User login
Try testosterone for some women with sexual dysfunction, but not others
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Poll: How much has the price of insulin risen in the past 15 years?
Choose your answer in the poll below. To check the accuracy of your answer, see Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
[polldaddy:10400221]
Click on page 2 below to find out what the correct answer is...
The correct answer is d.) 500%
To learn more, see this month's Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
Choose your answer in the poll below. To check the accuracy of your answer, see Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
[polldaddy:10400221]
Click on page 2 below to find out what the correct answer is...
The correct answer is d.) 500%
To learn more, see this month's Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
Choose your answer in the poll below. To check the accuracy of your answer, see Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
[polldaddy:10400221]
Click on page 2 below to find out what the correct answer is...
The correct answer is d.) 500%
To learn more, see this month's Endocrine Consult: 10 (Safe) Ways to Reduce Patients’ Insulin Costs.
10 (Safe) Ways to Reduce Patients’ Insulin Costs
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
Clinical outcomes in diabetes: It’s not just the glucose (and it’s not so simple)
There has been increasing emphasis from drug regulatory agencies on collecting robust data on multiple outcomes from clinical trials in addition to the efficacy outcomes and usual safety data. For about a decade, the US Food and Drug Administration has required the collection of cardiovascular outcome data during the testing of new antidiabetic therapies. There are several potential consequences of this mandate, in addition to our now having a better understanding of cardiovascular risk. Studies are likely to be larger, longer, and more expensive. Patient cohorts are selected with this in mind, meaning that studies may be harder to compare, and labeled indications may be more specific. And we now have several drugs carrying a specific indication to reduce cardiovascular death in patients with diabetes!
But as we dig deeper into the reduction in cardiovascular deaths seen in clinical trials with some of the sodium-glucose cotransporter 2 (SGLT2) inhibitors, several questions arise. Why is their effect on mortality and cardiovascular events (and preservation of renal function) not a consistent drug class effect? All of these inhibitors decrease glucose reabsorption and thus cause glucosuria, resulting in lower blood glucose levels with modest caloric wasting and weight loss, as well as natriuresis with mild volume depletion. But the individual drugs behaved slightly differently in clinical trials. Perhaps this was due to slightly different trial populations, or chance (despite large trial numbers), or maybe molecular differences in the drugs despite their shared effect on glucosuria, resulting in distinct “off-target” effects. Perhaps the drugs differentially affect other transporters, on cells other than renal tubular cells, altering their function. An additional known effect of the drug class is uricosuria and mild relative hypouricemia. The differential effects of these drugs on urate transport into and out of different cells that may influence components of the metabolic syndrome and cardiovascular and renal outcomes has yet to be fully explored.
But one thing that seems to be true is that the effect of empagliflozin and canagliflozin on cardiac mortality is not due to simply lowering the blood glucose. Trials like the UK Prospective Diabetes Study1 demonstrated that better glucose control reduced microvascular complications, but they did not initially show a reduction in myocardial infarction. It took long-term follow-up studies to indicate that more intensive initial glucose control could reduce cardiovascular events. But a beneficial effect of empagliflozin (compared with placebo) on cardiovascular mortality (but interestingly not on stroke or nonfatal myocardial infarction) was seen within 3 months.2 This observation suggests unique properties of this drug and some others in the class, in addition to their glucose-lowering effect. Puzzling to me, looking at several of the SGLT2 inhibitor drug studies, is why they seemed to behave differently in terms of different cardiovascular outcomes (eg, heart failure, stroke, nonfatal myocardial infarction, need for limb amputation). While some of these seemingly paradoxical outcomes have also been seen in studies of other drugs, these differences are hard for me to understand on a biological basis: they do not seem consistent with simply differential drug effects on either acute thrombosis or chronic hypoperfusion. We have much more to learn.
For the moment, I suppose we should let our practice be guided by the results of specific clinical trials, hoping that at some point head-to-head comparator drug trials will be undertaken to provide us with even better guidance in drug selection.
We can also hope that our patients with diabetes will somehow be able to afford our increasingly complex and evidence-supported pharmacotherapy, as now not only can we lower the levels of blood glucose and biomarkers of comorbidity, we can also reduce adverse cardiovascular outcomes.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OuTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
There has been increasing emphasis from drug regulatory agencies on collecting robust data on multiple outcomes from clinical trials in addition to the efficacy outcomes and usual safety data. For about a decade, the US Food and Drug Administration has required the collection of cardiovascular outcome data during the testing of new antidiabetic therapies. There are several potential consequences of this mandate, in addition to our now having a better understanding of cardiovascular risk. Studies are likely to be larger, longer, and more expensive. Patient cohorts are selected with this in mind, meaning that studies may be harder to compare, and labeled indications may be more specific. And we now have several drugs carrying a specific indication to reduce cardiovascular death in patients with diabetes!
But as we dig deeper into the reduction in cardiovascular deaths seen in clinical trials with some of the sodium-glucose cotransporter 2 (SGLT2) inhibitors, several questions arise. Why is their effect on mortality and cardiovascular events (and preservation of renal function) not a consistent drug class effect? All of these inhibitors decrease glucose reabsorption and thus cause glucosuria, resulting in lower blood glucose levels with modest caloric wasting and weight loss, as well as natriuresis with mild volume depletion. But the individual drugs behaved slightly differently in clinical trials. Perhaps this was due to slightly different trial populations, or chance (despite large trial numbers), or maybe molecular differences in the drugs despite their shared effect on glucosuria, resulting in distinct “off-target” effects. Perhaps the drugs differentially affect other transporters, on cells other than renal tubular cells, altering their function. An additional known effect of the drug class is uricosuria and mild relative hypouricemia. The differential effects of these drugs on urate transport into and out of different cells that may influence components of the metabolic syndrome and cardiovascular and renal outcomes has yet to be fully explored.
But one thing that seems to be true is that the effect of empagliflozin and canagliflozin on cardiac mortality is not due to simply lowering the blood glucose. Trials like the UK Prospective Diabetes Study1 demonstrated that better glucose control reduced microvascular complications, but they did not initially show a reduction in myocardial infarction. It took long-term follow-up studies to indicate that more intensive initial glucose control could reduce cardiovascular events. But a beneficial effect of empagliflozin (compared with placebo) on cardiovascular mortality (but interestingly not on stroke or nonfatal myocardial infarction) was seen within 3 months.2 This observation suggests unique properties of this drug and some others in the class, in addition to their glucose-lowering effect. Puzzling to me, looking at several of the SGLT2 inhibitor drug studies, is why they seemed to behave differently in terms of different cardiovascular outcomes (eg, heart failure, stroke, nonfatal myocardial infarction, need for limb amputation). While some of these seemingly paradoxical outcomes have also been seen in studies of other drugs, these differences are hard for me to understand on a biological basis: they do not seem consistent with simply differential drug effects on either acute thrombosis or chronic hypoperfusion. We have much more to learn.
For the moment, I suppose we should let our practice be guided by the results of specific clinical trials, hoping that at some point head-to-head comparator drug trials will be undertaken to provide us with even better guidance in drug selection.
We can also hope that our patients with diabetes will somehow be able to afford our increasingly complex and evidence-supported pharmacotherapy, as now not only can we lower the levels of blood glucose and biomarkers of comorbidity, we can also reduce adverse cardiovascular outcomes.
There has been increasing emphasis from drug regulatory agencies on collecting robust data on multiple outcomes from clinical trials in addition to the efficacy outcomes and usual safety data. For about a decade, the US Food and Drug Administration has required the collection of cardiovascular outcome data during the testing of new antidiabetic therapies. There are several potential consequences of this mandate, in addition to our now having a better understanding of cardiovascular risk. Studies are likely to be larger, longer, and more expensive. Patient cohorts are selected with this in mind, meaning that studies may be harder to compare, and labeled indications may be more specific. And we now have several drugs carrying a specific indication to reduce cardiovascular death in patients with diabetes!
But as we dig deeper into the reduction in cardiovascular deaths seen in clinical trials with some of the sodium-glucose cotransporter 2 (SGLT2) inhibitors, several questions arise. Why is their effect on mortality and cardiovascular events (and preservation of renal function) not a consistent drug class effect? All of these inhibitors decrease glucose reabsorption and thus cause glucosuria, resulting in lower blood glucose levels with modest caloric wasting and weight loss, as well as natriuresis with mild volume depletion. But the individual drugs behaved slightly differently in clinical trials. Perhaps this was due to slightly different trial populations, or chance (despite large trial numbers), or maybe molecular differences in the drugs despite their shared effect on glucosuria, resulting in distinct “off-target” effects. Perhaps the drugs differentially affect other transporters, on cells other than renal tubular cells, altering their function. An additional known effect of the drug class is uricosuria and mild relative hypouricemia. The differential effects of these drugs on urate transport into and out of different cells that may influence components of the metabolic syndrome and cardiovascular and renal outcomes has yet to be fully explored.
But one thing that seems to be true is that the effect of empagliflozin and canagliflozin on cardiac mortality is not due to simply lowering the blood glucose. Trials like the UK Prospective Diabetes Study1 demonstrated that better glucose control reduced microvascular complications, but they did not initially show a reduction in myocardial infarction. It took long-term follow-up studies to indicate that more intensive initial glucose control could reduce cardiovascular events. But a beneficial effect of empagliflozin (compared with placebo) on cardiovascular mortality (but interestingly not on stroke or nonfatal myocardial infarction) was seen within 3 months.2 This observation suggests unique properties of this drug and some others in the class, in addition to their glucose-lowering effect. Puzzling to me, looking at several of the SGLT2 inhibitor drug studies, is why they seemed to behave differently in terms of different cardiovascular outcomes (eg, heart failure, stroke, nonfatal myocardial infarction, need for limb amputation). While some of these seemingly paradoxical outcomes have also been seen in studies of other drugs, these differences are hard for me to understand on a biological basis: they do not seem consistent with simply differential drug effects on either acute thrombosis or chronic hypoperfusion. We have much more to learn.
For the moment, I suppose we should let our practice be guided by the results of specific clinical trials, hoping that at some point head-to-head comparator drug trials will be undertaken to provide us with even better guidance in drug selection.
