User login
Cell therapy promising as long-term limb-saving treatment in diabetes
Bone marrow derived autologous cell therapy (ACT) has been shown to significantly reduce the rate of major amputation at 5 years in people with diabetes who developed critical limb-threatening ischemia (CLTI).
In a study of 130 patients, 64% of 42 patients who were treated conservatively needed a major amputation at 5 years versus just 30% of 45 patients who had been treated with ACT (P = .011).
This compared favorably to the results seen with repeated percutaneous angioplasty (re-PTA), where just 20.9% of 43 patients underwent limb salvage (P = .002 vs. conservative therapy).
Furthermore, amputation-free survival was significantly longer in both active groups, Michal Dubský, MD, PhD, FRSPH, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Dubský, of the Institute for Clinical and Experimental Medicine and Charles University in Prague, also reported that fewer patients who had undergone re-PTA or ACT than conservative treatment had died by 5 years (25.8% and 35.6%, respectively, vs. 61.9%), but that the difference was significant only for the revascularization procedure (P = .012).
Based on these findings, “we believe that autologous cell therapy seems to be an appropriate alternative to repeated PTA even for patients with no-option chronic limb-threatening ischemia,” he said.
“This is a very important area,” said Andrew J.M. Boulton, MBBS, MD, FRCP, who chaired the oral abstract presentation session during which the findings were presented.
“It is very difficult to get an evidence base from randomized studies in this area, because of the nature of the patients: They’re very sick and we all deal with them in our clinics very regularly,” added Dr. Boulton, professor of medicine within the division of diabetes, endocrinology and gastroenterology at the University of Manchester (England).
Dr. Boulton called the findings a “very important addition to what we know.”
New option for no-option CLTI
CLTI is associated with persistent pain at rest, ulcers, and gangrene, and can be the end result of longstanding peripheral arterial disease. Within the first year of presentation, there’s a 30% chance of having a major amputation and a 25% chance of dying.
Importantly, said Dr. Dubský, “there is a big difference in this diagnosis” between patients with diabetes and those without. For instance, CLTI is more diffuse in patients with diabetes than in those without, different arteries are affected and the sclerosis seen can be more rigid and “full of calcium.”
While surgery to improve blood flow is the standard of care, not everyone is suitable. Bypass surgery or endovascular procedures can be performed in only 40%-50% of patients, and even then a therapeutic effect may be seen in only a quarter of patients.
“We need some new therapeutic modalities for this diagnosis, and one of them could be autologous cell therapy,” said Dr. Dubský.
Study details
Dr. Dubský and coinvestigators consecutively recruited 130 patients with diabetic foot and CLTI who had been seen at their clinic over a 5-year period. Of these, 87 had not been eligible for standard revascularization and underwent ACT or were treated conservatively.
Of the patients who were not eligible for standard revascularization (‘no-option CLTI), 45 had undergone ACT and 42 had been treated conservatively. Dr. Dubský acknowledged that “his study was not prospective and randomized.”
All patients in the study had at least one unsuccessful revascularization procedure and diabetic foot ulcers, and low tissue oxygenation. The latter was defined as transcutaneous oxygen pressure (TcPO2) of below 30 mm Hg.
There were little differences in demographic characteristics between the treatment groups, the average age ranged from 62 to 67 years, there were more men (70%-80%) than women; most patients (90%) had type 2 diabetes for at least 20 years. There were similar rates of ischemic heart disease, hypertension, dialysis, and immunosuppressive therapy.
There were no differences in baseline values of TcPO2 between the groups, and similar improvements were seen in both the ACT and re-PTA groups versus conservative group.
ACT in practice
With such promising results, what about the practicalities of harvesting a patient’s bone marrow to make the ACT?
“Bone marrow harvesting usually takes about 20 minutes,” Dr. Dubský said. It then takes another 45 minutes to separate the cells and make the cell suspension, and then maybe another 10 minutes or so to administer this to the patient, which is done by injecting into the calf muscles and small muscles of the foot, aided by computed tomography. The whole process may take up to 2 hours, he said.
“Patients are under local or general anesthesia, so there is no pain during the procedure,” Dr. Dubský reassured. “Afterwards we sometimes see small hematoma[s], with low-intensity pain that responds well to usual analgesic therapy.”
Computed tomography was used to help guide the injections, which was advantageous, Dr. Boulton pointed out, because it was “less invasive than angioplasty in these very sick people with very distal lesions, many of whom already have renal problems.”
“It is surprising though, that everybody had re-PTA and not one had vascular surgery,” he suggested. Dr. Boulton added, however: “These are very important observations; they help us a lot in an area where there’s unlikely to be a full RCT.”
The next step in this research is to see if combining ACT and re-PTA could lead to even better results.
The study was funded by the Czech Republic Ministry of Health. Dr. Dubský had nothing to disclose. Dr. Boulton made no statement about his conflicts of interest.
Bone marrow derived autologous cell therapy (ACT) has been shown to significantly reduce the rate of major amputation at 5 years in people with diabetes who developed critical limb-threatening ischemia (CLTI).
In a study of 130 patients, 64% of 42 patients who were treated conservatively needed a major amputation at 5 years versus just 30% of 45 patients who had been treated with ACT (P = .011).
This compared favorably to the results seen with repeated percutaneous angioplasty (re-PTA), where just 20.9% of 43 patients underwent limb salvage (P = .002 vs. conservative therapy).
Furthermore, amputation-free survival was significantly longer in both active groups, Michal Dubský, MD, PhD, FRSPH, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Dubský, of the Institute for Clinical and Experimental Medicine and Charles University in Prague, also reported that fewer patients who had undergone re-PTA or ACT than conservative treatment had died by 5 years (25.8% and 35.6%, respectively, vs. 61.9%), but that the difference was significant only for the revascularization procedure (P = .012).
Based on these findings, “we believe that autologous cell therapy seems to be an appropriate alternative to repeated PTA even for patients with no-option chronic limb-threatening ischemia,” he said.
“This is a very important area,” said Andrew J.M. Boulton, MBBS, MD, FRCP, who chaired the oral abstract presentation session during which the findings were presented.
“It is very difficult to get an evidence base from randomized studies in this area, because of the nature of the patients: They’re very sick and we all deal with them in our clinics very regularly,” added Dr. Boulton, professor of medicine within the division of diabetes, endocrinology and gastroenterology at the University of Manchester (England).
Dr. Boulton called the findings a “very important addition to what we know.”
New option for no-option CLTI
CLTI is associated with persistent pain at rest, ulcers, and gangrene, and can be the end result of longstanding peripheral arterial disease. Within the first year of presentation, there’s a 30% chance of having a major amputation and a 25% chance of dying.
Importantly, said Dr. Dubský, “there is a big difference in this diagnosis” between patients with diabetes and those without. For instance, CLTI is more diffuse in patients with diabetes than in those without, different arteries are affected and the sclerosis seen can be more rigid and “full of calcium.”
While surgery to improve blood flow is the standard of care, not everyone is suitable. Bypass surgery or endovascular procedures can be performed in only 40%-50% of patients, and even then a therapeutic effect may be seen in only a quarter of patients.
“We need some new therapeutic modalities for this diagnosis, and one of them could be autologous cell therapy,” said Dr. Dubský.
Study details
Dr. Dubský and coinvestigators consecutively recruited 130 patients with diabetic foot and CLTI who had been seen at their clinic over a 5-year period. Of these, 87 had not been eligible for standard revascularization and underwent ACT or were treated conservatively.
Of the patients who were not eligible for standard revascularization (‘no-option CLTI), 45 had undergone ACT and 42 had been treated conservatively. Dr. Dubský acknowledged that “his study was not prospective and randomized.”
All patients in the study had at least one unsuccessful revascularization procedure and diabetic foot ulcers, and low tissue oxygenation. The latter was defined as transcutaneous oxygen pressure (TcPO2) of below 30 mm Hg.
There were little differences in demographic characteristics between the treatment groups, the average age ranged from 62 to 67 years, there were more men (70%-80%) than women; most patients (90%) had type 2 diabetes for at least 20 years. There were similar rates of ischemic heart disease, hypertension, dialysis, and immunosuppressive therapy.
There were no differences in baseline values of TcPO2 between the groups, and similar improvements were seen in both the ACT and re-PTA groups versus conservative group.
ACT in practice
With such promising results, what about the practicalities of harvesting a patient’s bone marrow to make the ACT?
“Bone marrow harvesting usually takes about 20 minutes,” Dr. Dubský said. It then takes another 45 minutes to separate the cells and make the cell suspension, and then maybe another 10 minutes or so to administer this to the patient, which is done by injecting into the calf muscles and small muscles of the foot, aided by computed tomography. The whole process may take up to 2 hours, he said.
“Patients are under local or general anesthesia, so there is no pain during the procedure,” Dr. Dubský reassured. “Afterwards we sometimes see small hematoma[s], with low-intensity pain that responds well to usual analgesic therapy.”
Computed tomography was used to help guide the injections, which was advantageous, Dr. Boulton pointed out, because it was “less invasive than angioplasty in these very sick people with very distal lesions, many of whom already have renal problems.”
“It is surprising though, that everybody had re-PTA and not one had vascular surgery,” he suggested. Dr. Boulton added, however: “These are very important observations; they help us a lot in an area where there’s unlikely to be a full RCT.”
The next step in this research is to see if combining ACT and re-PTA could lead to even better results.
The study was funded by the Czech Republic Ministry of Health. Dr. Dubský had nothing to disclose. Dr. Boulton made no statement about his conflicts of interest.
Bone marrow derived autologous cell therapy (ACT) has been shown to significantly reduce the rate of major amputation at 5 years in people with diabetes who developed critical limb-threatening ischemia (CLTI).
In a study of 130 patients, 64% of 42 patients who were treated conservatively needed a major amputation at 5 years versus just 30% of 45 patients who had been treated with ACT (P = .011).
This compared favorably to the results seen with repeated percutaneous angioplasty (re-PTA), where just 20.9% of 43 patients underwent limb salvage (P = .002 vs. conservative therapy).
Furthermore, amputation-free survival was significantly longer in both active groups, Michal Dubský, MD, PhD, FRSPH, reported at the annual meeting of the European Association for the Study of Diabetes.
Dr. Dubský, of the Institute for Clinical and Experimental Medicine and Charles University in Prague, also reported that fewer patients who had undergone re-PTA or ACT than conservative treatment had died by 5 years (25.8% and 35.6%, respectively, vs. 61.9%), but that the difference was significant only for the revascularization procedure (P = .012).
Based on these findings, “we believe that autologous cell therapy seems to be an appropriate alternative to repeated PTA even for patients with no-option chronic limb-threatening ischemia,” he said.
“This is a very important area,” said Andrew J.M. Boulton, MBBS, MD, FRCP, who chaired the oral abstract presentation session during which the findings were presented.
“It is very difficult to get an evidence base from randomized studies in this area, because of the nature of the patients: They’re very sick and we all deal with them in our clinics very regularly,” added Dr. Boulton, professor of medicine within the division of diabetes, endocrinology and gastroenterology at the University of Manchester (England).
Dr. Boulton called the findings a “very important addition to what we know.”
New option for no-option CLTI
CLTI is associated with persistent pain at rest, ulcers, and gangrene, and can be the end result of longstanding peripheral arterial disease. Within the first year of presentation, there’s a 30% chance of having a major amputation and a 25% chance of dying.
Importantly, said Dr. Dubský, “there is a big difference in this diagnosis” between patients with diabetes and those without. For instance, CLTI is more diffuse in patients with diabetes than in those without, different arteries are affected and the sclerosis seen can be more rigid and “full of calcium.”
While surgery to improve blood flow is the standard of care, not everyone is suitable. Bypass surgery or endovascular procedures can be performed in only 40%-50% of patients, and even then a therapeutic effect may be seen in only a quarter of patients.
“We need some new therapeutic modalities for this diagnosis, and one of them could be autologous cell therapy,” said Dr. Dubský.
Study details
Dr. Dubský and coinvestigators consecutively recruited 130 patients with diabetic foot and CLTI who had been seen at their clinic over a 5-year period. Of these, 87 had not been eligible for standard revascularization and underwent ACT or were treated conservatively.
Of the patients who were not eligible for standard revascularization (‘no-option CLTI), 45 had undergone ACT and 42 had been treated conservatively. Dr. Dubský acknowledged that “his study was not prospective and randomized.”
All patients in the study had at least one unsuccessful revascularization procedure and diabetic foot ulcers, and low tissue oxygenation. The latter was defined as transcutaneous oxygen pressure (TcPO2) of below 30 mm Hg.
