User login
Novel oral drug shows early promise for IBD
SAN DIEGO – A novel oral drug for inflammatory bowel disease showed good safety and efficacy data in preliminary clinical trial results.
Among a group of 32 patients with ulcerative colitis, the investigative drug ABX464 showed a decrease in Mayo score of over 50% and a drop in fecal calprotectin to near-normal levels. The safety profile was reassuring, and results were durable at the 9-month mark.
Coauthor Jean-Marc Steens, MD, presented results of the randomized, double-blind, placebo-controlled phase 2a study at the annual Digestive Disease Week®, and noted that ABX464 is also being investigated as antiviral therapy for individuals with HIV/AIDS.
“Despite the major advances in the last 10 years with the introduction of biologics and [Janus kinase] inhibitors, there is still a huge unmet medical need for these patients,” said Dr. Steens, chief medical officer of Abivax (Paris), in an interview. “Large phase 3 studies with these recent drugs have shown that about two-thirds of the patients show a clinical response during induction, but that half of these responders will lose their response within the next 6-12 months. The safety profile of these drugs also includes severe infections, which is a major concern,” he said.
Dr. Steens presented the findings on behalf of first author Severine Vermeire, MD, chair of the department of chronic diseases, metabolism, and aging at Catholic University, Leuven, Belgium.
ABX464, a small-molecule oral medication, has been evaluated for safety among more than 180 patients with HIV as well as the patients with ulcerative colitis (UC) studied in the current trial. The drug increases expression of the microRNA precursor miR-124, with the result that “the inflammatory brake is applied,” explained Dr. Steens.
In the present study, whose primary outcome was safety, 23 patients with moderate to severe active UC were randomized to ABX464 50 mg once daily, and 9 to placebo. Patients were included if they had failed or were intolerant to immunomodulators, anti–tumor necrosis factor–alpha therapies, vedolizumab, or corticosteroids; the two groups had balanced disease and demographic characteristics. At baseline, patients had a total Mayo score of 6-12, and an endoscopic subscore of 2 or 3.
Three patients withdrew from the ABX464 arm by the end of 8 weeks: one because of adverse events (AEs), one withdrew consent, and the third declined to undergo endoscopy at the 8-week mark.
All treatment-emergent AEs were mild or moderate, with gastrointestinal disorders occurring in eight of the ABX464 patients and two placebo patients (34.8% and 22.2%, respectively.) Five ABX-464 patients (21.7%) experienced nervous system symptoms – mostly headaches, said Dr. Steens. No patients in the placebo arm had headache or other neurological AEs.
By the end of 8 weeks, 30% of the intention-to-treat ABX464 group was in clinical remission, compared with 11% of the placebo group; this was not a statistically significant difference (P = .16). The proportion of ABX464 patients who had a clinical response just missed statistical significance, compared with placebo (61% versus 33%; P = 06).
However, significant endoscopic improvement was seen in the ABX464 arm, with 43% having a Mayo endoscopy subscore of 0 or 1, compared with 11% in the placebo arm (P = .03).
The total Mayo score dropped by 53% in the ABX464 group, compared with 27% in the placebo group (P = .03); a partial Mayo score dropped by 62% for those in the active arm, compared with 32% in the placebo arm (P = .02).
“The major finding from the induction study was that all endpoints were going in the same direction in favor of ABX464, even reaching statistical significance for endoscopy as well, and total and partial Mayo score,” said Dr. Steens.
Patients underwent rectal biopsies at the end of 8 weeks, and miR-124 expression increased more than sevenfold from baseline for those taking ABX464, compared with a small increase in the placebo group (7.69- versus 1.46-fold; P = .004). Expression of miR-124 in total blood also increased – by over 800-fold – at study day 28 for the ABX464 arm. Levels were sustained at more than 700-fold at study day 56 in this group. Placebo arm participants saw an insignificant rise in miR-124 blood levels.
Dr. Steens reported that 22 patients, including 7 who had originally been placebo arm participants, continued into the maintenance phase of the study. Nineteen patients have now had a median of over 400 days of exposure to ABX464, with sustained significant improvement in partial Mayo scores from a baseline of 6 to scores below 2 at 6 and 9 months. Fecal calprotectin scores have dropped from a median 1,044 mcg/g at baseline to 23.5 mcg/g at 9 months.
Next steps include the 12-month assessment, which includes another endoscopy, said Dr. Steens. Also, a phase 2b study is seeking to enroll 232 patients who have moderate to severe ulcerative colitis, with room within the enrollment scheme for new study sites, said Dr. Steens. This larger study will have arms in which the current 50-mg oral dose is doubled and halved, as well as a placebo arm, he said. The medication will also be trialed for Crohn’s disease and rheumatoid arthritis.
The small sample size is an inherent limitation of this early-stage clinical trial, noted Dr. Steens.
Dr. Steens reported being an employee and holding shares in Abivax, which funded the study.
SOURCE: Vermeire S et al. DDW 2019, Abstract 1007.
SAN DIEGO – A novel oral drug for inflammatory bowel disease showed good safety and efficacy data in preliminary clinical trial results.
Among a group of 32 patients with ulcerative colitis, the investigative drug ABX464 showed a decrease in Mayo score of over 50% and a drop in fecal calprotectin to near-normal levels. The safety profile was reassuring, and results were durable at the 9-month mark.
Coauthor Jean-Marc Steens, MD, presented results of the randomized, double-blind, placebo-controlled phase 2a study at the annual Digestive Disease Week®, and noted that ABX464 is also being investigated as antiviral therapy for individuals with HIV/AIDS.
“Despite the major advances in the last 10 years with the introduction of biologics and [Janus kinase] inhibitors, there is still a huge unmet medical need for these patients,” said Dr. Steens, chief medical officer of Abivax (Paris), in an interview. “Large phase 3 studies with these recent drugs have shown that about two-thirds of the patients show a clinical response during induction, but that half of these responders will lose their response within the next 6-12 months. The safety profile of these drugs also includes severe infections, which is a major concern,” he said.
Dr. Steens presented the findings on behalf of first author Severine Vermeire, MD, chair of the department of chronic diseases, metabolism, and aging at Catholic University, Leuven, Belgium.
ABX464, a small-molecule oral medication, has been evaluated for safety among more than 180 patients with HIV as well as the patients with ulcerative colitis (UC) studied in the current trial. The drug increases expression of the microRNA precursor miR-124, with the result that “the inflammatory brake is applied,” explained Dr. Steens.
In the present study, whose primary outcome was safety, 23 patients with moderate to severe active UC were randomized to ABX464 50 mg once daily, and 9 to placebo. Patients were included if they had failed or were intolerant to immunomodulators, anti–tumor necrosis factor–alpha therapies, vedolizumab, or corticosteroids; the two groups had balanced disease and demographic characteristics. At baseline, patients had a total Mayo score of 6-12, and an endoscopic subscore of 2 or 3.
Three patients withdrew from the ABX464 arm by the end of 8 weeks: one because of adverse events (AEs), one withdrew consent, and the third declined to undergo endoscopy at the 8-week mark.
All treatment-emergent AEs were mild or moderate, with gastrointestinal disorders occurring in eight of the ABX464 patients and two placebo patients (34.8% and 22.2%, respectively.) Five ABX-464 patients (21.7%) experienced nervous system symptoms – mostly headaches, said Dr. Steens. No patients in the placebo arm had headache or other neurological AEs.
By the end of 8 weeks, 30% of the intention-to-treat ABX464 group was in clinical remission, compared with 11% of the placebo group; this was not a statistically significant difference (P = .16). The proportion of ABX464 patients who had a clinical response just missed statistical significance, compared with placebo (61% versus 33%; P = 06).
However, significant endoscopic improvement was seen in the ABX464 arm, with 43% having a Mayo endoscopy subscore of 0 or 1, compared with 11% in the placebo arm (P = .03).
The total Mayo score dropped by 53% in the ABX464 group, compared with 27% in the placebo group (P = .03); a partial Mayo score dropped by 62% for those in the active arm, compared with 32% in the placebo arm (P = .02).
“The major finding from the induction study was that all endpoints were going in the same direction in favor of ABX464, even reaching statistical significance for endoscopy as well, and total and partial Mayo score,” said Dr. Steens.
Patients underwent rectal biopsies at the end of 8 weeks, and miR-124 expression increased more than sevenfold from baseline for those taking ABX464, compared with a small increase in the placebo group (7.69- versus 1.46-fold; P = .004). Expression of miR-124 in total blood also increased – by over 800-fold – at study day 28 for the ABX464 arm. Levels were sustained at more than 700-fold at study day 56 in this group. Placebo arm participants saw an insignificant rise in miR-124 blood levels.
Dr. Steens reported that 22 patients, including 7 who had originally been placebo arm participants, continued into the maintenance phase of the study. Nineteen patients have now had a median of over 400 days of exposure to ABX464, with sustained significant improvement in partial Mayo scores from a baseline of 6 to scores below 2 at 6 and 9 months. Fecal calprotectin scores have dropped from a median 1,044 mcg/g at baseline to 23.5 mcg/g at 9 months.
Next steps include the 12-month assessment, which includes another endoscopy, said Dr. Steens. Also, a phase 2b study is seeking to enroll 232 patients who have moderate to severe ulcerative colitis, with room within the enrollment scheme for new study sites, said Dr. Steens. This larger study will have arms in which the current 50-mg oral dose is doubled and halved, as well as a placebo arm, he said. The medication will also be trialed for Crohn’s disease and rheumatoid arthritis.
The small sample size is an inherent limitation of this early-stage clinical trial, noted Dr. Steens.
Dr. Steens reported being an employee and holding shares in Abivax, which funded the study.
SOURCE: Vermeire S et al. DDW 2019, Abstract 1007.
SAN DIEGO – A novel oral drug for inflammatory bowel disease showed good safety and efficacy data in preliminary clinical trial results.
Among a group of 32 patients with ulcerative colitis, the investigative drug ABX464 showed a decrease in Mayo score of over 50% and a drop in fecal calprotectin to near-normal levels. The safety profile was reassuring, and results were durable at the 9-month mark.
Coauthor Jean-Marc Steens, MD, presented results of the randomized, double-blind, placebo-controlled phase 2a study at the annual Digestive Disease Week®, and noted that ABX464 is also being investigated as antiviral therapy for individuals with HIV/AIDS.
“Despite the major advances in the last 10 years with the introduction of biologics and [Janus kinase] inhibitors, there is still a huge unmet medical need for these patients,” said Dr. Steens, chief medical officer of Abivax (Paris), in an interview. “Large phase 3 studies with these recent drugs have shown that about two-thirds of the patients show a clinical response during induction, but that half of these responders will lose their response within the next 6-12 months. The safety profile of these drugs also includes severe infections, which is a major concern,” he said.
Dr. Steens presented the findings on behalf of first author Severine Vermeire, MD, chair of the department of chronic diseases, metabolism, and aging at Catholic University, Leuven, Belgium.
ABX464, a small-molecule oral medication, has been evaluated for safety among more than 180 patients with HIV as well as the patients with ulcerative colitis (UC) studied in the current trial. The drug increases expression of the microRNA precursor miR-124, with the result that “the inflammatory brake is applied,” explained Dr. Steens.
In the present study, whose primary outcome was safety, 23 patients with moderate to severe active UC were randomized to ABX464 50 mg once daily, and 9 to placebo. Patients were included if they had failed or were intolerant to immunomodulators, anti–tumor necrosis factor–alpha therapies, vedolizumab, or corticosteroids; the two groups had balanced disease and demographic characteristics. At baseline, patients had a total Mayo score of 6-12, and an endoscopic subscore of 2 or 3.
Three patients withdrew from the ABX464 arm by the end of 8 weeks: one because of adverse events (AEs), one withdrew consent, and the third declined to undergo endoscopy at the 8-week mark.
