User login
Antimicrobial, pH-modulating gel shows promise in preventing common STIs
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
FDA supports robotic device as hysterectomy helper
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Surgeons have a new tool for use in benign hysterectomies with the Food & Drug Administration’s authorization for marketing of the Hominis Surgical System, a robotic-assisted surgical device. The marketing authorization was granted to Memic Innovative Surgery.
The FDA reviewed the device through the De Novo classification review process, a regulatory pathway for low- to moderate-risk devices of a new type.
The robotically assisted surgical device (RASD) is designed to facilitate transvaginal hysterectomy procedures and salpingo-oophorectomy procedures in patients without cancer.
RASDs are not robots and require human control, but they allow a surgeon to use computer technology to control and move surgical instruments inserted through incisions or orifices. “RASD technology facilitates performing minimally invasive surgery and complex tasks in confined areas inside the body,” according to an FDA press release announcing the authorization.
“The FDA continues to support advancements in safe and effective medical devices that can improve patient experiences when undergoing surgical procedures,” Binita Ashar, MD, of the Office of Surgical and Infection Control Devices in the FDA’s Center for Devices and Radiological Health, said in the press release. The device represents another minimally invasive option for noncancerous conditions requiring gynecologic surgery.
The FDA also is establishing controls to ensure safety and effectiveness for RASDs, including labeling and performance testing requirements. “When met, the special controls, along with general controls, provide reasonable assurance of safety and effectiveness for devices of this type,” according to the press release.
The Hominis Surgical System involves the use of minimally invasive surgical instruments inserted through the vagina. A video camera is inserted laparoscopically through an abdominal incision; the camera allows the surgeon to visualize the instruments inside the patient.
“The FDA will require the manufacturer to develop and provide a comprehensive training program for surgeons and operating room staff to complete before operation of the device,” according to the press release.
The FDA reviewed data from a clinical study of 30 patients aged 37-79 years who underwent transvaginal total hysterectomy with salpingo-oophorectomy or salpingectomy for benign conditions.
Observed adverse events included minor blood loss, urinary tract infection and delayed healing of the closure made at the top of the vagina (vaginal cuff) that is done as part of a hysterectomy, according to the FDA. However, all 30 procedures were completed with no need for conversion to an open or other procedure.
Cesarean myomectomy: Safe operation or surgical folly?

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
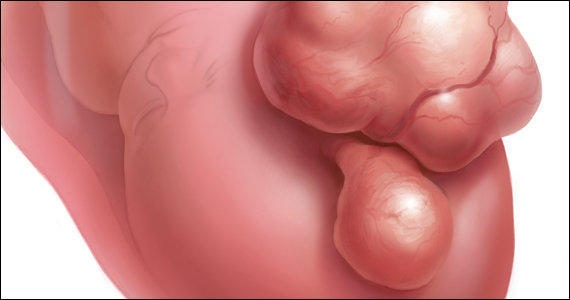
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
Prophylactic antibiotics for myomectomy?
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
Endometriosis, surgical approach impact risk of bowel injury in hysterectomy
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
Hysterectomies performed using an abdominal surgical approach or in women with endometriosis are more likely to carry an increased risk of bowel injury, according to recent results published in Obstetrics & Gynecology.
Cici R. Zhu, MD, of the department of obstetrics and gynecology at the University of Ottawa, and colleagues retrospectively studied the incidence of bowel injury in women participating in the American College of Surgeons National Surgical Quality Improvement Program who underwent hysterectomy for a benign surgical indication between 2012 and 2016.
“Although the absolute incidence is low, bowel injuries are among the most devastating complications of hysterectomy, as they can lead to a wide range of complications, including peritonitis, abscess formation, enterocutaneous fistula, sepsis, and even death,” Dr. Zhu and colleagues wrote. “Secondary bowel surgeries are often required, and associated ileostomies and colostomies can be distressing to patients. This not only severely affects quality of life, but the resultant readmissions, reoperations, and prolonged hospitalizations can impose a substantial economic toll on the health care system.”
