User login
Zoledronate promotes postdenosumab bone retention
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
Right hip and pelvic pain
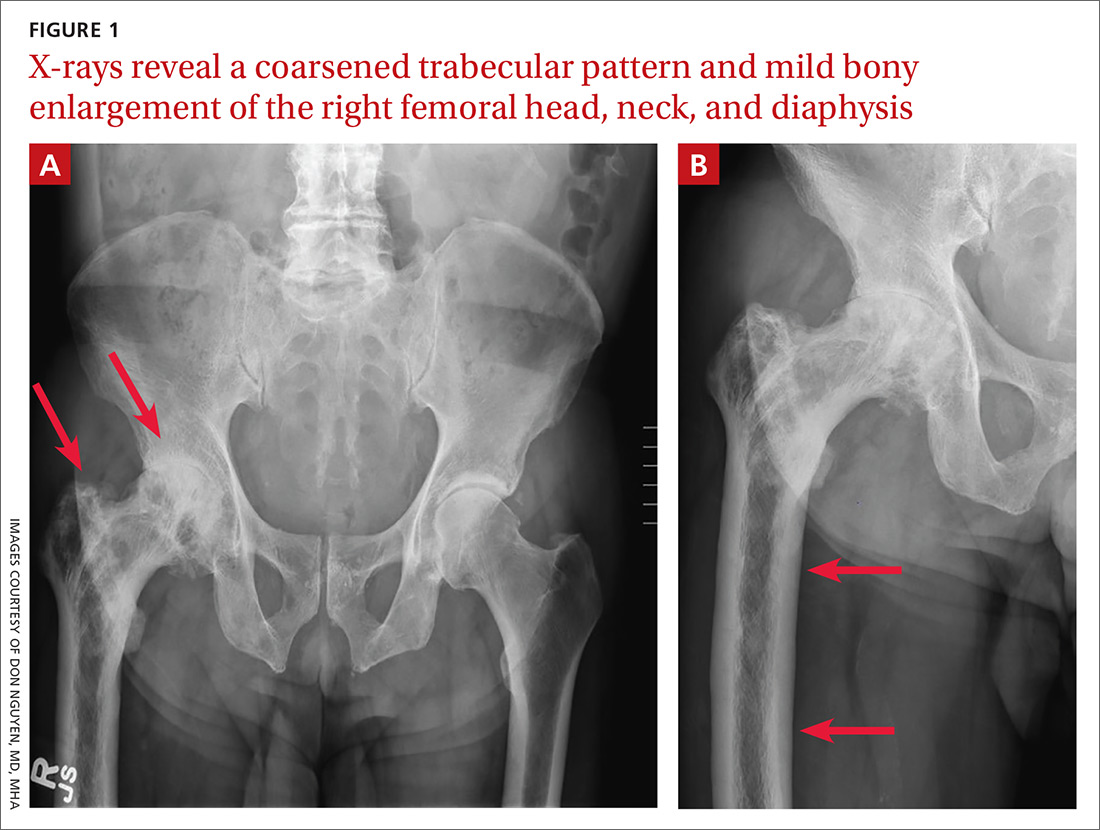
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
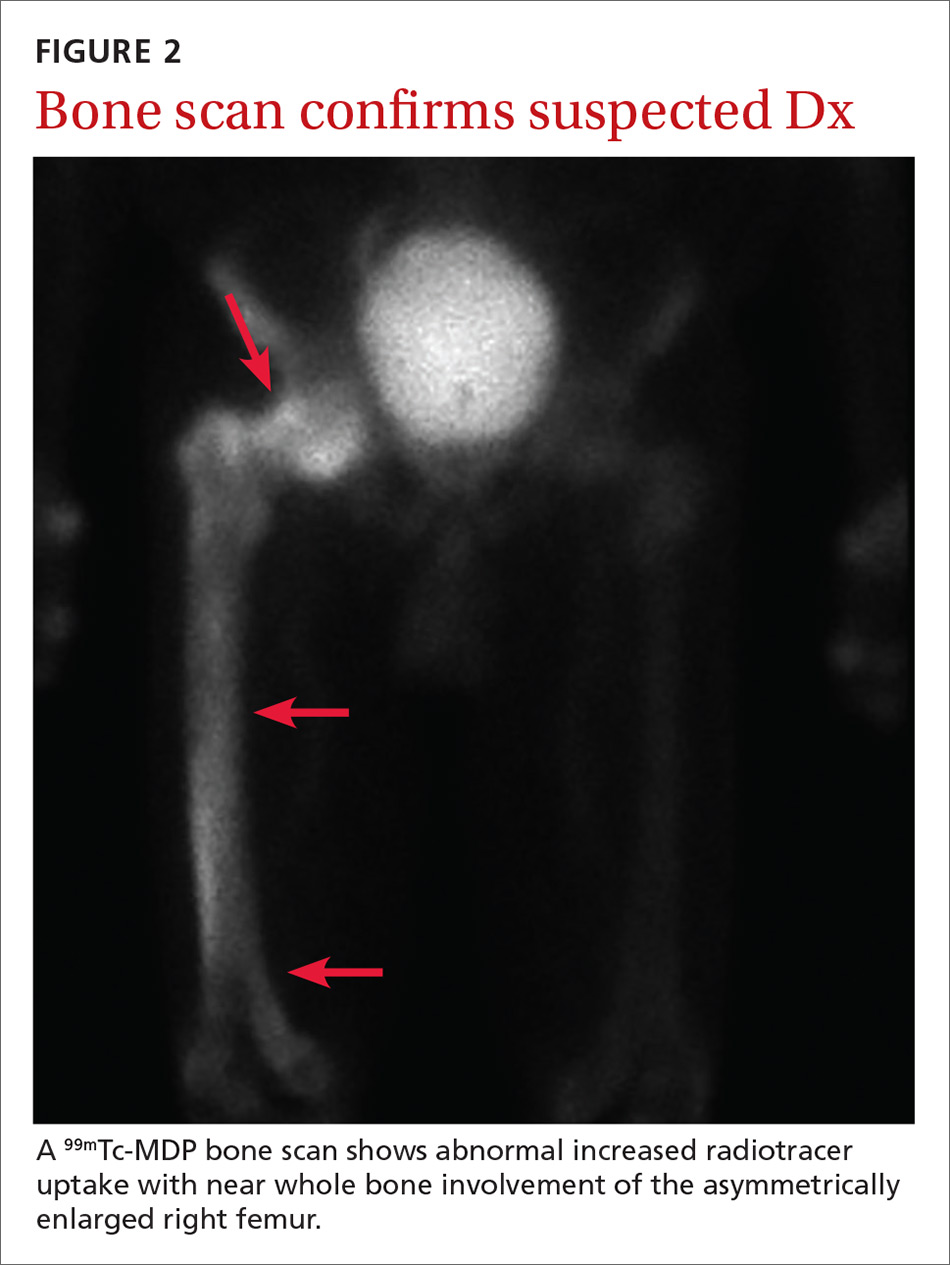
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.
Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; [email protected]
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.
Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; [email protected]
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.
Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; [email protected]
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
33-year-old man • flaccid paralysis in limbs • 30-lb weight loss • thyromegaly without nodules • Dx?
THE CASE
A 33-year-old Hispanic man with no significant past medical history presented to the emergency department with generalized flaccid paralysis in both arms and legs. Two days before, he had been working on a construction site in hot weather. The following day, he woke up with very little energy or strength to perform his daily activities, and he had pain in the inguinal area and both calves. He denied taking any medications or supplements.
The patient had complete muscle weakness and was unable to move his arms and legs. He reported dysphagia and an unintentional weight loss of 30 lb during the previous month.
On physical examination, the patient’s vital signs were within the normal range, and mild thyromegaly without nodules was present. Neurologic examination revealed decreased deep tendon reflexes with intact sensation. Muscle strength in his arms and legs was 0/5.
Initial laboratory test results included a potassium level of 2.2 mEq/L (normal range, 3.5–5 mEq/L) and normal acid-basic status that was confirmed by an arterial blood gas measurement. Serum magnesium was 1.6 mg/dL (normal range, 1.6–2.5 mg/dL); phosphorus, 1.9 mg/dL (normal range, 2.7–4.5 mg/dL); and random urinary potassium, 16 mEq/L (normal range, 25–125 mEq/L). An initial chest x-ray was normal, and an electrocardiogram showed a prolonged QT interval, flattening of the T wave, and a prominent U wave consistent with hypokalemia.
THE DIAGNOSIS
The initial clinical diagnosis was hypokalemic paralysis. The patient was treated with intravenous (IV) potassium chloride 40 mEq
Evaluation of the patient’s hypokalemia revealed the following: thyroid-stimulating hormone (TSH) level, < 0.01 microIU/mL (normal range, 0.27–4.2 microIU/mL); free T4 (thyroxine) level, 4.47 ng/dL (normal range, 0.08–1.70 ng/dL); total T3 (triiodothyronine) level, 17.5 ng/dL (normal range, 2.6–4.4 ng/dL).
The patient was diagnosed with hypokalemic periodic paralysis (HPP) secondary to thyrotoxicosis, also known as thyrotoxicosis periodic paralysis (TPP). His hyperthyroidism was treated with oral atenolol 25 mg/d and oral methimazole 10 mg tid.
Continue to: Within a few hours...
Within a few hours of this treatment, the patient experienced significant improvement in muscle strength and complete resolution of weakness in his arms and legs. Serial measurements of potassium levels normalized.
Further workup revealed that the patient’s thyroid-stimulating immunoglobulin (TSI) was 4.2 on the TSI index (normal, ≤ 1.3) and his thyroid peroxidase (TPO) antibody level was 133.4 IU/mL (normal, < 34 IU/mL). Ultrasonography showed decreased echogenicity of the thyroid gland, consistent with the acute phase of Hashimoto thyroiditis or Graves disease.
The patient was unaware that he had any thyroid disorder previously. He was a private-pay, undocumented immigrant and did not have a regular primary care physician. On discharge, he was referred to a local primary care physician as well as an endocrinologist. He was discharged on atenolol and methimazole.
