User login
Dietary interventions can support IBD treatment
For Crohn’s disease, a diet low in refined carbohydrates and a symptoms-guided diet appeared to help with remission, yet reduction of refined carbohydrates or red meat didn’t reduce the risk of relapse. For ulcerative colitis, solid food diets were similar to control measures.
“The Internet has a dizzying array of diet variants touted to benefit inflammation and IBD, which has led to much confusion among patients, and even clinicians, over what is truly effective or not,” Berkeley Limketkai, MD, PhD, director of clinical research at the Center for Inflammatory Bowel Disease at the University of California, Los Angeles, said in an interview.
“Even experiences shared by well-meaning individuals might not be generalizable to others,” he said. “The lack of clarity on what is or is not effective motivated us to perform this systematic review and meta-analysis.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing diets
Some nutritional therapies, such as exclusive enteral nutrition, have good evidence to support their use in the treatment of IBD, Dr. Limketkai said. However, patients often find maintaining a liquid diet difficult, particularly over a long period of time, so clinicians and patients have been interested in solid food diets as a treatment for IBD.
In 2019, Dr. Limketkai and colleagues conducted a systematic review and meta-analysis of randomized controlled trials focused on solid food diets for IBD that was published with the Cochrane Collaboration. At that time, the data were considered sparse, and the certainty of evidence was very low or low. Since then, several high-quality trials have been published.
For this study, Dr. Limketkai and colleagues conducted an updated review of 36 studies and a meta-analysis of 27 studies that compared a solid food diet with a control diet in patients with Crohn’s disease or ulcerative colitis. The intervention arm had to involve a well-defined diet, not merely a “usual” diet.
Among the studies, 12 evaluated dietary interventions for inducing clinical remission in patients with active Crohn’s disease, and 639 patients were involved. Overall, a low–refined carbohydrate diet was superior to a high-carbohydrate diet or a low-fiber diet. In addition, a symptoms-guided diet, which sequentially eliminated foods that aggravated a patient’s symptoms, was superior to conventional nutrition advice. However, the studies had serious imprecisions and very low certainty of evidence.
Compared with respective controls, a highly restrictive organic diet, a low-microparticle diet, and a low-calcium diet were ineffective at inducing remission of Crohn’s disease. Studies focused on immunoglobulin G-based measures were also inconsistent.
When comparing diets touted to benefit patients with Crohn’s disease, the Specific Carbohydrate Diet was similar to the Mediterranean diet and the whole-food diet, though the certainty of evidence was low. Partial enteral nutrition was similar to exclusive enteral nutrition, though there was substantial statistical heterogeneity between studies and very low certainty of evidence.
For maintenance of Crohn’s disease remission, researchers evaluated 14 studies that included 1,211 patients with inactive disease. Partial enteral nutrition appeared to reduce the risk of relapse, although evidence certainty was very low. In contrast, reducing red meat or refined carbohydrates did not lower the risk of relapse.
“These findings seemingly contradict our belief that red meat and refined carbohydrates have proinflammatory effects, although there are other studies that appear to show inconsistent, weak, or no association between consumption of unprocessed red meat and disease,” Dr. Limketkai said. “The caveat is that our findings are based on weak evidence, which may change as more studies are performed over time.”
For induction of remission in ulcerative colitis, researchers evaluated three studies that included 124 participants with active disease. When compared with participants’ usual diet, there was no benefit from a diet that excluded symptom-provoking foods, fried foods, refined carbohydrates, additives, preservatives, most condiments, spices, and beverages other than boiled water. Other studies found no benefit from eliminating cow milk protein or gluten.
For maintenance of ulcerative colitis remission, they looked at four studies that included 101 patients with inactive disease. Overall, there was no benefit from a carrageenan-free diet, anti-inflammatory diet, or cow milk protein elimination diet.
Helping patients
Although the certainty of evidence remains very low or low for most dietary trials in IBD, the emerging data suggest that nutrition plays an important role in IBD management and should be considered in the overall treatment plan for patients, the study authors wrote.
“Patients continue to look for ways to control their IBD, particularly with diet. Providers continue to struggle with making evidence-based recommendations about dietary interventions for IBD. This systematic review is a useful tool for providers to advise their patients,” James D. Lewis, MD, associate director of the inflammatory bowel diseases program at the University of Pennsylvania, Philadelphia, said in an interview.
Dr. Lewis, who wasn’t involved with this study, has researched dietary interventions for IBD. He and his colleagues have found that reducing red meat does not lower the rate of Crohn’s disease flares and that the Mediterranean diet and Specific Carbohydrate Diet appear to be similar for inducing clinical remission.
Based on this review, partial enteral nutrition could be an option for patients with Crohn’s disease, Dr. Lewis said.
“Partial enteral nutrition is much easier than exclusive enteral nutrition for patients,” he said. “However, there remains uncertainty as to whether the solid food component of a partial enteral nutrition approach impacts outcomes.”
As more dietary studies become available, the certainty of evidence could improve and lead to better recommendations for patients, Dr. Limketkai and colleagues wrote. They are conducting several studies focused on the concept of precision nutrition.
“While certain diets may be helpful and effective for IBD, different diets work differently in different people. This concept is no different than the fact that different IBD medications work differently in different individuals,” Dr. Limketkai said. “However, given the current state of evidence for dietary interventions in IBD, we still have a long path of research ahead of us.”
The study received no funding. The study authors reported no conflicts of interest. Dr. Lewis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
For Crohn’s disease, a diet low in refined carbohydrates and a symptoms-guided diet appeared to help with remission, yet reduction of refined carbohydrates or red meat didn’t reduce the risk of relapse. For ulcerative colitis, solid food diets were similar to control measures.
“The Internet has a dizzying array of diet variants touted to benefit inflammation and IBD, which has led to much confusion among patients, and even clinicians, over what is truly effective or not,” Berkeley Limketkai, MD, PhD, director of clinical research at the Center for Inflammatory Bowel Disease at the University of California, Los Angeles, said in an interview.
“Even experiences shared by well-meaning individuals might not be generalizable to others,” he said. “The lack of clarity on what is or is not effective motivated us to perform this systematic review and meta-analysis.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing diets
Some nutritional therapies, such as exclusive enteral nutrition, have good evidence to support their use in the treatment of IBD, Dr. Limketkai said. However, patients often find maintaining a liquid diet difficult, particularly over a long period of time, so clinicians and patients have been interested in solid food diets as a treatment for IBD.
In 2019, Dr. Limketkai and colleagues conducted a systematic review and meta-analysis of randomized controlled trials focused on solid food diets for IBD that was published with the Cochrane Collaboration. At that time, the data were considered sparse, and the certainty of evidence was very low or low. Since then, several high-quality trials have been published.
For this study, Dr. Limketkai and colleagues conducted an updated review of 36 studies and a meta-analysis of 27 studies that compared a solid food diet with a control diet in patients with Crohn’s disease or ulcerative colitis. The intervention arm had to involve a well-defined diet, not merely a “usual” diet.
Among the studies, 12 evaluated dietary interventions for inducing clinical remission in patients with active Crohn’s disease, and 639 patients were involved. Overall, a low–refined carbohydrate diet was superior to a high-carbohydrate diet or a low-fiber diet. In addition, a symptoms-guided diet, which sequentially eliminated foods that aggravated a patient’s symptoms, was superior to conventional nutrition advice. However, the studies had serious imprecisions and very low certainty of evidence.
Compared with respective controls, a highly restrictive organic diet, a low-microparticle diet, and a low-calcium diet were ineffective at inducing remission of Crohn’s disease. Studies focused on immunoglobulin G-based measures were also inconsistent.
When comparing diets touted to benefit patients with Crohn’s disease, the Specific Carbohydrate Diet was similar to the Mediterranean diet and the whole-food diet, though the certainty of evidence was low. Partial enteral nutrition was similar to exclusive enteral nutrition, though there was substantial statistical heterogeneity between studies and very low certainty of evidence.
For maintenance of Crohn’s disease remission, researchers evaluated 14 studies that included 1,211 patients with inactive disease. Partial enteral nutrition appeared to reduce the risk of relapse, although evidence certainty was very low. In contrast, reducing red meat or refined carbohydrates did not lower the risk of relapse.
“These findings seemingly contradict our belief that red meat and refined carbohydrates have proinflammatory effects, although there are other studies that appear to show inconsistent, weak, or no association between consumption of unprocessed red meat and disease,” Dr. Limketkai said. “The caveat is that our findings are based on weak evidence, which may change as more studies are performed over time.”
For induction of remission in ulcerative colitis, researchers evaluated three studies that included 124 participants with active disease. When compared with participants’ usual diet, there was no benefit from a diet that excluded symptom-provoking foods, fried foods, refined carbohydrates, additives, preservatives, most condiments, spices, and beverages other than boiled water. Other studies found no benefit from eliminating cow milk protein or gluten.
For maintenance of ulcerative colitis remission, they looked at four studies that included 101 patients with inactive disease. Overall, there was no benefit from a carrageenan-free diet, anti-inflammatory diet, or cow milk protein elimination diet.
Helping patients
Although the certainty of evidence remains very low or low for most dietary trials in IBD, the emerging data suggest that nutrition plays an important role in IBD management and should be considered in the overall treatment plan for patients, the study authors wrote.
“Patients continue to look for ways to control their IBD, particularly with diet. Providers continue to struggle with making evidence-based recommendations about dietary interventions for IBD. This systematic review is a useful tool for providers to advise their patients,” James D. Lewis, MD, associate director of the inflammatory bowel diseases program at the University of Pennsylvania, Philadelphia, said in an interview.
Dr. Lewis, who wasn’t involved with this study, has researched dietary interventions for IBD. He and his colleagues have found that reducing red meat does not lower the rate of Crohn’s disease flares and that the Mediterranean diet and Specific Carbohydrate Diet appear to be similar for inducing clinical remission.
Based on this review, partial enteral nutrition could be an option for patients with Crohn’s disease, Dr. Lewis said.
“Partial enteral nutrition is much easier than exclusive enteral nutrition for patients,” he said. “However, there remains uncertainty as to whether the solid food component of a partial enteral nutrition approach impacts outcomes.”
As more dietary studies become available, the certainty of evidence could improve and lead to better recommendations for patients, Dr. Limketkai and colleagues wrote. They are conducting several studies focused on the concept of precision nutrition.
“While certain diets may be helpful and effective for IBD, different diets work differently in different people. This concept is no different than the fact that different IBD medications work differently in different individuals,” Dr. Limketkai said. “However, given the current state of evidence for dietary interventions in IBD, we still have a long path of research ahead of us.”
The study received no funding. The study authors reported no conflicts of interest. Dr. Lewis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
For Crohn’s disease, a diet low in refined carbohydrates and a symptoms-guided diet appeared to help with remission, yet reduction of refined carbohydrates or red meat didn’t reduce the risk of relapse. For ulcerative colitis, solid food diets were similar to control measures.
“The Internet has a dizzying array of diet variants touted to benefit inflammation and IBD, which has led to much confusion among patients, and even clinicians, over what is truly effective or not,” Berkeley Limketkai, MD, PhD, director of clinical research at the Center for Inflammatory Bowel Disease at the University of California, Los Angeles, said in an interview.
“Even experiences shared by well-meaning individuals might not be generalizable to others,” he said. “The lack of clarity on what is or is not effective motivated us to perform this systematic review and meta-analysis.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing diets
Some nutritional therapies, such as exclusive enteral nutrition, have good evidence to support their use in the treatment of IBD, Dr. Limketkai said. However, patients often find maintaining a liquid diet difficult, particularly over a long period of time, so clinicians and patients have been interested in solid food diets as a treatment for IBD.
In 2019, Dr. Limketkai and colleagues conducted a systematic review and meta-analysis of randomized controlled trials focused on solid food diets for IBD that was published with the Cochrane Collaboration. At that time, the data were considered sparse, and the certainty of evidence was very low or low. Since then, several high-quality trials have been published.
For this study, Dr. Limketkai and colleagues conducted an updated review of 36 studies and a meta-analysis of 27 studies that compared a solid food diet with a control diet in patients with Crohn’s disease or ulcerative colitis. The intervention arm had to involve a well-defined diet, not merely a “usual” diet.
