User login
Maternal depressive symptoms may start at pregnancy
, new research suggests.
The analysis of more than 11,000 pregnant women with depressive symptoms from seven prospective cohorts in Canada, the United Kingdom, and Singapore suggests that depressive symptoms (low, mild, or high levels) start sooner and last longer than is commonly thought.
The term “postnatal depression” is “at odds with existing scientific literature and the experience of clinicians who treat mental disorders in the context of obstetric practice,” said Michael J. Meaney, PhD, professor at McGill University, Montreal, and director of the Translational Neuroscience Program at the Agency for Science, Technology and Research (A*STAR), Singapore.
“Although we anticipated that the prenatal period would be the primary time of onset and that symptom levels would be largely stable, I was nevertheless surprised at how this pattern was so universal across so many studies,” he said in an interview. “In truth, we saw very little evidence for a postnatal onset.”
This suggests that depressive symptoms start earlier than previously thought, and “that the relevant clinical settings for prevention are those treating women in routine health care, including family medicine,” he added.
Start screening sooner
The investigators examined the course and stability of self-reported depressive symptoms at multiple time points across the perinatal period among 11,563 pregnant women in seven cohorts from the United Kingdom, Canada, and Singapore. Participants’ mean age was 29 years; 87.6% were White, 4.9% were East Asian, and 2.6% were Southeast Asian.
The analysis tracked depressive symptoms from preconception through pregnancy to 2 years after childbirth. Three groups of mothers were identified in each cohort on the basis of their level of depressive symptoms (low, mild, or high) as assessed by the Edinburgh Postnatal Depression Scale (EPDS) or the Center for Epidemiological Studies Depression (CES-D).
The team found that all mothers within and across all cohorts had stable trajectories of maternal depressive symptoms from pregnancy onward. Trajectories for mothers who passed clinically validated cutoffs for “probable” depression also showed stable trajectories from pregnancy into the postnatal period.
“Taken together, these findings suggest that maternal depressive symptom levels in community-based cohort studies are apparent during pregnancy and remain stable into the postnatal period,” the authors write. “The results point to the early antenatal period as a timepoint for the identification of stable trajectories of maternal depressive symptoms. Public health policies should emphasize the early antenatal period as the optimal timing for interventions targeting maternal depressive symptoms.”
The findings, they note, “underscore the American Psychiatric Association’s recent approach in renaming postpartum depression as peripartum depression.”
Furthermore, a recent paper of the group’s findings details that depressive symptoms may often predate conception.
“Our findings should serve to universally align practice to prenatal screening,” even though depression screening often takes place in a mid-gestational visit during the second trimester, Dr. Meaney said. “Our findings and those on the effects on child development strongly suggest the timing of the screening must be advanced into the first confirmation of pregnancy.”
Depression is likely worse in the United States
Catherine Monk, PhD, chief of the Division of Women’s Mental Health and professor of medical psychology at Columbia University, Vagelos College of Physicians and Surgeons, New York, said in an interview that the results of the study “amplify similar research findings and the experience of most perinatal clinicians: Depression is stable from pregnancy onwards.”
Dr. Monk, who was not involved in the research, said that “as the authors note, the common focus on postpartum depression misses the months of prior suffering and an opportunity for earlier intervention.” Dr. Monk said she would have liked the results to have been examined further by race and ethnicity and socioeconomic factors. “Also, the combined sample does not include a U.S. cohort. This is significant as the U.S. has the highest maternal morbidity and mortality rate of developed nations, and some reports identify mental health factors as the number-one cause of maternal mortality.”
“Given the tremendous economic, racial, and ethnic inequities in health care – the lack of any kind of health justice – it is quite possible that in the U.S., depression that starts in pregnancy worsens over time, at least for some demographic groups,” Dr. Monk said. “Rates of depression, levels of depression, and the course of it during the peripartum period may be even more dire [in the U.S.] than what is represented in this article.” “What should be practice-changing about this article, and so many others demonstrating the persistent and often high levels of life-threatening depression during pregnancy, is the need for mental health providers to advocate for changes to the low rates of insurance reimbursement that push providers away from accepting insurance and into private practice, making access to affordable mental care nearly impossible for most,” she concluded.
This study was supported by the Singapore Institute for Clinical Sciences, Agency for Science, Technology, and Research; the Toxic Stress Network of the JPB Foundation; the Hope for Depression Research Foundation; and the Jacob’s Foundation. Dr. Meaney and Dr. Monk report no conflicts of interest.
A version of this article appeared on Medscape.com.
, new research suggests.
The analysis of more than 11,000 pregnant women with depressive symptoms from seven prospective cohorts in Canada, the United Kingdom, and Singapore suggests that depressive symptoms (low, mild, or high levels) start sooner and last longer than is commonly thought.
The term “postnatal depression” is “at odds with existing scientific literature and the experience of clinicians who treat mental disorders in the context of obstetric practice,” said Michael J. Meaney, PhD, professor at McGill University, Montreal, and director of the Translational Neuroscience Program at the Agency for Science, Technology and Research (A*STAR), Singapore.
“Although we anticipated that the prenatal period would be the primary time of onset and that symptom levels would be largely stable, I was nevertheless surprised at how this pattern was so universal across so many studies,” he said in an interview. “In truth, we saw very little evidence for a postnatal onset.”
This suggests that depressive symptoms start earlier than previously thought, and “that the relevant clinical settings for prevention are those treating women in routine health care, including family medicine,” he added.
Start screening sooner
The investigators examined the course and stability of self-reported depressive symptoms at multiple time points across the perinatal period among 11,563 pregnant women in seven cohorts from the United Kingdom, Canada, and Singapore. Participants’ mean age was 29 years; 87.6% were White, 4.9% were East Asian, and 2.6% were Southeast Asian.
The analysis tracked depressive symptoms from preconception through pregnancy to 2 years after childbirth. Three groups of mothers were identified in each cohort on the basis of their level of depressive symptoms (low, mild, or high) as assessed by the Edinburgh Postnatal Depression Scale (EPDS) or the Center for Epidemiological Studies Depression (CES-D).
The team found that all mothers within and across all cohorts had stable trajectories of maternal depressive symptoms from pregnancy onward. Trajectories for mothers who passed clinically validated cutoffs for “probable” depression also showed stable trajectories from pregnancy into the postnatal period.
“Taken together, these findings suggest that maternal depressive symptom levels in community-based cohort studies are apparent during pregnancy and remain stable into the postnatal period,” the authors write. “The results point to the early antenatal period as a timepoint for the identification of stable trajectories of maternal depressive symptoms. Public health policies should emphasize the early antenatal period as the optimal timing for interventions targeting maternal depressive symptoms.”
The findings, they note, “underscore the American Psychiatric Association’s recent approach in renaming postpartum depression as peripartum depression.”
Furthermore, a recent paper of the group’s findings details that depressive symptoms may often predate conception.
“Our findings should serve to universally align practice to prenatal screening,” even though depression screening often takes place in a mid-gestational visit during the second trimester, Dr. Meaney said. “Our findings and those on the effects on child development strongly suggest the timing of the screening must be advanced into the first confirmation of pregnancy.”
Depression is likely worse in the United States
Catherine Monk, PhD, chief of the Division of Women’s Mental Health and professor of medical psychology at Columbia University, Vagelos College of Physicians and Surgeons, New York, said in an interview that the results of the study “amplify similar research findings and the experience of most perinatal clinicians: Depression is stable from pregnancy onwards.”
Dr. Monk, who was not involved in the research, said that “as the authors note, the common focus on postpartum depression misses the months of prior suffering and an opportunity for earlier intervention.” Dr. Monk said she would have liked the results to have been examined further by race and ethnicity and socioeconomic factors. “Also, the combined sample does not include a U.S. cohort. This is significant as the U.S. has the highest maternal morbidity and mortality rate of developed nations, and some reports identify mental health factors as the number-one cause of maternal mortality.”
“Given the tremendous economic, racial, and ethnic inequities in health care – the lack of any kind of health justice – it is quite possible that in the U.S., depression that starts in pregnancy worsens over time, at least for some demographic groups,” Dr. Monk said. “Rates of depression, levels of depression, and the course of it during the peripartum period may be even more dire [in the U.S.] than what is represented in this article.” “What should be practice-changing about this article, and so many others demonstrating the persistent and often high levels of life-threatening depression during pregnancy, is the need for mental health providers to advocate for changes to the low rates of insurance reimbursement that push providers away from accepting insurance and into private practice, making access to affordable mental care nearly impossible for most,” she concluded.
This study was supported by the Singapore Institute for Clinical Sciences, Agency for Science, Technology, and Research; the Toxic Stress Network of the JPB Foundation; the Hope for Depression Research Foundation; and the Jacob’s Foundation. Dr. Meaney and Dr. Monk report no conflicts of interest.
A version of this article appeared on Medscape.com.
, new research suggests.
The analysis of more than 11,000 pregnant women with depressive symptoms from seven prospective cohorts in Canada, the United Kingdom, and Singapore suggests that depressive symptoms (low, mild, or high levels) start sooner and last longer than is commonly thought.
The term “postnatal depression” is “at odds with existing scientific literature and the experience of clinicians who treat mental disorders in the context of obstetric practice,” said Michael J. Meaney, PhD, professor at McGill University, Montreal, and director of the Translational Neuroscience Program at the Agency for Science, Technology and Research (A*STAR), Singapore.
“Although we anticipated that the prenatal period would be the primary time of onset and that symptom levels would be largely stable, I was nevertheless surprised at how this pattern was so universal across so many studies,” he said in an interview. “In truth, we saw very little evidence for a postnatal onset.”
This suggests that depressive symptoms start earlier than previously thought, and “that the relevant clinical settings for prevention are those treating women in routine health care, including family medicine,” he added.
Start screening sooner
The investigators examined the course and stability of self-reported depressive symptoms at multiple time points across the perinatal period among 11,563 pregnant women in seven cohorts from the United Kingdom, Canada, and Singapore. Participants’ mean age was 29 years; 87.6% were White, 4.9% were East Asian, and 2.6% were Southeast Asian.
The analysis tracked depressive symptoms from preconception through pregnancy to 2 years after childbirth. Three groups of mothers were identified in each cohort on the basis of their level of depressive symptoms (low, mild, or high) as assessed by the Edinburgh Postnatal Depression Scale (EPDS) or the Center for Epidemiological Studies Depression (CES-D).
The team found that all mothers within and across all cohorts had stable trajectories of maternal depressive symptoms from pregnancy onward. Trajectories for mothers who passed clinically validated cutoffs for “probable” depression also showed stable trajectories from pregnancy into the postnatal period.
“Taken together, these findings suggest that maternal depressive symptom levels in community-based cohort studies are apparent during pregnancy and remain stable into the postnatal period,” the authors write. “The results point to the early antenatal period as a timepoint for the identification of stable trajectories of maternal depressive symptoms. Public health policies should emphasize the early antenatal period as the optimal timing for interventions targeting maternal depressive symptoms.”
The findings, they note, “underscore the American Psychiatric Association’s recent approach in renaming postpartum depression as peripartum depression.”
Furthermore, a recent paper of the group’s findings details that depressive symptoms may often predate conception.
“Our findings should serve to universally align practice to prenatal screening,” even though depression screening often takes place in a mid-gestational visit during the second trimester, Dr. Meaney said. “Our findings and those on the effects on child development strongly suggest the timing of the screening must be advanced into the first confirmation of pregnancy.”
