User login
AI Tool Helps Detect, Differentiate Pancreatic Lesions During Endoscopic Ultrasound
PHILADELPHIA —
This was a transatlantic collaborative effort involving researchers in Portugal, Spain, the United States, and Brazil, and the AI tool “works on different platforms and different devices,” Miguel Mascarenhas, MD, PhD, with Centro Hospitalar Universitário de São João, Porto, Portugal, said in a presentation at the annual meeting of the American College of Gastroenterology.
Mascarenhas noted that pancreatic cystic lesions (PCLs) are a common incidental finding during imaging and are differentiated by whether they’re mucinous PCLs (M-PCLs) or non-mucinous PCLs (NM-PCLs). The malignancy risk is almost exclusive of PCL with a mucinous phenotype.
Pancreatic solid lesions are also prevalent, and differentiation is challenging. Pancreatic ductal adenocarcinoma (P-DAC) is the most common pancreatic solid lesion and has a poor prognosis because of late-stage disease at diagnosis. Pancreatic neuroendocrine tumors (P-NETs) are less common but have malignant potential.
EUS is the “gold standard” for pancreatic lesion evaluation, but its diagnostic accuracy is suboptimal, particularly for lesions < 10 mm, Mascarenhas noted.
With an eye toward improving diagnostic accuracy, he and colleagues developed a convolutional neural network for detecting and differentiating cystic (M-PCL and NM-PCL) and solid (P-DAC and P-NET) pancreatic lesions.
They leveraged data from 378 EUS exams with 126,000 still images — 19,528 M-PCL, 8175 NM-PCL, 64,286 P-DAC, 29,153 P-NET, and 4858 normal pancreas images.
The AI tool demonstrated 99.1% accuracy for identifying normal pancreatic tissue, and it showed 99% and 99.8% accuracy for M-PCL and NM-PCL, respectively.
For pancreatic solid lesions, P-DAC and P-NET were distinguished with 94% accuracy, with 98.7% and 83.6% sensitivity for P-DAC and P-NET, respectively.
Real-Time Validation Next
“AI is delivering promising results throughout medicine, but particularly in gastroenterology, which is one of the most fertile areas of AI research. This comes mostly from the deployment of deep-learning models, most of them convolutional neural networks, which are highly efficient for image analysis,” Mascarenhas told attendees.
This is the “first worldwide convolutional neural network” capable of detecting and differentiating both cystic and solid pancreatic lesions. The use of a large dataset from four centers in two continents helps minimize the impact of demographic bias, Mascarenhas added.
The study is based on still images, not full videos, he noted. As a next step, the team is conducting a multicenter study focused on real-time clinical validation of the model during EUS procedures.
“AI has the potential to improve the diagnostic accuracy of endoscopic ultrasound. We’re just on the tip of the iceberg. There is enormous potential to harness AI, and we welcome all the groups that might want to join our research,” Mascarenhas said.
Brennan Spiegel, MD, MSHS, AGAF, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, who wasn’t involved in the study, is optimistic about emerging applications for AI.
“AI holds incredible promise in gastroenterology, especially for diagnosing complex pancreatic lesions where early, accurate differentiation can be lifesaving,” Spiegel said in an interview.
“This study’s high accuracy across diverse datasets is encouraging; however, as a retrospective analysis, it leaves the real-time clinical impact still to be proven. Prospective studies will be essential to confirm AI’s role in enhancing our diagnostic capabilities,” Spiegel cautioned.
“More generally, AI is rapidly transforming gastroenterology by enhancing our ability to detect, differentiate, and monitor conditions with unprecedented precision. From improving early cancer detection to guiding complex diagnostic procedures, AI stands to become an invaluable tool that complements clinical expertise. As we refine these technologies, the potential for AI to elevate both diagnostic accuracy and patient outcomes in GI is truly remarkable,” Spiegel said.
The study had no specific funding. Mascarenhas and Spiegel have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
PHILADELPHIA —
This was a transatlantic collaborative effort involving researchers in Portugal, Spain, the United States, and Brazil, and the AI tool “works on different platforms and different devices,” Miguel Mascarenhas, MD, PhD, with Centro Hospitalar Universitário de São João, Porto, Portugal, said in a presentation at the annual meeting of the American College of Gastroenterology.
Mascarenhas noted that pancreatic cystic lesions (PCLs) are a common incidental finding during imaging and are differentiated by whether they’re mucinous PCLs (M-PCLs) or non-mucinous PCLs (NM-PCLs). The malignancy risk is almost exclusive of PCL with a mucinous phenotype.
Pancreatic solid lesions are also prevalent, and differentiation is challenging. Pancreatic ductal adenocarcinoma (P-DAC) is the most common pancreatic solid lesion and has a poor prognosis because of late-stage disease at diagnosis. Pancreatic neuroendocrine tumors (P-NETs) are less common but have malignant potential.
EUS is the “gold standard” for pancreatic lesion evaluation, but its diagnostic accuracy is suboptimal, particularly for lesions < 10 mm, Mascarenhas noted.
With an eye toward improving diagnostic accuracy, he and colleagues developed a convolutional neural network for detecting and differentiating cystic (M-PCL and NM-PCL) and solid (P-DAC and P-NET) pancreatic lesions.
They leveraged data from 378 EUS exams with 126,000 still images — 19,528 M-PCL, 8175 NM-PCL, 64,286 P-DAC, 29,153 P-NET, and 4858 normal pancreas images.
The AI tool demonstrated 99.1% accuracy for identifying normal pancreatic tissue, and it showed 99% and 99.8% accuracy for M-PCL and NM-PCL, respectively.
For pancreatic solid lesions, P-DAC and P-NET were distinguished with 94% accuracy, with 98.7% and 83.6% sensitivity for P-DAC and P-NET, respectively.
Real-Time Validation Next
“AI is delivering promising results throughout medicine, but particularly in gastroenterology, which is one of the most fertile areas of AI research. This comes mostly from the deployment of deep-learning models, most of them convolutional neural networks, which are highly efficient for image analysis,” Mascarenhas told attendees.
This is the “first worldwide convolutional neural network” capable of detecting and differentiating both cystic and solid pancreatic lesions. The use of a large dataset from four centers in two continents helps minimize the impact of demographic bias, Mascarenhas added.
The study is based on still images, not full videos, he noted. As a next step, the team is conducting a multicenter study focused on real-time clinical validation of the model during EUS procedures.
“AI has the potential to improve the diagnostic accuracy of endoscopic ultrasound. We’re just on the tip of the iceberg. There is enormous potential to harness AI, and we welcome all the groups that might want to join our research,” Mascarenhas said.
Brennan Spiegel, MD, MSHS, AGAF, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, who wasn’t involved in the study, is optimistic about emerging applications for AI.
“AI holds incredible promise in gastroenterology, especially for diagnosing complex pancreatic lesions where early, accurate differentiation can be lifesaving,” Spiegel said in an interview.
“This study’s high accuracy across diverse datasets is encouraging; however, as a retrospective analysis, it leaves the real-time clinical impact still to be proven. Prospective studies will be essential to confirm AI’s role in enhancing our diagnostic capabilities,” Spiegel cautioned.
“More generally, AI is rapidly transforming gastroenterology by enhancing our ability to detect, differentiate, and monitor conditions with unprecedented precision. From improving early cancer detection to guiding complex diagnostic procedures, AI stands to become an invaluable tool that complements clinical expertise. As we refine these technologies, the potential for AI to elevate both diagnostic accuracy and patient outcomes in GI is truly remarkable,” Spiegel said.
The study had no specific funding. Mascarenhas and Spiegel have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
PHILADELPHIA —
This was a transatlantic collaborative effort involving researchers in Portugal, Spain, the United States, and Brazil, and the AI tool “works on different platforms and different devices,” Miguel Mascarenhas, MD, PhD, with Centro Hospitalar Universitário de São João, Porto, Portugal, said in a presentation at the annual meeting of the American College of Gastroenterology.
Mascarenhas noted that pancreatic cystic lesions (PCLs) are a common incidental finding during imaging and are differentiated by whether they’re mucinous PCLs (M-PCLs) or non-mucinous PCLs (NM-PCLs). The malignancy risk is almost exclusive of PCL with a mucinous phenotype.
Pancreatic solid lesions are also prevalent, and differentiation is challenging. Pancreatic ductal adenocarcinoma (P-DAC) is the most common pancreatic solid lesion and has a poor prognosis because of late-stage disease at diagnosis. Pancreatic neuroendocrine tumors (P-NETs) are less common but have malignant potential.
EUS is the “gold standard” for pancreatic lesion evaluation, but its diagnostic accuracy is suboptimal, particularly for lesions < 10 mm, Mascarenhas noted.
With an eye toward improving diagnostic accuracy, he and colleagues developed a convolutional neural network for detecting and differentiating cystic (M-PCL and NM-PCL) and solid (P-DAC and P-NET) pancreatic lesions.
They leveraged data from 378 EUS exams with 126,000 still images — 19,528 M-PCL, 8175 NM-PCL, 64,286 P-DAC, 29,153 P-NET, and 4858 normal pancreas images.
The AI tool demonstrated 99.1% accuracy for identifying normal pancreatic tissue, and it showed 99% and 99.8% accuracy for M-PCL and NM-PCL, respectively.
For pancreatic solid lesions, P-DAC and P-NET were distinguished with 94% accuracy, with 98.7% and 83.6% sensitivity for P-DAC and P-NET, respectively.
Real-Time Validation Next
“AI is delivering promising results throughout medicine, but particularly in gastroenterology, which is one of the most fertile areas of AI research. This comes mostly from the deployment of deep-learning models, most of them convolutional neural networks, which are highly efficient for image analysis,” Mascarenhas told attendees.
This is the “first worldwide convolutional neural network” capable of detecting and differentiating both cystic and solid pancreatic lesions. The use of a large dataset from four centers in two continents helps minimize the impact of demographic bias, Mascarenhas added.
The study is based on still images, not full videos, he noted. As a next step, the team is conducting a multicenter study focused on real-time clinical validation of the model during EUS procedures.
“AI has the potential to improve the diagnostic accuracy of endoscopic ultrasound. We’re just on the tip of the iceberg. There is enormous potential to harness AI, and we welcome all the groups that might want to join our research,” Mascarenhas said.
Brennan Spiegel, MD, MSHS, AGAF, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, who wasn’t involved in the study, is optimistic about emerging applications for AI.
“AI holds incredible promise in gastroenterology, especially for diagnosing complex pancreatic lesions where early, accurate differentiation can be lifesaving,” Spiegel said in an interview.
“This study’s high accuracy across diverse datasets is encouraging; however, as a retrospective analysis, it leaves the real-time clinical impact still to be proven. Prospective studies will be essential to confirm AI’s role in enhancing our diagnostic capabilities,” Spiegel cautioned.
“More generally, AI is rapidly transforming gastroenterology by enhancing our ability to detect, differentiate, and monitor conditions with unprecedented precision. From improving early cancer detection to guiding complex diagnostic procedures, AI stands to become an invaluable tool that complements clinical expertise. As we refine these technologies, the potential for AI to elevate both diagnostic accuracy and patient outcomes in GI is truly remarkable,” Spiegel said.
The study had no specific funding. Mascarenhas and Spiegel have declared no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM ACG 2024
Baveno VI Criteria Appear Cost-Effective for Detecting Varices in Cirrhosis
Compared with endoscopy, , according to new research.
Although upper gastrointestinal endoscopy continues to be the gold standard for detecting varices, the Baveno VI criteria combine liver stiffness and platelet count values to rule out high-risk varices, which can save on endoscopy costs.
“The Baveno VI criteria can reduce the need for endoscopies in patients with cirrhosis, but it is important to ascertain if they are also cost-effective,” said senior author Emmanuel Tsochatzis, MD, professor of hepatology at the University College London Institute for Liver and Digestive Health and Royal Free Hospital in London.
“Our findings confirm that the application of these criteria is highly cost-effective, and given the fact that they are also safe, should be considered for widespread implementation,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Baveno VI Criteria Analysis
On the basis of the Baveno VI Consensus, endoscopy screening can be avoided in patients with compensated advanced chronic liver disease and Child-Pugh A cirrhosis who have a platelet count > 150,000/mm3 and a liver stiffness measurement < 20 kPa.
In addition, expanded Baveno VI criteria have suggested optimized cut-off values to avoid even more endoscopies — at a platelet value of > 110,000/mm3 and a liver stiffness < 25 kPa.
Previous research indicates that the expanded criteria could avoid double the number of endoscopies, the authors wrote, with a risk of missing high-risk varices in 1.6% of patients with the criteria and 0.6% of overall study participants. Both criteria have been validated in large groups of patients with compensated cirrhosis of different etiologies, but the cost-effectiveness hasn’t been analyzed.
Dr. Tsochatzis and colleagues created an analytical decision model to estimate the costs and benefits of using the Baveno VI criteria as compared with endoscopy as the standard of care among a hypothetical cohort of 1000 patients with Child-Pugh A cirrhosis. The research team looked at costs and clinical outcomes based on the United Kingdom National Health Service perspective at 1 year from diagnosis and then estimated the expected costs and outcomes at 5 years and 20 years, including factors such as liver disease progression and variceal bleeding.
