User login
MACE benefits with dapagliflozin improve with disease duration
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
Treatment with the sodium-glucose transporter 2 inhibitor dapagliflozin reduced the risk for cardiovascular disease or hospitalization for heart failure (CVD/HHF) in patients with diabetes, regardless of the duration of the disease, but had a greater protective benefit against major adverse cardiovascular events (MACE) and renal events in patients with longer disease duration, according to new findings from a post hoc analysis of the DECLARE-TIMI 58 trial.
The positive effect of dapagliflozin in patients with MACE – which includes myocardial infarction (MI), CVD, and ischemic stroke – may have been driven by lower rates of MI and ischemic stroke with the drug, compared with placebo, in patients with longer disease duration, wrote Harpreet S. Bajaj, MD, and colleagues. Their report is in Diabetes, Obesity and Metabolism (2020 Feb 23. doi: 10.1111/dom.14011).
It has been previously reported that the risk for complications in diabetes increases with increasing duration of the disease. Recent studies with SGLT-2 inhibitors have shown that the drugs improve cardiovascular and renal outcomes in diabetes, and they are recommended by the American Diabetes Association as second-line therapy in patients with atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure. The European Society of Cardiology and the European Association for the Study of Diabetes recommend that patients with diabetes patients who have three or more risk factors, or those with a disease duration of more than 20 years, should be deemed very high risk and be considered for early treatment with SGLT2 inhibitors.
“The MACE benefit observed with dapagliflozin in this study in patients with diabetes duration of [more than] 20 years, clearly supports that notion,” the authors wrote.
In DECLARE-TIMI 58, 17,160 patients with type 2 diabetes received dapagliflozin or placebo and were followed for a median of 4.2 years. Of those patients, 22.4% had a disease duration of fewer than 5 years; 27.6%, a duration of 5-10 years; 23.0%, 10-15 years; 14.2%, 10-15 years; and 12.9%, more than 20 years. The median duration of disease was 11 years.
Patients in all the age groups had similar reductions in CVD/HHF, compared with placebo, with hazard ratios of 0.79 (disease duration of 5 or fewer years), 0.86, 0.92, 0.81, and 0.75 (duration of 20 years), respectively (interaction trend P = .760).
Treatment with dapagliflozin reduced the incidence of MACE, but the benefit was more apparent in patients with longer-term disease: HR, 1.08; 1.02; 0.94; 0.92; and 0.67, respectively (interaction trend P = .004). Similar trends were seen with MI (interaction trend P = .019) and ischemic stroke (interaction trend P = .015).
The researchers also reported improved benefits in renal-specific outcome with increasing disease duration, with HRs ranging from 0.79 in patients with diabetes duration of fewer than 5 years, to 0.42 in those with a duration of more than 20 years (interaction trend P = .084).
Limitations of the study include the fact that the information about diabetes duration relied on patient reports, and that the original trial was not powered for all subgroup interactions. This authors emphasized that this was a post hoc analysis and as such, should be considered hypothesis generating.
All but two of the authors reported relationships with Astra Zeneca, which funded the study, and other drug companies.
SOURCE: Bajaj HS et al. Diabetes Obes Metab. 2020 Feb 23. doi: 10.1111/dom.14011.
FROM DIABETES, OBESITY AND METABOLISM
RA magnifies fragility fracture risk in ESRD
MAUI, HAWAII – Comorbid rheumatoid arthritis is a force multiplier for fragility fracture risk in patients with end-stage renal disease, Renée Peterkin-McCalman, MD, reported at the 2020 Rheumatology Winter Clinical Symposium.
“Patients with RA and ESRD are at substantially increased risk of osteoporotic fragility fractures compared to the overall population of ESRD patients. So fracture prevention prior to initiation of dialysis should be a focus of care in patients with RA,” said Dr. Peterkin-McCalman, a rheumatology fellow at the Medical College of Georgia, Augusta.
She presented a retrospective cohort study of 10,706 adults who initiated hemodialysis or peritoneal dialysis for ESRD during 2005-2008, including 1,040 who also had RA. All subjects were drawn from the United States Renal Data System. The impetus for the study, Dr. Peterkin-McCalman explained in an interview, was that although prior studies have established that RA and ESRD are independent risk factors for osteoporotic fractures, the interplay between the two was previously unknown.
The risk of incident osteoporotic fractures during the first 3 years after going on renal dialysis was 14.7% in patients with ESRD only, vaulting to 25.6% in those with comorbid RA. Individuals with both RA and ESRD were at an adjusted 1.83-fold increased overall risk for new fragility fractures and at 1.85-fold increased risk for hip fracture, compared to those without RA.
Far and away the strongest risk factor for incident osteoporotic fractures in the group with RA plus ESRD was a history of a fracture sustained within 5 years prior to initiation of dialysis, with an associated 11.5-fold increased fracture risk overall and an 8.2-fold increased risk of hip fracture.
“The reason that’s important is we don’t really have any medications to reduce fracture risk once you get to ESRD. Of course, we have bisphosphonates and Prolia (denosumab) and things like that, but that’s in patients with milder CKD [chronic kidney disease] or no renal disease at all. So the goal is to identify the patients early who are at higher risk so that we can protect those bones before they get to ESRD and we have nothing left to treat them with,” she said.
In addition to a history of prevalent fracture prior to starting ESRD, the other risk factors for fracture in patients with ESRD and comorbid RA Dr. Peterkin-McCalman identified in her study included age greater than 50 years at the start of dialysis and female gender, which was associated with a twofold greater fracture risk than in men. Black patients with ESRD and RA were 64% less likely than whites to experience an incident fragility fracture. And the fracture risk was higher in patients on hemodialysis than with peritoneal dialysis.
Her study was supported by the Medical College of Georgia and a research grant from Dialysis Clinic Inc.
SOURCE: Peterkin-McCalman R et al. RWCS 2020.
MAUI, HAWAII – Comorbid rheumatoid arthritis is a force multiplier for fragility fracture risk in patients with end-stage renal disease, Renée Peterkin-McCalman, MD, reported at the 2020 Rheumatology Winter Clinical Symposium.
“Patients with RA and ESRD are at substantially increased risk of osteoporotic fragility fractures compared to the overall population of ESRD patients. So fracture prevention prior to initiation of dialysis should be a focus of care in patients with RA,” said Dr. Peterkin-McCalman, a rheumatology fellow at the Medical College of Georgia, Augusta.
She presented a retrospective cohort study of 10,706 adults who initiated hemodialysis or peritoneal dialysis for ESRD during 2005-2008, including 1,040 who also had RA. All subjects were drawn from the United States Renal Data System. The impetus for the study, Dr. Peterkin-McCalman explained in an interview, was that although prior studies have established that RA and ESRD are independent risk factors for osteoporotic fractures, the interplay between the two was previously unknown.
The risk of incident osteoporotic fractures during the first 3 years after going on renal dialysis was 14.7% in patients with ESRD only, vaulting to 25.6% in those with comorbid RA. Individuals with both RA and ESRD were at an adjusted 1.83-fold increased overall risk for new fragility fractures and at 1.85-fold increased risk for hip fracture, compared to those without RA.
Far and away the strongest risk factor for incident osteoporotic fractures in the group with RA plus ESRD was a history of a fracture sustained within 5 years prior to initiation of dialysis, with an associated 11.5-fold increased fracture risk overall and an 8.2-fold increased risk of hip fracture.
“The reason that’s important is we don’t really have any medications to reduce fracture risk once you get to ESRD. Of course, we have bisphosphonates and Prolia (denosumab) and things like that, but that’s in patients with milder CKD [chronic kidney disease] or no renal disease at all. So the goal is to identify the patients early who are at higher risk so that we can protect those bones before they get to ESRD and we have nothing left to treat them with,” she said.
In addition to a history of prevalent fracture prior to starting ESRD, the other risk factors for fracture in patients with ESRD and comorbid RA Dr. Peterkin-McCalman identified in her study included age greater than 50 years at the start of dialysis and female gender, which was associated with a twofold greater fracture risk than in men. Black patients with ESRD and RA were 64% less likely than whites to experience an incident fragility fracture. And the fracture risk was higher in patients on hemodialysis than with peritoneal dialysis.
Her study was supported by the Medical College of Georgia and a research grant from Dialysis Clinic Inc.
SOURCE: Peterkin-McCalman R et al. RWCS 2020.
MAUI, HAWAII – Comorbid rheumatoid arthritis is a force multiplier for fragility fracture risk in patients with end-stage renal disease, Renée Peterkin-McCalman, MD, reported at the 2020 Rheumatology Winter Clinical Symposium.
“Patients with RA and ESRD are at substantially increased risk of osteoporotic fragility fractures compared to the overall population of ESRD patients. So fracture prevention prior to initiation of dialysis should be a focus of care in patients with RA,” said Dr. Peterkin-McCalman, a rheumatology fellow at the Medical College of Georgia, Augusta.
She presented a retrospective cohort study of 10,706 adults who initiated hemodialysis or peritoneal dialysis for ESRD during 2005-2008, including 1,040 who also had RA. All subjects were drawn from the United States Renal Data System. The impetus for the study, Dr. Peterkin-McCalman explained in an interview, was that although prior studies have established that RA and ESRD are independent risk factors for osteoporotic fractures, the interplay between the two was previously unknown.
The risk of incident osteoporotic fractures during the first 3 years after going on renal dialysis was 14.7% in patients with ESRD only, vaulting to 25.6% in those with comorbid RA. Individuals with both RA and ESRD were at an adjusted 1.83-fold increased overall risk for new fragility fractures and at 1.85-fold increased risk for hip fracture, compared to those without RA.
Far and away the strongest risk factor for incident osteoporotic fractures in the group with RA plus ESRD was a history of a fracture sustained within 5 years prior to initiation of dialysis, with an associated 11.5-fold increased fracture risk overall and an 8.2-fold increased risk of hip fracture.
“The reason that’s important is we don’t really have any medications to reduce fracture risk once you get to ESRD. Of course, we have bisphosphonates and Prolia (denosumab) and things like that, but that’s in patients with milder CKD [chronic kidney disease] or no renal disease at all. So the goal is to identify the patients early who are at higher risk so that we can protect those bones before they get to ESRD and we have nothing left to treat them with,” she said.
In addition to a history of prevalent fracture prior to starting ESRD, the other risk factors for fracture in patients with ESRD and comorbid RA Dr. Peterkin-McCalman identified in her study included age greater than 50 years at the start of dialysis and female gender, which was associated with a twofold greater fracture risk than in men. Black patients with ESRD and RA were 64% less likely than whites to experience an incident fragility fracture. And the fracture risk was higher in patients on hemodialysis than with peritoneal dialysis.
Her study was supported by the Medical College of Georgia and a research grant from Dialysis Clinic Inc.
SOURCE: Peterkin-McCalman R et al. RWCS 2020.
REPORTING FROM RWCS 2020
Refining your approach to hypothyroidism treatment
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4

Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3

Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.

Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4

Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3

Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.

Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; [email protected]
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine 1.6 mcg/kg/d for healthy adult patients < 50 years of age with overt hypothyroidism. B
› Consider lower initial doses of levothyroxine in patients with cardiac disease (12.5-50 mcg/d) or subclinical hypothyroidism (25-75 mcg/d). B
› Titrate levothyroxine by 12.5 to 25 mcg/d at 6- to 8-week intervals based on thyroid-stimulating hormone measurements, comorbidities, and symptoms. C
› Closely monitor and provide thyroid supplementation to female patients who are pregnant or of reproductive age with concomitant hypothyroidism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Can this patient get IV contrast?
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
A 59-year-old man is admitted with abdominal pain. He has a history of pancreatitis. A contrast CT scan is ordered. He reports a history of severe shellfish allergy when the radiology tech checks him in for the procedure. You are paged regarding what to do:
A) Continue with scan as ordered.
B) Switch to MRI scan.
C) Switch to MRI scan with gadolinium.
D) Continue with CT with contrast, give dose of Solu-Medrol.
E) Continue with CT with contrast give IV diphenhydramine.
The correct answer here is A, This patient can receive his scan and receive contrast as ordered.
The mistaken thought was that shellfish contains iodine, so allergy to shellfish was likely to portend allergy to iodine.
Allergy to shellfish is caused by individual proteins that are definitely not in iodine-containing contrast.1 Beaty et al. studied the prevalence of the belief that allergy to shellfish is tied to iodine allergy in a survey given to 231 faculty radiologists and interventional cardiologists.2 Almost 70% responded that they inquire about seafood allergy before procedures that require iodine contrast, and 37% reported they would withhold the contrast or premedicate patients if they had a seafood allergy.
