User login
Outpatient costs soar for Medicare patients with chronic hepatitis B
The average cost of outpatient care for Medicare recipients with chronic hepatitis B (CH-B) rose by 400% from 2005 to 2014, according to investigators.
Inpatient costs also increased, although less dramatically, reported lead author Min Kim, MD, of the Inova Fairfax Hospital Center for Liver Diseases in Falls Church, Virginia, and her colleagues. The causes of these spending hikes may range from policy changes and expanded screening to an aging immigrant population.
“According to the National Health and Nutrition Examination Survey, from 1988 to 2012 most people with CH-B in the United States were foreign born and accounted for up to 70% of all CH-B infections,” the authors wrote in the Journal of Clinical Gastroenterology. “The Centers for Disease Control [and Prevention] estimates that Asians, who comprise 5% of the U.S. population, account for 50% of all chronic CH-B infections.” Despite these statistics, the clinical and economic impacts of an aging immigrant population are unknown. The investigators therefore assessed patient characteristics associated with increased 1-year mortality and the impact of demographic changes on Medicare costs.
The retrospective study began with a random sample of Medicare beneficiaries from 2005 to 2014. From this group, 18,603 patients with CH-B were identified by ICD-9 codes V02.61, 070.2, 070.3, 070.42, and 070.52. Patients with ICD-9-CM codes of 197.7, 155.1, or 155.2 were excluded, as were records containing insufficient information about year, region, or race. Patients were analyzed collectively and as inpatients (n = 6,550) or outpatients (n = 13,648).
Cost of care (per patient, per year) and 1-year mortality were evaluated. Patient characteristics included age, sex, race/ethnicity, geographic region, type of Medicare eligibility, length of stay, Charlson comorbidity index, presence of decompensated cirrhosis, and/or hepatocellular carcinoma (HCC).
Most dramatically, outpatient charges rose more than 400% during the study period, from $9,257 in 2005 to $47,864 in 2014 (P less than .001). Inpatient charges increased by almost 50%, from $66,610 to $94,221 (P less than .001). (All values converted to 2016 dollars.)
Although the increase in outpatient costs appears seismic, the authors noted that costs held steady from 2005 to 2010 before spiking dramatically, reaching a peak of $58,450 in 2013 before settling down to $47,864 the following year. This spike may be caused by changes in screening measures and policies. In 2009, the American Association for the Study of Liver Diseases expanded screening guidelines to include previously ineligible patients with CH-B, and in 2010, the Centers for Medicare & Medicaid Services expanded ICD-9 and ICD-10 codes for CH-B from 9 to 25.
“It seems plausible that the increase in CH-B prevalence, and its associated costs, might actually be a reflection of [these factors],” the authors noted. Still, “additional studies are needed to clarify this observation.”
Turning to patient characteristics, the authors reported that 1-year mortality was independently associated most strongly with decompensated cirrhosis (odds ratio, 3.02) and hepatocellular carcinoma (OR, 2.64). In comparison with white patients, Asians were less likely to die (OR, 0.47).
“It is possible that this can be explained through differences in transmission mode and disease progression of CH-B between these two demographics,” the authors wrote. “A majority of Asian Medicare recipients with CH-B likely acquired it perinatally and did not develop significant liver disease. In contrast, whites with CH-B generally acquired it in adulthood, increasing the chance of developing liver disease.”
Over the 10-year study period, Medicare beneficiaries with CH-B became more frequently Asian and less frequently male. While the number of outpatient visits and average Charlson comorbidity index increased, decreases were reported for length of stay, rates of 1-year mortality, hospitalization, and HCC – the latter of which is most closely associated with higher costs of care.
The investigators suggested that the decreased incidence of HCC was caused by “better screening programs for HCC and/or more widespread use of antiviral treatment for CH-B.”
“Although advances in antiviral treatment have effectively reduced hospitalization and disease progression,” the authors wrote, “vulnerable groups – especially immigrants and individuals living in poverty – present an important challenge for better identification of infected individuals and their linkage to care. In this context, it is vital to target these cohorts to reduce further mortality and resource utilization, as well as optimize long-term public health and financial benefits.”
Study funding was provided by Seattle Genetics. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
SOURCE: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
The average cost of outpatient care for Medicare recipients with chronic hepatitis B (CH-B) rose by 400% from 2005 to 2014, according to investigators.
Inpatient costs also increased, although less dramatically, reported lead author Min Kim, MD, of the Inova Fairfax Hospital Center for Liver Diseases in Falls Church, Virginia, and her colleagues. The causes of these spending hikes may range from policy changes and expanded screening to an aging immigrant population.
“According to the National Health and Nutrition Examination Survey, from 1988 to 2012 most people with CH-B in the United States were foreign born and accounted for up to 70% of all CH-B infections,” the authors wrote in the Journal of Clinical Gastroenterology. “The Centers for Disease Control [and Prevention] estimates that Asians, who comprise 5% of the U.S. population, account for 50% of all chronic CH-B infections.” Despite these statistics, the clinical and economic impacts of an aging immigrant population are unknown. The investigators therefore assessed patient characteristics associated with increased 1-year mortality and the impact of demographic changes on Medicare costs.
The retrospective study began with a random sample of Medicare beneficiaries from 2005 to 2014. From this group, 18,603 patients with CH-B were identified by ICD-9 codes V02.61, 070.2, 070.3, 070.42, and 070.52. Patients with ICD-9-CM codes of 197.7, 155.1, or 155.2 were excluded, as were records containing insufficient information about year, region, or race. Patients were analyzed collectively and as inpatients (n = 6,550) or outpatients (n = 13,648).
Cost of care (per patient, per year) and 1-year mortality were evaluated. Patient characteristics included age, sex, race/ethnicity, geographic region, type of Medicare eligibility, length of stay, Charlson comorbidity index, presence of decompensated cirrhosis, and/or hepatocellular carcinoma (HCC).
Most dramatically, outpatient charges rose more than 400% during the study period, from $9,257 in 2005 to $47,864 in 2014 (P less than .001). Inpatient charges increased by almost 50%, from $66,610 to $94,221 (P less than .001). (All values converted to 2016 dollars.)
Although the increase in outpatient costs appears seismic, the authors noted that costs held steady from 2005 to 2010 before spiking dramatically, reaching a peak of $58,450 in 2013 before settling down to $47,864 the following year. This spike may be caused by changes in screening measures and policies. In 2009, the American Association for the Study of Liver Diseases expanded screening guidelines to include previously ineligible patients with CH-B, and in 2010, the Centers for Medicare & Medicaid Services expanded ICD-9 and ICD-10 codes for CH-B from 9 to 25.
“It seems plausible that the increase in CH-B prevalence, and its associated costs, might actually be a reflection of [these factors],” the authors noted. Still, “additional studies are needed to clarify this observation.”
