User login
Thyroid cancer outcomes worse for black and Hispanic young adults
African American and Hispanic adolescents and adults under the age of 40 years were more likely to die from differentiated thyroid cancer than were non-Hispanic whites from the same age range, said the authors of a newly published study in Thyroid.
Lead author Theresa H.M. Keegan, Ph.D., of Stanford (Calif.) University and her associates used the California Cancer Registry to obtain data on 16,827 adolescents and young adults who had a diagnosis of differentiated thyroid cancer between 1988 and 2010. Older young adults aged 35-39 years (versus 15- to 29-year-olds), men (hazard ratio, 2.77; 95% confidence interval, 1.62-4.72), and adolescents and young adults of African American or Hispanic race/ethnicity (versus non-Hispanic whites) had worse thyroid cancer–specific survival than did non-Hispanic whites, judging from findings of multivariate analyses using Cox proportional hazards regression.
In addition, residence in low-socioeconomic-status neighborhoods (HR, 3.11; 95% CI, 1.28-7.56) and nonmetropolitan areas (HR, 5.53; 95% CI, 2.07-14.78) was associated with worse thyroid cancer–specific survival among adolescent and young adult men but not adolescent and young adult women.
“Our study is one of the first to simultaneously consider the impact of small-area neighborhood [socioeconomic status], health insurance, marital status, diagnosis of subsequent cancers, and a number of tumor characteristics on survival after” differentiated thyroid cancer in adolescents and young adults, the authors noted.
Read the full article here (Thyroid 2015;25:635-48 [doi:10.1089/thy.2015.0021]).
The authors reported that they did not have any competing financial interests.
African American and Hispanic adolescents and adults under the age of 40 years were more likely to die from differentiated thyroid cancer than were non-Hispanic whites from the same age range, said the authors of a newly published study in Thyroid.
Lead author Theresa H.M. Keegan, Ph.D., of Stanford (Calif.) University and her associates used the California Cancer Registry to obtain data on 16,827 adolescents and young adults who had a diagnosis of differentiated thyroid cancer between 1988 and 2010. Older young adults aged 35-39 years (versus 15- to 29-year-olds), men (hazard ratio, 2.77; 95% confidence interval, 1.62-4.72), and adolescents and young adults of African American or Hispanic race/ethnicity (versus non-Hispanic whites) had worse thyroid cancer–specific survival than did non-Hispanic whites, judging from findings of multivariate analyses using Cox proportional hazards regression.
In addition, residence in low-socioeconomic-status neighborhoods (HR, 3.11; 95% CI, 1.28-7.56) and nonmetropolitan areas (HR, 5.53; 95% CI, 2.07-14.78) was associated with worse thyroid cancer–specific survival among adolescent and young adult men but not adolescent and young adult women.
“Our study is one of the first to simultaneously consider the impact of small-area neighborhood [socioeconomic status], health insurance, marital status, diagnosis of subsequent cancers, and a number of tumor characteristics on survival after” differentiated thyroid cancer in adolescents and young adults, the authors noted.
Read the full article here (Thyroid 2015;25:635-48 [doi:10.1089/thy.2015.0021]).
The authors reported that they did not have any competing financial interests.
African American and Hispanic adolescents and adults under the age of 40 years were more likely to die from differentiated thyroid cancer than were non-Hispanic whites from the same age range, said the authors of a newly published study in Thyroid.
Lead author Theresa H.M. Keegan, Ph.D., of Stanford (Calif.) University and her associates used the California Cancer Registry to obtain data on 16,827 adolescents and young adults who had a diagnosis of differentiated thyroid cancer between 1988 and 2010. Older young adults aged 35-39 years (versus 15- to 29-year-olds), men (hazard ratio, 2.77; 95% confidence interval, 1.62-4.72), and adolescents and young adults of African American or Hispanic race/ethnicity (versus non-Hispanic whites) had worse thyroid cancer–specific survival than did non-Hispanic whites, judging from findings of multivariate analyses using Cox proportional hazards regression.
In addition, residence in low-socioeconomic-status neighborhoods (HR, 3.11; 95% CI, 1.28-7.56) and nonmetropolitan areas (HR, 5.53; 95% CI, 2.07-14.78) was associated with worse thyroid cancer–specific survival among adolescent and young adult men but not adolescent and young adult women.
“Our study is one of the first to simultaneously consider the impact of small-area neighborhood [socioeconomic status], health insurance, marital status, diagnosis of subsequent cancers, and a number of tumor characteristics on survival after” differentiated thyroid cancer in adolescents and young adults, the authors noted.
Read the full article here (Thyroid 2015;25:635-48 [doi:10.1089/thy.2015.0021]).
The authors reported that they did not have any competing financial interests.
FROM THYROID
ASA: Mutation testing aids decision making in thyroid cancer
SAN DIEGO – Routine preoperative use of genetic testing to detect mutations implicated in thyroid carcinogenesis can help guide perioperative decision making, though risks associated with mutations are not always clear-cut.
For individuals with thyroid cancer (TC), the presence of certain mutations was associated with higher risk of early recurrence of cancer as well as distant metastases, according to a recent study presented by Dr. Linwah Yip at the annual meeting of the American Surgical Association. She and her colleagues at the University of Pittsburgh built on their previous work to characterize how thyroid cancer genotype relates both to cancer histology and to disease-related outcomes.
Using data from the electronic medical record of a single institution, Dr. Yip and her colleagues examined data from consecutive patients who had initial surgery for histologically confirmed TC. Of the 1,510 patients in the study cohort, 77% were women, and patients had a mean age of 49 years. All of the cancers in the study were tested for mutations in seven genes known to be associated with thyroid carcinogenesis. Mutation testing was a routine part of preoperative care for thyroid cancer patients, often performed on preoperative fine needle aspiration (FNA) biopsy.
Outcomes tracked in the study, Dr. Yip said, included the type and stage of thyroid cancer identified and whether the cancer recurred.
Mutations were found in 1,039 patients (69%), and no more than one mutation was found in any one tumor. No tumor genotype was specifically associated with tumor size or whether the tumor was multifocal.
Overall, BRAF V600E was the most common mutation associated with TC, and patients with this mutation were the ones most likely to have a recurrence (P = .001). However, Dr. Yip noted that there is phenotypic heterogeneity in how the recurrences present. More distant metastatic disease and lateral lymph node metastases were most likely with RET/PTC1 and three mutations (P = .02).
By contrast, about 25% of thyroid cancers in the study showed mutations in RAS, PAX8/PPARG, or BRAF K601E. These mutations were associated with a more indolent disease course, with more encapsulated tumors and an overall disease-free survival of nearly 100% at 5 years after diagnosis. Dr. Yip said, “However, RAS variations can be associated with any histologic type of thyroid cancer, including anaplastic.”
Dr. Yip said that clinicians should consider conducting perioperative neck ultrasound with lymph node mapping if BRAF V600E or RET/PTC mutations are found. Her recommendation for these patients was a total thyroidectomy, with consideration of a central compartment neck dissection performed prophylactically, in light of the > 50% chance for lymph node involvement. Additionally, surveillance for distant metastases in the form of a chest CT should be considered when tumors are REC/PTC positive.
Study limitations include its retrospective nature and the fact that the treating physicians were not blinded to mutation testing results. Additionally, Dr. Yip noted, in patients with multifocal disease, only the most aggressive tumor was included.
Dr. Chris McHenry of Case Western Reserve University, Cleveland, noted in discussion that disease-specific survival was not related to mutation testing in this study. For patients with advanced thyroid cancer who have limited treatment options, however, mutation testing may help direct specific adjuvant therapies based on risk.
Dr. Nikiforov is a consultant for Quest Diagnostics. The others reported no disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
SAN DIEGO – Routine preoperative use of genetic testing to detect mutations implicated in thyroid carcinogenesis can help guide perioperative decision making, though risks associated with mutations are not always clear-cut.
For individuals with thyroid cancer (TC), the presence of certain mutations was associated with higher risk of early recurrence of cancer as well as distant metastases, according to a recent study presented by Dr. Linwah Yip at the annual meeting of the American Surgical Association. She and her colleagues at the University of Pittsburgh built on their previous work to characterize how thyroid cancer genotype relates both to cancer histology and to disease-related outcomes.
Using data from the electronic medical record of a single institution, Dr. Yip and her colleagues examined data from consecutive patients who had initial surgery for histologically confirmed TC. Of the 1,510 patients in the study cohort, 77% were women, and patients had a mean age of 49 years. All of the cancers in the study were tested for mutations in seven genes known to be associated with thyroid carcinogenesis. Mutation testing was a routine part of preoperative care for thyroid cancer patients, often performed on preoperative fine needle aspiration (FNA) biopsy.
Outcomes tracked in the study, Dr. Yip said, included the type and stage of thyroid cancer identified and whether the cancer recurred.