In a more recent study, Westermann-Clark and colleagues surveyed 252 health professionals before and after an educational intervention to dispel the myth of shellfish allergy and iodinated contrast reactions.3 Before the intervention, 66% of participants felt it was important to ask about shellfish allergies and 93% felt it was important to ask about iodine allergies; 26% responded that they would withhold iodinated contrast material in patients with a shellfish allergy, and 56% would withhold in patients with an iodine allergy. A total of 62% reported they would premedicate patients with a shellfish allergy and 75% would premedicate patients with an iodine allergy. The numbers declined dramatically after the educational intervention.
Patients who have seafood allergy have a higher rate of reactions to iodinated contrast, but not at a higher rate than do patients with other food allergies or asthma.4 Most radiology departments do not screen for other food allergies despite the fact these allergies have the same increased risk as for patients with a seafood/shellfish allergy. These patients are more allergic, and in general, are more likely to have reactions. The American Academy of Allergy, Asthma, and Immunology recommends not routinely ordering low- or iso-osmolar radiocontrast media or pretreating with either antihistamines or steroids in patients with a history of seafood allergy.5
There is no evidence that iodine causes allergic reactions. It makes sense that iodine does not cause allergic reactions, as it is an essential component in the human body, in thyroid hormone and in amino acids.6 Patients with dermatitis following topical application of iodine preparations such as povidone-iodide are not reacting to the iodine.
Van Ketel and van den Berg patch-tested patients with a history of dermatitis after exposure to povidone-iodine.7 All patients reacted to patch testing with povidone-iodine, but none reacted to direct testing to iodine (0/5 with patch testing of potassium iodide and 0/3 with testing with iodine tincture).
Take home points:
- It is unnecessary and unhelpful to ask patients about seafood allergies before ordering radiologic studies involving contrast.
- Iodine allergy does not exist.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Narayan AK et al. Avoiding contrast-enhanced computed tomography scans in patients with shellfish allergies. J Hosp Med. 2016 Jun;11(6):435-7.
2. Beaty AD et al. Seafood allergy and radiocontrast media: Are physicians propagating a myth? Am J Med. 2008 Feb;121(2):158.e1-4.
3. Westermann-Clark E et al. Debunking myths about “allergy” to radiocontrast media in an academic institution. Postgrad Med. 2015 Apr;127(3):295-300.
4. Coakley FV and DM Panicek. Iodine allergy: An oyster without a pearl? AJR Am J Roentgenol. 1997 Oct;169(4):951-2.
5. American Academy of Allergy, Asthma & Immunology recommendations on low- or iso-osmolar radiocontrast media.
6. Schabelman E and M Witting. The relationship of radiocontrast, iodine, and seafood allergies: A medical myth exposed. J Emerg Med. 2010 Nov;39(5):701-7.
7. van Ketel WG and WH van den Berg. Sensitization to povidone-iodine. Dermatol Clin. 1990 Jan;8(1):107-9.
In gestational diabetes, early postpartum glucose testing is a winner
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
GRAPEVINE, TEX. – Early postpartum glucose tolerance testing for women with gestational diabetes resulted in a 99% adherence rate, with similar sensitivity and specificity as the currently recommended 4- to 12-week postpartum testing schedule.
“Two-day postpartum glucose tolerance testing has similar diagnostic utility as the 4- to 12-week postpartum glucose tolerance test to identify impaired glucose metabolism and diabetes at 1 year postpartum,” said Erika Werner, MD, speaking at the meeting sponsored by the Society for Maternal-Fetal Medicine.
Overall, 29% of women studied had impaired glucose metabolism at 2 days postpartum, as did 25% in the 4- to 12-weeks postpartum window. At 1 year, that figure was 35%. The number of women meeting diagnostic criteria for diabetes held steady at 4% for all three time points.
The findings warrant “consideration for the 2-day postpartum glucose tolerance test (GTT) as the initial postpartum test for women who have gestational diabetes, with repeat testing at 1 year,” said Dr. Werner, a maternal-fetal medicine physician at Brown University, Providence, R.I.
Glucose testing for women with gestational diabetes mellitus (GDM) is recommended at 4-12 weeks postpartum by both the American Diabetes Association and the American College of Obstetricians and Gynecologists.
Testing can allow detection and treatment of impaired glucose metabolism, seen in 15%-40% of women with a history of GDM. Up to 1 in 20 women with GDM will receive a postpartum diagnosis of type 2 diabetes.
However, fewer than one in five women will actually have postpartum glucose testing, representing a large missed opportunity, said Dr. Werner.
Several factors likely contribute to those screening failures, she added. In addition to the potential for public insurance to lapse at 6 weeks postpartum, the logistical realities and time demands of parenting a newborn are themselves a significant barrier.
“What if we changed the timing?” and shifted glucose testing to the early postpartum days, before hospital discharge, asked Dr. Werner. Several pilot studies had already compared glucose screening in the first few days postpartum with the routine schedule, finding good correlation between the early and routine GTT schedule.
Importantly, the earlier studies achieved an adherence rate of more than 90% for early GTT. By contrast, fewer than half of the participants in the usual-care arms actually returned for postpartum GTT in the 4- to 12-week postpartum window, even under the optimized conditions associated with a medical study.
The single-center prospective cohort study conducted by Dr. Werner and collaborators enrolled 300 women with GDM. Women agreed to participate in glucose tolerance testing as inpatients, at 2 days postpartum, in addition to receiving a GTT between 4 and 12 weeks postpartum, and additional screening that included a glycosylated hemoglobin (HbA1c) test at 1 year postpartum.
The investigators obtained postpartum day 2 GTTs for all but four of the patients. A total of 201 patients returned in the 4- to 12-week postpartum window, and 168 of those participants returned for HbA1c testing at 1 year. Of the 95 patients who didn’t come back for the 4- to 12-week test, 33 did return at 1 year for HbA1c testing.
Dr. Werner and her coinvestigators included adult women who spoke either fluent Spanish or English and had GDM diagnosed by the Carpenter-Coustan criteria, or by having a blood glucose level of 200 mg/dL or more in a 1-hour glucose challenge test.
The early GTT results weren’t shared with patients or their health care providers. For outpatient visits, participants were offered financial incentives and received multiple reminder phone calls and the offer of free transportation.
For the purposes of the study, impaired glucose metabolism was defined as fasting blood glucose of 100 mg/dL or greater, a 2-hour GTT blood glucose level of 140 mg/dL or greater, or HbA1c of 5.7% or greater.
Participants were diagnosed with diabetes if they had a fasting blood glucose of 126 mg/dL or greater, a 2-hour GTT blood glucose level of 200 mg/dL or greater, or HbA1c of 6.5% or greater.
Dr. Werner and colleagues conducted two analyses of their results. In the first, they included only women in both arms who had complete data. In the second analysis, they looked at all women who had data for the 1-year postpartum mark, assuming that interval GTTs were negative for women who were missing these values.
The statistical analysis showed that, for women with complete data, both early and later postpartum GTTs were similar in predicting impaired glucose metabolism at 1 year postpartum (areas under the receiver operating curve [AUC], 0.63 and 0.60, respectively).
For identifying diabetes at 1 year, both early and late testing had high negative predictive value (98% and 99%, respectively), but the later testing strategy had higher sensitivity and specificity, yielding an AUC of 0.83, compared with 0.65 for early testing.
Turning to the second analysis that included all women who had 1-year postpartum HbA1c values, negative predictive values for diabetes were similarly high (98%) for both the early and late testing strategies. For identifying impaired glucose metabolism at 1 year in this group, both the positive and negative predictive value of the early and late strategies were similar.
Patients were about 32 years old at baseline, with a mean body mass index of 31.7 kg/m2. More than half of patients (52.3%) had private insurance, and 22% had GDM in a pregnancy prior to the index pregnancy. Black patients made up about 9% of the study population; 54% of participants were white, and 23% Hispanic. About one-third of patients were nulliparous, and two-thirds had education beyond high school.
During their pregnancies, about 44% of patients managed GDM by diet alone, 40% required insulin, with an additional 1% also requiring an oral agent. The remainder required oral agents alone. Patients delivered at a mean 38.3 weeks gestation, with about 40% receiving cesarean deliveries.
Some of the study’s strengths included its prospective nature, the diverse population recruited, and the fact that participants and providers were both blinded to the 2-day GTT results. Although more than half of participants completed the study – besting the previous pilots – 44% of patients still had incomplete data, noted Dr. Werner.
The American Diabetes Association sponsored the study. Dr. Werner reported no other conflicts of interest.
SOURCE: Werner E et al. SMFM 2020. Abstract 72.
REPORTING FROM THE PREGNANCY MEETING
Endocrine Society advises on use of romosozumab for osteoporosis
Latest guidelines on the treatment of osteoporosis have been released that include new recommendations for the use of romosozumab (Evenity) in postmenopausal women with severe osteoporosis, but they contain caveats as to which women should – and should not – receive the drug.
The updated clinical practice guideline from the Endocrine Society is in response to the approval of romosozumab by the Food and Drug Administration (FDA) in April 2019, and more recently, by the European Medicines Agency.
It was published online February 18 in the Journal of Clinical Endocrinology & Metabolism.
In the new guidelines, committee members recommend the use of romosozumab for postmenopausal women with osteoporosis at very high risk of fracture. Candidates would include women with severe osteoporosis (T-score of less than –2.5 and a prior fracture) or women with a history of multiple vertebral fractures.
Women should be treated with romosozumab for up to 1 year, followed by an antiresorptive agent to maintain bone mineral density gains and further reduce fracture risk.
“The recommended dosage is 210 mg monthly by subcutaneous injection for 12 months,” the authors wrote.
However, and very importantly, romosozumab should not be considered for women at high risk of cardiovascular disease (CVD) or cerebrovascular disease. A high risk of CVD includes women who have had a previous myocardial infarction (MI) or stroke.
Experts questioned by Medscape Medical News stressed that romosozumab should not be a first-line, or even generally a second-line, option for osteoporosis, but it can be a considered for select patients with severe osteoporosis, taking into account CV risk.
Boxed warning
In the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH), there were more major adverse cardiovascular events (MACE) in the first year of the trial with romosozumab, and patients had a 31% higher risk of MACE with romosozumab, compared with the bisphosphonate alendronate.
As a result, the drug was initially rejected by a number of regulatory agencies.
In the United States and Canada, it was eventually approved with a boxed warning, which cautions against the use of the drug in patients at risk for myocardial infarction, stroke, and CVD-related death.
“Romosozumab offers promising results for postmenopausal women with severe osteoporosis or who have a history of fractures,” Clifford Rosen, MD, Maine Medical Center Research Institute in Scarborough and chair of the writing committee, said in an Endocrine Society statement. “It does, however, come with a risk of heart disease, so clinicians need to be careful when selecting patients for this therapy.”
Exact risk unknown
Asked by Medscape Medical News to comment, Kenneth Saag, MD, professor of medicine, University of Alabama at Birmingham and principle investigator of the ARCH study, said that physicians needed more data from real-world studies to resolve the issue around whether romosozumab heightens the risk of CV events in women with osteoporosis or whether that particular finding from ARCH was an artifact.
“Women who have had a recent cardiovascular event should not receive the drug,” he said, agreeing with the new guidelines.
But it remains unclear, for example, whether women who are at slightly higher risk of having a CV event by virtue of their age alone, are also at risk, he noted.
In the meantime, results from the ARCH study clearly showed that not only was romosozumab more effective than alendronate, “but it is more effective than other bone-building drugs as well,” Dr. Saag observed, and it leads to a significantly greater reduction in vertebral, nonvertebral, and hip fractures, compared with the alendronate, the current standard of care in osteoporosis.
“In patients who have very severe osteoporosis and who have had a recent fracture or who are at risk for imminent future fracture, physicians need to balance the benefit versus the risk in favor of using romosozumab,” Dr. Saag suggested.
“And while I would say most women prefer not to inject themselves, the women I have put on this medicine have all had recent fractures and they are very aware of the pain and the disability of having a broken bone, so it is something they are willing to do,” he added.
Not for all women
Giving his opinion, Bart Clarke, MD, of the Mayo Clinic in Rochester, Minnesota, underscored the fact that the Fracture Study in Postmenopausal Women With Osteoporosis (FRAME), again conducted in postmenopausal women, showed no increase in CV events in patients treated with romosozumab compared with placebo.