Depression is likely worse in the United States
Catherine Monk, PhD, chief of the Division of Women’s Mental Health and professor of medical psychology at Columbia University, Vagelos College of Physicians and Surgeons, New York, said in an interview that the results of the study “amplify similar research findings and the experience of most perinatal clinicians: Depression is stable from pregnancy onwards.”
Dr. Monk, who was not involved in the research, said that “as the authors note, the common focus on postpartum depression misses the months of prior suffering and an opportunity for earlier intervention.” Dr. Monk said she would have liked the results to have been examined further by race and ethnicity and socioeconomic factors. “Also, the combined sample does not include a U.S. cohort. This is significant as the U.S. has the highest maternal morbidity and mortality rate of developed nations, and some reports identify mental health factors as the number-one cause of maternal mortality.”
“Given the tremendous economic, racial, and ethnic inequities in health care – the lack of any kind of health justice – it is quite possible that in the U.S., depression that starts in pregnancy worsens over time, at least for some demographic groups,” Dr. Monk said. “Rates of depression, levels of depression, and the course of it during the peripartum period may be even more dire [in the U.S.] than what is represented in this article.” “What should be practice-changing about this article, and so many others demonstrating the persistent and often high levels of life-threatening depression during pregnancy, is the need for mental health providers to advocate for changes to the low rates of insurance reimbursement that push providers away from accepting insurance and into private practice, making access to affordable mental care nearly impossible for most,” she concluded.
This study was supported by the Singapore Institute for Clinical Sciences, Agency for Science, Technology, and Research; the Toxic Stress Network of the JPB Foundation; the Hope for Depression Research Foundation; and the Jacob’s Foundation. Dr. Meaney and Dr. Monk report no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
‘Love more’: Why doctors should promote social connection
Those who embrace lifestyle medicine are familiar with the slogan Dean Ornish, MD, likes to use: Eat well, move more, stress less, love more.
That last one, love, was the renowned physician and author’s focus at the recent American College of Lifestyle Medicine Conference in Denver. That’s because love – essentially the support, connectedness, and caring that patients feel when they join a lifestyle-change program – is “where healing occurs at the deepest level.”
Indeed, social connectedness is emerging as a vital pillar in the burgeoning field of lifestyle medicine, a specialty that uses lifestyle interventions to treat chronic conditions. About 300 lifestyle medicine programs are now integrated into residencies in medical schools across the country, up from a handful just 5 years ago, said Meagan Grega, MD, the conference chair.
“The energy and growth in American lifestyle medicine is unparalleled by anything else I see in the health care world right now,” said Dr. Grega, a family physician for 25 years in eastern Pennsylvania.
The field applies volumes of research, from the 1990s to today, demonstrating the healing effects of lifestyle changes. Dr. Ornish’s Preventive Medicine Research Institute has published research on small changes (like pomegranate juice helping blood flow in the heart) and huge ones: Coronary heart patients reversed the narrowing of arteries without lipid-lowering drugs after 1 year of lifestyle changes, including a vegetarian diet, aerobic exercise, stress management, and group support.
Ranking alongside bedrocks such as healthy diet, sleep, exercise, and stress management is positive social connection. That part, the “love more” part, often draws skepticism but is vital, said Dr. Ornish, who is sometimes referred to as the father of lifestyle medicine.
It’s “invariably the part that’s the most meaningful – that sense of connection to community that can come when you bring total strangers together,” Dr. Ornish said. “The ‘love more’ part, in many ways, is not only as important, but in some ways even more because everything really flows from that.”
Patients in a support group, who can “let down their emotional defenses and talk openly and authentically,” are much more likely to make and maintain healthy changes, Dr. Ornish said.
Love as medicine
Mounting evidence links loneliness and isolation with a range of health issues, from mood disorders such as depression to chronic conditions such as cardiovascular disease. What’s more, data suggest that loneliness and social isolation in the United States are on the rise, and the COVID pandemic made that more clear. In May 2023, Surgeon General Vivek Murthy, MD, called loneliness, isolation, and lack of connection in the United States a “public health crisis.”
“Good relationships keep us happier and healthier,” said Robert Waldinger, MD, a psychiatrist at Massachusetts General Hospital, Boston.
Dr. Waldinger, who was not affiliated with the conference, is head of the Harvard Study of Adult Development, one of the longest studies of adult life. Beginning in 1938, the study has tracked 724 people plus more than 1,300 of their descendants and found that embracing community and close relationships helps us live longer and be happier.
In the study, the people who were most satisfied with their relationships at age 50 years were the healthiest at age 80 years. Knowing you have someone to rely on protects the brain: “Those people’s memories stay sharper longer,” Dr. Waldinger said.
He draws a distinction between connection and love. “Love is, I think, more of a feeling,” Dr. Waldinger noted. “Connection is a feeling, but it’s also an activity.”
One in five Americans say they’re lonely, he said, “and loneliness is a stressor.” People who are isolated don’t sleep as well, he added. Their health declines earlier in midlife, brain function slips sooner, and their lives are shorter.
“You don’t have anyone to complain to,” he said. If you do, “you can feel your body start to calm down.” Those without social connections may stay in a low-level “fight-or-flight mode.”
“What we think happens is that you have low levels of inflammation chronically, and those can gradually break down body systems.” Moreover, higher rates of cardiac reactivity, for instance, a racing heartbeat when upset, can lead to high blood pressure and lower immune function.
In his talk, Dr. Ornish said, “Anger is that one emotion that has consistently been shown to make heart disease worse.”
Helping people in those straits is gratifying, Dr. Ornish said. “If we can work with people as lifestyle medicine practitioners when they’re suffering, there’s an opportunity for transformation.”
Future
Of course, that can be easier said than done. Dr. Ornish relayed a patient’s typical reaction to a lifestyle program: “This is kind of weird stuff. Like, I get diet. But a plant-based diet, really? Meditation? Loving more? Really?”
He told the conference, “Part of our job as lifestyle medicine practitioners is to spend a little extra time with them. It doesn’t even take that much time. And to really help them understand what brings them a sense of hope and meaning and purpose.”
The results can be motivating. “Most people feel so much better so quickly,” Dr. Ornish said. “It reframes the reason for change from fear of dying to joy of living.”
Dr. Grega, for one, is optimistic for the future, citing survey results showing that 95% of medical students think that they›d be better counselors with lifestyle training. ‘They passionately want this type of thing,” she said.
A version of this article first appeared on Medscape.com.
Those who embrace lifestyle medicine are familiar with the slogan Dean Ornish, MD, likes to use: Eat well, move more, stress less, love more.
That last one, love, was the renowned physician and author’s focus at the recent American College of Lifestyle Medicine Conference in Denver. That’s because love – essentially the support, connectedness, and caring that patients feel when they join a lifestyle-change program – is “where healing occurs at the deepest level.”
Indeed, social connectedness is emerging as a vital pillar in the burgeoning field of lifestyle medicine, a specialty that uses lifestyle interventions to treat chronic conditions. About 300 lifestyle medicine programs are now integrated into residencies in medical schools across the country, up from a handful just 5 years ago, said Meagan Grega, MD, the conference chair.
“The energy and growth in American lifestyle medicine is unparalleled by anything else I see in the health care world right now,” said Dr. Grega, a family physician for 25 years in eastern Pennsylvania.
The field applies volumes of research, from the 1990s to today, demonstrating the healing effects of lifestyle changes. Dr. Ornish’s Preventive Medicine Research Institute has published research on small changes (like pomegranate juice helping blood flow in the heart) and huge ones: Coronary heart patients reversed the narrowing of arteries without lipid-lowering drugs after 1 year of lifestyle changes, including a vegetarian diet, aerobic exercise, stress management, and group support.
Ranking alongside bedrocks such as healthy diet, sleep, exercise, and stress management is positive social connection. That part, the “love more” part, often draws skepticism but is vital, said Dr. Ornish, who is sometimes referred to as the father of lifestyle medicine.
It’s “invariably the part that’s the most meaningful – that sense of connection to community that can come when you bring total strangers together,” Dr. Ornish said. “The ‘love more’ part, in many ways, is not only as important, but in some ways even more because everything really flows from that.”
Patients in a support group, who can “let down their emotional defenses and talk openly and authentically,” are much more likely to make and maintain healthy changes, Dr. Ornish said.
Love as medicine
Mounting evidence links loneliness and isolation with a range of health issues, from mood disorders such as depression to chronic conditions such as cardiovascular disease. What’s more, data suggest that loneliness and social isolation in the United States are on the rise, and the COVID pandemic made that more clear. In May 2023, Surgeon General Vivek Murthy, MD, called loneliness, isolation, and lack of connection in the United States a “public health crisis.”
“Good relationships keep us happier and healthier,” said Robert Waldinger, MD, a psychiatrist at Massachusetts General Hospital, Boston.
Dr. Waldinger, who was not affiliated with the conference, is head of the Harvard Study of Adult Development, one of the longest studies of adult life. Beginning in 1938, the study has tracked 724 people plus more than 1,300 of their descendants and found that embracing community and close relationships helps us live longer and be happier.
In the study, the people who were most satisfied with their relationships at age 50 years were the healthiest at age 80 years. Knowing you have someone to rely on protects the brain: “Those people’s memories stay sharper longer,” Dr. Waldinger said.
He draws a distinction between connection and love. “Love is, I think, more of a feeling,” Dr. Waldinger noted. “Connection is a feeling, but it’s also an activity.”
One in five Americans say they’re lonely, he said, “and loneliness is a stressor.” People who are isolated don’t sleep as well, he added. Their health declines earlier in midlife, brain function slips sooner, and their lives are shorter.
“You don’t have anyone to complain to,” he said. If you do, “you can feel your body start to calm down.” Those without social connections may stay in a low-level “fight-or-flight mode.”
“What we think happens is that you have low levels of inflammation chronically, and those can gradually break down body systems.” Moreover, higher rates of cardiac reactivity, for instance, a racing heartbeat when upset, can lead to high blood pressure and lower immune function.
In his talk, Dr. Ornish said, “Anger is that one emotion that has consistently been shown to make heart disease worse.”
Helping people in those straits is gratifying, Dr. Ornish said. “If we can work with people as lifestyle medicine practitioners when they’re suffering, there’s an opportunity for transformation.”
Future
Of course, that can be easier said than done. Dr. Ornish relayed a patient’s typical reaction to a lifestyle program: “This is kind of weird stuff. Like, I get diet. But a plant-based diet, really? Meditation? Loving more? Really?”
He told the conference, “Part of our job as lifestyle medicine practitioners is to spend a little extra time with them. It doesn’t even take that much time. And to really help them understand what brings them a sense of hope and meaning and purpose.”
The results can be motivating. “Most people feel so much better so quickly,” Dr. Ornish said. “It reframes the reason for change from fear of dying to joy of living.”
Dr. Grega, for one, is optimistic for the future, citing survey results showing that 95% of medical students think that they›d be better counselors with lifestyle training. ‘They passionately want this type of thing,” she said.
A version of this article first appeared on Medscape.com.
Those who embrace lifestyle medicine are familiar with the slogan Dean Ornish, MD, likes to use: Eat well, move more, stress less, love more.
That last one, love, was the renowned physician and author’s focus at the recent American College of Lifestyle Medicine Conference in Denver. That’s because love – essentially the support, connectedness, and caring that patients feel when they join a lifestyle-change program – is “where healing occurs at the deepest level.”
Indeed, social connectedness is emerging as a vital pillar in the burgeoning field of lifestyle medicine, a specialty that uses lifestyle interventions to treat chronic conditions. About 300 lifestyle medicine programs are now integrated into residencies in medical schools across the country, up from a handful just 5 years ago, said Meagan Grega, MD, the conference chair.
