User login
Long COVID ‘brain fog’ confounds doctors, but new research offers hope
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Postacute effects of COVID on par with those of sepsis, flu
A large observational study examined population-wide data for 13 postacute conditions in patients who had been hospitalized with a COVID-19 infection and found that all but one of these conditions, venous thromboembolism, occurred at comparable rates in those hospitalized for sepsis and influenza.
“For us, the main takeaway was that patients hospitalized for severe illness in general really require ongoing treatment and support after they’re discharged. That type of care is often very challenging to coordinate for people in a sometimes siloed and fragmented health care system,” study author Kieran Quinn, MD, PhD, a clinician at Sinai Health in Toronto, and assistant professor at the University of Toronto, said in an interview.
The study was published in JAMA Internal Medicine.
Postacute effects
The investigators compared clinical and health administrative data from 26,499 Ontarians hospitalized with COVID-19 with data from three additional cohorts who had been hospitalized with influenza (17,516 patients) and sepsis. The sepsis cohort was divided into two groups, those hospitalized during the COVID-19 pandemic (52,878 patients) and a historical control population (282,473 patients).
These comparators allowed the researchers to compare COVID-19 with other severe infectious illnesses and control for any changes in health care delivery that may have occurred during the pandemic. The addition of sepsis cohorts was needed for the latter purpose, since influenza rates dropped significantly after the onset of the pandemic.
The study outcomes (including cardiovascular, neurological, and mental health conditions and rheumatoid arthritis) were selected based on previous associations with COVID-19 infections, as well as their availability in the data, according to Dr. Quinn. The investigators used diagnostic codes recorded in Ontario’s Institute for Clinical Evaluative Sciences database. The investigators observed some of the studied conditions in their own patients. “Many of us on the research team are practicing clinicians who care for people living with long COVID,” said Dr. Quinn.
Compared with cohorts with other serious infections, those hospitalized with COVID-19 were not at increased risk for selected cardiovascular or neurological disorders, rheumatoid arthritis, or mental health conditions within 1 year following hospitalization. Incident venous thromboembolic disease, however, was more common after hospitalization for COVID-19 than after hospitalization for influenza (adjusted hazard ratio, 1.77).
The study results corroborate previous findings that influenza and sepsis can have serious long-term health effects, such as heart failure, dementia, and depression, and found that the same was true for COVID-19 infections. For all three infections, patients at high risk require additional support after their initial discharge.
Defining long COVID
Although there was no increased risk with COVID-19 for most conditions, these results do not mean that the postacute effects of the infection, often called “long COVID,” are not significant, Dr. Quinn emphasized. The researcher believes that it’s important to listen to the many patients reporting symptoms and validate their experiences.
There needs to be greater consensus among the global health community on what constitutes long COVID. While the research led by Dr. Quinn focuses on postacute health conditions, some definitions of long COVID, such as that of the World Health Organization, refer only to ongoing symptoms of the original infection.
While there is now a diagnostic code for treating long COVID in Ontario, the data available to the researchers did not include information on some common symptoms of post-COVID condition, like chronic fatigue. In the data used, there was not an accurate way to identify patients who had developed conditions like myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome, said Dr. Quinn.
In addition to creating clear definitions and determining the best treatments, prevention is essential, said Dr. Quinn. Prior studies have shown that vaccination helps prevent ICU admission for COVID-19.
‘Important questions remain’
Commenting on the finding, Aravind Ganesh, MD, DPhil, a neurologist at the University of Calgary (Alta.), said that by including control populations, the study addressed an important limitation of previous research. Dr. Ganesh, who was not involved in the study, said that the controls help to determine the cause of associations found in other studies, including his own research on long-term symptoms following outpatient care for COVID-19.
“I think what this tells us is that maybe a lot of the issues that we’ve been seeing as complications attributable to COVID are, in fact, complications attributable to serious illness,” said Dr. Ganesh. He also found the association with venous thromboembolism interesting because the condition is recognized as a key risk factor for COVID-19 outcomes.
Compared with smaller randomized control trials, the population-level data provided a much larger sample size for the study. However, this design comes with limitations as well, Dr. Ganesh noted. The study relies on the administrative data of diagnostic codes and misses symptoms that aren’t associated with a diagnosis. In addition, because the cohorts were not assigned randomly, it may not account for preexisting risk factors.
While the study demonstrates associations with physical and mental health conditions, the cause of postacute effects from COVID-19, influenza, and sepsis is still unclear. “Important questions remain,” said Dr. Ganesh. “Why is it that these patients are experiencing these symptoms?”
The study was supported by ICES and the Canadian Institutes of Health Research. Dr. Quinn reported part-time employment at Public Health Ontario and stock in Pfizer and BioNTech. Dr. Ganesh reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large observational study examined population-wide data for 13 postacute conditions in patients who had been hospitalized with a COVID-19 infection and found that all but one of these conditions, venous thromboembolism, occurred at comparable rates in those hospitalized for sepsis and influenza.
“For us, the main takeaway was that patients hospitalized for severe illness in general really require ongoing treatment and support after they’re discharged. That type of care is often very challenging to coordinate for people in a sometimes siloed and fragmented health care system,” study author Kieran Quinn, MD, PhD, a clinician at Sinai Health in Toronto, and assistant professor at the University of Toronto, said in an interview.
The study was published in JAMA Internal Medicine.
Postacute effects
The investigators compared clinical and health administrative data from 26,499 Ontarians hospitalized with COVID-19 with data from three additional cohorts who had been hospitalized with influenza (17,516 patients) and sepsis. The sepsis cohort was divided into two groups, those hospitalized during the COVID-19 pandemic (52,878 patients) and a historical control population (282,473 patients).
These comparators allowed the researchers to compare COVID-19 with other severe infectious illnesses and control for any changes in health care delivery that may have occurred during the pandemic. The addition of sepsis cohorts was needed for the latter purpose, since influenza rates dropped significantly after the onset of the pandemic.
The study outcomes (including cardiovascular, neurological, and mental health conditions and rheumatoid arthritis) were selected based on previous associations with COVID-19 infections, as well as their availability in the data, according to Dr. Quinn. The investigators used diagnostic codes recorded in Ontario’s Institute for Clinical Evaluative Sciences database. The investigators observed some of the studied conditions in their own patients. “Many of us on the research team are practicing clinicians who care for people living with long COVID,” said Dr. Quinn.
Compared with cohorts with other serious infections, those hospitalized with COVID-19 were not at increased risk for selected cardiovascular or neurological disorders, rheumatoid arthritis, or mental health conditions within 1 year following hospitalization. Incident venous thromboembolic disease, however, was more common after hospitalization for COVID-19 than after hospitalization for influenza (adjusted hazard ratio, 1.77).
The study results corroborate previous findings that influenza and sepsis can have serious long-term health effects, such as heart failure, dementia, and depression, and found that the same was true for COVID-19 infections. For all three infections, patients at high risk require additional support after their initial discharge.
Defining long COVID
Although there was no increased risk with COVID-19 for most conditions, these results do not mean that the postacute effects of the infection, often called “long COVID,” are not significant, Dr. Quinn emphasized. The researcher believes that it’s important to listen to the many patients reporting symptoms and validate their experiences.
There needs to be greater consensus among the global health community on what constitutes long COVID. While the research led by Dr. Quinn focuses on postacute health conditions, some definitions of long COVID, such as that of the World Health Organization, refer only to ongoing symptoms of the original infection.
While there is now a diagnostic code for treating long COVID in Ontario, the data available to the researchers did not include information on some common symptoms of post-COVID condition, like chronic fatigue. In the data used, there was not an accurate way to identify patients who had developed conditions like myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome, said Dr. Quinn.
In addition to creating clear definitions and determining the best treatments, prevention is essential, said Dr. Quinn. Prior studies have shown that vaccination helps prevent ICU admission for COVID-19.
‘Important questions remain’
Commenting on the finding, Aravind Ganesh, MD, DPhil, a neurologist at the University of Calgary (Alta.), said that by including control populations, the study addressed an important limitation of previous research. Dr. Ganesh, who was not involved in the study, said that the controls help to determine the cause of associations found in other studies, including his own research on long-term symptoms following outpatient care for COVID-19.
“I think what this tells us is that maybe a lot of the issues that we’ve been seeing as complications attributable to COVID are, in fact, complications attributable to serious illness,” said Dr. Ganesh. He also found the association with venous thromboembolism interesting because the condition is recognized as a key risk factor for COVID-19 outcomes.
Compared with smaller randomized control trials, the population-level data provided a much larger sample size for the study. However, this design comes with limitations as well, Dr. Ganesh noted. The study relies on the administrative data of diagnostic codes and misses symptoms that aren’t associated with a diagnosis. In addition, because the cohorts were not assigned randomly, it may not account for preexisting risk factors.
While the study demonstrates associations with physical and mental health conditions, the cause of postacute effects from COVID-19, influenza, and sepsis is still unclear. “Important questions remain,” said Dr. Ganesh. “Why is it that these patients are experiencing these symptoms?”
The study was supported by ICES and the Canadian Institutes of Health Research. Dr. Quinn reported part-time employment at Public Health Ontario and stock in Pfizer and BioNTech. Dr. Ganesh reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A large observational study examined population-wide data for 13 postacute conditions in patients who had been hospitalized with a COVID-19 infection and found that all but one of these conditions, venous thromboembolism, occurred at comparable rates in those hospitalized for sepsis and influenza.
“For us, the main takeaway was that patients hospitalized for severe illness in general really require ongoing treatment and support after they’re discharged. That type of care is often very challenging to coordinate for people in a sometimes siloed and fragmented health care system,” study author Kieran Quinn, MD, PhD, a clinician at Sinai Health in Toronto, and assistant professor at the University of Toronto, said in an interview.
The study was published in JAMA Internal Medicine.
Postacute effects
The investigators compared clinical and health administrative data from 26,499 Ontarians hospitalized with COVID-19 with data from three additional cohorts who had been hospitalized with influenza (17,516 patients) and sepsis. The sepsis cohort was divided into two groups, those hospitalized during the COVID-19 pandemic (52,878 patients) and a historical control population (282,473 patients).
