User login
Sunitinib elicits “exceptional” response in refractory thymic carcinoma
Sunitinib elicited an “exceptional” treatment response from refractory thymic carcinoma in an open-label phase II clinical trial involving 40 patients, investigators reported online in Lancet Oncology.
The oral tyrosine kinase inhibitor produced a 26% rate of complete or partial response for thymic epithelial tumors overall and a 91% response rate in the subset of patients with thymic carcinoma. That response was rapid and durable with continued administration of the agent. This is particularly important because most of the study participants had failed on two or more previous treatments including platinum-based chemotherapy, and no other standard treatments are available for patients who have this aggressive cancer. “Our trial is the first to show robust and durable clinical activity of a targeted agent in previously treated patients with thymic carcinoma,” said Dr. Anish Thomas of the Thoracic and Gastrointestinal Oncology Branch, National Cancer Institute, Bethesda, Md., and his associates.
The trial, performed at two U.S. cancer centers during an 18-month period, involved 24 patients with thymic carcinoma and 16 with advanced thymoma whose disease had progressed despite at least one line of platinum-based chemotherapy. All the participants were given oral sunitinib once daily in 6-week cycles until further progression or unacceptable adverse events occurred. A total of 21 of the 23 assessable patients with thymic carcinoma (91%) achieved disease control (partial response or stable disease), as did 13 of the 16 patients who had thymoma (81%), and the median duration of response was 16.4 months. Median progression-free survival was 7.2 months for thymic carcinoma and 8.5 months for thymoma, and 1-year estimated survival was 78% and 86%, respectively.
Sunitinib was generally well tolerated, though many patients required dose reductions. “Considering concurrent cardiac risk factors in patients with thymic epithelial tumors – e.g., previous exposure to anthracyclines, radiation, and high rates of subclinical cardiac tumor involvement – careful monitoring of cardiac function is needed,” Dr. Thomas and his associates said (Lancet Oncol. 2015 Jan. 13 [doi:10.1016/S1470-2045(14)71181-7]).
They noted that these exploratory findings should be viewed with caution and must be confirmed in larger cohorts. At least one such trial (NCT01621568) is currently underway.
Sunitinib elicited an “exceptional” treatment response from refractory thymic carcinoma in an open-label phase II clinical trial involving 40 patients, investigators reported online in Lancet Oncology.
The oral tyrosine kinase inhibitor produced a 26% rate of complete or partial response for thymic epithelial tumors overall and a 91% response rate in the subset of patients with thymic carcinoma. That response was rapid and durable with continued administration of the agent. This is particularly important because most of the study participants had failed on two or more previous treatments including platinum-based chemotherapy, and no other standard treatments are available for patients who have this aggressive cancer. “Our trial is the first to show robust and durable clinical activity of a targeted agent in previously treated patients with thymic carcinoma,” said Dr. Anish Thomas of the Thoracic and Gastrointestinal Oncology Branch, National Cancer Institute, Bethesda, Md., and his associates.
The trial, performed at two U.S. cancer centers during an 18-month period, involved 24 patients with thymic carcinoma and 16 with advanced thymoma whose disease had progressed despite at least one line of platinum-based chemotherapy. All the participants were given oral sunitinib once daily in 6-week cycles until further progression or unacceptable adverse events occurred. A total of 21 of the 23 assessable patients with thymic carcinoma (91%) achieved disease control (partial response or stable disease), as did 13 of the 16 patients who had thymoma (81%), and the median duration of response was 16.4 months. Median progression-free survival was 7.2 months for thymic carcinoma and 8.5 months for thymoma, and 1-year estimated survival was 78% and 86%, respectively.
Sunitinib was generally well tolerated, though many patients required dose reductions. “Considering concurrent cardiac risk factors in patients with thymic epithelial tumors – e.g., previous exposure to anthracyclines, radiation, and high rates of subclinical cardiac tumor involvement – careful monitoring of cardiac function is needed,” Dr. Thomas and his associates said (Lancet Oncol. 2015 Jan. 13 [doi:10.1016/S1470-2045(14)71181-7]).
They noted that these exploratory findings should be viewed with caution and must be confirmed in larger cohorts. At least one such trial (NCT01621568) is currently underway.
Sunitinib elicited an “exceptional” treatment response from refractory thymic carcinoma in an open-label phase II clinical trial involving 40 patients, investigators reported online in Lancet Oncology.
The oral tyrosine kinase inhibitor produced a 26% rate of complete or partial response for thymic epithelial tumors overall and a 91% response rate in the subset of patients with thymic carcinoma. That response was rapid and durable with continued administration of the agent. This is particularly important because most of the study participants had failed on two or more previous treatments including platinum-based chemotherapy, and no other standard treatments are available for patients who have this aggressive cancer. “Our trial is the first to show robust and durable clinical activity of a targeted agent in previously treated patients with thymic carcinoma,” said Dr. Anish Thomas of the Thoracic and Gastrointestinal Oncology Branch, National Cancer Institute, Bethesda, Md., and his associates.
The trial, performed at two U.S. cancer centers during an 18-month period, involved 24 patients with thymic carcinoma and 16 with advanced thymoma whose disease had progressed despite at least one line of platinum-based chemotherapy. All the participants were given oral sunitinib once daily in 6-week cycles until further progression or unacceptable adverse events occurred. A total of 21 of the 23 assessable patients with thymic carcinoma (91%) achieved disease control (partial response or stable disease), as did 13 of the 16 patients who had thymoma (81%), and the median duration of response was 16.4 months. Median progression-free survival was 7.2 months for thymic carcinoma and 8.5 months for thymoma, and 1-year estimated survival was 78% and 86%, respectively.
Sunitinib was generally well tolerated, though many patients required dose reductions. “Considering concurrent cardiac risk factors in patients with thymic epithelial tumors – e.g., previous exposure to anthracyclines, radiation, and high rates of subclinical cardiac tumor involvement – careful monitoring of cardiac function is needed,” Dr. Thomas and his associates said (Lancet Oncol. 2015 Jan. 13 [doi:10.1016/S1470-2045(14)71181-7]).
They noted that these exploratory findings should be viewed with caution and must be confirmed in larger cohorts. At least one such trial (NCT01621568) is currently underway.
FROM LANCET ONCOLOGY
Key clinical point: The oral tyrosine kinase inhibitor sunitinib shows “exceptional” activity against thymic carcinoma.
Major finding: 21 of the 23 assessable patients with thymic carcinoma (91%) achieved disease control (partial response or stable disease), and the median duration of response was 16.4 months.
Data source: An open-label, uncontrolled phase II clinical trial involving 40 patients treated at two U.S. cancer centers.
Disclosures: This trial was supported by the National Institutes of Health and the National Cancer Institute. Pfizer provided the sunitinib used in the study through the Cancer Therapy Evaluation Program. Dr. Thomas and his associates reported having no relevant financial disclosures.
Study aims to determine prognostic factors for subset of thyroid cancer patients
CORONADO, CALIF. – In patients with radioactive iodine–refractory differentiated thyroid cancer, those with target lesions less than 1.5 cm in size appeared to derive less benefit from sorafenib in terms of progression-free survival, results from an international study showed.
In addition, papillary histology was a positive predictive factor and a predictive factor for benefit from sorafenib.