“The energy and growth in American lifestyle medicine is unparalleled by anything else I see in the health care world right now,” said Dr. Grega, a family physician for 25 years in eastern Pennsylvania.
The field applies volumes of research, from the 1990s to today, demonstrating the healing effects of lifestyle changes. Dr. Ornish’s Preventive Medicine Research Institute has published research on small changes (like pomegranate juice helping blood flow in the heart) and huge ones: Coronary heart patients reversed the narrowing of arteries without lipid-lowering drugs after 1 year of lifestyle changes, including a vegetarian diet, aerobic exercise, stress management, and group support.
Ranking alongside bedrocks such as healthy diet, sleep, exercise, and stress management is positive social connection. That part, the “love more” part, often draws skepticism but is vital, said Dr. Ornish, who is sometimes referred to as the father of lifestyle medicine.
It’s “invariably the part that’s the most meaningful – that sense of connection to community that can come when you bring total strangers together,” Dr. Ornish said. “The ‘love more’ part, in many ways, is not only as important, but in some ways even more because everything really flows from that.”
Patients in a support group, who can “let down their emotional defenses and talk openly and authentically,” are much more likely to make and maintain healthy changes, Dr. Ornish said.
Love as medicine
Mounting evidence links loneliness and isolation with a range of health issues, from mood disorders such as depression to chronic conditions such as cardiovascular disease. What’s more, data suggest that loneliness and social isolation in the United States are on the rise, and the COVID pandemic made that more clear. In May 2023, Surgeon General Vivek Murthy, MD, called loneliness, isolation, and lack of connection in the United States a “public health crisis.”
“Good relationships keep us happier and healthier,” said Robert Waldinger, MD, a psychiatrist at Massachusetts General Hospital, Boston.
Dr. Waldinger, who was not affiliated with the conference, is head of the Harvard Study of Adult Development, one of the longest studies of adult life. Beginning in 1938, the study has tracked 724 people plus more than 1,300 of their descendants and found that embracing community and close relationships helps us live longer and be happier.
In the study, the people who were most satisfied with their relationships at age 50 years were the healthiest at age 80 years. Knowing you have someone to rely on protects the brain: “Those people’s memories stay sharper longer,” Dr. Waldinger said.
He draws a distinction between connection and love. “Love is, I think, more of a feeling,” Dr. Waldinger noted. “Connection is a feeling, but it’s also an activity.”
One in five Americans say they’re lonely, he said, “and loneliness is a stressor.” People who are isolated don’t sleep as well, he added. Their health declines earlier in midlife, brain function slips sooner, and their lives are shorter.
“You don’t have anyone to complain to,” he said. If you do, “you can feel your body start to calm down.” Those without social connections may stay in a low-level “fight-or-flight mode.”
“What we think happens is that you have low levels of inflammation chronically, and those can gradually break down body systems.” Moreover, higher rates of cardiac reactivity, for instance, a racing heartbeat when upset, can lead to high blood pressure and lower immune function.
In his talk, Dr. Ornish said, “Anger is that one emotion that has consistently been shown to make heart disease worse.”
Helping people in those straits is gratifying, Dr. Ornish said. “If we can work with people as lifestyle medicine practitioners when they’re suffering, there’s an opportunity for transformation.”
Future
Of course, that can be easier said than done. Dr. Ornish relayed a patient’s typical reaction to a lifestyle program: “This is kind of weird stuff. Like, I get diet. But a plant-based diet, really? Meditation? Loving more? Really?”
He told the conference, “Part of our job as lifestyle medicine practitioners is to spend a little extra time with them. It doesn’t even take that much time. And to really help them understand what brings them a sense of hope and meaning and purpose.”
The results can be motivating. “Most people feel so much better so quickly,” Dr. Ornish said. “It reframes the reason for change from fear of dying to joy of living.”
Dr. Grega, for one, is optimistic for the future, citing survey results showing that 95% of medical students think that they›d be better counselors with lifestyle training. ‘They passionately want this type of thing,” she said.
A version of this article first appeared on Medscape.com.
Depression: Differential Diagnosis
Stroke patients benefit from neurologic music therapy
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
Neurologic music therapy (NMT), a specially designed intervention targeting movement, balance, and cognitive functioning, improves depressive symptoms and increases brain-derived neurotrophic factor (BDNF), early results of a small study suggest.
“We’re really happy with the results,” said lead study author psychotherapist Honey Bryant, a PhD candidate and research assistant at the Centre for Neuroscience Studies, Queen’s University, Kingston, Ont.
“We showed ”
The findings were presented at the virtual XXVI World Congress of Neurology.
Moving with music
With improved stroke survival rates and longer life expectancy, there’s an increasing need for effective post-stroke interventions for neurocognitive impairments and mood disorders, the authors noted.
NMT is an evidence-based treatment system that uses elements of music such as rhythm, melody, and tempo to treat various brain conditions. A trained NMT therapist uses standardized techniques to address goals in the areas of speech, movement, and cognition.
The intervention is not new – it’s been around for a few decades – but there are “minimal papers on NMT and nothing on stroke rehabilitation used in the way we did it,” said Ms. Bryant.
The study included 57 patients, mean age 75 years, receiving rehabilitation following a stroke who were randomly assigned to NMT or passive music listening.
In the NMT group, a music therapist asked participants to choose music beforehand and integrated this into each session.
“Each day was different,” said Ms. Bryant. “For example, if it involved motor movement, the music therapist would say, ‘When I sing this word, raise your arm up.’ For Johnny Cash’s ‘Ring of Fire,’ we made our arms into a circle.”
She explained that the rhythm and timing of the music can affect the motor system and other areas of the brain.
Those in the passive music group listened to a curated list of calming classical and relaxing spa music.
Both groups were offered five 45-minute sessions per week for 2 weeks.
Among other things, researchers used the Hospital Anxiety and Depression Scale (HADS), administered a semistructured interview, and collected blood samples to determine levels of cortisol and BDNF.
After the 2-week intervention, the researchers found participants in the NMT group had a significant mean decrease in depression.
They also had increased cortisol levels, which is not unexpected after a stroke, especially with increased anxiety linked to financial and other stressors, said Ms. Bryant, adding these levels should decrease with treatment.
Recipients of the NMT had significant increases in BDNF, a neurotrophin that plays an important role in neuronal survival and growth, but only in those who attended several consecutive sessions.
Increased plasticity
“We see greater increases in plasticity when the therapy is used intensively, meaning at least four treatments consecutively,” said Ms. Bryant. Participants in the NMT group also reported they “overall felt well,” she added.
She noted NMT can be tailored to individual deficit, “so you can make it solely for motor movement or you can make it solely for language.”
Next steps could include more closely targeting the music to individual preferences and investigating whether the benefits of the intervention extend to other types of brain injury, for example traumatic brain injury, which typically affects younger people, said Ms. Bryant.
“In this study, participants were older and there was an unknown; a lot of them were going back into the community but didn’t know if it was into a retirement home or long-term care.”
It’s unclear if the benefits are sustained after the intervention stops, she said.
There are also the issues of cost and accessibility; in Kingston, there are few music therapists certified in the area of NMT.
Ms. Bryant hopes NMT is eventually included in stroke rehabilitation. “Stroke therapy is typically very intensive on its own; you’re doing it every single day for about a month or 6 weeks,” she said. “It would be interesting to see whether we would see a shorter hospital stay if this is included in stroke rehab.”
Asked to comment, Michael H. Thaut, PhD, professor, faculty of music and faculty of medicine, and Canada research chair in music, neuroscience and health at the University of Toronto, said while these data are preliminary, “they do extend the benefits of NMT in stroke rehabilitation, especially measuring BDNF in addition to having behavioral data.”
However, it’s “unfortunate” the poster didn’t specify which cognitive intervention techniques were used in the study, said Dr. Thaut. “There are nine coded techniques in NMT, including for attention, memory, psychosocial function, and executive function.”
His own study, published in NeuroRehabilitation, focused on training for motor goals in stroke patients. It showed that NMT benefited cognitive functioning and affective responses.
The study was funded by a Queen’s University Research Initiation Grant. Ms. Bryant and Dr. Thaut have not disclosed any relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM WCN 2023
Suicide prevention and the pediatrician
Suicide is among the top three causes of death for young people in the United States. According to the Centers for Disease Control and Prevention, the rate of suicide deaths has climbed from 4.4 per 100,000 American 12- to 17-year-olds in 2011 to 6.5 per 100,000 in 2021, an increase of almost 50%. As with accidents and homicides, we hope these are preventable deaths, although the factors contributing to them are complex.
We do know that
Suicide screening
In 2022, the American Academy of Pediatrics (AAP) recommended that all adolescents get screened for suicide risk annually. Given that less than 1 in 10,000 adolescents commit suicide and that there is no definitive data on how to prevent suicide in any individual, the goal of suicide screening is much broader than preventing suicide. Beyond universal screening, we will review how being open and curious with all of your patients can be the most extraordinary screening instrument.
There is extensive data that tells us that far from causing suicide, asking about suicidal thoughts is protective. When you make suicidal thoughts discussable, you directly counteract the isolation, stigma, and shame that are strong predictors of actual suicide attempts. You model the value of bringing difficult or frightening thoughts to the attention of caring adults, and you model calm listening rather than emotional overreaction for their parents. The resulting connectedness can lower the risk for vulnerable patients and enhance resilience for all of your patients.
Who is at greater risk?
We have robust data to guide our understanding of which youth have suicidal ideation, which is distinct from those who attempt suicide, which also may be quite distinct from those who complete. The CDC reports that the rate of suicidal thoughts (“seriously considering suicide”) in high school students climbed from 16% in 2011 to 22% in 2021. In that decade, the number of high schoolers with a suicide plan climbed from 13% to 18%, and those with suicide attempts climbed from 8% to 10%. Girls are at higher risk for suicidal thoughts and attempts, but boys are at greater risk for suicide completion. Black youth were more likely to attempt suicide than were their Asian, Hispanic, or White peers and LGBTQ+ youth are at particular risk; in 2021, they were three times as likely as were their heterosexual peers to have suicidal thoughts and attempts. Youth with psychiatric illness (particularly PTSD, mood or thought disorders), a family history of suicide, a history of risk-taking behavior (including sexual activity, smoking, drinking, and drug use) and those with prior suicide attempts are at the highest risk for suicide. Adding all these risk factors together means that many, if not the majority, of teenagers have risk factors.
Focus on the patient
In your office, though, a public health approach should give way to curiosity about your individual patient. Suicidal thoughts usually follow a substantial stress. Pay attention to exceptional stresses, especially if they have a component of social stigma or isolation. Did your patient report another student for an assault? Are they now being bullied or ostracized by friends? Have they lost an especially important relationship? Some other stresses may seem minor, such as a poor grade on a test. But for a very driven, perfectionistic teenager who believes that a perfect 4.0 GPA is essential to college admission and future success and happiness, one poor grade may feel like a catastrophe.
When your patients tell you about a challenge or setback, slow down and be curious. Listen to the importance they give it. How have they managed it? Are they finding it hard to go to school or back to practice? Do they feel discouraged or even hopeless? Discouragement is a normal response to adversity, but it should be temporary. This approach can make it easy to ask if they have ever wished they were dead, or made a suicide plan or an attempt. When you calmly and supportively learn about their inner experience, it will be easy for young people to be honest with you.
