User login
TMS May Be a Good Alternative to ECT in Depression
DENVER — , according to results from a retrospective study of patients treated in the past 20 years.
“We always learn in our textbooks that after about two or three medication trials is when you can start exploring more serious treatment protocols, such as ECT or TMS, but a lot of these patients weren’t going forward with it, and I was curious about it. I figured that TMS, which is a less expensive, less scary procedure that patients would more likely be open to, that is also approved for treatment resistant depression, would be a good alternative to ECT,” said Anuttham Kandhadai, a third-year medical student at University of Texas Medical Branch at Galveston, who presented the study at the 2024 annual meeting of the American Academy of Neurology.
Study Findings Lead to More Questions
The researchers found lower rates of depressive episodes, suicidal attempts, and suicidal ideation among patients treated with TMS, but an important limitation was that the researchers did not know the severity of the depression in the two patient groups, according to Branch Coslett, MD, who attended the session and has performed research with TMS to treat aphasia in stroke patients. “I think it’s a very interesting study, and certainly something worth pursuing, but given that ECT is only used as a last resort, whereas TMS is often used as a second-line therapy, I think you’re really talking about very different populations that have had these treatments,” said Dr. Coslett.
Mr. Kandhadai recognized the limitations of the study and looks forward to expanding the research. “I’d love to explore cost effectiveness of the treatments. I’d love to explore patient familiarity and patient comfort with different treatments. And I’d also love to explore a more controlled study that can determine how severe someone’s depression is, and then be able to control for that and explore the outcomes based on the treatment protocol,” he said.
The ideal comparative study would be prospective, “but that will never be done. One Flew Over the Cuckoo’s Nest and similar sources of information have really poisoned the well,” said Dr. Coslett. However, he noted that advances have been made in ECT, and that targeting the right hemisphere produces fewer side effects: “The outcomes from unilateral right hemisphere stimulation are said to be every bit as good or maybe better, and you don’t get the confusion, you don’t get the memory loss, you don’t get all that sort of stuff that you’d expect when somebody has a prolonged, generalized tonic-clonic seizure.”
Still, people are naturally reluctant to undergo ECT. “I’ve seen it. It’s pretty barbaric. It’s better now and at my institution, people do get it, but they really, really have to be intractable,” he said.
Comparing Treatment Options
Mr. Kandhadai and his co-authors used the TriNetX database to identify patients with treatment-resistant major depressive disorder who received TMS or ECT in the past 20 years. There were 2,916 patients in both cohorts, who were matched by age, sex, ethnicity, mood and behavioral disorders, endocrine disorders, intellectual disabilities, cerebrovascular disease, and other nervous system disorders. The mean age at treatment was 48.2 years, 38.5% were male, and 3.1% were Black or African American.
Short-term outcomes favored TMS, including the frequency of disorientation (0.41% vs 2.81%), retrograde amnesia (0.34% vs 0.65%), and headache (4.36% vs 7.20%). Long-term outcomes from 1 month to 5 years post treatment were also better in the TMS group, including depressive episodes (44.99% vs 53.77%), suicide attempts (3.98% vs 6.86%), and suicidal ideation (12.38% vs 23.49%). Kaplan-Meier curve analysis between 1 month and 5 years showed a benefit to TMS in probability of not experiencing a depressive episode, and not experiencing suicidal ideation.
“ECT has been the gold standard of treatment resistant depression for a long time, and it deserves to be. I think it’s something you should offer your patients. Not everyone might be comfortable with it, and if they’re not, I think it’s important to not stop the conversation there, but to offer something like TMS because TMS is something that might be more accessible to patients. It might be more affordable, and it might be less scary,” said Mr. Kandhadai
Mr. Kandhadai and Dr. Coslett have no relevant financial disclosures.
DENVER — , according to results from a retrospective study of patients treated in the past 20 years.
“We always learn in our textbooks that after about two or three medication trials is when you can start exploring more serious treatment protocols, such as ECT or TMS, but a lot of these patients weren’t going forward with it, and I was curious about it. I figured that TMS, which is a less expensive, less scary procedure that patients would more likely be open to, that is also approved for treatment resistant depression, would be a good alternative to ECT,” said Anuttham Kandhadai, a third-year medical student at University of Texas Medical Branch at Galveston, who presented the study at the 2024 annual meeting of the American Academy of Neurology.
Study Findings Lead to More Questions
The researchers found lower rates of depressive episodes, suicidal attempts, and suicidal ideation among patients treated with TMS, but an important limitation was that the researchers did not know the severity of the depression in the two patient groups, according to Branch Coslett, MD, who attended the session and has performed research with TMS to treat aphasia in stroke patients. “I think it’s a very interesting study, and certainly something worth pursuing, but given that ECT is only used as a last resort, whereas TMS is often used as a second-line therapy, I think you’re really talking about very different populations that have had these treatments,” said Dr. Coslett.
Mr. Kandhadai recognized the limitations of the study and looks forward to expanding the research. “I’d love to explore cost effectiveness of the treatments. I’d love to explore patient familiarity and patient comfort with different treatments. And I’d also love to explore a more controlled study that can determine how severe someone’s depression is, and then be able to control for that and explore the outcomes based on the treatment protocol,” he said.
The ideal comparative study would be prospective, “but that will never be done. One Flew Over the Cuckoo’s Nest and similar sources of information have really poisoned the well,” said Dr. Coslett. However, he noted that advances have been made in ECT, and that targeting the right hemisphere produces fewer side effects: “The outcomes from unilateral right hemisphere stimulation are said to be every bit as good or maybe better, and you don’t get the confusion, you don’t get the memory loss, you don’t get all that sort of stuff that you’d expect when somebody has a prolonged, generalized tonic-clonic seizure.”
