User login
Inflammation diminishes quality of life in NAFLD, not fibrosis
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Spleen/liver stiffness ratio differentiates HCV, ALD
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
FROM JHEP REPORTS
HCC surveillance after anti-HCV therapy cost effective only for patients with cirrhosis
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Louisiana HCV program cuts costs – and hassles
Beginning July 15, physicians will no longer have to seek prior authorization or preauthorization to prescribe the authorized generic version of Epclusa (sofosbuvir/velpatasvir) to any Medicaid patient with hepatitis C. There will be no forms to file.
The change comes as part of a supplemental rebate agreement approved June 26 by CMS. That same day, Louisiana announced a deal with Asegua Therapeutics, a wholly owned subsidiary of Epclusa maker Gilead, that essentially caps the annual cost to the state for treating hepatitis C in incarcerated patients and Medicaid recipients.
State officials estimate about 39,000 Louisianans fit those criteria; the goal of the program is to cure at least 31,000 of them by the time the 5-year agreement expires.
“This new model has the potential to save many lives and improve the health of our citizens,” Louisiana Gov. John Bel Edwards (D) said in a statement. “Asegua was willing to come to the table to work with us to help Louisiana residents and we are pleased to initiate this 5-year partnership. Ultimately our goal is to eliminate this disease in Louisiana, and we have taken a big step forward in that effort.”
The agreement was designed to change very little in terms of the mechanics of how Medicaid managed care organizations, which cover most of the state’s Medicaid population and handle coverage and claims. The biggest change is that, when a spending cap is reached, Asegua will rebate 100% excess costs to the state. Louisiana officials did not disclose what the annual financial caps were as part of the agreement.
“We really thought it was important to leave the system – as much as possible – intact because we think that is going to make us most successful,” Alex Billioux, MD, of the Louisiana Department of Health said in an interview. “We think it leverages existing patient relationships and existing [Medicaid managed care organization] care management responsibilities.”
He added that, by keeping current processes unchanged, “it takes what is an otherwise very complicated arrangement with the state and makes it a little simpler.”
Patients will see no change in terms of copayments for the approved generic topping out at $3 depending on income level as they would have prior to the agreement. The biggest difference for them is that “people who couldn’t be treated are now going to have access to those prescriptions,” Dr. Billioux said.
Some cautious optimism surrounds this kind of arrangement and the potential effect it can have on the affected population.
“Innovation geared to improve access to hepatitis C treatment is critical, particularly in areas like Louisiana where treatment rates for Medicaid patients have been very low,” Robert Brown, MD, member of the American Liver Foundation’s National Medical Advisory Committee and hepatologist at Weill Cornell Medical College, New York, said. “If we can enhance patient access to treatment, we know we will improve health outcomes. However, it is too early to tell if this innovation will be a success. At the end of the day, the number of additional patients cured will determine if this was the right approach.”
Beginning July 15, physicians will no longer have to seek prior authorization or preauthorization to prescribe the authorized generic version of Epclusa (sofosbuvir/velpatasvir) to any Medicaid patient with hepatitis C. There will be no forms to file.
The change comes as part of a supplemental rebate agreement approved June 26 by CMS. That same day, Louisiana announced a deal with Asegua Therapeutics, a wholly owned subsidiary of Epclusa maker Gilead, that essentially caps the annual cost to the state for treating hepatitis C in incarcerated patients and Medicaid recipients.
State officials estimate about 39,000 Louisianans fit those criteria; the goal of the program is to cure at least 31,000 of them by the time the 5-year agreement expires.
“This new model has the potential to save many lives and improve the health of our citizens,” Louisiana Gov. John Bel Edwards (D) said in a statement. “Asegua was willing to come to the table to work with us to help Louisiana residents and we are pleased to initiate this 5-year partnership. Ultimately our goal is to eliminate this disease in Louisiana, and we have taken a big step forward in that effort.”
The agreement was designed to change very little in terms of the mechanics of how Medicaid managed care organizations, which cover most of the state’s Medicaid population and handle coverage and claims. The biggest change is that, when a spending cap is reached, Asegua will rebate 100% excess costs to the state. Louisiana officials did not disclose what the annual financial caps were as part of the agreement.
“We really thought it was important to leave the system – as much as possible – intact because we think that is going to make us most successful,” Alex Billioux, MD, of the Louisiana Department of Health said in an interview. “We think it leverages existing patient relationships and existing [Medicaid managed care organization] care management responsibilities.”
He added that, by keeping current processes unchanged, “it takes what is an otherwise very complicated arrangement with the state and makes it a little simpler.”
Patients will see no change in terms of copayments for the approved generic topping out at $3 depending on income level as they would have prior to the agreement. The biggest difference for them is that “people who couldn’t be treated are now going to have access to those prescriptions,” Dr. Billioux said.
Some cautious optimism surrounds this kind of arrangement and the potential effect it can have on the affected population.
“Innovation geared to improve access to hepatitis C treatment is critical, particularly in areas like Louisiana where treatment rates for Medicaid patients have been very low,” Robert Brown, MD, member of the American Liver Foundation’s National Medical Advisory Committee and hepatologist at Weill Cornell Medical College, New York, said. “If we can enhance patient access to treatment, we know we will improve health outcomes. However, it is too early to tell if this innovation will be a success. At the end of the day, the number of additional patients cured will determine if this was the right approach.”
Beginning July 15, physicians will no longer have to seek prior authorization or preauthorization to prescribe the authorized generic version of Epclusa (sofosbuvir/velpatasvir) to any Medicaid patient with hepatitis C. There will be no forms to file.
The change comes as part of a supplemental rebate agreement approved June 26 by CMS. That same day, Louisiana announced a deal with Asegua Therapeutics, a wholly owned subsidiary of Epclusa maker Gilead, that essentially caps the annual cost to the state for treating hepatitis C in incarcerated patients and Medicaid recipients.
State officials estimate about 39,000 Louisianans fit those criteria; the goal of the program is to cure at least 31,000 of them by the time the 5-year agreement expires.
“This new model has the potential to save many lives and improve the health of our citizens,” Louisiana Gov. John Bel Edwards (D) said in a statement. “Asegua was willing to come to the table to work with us to help Louisiana residents and we are pleased to initiate this 5-year partnership. Ultimately our goal is to eliminate this disease in Louisiana, and we have taken a big step forward in that effort.”
The agreement was designed to change very little in terms of the mechanics of how Medicaid managed care organizations, which cover most of the state’s Medicaid population and handle coverage and claims. The biggest change is that, when a spending cap is reached, Asegua will rebate 100% excess costs to the state. Louisiana officials did not disclose what the annual financial caps were as part of the agreement.
“We really thought it was important to leave the system – as much as possible – intact because we think that is going to make us most successful,” Alex Billioux, MD, of the Louisiana Department of Health said in an interview. “We think it leverages existing patient relationships and existing [Medicaid managed care organization] care management responsibilities.”
He added that, by keeping current processes unchanged, “it takes what is an otherwise very complicated arrangement with the state and makes it a little simpler.”
Patients will see no change in terms of copayments for the approved generic topping out at $3 depending on income level as they would have prior to the agreement. The biggest difference for them is that “people who couldn’t be treated are now going to have access to those prescriptions,” Dr. Billioux said.
Some cautious optimism surrounds this kind of arrangement and the potential effect it can have on the affected population.