There were little differences in demographic characteristics between the treatment groups, the average age ranged from 62 to 67 years, there were more men (70%-80%) than women; most patients (90%) had type 2 diabetes for at least 20 years. There were similar rates of ischemic heart disease, hypertension, dialysis, and immunosuppressive therapy.
There were no differences in baseline values of TcPO2 between the groups, and similar improvements were seen in both the ACT and re-PTA groups versus conservative group.
ACT in practice
With such promising results, what about the practicalities of harvesting a patient’s bone marrow to make the ACT?
“Bone marrow harvesting usually takes about 20 minutes,” Dr. Dubský said. It then takes another 45 minutes to separate the cells and make the cell suspension, and then maybe another 10 minutes or so to administer this to the patient, which is done by injecting into the calf muscles and small muscles of the foot, aided by computed tomography. The whole process may take up to 2 hours, he said.
“Patients are under local or general anesthesia, so there is no pain during the procedure,” Dr. Dubský reassured. “Afterwards we sometimes see small hematoma[s], with low-intensity pain that responds well to usual analgesic therapy.”
Computed tomography was used to help guide the injections, which was advantageous, Dr. Boulton pointed out, because it was “less invasive than angioplasty in these very sick people with very distal lesions, many of whom already have renal problems.”
“It is surprising though, that everybody had re-PTA and not one had vascular surgery,” he suggested. Dr. Boulton added, however: “These are very important observations; they help us a lot in an area where there’s unlikely to be a full RCT.”
The next step in this research is to see if combining ACT and re-PTA could lead to even better results.
The study was funded by the Czech Republic Ministry of Health. Dr. Dubský had nothing to disclose. Dr. Boulton made no statement about his conflicts of interest.
FROM EASD 2021
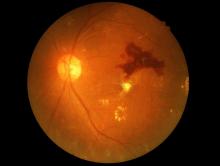
Retinopathy risk higher in young-onset T2D, more so in men
Men diagnosed with type 2 diabetes (T2D) by the age of 40 years appear significantly more likely to develop retinopathy than men who are diagnosed at an older age, Norwegian researchers report.
In a cross-sectional study of about 10,000 people, men with young-onset T2D were 72% more likely than men aged 50 years or older to have retinopathy.
While an increased retinopathy risk was also seen in women with young-onset T2D versus older women at first, this difference was not significant after adjusting for various confounding factors.
The effect of young-onset diabetes on retinopathy seems to be gender specific, Katrina Tibballs, MD, of the department of general practice at the University of Oslo, reported at the annual meeting of the European Association for the Study of Diabetes.
“In the unadjusted analysis, the odds ratio for retinopathy was substantially higher in both [young-onset] men [odds ratio, 3.0] and women [OR, 2.46], compared with those 50 or older at diabetes diagnosis,” Dr. Tibballs said.
That relationship was not substantially altered after adjustment for variables such as level of education, country background, gender, and body mass index, with adjusted ORs of 2.56 and 2.55 for men and women, respectively.
However, further adjustment to include current age, duration of diabetes, and blood lipids and glycated hemoglobin levels, led to the difference no longer holding for women (OR, 1.34; 95% confidence interval, 0.95-1.89) as it did for men (OR 1.72; 95% CI, 1.29-2.29).
First data in Norwegian population
Cross-sectional data on more than 10,000 people with T2D were used for the analysis. These came from the ROSA4 study, a general practice study conducted across Norway in 2014.
Just over 10% of the study population used in the analysis was under the age of 40 years at diagnosis of T2D; 21% were aged between 40 and 49 years, and 69% were at least 50 years old.
The mean age of those with young-onset T2D, defined as a diagnosis before the age of 40 years, was 33 years. These individuals had a longer disease duration than those in the other age groups (11.4 vs. 10.0 vs. 7.8 years).
“Looking at clinical characteristics, we say that individuals [with young-onset T2D] have a higher level of hemoglobin A1c than those with diabetes onset later in life,” Dr. Tibballs said.
“This is despite a substantially higher proportion [being] treated with insulin and fewer on lifestyle interventions alone.”
Gender differences were seen in A1c levels, with men with young-onset T2D having consistently higher levels than women, with levels increasing with diabetes duration.
Rise in retinopathy faster in men than in women
Dr. Tibballs reported that, not only did the prevalence of retinopathy rise faster in those of a younger age, but it also rose more quickly in men with young-onset T2D than it in their female counterparts.
“Comparing that [young-onset diabetes] and later-onset diabetes in men and women separately, we see a clearly higher prevalence of retinopathy with increasing diabetes duration for [young-onset] men,” she said.
In women, on the other hand, there was “no clear indication of a higher retinopathy prevalence in [young-onset diabetes], except in those with the longest diabetes duration.”
So, what do the results mean for practice? First, they confirm prior work showing that there is a strong association between retinopathy and age at diagnosis of T2D. Second, they suggest that this is despite intensive glucose-lowering treatment.
She speculated that men with young-onset T2D may have had a delayed diagnosis when compared with women and individuals with later onset diabetes, Dr. Tibballs said.
“This may in turn lead to delayed onset of glucose-lowering treatment, allowing for more time with high glycemic exposure and increased risk of acquiring complications, such as retinopathy at the time of diagnosis, or in the first years after,” said Dr. Tibballs.
These are cross-sectional data, “so we can’t say anything about whether this treatment is sufficient, but it is obviously not reducing HbA1c levels as much as we would like” added Dr. Tibballs, who is a primary care physician and PhD student.
The study was supported by The Norwegian Research Fund for General Practice. Dr. Tibballs had no conflicts of interest to disclose.
Men diagnosed with type 2 diabetes (T2D) by the age of 40 years appear significantly more likely to develop retinopathy than men who are diagnosed at an older age, Norwegian researchers report.
In a cross-sectional study of about 10,000 people, men with young-onset T2D were 72% more likely than men aged 50 years or older to have retinopathy.
While an increased retinopathy risk was also seen in women with young-onset T2D versus older women at first, this difference was not significant after adjusting for various confounding factors.
The effect of young-onset diabetes on retinopathy seems to be gender specific, Katrina Tibballs, MD, of the department of general practice at the University of Oslo, reported at the annual meeting of the European Association for the Study of Diabetes.
“In the unadjusted analysis, the odds ratio for retinopathy was substantially higher in both [young-onset] men [odds ratio, 3.0] and women [OR, 2.46], compared with those 50 or older at diabetes diagnosis,” Dr. Tibballs said.
That relationship was not substantially altered after adjustment for variables such as level of education, country background, gender, and body mass index, with adjusted ORs of 2.56 and 2.55 for men and women, respectively.
However, further adjustment to include current age, duration of diabetes, and blood lipids and glycated hemoglobin levels, led to the difference no longer holding for women (OR, 1.34; 95% confidence interval, 0.95-1.89) as it did for men (OR 1.72; 95% CI, 1.29-2.29).
First data in Norwegian population
Cross-sectional data on more than 10,000 people with T2D were used for the analysis. These came from the ROSA4 study, a general practice study conducted across Norway in 2014.
Just over 10% of the study population used in the analysis was under the age of 40 years at diagnosis of T2D; 21% were aged between 40 and 49 years, and 69% were at least 50 years old.
The mean age of those with young-onset T2D, defined as a diagnosis before the age of 40 years, was 33 years. These individuals had a longer disease duration than those in the other age groups (11.4 vs. 10.0 vs. 7.8 years).
“Looking at clinical characteristics, we say that individuals [with young-onset T2D] have a higher level of hemoglobin A1c than those with diabetes onset later in life,” Dr. Tibballs said.
“This is despite a substantially higher proportion [being] treated with insulin and fewer on lifestyle interventions alone.”
Gender differences were seen in A1c levels, with men with young-onset T2D having consistently higher levels than women, with levels increasing with diabetes duration.
Rise in retinopathy faster in men than in women
Dr. Tibballs reported that, not only did the prevalence of retinopathy rise faster in those of a younger age, but it also rose more quickly in men with young-onset T2D than it in their female counterparts.
“Comparing that [young-onset diabetes] and later-onset diabetes in men and women separately, we see a clearly higher prevalence of retinopathy with increasing diabetes duration for [young-onset] men,” she said.
In women, on the other hand, there was “no clear indication of a higher retinopathy prevalence in [young-onset diabetes], except in those with the longest diabetes duration.”
So, what do the results mean for practice? First, they confirm prior work showing that there is a strong association between retinopathy and age at diagnosis of T2D. Second, they suggest that this is despite intensive glucose-lowering treatment.
She speculated that men with young-onset T2D may have had a delayed diagnosis when compared with women and individuals with later onset diabetes, Dr. Tibballs said.
“This may in turn lead to delayed onset of glucose-lowering treatment, allowing for more time with high glycemic exposure and increased risk of acquiring complications, such as retinopathy at the time of diagnosis, or in the first years after,” said Dr. Tibballs.
These are cross-sectional data, “so we can’t say anything about whether this treatment is sufficient, but it is obviously not reducing HbA1c levels as much as we would like” added Dr. Tibballs, who is a primary care physician and PhD student.
The study was supported by The Norwegian Research Fund for General Practice. Dr. Tibballs had no conflicts of interest to disclose.
Men diagnosed with type 2 diabetes (T2D) by the age of 40 years appear significantly more likely to develop retinopathy than men who are diagnosed at an older age, Norwegian researchers report.
In a cross-sectional study of about 10,000 people, men with young-onset T2D were 72% more likely than men aged 50 years or older to have retinopathy.
While an increased retinopathy risk was also seen in women with young-onset T2D versus older women at first, this difference was not significant after adjusting for various confounding factors.
The effect of young-onset diabetes on retinopathy seems to be gender specific, Katrina Tibballs, MD, of the department of general practice at the University of Oslo, reported at the annual meeting of the European Association for the Study of Diabetes.
“In the unadjusted analysis, the odds ratio for retinopathy was substantially higher in both [young-onset] men [odds ratio, 3.0] and women [OR, 2.46], compared with those 50 or older at diabetes diagnosis,” Dr. Tibballs said.
That relationship was not substantially altered after adjustment for variables such as level of education, country background, gender, and body mass index, with adjusted ORs of 2.56 and 2.55 for men and women, respectively.
However, further adjustment to include current age, duration of diabetes, and blood lipids and glycated hemoglobin levels, led to the difference no longer holding for women (OR, 1.34; 95% confidence interval, 0.95-1.89) as it did for men (OR 1.72; 95% CI, 1.29-2.29).
First data in Norwegian population
Cross-sectional data on more than 10,000 people with T2D were used for the analysis. These came from the ROSA4 study, a general practice study conducted across Norway in 2014.
Just over 10% of the study population used in the analysis was under the age of 40 years at diagnosis of T2D; 21% were aged between 40 and 49 years, and 69% were at least 50 years old.
The mean age of those with young-onset T2D, defined as a diagnosis before the age of 40 years, was 33 years. These individuals had a longer disease duration than those in the other age groups (11.4 vs. 10.0 vs. 7.8 years).
“Looking at clinical characteristics, we say that individuals [with young-onset T2D] have a higher level of hemoglobin A1c than those with diabetes onset later in life,” Dr. Tibballs said.
“This is despite a substantially higher proportion [being] treated with insulin and fewer on lifestyle interventions alone.”
Gender differences were seen in A1c levels, with men with young-onset T2D having consistently higher levels than women, with levels increasing with diabetes duration.
Rise in retinopathy faster in men than in women
Dr. Tibballs reported that, not only did the prevalence of retinopathy rise faster in those of a younger age, but it also rose more quickly in men with young-onset T2D than it in their female counterparts.
“Comparing that [young-onset diabetes] and later-onset diabetes in men and women separately, we see a clearly higher prevalence of retinopathy with increasing diabetes duration for [young-onset] men,” she said.
In women, on the other hand, there was “no clear indication of a higher retinopathy prevalence in [young-onset diabetes], except in those with the longest diabetes duration.”
So, what do the results mean for practice? First, they confirm prior work showing that there is a strong association between retinopathy and age at diagnosis of T2D. Second, they suggest that this is despite intensive glucose-lowering treatment.
She speculated that men with young-onset T2D may have had a delayed diagnosis when compared with women and individuals with later onset diabetes, Dr. Tibballs said.
“This may in turn lead to delayed onset of glucose-lowering treatment, allowing for more time with high glycemic exposure and increased risk of acquiring complications, such as retinopathy at the time of diagnosis, or in the first years after,” said Dr. Tibballs.
These are cross-sectional data, “so we can’t say anything about whether this treatment is sufficient, but it is obviously not reducing HbA1c levels as much as we would like” added Dr. Tibballs, who is a primary care physician and PhD student.
