a consideration that is particularly important in treatment of pediatric cancers.
Dr. David Grosshans is at the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston. These remarks were part of an editorial accompanying the report by Dr. Yock and colleagues (Lancet Onc. 2016 Jan 29. doi: 10.1016/S1470-2045(15)00217-X. Dr. Grosshans reported having no disclosures.
The prospective study by Dr. Yock and colleagues sets a new benchmark for treatment of medulloblastoma in pediatric patients and alludes to the clinical benefits of advanced radiation treatment. It becomes increasingly important for radiation oncologists to incorporate new findings in genomics and molecular subtyping for diseases such as medulloblastoma in the design of prospective studies, and to implement strategies to prevent cognitive decline in pediatric patients. The investigators demonstrate benefits of low-dose sparing afforded by proton therapy, but further improvements are possible. With newer delivery techniques, such as spot scanning proton therapy for the craniospinal component of treatment, more improvements in hearing outcomes can be expected.
The rarity of the disease, combined with the compelling results of Dr. Yock and colleagues, make randomized trials of photons versus protons for medulloblastoma unlikely. Without randomized trial data, some states require that all pediatric patients be treated with photon therapy, a requirement that could result higher rates of cardiovascular disease and other adverse effects. Radiation oncologists understand the potential for severe adverse effects of treatment, and many embrace new technologies that mitigate effects of radiation therapy on patients’ quality of life, a consideration that is particularly important in treatment of pediatric cancers.
Dr. David Grosshans is at the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston. These remarks were part of an editorial accompanying the report by Dr. Yock and colleagues (Lancet Onc. 2016 Jan 29. doi: 10.1016/S1470-2045(15)00217-X. Dr. Grosshans reported having no disclosures.
The prospective study by Dr. Yock and colleagues sets a new benchmark for treatment of medulloblastoma in pediatric patients and alludes to the clinical benefits of advanced radiation treatment. It becomes increasingly important for radiation oncologists to incorporate new findings in genomics and molecular subtyping for diseases such as medulloblastoma in the design of prospective studies, and to implement strategies to prevent cognitive decline in pediatric patients. The investigators demonstrate benefits of low-dose sparing afforded by proton therapy, but further improvements are possible. With newer delivery techniques, such as spot scanning proton therapy for the craniospinal component of treatment, more improvements in hearing outcomes can be expected.
The rarity of the disease, combined with the compelling results of Dr. Yock and colleagues, make randomized trials of photons versus protons for medulloblastoma unlikely. Without randomized trial data, some states require that all pediatric patients be treated with photon therapy, a requirement that could result higher rates of cardiovascular disease and other adverse effects. Radiation oncologists understand the potential for severe adverse effects of treatment, and many embrace new technologies that mitigate effects of radiation therapy on patients’ quality of life, a consideration that is particularly important in treatment of pediatric cancers.
Dr. David Grosshans is at the department of radiation oncology, University of Texas MD Anderson Cancer Center, Houston. These remarks were part of an editorial accompanying the report by Dr. Yock and colleagues (Lancet Onc. 2016 Jan 29. doi: 10.1016/S1470-2045(15)00217-X. Dr. Grosshans reported having no disclosures.
Treatment of childhood medulloblastoma with proton radiotherapy resulted in acceptable toxicity, with no observed cardiac, pulmonary, or gastrointestinal late effects, and achieved outcomes that were similar to those of photon (x-ray)-based therapy.
At a median follow up of 5 years, the cumulative incidence of grade 3 to 4 hearing loss was 16% (95% confidence interval, 6-29). The Full Scale Intelligence Quotient decreased significantly, particularly in children younger than 8 years, driven mostly by drops in processing speed and verbal comprehension. The cumulative incidence of any hormone deficit at 7 years was 63% (95% CI, 48-75).