“So there are questions about what this means, because if these events really were a drug effect, then that effect would be even more evident compared with placebo and they did not see any signal of CV events [in FRAME],” said Dr. Clarke, past president of the American Society of Bone and Mineral Research.
Like everything else in medicine, “there is always some risk,” Dr. Clarke observed.
However, what physicians should do is talk to women, ensure they have not had either an MI or stroke in the last year, and if patients have severe osteoporosis and a high risk of fracture, “then we can say, here’s another option,” he suggested.
“Then, if a woman develops chest pain or shortness of breath while on the drug, [she needs] to let us know ,and then we’ll stop the drug and reassess the situation,” he added.
Dr. Clarke also pointed out that if the FDA had received further reports of CV events linked to romosozumab, physicians would know about it by now, but to his knowledge, there has been no change to the drug’s current warning label.
Furthermore, neither he nor any of his colleagues who treat metabolic bone disease at the Mayo Clinic has seen a single CV event in patients prescribed the agent.
“This is not a drug we would use as first-line for most patients, and we don’t even use it as second-line for most patients, but in people who have not responded to other drugs or who have had terrible things happen to them already, hip fracture especially, then we are saying, you can consider this, he concluded.
The guidelines were supported by the Endocrine Society. Dr. Rosen and Dr. Clarke reported no relevant financial relationships. Dr. Saag reported receiving grants and personal fees from Amgen, personal fees from Radius, and is a consultant for Amgen, Radius, Roche, and Daiichi Sankyo.
This article first appeared on Medscape.com.
Latest guidelines on the treatment of osteoporosis have been released that include new recommendations for the use of romosozumab (Evenity) in postmenopausal women with severe osteoporosis, but they contain caveats as to which women should – and should not – receive the drug.
The updated clinical practice guideline from the Endocrine Society is in response to the approval of romosozumab by the Food and Drug Administration (FDA) in April 2019, and more recently, by the European Medicines Agency.
It was published online February 18 in the Journal of Clinical Endocrinology & Metabolism.
In the new guidelines, committee members recommend the use of romosozumab for postmenopausal women with osteoporosis at very high risk of fracture. Candidates would include women with severe osteoporosis (T-score of less than –2.5 and a prior fracture) or women with a history of multiple vertebral fractures.
Women should be treated with romosozumab for up to 1 year, followed by an antiresorptive agent to maintain bone mineral density gains and further reduce fracture risk.
“The recommended dosage is 210 mg monthly by subcutaneous injection for 12 months,” the authors wrote.
However, and very importantly, romosozumab should not be considered for women at high risk of cardiovascular disease (CVD) or cerebrovascular disease. A high risk of CVD includes women who have had a previous myocardial infarction (MI) or stroke.
Experts questioned by Medscape Medical News stressed that romosozumab should not be a first-line, or even generally a second-line, option for osteoporosis, but it can be a considered for select patients with severe osteoporosis, taking into account CV risk.
Boxed warning
In the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH), there were more major adverse cardiovascular events (MACE) in the first year of the trial with romosozumab, and patients had a 31% higher risk of MACE with romosozumab, compared with the bisphosphonate alendronate.
As a result, the drug was initially rejected by a number of regulatory agencies.
In the United States and Canada, it was eventually approved with a boxed warning, which cautions against the use of the drug in patients at risk for myocardial infarction, stroke, and CVD-related death.
“Romosozumab offers promising results for postmenopausal women with severe osteoporosis or who have a history of fractures,” Clifford Rosen, MD, Maine Medical Center Research Institute in Scarborough and chair of the writing committee, said in an Endocrine Society statement. “It does, however, come with a risk of heart disease, so clinicians need to be careful when selecting patients for this therapy.”
Exact risk unknown
Asked by Medscape Medical News to comment, Kenneth Saag, MD, professor of medicine, University of Alabama at Birmingham and principle investigator of the ARCH study, said that physicians needed more data from real-world studies to resolve the issue around whether romosozumab heightens the risk of CV events in women with osteoporosis or whether that particular finding from ARCH was an artifact.
“Women who have had a recent cardiovascular event should not receive the drug,” he said, agreeing with the new guidelines.
But it remains unclear, for example, whether women who are at slightly higher risk of having a CV event by virtue of their age alone, are also at risk, he noted.
In the meantime, results from the ARCH study clearly showed that not only was romosozumab more effective than alendronate, “but it is more effective than other bone-building drugs as well,” Dr. Saag observed, and it leads to a significantly greater reduction in vertebral, nonvertebral, and hip fractures, compared with the alendronate, the current standard of care in osteoporosis.
“In patients who have very severe osteoporosis and who have had a recent fracture or who are at risk for imminent future fracture, physicians need to balance the benefit versus the risk in favor of using romosozumab,” Dr. Saag suggested.
“And while I would say most women prefer not to inject themselves, the women I have put on this medicine have all had recent fractures and they are very aware of the pain and the disability of having a broken bone, so it is something they are willing to do,” he added.
Not for all women
Giving his opinion, Bart Clarke, MD, of the Mayo Clinic in Rochester, Minnesota, underscored the fact that the Fracture Study in Postmenopausal Women With Osteoporosis (FRAME), again conducted in postmenopausal women, showed no increase in CV events in patients treated with romosozumab compared with placebo.
“So there are questions about what this means, because if these events really were a drug effect, then that effect would be even more evident compared with placebo and they did not see any signal of CV events [in FRAME],” said Dr. Clarke, past president of the American Society of Bone and Mineral Research.
Like everything else in medicine, “there is always some risk,” Dr. Clarke observed.
However, what physicians should do is talk to women, ensure they have not had either an MI or stroke in the last year, and if patients have severe osteoporosis and a high risk of fracture, “then we can say, here’s another option,” he suggested.
“Then, if a woman develops chest pain or shortness of breath while on the drug, [she needs] to let us know ,and then we’ll stop the drug and reassess the situation,” he added.
Dr. Clarke also pointed out that if the FDA had received further reports of CV events linked to romosozumab, physicians would know about it by now, but to his knowledge, there has been no change to the drug’s current warning label.
Furthermore, neither he nor any of his colleagues who treat metabolic bone disease at the Mayo Clinic has seen a single CV event in patients prescribed the agent.
“This is not a drug we would use as first-line for most patients, and we don’t even use it as second-line for most patients, but in people who have not responded to other drugs or who have had terrible things happen to them already, hip fracture especially, then we are saying, you can consider this, he concluded.
The guidelines were supported by the Endocrine Society. Dr. Rosen and Dr. Clarke reported no relevant financial relationships. Dr. Saag reported receiving grants and personal fees from Amgen, personal fees from Radius, and is a consultant for Amgen, Radius, Roche, and Daiichi Sankyo.
This article first appeared on Medscape.com.
Latest guidelines on the treatment of osteoporosis have been released that include new recommendations for the use of romosozumab (Evenity) in postmenopausal women with severe osteoporosis, but they contain caveats as to which women should – and should not – receive the drug.
The updated clinical practice guideline from the Endocrine Society is in response to the approval of romosozumab by the Food and Drug Administration (FDA) in April 2019, and more recently, by the European Medicines Agency.
It was published online February 18 in the Journal of Clinical Endocrinology & Metabolism.
In the new guidelines, committee members recommend the use of romosozumab for postmenopausal women with osteoporosis at very high risk of fracture. Candidates would include women with severe osteoporosis (T-score of less than –2.5 and a prior fracture) or women with a history of multiple vertebral fractures.
Women should be treated with romosozumab for up to 1 year, followed by an antiresorptive agent to maintain bone mineral density gains and further reduce fracture risk.
“The recommended dosage is 210 mg monthly by subcutaneous injection for 12 months,” the authors wrote.
However, and very importantly, romosozumab should not be considered for women at high risk of cardiovascular disease (CVD) or cerebrovascular disease. A high risk of CVD includes women who have had a previous myocardial infarction (MI) or stroke.
Experts questioned by Medscape Medical News stressed that romosozumab should not be a first-line, or even generally a second-line, option for osteoporosis, but it can be a considered for select patients with severe osteoporosis, taking into account CV risk.
Boxed warning
In the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH), there were more major adverse cardiovascular events (MACE) in the first year of the trial with romosozumab, and patients had a 31% higher risk of MACE with romosozumab, compared with the bisphosphonate alendronate.
As a result, the drug was initially rejected by a number of regulatory agencies.
In the United States and Canada, it was eventually approved with a boxed warning, which cautions against the use of the drug in patients at risk for myocardial infarction, stroke, and CVD-related death.
“Romosozumab offers promising results for postmenopausal women with severe osteoporosis or who have a history of fractures,” Clifford Rosen, MD, Maine Medical Center Research Institute in Scarborough and chair of the writing committee, said in an Endocrine Society statement. “It does, however, come with a risk of heart disease, so clinicians need to be careful when selecting patients for this therapy.”
Exact risk unknown
Asked by Medscape Medical News to comment, Kenneth Saag, MD, professor of medicine, University of Alabama at Birmingham and principle investigator of the ARCH study, said that physicians needed more data from real-world studies to resolve the issue around whether romosozumab heightens the risk of CV events in women with osteoporosis or whether that particular finding from ARCH was an artifact.
“Women who have had a recent cardiovascular event should not receive the drug,” he said, agreeing with the new guidelines.
But it remains unclear, for example, whether women who are at slightly higher risk of having a CV event by virtue of their age alone, are also at risk, he noted.
In the meantime, results from the ARCH study clearly showed that not only was romosozumab more effective than alendronate, “but it is more effective than other bone-building drugs as well,” Dr. Saag observed, and it leads to a significantly greater reduction in vertebral, nonvertebral, and hip fractures, compared with the alendronate, the current standard of care in osteoporosis.
“In patients who have very severe osteoporosis and who have had a recent fracture or who are at risk for imminent future fracture, physicians need to balance the benefit versus the risk in favor of using romosozumab,” Dr. Saag suggested.
“And while I would say most women prefer not to inject themselves, the women I have put on this medicine have all had recent fractures and they are very aware of the pain and the disability of having a broken bone, so it is something they are willing to do,” he added.
Not for all women
Giving his opinion, Bart Clarke, MD, of the Mayo Clinic in Rochester, Minnesota, underscored the fact that the Fracture Study in Postmenopausal Women With Osteoporosis (FRAME), again conducted in postmenopausal women, showed no increase in CV events in patients treated with romosozumab compared with placebo.
“So there are questions about what this means, because if these events really were a drug effect, then that effect would be even more evident compared with placebo and they did not see any signal of CV events [in FRAME],” said Dr. Clarke, past president of the American Society of Bone and Mineral Research.
Like everything else in medicine, “there is always some risk,” Dr. Clarke observed.
However, what physicians should do is talk to women, ensure they have not had either an MI or stroke in the last year, and if patients have severe osteoporosis and a high risk of fracture, “then we can say, here’s another option,” he suggested.
“Then, if a woman develops chest pain or shortness of breath while on the drug, [she needs] to let us know ,and then we’ll stop the drug and reassess the situation,” he added.
Dr. Clarke also pointed out that if the FDA had received further reports of CV events linked to romosozumab, physicians would know about it by now, but to his knowledge, there has been no change to the drug’s current warning label.
Furthermore, neither he nor any of his colleagues who treat metabolic bone disease at the Mayo Clinic has seen a single CV event in patients prescribed the agent.
“This is not a drug we would use as first-line for most patients, and we don’t even use it as second-line for most patients, but in people who have not responded to other drugs or who have had terrible things happen to them already, hip fracture especially, then we are saying, you can consider this, he concluded.
The guidelines were supported by the Endocrine Society. Dr. Rosen and Dr. Clarke reported no relevant financial relationships. Dr. Saag reported receiving grants and personal fees from Amgen, personal fees from Radius, and is a consultant for Amgen, Radius, Roche, and Daiichi Sankyo.
This article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Thyroid dysfunction is common in patients with diabetes
according to a new analysis of an Australian population.
The results add to the debate over whether patients with diabetes should undergo clinical or biochemical screening for thyroid dysfunction. The American Diabetes Association and U.K. National Institute for Health and Care Excellence guidelines do not recommend general thyroid function monitoring in type 2 diabetes, but the authors of the new study noted concerns that thyroid dysfunction could have metabolic consequences in type 2 disease.