Overall, 155,557 women were included in the study. The cohort consisted of women who were a mean age of 48 years and had a mean body mass index (BMI) of 31 kg/m2. The researchers evaluated whether baseline characteristics, clinical, and surgical variables impacted the incidence of bowel injury. They analyzed data of participant age, race (White vs. non-White), BMI, comorbid conditions (smoking, diabetes, chronic obstructive pulmonary disease, hypertension, and bleeding disorder), American Society of Anesthesiologists (ASA) classification, surgical approach (abdominal, laparoscopic, or vaginal), hysterectomy type (total or subtotal), lysis of adhesions, operation time, and admission type. Indication for hysterectomy was also evaluated, which included uterine leiomyoma (32.9%), menstrual disorders (22.0%), genital prolapse (13.1%), endometriosis (6.8%) and pelvic pain (3.8%).
Endometriosis, abdominal approach raise risk
There were 610 cases of bowel injury observed in the study, for an overall injury rate of 0.39%. A majority of the repairs were done during surgery (82.3%), with the remainder performed within 30 days of hysterectomy. Women with endometriosis had the most frequent incidence of bowel injury (0.59%), but it also occurred in women with uterine leiomyomas (0.47%), pain (0.24%), menstrual disorders (0.20%), genital prolapse (0.18%) and other indications (0.56%).
Dr. Zhu and colleagues found risk of bowel injury was higher among women 55 years and older, compared with women aged younger than 40 years (odds ratio, 1.66; 95% confidence interval, 1.28-2.15); in non-White women, compared with White women (OR, 1.92; 95% CI, 1.62-2.28); and in women with class 3 obesity, compared with women at a normal BMI (OR, 1.81; 95 CI, 1.40-2.34). Other risk factors for bowel injury included hypertension (OR, 1.39; 95% CI, 1.17-1.64) and ASA III, IV, and V classification, compared with ASA I classification (OR, 1.92; 95% CI, 1.43-2.58).
Researchers noted there was a statistically significant difference in rates of bowel injury between hysterectomy indications (P < .001). When compared with endometriosis, there were lower odds of bowel injury among women with uterine leiomyomas (adjusted odds ratio, 0.44; 95% confidence interval, 0.33-0.59), genital prolapse (aOR, 0.41; 95% CI, 0.25-0.67), and menstrual disorder (aOR, 0.33; 95% CI, 0.23-0.48).
Surgical factors also impacted the risk for bowel injury. In hysterectomies where the abdominal approach was used, there was an over-tenfold risk of bowel injury, compared with when a vaginal approach was used (OR, 10.80; 95% CI, 7.31-15.95). Lysis of lesions carried an increased risk of bowel injury (OR, 3.11; 95% CI, 2.20-4.40), and a subtotal hysterectomy increased the risk of bowel injury, compared with when a total hysterectomy was performed (OR, 1.76; 95% CI, 1.42-2.18).
The researchers acknowledged the lack of detailed clinical information on surgical indications, severity of bowel injury, and training of the surgeons and surgical team, and potential for missing information may limit the application of the study findings.
Findings must be cautiously interpreted
Kate Stampler, DO, assistant program director of minimally invasive gynecologic robotic surgery at Einstein Healthcare Network in Philadelphia, said in an interview that the study by Zhu et al. is a good reminder of the patient and surgical risk factors that can occur that affect outcomes of hysterectomy.
“In my clinical practice, I have not seen a significant difference in route of hysterectomy and bowel injury, however, this must be interpreted carefully in the context of an infrequent complication and as an MIS [minimally invasive surgery]-trained surgeon performing various complex cases,” she said. Other reports in the literature have not identified a difference in the rate of bowel injury based on surgical approach, but the study by Zhu et al. is “unique to the literature in its large sample size,” she explained.