Turning to patient characteristics, the authors reported that 1-year mortality was independently associated most strongly with decompensated cirrhosis (odds ratio, 3.02) and hepatocellular carcinoma (OR, 2.64). In comparison with white patients, Asians were less likely to die (OR, 0.47).
“It is possible that this can be explained through differences in transmission mode and disease progression of CH-B between these two demographics,” the authors wrote. “A majority of Asian Medicare recipients with CH-B likely acquired it perinatally and did not develop significant liver disease. In contrast, whites with CH-B generally acquired it in adulthood, increasing the chance of developing liver disease.”
Over the 10-year study period, Medicare beneficiaries with CH-B became more frequently Asian and less frequently male. While the number of outpatient visits and average Charlson comorbidity index increased, decreases were reported for length of stay, rates of 1-year mortality, hospitalization, and HCC – the latter of which is most closely associated with higher costs of care.
The investigators suggested that the decreased incidence of HCC was caused by “better screening programs for HCC and/or more widespread use of antiviral treatment for CH-B.”
“Although advances in antiviral treatment have effectively reduced hospitalization and disease progression,” the authors wrote, “vulnerable groups – especially immigrants and individuals living in poverty – present an important challenge for better identification of infected individuals and their linkage to care. In this context, it is vital to target these cohorts to reduce further mortality and resource utilization, as well as optimize long-term public health and financial benefits.”
Study funding was provided by Seattle Genetics. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
SOURCE: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
The average cost of outpatient care for Medicare recipients with chronic hepatitis B (CH-B) rose by 400% from 2005 to 2014, according to investigators.
Inpatient costs also increased, although less dramatically, reported lead author Min Kim, MD, of the Inova Fairfax Hospital Center for Liver Diseases in Falls Church, Virginia, and her colleagues. The causes of these spending hikes may range from policy changes and expanded screening to an aging immigrant population.
“According to the National Health and Nutrition Examination Survey, from 1988 to 2012 most people with CH-B in the United States were foreign born and accounted for up to 70% of all CH-B infections,” the authors wrote in the Journal of Clinical Gastroenterology. “The Centers for Disease Control [and Prevention] estimates that Asians, who comprise 5% of the U.S. population, account for 50% of all chronic CH-B infections.” Despite these statistics, the clinical and economic impacts of an aging immigrant population are unknown. The investigators therefore assessed patient characteristics associated with increased 1-year mortality and the impact of demographic changes on Medicare costs.
The retrospective study began with a random sample of Medicare beneficiaries from 2005 to 2014. From this group, 18,603 patients with CH-B were identified by ICD-9 codes V02.61, 070.2, 070.3, 070.42, and 070.52. Patients with ICD-9-CM codes of 197.7, 155.1, or 155.2 were excluded, as were records containing insufficient information about year, region, or race. Patients were analyzed collectively and as inpatients (n = 6,550) or outpatients (n = 13,648).
Cost of care (per patient, per year) and 1-year mortality were evaluated. Patient characteristics included age, sex, race/ethnicity, geographic region, type of Medicare eligibility, length of stay, Charlson comorbidity index, presence of decompensated cirrhosis, and/or hepatocellular carcinoma (HCC).
Most dramatically, outpatient charges rose more than 400% during the study period, from $9,257 in 2005 to $47,864 in 2014 (P less than .001). Inpatient charges increased by almost 50%, from $66,610 to $94,221 (P less than .001). (All values converted to 2016 dollars.)
Although the increase in outpatient costs appears seismic, the authors noted that costs held steady from 2005 to 2010 before spiking dramatically, reaching a peak of $58,450 in 2013 before settling down to $47,864 the following year. This spike may be caused by changes in screening measures and policies. In 2009, the American Association for the Study of Liver Diseases expanded screening guidelines to include previously ineligible patients with CH-B, and in 2010, the Centers for Medicare & Medicaid Services expanded ICD-9 and ICD-10 codes for CH-B from 9 to 25.
“It seems plausible that the increase in CH-B prevalence, and its associated costs, might actually be a reflection of [these factors],” the authors noted. Still, “additional studies are needed to clarify this observation.”
Turning to patient characteristics, the authors reported that 1-year mortality was independently associated most strongly with decompensated cirrhosis (odds ratio, 3.02) and hepatocellular carcinoma (OR, 2.64). In comparison with white patients, Asians were less likely to die (OR, 0.47).
“It is possible that this can be explained through differences in transmission mode and disease progression of CH-B between these two demographics,” the authors wrote. “A majority of Asian Medicare recipients with CH-B likely acquired it perinatally and did not develop significant liver disease. In contrast, whites with CH-B generally acquired it in adulthood, increasing the chance of developing liver disease.”
Over the 10-year study period, Medicare beneficiaries with CH-B became more frequently Asian and less frequently male. While the number of outpatient visits and average Charlson comorbidity index increased, decreases were reported for length of stay, rates of 1-year mortality, hospitalization, and HCC – the latter of which is most closely associated with higher costs of care.
The investigators suggested that the decreased incidence of HCC was caused by “better screening programs for HCC and/or more widespread use of antiviral treatment for CH-B.”
“Although advances in antiviral treatment have effectively reduced hospitalization and disease progression,” the authors wrote, “vulnerable groups – especially immigrants and individuals living in poverty – present an important challenge for better identification of infected individuals and their linkage to care. In this context, it is vital to target these cohorts to reduce further mortality and resource utilization, as well as optimize long-term public health and financial benefits.”
Study funding was provided by Seattle Genetics. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
SOURCE: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: Outpatient care for patients with chronic hepatitis B is becoming more expensive; the trend may be tied to an aging immigrant population.
Major finding: The average Medicare charge for outpatient care per patient increased from $9,257 in 2005 to $47,864 in 2014 (P less than .001).
Study details: A retrospective study involving 18,603 Medicare recipients with chronic hepatitis B who filed claims between 2005 and 2014.
Disclosures: Study funding was provided by the Beatty Center for Integrated Research. One coauthor reported compensation from Gilead Sciences, AbbVie, Intercept Pharmaceuticals, GlaxoSmithKline, and Bristol-Myers Squibb.
Source: Kim M et al. J Clin Gastro. 2018 Aug 13. doi: 10.1097/MCG.0000000000001110.