Still, people are naturally reluctant to undergo ECT. “I’ve seen it. It’s pretty barbaric. It’s better now and at my institution, people do get it, but they really, really have to be intractable,” he said.
Comparing Treatment Options
Mr. Kandhadai and his co-authors used the TriNetX database to identify patients with treatment-resistant major depressive disorder who received TMS or ECT in the past 20 years. There were 2,916 patients in both cohorts, who were matched by age, sex, ethnicity, mood and behavioral disorders, endocrine disorders, intellectual disabilities, cerebrovascular disease, and other nervous system disorders. The mean age at treatment was 48.2 years, 38.5% were male, and 3.1% were Black or African American.
Short-term outcomes favored TMS, including the frequency of disorientation (0.41% vs 2.81%), retrograde amnesia (0.34% vs 0.65%), and headache (4.36% vs 7.20%). Long-term outcomes from 1 month to 5 years post treatment were also better in the TMS group, including depressive episodes (44.99% vs 53.77%), suicide attempts (3.98% vs 6.86%), and suicidal ideation (12.38% vs 23.49%). Kaplan-Meier curve analysis between 1 month and 5 years showed a benefit to TMS in probability of not experiencing a depressive episode, and not experiencing suicidal ideation.
“ECT has been the gold standard of treatment resistant depression for a long time, and it deserves to be. I think it’s something you should offer your patients. Not everyone might be comfortable with it, and if they’re not, I think it’s important to not stop the conversation there, but to offer something like TMS because TMS is something that might be more accessible to patients. It might be more affordable, and it might be less scary,” said Mr. Kandhadai
Mr. Kandhadai and Dr. Coslett have no relevant financial disclosures.
DENVER — , according to results from a retrospective study of patients treated in the past 20 years.
“We always learn in our textbooks that after about two or three medication trials is when you can start exploring more serious treatment protocols, such as ECT or TMS, but a lot of these patients weren’t going forward with it, and I was curious about it. I figured that TMS, which is a less expensive, less scary procedure that patients would more likely be open to, that is also approved for treatment resistant depression, would be a good alternative to ECT,” said Anuttham Kandhadai, a third-year medical student at University of Texas Medical Branch at Galveston, who presented the study at the 2024 annual meeting of the American Academy of Neurology.
Study Findings Lead to More Questions
The researchers found lower rates of depressive episodes, suicidal attempts, and suicidal ideation among patients treated with TMS, but an important limitation was that the researchers did not know the severity of the depression in the two patient groups, according to Branch Coslett, MD, who attended the session and has performed research with TMS to treat aphasia in stroke patients. “I think it’s a very interesting study, and certainly something worth pursuing, but given that ECT is only used as a last resort, whereas TMS is often used as a second-line therapy, I think you’re really talking about very different populations that have had these treatments,” said Dr. Coslett.
Mr. Kandhadai recognized the limitations of the study and looks forward to expanding the research. “I’d love to explore cost effectiveness of the treatments. I’d love to explore patient familiarity and patient comfort with different treatments. And I’d also love to explore a more controlled study that can determine how severe someone’s depression is, and then be able to control for that and explore the outcomes based on the treatment protocol,” he said.
The ideal comparative study would be prospective, “but that will never be done. One Flew Over the Cuckoo’s Nest and similar sources of information have really poisoned the well,” said Dr. Coslett. However, he noted that advances have been made in ECT, and that targeting the right hemisphere produces fewer side effects: “The outcomes from unilateral right hemisphere stimulation are said to be every bit as good or maybe better, and you don’t get the confusion, you don’t get the memory loss, you don’t get all that sort of stuff that you’d expect when somebody has a prolonged, generalized tonic-clonic seizure.”
Still, people are naturally reluctant to undergo ECT. “I’ve seen it. It’s pretty barbaric. It’s better now and at my institution, people do get it, but they really, really have to be intractable,” he said.
Comparing Treatment Options
Mr. Kandhadai and his co-authors used the TriNetX database to identify patients with treatment-resistant major depressive disorder who received TMS or ECT in the past 20 years. There were 2,916 patients in both cohorts, who were matched by age, sex, ethnicity, mood and behavioral disorders, endocrine disorders, intellectual disabilities, cerebrovascular disease, and other nervous system disorders. The mean age at treatment was 48.2 years, 38.5% were male, and 3.1% were Black or African American.
Short-term outcomes favored TMS, including the frequency of disorientation (0.41% vs 2.81%), retrograde amnesia (0.34% vs 0.65%), and headache (4.36% vs 7.20%). Long-term outcomes from 1 month to 5 years post treatment were also better in the TMS group, including depressive episodes (44.99% vs 53.77%), suicide attempts (3.98% vs 6.86%), and suicidal ideation (12.38% vs 23.49%). Kaplan-Meier curve analysis between 1 month and 5 years showed a benefit to TMS in probability of not experiencing a depressive episode, and not experiencing suicidal ideation.
“ECT has been the gold standard of treatment resistant depression for a long time, and it deserves to be. I think it’s something you should offer your patients. Not everyone might be comfortable with it, and if they’re not, I think it’s important to not stop the conversation there, but to offer something like TMS because TMS is something that might be more accessible to patients. It might be more affordable, and it might be less scary,” said Mr. Kandhadai
Mr. Kandhadai and Dr. Coslett have no relevant financial disclosures.