DISCUSSION
A rare neuromuscular disorder known as periodic paralysis can be precipitated by a hypokalemic or hyperkalemic state; HPP is more common and can be either familial (a defect in the gene) or acquired (secondary to thyrotoxicosis; TPP).1,2 In both forms of periodic paralysis, patients present with hypokalemia and paralysis. Physicians need to look closely at thyroid lab test results so as not to miss the cause of the paralysis.
TPP is most commonly seen in Asian populations, and 95% of cases reported occur in males, despite the higher incidence of hyperthyroidism in females.3 TPP can be precipitated by emotional stress, steroid use, beta-adrenergic bronchodilators, heavy exercise, fasting, or high-carbohydrate meals.2-4 In our patient, heavy exercise and fasting likely were the triggers.
Continue to: The pathophysiology for the hypokalemia...
The pathophysiology for the hypokalemia in TPP is thought to involve the sodium/potassium–adenosine triphosphatase (Na+/K+–ATPase) pump. This pump activity is increased in skeletal muscle and platelets in patients with TPP vs patients with thyrotoxicosis alone.3,5
The role of Hashimoto thyrotoxicosis. Most acquired cases of TPP are mainly secondary to Graves disease with elevated levels of TSI and mildly elevated or normal levels of TPO. In this case, the patient was in the acute phase of Hashimoto thyrotoxicosis (“hashitoxicosis”) with elevated levels of TPO and only mildly elevated TSI.Imaging studies to support the diagnosis, such as a thyroid uptake scan or ultrasonography, are not necessary to determine the cause of thyrotoxicosis. In the absence of test results for TPO and TSI antibodies, however, a scan can be helpful.6,7
Treatment of TPP consists of early recognition and supportive management by correcting the potassium deficit; failure to do so could cause severe complications, such as respiratory failure and psychosis.8 Because of the risk for rebound hyperkalemia, serial potassium levels must be measured until a stable potassium level in the normal range is achieved.
Nonselective beta-blockers, such as propranolol (3 mg/kg) 4 times per day, have been reported to ameliorate the periodic paralysis and prevent rebound hyperkalemia.9 Finally, restoring a euthyroid state will prevent the patient from experiencing future attacks.
THE TAKEAWAY
Few medical conditions result in complete muscle paralysis in a matter of hours. Clinicians should consider the possibility of TPP in any patient who presents with acute onset of paralysis.
CORRESPONDENCE
Jorge Luis Chavez, MD; 8405 E. San Pedro Drive, Scottsdale, AZ 85258; [email protected].
1. Fontaine B. Periodic paralysis. Adv Genet. 2008;63:3-23.
2. Ober KP. Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of the literature. Medicine (Baltimore).1992;71:109-120.
3. Lin YF, Wu CC, Pei D, et al. Diagnosing thyrotoxic periodic paralysis in the ED. Am J Emerg Med. 2003;21:339-342.
4. Yu TS, Tseng CF, Chuang YY, et al. Potassium chloride supplementation alone may not improve hypokalemia in thyrotoxic hypokalemic periodic paralysis. J Emerg Med. 2007;32:263-265.
5. Chan A, Shinde R, Chow CC, et al. In vivo and in vitro sodium pump activity in subjects with thyrotoxic periodic paralysis. BMJ. 1991;303:1096-1099.
6. Harsch IA, Hahn EG, Strobel D. Hashitoxicosis—three cases and a review of the literature. Eur Endocrinol. 2008;4:70-72. 7. Pou Ucha JL. Imaging in hyperthyroidism. In: Díaz-Soto G, ed. Thyroid Disorders: Focus on Hyperthyroidism. InTechOpen; 2014. www.intechopen.com/books/thyroid-disorders-focus-on-hyperthyroidism/imaging-in-hyperthyroidism. Accessed January 14, 2020.
8. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci. 2010;340:147-153.
9. Lin SH, Lin YF. Propranolol rapidly reverses paralysis, hypokalemia, and hypophosphatemia in thyrotoxic periodic paralysis. Am J Kidney Dis. 2001;37:620-623.
THE CASE
A 33-year-old Hispanic man with no significant past medical history presented to the emergency department with generalized flaccid paralysis in both arms and legs. Two days before, he had been working on a construction site in hot weather. The following day, he woke up with very little energy or strength to perform his daily activities, and he had pain in the inguinal area and both calves. He denied taking any medications or supplements.
The patient had complete muscle weakness and was unable to move his arms and legs. He reported dysphagia and an unintentional weight loss of 30 lb during the previous month.
On physical examination, the patient’s vital signs were within the normal range, and mild thyromegaly without nodules was present. Neurologic examination revealed decreased deep tendon reflexes with intact sensation. Muscle strength in his arms and legs was 0/5.
Initial laboratory test results included a potassium level of 2.2 mEq/L (normal range, 3.5–5 mEq/L) and normal acid-basic status that was confirmed by an arterial blood gas measurement. Serum magnesium was 1.6 mg/dL (normal range, 1.6–2.5 mg/dL); phosphorus, 1.9 mg/dL (normal range, 2.7–4.5 mg/dL); and random urinary potassium, 16 mEq/L (normal range, 25–125 mEq/L). An initial chest x-ray was normal, and an electrocardiogram showed a prolonged QT interval, flattening of the T wave, and a prominent U wave consistent with hypokalemia.
THE DIAGNOSIS
The initial clinical diagnosis was hypokalemic paralysis. The patient was treated with intravenous (IV) potassium chloride 40 mEq
Evaluation of the patient’s hypokalemia revealed the following: thyroid-stimulating hormone (TSH) level, < 0.01 microIU/mL (normal range, 0.27–4.2 microIU/mL); free T4 (thyroxine) level, 4.47 ng/dL (normal range, 0.08–1.70 ng/dL); total T3 (triiodothyronine) level, 17.5 ng/dL (normal range, 2.6–4.4 ng/dL).
The patient was diagnosed with hypokalemic periodic paralysis (HPP) secondary to thyrotoxicosis, also known as thyrotoxicosis periodic paralysis (TPP). His hyperthyroidism was treated with oral atenolol 25 mg/d and oral methimazole 10 mg tid.
Continue to: Within a few hours...
Within a few hours of this treatment, the patient experienced significant improvement in muscle strength and complete resolution of weakness in his arms and legs. Serial measurements of potassium levels normalized.
Further workup revealed that the patient’s thyroid-stimulating immunoglobulin (TSI) was 4.2 on the TSI index (normal, ≤ 1.3) and his thyroid peroxidase (TPO) antibody level was 133.4 IU/mL (normal, < 34 IU/mL). Ultrasonography showed decreased echogenicity of the thyroid gland, consistent with the acute phase of Hashimoto thyroiditis or Graves disease.
The patient was unaware that he had any thyroid disorder previously. He was a private-pay, undocumented immigrant and did not have a regular primary care physician. On discharge, he was referred to a local primary care physician as well as an endocrinologist. He was discharged on atenolol and methimazole.
DISCUSSION
A rare neuromuscular disorder known as periodic paralysis can be precipitated by a hypokalemic or hyperkalemic state; HPP is more common and can be either familial (a defect in the gene) or acquired (secondary to thyrotoxicosis; TPP).1,2 In both forms of periodic paralysis, patients present with hypokalemia and paralysis. Physicians need to look closely at thyroid lab test results so as not to miss the cause of the paralysis.
TPP is most commonly seen in Asian populations, and 95% of cases reported occur in males, despite the higher incidence of hyperthyroidism in females.3 TPP can be precipitated by emotional stress, steroid use, beta-adrenergic bronchodilators, heavy exercise, fasting, or high-carbohydrate meals.2-4 In our patient, heavy exercise and fasting likely were the triggers.
Continue to: The pathophysiology for the hypokalemia...
The pathophysiology for the hypokalemia in TPP is thought to involve the sodium/potassium–adenosine triphosphatase (Na+/K+–ATPase) pump. This pump activity is increased in skeletal muscle and platelets in patients with TPP vs patients with thyrotoxicosis alone.3,5
The role of Hashimoto thyrotoxicosis. Most acquired cases of TPP are mainly secondary to Graves disease with elevated levels of TSI and mildly elevated or normal levels of TPO. In this case, the patient was in the acute phase of Hashimoto thyrotoxicosis (“hashitoxicosis”) with elevated levels of TPO and only mildly elevated TSI.Imaging studies to support the diagnosis, such as a thyroid uptake scan or ultrasonography, are not necessary to determine the cause of thyrotoxicosis. In the absence of test results for TPO and TSI antibodies, however, a scan can be helpful.6,7
Treatment of TPP consists of early recognition and supportive management by correcting the potassium deficit; failure to do so could cause severe complications, such as respiratory failure and psychosis.8 Because of the risk for rebound hyperkalemia, serial potassium levels must be measured until a stable potassium level in the normal range is achieved.
Nonselective beta-blockers, such as propranolol (3 mg/kg) 4 times per day, have been reported to ameliorate the periodic paralysis and prevent rebound hyperkalemia.9 Finally, restoring a euthyroid state will prevent the patient from experiencing future attacks.
THE TAKEAWAY
Few medical conditions result in complete muscle paralysis in a matter of hours. Clinicians should consider the possibility of TPP in any patient who presents with acute onset of paralysis.
CORRESPONDENCE
Jorge Luis Chavez, MD; 8405 E. San Pedro Drive, Scottsdale, AZ 85258; [email protected].
THE CASE
A 33-year-old Hispanic man with no significant past medical history presented to the emergency department with generalized flaccid paralysis in both arms and legs. Two days before, he had been working on a construction site in hot weather. The following day, he woke up with very little energy or strength to perform his daily activities, and he had pain in the inguinal area and both calves. He denied taking any medications or supplements.
The patient had complete muscle weakness and was unable to move his arms and legs. He reported dysphagia and an unintentional weight loss of 30 lb during the previous month.
On physical examination, the patient’s vital signs were within the normal range, and mild thyromegaly without nodules was present. Neurologic examination revealed decreased deep tendon reflexes with intact sensation. Muscle strength in his arms and legs was 0/5.
Initial laboratory test results included a potassium level of 2.2 mEq/L (normal range, 3.5–5 mEq/L) and normal acid-basic status that was confirmed by an arterial blood gas measurement. Serum magnesium was 1.6 mg/dL (normal range, 1.6–2.5 mg/dL); phosphorus, 1.9 mg/dL (normal range, 2.7–4.5 mg/dL); and random urinary potassium, 16 mEq/L (normal range, 25–125 mEq/L). An initial chest x-ray was normal, and an electrocardiogram showed a prolonged QT interval, flattening of the T wave, and a prominent U wave consistent with hypokalemia.
