User login
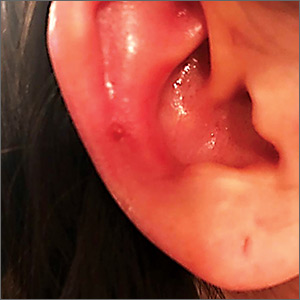
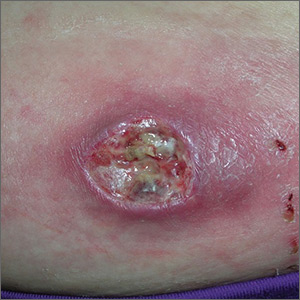
Widespread hyperpigmented plaques
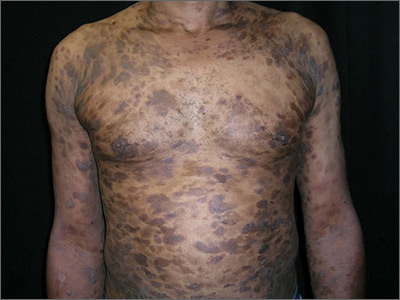
The differential diagnosis included psoriasis, drug eruption, and a cutaneous T-cell lymphoma.
A drug eruption could have been due to an over-the-counter medication or supplement, so the lack of improvement from stopping the antihypertensive medication did not rule out this diagnosis. Psoriasis does not always show erythema in persons of color, but these plaques were not typical of psoriasis. (There also were some flat patches that were even less typical of psoriasis.)
The FP performed a 4-mm punch biopsy on one of the hyperpigmented plaques on the abdomen. A 4-mm punch biopsy is generally an ideal method for determining the cause of an unknown skin rash, and it is usually better to choose a lesion on the upper body rather than below the waist if the rash is widespread. (See the Watch & Learn video on “Punch biopsy.”)
The FP also prescribed a 1-pound tub of 0.1% triamcinolone ointment for symptomatic relief as this could help any of the possible diagnoses being considered. The pathology report came back as mycosis fungoides, the most common type of cutaneous T-cell lymphoma.
The patient was sent to Hematology/Oncology for further evaluation and treatment. Mycosis fungoides can have both patches and plaques and frequently involves the trunk more than the extremities (which was the situation in this case). It is important to consider uncommon diagnoses like this in the differential when the initial diagnosis does not appear to be responding to treatment or there is something atypical about the presentation of an expected diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The differential diagnosis included psoriasis, drug eruption, and a cutaneous T-cell lymphoma.
A drug eruption could have been due to an over-the-counter medication or supplement, so the lack of improvement from stopping the antihypertensive medication did not rule out this diagnosis. Psoriasis does not always show erythema in persons of color, but these plaques were not typical of psoriasis. (There also were some flat patches that were even less typical of psoriasis.)
The FP performed a 4-mm punch biopsy on one of the hyperpigmented plaques on the abdomen. A 4-mm punch biopsy is generally an ideal method for determining the cause of an unknown skin rash, and it is usually better to choose a lesion on the upper body rather than below the waist if the rash is widespread. (See the Watch & Learn video on “Punch biopsy.”)
The FP also prescribed a 1-pound tub of 0.1% triamcinolone ointment for symptomatic relief as this could help any of the possible diagnoses being considered. The pathology report came back as mycosis fungoides, the most common type of cutaneous T-cell lymphoma.
The patient was sent to Hematology/Oncology for further evaluation and treatment. Mycosis fungoides can have both patches and plaques and frequently involves the trunk more than the extremities (which was the situation in this case). It is important to consider uncommon diagnoses like this in the differential when the initial diagnosis does not appear to be responding to treatment or there is something atypical about the presentation of an expected diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The differential diagnosis included psoriasis, drug eruption, and a cutaneous T-cell lymphoma.
A drug eruption could have been due to an over-the-counter medication or supplement, so the lack of improvement from stopping the antihypertensive medication did not rule out this diagnosis. Psoriasis does not always show erythema in persons of color, but these plaques were not typical of psoriasis. (There also were some flat patches that were even less typical of psoriasis.)
The FP performed a 4-mm punch biopsy on one of the hyperpigmented plaques on the abdomen. A 4-mm punch biopsy is generally an ideal method for determining the cause of an unknown skin rash, and it is usually better to choose a lesion on the upper body rather than below the waist if the rash is widespread. (See the Watch & Learn video on “Punch biopsy.”)
The FP also prescribed a 1-pound tub of 0.1% triamcinolone ointment for symptomatic relief as this could help any of the possible diagnoses being considered. The pathology report came back as mycosis fungoides, the most common type of cutaneous T-cell lymphoma.
The patient was sent to Hematology/Oncology for further evaluation and treatment. Mycosis fungoides can have both patches and plaques and frequently involves the trunk more than the extremities (which was the situation in this case). It is important to consider uncommon diagnoses like this in the differential when the initial diagnosis does not appear to be responding to treatment or there is something atypical about the presentation of an expected diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
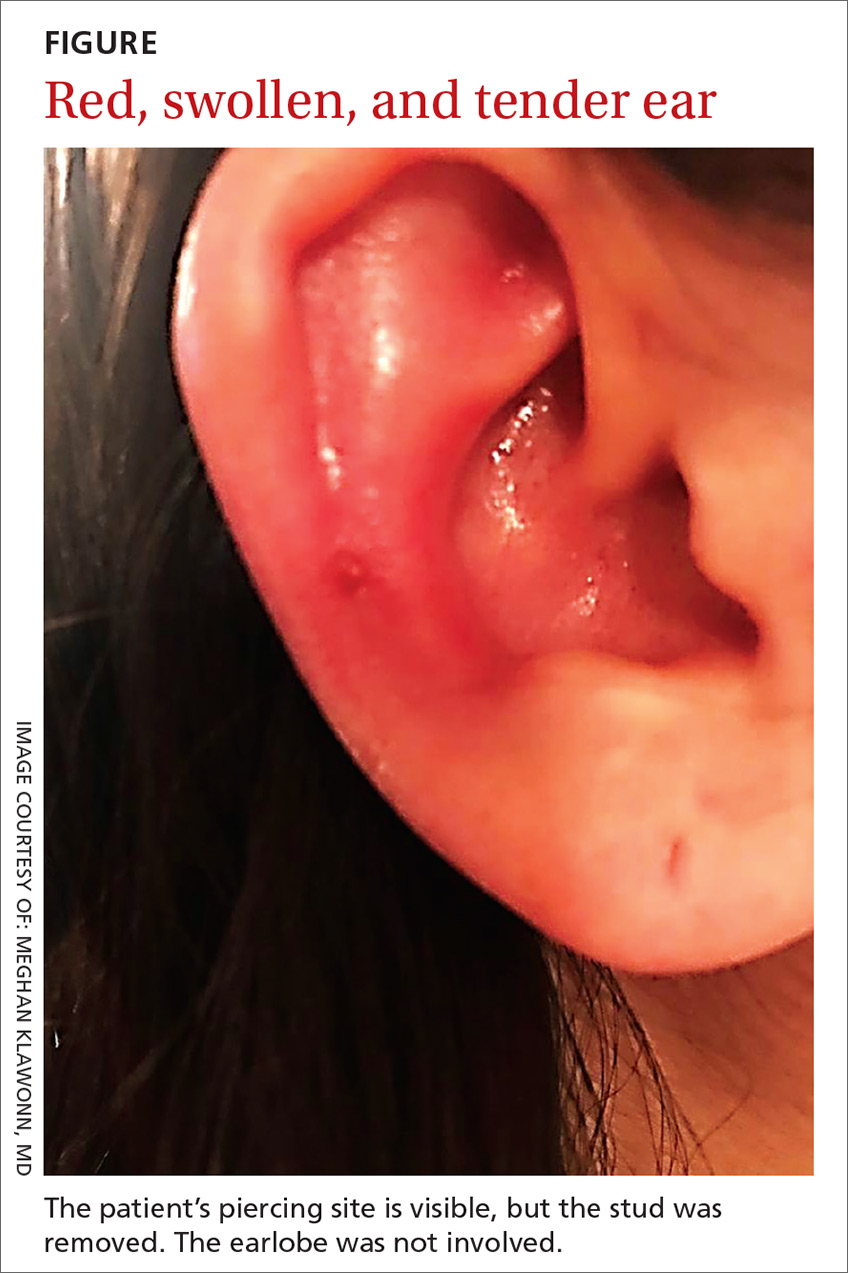
Erythematous swollen ear
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; [email protected]
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; [email protected]
A 25-year-old woman presented with an exceedingly tender right ear. She’d had the helix of her ear pierced 3 days prior to presentation and 2 days after that, the ear had become tender. The tenderness was progressively worsening and associated with throbbing pain. The patient, who’d had her ears pierced before, was otherwise in good health and denied fever, chills, or travel outside of the country. She had been going to the gym regularly and took frequent showers. Physical examination revealed an erythematous swollen ear that was tender to the touch (FIGURE). The entire auricle was swollen except for the earlobe. The patient also reported purulent material draining from the helical piercing site.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Auricular perichondritis
Auricular perichondritis is an inflammation of the connective tissue surrounding the cartilage of the ear. Infectious and autoimmune factors may play a role. The underlying cartilage also may become involved. A useful clinical clue to the diagnosis of auricular perichondritis is sparing of the earlobe, which does not contain cartilage. Autoimmune causes typically have bilateral involvement. Infectious causes are usually associated with trauma and purulent drainage at the wound site. Ear piercings are an increasingly common cause, but perichondritis due to minor trauma, as a surgical complication, or in the absence of an obvious inciting trigger can occur. A careful history usually will reveal the cause.
In this case, the patient indicated that an open piercing gun at a shopping mall kiosk had been used to pierce her ear. Piercing with a sterile straight needle would have been preferable and less likely to be associated with secondary infection, as the shearing trauma to the perichondrium experienced with a piercing gun is thought to predispose to infection.1 Exposure to fresh water from the shower could have been a source for Pseudomonas infection.1
Differential: Pinpointing the diagnosis early is vital
A red and tender ear can raise a differential diagnosis that includes erysipelas, relapsing polychondritis, and auricular perichondritis. Erysipelas is a bacterial infection that spreads through the lymphatic system and is associated with intense and well-demarcated erythema. Erysipelas typically involves the face or lower legs. Infection after piercing or traumatic injury should raise suspicion of pseudomonal infection.2-5 Untreated infection can spread quickly and lead to permanent ear deformity. Although the same pattern of inflammation can be seen in relapsing polychondritis, relapsing polychondritis typically involves both ears as well as the eyes and joints.