The study was supported by The Norwegian Research Fund for General Practice. Dr. Tibballs had no conflicts of interest to disclose.
FROM EASD 2021
Could the osteoporosis drug alendronate ward off diabetes?
A nationwide, retrospective, case-control study of older adults in Denmark suggests that the bisphosphonate alendronate that is widely used to treat osteoporosis may protect against new-onset type 2 diabetes. But these preliminary findings need to be confirmed in a randomized controlled trial, experts said.
The registry study showed that from 2008 to 2018, among individuals in Denmark age 50 and older (with a mean age of 67), those who were taking alendronate were 36% less likely to have new-onset type 2 diabetes than age- and sex-matched individuals who were not taking the drug, after controlling for multiple risk factors.
The results also suggest that longer alendronate use and higher compliance might be more protective.
Rikke Viggers, MD, a PhD student in the department of clinical medicine, Aalborg (Denmark) University, presented the findings during an oral session at the annual meeting of the European Association for the Study of Diabetes.
“Excitingly, our research suggests that alendronate, an inexpensive medicine widely used to treat osteoporosis, may also protect against type 2 diabetes,” Dr. Viggers summarized in a press release issued by the EASD.
“Type 2 diabetes is a serious lifelong condition that can lead to other serious health issues such as stroke, heart disease, blindness, and limb amputation,” she noted, “and anything that prevents or even delays it will also reduce a person’s risk of all these other conditions.”
“We believe that doctors should consider this when prescribing osteoporosis drugs to those with prediabetes or at high risk of type 2 diabetes,” she added.
Preliminary results, need for RCT
However, these are preliminary results, Dr. Viggers cautioned during the oral presentation and in an email. “This is a registry-based study,” she stressed, “and we cannot conclude causality.”
“We do not know if this effect [of decreased risk of developing diabetes among people taking alendronate] is ‘real’ and what the mechanisms are.”
“It could be a direct effect on peripheral tissues, for example, muscle and adipose tissue,” Dr. Viggers speculated, “or an indirect effect through bone metabolites that may impact glucose metabolism.”
The group is now conducting a randomized controlled trial in patients with diabetes and osteopenia or osteoporosis to examine the relationship between alendronate and insulin sensitivity, bone indices, and glycemic control.
They also aim to investigate whether alendronate is the optimal antiosteoporotic therapy for patients with type 2 diabetes. Preliminary results suggest that other bisphosphonates have similar effects.
“Alendronate decreases bone turnover and may not be beneficial in healthy bones,” Dr. Viggers noted. “However, as far as I know, potential other side effects have not been tested in healthy bones,” so further research is needed.
Invited to comment, Charles P. Vega, MD, who presented a case and a crowd-sourced opinion about deprescribing bisphosphonates, noted that type 2 diabetes is most often diagnosed between age 40 and 60, although a few cases are diagnosed after age 65, and the study by Dr. Viggers and colleagues suggests that alendronate might help lower the risk of diabetes onset in these older adults.
“This is an interesting retrospective analysis,” said Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, but like the study authors, he cautioned that “it should be verified with other data.”
“A meta-analysis from clinical trials of bisphosphonates which followed blood glucose levels would be helpful,” he said.
Current registry study findings
Glucose homeostasis has been linked to bone metabolism, Dr. Viggers said, and bisphosphonates were associated with increased insulin sensitivity and decreased risk of diabetes risk in two registry studies from Denmark and Taiwan.
The researchers aimed to investigate if the risk of developing type 2 diabetes was altered by previous use of alendronate.
Using data from the national Danish Patient Registry, they identified 163,588 individuals age 50 and older newly diagnosed with type 2 diabetes in 2008-2018.
They matched each patient with three individuals of the same gender and age range who did not have diabetes, for a total of 490,764 controls.
Roughly two-thirds of participants were in their 50s or 60s, a quarter were in their 70s, and 10% were 80 or older. About half of participants were women (45%).
Compared to the patients with new-onset type 2 diabetes, the control participants were healthier: they were less likely to have obesity (6% vs. 17%) and had a lower mean Charlson Comorbidity Index (0.38 vs. 0.88).
Using data from the national Danish Health Service Prescription Registry, the researchers identified individuals who filled prescriptions for alendronate in 2008-2018.
After controlling for heavy smoking, alcohol abuse, obesity, pancreatitis, hyperthyroidism, hypothyroidism, glucocorticoid use, marital status, household income, and Charlson Comorbidity Index, people taking alendronate were less likely to have new-onset diabetes than those not taking this drug (odds ratio, 0.64; 95% confidence interval, 0.62-0.66).
The odds of developing type 2 diabetes were even lower among those who took alendronate for 8 years or more versus never-users (OR, 0.47; 95% CI, 0.40-0.56), after controlling for the same variables.
Session Chair Zhila Semnani-Azad, a PhD student in nutritional science, University of Toronto, wanted to know if the researchers accounted for physical activity and vitamin D use. Dr. Viggers replied that the registries did not have this information.
The study was funded by a Steno Collaborative Project grant from the Novo Nordisk Foundation, Denmark. Dr. Viggers has disclosed receiving a grant from the foundation. Dr. Vega has disclosed serving as a consultant for Johnson & Johnson.
A version of this article first appeared on Medscape.com.
A nationwide, retrospective, case-control study of older adults in Denmark suggests that the bisphosphonate alendronate that is widely used to treat osteoporosis may protect against new-onset type 2 diabetes. But these preliminary findings need to be confirmed in a randomized controlled trial, experts said.
The registry study showed that from 2008 to 2018, among individuals in Denmark age 50 and older (with a mean age of 67), those who were taking alendronate were 36% less likely to have new-onset type 2 diabetes than age- and sex-matched individuals who were not taking the drug, after controlling for multiple risk factors.
The results also suggest that longer alendronate use and higher compliance might be more protective.
Rikke Viggers, MD, a PhD student in the department of clinical medicine, Aalborg (Denmark) University, presented the findings during an oral session at the annual meeting of the European Association for the Study of Diabetes.
“Excitingly, our research suggests that alendronate, an inexpensive medicine widely used to treat osteoporosis, may also protect against type 2 diabetes,” Dr. Viggers summarized in a press release issued by the EASD.
“Type 2 diabetes is a serious lifelong condition that can lead to other serious health issues such as stroke, heart disease, blindness, and limb amputation,” she noted, “and anything that prevents or even delays it will also reduce a person’s risk of all these other conditions.”
“We believe that doctors should consider this when prescribing osteoporosis drugs to those with prediabetes or at high risk of type 2 diabetes,” she added.
Preliminary results, need for RCT
However, these are preliminary results, Dr. Viggers cautioned during the oral presentation and in an email. “This is a registry-based study,” she stressed, “and we cannot conclude causality.”
“We do not know if this effect [of decreased risk of developing diabetes among people taking alendronate] is ‘real’ and what the mechanisms are.”
“It could be a direct effect on peripheral tissues, for example, muscle and adipose tissue,” Dr. Viggers speculated, “or an indirect effect through bone metabolites that may impact glucose metabolism.”
The group is now conducting a randomized controlled trial in patients with diabetes and osteopenia or osteoporosis to examine the relationship between alendronate and insulin sensitivity, bone indices, and glycemic control.
They also aim to investigate whether alendronate is the optimal antiosteoporotic therapy for patients with type 2 diabetes. Preliminary results suggest that other bisphosphonates have similar effects.
“Alendronate decreases bone turnover and may not be beneficial in healthy bones,” Dr. Viggers noted. “However, as far as I know, potential other side effects have not been tested in healthy bones,” so further research is needed.
Invited to comment, Charles P. Vega, MD, who presented a case and a crowd-sourced opinion about deprescribing bisphosphonates, noted that type 2 diabetes is most often diagnosed between age 40 and 60, although a few cases are diagnosed after age 65, and the study by Dr. Viggers and colleagues suggests that alendronate might help lower the risk of diabetes onset in these older adults.
“This is an interesting retrospective analysis,” said Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, but like the study authors, he cautioned that “it should be verified with other data.”
“A meta-analysis from clinical trials of bisphosphonates which followed blood glucose levels would be helpful,” he said.
Current registry study findings
Glucose homeostasis has been linked to bone metabolism, Dr. Viggers said, and bisphosphonates were associated with increased insulin sensitivity and decreased risk of diabetes risk in two registry studies from Denmark and Taiwan.
The researchers aimed to investigate if the risk of developing type 2 diabetes was altered by previous use of alendronate.
Using data from the national Danish Patient Registry, they identified 163,588 individuals age 50 and older newly diagnosed with type 2 diabetes in 2008-2018.
They matched each patient with three individuals of the same gender and age range who did not have diabetes, for a total of 490,764 controls.
Roughly two-thirds of participants were in their 50s or 60s, a quarter were in their 70s, and 10% were 80 or older. About half of participants were women (45%).
Compared to the patients with new-onset type 2 diabetes, the control participants were healthier: they were less likely to have obesity (6% vs. 17%) and had a lower mean Charlson Comorbidity Index (0.38 vs. 0.88).
Using data from the national Danish Health Service Prescription Registry, the researchers identified individuals who filled prescriptions for alendronate in 2008-2018.
After controlling for heavy smoking, alcohol abuse, obesity, pancreatitis, hyperthyroidism, hypothyroidism, glucocorticoid use, marital status, household income, and Charlson Comorbidity Index, people taking alendronate were less likely to have new-onset diabetes than those not taking this drug (odds ratio, 0.64; 95% confidence interval, 0.62-0.66).
The odds of developing type 2 diabetes were even lower among those who took alendronate for 8 years or more versus never-users (OR, 0.47; 95% CI, 0.40-0.56), after controlling for the same variables.
Session Chair Zhila Semnani-Azad, a PhD student in nutritional science, University of Toronto, wanted to know if the researchers accounted for physical activity and vitamin D use. Dr. Viggers replied that the registries did not have this information.
The study was funded by a Steno Collaborative Project grant from the Novo Nordisk Foundation, Denmark. Dr. Viggers has disclosed receiving a grant from the foundation. Dr. Vega has disclosed serving as a consultant for Johnson & Johnson.
A version of this article first appeared on Medscape.com.
A nationwide, retrospective, case-control study of older adults in Denmark suggests that the bisphosphonate alendronate that is widely used to treat osteoporosis may protect against new-onset type 2 diabetes. But these preliminary findings need to be confirmed in a randomized controlled trial, experts said.
The registry study showed that from 2008 to 2018, among individuals in Denmark age 50 and older (with a mean age of 67), those who were taking alendronate were 36% less likely to have new-onset type 2 diabetes than age- and sex-matched individuals who were not taking the drug, after controlling for multiple risk factors.
The results also suggest that longer alendronate use and higher compliance might be more protective.
Rikke Viggers, MD, a PhD student in the department of clinical medicine, Aalborg (Denmark) University, presented the findings during an oral session at the annual meeting of the European Association for the Study of Diabetes.
“Excitingly, our research suggests that alendronate, an inexpensive medicine widely used to treat osteoporosis, may also protect against type 2 diabetes,” Dr. Viggers summarized in a press release issued by the EASD.
“Type 2 diabetes is a serious lifelong condition that can lead to other serious health issues such as stroke, heart disease, blindness, and limb amputation,” she noted, “and anything that prevents or even delays it will also reduce a person’s risk of all these other conditions.”
“We believe that doctors should consider this when prescribing osteoporosis drugs to those with prediabetes or at high risk of type 2 diabetes,” she added.
Preliminary results, need for RCT
However, these are preliminary results, Dr. Viggers cautioned during the oral presentation and in an email. “This is a registry-based study,” she stressed, “and we cannot conclude causality.”
“We do not know if this effect [of decreased risk of developing diabetes among people taking alendronate] is ‘real’ and what the mechanisms are.”
“It could be a direct effect on peripheral tissues, for example, muscle and adipose tissue,” Dr. Viggers speculated, “or an indirect effect through bone metabolites that may impact glucose metabolism.”
The group is now conducting a randomized controlled trial in patients with diabetes and osteopenia or osteoporosis to examine the relationship between alendronate and insulin sensitivity, bone indices, and glycemic control.
They also aim to investigate whether alendronate is the optimal antiosteoporotic therapy for patients with type 2 diabetes. Preliminary results suggest that other bisphosphonates have similar effects.
“Alendronate decreases bone turnover and may not be beneficial in healthy bones,” Dr. Viggers noted. “However, as far as I know, potential other side effects have not been tested in healthy bones,” so further research is needed.