Mutations were found in 1,039 patients (69%), and no more than one mutation was found in any one tumor. No tumor genotype was specifically associated with tumor size or whether the tumor was multifocal.
Overall, BRAF V600E was the most common mutation associated with TC, and patients with this mutation were the ones most likely to have a recurrence (P = .001). However, Dr. Yip noted that there is phenotypic heterogeneity in how the recurrences present. More distant metastatic disease and lateral lymph node metastases were most likely with RET/PTC1 and three mutations (P = .02).
By contrast, about 25% of thyroid cancers in the study showed mutations in RAS, PAX8/PPARG, or BRAF K601E. These mutations were associated with a more indolent disease course, with more encapsulated tumors and an overall disease-free survival of nearly 100% at 5 years after diagnosis. Dr. Yip said, “However, RAS variations can be associated with any histologic type of thyroid cancer, including anaplastic.”
Dr. Yip said that clinicians should consider conducting perioperative neck ultrasound with lymph node mapping if BRAF V600E or RET/PTC mutations are found. Her recommendation for these patients was a total thyroidectomy, with consideration of a central compartment neck dissection performed prophylactically, in light of the > 50% chance for lymph node involvement. Additionally, surveillance for distant metastases in the form of a chest CT should be considered when tumors are REC/PTC positive.
Study limitations include its retrospective nature and the fact that the treating physicians were not blinded to mutation testing results. Additionally, Dr. Yip noted, in patients with multifocal disease, only the most aggressive tumor was included.
Dr. Chris McHenry of Case Western Reserve University, Cleveland, noted in discussion that disease-specific survival was not related to mutation testing in this study. For patients with advanced thyroid cancer who have limited treatment options, however, mutation testing may help direct specific adjuvant therapies based on risk.
Dr. Nikiforov is a consultant for Quest Diagnostics. The others reported no disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
SAN DIEGO – Routine preoperative use of genetic testing to detect mutations implicated in thyroid carcinogenesis can help guide perioperative decision making, though risks associated with mutations are not always clear-cut.
For individuals with thyroid cancer (TC), the presence of certain mutations was associated with higher risk of early recurrence of cancer as well as distant metastases, according to a recent study presented by Dr. Linwah Yip at the annual meeting of the American Surgical Association. She and her colleagues at the University of Pittsburgh built on their previous work to characterize how thyroid cancer genotype relates both to cancer histology and to disease-related outcomes.
Using data from the electronic medical record of a single institution, Dr. Yip and her colleagues examined data from consecutive patients who had initial surgery for histologically confirmed TC. Of the 1,510 patients in the study cohort, 77% were women, and patients had a mean age of 49 years. All of the cancers in the study were tested for mutations in seven genes known to be associated with thyroid carcinogenesis. Mutation testing was a routine part of preoperative care for thyroid cancer patients, often performed on preoperative fine needle aspiration (FNA) biopsy.
Outcomes tracked in the study, Dr. Yip said, included the type and stage of thyroid cancer identified and whether the cancer recurred.
Mutations were found in 1,039 patients (69%), and no more than one mutation was found in any one tumor. No tumor genotype was specifically associated with tumor size or whether the tumor was multifocal.
Overall, BRAF V600E was the most common mutation associated with TC, and patients with this mutation were the ones most likely to have a recurrence (P = .001). However, Dr. Yip noted that there is phenotypic heterogeneity in how the recurrences present. More distant metastatic disease and lateral lymph node metastases were most likely with RET/PTC1 and three mutations (P = .02).
By contrast, about 25% of thyroid cancers in the study showed mutations in RAS, PAX8/PPARG, or BRAF K601E. These mutations were associated with a more indolent disease course, with more encapsulated tumors and an overall disease-free survival of nearly 100% at 5 years after diagnosis. Dr. Yip said, “However, RAS variations can be associated with any histologic type of thyroid cancer, including anaplastic.”
Dr. Yip said that clinicians should consider conducting perioperative neck ultrasound with lymph node mapping if BRAF V600E or RET/PTC mutations are found. Her recommendation for these patients was a total thyroidectomy, with consideration of a central compartment neck dissection performed prophylactically, in light of the > 50% chance for lymph node involvement. Additionally, surveillance for distant metastases in the form of a chest CT should be considered when tumors are REC/PTC positive.
Study limitations include its retrospective nature and the fact that the treating physicians were not blinded to mutation testing results. Additionally, Dr. Yip noted, in patients with multifocal disease, only the most aggressive tumor was included.
Dr. Chris McHenry of Case Western Reserve University, Cleveland, noted in discussion that disease-specific survival was not related to mutation testing in this study. For patients with advanced thyroid cancer who have limited treatment options, however, mutation testing may help direct specific adjuvant therapies based on risk.
Dr. Nikiforov is a consultant for Quest Diagnostics. The others reported no disclosures.
The complete manuscript of this study and its presentation at the American Surgical Association’s 135th Annual Meeting, April 2015, in San Diego, California, are anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Genetic testing in those with thyroid cancer may aid perioperative decision making.
Major finding: Distant metastases were more common in thyroid cancer patients who were positive for the RET/PTC mutation (P = .02), while thyroid cancer expressing BRAF V600E or RET/PTC was associated with higher-grade cancer on presentation (P < .001) and early recurrence (P < .001).
Data source: Retrospective review of a consecutive series of 1,510 patients with thyroidectomy for thyroid cancer and testing for thyroid cancer-specific genetic alterations.
Disclosures: One of the researchers is a consultant for Quest Diagnostics. The others reported no disclosures.
AACE: How to safely skip radioactive iodine for low-grade thyroid cancer
NASHVILLE, TENN. – Patients with stage I or II differentiated thyroid cancers do not need radioactive iodine treatment if their nonsuppressed thyroglobulin level is less than 2 ng/mL 2 weeks after surgery, according to Dr. Kathleen Hands.
When that’s the case, “I know the patient had an excellent surgery and will have an excellent prognosis with an extremely low likelihood of recurrence over the next 10 years without radioactive iodine. These patients can be managed safely and effectively without radioactive iodine in a community setting,” said Dr. Hands, a thyroidologist who practices in San Antonio.
It’s common for patients in the United States to receive iodine-131 (I-131) after surgery for low-risk thyroid cancers “despite the abundance of evidence” showing that it does them no good and may cause harm and despite guidelines calling for conservative use of I-131, she said (World. J. Surg. 2002;26:879-85).
“It’s a habit,” a holdover from decades ago “when we didn’t actually have good surgical technique. We need to [heed recent data] and step away from what we did in the 60s, 70s, and 80s and get into the 21st century. We should stop using radioactive iodine in these low-risk patients,” Dr. Hands said at the American Association of Clinical Endocrinologists annual meeting.
Among radioactive iodine’s drawbacks are its expense and sometimes salivary and lacrimal problems associated with its use. Earlier in her career, “I personally had two of my cases” – 19 and 22 years old – “develop acute myelogenous leukemia [shortly] after I-131, one of whom succumbed. I took that very seriously. I’ve become very conservative in the use of this drug. Ablation should be restricted to patients with incomplete surgical excision or poor prognostic factors for recurrence or death,” she said.
This advice is backed up by findings from her review of 378 patients who underwent surgery for differentiated thyroid cancer, with MACIS (metastasis, age, completeness of resection, invasion, and size) scores below 7, meaning low-intermediate-risk disease. Patients ranged from 18 to 79 years old. The majority were women, and about a third had multifocal disease. Tumor sizes ranged from 0.8 mm to 4.0 cm. Twenty-one patients under 45 years old had lymph node metastases of less than 5 mm.
The patients had nonsuppressed thyroglobulin levels below 2 ng/mL 2 weeks after surgery. They opted against I-131, and were started on levothyroxine. There’s been no recurrence of disease in the group after 8 years’ follow-up; thyroglobulin was undetectable in 72% by 2 years. Those in whom thyroglobulin remained detectable had thyroglobulin velocities below 10% over a period of 5 years.
“Nonsuppressed thyroglobulin” means that the patients were not put on thyroxine right after surgery, so that Dr. Hands could get an idea if any tumor was left 2 weeks later. They also weren’t put on low-iodine diets in the interim, she said, because she had no intention of giving them I-131.
To get the most out of the approach, patients need excellent and complete surgeries. That means that endocrinologists should learn to perform preoperative neck ultrasounds – or refer to someone who can – to give surgeons a heads-up about tumor location, size, shape, and invasiveness, as well as lymph node involvement, calcifications, and other issues. “This is the kind of information your surgeon needs” to do a good job, Dr. Hands said.
She said she doesn’t worry about hypothyroidism when patients don’t get thyroxine right after surgery. Manipulation of the thyroid during surgery releases hormone into the system, and “I think that tides them over; It’s a long-acting hormone. Patients tolerate not having replacement immediately [after surgery],” Dr. Hands said.
There was no funding for the project, and Dr. Hands said she had no relevant financial disclosures.