Prompt treatment is necessary to avoid cosmetic disfigurement
The timing of the reaction in our patient made infection obvious because Pseudomonas aeruginosa seems to have a particular affinity for damaged cartilage.2
Ciprofloxacin 500 mg twice daily is the treatment of choice. Although many skin infections can be empirically treated with oral cephalosporin, penicillin, or erythromycin, it is important to recognize that infected piercing sites and auricular perichondritis due to pseudomonal infection will not respond to these agents. That’s because these agents do not provide as good coverage for Pseudomonas as they do for Staphylococci or other bacteria more often associated with skin infection. Treatment with an agent such as amoxicillin and clavulanic acid or oral cephalexin can mean the loss of valuable time and subsequent cosmetic disfigurement.6
Continue to: When fluctuance is present...
When fluctuance is present, incision and drainage, or even debridement, may be necessary. When extensive infection leads to cartilage necrosis and liquefaction, treatment is difficult and may result in lasting disfigurement. Prompt empiric treatment currently is considered the best option.6
Our patient was prescribed a course of ciprofloxacin 500 mg every 12 hours for 10 days. She noted improvement within 2 days, and the infection resolved without complication.
CORRESPONDENCE
Matthew F. Helm, MD, Penn State Health Hershey Medical Center, 500 University Dr, HU14, Hershey, PA 17033; [email protected]
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
1. Sandhu A, Gross M, Wylie J, et al. Pseudomonas aeruginosa necrotizing chondritis complicating high helical ear piercing case report: clinical and public health perspectives. Can J Public Health. 2007;98:74-77.
2. Prasad HK, Sreedharan S, Prasad HS, et al. Perichondritis of the auricle and its management. J Laryngol Otol. 2007;121:530-534.
3. Fisher CG, Kacica MA, Bennett NM. Risk factors for cartilage infections of the ear. Am J Prev Med. 2005;29:204-209.
4. Lee TC, Gold WL. Necrotizing Pseudomonas chondritis after piercing of the upper ear. CMAJ. 2011;183:819-821.
5. Rowshan HH, Keith K, Baur D, et al. Pseudomonas aeruginosa infection of the auricular cartilage caused by “high ear piercing”: a case report and review of the literature. J Oral Maxillofac Surg. 2008;66:543-546.
6. Liu ZW, Chokkalingam P. Piercing associated perichondritis of the pinna: are we treating it correctly? J Laryngol Otol. 2013;127:505-508.
Failure to thrive in a 6-day-old neonate • intermittent retractions with inspiratory stridor • Dx?
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
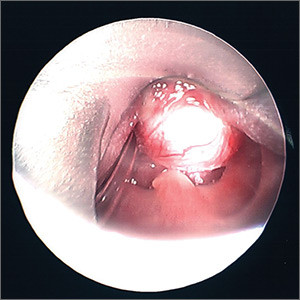
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).
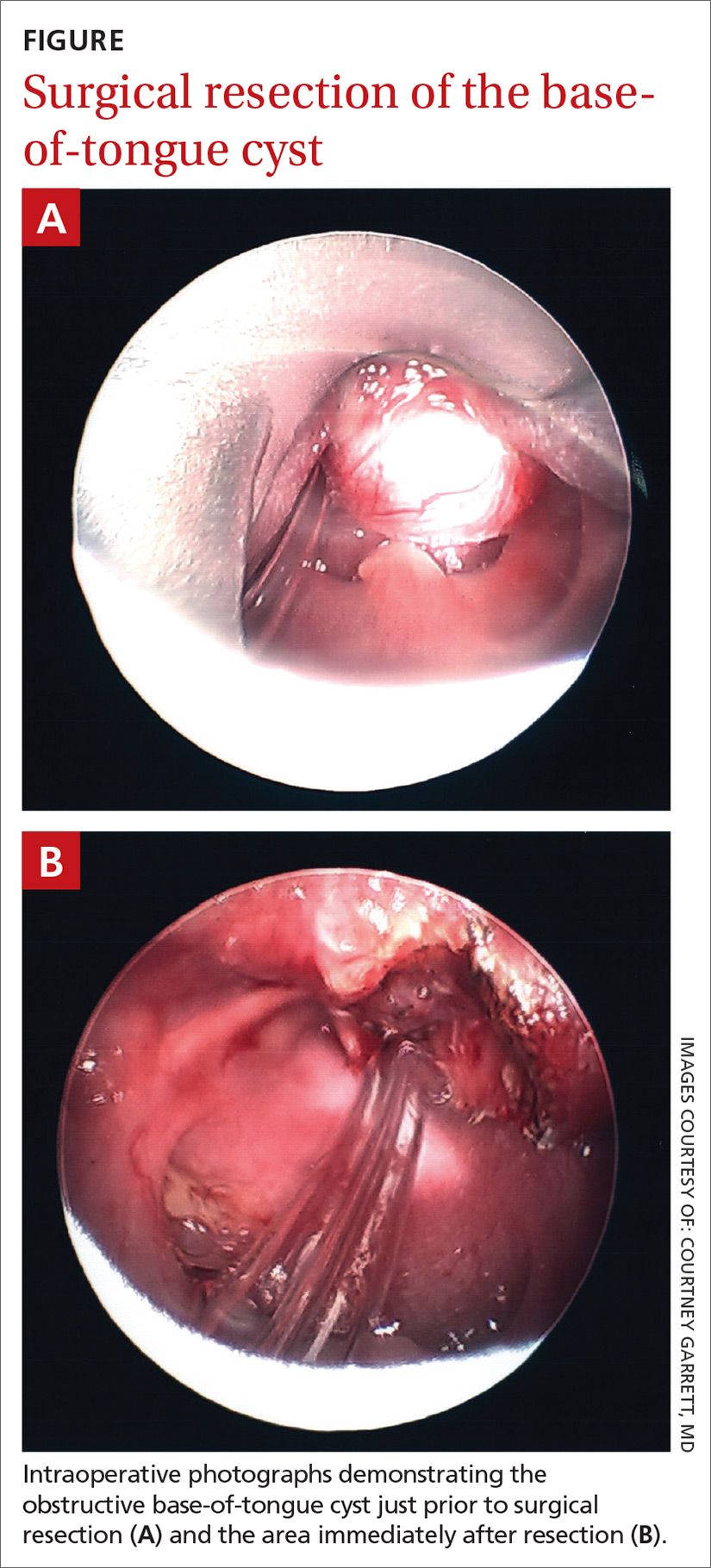
Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; [email protected]
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).

Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; [email protected]
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).

Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; [email protected]
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
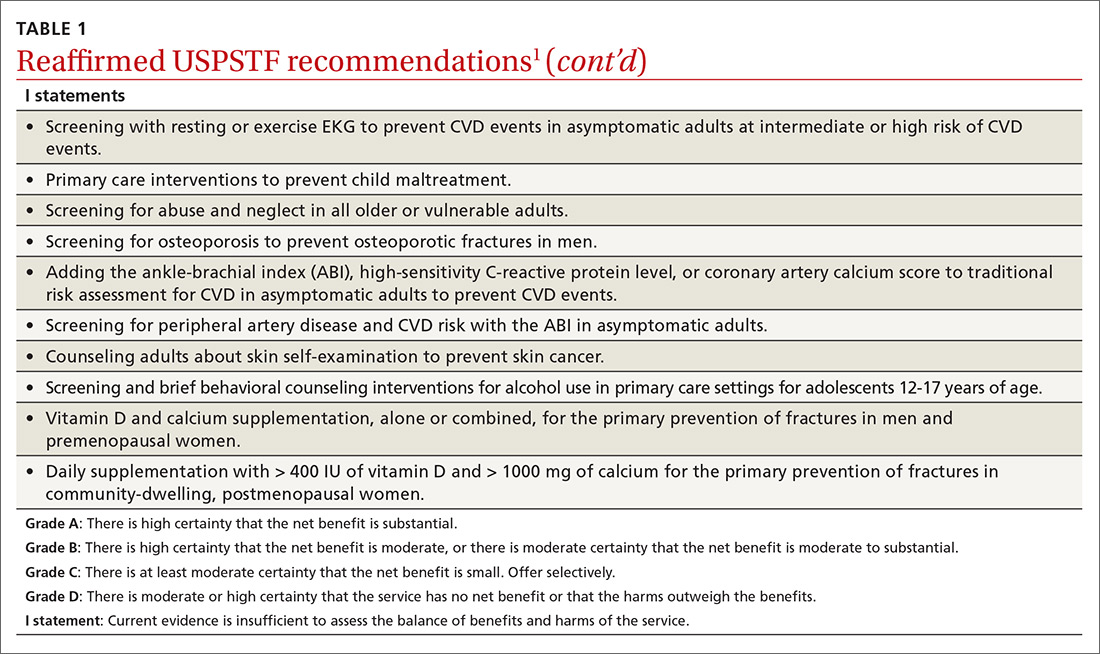
2019 USPSTF update
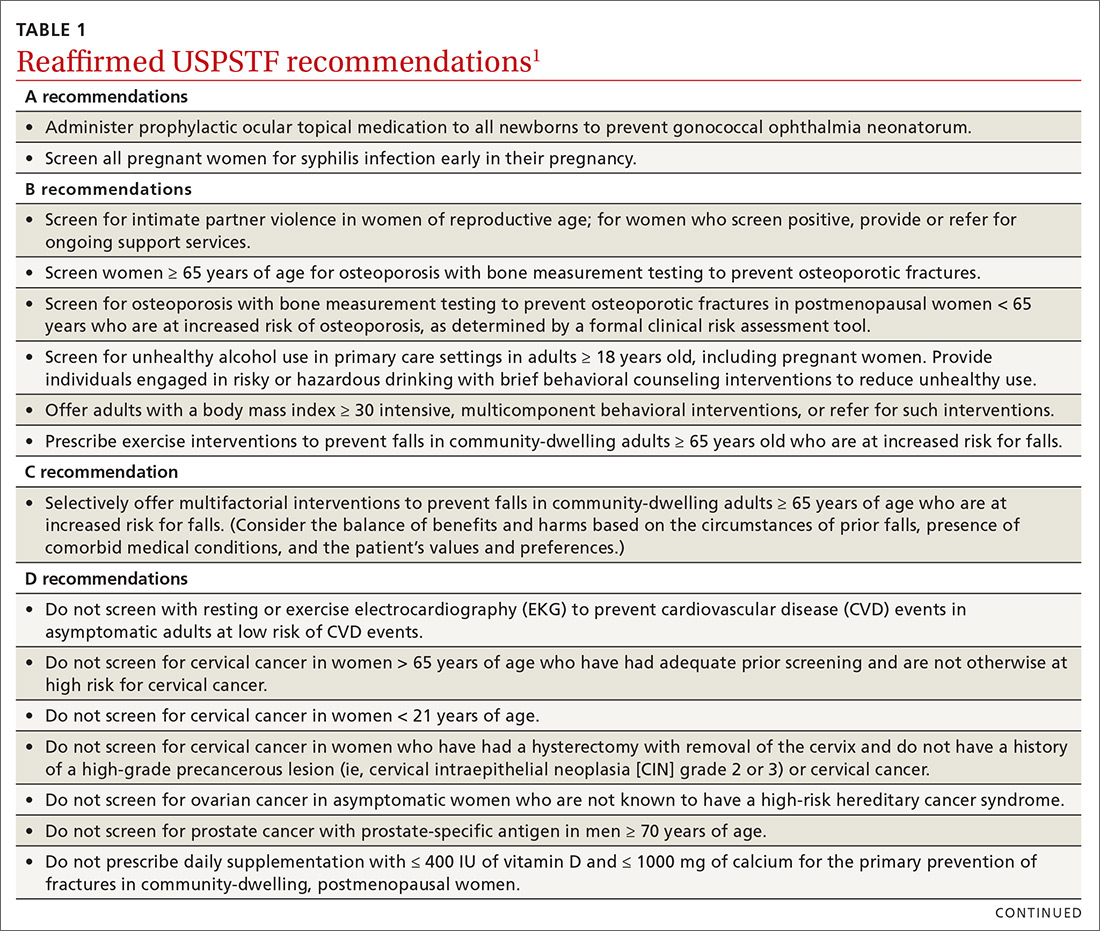
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).
