User login
Intrinsic Healing of the Anterior Cruciate Ligament in an Adolescent
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
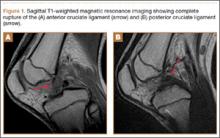
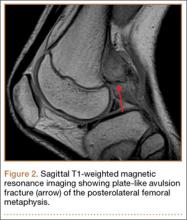
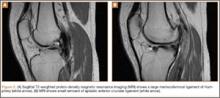
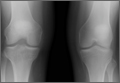
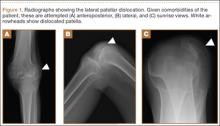
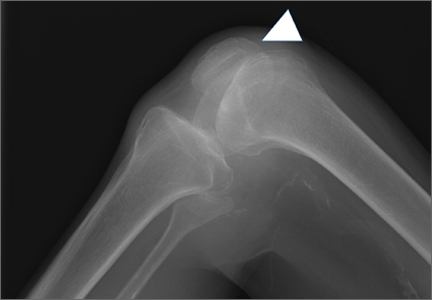
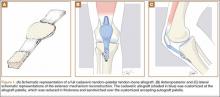
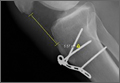
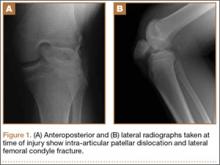
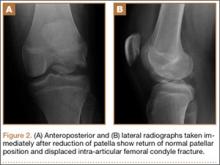
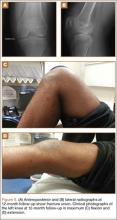
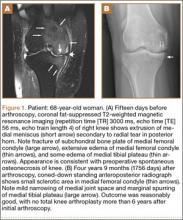
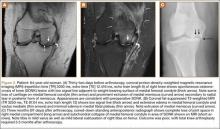
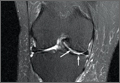
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
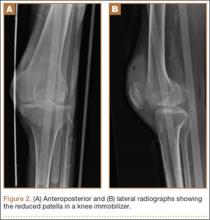
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
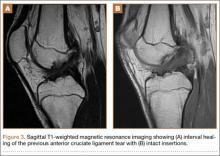
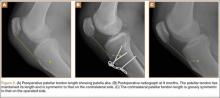
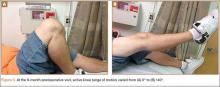
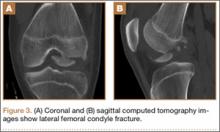
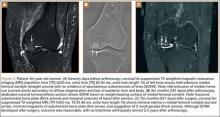
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
Fracture Blisters After Primary Total Knee Arthroplasty
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
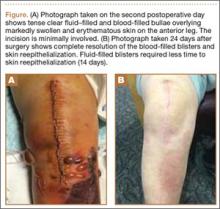
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
Congenital Absence of the Anterior Cruciate Ligament
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
CMS Proposes Major Initiative for Hip and Knee Replacements
In an attempt to reduce the rate and cost of complications following hip and knee replacements among Medicare beneficiaries, the Centers for Medicare & Medicaid Services (CMS) announced a new Comprehensive Care for Joint Replacement payment model. With this new measure, the CMS proposes to hold hospitals accountable for the quality of care they deliver to Medicare fee-for-service beneficiaries for hip and knee replacements from surgery through recovery.
“We are committed to changing our health care system to pay for quality over quantity, so that we spend our dollars more wisely and improve care for patients,” said Sylvia M. Burwell, Secretary of Health and Human Services.
Through the proposed 5-year payment model, health care providers in 75 geographic areas would continue to be paid under existing Medicare payment systems. However, the hospital where the hip or knee replacement is performed would be held liable for the quality and costs of care for the duration of care, from the time of the surgery through 90 days after discharge.