We can also hope that our patients with diabetes will somehow be able to afford our increasingly complex and evidence-supported pharmacotherapy, as now not only can we lower the levels of blood glucose and biomarkers of comorbidity, we can also reduce adverse cardiovascular outcomes.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OuTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil AW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OuTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
Diabetic dyslipidemia with eruptive xanthoma
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
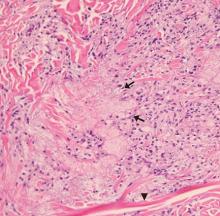
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
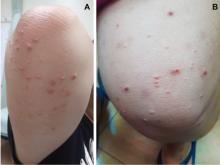
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
Diabetes management: Beyond hemoglobin A1c
When scientists discovered the band of hemoglobin A1c during electrophoresis in the 1950s and 1960s and discerned it was elevated in patients with diabetes, little did they know the important role it would play in the diagnosis and treatment of diabetes in the decades to come.1–3 Despite some caveats, a hemoglobin A1c level of 6.5% or higher is diagnostic of diabetes across most populations, and hemoglobin A1c goals ranging from 6.5% to 7.5% have been set for different subsets of patients depending on comorbidities, complications, risk of hypoglycemia, life expectancy, disease duration, patient preferences, and available resources.4
With a growing number of medications for diabetes—insulin in its various formulations and 11 other classes—hemoglobin A1c targets can now be tailored to fit individual patient profiles. Although helping patients attain their glycemic goals is paramount, other factors should be considered when prescribing or changing a drug treatment regimen, such as cardiovascular risk reduction, weight control, avoidance of hypoglycemia, and minimizing out-of-pocket drug costs (Table 1).
CARDIOVASCULAR BENEFIT
Patients with type 2 diabetes have a 2 to 3 times higher risk of clinical atherosclerotic disease, according to 20 years of surveillance data from the Framingham cohort.5
Mixed results with intensive treatment
Reducing cardiovascular risk remains an important goal in diabetes management, but unfortunately, data from the long-term clinical trials aimed at reducing macrovascular risk with intensive glycemic management have been conflicting.
The United Kingdom Prospective Diabetes Study (UKPDS),6 which enrolled more than 4,000 patients with newly diagnosed type 2 diabetes, did not initially show a statistically significant difference in the incidence of myocardial infarction with intensive control vs conventional control, although intensive treatment did reduce the incidence of microvascular disease. However, 10 years after the trial ended, the incidence was 15% lower in the intensive-treatment group than in the conventional-treatment group, and the difference was statistically significant.7
A 10-year follow-up analysis of the Veterans Affairs Diabetes Trial (VADT)8 showed that patients who had been randomly assigned to intensive glucose control for 5.6 years had 8.6 fewer major cardiovascular events per 1,000 person-years than those assigned to standard therapy, but no improvement in median overall survival. The hemoglobin A1c levels achieved during the trial were 6.9% and 8.4%, respectively.
In 2008, the US Food and Drug Administration (FDA)9 mandated that all new applications for diabetes drugs must include cardiovascular outcome studies. Therefore, we now have data on the cardiovascular benefits of two antihyperglycemic drug classes—incretins and sodium-glucose cotransporter 2 (SGLT2) inhibitors, making them attractive medications to target both cardiac and glucose concerns.
Incretins
The incretin drugs comprise 2 classes, glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors.
Liraglutide. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial10 compared liraglutide (a GLP-1 receptor agonist) and placebo in 9,000 patients with diabetes who either had or were at high risk of cardiovascular disease. Patients in the liraglutide group had a lower risk of the primary composite end point of death from cardiovascular causes or the first episode of nonfatal (including silent) myocardial infarction or nonfatal stroke, and a lower risk of cardiovascular death, all-cause mortality, and microvascular events than those in the placebo group. The number of patients who would need to be treated to prevent 1 event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause.9
Lixisenatide. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial11 studied the effect of the once-daily GLP-1 receptor agonist lixisenatide on cardiovascular outcomes in 6,000 patients with type 2 diabetes with a recent coronary event. In contrast to LEADER, ELIXA did not show a cardiovascular benefit over placebo.
Exenatide. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL)12 assessed another GLP-1 extended-release drug, exenatide, in 14,000 patients, 73% of whom had established cardiovascular disease. In those patients, the drug had a modest benefit in terms of first occurrence of any component of the composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (3-component major adverse cardiac event [MACE] outcome) in a time-to-event analysis, but the results were not statistically significant. However, the drug did significantly reduce all-cause mortality.
Semaglutide, another GLP-1 receptor agonist recently approved by the FDA, also showed benefit in patients who had cardiovascular disease or were at high risk, with significant reduction in the primary composite end point of death from cardiovascular causes or the first occurrence of nonfatal myocardial infarction (including silent) or nonfatal stroke.13
Dulaglutide, a newer GLP-1 drug, was associated with significantly reduced major adverse cardiovascular events (a composite end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in about 9,900 patients with diabetes, with a median follow-up of more than 5 years. Only 31% of the patients in the trial had established cardiovascular disease.14
Comment. GLP-1 drugs as a class are a good option for patients with diabetes who require weight loss, and liraglutide is now FDA-approved for reduction of cardiovascular events in patients with type 2 diabetes with established cardiovascular disease. However, other factors should be considered when prescribing these drugs: they have adverse gastrointestinal effects, the cardiovascular benefit was not a class effect, they are relatively expensive, and they must be injected. Also, they should not be prescribed concurrently with a DPP-4 inhibitor because they target the same pathway.
SGLT2 inhibitors
The other class of diabetes drugs that have shown cardiovascular benefit are the SGLT2 inhibitors.
Empagliflozin. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG)15 compared the efficacy of empagliflozin vs placebo in 7,000 patients with diabetes and cardiovascular disease and showed relative risk reductions of 38% in death from cardiovascular death, 31% in sudden death, and 35% in heart failure hospitalizations. Empagliflozin also showed benefit in terms of progression of kidney disease and occurrence of clinically relevant renal events in this population.16
Canagliflozin also has cardiovascular outcome data and showed significant benefit when compared with placebo in the primary outcome of the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, but no significant effects on cardiovascular death or all-cause mortality.17 Data from this trial also suggested a nonsignificant benefit of canagliflozin in decreasing progression of albuminuria and in the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate (eGFR), the need for renal replacement therapy, or death from renal causes.
The above data led to an additional indication from the FDA for empagliflozin—and recently, canagliflozin—to prevent cardiovascular death in patients with diabetes with established disease, but other factors should be considered when prescribing them. Patients taking canagliflozin showed a significantly increased risk of amputation. SGLT2 inhibitors as a class also increase the risk of genital infections in men and women; this is an important consideration since patients with diabetes complain of vaginal fungal and urinary tract infections even without the use of these drugs. A higher incidence of fractures with canagliflozin should also be considered when using these medications in elderly and osteoporosis-prone patients at high risk of falling.
Dapagliflozin, the third drug in this class, was associated with a lower rate of hospitalization for heart failure in about 17,160 patients—including 10,186 without atherosclerotic cardiovascular disease—who were followed for a median of 4.2 years.18 It did not show benefit for the primary safety outcome, a composite of major adverse cardiovascular events defined as cardiovascular death, myocardial infarction, or ischemic stroke.
WEIGHT MANAGEMENT
Weight loss can help overweight patients reach their hemoglobin A1c target.
Metformin should be continued as other drugs are added because it does not induce weight gain and may help with weight loss of up to 2 kg as shown in the Diabetes Prevention Program Outcomes Study.19
GLP-1 receptor agonists and SGLT2 inhibitors help with weight loss and are good additions to a basal insulin regimen to minimize weight gain.
Liraglutide was associated with a mean weight loss of 2.3 kg over 36 months of treatment compared with placebo in the LEADER trial.10
In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6),20 the mean body weight in the semaglutide group, compared with the placebo group, was 2.9 kg lower in the group receiving a lower dose and 4.3 kg lower in the group receiving a higher dose of the drug.
In a 24-week trial in 182 patients with type 2 diabetes inadequately controlled on metformin, dapagliflozin produced a statistically significant weight reduction of 2.08 kg (95% confidence interval 2.84–1.31; P < .0001) compared with placebo.21
Lifestyle changes aimed at weight management should be emphasized and discussed at every visit.
HYPOGLYCEMIA RISK
Hypoglycemia is a major consideration when tailoring hemoglobin A1c targets. In the Action to Control Cardiovascular Risk (ACCORD) trial,22 severe, symptomatic hypoglycemia increased the risk of death in both the intensive and conventional treatment groups. In VADT, the occurrence of a recent severe hypoglycemic event was the strongest independent predictor of death within 90 days. Further analysis showed that even though serious hypoglycemia occurred more often in the intensive therapy group, it was associated with progression of coronary artery calcification in the standard therapy group.23 Hence, it is imperative that tight glycemic control not be achieved at the cost of severe or recurrent hypoglycemia.
In terms of hypoglycemia, metformin is an excellent medication. The American Diabetes Association24 recommends metformin as the first-line therapy for newly diagnosed diabetes. Long-term follow-up data from UKPDS showed that metformin decreased mortality and the incidence of myocardial infarction and lowered treatment costs as well as the overall risk of hypoglycemia.25 When prescribed, it should be titrated to the highest dose.
The FDA26 has changed the prescribing information for metformin in patients with renal impairment. Metformin should not be started if the eGFR is less than 45 mL/min/1.73 m2, but it can be continued if the patient is already receiving it and the eGFR is between 30 and 45. Previously, creatinine levels were used to define renal impairment and suitability for metformin. This change has increased the number of patients who can benefit from this medication.
In patients who have a contraindication to metformin, DPP-4 inhibitors can be considered, as they carry a low risk of hypoglycemia as well. Sulfonylureas should be used with caution in these patients, especially if their oral intake is variable. When sulfonylureas were compared to the DPP-4 inhibitor sitagliptin as an add-on to metformin, the rate of hypoglycemia was 32% in the sulfonylurea group vs 5% in the sitagliptin group.27
Of the sulfonylureas, glipizide and glimepiride are better than glyburide because of a comparatively lower risk of hypoglycemia and a higher selectivity for binding the KATP channel on the pancreatic beta cell.28
Meglitinides can be a good option for patients who skip meals, but they are more expensive than other generic oral hypoglycemic agents and require multiple daily dosing.
GLP-1 analogues also have a low risk of hypoglycemia but are only available in injectable formulations. Patients must be willing and able to perform the injections themselves.29
LOOSER TARGETS FOR OLDER PATIENTS
In 2010, among US residents age 65 and older, 10.9 million (about 27%) had diabetes,30 and this number is projected to increase to 26.7 million by 2050.31 This population is prone to hypoglycemia when treated with insulin and sulfonylureas. An injury sustained by a fall induced by hypoglycemia can be life-altering. In addition, no randomized clinical trials show the effect of tight glycemic control on complications in older patients with diabetes because patients older than 80 are often excluded.
A reasonable goal suggested by the European Diabetes Working Party for Older People 201132 and reiterated by the American Geriatrics Society in 201333 is a hemoglobin A1c between 7% and 7.5% for relatively healthy older patients and 7.5% to 8% or 8.5% in frail elderly patients with diabetes.
