User login
Access to specialized treatment by adult Hispanic brain tumor patients: findings from a single-institution retrospective study
Background: The Hispanic population accounts for 15% of the population of the United States, and for as much as 75% in cities throughout California. Racial disparities that are reflected by limited access to health care and worse disease outcomes are well documented for adult Hispanic cancer patients.
Objective: To determine whether there are similar disparities—including delays in accessing surgery, radiation, and oncologic care—for adult Hispanic non English-speaking (HNES) neuro-oncology patients and white English-only–speaking (WES) patients in an academic, tertiary care center with a multidisciplinary neuro-oncology team.
Methods: This retrospective study was conducted at the Chao Family Comprehensive Cancer Center of the University of California, Irvine. All patients who were diagnosed with a primary brain tumor during January 1, 2003, to December 31, 2008, were identified and data were collected on their age, sex, ethnicity, languages spoken, diagnosis, and insurance status. The times from the date of diagnosis to the date of surgery, from the date of surgery to the date of starting radiation (if indicated), and from the date of finishing radiation to the date of starting chemotherapy (if indicated) were also recorded.
Results: Most of the HNES patients (56.4%) had state insurance for the indigent, whereas most of the WES patients (41.8%) had private insurance from a health maintenance organization. Moreover, 12.8% of HNES patients were uninsured, compared with 4.5% of WES patients. There were no significant delays in the time from diagnosis to surgery, but there was a significant delay in access to radiation treatment (P .023). There were no differences on overall survival between the 2 groups of patients.
Limitations: This is a retrospective study of a relatively small number of patients. Larger studies are needed to corroborate these findings
Conclusions: The findings demonstrate that there are disparities in insurance status and access to radiation therapy between HNES and WES neuro-oncology patients.
*To read the the full article, click on the link at the top of this introduction.
Background: The Hispanic population accounts for 15% of the population of the United States, and for as much as 75% in cities throughout California. Racial disparities that are reflected by limited access to health care and worse disease outcomes are well documented for adult Hispanic cancer patients.
Objective: To determine whether there are similar disparities—including delays in accessing surgery, radiation, and oncologic care—for adult Hispanic non English-speaking (HNES) neuro-oncology patients and white English-only–speaking (WES) patients in an academic, tertiary care center with a multidisciplinary neuro-oncology team.
Methods: This retrospective study was conducted at the Chao Family Comprehensive Cancer Center of the University of California, Irvine. All patients who were diagnosed with a primary brain tumor during January 1, 2003, to December 31, 2008, were identified and data were collected on their age, sex, ethnicity, languages spoken, diagnosis, and insurance status. The times from the date of diagnosis to the date of surgery, from the date of surgery to the date of starting radiation (if indicated), and from the date of finishing radiation to the date of starting chemotherapy (if indicated) were also recorded.
Results: Most of the HNES patients (56.4%) had state insurance for the indigent, whereas most of the WES patients (41.8%) had private insurance from a health maintenance organization. Moreover, 12.8% of HNES patients were uninsured, compared with 4.5% of WES patients. There were no significant delays in the time from diagnosis to surgery, but there was a significant delay in access to radiation treatment (P .023). There were no differences on overall survival between the 2 groups of patients.
Limitations: This is a retrospective study of a relatively small number of patients. Larger studies are needed to corroborate these findings
Conclusions: The findings demonstrate that there are disparities in insurance status and access to radiation therapy between HNES and WES neuro-oncology patients.
*To read the the full article, click on the link at the top of this introduction.
Background: The Hispanic population accounts for 15% of the population of the United States, and for as much as 75% in cities throughout California. Racial disparities that are reflected by limited access to health care and worse disease outcomes are well documented for adult Hispanic cancer patients.
Objective: To determine whether there are similar disparities—including delays in accessing surgery, radiation, and oncologic care—for adult Hispanic non English-speaking (HNES) neuro-oncology patients and white English-only–speaking (WES) patients in an academic, tertiary care center with a multidisciplinary neuro-oncology team.
Methods: This retrospective study was conducted at the Chao Family Comprehensive Cancer Center of the University of California, Irvine. All patients who were diagnosed with a primary brain tumor during January 1, 2003, to December 31, 2008, were identified and data were collected on their age, sex, ethnicity, languages spoken, diagnosis, and insurance status. The times from the date of diagnosis to the date of surgery, from the date of surgery to the date of starting radiation (if indicated), and from the date of finishing radiation to the date of starting chemotherapy (if indicated) were also recorded.
Results: Most of the HNES patients (56.4%) had state insurance for the indigent, whereas most of the WES patients (41.8%) had private insurance from a health maintenance organization. Moreover, 12.8% of HNES patients were uninsured, compared with 4.5% of WES patients. There were no significant delays in the time from diagnosis to surgery, but there was a significant delay in access to radiation treatment (P .023). There were no differences on overall survival between the 2 groups of patients.
Limitations: This is a retrospective study of a relatively small number of patients. Larger studies are needed to corroborate these findings
Conclusions: The findings demonstrate that there are disparities in insurance status and access to radiation therapy between HNES and WES neuro-oncology patients.
*To read the the full article, click on the link at the top of this introduction.
Labs Find Evidence of Cancer Stem Cells
In an era of targeted cancer therapies, laboratory scientists working with mice may have found the ultimate target – a reservoir of stem cells that drive cancers to grow and metastasize.
Separate reports in the journals Science and Nature document the presence of cancer stem cells in intestinal adenomas (Science 2012 Aug. 1 [doi:10.1126/science.1224676]), squamous skin cancer, (Nature 2012 Aug. 1 [doi:10.1038/nature11344]), and glioblastoma multiforme (Nature 2012 Aug. 1 [doi:10.1038/nature11287]).
In the last study, mice with these highly lethal brain tumors were given temozolomide (Temodar), an approved treatment in humans, along with ganciclovir, an antiviral. Despite a transient therapeutic response to chemotherapy, the cancers continued to grow, driven by "a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for cancer stem cells," the authors wrote.
Whether these reports will resolve controversy over the existence of stem cells or lead to clinically meaningful treatments remains to be seen. There is no doubt, however, that they will lead to further investigation.
In an era of targeted cancer therapies, laboratory scientists working with mice may have found the ultimate target – a reservoir of stem cells that drive cancers to grow and metastasize.
Separate reports in the journals Science and Nature document the presence of cancer stem cells in intestinal adenomas (Science 2012 Aug. 1 [doi:10.1126/science.1224676]), squamous skin cancer, (Nature 2012 Aug. 1 [doi:10.1038/nature11344]), and glioblastoma multiforme (Nature 2012 Aug. 1 [doi:10.1038/nature11287]).
In the last study, mice with these highly lethal brain tumors were given temozolomide (Temodar), an approved treatment in humans, along with ganciclovir, an antiviral. Despite a transient therapeutic response to chemotherapy, the cancers continued to grow, driven by "a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for cancer stem cells," the authors wrote.
Whether these reports will resolve controversy over the existence of stem cells or lead to clinically meaningful treatments remains to be seen. There is no doubt, however, that they will lead to further investigation.
In an era of targeted cancer therapies, laboratory scientists working with mice may have found the ultimate target – a reservoir of stem cells that drive cancers to grow and metastasize.
