User login
U.S. kidney transplants grow in number and success
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
During 2016-2019, U.S. centers performed kidney transplants in nearly 77,000 patients, a jump of almost 25% compared with 4-year averages of about 62,000 patients throughout 2004-2015. That works out to about 15,000 more patients receiving donor kidneys, Sundaram Hariharan, MD, and associates reported in the New England Journal of Medicine in a review of all U.S. renal transplantations performed during 1996-2019.
Coupled with the volume uptick during this 24-year period were new lows in graft losses and patient deaths. By 2018, mortality during the first year following transplantation occurred at about a 1% rate among patients who had received a kidney from a living donor, and at about a 3% rate when the organ came from a deceased donor, nearly half the rate of 2 decades earlier, in 1996. Rates of first-year graft loss during 2017 were also about half of what they had been in 1996, occurring in about 2% of patients who received a living donor organ and in about 6% of those who got a kidney from a deceased donor during 2017.
“Twenty years ago, kidney transplantation was the preferred option compared with dialysis, and even more so now,” summed up Dr. Hariharan, a senior transplant nephrologist and professor of medicine and surgery at the University of Pittsburgh Medical Center and first author of the report. Kidney transplantation survival at U.S. centers “improved steadily over the past 24 years, despite patient variables becoming worse,” he said in an interview.
Kidney recipients are older, more obese, and have more prevalent diabetes
During the period studied, kidney transplant recipients became on average older and more obese, and had a higher prevalence of diabetes; the age of organ donors grew as well. The prevalence of diabetes among patients who received a kidney from a deceased donor increased from 24% during 1996-1999 to 36% during 2016-2019, while diabetes prevalence among recipients of an organ from a living donor rose from 25% in 1996-1999 to 29% during 2016-2019.
The improved graft and patient survival numbers “are very encouraging trends,” said Michelle A. Josephson, MD, professor and medical director of kidney transplantation at the University of Chicago, who was not involved with the report. “We have been hearing for a number of years that short-term graft survival had improved, but I’m thrilled to learn that long-term survival has also improved.”
The report documented 10-year survival of graft recipients during 2008-2011 of 67%, up from 61% during 1996-1999, and a 10-year overall graft survival rate of 54% in the 2008-2011 cohort, an improvement from the 42% rate in patients who received their organs in 1996-1999, changes Dr. Hariharan characterized as “modest.”
These improvements in long-term graft and patient survival are “meaningful, and particularly notable that outcomes improved despite increased complexity of the transplant population,” said Krista L. Lentine, MD, PhD, professor and medical director of living donation at Saint Louis University. But “despite these improvements, long-term graft survival remains limited,” she cautioned, especially because of risks for substantial complications from chronic immunosuppressive treatment including infection, cancer, glucose intolerance, and dyslipidemia.
The analysis reported by Dr. Hariharan and his associates used data collected by the Scientific Registry of Transplant Patients, run under contract with the U.S. Department of Health and Human Services, which has tracked all patients who have had kidney transplants at U.S. centers since the late 1980s, said Dr. Hariharan. The database included just over 362,000 total transplants during the 24-year period studied, with 36% of all transplants involving organs from living donors with the remaining patients receiving kidneys from deceased donors.
Living donations still stagnant; deceased-donor kidneys rise
The data showed that the rate of transplants from living donors was stagnant for 2 decades, with 22,525 patients transplanted during 2000-2003, and 23,746 transplanted during 2016-2019, with very similar rates during the intervening years. The recent spurt in transplants during 2016-2019 compared with the preceding decade depended almost entirely on kidneys from deceased donors. This rate jumped from the steady, slow rise it showed during 1996-2015, when deceased-donor transplants rose from about 30,000 during 1996-1999 to about 41,000 during 2012-2015, to a more dramatic increase of about 12,000 additional transplants during the most recent period, adding up to a total of more than 53,000 transplants from deceased donors during 2016-2019.
“I strongly recommend organs from living donors” when feasible, said Dr. Hariharan. “At some centers, a high proportion of transplants use living donors, but not at other centers,” he said.
It’s unknown why transplants using organs from deceased donors has shown this growth, but Dr. Hariharan suggested a multifactorial explanation. Those factors include growth in the number of patients with end-stage renal disease who require dialysis, increased numbers of patients listed for kidney transplant, new approaches that allow organs from older donors and those infected with pathogens such as hepatitis C virus or HIV, greater numbers of people and families agreeing to donate organs, and possibly the opioid crisis that may have led to increased organ donation. The number of U.S. centers performing kidney transplants rose from fewer than 200 about a quarter of a century ago to about 250 today, he added.
‘Immuno Bill’ guarantees Medicare coverage for immunosuppression
Dr. Hariharan voiced optimism that graft and patient survival rates will continue to improve going forward. One factor will likely be the passage in late 2020 of the “Immuno Bill” by the U.S. Congress, which among other things mandated ongoing coverage starting in 2023 for immunosuppressive drugs for all Medicare beneficiaries with a kidney transplant. Until then, Medicare provides coverage for only 36 months, a time limit that has resulted in nearly 400 kidney recipients annually losing coverage of their immunosuppression medications.
Dr. Hariharan and coauthors called the existing potential for discontinuation of immunosuppressive drug an “unnecessary impediment to long-term survival for which patients and society paid a heavy price.”
“Kidney transplantation, especially from living donors, offers patients with kidney failure the best chance for long-term survival and improved quality of life, with lower cost to the health care system,” Dr. Lentine said in an interview. Despite the many positive trends detailed in the report from Dr. Hariharan and coauthors, “the vast majority of the more than 700,000 people in the United States with kidney failure will not have an opportunity to receive a transplant due to limitations in organ supply.” And many patients who receive a kidney transplant eventually must resume dialysis because of “limited long-term graft survival resulting from allograft nephropathy, recurrent native disease, medication nonadherence, or other causes.” Plus many potentially transplantable organs go unused.
Dr. Lentine cited a position statement issued in July 2021 by the National Kidney Foundation that made several recommendations on how to improve access to kidney transplants and improve outcomes. “Expanding opportunities for safe living donation, eliminating racial disparities in living-donor access, improving wait-list access and transport readiness, maximizing use of deceased-donor organs, and extending graft longevity are critical priorities,” said Dr. Lentine, lead author on the statement.
“For many or even most patients with kidney failure transplantation is the optimal form of renal replacement. The better recent outcomes and evolving management strategies make transplantation an even more attractive option,” said Dr. Josephson. Improved outcomes among U.S. transplant patients also highlights the “importance of increasing access to kidney transplantation” for all people with kidney failure who could benefit from this treatment, she added.
Dr. Hariharan and Dr. Lentine had no relevant disclosures. Dr. Josephson has been a consultant to UCB and has an ownership interest in Seagen.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
US Preventive Services Task Force lowers diabetes screening age for overweight
The United States Preventive Services Task Force has updated its recommendation on the age of screening for prediabetes and type 2 diabetes in the primary care setting – lowering the age from 40 to 35 years for asymptomatic patients who are overweight or obese and encouraging greater interventions when patients do show a risk.
“The USPSTF concludes with moderate certainty that screening for prediabetes and type 2 diabetes and offering or referring patients with prediabetes to effective preventive interventions has a moderate net benefit,” the task force concludes in its recommendation, published Aug. 24 in JAMA.
“Clinicians should offer or refer patients with prediabetes to effective preventive interventions,” they write.
Experts commenting on the issue strongly emphasize that it’s not just the screening, but the subsequent intervention that is needed to make a difference.
“If young adults newly identified with abnormal glucose metabolism do not receive the needed intensive behavioral change support, screening may provide no benefit,” write Richard W. Grant, MD, MPH, and colleagues in an editorial published with the recommendation.
“Given the role of our obesogenic and physically inactive society in the shift toward earlier onset of diabetes, efforts to increase screening and recognition of abnormal glucose metabolism must be coupled with robust public health measures to address the underlying contributors.”
BMI cutoff lower for at-risk ethnic populations
The recommendation, which updates the task force’s 2015 guideline, carries a “B” classification, meaning the USPSTF has high certainty that the net benefit is moderate. It now specifies screening from age 35to 70 for persons classified as overweight (body mass index at least 25) or obese (BMI at least 30) and recommends referral to preventive interventions when patients are found to have prediabetes.
In addition to recommendations of lifestyle changes, such as diet and physical activity, the task force also endorses the diabetes drug metformin as a beneficial intervention in the prevention or delay of diabetes, while noting fewer overall health benefits from metformin than from the lifestyle changes.
A lower BMI cutoff of at least 23 is recommended for diabetes screening of Asian Americans, and, importantly, screening for prediabetes and diabetes should be considered at an even earlier age if the patient is from a population with a disproportionately high prevalence of diabetes, including American Indian/Alaska Native, Black, Hawaiian/Pacific Islander, Hispanic/Latino, the task force recommends.
Screening tests should include fasting plasma glucose, hemoglobin A1c, or an oral glucose tolerance test. Although screening every 3 years “may be a reasonable approach for adults with normal blood glucose levels,” the task force adds that “the optimal screening interval for adults with an initial normal glucose test result is uncertain.”
Data review: Few with prediabetes know they have it
The need for the update was prompted by troubling data showing increasing diabetes rates despite early signs that can and should be identified and acted upon in the primary care setting to prevent disease progression.
Data from the Centers for Disease Control and Prevention, for instance, show that while 13% of all U.S. adults 18 years or older have diabetes and 35% meet criteria for prediabetes, as many as 21% of those with diabetes were not aware of or did not report having the disease. Furthermore, only a small fraction – 15% of those with prediabetes – said they had been told by a health professional that they had this condition, the task force notes.
The task force’s final recommendation was based on a systematic review of evidence regarding the screening of asymptomatic, nonpregnant adults and the harms and benefits of interventions, such as physical activity, behavioral counseling, or pharmacotherapy.
Among key evidence supporting the lower age was a 2014 study showing that the number of people necessary to obtain one positive test for diabetes with screening sharply drops from 80 among those aged 30-34 years to just 31 among those aged 36-39.
Opportunistic universal screening of eligible people aged 35 and older would yield a ratio of 1 out of just 15 to spot a positive test, the authors of that study reported.
In addition, a large cohort study in more than 77,000 people with prediabetes strongly links the risk of developing diabetes with increases in A1c level and with increasing BMI.
ADA recommendations differ
The new recommendations differ from American Diabetes Association guidelines, which call for diabetes screening at all ages for people who are overweight or obese and who have one or more risk factors, such as physical inactivity or a first-degree relative with diabetes. If results are normal, repeat screening at least every 3 years is recommended.
The ADA further recommends universal screening for all adults 45 years and older, regardless of their risk factors.
For the screening of adults over 45, the ADA recommends using a fasting plasma glucose level, 2-hour plasma glucose level during a 75-g oral glucose tolerance test, or A1c level, regardless of risk factors.
The American Association of Clinical Endocrinology also recommends universal screening for prediabetes and diabetes for all adults 45 years or older, regardless of risk factors, and also advises screening those who have risk factors for diabetes regardless of age.
Screening of little benefit without behavior change support
In an interview, Dr. Grant added that broad efforts are essential as those at the practice level have clearly not succeeded.
“The medical model of individual counseling and referral has not really been effective, and so we really need to think in terms of large-scale public health action,” said Dr. Grant, of the division of research, Kaiser Permanente Northern California, Oakland.
His editorial details the sweeping, multifactorial efforts that are needed.
“To turn this recommendation into action – that is, to translate screening activities into improved clinical outcomes – change is needed at the patient-clinician level (recognizing and encouraging eligible individuals to be screened), health care system level (reducing screening barriers and ensuring access to robust lifestyle programs), and societal level (applying effective public health interventions to reduce obesity and increase exercise),” they write.
A top priority has to be a focus on individuals of diverse backgrounds and issues such as access to healthy programs in minority communities, Dr. Grant noted.
“Newly diagnosed adults are more likely to be African-American and Latinx,” he said.
“We really need to invest in healthier communities for low-income, non-White communities to reverse the persistent health care disparities in these communities.”
While the challenges may appear daunting, history shows they are not necessarily insurmountable – as evidenced in the campaign to discourage tobacco smoking.
“National smoking cessation efforts are one example of a mostly successful public health campaign that has made a difference in health behaviors,” Grant noted.
The recommendation is also posted on the USPSTF web site .
Dr. Grant reports receiving grants from the National Institutes of Health and the Patient-Centered Outcomes Research Institute.
The United States Preventive Services Task Force has updated its recommendation on the age of screening for prediabetes and type 2 diabetes in the primary care setting – lowering the age from 40 to 35 years for asymptomatic patients who are overweight or obese and encouraging greater interventions when patients do show a risk.
“The USPSTF concludes with moderate certainty that screening for prediabetes and type 2 diabetes and offering or referring patients with prediabetes to effective preventive interventions has a moderate net benefit,” the task force concludes in its recommendation, published Aug. 24 in JAMA.
“Clinicians should offer or refer patients with prediabetes to effective preventive interventions,” they write.