“I would encourage less experienced surgeons to operate with a higher-volume assistant surgeon if the end result means being able to perform an MIS approach, or appropriately offer referral if feasible to another surgeon for best practices. A thorough informed consent of the available route of hysterectomy is integral to good surgical care and allows for shared decision making for the patient,” Dr. Stampler said. “Additionally, participation in a large quality reporting system such as ACS National Surgical Quality Improvement Program database should be considered broadly and we should strive for overall high-value care.”
Regarding endometriosis being a risk factor for bowel injury during hysterectomy, Dr. Stampler noted that severe endometriosis poses a significant challenge for gynecologic surgeons. “Loss of anatomic planes due to dense adhesions and fibrosis, in addition to deep infiltrating lesions, can add significant time, complexity, and risk to the procedure. This can be compounded in a scenario with less experienced surgeons and unplanned disease at the time of surgery.”
Dr. Stampler also applauded the paper for highlighting the differences in White and non-White patient outcomes for hysterectomy, and emphasized that it is not new information. “Their call to continue to address the social determinants of health in an effort to minimize risk and maximize safety for our patients of color is of critical importance now more than ever. While the hypothesis for this study was not meant to address this challenge specifically, the data should serve as a striking reminder that while several factors may be playing a role in surgical complications, ongoing systemic racism is a component that needs dedicated time and attention.”
Dr. Zhu and three coauthors reported no relevant financial disclosures. One coauthor received support from the University of Ottawa Clinical Research Chair in Reproductive Population Health and Health Services, the Canadian Institutes for Health Research, and Physicians’ Services Incorporated Foundation to conduct this research. Two other coauthors reported financial relationships with various pharmaceutical and medical technology companies. Dr. Stampler reported no relevant conflicts of interest.
SOURCE: Zhu CR et al. Obstet Gynecol. 2020 Oct. doi: 10.1097/AOG.0000000000004007.
FROM OBSTETRICS & GYNECOLOGY
Even in a virtual environment, the Society of Gynecologic Surgeons delivers without a “glitch”
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Earlier this year, I was honored to serve as the Scientific Program Chair for the 46th Annual Scientific Meeting of the Society of Gynecologic Surgeons (SGS). This year’s meeting was the first ever (and hopefully last) “virtual” scientific meeting, which consisted of a hybrid of prerecorded and live presentations. Although faculty and attendees were not able to be together physically, the essence of the lively SGS meetings came through loud and clear. We still had “discussants” comment on the oral presentations and ask questions of the presenters. These questions and answers were all done live—without a glitch! Many thanks to all who made this meeting possible.
In addition to the outstanding abstract and video presentations, there were 4 superb postgraduate courses:
- Mikio Nihira, MD, chaired “Enhanced recovery after surgery: Overcoming barriers to implementation.”
- Charles Hanes, MD, headed up “It’s all about the apex: The key to successful POP surgery.”
- Cara King, DO, MS, led “Total laparoscopic hysterectomy: Pushing the envelope.”
- Vincent Lucente, MD, chaired “Transvaginal reconstructive pelvic surgery using graft augmentation post-FDA.”
Many special thanks to Dr. Lucente who transformed his course into a wonderful article for this special section of
One of our exceptional keynote speakers was Marc Beer (a serial entrepreneur and cofounder, chairman, and CEO of Renovia, Inc.), whose talk was entitled “A primer on medical device innovation—How to avoid common pitfalls while realizing your vision.” Mr. Beer has turned this topic into a unique article for this special section (see next month’s issue for Part 2).
Our TeLinde Lecture, entitled “Artificial intelligence in surgery,” was delivered by the dynamic Vicente Gracias, MD, professor of surgery at Robert Wood Johnson University Hospital, New Brunswick, New Jersey. We also held 2 live panel discussions that were very popular. The first, “Work-life balance and gynecologic surgery,” featured various perspectives from Drs. Kristie Green, Sally Huber, Catherine Matthews, and Charles Rardin. The second panel discussion, entitled “Understanding, managing, and benefiting from your e-presence,” by experts Heather Schueppert; Chief Marketing Officer at Unified Physician Management, Brad Bowman, MD; and Peter Lotze, MD. Both of these panel discussions are included in this special section as well.