Separate reports in the journals Science and Nature document the presence of cancer stem cells in intestinal adenomas (Science 2012 Aug. 1 [doi:10.1126/science.1224676]), squamous skin cancer, (Nature 2012 Aug. 1 [doi:10.1038/nature11344]), and glioblastoma multiforme (Nature 2012 Aug. 1 [doi:10.1038/nature11287]).
In the last study, mice with these highly lethal brain tumors were given temozolomide (Temodar), an approved treatment in humans, along with ganciclovir, an antiviral. Despite a transient therapeutic response to chemotherapy, the cancers continued to grow, driven by "a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for cancer stem cells," the authors wrote.
Whether these reports will resolve controversy over the existence of stem cells or lead to clinically meaningful treatments remains to be seen. There is no doubt, however, that they will lead to further investigation.
Radiation Oncologists Say Medicare Cuts Could Shutter Practices
Radiation oncologists say they could be forced to shut their doors or consolidate their practices, if the proposed cuts to radiation therapy in the 2013 Medicare physician fee schedule are allowed to stay in place.
And this is likely to hasten a shift of services out of the community and into the hospital, according to officials at the American Society for Radiation Oncology (ASTRO), which surveyed its membership in the wake of the Centers for Medicare and Medicaid Services’ proposed rule in early July.
"Some patients will likely receive their care in radiation therapy centers at a far greater distance from their home in both hospital-based facilities and larger freestanding centers that survive these cuts," said Dr. Michael L. Steinberg, president of the ASTRO’s board of directors, in an interview.
With the closure of radiation therapy centers, physicians would seek employment in surviving freestanding or hospital-based facilities, he added,
The CMS is proposing a 15% reduction in payment for IMRT (Intensity Modulated Radiation Therapy) and SBRT (Stereotactic Body Radiation Therapy). The agency also proposed a 19% cut for community-based radiation therapy centers.
IMRT and SBRT account for about a third of Medicare spending on radiation therapy, according to a spokeswoman for the ASTRO. Medicare and Medicaid are the predominant payers for radiation therapy, which is delivered to two-thirds of the nation’s 1.5 million cancer patients annually.
The ASTRO survey, which was conducted online, received about 600 responses. (There are about 4,500 radiation oncologists in the United States.) Of the 599 who participated, almost 60% were from community practices or combined community- and hospital-based practices. The results reported were only for that 60%.
Physicians were not asked about the impact of the almost-29% overall cut in physician pay called for by Medicare’s Sustainable Growth Rate (SGR) formula.
If just the close-to-20% cut for radiation therapy centers goes into effect, 35% of practices said they would probably have to close their doors, and 64% said they would consolidate. Both actions would lead to longer wait times for treatment, said ASTRO CEO Laura Thevenot in a briefing with reporters.
The consequences may also mean that physicians will spend less time with patients – that is, with those patients who would still be able to access a radiation oncologist. Some 70% of respondents said they would limit Medicare patients if the cuts go through, and 49% said they would stop accepting Medicare patients altogether.
Radiation oncologists also said they would cut back on purchases of new technology and lay off support staff and other nonphysician employees.
"The likely end result for limited access to radiation therapy – an integral form of care used in nearly 70% of all cancer patients – would be an erosion in the gains in cure rates, quality of life, and other clinical outcomes that have been achieved in our country over the last 20 years," said Dr. Constantine A. Mantz, a radiation oncologist in Ft. Myers, Fla., during the press briefing.
Ms. Thevenot said that the CMS had used faulty information to calculate the true costs of IMRT and SBRT, and that the agency should revisit the codes for both procedures.
Added Dr. Steinberg in a statement, "ASTRO welcomes a comprehensive review of these procedure codes and supports the necessary sophistication of a process, such as provided by the [American Medical Association’s Relative Value Scale Update Committee (RUC)], to value complex medical procedures including IMRT and SBRT." He is also professor and chairman of radiation oncology at the University of California, Los Angeles.
According to Ms. Thevenot, IMRT was reviewed by AMA’s RUC in fall 2010, and SBRT was reviewed in February 2011.
The ASTRO has also enlisted the support of Congress to overturn the cuts. Representatives Joe Pitts (R-Penn.) and Frank Pallone (D-N.J.), along with Rep. Mike Rogers (R-Mich.), have been circulating a letter decrying the cuts that will eventually be sent to officials at the CMS. The organization says that a "similar bipartisan letter is being drafted in the Senate."
Radiation oncologists say they could be forced to shut their doors or consolidate their practices, if the proposed cuts to radiation therapy in the 2013 Medicare physician fee schedule are allowed to stay in place.
And this is likely to hasten a shift of services out of the community and into the hospital, according to officials at the American Society for Radiation Oncology (ASTRO), which surveyed its membership in the wake of the Centers for Medicare and Medicaid Services’ proposed rule in early July.
"Some patients will likely receive their care in radiation therapy centers at a far greater distance from their home in both hospital-based facilities and larger freestanding centers that survive these cuts," said Dr. Michael L. Steinberg, president of the ASTRO’s board of directors, in an interview.
With the closure of radiation therapy centers, physicians would seek employment in surviving freestanding or hospital-based facilities, he added,
The CMS is proposing a 15% reduction in payment for IMRT (Intensity Modulated Radiation Therapy) and SBRT (Stereotactic Body Radiation Therapy). The agency also proposed a 19% cut for community-based radiation therapy centers.
IMRT and SBRT account for about a third of Medicare spending on radiation therapy, according to a spokeswoman for the ASTRO. Medicare and Medicaid are the predominant payers for radiation therapy, which is delivered to two-thirds of the nation’s 1.5 million cancer patients annually.
The ASTRO survey, which was conducted online, received about 600 responses. (There are about 4,500 radiation oncologists in the United States.) Of the 599 who participated, almost 60% were from community practices or combined community- and hospital-based practices. The results reported were only for that 60%.
Physicians were not asked about the impact of the almost-29% overall cut in physician pay called for by Medicare’s Sustainable Growth Rate (SGR) formula.
If just the close-to-20% cut for radiation therapy centers goes into effect, 35% of practices said they would probably have to close their doors, and 64% said they would consolidate. Both actions would lead to longer wait times for treatment, said ASTRO CEO Laura Thevenot in a briefing with reporters.
The consequences may also mean that physicians will spend less time with patients – that is, with those patients who would still be able to access a radiation oncologist. Some 70% of respondents said they would limit Medicare patients if the cuts go through, and 49% said they would stop accepting Medicare patients altogether.
Radiation oncologists also said they would cut back on purchases of new technology and lay off support staff and other nonphysician employees.
"The likely end result for limited access to radiation therapy – an integral form of care used in nearly 70% of all cancer patients – would be an erosion in the gains in cure rates, quality of life, and other clinical outcomes that have been achieved in our country over the last 20 years," said Dr. Constantine A. Mantz, a radiation oncologist in Ft. Myers, Fla., during the press briefing.
Ms. Thevenot said that the CMS had used faulty information to calculate the true costs of IMRT and SBRT, and that the agency should revisit the codes for both procedures.
Added Dr. Steinberg in a statement, "ASTRO welcomes a comprehensive review of these procedure codes and supports the necessary sophistication of a process, such as provided by the [American Medical Association’s Relative Value Scale Update Committee (RUC)], to value complex medical procedures including IMRT and SBRT." He is also professor and chairman of radiation oncology at the University of California, Los Angeles.