For all patients, progression-free survival (PFS) at 5 years was 80% (95% CI, 67-88) and overall survival (OS) was 83% (95% CI, 70-90). For patients with standard-risk disease, PFS was 85% (95% CI, 69-93) and OS was 86% (95% CI, 70-94); for intermediate-risk disease, PFS was 67% (95% CI, 19-90) and OS was 67% (95% CI, 19-90); for high-risk disease, PFS was 71% (95% CI, 41-88) and OS was 79% (95% CI, 47-93). These rates are similar to previously published outcomes of 81%-83% for PFS and 85%-86% for OS (Lancet Onc. 2016 Jan 29. doi: 10.1016/S1470-2045(15)00167-9).
“Therefore, the similar disease control coupled with similar patterns of failure should quell concerns raised about the differences in relative biologic effectiveness of passively scattered proton radiotherapy,” wrote Dr. Torunn Yock, chief of pediatric radiation oncology at Massachusetts General Hospital, Boston, and colleagues.
“Although there remain some effects of treatment on hearing, endocrine, and neurocognitive outcomes – particularly in younger patients – other late effects common in photon-treated patients, such as cardiac, pulmonary, and gastrointestinal toxic effects, were absent,” they said.
Medulloblastoma survivors often have treatment-related adverse late effects, and proton radiotherapy is used to mitigate late effects by decreasing the volume of normal tissue irradiated.
The estimated mean loss per year of IQ points, at –1.5, was less than IQ differences reported in previous studies, which ranged from –1.9 to –5.8 depending on age, craniospinal irradiation dosing, boost volumes, and length of follow-up.
There were no observed cardiac effects, and no patients had restrictive lung disease, which can occur in 24%-50% of long-term survivors treated with craniospinal photon irradiation. In addition, there were no new cases of gastrointestinal toxic effects that have occurred in up to 44% of photon-treated patients.
The prospective, nonrandomized phase II study carried out at Massachusetts General Hospital, Boston, evaluated 59 patients aged 3-21 years (median age 6.6 years) who had medulloblastoma (39 standard risk, 6 intermediate risk, and 14 high risk). All patients received chemotherapy and 55 had a near or gross total resection.
Treatment of childhood medulloblastoma with proton radiotherapy resulted in acceptable toxicity, with no observed cardiac, pulmonary, or gastrointestinal late effects, and achieved outcomes that were similar to those of photon (x-ray)-based therapy.
At a median follow up of 5 years, the cumulative incidence of grade 3 to 4 hearing loss was 16% (95% confidence interval, 6-29). The Full Scale Intelligence Quotient decreased significantly, particularly in children younger than 8 years, driven mostly by drops in processing speed and verbal comprehension. The cumulative incidence of any hormone deficit at 7 years was 63% (95% CI, 48-75).
For all patients, progression-free survival (PFS) at 5 years was 80% (95% CI, 67-88) and overall survival (OS) was 83% (95% CI, 70-90). For patients with standard-risk disease, PFS was 85% (95% CI, 69-93) and OS was 86% (95% CI, 70-94); for intermediate-risk disease, PFS was 67% (95% CI, 19-90) and OS was 67% (95% CI, 19-90); for high-risk disease, PFS was 71% (95% CI, 41-88) and OS was 79% (95% CI, 47-93). These rates are similar to previously published outcomes of 81%-83% for PFS and 85%-86% for OS (Lancet Onc. 2016 Jan 29. doi: 10.1016/S1470-2045(15)00167-9).
“Therefore, the similar disease control coupled with similar patterns of failure should quell concerns raised about the differences in relative biologic effectiveness of passively scattered proton radiotherapy,” wrote Dr. Torunn Yock, chief of pediatric radiation oncology at Massachusetts General Hospital, Boston, and colleagues.
“Although there remain some effects of treatment on hearing, endocrine, and neurocognitive outcomes – particularly in younger patients – other late effects common in photon-treated patients, such as cardiac, pulmonary, and gastrointestinal toxic effects, were absent,” they said.
Medulloblastoma survivors often have treatment-related adverse late effects, and proton radiotherapy is used to mitigate late effects by decreasing the volume of normal tissue irradiated.
The estimated mean loss per year of IQ points, at –1.5, was less than IQ differences reported in previous studies, which ranged from –1.9 to –5.8 depending on age, craniospinal irradiation dosing, boost volumes, and length of follow-up.