This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1
New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.
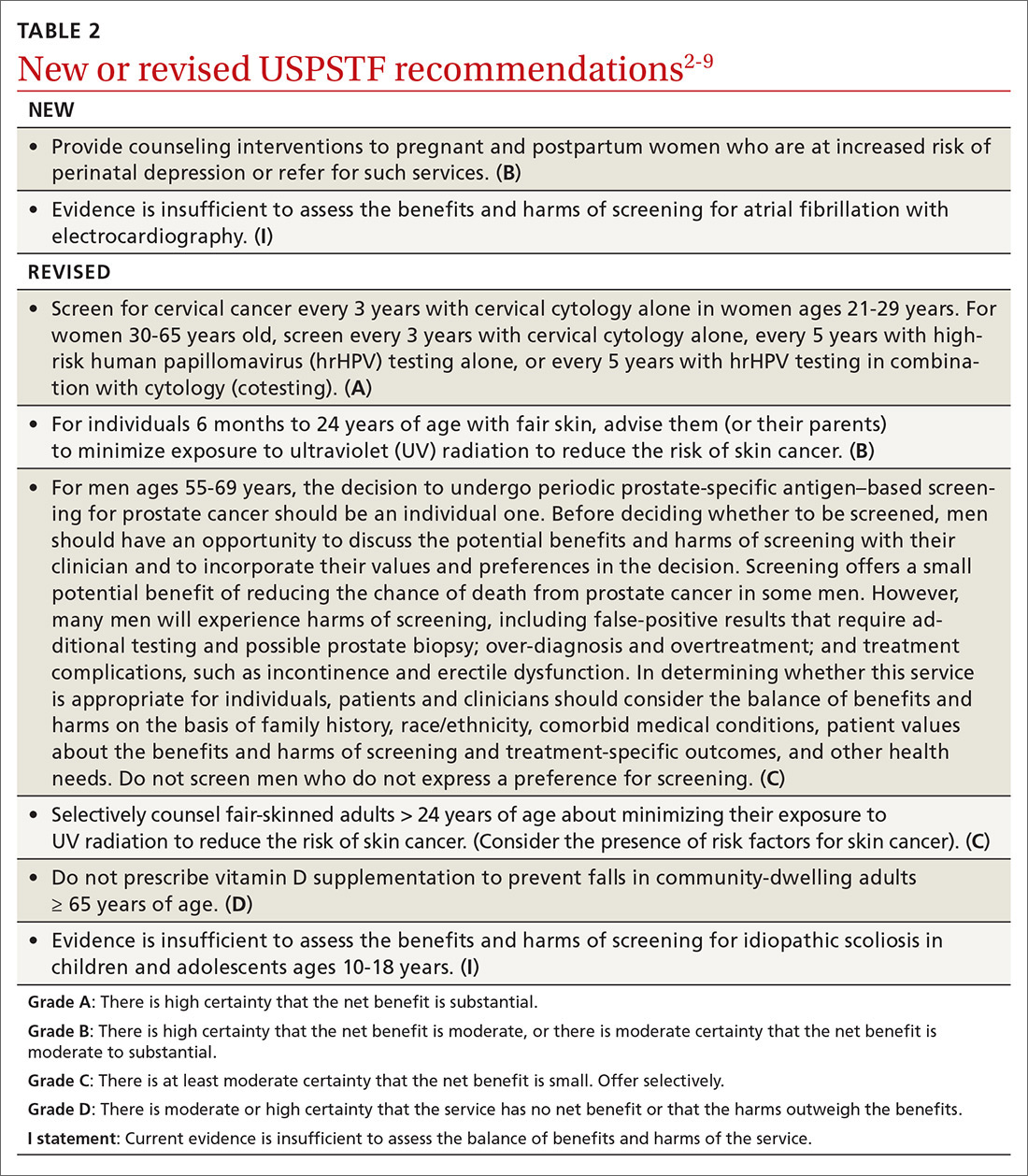
Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
Over the past year through early 2019, the US Preventive Services Task Force made 34 recommendations on 19 different topics. Twenty-six were reaffirmations of recommendations made in previous years (TABLE 11); the Task Force attempts to reassess topics every 7 years. Two new topics were addressed with 2 new recommendations, and 6 previous recommendations were revised or reversed (TABLE 22-9).

This Practice Alert discusses the new and the changed recommendations. (In 2018, the Practice Alert podcast series covered screening for ovarian cancer [April], prostate cancer [June], and cervical cancer [October], and EKG screening for cardiovascular disease [November].) All current Task Force recommendations are available on the USPSTF Web site.1

New topics
Perinatal depression prevention
The Task Force recommends that clinicians counsel pregnant women and women in the first year postpartum who are at increased risk for perinatal depression, or refer for such services. The recommendation applies to those who are not diagnosed with depression but are at increased risk.

Perinatal depression can negatively affect both mother and child in several ways and occurs at a rate close to 9% during pregnancy and 37% during the first year postpartum.2 The interventions studied by the Task Force included cognitive behavioral therapy and interpersonal therapy; most sessions were initiated in the second trimester of pregnancy and varied in number of sessions and intensity. The Task Force includes the following in the list of risks that should prompt a referral: a history of depression, current depressive symptoms that fall short of that needed for a depression diagnosis, low income, adolescent or single parenthood, recent intimate partner violence, elevated anxiety symptoms, physical or sexual abuse, or a history of significant negative life events. (See “Postpartum anxiety: More common than you think,” in the April issue.)
Atrial fibrillation
The Task Force found insufficient evidence to recommend for or against the use of electrocardiography (EKG) to screen for atrial fibrillation (AF).3
Revisions of previous recommendations
Cervical cancer screening
Skin cancer prevention
The Task Force made 2 revisions to the 2012 recommendation on preventing skin cancer through behavioral counseling to avoid ultraviolet (UV) radiation.6 These recommendations continue to focus on those with fair skin. The first revision: The earliest age at which children (through their guardians) can benefit from counseling on UV avoidance has been lowered from age 10 years to 6 months. The second revision: Some adults older than age 24 can also benefit from such counseling if they have fair skin and other skin cancer risks such as using tanning beds, having a history of sunburns or previous skin cancer, having an increased number of nevi (moles) and atypical nevi, having human immunodeficiency virus (HIV) infection, having received an organ transplant, or having a family history of skin cancer.
Continue to: Those at risk...
Those at risk can reduce their chances of skin cancer by using broad-spectrum sunscreens and sun-protective clothing, and by avoiding sun exposure and indoor tanning beds.
Fall prevention
In a reversal of its 2012 recommendation, the Task Force now recommends against the use of vitamin D supplementation to prevent falls in community-dwelling adults 65 years or older.7 In a reanalysis of previous studies on this topic, along with new evidence, the Task Force concluded that vitamin D supplementation offers no benefit for preventing falls in adults who are not vitamin D deficient.
Screening for scoliosis in adolescents
In 2004 the USPSTF recommended against screening for idiopathic scoliosis in children and adolescents 10 to 18 years of age. In its most recent review, the Task Force continued to find no direct evidence of the benefit of screening and inadequate evidence on the long-term benefits of reduction in spinal curvature through exercise, surgery, and bracing. However, following a reanalysis of the potential harms of these treatments and the use of a new analytic framework, the Task Force concluded it is not possible at this time to assess the balance of benefits and harms of screening.8
Prostate cancer screening
In its most controversial action, the Task Force reversed its 2012 recommendation against routine prostate-specific antigen–based screening for prostate cancer in men ages 55 to 69 years and now lists this as a “C” recommendation.9 The potential benefits of screening include preventing 1.3 deaths from prostate cancer per 1000 men screened over 13 years and approximately 3 cases of metastatic prostate cancer. However, no trials have found a reduction in all-cause mortality from screening. Contrast that with the known harms of screening: 15% false positive results over 10 years; 1% hospitalization rate among those undergoing a prostate biopsy; over-diagnosis and resultant treatment of 20% to 50% of men diagnosed with prostate cancer through screening; and incontinence and erectile dysfunction in 20% and 67%, respectively, of men following prostatectomy.9
Based on these outcomes, the Task Force “does not recommend screening for prostate cancer unless men express a preference for screening after being informed of and understanding the benefits and risks.”9 The Task Force continues to recommend against screening men ages 70 years and older.
