User login
Docs used permanent, not temporary stitches; lawsuits result
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
The first in what have come to be known as the “wrong stitches” cases has been settled, a story in The Ledger reports.
The former plaintiff in the now-settled suit is Carrie Monk, a Lakeland, Fla., resident who underwent total laparoscopic hysterectomy at Lakeland Regional Health Medical Center several years ago. (The medical center is managed by Lakeland Regional Health Systems.) D As a result, over the next 19 months, she experienced abdominal pain and constant bleeding, which in turn affected her personal life as well as her work as a nurse in the intensive care unit. She underwent follow-up surgery to have the permanent sutures removed, but two could not be identified and excised.
In July 2020, Ms. Monk filed a medical malpractice claim against Lakeland Regional Health, its medical center, and the ob-gyns who had performed her surgery. She was among the first of the women who had received the permanent sutures to do so.
On February 28, 2021, The Ledger ran a story on Ms. Monk’s suit. Less than 2 weeks later, Lakeland Regional Health sent letters to patients who had undergone “wrong stitch” surgeries, cautioning of possible postsurgical complications. The company reportedly kept secret how many letters it had sent out.
Since then, at least nine similar suits have been filed against Lakeland Regional Health, bringing the total number of such suits to 12. Four of these suits have been settled, including Ms. Monk’s. Of the remaining eight cases, several are in various pretrial stages.
Under the terms of her settlement, neither Ms. Monk nor her attorney may disclose what financial compensation or other awards she’s received. The attorney, however, referred to the settlement as “amicable.”
The content contained in this article is for informational purposes only and does not constitute legal advice. Reliance on any information provided in this article is solely at your own risk.
A version of this article first appeared on Medscape.com.
Hair straighteners’ risk too small for docs to advise against their use
Clarissa Ghazi gets lye relaxers, which contain the chemical sodium hydroxide, applied to her hair two to three times a year.
A recent study that made headlines over a potential link between hair straighteners and uterine cancer is not going to make her stop.
“This study is not enough to cause me to say I’ll stay away from this because [the researchers] don’t prove that using relaxers causes cancer,” Ms. Ghazi said.
Indeed, primary care doctors are unlikely to address the increased risk of uterine cancer in women who frequently use hair straighteners that the study reported.
In the recently published paper on this research, the authors said that they found an 80% higher adjusted risk of uterine cancer among women who had ever “straightened,” “relaxed,” or used “hair pressing products” in the 12 months before enrolling in their study.
This finding is “real, but small,” says internist Douglas S. Paauw, MD, professor of medicine at the University of Washington in Seattle.
Dr. Paauw is among several primary care doctors interviewed for this story who expressed little concern about the implications of this research for their patients.
“Since we have hundreds of things we are supposed to discuss at our 20-minute clinic visits, this would not make the cut,” Dr. Paauw said.
While it’s good to be able to answer questions a patient might ask about this new research, the study does not prove anything, he said.
Alan Nelson, MD, an internist-endocrinologist and former special adviser to the CEO of the American College of Physicians, said while the study is well done, the number of actual cases of uterine cancer found was small.
One of the reasons he would not recommend discussing the study with patients is that the brands of hair products used to straighten hair in the study were not identified.
Alexandra White, PhD, lead author of the study, said participants were simply asked, “In the past 12 months, how frequently have you or someone else straightened or relaxed your hair, or used hair pressing products?”
The terms “straightened,” “relaxed,” and “hair pressing products” were not defined, and “some women may have interpreted the term ‘pressing products’ to mean nonchemical products” such as flat irons, Dr. White, head of the National Institute of Environmental Health Sciences’ Environment and Cancer Epidemiology group, said in an email.
Dermatologist Crystal Aguh, MD, associate professor of dermatology at Johns Hopkins University, Baltimore, tweeted the following advice in light of the new findings: “The overall risk of uterine cancer is quite low so it’s important to remember that. For now, if you want to change your routine, there’s no downside to decreasing your frequency of hair straightening to every 12 weeks or more, as that may lessen your risk.”
She also noted that “styles like relaxer, silk pressing, and keratin treatments should only be done by a professional, as this will decrease the likelihood of hair damage and scalp irritation.
“I also encourage women to look for hair products free of parabens and phthalates (which are generically listed as “fragrance”) on products to minimize exposure to hormone disrupting chemicals.”
Not ready to go curly
Ms. Ghazi said she decided to stop using keratin straighteners years ago after she learned they are made with several added ingredients. That includes the chemical formaldehyde, a known carcinogen, according to the American Cancer Society.
“People have been relaxing their hair for a very long time, and I feel more comfortable using [a relaxer] to straighten my hair than any of the others out there,” Ms. Ghazi said.
Janaki Ram, who has had her hair chemically straightened several times, said the findings have not made her worried that straightening will cause her to get uterine cancer specifically, but that they are a reminder that the chemicals in these products could harm her in some other way.
She said the new study findings, her knowledge of the damage straightening causes to hair, and the lengthy amount of time receiving a keratin treatment takes will lead her to reduce the frequency with which she gets her hair straightened.
“Going forward, I will have this done once a year instead of twice a year,” she said.
Dr. White, the author of the paper, said in an interview that the takeaway for consumers is that women who reported frequent use of hair straighteners/relaxers and pressing products were more than twice as likely to go on to develop uterine cancer compared to women who reported no use of these products in the previous year.
“However, uterine cancer is relatively rare, so these increases in risks are small,” she said. “Less frequent use of these products was not as strongly associated with risk, suggesting that decreasing use may be an option to reduce harmful exposure. Black women were the most frequent users of these products and therefore these findings are more relevant for Black women.”
In a statement, Dr. White noted, “We estimated that 1.64% of women who never used hair straighteners would go on to develop uterine cancer by the age of 70; but for frequent users, that risk goes up to 4.05%.”
The findings were based on the Sister Study, which enrolled women living in the United States, including Puerto Rico, between 2003 and 2009. Participants needed to have at least one sister who had been diagnosed with breast cancer, been breast cancer-free themselves, and aged 35-74 years. Women who reported a diagnosis of uterine cancer before enrollment, had an uncertain uterine cancer history, or had a hysterectomy were excluded from the study.
The researchers examined hair product usage and uterine cancer incidence during an 11-year period among 33 ,947 women. The analysis controlled for variables such as age, race, and risk factors. At baseline, participants were asked to complete a questionnaire on hair products use in the previous 12 months.
