User login
2019 Update on minimally invasive gynecologic surgery
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).
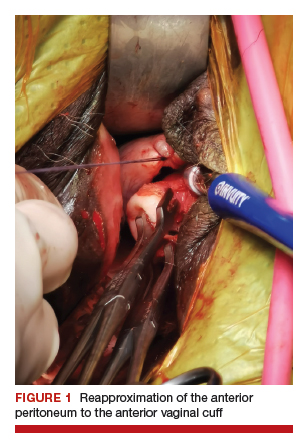
2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
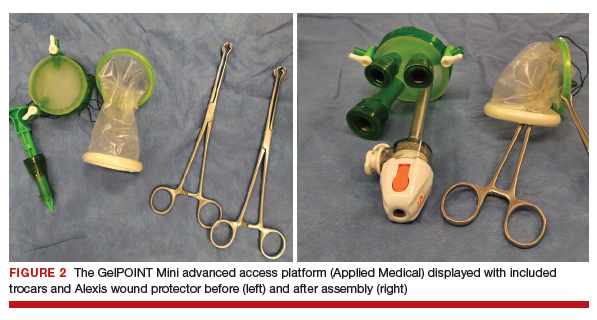
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).

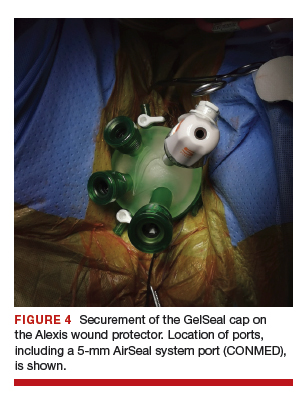
After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).
Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
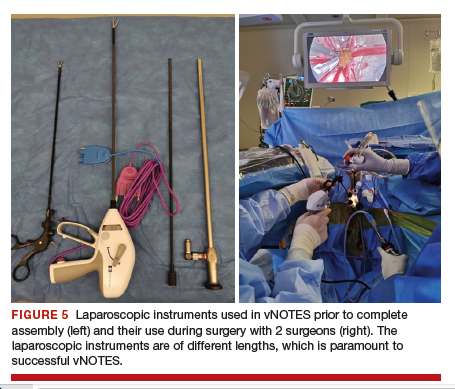
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).
vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
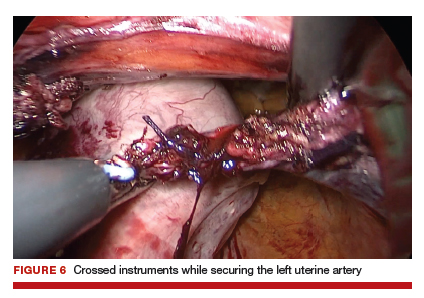
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).
This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
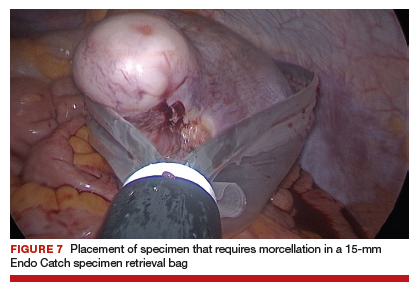
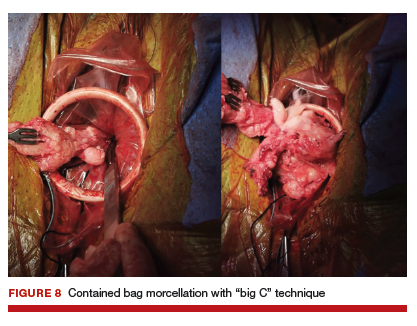
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.
Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).

2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).


After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).

Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).

vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).

This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.


Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).

2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).


After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).

Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).

vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).

This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.


Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
Hysterectomy in patients with history of prior cesarean delivery: A reverse dissection technique for vesicouterine adhesions
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
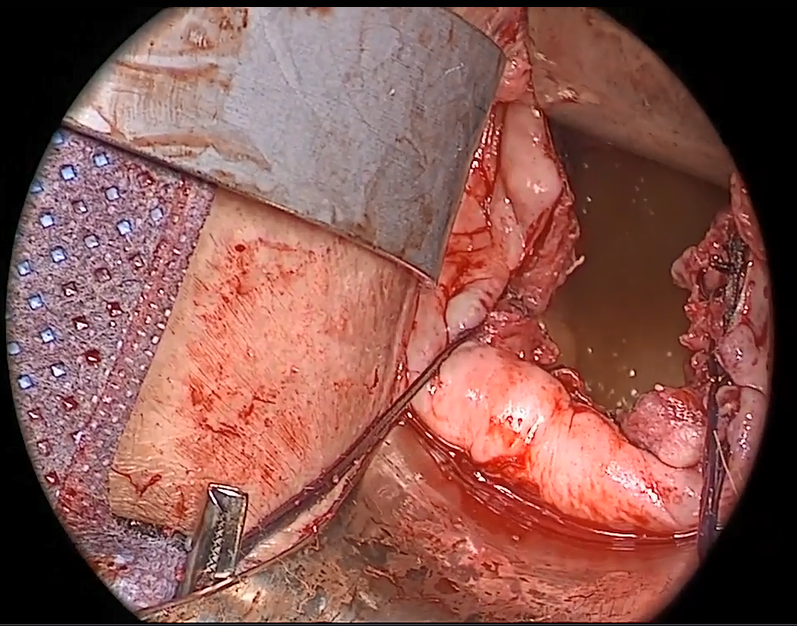
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).
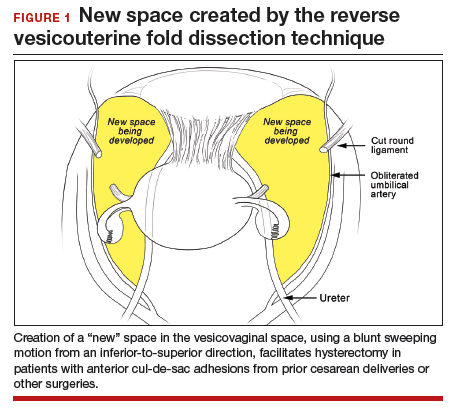
Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).
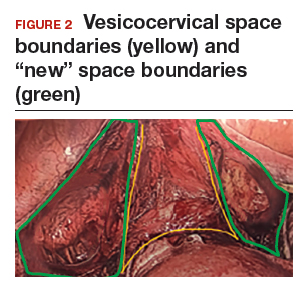
Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).
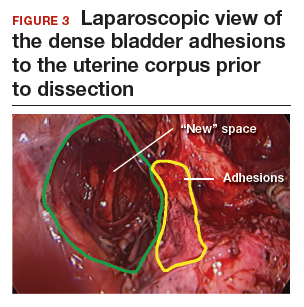
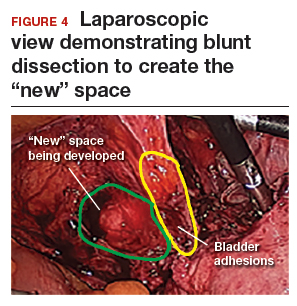
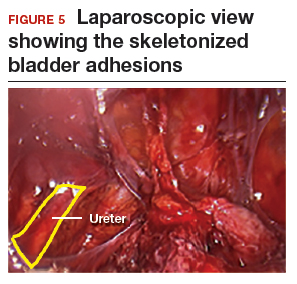
Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).

Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).

Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).



Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).

Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).

Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).



Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
Medical management of abnormal uterine bleeding in reproductive-age women
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).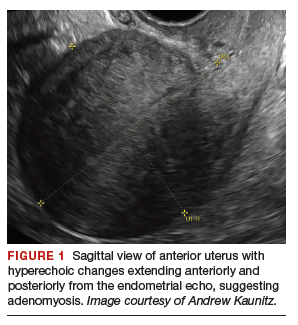
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).
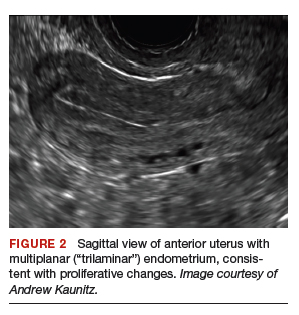

After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6
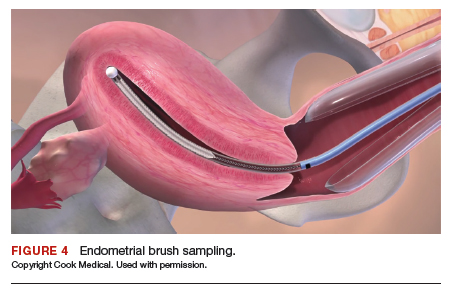
Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.
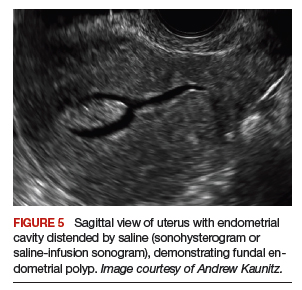
Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16
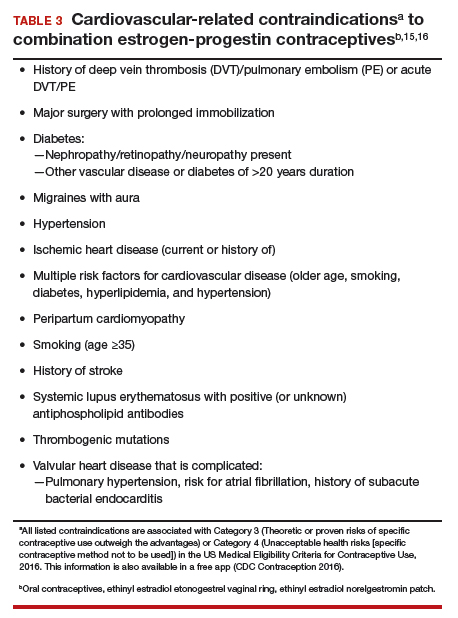
Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Trial of mesh vs. hysterectomy for prolapse yields inconclusive results
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
FROM JAMA
Native tissue repair of POP: Surgical techniques to improve outcomes
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.
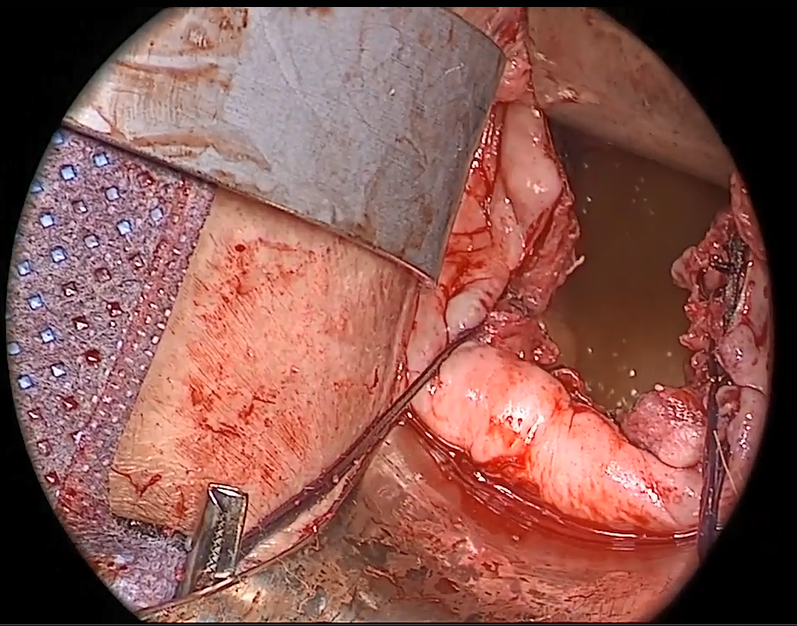
Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.
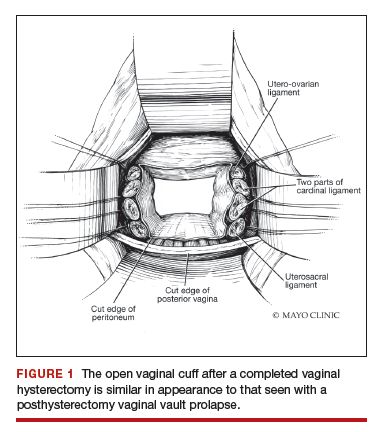
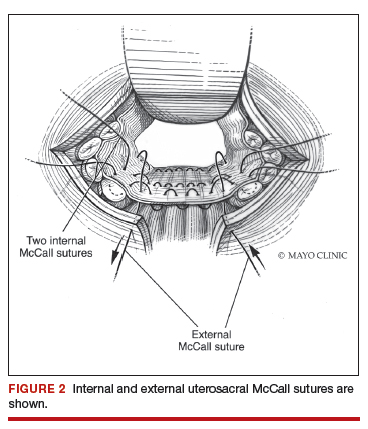
Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.
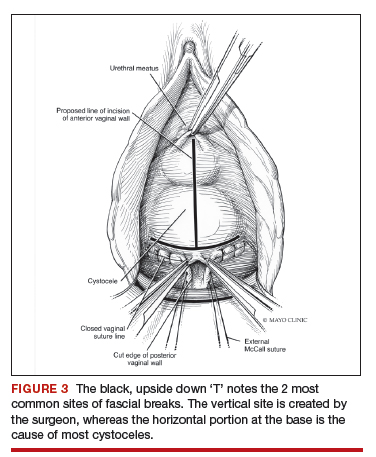
Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.
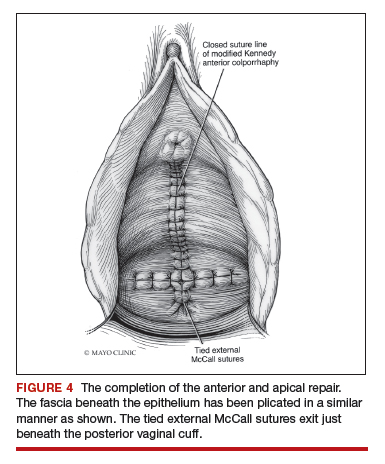
Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.
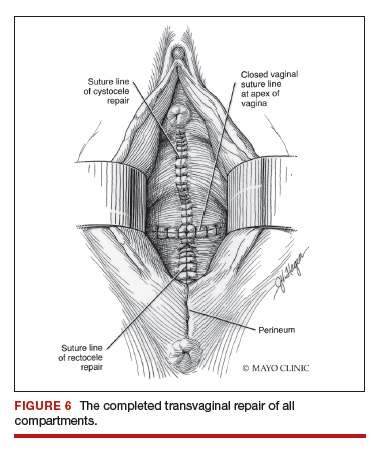
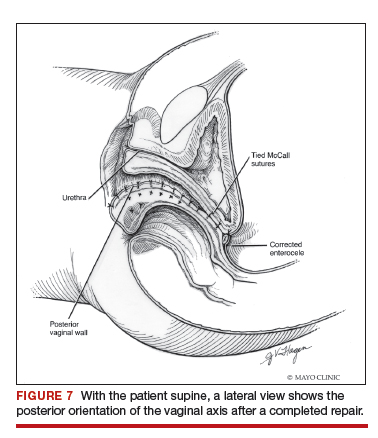
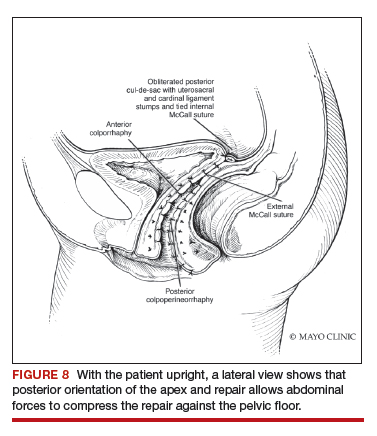
Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.

Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
“Take pride in your surgical work. Do it in such a way that you would be willing to sign your name to it…the operation was performed by me.”
—Raymond A. Lee, MD
The US Food and Drug Administration (FDA) recently ordered companies to cease selling transvaginal mesh intended for pelvic organ prolapse (POP) repair (but not for the treatment of stress urinary incontinence [SUI] or for abdominal sacrocolpopexy).1,2 The FDA is also requiring companies preparing premarket approval applications for mesh products for the treatment of transvaginal POP to continue safety and efficacy follow-up in existing section 522 postmarket surveillance studies.3
It is, therefore, incumbent upon gynecologic surgeons to understand the surgical options that remain and perfect their surgical approach to POP to optimize patient outcomes. POP may be performed transvaginally or transabdominally, with each approach offering its own set of risks and benefits. The ability to perform both effectively allows the surgeon to tailor the approach to the condition and circumstances encountered. It is also important to realize that “cures” are elusive in POP surgery. While we can frequently alleviate patient symptoms and improve quality of life, a lifelong “cure” is an unrealistic goal for most prolapse procedures.
This article focuses on transvaginal native tissue repair,4 specifically the Mayo approach.

Watch video here
Vaginal surgery fundamentals
Before we explore the details of the Mayo technique, let’s review some basic principles of vaginal surgery. First, it is important to make a good clinical diagnosis so that you know which compartments (apex, anterior, or posterior) are involved. Although single compartment defects exist, multicompartment defects are far more common. Failing to recognize all compartment defects often results in incomplete repair, which can mean recurrent prolapse and additional interventions.
Second, exposure is critical when performing surgery by any route. You must be able to see your surgical field completely in order to properly execute your surgical approach. Table height, lighting, and retraction are all important to surgical success.
Lastly, it is important to know how to effectively execute your intended procedure. Native tissue repair is often criticized for having a high failure rate. It makes sense that mesh augmentation offers greater durability of a repair, but an effective native tissue repair will also effectively treat the majority of patients. An ineffective repair does not benefit the patient and contributes to high failure rates.
- Mesh slings for urinary incontinence and mesh use in sacrocolpopexy have not been banned by the FDA.
- Apical support is helpful to all other compartment support.
- Fixing the fascial defect between the base of the bladder and the apex will improve your anterior compartment outcomes.
- Monitor vaginal caliber throughout your posterior compartment repair.
Vaginal apex repairs
Data from the OPTIMAL trial suggest that uterosacral ligament suspension and sacrospinous ligament fixation are equally effective in treating apical prolapse.5 Our preference is a McCall culdoplasty (uterosacral ligament plication). It allows direct visualization (internally or externally) to place apical support stitches and plicates the ligaments in the midline of the vaginal cuff to help prevent enterocele protrusion. DeLancey has described the levels of support in the female pelvis and places importance on apical support.6 Keep in mind that anterior and posterior compartment prolapse is often accompanied by apical prolapse. Therefore, treating the apex is critical for overall success.
External vs internal McCall sutures: My technique. Envision the open vaginal cuff after completing a vaginal hysterectomy or after opening the vaginal cuff for a posthysterectomy vaginal vault prolapse (FIGURE 1). External (suture placed through the vaginal cuff epithelium into the peritoneal cavity, incorporating the uterosacral ligaments and intervening peritoneum, and ultimately brought back out through the posterior cuff and tied) or internal (suture placed in the intraperitoneal space, incorporating the uterosacral ligaments and intervening peritoneum, and tied internally) McCall sutures can be utilized (FIGURE 2). I prefer a combination of both. I use 0-polyglactin for external sutures, as the sutures will ultimately dissolve and not remain in the vaginal cavity. I usually place at least 2 external sutures with the lowest suture on the vaginal cuff being the deepest uterosacral stitch. Each subsequent suture is placed closer to the vaginal cuff and closer to the ends of the ligamentous stumps, starting deepest and working back toward the cuff with each stitch. I place 1 or 2 internal sutures (delayed absorbable or permanent) between my 2 external sutures. Because these sutures will be tied internally and located in the intraperitoneal space, permanent sutures may be used.


Avoiding ureteral injury: Tips for cystoscopy. A known risk of performing uterosacral ligament stitches is kinking or injury to the ureter. Therefore, cystoscopy is mandatory when performing this procedure. I tie one suture at a time starting with the internal sutures. I then perform cystoscopy after each suture tying. If I do not get ureteral spill after tying the suture, I remove and replace the suture and repeat cystoscopy until normal bilateral ureteral spill is achieved.
Key points for uterosacral ligament suspension. Achieving apical support at this point gives me the ability to build my anterior and posterior repair procedures off of this support. It is critical when performing uterosacral ligament suspension that you define the space between the ureter and rectum on each side. (Elevation of the cardinal pedicle and medial retraction of the rectum facilitate this.) The ligament runs down toward the sacrum when the patient is supine. You must follow that trajectory to be successful and avoid injury. One must also be careful not to be too deep on the ligament, as plication at that level may cause defecatory dysfunction.
Continue to: Anterior compartment repairs...
Anterior compartment repairs
The anterior compartment seems the most susceptible to forces within the pelvis and is a common site of prolapse. Many theories exist as to what causes a cystocele—distension, displacement, detachment, etc. While paravaginal defects exist, I believe that most cystoceles arise horizontally at the base of the bladder as the anterior endopelvic fascia detaches from the apex or cervix. The tissue then attenuates as the hernia progresses.
For surgical success: Make certain your repair addresses re-establishing continuity of the anterior endopelvic fascia with the fascia and ligaments at the vaginal apex; it will increase your success in treating anterior compartment prolapse.
We prefer to mobilize the epithelium in the midline from the vaginal apex to the mid‑urethra (if performing a midurethral sling, we stop short of the bladder neck and perform a separate suburethral incision). When incising the epithelium in the midline, the underlying fascia is also split in the midline, creating a midline defect. Once the epithelium is split and mobilized laterally off the underlying fascia, we can begin reconstruction.
The midline fascial defect that was just created is closed with a running 2-0 polyglactin from just beneath the bladder neck down to and including the fascia and uterosacral ligaments at the apex. This is accomplished in an upside down ‘T’ orientation (FIGURE 3). It is critical that the fascia is reunited at the base or you will leave the patient with a hernia.
For surgical success: To check intraoperatively that the fascia is reunited at the base, try to place an index finger between the base of the cystocele repair and the apex. If you can insert your finger, that is where the hernia still exists. If you meet resistance with your finger, you are palpating reunification of the anterior and apical fascia.

Technique for Kelly-Kennedy bladder neck plication. If the patient has mild incontinence that does not require a sling procedure, we now complete the second portion of the anterior repair starting with a Kelly-Kennedy bladder neck plication. Utilizing interrupted 1-0 polyglactin suture, vertical bites are taken periurethrally, starting at the midurethra and then the bladder neck. This nicely supports the urethra and proximal bladder neck and is very helpful for mild incontinence or for prophylactic benefit. Then starting beneath the bladder neck, the fascia is plicated again in the midline, reinforcing the suture line of the inverse ‘T’ with 2-0 polyglactin. The redundant epithelium is trimmed and reapproximated with interrupted 2-0 polyglactin (FIGURE 4). We tend to be more aggressive by adding the Kelly-Kennedy plication, which can lead to temporary voiding delay. We offer placement of a suprapubic catheter at the time of surgery or self-intermittent catherization.
Lastly, given that we have just dissected and then plicated the tissues beneath the bladder, I like to perform cystoscopy to be certain the bladder has not been violated. It is also important not to over-plicate the anterior fascia so that the sutures shear through the fascia and weaken the support or narrow the vaginal lumen.