FROM AAN 2024
Commentary: Diet and Lifestyle in Migraine, May 2024
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events. To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events. To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
Migraine and other headache types are common ailments, and there are many stereotypes and stigmas associated with these conditions. One of the prevailing beliefs about headaches and migraines is that they are linked with internalizing mental health conditions — anxiety and depression. These associations can affect pediatric migraine patients and their parents in complicated ways, potentially hindering adequate diagnosis and treatment. Results of a recent prospective study, published in the journal Headache, provided results that challenge the widespread belief that people who have migraines have a higher-than-average rate of internalizing mental health disorders. The authors provided a discussion and data to explain that their initial hypothesis of a relationship between migraine and mental health was disproven. The study included 123 participants age 8-18 years who had been previously diagnosed with migraine. The patients, who were seen in a pediatric neurology clinic, completed headache questionnaires and validated measures of anxiety and depressive symptoms. The final analysis showed no significant association between migraines or headaches with anxiety or depression.
Why does this matter? Stigma can prevent patients and parents from seeking care if parents feel that they will be judged as bad parents for contributing to their children's anxiety, depression, headaches, and migraines. In fact, beyond mental health stigma, children who have migraine can be blamed for having an unhealthy lifestyle.[1] While advice to get enough sleep, eat healthy, and stay active is worthwhile, there can be an implication that pediatric migraine patients are causing their migraines by living an unhealthy lifestyle.[1] Additionally, the implication that parents are not properly taking care of their children's health can inhibit an accurate symptom history. Releasing pediatric migraine patients and their parents from myths about migraines and headaches can be a beneficial component of doctor-patient communication regarding migraine care.
It is possible that dietary adjustments or supplements could help improve migraine frequency and severity. Maintaining a healthy diet is a frequent recommendation for people who have headaches, but it can be frustrating for patients to receive general recommendations to follow a healthy lifestyle. Specific direction regarding which foods to avoid and which foods to add to a diet can be helpful for patients as they try to navigate the challenge of adopting migraine-friendly lifestyle changes.
Eicosapentaenoic acid (EPA) is one of the omega-3 fatty acids. A recent study, with results published in Brain, Behavior, and Immunity, examined the effects of EPA on migraines. The 12-week randomized, double-blind, placebo-controlled trial included 70 participants who had been diagnosed with episodic migraine. Participants were randomly assigned to either EPA (2 g fish oil with 1.8 g of EPA/day) or placebo (2 g soybean oil/day). Migraine frequency and severity were assessed using standardized scales. According to the authors, the high-dose-EPA group had significantly reduced migraine frequency and severity, fewer number of days using acute treatment, reduced migraine-associated disability, improved anxiety and depression, and improved quality of life in comparison to the placebo group. The EPA group did not experience notable adverse events. To provide a sense of scale regarding dietary EPA, 3 oz of cooked wild salmon has 0.35 g of EPA, 3 oz of cooked shrimp has 0.2 g of EPA, and 3 oz of light canned tuna has 0.02 g of EPA.[2] Thus, it's important to note that the amount of EPA used in this study was higher than what would be expected of dietary EPA.
An observational prospective study published in Scientific Reports examined the effects of dietary phytochemical index (DPI) on migraine. DPI is defined as the proportion of daily energy intake derived from foods rich in phytochemicals. Consumption of phytochemical-rich foods has been associated with cardiovascular and metabolic diseases prevention in various populations. These foods include fruits, vegetables, whole grains, seeds, nuts, and legumes. The study included 265 adults age 20-50 who had a diagnosis of migraine. Participants were asked to fill out a questionnaire, which was used to evaluate their diet in the preceding year, and they were asked to complete a diary to track their migraine symptoms. The results showed an inverse relationship between DPI index and migraine frequency. Participants who had the highest DPI had the lowest migraine frequency.[3] While the authors found the results to be statistically significant, they did not point to a cause and effect. Migraine-associated symptoms such as nausea can have an effect on dietary choices, so patients who experience migraine symptoms may avoid certain foods before, during, or after a migraine episode. They also may consistently avoid foods that they have experienced as migraine triggers.
Diet and lifestyle can have an effect on migraine frequency, severity, and overall migraine-associated quality of life. Beyond general recommendations, however, it is not yet well established which foods or supplements could potentially help alleviate migraines. Advice to maintain a healthy lifestyle is definitely worthwhile for migraine patients, but it is important to avoid conveying blame or stigma when it comes to communication about the effect of lifestyle on migraine. This is especially important for pediatric migraine patients because the stigma extends beyond children to parents and could potentially interfere with clear communication and adequate care.