“One of the original aims of the study was to better understand the environmental and genetic causes of breast cancer, but we are also interested in studying ovarian cancer, uterine cancer, and many other cancers and chronic diseases,” Dr. White said.
A version of this article first appeared on WebMD.com.
Clarissa Ghazi gets lye relaxers, which contain the chemical sodium hydroxide, applied to her hair two to three times a year.
A recent study that made headlines over a potential link between hair straighteners and uterine cancer is not going to make her stop.
“This study is not enough to cause me to say I’ll stay away from this because [the researchers] don’t prove that using relaxers causes cancer,” Ms. Ghazi said.
Indeed, primary care doctors are unlikely to address the increased risk of uterine cancer in women who frequently use hair straighteners that the study reported.
In the recently published paper on this research, the authors said that they found an 80% higher adjusted risk of uterine cancer among women who had ever “straightened,” “relaxed,” or used “hair pressing products” in the 12 months before enrolling in their study.
This finding is “real, but small,” says internist Douglas S. Paauw, MD, professor of medicine at the University of Washington in Seattle.
Dr. Paauw is among several primary care doctors interviewed for this story who expressed little concern about the implications of this research for their patients.
“Since we have hundreds of things we are supposed to discuss at our 20-minute clinic visits, this would not make the cut,” Dr. Paauw said.
While it’s good to be able to answer questions a patient might ask about this new research, the study does not prove anything, he said.
Alan Nelson, MD, an internist-endocrinologist and former special adviser to the CEO of the American College of Physicians, said while the study is well done, the number of actual cases of uterine cancer found was small.
One of the reasons he would not recommend discussing the study with patients is that the brands of hair products used to straighten hair in the study were not identified.
Alexandra White, PhD, lead author of the study, said participants were simply asked, “In the past 12 months, how frequently have you or someone else straightened or relaxed your hair, or used hair pressing products?”
The terms “straightened,” “relaxed,” and “hair pressing products” were not defined, and “some women may have interpreted the term ‘pressing products’ to mean nonchemical products” such as flat irons, Dr. White, head of the National Institute of Environmental Health Sciences’ Environment and Cancer Epidemiology group, said in an email.
Dermatologist Crystal Aguh, MD, associate professor of dermatology at Johns Hopkins University, Baltimore, tweeted the following advice in light of the new findings: “The overall risk of uterine cancer is quite low so it’s important to remember that. For now, if you want to change your routine, there’s no downside to decreasing your frequency of hair straightening to every 12 weeks or more, as that may lessen your risk.”
She also noted that “styles like relaxer, silk pressing, and keratin treatments should only be done by a professional, as this will decrease the likelihood of hair damage and scalp irritation.
“I also encourage women to look for hair products free of parabens and phthalates (which are generically listed as “fragrance”) on products to minimize exposure to hormone disrupting chemicals.”
Not ready to go curly
Ms. Ghazi said she decided to stop using keratin straighteners years ago after she learned they are made with several added ingredients. That includes the chemical formaldehyde, a known carcinogen, according to the American Cancer Society.
“People have been relaxing their hair for a very long time, and I feel more comfortable using [a relaxer] to straighten my hair than any of the others out there,” Ms. Ghazi said.
Janaki Ram, who has had her hair chemically straightened several times, said the findings have not made her worried that straightening will cause her to get uterine cancer specifically, but that they are a reminder that the chemicals in these products could harm her in some other way.
She said the new study findings, her knowledge of the damage straightening causes to hair, and the lengthy amount of time receiving a keratin treatment takes will lead her to reduce the frequency with which she gets her hair straightened.
“Going forward, I will have this done once a year instead of twice a year,” she said.
Dr. White, the author of the paper, said in an interview that the takeaway for consumers is that women who reported frequent use of hair straighteners/relaxers and pressing products were more than twice as likely to go on to develop uterine cancer compared to women who reported no use of these products in the previous year.
“However, uterine cancer is relatively rare, so these increases in risks are small,” she said. “Less frequent use of these products was not as strongly associated with risk, suggesting that decreasing use may be an option to reduce harmful exposure. Black women were the most frequent users of these products and therefore these findings are more relevant for Black women.”
In a statement, Dr. White noted, “We estimated that 1.64% of women who never used hair straighteners would go on to develop uterine cancer by the age of 70; but for frequent users, that risk goes up to 4.05%.”
The findings were based on the Sister Study, which enrolled women living in the United States, including Puerto Rico, between 2003 and 2009. Participants needed to have at least one sister who had been diagnosed with breast cancer, been breast cancer-free themselves, and aged 35-74 years. Women who reported a diagnosis of uterine cancer before enrollment, had an uncertain uterine cancer history, or had a hysterectomy were excluded from the study.
The researchers examined hair product usage and uterine cancer incidence during an 11-year period among 33 ,947 women. The analysis controlled for variables such as age, race, and risk factors. At baseline, participants were asked to complete a questionnaire on hair products use in the previous 12 months.
“One of the original aims of the study was to better understand the environmental and genetic causes of breast cancer, but we are also interested in studying ovarian cancer, uterine cancer, and many other cancers and chronic diseases,” Dr. White said.
A version of this article first appeared on WebMD.com.
Clarissa Ghazi gets lye relaxers, which contain the chemical sodium hydroxide, applied to her hair two to three times a year.
A recent study that made headlines over a potential link between hair straighteners and uterine cancer is not going to make her stop.
“This study is not enough to cause me to say I’ll stay away from this because [the researchers] don’t prove that using relaxers causes cancer,” Ms. Ghazi said.
Indeed, primary care doctors are unlikely to address the increased risk of uterine cancer in women who frequently use hair straighteners that the study reported.
In the recently published paper on this research, the authors said that they found an 80% higher adjusted risk of uterine cancer among women who had ever “straightened,” “relaxed,” or used “hair pressing products” in the 12 months before enrolling in their study.
This finding is “real, but small,” says internist Douglas S. Paauw, MD, professor of medicine at the University of Washington in Seattle.
Dr. Paauw is among several primary care doctors interviewed for this story who expressed little concern about the implications of this research for their patients.
“Since we have hundreds of things we are supposed to discuss at our 20-minute clinic visits, this would not make the cut,” Dr. Paauw said.
While it’s good to be able to answer questions a patient might ask about this new research, the study does not prove anything, he said.