I hope you enjoy the content of this special section of
Hysteroscopy and COVID-19: Have recommended techniques changed due to the pandemic?
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
New hormonal medical treatment is an important advance for AUB caused by uterine fibroids
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
Uterine leiomyomata (fibroids) are the most common pelvic tumor diagnosed in women.1 Women with symptomatic fibroids often report abnormal uterine bleeding (AUB) and pelvic cramping, fullness, or pain. Fibroids also may cause frequency of urination and contribute to fertility and pregnancy problems. Treatment options for the AUB caused by fibroids include, but are not limited to, hysterectomy, myomectomy, uterine artery embolization, endometrial ablation, insertion of a levonorgestrel intrauterine device, focused ultrasound surgery, radiofrequency ablation, leuprolide acetate, and elagolix plus low-dose hormone add-back (Oriahnn; AbbVie, North Chicago, Illinois).1 Oriahnn is the most recent addition to our treatment armamentarium for fibroids and represents the first US Food and Drug Administration (FDA)-approved long-term hormonal option for AUB caused by fibroids.
Gene dysregulation contributes to fibroid development
Most uterine fibroids are clonal tumors, which develop following a somatic mutation in a precursor uterine myocyte. The somatic mutation causes gene dysregulation that stimulates cell growth resulting in a benign tumor mass. The majority of fibroids contain a mutation in one of the following 6 genes: mediator complex subunit 12 (MED12), high mobility group AT-hook (HMGA2 or HMGA1), RAD51B, fumarate hydratase (FH), collagen type IV, alpha 5 chain (COL4A5), or collagen type IV alpha 6 chain (COL4A6).2
Gene dysregulation in fibroids may arise following chromothripsis of the uterine myocyte genome
Chromothripsis is a catastrophic intracellular genetic event in which one or more chromosomes are broken and reassemble in a new nucleic acid sequence, producing a derivative chromosome that contains complex genetic rearrangements.3 Chromothripsis is believed to occur frequently in uterine myocytes. It is unknown why uterine myocytes are susceptible to chromothripsis,3 or why a catastrophic intracellular event such as chromothripsis results in preferential mutations in the 6 genes that are associated with myoma formation.
Estrogen and progesterone influence fibroid size and cell activity
Although uterine fibroids are clonal tumors containing broken genes, they are also exquisitely responsive to estradiol and progesterone. Estradiol and progesterone play an important role in regulating fibroid size and function.4 Estrogen stimulates uterine fibroids to increase in size. In a hypoestrogenic state, uterine fibroids decrease in size. In addition, a hypoestrogenic state results in an atrophic endometrium and thereby reduces AUB. For women with uterine fibroids and AUB, a reversible hypoestrogenic state can be induced either with a parenteral GnRH-agonist analogue (leuprolide) or an oral GnRH-antagonist (elagolix). Both leuprolide and elagolix are approved for the treatment of uterine fibroids (see below).
Surprisingly, progesterone stimulates cell division in normal uterine myocytes and fibroid cells.5 In the luteal phase of the menstrual cycle, uterine myocyte mitoses are more frequent than in the follicular phase. In addition, synthetic progestins appear to maintain fibroid size in a hypoestrogenic environment. In one randomized trial, women with uterine fibroids treated with leuprolide acetate plus a placebo pill for 24 weeks had a 51% reduction in uterine volume as measured by ultrasound.6 Women with uterine fibroids treated with leuprolide acetate plus the synthetic progestin, oral medroxyprogesterone acetate 20 mg daily, had only a 15% reduction in uterine volume.6 This finding suggests that synthetic progestins partially block the decrease in uterine volume that occurs in a hypoestrogenic state.