“Patients with radioactive iodine–refractory differentiated thyroid cancer have a poor prognosis, and there is a lack of effective treatments,” Dr. Martin Schlumberger said at the annual meeting of the American Thyroid Association. “The median survival for this subset is estimated to be 2.5-5 years.”
Sorafenib was approved by the Food and Drug Administration in November 2013 for the treatment of radioactive iodine–refractory differentiated thyroid cancer based on results from the randomized, controlled, double-blind phase III DECISION trial (Lancet 2014;384:319-28). Investigators found that the use of sorafenib extended median progression-free survival by 5 months, compared with placebo (10.8 vs 5.8 months; P < .0001). The purpose of the current analysis was to determine which demographic baseline or disease-related characteristics are prognostic for better outcomes in this patient population. To do so, Dr. Schlumberger of the department of nuclear medicine and endocrine oncology at Gustave Roussy, Villejuif, France, and his associates performed multivariate Cox proportional hazards models adjusted for treatment effect.
He reported findings from 417 patients. Of these, 210 were randomized to receive placebo and 207 were randomized to receive sorafenib. Variables found to be prognostic factors for progression-free survival in placebo patients, and in all patients when adjusted for sorafenib treatment, included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia vs. Europe and North America. Subgroup analyses of patients in the sorafenib arm revealed that the following baseline or disease-related variables were predictive of progression-free survival: papillary histology, tumor size of at least 1.5 cm, and having only lung metastases.
In a post-hoc exploratory analysis of progression-free survival by thyroid cancer symptoms among all 417 patients at study entry, the researchers found that both symptomatic and asymptomatic patients had improved progression-free survival following treatment with sorafenib.
On the basis of these findings, radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm “appear to have a good prognosis and may be candidates for a ‘watch and wait’ approach before initiating treatment with sorafenib,” Dr. Schlumberger concluded.
Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
On Twitter @dougbrunk
CORONADO, CALIF. – In patients with radioactive iodine–refractory differentiated thyroid cancer, those with target lesions less than 1.5 cm in size appeared to derive less benefit from sorafenib in terms of progression-free survival, results from an international study showed.
In addition, papillary histology was a positive predictive factor and a predictive factor for benefit from sorafenib.
“Patients with radioactive iodine–refractory differentiated thyroid cancer have a poor prognosis, and there is a lack of effective treatments,” Dr. Martin Schlumberger said at the annual meeting of the American Thyroid Association. “The median survival for this subset is estimated to be 2.5-5 years.”
Sorafenib was approved by the Food and Drug Administration in November 2013 for the treatment of radioactive iodine–refractory differentiated thyroid cancer based on results from the randomized, controlled, double-blind phase III DECISION trial (Lancet 2014;384:319-28). Investigators found that the use of sorafenib extended median progression-free survival by 5 months, compared with placebo (10.8 vs 5.8 months; P < .0001). The purpose of the current analysis was to determine which demographic baseline or disease-related characteristics are prognostic for better outcomes in this patient population. To do so, Dr. Schlumberger of the department of nuclear medicine and endocrine oncology at Gustave Roussy, Villejuif, France, and his associates performed multivariate Cox proportional hazards models adjusted for treatment effect.
He reported findings from 417 patients. Of these, 210 were randomized to receive placebo and 207 were randomized to receive sorafenib. Variables found to be prognostic factors for progression-free survival in placebo patients, and in all patients when adjusted for sorafenib treatment, included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia vs. Europe and North America. Subgroup analyses of patients in the sorafenib arm revealed that the following baseline or disease-related variables were predictive of progression-free survival: papillary histology, tumor size of at least 1.5 cm, and having only lung metastases.
In a post-hoc exploratory analysis of progression-free survival by thyroid cancer symptoms among all 417 patients at study entry, the researchers found that both symptomatic and asymptomatic patients had improved progression-free survival following treatment with sorafenib.
On the basis of these findings, radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm “appear to have a good prognosis and may be candidates for a ‘watch and wait’ approach before initiating treatment with sorafenib,” Dr. Schlumberger concluded.
Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
On Twitter @dougbrunk
CORONADO, CALIF. – In patients with radioactive iodine–refractory differentiated thyroid cancer, those with target lesions less than 1.5 cm in size appeared to derive less benefit from sorafenib in terms of progression-free survival, results from an international study showed.
In addition, papillary histology was a positive predictive factor and a predictive factor for benefit from sorafenib.
“Patients with radioactive iodine–refractory differentiated thyroid cancer have a poor prognosis, and there is a lack of effective treatments,” Dr. Martin Schlumberger said at the annual meeting of the American Thyroid Association. “The median survival for this subset is estimated to be 2.5-5 years.”
Sorafenib was approved by the Food and Drug Administration in November 2013 for the treatment of radioactive iodine–refractory differentiated thyroid cancer based on results from the randomized, controlled, double-blind phase III DECISION trial (Lancet 2014;384:319-28). Investigators found that the use of sorafenib extended median progression-free survival by 5 months, compared with placebo (10.8 vs 5.8 months; P < .0001). The purpose of the current analysis was to determine which demographic baseline or disease-related characteristics are prognostic for better outcomes in this patient population. To do so, Dr. Schlumberger of the department of nuclear medicine and endocrine oncology at Gustave Roussy, Villejuif, France, and his associates performed multivariate Cox proportional hazards models adjusted for treatment effect.
He reported findings from 417 patients. Of these, 210 were randomized to receive placebo and 207 were randomized to receive sorafenib. Variables found to be prognostic factors for progression-free survival in placebo patients, and in all patients when adjusted for sorafenib treatment, included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia vs. Europe and North America. Subgroup analyses of patients in the sorafenib arm revealed that the following baseline or disease-related variables were predictive of progression-free survival: papillary histology, tumor size of at least 1.5 cm, and having only lung metastases.
In a post-hoc exploratory analysis of progression-free survival by thyroid cancer symptoms among all 417 patients at study entry, the researchers found that both symptomatic and asymptomatic patients had improved progression-free survival following treatment with sorafenib.
On the basis of these findings, radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm “appear to have a good prognosis and may be candidates for a ‘watch and wait’ approach before initiating treatment with sorafenib,” Dr. Schlumberger concluded.
Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: Radioactive iodine–refractory differentiated thyroid cancer patients with no progressive disease and a tumor size of less than 1.5 cm may be candidates for a “watch and wait” approach before initiating treatment with sorafenib.
Major finding: Baseline or disease-related variables found to be prognostic factors for progression-free survival in placebo patients and in all patients when adjusted for sorafenib treatment included papillary histology, lower targeted tumor size, baseline thyroglobulin less than 486 ng/mL, lower number of lesions, and residing in Asia versus Europe and North America.
Data source: An analysis of 417 patients from the randomized, controlled, double-blind, phase III DECISION trial.
Disclosures: Dr. Schlumberger is an adviser to AstraZeneca, Bayer, Eisai, Exelixis, and Genzyme. He has also received research support from Genzyme and Bayer.
Postdiagnosis imaging increasing for patients with all stages of thyroid cancer
Diagnosis of low-risk thyroid cancer has increased, yet researchers have unexpectedly found an increase in the use of postdiagnosis imaging among patients with all stages of disease, according to a report published online in Cancer.
“Greater imaging use clearly contributes to increased costs. Specific to thyroid cancer, increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions,” Dr. Jaime L. Wiebel and associates at the University of Michigan wrote.