There were no observed cardiac effects, and no patients had restrictive lung disease, which can occur in 24%-50% of long-term survivors treated with craniospinal photon irradiation. In addition, there were no new cases of gastrointestinal toxic effects that have occurred in up to 44% of photon-treated patients.
The prospective, nonrandomized phase II study carried out at Massachusetts General Hospital, Boston, evaluated 59 patients aged 3-21 years (median age 6.6 years) who had medulloblastoma (39 standard risk, 6 intermediate risk, and 14 high risk). All patients received chemotherapy and 55 had a near or gross total resection.
FROM THE LANCET ONCOLOGY
Key clinical point: Proton radiotherapy for childhood medulloblastoma resulted in similar survival outcomes to those of photon-based therapy and had acceptable toxicity.
Major finding: At 5 years, the cumulative incidence of grade 3 to 4 hearing loss was 16%; 5-year progression-free and overall survival for patients with standard risk were 85% and 86%, respectively, and for those with high to intermediate risk, 70% and 75%, respectively.
Data source: A prospective, nonrandomized, phase II study with 59 patients aged 3-21 years who had medulloblastoma (39 standard risk, 6 intermediate risk, and 14 high risk).
Disclosures: Dr. Yock and coauthors reported having no disclosures.
Tumor-treating fields may improve outcomes in glioblastoma
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
FROM JAMA
Key clinical point: Maintenance therapy with tumor-treating fields (TTFields) plus temozolomide resulted in longer survival, compared with temozolomide alone, for patients with glioblastoma.
Major finding: Median progression-free survival for TTFields plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (HR, 0.62; 98.7% CI, 0.43-0.89; P = .001).
Data sources: Interim analysis of the randomized trial included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group.
Disclosures: The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck, and Novartis. Several of his coauthors reported ties to industry.
IDH1 mutant inhibitor targets gliomas, chondrosarcomas
BOSTON – An investigational agent targeted against tumors carrying mutant forms of the metabolic protein IDH1 has shown clinical activity against advanced solid tumors, including gliomas and chondrosarcomas, investigators reported.
In a phase I trial in 62 patients with advanced IDH1 mutation-positive solid tumors that had recurred or progressed on a median three prior lines of therapy, 7 of 11 patients with chondrosarcomas treated with AG-120 had stable disease, with five of the responses maintained beyond 6 months, and 10 of 20 patients with glioma had stable disease, with 4 having responses longer than 6 months, reported Dr. Howard A. Burris III, chief medical officer and executive director, drug development program, at the Sarah Cannon Research Institute, Nashville, Tenn.
“These tumors don’t respond to any of our systemic therapies, so it’s pretty remarkable that you get patients who don’t have treatment options that do so well for so long. We’ve just not seen that in the past,” commented Dr. Lee J. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who was not involved in the study.
A related compound, AG-221, which inhibits IDH2, has previously been shown to produce durable objective responses in 30%-50% of patients with acute myeloid leukemia. Here at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics, Dr. Burris presented the first clinical results with the IHD1-inhibiting compound in solid tumors.
IDH1 is normally involved in regulation of the Krebs cycle, the central metabolic pathway. IDH1 mutations produce the metabolite 2-hydroxyglutarate (2-HG), which causes genetic and epigenetic dysregulation, leading to oncogenesis via unchecked cell proliferation. AG-120 blocks the accumulation of 2-HG, thereby allowing cells to differentiate and undergo apoptosis as normal, thereby restoring homeostasis.
IDH1 mutations are found in an estimated 68%-74% of gliomas, 11%-24% of intrahepatic cholangiocarcinomas (IHCC), and in 40%-52% of chondrosarcomas, Dr. Burris said.
Early efficacy promising
He reported early safety and clinical data from a single-arm, dose escalation study of single-agent AG-210 dosed orally once or twice daily in 28-day cycles in one of eight dose levels. The dose escalation phase has been completed and a 500-mg q.i.d. dose has been selected.