Depending on the hospital’s quality and cost performance during the episode, the hospital may receive an additional payment or be required to repay Medicare for a portion of the episode costs. As a result, hospitals would have an incentive to work with physicians, home health agencies, and nursing facilities to ensure that beneficiaries receive the coordinated care they need, with the goal of reducing avoidable hospitalizations and complications. Hospitals would receive tools, such as spending and utilization data and sharing of best practices, to improve the effectiveness of care coordination.
These bundled payments for joint replacement surgeries would build upon successful demonstration programs already underway in Medicare. This model is also consistent with the private sector, where major employers and leading providers and care systems are moving towards bundled payments for orthopedic services.
“Today, we are taking another important step to improve the quality of care for the hundreds of thousands of Americans who have hip and knee replacements through Medicare every year. By focusing on episodes of care, rather than a piecemeal system, hospitals and physicians have an incentive to work together to deliver more effective and efficient care. This model will incentivize providing patients with the right care the first time and finding better ways to help them recover successfully. It will reward providers and doctors for helping patients get and stay healthy, ” stated Ms. Burwell.
In an attempt to reduce the rate and cost of complications following hip and knee replacements among Medicare beneficiaries, the Centers for Medicare & Medicaid Services (CMS) announced a new Comprehensive Care for Joint Replacement payment model. With this new measure, the CMS proposes to hold hospitals accountable for the quality of care they deliver to Medicare fee-for-service beneficiaries for hip and knee replacements from surgery through recovery.
“We are committed to changing our health care system to pay for quality over quantity, so that we spend our dollars more wisely and improve care for patients,” said Sylvia M. Burwell, Secretary of Health and Human Services.
Through the proposed 5-year payment model, health care providers in 75 geographic areas would continue to be paid under existing Medicare payment systems. However, the hospital where the hip or knee replacement is performed would be held liable for the quality and costs of care for the duration of care, from the time of the surgery through 90 days after discharge.
Depending on the hospital’s quality and cost performance during the episode, the hospital may receive an additional payment or be required to repay Medicare for a portion of the episode costs. As a result, hospitals would have an incentive to work with physicians, home health agencies, and nursing facilities to ensure that beneficiaries receive the coordinated care they need, with the goal of reducing avoidable hospitalizations and complications. Hospitals would receive tools, such as spending and utilization data and sharing of best practices, to improve the effectiveness of care coordination.
These bundled payments for joint replacement surgeries would build upon successful demonstration programs already underway in Medicare. This model is also consistent with the private sector, where major employers and leading providers and care systems are moving towards bundled payments for orthopedic services.
“Today, we are taking another important step to improve the quality of care for the hundreds of thousands of Americans who have hip and knee replacements through Medicare every year. By focusing on episodes of care, rather than a piecemeal system, hospitals and physicians have an incentive to work together to deliver more effective and efficient care. This model will incentivize providing patients with the right care the first time and finding better ways to help them recover successfully. It will reward providers and doctors for helping patients get and stay healthy, ” stated Ms. Burwell.
In an attempt to reduce the rate and cost of complications following hip and knee replacements among Medicare beneficiaries, the Centers for Medicare & Medicaid Services (CMS) announced a new Comprehensive Care for Joint Replacement payment model. With this new measure, the CMS proposes to hold hospitals accountable for the quality of care they deliver to Medicare fee-for-service beneficiaries for hip and knee replacements from surgery through recovery.
“We are committed to changing our health care system to pay for quality over quantity, so that we spend our dollars more wisely and improve care for patients,” said Sylvia M. Burwell, Secretary of Health and Human Services.
Through the proposed 5-year payment model, health care providers in 75 geographic areas would continue to be paid under existing Medicare payment systems. However, the hospital where the hip or knee replacement is performed would be held liable for the quality and costs of care for the duration of care, from the time of the surgery through 90 days after discharge.