NASHVILLE, TENN. – Patients with stage I or II differentiated thyroid cancers do not need radioactive iodine treatment if their nonsuppressed thyroglobulin level is less than 2 ng/mL 2 weeks after surgery, according to Dr. Kathleen Hands.
When that’s the case, “I know the patient had an excellent surgery and will have an excellent prognosis with an extremely low likelihood of recurrence over the next 10 years without radioactive iodine. These patients can be managed safely and effectively without radioactive iodine in a community setting,” said Dr. Hands, a thyroidologist who practices in San Antonio.
It’s common for patients in the United States to receive iodine-131 (I-131) after surgery for low-risk thyroid cancers “despite the abundance of evidence” showing that it does them no good and may cause harm and despite guidelines calling for conservative use of I-131, she said (World. J. Surg. 2002;26:879-85).
“It’s a habit,” a holdover from decades ago “when we didn’t actually have good surgical technique. We need to [heed recent data] and step away from what we did in the 60s, 70s, and 80s and get into the 21st century. We should stop using radioactive iodine in these low-risk patients,” Dr. Hands said at the American Association of Clinical Endocrinologists annual meeting.
Among radioactive iodine’s drawbacks are its expense and sometimes salivary and lacrimal problems associated with its use. Earlier in her career, “I personally had two of my cases” – 19 and 22 years old – “develop acute myelogenous leukemia [shortly] after I-131, one of whom succumbed. I took that very seriously. I’ve become very conservative in the use of this drug. Ablation should be restricted to patients with incomplete surgical excision or poor prognostic factors for recurrence or death,” she said.
This advice is backed up by findings from her review of 378 patients who underwent surgery for differentiated thyroid cancer, with MACIS (metastasis, age, completeness of resection, invasion, and size) scores below 7, meaning low-intermediate-risk disease. Patients ranged from 18 to 79 years old. The majority were women, and about a third had multifocal disease. Tumor sizes ranged from 0.8 mm to 4.0 cm. Twenty-one patients under 45 years old had lymph node metastases of less than 5 mm.
The patients had nonsuppressed thyroglobulin levels below 2 ng/mL 2 weeks after surgery. They opted against I-131, and were started on levothyroxine. There’s been no recurrence of disease in the group after 8 years’ follow-up; thyroglobulin was undetectable in 72% by 2 years. Those in whom thyroglobulin remained detectable had thyroglobulin velocities below 10% over a period of 5 years.
“Nonsuppressed thyroglobulin” means that the patients were not put on thyroxine right after surgery, so that Dr. Hands could get an idea if any tumor was left 2 weeks later. They also weren’t put on low-iodine diets in the interim, she said, because she had no intention of giving them I-131.
To get the most out of the approach, patients need excellent and complete surgeries. That means that endocrinologists should learn to perform preoperative neck ultrasounds – or refer to someone who can – to give surgeons a heads-up about tumor location, size, shape, and invasiveness, as well as lymph node involvement, calcifications, and other issues. “This is the kind of information your surgeon needs” to do a good job, Dr. Hands said.
She said she doesn’t worry about hypothyroidism when patients don’t get thyroxine right after surgery. Manipulation of the thyroid during surgery releases hormone into the system, and “I think that tides them over; It’s a long-acting hormone. Patients tolerate not having replacement immediately [after surgery],” Dr. Hands said.
There was no funding for the project, and Dr. Hands said she had no relevant financial disclosures.
NASHVILLE, TENN. – Patients with stage I or II differentiated thyroid cancers do not need radioactive iodine treatment if their nonsuppressed thyroglobulin level is less than 2 ng/mL 2 weeks after surgery, according to Dr. Kathleen Hands.
When that’s the case, “I know the patient had an excellent surgery and will have an excellent prognosis with an extremely low likelihood of recurrence over the next 10 years without radioactive iodine. These patients can be managed safely and effectively without radioactive iodine in a community setting,” said Dr. Hands, a thyroidologist who practices in San Antonio.
It’s common for patients in the United States to receive iodine-131 (I-131) after surgery for low-risk thyroid cancers “despite the abundance of evidence” showing that it does them no good and may cause harm and despite guidelines calling for conservative use of I-131, she said (World. J. Surg. 2002;26:879-85).
“It’s a habit,” a holdover from decades ago “when we didn’t actually have good surgical technique. We need to [heed recent data] and step away from what we did in the 60s, 70s, and 80s and get into the 21st century. We should stop using radioactive iodine in these low-risk patients,” Dr. Hands said at the American Association of Clinical Endocrinologists annual meeting.
Among radioactive iodine’s drawbacks are its expense and sometimes salivary and lacrimal problems associated with its use. Earlier in her career, “I personally had two of my cases” – 19 and 22 years old – “develop acute myelogenous leukemia [shortly] after I-131, one of whom succumbed. I took that very seriously. I’ve become very conservative in the use of this drug. Ablation should be restricted to patients with incomplete surgical excision or poor prognostic factors for recurrence or death,” she said.
This advice is backed up by findings from her review of 378 patients who underwent surgery for differentiated thyroid cancer, with MACIS (metastasis, age, completeness of resection, invasion, and size) scores below 7, meaning low-intermediate-risk disease. Patients ranged from 18 to 79 years old. The majority were women, and about a third had multifocal disease. Tumor sizes ranged from 0.8 mm to 4.0 cm. Twenty-one patients under 45 years old had lymph node metastases of less than 5 mm.
The patients had nonsuppressed thyroglobulin levels below 2 ng/mL 2 weeks after surgery. They opted against I-131, and were started on levothyroxine. There’s been no recurrence of disease in the group after 8 years’ follow-up; thyroglobulin was undetectable in 72% by 2 years. Those in whom thyroglobulin remained detectable had thyroglobulin velocities below 10% over a period of 5 years.
“Nonsuppressed thyroglobulin” means that the patients were not put on thyroxine right after surgery, so that Dr. Hands could get an idea if any tumor was left 2 weeks later. They also weren’t put on low-iodine diets in the interim, she said, because she had no intention of giving them I-131.
To get the most out of the approach, patients need excellent and complete surgeries. That means that endocrinologists should learn to perform preoperative neck ultrasounds – or refer to someone who can – to give surgeons a heads-up about tumor location, size, shape, and invasiveness, as well as lymph node involvement, calcifications, and other issues. “This is the kind of information your surgeon needs” to do a good job, Dr. Hands said.
She said she doesn’t worry about hypothyroidism when patients don’t get thyroxine right after surgery. Manipulation of the thyroid during surgery releases hormone into the system, and “I think that tides them over; It’s a long-acting hormone. Patients tolerate not having replacement immediately [after surgery],” Dr. Hands said.
There was no funding for the project, and Dr. Hands said she had no relevant financial disclosures.
AT AACE 2015
Key clinical point: Thyroid cancer patients do not need radioactive iodine treatment if their nonsuppressed thyroglobulin is less than 2 ng/mL 2 weeks after surgery.
Major finding: Among 378 patients whose nonsuppressed thyroglobulin levels were below 2 ng/mL 2 weeks after removal of low-risk differentiated thyroid cancers, there were zero recurrences over 8 years of follow-up.
Data source: A single-center, retrospective study.
Disclosures: The investigator said she had no relevant financial disclosures and no outside funding.
VIDEO: Elective neck dissection during primary surgery improves oral cancer survival
CHICAGO – Patients who had elective neck dissection at the time of primary surgery for oral cancers had a 12.5% better overall survival rate than did patients who had therapeutic neck dissections at the time of recurrence.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil D’Cruz of the head and neck service of Tata Memorial Centre, Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study,” he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Patients who had elective neck dissection at the time of primary surgery for oral cancers had a 12.5% better overall survival rate than did patients who had therapeutic neck dissections at the time of recurrence.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil D’Cruz of the head and neck service of Tata Memorial Centre, Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study,” he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Patients who had elective neck dissection at the time of primary surgery for oral cancers had a 12.5% better overall survival rate than did patients who had therapeutic neck dissections at the time of recurrence.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil D’Cruz of the head and neck service of Tata Memorial Centre, Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study,” he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE 2015 ASCO ANNUAL MEETING
Upfront neck dissection boosts oral cancer survival
CHICAGO – Elective neck dissection at the time of primary surgery for early oral cancers increased overall survival by 12.5% compared with a watch-and-wait approach.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil K. D’Cruz from the head and neck service of the Tata Memorial Centre in Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study. For every eight patients undergoing elective neck dissection, one death is prevented, and for every four patients who undergo elective neck dissection, one recurrence is prevented,” he said at a briefing prior to his presentation of the data in a plenary session at the annual meeting of the American Society of Clinical Oncology.
“Dr. D’Cruz should be congratulated on such a robust study that will impact the lives of potentially over 300,000 people with oral cancer globally. This is particularly important in countries and in populations where there are multiple barriers to health care. This ‘one and done’ approach we know now definitively improves survival,” commented ASCO Expert Dr. Jyoti D. Patel from the Feinberg School of Medicine at Northwestern University, Chicago.