As part of the model, the Baveno VI criteria were implemented at annual screenings with targeted endoscopy for patients who met the criteria, as compared with endoscopy as a biannual screening using esophagogastroduodenoscopy for everyone.
In general, the Baveno VI criteria were cost-effective compared with endoscopy in all analyses, including all time points, as well as deterministic and probabilistic sensitivity analyses. The cost of using the criteria was £67 per patient, as compared with £411 per patient for esophagogastroduodenoscopy.
For the 1000 patients, the criteria produced 0.16 additional quality-adjusted life years (QALYs) per patient at an incremental cost of £326, or about $443, over 5 years. This resulted in an incremental cost-effectiveness ratio (ICER) of £2081, or $2830, per additional QALY gained.
In addition, the incremental net monetary benefit of the Baveno VI criteria was £2808, or $3819, over 5 years per patient.
The results were also consistent and cost-effective in Canada and Spain using relevant cost inputs from those countries. In Canada, the ICER per QALY estimates were €3535, or $3712, over 5 years and €4610, or $4841, over 20 years. In Spain, the ICER per QALY estimates were €1966, or $2064, over 5 years and €2225, or $2336, over 20 years.
Baveno VI Considerations
Despite the small risk of false negatives, the Baveno VI criteria could avoid unnecessary endoscopies and provide significant cost savings, the study authors wrote.
“It should be mentioned, however, that sparing endoscopies could result in missing the incidental detection of esophageal and gastric cancers, particularly in patients with higher risk, such as those who misuse alcohol,” Dr. Tsochatzis said.
Future studies could investigate ways to broaden the applicability of the Baveno VI criteria to other patient subgroups, identify optimal cut-off points, and incorporate patients with systemic therapies.
“Baveno VI criteria can be safely used to avoid endoscopy in a substantial proportion of patients with compensated cirrhosis,” said Wayne Bai, MBChB, a gastroenterologist at Waikato Hospital and the University of Auckland in New Zealand.
Dr. Bai, who wasn’t involved with this study, has researched the Baveno VI criteria and participated in Baveno VII criteria meetings. In an analysis of more than two dozen studies, he and colleagues found that the Baveno VI criteria had a pooled 99% negative predictive value for ruling out high-risk varices and weren’t affected by the cause of cirrhosis. However, expanding the criteria had suboptimal performance in some cases.
“The progressive change in approach to the management of compensated cirrhosis, progressively focusing on treating portal hypertension with beta-blockers independently of the presence of varices, might render these criteria less relevant,” he said.
The authors were supported by funds from the National Institute for Health and Care Research Applied Research Collaboration North Thames, the Instituto de Salud Carlos III, and the European Union’s European Regional Development Fund and European Social Fund. Dr Bai reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Compared with endoscopy, , according to new research.
Although upper gastrointestinal endoscopy continues to be the gold standard for detecting varices, the Baveno VI criteria combine liver stiffness and platelet count values to rule out high-risk varices, which can save on endoscopy costs.
“The Baveno VI criteria can reduce the need for endoscopies in patients with cirrhosis, but it is important to ascertain if they are also cost-effective,” said senior author Emmanuel Tsochatzis, MD, professor of hepatology at the University College London Institute for Liver and Digestive Health and Royal Free Hospital in London.
“Our findings confirm that the application of these criteria is highly cost-effective, and given the fact that they are also safe, should be considered for widespread implementation,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Baveno VI Criteria Analysis
On the basis of the Baveno VI Consensus, endoscopy screening can be avoided in patients with compensated advanced chronic liver disease and Child-Pugh A cirrhosis who have a platelet count > 150,000/mm3 and a liver stiffness measurement < 20 kPa.
In addition, expanded Baveno VI criteria have suggested optimized cut-off values to avoid even more endoscopies — at a platelet value of > 110,000/mm3 and a liver stiffness < 25 kPa.
Previous research indicates that the expanded criteria could avoid double the number of endoscopies, the authors wrote, with a risk of missing high-risk varices in 1.6% of patients with the criteria and 0.6% of overall study participants. Both criteria have been validated in large groups of patients with compensated cirrhosis of different etiologies, but the cost-effectiveness hasn’t been analyzed.
Dr. Tsochatzis and colleagues created an analytical decision model to estimate the costs and benefits of using the Baveno VI criteria as compared with endoscopy as the standard of care among a hypothetical cohort of 1000 patients with Child-Pugh A cirrhosis. The research team looked at costs and clinical outcomes based on the United Kingdom National Health Service perspective at 1 year from diagnosis and then estimated the expected costs and outcomes at 5 years and 20 years, including factors such as liver disease progression and variceal bleeding.
As part of the model, the Baveno VI criteria were implemented at annual screenings with targeted endoscopy for patients who met the criteria, as compared with endoscopy as a biannual screening using esophagogastroduodenoscopy for everyone.
In general, the Baveno VI criteria were cost-effective compared with endoscopy in all analyses, including all time points, as well as deterministic and probabilistic sensitivity analyses. The cost of using the criteria was £67 per patient, as compared with £411 per patient for esophagogastroduodenoscopy.
For the 1000 patients, the criteria produced 0.16 additional quality-adjusted life years (QALYs) per patient at an incremental cost of £326, or about $443, over 5 years. This resulted in an incremental cost-effectiveness ratio (ICER) of £2081, or $2830, per additional QALY gained.
In addition, the incremental net monetary benefit of the Baveno VI criteria was £2808, or $3819, over 5 years per patient.
The results were also consistent and cost-effective in Canada and Spain using relevant cost inputs from those countries. In Canada, the ICER per QALY estimates were €3535, or $3712, over 5 years and €4610, or $4841, over 20 years. In Spain, the ICER per QALY estimates were €1966, or $2064, over 5 years and €2225, or $2336, over 20 years.
Baveno VI Considerations
Despite the small risk of false negatives, the Baveno VI criteria could avoid unnecessary endoscopies and provide significant cost savings, the study authors wrote.
“It should be mentioned, however, that sparing endoscopies could result in missing the incidental detection of esophageal and gastric cancers, particularly in patients with higher risk, such as those who misuse alcohol,” Dr. Tsochatzis said.
Future studies could investigate ways to broaden the applicability of the Baveno VI criteria to other patient subgroups, identify optimal cut-off points, and incorporate patients with systemic therapies.
“Baveno VI criteria can be safely used to avoid endoscopy in a substantial proportion of patients with compensated cirrhosis,” said Wayne Bai, MBChB, a gastroenterologist at Waikato Hospital and the University of Auckland in New Zealand.
Dr. Bai, who wasn’t involved with this study, has researched the Baveno VI criteria and participated in Baveno VII criteria meetings. In an analysis of more than two dozen studies, he and colleagues found that the Baveno VI criteria had a pooled 99% negative predictive value for ruling out high-risk varices and weren’t affected by the cause of cirrhosis. However, expanding the criteria had suboptimal performance in some cases.
“The progressive change in approach to the management of compensated cirrhosis, progressively focusing on treating portal hypertension with beta-blockers independently of the presence of varices, might render these criteria less relevant,” he said.
The authors were supported by funds from the National Institute for Health and Care Research Applied Research Collaboration North Thames, the Instituto de Salud Carlos III, and the European Union’s European Regional Development Fund and European Social Fund. Dr Bai reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Compared with endoscopy, , according to new research.
Although upper gastrointestinal endoscopy continues to be the gold standard for detecting varices, the Baveno VI criteria combine liver stiffness and platelet count values to rule out high-risk varices, which can save on endoscopy costs.
“The Baveno VI criteria can reduce the need for endoscopies in patients with cirrhosis, but it is important to ascertain if they are also cost-effective,” said senior author Emmanuel Tsochatzis, MD, professor of hepatology at the University College London Institute for Liver and Digestive Health and Royal Free Hospital in London.
“Our findings confirm that the application of these criteria is highly cost-effective, and given the fact that they are also safe, should be considered for widespread implementation,” he said.
The study was published online in Clinical Gastroenterology and Hepatology.
Baveno VI Criteria Analysis
On the basis of the Baveno VI Consensus, endoscopy screening can be avoided in patients with compensated advanced chronic liver disease and Child-Pugh A cirrhosis who have a platelet count > 150,000/mm3 and a liver stiffness measurement < 20 kPa.
In addition, expanded Baveno VI criteria have suggested optimized cut-off values to avoid even more endoscopies — at a platelet value of > 110,000/mm3 and a liver stiffness < 25 kPa.
Previous research indicates that the expanded criteria could avoid double the number of endoscopies, the authors wrote, with a risk of missing high-risk varices in 1.6% of patients with the criteria and 0.6% of overall study participants. Both criteria have been validated in large groups of patients with compensated cirrhosis of different etiologies, but the cost-effectiveness hasn’t been analyzed.
Dr. Tsochatzis and colleagues created an analytical decision model to estimate the costs and benefits of using the Baveno VI criteria as compared with endoscopy as the standard of care among a hypothetical cohort of 1000 patients with Child-Pugh A cirrhosis. The research team looked at costs and clinical outcomes based on the United Kingdom National Health Service perspective at 1 year from diagnosis and then estimated the expected costs and outcomes at 5 years and 20 years, including factors such as liver disease progression and variceal bleeding.
As part of the model, the Baveno VI criteria were implemented at annual screenings with targeted endoscopy for patients who met the criteria, as compared with endoscopy as a biannual screening using esophagogastroduodenoscopy for everyone.
In general, the Baveno VI criteria were cost-effective compared with endoscopy in all analyses, including all time points, as well as deterministic and probabilistic sensitivity analyses. The cost of using the criteria was £67 per patient, as compared with £411 per patient for esophagogastroduodenoscopy.
For the 1000 patients, the criteria produced 0.16 additional quality-adjusted life years (QALYs) per patient at an incremental cost of £326, or about $443, over 5 years. This resulted in an incremental cost-effectiveness ratio (ICER) of £2081, or $2830, per additional QALY gained.
In addition, the incremental net monetary benefit of the Baveno VI criteria was £2808, or $3819, over 5 years per patient.
The results were also consistent and cost-effective in Canada and Spain using relevant cost inputs from those countries. In Canada, the ICER per QALY estimates were €3535, or $3712, over 5 years and €4610, or $4841, over 20 years. In Spain, the ICER per QALY estimates were €1966, or $2064, over 5 years and €2225, or $2336, over 20 years.
Baveno VI Considerations
Despite the small risk of false negatives, the Baveno VI criteria could avoid unnecessary endoscopies and provide significant cost savings, the study authors wrote.
“It should be mentioned, however, that sparing endoscopies could result in missing the incidental detection of esophageal and gastric cancers, particularly in patients with higher risk, such as those who misuse alcohol,” Dr. Tsochatzis said.
Future studies could investigate ways to broaden the applicability of the Baveno VI criteria to other patient subgroups, identify optimal cut-off points, and incorporate patients with systemic therapies.
“Baveno VI criteria can be safely used to avoid endoscopy in a substantial proportion of patients with compensated cirrhosis,” said Wayne Bai, MBChB, a gastroenterologist at Waikato Hospital and the University of Auckland in New Zealand.
Dr. Bai, who wasn’t involved with this study, has researched the Baveno VI criteria and participated in Baveno VII criteria meetings. In an analysis of more than two dozen studies, he and colleagues found that the Baveno VI criteria had a pooled 99% negative predictive value for ruling out high-risk varices and weren’t affected by the cause of cirrhosis. However, expanding the criteria had suboptimal performance in some cases.
“The progressive change in approach to the management of compensated cirrhosis, progressively focusing on treating portal hypertension with beta-blockers independently of the presence of varices, might render these criteria less relevant,” he said.
The authors were supported by funds from the National Institute for Health and Care Research Applied Research Collaboration North Thames, the Instituto de Salud Carlos III, and the European Union’s European Regional Development Fund and European Social Fund. Dr Bai reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
ACG/ASGE Task Force Identifies 19 Indicators for Achieving Quality GI Endoscopy
— most of which have a performance target > 98%, implying they should be achieved in nearly every case.
The task force’s work was published online in The American Journal of Gastroenterology.
“The purpose of this paper is to delineate all of the steps that the endoscopist should be thinking about before they perform any endoscopy,” task force member Nicholas Shaheen, MD, MPH, Division of Gastroenterology and Hepatology, the University of North Carolina at Chapel Hill, said in an interview.
“Some of these are straightforward — for instance, did we get informed consent? Others are more nuanced — did we appropriately plan for sedation for the procedure, or did we give the right antibiotics before the procedure to prevent an infectious complication after?
“While the vast majority of endoscopists do these measures with every procedure, especially in unusual circumstances or when the procedure is an emergency, they can be overlooked. Having these quality indicators listed in one place should minimize these omissions,” Dr. Shaheen said.
Four Priority Indicators
The update represents the third iteration of the ACG/ASGE quality indicators on GI endoscopic procedures, the most recent of which was published nearly a decade ago.
As in preceding versions, the task force “prioritized indicators that have wide-ranging clinical implications and have been validated in clinical studies.” There are 19 in total, divided into three time periods: Preprocedure (8), intraprocedure (4), and postprocedure (7).