Consider prescribing medications that carry a low risk of hypoglycemia, can be dose-adjusted for kidney function, and do not rely on manual dexterity for administration (ie, do not require patients to give themselves injections). These include metformin and DPP-4 inhibitors.
DRUG COMBINATIONS
Polypharmacy is a concern for all patients with diabetes, especially since it increases the risk of drug interactions and adverse effects, increases out-of-pocket costs, and decreases the likelihood that patients will remain adherent to their treatment regimen. The use of combination medications can reduce the number of pills or injections required, as well as copayments.
Due to concern for multiple drug-drug interactions (and also due to the progressive nature of diabetes), many people with type 2 diabetes are given insulin in lieu of pills to lower their blood glucose. In addition to premixed insulin combinations (such as combinations of neutral protamine Hagedorn and regular insulin or combinations of insulin analogues), long-acting basal insulins can now be prescribed with a GLP-1 drug in fixed-dose combinations such as insulin glargine plus lixisenatide and insulin degludec plus liraglutide.
COST CONSIDERATIONS
It is important to discuss medication cost with patients, because many newer diabetic drugs are expensive and add to the financial burden of patients already paying for multiple medications, such as antihypertensives and statins.
Metformin and sulfonylureas are less expensive alternatives for patients who cannot afford GLP-1 analogues or SGLT2 inhibitors. Even within the same drug class, the formulary-preferred drug may be cheaper than the nonformulary alternative. Thus, it is helpful to research formulary alternatives before discussing treatment regimens with patients.
- Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin: a study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Amer Chem Soc 1958; 80(7):1628–1634. doi:10.1021/ja01540a030
- Huisman TH, Dozy AM. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med 1962; 60:302–319. pmid:14449875
- Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun 1969; 36(5):838–843. pmid:5808299
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S55–S64. doi:10.2337/dc18-S006
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241(19):2035–2038. pmid:430798
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837–853. [Erratum in Lancet 1999; 354:602.] pmid:9742976
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372(23):2197–2206. doi:10.1056/NEJMoa1414266
- US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.govinfo.gov/content/pkg/FR-2008-12-19/pdf/E8-30086.pdf. Accessed August 6, 2019.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377(13):1228–1239. doi:10.1056/NEJMoa1612917
- Cosmi F, Laini R, Nicolucci A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376(9):890. doi:10.1056/NEJMc1615712
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193):121–130. doi:10.1016/S0140-6736(19)31149-3
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375(4):323–334. doi:10.1056/NEJMoa1515920
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018. [Epub ahead of print] doi:10.1056/NEJMoa1812389
- Diabetes Prevention Program Research Group; Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374(9702):1677–1686. doi:10.1016/S0140-6736(09)61457-4
- Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. doi:10.1056/NEJMoa1607141
- Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97(3):1020–1031. doi:10.1210/jc.2011-2260
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Saremi A, Bahn GD, Reaven PD; Veterans Affairs Diabetes Trial (VADT). A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016; 39(3):448–454. doi:10.2337/dc15-2107
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed August 5, 2019.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9(2):194–205. doi:10.1111/j.1463-1326.2006.00704.x
- Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007; 30(2):389–394. doi:10.2337/dc06-1789
- Nauck M, Frid A, Hermansen K, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32(1):84–90. doi:10.2337/dc08-1355
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 5, 2019.
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010; 8:29. doi:10.1186/1478-7954-8-29
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37(suppl 3):S27–S38. doi:10.1016/S1262-3636(11)70962-4
- American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013; 61(11):2020–2026. doi:10.1111/jgs.12514
When scientists discovered the band of hemoglobin A1c during electrophoresis in the 1950s and 1960s and discerned it was elevated in patients with diabetes, little did they know the important role it would play in the diagnosis and treatment of diabetes in the decades to come.1–3 Despite some caveats, a hemoglobin A1c level of 6.5% or higher is diagnostic of diabetes across most populations, and hemoglobin A1c goals ranging from 6.5% to 7.5% have been set for different subsets of patients depending on comorbidities, complications, risk of hypoglycemia, life expectancy, disease duration, patient preferences, and available resources.4
With a growing number of medications for diabetes—insulin in its various formulations and 11 other classes—hemoglobin A1c targets can now be tailored to fit individual patient profiles. Although helping patients attain their glycemic goals is paramount, other factors should be considered when prescribing or changing a drug treatment regimen, such as cardiovascular risk reduction, weight control, avoidance of hypoglycemia, and minimizing out-of-pocket drug costs (Table 1).
CARDIOVASCULAR BENEFIT
Patients with type 2 diabetes have a 2 to 3 times higher risk of clinical atherosclerotic disease, according to 20 years of surveillance data from the Framingham cohort.5
Mixed results with intensive treatment
Reducing cardiovascular risk remains an important goal in diabetes management, but unfortunately, data from the long-term clinical trials aimed at reducing macrovascular risk with intensive glycemic management have been conflicting.
The United Kingdom Prospective Diabetes Study (UKPDS),6 which enrolled more than 4,000 patients with newly diagnosed type 2 diabetes, did not initially show a statistically significant difference in the incidence of myocardial infarction with intensive control vs conventional control, although intensive treatment did reduce the incidence of microvascular disease. However, 10 years after the trial ended, the incidence was 15% lower in the intensive-treatment group than in the conventional-treatment group, and the difference was statistically significant.7
A 10-year follow-up analysis of the Veterans Affairs Diabetes Trial (VADT)8 showed that patients who had been randomly assigned to intensive glucose control for 5.6 years had 8.6 fewer major cardiovascular events per 1,000 person-years than those assigned to standard therapy, but no improvement in median overall survival. The hemoglobin A1c levels achieved during the trial were 6.9% and 8.4%, respectively.
In 2008, the US Food and Drug Administration (FDA)9 mandated that all new applications for diabetes drugs must include cardiovascular outcome studies. Therefore, we now have data on the cardiovascular benefits of two antihyperglycemic drug classes—incretins and sodium-glucose cotransporter 2 (SGLT2) inhibitors, making them attractive medications to target both cardiac and glucose concerns.
Incretins
The incretin drugs comprise 2 classes, glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors.
Liraglutide. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial10 compared liraglutide (a GLP-1 receptor agonist) and placebo in 9,000 patients with diabetes who either had or were at high risk of cardiovascular disease. Patients in the liraglutide group had a lower risk of the primary composite end point of death from cardiovascular causes or the first episode of nonfatal (including silent) myocardial infarction or nonfatal stroke, and a lower risk of cardiovascular death, all-cause mortality, and microvascular events than those in the placebo group. The number of patients who would need to be treated to prevent 1 event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause.9
Lixisenatide. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial11 studied the effect of the once-daily GLP-1 receptor agonist lixisenatide on cardiovascular outcomes in 6,000 patients with type 2 diabetes with a recent coronary event. In contrast to LEADER, ELIXA did not show a cardiovascular benefit over placebo.
Exenatide. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL)12 assessed another GLP-1 extended-release drug, exenatide, in 14,000 patients, 73% of whom had established cardiovascular disease. In those patients, the drug had a modest benefit in terms of first occurrence of any component of the composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (3-component major adverse cardiac event [MACE] outcome) in a time-to-event analysis, but the results were not statistically significant. However, the drug did significantly reduce all-cause mortality.
Semaglutide, another GLP-1 receptor agonist recently approved by the FDA, also showed benefit in patients who had cardiovascular disease or were at high risk, with significant reduction in the primary composite end point of death from cardiovascular causes or the first occurrence of nonfatal myocardial infarction (including silent) or nonfatal stroke.13
Dulaglutide, a newer GLP-1 drug, was associated with significantly reduced major adverse cardiovascular events (a composite end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in about 9,900 patients with diabetes, with a median follow-up of more than 5 years. Only 31% of the patients in the trial had established cardiovascular disease.14
Comment. GLP-1 drugs as a class are a good option for patients with diabetes who require weight loss, and liraglutide is now FDA-approved for reduction of cardiovascular events in patients with type 2 diabetes with established cardiovascular disease. However, other factors should be considered when prescribing these drugs: they have adverse gastrointestinal effects, the cardiovascular benefit was not a class effect, they are relatively expensive, and they must be injected. Also, they should not be prescribed concurrently with a DPP-4 inhibitor because they target the same pathway.
SGLT2 inhibitors
The other class of diabetes drugs that have shown cardiovascular benefit are the SGLT2 inhibitors.
Empagliflozin. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG)15 compared the efficacy of empagliflozin vs placebo in 7,000 patients with diabetes and cardiovascular disease and showed relative risk reductions of 38% in death from cardiovascular death, 31% in sudden death, and 35% in heart failure hospitalizations. Empagliflozin also showed benefit in terms of progression of kidney disease and occurrence of clinically relevant renal events in this population.16
Canagliflozin also has cardiovascular outcome data and showed significant benefit when compared with placebo in the primary outcome of the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, but no significant effects on cardiovascular death or all-cause mortality.17 Data from this trial also suggested a nonsignificant benefit of canagliflozin in decreasing progression of albuminuria and in the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate (eGFR), the need for renal replacement therapy, or death from renal causes.
The above data led to an additional indication from the FDA for empagliflozin—and recently, canagliflozin—to prevent cardiovascular death in patients with diabetes with established disease, but other factors should be considered when prescribing them. Patients taking canagliflozin showed a significantly increased risk of amputation. SGLT2 inhibitors as a class also increase the risk of genital infections in men and women; this is an important consideration since patients with diabetes complain of vaginal fungal and urinary tract infections even without the use of these drugs. A higher incidence of fractures with canagliflozin should also be considered when using these medications in elderly and osteoporosis-prone patients at high risk of falling.
Dapagliflozin, the third drug in this class, was associated with a lower rate of hospitalization for heart failure in about 17,160 patients—including 10,186 without atherosclerotic cardiovascular disease—who were followed for a median of 4.2 years.18 It did not show benefit for the primary safety outcome, a composite of major adverse cardiovascular events defined as cardiovascular death, myocardial infarction, or ischemic stroke.
WEIGHT MANAGEMENT
Weight loss can help overweight patients reach their hemoglobin A1c target.
Metformin should be continued as other drugs are added because it does not induce weight gain and may help with weight loss of up to 2 kg as shown in the Diabetes Prevention Program Outcomes Study.19
GLP-1 receptor agonists and SGLT2 inhibitors help with weight loss and are good additions to a basal insulin regimen to minimize weight gain.