Among the studies, 12 evaluated dietary interventions for inducing clinical remission in patients with active Crohn’s disease, and 639 patients were involved. Overall, a low–refined carbohydrate diet was superior to a high-carbohydrate diet or a low-fiber diet. In addition, a symptoms-guided diet, which sequentially eliminated foods that aggravated a patient’s symptoms, was superior to conventional nutrition advice. However, the studies had serious imprecisions and very low certainty of evidence.
Compared with respective controls, a highly restrictive organic diet, a low-microparticle diet, and a low-calcium diet were ineffective at inducing remission of Crohn’s disease. Studies focused on immunoglobulin G-based measures were also inconsistent.
When comparing diets touted to benefit patients with Crohn’s disease, the Specific Carbohydrate Diet was similar to the Mediterranean diet and the whole-food diet, though the certainty of evidence was low. Partial enteral nutrition was similar to exclusive enteral nutrition, though there was substantial statistical heterogeneity between studies and very low certainty of evidence.
For maintenance of Crohn’s disease remission, researchers evaluated 14 studies that included 1,211 patients with inactive disease. Partial enteral nutrition appeared to reduce the risk of relapse, although evidence certainty was very low. In contrast, reducing red meat or refined carbohydrates did not lower the risk of relapse.
“These findings seemingly contradict our belief that red meat and refined carbohydrates have proinflammatory effects, although there are other studies that appear to show inconsistent, weak, or no association between consumption of unprocessed red meat and disease,” Dr. Limketkai said. “The caveat is that our findings are based on weak evidence, which may change as more studies are performed over time.”
For induction of remission in ulcerative colitis, researchers evaluated three studies that included 124 participants with active disease. When compared with participants’ usual diet, there was no benefit from a diet that excluded symptom-provoking foods, fried foods, refined carbohydrates, additives, preservatives, most condiments, spices, and beverages other than boiled water. Other studies found no benefit from eliminating cow milk protein or gluten.
For maintenance of ulcerative colitis remission, they looked at four studies that included 101 patients with inactive disease. Overall, there was no benefit from a carrageenan-free diet, anti-inflammatory diet, or cow milk protein elimination diet.
Helping patients
Although the certainty of evidence remains very low or low for most dietary trials in IBD, the emerging data suggest that nutrition plays an important role in IBD management and should be considered in the overall treatment plan for patients, the study authors wrote.
“Patients continue to look for ways to control their IBD, particularly with diet. Providers continue to struggle with making evidence-based recommendations about dietary interventions for IBD. This systematic review is a useful tool for providers to advise their patients,” James D. Lewis, MD, associate director of the inflammatory bowel diseases program at the University of Pennsylvania, Philadelphia, said in an interview.
Dr. Lewis, who wasn’t involved with this study, has researched dietary interventions for IBD. He and his colleagues have found that reducing red meat does not lower the rate of Crohn’s disease flares and that the Mediterranean diet and Specific Carbohydrate Diet appear to be similar for inducing clinical remission.
Based on this review, partial enteral nutrition could be an option for patients with Crohn’s disease, Dr. Lewis said.
“Partial enteral nutrition is much easier than exclusive enteral nutrition for patients,” he said. “However, there remains uncertainty as to whether the solid food component of a partial enteral nutrition approach impacts outcomes.”
As more dietary studies become available, the certainty of evidence could improve and lead to better recommendations for patients, Dr. Limketkai and colleagues wrote. They are conducting several studies focused on the concept of precision nutrition.
“While certain diets may be helpful and effective for IBD, different diets work differently in different people. This concept is no different than the fact that different IBD medications work differently in different individuals,” Dr. Limketkai said. “However, given the current state of evidence for dietary interventions in IBD, we still have a long path of research ahead of us.”
The study received no funding. The study authors reported no conflicts of interest. Dr. Lewis reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Can nanotechnology help cure IBD?
Finding a cure for inflammatory bowel disease is a big goal. But the key to achieving it might be to think small.
University of Wisconsin–Madison researchers are developing nanoparticles – particles measuring between 1 and 100 nanometers (one-billionth of a meter) – designed to treat IBD, including Crohn’s disease and ulcerative colitis. (For context: A sheet of paper is about 100,000 nanometers thick.)
Described in a paper in Science Advances, these , a compatible compound commonly used in medicine.
The nanoparticles – the researchers call them “backpacks” – can be attached to probiotics, which deliver them to the gut.
“Due to the colonizing property of probiotics in colon tissues, the nanoparticles could be delivered to colon tissues by probiotics and released slowly,” says study author Quanyin Hu, PhD, a biomedical engineer and assistant professor at the University of Wisconsin–Madison School of Pharmacy.
This helps give the nanoparticles time to bring the ROS level back down to normal. But that’s only part of the IBD treatment the researchers envision.
The technology builds on a previous development from Dr. Hu and his team – a protective probiotic shell coating. The coating, which is about 330 nanometers thick, helps probiotics survive long enough to establish and multiply in the gut.
“The harsh environment of gastric acid and bile salt would kill most probiotics,” Dr. Hu says. “Moreover, antibiotics usually used in inflammatory bowel disease treatment also harm probiotic growth.”
Early results are promising, he says. Mice with IBD that received the full treatment – combining the ROS-targeting nanoparticles with the coated probiotics – had fewer IBD symptoms, like less weight loss and colon shortening, than those treated with the encapsulated probiotics alone.
By attacking the disease on multiple fronts – reducing the ROS and improving the balance of gut microbiota – a healthy gut environment could be restored, Dr. Hu says. In other words: “[It] might be possible to finally cure inflammatory bowel disease.”
Nanotechnology offers all kinds of unique advantages over traditional IBD treatments, he says. Nanoparticles can be designed to target specific tissues, like colon tissues. And, compared with small molecules, they can circulate throughout the body longer, so they have more time to build up and do their job.
The next steps will be to test the treatment in large animals and “to develop a stable formulation that can be stored for a long time and produced in a scalable and economical manner,” Dr. Hu says.
Current IBD treatments “can only relieve symptoms,” not cure the disease, he says.
“This study is our first try to fundamentally treat inflammatory bowel disease by recovering a healthy microenvironment in the intestines, and our preliminary data demonstrated that this strategy is delivering promises to pave a new treatment strategy for IBD,” Dr. Hu says.
A version of this article first appeared on WebMD.com.
Finding a cure for inflammatory bowel disease is a big goal. But the key to achieving it might be to think small.
University of Wisconsin–Madison researchers are developing nanoparticles – particles measuring between 1 and 100 nanometers (one-billionth of a meter) – designed to treat IBD, including Crohn’s disease and ulcerative colitis. (For context: A sheet of paper is about 100,000 nanometers thick.)
Described in a paper in Science Advances, these , a compatible compound commonly used in medicine.
The nanoparticles – the researchers call them “backpacks” – can be attached to probiotics, which deliver them to the gut.
“Due to the colonizing property of probiotics in colon tissues, the nanoparticles could be delivered to colon tissues by probiotics and released slowly,” says study author Quanyin Hu, PhD, a biomedical engineer and assistant professor at the University of Wisconsin–Madison School of Pharmacy.
This helps give the nanoparticles time to bring the ROS level back down to normal. But that’s only part of the IBD treatment the researchers envision.
The technology builds on a previous development from Dr. Hu and his team – a protective probiotic shell coating. The coating, which is about 330 nanometers thick, helps probiotics survive long enough to establish and multiply in the gut.
“The harsh environment of gastric acid and bile salt would kill most probiotics,” Dr. Hu says. “Moreover, antibiotics usually used in inflammatory bowel disease treatment also harm probiotic growth.”
Early results are promising, he says. Mice with IBD that received the full treatment – combining the ROS-targeting nanoparticles with the coated probiotics – had fewer IBD symptoms, like less weight loss and colon shortening, than those treated with the encapsulated probiotics alone.
By attacking the disease on multiple fronts – reducing the ROS and improving the balance of gut microbiota – a healthy gut environment could be restored, Dr. Hu says. In other words: “[It] might be possible to finally cure inflammatory bowel disease.”
Nanotechnology offers all kinds of unique advantages over traditional IBD treatments, he says. Nanoparticles can be designed to target specific tissues, like colon tissues. And, compared with small molecules, they can circulate throughout the body longer, so they have more time to build up and do their job.
The next steps will be to test the treatment in large animals and “to develop a stable formulation that can be stored for a long time and produced in a scalable and economical manner,” Dr. Hu says.
Current IBD treatments “can only relieve symptoms,” not cure the disease, he says.
“This study is our first try to fundamentally treat inflammatory bowel disease by recovering a healthy microenvironment in the intestines, and our preliminary data demonstrated that this strategy is delivering promises to pave a new treatment strategy for IBD,” Dr. Hu says.
A version of this article first appeared on WebMD.com.
Finding a cure for inflammatory bowel disease is a big goal. But the key to achieving it might be to think small.
University of Wisconsin–Madison researchers are developing nanoparticles – particles measuring between 1 and 100 nanometers (one-billionth of a meter) – designed to treat IBD, including Crohn’s disease and ulcerative colitis. (For context: A sheet of paper is about 100,000 nanometers thick.)
Described in a paper in Science Advances, these , a compatible compound commonly used in medicine.
The nanoparticles – the researchers call them “backpacks” – can be attached to probiotics, which deliver them to the gut.
“Due to the colonizing property of probiotics in colon tissues, the nanoparticles could be delivered to colon tissues by probiotics and released slowly,” says study author Quanyin Hu, PhD, a biomedical engineer and assistant professor at the University of Wisconsin–Madison School of Pharmacy.
This helps give the nanoparticles time to bring the ROS level back down to normal. But that’s only part of the IBD treatment the researchers envision.
The technology builds on a previous development from Dr. Hu and his team – a protective probiotic shell coating. The coating, which is about 330 nanometers thick, helps probiotics survive long enough to establish and multiply in the gut.
“The harsh environment of gastric acid and bile salt would kill most probiotics,” Dr. Hu says. “Moreover, antibiotics usually used in inflammatory bowel disease treatment also harm probiotic growth.”
Early results are promising, he says. Mice with IBD that received the full treatment – combining the ROS-targeting nanoparticles with the coated probiotics – had fewer IBD symptoms, like less weight loss and colon shortening, than those treated with the encapsulated probiotics alone.
By attacking the disease on multiple fronts – reducing the ROS and improving the balance of gut microbiota – a healthy gut environment could be restored, Dr. Hu says. In other words: “[It] might be possible to finally cure inflammatory bowel disease.”
Nanotechnology offers all kinds of unique advantages over traditional IBD treatments, he says. Nanoparticles can be designed to target specific tissues, like colon tissues. And, compared with small molecules, they can circulate throughout the body longer, so they have more time to build up and do their job.
The next steps will be to test the treatment in large animals and “to develop a stable formulation that can be stored for a long time and produced in a scalable and economical manner,” Dr. Hu says.
Current IBD treatments “can only relieve symptoms,” not cure the disease, he says.
“This study is our first try to fundamentally treat inflammatory bowel disease by recovering a healthy microenvironment in the intestines, and our preliminary data demonstrated that this strategy is delivering promises to pave a new treatment strategy for IBD,” Dr. Hu says.
A version of this article first appeared on WebMD.com.
FROM SCIENCE ADVANCES
Upadacitinib curbed UC symptoms from Day 1 in study
Patients with ulcerative colitis who were treated with upadacitinib showed significant improvement in symptoms compared to placebo within days of starting treatment, according to results of a new study.
Immunosuppressants and biologics used to treat moderate to severe disease can vary in their response times from weeks to months, and some ambulatory patients fail to respond to these treatments, wrote Edward V. Loftus Jr., MD, of the Mayo Clinic, Rochester, Minn., and colleagues.
“The lack of effective, quick-acting therapies may lead patients to experience prolonged relapses, reduced quality of life, and a significant burden of illness,” the researchers said.
Upadacitinib, an oral, once-daily reversible JAK inhibitor, is approved by the Food and Drug Administration for patients with moderate to severe ulcerative colitis (UC) who have not responded to at least one tumor necrosis factor (TNF) blocker, but the time frame for response to upadacitinib has not been well studied, they said.