Although the prevalence of undiagnosed thyroid disease in participants with type 2 diabetes was similar to that found in the general Australian population, the researchers, led by Kristen E. Peters, PhD, MedSc, and Timothy M.E. Davis, BMedSc MB, DPhil, of the University of Western Australia, Perth, noted in an article in Clinical Endocrinology that thyroid disease may worsen cardiometabolic risk factors, potentially giving it more significance in this population.
For their study, the researchers analyzed longitudinal data from 1,617 participants in the Fremantle Diabetes Study Phase II, of whom 8.0% had type 1 diabetes, 87.1% had type 2 diabetes, and 4.9% had latent autoimmune diabetes of adults (LADA).
All of the participants filled out questionnaires and underwent baseline fasting biochemical tests and were invited to return for biennial clinical and biochemical measures and to complete biennial questionnaires that were mailed to them. The participants were followed from 2008-2011 (baseline/recruitment period) to 2016. At baseline, 11.7% of the sample (189 of 1,617) had known thyroid disease, based on previous hospitalization of self-reported thyroid medication. Thyroid disease was more prevalent in women than in men (20.4% vs. 3.8%; P less than .001), and there was a nonsignificant trend for a higher prevalence of thyroid disease in participants with type 1 disease, compared with LADA and type 2 (16.9%, 11.4%, and 11.2%, respectively).
Among the 1,428 participants with no documented thyroid disease, 93 (6.5%) were found to have an abnormal thyroid-stimulating hormone (TSH) at baseline testing. Of those 93 participants, 79 had type 2 diabetes, 9 had type 1, and 5 had LADA; across all diabetes types, 5.1% of the participants had subclinical hypothyroidism, 1.1% had overt hypothyroidism, 0.1% had subclinical hyperthyroidism, and 0.2% had overt hyperthyroidism.
Overall, the baseline prevalence of any thyroid disease, known or previously undiagnosed, was 17.4% – 282 of 1,617 participants, of whom 23.8% had type 1 diabetes, 17.7% had LADA, and 16.8% had type 2.
At the end of year 4, serum concentrations were available for 844 participants who had no history of thyroid disease and normal baseline TSH. Over the course of the follow-up period (5,694 patient-years), 25 participants (3%) with normal baseline TSH levels had a first hospitalization for thyroid disease or started thyroid medication, including 2.8% of those with type 1 diabetes and 3.1% of those with type 2. Of the remaining 819 participants who did not have baseline thyroid disease, 3.4% developed subclinical hypothyroidism, 0.2% developed overt hypothyroidism, and 0.5% developed subclinical hyperthyroidism. In each case, there was no statistically significant difference in risk of developing thyroid dysfunction by diabetes type.
“The incidence of any new thyroid disease, including those [participants] with an abnormal follow-up TSH and those with known incident thyroid disease, was 59/844 (7.0%) during [4 years] of follow-up,” the authors noted. “The total incidence of new overt disease was 3.2% (27/844), with 7.4% (2/27) of these patients unaware of the condition. All of the patients with LADA remained euthyroid during follow-up.”
Among the limitations of the study, the authors noted, were that the identification of participants with thyroid disease depended on the available documentation in the databases the researchers used, they were not able to establish pretreatment of thyroid function in most participants with incident thyroid dysfunction, and they did not record free T3.
“Thyroid dysfunction, whether diagnosed or detected on biochemical screening, is common in diabetes regardless of type,” the authors wrote. “Subclinical hypothyroidism is the commonest form [of thyroid dysfunction], and it has a variable course. This latter observation alone makes an argument for periodic biochemical screening in all people with diabetes (not just type 1) as part of routine management.”
However, in an interview, Robert Eckel, MD, professor of medicine at University of Colorado at Denver, Aurora, said that the authors’ suggestion for routine thyroid function testing of patients with any type of diabetes may be premature. He noted that the study lacked a control group, making it difficult to justify a change in practice.
“My take-home message is that the current guidelines are probably not modified by [these findings], but the idea that 17% [of individuals with diabetes] have thyroid disease is worth noting. However, the absence of a control group limits changing recommendations based on this particular study,” said Dr. Eckel, who is also current president, medicine and science, of the ADA.
The study was supported by the National Health and Medical Research Council of Australia and the Spinnaker Health Research Foundation. The study authors did not disclose an financial conflicts.
SOURCE: Peters KE et al. Clin Endocrinol. 2020 Jan 27. doi: 10.1111/cen.14164.
according to a new analysis of an Australian population.
The results add to the debate over whether patients with diabetes should undergo clinical or biochemical screening for thyroid dysfunction. The American Diabetes Association and U.K. National Institute for Health and Care Excellence guidelines do not recommend general thyroid function monitoring in type 2 diabetes, but the authors of the new study noted concerns that thyroid dysfunction could have metabolic consequences in type 2 disease.
Although the prevalence of undiagnosed thyroid disease in participants with type 2 diabetes was similar to that found in the general Australian population, the researchers, led by Kristen E. Peters, PhD, MedSc, and Timothy M.E. Davis, BMedSc MB, DPhil, of the University of Western Australia, Perth, noted in an article in Clinical Endocrinology that thyroid disease may worsen cardiometabolic risk factors, potentially giving it more significance in this population.
For their study, the researchers analyzed longitudinal data from 1,617 participants in the Fremantle Diabetes Study Phase II, of whom 8.0% had type 1 diabetes, 87.1% had type 2 diabetes, and 4.9% had latent autoimmune diabetes of adults (LADA).
All of the participants filled out questionnaires and underwent baseline fasting biochemical tests and were invited to return for biennial clinical and biochemical measures and to complete biennial questionnaires that were mailed to them. The participants were followed from 2008-2011 (baseline/recruitment period) to 2016. At baseline, 11.7% of the sample (189 of 1,617) had known thyroid disease, based on previous hospitalization of self-reported thyroid medication. Thyroid disease was more prevalent in women than in men (20.4% vs. 3.8%; P less than .001), and there was a nonsignificant trend for a higher prevalence of thyroid disease in participants with type 1 disease, compared with LADA and type 2 (16.9%, 11.4%, and 11.2%, respectively).
Among the 1,428 participants with no documented thyroid disease, 93 (6.5%) were found to have an abnormal thyroid-stimulating hormone (TSH) at baseline testing. Of those 93 participants, 79 had type 2 diabetes, 9 had type 1, and 5 had LADA; across all diabetes types, 5.1% of the participants had subclinical hypothyroidism, 1.1% had overt hypothyroidism, 0.1% had subclinical hyperthyroidism, and 0.2% had overt hyperthyroidism.
Overall, the baseline prevalence of any thyroid disease, known or previously undiagnosed, was 17.4% – 282 of 1,617 participants, of whom 23.8% had type 1 diabetes, 17.7% had LADA, and 16.8% had type 2.
At the end of year 4, serum concentrations were available for 844 participants who had no history of thyroid disease and normal baseline TSH. Over the course of the follow-up period (5,694 patient-years), 25 participants (3%) with normal baseline TSH levels had a first hospitalization for thyroid disease or started thyroid medication, including 2.8% of those with type 1 diabetes and 3.1% of those with type 2. Of the remaining 819 participants who did not have baseline thyroid disease, 3.4% developed subclinical hypothyroidism, 0.2% developed overt hypothyroidism, and 0.5% developed subclinical hyperthyroidism. In each case, there was no statistically significant difference in risk of developing thyroid dysfunction by diabetes type.
“The incidence of any new thyroid disease, including those [participants] with an abnormal follow-up TSH and those with known incident thyroid disease, was 59/844 (7.0%) during [4 years] of follow-up,” the authors noted. “The total incidence of new overt disease was 3.2% (27/844), with 7.4% (2/27) of these patients unaware of the condition. All of the patients with LADA remained euthyroid during follow-up.”
Among the limitations of the study, the authors noted, were that the identification of participants with thyroid disease depended on the available documentation in the databases the researchers used, they were not able to establish pretreatment of thyroid function in most participants with incident thyroid dysfunction, and they did not record free T3.
“Thyroid dysfunction, whether diagnosed or detected on biochemical screening, is common in diabetes regardless of type,” the authors wrote. “Subclinical hypothyroidism is the commonest form [of thyroid dysfunction], and it has a variable course. This latter observation alone makes an argument for periodic biochemical screening in all people with diabetes (not just type 1) as part of routine management.”
However, in an interview, Robert Eckel, MD, professor of medicine at University of Colorado at Denver, Aurora, said that the authors’ suggestion for routine thyroid function testing of patients with any type of diabetes may be premature. He noted that the study lacked a control group, making it difficult to justify a change in practice.
“My take-home message is that the current guidelines are probably not modified by [these findings], but the idea that 17% [of individuals with diabetes] have thyroid disease is worth noting. However, the absence of a control group limits changing recommendations based on this particular study,” said Dr. Eckel, who is also current president, medicine and science, of the ADA.
The study was supported by the National Health and Medical Research Council of Australia and the Spinnaker Health Research Foundation. The study authors did not disclose an financial conflicts.
SOURCE: Peters KE et al. Clin Endocrinol. 2020 Jan 27. doi: 10.1111/cen.14164.
according to a new analysis of an Australian population.
The results add to the debate over whether patients with diabetes should undergo clinical or biochemical screening for thyroid dysfunction. The American Diabetes Association and U.K. National Institute for Health and Care Excellence guidelines do not recommend general thyroid function monitoring in type 2 diabetes, but the authors of the new study noted concerns that thyroid dysfunction could have metabolic consequences in type 2 disease.
Although the prevalence of undiagnosed thyroid disease in participants with type 2 diabetes was similar to that found in the general Australian population, the researchers, led by Kristen E. Peters, PhD, MedSc, and Timothy M.E. Davis, BMedSc MB, DPhil, of the University of Western Australia, Perth, noted in an article in Clinical Endocrinology that thyroid disease may worsen cardiometabolic risk factors, potentially giving it more significance in this population.
For their study, the researchers analyzed longitudinal data from 1,617 participants in the Fremantle Diabetes Study Phase II, of whom 8.0% had type 1 diabetes, 87.1% had type 2 diabetes, and 4.9% had latent autoimmune diabetes of adults (LADA).
All of the participants filled out questionnaires and underwent baseline fasting biochemical tests and were invited to return for biennial clinical and biochemical measures and to complete biennial questionnaires that were mailed to them. The participants were followed from 2008-2011 (baseline/recruitment period) to 2016. At baseline, 11.7% of the sample (189 of 1,617) had known thyroid disease, based on previous hospitalization of self-reported thyroid medication. Thyroid disease was more prevalent in women than in men (20.4% vs. 3.8%; P less than .001), and there was a nonsignificant trend for a higher prevalence of thyroid disease in participants with type 1 disease, compared with LADA and type 2 (16.9%, 11.4%, and 11.2%, respectively).
Among the 1,428 participants with no documented thyroid disease, 93 (6.5%) were found to have an abnormal thyroid-stimulating hormone (TSH) at baseline testing. Of those 93 participants, 79 had type 2 diabetes, 9 had type 1, and 5 had LADA; across all diabetes types, 5.1% of the participants had subclinical hypothyroidism, 1.1% had overt hypothyroidism, 0.1% had subclinical hyperthyroidism, and 0.2% had overt hyperthyroidism.
Overall, the baseline prevalence of any thyroid disease, known or previously undiagnosed, was 17.4% – 282 of 1,617 participants, of whom 23.8% had type 1 diabetes, 17.7% had LADA, and 16.8% had type 2.
At the end of year 4, serum concentrations were available for 844 participants who had no history of thyroid disease and normal baseline TSH. Over the course of the follow-up period (5,694 patient-years), 25 participants (3%) with normal baseline TSH levels had a first hospitalization for thyroid disease or started thyroid medication, including 2.8% of those with type 1 diabetes and 3.1% of those with type 2. Of the remaining 819 participants who did not have baseline thyroid disease, 3.4% developed subclinical hypothyroidism, 0.2% developed overt hypothyroidism, and 0.5% developed subclinical hyperthyroidism. In each case, there was no statistically significant difference in risk of developing thyroid dysfunction by diabetes type.
“The incidence of any new thyroid disease, including those [participants] with an abnormal follow-up TSH and those with known incident thyroid disease, was 59/844 (7.0%) during [4 years] of follow-up,” the authors noted. “The total incidence of new overt disease was 3.2% (27/844), with 7.4% (2/27) of these patients unaware of the condition. All of the patients with LADA remained euthyroid during follow-up.”