There were no dose-limiting toxicities, and the maximum tolerated dose was not reached. Serious adverse events, occurring in one patient each (18 of 62 patients) were acute kidney injury, acute respiratory failure, anemia, ataxia, brain herniation, confusional state, cystitis, urinary tract infection, headache, hyponatremia, joint effusion, esophageal varices hemorrhage, partial seizures, seizure, bacteremia, superior vena cava syndrome, vertebral fracture, and urosepsis. There were no deaths judged to be treatment related.
Data on 55 patients were available for the efficacy analysis. Among 11 patients with chondrosarcoma, 7 had stable disease, 2 had progressive disease, and 2 had unknown status. Among 20 patients with IHCC, there was 1 partial response, 11 cases of stable disease, 6 of progressive disease, and 2 unknown. Among patients with glioma, 10 had stable disease, and 10 had progressive disease.
Of four remaining patients (with adenocarcinoma and colitis-associated neuroendocrine, small intestine, and ovarian cancers), one had stable disease, and three had progression.
The patient with IHCC who had a partial response was a 65-year-old woman who had previously been treated with multiple lines of chemotherapy, including combinations of cisplatin and gemcitabine, gemcitabine and oxaliplatin, and cisplatin and docetaxel. Following treatment with AG-120, she had a 98.7% reduction in 2-HG level, and an 81% reduction in Ki-67 staining, indicating marked tumor reduction.
“This patient in fact was on the trial for more than 9 months. By protocol criteria, she had to come off study for development of a solitary new, small lesion that was felt to be a new metastatic spot, but had no change – no further regrowth of the tumor that had shrunk,” Dr. Burris said in a briefing following his presentation of the data in a plenary session.
In a 38-year-old man with grade II glioma, investigators saw volumetric changes consistent with 2-HG reduction on magnetic resonance spectroscopy, he added.
For the expansion phase, investigators are currently enrolling four cohorts each of 25 patients with low-grade glioma (with at least 6 months of prior scans), IHCC following progression on first-line therapy, high-grade metastatic chondrosarcoma, and patients with other solid tumors with IDH1 mutations. They are also planning a randomized phase II study in patients with IHCC beginning in 2016.
BOSTON – An investigational agent targeted against tumors carrying mutant forms of the metabolic protein IDH1 has shown clinical activity against advanced solid tumors, including gliomas and chondrosarcomas, investigators reported.
In a phase I trial in 62 patients with advanced IDH1 mutation-positive solid tumors that had recurred or progressed on a median three prior lines of therapy, 7 of 11 patients with chondrosarcomas treated with AG-120 had stable disease, with five of the responses maintained beyond 6 months, and 10 of 20 patients with glioma had stable disease, with 4 having responses longer than 6 months, reported Dr. Howard A. Burris III, chief medical officer and executive director, drug development program, at the Sarah Cannon Research Institute, Nashville, Tenn.
“These tumors don’t respond to any of our systemic therapies, so it’s pretty remarkable that you get patients who don’t have treatment options that do so well for so long. We’ve just not seen that in the past,” commented Dr. Lee J. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who was not involved in the study.
A related compound, AG-221, which inhibits IDH2, has previously been shown to produce durable objective responses in 30%-50% of patients with acute myeloid leukemia. Here at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics, Dr. Burris presented the first clinical results with the IHD1-inhibiting compound in solid tumors.
IDH1 is normally involved in regulation of the Krebs cycle, the central metabolic pathway. IDH1 mutations produce the metabolite 2-hydroxyglutarate (2-HG), which causes genetic and epigenetic dysregulation, leading to oncogenesis via unchecked cell proliferation. AG-120 blocks the accumulation of 2-HG, thereby allowing cells to differentiate and undergo apoptosis as normal, thereby restoring homeostasis.
IDH1 mutations are found in an estimated 68%-74% of gliomas, 11%-24% of intrahepatic cholangiocarcinomas (IHCC), and in 40%-52% of chondrosarcomas, Dr. Burris said.