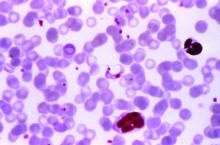
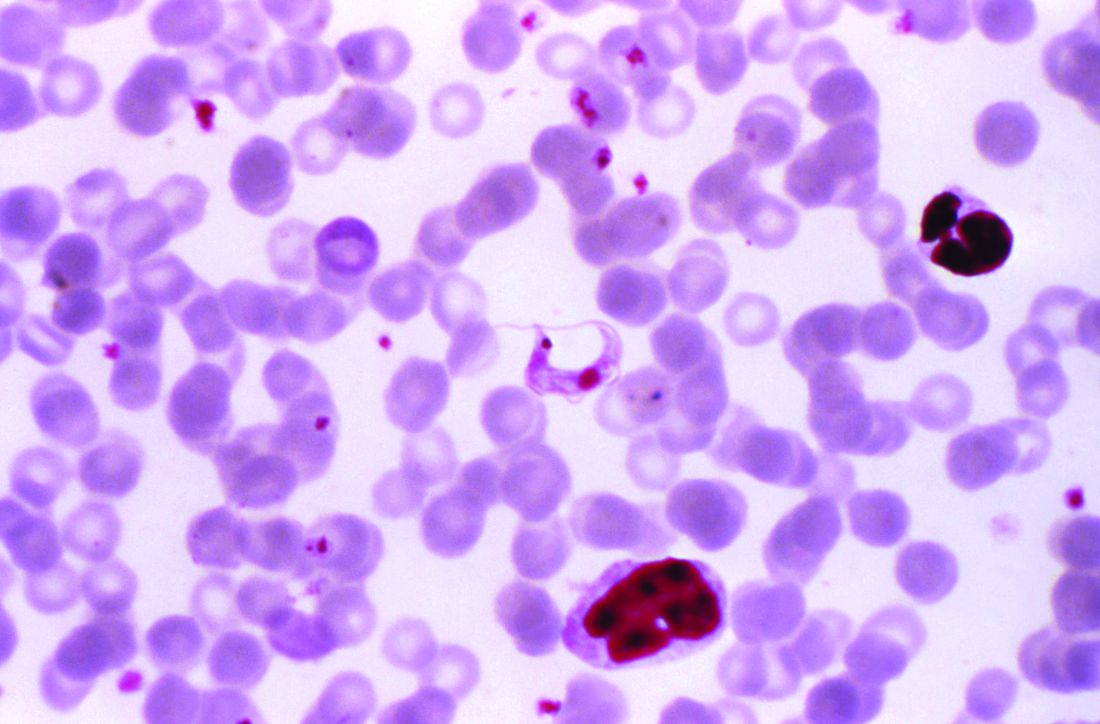
AHA: Chagas disease and its heart effects have come to the U.S.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
Chagas disease, a cause of serious cardiovascular problems and sudden death, was previously localized mainly in the tropics, but now affects at least 300,000 people in the United States and is growing in prevalence in other traditionally nonendemic areas, including Europe, Australia, and Japan. The American Heart Association and the Inter-American Society of Cardiology have released a statement to “increase global awareness among providers who may encounter patients with Chagas disease outside of traditionally endemic environments.”
The document summarizes the most up-to-date information on diagnosis, screening, and treatment of Trypanosoma cruzi (the protozoan cause of Chagas) infection, focusing primarily on its cardiovascular aspects, and was developed by Maria Carmo Pereira Nunes, MD, chair, and her colleagues on the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee.
Chagas disease is transmitted by a blood-sucking insect vector Triatoma infestans and, less frequently, from mother to fetus or by contaminated food or drink, and about one third of infected individuals develop chronic heart disease.
Although 60%-70% of people infected with T. cruzi never develop any symptoms, those who do can develop heart disease, including heart failure, stroke, life threatening ventricular arrhythmias, and cardiac arrest, according to the statement published in Circulation.
Chronic Chagas-related heart disease develops after several decades of the indeterminate, or subclinical, form of the disease following the initial acute infection. Potential risk factors for progression to the chronic stage include African ancestry, age, severity of acute infection, nutritional status, alcoholism, and their concomitant diseases.
In most studies, sudden death is the most common overall cause of death in patients with Chagas-related cardiomyopathy (55%-60%), followed by heart failure (25%-30%) and embolic events (10%-15%), according to the authors.
Benznidazole and nifurtimox are the only drugs with proven efficacy against Chagas disease, with benznidazole as the first-line treatment because it has better tolerance, is more widely available, and has more published data published on its efficacy. Furthermore, it is available in the United States, after the Food and Drug Administration granted fast-track approved 2017 for treatment of Chagas disease. Use of nifurtimox in the United States entails consultation with the Centers for Disease Control and prevention, according to the statement.
“More data are needed on the best practices for the treatment of Chagas cardiomyopathy. Because no specific clinical trials have been conducted, care for
patients with Chagas-induced ventricular dysfunction is extrapolated from general heart failure recommendations with unclear efficacy (and potential harm),” Dr. Pereira Nunes and her colleagues concluded.
One author disclosed receiving a research grant from Merck and speakers’ bureau and/or honoraria from Bayer; Biotronik, and Medtronic. The others had no relevant disclosures.
SOURCE: Nunes, MCP, et al., Circulation. 2018 Aug 20; doi: 10.1161/CIR.0000000000000599.
FROM CIRCULATION
Zika virus vaccine trial launches
A first-in-human trial of a live, attenuated Zika virus vaccine has begun, according to an announcement by the National Institutes of Health.
The vaccine, developed by scientists at the National Institute of Allergy and Infectious Diseases will be tested in 28 healthy, nonpregnant adults aged 18-50 years at two centers, the Johns Hopkins Bloomberg School of Public Health Center for Immunization Research in Baltimore, and the Vaccine Testing Center at the University of Vermont in Burlington.
The challenge virus in the vaccine is an attenuated genetic chimera consisting of a dengue virus 4 backbone that expresses Zika virus surface proteins designed to elicit an immune response. The virus was previously tested in rhesus macaque monkeys.
Study participants for the phase 1 trial, Evaluation of the Safety and Immunogenicity of the Live Attenuated Zika Vaccine rZIKV/D4Δ30-713 in Flavivirus-Naive Adults, (NCT03611946) will be assessed based on local and general adverse events to the vaccine and peak neutralizing antibody titer to Zika virus as measured up to 90 days after vaccination. The trial is expected to be reach primary completion by Dec. 31, 2018.
If the phase 1 trial is successful, the goal is to integrate the vaccine with a live, attenuated dengue vaccine candidate called TV003, which is designed to elicit antibodies against all four dengue virus serotypes. The TV003 experimental vaccine is currently under evaluation in a phase 3 clinical trial (NCT02406729) underway in Brazil. Both Zika and dengue viruses frequently are endemic in the same regions and a single vaccine against both diseases would be valued. Stephen Whitehead, PhD of NIAID’s Laboratory of Viral Diseases led the efforts to develop both experimental vaccines.
SOURCE: NIH, August 16, 2018. News Release.
A first-in-human trial of a live, attenuated Zika virus vaccine has begun, according to an announcement by the National Institutes of Health.
The vaccine, developed by scientists at the National Institute of Allergy and Infectious Diseases will be tested in 28 healthy, nonpregnant adults aged 18-50 years at two centers, the Johns Hopkins Bloomberg School of Public Health Center for Immunization Research in Baltimore, and the Vaccine Testing Center at the University of Vermont in Burlington.