These comparators allowed the researchers to compare COVID-19 with other severe infectious illnesses and control for any changes in health care delivery that may have occurred during the pandemic. The addition of sepsis cohorts was needed for the latter purpose, since influenza rates dropped significantly after the onset of the pandemic.
The study outcomes (including cardiovascular, neurological, and mental health conditions and rheumatoid arthritis) were selected based on previous associations with COVID-19 infections, as well as their availability in the data, according to Dr. Quinn. The investigators used diagnostic codes recorded in Ontario’s Institute for Clinical Evaluative Sciences database. The investigators observed some of the studied conditions in their own patients. “Many of us on the research team are practicing clinicians who care for people living with long COVID,” said Dr. Quinn.
Compared with cohorts with other serious infections, those hospitalized with COVID-19 were not at increased risk for selected cardiovascular or neurological disorders, rheumatoid arthritis, or mental health conditions within 1 year following hospitalization. Incident venous thromboembolic disease, however, was more common after hospitalization for COVID-19 than after hospitalization for influenza (adjusted hazard ratio, 1.77).
The study results corroborate previous findings that influenza and sepsis can have serious long-term health effects, such as heart failure, dementia, and depression, and found that the same was true for COVID-19 infections. For all three infections, patients at high risk require additional support after their initial discharge.
Defining long COVID
Although there was no increased risk with COVID-19 for most conditions, these results do not mean that the postacute effects of the infection, often called “long COVID,” are not significant, Dr. Quinn emphasized. The researcher believes that it’s important to listen to the many patients reporting symptoms and validate their experiences.
There needs to be greater consensus among the global health community on what constitutes long COVID. While the research led by Dr. Quinn focuses on postacute health conditions, some definitions of long COVID, such as that of the World Health Organization, refer only to ongoing symptoms of the original infection.
While there is now a diagnostic code for treating long COVID in Ontario, the data available to the researchers did not include information on some common symptoms of post-COVID condition, like chronic fatigue. In the data used, there was not an accurate way to identify patients who had developed conditions like myalgic encephalomyelitis/chronic fatigue syndrome and postural orthostatic tachycardia syndrome, said Dr. Quinn.
In addition to creating clear definitions and determining the best treatments, prevention is essential, said Dr. Quinn. Prior studies have shown that vaccination helps prevent ICU admission for COVID-19.
‘Important questions remain’
Commenting on the finding, Aravind Ganesh, MD, DPhil, a neurologist at the University of Calgary (Alta.), said that by including control populations, the study addressed an important limitation of previous research. Dr. Ganesh, who was not involved in the study, said that the controls help to determine the cause of associations found in other studies, including his own research on long-term symptoms following outpatient care for COVID-19.
“I think what this tells us is that maybe a lot of the issues that we’ve been seeing as complications attributable to COVID are, in fact, complications attributable to serious illness,” said Dr. Ganesh. He also found the association with venous thromboembolism interesting because the condition is recognized as a key risk factor for COVID-19 outcomes.
Compared with smaller randomized control trials, the population-level data provided a much larger sample size for the study. However, this design comes with limitations as well, Dr. Ganesh noted. The study relies on the administrative data of diagnostic codes and misses symptoms that aren’t associated with a diagnosis. In addition, because the cohorts were not assigned randomly, it may not account for preexisting risk factors.
While the study demonstrates associations with physical and mental health conditions, the cause of postacute effects from COVID-19, influenza, and sepsis is still unclear. “Important questions remain,” said Dr. Ganesh. “Why is it that these patients are experiencing these symptoms?”
The study was supported by ICES and the Canadian Institutes of Health Research. Dr. Quinn reported part-time employment at Public Health Ontario and stock in Pfizer and BioNTech. Dr. Ganesh reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
COVID-19 Incidence After Emergency Department Visit
At the onset of the COVID-19 pandemic, patient encounters with the health care system plummeted.1-3 The perceived increased risk of contracting COVID-19 while obtaining care was thought to be a contributing factor. In outpatient settings, one study noted a 63% decrease in visits to otolaryngology visits in Massachusetts, and another noted a 33% decrease in dental office visits at the onset of the pandemic in 2020 compared with the same time frame in 2019.2,4 Along with mask mandates and stay-at-home orders, various institutions sought to mitigate the spread of COVID-19 through different protocols, including the use of social distancing, limitation of visitors, and telehealth. Despite some of these measures, nosocomial infections were not uncommon. For example, one hospital in the United Kingdom reported that 15% of COVID-19 inpatient cases in a 6-week period in 2020 were probably or definitely hospital acquired. These patients had a 36% case fatality rate.5
Unlike outpatient treatment centers, however, the emergency department (ED) is mandated by the Emergency Medical Treatment and Labor Act to provide a medical screening examination and to stabilize emergency medical conditions to all patients presenting to the ED. Thus, high numbers of undifferentiated and symptomatic patients are forced to congregate in EDs, increasing the risk of transmission of COVID-19. This perception of increased risk led to a 42% decrease in ED visits during March and April 2020 at the onset of the COVID-19 pandemic.1 Correspondingly, there was a 20% decrease in code stroke activations at a hospital in Canada and a 38% decrease in ST-elevation myocardial infarction activations across 9 United States hospital systems.6,7
Limited studies have been conducted to date to determine whether contracting COVID-19 while in the ED is a risk. One retrospective case-control study evaluating 39 EDs in the US showed that ED colocation with known patients with COVID-19 was not associated with an increased risk of COVID-19 transmission.5 However, this study also recognized that infection control strategies widely varied by location and date.
In this study, we report the incidence of COVID-19 infections within 21 days after the initial visit for symptoms not associated with COVID-19 infection to the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) ED and compared it with that of COVID-19 infections for tests performed within the VAGLAHS.
Program Description
As a quality improvement measure, the
Patients with specific symptoms noted during triage, such as those associated with COVID-19 diagnosis, respiratory infections, fever, and/or myalgias, were isolated in their own patient room. Electronic tablets were used for persons under investigation and patients with COVID-19 to communicate with family and/or medical staff who did not need to enter the patient’s room. Two-hour disinfection protocols were instituted for high-risk patients who were moved during the course of their treatment (ie, transfer to another bed for admission or discharge). All staff was specifically trained in personal protective equipment (PPE) donning and doffing, and 2-physician airway teams were implemented to ensure proper PPE use and safe COVID-19 intubations.
COVID-19 Infections
Electronic health records of patients who visited the VAGLAHS ED for symptoms not related to COVID-19 were reviewed from
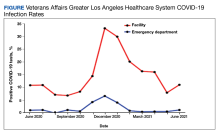
A total of 8708 patients who came to the ED with symptoms not associated with COVID-19 infection and had a COVID-19 test within 21 days of the ED visit met the inclusion criteria. The overall average positivity rate at the VAGLAHS ED for symptoms not associated with COVID-19 infection was 1.1% from June 1, 2020, to June 30, 2021. The positivity rate by month ranged from 0% to 6.7% for this period (Figure).
Discussion
Implementing COVID-19 mitigation measures in the VAGLAHS ED helped minimize exposure and subsequent infection of COVID-19 for veterans who visited the VAGLAHS ED with symptoms not associated with COVID-19 infection. Contextualizing this with the overall average monthly positivity rate of veterans in the VAGLAHS catchment area (10.9%) or Los Angeles County (7.9%) between June 1, 2020, to June 30, 2021, veterans who visited the VAGLAHS ED for symptoms not associated with COVID-19 infection were less likely to test positive for COVID-19 within 21 days (1.1%), suggesting that the extensive measures taken at the VAGLAHS ED were effective.8
Many health care systems in the US and abroad have experimented with different transmission mitigation strategies in the ED. These tactics have included careful resource allocation when PPE shortages occur, incorporation of airway teams with appropriate safety measures to reduce nosocomial spread to health care workers, and use of a cohorting plan to separate persons under investigation and patients with COVID-19 from other patients.9-15 Additionally, forward screening areas were incorporated similar to the COVID-19 tent that was instituted at the VAGLAHS ED to manage patients who were referred to the ED for COVID-19 testing during the beginning of the pandemic, which prevented symptomatic patients from congregating with asymptomatic patients.14,15
Encouragingly, some of these studies reported no cases of nosocomial transmission in the ED.11,13 In a separate study, 14 clusters of COVID-19 cases were identified at one VA health care system in which nosocomial transmission was suspected, including one in the ED.16 Using contact tracing, no patients and 9 employees were found to have contracted COVID-19 in that cluster. Overall, among all clusters examined within the health care system, either by contact tracing or by whole-genome sequencing, the authors found that transmission from health care personnel to patients was rare. Despite different methodologies, we also similarly found that ED patients in our VA facility were unlikely to become infected with COVID-19.
While the low incidence of positive COVID-19 tests cannot be attributed to any one method, our data provide a working blueprint for enhanced ED precautions in future surges of COVID-19 or other airborne diseases, including that of future pandemics.
Limitations
Notably, although the VA is the largest health care system in the US, a considerable number of veterans may present to non-VA EDs to seek care, and thus their data are not included here; these veterans may live farther from a VA facility or experience higher barriers to care than veterans who exclusively or almost exclusively seek care within the VA. As a result, we are unable to account for COVID-19 tests completed outside the VA. Moreover, the wild type SARS-CoV-2 virus was dominant during the time frame chosen for this assessment, and data may not be generalizable to other variants (eg, omicron) that are known to be more highly transmissible.17 Lastly, although our observation was performed at a single VA ED and may not apply to other facilities, especially in light of different mitigation strategies, our findings still provide support for approaches to minimizing patient and staff exposure to COVID-19 in ED settings.
Conclusions
Implementation of COVID-19 mitigation measures in the VAGLAHS ED may have minimized exposure to COVID-19 for veterans who visited the VAGLAHS ED for symptoms not associated with COVID-19 and did not put one at higher risk of contracting COVID-19. Taken together, our data suggest that patients should not avoid seeking emergency care out of fear of contracting COVID-19 if EDs have adequately instituted mitigation techniques.
1. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits—United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. doi:10.15585/mmwr.mm6923e1
2. Fan T, Workman AD, Miller LE, et al. The impact of COVID-19 on otolaryngology community practice in Massachusetts. Otolaryngol Head Neck Surg. 2021;165(3):424-430. doi:10.1177/0194599820983732
3. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
4. Kranz AM, Chen A, Gahlon G, Stein BD. 2020 trends in dental office visits during the COVID-19 pandemic. J Am Dent Assoc. 2021;152(7):535-541,e1. doi:10.1016/j.adaj.2021.02.01
5. Ridgway JP, Robicsek AA. Risk of coronavirus disease 2019 (COVID-19) acquisition among emergency department patients: a retrospective case control study. Infect Control Hosp Epidemiol. 2021;42(1):105-107. doi:10.1017/ice.2020.1224
6. Bres Bullrich M, Fridman S, Mandzia JL, et al. COVID-19: stroke admissions, emergency department visits, and prevention clinic referrals. Can J Neurol Sci. 2020;47(5):693-696. doi:10.1017/cjn.2020.101
7. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871-2872. doi:10.1016/j.jacc.2020.04.011
8. LA County COVID-19 Surveillance Dashboard. Accessed July 25, 2022. https://covid19.lacounty.gov/dashboards
9. Wallace DW, Burleson SL, Heimann MA, et al. An adapted emergency department triage algorithm for the COVID-19 pandemic. J Am Coll Emerg Physicians Open. 2020;1:1374-1379. doi:10.1002/emp2.12210
10. Montrief T, Ramzy M, Long B, Gottlieb M, Hercz D. COVID-19 respiratory support in the emergency department setting. Am Journal Emerg Med. 2020;38(10):2160-2168. doi:10.1016/j.ajem.2020.08.001
11. Alqahtani F, Alanazi M, Alassaf W, et al. Preventing SARS-CoV-2 transmission in the emergency department by implementing a separate pathway for patients with respiratory conditions. J Complement Integr Med. 2022;19(2):383-388. doi:10.1515/jcim-2020-0422
12. Odorizzi S, Clark E, Nemnom MJ, et al. Flow impacts of hot/cold zone infection control procedures during the COVID-19 pandemic in the emergency department. CJEM. 2022;24(4):390-396. doi:10.1007/s43678-022-00278-0
13. Wee LE, Fua TP, Chua YY, et al. Containing COVID-19 in the emergency department: the role of improved case detection and segregation of suspect cases. Acad Emerg Med. 2020;27(5):379-387. doi:10.1111/acem.13984
14. Tan RMR, Ong GYK, Chong SL, Ganapathy S, Tyebally A, Lee KP. Dynamic adaptation to COVID-19 in a Singapore paediatric emergency department. Emerg Med J. 2020;37(5):252-254. doi:10.1136/emermed-2020-20963
15. Quah LJJ, Tan BKK, Fua TP, et al. Reorganising the emergency department to manage the COVID-19 outbreak. Int J Emerg Med. 2020;13(1):32. doi:10.1186/s12245-020-00294-w
16. Jinadatha C, Jones LD, Choi H, et al. Transmission of SARS-CoV-2 in inpatient and outpatient settings in a Veterans Affairs health care system. Open Forum Infect Dis. 2021;8(8):ofab328. doi:10.1093/ofid/ofab328
17. Riediker M, Briceno-Ayala L, Ichihara G, et al. Higher viral load and infectivity increase risk of aerosol transmission for Delta and Omicron variants of SARS-CoV-2. Swiss Med Wkly. 2022;152:w30133. doi:10.4414/smw.2022.w30133
At the onset of the COVID-19 pandemic, patient encounters with the health care system plummeted.1-3 The perceived increased risk of contracting COVID-19 while obtaining care was thought to be a contributing factor. In outpatient settings, one study noted a 63% decrease in visits to otolaryngology visits in Massachusetts, and another noted a 33% decrease in dental office visits at the onset of the pandemic in 2020 compared with the same time frame in 2019.2,4 Along with mask mandates and stay-at-home orders, various institutions sought to mitigate the spread of COVID-19 through different protocols, including the use of social distancing, limitation of visitors, and telehealth. Despite some of these measures, nosocomial infections were not uncommon. For example, one hospital in the United Kingdom reported that 15% of COVID-19 inpatient cases in a 6-week period in 2020 were probably or definitely hospital acquired. These patients had a 36% case fatality rate.5
Unlike outpatient treatment centers, however, the emergency department (ED) is mandated by the Emergency Medical Treatment and Labor Act to provide a medical screening examination and to stabilize emergency medical conditions to all patients presenting to the ED. Thus, high numbers of undifferentiated and symptomatic patients are forced to congregate in EDs, increasing the risk of transmission of COVID-19. This perception of increased risk led to a 42% decrease in ED visits during March and April 2020 at the onset of the COVID-19 pandemic.1 Correspondingly, there was a 20% decrease in code stroke activations at a hospital in Canada and a 38% decrease in ST-elevation myocardial infarction activations across 9 United States hospital systems.6,7
Limited studies have been conducted to date to determine whether contracting COVID-19 while in the ED is a risk. One retrospective case-control study evaluating 39 EDs in the US showed that ED colocation with known patients with COVID-19 was not associated with an increased risk of COVID-19 transmission.5 However, this study also recognized that infection control strategies widely varied by location and date.
In this study, we report the incidence of COVID-19 infections within 21 days after the initial visit for symptoms not associated with COVID-19 infection to the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) ED and compared it with that of COVID-19 infections for tests performed within the VAGLAHS.
Program Description
As a quality improvement measure, the
Patients with specific symptoms noted during triage, such as those associated with COVID-19 diagnosis, respiratory infections, fever, and/or myalgias, were isolated in their own patient room. Electronic tablets were used for persons under investigation and patients with COVID-19 to communicate with family and/or medical staff who did not need to enter the patient’s room. Two-hour disinfection protocols were instituted for high-risk patients who were moved during the course of their treatment (ie, transfer to another bed for admission or discharge). All staff was specifically trained in personal protective equipment (PPE) donning and doffing, and 2-physician airway teams were implemented to ensure proper PPE use and safe COVID-19 intubations.
COVID-19 Infections
Electronic health records of patients who visited the VAGLAHS ED for symptoms not related to COVID-19 were reviewed from
A total of 8708 patients who came to the ED with symptoms not associated with COVID-19 infection and had a COVID-19 test within 21 days of the ED visit met the inclusion criteria. The overall average positivity rate at the VAGLAHS ED for symptoms not associated with COVID-19 infection was 1.1% from June 1, 2020, to June 30, 2021. The positivity rate by month ranged from 0% to 6.7% for this period (Figure).
Discussion
Implementing COVID-19 mitigation measures in the VAGLAHS ED helped minimize exposure and subsequent infection of COVID-19 for veterans who visited the VAGLAHS ED with symptoms not associated with COVID-19 infection. Contextualizing this with the overall average monthly positivity rate of veterans in the VAGLAHS catchment area (10.9%) or Los Angeles County (7.9%) between June 1, 2020, to June 30, 2021, veterans who visited the VAGLAHS ED for symptoms not associated with COVID-19 infection were less likely to test positive for COVID-19 within 21 days (1.1%), suggesting that the extensive measures taken at the VAGLAHS ED were effective.8
Many health care systems in the US and abroad have experimented with different transmission mitigation strategies in the ED. These tactics have included careful resource allocation when PPE shortages occur, incorporation of airway teams with appropriate safety measures to reduce nosocomial spread to health care workers, and use of a cohorting plan to separate persons under investigation and patients with COVID-19 from other patients.9-15 Additionally, forward screening areas were incorporated similar to the COVID-19 tent that was instituted at the VAGLAHS ED to manage patients who were referred to the ED for COVID-19 testing during the beginning of the pandemic, which prevented symptomatic patients from congregating with asymptomatic patients.14,15
Encouragingly, some of these studies reported no cases of nosocomial transmission in the ED.11,13 In a separate study, 14 clusters of COVID-19 cases were identified at one VA health care system in which nosocomial transmission was suspected, including one in the ED.16 Using contact tracing, no patients and 9 employees were found to have contracted COVID-19 in that cluster. Overall, among all clusters examined within the health care system, either by contact tracing or by whole-genome sequencing, the authors found that transmission from health care personnel to patients was rare. Despite different methodologies, we also similarly found that ED patients in our VA facility were unlikely to become infected with COVID-19.
While the low incidence of positive COVID-19 tests cannot be attributed to any one method, our data provide a working blueprint for enhanced ED precautions in future surges of COVID-19 or other airborne diseases, including that of future pandemics.
Limitations
Notably, although the VA is the largest health care system in the US, a considerable number of veterans may present to non-VA EDs to seek care, and thus their data are not included here; these veterans may live farther from a VA facility or experience higher barriers to care than veterans who exclusively or almost exclusively seek care within the VA. As a result, we are unable to account for COVID-19 tests completed outside the VA. Moreover, the wild type SARS-CoV-2 virus was dominant during the time frame chosen for this assessment, and data may not be generalizable to other variants (eg, omicron) that are known to be more highly transmissible.17 Lastly, although our observation was performed at a single VA ED and may not apply to other facilities, especially in light of different mitigation strategies, our findings still provide support for approaches to minimizing patient and staff exposure to COVID-19 in ED settings.
Conclusions
Implementation of COVID-19 mitigation measures in the VAGLAHS ED may have minimized exposure to COVID-19 for veterans who visited the VAGLAHS ED for symptoms not associated with COVID-19 and did not put one at higher risk of contracting COVID-19. Taken together, our data suggest that patients should not avoid seeking emergency care out of fear of contracting COVID-19 if EDs have adequately instituted mitigation techniques.