“Innovation geared to improve access to hepatitis C treatment is critical, particularly in areas like Louisiana where treatment rates for Medicaid patients have been very low,” Robert Brown, MD, member of the American Liver Foundation’s National Medical Advisory Committee and hepatologist at Weill Cornell Medical College, New York, said. “If we can enhance patient access to treatment, we know we will improve health outcomes. However, it is too early to tell if this innovation will be a success. At the end of the day, the number of additional patients cured will determine if this was the right approach.”
Formal weight loss programs improve NAFLD
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues. “Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine. “However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease. The literature search returned 22 eligible studies involving 2,588 patients.
The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
Past studies have attempted to investigate the relationship between weight loss and nonalcoholic fatty liver disease (NAFLD), but they did so with various interventions and outcomes measures. Fortunately, the study by Dr. Koutoukidis and colleagues helps clear up this variability with a well-conducted systematic review. The results offer a convincing case that formal weight loss programs should be a cornerstone of NALFD treatment, based on improvements in blood, histologic, and radiologic biomarkers of liver disease. Since pharmacologic options for NAFLD are limited, these findings are particularly important.
Although the study did not reveal improvements in fibrosis or inflammation with weight loss, this is likely due to the scarcity of trials with histologic measures or long-term follow-up. Where long-term follow-up was available, weight loss was not maintained, disallowing clear conclusions. Still, other studies have shown that sustained weight loss is associated with improvements in fibrosis and mortality, so clinicians should feel encouraged that formal weight loss programs for patients with NAFLD likely have life-saving consequences.
Elizabeth Adler, MD and Danielle Brandman, MD , are with the University of California, San Francisco. Dr. Brandman reported financial affiliations with Conatus, Gilead, and Allergan. Their remarks are adapted from an accompanying editorial (JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2244 ).
Past studies have attempted to investigate the relationship between weight loss and nonalcoholic fatty liver disease (NAFLD), but they did so with various interventions and outcomes measures. Fortunately, the study by Dr. Koutoukidis and colleagues helps clear up this variability with a well-conducted systematic review. The results offer a convincing case that formal weight loss programs should be a cornerstone of NALFD treatment, based on improvements in blood, histologic, and radiologic biomarkers of liver disease. Since pharmacologic options for NAFLD are limited, these findings are particularly important.
Although the study did not reveal improvements in fibrosis or inflammation with weight loss, this is likely due to the scarcity of trials with histologic measures or long-term follow-up. Where long-term follow-up was available, weight loss was not maintained, disallowing clear conclusions. Still, other studies have shown that sustained weight loss is associated with improvements in fibrosis and mortality, so clinicians should feel encouraged that formal weight loss programs for patients with NAFLD likely have life-saving consequences.
Elizabeth Adler, MD and Danielle Brandman, MD , are with the University of California, San Francisco. Dr. Brandman reported financial affiliations with Conatus, Gilead, and Allergan. Their remarks are adapted from an accompanying editorial (JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2244 ).
Past studies have attempted to investigate the relationship between weight loss and nonalcoholic fatty liver disease (NAFLD), but they did so with various interventions and outcomes measures. Fortunately, the study by Dr. Koutoukidis and colleagues helps clear up this variability with a well-conducted systematic review. The results offer a convincing case that formal weight loss programs should be a cornerstone of NALFD treatment, based on improvements in blood, histologic, and radiologic biomarkers of liver disease. Since pharmacologic options for NAFLD are limited, these findings are particularly important.
Although the study did not reveal improvements in fibrosis or inflammation with weight loss, this is likely due to the scarcity of trials with histologic measures or long-term follow-up. Where long-term follow-up was available, weight loss was not maintained, disallowing clear conclusions. Still, other studies have shown that sustained weight loss is associated with improvements in fibrosis and mortality, so clinicians should feel encouraged that formal weight loss programs for patients with NAFLD likely have life-saving consequences.
Elizabeth Adler, MD and Danielle Brandman, MD , are with the University of California, San Francisco. Dr. Brandman reported financial affiliations with Conatus, Gilead, and Allergan. Their remarks are adapted from an accompanying editorial (JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2244 ).
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues. “Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine. “However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease. The literature search returned 22 eligible studies involving 2,588 patients.
The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
For patients with nonalcoholic fatty liver disease (NAFLD), formal weight loss programs lead to statistically and clinically significant improvements in biomarkers of liver disease, based on a recent meta-analysis.
The findings support changing NAFLD guidelines to recommend weight loss interventions, according to lead author Dimitrios A. Koutoukidis, PhD, of the University of Oxford, UK, and colleagues. “Clinical guidelines around the world recommend physicians offer advice on lifestyle modification, which mostly includes weight loss through hypoenergetic diets and increased physical activity,” the investigators wrote in JAMA Internal Medicine. “However, whether clinicians provide advice and the type of advice they give vary greatly, and guidelines rarely specifically recommend treatment programs to support weight loss,” they added.
To investigate associations between methods of weight loss and improvements in NAFLD, the investigators screened for studies involving behavioral weight loss programs, pharmacotherapy, or bariatric surgery, alone or in combination. To limit confounding, studies combining weight loss with other potential treatments, such as medications, were excluded. Weight loss interventions were compared to liver disease outcomes associated with lower-intensity weight loss intervention or none or minimal weight loss support, using at least 1 reported biomarker of liver disease. The literature search returned 22 eligible studies involving 2,588 patients.
The investigators found that more intensive weight loss programs were associated with greater weight loss than lower intensity methods (-3.61 kg; I2 = 95%). Multiple biomarkers of liver disease showed significant improvements in association with formal weight loss programs, including histologically or radiologically measured liver steatosis (standardized mean difference: -1.48; I2 = 94%), histologic NAFLD activity score (-0.92; I2= 95%), presence of nonalcoholic steatohepatitis (OR, 0.14; I2 =0%), alanine aminotransferase (-9.81 U/L; I2= 97%), aspartate transaminase (-4.84 U/L; I2 = 96%), alkaline phosphatase (-5.53 U/L; I2 = 96%), and gamma-glutamyl transferase (-4.35 U/L; I2 = 92%). Weight loss interventions were not significantly associated with histologic liver fibrosis or inflammation, the investigators noted.
“The advantages [of weight loss interventions] seem to be greater in people who are overweight and with NAFLD, but our exploratory results suggest that weight loss interventions might still be beneficial in the minority of people with healthy weight and NAFLD,” the investigators wrote. “Clinicians may use these findings to counsel people with NAFLD on the expected clinically significant improvements in liver biomarkers after weight loss and direct the patients toward valuable interventions.”
“The accumulated evidence supports changing the clinical guidelines and routine practice to recommend formal weight loss programs to treat people with NAFLD,” the investigators concluded.
The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
SOURCE: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Weight loss interventions were associated with significantly decreased alanine aminotransferase (-9.81 U/L; I2 = 97%).
Study details: A meta-analysis of randomized clinicals involving weight loss interventions for patients with nonalcoholic fatty liver disease (NAFLD).
Disclosures: The study was funded by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the Oxford NIHR Collaboration and Leadership in Applied Health Research. The investigators reported grants for other research from Cambridge Weight Plan.
Source: Koutoukidis et al. JAMA Int Med. 2019 Jul 1. doi: 10.1001/jamainternmed.2019.2248.