The challenge virus in the vaccine is an attenuated genetic chimera consisting of a dengue virus 4 backbone that expresses Zika virus surface proteins designed to elicit an immune response. The virus was previously tested in rhesus macaque monkeys.
Study participants for the phase 1 trial, Evaluation of the Safety and Immunogenicity of the Live Attenuated Zika Vaccine rZIKV/D4Δ30-713 in Flavivirus-Naive Adults, (NCT03611946) will be assessed based on local and general adverse events to the vaccine and peak neutralizing antibody titer to Zika virus as measured up to 90 days after vaccination. The trial is expected to be reach primary completion by Dec. 31, 2018.
If the phase 1 trial is successful, the goal is to integrate the vaccine with a live, attenuated dengue vaccine candidate called TV003, which is designed to elicit antibodies against all four dengue virus serotypes. The TV003 experimental vaccine is currently under evaluation in a phase 3 clinical trial (NCT02406729) underway in Brazil. Both Zika and dengue viruses frequently are endemic in the same regions and a single vaccine against both diseases would be valued. Stephen Whitehead, PhD of NIAID’s Laboratory of Viral Diseases led the efforts to develop both experimental vaccines.
SOURCE: NIH, August 16, 2018. News Release.
A first-in-human trial of a live, attenuated Zika virus vaccine has begun, according to an announcement by the National Institutes of Health.
The vaccine, developed by scientists at the National Institute of Allergy and Infectious Diseases will be tested in 28 healthy, nonpregnant adults aged 18-50 years at two centers, the Johns Hopkins Bloomberg School of Public Health Center for Immunization Research in Baltimore, and the Vaccine Testing Center at the University of Vermont in Burlington.
The challenge virus in the vaccine is an attenuated genetic chimera consisting of a dengue virus 4 backbone that expresses Zika virus surface proteins designed to elicit an immune response. The virus was previously tested in rhesus macaque monkeys.
Study participants for the phase 1 trial, Evaluation of the Safety and Immunogenicity of the Live Attenuated Zika Vaccine rZIKV/D4Δ30-713 in Flavivirus-Naive Adults, (NCT03611946) will be assessed based on local and general adverse events to the vaccine and peak neutralizing antibody titer to Zika virus as measured up to 90 days after vaccination. The trial is expected to be reach primary completion by Dec. 31, 2018.
If the phase 1 trial is successful, the goal is to integrate the vaccine with a live, attenuated dengue vaccine candidate called TV003, which is designed to elicit antibodies against all four dengue virus serotypes. The TV003 experimental vaccine is currently under evaluation in a phase 3 clinical trial (NCT02406729) underway in Brazil. Both Zika and dengue viruses frequently are endemic in the same regions and a single vaccine against both diseases would be valued. Stephen Whitehead, PhD of NIAID’s Laboratory of Viral Diseases led the efforts to develop both experimental vaccines.
SOURCE: NIH, August 16, 2018. News Release.
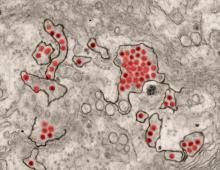
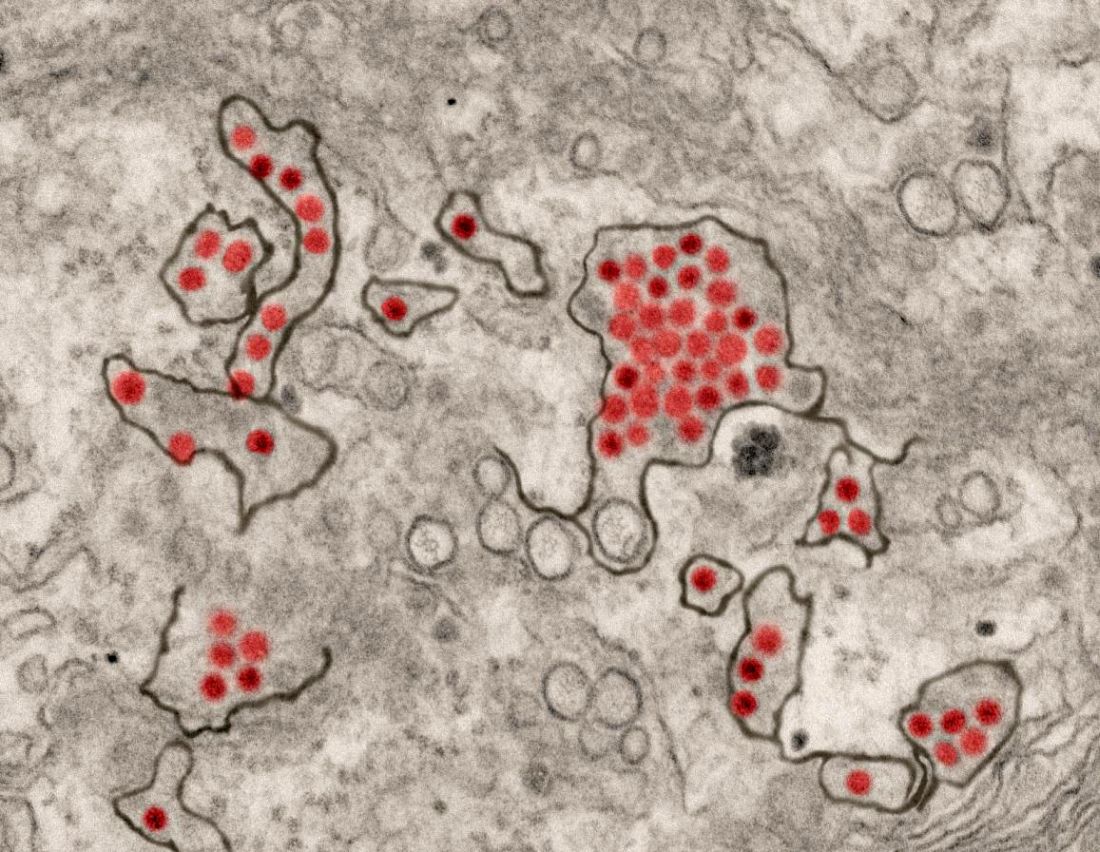
CDC supports Ebola response in DRC
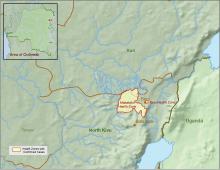
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
Next-gen sputum PCR panel boosts CAP diagnostics
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
NEW ORLEANS – A next-generation lower respiratory tract sputum polymerase chain reaction (PCR) film array panel identified etiologic pathogens in 100% of a group of patients hospitalized for community-acquired pneumonia, Kathryn Hendrickson, MD, reported at the annual meeting of the American College of Physicians.
The investigational new diagnostic assay, the BioFire Pneumonia Panel, is now under Food and Drug Administration review for marketing clearance. (CAP), observed Dr. Hendrickson, an internal medicine resident at Providence Portland (Ore.) Medical Center. The new product is designed to complement the currently available respiratory panels from BioFire.
