User login
Aleukemic leukemia cutis
To the Editor: I read with great interest the article “Aleukemic leukemia cutis” by Abraham et al,1 as we recently had a case of this at my institution. The case is unique and quite intriguing; however, I found the pathologic description confusing and imprecise.
The authors state, “The findings were consistent with leukemic T cells with monocytic differentiation.”1 This is based on their findings that the tumor cells expressed CD4, CD43, CD68, and lysozyme. However, the cells were negative for CD30, ALK-1, CD2, and CD3.
First, I must contest the authors’ claim that “the cells co-expressed T-cell markers (CD4 and CD43)”: CD4 and CD43 are not specific for T cells and are almost invariably seen on monocytes, especially in acute monoblastic/monocytic leukemia (AMoL; also known as M5 in the French-American-British classification system).2,3 Therefore, the immunophenotype is perfect for an AMoL, but since there was no significant blood or bone marrow involvement and it was limited to the skin, this would best fit with a myeloid sarcoma, which frequently has a monocytic immunoprofile.3,4
Additionally, this would not be a mixed-phenotype acute leukemia, T/myeloid, not otherwise specified, as that requires positivity for cytoplasmic CD3 or surface CD3, and that was conspicuously absent.5 Therefore, the appropriate workup and treatment should have essentially followed the course for acute myeloid leukemia,4 which is unclear from the present report as there is no mention of a molecular workup (eg, for FLT3 and NPM1 mutations). This would, in turn, have important treatment and prognostic implications.6
The reason for my comments is to bring to light the importance of exact pathologic diagnosis, especially when dealing with leukemia. We currently have a host of treatment options and prognostic tools for the various types of acute myeloid leukemia, but only when a clear and precise pathologic diagnosis is given.5
- Abraham TN, Morawiecki P, Flischel A, Agrawal B. Aleukemic leukemia cutis. Cleve Clin J Med 2019; 86(2):85–86. doi:10.3949/ccjm.86a.18057
- Xu Y, McKenna RW, Wilson KS, Karandikar NJ, Schultz RA, Kroft SH. Immunophenotypic identification of acute myeloid leukemia with monocytic differentiation. Leukemia 2006; 20(7):1321–1324. doi:10.1038/sj.leu.2404242
- Cronin DMP, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol 2009; 132(1):101–110. doi:10.1309/AJCP6GR8BDEXPKHR
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol 2011; 2(5):309–316. doi:10.1177/2040620711410774
- Weir EG, Ali Ansari-Lari M, Batista DAS, et al. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia 2007; 21(11):2264–2270. doi:10.1038/sj.leu.2404848
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 2016; 6(7):e441. doi:10.1038/bcj.2016.50
To the Editor: I read with great interest the article “Aleukemic leukemia cutis” by Abraham et al,1 as we recently had a case of this at my institution. The case is unique and quite intriguing; however, I found the pathologic description confusing and imprecise.
The authors state, “The findings were consistent with leukemic T cells with monocytic differentiation.”1 This is based on their findings that the tumor cells expressed CD4, CD43, CD68, and lysozyme. However, the cells were negative for CD30, ALK-1, CD2, and CD3.
First, I must contest the authors’ claim that “the cells co-expressed T-cell markers (CD4 and CD43)”: CD4 and CD43 are not specific for T cells and are almost invariably seen on monocytes, especially in acute monoblastic/monocytic leukemia (AMoL; also known as M5 in the French-American-British classification system).2,3 Therefore, the immunophenotype is perfect for an AMoL, but since there was no significant blood or bone marrow involvement and it was limited to the skin, this would best fit with a myeloid sarcoma, which frequently has a monocytic immunoprofile.3,4
Additionally, this would not be a mixed-phenotype acute leukemia, T/myeloid, not otherwise specified, as that requires positivity for cytoplasmic CD3 or surface CD3, and that was conspicuously absent.5 Therefore, the appropriate workup and treatment should have essentially followed the course for acute myeloid leukemia,4 which is unclear from the present report as there is no mention of a molecular workup (eg, for FLT3 and NPM1 mutations). This would, in turn, have important treatment and prognostic implications.6
The reason for my comments is to bring to light the importance of exact pathologic diagnosis, especially when dealing with leukemia. We currently have a host of treatment options and prognostic tools for the various types of acute myeloid leukemia, but only when a clear and precise pathologic diagnosis is given.5
To the Editor: I read with great interest the article “Aleukemic leukemia cutis” by Abraham et al,1 as we recently had a case of this at my institution. The case is unique and quite intriguing; however, I found the pathologic description confusing and imprecise.
The authors state, “The findings were consistent with leukemic T cells with monocytic differentiation.”1 This is based on their findings that the tumor cells expressed CD4, CD43, CD68, and lysozyme. However, the cells were negative for CD30, ALK-1, CD2, and CD3.