Investigators analyzed the records of 23,669 patients diagnosed with differentiated thyroid cancer from 1991-2009 in the Surveillance, Epidemiology, and End Results-Medicare database, and identified the percentage of patients who underwent a neck ultrasound, I-131 scan, or PET scan during the first 3 years after diagnosis.
“Using the SEER-Medicare database, we unexpectedly found that there was a significant increase in the use of surveillance imaging studies over the past 20 years across all stages of disease,” Dr. Wiebel and her associates wrote.
Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001), compared with those diagnosed between 1991 and 2000, and were more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Compared with 1996 through 2004, PET scan use from 2005 to 2009 increased 32.4-fold (P = .001) in patients with localized disease, 13.1-fold (P < .001) in patients with regional disease, and 33.4-fold (P < .001) in patients with distant differentiated thyroid cancer, Dr. Wiebel and her associates reported (Cancer 2015 Jan. 6 [doi: 10.1002/cncr.29210]).
“We demonstrated the increased use of imaging over time despite the diagnosis of smaller, more limited, low-risk thyroid cancer. Although many of the themes presented in the current study are true for other malignancies, the overuse of imaging is highlighted by the increased use in patients with this relatively indolent malignancy,” the researchers wrote.
On Twitter @nikolaideslaura
Diagnosis of low-risk thyroid cancer has increased, yet researchers have unexpectedly found an increase in the use of postdiagnosis imaging among patients with all stages of disease, according to a report published online in Cancer.
“Greater imaging use clearly contributes to increased costs. Specific to thyroid cancer, increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions,” Dr. Jaime L. Wiebel and associates at the University of Michigan wrote.
Investigators analyzed the records of 23,669 patients diagnosed with differentiated thyroid cancer from 1991-2009 in the Surveillance, Epidemiology, and End Results-Medicare database, and identified the percentage of patients who underwent a neck ultrasound, I-131 scan, or PET scan during the first 3 years after diagnosis.
“Using the SEER-Medicare database, we unexpectedly found that there was a significant increase in the use of surveillance imaging studies over the past 20 years across all stages of disease,” Dr. Wiebel and her associates wrote.
Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001), compared with those diagnosed between 1991 and 2000, and were more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Compared with 1996 through 2004, PET scan use from 2005 to 2009 increased 32.4-fold (P = .001) in patients with localized disease, 13.1-fold (P < .001) in patients with regional disease, and 33.4-fold (P < .001) in patients with distant differentiated thyroid cancer, Dr. Wiebel and her associates reported (Cancer 2015 Jan. 6 [doi: 10.1002/cncr.29210]).
“We demonstrated the increased use of imaging over time despite the diagnosis of smaller, more limited, low-risk thyroid cancer. Although many of the themes presented in the current study are true for other malignancies, the overuse of imaging is highlighted by the increased use in patients with this relatively indolent malignancy,” the researchers wrote.
On Twitter @nikolaideslaura
Diagnosis of low-risk thyroid cancer has increased, yet researchers have unexpectedly found an increase in the use of postdiagnosis imaging among patients with all stages of disease, according to a report published online in Cancer.
“Greater imaging use clearly contributes to increased costs. Specific to thyroid cancer, increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions,” Dr. Jaime L. Wiebel and associates at the University of Michigan wrote.
Investigators analyzed the records of 23,669 patients diagnosed with differentiated thyroid cancer from 1991-2009 in the Surveillance, Epidemiology, and End Results-Medicare database, and identified the percentage of patients who underwent a neck ultrasound, I-131 scan, or PET scan during the first 3 years after diagnosis.
“Using the SEER-Medicare database, we unexpectedly found that there was a significant increase in the use of surveillance imaging studies over the past 20 years across all stages of disease,” Dr. Wiebel and her associates wrote.
Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001), compared with those diagnosed between 1991 and 2000, and were more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Compared with 1996 through 2004, PET scan use from 2005 to 2009 increased 32.4-fold (P = .001) in patients with localized disease, 13.1-fold (P < .001) in patients with regional disease, and 33.4-fold (P < .001) in patients with distant differentiated thyroid cancer, Dr. Wiebel and her associates reported (Cancer 2015 Jan. 6 [doi: 10.1002/cncr.29210]).
“We demonstrated the increased use of imaging over time despite the diagnosis of smaller, more limited, low-risk thyroid cancer. Although many of the themes presented in the current study are true for other malignancies, the overuse of imaging is highlighted by the increased use in patients with this relatively indolent malignancy,” the researchers wrote.
On Twitter @nikolaideslaura
FROM CANCER
Key clinical point: Increased imaging may identify low-volume recurrent disease that is unlikely to be clinically significant, leading to heightened patient anxiety and potentially unnecessary interventions.
Major finding: Patients diagnosed after 2000 were more likely to have localized disease (P < .001) and tumors measuring less than 1 cm (P < .001) and more likely to undergo neck ultrasound (odds ratio, 2.15; 95% confidence interval, 2.02 to 2.28) and I-131 scan (odds ratio, 1.44; 95% confidence interval, 1.35 to 1.54).
Data source: Records of 23,669 patients diagnosed with differentiated thyroid cancer from January 1, 1991 to December 31, 2009 in the Surveillance, Epidemiology, and End Results-Medicare database.
Disclosures: The study was funded by the University of Michigan MCubed Seed Funding Program and the Punya Foundation for Thyroid Cancer Research. Dr. Wiebel reported no disclosures. Other authors reported financial support from NIH or NCI grants.
HIM Study: Prevalent HVP-16 infections tend to persist
Prevalent oral human papillomavirus 16 infections in men persist longer than newly acquired infections, and the rate of persistence for incident infections increases with age, according to findings from the HPV Infection in Men (HIM) study.
Of 23 oral HPV-16–positive men aged 18 to 64 years who provided an oral gargle sample during at least two visits during the course of the ongoing multinational cohort study and who were followed for a median of 44.4 months, 13 acquired a new incident infection and 10 entered the study with a prevalent infection. Of the incident infections, 38.5% persisted for at least 1 month, and 10% persisted for at least 24 months; none persisted for more than 36 months (median duration, 7 months), but the rate of persistence for 12 months or longer increased significantly with age (100% of men aged 45 years or older had persistent infection vs. 50% of those aged 31-44 years, and 0% of those aged 18-30 years), Dr. Christine M. Pierce Campbell of the Moffitt Cancer Center and Research Institute, Tampa, Fla., and her colleagues reported online Jan. 9 in Cancer Prevention Research.
Of the prevalent infections, 90% persisted for at least 12 months, 80% persisted for at least 24 months, 57% persisted for at least 36 months, and 40% persisted for 48 months or longer, the investigators said, noting that persistence of prevalent infections also increased with age, but this finding was not statistically significant (Cancer Prev. Res. 2015 Jan. 9 [doi:10.1158/1940-6207.CAPR-14-0296]).
The findings may explain the high prevalence of oral HPV among older patients and could have implications for identifying men at high risk for developing HPV-related oropharyngeal cancer, they said, noting that evidence increasingly suggests that most oropharyngeal cancers among men are caused by HPV infection, and that most cases are diagnosed at an advanced clinical stage.