Pediatric cholestatic liver disease: Successful transition of care
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
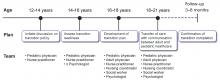
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
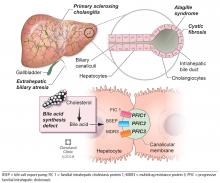
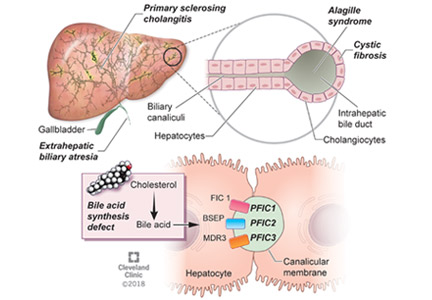
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
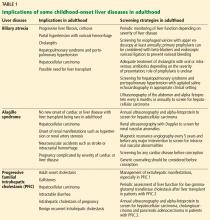
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
KEY POINTS
- The causes of cholestasis in children are different from those in adults, with genetic inherited causes more common in childhood.
- Cholestasis in children can be caused by biliary tract obstruction such as in biliary atresia or defects in forming and excreting bile acids and other components of bile.
- With the growing number of people with childhood-onset liver disease surviving into adulthood, it is important for internists to be aware of unique problems and challenges in continuing management of this population.
- In addition to medical comorbidities, these patients may also have impaired psychosocial functioning and quality of life.
AGA Clinical Practice Update: Coagulation in cirrhosis
Cirrhosis can involve “precarious” changes in hemostatic pathways that tip the scales toward either bleeding or hypercoagulation, experts wrote in an American Gastroenterological Association Clinical Practice Update.
Based on current evidence, clinicians should not routinely correct thrombocytopenia and coagulopathy in patients with cirrhosis prior to low-risk procedures, such as therapeutic paracentesis, thoracentesis, and routine upper endoscopy for variceal ligation, Jacqueline G. O’Leary, MD, of Dallas VA Medical Center and her three coreviewers wrote in Gastroenterology.
To optimize clot formation prior to high-risk procedures, and in patients with active bleeding, a platelet count above 50,000 per mcL is still recommended. However, it may be more meaningful to couple that platelet target with a fibrinogen level above 120 mg/dL rather than rely on the international normalized ratio (INR), the experts wrote. Not only does INR vary significantly depending on which thromboplastin is used in the test, but “correcting” INR with a fresh frozen plasma infusion does not affect thrombin production and worsens portal hypertension. Using cryoprecipitate to replenish fibrinogen has less impact on portal hypertension. “Global tests of clot formation, such as rotational thromboelastometry (ROTEM), thromboelastography (TEG), sonorheometry, and thrombin generation may eventually have a role in the evaluation of clotting in patients with cirrhosis but currently lack validated target levels,” the experts wrote.
They advised clinicians to limit the use of blood products (such as fresh frozen plasma and pooled platelet transfusions) because of cost and the risk of exacerbated portal hypertension, infection, and immunologic complications. For severe anemia and uremia, red blood cell transfusion (250 mL) can be considered. Platelet-rich plasma from one donor is less immunologically risky than a pooled platelet transfusion. Thrombopoietin agonists are “a good alternative” to platelet transfusion but require about 10 days for response. Alternative prothrombotic therapies include oral thrombopoietin receptor agonists (avatrombopag and lusutrombopag) to boost platelet count before an invasive procedure, antifibrinolytic therapy (aminocaproic acid and tranexamic acid) for persistent bleeding from mucosal oozing or puncture wounds. Desmopressin should only be considered for patients with comorbid renal failure.
For anticoagulation, the practice update recommends considering systemic heparin infusion for cirrhotic patients with symptomatic deep venous thrombosis (DVT) or portal vein thrombosis (PVT). However, the anti–factor Xa assay will not reliably monitor response if patients have low liver-derived antithrombin III (heparin cofactor). “With newly diagnosed PVT, the decision to intervene with directed therapy rests on the extent of the thrombosis, presence or absence of attributable symptoms, and the risk of bleeding and falls,” the experts stated.
Six-month follow-up imaging is recommended to assess anticoagulation efficacy. More frequent imaging can be considered for PVT patients considered at high risk for therapeutic anticoagulation. If clots do not fully resolve after 6 months of treatment, options including extending therapy with the same agent, switching to a different anticoagulant class, or receiving transjugular intrahepatic portosystemic shunt (TIPS). “The role for TIPS in PVT is evolving and may address complications like portal hypertensive bleeding, medically refractory clot, and the need for repeated banding after variceal bleeding,” the experts noted.
Prophylaxis of DVT is recommended for all hospitalized patients with cirrhosis. Vitamin K antagonists and direct-acting oral anticoagulants (dabigatran, apixaban, rivaroxaban, and edoxaban) are alternatives to heparin for anticoagulation of cirrhotic patients with either PVT and DVT, the experts wrote. However, DOACs are not recommended for most Child-Pugh B patients or for any Child-Pugh C patients.
No funding sources or conflicts of interest were reported.
SOURCE: O’Leary JG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
Cirrhosis can involve “precarious” changes in hemostatic pathways that tip the scales toward either bleeding or hypercoagulation, experts wrote in an American Gastroenterological Association Clinical Practice Update.
Based on current evidence, clinicians should not routinely correct thrombocytopenia and coagulopathy in patients with cirrhosis prior to low-risk procedures, such as therapeutic paracentesis, thoracentesis, and routine upper endoscopy for variceal ligation, Jacqueline G. O’Leary, MD, of Dallas VA Medical Center and her three coreviewers wrote in Gastroenterology.
To optimize clot formation prior to high-risk procedures, and in patients with active bleeding, a platelet count above 50,000 per mcL is still recommended. However, it may be more meaningful to couple that platelet target with a fibrinogen level above 120 mg/dL rather than rely on the international normalized ratio (INR), the experts wrote. Not only does INR vary significantly depending on which thromboplastin is used in the test, but “correcting” INR with a fresh frozen plasma infusion does not affect thrombin production and worsens portal hypertension. Using cryoprecipitate to replenish fibrinogen has less impact on portal hypertension. “Global tests of clot formation, such as rotational thromboelastometry (ROTEM), thromboelastography (TEG), sonorheometry, and thrombin generation may eventually have a role in the evaluation of clotting in patients with cirrhosis but currently lack validated target levels,” the experts wrote.
They advised clinicians to limit the use of blood products (such as fresh frozen plasma and pooled platelet transfusions) because of cost and the risk of exacerbated portal hypertension, infection, and immunologic complications. For severe anemia and uremia, red blood cell transfusion (250 mL) can be considered. Platelet-rich plasma from one donor is less immunologically risky than a pooled platelet transfusion. Thrombopoietin agonists are “a good alternative” to platelet transfusion but require about 10 days for response. Alternative prothrombotic therapies include oral thrombopoietin receptor agonists (avatrombopag and lusutrombopag) to boost platelet count before an invasive procedure, antifibrinolytic therapy (aminocaproic acid and tranexamic acid) for persistent bleeding from mucosal oozing or puncture wounds. Desmopressin should only be considered for patients with comorbid renal failure.