Early efficacy promising
He reported early safety and clinical data from a single-arm, dose escalation study of single-agent AG-210 dosed orally once or twice daily in 28-day cycles in one of eight dose levels. The dose escalation phase has been completed and a 500-mg q.i.d. dose has been selected.
There were no dose-limiting toxicities, and the maximum tolerated dose was not reached. Serious adverse events, occurring in one patient each (18 of 62 patients) were acute kidney injury, acute respiratory failure, anemia, ataxia, brain herniation, confusional state, cystitis, urinary tract infection, headache, hyponatremia, joint effusion, esophageal varices hemorrhage, partial seizures, seizure, bacteremia, superior vena cava syndrome, vertebral fracture, and urosepsis. There were no deaths judged to be treatment related.
Data on 55 patients were available for the efficacy analysis. Among 11 patients with chondrosarcoma, 7 had stable disease, 2 had progressive disease, and 2 had unknown status. Among 20 patients with IHCC, there was 1 partial response, 11 cases of stable disease, 6 of progressive disease, and 2 unknown. Among patients with glioma, 10 had stable disease, and 10 had progressive disease.
Of four remaining patients (with adenocarcinoma and colitis-associated neuroendocrine, small intestine, and ovarian cancers), one had stable disease, and three had progression.
The patient with IHCC who had a partial response was a 65-year-old woman who had previously been treated with multiple lines of chemotherapy, including combinations of cisplatin and gemcitabine, gemcitabine and oxaliplatin, and cisplatin and docetaxel. Following treatment with AG-120, she had a 98.7% reduction in 2-HG level, and an 81% reduction in Ki-67 staining, indicating marked tumor reduction.
“This patient in fact was on the trial for more than 9 months. By protocol criteria, she had to come off study for development of a solitary new, small lesion that was felt to be a new metastatic spot, but had no change – no further regrowth of the tumor that had shrunk,” Dr. Burris said in a briefing following his presentation of the data in a plenary session.
In a 38-year-old man with grade II glioma, investigators saw volumetric changes consistent with 2-HG reduction on magnetic resonance spectroscopy, he added.
For the expansion phase, investigators are currently enrolling four cohorts each of 25 patients with low-grade glioma (with at least 6 months of prior scans), IHCC following progression on first-line therapy, high-grade metastatic chondrosarcoma, and patients with other solid tumors with IDH1 mutations. They are also planning a randomized phase II study in patients with IHCC beginning in 2016.
BOSTON – An investigational agent targeted against tumors carrying mutant forms of the metabolic protein IDH1 has shown clinical activity against advanced solid tumors, including gliomas and chondrosarcomas, investigators reported.
In a phase I trial in 62 patients with advanced IDH1 mutation-positive solid tumors that had recurred or progressed on a median three prior lines of therapy, 7 of 11 patients with chondrosarcomas treated with AG-120 had stable disease, with five of the responses maintained beyond 6 months, and 10 of 20 patients with glioma had stable disease, with 4 having responses longer than 6 months, reported Dr. Howard A. Burris III, chief medical officer and executive director, drug development program, at the Sarah Cannon Research Institute, Nashville, Tenn.
“These tumors don’t respond to any of our systemic therapies, so it’s pretty remarkable that you get patients who don’t have treatment options that do so well for so long. We’ve just not seen that in the past,” commented Dr. Lee J. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who was not involved in the study.
A related compound, AG-221, which inhibits IDH2, has previously been shown to produce durable objective responses in 30%-50% of patients with acute myeloid leukemia. Here at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics, Dr. Burris presented the first clinical results with the IHD1-inhibiting compound in solid tumors.
IDH1 is normally involved in regulation of the Krebs cycle, the central metabolic pathway. IDH1 mutations produce the metabolite 2-hydroxyglutarate (2-HG), which causes genetic and epigenetic dysregulation, leading to oncogenesis via unchecked cell proliferation. AG-120 blocks the accumulation of 2-HG, thereby allowing cells to differentiate and undergo apoptosis as normal, thereby restoring homeostasis.