“Given that the overwhelming majority of prevalent oral HPV-16 infections detected here persisted beyond 1 year, and 40% persisted beyond 4 years, there is a clear need to evaluate whether long-term persistent oral HPV-16 infection can predict future oropharyngeal cancer risk. … mid-adult and older men appear to be at the highest risk of oral HPV infection and should be the focus of prevention interventions, they concluded.
The HIM study cohort was supported by grants from the National Cancer Institute and Merck Sharp & Dohme to individual authors. Dr. Campbell was supported, in part, by a postdoctoral fellowship and reported having no other disclosures.
Prevalent oral human papillomavirus 16 infections in men persist longer than newly acquired infections, and the rate of persistence for incident infections increases with age, according to findings from the HPV Infection in Men (HIM) study.
Of 23 oral HPV-16–positive men aged 18 to 64 years who provided an oral gargle sample during at least two visits during the course of the ongoing multinational cohort study and who were followed for a median of 44.4 months, 13 acquired a new incident infection and 10 entered the study with a prevalent infection. Of the incident infections, 38.5% persisted for at least 1 month, and 10% persisted for at least 24 months; none persisted for more than 36 months (median duration, 7 months), but the rate of persistence for 12 months or longer increased significantly with age (100% of men aged 45 years or older had persistent infection vs. 50% of those aged 31-44 years, and 0% of those aged 18-30 years), Dr. Christine M. Pierce Campbell of the Moffitt Cancer Center and Research Institute, Tampa, Fla., and her colleagues reported online Jan. 9 in Cancer Prevention Research.
Of the prevalent infections, 90% persisted for at least 12 months, 80% persisted for at least 24 months, 57% persisted for at least 36 months, and 40% persisted for 48 months or longer, the investigators said, noting that persistence of prevalent infections also increased with age, but this finding was not statistically significant (Cancer Prev. Res. 2015 Jan. 9 [doi:10.1158/1940-6207.CAPR-14-0296]).
The findings may explain the high prevalence of oral HPV among older patients and could have implications for identifying men at high risk for developing HPV-related oropharyngeal cancer, they said, noting that evidence increasingly suggests that most oropharyngeal cancers among men are caused by HPV infection, and that most cases are diagnosed at an advanced clinical stage.
“Given that the overwhelming majority of prevalent oral HPV-16 infections detected here persisted beyond 1 year, and 40% persisted beyond 4 years, there is a clear need to evaluate whether long-term persistent oral HPV-16 infection can predict future oropharyngeal cancer risk. … mid-adult and older men appear to be at the highest risk of oral HPV infection and should be the focus of prevention interventions, they concluded.
The HIM study cohort was supported by grants from the National Cancer Institute and Merck Sharp & Dohme to individual authors. Dr. Campbell was supported, in part, by a postdoctoral fellowship and reported having no other disclosures.
Prevalent oral human papillomavirus 16 infections in men persist longer than newly acquired infections, and the rate of persistence for incident infections increases with age, according to findings from the HPV Infection in Men (HIM) study.
Of 23 oral HPV-16–positive men aged 18 to 64 years who provided an oral gargle sample during at least two visits during the course of the ongoing multinational cohort study and who were followed for a median of 44.4 months, 13 acquired a new incident infection and 10 entered the study with a prevalent infection. Of the incident infections, 38.5% persisted for at least 1 month, and 10% persisted for at least 24 months; none persisted for more than 36 months (median duration, 7 months), but the rate of persistence for 12 months or longer increased significantly with age (100% of men aged 45 years or older had persistent infection vs. 50% of those aged 31-44 years, and 0% of those aged 18-30 years), Dr. Christine M. Pierce Campbell of the Moffitt Cancer Center and Research Institute, Tampa, Fla., and her colleagues reported online Jan. 9 in Cancer Prevention Research.
Of the prevalent infections, 90% persisted for at least 12 months, 80% persisted for at least 24 months, 57% persisted for at least 36 months, and 40% persisted for 48 months or longer, the investigators said, noting that persistence of prevalent infections also increased with age, but this finding was not statistically significant (Cancer Prev. Res. 2015 Jan. 9 [doi:10.1158/1940-6207.CAPR-14-0296]).
The findings may explain the high prevalence of oral HPV among older patients and could have implications for identifying men at high risk for developing HPV-related oropharyngeal cancer, they said, noting that evidence increasingly suggests that most oropharyngeal cancers among men are caused by HPV infection, and that most cases are diagnosed at an advanced clinical stage.
“Given that the overwhelming majority of prevalent oral HPV-16 infections detected here persisted beyond 1 year, and 40% persisted beyond 4 years, there is a clear need to evaluate whether long-term persistent oral HPV-16 infection can predict future oropharyngeal cancer risk. … mid-adult and older men appear to be at the highest risk of oral HPV infection and should be the focus of prevention interventions, they concluded.
The HIM study cohort was supported by grants from the National Cancer Institute and Merck Sharp & Dohme to individual authors. Dr. Campbell was supported, in part, by a postdoctoral fellowship and reported having no other disclosures.
Key clinical point: Prevalent oral HPV-16 infections tend to be persistent, and infection risk is highest for middle-aged and older men.
Major finding: 90% of prevalent infections persisted for at least 12 months; 40% persisted for at least 48 months.
Data source: An analysis of data from 23 patients in a multinational cohort study (HIM study).
Disclosures: The HIM study cohort was supported by grants from the National Cancer Institute and Merck Sharp & Dohme to individual authors. Dr. Campbell was supported, in part, by a postdoctoral fellowship and reported having no other disclosures.
AHRQ releases update on radiotherapy for head and neck cancer
A new guideline update on radiotherapy treatments for head and neck cancer strengthened the previous guideline’s findings but did not find any new significant evidence on the effectiveness of other procedures.
The guideline, prepared by the Blue Cross and Blue Shield Association and published by the Agency for Healthcare Research and Quality, updates Comparative Effectiveness Review (CER) No. 20, published in 2010. The update includes three-dimensional conformal radiotherapy (3DCRT), intensity-modulated RT (IMRT), and proton-beam RT (PBT), which were in the previous guideline, but also includes stereotactic body RT (SBRT) and excludes two-dimensional RT (2DRT). The search included studies published from September 2009 to April 2013, except for SBRT, where studies from January 1, 1990, through April 2013 were included. Fourteen studies and one randomized controlled trial met inclusion criteria.
The update found new evidence that IMRT reduced xerostomia more than did 3DCRT or 2DRT, but no new evidence was found on how quality of life domains were improved, which were the primary findings of the CER No. 20. Evidence toward other radiotherapy comparisons was limited and insufficient to draw any new conclusions, and no evidence was found for PBT.
A new guideline update on radiotherapy treatments for head and neck cancer strengthened the previous guideline’s findings but did not find any new significant evidence on the effectiveness of other procedures.
The guideline, prepared by the Blue Cross and Blue Shield Association and published by the Agency for Healthcare Research and Quality, updates Comparative Effectiveness Review (CER) No. 20, published in 2010. The update includes three-dimensional conformal radiotherapy (3DCRT), intensity-modulated RT (IMRT), and proton-beam RT (PBT), which were in the previous guideline, but also includes stereotactic body RT (SBRT) and excludes two-dimensional RT (2DRT). The search included studies published from September 2009 to April 2013, except for SBRT, where studies from January 1, 1990, through April 2013 were included. Fourteen studies and one randomized controlled trial met inclusion criteria.