Further evidence that progesterone plays a role in fibroid biology is the observation that treatment of women with uterine fibroids with the antiprogestin ulipristal decreases fibroid size and reduces AUB.7-9 Ulipristal was approved for the treatment of fibroids in many countries but not the United States. Reports of severe, life-threatening liver injury—some necessitating liver transplantation—among women using ulipristal prompted the European Medicines Agency (EMA) in 2020 to recommend that women stop taking ulipristal. In addition, the EMA recommended that no woman should initiate ulipristal treatment at this time.10
Continue to: Leuprolide acetate...
Leuprolide acetate
Leuprolide acetate is a peptide GnRH-agonist analogue. Initiation of leuprolide treatment stimulates gonadotropin release, but with chronic administration pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) decreases, resulting in reduced ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Leuprolide treatment concomitant with iron therapy is approved by the FDA for improving red blood cell volume prior to surgery in women with fibroids, AUB, and anemia.11 Among women with fibroids, AUB, and anemia, after 12 weeks of treatment, the hemoglobin concentration was ≥12 g/dL in 79% treated with leuprolide plus iron and 56% treated with iron alone.11 The FDA recommends limiting preoperative leuprolide treatment to no more than 3 months. The approved leuprolide regimens are a maximum of 3 monthly injections of leuprolide 3.75 mg or a single injection of leuprolide 11.25 mg. Leuprolide treatment prior to hysterectomy surgery for uterine fibroids usually will result in a decrease in uterine size and may facilitate vaginal hysterectomy.
Elagolix plus estradiol plus norethindrone acetate (Oriahnn)
GnRH analogues cause a hypoestrogenic state resulting in adverse effects, including moderate to severe hot flashes and a reduction in bone mineral density. One approach to reducing the unwanted effects of hot flashes and decreased bone density is to combine a GnRH analogue with low-dose steroid hormone add-back therapy. Combining a GnRH analogue with low-dose steroid hormone add-back permits long-term treatment of AUB caused by fibroids, with few hot flashes and a minimal decrease in bone mineral density. The FDA recently has approved the combination of elagolix plus low-dose estradiol and norethindrone acetate (Oriahnn) for the long-term treatment of AUB caused by fibroids.
Elagolix is a nonpeptide oral GnRH antagonist that reduces pituitary secretion of LH and FSH, resulting in a decrease in ovarian follicular activity, anovulation, and low serum concentration of estradiol and progesterone. Unlike leuprolide, which causes an initial increase in LH and FSH secretion, the initiation of elagolix treatment causes an immediate and sustained reduction in LH and FSH secretion. Combining elagolix with a low dose of estradiol and norethindrone acetate reduces the side effects of hot flashes and decreased bone density. Clinical trials have reported that the combination of elagolix (300 mg) twice daily plus estradiol (1 mg) and norethindrone acetate (0.5 mg) once daily is an effective long-term treatment of AUB caused by uterine fibroids.