There will be teenagers in your practice who are sensation-seeking and impulsive, and you should pay special attention to this group. They may not be classically depressed, but in the aftermath of a stressful experience that they find humiliating or shameful, they are at risk for an impulsive act that could still be lethal. Be curious with these patients after they feel they have let down their team or their family, or if they have been caught in a crime or cheating, or even if their girlfriend breaks up with them. Find out how they are managing, and where their support comes from. Ask them in a nonjudgmental manner about whether they are having thoughts about death or suicide, and if those thoughts are troubling, frequent, or feel like a relief. What has stopped them from acting on these thoughts? Offer your patient the perspective that such thoughts may be normal in the face of a large stress, but that the pain of stress always subsides, whereas suicide is irreversible.
There will also be patients in your practice who cut themselves. This is sometimes called “nonsuicidal self-injury,” and it often raises concern about suicide risk. While accelerating frequency of self-injury in a teenager who is suicidal can indicate growing risk, this behavior alone is usually a mechanism for regulating emotion. Ask your patient about when they cut themselves. What are the triggers? How do they feel afterward? Are their friends all doing it? Is it only after fighting with their parents? Or does it make their parents worry instead of getting angry? As you learn about the nature of the behavior, you will be able to offer thoughtful guidance about better strategies for stress management or to pursue further assessment and support.
Next steps
Speaking comfortably with your patients about suicidal thoughts and behaviors requires that you also feel comfortable with what comes next. As in the ASQ screening instrument recommended by the AAP, you should always follow affirmative answers about suicidal thoughts with more questions. Do they have a plan? Do they have access to lethal means including any guns in the home? Have they ever made an attempt? Are they thinking about killing themselves now? If the thoughts are current, they have access, and they have tried before, it is clear that they need an urgent assessment, probably in an emergency department. But when the thoughts were in the past or have never been connected to plans or intent, there is an opportunity to enhance their connectedness. You can diminish the potential for shame, stigma and isolation by reminding them that such thoughts and feelings are normal in the face of difficulty. They deserve support to help them face and manage their adversity, whether that stress comes from an internal or external source. How do they feel now that they have shared these thoughts with you? Most will describe feeling better, relieved, even hopeful once they are not facing intense thoughts and feelings alone.
You should tell them that you would like to bring their parents into the conversation. You want them to know they can turn to their parents if they are having these thoughts, so they are never alone in facing them. Parents can learn from your model of calm and supportive listening to fully understand the situation before turning together to talk about what might be helpful next steps. It is always prudent to create “speed bumps” between thought and action with impulsive teens, so recommend limiting access to any lethal means (firearms especially). But the strongest protective intervention is for the child to feel confident in and connected to their support network, trusting you and their parents to listen and understand before figuring out together what else is needed to address the situation.
Lastly, recognize that talking about difficult issues with teenagers is among the most stressful and demanding aspects of pediatric primary care. Talk to colleagues, never worry alone, and recognize and manage your own stress. This is among the best ways to model for your patients and their parents that every challenge can be met, but we often need support.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Suicide is among the top three causes of death for young people in the United States. According to the Centers for Disease Control and Prevention, the rate of suicide deaths has climbed from 4.4 per 100,000 American 12- to 17-year-olds in 2011 to 6.5 per 100,000 in 2021, an increase of almost 50%. As with accidents and homicides, we hope these are preventable deaths, although the factors contributing to them are complex.
We do know that
Suicide screening
In 2022, the American Academy of Pediatrics (AAP) recommended that all adolescents get screened for suicide risk annually. Given that less than 1 in 10,000 adolescents commit suicide and that there is no definitive data on how to prevent suicide in any individual, the goal of suicide screening is much broader than preventing suicide. Beyond universal screening, we will review how being open and curious with all of your patients can be the most extraordinary screening instrument.
There is extensive data that tells us that far from causing suicide, asking about suicidal thoughts is protective. When you make suicidal thoughts discussable, you directly counteract the isolation, stigma, and shame that are strong predictors of actual suicide attempts. You model the value of bringing difficult or frightening thoughts to the attention of caring adults, and you model calm listening rather than emotional overreaction for their parents. The resulting connectedness can lower the risk for vulnerable patients and enhance resilience for all of your patients.
Who is at greater risk?
We have robust data to guide our understanding of which youth have suicidal ideation, which is distinct from those who attempt suicide, which also may be quite distinct from those who complete. The CDC reports that the rate of suicidal thoughts (“seriously considering suicide”) in high school students climbed from 16% in 2011 to 22% in 2021. In that decade, the number of high schoolers with a suicide plan climbed from 13% to 18%, and those with suicide attempts climbed from 8% to 10%. Girls are at higher risk for suicidal thoughts and attempts, but boys are at greater risk for suicide completion. Black youth were more likely to attempt suicide than were their Asian, Hispanic, or White peers and LGBTQ+ youth are at particular risk; in 2021, they were three times as likely as were their heterosexual peers to have suicidal thoughts and attempts. Youth with psychiatric illness (particularly PTSD, mood or thought disorders), a family history of suicide, a history of risk-taking behavior (including sexual activity, smoking, drinking, and drug use) and those with prior suicide attempts are at the highest risk for suicide. Adding all these risk factors together means that many, if not the majority, of teenagers have risk factors.
Focus on the patient
In your office, though, a public health approach should give way to curiosity about your individual patient. Suicidal thoughts usually follow a substantial stress. Pay attention to exceptional stresses, especially if they have a component of social stigma or isolation. Did your patient report another student for an assault? Are they now being bullied or ostracized by friends? Have they lost an especially important relationship? Some other stresses may seem minor, such as a poor grade on a test. But for a very driven, perfectionistic teenager who believes that a perfect 4.0 GPA is essential to college admission and future success and happiness, one poor grade may feel like a catastrophe.
When your patients tell you about a challenge or setback, slow down and be curious. Listen to the importance they give it. How have they managed it? Are they finding it hard to go to school or back to practice? Do they feel discouraged or even hopeless? Discouragement is a normal response to adversity, but it should be temporary. This approach can make it easy to ask if they have ever wished they were dead, or made a suicide plan or an attempt. When you calmly and supportively learn about their inner experience, it will be easy for young people to be honest with you.
There will be teenagers in your practice who are sensation-seeking and impulsive, and you should pay special attention to this group. They may not be classically depressed, but in the aftermath of a stressful experience that they find humiliating or shameful, they are at risk for an impulsive act that could still be lethal. Be curious with these patients after they feel they have let down their team or their family, or if they have been caught in a crime or cheating, or even if their girlfriend breaks up with them. Find out how they are managing, and where their support comes from. Ask them in a nonjudgmental manner about whether they are having thoughts about death or suicide, and if those thoughts are troubling, frequent, or feel like a relief. What has stopped them from acting on these thoughts? Offer your patient the perspective that such thoughts may be normal in the face of a large stress, but that the pain of stress always subsides, whereas suicide is irreversible.
There will also be patients in your practice who cut themselves. This is sometimes called “nonsuicidal self-injury,” and it often raises concern about suicide risk. While accelerating frequency of self-injury in a teenager who is suicidal can indicate growing risk, this behavior alone is usually a mechanism for regulating emotion. Ask your patient about when they cut themselves. What are the triggers? How do they feel afterward? Are their friends all doing it? Is it only after fighting with their parents? Or does it make their parents worry instead of getting angry? As you learn about the nature of the behavior, you will be able to offer thoughtful guidance about better strategies for stress management or to pursue further assessment and support.
Next steps
Speaking comfortably with your patients about suicidal thoughts and behaviors requires that you also feel comfortable with what comes next. As in the ASQ screening instrument recommended by the AAP, you should always follow affirmative answers about suicidal thoughts with more questions. Do they have a plan? Do they have access to lethal means including any guns in the home? Have they ever made an attempt? Are they thinking about killing themselves now? If the thoughts are current, they have access, and they have tried before, it is clear that they need an urgent assessment, probably in an emergency department. But when the thoughts were in the past or have never been connected to plans or intent, there is an opportunity to enhance their connectedness. You can diminish the potential for shame, stigma and isolation by reminding them that such thoughts and feelings are normal in the face of difficulty. They deserve support to help them face and manage their adversity, whether that stress comes from an internal or external source. How do they feel now that they have shared these thoughts with you? Most will describe feeling better, relieved, even hopeful once they are not facing intense thoughts and feelings alone.
You should tell them that you would like to bring their parents into the conversation. You want them to know they can turn to their parents if they are having these thoughts, so they are never alone in facing them. Parents can learn from your model of calm and supportive listening to fully understand the situation before turning together to talk about what might be helpful next steps. It is always prudent to create “speed bumps” between thought and action with impulsive teens, so recommend limiting access to any lethal means (firearms especially). But the strongest protective intervention is for the child to feel confident in and connected to their support network, trusting you and their parents to listen and understand before figuring out together what else is needed to address the situation.
Lastly, recognize that talking about difficult issues with teenagers is among the most stressful and demanding aspects of pediatric primary care. Talk to colleagues, never worry alone, and recognize and manage your own stress. This is among the best ways to model for your patients and their parents that every challenge can be met, but we often need support.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Suicide is among the top three causes of death for young people in the United States. According to the Centers for Disease Control and Prevention, the rate of suicide deaths has climbed from 4.4 per 100,000 American 12- to 17-year-olds in 2011 to 6.5 per 100,000 in 2021, an increase of almost 50%. As with accidents and homicides, we hope these are preventable deaths, although the factors contributing to them are complex.
We do know that
Suicide screening
In 2022, the American Academy of Pediatrics (AAP) recommended that all adolescents get screened for suicide risk annually. Given that less than 1 in 10,000 adolescents commit suicide and that there is no definitive data on how to prevent suicide in any individual, the goal of suicide screening is much broader than preventing suicide. Beyond universal screening, we will review how being open and curious with all of your patients can be the most extraordinary screening instrument.
There is extensive data that tells us that far from causing suicide, asking about suicidal thoughts is protective. When you make suicidal thoughts discussable, you directly counteract the isolation, stigma, and shame that are strong predictors of actual suicide attempts. You model the value of bringing difficult or frightening thoughts to the attention of caring adults, and you model calm listening rather than emotional overreaction for their parents. The resulting connectedness can lower the risk for vulnerable patients and enhance resilience for all of your patients.
Who is at greater risk?
We have robust data to guide our understanding of which youth have suicidal ideation, which is distinct from those who attempt suicide, which also may be quite distinct from those who complete. The CDC reports that the rate of suicidal thoughts (“seriously considering suicide”) in high school students climbed from 16% in 2011 to 22% in 2021. In that decade, the number of high schoolers with a suicide plan climbed from 13% to 18%, and those with suicide attempts climbed from 8% to 10%. Girls are at higher risk for suicidal thoughts and attempts, but boys are at greater risk for suicide completion. Black youth were more likely to attempt suicide than were their Asian, Hispanic, or White peers and LGBTQ+ youth are at particular risk; in 2021, they were three times as likely as were their heterosexual peers to have suicidal thoughts and attempts. Youth with psychiatric illness (particularly PTSD, mood or thought disorders), a family history of suicide, a history of risk-taking behavior (including sexual activity, smoking, drinking, and drug use) and those with prior suicide attempts are at the highest risk for suicide. Adding all these risk factors together means that many, if not the majority, of teenagers have risk factors.
Focus on the patient
In your office, though, a public health approach should give way to curiosity about your individual patient. Suicidal thoughts usually follow a substantial stress. Pay attention to exceptional stresses, especially if they have a component of social stigma or isolation. Did your patient report another student for an assault? Are they now being bullied or ostracized by friends? Have they lost an especially important relationship? Some other stresses may seem minor, such as a poor grade on a test. But for a very driven, perfectionistic teenager who believes that a perfect 4.0 GPA is essential to college admission and future success and happiness, one poor grade may feel like a catastrophe.