First, I must contest the authors’ claim that “the cells co-expressed T-cell markers (CD4 and CD43)”: CD4 and CD43 are not specific for T cells and are almost invariably seen on monocytes, especially in acute monoblastic/monocytic leukemia (AMoL; also known as M5 in the French-American-British classification system).2,3 Therefore, the immunophenotype is perfect for an AMoL, but since there was no significant blood or bone marrow involvement and it was limited to the skin, this would best fit with a myeloid sarcoma, which frequently has a monocytic immunoprofile.3,4
Additionally, this would not be a mixed-phenotype acute leukemia, T/myeloid, not otherwise specified, as that requires positivity for cytoplasmic CD3 or surface CD3, and that was conspicuously absent.5 Therefore, the appropriate workup and treatment should have essentially followed the course for acute myeloid leukemia,4 which is unclear from the present report as there is no mention of a molecular workup (eg, for FLT3 and NPM1 mutations). This would, in turn, have important treatment and prognostic implications.6
The reason for my comments is to bring to light the importance of exact pathologic diagnosis, especially when dealing with leukemia. We currently have a host of treatment options and prognostic tools for the various types of acute myeloid leukemia, but only when a clear and precise pathologic diagnosis is given.5
- Abraham TN, Morawiecki P, Flischel A, Agrawal B. Aleukemic leukemia cutis. Cleve Clin J Med 2019; 86(2):85–86. doi:10.3949/ccjm.86a.18057
- Xu Y, McKenna RW, Wilson KS, Karandikar NJ, Schultz RA, Kroft SH. Immunophenotypic identification of acute myeloid leukemia with monocytic differentiation. Leukemia 2006; 20(7):1321–1324. doi:10.1038/sj.leu.2404242
- Cronin DMP, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol 2009; 132(1):101–110. doi:10.1309/AJCP6GR8BDEXPKHR
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol 2011; 2(5):309–316. doi:10.1177/2040620711410774
- Weir EG, Ali Ansari-Lari M, Batista DAS, et al. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia 2007; 21(11):2264–2270. doi:10.1038/sj.leu.2404848
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 2016; 6(7):e441. doi:10.1038/bcj.2016.50
- Abraham TN, Morawiecki P, Flischel A, Agrawal B. Aleukemic leukemia cutis. Cleve Clin J Med 2019; 86(2):85–86. doi:10.3949/ccjm.86a.18057
- Xu Y, McKenna RW, Wilson KS, Karandikar NJ, Schultz RA, Kroft SH. Immunophenotypic identification of acute myeloid leukemia with monocytic differentiation. Leukemia 2006; 20(7):1321–1324. doi:10.1038/sj.leu.2404242
- Cronin DMP, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol 2009; 132(1):101–110. doi:10.1309/AJCP6GR8BDEXPKHR
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol 2011; 2(5):309–316. doi:10.1177/2040620711410774
- Weir EG, Ali Ansari-Lari M, Batista DAS, et al. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia 2007; 21(11):2264–2270. doi:10.1038/sj.leu.2404848
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J 2016; 6(7):e441. doi:10.1038/bcj.2016.50
In reply: Aleukemic leukemia cutis
In Reply: We greatly appreciate our reader’s interest and response. He brings up a very good point. We have reviewed the reports and discussed it with our pathologists. On page 85, the sentence that begins, “The findings were consistent with leukemic T cells with monocytic differentiation” should actually read, “The findings were consistent with leukemic cells with monocytic differentiation.” The patient was appropriately treated for acute myeloid leukemia.
In Reply: We greatly appreciate our reader’s interest and response. He brings up a very good point. We have reviewed the reports and discussed it with our pathologists. On page 85, the sentence that begins, “The findings were consistent with leukemic T cells with monocytic differentiation” should actually read, “The findings were consistent with leukemic cells with monocytic differentiation.” The patient was appropriately treated for acute myeloid leukemia.
In Reply: We greatly appreciate our reader’s interest and response. He brings up a very good point. We have reviewed the reports and discussed it with our pathologists. On page 85, the sentence that begins, “The findings were consistent with leukemic T cells with monocytic differentiation” should actually read, “The findings were consistent with leukemic cells with monocytic differentiation.” The patient was appropriately treated for acute myeloid leukemia.
Positive psoriatic arthritis screens occur often in psoriasis patients
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
One out of eight patients with psoriasis had a positive screen for possibly undiagnosed psoriatic arthritis, according to an analysis of data from a prospective registry.
The finding highlights the need for better psoriatic arthritis screening among patients with psoriasis, said Philip J. Mease, MD, of the University of Washington, Seattle, and associates. The simple, five-question Psoriasis Epidemiology Screening Tool (PEST) used in this study could be deployed in general or dermatology practices to identify psoriasis patients who might need a rheumatology referral, they wrote. The report is in the Journal of the European Academy of Dermatology and Venereology.
Up to 30% of patients with psoriasis have comorbid psoriatic arthritis, but many such cases go undiagnosed, and even a 6-month diagnostic delay can worsen peripheral joint erosion and physical disability.
This study included 1,516 patients with psoriasis seen at 114 private and academic practices in 34 states that participate in the independent, prospective Corrona Psoriasis Registry. A total of 904 patients without dermatologist-reported psoriatic arthritis responded to the validated PEST, which assesses risk of psoriatic arthritis by asking whether the test taker has been told by a doctor that he or she has arthritis and whether they have experienced swollen joints, heel pain, pronounced and unexplained swelling of a finger or toe, and pitting of the fingernails or toenails. Each “yes” response is worth 1 point, and total scores of 3 or higher indicate risk of psoriatic arthritis. A total of 112 (12.4%) had a score of 3 or higher.
The average age of patients who met this threshold was 53 years, 4 years older than those who did not (P = .02). Patients with PEST scores of 3 or more also had a significantly longer duration of psoriasis and were significantly more likely to have nail disease and a family history of psoriasis. Demographically, they were more likely to be white, female, and unemployed. They had significantly higher rates of several comorbidities, including depression and anxiety, cardiovascular disease, obesity, and serious infections. Finally, they reported having significantly more pain and fatigue and significantly worse health-related quality of life.
The study did not account for possible confounding. “Further research is needed to characterize patients by individual PEST score and to assess outcomes over time,” the researchers wrote. “The use of screening tools can be beneficial in the detection of psoriatic arthritis, and comprehensive efforts to validate them in multiple clinical settings must continue, along with collection of critical feedback from patients and clinicians.”
Corrona and Novartis designed and helped conduct the study. Novartis, the chief funder, participated in data analysis and manuscript review. Dr. Mease disclosed research funding from Novartis and several other pharmaceutical companies. He also disclosed consulting and speakers bureau fees from Novartis, Corrona, and several other companies.
SOURCE: Mease PJ et al. J Eur Acad Dermatol Venereol. 2019 Mar 5. doi: 10.1111/jdv.15443.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Using Social Media to Talk About Public Health Issues
Public health organizations have learned that when it comes to sharing important information, it pays to capitalize on social media. Platforms like Facebook can not only reach multitudes, but also spread a message far more widely than conventional media can. But what is the best way to leverage social media for public health messages? Researchers from University of Sydney in Australia analyzed 20 Facebook pages on skin cancer, smoking, and other public health issues to find out the most effective strategies for getting users to engage.
The researchers coded 360 days of posts for each page, ending up with 5,356 posts. They categorized the communication techniques as informative, call-to-action, instructive, positive emotive appeal, fear appeal, testimonial, and humor. They also looked at marketing elements, such as whether the page used branding, celebrities or persons of authority, mascots, competitions or giveaways, sponsorships, or vouchers and other offers.
Almost all pages were administered by a nongovernment organization. Mental health and cancer prevention were the most common public health issues. Most posts were photos; the next most common were links (but only 1% of users actually clicked on the links). The most common communication techniques were positive emotional appeal and testimonial. Fear appeal was the least common.