Dr. Patel moderated the briefing, but was not involved in the study.
Results of the study are also published online in the New England Journal of Medicine.
Cancers of the lips and oral cavity are especially prevalent in countries where tobacco use and excessive alcohol consumption are common, with these two risk factors alone accounting for an estimated 90% of oral cancer diagnoses.
Until now, management of neck lymph nodes at the time of primary surgery for oral cancers has been controversial, due to a lack of clear evidence of a survival disadvantage to waiting until recurrence before performing a neck dissection, and to concerns about additional surgical procedures with their associated morbidities, including nerve injury and shoulder dysfunction, Dr. D’Cruz said in an interview.
In an attempt to settle the question, Dr. D’Cruz and colleagues conducted a randomized trial in which patients with early stage oral squamous cell carcinomas (stage T1-T2,N0) underwent perioral excision of the primary tumor and were then assigned to either elective neck dissection, or an observation (watch and wait) strategy to be followed by therapeutic neck dissection for nodal relapses.
The trial was terminated early because of evident benefit after 596 patients had been recruited. Dr. D’Cruz presented data from the second interim analysis of 500 patients with a least 9 months of follow-up, 245 of whom had been randomized to upfront neck dissection, and 255 of whom were assigned to watch-and-wait. There were 427 cancers of the tongue, 68 of the buccal mucosa, and 5 of the floor of the mouth.
At a median follow-up of 39 months, 50 patients treated with elective neck dissection had died, compared with 79 patients treated with observation, translating into a 12.5% improvement in overall survival for the elective strategy.
The upfront neck dissection strategy was associated with a 23.6% absolute increase in 3-year overall survival (80% vs. 67.5%, hazard ratio [HR] 0.63, P = .01). The benefits of elective dissection on both overall survival and disease-free survival remained after adjustment for stratification factors, Dr. D’Cruz said.
For the secondary endpoint of disease-free survival, there were 81 recurrences among patients treated with elective dissection, compared with 146 for those assigned to watch and wait. This translated into respective DFS rates of 69.5% and 45.9% (HR 0.45 P < .001).
The rates of adverse events were 6.6% for patients treated with elective dissection, compared with 3.6% for patients who underwent therapeutic dissection.
Dr. Hisham Mehanna, from the Institute of Head and Neck Studies and Eduation at the University of Birmingham, United Kingdom, the invited discussant, commented in the plenary that sentinel node biopsy might be an effective method for monitoring patients for recurrence, thus sparing the possible need for upfront dissection with its attendant morbidities. Surveillance by clinical follow-up alone, however, is not adequate, and in settings where only clinical follow-up is available (such as in low resource settings), elective neck dissection should become the standard of care, he recommended.
The study was sponsored by India’s Department of Atomic Energy Clinical Trial Center. Dr. D’Cruz and Dr. Patel reported having no disclosures relevant to the study.
CHICAGO – Elective neck dissection at the time of primary surgery for early oral cancers increased overall survival by 12.5% compared with a watch-and-wait approach.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil K. D’Cruz from the head and neck service of the Tata Memorial Centre in Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study. For every eight patients undergoing elective neck dissection, one death is prevented, and for every four patients who undergo elective neck dissection, one recurrence is prevented,” he said at a briefing prior to his presentation of the data in a plenary session at the annual meeting of the American Society of Clinical Oncology.
“Dr. D’Cruz should be congratulated on such a robust study that will impact the lives of potentially over 300,000 people with oral cancer globally. This is particularly important in countries and in populations where there are multiple barriers to health care. This ‘one and done’ approach we know now definitively improves survival,” commented ASCO Expert Dr. Jyoti D. Patel from the Feinberg School of Medicine at Northwestern University, Chicago.
Dr. Patel moderated the briefing, but was not involved in the study.
Results of the study are also published online in the New England Journal of Medicine.
Cancers of the lips and oral cavity are especially prevalent in countries where tobacco use and excessive alcohol consumption are common, with these two risk factors alone accounting for an estimated 90% of oral cancer diagnoses.
Until now, management of neck lymph nodes at the time of primary surgery for oral cancers has been controversial, due to a lack of clear evidence of a survival disadvantage to waiting until recurrence before performing a neck dissection, and to concerns about additional surgical procedures with their associated morbidities, including nerve injury and shoulder dysfunction, Dr. D’Cruz said in an interview.
In an attempt to settle the question, Dr. D’Cruz and colleagues conducted a randomized trial in which patients with early stage oral squamous cell carcinomas (stage T1-T2,N0) underwent perioral excision of the primary tumor and were then assigned to either elective neck dissection, or an observation (watch and wait) strategy to be followed by therapeutic neck dissection for nodal relapses.
The trial was terminated early because of evident benefit after 596 patients had been recruited. Dr. D’Cruz presented data from the second interim analysis of 500 patients with a least 9 months of follow-up, 245 of whom had been randomized to upfront neck dissection, and 255 of whom were assigned to watch-and-wait. There were 427 cancers of the tongue, 68 of the buccal mucosa, and 5 of the floor of the mouth.
At a median follow-up of 39 months, 50 patients treated with elective neck dissection had died, compared with 79 patients treated with observation, translating into a 12.5% improvement in overall survival for the elective strategy.
The upfront neck dissection strategy was associated with a 23.6% absolute increase in 3-year overall survival (80% vs. 67.5%, hazard ratio [HR] 0.63, P = .01). The benefits of elective dissection on both overall survival and disease-free survival remained after adjustment for stratification factors, Dr. D’Cruz said.
For the secondary endpoint of disease-free survival, there were 81 recurrences among patients treated with elective dissection, compared with 146 for those assigned to watch and wait. This translated into respective DFS rates of 69.5% and 45.9% (HR 0.45 P < .001).
The rates of adverse events were 6.6% for patients treated with elective dissection, compared with 3.6% for patients who underwent therapeutic dissection.
Dr. Hisham Mehanna, from the Institute of Head and Neck Studies and Eduation at the University of Birmingham, United Kingdom, the invited discussant, commented in the plenary that sentinel node biopsy might be an effective method for monitoring patients for recurrence, thus sparing the possible need for upfront dissection with its attendant morbidities. Surveillance by clinical follow-up alone, however, is not adequate, and in settings where only clinical follow-up is available (such as in low resource settings), elective neck dissection should become the standard of care, he recommended.
The study was sponsored by India’s Department of Atomic Energy Clinical Trial Center. Dr. D’Cruz and Dr. Patel reported having no disclosures relevant to the study.
CHICAGO – Elective neck dissection at the time of primary surgery for early oral cancers increased overall survival by 12.5% compared with a watch-and-wait approach.
The risk of death was reduced by 36% among patients randomized in a phase III trial to neck lymph node dissection at the time of primary surgery, and the risk of recurrences was reduced by 55%, reported Dr. Anil K. D’Cruz from the head and neck service of the Tata Memorial Centre in Mumbai, India.
“Elective neck dissection should be the standard of care for early oral, node-negative squamous cell cancers, based on the findings of our study. For every eight patients undergoing elective neck dissection, one death is prevented, and for every four patients who undergo elective neck dissection, one recurrence is prevented,” he said at a briefing prior to his presentation of the data in a plenary session at the annual meeting of the American Society of Clinical Oncology.
“Dr. D’Cruz should be congratulated on such a robust study that will impact the lives of potentially over 300,000 people with oral cancer globally. This is particularly important in countries and in populations where there are multiple barriers to health care. This ‘one and done’ approach we know now definitively improves survival,” commented ASCO Expert Dr. Jyoti D. Patel from the Feinberg School of Medicine at Northwestern University, Chicago.
Dr. Patel moderated the briefing, but was not involved in the study.
Results of the study are also published online in the New England Journal of Medicine.
Cancers of the lips and oral cavity are especially prevalent in countries where tobacco use and excessive alcohol consumption are common, with these two risk factors alone accounting for an estimated 90% of oral cancer diagnoses.
Until now, management of neck lymph nodes at the time of primary surgery for oral cancers has been controversial, due to a lack of clear evidence of a survival disadvantage to waiting until recurrence before performing a neck dissection, and to concerns about additional surgical procedures with their associated morbidities, including nerve injury and shoulder dysfunction, Dr. D’Cruz said in an interview.
In an attempt to settle the question, Dr. D’Cruz and colleagues conducted a randomized trial in which patients with early stage oral squamous cell carcinomas (stage T1-T2,N0) underwent perioral excision of the primary tumor and were then assigned to either elective neck dissection, or an observation (watch and wait) strategy to be followed by therapeutic neck dissection for nodal relapses.
The trial was terminated early because of evident benefit after 596 patients had been recruited. Dr. D’Cruz presented data from the second interim analysis of 500 patients with a least 9 months of follow-up, 245 of whom had been randomized to upfront neck dissection, and 255 of whom were assigned to watch-and-wait. There were 427 cancers of the tongue, 68 of the buccal mucosa, and 5 of the floor of the mouth.