“Rapid-detection results in less empiric antibiotic use in hospitalized patients. When it’s FDA approved, this investigational sputum PCR panel will simplify the diagnostic bundle while improving antibiotic stewardship,” she observed.
She presented a prospective study of 63 patients with CAP hospitalized at the medical center, all of whom were evaluated by two laboratory methods: the hospital’s standard bundle of diagnostic tests and the new BioFire film array panel. The purpose was to determine if there was a difference between the two tests in the detection rate of viral and/or bacterial pathogens as well as the clinical significance of any such differences; that is, was there an impact on days of treatment and length of hospital stay?
Traditional diagnostic methods detect an etiologic pathogen in at best half of hospitalized CAP patients, and the results take too much time. So Providence Portland Medical Center adopted as its standard diagnostic bundle a nasopharyngeal swab and a BioFire film array PCR that’s currently on the market and can detect nine viruses and three bacteria, along with urine antigens for Legionella sp. and Streptococcus pneumoniae, nucleic acid amplification testing for S. pneumoniae and Staphylococcus aureus, and blood and sputum cultures. In contrast, the investigational panel probes for 17 viruses, 18 bacterial pathogens, and seven antibiotic-resistant genes; it also measures procalcitonin levels in order to distinguish between bacterial colonization and invasion.
The new BioFire Pneumonia Panel detected a mean of 1.4 species of pathogenic bacteria in 79% of patients, while the standard diagnostic bundle detected 0.7 species in 59% of patients. The investigational panel identified a mean of 1.0 species of viral pathogens in 86% of the CAP patients; the standard bundle detected a mean of 0.6 species in 56%.
All told, any CAP pathogen was detected in 100% of patients using the new panel, with a mean of 2.5 different pathogens identified. The standard bundle detected any pathogen in 84% of patients, with half as many different pathogens found, according to Dr. Hendrickson.
A peak procalcitonin level of 0.25 ng/mL or less, which was defined as bacterial colonization, was associated with a mean 7 days of treatment, while a level above that threshold was associated with 11.3 days of treatment. Patients with a peak procalcitonin of 0.25 ng/mL or less had an average hospital length of stay of 5.9 days, versus 7.8 days for those with a higher procalcitonin indicative of bacterial invasion.
The new biofilm assay reports information about the abundance of 15 of the 18 bacterial targets in the sample. However, in contrast to peak procalcitonin, Dr. Hendrickson and her coinvestigators didn’t find this bacterial quantitation feature to be substantially more useful than a coin flip in distinguishing bacterial colonization from invasion.
She reported having no financial conflicts regarding the head-to-head comparative study, which was supported by BioFire Diagnostics.
REPORTING FROM ACP INTERNAL MEDICINE
Key clinical point: A new CAP diagnostic panel represents a significant advance in clinical care.
Major finding: The investigational BioFire Pneumonia Panel identified specific pathogens in 100% of patients hospitalized for CAP, compared with 84% using the hospital’s standard test bundle.
Study details: This was a prospective head-to-head study comparing two approaches to identification of specific pathogens in 63 patients hospitalized for CAP.
Disclosures: The study was supported by BioFire Diagnostics. The presenter reported having no financial conflicts.
FDA issues Ebola preparedness statement
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
MDedge Daily News: Keeping patients summer safe
Memorial Day is the traditional kickoff to summer, so patients will need information on some important topics. To that end, the Food and Drug Administration reminds that there is no such thing as a supplement that prevents sun damage. The CDC warns that nasty things happen when people (kids) drink the pool water. And the federal government advises on what should be included in an approved insect repellent.
Listen to the MDedge Daily News podcast for all the details.
Memorial Day is the traditional kickoff to summer, so patients will need information on some important topics. To that end, the Food and Drug Administration reminds that there is no such thing as a supplement that prevents sun damage. The CDC warns that nasty things happen when people (kids) drink the pool water. And the federal government advises on what should be included in an approved insect repellent.
Listen to the MDedge Daily News podcast for all the details.
Memorial Day is the traditional kickoff to summer, so patients will need information on some important topics. To that end, the Food and Drug Administration reminds that there is no such thing as a supplement that prevents sun damage. The CDC warns that nasty things happen when people (kids) drink the pool water. And the federal government advises on what should be included in an approved insect repellent.
Listen to the MDedge Daily News podcast for all the details.
Simvastatin, atorvastatin cut mortality risk for sepsis patients
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
The statin-sepsis mortality link will probably never be definitively proven, but the study by Lee and colleagues gives us the best data so far on this intriguing connection, Steven Q. Simpson, MD and Joel D. Mermis, MD wrote in an accompanying editorial.
“It is unlikely that prospective randomized trials of statins for prevention of sepsis mortality will ever be undertaken, owing to the sheer number of patients that would require randomization in order to have adequate numbers who actually develop sepsis,” the colleagues wrote. “We believe that the next best thing to randomization and a prospective trial is exactly what the authors have done – identify a cohort, track them through time, even if nonconcurrently, and match cases to controls by propensity matching on important clinical characteristics.”
Nevertheless, the two said, “This brings us to one aspect of the study that leaves open a window for some doubt.”
Lee et al. extracted their data from a large national insurance claims database. These systems “are commonly believed to overestimate sepsis incidence,” Dr. Simpson and Dr. Mermis wrote. A 2009 U.S. study bore this out, they said. “That study showed that in the U.S in 2014, there were approximately 1.7 million cases of sepsis in a population of 330 million, for an annual incidence rate of five sepsis cases per 1,000 patient-years.”
However, a “quick calculation” of the Taiwan data suggests that the annual sepsis caseload is about 5,200 per year in a population of 23 million at risk – an annual incidence of only 0.2 cases per 1,000 patient-years.
“This represents an order of magnitude difference in sepsis incidence between the U.S. and Taiwan, providing some issues to ponder. Does Taiwan indeed have a lower incidence of sepsis by that much? If so, is the lower incidence related to genetics, environment, health care access, or other factors?
“Although Lee et al. have provided us with data of the highest quality that we can likely hope for, the book may not be quite closed, yet.”
Dr. Mermis and Dr. Simpson are pulmonologists at the University of Kansas, Kansas City. They made their comments in an editorial published in the April issue of CHEST® (Mermis JD and Simpson SQ. CHEST. 2018 April. doi: 10.1016/j.chest.2017.12.004.)
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
a large health care database review has determined.
Among almost 53,000 sepsis patients, those who had been taking simvastatin were 28% less likely to die within 30 days of a sepsis admission than were patients not taking a statin. Atorvastatin conferred a similar significant survival benefit, reducing the risk of death by 22%, Chien-Chang Lee, MD and his colleagues wrote in the April issue of the journal CHEST®.