Experts commenting on the issue strongly emphasize that it’s not just the screening, but the subsequent intervention that is needed to make a difference.
“If young adults newly identified with abnormal glucose metabolism do not receive the needed intensive behavioral change support, screening may provide no benefit,” write Richard W. Grant, MD, MPH, and colleagues in an editorial published with the recommendation.
“Given the role of our obesogenic and physically inactive society in the shift toward earlier onset of diabetes, efforts to increase screening and recognition of abnormal glucose metabolism must be coupled with robust public health measures to address the underlying contributors.”
BMI cutoff lower for at-risk ethnic populations
The recommendation, which updates the task force’s 2015 guideline, carries a “B” classification, meaning the USPSTF has high certainty that the net benefit is moderate. It now specifies screening from age 35to 70 for persons classified as overweight (body mass index at least 25) or obese (BMI at least 30) and recommends referral to preventive interventions when patients are found to have prediabetes.
In addition to recommendations of lifestyle changes, such as diet and physical activity, the task force also endorses the diabetes drug metformin as a beneficial intervention in the prevention or delay of diabetes, while noting fewer overall health benefits from metformin than from the lifestyle changes.
A lower BMI cutoff of at least 23 is recommended for diabetes screening of Asian Americans, and, importantly, screening for prediabetes and diabetes should be considered at an even earlier age if the patient is from a population with a disproportionately high prevalence of diabetes, including American Indian/Alaska Native, Black, Hawaiian/Pacific Islander, Hispanic/Latino, the task force recommends.
Screening tests should include fasting plasma glucose, hemoglobin A1c, or an oral glucose tolerance test. Although screening every 3 years “may be a reasonable approach for adults with normal blood glucose levels,” the task force adds that “the optimal screening interval for adults with an initial normal glucose test result is uncertain.”
Data review: Few with prediabetes know they have it
The need for the update was prompted by troubling data showing increasing diabetes rates despite early signs that can and should be identified and acted upon in the primary care setting to prevent disease progression.
Data from the Centers for Disease Control and Prevention, for instance, show that while 13% of all U.S. adults 18 years or older have diabetes and 35% meet criteria for prediabetes, as many as 21% of those with diabetes were not aware of or did not report having the disease. Furthermore, only a small fraction – 15% of those with prediabetes – said they had been told by a health professional that they had this condition, the task force notes.
The task force’s final recommendation was based on a systematic review of evidence regarding the screening of asymptomatic, nonpregnant adults and the harms and benefits of interventions, such as physical activity, behavioral counseling, or pharmacotherapy.
Among key evidence supporting the lower age was a 2014 study showing that the number of people necessary to obtain one positive test for diabetes with screening sharply drops from 80 among those aged 30-34 years to just 31 among those aged 36-39.
Opportunistic universal screening of eligible people aged 35 and older would yield a ratio of 1 out of just 15 to spot a positive test, the authors of that study reported.
In addition, a large cohort study in more than 77,000 people with prediabetes strongly links the risk of developing diabetes with increases in A1c level and with increasing BMI.
ADA recommendations differ
The new recommendations differ from American Diabetes Association guidelines, which call for diabetes screening at all ages for people who are overweight or obese and who have one or more risk factors, such as physical inactivity or a first-degree relative with diabetes. If results are normal, repeat screening at least every 3 years is recommended.
The ADA further recommends universal screening for all adults 45 years and older, regardless of their risk factors.
For the screening of adults over 45, the ADA recommends using a fasting plasma glucose level, 2-hour plasma glucose level during a 75-g oral glucose tolerance test, or A1c level, regardless of risk factors.
The American Association of Clinical Endocrinology also recommends universal screening for prediabetes and diabetes for all adults 45 years or older, regardless of risk factors, and also advises screening those who have risk factors for diabetes regardless of age.
Screening of little benefit without behavior change support
In an interview, Dr. Grant added that broad efforts are essential as those at the practice level have clearly not succeeded.
“The medical model of individual counseling and referral has not really been effective, and so we really need to think in terms of large-scale public health action,” said Dr. Grant, of the division of research, Kaiser Permanente Northern California, Oakland.
His editorial details the sweeping, multifactorial efforts that are needed.
“To turn this recommendation into action – that is, to translate screening activities into improved clinical outcomes – change is needed at the patient-clinician level (recognizing and encouraging eligible individuals to be screened), health care system level (reducing screening barriers and ensuring access to robust lifestyle programs), and societal level (applying effective public health interventions to reduce obesity and increase exercise),” they write.
A top priority has to be a focus on individuals of diverse backgrounds and issues such as access to healthy programs in minority communities, Dr. Grant noted.
“Newly diagnosed adults are more likely to be African-American and Latinx,” he said.
“We really need to invest in healthier communities for low-income, non-White communities to reverse the persistent health care disparities in these communities.”
While the challenges may appear daunting, history shows they are not necessarily insurmountable – as evidenced in the campaign to discourage tobacco smoking.
“National smoking cessation efforts are one example of a mostly successful public health campaign that has made a difference in health behaviors,” Grant noted.
The recommendation is also posted on the USPSTF web site .
Dr. Grant reports receiving grants from the National Institutes of Health and the Patient-Centered Outcomes Research Institute.
The United States Preventive Services Task Force has updated its recommendation on the age of screening for prediabetes and type 2 diabetes in the primary care setting – lowering the age from 40 to 35 years for asymptomatic patients who are overweight or obese and encouraging greater interventions when patients do show a risk.
“The USPSTF concludes with moderate certainty that screening for prediabetes and type 2 diabetes and offering or referring patients with prediabetes to effective preventive interventions has a moderate net benefit,” the task force concludes in its recommendation, published Aug. 24 in JAMA.
“Clinicians should offer or refer patients with prediabetes to effective preventive interventions,” they write.
Experts commenting on the issue strongly emphasize that it’s not just the screening, but the subsequent intervention that is needed to make a difference.
“If young adults newly identified with abnormal glucose metabolism do not receive the needed intensive behavioral change support, screening may provide no benefit,” write Richard W. Grant, MD, MPH, and colleagues in an editorial published with the recommendation.
“Given the role of our obesogenic and physically inactive society in the shift toward earlier onset of diabetes, efforts to increase screening and recognition of abnormal glucose metabolism must be coupled with robust public health measures to address the underlying contributors.”
BMI cutoff lower for at-risk ethnic populations
The recommendation, which updates the task force’s 2015 guideline, carries a “B” classification, meaning the USPSTF has high certainty that the net benefit is moderate. It now specifies screening from age 35to 70 for persons classified as overweight (body mass index at least 25) or obese (BMI at least 30) and recommends referral to preventive interventions when patients are found to have prediabetes.
In addition to recommendations of lifestyle changes, such as diet and physical activity, the task force also endorses the diabetes drug metformin as a beneficial intervention in the prevention or delay of diabetes, while noting fewer overall health benefits from metformin than from the lifestyle changes.
A lower BMI cutoff of at least 23 is recommended for diabetes screening of Asian Americans, and, importantly, screening for prediabetes and diabetes should be considered at an even earlier age if the patient is from a population with a disproportionately high prevalence of diabetes, including American Indian/Alaska Native, Black, Hawaiian/Pacific Islander, Hispanic/Latino, the task force recommends.
Screening tests should include fasting plasma glucose, hemoglobin A1c, or an oral glucose tolerance test. Although screening every 3 years “may be a reasonable approach for adults with normal blood glucose levels,” the task force adds that “the optimal screening interval for adults with an initial normal glucose test result is uncertain.”
Data review: Few with prediabetes know they have it
The need for the update was prompted by troubling data showing increasing diabetes rates despite early signs that can and should be identified and acted upon in the primary care setting to prevent disease progression.
Data from the Centers for Disease Control and Prevention, for instance, show that while 13% of all U.S. adults 18 years or older have diabetes and 35% meet criteria for prediabetes, as many as 21% of those with diabetes were not aware of or did not report having the disease. Furthermore, only a small fraction – 15% of those with prediabetes – said they had been told by a health professional that they had this condition, the task force notes.
The task force’s final recommendation was based on a systematic review of evidence regarding the screening of asymptomatic, nonpregnant adults and the harms and benefits of interventions, such as physical activity, behavioral counseling, or pharmacotherapy.
Among key evidence supporting the lower age was a 2014 study showing that the number of people necessary to obtain one positive test for diabetes with screening sharply drops from 80 among those aged 30-34 years to just 31 among those aged 36-39.
Opportunistic universal screening of eligible people aged 35 and older would yield a ratio of 1 out of just 15 to spot a positive test, the authors of that study reported.
In addition, a large cohort study in more than 77,000 people with prediabetes strongly links the risk of developing diabetes with increases in A1c level and with increasing BMI.
ADA recommendations differ
The new recommendations differ from American Diabetes Association guidelines, which call for diabetes screening at all ages for people who are overweight or obese and who have one or more risk factors, such as physical inactivity or a first-degree relative with diabetes. If results are normal, repeat screening at least every 3 years is recommended.
The ADA further recommends universal screening for all adults 45 years and older, regardless of their risk factors.
For the screening of adults over 45, the ADA recommends using a fasting plasma glucose level, 2-hour plasma glucose level during a 75-g oral glucose tolerance test, or A1c level, regardless of risk factors.
The American Association of Clinical Endocrinology also recommends universal screening for prediabetes and diabetes for all adults 45 years or older, regardless of risk factors, and also advises screening those who have risk factors for diabetes regardless of age.
Screening of little benefit without behavior change support
In an interview, Dr. Grant added that broad efforts are essential as those at the practice level have clearly not succeeded.
“The medical model of individual counseling and referral has not really been effective, and so we really need to think in terms of large-scale public health action,” said Dr. Grant, of the division of research, Kaiser Permanente Northern California, Oakland.
His editorial details the sweeping, multifactorial efforts that are needed.
“To turn this recommendation into action – that is, to translate screening activities into improved clinical outcomes – change is needed at the patient-clinician level (recognizing and encouraging eligible individuals to be screened), health care system level (reducing screening barriers and ensuring access to robust lifestyle programs), and societal level (applying effective public health interventions to reduce obesity and increase exercise),” they write.
A top priority has to be a focus on individuals of diverse backgrounds and issues such as access to healthy programs in minority communities, Dr. Grant noted.
“Newly diagnosed adults are more likely to be African-American and Latinx,” he said.
“We really need to invest in healthier communities for low-income, non-White communities to reverse the persistent health care disparities in these communities.”
While the challenges may appear daunting, history shows they are not necessarily insurmountable – as evidenced in the campaign to discourage tobacco smoking.
“National smoking cessation efforts are one example of a mostly successful public health campaign that has made a difference in health behaviors,” Grant noted.
The recommendation is also posted on the USPSTF web site .
Dr. Grant reports receiving grants from the National Institutes of Health and the Patient-Centered Outcomes Research Institute.
FROM JAMA
SGLT2 inhibitor use rising in patients with DKD
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
U.S. prescribing data from 160,000 adults with type 2 diabetes and diabetic kidney disease showed a notable uptick in new prescriptions for sodium-glucose cotransporter 2 inhibitors and less dramatic gains for glucagonlike peptide–1 receptor agonists during 2019 and continuing into early 2020, compared with prior years, with usage levels of both classes during the first quarter of 2020 rivaling those of more traditional agents including metformin and insulin.
During the first 3 months of 2020, initiation of a SGLT2 inhibitor constituted 13% of all new starts of an antidiabetes drug among adults with type 2 diabetes and diabetic kidney disease (DKD). This compared with initiation rates during the same early 2020 period of 17% for GLP-1 receptor agonists, 19% for metformin, 16% for sulfonylureas, 15% for insulins, 14% for thiazolidinediones, and 6% for dipeptidyl peptidase–4 inhibitors, the seven drug classes examined in a study published in Diabetes Care.
Early 2020 was the first time that starts of a GLP-1 receptor agonist ranked second (behind only metformin) among these seven drug classes in the studied U.S. population, and early 2020 also marked an unprecedentedly high start rate for SGLT2 inhibitors that nearly tripled the roughly 5% rate in place as recently as 2018.
Rises are ‘what we expected’
The recent rise of SGLT2 inhibitors and GLP-1 receptor agonists in these patients “was what we expected,” given the evidence for both classes in slowing progression of DKD, said Julie M. Paik, MD, senior author on the study and a nephrologist and pharmacoepidemiologist at Brigham and Women’s Hospital in Boston.
“We’ve seen other beneficial drugs slow on the uptake, so it’s not surprising to see it here, and I’m optimistic” about further increases going forward, she said in an interview.
Both drug classes “were originally marketed as diabetes drugs,” and it is only since 2019, with the publication of trials showing dramatic renal benefits from canagliflozin (Invokana) in CREDENCE, and from dapagliflozin (Farxiga) in DAPA-CKD in 2020 that the evidence became truly compelling for SGLT2 inhibitors. This evidence also led to new renal-protection indications approved by the Food and Drug Administration for canagliflozin and for dapagliflozin, noted Dr. Paik.