Liraglutide was associated with a mean weight loss of 2.3 kg over 36 months of treatment compared with placebo in the LEADER trial.10
In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6),20 the mean body weight in the semaglutide group, compared with the placebo group, was 2.9 kg lower in the group receiving a lower dose and 4.3 kg lower in the group receiving a higher dose of the drug.
In a 24-week trial in 182 patients with type 2 diabetes inadequately controlled on metformin, dapagliflozin produced a statistically significant weight reduction of 2.08 kg (95% confidence interval 2.84–1.31; P < .0001) compared with placebo.21
Lifestyle changes aimed at weight management should be emphasized and discussed at every visit.
HYPOGLYCEMIA RISK
Hypoglycemia is a major consideration when tailoring hemoglobin A1c targets. In the Action to Control Cardiovascular Risk (ACCORD) trial,22 severe, symptomatic hypoglycemia increased the risk of death in both the intensive and conventional treatment groups. In VADT, the occurrence of a recent severe hypoglycemic event was the strongest independent predictor of death within 90 days. Further analysis showed that even though serious hypoglycemia occurred more often in the intensive therapy group, it was associated with progression of coronary artery calcification in the standard therapy group.23 Hence, it is imperative that tight glycemic control not be achieved at the cost of severe or recurrent hypoglycemia.
In terms of hypoglycemia, metformin is an excellent medication. The American Diabetes Association24 recommends metformin as the first-line therapy for newly diagnosed diabetes. Long-term follow-up data from UKPDS showed that metformin decreased mortality and the incidence of myocardial infarction and lowered treatment costs as well as the overall risk of hypoglycemia.25 When prescribed, it should be titrated to the highest dose.
The FDA26 has changed the prescribing information for metformin in patients with renal impairment. Metformin should not be started if the eGFR is less than 45 mL/min/1.73 m2, but it can be continued if the patient is already receiving it and the eGFR is between 30 and 45. Previously, creatinine levels were used to define renal impairment and suitability for metformin. This change has increased the number of patients who can benefit from this medication.
In patients who have a contraindication to metformin, DPP-4 inhibitors can be considered, as they carry a low risk of hypoglycemia as well. Sulfonylureas should be used with caution in these patients, especially if their oral intake is variable. When sulfonylureas were compared to the DPP-4 inhibitor sitagliptin as an add-on to metformin, the rate of hypoglycemia was 32% in the sulfonylurea group vs 5% in the sitagliptin group.27
Of the sulfonylureas, glipizide and glimepiride are better than glyburide because of a comparatively lower risk of hypoglycemia and a higher selectivity for binding the KATP channel on the pancreatic beta cell.28
Meglitinides can be a good option for patients who skip meals, but they are more expensive than other generic oral hypoglycemic agents and require multiple daily dosing.
GLP-1 analogues also have a low risk of hypoglycemia but are only available in injectable formulations. Patients must be willing and able to perform the injections themselves.29
LOOSER TARGETS FOR OLDER PATIENTS
In 2010, among US residents age 65 and older, 10.9 million (about 27%) had diabetes,30 and this number is projected to increase to 26.7 million by 2050.31 This population is prone to hypoglycemia when treated with insulin and sulfonylureas. An injury sustained by a fall induced by hypoglycemia can be life-altering. In addition, no randomized clinical trials show the effect of tight glycemic control on complications in older patients with diabetes because patients older than 80 are often excluded.
A reasonable goal suggested by the European Diabetes Working Party for Older People 201132 and reiterated by the American Geriatrics Society in 201333 is a hemoglobin A1c between 7% and 7.5% for relatively healthy older patients and 7.5% to 8% or 8.5% in frail elderly patients with diabetes.
Consider prescribing medications that carry a low risk of hypoglycemia, can be dose-adjusted for kidney function, and do not rely on manual dexterity for administration (ie, do not require patients to give themselves injections). These include metformin and DPP-4 inhibitors.
DRUG COMBINATIONS
Polypharmacy is a concern for all patients with diabetes, especially since it increases the risk of drug interactions and adverse effects, increases out-of-pocket costs, and decreases the likelihood that patients will remain adherent to their treatment regimen. The use of combination medications can reduce the number of pills or injections required, as well as copayments.
Due to concern for multiple drug-drug interactions (and also due to the progressive nature of diabetes), many people with type 2 diabetes are given insulin in lieu of pills to lower their blood glucose. In addition to premixed insulin combinations (such as combinations of neutral protamine Hagedorn and regular insulin or combinations of insulin analogues), long-acting basal insulins can now be prescribed with a GLP-1 drug in fixed-dose combinations such as insulin glargine plus lixisenatide and insulin degludec plus liraglutide.
COST CONSIDERATIONS
It is important to discuss medication cost with patients, because many newer diabetic drugs are expensive and add to the financial burden of patients already paying for multiple medications, such as antihypertensives and statins.
Metformin and sulfonylureas are less expensive alternatives for patients who cannot afford GLP-1 analogues or SGLT2 inhibitors. Even within the same drug class, the formulary-preferred drug may be cheaper than the nonformulary alternative. Thus, it is helpful to research formulary alternatives before discussing treatment regimens with patients.
When scientists discovered the band of hemoglobin A1c during electrophoresis in the 1950s and 1960s and discerned it was elevated in patients with diabetes, little did they know the important role it would play in the diagnosis and treatment of diabetes in the decades to come.1–3 Despite some caveats, a hemoglobin A1c level of 6.5% or higher is diagnostic of diabetes across most populations, and hemoglobin A1c goals ranging from 6.5% to 7.5% have been set for different subsets of patients depending on comorbidities, complications, risk of hypoglycemia, life expectancy, disease duration, patient preferences, and available resources.4
With a growing number of medications for diabetes—insulin in its various formulations and 11 other classes—hemoglobin A1c targets can now be tailored to fit individual patient profiles. Although helping patients attain their glycemic goals is paramount, other factors should be considered when prescribing or changing a drug treatment regimen, such as cardiovascular risk reduction, weight control, avoidance of hypoglycemia, and minimizing out-of-pocket drug costs (Table 1).
CARDIOVASCULAR BENEFIT
Patients with type 2 diabetes have a 2 to 3 times higher risk of clinical atherosclerotic disease, according to 20 years of surveillance data from the Framingham cohort.5
Mixed results with intensive treatment
Reducing cardiovascular risk remains an important goal in diabetes management, but unfortunately, data from the long-term clinical trials aimed at reducing macrovascular risk with intensive glycemic management have been conflicting.
The United Kingdom Prospective Diabetes Study (UKPDS),6 which enrolled more than 4,000 patients with newly diagnosed type 2 diabetes, did not initially show a statistically significant difference in the incidence of myocardial infarction with intensive control vs conventional control, although intensive treatment did reduce the incidence of microvascular disease. However, 10 years after the trial ended, the incidence was 15% lower in the intensive-treatment group than in the conventional-treatment group, and the difference was statistically significant.7
A 10-year follow-up analysis of the Veterans Affairs Diabetes Trial (VADT)8 showed that patients who had been randomly assigned to intensive glucose control for 5.6 years had 8.6 fewer major cardiovascular events per 1,000 person-years than those assigned to standard therapy, but no improvement in median overall survival. The hemoglobin A1c levels achieved during the trial were 6.9% and 8.4%, respectively.
In 2008, the US Food and Drug Administration (FDA)9 mandated that all new applications for diabetes drugs must include cardiovascular outcome studies. Therefore, we now have data on the cardiovascular benefits of two antihyperglycemic drug classes—incretins and sodium-glucose cotransporter 2 (SGLT2) inhibitors, making them attractive medications to target both cardiac and glucose concerns.
Incretins
The incretin drugs comprise 2 classes, glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP-4) inhibitors.
Liraglutide. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial10 compared liraglutide (a GLP-1 receptor agonist) and placebo in 9,000 patients with diabetes who either had or were at high risk of cardiovascular disease. Patients in the liraglutide group had a lower risk of the primary composite end point of death from cardiovascular causes or the first episode of nonfatal (including silent) myocardial infarction or nonfatal stroke, and a lower risk of cardiovascular death, all-cause mortality, and microvascular events than those in the placebo group. The number of patients who would need to be treated to prevent 1 event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause.9
Lixisenatide. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial11 studied the effect of the once-daily GLP-1 receptor agonist lixisenatide on cardiovascular outcomes in 6,000 patients with type 2 diabetes with a recent coronary event. In contrast to LEADER, ELIXA did not show a cardiovascular benefit over placebo.
Exenatide. The Exenatide Study of Cardiovascular Event Lowering (EXSCEL)12 assessed another GLP-1 extended-release drug, exenatide, in 14,000 patients, 73% of whom had established cardiovascular disease. In those patients, the drug had a modest benefit in terms of first occurrence of any component of the composite outcome of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke (3-component major adverse cardiac event [MACE] outcome) in a time-to-event analysis, but the results were not statistically significant. However, the drug did significantly reduce all-cause mortality.
Semaglutide, another GLP-1 receptor agonist recently approved by the FDA, also showed benefit in patients who had cardiovascular disease or were at high risk, with significant reduction in the primary composite end point of death from cardiovascular causes or the first occurrence of nonfatal myocardial infarction (including silent) or nonfatal stroke.13
Dulaglutide, a newer GLP-1 drug, was associated with significantly reduced major adverse cardiovascular events (a composite end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) in about 9,900 patients with diabetes, with a median follow-up of more than 5 years. Only 31% of the patients in the trial had established cardiovascular disease.14
Comment. GLP-1 drugs as a class are a good option for patients with diabetes who require weight loss, and liraglutide is now FDA-approved for reduction of cardiovascular events in patients with type 2 diabetes with established cardiovascular disease. However, other factors should be considered when prescribing these drugs: they have adverse gastrointestinal effects, the cardiovascular benefit was not a class effect, they are relatively expensive, and they must be injected. Also, they should not be prescribed concurrently with a DPP-4 inhibitor because they target the same pathway.
SGLT2 inhibitors
The other class of diabetes drugs that have shown cardiovascular benefit are the SGLT2 inhibitors.
Empagliflozin. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG)15 compared the efficacy of empagliflozin vs placebo in 7,000 patients with diabetes and cardiovascular disease and showed relative risk reductions of 38% in death from cardiovascular death, 31% in sudden death, and 35% in heart failure hospitalizations. Empagliflozin also showed benefit in terms of progression of kidney disease and occurrence of clinically relevant renal events in this population.16
Canagliflozin also has cardiovascular outcome data and showed significant benefit when compared with placebo in the primary outcome of the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, but no significant effects on cardiovascular death or all-cause mortality.17 Data from this trial also suggested a nonsignificant benefit of canagliflozin in decreasing progression of albuminuria and in the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate (eGFR), the need for renal replacement therapy, or death from renal causes.