THE DIAGNOSIS
The initial clinical diagnosis was hypokalemic paralysis. The patient was treated with intravenous (IV) potassium chloride 40 mEq
Evaluation of the patient’s hypokalemia revealed the following: thyroid-stimulating hormone (TSH) level, < 0.01 microIU/mL (normal range, 0.27–4.2 microIU/mL); free T4 (thyroxine) level, 4.47 ng/dL (normal range, 0.08–1.70 ng/dL); total T3 (triiodothyronine) level, 17.5 ng/dL (normal range, 2.6–4.4 ng/dL).
The patient was diagnosed with hypokalemic periodic paralysis (HPP) secondary to thyrotoxicosis, also known as thyrotoxicosis periodic paralysis (TPP). His hyperthyroidism was treated with oral atenolol 25 mg/d and oral methimazole 10 mg tid.
Continue to: Within a few hours...
Within a few hours of this treatment, the patient experienced significant improvement in muscle strength and complete resolution of weakness in his arms and legs. Serial measurements of potassium levels normalized.
Further workup revealed that the patient’s thyroid-stimulating immunoglobulin (TSI) was 4.2 on the TSI index (normal, ≤ 1.3) and his thyroid peroxidase (TPO) antibody level was 133.4 IU/mL (normal, < 34 IU/mL). Ultrasonography showed decreased echogenicity of the thyroid gland, consistent with the acute phase of Hashimoto thyroiditis or Graves disease.
The patient was unaware that he had any thyroid disorder previously. He was a private-pay, undocumented immigrant and did not have a regular primary care physician. On discharge, he was referred to a local primary care physician as well as an endocrinologist. He was discharged on atenolol and methimazole.
DISCUSSION
A rare neuromuscular disorder known as periodic paralysis can be precipitated by a hypokalemic or hyperkalemic state; HPP is more common and can be either familial (a defect in the gene) or acquired (secondary to thyrotoxicosis; TPP).1,2 In both forms of periodic paralysis, patients present with hypokalemia and paralysis. Physicians need to look closely at thyroid lab test results so as not to miss the cause of the paralysis.
TPP is most commonly seen in Asian populations, and 95% of cases reported occur in males, despite the higher incidence of hyperthyroidism in females.3 TPP can be precipitated by emotional stress, steroid use, beta-adrenergic bronchodilators, heavy exercise, fasting, or high-carbohydrate meals.2-4 In our patient, heavy exercise and fasting likely were the triggers.
Continue to: The pathophysiology for the hypokalemia...
The pathophysiology for the hypokalemia in TPP is thought to involve the sodium/potassium–adenosine triphosphatase (Na+/K+–ATPase) pump. This pump activity is increased in skeletal muscle and platelets in patients with TPP vs patients with thyrotoxicosis alone.3,5
The role of Hashimoto thyrotoxicosis. Most acquired cases of TPP are mainly secondary to Graves disease with elevated levels of TSI and mildly elevated or normal levels of TPO. In this case, the patient was in the acute phase of Hashimoto thyrotoxicosis (“hashitoxicosis”) with elevated levels of TPO and only mildly elevated TSI.Imaging studies to support the diagnosis, such as a thyroid uptake scan or ultrasonography, are not necessary to determine the cause of thyrotoxicosis. In the absence of test results for TPO and TSI antibodies, however, a scan can be helpful.6,7
Treatment of TPP consists of early recognition and supportive management by correcting the potassium deficit; failure to do so could cause severe complications, such as respiratory failure and psychosis.8 Because of the risk for rebound hyperkalemia, serial potassium levels must be measured until a stable potassium level in the normal range is achieved.
Nonselective beta-blockers, such as propranolol (3 mg/kg) 4 times per day, have been reported to ameliorate the periodic paralysis and prevent rebound hyperkalemia.9 Finally, restoring a euthyroid state will prevent the patient from experiencing future attacks.
THE TAKEAWAY
Few medical conditions result in complete muscle paralysis in a matter of hours. Clinicians should consider the possibility of TPP in any patient who presents with acute onset of paralysis.
CORRESPONDENCE
Jorge Luis Chavez, MD; 8405 E. San Pedro Drive, Scottsdale, AZ 85258; [email protected].
1. Fontaine B. Periodic paralysis. Adv Genet. 2008;63:3-23.
2. Ober KP. Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of the literature. Medicine (Baltimore).1992;71:109-120.
3. Lin YF, Wu CC, Pei D, et al. Diagnosing thyrotoxic periodic paralysis in the ED. Am J Emerg Med. 2003;21:339-342.
4. Yu TS, Tseng CF, Chuang YY, et al. Potassium chloride supplementation alone may not improve hypokalemia in thyrotoxic hypokalemic periodic paralysis. J Emerg Med. 2007;32:263-265.
5. Chan A, Shinde R, Chow CC, et al. In vivo and in vitro sodium pump activity in subjects with thyrotoxic periodic paralysis. BMJ. 1991;303:1096-1099.
6. Harsch IA, Hahn EG, Strobel D. Hashitoxicosis—three cases and a review of the literature. Eur Endocrinol. 2008;4:70-72. 7. Pou Ucha JL. Imaging in hyperthyroidism. In: Díaz-Soto G, ed. Thyroid Disorders: Focus on Hyperthyroidism. InTechOpen; 2014. www.intechopen.com/books/thyroid-disorders-focus-on-hyperthyroidism/imaging-in-hyperthyroidism. Accessed January 14, 2020.
8. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci. 2010;340:147-153.
9. Lin SH, Lin YF. Propranolol rapidly reverses paralysis, hypokalemia, and hypophosphatemia in thyrotoxic periodic paralysis. Am J Kidney Dis. 2001;37:620-623.
1. Fontaine B. Periodic paralysis. Adv Genet. 2008;63:3-23.
2. Ober KP. Thyrotoxic periodic paralysis in the United States. Report of 7 cases and review of the literature. Medicine (Baltimore).1992;71:109-120.
3. Lin YF, Wu CC, Pei D, et al. Diagnosing thyrotoxic periodic paralysis in the ED. Am J Emerg Med. 2003;21:339-342.
4. Yu TS, Tseng CF, Chuang YY, et al. Potassium chloride supplementation alone may not improve hypokalemia in thyrotoxic hypokalemic periodic paralysis. J Emerg Med. 2007;32:263-265.
5. Chan A, Shinde R, Chow CC, et al. In vivo and in vitro sodium pump activity in subjects with thyrotoxic periodic paralysis. BMJ. 1991;303:1096-1099.
6. Harsch IA, Hahn EG, Strobel D. Hashitoxicosis—three cases and a review of the literature. Eur Endocrinol. 2008;4:70-72. 7. Pou Ucha JL. Imaging in hyperthyroidism. In: Díaz-Soto G, ed. Thyroid Disorders: Focus on Hyperthyroidism. InTechOpen; 2014. www.intechopen.com/books/thyroid-disorders-focus-on-hyperthyroidism/imaging-in-hyperthyroidism. Accessed January 14, 2020.
8. Abbasi B, Sharif Z, Sprabery LR. Hypokalemic thyrotoxic periodic paralysis with thyrotoxic psychosis and hypercapnic respiratory failure. Am J Med Sci. 2010;340:147-153.
9. Lin SH, Lin YF. Propranolol rapidly reverses paralysis, hypokalemia, and hypophosphatemia in thyrotoxic periodic paralysis. Am J Kidney Dis. 2001;37:620-623.
Teprotumumab gets FDA go-ahead for thyroid eye disease
according to a press release.
Thyroid eye disease is a rare, progressive, autoimmune condition that causes the eyes to bulge (proptosis) and can lead to blindness. Until now, treatment has focused on managing its symptoms – which can include eye pain, double vision, or sensitivity to light – with steroids, and in some cases, multiple invasive surgeries.
The human monoclonal antibody and a targeted inhibitor of the insulinlike growth factor-1 receptor is administered to patients once every 3 weeks, for a total of eight infusions, according to a statement from Horizon Therapeutics, which manufactures the drug.
The approval was based on the findings from two similarly designed, parallel-group studies (Studies 1 and 2) involving 170 patients with thyroid eye disease who were randomized to receive either teprotumumab or placebo. Of those receiving the study drug, 71% in Study 1 and 83% in Study 2 had a reduction of more than 2 mm in eye protrusion, compared with 20% and 10%, respectively, among the placebo participants.
The most common adverse reactions in patients receiving teprotumumab were muscle spasm, nausea, alopecia, diarrhea, fatigue, and hyperglycemia. The treatment is contraindicated for pregnancy.
according to a press release.
Thyroid eye disease is a rare, progressive, autoimmune condition that causes the eyes to bulge (proptosis) and can lead to blindness. Until now, treatment has focused on managing its symptoms – which can include eye pain, double vision, or sensitivity to light – with steroids, and in some cases, multiple invasive surgeries.
The human monoclonal antibody and a targeted inhibitor of the insulinlike growth factor-1 receptor is administered to patients once every 3 weeks, for a total of eight infusions, according to a statement from Horizon Therapeutics, which manufactures the drug.
The approval was based on the findings from two similarly designed, parallel-group studies (Studies 1 and 2) involving 170 patients with thyroid eye disease who were randomized to receive either teprotumumab or placebo. Of those receiving the study drug, 71% in Study 1 and 83% in Study 2 had a reduction of more than 2 mm in eye protrusion, compared with 20% and 10%, respectively, among the placebo participants.
The most common adverse reactions in patients receiving teprotumumab were muscle spasm, nausea, alopecia, diarrhea, fatigue, and hyperglycemia. The treatment is contraindicated for pregnancy.
according to a press release.
Thyroid eye disease is a rare, progressive, autoimmune condition that causes the eyes to bulge (proptosis) and can lead to blindness. Until now, treatment has focused on managing its symptoms – which can include eye pain, double vision, or sensitivity to light – with steroids, and in some cases, multiple invasive surgeries.
The human monoclonal antibody and a targeted inhibitor of the insulinlike growth factor-1 receptor is administered to patients once every 3 weeks, for a total of eight infusions, according to a statement from Horizon Therapeutics, which manufactures the drug.