The drugs also exert a direct antimicrobial effect, he asserted.
“Of note, simvastatin was shown by several reports to have the most potent antibacterial activity,” targeting both methicillin-resistant and -sensitive Staphylococcus aureus, as well as gram negative and positive bacteria.
Dr. Lee and his colleagues extracted mortality and statin prescription data from the Taiwan National Health Insurance Database from 2000-2011. They looked at 30- and 90-day mortality in 52,737 patients who developed sepsis; the statins of interest were atorvastatin, simvastatin, and rosuvastatin. Patients had to have been taking the medication for at least 30 days before sepsis onset to be included, and patients taking more than one statin were excluded from the analysis.
Patients were a mean of 69 years old. About half had a lower respiratory infection. The remainder had infections within the abdomen, the biliary or urinary tract, skin, or orthopedic infections. There were no significant differences in comorbidities or in other medications taken among the three statin groups or the nonusers.
Of the entire cohort, 17% died by 30 days and nearly 23% by 90 days. Compared with those who had never received a statin, the statin users were 12% less likely to die by 30 days (hazard ratio, 0.88). Mortality at 90 days was also decreased, when compared with nonusers (HR, 0.93).
Simvastatin demonstrated the greatest benefit, with a 28% decreased risk of 30-day mortality (HR, 0.72). Atorvastatin followed, with a 22% risk reduction (HR, 0.78). Rosuvastatin exerted a nonsignificant 13% benefit.
The authors then examined 90-day mortality risks for the patients with a propensity matching score using a subgroup comprising 536 simvastatin users, 536 atorvastatin users, and 536 rosuvastatin users. Simvastatin was associated with a 23% reduction in 30-day mortality risk (HR, 0.77) and atorvastatin with a 21% reduction (HR, 0.79), when compared with rosuvastatin.
Statins’ antimicrobial properties are probably partially caused by their inactivation of the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase pathway, Dr. Lee and his colleagues noted. In addition to being vital for cholesterol synthesis, this pathway “also contributes to the production of isoprenoids and lipid compounds that are essential for cell signaling and structure in the pathogen. Secondly, the chemical property of different types of statins may affect their targeting to bacteria. The lipophilic properties of simvastatin or atorvastatin may allow better binding to bacteria cell walls than the hydrophilic properties of rosuvastatin.”
The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
FROM CHEST
Key clinical point: Simvastatin and atorvastatin were associated with decreased mortality risk among sepsis patients.
Major finding: Compared with those not taking the drugs, those taking simvastatin were 28% less likely to die by 30 days, and those taking atorvastatin were 22% less likely.
Study details: The database study comprised almost 54,000 sepsis cases over 11 years.
Disclosures: The study was funded by the Taiwan National Science Foundation and Taiwan National Ministry of Science and Technology. Dr. Lee had no financial conflicts.
Source: Lee C-C et al. CHEST. 2018 April;153(4):769-70.
CDC: Beware Brazil yellow fever outbreak
The Centers for Disease Control and Prevention recommends that travelers who haven’t been vaccinated against yellow fever should avoid travel to Brazil, according to a media teleconference by CDC officials.
“The most important new recommendation ... is that travelers should not go to these yellow fever hot spots in Brazil, unless they are vaccinated,” stated Martin Cetron, MD, director of the Division of Global Migration and Quarantine at the CDC. “Health officials in Brazil recently confirmed more than 920 cases of yellow fever, including more than 300 deaths, during this outbreak” he added.
Since the beginning of 2018, 10 travel-related cases of yellow fever have been reported among international travelers returning from Brazil. Four of these travelers died. All 10 travelers had not received the yellow fever vaccine. Of these 10 travelers, 8 acquired the disease on Ilha Grande, an island off the coast of Rio De Janeiro that is popular among tourists.
The CDC is urging travelers to get vaccinated because of the potentially fatal effects of yellow fever. Vaccinations are recommended for any eligible person 9 months of age or older traveling to Brazil, specifically Espírito Santo, São Paulo, and Rio de Janeiro states, and certain cities in Bahia state, as well as Ilha Grande in particular.
Individuals heading to Brazil should receive the vaccination at least 10 days before travel. If a traveler is unvaccinated and cannot get the vaccination in the appropriate amount of time, areas where vaccination is recommended should be avoided.
The Food and Drug Administration–approved yellow fever vaccine, YF-VAX, is not currently available because of manufacturing issues. Stamaril, another yellow fever vaccine, is available at a limited number of yellow fever vaccination clinics in the United States.
In light of these supply issues, the CDC has provided resources to locate yellow fever vaccination clinics.
Dr. Cetron reemphasized that unvaccinated individuals planning to vacation in Brazil may want to reconsider their travel plans.
“People who have never been vaccinated against yellow fever should not travel to the areas in Brazil affected by the outbreak. Particularly the hot spot of Ilha Grande.”
Information for clinicians and travelers is available on the travel notice portion of the CDC site. The travel notice for Brazil includes a map of the yellow fever–affected areas in Brazil, as well as other informational resources.
The related CDC Morbidity and Mortality Weekly Report is available online.
The Centers for Disease Control and Prevention recommends that travelers who haven’t been vaccinated against yellow fever should avoid travel to Brazil, according to a media teleconference by CDC officials.
“The most important new recommendation ... is that travelers should not go to these yellow fever hot spots in Brazil, unless they are vaccinated,” stated Martin Cetron, MD, director of the Division of Global Migration and Quarantine at the CDC. “Health officials in Brazil recently confirmed more than 920 cases of yellow fever, including more than 300 deaths, during this outbreak” he added.
Since the beginning of 2018, 10 travel-related cases of yellow fever have been reported among international travelers returning from Brazil. Four of these travelers died. All 10 travelers had not received the yellow fever vaccine. Of these 10 travelers, 8 acquired the disease on Ilha Grande, an island off the coast of Rio De Janeiro that is popular among tourists.
The CDC is urging travelers to get vaccinated because of the potentially fatal effects of yellow fever. Vaccinations are recommended for any eligible person 9 months of age or older traveling to Brazil, specifically Espírito Santo, São Paulo, and Rio de Janeiro states, and certain cities in Bahia state, as well as Ilha Grande in particular.
Individuals heading to Brazil should receive the vaccination at least 10 days before travel. If a traveler is unvaccinated and cannot get the vaccination in the appropriate amount of time, areas where vaccination is recommended should be avoided.
The Food and Drug Administration–approved yellow fever vaccine, YF-VAX, is not currently available because of manufacturing issues. Stamaril, another yellow fever vaccine, is available at a limited number of yellow fever vaccination clinics in the United States.
In light of these supply issues, the CDC has provided resources to locate yellow fever vaccination clinics.
Dr. Cetron reemphasized that unvaccinated individuals planning to vacation in Brazil may want to reconsider their travel plans.