When your patients tell you about a challenge or setback, slow down and be curious. Listen to the importance they give it. How have they managed it? Are they finding it hard to go to school or back to practice? Do they feel discouraged or even hopeless? Discouragement is a normal response to adversity, but it should be temporary. This approach can make it easy to ask if they have ever wished they were dead, or made a suicide plan or an attempt. When you calmly and supportively learn about their inner experience, it will be easy for young people to be honest with you.
There will be teenagers in your practice who are sensation-seeking and impulsive, and you should pay special attention to this group. They may not be classically depressed, but in the aftermath of a stressful experience that they find humiliating or shameful, they are at risk for an impulsive act that could still be lethal. Be curious with these patients after they feel they have let down their team or their family, or if they have been caught in a crime or cheating, or even if their girlfriend breaks up with them. Find out how they are managing, and where their support comes from. Ask them in a nonjudgmental manner about whether they are having thoughts about death or suicide, and if those thoughts are troubling, frequent, or feel like a relief. What has stopped them from acting on these thoughts? Offer your patient the perspective that such thoughts may be normal in the face of a large stress, but that the pain of stress always subsides, whereas suicide is irreversible.
There will also be patients in your practice who cut themselves. This is sometimes called “nonsuicidal self-injury,” and it often raises concern about suicide risk. While accelerating frequency of self-injury in a teenager who is suicidal can indicate growing risk, this behavior alone is usually a mechanism for regulating emotion. Ask your patient about when they cut themselves. What are the triggers? How do they feel afterward? Are their friends all doing it? Is it only after fighting with their parents? Or does it make their parents worry instead of getting angry? As you learn about the nature of the behavior, you will be able to offer thoughtful guidance about better strategies for stress management or to pursue further assessment and support.
Next steps
Speaking comfortably with your patients about suicidal thoughts and behaviors requires that you also feel comfortable with what comes next. As in the ASQ screening instrument recommended by the AAP, you should always follow affirmative answers about suicidal thoughts with more questions. Do they have a plan? Do they have access to lethal means including any guns in the home? Have they ever made an attempt? Are they thinking about killing themselves now? If the thoughts are current, they have access, and they have tried before, it is clear that they need an urgent assessment, probably in an emergency department. But when the thoughts were in the past or have never been connected to plans or intent, there is an opportunity to enhance their connectedness. You can diminish the potential for shame, stigma and isolation by reminding them that such thoughts and feelings are normal in the face of difficulty. They deserve support to help them face and manage their adversity, whether that stress comes from an internal or external source. How do they feel now that they have shared these thoughts with you? Most will describe feeling better, relieved, even hopeful once they are not facing intense thoughts and feelings alone.
You should tell them that you would like to bring their parents into the conversation. You want them to know they can turn to their parents if they are having these thoughts, so they are never alone in facing them. Parents can learn from your model of calm and supportive listening to fully understand the situation before turning together to talk about what might be helpful next steps. It is always prudent to create “speed bumps” between thought and action with impulsive teens, so recommend limiting access to any lethal means (firearms especially). But the strongest protective intervention is for the child to feel confident in and connected to their support network, trusting you and their parents to listen and understand before figuring out together what else is needed to address the situation.
Lastly, recognize that talking about difficult issues with teenagers is among the most stressful and demanding aspects of pediatric primary care. Talk to colleagues, never worry alone, and recognize and manage your own stress. This is among the best ways to model for your patients and their parents that every challenge can be met, but we often need support.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Five hours or less of sleep per night tied to subsequent depression
TOPLINE:
, new research shows.
METHODOLOGY:
- The analysis included participants in the English Longitudinal Study of Ageing (ELSA), a prospective cohort study of a representative U.K. sample (mean age, 65 years) that is assessed biennially.
- Researchers collected data on sleep duration and depression through nursing home visits and computer-assisted personal interviews and used combined ELSA waves from 2004 to 2008, when collection of genetic data began.
- Using genome-wide association studies from the U.K. Biobank, the authors constructed polygenic scores (PGSs) to predict an individual’s genetic risk over an average of 8 years for a disease or outcome, overall sleep duration, short sleep (≤ 5 hours nightly), long sleep (≥ 9 hours of sleep nightly), and depression.
- The analysis included two analytic samples; one involved 6,521 persons to determine the role of baseline sleep on depression (assessed using the Center for Epidemiologic Studies Depression Scale) at follow-up, and the other involved 6,070 persons to determine the role of baseline depression on suboptimal sleep at follow-up.
TAKEAWAY:
- After adjustments, including for age and sex, a 1–standard deviation increase in PGS for short sleep was associated with an increase of 14% in odds of developing depression during the follow-up period (odds ratio, 1.14; P = .008).
- There was no significant association of the PGS for sleep duration (P = .053) or long sleep (P = .544) with the onset of depression.
- There were no significant associations between PGS for depression and future overall sleep duration, short sleep, and long sleep by the end of the follow-up, suggesting that different mechanisms underlie the relationship between depression and subsequent onset of suboptimal sleep in older adults.
- Several sensitivity analyses – including additional adjustment for socioeconomic, environmental, and behavioral factors – upheld the findings of the main analysis, highlighting the robustness of the results.
IN PRACTICE:
The study showed that common genetic markers for short sleep play an important role in the incidence of depression in older adults, the authors note, adding that the new findings “support a growing view that short-sleep is more salient to the experience of depression than long sleep” across the lifespan.
SOURCE:
The study was led by Odessa S. Hamilton, department of behavioral science and health, University College London. It was published online in Translational Psychiatry.
LIMITATIONS:
There are probably intraindividual differences in sleep duration that were not assessed in the study. The depression scale used may be indicative of subclinical depression and not major depressive disorder. The phenotypic sensitivity analyses did not account for comorbidities or medications that can affect sleep duration and depression.
DISCLOSURES:
ELSA is funded by the National Institute on Aging and by a consortium of U.K. government departments coordinated by the National Institute for Health and Care Research. The authors report no relevant conflicts of interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- The analysis included participants in the English Longitudinal Study of Ageing (ELSA), a prospective cohort study of a representative U.K. sample (mean age, 65 years) that is assessed biennially.
- Researchers collected data on sleep duration and depression through nursing home visits and computer-assisted personal interviews and used combined ELSA waves from 2004 to 2008, when collection of genetic data began.
- Using genome-wide association studies from the U.K. Biobank, the authors constructed polygenic scores (PGSs) to predict an individual’s genetic risk over an average of 8 years for a disease or outcome, overall sleep duration, short sleep (≤ 5 hours nightly), long sleep (≥ 9 hours of sleep nightly), and depression.
- The analysis included two analytic samples; one involved 6,521 persons to determine the role of baseline sleep on depression (assessed using the Center for Epidemiologic Studies Depression Scale) at follow-up, and the other involved 6,070 persons to determine the role of baseline depression on suboptimal sleep at follow-up.
TAKEAWAY:
- After adjustments, including for age and sex, a 1–standard deviation increase in PGS for short sleep was associated with an increase of 14% in odds of developing depression during the follow-up period (odds ratio, 1.14; P = .008).
- There was no significant association of the PGS for sleep duration (P = .053) or long sleep (P = .544) with the onset of depression.
- There were no significant associations between PGS for depression and future overall sleep duration, short sleep, and long sleep by the end of the follow-up, suggesting that different mechanisms underlie the relationship between depression and subsequent onset of suboptimal sleep in older adults.
- Several sensitivity analyses – including additional adjustment for socioeconomic, environmental, and behavioral factors – upheld the findings of the main analysis, highlighting the robustness of the results.
IN PRACTICE:
The study showed that common genetic markers for short sleep play an important role in the incidence of depression in older adults, the authors note, adding that the new findings “support a growing view that short-sleep is more salient to the experience of depression than long sleep” across the lifespan.
SOURCE:
The study was led by Odessa S. Hamilton, department of behavioral science and health, University College London. It was published online in Translational Psychiatry.
LIMITATIONS:
There are probably intraindividual differences in sleep duration that were not assessed in the study. The depression scale used may be indicative of subclinical depression and not major depressive disorder. The phenotypic sensitivity analyses did not account for comorbidities or medications that can affect sleep duration and depression.
DISCLOSURES:
ELSA is funded by the National Institute on Aging and by a consortium of U.K. government departments coordinated by the National Institute for Health and Care Research. The authors report no relevant conflicts of interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
, new research shows.
METHODOLOGY:
- The analysis included participants in the English Longitudinal Study of Ageing (ELSA), a prospective cohort study of a representative U.K. sample (mean age, 65 years) that is assessed biennially.
- Researchers collected data on sleep duration and depression through nursing home visits and computer-assisted personal interviews and used combined ELSA waves from 2004 to 2008, when collection of genetic data began.
- Using genome-wide association studies from the U.K. Biobank, the authors constructed polygenic scores (PGSs) to predict an individual’s genetic risk over an average of 8 years for a disease or outcome, overall sleep duration, short sleep (≤ 5 hours nightly), long sleep (≥ 9 hours of sleep nightly), and depression.
- The analysis included two analytic samples; one involved 6,521 persons to determine the role of baseline sleep on depression (assessed using the Center for Epidemiologic Studies Depression Scale) at follow-up, and the other involved 6,070 persons to determine the role of baseline depression on suboptimal sleep at follow-up.
TAKEAWAY:
- After adjustments, including for age and sex, a 1–standard deviation increase in PGS for short sleep was associated with an increase of 14% in odds of developing depression during the follow-up period (odds ratio, 1.14; P = .008).
- There was no significant association of the PGS for sleep duration (P = .053) or long sleep (P = .544) with the onset of depression.
- There were no significant associations between PGS for depression and future overall sleep duration, short sleep, and long sleep by the end of the follow-up, suggesting that different mechanisms underlie the relationship between depression and subsequent onset of suboptimal sleep in older adults.
- Several sensitivity analyses – including additional adjustment for socioeconomic, environmental, and behavioral factors – upheld the findings of the main analysis, highlighting the robustness of the results.
IN PRACTICE:
The study showed that common genetic markers for short sleep play an important role in the incidence of depression in older adults, the authors note, adding that the new findings “support a growing view that short-sleep is more salient to the experience of depression than long sleep” across the lifespan.
SOURCE:
The study was led by Odessa S. Hamilton, department of behavioral science and health, University College London. It was published online in Translational Psychiatry.
LIMITATIONS:
There are probably intraindividual differences in sleep duration that were not assessed in the study. The depression scale used may be indicative of subclinical depression and not major depressive disorder. The phenotypic sensitivity analyses did not account for comorbidities or medications that can affect sleep duration and depression.
DISCLOSURES:
ELSA is funded by the National Institute on Aging and by a consortium of U.K. government departments coordinated by the National Institute for Health and Care Research. The authors report no relevant conflicts of interests.
A version of this article first appeared on Medscape.com.
FROM TRANSLATIONAL PSYCHIATRY
Perinatal depression rarely stands alone
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Mental health conditions are the leading cause of pregnancy-related death in Illinois (40%) and across the United States (21%).1,2 Funding bodies, such as the Agency for Healthcare Research and Quality3 and the Health Resources and Service Administration,4 have spotlights on improving screening and access to care for depression and substance use disorders (SUDs). However, the needs of individuals with multiple mental health conditions still often go unrecognized and unaddressed in perinatal health settings.