The approval was based on the findings from two similarly designed, parallel-group studies (Studies 1 and 2) involving 170 patients with thyroid eye disease who were randomized to receive either teprotumumab or placebo. Of those receiving the study drug, 71% in Study 1 and 83% in Study 2 had a reduction of more than 2 mm in eye protrusion, compared with 20% and 10%, respectively, among the placebo participants.
The most common adverse reactions in patients receiving teprotumumab were muscle spasm, nausea, alopecia, diarrhea, fatigue, and hyperglycemia. The treatment is contraindicated for pregnancy.
FROM THE FDA
Testosterone gel increases LV mass in older men
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
REPORTING FROM AHA 2019
Treatment of heart failure with preserved ejection fraction is a work in progress
LOS ANGELES – When it comes to the optimal treatment of patients with heart failure with preserved ejection fraction and diabetes, cardiologists like Mark T. Kearney, MB ChB, MD, remain stumped.
“Over the years, the diagnosis of heart failure with preserved ejection fraction has been notoriously difficult [to treat], controversial, and ultimately involves aggressive catheterization of the heart to assess diastolic dysfunction, complex echocardiography, and invasive tests,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease. “These patients have an ejection fraction of over 50% and classic signs and symptoms of heart failure. Studies of beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers have been unsuccessful in this group of patients. We’re at the beginning of a journey in understanding this disorder, and it’s important, because more and more patients present to us with signs and symptoms of heart failure with an ejection fraction greater than 50%.”
In a recent analysis of 1,797 patients with chronic heart failure, Dr. Kearney, British Heart Foundation Professor of Cardiovascular and Diabetes Research at the Leeds (England) Institute of Cardiovascular and Metabolic Medicine, and colleagues examined whether beta-blockers and ACE inhibitors were associated with differential effects on mortality in patients with and without diabetes (Diabetes Care. 2018;41:136-42). Mean follow-up was 4 years.
For the ACE inhibitor component of the trial, the researchers correlated the dose of ramipril to outcomes and found that each milligram increase of ramipril reduced the risk of death by about 3%. “In the nondiabetic patients who did not receive an ACE inhibitor, mortality was about 60% – worse than most cancers,” Dr. Kearney said. “In patients with diabetes, there was a similar pattern. If you didn’t get an ACE inhibitor, mortality was 70%. So, if you get patients on an optimal dose of an ACE inhibitor, you improve their mortality substantially, whether they have diabetes or not.”
The beta-blocker component of the trial yielded similar results. “Among patients who did not receive a beta-blocker, the mortality was about 70% at 5 years – really terrible,” he said. “Every milligram of bisoprolol was associated with a reduction in mortality of about 9%. So, if a patient gets on an optimal dose of a beta-blocker and they have diabetes, it’s associated with prolongation of life over a year.”
Dr. Kearney said that patients often do not want to take an increased dose of a beta-blocker because of concerns about side effects, such as tiredness. “They ask me what the side effects of an increased dose would be. My answer is: ‘It will make you live longer.’ Usually, they’ll respond by agreeing to have a little bit more of the beta-blocker. The message here is, if you have a patient with ejection fraction heart failure and diabetes, get them on the optimal dose of a beta-blocker, even at the expense of an ACE inhibitor.”
In 2016, the European Society of Cardiology introduced guidelines for physicians to make a diagnosis of heart failure with preserved ejection fraction. The guidelines mandate that a diagnosis requires signs and symptoms of heart failure, elevated levels of natriuretic peptide, and echocardiographic abnormalities of cardiac structure and/or function in the presence of a left ventricular ejection fraction of 50% or more (Eur J Heart Fail. 2016;18[8]:891-975).
“Signs and symptoms of heart failure, elevated BNP [brain natriuretic peptide], and echocardiography allow us to make a diagnosis of heart failure with preserved ejection fraction,” Dr. Kearney, who is also dean of the Leeds University School of Medicine. “But we don’t know the outcome of these patients, we don’t know how to treat them, and we don’t know the impact on hospitalizations.”
In a large, unpublished cohort study conducted at Leeds, Dr. Kearney and colleagues evaluated how many patients met criteria for heart failure with reduced ejection fraction or heart failure with preserved ejection fraction after undergoing a BNP measurement. Ultimately, 959 patients met criteria. After assessment, 23% had no heart failure, 44% had heart failure with preserved ejection fraction, and 33% had heart failure with reduced ejection fraction. They found that patients with preserved ejection fraction were older (mean age, 84 years); were more likely to be female; and had less ischemia, less diabetes, and more hypertension. In addition, patients with preserved ejection fraction had significantly better survival than patients with reduced ejection fraction over 5 years follow-up.
“What was really interesting were the findings related to hospitalization,” he said. “All 959 patients accounted for 20,517 days in the hospital over 5 years, which is the equivalent of 1 patient occupying a hospital bed for 56 years. This disorder [heart failure with preserved ejection fraction], despite having a lower mortality than heart failure with reduced ejection fraction, leads to a significant burden on health care systems.”
Among patients with preserved ejection fraction, 82% were hospitalized for a noncardiovascular cause, 6.9% because of heart failure, and 11% were caused by other cardiovascular causes. Most of the hospital admissions were because of chest infections, falls, and other frailty-linked causes. “This link between systemic frailty and heart failure with preserved ejection fraction warrants further investigation,” Dr. Kearney said. “This is a major burden on patient hospital care.”
When the researchers examined outcomes in patients with and without diabetes, those with diabetes were younger, more likely to be male, and have a higher body mass index. They found that, in the presence of diabetes, mortality was increased in heart failure with preserved and reduced ejection fraction. “So, even at the age of 81 or 82, diabetes changes the pathophysiology of mortality in what was previously believed to be a benign disease,” he said.
In a subset analysis of patients with and without diabetes who were not taking a beta-blocker, there did not seem to be increased sympathetic activation in the patients with diabetes and heart failure with preserved ejection fraction, nor a difference in heart rate between the nondiabetic patients and patients with diabetes. However, among patients with heart failure with reduced ejection fraction, those with diabetes had an increased heart rate.
“Is heart failure with preserved ejection fraction in diabetes benign? I think the answer is no,” Dr. Kearney said. “It increases hospitalization and is a major burden on health care systems. What should we do? We deal with comorbidity and fall risk. It’s good old-fashioned doctoring, really. We address frailty and respiratory tract infections, but the key thing here is that we need more research.”
Dr. Kearney reported having no relevant financial disclosures.
LOS ANGELES – When it comes to the optimal treatment of patients with heart failure with preserved ejection fraction and diabetes, cardiologists like Mark T. Kearney, MB ChB, MD, remain stumped.
“Over the years, the diagnosis of heart failure with preserved ejection fraction has been notoriously difficult [to treat], controversial, and ultimately involves aggressive catheterization of the heart to assess diastolic dysfunction, complex echocardiography, and invasive tests,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease. “These patients have an ejection fraction of over 50% and classic signs and symptoms of heart failure. Studies of beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers have been unsuccessful in this group of patients. We’re at the beginning of a journey in understanding this disorder, and it’s important, because more and more patients present to us with signs and symptoms of heart failure with an ejection fraction greater than 50%.”
In a recent analysis of 1,797 patients with chronic heart failure, Dr. Kearney, British Heart Foundation Professor of Cardiovascular and Diabetes Research at the Leeds (England) Institute of Cardiovascular and Metabolic Medicine, and colleagues examined whether beta-blockers and ACE inhibitors were associated with differential effects on mortality in patients with and without diabetes (Diabetes Care. 2018;41:136-42). Mean follow-up was 4 years.
For the ACE inhibitor component of the trial, the researchers correlated the dose of ramipril to outcomes and found that each milligram increase of ramipril reduced the risk of death by about 3%. “In the nondiabetic patients who did not receive an ACE inhibitor, mortality was about 60% – worse than most cancers,” Dr. Kearney said. “In patients with diabetes, there was a similar pattern. If you didn’t get an ACE inhibitor, mortality was 70%. So, if you get patients on an optimal dose of an ACE inhibitor, you improve their mortality substantially, whether they have diabetes or not.”
The beta-blocker component of the trial yielded similar results. “Among patients who did not receive a beta-blocker, the mortality was about 70% at 5 years – really terrible,” he said. “Every milligram of bisoprolol was associated with a reduction in mortality of about 9%. So, if a patient gets on an optimal dose of a beta-blocker and they have diabetes, it’s associated with prolongation of life over a year.”
Dr. Kearney said that patients often do not want to take an increased dose of a beta-blocker because of concerns about side effects, such as tiredness. “They ask me what the side effects of an increased dose would be. My answer is: ‘It will make you live longer.’ Usually, they’ll respond by agreeing to have a little bit more of the beta-blocker. The message here is, if you have a patient with ejection fraction heart failure and diabetes, get them on the optimal dose of a beta-blocker, even at the expense of an ACE inhibitor.”
In 2016, the European Society of Cardiology introduced guidelines for physicians to make a diagnosis of heart failure with preserved ejection fraction. The guidelines mandate that a diagnosis requires signs and symptoms of heart failure, elevated levels of natriuretic peptide, and echocardiographic abnormalities of cardiac structure and/or function in the presence of a left ventricular ejection fraction of 50% or more (Eur J Heart Fail. 2016;18[8]:891-975).
“Signs and symptoms of heart failure, elevated BNP [brain natriuretic peptide], and echocardiography allow us to make a diagnosis of heart failure with preserved ejection fraction,” Dr. Kearney, who is also dean of the Leeds University School of Medicine. “But we don’t know the outcome of these patients, we don’t know how to treat them, and we don’t know the impact on hospitalizations.”
In a large, unpublished cohort study conducted at Leeds, Dr. Kearney and colleagues evaluated how many patients met criteria for heart failure with reduced ejection fraction or heart failure with preserved ejection fraction after undergoing a BNP measurement. Ultimately, 959 patients met criteria. After assessment, 23% had no heart failure, 44% had heart failure with preserved ejection fraction, and 33% had heart failure with reduced ejection fraction. They found that patients with preserved ejection fraction were older (mean age, 84 years); were more likely to be female; and had less ischemia, less diabetes, and more hypertension. In addition, patients with preserved ejection fraction had significantly better survival than patients with reduced ejection fraction over 5 years follow-up.