For anticoagulation, the practice update recommends considering systemic heparin infusion for cirrhotic patients with symptomatic deep venous thrombosis (DVT) or portal vein thrombosis (PVT). However, the anti–factor Xa assay will not reliably monitor response if patients have low liver-derived antithrombin III (heparin cofactor). “With newly diagnosed PVT, the decision to intervene with directed therapy rests on the extent of the thrombosis, presence or absence of attributable symptoms, and the risk of bleeding and falls,” the experts stated.
Six-month follow-up imaging is recommended to assess anticoagulation efficacy. More frequent imaging can be considered for PVT patients considered at high risk for therapeutic anticoagulation. If clots do not fully resolve after 6 months of treatment, options including extending therapy with the same agent, switching to a different anticoagulant class, or receiving transjugular intrahepatic portosystemic shunt (TIPS). “The role for TIPS in PVT is evolving and may address complications like portal hypertensive bleeding, medically refractory clot, and the need for repeated banding after variceal bleeding,” the experts noted.
Prophylaxis of DVT is recommended for all hospitalized patients with cirrhosis. Vitamin K antagonists and direct-acting oral anticoagulants (dabigatran, apixaban, rivaroxaban, and edoxaban) are alternatives to heparin for anticoagulation of cirrhotic patients with either PVT and DVT, the experts wrote. However, DOACs are not recommended for most Child-Pugh B patients or for any Child-Pugh C patients.
No funding sources or conflicts of interest were reported.
SOURCE: O’Leary JG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
Cirrhosis can involve “precarious” changes in hemostatic pathways that tip the scales toward either bleeding or hypercoagulation, experts wrote in an American Gastroenterological Association Clinical Practice Update.
Based on current evidence, clinicians should not routinely correct thrombocytopenia and coagulopathy in patients with cirrhosis prior to low-risk procedures, such as therapeutic paracentesis, thoracentesis, and routine upper endoscopy for variceal ligation, Jacqueline G. O’Leary, MD, of Dallas VA Medical Center and her three coreviewers wrote in Gastroenterology.
To optimize clot formation prior to high-risk procedures, and in patients with active bleeding, a platelet count above 50,000 per mcL is still recommended. However, it may be more meaningful to couple that platelet target with a fibrinogen level above 120 mg/dL rather than rely on the international normalized ratio (INR), the experts wrote. Not only does INR vary significantly depending on which thromboplastin is used in the test, but “correcting” INR with a fresh frozen plasma infusion does not affect thrombin production and worsens portal hypertension. Using cryoprecipitate to replenish fibrinogen has less impact on portal hypertension. “Global tests of clot formation, such as rotational thromboelastometry (ROTEM), thromboelastography (TEG), sonorheometry, and thrombin generation may eventually have a role in the evaluation of clotting in patients with cirrhosis but currently lack validated target levels,” the experts wrote.
They advised clinicians to limit the use of blood products (such as fresh frozen plasma and pooled platelet transfusions) because of cost and the risk of exacerbated portal hypertension, infection, and immunologic complications. For severe anemia and uremia, red blood cell transfusion (250 mL) can be considered. Platelet-rich plasma from one donor is less immunologically risky than a pooled platelet transfusion. Thrombopoietin agonists are “a good alternative” to platelet transfusion but require about 10 days for response. Alternative prothrombotic therapies include oral thrombopoietin receptor agonists (avatrombopag and lusutrombopag) to boost platelet count before an invasive procedure, antifibrinolytic therapy (aminocaproic acid and tranexamic acid) for persistent bleeding from mucosal oozing or puncture wounds. Desmopressin should only be considered for patients with comorbid renal failure.
For anticoagulation, the practice update recommends considering systemic heparin infusion for cirrhotic patients with symptomatic deep venous thrombosis (DVT) or portal vein thrombosis (PVT). However, the anti–factor Xa assay will not reliably monitor response if patients have low liver-derived antithrombin III (heparin cofactor). “With newly diagnosed PVT, the decision to intervene with directed therapy rests on the extent of the thrombosis, presence or absence of attributable symptoms, and the risk of bleeding and falls,” the experts stated.
Six-month follow-up imaging is recommended to assess anticoagulation efficacy. More frequent imaging can be considered for PVT patients considered at high risk for therapeutic anticoagulation. If clots do not fully resolve after 6 months of treatment, options including extending therapy with the same agent, switching to a different anticoagulant class, or receiving transjugular intrahepatic portosystemic shunt (TIPS). “The role for TIPS in PVT is evolving and may address complications like portal hypertensive bleeding, medically refractory clot, and the need for repeated banding after variceal bleeding,” the experts noted.
Prophylaxis of DVT is recommended for all hospitalized patients with cirrhosis. Vitamin K antagonists and direct-acting oral anticoagulants (dabigatran, apixaban, rivaroxaban, and edoxaban) are alternatives to heparin for anticoagulation of cirrhotic patients with either PVT and DVT, the experts wrote. However, DOACs are not recommended for most Child-Pugh B patients or for any Child-Pugh C patients.
No funding sources or conflicts of interest were reported.
SOURCE: O’Leary JG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.070.
FROM GASTROENTEROLOGY
Treatment for hepatitis C reduces risk of Parkinson’s disease
, according to a cohort study published online June 5 in JAMA Neurology. The results provide evidence that hepatitis C virus is a risk factor for Parkinson’s disease.
In the past several years, epidemiologic studies have suggested an association between hepatitis C virus infection and Parkinson’s disease. A study published in 2017, however, found no association between the two. In addition, these investigations did not consider antiviral therapy as a potential modifying factor.
Wey-Yil Lin, MD, a neurologist at Landseed International Hospital in Taoyuan, Taiwan, and colleagues examined claims data from the Taiwan National Health Insurance Research Database to identify the risk of incident Parkinson’s disease in patients with hepatitis C virus infection who received antiviral treatment, compared with those who did not receive treatment.
The investigators selected all patients with a new diagnosis of hepatitis C virus infection with or without hepatitis from January 1, 2003, to December 31, 2013. They excluded patients who were aged 20 years or younger; had Parkinson’s disease, dementia, or stroke; or had had major hepatic diseases on the index date. To ensure that treated patients had had an effective course of therapy, the researchers excluded patients who were lost to follow-up within 6 months of the index date, received antiviral therapy for fewer than 16 weeks, or developed Parkinson’s disease within 6 months of the index date.
The primary outcome was incident Parkinson’s disease. Dr. Lin and colleagues excluded participants with a diagnosis of stroke and dementia before the index date to reduce the possibility of enrolling participants with secondary and atypical parkinsonism.
To minimize the potential selection bias to which observational studies are subject, the investigators performed propensity score matching with sex, age, comorbidities, and medication as covariates. This method was intended to create treated and untreated cohorts with comparable characteristics.
Dr. Lin and colleagues included 188,152 patients in their analysis. After matching, each group included 39,936 participants. In the group that received antiviral treatment, 45.0% of participants were female, and mean age was 52.8 years. In the untreated group, 44.4% of participants were female, and mean age was 52.5 years.
The incidence density of Parkinson’s disease per 1,000 person-years was 1.00 in the treated group and 1.39 in the untreated group. The difference in risk of Parkinson’s disease between the treated and untreated groups was statistically significant at year 5 of follow-up (hazard ratio [HR], 0.75) and at the end of the cohort (HR, 0.71). The risk did not differ significantly at year 1 and year 3, however. A subgroup analysis found a greater benefit of antiviral therapy among patients who concurrently used dihydropyridine calcium channel blockers.