All treatment-emergent AEs were mild or moderate, with gastrointestinal disorders occurring in eight of the ABX464 patients and two placebo patients (34.8% and 22.2%, respectively.) Five ABX-464 patients (21.7%) experienced nervous system symptoms – mostly headaches, said Dr. Steens. No patients in the placebo arm had headache or other neurological AEs.
By the end of 8 weeks, 30% of the intention-to-treat ABX464 group was in clinical remission, compared with 11% of the placebo group; this was not a statistically significant difference (P = .16). The proportion of ABX464 patients who had a clinical response just missed statistical significance, compared with placebo (61% versus 33%; P = 06).
However, significant endoscopic improvement was seen in the ABX464 arm, with 43% having a Mayo endoscopy subscore of 0 or 1, compared with 11% in the placebo arm (P = .03).
The total Mayo score dropped by 53% in the ABX464 group, compared with 27% in the placebo group (P = .03); a partial Mayo score dropped by 62% for those in the active arm, compared with 32% in the placebo arm (P = .02).
“The major finding from the induction study was that all endpoints were going in the same direction in favor of ABX464, even reaching statistical significance for endoscopy as well, and total and partial Mayo score,” said Dr. Steens.
Patients underwent rectal biopsies at the end of 8 weeks, and miR-124 expression increased more than sevenfold from baseline for those taking ABX464, compared with a small increase in the placebo group (7.69- versus 1.46-fold; P = .004). Expression of miR-124 in total blood also increased – by over 800-fold – at study day 28 for the ABX464 arm. Levels were sustained at more than 700-fold at study day 56 in this group. Placebo arm participants saw an insignificant rise in miR-124 blood levels.
Dr. Steens reported that 22 patients, including 7 who had originally been placebo arm participants, continued into the maintenance phase of the study. Nineteen patients have now had a median of over 400 days of exposure to ABX464, with sustained significant improvement in partial Mayo scores from a baseline of 6 to scores below 2 at 6 and 9 months. Fecal calprotectin scores have dropped from a median 1,044 mcg/g at baseline to 23.5 mcg/g at 9 months.
Next steps include the 12-month assessment, which includes another endoscopy, said Dr. Steens. Also, a phase 2b study is seeking to enroll 232 patients who have moderate to severe ulcerative colitis, with room within the enrollment scheme for new study sites, said Dr. Steens. This larger study will have arms in which the current 50-mg oral dose is doubled and halved, as well as a placebo arm, he said. The medication will also be trialed for Crohn’s disease and rheumatoid arthritis.
The small sample size is an inherent limitation of this early-stage clinical trial, noted Dr. Steens.
Dr. Steens reported being an employee and holding shares in Abivax, which funded the study.
SOURCE: Vermeire S et al. DDW 2019, Abstract 1007.
REPORTING FROM DDW 2019
Key clinical point: A novel oral small molecule’s potent anti-inflammatory effect over 8 weeks was associated with significant endoscopic improvement, reduced Mayo scores, and a trend toward clinical response, compared with placebo.
Major finding: Study details: Randomized, double-blind, placebo-controlled study of 32 patients with moderate to severe ulcerative colitis.
Disclosures: The study was sponsored by Abivax. Dr. Steens is an employee of and holds shares of Abivax.
Source: Vermeire S et al. DDW, Abstract 1007.
AGA introduces pathway to navigate IBD care
Inflammatory bowel disease (IBD) treatment remains a challenge in part because care is often fragmented among providers in different specialties, according to the American Gastroenterological Association. To address the need for provider coordination, the AGA has issued a new referral pathway for IBD care, published in Gastroenterology.
“The goal of this pathway is to offer guidance to primary care, emergency department, and gastroenterology providers, by helping identify patients at risk of or diagnosed with IBD and provide direction on initiating appropriate patient referrals,” wrote lead author Jami Kinnucan, MD, of the University of Michigan, Ann Arbor, and members of the AGA workgroup.
In particular, the pathway focuses on gaps in IBD care related to inflammatory issues, mental health, and nutrition. The work group included not only gastroenterologists, but also a primary care physician, mental/behavioral health specialist, registered dietitian/nutritionist, critical care specialist, nurse practitioner, physician group representative, and a patient advocacy representative.
The pathway identifies the top three areas where IBD patients usually present with symptoms: the emergency department, primary care office, and gastroenterology office.
The work group developed a list of key characteristics associated with increased morbidity, established IBD, or IBD-related complications that can be separated into high-risk, moderate-risk, and low-risk groups to help clinicians determine the timing of and need for referrals.
The pathway uses a sample patient presenting with GI symptoms such as bloody diarrhea; GI bleeding; anemia; fecal urgency; fever; abdominal pain; weight loss; and pain, swelling, or redness in the joints. Clinicians then apply the key characteristics to triage the patients into the risk groups.
High-risk characteristics include history of perianal or severe rectal disease, or deep ulcers in the GI mucosa; two or more emergency department visits for GI problems within the past 6 months, severe anemia, inadequate response to outpatient IBD therapy, history of IBD-related surgery, and malnourishment.
Moderate-risk characteristics include anemia without clinical symptoms, chronic corticosteroid use, and no emergency department or other GI medical visits within the past year.
Low-risk characteristics include chronic narcotic use, one or more comorbidities (such as heart failure, active hepatitis B, oncologic malignancy, lupus, GI infections, primary sclerosing cholangitis, viral hepatitis, and celiac disease), one or more relevant mental health conditions (such as depression, anxiety, or chronic pain), and nonadherence to IBD medical therapies.
“Referrals should be based on the highest level of risk present, in the event that a patient has characteristics that fall in more than one risk category,” the work group wrote.
To further guide clinicians in referring patients with possible or diagnosed IBD to gastroenterology specialists and to mental health and nutrition specialists, the work group developed an IBD Characteristics Assessment Checklist and a Referral Feedback form to accompany the pathway.
The checklist is designed for use by any health care professional to help identify whether a patient needs to be referred based on the key characteristics; the feedback form gives gastroenterologists a template to communicate with referring physicians about comanagement strategies for the patient.
The pathway also includes more details on how clinicians can tackle barriers to mental health and nutrition care for IBD patients.
“Until further evaluations are conducted, the work group encourages the immediate use of the pathway to begin addressing the needed improvements for IBD care coordination and communication between the different IBD providers,” the authors wrote.
Dr. Kinnucan disclosed serving as a consultant for AbbVie, Janssen, and Pfizer and serving on the Patient Education Committee of the Crohn’s and Colitis Foundation.
SOURCE: Kinnucan J et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.064.
Inflammatory bowel disease (IBD) treatment remains a challenge in part because care is often fragmented among providers in different specialties, according to the American Gastroenterological Association. To address the need for provider coordination, the AGA has issued a new referral pathway for IBD care, published in Gastroenterology.
“The goal of this pathway is to offer guidance to primary care, emergency department, and gastroenterology providers, by helping identify patients at risk of or diagnosed with IBD and provide direction on initiating appropriate patient referrals,” wrote lead author Jami Kinnucan, MD, of the University of Michigan, Ann Arbor, and members of the AGA workgroup.
In particular, the pathway focuses on gaps in IBD care related to inflammatory issues, mental health, and nutrition. The work group included not only gastroenterologists, but also a primary care physician, mental/behavioral health specialist, registered dietitian/nutritionist, critical care specialist, nurse practitioner, physician group representative, and a patient advocacy representative.
The pathway identifies the top three areas where IBD patients usually present with symptoms: the emergency department, primary care office, and gastroenterology office.
The work group developed a list of key characteristics associated with increased morbidity, established IBD, or IBD-related complications that can be separated into high-risk, moderate-risk, and low-risk groups to help clinicians determine the timing of and need for referrals.
The pathway uses a sample patient presenting with GI symptoms such as bloody diarrhea; GI bleeding; anemia; fecal urgency; fever; abdominal pain; weight loss; and pain, swelling, or redness in the joints. Clinicians then apply the key characteristics to triage the patients into the risk groups.
High-risk characteristics include history of perianal or severe rectal disease, or deep ulcers in the GI mucosa; two or more emergency department visits for GI problems within the past 6 months, severe anemia, inadequate response to outpatient IBD therapy, history of IBD-related surgery, and malnourishment.
Moderate-risk characteristics include anemia without clinical symptoms, chronic corticosteroid use, and no emergency department or other GI medical visits within the past year.
Low-risk characteristics include chronic narcotic use, one or more comorbidities (such as heart failure, active hepatitis B, oncologic malignancy, lupus, GI infections, primary sclerosing cholangitis, viral hepatitis, and celiac disease), one or more relevant mental health conditions (such as depression, anxiety, or chronic pain), and nonadherence to IBD medical therapies.
“Referrals should be based on the highest level of risk present, in the event that a patient has characteristics that fall in more than one risk category,” the work group wrote.
To further guide clinicians in referring patients with possible or diagnosed IBD to gastroenterology specialists and to mental health and nutrition specialists, the work group developed an IBD Characteristics Assessment Checklist and a Referral Feedback form to accompany the pathway.
The checklist is designed for use by any health care professional to help identify whether a patient needs to be referred based on the key characteristics; the feedback form gives gastroenterologists a template to communicate with referring physicians about comanagement strategies for the patient.
The pathway also includes more details on how clinicians can tackle barriers to mental health and nutrition care for IBD patients.
“Until further evaluations are conducted, the work group encourages the immediate use of the pathway to begin addressing the needed improvements for IBD care coordination and communication between the different IBD providers,” the authors wrote.
Dr. Kinnucan disclosed serving as a consultant for AbbVie, Janssen, and Pfizer and serving on the Patient Education Committee of the Crohn’s and Colitis Foundation.
SOURCE: Kinnucan J et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.064.
Inflammatory bowel disease (IBD) treatment remains a challenge in part because care is often fragmented among providers in different specialties, according to the American Gastroenterological Association. To address the need for provider coordination, the AGA has issued a new referral pathway for IBD care, published in Gastroenterology.
“The goal of this pathway is to offer guidance to primary care, emergency department, and gastroenterology providers, by helping identify patients at risk of or diagnosed with IBD and provide direction on initiating appropriate patient referrals,” wrote lead author Jami Kinnucan, MD, of the University of Michigan, Ann Arbor, and members of the AGA workgroup.
In particular, the pathway focuses on gaps in IBD care related to inflammatory issues, mental health, and nutrition. The work group included not only gastroenterologists, but also a primary care physician, mental/behavioral health specialist, registered dietitian/nutritionist, critical care specialist, nurse practitioner, physician group representative, and a patient advocacy representative.
The pathway identifies the top three areas where IBD patients usually present with symptoms: the emergency department, primary care office, and gastroenterology office.
The work group developed a list of key characteristics associated with increased morbidity, established IBD, or IBD-related complications that can be separated into high-risk, moderate-risk, and low-risk groups to help clinicians determine the timing of and need for referrals.
The pathway uses a sample patient presenting with GI symptoms such as bloody diarrhea; GI bleeding; anemia; fecal urgency; fever; abdominal pain; weight loss; and pain, swelling, or redness in the joints. Clinicians then apply the key characteristics to triage the patients into the risk groups.
High-risk characteristics include history of perianal or severe rectal disease, or deep ulcers in the GI mucosa; two or more emergency department visits for GI problems within the past 6 months, severe anemia, inadequate response to outpatient IBD therapy, history of IBD-related surgery, and malnourishment.
Moderate-risk characteristics include anemia without clinical symptoms, chronic corticosteroid use, and no emergency department or other GI medical visits within the past year.
Low-risk characteristics include chronic narcotic use, one or more comorbidities (such as heart failure, active hepatitis B, oncologic malignancy, lupus, GI infections, primary sclerosing cholangitis, viral hepatitis, and celiac disease), one or more relevant mental health conditions (such as depression, anxiety, or chronic pain), and nonadherence to IBD medical therapies.
“Referrals should be based on the highest level of risk present, in the event that a patient has characteristics that fall in more than one risk category,” the work group wrote.