Depending on the hospital’s quality and cost performance during the episode, the hospital may receive an additional payment or be required to repay Medicare for a portion of the episode costs. As a result, hospitals would have an incentive to work with physicians, home health agencies, and nursing facilities to ensure that beneficiaries receive the coordinated care they need, with the goal of reducing avoidable hospitalizations and complications. Hospitals would receive tools, such as spending and utilization data and sharing of best practices, to improve the effectiveness of care coordination.
These bundled payments for joint replacement surgeries would build upon successful demonstration programs already underway in Medicare. This model is also consistent with the private sector, where major employers and leading providers and care systems are moving towards bundled payments for orthopedic services.
“Today, we are taking another important step to improve the quality of care for the hundreds of thousands of Americans who have hip and knee replacements through Medicare every year. By focusing on episodes of care, rather than a piecemeal system, hospitals and physicians have an incentive to work together to deliver more effective and efficient care. This model will incentivize providing patients with the right care the first time and finding better ways to help them recover successfully. It will reward providers and doctors for helping patients get and stay healthy, ” stated Ms. Burwell.
Evaluation of 3 Fixation Devices for Tibial-Sided Anterior Cruciate Ligament Graft Backup Fixation
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
Restoration of stability with return to activity is generally expected after anterior cruciate ligament (ACL) reconstruction; long-term success rates range from 75% to 95%.1 However, graft failure occurs most frequently with soft-tissue grafts fixated only with interference screws.2,3 Fixation failure also occurs more frequently at the tibial site.2 This failure has been attributed to extensive graft slippage in cases of soft-tissue fixation with interference screws.2 Interference screw fixation alone, with a double-looped hamstring tendon graft, fails at 350 N in young human tibiae.4,5 However, failure is limited with use of a bone–tendon–bone graft or with backup fixation, particularly at the tibial site.3 The superiority of bicortical fixation has also been proven.5-7
In addition, as shown in a goat model, ACL graft fixation is a major cause of failure in the immediate postoperative period, before biological incorporation of the graft.8 Fixation techniques for soft-tissue grafts must withstand stresses during the healing period (grafts may take up to 12 weeks to incorporate).9 Failures may result from forces exerted on the graft—forces that may be as high as 450 to 700 N during daily activities.10,11 Within the tibial tunnel, various fixation devices are used, including interference screws, staples, pins, buttons, and interference screw/sheath constructs.12,13 Primary fixation is commonly achieved with interference screws because of their ease of insertion and greater stiffness. However, fixation of the soft-tissue graft is influenced by several variables, including bone density, insertion torque, thread geometry, and interference screw material.14-16 Many of these variables, which are a source of inconsistency and concern during the immediate postoperative period, have led surgeons to seek alternative methods of backup fixation at the tibial site. Nevertheless, good clinical and subjective results have been found after ACL reconstruction with a 4-stranded semitendinosus tendon at 10-year follow-up.17
An anchor used in rotator cuff repair is the SwiveLock system (Arthrex). Major advantages of this system include ease and speed of insertion, good strength, and reduced need for later hardware removal.
We conducted a study to biomechanically evaluate 3 methods of tibial-sided fixation for ACL reconstruction: fully threaded interference screw only, interference screw backed with 4.75-mm SwiveLock anchor, and fully threaded bio-interference screw backed with 4.5-mm bicortical screw. We hypothesized that a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanical strength no different from that provided by backup fixation with a bicortical post at the tibial site. We further hypothesized that SwiveLock backup fixation would provide more strength than fixation with bio-interference screw alone.
Materials and Methods
The design of this study was adapted from one used by Walsh and colleagues,3 who compared 3 fixation methods: retrograde interference screw, suture button, and combined fixation. Tibiae inspected before selection showed no signs of injury or abnormality. Bovine extensor tendons, which lacked any defects along their entire length, were stored in saline 0.9% solution. Both the tibiae and the extensor tendons were stored at –20°C before completion of the tibial-sided ACL reconstruction. Thirty fresh-frozen, skeletally mature porcine proximal tibiae were selected and thawed at 4°C before preparation. Specimens were prepared by potting the diaphysis in fiberglass resin, and a tunnel 9 mm in diameter was drilled through the anteromedial aspect of the tibia.