“To our knowledge, this is the first cohort study to investigate the association between antiviral therapy and risk of Parkinson’s disease in patients with chronic hepatitis C viral infection,” said Dr. Lin and colleagues. Although it is possible that interferon-based antiviral therapy directly protected against the development of Parkinson’s disease, the short time of exposure to the antiviral agent “makes protecting against Parkinson’s disease development in 5 years less likely,” they added.
Among the study limitations that the authors acknowledged was the lack of data about hepatic function profile, serum virologic response, viral genotype, and hepatitis C virus RNA-level. The database that the investigators used also lacked data about behavioral factors (e.g., smoking status, coffee consumption, and alcohol consumption) that may have affected the incidence of Parkinson’s disease in the cohort. Investigations with longer follow-up periods will be needed to provide clearer information, they concluded.
The authors reported no conflicts of interest. The study was funded by grants from Chang Gung Medical Research Fund and from Chang Gung Memorial Hospital.
SOURCE: Lin W-Y et al. JAMA Neurol. 2019 Jun 5. doi: 10.1001/jamaneurol.2019.1368.
The findings of Lin et al. suggest a potentially modifiable hepatologic risk factor for Parkinson’s disease, Adolfo Ramirez-Zamora, MD, associate professor of neurology; Christopher W. Hess, MD, assistant professor of neurology; and David R. Nelson, MD, senior vice president for health affairs, all at the University of Florida in Gainesville, wrote in an accompanying editorial. Hepatitis C virus infection might enter the brain through the microvasculature and might induce microglial and macrophage-related inflammatory changes (JAMA Neurol. 2019 June 5. doi: 10.1001/jamaneurol.2019.1377).
Lin et al. estimated high diagnostic accuracy for Parkinson’s disease in their study. Nevertheless, clinical, neuroimaging, and pathological confirmation was unavailable, which is a limitation of their investigation, said Dr. Ramirez-Zamora and colleagues. “The diagnosis of Parkinson’s disease in early stages can be challenging, as other related conditions can mimic Parkinson’s disease, including cirrhosis-related parkinsonism. Moreover, using record-linkage systems excludes patients who did not seek medical advice or those who were misdiagnosed by symptoms alone, which may also underestimate the prevalence of Parkinson’s disease. Using population-based studies would be a more accurate method.”
Because interferon, which was the antiviral therapy used in this study, greatly affects the immune system and has a modest rate of eradicating viral hepatitis C infection, future research should examine the association between Parkinson’s disease and patients who cleared the virus, as well as patients who did not, said Dr. Ramirez-Zamora and colleagues. Such research could shed light on potential mechanisms of treatment response. Lin et al. did not examine the newer direct-acting antiviral therapies for hepatitis C virus infection, which cure more than 90% of patients. Nor did they analyze other well established lifestyle and demographic risk factors for developing the disease. In addition, “the authors could not generalize the results to those aged 75 years or older because of the substantially smaller number of patients in this age group,” said Dr. Ramirez-Zamora and colleagues.
Still, “identification of potentially treatable Parkinson’s disease risk factors presents a unique opportunity for treatment. Additional studies with detailed viral analysis and exposure are needed, including in other geographic and ethnic distributions,” they concluded.
The findings of Lin et al. suggest a potentially modifiable hepatologic risk factor for Parkinson’s disease, Adolfo Ramirez-Zamora, MD, associate professor of neurology; Christopher W. Hess, MD, assistant professor of neurology; and David R. Nelson, MD, senior vice president for health affairs, all at the University of Florida in Gainesville, wrote in an accompanying editorial. Hepatitis C virus infection might enter the brain through the microvasculature and might induce microglial and macrophage-related inflammatory changes (JAMA Neurol. 2019 June 5. doi: 10.1001/jamaneurol.2019.1377).
Lin et al. estimated high diagnostic accuracy for Parkinson’s disease in their study. Nevertheless, clinical, neuroimaging, and pathological confirmation was unavailable, which is a limitation of their investigation, said Dr. Ramirez-Zamora and colleagues. “The diagnosis of Parkinson’s disease in early stages can be challenging, as other related conditions can mimic Parkinson’s disease, including cirrhosis-related parkinsonism. Moreover, using record-linkage systems excludes patients who did not seek medical advice or those who were misdiagnosed by symptoms alone, which may also underestimate the prevalence of Parkinson’s disease. Using population-based studies would be a more accurate method.”
Because interferon, which was the antiviral therapy used in this study, greatly affects the immune system and has a modest rate of eradicating viral hepatitis C infection, future research should examine the association between Parkinson’s disease and patients who cleared the virus, as well as patients who did not, said Dr. Ramirez-Zamora and colleagues. Such research could shed light on potential mechanisms of treatment response. Lin et al. did not examine the newer direct-acting antiviral therapies for hepatitis C virus infection, which cure more than 90% of patients. Nor did they analyze other well established lifestyle and demographic risk factors for developing the disease. In addition, “the authors could not generalize the results to those aged 75 years or older because of the substantially smaller number of patients in this age group,” said Dr. Ramirez-Zamora and colleagues.
Still, “identification of potentially treatable Parkinson’s disease risk factors presents a unique opportunity for treatment. Additional studies with detailed viral analysis and exposure are needed, including in other geographic and ethnic distributions,” they concluded.
The findings of Lin et al. suggest a potentially modifiable hepatologic risk factor for Parkinson’s disease, Adolfo Ramirez-Zamora, MD, associate professor of neurology; Christopher W. Hess, MD, assistant professor of neurology; and David R. Nelson, MD, senior vice president for health affairs, all at the University of Florida in Gainesville, wrote in an accompanying editorial. Hepatitis C virus infection might enter the brain through the microvasculature and might induce microglial and macrophage-related inflammatory changes (JAMA Neurol. 2019 June 5. doi: 10.1001/jamaneurol.2019.1377).
Lin et al. estimated high diagnostic accuracy for Parkinson’s disease in their study. Nevertheless, clinical, neuroimaging, and pathological confirmation was unavailable, which is a limitation of their investigation, said Dr. Ramirez-Zamora and colleagues. “The diagnosis of Parkinson’s disease in early stages can be challenging, as other related conditions can mimic Parkinson’s disease, including cirrhosis-related parkinsonism. Moreover, using record-linkage systems excludes patients who did not seek medical advice or those who were misdiagnosed by symptoms alone, which may also underestimate the prevalence of Parkinson’s disease. Using population-based studies would be a more accurate method.”
Because interferon, which was the antiviral therapy used in this study, greatly affects the immune system and has a modest rate of eradicating viral hepatitis C infection, future research should examine the association between Parkinson’s disease and patients who cleared the virus, as well as patients who did not, said Dr. Ramirez-Zamora and colleagues. Such research could shed light on potential mechanisms of treatment response. Lin et al. did not examine the newer direct-acting antiviral therapies for hepatitis C virus infection, which cure more than 90% of patients. Nor did they analyze other well established lifestyle and demographic risk factors for developing the disease. In addition, “the authors could not generalize the results to those aged 75 years or older because of the substantially smaller number of patients in this age group,” said Dr. Ramirez-Zamora and colleagues.
Still, “identification of potentially treatable Parkinson’s disease risk factors presents a unique opportunity for treatment. Additional studies with detailed viral analysis and exposure are needed, including in other geographic and ethnic distributions,” they concluded.