In a study published online in Clinical Gastroenterology and Hepatology, the researchers reviewed data from two phase 3 randomized, placebo-controlled induction trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026), that assessed the safety and efficacy of a 45-mg daily dose of upadacitinib for managing symptoms of moderate to severe UC over an 8-week period.
The intent-to-treat analysis included 660 patients who had been randomized to received upadacitinib and 328 in the placebo group. Demographics were similar between the groups. Symptom improvement was based on changes in inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) measured at week 2. Quality of life was assessed at 2 and 8 weeks.
Between 1 and 3 days after starting treatment, significantly greater percentages of patients in the treatment arm achieved a reduction of at least 50% from baseline in hs-CRP (75.7% vs. 21.9%) and FCP (48.2% vs. 20.2%).
The significant differences in symptom improvement persisted for 14 days from the first dose.
Patients in the upadacitinib group also showed increased rates of clinical remission/response (26.9%/59.4%) based on Partial Mayo scores at week 2 compared with placebo patients (4.3%/22.3%). Treated patients showed significant improvements in quality of life at weeks 2 and 8.
In addition, patient-reported UC symptoms of stool frequency, rectal bleeding, abdominal pain, and bowel urgency improved significantly compared with placebo by day 3, the researchers noted. “In this study, upadacitinib led to absence of rectal bleeding by day 1 and absence of abdominal pain and bowel urgency by day 3 in a significant proportion of patients vs. placebo (all P < .05),” they said.
The findings were limited by the post hoc design, but reflect results of other studies, including those of the JAK inhibitor tofacitinib, the researchers noted. The results of the current study demonstrate the ability of upadacitinib to alleviate key UC symptoms as early as 1 day after the first dose, they concluded.
Need for rapid onset treatment
A majority of biologics used to treat IBD take weeks to months to exert an effect, “so it is crucial that a drug with more rapid onset of action become available,” Atsushi Sakuraba, MD, director of clinical trial/IBD research at the University of Chicago, said in an interview.
Dr. Sakuraba, who was not involved in the study, said he was surprised by the findings.
“It is amazing that upadacitinib started to show response in 1-3 days and showed superior rates of remission/response versus placebo by week 2,” he said.
“Clinicians treating IBD patients should take the time to response into consideration, because it leads to rapid improvement in quality of life and reduced need for steroids,” Dr. Sakuraba said. The current study findings need to be confirmed in real life studies, he cautioned.
Expanding clinical options
Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, who was not involved with the study, said in an interview that he was not surprised by upadacitinib’s quick action, which supports what he has seen in clinical practice. “We now have post hoc data from the phase 3, multicenter induction trials ... to provide us with hard evidence to support our observations,” he noted.
The current study is important because many patients with UC can be very symptomatic, with rectal bleeding, diarrhea, urgency, and abdominal pain, he said in an interview.
“It is often critical to have a fast-acting medication to avoid worsening symptoms, hospitalization, and severe complications. This study shows that upadacitinib, a new oral small molecule that selectively inhibits JAK-1, works within 1 day to improve symptoms and within 2 weeks to improve CRP and fecal calprotectin,” he said.
The takeaway message to clinicians is that upadacitinib is effective, relatively safe, and fast-acting option for patients with UC who have previously failed an anti-TNF agent, he said.
Challenges remain, however.
“Despite this exciting new therapeutic, there is still significant research needed to break our current therapeutic efficacy ceiling and get more IBD patients into a stable and durable remission,” Dr. Berinstein said. “Additional research is needed to develop personalized treatment strategies to address the unique needs of individual patients and to guide optimal medical management.”
Dr. Berinstein and his team are exploring the use of upadacitinib on the sickest IBD patients, “those admitted to the hospital with acute severe ulcerative colitis,” he said. Emerging data from multiple academic centers suggest that “upadacitinib induction may be a viable treatment strategy for these very high-risk patients. Of course, large prospective trials will be needed to confirm this,” he added.
The study was funded by AbbVie, which markets upadacitinib (Rinvoq). Lead author Dr. Loftus and several study authors disclosed financial relationships with AbbVie and other companies. Dr. Sakuraba and Dr. Berinstein reported having no relevant financial conflicts.
Patients with ulcerative colitis who were treated with upadacitinib showed significant improvement in symptoms compared to placebo within days of starting treatment, according to results of a new study.
Immunosuppressants and biologics used to treat moderate to severe disease can vary in their response times from weeks to months, and some ambulatory patients fail to respond to these treatments, wrote Edward V. Loftus Jr., MD, of the Mayo Clinic, Rochester, Minn., and colleagues.
“The lack of effective, quick-acting therapies may lead patients to experience prolonged relapses, reduced quality of life, and a significant burden of illness,” the researchers said.
Upadacitinib, an oral, once-daily reversible JAK inhibitor, is approved by the Food and Drug Administration for patients with moderate to severe ulcerative colitis (UC) who have not responded to at least one tumor necrosis factor (TNF) blocker, but the time frame for response to upadacitinib has not been well studied, they said.
In a study published online in Clinical Gastroenterology and Hepatology, the researchers reviewed data from two phase 3 randomized, placebo-controlled induction trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026), that assessed the safety and efficacy of a 45-mg daily dose of upadacitinib for managing symptoms of moderate to severe UC over an 8-week period.
The intent-to-treat analysis included 660 patients who had been randomized to received upadacitinib and 328 in the placebo group. Demographics were similar between the groups. Symptom improvement was based on changes in inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) measured at week 2. Quality of life was assessed at 2 and 8 weeks.
Between 1 and 3 days after starting treatment, significantly greater percentages of patients in the treatment arm achieved a reduction of at least 50% from baseline in hs-CRP (75.7% vs. 21.9%) and FCP (48.2% vs. 20.2%).
The significant differences in symptom improvement persisted for 14 days from the first dose.
Patients in the upadacitinib group also showed increased rates of clinical remission/response (26.9%/59.4%) based on Partial Mayo scores at week 2 compared with placebo patients (4.3%/22.3%). Treated patients showed significant improvements in quality of life at weeks 2 and 8.
In addition, patient-reported UC symptoms of stool frequency, rectal bleeding, abdominal pain, and bowel urgency improved significantly compared with placebo by day 3, the researchers noted. “In this study, upadacitinib led to absence of rectal bleeding by day 1 and absence of abdominal pain and bowel urgency by day 3 in a significant proportion of patients vs. placebo (all P < .05),” they said.
The findings were limited by the post hoc design, but reflect results of other studies, including those of the JAK inhibitor tofacitinib, the researchers noted. The results of the current study demonstrate the ability of upadacitinib to alleviate key UC symptoms as early as 1 day after the first dose, they concluded.
Need for rapid onset treatment
A majority of biologics used to treat IBD take weeks to months to exert an effect, “so it is crucial that a drug with more rapid onset of action become available,” Atsushi Sakuraba, MD, director of clinical trial/IBD research at the University of Chicago, said in an interview.
Dr. Sakuraba, who was not involved in the study, said he was surprised by the findings.
“It is amazing that upadacitinib started to show response in 1-3 days and showed superior rates of remission/response versus placebo by week 2,” he said.
“Clinicians treating IBD patients should take the time to response into consideration, because it leads to rapid improvement in quality of life and reduced need for steroids,” Dr. Sakuraba said. The current study findings need to be confirmed in real life studies, he cautioned.
Expanding clinical options
Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, who was not involved with the study, said in an interview that he was not surprised by upadacitinib’s quick action, which supports what he has seen in clinical practice. “We now have post hoc data from the phase 3, multicenter induction trials ... to provide us with hard evidence to support our observations,” he noted.
The current study is important because many patients with UC can be very symptomatic, with rectal bleeding, diarrhea, urgency, and abdominal pain, he said in an interview.
“It is often critical to have a fast-acting medication to avoid worsening symptoms, hospitalization, and severe complications. This study shows that upadacitinib, a new oral small molecule that selectively inhibits JAK-1, works within 1 day to improve symptoms and within 2 weeks to improve CRP and fecal calprotectin,” he said.
The takeaway message to clinicians is that upadacitinib is effective, relatively safe, and fast-acting option for patients with UC who have previously failed an anti-TNF agent, he said.
Challenges remain, however.
“Despite this exciting new therapeutic, there is still significant research needed to break our current therapeutic efficacy ceiling and get more IBD patients into a stable and durable remission,” Dr. Berinstein said. “Additional research is needed to develop personalized treatment strategies to address the unique needs of individual patients and to guide optimal medical management.”
Dr. Berinstein and his team are exploring the use of upadacitinib on the sickest IBD patients, “those admitted to the hospital with acute severe ulcerative colitis,” he said. Emerging data from multiple academic centers suggest that “upadacitinib induction may be a viable treatment strategy for these very high-risk patients. Of course, large prospective trials will be needed to confirm this,” he added.
The study was funded by AbbVie, which markets upadacitinib (Rinvoq). Lead author Dr. Loftus and several study authors disclosed financial relationships with AbbVie and other companies. Dr. Sakuraba and Dr. Berinstein reported having no relevant financial conflicts.
Patients with ulcerative colitis who were treated with upadacitinib showed significant improvement in symptoms compared to placebo within days of starting treatment, according to results of a new study.
Immunosuppressants and biologics used to treat moderate to severe disease can vary in their response times from weeks to months, and some ambulatory patients fail to respond to these treatments, wrote Edward V. Loftus Jr., MD, of the Mayo Clinic, Rochester, Minn., and colleagues.
“The lack of effective, quick-acting therapies may lead patients to experience prolonged relapses, reduced quality of life, and a significant burden of illness,” the researchers said.
Upadacitinib, an oral, once-daily reversible JAK inhibitor, is approved by the Food and Drug Administration for patients with moderate to severe ulcerative colitis (UC) who have not responded to at least one tumor necrosis factor (TNF) blocker, but the time frame for response to upadacitinib has not been well studied, they said.
In a study published online in Clinical Gastroenterology and Hepatology, the researchers reviewed data from two phase 3 randomized, placebo-controlled induction trials, U-ACHIEVE Induction (NCT02819635) and U-ACCOMPLISH (NCT03653026), that assessed the safety and efficacy of a 45-mg daily dose of upadacitinib for managing symptoms of moderate to severe UC over an 8-week period.
The intent-to-treat analysis included 660 patients who had been randomized to received upadacitinib and 328 in the placebo group. Demographics were similar between the groups. Symptom improvement was based on changes in inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and fecal calprotectin (FCP) measured at week 2. Quality of life was assessed at 2 and 8 weeks.
Between 1 and 3 days after starting treatment, significantly greater percentages of patients in the treatment arm achieved a reduction of at least 50% from baseline in hs-CRP (75.7% vs. 21.9%) and FCP (48.2% vs. 20.2%).
The significant differences in symptom improvement persisted for 14 days from the first dose.
Patients in the upadacitinib group also showed increased rates of clinical remission/response (26.9%/59.4%) based on Partial Mayo scores at week 2 compared with placebo patients (4.3%/22.3%). Treated patients showed significant improvements in quality of life at weeks 2 and 8.
In addition, patient-reported UC symptoms of stool frequency, rectal bleeding, abdominal pain, and bowel urgency improved significantly compared with placebo by day 3, the researchers noted. “In this study, upadacitinib led to absence of rectal bleeding by day 1 and absence of abdominal pain and bowel urgency by day 3 in a significant proportion of patients vs. placebo (all P < .05),” they said.
The findings were limited by the post hoc design, but reflect results of other studies, including those of the JAK inhibitor tofacitinib, the researchers noted. The results of the current study demonstrate the ability of upadacitinib to alleviate key UC symptoms as early as 1 day after the first dose, they concluded.
Need for rapid onset treatment
A majority of biologics used to treat IBD take weeks to months to exert an effect, “so it is crucial that a drug with more rapid onset of action become available,” Atsushi Sakuraba, MD, director of clinical trial/IBD research at the University of Chicago, said in an interview.
Dr. Sakuraba, who was not involved in the study, said he was surprised by the findings.
“It is amazing that upadacitinib started to show response in 1-3 days and showed superior rates of remission/response versus placebo by week 2,” he said.
“Clinicians treating IBD patients should take the time to response into consideration, because it leads to rapid improvement in quality of life and reduced need for steroids,” Dr. Sakuraba said. The current study findings need to be confirmed in real life studies, he cautioned.