At the onset of the COVID-19 pandemic, patient encounters with the health care system plummeted.1-3 The perceived increased risk of contracting COVID-19 while obtaining care was thought to be a contributing factor. In outpatient settings, one study noted a 63% decrease in visits to otolaryngology visits in Massachusetts, and another noted a 33% decrease in dental office visits at the onset of the pandemic in 2020 compared with the same time frame in 2019.2,4 Along with mask mandates and stay-at-home orders, various institutions sought to mitigate the spread of COVID-19 through different protocols, including the use of social distancing, limitation of visitors, and telehealth. Despite some of these measures, nosocomial infections were not uncommon. For example, one hospital in the United Kingdom reported that 15% of COVID-19 inpatient cases in a 6-week period in 2020 were probably or definitely hospital acquired. These patients had a 36% case fatality rate.5
Unlike outpatient treatment centers, however, the emergency department (ED) is mandated by the Emergency Medical Treatment and Labor Act to provide a medical screening examination and to stabilize emergency medical conditions to all patients presenting to the ED. Thus, high numbers of undifferentiated and symptomatic patients are forced to congregate in EDs, increasing the risk of transmission of COVID-19. This perception of increased risk led to a 42% decrease in ED visits during March and April 2020 at the onset of the COVID-19 pandemic.1 Correspondingly, there was a 20% decrease in code stroke activations at a hospital in Canada and a 38% decrease in ST-elevation myocardial infarction activations across 9 United States hospital systems.6,7
Limited studies have been conducted to date to determine whether contracting COVID-19 while in the ED is a risk. One retrospective case-control study evaluating 39 EDs in the US showed that ED colocation with known patients with COVID-19 was not associated with an increased risk of COVID-19 transmission.5 However, this study also recognized that infection control strategies widely varied by location and date.
In this study, we report the incidence of COVID-19 infections within 21 days after the initial visit for symptoms not associated with COVID-19 infection to the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS) ED and compared it with that of COVID-19 infections for tests performed within the VAGLAHS.
Program Description
As a quality improvement measure, the
Patients with specific symptoms noted during triage, such as those associated with COVID-19 diagnosis, respiratory infections, fever, and/or myalgias, were isolated in their own patient room. Electronic tablets were used for persons under investigation and patients with COVID-19 to communicate with family and/or medical staff who did not need to enter the patient’s room. Two-hour disinfection protocols were instituted for high-risk patients who were moved during the course of their treatment (ie, transfer to another bed for admission or discharge). All staff was specifically trained in personal protective equipment (PPE) donning and doffing, and 2-physician airway teams were implemented to ensure proper PPE use and safe COVID-19 intubations.
COVID-19 Infections
Electronic health records of patients who visited the VAGLAHS ED for symptoms not related to COVID-19 were reviewed from
A total of 8708 patients who came to the ED with symptoms not associated with COVID-19 infection and had a COVID-19 test within 21 days of the ED visit met the inclusion criteria. The overall average positivity rate at the VAGLAHS ED for symptoms not associated with COVID-19 infection was 1.1% from June 1, 2020, to June 30, 2021. The positivity rate by month ranged from 0% to 6.7% for this period (Figure).
Discussion
Implementing COVID-19 mitigation measures in the VAGLAHS ED helped minimize exposure and subsequent infection of COVID-19 for veterans who visited the VAGLAHS ED with symptoms not associated with COVID-19 infection. Contextualizing this with the overall average monthly positivity rate of veterans in the VAGLAHS catchment area (10.9%) or Los Angeles County (7.9%) between June 1, 2020, to June 30, 2021, veterans who visited the VAGLAHS ED for symptoms not associated with COVID-19 infection were less likely to test positive for COVID-19 within 21 days (1.1%), suggesting that the extensive measures taken at the VAGLAHS ED were effective.8
Many health care systems in the US and abroad have experimented with different transmission mitigation strategies in the ED. These tactics have included careful resource allocation when PPE shortages occur, incorporation of airway teams with appropriate safety measures to reduce nosocomial spread to health care workers, and use of a cohorting plan to separate persons under investigation and patients with COVID-19 from other patients.9-15 Additionally, forward screening areas were incorporated similar to the COVID-19 tent that was instituted at the VAGLAHS ED to manage patients who were referred to the ED for COVID-19 testing during the beginning of the pandemic, which prevented symptomatic patients from congregating with asymptomatic patients.14,15
Encouragingly, some of these studies reported no cases of nosocomial transmission in the ED.11,13 In a separate study, 14 clusters of COVID-19 cases were identified at one VA health care system in which nosocomial transmission was suspected, including one in the ED.16 Using contact tracing, no patients and 9 employees were found to have contracted COVID-19 in that cluster. Overall, among all clusters examined within the health care system, either by contact tracing or by whole-genome sequencing, the authors found that transmission from health care personnel to patients was rare. Despite different methodologies, we also similarly found that ED patients in our VA facility were unlikely to become infected with COVID-19.
While the low incidence of positive COVID-19 tests cannot be attributed to any one method, our data provide a working blueprint for enhanced ED precautions in future surges of COVID-19 or other airborne diseases, including that of future pandemics.
Limitations
Notably, although the VA is the largest health care system in the US, a considerable number of veterans may present to non-VA EDs to seek care, and thus their data are not included here; these veterans may live farther from a VA facility or experience higher barriers to care than veterans who exclusively or almost exclusively seek care within the VA. As a result, we are unable to account for COVID-19 tests completed outside the VA. Moreover, the wild type SARS-CoV-2 virus was dominant during the time frame chosen for this assessment, and data may not be generalizable to other variants (eg, omicron) that are known to be more highly transmissible.17 Lastly, although our observation was performed at a single VA ED and may not apply to other facilities, especially in light of different mitigation strategies, our findings still provide support for approaches to minimizing patient and staff exposure to COVID-19 in ED settings.
Conclusions
Implementation of COVID-19 mitigation measures in the VAGLAHS ED may have minimized exposure to COVID-19 for veterans who visited the VAGLAHS ED for symptoms not associated with COVID-19 and did not put one at higher risk of contracting COVID-19. Taken together, our data suggest that patients should not avoid seeking emergency care out of fear of contracting COVID-19 if EDs have adequately instituted mitigation techniques.
1. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits—United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. doi:10.15585/mmwr.mm6923e1
2. Fan T, Workman AD, Miller LE, et al. The impact of COVID-19 on otolaryngology community practice in Massachusetts. Otolaryngol Head Neck Surg. 2021;165(3):424-430. doi:10.1177/0194599820983732
3. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
4. Kranz AM, Chen A, Gahlon G, Stein BD. 2020 trends in dental office visits during the COVID-19 pandemic. J Am Dent Assoc. 2021;152(7):535-541,e1. doi:10.1016/j.adaj.2021.02.01
5. Ridgway JP, Robicsek AA. Risk of coronavirus disease 2019 (COVID-19) acquisition among emergency department patients: a retrospective case control study. Infect Control Hosp Epidemiol. 2021;42(1):105-107. doi:10.1017/ice.2020.1224
6. Bres Bullrich M, Fridman S, Mandzia JL, et al. COVID-19: stroke admissions, emergency department visits, and prevention clinic referrals. Can J Neurol Sci. 2020;47(5):693-696. doi:10.1017/cjn.2020.101
7. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871-2872. doi:10.1016/j.jacc.2020.04.011
8. LA County COVID-19 Surveillance Dashboard. Accessed July 25, 2022. https://covid19.lacounty.gov/dashboards
9. Wallace DW, Burleson SL, Heimann MA, et al. An adapted emergency department triage algorithm for the COVID-19 pandemic. J Am Coll Emerg Physicians Open. 2020;1:1374-1379. doi:10.1002/emp2.12210
10. Montrief T, Ramzy M, Long B, Gottlieb M, Hercz D. COVID-19 respiratory support in the emergency department setting. Am Journal Emerg Med. 2020;38(10):2160-2168. doi:10.1016/j.ajem.2020.08.001
11. Alqahtani F, Alanazi M, Alassaf W, et al. Preventing SARS-CoV-2 transmission in the emergency department by implementing a separate pathway for patients with respiratory conditions. J Complement Integr Med. 2022;19(2):383-388. doi:10.1515/jcim-2020-0422
12. Odorizzi S, Clark E, Nemnom MJ, et al. Flow impacts of hot/cold zone infection control procedures during the COVID-19 pandemic in the emergency department. CJEM. 2022;24(4):390-396. doi:10.1007/s43678-022-00278-0
13. Wee LE, Fua TP, Chua YY, et al. Containing COVID-19 in the emergency department: the role of improved case detection and segregation of suspect cases. Acad Emerg Med. 2020;27(5):379-387. doi:10.1111/acem.13984
14. Tan RMR, Ong GYK, Chong SL, Ganapathy S, Tyebally A, Lee KP. Dynamic adaptation to COVID-19 in a Singapore paediatric emergency department. Emerg Med J. 2020;37(5):252-254. doi:10.1136/emermed-2020-20963
15. Quah LJJ, Tan BKK, Fua TP, et al. Reorganising the emergency department to manage the COVID-19 outbreak. Int J Emerg Med. 2020;13(1):32. doi:10.1186/s12245-020-00294-w
16. Jinadatha C, Jones LD, Choi H, et al. Transmission of SARS-CoV-2 in inpatient and outpatient settings in a Veterans Affairs health care system. Open Forum Infect Dis. 2021;8(8):ofab328. doi:10.1093/ofid/ofab328
17. Riediker M, Briceno-Ayala L, Ichihara G, et al. Higher viral load and infectivity increase risk of aerosol transmission for Delta and Omicron variants of SARS-CoV-2. Swiss Med Wkly. 2022;152:w30133. doi:10.4414/smw.2022.w30133
1. Hartnett KP, Kite-Powell A, DeVies J, et al; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 pandemic on emergency department visits—United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(23):699-704. doi:10.15585/mmwr.mm6923e1
2. Fan T, Workman AD, Miller LE, et al. The impact of COVID-19 on otolaryngology community practice in Massachusetts. Otolaryngol Head Neck Surg. 2021;165(3):424-430. doi:10.1177/0194599820983732
3. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
4. Kranz AM, Chen A, Gahlon G, Stein BD. 2020 trends in dental office visits during the COVID-19 pandemic. J Am Dent Assoc. 2021;152(7):535-541,e1. doi:10.1016/j.adaj.2021.02.01
5. Ridgway JP, Robicsek AA. Risk of coronavirus disease 2019 (COVID-19) acquisition among emergency department patients: a retrospective case control study. Infect Control Hosp Epidemiol. 2021;42(1):105-107. doi:10.1017/ice.2020.1224
6. Bres Bullrich M, Fridman S, Mandzia JL, et al. COVID-19: stroke admissions, emergency department visits, and prevention clinic referrals. Can J Neurol Sci. 2020;47(5):693-696. doi:10.1017/cjn.2020.101
7. Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75(22):2871-2872. doi:10.1016/j.jacc.2020.04.011
8. LA County COVID-19 Surveillance Dashboard. Accessed July 25, 2022. https://covid19.lacounty.gov/dashboards
9. Wallace DW, Burleson SL, Heimann MA, et al. An adapted emergency department triage algorithm for the COVID-19 pandemic. J Am Coll Emerg Physicians Open. 2020;1:1374-1379. doi:10.1002/emp2.12210
10. Montrief T, Ramzy M, Long B, Gottlieb M, Hercz D. COVID-19 respiratory support in the emergency department setting. Am Journal Emerg Med. 2020;38(10):2160-2168. doi:10.1016/j.ajem.2020.08.001
11. Alqahtani F, Alanazi M, Alassaf W, et al. Preventing SARS-CoV-2 transmission in the emergency department by implementing a separate pathway for patients with respiratory conditions. J Complement Integr Med. 2022;19(2):383-388. doi:10.1515/jcim-2020-0422
12. Odorizzi S, Clark E, Nemnom MJ, et al. Flow impacts of hot/cold zone infection control procedures during the COVID-19 pandemic in the emergency department. CJEM. 2022;24(4):390-396. doi:10.1007/s43678-022-00278-0
13. Wee LE, Fua TP, Chua YY, et al. Containing COVID-19 in the emergency department: the role of improved case detection and segregation of suspect cases. Acad Emerg Med. 2020;27(5):379-387. doi:10.1111/acem.13984
14. Tan RMR, Ong GYK, Chong SL, Ganapathy S, Tyebally A, Lee KP. Dynamic adaptation to COVID-19 in a Singapore paediatric emergency department. Emerg Med J. 2020;37(5):252-254. doi:10.1136/emermed-2020-20963
15. Quah LJJ, Tan BKK, Fua TP, et al. Reorganising the emergency department to manage the COVID-19 outbreak. Int J Emerg Med. 2020;13(1):32. doi:10.1186/s12245-020-00294-w
16. Jinadatha C, Jones LD, Choi H, et al. Transmission of SARS-CoV-2 in inpatient and outpatient settings in a Veterans Affairs health care system. Open Forum Infect Dis. 2021;8(8):ofab328. doi:10.1093/ofid/ofab328
17. Riediker M, Briceno-Ayala L, Ichihara G, et al. Higher viral load and infectivity increase risk of aerosol transmission for Delta and Omicron variants of SARS-CoV-2. Swiss Med Wkly. 2022;152:w30133. doi:10.4414/smw.2022.w30133
Dermatology Author Gender Trends During the COVID-19 Pandemic
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.