“People who have never been vaccinated against yellow fever should not travel to the areas in Brazil affected by the outbreak. Particularly the hot spot of Ilha Grande.”
Information for clinicians and travelers is available on the travel notice portion of the CDC site. The travel notice for Brazil includes a map of the yellow fever–affected areas in Brazil, as well as other informational resources.
The related CDC Morbidity and Mortality Weekly Report is available online.
The Centers for Disease Control and Prevention recommends that travelers who haven’t been vaccinated against yellow fever should avoid travel to Brazil, according to a media teleconference by CDC officials.
“The most important new recommendation ... is that travelers should not go to these yellow fever hot spots in Brazil, unless they are vaccinated,” stated Martin Cetron, MD, director of the Division of Global Migration and Quarantine at the CDC. “Health officials in Brazil recently confirmed more than 920 cases of yellow fever, including more than 300 deaths, during this outbreak” he added.
Since the beginning of 2018, 10 travel-related cases of yellow fever have been reported among international travelers returning from Brazil. Four of these travelers died. All 10 travelers had not received the yellow fever vaccine. Of these 10 travelers, 8 acquired the disease on Ilha Grande, an island off the coast of Rio De Janeiro that is popular among tourists.
The CDC is urging travelers to get vaccinated because of the potentially fatal effects of yellow fever. Vaccinations are recommended for any eligible person 9 months of age or older traveling to Brazil, specifically Espírito Santo, São Paulo, and Rio de Janeiro states, and certain cities in Bahia state, as well as Ilha Grande in particular.
Individuals heading to Brazil should receive the vaccination at least 10 days before travel. If a traveler is unvaccinated and cannot get the vaccination in the appropriate amount of time, areas where vaccination is recommended should be avoided.
The Food and Drug Administration–approved yellow fever vaccine, YF-VAX, is not currently available because of manufacturing issues. Stamaril, another yellow fever vaccine, is available at a limited number of yellow fever vaccination clinics in the United States.
In light of these supply issues, the CDC has provided resources to locate yellow fever vaccination clinics.
Dr. Cetron reemphasized that unvaccinated individuals planning to vacation in Brazil may want to reconsider their travel plans.
“People who have never been vaccinated against yellow fever should not travel to the areas in Brazil affected by the outbreak. Particularly the hot spot of Ilha Grande.”
Information for clinicians and travelers is available on the travel notice portion of the CDC site. The travel notice for Brazil includes a map of the yellow fever–affected areas in Brazil, as well as other informational resources.
The related CDC Morbidity and Mortality Weekly Report is available online.
Dr. Paul E. Marik proclaims end to corticosteroid monotherapy for sepsis
SAN ANTONIO – While critical care specialists await more data on a so-called sepsis cocktail with varying degrees of hope and skepticism, Paul E. Marik, MD, FCCP, has proclaimed the dawning of a new era.
Dr. Marik became a celebrity in the critical care medicine community after he and his colleagues reported the results of his retrospective study evaluating the combination of hydrocortisone, vitamin C, and thiamine for treatment of severe sepsis and septic shock (Chest. 2017 June. doi: 10.1016/j.chest.2016.11.036).
Since this study, several physicians have already been putting Dr. Marik’s method to practice, the investigator and audience members noted during a session at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
in his presentation at the meeting.
These comments echoed Dr. Marik’s May 2017 editorial in Critical Care Medicine, in which he suggested that critically ill and injured patients may benefit from combination therapy with hydrocortisone and vitamin C (Crit Care Med 2017 May;45[5]910-1).
That editorial was quickly followed by the report on Dr. Marik and colleagues’ before-after study, in which hospital mortality was 8.5%, versus 0.4% in the treatment and control groups, respectively (P less than .001). This finding led the investigators to suggest that intravenous vitamin C administered along with corticosteroids and thiamine is “effective” in reducing mortality, in their paper published in CHEST®.
During Dr. Marik’s presentation at the meeting, he noted that he had been “misquoted” with regard to the finality of his study’s results. The final line of the CHEST® paper reads, “Additional studies are required to confirm these preliminary findings,” he emphasized.
Nevertheless, Dr. Marik alluded to a “big paradigm shift” in the treatment of sepsis.
“Our experience has been echoed by now hundreds, if not thousands, of clinicians across the world,” said Dr. Marik, chief of the division of pulmonary and critical care medicine, Eastern Virginia Medical School, Norfolk.
He recounted an anecdotal case submitted by “Josh from Ohio” describing an elderly man who was “started on cocktail and within a day his pressor requirements melted away and he was extubated.” Quoting “Josh from Ohio,” Dr. Mark continued, “Tomorrow he will probably leave the ICU with no residual organ dysfunction, no volume overload, (and) no ICU complications.”
Eddy Gutierrez, MD, of Jacksonville, Fla., noted in a question-and-answer period that he has had “positive results” with a similar approach.
“When we first learned about the vitamin C and the ‘Marik protocol,’ so to speak, I was in fellowship and I got laughed at,” Dr. Gutierrez said. “Nobody would let me try it.”
Others are taking a wait-and-see approach.
Greg S. Martin, MD, secretary of the Society of Critical Care Medicine, said in an interview that there are “at least two schools of thought” among critical care specialists regarding the use of hydrocortisone, vitamin C, and thiamine for treatment of sepsis and septic shock.
“One school of thought is that this is incredibly important if this is even fractionally as effective as what [Dr. Marik] showed, because we have not found an effective therapy for sepsis,” said Dr. Martin, associate professor of medicine at Grady Memorial Hospital, Atlanta.
“The contrarian approach is to say, ‘yes, but this seems remarkably unlikely to be as effective as what he has shown,’ ” Dr. Martin added. “Particularly in sepsis, people are very skeptical of whether a drug or a drug combination is going to be as effective when you really get down to a high-quality randomized controlled trial that would be the definitive level of evidence.”
The wait may not be long for at least some data. Multiple clinical trials are recruiting or planned, according to Dr. Marik. These included a 140-patient U.S. randomized, double-blind trial of vitamin C, hydrocortisone, and thiamine vs. placebo that started in February 2018 and is expected to be completed by February 2019, according to the study’s ClinicalTrials.gov listing.
“The good news is some people think this is of value,” Dr. Marik said.
As part of his presentation, Dr. Marik reported a disclosure related to Baxter (advisory board).
SAN ANTONIO – While critical care specialists await more data on a so-called sepsis cocktail with varying degrees of hope and skepticism, Paul E. Marik, MD, FCCP, has proclaimed the dawning of a new era.
Dr. Marik became a celebrity in the critical care medicine community after he and his colleagues reported the results of his retrospective study evaluating the combination of hydrocortisone, vitamin C, and thiamine for treatment of severe sepsis and septic shock (Chest. 2017 June. doi: 10.1016/j.chest.2016.11.036).
Since this study, several physicians have already been putting Dr. Marik’s method to practice, the investigator and audience members noted during a session at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
in his presentation at the meeting.