Evidence for renal protection also emerged in 2017 for the GLP-1 receptor agonist liraglutide (Victoza) in the LEADER trial, and for dulaglutide (Trulicity) in the AWARD-7 trial, although neither drug has received a renal indication in its labeling.
By 2020, guidelines for managing patients with type 2 diabetes and chronic kidney disease from the influential Kidney Disease: Improving Global Outcomes organization had identified agents from the SGLT2 inhibitor class as top-tier options, along with metformin, for treating these patients, with agents from the GLP-1 receptor agonist class as the top third class to add in patients who require additional glycemic control.
Additional analyses Dr. Paik and associates ran showed how this played out in terms of which specialists prescribed these drugs during the full period studied beginning in 2013. Throughout this roughly 7-year span, about 70% of the prescriptions written for either SGLT2 inhibitors or for GLP-1 receptor agonists were from internal medicine physicians, followed by about 20% written by endocrinologists. Prescriptions from nephrologists, as well as from cardiologists, have hovered at about 5% each, but seem poised to start rising based on the recently added indications and newer treatment recommendations.
“It’s good to see the recent uptick in use since 2019,” Katherine R. Tuttle, MD, commented in an interview. It’s a positive development for U.S. public health, “but we need to do more to disseminate and implement these life-, kidney-, and heart-saving therapies.”
Future use could approach 80% of DKD patients
Dr. Tuttle estimated that “target” levels of use for SGLT2 inhibitors and for GLP-1 receptor agonists “could reasonably approach 80%” for patients with type 2 diabetes and diabetic kidney disease.
“We will likely move to combination therapy” with simultaneous use of agents from both classes in a targeted way using “precision phenotyping based on clinical characteristics, and eventually perhaps by biomarkers, kidney biopsies, or both.” Combined treatment with both an SGLT2 inhibitor and a GLP-1 receptor agonist may be especially suited to patients with type 2 diabetes, atherosclerotic cardiovascular disease, low estimated glomerular filtration rate, and need for better glycemic control and weight loss, a profile that is “pretty typical” in real-world practice, said Dr. Tuttle, a nephrologist and endocrinologist and executive director for research at Providence Healthcare in Spokane, Wash.
Study included patients with commercial or Medicare Advantage coverage
The study used information in an Optum database that included patients enrolled in either commercial or in Medicare Advantage health insurance plans from 2013 to the first quarter of 2020. This included 160,489 adults with type 2 diabetes and DKD who started during that period at least one agent from any of the seven included drug classes.
This focus may have biased the findings because, overall, U.S. coverage of the relatively expensive agents from the SGLT2 inhibitor and GLP-1 receptor agonist classes has often been problematic.
“There are issues of cost, coverage, and access” using these medications, as well as limited data on cost-effectiveness, Dr. Paik acknowledged. Additional issues that have helped generate prescribing lags include concerns about possible adverse effects, low familiarity by providers with these drugs early on, and limited trial experience using them in older patients. The process of clinicians growing more comfortable prescribing these new agents has depended on their “working through the evidence,” she explained.
The FDA’s approval in July 2021 of finerenone (Kerendia) for treating patients with type 2 diabetes and chronic kidney disease threw yet another new variable into the prescribing mix for these patients.
“SGLT2 inhibitors are here to stay as a new standard of care for patients with diabetic kidney disease, but combination with finerenone might be especially useful for patients with diabetic kidney disease and heart failure,” Dr. Tuttle suggested. A new generation of clinical trials will likely soon launch to test these combinations, she predicted.
Dr. Paik had no disclosures. Dr. Tuttle has been a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Eli Lilly, and Novo Nordisk.
FROM DIABETES CARE
Empagliflozin gets HFrEF approval from FDA
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
The U.S. Food and Drug Administration approved empagliflozin (Jardiance) as a treatment for adults with heart failure with reduced ejection fraction (HFrEF) regardless of whether patients have diabetes on Aug. 18, making it the second agent from the sodium-glucose transporter 2 inhibitor class to received this indication.
Empagliflozin first received FDA marketing approval in 2014 for improving glycemic control in patients with type 2 diabetes, and in 2016 the agency added a second indication of reducing cardiovascular death in patients with type 2 diabetes and cardiovascular disease. The newly granted indication for patients with HFrEF without regard to glycemic status was for reducing the risk for cardiovascular death and hospitalization for heart failure, according to a statement from Boehringer Ingelheim and Lilly, the two companies that together market empagliflozin.
The statement also said that the approval allowed for empagliflozin treatment in patients with HFrEF and an estimated glomerular filtration rate (eGFR) as low as 20 mL/min per 1.73 m2, in contrast to its indication for improving glycemic control in patients with type 2 diabetes that limits use to patients with an eGFR of at least 30 mL per 1.73 m2.
EMPEROR-Reduced results drive approval
The FDA based its decision on results from the EMPEROR-Reduced study, first reported in August 2020, that showed treatment of patients with HFrEF with empagliflozin on top of standard therapy for a median of 16 months cut the incidence of cardiovascular death or hospitalization for worsening heart failure by 25% relative to placebo, and by an absolute 5.3%, compared with placebo-treated patients.
Patients enrolled in EMPEROR-Reduced had chronic heart failure in New York Heart Association functional class II-IV and with a left ventricular ejection fraction of 40% or less, the standard ejection fraction criterion for defining HFrEF. Half the enrolled patients had diabetes, and analysis showed no heterogeneity in the primary outcome response based on diabetes status at enrollment.
Empagliflozin joins dapagliflozin for treating HFrEF
Dapagliflozin (Farxiga) was the first agent from the SGLT2 inhibitor class to receive an FDA indication, in 2020, for treating patients with HFrEF regardless of their diabetes status, a decision based on results from the DAPA-HF trial. Results from DAPA-HF showed that treatment with dapagliflozin in patients with HFrEF for a median of 18 months led to a 26% relative reduction in the incidence of cardiovascular death or worsening heart failure and a 4.9% absolute reduction, compared with placebo when added to standard treatment. DAPA-HF enrolled patients using similar criteria to EMPEROR-Reduced, and 42% of enrolled patients had diabetes with no heterogeneity in the primary outcome related to baseline diabetes status.
Subsequent to the report of results from the EMPEROR-Reduced trial nearly a year ago, heart failure experts declared that treatment with an agent from the SGLT2 inhibitor class had become a “new pillar of foundational therapy for HFrEF,” and they urged rapid initiation of an SGLT2 inhibitor (along with other appropriate medications) at the time of initial diagnosis of HFrEF.
Pandemic derails small success in lowering diabetes-related amputations
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.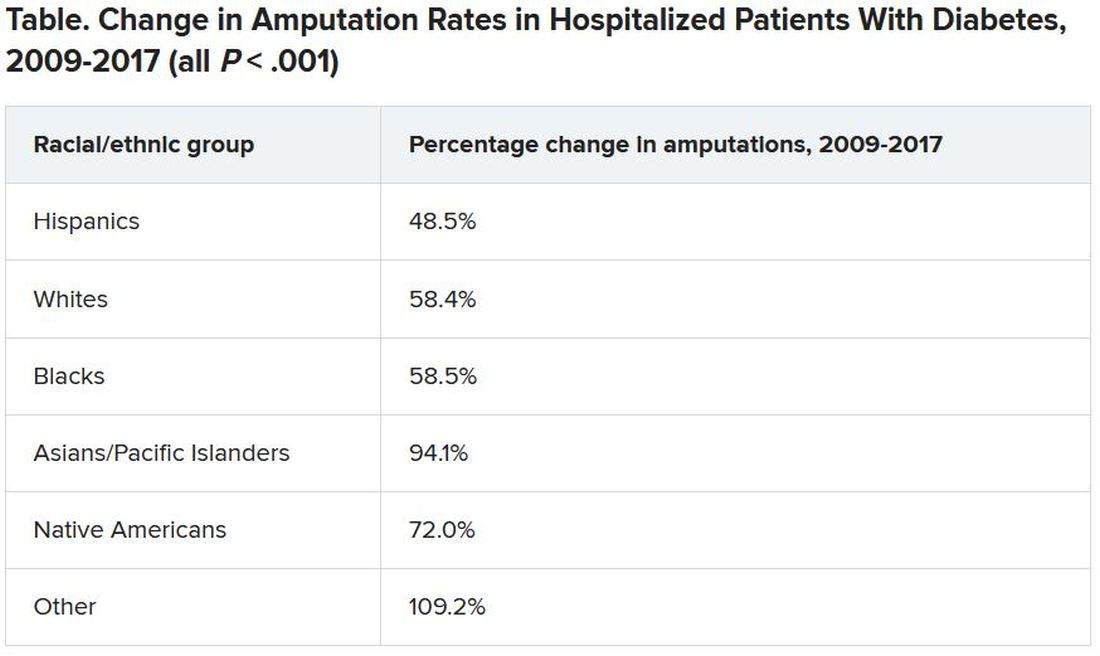
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves rapid-acting insulin, Lyumjev, for pump use
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded the label for Eli Lilly’s ultra–rapid-acting insulin lispro-aabc injection 100 units/mL (Lyumjev) for use in insulin pumps.
Lyumjev (insulin lispro-aabc injection 100 and 200 units/mL) was initially approved in June 2020 to improve glycemic control in adults with type 1 or type 2 diabetes. That formulation is administered by injection from a pen or syringe. Now, the 100 units/mL formulation can also be delivered via continuous subcutaneous insulin infusion with an insulin pump.
Lyumjev will compete with Novo Nordisk’s fast-acting insulin aspart injection 100 units/mL (Fiasp). Fiasp had a head start: it was approved for use in adults in the United States in September 2017. It was approved for use in insulin pumps in October 2019 and for use in children with diabetes in January 2020.
The new approval for Lyumjev was based on data from a phase 3 trial, PRONTO-Pump-2. That trial, which included 432 participants with type 1 diabetes, confirmed the drug’s safety and efficacy when used in pumps.
The study met the primary endpoint of noninferiority in reduction of hemoglobin A1c from baseline to week 16, compared with insulin lispro (Humalog 100 units/mL). It was superior in both 1-hour and 2-hour postprandial glucose reduction when delivered 0-2 minutes before meals, according to a Lilly statement.
Patients who cannot afford the drug can go to www.insulinaffordability.com for assistance. Those with commercial insurance can also visit www.Lyumjev.com to access the Lyumjev Savings Card.
Lyumjev is available in several global markets, including Japan and the European Union, where it is also approved for use in insulin pumps.
A version of this article first appeared on Medscape.com.
Brain memory signals appear to regulate metabolism
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rhythmic brain signals that help encode memories also appear to influence blood sugar levels and may regulate the timing of the release of hormones, early, pre-clinical research shows.
“Our study is the first to show how clusters of brain cell firing in the hippocampus may directly regulate metabolism,” senior author György Buzsáki, MD, PhD, professor, department of neuroscience and physiology, NYU Grossman School of Medicine and NYU Langone Health, said in a news release.
“Evidence suggests that the brain evolved, for reasons of efficiency, to use the same signals to achieve two very different functions in terms of memory and hormonal regulation,” added corresponding author David Tingley, PhD, a post-doctoral scholar in Dr. Buzsáki’s lab.
The study was published online August 11 in Nature.
It’s recently been discovered that populations of hippocampal neurons fire within milliseconds of each other in cycles. This firing pattern is called a “sharp wave ripple” for the shape it takes when captured graphically by electroencephalogram.
In their study, Dr. Buzsáki, Dr. Tingley, and colleagues observed that clusters of sharp wave ripples recorded from the hippocampus of rats were “reliably” and rapidly, followed by decreases in blood sugar concentrations in the animals.
“This correlation was not dependent on circadian, ultradian, or meal-triggered fluctuations; it could be mimicked with optogenetically induced ripples in the hippocampus, but not in the parietal cortex, and was attenuated to chance levels by pharmacogenetically suppressing activity of the lateral septum (LS), the major conduit between the hippocampus and hypothalamus,” the researchers report.
These observations suggest that hippocampal sharp wave ripples may regulate the timing of the release of hormones, possibly including insulin, by the pancreas and liver, as well as other hormones by the pituitary gland, the researchers note.
As sharp wave ripples mostly occur during non-rapid eye movement sleep, the impact of sleep disturbance on sharp wave ripples may provide a mechanistic link between poor sleep and high blood sugar levels seen in type 2 diabetes, they suggest.
“There are a couple of experimental studies showing that if you deprive a young healthy person from sleep [for 48 hours], their glucose tolerance resembles” that of a person with diabetes, Dr. Buzsáki noted in an interview.
Moving forward, the researchers will seek to extend their theory that several hormones could be affected by nightly sharp wave ripples.
The research was funded by National Institutes of Health. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low glycemic diet improves A1c, other risk factors in diabetes
A diet rich in vegetables and low in carbs – a so-called low glycemic index (GI) diet – is associated with clinically significant benefits beyond those provided by existing medications for people with type 1 and type 2 diabetes, compared with a higher glycemic diet, findings from a new meta-analysis show.