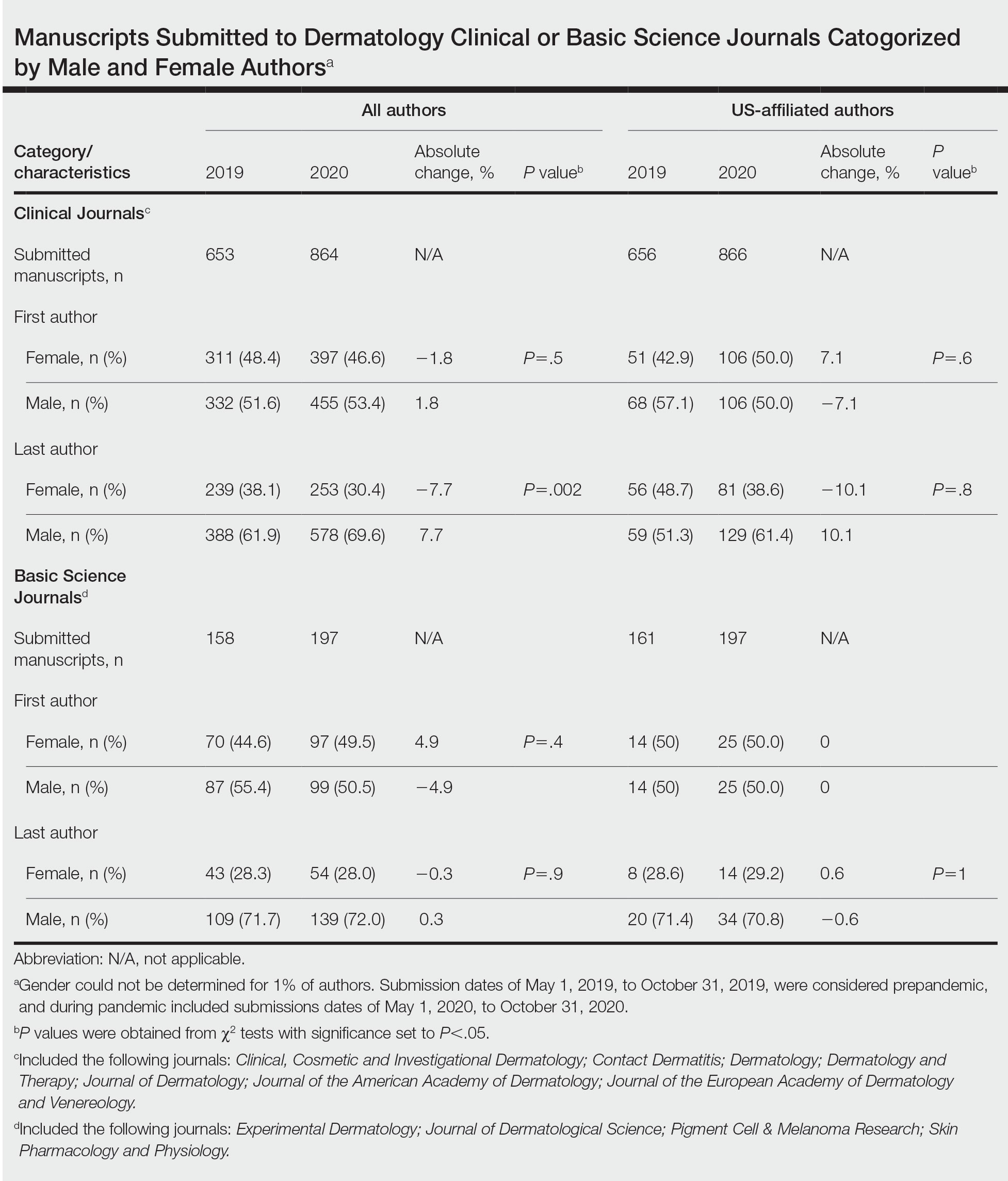
Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.

Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
To the Editor:
Peer-reviewed publications are important determinants for promotions, academic leadership, and grants in dermatology.1 The impact of the COVID-19 pandemic on dermatology research productivity remains an area of investigation. We sought to determine authorship trends for males and females during the pandemic.
A cross-sectional retrospective study of the top 20 dermatology journals—determined by impact factor and Google Scholar H5-index—was conducted to identify manuscripts with submission date specified prepandemic (May 1, 2019–October 31, 2019) and during the pandemic (May 1, 2020–October 31, 2020). Submission date, first/last author name, sex, and affiliated country were extracted. Single authors were designated as first authors. Gender API (https://gender-api.com/en/) classified gender. A χ2 test (P<.05) compared differences in proportions of female first/last authors from 2019 to 2020.
Overall, 811 and 1061 articles submitted in 2019 and 2020, respectively, were included. There were 1517 articles submitted to clinical journals and 355 articles submitted to basic science journals (Table). For the 7 clinical journals included, there was a 7.7% decrease in the proportion of female last authors in 2020 vs 2019 (P=.002), with the largest decrease between August and September 2020. Although other comparisons did not yield statistically significant differences (P>.05 all)(Table), several trends were observed. For clinical journals, there was a 1.8% decrease in the proportion of female first authors. For the 4 basic science journals included, there was a 4.9% increase and a 0.3% decrease in percentages of female first and last authors, respectively, for 2020 vs 2019.

Our findings indicate that the COVID-19 pandemic may have impacted female authors’ productivity in clinical dermatology publications. In a survey-based study for 2010 to 2011, female physician-researchers (n=437) spent 8.5 more hours per week on domestic activities and childcare and were more likely to take time off for childcare if their partner worked full time compared with males (n=612)(42.6% vs 12.4%, respectively).2 Our observation that female last authors had a significant decrease in publications may suggest that this population had a disproportionate burden of domestic labor and childcare during the pandemic. It is possible that last authors, who generally are more senior researchers, may be more likely to have childcare, eldercare, and other types of domestic responsibilities. Similarly, in a study of surgery submissions (n=1068), there were 6%, 7%, and 4% decreases in percentages of female last, corresponding, and first authors, respectively, from 2019 to 2020.3Our study had limitations. Only 11 journals were analyzed because others did not have specified submission dates. Some journals only provided submission information for a subset of articles (eg, those published in the In Press section), which may have accounted for the large discrepancy in submission numbers for 2019 to 2020. Gender could not be determined for 1% of authors and was limited to female and male. Although our study submission time frame (May–October 2020) aimed at identifying research conducted during the height of the COVID-19 pandemic, some of these studies may have been conducted months or years before the pandemic. Future studies should focus on longer and more comprehensive time frames. Finally, estimated dates of stay-at-home orders fail to consider differences within countries.
The proportion of female US-affiliated first and last authors publishing in dermatology journals increased from 12% to 48% in 1976 and from 6% to 31% in 2006,4 which is encouraging. However, a gender gap persists, with one-third of National Institutes of Health grants in dermatology and one-fourth of research project grants in dermatology awarded to women.5 Consequences of the pandemic on academic productivity may include fewer women represented in higher academic ranks, lower compensation, and lower career satisfaction compared with men.1 We urge academic institutions and funding agencies to recognize and take action to mitigate long-term sequelae. Extended grant end dates and submission periods, funding opportunities dedicated to women, and prioritization of female-authored submissions are some strategies that can safeguard equitable career progression in dermatology research.