Expanding clinical options
Jeffrey Berinstein, MD, of the University of Michigan, Ann Arbor, who was not involved with the study, said in an interview that he was not surprised by upadacitinib’s quick action, which supports what he has seen in clinical practice. “We now have post hoc data from the phase 3, multicenter induction trials ... to provide us with hard evidence to support our observations,” he noted.
The current study is important because many patients with UC can be very symptomatic, with rectal bleeding, diarrhea, urgency, and abdominal pain, he said in an interview.
“It is often critical to have a fast-acting medication to avoid worsening symptoms, hospitalization, and severe complications. This study shows that upadacitinib, a new oral small molecule that selectively inhibits JAK-1, works within 1 day to improve symptoms and within 2 weeks to improve CRP and fecal calprotectin,” he said.
The takeaway message to clinicians is that upadacitinib is effective, relatively safe, and fast-acting option for patients with UC who have previously failed an anti-TNF agent, he said.
Challenges remain, however.
“Despite this exciting new therapeutic, there is still significant research needed to break our current therapeutic efficacy ceiling and get more IBD patients into a stable and durable remission,” Dr. Berinstein said. “Additional research is needed to develop personalized treatment strategies to address the unique needs of individual patients and to guide optimal medical management.”
Dr. Berinstein and his team are exploring the use of upadacitinib on the sickest IBD patients, “those admitted to the hospital with acute severe ulcerative colitis,” he said. Emerging data from multiple academic centers suggest that “upadacitinib induction may be a viable treatment strategy for these very high-risk patients. Of course, large prospective trials will be needed to confirm this,” he added.
The study was funded by AbbVie, which markets upadacitinib (Rinvoq). Lead author Dr. Loftus and several study authors disclosed financial relationships with AbbVie and other companies. Dr. Sakuraba and Dr. Berinstein reported having no relevant financial conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Blame IBS on gravity intolerance?
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The precise cause of irritable bowel syndrome (IBS) remains a mystery. A novel new hypothesis suggests that IBS could result from the body’s inability to manage gravity.
Gravity may be the “unifying factor in multiple seemingly disparate and mutually incompatible theories of IBS,” Brennan Spiegel, MD, director of Health Services Research at Cedars-Sinai Medical Center, Los Angeles, told this news organization. Dr. Spiegel’s gravity hypothesis of IBS is described in an article published in the December issue of American Journal of Gastroenterology.
A human being’s relationship to gravity is not unlike the relationship of a fish to water, he explained.
“We live our entire life in it, are shaped by it, yet hardly notice its ever-present influence on our body. Every fiber of our body is affected by gravity every day, including our gastrointestinal tract,” said Dr. Spiegel.
The abdominal contents are like a sack of heavy potatoes that we’re destined to carry around for our entire lives. To meet this demand, our body evolved to support the abdominal load with a set of mechanisms that hoist the viscera against gravity in an upright posture, Dr. Spiegel explained.
A failure of these mechanisms could lead to a host of problems, including motility problems or bacterial overgrowth in the gut and symptoms of IBS.
Dr. Spiegel’s gravity hypothesis, however, goes beyond the gastrointestinal tract.
“Our nervous system has evolved its own ways of managing gravity and how gut feelings arise when our nervous system detects gravity challenges, like getting ‘butterflies’ when falling on a roller coaster or in a turbulent airplane,” Dr. Spiegel said.
“Even our neuropsychological orientation to gravity is found in our language, like when people talk about feeling down in the dumps, feeling low, [or say they] can’t get out of bed. These are directional metaphors that we use that refer to the fact that there is something about getting pulled down that’s obviously negative,” he noted.
‘A big ask’
Dr. Spiegel said his gravity theory of IBS draws from “extensive literature to build a hypothesis that IBS may result from ineffective anatomical, physiological, and neuropsychological gravity-management systems designed to optimize GI form and function, protect body integrity, and maximize survival in a gravity-bound world.”
He acknowledged that it’s “a big ask” to get people to consider a unifying theory of anything. “But when we dig down deep, it’s not terribly controversial to me to suggest that our health has something to do with gravity. How could it not?” he said.
Dr. Spiegel also thinks this line of thinking has clinical implications.
“While we can’t change gravity, we can change our relationship to gravity in different ways,” he said.
“For starters, we can bolster our body to manage gravity better, through losing weight, exercise, and strengthening the anti-gravity extensor muscles along the back, which supports the spine, which is the chassis that holds up the whole body and includes maintaining the shape of the abdominal cavity,” Dr. Spiegel said.
The reason physical therapy and exercise are effective for IBS could be because these interventions strengthen the GI support systems, he said.
Testable theory
Before Dr. Spiegel “got up the courage” to submit his paper, he sent it to leading IBS researchers in the United States to get their honest opinion, he said.
“To my surprise, they wrote back and said this makes sense. And a few said this could have implications for other diseases,” he said in an interview.
Some of his patients with IBS have told him how the paper resonates and the specific ways they have noticed the impact of gravity and related air pressure on their IBS symptoms.
Some have reported that their symptoms get better when they scuba dive but worsen when they get out of the ocean; others said they feel much better up in the mountains versus at sea level; another said doing a headstand during yoga eases their GI symptoms.
“These may just be anecdotes, but they’re really striking,” Dr. Spiegel said.
His theory is not meant to replace any of the many existing theories of IBS, Dr. Spiegel emphasized. Rather, it’s an attempt to pull together the different theories under a single, potentially unifying explanation.
His paper includes a list of research projects that might help explore the gravity theory of IBS.
“It may be that none of this ends up being true, or bits and pieces of it are kind of true,” Dr. Spiegel said.
A research challenge
Dr. Spiegel has given researchers an “intriguing and interesting thought experiment and kind of a challenge to go out there and determine whether or not this hypothesis may actually be true,” Millie Long, MD, co–editor-in-chief of the American Journal of Gastroenterology, said in a podcast.
Dr. Long, a gastroenterologist and professor at the University of North Carolina at Chapel Hill School of Medicine, encouraged listeners to “dig deep into this hypothesis.”
The gravity hypothesis is provocative, “but the best thing about it is that it is testable,” Shelly Lu, MD, director of the Division of Digestive and Liver Diseases at Cedars-Sinai, Los Angeles, said in a news release issued by the medical center.
“If proved correct, it is a major paradigm shift in the way we think about IBS and possibly treatment as well,” said Dr. Lu.
Also weighing in, Brian Lacy, MD, PhD, a gastroenterologist with the Mayo Clinic, Jacksonville, Fla., noted that “our understanding of the etiopathophysiology of IBS has evolved over the past 50 years.”
“Once thought to be a psychiatric disorder (‘nervous colitis’) or a disorder simply of gut spasms (‘spastic colitis’), we now understand that symptoms of IBS develop for a multitude of reasons, including alterations in the gut microbiome, changes in gut sensation and motility, and modulation of the brain-gut axis, to name just a few,” Dr. Lacy said in an interview.
“Dr. Spiegel’s intriguing manuscript opens the door for us to think about IBS in a completely different way,” said Dr. Lacy.
“His novel hypothesis is a superb challenge to researchers and clinicians who can directly test his theory with a number of intriguing experiments. The results of these experiments may completely change the treatment paradigm for IBS patients,” Dr. Lacy added.
This research had no financial support. Dr. Spiegel and Dr. Lacy report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
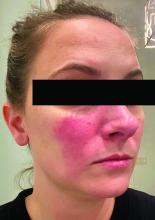
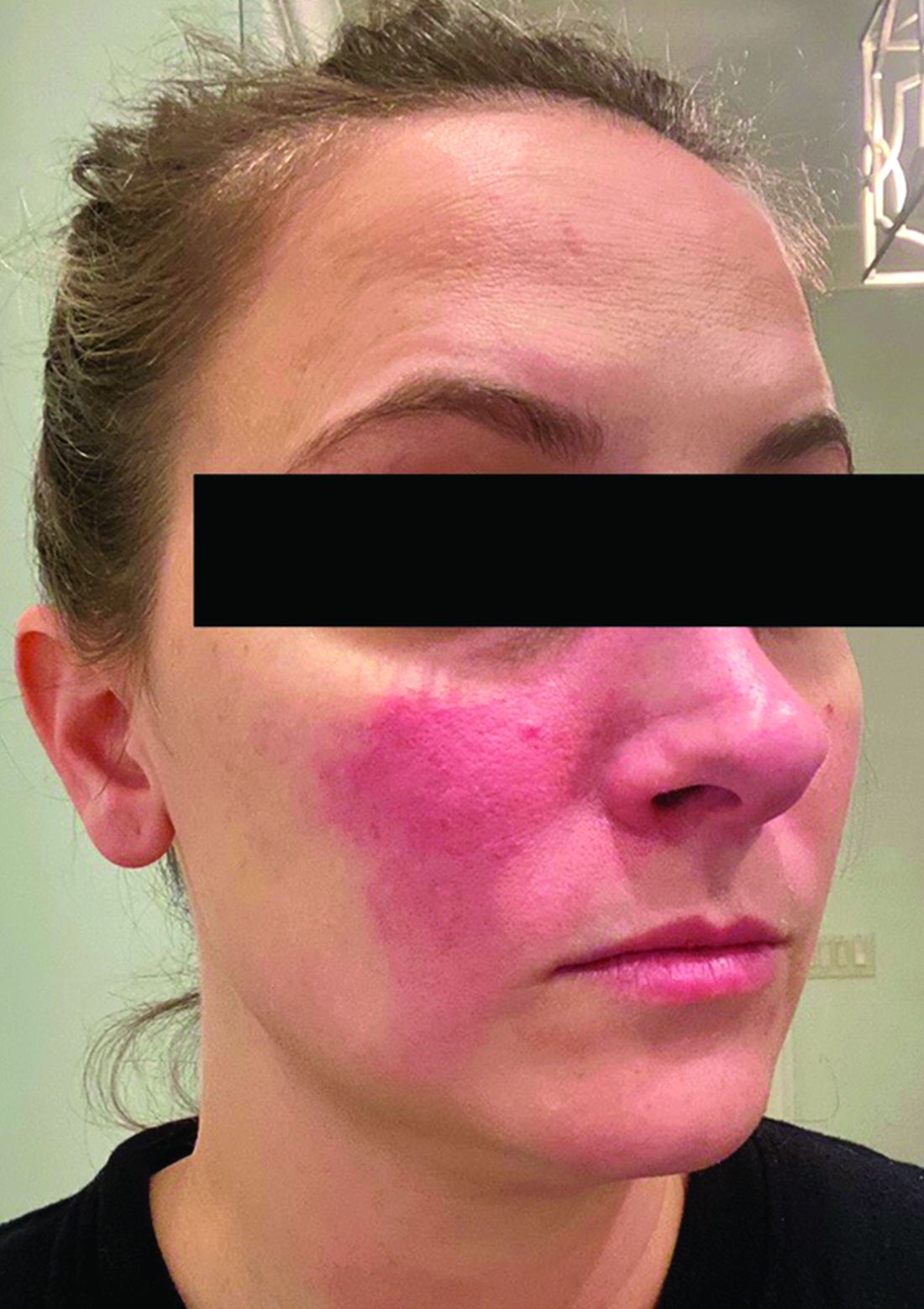
Rosacea and the gut: Looking into SIBO
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
REPORTING FROM IDS 2022
FDA OKs first fecal microbiota therapy for recurrent C. difficile
Rebyota (fecal microbiota, live-jslm), from Ferring Pharmaceuticals, is intended for use after an individual has completed antibiotic treatment for recurrent CDI. It is not indicated for the first occurrence of CDI.
“Recurrent CDI impacts an individual’s quality of life and can also potentially be life-threatening,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing approval.
As the first FDA-approved fecal microbiota product, this approval “represents an important milestone, as it provides an additional approved option to prevent recurrent CDI,” Dr. Marks added.
A panel of FDA advisors recommended approval of Rebyota in September.
The application for Rebyota received priority review and had orphan drug and breakthrough therapy designation.
A vicious cycle
Treatment options for recurrent CDI are limited. It’s been estimated that up to one-third of CDI cases recur, and people who suffer a recurrent bout of CDI are at a significantly higher risk for further infections.