“Although the effects were small, which is not surprising in clinical trials in nutrition, they were clinically meaningful improvements for which our certainty in the effects were moderate to high,” first author Laura Chiavaroli, PhD, of the department of nutritional sciences, Temerty Faculty of Medicine, University of Toronto, said in an interview.
The GI rates foods on the basis of how quickly they affect blood glucose levels.
Fruits, vegetables, and whole grains have a low GI. They also help to regulate blood sugar levels. Such foods are linked to a reduced risk for heart disease among people with diabetes.
But guidelines on this – such as those from the European Association for the Study of Diabetes – reflect research published more than 15 years ago, before several key trials were published.
Dr. Chiavaroli and colleagues identified 27 randomized controlled trials – the most recent of which was published in May 2021 – that involved a total of 1,617 adults with type 1 or 2 diabetes. For the patients in these trials, diabetes was moderately controlled with glucose-lowering drugs or insulin. All of the included trials examined the effects of a low GI diet or a low glycemic load (GL) diet for people with diabetes over a period 3 or more weeks. The majority of patients in the studies were overweight or had obesity, and they were largely middle-aged.
The meta-analysis, which included new data, was published Aug. 5 in The BMJ. The study “expands the number of relevant intermediate cardiometabolic outcomes, and assesses the certainty of the evidence using GRADE [grading of recommendations assessment, development, and evaluation],” Dr. Chiavaroli and colleagues noted.
“The available evidence provides a good indication of the likely benefit in this population and supports existing recommendations for the use of low GI dietary patterns in the management of diabetes,” they emphasized.
Improvements in A1c, fasting glucose, cholesterol, and triglycerides
Overall, compared with people who consumed diets with higher GI/GL ratings, for those who consumed lower glycemic diets, glycemic control was significantly improved, as reflected in A1c level, which was the primary outcome of the study (mean difference, –0.31%; P < .001).
This “would meet the threshold of ≥ 0.3% reduction in HbA1c proposed by the European Medicines Agency as clinically relevant for risk reduction of diabetic complications,” the authors noted.
Those who consumed low glycemic diets also showed improvements in secondary outcomes, including fasting glucose level, which was reduced by 0.36 mmol/L (–6.5 mg/dL), a 6% reduction in low-density cholesterol (LDL-C) (–0.17 mmol/L), and a fall in triglyceride levels (–0.09 mmol/L).
They also lost marginally more body weight, at –0.66 kg (–1.5 pounds). Body mass index was lower by –0.38, and inflammation was reduced (C-reactive protein, –.41 mg/L; all P < .05).
No significant differences were observed between the groups in blood insulin level, high-density lipoprotein cholesterol level, waist circumference, or blood pressure.
Three of the studies showed that participants developed a preference for the low GI diet. “In recent years, there has been a growing interest in whole-food plant-based diets, and there are more options, for example, for pulse-based products,” Dr. Chiavaroli said.
This meta-analysis should support the recommendation of the low-glycemic diet, particularly among people with diabetes, she reiterated.
Will larger randomized trial show effect on outcomes?
The authors noted, however, that to determine whether these small improvements in intermediate cardiometabolic risk factors observed with low GI diets translate to reductions in cardiovascular disease, nephropathy, and retinopathy among people with diabetes, larger randomized trials are needed.
One such trial, the Low Glycemic Index Diet for Type 2 Diabetics, includes 169 high-risk patients with type 2 diabetes and subclinical atherosclerosis. The investigators are evaluating the effect of a low GI diet on the progression of atherosclerosis, as assessed by vascular MRI over 3 years.
“We await the results,” they said.
The study received funding from the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD) as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. The study was also supported by the Canadian Institutes of Health Research through the Canada-wide Human Nutrition Trialists’ Network. The Diet, Digestive Tract, and Disease (3D) Center, which is funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the study.
A version of this article first appeared on Medscape.com.
A diet rich in vegetables and low in carbs – a so-called low glycemic index (GI) diet – is associated with clinically significant benefits beyond those provided by existing medications for people with type 1 and type 2 diabetes, compared with a higher glycemic diet, findings from a new meta-analysis show.
“Although the effects were small, which is not surprising in clinical trials in nutrition, they were clinically meaningful improvements for which our certainty in the effects were moderate to high,” first author Laura Chiavaroli, PhD, of the department of nutritional sciences, Temerty Faculty of Medicine, University of Toronto, said in an interview.
The GI rates foods on the basis of how quickly they affect blood glucose levels.
Fruits, vegetables, and whole grains have a low GI. They also help to regulate blood sugar levels. Such foods are linked to a reduced risk for heart disease among people with diabetes.
But guidelines on this – such as those from the European Association for the Study of Diabetes – reflect research published more than 15 years ago, before several key trials were published.
Dr. Chiavaroli and colleagues identified 27 randomized controlled trials – the most recent of which was published in May 2021 – that involved a total of 1,617 adults with type 1 or 2 diabetes. For the patients in these trials, diabetes was moderately controlled with glucose-lowering drugs or insulin. All of the included trials examined the effects of a low GI diet or a low glycemic load (GL) diet for people with diabetes over a period 3 or more weeks. The majority of patients in the studies were overweight or had obesity, and they were largely middle-aged.
The meta-analysis, which included new data, was published Aug. 5 in The BMJ. The study “expands the number of relevant intermediate cardiometabolic outcomes, and assesses the certainty of the evidence using GRADE [grading of recommendations assessment, development, and evaluation],” Dr. Chiavaroli and colleagues noted.
“The available evidence provides a good indication of the likely benefit in this population and supports existing recommendations for the use of low GI dietary patterns in the management of diabetes,” they emphasized.
Improvements in A1c, fasting glucose, cholesterol, and triglycerides
Overall, compared with people who consumed diets with higher GI/GL ratings, for those who consumed lower glycemic diets, glycemic control was significantly improved, as reflected in A1c level, which was the primary outcome of the study (mean difference, –0.31%; P < .001).
This “would meet the threshold of ≥ 0.3% reduction in HbA1c proposed by the European Medicines Agency as clinically relevant for risk reduction of diabetic complications,” the authors noted.
Those who consumed low glycemic diets also showed improvements in secondary outcomes, including fasting glucose level, which was reduced by 0.36 mmol/L (–6.5 mg/dL), a 6% reduction in low-density cholesterol (LDL-C) (–0.17 mmol/L), and a fall in triglyceride levels (–0.09 mmol/L).
They also lost marginally more body weight, at –0.66 kg (–1.5 pounds). Body mass index was lower by –0.38, and inflammation was reduced (C-reactive protein, –.41 mg/L; all P < .05).
No significant differences were observed between the groups in blood insulin level, high-density lipoprotein cholesterol level, waist circumference, or blood pressure.
Three of the studies showed that participants developed a preference for the low GI diet. “In recent years, there has been a growing interest in whole-food plant-based diets, and there are more options, for example, for pulse-based products,” Dr. Chiavaroli said.
This meta-analysis should support the recommendation of the low-glycemic diet, particularly among people with diabetes, she reiterated.
Will larger randomized trial show effect on outcomes?
The authors noted, however, that to determine whether these small improvements in intermediate cardiometabolic risk factors observed with low GI diets translate to reductions in cardiovascular disease, nephropathy, and retinopathy among people with diabetes, larger randomized trials are needed.
One such trial, the Low Glycemic Index Diet for Type 2 Diabetics, includes 169 high-risk patients with type 2 diabetes and subclinical atherosclerosis. The investigators are evaluating the effect of a low GI diet on the progression of atherosclerosis, as assessed by vascular MRI over 3 years.
“We await the results,” they said.
The study received funding from the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD) as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. The study was also supported by the Canadian Institutes of Health Research through the Canada-wide Human Nutrition Trialists’ Network. The Diet, Digestive Tract, and Disease (3D) Center, which is funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the study.
A version of this article first appeared on Medscape.com.
A diet rich in vegetables and low in carbs – a so-called low glycemic index (GI) diet – is associated with clinically significant benefits beyond those provided by existing medications for people with type 1 and type 2 diabetes, compared with a higher glycemic diet, findings from a new meta-analysis show.
“Although the effects were small, which is not surprising in clinical trials in nutrition, they were clinically meaningful improvements for which our certainty in the effects were moderate to high,” first author Laura Chiavaroli, PhD, of the department of nutritional sciences, Temerty Faculty of Medicine, University of Toronto, said in an interview.
The GI rates foods on the basis of how quickly they affect blood glucose levels.
Fruits, vegetables, and whole grains have a low GI. They also help to regulate blood sugar levels. Such foods are linked to a reduced risk for heart disease among people with diabetes.
But guidelines on this – such as those from the European Association for the Study of Diabetes – reflect research published more than 15 years ago, before several key trials were published.
Dr. Chiavaroli and colleagues identified 27 randomized controlled trials – the most recent of which was published in May 2021 – that involved a total of 1,617 adults with type 1 or 2 diabetes. For the patients in these trials, diabetes was moderately controlled with glucose-lowering drugs or insulin. All of the included trials examined the effects of a low GI diet or a low glycemic load (GL) diet for people with diabetes over a period 3 or more weeks. The majority of patients in the studies were overweight or had obesity, and they were largely middle-aged.
The meta-analysis, which included new data, was published Aug. 5 in The BMJ. The study “expands the number of relevant intermediate cardiometabolic outcomes, and assesses the certainty of the evidence using GRADE [grading of recommendations assessment, development, and evaluation],” Dr. Chiavaroli and colleagues noted.
“The available evidence provides a good indication of the likely benefit in this population and supports existing recommendations for the use of low GI dietary patterns in the management of diabetes,” they emphasized.
Improvements in A1c, fasting glucose, cholesterol, and triglycerides
Overall, compared with people who consumed diets with higher GI/GL ratings, for those who consumed lower glycemic diets, glycemic control was significantly improved, as reflected in A1c level, which was the primary outcome of the study (mean difference, –0.31%; P < .001).
This “would meet the threshold of ≥ 0.3% reduction in HbA1c proposed by the European Medicines Agency as clinically relevant for risk reduction of diabetic complications,” the authors noted.
Those who consumed low glycemic diets also showed improvements in secondary outcomes, including fasting glucose level, which was reduced by 0.36 mmol/L (–6.5 mg/dL), a 6% reduction in low-density cholesterol (LDL-C) (–0.17 mmol/L), and a fall in triglyceride levels (–0.09 mmol/L).
They also lost marginally more body weight, at –0.66 kg (–1.5 pounds). Body mass index was lower by –0.38, and inflammation was reduced (C-reactive protein, –.41 mg/L; all P < .05).
No significant differences were observed between the groups in blood insulin level, high-density lipoprotein cholesterol level, waist circumference, or blood pressure.
Three of the studies showed that participants developed a preference for the low GI diet. “In recent years, there has been a growing interest in whole-food plant-based diets, and there are more options, for example, for pulse-based products,” Dr. Chiavaroli said.
This meta-analysis should support the recommendation of the low-glycemic diet, particularly among people with diabetes, she reiterated.
Will larger randomized trial show effect on outcomes?
The authors noted, however, that to determine whether these small improvements in intermediate cardiometabolic risk factors observed with low GI diets translate to reductions in cardiovascular disease, nephropathy, and retinopathy among people with diabetes, larger randomized trials are needed.
One such trial, the Low Glycemic Index Diet for Type 2 Diabetics, includes 169 high-risk patients with type 2 diabetes and subclinical atherosclerosis. The investigators are evaluating the effect of a low GI diet on the progression of atherosclerosis, as assessed by vascular MRI over 3 years.
“We await the results,” they said.
The study received funding from the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD) as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. The study was also supported by the Canadian Institutes of Health Research through the Canada-wide Human Nutrition Trialists’ Network. The Diet, Digestive Tract, and Disease (3D) Center, which is funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation’s Ontario Research Fund, provided the infrastructure for the study.
A version of this article first appeared on Medscape.com.
Recent Trends in Diabetes Treatment and Control in US Adults: A Geriatrician’s Point of View
Study Overview
Objective. To update national trends in the treatment and risk factor control of diabetic patients from 1999 through 2018 in the US using data from the National Health and Nutrition Examination Survey (NHANES) with the goal of identifying population subgroups with the highest probability of having untreated risk factors.
Design. The authors conducted a cross-sectional analysis of data from NHANES focusing on adults with diabetes. They examined patient characteristics and medication use over time and estimated the prevalence of risk factor control and medication use. To minimize the effects of a small sample size, the survey years were pooled into 4-year intervals. The variables studied included glycated hemoglobin (HbA1c), blood pressure, serum cholesterol, medication use, sociodemographic characteristics, and weight status. For statistical analysis, logistic and multinomial logistic regression models were used to examine factors associated with treatment in participants who did not achieve targets for glycemic, blood pressure, and lipid control. Temporal trends were estimated using 2-piece linear spline models with 1 knot at inflection points.