While all 19 indicators are intended to serve as a framework for continual quality improvement efforts among endoscopists and units, the task force recognized a subset of 4 they identified as being a particular priority:
- Frequency with which endoscopy is performed for an indication that is included in a published standard list of appropriate indications and the indication is documented (performance target > 95%)
- Frequency with which prophylactic antibiotics are administered for appropriate indications (performance target > 98%)
- Frequency with which a plan for the management of antithrombotic therapy is formulated and documented before the procedure (performance target = 95%)
- Frequency with which adverse events are documented (performance target > 98%)
Room for Improvement
There remains a lack of compliance with some of these indicators, the task force said.
“Procedures are still performed for questionable indications, adverse events are not always captured and documented, and communication between the endoscopist and patient and/or involved clinicians is sometimes lacking.
“For these reasons, strict attention to the quality indicators in this document and an active plan for improvement in areas of measured deficiency should be a central pillar of the successful practice of endoscopy,” they wrote.
The task force advised that quality improvement efforts initially focus on the four priority indicators and then progress to include other indicators once it is determined that endoscopists are performing above recommended thresholds, either at baseline or after corrective interventions.
Reached for comment, Ashwin N. Ananthakrishnan, MD, MPH, AGAF, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts, said in an interview that these updated recommendations are “important and commonsense standard procedures that should be followed for all procedures.”
“We recognize endoscopic evaluation plays an important role in the assessment of GI illnesses, but there are also both risks and costs to this as a diagnostic and therapeutic intervention. Thus, it is important to make sure these standards are met, to optimize the outcomes of our patients,” said Dr. Ananthakrishnan, who was not involved in this work.
In a separate statement, the American Gastroenterological Association affirmed that is committed to supporting gastroenterologists in providing high-quality care via improved patients outcomes, increased efficiency and cost-effectiveness. AGA encouraged GIs to visit gastro.org/quality to learn more and find quality measures on topics including Barrett’s esophagus, inflammatory bowel disease, acute pancreatitis, and gastric intestinal metaplasia.
This work had no financial support. Dr. Shaheen and Dr. Ananthakrishnan disclosed having no relevant competing interests.
A version of this article first appeared on Medscape.com.
— most of which have a performance target > 98%, implying they should be achieved in nearly every case.
The task force’s work was published online in The American Journal of Gastroenterology.
“The purpose of this paper is to delineate all of the steps that the endoscopist should be thinking about before they perform any endoscopy,” task force member Nicholas Shaheen, MD, MPH, Division of Gastroenterology and Hepatology, the University of North Carolina at Chapel Hill, said in an interview.
“Some of these are straightforward — for instance, did we get informed consent? Others are more nuanced — did we appropriately plan for sedation for the procedure, or did we give the right antibiotics before the procedure to prevent an infectious complication after?
“While the vast majority of endoscopists do these measures with every procedure, especially in unusual circumstances or when the procedure is an emergency, they can be overlooked. Having these quality indicators listed in one place should minimize these omissions,” Dr. Shaheen said.
Four Priority Indicators
The update represents the third iteration of the ACG/ASGE quality indicators on GI endoscopic procedures, the most recent of which was published nearly a decade ago.
As in preceding versions, the task force “prioritized indicators that have wide-ranging clinical implications and have been validated in clinical studies.” There are 19 in total, divided into three time periods: Preprocedure (8), intraprocedure (4), and postprocedure (7).
While all 19 indicators are intended to serve as a framework for continual quality improvement efforts among endoscopists and units, the task force recognized a subset of 4 they identified as being a particular priority:
- Frequency with which endoscopy is performed for an indication that is included in a published standard list of appropriate indications and the indication is documented (performance target > 95%)
- Frequency with which prophylactic antibiotics are administered for appropriate indications (performance target > 98%)
- Frequency with which a plan for the management of antithrombotic therapy is formulated and documented before the procedure (performance target = 95%)
- Frequency with which adverse events are documented (performance target > 98%)
Room for Improvement
There remains a lack of compliance with some of these indicators, the task force said.
“Procedures are still performed for questionable indications, adverse events are not always captured and documented, and communication between the endoscopist and patient and/or involved clinicians is sometimes lacking.
“For these reasons, strict attention to the quality indicators in this document and an active plan for improvement in areas of measured deficiency should be a central pillar of the successful practice of endoscopy,” they wrote.
The task force advised that quality improvement efforts initially focus on the four priority indicators and then progress to include other indicators once it is determined that endoscopists are performing above recommended thresholds, either at baseline or after corrective interventions.
Reached for comment, Ashwin N. Ananthakrishnan, MD, MPH, AGAF, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts, said in an interview that these updated recommendations are “important and commonsense standard procedures that should be followed for all procedures.”
“We recognize endoscopic evaluation plays an important role in the assessment of GI illnesses, but there are also both risks and costs to this as a diagnostic and therapeutic intervention. Thus, it is important to make sure these standards are met, to optimize the outcomes of our patients,” said Dr. Ananthakrishnan, who was not involved in this work.
In a separate statement, the American Gastroenterological Association affirmed that is committed to supporting gastroenterologists in providing high-quality care via improved patients outcomes, increased efficiency and cost-effectiveness. AGA encouraged GIs to visit gastro.org/quality to learn more and find quality measures on topics including Barrett’s esophagus, inflammatory bowel disease, acute pancreatitis, and gastric intestinal metaplasia.
This work had no financial support. Dr. Shaheen and Dr. Ananthakrishnan disclosed having no relevant competing interests.
A version of this article first appeared on Medscape.com.
— most of which have a performance target > 98%, implying they should be achieved in nearly every case.
The task force’s work was published online in The American Journal of Gastroenterology.
“The purpose of this paper is to delineate all of the steps that the endoscopist should be thinking about before they perform any endoscopy,” task force member Nicholas Shaheen, MD, MPH, Division of Gastroenterology and Hepatology, the University of North Carolina at Chapel Hill, said in an interview.
“Some of these are straightforward — for instance, did we get informed consent? Others are more nuanced — did we appropriately plan for sedation for the procedure, or did we give the right antibiotics before the procedure to prevent an infectious complication after?
“While the vast majority of endoscopists do these measures with every procedure, especially in unusual circumstances or when the procedure is an emergency, they can be overlooked. Having these quality indicators listed in one place should minimize these omissions,” Dr. Shaheen said.
Four Priority Indicators
The update represents the third iteration of the ACG/ASGE quality indicators on GI endoscopic procedures, the most recent of which was published nearly a decade ago.
As in preceding versions, the task force “prioritized indicators that have wide-ranging clinical implications and have been validated in clinical studies.” There are 19 in total, divided into three time periods: Preprocedure (8), intraprocedure (4), and postprocedure (7).
While all 19 indicators are intended to serve as a framework for continual quality improvement efforts among endoscopists and units, the task force recognized a subset of 4 they identified as being a particular priority:
- Frequency with which endoscopy is performed for an indication that is included in a published standard list of appropriate indications and the indication is documented (performance target > 95%)
- Frequency with which prophylactic antibiotics are administered for appropriate indications (performance target > 98%)
- Frequency with which a plan for the management of antithrombotic therapy is formulated and documented before the procedure (performance target = 95%)
- Frequency with which adverse events are documented (performance target > 98%)
Room for Improvement
There remains a lack of compliance with some of these indicators, the task force said.
“Procedures are still performed for questionable indications, adverse events are not always captured and documented, and communication between the endoscopist and patient and/or involved clinicians is sometimes lacking.
“For these reasons, strict attention to the quality indicators in this document and an active plan for improvement in areas of measured deficiency should be a central pillar of the successful practice of endoscopy,” they wrote.
The task force advised that quality improvement efforts initially focus on the four priority indicators and then progress to include other indicators once it is determined that endoscopists are performing above recommended thresholds, either at baseline or after corrective interventions.
Reached for comment, Ashwin N. Ananthakrishnan, MD, MPH, AGAF, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, both in Boston, Massachusetts, said in an interview that these updated recommendations are “important and commonsense standard procedures that should be followed for all procedures.”
“We recognize endoscopic evaluation plays an important role in the assessment of GI illnesses, but there are also both risks and costs to this as a diagnostic and therapeutic intervention. Thus, it is important to make sure these standards are met, to optimize the outcomes of our patients,” said Dr. Ananthakrishnan, who was not involved in this work.
In a separate statement, the American Gastroenterological Association affirmed that is committed to supporting gastroenterologists in providing high-quality care via improved patients outcomes, increased efficiency and cost-effectiveness. AGA encouraged GIs to visit gastro.org/quality to learn more and find quality measures on topics including Barrett’s esophagus, inflammatory bowel disease, acute pancreatitis, and gastric intestinal metaplasia.
This work had no financial support. Dr. Shaheen and Dr. Ananthakrishnan disclosed having no relevant competing interests.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Automated ERCP Report Card Offers High Accuracy, Minimal Work
offering a real-time gauge of both individual- and institutional-level quality indicators, according to the developers.
The tool boasts an accuracy level exceeding 96%, integrates with multiple electronic health records, and requires minimal additional work time, reported Anmol Singh, MD, of TriStar Centennial Medical Center, Nashville, Tennessee, and colleagues.
“Implementation of quality indicator tracking remains difficult due to the complexity of ERCP as compared with other endoscopic procedures, resulting in significant limitations in the extraction and synthesis of these data,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Manual extraction methods such as self-assessment forms and chart reviews are both time intensive and error prone, and current automated extraction methods, such as natural language processing, can require substantial resources to implement and undesirably complicate the endoscopy work flow.”
To overcome these challenges, Dr. Singh and colleagues designed an analytics tool that automatically collects ERCP quality indicators from endoscopy reports with “minimal input” from the endoscopist, and is compatible with “any electronic reporting system.”
Development relied upon endoscopy records from 2,146 ERCPs performed by 12 endoscopists at four facilities. The most common reason for ERCP was choledocholithiasis, followed by malignant and benign biliary stricture. Most common procedures were stent placement and sphincterotomy.
Data were aggregated in a Health Level–7 (HL-7) interface, an international standard system that enables compatibility between different types of electronic health records. Some inputs were entered by the performing endoscopist via drop-down menus.
Next, data were shifted into an analytics suite, which evaluated quality indicators, including cannulation difficulty and success rate, and administration of post-ERCP pancreatitis prophylaxis.
Manual review showed that this approach yielded an accuracy of 96.5%-100%.
Beyond this high level of accuracy, Dr. Singh and colleagues described several reasons why their tool may be superior to previous attempts at an automated ERCP report card.
“Our HL-7–based tool offers several advantages, including versatility via compatibility with multiple types of commercial reporting software and flexibility in customizing the type and aesthetic of the data displayed,” they wrote. “These features improve the user interface, keep costs down, and allow for integration into smaller or nonacademic practice settings.”
They also highlighted how the tool measures quality in relation to procedure indication and difficulty at the provider level.
“Unlike in colonoscopy, where metrics such as adenoma detection rate can be ubiquitously applied to all screening procedures, the difficulty and risk profile of ERCP is inextricably dependent on patient and procedural factors such as indication of the procedure, history of interventions, or history of altered anatomy,” Dr. Singh and colleagues wrote. “Prior studies have shown that both the cost-effectiveness and complication rates of procedures are influenced by procedural indication and complexity. As such, benchmarking an individual provider’s performance necessarily requires the correct procedural context.”
With further optimization, this tool can be integrated into various types of existing endoscopy reporting software at a reasonable cost, and with minimal impact on routine work flow, the investigators concluded.
The investigators disclosed relationships with AbbVie, Boston Scientific, Organon, and others.
offering a real-time gauge of both individual- and institutional-level quality indicators, according to the developers.
The tool boasts an accuracy level exceeding 96%, integrates with multiple electronic health records, and requires minimal additional work time, reported Anmol Singh, MD, of TriStar Centennial Medical Center, Nashville, Tennessee, and colleagues.
“Implementation of quality indicator tracking remains difficult due to the complexity of ERCP as compared with other endoscopic procedures, resulting in significant limitations in the extraction and synthesis of these data,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Manual extraction methods such as self-assessment forms and chart reviews are both time intensive and error prone, and current automated extraction methods, such as natural language processing, can require substantial resources to implement and undesirably complicate the endoscopy work flow.”
To overcome these challenges, Dr. Singh and colleagues designed an analytics tool that automatically collects ERCP quality indicators from endoscopy reports with “minimal input” from the endoscopist, and is compatible with “any electronic reporting system.”
Development relied upon endoscopy records from 2,146 ERCPs performed by 12 endoscopists at four facilities. The most common reason for ERCP was choledocholithiasis, followed by malignant and benign biliary stricture. Most common procedures were stent placement and sphincterotomy.
Data were aggregated in a Health Level–7 (HL-7) interface, an international standard system that enables compatibility between different types of electronic health records. Some inputs were entered by the performing endoscopist via drop-down menus.
Next, data were shifted into an analytics suite, which evaluated quality indicators, including cannulation difficulty and success rate, and administration of post-ERCP pancreatitis prophylaxis.
Manual review showed that this approach yielded an accuracy of 96.5%-100%.
Beyond this high level of accuracy, Dr. Singh and colleagues described several reasons why their tool may be superior to previous attempts at an automated ERCP report card.
“Our HL-7–based tool offers several advantages, including versatility via compatibility with multiple types of commercial reporting software and flexibility in customizing the type and aesthetic of the data displayed,” they wrote. “These features improve the user interface, keep costs down, and allow for integration into smaller or nonacademic practice settings.”