Invited to comment, Charles P. Vega, MD, who presented a case and a crowd-sourced opinion about deprescribing bisphosphonates, noted that type 2 diabetes is most often diagnosed between age 40 and 60, although a few cases are diagnosed after age 65, and the study by Dr. Viggers and colleagues suggests that alendronate might help lower the risk of diabetes onset in these older adults.
“This is an interesting retrospective analysis,” said Dr. Vega, health sciences clinical professor, family medicine, University of California, Irvine, but like the study authors, he cautioned that “it should be verified with other data.”
“A meta-analysis from clinical trials of bisphosphonates which followed blood glucose levels would be helpful,” he said.
Current registry study findings
Glucose homeostasis has been linked to bone metabolism, Dr. Viggers said, and bisphosphonates were associated with increased insulin sensitivity and decreased risk of diabetes risk in two registry studies from Denmark and Taiwan.
The researchers aimed to investigate if the risk of developing type 2 diabetes was altered by previous use of alendronate.
Using data from the national Danish Patient Registry, they identified 163,588 individuals age 50 and older newly diagnosed with type 2 diabetes in 2008-2018.
They matched each patient with three individuals of the same gender and age range who did not have diabetes, for a total of 490,764 controls.
Roughly two-thirds of participants were in their 50s or 60s, a quarter were in their 70s, and 10% were 80 or older. About half of participants were women (45%).
Compared to the patients with new-onset type 2 diabetes, the control participants were healthier: they were less likely to have obesity (6% vs. 17%) and had a lower mean Charlson Comorbidity Index (0.38 vs. 0.88).
Using data from the national Danish Health Service Prescription Registry, the researchers identified individuals who filled prescriptions for alendronate in 2008-2018.
After controlling for heavy smoking, alcohol abuse, obesity, pancreatitis, hyperthyroidism, hypothyroidism, glucocorticoid use, marital status, household income, and Charlson Comorbidity Index, people taking alendronate were less likely to have new-onset diabetes than those not taking this drug (odds ratio, 0.64; 95% confidence interval, 0.62-0.66).
The odds of developing type 2 diabetes were even lower among those who took alendronate for 8 years or more versus never-users (OR, 0.47; 95% CI, 0.40-0.56), after controlling for the same variables.
Session Chair Zhila Semnani-Azad, a PhD student in nutritional science, University of Toronto, wanted to know if the researchers accounted for physical activity and vitamin D use. Dr. Viggers replied that the registries did not have this information.
The study was funded by a Steno Collaborative Project grant from the Novo Nordisk Foundation, Denmark. Dr. Viggers has disclosed receiving a grant from the foundation. Dr. Vega has disclosed serving as a consultant for Johnson & Johnson.
A version of this article first appeared on Medscape.com.
FROM EASD 2021
USPSTF updates diabetes recs, lowers screening age
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
Age, C-reactive protein predict COVID-19 death in diabetes
The data, from the retrospective ACCREDIT cohort study, were presented at the virtual annual meeting of the European Association for the Study of Diabetes (EASD 2021) by Daniel Kevin Llanera, MD.
The combination of older age and high levels of the inflammatory marker CRP were linked to a tripled risk for death by day 7 after hospitalization for COVID-19 among people with diabetes. But, in contrast to other studies, recent A1c and body mass index did not predict COVID-19 outcomes.
“Both of these variables are easily available upon admission to hospital,” Dr. Llanera, who now works at Imperial College, London, said in an EASD press release.
“This means we can easily identify patients early on in their hospital stay who will likely require more aggressive interventions to try and improve survival.”
“It makes sense that CRP and age are important,” said Simon Heller, MB BChir, DM, of the University of Sheffield, England. “It may be that diabetes alone overwhelmed the additional effects of obesity and A1c.
“Certainly in other studies, age was the overwhelming bad prognostic sign among people with diabetes, and perhaps long-term diabetes has effects on the immune system which we haven’t yet identified.”
Kidney disease in younger patients also linked to poorer outcomes
The study, conducted when Dr. Llanera worked for the Countess of Chester NHS Foundation Trust, involved 1,004 patients with diabetes admitted with COVID-19 to seven hospitals in northwest England from Jan. 1 through June 30, 2020. The patients were a mean age of 74.1 years, 60.7% were male, and 45% were in the most deprived quintile based on the U.K. government deprivation index. Overall, 56.2% had macrovascular complications and 49.6% had microvascular complications.
They had a median BMI of 27.6 kg/m2, which is lower than that reported in previous studies and might explain the difference, Dr. Llanera noted.
The primary outcome, death within 7 days of admission, occurred in 24%. By day 30, 33% had died. These rates are higher than the rate found in previous studies, possibly because of greater socioeconomic deprivation and older age of the population, Dr. Llanera speculated.
A total of 7.5% of patients received intensive care by day 7 and 9.8% required intravenous insulin infusions.
On univariate analysis, insulin infusion was found to be protective, with those receiving it half as likely to die as those who didn’t need IV insulin (odds ratio [OR], 0.5).
In contrast, chronic kidney disease in people younger than 70 years increased the risk of death more than twofold (OR, 2.74), as did type 2 diabetes compared with other diabetes types (OR, 2.52).
As in previous studies, use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers were not associated with COVID-19 outcomes, nor was the presence of diabetes-related complications.
In multivariate analysis, CRP and age emerged as the most significant predictors of the primary outcome, with those deemed high risk by a logistic regression model having an OR of 3.44 for death by day 7 compared with those at lower risk based on the two factors.
Data for glycemic control during the time of hospitalization weren’t available for this study, Dr. Llanera said in response to a question.
“We didn’t look into glycemic control during admission, just at entry, so I can’t answer whether strict glucose control is of benefit. I think it’s worth exploring further whether the use of IV insulin may be of benefit.”
Dr. Llanera also pointed out that people with diabetic kidney disease are in a chronic proinflammatory state and have immune dysregulation, thus potentially hindering their ability to “fight off” the virus.
“In addition, ACE2 receptors are upregulated in the kidneys of patients with diabetic kidney disease. These are molecules that facilitate entry of SARS-CoV-2 into the cells. This may lead to direct attack of the kidneys by the virus, possibly leading to worse overall outcomes,” he said.
Dr. Llanera has reported no relevant financial relationships. Dr. Heller has reported serving as consultant or speaker for Novo Nordisk, Eli Lilly, Sanofi Aventis, Mannkind, Zealand, MSD, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The data, from the retrospective ACCREDIT cohort study, were presented at the virtual annual meeting of the European Association for the Study of Diabetes (EASD 2021) by Daniel Kevin Llanera, MD.
The combination of older age and high levels of the inflammatory marker CRP were linked to a tripled risk for death by day 7 after hospitalization for COVID-19 among people with diabetes. But, in contrast to other studies, recent A1c and body mass index did not predict COVID-19 outcomes.
“Both of these variables are easily available upon admission to hospital,” Dr. Llanera, who now works at Imperial College, London, said in an EASD press release.
“This means we can easily identify patients early on in their hospital stay who will likely require more aggressive interventions to try and improve survival.”
“It makes sense that CRP and age are important,” said Simon Heller, MB BChir, DM, of the University of Sheffield, England. “It may be that diabetes alone overwhelmed the additional effects of obesity and A1c.
“Certainly in other studies, age was the overwhelming bad prognostic sign among people with diabetes, and perhaps long-term diabetes has effects on the immune system which we haven’t yet identified.”
Kidney disease in younger patients also linked to poorer outcomes
The study, conducted when Dr. Llanera worked for the Countess of Chester NHS Foundation Trust, involved 1,004 patients with diabetes admitted with COVID-19 to seven hospitals in northwest England from Jan. 1 through June 30, 2020. The patients were a mean age of 74.1 years, 60.7% were male, and 45% were in the most deprived quintile based on the U.K. government deprivation index. Overall, 56.2% had macrovascular complications and 49.6% had microvascular complications.
They had a median BMI of 27.6 kg/m2, which is lower than that reported in previous studies and might explain the difference, Dr. Llanera noted.
The primary outcome, death within 7 days of admission, occurred in 24%. By day 30, 33% had died. These rates are higher than the rate found in previous studies, possibly because of greater socioeconomic deprivation and older age of the population, Dr. Llanera speculated.
A total of 7.5% of patients received intensive care by day 7 and 9.8% required intravenous insulin infusions.
On univariate analysis, insulin infusion was found to be protective, with those receiving it half as likely to die as those who didn’t need IV insulin (odds ratio [OR], 0.5).
In contrast, chronic kidney disease in people younger than 70 years increased the risk of death more than twofold (OR, 2.74), as did type 2 diabetes compared with other diabetes types (OR, 2.52).
As in previous studies, use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers were not associated with COVID-19 outcomes, nor was the presence of diabetes-related complications.
In multivariate analysis, CRP and age emerged as the most significant predictors of the primary outcome, with those deemed high risk by a logistic regression model having an OR of 3.44 for death by day 7 compared with those at lower risk based on the two factors.
Data for glycemic control during the time of hospitalization weren’t available for this study, Dr. Llanera said in response to a question.
“We didn’t look into glycemic control during admission, just at entry, so I can’t answer whether strict glucose control is of benefit. I think it’s worth exploring further whether the use of IV insulin may be of benefit.”
Dr. Llanera also pointed out that people with diabetic kidney disease are in a chronic proinflammatory state and have immune dysregulation, thus potentially hindering their ability to “fight off” the virus.
“In addition, ACE2 receptors are upregulated in the kidneys of patients with diabetic kidney disease. These are molecules that facilitate entry of SARS-CoV-2 into the cells. This may lead to direct attack of the kidneys by the virus, possibly leading to worse overall outcomes,” he said.
Dr. Llanera has reported no relevant financial relationships. Dr. Heller has reported serving as consultant or speaker for Novo Nordisk, Eli Lilly, Sanofi Aventis, Mannkind, Zealand, MSD, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The data, from the retrospective ACCREDIT cohort study, were presented at the virtual annual meeting of the European Association for the Study of Diabetes (EASD 2021) by Daniel Kevin Llanera, MD.
The combination of older age and high levels of the inflammatory marker CRP were linked to a tripled risk for death by day 7 after hospitalization for COVID-19 among people with diabetes. But, in contrast to other studies, recent A1c and body mass index did not predict COVID-19 outcomes.
“Both of these variables are easily available upon admission to hospital,” Dr. Llanera, who now works at Imperial College, London, said in an EASD press release.
“This means we can easily identify patients early on in their hospital stay who will likely require more aggressive interventions to try and improve survival.”
“It makes sense that CRP and age are important,” said Simon Heller, MB BChir, DM, of the University of Sheffield, England. “It may be that diabetes alone overwhelmed the additional effects of obesity and A1c.
“Certainly in other studies, age was the overwhelming bad prognostic sign among people with diabetes, and perhaps long-term diabetes has effects on the immune system which we haven’t yet identified.”
Kidney disease in younger patients also linked to poorer outcomes
The study, conducted when Dr. Llanera worked for the Countess of Chester NHS Foundation Trust, involved 1,004 patients with diabetes admitted with COVID-19 to seven hospitals in northwest England from Jan. 1 through June 30, 2020. The patients were a mean age of 74.1 years, 60.7% were male, and 45% were in the most deprived quintile based on the U.K. government deprivation index. Overall, 56.2% had macrovascular complications and 49.6% had microvascular complications.
They had a median BMI of 27.6 kg/m2, which is lower than that reported in previous studies and might explain the difference, Dr. Llanera noted.
The primary outcome, death within 7 days of admission, occurred in 24%. By day 30, 33% had died. These rates are higher than the rate found in previous studies, possibly because of greater socioeconomic deprivation and older age of the population, Dr. Llanera speculated.
A total of 7.5% of patients received intensive care by day 7 and 9.8% required intravenous insulin infusions.
On univariate analysis, insulin infusion was found to be protective, with those receiving it half as likely to die as those who didn’t need IV insulin (odds ratio [OR], 0.5).
In contrast, chronic kidney disease in people younger than 70 years increased the risk of death more than twofold (OR, 2.74), as did type 2 diabetes compared with other diabetes types (OR, 2.52).
As in previous studies, use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers were not associated with COVID-19 outcomes, nor was the presence of diabetes-related complications.
In multivariate analysis, CRP and age emerged as the most significant predictors of the primary outcome, with those deemed high risk by a logistic regression model having an OR of 3.44 for death by day 7 compared with those at lower risk based on the two factors.
Data for glycemic control during the time of hospitalization weren’t available for this study, Dr. Llanera said in response to a question.
“We didn’t look into glycemic control during admission, just at entry, so I can’t answer whether strict glucose control is of benefit. I think it’s worth exploring further whether the use of IV insulin may be of benefit.”