According to Ms. Thevenot, IMRT was reviewed by AMA’s RUC in fall 2010, and SBRT was reviewed in February 2011.
The ASTRO has also enlisted the support of Congress to overturn the cuts. Representatives Joe Pitts (R-Penn.) and Frank Pallone (D-N.J.), along with Rep. Mike Rogers (R-Mich.), have been circulating a letter decrying the cuts that will eventually be sent to officials at the CMS. The organization says that a "similar bipartisan letter is being drafted in the Senate."
Radiation oncologists say they could be forced to shut their doors or consolidate their practices, if the proposed cuts to radiation therapy in the 2013 Medicare physician fee schedule are allowed to stay in place.
And this is likely to hasten a shift of services out of the community and into the hospital, according to officials at the American Society for Radiation Oncology (ASTRO), which surveyed its membership in the wake of the Centers for Medicare and Medicaid Services’ proposed rule in early July.
"Some patients will likely receive their care in radiation therapy centers at a far greater distance from their home in both hospital-based facilities and larger freestanding centers that survive these cuts," said Dr. Michael L. Steinberg, president of the ASTRO’s board of directors, in an interview.
With the closure of radiation therapy centers, physicians would seek employment in surviving freestanding or hospital-based facilities, he added,
The CMS is proposing a 15% reduction in payment for IMRT (Intensity Modulated Radiation Therapy) and SBRT (Stereotactic Body Radiation Therapy). The agency also proposed a 19% cut for community-based radiation therapy centers.
IMRT and SBRT account for about a third of Medicare spending on radiation therapy, according to a spokeswoman for the ASTRO. Medicare and Medicaid are the predominant payers for radiation therapy, which is delivered to two-thirds of the nation’s 1.5 million cancer patients annually.
The ASTRO survey, which was conducted online, received about 600 responses. (There are about 4,500 radiation oncologists in the United States.) Of the 599 who participated, almost 60% were from community practices or combined community- and hospital-based practices. The results reported were only for that 60%.
Physicians were not asked about the impact of the almost-29% overall cut in physician pay called for by Medicare’s Sustainable Growth Rate (SGR) formula.
If just the close-to-20% cut for radiation therapy centers goes into effect, 35% of practices said they would probably have to close their doors, and 64% said they would consolidate. Both actions would lead to longer wait times for treatment, said ASTRO CEO Laura Thevenot in a briefing with reporters.
The consequences may also mean that physicians will spend less time with patients – that is, with those patients who would still be able to access a radiation oncologist. Some 70% of respondents said they would limit Medicare patients if the cuts go through, and 49% said they would stop accepting Medicare patients altogether.
Radiation oncologists also said they would cut back on purchases of new technology and lay off support staff and other nonphysician employees.
"The likely end result for limited access to radiation therapy – an integral form of care used in nearly 70% of all cancer patients – would be an erosion in the gains in cure rates, quality of life, and other clinical outcomes that have been achieved in our country over the last 20 years," said Dr. Constantine A. Mantz, a radiation oncologist in Ft. Myers, Fla., during the press briefing.
Ms. Thevenot said that the CMS had used faulty information to calculate the true costs of IMRT and SBRT, and that the agency should revisit the codes for both procedures.
Added Dr. Steinberg in a statement, "ASTRO welcomes a comprehensive review of these procedure codes and supports the necessary sophistication of a process, such as provided by the [American Medical Association’s Relative Value Scale Update Committee (RUC)], to value complex medical procedures including IMRT and SBRT." He is also professor and chairman of radiation oncology at the University of California, Los Angeles.
According to Ms. Thevenot, IMRT was reviewed by AMA’s RUC in fall 2010, and SBRT was reviewed in February 2011.
The ASTRO has also enlisted the support of Congress to overturn the cuts. Representatives Joe Pitts (R-Penn.) and Frank Pallone (D-N.J.), along with Rep. Mike Rogers (R-Mich.), have been circulating a letter decrying the cuts that will eventually be sent to officials at the CMS. The organization says that a "similar bipartisan letter is being drafted in the Senate."
Treating Brain Tumors With Bacteria Gets Neurosurgeons Banned
Are a few animal studies and a handful of human case reports enough to let physicians skirt institutional review boards?
Two neurosurgeons in California did just that when they used Enterobacter aerogenes to infect the surgical wounds of three terminally ill glioblastoma patients. Two of the patients died from the infections.
Dr. J. Paul Muizelaar and Dr. Rudolph J. Schrot of the University of California, Davis, said their attempt to stimulate their patients’ immune response was not research but "a one-time procedure" – exempt from review, according to a report in the Sacramento Bee.
Now both are banned from human research projects and the institutional review board is the subject of its own investigation.
For an account of the scientific thinking behind the deployment of bacteria in these patients and of ongoing efforts to develop immunotherapies against cancer, see the journal Nature (2012 July 27 [doi:10.1038/nature.2012.11080]).
Are a few animal studies and a handful of human case reports enough to let physicians skirt institutional review boards?
Two neurosurgeons in California did just that when they used Enterobacter aerogenes to infect the surgical wounds of three terminally ill glioblastoma patients. Two of the patients died from the infections.
Dr. J. Paul Muizelaar and Dr. Rudolph J. Schrot of the University of California, Davis, said their attempt to stimulate their patients’ immune response was not research but "a one-time procedure" – exempt from review, according to a report in the Sacramento Bee.
Now both are banned from human research projects and the institutional review board is the subject of its own investigation.
For an account of the scientific thinking behind the deployment of bacteria in these patients and of ongoing efforts to develop immunotherapies against cancer, see the journal Nature (2012 July 27 [doi:10.1038/nature.2012.11080]).
Are a few animal studies and a handful of human case reports enough to let physicians skirt institutional review boards?
Two neurosurgeons in California did just that when they used Enterobacter aerogenes to infect the surgical wounds of three terminally ill glioblastoma patients. Two of the patients died from the infections.
Dr. J. Paul Muizelaar and Dr. Rudolph J. Schrot of the University of California, Davis, said their attempt to stimulate their patients’ immune response was not research but "a one-time procedure" – exempt from review, according to a report in the Sacramento Bee.
Now both are banned from human research projects and the institutional review board is the subject of its own investigation.
For an account of the scientific thinking behind the deployment of bacteria in these patients and of ongoing efforts to develop immunotherapies against cancer, see the journal Nature (2012 July 27 [doi:10.1038/nature.2012.11080]).
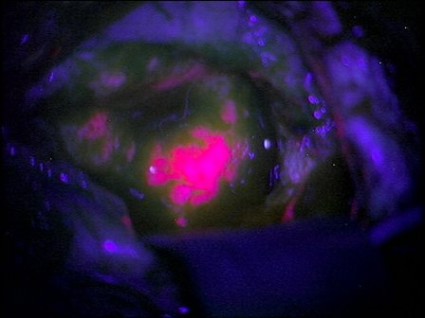
Brain Tumors Glow 'Like Lava' With New Surgical Probe
Neurosurgeons can now follow a glowing road map that points the way to cancerous brain tissue, leading thereby to a more effective surgical excision.