Continue to: Posterior compartment repairs...
Posterior compartment repairs
Like with the anterior compartment, opinions differ as to the site of posterior compartment prolapse. Midline, lateral, distal, and site-specific defects and surgical approaches have been described. Research suggests that there is no benefit to the use of mesh in the posterior compartment.7 It is very important to recognize that over-plication of the posterior compartment can lead to narrowing/stricture and dyspareunia. Therefore, monitor vaginal caliber throughout repair of the posterior compartment.
Although we believe that a midline defect in the endopelvic fascia is primarily responsible for rectoceles, we also appreciate that the fascia must be reconstructed all the way to the perineal body and that narrowing the genital hiatus is very important and often underappreciated (FIGURE 5). Thus, perineal reconstruction is universally performed. I will emphasize again that reconstruction must be performed while also monitoring vaginal caliber. If it is too tight with the patient under anesthesia, it will be too tight when the patient recovers. Avoidance is the best option. If the patient does not desire a functional vagina (eg, an elderly patient), then narrowing is a desired goal.

Perineal reconstruction technique and tips for success
A retractor at 12 o’clock to support the apex and anterior wall can be helpful for visualization in the posterior compartment. We start with a v-shaped incision on the perineum. The width is determined by how much you want to build up the perineum and narrow the vagina (the wider the incision, the more building up of the perineal body and vaginal narrowing). A strip of epithelium is then mobilized in the midline (be careful not to excise too much). This dissection is carried all the way up the midline to just short of the tied apical suspension sutures at the posterior vaginal apex. The posterior dissection tends to be the most vascular in my experience.
Utilize cautery to obtain hemostasis along your dissection margins while protecting the underlying rectum. We have not found it necessary to dissect the posterior epithelium off the underlying fascia (that is an option at this point, however, if you feel more comfortable doing this). With an index finger in the vagina, compressing the rectum posteriorly, interrupted 1-0 polyglactin suture is placed through the epithelium and underlying fascia (avoiding the rectum) on one side, then the other, and then tied. The next sutures are placed utilizing the same technique, and the caliber of the vagina is noted with the placement of each suture (if it is too tight, then remove and replace the suture and recheck). It is important to realize you want to plicate the fascia in the midline and not perform an aggressive levatorplasty that could lead to muscle pain. Additionally, each suture should get the same purchase of tissue on each side, and the spacing of each suture should be uniform, like rungs on a ladder. Ultimately, the repair is carried down to the hymenal ring. At this point, the perineal reconstruction is performed, plicating the perineal body in the midline with deeper horizontal sutures and then closing the perineal skin with interrupted or subcuticular sutures (FIGURE 6). Completion of these repairs should orient the vagina toward the hollow of the sacrum (FIGURE 7), allowing downward forces to compress the vaginal supports posteriorly onto the pelvic floor instead of forcing it out the vaginal lumen (FIGURE 8).
Our patients generally stay in the hospital overnight, and we place a vaginal pack to provide topical pressure throughout the vagina overnight. We tell patients no lifting more than 15 lb and no intercourse for 6 weeks. While we do not tend to use hydrodissection in our repairs, it is a perfectly acceptable option.