Among the limitations of the study, the authors noted, were that the identification of participants with thyroid disease depended on the available documentation in the databases the researchers used, they were not able to establish pretreatment of thyroid function in most participants with incident thyroid dysfunction, and they did not record free T3.
“Thyroid dysfunction, whether diagnosed or detected on biochemical screening, is common in diabetes regardless of type,” the authors wrote. “Subclinical hypothyroidism is the commonest form [of thyroid dysfunction], and it has a variable course. This latter observation alone makes an argument for periodic biochemical screening in all people with diabetes (not just type 1) as part of routine management.”
However, in an interview, Robert Eckel, MD, professor of medicine at University of Colorado at Denver, Aurora, said that the authors’ suggestion for routine thyroid function testing of patients with any type of diabetes may be premature. He noted that the study lacked a control group, making it difficult to justify a change in practice.
“My take-home message is that the current guidelines are probably not modified by [these findings], but the idea that 17% [of individuals with diabetes] have thyroid disease is worth noting. However, the absence of a control group limits changing recommendations based on this particular study,” said Dr. Eckel, who is also current president, medicine and science, of the ADA.
The study was supported by the National Health and Medical Research Council of Australia and the Spinnaker Health Research Foundation. The study authors did not disclose an financial conflicts.
SOURCE: Peters KE et al. Clin Endocrinol. 2020 Jan 27. doi: 10.1111/cen.14164.
FROM CLINICAL ENDOCRINOLOGY
FDA approves weekly contraceptive patch Twirla
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
Radioactive iodine can be first-line for hyperthyroidism, says UK
New UK guidelines for the treatment of hyperthyroidism, including Graves’ disease, place heavier emphasis on the use of radioactive iodine as the frontline treatment for patients unlikely to remain remission-free on the medications, as opposed to the alternative of antithyroid medications as a first choice.
senior author Kristien Boelaert, MD, PhD, who led the guideline committee, said in an interview.
“Recommending the use of radioactive iodine as first-line treatment for adults with Graves’ disease is a change to current practice and should reduce the variation between centers as to when radioactive iodine is considered appropriate,” the guidelines further state.
The new recommendations on hyperthyroidism are part of broader guidelines on thyroid disease by the UK National Institute for Health and Care Excellence (NICE), which concludes that radioactive iodine results in cure in as many as 90% of hyperthyroidism cases.
The recommendations were published in a guideline summary in BMJ by research fellow Melina Vasileiou of the National Guideline Centre, Royal College of Physicians, London, and colleagues.
Current guidelines in the United Kingdom and Europe typically call for radioactive iodine to be reserved for use as a definitive treatment only after relapse following antithyroid medication treatment. The latest European Thyroid Association guidelines were published in 2018.
Elsewhere guidelines vary, with many, including those by the American Thyroid Association (ATA) – the most recent published in 2016 – generally calling for treatment with either antithyroid medications, radioactive iodine, or total thyroidectomy, in the absence of any contraindications to each treatment option.
“The U.S. tends to use more radioactive iodine, while Europe, Latin America, and Japan lean more toward (perhaps longer) use of antithyroid medications,” Angela Leung, MD, associate clinical professor of medicine in the division of endocrinology, diabetes, and metabolism, department of medicine, University of California, Los Angeles, said in an interview.
“Preferences of deciding which treatment option, which may involve more than one option if antithyroid medications are used initially, depend on a variety of factors related to patient desire, comorbidities, and availability of the therapy,” she explained.
Concerns including worsening thyroid eye disease, cardiovascular disease, and development of secondary cancers have caused some hesitation in the use of frontline radioiodine therapy.
And one notably controversial article, published last year, suggested a link between radioactive iodine therapy and an increased risk of cancer mortality. However, as reported by Medscape Medical News, the article spurred debate, with the Society for Endocrinology and British Thyroid Association issuing a joint statement urging caution in interpretation of the findings.
Evidence supporting first-line radioactive iodine
Patients treated with radioactive iodine take a single tablet that contains iodine and a low dose of radiation, which is absorbed by the thyroid. After taking the treatment patients are advised to avoid prolonged close contact with children and pregnant women for a few days or weeks and to avoid getting pregnant or fathering a child for several months. The treatment is likely to lead to an underactive thyroid gland that will require ongoing treatment with thyroid hormone replacement.
In providing evidence in favor of the benefits of radioactive iodine over the risks, the new NICE guidelines cite five randomized controlled trials of people with hyperthyroid disease, which, though defined as “low quality” evidence, collectively indicate that long-term outcomes were improved with radioactive iodine treatment compared with antithyroid drugs – despite the former having a higher risk of thyroid eye disease (also known as Graves’ ophthalmopathy).
In addition, eight nonrandomized studies show no evidence of a clinically important increase in cancer diagnoses or deaths between people treated with radioactive iodine or surgery, or between people treated with radioactive iodine and healthy controls, the guideline committee notes.
“The strongest arguments (in favor of radioactive iodine as a first-line therapy) were the likelihood of inducing remission of Graves’ disease with radioactive iodine, the finding that radioiodine is a safe treatment (confirmed in the safety review undertaken by NICE), and the reduction in the need for patients to remain on antithyroid drugs, which may have significant side effects and treatment which usually requires repeated hospital visits or follow-up under a hospital service,” said Dr. Boelaert.
The new guideline does recommend that antithyroid medication is acceptable as the first-line treatment among patients considered likely to achieve remission.
Dr. Leung explains that the percentage of patients with Graves’ disease who can achieve remission with antithyroid drugs ranges from 30% to 50%. She noted some evidence does suggest the long-term use of the drugs may be acceptable.
“There are some data that ... report the relative safety of long-term use of antithyroid drugs (beyond 24 months) for both Graves’ disease and autonomous thyroid nodules,” Dr. Leung elaborated.
Pregnancy concerns and cost-effectiveness of radioactive iodine
Radioactive iodine therapy is meanwhile not suitable if malignancy is suspected, if the patient is pregnant or trying to become pregnant, or if the patient has active thyroid eye disease, the experts agree.
Dr. Leung noted that although “it is generally advised to not treat Graves’ disease with radioiodine if there is concurrent thyroid eye disease, steroids are a proven effective therapy to decrease this risk in select patients.”
And among pregnant patients, “antithyroid medications should be minimally used in the lowest possible doses,” Dr. Leung said, although she added that, despite their potential risks, the drugs “represent a viable option” for this patient population.
“Also, many would actually advocate for total thyroidectomy in women who are thinking of pregnancy in the near future,” she noted.
Another factor of relevance in the guideline recommendations – cost – also favors radioactive iodine, the committee noted.
“Economic evidence showed that radioactive iodine was the most cost-effective intervention,” the committee pointed out.
Trabs advised for determination of hyperthyroidism cause
The new U.K. guidelines further underscore the importance of establishing the underlying cause of hyperthyroidism to ensure appropriate treatment, and the preferred method for doing so is the measurement of thyroid-stimulating hormone receptor antibodies (TRAbs).
“It is important to identify the underlying cause of thyrotoxicosis through measurement of TRAbs, or radioisotope scanning, in order to distinguish hyperthyroidism from transient causes of thyrotoxicosis such as transient thyroiditis, which only requires supportive treatment,” explained Dr. Boelaert, consultant endocrinologist and director of the National Institute for Health Research Integrated Academic Training Program at the Institute of Applied Health Research, University of Birmingham (England).
“In addition, this will help distinguish Graves’ disease from toxic nodular hyperthyroidism, which is important as antithyroid drugs are not effective in inducing a cure in the latter,” she explained.
Meanwhile, the new guidelines further note that although use of diagnostic ultrasound is informative when palpation suggests thyroid nodules, it is of limited diagnostic value for Graves’ disease.
“The recommendation (suggests that) thyroid ultrasonography should only be offered if there is a palpable thyroid nodule,” Dr. Boelaert noted.
She concluded: “There has been uncertainty in the U.K. about the best treatment for hyperthyroidism despite radioactive iodine being the most common first-line treatment for this condition in the United States. We are very pleased to have been able to work with NICE to provide clear new guidance which we hope will improve outcomes for patients with this condition.”
The National Guideline Centre was commissioned and funded by NICE to develop the guideline. No authors received specific funding to write the summary. Dr. Boelaert has reported no relevant financial relationships. Disclosures for the other authors are listed in the article.
This article first appeared on Medscape.com.
New UK guidelines for the treatment of hyperthyroidism, including Graves’ disease, place heavier emphasis on the use of radioactive iodine as the frontline treatment for patients unlikely to remain remission-free on the medications, as opposed to the alternative of antithyroid medications as a first choice.
senior author Kristien Boelaert, MD, PhD, who led the guideline committee, said in an interview.
“Recommending the use of radioactive iodine as first-line treatment for adults with Graves’ disease is a change to current practice and should reduce the variation between centers as to when radioactive iodine is considered appropriate,” the guidelines further state.
The new recommendations on hyperthyroidism are part of broader guidelines on thyroid disease by the UK National Institute for Health and Care Excellence (NICE), which concludes that radioactive iodine results in cure in as many as 90% of hyperthyroidism cases.
The recommendations were published in a guideline summary in BMJ by research fellow Melina Vasileiou of the National Guideline Centre, Royal College of Physicians, London, and colleagues.
Current guidelines in the United Kingdom and Europe typically call for radioactive iodine to be reserved for use as a definitive treatment only after relapse following antithyroid medication treatment. The latest European Thyroid Association guidelines were published in 2018.
Elsewhere guidelines vary, with many, including those by the American Thyroid Association (ATA) – the most recent published in 2016 – generally calling for treatment with either antithyroid medications, radioactive iodine, or total thyroidectomy, in the absence of any contraindications to each treatment option.
“The U.S. tends to use more radioactive iodine, while Europe, Latin America, and Japan lean more toward (perhaps longer) use of antithyroid medications,” Angela Leung, MD, associate clinical professor of medicine in the division of endocrinology, diabetes, and metabolism, department of medicine, University of California, Los Angeles, said in an interview.
“Preferences of deciding which treatment option, which may involve more than one option if antithyroid medications are used initially, depend on a variety of factors related to patient desire, comorbidities, and availability of the therapy,” she explained.
Concerns including worsening thyroid eye disease, cardiovascular disease, and development of secondary cancers have caused some hesitation in the use of frontline radioiodine therapy.
And one notably controversial article, published last year, suggested a link between radioactive iodine therapy and an increased risk of cancer mortality. However, as reported by Medscape Medical News, the article spurred debate, with the Society for Endocrinology and British Thyroid Association issuing a joint statement urging caution in interpretation of the findings.
Evidence supporting first-line radioactive iodine
Patients treated with radioactive iodine take a single tablet that contains iodine and a low dose of radiation, which is absorbed by the thyroid. After taking the treatment patients are advised to avoid prolonged close contact with children and pregnant women for a few days or weeks and to avoid getting pregnant or fathering a child for several months. The treatment is likely to lead to an underactive thyroid gland that will require ongoing treatment with thyroid hormone replacement.
In providing evidence in favor of the benefits of radioactive iodine over the risks, the new NICE guidelines cite five randomized controlled trials of people with hyperthyroid disease, which, though defined as “low quality” evidence, collectively indicate that long-term outcomes were improved with radioactive iodine treatment compared with antithyroid drugs – despite the former having a higher risk of thyroid eye disease (also known as Graves’ ophthalmopathy).
In addition, eight nonrandomized studies show no evidence of a clinically important increase in cancer diagnoses or deaths between people treated with radioactive iodine or surgery, or between people treated with radioactive iodine and healthy controls, the guideline committee notes.
“The strongest arguments (in favor of radioactive iodine as a first-line therapy) were the likelihood of inducing remission of Graves’ disease with radioactive iodine, the finding that radioiodine is a safe treatment (confirmed in the safety review undertaken by NICE), and the reduction in the need for patients to remain on antithyroid drugs, which may have significant side effects and treatment which usually requires repeated hospital visits or follow-up under a hospital service,” said Dr. Boelaert.
The new guideline does recommend that antithyroid medication is acceptable as the first-line treatment among patients considered likely to achieve remission.
Dr. Leung explains that the percentage of patients with Graves’ disease who can achieve remission with antithyroid drugs ranges from 30% to 50%. She noted some evidence does suggest the long-term use of the drugs may be acceptable.
“There are some data that ... report the relative safety of long-term use of antithyroid drugs (beyond 24 months) for both Graves’ disease and autonomous thyroid nodules,” Dr. Leung elaborated.