These comments echoed Dr. Marik’s May 2017 editorial in Critical Care Medicine, in which he suggested that critically ill and injured patients may benefit from combination therapy with hydrocortisone and vitamin C (Crit Care Med 2017 May;45[5]910-1).
That editorial was quickly followed by the report on Dr. Marik and colleagues’ before-after study, in which hospital mortality was 8.5%, versus 0.4% in the treatment and control groups, respectively (P less than .001). This finding led the investigators to suggest that intravenous vitamin C administered along with corticosteroids and thiamine is “effective” in reducing mortality, in their paper published in CHEST®.
During Dr. Marik’s presentation at the meeting, he noted that he had been “misquoted” with regard to the finality of his study’s results. The final line of the CHEST® paper reads, “Additional studies are required to confirm these preliminary findings,” he emphasized.
Nevertheless, Dr. Marik alluded to a “big paradigm shift” in the treatment of sepsis.
“Our experience has been echoed by now hundreds, if not thousands, of clinicians across the world,” said Dr. Marik, chief of the division of pulmonary and critical care medicine, Eastern Virginia Medical School, Norfolk.
He recounted an anecdotal case submitted by “Josh from Ohio” describing an elderly man who was “started on cocktail and within a day his pressor requirements melted away and he was extubated.” Quoting “Josh from Ohio,” Dr. Mark continued, “Tomorrow he will probably leave the ICU with no residual organ dysfunction, no volume overload, (and) no ICU complications.”
Eddy Gutierrez, MD, of Jacksonville, Fla., noted in a question-and-answer period that he has had “positive results” with a similar approach.
“When we first learned about the vitamin C and the ‘Marik protocol,’ so to speak, I was in fellowship and I got laughed at,” Dr. Gutierrez said. “Nobody would let me try it.”
Others are taking a wait-and-see approach.
Greg S. Martin, MD, secretary of the Society of Critical Care Medicine, said in an interview that there are “at least two schools of thought” among critical care specialists regarding the use of hydrocortisone, vitamin C, and thiamine for treatment of sepsis and septic shock.
“One school of thought is that this is incredibly important if this is even fractionally as effective as what [Dr. Marik] showed, because we have not found an effective therapy for sepsis,” said Dr. Martin, associate professor of medicine at Grady Memorial Hospital, Atlanta.
“The contrarian approach is to say, ‘yes, but this seems remarkably unlikely to be as effective as what he has shown,’ ” Dr. Martin added. “Particularly in sepsis, people are very skeptical of whether a drug or a drug combination is going to be as effective when you really get down to a high-quality randomized controlled trial that would be the definitive level of evidence.”
The wait may not be long for at least some data. Multiple clinical trials are recruiting or planned, according to Dr. Marik. These included a 140-patient U.S. randomized, double-blind trial of vitamin C, hydrocortisone, and thiamine vs. placebo that started in February 2018 and is expected to be completed by February 2019, according to the study’s ClinicalTrials.gov listing.
“The good news is some people think this is of value,” Dr. Marik said.
As part of his presentation, Dr. Marik reported a disclosure related to Baxter (advisory board).
SAN ANTONIO – While critical care specialists await more data on a so-called sepsis cocktail with varying degrees of hope and skepticism, Paul E. Marik, MD, FCCP, has proclaimed the dawning of a new era.
Dr. Marik became a celebrity in the critical care medicine community after he and his colleagues reported the results of his retrospective study evaluating the combination of hydrocortisone, vitamin C, and thiamine for treatment of severe sepsis and septic shock (Chest. 2017 June. doi: 10.1016/j.chest.2016.11.036).
Since this study, several physicians have already been putting Dr. Marik’s method to practice, the investigator and audience members noted during a session at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
in his presentation at the meeting.
These comments echoed Dr. Marik’s May 2017 editorial in Critical Care Medicine, in which he suggested that critically ill and injured patients may benefit from combination therapy with hydrocortisone and vitamin C (Crit Care Med 2017 May;45[5]910-1).
That editorial was quickly followed by the report on Dr. Marik and colleagues’ before-after study, in which hospital mortality was 8.5%, versus 0.4% in the treatment and control groups, respectively (P less than .001). This finding led the investigators to suggest that intravenous vitamin C administered along with corticosteroids and thiamine is “effective” in reducing mortality, in their paper published in CHEST®.
During Dr. Marik’s presentation at the meeting, he noted that he had been “misquoted” with regard to the finality of his study’s results. The final line of the CHEST® paper reads, “Additional studies are required to confirm these preliminary findings,” he emphasized.
Nevertheless, Dr. Marik alluded to a “big paradigm shift” in the treatment of sepsis.
“Our experience has been echoed by now hundreds, if not thousands, of clinicians across the world,” said Dr. Marik, chief of the division of pulmonary and critical care medicine, Eastern Virginia Medical School, Norfolk.
He recounted an anecdotal case submitted by “Josh from Ohio” describing an elderly man who was “started on cocktail and within a day his pressor requirements melted away and he was extubated.” Quoting “Josh from Ohio,” Dr. Mark continued, “Tomorrow he will probably leave the ICU with no residual organ dysfunction, no volume overload, (and) no ICU complications.”
Eddy Gutierrez, MD, of Jacksonville, Fla., noted in a question-and-answer period that he has had “positive results” with a similar approach.
“When we first learned about the vitamin C and the ‘Marik protocol,’ so to speak, I was in fellowship and I got laughed at,” Dr. Gutierrez said. “Nobody would let me try it.”
Others are taking a wait-and-see approach.
Greg S. Martin, MD, secretary of the Society of Critical Care Medicine, said in an interview that there are “at least two schools of thought” among critical care specialists regarding the use of hydrocortisone, vitamin C, and thiamine for treatment of sepsis and septic shock.
“One school of thought is that this is incredibly important if this is even fractionally as effective as what [Dr. Marik] showed, because we have not found an effective therapy for sepsis,” said Dr. Martin, associate professor of medicine at Grady Memorial Hospital, Atlanta.
“The contrarian approach is to say, ‘yes, but this seems remarkably unlikely to be as effective as what he has shown,’ ” Dr. Martin added. “Particularly in sepsis, people are very skeptical of whether a drug or a drug combination is going to be as effective when you really get down to a high-quality randomized controlled trial that would be the definitive level of evidence.”
The wait may not be long for at least some data. Multiple clinical trials are recruiting or planned, according to Dr. Marik. These included a 140-patient U.S. randomized, double-blind trial of vitamin C, hydrocortisone, and thiamine vs. placebo that started in February 2018 and is expected to be completed by February 2019, according to the study’s ClinicalTrials.gov listing.
“The good news is some people think this is of value,” Dr. Marik said.
As part of his presentation, Dr. Marik reported a disclosure related to Baxter (advisory board).
REPORTING FROM CCC47