Researchers at the Norris Cotton Cancer Center and the Thayer School of Engineering at Dartmouth College, Hanover, N.H., have developed a probe that uses protoporphyrin IX fluorescence, oxygen saturation, hemoglobin concentration, and cell morphology to differentiate cancerous tissue from normal.
The probe identified 94% of glioma tissue in a small pilot study (J. Biomed. Opt. 2012 May 4 [doi:10.1117/1.JBO.17.5.056008]).
Research in Germany 15 years ago suggested that such a tool would identify only highly metabolic primary tumors. But augmenting the fluorescence technique with a computer algorithm that added the other cellular features gave surgeons a "jaw-dropping" view of low-grade tumors.
"The tumor glowed like lava," said Keith Paulsen, Ph.D., a professor of biomedical engineering at the school of engineering and a member of the cancer imaging and radiobiology research program at Norris Cotton Cancer Center.
The team will next evaluate their technique on lung cancers, with investigations of other tumor types to follow.
Neurosurgeons can now follow a glowing road map that points the way to cancerous brain tissue, leading thereby to a more effective surgical excision.
Researchers at the Norris Cotton Cancer Center and the Thayer School of Engineering at Dartmouth College, Hanover, N.H., have developed a probe that uses protoporphyrin IX fluorescence, oxygen saturation, hemoglobin concentration, and cell morphology to differentiate cancerous tissue from normal.
The probe identified 94% of glioma tissue in a small pilot study (J. Biomed. Opt. 2012 May 4 [doi:10.1117/1.JBO.17.5.056008]).
Research in Germany 15 years ago suggested that such a tool would identify only highly metabolic primary tumors. But augmenting the fluorescence technique with a computer algorithm that added the other cellular features gave surgeons a "jaw-dropping" view of low-grade tumors.
"The tumor glowed like lava," said Keith Paulsen, Ph.D., a professor of biomedical engineering at the school of engineering and a member of the cancer imaging and radiobiology research program at Norris Cotton Cancer Center.
The team will next evaluate their technique on lung cancers, with investigations of other tumor types to follow.
Neurosurgeons can now follow a glowing road map that points the way to cancerous brain tissue, leading thereby to a more effective surgical excision.
Researchers at the Norris Cotton Cancer Center and the Thayer School of Engineering at Dartmouth College, Hanover, N.H., have developed a probe that uses protoporphyrin IX fluorescence, oxygen saturation, hemoglobin concentration, and cell morphology to differentiate cancerous tissue from normal.
The probe identified 94% of glioma tissue in a small pilot study (J. Biomed. Opt. 2012 May 4 [doi:10.1117/1.JBO.17.5.056008]).
Research in Germany 15 years ago suggested that such a tool would identify only highly metabolic primary tumors. But augmenting the fluorescence technique with a computer algorithm that added the other cellular features gave surgeons a "jaw-dropping" view of low-grade tumors.
"The tumor glowed like lava," said Keith Paulsen, Ph.D., a professor of biomedical engineering at the school of engineering and a member of the cancer imaging and radiobiology research program at Norris Cotton Cancer Center.
The team will next evaluate their technique on lung cancers, with investigations of other tumor types to follow.
FROM THE JOURNAL OF BIOMEDICAL OPTICS
Washington Post Blasts Proliferation of ESAs for Anemia
Anemia drugs sold under the brand names of Procrit, Aranesp, and Epogen come under new and scathing scrutiny in an exclusive report published July 20 in the Washington Post.
The investigative article by Peter Whoriskey alleges that pharmaceutical giants Amgen and Johnson & Johnson "wildly overstated" benefits while understating potentially lethal side effects of these erythropoiesis-stimulating agents (ESAs).
While safety trials required by the Food and Drug Administration lagged for more than a decade, the companies successfully lobbied for a payment system that rewarded physicians for giving large doses of their high-priced drugs, according to the report.
Use of the drugs declined in recent years after studies showed higher mortality rates in patients given ESAs. Epoetin-alfa (Procrit and Epogen) and darbepoetin alfa (Aranesp) are used to treat anemia in patients undergoing cancer chemotherapy or dialysis for chronic kidney disease.
Anemia drugs sold under the brand names of Procrit, Aranesp, and Epogen come under new and scathing scrutiny in an exclusive report published July 20 in the Washington Post.
The investigative article by Peter Whoriskey alleges that pharmaceutical giants Amgen and Johnson & Johnson "wildly overstated" benefits while understating potentially lethal side effects of these erythropoiesis-stimulating agents (ESAs).
While safety trials required by the Food and Drug Administration lagged for more than a decade, the companies successfully lobbied for a payment system that rewarded physicians for giving large doses of their high-priced drugs, according to the report.
Use of the drugs declined in recent years after studies showed higher mortality rates in patients given ESAs. Epoetin-alfa (Procrit and Epogen) and darbepoetin alfa (Aranesp) are used to treat anemia in patients undergoing cancer chemotherapy or dialysis for chronic kidney disease.
Anemia drugs sold under the brand names of Procrit, Aranesp, and Epogen come under new and scathing scrutiny in an exclusive report published July 20 in the Washington Post.
The investigative article by Peter Whoriskey alleges that pharmaceutical giants Amgen and Johnson & Johnson "wildly overstated" benefits while understating potentially lethal side effects of these erythropoiesis-stimulating agents (ESAs).
While safety trials required by the Food and Drug Administration lagged for more than a decade, the companies successfully lobbied for a payment system that rewarded physicians for giving large doses of their high-priced drugs, according to the report.
Use of the drugs declined in recent years after studies showed higher mortality rates in patients given ESAs. Epoetin-alfa (Procrit and Epogen) and darbepoetin alfa (Aranesp) are used to treat anemia in patients undergoing cancer chemotherapy or dialysis for chronic kidney disease.
Erlotinib Fails as Maintenance Therapy for Ovarian Cancer
CHICAGO – Maintenance erlotinib was no more efficacious than observation when given to women with ovarian or related cancers who had no evidence of progression after receiving first-line platinum-based chemotherapy.
The 835 women studied in a randomized, phase III trial had a median progression-free survival of about 1 year and an overall survival median of about 4.5 years regardless of whether they were assigned to erlotinib – an oral inhibitor of the EGFR (epidermal growth factor receptor) tyrosine kinase – or simple observation.
The findings were similar in patient subgroups who were stratified according to a wide range of factors, such as stage and (in preliminary analyses) tumor EGFR positivity, investigators reported at the annual meeting of the American Society of Clinical Oncology.
"Maintenance erlotinib after first-line chemotherapy in patients with ovarian, peritoneal, or fallopian tube cancer did not increase progression-free survival nor overall survival," said lead investigator Dr. Ignace B. Vergote. Moreover, a quarter of patients stopped the drug early because of adverse effects.
"At this moment, we cannot identify a subgroup that might benefit from erlotinib maintenance therapy after first-line chemotherapy for ovarian cancer, but we are continuing to look at immunohistochemistry and FISH [fluorescence in situ hybridization] analysis," added Dr. Vergote, chairman of the Leuven (Belgium) Cancer Institute and head of the department of obstetrics and gynecology and gynecologic oncology at the Catholic University of Leuven.