Continue to: Commit to knowledge of native tissue techniques...
Commit to knowledge of native tissue techniques
Given the recent FDA ban on the sale of transvaginal mesh for POP and the public’s negative perception of mesh (based often on misleading information in the media), it is incumbent upon gynecologic surgeons to invest in learning or relearning effective native tissue techniques for the transvaginal treatment of POP. While not perfect, they offer an effective nonmesh treatment option for many of our patients.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
- US Food and Drug Administration. FDA takes action to protect women’s health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. . Published April 16, 2019. Accessed August 6, 2019.
- US Food and Drug Administration. Urogynecological surgical mesh implants. . Published July 10, 2019. Accessed August 5, 2019.
- US Food and Drug Administration. Effective date of requirement for premarket approval for surgical mesh for transvaginal pelvic organ prolapse repair. https://www.federalregister.gov/documents/2016/01/05/2015-33163/effective-date-of-requirement-for-premarket-approval-for-surgical-mesh-for-transvaginal-pelvic-organ. Published January 5, 2016. Accessed August 5, 2019.
- Lee RA. Atlas of Gynecologic Surgery. W.B. Saunders: Philadelphia, PA; 1992.
- Jelovsek JE, Barber MD, Brubaker L, et al. Effect of uterosacral ligament suspension vs sacrospinous ligament fixation with or without perioperative behavioral therapy for pelvic organ vaginal prolapse on surgical outcomes and prolapse symptoms at 5 years in the OPTIMAL randomized clinical trial. JAMA. 2018;319:1554-1565.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 part 1):1717-1728.
- Paraiso MF, Barber MD, Muir TW, et al. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006;195:1762- 1771.
Minimally invasive surgery for cervical cancer: Is surgeon volume a factor?
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
The role of minimally invasive surgery for early-stage cervical cancer has been the subject of heated debate since the presentation of the results of the Laparoscopic Approach to Cervical Cancer (LACC) Trial at the Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in 2018. This was an international, randomized, phase 3 trial comparing minimally invasive radical hysterectomy (MH) to open radical hysterectomy (OH) in the treatment of early-stage cervical cancer. The trial was closed early by the study’s Data and Safety Monitoring Committee due to an imbalance of deaths between the groups, with a higher rate in the minimally invasive arm. The final results, which were largely unexpected by the medical community, showed that the disease-free survival (DFS) at 4.5 years was 86.0% in the MH arm and 96.5% in the OH arm, which was a larger difference than their noninferiority cutoff of -7.2 percentage points.1 Results of an epidemiologic study, which used data from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database, also were presented at this meeting, and they reinforced the findings of the LACC trial.2
The combined results have caused significant concern and confusion from the medical community regarding the clinical implication that minimally invasive surgery may be an unacceptable approach for radical hysterectomy in cervical cancer. Prior to this study, retrospective data supported similar outcomes between the two approaches.3 Additionally, robotic surgery has made radical hysterectomy an option for those with a higher body mass index, as an open radical hysterectomy can be technically challenging in larger patients and result in a higher rate of adverse outcomes.
LACC trial questioned by US surgeons
Many in the United States have questioned the design and conclusions of the LACC trial. This trial was conducted primarily outside of North America and utilized conventional laparoscopic surgery 85% of the time as opposed to robotic surgery. Additionally, the found difference in DFS between MH and OH may have been driven more by the superior performance of the OH group (compared with historical data) than the poorly performing MH group.4 Other criticisms have touched on the low number of overall survival events, the low bar for surgeon volume or skill assessment, and the inability to make conclusions regarding “low-risk” lesions (<2 cm, no lymphovascular space invasion, <1 cm depth of invasion).
Were requirements for surgical skill adequate? Regarding surgeon skill, the LACC trial required documentation of the perioperative outcomes from 10 laparoscopic or robotic radical hysterectomies, as well as 2 unedited videos of each surgeon participating in the study to verify their technique, which some have considered inadequate to sufficiently vet a surgeon’s ability. Additionally, 14 of the 33 centers enrolled in the study accrued 71% of the patients, and concerns about the surgeon volume of the remaining 19 centers have been raised. Finally, there has been discussion about whether the variance in surgical approach can even be adequately assessed in a trial of this nature, as surgical skill is not a binary variable that is easily amenable to randomization. Unlike other trials, which have clear exposure and control arms, no 2 surgeries are exactly alike, and surgical technique is highly variable between surgeons, institutions, and countries.
Continue to: New data evaluate for surgeon volume
New data evaluate for surgeon volume
In an effort to address the concerns regarding surgical approach and expertise, the recently published study by Cusimano and colleagues uses population-based data from Ontario for all women undergoing radical hysterectomy for cervical cancer over a 10-year period from 2006 through 2016.5 The primary outcome was all-cause death, but the study also sought to address whether surgeon volume has an impact on recurrence rates for patients undergoing MH versus OH. To measure this impact the authors stratified surgeon characteristics by technique-specific volume and cervical cancer volume, splitting these volumes at the 50% percentile for low- and high-volume surgeons. They defined technique-specific volume as the number of simple and radical hysterectomies performed in the prior year using the selected approach (MH or OH). Cervical cancer volume was calculated as the number of hysterectomies of any type for cervical cancer in the previous 2 years. The technique-specific volume variable was subsequently re-categorized into tertiles, examined as a continuous variable, and analyzed at the 50th percentile for each year of the study.
Death and recurrence rates better in the OH group. The final cohort included 958 women that were relatively evenly split between MH and OH procedures. Results from their analysis show no difference in terms of all-cause death, cervical cancer–specific death, or recurrence. However, all 3 of these parameters were significantly different in favor of the OH group in women with Stage IB disease, which comprised over half of the overall cohort. Importantly, neither technique-specific volume nor cervical cancer volume had an effect on death or recurrence in Stage IB patients in any of the investigators’ analyses.
Important limitations. There are several limitations to this study that have to be taken into account before drawing any conclusions. Pathologic data were obtained from the database and did not include some important details about the tumor specimens (including specifying subgroups of Stage IA and IB disease, tumor size, presence of lymphovascular space invasion, and depth of stromal invasion). All of these details have been shown to be important prognostic variables in early-stage cervical cancer. Additionally, the MH group included a predominantly laparoscopic approach with only 10% of cases performed robotically, which again brings into question the generalizability of the data.
However, despite some of these shortcomings, the study authors do make a compelling argument that surgeon volume alone does not seem to play a significant role in cancer outcomes after MH.
With surgical approaches hard to compare, turn to careful patient counseling
Definitive assessment of the impact of surgical skill and experience on cervical cancer outcomes is probably an impossible task, as even a perfectly designed trial cannot entirely account for the intricacies of a complex surgical procedure. Variations in tumor characteristics and patient anatomy that affect operative decision making are not likely to be reflected when a patient’s outcome is plugged into a database. As a result, some surgeons and departments have turned to reporting personal or institutional recurrence rates for MH, which they believe may b
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Wang Y, Deng L, Cao L, et al. The outcome of laparoscopy versus laparotomy for the management of early stage cervical cancer-meta analysis. J Minim Invasive Gynecol. 2015;22:S4-S5.
- Leitao MM Jr. The LACC Trial: has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248-1250.
- Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. July 6, 2019. doi:10.1016/j.ajog.2019.07.009.
Vaginal approach is the most cost-effective route
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
Vaginal approach is the most cost-effective route
I applaud Drs. Kotha and Sanfilippo for addressing the “elephant in the room.” At the hospitals where I work, surgeons pay little or no attention to the cost of the disposables and operating room time used for their laparoscopic procedures. I believe the authors were remiss, though, to not at least mention the most minimally invasive approach to hysterectomy—the vaginal approach—which is by far the most cost-effective and safest route.
Thomas Powers, MD
Arcadia, California
Drs. Kotha and Sanfilippo respond
We appreciate Dr. Powers’ comments regarding our article on cost-conscious choices for minimally invasive gynecologic surgery. Indeed, he provides an important point. As the article’s overall focus, however, was on minimally invasive gynecologic surgery, we did not include a comparison with either abdominal or vaginal hysterectomy. Of interest, Warren and colleagues conducted a retrospective analysis of claims data in which expenditures for minimally invasive procedures (MIP) were compared with those of vaginal hysterectomy.1 For 15,404 patients who underwent surgery, costs were compared between MIP and abdominal as well as vaginal hysterectomy. Costs were as follows:
- abdominal hysterectomy, $12,086
- MIP, $10,868
- vaginal hysterectomy, $9,544.