Dr. Llanera also pointed out that people with diabetic kidney disease are in a chronic proinflammatory state and have immune dysregulation, thus potentially hindering their ability to “fight off” the virus.
“In addition, ACE2 receptors are upregulated in the kidneys of patients with diabetic kidney disease. These are molecules that facilitate entry of SARS-CoV-2 into the cells. This may lead to direct attack of the kidneys by the virus, possibly leading to worse overall outcomes,” he said.
Dr. Llanera has reported no relevant financial relationships. Dr. Heller has reported serving as consultant or speaker for Novo Nordisk, Eli Lilly, Sanofi Aventis, Mannkind, Zealand, MSD, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Women with type 2 diabetes get fewer cardioprotective drugs than do men
At study entry, significantly fewer women received a statin, at 73%, or daily aspirin, at 44%, compared with men, who had treatment rates of 81% and 58%, respectively, Giulia Ferrannini, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
The data also show that significantly fewer women received treatment with an ACE inhibitor or angiotensin-receptor blocker (ARB), at 80%, than men, at 83%, although the absolute between-group difference was modest. Rates of a fourth metric of appropriate treatment, receipt of antihypertensive medications if systolic blood pressure was at least 130 mm Hg, were nearly identical among women and men.
Cardiovascular risk in women “less well managed”
“This is confirmation that women are less well managed than men when it comes to cardiovascular risk, especially if they have [type 2] diabetes,” Dr. Ferrannini said in an interview.
Similar observations have been documented before, including in a report in 2019.
The treatment disparity by sex among the 9901 women and men with type 2 diabetes enrolled in REWIND is particularly striking because in clinical trials “patients are generally better managed than in the real world,” Dr. Ferrannini noted. “Despite this, the pattern of disadvantage to women was still evident,” she added.
“In cardiovascular protection the gender issue is preponderant. Women are less well treated,” she said.
REWIND is the cardiovascular outcomes trial for the once-weekly injectable glucagonlike peptide–1 receptor agonist dulaglutide (Trulicity, Lilly) in patients with type 2 diabetes.
The primary results, reported at the 2019 scientific sessions of the American Diabetes Association and simultaneously published in The Lancet, showed dulaglutide significantly reduced major adverse cardiovascular events (MACE) by 12%, compared with placebo. The study ran at about 300 centers worldwide, including many U.S. and Canadian sites, and 46% of enrolled patients were women.
But despite undertreatment, women had significantly better outcomes in terms of MACE, the primary endpoint, during a median 5.4 years of follow-up compared with men. After adjustment for sex, other baseline characteristics, and study-treatment assignment, women had a significant 27% lower composite rate of nonfatal MI, nonfatal stroke, or death from either cardiovascular or unknown causes, compared with men, said Dr. Ferrannini, a researcher at the Karolinska Institute in Stockholm.
The analysis by sex also showed that women had a significant outcome advantage, compared with men, for three of the four components of the combined MACE outcome: nonfatal MI, cardiovascular death, and all-cause death, as well as for the outcome of hospitalization for heart failure, which was not part of the composite MACE outcome. The only MACE outcome component that showed no significant between-group difference was nonfatal stroke, which had roughly equal incidence rates among women and men.
Women had half the prevalence of CVD at baseline
The results also showed that the women with type 2 diabetes enrolled in REWIND had a prevalence of existing cardiovascular disease of 20%, which was half the rate of men at study entry, at 41%. However, the between-sex differences in the primary outcome, as well as each of the individual cardiovascular disease outcomes, didn’t change based on whether or not patients had a history of cardiovascular disease at baseline.
Only one outcome showed a between-sex difference linked to prevalent cardiovascular disease at study entry, the rate of all-cause mortality, which was not significantly different between men and women with a history of cardiovascular disease, but was 39% lower in women compared with men without such a history.
“The good news is that, at baseline and after 2 years, the majority of participants were meeting the relevant treatment targets regardless of sex,” commented Peter Novodvorsky, MUDr, a diabetes researcher at the University of Sheffield (England), who chaired the session during which Dr. Ferrannini presented her findings.
A role for geography, or selection bias?
The new analyses did not examine whether the overall pattern of undertreatment of women differed among each of the 24 participating countries, or by region of the world.
“We have to assume that these results reflect current [routine] practice” in the 24 countries that contributed patients to the trial, noted Dr. Novodvorsky.
There is also “the well-known issue of selection bias” in randomized trials. The current findings raise the question of whether the women willing to take part in the trial somehow differed from the men, he suggested.
Dr. Ferrannini added: “Even if we do observe a gender difference in management, if the majority of women with type 2 diabetes are appropriately treated, this ‘restores’ their cardiovascular risk advantage, compared with men, with the exception of stroke.”
The main hypothesis generated by the post hoc analysis of REWIND is that “women with diabetes have better outcomes than men if they are treated properly,” she stressed, noting that this “would have to be tested in a trial designed to ascertain gender differences.”
REWIND was sponsored by Eli Lilly. Dr. Ferrannini has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At study entry, significantly fewer women received a statin, at 73%, or daily aspirin, at 44%, compared with men, who had treatment rates of 81% and 58%, respectively, Giulia Ferrannini, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
The data also show that significantly fewer women received treatment with an ACE inhibitor or angiotensin-receptor blocker (ARB), at 80%, than men, at 83%, although the absolute between-group difference was modest. Rates of a fourth metric of appropriate treatment, receipt of antihypertensive medications if systolic blood pressure was at least 130 mm Hg, were nearly identical among women and men.
Cardiovascular risk in women “less well managed”
“This is confirmation that women are less well managed than men when it comes to cardiovascular risk, especially if they have [type 2] diabetes,” Dr. Ferrannini said in an interview.
Similar observations have been documented before, including in a report in 2019.
The treatment disparity by sex among the 9901 women and men with type 2 diabetes enrolled in REWIND is particularly striking because in clinical trials “patients are generally better managed than in the real world,” Dr. Ferrannini noted. “Despite this, the pattern of disadvantage to women was still evident,” she added.
“In cardiovascular protection the gender issue is preponderant. Women are less well treated,” she said.
REWIND is the cardiovascular outcomes trial for the once-weekly injectable glucagonlike peptide–1 receptor agonist dulaglutide (Trulicity, Lilly) in patients with type 2 diabetes.
The primary results, reported at the 2019 scientific sessions of the American Diabetes Association and simultaneously published in The Lancet, showed dulaglutide significantly reduced major adverse cardiovascular events (MACE) by 12%, compared with placebo. The study ran at about 300 centers worldwide, including many U.S. and Canadian sites, and 46% of enrolled patients were women.
But despite undertreatment, women had significantly better outcomes in terms of MACE, the primary endpoint, during a median 5.4 years of follow-up compared with men. After adjustment for sex, other baseline characteristics, and study-treatment assignment, women had a significant 27% lower composite rate of nonfatal MI, nonfatal stroke, or death from either cardiovascular or unknown causes, compared with men, said Dr. Ferrannini, a researcher at the Karolinska Institute in Stockholm.
The analysis by sex also showed that women had a significant outcome advantage, compared with men, for three of the four components of the combined MACE outcome: nonfatal MI, cardiovascular death, and all-cause death, as well as for the outcome of hospitalization for heart failure, which was not part of the composite MACE outcome. The only MACE outcome component that showed no significant between-group difference was nonfatal stroke, which had roughly equal incidence rates among women and men.
Women had half the prevalence of CVD at baseline
The results also showed that the women with type 2 diabetes enrolled in REWIND had a prevalence of existing cardiovascular disease of 20%, which was half the rate of men at study entry, at 41%. However, the between-sex differences in the primary outcome, as well as each of the individual cardiovascular disease outcomes, didn’t change based on whether or not patients had a history of cardiovascular disease at baseline.
Only one outcome showed a between-sex difference linked to prevalent cardiovascular disease at study entry, the rate of all-cause mortality, which was not significantly different between men and women with a history of cardiovascular disease, but was 39% lower in women compared with men without such a history.
“The good news is that, at baseline and after 2 years, the majority of participants were meeting the relevant treatment targets regardless of sex,” commented Peter Novodvorsky, MUDr, a diabetes researcher at the University of Sheffield (England), who chaired the session during which Dr. Ferrannini presented her findings.
A role for geography, or selection bias?
The new analyses did not examine whether the overall pattern of undertreatment of women differed among each of the 24 participating countries, or by region of the world.
“We have to assume that these results reflect current [routine] practice” in the 24 countries that contributed patients to the trial, noted Dr. Novodvorsky.
There is also “the well-known issue of selection bias” in randomized trials. The current findings raise the question of whether the women willing to take part in the trial somehow differed from the men, he suggested.
Dr. Ferrannini added: “Even if we do observe a gender difference in management, if the majority of women with type 2 diabetes are appropriately treated, this ‘restores’ their cardiovascular risk advantage, compared with men, with the exception of stroke.”
The main hypothesis generated by the post hoc analysis of REWIND is that “women with diabetes have better outcomes than men if they are treated properly,” she stressed, noting that this “would have to be tested in a trial designed to ascertain gender differences.”
REWIND was sponsored by Eli Lilly. Dr. Ferrannini has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At study entry, significantly fewer women received a statin, at 73%, or daily aspirin, at 44%, compared with men, who had treatment rates of 81% and 58%, respectively, Giulia Ferrannini, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
The data also show that significantly fewer women received treatment with an ACE inhibitor or angiotensin-receptor blocker (ARB), at 80%, than men, at 83%, although the absolute between-group difference was modest. Rates of a fourth metric of appropriate treatment, receipt of antihypertensive medications if systolic blood pressure was at least 130 mm Hg, were nearly identical among women and men.
Cardiovascular risk in women “less well managed”
“This is confirmation that women are less well managed than men when it comes to cardiovascular risk, especially if they have [type 2] diabetes,” Dr. Ferrannini said in an interview.
Similar observations have been documented before, including in a report in 2019.
The treatment disparity by sex among the 9901 women and men with type 2 diabetes enrolled in REWIND is particularly striking because in clinical trials “patients are generally better managed than in the real world,” Dr. Ferrannini noted. “Despite this, the pattern of disadvantage to women was still evident,” she added.
“In cardiovascular protection the gender issue is preponderant. Women are less well treated,” she said.
REWIND is the cardiovascular outcomes trial for the once-weekly injectable glucagonlike peptide–1 receptor agonist dulaglutide (Trulicity, Lilly) in patients with type 2 diabetes.
The primary results, reported at the 2019 scientific sessions of the American Diabetes Association and simultaneously published in The Lancet, showed dulaglutide significantly reduced major adverse cardiovascular events (MACE) by 12%, compared with placebo. The study ran at about 300 centers worldwide, including many U.S. and Canadian sites, and 46% of enrolled patients were women.
But despite undertreatment, women had significantly better outcomes in terms of MACE, the primary endpoint, during a median 5.4 years of follow-up compared with men. After adjustment for sex, other baseline characteristics, and study-treatment assignment, women had a significant 27% lower composite rate of nonfatal MI, nonfatal stroke, or death from either cardiovascular or unknown causes, compared with men, said Dr. Ferrannini, a researcher at the Karolinska Institute in Stockholm.
The analysis by sex also showed that women had a significant outcome advantage, compared with men, for three of the four components of the combined MACE outcome: nonfatal MI, cardiovascular death, and all-cause death, as well as for the outcome of hospitalization for heart failure, which was not part of the composite MACE outcome. The only MACE outcome component that showed no significant between-group difference was nonfatal stroke, which had roughly equal incidence rates among women and men.
Women had half the prevalence of CVD at baseline
The results also showed that the women with type 2 diabetes enrolled in REWIND had a prevalence of existing cardiovascular disease of 20%, which was half the rate of men at study entry, at 41%. However, the between-sex differences in the primary outcome, as well as each of the individual cardiovascular disease outcomes, didn’t change based on whether or not patients had a history of cardiovascular disease at baseline.
Only one outcome showed a between-sex difference linked to prevalent cardiovascular disease at study entry, the rate of all-cause mortality, which was not significantly different between men and women with a history of cardiovascular disease, but was 39% lower in women compared with men without such a history.
“The good news is that, at baseline and after 2 years, the majority of participants were meeting the relevant treatment targets regardless of sex,” commented Peter Novodvorsky, MUDr, a diabetes researcher at the University of Sheffield (England), who chaired the session during which Dr. Ferrannini presented her findings.
A role for geography, or selection bias?
The new analyses did not examine whether the overall pattern of undertreatment of women differed among each of the 24 participating countries, or by region of the world.