At a median follow-up of 39 months, 50 patients treated with elective neck dissection had died, compared with 79 patients treated with observation, translating into a 12.5% improvement in overall survival for the elective strategy.
The upfront neck dissection strategy was associated with a 23.6% absolute increase in 3-year overall survival (80% vs. 67.5%, hazard ratio [HR] 0.63, P = .01). The benefits of elective dissection on both overall survival and disease-free survival remained after adjustment for stratification factors, Dr. D’Cruz said.
For the secondary endpoint of disease-free survival, there were 81 recurrences among patients treated with elective dissection, compared with 146 for those assigned to watch and wait. This translated into respective DFS rates of 69.5% and 45.9% (HR 0.45 P < .001).
The rates of adverse events were 6.6% for patients treated with elective dissection, compared with 3.6% for patients who underwent therapeutic dissection.
Dr. Hisham Mehanna, from the Institute of Head and Neck Studies and Eduation at the University of Birmingham, United Kingdom, the invited discussant, commented in the plenary that sentinel node biopsy might be an effective method for monitoring patients for recurrence, thus sparing the possible need for upfront dissection with its attendant morbidities. Surveillance by clinical follow-up alone, however, is not adequate, and in settings where only clinical follow-up is available (such as in low resource settings), elective neck dissection should become the standard of care, he recommended.
The study was sponsored by India’s Department of Atomic Energy Clinical Trial Center. Dr. D’Cruz and Dr. Patel reported having no disclosures relevant to the study.
AT ASCO 2015
Key clinical point: Elective neck dissection at the time of primary surgery for oral cancers improves overall survival.
Major finding: Elective neck dissection improved overall survival by 12.5% compared with therapeutic dissection at the time of recurrence.
Data source: Randomized clinical trial halted early for efficacy; interim analysis of the first 500 patients with cancers of the tongue, buccosal mucosa, and floor of the mouth.
Disclosures: The study was sponsored by India’s Department of Atomic Energy Clinical Trial Center. Dr. D’Cruz and Dr. Patel reported having no disclosures relavent to the study.
Pembrolizumab active in head and neck cancer, regardless of HPV status
CHICAGO – One in four patients with recurrent or metastatic head and neck cancer respond to anti-PD-1 immunotherapy with pembrolizumab, according to preliminary expanded cohort results from KEYNOTE-012.
Among 117 evaluable patients, the objective response rate with pembrolizumab (Keytruda) was 24.8%, including 1 complete response and 28 partial responses.
Pembrolizumab was active in both human papillomavirus-negative and HPV-positive tumors, with response rates of 27.2% and 20.6%.
The efficacy is remarkable in this setting and when measured by response, pembrolizumab seems to be roughly twice as effective as cetuximab, our only targeted therapy, study author Dr. Tanguy Seiwert said during a press briefing in advance of his presentation at the annual meeting of the American Society of Clinical Oncology.
In the pivotal EXTREME trial leading to cetuximab’s approval, 36% of patients responded to cetuximab (Erbitux), when the epidermal growth factor receptor inhibitor was added to platinum-based chemotherapy in recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) . Only 10%-13% of patients, however, respond to single-agent cetuximab. Also, several recent studies, with the exception of a retrospective EXTREME analysis, suggest cetuximab efficacy varies with HPV status, Dr. Seiwert, from the University of Chicago, said in an interview.
Pembrolizumab was the first anti-programmed death (PD)-1 therapy to reach the market, following its September 2014 approval for use in metastatic melanoma.
The phase Ib, multi-cohort KEYNOTE-012 enrolled patients with advanced solid tumors and previously reported a 20% response rate with pembrolizumab 10 mg/kg every 2 weeks in recurrent or metastatic SCCHN enriched for PD-L1-positive tumors.
For the expansion cohort, 132 patients with recurrent or metastatic SCCHN were enrolled, irrespective of PD-L1 expression or HPV status, and pembrolizumab was given at a fixed dose of 200 mg every 3 weeks. Their mean age was 59 years and nearly 60% had received two or more prior lines of therapy. The primary end point was objective response rate per investigator assessment using RECIST v1.1.
Overall, 56% of patents experienced some tumor shrinkage. The median time to response was 9 weeks (range, 7.6-18 weeks).
Responses were durable, with 86% of responding patients remaining in response, Dr. Seiwert said. Overall, 40 patients are still on therapy, Dr. Seiwert said.
Data reported in a separate study at the meeting suggest that a novel interferon-gamma expression signature may be useful in predicting which patients are likely to benefit from therapy, with a negative predictive value of 95% and positive predictive value of 40%, he said.
Adverse events were reported in 60% of all 132 patients, most commonly fatigue, hypothyroidism, and decreased appetite. Serious grade 3-4 drug-related events were reported in 13 patients and included pneumonitis in 2 and facial swelling in 2.
ASCO expert Dr. Gregory Masters, of Christiana Care Health System in Newark, DE., commented in a statement that, “This is yet another example where PD-1 immunotherapy might work better and more reliably than existing drugs, and with fewer side effects. The diversity of patients who responded is greater than in any previous trials.”
Dr. Masters added that larger studies and longer follow-up are needed to assess the impact of treatment on patient survival.
Pembrolizumab is being evaluated against standard therapy in recurrent or metastatic head and neck cancer in two phase III trials, KEYNOTE-040 and KEYNOTE-048.
CHICAGO – One in four patients with recurrent or metastatic head and neck cancer respond to anti-PD-1 immunotherapy with pembrolizumab, according to preliminary expanded cohort results from KEYNOTE-012.
Among 117 evaluable patients, the objective response rate with pembrolizumab (Keytruda) was 24.8%, including 1 complete response and 28 partial responses.
Pembrolizumab was active in both human papillomavirus-negative and HPV-positive tumors, with response rates of 27.2% and 20.6%.
The efficacy is remarkable in this setting and when measured by response, pembrolizumab seems to be roughly twice as effective as cetuximab, our only targeted therapy, study author Dr. Tanguy Seiwert said during a press briefing in advance of his presentation at the annual meeting of the American Society of Clinical Oncology.
In the pivotal EXTREME trial leading to cetuximab’s approval, 36% of patients responded to cetuximab (Erbitux), when the epidermal growth factor receptor inhibitor was added to platinum-based chemotherapy in recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) . Only 10%-13% of patients, however, respond to single-agent cetuximab. Also, several recent studies, with the exception of a retrospective EXTREME analysis, suggest cetuximab efficacy varies with HPV status, Dr. Seiwert, from the University of Chicago, said in an interview.
Pembrolizumab was the first anti-programmed death (PD)-1 therapy to reach the market, following its September 2014 approval for use in metastatic melanoma.
The phase Ib, multi-cohort KEYNOTE-012 enrolled patients with advanced solid tumors and previously reported a 20% response rate with pembrolizumab 10 mg/kg every 2 weeks in recurrent or metastatic SCCHN enriched for PD-L1-positive tumors.
For the expansion cohort, 132 patients with recurrent or metastatic SCCHN were enrolled, irrespective of PD-L1 expression or HPV status, and pembrolizumab was given at a fixed dose of 200 mg every 3 weeks. Their mean age was 59 years and nearly 60% had received two or more prior lines of therapy. The primary end point was objective response rate per investigator assessment using RECIST v1.1.
Overall, 56% of patents experienced some tumor shrinkage. The median time to response was 9 weeks (range, 7.6-18 weeks).
Responses were durable, with 86% of responding patients remaining in response, Dr. Seiwert said. Overall, 40 patients are still on therapy, Dr. Seiwert said.
Data reported in a separate study at the meeting suggest that a novel interferon-gamma expression signature may be useful in predicting which patients are likely to benefit from therapy, with a negative predictive value of 95% and positive predictive value of 40%, he said.
Adverse events were reported in 60% of all 132 patients, most commonly fatigue, hypothyroidism, and decreased appetite. Serious grade 3-4 drug-related events were reported in 13 patients and included pneumonitis in 2 and facial swelling in 2.
ASCO expert Dr. Gregory Masters, of Christiana Care Health System in Newark, DE., commented in a statement that, “This is yet another example where PD-1 immunotherapy might work better and more reliably than existing drugs, and with fewer side effects. The diversity of patients who responded is greater than in any previous trials.”
Dr. Masters added that larger studies and longer follow-up are needed to assess the impact of treatment on patient survival.
Pembrolizumab is being evaluated against standard therapy in recurrent or metastatic head and neck cancer in two phase III trials, KEYNOTE-040 and KEYNOTE-048.
CHICAGO – One in four patients with recurrent or metastatic head and neck cancer respond to anti-PD-1 immunotherapy with pembrolizumab, according to preliminary expanded cohort results from KEYNOTE-012.