Continue to: The change in this recommendation...
The change in this recommendation and its wording present dilemmas for family physicians: whether to discuss potential screening with all men ages 55 to 69; to selectively discuss it with those at high risk (principally African Americans and those with a strong family history of prostate cancer); or to address the issue only if a patient asks about it. In addition, if a man requests screening, how often should it be performed? Most clinical trials have found equal benefit from testing less frequently than every year, with fewer harms. The Task Force provided little or no guidance on these issues.
Final advice: D recommendations
The Task Force reaffirmed that 7 services have either no benefit or cause more harm than benefit (TABLE 11). Family physicians should be familiar with these services, as well as all Task Force D recommendations, and avoid recommending them or providing them. High quality preventive care involves both providing services of proven benefit and avoiding those that do not.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
1. USPSTF. Published recommendations. https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 25, 2019.
2. USPSTF. Final recommendation statement. Perinatal depression: preventive interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/perinatal-depression-preventive-interventions. Accessed March 25, 2019.
3. USPSTF. Atrial fibrillation: screening with electrocardiography. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/atrial-fibrillation-screening-with-electrocardiography. Accessed March 25, 2019.
4. USPSTF. Screening for atrial fibrillation with electrocardiography. JAMA. 2018;320:478-484.
5. USPSTF. Cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening2. Accessed March 25, 2019.
6. USPSTF. Skin cancer prevention: behavioral counseling. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancer-counseling2. Accessed March 25, 2019.
7. USPSTF. Falls prevention in community-dwelling older adults: interventions. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/falls-prevention-in-older-adults-interventions1. Accessed March 25, 2019.
8. USPSTF. Adolescent idiopathic scoliosis: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 25, 2019.
9. USPSTF. Prostate cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening1#consider. Accessed March 25, 2019.
A practical guide to the care of ingrown toenails
CASE
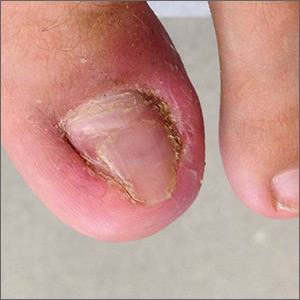
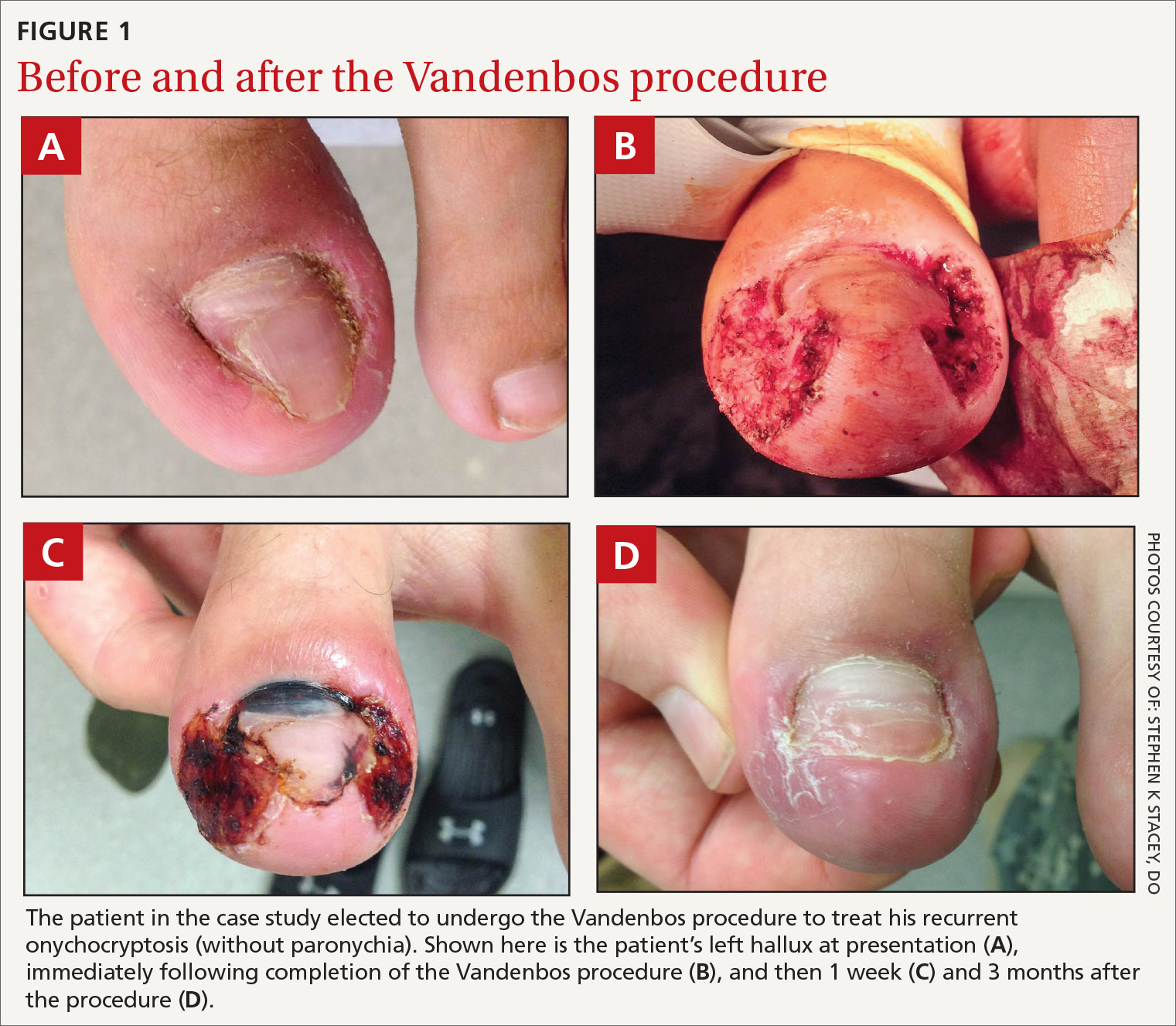
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.
How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.

How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
CASE
A 22-year-old active-duty man presented with left hallux pain, which he had experienced for several years due to an “ingrown toenail.” During the 3 to 4 months prior to presentation, his pain had progressed to the point that he had difficulty with weight-bearing activities. Several weeks prior to evaluation, he tried removing a portion of the nail himself with nail clippers and a pocket knife, but the symptoms persisted.
A skin exam revealed inflamed hypertrophic skin on the medial and lateral border of the toenail without exudate (FIGURE 1A). The patient was given a diagnosis of recurrent onychocryptosis without paronychia. He reported having a similar occurrence 1 to 2 years earlier, which had been treated by his primary care physician via total nail avulsion.

How would you proceed with his care?
Onychocryptosis, also known as an ingrown toenail, is a relatively common condition that can be treated with several nonsurgical and surgical approaches. It occurs when the nail plate punctures the periungual skin, usually on the hallux. Onychocryptosis may be caused by close-trimmed nails with a free edge that are allowed to enter the lateral nail fold. This results in a cascade of inflammatory and infectious processes and may result in paronychia. The inflamed toe skin will often grow over the lateral nail, which further exacerbates the condition. Mild to moderate lesions have limited pain, redness, and swelling with little or no discharge. Moderate to severe lesions have significant pain, redness, swelling, discharge, and/or persistent symptoms despite appropriate conservative therapies.
The condition may manifest at any age, although it is more common in adolescents and young adults. Onychocryptosis is slightly more common in males.1 It may present as a chief complaint, although many cases will likely be discovered incidentally on a skin exam. Although there is no firm evidence of causative factors, possible risk factors include tight-fitting shoes, repetitive activities/sports, poor foot hygiene, hyperhidrosis, genetic predisposition, obesity, and lower-extremity edema.2 Patients often exacerbate the problem with home treatments designed to trim the nail as short as possible. Comparison of symptomatic vs control patients has failed to demonstrate any systematic difference between the nails themselves. This suggests that treatment may not be effective if it is simply directed at controlling nail abnormalities.3,4
Conservative therapy
Conservative therapy should be considered first-line treatment for mild to moderate cases of onychocryptosis. The following are conservative therapy options.5
Proper nail trimming. Advise the patient to allow the nail to grow past the lateral nail fold and to keep it trimmed long so that the overgrowing toe skin cannot encroach on the free edge of the nail. The growth rate of the toenail is approximately 1.62 mm/month—something you may want to mention to the patient so that he or she will have a sense of the estimated duration of therapy.6 Also, the patient may need to implement the following other measures, while the nail is allowed to grow.
Continue to: Skin-softening techniques
Skin-softening techniques. Encourage the patient to apply warm compresses or to soak the toe in warm water for 10 to 20 minutes a day.
Barriers may be inserted between the nail and the periungual skin. Daily intermittent barriers may be used to lift the nail away from the lateral nail fold during regular hygiene activities. Tell the patient that a continuous barrier may be created using gauze or any variety of dental floss placed between the nail and the lateral nail fold, then secured in place with tape and changed daily.