The update found new evidence that IMRT reduced xerostomia more than did 3DCRT or 2DRT, but no new evidence was found on how quality of life domains were improved, which were the primary findings of the CER No. 20. Evidence toward other radiotherapy comparisons was limited and insufficient to draw any new conclusions, and no evidence was found for PBT.
A new guideline update on radiotherapy treatments for head and neck cancer strengthened the previous guideline’s findings but did not find any new significant evidence on the effectiveness of other procedures.
The guideline, prepared by the Blue Cross and Blue Shield Association and published by the Agency for Healthcare Research and Quality, updates Comparative Effectiveness Review (CER) No. 20, published in 2010. The update includes three-dimensional conformal radiotherapy (3DCRT), intensity-modulated RT (IMRT), and proton-beam RT (PBT), which were in the previous guideline, but also includes stereotactic body RT (SBRT) and excludes two-dimensional RT (2DRT). The search included studies published from September 2009 to April 2013, except for SBRT, where studies from January 1, 1990, through April 2013 were included. Fourteen studies and one randomized controlled trial met inclusion criteria.
The update found new evidence that IMRT reduced xerostomia more than did 3DCRT or 2DRT, but no new evidence was found on how quality of life domains were improved, which were the primary findings of the CER No. 20. Evidence toward other radiotherapy comparisons was limited and insufficient to draw any new conclusions, and no evidence was found for PBT.
High failure rate seen with limited parathyroidectomy in patients with MEN-1
SAN FRANCISCO – Patients with hyperparathyroidism due to multiple endocrine neoplasia type 1 (MEN-1) have a 4 in 10 chance of persistent hyperparathyroidism if they undergo surgery that leaves at least one gland in place, according to a retrospective cohort study presented at the annual clinical congress of the American College of Surgeons.
“Limited initial parathyroidectomy in patients with MEN-1–associated primary hyperparathyroidism results in a high failure rate. Additional enlarged contralateral parathyroid glands are frequently missed by preoperative localizing studies,” commented lead investigator Dr. Naris Nilubol, a staff clinician with the endocrine oncology branch of the Center for Cancer Research, National Cancer Institute, Bethesda, Md.
“We conclude that limited parathyroidectomy in MEN-1 guided by preoperative localizing studies is associated with high failure rates and therefore should not be performed,” he maintained.
In an interview, session comoderator Dr. Marybeth S. Hughes, a staff clinician with the thoracic and gastrointestinal oncology branch, Center for Cancer Research, National Cancer Institute, commented, “In general, I would say that the data presented just reiterates the standard of care, that MEN-1 patients should have bilateral neck exploration with [removal of] three and half glands, or four glands with autotransplantation. So it just basically solidifies what is being done standardly. I don’t think there is a compelling argument to change the standard.”
Dr. Nilubol and colleagues reviewed the charts of 99 patients with MEN-1 who underwent at least one parathyroidectomy at the National Institutes of Health (NIH).
Of the 64 patients who had initial surgery at NIH and had preoperative localizing studies done, 32 had only a single enlarged gland identified by the tests, suggesting they would be good candidates for limited surgery, according to Dr. Nilubol. Bilateral neck dissection at the time of parathyroidectomy showed that in 22 (69%) of these 32 patients, the studies had correctly identified the largest gland; however, in 19 (87%) of those 22, it missed another enlarged gland on the contralateral side. Furthermore, in 5 (16%) of the 32, the largest gland was found on the contralateral side.
With a median follow-up of 23 months, the risk of persistent hyperparathyroidism was 41% for patients who had limited parathyroidectomy (three or fewer glands removed) at initial surgery, significantly and sharply higher than the 6% seen in patients who had subtotal parathyroidectomy or more extensive surgery (at least three and a half glands removed).
Looking at the cumulative number of glands removed during initial and subsequent surgeries, 57% of patients having two or fewer glands removed and 45% of those having two and a half to three glands removed had persistent hyperparathyroidism – both significantly higher than the 5% of patients having at least three and a half glands removed.
Regarding complications, 10% of the patients who had their initial surgery at NIH developed permanent hypoparathyroidism, reported Dr. Nilubol, who disclosed that he had no relevant conflicts of interest.
Session attendees asked about the use of parathyroid hormone levels intraoperatively to guide surgery and what strategy surgeons follow at his institution in this patient population.
Previous research has suggested that intraoperative parathyroid hormone levels do not add any information that would change the operative plan, Dr. Nilubol replied. “Everybody at NIH has preop localizing studies as part of the clinical investigation, but it doesn’t change the way we approach it. Everybody gets a bilateral neck exploration and three and a half–gland removal,” provided all glands can be found, he said.
Session attendee Dr. Michael J. Campbell, a surgeon at the University California, Davis, commented, “A 10% permanent hypoparathyroidism rate in these patients – and they have a tendency to be young, most of them in their late teens, early 20s – that’s a major complication. So could you take your data and make exactly the opposite argument, that maybe you should be doing less to these patients to limit that fairly life-altering complication?” Permanent hypothyroidism at that age is “a significant medical problem,” Dr. Nilubol agreed. However, “at the NIH, we don’t operate on everybody just because they have primary hyperparathyroidism. They have to fulfill metabolic complications before we choose to operate on them. We want to delay the surgeries and [time] between the surgeries because if they live long enough, it will recur, so we want to operate when we can make the most difference, meaning [addressing] kidney stone, bone loss, etc. The most common reason for young patients is they have kidney stones, which leads to surgery.”
SAN FRANCISCO – Patients with hyperparathyroidism due to multiple endocrine neoplasia type 1 (MEN-1) have a 4 in 10 chance of persistent hyperparathyroidism if they undergo surgery that leaves at least one gland in place, according to a retrospective cohort study presented at the annual clinical congress of the American College of Surgeons.
“Limited initial parathyroidectomy in patients with MEN-1–associated primary hyperparathyroidism results in a high failure rate. Additional enlarged contralateral parathyroid glands are frequently missed by preoperative localizing studies,” commented lead investigator Dr. Naris Nilubol, a staff clinician with the endocrine oncology branch of the Center for Cancer Research, National Cancer Institute, Bethesda, Md.
“We conclude that limited parathyroidectomy in MEN-1 guided by preoperative localizing studies is associated with high failure rates and therefore should not be performed,” he maintained.
In an interview, session comoderator Dr. Marybeth S. Hughes, a staff clinician with the thoracic and gastrointestinal oncology branch, Center for Cancer Research, National Cancer Institute, commented, “In general, I would say that the data presented just reiterates the standard of care, that MEN-1 patients should have bilateral neck exploration with [removal of] three and half glands, or four glands with autotransplantation. So it just basically solidifies what is being done standardly. I don’t think there is a compelling argument to change the standard.”
Dr. Nilubol and colleagues reviewed the charts of 99 patients with MEN-1 who underwent at least one parathyroidectomy at the National Institutes of Health (NIH).
Of the 64 patients who had initial surgery at NIH and had preoperative localizing studies done, 32 had only a single enlarged gland identified by the tests, suggesting they would be good candidates for limited surgery, according to Dr. Nilubol. Bilateral neck dissection at the time of parathyroidectomy showed that in 22 (69%) of these 32 patients, the studies had correctly identified the largest gland; however, in 19 (87%) of those 22, it missed another enlarged gland on the contralateral side. Furthermore, in 5 (16%) of the 32, the largest gland was found on the contralateral side.