, according to a cohort study published online June 5 in JAMA Neurology. The results provide evidence that hepatitis C virus is a risk factor for Parkinson’s disease.
In the past several years, epidemiologic studies have suggested an association between hepatitis C virus infection and Parkinson’s disease. A study published in 2017, however, found no association between the two. In addition, these investigations did not consider antiviral therapy as a potential modifying factor.
Wey-Yil Lin, MD, a neurologist at Landseed International Hospital in Taoyuan, Taiwan, and colleagues examined claims data from the Taiwan National Health Insurance Research Database to identify the risk of incident Parkinson’s disease in patients with hepatitis C virus infection who received antiviral treatment, compared with those who did not receive treatment.
The investigators selected all patients with a new diagnosis of hepatitis C virus infection with or without hepatitis from January 1, 2003, to December 31, 2013. They excluded patients who were aged 20 years or younger; had Parkinson’s disease, dementia, or stroke; or had had major hepatic diseases on the index date. To ensure that treated patients had had an effective course of therapy, the researchers excluded patients who were lost to follow-up within 6 months of the index date, received antiviral therapy for fewer than 16 weeks, or developed Parkinson’s disease within 6 months of the index date.
The primary outcome was incident Parkinson’s disease. Dr. Lin and colleagues excluded participants with a diagnosis of stroke and dementia before the index date to reduce the possibility of enrolling participants with secondary and atypical parkinsonism.
To minimize the potential selection bias to which observational studies are subject, the investigators performed propensity score matching with sex, age, comorbidities, and medication as covariates. This method was intended to create treated and untreated cohorts with comparable characteristics.
Dr. Lin and colleagues included 188,152 patients in their analysis. After matching, each group included 39,936 participants. In the group that received antiviral treatment, 45.0% of participants were female, and mean age was 52.8 years. In the untreated group, 44.4% of participants were female, and mean age was 52.5 years.
The incidence density of Parkinson’s disease per 1,000 person-years was 1.00 in the treated group and 1.39 in the untreated group. The difference in risk of Parkinson’s disease between the treated and untreated groups was statistically significant at year 5 of follow-up (hazard ratio [HR], 0.75) and at the end of the cohort (HR, 0.71). The risk did not differ significantly at year 1 and year 3, however. A subgroup analysis found a greater benefit of antiviral therapy among patients who concurrently used dihydropyridine calcium channel blockers.
“To our knowledge, this is the first cohort study to investigate the association between antiviral therapy and risk of Parkinson’s disease in patients with chronic hepatitis C viral infection,” said Dr. Lin and colleagues. Although it is possible that interferon-based antiviral therapy directly protected against the development of Parkinson’s disease, the short time of exposure to the antiviral agent “makes protecting against Parkinson’s disease development in 5 years less likely,” they added.
Among the study limitations that the authors acknowledged was the lack of data about hepatic function profile, serum virologic response, viral genotype, and hepatitis C virus RNA-level. The database that the investigators used also lacked data about behavioral factors (e.g., smoking status, coffee consumption, and alcohol consumption) that may have affected the incidence of Parkinson’s disease in the cohort. Investigations with longer follow-up periods will be needed to provide clearer information, they concluded.
The authors reported no conflicts of interest. The study was funded by grants from Chang Gung Medical Research Fund and from Chang Gung Memorial Hospital.
SOURCE: Lin W-Y et al. JAMA Neurol. 2019 Jun 5. doi: 10.1001/jamaneurol.2019.1368.
, according to a cohort study published online June 5 in JAMA Neurology. The results provide evidence that hepatitis C virus is a risk factor for Parkinson’s disease.
In the past several years, epidemiologic studies have suggested an association between hepatitis C virus infection and Parkinson’s disease. A study published in 2017, however, found no association between the two. In addition, these investigations did not consider antiviral therapy as a potential modifying factor.
Wey-Yil Lin, MD, a neurologist at Landseed International Hospital in Taoyuan, Taiwan, and colleagues examined claims data from the Taiwan National Health Insurance Research Database to identify the risk of incident Parkinson’s disease in patients with hepatitis C virus infection who received antiviral treatment, compared with those who did not receive treatment.
The investigators selected all patients with a new diagnosis of hepatitis C virus infection with or without hepatitis from January 1, 2003, to December 31, 2013. They excluded patients who were aged 20 years or younger; had Parkinson’s disease, dementia, or stroke; or had had major hepatic diseases on the index date. To ensure that treated patients had had an effective course of therapy, the researchers excluded patients who were lost to follow-up within 6 months of the index date, received antiviral therapy for fewer than 16 weeks, or developed Parkinson’s disease within 6 months of the index date.
The primary outcome was incident Parkinson’s disease. Dr. Lin and colleagues excluded participants with a diagnosis of stroke and dementia before the index date to reduce the possibility of enrolling participants with secondary and atypical parkinsonism.
To minimize the potential selection bias to which observational studies are subject, the investigators performed propensity score matching with sex, age, comorbidities, and medication as covariates. This method was intended to create treated and untreated cohorts with comparable characteristics.
Dr. Lin and colleagues included 188,152 patients in their analysis. After matching, each group included 39,936 participants. In the group that received antiviral treatment, 45.0% of participants were female, and mean age was 52.8 years. In the untreated group, 44.4% of participants were female, and mean age was 52.5 years.
The incidence density of Parkinson’s disease per 1,000 person-years was 1.00 in the treated group and 1.39 in the untreated group. The difference in risk of Parkinson’s disease between the treated and untreated groups was statistically significant at year 5 of follow-up (hazard ratio [HR], 0.75) and at the end of the cohort (HR, 0.71). The risk did not differ significantly at year 1 and year 3, however. A subgroup analysis found a greater benefit of antiviral therapy among patients who concurrently used dihydropyridine calcium channel blockers.
“To our knowledge, this is the first cohort study to investigate the association between antiviral therapy and risk of Parkinson’s disease in patients with chronic hepatitis C viral infection,” said Dr. Lin and colleagues. Although it is possible that interferon-based antiviral therapy directly protected against the development of Parkinson’s disease, the short time of exposure to the antiviral agent “makes protecting against Parkinson’s disease development in 5 years less likely,” they added.
Among the study limitations that the authors acknowledged was the lack of data about hepatic function profile, serum virologic response, viral genotype, and hepatitis C virus RNA-level. The database that the investigators used also lacked data about behavioral factors (e.g., smoking status, coffee consumption, and alcohol consumption) that may have affected the incidence of Parkinson’s disease in the cohort. Investigations with longer follow-up periods will be needed to provide clearer information, they concluded.
The authors reported no conflicts of interest. The study was funded by grants from Chang Gung Medical Research Fund and from Chang Gung Memorial Hospital.
SOURCE: Lin W-Y et al. JAMA Neurol. 2019 Jun 5. doi: 10.1001/jamaneurol.2019.1368.
FROM JAMA NEUROLOGY
TEG-guided topped conventional transfusion in cirrhotic patients with variceal bleeding
For patients with cirrhosis, variceal bleeding, and severe coagulopathies, the use of thromboelastography (TEG) to guide transfusion decisions significantly reduced both transfusions and rates of late rebleeding, according to the results of a randomized, open-label trial.