For consistency, one author (CAV) prepared all 30 specimens. Both tails of all 30 bovine extensor tendons were whip-stitched with No. 2 FiberLoop (Arthrex) 9 mm in diameter. Grafts and tibiae were randomly divided into 3 sample groups. The first group was prepared by antegrade graft fixation within the tibial tunnel using a fully threaded 9×28-mm BioComposite interference screw (Arthrex). The second and third groups used the same primary fixation within the tibial tunnel along with 2 types of secondary fixation. These backup fixation groups included a 4.5-mm titanium bicortical post (Arthrex) and a 4.75-mm BioComposite SwiveLock C anchor (Arthrex) (Figure 1). The FiberLoop at the ends of the distal graft tails for backup groups were fixated 1 cm distal to the tibial tunnel and tapped before insertion of backup devices (Figures 2A, 2B). Insertion was completed after 4.5-mm bicortical and 4.75-mm unicortical drilling and tapping of the anteromedial cortices for the titanium posts and SwiveLocks, respectively. The free ends of the whip-stitched No. 2 FiberLoop were tied to the proximal end of the titanium post with a single surgical knot followed by 5 square knots.3 The free ends of the No. 2 FiberLoop were inserted into the eyelet of the 4.75-mm SwiveLock and 1 cm directly inferior to the tibial tunnel. Interference fit of FiberLoop with SwiveLock was achieved within the corticocancellous bone of the tibiae. All samples retained a 30-mm tendon loop superior to the tibial plateau to simulate intra-articular ACL length. Specimens were then stored at –20°C and thawed at 4°C before biomechanical testing.
Each of the 30 tibiae was tested once. Each testing group consisted of 10 porcine tibiae. The tendons were kept moist during the entire testing procedure by spraying them thoroughly with saline 0.9% solution. Mechanical testing was performed with an Instron 8871 system with a 5-kN load cell secured to the crosshead. A fixed-angle aperture, attached to the testing surface, was adjusted so that the tendon would be pulled in line with the tibial tunnel. A hook fixture suspended from clevis and dowel was used to secure the tendon to the crosshead (Figure 3). A small preload of 5 N manually applied to each sample was followed by a precycling regimen of 10 cycles between 10 N and 50 N at 1 Hz. Precycling was performed to remove slack from the system. Mechanical testing consisted of 500 cycles between 50 N and 250 N at 1 Hz followed by pull to failure at 20 mm per minute. Load and displacement data were recorded at 500 Hz.
An a priori power analysis was not performed because 6 specimens per group in the study from which the testing protocol was adapted demonstrated sufficient power among 3 testing categories.3 In addition, other studies have demonstrated similar testing protocols using 10 specimens per testing group.7,12,13,18 The data for each sample were analyzed with OriginPro 8.0 software (OriginLab). Ultimate load, yield load, stiffness, and cyclic displacement of the 3 sample groups were compared with 1-way analysis of variance (α = 0.05). Holm-Sidak tests were used for post hoc analysis.19P < .05 was statistically significant.
Results
None of the 30 specimens failed during preloading. Modes of failure were consistent among groups. All 10 specimens in the interference-screw-only group failed by graft slippage past the screw in the tibial tunnel. Nineteen of the 20 specimens in the backup-fixation groups failed by graft slippage past the screw and suture cutout through the distal graft tail. In the bicortical-post backup group, 1 failure was attributed to tendon tearing proximal to whip-stitching. There were no instances of hardware breakage or failure of either titanium screw or SwiveLock anchor.