Among 117 evaluable patients, the objective response rate with pembrolizumab (Keytruda) was 24.8%, including 1 complete response and 28 partial responses.
Pembrolizumab was active in both human papillomavirus-negative and HPV-positive tumors, with response rates of 27.2% and 20.6%.
The efficacy is remarkable in this setting and when measured by response, pembrolizumab seems to be roughly twice as effective as cetuximab, our only targeted therapy, study author Dr. Tanguy Seiwert said during a press briefing in advance of his presentation at the annual meeting of the American Society of Clinical Oncology.
In the pivotal EXTREME trial leading to cetuximab’s approval, 36% of patients responded to cetuximab (Erbitux), when the epidermal growth factor receptor inhibitor was added to platinum-based chemotherapy in recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) . Only 10%-13% of patients, however, respond to single-agent cetuximab. Also, several recent studies, with the exception of a retrospective EXTREME analysis, suggest cetuximab efficacy varies with HPV status, Dr. Seiwert, from the University of Chicago, said in an interview.
Pembrolizumab was the first anti-programmed death (PD)-1 therapy to reach the market, following its September 2014 approval for use in metastatic melanoma.
The phase Ib, multi-cohort KEYNOTE-012 enrolled patients with advanced solid tumors and previously reported a 20% response rate with pembrolizumab 10 mg/kg every 2 weeks in recurrent or metastatic SCCHN enriched for PD-L1-positive tumors.
For the expansion cohort, 132 patients with recurrent or metastatic SCCHN were enrolled, irrespective of PD-L1 expression or HPV status, and pembrolizumab was given at a fixed dose of 200 mg every 3 weeks. Their mean age was 59 years and nearly 60% had received two or more prior lines of therapy. The primary end point was objective response rate per investigator assessment using RECIST v1.1.
Overall, 56% of patents experienced some tumor shrinkage. The median time to response was 9 weeks (range, 7.6-18 weeks).
Responses were durable, with 86% of responding patients remaining in response, Dr. Seiwert said. Overall, 40 patients are still on therapy, Dr. Seiwert said.
Data reported in a separate study at the meeting suggest that a novel interferon-gamma expression signature may be useful in predicting which patients are likely to benefit from therapy, with a negative predictive value of 95% and positive predictive value of 40%, he said.
Adverse events were reported in 60% of all 132 patients, most commonly fatigue, hypothyroidism, and decreased appetite. Serious grade 3-4 drug-related events were reported in 13 patients and included pneumonitis in 2 and facial swelling in 2.
ASCO expert Dr. Gregory Masters, of Christiana Care Health System in Newark, DE., commented in a statement that, “This is yet another example where PD-1 immunotherapy might work better and more reliably than existing drugs, and with fewer side effects. The diversity of patients who responded is greater than in any previous trials.”
Dr. Masters added that larger studies and longer follow-up are needed to assess the impact of treatment on patient survival.
Pembrolizumab is being evaluated against standard therapy in recurrent or metastatic head and neck cancer in two phase III trials, KEYNOTE-040 and KEYNOTE-048.
AT ASCO 2015
Key clinical point: Immunotherapy with pembrolizumab is active in patients with recurrent or metastatic head and neck cancer.
Major finding: The objective response rate was 24.8% overall, 27.2% in HPV-negative patients, and 20.6% in HPV-positive patients.
Data source: Expansion cohort of 132 patients with recurrent or metastatic head and neck cancer from the phase Ib KEYSTONE-012 study.
Disclosures: Merck, Sharp & Dohme funded the study. Dr. Seiwert reported honoraria from Novartis, Bayer/Onyx, and Merck and institutional research funding from Genentech/Roche and Boehringer Ingelheim. Several co-authors reported financial relationships including employment with MSD or its parent company, Merck. Dr. Masters reported having no conflicts.
Oral cancer survival lower with positive margins, public insurance
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
In patients who underwent surgical treatment for stage I or II oral cavity squamous cell cancer, positive tumor margin, the use of radiation or chemotherapy, treatment in a nonacademic facility, and having public health insurance were significantly associated with lower 5-year survival rates, according to a retrospective analysis published online in the JAMA Otolaryngology–Head & Neck Surgery.
The findings suggest that some factors associated with lower 5-year survival rates “may be targets for quality improvement efforts,” wrote Alexander L. Luryi of Yale University, New Haven, Conn., and colleagues.
Seventy percent of 6,830 patients who underwent surgery for stage I or II oral cavity squamous cell cancer (OCSCC) from 2003 to 2006 survived 5 years, according to information from the National Cancer Data Base.
Multivariate analysis showed higher survival rates were significantly associated with neck dissection (hazard ratio, 0.85; P = .003). Lower survival rates were significantly associated with radiation therapy (HR, 1.31; P < .001), chemotherapy (HR, 1.34; P = .03), nonprivate insurance (HR Medicaid, 1.96; HR Medicare, 1.45; P < .001), and nonacademic treatment facility (HR, 1.13; P = .03).
Care at academic centers compared with nonacademic centers was associated with improved survival, possibly due to health care provider expertise, the study authors noted (JAMA Otolaryngol. Head Neck Surg. 2015 May 14 [doi:10.1001/jamaoto.2015.0719]).
Survival rates were lower in patients treated at nonacademic cancer centers, but multivariate analysis showed no association between facility-based case volume and survival. Patients insured through Medicaid and Medicare had significantly lower 5-year survival rates (P < .001 for both). That finding may be the result of inconsistent treatment and follow-up, the investigators said, or worse baseline health among that patient population.
Controversy exists over the relationship between positive margins and outcomes, and the implications for aggressiveness of surgery. The study found positive margins were significantly associated with poorer outcomes, the researchers noted, which supports the use of aggressive surgery in early OCSCC to achieve negative margins.
Radiation and chemotherapy were linked to worse outcomes, and those therapies were possibly indicators of less aggressive resection in localized disease. The analysis could not adjust for potential confounding effects of perineural and lymphovascular invasion, because the information was not recorded in the National Cancer Data Base.
The study indicated a positive impact by neck dissection on survival. Patients with occult neck disease who underwent neck dissection likely would have been restaged to stage III or higher and removed from the early stage sample, the authors explained, which would account for higher survival rates for those remaining. Prospective trials are needed to determine the role of elective neck dissection in early OCSCC, the researchers added.
The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
FROM JAMA OTOLARYNGOLOGY–HEAD & NECK SURGERY
Key clinical point: Treatment factors such as neck dissection, tumor margins, type of insurance, and health care facility impact 5-year survival after surgery for early stage oral cavity cancer.
Major finding: Radiation, chemotherapy, positive tumor margin, nonacademic facility, and nonprivate insurance were significantly associated with lower 5-year survival.
Data source: Retrospective study of 6,830 patients in National Cancer Data Base who underwent surgery to treat stage I or II oral cavity squamous cell cancer.
Disclosures: The William U. Gardner Memorial Research Fund at Yale University supported the study. Dr. Luryi and coauthors reported having no disclosures.