To further guide clinicians in referring patients with possible or diagnosed IBD to gastroenterology specialists and to mental health and nutrition specialists, the work group developed an IBD Characteristics Assessment Checklist and a Referral Feedback form to accompany the pathway.
The checklist is designed for use by any health care professional to help identify whether a patient needs to be referred based on the key characteristics; the feedback form gives gastroenterologists a template to communicate with referring physicians about comanagement strategies for the patient.
The pathway also includes more details on how clinicians can tackle barriers to mental health and nutrition care for IBD patients.
“Until further evaluations are conducted, the work group encourages the immediate use of the pathway to begin addressing the needed improvements for IBD care coordination and communication between the different IBD providers,” the authors wrote.
Dr. Kinnucan disclosed serving as a consultant for AbbVie, Janssen, and Pfizer and serving on the Patient Education Committee of the Crohn’s and Colitis Foundation.
SOURCE: Kinnucan J et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.03.064.
FROM GASTROENTEROLOGY
FDA approves IB-Stim device for abdominal pain in adolescents with IBS
The IB-Stim device has been approved to aid in the reduction of functional abdominal pain in patients 11-18 years of age with irritable bowel syndrome (IBS), according to the U.S. Food and Drug Administration.
“This device offers a safe option for treatment of adolescents experiencing pain from IBS through the use of mild nerve stimulation,” Carlos Peña, PhD, director of the Office of Neurological and Physical Medicine Devices in the FDA’s Center for Devices and Radiological Health, said in a press release.
The prescription-only device has a single-use electrical nerve stimulator that is placed behind the patient’s ear. Stimulating nerve bundles in and around the ear is thought to provide pain relief. The battery-powered chip of the device emits low-frequency electrical pulses continuously for 5 days, at which time it is replaced. Patients can use the device for up to 3 consecutive weeks to reduce functional abdominal pain associated with IBS.
The FDA reviewed data from 50 patients, aged 11-18 years, with IBS; 27 patients were treated with the device and 23 patients received a placebo device. The study measured change from baseline to the end of the third week in worst abdominal pain, usual pain, and Pain Frequency Severity Duration (PFSD) scores. Patients were allowed to continue stable doses of medication to treat chronic abdominal pain.
IB-Stim treatment resulted in at least a 30% decrease in usual pain at the end of 3 weeks in 52% of treated patients, compared with 30% of patients who received the placebo, and at least a 30% decrease in worst pain in 59% of treated patients, compared with 26% of patients who received the placebo.
The treatment group also had greater changes in composite PFSD scores at the end of three weeks. During the study, six patients reported mild ear discomfort, and three patients reported adhesive allergy at the site of application, according to the press release.
The device is contraindicated for patients with hemophilia, patients with cardiac pacemakers, or those diagnosed with psoriasis vulgaris.
The FDA granted marketing authorization of the IB-Stim to Innovative Health Solutions.
The IB-Stim device has been approved to aid in the reduction of functional abdominal pain in patients 11-18 years of age with irritable bowel syndrome (IBS), according to the U.S. Food and Drug Administration.
“This device offers a safe option for treatment of adolescents experiencing pain from IBS through the use of mild nerve stimulation,” Carlos Peña, PhD, director of the Office of Neurological and Physical Medicine Devices in the FDA’s Center for Devices and Radiological Health, said in a press release.
The prescription-only device has a single-use electrical nerve stimulator that is placed behind the patient’s ear. Stimulating nerve bundles in and around the ear is thought to provide pain relief. The battery-powered chip of the device emits low-frequency electrical pulses continuously for 5 days, at which time it is replaced. Patients can use the device for up to 3 consecutive weeks to reduce functional abdominal pain associated with IBS.
The FDA reviewed data from 50 patients, aged 11-18 years, with IBS; 27 patients were treated with the device and 23 patients received a placebo device. The study measured change from baseline to the end of the third week in worst abdominal pain, usual pain, and Pain Frequency Severity Duration (PFSD) scores. Patients were allowed to continue stable doses of medication to treat chronic abdominal pain.
IB-Stim treatment resulted in at least a 30% decrease in usual pain at the end of 3 weeks in 52% of treated patients, compared with 30% of patients who received the placebo, and at least a 30% decrease in worst pain in 59% of treated patients, compared with 26% of patients who received the placebo.
The treatment group also had greater changes in composite PFSD scores at the end of three weeks. During the study, six patients reported mild ear discomfort, and three patients reported adhesive allergy at the site of application, according to the press release.
The device is contraindicated for patients with hemophilia, patients with cardiac pacemakers, or those diagnosed with psoriasis vulgaris.
The FDA granted marketing authorization of the IB-Stim to Innovative Health Solutions.
The IB-Stim device has been approved to aid in the reduction of functional abdominal pain in patients 11-18 years of age with irritable bowel syndrome (IBS), according to the U.S. Food and Drug Administration.
“This device offers a safe option for treatment of adolescents experiencing pain from IBS through the use of mild nerve stimulation,” Carlos Peña, PhD, director of the Office of Neurological and Physical Medicine Devices in the FDA’s Center for Devices and Radiological Health, said in a press release.
The prescription-only device has a single-use electrical nerve stimulator that is placed behind the patient’s ear. Stimulating nerve bundles in and around the ear is thought to provide pain relief. The battery-powered chip of the device emits low-frequency electrical pulses continuously for 5 days, at which time it is replaced. Patients can use the device for up to 3 consecutive weeks to reduce functional abdominal pain associated with IBS.
The FDA reviewed data from 50 patients, aged 11-18 years, with IBS; 27 patients were treated with the device and 23 patients received a placebo device. The study measured change from baseline to the end of the third week in worst abdominal pain, usual pain, and Pain Frequency Severity Duration (PFSD) scores. Patients were allowed to continue stable doses of medication to treat chronic abdominal pain.
IB-Stim treatment resulted in at least a 30% decrease in usual pain at the end of 3 weeks in 52% of treated patients, compared with 30% of patients who received the placebo, and at least a 30% decrease in worst pain in 59% of treated patients, compared with 26% of patients who received the placebo.
The treatment group also had greater changes in composite PFSD scores at the end of three weeks. During the study, six patients reported mild ear discomfort, and three patients reported adhesive allergy at the site of application, according to the press release.
The device is contraindicated for patients with hemophilia, patients with cardiac pacemakers, or those diagnosed with psoriasis vulgaris.
The FDA granted marketing authorization of the IB-Stim to Innovative Health Solutions.
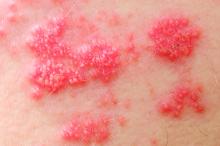
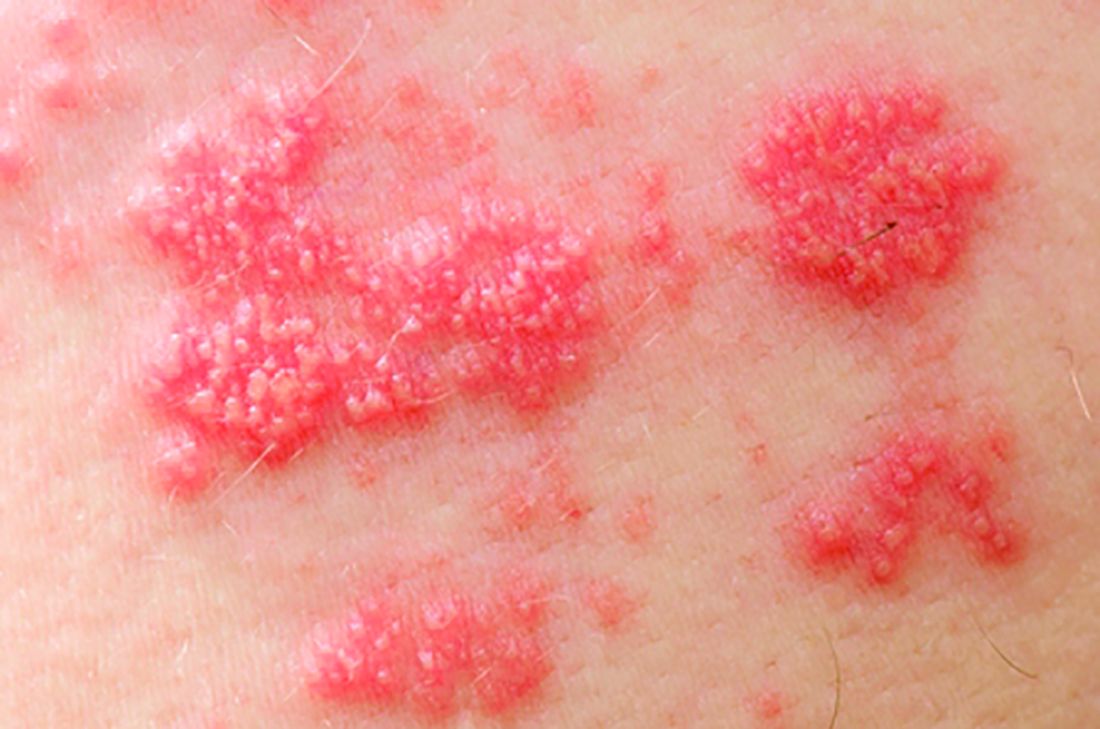
Tofacitinib upped herpes zoster risk in ulcerative colitis
Among patients with moderate to severe ulcerative colitis, a median of 1.4 years and up to 4.4 years of tofacitinib therapy was safe apart from a dose-related increase in risk of herpes zoster infection, according to an integrated analysis of data from five clinical trials.
Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib (Xeljanz) produced a 2.1-fold greater risk of herpes zoster infection (95% confidence interval, 0.4-6.0), while a 10-mg, twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2-12.2).
With the exception of the higher incidence rate of herpes zoster, “in the overall cohort, the safety profile of tofacitinib was generally similar to that of tumor necrosis factor inhibitor therapies,” wrote William J. Sandborn, MD, director of the inflammatory bowel disease center and professor of medicine, at the University of California, San Diego, and associates. The findings were published in Clinical Gastroenterology and Hepatology.
Tofacitinib is an oral, small-molecular Janus kinase inhibitor approved in the United States for treating moderate to severe ulcerative colitis, as well as rheumatoid and psoriatic arthritis. The recommended ulcerative colitis dose is 10 mg twice daily for at least 8 weeks (induction therapy) followed by 5 or 10 mg twice daily (maintenance). The safety of tofacitinib has been studied in patients with rheumatoid arthritis through 9 years of treatment. To begin a similar undertaking in ulcerative colitis, Dr. Sandborn and associates pooled data from three 8-week, double-blind, placebo-controlled induction trials, as well as one 52-week, double-blind, placebo-controlled maintenance trial and one ongoing open-label trial. All patients received twice-daily tofacitinib (5 mg or 10 mg) or placebo.
Among 1,157 tofacitinib recipients in the pooled analysis, 84% received an average of 10 mg twice daily. For every 100 person-years of tofacitinib exposure, there were an estimated 2.0 serious infections, 1.3 opportunistic infections, 4.1 herpes zoster infections, 1.4 malignancies (including nonmelanoma skin cancer, which had an incidence of 0.7), 0.2 major adverse cardiovascular events, and 0.2 gastrointestinal perforations. The likelihood of these events did not increase with time on tofacitinib, the researchers said.
Worsening ulcerative colitis was the most common serious adverse event for patients who received both induction and maintenance therapy. For patients on maintenance therapy, only herpes zoster infection had a higher incidence than placebo, which reached statistical significance at the 10-mg dose. These safety findings resemble those in rheumatoid arthritis trials of tofacitinib, and apart from herpes zoster, they also resemble safety data for vedolizumab (an integrin receptor antagonist), and anti-tumor necrosis factor agents in ulcerative colitis, the researchers wrote.