Following the first recurrence, up to two-thirds of patients may experience a subsequent recurrence. Antibiotics used to treat CDI may contribute to a cycle of recurrence by altering the gut flora. The administration of fecal microbiota helps restore the gut flora to prevent further episodes of CDI.
“This is a major milestone in the translation of gut microbiome science to clinical solutions for patients,” Phillip I. Tarr, MD, chair of the American Gastroenterological Association’s Center for Gut Microbiome Research and Education Scientific Advisory Board, said in a written statement issued by the AGA. “This accomplishment is based on decades of work on the gut microbiome by gastroenterologists and collaborators. AGA applauds FDA for recognizing the demonstrated and conceptual merit of microbiota-based therapies.”
Rebyota is a microbiota-based live biotherapeutic prepared from human stool collected from prescreened, qualified donors. It comes prepackaged in a single dose that is administered rectally.
The safety and efficacy of Rebyota were assessed in five clinical trials with more than 1,000 participants, the company notes in a press release.
In one trial, following a standard course of antibiotics, a one-time treatment with Rebyota was successful for three-quarters of participants at 8 weeks.
The treatment also prevented additional bouts; 84% of these initial responders remaining free of CDI at 6 months.
Two-thirds of participants reported treatment-emergent adverse events. Most events were mild to moderate in severity. Diarrhea and abdominal pain were the most common.
The data, from the ongoing PUNCH CD3-OLS study, were presented in October at the annual meeting of the American College of Gastroenterology and were published simultaneously in the journal Drugs.
“This is a positive adjunct to our current therapies for C. difficile in terms of trying to knock it out once a standard course of antibiotics has been administered,” Lisa Malter, MD, a gastroenterologist and professor of medicine at New York University Langone Health, said in an interview.
Dr. Malter acknowledged that, because it’s delivered rectally, there could be “some hesitation” on the patient’s part to undergo the therapy.
However, C. difficile can be “excruciating” for patients, and they “may be more than willing to take [this agent] because it gets them feeling better,” said Dr. Malter said, who reported no relevant financial relationships.
The AGA will continue to follow the long-term effectiveness and safety of patients receiving Rebyota, fecal microbiota transplant, and other microbiota-based therapies through its FMT National Registry, according to the AGA statement.
Full prescribing information for Rebyota is available online.
For more information about CDI and FMT, visit patient.gastro.org.
Rebyota (fecal microbiota, live-jslm), from Ferring Pharmaceuticals, is intended for use after an individual has completed antibiotic treatment for recurrent CDI. It is not indicated for the first occurrence of CDI.
“Recurrent CDI impacts an individual’s quality of life and can also potentially be life-threatening,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing approval.
As the first FDA-approved fecal microbiota product, this approval “represents an important milestone, as it provides an additional approved option to prevent recurrent CDI,” Dr. Marks added.
A panel of FDA advisors recommended approval of Rebyota in September.
The application for Rebyota received priority review and had orphan drug and breakthrough therapy designation.
A vicious cycle
Treatment options for recurrent CDI are limited. It’s been estimated that up to one-third of CDI cases recur, and people who suffer a recurrent bout of CDI are at a significantly higher risk for further infections.
Following the first recurrence, up to two-thirds of patients may experience a subsequent recurrence. Antibiotics used to treat CDI may contribute to a cycle of recurrence by altering the gut flora. The administration of fecal microbiota helps restore the gut flora to prevent further episodes of CDI.
“This is a major milestone in the translation of gut microbiome science to clinical solutions for patients,” Phillip I. Tarr, MD, chair of the American Gastroenterological Association’s Center for Gut Microbiome Research and Education Scientific Advisory Board, said in a written statement issued by the AGA. “This accomplishment is based on decades of work on the gut microbiome by gastroenterologists and collaborators. AGA applauds FDA for recognizing the demonstrated and conceptual merit of microbiota-based therapies.”
Rebyota is a microbiota-based live biotherapeutic prepared from human stool collected from prescreened, qualified donors. It comes prepackaged in a single dose that is administered rectally.
The safety and efficacy of Rebyota were assessed in five clinical trials with more than 1,000 participants, the company notes in a press release.
In one trial, following a standard course of antibiotics, a one-time treatment with Rebyota was successful for three-quarters of participants at 8 weeks.
The treatment also prevented additional bouts; 84% of these initial responders remaining free of CDI at 6 months.
Two-thirds of participants reported treatment-emergent adverse events. Most events were mild to moderate in severity. Diarrhea and abdominal pain were the most common.
The data, from the ongoing PUNCH CD3-OLS study, were presented in October at the annual meeting of the American College of Gastroenterology and were published simultaneously in the journal Drugs.
“This is a positive adjunct to our current therapies for C. difficile in terms of trying to knock it out once a standard course of antibiotics has been administered,” Lisa Malter, MD, a gastroenterologist and professor of medicine at New York University Langone Health, said in an interview.
Dr. Malter acknowledged that, because it’s delivered rectally, there could be “some hesitation” on the patient’s part to undergo the therapy.
However, C. difficile can be “excruciating” for patients, and they “may be more than willing to take [this agent] because it gets them feeling better,” said Dr. Malter said, who reported no relevant financial relationships.
The AGA will continue to follow the long-term effectiveness and safety of patients receiving Rebyota, fecal microbiota transplant, and other microbiota-based therapies through its FMT National Registry, according to the AGA statement.
Full prescribing information for Rebyota is available online.
For more information about CDI and FMT, visit patient.gastro.org.
Rebyota (fecal microbiota, live-jslm), from Ferring Pharmaceuticals, is intended for use after an individual has completed antibiotic treatment for recurrent CDI. It is not indicated for the first occurrence of CDI.
“Recurrent CDI impacts an individual’s quality of life and can also potentially be life-threatening,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing approval.
As the first FDA-approved fecal microbiota product, this approval “represents an important milestone, as it provides an additional approved option to prevent recurrent CDI,” Dr. Marks added.
A panel of FDA advisors recommended approval of Rebyota in September.
The application for Rebyota received priority review and had orphan drug and breakthrough therapy designation.
A vicious cycle
Treatment options for recurrent CDI are limited. It’s been estimated that up to one-third of CDI cases recur, and people who suffer a recurrent bout of CDI are at a significantly higher risk for further infections.
Following the first recurrence, up to two-thirds of patients may experience a subsequent recurrence. Antibiotics used to treat CDI may contribute to a cycle of recurrence by altering the gut flora. The administration of fecal microbiota helps restore the gut flora to prevent further episodes of CDI.
“This is a major milestone in the translation of gut microbiome science to clinical solutions for patients,” Phillip I. Tarr, MD, chair of the American Gastroenterological Association’s Center for Gut Microbiome Research and Education Scientific Advisory Board, said in a written statement issued by the AGA. “This accomplishment is based on decades of work on the gut microbiome by gastroenterologists and collaborators. AGA applauds FDA for recognizing the demonstrated and conceptual merit of microbiota-based therapies.”
Rebyota is a microbiota-based live biotherapeutic prepared from human stool collected from prescreened, qualified donors. It comes prepackaged in a single dose that is administered rectally.
The safety and efficacy of Rebyota were assessed in five clinical trials with more than 1,000 participants, the company notes in a press release.
In one trial, following a standard course of antibiotics, a one-time treatment with Rebyota was successful for three-quarters of participants at 8 weeks.
The treatment also prevented additional bouts; 84% of these initial responders remaining free of CDI at 6 months.
Two-thirds of participants reported treatment-emergent adverse events. Most events were mild to moderate in severity. Diarrhea and abdominal pain were the most common.
The data, from the ongoing PUNCH CD3-OLS study, were presented in October at the annual meeting of the American College of Gastroenterology and were published simultaneously in the journal Drugs.
“This is a positive adjunct to our current therapies for C. difficile in terms of trying to knock it out once a standard course of antibiotics has been administered,” Lisa Malter, MD, a gastroenterologist and professor of medicine at New York University Langone Health, said in an interview.
Dr. Malter acknowledged that, because it’s delivered rectally, there could be “some hesitation” on the patient’s part to undergo the therapy.
However, C. difficile can be “excruciating” for patients, and they “may be more than willing to take [this agent] because it gets them feeling better,” said Dr. Malter said, who reported no relevant financial relationships.
The AGA will continue to follow the long-term effectiveness and safety of patients receiving Rebyota, fecal microbiota transplant, and other microbiota-based therapies through its FMT National Registry, according to the AGA statement.
Full prescribing information for Rebyota is available online.
For more information about CDI and FMT, visit patient.gastro.org.
Virtual yoga program appears to improve IBS symptoms, fatigue, stress
Participants reported a decrease in IBS-related symptoms and improvements in quality of life, fatigue, and perceived stress.
“IBS affects upwards of 15%-20% of the North American population, and despite our advances in the area, we have very limited options to offer our patients,” Maitreyi Raman, MD, an associate professor of medicine at the University of Calgary (Alta.), said in an interview.
“Often, we are focused on treating symptoms but not addressing the underlying cause,” said Dr. Raman, who is director of Alberta’s Collaboration of Excellence for Nutrition in Digestive Diseases. “With advances around the gut microbiome and the evolving science on the brain-gut axis, mind-body interventions could offer a therapeutic option that patients can use to improve the overall course of their disease.”
The study was published online in the American Journal of Gastroenterology.
Online yoga program vs. IBS advice only
IBS often involves alterations of the gut-brain axis and can be affected by psychological or physiological stress, the study authors write. Previous studies have found that in-person yoga programs can manage IBS symptoms and improve physiological, psychological, and emotional health.
During the COVID-19 pandemic, yoga programs had to switch to a virtual format – a delivery method that could remain relevant due to limited health care resources. However, the efficacy, feasibility, and safety of virtual yoga for people with IBS were unknown.
Dr. Raman and colleagues conducted a randomized, two-group, controlled clinical trial at the University of Calgary (Alta.) between March 2021 and December 2022. The 79 participants weren’t blinded to the trial arms – an online yoga program or an advice-only control group.
The eligible participants had a diagnosis of IBS, scored at least 75 out of 500 points on the IBS Symptoms Severity Scale (IBS-SSS) for mild IBS, and were on stable doses of medications for IBS. They were instructed to continue with their current therapies during the study but didn’t start new medications or make major changes to their diet or physical patterns.
The yoga program was based on Upa Yoga, a subtype of Hatha Yoga developed by the Isha Foundation of Inner Sciences. The program was delivered by a certified yoga facilitator from the Isha Foundation and included directional movements, neck rotations, breathing practices, breath watching, and mantra meditation with aum/om chanting.
The online classes of three to seven participants were delivered in 60-minute sessions for 8 weeks. The participants were also asked to practice at home daily with the support of yoga videos.
The advice-only control group included a 10-minute video with general education on IBS, the mind-gut connection in IBS, and the role of mind-body therapies in managing IBS. The participants received a list of IBS-related resources from the Canadian Digestive Health Foundation, a link to an IBS patient support group, and information about physical activity guidelines from the World Health Organization.
The research team looked for a primary endpoint of at least a 50-point reduction on the IBS-SSS, which is considered clinically meaningful.
They also measured for secondary outcomes, such as quality of life, anxiety, depression, perceived stress, COVID-19–related stress, fatigue, somatic symptoms, self-compassion, and intention to practice yoga.
Among the 79 participants, 38 were randomized to the yoga program and 41 were randomized to the advice-only control group. The average age was 45 years. Most (92%) were women, and 81% were White. The average IBS duration since diagnosis was 11.5 years.
The overall average IBS-SSS was moderate, at 245.3, at the beginning of the program, and dropped to 207.9 at week 8. The score decreased from 255.2 to 200.5 in the yoga group and from 236.1 to 213.5 in the control group. The difference between the groups was 32 points, which wasn’t statistically significant, though symptom improvement began after 4 weeks in the yoga group.
In the yoga group, 14 participants (37%) met the target decrease of 50 points or more, compared with eight participants (20%) in the control group. These 22 “responders” reported improvements in IBS symptoms, quality of life, perceived stress, and COVID-19–related stress.
Specifically, among the 14 responders in the yoga group, there were significant improvements in IBS symptoms, quality of life, fatigue, somatic symptoms, self-compassion, and COVID-19–related stress. In the control group, there were significant improvements in IBS symptoms and COVID-19–related stress.