To study the efficacy of elagolix (alone or with estrogen-progestin add-back therapy) for the treatment of AUB caused by uterine fibroids, two identical trials were performed,12 in which 790 women participated. The participants had a mean age of 42 years and were documented to have heavy menstrual bleeding (>80 mL blood loss per cycle) and ultrasound-diagnosed uterine fibroids. The participants were randomized to one of 3 groups:
- elagolix (300 mg twice daily) plus low-dose steroid add-back (1 mg estradiol and 0.5 mg norethindrone acetate once daily),
- elagolix 300 mg twice daily with no steroid add-back (elagolix alone), or
- placebo for 6 months.12
Menstrual blood loss was quantified using the alkaline hematin method on collected sanitary products. The primary endpoint was menstrual blood loss <80 mL per cycle as well as a ≥50% reduction in quantified blood loss from baseline during the final month of treatment. At 6 months, the percentage of women achieving the primary endpoint in the first trial was 84% (elagolix alone), 69% (elagolix plus add-back), and 9% (placebo). Mean changes from baseline in lumbar spine bone density were −2.95% (elagolix alone), −0.76% (elagolix plus add-back), and −0.21% (placebo). The percentage of women reporting hot flashes was 64% in the elagolix group, 20% in the elagolix plus low-dose steroid add-back group, and 9% in the placebo group. Results were similar in the second trial.12
The initial trials were extended to 12 months with two groups: elagolix 300 mg twice daily plus low-dose hormone add-back with 1 mg estradiol and 0.5 mg norethindrone acetate once daily (n = 218) or elagolix 300 mg twice daily (elagolix alone) (n = 98).13 Following 12 months of treatment, heavy menstrual bleeding was controlled in 88% and 89% of women treated with elagolix plus add-back and elagolix alone, respectively. Amenorrhea was reported by 65% of the women in the elagolix plus add-back group. Compared with baseline bone density, at the end of 12 months of treatment, bone mineral density in the lumbar spine was reduced by -1.5% and -4.8% in the women treated with elagolix plus add-back and elagolix alone, respectively. Compared with baseline bone density, at 1 year following completion of treatment, bone mineral density in the lumbar spine was reduced by -0.6% and -2.0% in the women treated with elagolix plus add-back and elagolix alone, respectively. Similar trends were observed in total hip and femoral neck bone density. During treatment with elagolix plus add-back, adverse effects were modest, including hot flushes (6%), night sweats (3.2%), headache (5.5%), and nausea (4.1%). Two women developed liver transaminase levels >3 times the upper limit of normal, resulting in one woman discontinuing treatment.13
Continue to: Contraindications to Oriahnn include known allergies...
Contraindications to Oriahnn include known allergies to the components of the medication (including the yellow dye tartrazine); high risk of arterial, venous thrombotic or thromboembolic disorders; pregnancy; known osteoporosis; current breast cancer or other hormonally-sensitive malignancies; known liver disease; and concurrent use of organic anion transporting polypeptide 1B1 inhibitors, which includes many HIV antiviral medications.14 Undiagnosed AUB is a contraindication, and all women prescribed Oriahnn should have endometrial sampling before initiating treatment. Oriahnn should not be used for more than 24 months due to the risk of irreversible bone loss.14 Systemic estrogen and progestin combinations, a component of Oriahnn, increases the risk for pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at increased risk for these events (such as women >35 years who smoke cigarettes and women with uncontrolled hypertension).14 In two studies there was a higher incidence of depression, depressed mood, and/or tearfulness in women taking Oriahnn (3%) compared with those taking a placebo (1%).14 The FDA recommends promptly evaluating women with depressive symptoms to determine the risks of initiating and continuing Oriahnn therapy. In two studies there was a higher risk of reported alopecia among women taking Oriahnn (3.5%) compared with placebo (1%).14
It should be noted that elagolix is approved for the treatment of pelvic pain caused by endometriosis at a dose of 150 mg daily for 24 months or 200 mg twice daily for 6 months. The elagolix dose for the treatment of AUB caused by fibroids is 300 mg twice daily for up to 24 months, necessitating the addition of low-dose estradiol-norethindrone add-back to reduce the frequency and severity of hot flashes and minimize the loss of bone density. Norethindrone acetate also protects the endometrium from the stimulatory effect of estradiol, reducing the risk of developing endometrial hyperplasia and cancer. Oriahnn is formulated as two different capsules. A yellow and white capsule contains elagolix 300 mg plus estradiol 1 mg and norethindrone acetate 0.5 mg to be taken in the morning, and a blue and white capsule contains elagolix 300 mg to be taken in the evening.