Video posts engaged the most users, getting the most likes and shares, the researchers say. Videos received nearly 4 times as many shares as photo posts; links and text received 30% and 69% fewer shares, respectively. Video and text-only posts received more comments than photo posts. However, the researchers add, this could reflect the Facebook algorithm, which may favor videos over other post types. They also note that only 3% of all posts they coded were videos, “suggesting that public health organizations are trailing behind conventional marketers.”
Posts with positive emotional appeal drew 18% more likes than call-to-action posts, but 27% fewer shares. Informative posts received more than twice as many shares. Fear appeal and humorous posts received more comments than call-to-action posts (perhaps because they are more controversial, the researchers suggest), and instructive posts received fewer.
Conventional marketing, such as using sponsorships or “persons of authority,” generally did not have much engagement. Celebrities and sports figures, though, got 62% more likes, more than double the shares, and 64% more comments than posts without celebrities and sportspeople.
Still, regardless of the post type, communication technique, or marketing element, the researchers say, only 2% to 6% of potential customers engaged with it in some way.
Public health organizations have learned that when it comes to sharing important information, it pays to capitalize on social media. Platforms like Facebook can not only reach multitudes, but also spread a message far more widely than conventional media can. But what is the best way to leverage social media for public health messages? Researchers from University of Sydney in Australia analyzed 20 Facebook pages on skin cancer, smoking, and other public health issues to find out the most effective strategies for getting users to engage.
The researchers coded 360 days of posts for each page, ending up with 5,356 posts. They categorized the communication techniques as informative, call-to-action, instructive, positive emotive appeal, fear appeal, testimonial, and humor. They also looked at marketing elements, such as whether the page used branding, celebrities or persons of authority, mascots, competitions or giveaways, sponsorships, or vouchers and other offers.
Almost all pages were administered by a nongovernment organization. Mental health and cancer prevention were the most common public health issues. Most posts were photos; the next most common were links (but only 1% of users actually clicked on the links). The most common communication techniques were positive emotional appeal and testimonial. Fear appeal was the least common.
Video posts engaged the most users, getting the most likes and shares, the researchers say. Videos received nearly 4 times as many shares as photo posts; links and text received 30% and 69% fewer shares, respectively. Video and text-only posts received more comments than photo posts. However, the researchers add, this could reflect the Facebook algorithm, which may favor videos over other post types. They also note that only 3% of all posts they coded were videos, “suggesting that public health organizations are trailing behind conventional marketers.”
Posts with positive emotional appeal drew 18% more likes than call-to-action posts, but 27% fewer shares. Informative posts received more than twice as many shares. Fear appeal and humorous posts received more comments than call-to-action posts (perhaps because they are more controversial, the researchers suggest), and instructive posts received fewer.
Conventional marketing, such as using sponsorships or “persons of authority,” generally did not have much engagement. Celebrities and sports figures, though, got 62% more likes, more than double the shares, and 64% more comments than posts without celebrities and sportspeople.
Still, regardless of the post type, communication technique, or marketing element, the researchers say, only 2% to 6% of potential customers engaged with it in some way.
Public health organizations have learned that when it comes to sharing important information, it pays to capitalize on social media. Platforms like Facebook can not only reach multitudes, but also spread a message far more widely than conventional media can. But what is the best way to leverage social media for public health messages? Researchers from University of Sydney in Australia analyzed 20 Facebook pages on skin cancer, smoking, and other public health issues to find out the most effective strategies for getting users to engage.
The researchers coded 360 days of posts for each page, ending up with 5,356 posts. They categorized the communication techniques as informative, call-to-action, instructive, positive emotive appeal, fear appeal, testimonial, and humor. They also looked at marketing elements, such as whether the page used branding, celebrities or persons of authority, mascots, competitions or giveaways, sponsorships, or vouchers and other offers.
Almost all pages were administered by a nongovernment organization. Mental health and cancer prevention were the most common public health issues. Most posts were photos; the next most common were links (but only 1% of users actually clicked on the links). The most common communication techniques were positive emotional appeal and testimonial. Fear appeal was the least common.
Video posts engaged the most users, getting the most likes and shares, the researchers say. Videos received nearly 4 times as many shares as photo posts; links and text received 30% and 69% fewer shares, respectively. Video and text-only posts received more comments than photo posts. However, the researchers add, this could reflect the Facebook algorithm, which may favor videos over other post types. They also note that only 3% of all posts they coded were videos, “suggesting that public health organizations are trailing behind conventional marketers.”
Posts with positive emotional appeal drew 18% more likes than call-to-action posts, but 27% fewer shares. Informative posts received more than twice as many shares. Fear appeal and humorous posts received more comments than call-to-action posts (perhaps because they are more controversial, the researchers suggest), and instructive posts received fewer.
Conventional marketing, such as using sponsorships or “persons of authority,” generally did not have much engagement. Celebrities and sports figures, though, got 62% more likes, more than double the shares, and 64% more comments than posts without celebrities and sportspeople.
Still, regardless of the post type, communication technique, or marketing element, the researchers say, only 2% to 6% of potential customers engaged with it in some way.
FDA approves new etanercept biosimilar, Eticovo
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
The Food and Drug Administration has approved Eticovo (etanercept-ykro), a biosimilar of Enbrel (etanercept), for the treatment of several different rheumatologic and dermatologic conditions.
FDA approval was based in part on the results of a phase 3 trial in which 596 patients with moderate to severe rheumatoid arthritis uncontrolled by methotrexate received either Eticovo or Enbrel. The American College of Rheumatology 20% response rate after 24 weeks was 78.1% for Eticovo and 80.3% for Enbrel; the two drugs were statistically equivalent. Both groups had statistically equivalent rates of treatment-emergent adverse events (55.2% vs. 58.2%).
According to the label, Eticovo is a tumor necrosis factor blocker approved for the treatment of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis in patients aged 4 years or older. The most common adverse events associated with the drug include infections and injection site reactions.
Eticovo is the second etanercept biosimilar approved by the FDA. The first FDA-approved etanercept biosimilar, etanercept-szzs (Erelzi), is currently facing a legal challenge from Amgen, the manufacturer of Enbrel.