Pregnancy concerns and cost-effectiveness of radioactive iodine
Radioactive iodine therapy is meanwhile not suitable if malignancy is suspected, if the patient is pregnant or trying to become pregnant, or if the patient has active thyroid eye disease, the experts agree.
Dr. Leung noted that although “it is generally advised to not treat Graves’ disease with radioiodine if there is concurrent thyroid eye disease, steroids are a proven effective therapy to decrease this risk in select patients.”
And among pregnant patients, “antithyroid medications should be minimally used in the lowest possible doses,” Dr. Leung said, although she added that, despite their potential risks, the drugs “represent a viable option” for this patient population.
“Also, many would actually advocate for total thyroidectomy in women who are thinking of pregnancy in the near future,” she noted.
Another factor of relevance in the guideline recommendations – cost – also favors radioactive iodine, the committee noted.
“Economic evidence showed that radioactive iodine was the most cost-effective intervention,” the committee pointed out.
Trabs advised for determination of hyperthyroidism cause
The new U.K. guidelines further underscore the importance of establishing the underlying cause of hyperthyroidism to ensure appropriate treatment, and the preferred method for doing so is the measurement of thyroid-stimulating hormone receptor antibodies (TRAbs).
“It is important to identify the underlying cause of thyrotoxicosis through measurement of TRAbs, or radioisotope scanning, in order to distinguish hyperthyroidism from transient causes of thyrotoxicosis such as transient thyroiditis, which only requires supportive treatment,” explained Dr. Boelaert, consultant endocrinologist and director of the National Institute for Health Research Integrated Academic Training Program at the Institute of Applied Health Research, University of Birmingham (England).
“In addition, this will help distinguish Graves’ disease from toxic nodular hyperthyroidism, which is important as antithyroid drugs are not effective in inducing a cure in the latter,” she explained.
Meanwhile, the new guidelines further note that although use of diagnostic ultrasound is informative when palpation suggests thyroid nodules, it is of limited diagnostic value for Graves’ disease.
“The recommendation (suggests that) thyroid ultrasonography should only be offered if there is a palpable thyroid nodule,” Dr. Boelaert noted.
She concluded: “There has been uncertainty in the U.K. about the best treatment for hyperthyroidism despite radioactive iodine being the most common first-line treatment for this condition in the United States. We are very pleased to have been able to work with NICE to provide clear new guidance which we hope will improve outcomes for patients with this condition.”
The National Guideline Centre was commissioned and funded by NICE to develop the guideline. No authors received specific funding to write the summary. Dr. Boelaert has reported no relevant financial relationships. Disclosures for the other authors are listed in the article.
This article first appeared on Medscape.com.
New UK guidelines for the treatment of hyperthyroidism, including Graves’ disease, place heavier emphasis on the use of radioactive iodine as the frontline treatment for patients unlikely to remain remission-free on the medications, as opposed to the alternative of antithyroid medications as a first choice.
senior author Kristien Boelaert, MD, PhD, who led the guideline committee, said in an interview.
“Recommending the use of radioactive iodine as first-line treatment for adults with Graves’ disease is a change to current practice and should reduce the variation between centers as to when radioactive iodine is considered appropriate,” the guidelines further state.
The new recommendations on hyperthyroidism are part of broader guidelines on thyroid disease by the UK National Institute for Health and Care Excellence (NICE), which concludes that radioactive iodine results in cure in as many as 90% of hyperthyroidism cases.
The recommendations were published in a guideline summary in BMJ by research fellow Melina Vasileiou of the National Guideline Centre, Royal College of Physicians, London, and colleagues.
Current guidelines in the United Kingdom and Europe typically call for radioactive iodine to be reserved for use as a definitive treatment only after relapse following antithyroid medication treatment. The latest European Thyroid Association guidelines were published in 2018.
Elsewhere guidelines vary, with many, including those by the American Thyroid Association (ATA) – the most recent published in 2016 – generally calling for treatment with either antithyroid medications, radioactive iodine, or total thyroidectomy, in the absence of any contraindications to each treatment option.
“The U.S. tends to use more radioactive iodine, while Europe, Latin America, and Japan lean more toward (perhaps longer) use of antithyroid medications,” Angela Leung, MD, associate clinical professor of medicine in the division of endocrinology, diabetes, and metabolism, department of medicine, University of California, Los Angeles, said in an interview.
“Preferences of deciding which treatment option, which may involve more than one option if antithyroid medications are used initially, depend on a variety of factors related to patient desire, comorbidities, and availability of the therapy,” she explained.
Concerns including worsening thyroid eye disease, cardiovascular disease, and development of secondary cancers have caused some hesitation in the use of frontline radioiodine therapy.
And one notably controversial article, published last year, suggested a link between radioactive iodine therapy and an increased risk of cancer mortality. However, as reported by Medscape Medical News, the article spurred debate, with the Society for Endocrinology and British Thyroid Association issuing a joint statement urging caution in interpretation of the findings.
Evidence supporting first-line radioactive iodine
Patients treated with radioactive iodine take a single tablet that contains iodine and a low dose of radiation, which is absorbed by the thyroid. After taking the treatment patients are advised to avoid prolonged close contact with children and pregnant women for a few days or weeks and to avoid getting pregnant or fathering a child for several months. The treatment is likely to lead to an underactive thyroid gland that will require ongoing treatment with thyroid hormone replacement.
In providing evidence in favor of the benefits of radioactive iodine over the risks, the new NICE guidelines cite five randomized controlled trials of people with hyperthyroid disease, which, though defined as “low quality” evidence, collectively indicate that long-term outcomes were improved with radioactive iodine treatment compared with antithyroid drugs – despite the former having a higher risk of thyroid eye disease (also known as Graves’ ophthalmopathy).
In addition, eight nonrandomized studies show no evidence of a clinically important increase in cancer diagnoses or deaths between people treated with radioactive iodine or surgery, or between people treated with radioactive iodine and healthy controls, the guideline committee notes.
“The strongest arguments (in favor of radioactive iodine as a first-line therapy) were the likelihood of inducing remission of Graves’ disease with radioactive iodine, the finding that radioiodine is a safe treatment (confirmed in the safety review undertaken by NICE), and the reduction in the need for patients to remain on antithyroid drugs, which may have significant side effects and treatment which usually requires repeated hospital visits or follow-up under a hospital service,” said Dr. Boelaert.
The new guideline does recommend that antithyroid medication is acceptable as the first-line treatment among patients considered likely to achieve remission.
Dr. Leung explains that the percentage of patients with Graves’ disease who can achieve remission with antithyroid drugs ranges from 30% to 50%. She noted some evidence does suggest the long-term use of the drugs may be acceptable.
“There are some data that ... report the relative safety of long-term use of antithyroid drugs (beyond 24 months) for both Graves’ disease and autonomous thyroid nodules,” Dr. Leung elaborated.
Pregnancy concerns and cost-effectiveness of radioactive iodine
Radioactive iodine therapy is meanwhile not suitable if malignancy is suspected, if the patient is pregnant or trying to become pregnant, or if the patient has active thyroid eye disease, the experts agree.
Dr. Leung noted that although “it is generally advised to not treat Graves’ disease with radioiodine if there is concurrent thyroid eye disease, steroids are a proven effective therapy to decrease this risk in select patients.”
And among pregnant patients, “antithyroid medications should be minimally used in the lowest possible doses,” Dr. Leung said, although she added that, despite their potential risks, the drugs “represent a viable option” for this patient population.
“Also, many would actually advocate for total thyroidectomy in women who are thinking of pregnancy in the near future,” she noted.
Another factor of relevance in the guideline recommendations – cost – also favors radioactive iodine, the committee noted.
“Economic evidence showed that radioactive iodine was the most cost-effective intervention,” the committee pointed out.
Trabs advised for determination of hyperthyroidism cause
The new U.K. guidelines further underscore the importance of establishing the underlying cause of hyperthyroidism to ensure appropriate treatment, and the preferred method for doing so is the measurement of thyroid-stimulating hormone receptor antibodies (TRAbs).
“It is important to identify the underlying cause of thyrotoxicosis through measurement of TRAbs, or radioisotope scanning, in order to distinguish hyperthyroidism from transient causes of thyrotoxicosis such as transient thyroiditis, which only requires supportive treatment,” explained Dr. Boelaert, consultant endocrinologist and director of the National Institute for Health Research Integrated Academic Training Program at the Institute of Applied Health Research, University of Birmingham (England).
“In addition, this will help distinguish Graves’ disease from toxic nodular hyperthyroidism, which is important as antithyroid drugs are not effective in inducing a cure in the latter,” she explained.
Meanwhile, the new guidelines further note that although use of diagnostic ultrasound is informative when palpation suggests thyroid nodules, it is of limited diagnostic value for Graves’ disease.
“The recommendation (suggests that) thyroid ultrasonography should only be offered if there is a palpable thyroid nodule,” Dr. Boelaert noted.
She concluded: “There has been uncertainty in the U.K. about the best treatment for hyperthyroidism despite radioactive iodine being the most common first-line treatment for this condition in the United States. We are very pleased to have been able to work with NICE to provide clear new guidance which we hope will improve outcomes for patients with this condition.”
The National Guideline Centre was commissioned and funded by NICE to develop the guideline. No authors received specific funding to write the summary. Dr. Boelaert has reported no relevant financial relationships. Disclosures for the other authors are listed in the article.
This article first appeared on Medscape.com.