The U.S. Preventive Services Task Force recommends that all adults be screened for depression, alcohol use, and drug use, and will be recommending screening for anxiety.5,6 The American College of Obstetrics and Gynecology recommends screening for perinatal mental health conditions including depression, anxiety, bipolar disorder, acute postpartum psychosis, and suicidality; however, despite these recommendations, screening and treatment for comorbid mental health disorders during pregnancy and the postpartum is not standard practice.7
Addressing perinatal mental health is critical because untreated mental health conditions during the perinatal period can cause long-term adverse psychiatric and medical outcomes for the birthing person, the baby, and the family.8 This commentary highlights the importance of recognizing and screening for perinatal mental health comorbidities, improving referral rates for mental health treatment, and raising awareness of the importance of addressing rural perinatal mental health.
Perinatal mental health comorbidities
Major depressive disorder is the most common mental health condition during the perinatal period9 and is often comorbid.10-12 In “Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities,” Craemer et al.13 reported that nearly half of the perinatal patients who screened positive for MDD also screened positive for at least one other mental health condition, among them general anxiety disorder (GAD), SUD, posttraumatic stress disorder (PTSD), and suicidality.
Many (9%) of the perinatal patients with MDD had a severe comorbidity profile characterized by four diagnoses – MDD, GAD, SUD, and PTSD. In routine medical care these comorbidities often go undetected even though the risk to mothers and babies increases with more severe mental health symptoms.8
The high frequency of perinatal mental health comorbidities Craemer et al.13 found demonstrates a compelling need for comorbid mental health screening during the perinatal period, particularly for low-income Black, Hispanic, and rural birthing persons. Positive screens for perinatal mental health disorders may reflect the onset of these disorders in pregnancy or the postpartum, or preexisting disorders that have gone undetected or untreated before pregnancy.
For many patients, the perinatal period is the first time they are screened for any mental health disorder; typically, they are screened solely for depression. Screening alone can have a positive impact on perinatal mental health. In fact, the USPSTF found that programs to screen perinatal patients, with or without treatment-related support, resulted in a 2%-9% absolute reduction in depression prevalence.14 However, screening for MDD is too infrequent for many reasons, including the logistics of integrating screening into the clinic workflow and limited provider availability, time, and training in mental health.
We recommend screening perinatal patients for mental health comorbidities. This recommendation may seem impractical given the lack of screening tools for comorbid mental health conditions; however, the Computerized Adaptive Test for Mental Health (CAT-MH), the validated tool15-17 used in this study, is an ideal option. CAT-MH is uniquely capable of screening for MDD, GAD, PTSD, SUD, and suicidality in one platform and is routinely used in diverse settings including the Veterans Administration,18 foster care,19 and universities.20 The main limitation of this more comprehensive screening is that it takes about 10 minutes per patient. However, CAT-MH is self-administered and can be done in the waiting room or on a mobile device prior to a clinic visit.
CAT-MH can also be easily integrated into clinical workflow when added to the Electronic Medical Record21, and is a more comprehensive tool than existing perinatal depression tools such as the Perinatal Health Questionaire-9 (PHQ-9) and Edinburgh Perinatal Depression Scale (EPDS).22 Another limitation is cost – currently $5.00 per assessment – however, this is less than routine blood work.23 If CAT-MH is not an option, we recommend a stepped approach of screening for GAD when perinatal patients screen positive for MDD, as this is the most common comorbidity profile. The GAD-7 is a free and widely available tool.24
Barriers to care
In Craemer et al,13 nearly two-thirds (64.9%) of perinatal patients with a positive screen did not receive a referral to follow-up care or a medication prescription. These low referral rates may reflect a variety of widely recognized barriers to care, including lack of referral options, provider and/or patient reluctance to pursue referrals, barriers to insurance coverage, or inadequate behavioral health infrastructure to ensure referral and diagnostic follow-up.
Further, rural residing perinatal patients are an underserved population that need more resources and screening. Despite an on-site behavioral specialist at the rural clinic, Craemer et al13 found a stark disparity in referral rates: referrals to treatment for a positive diagnosis was over two times less at the rural clinic (23.9%), compared with the urban clinics (51.6%). The most common treatment offered at the rural clinic was a prescription for medication (17.4%), while referral to follow-up care was the most common at the urban clinics (35.5%). Rural areas not only have a shortage of health care providers, but community members seeking mental health care often encounter greater stigma, compared with urban residents.25,26
These data highlight an unmet need for referrals to treatment for patients in rural communities, particularly in Illinois where the pregnancy-related mortality ratio attributable to mental health conditions is three times greater in rural areas, compared with those residing in urban Cook County (Chicago).2 Increasing access and availability to mental health treatment and prevention resources in Illinois, especially in rural areas, is an opportunity to prevent pregnancy-related mortality attributable to mental health conditions.
Overall, there is a critical need for screening for perinatal mental health comorbidities, increased attention to low rates of referral to mental health treatment, and investing in rural perinatal mental health. Addressing perinatal mental health disorders is key to decreasing the burden of maternal mortality, particularly in Illinois.
Ms. Craemer and Ms. Sayah are senior research specialists at the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Duffecy is a professor of clinical psychiatry at the University of Illinois at Chicago. Dr. Geller is a professor of obstetrics & gynecology and director of the Center for Research on Women & Gender, University of Illinois at Chicago. Dr. Maki is a professor of psychiatry, psychology, and obstetrics & gynecology at the University of Illinois at Chicago.
References
1. Trost S et al. Pregnancy-related deaths: Data from maternal mortality review committees in 36 states, 2017-2019. Atlanta: Centers for Disease Control and Prevention, U.S. Department of Health & Human Services, 2022.
2. Illinois Department of Public Health. Illinois maternal morbidity and mortality report 2016-2017. 2021.
3. AHRQ. Funding opportunities to address opioid and other substance use disorders. Updated 2023.
4. HRSA. Screening and treatment for maternal mental health and substance use disorders.
5. U.S. Preventive Services Task Force. Recommendations for primary care practice. Accessed May 26, 2023.
6. U.S. Preventive Services Task Force. Draft recommendation statement: Anxiety in adults: Screening. 2022.
7. ACOG. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline. Number 4. 2023 June.
8. Meltzer-Brody S and Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol. 2014 Jan. doi: 10.1016/j.bpobgyn.2013.08.009.
9. Van Niel MS and Payne JL. Perinatal depression: A review. Cleve Clin J Med. 2020 May. doi: 10.3949/ccjm.87a.19054.
10. Wisner KL et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. 2013 May. doi: 10.1001/jamapsychiatry.2013.87.
11. Falah-Hassani K et al. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol Med. 2017 Sep. doi: 10.1017/S0033291717000617.
12. Pentecost R et al. Scoping review of the associations between perinatal substance use and perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2021 Jul. doi: 10.1016/j.jogn.2021.02.008.
13. Craemer KA et al. Perinatal mental health in low-income urban and rural patients: The importance of screening for comorbidities. Gen Hosp Psychiatry. 2023 Jul-Aug. doi: 10.1016/j.genhosppsych.2023.05.007.
14. O’Connor E et al. Primary care screening for and treatment of depression in pregnant and postpartum women: Evidence report and systematic review for the U.S. Preventive Services Task Force. JAMA. 2016 Jan 26. doi: 10.1001/jama.2015.18948.
15. Kozhimannil KB et al. Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv. 2011 Jun. doi: 10.1176/ps.62.6.pss6206_0619.
16. Wenzel ES et al. Depression and anxiety symptoms across pregnancy and the postpartum in low-income Black and Latina women. Arch Womens Ment Health. 2021 Dec. doi: 10.1007/s00737-021-01139-y.
17. Gibbons RD et al. Development of a computerized adaptive substance use disorder scale for screening and measurement: The CAT‐SUD. Addiction. 2020 Jul. doi: 10.1111/add.14938.
18. Brenner LA et al. Validation of a computerized adaptive test suicide scale (CAT-SS) among united states military veterans. PloS One. 2022 Jan 21. doi: 10.1371/journal.pone.0261920.
19. The Center for State Child Welfare Data. Using technology to diagnose and report on behavioral health challenges facing foster youth. 2018.
20. Kim JJ et al. The experience of depression, anxiety, and mania among perinatal women. Arch Womens Ment Health. 2016 Oct. doi: 10.1007/s00737-016-0632-6.
21. Tepper MC et al. Toward population health: Using a learning behavioral health system and measurement-based care to improve access, care, outcomes, and disparities. Community Ment Health J. 2022 Nov. doi: 10.1007/s10597-022-00957-3.
22. Wenzel E et al. Using computerised adaptive tests to screen for perinatal depression in underserved women of colour. Evid Based Ment Health. 2022 Feb. doi: 10.1136/ebmental-2021-300262.
23. Sanger-Katz M. They want it to be secret: How a common blood test can cost $11 or almost $1,000. New York Times. 2019 Apr 19.
24. Spitzer RL et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006 May 22. doi: 10.1001/archinte.166.10.1092.
25. Mollard E et al. An integrative review of postpartum depression in rural US communities. Arch Psychiatr Nurs. 2016 Jun. doi: 10.1016/j.apnu.2015.12.003.
26. Anglim AJ and Radke SM. Rural maternal health care outcomes, drivers, and patient perspectives. Clin Obstet Gynecol. 2022 Dec 1. doi: 10.1097/GRF.0000000000000753.
Serious mental illness tied to 50% higher all-cause mortality risk after COVID
TOPLINE:
METHODOLOGY:
- Investigators analyzed data from the Clinical Practice Research Datalink database, which contains health information on 13.5 million patients receiving care from family practices in England and Northern Ireland.
- The study included participants with SMI, including schizophrenia, schizoaffective disorder, and bipolar disorder.
- Participants were aged 5 years or older with a SARS-CoV-2 infection recorded between Feb. 1, 2020, and March 31, 2021, spanning two waves of the pandemic.
- Death rates among participants with SMI and COVID-19 (n = 7,150; 56% female) were compared with those in a control group of participants without SMI who had been diagnosed with COVID-19 (n = 650,000; 55% female).
TAKEAWAY:
- Participants with SMI and COVID-19 had a 53% higher risk for death than those in the non-SMI control group (adjusted hazard ratio, 1.53; 95% confidence interval, 1.39-1.68).
- Black Caribbean/Black African participants were more likely than White participants to die of COVID-19 (aHR, 1.22; 95% CI, 1.12-1.34), although ethnicity was not recorded in 30% of participants.
- After SARS-CoV-2 infection, for every additional multimorbid condition, the aHR for death increased by 6% in the SMI group and 16% in the non-SMI group (P = .001). Some of these conditions included hypertension, heart disease, diabetes, kidney disease, depression, and anxiety.
IN PRACTICE:
“From a public health perspective, our study has emphasized the need for early and timely preventative interventions (e.g. vaccination) for the SMI population. Future studies are needed to disentangle the complex biological and psychosocial factors, and health care pathways, that have led to the greater mortality rates in the SMI population,” the authors write.
SOURCE:
Jayati Das-Munshi, MD, of Kings College London, led the study, which was published online in the British Journal of Psychiatry. The study was funded by the Health Foundation.
LIMITATIONS:
COVID-19 may have been underdiagnosed or underreported in the records studied. Also, investigators did not have information about cause of death.
DISCLOSURES:
One author received funding from Janssen, GSK, and Takeda. All other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators analyzed data from the Clinical Practice Research Datalink database, which contains health information on 13.5 million patients receiving care from family practices in England and Northern Ireland.