Chronic urticaria population identified
Half a million people. That’s pretty close to the population of Sacramento. It’s also the estimated number of adults living with chronic urticaria in the United States, according to analysis of a database including over 55 million individuals.
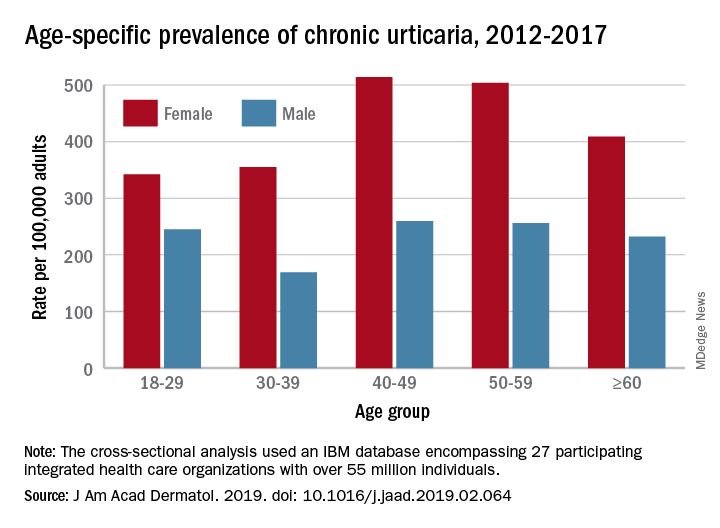
That cross-sectional analysis put the overall standardized at 309.3 per 100,000 (0.31%) and men well below at 145.5 per 100,000 (0.15%), Sara Wertenteil, BA, and her associates at Hofstra University, Hempstead, N.Y., wrote in the Journal of the American Academy of Dermatology.
Overall prevalence of chronic urticaria was similar for all age groups, ranging from 0.21% for those aged 18-29 years and those aged 30-39 years to 0.26% for those aged 40-49, and prevalence was higher for females than males in all age groups, the investigators reported.
“Epidemiologic studies estimating disease burden for chronic urticaria are sparse, [but this study] is based on one of the largest and most ethnically diversified population samples in the United States. It is also drawn from patients with all insurance types and self-pay patients across various types of health care settings and from all census regions,” Ms. Wertenteil and her associates wrote.
The study involved an IBM Watson Health database encompassing 27 participating integrated health care organizations and representing approximately 17% of the population. The analysis identified 69,570 adult patients with chronic urticaria, and the ratio of women to men was 2.7:1.
The senior author, Amit Garg, MD, has served as an advisor for AbbVie, Pfizer, Janssen, and Asana Biosciences.
SOURCE: Wertenteil S et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.064.
Half a million people. That’s pretty close to the population of Sacramento. It’s also the estimated number of adults living with chronic urticaria in the United States, according to analysis of a database including over 55 million individuals.

That cross-sectional analysis put the overall standardized at 309.3 per 100,000 (0.31%) and men well below at 145.5 per 100,000 (0.15%), Sara Wertenteil, BA, and her associates at Hofstra University, Hempstead, N.Y., wrote in the Journal of the American Academy of Dermatology.
Overall prevalence of chronic urticaria was similar for all age groups, ranging from 0.21% for those aged 18-29 years and those aged 30-39 years to 0.26% for those aged 40-49, and prevalence was higher for females than males in all age groups, the investigators reported.
“Epidemiologic studies estimating disease burden for chronic urticaria are sparse, [but this study] is based on one of the largest and most ethnically diversified population samples in the United States. It is also drawn from patients with all insurance types and self-pay patients across various types of health care settings and from all census regions,” Ms. Wertenteil and her associates wrote.
The study involved an IBM Watson Health database encompassing 27 participating integrated health care organizations and representing approximately 17% of the population. The analysis identified 69,570 adult patients with chronic urticaria, and the ratio of women to men was 2.7:1.
The senior author, Amit Garg, MD, has served as an advisor for AbbVie, Pfizer, Janssen, and Asana Biosciences.
SOURCE: Wertenteil S et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.064.
Half a million people. That’s pretty close to the population of Sacramento. It’s also the estimated number of adults living with chronic urticaria in the United States, according to analysis of a database including over 55 million individuals.

That cross-sectional analysis put the overall standardized at 309.3 per 100,000 (0.31%) and men well below at 145.5 per 100,000 (0.15%), Sara Wertenteil, BA, and her associates at Hofstra University, Hempstead, N.Y., wrote in the Journal of the American Academy of Dermatology.
Overall prevalence of chronic urticaria was similar for all age groups, ranging from 0.21% for those aged 18-29 years and those aged 30-39 years to 0.26% for those aged 40-49, and prevalence was higher for females than males in all age groups, the investigators reported.
“Epidemiologic studies estimating disease burden for chronic urticaria are sparse, [but this study] is based on one of the largest and most ethnically diversified population samples in the United States. It is also drawn from patients with all insurance types and self-pay patients across various types of health care settings and from all census regions,” Ms. Wertenteil and her associates wrote.
The study involved an IBM Watson Health database encompassing 27 participating integrated health care organizations and representing approximately 17% of the population. The analysis identified 69,570 adult patients with chronic urticaria, and the ratio of women to men was 2.7:1.
The senior author, Amit Garg, MD, has served as an advisor for AbbVie, Pfizer, Janssen, and Asana Biosciences.
SOURCE: Wertenteil S et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.064.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Indoor Tanning: Turning First-Time Clients Into Repeat Customers
Nearly 10 million people use indoor tanning (IT) even though it increases the risk of skin cancer. Young white women are particularly at risk—almost 1 in 3 reports using indoor tanning in the past year, and nearly 1 in 5 reports regular use (that is, > 10 times in the past year), according to researchers from Rutgers University in New Brunswick, New Jersey.
Research has already shown that most people use IT to enhance their appearance. But a tan is not only seen as attractive: It “plays an important part of youth culture,” the researchers note, especially when it comes to special events, like high school proms. Still, some IT users might remain “special event” users, not regular clients. What makes the difference? To find out, the researchers conducted 6 interviews with a salon employee who also used tanning beds. Their purpose was not to produce “generalizable knowledge of the experiences of many users” but to provide insights into the behavior and to propose working hypotheses for future examination.