Alan Nelson, MD, an internist-endocrinologist and former special adviser to the CEO of the American College of Physicians, said while the study is well done, the number of actual cases of uterine cancer found was small.
One of the reasons he would not recommend discussing the study with patients is that the brands of hair products used to straighten hair in the study were not identified.
Alexandra White, PhD, lead author of the study, said participants were simply asked, “In the past 12 months, how frequently have you or someone else straightened or relaxed your hair, or used hair pressing products?”
The terms “straightened,” “relaxed,” and “hair pressing products” were not defined, and “some women may have interpreted the term ‘pressing products’ to mean nonchemical products” such as flat irons, Dr. White, head of the National Institute of Environmental Health Sciences’ Environment and Cancer Epidemiology group, said in an email.
Dermatologist Crystal Aguh, MD, associate professor of dermatology at Johns Hopkins University, Baltimore, tweeted the following advice in light of the new findings: “The overall risk of uterine cancer is quite low so it’s important to remember that. For now, if you want to change your routine, there’s no downside to decreasing your frequency of hair straightening to every 12 weeks or more, as that may lessen your risk.”
She also noted that “styles like relaxer, silk pressing, and keratin treatments should only be done by a professional, as this will decrease the likelihood of hair damage and scalp irritation.
“I also encourage women to look for hair products free of parabens and phthalates (which are generically listed as “fragrance”) on products to minimize exposure to hormone disrupting chemicals.”
Not ready to go curly
Ms. Ghazi said she decided to stop using keratin straighteners years ago after she learned they are made with several added ingredients. That includes the chemical formaldehyde, a known carcinogen, according to the American Cancer Society.
“People have been relaxing their hair for a very long time, and I feel more comfortable using [a relaxer] to straighten my hair than any of the others out there,” Ms. Ghazi said.
Janaki Ram, who has had her hair chemically straightened several times, said the findings have not made her worried that straightening will cause her to get uterine cancer specifically, but that they are a reminder that the chemicals in these products could harm her in some other way.
She said the new study findings, her knowledge of the damage straightening causes to hair, and the lengthy amount of time receiving a keratin treatment takes will lead her to reduce the frequency with which she gets her hair straightened.
“Going forward, I will have this done once a year instead of twice a year,” she said.
Dr. White, the author of the paper, said in an interview that the takeaway for consumers is that women who reported frequent use of hair straighteners/relaxers and pressing products were more than twice as likely to go on to develop uterine cancer compared to women who reported no use of these products in the previous year.
“However, uterine cancer is relatively rare, so these increases in risks are small,” she said. “Less frequent use of these products was not as strongly associated with risk, suggesting that decreasing use may be an option to reduce harmful exposure. Black women were the most frequent users of these products and therefore these findings are more relevant for Black women.”
In a statement, Dr. White noted, “We estimated that 1.64% of women who never used hair straighteners would go on to develop uterine cancer by the age of 70; but for frequent users, that risk goes up to 4.05%.”
The findings were based on the Sister Study, which enrolled women living in the United States, including Puerto Rico, between 2003 and 2009. Participants needed to have at least one sister who had been diagnosed with breast cancer, been breast cancer-free themselves, and aged 35-74 years. Women who reported a diagnosis of uterine cancer before enrollment, had an uncertain uterine cancer history, or had a hysterectomy were excluded from the study.
The researchers examined hair product usage and uterine cancer incidence during an 11-year period among 33 ,947 women. The analysis controlled for variables such as age, race, and risk factors. At baseline, participants were asked to complete a questionnaire on hair products use in the previous 12 months.
“One of the original aims of the study was to better understand the environmental and genetic causes of breast cancer, but we are also interested in studying ovarian cancer, uterine cancer, and many other cancers and chronic diseases,” Dr. White said.
A version of this article first appeared on WebMD.com.
Models stratify hysterectomy risk with benign conditions
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
New models can help predict whether women having a hysterectomy for benign conditions are likely to have major complications, according to researchers.
The models, which use routinely collected data, are meant to aid surgeons in counseling women before surgery and help guide shared decision-making. The tools may lead to referrals for centers with greater surgical experience or may result in seeking nonsurgical treatment options, the researchers indicate.
The tools are not applicable to patients having hysterectomy for malignant disease.
Findings of the study, led by Krupa Madhvani, MD, of Barts and the London School of Medicine and Dentistry in London, are published online in the Canadian Medical Association Journal.
Calculators complement surgeons’ intuition
“Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counseling and match the advances made in the technical aspects of surgery,” the authors write.
“Internal–external cross-validation and external validation showed moderate discrimination,” they note.
The study included 68,599 patients who had laparoscopic hysterectomies and 125,971 patients who had an abdominal hysterectomy, all English National Health System patients between 2011 and 2018.
Among their findings were that major complications occurred in 4.4% of laparoscopic and 4.9% of abdominal hysterectomies. Major complications in this study included ureteric, gastrointestinal, and vascular injury and wound complications.
Adhesions biggest predictors of complications
Adhesions were most predictive of complications – with double the odds – in both models (laparoscopic: odds ratio, 1.92; 95% confidence interval, 1.73-2.13; abdominal: OR, 2.46; 95% CI, 2.27-2.66). That finding was consistent with previous literature.
“Adhesions should be suspected if there is a previous history of laparotomy, cesarean section, pelvic infection, or endometriosis, and can be reliably diagnosed preoperatively using ultrasonography,” the authors write. “As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.”
Other factors that best predicted complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Diabetes was not a predictive factor for complications in laparoscopic hysterectomy as it was in a previous study.
Obesity was not a significant predictor of major complications for either form of hysterectomy.
Factors protective against major complications included younger age and diagnosed menstrual disorders or benign adnexal mass (both models) and diagnosis of fibroids in the abdominal model.
Models miss surgeon experience
Jon Ivar Einarsson MD, PhD, MPH, founder of the division of minimally invasive gynecologic surgery at Brigham and Women’s Hospital, Boston, said it’s good to have these models to estimate risk as “there’s possibly a tendency to underestimate the risk by the surgeon.”
However, he told this publication that, though these models are based on a very large data set, the models are missing some key variables – often a problem with database studies – that are more indicative of complications. The most important factor missing, he said, is surgeon experience.
“We’ve shown in our publications that there’s a correlation between that and the risk of complications,“ Dr. Einarsson said.