“What was really interesting were the findings related to hospitalization,” he said. “All 959 patients accounted for 20,517 days in the hospital over 5 years, which is the equivalent of 1 patient occupying a hospital bed for 56 years. This disorder [heart failure with preserved ejection fraction], despite having a lower mortality than heart failure with reduced ejection fraction, leads to a significant burden on health care systems.”
Among patients with preserved ejection fraction, 82% were hospitalized for a noncardiovascular cause, 6.9% because of heart failure, and 11% were caused by other cardiovascular causes. Most of the hospital admissions were because of chest infections, falls, and other frailty-linked causes. “This link between systemic frailty and heart failure with preserved ejection fraction warrants further investigation,” Dr. Kearney said. “This is a major burden on patient hospital care.”
When the researchers examined outcomes in patients with and without diabetes, those with diabetes were younger, more likely to be male, and have a higher body mass index. They found that, in the presence of diabetes, mortality was increased in heart failure with preserved and reduced ejection fraction. “So, even at the age of 81 or 82, diabetes changes the pathophysiology of mortality in what was previously believed to be a benign disease,” he said.
In a subset analysis of patients with and without diabetes who were not taking a beta-blocker, there did not seem to be increased sympathetic activation in the patients with diabetes and heart failure with preserved ejection fraction, nor a difference in heart rate between the nondiabetic patients and patients with diabetes. However, among patients with heart failure with reduced ejection fraction, those with diabetes had an increased heart rate.
“Is heart failure with preserved ejection fraction in diabetes benign? I think the answer is no,” Dr. Kearney said. “It increases hospitalization and is a major burden on health care systems. What should we do? We deal with comorbidity and fall risk. It’s good old-fashioned doctoring, really. We address frailty and respiratory tract infections, but the key thing here is that we need more research.”
Dr. Kearney reported having no relevant financial disclosures.
LOS ANGELES – When it comes to the optimal treatment of patients with heart failure with preserved ejection fraction and diabetes, cardiologists like Mark T. Kearney, MB ChB, MD, remain stumped.
“Over the years, the diagnosis of heart failure with preserved ejection fraction has been notoriously difficult [to treat], controversial, and ultimately involves aggressive catheterization of the heart to assess diastolic dysfunction, complex echocardiography, and invasive tests,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease. “These patients have an ejection fraction of over 50% and classic signs and symptoms of heart failure. Studies of beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers have been unsuccessful in this group of patients. We’re at the beginning of a journey in understanding this disorder, and it’s important, because more and more patients present to us with signs and symptoms of heart failure with an ejection fraction greater than 50%.”
In a recent analysis of 1,797 patients with chronic heart failure, Dr. Kearney, British Heart Foundation Professor of Cardiovascular and Diabetes Research at the Leeds (England) Institute of Cardiovascular and Metabolic Medicine, and colleagues examined whether beta-blockers and ACE inhibitors were associated with differential effects on mortality in patients with and without diabetes (Diabetes Care. 2018;41:136-42). Mean follow-up was 4 years.
For the ACE inhibitor component of the trial, the researchers correlated the dose of ramipril to outcomes and found that each milligram increase of ramipril reduced the risk of death by about 3%. “In the nondiabetic patients who did not receive an ACE inhibitor, mortality was about 60% – worse than most cancers,” Dr. Kearney said. “In patients with diabetes, there was a similar pattern. If you didn’t get an ACE inhibitor, mortality was 70%. So, if you get patients on an optimal dose of an ACE inhibitor, you improve their mortality substantially, whether they have diabetes or not.”
The beta-blocker component of the trial yielded similar results. “Among patients who did not receive a beta-blocker, the mortality was about 70% at 5 years – really terrible,” he said. “Every milligram of bisoprolol was associated with a reduction in mortality of about 9%. So, if a patient gets on an optimal dose of a beta-blocker and they have diabetes, it’s associated with prolongation of life over a year.”
Dr. Kearney said that patients often do not want to take an increased dose of a beta-blocker because of concerns about side effects, such as tiredness. “They ask me what the side effects of an increased dose would be. My answer is: ‘It will make you live longer.’ Usually, they’ll respond by agreeing to have a little bit more of the beta-blocker. The message here is, if you have a patient with ejection fraction heart failure and diabetes, get them on the optimal dose of a beta-blocker, even at the expense of an ACE inhibitor.”
In 2016, the European Society of Cardiology introduced guidelines for physicians to make a diagnosis of heart failure with preserved ejection fraction. The guidelines mandate that a diagnosis requires signs and symptoms of heart failure, elevated levels of natriuretic peptide, and echocardiographic abnormalities of cardiac structure and/or function in the presence of a left ventricular ejection fraction of 50% or more (Eur J Heart Fail. 2016;18[8]:891-975).
“Signs and symptoms of heart failure, elevated BNP [brain natriuretic peptide], and echocardiography allow us to make a diagnosis of heart failure with preserved ejection fraction,” Dr. Kearney, who is also dean of the Leeds University School of Medicine. “But we don’t know the outcome of these patients, we don’t know how to treat them, and we don’t know the impact on hospitalizations.”
In a large, unpublished cohort study conducted at Leeds, Dr. Kearney and colleagues evaluated how many patients met criteria for heart failure with reduced ejection fraction or heart failure with preserved ejection fraction after undergoing a BNP measurement. Ultimately, 959 patients met criteria. After assessment, 23% had no heart failure, 44% had heart failure with preserved ejection fraction, and 33% had heart failure with reduced ejection fraction. They found that patients with preserved ejection fraction were older (mean age, 84 years); were more likely to be female; and had less ischemia, less diabetes, and more hypertension. In addition, patients with preserved ejection fraction had significantly better survival than patients with reduced ejection fraction over 5 years follow-up.
“What was really interesting were the findings related to hospitalization,” he said. “All 959 patients accounted for 20,517 days in the hospital over 5 years, which is the equivalent of 1 patient occupying a hospital bed for 56 years. This disorder [heart failure with preserved ejection fraction], despite having a lower mortality than heart failure with reduced ejection fraction, leads to a significant burden on health care systems.”
Among patients with preserved ejection fraction, 82% were hospitalized for a noncardiovascular cause, 6.9% because of heart failure, and 11% were caused by other cardiovascular causes. Most of the hospital admissions were because of chest infections, falls, and other frailty-linked causes. “This link between systemic frailty and heart failure with preserved ejection fraction warrants further investigation,” Dr. Kearney said. “This is a major burden on patient hospital care.”
When the researchers examined outcomes in patients with and without diabetes, those with diabetes were younger, more likely to be male, and have a higher body mass index. They found that, in the presence of diabetes, mortality was increased in heart failure with preserved and reduced ejection fraction. “So, even at the age of 81 or 82, diabetes changes the pathophysiology of mortality in what was previously believed to be a benign disease,” he said.
In a subset analysis of patients with and without diabetes who were not taking a beta-blocker, there did not seem to be increased sympathetic activation in the patients with diabetes and heart failure with preserved ejection fraction, nor a difference in heart rate between the nondiabetic patients and patients with diabetes. However, among patients with heart failure with reduced ejection fraction, those with diabetes had an increased heart rate.
“Is heart failure with preserved ejection fraction in diabetes benign? I think the answer is no,” Dr. Kearney said. “It increases hospitalization and is a major burden on health care systems. What should we do? We deal with comorbidity and fall risk. It’s good old-fashioned doctoring, really. We address frailty and respiratory tract infections, but the key thing here is that we need more research.”
Dr. Kearney reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM WCIRDC 2019
New guideline for testosterone treatment in men with ‘low T’
The American College of Physicians has released new clinical guidelines providing practical recommendations for testosterone therapy in adult men with age-related low testosterone.
The evidence-based recommendations target all clinicians and were published online January 6, 2020, in Annals of Internal Medicine, highlighting data from a systematic review of evidence on the efficacy and safety of testosterone treatment in adult men with age-related low testosterone.
Serum testosterone levels drop as men age, starting in their mid-30s, and approximately 20% of American men older than 60 years have low testosterone.
However, no widely accepted testosterone threshold level exists that represents a measure below which symptoms of androgen deficiency and adverse health outcomes occur.
In addition, the role of testosterone therapy in managing this patient population is controversial.
“The purpose of this American College of Physicians guideline is to present recommendations based on the best available evidence on the benefits, harms, and costs of testosterone treatment in adult men with age-related low testosterone,” write Amir Qaseem, MD, PhD, MHA, from the American College of Physicians, Philadelphia, and colleagues.
“This guideline does not address screening or diagnosis of hypogonadism or monitoring of testosterone levels,” the authors note.
In particular, the recommendations suggest that clinicians should initiate testosterone treatment in these patients only to help them improve their sexual function.
According to the authors, moderate-certainty evidence from seven trials involving testosterone treatment in adult men with age-related low testosterone showed a small improvement in global sexual function, whereas low-certainty evidence from seven trials showed a small improvement in erectile function.
By contrast, the guideline emphasizes that clinicians should avoid prescribing testosterone treatment for any other concern in this population. Available evidence demonstrates little to no improvement in physical function, depressive symptoms, energy and vitality, or cognition among these men after receiving testosterone treatment, the authors stress.
ACP recommends that clinicians should reassess men’s symptoms within 12 months of testosterone treatment initiation, with regular reevaluations during subsequent follow up. Clinicians should discontinue treatment in men if sexual function fails to improve.
The guideline also recommends using intramuscular formulations of testosterone treatment for this patient population instead of transdermal ones, because intramuscular formulations cost less and have similar clinical effectiveness and harms.
“The annual cost in 2016 per beneficiary for TRT [testosterone replacement therapy] was $2,135.32 for the transdermal and $156.24 for the intramuscular formulation, according to paid pharmaceutical claims provided in the 2016 Medicare Part D Drug Claims data,” the authors write.
In an accompanying editorial, E. Victor Adlin, MD, of Temple University, Philadelphia, notes that these new ACP guidelines mostly mirror those recently proposed by both the Endocrine Society and the American Urological Association.
However, he predicts that many clinicians will question the ACP’s recommendation to favor use of intramuscular over transdermal formulations of testosterone.