Sleep disorders in patients with cancer
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
disorder
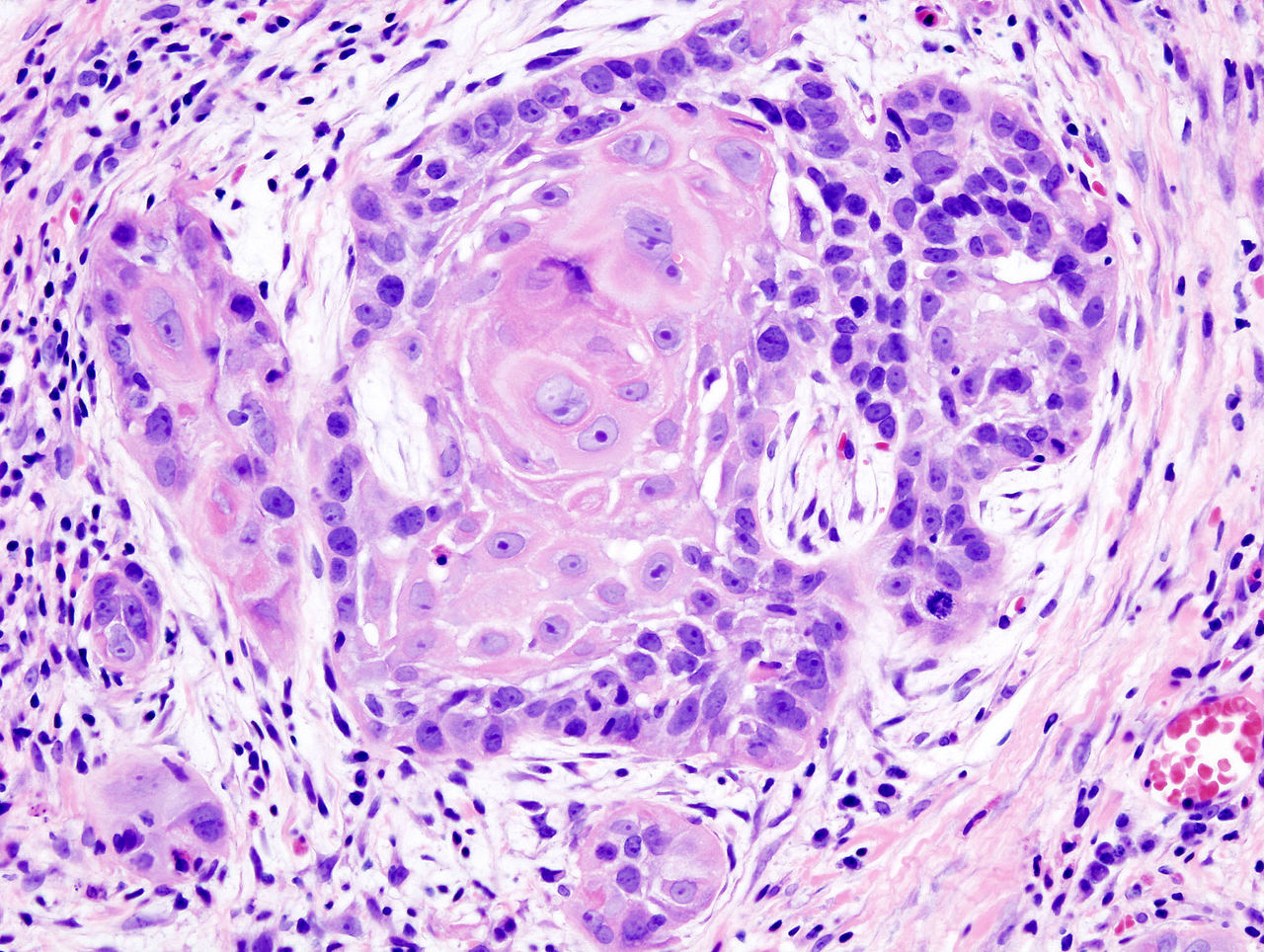
Several genetic variants linked to salivary gland cancer risk
Several single nucleotide polymorphisms were found to be significantly associated with salivary gland carcinoma in a genomewide association study, Li Xu, Ph.D., and associates at the University of Texas MD Anderson Cancer Center, Houston, reported online in Cancer.
The results of what they believe is the first such study conducted to identify common genetic variants associated with salivary gland carcinoma (SGC) need to be confirmed, but indicate that these single nucleotide polymorphisms (SNPs) could be further evaluated as possible screening tools for the rare cancer, they wrote (Cancer 2015 March 30 [doi:10.1002/cncr.29381]).
The study involved genotyping analyses of 309 cases of SGC in patients (mean age, 54 years) who had been treated at MD Anderson from September 2001 through February 2014; and 535 controls (mean age, 51 years), patients with no cancer.
Five SNPs were found to be associated with SGC risk. “The finding that the five novel SNPs associated with SGC risk are coding SNPs with functional potential, the finding that the genetic effects were considerable,” and the finding that the two SNPs with the strongest SGC risk were relatively rare among controls, “support that these five SNPs may be good candidate SNPs for SGC screening and prevention,” the authors concluded. “These findings support the existence of genetic heterogeneity between histological subtypes of SGC and provide a set of candidate SNPs and genes worthy of in-depth evaluation in future studies,” they added.
The small size of the study was among the limitations, and the results need to be confirmed in another study that also analyzes the function of the SNPs identified in the study, they noted.
In the United States, salivary gland carcinomas are rare, and account for about 0.3% of all malignancies, with an annual incidence of about 1 case per 100,000 people, Dr. Xu and associates said. A small proportion of SGCs are associated with high-dose ionizing radiation, the only well-documented environmental factor associated with the cancer, “suggesting a genetic role in the development of SGC,” they pointed out.
The authors had no disclosures. The study was partly supported by funds from the University of Texas MD Anderson Cancer Center, tge National Institutes of Health grants, and a Cancer Center Support grant.
Several single nucleotide polymorphisms were found to be significantly associated with salivary gland carcinoma in a genomewide association study, Li Xu, Ph.D., and associates at the University of Texas MD Anderson Cancer Center, Houston, reported online in Cancer.
The results of what they believe is the first such study conducted to identify common genetic variants associated with salivary gland carcinoma (SGC) need to be confirmed, but indicate that these single nucleotide polymorphisms (SNPs) could be further evaluated as possible screening tools for the rare cancer, they wrote (Cancer 2015 March 30 [doi:10.1002/cncr.29381]).
The study involved genotyping analyses of 309 cases of SGC in patients (mean age, 54 years) who had been treated at MD Anderson from September 2001 through February 2014; and 535 controls (mean age, 51 years), patients with no cancer.
Five SNPs were found to be associated with SGC risk. “The finding that the five novel SNPs associated with SGC risk are coding SNPs with functional potential, the finding that the genetic effects were considerable,” and the finding that the two SNPs with the strongest SGC risk were relatively rare among controls, “support that these five SNPs may be good candidate SNPs for SGC screening and prevention,” the authors concluded. “These findings support the existence of genetic heterogeneity between histological subtypes of SGC and provide a set of candidate SNPs and genes worthy of in-depth evaluation in future studies,” they added.
The small size of the study was among the limitations, and the results need to be confirmed in another study that also analyzes the function of the SNPs identified in the study, they noted.
In the United States, salivary gland carcinomas are rare, and account for about 0.3% of all malignancies, with an annual incidence of about 1 case per 100,000 people, Dr. Xu and associates said. A small proportion of SGCs are associated with high-dose ionizing radiation, the only well-documented environmental factor associated with the cancer, “suggesting a genetic role in the development of SGC,” they pointed out.
The authors had no disclosures. The study was partly supported by funds from the University of Texas MD Anderson Cancer Center, tge National Institutes of Health grants, and a Cancer Center Support grant.
Several single nucleotide polymorphisms were found to be significantly associated with salivary gland carcinoma in a genomewide association study, Li Xu, Ph.D., and associates at the University of Texas MD Anderson Cancer Center, Houston, reported online in Cancer.
The results of what they believe is the first such study conducted to identify common genetic variants associated with salivary gland carcinoma (SGC) need to be confirmed, but indicate that these single nucleotide polymorphisms (SNPs) could be further evaluated as possible screening tools for the rare cancer, they wrote (Cancer 2015 March 30 [doi:10.1002/cncr.29381]).
The study involved genotyping analyses of 309 cases of SGC in patients (mean age, 54 years) who had been treated at MD Anderson from September 2001 through February 2014; and 535 controls (mean age, 51 years), patients with no cancer.
Five SNPs were found to be associated with SGC risk. “The finding that the five novel SNPs associated with SGC risk are coding SNPs with functional potential, the finding that the genetic effects were considerable,” and the finding that the two SNPs with the strongest SGC risk were relatively rare among controls, “support that these five SNPs may be good candidate SNPs for SGC screening and prevention,” the authors concluded. “These findings support the existence of genetic heterogeneity between histological subtypes of SGC and provide a set of candidate SNPs and genes worthy of in-depth evaluation in future studies,” they added.
The small size of the study was among the limitations, and the results need to be confirmed in another study that also analyzes the function of the SNPs identified in the study, they noted.
In the United States, salivary gland carcinomas are rare, and account for about 0.3% of all malignancies, with an annual incidence of about 1 case per 100,000 people, Dr. Xu and associates said. A small proportion of SGCs are associated with high-dose ionizing radiation, the only well-documented environmental factor associated with the cancer, “suggesting a genetic role in the development of SGC,” they pointed out.
The authors had no disclosures. The study was partly supported by funds from the University of Texas MD Anderson Cancer Center, tge National Institutes of Health grants, and a Cancer Center Support grant.
Key clinical point: The identification of several gene variants associated with salivary gland carcinoma could eventually lead to screening tests for people at high risk for developing this rare cancer.
Major finding: Five different single nucleotide polymorphisms (SNPs) were significantly associated with salivary gland carcinomas.
Data source: A case-control genomewide association study looked for associations with salivary gland carcinomas, comparing results in 309 patients with salivary gland carcinoma and 535 controls with no cancer.
Disclosures: The authors had no disclosures. The study was partly supported by funds from the University of Texas MD Anderson Cancer Center, grants from the National Institutes of Health, and a Cancer Center Support grant.
Lateral neck dissection morbidity high, but transient
CHICAGO – Lateral neck dissection for thyroid cancer is associated with significant early postoperative morbidity of 20%, even in the hands of experienced endocrine surgeons at a high-volume medical center.
Among 99 procedures, 20 patients had 26 complications, including surgical site infection in 10, chyle leak in 7, spinal accessory nerve dysfunction in 7, and seroma in 2.
Long-term complications were rare, however, occurring in just one patient with a spinal accessory nerve injury, Dr. Jason A. Glenn said at the annual meeting of the Central Surgical Association.