There were four deaths during the entire tofacitinib ulcerative colitis program, for an incidence rate of 0.2 per 100 person-years of exposure. All occurred in patients receiving 10 mg twice daily. Causes of death were dissecting aortic aneurysm, hepatic angiosarcoma, acute myeloid leukemia, and pulmonary embolism in a patient with cholangiocarcinoma that had metastasized to the peritoneum. Recently, concerns about pulmonary embolism have led the European Medicines Agency (EMA) to recommend against the use of 10-mg twice daily tofacitinib dose in patients at increased risk for pulmonary embolism.
“Compared with prior experience with tofacitinib in rheumatoid arthritis, no new or unexpected safety signals were identified,” the researchers concluded. “These
Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
SOURCE: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
As new mechanisms of action become available for ulcerative colitis (UC) drugs, clinicians must weigh the risks versus benefits (i.e., safety vs. efficacy). In this article, Sandborn and colleagues provide additional information on the safety profile of tofacitinib. They report an increased risk of herpes zoster that was dose dependent (sixfold increase on 10 mg twice daily). The overall safety profile was reassuring, is similar to the rheumatoid arthritis population treated with tofacitinib, and is in line with the safety profile of anti-TNF antibodies (excluding the increase risk of zoster). With a nonlive zoster vaccine now available, some have advocated vaccinating all patients being started on tofacitinib. However, there is a theoretical risk of disease exacerbation and ongoing studies that will hopefully answer this important question.
David A. Schwartz, MD, professor of medicine, division of gastroenterology, hepatology and nutrition, Inflammatory Bowel Disease Center, Vanderbilt University, Nashville.
As new mechanisms of action become available for ulcerative colitis (UC) drugs, clinicians must weigh the risks versus benefits (i.e., safety vs. efficacy). In this article, Sandborn and colleagues provide additional information on the safety profile of tofacitinib. They report an increased risk of herpes zoster that was dose dependent (sixfold increase on 10 mg twice daily). The overall safety profile was reassuring, is similar to the rheumatoid arthritis population treated with tofacitinib, and is in line with the safety profile of anti-TNF antibodies (excluding the increase risk of zoster). With a nonlive zoster vaccine now available, some have advocated vaccinating all patients being started on tofacitinib. However, there is a theoretical risk of disease exacerbation and ongoing studies that will hopefully answer this important question.
David A. Schwartz, MD, professor of medicine, division of gastroenterology, hepatology and nutrition, Inflammatory Bowel Disease Center, Vanderbilt University, Nashville.
As new mechanisms of action become available for ulcerative colitis (UC) drugs, clinicians must weigh the risks versus benefits (i.e., safety vs. efficacy). In this article, Sandborn and colleagues provide additional information on the safety profile of tofacitinib. They report an increased risk of herpes zoster that was dose dependent (sixfold increase on 10 mg twice daily). The overall safety profile was reassuring, is similar to the rheumatoid arthritis population treated with tofacitinib, and is in line with the safety profile of anti-TNF antibodies (excluding the increase risk of zoster). With a nonlive zoster vaccine now available, some have advocated vaccinating all patients being started on tofacitinib. However, there is a theoretical risk of disease exacerbation and ongoing studies that will hopefully answer this important question.
David A. Schwartz, MD, professor of medicine, division of gastroenterology, hepatology and nutrition, Inflammatory Bowel Disease Center, Vanderbilt University, Nashville.
Among patients with moderate to severe ulcerative colitis, a median of 1.4 years and up to 4.4 years of tofacitinib therapy was safe apart from a dose-related increase in risk of herpes zoster infection, according to an integrated analysis of data from five clinical trials.
Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib (Xeljanz) produced a 2.1-fold greater risk of herpes zoster infection (95% confidence interval, 0.4-6.0), while a 10-mg, twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2-12.2).
With the exception of the higher incidence rate of herpes zoster, “in the overall cohort, the safety profile of tofacitinib was generally similar to that of tumor necrosis factor inhibitor therapies,” wrote William J. Sandborn, MD, director of the inflammatory bowel disease center and professor of medicine, at the University of California, San Diego, and associates. The findings were published in Clinical Gastroenterology and Hepatology.
Tofacitinib is an oral, small-molecular Janus kinase inhibitor approved in the United States for treating moderate to severe ulcerative colitis, as well as rheumatoid and psoriatic arthritis. The recommended ulcerative colitis dose is 10 mg twice daily for at least 8 weeks (induction therapy) followed by 5 or 10 mg twice daily (maintenance). The safety of tofacitinib has been studied in patients with rheumatoid arthritis through 9 years of treatment. To begin a similar undertaking in ulcerative colitis, Dr. Sandborn and associates pooled data from three 8-week, double-blind, placebo-controlled induction trials, as well as one 52-week, double-blind, placebo-controlled maintenance trial and one ongoing open-label trial. All patients received twice-daily tofacitinib (5 mg or 10 mg) or placebo.
Among 1,157 tofacitinib recipients in the pooled analysis, 84% received an average of 10 mg twice daily. For every 100 person-years of tofacitinib exposure, there were an estimated 2.0 serious infections, 1.3 opportunistic infections, 4.1 herpes zoster infections, 1.4 malignancies (including nonmelanoma skin cancer, which had an incidence of 0.7), 0.2 major adverse cardiovascular events, and 0.2 gastrointestinal perforations. The likelihood of these events did not increase with time on tofacitinib, the researchers said.
Worsening ulcerative colitis was the most common serious adverse event for patients who received both induction and maintenance therapy. For patients on maintenance therapy, only herpes zoster infection had a higher incidence than placebo, which reached statistical significance at the 10-mg dose. These safety findings resemble those in rheumatoid arthritis trials of tofacitinib, and apart from herpes zoster, they also resemble safety data for vedolizumab (an integrin receptor antagonist), and anti-tumor necrosis factor agents in ulcerative colitis, the researchers wrote.
There were four deaths during the entire tofacitinib ulcerative colitis program, for an incidence rate of 0.2 per 100 person-years of exposure. All occurred in patients receiving 10 mg twice daily. Causes of death were dissecting aortic aneurysm, hepatic angiosarcoma, acute myeloid leukemia, and pulmonary embolism in a patient with cholangiocarcinoma that had metastasized to the peritoneum. Recently, concerns about pulmonary embolism have led the European Medicines Agency (EMA) to recommend against the use of 10-mg twice daily tofacitinib dose in patients at increased risk for pulmonary embolism.
“Compared with prior experience with tofacitinib in rheumatoid arthritis, no new or unexpected safety signals were identified,” the researchers concluded. “These
Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
SOURCE: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
Among patients with moderate to severe ulcerative colitis, a median of 1.4 years and up to 4.4 years of tofacitinib therapy was safe apart from a dose-related increase in risk of herpes zoster infection, according to an integrated analysis of data from five clinical trials.
Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib (Xeljanz) produced a 2.1-fold greater risk of herpes zoster infection (95% confidence interval, 0.4-6.0), while a 10-mg, twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2-12.2).
With the exception of the higher incidence rate of herpes zoster, “in the overall cohort, the safety profile of tofacitinib was generally similar to that of tumor necrosis factor inhibitor therapies,” wrote William J. Sandborn, MD, director of the inflammatory bowel disease center and professor of medicine, at the University of California, San Diego, and associates. The findings were published in Clinical Gastroenterology and Hepatology.
Tofacitinib is an oral, small-molecular Janus kinase inhibitor approved in the United States for treating moderate to severe ulcerative colitis, as well as rheumatoid and psoriatic arthritis. The recommended ulcerative colitis dose is 10 mg twice daily for at least 8 weeks (induction therapy) followed by 5 or 10 mg twice daily (maintenance). The safety of tofacitinib has been studied in patients with rheumatoid arthritis through 9 years of treatment. To begin a similar undertaking in ulcerative colitis, Dr. Sandborn and associates pooled data from three 8-week, double-blind, placebo-controlled induction trials, as well as one 52-week, double-blind, placebo-controlled maintenance trial and one ongoing open-label trial. All patients received twice-daily tofacitinib (5 mg or 10 mg) or placebo.
Among 1,157 tofacitinib recipients in the pooled analysis, 84% received an average of 10 mg twice daily. For every 100 person-years of tofacitinib exposure, there were an estimated 2.0 serious infections, 1.3 opportunistic infections, 4.1 herpes zoster infections, 1.4 malignancies (including nonmelanoma skin cancer, which had an incidence of 0.7), 0.2 major adverse cardiovascular events, and 0.2 gastrointestinal perforations. The likelihood of these events did not increase with time on tofacitinib, the researchers said.
Worsening ulcerative colitis was the most common serious adverse event for patients who received both induction and maintenance therapy. For patients on maintenance therapy, only herpes zoster infection had a higher incidence than placebo, which reached statistical significance at the 10-mg dose. These safety findings resemble those in rheumatoid arthritis trials of tofacitinib, and apart from herpes zoster, they also resemble safety data for vedolizumab (an integrin receptor antagonist), and anti-tumor necrosis factor agents in ulcerative colitis, the researchers wrote.
There were four deaths during the entire tofacitinib ulcerative colitis program, for an incidence rate of 0.2 per 100 person-years of exposure. All occurred in patients receiving 10 mg twice daily. Causes of death were dissecting aortic aneurysm, hepatic angiosarcoma, acute myeloid leukemia, and pulmonary embolism in a patient with cholangiocarcinoma that had metastasized to the peritoneum. Recently, concerns about pulmonary embolism have led the European Medicines Agency (EMA) to recommend against the use of 10-mg twice daily tofacitinib dose in patients at increased risk for pulmonary embolism.
“Compared with prior experience with tofacitinib in rheumatoid arthritis, no new or unexpected safety signals were identified,” the researchers concluded. “These
Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
SOURCE: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Tofacitinib therapy shows a dose-related increase in risk of herpes zoster in patients with ulcerative colitis.
Major finding: Compared with placebo, a 5-mg twice-daily maintenance dose of tofacitinib produced a 2.1-fold greater risk of herpes zoster infection (95% CI, 0.4-6.0), while a 10-mg twice-daily dose produced a statistically significant 6.6-fold increase in incidence (95% CI, 3.2–12.2).
Study details: Integrated safety analysis of five clinical trials (four randomized, double-blinded, and placebo-controlled) with 1,612.8 total years of exposure (median treatment duration, 1.4 years).
Disclosures: Pfizer makes tofacitinib, funded the individual trials, and paid for medical writing. Dr. Sandborn disclosed grants, personal fees, and nonfinancial support from Pfizer and many other pharmaceutical companies.
Source: Sandborn WJ et al. Clin Gastroenterol Hepatol. 2018 Nov 23. doi: 10.1016/j.cgh.2018.11.035.
Persistent fatigue plagues many IBD patients
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
SAN DIEGO – .
“Fatigue is one of the most heard complaints in the clinic,” lead study author Nynke Z. Borren, MD, said in an interview at the annual Digestive Disease Week.® “In the past few years there has been more interest because we know there is a communication system between the gut and the brain. Some studies suggest that biologic therapy improves fatigue symptoms, but it’s really correlated with disease activity.”
In an effort to define the longitudinal trajectory of fatigue in IBD patients initiating treatment with infliximab, adalimumab, vedolizumab, or ustekinumab, Dr. Borren, a research fellow at the Massachusetts General Hospital Crohn’s and Colitis Center, Boston, and colleagues prospectively enrolled 206 patients with Crohn’s disease and 120 patients with ulcerative colitis. They used the seven-point fatigue question in the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) to define fatigue. A score of four or less for this question was used to define fatigue. To validate this question, the researchers used two widely used questionnaires: the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F), and the Multidimensional Fatigue inventory (MFI). Next, they used multivariable regression models to examine the independent association between attainment of clinical remission and the resolution of fatigue.
Of the 326 patients, 134 initiated biologic therapy with infliximab or adalimumab, 129 with vedolizumab, and 63 with ustekinumab. Nearly two-thirds of all patients (198, or 61%) reported significant fatigue at baseline, which was associated with female sex, depressive symptoms, and disturbed sleep (P less than .001). Those reporting significant fatigue at baseline also had higher disease activity scores, compared with those without fatigue (P less than .001).