Vaginal hysterectomy cost was statistically significantly less (P<.05) compared with other methods. The authors concluded that the laparoscopic approach should be considered when the option of an abdominal versus laparoscopic procedure is entertained. For the gynecologic surgeon considering a laparoscopic approach, the information we provided in our article merits strong consideration.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
- Warren L, Ladapo JA, Borah BJ, et al. Open abdominal versus laparoscopic and vaginal hysterectomy: analysis of a large United States payer measuring quality and cost of care. J Minim Invasive Gynecol. 2009;16:581-588.
Woman loses hands and feet after cystectomy
Woman loses hands and feet after cystectomy: $109M award
On November 1, a 45-year-old woman underwent laparoscopic excision of a benign ovarian cyst performed by a minimally invasive gynecologic (MIG) surgeon. After surgery, the patient’s blood pressure (BP) declined. She was given fluids, but her BP remained low. The next day, she became incoherent and her BP could not be stabilized. Twenty-seven hours after surgery, the 5-cm umbilical incision opened while the patient was attempting to stand up from the commode. A large amount of bloody discharge drained.
At 11:00
At 4:30
The patient remained unconscious from the time of the exploratory operation until the end of January. She required additional surgeries to control the bacteria as well as amputation of both hands above the wrists and both feet above the ankles due to gangrene. Because she no longer had an abdominal wall, a skin sac was created to hold her intestines outside of her body. When a fistula developed, a colostomy was performed.
She went to a Maryland hospital for rehab, where she learned to walk with prosthetic feet and to use her prosthetic hands. Currently, she has constant abdominal pain, can walk a short distance, and uses a wheelchair. She requires 24/7 assistance for everyday tasks. She can no longer work and is on disability.
PATIENT’S CLAIM: The patient sued the university health system that employed the MIG surgeon. During the cystectomy, he almost completely transected her small intestine, but did not find the injury during surgery. This allowed bacteria to enter the abdominal cavity, causing sepsis and necrotizing fasciitis. The trauma surgeon referred to the injury as an enterotomy, not a tear.
During the procedure, the surgeon used ADEPT, a solution to prevent the formation of adhesions. The patient’s ObGyn expert concluded that ADEPT created an environment that allowed the necrotizing fasciitis to flourish.
The ICU physicians concluded that the patient was stable enough to be transported for a CT scan, but the surgeon repeatedly delayed the procedure and did not call for a surgical consult until 12 hours later. Had the CT scan or exploratory surgery occurred earlier, the diagnosis would have been discovered, and the bacteria would have been prevented from spreading. She would not have required extensive doses of vasopressors, which increase BP by cutting off blood circulation to the 4 extremities. In this case, use of vasopressors led to gangrene and the subsequent amputations.
Continue to: DEFENDANTS’ DEFENSE...
DEFENDANTS’ DEFENSE: The defendants denied all allegations. The expert witness for the defense opined that the surgeon had only nicked the intestine and that the main injury was a tear that had occurred on its own. The defense also claimed that the surgeon did not call for a CT scan because it would not have shown the source of the patient’s condition.
VERDICT: After 2 trials ended with hung juries, a $109 million Florida verdict was returned against the university health system. Under Florida’s sovereign immunity statute, the patient must seek recovery of all but $100,000 of the award through the Florida legislature in a separate claims bill.
Child has hypoxic brain injury: $7.75M settlement
At 41 weeks’ gestation, a mother presented to the emergency department (ED) for delivery after an unremarkable pregnancy. During the last 90 minutes of labor, fetal heart-rate (FHR) monitoring showed nonreassuring findings. After a vaginal delivery, the infant was found to have a hypoxic brain injury.
PARENT’S CLAIM: Even though nonreassuring FHR monitoring findings occurred, the physicians did not offer cesarean delivery (CD). The pediatrician and ED physician were negligent in failing to provide proper neonatal resuscitation and in recognizing a problem with the infants’ intubation. The delay in delivery and poor resuscitation procedure caused the child’s injury.
DEFENDANTS’ DEFENSE: All allegations were denied. There was no deviation from the standard of care.
VERDICT: A $7.75 million Massachusetts settlement was reached.
Kidney failed after hysterectomy
A 46-year-old woman underwent a hysterectomy performed by her ObGyn. Surgery went well but the patient continued to report symptoms. A year later, she underwent an oophorectomy. Two years later, the patient reported blood in her urine and underwent a computed tomography scan, which revealed an obstructed left ureter that had caused injury to the left kidney. Seven months later, the kidney was removed.
PATIENT’S CLAIM: Her kidney loss was a direct result of the ObGyn’s initial surgical procedure. He had placed several clips near the ureter and did not verify their position or protect the ureter. He also failed to address her reported symptoms in a timely manner.
PHYSICIAN’S DEFENSE: The damage to the ureter is a known risk of hysterectomy and oophorectomy. The obstruction developed over time, not as an immediate result of the surgery.
VERDICT: A Kentucky defense verdict was returned.
History of shoulder dystocia, Erb's palsy: $1.2M settlement
An obese mother was admitted to the hospital at 39 weeks’ gestation with signs of labor. She requested a CD and was advised that she had progressed too far for that to be an option, and that vaginal delivery would be safe. During the second stage of labor, shoulder dystocia was encountered. The ObGyn made several attempts to deliver using downward traction, but was unsuccessful. A second ObGyn swept the shoulder with an internal maneuver of his hand and delivered the baby. The child has a severe brachial plexus injury at multiple spinal levels resulting in Erb’s palsy.
PARENT’S CLAIM: A CD should have been performed. The first ObGyn failed to provide a CD and repeatedly applied excessive downward traction, causing the infant’s injury.
Continue to: PHYSICIAN’S DEFENSE...
PHYSICIAN’S DEFENSE: Shoulder dystocia is unpredictable and an unpreventable obstetric emergency. The ObGyn used proper maneuvers to release the shoulder dystocia.
VERDICT: A $1.2 million Virginia settlement was reached.
Ureter injured during hysterectomy
When a patient was found to have multiple, symptomatic fibroids and an enlarged uterus, her gynecologist suggested a total laparoscopic hysterectomy. During the procedure, when he inspected the pelvis and found multiple fibroids in and around the uterus, the gynecologist converted to a supracervical hysterectomy. Surgery was difficult because of a large myoma on the right broad ligament.
The patient tolerated surgery well and was released home the next day. At follow-up one week later, she had no signs or symptoms of ureter injury. Later that same evening, she experienced sharp flank pain and nausea. When she called the gynecologist, he sent her to the emergency department. A computed tomography scan showed extravasation of the right ureter. She underwent months of stent placements and replacements, nephrostomies, and ultimately ureteral reimplantation surgery.
PATIENT’S CLAIM: The gynecologist caused a thermal injury to her right ureter during the hysterectomy by misusing an electrocautery device. There was a delay in timely diagnosis postsurgery.
PHYSICIAN’S DEFENSE: The gynecologist contended that he employed proper surgical technique, and that he reacted properly when the patient reported the pain.
VERDICT: A Virginia defense verdict was returned.
Woman loses hands and feet after cystectomy: $109M award
On November 1, a 45-year-old woman underwent laparoscopic excision of a benign ovarian cyst performed by a minimally invasive gynecologic (MIG) surgeon. After surgery, the patient’s blood pressure (BP) declined. She was given fluids, but her BP remained low. The next day, she became incoherent and her BP could not be stabilized. Twenty-seven hours after surgery, the 5-cm umbilical incision opened while the patient was attempting to stand up from the commode. A large amount of bloody discharge drained.
At 11:00
At 4:30
The patient remained unconscious from the time of the exploratory operation until the end of January. She required additional surgeries to control the bacteria as well as amputation of both hands above the wrists and both feet above the ankles due to gangrene. Because she no longer had an abdominal wall, a skin sac was created to hold her intestines outside of her body. When a fistula developed, a colostomy was performed.
She went to a Maryland hospital for rehab, where she learned to walk with prosthetic feet and to use her prosthetic hands. Currently, she has constant abdominal pain, can walk a short distance, and uses a wheelchair. She requires 24/7 assistance for everyday tasks. She can no longer work and is on disability.
PATIENT’S CLAIM: The patient sued the university health system that employed the MIG surgeon. During the cystectomy, he almost completely transected her small intestine, but did not find the injury during surgery. This allowed bacteria to enter the abdominal cavity, causing sepsis and necrotizing fasciitis. The trauma surgeon referred to the injury as an enterotomy, not a tear.
During the procedure, the surgeon used ADEPT, a solution to prevent the formation of adhesions. The patient’s ObGyn expert concluded that ADEPT created an environment that allowed the necrotizing fasciitis to flourish.
The ICU physicians concluded that the patient was stable enough to be transported for a CT scan, but the surgeon repeatedly delayed the procedure and did not call for a surgical consult until 12 hours later. Had the CT scan or exploratory surgery occurred earlier, the diagnosis would have been discovered, and the bacteria would have been prevented from spreading. She would not have required extensive doses of vasopressors, which increase BP by cutting off blood circulation to the 4 extremities. In this case, use of vasopressors led to gangrene and the subsequent amputations.
Continue to: DEFENDANTS’ DEFENSE...
DEFENDANTS’ DEFENSE: The defendants denied all allegations. The expert witness for the defense opined that the surgeon had only nicked the intestine and that the main injury was a tear that had occurred on its own. The defense also claimed that the surgeon did not call for a CT scan because it would not have shown the source of the patient’s condition.