The researchers found that the incentive to use IT mostly comes down to—as many health-related decisions do—how it is advertised. The first encounter is likely to be the most important one. That is when the sell begins, designed to “guide” the patron into coming back, and back again. For instance, the salon employee may be trained to establish rapport, to personalize the interaction, and to ask about “tan goals,” setting the stage for a process, rather than a 1-time purchase. The employee describes the steps of creating a “base tan,” maintaining the tan, deepening the tan. Framing tanning as a process sends the message that frequent visits are needed. The researchers cite self-regulation theories that posit for a habit to take hold, the individual must develop a mental model or plans for how to use the habitual behavior to achieve desired goals.
The US Federal Trade Commission and other agencies have enacted restrictions on IT industry advertisements, the researchers say. But the policy efforts have not addressed greater regulation at the point-of-purchase, other than requiring the provision of standardized risk warnings. The interview findings suggest ways to help reduce IT use. Pricing controls, for instance: If patrons had to buy single sessions—instead of in bulk—they might feel less pressured to “get their money’s worth.” Restrictions on advertisement might require salon employees also to provide information on unnecessary exposure. The researchers contrast the salon employee to a convenience store clerk who “simply serves as a cashier for purchasing cigarettes or unhealthy food options.”
The researchers suggest that their findings be followed up in larger, more representational samples.
Nearly 10 million people use indoor tanning (IT) even though it increases the risk of skin cancer. Young white women are particularly at risk—almost 1 in 3 reports using indoor tanning in the past year, and nearly 1 in 5 reports regular use (that is, > 10 times in the past year), according to researchers from Rutgers University in New Brunswick, New Jersey.
Research has already shown that most people use IT to enhance their appearance. But a tan is not only seen as attractive: It “plays an important part of youth culture,” the researchers note, especially when it comes to special events, like high school proms. Still, some IT users might remain “special event” users, not regular clients. What makes the difference? To find out, the researchers conducted 6 interviews with a salon employee who also used tanning beds. Their purpose was not to produce “generalizable knowledge of the experiences of many users” but to provide insights into the behavior and to propose working hypotheses for future examination.
The researchers found that the incentive to use IT mostly comes down to—as many health-related decisions do—how it is advertised. The first encounter is likely to be the most important one. That is when the sell begins, designed to “guide” the patron into coming back, and back again. For instance, the salon employee may be trained to establish rapport, to personalize the interaction, and to ask about “tan goals,” setting the stage for a process, rather than a 1-time purchase. The employee describes the steps of creating a “base tan,” maintaining the tan, deepening the tan. Framing tanning as a process sends the message that frequent visits are needed. The researchers cite self-regulation theories that posit for a habit to take hold, the individual must develop a mental model or plans for how to use the habitual behavior to achieve desired goals.
The US Federal Trade Commission and other agencies have enacted restrictions on IT industry advertisements, the researchers say. But the policy efforts have not addressed greater regulation at the point-of-purchase, other than requiring the provision of standardized risk warnings. The interview findings suggest ways to help reduce IT use. Pricing controls, for instance: If patrons had to buy single sessions—instead of in bulk—they might feel less pressured to “get their money’s worth.” Restrictions on advertisement might require salon employees also to provide information on unnecessary exposure. The researchers contrast the salon employee to a convenience store clerk who “simply serves as a cashier for purchasing cigarettes or unhealthy food options.”
The researchers suggest that their findings be followed up in larger, more representational samples.
Nearly 10 million people use indoor tanning (IT) even though it increases the risk of skin cancer. Young white women are particularly at risk—almost 1 in 3 reports using indoor tanning in the past year, and nearly 1 in 5 reports regular use (that is, > 10 times in the past year), according to researchers from Rutgers University in New Brunswick, New Jersey.
Research has already shown that most people use IT to enhance their appearance. But a tan is not only seen as attractive: It “plays an important part of youth culture,” the researchers note, especially when it comes to special events, like high school proms. Still, some IT users might remain “special event” users, not regular clients. What makes the difference? To find out, the researchers conducted 6 interviews with a salon employee who also used tanning beds. Their purpose was not to produce “generalizable knowledge of the experiences of many users” but to provide insights into the behavior and to propose working hypotheses for future examination.
The researchers found that the incentive to use IT mostly comes down to—as many health-related decisions do—how it is advertised. The first encounter is likely to be the most important one. That is when the sell begins, designed to “guide” the patron into coming back, and back again. For instance, the salon employee may be trained to establish rapport, to personalize the interaction, and to ask about “tan goals,” setting the stage for a process, rather than a 1-time purchase. The employee describes the steps of creating a “base tan,” maintaining the tan, deepening the tan. Framing tanning as a process sends the message that frequent visits are needed. The researchers cite self-regulation theories that posit for a habit to take hold, the individual must develop a mental model or plans for how to use the habitual behavior to achieve desired goals.
The US Federal Trade Commission and other agencies have enacted restrictions on IT industry advertisements, the researchers say. But the policy efforts have not addressed greater regulation at the point-of-purchase, other than requiring the provision of standardized risk warnings. The interview findings suggest ways to help reduce IT use. Pricing controls, for instance: If patrons had to buy single sessions—instead of in bulk—they might feel less pressured to “get their money’s worth.” Restrictions on advertisement might require salon employees also to provide information on unnecessary exposure. The researchers contrast the salon employee to a convenience store clerk who “simply serves as a cashier for purchasing cigarettes or unhealthy food options.”
The researchers suggest that their findings be followed up in larger, more representational samples.
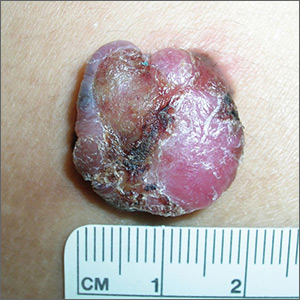
Painful lump on back

The FP suspected that this could be a nodular melanoma that was mostly hypomelanotic (with minimal melanin visible, which explained why it was so pink). It looked like there was a flat nevus with brown coloration at one side of the base. The FP asked the patient whether she had a mole there in the past. The patient thought she did have a mole there since childhood, but had not thought about it. The light brown hyperpigmentation lateral to the lesion was likely secondary to scratching.