Among the 198 patients who reported fatigue at baseline, 70% remained fatigued at week 14, while 63% remained fatigued at week 30, and 61% remained fatigued at week 54. Dr. Borren and associates observed that at each of these time points, achieving clinical remission was associated with threefold lower likelihood of remaining fatigued. However, 35% of patients who achieved clinical remission experienced persistent fatigue at week 14, compared with 37% of patients at week 30 and 35% of patients at week 54.
The researchers observed no significant differences between the different therapies in the proportion of patients who remained fatigued. In addition to disease activity, disturbed sleep at baseline was associated with persistent fatigue at week 14 (OR 9.7) and at week 30 (OR 3.7).
“We think that gut dysbiosis might be involved in inducing fatigue,” Dr. Borren said. “In the beginning, we thought that it might be due to ongoing inflammation, but our research has shown that we find a less diverse gut microbiome in those patients with fatigue compared to patients without fatigue while they were in remission. There is something in the gut that influences the central nervous system. We are still exploring this.”
The researchers reported having no financial disclosures. The abstract received a “poster of distinction” honor at the meeting.
REPORTING FROM DDW 2019
Crohn’s: Red meat avoidance won’t prevent flares
For adults with Crohn’s disease in remission at baseline, eating red and processed meat no more than once per month did not reduce risk of relapse in a randomized control trial.
After 49 weeks, there were no significant differences in time to relapse, time to moderate or severe relapse, or time to persistent relapse between the low- and high-meat groups, reported Lindsey G. Aldenberg, DO, of the Children’s Hospital of Philadelphia and coinvestigators. The findings were published in Gastroenterology.
The randomized study included 213 adults with Crohn’s disease whose short Crohn’s Disease Activity Index (sCDAI) score was 150 or less at baseline and who consumed red meat at least once weekly. They were instructed to consume one serving (3 ounces) of red meat or any processed (smoked, salted, or otherwise preserved) meat at least twice weekly (high-meat group) or no more than once monthly (low-meat group). To create a placebo-like effect, all patients were told to drink at least 16 ounces of water daily. Each week, patients were emailed a web-based survey of disease status and dietary adherence. At baseline and during six other weeks, they also received a daily survey of disease activity and current medications. The primary outcome was symptomatic relapse, defined as at least a 70-point rise such that sCDAI score exceeded 150, surgery for Crohn’s disease flare, or self-reported initiation or dose increase of mesalamine, thiopurine, methotrexate, corticosteroid, anti–tumor necrosis factor-alpha therapy, or natalizumab.
In all, 78% (166) of patients either completed the study or experienced an outcome. Symptomatic relapse occurred in 62% of these 166 patients, while 42% and 35% had moderate to severe or persistent relapses, respectively. “There were no significant differences in time to relapse for any of the outcomes (P greater than .3 for all outcomes),” the researchers wrote. Results were similar when they assumed that patients who completed no surveys all relapsed at week 1.
At week 20, median fecal calprotectin levels were higher in the high-meat arm (74.5 mcg/g) than in the low-meat arm (36.0 mcg/g), but the difference was not statistically significant. Proportions of patients with fecal calprotectin levels above 150 or 250 mcg/g also did not significantly differ between arms.
Adherence to the diets was reasonable: Patients in the high-meat group reported consuming at least two servings of red or processed meat during 98.5% of weeks, while patients in the low-meat arm completely abstained from red or processed meat during 57.3% of weeks. A logistic regression model showed that the high-meat group was much more likely to consume a least two servings of red or processed meat in the prior week than the low-meat group (P less than .0001). Approximately 90% of patients in both arms drank the recommended amount of water.
Study participants were part of IBD Partners, an Internet-based cohort of more than 15,000 patients with inflammatory bowel disease. Recruitment into the trial occurred through emails, social media, educational and fundraising events, and the Crohn’s & Colitis Foundation website, the researchers said.
“Based on these results, although there may be some benefit for other health conditions,” Dr. Aldenberg and associates concluded.
The Crohn’s and Colitis Foundation and the National Institutes of Health supported the work. Dr. Aldenberg disclosed receiving research funding from Seres Therapeutics. Two of six coinvestigators disclosed ties to Nestle Health Science, AbbVie, Pfizer, Eli Lilly, and several other pharmaceutical companies.
SOURCE: Aldenberg L et al. Gastroenterology. 2019 Mar 11. doi: 10.1053/j.gastro.2019.03.01.
For understandable reasons, many patients believe that their symptoms or gastrointestinal disorders emanate from some interaction with a component of their diet. Crohn’s disease is no exception; various dietary factors have been incriminated in disease pathogenesis and the induction of relapse among those already affected. Furthermore, a number of dietary strategies or interventions have been recommended as therapeutic. For the induction of relapse, meat and related dietary components, such as fat, have been primary suspects.
This association was examined in this study by comparing the effects of low- or high-meat intakes (red meat and processed meat) over 49 weeks on clinical relapse rates in Crohn’s patients in remission at baseline. Sixty-two percent relapsed, and 42% had a moderate to severe relapse. However, there was no difference in time to relapse or rates of moderate/severe relapse between the two dietary groups.
Dietary intervention studies are notoriously difficult to perform; what is remarkable was that the investigators were able to complete the study with high rates of compliance over almost a year! Whether dietary patterns earlier in life (when the microbiota is more susceptible) or over longer periods could affect the natural history of inflammatory bowel disease remains to be determined. For now, this study has shown us that high-quality dietary studies can be performed and that variations in meat intake, within the range of those likely to occur in real life, do not affect relapse rates in Crohn’s disease.
Eamonn M. Quigley, MD, is the David M. Underwood Chair of Medicine in Digestive Disorders, Institute for Academic Medicine; director, Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. He has no relevant conflicts of interest.
For understandable reasons, many patients believe that their symptoms or gastrointestinal disorders emanate from some interaction with a component of their diet. Crohn’s disease is no exception; various dietary factors have been incriminated in disease pathogenesis and the induction of relapse among those already affected. Furthermore, a number of dietary strategies or interventions have been recommended as therapeutic. For the induction of relapse, meat and related dietary components, such as fat, have been primary suspects.
This association was examined in this study by comparing the effects of low- or high-meat intakes (red meat and processed meat) over 49 weeks on clinical relapse rates in Crohn’s patients in remission at baseline. Sixty-two percent relapsed, and 42% had a moderate to severe relapse. However, there was no difference in time to relapse or rates of moderate/severe relapse between the two dietary groups.
Dietary intervention studies are notoriously difficult to perform; what is remarkable was that the investigators were able to complete the study with high rates of compliance over almost a year! Whether dietary patterns earlier in life (when the microbiota is more susceptible) or over longer periods could affect the natural history of inflammatory bowel disease remains to be determined. For now, this study has shown us that high-quality dietary studies can be performed and that variations in meat intake, within the range of those likely to occur in real life, do not affect relapse rates in Crohn’s disease.
Eamonn M. Quigley, MD, is the David M. Underwood Chair of Medicine in Digestive Disorders, Institute for Academic Medicine; director, Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. He has no relevant conflicts of interest.
For understandable reasons, many patients believe that their symptoms or gastrointestinal disorders emanate from some interaction with a component of their diet. Crohn’s disease is no exception; various dietary factors have been incriminated in disease pathogenesis and the induction of relapse among those already affected. Furthermore, a number of dietary strategies or interventions have been recommended as therapeutic. For the induction of relapse, meat and related dietary components, such as fat, have been primary suspects.
This association was examined in this study by comparing the effects of low- or high-meat intakes (red meat and processed meat) over 49 weeks on clinical relapse rates in Crohn’s patients in remission at baseline. Sixty-two percent relapsed, and 42% had a moderate to severe relapse. However, there was no difference in time to relapse or rates of moderate/severe relapse between the two dietary groups.
Dietary intervention studies are notoriously difficult to perform; what is remarkable was that the investigators were able to complete the study with high rates of compliance over almost a year! Whether dietary patterns earlier in life (when the microbiota is more susceptible) or over longer periods could affect the natural history of inflammatory bowel disease remains to be determined. For now, this study has shown us that high-quality dietary studies can be performed and that variations in meat intake, within the range of those likely to occur in real life, do not affect relapse rates in Crohn’s disease.
Eamonn M. Quigley, MD, is the David M. Underwood Chair of Medicine in Digestive Disorders, Institute for Academic Medicine; director, Lynda K. and David M. Underwood Center for Digestive Disorders, Houston Methodist Hospital. He has no relevant conflicts of interest.
For adults with Crohn’s disease in remission at baseline, eating red and processed meat no more than once per month did not reduce risk of relapse in a randomized control trial.
After 49 weeks, there were no significant differences in time to relapse, time to moderate or severe relapse, or time to persistent relapse between the low- and high-meat groups, reported Lindsey G. Aldenberg, DO, of the Children’s Hospital of Philadelphia and coinvestigators. The findings were published in Gastroenterology.
The randomized study included 213 adults with Crohn’s disease whose short Crohn’s Disease Activity Index (sCDAI) score was 150 or less at baseline and who consumed red meat at least once weekly. They were instructed to consume one serving (3 ounces) of red meat or any processed (smoked, salted, or otherwise preserved) meat at least twice weekly (high-meat group) or no more than once monthly (low-meat group). To create a placebo-like effect, all patients were told to drink at least 16 ounces of water daily. Each week, patients were emailed a web-based survey of disease status and dietary adherence. At baseline and during six other weeks, they also received a daily survey of disease activity and current medications. The primary outcome was symptomatic relapse, defined as at least a 70-point rise such that sCDAI score exceeded 150, surgery for Crohn’s disease flare, or self-reported initiation or dose increase of mesalamine, thiopurine, methotrexate, corticosteroid, anti–tumor necrosis factor-alpha therapy, or natalizumab.
In all, 78% (166) of patients either completed the study or experienced an outcome. Symptomatic relapse occurred in 62% of these 166 patients, while 42% and 35% had moderate to severe or persistent relapses, respectively. “There were no significant differences in time to relapse for any of the outcomes (P greater than .3 for all outcomes),” the researchers wrote. Results were similar when they assumed that patients who completed no surveys all relapsed at week 1.
At week 20, median fecal calprotectin levels were higher in the high-meat arm (74.5 mcg/g) than in the low-meat arm (36.0 mcg/g), but the difference was not statistically significant. Proportions of patients with fecal calprotectin levels above 150 or 250 mcg/g also did not significantly differ between arms.
Adherence to the diets was reasonable: Patients in the high-meat group reported consuming at least two servings of red or processed meat during 98.5% of weeks, while patients in the low-meat arm completely abstained from red or processed meat during 57.3% of weeks. A logistic regression model showed that the high-meat group was much more likely to consume a least two servings of red or processed meat in the prior week than the low-meat group (P less than .0001). Approximately 90% of patients in both arms drank the recommended amount of water.
Study participants were part of IBD Partners, an Internet-based cohort of more than 15,000 patients with inflammatory bowel disease. Recruitment into the trial occurred through emails, social media, educational and fundraising events, and the Crohn’s & Colitis Foundation website, the researchers said.
“Based on these results, although there may be some benefit for other health conditions,” Dr. Aldenberg and associates concluded.
The Crohn’s and Colitis Foundation and the National Institutes of Health supported the work. Dr. Aldenberg disclosed receiving research funding from Seres Therapeutics. Two of six coinvestigators disclosed ties to Nestle Health Science, AbbVie, Pfizer, Eli Lilly, and several other pharmaceutical companies.
SOURCE: Aldenberg L et al. Gastroenterology. 2019 Mar 11. doi: 10.1053/j.gastro.2019.03.01.
For adults with Crohn’s disease in remission at baseline, eating red and processed meat no more than once per month did not reduce risk of relapse in a randomized control trial.