Using a prospectively collected thyroid database, the investigators reviewed 96 patients who underwent lateral neck dissection (LND) for suspicion of initial or recurrent lateral neck metastases by one of four experienced endocrine surgeons at the Medical College of Wisconsin in Milwaukee.
Three patients had reoperations during the study period of February 2009 and June 2014, resulting in 99 procedures and 198 lateral necks evaluated preoperatively. Most patients were women (73%) and their median age was 45 years.
LND was performed on 127 necks and metastatic disease was confirmed in 111 (87%). This included all 82 patients who had positive preoperative fine needle aspiration (FNA), 25 of 37 patients operated on without FNA, and 4 of 8 patients with a negative or nondiagnostic FNA, Dr. Glenn said.
The median number of lymph nodes excised was 22 (range 1-122), with a median of 3 (range 0-39) malignant nodes per lateral neck.
“FNA is an important adjunct in the preoperative evaluation, especially when it returns a positive result,” he said. “However, when FNA is negative, not available, or not performed, you really must consider the entire clinical picture, as 64% of these patients were found to have lymph node metastases in our study.”
Surgical drains were placed in 94% of the 127 lateral neck dissections and remained in place for a median of 6 days. The median length of stay was 1 day.
There was no association between drain duration and surgical site infection, although chyle leak was associated with a significantly longer median drain duration (12 days vs. 6 days; P value < .01), Dr. Glenn said.
Two of the seven patients with chyle leak, defined by drain output that was milky white and/or exceeded 1,000 cc in 24 hours, underwent reoperation with ligation of the cervical thoracic duct and fibrin sealant application. Both leaks resolved and patients were discharge on postoperative day 2.
“Surgical drains allow for early leak recognition and monitoring of leak resolution,” he said. “Most of these complications were diagnosed and managed on an outpatient basis, highlighting the importance of continuity of care between the inpatient and outpatient setting for the treatment of thyroid cancer.”
Discussant Janice L. Pasieka, head of general surgery and a clinical professor of surgery and oncology at the University of Calgary (Alberta), said the retrospective review is a very valuable contribution to the literature because of its comprehensive follow-up.
“Today, most patients with this type of procedure are discharged within the 23 hours, and as such, complications such as nerve palsies, chyle leaks, and surgical site infections are not apparent for the majority of patients during their hospital stay,” Dr. Pasieka said. “Many times, the true incidences are lost unless the patient re-presents to the health care system, thus introducing your bias of only those significant enough to require intervention.”
Dr. Glenn and his coauthors reported no financial disclosures.
CHICAGO – Lateral neck dissection for thyroid cancer is associated with significant early postoperative morbidity of 20%, even in the hands of experienced endocrine surgeons at a high-volume medical center.
Among 99 procedures, 20 patients had 26 complications, including surgical site infection in 10, chyle leak in 7, spinal accessory nerve dysfunction in 7, and seroma in 2.
Long-term complications were rare, however, occurring in just one patient with a spinal accessory nerve injury, Dr. Jason A. Glenn said at the annual meeting of the Central Surgical Association.
Using a prospectively collected thyroid database, the investigators reviewed 96 patients who underwent lateral neck dissection (LND) for suspicion of initial or recurrent lateral neck metastases by one of four experienced endocrine surgeons at the Medical College of Wisconsin in Milwaukee.
Three patients had reoperations during the study period of February 2009 and June 2014, resulting in 99 procedures and 198 lateral necks evaluated preoperatively. Most patients were women (73%) and their median age was 45 years.
LND was performed on 127 necks and metastatic disease was confirmed in 111 (87%). This included all 82 patients who had positive preoperative fine needle aspiration (FNA), 25 of 37 patients operated on without FNA, and 4 of 8 patients with a negative or nondiagnostic FNA, Dr. Glenn said.
The median number of lymph nodes excised was 22 (range 1-122), with a median of 3 (range 0-39) malignant nodes per lateral neck.
“FNA is an important adjunct in the preoperative evaluation, especially when it returns a positive result,” he said. “However, when FNA is negative, not available, or not performed, you really must consider the entire clinical picture, as 64% of these patients were found to have lymph node metastases in our study.”
Surgical drains were placed in 94% of the 127 lateral neck dissections and remained in place for a median of 6 days. The median length of stay was 1 day.
There was no association between drain duration and surgical site infection, although chyle leak was associated with a significantly longer median drain duration (12 days vs. 6 days; P value < .01), Dr. Glenn said.
Two of the seven patients with chyle leak, defined by drain output that was milky white and/or exceeded 1,000 cc in 24 hours, underwent reoperation with ligation of the cervical thoracic duct and fibrin sealant application. Both leaks resolved and patients were discharge on postoperative day 2.
“Surgical drains allow for early leak recognition and monitoring of leak resolution,” he said. “Most of these complications were diagnosed and managed on an outpatient basis, highlighting the importance of continuity of care between the inpatient and outpatient setting for the treatment of thyroid cancer.”
Discussant Janice L. Pasieka, head of general surgery and a clinical professor of surgery and oncology at the University of Calgary (Alberta), said the retrospective review is a very valuable contribution to the literature because of its comprehensive follow-up.
“Today, most patients with this type of procedure are discharged within the 23 hours, and as such, complications such as nerve palsies, chyle leaks, and surgical site infections are not apparent for the majority of patients during their hospital stay,” Dr. Pasieka said. “Many times, the true incidences are lost unless the patient re-presents to the health care system, thus introducing your bias of only those significant enough to require intervention.”
Dr. Glenn and his coauthors reported no financial disclosures.
CHICAGO – Lateral neck dissection for thyroid cancer is associated with significant early postoperative morbidity of 20%, even in the hands of experienced endocrine surgeons at a high-volume medical center.
Among 99 procedures, 20 patients had 26 complications, including surgical site infection in 10, chyle leak in 7, spinal accessory nerve dysfunction in 7, and seroma in 2.
Long-term complications were rare, however, occurring in just one patient with a spinal accessory nerve injury, Dr. Jason A. Glenn said at the annual meeting of the Central Surgical Association.
Using a prospectively collected thyroid database, the investigators reviewed 96 patients who underwent lateral neck dissection (LND) for suspicion of initial or recurrent lateral neck metastases by one of four experienced endocrine surgeons at the Medical College of Wisconsin in Milwaukee.
Three patients had reoperations during the study period of February 2009 and June 2014, resulting in 99 procedures and 198 lateral necks evaluated preoperatively. Most patients were women (73%) and their median age was 45 years.
LND was performed on 127 necks and metastatic disease was confirmed in 111 (87%). This included all 82 patients who had positive preoperative fine needle aspiration (FNA), 25 of 37 patients operated on without FNA, and 4 of 8 patients with a negative or nondiagnostic FNA, Dr. Glenn said.
The median number of lymph nodes excised was 22 (range 1-122), with a median of 3 (range 0-39) malignant nodes per lateral neck.
“FNA is an important adjunct in the preoperative evaluation, especially when it returns a positive result,” he said. “However, when FNA is negative, not available, or not performed, you really must consider the entire clinical picture, as 64% of these patients were found to have lymph node metastases in our study.”
Surgical drains were placed in 94% of the 127 lateral neck dissections and remained in place for a median of 6 days. The median length of stay was 1 day.
There was no association between drain duration and surgical site infection, although chyle leak was associated with a significantly longer median drain duration (12 days vs. 6 days; P value < .01), Dr. Glenn said.
Two of the seven patients with chyle leak, defined by drain output that was milky white and/or exceeded 1,000 cc in 24 hours, underwent reoperation with ligation of the cervical thoracic duct and fibrin sealant application. Both leaks resolved and patients were discharge on postoperative day 2.
“Surgical drains allow for early leak recognition and monitoring of leak resolution,” he said. “Most of these complications were diagnosed and managed on an outpatient basis, highlighting the importance of continuity of care between the inpatient and outpatient setting for the treatment of thyroid cancer.”
Discussant Janice L. Pasieka, head of general surgery and a clinical professor of surgery and oncology at the University of Calgary (Alberta), said the retrospective review is a very valuable contribution to the literature because of its comprehensive follow-up.
“Today, most patients with this type of procedure are discharged within the 23 hours, and as such, complications such as nerve palsies, chyle leaks, and surgical site infections are not apparent for the majority of patients during their hospital stay,” Dr. Pasieka said. “Many times, the true incidences are lost unless the patient re-presents to the health care system, thus introducing your bias of only those significant enough to require intervention.”
Dr. Glenn and his coauthors reported no financial disclosures.
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Lateral neck dissections for thyroid cancer are associated with high early morbidity but few long-term complications.
Major finding: The overall complication rate was 20%, however, most were transient.
Data source: Retrospective observational series of 96 patients undergoing lateral neck dissection.
Disclosures: Dr. Glenn and his coauthors reported no financial disclosures.