Setting and participants. The NHANES program began in the early 1960s to monitor the health of the US population. In 1999, the survey became a continuous program combining interviews and physical examinations. The survey examines a nationally representative sample of about 5000 persons each year. This study included 6653 participants who were nonpregnant, aged older than 20 years, reported a diagnosis of diabetes from a physician, and participated in NHANES from 1999 through 2018.
Main outcome measures. The main outcome measures were temporal trends in risk factor control (glycemic, blood pressure, or lipid levels) and medication use (glucose lowering, blood pressure lowering, or lipid lowering medications), and number as well as class of drug used, from 1999 through 2018 in diabetic adults from the US participating in NHANES.
Results. Sociodemographic characteristics of the studied diabetes population—The age and racial or ethnic distribution of participants with diabetes were stable from 1999 through 2018, whereas participants with a college degree, higher income, health insurance, obesity, or long-standing diabetes increased during the same period.
Trends in diabetes risk factor control—The trends for glycemic, blood pressure, and lipid control were nonlinear, with an inflection point around 2010. Glycemic control was defined as HbA1c less than 7%, blood pressure was considered controlled if less than 140/90 mmHg, and lipid was controlled if non-HDL cholesterol level was less than 130 mg/dL. Although these chosen targets were based on the most recent clinical guidelines, the authors declared that they observed similar trends when alternative targets were used. The level of risk factor control improved in all diabetic patients from 1999 through 2010. However, the percentage of adult diabetic participants for whom glycemic control was achieved declined from 57.4% (95% CI, 52.9-61.8) in 2007-2010 to 50.5% (95% CI, 45.8-55.3) in 2015-2018. Blood pressure control was achieved in 74.2% of participants (95% CI, 70.7-77.4) in 2011-2014 but declined to 70.4% (95% CI, 66.7-73.8) in 2015-2018. Control in lipid levels improved during the entire study period; however, the rate of improvement heavily declined after 2007 with lipid target levels attained in 52.3% of participants (95% CI, 49.2-55.3) in 2007-2014 and 55.7% (95% CI, 50.8-60.5) in 2015-2018. Finally, the percentage of participants in whom targets for all 3 risk factors were simultaneously achieved plateaued after 2010 and was 22.2% (95% CI, 17.9-27.3) in 2015-2018.
Trends in diabetes treatment—The use of glucose lowering drugs increased from 74.1% in 1999-2002 to 82.7% in 2007-2010 and then stabilized. A shift toward a safer glucose lowering treatment choice was observed with a decline in the use of older glucose lowering medications such as sulfonylureas, which increases the risk of hypoglycemia, and an increase in the use of metformin, insulin, and newer agents such as sodium-glucose cotransporter 2 inhibitors.
Similarly, blood pressure lowering medication use rose from 1999-2002 to 2007-2010 and then stabilized, with increased use of first-line recommended treatments including angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers. Likewise, statin use rose from 28.4% in 1999-2002 to 56% in 2011-2014 and then stabilized. The total number of drugs used culminated in 2011-2014 with 60% of participants using more than 5 drugs and then leveled off to 57.2% in 2015-2018. Lastly, health insurance status and race or ethnicity impacted the likelihood of receiving monotherapy or combination drug therapy when targets for glycemic, blood pressure, or lipid control were not achieved.
Conclusion. Despite great progress in the control of diabetes and its associated risk factors between 1999 and 2010, this trend declined for glycemic and blood pressure control and leveled off for lipid control in adult NHANES participants with diabetes after 2010. First-line treatments for diabetes and associated risk factors remain underused, and treatment intensification may not be sufficiently considered in patients with uncontrolled risk factors despite clinical guideline recommendations. The findings of this study may portend a possible population-level increase in diabetes-related illnesses in the years to come.
Commentary
The thorough understanding of trends in management of diseases is critical to inform public health policies and planning. Well designed clinical studies heavily influence the development of public health policies and clinical guidelines, which in turn drive real-world clinical practice. In a recent analysis utilizing data from NHANES, Fang et al1 showed evidence of a general shift toward less intensive treatment of diabetes, hypertension, and hypercholesterolemia in adults living in the US during the last decade.
Similarly, in a separate study using NHANES data collected between 1999 and 2018 published in JAMA just 2 weeks after the current report, Wang et al2 confirms this declining trend in diabetes management with only 21.2% of diabetic adults simultaneously attaining glycemic, blood pressure, and lipid level targets during the same period. What led to the decline in more stringent risk factor and diabetes management since 2010 observed in these studies? One possible explanation, as suggested by Fang et al, is that major clinical trials from the late 2000s—including Action to Control Cardiovascular Risk in Diabetes, UK Prospective Diabetes Study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation, and Veterans Affairs Diabetes Trial—that assessed the effects of intensive glycemic control (with target HbA1c < 6.5%) found that intensive treatment of diabetes compared to standard care had no cardiovascular benefit albeit increasing the risk of hypoglycemia. Thus, these trial findings may have translated into suboptimal diabetes treatment observed in some NHANES participants. Wang et al propose that effective tailored approaches are needed to improve risk factor control in diabetic patients, such as enhance and maintain adherence to medications and healthy lifestyle behaviors, as well as better access to health care and therapeutic education.
The changes in recent trends in diabetes management have immense clinical implications. The authors of this study suggest a link between the recent relaxation of glycemic targets, as well as risk factor control, and a resurgence of diabetic complications such as lower limb amputation or stroke. Indeed, several recent studies indicate an upward trend or plateau in diabetic complications which had been decreasing in prevalence prior to 2010.3 For example, lower extremity amputation has surged by more than 25% between 2010 and 2015, especially in young and middle-aged adults.4 Among the arguments brought forward that this recent resurgence in amputations is directly linked to worsening glycemic control is the fact that between 2007 and 2010, when glucose levels were best controlled within the previous 30-year period, amputations were also at the lowest levels. Moreover, data from the Centers for Disease Control and Prevention also show a 55% increase in mortality (from 15.7 to 24.2 per 1000) among diabetic patients between 2010 and 2015.14 On the other hand, a growing number of studies show that an increase of inappropriate treatment intensification—reaching HbA1c levels that are way below the recommended targets—is associated with adverse consequences in diabetic patients particularly in those aged more than 65 years.5-7 These seemingly contradictory findings highlight the importance of a personalized and thoughtful approach to the management of diabetes and its risk factors. As an example, an increase in the use of newer and safer glucose lowering drugs (eg, sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and dipeptidyl peptidase 4 inhibitors) can help achieve better HbA1c goals with a reduced risk of hypoglycemic episodes as recently shown by a Danish study.8 In this study, the authors concluded that the reduction of the rate of hypoglycemic episodes leading to hospitalization in Denmark was directly linked to the use of these safer and newer glucose lowering drugs.
A discussion on the specifics of trends in diabetes treatment and control must include considerations in older adults aged more than 65 years who constitute more than 40% of the diabetic population. Despite the high prevalence of diabetes in this vulnerable population, such data are still insufficient in the literature and are critically needed to inform public health policies and clinical guidelines. In epidemiological studies focusing on diabetic complications from the last 10 years, concerning increases have been observed in younger9 and middle-aged adults while remaining stable in older adults. However, the risk of hypoglycemia or severe hypoglycemia remains high in older adults living in nursing facilities, even in those with an elevated HbA1c of greater than 8%.7 Moreover, in light of more relaxed HbA1c treatment goals for older frail adults as recommended by international guidelines since 2010,10,11 recent findings from the French GERODIAB cohort show an increased mortality (hazard ratio, 1.76) in type 2 diabetics aged 70 years and older with HbA1c greater than or equal to 8.6%.12 Similarly, a 5-year retrospective British study from 2018 which included patients aged 70 years and older, shows an increased overall mortality in those with HbA1c greater than 8.5%.13 Taken together, further age-stratified analysis utilizing data from large cohort studies including NHANES may help to clarify national trends in diabetes treatment and risk factor control as well as diabetic complications specific to the geriatric population. By being better informed of such trends, clinicians could then develop treatment strategies that minimize complications (eg, hypoglycemia, falls) while achieving favorable outcomes (eg, reduce hyperglycemic emergencies, improve survival) in frail older patients.
Applications for Clinical Practice
The understanding of population-wide trends in diabetes control is critical to planning public health approaches for the prevention and treatment of this disease and its complications. In older adults, the high risk of hypoglycemic events and insufficient epidemiological data on trends of diabetes control hinder diabetes management. Personalized treatment targets taking into account geriatric syndromes and general health status, as well as multidisciplinary management involving endocrinologists, geriatricians, and clinical pharmacists, are necessary to optimize care in older adults with diabetes.
1. Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999-2018. N Engl J Med. 2021;384(23):2219-28. doi:10.1056/NEJMsa2032271
2. Wang L, Li X, Wang Z, et al. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. JAMA. 2021. doi:10.1001/jama.2021.9883
3. Gregg EW, Hora I, Benoit SR. Resurgence in Diabetes-Related Complications. JAMA. 2019;321(19):1867-8. doi:10.1001/jama.2019.3471
4. Caruso P, Scappaticcio L, Maiorino MI, et al. Up and down waves of glycemic control and lower-extremity amputation in diabetes. Cardiovasc Diabetol. 2021;20(1):135. doi:10.1186/s12933-021-01325-3
5. Bongaerts B, Arnold SV, Charbonnel BH, et al. Inappropriate intensification of glucose-lowering treatment in older patients with type 2 diabetes: the global DISCOVER study. BMJ Open Diabetes Res Care. 2021;9(1)e001585. doi:10.1136/bmjdrc-2020-001585
6. Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116-1124. doi: 10.1001/jamainternmed.2014.1824
7. Bouillet B, Tscherter P, Vaillard L, et al. Frequent and severe hypoglycaemia detected with continuous glucose monitoring in older institutionalised patients with diabetes. Age Ageing. 2021;afab128. doi: 10.1093/ageing/afab128
8. Jensen MH, Hejlesen O, Vestergaard P. Epidemiology of hypoglycaemic episodes leading to hospitalisations in Denmark in 1998-2018. Diabetologia. 2021. doi: 10.1007/s00125-021-05507-2
9. TODAY Study Group, Bjornstad P, Drews KL, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385(5):416-426. doi: 10.1056/NEJMoa2100165
10. Sinclair AJ, Paolisso G, Castro M, et al. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37 Suppl 3:S27-S38. doi:10.1016/S1262-3636(11)70962-4
11. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650-2664. doi: 10.2337/dc12-1801
12. Doucet J, Verny C, Balkau B, et al. Haemoglobin A1c and 5-year all-cause mortality in French type 2 diabetic patients aged 70 years and older: The GERODIAB observational cohort. Diabetes Metab. 2018;44(6):465-472. doi: 10.1016/j.diabet.2018.05.003
13. Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):476-486. doi: 10.1016/S2213-8587(18)30048-2
14. US Centers for Disease Control and Prevention. US diabetes surveillance system and diabetes atlas, 2019. https://www.cdc.gov/diabetes/data
Study Overview
Objective. To update national trends in the treatment and risk factor control of diabetic patients from 1999 through 2018 in the US using data from the National Health and Nutrition Examination Survey (NHANES) with the goal of identifying population subgroups with the highest probability of having untreated risk factors.
Design. The authors conducted a cross-sectional analysis of data from NHANES focusing on adults with diabetes. They examined patient characteristics and medication use over time and estimated the prevalence of risk factor control and medication use. To minimize the effects of a small sample size, the survey years were pooled into 4-year intervals. The variables studied included glycated hemoglobin (HbA1c), blood pressure, serum cholesterol, medication use, sociodemographic characteristics, and weight status. For statistical analysis, logistic and multinomial logistic regression models were used to examine factors associated with treatment in participants who did not achieve targets for glycemic, blood pressure, and lipid control. Temporal trends were estimated using 2-piece linear spline models with 1 knot at inflection points.
Setting and participants. The NHANES program began in the early 1960s to monitor the health of the US population. In 1999, the survey became a continuous program combining interviews and physical examinations. The survey examines a nationally representative sample of about 5000 persons each year. This study included 6653 participants who were nonpregnant, aged older than 20 years, reported a diagnosis of diabetes from a physician, and participated in NHANES from 1999 through 2018.
Main outcome measures. The main outcome measures were temporal trends in risk factor control (glycemic, blood pressure, or lipid levels) and medication use (glucose lowering, blood pressure lowering, or lipid lowering medications), and number as well as class of drug used, from 1999 through 2018 in diabetic adults from the US participating in NHANES.
Results. Sociodemographic characteristics of the studied diabetes population—The age and racial or ethnic distribution of participants with diabetes were stable from 1999 through 2018, whereas participants with a college degree, higher income, health insurance, obesity, or long-standing diabetes increased during the same period.