“With the use of TEG, only 13.3% of patients received any blood product, as compared with all patients in the conventional transfusion group,” wrote Gyanranjan Rout, MD, and associates at the All India Institute of Medical Sciences, a tertiary care center in New Delhi. The rate of rebleeding at 6 weeks was more than two-thirds lower with TEG versus the comparator group. The findings were published in the Journal of Clinical Gastroenterology.
Mortality remains high in patients with hepatic cirrhosis and variceal bleeding. Rebleeding is a major concern for these patients, and guidelines disagree on how to correct their coagulopathies so that they can undergo endoscopic treatment of varices. TEG “provides a global assessment of various factors promoting coagulation [platelets and clotting factors] and anticoagulation [fibrinolysis] in a single test,” the researchers noted.
Hence, they randomly assigned 60 adults with hepatic cirrhosis, acute variceal bleeding based on the Baveno VI consensus criteria, and significant coagulopathy (less than 50,000 platelets per mm3 or international normalized ratio under 1.8) to either conventional or TEG-guided transfusion. TEG of fresh blood was performed within 6 hours of hospital admission by using a MonoTEM-A automated thromboelastometer (Framar Hemologix, Rome).
Patients in the TEG group whose blood samples took more than 15 minutes to start forming fibrin received fresh frozen plasma (5 mL/kg of ideal body weight based on the Devine formula). Those whose maximum amplitude (an indicator of clot strength) was less than 30 mm received three units of platelets. Conventionally transfused patients received the same dose of fresh frozen plasma if their international normalized ratio was under 1.8 and the same amount of platelets if their platelet count was under 50,000 per mm3.
The groups had comparable baseline endoscopic findings, international normalized ratios, and hemoglobin and platelet levels. In the TEG group, only four (13.3%) patients underwent blood product transfusions, compared with every patient in the comparator group (P less than .001). Initial endoscopy showed similar control of bleeding between groups. At 5 days, rebleeding was noted in one (3.3%) TEG patient and four (13.3%) conventional transfusion patients (P = .167). At 42 days, this difference reached statistical significance (10% vs. 36.7%; P = .012).
The 6-week mortality rates were 13.3% in the TEG group and 26.7% in the conventional transfusion group (P = .176). The lack of statistical significance “can be explained by the fact that our study was not adequately powered to address the difference in mortality between the two study arms,” the researchers wrote.
The study excluded patients with sepsis, a major reason for coagulopathy in patients with cirrhosis. It also did not assess fibrinogen levels, and no patient received cryoprecipitate. “Our study provides initial data [supporting] the concept that TEG may help to decrease unnecessary blood transfusion and may even decrease 6-week rebleeding rate,” the researchers concluded. “However, this needs to be validated in a larger cohort of patients.”
The investigators did not disclose funding sources. They reported having no conflicts of interest.
SOURCE: Rout G et al. J Clin Gastroenterol. 2019 Apr 17. doi: 10.1097/MCG.000000000000121.
For patients with cirrhosis, variceal bleeding, and severe coagulopathies, the use of thromboelastography (TEG) to guide transfusion decisions significantly reduced both transfusions and rates of late rebleeding, according to the results of a randomized, open-label trial.
“With the use of TEG, only 13.3% of patients received any blood product, as compared with all patients in the conventional transfusion group,” wrote Gyanranjan Rout, MD, and associates at the All India Institute of Medical Sciences, a tertiary care center in New Delhi. The rate of rebleeding at 6 weeks was more than two-thirds lower with TEG versus the comparator group. The findings were published in the Journal of Clinical Gastroenterology.
Mortality remains high in patients with hepatic cirrhosis and variceal bleeding. Rebleeding is a major concern for these patients, and guidelines disagree on how to correct their coagulopathies so that they can undergo endoscopic treatment of varices. TEG “provides a global assessment of various factors promoting coagulation [platelets and clotting factors] and anticoagulation [fibrinolysis] in a single test,” the researchers noted.
Hence, they randomly assigned 60 adults with hepatic cirrhosis, acute variceal bleeding based on the Baveno VI consensus criteria, and significant coagulopathy (less than 50,000 platelets per mm3 or international normalized ratio under 1.8) to either conventional or TEG-guided transfusion. TEG of fresh blood was performed within 6 hours of hospital admission by using a MonoTEM-A automated thromboelastometer (Framar Hemologix, Rome).
Patients in the TEG group whose blood samples took more than 15 minutes to start forming fibrin received fresh frozen plasma (5 mL/kg of ideal body weight based on the Devine formula). Those whose maximum amplitude (an indicator of clot strength) was less than 30 mm received three units of platelets. Conventionally transfused patients received the same dose of fresh frozen plasma if their international normalized ratio was under 1.8 and the same amount of platelets if their platelet count was under 50,000 per mm3.
The groups had comparable baseline endoscopic findings, international normalized ratios, and hemoglobin and platelet levels. In the TEG group, only four (13.3%) patients underwent blood product transfusions, compared with every patient in the comparator group (P less than .001). Initial endoscopy showed similar control of bleeding between groups. At 5 days, rebleeding was noted in one (3.3%) TEG patient and four (13.3%) conventional transfusion patients (P = .167). At 42 days, this difference reached statistical significance (10% vs. 36.7%; P = .012).
The 6-week mortality rates were 13.3% in the TEG group and 26.7% in the conventional transfusion group (P = .176). The lack of statistical significance “can be explained by the fact that our study was not adequately powered to address the difference in mortality between the two study arms,” the researchers wrote.
The study excluded patients with sepsis, a major reason for coagulopathy in patients with cirrhosis. It also did not assess fibrinogen levels, and no patient received cryoprecipitate. “Our study provides initial data [supporting] the concept that TEG may help to decrease unnecessary blood transfusion and may even decrease 6-week rebleeding rate,” the researchers concluded. “However, this needs to be validated in a larger cohort of patients.”
The investigators did not disclose funding sources. They reported having no conflicts of interest.
SOURCE: Rout G et al. J Clin Gastroenterol. 2019 Apr 17. doi: 10.1097/MCG.000000000000121.
For patients with cirrhosis, variceal bleeding, and severe coagulopathies, the use of thromboelastography (TEG) to guide transfusion decisions significantly reduced both transfusions and rates of late rebleeding, according to the results of a randomized, open-label trial.
“With the use of TEG, only 13.3% of patients received any blood product, as compared with all patients in the conventional transfusion group,” wrote Gyanranjan Rout, MD, and associates at the All India Institute of Medical Sciences, a tertiary care center in New Delhi. The rate of rebleeding at 6 weeks was more than two-thirds lower with TEG versus the comparator group. The findings were published in the Journal of Clinical Gastroenterology.
Mortality remains high in patients with hepatic cirrhosis and variceal bleeding. Rebleeding is a major concern for these patients, and guidelines disagree on how to correct their coagulopathies so that they can undergo endoscopic treatment of varices. TEG “provides a global assessment of various factors promoting coagulation [platelets and clotting factors] and anticoagulation [fibrinolysis] in a single test,” the researchers noted.
Hence, they randomly assigned 60 adults with hepatic cirrhosis, acute variceal bleeding based on the Baveno VI consensus criteria, and significant coagulopathy (less than 50,000 platelets per mm3 or international normalized ratio under 1.8) to either conventional or TEG-guided transfusion. TEG of fresh blood was performed within 6 hours of hospital admission by using a MonoTEM-A automated thromboelastometer (Framar Hemologix, Rome).