Additional References
1. Gelfand AA, Irwin SL. Lifestyle advice for pediatric migraine: Blaming the patient, or evidence based? Semin Neurol. 2020;40:277-285. doi: 10.1055/s-0040-1708868 Source
2. National Institutes of Health. Office of Dietary Supplements. Omega-3 fatty acids. Updated February 15, 2023. Source
3. Hamedi-Shahraki S, Jowshan M-R, Zolghadrpour M-A, et al. Dietary phytochemical index is favorably associated with oxidative stress status and cardiovascular risk factors in adults with obesity. Sci Rep. 2023;13:7035. doi: 10.1038/s41598-023-34064-4 Source
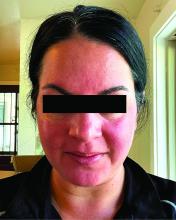
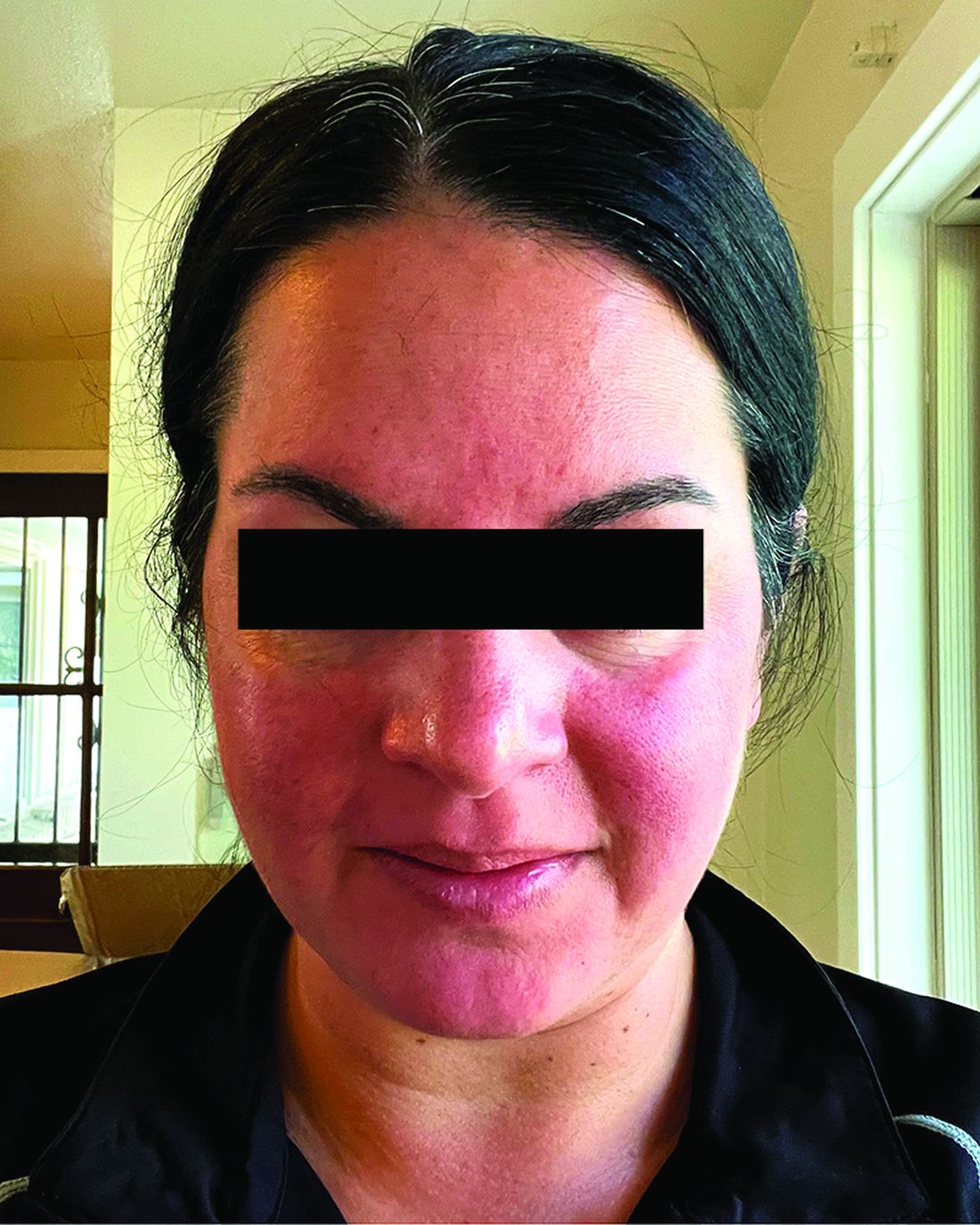
Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Anti-CGRP mAb Superior to Botulinum Toxin A in Reducing Migraine Burden
Key clinical point: Anti-calcitonin gene-related peptide monoclonal antibodies (anti-CGRP mAb) showed superior efficacy to botulinum toxin A (BoNT-A) in reducing the migraine burden in patients with chronic migraine (CM).
Major finding: Anti-CGRP mAbs vs BoNT-A led to significant reductions in mean Headache Impact Test-6 and Allodynia Symptoms Checklist-12 scores at 6 months (mean change −11.1 vs −3.2 points, P < .0001; and −5.2 vs −0.5 points, P = .0056, respectively) and 12 months (mean change −11.4 vs −3.6 points, P = .0042; and −6.0 vs −0.9 points, P = .0011, respectively).
Study details: This exploratory analysis of the real-world effectiveness of anti-CGRP mAb vs BoNT-A included 126 patients with CM who were treated with anti-CGRP mAb (n = 36) or BoNT-A (n = 90).
Disclosures: The study was supported by the Italian Ministry of Health. Some authors declared receiving funding, travel grants, honoraria, or personal fees for participation in advisory boards, speaker panels, and clinical investigation studies from various sources.
Source: Montisano DA, Giossi R, Canella M, et al. Reducing the impact of headache and allodynia score in chronic migraine: An exploratory analysis from the Real-world Effectiveness of Anti-CGRP Monoclonal Antibodies Compared to Onabotulinum Toxin A (RAMO) Study. Toxins. 2024;16(4):178. doi: 10.3390/toxins16040178 Source
Key clinical point: Anti-calcitonin gene-related peptide monoclonal antibodies (anti-CGRP mAb) showed superior efficacy to botulinum toxin A (BoNT-A) in reducing the migraine burden in patients with chronic migraine (CM).
Major finding: Anti-CGRP mAbs vs BoNT-A led to significant reductions in mean Headache Impact Test-6 and Allodynia Symptoms Checklist-12 scores at 6 months (mean change −11.1 vs −3.2 points, P < .0001; and −5.2 vs −0.5 points, P = .0056, respectively) and 12 months (mean change −11.4 vs −3.6 points, P = .0042; and −6.0 vs −0.9 points, P = .0011, respectively).