Trends in diabetes risk factor control—The trends for glycemic, blood pressure, and lipid control were nonlinear, with an inflection point around 2010. Glycemic control was defined as HbA1c less than 7%, blood pressure was considered controlled if less than 140/90 mmHg, and lipid was controlled if non-HDL cholesterol level was less than 130 mg/dL. Although these chosen targets were based on the most recent clinical guidelines, the authors declared that they observed similar trends when alternative targets were used. The level of risk factor control improved in all diabetic patients from 1999 through 2010. However, the percentage of adult diabetic participants for whom glycemic control was achieved declined from 57.4% (95% CI, 52.9-61.8) in 2007-2010 to 50.5% (95% CI, 45.8-55.3) in 2015-2018. Blood pressure control was achieved in 74.2% of participants (95% CI, 70.7-77.4) in 2011-2014 but declined to 70.4% (95% CI, 66.7-73.8) in 2015-2018. Control in lipid levels improved during the entire study period; however, the rate of improvement heavily declined after 2007 with lipid target levels attained in 52.3% of participants (95% CI, 49.2-55.3) in 2007-2014 and 55.7% (95% CI, 50.8-60.5) in 2015-2018. Finally, the percentage of participants in whom targets for all 3 risk factors were simultaneously achieved plateaued after 2010 and was 22.2% (95% CI, 17.9-27.3) in 2015-2018.
Trends in diabetes treatment—The use of glucose lowering drugs increased from 74.1% in 1999-2002 to 82.7% in 2007-2010 and then stabilized. A shift toward a safer glucose lowering treatment choice was observed with a decline in the use of older glucose lowering medications such as sulfonylureas, which increases the risk of hypoglycemia, and an increase in the use of metformin, insulin, and newer agents such as sodium-glucose cotransporter 2 inhibitors.
Similarly, blood pressure lowering medication use rose from 1999-2002 to 2007-2010 and then stabilized, with increased use of first-line recommended treatments including angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers. Likewise, statin use rose from 28.4% in 1999-2002 to 56% in 2011-2014 and then stabilized. The total number of drugs used culminated in 2011-2014 with 60% of participants using more than 5 drugs and then leveled off to 57.2% in 2015-2018. Lastly, health insurance status and race or ethnicity impacted the likelihood of receiving monotherapy or combination drug therapy when targets for glycemic, blood pressure, or lipid control were not achieved.
Conclusion. Despite great progress in the control of diabetes and its associated risk factors between 1999 and 2010, this trend declined for glycemic and blood pressure control and leveled off for lipid control in adult NHANES participants with diabetes after 2010. First-line treatments for diabetes and associated risk factors remain underused, and treatment intensification may not be sufficiently considered in patients with uncontrolled risk factors despite clinical guideline recommendations. The findings of this study may portend a possible population-level increase in diabetes-related illnesses in the years to come.
Commentary
The thorough understanding of trends in management of diseases is critical to inform public health policies and planning. Well designed clinical studies heavily influence the development of public health policies and clinical guidelines, which in turn drive real-world clinical practice. In a recent analysis utilizing data from NHANES, Fang et al1 showed evidence of a general shift toward less intensive treatment of diabetes, hypertension, and hypercholesterolemia in adults living in the US during the last decade.
Similarly, in a separate study using NHANES data collected between 1999 and 2018 published in JAMA just 2 weeks after the current report, Wang et al2 confirms this declining trend in diabetes management with only 21.2% of diabetic adults simultaneously attaining glycemic, blood pressure, and lipid level targets during the same period. What led to the decline in more stringent risk factor and diabetes management since 2010 observed in these studies? One possible explanation, as suggested by Fang et al, is that major clinical trials from the late 2000s—including Action to Control Cardiovascular Risk in Diabetes, UK Prospective Diabetes Study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation, and Veterans Affairs Diabetes Trial—that assessed the effects of intensive glycemic control (with target HbA1c < 6.5%) found that intensive treatment of diabetes compared to standard care had no cardiovascular benefit albeit increasing the risk of hypoglycemia. Thus, these trial findings may have translated into suboptimal diabetes treatment observed in some NHANES participants. Wang et al propose that effective tailored approaches are needed to improve risk factor control in diabetic patients, such as enhance and maintain adherence to medications and healthy lifestyle behaviors, as well as better access to health care and therapeutic education.
The changes in recent trends in diabetes management have immense clinical implications. The authors of this study suggest a link between the recent relaxation of glycemic targets, as well as risk factor control, and a resurgence of diabetic complications such as lower limb amputation or stroke. Indeed, several recent studies indicate an upward trend or plateau in diabetic complications which had been decreasing in prevalence prior to 2010.3 For example, lower extremity amputation has surged by more than 25% between 2010 and 2015, especially in young and middle-aged adults.4 Among the arguments brought forward that this recent resurgence in amputations is directly linked to worsening glycemic control is the fact that between 2007 and 2010, when glucose levels were best controlled within the previous 30-year period, amputations were also at the lowest levels. Moreover, data from the Centers for Disease Control and Prevention also show a 55% increase in mortality (from 15.7 to 24.2 per 1000) among diabetic patients between 2010 and 2015.14 On the other hand, a growing number of studies show that an increase of inappropriate treatment intensification—reaching HbA1c levels that are way below the recommended targets—is associated with adverse consequences in diabetic patients particularly in those aged more than 65 years.5-7 These seemingly contradictory findings highlight the importance of a personalized and thoughtful approach to the management of diabetes and its risk factors. As an example, an increase in the use of newer and safer glucose lowering drugs (eg, sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and dipeptidyl peptidase 4 inhibitors) can help achieve better HbA1c goals with a reduced risk of hypoglycemic episodes as recently shown by a Danish study.8 In this study, the authors concluded that the reduction of the rate of hypoglycemic episodes leading to hospitalization in Denmark was directly linked to the use of these safer and newer glucose lowering drugs.
A discussion on the specifics of trends in diabetes treatment and control must include considerations in older adults aged more than 65 years who constitute more than 40% of the diabetic population. Despite the high prevalence of diabetes in this vulnerable population, such data are still insufficient in the literature and are critically needed to inform public health policies and clinical guidelines. In epidemiological studies focusing on diabetic complications from the last 10 years, concerning increases have been observed in younger9 and middle-aged adults while remaining stable in older adults. However, the risk of hypoglycemia or severe hypoglycemia remains high in older adults living in nursing facilities, even in those with an elevated HbA1c of greater than 8%.7 Moreover, in light of more relaxed HbA1c treatment goals for older frail adults as recommended by international guidelines since 2010,10,11 recent findings from the French GERODIAB cohort show an increased mortality (hazard ratio, 1.76) in type 2 diabetics aged 70 years and older with HbA1c greater than or equal to 8.6%.12 Similarly, a 5-year retrospective British study from 2018 which included patients aged 70 years and older, shows an increased overall mortality in those with HbA1c greater than 8.5%.13 Taken together, further age-stratified analysis utilizing data from large cohort studies including NHANES may help to clarify national trends in diabetes treatment and risk factor control as well as diabetic complications specific to the geriatric population. By being better informed of such trends, clinicians could then develop treatment strategies that minimize complications (eg, hypoglycemia, falls) while achieving favorable outcomes (eg, reduce hyperglycemic emergencies, improve survival) in frail older patients.
Applications for Clinical Practice
The understanding of population-wide trends in diabetes control is critical to planning public health approaches for the prevention and treatment of this disease and its complications. In older adults, the high risk of hypoglycemic events and insufficient epidemiological data on trends of diabetes control hinder diabetes management. Personalized treatment targets taking into account geriatric syndromes and general health status, as well as multidisciplinary management involving endocrinologists, geriatricians, and clinical pharmacists, are necessary to optimize care in older adults with diabetes.
Study Overview
Objective. To update national trends in the treatment and risk factor control of diabetic patients from 1999 through 2018 in the US using data from the National Health and Nutrition Examination Survey (NHANES) with the goal of identifying population subgroups with the highest probability of having untreated risk factors.
Design. The authors conducted a cross-sectional analysis of data from NHANES focusing on adults with diabetes. They examined patient characteristics and medication use over time and estimated the prevalence of risk factor control and medication use. To minimize the effects of a small sample size, the survey years were pooled into 4-year intervals. The variables studied included glycated hemoglobin (HbA1c), blood pressure, serum cholesterol, medication use, sociodemographic characteristics, and weight status. For statistical analysis, logistic and multinomial logistic regression models were used to examine factors associated with treatment in participants who did not achieve targets for glycemic, blood pressure, and lipid control. Temporal trends were estimated using 2-piece linear spline models with 1 knot at inflection points.
Setting and participants. The NHANES program began in the early 1960s to monitor the health of the US population. In 1999, the survey became a continuous program combining interviews and physical examinations. The survey examines a nationally representative sample of about 5000 persons each year. This study included 6653 participants who were nonpregnant, aged older than 20 years, reported a diagnosis of diabetes from a physician, and participated in NHANES from 1999 through 2018.
Main outcome measures. The main outcome measures were temporal trends in risk factor control (glycemic, blood pressure, or lipid levels) and medication use (glucose lowering, blood pressure lowering, or lipid lowering medications), and number as well as class of drug used, from 1999 through 2018 in diabetic adults from the US participating in NHANES.
Results. Sociodemographic characteristics of the studied diabetes population—The age and racial or ethnic distribution of participants with diabetes were stable from 1999 through 2018, whereas participants with a college degree, higher income, health insurance, obesity, or long-standing diabetes increased during the same period.
Trends in diabetes risk factor control—The trends for glycemic, blood pressure, and lipid control were nonlinear, with an inflection point around 2010. Glycemic control was defined as HbA1c less than 7%, blood pressure was considered controlled if less than 140/90 mmHg, and lipid was controlled if non-HDL cholesterol level was less than 130 mg/dL. Although these chosen targets were based on the most recent clinical guidelines, the authors declared that they observed similar trends when alternative targets were used. The level of risk factor control improved in all diabetic patients from 1999 through 2010. However, the percentage of adult diabetic participants for whom glycemic control was achieved declined from 57.4% (95% CI, 52.9-61.8) in 2007-2010 to 50.5% (95% CI, 45.8-55.3) in 2015-2018. Blood pressure control was achieved in 74.2% of participants (95% CI, 70.7-77.4) in 2011-2014 but declined to 70.4% (95% CI, 66.7-73.8) in 2015-2018. Control in lipid levels improved during the entire study period; however, the rate of improvement heavily declined after 2007 with lipid target levels attained in 52.3% of participants (95% CI, 49.2-55.3) in 2007-2014 and 55.7% (95% CI, 50.8-60.5) in 2015-2018. Finally, the percentage of participants in whom targets for all 3 risk factors were simultaneously achieved plateaued after 2010 and was 22.2% (95% CI, 17.9-27.3) in 2015-2018.
Trends in diabetes treatment—The use of glucose lowering drugs increased from 74.1% in 1999-2002 to 82.7% in 2007-2010 and then stabilized. A shift toward a safer glucose lowering treatment choice was observed with a decline in the use of older glucose lowering medications such as sulfonylureas, which increases the risk of hypoglycemia, and an increase in the use of metformin, insulin, and newer agents such as sodium-glucose cotransporter 2 inhibitors.
Similarly, blood pressure lowering medication use rose from 1999-2002 to 2007-2010 and then stabilized, with increased use of first-line recommended treatments including angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers. Likewise, statin use rose from 28.4% in 1999-2002 to 56% in 2011-2014 and then stabilized. The total number of drugs used culminated in 2011-2014 with 60% of participants using more than 5 drugs and then leveled off to 57.2% in 2015-2018. Lastly, health insurance status and race or ethnicity impacted the likelihood of receiving monotherapy or combination drug therapy when targets for glycemic, blood pressure, or lipid control were not achieved.
Conclusion. Despite great progress in the control of diabetes and its associated risk factors between 1999 and 2010, this trend declined for glycemic and blood pressure control and leveled off for lipid control in adult NHANES participants with diabetes after 2010. First-line treatments for diabetes and associated risk factors remain underused, and treatment intensification may not be sufficiently considered in patients with uncontrolled risk factors despite clinical guideline recommendations. The findings of this study may portend a possible population-level increase in diabetes-related illnesses in the years to come.
Commentary
The thorough understanding of trends in management of diseases is critical to inform public health policies and planning. Well designed clinical studies heavily influence the development of public health policies and clinical guidelines, which in turn drive real-world clinical practice. In a recent analysis utilizing data from NHANES, Fang et al1 showed evidence of a general shift toward less intensive treatment of diabetes, hypertension, and hypercholesterolemia in adults living in the US during the last decade.
Similarly, in a separate study using NHANES data collected between 1999 and 2018 published in JAMA just 2 weeks after the current report, Wang et al2 confirms this declining trend in diabetes management with only 21.2% of diabetic adults simultaneously attaining glycemic, blood pressure, and lipid level targets during the same period. What led to the decline in more stringent risk factor and diabetes management since 2010 observed in these studies? One possible explanation, as suggested by Fang et al, is that major clinical trials from the late 2000s—including Action to Control Cardiovascular Risk in Diabetes, UK Prospective Diabetes Study, Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation, and Veterans Affairs Diabetes Trial—that assessed the effects of intensive glycemic control (with target HbA1c < 6.5%) found that intensive treatment of diabetes compared to standard care had no cardiovascular benefit albeit increasing the risk of hypoglycemia. Thus, these trial findings may have translated into suboptimal diabetes treatment observed in some NHANES participants. Wang et al propose that effective tailored approaches are needed to improve risk factor control in diabetic patients, such as enhance and maintain adherence to medications and healthy lifestyle behaviors, as well as better access to health care and therapeutic education.