Using an intent-to-treat analysis, the research team found that the yoga group had improved quality of life, fatigue, and perceived stress. In the control group, improvements were seen only in COVID-19–related stress.
No significant improvements were found in anxiety or depression between the groups, although the changes in depression scores were in favor of the yoga group. The intention to practice yoga dropped in both groups during the study period, but it wasn’t associated with the actual yoga practice minutes or change in IBS-SSS scores.
“We saw a surprising improvement in quality of life,” Dr. Raman said. “Although we talk about quality of life as an important endpoint, it can be hard to show in studies, so that was a nice finding to demonstrate in this study.”
The yoga intervention was feasible in terms of adherence (79%), attrition rate (20%), and high program satisfaction, the researchers write. Safety was demonstrated by the absence of any adverse events.
Future program considerations
Dr. Raman and colleagues are interested in understanding the mechanisms that underlie the efficacy of mind-body interventions. They also plan to test the virtual yoga program in a mobile app, called LyfeMD, which is intended to support patients with digestive diseases through evidence-based dietary programs and mind-body interventions, such as guided meditation, breathing exercises, and cognitive behavioral therapy.
“We know that patients are looking for all possible resources,” Dr. Raman said. “Our next goal is to better understand how an app-based intervention can be effective, even without a live instructor.”
Future studies should also consider clinicians’ perspectives, she noted. In previous studies, Dr. Raman and colleagues have found that physicians are open to recommending yoga as a therapeutic option for patients, but some are unsure how to prescribe a recommended dose, frequency, or type of yoga.
“When treating patients with IBS, it is important to think broadly and creatively about all our treatment options,” said Elyse Thakur, PhD, a clinical health psychologist at Atrium Health Gastroenterology and Hepatology, Charlotte, N.C.
Dr. Thakur, who wasn’t involved with this study, specializes in gastrointestinal health psychology. She and colleagues use numerous complementary and alternative medicine options with patients.
“We have to remember that people may respond differently to available treatment options,” she said. “It is imperative to understand the evidence so we can have productive conversations with our patients about the pros and cons and the potential benefits and limitations.”
The study did not receive a specific grant from a funding agency. The authors and Dr. Thakur declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Participants reported a decrease in IBS-related symptoms and improvements in quality of life, fatigue, and perceived stress.
“IBS affects upwards of 15%-20% of the North American population, and despite our advances in the area, we have very limited options to offer our patients,” Maitreyi Raman, MD, an associate professor of medicine at the University of Calgary (Alta.), said in an interview.
“Often, we are focused on treating symptoms but not addressing the underlying cause,” said Dr. Raman, who is director of Alberta’s Collaboration of Excellence for Nutrition in Digestive Diseases. “With advances around the gut microbiome and the evolving science on the brain-gut axis, mind-body interventions could offer a therapeutic option that patients can use to improve the overall course of their disease.”
The study was published online in the American Journal of Gastroenterology.
Online yoga program vs. IBS advice only
IBS often involves alterations of the gut-brain axis and can be affected by psychological or physiological stress, the study authors write. Previous studies have found that in-person yoga programs can manage IBS symptoms and improve physiological, psychological, and emotional health.
During the COVID-19 pandemic, yoga programs had to switch to a virtual format – a delivery method that could remain relevant due to limited health care resources. However, the efficacy, feasibility, and safety of virtual yoga for people with IBS were unknown.
Dr. Raman and colleagues conducted a randomized, two-group, controlled clinical trial at the University of Calgary (Alta.) between March 2021 and December 2022. The 79 participants weren’t blinded to the trial arms – an online yoga program or an advice-only control group.
The eligible participants had a diagnosis of IBS, scored at least 75 out of 500 points on the IBS Symptoms Severity Scale (IBS-SSS) for mild IBS, and were on stable doses of medications for IBS. They were instructed to continue with their current therapies during the study but didn’t start new medications or make major changes to their diet or physical patterns.
The yoga program was based on Upa Yoga, a subtype of Hatha Yoga developed by the Isha Foundation of Inner Sciences. The program was delivered by a certified yoga facilitator from the Isha Foundation and included directional movements, neck rotations, breathing practices, breath watching, and mantra meditation with aum/om chanting.
The online classes of three to seven participants were delivered in 60-minute sessions for 8 weeks. The participants were also asked to practice at home daily with the support of yoga videos.
The advice-only control group included a 10-minute video with general education on IBS, the mind-gut connection in IBS, and the role of mind-body therapies in managing IBS. The participants received a list of IBS-related resources from the Canadian Digestive Health Foundation, a link to an IBS patient support group, and information about physical activity guidelines from the World Health Organization.
The research team looked for a primary endpoint of at least a 50-point reduction on the IBS-SSS, which is considered clinically meaningful.
They also measured for secondary outcomes, such as quality of life, anxiety, depression, perceived stress, COVID-19–related stress, fatigue, somatic symptoms, self-compassion, and intention to practice yoga.
Among the 79 participants, 38 were randomized to the yoga program and 41 were randomized to the advice-only control group. The average age was 45 years. Most (92%) were women, and 81% were White. The average IBS duration since diagnosis was 11.5 years.
The overall average IBS-SSS was moderate, at 245.3, at the beginning of the program, and dropped to 207.9 at week 8. The score decreased from 255.2 to 200.5 in the yoga group and from 236.1 to 213.5 in the control group. The difference between the groups was 32 points, which wasn’t statistically significant, though symptom improvement began after 4 weeks in the yoga group.
In the yoga group, 14 participants (37%) met the target decrease of 50 points or more, compared with eight participants (20%) in the control group. These 22 “responders” reported improvements in IBS symptoms, quality of life, perceived stress, and COVID-19–related stress.
Specifically, among the 14 responders in the yoga group, there were significant improvements in IBS symptoms, quality of life, fatigue, somatic symptoms, self-compassion, and COVID-19–related stress. In the control group, there were significant improvements in IBS symptoms and COVID-19–related stress.
Using an intent-to-treat analysis, the research team found that the yoga group had improved quality of life, fatigue, and perceived stress. In the control group, improvements were seen only in COVID-19–related stress.
No significant improvements were found in anxiety or depression between the groups, although the changes in depression scores were in favor of the yoga group. The intention to practice yoga dropped in both groups during the study period, but it wasn’t associated with the actual yoga practice minutes or change in IBS-SSS scores.
“We saw a surprising improvement in quality of life,” Dr. Raman said. “Although we talk about quality of life as an important endpoint, it can be hard to show in studies, so that was a nice finding to demonstrate in this study.”
The yoga intervention was feasible in terms of adherence (79%), attrition rate (20%), and high program satisfaction, the researchers write. Safety was demonstrated by the absence of any adverse events.
Future program considerations
Dr. Raman and colleagues are interested in understanding the mechanisms that underlie the efficacy of mind-body interventions. They also plan to test the virtual yoga program in a mobile app, called LyfeMD, which is intended to support patients with digestive diseases through evidence-based dietary programs and mind-body interventions, such as guided meditation, breathing exercises, and cognitive behavioral therapy.
“We know that patients are looking for all possible resources,” Dr. Raman said. “Our next goal is to better understand how an app-based intervention can be effective, even without a live instructor.”
Future studies should also consider clinicians’ perspectives, she noted. In previous studies, Dr. Raman and colleagues have found that physicians are open to recommending yoga as a therapeutic option for patients, but some are unsure how to prescribe a recommended dose, frequency, or type of yoga.
“When treating patients with IBS, it is important to think broadly and creatively about all our treatment options,” said Elyse Thakur, PhD, a clinical health psychologist at Atrium Health Gastroenterology and Hepatology, Charlotte, N.C.
Dr. Thakur, who wasn’t involved with this study, specializes in gastrointestinal health psychology. She and colleagues use numerous complementary and alternative medicine options with patients.
“We have to remember that people may respond differently to available treatment options,” she said. “It is imperative to understand the evidence so we can have productive conversations with our patients about the pros and cons and the potential benefits and limitations.”
The study did not receive a specific grant from a funding agency. The authors and Dr. Thakur declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Participants reported a decrease in IBS-related symptoms and improvements in quality of life, fatigue, and perceived stress.
“IBS affects upwards of 15%-20% of the North American population, and despite our advances in the area, we have very limited options to offer our patients,” Maitreyi Raman, MD, an associate professor of medicine at the University of Calgary (Alta.), said in an interview.
“Often, we are focused on treating symptoms but not addressing the underlying cause,” said Dr. Raman, who is director of Alberta’s Collaboration of Excellence for Nutrition in Digestive Diseases. “With advances around the gut microbiome and the evolving science on the brain-gut axis, mind-body interventions could offer a therapeutic option that patients can use to improve the overall course of their disease.”
The study was published online in the American Journal of Gastroenterology.
Online yoga program vs. IBS advice only
IBS often involves alterations of the gut-brain axis and can be affected by psychological or physiological stress, the study authors write. Previous studies have found that in-person yoga programs can manage IBS symptoms and improve physiological, psychological, and emotional health.
During the COVID-19 pandemic, yoga programs had to switch to a virtual format – a delivery method that could remain relevant due to limited health care resources. However, the efficacy, feasibility, and safety of virtual yoga for people with IBS were unknown.
Dr. Raman and colleagues conducted a randomized, two-group, controlled clinical trial at the University of Calgary (Alta.) between March 2021 and December 2022. The 79 participants weren’t blinded to the trial arms – an online yoga program or an advice-only control group.
The eligible participants had a diagnosis of IBS, scored at least 75 out of 500 points on the IBS Symptoms Severity Scale (IBS-SSS) for mild IBS, and were on stable doses of medications for IBS. They were instructed to continue with their current therapies during the study but didn’t start new medications or make major changes to their diet or physical patterns.
The yoga program was based on Upa Yoga, a subtype of Hatha Yoga developed by the Isha Foundation of Inner Sciences. The program was delivered by a certified yoga facilitator from the Isha Foundation and included directional movements, neck rotations, breathing practices, breath watching, and mantra meditation with aum/om chanting.
The online classes of three to seven participants were delivered in 60-minute sessions for 8 weeks. The participants were also asked to practice at home daily with the support of yoga videos.
The advice-only control group included a 10-minute video with general education on IBS, the mind-gut connection in IBS, and the role of mind-body therapies in managing IBS. The participants received a list of IBS-related resources from the Canadian Digestive Health Foundation, a link to an IBS patient support group, and information about physical activity guidelines from the World Health Organization.
The research team looked for a primary endpoint of at least a 50-point reduction on the IBS-SSS, which is considered clinically meaningful.
They also measured for secondary outcomes, such as quality of life, anxiety, depression, perceived stress, COVID-19–related stress, fatigue, somatic symptoms, self-compassion, and intention to practice yoga.
Among the 79 participants, 38 were randomized to the yoga program and 41 were randomized to the advice-only control group. The average age was 45 years. Most (92%) were women, and 81% were White. The average IBS duration since diagnosis was 11.5 years.
The overall average IBS-SSS was moderate, at 245.3, at the beginning of the program, and dropped to 207.9 at week 8. The score decreased from 255.2 to 200.5 in the yoga group and from 236.1 to 213.5 in the control group. The difference between the groups was 32 points, which wasn’t statistically significant, though symptom improvement began after 4 weeks in the yoga group.
In the yoga group, 14 participants (37%) met the target decrease of 50 points or more, compared with eight participants (20%) in the control group. These 22 “responders” reported improvements in IBS symptoms, quality of life, perceived stress, and COVID-19–related stress.
Specifically, among the 14 responders in the yoga group, there were significant improvements in IBS symptoms, quality of life, fatigue, somatic symptoms, self-compassion, and COVID-19–related stress. In the control group, there were significant improvements in IBS symptoms and COVID-19–related stress.
Using an intent-to-treat analysis, the research team found that the yoga group had improved quality of life, fatigue, and perceived stress. In the control group, improvements were seen only in COVID-19–related stress.
No significant improvements were found in anxiety or depression between the groups, although the changes in depression scores were in favor of the yoga group. The intention to practice yoga dropped in both groups during the study period, but it wasn’t associated with the actual yoga practice minutes or change in IBS-SSS scores.