Improving Nephropathy Screening in Appalachian Patients With Diabetes Using Practice-Wide Outreach
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively. 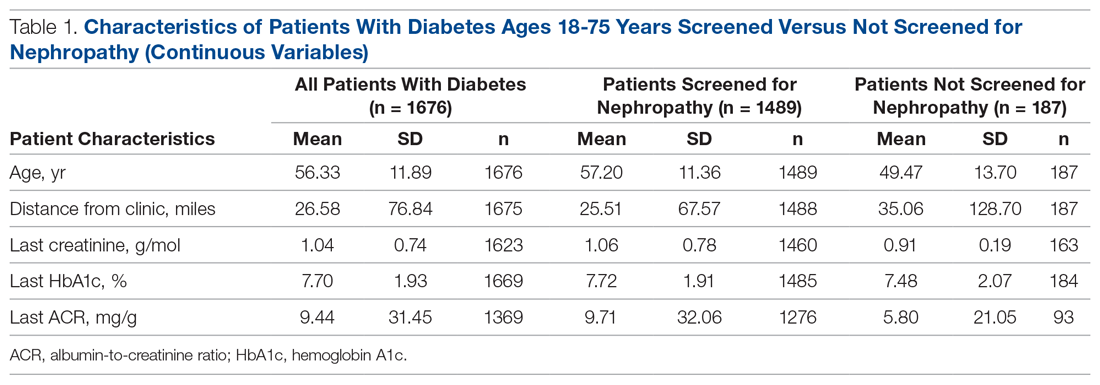
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.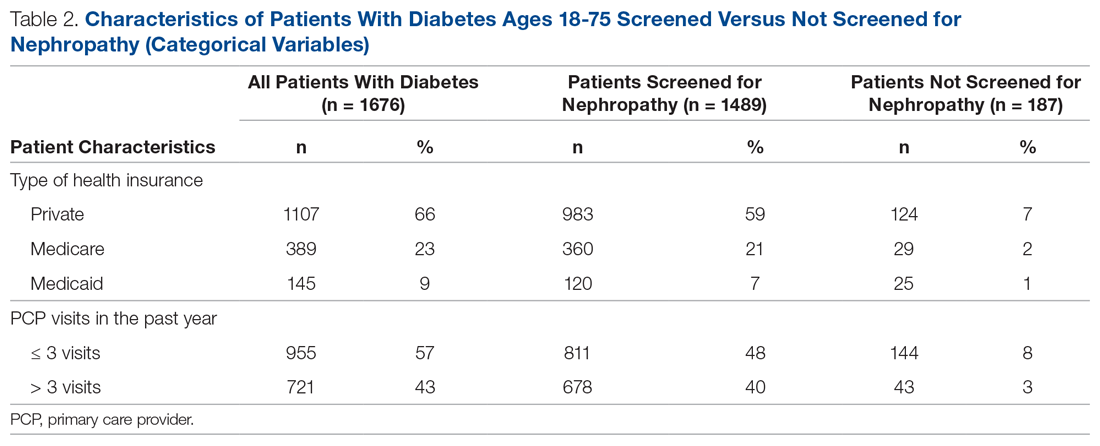
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4). 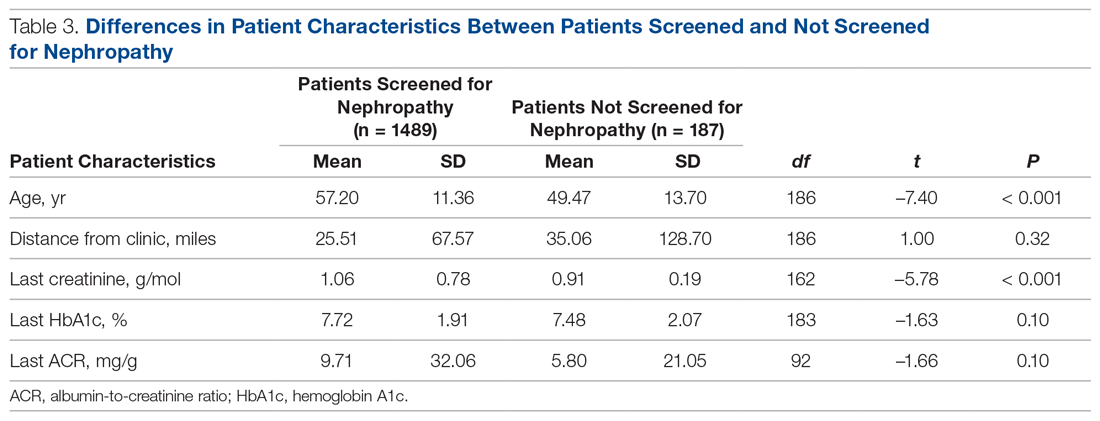
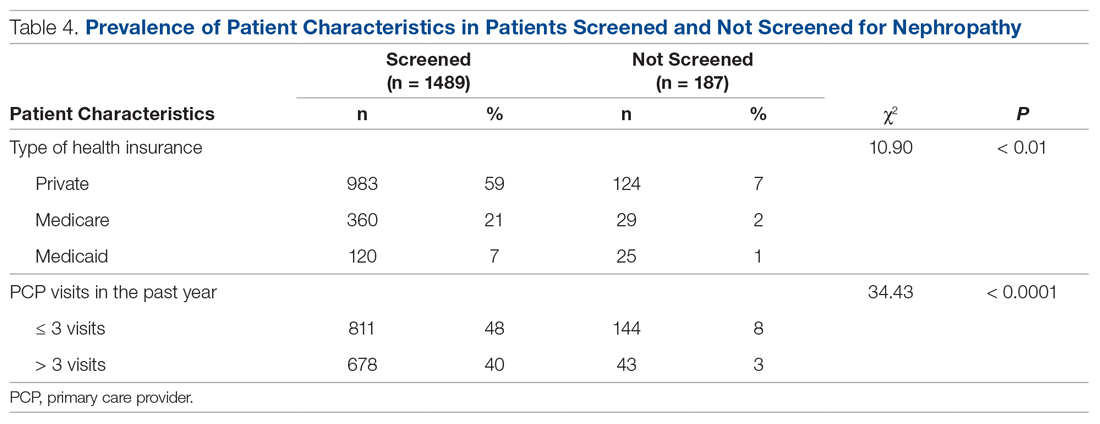
Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.
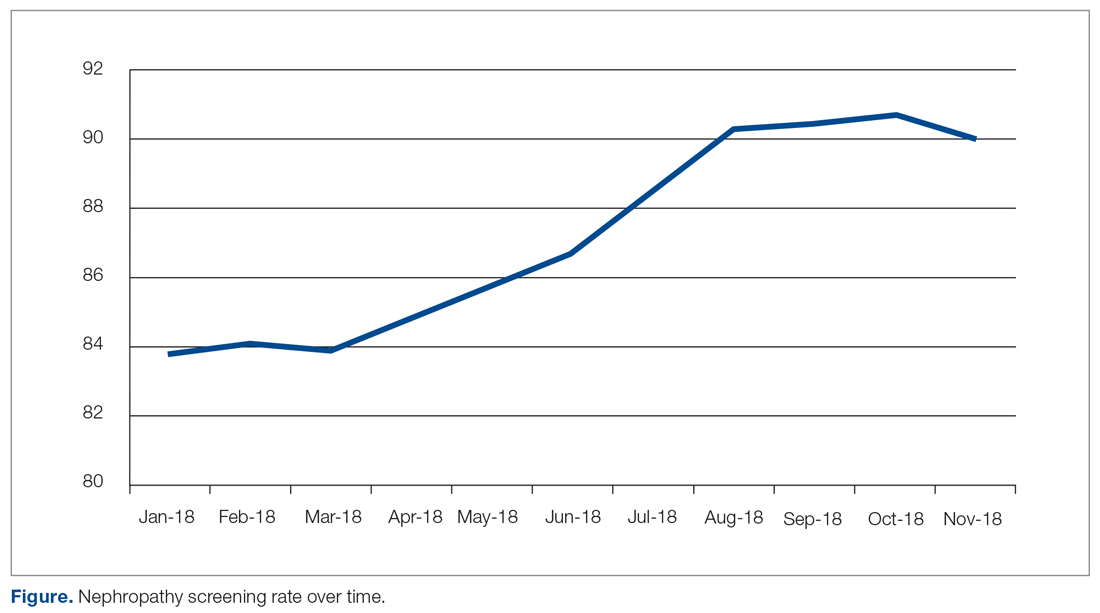
Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.
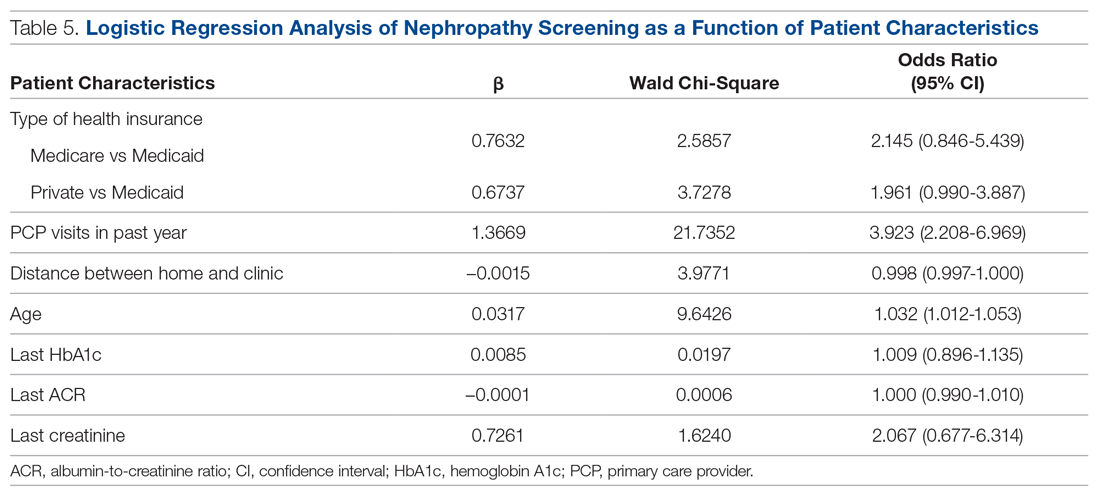
Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; [email protected].
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively. 
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4). 

Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.

Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.

Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; [email protected].
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
From West Virginia University, Morgantown, WV.
Abstract
Objective: To describe the strategies a family medicine clinic in Appalachia utilized to increase nephropathy screening rates as well as to explore the factors predictive of nephropathy screening in patients with diabetes.
Design: This quality improvement project targeted the points in the care process when patients are lost to follow-up for nephropathy screening.
Setting and participants: Patients with diabetes cared for by a primary care provider (PCP) at an academic family medicine practice in Appalachia from January 2018 to November 2018.
Interventions: Bulk orders for albumin-to-creatinine (ACR) testing and urine collection during clinic visit, enhanced patient communication through bulk communication reminders and individual patient outreach, and education of clinic providers.
Measurements: Demographic data and monthly nephropathy screening rates.
Results: The nephropathy screening rate increased by 6.2% during the project. Older patients living closer to the clinic who visited their PCP 3 or more times per year were the most likely to be screened.
Conclusion: Combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy treatment and screening in rural patients with diabetes at a family medicine clinic.
Keywords: rural; kidney disease; albumin-to-creatinine ratio; electronic health record.
According to the Centers for Disease Control and Prevention (CDC), an estimated 30.3 million people in the United States—about 9.4% of the population—have been diagnosed with diabetes.1 Diabetes is the seventh leading cause of death in the United States, and it contributes to other leading causes of death: heart disease and stroke.1 Diabetes also is related to high morbidity risk and is a leading cause of chronic kidney disease.1 The total cost of diagnosed diabetes was estimated at $327 billion in direct medical costs and reduced productivity.2
Residents of Appalachia bear a disproportionate burden of diabetes and other related negative health outcomes; these outcomes are influenced by a number of factors, including socioeconomic status, poverty, rurality, and health care access. Rates of chronic disease, such as diabetes, are most pronounced in Appalachia’s most economically distressed counties.3-5 In 2011, the CDC labeled a 644-county area the “diabetes belt,” which included most of Appalachia.6 As a result of this elevated prevalence of diabetes in Appalachia as compared to the rest of the country, complications directly associated with diabetes are more commonly observed in Appalachian residents. One of the most damaging complications is diabetic nephropathy.
Diabetic nephropathy results from damage to the microvasculature of the kidney due to inadequately controlled blood glucose. This, in turn, leads to decreased renal function, eventually leading to clinically significant renal disease. The long-term complications associated with nephropathy can include many comorbid conditions, the most serious of which are progression to end-stage renal disease, dialysis requirement, and early mortality. Diabetic nephropathy affects approximately 40% of patients with type 1 and type 2 diabetes.7,8
One way to prevent complications of diabetic nephropathy, in addition to good glycemic control in patients with diabetes, is early and regular screening. Currently, the American Diabetes Association (ADA) recommends yearly screening for diabetic nephropathy in the form of a urine albumin-to-creatinine ratio (ACR) for patients 18 to 75 years of age.2 This screening to detect diabetic nephropathy is recognized as a marker of quality care by many public and private insurance agencies and medical specialty associations, such as the Centers for Medicare and Medicaid Services.
Many patients with diabetes are cared for by primary care providers (PCP), and these PCP appointments provide an opportune time to screen and appropriately treat nephropathy. Screening opportunities are often missed, however, due to time constraints and competing health priorities. There are also a number of other factors specific to the Appalachian region that reduce the likelihood of screening for diabetic nephropathy, such as a lack of health insurance, the need to travel long distances to see a PCP, work and household responsibilities, low levels of education and health literacy, and a mistrust of outsiders regarding personal matters, including health.9-11 While nephropathy can have a detrimental impact on patients across populations, it is of particular concern for a state located in the heart of Appalachia, such as West Virginia.
Given the disproportionate burden of diabetes in this region and the potentially severe consequences of undetected nephropathy, clinicians from an academic family medicine clinic in West Virginia undertook a quality improvement project to increase the rate of nephropathy screening and treatment among patients with diabetes. This article describes the intervention strategies the team utilized to increase nephropathy screening and treatment in patients 18 to 75 years of age who met quality measures for nephropathy screening or treatment in the previous 12 months and explores the factors most predictive of nephropathy screening in Appalachian patients in this age group. It also reports the challenges and opportunities encountered and offers suggestions for other providers and clinics attempting to increase their nephropathy screening rates.
Methods
Setting and Study Population
The study population included patients ages 18 to 75 years under the care of providers in an academic family medicine practice in West Virginia who had been diagnosed with diabetes mellitus. The study focused on those patients overdue for diabetic nephropathy screening (ie, had not been screened in previous 12 months). The project began in January 2018 with a screening rate of 83.8%. The goal of this project was to increase this compliance metric by at least 5%. The project protocol was submitted to the West Virginia University Institutional Review Board, and, because it is a quality improvement project, permission was given to proceed without a board review.
Interventions
The team identified and implemented several interventions intended to reduce screening barriers and increase the screening rate.
Bulk orders for ACR and urine collection during clinic visits. Prior to initiation of this project, it was left to individual clinic providers to order nephropathy screening for patients with diabetes during a clinic visit; after receiving the order for “random urine microalbumin/creatinine ratio,” patients then had to travel to a lab to provide a urine sample. For this project and moving forward, the team changed to the procedure of initiating bulk ACR orders and collecting urine samples during clinic visits from all patients ages 18 to 75 years who have diabetes.