Although Dr. Adlin acknowledges the lower cost of intramuscular preparations as a major consideration, he explains that “the need for an intramuscular injection every 1-4 weeks is a potential barrier to adherence, and some patients require visits to a health care facility for the injections, which may add to the expense.”
Fluctuating blood testosterone levels after each injection may also result in irregular symptom relief and difficulty achieving the desired blood level, he adds. “Individual preference may vary widely in the choice of testosterone therapy.”
Overall, Dr. Adlin stresses that a patient-clinician discussion should serve as the foundation for starting testosterone therapy in men with age-related low testosterone, with the patient playing a central role in treatment decision making.
This guideline was developed with financial support from the American College of Physicians’ operating budget. Study author Carrie Horwitch reports serving as a fiduciary officer for the Washington State Medical Association. Jennifer S. Lin, a member of the ACP Clinical Guidelines Committee, reports being an employee of Kaiser Permanente. Robert McLean, another member of the committee, reports being an employee of Northeast Medical Group. The remaining authors and the editorialist have disclosed no relevant financial relationships.
A version of this story appeared on Medscape.com.
The American College of Physicians has released new clinical guidelines providing practical recommendations for testosterone therapy in adult men with age-related low testosterone.
The evidence-based recommendations target all clinicians and were published online January 6, 2020, in Annals of Internal Medicine, highlighting data from a systematic review of evidence on the efficacy and safety of testosterone treatment in adult men with age-related low testosterone.
Serum testosterone levels drop as men age, starting in their mid-30s, and approximately 20% of American men older than 60 years have low testosterone.
However, no widely accepted testosterone threshold level exists that represents a measure below which symptoms of androgen deficiency and adverse health outcomes occur.
In addition, the role of testosterone therapy in managing this patient population is controversial.
“The purpose of this American College of Physicians guideline is to present recommendations based on the best available evidence on the benefits, harms, and costs of testosterone treatment in adult men with age-related low testosterone,” write Amir Qaseem, MD, PhD, MHA, from the American College of Physicians, Philadelphia, and colleagues.
“This guideline does not address screening or diagnosis of hypogonadism or monitoring of testosterone levels,” the authors note.
In particular, the recommendations suggest that clinicians should initiate testosterone treatment in these patients only to help them improve their sexual function.
According to the authors, moderate-certainty evidence from seven trials involving testosterone treatment in adult men with age-related low testosterone showed a small improvement in global sexual function, whereas low-certainty evidence from seven trials showed a small improvement in erectile function.
By contrast, the guideline emphasizes that clinicians should avoid prescribing testosterone treatment for any other concern in this population. Available evidence demonstrates little to no improvement in physical function, depressive symptoms, energy and vitality, or cognition among these men after receiving testosterone treatment, the authors stress.
ACP recommends that clinicians should reassess men’s symptoms within 12 months of testosterone treatment initiation, with regular reevaluations during subsequent follow up. Clinicians should discontinue treatment in men if sexual function fails to improve.
The guideline also recommends using intramuscular formulations of testosterone treatment for this patient population instead of transdermal ones, because intramuscular formulations cost less and have similar clinical effectiveness and harms.
“The annual cost in 2016 per beneficiary for TRT [testosterone replacement therapy] was $2,135.32 for the transdermal and $156.24 for the intramuscular formulation, according to paid pharmaceutical claims provided in the 2016 Medicare Part D Drug Claims data,” the authors write.
In an accompanying editorial, E. Victor Adlin, MD, of Temple University, Philadelphia, notes that these new ACP guidelines mostly mirror those recently proposed by both the Endocrine Society and the American Urological Association.
However, he predicts that many clinicians will question the ACP’s recommendation to favor use of intramuscular over transdermal formulations of testosterone.
Although Dr. Adlin acknowledges the lower cost of intramuscular preparations as a major consideration, he explains that “the need for an intramuscular injection every 1-4 weeks is a potential barrier to adherence, and some patients require visits to a health care facility for the injections, which may add to the expense.”
Fluctuating blood testosterone levels after each injection may also result in irregular symptom relief and difficulty achieving the desired blood level, he adds. “Individual preference may vary widely in the choice of testosterone therapy.”
Overall, Dr. Adlin stresses that a patient-clinician discussion should serve as the foundation for starting testosterone therapy in men with age-related low testosterone, with the patient playing a central role in treatment decision making.
This guideline was developed with financial support from the American College of Physicians’ operating budget. Study author Carrie Horwitch reports serving as a fiduciary officer for the Washington State Medical Association. Jennifer S. Lin, a member of the ACP Clinical Guidelines Committee, reports being an employee of Kaiser Permanente. Robert McLean, another member of the committee, reports being an employee of Northeast Medical Group. The remaining authors and the editorialist have disclosed no relevant financial relationships.
A version of this story appeared on Medscape.com.
The American College of Physicians has released new clinical guidelines providing practical recommendations for testosterone therapy in adult men with age-related low testosterone.
The evidence-based recommendations target all clinicians and were published online January 6, 2020, in Annals of Internal Medicine, highlighting data from a systematic review of evidence on the efficacy and safety of testosterone treatment in adult men with age-related low testosterone.
Serum testosterone levels drop as men age, starting in their mid-30s, and approximately 20% of American men older than 60 years have low testosterone.
However, no widely accepted testosterone threshold level exists that represents a measure below which symptoms of androgen deficiency and adverse health outcomes occur.
In addition, the role of testosterone therapy in managing this patient population is controversial.
“The purpose of this American College of Physicians guideline is to present recommendations based on the best available evidence on the benefits, harms, and costs of testosterone treatment in adult men with age-related low testosterone,” write Amir Qaseem, MD, PhD, MHA, from the American College of Physicians, Philadelphia, and colleagues.
“This guideline does not address screening or diagnosis of hypogonadism or monitoring of testosterone levels,” the authors note.
In particular, the recommendations suggest that clinicians should initiate testosterone treatment in these patients only to help them improve their sexual function.
According to the authors, moderate-certainty evidence from seven trials involving testosterone treatment in adult men with age-related low testosterone showed a small improvement in global sexual function, whereas low-certainty evidence from seven trials showed a small improvement in erectile function.
By contrast, the guideline emphasizes that clinicians should avoid prescribing testosterone treatment for any other concern in this population. Available evidence demonstrates little to no improvement in physical function, depressive symptoms, energy and vitality, or cognition among these men after receiving testosterone treatment, the authors stress.
ACP recommends that clinicians should reassess men’s symptoms within 12 months of testosterone treatment initiation, with regular reevaluations during subsequent follow up. Clinicians should discontinue treatment in men if sexual function fails to improve.
The guideline also recommends using intramuscular formulations of testosterone treatment for this patient population instead of transdermal ones, because intramuscular formulations cost less and have similar clinical effectiveness and harms.
“The annual cost in 2016 per beneficiary for TRT [testosterone replacement therapy] was $2,135.32 for the transdermal and $156.24 for the intramuscular formulation, according to paid pharmaceutical claims provided in the 2016 Medicare Part D Drug Claims data,” the authors write.
In an accompanying editorial, E. Victor Adlin, MD, of Temple University, Philadelphia, notes that these new ACP guidelines mostly mirror those recently proposed by both the Endocrine Society and the American Urological Association.
However, he predicts that many clinicians will question the ACP’s recommendation to favor use of intramuscular over transdermal formulations of testosterone.
Although Dr. Adlin acknowledges the lower cost of intramuscular preparations as a major consideration, he explains that “the need for an intramuscular injection every 1-4 weeks is a potential barrier to adherence, and some patients require visits to a health care facility for the injections, which may add to the expense.”
Fluctuating blood testosterone levels after each injection may also result in irregular symptom relief and difficulty achieving the desired blood level, he adds. “Individual preference may vary widely in the choice of testosterone therapy.”
Overall, Dr. Adlin stresses that a patient-clinician discussion should serve as the foundation for starting testosterone therapy in men with age-related low testosterone, with the patient playing a central role in treatment decision making.
This guideline was developed with financial support from the American College of Physicians’ operating budget. Study author Carrie Horwitch reports serving as a fiduciary officer for the Washington State Medical Association. Jennifer S. Lin, a member of the ACP Clinical Guidelines Committee, reports being an employee of Kaiser Permanente. Robert McLean, another member of the committee, reports being an employee of Northeast Medical Group. The remaining authors and the editorialist have disclosed no relevant financial relationships.
A version of this story appeared on Medscape.com.
Anorexia linked to low bone density, osteoporosis
A new study has reinforced the link between anorexia nervosa and reduced bone mineral density (BMD), especially in patients with lower body mass index.
“Our large study raises further concerns that [anorexia nervosa] has significant deleterious effects on BMD,” wrote Cassandra Workman, MD, of the Eating Recovery Center in Denver and coauthors. The study was published in Bone.
To determine the degree of low BMD in patients with certain severe eating disorders, the researchers reviewed the medical records of 336 patients with either anorexia nervosa–restricting subtype (AN-R) or anorexia nervosa–binge/purge subtype (AN-BP) who had been admitted to a treatment facility in Denver. Bone density was assessed using dual-energy x-ray absorptiometry, with osteopenia being diagnosed for an average BMD z score between –1.0 and –2.0 and osteoporosis being diagnosed for an average BMD z score of less than –2.0. The average age of the patients was 27 years (standard deviation, 9.12; range, 18-69), and 91% (n = 305) were women.
Across the sample, the average BMD z score was –1.67 (SD, 1.21), and 43.5% of the sample met the established criteria for low BMD.
Patients with AN-R had slightly lower z scores (–1.79; SD, 1.31), compared with patients with AN-BP (–1.54; SD, 1.08; P = .06), but the severity of osteoporosis was greater in patients with AN-R, compared with patients with AN-BP (chi-square, 7.40; P less than .01).
The authors acknowledged their study’s limitations, including the use of retrospective data from the patient charts, which did not allow for assessment of follow-up improvements or longer-term effects. In addition, they noted that extrapolation of their findings may be problematic because all the patients were from a single site and the data might be representative of “a more ill population than a true cross section of the eating disorder population.”
The authors reported no conflicts of interest.
SOURCE: Workman C et al. Bone. 2019 Nov 23. doi: 10.1016/j.bone.2019.115161.