They also highlighted how the tool measures quality in relation to procedure indication and difficulty at the provider level.
“Unlike in colonoscopy, where metrics such as adenoma detection rate can be ubiquitously applied to all screening procedures, the difficulty and risk profile of ERCP is inextricably dependent on patient and procedural factors such as indication of the procedure, history of interventions, or history of altered anatomy,” Dr. Singh and colleagues wrote. “Prior studies have shown that both the cost-effectiveness and complication rates of procedures are influenced by procedural indication and complexity. As such, benchmarking an individual provider’s performance necessarily requires the correct procedural context.”
With further optimization, this tool can be integrated into various types of existing endoscopy reporting software at a reasonable cost, and with minimal impact on routine work flow, the investigators concluded.
The investigators disclosed relationships with AbbVie, Boston Scientific, Organon, and others.
offering a real-time gauge of both individual- and institutional-level quality indicators, according to the developers.
The tool boasts an accuracy level exceeding 96%, integrates with multiple electronic health records, and requires minimal additional work time, reported Anmol Singh, MD, of TriStar Centennial Medical Center, Nashville, Tennessee, and colleagues.
“Implementation of quality indicator tracking remains difficult due to the complexity of ERCP as compared with other endoscopic procedures, resulting in significant limitations in the extraction and synthesis of these data,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy. “Manual extraction methods such as self-assessment forms and chart reviews are both time intensive and error prone, and current automated extraction methods, such as natural language processing, can require substantial resources to implement and undesirably complicate the endoscopy work flow.”
To overcome these challenges, Dr. Singh and colleagues designed an analytics tool that automatically collects ERCP quality indicators from endoscopy reports with “minimal input” from the endoscopist, and is compatible with “any electronic reporting system.”
Development relied upon endoscopy records from 2,146 ERCPs performed by 12 endoscopists at four facilities. The most common reason for ERCP was choledocholithiasis, followed by malignant and benign biliary stricture. Most common procedures were stent placement and sphincterotomy.
Data were aggregated in a Health Level–7 (HL-7) interface, an international standard system that enables compatibility between different types of electronic health records. Some inputs were entered by the performing endoscopist via drop-down menus.
Next, data were shifted into an analytics suite, which evaluated quality indicators, including cannulation difficulty and success rate, and administration of post-ERCP pancreatitis prophylaxis.
Manual review showed that this approach yielded an accuracy of 96.5%-100%.
Beyond this high level of accuracy, Dr. Singh and colleagues described several reasons why their tool may be superior to previous attempts at an automated ERCP report card.
“Our HL-7–based tool offers several advantages, including versatility via compatibility with multiple types of commercial reporting software and flexibility in customizing the type and aesthetic of the data displayed,” they wrote. “These features improve the user interface, keep costs down, and allow for integration into smaller or nonacademic practice settings.”
They also highlighted how the tool measures quality in relation to procedure indication and difficulty at the provider level.
“Unlike in colonoscopy, where metrics such as adenoma detection rate can be ubiquitously applied to all screening procedures, the difficulty and risk profile of ERCP is inextricably dependent on patient and procedural factors such as indication of the procedure, history of interventions, or history of altered anatomy,” Dr. Singh and colleagues wrote. “Prior studies have shown that both the cost-effectiveness and complication rates of procedures are influenced by procedural indication and complexity. As such, benchmarking an individual provider’s performance necessarily requires the correct procedural context.”
With further optimization, this tool can be integrated into various types of existing endoscopy reporting software at a reasonable cost, and with minimal impact on routine work flow, the investigators concluded.
The investigators disclosed relationships with AbbVie, Boston Scientific, Organon, and others.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Advanced Tissue Resection in Gastroenterology: Indications, Role, and Outcomes
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 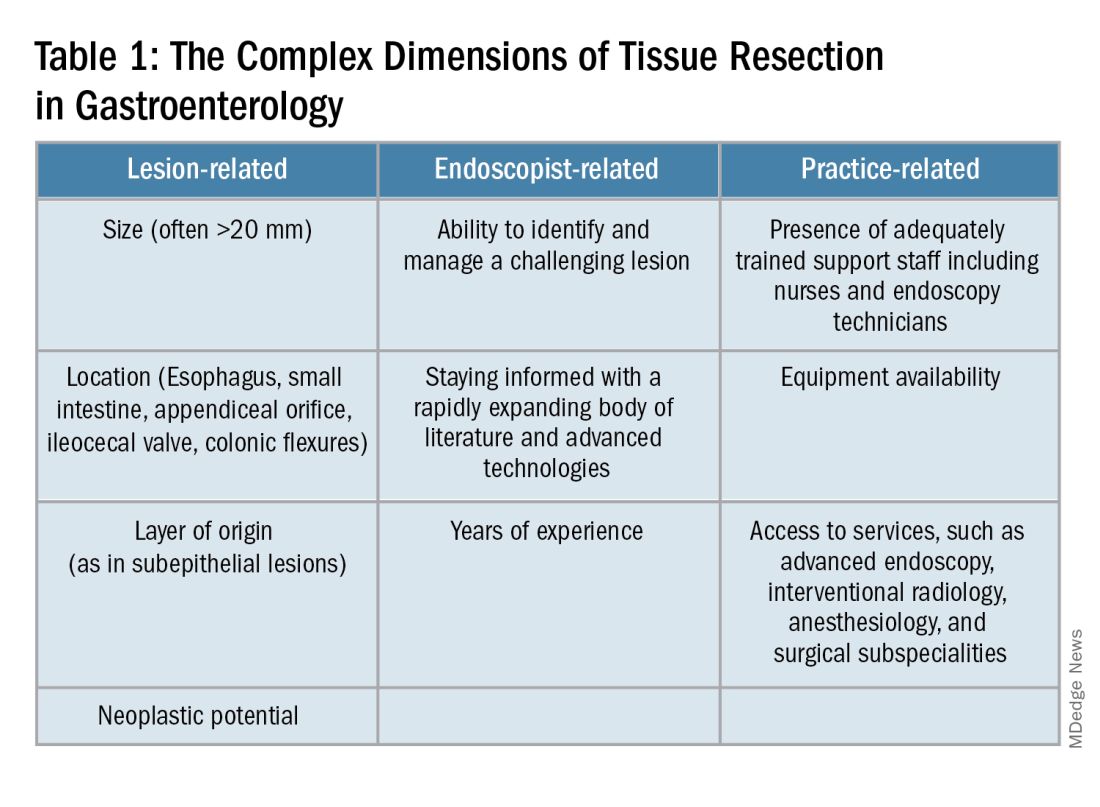
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1).
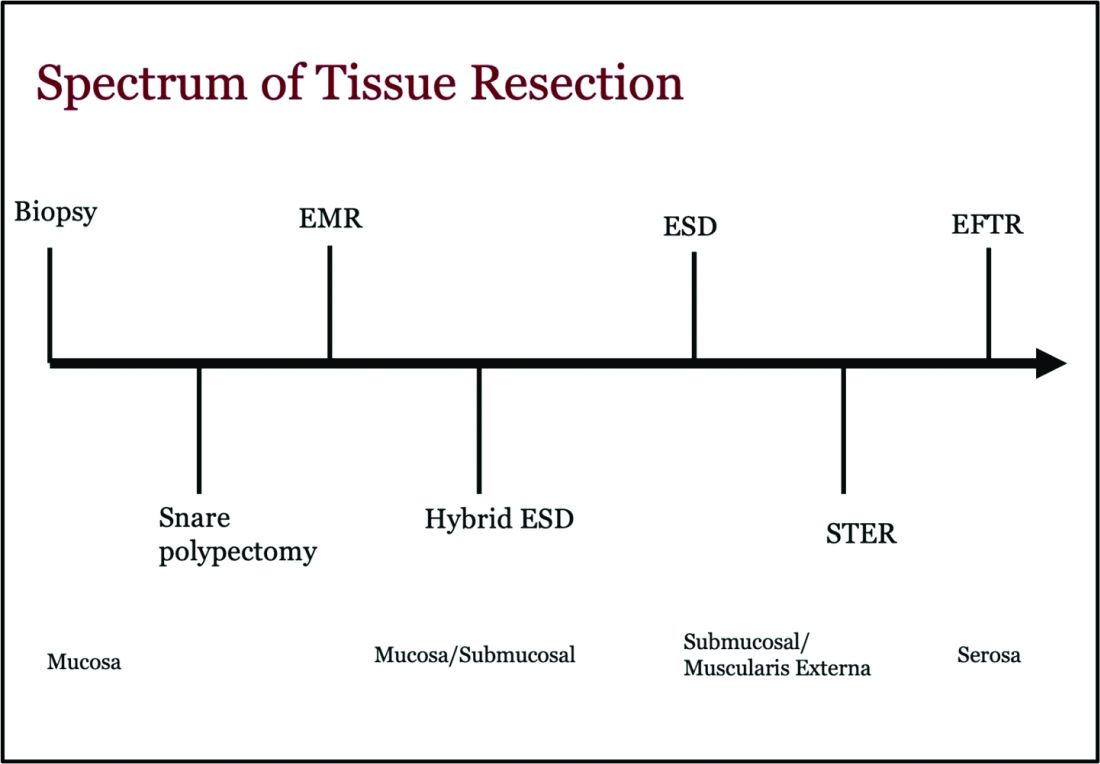
ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1).

ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
Endoscopists are often faced with unique challenges in the management and resection of various gastrointestinal tract lesions. These challenges could be lesion-related, endoscopist-related, or practice-related (see Table 1). (ATR). Not only does this organ-sparing approach offer a less invasive alternative to surgery, but it has also proved to have outcomes comparable to those of surgical standard of practice in specific scenarios. 
When Do You Refer to an Advanced Endoscopist?
One of the most critical steps in caring for patients with complex lesions is the ability to accurately determine whether a referral to an advanced endoscopist is warranted. The initial assessment of a lesion should always involve a careful assessment that risk stratifies the lesion depending on the location, size, neoplastic potential, and the feasibility of standard endoscopic resection compared to the need for surgical input.
A practical example in the case of colonic polyps is highlighted by the American Gastroenterology Association (AGA) guidelines recommending the referral of patients with polyps’ size ≥ 20 mm, challenging polypectomy location, or recurrent polyp at a prior polypectomy site to an endoscopic referral center.1 In the case of subepithelial lesions without endoscopic characteristics of benign etiology (i.e., lipomas, pancreatic rests, etc.), the threshold for referral to advanced endoscopists for further diagnostic testing by means of endoscopic ultrasonography or for therapeutic ATR should be lower.
Endoscopic tissue resection follows a spectrum, which often involves deeper layers of the gastrointestinal tract (GIT) as we progress along this spectrum (see Figure 1).

ATR, a term encompassing a variety of endoscopic techniques ranging from endoscopic mucosal resection to full thickness resection, has gained traction over the last years given the ability to effectively remove various lesions in a precise time and cost-effective manner while maintaining the integrity of the GIT and avoiding major surgery. The indications for ATR vary depending on the technique, but generally include the presence of large or poorly positioned lesions, particularly in high-risk areas of the GIT such as the esophagus and small intestine, lesions extending beyond the mucosal layer or originating from deeper layers, and when en bloc resection of select lesions is necessary.
For providers referring patients for ATR, we recommend a few important endoscopic pearls when caring for these patients.
1) Biopsy the lesion if there is concern for malignancy — While some studies have noted increased fibrosis during endoscopic submucosal dissection (ESD) and some guidelines recommend against biopsies pre ESD, we believe that when there is high pretest probability for malignancy, a biopsy should be obtained. This should involve the area that is most concerning for malignancy (at the margin or center).2
2) While marking a lesion with tattoo is helpful for surgical planning and for lesions difficult to locate endoscopically, we stress the importance of placing tattoos 3 to 5 centimeters distal to the lesion and avoiding tattooing the lesion itself, which has been shown to induce fibrosis and can make resection challenging. Based on an international Delphi consensus, expert recommendations on when and how to endoscopically tattoo a lesion can be instrumental in adequately localizing the lesion, allowing for endoscopic resection, and preventing unnecessary surgeries.3
3) If you encounter a lesion that you are not sure can be resected safely and efficaciously, we recommend against attempting resection that may result in partial resection. This can also induce fibrosis and scarring and limit future attempts at resection.
Endoscopic Mucosal Resection (EMR)
EMR is currently utilized for curative treatment of a wide array of GIT lesions limited to the mucosal layer, whether metaplastic, dysplastic, or even in cases with early mucosal cancer, where the risk of submucosal and lymphatic invasion is minimal.4 This makes EMR a versatile and proven therapy, often serving as the first-line treatment for many GIT lesions.