Bulk communication reminders. Since many patients with diabetes may not have realized they were overdue for nephropathy screening, the team began sending out bulk communication reminders through either the institution’s electronic health record (EHR; MyChart) or postal service–delivered physical letters (according to patient communication preferences) to remind patients that they were due for screening and to encourage them to schedule an appointment or keep a previously scheduled appointment with their PCP.
Individual patient outreach. A team of pharmacy students led by a licensed pharmacist in the family medicine clinic contacted patients overdue for screening even after bulk communication reminders went out. The students telephoned patients 2 to 3 months following the bulk communication. The students obtained an updated list of patients with diabetes ages 18 to 75 years from an EHR quality report. They began by prescreening the patients on the overdue list for potential candidacy for an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB). Screening for candidacy included evaluation of recent blood pressure readings, electrolytes (ie, basic metabolic panel), and ACR. If the students determined a patient was a candidate, they presented the patient to the preceptor for verification and then reached out to the provider with a recommendation. If the provider agreed, the student contacted the patient by telephone for medication counseling and education. The remaining patients determined not to be candidates for ACE inhibitors or ARBs were contacted by the pharmacy students by telephone to remind them that laboratory work was pending. Up to 3 phone call attempts were made before patients were determined to be unreachable. Students left voice mails with generic reminders if a patient could not be reached. If a patient answered, the student provided a reminder but also reviewed indications for lab work, the reason why the provider wished for follow-up, and updated lab hours. Students also followed up with the results of the work-up, as appropriate. During this outreach process, the student team encountered a number of patients who had moved or changed to a PCP outside of the family medicine clinic. In these cases, the EHR was updated and those patients were removed from the list of patients altogether.
Education of clinic providers. Clinic providers were educated during faculty and resident meetings and didactic learning sessions on identifying patients within the EHR who are due for nephropathy screening. They also received instruction on how to update the EHR to reflect completed screenings.
Data Analysis
All analyses in this study were conducted using SAS (version 9.4, 2013, SAS Institute Inc., Cary, NC). Descriptive analyses were conducted to summarize basic patient demographic information. To compare patients screened within the previous 12 months to those patients overdue for screening, 2-sample t-tests were used to examine differences in patients’ age, HbA1c, ACR, and creatinine level and the distance (in miles) between the patient’s home and the clinic. Chi-square analyses were used to examine the relationship between whether a patient was recently screened for nephropathy and the patient’s insurance, number of patient visits in the previous 12 months, and provider level. Logistic regression analyses were conducted to control for covariates and to explore which factors were most predictive of nephropathy screening. All tests were 2-tailed, and P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
There were 1676 family medicine clinic patients with diabetes between 18 and 75 years of age (Table 1 and Table 2). Of the total sample, 1489 (88.8%) had completed screening for nephropathy in the 12 months prior to evaluation, and 67.5%, 23.7%, and 8.8% of patients had private insurance, Medicare, and Medicaid, respectively. 
The mean (SD) age of the patients was 56.3 (11.9) years. The mean distance between the patient’s home and the clinic was 26.6 (76.8) miles. The mean number of visits was 3.6 (2.9) per year, and 43.0% of the patientvisited the clinic more than 3 times in a year. The mean values for HbA1c (%), creatinine (g/mol), and ACR (mg/g) were 7.7 (1.9), 1.0 (0.7), and 9.4 (31.4), respectively.
Screening of Patients for Nephropathy
Patients with Medicare and private insurance were more likely to have completed the nephropathy screening than those with Medicaid (92.5% versus 88.8% versus 82.8%, P = 0.004; Table 3 and Table 4). 

Changes in Screening Rate
The practice-wide screening rate was 83.8% at the start of this project in January 2018. The screening rate steadily increased throughout 2018, reaching 90.3% in August 2018, and then leveled off around 90% when the project was concluded at the end of November 2018 (Figure). As an added benefit of the increased screening rates, a number of patients were initiated on an ACE inhibitor or ARB based on the team’s screening efforts.

Predictors of Nephropathy Screening
A logistic regression analysis was conducted with nephropathy screening (screened or not screened) as the outcome and 7 patient characteristics as predictors: type of insurance (private, Medicare, or Medicaid), PCP visits in the past 12 months (≤ 3 or > 3), distance in miles of the patient’s residence from the clinic, age, last HbA1c value, last ACR value, and last creatinine value. A test of the full model with all 7 predictors was statistically significant (χ2 (8) = 57.77, P < 0.001). Table 5 shows regression coefficients, Wald statistics, and 95% confidence intervals for odds ratios for each of the 7 predictors. According to the Wald criterion, 3 patient characteristics were significant predictors of nephropathy screening: age, distance between the patient’s home and clinic, and number of PCP visits in the past 12 months. After adjusting for the covariates, there were still significant associations between the nephropathy screening status and age ( χ2(1) = 9.64, P < 0.01); distance between the patient’s home and the clinic (χ2(1) = 3.98, P < 0.05); and the number of PCP visits in the previous year (χ2(1) = 21.74, P < 0.001). With each 1-year increment in age, the odds of completing the nephropathy screening increased by 3.2%. With each 1-mile increase in the distance between the patient’s home and clinic, the odds of completing the nephropathy screening decreased by 0.2%. Patients who visited the clinic more than 3 times in a year were 3.9 times (95% confidence interval, 2.2-7.0) more likely to complete the nephropathy screening than their counterparts who visited fewer than 3 times per year.
In summary, older patients living within about 164 miles of the clinic (ie, within 1 standard deviation from the average miles between patient’s homes and the clinic) who visited their PCP 3 or more times per year were the most likely to be screened.

Discussion
Diabetic nephropathy is a critical issue facing family medicine providers and patients. The morbidity and mortality costs are significant, as diabetic nephropathy is the leading cause of end-stage renal disease. While the ADA recommends annual ACR screening in patients with diabetes and prescription of ACE inhibitors or ARBs in patients who qualify, many patients do not receive these interventions, despite following up with a provider.12-15 There is no current literature that indicates the compliance rates in the rural setting. Due to health disparities in the rural setting noted in the literature, it could be hypothesized that these individuals are at high risk of not meeting these screening and treatment recommendations.16,17 Limited access to care and resources, gaps in insurance coverage, and lower health literacy are a few barriers identified in the rural population that may influence whether these measures are met.17
Considering the disease burden of diabetes and its related complications, including nephropathy, consistent screening is necessary to reduce diabetes-related burdens and cost, while also increasing the quality of life for patients with diabetes. All parties must be involved to ensure appropriate compliance and treatment. Our institution’s implementation of quality improvement strategies has key implications for nephropathy screening and treatment efforts in rural settings.
An additional step of having a health care provider (other than the PCP) screen all patients who are not meeting the standard allows for identification of gaps in care. In our quality improvement workflow, the clinical pharmacist screened all patients for candidacy for ACE inhibitor/ARB therapy. While only a small percentage of patients qualified, many of these patients had previously been on therapy and were discontinued for an unknown reason or were stopped due to an acute condition (eg, acute kidney injury) and never restarted after recovery. Other patients required additional education that therapy would be utilized for nephroprotection versus blood pressure management (secondary to an elevated ACR). This highlights the importance of transitions of care and ongoing, intensive education, not only during initial diagnosis but also throughout the disease-state progression.
Utilization of EHRs and telephone outreach are additional aspects of care that can be provided. Our improved rates of compliance with these care interventions parallel findings from previous studies.15,18 Optimization of an institution’s EHR can aid in standardization of care, workflow management, and communication with patients, as well as alert nursing or support staff of screening needs. Techniques such as best practice reminders, patient chart messages, and nursing-entered physician alerts on daily schedules have been shown to increase rates of compliance with nephropathy standards. These findings underscore an additional opportunity for nursing and support staff to be better integrated into care.
Despite the success of this quality improvement initiative, there remain some limitations. The processes we used in this project may not be applicable to every institution and may have limited external validity. Primarily, while these processes may be implemented at some sites, without additional support staff (ie, extra nursing staff, pharmacists) and students to aid in patient outreach, success may be limited due to provider time constraints. Additionally, our workflow process demonstrates significant incorporation of an EHR system for patient outreach. Institutions and/or clinics that heavily rely on paper charts and paper outreach may face barriers with bulk orders (eg, ACR) and messages, interventions that streamlined our population health management. Finally, this project focuses on only 1 aspect of population health management for patients with diabetes. While nephropathy is a critical aspect of caring for individuals with diabetes, this patient outreach does not address retinopathy screening, HbA1c control, or vaccination rates, which are other components of care.
Conclusion
Although this evaluation does not provide insight into why patients were not treated or screened, it demonstrates processes to improve compliance in patients with diabetic nephropathy. Rural health care facilities require an ongoing program of change and evaluation, with the aim to improve the provision of services, increase screening, and encourage team member involvement in health promotion. This study demonstrates that combining team-based interventions with quality control monitoring can significantly improve compliance with recommended nephropathy screening and treatment in rural patients with diabetes at a family medicine clinic.
Corresponding author: Amie M. Ashcraft, West Virginia University, Department of Family Medicine, 1 Medical Center Drive, Box 9152, Morgantown, WV 26506; [email protected].
Financial disclosures: None.
Acknowledgment: The authors thank the faculty, residents, nurses, and clinic staff for their hard work and dedication to this effort: Umama Sadia, Michelle Prestoza, Richard Dattola, Greg Doyle, Dana King, Mike Maroon, Kendra Under, Judy Siebert, Christine Snyder, Rachel Burge, Meagan Gribble, Lisa Metts, Kelsey Samek, Sarah Deavers, Amber Kitzmiller, Angela Lamp, Tina Waldeck, and Andrea Sukeruksa.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.
1. Centers for Disease Control and Prevention (CDC). National diabetes statistics report. Estimates of diabetes and its burden in the United States. Atlanta, GA: CDC; 2017www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 20, 2020.
2. American Diabetes Association (ADA). Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928.
3. Wood L. Trends in national and regional economic distress, 1960-2000. Washington, DC: Appalachian Regional Commission; 2005.
4. Barker L, Crespo R, Gerzoff RB, et al. Residence in a distressed county in Appalachia as a risk factor for diabetes, Behavioral Risk Factor Surveillance System, 2006-2007. Prev Chronic Dis. 2010;7:A104.
5. Barker L, Kirtland KA, Gregg E, et al. Geographic distribution of diagnosed diabetes in the United States: A diabetes belt. Am J Prev Med. 2011;40:434-439.
6. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176.
7. United States Renal Data System (USRDS). Annual data report. Ann Arbor, MI: USRDS; 2018. www.usrds.org/2018/view/Default.aspx. Accessed December 20, 2020.
8. Halverson JA, Bichak G. Underlying socioeconomic factors influencing health disparities in the Appalachian region. Washington, DC: Appalachian Regional Commission; 2008.
9. Shell R, Tudiver F. Barriers to cancer screening by rural Appalachian primary care providers. J Rural Health. 2004;20:368-373.
10. Hatcher J, Dignan MB, Schoenberg N. How do rural health care providers and patients view barriers to colorectal cancer screening? Insights from Appalachian Kentucky. Nurs Clin North Am. 2011;46:181-192.
11. Scott S, McSpirit S. The suspicious, untrusting hillbilly in political-economic contexts: Stereotypes and social trust in the Appalachian coalfields. Pract Anthropol. 2014;36:42-46.
12. Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25:1946-1951.
13. Byun SH, Ma SH, Jun JK, et al. Screening for diabetic retinopathy and nephropathy in patients with diabetes: A nationwide survey in Korea. PLoS One. 2013;8:e62991.
14. Flood D, Garcia P, Douglas K, et al. Screening for chronic kidney disease in a community-based diabetes cohort in rural Guatemala: A cross-sectional study. BMJ Open. 2018;8:e019778.
15. Anabtawi A, Mathew LM. Improving compliance with screening of diabetic patients for microalbuminuria in primary care practice. ISRN Endocrinology. 2013:893913.
16. Tonks SA, Makwana S, Salanitro AH, et al. Quality of diabetes mellitus care by rural primary care physicians. J Rural Health. 2012;28:364-371.
17. Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public Health. 2015;129:611-620.
18. Weber V, Bloom F, Pierdon S, Wood C. Employing the electronic health record to improve diabetes care: a multifaceted intervention in an integrated delivery system. J Gen Intern Med. 2008;23:379-382.