The above data led to an additional indication from the FDA for empagliflozin—and recently, canagliflozin—to prevent cardiovascular death in patients with diabetes with established disease, but other factors should be considered when prescribing them. Patients taking canagliflozin showed a significantly increased risk of amputation. SGLT2 inhibitors as a class also increase the risk of genital infections in men and women; this is an important consideration since patients with diabetes complain of vaginal fungal and urinary tract infections even without the use of these drugs. A higher incidence of fractures with canagliflozin should also be considered when using these medications in elderly and osteoporosis-prone patients at high risk of falling.
Dapagliflozin, the third drug in this class, was associated with a lower rate of hospitalization for heart failure in about 17,160 patients—including 10,186 without atherosclerotic cardiovascular disease—who were followed for a median of 4.2 years.18 It did not show benefit for the primary safety outcome, a composite of major adverse cardiovascular events defined as cardiovascular death, myocardial infarction, or ischemic stroke.
WEIGHT MANAGEMENT
Weight loss can help overweight patients reach their hemoglobin A1c target.
Metformin should be continued as other drugs are added because it does not induce weight gain and may help with weight loss of up to 2 kg as shown in the Diabetes Prevention Program Outcomes Study.19
GLP-1 receptor agonists and SGLT2 inhibitors help with weight loss and are good additions to a basal insulin regimen to minimize weight gain.
Liraglutide was associated with a mean weight loss of 2.3 kg over 36 months of treatment compared with placebo in the LEADER trial.10
In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6),20 the mean body weight in the semaglutide group, compared with the placebo group, was 2.9 kg lower in the group receiving a lower dose and 4.3 kg lower in the group receiving a higher dose of the drug.
In a 24-week trial in 182 patients with type 2 diabetes inadequately controlled on metformin, dapagliflozin produced a statistically significant weight reduction of 2.08 kg (95% confidence interval 2.84–1.31; P < .0001) compared with placebo.21
Lifestyle changes aimed at weight management should be emphasized and discussed at every visit.
HYPOGLYCEMIA RISK
Hypoglycemia is a major consideration when tailoring hemoglobin A1c targets. In the Action to Control Cardiovascular Risk (ACCORD) trial,22 severe, symptomatic hypoglycemia increased the risk of death in both the intensive and conventional treatment groups. In VADT, the occurrence of a recent severe hypoglycemic event was the strongest independent predictor of death within 90 days. Further analysis showed that even though serious hypoglycemia occurred more often in the intensive therapy group, it was associated with progression of coronary artery calcification in the standard therapy group.23 Hence, it is imperative that tight glycemic control not be achieved at the cost of severe or recurrent hypoglycemia.
In terms of hypoglycemia, metformin is an excellent medication. The American Diabetes Association24 recommends metformin as the first-line therapy for newly diagnosed diabetes. Long-term follow-up data from UKPDS showed that metformin decreased mortality and the incidence of myocardial infarction and lowered treatment costs as well as the overall risk of hypoglycemia.25 When prescribed, it should be titrated to the highest dose.
The FDA26 has changed the prescribing information for metformin in patients with renal impairment. Metformin should not be started if the eGFR is less than 45 mL/min/1.73 m2, but it can be continued if the patient is already receiving it and the eGFR is between 30 and 45. Previously, creatinine levels were used to define renal impairment and suitability for metformin. This change has increased the number of patients who can benefit from this medication.
In patients who have a contraindication to metformin, DPP-4 inhibitors can be considered, as they carry a low risk of hypoglycemia as well. Sulfonylureas should be used with caution in these patients, especially if their oral intake is variable. When sulfonylureas were compared to the DPP-4 inhibitor sitagliptin as an add-on to metformin, the rate of hypoglycemia was 32% in the sulfonylurea group vs 5% in the sitagliptin group.27
Of the sulfonylureas, glipizide and glimepiride are better than glyburide because of a comparatively lower risk of hypoglycemia and a higher selectivity for binding the KATP channel on the pancreatic beta cell.28
Meglitinides can be a good option for patients who skip meals, but they are more expensive than other generic oral hypoglycemic agents and require multiple daily dosing.
GLP-1 analogues also have a low risk of hypoglycemia but are only available in injectable formulations. Patients must be willing and able to perform the injections themselves.29
LOOSER TARGETS FOR OLDER PATIENTS
In 2010, among US residents age 65 and older, 10.9 million (about 27%) had diabetes,30 and this number is projected to increase to 26.7 million by 2050.31 This population is prone to hypoglycemia when treated with insulin and sulfonylureas. An injury sustained by a fall induced by hypoglycemia can be life-altering. In addition, no randomized clinical trials show the effect of tight glycemic control on complications in older patients with diabetes because patients older than 80 are often excluded.
A reasonable goal suggested by the European Diabetes Working Party for Older People 201132 and reiterated by the American Geriatrics Society in 201333 is a hemoglobin A1c between 7% and 7.5% for relatively healthy older patients and 7.5% to 8% or 8.5% in frail elderly patients with diabetes.
Consider prescribing medications that carry a low risk of hypoglycemia, can be dose-adjusted for kidney function, and do not rely on manual dexterity for administration (ie, do not require patients to give themselves injections). These include metformin and DPP-4 inhibitors.
DRUG COMBINATIONS
Polypharmacy is a concern for all patients with diabetes, especially since it increases the risk of drug interactions and adverse effects, increases out-of-pocket costs, and decreases the likelihood that patients will remain adherent to their treatment regimen. The use of combination medications can reduce the number of pills or injections required, as well as copayments.
Due to concern for multiple drug-drug interactions (and also due to the progressive nature of diabetes), many people with type 2 diabetes are given insulin in lieu of pills to lower their blood glucose. In addition to premixed insulin combinations (such as combinations of neutral protamine Hagedorn and regular insulin or combinations of insulin analogues), long-acting basal insulins can now be prescribed with a GLP-1 drug in fixed-dose combinations such as insulin glargine plus lixisenatide and insulin degludec plus liraglutide.
COST CONSIDERATIONS
It is important to discuss medication cost with patients, because many newer diabetic drugs are expensive and add to the financial burden of patients already paying for multiple medications, such as antihypertensives and statins.
Metformin and sulfonylureas are less expensive alternatives for patients who cannot afford GLP-1 analogues or SGLT2 inhibitors. Even within the same drug class, the formulary-preferred drug may be cheaper than the nonformulary alternative. Thus, it is helpful to research formulary alternatives before discussing treatment regimens with patients.
- Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin: a study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Amer Chem Soc 1958; 80(7):1628–1634. doi:10.1021/ja01540a030
- Huisman TH, Dozy AM. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med 1962; 60:302–319. pmid:14449875
- Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun 1969; 36(5):838–843. pmid:5808299
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S55–S64. doi:10.2337/dc18-S006
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241(19):2035–2038. pmid:430798
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837–853. [Erratum in Lancet 1999; 354:602.] pmid:9742976
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372(23):2197–2206. doi:10.1056/NEJMoa1414266
- US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.govinfo.gov/content/pkg/FR-2008-12-19/pdf/E8-30086.pdf. Accessed August 6, 2019.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377(13):1228–1239. doi:10.1056/NEJMoa1612917
- Cosmi F, Laini R, Nicolucci A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376(9):890. doi:10.1056/NEJMc1615712
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193):121–130. doi:10.1016/S0140-6736(19)31149-3
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375(4):323–334. doi:10.1056/NEJMoa1515920
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018. [Epub ahead of print] doi:10.1056/NEJMoa1812389
- Diabetes Prevention Program Research Group; Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374(9702):1677–1686. doi:10.1016/S0140-6736(09)61457-4
- Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. doi:10.1056/NEJMoa1607141
- Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97(3):1020–1031. doi:10.1210/jc.2011-2260
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Saremi A, Bahn GD, Reaven PD; Veterans Affairs Diabetes Trial (VADT). A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016; 39(3):448–454. doi:10.2337/dc15-2107
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed August 5, 2019.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9(2):194–205. doi:10.1111/j.1463-1326.2006.00704.x
- Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007; 30(2):389–394. doi:10.2337/dc06-1789
- Nauck M, Frid A, Hermansen K, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32(1):84–90. doi:10.2337/dc08-1355
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 5, 2019.
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010; 8:29. doi:10.1186/1478-7954-8-29
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37(suppl 3):S27–S38. doi:10.1016/S1262-3636(11)70962-4
- American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013; 61(11):2020–2026. doi:10.1111/jgs.12514
- Allen DW, Schroeder WA, Balog J. Observations on the chromatographic heterogeneity of normal adult and fetal human hemoglobin: a study of the effects of crystallization and chromatography on the heterogeneity and isoleucine content. J Amer Chem Soc 1958; 80(7):1628–1634. doi:10.1021/ja01540a030
- Huisman TH, Dozy AM. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med 1962; 60:302–319. pmid:14449875
- Rahbar S, Blumenfeld O, Ranney HM. Studies of an unusual hemoglobin in patients with diabetes mellitus. Biochem Biophys Res Commun 1969; 36(5):838–843. pmid:5808299
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S55–S64. doi:10.2337/dc18-S006
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241(19):2035–2038. pmid:430798
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352(9131):837–853. [Erratum in Lancet 1999; 354:602.] pmid:9742976
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- Hayward RA, Reaven PD, Wiitala WL, et al; VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372(23):2197–2206. doi:10.1056/NEJMoa1414266
- US Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. https://www.govinfo.gov/content/pkg/FR-2008-12-19/pdf/E8-30086.pdf. Accessed August 6, 2019.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311–322. doi:10.1056/NEJMoa1603827
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373(23):2247–2257. doi:10.1056/NEJMoa1509225
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377(13):1228–1239. doi:10.1056/NEJMoa1612917
- Cosmi F, Laini R, Nicolucci A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2017; 376(9):890. doi:10.1056/NEJMc1615712
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193):121–130. doi:10.1016/S0140-6736(19)31149-3
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373(22):2117–2128. doi:10.1056/NEJMoa1504720
- Wanner C, Inzucchi SE, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375(4):323–334. doi:10.1056/NEJMoa1515920
- Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7):644–657. doi:10.1056/NEJMoa1611925
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018. [Epub ahead of print] doi:10.1056/NEJMoa1812389
- Diabetes Prevention Program Research Group; Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009; 374(9702):1677–1686. doi:10.1016/S0140-6736(09)61457-4
- Marso SP, Bain SC, Consoli A, et al, for the SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375:1834–1844. doi:10.1056/NEJMoa1607141
- Bolinder J, Ljunggren Ö, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97(3):1020–1031. doi:10.1210/jc.2011-2260
- Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340:b4909. doi:10.1136/bmj.b4909
- Saremi A, Bahn GD, Reaven PD; Veterans Affairs Diabetes Trial (VADT). A link between hypoglycemia and progression of atherosclerosis in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2016; 39(3):448–454. doi:10.2337/dc15-2107
- American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(suppl 1):S73–S85. doi:10.2337/dc18-S008
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15):1577–1589. doi:10.1056/NEJMoa0806470
- US Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed August 5, 2019.
- Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9(2):194–205. doi:10.1111/j.1463-1326.2006.00704.x
- Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 2007; 30(2):389–394. doi:10.2337/dc06-1789
- Nauck M, Frid A, Hermansen K, et al; LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32(1):84–90. doi:10.2337/dc08-1355
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 5, 2019.
- Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010; 8:29. doi:10.1186/1478-7954-8-29
- Sinclair AJ, Paolisso G, Castro M, Bourdel-Marchasson I, Gadsby R, Rodriguez Mañas L; European Diabetes Working Party for Older People. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab 2011; 37(suppl 3):S27–S38. doi:10.1016/S1262-3636(11)70962-4
- American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus; Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013; 61(11):2020–2026. doi:10.1111/jgs.12514
KEY POINTS
- Some glucagon-like peptide 1 (GLP-1) receptor agonists have been shown to reduce cardiovascular risk, and liraglutide carries an indication for this use.
- The sodium-glucose cotransporter 2 inhibitors empaglifozin and canaglifozin carry indications to prevent cardiovascular death in patients with diabetes with established cardiovascular disease.
- Metformin, GLP-1 receptor agonists, and dipeptidyl peptidase 4 inhibitors are beneficial in terms of promoting weight loss—or at least not causing weight gain.
- Disadvantages and adverse effects of various drugs must also be considered.
DAPA-HF results transform dapagliflozin from antidiabetic to heart failure drug
PARIS – Treatment with the SGLT2 inhibitor dapagliflozin produced a statistically significant 27% drop in cardiovascular death or heart failure events in patients with existing heart failure with reduced ejection fraction and no diabetes, results that in a stroke changed the status of dapagliflozin from fundamentally a drug that treats diabetes to a drug that treats heart failure.

“Dapagliflozin offers a new approach to the treatment of heart failure with reduced ejection fraction” (HFrEF), John McMurray, MD, said at the annual congress of the European Society of Cardiology.
The results he reported from the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial showed statistically significant benefits when adding dapagliflozin to guideline-directed therapy for a list of outcomes that include a 17% drop in all-cause death compared with placebo, an 18% fall in cardiovascular death, and a 25% relative reduction in total heart failure hospitalizations plus cardiovascular deaths during a median follow-up of just over 18 months. The primary endpoint of the reduction in cardiovascular death, first heart failure hospitalization, or an urgent heart failure visit fell by 25% in the enrolled patients with diabetes (45% of the study population, all with type 2 diabetes), and by 27% in the remaining patients who had no diabetes, showing that the presence of diabetes had no impact on the heart failure benefit from dapagliflozin (Farxiga). The absolute reduction in the primary endpoint was about 5%, with a number needed to treat of 21 to prevent one primary endpoint during 18 months of treatment.
Dr. McMurray’s report of the primary endpoint as well as the finding that the drug was as effective in patients without diabetes as in those with diabetes were both met with loud applause by the packed congress audience.
The efficacy results also showed that 58% of patients on dapagliflozin had a clinically meaningful (5 point or greater) increase in their quality of life score on the Kansas City Cardiomyopathy Questionnaire after 8 months on treatment compared with a 51% rate in the placebo patients, a statistically significant difference.
The safety results showed no new signals for a drug that already has regulatory approval but was being used in a novel population. The rate of major hypoglycemia was virtually nonexistent, 0.2%, and identical in both treatment arms. All adverse events occurred at roughly equal rates in the dapagliflozin and placebo groups, with a 5% rate of adverse events leading to study discontinuation in both arms, and a serious adverse event rate of 38% in the dapaglifolzin patients and 42% in the placebo patients. The rate of worsening renal function was less than 2% in both arms and not statistically different.
“This is as close to a home run as you see in heart failure treatment,” commented Douglas L. Mann, MD, professor of medicine at Washington University, St. Louis, and a heart failure clinician and researcher.
DAPA-HF “is a landmark trial. It took a diabetes drug and used it in patients without diabetes, a concept that would have been considered outlandish 5 years ago. Scientifically it’s huge,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston.
The DAPA-HF results were another step in the remarkable journey toward heart failure intervention taken by the SGLT2 (sodium glucose cotransport 2) inhibitor class of drugs that includes dapagliflozin as well as canagliflozin (Invokana) and empagliflozin(Jardiance), a path that began 4 years ago with the report of empagliflozin’s unexpected efficacy for reducing cardiovascular death and heart failure hospitalizations in a large cardiovascular-safety study, EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28). Subsequent reports showed similar effects benefiting heart failure and survival for canagliflozin and dapagliflozin, and now with DAPA-HF the evidence extended the benefit to heart failure patients regardless of whether they have diabetes. Additional studies now in progress are exploring the same question for empagliflozin and canagliflozin.
The results from DAPA-HF are likely a class effect for all these SGLT2 inhibitors, suggested Dr. McMurray in a video interview, a view shared by several other experts. He cautioned clinicians against using dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) but without diabetes until this indication receives regulatory approval, and even then using dapagliflozin or other SGLT2 inhibitors this way may take some getting used to on the part of cardiologists and other clinicians.
“The results put dapagliflozin in the same league as [standard HFrEF drugs], but using it will require a shift in thinking. Most physicians will initially say “aren’t SGLT2 inhibitors used for treating diabetes?” Dr. Bhatt said.
“I’m sure most cardiologists are not familiar with the SGLT2 inhibitors; we’ll have to educate them,” conceded Dr. McMurray, professor of medical cardiology at the University of Glasgow. However, other aspects of dapagliflozin and this drug class in general may make the SGLT2 inhibitors particularly attractive and spur their use once labeling changes.
The adverse-event profile seen in DAPA-HF looked very “clean,” said Dr. Mann, especially compared with the other medical classes recommended in guidelines for patients with HFrEF: the angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid-receptor antagonists such as spironolactone, and the angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril-valsartan (Entresto). As used in DAPA-HF dapagliflozin also had the advantages of not needing dose titration or laboratory follow-up, as do several of these other drug classes.
“I think dapagliflozin will have a huge uptake [for treating HFrEF], because it will be easy for primary care physicians to prescribe. It will be easier to use than traditional heart failure medications.” Once approved for heart failure use, Dr. Mann predicted a standard dosing regimen for HFrEF patients of an ACE inhibitor, ARB or ARNI, a beta-blocker, a mineralocorticoid-receptor antagonist, and an SGLT2 inhibitor. He suggested that this large and cumbersome collection of medications could conceivably be simplified into a polypill.
He also saw a suggestion in the DAPA-HF results that combining dapagliflozin with the ARB valsartan might have similar efficacy to dapaglifozin plus sacubitril-valsartan, which might also help simplify heart failure treatment. In the trial, 11% of patients received sacubritril-valsartan, and the primary-endpoint reduction compared with placebo in this subgroup was 26%, compared with 25% for patients treated with an ACE inhibitor or ARB. Currently, labeling for sacubitril-valsartan calls for starting a patients on an ACE inhibitor or ARB, titrating them to a stable and effective dosage, and then stopping this regimen to switch to the ARNI. If dapagliflozin is also added, then a simpler approach would be to just start a patient on valsartan, optimize the dosage, and then start dapagliflozin and achieve the same benefit as from sacubitril-valsartan plus dapagliflozin. While an attractive scenario, it needs validation, Dr. Mann said in an interview.
One additional, notable finding from DAPA-HF was that the primary endpoint benefit appeared much stronger in patients with New York Heart Association class II heart failure at entry, two-thirds of the study population, compared with patients with class III or IV HFrEF. Compared with placebo the primary endpoint fell by 37% among the class II patients, a statistically significant difference, but by just 10% in the class III and IV patients, a reduction that was not significant compared with placebo. This too needs more study, commented Dr. Mann, as does the ways by which dapagliflozin and the other SGLT2 inhibitors benefit heart failure patients. Currently the ways by which dapagliflozin produced these results remain unknown.
DAPA-HF randomized a total of 4,744 patients at 410 sites in 20 countries. About 10% of enrolled patients were in the United States.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF. Dr. McMurray had no other relevant disclosures. Dr. Mann has been a consultant to Bristol-Myers Squibb, LivaNova, Novartis, and Tenaya Therapeutics. Dr. Bhatt has received research funding from AstraZeneca, and he has served as a consultant to or received research funding from several other companies.
PARIS – Treatment with the SGLT2 inhibitor dapagliflozin produced a statistically significant 27% drop in cardiovascular death or heart failure events in patients with existing heart failure with reduced ejection fraction and no diabetes, results that in a stroke changed the status of dapagliflozin from fundamentally a drug that treats diabetes to a drug that treats heart failure.

“Dapagliflozin offers a new approach to the treatment of heart failure with reduced ejection fraction” (HFrEF), John McMurray, MD, said at the annual congress of the European Society of Cardiology.
The results he reported from the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial showed statistically significant benefits when adding dapagliflozin to guideline-directed therapy for a list of outcomes that include a 17% drop in all-cause death compared with placebo, an 18% fall in cardiovascular death, and a 25% relative reduction in total heart failure hospitalizations plus cardiovascular deaths during a median follow-up of just over 18 months. The primary endpoint of the reduction in cardiovascular death, first heart failure hospitalization, or an urgent heart failure visit fell by 25% in the enrolled patients with diabetes (45% of the study population, all with type 2 diabetes), and by 27% in the remaining patients who had no diabetes, showing that the presence of diabetes had no impact on the heart failure benefit from dapagliflozin (Farxiga). The absolute reduction in the primary endpoint was about 5%, with a number needed to treat of 21 to prevent one primary endpoint during 18 months of treatment.
Dr. McMurray’s report of the primary endpoint as well as the finding that the drug was as effective in patients without diabetes as in those with diabetes were both met with loud applause by the packed congress audience.
The efficacy results also showed that 58% of patients on dapagliflozin had a clinically meaningful (5 point or greater) increase in their quality of life score on the Kansas City Cardiomyopathy Questionnaire after 8 months on treatment compared with a 51% rate in the placebo patients, a statistically significant difference.
The safety results showed no new signals for a drug that already has regulatory approval but was being used in a novel population. The rate of major hypoglycemia was virtually nonexistent, 0.2%, and identical in both treatment arms. All adverse events occurred at roughly equal rates in the dapagliflozin and placebo groups, with a 5% rate of adverse events leading to study discontinuation in both arms, and a serious adverse event rate of 38% in the dapaglifolzin patients and 42% in the placebo patients. The rate of worsening renal function was less than 2% in both arms and not statistically different.