Among other variables missing, he noted, are some that the authors list when acknowledging the limitations: severity of endometriosis and severity of adhesions.
He said his team wouldn’t use such models because they rely on their own data for gauging risk. He encourages other surgeons to track their own data and outcomes as well.
“I think the external validity here is nonexistent because we’re dealing with a different patient population in a different country with different surgeons [who] have various degrees of expertise,” Dr. Einarsson said.
“But if surgeons have not collected their own data, then this could be useful,” he said.
Links to online calculators
The online calculator can be found at www.evidencio.com (laparoscopic, www.evidencio.com/models/show/2551; abdominal, www.evidencio.com/models/show/2552).
The large, national multi-institutional database helps with generalizability of findings, the authors write. Additionally, patients had a unique identifier number so if patients were admitted to a different hospital after surgery, they were not lost to follow-up.
Limitations, in addition to those mentioned, include gaps in detailed clinical information, such as exact body mass index, and location, type, and size of leiomyoma, the authors write.
“Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications,” they write.
Dr. Madhvani has received article-processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared. Dr. Einarsson reports no relevant financial relationships. The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report, or the decision to submit the article for publication. Coauthor Khalid Khan, MD is a distinguished investigator funded by the Beatriz Galindo Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
FROM CANADIAN MEDICAL ASSOCIATION JOURNAL
Steps to minimize morbidity from unanticipated placenta accreta spectrum
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
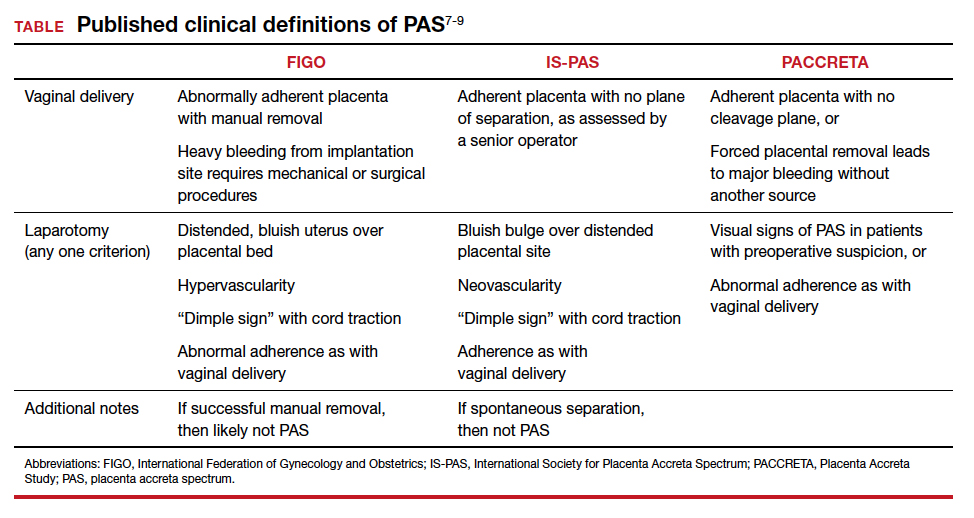
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.
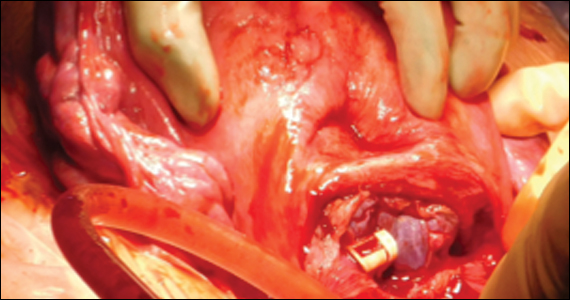
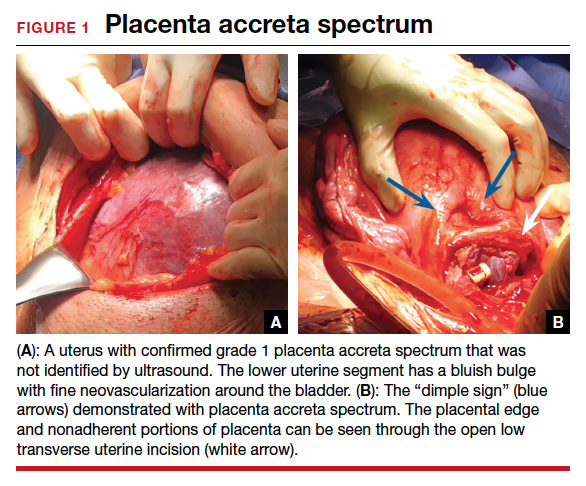
At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8
Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
CASE Placenta accreta spectrum following uncomplicated vaginal delivery
Imagine you are an obstetric hospitalist taking call at a level II maternal level of care hospital. Your patient is a 35-year-old woman, gravida 2, para 1, with a past history of retained placenta requiring dilation and curettage and intravenous antibiotics for endomyometritis. This is an in vitro fertilization pregnancy that has progressed normally, and the patient labored spontaneously at 38 weeks’ gestation. Following an uncomplicated vaginal delivery, the placenta has not delivered, and you attempt a manual placental extraction after a 40-minute third stage. While there is epidural analgesia and you can reach the uterine fundus, you are unable to create a separation plane between the placenta and uterus.
What do you do next?
Placenta accreta spectrum (PAS) includes a broad range of clinical scenarios with abnormal placental attachment as their common denominator. The condition has classically been defined pathologically, with chorionic villi attaching directly to the myometrium (“accreta”) or extending more deeply into the myometrium (“increta”) or attaching to surrounding tissues and structures (“percreta”).1 It is most commonly encountered in patients with low placental implantation on a prior cesarean section scar; indeed, placenta previa, particularly with a history of cesarean delivery, is the strongest risk factor for the development of PAS.2 In addition to abnormal placental attachment, these placental attachments are often hypervascular and can lead to catastrophic hemorrhage if not managed appropriately. For this reason, patients with sonographic or radiologic signs of PAS should be referred to specialized centers for further workup, counseling, and delivery planning.3
Although delivery at a specialized PAS center has been associated with improved patient outcomes,4 not all patients with PAS will be identified in the antepartum period. Ultrasonography may miss up to 40% to 50% of PAS cases, particularly when the sonologist has not been advised to look for the condition,5 and not all patients with PAS will have a previa implanted in a prior cesarean scar. A recent study found that these patients with nonprevia PAS were identified by imaging less than 40% of the time and were significantly less likely to be managed by a specialized team of clinicians.6 Thus, it falls upon every obstetric care provider to be aware of this diagnosis, promptly recognize its unanticipated presentations, and have a plan to optimize patient safety.