IDH1 mutations are found in an estimated 68%-74% of gliomas, 11%-24% of intrahepatic cholangiocarcinomas (IHCC), and in 40%-52% of chondrosarcomas, Dr. Burris said.
Early efficacy promising
He reported early safety and clinical data from a single-arm, dose escalation study of single-agent AG-210 dosed orally once or twice daily in 28-day cycles in one of eight dose levels. The dose escalation phase has been completed and a 500-mg q.i.d. dose has been selected.
There were no dose-limiting toxicities, and the maximum tolerated dose was not reached. Serious adverse events, occurring in one patient each (18 of 62 patients) were acute kidney injury, acute respiratory failure, anemia, ataxia, brain herniation, confusional state, cystitis, urinary tract infection, headache, hyponatremia, joint effusion, esophageal varices hemorrhage, partial seizures, seizure, bacteremia, superior vena cava syndrome, vertebral fracture, and urosepsis. There were no deaths judged to be treatment related.
Data on 55 patients were available for the efficacy analysis. Among 11 patients with chondrosarcoma, 7 had stable disease, 2 had progressive disease, and 2 had unknown status. Among 20 patients with IHCC, there was 1 partial response, 11 cases of stable disease, 6 of progressive disease, and 2 unknown. Among patients with glioma, 10 had stable disease, and 10 had progressive disease.
Of four remaining patients (with adenocarcinoma and colitis-associated neuroendocrine, small intestine, and ovarian cancers), one had stable disease, and three had progression.
The patient with IHCC who had a partial response was a 65-year-old woman who had previously been treated with multiple lines of chemotherapy, including combinations of cisplatin and gemcitabine, gemcitabine and oxaliplatin, and cisplatin and docetaxel. Following treatment with AG-120, she had a 98.7% reduction in 2-HG level, and an 81% reduction in Ki-67 staining, indicating marked tumor reduction.
“This patient in fact was on the trial for more than 9 months. By protocol criteria, she had to come off study for development of a solitary new, small lesion that was felt to be a new metastatic spot, but had no change – no further regrowth of the tumor that had shrunk,” Dr. Burris said in a briefing following his presentation of the data in a plenary session.
In a 38-year-old man with grade II glioma, investigators saw volumetric changes consistent with 2-HG reduction on magnetic resonance spectroscopy, he added.
For the expansion phase, investigators are currently enrolling four cohorts each of 25 patients with low-grade glioma (with at least 6 months of prior scans), IHCC following progression on first-line therapy, high-grade metastatic chondrosarcoma, and patients with other solid tumors with IDH1 mutations. They are also planning a randomized phase II study in patients with IHCC beginning in 2016.
AT THE AACR–NCI–EORTC
Key clinical point: AG-120 is a first-in-class inhibitor of IDH1 mutations that may drive oncogenesis.
Major finding: 10 of 20 patients with gliomas and 7 of 11 with chondrosarcomas had stable disease on AG-120.
Data source: Phase I dose-escalation trial in 62 patients (55 available for efficacy analysis).
Disclosures: The trial is supported by Agios Pharmaceuticals. Dr. Burris and Dr. Helman reported no conflicts of interest.
Tumor location, radiotherapy predict neuroendocrine dysfunction after pediatric glioma
Tumor location and radiotherapy, respectively, predict the speed of onset and density of long-term neuroendocrinopathy following pediatric glioma, report Dr. Hoong-Wei Gan and coauthors at University College London Institute of Child Health.
In a longitudinal study of 166 children with a median age of 4.9 years at diagnosis, progression-free status and endocrine event–free survival (EEFS) were 47.2% and 20.8%, respectively, despite high overall survival of 81%.
Growth hormone deficiency was the most common disorder (40.3%), followed by central precocious puberty (26%), gonadotropin (20.4%), thyroid-stimulating hormone (13.3%), and adrenocorticotropic hormone (13.3%) deficiencies. Hypothalamic involvement was associated with earlier onset of dysfunction (P < .001), whereas radiotherapy predicted density (P < .001), Dr. Gan and colleagues reported.
The reduction in EEFS is “concerning given the lack of a corresponding improvement in survival,” the authors said.