The changes in recent trends in diabetes management have immense clinical implications. The authors of this study suggest a link between the recent relaxation of glycemic targets, as well as risk factor control, and a resurgence of diabetic complications such as lower limb amputation or stroke. Indeed, several recent studies indicate an upward trend or plateau in diabetic complications which had been decreasing in prevalence prior to 2010.3 For example, lower extremity amputation has surged by more than 25% between 2010 and 2015, especially in young and middle-aged adults.4 Among the arguments brought forward that this recent resurgence in amputations is directly linked to worsening glycemic control is the fact that between 2007 and 2010, when glucose levels were best controlled within the previous 30-year period, amputations were also at the lowest levels. Moreover, data from the Centers for Disease Control and Prevention also show a 55% increase in mortality (from 15.7 to 24.2 per 1000) among diabetic patients between 2010 and 2015.14 On the other hand, a growing number of studies show that an increase of inappropriate treatment intensification—reaching HbA1c levels that are way below the recommended targets—is associated with adverse consequences in diabetic patients particularly in those aged more than 65 years.5-7 These seemingly contradictory findings highlight the importance of a personalized and thoughtful approach to the management of diabetes and its risk factors. As an example, an increase in the use of newer and safer glucose lowering drugs (eg, sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, and dipeptidyl peptidase 4 inhibitors) can help achieve better HbA1c goals with a reduced risk of hypoglycemic episodes as recently shown by a Danish study.8 In this study, the authors concluded that the reduction of the rate of hypoglycemic episodes leading to hospitalization in Denmark was directly linked to the use of these safer and newer glucose lowering drugs.
A discussion on the specifics of trends in diabetes treatment and control must include considerations in older adults aged more than 65 years who constitute more than 40% of the diabetic population. Despite the high prevalence of diabetes in this vulnerable population, such data are still insufficient in the literature and are critically needed to inform public health policies and clinical guidelines. In epidemiological studies focusing on diabetic complications from the last 10 years, concerning increases have been observed in younger9 and middle-aged adults while remaining stable in older adults. However, the risk of hypoglycemia or severe hypoglycemia remains high in older adults living in nursing facilities, even in those with an elevated HbA1c of greater than 8%.7 Moreover, in light of more relaxed HbA1c treatment goals for older frail adults as recommended by international guidelines since 2010,10,11 recent findings from the French GERODIAB cohort show an increased mortality (hazard ratio, 1.76) in type 2 diabetics aged 70 years and older with HbA1c greater than or equal to 8.6%.12 Similarly, a 5-year retrospective British study from 2018 which included patients aged 70 years and older, shows an increased overall mortality in those with HbA1c greater than 8.5%.13 Taken together, further age-stratified analysis utilizing data from large cohort studies including NHANES may help to clarify national trends in diabetes treatment and risk factor control as well as diabetic complications specific to the geriatric population. By being better informed of such trends, clinicians could then develop treatment strategies that minimize complications (eg, hypoglycemia, falls) while achieving favorable outcomes (eg, reduce hyperglycemic emergencies, improve survival) in frail older patients.
Applications for Clinical Practice
The understanding of population-wide trends in diabetes control is critical to planning public health approaches for the prevention and treatment of this disease and its complications. In older adults, the high risk of hypoglycemic events and insufficient epidemiological data on trends of diabetes control hinder diabetes management. Personalized treatment targets taking into account geriatric syndromes and general health status, as well as multidisciplinary management involving endocrinologists, geriatricians, and clinical pharmacists, are necessary to optimize care in older adults with diabetes.
1. Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999-2018. N Engl J Med. 2021;384(23):2219-28. doi:10.1056/NEJMsa2032271
2. Wang L, Li X, Wang Z, et al. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. JAMA. 2021. doi:10.1001/jama.2021.9883
3. Gregg EW, Hora I, Benoit SR. Resurgence in Diabetes-Related Complications. JAMA. 2019;321(19):1867-8. doi:10.1001/jama.2019.3471
4. Caruso P, Scappaticcio L, Maiorino MI, et al. Up and down waves of glycemic control and lower-extremity amputation in diabetes. Cardiovasc Diabetol. 2021;20(1):135. doi:10.1186/s12933-021-01325-3
5. Bongaerts B, Arnold SV, Charbonnel BH, et al. Inappropriate intensification of glucose-lowering treatment in older patients with type 2 diabetes: the global DISCOVER study. BMJ Open Diabetes Res Care. 2021;9(1)e001585. doi:10.1136/bmjdrc-2020-001585
6. Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116-1124. doi: 10.1001/jamainternmed.2014.1824
7. Bouillet B, Tscherter P, Vaillard L, et al. Frequent and severe hypoglycaemia detected with continuous glucose monitoring in older institutionalised patients with diabetes. Age Ageing. 2021;afab128. doi: 10.1093/ageing/afab128
8. Jensen MH, Hejlesen O, Vestergaard P. Epidemiology of hypoglycaemic episodes leading to hospitalisations in Denmark in 1998-2018. Diabetologia. 2021. doi: 10.1007/s00125-021-05507-2
9. TODAY Study Group, Bjornstad P, Drews KL, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385(5):416-426. doi: 10.1056/NEJMoa2100165
10. Sinclair AJ, Paolisso G, Castro M, et al. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37 Suppl 3:S27-S38. doi:10.1016/S1262-3636(11)70962-4
11. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650-2664. doi: 10.2337/dc12-1801
12. Doucet J, Verny C, Balkau B, et al. Haemoglobin A1c and 5-year all-cause mortality in French type 2 diabetic patients aged 70 years and older: The GERODIAB observational cohort. Diabetes Metab. 2018;44(6):465-472. doi: 10.1016/j.diabet.2018.05.003
13. Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):476-486. doi: 10.1016/S2213-8587(18)30048-2
14. US Centers for Disease Control and Prevention. US diabetes surveillance system and diabetes atlas, 2019. https://www.cdc.gov/diabetes/data
1. Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999-2018. N Engl J Med. 2021;384(23):2219-28. doi:10.1056/NEJMsa2032271
2. Wang L, Li X, Wang Z, et al. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999-2018. JAMA. 2021. doi:10.1001/jama.2021.9883
3. Gregg EW, Hora I, Benoit SR. Resurgence in Diabetes-Related Complications. JAMA. 2019;321(19):1867-8. doi:10.1001/jama.2019.3471
4. Caruso P, Scappaticcio L, Maiorino MI, et al. Up and down waves of glycemic control and lower-extremity amputation in diabetes. Cardiovasc Diabetol. 2021;20(1):135. doi:10.1186/s12933-021-01325-3
5. Bongaerts B, Arnold SV, Charbonnel BH, et al. Inappropriate intensification of glucose-lowering treatment in older patients with type 2 diabetes: the global DISCOVER study. BMJ Open Diabetes Res Care. 2021;9(1)e001585. doi:10.1136/bmjdrc-2020-001585
6. Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116-1124. doi: 10.1001/jamainternmed.2014.1824
7. Bouillet B, Tscherter P, Vaillard L, et al. Frequent and severe hypoglycaemia detected with continuous glucose monitoring in older institutionalised patients with diabetes. Age Ageing. 2021;afab128. doi: 10.1093/ageing/afab128
8. Jensen MH, Hejlesen O, Vestergaard P. Epidemiology of hypoglycaemic episodes leading to hospitalisations in Denmark in 1998-2018. Diabetologia. 2021. doi: 10.1007/s00125-021-05507-2
9. TODAY Study Group, Bjornstad P, Drews KL, et al. Long-Term Complications in Youth-Onset Type 2 Diabetes. N Engl J Med. 2021;385(5):416-426. doi: 10.1056/NEJMoa2100165
10. Sinclair AJ, Paolisso G, Castro M, et al. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37 Suppl 3:S27-S38. doi:10.1016/S1262-3636(11)70962-4
11. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650-2664. doi: 10.2337/dc12-1801
12. Doucet J, Verny C, Balkau B, et al. Haemoglobin A1c and 5-year all-cause mortality in French type 2 diabetic patients aged 70 years and older: The GERODIAB observational cohort. Diabetes Metab. 2018;44(6):465-472. doi: 10.1016/j.diabet.2018.05.003
13. Forbes A, Murrells T, Mulnier H, Sinclair AJ. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(6):476-486. doi: 10.1016/S2213-8587(18)30048-2
14. US Centers for Disease Control and Prevention. US diabetes surveillance system and diabetes atlas, 2019. https://www.cdc.gov/diabetes/data
Patients with diabetes more likely to be hospitalized, especially with foot infection
People with diabetes are at increased risk of hospitalization for infection, as well as infection-related mortality, shows a large U.S. study that suggests the risk is even higher in younger and Black individuals.
Michael Fang, PhD, Johns Hopkins University, Baltimore, and colleagues studied more than 12,000 participants in a community cohort study who were followed for an average of 24 years, between 1987-1989 and 2019.
Participants with diabetes faced a 67% increase risk of infection-related hospitalization, compared with those without diabetes.
Of particular note, the risk of hospitalization with foot infection was almost sixfold higher for people with diabetes than those without.
The research, published in Diabetologia on August 4, also suggests that diabetes may be associated with a 72% increased risk of infection-related mortality, although the absolute numbers were small.
Dr. Fang explained to this news organization that they focused on infection-related hospitalization and mortality “because these are comprehensively tracked in administrative data and ... are the most severe types of outcomes.”
However, this is probably just the tip of the iceberg, as people with diabetes are “likely at increased risk for milder infection too,” which can have a “significant adverse impact on people’s well-being and quality of life.”
As a result of their findings, the authors call for “broader guidance on infection prevention and management” in people with diabetes. To achieve this, Dr. Fang said, “we need to better understand why diabetes is associated with an increased risk of infection-related complications.”
“One likely factor is glycemic control: Emerging research suggests patients with diabetes with better glycemic control may be at significantly lower risk of infection-related complications.”
He continued that, in younger patients, a factor for worse outcomes could be that “diabetes tends to be more aggressive when it emerges early in life,” while in Black patients “there is research highlighting Black-White differences in glycemic control, access to care, and beliefs around vaccines.”
Overall, their findings – coupled with recent data showing that diabetes is an important risk factor for adverse outcomes with COVID-19 infection – paint “a common picture,” Dr. Fang said.
“People with diabetes are much more susceptible to infection-related complications, including COVID-related hospitalization and mortality,” which suggests people with diabetes “may need to be especially cautious.”
Adds to existing literature; amputations begin with infections
Robert A. Gabbay, MD, PhD, chief scientific and medical officer for the American Diabetes Association (ADA), said the study “does add to the existing literature by having followed a larger number of people over time and linking them to serious complications from infections.”
“Sadly, we have seen this play out in real-time during the COVID-19 pandemic.”
“One of the sobering bits of data is the significant health disparities that exist in Black Americans and the fact that foot infections remain a significant problem,” he said in an interview.
“Given that amputation rates for [Black Americans] are three times higher than White Americans, amputations begin with infections,” Dr. Gabbay added, noting the ADA “has been taking a strong stand to prevent amputations and address the inequities in health that exist.”
Jamie Hartmann-Boyce, PhD, from the University of Oxford, U.K., who was not involved in the study, commented that diabetes is a “well-known risk factor for worse outcomes from all kinds of infection,” which is why they “are prioritized for flu vaccination every year.”
She told this news organization that the current study “further confirms that people with diabetes are more likely to be hospitalized for infection of any type and most markedly for foot infection.”
“These new data further highlight the need for public health interventions to prevent type 2 diabetes, and for preventive health care in people with diabetes, including access to diabetes medications and support and to vaccinations to prevent infection,” added Dr. Hartmann-Boyce, who is a senior research fellow in health behaviors.
Diabetes is thought to be associated with susceptibility to infection via mechanisms such as impaired neutrophil functioning and humoral immune responses, and studies have shown a link with both common and rare infections.
However, the authors point out that “most” of those included “small clinical populations and were cross-sectional or had short follow-up.”
Guidelines for diabetes management, they note, also “pay less attention” to infectious diseases than they do to the prevention of micro- and macrovascular complications.
ARIC data mined for infections in those with diabetes
The team analyzed data from the ongoing U.S. community-based Atherosclerosis Risk in Communities (ARIC) study.
The National Heart, Lung, and Blood Institute–sponsored cohort was comprised of adults aged 45-64 years from four U.S. communities, recruited between 1987 and 1989 for clinical examinations, medical interviews, and laboratory tests, repeated over five more visits up to 2018-2019.
For the current analysis, the team included 12,739 individuals with a mean age of 54.5 years, of whom 54.3% were female and 24.7% were Black.