Discussant Dr. Michael V. Seiden of the Fox Chase Cancer Center in Philadelphia noted that there was "a compelling body of basic and preclinical evidence that made the advent or launch of this study very logical." For example, research had shown a correlation between high tumor EGFR levels and poor prognosis in ovarian cancer, as well as an association– in various cancers – of EGFR mutations or expression with response to therapies targeting this receptor.
"Unfortunately, the addition of erlotinib as part of a maintenance schedule did not budge progression-free survival or overall survival," he said. "Perhaps now, in 2012, with the value of ... hindsight, one might have made this prediction, in that there have been now numerous negative single-arm studies or nearly negative single-arm studies with both small-molecule inhibitors and monoclonal antibodies" against EGFR.
Women enrolled in the EORTC (European Organisation for Research and Treatment of Cancer) 55041 trial – a joint effort of six cooperative groups – had high-risk stage I, or stages II to IV epithelial ovarian, primary peritoneal, or fallopian tube cancer and at least stable disease as of the end of first-line platinum-based chemotherapy.
They were assigned to either erlotinib (Tarceva) 150 mg daily for 2 years or observation. Erlotinib is currently approved by the Food and Drug Administration for the treatment of non–small cell lung cancer and pancreatic cancer.
Demographic and clinical data showed that about 62% of patients had serous tumor histology. Nearly all (96%) had received carboplatin and paclitaxel as their first-line therapy. Roughly 70% had had primary debulking surgery, whereas 30% had received neoadjuvant therapy and interval debulking surgery; there was no evidence of disease at surgery in 48% of the former and 62% of the latter.
Overall, 25% of patients in the erlotinib group stopped therapy early because of unacceptable adverse events (mainly rash), Dr. Vergote reported.
Main results showed that with a median follow-up of 4.3 years, erlotinib and observation were statistically indistinguishable in terms of median progression-free survival (12.7 vs. 12.4 months), with progression defined according to RECIST criteria or CA (cancer antigen) 125 levels, and overall survival (51 vs. 59 months). The findings were similar in subgroups stratified by stage, age, performance status, and response at the end of first-line chemotherapy.
Patients in the erlotinib group were more likely to experience grade 3/4 diarrhea (5% vs. 1%) and rash (13% vs. 0%).
Preliminary tumor mutational analysis in 318 patients showed that the most common was PI3KCA mutation (present in about 4% of cases), followed by KRAS mutation (present in about 3%). Only about 1% had an EGFR mutation, and mutations of NRAS and BRAF were similarly uncommon.
Progression-free survival was better for patients having any of these mutations than for those having none (P = .04). But among the former, there was no significant benefit of erlotinib over observation.
Progression-free survival was also similar across subgroups of patients whose tumors were EGFR positive and negative according to either immunohistochemistry or FISH.
Analyses failed to identify any significant relationship between the development of rash during erlotinib therapy and progression-free survival. The investigators plan to analyze quality of life data.
Dr. Vergote disclosed no relevant conflicts of interest. Dr. Seiden disclosed no relevant conflicts of interest.
CHICAGO – Maintenance erlotinib was no more efficacious than observation when given to women with ovarian or related cancers who had no evidence of progression after receiving first-line platinum-based chemotherapy.
The 835 women studied in a randomized, phase III trial had a median progression-free survival of about 1 year and an overall survival median of about 4.5 years regardless of whether they were assigned to erlotinib – an oral inhibitor of the EGFR (epidermal growth factor receptor) tyrosine kinase – or simple observation.
The findings were similar in patient subgroups who were stratified according to a wide range of factors, such as stage and (in preliminary analyses) tumor EGFR positivity, investigators reported at the annual meeting of the American Society of Clinical Oncology.
"Maintenance erlotinib after first-line chemotherapy in patients with ovarian, peritoneal, or fallopian tube cancer did not increase progression-free survival nor overall survival," said lead investigator Dr. Ignace B. Vergote. Moreover, a quarter of patients stopped the drug early because of adverse effects.
"At this moment, we cannot identify a subgroup that might benefit from erlotinib maintenance therapy after first-line chemotherapy for ovarian cancer, but we are continuing to look at immunohistochemistry and FISH [fluorescence in situ hybridization] analysis," added Dr. Vergote, chairman of the Leuven (Belgium) Cancer Institute and head of the department of obstetrics and gynecology and gynecologic oncology at the Catholic University of Leuven.
Discussant Dr. Michael V. Seiden of the Fox Chase Cancer Center in Philadelphia noted that there was "a compelling body of basic and preclinical evidence that made the advent or launch of this study very logical." For example, research had shown a correlation between high tumor EGFR levels and poor prognosis in ovarian cancer, as well as an association– in various cancers – of EGFR mutations or expression with response to therapies targeting this receptor.
"Unfortunately, the addition of erlotinib as part of a maintenance schedule did not budge progression-free survival or overall survival," he said. "Perhaps now, in 2012, with the value of ... hindsight, one might have made this prediction, in that there have been now numerous negative single-arm studies or nearly negative single-arm studies with both small-molecule inhibitors and monoclonal antibodies" against EGFR.
Women enrolled in the EORTC (European Organisation for Research and Treatment of Cancer) 55041 trial – a joint effort of six cooperative groups – had high-risk stage I, or stages II to IV epithelial ovarian, primary peritoneal, or fallopian tube cancer and at least stable disease as of the end of first-line platinum-based chemotherapy.
They were assigned to either erlotinib (Tarceva) 150 mg daily for 2 years or observation. Erlotinib is currently approved by the Food and Drug Administration for the treatment of non–small cell lung cancer and pancreatic cancer.
Demographic and clinical data showed that about 62% of patients had serous tumor histology. Nearly all (96%) had received carboplatin and paclitaxel as their first-line therapy. Roughly 70% had had primary debulking surgery, whereas 30% had received neoadjuvant therapy and interval debulking surgery; there was no evidence of disease at surgery in 48% of the former and 62% of the latter.
Overall, 25% of patients in the erlotinib group stopped therapy early because of unacceptable adverse events (mainly rash), Dr. Vergote reported.
Main results showed that with a median follow-up of 4.3 years, erlotinib and observation were statistically indistinguishable in terms of median progression-free survival (12.7 vs. 12.4 months), with progression defined according to RECIST criteria or CA (cancer antigen) 125 levels, and overall survival (51 vs. 59 months). The findings were similar in subgroups stratified by stage, age, performance status, and response at the end of first-line chemotherapy.
Patients in the erlotinib group were more likely to experience grade 3/4 diarrhea (5% vs. 1%) and rash (13% vs. 0%).
Preliminary tumor mutational analysis in 318 patients showed that the most common was PI3KCA mutation (present in about 4% of cases), followed by KRAS mutation (present in about 3%). Only about 1% had an EGFR mutation, and mutations of NRAS and BRAF were similarly uncommon.
Progression-free survival was better for patients having any of these mutations than for those having none (P = .04). But among the former, there was no significant benefit of erlotinib over observation.
Progression-free survival was also similar across subgroups of patients whose tumors were EGFR positive and negative according to either immunohistochemistry or FISH.
Analyses failed to identify any significant relationship between the development of rash during erlotinib therapy and progression-free survival. The investigators plan to analyze quality of life data.