With a median follow-up of 23 months, the risk of persistent hyperparathyroidism was 41% for patients who had limited parathyroidectomy (three or fewer glands removed) at initial surgery, significantly and sharply higher than the 6% seen in patients who had subtotal parathyroidectomy or more extensive surgery (at least three and a half glands removed).
Looking at the cumulative number of glands removed during initial and subsequent surgeries, 57% of patients having two or fewer glands removed and 45% of those having two and a half to three glands removed had persistent hyperparathyroidism – both significantly higher than the 5% of patients having at least three and a half glands removed.
Regarding complications, 10% of the patients who had their initial surgery at NIH developed permanent hypoparathyroidism, reported Dr. Nilubol, who disclosed that he had no relevant conflicts of interest.
Session attendees asked about the use of parathyroid hormone levels intraoperatively to guide surgery and what strategy surgeons follow at his institution in this patient population.
Previous research has suggested that intraoperative parathyroid hormone levels do not add any information that would change the operative plan, Dr. Nilubol replied. “Everybody at NIH has preop localizing studies as part of the clinical investigation, but it doesn’t change the way we approach it. Everybody gets a bilateral neck exploration and three and a half–gland removal,” provided all glands can be found, he said.
Session attendee Dr. Michael J. Campbell, a surgeon at the University California, Davis, commented, “A 10% permanent hypoparathyroidism rate in these patients – and they have a tendency to be young, most of them in their late teens, early 20s – that’s a major complication. So could you take your data and make exactly the opposite argument, that maybe you should be doing less to these patients to limit that fairly life-altering complication?” Permanent hypothyroidism at that age is “a significant medical problem,” Dr. Nilubol agreed. However, “at the NIH, we don’t operate on everybody just because they have primary hyperparathyroidism. They have to fulfill metabolic complications before we choose to operate on them. We want to delay the surgeries and [time] between the surgeries because if they live long enough, it will recur, so we want to operate when we can make the most difference, meaning [addressing] kidney stone, bone loss, etc. The most common reason for young patients is they have kidney stones, which leads to surgery.”
SAN FRANCISCO – Patients with hyperparathyroidism due to multiple endocrine neoplasia type 1 (MEN-1) have a 4 in 10 chance of persistent hyperparathyroidism if they undergo surgery that leaves at least one gland in place, according to a retrospective cohort study presented at the annual clinical congress of the American College of Surgeons.
“Limited initial parathyroidectomy in patients with MEN-1–associated primary hyperparathyroidism results in a high failure rate. Additional enlarged contralateral parathyroid glands are frequently missed by preoperative localizing studies,” commented lead investigator Dr. Naris Nilubol, a staff clinician with the endocrine oncology branch of the Center for Cancer Research, National Cancer Institute, Bethesda, Md.
“We conclude that limited parathyroidectomy in MEN-1 guided by preoperative localizing studies is associated with high failure rates and therefore should not be performed,” he maintained.
In an interview, session comoderator Dr. Marybeth S. Hughes, a staff clinician with the thoracic and gastrointestinal oncology branch, Center for Cancer Research, National Cancer Institute, commented, “In general, I would say that the data presented just reiterates the standard of care, that MEN-1 patients should have bilateral neck exploration with [removal of] three and half glands, or four glands with autotransplantation. So it just basically solidifies what is being done standardly. I don’t think there is a compelling argument to change the standard.”
Dr. Nilubol and colleagues reviewed the charts of 99 patients with MEN-1 who underwent at least one parathyroidectomy at the National Institutes of Health (NIH).
Of the 64 patients who had initial surgery at NIH and had preoperative localizing studies done, 32 had only a single enlarged gland identified by the tests, suggesting they would be good candidates for limited surgery, according to Dr. Nilubol. Bilateral neck dissection at the time of parathyroidectomy showed that in 22 (69%) of these 32 patients, the studies had correctly identified the largest gland; however, in 19 (87%) of those 22, it missed another enlarged gland on the contralateral side. Furthermore, in 5 (16%) of the 32, the largest gland was found on the contralateral side.
With a median follow-up of 23 months, the risk of persistent hyperparathyroidism was 41% for patients who had limited parathyroidectomy (three or fewer glands removed) at initial surgery, significantly and sharply higher than the 6% seen in patients who had subtotal parathyroidectomy or more extensive surgery (at least three and a half glands removed).
Looking at the cumulative number of glands removed during initial and subsequent surgeries, 57% of patients having two or fewer glands removed and 45% of those having two and a half to three glands removed had persistent hyperparathyroidism – both significantly higher than the 5% of patients having at least three and a half glands removed.
Regarding complications, 10% of the patients who had their initial surgery at NIH developed permanent hypoparathyroidism, reported Dr. Nilubol, who disclosed that he had no relevant conflicts of interest.
Session attendees asked about the use of parathyroid hormone levels intraoperatively to guide surgery and what strategy surgeons follow at his institution in this patient population.
Previous research has suggested that intraoperative parathyroid hormone levels do not add any information that would change the operative plan, Dr. Nilubol replied. “Everybody at NIH has preop localizing studies as part of the clinical investigation, but it doesn’t change the way we approach it. Everybody gets a bilateral neck exploration and three and a half–gland removal,” provided all glands can be found, he said.
Session attendee Dr. Michael J. Campbell, a surgeon at the University California, Davis, commented, “A 10% permanent hypoparathyroidism rate in these patients – and they have a tendency to be young, most of them in their late teens, early 20s – that’s a major complication. So could you take your data and make exactly the opposite argument, that maybe you should be doing less to these patients to limit that fairly life-altering complication?” Permanent hypothyroidism at that age is “a significant medical problem,” Dr. Nilubol agreed. However, “at the NIH, we don’t operate on everybody just because they have primary hyperparathyroidism. They have to fulfill metabolic complications before we choose to operate on them. We want to delay the surgeries and [time] between the surgeries because if they live long enough, it will recur, so we want to operate when we can make the most difference, meaning [addressing] kidney stone, bone loss, etc. The most common reason for young patients is they have kidney stones, which leads to surgery.”
AT THE ACS CLINICAL CONGRESS
Key clinical point: Patients are more likely to have persistent hyperparathyroidism if a gland is left behind.
Major finding: The failure rate after initial parathyroidectomy was 41% with limited surgery versus 6% with subtotal or more extensive surgery.
Data source: A retrospective chart review of 99 patients with MEN-1–associated hyperparathyroidism.
Disclosures: Dr. Nilubol disclosed that he had no relevant conflicts of interest.
Total thyroidectomy more likely with younger thyroid cancer patients
CORONADO, CALIF. – Patients with differentiated thyroid cancer who were younger than age 45 years were more likely to undergo total or near-total thyroidectomy and to receive radioactive iodine, compared with their older counterparts, a large registry analysis demonstrated.
In addition, younger patients were more likely to be Hispanic and female and to have papillary carcinoma, lead study author Dr. Thomas J. Semrad reported during the annual meeting of the American Thyroid Association.
“Not much is known about how treatment administration differs between younger and older patients with thyroid cancer,” Dr. Semrad of the division of hematology/oncology at the University of California, Davis, Comprehensive Cancer Center, Sacramento, said in an interview. “Some data suggest that perhaps patients younger than age 15 years may respond better to radioactive iodine and may present with more advanced disease. But not much is known about how they’re treated.”