Step 1: Recognition
While PAS is classically defined as a pathologic condition, no clinician has the luxury of histology in the delivery room. Researchers have variously defined PAS clinically, with the common trait of abnormal placental adherence.7-9 The TABLE compares published definitions that have been used in the literature. While some definitions include hemorrhage, no clinician wants to induce significant hemorrhage to confirm their patient’s diagnosis. Thus, practically, the clinical PAS diagnosis comes down to abnormal placental attachment: If it is apparent that some or all of the placenta will not separate from the uterine wall with digital manipulation or careful curettage, then PAS should be suspected, and appropriate steps should be taken before further removal attempts.

At cesarean delivery, the PAS diagnosis may be aided by visual cues. With placenta previa, the lower uterine segment may bulge and take on a bluish hue, distinctly different from the upper healthy myometrium. PAS may also manifest with neovascularization, particularly behind the bladder. As with vaginal births, the placenta will fail to separate after the delivery, and controlled traction on the umbilical cord can produce a “dimple sign,” or visible myometrial retraction at the site of implantation (FIGURE 1). Finally, if the diagnosis is still in doubt, attempts to gently form a cleavage plane between the placenta and myometrium will be unsuccessful if PAS is present.8

Step 2: Initial management—pause, plan
Most importantly, do not attempt to forcibly remove the placenta. It can be left attached to the uterus until appropriate resources are secured. Efforts to forcibly remove an adherent placenta may well lead to major hemorrhage, and thus it falls on the patient’s care team to pause and plan for PAS care at this point. FIGURE 2 displays an algorithm for patient management. Further steps depend primarily on whether or not the patient is already hemorrhaging. In a stable situation, the patient should be counseled regarding the abnormal findings and the suspected PAS diagnosis. This includes the possibility of further procedures, blood transfusion, and hysterectomy. Local resources, including nursing, anesthesia, and the blood bank, should be notified about the situation and for the potential to call in specialized services. If on-site experienced specialists are not available, then patient transfer to a PAS specialty center should be strongly considered. While awaiting additional help or transport, the patient requires close monitoring for gross and physiologic signs of hemorrhage. If pursued, transport to a PAS specialty center should be expedited.

If the patient is already hemorrhaging or unstable, then appropriate local resources must be activated. At a minimum, this requires an obstetrician and anesthesiologist at the bedside and activation of hemorrhage protocols (eg, a massive transfusion protocol). If blood products are unavailable, consider whether they can be transported from other nearby blood banks, and start that process promptly. Next, contact backup services. Based on local resources and clinical severity, this may include maternal-fetal medicine specialists, pelvic surgeons, general and trauma surgeons, intensivists, interventional radiologists, and transfusion specialists. Even if the patient cannot be safely transferred to another hospital, the obstetrician can call an outside PAS specialist to discuss next steps in care and begin transfer plans, assuming the patient can be stabilized. Based on the Maternal Levels of Care definitions published by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine,10 patients with PAS should be managed at level III or level IV centers. However, delivery units at every level of maternal care should have a protocol for securing local help and reaching an appropriate consultant if a PAS case is encountered. Know which center in your area specializes in PAS so that when an unanticipated case arises, you know who to call.
Continue to: Step 3: Ultimate management—mobilize and prepare for bleeding...
Step 3: Ultimate management—mobilize and prepare for bleeding
If diagnosis occurs intraoperatively at a PAS specialty center, or if safe transport is not possible, then the team should mobilize for the possibility of hysterectomy and prepare for massive bleeding, which can occur regardless of the treatment chosen. Many patients require or will opt for hysterectomy. For example, a patient who has finished childbearing may consent to a hysterectomy upon hearing she likely has PAS. In patients with suspected PAS who are actively hemorrhaging or are unstable, hysterectomy is required.
Uterine conservation may be considered in stable patients who strongly desire future childbearing or uterine retention. This often requires leaving densely adherent placental tissue in situ and thus requires thorough counseling regarding the risks of delayed hemorrhage, infection, and emergent hysterectomy.11 This may not be desirable or safe for some patients, so informed consent is crucial. In such cases, we strongly recommend consultation with a PAS specialist, even if that requires immediate control of the placental blood supply (such as with arterial embolization), and transfer to a PAS specialty center.
Clinical scenarios
Vaginal delivery
The patient in the opening case was never expected to have PAS given her normal placental location and absence of a uterine scar. Even though she had some possible PAS risk factors (past retained placenta with instrumentation and in vitro fertilization), her absolute risk for the condition was low. Nevertheless, inability to create a separation plane should be considered PAS until proven otherwise. Although at this point many obstetricians would move to an operating room for uterine curettage, we recommend that the care team pause and put measures in place for possible PAS and hemorrhage. This involves notification of the blood bank, crossmatching of blood products, alerting the anesthesia team, and having a clear plan in place should a major hemorrhage ensue. This may involve use of balloon tamponade, activation of an interventional radiology team, or possible laparotomy with arterial ligations or hysterectomy. Avoidance of a prolonged third stage should be balanced against the need for preparation with these cases.
It is important for clinicians to bear in mind, and communicate to the patient, that hysterectomy is the standard of care for PAS. Significant delays in performing an indicated hysterectomy can lead to coagulopathy and patient instability. Timeliness is key; we find that delays in the decision to perform an indicated hysterectomy are often at the root of the cause for worsened morbidity in patients with unanticipated PAS. With an unscarred uterus and no placenta previa, a postpartum hysterectomy can be performed by many obstetrician-gynecologists experienced in this abdominal procedure.
Cesarean delivery
Undiagnosed PAS may present at cesarean delivery with or without placenta previa and a prior uterine scar. With this combination, PAS is often visually apparent upon opening the abdominal cavity (TABLE and FIGURE 1). Such surgical findings call for a clinical pause, as further actions at this point can lead to catastrophic hemorrhage. The obstetrician should consider a series of questions:
1. Are appropriate surgical and transfusion resources immediately available? If yes, they should be notified in case they are needed urgently. If not, then the obstetrician should ask whether the delivery must occur now.