Patients in the TEG group whose blood samples took more than 15 minutes to start forming fibrin received fresh frozen plasma (5 mL/kg of ideal body weight based on the Devine formula). Those whose maximum amplitude (an indicator of clot strength) was less than 30 mm received three units of platelets. Conventionally transfused patients received the same dose of fresh frozen plasma if their international normalized ratio was under 1.8 and the same amount of platelets if their platelet count was under 50,000 per mm3.
The groups had comparable baseline endoscopic findings, international normalized ratios, and hemoglobin and platelet levels. In the TEG group, only four (13.3%) patients underwent blood product transfusions, compared with every patient in the comparator group (P less than .001). Initial endoscopy showed similar control of bleeding between groups. At 5 days, rebleeding was noted in one (3.3%) TEG patient and four (13.3%) conventional transfusion patients (P = .167). At 42 days, this difference reached statistical significance (10% vs. 36.7%; P = .012).
The 6-week mortality rates were 13.3% in the TEG group and 26.7% in the conventional transfusion group (P = .176). The lack of statistical significance “can be explained by the fact that our study was not adequately powered to address the difference in mortality between the two study arms,” the researchers wrote.
The study excluded patients with sepsis, a major reason for coagulopathy in patients with cirrhosis. It also did not assess fibrinogen levels, and no patient received cryoprecipitate. “Our study provides initial data [supporting] the concept that TEG may help to decrease unnecessary blood transfusion and may even decrease 6-week rebleeding rate,” the researchers concluded. “However, this needs to be validated in a larger cohort of patients.”
The investigators did not disclose funding sources. They reported having no conflicts of interest.
SOURCE: Rout G et al. J Clin Gastroenterol. 2019 Apr 17. doi: 10.1097/MCG.000000000000121.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Despite HCV cure, liver cancer-associated genetic changes persist
A new study showed that liver tissue from hepatitis C virus (HCV)–infected humans with and without sustained virologic response found epigenetic and gene expression alterations associated with the risk for hepatocellular carcinoma (HCC), according to Nourdine Hamdane, PHD, of the Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France, and colleagues.
The researchers analyzed liver tissue from 6 noninfected control patients, 18 patients with chronic HCV infection, 21 patients with cured chronic HCV, 4 patients with hepatitis B virus (HBV) infection, and 7 patients with nonalcoholic steatohepatitis (NASH), as well as 8 paired HCC samples with HCV-induced liver disease (Gastroenterology 2019;156:2313–29).
They found that several altered pathways related to carcinogenesis persisted after cure, including TNF-alpha signaling, inflammatory response, G2M checkpoint, epithelial–mesenchymal transition, and phosphoinositide 3-kinase, Akt, and mammalian target of rapamycin.
They also observed lower levels of H3K27ac mapping to genes related to oxidative phosphorylation pathways, providing evidence supporting a functional role for H3K27ac changes in establishing gene expression patterns that persist after cure and contribute to carcinogenesis, according to the authors.
“Our study exposes a previously undiscovered paradigm showing that chronic HCV infection induces H3K27ac modifications that are associated with HCC risk and that persist after HCV cure,” the authors wrote. “[This study] provides a unique opportunity to uncover novel biomarkers for HCC risk, that is, from plasma through the detection of epigenetic changes of histones bound to circulating DNA complexes. Furthermore, by uncovering virus-induced epigenetic changes as therapeutic targets, our findings offer novel perspectives for HCC prevention – a key unmet medical need,” the researchers concluded.
The authors declared that they had no conflicts.
SOURCE: Hamdane N, et al. 2019; Gastroenterology 156:2313–29.
A new study showed that liver tissue from hepatitis C virus (HCV)–infected humans with and without sustained virologic response found epigenetic and gene expression alterations associated with the risk for hepatocellular carcinoma (HCC), according to Nourdine Hamdane, PHD, of the Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France, and colleagues.
The researchers analyzed liver tissue from 6 noninfected control patients, 18 patients with chronic HCV infection, 21 patients with cured chronic HCV, 4 patients with hepatitis B virus (HBV) infection, and 7 patients with nonalcoholic steatohepatitis (NASH), as well as 8 paired HCC samples with HCV-induced liver disease (Gastroenterology 2019;156:2313–29).
They found that several altered pathways related to carcinogenesis persisted after cure, including TNF-alpha signaling, inflammatory response, G2M checkpoint, epithelial–mesenchymal transition, and phosphoinositide 3-kinase, Akt, and mammalian target of rapamycin.
They also observed lower levels of H3K27ac mapping to genes related to oxidative phosphorylation pathways, providing evidence supporting a functional role for H3K27ac changes in establishing gene expression patterns that persist after cure and contribute to carcinogenesis, according to the authors.
“Our study exposes a previously undiscovered paradigm showing that chronic HCV infection induces H3K27ac modifications that are associated with HCC risk and that persist after HCV cure,” the authors wrote. “[This study] provides a unique opportunity to uncover novel biomarkers for HCC risk, that is, from plasma through the detection of epigenetic changes of histones bound to circulating DNA complexes. Furthermore, by uncovering virus-induced epigenetic changes as therapeutic targets, our findings offer novel perspectives for HCC prevention – a key unmet medical need,” the researchers concluded.
The authors declared that they had no conflicts.
SOURCE: Hamdane N, et al. 2019; Gastroenterology 156:2313–29.
A new study showed that liver tissue from hepatitis C virus (HCV)–infected humans with and without sustained virologic response found epigenetic and gene expression alterations associated with the risk for hepatocellular carcinoma (HCC), according to Nourdine Hamdane, PHD, of the Institut de Recherche sur les Maladies Virales et Hépatiques, Strasbourg, France, and colleagues.
The researchers analyzed liver tissue from 6 noninfected control patients, 18 patients with chronic HCV infection, 21 patients with cured chronic HCV, 4 patients with hepatitis B virus (HBV) infection, and 7 patients with nonalcoholic steatohepatitis (NASH), as well as 8 paired HCC samples with HCV-induced liver disease (Gastroenterology 2019;156:2313–29).
They found that several altered pathways related to carcinogenesis persisted after cure, including TNF-alpha signaling, inflammatory response, G2M checkpoint, epithelial–mesenchymal transition, and phosphoinositide 3-kinase, Akt, and mammalian target of rapamycin.
They also observed lower levels of H3K27ac mapping to genes related to oxidative phosphorylation pathways, providing evidence supporting a functional role for H3K27ac changes in establishing gene expression patterns that persist after cure and contribute to carcinogenesis, according to the authors.
“Our study exposes a previously undiscovered paradigm showing that chronic HCV infection induces H3K27ac modifications that are associated with HCC risk and that persist after HCV cure,” the authors wrote. “[This study] provides a unique opportunity to uncover novel biomarkers for HCC risk, that is, from plasma through the detection of epigenetic changes of histones bound to circulating DNA complexes. Furthermore, by uncovering virus-induced epigenetic changes as therapeutic targets, our findings offer novel perspectives for HCC prevention – a key unmet medical need,” the researchers concluded.
The authors declared that they had no conflicts.
SOURCE: Hamdane N, et al. 2019; Gastroenterology 156:2313–29.
FROM GASTROENTEROLOGY