Study details: This exploratory analysis of the real-world effectiveness of anti-CGRP mAb vs BoNT-A included 126 patients with CM who were treated with anti-CGRP mAb (n = 36) or BoNT-A (n = 90).
Disclosures: The study was supported by the Italian Ministry of Health. Some authors declared receiving funding, travel grants, honoraria, or personal fees for participation in advisory boards, speaker panels, and clinical investigation studies from various sources.
Source: Montisano DA, Giossi R, Canella M, et al. Reducing the impact of headache and allodynia score in chronic migraine: An exploratory analysis from the Real-world Effectiveness of Anti-CGRP Monoclonal Antibodies Compared to Onabotulinum Toxin A (RAMO) Study. Toxins. 2024;16(4):178. doi: 10.3390/toxins16040178 Source
Key clinical point: Anti-calcitonin gene-related peptide monoclonal antibodies (anti-CGRP mAb) showed superior efficacy to botulinum toxin A (BoNT-A) in reducing the migraine burden in patients with chronic migraine (CM).
Major finding: Anti-CGRP mAbs vs BoNT-A led to significant reductions in mean Headache Impact Test-6 and Allodynia Symptoms Checklist-12 scores at 6 months (mean change −11.1 vs −3.2 points, P < .0001; and −5.2 vs −0.5 points, P = .0056, respectively) and 12 months (mean change −11.4 vs −3.6 points, P = .0042; and −6.0 vs −0.9 points, P = .0011, respectively).
Study details: This exploratory analysis of the real-world effectiveness of anti-CGRP mAb vs BoNT-A included 126 patients with CM who were treated with anti-CGRP mAb (n = 36) or BoNT-A (n = 90).
Disclosures: The study was supported by the Italian Ministry of Health. Some authors declared receiving funding, travel grants, honoraria, or personal fees for participation in advisory boards, speaker panels, and clinical investigation studies from various sources.
Source: Montisano DA, Giossi R, Canella M, et al. Reducing the impact of headache and allodynia score in chronic migraine: An exploratory analysis from the Real-world Effectiveness of Anti-CGRP Monoclonal Antibodies Compared to Onabotulinum Toxin A (RAMO) Study. Toxins. 2024;16(4):178. doi: 10.3390/toxins16040178 Source
Anxiety or Depressive Symptoms Do Not Predict Migraine-Related Outcomes in Youth
Key clinical point: Contrary to common beliefs, neither anxiety nor depressive symptoms were associated with migraine-related outcomes in children and adolescents.
Major finding: Headache frequency and migraine-related disability had no significant association with anxiety (headache frequency P = .639; migraine-related disability P = .470) and depressive symptoms (headache frequency P = .209; migraine-related disability P = .796) at baseline. Similarly, no significant association was observed between longitudinal changes in anxiety and depressive symptoms and migraine-related outcomes.
Study details: Findings are from a prospective clinical cohort study including 123 children and adolescents with migraine who responded to a headache questionnaire and were assessed for anxiety and depressive symptoms.
Disclosures: This study was supported by the Program of Undergraduate Research Experience, University of Calgary, Canada, and others. Serena Laura Orr declared receiving royalties and research funding from various sources and serving on the editorial boards of Headache, Neurology, and the American Migraine Foundation. The other authors declared no conflicts of interest.
Source: Rizvi BA, Kuziek J, Cho LY, et al. Anxiety and depressive symptoms and migraine-related outcomes in children and adolescents. Headache. 2024 (Apr 6). doi: 10.1111/head.14701 Source
Key clinical point: Contrary to common beliefs, neither anxiety nor depressive symptoms were associated with migraine-related outcomes in children and adolescents.
Major finding: Headache frequency and migraine-related disability had no significant association with anxiety (headache frequency P = .639; migraine-related disability P = .470) and depressive symptoms (headache frequency P = .209; migraine-related disability P = .796) at baseline. Similarly, no significant association was observed between longitudinal changes in anxiety and depressive symptoms and migraine-related outcomes.
Study details: Findings are from a prospective clinical cohort study including 123 children and adolescents with migraine who responded to a headache questionnaire and were assessed for anxiety and depressive symptoms.
Disclosures: This study was supported by the Program of Undergraduate Research Experience, University of Calgary, Canada, and others. Serena Laura Orr declared receiving royalties and research funding from various sources and serving on the editorial boards of Headache, Neurology, and the American Migraine Foundation. The other authors declared no conflicts of interest.
Source: Rizvi BA, Kuziek J, Cho LY, et al. Anxiety and depressive symptoms and migraine-related outcomes in children and adolescents. Headache. 2024 (Apr 6). doi: 10.1111/head.14701 Source
Key clinical point: Contrary to common beliefs, neither anxiety nor depressive symptoms were associated with migraine-related outcomes in children and adolescents.
Major finding: Headache frequency and migraine-related disability had no significant association with anxiety (headache frequency P = .639; migraine-related disability P = .470) and depressive symptoms (headache frequency P = .209; migraine-related disability P = .796) at baseline. Similarly, no significant association was observed between longitudinal changes in anxiety and depressive symptoms and migraine-related outcomes.
Study details: Findings are from a prospective clinical cohort study including 123 children and adolescents with migraine who responded to a headache questionnaire and were assessed for anxiety and depressive symptoms.
Disclosures: This study was supported by the Program of Undergraduate Research Experience, University of Calgary, Canada, and others. Serena Laura Orr declared receiving royalties and research funding from various sources and serving on the editorial boards of Headache, Neurology, and the American Migraine Foundation. The other authors declared no conflicts of interest.
Source: Rizvi BA, Kuziek J, Cho LY, et al. Anxiety and depressive symptoms and migraine-related outcomes in children and adolescents. Headache. 2024 (Apr 6). doi: 10.1111/head.14701 Source
Anti-CGRP mAb and Onabot Combination More Effective Than Either Therapy in Chronic Migraine
Key clinical point: Treatment with a combination of anti-calcitonin gene-related peptide (anti-CGRP) monoclonal antibodies (mAb) and onabotulinumtoxinA (onabot) was more effective than monotherapy with either anti-CGRP alone or onabot in reducing monthly migraine days (MMD) in patients with chronic migraine.
Major finding: Initiating therapy with either anti-CGRP mAbs or onabot significantly reduced the median MMD from 30 to 15 days, which further decreased to 8 days after initiating dual therapy with anti-CGRP mAb and onabot (both P < .0001). Overall, 68% of patients receiving dual therapy achieved 50% or greater reduction in MMD.
Study details: This real-world retrospective chart review included 423 patients with chronic migraine (age ≥ 18 years) who received monotherapy with either anti-CGRP mAb or onabot (n = 229) followed by dual therapy (concurrent treatment with anti-CGRP mAb and onabot; n = 194) for at least three consecutive months.
Disclosures: This study was funded by a Cleveland Clinic institutional grant. The authors declared no conflicts of interest.
Source: Salim A, Hennessy E, Sonneborn C, et al. Synergism of anti-CGRP monoclonal antibodies and onabotulinumtoxinA in the treatment of chronic migraine: A real-world retrospective chart review. CNS Drugs. 2024 (Apr 7). doi: 10.1007/s40263-024-01086-z Source
Key clinical point: Treatment with a combination of anti-calcitonin gene-related peptide (anti-CGRP) monoclonal antibodies (mAb) and onabotulinumtoxinA (onabot) was more effective than monotherapy with either anti-CGRP alone or onabot in reducing monthly migraine days (MMD) in patients with chronic migraine.
Major finding: Initiating therapy with either anti-CGRP mAbs or onabot significantly reduced the median MMD from 30 to 15 days, which further decreased to 8 days after initiating dual therapy with anti-CGRP mAb and onabot (both P < .0001). Overall, 68% of patients receiving dual therapy achieved 50% or greater reduction in MMD.
Study details: This real-world retrospective chart review included 423 patients with chronic migraine (age ≥ 18 years) who received monotherapy with either anti-CGRP mAb or onabot (n = 229) followed by dual therapy (concurrent treatment with anti-CGRP mAb and onabot; n = 194) for at least three consecutive months.
Disclosures: This study was funded by a Cleveland Clinic institutional grant. The authors declared no conflicts of interest.
Source: Salim A, Hennessy E, Sonneborn C, et al. Synergism of anti-CGRP monoclonal antibodies and onabotulinumtoxinA in the treatment of chronic migraine: A real-world retrospective chart review. CNS Drugs. 2024 (Apr 7). doi: 10.1007/s40263-024-01086-z Source
Key clinical point: Treatment with a combination of anti-calcitonin gene-related peptide (anti-CGRP) monoclonal antibodies (mAb) and onabotulinumtoxinA (onabot) was more effective than monotherapy with either anti-CGRP alone or onabot in reducing monthly migraine days (MMD) in patients with chronic migraine.
Major finding: Initiating therapy with either anti-CGRP mAbs or onabot significantly reduced the median MMD from 30 to 15 days, which further decreased to 8 days after initiating dual therapy with anti-CGRP mAb and onabot (both P < .0001). Overall, 68% of patients receiving dual therapy achieved 50% or greater reduction in MMD.
Study details: This real-world retrospective chart review included 423 patients with chronic migraine (age ≥ 18 years) who received monotherapy with either anti-CGRP mAb or onabot (n = 229) followed by dual therapy (concurrent treatment with anti-CGRP mAb and onabot; n = 194) for at least three consecutive months.
Disclosures: This study was funded by a Cleveland Clinic institutional grant. The authors declared no conflicts of interest.
Source: Salim A, Hennessy E, Sonneborn C, et al. Synergism of anti-CGRP monoclonal antibodies and onabotulinumtoxinA in the treatment of chronic migraine: A real-world retrospective chart review. CNS Drugs. 2024 (Apr 7). doi: 10.1007/s40263-024-01086-z Source
BMI Tied to Medication-Overuse Headache Risk in Migraine
Key clinical point: Higher body mass index (BMI) was positively associated with an increased risk for medication-overuse headache (MOH) in patients with migraine.
Major finding: Higher BMI was positively associated with a higher risk for MOH (adjusted odds ratio [aOR] 1.05; 95% CI 1.01-1.11; P = .031), with the risk being significantly higher in patients with migraine who had a BMI ≥ 28.0 kg/m2 vs those with BMI of 18.5-23.9 kg/m2 (aOR 1.81; 95% CI 1.04-3.17; P = .037).
Study details: This secondary analysis of the Survey of Fibromyalgia Comorbidity with Headache study included 2251 patients with migraine, of whom 195 (8.7%) had concomitant MOH.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Liu H, Zhao H, Liu K, et al. Association between body mass index and medication-overuse headache among individuals with migraine: A cross-sectional study. Obes Facts. 2024 (Apr 3). doi: 10.1159/000538528 Source
Key clinical point: Higher body mass index (BMI) was positively associated with an increased risk for medication-overuse headache (MOH) in patients with migraine.
Major finding: Higher BMI was positively associated with a higher risk for MOH (adjusted odds ratio [aOR] 1.05; 95% CI 1.01-1.11; P = .031), with the risk being significantly higher in patients with migraine who had a BMI ≥ 28.0 kg/m2 vs those with BMI of 18.5-23.9 kg/m2 (aOR 1.81; 95% CI 1.04-3.17; P = .037).
Study details: This secondary analysis of the Survey of Fibromyalgia Comorbidity with Headache study included 2251 patients with migraine, of whom 195 (8.7%) had concomitant MOH.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Liu H, Zhao H, Liu K, et al. Association between body mass index and medication-overuse headache among individuals with migraine: A cross-sectional study. Obes Facts. 2024 (Apr 3). doi: 10.1159/000538528 Source
Key clinical point: Higher body mass index (BMI) was positively associated with an increased risk for medication-overuse headache (MOH) in patients with migraine.
Major finding: Higher BMI was positively associated with a higher risk for MOH (adjusted odds ratio [aOR] 1.05; 95% CI 1.01-1.11; P = .031), with the risk being significantly higher in patients with migraine who had a BMI ≥ 28.0 kg/m2 vs those with BMI of 18.5-23.9 kg/m2 (aOR 1.81; 95% CI 1.04-3.17; P = .037).
Study details: This secondary analysis of the Survey of Fibromyalgia Comorbidity with Headache study included 2251 patients with migraine, of whom 195 (8.7%) had concomitant MOH.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Liu H, Zhao H, Liu K, et al. Association between body mass index and medication-overuse headache among individuals with migraine: A cross-sectional study. Obes Facts. 2024 (Apr 3). doi: 10.1159/000538528 Source
Dietary Phytochemical Index and Migraine Headache: Is There a Link?
Key clinical point: A higher dietary phytochemical index (DPI) score is associated with lower migraine frequency and improved migraine-related disability in patients with migraine.
Major finding: Patients with migraine in the third vs first tertile of the DPI showed a significant reduction in migraine frequency (incidence rate ratio 0.84; Ptrend = .009) and an improvement in migraine-related disability (β −2.48; Ptrend = .046). However, no significant association was seen between DPI and headache duration (Ptrend = .439), headache severity (Ptrend = .239), depression (Ptrend = .480), anxiety (Ptrend = .655), and stress (Ptrend = .876).
Study details: This cross-sectional study evaluated the association between DPI and the clinical and psychological characteristics of migraine headaches in 262 patients with migraine (age 20-50 years).
Disclosures: This study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran. The authors declared no conflicts of interest.
Source: Amani Tirani S, Balali A, Kazemi M, et al. The predictive role of the dietary phytochemical index in relation to the clinical and psychological traits of migraine headaches. Sci Rep. 2024;14:6886 (Mar 22). doi: 10.1038/s41598-024-57536-7 Source
Key clinical point: A higher dietary phytochemical index (DPI) score is associated with lower migraine frequency and improved migraine-related disability in patients with migraine.
Major finding: Patients with migraine in the third vs first tertile of the DPI showed a significant reduction in migraine frequency (incidence rate ratio 0.84; Ptrend = .009) and an improvement in migraine-related disability (β −2.48; Ptrend = .046). However, no significant association was seen between DPI and headache duration (Ptrend = .439), headache severity (Ptrend = .239), depression (Ptrend = .480), anxiety (Ptrend = .655), and stress (Ptrend = .876).
Study details: This cross-sectional study evaluated the association between DPI and the clinical and psychological characteristics of migraine headaches in 262 patients with migraine (age 20-50 years).
Disclosures: This study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran. The authors declared no conflicts of interest.
Source: Amani Tirani S, Balali A, Kazemi M, et al. The predictive role of the dietary phytochemical index in relation to the clinical and psychological traits of migraine headaches. Sci Rep. 2024;14:6886 (Mar 22). doi: 10.1038/s41598-024-57536-7 Source
Key clinical point: A higher dietary phytochemical index (DPI) score is associated with lower migraine frequency and improved migraine-related disability in patients with migraine.
Major finding: Patients with migraine in the third vs first tertile of the DPI showed a significant reduction in migraine frequency (incidence rate ratio 0.84; Ptrend = .009) and an improvement in migraine-related disability (β −2.48; Ptrend = .046). However, no significant association was seen between DPI and headache duration (Ptrend = .439), headache severity (Ptrend = .239), depression (Ptrend = .480), anxiety (Ptrend = .655), and stress (Ptrend = .876).
Study details: This cross-sectional study evaluated the association between DPI and the clinical and psychological characteristics of migraine headaches in 262 patients with migraine (age 20-50 years).
Disclosures: This study was supported by the Isfahan University of Medical Sciences, Isfahan, Iran. The authors declared no conflicts of interest.
Source: Amani Tirani S, Balali A, Kazemi M, et al. The predictive role of the dietary phytochemical index in relation to the clinical and psychological traits of migraine headaches. Sci Rep. 2024;14:6886 (Mar 22). doi: 10.1038/s41598-024-57536-7 Source
Meta-analysis Identifies Atopic Dermatitis as Potential Risk Factor for Headache Disorder or Migraine
Key clinical point: Atopic dermatitis (AD) is significantly associated with a higher risk for headache disorders or migraine.
Major finding: Patients with AD had a significantly higher risk for headache disorder (odds ratio [OR] 1.46; 95% CI 1.36-1.56) or migraine (OR 1.32; 95% CI 1.18-1.47; both P < .001). This finding was consistent across the different subgroup analyses.
Study details: The data come from a meta-analysis of 10 observational studies that evaluated the risk for headache disorders in 12,717,747 patients with AD, of which six studies evaluated migraine risk in 11,090,412 patients with AD.
Disclosures: This study was supported by the project of training talent for Zhejiang province young and middle-aged clinical traditional Chinese medicine experts and Hangzhou Municipal Health and Family Planning Commission Science and Technology Project, China. The authors declared no conflicts of interest.
Source: Yang W, Dai H, Xu X-F, et al. Association of atopic dermatitis and headache disorder: A systematic review and meta-analyses. Front Neurol. 2024;15:1383832. doi: 10.3389/fneur.2024.1383832 Source
Key clinical point: Atopic dermatitis (AD) is significantly associated with a higher risk for headache disorders or migraine.
Major finding: Patients with AD had a significantly higher risk for headache disorder (odds ratio [OR] 1.46; 95% CI 1.36-1.56) or migraine (OR 1.32; 95% CI 1.18-1.47; both P < .001). This finding was consistent across the different subgroup analyses.
Study details: The data come from a meta-analysis of 10 observational studies that evaluated the risk for headache disorders in 12,717,747 patients with AD, of which six studies evaluated migraine risk in 11,090,412 patients with AD.
Disclosures: This study was supported by the project of training talent for Zhejiang province young and middle-aged clinical traditional Chinese medicine experts and Hangzhou Municipal Health and Family Planning Commission Science and Technology Project, China. The authors declared no conflicts of interest.
Source: Yang W, Dai H, Xu X-F, et al. Association of atopic dermatitis and headache disorder: A systematic review and meta-analyses. Front Neurol. 2024;15:1383832. doi: 10.3389/fneur.2024.1383832 Source
Key clinical point: Atopic dermatitis (AD) is significantly associated with a higher risk for headache disorders or migraine.
Major finding: Patients with AD had a significantly higher risk for headache disorder (odds ratio [OR] 1.46; 95% CI 1.36-1.56) or migraine (OR 1.32; 95% CI 1.18-1.47; both P < .001). This finding was consistent across the different subgroup analyses.
Study details: The data come from a meta-analysis of 10 observational studies that evaluated the risk for headache disorders in 12,717,747 patients with AD, of which six studies evaluated migraine risk in 11,090,412 patients with AD.
Disclosures: This study was supported by the project of training talent for Zhejiang province young and middle-aged clinical traditional Chinese medicine experts and Hangzhou Municipal Health and Family Planning Commission Science and Technology Project, China. The authors declared no conflicts of interest.
Source: Yang W, Dai H, Xu X-F, et al. Association of atopic dermatitis and headache disorder: A systematic review and meta-analyses. Front Neurol. 2024;15:1383832. doi: 10.3389/fneur.2024.1383832 Source
Patients With Chronic Kidney Disease Experiences Modestly Lower Risk for Migraine
Key clinical point: Patients with chronic kidney disease (CKD), particularly older adults and women, had a modestly reduced risk of developing migraine over a 16-year follow-up period.
Major finding: During the 16-year follow-up, patients with vs without CKD had an 11% lower risk for migraine (adjusted hazard ratio [aHR] 0.89; P = .006), with the risk being prominently lower among older adults (age ≥ 70 years; aHR 0.69; P < .001) and women (aHR 0.84; P = .006).
Study details: This nationwide, 16-year longitudinal follow-up study included 15,443 participants with CKD, of whom 349 (2.26%) had migraine, and 61,772 participants without CKD, of whom 1901 (3.08%) had migraine.
Disclosures: This study was supported by Bracco Imaging Korea and the National Research Foundation of Korea. The authors declared no conflicts of interest.
Source: Kwon MJ, Kim J-K, Kim M-J, et al. Associations between chronic kidney disease and migraine incidence: Findings from a Korean longitudinal big data study. J Pers Med. 2024;14(4):356 (Mar 28). doi: 10.3390/jpm14040356 Source
Key clinical point: Patients with chronic kidney disease (CKD), particularly older adults and women, had a modestly reduced risk of developing migraine over a 16-year follow-up period.
Major finding: During the 16-year follow-up, patients with vs without CKD had an 11% lower risk for migraine (adjusted hazard ratio [aHR] 0.89; P = .006), with the risk being prominently lower among older adults (age ≥ 70 years; aHR 0.69; P < .001) and women (aHR 0.84; P = .006).
Study details: This nationwide, 16-year longitudinal follow-up study included 15,443 participants with CKD, of whom 349 (2.26%) had migraine, and 61,772 participants without CKD, of whom 1901 (3.08%) had migraine.
Disclosures: This study was supported by Bracco Imaging Korea and the National Research Foundation of Korea. The authors declared no conflicts of interest.
Source: Kwon MJ, Kim J-K, Kim M-J, et al. Associations between chronic kidney disease and migraine incidence: Findings from a Korean longitudinal big data study. J Pers Med. 2024;14(4):356 (Mar 28). doi: 10.3390/jpm14040356 Source
Key clinical point: Patients with chronic kidney disease (CKD), particularly older adults and women, had a modestly reduced risk of developing migraine over a 16-year follow-up period.
Major finding: During the 16-year follow-up, patients with vs without CKD had an 11% lower risk for migraine (adjusted hazard ratio [aHR] 0.89; P = .006), with the risk being prominently lower among older adults (age ≥ 70 years; aHR 0.69; P < .001) and women (aHR 0.84; P = .006).
Study details: This nationwide, 16-year longitudinal follow-up study included 15,443 participants with CKD, of whom 349 (2.26%) had migraine, and 61,772 participants without CKD, of whom 1901 (3.08%) had migraine.
Disclosures: This study was supported by Bracco Imaging Korea and the National Research Foundation of Korea. The authors declared no conflicts of interest.
Source: Kwon MJ, Kim J-K, Kim M-J, et al. Associations between chronic kidney disease and migraine incidence: Findings from a Korean longitudinal big data study. J Pers Med. 2024;14(4):356 (Mar 28). doi: 10.3390/jpm14040356 Source