The differential diagnosis included melanoma, squamous cell carcinoma, and basal cell carcinoma. Suspecting that it was most likely a nodular melanoma, the FP knew that a rapid diagnosis would be essential to an improved prognosis. Nodular melanomas are fast-growing melanomas that grow vertically, thereby making them one of the deadliest melanomas. A delay in the diagnosis of a nodular melanoma by even 3 to 6 months can change the prognosis from favorable to fatal.
The FP considered the options for biopsy but realized that cutting out the whole lesion would be time-consuming and require rescheduling for a different time. Getting a good sampling of the tumor with either a deep shave or a large punch biopsy would most likely provide the diagnosis. The FP presented the options to the patient, who indicated that the FP should do whatever he thought would be best. The FP performed a deep shave biopsy below the pigment on the edge and acquired a good-sized portion of the tumor. Aluminum chloride was initially used for hemostasis, but electrosurgery was ultimately required because of the vascular nature of the tumor. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular melanoma with a depth of 4.1 mm. The patient was referred to Surgical Oncology for a wide local excision and a sentinel lymph node biopsy. The sentinel node biopsy was positive for metastasis. The patient was then sent to Medical Oncology to discuss further evaluation and treatment of her melanoma. The FP was saddened by the worrisome prognosis for this young mother.
He reflected that this nodular melanoma should have been diagnosed at least 6 to 12 months earlier when this patient was seeing an obstetrician regularly for health care. It was unfortunate that no one in the health care team during her pregnancy, labor, delivery, or postpartum care noted the melanoma and encouraged her to get evaluated. This supports the practice that we should not listen to lungs over the shirt. While every health care provider is not a dermatologist, the skin should not be ignored.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this could be a nodular melanoma that was mostly hypomelanotic (with minimal melanin visible, which explained why it was so pink). It looked like there was a flat nevus with brown coloration at one side of the base. The FP asked the patient whether she had a mole there in the past. The patient thought she did have a mole there since childhood, but had not thought about it. The light brown hyperpigmentation lateral to the lesion was likely secondary to scratching.
The differential diagnosis included melanoma, squamous cell carcinoma, and basal cell carcinoma. Suspecting that it was most likely a nodular melanoma, the FP knew that a rapid diagnosis would be essential to an improved prognosis. Nodular melanomas are fast-growing melanomas that grow vertically, thereby making them one of the deadliest melanomas. A delay in the diagnosis of a nodular melanoma by even 3 to 6 months can change the prognosis from favorable to fatal.
The FP considered the options for biopsy but realized that cutting out the whole lesion would be time-consuming and require rescheduling for a different time. Getting a good sampling of the tumor with either a deep shave or a large punch biopsy would most likely provide the diagnosis. The FP presented the options to the patient, who indicated that the FP should do whatever he thought would be best. The FP performed a deep shave biopsy below the pigment on the edge and acquired a good-sized portion of the tumor. Aluminum chloride was initially used for hemostasis, but electrosurgery was ultimately required because of the vascular nature of the tumor. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular melanoma with a depth of 4.1 mm. The patient was referred to Surgical Oncology for a wide local excision and a sentinel lymph node biopsy. The sentinel node biopsy was positive for metastasis. The patient was then sent to Medical Oncology to discuss further evaluation and treatment of her melanoma. The FP was saddened by the worrisome prognosis for this young mother.
He reflected that this nodular melanoma should have been diagnosed at least 6 to 12 months earlier when this patient was seeing an obstetrician regularly for health care. It was unfortunate that no one in the health care team during her pregnancy, labor, delivery, or postpartum care noted the melanoma and encouraged her to get evaluated. This supports the practice that we should not listen to lungs over the shirt. While every health care provider is not a dermatologist, the skin should not be ignored.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected that this could be a nodular melanoma that was mostly hypomelanotic (with minimal melanin visible, which explained why it was so pink). It looked like there was a flat nevus with brown coloration at one side of the base. The FP asked the patient whether she had a mole there in the past. The patient thought she did have a mole there since childhood, but had not thought about it. The light brown hyperpigmentation lateral to the lesion was likely secondary to scratching.
The differential diagnosis included melanoma, squamous cell carcinoma, and basal cell carcinoma. Suspecting that it was most likely a nodular melanoma, the FP knew that a rapid diagnosis would be essential to an improved prognosis. Nodular melanomas are fast-growing melanomas that grow vertically, thereby making them one of the deadliest melanomas. A delay in the diagnosis of a nodular melanoma by even 3 to 6 months can change the prognosis from favorable to fatal.
The FP considered the options for biopsy but realized that cutting out the whole lesion would be time-consuming and require rescheduling for a different time. Getting a good sampling of the tumor with either a deep shave or a large punch biopsy would most likely provide the diagnosis. The FP presented the options to the patient, who indicated that the FP should do whatever he thought would be best. The FP performed a deep shave biopsy below the pigment on the edge and acquired a good-sized portion of the tumor. Aluminum chloride was initially used for hemostasis, but electrosurgery was ultimately required because of the vascular nature of the tumor. (See the Watch & Learn video on “Shave biopsy.”)
The pathology report came back as a nodular melanoma with a depth of 4.1 mm. The patient was referred to Surgical Oncology for a wide local excision and a sentinel lymph node biopsy. The sentinel node biopsy was positive for metastasis. The patient was then sent to Medical Oncology to discuss further evaluation and treatment of her melanoma. The FP was saddened by the worrisome prognosis for this young mother.
He reflected that this nodular melanoma should have been diagnosed at least 6 to 12 months earlier when this patient was seeing an obstetrician regularly for health care. It was unfortunate that no one in the health care team during her pregnancy, labor, delivery, or postpartum care noted the melanoma and encouraged her to get evaluated. This supports the practice that we should not listen to lungs over the shirt. While every health care provider is not a dermatologist, the skin should not be ignored.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill;2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Misleading information, reimbursement among the barriers to teledermatology progress
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Even with disclaimers, there are people who want a “quick and easy answer,” and these apps can provide misleading information that “can lead them down a wrong diagnostic pathway,” said Dr. Chen, professor of dermatology and director of the teledermatology service at Emory University, Atlanta. Users not only include lower income or uninsured patients, but busy, high-powered executives.
Apps focused on photo storage are used to help patients track lesions for changes, with some apps dedicated to total body mole mapping. However, while these apps may empower patients to perform regular self skin checks, there is a question of whether they are HIPAA secure, Dr. Chen said. Another issue is that the many different app choices on the market may make it difficult for providers to keep up with which app a particular patient is using, she added. “If you have 10 different patients coming in with 10 different apps, it’s going to be really hard for you to learn all of those and be able to manipulate that easily, especially in the 15-minute slot.”