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
- Stewart C, Lipner SR. Gender and race trends in academic rank of dermatologists at top U.S. institutions: a cross-sectional study. Int J Womens Dermatol. 2020;6:283-285. doi:10.1016/j .ijwd.2020.04.010
- Jolly S, Griffith KA, DeCastro R, et al. Gender differences in time spent on parenting and domestic responsibilities by highachieving young physician-researchers. Ann Intern Med. 2014; 160:344-353. doi:10.7326/M13-0974
- Kibbe MR. Consequences of the COVID-19 pandemic on manuscript submissions by women. JAMA Surg. 2020;155:803-804. doi:10.1001/jamasurg.2020.3917
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;6:63-69. doi:10.1016/j.jaad.2008.06.044
- Cheng MY, Sukhov A, Sultani H, et al. Trends in national institutes of health funding of principal investigators in dermatology research by academic degree and sex. JAMA Dermatol. 2016;152:883-888. doi:10.1001/jamadermatol.2016.0271
Practice Points
- The academic productivity of female dermatologists as last authors in dermatology clinical journals has potentially been impacted by the COVID-19 pandemic.
- To potentially aid in the resurgence of female dermatologist authors impacted by the pandemic, academic institutions and funding agencies may consider implementing strategies such as extending grant end dates, providing dedicated funding opportunities, and prioritizing female-authored submissions in dermatology research.
Agency issues advisory on mental health symptoms of long COVID
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
The nine mental health symptoms highlighted in the advisory are fatigue; cognitive impairment, including brain fog; anxiety; depression; obsessive-compulsive disorder; sleep disorders; PTSD; psychotic disorder; and start of a substance use disorder.
The advisory noted that social factors can contribute to the mental health problems for racial and ethnic minorities; people with limited access to health care; people who already have behavioral health conditions and physical disabilities; and people who are lesbian, gay, bisexual, transgender, queer, or intersex.
“Long COVID has a range of burdensome physical symptoms and can take a toll on a person’s mental health. It can be very challenging for a person, whether they are impacted themselves, or they are a caregiver for someone who is affected,” Health and Human Services Secretary Xavier Becerra said in a statement. “This advisory helps to raise awareness, especially among primary care practitioners and clinicians who are often the ones treating patients with long COVID.”
The department says about 10% of people infected with COVID have at least one long COVID symptom. Physical symptoms include dizziness, stomach upset, heart palpitations, issues with sexual desire or capacity, loss of smell or taste, thirst, chronic coughing, chest pain, and abnormal movements.
“We know that people living with long COVID need help today, and providers need help understanding what long COVID is and how to treat it,” Admiral Rachel Levine, MD, assistant secretary for health, said in the statement. “This advisory helps bridge that gap for the behavioral health impacts of long COVID.”
A version of this article first appeared on WebMD.com.
International rights group calls out United States for allowing hospitals to push millions into debt
Human Rights Watch, the nonprofit that for decades has called attention to the victims of war, famine, and political repression around the world, is taking aim at U.S. hospitals for pushing millions of American patients into debt.
In a new report, the group calls for stronger government action to protect Americans from aggressive billing and debt collection by nonprofit hospitals, which Human Rights Watch said are systematically undermining patients’ human rights.
“Given the high prevalence of hospital-related medical debt in the U.S., this system is clearly not working,” concludes the report, which draws extensively on an ongoing investigation of medical debt by KFF Health News and NPR.
The report continues: “The U.S. model of subsidizing privately operated hospitals with tax exemptions in the hope that they will increase the accessibility of hospital care for un- and underinsured patients allows for abusive medical billing and debt collection practices and undermines human rights, including the right to health.”
Nationwide, about 100 million people – or 41% of adults – have some form of health care debt, a KFF survey conducted for the KFF Health News–NPR project found. And while patient debt is being driven by a range of medical and dental bills, polls and studies suggest hospitals are a major contributor.
About a third of U.S. adults with health care debt owed money for hospitalization, KFF’s polling found. Close to half of those owed at least $5,000. About a quarter owed $10,000 or more.
The scale of this crisis – which is unparalleled among wealthy nations – compelled Human Rights Watch to release the new report, said researcher Matt McConnell, its author. “Historically, Human Rights Watch has been an organization that has focused on international human rights issues,” he said. “But on medical debt, the U.S. is a real outlier. What you see is a system that privileges a few but creates large barriers to people accessing basic health rights.”
Hospital industry officials defend their work, citing hospitals’ broader work to help the communities they serve. “As a field, hospitals provide more benefit to their communities than any other sector in health care,” Melinda Hatton, general counsel at the American Hospital Association, wrote in a response to the Human Right Watch report.
Federal law requires private, tax-exempt hospitals – which make up more than half the nation’s medical centers – to provide care at no cost or at a discount to low-income patients. But reporting by KFF Health News and others has found that many hospitals make this aid difficult for patients to get.
At the same time, thousands of medical centers – including many tax-exempt ones – engage in aggressive debt collection tactics to pursue patients, including garnishing patients’ wages, placing liens on their homes, or selling their debt to third-party debt collectors.
Overall, KFF Health News found that most of the nation’s approximately 5,100 hospitals serving the general public have policies to use legal action or other aggressive tactics against patients. And one in five will deny nonemergency care to people with outstanding debt.
“Medical debt is drowning many low-income and working families while hospitals continue to benefit from nonprofit tax status as they pursue families for medical debt,” said Marceline White, executive director of Economic Action Maryland. The advocacy group has helped enact tighter rules to ensure Maryland hospitals make financial assistance more easily accessible and to restrict hospitals from some aggressive debt collection tactics, such as placing liens on patients’ homes.
Similar efforts are underway in other states, including Colorado, New Mexico, New York, Oregon, and Washington. But many patient and consumer advocates say stronger federal action is needed to expand patient protections.
The Human Rights Watch report – titled “In Sheep’s Clothing: United States’ Poorly Regulated Nonprofit Hospitals Undermine Health Care Access” – lists more than a dozen recommendations. These include:
- Congress should pass legislation to ensure that hospitals provide at least the same amount of charity care as they receive in public subsidies.
- The IRS should set uniform national standards on patients’ eligibility for financial assistance at nonprofit hospitals. Currently, hospitals are free to set their own standards, resulting in widespread variation, which can confuse patients.
- The Consumer Financial Protection Bureau, a federal watchdog agency, should crack down on debt collectors that do not ensure that patients have been screened for financial assistance before being pursued.
- The federal Centers for Medicare & Medicaid Services, which administers the two mammoth public insurance programs, should penalize hospitals that do not provide adequate financial assistance to patients.
“Nonprofit hospitals are contributing to medical debt and engaging in abusive billing and debt collection practices,” Mr. McConnell said. “The reason this keeps happening is the absence of clear guidelines and the federal government’s inadequate enforcement of existing regulations.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Human Rights Watch, the nonprofit that for decades has called attention to the victims of war, famine, and political repression around the world, is taking aim at U.S. hospitals for pushing millions of American patients into debt.
In a new report, the group calls for stronger government action to protect Americans from aggressive billing and debt collection by nonprofit hospitals, which Human Rights Watch said are systematically undermining patients’ human rights.
“Given the high prevalence of hospital-related medical debt in the U.S., this system is clearly not working,” concludes the report, which draws extensively on an ongoing investigation of medical debt by KFF Health News and NPR.
The report continues: “The U.S. model of subsidizing privately operated hospitals with tax exemptions in the hope that they will increase the accessibility of hospital care for un- and underinsured patients allows for abusive medical billing and debt collection practices and undermines human rights, including the right to health.”
Nationwide, about 100 million people – or 41% of adults – have some form of health care debt, a KFF survey conducted for the KFF Health News–NPR project found. And while patient debt is being driven by a range of medical and dental bills, polls and studies suggest hospitals are a major contributor.
About a third of U.S. adults with health care debt owed money for hospitalization, KFF’s polling found. Close to half of those owed at least $5,000. About a quarter owed $10,000 or more.
The scale of this crisis – which is unparalleled among wealthy nations – compelled Human Rights Watch to release the new report, said researcher Matt McConnell, its author. “Historically, Human Rights Watch has been an organization that has focused on international human rights issues,” he said. “But on medical debt, the U.S. is a real outlier. What you see is a system that privileges a few but creates large barriers to people accessing basic health rights.”
Hospital industry officials defend their work, citing hospitals’ broader work to help the communities they serve. “As a field, hospitals provide more benefit to their communities than any other sector in health care,” Melinda Hatton, general counsel at the American Hospital Association, wrote in a response to the Human Right Watch report.
Federal law requires private, tax-exempt hospitals – which make up more than half the nation’s medical centers – to provide care at no cost or at a discount to low-income patients. But reporting by KFF Health News and others has found that many hospitals make this aid difficult for patients to get.
At the same time, thousands of medical centers – including many tax-exempt ones – engage in aggressive debt collection tactics to pursue patients, including garnishing patients’ wages, placing liens on their homes, or selling their debt to third-party debt collectors.
Overall, KFF Health News found that most of the nation’s approximately 5,100 hospitals serving the general public have policies to use legal action or other aggressive tactics against patients. And one in five will deny nonemergency care to people with outstanding debt.
“Medical debt is drowning many low-income and working families while hospitals continue to benefit from nonprofit tax status as they pursue families for medical debt,” said Marceline White, executive director of Economic Action Maryland. The advocacy group has helped enact tighter rules to ensure Maryland hospitals make financial assistance more easily accessible and to restrict hospitals from some aggressive debt collection tactics, such as placing liens on patients’ homes.
Similar efforts are underway in other states, including Colorado, New Mexico, New York, Oregon, and Washington. But many patient and consumer advocates say stronger federal action is needed to expand patient protections.
The Human Rights Watch report – titled “In Sheep’s Clothing: United States’ Poorly Regulated Nonprofit Hospitals Undermine Health Care Access” – lists more than a dozen recommendations. These include:
- Congress should pass legislation to ensure that hospitals provide at least the same amount of charity care as they receive in public subsidies.
- The IRS should set uniform national standards on patients’ eligibility for financial assistance at nonprofit hospitals. Currently, hospitals are free to set their own standards, resulting in widespread variation, which can confuse patients.
- The Consumer Financial Protection Bureau, a federal watchdog agency, should crack down on debt collectors that do not ensure that patients have been screened for financial assistance before being pursued.
- The federal Centers for Medicare & Medicaid Services, which administers the two mammoth public insurance programs, should penalize hospitals that do not provide adequate financial assistance to patients.