After 49 weeks, there were no significant differences in time to relapse, time to moderate or severe relapse, or time to persistent relapse between the low- and high-meat groups, reported Lindsey G. Aldenberg, DO, of the Children’s Hospital of Philadelphia and coinvestigators. The findings were published in Gastroenterology.
The randomized study included 213 adults with Crohn’s disease whose short Crohn’s Disease Activity Index (sCDAI) score was 150 or less at baseline and who consumed red meat at least once weekly. They were instructed to consume one serving (3 ounces) of red meat or any processed (smoked, salted, or otherwise preserved) meat at least twice weekly (high-meat group) or no more than once monthly (low-meat group). To create a placebo-like effect, all patients were told to drink at least 16 ounces of water daily. Each week, patients were emailed a web-based survey of disease status and dietary adherence. At baseline and during six other weeks, they also received a daily survey of disease activity and current medications. The primary outcome was symptomatic relapse, defined as at least a 70-point rise such that sCDAI score exceeded 150, surgery for Crohn’s disease flare, or self-reported initiation or dose increase of mesalamine, thiopurine, methotrexate, corticosteroid, anti–tumor necrosis factor-alpha therapy, or natalizumab.
In all, 78% (166) of patients either completed the study or experienced an outcome. Symptomatic relapse occurred in 62% of these 166 patients, while 42% and 35% had moderate to severe or persistent relapses, respectively. “There were no significant differences in time to relapse for any of the outcomes (P greater than .3 for all outcomes),” the researchers wrote. Results were similar when they assumed that patients who completed no surveys all relapsed at week 1.
At week 20, median fecal calprotectin levels were higher in the high-meat arm (74.5 mcg/g) than in the low-meat arm (36.0 mcg/g), but the difference was not statistically significant. Proportions of patients with fecal calprotectin levels above 150 or 250 mcg/g also did not significantly differ between arms.
Adherence to the diets was reasonable: Patients in the high-meat group reported consuming at least two servings of red or processed meat during 98.5% of weeks, while patients in the low-meat arm completely abstained from red or processed meat during 57.3% of weeks. A logistic regression model showed that the high-meat group was much more likely to consume a least two servings of red or processed meat in the prior week than the low-meat group (P less than .0001). Approximately 90% of patients in both arms drank the recommended amount of water.
Study participants were part of IBD Partners, an Internet-based cohort of more than 15,000 patients with inflammatory bowel disease. Recruitment into the trial occurred through emails, social media, educational and fundraising events, and the Crohn’s & Colitis Foundation website, the researchers said.
“Based on these results, although there may be some benefit for other health conditions,” Dr. Aldenberg and associates concluded.
The Crohn’s and Colitis Foundation and the National Institutes of Health supported the work. Dr. Aldenberg disclosed receiving research funding from Seres Therapeutics. Two of six coinvestigators disclosed ties to Nestle Health Science, AbbVie, Pfizer, Eli Lilly, and several other pharmaceutical companies.
SOURCE: Aldenberg L et al. Gastroenterology. 2019 Mar 11. doi: 10.1053/j.gastro.2019.03.01.
FROM GASTROENTEROLOGY
Zoster vaccination is underused but looks effective in IBD
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
Preventive care is an underemphasized component of IBD management because the primary focus tends to be control of active symptoms. However, as patients are treated with immunosuppression, particularly combinations of therapies and newer mechanisms of action such as the Janus kinase inhibitors, the risk of infections increases, including those that are vaccine preventable including shingles and its related complications.
This study by Khan et al. highlights several important messages for patients and providers. First, in this large older IBD cohort, the vaccination rates were very low at 18% even though more than 80% of patients had more than six annual visits to the VA Health Systems during the study period. These represent multiple missed opportunities to discuss and administer vaccinations. Second, the authors highlighted the vaccine’s efficacy: Persons receiving herpes zoster vaccination had a clearly decreased risk of subsequent infection. While the number of vaccinated patients on immunosuppression was too small to draw conclusions about efficacy, the live attenuated vaccination is contraindicated for immunosuppressed patients. However, the newer recombinant shingles vaccine offers the opportunity to extend the reach of shingles vaccination to include those on immunosuppression. As utilization of the newer vaccine series increases, we will be able to evaluate the efficacy for immunosuppressed IBD patients, although studies from other disease states suggest efficacy. However, vaccinations will never work if they aren’t administered. Counseling patients and providers regarding the importance of vaccinations is a low-risk, efficacious means to decrease infection and associated morbidity.
Christina Ha, MD, AGAF, associate professor of medicine, Inflammatory Bowel Disease Center, division of digestive diseases, Cedars-Sinai Medical Center, Los Angeles. She is a speaker, consultant, or on the advisory board for AbbVie, Janssen, Genentech, Samsung Bioepis, and Takeda. She received grant funding from Pfizer.
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
For men with inflammatory bowel disease, herpes zoster vaccination was associated with about a 46% decrease in risk of associated infection, according to the results of a retrospective study from the national Veterans Affairs Healthcare System.
Crude rates of herpes zoster infection were 4.09 cases per 1,000 person-years among vaccinated patients versus 6.97 cases per 1,000 person-years among unvaccinated patients, for an adjusted hazard ratio of 0.54 (95% confidence interval, 0.44-0.68), reported Nabeel Khan, MD, of the University of Pennsylvania, Philadelphia, and associates. “This vaccine is therefore effective in patients with IBD, but underused,” they wrote in Clinical Gastroenterology and Hepatology.
Studies have linked IBD with a 1.2- to 1.8-fold increased risk of herpes zoster infection, the researchers noted. Relevant risk factors include older age, disease flare, recent use or high cumulative use of prednisone, and use of thiopurines, either alone or in combination with a tumor necrosis factor (TNF) inhibitor. Although the American College of Gastroenterology recommends that all patients with IBD receive the herpes zoster vaccine by age 50 years, the efficacy of the vaccine in these patients remains unclear.
For their study, Dr. Khan and associates analyzed International Classification of Diseases (ICD) codes and other medical record data from 39,983 veterans with IBD who had not received the herpes zoster vaccine by age 60 years. In all, 97% of patients were male, and 94% were white. Most patients had high rates of health care utilization: Approximately half visited VA clinics or hospitals at least 13 times per year, and another third made 6-12 annual visits.
Despite their many contacts with VA health care systems, only 7,170 (17.9%) patients received the herpes zoster vaccine during 2000-2016, the researchers found. Vaccination rates varied substantially by region – they were highest in the Midwest (35%) and North Atlantic states (29%) but reached only 9% in Montana, Utah, Wyoming, Colorado, Oklahoma, Texas, Arkansas, and Louisiana, collectively.
The crude rate of herpes zoster infection among unvaccinated patients with IBD resembled the incidence reported in prior studies, the researchers said. After researchers accounted for differences in geography, demographics, and health care utilization between vaccinated and unvaccinated veterans with IBD, they found that vaccination was associated with an approximately 46% decrease in the risk of herpes zoster infection.
Very few patients were vaccinated for herpes zoster while on a TNF inhibitor, precluding the ability to study this subgroup. However, the vaccine showed a protective effect (adjusted HR, 0.63) among patients who received thiopurines without a TNF inhibitor. This effect did not reach statistical significance, perhaps because of lack of power, the researchers noted. “Among the 315 patients who were [vaccinated while] on thiopurines, none developed a documented painful or painless vesicular rash within 42 days of herpes zoster vaccination,” they added. One patient developed a painful blister 20 days post vaccination without vesicles or long-term sequelae.
Pfizer provided funding. Dr. Khan disclosed research funding from Pfizer, Luitpold, and Takeda Pharmaceuticals. One coinvestigator disclosed ties to Pfizer, Gilead, Merck, AbbVie, Lilly, Janssen, Johnson & Johnson, UCB, and Nestle Health Science. The remaining researchers disclosed no conflicts.
SOURCE: Khan N et al. Clin Gastroenterol Hepatol. 2018 Oct 13. doi: 10.1016/j.cgh.2018.10.016.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Infections within first year of life predicted IBD
according to the findings of a large population-based study.
It remains unclear whether the risk reflects infections in themselves or the use of antibiotic therapy, wrote Charles N. Bernstein, MD, of the University of Manitoba, Winnipeg, and associates. Infections did not appear to be a proxy for immunodeficiency disorders, which were similarly infrequent among cases and controls, they noted. Limiting antibiotic usage, while desirable, would be difficult to do for infections as serious as many in the study. Hence, they suggested research to determine “exactly what antibiotic intake does to infant gut microflora or intestinal or systemic immune responses,” and whether giving probiotics or prebiotics after antibiotic therapy helps attenuate the risk of inflammatory bowel disease (IBD) and other autoimmune disorders. The findings were published in Gastroenterology.
IBD is probably multifactorial, but specific causal factors remain unclear. Based on mounting evidence for the role of gut dysbiosis, the researchers explored whether IBD is associated with higher rates of infections and other critical events during the neonatal period and the first year of life by comparing 825 patients with IBD and 5,999 controls matched by age, sex, and area of residence. The data source was the University of Manitoba IBD Epidemiology Database, which includes all Manitobans diagnosed with IBD from 1984 to 2010. The researchers also compared patients with 1,740 unaffected siblings.
Gastrointestinal infections, gastrointestinal disease, and abdominal pain during the first year of life did not predict subsequent IBD. Maternal IBD was the strongest risk factor (odds ratio, 4.5; 95% confidence interval, 3.1-6.7). Among neonatal events, the only significant risk factor was being in the highest versus the lowest socioeconomic quintile (OR, 1.35; 95% CI, 1.01-1.79). This association persisted during the first year of life.
Infections during the first year of life were a significant risk factor for IBD before age 10 (OR, 3.1; 95% CI, 1.1-8.8) and age 20 years (OR, 1.6; 95% CI, 1.2-2.2) in the population-based analysis. In contrast, patients and their unaffected siblings had similar rates of infection during early life. The study may have missed differences in exposures between these groups, or perhaps patients lack certain protective genes possessed by healthy siblings, the researchers wrote.
Numbers of antibiotic prescriptions during the first year and the first decade of life did not significantly differ between 33 cases and 270 controls with available data. However, there was a trend toward more antibiotics prescribed to patients versus controls.
“Together with our past reports that neither cesarean section birth nor antenatal or perinatal maternal use of antibiotics predict ultimate development of IBD, it seems that neonatal changes to the microbiome are subsumed by those occurring in the first year of life,” the investigators concluded. They recommended studying the infant gut microbiome before and for several months after infections and antibiotic exposure to determine which shifts in microbiota predict IBD onset.
The Manitoba Centre for Health Policy provided access to the Population Health Research Data Repository. Dr. Bernstein is supported by the Bingham Chair in Gastroenterology. He reported ties to AbbVie Canada, Ferring Canada, Janssen Canada, Shire Canada, Takeda Canada, Pfizer Canada, Napo Pharmaceuticals, 4D Pharma, and Mylan.
SOURCE: Bernstein CN et al. Gastroenterology. 2019 Feb 14. doi: 10.1053/j.gastro.2019.02.004.
Understanding and exploring factors that could impact inflammatory bowel disease (IBD) development is imperative. This study by Bernstein et al. evaluated whether environmental factors in the first year of life may impact subsequent diagnosis of IBD using population-based cohort data with robust and detailed health information. Maternal history of IBD was the most predictive factor in development of IBD, further evidence of a genetic component to disease pathogenesis. However, environmental factors such as high socioeconomic status within the first year of life were predictive of diagnosis of IBD later in life, possibly lending further support to the “hygiene hypothesis.”
Also, significant infections identified in the clinical setting or requiring hospitalization were predictive of subsequent IBD diagnosis. This is particularly interesting as gut microbiome perturbations increasingly take the stage as a possible pathway of significance in IBD. Could infection within the first year of life or the subsequent antibiotic use required affect the gut microbiome so significantly and perhaps permanently to affect development of later childhood or adult IBD?