“Minimizing future endocrine, visual, and cognitive morbidity remains an important therapeutic goal in managing these tumors,” they added. “Optimal treatment strategy for these benign lesions remains elusive; while the absence of longitudinal neuroendocrine morbidity data limits our understanding of their etiology and evolution.”
Read the full report at J Clin Endocrinol Metab. 2015 Jul 28. doi: 10.1210/jc.2015-2028.
Tumor location and radiotherapy, respectively, predict the speed of onset and density of long-term neuroendocrinopathy following pediatric glioma, report Dr. Hoong-Wei Gan and coauthors at University College London Institute of Child Health.
In a longitudinal study of 166 children with a median age of 4.9 years at diagnosis, progression-free status and endocrine event–free survival (EEFS) were 47.2% and 20.8%, respectively, despite high overall survival of 81%.
Growth hormone deficiency was the most common disorder (40.3%), followed by central precocious puberty (26%), gonadotropin (20.4%), thyroid-stimulating hormone (13.3%), and adrenocorticotropic hormone (13.3%) deficiencies. Hypothalamic involvement was associated with earlier onset of dysfunction (P < .001), whereas radiotherapy predicted density (P < .001), Dr. Gan and colleagues reported.
The reduction in EEFS is “concerning given the lack of a corresponding improvement in survival,” the authors said.
“Minimizing future endocrine, visual, and cognitive morbidity remains an important therapeutic goal in managing these tumors,” they added. “Optimal treatment strategy for these benign lesions remains elusive; while the absence of longitudinal neuroendocrine morbidity data limits our understanding of their etiology and evolution.”
Read the full report at J Clin Endocrinol Metab. 2015 Jul 28. doi: 10.1210/jc.2015-2028.
Tumor location and radiotherapy, respectively, predict the speed of onset and density of long-term neuroendocrinopathy following pediatric glioma, report Dr. Hoong-Wei Gan and coauthors at University College London Institute of Child Health.
In a longitudinal study of 166 children with a median age of 4.9 years at diagnosis, progression-free status and endocrine event–free survival (EEFS) were 47.2% and 20.8%, respectively, despite high overall survival of 81%.
Growth hormone deficiency was the most common disorder (40.3%), followed by central precocious puberty (26%), gonadotropin (20.4%), thyroid-stimulating hormone (13.3%), and adrenocorticotropic hormone (13.3%) deficiencies. Hypothalamic involvement was associated with earlier onset of dysfunction (P < .001), whereas radiotherapy predicted density (P < .001), Dr. Gan and colleagues reported.
The reduction in EEFS is “concerning given the lack of a corresponding improvement in survival,” the authors said.
“Minimizing future endocrine, visual, and cognitive morbidity remains an important therapeutic goal in managing these tumors,” they added. “Optimal treatment strategy for these benign lesions remains elusive; while the absence of longitudinal neuroendocrine morbidity data limits our understanding of their etiology and evolution.”
Read the full report at J Clin Endocrinol Metab. 2015 Jul 28. doi: 10.1210/jc.2015-2028.
FDA investigating risk of gadolinium contrast agent brain deposits
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
The Food and Drug Administration is investigating the risk of brain deposits after recurring use of gadolinium-based contrast agents for MRI, the agency announced in a statement.
Studies suggest that gadolinium-based contrast agent (GBCA) deposits may stay in the brains of patients who have four or more contrast MRI scans, though it is unknown whether these deposits cause adverse effects, the FDA said.
GBCAs are usually expelled through the kidneys, but may remain in the brain after repeated exposure. FDA’s National Center for Toxicological
Research will further investigate safety risks in consultation with researchers and industry, the statement said.
The FDA is not requiring manufacturers to change the labels of GBCA products until more information is known. The agency is, however, recommending that clinicians limit GBCA use to situations in which it would be necessary for patient care.
“Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols,” the FDA said.
Patients may report side effects and adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Anticoagulant therapy not contraindicated in brain metastases
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
FROM BLOOD
Key clinical point: Therapeutic anticoagulation does not increase the risk for intracranial hemorrhage in patients with brain metastases.