- The study included participants with SMI, including schizophrenia, schizoaffective disorder, and bipolar disorder.
- Participants were aged 5 years or older with a SARS-CoV-2 infection recorded between Feb. 1, 2020, and March 31, 2021, spanning two waves of the pandemic.
- Death rates among participants with SMI and COVID-19 (n = 7,150; 56% female) were compared with those in a control group of participants without SMI who had been diagnosed with COVID-19 (n = 650,000; 55% female).
TAKEAWAY:
- Participants with SMI and COVID-19 had a 53% higher risk for death than those in the non-SMI control group (adjusted hazard ratio, 1.53; 95% confidence interval, 1.39-1.68).
- Black Caribbean/Black African participants were more likely than White participants to die of COVID-19 (aHR, 1.22; 95% CI, 1.12-1.34), although ethnicity was not recorded in 30% of participants.
- After SARS-CoV-2 infection, for every additional multimorbid condition, the aHR for death increased by 6% in the SMI group and 16% in the non-SMI group (P = .001). Some of these conditions included hypertension, heart disease, diabetes, kidney disease, depression, and anxiety.
IN PRACTICE:
“From a public health perspective, our study has emphasized the need for early and timely preventative interventions (e.g. vaccination) for the SMI population. Future studies are needed to disentangle the complex biological and psychosocial factors, and health care pathways, that have led to the greater mortality rates in the SMI population,” the authors write.
SOURCE:
Jayati Das-Munshi, MD, of Kings College London, led the study, which was published online in the British Journal of Psychiatry. The study was funded by the Health Foundation.
LIMITATIONS:
COVID-19 may have been underdiagnosed or underreported in the records studied. Also, investigators did not have information about cause of death.
DISCLOSURES:
One author received funding from Janssen, GSK, and Takeda. All other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators analyzed data from the Clinical Practice Research Datalink database, which contains health information on 13.5 million patients receiving care from family practices in England and Northern Ireland.
- The study included participants with SMI, including schizophrenia, schizoaffective disorder, and bipolar disorder.
- Participants were aged 5 years or older with a SARS-CoV-2 infection recorded between Feb. 1, 2020, and March 31, 2021, spanning two waves of the pandemic.
- Death rates among participants with SMI and COVID-19 (n = 7,150; 56% female) were compared with those in a control group of participants without SMI who had been diagnosed with COVID-19 (n = 650,000; 55% female).
TAKEAWAY:
- Participants with SMI and COVID-19 had a 53% higher risk for death than those in the non-SMI control group (adjusted hazard ratio, 1.53; 95% confidence interval, 1.39-1.68).
- Black Caribbean/Black African participants were more likely than White participants to die of COVID-19 (aHR, 1.22; 95% CI, 1.12-1.34), although ethnicity was not recorded in 30% of participants.
- After SARS-CoV-2 infection, for every additional multimorbid condition, the aHR for death increased by 6% in the SMI group and 16% in the non-SMI group (P = .001). Some of these conditions included hypertension, heart disease, diabetes, kidney disease, depression, and anxiety.
IN PRACTICE:
“From a public health perspective, our study has emphasized the need for early and timely preventative interventions (e.g. vaccination) for the SMI population. Future studies are needed to disentangle the complex biological and psychosocial factors, and health care pathways, that have led to the greater mortality rates in the SMI population,” the authors write.
SOURCE:
Jayati Das-Munshi, MD, of Kings College London, led the study, which was published online in the British Journal of Psychiatry. The study was funded by the Health Foundation.
LIMITATIONS:
COVID-19 may have been underdiagnosed or underreported in the records studied. Also, investigators did not have information about cause of death.
DISCLOSURES:
One author received funding from Janssen, GSK, and Takeda. All other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Managing psychotropic-induced hyperhidrosis
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.
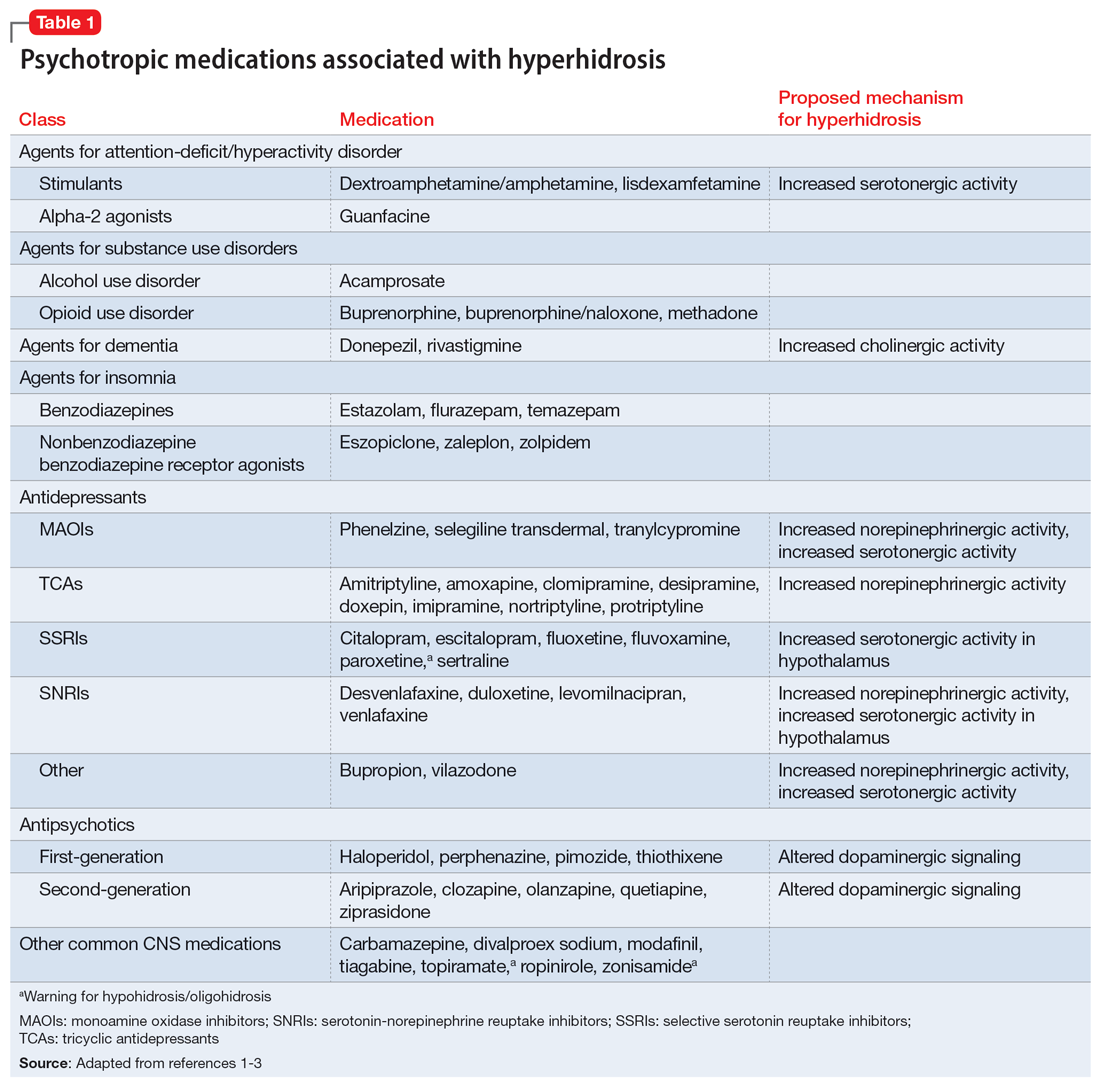
The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.
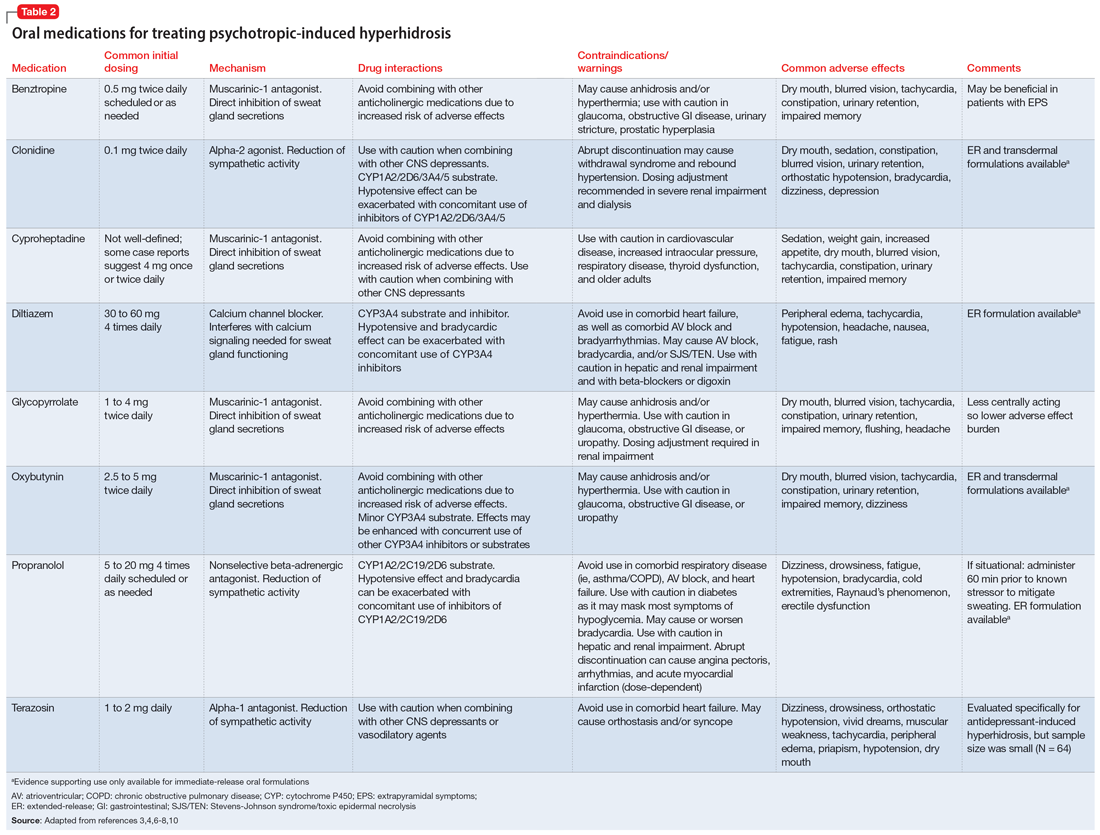
Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
Ms. K, age 32, presents to the psychiatric clinic for a routine follow-up. Her history includes agoraphobia, attention-deficit/hyperactivity disorder, and schizoaffective disorder. Ms. K’s current medications are oral hydroxyzine 50 mg 4 times daily as needed for anxiety and paliperidone palmitate 234 mg IM monthly. Since her last follow-up, she has been switched from oral sertraline 150 mg/d to oral paroxetine 20 mg/d. Ms. K reports having constipation (which improves by taking oral docusate 100 mg twice daily) and generalized hyperhidrosis. She wants to alleviate the hyperhidrosis without changing her paroxetine because that medication improved her symptoms.
Hyperhidrosis—excessive sweating not needed to maintain a normal body temperature—is an uncommon and uncomfortable adverse effect of many medications, including psychotropics.1 This long-term adverse effect typically is not dose-related and does not remit with continued therapy.2 Table 11-3 lists psychotropic medications associated with hyperhidrosis as well as postulated mechanisms.