“This is as close to a home run as you see in heart failure treatment,” commented Douglas L. Mann, MD, professor of medicine at Washington University, St. Louis, and a heart failure clinician and researcher.
DAPA-HF “is a landmark trial. It took a diabetes drug and used it in patients without diabetes, a concept that would have been considered outlandish 5 years ago. Scientifically it’s huge,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston.
The DAPA-HF results were another step in the remarkable journey toward heart failure intervention taken by the SGLT2 (sodium glucose cotransport 2) inhibitor class of drugs that includes dapagliflozin as well as canagliflozin (Invokana) and empagliflozin(Jardiance), a path that began 4 years ago with the report of empagliflozin’s unexpected efficacy for reducing cardiovascular death and heart failure hospitalizations in a large cardiovascular-safety study, EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28). Subsequent reports showed similar effects benefiting heart failure and survival for canagliflozin and dapagliflozin, and now with DAPA-HF the evidence extended the benefit to heart failure patients regardless of whether they have diabetes. Additional studies now in progress are exploring the same question for empagliflozin and canagliflozin.
The results from DAPA-HF are likely a class effect for all these SGLT2 inhibitors, suggested Dr. McMurray in a video interview, a view shared by several other experts. He cautioned clinicians against using dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) but without diabetes until this indication receives regulatory approval, and even then using dapagliflozin or other SGLT2 inhibitors this way may take some getting used to on the part of cardiologists and other clinicians.
“The results put dapagliflozin in the same league as [standard HFrEF drugs], but using it will require a shift in thinking. Most physicians will initially say “aren’t SGLT2 inhibitors used for treating diabetes?” Dr. Bhatt said.
“I’m sure most cardiologists are not familiar with the SGLT2 inhibitors; we’ll have to educate them,” conceded Dr. McMurray, professor of medical cardiology at the University of Glasgow. However, other aspects of dapagliflozin and this drug class in general may make the SGLT2 inhibitors particularly attractive and spur their use once labeling changes.
The adverse-event profile seen in DAPA-HF looked very “clean,” said Dr. Mann, especially compared with the other medical classes recommended in guidelines for patients with HFrEF: the angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid-receptor antagonists such as spironolactone, and the angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril-valsartan (Entresto). As used in DAPA-HF dapagliflozin also had the advantages of not needing dose titration or laboratory follow-up, as do several of these other drug classes.
“I think dapagliflozin will have a huge uptake [for treating HFrEF], because it will be easy for primary care physicians to prescribe. It will be easier to use than traditional heart failure medications.” Once approved for heart failure use, Dr. Mann predicted a standard dosing regimen for HFrEF patients of an ACE inhibitor, ARB or ARNI, a beta-blocker, a mineralocorticoid-receptor antagonist, and an SGLT2 inhibitor. He suggested that this large and cumbersome collection of medications could conceivably be simplified into a polypill.
He also saw a suggestion in the DAPA-HF results that combining dapagliflozin with the ARB valsartan might have similar efficacy to dapaglifozin plus sacubitril-valsartan, which might also help simplify heart failure treatment. In the trial, 11% of patients received sacubritril-valsartan, and the primary-endpoint reduction compared with placebo in this subgroup was 26%, compared with 25% for patients treated with an ACE inhibitor or ARB. Currently, labeling for sacubitril-valsartan calls for starting a patients on an ACE inhibitor or ARB, titrating them to a stable and effective dosage, and then stopping this regimen to switch to the ARNI. If dapagliflozin is also added, then a simpler approach would be to just start a patient on valsartan, optimize the dosage, and then start dapagliflozin and achieve the same benefit as from sacubitril-valsartan plus dapagliflozin. While an attractive scenario, it needs validation, Dr. Mann said in an interview.
One additional, notable finding from DAPA-HF was that the primary endpoint benefit appeared much stronger in patients with New York Heart Association class II heart failure at entry, two-thirds of the study population, compared with patients with class III or IV HFrEF. Compared with placebo the primary endpoint fell by 37% among the class II patients, a statistically significant difference, but by just 10% in the class III and IV patients, a reduction that was not significant compared with placebo. This too needs more study, commented Dr. Mann, as does the ways by which dapagliflozin and the other SGLT2 inhibitors benefit heart failure patients. Currently the ways by which dapagliflozin produced these results remain unknown.
DAPA-HF randomized a total of 4,744 patients at 410 sites in 20 countries. About 10% of enrolled patients were in the United States.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF. Dr. McMurray had no other relevant disclosures. Dr. Mann has been a consultant to Bristol-Myers Squibb, LivaNova, Novartis, and Tenaya Therapeutics. Dr. Bhatt has received research funding from AstraZeneca, and he has served as a consultant to or received research funding from several other companies.
PARIS – Treatment with the SGLT2 inhibitor dapagliflozin produced a statistically significant 27% drop in cardiovascular death or heart failure events in patients with existing heart failure with reduced ejection fraction and no diabetes, results that in a stroke changed the status of dapagliflozin from fundamentally a drug that treats diabetes to a drug that treats heart failure.

“Dapagliflozin offers a new approach to the treatment of heart failure with reduced ejection fraction” (HFrEF), John McMurray, MD, said at the annual congress of the European Society of Cardiology.
The results he reported from the DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trial showed statistically significant benefits when adding dapagliflozin to guideline-directed therapy for a list of outcomes that include a 17% drop in all-cause death compared with placebo, an 18% fall in cardiovascular death, and a 25% relative reduction in total heart failure hospitalizations plus cardiovascular deaths during a median follow-up of just over 18 months. The primary endpoint of the reduction in cardiovascular death, first heart failure hospitalization, or an urgent heart failure visit fell by 25% in the enrolled patients with diabetes (45% of the study population, all with type 2 diabetes), and by 27% in the remaining patients who had no diabetes, showing that the presence of diabetes had no impact on the heart failure benefit from dapagliflozin (Farxiga). The absolute reduction in the primary endpoint was about 5%, with a number needed to treat of 21 to prevent one primary endpoint during 18 months of treatment.
Dr. McMurray’s report of the primary endpoint as well as the finding that the drug was as effective in patients without diabetes as in those with diabetes were both met with loud applause by the packed congress audience.
The efficacy results also showed that 58% of patients on dapagliflozin had a clinically meaningful (5 point or greater) increase in their quality of life score on the Kansas City Cardiomyopathy Questionnaire after 8 months on treatment compared with a 51% rate in the placebo patients, a statistically significant difference.
The safety results showed no new signals for a drug that already has regulatory approval but was being used in a novel population. The rate of major hypoglycemia was virtually nonexistent, 0.2%, and identical in both treatment arms. All adverse events occurred at roughly equal rates in the dapagliflozin and placebo groups, with a 5% rate of adverse events leading to study discontinuation in both arms, and a serious adverse event rate of 38% in the dapaglifolzin patients and 42% in the placebo patients. The rate of worsening renal function was less than 2% in both arms and not statistically different.
“This is as close to a home run as you see in heart failure treatment,” commented Douglas L. Mann, MD, professor of medicine at Washington University, St. Louis, and a heart failure clinician and researcher.
DAPA-HF “is a landmark trial. It took a diabetes drug and used it in patients without diabetes, a concept that would have been considered outlandish 5 years ago. Scientifically it’s huge,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston.
The DAPA-HF results were another step in the remarkable journey toward heart failure intervention taken by the SGLT2 (sodium glucose cotransport 2) inhibitor class of drugs that includes dapagliflozin as well as canagliflozin (Invokana) and empagliflozin(Jardiance), a path that began 4 years ago with the report of empagliflozin’s unexpected efficacy for reducing cardiovascular death and heart failure hospitalizations in a large cardiovascular-safety study, EMPA-REG OUTCOME (N Engl J Med. 2015 Nov 26;373[22]:2117-28). Subsequent reports showed similar effects benefiting heart failure and survival for canagliflozin and dapagliflozin, and now with DAPA-HF the evidence extended the benefit to heart failure patients regardless of whether they have diabetes. Additional studies now in progress are exploring the same question for empagliflozin and canagliflozin.
The results from DAPA-HF are likely a class effect for all these SGLT2 inhibitors, suggested Dr. McMurray in a video interview, a view shared by several other experts. He cautioned clinicians against using dapagliflozin to treat patients with heart failure with reduced ejection fraction (HFrEF) but without diabetes until this indication receives regulatory approval, and even then using dapagliflozin or other SGLT2 inhibitors this way may take some getting used to on the part of cardiologists and other clinicians.
“The results put dapagliflozin in the same league as [standard HFrEF drugs], but using it will require a shift in thinking. Most physicians will initially say “aren’t SGLT2 inhibitors used for treating diabetes?” Dr. Bhatt said.
“I’m sure most cardiologists are not familiar with the SGLT2 inhibitors; we’ll have to educate them,” conceded Dr. McMurray, professor of medical cardiology at the University of Glasgow. However, other aspects of dapagliflozin and this drug class in general may make the SGLT2 inhibitors particularly attractive and spur their use once labeling changes.
The adverse-event profile seen in DAPA-HF looked very “clean,” said Dr. Mann, especially compared with the other medical classes recommended in guidelines for patients with HFrEF: the angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), beta-blockers, and mineralocorticoid-receptor antagonists such as spironolactone, and the angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril-valsartan (Entresto). As used in DAPA-HF dapagliflozin also had the advantages of not needing dose titration or laboratory follow-up, as do several of these other drug classes.
“I think dapagliflozin will have a huge uptake [for treating HFrEF], because it will be easy for primary care physicians to prescribe. It will be easier to use than traditional heart failure medications.” Once approved for heart failure use, Dr. Mann predicted a standard dosing regimen for HFrEF patients of an ACE inhibitor, ARB or ARNI, a beta-blocker, a mineralocorticoid-receptor antagonist, and an SGLT2 inhibitor. He suggested that this large and cumbersome collection of medications could conceivably be simplified into a polypill.
He also saw a suggestion in the DAPA-HF results that combining dapagliflozin with the ARB valsartan might have similar efficacy to dapaglifozin plus sacubitril-valsartan, which might also help simplify heart failure treatment. In the trial, 11% of patients received sacubritril-valsartan, and the primary-endpoint reduction compared with placebo in this subgroup was 26%, compared with 25% for patients treated with an ACE inhibitor or ARB. Currently, labeling for sacubitril-valsartan calls for starting a patients on an ACE inhibitor or ARB, titrating them to a stable and effective dosage, and then stopping this regimen to switch to the ARNI. If dapagliflozin is also added, then a simpler approach would be to just start a patient on valsartan, optimize the dosage, and then start dapagliflozin and achieve the same benefit as from sacubitril-valsartan plus dapagliflozin. While an attractive scenario, it needs validation, Dr. Mann said in an interview.