Mean (SD) cyclic displacement was higher in the interference-screw-only group, 3.5 (2.2) mm, than in the SwiveLock backup group, 2.6 (0.5) mm, and the bicortical-post backup group, 2.1 (0.6) mm; no statistical significance was demonstrated between any 2 of these groups alone (P = .12) (Figure 4). Mean (SD) pullout stiffness was higher in the bicortical-post backup group, 192 (48) N/mm, than in the SwiveLock backup group, 164 (53) N/mm, and the screw-only group, 163 (64) N/mm (P = .42) (Figure 5). Mean (SD) initial load at 5 mm of displacement was higher in the bicortical-post backup group, 482 (156) N, and the SwiveLock backup group, 423 (94) N, than in the screw-only group, 381 (169) N (P = .30).
Mean (SD) yield load was higher in the bicortical-post backup group, 829 (253) N, than in the SwiveLock backup group, 642 (172) N, and the interference-screw-only group, 496 (133) N (P = .003). Statistical significance was demonstrated between the screw-only and bicortical-post groups (P = .002) and between the screw-only and SwiveLock groups (P = .048). There was no statistical difference between the bicortical-post and SwiveLock groups (P = .07).
Mean (SD) ultimate load to failure was higher in the bicortical-post backup group, 1148 (186) N, than in the SwiveLock backup group, 1007 (176) N, and the interference-screw-only group, 778 (139) N (Figure 6). The difference was statistically significant, whereby the screw-only group failed at a lower load compared with the bicortical-post group (P < .001) and the SwiveLock group (P = .005). The 2 backup groups were not statistically different (P = .1).
Discussion
We investigated whether a fully threaded bio-interference screw backed with a 4.75-mm SwiveLock anchor would provide mechanically equivalent pullout strength within the tibial tunnel during ACL reconstruction with soft-tissue allografts in comparison either with a fully threaded bio-interference screw backed with a bicortical post or with a fully threaded bio-interference screw without backup fixation. The results of the study support this hypothesis. With SwiveLock used for backup fixation, there was no significant difference in stiffness or cyclic load displacement between the screw-only, SwiveLock, and bicortical-post groups. However, adding backup fixation could particularly help improve fixation consistency. Specifically, although after only 500 cycles there was no statistically significant difference in cyclic displacement, continued cycling may be clinically relevant if graft slippage exceeded limits to allow for healing within the tibial tunnel. Conversely, a significantly larger difference was found between the SwiveLock, bicortical-post, and screw-only groups in yield load and ultimate load to failure. However, there was no significant difference between the SwiveLock and bicortical-post groups.
In this study, interference screw with SwiveLock backup demonstrated a mean (SD) ultimate load to failure of 1007 (176) N, comparable to that found by Walsh and colleagues3 for retrograde bio-interference screw with suture button, 1027 (157.11) N. In a study comparing quadrupled hamstring tibial graft fixation, Intrafix (DePuy Mitek) and an 8×25-mm Bioscrew (Linvatec) demonstrated mean (SD) single-cycle yield loads of 1332 (304) N and 612 (176) N, respectively.13 These results are similar to the ultimate yield loads in the present study: bicortical-post group, 1148 (186) N; SwiveLock group, 1007 (176) N; screw-only group, 778 (139) N. Differences may be attributed to hamstring tendons used in a quadrupled manner in the aforementioned study.12,13 Last, mean (SD) ultimate load to failure in a study that used only a retrograde bio-interference screw (9×20 mm) was 679.00 (109.44) N,3 similar to the 778 (139) N found for interference-screw-only in the present study. The difference is likely attributable to the longer screw (9×28 mm) in our study. Using SwiveLock C in cortical bone, Barber and colleagues18 found mean (SD) loads to failure up to 711.9 (89.1) N.
Clinically, it has been shown that a statistically significant increase in anterior laxity occurred between 4 months and 2 years in 10.7% of patients who underwent hamstring ACL reconstruction.20 The knees were clinically categorized as unstable or severely abnormal. The authors concluded that the clinical outcome was more likely influenced by the methods used to fix the free ends of the graft, specifically with 2 staples or a washer. To simulate early postoperative rehabilitation in the present study, cyclic loading of the graft was performed. Ultimate load to failure was then determined in order to evaluate catastrophic failure strength of the backup fixation devices in comparison with the interference-screw-only group without supplementary fixation.