Dr. Vergote disclosed no relevant conflicts of interest. Dr. Seiden disclosed no relevant conflicts of interest.
CHICAGO – Maintenance erlotinib was no more efficacious than observation when given to women with ovarian or related cancers who had no evidence of progression after receiving first-line platinum-based chemotherapy.
The 835 women studied in a randomized, phase III trial had a median progression-free survival of about 1 year and an overall survival median of about 4.5 years regardless of whether they were assigned to erlotinib – an oral inhibitor of the EGFR (epidermal growth factor receptor) tyrosine kinase – or simple observation.
The findings were similar in patient subgroups who were stratified according to a wide range of factors, such as stage and (in preliminary analyses) tumor EGFR positivity, investigators reported at the annual meeting of the American Society of Clinical Oncology.
"Maintenance erlotinib after first-line chemotherapy in patients with ovarian, peritoneal, or fallopian tube cancer did not increase progression-free survival nor overall survival," said lead investigator Dr. Ignace B. Vergote. Moreover, a quarter of patients stopped the drug early because of adverse effects.
"At this moment, we cannot identify a subgroup that might benefit from erlotinib maintenance therapy after first-line chemotherapy for ovarian cancer, but we are continuing to look at immunohistochemistry and FISH [fluorescence in situ hybridization] analysis," added Dr. Vergote, chairman of the Leuven (Belgium) Cancer Institute and head of the department of obstetrics and gynecology and gynecologic oncology at the Catholic University of Leuven.
Discussant Dr. Michael V. Seiden of the Fox Chase Cancer Center in Philadelphia noted that there was "a compelling body of basic and preclinical evidence that made the advent or launch of this study very logical." For example, research had shown a correlation between high tumor EGFR levels and poor prognosis in ovarian cancer, as well as an association– in various cancers – of EGFR mutations or expression with response to therapies targeting this receptor.
"Unfortunately, the addition of erlotinib as part of a maintenance schedule did not budge progression-free survival or overall survival," he said. "Perhaps now, in 2012, with the value of ... hindsight, one might have made this prediction, in that there have been now numerous negative single-arm studies or nearly negative single-arm studies with both small-molecule inhibitors and monoclonal antibodies" against EGFR.
Women enrolled in the EORTC (European Organisation for Research and Treatment of Cancer) 55041 trial – a joint effort of six cooperative groups – had high-risk stage I, or stages II to IV epithelial ovarian, primary peritoneal, or fallopian tube cancer and at least stable disease as of the end of first-line platinum-based chemotherapy.
They were assigned to either erlotinib (Tarceva) 150 mg daily for 2 years or observation. Erlotinib is currently approved by the Food and Drug Administration for the treatment of non–small cell lung cancer and pancreatic cancer.
Demographic and clinical data showed that about 62% of patients had serous tumor histology. Nearly all (96%) had received carboplatin and paclitaxel as their first-line therapy. Roughly 70% had had primary debulking surgery, whereas 30% had received neoadjuvant therapy and interval debulking surgery; there was no evidence of disease at surgery in 48% of the former and 62% of the latter.
Overall, 25% of patients in the erlotinib group stopped therapy early because of unacceptable adverse events (mainly rash), Dr. Vergote reported.
Main results showed that with a median follow-up of 4.3 years, erlotinib and observation were statistically indistinguishable in terms of median progression-free survival (12.7 vs. 12.4 months), with progression defined according to RECIST criteria or CA (cancer antigen) 125 levels, and overall survival (51 vs. 59 months). The findings were similar in subgroups stratified by stage, age, performance status, and response at the end of first-line chemotherapy.
Patients in the erlotinib group were more likely to experience grade 3/4 diarrhea (5% vs. 1%) and rash (13% vs. 0%).
Preliminary tumor mutational analysis in 318 patients showed that the most common was PI3KCA mutation (present in about 4% of cases), followed by KRAS mutation (present in about 3%). Only about 1% had an EGFR mutation, and mutations of NRAS and BRAF were similarly uncommon.
Progression-free survival was better for patients having any of these mutations than for those having none (P = .04). But among the former, there was no significant benefit of erlotinib over observation.
Progression-free survival was also similar across subgroups of patients whose tumors were EGFR positive and negative according to either immunohistochemistry or FISH.
Analyses failed to identify any significant relationship between the development of rash during erlotinib therapy and progression-free survival. The investigators plan to analyze quality of life data.
Dr. Vergote disclosed no relevant conflicts of interest. Dr. Seiden disclosed no relevant conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: Compared with observation, erlotinib did not significantly improve either progression-free survival (12.7 vs. 12.4 months) or overall survival (51 vs. 59 months).
Data Source: The phase III EORTC 55041A trial involved 835 women with high-risk stage I or stage II-IV ovarian, peritoneal, or tubal cancer who had no evidence of progression after first-line, platinum-based chemotherapy.
Disclosures: Dr. Vergote and Dr. Seiden disclosed no relevant conflicts of interest.
FDA Approves REMS for Long-Acting Opioids
A risk evaluation and mitigation strategy for extended-release and long-acting opioid medications has received Food and Drug Administration approval.
The move has been anticipated as an important element in an attempt to stem the swelling tide of abuse, misuse, and overdose of these prescription drugs while assuring continued access to the highly potent analgesics for patients with moderate to severe persistent pain, Dr. Margaret A. Hamburg said in a media briefing.
The new safety measures, which pertain to approximately 30 currently available medications, will require the manufacturers of the drugs to make FDA-approved education materials available to prescribers no later than March 1, 2013, via development grants to continuing education providers who will develop and deliver the training, said Dr. Hamburg, commissioner of the FDA. In addition, the risk evaluation and mitigation strategy (REMS) will include the distribution of a patient counseling leaflet to prescribers regarding the safe and effective use of the drugs; an updated, single-page medication guide for consumers, an implementation plan, and periodic assessments to evaluate the impact of the program on the safe use of these products, which the FDA will review and use to tweak the program as needed, she said.
"It is important to note that we are focusing on extended-release and long-acting opioid medications ... because these have very specific safety problems that have to be addressed very carefully," according to Dr. John Jenkins, director of the Office of New Drugs within the FDA’s Center for Drug Evaluation and Research. These drugs, including hydromorphone, oxycodone, morphine, oxymorphone, methadone, transdermal fentanyl, and transdermal buprenorphine, "can cause problems even when prescribed appropriately."
The prescriber education component of the REMS will include information regarding the risks and benefits of opioid therapy for individual patients, choosing patients appropriately, patient management and monitoring, and patient counseling, Dr. Jenkins explained. It will also include guidance on the potential for misuse, abuse, and addition, as well as recognizing evidence for these outcomes. The updated medication guide and patient counseling document will include information on how to safely use, store, and dispose of these analgesics, specific instructions for recognizing the signs of potential overdose and advice for preventing accidental exposure to family and household visitors, he said.
With respect to the assessment and auditing component of the program, the FDA has established goals, which the manufacturers are expected to achieve, for the percentage of prescribers who complete the training and for assessing prescribers’ understanding of the risk information. Participation in the educational programs is not mandatory for prescribers, Dr. Hamburg stated. The assessments are also required to evaluate whether the [REMS] adversely affect patient access to these drugs, she said. "Patients in pain must have continued assess to medications they need," she added.