Sara Horst, MD, MPH, is an associate professor of medicine in the department of gastroenterology, hepatology, and medicine at Vanderbilt University, Nashville, Tenn. She has consulted for Janssen, UCB, and Boehringer Ingelheim.
Understanding and exploring factors that could impact inflammatory bowel disease (IBD) development is imperative. This study by Bernstein et al. evaluated whether environmental factors in the first year of life may impact subsequent diagnosis of IBD using population-based cohort data with robust and detailed health information. Maternal history of IBD was the most predictive factor in development of IBD, further evidence of a genetic component to disease pathogenesis. However, environmental factors such as high socioeconomic status within the first year of life were predictive of diagnosis of IBD later in life, possibly lending further support to the “hygiene hypothesis.”
Also, significant infections identified in the clinical setting or requiring hospitalization were predictive of subsequent IBD diagnosis. This is particularly interesting as gut microbiome perturbations increasingly take the stage as a possible pathway of significance in IBD. Could infection within the first year of life or the subsequent antibiotic use required affect the gut microbiome so significantly and perhaps permanently to affect development of later childhood or adult IBD?
Sara Horst, MD, MPH, is an associate professor of medicine in the department of gastroenterology, hepatology, and medicine at Vanderbilt University, Nashville, Tenn. She has consulted for Janssen, UCB, and Boehringer Ingelheim.
Understanding and exploring factors that could impact inflammatory bowel disease (IBD) development is imperative. This study by Bernstein et al. evaluated whether environmental factors in the first year of life may impact subsequent diagnosis of IBD using population-based cohort data with robust and detailed health information. Maternal history of IBD was the most predictive factor in development of IBD, further evidence of a genetic component to disease pathogenesis. However, environmental factors such as high socioeconomic status within the first year of life were predictive of diagnosis of IBD later in life, possibly lending further support to the “hygiene hypothesis.”
Also, significant infections identified in the clinical setting or requiring hospitalization were predictive of subsequent IBD diagnosis. This is particularly interesting as gut microbiome perturbations increasingly take the stage as a possible pathway of significance in IBD. Could infection within the first year of life or the subsequent antibiotic use required affect the gut microbiome so significantly and perhaps permanently to affect development of later childhood or adult IBD?
Sara Horst, MD, MPH, is an associate professor of medicine in the department of gastroenterology, hepatology, and medicine at Vanderbilt University, Nashville, Tenn. She has consulted for Janssen, UCB, and Boehringer Ingelheim.
according to the findings of a large population-based study.
It remains unclear whether the risk reflects infections in themselves or the use of antibiotic therapy, wrote Charles N. Bernstein, MD, of the University of Manitoba, Winnipeg, and associates. Infections did not appear to be a proxy for immunodeficiency disorders, which were similarly infrequent among cases and controls, they noted. Limiting antibiotic usage, while desirable, would be difficult to do for infections as serious as many in the study. Hence, they suggested research to determine “exactly what antibiotic intake does to infant gut microflora or intestinal or systemic immune responses,” and whether giving probiotics or prebiotics after antibiotic therapy helps attenuate the risk of inflammatory bowel disease (IBD) and other autoimmune disorders. The findings were published in Gastroenterology.
IBD is probably multifactorial, but specific causal factors remain unclear. Based on mounting evidence for the role of gut dysbiosis, the researchers explored whether IBD is associated with higher rates of infections and other critical events during the neonatal period and the first year of life by comparing 825 patients with IBD and 5,999 controls matched by age, sex, and area of residence. The data source was the University of Manitoba IBD Epidemiology Database, which includes all Manitobans diagnosed with IBD from 1984 to 2010. The researchers also compared patients with 1,740 unaffected siblings.
Gastrointestinal infections, gastrointestinal disease, and abdominal pain during the first year of life did not predict subsequent IBD. Maternal IBD was the strongest risk factor (odds ratio, 4.5; 95% confidence interval, 3.1-6.7). Among neonatal events, the only significant risk factor was being in the highest versus the lowest socioeconomic quintile (OR, 1.35; 95% CI, 1.01-1.79). This association persisted during the first year of life.
Infections during the first year of life were a significant risk factor for IBD before age 10 (OR, 3.1; 95% CI, 1.1-8.8) and age 20 years (OR, 1.6; 95% CI, 1.2-2.2) in the population-based analysis. In contrast, patients and their unaffected siblings had similar rates of infection during early life. The study may have missed differences in exposures between these groups, or perhaps patients lack certain protective genes possessed by healthy siblings, the researchers wrote.
Numbers of antibiotic prescriptions during the first year and the first decade of life did not significantly differ between 33 cases and 270 controls with available data. However, there was a trend toward more antibiotics prescribed to patients versus controls.
“Together with our past reports that neither cesarean section birth nor antenatal or perinatal maternal use of antibiotics predict ultimate development of IBD, it seems that neonatal changes to the microbiome are subsumed by those occurring in the first year of life,” the investigators concluded. They recommended studying the infant gut microbiome before and for several months after infections and antibiotic exposure to determine which shifts in microbiota predict IBD onset.
The Manitoba Centre for Health Policy provided access to the Population Health Research Data Repository. Dr. Bernstein is supported by the Bingham Chair in Gastroenterology. He reported ties to AbbVie Canada, Ferring Canada, Janssen Canada, Shire Canada, Takeda Canada, Pfizer Canada, Napo Pharmaceuticals, 4D Pharma, and Mylan.
SOURCE: Bernstein CN et al. Gastroenterology. 2019 Feb 14. doi: 10.1053/j.gastro.2019.02.004.
according to the findings of a large population-based study.
It remains unclear whether the risk reflects infections in themselves or the use of antibiotic therapy, wrote Charles N. Bernstein, MD, of the University of Manitoba, Winnipeg, and associates. Infections did not appear to be a proxy for immunodeficiency disorders, which were similarly infrequent among cases and controls, they noted. Limiting antibiotic usage, while desirable, would be difficult to do for infections as serious as many in the study. Hence, they suggested research to determine “exactly what antibiotic intake does to infant gut microflora or intestinal or systemic immune responses,” and whether giving probiotics or prebiotics after antibiotic therapy helps attenuate the risk of inflammatory bowel disease (IBD) and other autoimmune disorders. The findings were published in Gastroenterology.
IBD is probably multifactorial, but specific causal factors remain unclear. Based on mounting evidence for the role of gut dysbiosis, the researchers explored whether IBD is associated with higher rates of infections and other critical events during the neonatal period and the first year of life by comparing 825 patients with IBD and 5,999 controls matched by age, sex, and area of residence. The data source was the University of Manitoba IBD Epidemiology Database, which includes all Manitobans diagnosed with IBD from 1984 to 2010. The researchers also compared patients with 1,740 unaffected siblings.
Gastrointestinal infections, gastrointestinal disease, and abdominal pain during the first year of life did not predict subsequent IBD. Maternal IBD was the strongest risk factor (odds ratio, 4.5; 95% confidence interval, 3.1-6.7). Among neonatal events, the only significant risk factor was being in the highest versus the lowest socioeconomic quintile (OR, 1.35; 95% CI, 1.01-1.79). This association persisted during the first year of life.
Infections during the first year of life were a significant risk factor for IBD before age 10 (OR, 3.1; 95% CI, 1.1-8.8) and age 20 years (OR, 1.6; 95% CI, 1.2-2.2) in the population-based analysis. In contrast, patients and their unaffected siblings had similar rates of infection during early life. The study may have missed differences in exposures between these groups, or perhaps patients lack certain protective genes possessed by healthy siblings, the researchers wrote.
Numbers of antibiotic prescriptions during the first year and the first decade of life did not significantly differ between 33 cases and 270 controls with available data. However, there was a trend toward more antibiotics prescribed to patients versus controls.
“Together with our past reports that neither cesarean section birth nor antenatal or perinatal maternal use of antibiotics predict ultimate development of IBD, it seems that neonatal changes to the microbiome are subsumed by those occurring in the first year of life,” the investigators concluded. They recommended studying the infant gut microbiome before and for several months after infections and antibiotic exposure to determine which shifts in microbiota predict IBD onset.
The Manitoba Centre for Health Policy provided access to the Population Health Research Data Repository. Dr. Bernstein is supported by the Bingham Chair in Gastroenterology. He reported ties to AbbVie Canada, Ferring Canada, Janssen Canada, Shire Canada, Takeda Canada, Pfizer Canada, Napo Pharmaceuticals, 4D Pharma, and Mylan.
SOURCE: Bernstein CN et al. Gastroenterology. 2019 Feb 14. doi: 10.1053/j.gastro.2019.02.004.
FROM GASTROENTEROLOGY
Appendectomy linked to increased risk of subsequent Parkinson’s
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
.
“One of the factors that’s seen in the brains of patients with Parkinson’s disease is accumulation of an abnormal protein known as alpha-synuclein,” one of the study authors, Gregory S. Cooper, MD, said during a media briefing in advance of the annual Digestive Disease Week. “It’s released by damaged nerve cells in the brain. Not only is alpha-synuclein found in the brain of patients with Parkinson’s disease; it’s also found in the GI tract. It’s thought that its accumulation in the GI tract occurs prior to the development of its accumulation in the brain.”
This has prompted scientists around the world to evaluate the GI tract, including the appendix, for evidence about the pathophysiology and onset of Parkinson’s disease, said Dr. Cooper, professor of medicine, oncology, and population and quantitative health sciences at Case Western Reserve University, Cleveland. “It’s thought that, potentially, in the presence of inflammation, [molecules] of this protein are released from damaged nerves in the gut and then are transported to the brain, where they accumulate,” he said. “Or, it could be that the appendix is a storage place for this protein and gets released at the time of appendectomy.”
To investigate if appendectomy increases the risk of Parkinson’s disease, Dr. Cooper and colleagues drew from the Explorys database, which contains EHRs from 26 integrated U.S. health care systems. They limited their search to patients who underwent appendectomies and those who were diagnosed with Parkinson’s disease based on Systematized Nomenclature of Medicine–Clinical Terms. The researchers chose a washout period of 6 months to the development of Parkinson’s disease after appendectomy, and compared the prevalence of Parkinson’s disease in the general population to those with appendectomies.
Of the 62,218,050 records in the database, Dr. Cooper and colleagues identified 488,190 patients who underwent appendectomies. In all, 4,470 cases of Parkinson’s disease were observed in patients with appendectomies, and 177,230 cases of Parkinson’s disease in patients without appendectomies. The overall relative risk of developing Parkinson’s disease in patients after appendectomies was 3.19 (95% confidence interval, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure. The relative risk was higher in patients aged 18-64 years (RR, 4.27; 95% CI, 3.99-4.57; P less than .0001), compared with those 65 years and older (RR, 2.20; 95% CI, 2.13-2.27; P less than .0001). “We know that Parkinson’s disease is more common in the elderly,” Dr. Cooper said. “But at virtually all ages, the prevalence of Parkinson’s disease was higher in patients who had an appendectomy, compared to those without an appendectomy.”
The overall relative risk of developing Parkinson’s disease in patients after appendectomies was slightly higher in females (RR, 3.86; 95% CI, 3.71-4.02; P less than .0001), compared with males (RR, 2.67; 95% CI, 2.56-2.79; P less than .0001). The researchers also observed a similar effect of appendectomy by race. The overall relative risk of developing Parkinson’s disease in patients after appendectomy was slightly higher in African Americans (RR, 3.11; 95% CI, 2.69-3.58; P less than .0001), compared with Asians (RR, 2.73; 95% CI, 2.19-3.41; P less than .0001), and whites (RR, 2.55; 95% CI, 2.48-2.63; P less than .0001).
“If these data get borne out, it may question the role of doing a discretionary appendectomy in a patient who’s having surgery for another reason,” Dr. Cooper said. “Our research does show a clear relationship between appendectomy and Parkinson’s disease. However, at this point, it’s only an association. As a next step, we’d like to conduct additional research to confirm this connection and better understand the mechanisms involved.”