“We saw a surprising improvement in quality of life,” Dr. Raman said. “Although we talk about quality of life as an important endpoint, it can be hard to show in studies, so that was a nice finding to demonstrate in this study.”
The yoga intervention was feasible in terms of adherence (79%), attrition rate (20%), and high program satisfaction, the researchers write. Safety was demonstrated by the absence of any adverse events.
Future program considerations
Dr. Raman and colleagues are interested in understanding the mechanisms that underlie the efficacy of mind-body interventions. They also plan to test the virtual yoga program in a mobile app, called LyfeMD, which is intended to support patients with digestive diseases through evidence-based dietary programs and mind-body interventions, such as guided meditation, breathing exercises, and cognitive behavioral therapy.
“We know that patients are looking for all possible resources,” Dr. Raman said. “Our next goal is to better understand how an app-based intervention can be effective, even without a live instructor.”
Future studies should also consider clinicians’ perspectives, she noted. In previous studies, Dr. Raman and colleagues have found that physicians are open to recommending yoga as a therapeutic option for patients, but some are unsure how to prescribe a recommended dose, frequency, or type of yoga.
“When treating patients with IBS, it is important to think broadly and creatively about all our treatment options,” said Elyse Thakur, PhD, a clinical health psychologist at Atrium Health Gastroenterology and Hepatology, Charlotte, N.C.
Dr. Thakur, who wasn’t involved with this study, specializes in gastrointestinal health psychology. She and colleagues use numerous complementary and alternative medicine options with patients.
“We have to remember that people may respond differently to available treatment options,” she said. “It is imperative to understand the evidence so we can have productive conversations with our patients about the pros and cons and the potential benefits and limitations.”
The study did not receive a specific grant from a funding agency. The authors and Dr. Thakur declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Bloating common but often ignored: Survey
, some of whom said they weren’t comfortable discussing it with their doctor, according to a large national survey.
The findings suggest doctors should “proactively” ask about bloating, especially in adults at increased risk, including women and those with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), the researchers say.
“Bloating is common because it usually has multifactorial causes and can also be a secondary symptom to another gastrointestinal (GI) symptom or condition. Its mechanisms are complex and individualized, making it difficult for providers to identify and treat each patient,” Janice E. Oh, MD, department of medicine, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“Thus, many adults may be persistently suffering without proper diagnosis or management,” Dr. Oh added.
Results of the survey are published online in Clinical Gastroenterology and Hepatology.
Common problem, incompletely understood
To get a better handle on the nationwide prevalence and health-related impact of bloating in the United States, Dr. Oh and her colleagues conducted an online survey of a nationally representative group of 88,795 adults aged 18 years or older.
Altogether, 12,324 (14%) respondents reported bloating in the past week.
The likelihood of bloating was significantly higher in women (odds ratio, 2.56) and in those with certain comorbid conditions, especially IBS, chronic constipation, and ulcerative colitis, the authors write.
The odds of bloating were also higher in adults with other concomitant GI symptoms, especially abdominal pain and excess gas.
Factors associated with more severe bloating included the presence of IBS, IBD, celiac disease, bowel incontinence, abdominal pain, constipation (functional and opioid-induced), and excess gas.
Bloating severity increased with age up to 59 years and then decreased in people aged 60 years or older.
Suffering in silence?
Notably, more than half (59%) of people who reported recent bloating never sought care for the problem. About one-third of them reported that bloating resolved on its own, and 30% said the symptoms were not bothersome.
About 1 in 5 adults who did not seek care said that they were managing symptoms on their own with over-the-counter medications or lifestyle modifications. And 9% of those who did not seek care said that they were uncomfortable discussing the problem with their doctor.
“The hesitancy in seeking health care or discussing bloating in patients may be attributed to lack of routine screening for bloating, lack of focus on bloating complaints by providers, or patients’ dissatisfaction with management of bloating symptoms,” the researchers say.
Adults most apt to seek care for bloating were those older than 29 years; non-Hispanic Black persons; those with comorbid conditions, such as celiac disease, IBD, and IBS; and those with more severe bloating symptoms.
A limitation is that individuals with GI symptoms or conditions may be more likely to participate in a GI-focused survey, leading to a possible overestimation of the prevalence of bloating.
Also, the survey was conducted during the COVID-19 pandemic, which has the potential to overestimate the prevalence or severity of bloating because COVID-19 is known to affect the GI system.
Despite these limitations, the researchers encourage health care professionals to routinely ask their patients about bloating as a first step in appropriate management.
“Bloating can be associated with nutrition/diet, the gut microbiome, anatomical issues, or underlying conditions that range from neurologic to gynecologic disorders. And, the majority of the time, it is usually more than one distinct issue that is attributing to the bloating,” Dr. Oh said.
“Understanding the patterns of bloating occurrence, psychosocial factors, past medical history, and nutrition can help providers determine the causes. We hope to identify a more standardized method to identify causes of bloating,” Dr. Oh added.
Support for the survey was provided by Ironwood Pharmaceuticals in the form of an institutional research grant to Cedars-Sinai. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, some of whom said they weren’t comfortable discussing it with their doctor, according to a large national survey.
The findings suggest doctors should “proactively” ask about bloating, especially in adults at increased risk, including women and those with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), the researchers say.
“Bloating is common because it usually has multifactorial causes and can also be a secondary symptom to another gastrointestinal (GI) symptom or condition. Its mechanisms are complex and individualized, making it difficult for providers to identify and treat each patient,” Janice E. Oh, MD, department of medicine, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“Thus, many adults may be persistently suffering without proper diagnosis or management,” Dr. Oh added.
Results of the survey are published online in Clinical Gastroenterology and Hepatology.
Common problem, incompletely understood
To get a better handle on the nationwide prevalence and health-related impact of bloating in the United States, Dr. Oh and her colleagues conducted an online survey of a nationally representative group of 88,795 adults aged 18 years or older.
Altogether, 12,324 (14%) respondents reported bloating in the past week.
The likelihood of bloating was significantly higher in women (odds ratio, 2.56) and in those with certain comorbid conditions, especially IBS, chronic constipation, and ulcerative colitis, the authors write.
The odds of bloating were also higher in adults with other concomitant GI symptoms, especially abdominal pain and excess gas.
Factors associated with more severe bloating included the presence of IBS, IBD, celiac disease, bowel incontinence, abdominal pain, constipation (functional and opioid-induced), and excess gas.
Bloating severity increased with age up to 59 years and then decreased in people aged 60 years or older.
Suffering in silence?
Notably, more than half (59%) of people who reported recent bloating never sought care for the problem. About one-third of them reported that bloating resolved on its own, and 30% said the symptoms were not bothersome.
About 1 in 5 adults who did not seek care said that they were managing symptoms on their own with over-the-counter medications or lifestyle modifications. And 9% of those who did not seek care said that they were uncomfortable discussing the problem with their doctor.
“The hesitancy in seeking health care or discussing bloating in patients may be attributed to lack of routine screening for bloating, lack of focus on bloating complaints by providers, or patients’ dissatisfaction with management of bloating symptoms,” the researchers say.
Adults most apt to seek care for bloating were those older than 29 years; non-Hispanic Black persons; those with comorbid conditions, such as celiac disease, IBD, and IBS; and those with more severe bloating symptoms.
A limitation is that individuals with GI symptoms or conditions may be more likely to participate in a GI-focused survey, leading to a possible overestimation of the prevalence of bloating.
Also, the survey was conducted during the COVID-19 pandemic, which has the potential to overestimate the prevalence or severity of bloating because COVID-19 is known to affect the GI system.
Despite these limitations, the researchers encourage health care professionals to routinely ask their patients about bloating as a first step in appropriate management.
“Bloating can be associated with nutrition/diet, the gut microbiome, anatomical issues, or underlying conditions that range from neurologic to gynecologic disorders. And, the majority of the time, it is usually more than one distinct issue that is attributing to the bloating,” Dr. Oh said.
“Understanding the patterns of bloating occurrence, psychosocial factors, past medical history, and nutrition can help providers determine the causes. We hope to identify a more standardized method to identify causes of bloating,” Dr. Oh added.
Support for the survey was provided by Ironwood Pharmaceuticals in the form of an institutional research grant to Cedars-Sinai. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, some of whom said they weren’t comfortable discussing it with their doctor, according to a large national survey.
The findings suggest doctors should “proactively” ask about bloating, especially in adults at increased risk, including women and those with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), the researchers say.
“Bloating is common because it usually has multifactorial causes and can also be a secondary symptom to another gastrointestinal (GI) symptom or condition. Its mechanisms are complex and individualized, making it difficult for providers to identify and treat each patient,” Janice E. Oh, MD, department of medicine, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“Thus, many adults may be persistently suffering without proper diagnosis or management,” Dr. Oh added.
Results of the survey are published online in Clinical Gastroenterology and Hepatology.
Common problem, incompletely understood
To get a better handle on the nationwide prevalence and health-related impact of bloating in the United States, Dr. Oh and her colleagues conducted an online survey of a nationally representative group of 88,795 adults aged 18 years or older.
Altogether, 12,324 (14%) respondents reported bloating in the past week.
The likelihood of bloating was significantly higher in women (odds ratio, 2.56) and in those with certain comorbid conditions, especially IBS, chronic constipation, and ulcerative colitis, the authors write.
The odds of bloating were also higher in adults with other concomitant GI symptoms, especially abdominal pain and excess gas.
Factors associated with more severe bloating included the presence of IBS, IBD, celiac disease, bowel incontinence, abdominal pain, constipation (functional and opioid-induced), and excess gas.
Bloating severity increased with age up to 59 years and then decreased in people aged 60 years or older.
Suffering in silence?
Notably, more than half (59%) of people who reported recent bloating never sought care for the problem. About one-third of them reported that bloating resolved on its own, and 30% said the symptoms were not bothersome.
About 1 in 5 adults who did not seek care said that they were managing symptoms on their own with over-the-counter medications or lifestyle modifications. And 9% of those who did not seek care said that they were uncomfortable discussing the problem with their doctor.
“The hesitancy in seeking health care or discussing bloating in patients may be attributed to lack of routine screening for bloating, lack of focus on bloating complaints by providers, or patients’ dissatisfaction with management of bloating symptoms,” the researchers say.
Adults most apt to seek care for bloating were those older than 29 years; non-Hispanic Black persons; those with comorbid conditions, such as celiac disease, IBD, and IBS; and those with more severe bloating symptoms.
A limitation is that individuals with GI symptoms or conditions may be more likely to participate in a GI-focused survey, leading to a possible overestimation of the prevalence of bloating.
Also, the survey was conducted during the COVID-19 pandemic, which has the potential to overestimate the prevalence or severity of bloating because COVID-19 is known to affect the GI system.
Despite these limitations, the researchers encourage health care professionals to routinely ask their patients about bloating as a first step in appropriate management.
“Bloating can be associated with nutrition/diet, the gut microbiome, anatomical issues, or underlying conditions that range from neurologic to gynecologic disorders. And, the majority of the time, it is usually more than one distinct issue that is attributing to the bloating,” Dr. Oh said.
“Understanding the patterns of bloating occurrence, psychosocial factors, past medical history, and nutrition can help providers determine the causes. We hope to identify a more standardized method to identify causes of bloating,” Dr. Oh added.
Support for the survey was provided by Ironwood Pharmaceuticals in the form of an institutional research grant to Cedars-Sinai. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Celiac disease linked to higher risk for rheumatoid arthritis, juvenile idiopathic arthritis
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Celiac disease is linked to juvenile idiopathic arthritis (JIA) in children and rheumatoid arthritis (RA) in adults, according to an analysis of nationwide data in Sweden.
“I hope that our study can ultimately change clinical practice by lowering the threshold to evaluate celiac disease patients for inflammatory joint diseases,” John B. Doyle, MD, a gastroenterology fellow at Columbia University Irving Medical Center in New York, told this news organization.
“Inflammatory joint diseases, such as JIA and RA, are notoriously difficult to diagnose given their variable presentations,” he said. “But if JIA or RA can be identified sooner by physicians, patients will ultimately benefit by starting disease-modifying therapy earlier in their disease course.”
The study was published online in The American Journal of Gastroenterology.