VERDICT: After 2 trials ended with hung juries, a $109 million Florida verdict was returned against the university health system. Under Florida’s sovereign immunity statute, the patient must seek recovery of all but $100,000 of the award through the Florida legislature in a separate claims bill.
Child has hypoxic brain injury: $7.75M settlement
At 41 weeks’ gestation, a mother presented to the emergency department (ED) for delivery after an unremarkable pregnancy. During the last 90 minutes of labor, fetal heart-rate (FHR) monitoring showed nonreassuring findings. After a vaginal delivery, the infant was found to have a hypoxic brain injury.
PARENT’S CLAIM: Even though nonreassuring FHR monitoring findings occurred, the physicians did not offer cesarean delivery (CD). The pediatrician and ED physician were negligent in failing to provide proper neonatal resuscitation and in recognizing a problem with the infants’ intubation. The delay in delivery and poor resuscitation procedure caused the child’s injury.
DEFENDANTS’ DEFENSE: All allegations were denied. There was no deviation from the standard of care.
VERDICT: A $7.75 million Massachusetts settlement was reached.
Kidney failed after hysterectomy
A 46-year-old woman underwent a hysterectomy performed by her ObGyn. Surgery went well but the patient continued to report symptoms. A year later, she underwent an oophorectomy. Two years later, the patient reported blood in her urine and underwent a computed tomography scan, which revealed an obstructed left ureter that had caused injury to the left kidney. Seven months later, the kidney was removed.
PATIENT’S CLAIM: Her kidney loss was a direct result of the ObGyn’s initial surgical procedure. He had placed several clips near the ureter and did not verify their position or protect the ureter. He also failed to address her reported symptoms in a timely manner.
PHYSICIAN’S DEFENSE: The damage to the ureter is a known risk of hysterectomy and oophorectomy. The obstruction developed over time, not as an immediate result of the surgery.
VERDICT: A Kentucky defense verdict was returned.
History of shoulder dystocia, Erb's palsy: $1.2M settlement
An obese mother was admitted to the hospital at 39 weeks’ gestation with signs of labor. She requested a CD and was advised that she had progressed too far for that to be an option, and that vaginal delivery would be safe. During the second stage of labor, shoulder dystocia was encountered. The ObGyn made several attempts to deliver using downward traction, but was unsuccessful. A second ObGyn swept the shoulder with an internal maneuver of his hand and delivered the baby. The child has a severe brachial plexus injury at multiple spinal levels resulting in Erb’s palsy.
PARENT’S CLAIM: A CD should have been performed. The first ObGyn failed to provide a CD and repeatedly applied excessive downward traction, causing the infant’s injury.
Continue to: PHYSICIAN’S DEFENSE...
PHYSICIAN’S DEFENSE: Shoulder dystocia is unpredictable and an unpreventable obstetric emergency. The ObGyn used proper maneuvers to release the shoulder dystocia.
VERDICT: A $1.2 million Virginia settlement was reached.
Ureter injured during hysterectomy
When a patient was found to have multiple, symptomatic fibroids and an enlarged uterus, her gynecologist suggested a total laparoscopic hysterectomy. During the procedure, when he inspected the pelvis and found multiple fibroids in and around the uterus, the gynecologist converted to a supracervical hysterectomy. Surgery was difficult because of a large myoma on the right broad ligament.
The patient tolerated surgery well and was released home the next day. At follow-up one week later, she had no signs or symptoms of ureter injury. Later that same evening, she experienced sharp flank pain and nausea. When she called the gynecologist, he sent her to the emergency department. A computed tomography scan showed extravasation of the right ureter. She underwent months of stent placements and replacements, nephrostomies, and ultimately ureteral reimplantation surgery.
PATIENT’S CLAIM: The gynecologist caused a thermal injury to her right ureter during the hysterectomy by misusing an electrocautery device. There was a delay in timely diagnosis postsurgery.
PHYSICIAN’S DEFENSE: The gynecologist contended that he employed proper surgical technique, and that he reacted properly when the patient reported the pain.
VERDICT: A Virginia defense verdict was returned.
Woman loses hands and feet after cystectomy: $109M award
On November 1, a 45-year-old woman underwent laparoscopic excision of a benign ovarian cyst performed by a minimally invasive gynecologic (MIG) surgeon. After surgery, the patient’s blood pressure (BP) declined. She was given fluids, but her BP remained low. The next day, she became incoherent and her BP could not be stabilized. Twenty-seven hours after surgery, the 5-cm umbilical incision opened while the patient was attempting to stand up from the commode. A large amount of bloody discharge drained.
At 11:00
At 4:30
The patient remained unconscious from the time of the exploratory operation until the end of January. She required additional surgeries to control the bacteria as well as amputation of both hands above the wrists and both feet above the ankles due to gangrene. Because she no longer had an abdominal wall, a skin sac was created to hold her intestines outside of her body. When a fistula developed, a colostomy was performed.
She went to a Maryland hospital for rehab, where she learned to walk with prosthetic feet and to use her prosthetic hands. Currently, she has constant abdominal pain, can walk a short distance, and uses a wheelchair. She requires 24/7 assistance for everyday tasks. She can no longer work and is on disability.
PATIENT’S CLAIM: The patient sued the university health system that employed the MIG surgeon. During the cystectomy, he almost completely transected her small intestine, but did not find the injury during surgery. This allowed bacteria to enter the abdominal cavity, causing sepsis and necrotizing fasciitis. The trauma surgeon referred to the injury as an enterotomy, not a tear.
During the procedure, the surgeon used ADEPT, a solution to prevent the formation of adhesions. The patient’s ObGyn expert concluded that ADEPT created an environment that allowed the necrotizing fasciitis to flourish.
The ICU physicians concluded that the patient was stable enough to be transported for a CT scan, but the surgeon repeatedly delayed the procedure and did not call for a surgical consult until 12 hours later. Had the CT scan or exploratory surgery occurred earlier, the diagnosis would have been discovered, and the bacteria would have been prevented from spreading. She would not have required extensive doses of vasopressors, which increase BP by cutting off blood circulation to the 4 extremities. In this case, use of vasopressors led to gangrene and the subsequent amputations.
Continue to: DEFENDANTS’ DEFENSE...
DEFENDANTS’ DEFENSE: The defendants denied all allegations. The expert witness for the defense opined that the surgeon had only nicked the intestine and that the main injury was a tear that had occurred on its own. The defense also claimed that the surgeon did not call for a CT scan because it would not have shown the source of the patient’s condition.
VERDICT: After 2 trials ended with hung juries, a $109 million Florida verdict was returned against the university health system. Under Florida’s sovereign immunity statute, the patient must seek recovery of all but $100,000 of the award through the Florida legislature in a separate claims bill.
Child has hypoxic brain injury: $7.75M settlement
At 41 weeks’ gestation, a mother presented to the emergency department (ED) for delivery after an unremarkable pregnancy. During the last 90 minutes of labor, fetal heart-rate (FHR) monitoring showed nonreassuring findings. After a vaginal delivery, the infant was found to have a hypoxic brain injury.
PARENT’S CLAIM: Even though nonreassuring FHR monitoring findings occurred, the physicians did not offer cesarean delivery (CD). The pediatrician and ED physician were negligent in failing to provide proper neonatal resuscitation and in recognizing a problem with the infants’ intubation. The delay in delivery and poor resuscitation procedure caused the child’s injury.
DEFENDANTS’ DEFENSE: All allegations were denied. There was no deviation from the standard of care.
VERDICT: A $7.75 million Massachusetts settlement was reached.
Kidney failed after hysterectomy
A 46-year-old woman underwent a hysterectomy performed by her ObGyn. Surgery went well but the patient continued to report symptoms. A year later, she underwent an oophorectomy. Two years later, the patient reported blood in her urine and underwent a computed tomography scan, which revealed an obstructed left ureter that had caused injury to the left kidney. Seven months later, the kidney was removed.
PATIENT’S CLAIM: Her kidney loss was a direct result of the ObGyn’s initial surgical procedure. He had placed several clips near the ureter and did not verify their position or protect the ureter. He also failed to address her reported symptoms in a timely manner.
PHYSICIAN’S DEFENSE: The damage to the ureter is a known risk of hysterectomy and oophorectomy. The obstruction developed over time, not as an immediate result of the surgery.
VERDICT: A Kentucky defense verdict was returned.
History of shoulder dystocia, Erb's palsy: $1.2M settlement
An obese mother was admitted to the hospital at 39 weeks’ gestation with signs of labor. She requested a CD and was advised that she had progressed too far for that to be an option, and that vaginal delivery would be safe. During the second stage of labor, shoulder dystocia was encountered. The ObGyn made several attempts to deliver using downward traction, but was unsuccessful. A second ObGyn swept the shoulder with an internal maneuver of his hand and delivered the baby. The child has a severe brachial plexus injury at multiple spinal levels resulting in Erb’s palsy.
PARENT’S CLAIM: A CD should have been performed. The first ObGyn failed to provide a CD and repeatedly applied excessive downward traction, causing the infant’s injury.
Continue to: PHYSICIAN’S DEFENSE...
PHYSICIAN’S DEFENSE: Shoulder dystocia is unpredictable and an unpreventable obstetric emergency. The ObGyn used proper maneuvers to release the shoulder dystocia.
VERDICT: A $1.2 million Virginia settlement was reached.
Ureter injured during hysterectomy