2. Is this a scheduled delivery with a stable patient and fetus? If so, then closing the abdominal incision, monitoring the patient and fetus, and either transferring the patient to a PAS center or awaiting appropriate local specialists may be a lifesaving step.
3. Is immediate delivery required? If the fetus must be delivered, then it is imperative to create a hysterotomy out of the way of the placenta. Disrupting the adherent placenta with either an incision or manual manipulation may trigger a massive hemorrhage and should be avoided. This may require rectus muscle transection or creating a “T” incision on the skin to reach the uterine fundus and creating a hysterotomy over the top or even the back of the uterus. Once the fetus is delivered and lack of uterine hemorrhage confirmed (both abdominally and vaginally), the hysterotomy and abdomen can be closed with anticipation of urgent patient transfer to a PAS team or center.
4. Is the patient hemorrhaging? If the patient is hemorrhaging and closure is not an option, then recruitment of local emergent surgical teams is warranted, even if that requires packing the abdomen until an appropriate surgeon can arrive.
Diagnosis at cesarean delivery requires expedited and complex patient counseling. A patient who is unstable or hemorrhaging needs to be told that hysterectomy is lifesaving in this situation. For patients who are stable, it may be appropriate to close the abdomen and leave the placenta in situ, perform comprehensive counseling, and assess the possibility of transfer to a specialty center.
Summary
All obstetric care providers should be familiar with the clinical presentation of undiagnosed accreta spectrum. While hemorrhage is often part of the diagnosis, recognition of abnormal placental adherence and PAS-focused management should ideally be undertaken before this occurs. Once PAS is suspected, avoidance of further placental disruption may save significant morbidity, even if that means leaving the placenta attached until appropriate resources can be obtained. A local protocol for consultation, emergency transfer, and deployment of local resources should be part of every delivery unit’s emergency preparedness plan.
CASE Outcome
This patient is stabilized, with an adherent, retained placenta and no signs of hemorrhage. You administer uterotonics and notify your anesthesiologist and backup obstetrician that you have a likely case of accreta spectrum. A second intravenous line is placed, and blood products are crossmatched. The closest level III hospital is called, and they accept your patient for transfer. There, she is counseled about PAS, and she expresses no desire for future childbearing. After again confirming no placental separation in the operating room, the patient is moved immediately to perform laparotomy and total abdominal hysterectomy through a Pfannenstiel incision. She does not require a blood transfusion, and the pathology returns with grade I placenta accreta spectrum. ●
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
- American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta accreta spectrum. Obstet Gynecol. 2018; 132:e259-e275. doi:10.1097/AOG.0000000000002983.
- Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. 2018;61:733-742. doi:10.1097/GRF.0000000000000391.
- Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561-568. doi:10.1016/j.ajog.2014.11.018.
- Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218.e1-9. doi:10.1016/j.ajog.2014.08.019.
- Bowman ZS, Eller AG, Kennedy AM, et al. Accuracy of ultrasound for the prediction of placenta accreta. Am J Obstet Gynecol. 2014;211:177.e1-7. doi:10.1016/j.ajog.2014.03.029.
- Carusi DA, Fox KA, Lyell DJ, et al. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136:458-465. doi:10.1097/AOG.0000000000003970.
- Kayem G, Seco A, Beucher G, et al. Clinical profiles of placenta accreta spectrum: the PACCRETA population-based study. BJOG. 2021;128:1646-1655. doi:10.1111/1471-0528.16647.
- Jauniaux E, Ayres-de-Campos D, Langhoff-Roos J, et al. FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet. 2019;146:20-24. doi:10.1002/ijgo.12761.
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511-526. doi:10.1016/j.ajog.2019.02.054.
- American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus. No. 7: placenta accreta spectrum. Obstet Gynecol. 2018;132:e259-e275. doi: 10.1097/AOG.0000000000002983.
- Sentilhes L, Kayem G, Silver RM. Conservative management of placenta accreta spectrum. Clin Obstet Gynecol. 2018; 61(4):783-794. doi:10.1097/GRF.0000000000000395.
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
Socioeconomic disparities persist in hysterectomy access
Black women undergoing hysterectomies were significantly more likely to be treated by low-volume surgeons than high-volume surgeons, and to experience perioperative complications as a result, based on data from more than 300,000 patients.
“Outcomes for hysterectomy, for both benign and malignant disease, are improved when the procedure is performed at high-volume hospitals and by high-volume surgeons,” Anne Knisely, MD, of Columbia University, New York, and colleagues wrote.
Historically, Black patients have been less likely to be referred to high-volume hospitals, the researchers noted. Recent efforts to regionalize surgical procedures to high-volume hospitals aim to reduce disparities and improve care for all patients, but the data on disparities in care within high-volume hospitals are limited, they said.
In a study published in Obstetrics & Gynecology, the researchers identified 300,586 women who underwent hysterectomy in New York state between 2000 and 2014. The researchers divided surgeons at these hospitals into volume groups based on average annual hysterectomy volume.
The women were treated by 5,505 surgeons at 59 hospitals. Overall, Black women comprised significantly more of the patients treated by low-volume surgeons compared with high-volume surgeons (19.4% vs. 14.3%; adjusted odds ratio, 1.26), and more women treated by low-volume surgeons had Medicare insurance compared with those treated by high-volume surgeons (20.6% vs. 14.5%; aOR, 1.22).
A majority of the patients (262,005 patients) were treated by a total of 1,377 high-volume surgeons, while 2,105 low-volume surgeons treated 2,900 patients. Abdominal hysterectomies accounted for 57.5% of the procedures, followed by laparoscopic (23.9%), vaginal (13.2%), and robotic assisted (5.3%). Approximately two-thirds (64.4%) of the patients were aged 40-59 years; 63.7% were White, 15.1% were Black, and 8.5% were Hispanic.
The overall complication rate was significantly higher in patients treated by low-volume surgeons, compared with high-volume surgeons (31.0% vs. 10.3%), including intraoperative complications, surgical-site complications, medical complications, and transfusions. The perioperative mortality rate also was significantly higher for patients of low-volume surgeons compared with high-volume surgeons (2.2% vs. 0.2%).
Low-volume surgeons were more likely to perform urgent or emergent procedures, compared with high-volume surgeons (26.1% vs 6.4%), and to perform abdominal hysterectomy versus minimally invasive hysterectomy compared with high-volume surgeons (77.8% vs. 54.7%), the researchers added.