“We have to assume that these results reflect current [routine] practice” in the 24 countries that contributed patients to the trial, noted Dr. Novodvorsky.
There is also “the well-known issue of selection bias” in randomized trials. The current findings raise the question of whether the women willing to take part in the trial somehow differed from the men, he suggested.
Dr. Ferrannini added: “Even if we do observe a gender difference in management, if the majority of women with type 2 diabetes are appropriately treated, this ‘restores’ their cardiovascular risk advantage, compared with men, with the exception of stroke.”
The main hypothesis generated by the post hoc analysis of REWIND is that “women with diabetes have better outcomes than men if they are treated properly,” she stressed, noting that this “would have to be tested in a trial designed to ascertain gender differences.”
REWIND was sponsored by Eli Lilly. Dr. Ferrannini has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time-restricted eating: An easy way to improve metabolic health?
Time-restricted eating – where caloric intake is restricted within a consistent interval of less than 12 hours without overtly attempting to reduce calories – has “generated impressive [animal] data in preventing or reversing metabolic diseases associated with obesity,” and “more rigorous human studies are needed,” conclude the authors of a new review.
“Time-restricted eating is an easy-to-follow and effective dietary strategy that requires less mental math than counting calories,” said senior author Satchidananda Panda, PhD, of the Panda Lab at the Salk Institute for Biological Studies, La Jolla, Calif.
he noted in a press release from the Endocrine Society.
“People who are trying to lose weight and live a healthier lifestyle should pay more attention to when they eat as well as what they eat,” Dr. Panda advised.
Moreover, “eating at random times breaks the synchrony of our internal program [circadian clock] and make us prone to diseases,” so it is important to eat at consistent times.
Furthermore, time-restricted eating, a type of intermittent fasting, “is a lifestyle that anyone can adopt,” he noted, which “can help eliminate health disparities and lets everyone live a healthy and fulfilling life.”
The article, by Emily N. Manoogian, PhD, a postdoctoral fellow in the same lab, and colleagues was published online Sept. 22 in Endocrine Reviews.
The authors suggest that health care providers should encourage high-risk patients (such as those with obesity) to monitor their eating and sleeping times and make easy-to-implement behavior changes, such as decreasing after-dinner snacking and going to bed at the same time each day.
Animal experiments, early studies in humans
In animal experiments, time-restricted feeding without reducing caloric intake prevented or attenuated the severity of metabolic diseases including obesity, glucose intolerance, hepatic steatosis, dyslipidemia, and age-related decline in cardiac function, Dr. Manoogian and colleagues report.
In pilot human studies, time-restricted eating with or without explicit calorie reduction was associated with reductions in body weight, glucose intolerance, hypertension, and dyslipidemia.
Most studies did not restrict calories or provide dietary recommendations, yet participants commonly reduced their caloric intake by 7%-22%.
39 published clinical trials, many upcoming ones
The authors identified 39 clinical trials of time-restricted eating, which were mostly published in the past 2 years, with the earliest one published in 2013.
Most studies were short and small (4-12 weeks, 10-20 participants) and were of people with obesity, healthy adults, and athletes. Most of the trials had an 8- to 10-hour daily “eating window.”
Body weight decreased in 24 of 39 studies, and “importantly,” time-restricted eating was feasible and safe in all studies, the authors note.
“Larger randomized controlled trials are needed as many of the studies to date are smaller pre-post or crossover trials,” Dr. Manoogian and colleagues summarize. “Yet, the replication of findings, even in diverse patient populations, speaks to the potential impact of [time-restricted eating] as a health intervention.”
The many ongoing international clinical trials of time-restricted eating that are listed on clinicaltrials.gov should improve our understanding of time-restricted eating, they add.
Some of the larger trials are in participants with prediabetes (344 participants, NCT03504683), diabetes (144 participants, NCT04155619), metabolic syndrome (118 participants, NCT04057339), and firefighters on 24-hour shifts (150 participants, NCT03533023). There are also smaller pilot studies in participants with cancer (NCT04243512) and polycystic ovary syndrome (NCT03792282).
Be consistent; do not eat within 3 hours of bedtime
In the meantime, the review authors offer several tips:
- Because high melatonin levels (late at night or early morning) can inhibit proper response to food, choose a time to eat that starts at least an hour after waking and stops at least 3 hours before bedtime. If you sleep 8 hours, that leaves 12 hours for the time-restricted eating window.
- Try to eat within the same time window each day.
- Some research suggests eating earlier in the eating phase is better than eating later.
The study received funding from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, the National Cancer Institute, the Larry l. Hillblom Foundation, the Wu Tsai Human Performance Alliance, the U.S. Department of Defense, and the Federal Emergency Management Agency. Dr. Panda has reported receiving royalties from his book, The Circadian Code. The other authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Time-restricted eating – where caloric intake is restricted within a consistent interval of less than 12 hours without overtly attempting to reduce calories – has “generated impressive [animal] data in preventing or reversing metabolic diseases associated with obesity,” and “more rigorous human studies are needed,” conclude the authors of a new review.
“Time-restricted eating is an easy-to-follow and effective dietary strategy that requires less mental math than counting calories,” said senior author Satchidananda Panda, PhD, of the Panda Lab at the Salk Institute for Biological Studies, La Jolla, Calif.
he noted in a press release from the Endocrine Society.
“People who are trying to lose weight and live a healthier lifestyle should pay more attention to when they eat as well as what they eat,” Dr. Panda advised.
Moreover, “eating at random times breaks the synchrony of our internal program [circadian clock] and make us prone to diseases,” so it is important to eat at consistent times.
Furthermore, time-restricted eating, a type of intermittent fasting, “is a lifestyle that anyone can adopt,” he noted, which “can help eliminate health disparities and lets everyone live a healthy and fulfilling life.”
The article, by Emily N. Manoogian, PhD, a postdoctoral fellow in the same lab, and colleagues was published online Sept. 22 in Endocrine Reviews.
The authors suggest that health care providers should encourage high-risk patients (such as those with obesity) to monitor their eating and sleeping times and make easy-to-implement behavior changes, such as decreasing after-dinner snacking and going to bed at the same time each day.
Animal experiments, early studies in humans
In animal experiments, time-restricted feeding without reducing caloric intake prevented or attenuated the severity of metabolic diseases including obesity, glucose intolerance, hepatic steatosis, dyslipidemia, and age-related decline in cardiac function, Dr. Manoogian and colleagues report.
In pilot human studies, time-restricted eating with or without explicit calorie reduction was associated with reductions in body weight, glucose intolerance, hypertension, and dyslipidemia.
Most studies did not restrict calories or provide dietary recommendations, yet participants commonly reduced their caloric intake by 7%-22%.
39 published clinical trials, many upcoming ones
The authors identified 39 clinical trials of time-restricted eating, which were mostly published in the past 2 years, with the earliest one published in 2013.
Most studies were short and small (4-12 weeks, 10-20 participants) and were of people with obesity, healthy adults, and athletes. Most of the trials had an 8- to 10-hour daily “eating window.”
Body weight decreased in 24 of 39 studies, and “importantly,” time-restricted eating was feasible and safe in all studies, the authors note.
“Larger randomized controlled trials are needed as many of the studies to date are smaller pre-post or crossover trials,” Dr. Manoogian and colleagues summarize. “Yet, the replication of findings, even in diverse patient populations, speaks to the potential impact of [time-restricted eating] as a health intervention.”
The many ongoing international clinical trials of time-restricted eating that are listed on clinicaltrials.gov should improve our understanding of time-restricted eating, they add.
Some of the larger trials are in participants with prediabetes (344 participants, NCT03504683), diabetes (144 participants, NCT04155619), metabolic syndrome (118 participants, NCT04057339), and firefighters on 24-hour shifts (150 participants, NCT03533023). There are also smaller pilot studies in participants with cancer (NCT04243512) and polycystic ovary syndrome (NCT03792282).
Be consistent; do not eat within 3 hours of bedtime
In the meantime, the review authors offer several tips:
- Because high melatonin levels (late at night or early morning) can inhibit proper response to food, choose a time to eat that starts at least an hour after waking and stops at least 3 hours before bedtime. If you sleep 8 hours, that leaves 12 hours for the time-restricted eating window.
- Try to eat within the same time window each day.
- Some research suggests eating earlier in the eating phase is better than eating later.
The study received funding from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, the National Cancer Institute, the Larry l. Hillblom Foundation, the Wu Tsai Human Performance Alliance, the U.S. Department of Defense, and the Federal Emergency Management Agency. Dr. Panda has reported receiving royalties from his book, The Circadian Code. The other authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Time-restricted eating – where caloric intake is restricted within a consistent interval of less than 12 hours without overtly attempting to reduce calories – has “generated impressive [animal] data in preventing or reversing metabolic diseases associated with obesity,” and “more rigorous human studies are needed,” conclude the authors of a new review.
“Time-restricted eating is an easy-to-follow and effective dietary strategy that requires less mental math than counting calories,” said senior author Satchidananda Panda, PhD, of the Panda Lab at the Salk Institute for Biological Studies, La Jolla, Calif.
he noted in a press release from the Endocrine Society.
“People who are trying to lose weight and live a healthier lifestyle should pay more attention to when they eat as well as what they eat,” Dr. Panda advised.
Moreover, “eating at random times breaks the synchrony of our internal program [circadian clock] and make us prone to diseases,” so it is important to eat at consistent times.
Furthermore, time-restricted eating, a type of intermittent fasting, “is a lifestyle that anyone can adopt,” he noted, which “can help eliminate health disparities and lets everyone live a healthy and fulfilling life.”
The article, by Emily N. Manoogian, PhD, a postdoctoral fellow in the same lab, and colleagues was published online Sept. 22 in Endocrine Reviews.
The authors suggest that health care providers should encourage high-risk patients (such as those with obesity) to monitor their eating and sleeping times and make easy-to-implement behavior changes, such as decreasing after-dinner snacking and going to bed at the same time each day.
Animal experiments, early studies in humans
In animal experiments, time-restricted feeding without reducing caloric intake prevented or attenuated the severity of metabolic diseases including obesity, glucose intolerance, hepatic steatosis, dyslipidemia, and age-related decline in cardiac function, Dr. Manoogian and colleagues report.
In pilot human studies, time-restricted eating with or without explicit calorie reduction was associated with reductions in body weight, glucose intolerance, hypertension, and dyslipidemia.
Most studies did not restrict calories or provide dietary recommendations, yet participants commonly reduced their caloric intake by 7%-22%.
39 published clinical trials, many upcoming ones
The authors identified 39 clinical trials of time-restricted eating, which were mostly published in the past 2 years, with the earliest one published in 2013.
Most studies were short and small (4-12 weeks, 10-20 participants) and were of people with obesity, healthy adults, and athletes. Most of the trials had an 8- to 10-hour daily “eating window.”
Body weight decreased in 24 of 39 studies, and “importantly,” time-restricted eating was feasible and safe in all studies, the authors note.
“Larger randomized controlled trials are needed as many of the studies to date are smaller pre-post or crossover trials,” Dr. Manoogian and colleagues summarize. “Yet, the replication of findings, even in diverse patient populations, speaks to the potential impact of [time-restricted eating] as a health intervention.”
The many ongoing international clinical trials of time-restricted eating that are listed on clinicaltrials.gov should improve our understanding of time-restricted eating, they add.
Some of the larger trials are in participants with prediabetes (344 participants, NCT03504683), diabetes (144 participants, NCT04155619), metabolic syndrome (118 participants, NCT04057339), and firefighters on 24-hour shifts (150 participants, NCT03533023). There are also smaller pilot studies in participants with cancer (NCT04243512) and polycystic ovary syndrome (NCT03792282).
Be consistent; do not eat within 3 hours of bedtime
In the meantime, the review authors offer several tips:
- Because high melatonin levels (late at night or early morning) can inhibit proper response to food, choose a time to eat that starts at least an hour after waking and stops at least 3 hours before bedtime. If you sleep 8 hours, that leaves 12 hours for the time-restricted eating window.
- Try to eat within the same time window each day.
- Some research suggests eating earlier in the eating phase is better than eating later.
The study received funding from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, the National Cancer Institute, the Larry l. Hillblom Foundation, the Wu Tsai Human Performance Alliance, the U.S. Department of Defense, and the Federal Emergency Management Agency. Dr. Panda has reported receiving royalties from his book, The Circadian Code. The other authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
PCOS linked to menopausal urogenital symptoms but not hot flashes
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
Women with a history of polycystic ovary syndrome (PCOS) are more likely to experience somatic and urogenital symptoms post menopause, but they were no more likely to experience severe hot flashes than were other women with similar characteristics, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society.