Major finding: There were no differences in the cumulative incidence of intracranial hemorrhage at 1 year in the enoxaparin and control cohorts for total (44% vs 37%; P = .13) intracranial hemorrhages.
Data source: A matched, retrospective cohort study of 293 cancer patients with brain metastases.
Disclosures: The Harvard Catalyst/The Harvard Clinical and Translational Science Center and a Dana-Farber/Harvard Cancer Center Core Grant supported the study. Dr. Zwicker received prior research funding from Sanofi, serves on advisory committees for Portola Pharmaceuticals and Merck, and receives consulting fees from Parexel. The remaining authors declared no financial conflicts.
Impact of nurse navigation on timeliness of diagnostic medical services in patients with newly diagnosed lung cancer
Background The Summa Cancer Institute in Akron, Ohio, sought to improve access to and the timeliness of lung cancer care by hiring an oncology-certified nurse navigator. The nurse navigator was charged with coordinating diagnostic procedures and specialty oncology consultations, and with facilitating a multidisciplinary thoracic oncology tumor board.
Objective To test the hypothesis that nurse navigation would improve the timeliness of and access to diagnostic medical services among men and women with newly diagnosed lung cancer.
Methods A conducted a retrospective review of 460 patients with lung cancer to evaluate access to care and the timeliness of the care received in the non-navigated and nurse-navigated cohorts.
Results During December 2009-September 2013, the time between the suspicion of cancer on chest X-ray to treatment was 64 days. During October 2013-March 2014, the nurse navigator helped reduce that timespan to 45 days (P < .001).
Limitations Long-term follow-up on clinical outcomes remains premature.
Conclusion This finding attests to the successful implementation of nurse navigation to improve access and timeliness of lung cancer care in a community oncology practice.
Click on the PDF icon at the top of this introduction to read the full article.
Background The Summa Cancer Institute in Akron, Ohio, sought to improve access to and the timeliness of lung cancer care by hiring an oncology-certified nurse navigator. The nurse navigator was charged with coordinating diagnostic procedures and specialty oncology consultations, and with facilitating a multidisciplinary thoracic oncology tumor board.
Objective To test the hypothesis that nurse navigation would improve the timeliness of and access to diagnostic medical services among men and women with newly diagnosed lung cancer.
Methods A conducted a retrospective review of 460 patients with lung cancer to evaluate access to care and the timeliness of the care received in the non-navigated and nurse-navigated cohorts.
Results During December 2009-September 2013, the time between the suspicion of cancer on chest X-ray to treatment was 64 days. During October 2013-March 2014, the nurse navigator helped reduce that timespan to 45 days (P < .001).
Limitations Long-term follow-up on clinical outcomes remains premature.
Conclusion This finding attests to the successful implementation of nurse navigation to improve access and timeliness of lung cancer care in a community oncology practice.
Click on the PDF icon at the top of this introduction to read the full article.
Background The Summa Cancer Institute in Akron, Ohio, sought to improve access to and the timeliness of lung cancer care by hiring an oncology-certified nurse navigator. The nurse navigator was charged with coordinating diagnostic procedures and specialty oncology consultations, and with facilitating a multidisciplinary thoracic oncology tumor board.
Objective To test the hypothesis that nurse navigation would improve the timeliness of and access to diagnostic medical services among men and women with newly diagnosed lung cancer.
Methods A conducted a retrospective review of 460 patients with lung cancer to evaluate access to care and the timeliness of the care received in the non-navigated and nurse-navigated cohorts.
Results During December 2009-September 2013, the time between the suspicion of cancer on chest X-ray to treatment was 64 days. During October 2013-March 2014, the nurse navigator helped reduce that timespan to 45 days (P < .001).
Limitations Long-term follow-up on clinical outcomes remains premature.
Conclusion This finding attests to the successful implementation of nurse navigation to improve access and timeliness of lung cancer care in a community oncology practice.
Click on the PDF icon at the top of this introduction to read the full article.