Gutter splint. The gutter splint consists of a plastic tube that has been slit longitudinally from bottom to top with iris scissors or a scalpel. One end is then cut diagonally for smooth insertion between the nail edge and the periungual skin. When placed, the gutter splint lies longitudinally along the edge of the nail, providing a barrier to protect the toe during nail growth. The tube may be obtained by trimming a sterilized vinyl intravenous drip infusion, the catheter from an 18-gauge or larger needle (with the needle removed), or a filter straw. This tube can be affixed with adhesive tape, sutures, or cyanoacrylate.7
Patient-controlled taping. An adhesive tape such as 1-inch silk tape is placed on the symptomatic edge of the lateral nail fold and traction is applied. The tape is then wrapped around the toe and affixed such that the lateral nail fold is pulled away from the nail.8
Medications. Many practitioners use high-potency topical steroids, although evidence for their effectiveness is lacking. Oral antibiotics are unnecessary.
Continue to: One disadvantage of conservative therapy is...
One disadvantage of conservative therapy is that the patient must wait for nail growth before symptom resolution is achieved. In cases where the patient requires immediate symptom resolution, surgical therapies can be used (such as nail edge excision).
Surgical therapy
Surgery is more effective than nonsurgical therapies in preventing recurrence2,9 and is indicated for severe cases of onychocryptosis or for patients who do not respond to a trial of at least 3 months of conservative care.
While there are no universally accepted contraindications to surgical toenail procedures, caution should be taken with patients who have poor healing potential of the feet (eg, chronic vasculopathy or neuropathy). That said, when patients with diabetes have undergone surgical toenail procedures, the research indicates that they have not had worse outcomes.10,11
The following options for surgical therapy of onychocryptosis are considered safe; however, each has variable effectiveness. Each procedure should be performed under local anesthesia, typically as a digital nerve block. The toe should be cleansed prior to any surgical intervention, and clean procedure precautions should be employed. Of the procedures listed here, only phenolization and the Vandenbos procedure are considered definitive treatments for onychocryptosis.5
Total nail removal without matricectomy. In this procedure, the nail is removed entirely, but the nail matrix is not destroyed. The nail regrows in the same dimensions as it had previously, but during the time it is absent the nail bed tends to contract longitudinally and transversely, increasing the likelihood that new nail growth will cause recurrence of symptoms.5 Due to a recurrence rate of > 70%, total nail removal without matricectomy is not recommended as monotherapy for ingrown toenails.9
Continue to: Nail edge excision without mactricectomy
Nail edge excision without matricectomy. This procedure involves removing one-quarter to one-third of the nail from the symptomatic edge. This procedure takes little time and is easy to perform. Recurrence rates are > 70% for the same reasons as outlined above.9 (Often during preparation for this procedure, a loose shard of nail is observed puncturing the periungual skin. Removal of this single aberrant portion of nail is frequently curative in and of itself.) Patients typically report rapid relief of symptoms, so this procedure may be favored when patients do not have the time or desire to attempt more definitive therapy. However, patients should be advised of the high recurrence rate.
Nail excision with matricectomy using phenol (ie, phenolization). In this procedure, the nail is avulsed, and the matrix is destroyed with phenol (80%-88%).9,12 Typically, this is performed only on the symptomatic edge of the nail. The phenol should be applied for 1 to 3 minutes using a cotton-tipped applicator saturated in the solution.
While phenolization is relatively quick and simple—and is associated with good cure rates—it causes pain and disability during the healing process and takes several weeks to heal. Phenolization also has a slightly increased risk for infection when compared to nail excision without matricectomy. Giving antibiotics before or following the procedure does not appear to reduce this risk.7 If the matrix is incompletely destroyed, a new nail spicule may grow along the lateral nail edge and a repeat procedure may be required.7 When properly performed, the nail will be narrower but should otherwise maintain a more-or-less normal appearance. The use of phenolization for the treatment of onychocryptosis in the pediatric population has been found to be successful, as well.14
The Vandenbos procedure. This procedure involves removing a large amount of skin from the lateral nail fold and allowing it to heal secondarily. When performed correctly, this procedure has a very low recurrence rate, with no cases of recurrence in nearly 1200 patients reported in the literature.15 The cosmetic results are generally superior to the other surgical methods described here5 and patient satisfaction is high.15 It has been used with similar effectiveness in children.16
Full recovery takes about 6 weeks. Overall, the Vandenbos procedure can definitively treat the condition with a good cosmetic outcome. (See “How to perform the Vandenbos procedure.”)
Continue to: SIDEBAR
SIDEBAR
How to perform the Vandenbox procedure
The Vandenbos procedure, also known as soft-tissue nail fold excision, was first described in 1958 by Kermit Q. Vandenbos, a surgeon for the US Air Force. He felt that overgrown toe skin was the primary causative factor in onychocryptosis.4
In the procedure, the hypertrophic skin is removed to such a degree that it cannot encroach on the growing nail. After the toe is fully healed, the toe and nail should have a fully normal appearance. Indications and contraindications are the same as for other surgical procedures for the treatment of onychocryptosis. Pain and disability following the procedure is similar to phenolization, and the recovery period takes several weeks for the patient to fully heal.
Equipment needed:
- alcohol swab
- tourniquet (optional)
- 3 mL to 5 mL of local anesthetic (eg, 2% lidocaine)
- topical antiseptic (eg, iodine or chlorhexidine)
- number 15 blade scalpel
- tissue forceps
- cautery device (electrocautery or thermocautery)
- dressing supplies (topical ointment, gauze, tape)
The steps15:
- Perform a digital nerve block using an alcohol swab and anesthetic. The anesthetic may be used with or without epinephrine.
- Place a tourniquet at the base of the toe if the anesthetic does not contain epinephrine. The tourniquet is not required if epinephrine is used during anesthesia.17
- Cleanse the toe with iodine, chlorhexidine, or a similar agent.
- Make a 5-mm incision proximally while leaving the nail bed intact. Begin approximately 3 mm from the lateral edge of the base of the nail. The incision should extend around the edge of the toe in an elliptical sweep towards the tip of the nail, remaining 3 mm from the edge of the nail. This is best accomplished in a single motion with a #15 blade. An adequate portion of skin must be removed, leaving a defect of approximately 1.5 × 3 cm (approximately the size of a cashew) (FIGURE 1B).
- Electrocauterize or thermocauterize along the edges and subcutaneous tissue of the wound. This reduces postoperative bleeding and pain. The matrix should not be damaged.
- Dress the wound with ample amounts of petrolatum followed by nonstick gauze. Profuse bleeding can be expected unless pressure is applied, so apply ample amounts of additional gauze to absorb any blood. The foot is elevated and the tourniquet (if used) removed. In order to reduce postoperative bleeding and pain, instruct the patient to lie with the foot elevated as much as possible for the first 24 to 48 hours.
- Advise the patient that moderate pain is expected for the first 2 to 3 days. Analgesia may be obtained with an acetaminophen/opiate combination (eg, hydrocodone/acetaminophen 5/325, 1 tablet every 4-6 hours as needed) for the first 2 to 3 days. This may be followed by acetaminophen or nonsteroidal anti-inflammatory drugs thereafter at usual dosing, which can either be prescribed or obtained over the counter.
Postoperative care
After 48 hours, the patient can remove the dressing and gently rinse the wound and reapply a new dressing as before. The dressing should be changed at least once daily and whenever it becomes soiled or wet. After 48 hours, while the dressing remains on the toe, the patient may begin taking brief showers. After showering, the toe should be gently rinsed with clean water and the dressing changed. Blood or crust should not be scrubbed off, as this will impair re-epithelialization, but it may be rinsed off if able. Otherwise, the wound should not be soaked until re-epithelialization has occurred.
Patient follow-up should occur after 1 to 2 weeks (FIGURE 1C). After approximately 6 weeks, the wound should be healed completely with the nail remaining above the skin. (FIGURE 1D shows wound healing after 3 months.)
Advise patients that erythema and drainage are expected, but the erythema should not extend proximally from the metatarsophalangeal joint. Prophylactic antibiotics are not required, although they may be used if infection is suspected. Despite the proximity of the procedure to the distal phalanx, there have been no reported cases of osteomyelitis.15
Stephen K. Stacey, DO, Chief Resident, Peak Vista Family Medicine Residency Program, 340 Printers Parkway, Colorado Springs, CO 80910; [email protected].
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
1. Bryant A, Knox A. Ingrown toenails: the role of the GP. Aust Fam Physician. 2015;44:102-105.
2. Eekhof JA, Van Wijk B, Knuistingh Neven A, et al. Interventions for ingrowing toenails. Cochrane Database Syst Rev. 2012;(4):CD001541. doi: 10.1002/14651858.
3. Pearson HJ, Bury RN, et al. Ingrowing toenails: is there a nail abnormality? A prospective study. J Bone Joint Surg Br. 1987;69:840-842.
4. Vandenbos KQ, Bowers WF. Ingrown toenail: a result of weight bearing on soft tissue. US Armed Forces Med J. 1959;10:1168-1173.
5. Haneke E. Controversies in the treatment of ingrown nails. Dermatol Res Pract. 2012;2012:783924. doi.org/10.1155/2012/783924.
6. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423.
7. Heidelbaugh JJ, Hobart L. Management of the ingrown toenail. Am Fam Physician. 2009;79:303-308.
8. Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555.
9. Rounding C, Bloomfield S. Surgical treatments for ingrowing toenails. Cochrane Database Syst Rev. 2005;(2):CD001541.
10. Felton PM, Weaver TD. Phenol and alcohol chemical matrixectomy in diabetic versus nondiabetic patients. A retrospective study. J Am Podiatr Med Assoc. 1999;89:410-412.
11. Giacalone VF. Phenol matricectomy in patients with diabetes. J Foot Ankle Surg. 1997;36:264-267; discussion 328.
12. Tatlican S, Yamangöktürk B, Eren C, et al. [Comparison of phenol applications of different durations for the cauterization of the germinal matrix: an efficacy and safety study]. Acta Orthop Traumatol Turc. 2009;43:298-302.
13. Grieg JD, Anderson JH, et al. The surgical treatment of ingrowing toenails. J Bone Joint Surg Br. 1991;73:131-133.
14. Islam S, Lin EM, Drongowski R, et al. The effect of phenol on ingrown toenail excision in children. J Pediatr Surg. 2005;40:290-292.
15. Chapeskie H. Ingrown toenail or overgrown toe skin?: Alternative treatment for onychocryptosis. Can Fam Physician. 2008;54:1561-1562.