EMR has various techniques that could be categorized into suction or non-suction (lift and cut) techniques. In the suction technique, devices like multiband mucosectomy (MBM) are commonly used, especially in nodular Barrett’s dysplasia, forming a pseudopolyp for subsequent resection. The procedure is characterized by its safety, efficacy, and cost-effectiveness, contributing to its widespread adoption in clinical practice. In the lift and cut approach, a submucosal injection is utilized to separate the muscularis propria from the lesion, thereby reducing the risk of perforation. Different solutions, such as normal saline, hypertonic saline, 50% dextrose, or proprietary submucosal injection solutions, are employed for submucosal injection.5
The non-suction technique using a snare to resect polyps after injection is more often used in colonic and small intestinal EMR. Resection can be done via thermal energy in the form of cut or coagulation; however, there is rising data on the use of piecemeal cold snare resection for select flat polyps of the colon.6 There is also promising data on the role of underwater EMR, a common technique employed for colonic lesions, particularly if the lesion does not lift well with submucosal injection.7
Adverse events associated with EMR include bleeding (7%-8%) and perforation (0.9%-2%).8-9 Adequate submucosal fluid injection is crucial to prevent perforations. However, the main limitation of EMR is the piecemeal nature of resections for lesions larger than 20 mm, leading to compromised histopathologic evaluation for complete excision, especially in cases with superficial submucosal invasion (SMI). This can result in residual or recurrent tissue, reportedly 8% to 20%.10 Despite this limitation, EMR remains a reliable strategy, and recurrent lesions are generally manageable through repeat sessions. The importance of EMR as a therapeutic modality lies in its role in addressing lesions with favorable characteristics, where the risk of SMI is low.
Endoscopic Submucosal Dissection (ESD)
ESD is an evolving technique that can be utilized for submucosal lesions of the GIT, lesions not amenable to EMR due to submucosal fibrosis, when en bloc removal of a lesion is needed for accurate histopathological diagnosis, and when other techniques fail.11-12
ESD was only recently adopted in the United States, requires specialized training, and usually is a lengthier procedure than EMR.13 Compared to EMR, it has higher en bloc resection rates and lower recurrence rates, making it curative for lesions with superficial SMI and favorable histologic features.4,14 The safety profile of ESD appears favorable, with most of the adverse events managed successfully by endoscopic methods. Major complications include intraoperative and delayed perforation, intraoperative and delayed bleeding, aspiration pneumonia, thromboembolism, and stricture formation in the case of circumferential lesions.15
Despite being technically challenging, ESD may provide a cost-effective long-term solution by avoiding surgery, reducing the need for additional interventions by minimizing recurrence rates. Given the technical complexity of ESD, particularly the submucosal dissection portion, techniques such as hybrid ESD developed. Hybrid ESD combines snaring with circumferential mucosal incision and partial submucosal dissection. Although it promises shorter procedure times, reduced complication rates like perforation, and similar recurrence rates compared to traditional ESD, studies have shown lower success rates in en bloc resection.16-17
Both EMR and ESD are considered complementary strategies, and the choice between them should be dictated by lesion characteristics, patient preferences, and local expertise.
Submucosal Tunneling Endoscopic Resection (STER)
STER has emerged as a well-established technique for the endoscopic resection of GI subepithelial tumors (SETs) originating from the muscularis propria layer. The standard STER procedure involves a series of steps including submucosal elevation proximal to the SET, mucosotomy, creation of a submucosal tunnel, dissection of the SET within the tunnel, enucleation from the deep muscle layer, and subsequent specimen retrieval followed by mucosal closure.
This technique is typically recommended for SETs smaller than 3.5 cm, particularly those located in the mid or distal esophagus, cardia, or along the greater curvature of the gastric body.18 However, STER may pose technical challenges for larger SETs or lesions in anatomically difficult locations, where surgical resection is recommended instead.19 Notably, recent large-scale meta-analyses have showcased the favorable complete resection and en bloc resection rates of STER in treating GI SETs.20
Endoscopic Full Thickness Resection (EFTR)
EFTR has emerged as a valuable technique in the endoscopic management of gastrointestinal lesions, particularly SETs and lesions not amenable to EMR or ESD due to fibrosis. EFTR involves the resection of all layers of the GIT from mucosa to serosa, and therefore is well-suited for SETs arising from the muscularis propria (MP).20
EFTR entails two main concepts: tissue resection and complete defect closure. Conventional EFTR consists of several steps, which include mucosal and submucosal pre-cutting, circumferential incision, and dissection through the MP or serosa. This results in a full thickness defect, for which closure of the wall defect is achieved using standard endoscopic clips or a combination of clips and endoloops or endoscopic suturing.21 For lesions less than 2 cm, EFTR can be performed in a single step using a cap-mounted full thickness resection device (FTRD). This results in deployment of over-the-scope clip over the target lesion followed by snaring the lesions above the clip.21
Location of the SET generally dictates the specific modality of ATR. For example, esophageal SETs may be more amenable to STER given that the lesion typically runs parallel with the lumen of the tubular esophagus, which allows for easier dissection without the need of full or partial retroflexion. While gastric SETs can be resected with STER, it may be challenging and more effectively addressed with EFTR, particularly when the entire lesion can be grasped into the full-thickness resection device.22 Limited data exists for duodenal EFTR, and colorectal SETs closure is particularly challenging.
Conclusion
It is key to emphasize that ATR cannot be safely established in practice without the incorporation of a multidisciplinary team (surgeons, radiologists, etc.), specialized tools, and trained personnel. This requires dedicated endoscopic rooms, careful patient selection, and a comprehensive approach to patient care before, during, and after these procedures.
Moreover, it is important to note that some patients may require post-procedure hospitalization for observation to ensure no early complications are encountered. Optimal surveillance strategies after ATR rely heavily on the potential for residual or recurrent disease, underlying pathology, and the expertise of the advanced endoscopist. As the field continues to evolve, ongoing research and technological advances of devices will further enhance the efficacy and safety of ATR in gastroenterology.
Dr. Madi (@MahMadi90) is based in the Division of Gastroenterology and Hepatology, Saint Louis University School of Medicine, Saint Louis, Missouri. Dr. Rengarajan (@ArvindRenga) and Dr. Bazarbashi (@AhmadBazarbashi) are based in the Division of Gastroenterology, Washington University in St. Louis. The authors have no conflicts of interest to disclose, and no funding was required for this project.
References
1. Copland AP, et al. AGA Clinical Practice Update on appropriate and tailored polypectomy: Expert review. Clin Gastroenterol Hepatol. 2024 Mar. doi: 10.1016/j.cgh.2023.10.012.
2. Lee SP, et al. Effect of preceding biopsy on the results of endoscopic submucosal dissection for colorectal laterally spreading tumor. Dig Dis Sci. 2019 Oct. doi: 10.1007/s10620-019-05625-3.
3. Medina-Prado L, et al. When and how to use endoscopic tattooing in the colon: An international Delphi agreement. Clin Gastroenterol Hepatol. 2021 May. doi: 10.1016/j.cgh.2021.01.024.
4. Rashid MU, et al. EMR and ESD: Indications, techniques and results. Surg Oncol. 2022 Aug. doi: 10.1016/j.suronc.2022.101742.
5. Castro R, et al. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019 Feb. doi: 10.3748/wjg.v25.i7.777.
6. Rex DK. Best practices for resection of diminutive and small polyps in the colorectum. Gastrointest Endosc Clin N Am. 2019 Oct. doi: 10.1016/j.giec.2019.06.004.
7. Lv XH, et al. Underwater EMR for nonpedunculated colorectal lesions. Gastrointest Endosc. 2023 Apr. doi: 10.1016/j.gie.2022.10.044.
8. Fujiya M, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015 Mar. doi: 10.1016/j.gie.2014.07.034.
9. Kandel P, Wallace MB. Colorectal endoscopic mucosal resection (EMR). Best Pract Res Clin Gastroenterol. 2017 Aug. doi: 10.1016/j.bpg.2017.05.006.
10. Kemper G, et al; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021 Oct. doi: 10.1007/s00464-021-08574-z.
11. Goto O, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am. 2014 Apr. doi: 10.1016/j.giec.2013.11.006.
12. Wang K, et al. Endoscopic full-thickness resection, indication, methods and perspectives. Dig Endosc. 2023 Jan. doi: 10.1111/den.14474.
13. Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014 Apr 16. doi: 10.4253/wjge.v6.i4.112.
14. Chiba H, et al. Safety and efficacy of simultaneous colorectal ESD for large synchronous colorectal lesions. Endosc Int Open. 2017 Jul. doi: 10.1055/s-0043-110567.
15. Mannath J, Ragunath K. Endoscopic mucosal resection: who and how? Therap Adv Gastroenterol. 2011 Sep. doi: 10.1177/1756283X10388683.
16. Wang XY, et al. Hybrid endoscopic submucosal dissection: An alternative resection modality for large laterally spreading tumors in the cecum? BMC Gastroenterol. 2021 May. doi: 10.1186/s12876-021-01766-w.
17. McCarty TR, et al. Hybrid endoscopic submucosal dissection (ESD) compared with conventional ESD for colorectal lesions: a systematic review and meta-analysis. Endoscopy. 2021 Oct. doi: 10.1055/a-1266-1855.
18. Jain D, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol. 2017 Feb. doi: 10.20524/aog.2017.0128.
19. Lv XH, et al. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc. 2017 Jan. doi: 10.1007/s00464-016-4978-7.
20. Cao B, et al. Efficacy and safety of submucosal tunneling endoscopic resection for gastric submucosal tumors: a systematic review and meta-analysis. Rev Esp Enferm Dig. 2021 Jan. doi: 10.17235/reed.2020.6989/2020.
21. Cai M, et al. Endoscopic full-thickness resection (EFTR) for gastrointestinal subepithelial tumors. Gastrointest Endosc Clin N Am. 2016 Apr. doi: 10.1016/j.giec.2015.12.013.
22. Brigic A, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc. 2013 Oct. doi: 10.1007/s00464-013-2946-z.
Fluid Management in Acute Pancreatitis
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
May 2024 – ICYMI
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Gastroenterology
January 2024
Hirano I, et al; ASCENT WORKING GROUP. Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis. Gastroenterology. 2024 Jan;166(1):1-10. doi: 10.1053/j.gastro.2023.09.004. Epub 2023 Sep 9. PMID: 37690772; PMCID: PMC10872872.
Åkerström JH, et al. Antireflux Surgery Versus Antireflux Medication and Risk of Esophageal Adenocarcinoma in Patients With Barrett’s Esophagus. Gastroenterology. 2024 Jan;166(1):132-138.e3. doi: 10.1053/j.gastro.2023.08.050. Epub 2023 Sep 9. PMID: 37690771.
Barnes EL, et al; AGA Clinical Guidelines Committee. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan;166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. PMID: 38128971.
February 2024
Yoo HW, et al. Helicobacter pylori Treatment and Gastric Cancer Risk After Endoscopic Resection of Dysplasia: A Nationwide Cohort Study. Gastroenterology. 2024 Feb;166(2):313-322.e3. doi: 10.1053/j.gastro.2023.10.013. Epub 2023 Oct 18. PMID: 37863270.
Yang J, et al. High Soluble Fiber Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites in Mice. Gastroenterology. 2024 Feb;166(2):323-337.e7. doi: 10.1053/j.gastro.2023.10.012. Epub 2023 Oct 18. PMID: 37858797.
Young E, et al. Texture and Color Enhancement Imaging Improves Colonic Adenoma Detection: A Multicenter Randomized Controlled Trial. Gastroenterology. 2024 Feb;166(2):338-340.e3. doi: 10.1053/j.gastro.2023.10.008. Epub 2023 Oct 14. PMID: 37839498.
Clinical Gastroenterology and Hepatology
January 2024
Overbeek KA, et al; Dutch Familial Pancreatic Cancer Surveillance Study work group. Intraductal Papillary Mucinous Neoplasms in High-Risk Individuals: Incidence, Growth Rate, and Malignancy Risk. Clin Gastroenterol Hepatol. 2024 Jan;22(1):62-71.e7. doi: 10.1016/j.cgh.2023.03.035. Epub 2023 Apr 7. PMID: 37031711.
Reddy CA, et al. Achalasia is Strongly Associated With Eosinophilic Esophagitis and Other Allergic Disorders. Clin Gastroenterol Hepatol. 2024 Jan;22(1):34-41.e2. doi: 10.1016/j.cgh.2023.06.013. Epub 2023 Jun 28. PMID: 37391057; PMCID: PMC10753026.
Thiruvengadam NR, et al. The Clinical Impact and Cost-Effectiveness of Surveillance of Incidentally Detected Gastric Intestinal Metaplasia: A Microsimulation Analysis. Clin Gastroenterol Hepatol. 2024 Jan;22(1):51-61. doi: 10.1016/j.cgh.2023.05.028. Epub 2023 Jun 9. Erratum in: Clin Gastroenterol Hepatol. 2024 Jan 19;: PMID: 37302442.
February 2024
Goodoory VC, et al. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):243-251.e5. doi: 10.1016/j.cgh.2023.02.014. Epub 2023 Feb 27. PMID: 36858143.
Brenner DM, et al. Development and Current State of Digital Therapeutics for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2024 Feb;22(2):222-234. doi: 10.1016/j.cgh.2023.09.013. Epub 2023 Sep 22. PMID: 37743035.
Techniques and Innovations in Gastrointestinal Endoscopy
January 2024
Ramirez PR, et al. Gaps and Improvement Opportunities in Post-Colonoscopy Communication. Tech Innov Gastrointest Endosc. 2024 Jan;26(1):90-92. doi: 10.1016/j.tige.2023.10.001. Epub 2023 Oct 22.
Gonzaga ER, et al. Gastric Peroral Endoscopic Myotomy (G-POEM) for the Management of Gastroparesis. Tech Innov Gastrointest Endosc. 2024 Jan; 26(1): 46-55. doi: 10.1016/j.tige.2023.09.002. Epub 2023 Oct 13.