It has been shown in autologous hamstring ACL reconstruction that a centrally placed polyethylene screw with sheath (Intrafix) performed as well as a standard, eccentrically placed metal interference screw with staple.10 It is therefore logical that backup fixation with use of a similar device (eg, SwiveLock, bicortical post) is necessary to ensure comparable clinical outcomes in relation to a screw/sheath device that has been shown to endure the highest yield loads.2,9,12,13,21-23 Potential benefits of using SwiveLock anchors for backup fixation include a statistically significant increased mean (SD) ultimate yield load of 229 (176) N over interference screw only. These results are similar to those in comparable studies: 218.3 (59.7) N24 and 165 (24.15) N25 in healthy bone with a reported bone mineral density (BMD) of 1.39 g/cm2, similar to that of skeletally mature porcine tibia (1.220-1.675 g/cm²).3 In addition, ease of insertion of this device over a bicortical post was demonstrated. The titanium post required bicortical drilling as well as measurement with a depth gauge to ensure adequate screw length. This process appeared to require more time during specimen preparation and theoretically could prove to be more dangerous clinically.7 However, caution in using a SwiveLock anchor in osteoporotic bone is advised because of reduced pullout strength.26 In this case, bicortical-post backup fixation may be more suitable. Moreover, although not demonstrated in this study, hardware prominence and irritation with a post may cause postoperative morbidity necessitating future removal.20 Hardware removal was the most common reason for additional surgery using hamstring tendons as graft.20 A second surgery for hardware removal was required in 21% of these patients.20 This is unlikely to occur with a SwiveLock, as the anchor is buried within cortical bone.
Limitations
Regarding use of nonhuman tissues in a biomechanical model, porcine tibiae and bovine extensor tendons were used because of availability, consistency among specimens, and cost-effectiveness. However, bovine extensor tendons have been shown to exhibit stiffness and viscoelastic properties similar to those of a human hamstring graft.27 In addition, the BMD of the porcine tibiae used in this study was not tested because of time involved and cost-efficiency. However, it has been shown that average BMD of porcine tibiae, 1.220-1.675 g/cm², is similar to that in a young athletic population, 1.24-1.62 g/cm2.3,28-31 We therefore assumed similarity to a young athletic population and uniformity of BMD of the porcine tibiae used in this study.
In addition, the biomechanical testing protocol did not simulate physiologic loading within the tibial tunnel. Moreover, the testing protocol used loads of only 250 N during cyclic testing for 500 cycles. This simulates only the early rehabilitation period and not the healing period, which may last up to 12 weeks.9 In addition, as previously mentioned, forces on the graft may be as high as 450 to 700 N.11,32 Pullout testing in line with the long axis of the tibia was performed in order to compare mechanical testing results with those of similar studies.3,12,13 Last, the P of .07 for the comparison of ultimate load to failure between the 2 backup fixation groups suggests that this study may have been underpowered.
Conclusion
This study demonstrated an effective, alternative, and equivalent backup fixation device that can help prevent graft slippage within the tibial tunnel during soft-tissue ACL reconstruction. Potential benefits of using SwiveLock anchors for backup fixation include a significantly increased ultimate yield load (229 N) when supplementing an interference screw, ease of insertion compared with a bicortical post, and the improbable need for future hardware removal. We support using SwiveLock for supplementary fixation at the tibial tunnel site when using soft-tissue grafts in ACL reconstruction.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
1. Wetzler MJ, Bartolozzi AR, Gillespie MJ, Rubenstein DL, Ciccotti MG, Miller LS. Revision anterior cruciate ligament reconstruction. Oper Tech Orthop. 1996;6(3):181-189.
2. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RF, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy. 2002;18(3):304-315.