Patients were defined as having diabetes if their baseline fasting blood glucose was greater than or equal to 7 mmol/L, or nonfasting glucose was greater than or equal to 11.1 mmol/l, they self-reported a diagnosis of diabetes by a physician, or they were taking glucose-lowering medication at the first study visit. The researchers weren’t able to distinguish between type 1 and type 2 diabetes.
In total, 1,485 individuals had diabetes at baseline. They were more likely to be older, Black, have a low socioeconomic status, and have worse cardiometabolic health than participants without diabetes.
Over an average follow-up of 23.8 years, there were 4,229 incident hospitalizations for infection, at an overall rate of 15.9 per 1,000 person-years.
Individuals with diabetes at baseline had a higher rate of hospitalizations than those without, at 25.4 per 1,000 person-years versus 15.2 per 1,000 person-years.
After taking into account sociodemographic characteristics, socioeconomic status, and cardiometabolic risk factors, this equated to a hazard ratio for hospitalization with any infection of 1.67 (P < .001).
The risk of hospitalization for any infection was significantly higher for younger patients with diabetes, defined as aged less than 55 years (P = .005), and for Black patients (P < .001).
While the increased risk was generally consistent across infection types, it was markedly increased for foot infection, at a hazard ratio of 5.99 (P < .001).
Overall, there were few deaths due to infection in the study, at just 362. The risk of infection mortality was nevertheless significantly increased in people with diabetes, at an adjusted hazard ratio of 1.72 (P < .001).
Dr. Fang has reported being supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Selvin has reported being supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Selvin is an associate editor for Diabetologia and had no role in the peer review of the manuscript.
A version of this article first appeared on Medscape.com.
People with diabetes are at increased risk of hospitalization for infection, as well as infection-related mortality, shows a large U.S. study that suggests the risk is even higher in younger and Black individuals.
Michael Fang, PhD, Johns Hopkins University, Baltimore, and colleagues studied more than 12,000 participants in a community cohort study who were followed for an average of 24 years, between 1987-1989 and 2019.
Participants with diabetes faced a 67% increase risk of infection-related hospitalization, compared with those without diabetes.
Of particular note, the risk of hospitalization with foot infection was almost sixfold higher for people with diabetes than those without.
The research, published in Diabetologia on August 4, also suggests that diabetes may be associated with a 72% increased risk of infection-related mortality, although the absolute numbers were small.
Dr. Fang explained to this news organization that they focused on infection-related hospitalization and mortality “because these are comprehensively tracked in administrative data and ... are the most severe types of outcomes.”
However, this is probably just the tip of the iceberg, as people with diabetes are “likely at increased risk for milder infection too,” which can have a “significant adverse impact on people’s well-being and quality of life.”
As a result of their findings, the authors call for “broader guidance on infection prevention and management” in people with diabetes. To achieve this, Dr. Fang said, “we need to better understand why diabetes is associated with an increased risk of infection-related complications.”
“One likely factor is glycemic control: Emerging research suggests patients with diabetes with better glycemic control may be at significantly lower risk of infection-related complications.”
He continued that, in younger patients, a factor for worse outcomes could be that “diabetes tends to be more aggressive when it emerges early in life,” while in Black patients “there is research highlighting Black-White differences in glycemic control, access to care, and beliefs around vaccines.”
Overall, their findings – coupled with recent data showing that diabetes is an important risk factor for adverse outcomes with COVID-19 infection – paint “a common picture,” Dr. Fang said.
“People with diabetes are much more susceptible to infection-related complications, including COVID-related hospitalization and mortality,” which suggests people with diabetes “may need to be especially cautious.”
Adds to existing literature; amputations begin with infections
Robert A. Gabbay, MD, PhD, chief scientific and medical officer for the American Diabetes Association (ADA), said the study “does add to the existing literature by having followed a larger number of people over time and linking them to serious complications from infections.”
“Sadly, we have seen this play out in real-time during the COVID-19 pandemic.”
“One of the sobering bits of data is the significant health disparities that exist in Black Americans and the fact that foot infections remain a significant problem,” he said in an interview.
“Given that amputation rates for [Black Americans] are three times higher than White Americans, amputations begin with infections,” Dr. Gabbay added, noting the ADA “has been taking a strong stand to prevent amputations and address the inequities in health that exist.”
Jamie Hartmann-Boyce, PhD, from the University of Oxford, U.K., who was not involved in the study, commented that diabetes is a “well-known risk factor for worse outcomes from all kinds of infection,” which is why they “are prioritized for flu vaccination every year.”
She told this news organization that the current study “further confirms that people with diabetes are more likely to be hospitalized for infection of any type and most markedly for foot infection.”
“These new data further highlight the need for public health interventions to prevent type 2 diabetes, and for preventive health care in people with diabetes, including access to diabetes medications and support and to vaccinations to prevent infection,” added Dr. Hartmann-Boyce, who is a senior research fellow in health behaviors.
Diabetes is thought to be associated with susceptibility to infection via mechanisms such as impaired neutrophil functioning and humoral immune responses, and studies have shown a link with both common and rare infections.
However, the authors point out that “most” of those included “small clinical populations and were cross-sectional or had short follow-up.”
Guidelines for diabetes management, they note, also “pay less attention” to infectious diseases than they do to the prevention of micro- and macrovascular complications.
ARIC data mined for infections in those with diabetes
The team analyzed data from the ongoing U.S. community-based Atherosclerosis Risk in Communities (ARIC) study.
The National Heart, Lung, and Blood Institute–sponsored cohort was comprised of adults aged 45-64 years from four U.S. communities, recruited between 1987 and 1989 for clinical examinations, medical interviews, and laboratory tests, repeated over five more visits up to 2018-2019.
For the current analysis, the team included 12,739 individuals with a mean age of 54.5 years, of whom 54.3% were female and 24.7% were Black.
Patients were defined as having diabetes if their baseline fasting blood glucose was greater than or equal to 7 mmol/L, or nonfasting glucose was greater than or equal to 11.1 mmol/l, they self-reported a diagnosis of diabetes by a physician, or they were taking glucose-lowering medication at the first study visit. The researchers weren’t able to distinguish between type 1 and type 2 diabetes.
In total, 1,485 individuals had diabetes at baseline. They were more likely to be older, Black, have a low socioeconomic status, and have worse cardiometabolic health than participants without diabetes.
Over an average follow-up of 23.8 years, there were 4,229 incident hospitalizations for infection, at an overall rate of 15.9 per 1,000 person-years.
Individuals with diabetes at baseline had a higher rate of hospitalizations than those without, at 25.4 per 1,000 person-years versus 15.2 per 1,000 person-years.
After taking into account sociodemographic characteristics, socioeconomic status, and cardiometabolic risk factors, this equated to a hazard ratio for hospitalization with any infection of 1.67 (P < .001).
The risk of hospitalization for any infection was significantly higher for younger patients with diabetes, defined as aged less than 55 years (P = .005), and for Black patients (P < .001).
While the increased risk was generally consistent across infection types, it was markedly increased for foot infection, at a hazard ratio of 5.99 (P < .001).
Overall, there were few deaths due to infection in the study, at just 362. The risk of infection mortality was nevertheless significantly increased in people with diabetes, at an adjusted hazard ratio of 1.72 (P < .001).
Dr. Fang has reported being supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Selvin has reported being supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Selvin is an associate editor for Diabetologia and had no role in the peer review of the manuscript.
A version of this article first appeared on Medscape.com.
People with diabetes are at increased risk of hospitalization for infection, as well as infection-related mortality, shows a large U.S. study that suggests the risk is even higher in younger and Black individuals.
Michael Fang, PhD, Johns Hopkins University, Baltimore, and colleagues studied more than 12,000 participants in a community cohort study who were followed for an average of 24 years, between 1987-1989 and 2019.
Participants with diabetes faced a 67% increase risk of infection-related hospitalization, compared with those without diabetes.
Of particular note, the risk of hospitalization with foot infection was almost sixfold higher for people with diabetes than those without.
The research, published in Diabetologia on August 4, also suggests that diabetes may be associated with a 72% increased risk of infection-related mortality, although the absolute numbers were small.
Dr. Fang explained to this news organization that they focused on infection-related hospitalization and mortality “because these are comprehensively tracked in administrative data and ... are the most severe types of outcomes.”
However, this is probably just the tip of the iceberg, as people with diabetes are “likely at increased risk for milder infection too,” which can have a “significant adverse impact on people’s well-being and quality of life.”
As a result of their findings, the authors call for “broader guidance on infection prevention and management” in people with diabetes. To achieve this, Dr. Fang said, “we need to better understand why diabetes is associated with an increased risk of infection-related complications.”
“One likely factor is glycemic control: Emerging research suggests patients with diabetes with better glycemic control may be at significantly lower risk of infection-related complications.”
He continued that, in younger patients, a factor for worse outcomes could be that “diabetes tends to be more aggressive when it emerges early in life,” while in Black patients “there is research highlighting Black-White differences in glycemic control, access to care, and beliefs around vaccines.”
Overall, their findings – coupled with recent data showing that diabetes is an important risk factor for adverse outcomes with COVID-19 infection – paint “a common picture,” Dr. Fang said.
“People with diabetes are much more susceptible to infection-related complications, including COVID-related hospitalization and mortality,” which suggests people with diabetes “may need to be especially cautious.”
Adds to existing literature; amputations begin with infections
Robert A. Gabbay, MD, PhD, chief scientific and medical officer for the American Diabetes Association (ADA), said the study “does add to the existing literature by having followed a larger number of people over time and linking them to serious complications from infections.”
“Sadly, we have seen this play out in real-time during the COVID-19 pandemic.”
“One of the sobering bits of data is the significant health disparities that exist in Black Americans and the fact that foot infections remain a significant problem,” he said in an interview.
“Given that amputation rates for [Black Americans] are three times higher than White Americans, amputations begin with infections,” Dr. Gabbay added, noting the ADA “has been taking a strong stand to prevent amputations and address the inequities in health that exist.”
Jamie Hartmann-Boyce, PhD, from the University of Oxford, U.K., who was not involved in the study, commented that diabetes is a “well-known risk factor for worse outcomes from all kinds of infection,” which is why they “are prioritized for flu vaccination every year.”
She told this news organization that the current study “further confirms that people with diabetes are more likely to be hospitalized for infection of any type and most markedly for foot infection.”
“These new data further highlight the need for public health interventions to prevent type 2 diabetes, and for preventive health care in people with diabetes, including access to diabetes medications and support and to vaccinations to prevent infection,” added Dr. Hartmann-Boyce, who is a senior research fellow in health behaviors.
Diabetes is thought to be associated with susceptibility to infection via mechanisms such as impaired neutrophil functioning and humoral immune responses, and studies have shown a link with both common and rare infections.
However, the authors point out that “most” of those included “small clinical populations and were cross-sectional or had short follow-up.”
Guidelines for diabetes management, they note, also “pay less attention” to infectious diseases than they do to the prevention of micro- and macrovascular complications.
ARIC data mined for infections in those with diabetes
The team analyzed data from the ongoing U.S. community-based Atherosclerosis Risk in Communities (ARIC) study.
The National Heart, Lung, and Blood Institute–sponsored cohort was comprised of adults aged 45-64 years from four U.S. communities, recruited between 1987 and 1989 for clinical examinations, medical interviews, and laboratory tests, repeated over five more visits up to 2018-2019.
For the current analysis, the team included 12,739 individuals with a mean age of 54.5 years, of whom 54.3% were female and 24.7% were Black.
Patients were defined as having diabetes if their baseline fasting blood glucose was greater than or equal to 7 mmol/L, or nonfasting glucose was greater than or equal to 11.1 mmol/l, they self-reported a diagnosis of diabetes by a physician, or they were taking glucose-lowering medication at the first study visit. The researchers weren’t able to distinguish between type 1 and type 2 diabetes.
In total, 1,485 individuals had diabetes at baseline. They were more likely to be older, Black, have a low socioeconomic status, and have worse cardiometabolic health than participants without diabetes.
Over an average follow-up of 23.8 years, there were 4,229 incident hospitalizations for infection, at an overall rate of 15.9 per 1,000 person-years.
Individuals with diabetes at baseline had a higher rate of hospitalizations than those without, at 25.4 per 1,000 person-years versus 15.2 per 1,000 person-years.
After taking into account sociodemographic characteristics, socioeconomic status, and cardiometabolic risk factors, this equated to a hazard ratio for hospitalization with any infection of 1.67 (P < .001).
The risk of hospitalization for any infection was significantly higher for younger patients with diabetes, defined as aged less than 55 years (P = .005), and for Black patients (P < .001).
While the increased risk was generally consistent across infection types, it was markedly increased for foot infection, at a hazard ratio of 5.99 (P < .001).
Overall, there were few deaths due to infection in the study, at just 362. The risk of infection mortality was nevertheless significantly increased in people with diabetes, at an adjusted hazard ratio of 1.72 (P < .001).
Dr. Fang has reported being supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute. Dr. Selvin has reported being supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute and National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Selvin is an associate editor for Diabetologia and had no role in the peer review of the manuscript.
A version of this article first appeared on Medscape.com.