Wang D, et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2024 Jan;26(1): 30-37. doi: 10.1016/j.tige.2023.10.003. Epub 2023 Nov 8.
Gastro Hep Advances
January 2024
Adeniran E, et al. Intense and Sustained Alcohol Consumption Associated With Acute Pancreatitis Warrants Early Intervention. Gastro Hep Advances. 2024 Jan;3(1):61-63. doi: 10.1016/j.gastha.2023.08.017. Epub 2023 Sep 2.
Alkhouri N, et al. A Novel Prescription Digital Therapeutic Option for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastro Hep Advances. 2024 Jan;3(1): 9-16. doi: 10.1016/j.gastha.2023.08.019. Epub 2023 Oct 1.
Artificial Intelligence in GI and Hepatology
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
Dear colleagues,
Since our prior Perspectives piece on artificial intelligence (AI) in GI and Hepatology in 2022, the field has seen almost exponential growth. Expectations are high that AI will revolutionize our field and significantly improve patient care. But as the global discussion on AI has shown, there are real challenges with adoption, including issues with accuracy, reliability, and privacy.
In this issue, Dr. Nabil M. Mansour and Dr. Thomas R. McCarty explore the current and future impact of AI on gastroenterology, while Dr. Basile Njei and Yazan A. Al Ajlouni assess its role in hepatology. We hope these pieces will help your discussions in incorporating or researching AI for use in your own practices. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Artificial Intelligence in Gastrointestinal Endoscopy
BY THOMAS R. MCCARTY, MD, MPH; NABIL M. MANSOUR, MD
The last few decades have seen an exponential increase and interest in the role of artificial intelligence (AI) and adoption of deep learning algorithms within healthcare and patient care services. The field of gastroenterology and endoscopy has similarly seen a tremendous uptake in acceptance and implementation of AI for a variety of gastrointestinal conditions. The spectrum of AI-based applications includes detection or diagnostic-based as well as therapeutic assistance tools. From the first US Food and Drug Administration (FDA)-approved device that uses machine learning to assist clinicians in detecting lesions during colonoscopy, to other more innovative machine learning techniques for small bowel, esophageal, and hepatobiliary conditions, AI has dramatically changed the landscape of gastrointestinal endoscopy.
Approved applications for colorectal cancer
In an attempt to improve colorectal cancer screening and outcomes related to screening and surveillance, efforts have been focused on procedural performance metrics, quality indicators, and tools to aid in lesion detection and improve quality of care. One such tool has been computer-aided detection (CADe), with early randomized controlled trial (RCT) data showing significantly increased adenoma detection rate (ADR) and adenomas per colonoscopy (APC).1-3
Ultimately, this data led to FDA approval of the CADe system GI Genius (Medtronic, Dublin, Ireland) in 2021.4 Additional systems have since been FDA approved or 510(k) cleared including Endoscreener (Wision AI, Shanghai, China), SKOUT (Iterative Health, Cambridge, Massachusetts), MAGENTIQ-COLO (MAGENTIQ-EYE LTD, Haifa, Israel), and CAD EYE (Fujifilm, Tokyo), all of which have shown increased ADR and/or increased APC and/or reduced adenoma miss rates in randomized trials.5
Yet despite the promise of improved quality and subsequent translation to better patient outcomes, there has been a noticeable disconnect between RCT data and more real-world literature.6 In a recent study, no improvement was seen in ADR after implementation of a CADe system for colorectal cancer screening — including both higher and lower-ADR performers. Looking at change over time after implementation, CADe had no positive effect in any group over time, divergent from early RCT data. In a more recent multicenter, community-based RCT study, again CADe did not result in a statistically significant difference in the number of adenomas detected.7 The differences between some of these more recent “real-world” studies vs the majority of data from RCTs raise important questions regarding the potential of bias (due to unblinding) in prospective trials, as well as the role of the human-AI interaction.
Importantly for RCT data, both cohorts in these studies met adequate ADR benchmarks, though it remains unclear whether a truly increased ADR necessitates better patient outcomes — is higher always better? In addition, an important consideration with evaluating any AI/CADe system is that they often undergo frequent updates, each promising improved accuracy, sensitivity, and specificity. This is an interesting dilemma and raises questions about the enduring relevance of studies conducted using an outdated version of a CADe system.
Additional unanswered questions regarding an ideal ADR for implementation, preferred patient populations for screening (especially for younger individuals), and the role and adoption of computer-aided polyp diagnosis/characterization (CADx) within the United States remain. Furthermore, questions regarding procedural withdrawal time, impact on sessile serrated lesion detection, cost-effectiveness, and preferred adoption strategies have begun to be explored, though require more data to better define a best practice approach. Ultimately, answers to some of these unknowns may explain the discordant results and help guide future implementation measures.
Innovative applications for alternative gastrointestinal conditions
Given the fervor and excitement, as well as the outcomes associated with AI-based colorectal screening, it is not surprising these techniques have been expanded to other gastrointestinal conditions. At this time, all of these are fledgling, mostly single-center tools, not yet ready for widespread adoption. Nonetheless, these represent a potentially important step forward for difficult-to-manage gastrointestinal diseases.
Machine learning CADe systems have been developed to help identify early Barrett’s neoplasia, depth and invasion of gastric cancer, as well as lesion detection in small bowel video capsule endoscopy.8-10 Endoscopic retrograde cholangiopancreatography (ERCP)-based applications for cholangiocarcinoma and indeterminate stricture diagnosis have also been studied.11 Additional AI-based algorithms have been employed for complex procedures such as endoscopic submucosal dissection (ESD) or peroral endoscopic myotomy (POEM) to delineate vessels, better define tissue planes for dissection, and visualize landmark structures.12,13 Furthermore, AI-based scope guidance/manipulation, bleeding detection, landmark identification, and lesion detection have the potential to revolutionize endoscopic training and education. The impact that generative AI can potentially have on clinical practice is also an exciting prospect that warrants further investigation.
Artificial intelligence adoption in clinical practice
Clinical practice with regard to AI and colorectal cancer screening largely mirrors the disconnect in the current literature, with “believers” and “non-believers” as well as innovators and early adopters alongside laggards. In our own academic practices, we continue to struggle with the adoption and standardized implementation of AI-based colorectal cancer CADe systems, despite the RCT data showing positive results. It is likely that AI uptake will follow the technology predictions of Amara’s Law — i.e., individuals tend to overestimate the short-term impact of new technologies while underestimating long-term effects. In the end, more widespread adoption in community practice and larger scale real-world clinical outcomes studies are likely to determine the true impact of these exciting technologies. For other, less established AI-based tools, more data are currently required.
Conclusions
Ultimately, AI-based algorithms are likely here to stay, with continued improvement and evolution to occur based on provider feedback and patient care needs. Current tools, while not all-encompassing, have the potential to dramatically change the landscape of endoscopic training, diagnostic evaluation, and therapeutic care. It is critically important that relevant stakeholders, both endoscopists and patients, be involved in future applications and design to improve efficiency and quality outcomes overall.
Dr. McCarty is based in the Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. Dr. Mansour is based in the section of gastroenterology, Baylor College of Medicine, Houston. Dr. McCarty reports no conflicts of interest. Dr. Mansour reports having been a consultant for Iterative Health.
References
1. Repici A, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020 Aug. doi: 10.1053/j.gastro.2020.04.062.
2. Repici A, et al. Artificial intelligence and colonoscopy experience: Lessons from two randomised trials. Gut. Apr 2022. doi: 10.1136/gutjnl-2021-324471.
3. Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022 Jul. doi: 10.1053/j.gastro.2022.03.007.
4. United States Food and Drug Administration (FDA). GI Genius FDA Approval [April 9, 2021]. Accessed January 5, 2022. Available at: www.accessdata.fda.gov/cdrh_docs/pdf21/K211951.pdf.
5. Maas MHJ, et al. A computer-aided polyp detection system in screening and surveillance colonoscopy: An international, multicentre, randomised, tandem trial. Lancet Digit Health. 2024 Mar. doi: 10.1016/S2589-7500(23)00242-X.
6. Ladabaum U, et al. Computer-aided detection of polyps does not improve colonoscopist performance in a pragmatic implementation trial. Gastroenterology. 2023 Mar. doi: 10.1053/j.gastro.2022.12.004.
7. Wei MT, et al. Evaluation of computer-aided detection during colonoscopy in the community (AI-SEE): A multicenter randomized clinical trial. Am J Gastroenterol. 2023 Oct. doi: 10.14309/ajg.0000000000002239.
8. de Groof J, et al. The Argos project: The development of a computer-aided detection system to improve detection of Barrett’s neoplasia on white light endoscopy. United European Gastroenterol J. 2019 May. doi: 10.1177/2050640619837443.
9. Kanesaka T, et al. Computer-aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow-band imaging. Gastrointest Endosc. 2018 May. doi: 10.1016/j.gie.2017.11.029.
10. Sahafi A, et al. Edge artificial intelligence wireless video capsule endoscopy. Sci Rep. 2022 Aug. doi: 10.1038/s41598-022-17502-7.
11. Njei B, et al. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann Gastroenterol. 2023 Mar-Apr. doi: 10.20524/aog.2023.0779.
12. Ebigbo A, et al. Vessel and tissue recognition during third-space endoscopy using a deep learning algorithm. Gut. 2022 Dec. doi: 10.1136/gutjnl-2021-326470.
13. Cao J, et al. Intelligent surgical workflow recognition for endoscopic submucosal dissection with real-time animal study. Nat Commun. 2023 Oct. doi: 10.1038/s41467-023-42451-8.
The Promise and Challenges of AI in Hepatology
BY BASILE NJEI, MD, MPH, PHD; YAZAN A. AL-AJLOUNI, MPHIL
In the dynamic realm of medicine, artificial intelligence (AI) emerges as a transformative force, notably within hepatology. The discipline of hepatology, dedicated to liver and related organ diseases, is ripe for AI’s promise to revolutionize diagnostics and treatment, pushing toward a future of precision medicine. Yet, the path to fully realizing AI’s potential in hepatology is laced with data, ethical, and integration challenges.
The application of AI, particularly in histopathology, significantly enhances disease diagnosis and staging in hepatology. AI-driven approaches remedy traditional histopathological challenges, such as interpretative variability, providing more consistent and accurate disease analyses. This is especially evident in conditions like metabolic dysfunction-associated steatohepatitis (MASH) and hepatocellular carcinoma (HCC), where AI aids in identifying critical gene signatures, thereby refining therapy selection.
Similarly, deep learning (DL), a branch of AI, has attracted significant interest globally, particularly in image recognition. AI’s incorporation into medical imaging marks a significant advancement, enabling early detection of malignancies like HCC and improving diagnostics in steatotic liver disease through enhanced imaging analyses using convolutional neural networks (CNN). The abundance of imaging data alongside clinical outcomes has catalyzed AI’s integration into radiology, leading to the swift growth of radiomics as a novel domain in medical research.
AI has also been shown to identify nuanced alterations in electrocardiograms (EKGs) associated with liver conditions, potentially detecting the progression of liver diseases at an earlier stage than currently possible. By leveraging complex algorithms and machine learning, AI can analyze EKG patterns with a precision and depth unattainable through traditional manual interpretation. Given that liver diseases, such as cirrhosis or hepatitis, can induce subtle cardiac changes long before other clinical symptoms manifest, early detection through AI-enhanced EKG analysis could lead to timely interventions, potentially halting or reversing disease progression. This approach further enriches our understanding of the intricate interplay between liver function and cardiac health, highlighting the potential for AI to transform not just liver disease diagnostics but also to foster a more integrated approach to patient care.
Beyond diagnostics, the burgeoning field of generative AI introduces groundbreaking possibilities in treatment planning and patient education, particularly for chronic conditions like cirrhosis. Generative AI produces original content, including text, visuals, and music, by identifying and learning patterns from its training data. When it leverages large language models (LLMs), it entails training on vast collections of textual data and using AI models characterized by many parameters. A notable instance of generative AI employing LLMs is ChatGPT (General Pretrained Transformers). By simulating disease progression and treatment outcomes, generative AI can foster personalized treatment strategies and empower patients with knowledge about their health trajectories. Yet, realizing these potential demands requires overcoming data quality and interpretability challenges, and ensuring AI outputs are accessible and actionable for clinicians and patients.
Despite these advancements, leveraging AI in hepatology is not devoid of hurdles. The development and training of AI models require extensive and diverse datasets, raising concerns about data privacy and ethical use. Addressing these concerns is paramount for successfully integrating AI into clinical hepatology practice, necessitating transparent algorithmic processes and stringent ethical standards. Ethical considerations are central to AI’s integration into hepatology. Algorithmic biases, patient privacy, and the impact of AI-driven decisions underscore the need for cautious AI deployment. Developing transparent, understandable algorithms and establishing ethical guidelines for AI use are critical steps towards ethically leveraging AI in patient care.
In conclusion, AI’s integration into hepatology holds tremendous promise for advancing patient care through enhanced diagnostics, treatment planning, and patient education. Overcoming the associated challenges, including ethical concerns, data diversity, and algorithm interpretability, is crucial. As the hepatology community navigates this technological evolution, a balanced approach that marries technological advancements with ethical stewardship will be key to harnessing AI’s full potential, ensuring it serves the best interests of patients and propels the field of hepatology into the future.