A new study has reinforced the link between anorexia nervosa and reduced bone mineral density (BMD), especially in patients with lower body mass index.
“Our large study raises further concerns that [anorexia nervosa] has significant deleterious effects on BMD,” wrote Cassandra Workman, MD, of the Eating Recovery Center in Denver and coauthors. The study was published in Bone.
To determine the degree of low BMD in patients with certain severe eating disorders, the researchers reviewed the medical records of 336 patients with either anorexia nervosa–restricting subtype (AN-R) or anorexia nervosa–binge/purge subtype (AN-BP) who had been admitted to a treatment facility in Denver. Bone density was assessed using dual-energy x-ray absorptiometry, with osteopenia being diagnosed for an average BMD z score between –1.0 and –2.0 and osteoporosis being diagnosed for an average BMD z score of less than –2.0. The average age of the patients was 27 years (standard deviation, 9.12; range, 18-69), and 91% (n = 305) were women.
Across the sample, the average BMD z score was –1.67 (SD, 1.21), and 43.5% of the sample met the established criteria for low BMD.
Patients with AN-R had slightly lower z scores (–1.79; SD, 1.31), compared with patients with AN-BP (–1.54; SD, 1.08; P = .06), but the severity of osteoporosis was greater in patients with AN-R, compared with patients with AN-BP (chi-square, 7.40; P less than .01).
The authors acknowledged their study’s limitations, including the use of retrospective data from the patient charts, which did not allow for assessment of follow-up improvements or longer-term effects. In addition, they noted that extrapolation of their findings may be problematic because all the patients were from a single site and the data might be representative of “a more ill population than a true cross section of the eating disorder population.”
The authors reported no conflicts of interest.
SOURCE: Workman C et al. Bone. 2019 Nov 23. doi: 10.1016/j.bone.2019.115161.
A new study has reinforced the link between anorexia nervosa and reduced bone mineral density (BMD), especially in patients with lower body mass index.
“Our large study raises further concerns that [anorexia nervosa] has significant deleterious effects on BMD,” wrote Cassandra Workman, MD, of the Eating Recovery Center in Denver and coauthors. The study was published in Bone.
To determine the degree of low BMD in patients with certain severe eating disorders, the researchers reviewed the medical records of 336 patients with either anorexia nervosa–restricting subtype (AN-R) or anorexia nervosa–binge/purge subtype (AN-BP) who had been admitted to a treatment facility in Denver. Bone density was assessed using dual-energy x-ray absorptiometry, with osteopenia being diagnosed for an average BMD z score between –1.0 and –2.0 and osteoporosis being diagnosed for an average BMD z score of less than –2.0. The average age of the patients was 27 years (standard deviation, 9.12; range, 18-69), and 91% (n = 305) were women.
Across the sample, the average BMD z score was –1.67 (SD, 1.21), and 43.5% of the sample met the established criteria for low BMD.
Patients with AN-R had slightly lower z scores (–1.79; SD, 1.31), compared with patients with AN-BP (–1.54; SD, 1.08; P = .06), but the severity of osteoporosis was greater in patients with AN-R, compared with patients with AN-BP (chi-square, 7.40; P less than .01).
The authors acknowledged their study’s limitations, including the use of retrospective data from the patient charts, which did not allow for assessment of follow-up improvements or longer-term effects. In addition, they noted that extrapolation of their findings may be problematic because all the patients were from a single site and the data might be representative of “a more ill population than a true cross section of the eating disorder population.”
The authors reported no conflicts of interest.
SOURCE: Workman C et al. Bone. 2019 Nov 23. doi: 10.1016/j.bone.2019.115161.
FROM BONE
Farxiga granted Priority Review for treatment of adults with HFrEF
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
The Food and Drug Administration has accepted a supplemental New Drug Application and granted Priority Review for dapagliflozin (Farxiga) for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients with heart failure with reduced ejection fraction (HFrEF).
The application was based on results from the landmark, phase 3 DAPA-HF trial, published in September 2019 in the New England Journal of Medicine. The study showed that dapagliflozin plus standard care reduced the incidence of cardiovascular death and worsening of heart failure versus placebo in patients with HFrEF.
Dapagliflozin was granted Fast Track designation for heart failure by the FDA in September 2019. In August 2019, the FDA also granted Fast Track designation to dapagliflozin for the delayed progression of renal failure and prevention of cardiovascular and renal death in patients with chronic kidney disease.
The drug is currently indicated for the improvement of glycemic control in adults with type 2 diabetes as either monotherapy or in combination. The FDA approved dapagliflozin in October 2019 for the reduction of heart failure hospitalization risk in patients with type 2 diabetes and cardiovascular risk factors.
“Farxiga is well established in the treatment of type 2 diabetes and this Priority Review shows its potential to also impact millions of patients with heart failure. If approved, Farxiga will be the first and only medicine of its kind indicated to treat patients with heart failure,” said Mene Pangalos, executive vice president of biopharmaceutical research and development at AstraZeneca.
Find the full press release on the AstraZeneca website.
FDA panel okays teprotumumab for thyroid eye disease
TED is a rare autoimmune disease that causes the eyes to bulge (proptosis) and can lead to blindness. It is also known as thyroid-associated ophthalmopathy, Graves ophthalmopathy, and Graves orbitopathy. Current treatment is aimed at relief of symptoms and includes corticosteroids and orbital decompression.
“TED can affect patients both physically and emotionally, limiting their ability to perform everyday activities like driving, working, reading, sleeping, and participating in social activities,” Jeff Todd, president and chief executive officer, Prevent Blindness, said in a news release.
“As an organization dedicated to helping patients with vision impairment and those who are at significant risk, we are extremely encouraged by today’s vote and hopeful this will change the future of TED treatment by giving patients an option that has been shown to improve the painful and vision-threatening aspects of the disease,” Mr. Todd said.
Teprotumumab was granted fast-track status in April 2015 and breakthrough therapy designation in July 2016. It received orphan drug designation on June 19, 2019. If approved, it would be the first approved treatment for this indication.
All 12 members of the Dermatologic and Ophthalmic Drugs Advisory Committee of the FDA voted to recommend approval of teprotumumab.
“It’s clearly a pleasure to participate in seeing a drug being designed and moving forward in a clinical trial for a disease that really has not been treatable for us in the past,” said voting committee member Timothy Murray, MD, MBA, of the Bascom Palmer Eye Institute, Miami.
Efficacy demonstrated
The FDA advisory committee considered data from two randomized, double-masked, placebo-controlled, parallel-group studies that were similar in design and that demonstrated efficacy.
The studies included a total of 171 patients, fewer than 90 of whom were treated with teprotumumab, the agency explained in a briefing document. “This is a considerably smaller database than the common safety database of greater than 300 patients treated with a course of therapy,” it observed.
The study required that patients have proptosis but did not require that they have “progressive forward motion of the globe,” it noted.
The primary objective – a reduction in proptosis of 2 or more mm – was met in 82% of patients who received teprotumumab, compared with 16% of those who received placebo.
Review study No. 1 (TED01RV) was a phase 2 study, and review study No. 2 (OPTIC) was a phase 3 study. Both trials included a 24-week treatment period during which participants received teprotumumab or placebo intravenously every 3 weeks for a total of eight doses. Patients in both studies underwent a follow-up period during which they received no further treatment.
Some patients experienced a reduction in proptosis as early as 6 weeks after they received the first infusion. In an extension of TED01RV, that reduction extended for at least 4 weeks after the last infusion. For about 60% of responders, no relapse had occurred by week 72. The extension of OPTIC and an open-label treatment period for those with no response to placebo or teprotumumab are ongoing.
“I welcome the addition of this drug to our armamentarium to treat this horrible, horrible disease,” said temporary voting committee member John F. Stamler, MD, PhD, clinical instructor, ophthalmology and visual sciences, University of Iowa, Iowa City.
Adverse events
Teprotumumab inhibits the insulinlike growth factor–1 receptor, which can interfere with the body’s ability to regulate glucose, particularly in patients with diabetes. In the study, some patients with diabetes required additional insulin for glycemic control.
In three study participants whose baseline fasting blood glucose levels were normal, blood glucose levels were found to be elevated at one or more visits during the treatment period. None had a history of diabetes mellitus, but for two, baseline hemoglobin A1c levels were elevated.
Five or more patients reported loss of hearing (hypoacusis), and others reported tinnitus. One of them experienced a spontaneous return of hearing the day after the hypoacusis developed, whereas for others, hearing did not return until after teprotumumab treatment was completed. The mechanism of action for hearing loss is unclear.
Panel members felt the potential for hearing loss is important and that patients should receive some type of monitoring, but they did not all agree on when that testing should occur or who should be responsible for getting it done.
“It strikes me that, if this drug were approved, there would be centers that would be interested in undertaking independent studies of hearing loss in treated patients, and that that could be done outside the sponsor’s responsibility and probably would be of interest to independent investigators,” noted committee chairperson James Chodosh, MD, MPH, of Massachusetts Eye and Ear and Harvard Medical School, Boston.
Benefits outweigh risks
More than one-third of patients (36%) experienced gastrointestinal complaints such as nausea and diarrhea (12% each) and abdominal pain (5%). None of the cases caused any patient to discontinue the study drug.
The overall incidence of muscle spasms was more than three times higher in the teprotumumab group (32%) than in the placebo group (9.5%).
One patient stopped taking teprotumumab after being hospitalized for Escherichia coli sepsis and dehydration, and another participant stopped after experiencing an episode of inflammatory bowel disease. It is not clear whether there is a causal association between teprotumumab and inflammatory bowel disease.
No study participants died.
Panel members expressed concern about safety in the longer term or in patients who receive multiple courses of teprotumumab, but they felt the potential benefits from teprotumumab outweigh the risks. The committee also wanted more information about the effects of teprotumumab with respect to glucose control, hearing loss, and other outcomes that are very important to patients, such as alopecia.
The panel favored including diarrhea in the list of adverse events in the label and felt that there should be a warning about inflammatory bowel disease.
“We’re finally going to be able to get a lot of people some help,” concluded voting committee member Sidney Gicheru, MD, LaserCare Eye Center, Irving, Texas.
A version of this story originally appeared on Medscape.com.