Smartphone and tablet apps that offer reminders to perform monthly skin checks or apply sunscreen when outdoors are plentiful. Dr. Chen noted that, while the efficacy of these apps are not known, they are similar to less high-tech technology like alarms or calendar reminders. “[They] are really kind of neat and fun. It’s kind of boring to just get a reminder, and you tune it out if you get a reminder on your calendars, so this may be a new way to help people,” she said.
Wearables also track users’ sun exposure, and range from a UV sensor on the thumb that measures sun exposure over a period of months to clip-on wearables and temporary tattoos that tell users when to apply or reapply sunscreen. Some devices allow entry of an individual’s Fitzpatrick skin type and can detect temperature and humidity, she noted.
Risk-calculating apps use images taken from smartphone cameras to determine the risk of melanoma, using algorithms that consider color and pattern recognition, but these apps are not as accurate as dermatologists, she said. In a study published in 2013, the app that sent images directly to a dermatologist was the most effective, compared with apps that relied on an automated algorithm to analyze the images (JAMA Dermatol. 2013 Apr;149[4]:422-6).
One of the conclusions the authors made was that feedback was slow for the one that required the image be sent to a dermatologist. “As opposed to just a minute and spitting out the result, it took 24 hours. My argument is 24 hours is still a lot faster than if you tried to call and get an appointment with a dermatologist,” Dr. Chen commented.
One step above teledermatology is teledermoscopy, or using a mobile, smartphone-attached device to send images to a dermatologist over a secure cloud service for review. “Most of us would agree that it would just take too long to do a live video with a patient,” Dr. Chen pointed out. “They may as well just come in anyway. It’ll take you 40 minutes to be able to take a look at that mole on the video, but to do it in a store-and-forward format can be quite efficient.”
However, she noted that one barrier to entry for teledermoscopy is defining the type of service, such as whether apps will offer provider-to-provider or patient-to-provider services. “That is fraught with its own details and issues, especially with photo quality.”
Another barrier, reimbursement from Centers for Medicare & Medicaid Services for teledermatology, is “the real sticking point,” Dr. Chen continued. Under a 2019 CMS Final Rule, telemedicine is only covered if the patient is already established within the practice, and reimbursement for Healthcare Common Procedure Coding System codes G2010 and G2012 relating to telemedicine ranges between $12 and $14.
Based on her back-of-the-envelope calculation, she added, “I would have to see 180 patients in a half-day session by this method in order to generate my salary, and that would just be impossible.”
Dr. Chen said that teledermatology is the “way of the future” and hopes the CMS Final Rule is reconsidered so the technology can be used to help solve some of the growing issues in the dermatology field. “There’s no way we can meet the demands of an increasingly aging population by an in-person brick and mortar sort of paradigm,” she said, noting that, even in an urban setting, it can be difficult to see a dermatologist.
Dr. Chen reports relationships with BioPharmX, Dermecular Therapeutics, Leo Pharma, Phoenix Tissue Repair, Trevi Therapeutics, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Even with disclaimers, there are people who want a “quick and easy answer,” and these apps can provide misleading information that “can lead them down a wrong diagnostic pathway,” said Dr. Chen, professor of dermatology and director of the teledermatology service at Emory University, Atlanta. Users not only include lower income or uninsured patients, but busy, high-powered executives.
Apps focused on photo storage are used to help patients track lesions for changes, with some apps dedicated to total body mole mapping. However, while these apps may empower patients to perform regular self skin checks, there is a question of whether they are HIPAA secure, Dr. Chen said. Another issue is that the many different app choices on the market may make it difficult for providers to keep up with which app a particular patient is using, she added. “If you have 10 different patients coming in with 10 different apps, it’s going to be really hard for you to learn all of those and be able to manipulate that easily, especially in the 15-minute slot.”
Smartphone and tablet apps that offer reminders to perform monthly skin checks or apply sunscreen when outdoors are plentiful. Dr. Chen noted that, while the efficacy of these apps are not known, they are similar to less high-tech technology like alarms or calendar reminders. “[They] are really kind of neat and fun. It’s kind of boring to just get a reminder, and you tune it out if you get a reminder on your calendars, so this may be a new way to help people,” she said.
Wearables also track users’ sun exposure, and range from a UV sensor on the thumb that measures sun exposure over a period of months to clip-on wearables and temporary tattoos that tell users when to apply or reapply sunscreen. Some devices allow entry of an individual’s Fitzpatrick skin type and can detect temperature and humidity, she noted.
Risk-calculating apps use images taken from smartphone cameras to determine the risk of melanoma, using algorithms that consider color and pattern recognition, but these apps are not as accurate as dermatologists, she said. In a study published in 2013, the app that sent images directly to a dermatologist was the most effective, compared with apps that relied on an automated algorithm to analyze the images (JAMA Dermatol. 2013 Apr;149[4]:422-6).
One of the conclusions the authors made was that feedback was slow for the one that required the image be sent to a dermatologist. “As opposed to just a minute and spitting out the result, it took 24 hours. My argument is 24 hours is still a lot faster than if you tried to call and get an appointment with a dermatologist,” Dr. Chen commented.
One step above teledermatology is teledermoscopy, or using a mobile, smartphone-attached device to send images to a dermatologist over a secure cloud service for review. “Most of us would agree that it would just take too long to do a live video with a patient,” Dr. Chen pointed out. “They may as well just come in anyway. It’ll take you 40 minutes to be able to take a look at that mole on the video, but to do it in a store-and-forward format can be quite efficient.”
However, she noted that one barrier to entry for teledermoscopy is defining the type of service, such as whether apps will offer provider-to-provider or patient-to-provider services. “That is fraught with its own details and issues, especially with photo quality.”
Another barrier, reimbursement from Centers for Medicare & Medicaid Services for teledermatology, is “the real sticking point,” Dr. Chen continued. Under a 2019 CMS Final Rule, telemedicine is only covered if the patient is already established within the practice, and reimbursement for Healthcare Common Procedure Coding System codes G2010 and G2012 relating to telemedicine ranges between $12 and $14.