The incidence of medication-induced hyperhidrosis is unknown, but for psychotropic medications it is estimated to be 5% to 20%.3 Patients may not report hyperhidrosis due to embarrassment; in clinical trials, reporting measures may be inconsistent and, in some cases, misleading. For example, it is possible hyperhidrosis that appears to be associated with buprenorphine is actually a symptom of the withdrawal syndrome rather than a direct effect of the medication. Also, some medications, including certain psychotropics (eg, paroxetine4 and topiramate3) may cause either hyperhidrosis or hypohidrosis (decreased sweating). Few medications carry labeled warnings for hypohidrosis; the condition generally is not of clinical concern unless patients experience heat intolerance or hyperthermia.3
Psychotropic-induced hyperhidrosis is likely an idiopathic effect. There are few known predisposing factors, but some medications carry a greater risk than others. In a meta-analysis, Beyer et al2 found certain selective serotonin reuptake inhibitors (SSRIs), such as sertraline and paroxetine, had a higher risk of causing hyperhidrosis. Fluvoxamine, bupropion, and vortioxetine had the lowest risk. The class risk for SSRIs was comparable to that of serotonin-norepinephrine reuptake inhibitors (SNRIs), which all carried a comparable risk. In this analysis, neither indication nor dose were reliable indicators of risk of causing hyperhidrosis. However, the study found that for both SSRIs and SNRIs, increased affinity for the dopamine transporter was correlated with an increased risk of hyperhidrosis.2
Treatment
Treatment of hyperhidrosis depends on its cause and presentation.5 Hyperhidrosis may be categorized as primary (idiopathic) or secondary (also termed diaphoresis), and either focal or generalized.6 Many treatment recommendations focus on primary or focal hyperhidrosis and prioritize topical therapies.5 Because medication-induced hyperhidrosis most commonly presents as generalized3 and thus affects a large body surface area, the use of topical therapies is precluded. Topical therapy for psychotropic-induced hyperhidrosis should be pursued only if the patient’s sweating is localized.
Treating medication-induced hyperhidrosis becomes more complicated if it is not possible to alter the inciting medication (ie, because the medication is effective or the patient is resistant to change). In such scenarios, discontinuing the medication and initiating an alternative therapy may not be effective or feasible.2 For generalized presentations of medication-induced hyperhidrosis, if the inciting medication cannot be altered, initiating an oral systemic therapy is the preferred treatment.3,5
Oral anticholinergic medications (eg, benztropine, glycopyrrolate, and oxybutynin),4-6 act directly on muscarinic receptors within the eccrine sweat glands to decrease or stop sweating. They are considered first-line for generalized hyperhidrosis but may be inappropriate for psychotropic-induced hyperhidrosis because many psychotropics (eg, tricyclic antidepressants, paroxetine, olanzapine, quetiapine, and clozapine) have anticholinergic properties. Adding an anticholinergic medication to these patients’ regimens may increase the adverse effect burden and worsen cognitive deficits. Additionally, approximately one-third of patients discontinue anticholinergic medications due to tolerability issues (eg, dry mouth).
Continue to: However, anticholinergic medications...
However, anticholinergic medications may still have a role in treating psychotropic-induced hyperhidrosis. Benztropine3,7,8 and cyproheptadine2,3,9 may be effective options, though their role in treating psychotropic-induced hyperhidrosis should be limited and reserved for patients who have another compelling indication for these medications (eg, extrapyramidal symptoms) or when other treatment options are ineffective or intolerable.
Avoiding anticholinergic medications can also be justified based on the proposed mechanism of psychotropic-induced hyperhidrosis as an extension of the medication’s toxic effects. Conceptualizing psychotropic-induced hyperhidrosis as similar to the diaphoresis and hyperthermia observed in neuroleptic malignant syndrome and serotonin syndrome offers a clearer target for treatment. Though the specifics of the mechanisms remain unknown,2 many medications that cause hyperhidrosis do so by increasing sweat gland secretions, either directly by increasing cholinergic activity or indirectly via increased sympathetic transmission.
Considering this pathophysiology, another target for psychotropic-induced hyperhidrosis may be altered and/or excessive catecholamine activity. The use of medications such as clonidine,3-6 propranolol,4-6 or terazosin2,3,10 should be considered given their beneficial effects on the activation of the sympathetic nervous system, although clonidine also possesses anticholinergic activity. The calcium channel blocker diltiazem can improve hyperhidrosis symptoms by interfering with the calcium signaling necessary for normal sweat gland function.4,5 Comorbid cardiovascular diseases and tachycardia, an adverse effect of many psychotropic medications, may also be managed with these treatment options. Some research suggests using benzodiazepines to treat psychotropic-induced hyperhidrosis.4-6 As is the case for anticholinergic medications, the use of benzodiazepines would require another compelling indication for long-term use.
Table 23,4,6-8,10 provides recommended dosing and caveats for the use of these medications and other potentially appropriate medications.

Research of investigational treatments for generalized hyperhidrosis is ongoing. It is possible some of these medications may have a future role in the treatment of psychotropic-induced hyperhidrosis, with improved efficacy and better tolerability.
Continue to: CASE CONTINUED
CASE CONTINUED
Because Ms. K’s medication-induced hyperhidrosis is generalized and therefore ineligible for topical therapies, and because the inciting medication (paroxetine) cannot be switched to an alternative, the treatment team considers adding an oral medication. Treatment with an anticholinergic medication, such as benztropine, is not preferred due to the anticholinergic activity associated with paroxetine and Ms. K’s history of constipation. After discussing other oral treatment options with Ms. K, the team ultimately decides to initiate propranolol at a low dose (5 mg twice daily) to minimize the chances of an interaction with paroxetine, and titrate based on efficacy and tolerability.
Related Resources
- International Hyperhidrosis Society. Hyperhidrosis treatment overview. www.sweathelp.org/hyperhidrosis-treatments/treatment-overview.html
Drug Brand Names
Acamprosate • Campral
Aripiprazole • Abilify
Buprenorphine • Sublocade
Buprenorphine/naloxone • Zubsolv
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clomipramine • Anafranil
Clonidine • Catapres
Clozapine • Clozaril
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextroamphetamine/amphetamine • Adderall
Diltiazem • Cardizem
Divalproex • Depakote
Donepezil • Aricept
Doxepin • Silenor
Duloxetine • Cymbalta
Escitalopram • Lexapro
Eszopiclone • Lunesta
Fluoxetine • Prozac
Fluvoxamine • Luvox
Guanfacine • Intuniv
Glycopyrrolate • Cuvposa
Hydroxyzine • Vistaril
Imipramine • Tofranil
Levomilnacipran • Fetzima
Lisdexamfetamine • Vyvanse
Methadone • Dolophine, Methadose
Modafinil • Provigil
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paliperidone palmitate • Invega Sustenna
Paroxetine • Paxil
Phenelzine • Nardil
Pimozide • Orap
Protriptyline • Vivactil
Quetiapine • Seroquel
Rivastigmine • Exelon
Selegiline transdermal • Emsam
Sertraline • Zoloft
Temazepam • Restoril
Thiothixene • Navane
Tiagabine • Gabitril
Topiramate • Topamax
Tranylcypromine • Parnate
Vilazodone • Viibryd
Vortioxetine • Trintellix
Zaleplon • Sonata
Ziprasidone • Geodon
Zolpidem • Ambien
Zonisamide • Zonegran
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
1. International Hyperhidrosis Society. Drugs/medications known to cause hyperhidrosis. Sweathelp.org. 2022. Accessed September 6, 2022. https://www.sweathelp.org/pdf/drugs_2009.pdf
2. Beyer C, Cappetta K, Johnson JA, et al. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134-1146. doi:10.1002/da.22680
3. Cheshire WP, Fealey RD. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109-126. doi:10.2165/00002018-200831020-00002
4. del Boz J. Systemic treatment of hyperhidrosis. Actas Dermosifiliogr. 2015;106(4):271-277. doi:10.1016/j.ad.2014.11.012
5. Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol. 2019;81(3):669-680. doi:10.1016/j.jaad2018.11.066
6. Glaser DA. Oral medications. Dermatol Clin. 2014;32(4):527-532. doi:10.1016/j.det.2014.06.002
7. Garber A, Gregory RJ. Benztropine in the treatment of venlafaxine-induced sweating. J Clin Psychiatry. 1997;58(4):176-177. doi:10.4088/jcp.v58n0407e
8. Kolli V, Ramaswamy S. Improvement of antidepressant-induced sweating with as-required benztropine. Innov Clin Neurosci. 2013;10(11-12):10-11.
9. Ashton AK, Weinstein WL. Cyproheptadine for drug-induced sweating. Am J Psychiatry. 2002;159(5):875. doi:10.1176/APPI.AJP.159.5.874-A
10. Ghaleiha A, Shahidi KM, Afzali S, et al. Effect of terazosin on sweating in patients with major depressive disorder receiving sertraline: a randomized controlled trial. Int J Psychiatry Clin Pract. 2013;17(1):44-47. doi:10.3109/13651501.2012.687449
Ketamine no better for depression than placebo?
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.
TOPLINE:
results of a new study suggest, contradicting prior research. Although symptoms improved in both study groups, investigators say participants’ expectations of an improvement from ketamine may be driving that result.
METHODOLOGY:
- The randomized, placebo-controlled trial included 40 patients who had previously been diagnosed with MDD and who were scheduled for elective noncardiac, nonintracranial surgery.
- Participants completed pre- and postsurgery depression screenings with the Patient Health Questionnaire–8 (inclusion score was ≥ 12) and the Montgomery-Åsberg Depression Rating Scale (MADRS).
- Patients received an infusion of 0.5 mg/kg of saline (placebo group; n = 20) or ketamine (n = 20) during surgery, along with general anesthesia.
- At the end of a 14-day follow-up, patients were asked to guess whether they had received ketamine or placebo.
TAKEAWAY:
- MADRS scores dropped by around half 1 day after treatment, indicating an improvement in depressive symptoms in both the group that received ketamine (mean decrease from 25 to 12.6 points) and the group that received placebo (mean decrease from 30 to 15.3 points). There was no significant difference between the two.
- Participants in the ketamine and placebo groups also reported high rates of clinical response (60% and 50%, respectively) and remission (50% and 35%, respectively), again with no significant difference based on treatment with ketamine or placebo.
- Only 36.8% of participants accurately guessed their treatment group. Those who guessed they had received ketamine had higher MADRS scores than those who guessed they had received placebo or said they didn’t know (10.1 vs. 19.2 vs. 23.0).
- The ketamine group had a significantly shorter hospital stay (1.9 days) than the placebo group (4 days) (P = .02).
IN PRACTICE:
“Our primary findings differ from those of previous antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine,” the authors wrote, adding that “regardless of the intervention being tested, participant expectations of a positive outcome – also known as hope – may drive large decreases in depression symptoms seen in antidepressant trials.”
SOURCE:
Boris D. Heifets, MD, PhD, led the study, which was published online in Nature Mental Health. The study was funded by the Society for Neuroscience in Anesthesiology and Critical Care, the National Institutes of Health, and the Stanford School of Medicine Research Office.
LIMITATIONS:
The investigators did not measure participants’ treatment expectations prior to randomization and could not determine what effect participant expectancy bias may have had on the results. In addition, there was no assessment of the blind for anesthesiologists who administered the ketamine or placebo to patients.
DISCLOSURES:
Dr. Heifets is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant to Clairvoyant Therapeutics and Vine Ventures.
A version of this article first appeared on Medscape.com.