TED is a rare autoimmune disease that causes the eyes to bulge (proptosis) and can lead to blindness. It is also known as thyroid-associated ophthalmopathy, Graves ophthalmopathy, and Graves orbitopathy. Current treatment is aimed at relief of symptoms and includes corticosteroids and orbital decompression.
“TED can affect patients both physically and emotionally, limiting their ability to perform everyday activities like driving, working, reading, sleeping, and participating in social activities,” Jeff Todd, president and chief executive officer, Prevent Blindness, said in a news release.
“As an organization dedicated to helping patients with vision impairment and those who are at significant risk, we are extremely encouraged by today’s vote and hopeful this will change the future of TED treatment by giving patients an option that has been shown to improve the painful and vision-threatening aspects of the disease,” Mr. Todd said.
Teprotumumab was granted fast-track status in April 2015 and breakthrough therapy designation in July 2016. It received orphan drug designation on June 19, 2019. If approved, it would be the first approved treatment for this indication.
All 12 members of the Dermatologic and Ophthalmic Drugs Advisory Committee of the FDA voted to recommend approval of teprotumumab.
“It’s clearly a pleasure to participate in seeing a drug being designed and moving forward in a clinical trial for a disease that really has not been treatable for us in the past,” said voting committee member Timothy Murray, MD, MBA, of the Bascom Palmer Eye Institute, Miami.
Efficacy demonstrated
The FDA advisory committee considered data from two randomized, double-masked, placebo-controlled, parallel-group studies that were similar in design and that demonstrated efficacy.
The studies included a total of 171 patients, fewer than 90 of whom were treated with teprotumumab, the agency explained in a briefing document. “This is a considerably smaller database than the common safety database of greater than 300 patients treated with a course of therapy,” it observed.
The study required that patients have proptosis but did not require that they have “progressive forward motion of the globe,” it noted.
The primary objective – a reduction in proptosis of 2 or more mm – was met in 82% of patients who received teprotumumab, compared with 16% of those who received placebo.
Review study No. 1 (TED01RV) was a phase 2 study, and review study No. 2 (OPTIC) was a phase 3 study. Both trials included a 24-week treatment period during which participants received teprotumumab or placebo intravenously every 3 weeks for a total of eight doses. Patients in both studies underwent a follow-up period during which they received no further treatment.
Some patients experienced a reduction in proptosis as early as 6 weeks after they received the first infusion. In an extension of TED01RV, that reduction extended for at least 4 weeks after the last infusion. For about 60% of responders, no relapse had occurred by week 72. The extension of OPTIC and an open-label treatment period for those with no response to placebo or teprotumumab are ongoing.
“I welcome the addition of this drug to our armamentarium to treat this horrible, horrible disease,” said temporary voting committee member John F. Stamler, MD, PhD, clinical instructor, ophthalmology and visual sciences, University of Iowa, Iowa City.
Adverse events
Teprotumumab inhibits the insulinlike growth factor–1 receptor, which can interfere with the body’s ability to regulate glucose, particularly in patients with diabetes. In the study, some patients with diabetes required additional insulin for glycemic control.
In three study participants whose baseline fasting blood glucose levels were normal, blood glucose levels were found to be elevated at one or more visits during the treatment period. None had a history of diabetes mellitus, but for two, baseline hemoglobin A1c levels were elevated.
Five or more patients reported loss of hearing (hypoacusis), and others reported tinnitus. One of them experienced a spontaneous return of hearing the day after the hypoacusis developed, whereas for others, hearing did not return until after teprotumumab treatment was completed. The mechanism of action for hearing loss is unclear.
Panel members felt the potential for hearing loss is important and that patients should receive some type of monitoring, but they did not all agree on when that testing should occur or who should be responsible for getting it done.
“It strikes me that, if this drug were approved, there would be centers that would be interested in undertaking independent studies of hearing loss in treated patients, and that that could be done outside the sponsor’s responsibility and probably would be of interest to independent investigators,” noted committee chairperson James Chodosh, MD, MPH, of Massachusetts Eye and Ear and Harvard Medical School, Boston.
Benefits outweigh risks
More than one-third of patients (36%) experienced gastrointestinal complaints such as nausea and diarrhea (12% each) and abdominal pain (5%). None of the cases caused any patient to discontinue the study drug.
The overall incidence of muscle spasms was more than three times higher in the teprotumumab group (32%) than in the placebo group (9.5%).
One patient stopped taking teprotumumab after being hospitalized for Escherichia coli sepsis and dehydration, and another participant stopped after experiencing an episode of inflammatory bowel disease. It is not clear whether there is a causal association between teprotumumab and inflammatory bowel disease.
No study participants died.
Panel members expressed concern about safety in the longer term or in patients who receive multiple courses of teprotumumab, but they felt the potential benefits from teprotumumab outweigh the risks. The committee also wanted more information about the effects of teprotumumab with respect to glucose control, hearing loss, and other outcomes that are very important to patients, such as alopecia.
The panel favored including diarrhea in the list of adverse events in the label and felt that there should be a warning about inflammatory bowel disease.
“We’re finally going to be able to get a lot of people some help,” concluded voting committee member Sidney Gicheru, MD, LaserCare Eye Center, Irving, Texas.
A version of this story originally appeared on Medscape.com.
TED is a rare autoimmune disease that causes the eyes to bulge (proptosis) and can lead to blindness. It is also known as thyroid-associated ophthalmopathy, Graves ophthalmopathy, and Graves orbitopathy. Current treatment is aimed at relief of symptoms and includes corticosteroids and orbital decompression.
“TED can affect patients both physically and emotionally, limiting their ability to perform everyday activities like driving, working, reading, sleeping, and participating in social activities,” Jeff Todd, president and chief executive officer, Prevent Blindness, said in a news release.
“As an organization dedicated to helping patients with vision impairment and those who are at significant risk, we are extremely encouraged by today’s vote and hopeful this will change the future of TED treatment by giving patients an option that has been shown to improve the painful and vision-threatening aspects of the disease,” Mr. Todd said.
Teprotumumab was granted fast-track status in April 2015 and breakthrough therapy designation in July 2016. It received orphan drug designation on June 19, 2019. If approved, it would be the first approved treatment for this indication.
All 12 members of the Dermatologic and Ophthalmic Drugs Advisory Committee of the FDA voted to recommend approval of teprotumumab.
“It’s clearly a pleasure to participate in seeing a drug being designed and moving forward in a clinical trial for a disease that really has not been treatable for us in the past,” said voting committee member Timothy Murray, MD, MBA, of the Bascom Palmer Eye Institute, Miami.
Efficacy demonstrated
The FDA advisory committee considered data from two randomized, double-masked, placebo-controlled, parallel-group studies that were similar in design and that demonstrated efficacy.
The studies included a total of 171 patients, fewer than 90 of whom were treated with teprotumumab, the agency explained in a briefing document. “This is a considerably smaller database than the common safety database of greater than 300 patients treated with a course of therapy,” it observed.
The study required that patients have proptosis but did not require that they have “progressive forward motion of the globe,” it noted.
The primary objective – a reduction in proptosis of 2 or more mm – was met in 82% of patients who received teprotumumab, compared with 16% of those who received placebo.
Review study No. 1 (TED01RV) was a phase 2 study, and review study No. 2 (OPTIC) was a phase 3 study. Both trials included a 24-week treatment period during which participants received teprotumumab or placebo intravenously every 3 weeks for a total of eight doses. Patients in both studies underwent a follow-up period during which they received no further treatment.
Some patients experienced a reduction in proptosis as early as 6 weeks after they received the first infusion. In an extension of TED01RV, that reduction extended for at least 4 weeks after the last infusion. For about 60% of responders, no relapse had occurred by week 72. The extension of OPTIC and an open-label treatment period for those with no response to placebo or teprotumumab are ongoing.
“I welcome the addition of this drug to our armamentarium to treat this horrible, horrible disease,” said temporary voting committee member John F. Stamler, MD, PhD, clinical instructor, ophthalmology and visual sciences, University of Iowa, Iowa City.
Adverse events
Teprotumumab inhibits the insulinlike growth factor–1 receptor, which can interfere with the body’s ability to regulate glucose, particularly in patients with diabetes. In the study, some patients with diabetes required additional insulin for glycemic control.
In three study participants whose baseline fasting blood glucose levels were normal, blood glucose levels were found to be elevated at one or more visits during the treatment period. None had a history of diabetes mellitus, but for two, baseline hemoglobin A1c levels were elevated.
Five or more patients reported loss of hearing (hypoacusis), and others reported tinnitus. One of them experienced a spontaneous return of hearing the day after the hypoacusis developed, whereas for others, hearing did not return until after teprotumumab treatment was completed. The mechanism of action for hearing loss is unclear.
Panel members felt the potential for hearing loss is important and that patients should receive some type of monitoring, but they did not all agree on when that testing should occur or who should be responsible for getting it done.
“It strikes me that, if this drug were approved, there would be centers that would be interested in undertaking independent studies of hearing loss in treated patients, and that that could be done outside the sponsor’s responsibility and probably would be of interest to independent investigators,” noted committee chairperson James Chodosh, MD, MPH, of Massachusetts Eye and Ear and Harvard Medical School, Boston.
Benefits outweigh risks
More than one-third of patients (36%) experienced gastrointestinal complaints such as nausea and diarrhea (12% each) and abdominal pain (5%). None of the cases caused any patient to discontinue the study drug.
The overall incidence of muscle spasms was more than three times higher in the teprotumumab group (32%) than in the placebo group (9.5%).
One patient stopped taking teprotumumab after being hospitalized for Escherichia coli sepsis and dehydration, and another participant stopped after experiencing an episode of inflammatory bowel disease. It is not clear whether there is a causal association between teprotumumab and inflammatory bowel disease.
No study participants died.
Panel members expressed concern about safety in the longer term or in patients who receive multiple courses of teprotumumab, but they felt the potential benefits from teprotumumab outweigh the risks. The committee also wanted more information about the effects of teprotumumab with respect to glucose control, hearing loss, and other outcomes that are very important to patients, such as alopecia.
The panel favored including diarrhea in the list of adverse events in the label and felt that there should be a warning about inflammatory bowel disease.
“We’re finally going to be able to get a lot of people some help,” concluded voting committee member Sidney Gicheru, MD, LaserCare Eye Center, Irving, Texas.
A version of this story originally appeared on Medscape.com.