To find out, Dr. Semrad and his associates used the California Cancer Registry to identify 23,629 patients who were diagnosed with differentiated thyroid cancer between 2004 and 2011. They divided the patients into two cohorts: younger (defined as those younger than 45 years) and older (those 45 years or older). Treatment variables of interest included total or near-total thyroidectomy, other types of thyroid surgery, and the administration of radioactive iodine (RAI). The researchers compared the descriptive statistics between the two groups and used univariate and multivariate logistic regression to identify predictors of the treatment administered.
Compared with older patients, younger patients were significantly more likely to be Hispanic (33% vs. 22%), to be female (83% vs. 75%), to have papillary carcinoma (93% vs. 91%), and to have lymph node involvement (32% vs. 20%, all P < .0001).
Overall, the majority of patients (86%) underwent total or near-total thyroidectomy, but the surgery was slightly and significantly more common in younger patients, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients also were significantly more likely to receive RAI (55% vs. 49%, P < .0001).
On multivariate analysis, statistically significant predictors of total thyroidectomy, compared with other thyroid surgery, included younger age (odds ratio, 1.193); higher socioeconomic status (OR, 1.263, for higher-middle SES and OR, 1.325, for highest SES); higher T stage (OR, 1.848, for T2; OR, 2.473, for T3; and OR, 2.908, for T4); and papillary histology (OR, 0.349).
At the same time, statistically significant predictors of RAI administration included younger age (OR, 1.116); higher SES (OR, 1.410, for higher-middle SES and OR, 1.307, for highest SES); more advanced T stage (OR, 2.194 for T2; OR, 2.084, for T3; and OR, 1.527, for T4); node positivity (OR, 0.481), and total thyroidectomy (OR, 3.76).
“As we expected, the younger population was more likely to be female, but we did find that the younger population was also more likely to be Hispanic,” Dr. Semrad said. “We don’t know if they were native Hispanics or if it has something to do with immigration rates.”
Dr. Semrad acknowledged certain limitations of the study, including the risk of misclassification bias in registry data, the lack of details about surgical procedures performed, and the fact that the radioiodine dose was not captured.
“We have data regarding the T stage, the nodal stage, and the number of lymph nodes examined, but we don’t have some of the finer histology data,” he said.
Even so, he characterized the findings as “provocative in suggesting that perhaps our treatment patterns in younger patients are different. With more aggressive surgery and more use of radioactive iodine, that can have potential implications in terms of long-term side effects and follow-up.”
The researchers said they plan to use linked administrative data to analyze initial and subsequent thyroid surgical procedures in this patient population.
The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – Patients with differentiated thyroid cancer who were younger than age 45 years were more likely to undergo total or near-total thyroidectomy and to receive radioactive iodine, compared with their older counterparts, a large registry analysis demonstrated.
In addition, younger patients were more likely to be Hispanic and female and to have papillary carcinoma, lead study author Dr. Thomas J. Semrad reported during the annual meeting of the American Thyroid Association.
“Not much is known about how treatment administration differs between younger and older patients with thyroid cancer,” Dr. Semrad of the division of hematology/oncology at the University of California, Davis, Comprehensive Cancer Center, Sacramento, said in an interview. “Some data suggest that perhaps patients younger than age 15 years may respond better to radioactive iodine and may present with more advanced disease. But not much is known about how they’re treated.”
To find out, Dr. Semrad and his associates used the California Cancer Registry to identify 23,629 patients who were diagnosed with differentiated thyroid cancer between 2004 and 2011. They divided the patients into two cohorts: younger (defined as those younger than 45 years) and older (those 45 years or older). Treatment variables of interest included total or near-total thyroidectomy, other types of thyroid surgery, and the administration of radioactive iodine (RAI). The researchers compared the descriptive statistics between the two groups and used univariate and multivariate logistic regression to identify predictors of the treatment administered.
Compared with older patients, younger patients were significantly more likely to be Hispanic (33% vs. 22%), to be female (83% vs. 75%), to have papillary carcinoma (93% vs. 91%), and to have lymph node involvement (32% vs. 20%, all P < .0001).
Overall, the majority of patients (86%) underwent total or near-total thyroidectomy, but the surgery was slightly and significantly more common in younger patients, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients also were significantly more likely to receive RAI (55% vs. 49%, P < .0001).
On multivariate analysis, statistically significant predictors of total thyroidectomy, compared with other thyroid surgery, included younger age (odds ratio, 1.193); higher socioeconomic status (OR, 1.263, for higher-middle SES and OR, 1.325, for highest SES); higher T stage (OR, 1.848, for T2; OR, 2.473, for T3; and OR, 2.908, for T4); and papillary histology (OR, 0.349).
At the same time, statistically significant predictors of RAI administration included younger age (OR, 1.116); higher SES (OR, 1.410, for higher-middle SES and OR, 1.307, for highest SES); more advanced T stage (OR, 2.194 for T2; OR, 2.084, for T3; and OR, 1.527, for T4); node positivity (OR, 0.481), and total thyroidectomy (OR, 3.76).
“As we expected, the younger population was more likely to be female, but we did find that the younger population was also more likely to be Hispanic,” Dr. Semrad said. “We don’t know if they were native Hispanics or if it has something to do with immigration rates.”
Dr. Semrad acknowledged certain limitations of the study, including the risk of misclassification bias in registry data, the lack of details about surgical procedures performed, and the fact that the radioiodine dose was not captured.
“We have data regarding the T stage, the nodal stage, and the number of lymph nodes examined, but we don’t have some of the finer histology data,” he said.
Even so, he characterized the findings as “provocative in suggesting that perhaps our treatment patterns in younger patients are different. With more aggressive surgery and more use of radioactive iodine, that can have potential implications in terms of long-term side effects and follow-up.”
The researchers said they plan to use linked administrative data to analyze initial and subsequent thyroid surgical procedures in this patient population.
The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – Patients with differentiated thyroid cancer who were younger than age 45 years were more likely to undergo total or near-total thyroidectomy and to receive radioactive iodine, compared with their older counterparts, a large registry analysis demonstrated.
In addition, younger patients were more likely to be Hispanic and female and to have papillary carcinoma, lead study author Dr. Thomas J. Semrad reported during the annual meeting of the American Thyroid Association.
“Not much is known about how treatment administration differs between younger and older patients with thyroid cancer,” Dr. Semrad of the division of hematology/oncology at the University of California, Davis, Comprehensive Cancer Center, Sacramento, said in an interview. “Some data suggest that perhaps patients younger than age 15 years may respond better to radioactive iodine and may present with more advanced disease. But not much is known about how they’re treated.”
To find out, Dr. Semrad and his associates used the California Cancer Registry to identify 23,629 patients who were diagnosed with differentiated thyroid cancer between 2004 and 2011. They divided the patients into two cohorts: younger (defined as those younger than 45 years) and older (those 45 years or older). Treatment variables of interest included total or near-total thyroidectomy, other types of thyroid surgery, and the administration of radioactive iodine (RAI). The researchers compared the descriptive statistics between the two groups and used univariate and multivariate logistic regression to identify predictors of the treatment administered.
Compared with older patients, younger patients were significantly more likely to be Hispanic (33% vs. 22%), to be female (83% vs. 75%), to have papillary carcinoma (93% vs. 91%), and to have lymph node involvement (32% vs. 20%, all P < .0001).