One additional, notable finding from DAPA-HF was that the primary endpoint benefit appeared much stronger in patients with New York Heart Association class II heart failure at entry, two-thirds of the study population, compared with patients with class III or IV HFrEF. Compared with placebo the primary endpoint fell by 37% among the class II patients, a statistically significant difference, but by just 10% in the class III and IV patients, a reduction that was not significant compared with placebo. This too needs more study, commented Dr. Mann, as does the ways by which dapagliflozin and the other SGLT2 inhibitors benefit heart failure patients. Currently the ways by which dapagliflozin produced these results remain unknown.
DAPA-HF randomized a total of 4,744 patients at 410 sites in 20 countries. About 10% of enrolled patients were in the United States.
DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF. Dr. McMurray had no other relevant disclosures. Dr. Mann has been a consultant to Bristol-Myers Squibb, LivaNova, Novartis, and Tenaya Therapeutics. Dr. Bhatt has received research funding from AstraZeneca, and he has served as a consultant to or received research funding from several other companies.
REPORTING FROM THE ESC CONGRESS 2019
Key clinical point: Dapagliflozin produced multiple, statistically significant benefits in heart failure patients on top of guideline-directed therapy.
Major finding: The study’s primary endpoint fell by a statistically significant 27% with dapagliflozin compared with placebo in patients without diabetes.
Study details: DAPA-HF, a multinational study with 4,744 patients at 410 sites.
Disclosures: DAPA-HF was sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). AstraZeneca paid Glasgow University to cover Dr. McMurray’s salary during the time he spent working as principal investigator of DAPA-HF.
Click for Credit: Fasting rules for surgery; Biomarkers for PSA vs OA; more
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Ticagrelor: Modest benefit, bigger bleed risk in diabetes plus stable CAD
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
PARIS – , though they also had more major bleeding events than patients receiving placebo plus aspirin.
The subset of patients who had received prior percutaneous coronary intervention (PCI) stood to benefit more from extended dual antiplatelet therapy (DAPT), according to clinical trial results presented to an overflow crowd at the annual congress of the European Society of Cardiology.
Findings from the full study, named The Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study (THEMIS), and from the PCI subgroup analysis were published concurrently with the presentation (N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2).
“This strategy of long-term dual antiplatelet therapy may be beneficial in selected patients at low risk of bleeding, but at high risk of ischemic events,” said the study’s co-principal investigator Deepak Bhatt, MD, professor of medicine at Harvard Medical School, Boston, and executive director of interventional cardiology programs at Boston’s Brigham and Women’s Hospital. In a video interview, he hypothesized that “prior PCI may serve as a sort of ‘stress test’ for bleeding,” thus identifying a subset of patients who might benefit from long-term DAPT.
Ischemic events, the primary efficacy outcome of THEMIS, occurred in 7.7% of patients taking the P2Y12 receptor antagonist ticagrelor and 8.5% of those receiving placebo, for a hazard ratio of 0.90 favoring ticagrelor (P = .04). Ischemic events included cardiovascular deaths, myocardial infarctions (MIs), and stroke.
Looking at secondary endpoints, Dr. Bhatt said that there was no difference in cardiovascular deaths between study arms, but that ischemic strokes, all MIs, and ST segment elevation MIs were all less common for patients taking ticagrelor. All-cause mortality was similar between study groups.
Though ischemic events dropped, “This benefit was achieved at the expense of more bleeding,” said Dr. Bhatt. Major bleeding, the primary safety outcome, was seen in 2.2% of those taking ticagrelor and 1.0% of the placebo group, for a hazard ratio of 2.32 (P less than .001). Dr. Bhatt and his collaborators used the Thrombolysis in Myocardial Infarction (TIMI) criteria for major bleeding for ascertainment of this outcome.
Intracranial hemorrhage was also more common for patients on ticagrelor, though incidence was low and the absolute difference was small between groups. This complication occurred in 0.7% of ticagrelor patients and 0.5% of placebo patients, yielding a hazard ratio of 1.71 (P = .0005). “This excess wasn’t in spontaneous or procedural intracranial bleeding, but rather in traumatic intracranial hemorrhage,” said Dr. Bhatt.
Fatal bleeds affected just 0.2% of those on ticagrelor and 0.1% of those receiving placebo; this difference wasn’t statistically significant.
THEMIS was an international multisite double-blind, placebo-controlled study randomizing 19,220 patients 1:1 to receive aspirin, plus placebo (N = 9,601) or ticagrelor (N = 9,619). Patients were followed for a median of 39.9 months; those with previous myocardial infarction or stroke were excluded. Patients had to be at least 50 years old and on anti-hyperglycemic medications for at least 6 months to participate. Patients in the overall study had a baseline age of 66 years, and 31% were female. Most patients were white (71%).
Stable coronary artery disease (CAD) was defined by having any of a previous history of PCI, coronary artery bypass grafting, or angiographically documented stenosis of at least 50% in at least one coronary artery.
During the study period, Dr. Bhatt explained, ticagrelor dosage was reduced from 90 to 60 mg daily as other studies yielded data about improved safety and tolerability without compromise in efficacy at the lower ticagrelor dose.
Permanent treatment discontinuation was common, but more common in patients taking ticagrelor, compared with placebo (34.5% vs. 25.4%). The most frequent reasons for ticagrelor discontinuation were dyspnea and bleeding. All patients who were randomized, save those at a study site that was closed before unblinding, were included in the modified intention-to-treat population for calculation of efficacy outcomes for both THEMIS and THEMIS-PCI.
Given the large number of patients who discontinued the study drug, an estimation was made of the number of events that would have occurred had patients remained in the trial, and outcomes were calculated using these estimations to account for missing data.
Safety outcomes were calculated by including all patients who received at least one dose of a study drug.
An exploratory composite outcome of “net irreversible harm” included all-cause death, myocardial infarction, and stroke, but also fatal bleeding and intracranial hemorrhage. In the full study population, this outcome was seen in 10.1% of the placebo group and 10.8% of the placebo group, for a nonsignificant hazard ratio of 0.93, said Dr. Bhatt.
An additional composite pre-specified exploratory outcome included acute limb ischemia or major amputation; here, the HR of 0.45 favored ticagrelor.
Dr. Bhatt made the point that these pragmatic, patient-centered outcomes are valuable tools when weighing the potential risks and benefits of therapy for a particular patient, and provide a discussion point for individualized, shared decision making.
Results of a pre-specified subgroup analysis of the 58% of THEMIS participants (n = 5,558) with prior PCI were presented by THEMIS’ co-principal investigator, Philippe Gabriel Steg, MD, of the University of Paris and the French National Institute of Health and Medical Research.
“In the history of PCI subgroup, 92% of patients had a history of receiving a stent, and 61% had received at least one drug-eluting stent,” said Dr. Steg.
Patients with PCI saw a slightly greater reduction in relative risk for ischemic events when they received ticagrelor, compared with placebo; the PCI group had a HR of 0.85 for ischemic events (P = .013), compared with a HR of 0.98 for those with no PCI history (P = .76). This meant that ticagrelor DAPT’s efficacy as measured by the primary endpoint of ischemic events lost significance when the non-PCI group was evaluated (P = .76, with P for interaction between the groups of .16).
Some secondary endpoints showed statistical significance for the interaction between PCI status and study drug status. These included the composite outcome of all-cause death, MI, or stroke (P for interaction, .021), and another “mega-composite ischemia” outcome that folded in major amputation of vascular etiology along with all-cause death, MI, and stroke (P = .023).
Looking at bleeding endpoints, there was no significant difference between the groups for TIMI major bleeding, the primary safety endpoint. Patients in the full study cohort as well as the PCI subgroup had significantly more TIMI major bleeding on ticagrelor.
Bleeding measured by Bleeding Academic Research Consortium (BARC) criteria was a secondary endpoint, and the P for interaction just reached statistical significance for the aggregate of all levels of BARC bleeding.
“But the two observations I would draw your attention to are the fact that in patients with a history of PCI, fatal bleeding occurred in the same number of patients in each group – 6 patients in each group,” added Dr. Steg. “And even more importantly, intracranial hemorrhage occurred in 33 patients in the ticagrelor group and 31 patients in the placebo group for patients with a history of PCI, whereas it was 37 and 15 for patients without a history of PCI.” This yielded a significant P value for the interaction of .036.
The exploratory net clinical benefit score favored the PCI group, for a P for interaction of .012. Dr. Steg also shared an analysis showing a net benefit for ticagrelor vs. placebo as a function of the time elapsed between PCI and trial randomization, showing patient benefit to 6 years post drug initiation for the PCI group.
“The subgroup analysis of THEMIS PCI was pre-specified, from a large, clinically meaningful population; it’s plausible and it can be easily explained from the action of dual antiplatelet therapy, and it shows a net benefit,” Dr. Steg said.
The discussant for the presentations was Colin Baigent, , and he wasn’t convinced by the THEMIS-PCI data. He pointed out that looking at the absolute numbers overall for THEMIS yields an absolute benefit of about 8 per 1,000 participants, and an absolute risk of about 12 per 1,000 participants.
“The natural instinct is to then go to the subgroups and try to find people who will see a net benefit,” he said. “Why pick out ‘history of PCI?’” among the 18 pre-specified subgroups, he asked, noting that there was not significant evidence of heterogeneity of hazard ratios among the subgroups.
Overall, “The main results of THEMIS are consistent” with previous investigations into the benefits of ticagrelor DAPT, showing modest efficacy at the expense of a two-fold rise in major bleeding events, said Dr. Baigent, professor of epidemiology at the University of Oxford (England).
The THEMIS study and the subpopulation analysis were funded by AstraZeneca, which markets ticagrelor. Dr. Bhatt reported financial relationships with AstraZeneca and multiple other pharmaceutical companies. In addition to reporting a financial relationship with AstraZeneca, Dr. Steg also reported relationships with multiple pharmaceutical companies. Dr. Baigent reported a financial relationship with Boehringer Engelheim.
Source: Steg PG et al. N Engl J Med. 2019 Sep 1: DOI: 10.1056/NEJMoa1908077; Bhatt DL et al.Lancet. 2019 Sep 1: DOI:https://doi.org/10.1016/S0140-6736(19)31887-2)
AT THE ESC CONGRESS 2019
Zoledronate maintains bone loss after denosumab discontinuation
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH