User login
Can Serum Free Light Chains Be Used for the Early Diagnosis of Monoclonal Immunoglobulin-Secreting B-Cell and Plasma-Cell Diseases? (FULL)
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
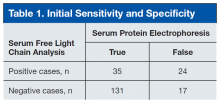
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
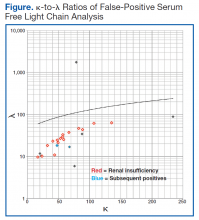
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
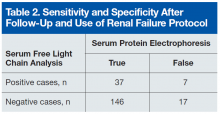
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Patients who are undergoing multiple myeloma screening with serum protein electrophoresis and immunofixation, especially those with renal failure, also should receive serum free light chain testing to increase specificity and reduce false-negatives.
Multiple myeloma (MM) is a devastating disease with an estimated 26,850 new cases in 2015 according to Surveillance, Epidemiology, and End Results data and no definitive chemotherapeutic cure.1 In 97% of cases, MM is defined by monoclonal hypergammaglobulinemia, in which a malignant plasma cell clone secretes a monoclonal globulin; the remaining cases are nonsecretors.2 Each pathologically produced clonal globulin contains 2 heavy chains attached by disulfide linkage and 2 light chains. Unchecked plasma cell production is what later causes the symptoms of renal failure, bone destruction, and anemia.
The rate of MM is disproportionately high in the veteran population, and the VA health care system provides care for many of these patients. The higher rate is likely secondary to the predominantly male population, which has higher MM rates, and has been linked to Agent Orange exposure in Vietnam. As MM is not easy to diagnose, any algorithm or testing method would be of great benefit to this population.
The gold standard for MM detection remains serum protein electrophoresis (SPEP) with immunofixation (IFE), but other detection methods have been emerging. The method of serum free light chain (SFLC) assay has become more readily available, and its incorporation into diagnostic guidelines has become more apparent but is not universal.3
In the case series reported in this article, SPEP/IFE and SFLC assays were used to test 207 patients from the VA New York Harbor Healthcare System (VANYHHS). All these patients had a clinical context for MM testing.
Methods
In this retrospective study, the authors reviewed the charts of VANYHHS patients who were being treated for conditions that prompted SPEP/IFE and λ and κ SFLC analysis between December 2013 and March 2014. The study was exempt from institutional review board approval.
The SPEP/IFE analysis was performed with an automated electrophoresis machine (Sebia Electrophoresis), and the SFLC analysis was performed with an automated SFLC assay (Freelite). Sensitivity, specificity, and positive and negative predictive values were calculated using SPEP/IFE as the gold standard and SFLC κ-to-λ ratio asthe test method. Patients with a positive κ-to-λ ratio but negative SPEP were considered false-positives. These patients’ SFLC analyses were further analyzed in an effort to evaluate use of the κ-to-λ ratio as an early tumor marker.
The κ reference range used was 3.3 to 19.4 mg/L, and the λ reference range used was 5.7 to 26.3 mg/L.4 The traditional reference range for the κ-to-λ ratio is 0.26 to 1.65.5
Results
Of the 207 patients in this study, 205 were men. Mean age was 69 years (range, 28-97 years). Mean serum urea nitrogen level was 8.75 mmol/L (range, 2.86-38.21 mmol/L), and mean creatinine level was 140.59 μmol/L (range, 44.21-1503.14 μmol/L). Mean κ was 49.82 mg/L (range, 4.6-700.96 mg/L), and mean λ was 54.27 mg/L (range, 3-1,750 mg/L). Table 1 compares the SPEP and SFLC data. Sensitivity was 67%, specificity was 85%, positive predictive value was 58%, and negative predictive value was 89%. Concordance of the 2 methods was 80%. The false-positive group was followed up 16 months later to check for diagnosis of disease. Two of the 24 patients in this quadrant were later diagnosed with MM (Table 1).
One of the patients with MM was an 82-year-old African American man with a history of hypertension, diabetes, and prostate cancer (Gleason 4 + 4 = 8/10). He presented to VANYHHS after a fall in which he sustained a pathologic fracture of the left acromion. Recurrent prostate cancer was initially suspected, and nuclear bone scintigraphy revealed increased uptake in the left shoulder and the posterior ninth rib. Results of computed tomography-guided biopsy showed the rib lesion packed with plasma cells and consistent with MM. Immunohistochemical analysis was positive for CD138 and κ in the malignant plasma cells. Initial SPEP performed before the biopsy showed an acute phase reaction with hypogammaglobulinemia, and SPEP after the biopsy showed an increased α-2 band but no monoclonal gammaglobulinopathy. The initial κ of 42.18 mg/L (κ-to-λ ratio, 4.01) was up to 67.53 mg/L 4 months later.
The other patient with MM was a 91-year-old man who had coronary artery disease after undergoing coronary artery bypass grafting in 1993, sick sinus syndrome after pacemaker implantation, hypertension, and anemia. He initially presented to the geriatrics clinic with polyneuropathy, which prompted SPEP and SFLC analysis. SPEP results showed a normal electrophoretic pattern, but κ increased to 47.52 mg/L (κ-to-λ ratio, 2.63). The decision was made to monitor the patient in the hematology clinic. Subsequent κ chain analysis revealed an increase to 59.50 mg/L. A repeat SPEP, performed 1 year after the first SPEP, revealed monoclonal immunoglobulin A on IFE.
Of the 24 patients with false-positive results, 16 had moderate-to-severe kidney disease (stage IIIa-IV).6All patients in this quadrant were men; their mean age was 75 years, and their mean creatinine level was 182.15 μmol/L. Further laboratory data are listed in Table 2.
The patient whose biopsy results led to an MM diagnosis and the patient whose IFE led to a gammopathy diagnosis both maintained a glomerular filtration rate within normal limits. The Figure shows the κ-to-λ ratios of this quadrant logarithmically.
Discussion
Use of SFLC analysis as a supplement to serum and urine protein electrophoresis has been investigated and accepted in the recent literature.3,4,7,8 Use of light chains as a method of earlier or alternative detection has not been proved. In the present study of 207 patients, comparisons showed that more traditional MM detection methods and SFLC analysis are largely concordant. The 2 patients with MM and negative electrophoretic patterns provided a clear indication of the potential benefit of SFLC analysis in the diagnosis of secretory and nonsecretory myeloma.
In 2014, Kim and colleagues compared 2 SFLC assays (Freelite, N Latex) to each other and to SPEP in a 120-patient population.9 The Freelite results in their study correlated closely with VA population findings (κ-to-λ ratio sensitivity and specificity: 72.2% and 93.6%, respectively). N Latex, the newer SFLC assay, had lower sensitivity (64.6%) and higher specificity (100%). With application of the extended reference range (0.37-3.1) proposed by Hutchison and colleagues for use in patients with renal failure, SFLC becomes a more statistically powerful tool.5
The patients who tested false-positive had higher mean creatinine levels, and 16 had renal insufficiency. The 2 false-positive patients were later found to have clinical myeloma and were within the normal range of renal function. Of the 16 patients with an abnormal κ-to-λ ratio and renal failure, 15 would be within the revised normal reference range, leaving 9 false-positives, 2 of whom eventually were found to have disease. With the application of the extended light chain range (as per Hutchison) for those patients with renal failure, 15 of the original 24 false-positives became true-negatives. Two of the false-positives become true-positives after they were subsequently diagnosed. Therefore, SFLC analysis detected disease in 22% of the revised false-positives when SPEP could not.
Table 2 lists the revised data after follow-up and renal failure correction. The strongest aspect of SFLC analysis remains its 95% specificity; its 69% sensitivity remains relatively constant. The test’s positive predictive value is 84%, and its negative predictive value is 90%. In veteran and other at-risk populations, SFLC analysis proves to be a very powerful tool on its own.
Conclusion
Both patient cases described in this article demonstrate the usefulness of SFLC analysis as an adjunct to SPEP. The authors propose SFLC testing for all patients who are undergoing MM screening with SPEP/IFE. In patients with renal failure, the expanded reference range seems to reduce erroneous false-positive results. Patients who have abnormal ratios should be followed up in clinic with repeat MM testing. It seems clear that, at the very least, SFLC analysis is a necessary adjunct to SPEP testing. However, SFLC stands on its own merit as well.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
1. National Cancer Institute, Surveillance, Epidemiology, and End Results (SEER) Program. SEER website. http://seer.cancer.gov/statfacts/html/mulmy.html. Accessed July 11, 2016.
2. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33.
3. Dimopoulos M, Kyle R, Fermand JP, et al; International Myeloma Workshop Consensus Panel 3. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705.
4. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48(9):1437-1444.
5. Hutchison CA, Plant T, Drayson M, et al. Serum free light chain measurement
aids the diagnosis of myeloma in patients with severe renal failure.
BMC Nephrol. 2008;9:11.
6. Levey AS, Stevens LA, Schmid CH, et al; CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration). A new equation to estimate glomerular filtration
rate. Ann Intern Med. 2009;150(9):604-612.
7. McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis
with serum free light chain analysis as a first-line test for detecting plasma
cell disorders offers increased diagnostic accuracy and potential health benefit
to patients. Am J Clin Pathol. 2013;140(6):890-897.
8. Abadie JM, Bankson DD. Assessment of serum free light chain assays for
plasma cell disorder screening in a Veterans Affairs population. Ann Clin Lab
Sci. 2006;36(2):157-162.
9. Kim HS, Kim HS, Shin KS, et al. Clinical comparisons of two free light chain
assays to immunofixation electrophoresis for detecting monoclonal gammopathy.
Biomed Res Int. 2014;2014:647238.
Note: Page numbers differ between the print issue and digital edition.
Pneumatic Tube-Induced Reverse Pseudohyperkalemia in a Patient With Chronic Lymphocytic Leukemia
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
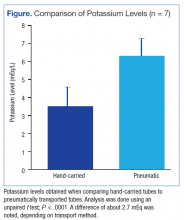
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Pseudohyperkalemia is a potentially dangerous phenomenon where falsely reported elevated potassium levels result in potentially unwarranted correction of potassium by sodium polystyrene or by dialysis in extreme cases. Overcorrection of potassium in a patient whose potassium is normal or low can lead to hypokalemia and potentially life-threatening consequences. Typical pseudohyperkalemia is thought to be a result of platelet-mediated release of potassium that occurs from the clotting process of a serum sample where no anticoagulant is present. As a result, pseudohyperkalemia is typically corrected when potassium is measured with a plasma sample where heparin and other preservatives are present in the collection tube.1
Reverse pseudohyperkalemia is seen in patients with leukemia and lymphoma with significant lymphocytosis when laboratory studies demonstrate falsely elevated potassium. In reverse pseudohyperkalemia the potassium level from a plasma sample is falsely elevated despite the presence of an anticoagulant, as the process is independent of platelet activation and occurs as a result of white blood cell (WBC) breakdown.2
For several decades, it has been suggested that the presence of heparin in tubes used to collect plasma is the cause of lysis of WBCs, presumably due to possible membrane fragility of these cells. Correction was recommended with the use of low-heparin-coated tubes.3 The other proposed theory for reverse pseudohyperkalemia is that lysis of WBCs is primarily due to procedural handling: Several case reports suggest that pneumatic tube transport likely plays a strong role, as well as other factors, such as the length of time to the laboratory.4-6
The authors report a case of a patient with chronic lymphocytic leukemia (CLL) who presented with significant reverse pseudohyperkalemia that later was determined to be dependent on pneumatic tube transport and independent of heparin.
Case Presentation
The patient, an 83-year-old man with a long history of asymptomatic CLL, was noted to have rapid WBC doubling time. His WBC counts had increased from 45 × 103/μL to 95 x 103/μL over the year preceding admission, then further increased to 300 x 103/μL in the month before admission.
A computed tomography (CT) scan of the chest, abdomen, and pelvis showed significant lymphadenopathy and splenomegaly. The patient presented to the hospital for treatment with a planned first cycle of bendamustine alone and subsequent cycles of bendamustine and rituximab. His medical history included Prinzmetal angina, coronary artery disease, wet macular degeneration, and benign prostatic hyperplasia. Notably, he had a documented history of hyperkalemia with potassium levels ranging from 4.7 mEq/L to 4.9 mEq/L over the previous year and was placed on a potassium-restricted diet.
On presentation, he reported no recent history of B symptoms of fever, night sweats, weight loss, and malaise. His labs oratory results showed an elevated potassium level of 6.1 mEq/L with repeated whole blood potassium of 8.2 mEq/L. An electrocardiogram (ECG) showed sinus rhythm, no noted T-wave abnormalities, and no conduction abnormalities. A physical exam was significant for normal muscle strength, cervical lymphadenopathy, and splenomegaly.
The patient was initially treated for hyperkalemia with insulin plus glucose and sodium polystyrene. He responded with mild improvement of his potassium level to 6.3 mEq/L, 5.6 mEq/L, and 5.1 mEq/L after receiving 5 doses of 30 g of polystyrene over multiple checks during a 24-hour period. Hemolysis results drawn at that time were unremarkable. It was noted that the patient had an elevated lactate dehydrogenase (LDH) level of 328 IU/L.
The following morning, his potassium level remained elevated at 6.2 mEq/L, but because the treatment team suspected pseudohyperkalemia, the decision at the time was to proceed with chemotherapy.
To evaluate this possibility, the authors attempted to correct for procedural handling resulting in unwanted WBC lysis. They reduced the lithium heparin in the collection from 81 IU of lithium heparin found in the green-mint collection tube and instead used an arterial blood gas (ABG) syringe that contained 23.5 IU of heparin and hand-carried the sample to the lab. The potassium value was 3.4 mEq/L in the sample collected in the ABG syringe, and a concurrent value collected by the standard method was 7.4 mEq/L. A repeated ECG was negative for any cardiac arrhythmias or conduction abnormalities. The subsequent 2 sets of potassium values were 3.9 mEq/L for the ABG syringe and 6.4 mEq/L for the standard heparinized tube, and 3.5 mEq/L and 5.8 mEq/L, respectively. The patient received the remainder of his chemotherapy, and there was no evidence of tumor lysis syndrome (TLS).
The following day, tumor lysis labs were collected in a low-heparin ABG syringe and a regular green-mint collection tube. Both samples were manually brought to the lab without pneumatic tube transport. Interestingly, the patient’s repeat potassium levels were 3.3 mEq/L and 3.1 mEq/L, respectively. Therefore, it was determined that the potassium level was not dependent on the presence of an anticoagulant. The following day the patient remained asymptomatic with normal potassium levels, and he was discharged on a normal cardiac diet. When he was evaluated in an outpatient setting a month later, the patient was found to have a normal potassium level at 4.3 mEq/L on a normal potassium diet.
Conclusion
In the hospital setting, pseudohyperkalemia is a potentially dangerous situation. Because the patient discussed here initially presented with potassium values as high as 8.2 mEq/L, treatment was warranted. However, given the presence of CLL with extreme leukocytosis and otherwise
normal clinical findings, suspicion for pseudohyperkalemia was high. Initial treatment of the elevated potassium levels, which were revealed to be borderline low later in his clinical course, may have had detrimental effects on his cardiac function if hypokalemia had been inadvertently exacerbated to a significant level. The authors bring this case to the attention of health care providers of patients with CLL because this patient had been chronically managed for hyperkalemia with a lowpotassium diet.
Further, this case confirms the importance of avoiding the use of pneumatic tubes to prevent WBC lysis in patients with significant malignant leukocytosis. Importantly, the authors were able to differentiate between postulated heparin-mediated lysis and pneumatictube usage. As the literature has suggested, the authors speculated that mechanical stress on chronic lymphocytic leukemia cells is the primary cause of pseudo-hyperkalemia.
Pneumatic tube use or mechanical manipulation seemed to cause unwanted WBC lysis in this case, as values in the standard 81 IU heparin tubes used in this case study could be corrected by manually transporting the tube to the lab. This suggests that the process is heparin-independent, although initial investigations on that effect focused on the use of low-heparin vials. The potassium correction also was supported by the correction of likely falsely elevated LDH, which normalized when samples were manually transported. This supports the mechanism of WBC lysis. The authors’ observations are in line with several recent reports where pneumatic tube use was suspected as the cause of reverse pseudohyperkalemia.4,5,7,8
During the authors’ monitoring of the patient for TLS, comparison of repeat values for potassium showed a significant difference of about 2.7 mEq/L between samples transported manually and samples sent via pneumatic tube (Figure). Similar elevations of values have been described in other case reports.1
Reverse pseudohyperkalemia is a phenomenon that should not be overlooked in the medical management of patients with CLL with leukocytosis, especially in asymptomatic chronic patients. Although initially the differences can be benign, as the tumor burden increases, the degree of falsely elevated potassium can increase to thresholds that lead to inappropriate management in an acute setting. To prevent mismanagement, the authors recommend placing precautionary flags with hospital laboratories so that if a patient with CLL has a high potassium draw, lab values are rechecked with hand-delivered samples. The authors hope that this case will highlight the importance of suspecting this diagnosis in patients with CLL and provide guidance on obtaining accurate labs to better manage these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
1. Avelar T. Reverse pseudohyperkalemia in a patient with chronic lymphocytic leukemia. Perm J. 2014;18(4):e150-e152.
2. Abraham B, Fakhar I, Tikaria A, et al. Reverse pseudohyperkalemia in a leukemic patient. Clin Chem. 2008;54(2):449-451.
3. Singh PJ, Zawada ET, Santella RN. A case of “reverse” pseudohyperkalemia. Miner Electrolyte Metab. 1997;23(1):58-61.
4. Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45(18):1700-1702.
5. Garwicz D, Karlman M, Øra I. Reverse pseudohyperkalemia in heparin plasma samples from a child with T cell acute lymphoblastic leukemia with hyperleukocytosis. Clin Chim Acta. 2011;412(3-4):396-397.
6. Kintzel PE, Scott WL. Pseudohyperkalemia in a patient with chronic lymphoblastic leukemia and tumor lysis syndrome. J Oncol Pharm Pract. 2012;18(4):432-435.
7. Sindhu SK, Hix JK, Fricke W. Pseudohyperkalemia in chronic lymphocytic leukemia: phlebotomy sites and pneumatic tubes. Am J Kidney Dis. 2011;57(2):354-355.
8. Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46(4):746-748
Note: Page numbers differ between the print issue and digital edition.
Daratumumab Effective in Combo Regimen
Adding daratumumab to bortezomib (a protease inhibitor [PI]) and dexamethasone reduces the risk of disease progression or death by 61% in patients with multiple myeloma (MM), compared with bortezomib and dexamethasone alone, according to an interim analysis of phase 3 trial results.
Related: Multiple Myeloma: Updates on Diagnosis and Management
Daratumumab is a biologic that targets CD38, a surface protein that is highly expressed across MM cells, regardless of disease stage. “CD38 is the most important tumor antigen on myeloma plasma cells,” said Antonio Palumbo, MD, University of Torino, Italy, who presented the study findings at the 2016 American Society of Clinical Oncology Annual Meeting in June. Daratumumab has more than 1 immune-mediated mechanism of action and both direct and indirect antimyeloma activity: It depletes CD38+ immunosuppressive regulatory cells and promotes T-cell expansion and activation, all while directly killing myeloma cells.
The multinational study (CASTOR) included 498 patients with MM who had received a median of 2 prior lines of therapy: bortezomib, an immunomodulatory agent, or a PI and immunomodulatory agent. Prior treatments had been unsuccessful in one-third of patients. In this study, researchers randomly assigned 251 patients to combination treatment with daratumumab.
Related: Treating Patients With Multiple Myeloma in the VA
Daratumumab significantly increased the overall response rate (83% vs 63%) and doubled rates of complete response or better (19% vs 9%). It also doubled the rates of very good partial response (59% vs 29%). The combination therapy met the primary endpoint of improved progression-free survival at a median follow-up of 7.4 months (60.7% in the combination arm, compared with 26.9%). The treatment was unblinded after meeting the primary endpoint.
The treatment benefits of the combination regimen were maintained across clinically relevant subgroups, the researchers said. The most common adverse effects were thrombocytopenia ,sensory peripheral neuropathy, anaemia, and diarrhea.
“These compelling phase 3 results demonstrate that a regimen built on daratumumab deepens clinical responses and help to underscore its potential for multiple myeloma patients who have been previously treated,” said Dr. Palumbo.
Related: A Mysterious Massive Hemorrhage
In a discussion of the study, Paul Richardson, MD, of the Dana-Farber Cancer Institute, praised the study as “outstanding” and “potentially practice changing.” However, another panel member noted that with an added cost of more than $10,000 a month, the treatment could be out of reach for many.
Adding daratumumab to bortezomib (a protease inhibitor [PI]) and dexamethasone reduces the risk of disease progression or death by 61% in patients with multiple myeloma (MM), compared with bortezomib and dexamethasone alone, according to an interim analysis of phase 3 trial results.
Related: Multiple Myeloma: Updates on Diagnosis and Management
Daratumumab is a biologic that targets CD38, a surface protein that is highly expressed across MM cells, regardless of disease stage. “CD38 is the most important tumor antigen on myeloma plasma cells,” said Antonio Palumbo, MD, University of Torino, Italy, who presented the study findings at the 2016 American Society of Clinical Oncology Annual Meeting in June. Daratumumab has more than 1 immune-mediated mechanism of action and both direct and indirect antimyeloma activity: It depletes CD38+ immunosuppressive regulatory cells and promotes T-cell expansion and activation, all while directly killing myeloma cells.
The multinational study (CASTOR) included 498 patients with MM who had received a median of 2 prior lines of therapy: bortezomib, an immunomodulatory agent, or a PI and immunomodulatory agent. Prior treatments had been unsuccessful in one-third of patients. In this study, researchers randomly assigned 251 patients to combination treatment with daratumumab.
Related: Treating Patients With Multiple Myeloma in the VA
Daratumumab significantly increased the overall response rate (83% vs 63%) and doubled rates of complete response or better (19% vs 9%). It also doubled the rates of very good partial response (59% vs 29%). The combination therapy met the primary endpoint of improved progression-free survival at a median follow-up of 7.4 months (60.7% in the combination arm, compared with 26.9%). The treatment was unblinded after meeting the primary endpoint.
The treatment benefits of the combination regimen were maintained across clinically relevant subgroups, the researchers said. The most common adverse effects were thrombocytopenia ,sensory peripheral neuropathy, anaemia, and diarrhea.
“These compelling phase 3 results demonstrate that a regimen built on daratumumab deepens clinical responses and help to underscore its potential for multiple myeloma patients who have been previously treated,” said Dr. Palumbo.
Related: A Mysterious Massive Hemorrhage
In a discussion of the study, Paul Richardson, MD, of the Dana-Farber Cancer Institute, praised the study as “outstanding” and “potentially practice changing.” However, another panel member noted that with an added cost of more than $10,000 a month, the treatment could be out of reach for many.
Adding daratumumab to bortezomib (a protease inhibitor [PI]) and dexamethasone reduces the risk of disease progression or death by 61% in patients with multiple myeloma (MM), compared with bortezomib and dexamethasone alone, according to an interim analysis of phase 3 trial results.
Related: Multiple Myeloma: Updates on Diagnosis and Management
Daratumumab is a biologic that targets CD38, a surface protein that is highly expressed across MM cells, regardless of disease stage. “CD38 is the most important tumor antigen on myeloma plasma cells,” said Antonio Palumbo, MD, University of Torino, Italy, who presented the study findings at the 2016 American Society of Clinical Oncology Annual Meeting in June. Daratumumab has more than 1 immune-mediated mechanism of action and both direct and indirect antimyeloma activity: It depletes CD38+ immunosuppressive regulatory cells and promotes T-cell expansion and activation, all while directly killing myeloma cells.
The multinational study (CASTOR) included 498 patients with MM who had received a median of 2 prior lines of therapy: bortezomib, an immunomodulatory agent, or a PI and immunomodulatory agent. Prior treatments had been unsuccessful in one-third of patients. In this study, researchers randomly assigned 251 patients to combination treatment with daratumumab.
Related: Treating Patients With Multiple Myeloma in the VA
Daratumumab significantly increased the overall response rate (83% vs 63%) and doubled rates of complete response or better (19% vs 9%). It also doubled the rates of very good partial response (59% vs 29%). The combination therapy met the primary endpoint of improved progression-free survival at a median follow-up of 7.4 months (60.7% in the combination arm, compared with 26.9%). The treatment was unblinded after meeting the primary endpoint.
The treatment benefits of the combination regimen were maintained across clinically relevant subgroups, the researchers said. The most common adverse effects were thrombocytopenia ,sensory peripheral neuropathy, anaemia, and diarrhea.
“These compelling phase 3 results demonstrate that a regimen built on daratumumab deepens clinical responses and help to underscore its potential for multiple myeloma patients who have been previously treated,” said Dr. Palumbo.
Related: A Mysterious Massive Hemorrhage
In a discussion of the study, Paul Richardson, MD, of the Dana-Farber Cancer Institute, praised the study as “outstanding” and “potentially practice changing.” However, another panel member noted that with an added cost of more than $10,000 a month, the treatment could be out of reach for many.
Sustained Remissions After Discontinuation of Ibrutinib in Relapsed/Refractory CLL: A Basis for Reducing Drug Toxicity and Treatment Costs?
In contrast to traditional chemotherapy, patients responding to biological or targeted therapies often are treated indefinitely until progression or toxicity. This therapeutic model, however, increases treatment costs, may induce greater toxicity and theoretically could select for earlier emergence of drug resistance. Moreover, little data are available regarding the outcomes of patients who discontinue targeted therapies after achieving remission. In this regard, we report 2 patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who chose to stop therapy unrelated to toxicity or disease status after the induction of remission by the BTK inhibitor ibrutinib.
Patient A started ibrutinib for progressive CLL at an absolute lymphocyte count (ALC) of 137,000 mm3 and recurrent hemolytic anemia. After 5 months, the hemolysis had resolved (Hgb 15.6 g/dL), while the ALC had declined to 9,200 mm3. Treatment was then interrupted due to patient preference. One month after drug discontinuation, the ALC was in the normal range at 1,400 mm3 and remained within or near the normal range for a total of 12 months. Two months later, the ALC was again markedly elevated at 68,000 mm3 and anemia recurred. The patient then agreed to restart ibrutinib. After 4 months of re-treatment, he has had prompt resolution of the anemia and achieved a partial remission thus far.
Patient B was started on ibrutinib for a rising ALC (26,000 mm3) and severe hemolytic anemia. After 9 months of treatment, the hemoglobin was 13 g/dL and the ALC was in the normal range at 3,300 mm3. Due to unrelated medical problems, ibrutinib therapy was stopped. Currently, 6 months since drug discontinuation, the ALC remains in the normal range, and no other signs of CLL are present.
These clinical observations suggest that interruption of ibrutinib may be feasible in at least some CLL patients who achieve remission. Even if flow cytometry were performed at monthly intervals to detect early recurrence and ensure prompt re-institution of therapy, the cost savings would still be considerable. Of course, clinical trials will be necessary to confirm equivalent long-term efficacy and overall survival for intermittent versus continuous ibrutinib therapy in CLL.
In contrast to traditional chemotherapy, patients responding to biological or targeted therapies often are treated indefinitely until progression or toxicity. This therapeutic model, however, increases treatment costs, may induce greater toxicity and theoretically could select for earlier emergence of drug resistance. Moreover, little data are available regarding the outcomes of patients who discontinue targeted therapies after achieving remission. In this regard, we report 2 patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who chose to stop therapy unrelated to toxicity or disease status after the induction of remission by the BTK inhibitor ibrutinib.
Patient A started ibrutinib for progressive CLL at an absolute lymphocyte count (ALC) of 137,000 mm3 and recurrent hemolytic anemia. After 5 months, the hemolysis had resolved (Hgb 15.6 g/dL), while the ALC had declined to 9,200 mm3. Treatment was then interrupted due to patient preference. One month after drug discontinuation, the ALC was in the normal range at 1,400 mm3 and remained within or near the normal range for a total of 12 months. Two months later, the ALC was again markedly elevated at 68,000 mm3 and anemia recurred. The patient then agreed to restart ibrutinib. After 4 months of re-treatment, he has had prompt resolution of the anemia and achieved a partial remission thus far.
Patient B was started on ibrutinib for a rising ALC (26,000 mm3) and severe hemolytic anemia. After 9 months of treatment, the hemoglobin was 13 g/dL and the ALC was in the normal range at 3,300 mm3. Due to unrelated medical problems, ibrutinib therapy was stopped. Currently, 6 months since drug discontinuation, the ALC remains in the normal range, and no other signs of CLL are present.
These clinical observations suggest that interruption of ibrutinib may be feasible in at least some CLL patients who achieve remission. Even if flow cytometry were performed at monthly intervals to detect early recurrence and ensure prompt re-institution of therapy, the cost savings would still be considerable. Of course, clinical trials will be necessary to confirm equivalent long-term efficacy and overall survival for intermittent versus continuous ibrutinib therapy in CLL.
In contrast to traditional chemotherapy, patients responding to biological or targeted therapies often are treated indefinitely until progression or toxicity. This therapeutic model, however, increases treatment costs, may induce greater toxicity and theoretically could select for earlier emergence of drug resistance. Moreover, little data are available regarding the outcomes of patients who discontinue targeted therapies after achieving remission. In this regard, we report 2 patients with relapsed/refractory chronic lymphocytic leukemia (CLL) who chose to stop therapy unrelated to toxicity or disease status after the induction of remission by the BTK inhibitor ibrutinib.
Patient A started ibrutinib for progressive CLL at an absolute lymphocyte count (ALC) of 137,000 mm3 and recurrent hemolytic anemia. After 5 months, the hemolysis had resolved (Hgb 15.6 g/dL), while the ALC had declined to 9,200 mm3. Treatment was then interrupted due to patient preference. One month after drug discontinuation, the ALC was in the normal range at 1,400 mm3 and remained within or near the normal range for a total of 12 months. Two months later, the ALC was again markedly elevated at 68,000 mm3 and anemia recurred. The patient then agreed to restart ibrutinib. After 4 months of re-treatment, he has had prompt resolution of the anemia and achieved a partial remission thus far.
Patient B was started on ibrutinib for a rising ALC (26,000 mm3) and severe hemolytic anemia. After 9 months of treatment, the hemoglobin was 13 g/dL and the ALC was in the normal range at 3,300 mm3. Due to unrelated medical problems, ibrutinib therapy was stopped. Currently, 6 months since drug discontinuation, the ALC remains in the normal range, and no other signs of CLL are present.
These clinical observations suggest that interruption of ibrutinib may be feasible in at least some CLL patients who achieve remission. Even if flow cytometry were performed at monthly intervals to detect early recurrence and ensure prompt re-institution of therapy, the cost savings would still be considerable. Of course, clinical trials will be necessary to confirm equivalent long-term efficacy and overall survival for intermittent versus continuous ibrutinib therapy in CLL.
Severe psoriasis upped lymphoma risk in large cohort study
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
SCOTTSDALE, ARIZ. – Psoriasis of all severities was linked to a 3.5-fold increase in risk of cutaneous T-cell lymphoma, and severe psoriasis upped the associated risk of Hodgkin lymphoma by about 2.5 times, in a large, longitudinal, population-based cohort study.
Psoriasis also was tied to a smaller but statistically significant increase in the risk of non-Hodgkin lymphoma, said Zelma Chiesa Fuxench, MD, of the department of dermatology, the University of Pennsylvania, Philadelphia. Overall, lymphoma risk was highest in people with severe psoriasis, independent of traditional risk factors and exposure to immunosuppressive medications, Dr. Fuxench said at the annual meeting of the Society for Investigative Dermatology.
Psoriasis affects more than 125 million people worldwide, and severe cases are a major cause of cancer-related mortality. “Prior studies have suggested an increased risk of lymphoma in psoriasis patients, but it is unclear if this due to chronic inflammation, exposure to immunosuppressive therapies, or a combination of both factors,” Dr. Fuxench said.
To further explore these links, she and her associates analyzed electronic medical records from THIN (The Health Information Network), which includes about 12 million patients across the United Kingdom. Adults with psoriasis were matched to up to five nonpsoriatic controls based on date and clinic location. Patients who needed systemic medications or phototherapy were categorized as having severe psoriasis. The final dataset included more than 12,000 such patients, as well as 184,000 patients with mild psoriasis and more than 965,000 patients without psoriasis.
Psoriasis patients were younger and more likely to be overweight, male, and smoke and drink alcohol than patients without psoriasis, Dr. Fuxench said. Almost 80% of patients with severe disease had received systemic therapies, most often methotrexate (70% of systemic treatments) or cyclosporine (10%), while only 1% had received biologics.
Patients with severe psoriasis were more likely to be diagnosed with Hodgkin disease, non-Hodgkin lymphoma, and cutaneous T-cell lymphoma than were patients with mild psoriasis or controls. Over a median follow-up of 5.3 years, 34 patients with severe psoriasis were diagnosed with any type of lymphoma, for an incidence of 5.2 cases per 10,000 person-years (95% confidence interval, 3.7-7.3). In contrast, incidence rates for patients with mild psoriasis and controls were 3.3 and 3.2 cases per 10,000 person-years, respectively, Dr. Fuxench said.
In the multivariable analysis, patients with psoriasis were about 18% more likely to develop any type of lymphoma than were controls, an association that reached statistical significance (adjusted hazard ratio, 1.18; 95% CI, 1.06-1.31). Mild psoriasis increased lymphoma risk by 14%, and severe psoriasis upped it by about 83%, and both associations were statistically significant.
The increase in risk of non-Hodgkin lymphoma was 13% greater with mild psoriasis and 56% greater with severe disease, compared with controls, and these associations also reached statistical significance. Mild psoriasis was not linked to Hodgkin lymphoma, but patients with severe psoriasis were about 250% more likely to develop it than controls, with a trend toward statistical significance (aHR, 2.54; 95% CI, 0.94-6.87).
Finally, severe psoriasis was linked to a more than ninefold increase in risk of cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4), while mild psoriasis was linked to about a threefold increase in risk.
“These results were robust in multiple sensitivity analyses, including analyses that excluded patients with rheumatoid arthritis, psoriatic arthritis, or a history of exposure to methotrexate, cyclosporine, or biologics,” Dr. Fuxench said. Future studies should explore the effect of treatment timing and selection on cancer risk, she added. “For those of us who care for these patients, we are increasingly using systemic agents that selectively target the immune system, and these questions will arise in clinics.”
The study’s design made it possible to pinpoint dates of diagnosis more effectively than investigators could estimate disease duration or confirm whether patients initially diagnosed with psoriasis actually had cutaneous T-cell lymphoma, Dr. Fuxench noted. “Ideally, we could have another cohort study of incident psoriasis with prospective follow-up, but lymphoma is so rare that there is currently not enough power [in the THIN database] to determine associations.”
The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research funding from Pfizer outside the submitted work.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis was identified as an independent risk factor for lymphoma, with the risk of lymphoma increasing with disease severity.
Major finding: The strongest association was between severe psoriasis and cutaneous T-cell lymphoma (aHR, 9.3; 95% CI, 4.1-21.4).
Data source: A population-based longitudinal cohort study of 12,198 patients with severe psoriasis, 184,870 patients with mild psoriasis, and 965,730 nonpsoriatic controls.
Disclosures: The study was funded by grants from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Fuxench disclosed unrestricted research support from Pfizer outside the submitted work.
New Treatments Offer Hope in Diffuse Large B-Cell Lymphoma
A new class of cancer drugs, SMAC mimetics, induces apoptosis by mimicking a protein that regulates cancer cell survival. The drugs have shown promising results in studies on many fronts. In animal and cell-line studies, the drugs killed cancer cells and shrank tumors. Now National Cancer Institute (NCI) researchers are hoping to put them to the test in clinical trials with patients who have the ABC subtype of diffuse large B-cell lymphoma (DLBCL).
Of the 2 distinct molecular DLBCL subtypes, ABC DLBCL is the least curable, the researchers note. A hallmark of the ABC subtype is the continuous activation of the B-cell receptor (BCR) signaling pathway, which promotes cell growth and survival by overriding signals for apoptosis. When other research suggested that blocking a protein in the NF-ΚB protein complex in the BCR pathway was effective, the NCI researchers also began to look at other potentially targetable proteins.
In preliminary studies, the researchers showed that the proteins cIAP1 and cIAP2 control the activity of the BCR signaling pathway in ABC DLBCL. Birinapant, one of the SMAC mimetics in clinical development, reduced cIAP1 and cIAP2 levels in ABC cell lines. Further research showed that birinapant had the effect only in those cancer cells that depend on BCR signaling for survival.
According to NCI, SMAC mimetics are well tolerated and seem to be safe. In animal studies, birinapant “substantially shrank tumors while causing no evident toxicity.”
A new class of cancer drugs, SMAC mimetics, induces apoptosis by mimicking a protein that regulates cancer cell survival. The drugs have shown promising results in studies on many fronts. In animal and cell-line studies, the drugs killed cancer cells and shrank tumors. Now National Cancer Institute (NCI) researchers are hoping to put them to the test in clinical trials with patients who have the ABC subtype of diffuse large B-cell lymphoma (DLBCL).
Of the 2 distinct molecular DLBCL subtypes, ABC DLBCL is the least curable, the researchers note. A hallmark of the ABC subtype is the continuous activation of the B-cell receptor (BCR) signaling pathway, which promotes cell growth and survival by overriding signals for apoptosis. When other research suggested that blocking a protein in the NF-ΚB protein complex in the BCR pathway was effective, the NCI researchers also began to look at other potentially targetable proteins.
In preliminary studies, the researchers showed that the proteins cIAP1 and cIAP2 control the activity of the BCR signaling pathway in ABC DLBCL. Birinapant, one of the SMAC mimetics in clinical development, reduced cIAP1 and cIAP2 levels in ABC cell lines. Further research showed that birinapant had the effect only in those cancer cells that depend on BCR signaling for survival.
According to NCI, SMAC mimetics are well tolerated and seem to be safe. In animal studies, birinapant “substantially shrank tumors while causing no evident toxicity.”
A new class of cancer drugs, SMAC mimetics, induces apoptosis by mimicking a protein that regulates cancer cell survival. The drugs have shown promising results in studies on many fronts. In animal and cell-line studies, the drugs killed cancer cells and shrank tumors. Now National Cancer Institute (NCI) researchers are hoping to put them to the test in clinical trials with patients who have the ABC subtype of diffuse large B-cell lymphoma (DLBCL).
Of the 2 distinct molecular DLBCL subtypes, ABC DLBCL is the least curable, the researchers note. A hallmark of the ABC subtype is the continuous activation of the B-cell receptor (BCR) signaling pathway, which promotes cell growth and survival by overriding signals for apoptosis. When other research suggested that blocking a protein in the NF-ΚB protein complex in the BCR pathway was effective, the NCI researchers also began to look at other potentially targetable proteins.
In preliminary studies, the researchers showed that the proteins cIAP1 and cIAP2 control the activity of the BCR signaling pathway in ABC DLBCL. Birinapant, one of the SMAC mimetics in clinical development, reduced cIAP1 and cIAP2 levels in ABC cell lines. Further research showed that birinapant had the effect only in those cancer cells that depend on BCR signaling for survival.
According to NCI, SMAC mimetics are well tolerated and seem to be safe. In animal studies, birinapant “substantially shrank tumors while causing no evident toxicity.”
Dronabinol: A Controversial Acute Leukemia Treatment
Researchers from University Hospital Tübingen, Germany, say that although the data for treating acute leukemia with dronabinol (THC), a cannabinoid derivative, are “controversial,” it has been shown to have antitumor potential for several cancers. When the researchers tested THC in several leukemia cell lines and native leukemia blasts cultured ex vivo, they found “meaningful” antiproliferative and proapoptotic effects.
Related: Lawmakers Urge VA to Reform Medical Marijuana Rules
From the data, they also found cannabinoid receptor agonists may be useful as low-toxic agents, especially for patients who are “heavily pretreated,” elderly, or have refractory disease. Evidence was cited that THC retained antileukemic activity in a sample from a patient with otherwise chemotherapy- and steroid-refractory acute lymphocytic leukemia (ALL).
Related: Veterans’ Use of Designer Cathinones and Cannabinoids
Due to the excellent safety profile of THC, the researchers say, effective doses are achievable in vivo, although tolerable doses may vary widely. They suggest starting with a subeffective dose and increasing gradually, which will help the patient build tolerance to the well-known psychoactive effects, which have been a drawback to widespread use of THC for patients with cancer.
They add that, due to sparse densities of cannabinoid receptors in lower brain stem areas, severe intoxications with THC rarely have been reported.
Related: Surgeon General Murthy Discusses Marijuana Efficacy
In addition to the direct antileukemic effects, the researchers suggest that therapeutic use of THC has many desirable adverse effects, such as general physical well-being, cachexia control, and relief of pain, anxiety and stress—which, they note, should “facilitate the decision process.”
Source:
Kampa-Schittenhelm KM, Salitzky O, Akmut F, et al. BMC Cancer. 2016;16(25)1-12.
doi: 10.1186/s12885-015-2029.
Researchers from University Hospital Tübingen, Germany, say that although the data for treating acute leukemia with dronabinol (THC), a cannabinoid derivative, are “controversial,” it has been shown to have antitumor potential for several cancers. When the researchers tested THC in several leukemia cell lines and native leukemia blasts cultured ex vivo, they found “meaningful” antiproliferative and proapoptotic effects.
Related: Lawmakers Urge VA to Reform Medical Marijuana Rules
From the data, they also found cannabinoid receptor agonists may be useful as low-toxic agents, especially for patients who are “heavily pretreated,” elderly, or have refractory disease. Evidence was cited that THC retained antileukemic activity in a sample from a patient with otherwise chemotherapy- and steroid-refractory acute lymphocytic leukemia (ALL).
Related: Veterans’ Use of Designer Cathinones and Cannabinoids
Due to the excellent safety profile of THC, the researchers say, effective doses are achievable in vivo, although tolerable doses may vary widely. They suggest starting with a subeffective dose and increasing gradually, which will help the patient build tolerance to the well-known psychoactive effects, which have been a drawback to widespread use of THC for patients with cancer.
They add that, due to sparse densities of cannabinoid receptors in lower brain stem areas, severe intoxications with THC rarely have been reported.
Related: Surgeon General Murthy Discusses Marijuana Efficacy
In addition to the direct antileukemic effects, the researchers suggest that therapeutic use of THC has many desirable adverse effects, such as general physical well-being, cachexia control, and relief of pain, anxiety and stress—which, they note, should “facilitate the decision process.”
Source:
Kampa-Schittenhelm KM, Salitzky O, Akmut F, et al. BMC Cancer. 2016;16(25)1-12.
doi: 10.1186/s12885-015-2029.
Researchers from University Hospital Tübingen, Germany, say that although the data for treating acute leukemia with dronabinol (THC), a cannabinoid derivative, are “controversial,” it has been shown to have antitumor potential for several cancers. When the researchers tested THC in several leukemia cell lines and native leukemia blasts cultured ex vivo, they found “meaningful” antiproliferative and proapoptotic effects.
Related: Lawmakers Urge VA to Reform Medical Marijuana Rules
From the data, they also found cannabinoid receptor agonists may be useful as low-toxic agents, especially for patients who are “heavily pretreated,” elderly, or have refractory disease. Evidence was cited that THC retained antileukemic activity in a sample from a patient with otherwise chemotherapy- and steroid-refractory acute lymphocytic leukemia (ALL).
Related: Veterans’ Use of Designer Cathinones and Cannabinoids
Due to the excellent safety profile of THC, the researchers say, effective doses are achievable in vivo, although tolerable doses may vary widely. They suggest starting with a subeffective dose and increasing gradually, which will help the patient build tolerance to the well-known psychoactive effects, which have been a drawback to widespread use of THC for patients with cancer.
They add that, due to sparse densities of cannabinoid receptors in lower brain stem areas, severe intoxications with THC rarely have been reported.
Related: Surgeon General Murthy Discusses Marijuana Efficacy
In addition to the direct antileukemic effects, the researchers suggest that therapeutic use of THC has many desirable adverse effects, such as general physical well-being, cachexia control, and relief of pain, anxiety and stress—which, they note, should “facilitate the decision process.”
Source:
Kampa-Schittenhelm KM, Salitzky O, Akmut F, et al. BMC Cancer. 2016;16(25)1-12.
doi: 10.1186/s12885-015-2029.
A Rare Case of Seronegative Cat Scratch Disease Masquerading as Relapsed Hodgkin Lymphoma
Background: Cat scratch disease is a benign, self-limiting but rare condition that causes significant lymphadenopathy. There is less than 1 case per 100,000 cases per year, mostly in children. Even rarer are cases presenting in association with malignancies, usually non-Hodgkin lymphoma. Our review of the literature revealed only 1 case of cat scratch disease as-sociated with Hodgkin lymphoma. We describe here a case of a 43-year-old white man who presented with a clinical picture of recurrent Hodgkin lymphoma but with negative serology for Bartonella antibodies, responding to a short course of antibiotic treatment.
Methods: This 43-year-old patient initially presented with stage IA Hodgkin lymphoma in January 2011. He had a me-diastinal mass, which was resected and followed by 4 cycles of ABVD (doxorubicin, bleomycin, vincristine, dexameth-asone) chemotherapy. In July 2011, he went into complete remission after 4 cycles of chemotherapy and remained so until October 2014, when he noted increasing left-sided in-guinal adenopathy accompanied by a constant stabbing pain. He had no other symptoms. On positron emission tomogra-phy/computed tomography (PET/CT) scanning he had sev-eral hypermetabolic splenic lesions and enlarged tonsillar, retroperitoneal, pelvic, axillary, and inguinal lymph nodes. His complete blood count and chemistries were normal. His sedimentation rate was also normal. Bilateral bone marrow biopsies were negative for Hodgkin disease recurrence. Bi-opsy of one of his inguinal nodes was positive for necrotiz-ing granulomas and stained positive for CD2, CD3, and CD5 with areas of high Ki-67 proliferation. Hodgkin disease, how-ever, was not confirmed. He mentioned a family history of cat scratch disease a decade ago when his 2 young children were diagnosed with cat scratch disease and treated with oral antibiotics, resulting in complete resolution of symptoms. Se-rologic testing for Bartonella antibodies was ordered for him but came back negative. An infectious disease consult was re-quested, and he was treated empirically with a 5-day course of azithromycin.
Results: After 2 months, he was seen in the clinic and found to have no palpable inguinal adenopathy. A repeat PET/CT scan at 3 months showed resolution of all areas of lymphadenop-athy with the exception of the left inguinal node basin. At 5 months, all areas of hypermetabolic adenopathy had resolved. A repeat serology for Bartonella antibodies remained negative.
Conclusions: Cat scratch disease is extremely rare but can occur in a patient with known hematologic malignancies. In this case, it was mistaken as a recurrent case of relapsed Hodgkin disease but fortunately resolved quickly. The pre-sentation can be acute; patients can have negative serologies for Bartonellosis, yet achievement of a complete clinical and radiographic response with a short course of oral antibiotics was successful.
Background: Cat scratch disease is a benign, self-limiting but rare condition that causes significant lymphadenopathy. There is less than 1 case per 100,000 cases per year, mostly in children. Even rarer are cases presenting in association with malignancies, usually non-Hodgkin lymphoma. Our review of the literature revealed only 1 case of cat scratch disease as-sociated with Hodgkin lymphoma. We describe here a case of a 43-year-old white man who presented with a clinical picture of recurrent Hodgkin lymphoma but with negative serology for Bartonella antibodies, responding to a short course of antibiotic treatment.
Methods: This 43-year-old patient initially presented with stage IA Hodgkin lymphoma in January 2011. He had a me-diastinal mass, which was resected and followed by 4 cycles of ABVD (doxorubicin, bleomycin, vincristine, dexameth-asone) chemotherapy. In July 2011, he went into complete remission after 4 cycles of chemotherapy and remained so until October 2014, when he noted increasing left-sided in-guinal adenopathy accompanied by a constant stabbing pain. He had no other symptoms. On positron emission tomogra-phy/computed tomography (PET/CT) scanning he had sev-eral hypermetabolic splenic lesions and enlarged tonsillar, retroperitoneal, pelvic, axillary, and inguinal lymph nodes. His complete blood count and chemistries were normal. His sedimentation rate was also normal. Bilateral bone marrow biopsies were negative for Hodgkin disease recurrence. Bi-opsy of one of his inguinal nodes was positive for necrotiz-ing granulomas and stained positive for CD2, CD3, and CD5 with areas of high Ki-67 proliferation. Hodgkin disease, how-ever, was not confirmed. He mentioned a family history of cat scratch disease a decade ago when his 2 young children were diagnosed with cat scratch disease and treated with oral antibiotics, resulting in complete resolution of symptoms. Se-rologic testing for Bartonella antibodies was ordered for him but came back negative. An infectious disease consult was re-quested, and he was treated empirically with a 5-day course of azithromycin.
Results: After 2 months, he was seen in the clinic and found to have no palpable inguinal adenopathy. A repeat PET/CT scan at 3 months showed resolution of all areas of lymphadenop-athy with the exception of the left inguinal node basin. At 5 months, all areas of hypermetabolic adenopathy had resolved. A repeat serology for Bartonella antibodies remained negative.
Conclusions: Cat scratch disease is extremely rare but can occur in a patient with known hematologic malignancies. In this case, it was mistaken as a recurrent case of relapsed Hodgkin disease but fortunately resolved quickly. The pre-sentation can be acute; patients can have negative serologies for Bartonellosis, yet achievement of a complete clinical and radiographic response with a short course of oral antibiotics was successful.
Background: Cat scratch disease is a benign, self-limiting but rare condition that causes significant lymphadenopathy. There is less than 1 case per 100,000 cases per year, mostly in children. Even rarer are cases presenting in association with malignancies, usually non-Hodgkin lymphoma. Our review of the literature revealed only 1 case of cat scratch disease as-sociated with Hodgkin lymphoma. We describe here a case of a 43-year-old white man who presented with a clinical picture of recurrent Hodgkin lymphoma but with negative serology for Bartonella antibodies, responding to a short course of antibiotic treatment.
Methods: This 43-year-old patient initially presented with stage IA Hodgkin lymphoma in January 2011. He had a me-diastinal mass, which was resected and followed by 4 cycles of ABVD (doxorubicin, bleomycin, vincristine, dexameth-asone) chemotherapy. In July 2011, he went into complete remission after 4 cycles of chemotherapy and remained so until October 2014, when he noted increasing left-sided in-guinal adenopathy accompanied by a constant stabbing pain. He had no other symptoms. On positron emission tomogra-phy/computed tomography (PET/CT) scanning he had sev-eral hypermetabolic splenic lesions and enlarged tonsillar, retroperitoneal, pelvic, axillary, and inguinal lymph nodes. His complete blood count and chemistries were normal. His sedimentation rate was also normal. Bilateral bone marrow biopsies were negative for Hodgkin disease recurrence. Bi-opsy of one of his inguinal nodes was positive for necrotiz-ing granulomas and stained positive for CD2, CD3, and CD5 with areas of high Ki-67 proliferation. Hodgkin disease, how-ever, was not confirmed. He mentioned a family history of cat scratch disease a decade ago when his 2 young children were diagnosed with cat scratch disease and treated with oral antibiotics, resulting in complete resolution of symptoms. Se-rologic testing for Bartonella antibodies was ordered for him but came back negative. An infectious disease consult was re-quested, and he was treated empirically with a 5-day course of azithromycin.
Results: After 2 months, he was seen in the clinic and found to have no palpable inguinal adenopathy. A repeat PET/CT scan at 3 months showed resolution of all areas of lymphadenop-athy with the exception of the left inguinal node basin. At 5 months, all areas of hypermetabolic adenopathy had resolved. A repeat serology for Bartonella antibodies remained negative.
Conclusions: Cat scratch disease is extremely rare but can occur in a patient with known hematologic malignancies. In this case, it was mistaken as a recurrent case of relapsed Hodgkin disease but fortunately resolved quickly. The pre-sentation can be acute; patients can have negative serologies for Bartonellosis, yet achievement of a complete clinical and radiographic response with a short course of oral antibiotics was successful.
Impact of Treatment Sequencing on Outcomes and Costs in Relapsed Low-Grade or Follicular B-Cell Non-Hodgkin Lymphoma
Purpose: Today, patients with relapsed low-grade or follicular B-cell non-Hodgkin lymphoma have more treatment options available to them than ever. These therapies include infused chemotherapy regimens, radioimmunotherapy, and oral agents. There is limited information available about the impact of available treatment sequences on clinical outcomes and costs.
Methods: A budget impact model was developed to represent the current treatment pathways for relapsed low-grade or follicular B-cell non-Hodgkin lymphoma. First-line treatment regimens were selected based on the Category 1 recommendations from the National Comprehensive Cancer Network guidelines for follicular lymphoma. Those included were the most commonly used: BR, RCHOP, and RCVP. For purposes of this model, the second-line regimens included Y90 ibritumomab (Y90), rituximab monotherapy with 4 weekly doses, and idelalisib. Treatment outcomes were based on the published literature that summarized the overall response rate, median duration of response (DOR), and toxicity. Total costs per treatment regimen were based on medical care, drugs administered, and associated adverse effects. The 2015 Medicare fee schedule rates were used to estimate the medical care costs. Total DOR and costs were determined for each treatment regimen.
Results: The treatment sequence, BR followed by Y90 at the time of relapse, had the longest predicted DOR (7.2 years). The associated treatment sequence costs were $157,712 for BR followed by Y90, compared with $224,628 and $145,047 for BR followed by idelalisib and BR followed by rituximab monotherapy, respectively, with a predicted DOR of 6.7 years. The predicted DOR for treatment sequences starting with RCVP and RCHOP and followed by Y90 was about 3 years less than BR followed by Y90 at a cost increase of $11,102 and $37,866, respectively. The use of idelalisib after RCVP or RCHOP had a predicted DOR of 3.6 years, respectively, with associated costs that were greater than BR followed by Y90.
Conclusions: The use of Y90 ibritumomab after BR demonstrated the optimal patient outcomes at one of the lowest cost profiles.
Purpose: Today, patients with relapsed low-grade or follicular B-cell non-Hodgkin lymphoma have more treatment options available to them than ever. These therapies include infused chemotherapy regimens, radioimmunotherapy, and oral agents. There is limited information available about the impact of available treatment sequences on clinical outcomes and costs.
Methods: A budget impact model was developed to represent the current treatment pathways for relapsed low-grade or follicular B-cell non-Hodgkin lymphoma. First-line treatment regimens were selected based on the Category 1 recommendations from the National Comprehensive Cancer Network guidelines for follicular lymphoma. Those included were the most commonly used: BR, RCHOP, and RCVP. For purposes of this model, the second-line regimens included Y90 ibritumomab (Y90), rituximab monotherapy with 4 weekly doses, and idelalisib. Treatment outcomes were based on the published literature that summarized the overall response rate, median duration of response (DOR), and toxicity. Total costs per treatment regimen were based on medical care, drugs administered, and associated adverse effects. The 2015 Medicare fee schedule rates were used to estimate the medical care costs. Total DOR and costs were determined for each treatment regimen.
Results: The treatment sequence, BR followed by Y90 at the time of relapse, had the longest predicted DOR (7.2 years). The associated treatment sequence costs were $157,712 for BR followed by Y90, compared with $224,628 and $145,047 for BR followed by idelalisib and BR followed by rituximab monotherapy, respectively, with a predicted DOR of 6.7 years. The predicted DOR for treatment sequences starting with RCVP and RCHOP and followed by Y90 was about 3 years less than BR followed by Y90 at a cost increase of $11,102 and $37,866, respectively. The use of idelalisib after RCVP or RCHOP had a predicted DOR of 3.6 years, respectively, with associated costs that were greater than BR followed by Y90.
Conclusions: The use of Y90 ibritumomab after BR demonstrated the optimal patient outcomes at one of the lowest cost profiles.
Purpose: Today, patients with relapsed low-grade or follicular B-cell non-Hodgkin lymphoma have more treatment options available to them than ever. These therapies include infused chemotherapy regimens, radioimmunotherapy, and oral agents. There is limited information available about the impact of available treatment sequences on clinical outcomes and costs.
Methods: A budget impact model was developed to represent the current treatment pathways for relapsed low-grade or follicular B-cell non-Hodgkin lymphoma. First-line treatment regimens were selected based on the Category 1 recommendations from the National Comprehensive Cancer Network guidelines for follicular lymphoma. Those included were the most commonly used: BR, RCHOP, and RCVP. For purposes of this model, the second-line regimens included Y90 ibritumomab (Y90), rituximab monotherapy with 4 weekly doses, and idelalisib. Treatment outcomes were based on the published literature that summarized the overall response rate, median duration of response (DOR), and toxicity. Total costs per treatment regimen were based on medical care, drugs administered, and associated adverse effects. The 2015 Medicare fee schedule rates were used to estimate the medical care costs. Total DOR and costs were determined for each treatment regimen.
Results: The treatment sequence, BR followed by Y90 at the time of relapse, had the longest predicted DOR (7.2 years). The associated treatment sequence costs were $157,712 for BR followed by Y90, compared with $224,628 and $145,047 for BR followed by idelalisib and BR followed by rituximab monotherapy, respectively, with a predicted DOR of 6.7 years. The predicted DOR for treatment sequences starting with RCVP and RCHOP and followed by Y90 was about 3 years less than BR followed by Y90 at a cost increase of $11,102 and $37,866, respectively. The use of idelalisib after RCVP or RCHOP had a predicted DOR of 3.6 years, respectively, with associated costs that were greater than BR followed by Y90.
Conclusions: The use of Y90 ibritumomab after BR demonstrated the optimal patient outcomes at one of the lowest cost profiles.
Cytarabine Combination: Long-term Effects
Low-dose cytarabine in combination with valproic acid and all-transretinoic acid (ATRA) is used in stabilizing treatment for patients with acute myeloid leukemia (AML) who aren’t candidates for intensive therapy. In vivo studies have shown that the triple-drug treatment has immunomodulatory effects. But researchers from University of Bergen, Norway, say little was known about both the acute and long-term effects of such treatment on the T-cell system.
Related: Signaling Pathways and Novel Inhibitors in Chronic Lymphocytic Leukemia
To find out, they conducted an in vitro study to examine the effects of cytarabine, valproic acid, and ATRA on activated T cells, testing cytarabine at concentrations reached during in vivo treatment with high doses, conventional doses, and low doses.
The researchers found that cytarabine—especially when combined with valproic acid and ATRA—can reduce T-cell viability and proliferation, alter the activation-induced expression of membrane molecules, and reduce the release of several cytokines. Cytarabine’s effects on T-cell activation were concentration dependent: Reduced viability was seen only at the higher concentrations. However, the researchers note that cytarabine had immunoregulatory effects even at lower levels. When cytarabine was combined with valproic acid and ATRA, the researchers observed no or minor effects on T-cell viability.
Related: Blast Phase Chronic Myelogenous Leukemia
Only cytarabine 44 μM had both antiproliferative and proapoptotic effects. The drug reduced AML cell viability only at 0.5 and 0.05 μM, not at the lowest concentration; but it inhibited AML cell proliferation even at 0.01 μM. By contrast, T-cell proliferation was inhibited only at concentrations ≥ 0.35 μM.
Based on the proliferation studies, the researchers conclude that primary AML cells are more susceptible to cytarabine than are normal T cells, which suggests, they add, a therapeutic window for cytarabine treatment that “makes it possible to achieve antileukemic effects in vivo before severe T-cell toxicity occurs.”
Related:HIV-Negative Patients at Risk for Pneumocystosis
The triple-drug combination’s direct effects on the T cells may be offset by other effects on immunocompetent cells; the researchers say the possible risk of immunosuppression should be further investigated.
Source
Ersvaer E, Brenner AK, Vetås K, Reikvam H, Bruserud Ø. BMC Pharmacol Toxicol. 2015;16:12.
doi: 10.1186/s40360-015-0012-2.
Low-dose cytarabine in combination with valproic acid and all-transretinoic acid (ATRA) is used in stabilizing treatment for patients with acute myeloid leukemia (AML) who aren’t candidates for intensive therapy. In vivo studies have shown that the triple-drug treatment has immunomodulatory effects. But researchers from University of Bergen, Norway, say little was known about both the acute and long-term effects of such treatment on the T-cell system.
Related: Signaling Pathways and Novel Inhibitors in Chronic Lymphocytic Leukemia
To find out, they conducted an in vitro study to examine the effects of cytarabine, valproic acid, and ATRA on activated T cells, testing cytarabine at concentrations reached during in vivo treatment with high doses, conventional doses, and low doses.
The researchers found that cytarabine—especially when combined with valproic acid and ATRA—can reduce T-cell viability and proliferation, alter the activation-induced expression of membrane molecules, and reduce the release of several cytokines. Cytarabine’s effects on T-cell activation were concentration dependent: Reduced viability was seen only at the higher concentrations. However, the researchers note that cytarabine had immunoregulatory effects even at lower levels. When cytarabine was combined with valproic acid and ATRA, the researchers observed no or minor effects on T-cell viability.
Related: Blast Phase Chronic Myelogenous Leukemia
Only cytarabine 44 μM had both antiproliferative and proapoptotic effects. The drug reduced AML cell viability only at 0.5 and 0.05 μM, not at the lowest concentration; but it inhibited AML cell proliferation even at 0.01 μM. By contrast, T-cell proliferation was inhibited only at concentrations ≥ 0.35 μM.
Based on the proliferation studies, the researchers conclude that primary AML cells are more susceptible to cytarabine than are normal T cells, which suggests, they add, a therapeutic window for cytarabine treatment that “makes it possible to achieve antileukemic effects in vivo before severe T-cell toxicity occurs.”
Related:HIV-Negative Patients at Risk for Pneumocystosis
The triple-drug combination’s direct effects on the T cells may be offset by other effects on immunocompetent cells; the researchers say the possible risk of immunosuppression should be further investigated.
Source
Ersvaer E, Brenner AK, Vetås K, Reikvam H, Bruserud Ø. BMC Pharmacol Toxicol. 2015;16:12.
doi: 10.1186/s40360-015-0012-2.
Low-dose cytarabine in combination with valproic acid and all-transretinoic acid (ATRA) is used in stabilizing treatment for patients with acute myeloid leukemia (AML) who aren’t candidates for intensive therapy. In vivo studies have shown that the triple-drug treatment has immunomodulatory effects. But researchers from University of Bergen, Norway, say little was known about both the acute and long-term effects of such treatment on the T-cell system.
Related: Signaling Pathways and Novel Inhibitors in Chronic Lymphocytic Leukemia
To find out, they conducted an in vitro study to examine the effects of cytarabine, valproic acid, and ATRA on activated T cells, testing cytarabine at concentrations reached during in vivo treatment with high doses, conventional doses, and low doses.
The researchers found that cytarabine—especially when combined with valproic acid and ATRA—can reduce T-cell viability and proliferation, alter the activation-induced expression of membrane molecules, and reduce the release of several cytokines. Cytarabine’s effects on T-cell activation were concentration dependent: Reduced viability was seen only at the higher concentrations. However, the researchers note that cytarabine had immunoregulatory effects even at lower levels. When cytarabine was combined with valproic acid and ATRA, the researchers observed no or minor effects on T-cell viability.
Related: Blast Phase Chronic Myelogenous Leukemia
Only cytarabine 44 μM had both antiproliferative and proapoptotic effects. The drug reduced AML cell viability only at 0.5 and 0.05 μM, not at the lowest concentration; but it inhibited AML cell proliferation even at 0.01 μM. By contrast, T-cell proliferation was inhibited only at concentrations ≥ 0.35 μM.
Based on the proliferation studies, the researchers conclude that primary AML cells are more susceptible to cytarabine than are normal T cells, which suggests, they add, a therapeutic window for cytarabine treatment that “makes it possible to achieve antileukemic effects in vivo before severe T-cell toxicity occurs.”
Related:HIV-Negative Patients at Risk for Pneumocystosis
The triple-drug combination’s direct effects on the T cells may be offset by other effects on immunocompetent cells; the researchers say the possible risk of immunosuppression should be further investigated.
Source
Ersvaer E, Brenner AK, Vetås K, Reikvam H, Bruserud Ø. BMC Pharmacol Toxicol. 2015;16:12.
doi: 10.1186/s40360-015-0012-2.