The new REMS program is one component of a multiagency, national strategy unveiled by the White House in 2011 to address prescription drug abuse, "which is this country’s fastest-growing drug problem," said Dr. Hamburg, noting that opioid overdoses in particular were the cause of approximately 15,000 deaths among Americans in 2008 and nearly 16,000 in 2009. Called Epidemic: Responding to America's Prescription Drug Abuse Crisis, the national plan suggests expansion of state-based prescription drug-monitoring programs; seeks creation of recommendations for convenient and environmentally responsible ways to dispose of unused medications; and calls for legislative action to reduce "doctor shopping" and "pill mills."
In the absence of the legislative changes needed to fulfill the comprehensive White House plan, the REMS "is an important and timely step by FDA to supplement prescriber training and consumer information," R. Gil Kerlikowske, director of the White House Office of National Drug Control Policy, said during the telebriefing.
A risk evaluation and mitigation strategy for extended-release and long-acting opioid medications has received Food and Drug Administration approval.
The move has been anticipated as an important element in an attempt to stem the swelling tide of abuse, misuse, and overdose of these prescription drugs while assuring continued access to the highly potent analgesics for patients with moderate to severe persistent pain, Dr. Margaret A. Hamburg said in a media briefing.
The new safety measures, which pertain to approximately 30 currently available medications, will require the manufacturers of the drugs to make FDA-approved education materials available to prescribers no later than March 1, 2013, via development grants to continuing education providers who will develop and deliver the training, said Dr. Hamburg, commissioner of the FDA. In addition, the risk evaluation and mitigation strategy (REMS) will include the distribution of a patient counseling leaflet to prescribers regarding the safe and effective use of the drugs; an updated, single-page medication guide for consumers, an implementation plan, and periodic assessments to evaluate the impact of the program on the safe use of these products, which the FDA will review and use to tweak the program as needed, she said.
"It is important to note that we are focusing on extended-release and long-acting opioid medications ... because these have very specific safety problems that have to be addressed very carefully," according to Dr. John Jenkins, director of the Office of New Drugs within the FDA’s Center for Drug Evaluation and Research. These drugs, including hydromorphone, oxycodone, morphine, oxymorphone, methadone, transdermal fentanyl, and transdermal buprenorphine, "can cause problems even when prescribed appropriately."
The prescriber education component of the REMS will include information regarding the risks and benefits of opioid therapy for individual patients, choosing patients appropriately, patient management and monitoring, and patient counseling, Dr. Jenkins explained. It will also include guidance on the potential for misuse, abuse, and addition, as well as recognizing evidence for these outcomes. The updated medication guide and patient counseling document will include information on how to safely use, store, and dispose of these analgesics, specific instructions for recognizing the signs of potential overdose and advice for preventing accidental exposure to family and household visitors, he said.
With respect to the assessment and auditing component of the program, the FDA has established goals, which the manufacturers are expected to achieve, for the percentage of prescribers who complete the training and for assessing prescribers’ understanding of the risk information. Participation in the educational programs is not mandatory for prescribers, Dr. Hamburg stated. The assessments are also required to evaluate whether the [REMS] adversely affect patient access to these drugs, she said. "Patients in pain must have continued assess to medications they need," she added.
The new REMS program is one component of a multiagency, national strategy unveiled by the White House in 2011 to address prescription drug abuse, "which is this country’s fastest-growing drug problem," said Dr. Hamburg, noting that opioid overdoses in particular were the cause of approximately 15,000 deaths among Americans in 2008 and nearly 16,000 in 2009. Called Epidemic: Responding to America's Prescription Drug Abuse Crisis, the national plan suggests expansion of state-based prescription drug-monitoring programs; seeks creation of recommendations for convenient and environmentally responsible ways to dispose of unused medications; and calls for legislative action to reduce "doctor shopping" and "pill mills."
In the absence of the legislative changes needed to fulfill the comprehensive White House plan, the REMS "is an important and timely step by FDA to supplement prescriber training and consumer information," R. Gil Kerlikowske, director of the White House Office of National Drug Control Policy, said during the telebriefing.
A risk evaluation and mitigation strategy for extended-release and long-acting opioid medications has received Food and Drug Administration approval.
The move has been anticipated as an important element in an attempt to stem the swelling tide of abuse, misuse, and overdose of these prescription drugs while assuring continued access to the highly potent analgesics for patients with moderate to severe persistent pain, Dr. Margaret A. Hamburg said in a media briefing.
The new safety measures, which pertain to approximately 30 currently available medications, will require the manufacturers of the drugs to make FDA-approved education materials available to prescribers no later than March 1, 2013, via development grants to continuing education providers who will develop and deliver the training, said Dr. Hamburg, commissioner of the FDA. In addition, the risk evaluation and mitigation strategy (REMS) will include the distribution of a patient counseling leaflet to prescribers regarding the safe and effective use of the drugs; an updated, single-page medication guide for consumers, an implementation plan, and periodic assessments to evaluate the impact of the program on the safe use of these products, which the FDA will review and use to tweak the program as needed, she said.
"It is important to note that we are focusing on extended-release and long-acting opioid medications ... because these have very specific safety problems that have to be addressed very carefully," according to Dr. John Jenkins, director of the Office of New Drugs within the FDA’s Center for Drug Evaluation and Research. These drugs, including hydromorphone, oxycodone, morphine, oxymorphone, methadone, transdermal fentanyl, and transdermal buprenorphine, "can cause problems even when prescribed appropriately."
The prescriber education component of the REMS will include information regarding the risks and benefits of opioid therapy for individual patients, choosing patients appropriately, patient management and monitoring, and patient counseling, Dr. Jenkins explained. It will also include guidance on the potential for misuse, abuse, and addition, as well as recognizing evidence for these outcomes. The updated medication guide and patient counseling document will include information on how to safely use, store, and dispose of these analgesics, specific instructions for recognizing the signs of potential overdose and advice for preventing accidental exposure to family and household visitors, he said.
With respect to the assessment and auditing component of the program, the FDA has established goals, which the manufacturers are expected to achieve, for the percentage of prescribers who complete the training and for assessing prescribers’ understanding of the risk information. Participation in the educational programs is not mandatory for prescribers, Dr. Hamburg stated. The assessments are also required to evaluate whether the [REMS] adversely affect patient access to these drugs, she said. "Patients in pain must have continued assess to medications they need," she added.
The new REMS program is one component of a multiagency, national strategy unveiled by the White House in 2011 to address prescription drug abuse, "which is this country’s fastest-growing drug problem," said Dr. Hamburg, noting that opioid overdoses in particular were the cause of approximately 15,000 deaths among Americans in 2008 and nearly 16,000 in 2009. Called Epidemic: Responding to America's Prescription Drug Abuse Crisis, the national plan suggests expansion of state-based prescription drug-monitoring programs; seeks creation of recommendations for convenient and environmentally responsible ways to dispose of unused medications; and calls for legislative action to reduce "doctor shopping" and "pill mills."
In the absence of the legislative changes needed to fulfill the comprehensive White House plan, the REMS "is an important and timely step by FDA to supplement prescriber training and consumer information," R. Gil Kerlikowske, director of the White House Office of National Drug Control Policy, said during the telebriefing.