Analyzing associations
Celiac disease has been linked to numerous autoimmune diseases, including type 1 diabetes, autoimmune thyroid disease, lupus, and inflammatory bowel disease (IBD), Dr. Doyle noted. However, a definitive epidemiologic association between celiac disease and inflammatory joint diseases such as JIA or RA hasn›t been established.
Dr. Doyle and colleagues conducted a nationwide population-based, retrospective matched cohort study using the Epidemiology Strengthened by Histopathology Reports in Sweden. They identified 24,014 patients diagnosed with biopsy-proven celiac disease between 2004 and 2017.
With these data, each patient was matched to five reference individuals in the general population by age, sex, calendar year, and geographic region, for a total of 117,397 people without a previous diagnosis of celiac disease. The researchers calculated the incidence and estimated the relative risk for JIA in patients younger than 18 years and RA in patients aged 18 years or older.
For those younger than 18 years, the incidence rate of JIA was 5.9 per 10,000 person-years among the 9,415 patients with celiac disease versus 2.2 per 10,000 person-years in the general population, over a follow-up of 7 years. Those with celiac disease were 2.7 times as likely to develop JIA.
The association between celiac disease and JIA remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. The incidence rate of JIA among patients with celiac disease was higher in both females and males, and across all age groups studied.
When 6,703 children with celiac disease were compared with their 9,089 siblings without celiac disease, the higher risk for JIA in patients with celiac disease fell slightly short of statistical significance.
For those aged 18 years or older, the incidence rate of RA was 8.4 per 10,000 person-years among the 14,599 patients with celiac disease versus 5.1 per 10,000 person-years in the general population, over a follow-up of 8.8 years. Those with celiac disease were 1.7 times as likely to develop RA.
As with the younger cohort, the association between celiac disease and RA in the adult group remained similar after adjustment for education, Nordic country of birth, type 1 diabetes, autoimmune thyroid disease, lupus, and IBD. Although both men and women with celiac disease had higher rates of RA, the risk was higher among those in whom disease was diagnosed at age 18-59 years compared with those who received a diagnosis at age 60 years or older.
When 9,578 adults with celiac disease were compared with their 17,067 siblings without celiac disease, the risk for RA remained higher in patients with celiac disease.
This suggests “that the association between celiac disease and RA is unlikely to be explained by environmental factors alone,” Dr. Doyle said.
Additional findings
Notably, the primary analysis excluded patients diagnosed with JIA or RA before their celiac disease diagnosis. In additional analyses, however, significant associations emerged.
Among children with celiac disease, 0.5% had a previous diagnosis of JIA, compared with 0.1% of matched comparators. Those with celiac disease were 3.5 times more likely to have a JIA diagnosis.
Among adults with celiac disease, 0.9% had a previous diagnosis of RA, compared with 0.6% of matched comparators. Those with celiac disease were 1.4 times more likely to have a RA diagnosis.
“We found that diagnoses of these types of arthritis were more common before a diagnosis of celiac disease compared to the general population,” Benjamin Lebwohl, MD, director of clinical research at the Celiac Disease Center at Columbia University, New York, told this news organization.
“This suggests that undiagnosed and untreated celiac disease might be contributing to these others autoimmune conditions,” he said.
Dr. Doyle and Dr. Lebwohl emphasized the practical implications for clinicians caring for patients with celiac disease. Among patients with celiac disease and inflammatory joint symptoms, clinicians should have a low threshold to evaluate for JIA or RA, they said.
“Particularly in pediatrics, we are trained to screen patients with JIA for celiac disease, but this study points to the possible bidirectional association and the importance of maintaining a clinical suspicion for JIA and RA among established celiac disease patients,” Marisa Stahl, MD, assistant professor of pediatrics and associate program director of the pediatric gastroenterology, hepatology, and nutrition fellowship training program at the University of Colorado at Denver, Aurora, said in an interview.
Dr. Stahl, who wasn’t involved with this study, conducts research at the Colorado Center for Celiac Disease. She and colleagues are focused on understanding the genetic and environmental factors that lead to the development of celiac disease and other autoimmune diseases.
Given the clear association between celiac disease and other autoimmune diseases, Dr. Stahl agreed that clinicians should have a low threshold for screening, with “additional workup for other autoimmune diseases once an autoimmune diagnosis is established.”
The study was supported by Karolinska Institutet and the Swedish Research Council. Dr. Lebwohl coordinates a study on behalf of the Swedish IBD quality register, which has received funding from Janssen. The other authors declared no conflicts of interest. Dr. Stahl reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Doctors urge screening for autoimmune disorders for patients with celiac disease
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.
Diagnosed at age 4, Dr. Mollo has been on a gluten-free diet for 41 years, which she says has kept her healthy and may also be why she hasn’t developed other autoimmune diseases. It’s also played a part in her thinking about screening patients with CD.
“I think [physicians] should definitely be screening people with celiac disease for autoimmune disorders, especially if they see things like anemia or if a child has dropped on the growth chart and has nutrient deficiencies,” said Dr. Mollo, whose daughter also has the disease. “I would recommend that they see someone who specializes in celiac disease so they can get monitored and have regular follow-up checks for nutrient deficiencies and other autoimmune disorders.”
Dr. Mollo’s views on screening are echoed by many CD specialists and physicians, who cite multiple studies that have found that people with the disease face higher risks for diabetes, thyroid conditions, arthritis, and other autoimmune disorders.
Gastroenterologist Alessio Fasano, MD, with Massachusetts General Hospital, Boston, said there has been a “shift in the paradigm in thinking” about cross-screening for CD and autoimmune disorders. As result, he believes the answer to the question of whether to routinely do so is a no-brainer.
“The bottom line is, if you have CD, it [should be] routine that during your annual follow-ups you check for the possibility of the onset of other autoimmune disease. And people with other autoimmune diseases, like type 1 diabetes, should also be screened for CD because of the comorbidity,” said Dr. Fasano, professor of pediatrics and gastroenterology at Harvard Medical School and professor of nutrition at the Harvard School of Public Health, both in Boston. “This is what we call good clinical practice.”
Screening, despite lack of consensus guidelines
Other CD specialists differ on the need for universal cross-screening but agree that, at least in some cases, people with one autoimmune disorder should be tested for others.
Jolanda Denham, MD, a pediatric gastroenterologist affiliated with Nemours Children’s Hospital in Orlando, routinely recommends that her patients with CD be screened for certain autoimmune disorders – such as type 1 diabetes and autoimmune thyroid and liver diseases – even though medical organizations have not developed clear consensus or standard guidelines on cross-screening.
“There currently is no evidence to support the screening of celiac patients for all autoimmune and rheumatologic disorders,” she said. “It is true that celiac disease is an autoimmune disorder, and as such, there is a definite increased risk of these disorders in patients with celiac disease and vice versa.”
Echoing Dr. Denham, New York–based gastroenterologist Benjamin Lebwohl, MD, president of the Society for the Study of Celiac Disease, urges physicians to look beyond consensus guidelines and to err on the side of caution and make the best decisions for their patients on a case-by-case basis.
“Given the increased risk of certain autoimmune conditions in people with celiac disease, it behooves physicians to have a low threshold to evaluate for these conditions if any suggestive symptoms are present,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University, New York.
“Whether to screen for these conditions among people who are entirely without symptoms is less certain, and there is no consensus on that. But it is reasonable and common to include some basic tests with annual blood work, such as thyroid function and a liver profile, since both autoimmune thyroid disease and autoimmune liver disease can be silent early on and the patient would potentially benefit from identification and treatment of these conditions,” he said.
The American Diabetes Association and the International Society of Pediatric and Adolescent Diabetes do recommend that people with diabetes be screened for CD years after diagnosis, noted Robert Rapaport, MD, a pediatric endocrinologist, with Kravis Children’s Hospital, New York. But in a study published in 2021, he and colleagues found that this wasn’t occurring, which prompted them to recommend yearly screening.
“There is a consensus that in children with type 1 diabetes, we screen them for other autoimmune disorders, specifically for thyroid disease and celiac disease,” said Dr. Rapaport, who is also Emma Elizabeth Sullivan Professor of Pediatric Endocrinology and Diabetes at Icahn School of Medicine at Mount Sinai, New York. “But there is no consensus going the other way – for patients with celiac disease, what other autoimmune conditions they should be screened for.”
This hasn’t kept some doctors from extending cross-screening efforts to their patients.
“At our center, we screen ... for thyroid disease and autoimmune liver disease as part of routine healthcare maintenance for our celiac disease patients. We discuss symptoms of diabetes and send screening with [hemoglobin] A1c for anyone who has symptoms,” said Lui Edwin, MD, a pediatric gastroenterologist with Children’s Hospital Colorado, Aurora, and director of the Colorado Center for Celiac Disease, who delivered a lecture on CD-autoimmune screening at the International Celiac Disease Symposium in October.
“It is definitely worth screening for celiac disease in [those with] other autoimmune disorders,” Dr. Edwin added.
“The symptoms can be very heterogeneous. Diagnosing and treating celiac disease can make a huge impact with respect to symptoms, quality of life, and preventing disease-related complications,” he said.
Mounting evidence linking CD to autoimmune disorders
Many studies have linked CD to a variety of other autoimmune disorders. The association could be due to common genetic factors or because CD might lead to such conditions. Researchers have found that people diagnosed with CD later in life are more likely to develop other autoimmune disorders.

Some studies have also found that people with certain autoimmune diseases are more likely to also have CD. In addition, some individuals develop what’s known as nonceliac gluten sensitivity, which is not an autoimmune disease but a gluten intolerance not unlike lactose intolerance.
In light of these coexisting conditions in many people with CD and other autoimmune disorders, as well as the fact that the prevalence of CD is on the rise, some specialists argue that the benefits of routine cross-screening outweigh the risks.
Going gluten free has preventive advantages
In a landmark 2012 study, researchers with the Celiac Disease Center at Columbia University stopped short of recommending routine screening for the general public or asymptomatic individuals in high-prevalence groups. But they concluded that more screening of symptomatic individuals – and close relatives – would speed treatment for those with more than one autoimmune disorder.
They also noted that some studies have found that a gluten-free diet might help prevent the development of other autoimmune disorders.
Marisa Gallant Stahl, MD, a gastroenterologist with Children’s Hospital Colorado, agreed that it is important that physicians keep gluten-free diets in mind when determining which patients to cross-screen.
“The literature is mixed, but some studies suggest that treating celiac disease with a gluten-free diet actually augments the treatment and control of other autoimmune disorders [and] adherence to a gluten-free diet does reduce the risk of cancer associated with celiac disease,” she said.
Dr. Denham agreed. “Strict adherence to a gluten-free diet definitely protects against the development of enteropathy-associated T-cell lymphoma but may be protective against non-Hodgkin’s lymphoma and adenocarcinoma of the small intestine as well. All three are associated with long-term nonadherence to a gluten-free diet.”
She also noted that a gluten-free diet may help people with CD manage other autoimmune disorders, which can be complicated by CD.
“Good control of celiac disease will help prevent complications that can worsen symptoms and outcomes of concomitant autoimmune and rheumatologic disorders,” she said.
Other factors to consider
Dr. Fasano added that autoimmune disorders can be complicated by CD in cases in which oral medications or healthful foods are not properly absorbed in the intestines.
“For example, with Hashimoto’s disease, if you have hormone replacement with oral treatments and your intestines are not 100% functional because you have inflammation, then you may have a problem [with] the absorption of medications like levothyroxine,” he said.
“It’s the same story with diabetes. You don’t take insulin by mouth, but glucose [control] strongly depends on several factors, mostly what comes from the diet, and if it’s erratic, that can be a problem. ... So, the treatment of autoimmune diseases can be influenced by celiac disease,” he said.
In addition, Dr. Fasano and others believe that people with CD and other autoimmune disorders should be managed by a team of experts who can personalize the care on the basis of specific needs of the individual patient. These should include specialists, dietitians, mental health counselors, and family social workers.
“It has to be a multidisciplinary approach to maintain the good health of an individual,” Dr. Fasano said. “Celiac disease is the quintessential example in which the primary care physician needs to be the quarterback of the team, the patient is active in his or her health, and [specialists] not only deliver personalized care but also preventive intervention, particularly the prevention of comorbidities.”
Financial disclosures for those quoted in this article were not available at the time of publication.
A version of this article first appeared on Medscape.com.