3. Walsh MP, Wijdicks CA, Parker JB, Hapa O, LaPrade RF. A comparison between a retrograde interference screw, suture button, and combined fixation on the tibial side in an all-inside anterior cruciate ligament reconstruction: a biomechanical study in a porcine model. Am J Sports Med. 2009;37(1):160-167.
4. Howell SM, Hull ML. Aggressive rehabilitation using hamstring tendons: graft construct, tibial tunnel placement, fixation properties, and clinical outcome. Am J Knee Surg. 1998;11(2):120-127.
5. Magen HE, Howell SM, Hull ML. Structural properties of six tibial fixation methods for anterior cruciate ligament soft tissue grafts. Am J Sports Med. 1999;27(1):35-43.
6. Beynnon BD, Meriam CM, Ryder SH, Fleming BC, Johnson RJ. The effect of screw insertion torque on tendons fixed with spiked washers. Am J Sports Med. 1998;26(4):536-539.
7. Post WR, King SS. Neurovascular risk of bicortical tibial drilling for screw and spiked washer fixation of soft-tissue anterior cruciate ligament graft. Arthroscopy. 2001;17(3):244-247.
8. Holden JP, Grood ES, Butler DL, et al. Biomechanics of fascia lata ligament replacements: early postoperative changes in the goat. J Orthop Res. 1988;6(5):639-647.
9. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75(12):1795-1803.
10. Frank CB, Jackson DW. The science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79(10):1556-1576.
11. Markolf KL, Willems MJ, Jackson SR, Finerman GA. In situ calibration of miniature sensors implanted into the anterior cruciate ligament. Part I: strain measurements. J Orthop Res. 1998;16(4):455-463.
12. Kousa P, Teppo LN, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: I. Femoral site. Am J Sports Med. 2003;3 (2)1:174-181.
13. Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction: II. Tibial site. Am J Sports Med. 2003;31(2):182-188.
14. Brand JC Jr, Pienkowski D, Steenlage E, Hamilton D, Johnson DL, Caborn DN. Interference screw fixation strength of a quadrupled hamstring tendon graft is directly related to bone mineral density and insertion torque. Am J Sports Med. 2000;28(5):705-710.
15. Weiler A, Hoffmann RF, Siepe CJ, Kolbeck SF, Südkamp NP. The influence of screw geometry on hamstring tendon interference fit fixation. Am J Sports Med. 2000;28(3):356-359.
16. Weiler A, Hoffmann RF, Stähelin AC, Bail HJ, Siepe CJ, Südkamp NP. Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy. 1998;14(1):29-37.
17. Streich NA, Reichenbacher S, Barié A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279-284.
18. Barber FA, Herbert MA, Beavis C, Barrera Oro F. Suture anchor materials, eyelets, and designs: update 2008. Arthroscopy. 2008;24(8):859-867.
19. Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728.
20. Howell SM, Deutsch ML. Comparison of endoscopic and two-incision techniques for reconstructing a torn anterior cruciate ligament using hamstring tendons. Arthroscopy. 1999;15(6):594-606.
21. Gwynne-Jones DP, Draffin J, Vane A, Craig R, McMahon S. Failure strengths of concentric and eccentric implants for hamstring graft fixation. ANZ J Surg. 2008;78(3):177-181.
22. Hayes DA, Watts MC, Tevelen GA, Crawford RW. Central versus peripheral tibial screw placement in hamstring anterior cruciate ligament reconstruction: in vitro biomechanics. Arthroscopy. 2005;21(6):703-706.
23. Shino K, Pflaster DS. Comparison of eccentric and concentric screw placement for hamstring graft fixation in the tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):73-75.
24. Prevrhal S, Fuerst T, Fan B, et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporosis Int. 2001;12(1):28-34.
25. Pietschmann MF, Gülecyüz MF, Fieseler S, et al. Biomechanical stability of knotless suture anchors used in rotator cuff repair in healthy and osteopenic bone. Arthroscopy. 2010;26(8):1035-1044.