Overall, the majority of patients (86%) underwent total or near-total thyroidectomy, but the surgery was slightly and significantly more common in younger patients, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients also were significantly more likely to receive RAI (55% vs. 49%, P < .0001).
On multivariate analysis, statistically significant predictors of total thyroidectomy, compared with other thyroid surgery, included younger age (odds ratio, 1.193); higher socioeconomic status (OR, 1.263, for higher-middle SES and OR, 1.325, for highest SES); higher T stage (OR, 1.848, for T2; OR, 2.473, for T3; and OR, 2.908, for T4); and papillary histology (OR, 0.349).
At the same time, statistically significant predictors of RAI administration included younger age (OR, 1.116); higher SES (OR, 1.410, for higher-middle SES and OR, 1.307, for highest SES); more advanced T stage (OR, 2.194 for T2; OR, 2.084, for T3; and OR, 1.527, for T4); node positivity (OR, 0.481), and total thyroidectomy (OR, 3.76).
“As we expected, the younger population was more likely to be female, but we did find that the younger population was also more likely to be Hispanic,” Dr. Semrad said. “We don’t know if they were native Hispanics or if it has something to do with immigration rates.”
Dr. Semrad acknowledged certain limitations of the study, including the risk of misclassification bias in registry data, the lack of details about surgical procedures performed, and the fact that the radioiodine dose was not captured.
“We have data regarding the T stage, the nodal stage, and the number of lymph nodes examined, but we don’t have some of the finer histology data,” he said.
Even so, he characterized the findings as “provocative in suggesting that perhaps our treatment patterns in younger patients are different. With more aggressive surgery and more use of radioactive iodine, that can have potential implications in terms of long-term side effects and follow-up.”
The researchers said they plan to use linked administrative data to analyze initial and subsequent thyroid surgical procedures in this patient population.
The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: Younger patients with differentiated thyroid cancer were more likely to undergo total thyroidectomy and receive radioactive iodine.
Major finding: Total or near-total thyroidectomy was slightly more common in patients younger than age 45 years, compared with their older counterparts (88% vs. 85%, P < .0001). Younger patients were also more likely to receive RAI (55% vs. 49%, P < .0001).
Data source: A study of 23,629 patients from the California Cancer Registry who were diagnosed with differentiated thyroid cancer between 2004 and 2011.
Disclosures: The study was supported by a grant from the National Institutes of Health. Dr. Semrad reported having no relevant financial disclosures.
Impact of aprepitant on emesis control, dose intensity, and recurrence-free survival in a population-based cohort of head and neck cancer patients receiving high-dose cisplatin chemotherapy
Objective To evaluate the impact of aprepitant on emesis control, DI, and RFS.
Methods HNC patients treated at the British Columbia Cancer Agency were analyzed. Kaplan-Meier method and adjusted Cox proportional hazard models were used to evaluate RFS in aprepitant users. To control for selection bias, a propensity score analysis was conducted.
Results A total of 192 HNC patients were included: 141 received aprepitant prophylaxis. The aprepitant-treated and untreated groups were comparable in mean age (56.3 vs 58.1 years), male gender (82.3% vs 86.3%), tumor location, and number of metastatic sites. However, more patients in the aprepitant group than in the untreated group had surgically resectable disease (31.2% vs 15.7%, respectively) and better performance status (ECOG 0/1, 87.9% vs 76.4%). Less emesis was reported in the aprepitant group (21.3% vs 28.0%). Patients in the treated group were also more likely to complete 3 cycles of high-dose cisplatin (OR, 2.3; P = .03). The propensity score adjusted Cox regression analysis suggested a reduced risk of disease recurrence in patients who received aprepitant (HR, 0.47; 95% CI, 0.17- 1.28).
Limitations Potential confounders such as other diseases or treatments that may have influenced the presence of nausea/emesis symptoms.
Conclusion Aprepitant contributed to improved emesis control, enhanced DI, and better adherence to cisplatin chemotherapy.
Funding/sponsorship The British Columbia Cancer Foundation and Canadian Cancer Society Research Institute.
Click on the PDF icon at the top of this introduction to read the full article.
Objective To evaluate the impact of aprepitant on emesis control, DI, and RFS.
Methods HNC patients treated at the British Columbia Cancer Agency were analyzed. Kaplan-Meier method and adjusted Cox proportional hazard models were used to evaluate RFS in aprepitant users. To control for selection bias, a propensity score analysis was conducted.
Results A total of 192 HNC patients were included: 141 received aprepitant prophylaxis. The aprepitant-treated and untreated groups were comparable in mean age (56.3 vs 58.1 years), male gender (82.3% vs 86.3%), tumor location, and number of metastatic sites. However, more patients in the aprepitant group than in the untreated group had surgically resectable disease (31.2% vs 15.7%, respectively) and better performance status (ECOG 0/1, 87.9% vs 76.4%). Less emesis was reported in the aprepitant group (21.3% vs 28.0%). Patients in the treated group were also more likely to complete 3 cycles of high-dose cisplatin (OR, 2.3; P = .03). The propensity score adjusted Cox regression analysis suggested a reduced risk of disease recurrence in patients who received aprepitant (HR, 0.47; 95% CI, 0.17- 1.28).
Limitations Potential confounders such as other diseases or treatments that may have influenced the presence of nausea/emesis symptoms.
Conclusion Aprepitant contributed to improved emesis control, enhanced DI, and better adherence to cisplatin chemotherapy.
Funding/sponsorship The British Columbia Cancer Foundation and Canadian Cancer Society Research Institute.
Click on the PDF icon at the top of this introduction to read the full article.
Objective To evaluate the impact of aprepitant on emesis control, DI, and RFS.
Methods HNC patients treated at the British Columbia Cancer Agency were analyzed. Kaplan-Meier method and adjusted Cox proportional hazard models were used to evaluate RFS in aprepitant users. To control for selection bias, a propensity score analysis was conducted.
Results A total of 192 HNC patients were included: 141 received aprepitant prophylaxis. The aprepitant-treated and untreated groups were comparable in mean age (56.3 vs 58.1 years), male gender (82.3% vs 86.3%), tumor location, and number of metastatic sites. However, more patients in the aprepitant group than in the untreated group had surgically resectable disease (31.2% vs 15.7%, respectively) and better performance status (ECOG 0/1, 87.9% vs 76.4%). Less emesis was reported in the aprepitant group (21.3% vs 28.0%). Patients in the treated group were also more likely to complete 3 cycles of high-dose cisplatin (OR, 2.3; P = .03). The propensity score adjusted Cox regression analysis suggested a reduced risk of disease recurrence in patients who received aprepitant (HR, 0.47; 95% CI, 0.17- 1.28).
Limitations Potential confounders such as other diseases or treatments that may have influenced the presence of nausea/emesis symptoms.
Conclusion Aprepitant contributed to improved emesis control, enhanced DI, and better adherence to cisplatin chemotherapy.
Funding/sponsorship The British Columbia Cancer Foundation and Canadian Cancer Society Research Institute.
Click on the PDF icon at the top of this introduction to read the full article.
A wonderful life
Radioactive Iodine Scintiphotos of a Man With Thyroid Cancer
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
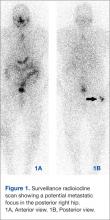
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.