The study findings were limited by several factors, including the observational design and possible undercoding of outcomes, inclusion only of New York state patients, lack of data on clinical characteristics such as surgical history and complexity, lack of data on surgeon characteristics, and changing practice patterns over time, the researchers noted.
However, “this study demonstrates increased perioperative morbidity and mortality for patients who underwent hysterectomy by low-volume surgeons, in comparison with high-volume surgeons, at high-volume hospitals,” and that Black patients were more likely to be treated by low-volume surgeons, they said. “Although centralization of complex surgical care to higher-volume hospitals may have benefit, there are additional surgeon-level factors that must be considered to address disparities in access to high-quality care for patients undergoing hysterectomy.”
Explore range of issues to improve access
“It is always beneficial to review morbidity and mortality statistics,” Constance Bohon, MD, a gynecologist in private practice in Washington, D.C., said in an interview. “With a heightened awareness of equity and equality, now is a good time to review the data with that focus in mind. Hospital committees review the data on a regular basis, but they may not have looked closely at demographics in the past.
“It was always my understanding that for many procedures, including surgery, volume impacts outcome, so the finding that low-volume surgeons had worse outcomes than high-volume surgeons was not particularly surprising,” said Dr. Bohon. However, the question of how hospitals might address disparities in access to high-volume surgeons “is a difficult question, because there are a variety of issues that may not be caused by disparities,” she added. “It may be that the high-volume surgeons do not take Medicare. It may be that some of the emergent/urgent surgeries come from patients seen in the ED and the high-volume surgeons may not take call or see new patients in the ED. There may be a difference in the preop testing done that may be more extensive with the high-volume surgeons as compared with the low-volume surgeons. It may be that it is easier to get an appointment with a low-volume rather than a high-volume surgeon.
“Additional research is needed to determine whether there is an algorithm that can be created to determine risk for morbidity or mortality based on factors such as the number of years in practice, the number of hysterectomies per year, and the age of the physician,” Dr. Bohon explained. “The patient data could include preexisting risk factors such as weight, preexisting medical conditions, prior surgeries, and current medications, along with demographics. It would be interesting to determine whether low-risk patients have similar outcomes with low- as compared with high-volume surgeons while high-risk patients do not. The demographics could then be evaluated to determine if disparities exist for both low- and high-risk patients.”
The study received no outside funding. One coauthor disclosed serving as a consultant for Clovis Oncology, receiving research funding from Merck, and receiving royalties from UpToDate. Lead author Dr. Knisely had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose, but serves on the Ob.Gyn. News editorial advisory board.
Black women undergoing hysterectomies were significantly more likely to be treated by low-volume surgeons than high-volume surgeons, and to experience perioperative complications as a result, based on data from more than 300,000 patients.
“Outcomes for hysterectomy, for both benign and malignant disease, are improved when the procedure is performed at high-volume hospitals and by high-volume surgeons,” Anne Knisely, MD, of Columbia University, New York, and colleagues wrote.
Historically, Black patients have been less likely to be referred to high-volume hospitals, the researchers noted. Recent efforts to regionalize surgical procedures to high-volume hospitals aim to reduce disparities and improve care for all patients, but the data on disparities in care within high-volume hospitals are limited, they said.
In a study published in Obstetrics & Gynecology, the researchers identified 300,586 women who underwent hysterectomy in New York state between 2000 and 2014. The researchers divided surgeons at these hospitals into volume groups based on average annual hysterectomy volume.
The women were treated by 5,505 surgeons at 59 hospitals. Overall, Black women comprised significantly more of the patients treated by low-volume surgeons compared with high-volume surgeons (19.4% vs. 14.3%; adjusted odds ratio, 1.26), and more women treated by low-volume surgeons had Medicare insurance compared with those treated by high-volume surgeons (20.6% vs. 14.5%; aOR, 1.22).
A majority of the patients (262,005 patients) were treated by a total of 1,377 high-volume surgeons, while 2,105 low-volume surgeons treated 2,900 patients. Abdominal hysterectomies accounted for 57.5% of the procedures, followed by laparoscopic (23.9%), vaginal (13.2%), and robotic assisted (5.3%). Approximately two-thirds (64.4%) of the patients were aged 40-59 years; 63.7% were White, 15.1% were Black, and 8.5% were Hispanic.
The overall complication rate was significantly higher in patients treated by low-volume surgeons, compared with high-volume surgeons (31.0% vs. 10.3%), including intraoperative complications, surgical-site complications, medical complications, and transfusions. The perioperative mortality rate also was significantly higher for patients of low-volume surgeons compared with high-volume surgeons (2.2% vs. 0.2%).
Low-volume surgeons were more likely to perform urgent or emergent procedures, compared with high-volume surgeons (26.1% vs 6.4%), and to perform abdominal hysterectomy versus minimally invasive hysterectomy compared with high-volume surgeons (77.8% vs. 54.7%), the researchers added.
The study findings were limited by several factors, including the observational design and possible undercoding of outcomes, inclusion only of New York state patients, lack of data on clinical characteristics such as surgical history and complexity, lack of data on surgeon characteristics, and changing practice patterns over time, the researchers noted.
However, “this study demonstrates increased perioperative morbidity and mortality for patients who underwent hysterectomy by low-volume surgeons, in comparison with high-volume surgeons, at high-volume hospitals,” and that Black patients were more likely to be treated by low-volume surgeons, they said. “Although centralization of complex surgical care to higher-volume hospitals may have benefit, there are additional surgeon-level factors that must be considered to address disparities in access to high-quality care for patients undergoing hysterectomy.”
Explore range of issues to improve access
“It is always beneficial to review morbidity and mortality statistics,” Constance Bohon, MD, a gynecologist in private practice in Washington, D.C., said in an interview. “With a heightened awareness of equity and equality, now is a good time to review the data with that focus in mind. Hospital committees review the data on a regular basis, but they may not have looked closely at demographics in the past.