He pointed out that, because of the nature of the Explorys database, he and his colleagues were unable to determine the length of time following appendectomy to the development of Parkinson’s disease.
The study’s lead author was Mohammed Z. Sheriff, MD, also of Case Western Reserve University, Cleveland. The researchers reported having no financial disclosures.
SOURCE: Sheriff MZ et al. DDW 2019, Abstract 739.
REPORTING FROM DDW 2019
Key clinical point: Appendectomy appears to increase the risk of Parkinson’s disease.
Major finding: The overall relative risk of developing Parkinson’s disease in patients after appendectomy was 3.19 (95% CI, 3.10-3.28; P less than .0001), compared with those who did not undergo the procedure.
Study details: A population-based study of more than 62 million medical records from a national database.
Disclosures: The researchers reported having no financial disclosures.
Source: Sheriff MZ et al. DDW 2019, Abstract 739.
Induction trough levels predicted ustekinumab response in Crohn’s disease
For patients with Crohn’s disease, therapeutic drug monitoring helped identify early primary nonresponders to induction with ustekinumab, according to researchers. The report is in Clinical Gastroenterology and Hepatology.
At week 8, median trough levels of ustekinumab were 6.0 mcg per mL (interquartile range, 3.1-8.0) among patients who achieved a primary response to induction at week 16, versus 1.3 mcg/mL (IQR, 0.9-5.6 ) among primary nonresponders (P = .03). An 8-week ustekinumab trough level cutoff of 2.0 mcg/mL distinguished week 16 responders from nonresponders with an area under the receiver operating curve (AUROC) of 0.75, wrote Ninon Soufflet of University Claude Bernard Lyon 1 in France, and associates. The researchers recommended “dedicated studies” to assess whether escalating the dose of ustekinumab can benefit patients with lower trough levels at week 8.
Few studies have explored biomarkers for response to ustekinumab induction therapy. Hence, the researchers assessed the relative utility of ustekinumab trough levels, C-reactive protein (CRP) levels, and fecal calprotectin levels for predicting early primary nonresponse. All 51 study participants had active luminal Crohn’s disease and received body weight–based intravenous infusions of ustekinumab at baseline, followed by subcutaneous injections of 90 mg. Primary nonresponders did not achieve steroid-free clinical and biochemical remission at week 16, defined as a Harvey-Bradshaw Index (HBI) of 4 points or less, a CRP level under 5 mg/L, and a fecal calprotectin level under 250 mcg/g. Week 16 was chosen to account for any delayed responders, the researchers noted.
A total of 32 patients (63%) achieved remission to ustekinumab induction therapy by week 16. An 8-week trough level of 2.0 mcg/mL was found to be optimal and distinguished primary nonresponders from responders with a sensitivity of 87%, a specificity of 66%, a positive predictive value of 82%, and a negative predictive value of 75%. In prior studies, optimal thresholds exceeded 3.3 mcg/mL for achieving remission and 4.5 mcg/mL at week 26 for achieving endoscopic response, the researchers noted. They said that this discrepancy might reflect different time points for evaluation, assays for measuring ustekinumab, patient populations, and a lack of endoscopic data in their study. “The relatively small sample size and the short period of follow-up evaluation [were] substantial limitations” they acknowledged.
In this study, levels of CRP did not change significantly between weeks 0 and 16 among either responders or nonresponders. In contrast, fecal calprotectin levels dropped rapidly and significantly over time only in responders. Median fecal calprotectin levels were 1,612 mcg/g of stools at week 0 versus 374 mcg/g at week 4 and 339 mcg/g at week 8. The finding “confirms the value of this biomarker, as previously shown in inflammatory bowel disease with anti–tumor necrosis factor,” the researchers wrote.
The investigators did not acknowledge external funding sources. Dr. Soufflet reported having no conflicts of interest. The senior author and three coinvestigators disclosed ties to MSD, AbbVie, Tillots, and several other pharmaceutical companies.
Learn more about therapeutic drug monitoring in IBD by reviewing the AGA Institute guideline at http://www.gastrojournal.org/article/S0016-5085(17)35963-2/fulltext.
SOURCE: Soufflet N et al. Clin Gastroenterol Hepatol. 2019 Mar 6. doi: 10.1016/j.cgh.2019.02.042. https://www.cghjournal.org/article/S1542-3565(19)30248-4/abstract
For patients with Crohn’s disease, therapeutic drug monitoring helped identify early primary nonresponders to induction with ustekinumab, according to researchers. The report is in Clinical Gastroenterology and Hepatology.
At week 8, median trough levels of ustekinumab were 6.0 mcg per mL (interquartile range, 3.1-8.0) among patients who achieved a primary response to induction at week 16, versus 1.3 mcg/mL (IQR, 0.9-5.6 ) among primary nonresponders (P = .03). An 8-week ustekinumab trough level cutoff of 2.0 mcg/mL distinguished week 16 responders from nonresponders with an area under the receiver operating curve (AUROC) of 0.75, wrote Ninon Soufflet of University Claude Bernard Lyon 1 in France, and associates. The researchers recommended “dedicated studies” to assess whether escalating the dose of ustekinumab can benefit patients with lower trough levels at week 8.
Few studies have explored biomarkers for response to ustekinumab induction therapy. Hence, the researchers assessed the relative utility of ustekinumab trough levels, C-reactive protein (CRP) levels, and fecal calprotectin levels for predicting early primary nonresponse. All 51 study participants had active luminal Crohn’s disease and received body weight–based intravenous infusions of ustekinumab at baseline, followed by subcutaneous injections of 90 mg. Primary nonresponders did not achieve steroid-free clinical and biochemical remission at week 16, defined as a Harvey-Bradshaw Index (HBI) of 4 points or less, a CRP level under 5 mg/L, and a fecal calprotectin level under 250 mcg/g. Week 16 was chosen to account for any delayed responders, the researchers noted.
A total of 32 patients (63%) achieved remission to ustekinumab induction therapy by week 16. An 8-week trough level of 2.0 mcg/mL was found to be optimal and distinguished primary nonresponders from responders with a sensitivity of 87%, a specificity of 66%, a positive predictive value of 82%, and a negative predictive value of 75%. In prior studies, optimal thresholds exceeded 3.3 mcg/mL for achieving remission and 4.5 mcg/mL at week 26 for achieving endoscopic response, the researchers noted. They said that this discrepancy might reflect different time points for evaluation, assays for measuring ustekinumab, patient populations, and a lack of endoscopic data in their study. “The relatively small sample size and the short period of follow-up evaluation [were] substantial limitations” they acknowledged.
In this study, levels of CRP did not change significantly between weeks 0 and 16 among either responders or nonresponders. In contrast, fecal calprotectin levels dropped rapidly and significantly over time only in responders. Median fecal calprotectin levels were 1,612 mcg/g of stools at week 0 versus 374 mcg/g at week 4 and 339 mcg/g at week 8. The finding “confirms the value of this biomarker, as previously shown in inflammatory bowel disease with anti–tumor necrosis factor,” the researchers wrote.
The investigators did not acknowledge external funding sources. Dr. Soufflet reported having no conflicts of interest. The senior author and three coinvestigators disclosed ties to MSD, AbbVie, Tillots, and several other pharmaceutical companies.
Learn more about therapeutic drug monitoring in IBD by reviewing the AGA Institute guideline at http://www.gastrojournal.org/article/S0016-5085(17)35963-2/fulltext.
SOURCE: Soufflet N et al. Clin Gastroenterol Hepatol. 2019 Mar 6. doi: 10.1016/j.cgh.2019.02.042. https://www.cghjournal.org/article/S1542-3565(19)30248-4/abstract
For patients with Crohn’s disease, therapeutic drug monitoring helped identify early primary nonresponders to induction with ustekinumab, according to researchers. The report is in Clinical Gastroenterology and Hepatology.
At week 8, median trough levels of ustekinumab were 6.0 mcg per mL (interquartile range, 3.1-8.0) among patients who achieved a primary response to induction at week 16, versus 1.3 mcg/mL (IQR, 0.9-5.6 ) among primary nonresponders (P = .03). An 8-week ustekinumab trough level cutoff of 2.0 mcg/mL distinguished week 16 responders from nonresponders with an area under the receiver operating curve (AUROC) of 0.75, wrote Ninon Soufflet of University Claude Bernard Lyon 1 in France, and associates. The researchers recommended “dedicated studies” to assess whether escalating the dose of ustekinumab can benefit patients with lower trough levels at week 8.
Few studies have explored biomarkers for response to ustekinumab induction therapy. Hence, the researchers assessed the relative utility of ustekinumab trough levels, C-reactive protein (CRP) levels, and fecal calprotectin levels for predicting early primary nonresponse. All 51 study participants had active luminal Crohn’s disease and received body weight–based intravenous infusions of ustekinumab at baseline, followed by subcutaneous injections of 90 mg. Primary nonresponders did not achieve steroid-free clinical and biochemical remission at week 16, defined as a Harvey-Bradshaw Index (HBI) of 4 points or less, a CRP level under 5 mg/L, and a fecal calprotectin level under 250 mcg/g. Week 16 was chosen to account for any delayed responders, the researchers noted.
A total of 32 patients (63%) achieved remission to ustekinumab induction therapy by week 16. An 8-week trough level of 2.0 mcg/mL was found to be optimal and distinguished primary nonresponders from responders with a sensitivity of 87%, a specificity of 66%, a positive predictive value of 82%, and a negative predictive value of 75%. In prior studies, optimal thresholds exceeded 3.3 mcg/mL for achieving remission and 4.5 mcg/mL at week 26 for achieving endoscopic response, the researchers noted. They said that this discrepancy might reflect different time points for evaluation, assays for measuring ustekinumab, patient populations, and a lack of endoscopic data in their study. “The relatively small sample size and the short period of follow-up evaluation [were] substantial limitations” they acknowledged.
In this study, levels of CRP did not change significantly between weeks 0 and 16 among either responders or nonresponders. In contrast, fecal calprotectin levels dropped rapidly and significantly over time only in responders. Median fecal calprotectin levels were 1,612 mcg/g of stools at week 0 versus 374 mcg/g at week 4 and 339 mcg/g at week 8. The finding “confirms the value of this biomarker, as previously shown in inflammatory bowel disease with anti–tumor necrosis factor,” the researchers wrote.
The investigators did not acknowledge external funding sources. Dr. Soufflet reported having no conflicts of interest. The senior author and three coinvestigators disclosed ties to MSD, AbbVie, Tillots, and several other pharmaceutical companies.
Learn more about therapeutic drug monitoring in IBD by reviewing the AGA Institute guideline at http://www.gastrojournal.org/article/S0016-5085(17)35963-2/fulltext.
SOURCE: Soufflet N et al. Clin Gastroenterol Hepatol. 2019 Mar 6. doi: 10.1016/j.cgh.2019.02.042. https://www.cghjournal.org/article/S1542-3565(19)30248-4/abstract
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: For patients with Crohn’s disease, therapeutic drug monitoring helped identify early nonresponse to ustekinumab induction.
Major finding: At week 8, median trough ustekinumab levels were 6 mcg/mL in week 16 responders and 1.3 mcg/mL in week 16 nonresponders (P = .03). An ustekinumab trough level of 2.0 mcg/mL or higher distinguished responders from nonresponders with an area under the receiver operating curve of 0.75.
Study details: Prospective study of 51 patients with active luminal Crohn’s disease.
Disclosures: The researchers did not acknowledge external funding sources. Dr. Soufflet reported having no conflicts of interest. The senior author and three coinvestigators disclosed ties to MSD, AbbVie, Tillots, and several other pharmaceutical companies.
Source: Soufflet N et al. Clin Gastroenterol Hepatol. 2019 Mar 6. doi: 10.1016/j.cgh.2019.02.042.