16. Haricharan RN, Masquijo J, Bettolli M. Nail-fold excision for the treatment of ingrown toenail in children. J Pediatr. 2013;162:398-402.
17. Córdoba-Fernández A, Rodríguez-Delgado FJ. Anaesthetic digital block with epinephrine vs. tourniquet in ingrown toenail surgery: a clinical trial on efficacy. J Eur Acad Dermatol Venereol. 2015;29:985-990.
Females with acne stay on spironolactone longer than antibiotics in real-world usage study
according to a retrospective study of women with acne published in the Journal of the American Academy of Dermatology.
Among those treated for at least a year, patients continued spironolactone for about 90 days longer on average, than those on antibiotic therapy, reported John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and associates. “The extended drug usage survival of spironolactone suggests that, in routine clinical practice, spironolactone may have good long-term effectiveness and tolerability,” they wrote. “Since female patients often have persistent acne into adulthood and given concerns regarding antibiotic overuse among acne patients, it is possible that using spironolactone as a first-line agent before oral antibiotics could improve outcomes for female patients with acne,” they added.
In the study, they pointed out that spironolactone is emerging as a possible alternative to oral antibiotic therapy, but “little is known about long-term outcomes with spironolactone for those who have an initial positive response and how it compares to other alternatives.”
To examine the duration of acne treatment with spironolactone versus oral antibiotics, the researchers analyzed data during 2010-2016 in the Optum Clinformatics Data Mart. They included data on female patients aged 12-40 years, with at least two diagnosis codes for acne, who received spironolactone or oral antibiotics for at least 12 months. They used multivariate Cox proportional hazard models to assess differences in duration of therapy for spironolactone, compared with oral antibiotics.
The mean duration of a treatment course was significantly longer among the 4,321 patients treated with spironolactone than among the 7,517 patients treated with oral tetracycline-class antibiotics (697.8 days vs. 604.4 days; P less than .001). Compared with treatment with oral tetracyclines, the hazard ratio for discontinuing spironolactone treatment was 0.74, after researchers controlled for the age at diagnosis and treatment, history of polycystic ovarian syndrome, and history of combined oral contraceptive or topical retinoid treatment.
Patients who receive spironolactone and patients who receive oral antibiotics may represent different populations, the authors noted. In addition, guidelines advise limiting antibiotic treatment to 3-6 months, and antibiotic discontinuations may have been related to these recommendations. “It is not possible to determine whether medication discontinuation occurred due to lack of efficacy, cost, side effects, resolution of acne, or other factors,” they said, adding that prospective studies are needed “to identify the optimal treatment approaches for female patients with moderate to severe acne.”
The study was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri is supported by NIAMS and receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania.
SOURCE: Barbieri JS et al. J Am Acad Dermatol. 2019 Mar 21. doi: 10.1016/j.jaad.2019.03.036.
according to a retrospective study of women with acne published in the Journal of the American Academy of Dermatology.
Among those treated for at least a year, patients continued spironolactone for about 90 days longer on average, than those on antibiotic therapy, reported John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and associates. “The extended drug usage survival of spironolactone suggests that, in routine clinical practice, spironolactone may have good long-term effectiveness and tolerability,” they wrote. “Since female patients often have persistent acne into adulthood and given concerns regarding antibiotic overuse among acne patients, it is possible that using spironolactone as a first-line agent before oral antibiotics could improve outcomes for female patients with acne,” they added.
In the study, they pointed out that spironolactone is emerging as a possible alternative to oral antibiotic therapy, but “little is known about long-term outcomes with spironolactone for those who have an initial positive response and how it compares to other alternatives.”
To examine the duration of acne treatment with spironolactone versus oral antibiotics, the researchers analyzed data during 2010-2016 in the Optum Clinformatics Data Mart. They included data on female patients aged 12-40 years, with at least two diagnosis codes for acne, who received spironolactone or oral antibiotics for at least 12 months. They used multivariate Cox proportional hazard models to assess differences in duration of therapy for spironolactone, compared with oral antibiotics.
The mean duration of a treatment course was significantly longer among the 4,321 patients treated with spironolactone than among the 7,517 patients treated with oral tetracycline-class antibiotics (697.8 days vs. 604.4 days; P less than .001). Compared with treatment with oral tetracyclines, the hazard ratio for discontinuing spironolactone treatment was 0.74, after researchers controlled for the age at diagnosis and treatment, history of polycystic ovarian syndrome, and history of combined oral contraceptive or topical retinoid treatment.
Patients who receive spironolactone and patients who receive oral antibiotics may represent different populations, the authors noted. In addition, guidelines advise limiting antibiotic treatment to 3-6 months, and antibiotic discontinuations may have been related to these recommendations. “It is not possible to determine whether medication discontinuation occurred due to lack of efficacy, cost, side effects, resolution of acne, or other factors,” they said, adding that prospective studies are needed “to identify the optimal treatment approaches for female patients with moderate to severe acne.”
The study was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri is supported by NIAMS and receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania.
SOURCE: Barbieri JS et al. J Am Acad Dermatol. 2019 Mar 21. doi: 10.1016/j.jaad.2019.03.036.
according to a retrospective study of women with acne published in the Journal of the American Academy of Dermatology.
Among those treated for at least a year, patients continued spironolactone for about 90 days longer on average, than those on antibiotic therapy, reported John S. Barbieri, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and associates. “The extended drug usage survival of spironolactone suggests that, in routine clinical practice, spironolactone may have good long-term effectiveness and tolerability,” they wrote. “Since female patients often have persistent acne into adulthood and given concerns regarding antibiotic overuse among acne patients, it is possible that using spironolactone as a first-line agent before oral antibiotics could improve outcomes for female patients with acne,” they added.
In the study, they pointed out that spironolactone is emerging as a possible alternative to oral antibiotic therapy, but “little is known about long-term outcomes with spironolactone for those who have an initial positive response and how it compares to other alternatives.”
To examine the duration of acne treatment with spironolactone versus oral antibiotics, the researchers analyzed data during 2010-2016 in the Optum Clinformatics Data Mart. They included data on female patients aged 12-40 years, with at least two diagnosis codes for acne, who received spironolactone or oral antibiotics for at least 12 months. They used multivariate Cox proportional hazard models to assess differences in duration of therapy for spironolactone, compared with oral antibiotics.
The mean duration of a treatment course was significantly longer among the 4,321 patients treated with spironolactone than among the 7,517 patients treated with oral tetracycline-class antibiotics (697.8 days vs. 604.4 days; P less than .001). Compared with treatment with oral tetracyclines, the hazard ratio for discontinuing spironolactone treatment was 0.74, after researchers controlled for the age at diagnosis and treatment, history of polycystic ovarian syndrome, and history of combined oral contraceptive or topical retinoid treatment.
Patients who receive spironolactone and patients who receive oral antibiotics may represent different populations, the authors noted. In addition, guidelines advise limiting antibiotic treatment to 3-6 months, and antibiotic discontinuations may have been related to these recommendations. “It is not possible to determine whether medication discontinuation occurred due to lack of efficacy, cost, side effects, resolution of acne, or other factors,” they said, adding that prospective studies are needed “to identify the optimal treatment approaches for female patients with moderate to severe acne.”
The study was funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri is supported by NIAMS and receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania.
SOURCE: Barbieri JS et al. J Am Acad Dermatol. 2019 Mar 21. doi: 10.1016/j.jaad.2019.03.036.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Patch testing in atopic dermatitis: when and how
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Painful, slow-growing recurrent nodules
A 67-year-old woman presented with multiple painful nodules that had developed on her scalp, face, and neck over the course of 1 year. She also had a few nodules on her trunk and hip. There was no associated bleeding, ulceration, or drainage from the lesions. She had no systemic symptoms. The patient reported that she’d had a similar lesion on her frontal scalp about 15 years earlier, and it was excised completely. (She was not aware of the diagnosis.) She indicated that her mother and son had similar lesions in the past.
Her medical history was remarkable for diabetes and hypertension, which were well controlled on metformin and lisinopril, respectively. The patient had cancer of the left breast that was treated with mastectomy and chemotherapy 3 years prior.