“Nonprofit hospitals are contributing to medical debt and engaging in abusive billing and debt collection practices,” Mr. McConnell said. “The reason this keeps happening is the absence of clear guidelines and the federal government’s inadequate enforcement of existing regulations.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Human Rights Watch, the nonprofit that for decades has called attention to the victims of war, famine, and political repression around the world, is taking aim at U.S. hospitals for pushing millions of American patients into debt.
In a new report, the group calls for stronger government action to protect Americans from aggressive billing and debt collection by nonprofit hospitals, which Human Rights Watch said are systematically undermining patients’ human rights.
“Given the high prevalence of hospital-related medical debt in the U.S., this system is clearly not working,” concludes the report, which draws extensively on an ongoing investigation of medical debt by KFF Health News and NPR.
The report continues: “The U.S. model of subsidizing privately operated hospitals with tax exemptions in the hope that they will increase the accessibility of hospital care for un- and underinsured patients allows for abusive medical billing and debt collection practices and undermines human rights, including the right to health.”
Nationwide, about 100 million people – or 41% of adults – have some form of health care debt, a KFF survey conducted for the KFF Health News–NPR project found. And while patient debt is being driven by a range of medical and dental bills, polls and studies suggest hospitals are a major contributor.
About a third of U.S. adults with health care debt owed money for hospitalization, KFF’s polling found. Close to half of those owed at least $5,000. About a quarter owed $10,000 or more.
The scale of this crisis – which is unparalleled among wealthy nations – compelled Human Rights Watch to release the new report, said researcher Matt McConnell, its author. “Historically, Human Rights Watch has been an organization that has focused on international human rights issues,” he said. “But on medical debt, the U.S. is a real outlier. What you see is a system that privileges a few but creates large barriers to people accessing basic health rights.”
Hospital industry officials defend their work, citing hospitals’ broader work to help the communities they serve. “As a field, hospitals provide more benefit to their communities than any other sector in health care,” Melinda Hatton, general counsel at the American Hospital Association, wrote in a response to the Human Right Watch report.
Federal law requires private, tax-exempt hospitals – which make up more than half the nation’s medical centers – to provide care at no cost or at a discount to low-income patients. But reporting by KFF Health News and others has found that many hospitals make this aid difficult for patients to get.
At the same time, thousands of medical centers – including many tax-exempt ones – engage in aggressive debt collection tactics to pursue patients, including garnishing patients’ wages, placing liens on their homes, or selling their debt to third-party debt collectors.
Overall, KFF Health News found that most of the nation’s approximately 5,100 hospitals serving the general public have policies to use legal action or other aggressive tactics against patients. And one in five will deny nonemergency care to people with outstanding debt.
“Medical debt is drowning many low-income and working families while hospitals continue to benefit from nonprofit tax status as they pursue families for medical debt,” said Marceline White, executive director of Economic Action Maryland. The advocacy group has helped enact tighter rules to ensure Maryland hospitals make financial assistance more easily accessible and to restrict hospitals from some aggressive debt collection tactics, such as placing liens on patients’ homes.
Similar efforts are underway in other states, including Colorado, New Mexico, New York, Oregon, and Washington. But many patient and consumer advocates say stronger federal action is needed to expand patient protections.
The Human Rights Watch report – titled “In Sheep’s Clothing: United States’ Poorly Regulated Nonprofit Hospitals Undermine Health Care Access” – lists more than a dozen recommendations. These include:
- Congress should pass legislation to ensure that hospitals provide at least the same amount of charity care as they receive in public subsidies.
- The IRS should set uniform national standards on patients’ eligibility for financial assistance at nonprofit hospitals. Currently, hospitals are free to set their own standards, resulting in widespread variation, which can confuse patients.
- The Consumer Financial Protection Bureau, a federal watchdog agency, should crack down on debt collectors that do not ensure that patients have been screened for financial assistance before being pursued.
- The federal Centers for Medicare & Medicaid Services, which administers the two mammoth public insurance programs, should penalize hospitals that do not provide adequate financial assistance to patients.
“Nonprofit hospitals are contributing to medical debt and engaging in abusive billing and debt collection practices,” Mr. McConnell said. “The reason this keeps happening is the absence of clear guidelines and the federal government’s inadequate enforcement of existing regulations.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Patients with post-COVID cognitive symptoms may have gliosis
In a case-control study of 40 patients who were treated at a tertiary care psychiatric hospital in Canada, the level of translocator protein total distribution volume (TSPO VT), a marker of gliosis, was 9.23 mL/cm3 among patients with COVID-DC and 7.72 mL/cm3 among control persons. Differences were particularly notable in the ventral striatum and dorsal putamen.
“Most theories assume there is inflammation in the brain [with] long COVID,” but that assumption had not been studied, author Jeffrey H. Meyer, MD, PhD, Canada Research Chair in Neurochemistry of Major Depressive Disorder at the University of Toronto, said in an interview. “Such information is pivotal to developing treatments.”
The study was published online in JAMA Psychiatry.
Quantifiable marker
The investigators sought to determine whether levels of TSPO VT, which are quantifiable with PET, are elevated in the dorsal putamen, ventral striatum, prefrontal cortex, anterior cingulate cortex, and hippocampus of patients with COVID-DC, compared with patients without this syndrome. These brain regions were chosen, according to the authors, “because injury in these regions, which can cause gliosis, also induces symptoms of COVID-DC.”
The study was conducted from April 2021 through June 30, 2022. The investigators compared levels of TSPO VT in the selected brain regions of 20 participants with COVID-DC (mean age, 32.7 years; 60% women) with that of 20 control persons (mean age, 33.3 years; 55% women). TSPO VT was measured with fluorine F18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide PET.
The difference in TSPO VT was most noticeable in the ventral striatum (mean difference, 1.97 mL/cm3) and dorsal putamen (mean difference, 1.70 mL/cm3). The study authors suggest that gliosis in these areas may explain some of the persistent symptoms reported in structured clinical interviews and assessed on neuropsychological and psychological testing.
For patients with COVID-DC, motor speed on the finger-tapping test was negatively associated with dorsal putamen TSPO VT (r, −0.53). The 10 participants with COVID-DC whose speed was lowest had higher mean dorsal putamen TSPO VT levels than those of control persons by 2.3 mL/cm3.
The investigators could not assess a possible association between the ventral striatum TSPO VT and anhedonia because all participants had these symptoms. No significant correlations were found between depression and TSPO VT in the prefrontal cortex or anterior cingulate cortex.
The authors acknowledged that the study was cross-sectional, and so the duration of persistently elevated TSPO VT is not yet known. In addition, elevation in TSPO VT is not completely specific to glial cells, and although correlations with finger-tapping test performance reflect associations between brain changes and symptoms, they do not prove cause and effect.
“Presently, clinicians can use treatments for symptoms in other illnesses that are [also] common with long COVID. We need better than this,” said Dr. Meyer. “Clients with long COVID should be able to state their symptoms, and the practitioner should have an evidence-based matching treatment to recommend.”
Research is ongoing. “We are acquiring more information regarding different types of inflammation in the brain, whether there is ongoing injury, and whether treatments that influence inflammation are helpful,” said Dr. Meyer.
Jigsaw puzzle
“While this is an important piece in the jigsaw puzzle of neuroinflammation in chronic neurological disease, it is important to keep in mind that we still lack understanding of the complex picture for several reasons,” Alexander Gerhard, MD, honorary senior lecturer in neuroscience at the University of Manchester, England, wrote in an accompanying editorial.
Among these reasons is that the PET technique used in the study is noisy and not restricted to glial cells, he wrote. TSPO expression is only one part of the brain’s neuroinflammatory response, but PET techniques “do not currently allow us to distinguish between different states of microglial activation.” In addition, “a much more detailed understanding of microglial activation at different time points” is needed before neuroinflammatory changes can be targeted therapeutically, Dr. Gerhard wrote.
In a comment, Vilma Gabbay, MD, professor of psychiatry and neuroscience and director of biomarkers and dimensional psychiatry in the Psychiatry Research Institute at Montefiore Einstein, Albert Einstein College of Medicine, New York, said that “this is an important initial step to better understand the neuropsychiatric consequences of COVID even in only a mild and moderate viral illness.” TSPO imaging through PET scanning has been used as an index for neuroinflammation and gliosis. Researchers have used it to study neurodegenerative diseases, but as the authors noted, the ligand is not specific for gliosis.
“Follow-up large cohort studies including other measures of neuroimaging modalities assessing circuitry and neurochemistry are needed,” she said. “Similarly, studying the blood-brain barrier will also allow us to better understand how the immune reaction in the blood transitions to the brain.”
This field of research is evolving, and clinical trials are ongoing, Dr. Gabbay added. Meanwhile, clinicians should monitor for, assess, and treat neuropsychiatric symptoms and “follow the literature for new research and management recommendations.”
The study was primarily funded by a Canadian Institutes of Health Research Project grant to the authors, with some funding from the Canadian Institute for Military and Veteran Health Research. Dr. Meyer received support from their Canada Research Chair awards and received grants and support from several pharmaceutical companies outside of the submitted work. Dr. Gerhard and Dr. Gabbay disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a case-control study of 40 patients who were treated at a tertiary care psychiatric hospital in Canada, the level of translocator protein total distribution volume (TSPO VT), a marker of gliosis, was 9.23 mL/cm3 among patients with COVID-DC and 7.72 mL/cm3 among control persons. Differences were particularly notable in the ventral striatum and dorsal putamen.
“Most theories assume there is inflammation in the brain [with] long COVID,” but that assumption had not been studied, author Jeffrey H. Meyer, MD, PhD, Canada Research Chair in Neurochemistry of Major Depressive Disorder at the University of Toronto, said in an interview. “Such information is pivotal to developing treatments.”
The study was published online in JAMA Psychiatry.
Quantifiable marker
The investigators sought to determine whether levels of TSPO VT, which are quantifiable with PET, are elevated in the dorsal putamen, ventral striatum, prefrontal cortex, anterior cingulate cortex, and hippocampus of patients with COVID-DC, compared with patients without this syndrome. These brain regions were chosen, according to the authors, “because injury in these regions, which can cause gliosis, also induces symptoms of COVID-DC.”