PCOS and vasomotor symptoms are each risk factors for cardiovascular disease, so researchers wanted to find out whether they were linked to one another, which might indicate that they are markers for the same underlying mechanisms that increase heart disease risk. The lack of an association, however, raises questions about how much each of these conditions might independently increase cardiovascular risk.
“Should we take a little more time to truly risk-assess these patients not just with their ASCVD risk score, but take into account that they have PCOS and they’re going through menopause, and how severe their hot flashes are?” asked Angie S. Lobo, MD, an internal medicine specialist at Mayo Clinic in Rochester, Minn., when she discussed her findings in an interview.
The association between PCOS and urogenital symptoms was surprising, Dr. Lobo said, but she said she suspects the reason for the finding may be the self-reported nature of the study.
“If you ask the question, you get the answer,” Dr. Lobo said. ”Are we just not asking the right questions to our patients? And should we be doing this more often? This is an exciting finding because there’s so much room to improve the clinical care of our patients.”
The researchers analyzed data from 3,308 women, ages 45-60, in a cross-sectional study from the Data Registry on the Experiences of Aging, Menopause, and Sexuality (DREAMS). The study occurred at Mayo Clinic locations between May 2015 and December 2019 in Rochester, Minn., in Scottsdale, Ariz., and in Jacksonville, Fla.
The women were an average 53 years old and were primarily White, educated, and postmenopausal. Among the 4.6% of women with a self-reported history of PCOS, 56% of them reported depression symptoms, compared to 42% of women without PCOS. Those with PCOS also had nearly twice the prevalence of obesity – 42% versus 22.5% among women without PCOS – and had a higher average overall score on the Menopause Rating Scale (17.7 vs. 14.7; P < .001).
Although women with PCOS initially had a greater burden of psychological symptoms on the same scale, that association disappeared after adjustment for menopause status, body mass index, depression, anxiety, and current use of hormone therapy. Even after adjustment, however, women with PCOS had higher average scores for somatic symptoms (6.7 vs. 5.6) and urogenital symptoms (5.2 vs. 4.3) than those of women without PCOS (P < .001).
Severe or very severe hot flashes were no more likely in women with a history of PCOS than in the other women in the study.
”The mechanisms underlying the correlation between PCOS and menopause symptoms in the psychological and urogenital symptom domains requires further study, although the well-known association between PCOS and mood disorders may explain the high psychological symptom burden in these women during the menopause transition,” the authors concluded.
Rachael B. Smith, DO, clinical assistant professor of ob.gyn. at the University of Arizona in Phoenix, said she was not surprised to see an association between PCOS and menopause symptoms overall, but she was surprised that PCOS did not correlate with severity of vasomotor symptoms. But Dr. Smith pointed out that the sample size of women with PCOS is fairly small (n = 151).
“Given that PCOS prevalence is about 6%-10%, I feel this association should be further studied to improve our counseling and treatment for this PCOS population,” Dr. Smith, who was not involved in the research, said in an interview. “The take-home message for physicians is improved patient-tailored counseling that takes into account patients’ prior medical history of PCOS.”
Although it will require more research to find out, Dr. Smith said she suspects that PCOS and vasomotor symptoms are additive risk factors for cardiovascular disease. She also noted that the study is limited by the homogeneity of the study population.
The research was funded by the National Institutes of Health. Dr. Lobo and Dr. Smith had no disclosures.
FROM NAMS 2021
Heart failure hospitalization risk lower with SGLT2 inhibitors than GLP-1 RAs
Around a 30% reduction in the risk for being hospitalized for heart failure was achieved in people with type 2 diabetes who were treated with a SGLT2 inhibitor over a GLP-1 RA regardless of whether the patients had a preexisting heart condition.
The findings, published in the Annals of Internal Medicine, also showed a 10% lower risk for myocardial infarction or stroke among those treated with a SGLT2 inhibitor who had preexisting cardiovascular disease (CVD), although there was no difference in risk between the two classes of drugs in those without preexisting CVD.
“These findings are important as they suggest that SGLT2 [inhibitors] and GLP-1 RA offer similar benefits in preventing myocardial infarction and stroke in patients with diabetes,” said study investigator Elisabetta Patorno, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School in Boston, in an interview.
They also show “that SGLT2 [inhibitors] offer greater efficacy in preventing heart failure, which supports the existing guidelines,” she added.
Paul S. Jellinger, MD, MACE, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said these data were likely to be “additive to guidelines but not transformative.” The overall analysis results were “not surprising.” It was not unexpected that SGLT2 inhibitors provided a robust chronic heart failure (CHF) benefit, particularly in individuals with history of CVD, he said.
Dr. Jellinger, a clinical endocrinologist and professor of clinical medicine on the voluntary faculty at the University of Miami, observed, however, that “the similar CVD benefit in both drug classes in patients without known CVD adds to our knowledge in this somewhat controversial area and may be useful to the clinician in evaluating therapy in a diabetic individual without evidence of or at high risk for CHF.”
Furthermore, “the study also reminds us that, as demonstrated in published meta-analysis, there is also a modest CHF benefit associated with GLP-1 RA treatment particularly in patients with a history of CVD.”
Addressing the knowledge gap
Thanks to the results of many large-scale, prospective, cardiovascular outcomes studies, both SGLT2 inhibitors and GLP1 RA have been recommended as treatment for people with diabetes who have established CVD. But with no direct head-to-head trials having been conducted, there is a gap in knowledge and there is currently little guidance for physicians on which drug class to choose for an individual patient.
To try to clarify things, Dr. Patorno and associates looked at data from more than 370,000 people with type 2 diabetes who had been treated between April 2013 and December 2017 with either a SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or GLP-1 RA (albiglutide, dulaglutide, exenatide, or liraglutide).
One-to-one propensity score matching was used to create the study groups: participants were first grouped according to their history of CVD, and then by the class of drug they had been prescribed. The primary outcomes were hospitalization for MI, stroke, or heart failure.
Comparing the initiation of a SGLT2 inhibitor with GLP-1 RA therapy, the hazard ratios (HRs) for MI or stroke in patients with and without a history of CVD were a respective 0.90 (95% CI, 0.82 to 0.98) and 1.07 (0.97 to 1.18).
The corresponding hazard ratios for heart failure hospitalizations were 0.71 (0.64 to 0.79) and 0.69 (0.56 to 0.85).
Real-world studies are of ‘increasing value’
“As in other not-randomized studies based on real-world data, residual confounding cannot be completely ruled out,” Dr. Patorno acknowledged. She added, however that “state-of-the-art methodological strategies were implemented to minimize this possibility.”
Limitations notwithstanding, “real world studies are demonstrating increasing value,” observed Dr. Jellinger. Further large-scale cardiovascular outcomes trials that directly compare these two drug classes “are unlikely given the depth of information available now,” Dr. Jellinger suggested.
“This head-to-head retrospective study may be as close as we get and does represent the first effort at a comparison of these two classes.”
Dr. Patorno said of the potential clinical implications: “Because the two classes are equally effective for stroke and myocardial infarction, but the SGLT2 inhibitors are superior for heart failure, when considered in aggregate, SGLT2 inhibitors are likely to prevent more of these adverse cardiovascular events than GLP-1 RA.”
The study received no commercial funding and was supported by the Brigham and Women’s Hospital and Harvard Medical School Division of Pharmacoepidemiology and Pharmacoeconomics.
Dr. Patorno reported no conflicts of interest. Dr. Jellinger is on the speaker’s bureau for Astra Zeneca, Amgen, and Esperion, and has served on advisory boards for Corcept and Regeneron.
Around a 30% reduction in the risk for being hospitalized for heart failure was achieved in people with type 2 diabetes who were treated with a SGLT2 inhibitor over a GLP-1 RA regardless of whether the patients had a preexisting heart condition.
The findings, published in the Annals of Internal Medicine, also showed a 10% lower risk for myocardial infarction or stroke among those treated with a SGLT2 inhibitor who had preexisting cardiovascular disease (CVD), although there was no difference in risk between the two classes of drugs in those without preexisting CVD.
“These findings are important as they suggest that SGLT2 [inhibitors] and GLP-1 RA offer similar benefits in preventing myocardial infarction and stroke in patients with diabetes,” said study investigator Elisabetta Patorno, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School in Boston, in an interview.
They also show “that SGLT2 [inhibitors] offer greater efficacy in preventing heart failure, which supports the existing guidelines,” she added.
Paul S. Jellinger, MD, MACE, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said these data were likely to be “additive to guidelines but not transformative.” The overall analysis results were “not surprising.” It was not unexpected that SGLT2 inhibitors provided a robust chronic heart failure (CHF) benefit, particularly in individuals with history of CVD, he said.
Dr. Jellinger, a clinical endocrinologist and professor of clinical medicine on the voluntary faculty at the University of Miami, observed, however, that “the similar CVD benefit in both drug classes in patients without known CVD adds to our knowledge in this somewhat controversial area and may be useful to the clinician in evaluating therapy in a diabetic individual without evidence of or at high risk for CHF.”
Furthermore, “the study also reminds us that, as demonstrated in published meta-analysis, there is also a modest CHF benefit associated with GLP-1 RA treatment particularly in patients with a history of CVD.”
Addressing the knowledge gap
Thanks to the results of many large-scale, prospective, cardiovascular outcomes studies, both SGLT2 inhibitors and GLP1 RA have been recommended as treatment for people with diabetes who have established CVD. But with no direct head-to-head trials having been conducted, there is a gap in knowledge and there is currently little guidance for physicians on which drug class to choose for an individual patient.
To try to clarify things, Dr. Patorno and associates looked at data from more than 370,000 people with type 2 diabetes who had been treated between April 2013 and December 2017 with either a SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or GLP-1 RA (albiglutide, dulaglutide, exenatide, or liraglutide).
One-to-one propensity score matching was used to create the study groups: participants were first grouped according to their history of CVD, and then by the class of drug they had been prescribed. The primary outcomes were hospitalization for MI, stroke, or heart failure.
Comparing the initiation of a SGLT2 inhibitor with GLP-1 RA therapy, the hazard ratios (HRs) for MI or stroke in patients with and without a history of CVD were a respective 0.90 (95% CI, 0.82 to 0.98) and 1.07 (0.97 to 1.18).
The corresponding hazard ratios for heart failure hospitalizations were 0.71 (0.64 to 0.79) and 0.69 (0.56 to 0.85).
Real-world studies are of ‘increasing value’
“As in other not-randomized studies based on real-world data, residual confounding cannot be completely ruled out,” Dr. Patorno acknowledged. She added, however that “state-of-the-art methodological strategies were implemented to minimize this possibility.”
Limitations notwithstanding, “real world studies are demonstrating increasing value,” observed Dr. Jellinger. Further large-scale cardiovascular outcomes trials that directly compare these two drug classes “are unlikely given the depth of information available now,” Dr. Jellinger suggested.
“This head-to-head retrospective study may be as close as we get and does represent the first effort at a comparison of these two classes.”
Dr. Patorno said of the potential clinical implications: “Because the two classes are equally effective for stroke and myocardial infarction, but the SGLT2 inhibitors are superior for heart failure, when considered in aggregate, SGLT2 inhibitors are likely to prevent more of these adverse cardiovascular events than GLP-1 RA.”
The study received no commercial funding and was supported by the Brigham and Women’s Hospital and Harvard Medical School Division of Pharmacoepidemiology and Pharmacoeconomics.
Dr. Patorno reported no conflicts of interest. Dr. Jellinger is on the speaker’s bureau for Astra Zeneca, Amgen, and Esperion, and has served on advisory boards for Corcept and Regeneron.
Around a 30% reduction in the risk for being hospitalized for heart failure was achieved in people with type 2 diabetes who were treated with a SGLT2 inhibitor over a GLP-1 RA regardless of whether the patients had a preexisting heart condition.
The findings, published in the Annals of Internal Medicine, also showed a 10% lower risk for myocardial infarction or stroke among those treated with a SGLT2 inhibitor who had preexisting cardiovascular disease (CVD), although there was no difference in risk between the two classes of drugs in those without preexisting CVD.
“These findings are important as they suggest that SGLT2 [inhibitors] and GLP-1 RA offer similar benefits in preventing myocardial infarction and stroke in patients with diabetes,” said study investigator Elisabetta Patorno, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School in Boston, in an interview.
They also show “that SGLT2 [inhibitors] offer greater efficacy in preventing heart failure, which supports the existing guidelines,” she added.
Paul S. Jellinger, MD, MACE, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said these data were likely to be “additive to guidelines but not transformative.” The overall analysis results were “not surprising.” It was not unexpected that SGLT2 inhibitors provided a robust chronic heart failure (CHF) benefit, particularly in individuals with history of CVD, he said.