On physical examination, the patient had multiple firm, rubbery, tender nodules with tan or pink hue, measuring 1 to 1.5 cm. The nodules were located on the left side of her chin and right preauricular area (FIGURE 1), as well as the right sides of her neck and hip. Most of the nodules were solitary; the preauricular area had 2 clustered pink lesions. The largest nodule was located on the patient’s chin and had overlying telangiectasias.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cylindroma
Definitive diagnosis was made by shave biopsy of the left hip lesion. Histopathology demonstrated various-sized discrete aggregates of basaloid cell nests in a jigsaw puzzle–like configuration (FIGURE 2), surrounded by rims of homogenous eosinophilic material. Histologic findings were consistent with cylindroma.1
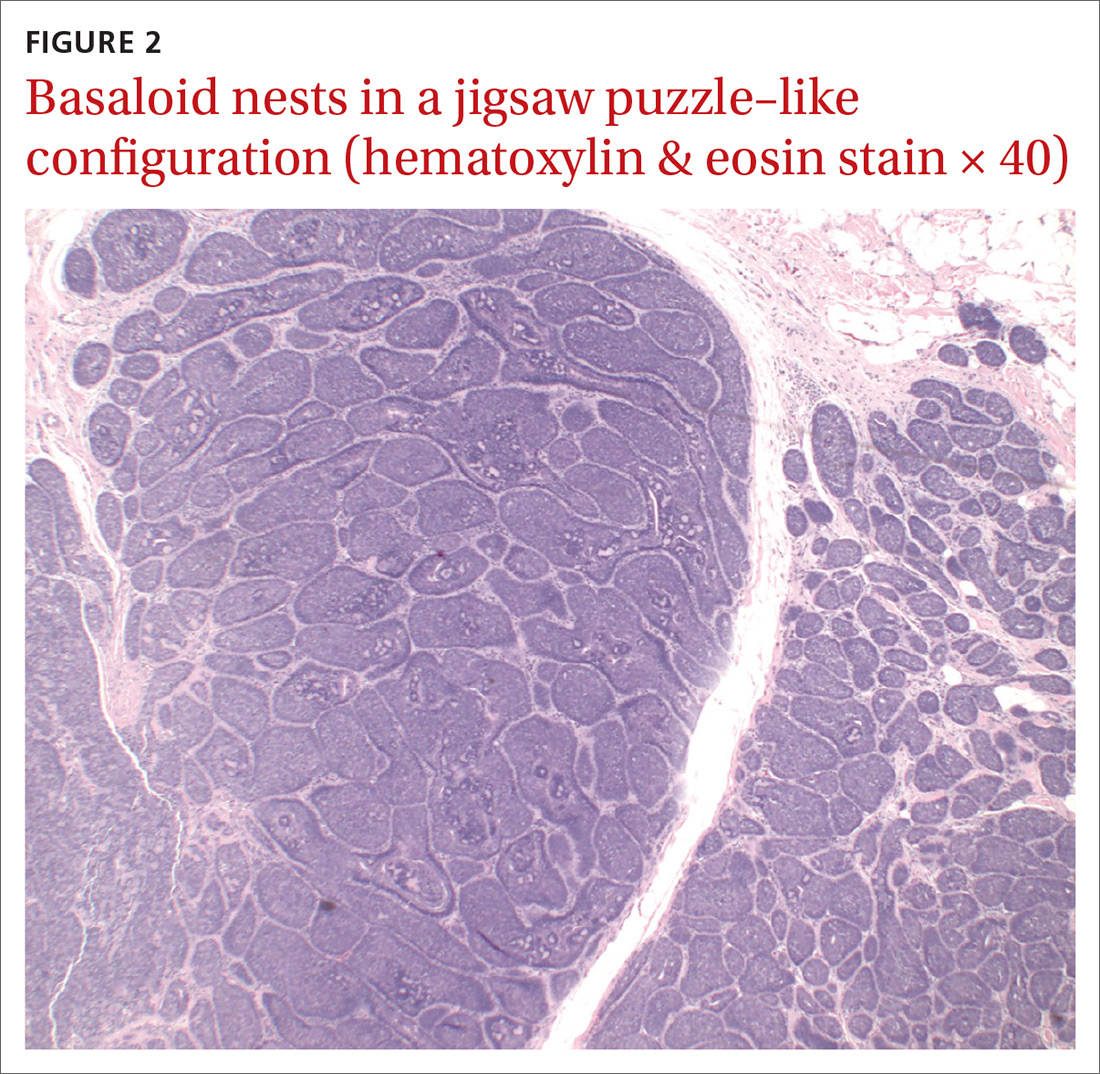
Rare with a female predominance. Solitary cylindromas occur sporadically and usually affect middle-aged and elderly patients. Incidence is rare, but there is a female predominance of 6 to 9:1.2 Clinical appearance shows a slow-growing, firm mass that can range from a few millimeters to a few centimeters in diameter. The masses can have a pink or blue hue and usually are nontender unless there is nerve impingement.2
If multiple tumors are present or the patient has a family history of similar lesions, the disorder is likely inherited in an autosomal dominant pattern (with variable expression), which can be associated with Brooke-Spiegler syndrome. This syndrome is related to a mutation of the cylindromatosis gene on chromosome 16. This is a tumor suppressor gene, inactivation of which can lead to uninhibited action of NF-
Rarely, cylindromas can undergo malignant transformation; signs include ulceration, bleeding, rapid growth, or color change.2 In these cases, appropriate imaging such as computed tomography, magnetic resonance imaging, or positron emission tomography should be sought as there have been case reports of cylindroma extension to bone, as well as metastases to sites including the lymph nodes, thyroid, liver, lungs, bones, and meninges.4
Lipomas and pilar cysts comprise the differential
Lipomas are soft, painless, flesh-colored masses that typically appear on the trunk and arms but are uncommon on the face. Telangiectasias are not seen.
Continue to: Pilar cysts
Pilar cysts can mimic cylindromas in clinical presentation. Derived from the root sheath of hair follicles, they typically appear on the scalp but rarely on the face. Pilar cysts are slow growing, firm, and whitish in color5; several cysts can appear at a time.
Pilomatricomas are firm skin masses—usually < 3 cm in size—that can vary in color. There may be an extrusion of calcified material within the nodules, which does not occur in cylindromas.
Sebaceous adenomas are yellowish papules, usually < 1 cm in size, that appear on the head and neck area.6
Making the diagnosis. The clinical appearance of cylindromas and their location will point to the diagnosis, as will a family history of similar lesions. Ultimately, the diagnosis is confirmed by biopsy.
Treatment mainstay includes excision
Complete excision of all lesions is key due to the possibility of malignant transformation and metastasis. Options for removal include electrodesiccation, curettage, cryosurgery,3 high-dose radiation, and the use of a CO2 laser.2 For multiple large tumors, or ones in cosmetically sensitive areas, consider referral to Dermatology or Plastic Surgery. Further imaging should be sought to rule out extension to bone or metastasis. Patients with multiple cylindromas and Brooke-Spiegler syndrome need lifelong follow-up to monitor for recurrence and malignant transformation.3,7,8
Continue to: Our patient...
Our patient underwent complete excision of the left hip lesion. Other trunk lesions were excised serially. The patient was referred to a surgeon specializing in Mohs micrographic surgery for excision of the facial lesions. The patient and her family members were referred for genetic testing.
CORRESPONDENCE
Zeeshan Afzal, MD, McAllen Family Medicine Residency Program, University of Texas Rio Grande Valley, 205 E Toronto Ave, McAllen, TX 78503; [email protected]
1. Elder D, Elenitsas R, Jaworsky CH, et al. Tumors of the epidermal appendages. Lever’s Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:775-777.
2. Singh DD, Naujoks C, Depprich R, et al. Cylindroma of head and neck: Review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516-521.
3. Sicinska J, Rakowska A, Czuwara-Ladykowska J, et al. Cylindroma transforming into basal cell carcinoma in a patient with Brooke-Spiegler syndrome. J Dermatol Case Rep. 2007;1:4-9.
4. Jordão C, de Magalhães TC, Cuzzi T, et al. Cylindroma: an update. Int J Dermatol. 2015;54:275-278.
5. Stone M. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1920.
6. McCalmont T, Pincus L. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1938-1942.
7. Gerretsen AL, Van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
8. Manicketh I, Singh R, Ghosh PK. Eccrine cylindroma of the face and scalp. Indian Dermatol Online J. 2016;7:203-205.
A 67-year-old woman presented with multiple painful nodules that had developed on her scalp, face, and neck over the course of 1 year. She also had a few nodules on her trunk and hip. There was no associated bleeding, ulceration, or drainage from the lesions. She had no systemic symptoms. The patient reported that she’d had a similar lesion on her frontal scalp about 15 years earlier, and it was excised completely. (She was not aware of the diagnosis.) She indicated that her mother and son had similar lesions in the past.
Her medical history was remarkable for diabetes and hypertension, which were well controlled on metformin and lisinopril, respectively. The patient had cancer of the left breast that was treated with mastectomy and chemotherapy 3 years prior.
On physical examination, the patient had multiple firm, rubbery, tender nodules with tan or pink hue, measuring 1 to 1.5 cm. The nodules were located on the left side of her chin and right preauricular area (FIGURE 1), as well as the right sides of her neck and hip. Most of the nodules were solitary; the preauricular area had 2 clustered pink lesions. The largest nodule was located on the patient’s chin and had overlying telangiectasias.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cylindroma
Definitive diagnosis was made by shave biopsy of the left hip lesion. Histopathology demonstrated various-sized discrete aggregates of basaloid cell nests in a jigsaw puzzle–like configuration (FIGURE 2), surrounded by rims of homogenous eosinophilic material. Histologic findings were consistent with cylindroma.1

Rare with a female predominance. Solitary cylindromas occur sporadically and usually affect middle-aged and elderly patients. Incidence is rare, but there is a female predominance of 6 to 9:1.2 Clinical appearance shows a slow-growing, firm mass that can range from a few millimeters to a few centimeters in diameter. The masses can have a pink or blue hue and usually are nontender unless there is nerve impingement.2
If multiple tumors are present or the patient has a family history of similar lesions, the disorder is likely inherited in an autosomal dominant pattern (with variable expression), which can be associated with Brooke-Spiegler syndrome. This syndrome is related to a mutation of the cylindromatosis gene on chromosome 16. This is a tumor suppressor gene, inactivation of which can lead to uninhibited action of NF-
Rarely, cylindromas can undergo malignant transformation; signs include ulceration, bleeding, rapid growth, or color change.2 In these cases, appropriate imaging such as computed tomography, magnetic resonance imaging, or positron emission tomography should be sought as there have been case reports of cylindroma extension to bone, as well as metastases to sites including the lymph nodes, thyroid, liver, lungs, bones, and meninges.4
Lipomas and pilar cysts comprise the differential
Lipomas are soft, painless, flesh-colored masses that typically appear on the trunk and arms but are uncommon on the face. Telangiectasias are not seen.
Continue to: Pilar cysts
Pilar cysts can mimic cylindromas in clinical presentation. Derived from the root sheath of hair follicles, they typically appear on the scalp but rarely on the face. Pilar cysts are slow growing, firm, and whitish in color5; several cysts can appear at a time.
Pilomatricomas are firm skin masses—usually < 3 cm in size—that can vary in color. There may be an extrusion of calcified material within the nodules, which does not occur in cylindromas.
Sebaceous adenomas are yellowish papules, usually < 1 cm in size, that appear on the head and neck area.6
Making the diagnosis. The clinical appearance of cylindromas and their location will point to the diagnosis, as will a family history of similar lesions. Ultimately, the diagnosis is confirmed by biopsy.
Treatment mainstay includes excision
Complete excision of all lesions is key due to the possibility of malignant transformation and metastasis. Options for removal include electrodesiccation, curettage, cryosurgery,3 high-dose radiation, and the use of a CO2 laser.2 For multiple large tumors, or ones in cosmetically sensitive areas, consider referral to Dermatology or Plastic Surgery. Further imaging should be sought to rule out extension to bone or metastasis. Patients with multiple cylindromas and Brooke-Spiegler syndrome need lifelong follow-up to monitor for recurrence and malignant transformation.3,7,8
Continue to: Our patient...