The study was conducted from April 2021 through June 30, 2022. The investigators compared levels of TSPO VT in the selected brain regions of 20 participants with COVID-DC (mean age, 32.7 years; 60% women) with that of 20 control persons (mean age, 33.3 years; 55% women). TSPO VT was measured with fluorine F18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide PET.
The difference in TSPO VT was most noticeable in the ventral striatum (mean difference, 1.97 mL/cm3) and dorsal putamen (mean difference, 1.70 mL/cm3). The study authors suggest that gliosis in these areas may explain some of the persistent symptoms reported in structured clinical interviews and assessed on neuropsychological and psychological testing.
For patients with COVID-DC, motor speed on the finger-tapping test was negatively associated with dorsal putamen TSPO VT (r, −0.53). The 10 participants with COVID-DC whose speed was lowest had higher mean dorsal putamen TSPO VT levels than those of control persons by 2.3 mL/cm3.
The investigators could not assess a possible association between the ventral striatum TSPO VT and anhedonia because all participants had these symptoms. No significant correlations were found between depression and TSPO VT in the prefrontal cortex or anterior cingulate cortex.
The authors acknowledged that the study was cross-sectional, and so the duration of persistently elevated TSPO VT is not yet known. In addition, elevation in TSPO VT is not completely specific to glial cells, and although correlations with finger-tapping test performance reflect associations between brain changes and symptoms, they do not prove cause and effect.
“Presently, clinicians can use treatments for symptoms in other illnesses that are [also] common with long COVID. We need better than this,” said Dr. Meyer. “Clients with long COVID should be able to state their symptoms, and the practitioner should have an evidence-based matching treatment to recommend.”
Research is ongoing. “We are acquiring more information regarding different types of inflammation in the brain, whether there is ongoing injury, and whether treatments that influence inflammation are helpful,” said Dr. Meyer.
Jigsaw puzzle
“While this is an important piece in the jigsaw puzzle of neuroinflammation in chronic neurological disease, it is important to keep in mind that we still lack understanding of the complex picture for several reasons,” Alexander Gerhard, MD, honorary senior lecturer in neuroscience at the University of Manchester, England, wrote in an accompanying editorial.
Among these reasons is that the PET technique used in the study is noisy and not restricted to glial cells, he wrote. TSPO expression is only one part of the brain’s neuroinflammatory response, but PET techniques “do not currently allow us to distinguish between different states of microglial activation.” In addition, “a much more detailed understanding of microglial activation at different time points” is needed before neuroinflammatory changes can be targeted therapeutically, Dr. Gerhard wrote.
In a comment, Vilma Gabbay, MD, professor of psychiatry and neuroscience and director of biomarkers and dimensional psychiatry in the Psychiatry Research Institute at Montefiore Einstein, Albert Einstein College of Medicine, New York, said that “this is an important initial step to better understand the neuropsychiatric consequences of COVID even in only a mild and moderate viral illness.” TSPO imaging through PET scanning has been used as an index for neuroinflammation and gliosis. Researchers have used it to study neurodegenerative diseases, but as the authors noted, the ligand is not specific for gliosis.
“Follow-up large cohort studies including other measures of neuroimaging modalities assessing circuitry and neurochemistry are needed,” she said. “Similarly, studying the blood-brain barrier will also allow us to better understand how the immune reaction in the blood transitions to the brain.”
This field of research is evolving, and clinical trials are ongoing, Dr. Gabbay added. Meanwhile, clinicians should monitor for, assess, and treat neuropsychiatric symptoms and “follow the literature for new research and management recommendations.”
The study was primarily funded by a Canadian Institutes of Health Research Project grant to the authors, with some funding from the Canadian Institute for Military and Veteran Health Research. Dr. Meyer received support from their Canada Research Chair awards and received grants and support from several pharmaceutical companies outside of the submitted work. Dr. Gerhard and Dr. Gabbay disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a case-control study of 40 patients who were treated at a tertiary care psychiatric hospital in Canada, the level of translocator protein total distribution volume (TSPO VT), a marker of gliosis, was 9.23 mL/cm3 among patients with COVID-DC and 7.72 mL/cm3 among control persons. Differences were particularly notable in the ventral striatum and dorsal putamen.
“Most theories assume there is inflammation in the brain [with] long COVID,” but that assumption had not been studied, author Jeffrey H. Meyer, MD, PhD, Canada Research Chair in Neurochemistry of Major Depressive Disorder at the University of Toronto, said in an interview. “Such information is pivotal to developing treatments.”
The study was published online in JAMA Psychiatry.
Quantifiable marker
The investigators sought to determine whether levels of TSPO VT, which are quantifiable with PET, are elevated in the dorsal putamen, ventral striatum, prefrontal cortex, anterior cingulate cortex, and hippocampus of patients with COVID-DC, compared with patients without this syndrome. These brain regions were chosen, according to the authors, “because injury in these regions, which can cause gliosis, also induces symptoms of COVID-DC.”
The study was conducted from April 2021 through June 30, 2022. The investigators compared levels of TSPO VT in the selected brain regions of 20 participants with COVID-DC (mean age, 32.7 years; 60% women) with that of 20 control persons (mean age, 33.3 years; 55% women). TSPO VT was measured with fluorine F18–labeled N-(2-(2-fluoroethoxy)benzyl)-N-(4-phenoxypyridin-3-yl)acetamide PET.
The difference in TSPO VT was most noticeable in the ventral striatum (mean difference, 1.97 mL/cm3) and dorsal putamen (mean difference, 1.70 mL/cm3). The study authors suggest that gliosis in these areas may explain some of the persistent symptoms reported in structured clinical interviews and assessed on neuropsychological and psychological testing.
For patients with COVID-DC, motor speed on the finger-tapping test was negatively associated with dorsal putamen TSPO VT (r, −0.53). The 10 participants with COVID-DC whose speed was lowest had higher mean dorsal putamen TSPO VT levels than those of control persons by 2.3 mL/cm3.
The investigators could not assess a possible association between the ventral striatum TSPO VT and anhedonia because all participants had these symptoms. No significant correlations were found between depression and TSPO VT in the prefrontal cortex or anterior cingulate cortex.
The authors acknowledged that the study was cross-sectional, and so the duration of persistently elevated TSPO VT is not yet known. In addition, elevation in TSPO VT is not completely specific to glial cells, and although correlations with finger-tapping test performance reflect associations between brain changes and symptoms, they do not prove cause and effect.
“Presently, clinicians can use treatments for symptoms in other illnesses that are [also] common with long COVID. We need better than this,” said Dr. Meyer. “Clients with long COVID should be able to state their symptoms, and the practitioner should have an evidence-based matching treatment to recommend.”
Research is ongoing. “We are acquiring more information regarding different types of inflammation in the brain, whether there is ongoing injury, and whether treatments that influence inflammation are helpful,” said Dr. Meyer.
Jigsaw puzzle
“While this is an important piece in the jigsaw puzzle of neuroinflammation in chronic neurological disease, it is important to keep in mind that we still lack understanding of the complex picture for several reasons,” Alexander Gerhard, MD, honorary senior lecturer in neuroscience at the University of Manchester, England, wrote in an accompanying editorial.
Among these reasons is that the PET technique used in the study is noisy and not restricted to glial cells, he wrote. TSPO expression is only one part of the brain’s neuroinflammatory response, but PET techniques “do not currently allow us to distinguish between different states of microglial activation.” In addition, “a much more detailed understanding of microglial activation at different time points” is needed before neuroinflammatory changes can be targeted therapeutically, Dr. Gerhard wrote.
In a comment, Vilma Gabbay, MD, professor of psychiatry and neuroscience and director of biomarkers and dimensional psychiatry in the Psychiatry Research Institute at Montefiore Einstein, Albert Einstein College of Medicine, New York, said that “this is an important initial step to better understand the neuropsychiatric consequences of COVID even in only a mild and moderate viral illness.” TSPO imaging through PET scanning has been used as an index for neuroinflammation and gliosis. Researchers have used it to study neurodegenerative diseases, but as the authors noted, the ligand is not specific for gliosis.
“Follow-up large cohort studies including other measures of neuroimaging modalities assessing circuitry and neurochemistry are needed,” she said. “Similarly, studying the blood-brain barrier will also allow us to better understand how the immune reaction in the blood transitions to the brain.”
This field of research is evolving, and clinical trials are ongoing, Dr. Gabbay added. Meanwhile, clinicians should monitor for, assess, and treat neuropsychiatric symptoms and “follow the literature for new research and management recommendations.”
The study was primarily funded by a Canadian Institutes of Health Research Project grant to the authors, with some funding from the Canadian Institute for Military and Veteran Health Research. Dr. Meyer received support from their Canada Research Chair awards and received grants and support from several pharmaceutical companies outside of the submitted work. Dr. Gerhard and Dr. Gabbay disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
FDA panel backs new COVID booster focusing only on variants
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
Latest data: COVID vaccine safety, protection, and breakthrough infections in inflammatory, autoimmune diseases
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
Millions who had COVID-19 still don’t have sense of smell, taste
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
Almost 36 million people were diagnosed in 2021, and 60% of them reported accompanying losses in smell or taste, according to the study by Mass Eye and Ear, which is affiliated with Harvard Medical School, Boston. The study was published in The Laryngoscope.
Most people fully regained the senses, but about 24% didn’t get smell back completely, and more than 3% had no recovery, the researchers reported. The numbers were similar among those who lost the sense of taste, they added.
“Many people never fully recovered,” Neil Bhattacharyya, MD, professor of otolaryngology and one of the study’s authors, told Fortune, estimating that up to 6 million people still have lingering symptoms. “If you lost your sense of smell, did you get it back? There’s about a one in four chance you didn’t. That’s terrible.”
Researchers looked at the records of 30,000 adults who had COVID-19 in 2021. They reported that patients who suffered more severe cases were less likely to regain some or all their senses.
Some patients said they lost appetite because they couldn’t smell food. There’s concern, too, about losing the ability to smell gas and smoke, spoiled food, and dirty diapers.
People with symptoms should see their doctors, Dr. Bhattacharyya said. The symptoms might be caused by something other than lingering COVID-19 effects and might be treatable.
A version of this article first appeared on WebMD.com.
FROM THE LARYNGOSCOPE