“It was always my understanding that for many procedures, including surgery, volume impacts outcome, so the finding that low-volume surgeons had worse outcomes than high-volume surgeons was not particularly surprising,” said Dr. Bohon. However, the question of how hospitals might address disparities in access to high-volume surgeons “is a difficult question, because there are a variety of issues that may not be caused by disparities,” she added. “It may be that the high-volume surgeons do not take Medicare. It may be that some of the emergent/urgent surgeries come from patients seen in the ED and the high-volume surgeons may not take call or see new patients in the ED. There may be a difference in the preop testing done that may be more extensive with the high-volume surgeons as compared with the low-volume surgeons. It may be that it is easier to get an appointment with a low-volume rather than a high-volume surgeon.
“Additional research is needed to determine whether there is an algorithm that can be created to determine risk for morbidity or mortality based on factors such as the number of years in practice, the number of hysterectomies per year, and the age of the physician,” Dr. Bohon explained. “The patient data could include preexisting risk factors such as weight, preexisting medical conditions, prior surgeries, and current medications, along with demographics. It would be interesting to determine whether low-risk patients have similar outcomes with low- as compared with high-volume surgeons while high-risk patients do not. The demographics could then be evaluated to determine if disparities exist for both low- and high-risk patients.”
The study received no outside funding. One coauthor disclosed serving as a consultant for Clovis Oncology, receiving research funding from Merck, and receiving royalties from UpToDate. Lead author Dr. Knisely had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose, but serves on the Ob.Gyn. News editorial advisory board.
Black women undergoing hysterectomies were significantly more likely to be treated by low-volume surgeons than high-volume surgeons, and to experience perioperative complications as a result, based on data from more than 300,000 patients.
“Outcomes for hysterectomy, for both benign and malignant disease, are improved when the procedure is performed at high-volume hospitals and by high-volume surgeons,” Anne Knisely, MD, of Columbia University, New York, and colleagues wrote.
Historically, Black patients have been less likely to be referred to high-volume hospitals, the researchers noted. Recent efforts to regionalize surgical procedures to high-volume hospitals aim to reduce disparities and improve care for all patients, but the data on disparities in care within high-volume hospitals are limited, they said.
In a study published in Obstetrics & Gynecology, the researchers identified 300,586 women who underwent hysterectomy in New York state between 2000 and 2014. The researchers divided surgeons at these hospitals into volume groups based on average annual hysterectomy volume.
The women were treated by 5,505 surgeons at 59 hospitals. Overall, Black women comprised significantly more of the patients treated by low-volume surgeons compared with high-volume surgeons (19.4% vs. 14.3%; adjusted odds ratio, 1.26), and more women treated by low-volume surgeons had Medicare insurance compared with those treated by high-volume surgeons (20.6% vs. 14.5%; aOR, 1.22).
A majority of the patients (262,005 patients) were treated by a total of 1,377 high-volume surgeons, while 2,105 low-volume surgeons treated 2,900 patients. Abdominal hysterectomies accounted for 57.5% of the procedures, followed by laparoscopic (23.9%), vaginal (13.2%), and robotic assisted (5.3%). Approximately two-thirds (64.4%) of the patients were aged 40-59 years; 63.7% were White, 15.1% were Black, and 8.5% were Hispanic.
The overall complication rate was significantly higher in patients treated by low-volume surgeons, compared with high-volume surgeons (31.0% vs. 10.3%), including intraoperative complications, surgical-site complications, medical complications, and transfusions. The perioperative mortality rate also was significantly higher for patients of low-volume surgeons compared with high-volume surgeons (2.2% vs. 0.2%).
Low-volume surgeons were more likely to perform urgent or emergent procedures, compared with high-volume surgeons (26.1% vs 6.4%), and to perform abdominal hysterectomy versus minimally invasive hysterectomy compared with high-volume surgeons (77.8% vs. 54.7%), the researchers added.
The study findings were limited by several factors, including the observational design and possible undercoding of outcomes, inclusion only of New York state patients, lack of data on clinical characteristics such as surgical history and complexity, lack of data on surgeon characteristics, and changing practice patterns over time, the researchers noted.
However, “this study demonstrates increased perioperative morbidity and mortality for patients who underwent hysterectomy by low-volume surgeons, in comparison with high-volume surgeons, at high-volume hospitals,” and that Black patients were more likely to be treated by low-volume surgeons, they said. “Although centralization of complex surgical care to higher-volume hospitals may have benefit, there are additional surgeon-level factors that must be considered to address disparities in access to high-quality care for patients undergoing hysterectomy.”
Explore range of issues to improve access
“It is always beneficial to review morbidity and mortality statistics,” Constance Bohon, MD, a gynecologist in private practice in Washington, D.C., said in an interview. “With a heightened awareness of equity and equality, now is a good time to review the data with that focus in mind. Hospital committees review the data on a regular basis, but they may not have looked closely at demographics in the past.
“It was always my understanding that for many procedures, including surgery, volume impacts outcome, so the finding that low-volume surgeons had worse outcomes than high-volume surgeons was not particularly surprising,” said Dr. Bohon. However, the question of how hospitals might address disparities in access to high-volume surgeons “is a difficult question, because there are a variety of issues that may not be caused by disparities,” she added. “It may be that the high-volume surgeons do not take Medicare. It may be that some of the emergent/urgent surgeries come from patients seen in the ED and the high-volume surgeons may not take call or see new patients in the ED. There may be a difference in the preop testing done that may be more extensive with the high-volume surgeons as compared with the low-volume surgeons. It may be that it is easier to get an appointment with a low-volume rather than a high-volume surgeon.
“Additional research is needed to determine whether there is an algorithm that can be created to determine risk for morbidity or mortality based on factors such as the number of years in practice, the number of hysterectomies per year, and the age of the physician,” Dr. Bohon explained. “The patient data could include preexisting risk factors such as weight, preexisting medical conditions, prior surgeries, and current medications, along with demographics. It would be interesting to determine whether low-risk patients have similar outcomes with low- as compared with high-volume surgeons while high-risk patients do not. The demographics could then be evaluated to determine if disparities exist for both low- and high-risk patients.”
The study received no outside funding. One coauthor disclosed serving as a consultant for Clovis Oncology, receiving research funding from Merck, and receiving royalties from UpToDate. Lead author Dr. Knisely had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose, but serves on the Ob.Gyn. News editorial advisory board.
FROM OBSTETRICS & GYNECOLOGY
Closing the racial gap in minimally invasive gyn hysterectomy and myomectomy
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
Can a once-daily oral formulation treat symptoms of uterine fibroids without causing hot flashes or bone loss?
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Optimize your treatment of endometriosis by using an FDA-approved hormonal medication
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.