Our patient underwent complete excision of the left hip lesion. Other trunk lesions were excised serially. The patient was referred to a surgeon specializing in Mohs micrographic surgery for excision of the facial lesions. The patient and her family members were referred for genetic testing.
CORRESPONDENCE
Zeeshan Afzal, MD, McAllen Family Medicine Residency Program, University of Texas Rio Grande Valley, 205 E Toronto Ave, McAllen, TX 78503; [email protected]
A 67-year-old woman presented with multiple painful nodules that had developed on her scalp, face, and neck over the course of 1 year. She also had a few nodules on her trunk and hip. There was no associated bleeding, ulceration, or drainage from the lesions. She had no systemic symptoms. The patient reported that she’d had a similar lesion on her frontal scalp about 15 years earlier, and it was excised completely. (She was not aware of the diagnosis.) She indicated that her mother and son had similar lesions in the past.
Her medical history was remarkable for diabetes and hypertension, which were well controlled on metformin and lisinopril, respectively. The patient had cancer of the left breast that was treated with mastectomy and chemotherapy 3 years prior.
On physical examination, the patient had multiple firm, rubbery, tender nodules with tan or pink hue, measuring 1 to 1.5 cm. The nodules were located on the left side of her chin and right preauricular area (FIGURE 1), as well as the right sides of her neck and hip. Most of the nodules were solitary; the preauricular area had 2 clustered pink lesions. The largest nodule was located on the patient’s chin and had overlying telangiectasias.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Cylindroma
Definitive diagnosis was made by shave biopsy of the left hip lesion. Histopathology demonstrated various-sized discrete aggregates of basaloid cell nests in a jigsaw puzzle–like configuration (FIGURE 2), surrounded by rims of homogenous eosinophilic material. Histologic findings were consistent with cylindroma.1

Rare with a female predominance. Solitary cylindromas occur sporadically and usually affect middle-aged and elderly patients. Incidence is rare, but there is a female predominance of 6 to 9:1.2 Clinical appearance shows a slow-growing, firm mass that can range from a few millimeters to a few centimeters in diameter. The masses can have a pink or blue hue and usually are nontender unless there is nerve impingement.2
If multiple tumors are present or the patient has a family history of similar lesions, the disorder is likely inherited in an autosomal dominant pattern (with variable expression), which can be associated with Brooke-Spiegler syndrome. This syndrome is related to a mutation of the cylindromatosis gene on chromosome 16. This is a tumor suppressor gene, inactivation of which can lead to uninhibited action of NF-
Rarely, cylindromas can undergo malignant transformation; signs include ulceration, bleeding, rapid growth, or color change.2 In these cases, appropriate imaging such as computed tomography, magnetic resonance imaging, or positron emission tomography should be sought as there have been case reports of cylindroma extension to bone, as well as metastases to sites including the lymph nodes, thyroid, liver, lungs, bones, and meninges.4
Lipomas and pilar cysts comprise the differential
Lipomas are soft, painless, flesh-colored masses that typically appear on the trunk and arms but are uncommon on the face. Telangiectasias are not seen.
Continue to: Pilar cysts
Pilar cysts can mimic cylindromas in clinical presentation. Derived from the root sheath of hair follicles, they typically appear on the scalp but rarely on the face. Pilar cysts are slow growing, firm, and whitish in color5; several cysts can appear at a time.
Pilomatricomas are firm skin masses—usually < 3 cm in size—that can vary in color. There may be an extrusion of calcified material within the nodules, which does not occur in cylindromas.
Sebaceous adenomas are yellowish papules, usually < 1 cm in size, that appear on the head and neck area.6
Making the diagnosis. The clinical appearance of cylindromas and their location will point to the diagnosis, as will a family history of similar lesions. Ultimately, the diagnosis is confirmed by biopsy.
Treatment mainstay includes excision
Complete excision of all lesions is key due to the possibility of malignant transformation and metastasis. Options for removal include electrodesiccation, curettage, cryosurgery,3 high-dose radiation, and the use of a CO2 laser.2 For multiple large tumors, or ones in cosmetically sensitive areas, consider referral to Dermatology or Plastic Surgery. Further imaging should be sought to rule out extension to bone or metastasis. Patients with multiple cylindromas and Brooke-Spiegler syndrome need lifelong follow-up to monitor for recurrence and malignant transformation.3,7,8
Continue to: Our patient...
Our patient underwent complete excision of the left hip lesion. Other trunk lesions were excised serially. The patient was referred to a surgeon specializing in Mohs micrographic surgery for excision of the facial lesions. The patient and her family members were referred for genetic testing.
CORRESPONDENCE
Zeeshan Afzal, MD, McAllen Family Medicine Residency Program, University of Texas Rio Grande Valley, 205 E Toronto Ave, McAllen, TX 78503; [email protected]
1. Elder D, Elenitsas R, Jaworsky CH, et al. Tumors of the epidermal appendages. Lever’s Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:775-777.
2. Singh DD, Naujoks C, Depprich R, et al. Cylindroma of head and neck: Review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516-521.
3. Sicinska J, Rakowska A, Czuwara-Ladykowska J, et al. Cylindroma transforming into basal cell carcinoma in a patient with Brooke-Spiegler syndrome. J Dermatol Case Rep. 2007;1:4-9.
4. Jordão C, de Magalhães TC, Cuzzi T, et al. Cylindroma: an update. Int J Dermatol. 2015;54:275-278.
5. Stone M. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1920.
6. McCalmont T, Pincus L. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1938-1942.
7. Gerretsen AL, Van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
8. Manicketh I, Singh R, Ghosh PK. Eccrine cylindroma of the face and scalp. Indian Dermatol Online J. 2016;7:203-205.
1. Elder D, Elenitsas R, Jaworsky CH, et al. Tumors of the epidermal appendages. Lever’s Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:775-777.
2. Singh DD, Naujoks C, Depprich R, et al. Cylindroma of head and neck: Review of the literature and report of two rare cases. J Craniomaxillofac Surg. 2013;41:516-521.
3. Sicinska J, Rakowska A, Czuwara-Ladykowska J, et al. Cylindroma transforming into basal cell carcinoma in a patient with Brooke-Spiegler syndrome. J Dermatol Case Rep. 2007;1:4-9.
4. Jordão C, de Magalhães TC, Cuzzi T, et al. Cylindroma: an update. Int J Dermatol. 2015;54:275-278.
5. Stone M. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1920.
6. McCalmont T, Pincus L. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1938-1942.
7. Gerretsen AL, Van der Putte SC, Deenstra W, et al. Cutaneous cylindroma with malignant transformation. Cancer. 1993;72:1618-1623.
8. Manicketh I, Singh R, Ghosh PK. Eccrine cylindroma of the face and scalp. Indian Dermatol Online J. 2016;7:203-205.
Erythema on abdomen
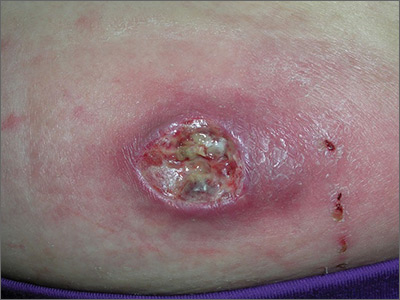
The FP was puzzled by the lack of response to treatment and decided to perform a 4-mm punch biopsy at the edge of the nonhealing ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). His differential diagnosis included pyoderma gangrenosum and a deep fungal infection. The pathologist called a week later FP with a surprising result: anaplastic large cell cutaneous T-cell lymphoma. (See the Watch & Learn video on “Punch biopsy.”)
Anaplastic large cell cutaneous T-cell lymphoma is a rare diagnosis—especially in a teenager—and it can’t be determined by appearance only. On follow-up, the FP explained the diagnosis to the patient and her mother. He called Hematology/Oncology to facilitate the referral.
The patient was treated by the specialist with weekly oral methotrexate and her skin cleared up completely. Although she would likely need treatment for years, the prognosis was good. This case is a reminder that when a treatment is not working for an expected diagnosis, it’s time to reconsider the diagnosis and do further testing to identify the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP was puzzled by the lack of response to treatment and decided to perform a 4-mm punch biopsy at the edge of the nonhealing ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). His differential diagnosis included pyoderma gangrenosum and a deep fungal infection. The pathologist called a week later FP with a surprising result: anaplastic large cell cutaneous T-cell lymphoma. (See the Watch & Learn video on “Punch biopsy.”)
Anaplastic large cell cutaneous T-cell lymphoma is a rare diagnosis—especially in a teenager—and it can’t be determined by appearance only. On follow-up, the FP explained the diagnosis to the patient and her mother. He called Hematology/Oncology to facilitate the referral.
The patient was treated by the specialist with weekly oral methotrexate and her skin cleared up completely. Although she would likely need treatment for years, the prognosis was good. This case is a reminder that when a treatment is not working for an expected diagnosis, it’s time to reconsider the diagnosis and do further testing to identify the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP was puzzled by the lack of response to treatment and decided to perform a 4-mm punch biopsy at the edge of the nonhealing ulcer. (Note that the correct location for a biopsy of an ulcer is on the edge, not in the middle). His differential diagnosis included pyoderma gangrenosum and a deep fungal infection. The pathologist called a week later FP with a surprising result: anaplastic large cell cutaneous T-cell lymphoma. (See the Watch & Learn video on “Punch biopsy.”)
Anaplastic large cell cutaneous T-cell lymphoma is a rare diagnosis—especially in a teenager—and it can’t be determined by appearance only. On follow-up, the FP explained the diagnosis to the patient and her mother. He called Hematology/Oncology to facilitate the referral.
The patient was treated by the specialist with weekly oral methotrexate and her skin cleared up completely. Although she would likely need treatment for years, the prognosis was good. This case is a reminder that when a treatment is not working for an expected diagnosis, it’s time to reconsider the diagnosis and do further testing to identify the correct diagnosis.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Chacon G, Nayar A, Usatine R, Smith M. Cutaneous T-cell lymphoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1124-1131.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Pyoderma gangrenosum mistaken for diabetic ulcer
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924