We predict a trajectory of increased use and adoption of AI in hepatology. AI in hepatology is likely to meet the test of pervasiveness, improvement, and innovation. The adoption of AI in routine hepatology diagnosis and management will likely follow Amara’s law and the five stages of the hype cycle. We believe that we are still in the infant stages of adopting AI technology in hepatology, and this phase may last 5 years before there is a peak of inflated expectations. The trough of disillusionment and slopes of enlightenment may only be observed in the next decades.
Dr. Njei is based in the Section of Digestive Diseases, Yale School of Medicine, New Haven, Conn. Mr. Al-Ajlouni is a senior medical student at New York Medical College School of Medicine, Valhalla, N.Y. They have no conflicts of interest to declare.
Sources
Taylor-Weiner A, et al. A Machine Learning Approach Enables Quantitative Measurement of Liver Histology and Disease Monitoring in NASH. Hepatology. 2021 Jul. doi: 10.1002/hep.31750.
Zeng Q, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. 2022 Jul. doi: 10.1016/j.jhep.2022.01.018.
Ahn JC, et al. Development of the AI-Cirrhosis-ECG Score: An Electrocardiogram-Based Deep Learning Model in Cirrhosis. Am J Gastroenterol. 2022 Mar. doi: 10.14309/ajg.0000000000001617.
Nduma BN, et al. The Application of Artificial Intelligence (AI)-Based Ultrasound for the Diagnosis of Fatty Liver Disease: A Systematic Review. Cureus. 2023 Dec 15. doi: 10.7759/cureus.50601.
AGA Guides Usage of GLP-1 Receptor Agonists Before Endoscopy
The American Gastroenterological Association (AGA) has issued a rapid clinical practice update on the use of glucagon-like peptide 1 (GLP-1) receptor agonists prior to endoscopy.
The update was partly prompted by consensus-based perioperative guidance issued by the American Society of Anesthesiologists in June 2023, which advises withholding GLP-1 receptor agonists before endoscopy. This recommendation has caused some anesthesia providers to cancel or postpone endoscopic procedures, or even elect general endotracheal intubation over standard sedation.
“Many facilities and medical centers are now struggling to revise preprocedural protocols for patients taking this class of medications despite the lack of high-level evidence regarding how to proceed,” the panelists wrote in Clinical Gastroenterology and Hepatology. “Important questions include whether these preprocedural changes are necessary, if they truly mitigate periprocedural aspiration, or if the delays instituted by following this guidance might further compound the major problem currently faced nationwide: that of large numbers of patients awaiting endoscopic procedures because of delays from the COVID-19 pandemic, reduction in the recommended age threshold to start colorectal cancer screening in 2018, and workforce challenges.”
The rapid clinical practice update, commissioned and approved by the AGA, includes background on the relationship between GLP-1 receptor agonists and endoscopic procedures, followed by clinical strategies for patients taking these medications.
Lead panelist Jana G. Al Hashash, MD, MSc, AGAF, of Mayo Clinic, Jacksonville, Florida, and colleagues began by noting that GLP-1 receptor agonists have been associated with increased gastric residue in patients with diabetes, and among nondiabetic patients, increased gastric retention of solids but not liquids. Delayed gastric emptying and increased residual gastric contents may be more common among patients on GLP-1 agonists who have vomiting, nausea, dyspepsia, or abdominal bloating, they added.
The above findings “imply an increased risk of aspiration in patients receiving GLP-1 receptor agonist medications who present for procedures that require sedation,” the panelists wrote, but more data is needed to support this hypothesis.
Yet the implications for endoscopic risk are still unclear.
Residual liquid in the stomach, at least, is “less of an issue,” according to the update, since “it is easily removed during an esophagogastroduodenoscopy, and this is the first maneuver performed by endoscopists on entering the stomach.”
While residual solids in the stomach could theoretically increase risk of aspiration, other patients with gastroparesis, such as those taking opioids, are not routinely given “special dietary precautions or medication adjustments” prior to endoscopy, Dr. Al Hashash and colleagues wrote. Even patients with severe gastroparesis who are undergoing gastric peroral endoscopic myotomy (which depends upon an empty stomach), are only required to stop ingesting solid foods the day before the procedure, they noted.
“It is appropriate that the ASA’s perioperative suggestions for patients on GLP-1 [receptor agonists] are labeled ‘consensus-based guidance on perioperative management,’ because there is clearly insufficient published evidence for a robust systematic review and guideline,” they wrote. “As such, the ASA’s suggestions are expert opinions, which may inform but should not replace clinical judgment.”
, for whom withholding these medications “might provide more risk than benefit.”
Withholding GLP-1 receptor agonists may be safe and reasonable for patients taking them solely for weight loss, but “this should not be considered mandatory or evidence-based,” as it remains unclear whether withholding one dose is enough to restore normal gastric motility.
“Generally, in patients on GLP-1 receptor agonists who have followed standard perioperative procedures (typically an 8-hour solid-food fast and a 2-hour liquid fast) and who do not have symptoms of nausea, vomiting, dyspepsia, or abdominal distention, we advise proceeding with upper and/or lower endoscopy,” the panelists concluded.
The rapid clinical practice update was commissioned and approved by the AGA. The update panelists disclosed relationships with Apollo Endosurgery, Medtronic, Boston Scientific, and others.
The American Gastroenterological Association (AGA) has issued a rapid clinical practice update on the use of glucagon-like peptide 1 (GLP-1) receptor agonists prior to endoscopy.
The update was partly prompted by consensus-based perioperative guidance issued by the American Society of Anesthesiologists in June 2023, which advises withholding GLP-1 receptor agonists before endoscopy. This recommendation has caused some anesthesia providers to cancel or postpone endoscopic procedures, or even elect general endotracheal intubation over standard sedation.
“Many facilities and medical centers are now struggling to revise preprocedural protocols for patients taking this class of medications despite the lack of high-level evidence regarding how to proceed,” the panelists wrote in Clinical Gastroenterology and Hepatology. “Important questions include whether these preprocedural changes are necessary, if they truly mitigate periprocedural aspiration, or if the delays instituted by following this guidance might further compound the major problem currently faced nationwide: that of large numbers of patients awaiting endoscopic procedures because of delays from the COVID-19 pandemic, reduction in the recommended age threshold to start colorectal cancer screening in 2018, and workforce challenges.”
The rapid clinical practice update, commissioned and approved by the AGA, includes background on the relationship between GLP-1 receptor agonists and endoscopic procedures, followed by clinical strategies for patients taking these medications.
Lead panelist Jana G. Al Hashash, MD, MSc, AGAF, of Mayo Clinic, Jacksonville, Florida, and colleagues began by noting that GLP-1 receptor agonists have been associated with increased gastric residue in patients with diabetes, and among nondiabetic patients, increased gastric retention of solids but not liquids. Delayed gastric emptying and increased residual gastric contents may be more common among patients on GLP-1 agonists who have vomiting, nausea, dyspepsia, or abdominal bloating, they added.
The above findings “imply an increased risk of aspiration in patients receiving GLP-1 receptor agonist medications who present for procedures that require sedation,” the panelists wrote, but more data is needed to support this hypothesis.
Yet the implications for endoscopic risk are still unclear.
Residual liquid in the stomach, at least, is “less of an issue,” according to the update, since “it is easily removed during an esophagogastroduodenoscopy, and this is the first maneuver performed by endoscopists on entering the stomach.”
While residual solids in the stomach could theoretically increase risk of aspiration, other patients with gastroparesis, such as those taking opioids, are not routinely given “special dietary precautions or medication adjustments” prior to endoscopy, Dr. Al Hashash and colleagues wrote. Even patients with severe gastroparesis who are undergoing gastric peroral endoscopic myotomy (which depends upon an empty stomach), are only required to stop ingesting solid foods the day before the procedure, they noted.
“It is appropriate that the ASA’s perioperative suggestions for patients on GLP-1 [receptor agonists] are labeled ‘consensus-based guidance on perioperative management,’ because there is clearly insufficient published evidence for a robust systematic review and guideline,” they wrote. “As such, the ASA’s suggestions are expert opinions, which may inform but should not replace clinical judgment.”
, for whom withholding these medications “might provide more risk than benefit.”
Withholding GLP-1 receptor agonists may be safe and reasonable for patients taking them solely for weight loss, but “this should not be considered mandatory or evidence-based,” as it remains unclear whether withholding one dose is enough to restore normal gastric motility.
“Generally, in patients on GLP-1 receptor agonists who have followed standard perioperative procedures (typically an 8-hour solid-food fast and a 2-hour liquid fast) and who do not have symptoms of nausea, vomiting, dyspepsia, or abdominal distention, we advise proceeding with upper and/or lower endoscopy,” the panelists concluded.
The rapid clinical practice update was commissioned and approved by the AGA. The update panelists disclosed relationships with Apollo Endosurgery, Medtronic, Boston Scientific, and others.
The American Gastroenterological Association (AGA) has issued a rapid clinical practice update on the use of glucagon-like peptide 1 (GLP-1) receptor agonists prior to endoscopy.
The update was partly prompted by consensus-based perioperative guidance issued by the American Society of Anesthesiologists in June 2023, which advises withholding GLP-1 receptor agonists before endoscopy. This recommendation has caused some anesthesia providers to cancel or postpone endoscopic procedures, or even elect general endotracheal intubation over standard sedation.
“Many facilities and medical centers are now struggling to revise preprocedural protocols for patients taking this class of medications despite the lack of high-level evidence regarding how to proceed,” the panelists wrote in Clinical Gastroenterology and Hepatology. “Important questions include whether these preprocedural changes are necessary, if they truly mitigate periprocedural aspiration, or if the delays instituted by following this guidance might further compound the major problem currently faced nationwide: that of large numbers of patients awaiting endoscopic procedures because of delays from the COVID-19 pandemic, reduction in the recommended age threshold to start colorectal cancer screening in 2018, and workforce challenges.”
The rapid clinical practice update, commissioned and approved by the AGA, includes background on the relationship between GLP-1 receptor agonists and endoscopic procedures, followed by clinical strategies for patients taking these medications.
Lead panelist Jana G. Al Hashash, MD, MSc, AGAF, of Mayo Clinic, Jacksonville, Florida, and colleagues began by noting that GLP-1 receptor agonists have been associated with increased gastric residue in patients with diabetes, and among nondiabetic patients, increased gastric retention of solids but not liquids. Delayed gastric emptying and increased residual gastric contents may be more common among patients on GLP-1 agonists who have vomiting, nausea, dyspepsia, or abdominal bloating, they added.
The above findings “imply an increased risk of aspiration in patients receiving GLP-1 receptor agonist medications who present for procedures that require sedation,” the panelists wrote, but more data is needed to support this hypothesis.
Yet the implications for endoscopic risk are still unclear.
Residual liquid in the stomach, at least, is “less of an issue,” according to the update, since “it is easily removed during an esophagogastroduodenoscopy, and this is the first maneuver performed by endoscopists on entering the stomach.”
While residual solids in the stomach could theoretically increase risk of aspiration, other patients with gastroparesis, such as those taking opioids, are not routinely given “special dietary precautions or medication adjustments” prior to endoscopy, Dr. Al Hashash and colleagues wrote. Even patients with severe gastroparesis who are undergoing gastric peroral endoscopic myotomy (which depends upon an empty stomach), are only required to stop ingesting solid foods the day before the procedure, they noted.
“It is appropriate that the ASA’s perioperative suggestions for patients on GLP-1 [receptor agonists] are labeled ‘consensus-based guidance on perioperative management,’ because there is clearly insufficient published evidence for a robust systematic review and guideline,” they wrote. “As such, the ASA’s suggestions are expert opinions, which may inform but should not replace clinical judgment.”
, for whom withholding these medications “might provide more risk than benefit.”
Withholding GLP-1 receptor agonists may be safe and reasonable for patients taking them solely for weight loss, but “this should not be considered mandatory or evidence-based,” as it remains unclear whether withholding one dose is enough to restore normal gastric motility.
“Generally, in patients on GLP-1 receptor agonists who have followed standard perioperative procedures (typically an 8-hour solid-food fast and a 2-hour liquid fast) and who do not have symptoms of nausea, vomiting, dyspepsia, or abdominal distention, we advise proceeding with upper and/or lower endoscopy,” the panelists concluded.
The rapid clinical practice update was commissioned and approved by the AGA. The update panelists disclosed relationships with Apollo Endosurgery, Medtronic, Boston Scientific, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
The Gamer Who Became a GI Hospitalist and Dedicated Endoscopist
Reflecting on his career in gastroenterology, Andy Tau, MD, (@DrBloodandGuts on X) claims the discipline chose him, in many ways.
“I love gaming, which my mom said would never pay off. Then one day she nearly died from a peptic ulcer, and endoscopy saved her,” said Dr. Tau, a GI hospitalist who practices with Austin Gastroenterology in Austin, Texas. One of his specialties is endoscopic hemostasis.
Endoscopy functions similarly to a game because the interface between the operator and the patient is a controller and a video screen, he explained. “Movements in my hands translate directly onto the screen. Obviously, endoscopy is serious business, but the tactile feel was very familiar and satisfying to me.”