AUB caused by fibroids is a common problem in gyn practice
There are many procedural interventions that are effective in reducing AUB caused by fibroids. However, prior to the approval of Oriahnn there were no hormonal medications that were FDA approved for the long-term treatment of AUB caused by fibroids. Hence, Oriahnn represents an important advance in the hormonal treatment of AUB caused by fibroids and expands the treatment options available to our patients. ●
Black women are more likely to develop fibroids and experience more severe fibroid symptoms. Obstetrician-gynecologists are experts in the diagnosis and treatment of fibroids. We play a key role in partnering with Black women to reduce fibroid disease burden.
Factors that increase the risk of developing fibroids include: increasing age, Black race, nulliparity, early menarche (<10 years of age), obesity, and consumption of red meat.1 The Nurses Health Study II is the largest prospective study of the factors that influence fibroid development.2 A total of 95,061 premenopausal nurses aged 25 to 44 years were followed from September 1989 through May 1993. Review of a sample of medical records demonstrated that the nurses participating in the study were reliable reporters of whether or not they had been diagnosed with fibroids. Based on a report of an ultrasound or hysterectomy diagnosis, the incidence rate for fibroids increased with age. Incidence rate per 1,000 women-years was 4.3 (age 25 to 29 years), 9.0 (30 to 34 years), 14.7 (age 35 to 39 years), and 22.5 (40 to 44 years). Compared with White race, Black race (but not Hispanic ethnicity or Asian race) was associated with an increased incidence of fibroids. Incidence rate per 1,000 women-years was 12.5 (White race), 37.9 (Black race), 14.5 (Hispanic ethnicity), and 10.4 (Asian race). The risk of developing fibroids was 3.25 times (95% CI, 2.71 to 3.88) greater among Black compared with White women after controlling for body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use, marital status, and current alcohol use.2
Other epidemiology studies also report an increased incidence of fibroids among Black women.3,4 The size of the uterus, the size and number of fibroids, and the severity of fibroid symptoms are greater among Black versus White women.5,6 The molecular factors that increase fibroid incidence among Black women are unknown. Given the burden of fibroid disease among Black women, obstetrician-gynecologists are best positioned to ensure early diagnosis and to develop an effective follow-up and treatment plan for affected women.
References
1. Stewart EA, Laughlin-Tommaso SK, Catherino WH, et al. Uterine fibroids. Nat Rev Dis Primers. 2016;2:16043.
2. Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973.
3. Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
4. Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. J Womens Health. 1997;6:309-316.
5. Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:1988719892.
6. Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168.e1-e9.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
- Stewart EA. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- Mehine M, Makinen N, Heinonen HR, et al. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. 2014;102:621-629.
- Mehine M, Kaasinen E, Makinen N, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369:43-53.
- Moravek MB, Bulun SE. Endocrinology of uterine fibroids: steroid hormones, stem cells and genetic contribution. Curr Opin Obstet Gynecol. 2015;27:276-283.
- Rein MS. Advances in uterine leiomyoma research: the progesterone hypothesis. Environ Health Perspect. 2000;108(suppl 5):791-793.
- Friedman AJ, Barbieri RL, Doubilet PM, et al. A randomized double-blind trial of a gonadotropin-releasing hormone agonist (leuprolide) with or without medroxyprogesterone acetate in the treatment of leiomyomata uteri. Fertil Steril. 1988;49:404-409.
- Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103:519-527.
- Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409-420.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432.
- European Medicines Agency. Suspension of ulipristal acetate for uterine fibroids during ongoing EMA review of liver injury risk. March 13, 2020. https://www.ema.europa.eu/en/news/suspension-ulipristal-acetate-uterine-fibroids-during-ongoing-ema-review-liver-injury-risk#:~:text=EMA's%20safety%20committee%20(PRAC)%20has,the%20EU%20during%20the%20review. Accessed July 24, 2020.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
- Oriahnn [package insert]. North Chicago, IL: AbbVie; 2020.
How effective is elagolix treatment in women with fibroids and HMB?
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
Can a drug FDA approved for endometriosis become a mainstay for nonsurgical treatment of HMB in women with fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.