FDA Warns of QT Prolongation with Ondansetron Dose
Preliminary data indicate that a single 32-mg intravenous dose of ondansetron should be avoided because it may increase the risk of QT prolongation, along with the potentially fatal arrhythmia torsades de pointes, the Food and Drug Administration has announced.
GlaxoSmithKline, which manufactures ondansetron (Zofran), is removing the 32-mg single IV dose from the antinausea and vomiting drug’s label, according to an FDA statement.
The updated label will say that ondansetron, a 5-HT3 receptor antagonist, can continue to be used to treat adults and children with chemotherapy-induced nausea and vomiting at the dose of 0.15 mg/kg administered every 4 hours for three doses. "However, no single intravenous dose of ondansetron should exceed 16 mg due to the risk of QT prolongation," the FDA said.
The new data do not affect recommendations for oral doses of ondansetron (including the single 24-mg oral dose) used for chemotherapy-induced nausea and vomiting, the FDA noted. Recommendations on lower IV doses that are used to prevent postoperative nausea and vomiting (the other approved indication for ondansetron) also are unaffected.
Preliminary results of a study conducted by GlaxoSmithKline showed that QT prolongation "occurs in a dose-dependent manner," the FDA said. At the highest dose tested (the single 32-mg IV dose), the maximum mean difference in QTcF from placebo after baseline-correction was 20 msec. At the lower single dose tested (8 mg), the maximum mean difference in QTcF from placebo after baseline correction was 6 msec.
The FDA, which required GlaxoSmithKline to conduct the study, "will evaluate the final study results when available, and will work with GSK to explore an alternative single dose regimen that is both safe and effective for the prevention of chemotherapy-induced nausea and vomiting in adults," the FDA said.
It pointed out that ECG changes, including QT interval prolongation and torsades de pointes, have been reported in patients treated with ondansetron. In September 2011, the agency announced that it was reviewing the potential for QT prolongation with ondansetron.
Patients who have congenital long QT syndrome, heart failure, or bradyarrhythmias, or who are taking other medications that prolong the QT interval "may be at particular risk for QT prolongation" with ondansetron, the FDA warned.
The statement is available at www.fda.gov/Drugs/DrugSafety/ucm310190.htm. Serious adverse events associated with ondansetron should be reported to the FDA at 800-332-1088 or www.fda.gov/medwatch.
Preliminary data indicate that a single 32-mg intravenous dose of ondansetron should be avoided because it may increase the risk of QT prolongation, along with the potentially fatal arrhythmia torsades de pointes, the Food and Drug Administration has announced.
GlaxoSmithKline, which manufactures ondansetron (Zofran), is removing the 32-mg single IV dose from the antinausea and vomiting drug’s label, according to an FDA statement.
The updated label will say that ondansetron, a 5-HT3 receptor antagonist, can continue to be used to treat adults and children with chemotherapy-induced nausea and vomiting at the dose of 0.15 mg/kg administered every 4 hours for three doses. "However, no single intravenous dose of ondansetron should exceed 16 mg due to the risk of QT prolongation," the FDA said.
The new data do not affect recommendations for oral doses of ondansetron (including the single 24-mg oral dose) used for chemotherapy-induced nausea and vomiting, the FDA noted. Recommendations on lower IV doses that are used to prevent postoperative nausea and vomiting (the other approved indication for ondansetron) also are unaffected.
Preliminary results of a study conducted by GlaxoSmithKline showed that QT prolongation "occurs in a dose-dependent manner," the FDA said. At the highest dose tested (the single 32-mg IV dose), the maximum mean difference in QTcF from placebo after baseline-correction was 20 msec. At the lower single dose tested (8 mg), the maximum mean difference in QTcF from placebo after baseline correction was 6 msec.
The FDA, which required GlaxoSmithKline to conduct the study, "will evaluate the final study results when available, and will work with GSK to explore an alternative single dose regimen that is both safe and effective for the prevention of chemotherapy-induced nausea and vomiting in adults," the FDA said.
It pointed out that ECG changes, including QT interval prolongation and torsades de pointes, have been reported in patients treated with ondansetron. In September 2011, the agency announced that it was reviewing the potential for QT prolongation with ondansetron.
Patients who have congenital long QT syndrome, heart failure, or bradyarrhythmias, or who are taking other medications that prolong the QT interval "may be at particular risk for QT prolongation" with ondansetron, the FDA warned.
The statement is available at www.fda.gov/Drugs/DrugSafety/ucm310190.htm. Serious adverse events associated with ondansetron should be reported to the FDA at 800-332-1088 or www.fda.gov/medwatch.
Preliminary data indicate that a single 32-mg intravenous dose of ondansetron should be avoided because it may increase the risk of QT prolongation, along with the potentially fatal arrhythmia torsades de pointes, the Food and Drug Administration has announced.
GlaxoSmithKline, which manufactures ondansetron (Zofran), is removing the 32-mg single IV dose from the antinausea and vomiting drug’s label, according to an FDA statement.
The updated label will say that ondansetron, a 5-HT3 receptor antagonist, can continue to be used to treat adults and children with chemotherapy-induced nausea and vomiting at the dose of 0.15 mg/kg administered every 4 hours for three doses. "However, no single intravenous dose of ondansetron should exceed 16 mg due to the risk of QT prolongation," the FDA said.
The new data do not affect recommendations for oral doses of ondansetron (including the single 24-mg oral dose) used for chemotherapy-induced nausea and vomiting, the FDA noted. Recommendations on lower IV doses that are used to prevent postoperative nausea and vomiting (the other approved indication for ondansetron) also are unaffected.
Preliminary results of a study conducted by GlaxoSmithKline showed that QT prolongation "occurs in a dose-dependent manner," the FDA said. At the highest dose tested (the single 32-mg IV dose), the maximum mean difference in QTcF from placebo after baseline-correction was 20 msec. At the lower single dose tested (8 mg), the maximum mean difference in QTcF from placebo after baseline correction was 6 msec.
The FDA, which required GlaxoSmithKline to conduct the study, "will evaluate the final study results when available, and will work with GSK to explore an alternative single dose regimen that is both safe and effective for the prevention of chemotherapy-induced nausea and vomiting in adults," the FDA said.
It pointed out that ECG changes, including QT interval prolongation and torsades de pointes, have been reported in patients treated with ondansetron. In September 2011, the agency announced that it was reviewing the potential for QT prolongation with ondansetron.
Patients who have congenital long QT syndrome, heart failure, or bradyarrhythmias, or who are taking other medications that prolong the QT interval "may be at particular risk for QT prolongation" with ondansetron, the FDA warned.
The statement is available at www.fda.gov/Drugs/DrugSafety/ucm310190.htm. Serious adverse events associated with ondansetron should be reported to the FDA at 800-332-1088 or www.fda.gov/medwatch.
PET Radiotracer Identifies Glioma Treatment Response
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY OF NUCLEAR MEDICINE AND MOLECULAR IMAGING
Major Finding: The 17 patients identified as responders to bevacizumab by 18F-FDOPA PET after 2 weeks of treatment survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders.
Data Source: This pilot study involved 28 patients with recurrent high-grade gliomas who underwent MRI and 18F-FDOPA PET at baseline and after 2 and 6 weeks.
Disclosures: The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.