Advocating for the GI hospitalist and the versatile role they play in hospital medicine, is another passion of his. “The dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist,” Dr. Tau wrote in an opinion piece in GI & Hepatology News .
He expounded more on this topic and others in an interview, recalling what he learned from one mentor about maintaining a sense of humor at the bedside.
Q: You’ve said that GI hospitalists are the future of patient care. Can you explain why you feel this way?
Dr. Tau: From a quality perspective, even though it’s hard to put into one word, the care of acute GI pathology and endoscopy can be seen as a specialty in and of itself. These skills include hemostasis, enteral access, percutaneous endoscopic gastrostomy (PEG), balloon-assisted enteroscopy, luminal stenting, advanced tissue closure, and endoscopic retrograde cholangiopancreatography. The greater availability of a GI hospitalist, as opposed to an outpatient GI doctor rounding at the ends of days, likely shortens admissions and improves the logistics of scheduling inpatient cases.
From a financial perspective, the landscape of GI practice is changing because of GI physician shortages relative to increased demand for outpatient procedures. Namely, the outpatient gastroenterologists simply have too much on their plate and inefficiencies abound when they have to juggle inpatient and outpatient work. Thus, two tracks are forming, especially in large busy hospitals. This is the same evolution of the pure outpatient internist and inpatient internist 20 years ago.
Q: What attributes does a GI hospitalist bring to the table?
Dr. Tau: A GI hospitalist is one who can multitask through interruptions, manage end-of-life issues, craves therapeutic endoscopy (even if that’s hemostasis), and can keep more erratic hours based on the number of consults that come in. She/he tends to want immediate gratification and doesn’t mind the lack of continuity of care. Lastly, the GI hospitalist has to be brave and yet careful as the patients are sicker and thus complications may be higher and certainly less well tolerated.
Q: Are there enough of them going into practice right now?
Dr. Tau: Not really! The demand seems to outstrip supply based on what I see. There is a definite financial lure as the market rate for them rises (because more GIs are leaving the hospital for pure outpatient practice), but burnout can be an issue. Interestingly, fellows are typically highly trained and familiar with inpatient work, but once in practice, most choose the outpatient track. I think it’s a combination of work-life balance, inefficiency of inpatient endoscopy, and perhaps the strain of daily, erratic consultation.
Q: You received the 2021 Travis County Medical Society (TCMS) Young Physician of the Year. What achievements led to this honor?
Dr. Tau: I am not sure I am deserving of that award, but I think it was related to personal risk and some long hours as a GI hospitalist during the COVID pandemic. I may have the unfortunate distinction of performing more procedures on COVID patients than any other physician in the city. My hospital was the largest COVID-designated site in the city. There were countless PEG tubes in COVID survivors and a lot of bleeders for some reason. A critical care physician on the front lines and health director of the city of Austin received Physician of the Year, deservedly.
Q: What teacher or mentor had the greatest impact on you?
Dr. Tau: David Y. Graham, MD, MACG, got me into GI as a medical student and taught me to never tolerate any loose ends when it came to patient care as a resident. He trained me at every level — from medical school, residency, and through my fellowship. His advice is often delivered sly and dry, but his humor-laden truths continue to ring true throughout my life. One story: my whole family tested positive for Helicobacter pylori after my mother survived peptic ulcer hemorrhage. I was the only one who tested negative! I asked Dr Graham about it and he quipped, “You’re lucky! It’s because your mother didn’t love (and kiss) you as much!”
Even to this moment I laugh about that. I share that with my patients when they ask about how they contracted H. pylori.
Lightning Round
Favorite junk food?
McDonalds fries
Favorite movie genre?
Psychological thriller
Cat person or dog person?
Dog
What was your favorite Halloween costume?
Ninja turtle
Favorite sport:
Football (played in college)
Introvert or extrovert?
Extrovert unless sleep deprived.
Favorite holiday:
Thanksgiving
The book you read over and over:
Swiss Family Robinson
Favorite travel destination:
Hawaii
Optimist or pessimist?
A happy pessimist.
Reflecting on his career in gastroenterology, Andy Tau, MD, (@DrBloodandGuts on X) claims the discipline chose him, in many ways.
“I love gaming, which my mom said would never pay off. Then one day she nearly died from a peptic ulcer, and endoscopy saved her,” said Dr. Tau, a GI hospitalist who practices with Austin Gastroenterology in Austin, Texas. One of his specialties is endoscopic hemostasis.
Endoscopy functions similarly to a game because the interface between the operator and the patient is a controller and a video screen, he explained. “Movements in my hands translate directly onto the screen. Obviously, endoscopy is serious business, but the tactile feel was very familiar and satisfying to me.”
Advocating for the GI hospitalist and the versatile role they play in hospital medicine, is another passion of his. “The dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist,” Dr. Tau wrote in an opinion piece in GI & Hepatology News .
He expounded more on this topic and others in an interview, recalling what he learned from one mentor about maintaining a sense of humor at the bedside.
Q: You’ve said that GI hospitalists are the future of patient care. Can you explain why you feel this way?
Dr. Tau: From a quality perspective, even though it’s hard to put into one word, the care of acute GI pathology and endoscopy can be seen as a specialty in and of itself. These skills include hemostasis, enteral access, percutaneous endoscopic gastrostomy (PEG), balloon-assisted enteroscopy, luminal stenting, advanced tissue closure, and endoscopic retrograde cholangiopancreatography. The greater availability of a GI hospitalist, as opposed to an outpatient GI doctor rounding at the ends of days, likely shortens admissions and improves the logistics of scheduling inpatient cases.
From a financial perspective, the landscape of GI practice is changing because of GI physician shortages relative to increased demand for outpatient procedures. Namely, the outpatient gastroenterologists simply have too much on their plate and inefficiencies abound when they have to juggle inpatient and outpatient work. Thus, two tracks are forming, especially in large busy hospitals. This is the same evolution of the pure outpatient internist and inpatient internist 20 years ago.
Q: What attributes does a GI hospitalist bring to the table?
Dr. Tau: A GI hospitalist is one who can multitask through interruptions, manage end-of-life issues, craves therapeutic endoscopy (even if that’s hemostasis), and can keep more erratic hours based on the number of consults that come in. She/he tends to want immediate gratification and doesn’t mind the lack of continuity of care. Lastly, the GI hospitalist has to be brave and yet careful as the patients are sicker and thus complications may be higher and certainly less well tolerated.
Q: Are there enough of them going into practice right now?
Dr. Tau: Not really! The demand seems to outstrip supply based on what I see. There is a definite financial lure as the market rate for them rises (because more GIs are leaving the hospital for pure outpatient practice), but burnout can be an issue. Interestingly, fellows are typically highly trained and familiar with inpatient work, but once in practice, most choose the outpatient track. I think it’s a combination of work-life balance, inefficiency of inpatient endoscopy, and perhaps the strain of daily, erratic consultation.
Q: You received the 2021 Travis County Medical Society (TCMS) Young Physician of the Year. What achievements led to this honor?
Dr. Tau: I am not sure I am deserving of that award, but I think it was related to personal risk and some long hours as a GI hospitalist during the COVID pandemic. I may have the unfortunate distinction of performing more procedures on COVID patients than any other physician in the city. My hospital was the largest COVID-designated site in the city. There were countless PEG tubes in COVID survivors and a lot of bleeders for some reason. A critical care physician on the front lines and health director of the city of Austin received Physician of the Year, deservedly.
Q: What teacher or mentor had the greatest impact on you?
Dr. Tau: David Y. Graham, MD, MACG, got me into GI as a medical student and taught me to never tolerate any loose ends when it came to patient care as a resident. He trained me at every level — from medical school, residency, and through my fellowship. His advice is often delivered sly and dry, but his humor-laden truths continue to ring true throughout my life. One story: my whole family tested positive for Helicobacter pylori after my mother survived peptic ulcer hemorrhage. I was the only one who tested negative! I asked Dr Graham about it and he quipped, “You’re lucky! It’s because your mother didn’t love (and kiss) you as much!”
Even to this moment I laugh about that. I share that with my patients when they ask about how they contracted H. pylori.
Lightning Round
Favorite junk food?
McDonalds fries
Favorite movie genre?
Psychological thriller
Cat person or dog person?
Dog
What was your favorite Halloween costume?
Ninja turtle
Favorite sport:
Football (played in college)
Introvert or extrovert?
Extrovert unless sleep deprived.
Favorite holiday:
Thanksgiving
The book you read over and over:
Swiss Family Robinson
Favorite travel destination:
Hawaii
Optimist or pessimist?
A happy pessimist.
Reflecting on his career in gastroenterology, Andy Tau, MD, (@DrBloodandGuts on X) claims the discipline chose him, in many ways.
“I love gaming, which my mom said would never pay off. Then one day she nearly died from a peptic ulcer, and endoscopy saved her,” said Dr. Tau, a GI hospitalist who practices with Austin Gastroenterology in Austin, Texas. One of his specialties is endoscopic hemostasis.
Endoscopy functions similarly to a game because the interface between the operator and the patient is a controller and a video screen, he explained. “Movements in my hands translate directly onto the screen. Obviously, endoscopy is serious business, but the tactile feel was very familiar and satisfying to me.”
Advocating for the GI hospitalist and the versatile role they play in hospital medicine, is another passion of his. “The dedicated GI hospitalist indirectly improves the efficiency of an outpatient practice, while directly improving inpatient outcomes, collegiality, and even one’s own skills as an endoscopist,” Dr. Tau wrote in an opinion piece in GI & Hepatology News .
He expounded more on this topic and others in an interview, recalling what he learned from one mentor about maintaining a sense of humor at the bedside.
Q: You’ve said that GI hospitalists are the future of patient care. Can you explain why you feel this way?
Dr. Tau: From a quality perspective, even though it’s hard to put into one word, the care of acute GI pathology and endoscopy can be seen as a specialty in and of itself. These skills include hemostasis, enteral access, percutaneous endoscopic gastrostomy (PEG), balloon-assisted enteroscopy, luminal stenting, advanced tissue closure, and endoscopic retrograde cholangiopancreatography. The greater availability of a GI hospitalist, as opposed to an outpatient GI doctor rounding at the ends of days, likely shortens admissions and improves the logistics of scheduling inpatient cases.
From a financial perspective, the landscape of GI practice is changing because of GI physician shortages relative to increased demand for outpatient procedures. Namely, the outpatient gastroenterologists simply have too much on their plate and inefficiencies abound when they have to juggle inpatient and outpatient work. Thus, two tracks are forming, especially in large busy hospitals. This is the same evolution of the pure outpatient internist and inpatient internist 20 years ago.
Q: What attributes does a GI hospitalist bring to the table?
Dr. Tau: A GI hospitalist is one who can multitask through interruptions, manage end-of-life issues, craves therapeutic endoscopy (even if that’s hemostasis), and can keep more erratic hours based on the number of consults that come in. She/he tends to want immediate gratification and doesn’t mind the lack of continuity of care. Lastly, the GI hospitalist has to be brave and yet careful as the patients are sicker and thus complications may be higher and certainly less well tolerated.
Q: Are there enough of them going into practice right now?
Dr. Tau: Not really! The demand seems to outstrip supply based on what I see. There is a definite financial lure as the market rate for them rises (because more GIs are leaving the hospital for pure outpatient practice), but burnout can be an issue. Interestingly, fellows are typically highly trained and familiar with inpatient work, but once in practice, most choose the outpatient track. I think it’s a combination of work-life balance, inefficiency of inpatient endoscopy, and perhaps the strain of daily, erratic consultation.
Q: You received the 2021 Travis County Medical Society (TCMS) Young Physician of the Year. What achievements led to this honor?
Dr. Tau: I am not sure I am deserving of that award, but I think it was related to personal risk and some long hours as a GI hospitalist during the COVID pandemic. I may have the unfortunate distinction of performing more procedures on COVID patients than any other physician in the city. My hospital was the largest COVID-designated site in the city. There were countless PEG tubes in COVID survivors and a lot of bleeders for some reason. A critical care physician on the front lines and health director of the city of Austin received Physician of the Year, deservedly.
Q: What teacher or mentor had the greatest impact on you?
Dr. Tau: David Y. Graham, MD, MACG, got me into GI as a medical student and taught me to never tolerate any loose ends when it came to patient care as a resident. He trained me at every level — from medical school, residency, and through my fellowship. His advice is often delivered sly and dry, but his humor-laden truths continue to ring true throughout my life. One story: my whole family tested positive for Helicobacter pylori after my mother survived peptic ulcer hemorrhage. I was the only one who tested negative! I asked Dr Graham about it and he quipped, “You’re lucky! It’s because your mother didn’t love (and kiss) you as much!”
Even to this moment I laugh about that. I share that with my patients when they ask about how they contracted H. pylori.
Lightning Round
Favorite junk food?
McDonalds fries
Favorite movie genre?
Psychological thriller
Cat person or dog person?
Dog
What was your favorite Halloween costume?
Ninja turtle
Favorite sport:
Football (played in college)
Introvert or extrovert?
Extrovert unless sleep deprived.
Favorite holiday:
Thanksgiving
The book you read over and over:
Swiss Family Robinson
Favorite travel destination:
Hawaii
Optimist or pessimist?
A happy pessimist.