When a patient was found to have multiple, symptomatic fibroids and an enlarged uterus, her gynecologist suggested a total laparoscopic hysterectomy. During the procedure, when he inspected the pelvis and found multiple fibroids in and around the uterus, the gynecologist converted to a supracervical hysterectomy. Surgery was difficult because of a large myoma on the right broad ligament.
The patient tolerated surgery well and was released home the next day. At follow-up one week later, she had no signs or symptoms of ureter injury. Later that same evening, she experienced sharp flank pain and nausea. When she called the gynecologist, he sent her to the emergency department. A computed tomography scan showed extravasation of the right ureter. She underwent months of stent placements and replacements, nephrostomies, and ultimately ureteral reimplantation surgery.
PATIENT’S CLAIM: The gynecologist caused a thermal injury to her right ureter during the hysterectomy by misusing an electrocautery device. There was a delay in timely diagnosis postsurgery.
PHYSICIAN’S DEFENSE: The gynecologist contended that he employed proper surgical technique, and that he reacted properly when the patient reported the pain.
VERDICT: A Virginia defense verdict was returned.
Nationwide implementation of MIS reduced complications and increased survival in early-stage endometrial cancer
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
To determine if a nationwide implementation of robotic minimally invasive surgery (MIS) influenced the risk of severe complications and survival among women with early-stage endometrial cancer, a group of researchers from the University of Southern Denmark studied the Danish Gynecological Cancer Database, a nationwide, mandatory prospective registration of new cases of women with endometrial cancer who received their surgical treatment in a public hospital.1 Siv Joergensen, MD, reported results at the 47th AAGL Global Congress on Minimally Invasive Gynecology annual meeting on November 13, 2018, in Las Vegas, Nevada.
The transition to robotic MIS was undertaken in Denmark from 2008 to 2013, with the centralization of endometrial cancer treatment in 2012. Over the span of 10 years, the surgical approach to treatment changed from 97% open access surgery to 95% MIS.
For the prospective cohort study, more than 7,000 women with endometrial cancer who received a hysterectomy from January 2005 to June 2015 were grouped by those receiving surgical care before (group 1) and after (group 2) robotic MIS implementation in Denmark. A total of 5,654 women with FIGO Stage I–II endometrial cancer were included in the final study.
Severe complications were 7.3% in group 1 and 6.2% in group 2 (odds ratio, 1.38; 95% confidence interval [CI], 1.10–1.73). Five-year survival rates were significantly lower before robotic MIS was implemented (hazard ratio, 1.22; 95% CI, 1.05–1.41), and no difference was found between laparoscopic and robotic MIS.
The authors concluded that nationwide implementation of robotic MIS enabled a shift toward all types of MIS (with a 73% reduction in hysterectomies performed by laparotomy) and translated into reduced risk of severe complications and increased survival.
How do these results compare with those in the United States?
According to Erica Dun, MD, MPH, who provided commentary for Dr. Joergensen’s study, the United States adopted robotic MIS in the early 2000s. Around 2008, 14% of hysterectomies performed for early-stage endometrial cancer were done through a MIS approach.2 In 2014, after a study in which Walker and colleagues found that laparoscopy was safe and feasible compared with laparotomy,3 the American College of Obstetricians and Gynecologists, jointly with the Society of Gynecologic Oncologists, stated that “MIS should be embraced as the standard surgical approach for comprehensive surgical staging in women with endometrial cancer.”4
Dr. Dun pointed out that Casarin and colleagues found in 2018 that 71.4% of surgeries performed in the United States for endometrial cancer were performed through MIS.5 That number rose to 86.5% MIS (72.5% robot-assisted) for centers of the National Comprehensive Cancer Network.6
Dr. Dun concluded that nationwide implementation of robotic MIS is feasible for gynecologic oncologists, and it is beneficial for patients.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
1. Joergensen SL. Nationwide implementation of robotic minimally invasive surgery for endometrial cancer increases survival and reduces complications. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
2. Jacoby VL, Autry A, Jacobson G, et al. Nationwide use of laparoscopic hysterectomy compared with abdominal and vaginal approaches. Obstet Gynecol. 2009;114:1041-1048.
3. Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
4. American College of Obstetricians and Gynecologists, Society of Gynecologic Oncologists. Practice bulletin no. 149: endometrial cancer. Obstet Gynecol. 2015;125:1006-1026.
5. Casarin J, et al. Adaptation of minimally invasive surgery and decrease in surgical morbidity for endometrial cancer treatment in the United States. Obstet Gynecol. 2018;131:304-311.
6. Bergstrom, Aloisi A, Armbruster S, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480-484.
Patient-centric pain management decision aid reduces opioid use posthysterectomy
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
Investigators at the University of Michigan, Ann Arbor, found that a simple patient decision aid can be a useful tool in providing adequate postsurgical pain control to patients while reducing the number of opioid tablets in the community. The shared decision-making aid focuses on educating the patient about opioid use and engages her in an appropriate postoperative pain management plan. Results from this prospective quality improvement study were presented in a poster at the 47th AAGL Global Congress on Minimally Invasive Gynecology (Las Vegas, Nevada, November 11–15, 2018).1
Annmarie Vilkins, DO, and colleagues’ aim was to evaluate the impact of shared decision-making through the use of a patient decision aid targeting posthysterectomy pain management and opioid use. Can such a targeted strategy help decrease posthysterectomy opioid distribution in the community without compromising patient pain control or satisfaction?
The authors noted that more than 46 people die each day from an overdose involving prescription opioids.2 Studies have shown that patients actually use significantly fewer opioid tablets than the amount clinicians generally prescribe following ObGyn surgeries.3,4 Unused prescription opioid availability has the potential for accidental use or intentional misuse of the unneeded drugs by others.
Study methods
The investigators included all English-speaking patients undergoing hysterectomy for benign disease at their institution from March 1 through July 31, 2018. Data were analyzed from women undergoing laparoscopic, vaginal, or abdominal hysterectomy before (n = 195) and after (n = 177) the decision aid was implemented.
Preoperative education. In the preoperative area, patients were uniformly educated regarding postoperative pain expectations (for example, it is normal to have some pain; the goal is to manage your pain so you can function; some women do not require opioid medications after surgery), risks of opioid medications (such as dependence or addiction; misuse of leftover pills by others), adverse effects (drowsiness; confusion), and the recommended postoperative pain management schedule.
Postoperatively, pain medications included ibuprofen around the clock, acetaminophen as needed (used with caution when hydrocodone with acetaminophen was also prescribed), and opioids only if needed.
Discharge medication planning. Using a visual scale, the investigators then educated patients regarding the maximum number of opioid tablets permitted to be prescribed according to department guidelines and the average number of opioid tablets that a typical patient uses. The number of opioid tablets prescribed varied based on route of hysterectomy (laparoscopic, abdominal, or vaginal). For example, for a laparoscopic hysterectomy, the maximum allowed prescription for oxycodone was 20 tablets, while patients used an average number of 10 tablets.
The patient was then asked to choose her desired number of tablets with which she would like to be discharged.
Structured telephone calls were made to patients 2 weeks postoperatively.
Impact of the decision aid on opioid prescribing
Before implementation of the decision aid, the average number of opioid pills prescribed at discharge was 25 (median, 20–35), while that number dropped to 10 (median, 10–15) after the aid’s implementation. Similarly, the average oral morphine equivalents (OMEs) at time of discharge was 150 (interquartile range [IQR], 120–200) before decision aid implementation and 75 (IQR, 25–150) after decision aid implementation. Similar reductions in average OMEs were observed before and after the aid’s implementation across the 3 hysterectomy routes.
Continue to: According to the type of opioid...
According to the type of opioid prescribed at discharge, hydrocodone 5 mg was prescribed in 99 cases (50.8%) before decision aid implementation and in 14 cases (7.9%) after implementation. By contrast, oxycodone 5 mg was prescribed in 85 cases (43.6%) before implementation and in 149 cases (84.2%) after implementation.
The number of refill requests was similar before (n = 11 [5.6%]) and after (n = 12 [6.8%]) the aid’s implementation.
Tool reduced opioid availability in the community
The use of a simple patient decision aid—which focuses on opioid education and engages patients in an appropriate postoperative pain management plan—can result in fewer opioid tablets in the community while still providing adequate pain control, the authors concluded.
Online resource. For more on targeted strategies to optimize opioid prescribing after surgery, visit the University of Michigan’s Opioid Prescribing Engagement Network (OPEN) at http://michigan-open.org.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.
- Vilkins A, Till S, Lim R, et al. The impact of shared decision making on post-hysterectomy opioid prescribing. Poster presented at: 47th AAGL Global Congress on Minimally Invasive Gynecology; November 11-15, 2018; Las Vegas, NV.
- Seth P, Scholl L, Rudd RA, et al. Overdose deaths involving opioids, cocaine, and psychostimulants—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:349-358.
- Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29-35.
- As-Sanie S, Till S, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261-1268.