Dr. Jellinger, a clinical endocrinologist and professor of clinical medicine on the voluntary faculty at the University of Miami, observed, however, that “the similar CVD benefit in both drug classes in patients without known CVD adds to our knowledge in this somewhat controversial area and may be useful to the clinician in evaluating therapy in a diabetic individual without evidence of or at high risk for CHF.”
Furthermore, “the study also reminds us that, as demonstrated in published meta-analysis, there is also a modest CHF benefit associated with GLP-1 RA treatment particularly in patients with a history of CVD.”
Addressing the knowledge gap
Thanks to the results of many large-scale, prospective, cardiovascular outcomes studies, both SGLT2 inhibitors and GLP1 RA have been recommended as treatment for people with diabetes who have established CVD. But with no direct head-to-head trials having been conducted, there is a gap in knowledge and there is currently little guidance for physicians on which drug class to choose for an individual patient.
To try to clarify things, Dr. Patorno and associates looked at data from more than 370,000 people with type 2 diabetes who had been treated between April 2013 and December 2017 with either a SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or GLP-1 RA (albiglutide, dulaglutide, exenatide, or liraglutide).
One-to-one propensity score matching was used to create the study groups: participants were first grouped according to their history of CVD, and then by the class of drug they had been prescribed. The primary outcomes were hospitalization for MI, stroke, or heart failure.
Comparing the initiation of a SGLT2 inhibitor with GLP-1 RA therapy, the hazard ratios (HRs) for MI or stroke in patients with and without a history of CVD were a respective 0.90 (95% CI, 0.82 to 0.98) and 1.07 (0.97 to 1.18).
The corresponding hazard ratios for heart failure hospitalizations were 0.71 (0.64 to 0.79) and 0.69 (0.56 to 0.85).
Real-world studies are of ‘increasing value’
“As in other not-randomized studies based on real-world data, residual confounding cannot be completely ruled out,” Dr. Patorno acknowledged. She added, however that “state-of-the-art methodological strategies were implemented to minimize this possibility.”
Limitations notwithstanding, “real world studies are demonstrating increasing value,” observed Dr. Jellinger. Further large-scale cardiovascular outcomes trials that directly compare these two drug classes “are unlikely given the depth of information available now,” Dr. Jellinger suggested.
“This head-to-head retrospective study may be as close as we get and does represent the first effort at a comparison of these two classes.”
Dr. Patorno said of the potential clinical implications: “Because the two classes are equally effective for stroke and myocardial infarction, but the SGLT2 inhibitors are superior for heart failure, when considered in aggregate, SGLT2 inhibitors are likely to prevent more of these adverse cardiovascular events than GLP-1 RA.”
The study received no commercial funding and was supported by the Brigham and Women’s Hospital and Harvard Medical School Division of Pharmacoepidemiology and Pharmacoeconomics.
Dr. Patorno reported no conflicts of interest. Dr. Jellinger is on the speaker’s bureau for Astra Zeneca, Amgen, and Esperion, and has served on advisory boards for Corcept and Regeneron.
FROM ANNALS OF INTERNAL MEDICINE
Diabetes drug may extend pregnancy in women with preeclampsia
New evidence suggests a drug used to lower blood sugar levels may also help extend the duration of preterm pregnancies in women with preeclampsia.
The findings from a small clinical trial, published Sept. 23 in the BMJ, showed that pregnant women who received the diabetes medication metformin prolonged their pregnancy by a week compared to those who received a placebo. Although this finding was not statistically significant, researchers said they are “cautiously optimistic” about the treatment of preterm preeclampsia.
“We hope that it will encourage others to test not only metformin but also other promising therapeutic candidates to treat and prevent preeclampsia,” study author Catherine Cluver, MBChB, FCOG, PhD, associate professor in the department of obstetrics and gynecology at Stellenbosch University in South Africa, said in an interview.
Preeclampsia, a condition that occurs about 1 in 25 pregnancies in the United States, happens when a woman develops high blood pressure and protein in her urine, according to the Centers for Disease Control and Prevention.
Preterm preeclampsia is a severe variant affecting 0.5% of all pregnancies, or 10% of those with preeclampsia, researchers wrote in the study. The condition is associated with more maternal and neonatal death and increases their risks of developing an illness.
Dr. Cluver said that when a mother develops preeclampsia, the lining of her blood vessels, or the endothelium, is affected and there are specific proteins in the blood that increase. Dr. Cluver’s preclinical study found that metformin improved endothelial function and decreased these biomarkers in laboratory work.
“We therefore set out to see if metformin could be used to prolong gestation in preterm preeclampsia,” she said.
For the study, Dr. Cluver and colleagues performed a double-blind, placebo-controlled clinical trial to compare the prolongation of pregnancies among women who were at least 26 months pregnant with preterm preeclampsia. They were treated with either 3 grams of extended-release metformin (90 women), or a matching placebo (90 women).*
In the treatment group, the average time from the start of the study to delivery was 17.7 days, compared to 10.1 days in the placebo group. The median difference was 7.6 days.
The researchers also found that 40% of women in the metformin group reached 34 weeks’ gestation compared with 28% of those in the placebo group. Fewer women in the metformin group delivered because of fetal indications such as fetal distress or other issues – 33% vs. 44%. However, the researchers said those results were not statistically significant.
They said they were cautiously optimistic when they found that the median time for prolongation of pregnancy in the metformin group was 17.5 days compared with 7.9 days in the placebo group, findings that were statistically significant.
Some adverse effects participants experienced while taking metformin during their pregnancy included diarrhea and an increase in nausea.
Although the study is important in maternal-fetal medicine and is a novel approach to preterm preeclampsia, the findings weren’t strong enough, but they point to the need for further study, said Victor Klein, MD, MBA, CPHRM, a specialist in high-risk pregnancy who was not involved in the study.
“Even though they did have an improved outcome, it wasn’t strong enough. It wasn’t long enough to prove that the medicine was useful or efficacious,” said Dr. Klein, vice chairman of obstetrics and gynecology at North Shore University Hospital, New York.
Metformin is also used to treat gestational diabetes, which is an “advantage of repurposing the drug is that it is likely to be safe,” the researchers wrote. They said longer term follow-up data might be worthwhile in future trials.
None of the experts had conflicts of interest to disclose.
*This story was updated on 10/6/2021.
New evidence suggests a drug used to lower blood sugar levels may also help extend the duration of preterm pregnancies in women with preeclampsia.
The findings from a small clinical trial, published Sept. 23 in the BMJ, showed that pregnant women who received the diabetes medication metformin prolonged their pregnancy by a week compared to those who received a placebo. Although this finding was not statistically significant, researchers said they are “cautiously optimistic” about the treatment of preterm preeclampsia.
“We hope that it will encourage others to test not only metformin but also other promising therapeutic candidates to treat and prevent preeclampsia,” study author Catherine Cluver, MBChB, FCOG, PhD, associate professor in the department of obstetrics and gynecology at Stellenbosch University in South Africa, said in an interview.
Preeclampsia, a condition that occurs about 1 in 25 pregnancies in the United States, happens when a woman develops high blood pressure and protein in her urine, according to the Centers for Disease Control and Prevention.
Preterm preeclampsia is a severe variant affecting 0.5% of all pregnancies, or 10% of those with preeclampsia, researchers wrote in the study. The condition is associated with more maternal and neonatal death and increases their risks of developing an illness.
Dr. Cluver said that when a mother develops preeclampsia, the lining of her blood vessels, or the endothelium, is affected and there are specific proteins in the blood that increase. Dr. Cluver’s preclinical study found that metformin improved endothelial function and decreased these biomarkers in laboratory work.
“We therefore set out to see if metformin could be used to prolong gestation in preterm preeclampsia,” she said.
For the study, Dr. Cluver and colleagues performed a double-blind, placebo-controlled clinical trial to compare the prolongation of pregnancies among women who were at least 26 months pregnant with preterm preeclampsia. They were treated with either 3 grams of extended-release metformin (90 women), or a matching placebo (90 women).*
In the treatment group, the average time from the start of the study to delivery was 17.7 days, compared to 10.1 days in the placebo group. The median difference was 7.6 days.
The researchers also found that 40% of women in the metformin group reached 34 weeks’ gestation compared with 28% of those in the placebo group. Fewer women in the metformin group delivered because of fetal indications such as fetal distress or other issues – 33% vs. 44%. However, the researchers said those results were not statistically significant.
They said they were cautiously optimistic when they found that the median time for prolongation of pregnancy in the metformin group was 17.5 days compared with 7.9 days in the placebo group, findings that were statistically significant.
Some adverse effects participants experienced while taking metformin during their pregnancy included diarrhea and an increase in nausea.
Although the study is important in maternal-fetal medicine and is a novel approach to preterm preeclampsia, the findings weren’t strong enough, but they point to the need for further study, said Victor Klein, MD, MBA, CPHRM, a specialist in high-risk pregnancy who was not involved in the study.
“Even though they did have an improved outcome, it wasn’t strong enough. It wasn’t long enough to prove that the medicine was useful or efficacious,” said Dr. Klein, vice chairman of obstetrics and gynecology at North Shore University Hospital, New York.
Metformin is also used to treat gestational diabetes, which is an “advantage of repurposing the drug is that it is likely to be safe,” the researchers wrote. They said longer term follow-up data might be worthwhile in future trials.
None of the experts had conflicts of interest to disclose.
*This story was updated on 10/6/2021.
New evidence suggests a drug used to lower blood sugar levels may also help extend the duration of preterm pregnancies in women with preeclampsia.
The findings from a small clinical trial, published Sept. 23 in the BMJ, showed that pregnant women who received the diabetes medication metformin prolonged their pregnancy by a week compared to those who received a placebo. Although this finding was not statistically significant, researchers said they are “cautiously optimistic” about the treatment of preterm preeclampsia.
“We hope that it will encourage others to test not only metformin but also other promising therapeutic candidates to treat and prevent preeclampsia,” study author Catherine Cluver, MBChB, FCOG, PhD, associate professor in the department of obstetrics and gynecology at Stellenbosch University in South Africa, said in an interview.
Preeclampsia, a condition that occurs about 1 in 25 pregnancies in the United States, happens when a woman develops high blood pressure and protein in her urine, according to the Centers for Disease Control and Prevention.
Preterm preeclampsia is a severe variant affecting 0.5% of all pregnancies, or 10% of those with preeclampsia, researchers wrote in the study. The condition is associated with more maternal and neonatal death and increases their risks of developing an illness.
Dr. Cluver said that when a mother develops preeclampsia, the lining of her blood vessels, or the endothelium, is affected and there are specific proteins in the blood that increase. Dr. Cluver’s preclinical study found that metformin improved endothelial function and decreased these biomarkers in laboratory work.
“We therefore set out to see if metformin could be used to prolong gestation in preterm preeclampsia,” she said.
For the study, Dr. Cluver and colleagues performed a double-blind, placebo-controlled clinical trial to compare the prolongation of pregnancies among women who were at least 26 months pregnant with preterm preeclampsia. They were treated with either 3 grams of extended-release metformin (90 women), or a matching placebo (90 women).*
In the treatment group, the average time from the start of the study to delivery was 17.7 days, compared to 10.1 days in the placebo group. The median difference was 7.6 days.
The researchers also found that 40% of women in the metformin group reached 34 weeks’ gestation compared with 28% of those in the placebo group. Fewer women in the metformin group delivered because of fetal indications such as fetal distress or other issues – 33% vs. 44%. However, the researchers said those results were not statistically significant.
They said they were cautiously optimistic when they found that the median time for prolongation of pregnancy in the metformin group was 17.5 days compared with 7.9 days in the placebo group, findings that were statistically significant.
Some adverse effects participants experienced while taking metformin during their pregnancy included diarrhea and an increase in nausea.
Although the study is important in maternal-fetal medicine and is a novel approach to preterm preeclampsia, the findings weren’t strong enough, but they point to the need for further study, said Victor Klein, MD, MBA, CPHRM, a specialist in high-risk pregnancy who was not involved in the study.
“Even though they did have an improved outcome, it wasn’t strong enough. It wasn’t long enough to prove that the medicine was useful or efficacious,” said Dr. Klein, vice chairman of obstetrics and gynecology at North Shore University Hospital, New York.
Metformin is also used to treat gestational diabetes, which is an “advantage of repurposing the drug is that it is likely to be safe,” the researchers wrote. They said longer term follow-up data might be worthwhile in future trials.
None of the experts had conflicts of interest to disclose.
*This story was updated on 10/6/2021.