Based on her back-of-the-envelope calculation, she added, “I would have to see 180 patients in a half-day session by this method in order to generate my salary, and that would just be impossible.”
Dr. Chen said that teledermatology is the “way of the future” and hopes the CMS Final Rule is reconsidered so the technology can be used to help solve some of the growing issues in the dermatology field. “There’s no way we can meet the demands of an increasingly aging population by an in-person brick and mortar sort of paradigm,” she said, noting that, even in an urban setting, it can be difficult to see a dermatologist.
Dr. Chen reports relationships with BioPharmX, Dermecular Therapeutics, Leo Pharma, Phoenix Tissue Repair, Trevi Therapeutics, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Even with disclaimers, there are people who want a “quick and easy answer,” and these apps can provide misleading information that “can lead them down a wrong diagnostic pathway,” said Dr. Chen, professor of dermatology and director of the teledermatology service at Emory University, Atlanta. Users not only include lower income or uninsured patients, but busy, high-powered executives.
Apps focused on photo storage are used to help patients track lesions for changes, with some apps dedicated to total body mole mapping. However, while these apps may empower patients to perform regular self skin checks, there is a question of whether they are HIPAA secure, Dr. Chen said. Another issue is that the many different app choices on the market may make it difficult for providers to keep up with which app a particular patient is using, she added. “If you have 10 different patients coming in with 10 different apps, it’s going to be really hard for you to learn all of those and be able to manipulate that easily, especially in the 15-minute slot.”
Smartphone and tablet apps that offer reminders to perform monthly skin checks or apply sunscreen when outdoors are plentiful. Dr. Chen noted that, while the efficacy of these apps are not known, they are similar to less high-tech technology like alarms or calendar reminders. “[They] are really kind of neat and fun. It’s kind of boring to just get a reminder, and you tune it out if you get a reminder on your calendars, so this may be a new way to help people,” she said.
Wearables also track users’ sun exposure, and range from a UV sensor on the thumb that measures sun exposure over a period of months to clip-on wearables and temporary tattoos that tell users when to apply or reapply sunscreen. Some devices allow entry of an individual’s Fitzpatrick skin type and can detect temperature and humidity, she noted.
Risk-calculating apps use images taken from smartphone cameras to determine the risk of melanoma, using algorithms that consider color and pattern recognition, but these apps are not as accurate as dermatologists, she said. In a study published in 2013, the app that sent images directly to a dermatologist was the most effective, compared with apps that relied on an automated algorithm to analyze the images (JAMA Dermatol. 2013 Apr;149[4]:422-6).
One of the conclusions the authors made was that feedback was slow for the one that required the image be sent to a dermatologist. “As opposed to just a minute and spitting out the result, it took 24 hours. My argument is 24 hours is still a lot faster than if you tried to call and get an appointment with a dermatologist,” Dr. Chen commented.
One step above teledermatology is teledermoscopy, or using a mobile, smartphone-attached device to send images to a dermatologist over a secure cloud service for review. “Most of us would agree that it would just take too long to do a live video with a patient,” Dr. Chen pointed out. “They may as well just come in anyway. It’ll take you 40 minutes to be able to take a look at that mole on the video, but to do it in a store-and-forward format can be quite efficient.”
However, she noted that one barrier to entry for teledermoscopy is defining the type of service, such as whether apps will offer provider-to-provider or patient-to-provider services. “That is fraught with its own details and issues, especially with photo quality.”
Another barrier, reimbursement from Centers for Medicare & Medicaid Services for teledermatology, is “the real sticking point,” Dr. Chen continued. Under a 2019 CMS Final Rule, telemedicine is only covered if the patient is already established within the practice, and reimbursement for Healthcare Common Procedure Coding System codes G2010 and G2012 relating to telemedicine ranges between $12 and $14.
Based on her back-of-the-envelope calculation, she added, “I would have to see 180 patients in a half-day session by this method in order to generate my salary, and that would just be impossible.”
Dr. Chen said that teledermatology is the “way of the future” and hopes the CMS Final Rule is reconsidered so the technology can be used to help solve some of the growing issues in the dermatology field. “There’s no way we can meet the demands of an increasingly aging population by an in-person brick and mortar sort of paradigm,” she said, noting that, even in an urban setting, it can be difficult to see a dermatologist.
Dr. Chen reports relationships with BioPharmX, Dermecular Therapeutics, Leo Pharma, Phoenix Tissue Repair, Trevi Therapeutics, and Unilever.
EXPERT ANALYSIS FROM AAD 2019
FDA approves IL-23 inhibitor risankizumab for treating plaque psoriasis
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.
Risankizumab, an interleukin-23 inhibitor, has been approved by the Food and Drug Administration for treating moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy, the manufacturer announced on April 23.
Risankizumab selectively inhibits interleukin-23 (IL-23), a key inflammatory protein, by binding to its p19 subunit. The drug is administered at a dose of 150 mg, in two subcutaneous injections, every 12 weeks, after starting doses at weeks 0 and 4. It will be available in early May, according to an AbbVie press release announcing the approval.
The approval was based in part on data from two phase 3, 2-year studies, In UltIMMA-1 and UltIMMA-2, at 16 weeks, 75% of risankizumab patients in both studies achieved a Psoriasis Area and Severity Index (PASI 90), compared with 5% and 2% of those on placebo, respectively. These results were published in 2018 (Lancet. 2018 Aug 25;392[10148]:650-61).
At 1 year, 82% and 81% of those treated with risankizumab in the two studies achieved a PASI 90, and 56% and 60% achieved a PASI 100, respectively, according to the company.
Approval was also based on additional phase 3 studies, IMMhance and IMMvent.
Upper respiratory infections were among the most common adverse events associated with risankizumab in trials, reported in 13%, according to the company. Other adverse events associated with treatment included headache (3.5 %), fatigue (2.5 %), injection site reactions (1.5%) and tinea infections (1.1%). The AbbVie release states that candidates for treatment should be evaluated for tuberculosis before starting therapy, and patients should be instructed to report signs and symptoms of infection.
Risankizumab, which will be marketed as Skyrizi, was recently approved in Canada for the same indication, and in Japan, for plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults. It currently is under review in Europe.
AbbVie and Boehringer Ingelheim are collaborating on the development of risankizumab, according to an AbbVie press release. Studies of risankizumab for treatment of psoriatic arthritis and Crohn’s disease are underway.