26. Burns JP, Snyder SJ, Albritton M. Arthroscopic rotator cuff repair using triple-loaded anchors, suture shuttles, and suture savers. J Am Acad Orthop Surg. 2007;15(7):432-444.
27. Tetsumura S, Fujita A, Nakajima M, Abe M. Biomechanical comparison of different fixation methods on the tibial side in anterior cruciate ligament reconstruction: a biomechanical study in porcine tibial bone. J Orthop Sci. 2006;11(3):278-282.
28. Alfredson H, Nordstrom P, Lorentzon R. Total and regional bone mass in female soccer players. Calcif Tissue Int. 1996;59(6):438-442.
29. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes’ peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone. 2003;32(1):62-68.
30. Nordström P, Lorentzon R. Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int. 1996;59(6):4443-4448.
31. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
32. De Wall M, Scholes CJ, Patel S, Coolican MR, Parker DA. Tibial fixation in anterior cruciate ligament reconstruction: a prospective randomized study comparing metal interference screw and staples with a centrally placed polyethylene screw and sheath. Am J Sports Med. 2011;39(9):1858-1864.
Poor Sleep, Negative Attitude, Amplify Pain in Knee Osteoarthritis
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Patients with knee osteoarthritis (OA) who have poor sleep habits display greater central sensitization of pain, according to a study published online ahead of print June 4 in Arthritis Care & Research. Study findings also showed that OA patients who catastrophize had increased central sensitization that was associated with greater pain.
“Our study is the largest and most comprehensive examination of the relationship between sleep disturbance, catastrophizing, and central sensitization in knee OA,” stated lead author Claudia Campbell, PhD, an Associate Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine in Baltimore.
The case-controlled study included 208 participants who were categorized according to 4 groups: patients who have OA and insomnia, patients who have OA and normal sleep habits, healthy controls with insomnia, and healthy controls without a pain syndrome and normal sleep. In all, 72% of the study’s participants were female.
Participants completed multimodal sleep assessments (eg, questionnaire, diary, actigraphy, and polysmnography) and extensive evaluation of pain using clinical measures and quantitative sensory testing to evaluate associations between central sensitization, catastrophizing, and insomnia.
Results showed that the participants with knee OA and insomnia had the greatest amount of central sensitization compared with controls. The team found patients with poor sleep and high catastrophizing scores reported increased levels of central sensitization. In turn, central sensitization was significantly associated with increased clinical pain.
“While no causal processes may be determined from this study, our data suggest that those with low sleep efficiency and higher catastrophizing have the greatest central sensitization. Understanding the intricate relationship between sleep, central sensitization, and catastrophizing has important clinical implications for treating those with chronic pain conditions such as knee OA,” Dr. Campbell stated.
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
Suggested Reading
Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res. 2015 June 4. [Epub ahead of print]
Closed Reduction of Subacute Patellar Dislocation Using Saline Joint Insufflation: A Technical Trick
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
Knee Extensor Mechanism Reconstruction With Complete Extensor Allograft After Failure of Patellar Tendon Repair
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
The extensor mechanism of the knee comprises the quadriceps tendon, the patella, and the patellar tendon. The extensor mechanism may be damaged by injury to these structures, with consequences such as the inability to actively extend the knee and hemarthrosis.1,2 Disruption of this mechanism is rare, and the most common injury pattern is an eccentric contraction of the quadriceps tendon on a flexed knee causing a tendon (quadriceps or patellar) rupture or a patella fracture.1,2
Patellar tendon ruptures are more common in persons younger than 40 years.1 Treatment is surgical, regardless of age and physical activity. In the acute setting, repair can be end-to-end suture or transosseous tunnel insertion. End-to-end suturing is difficult in chronic patellar tendon ruptures because of patella alta secondary to quadriceps contraction.3 Treatment options for chronic ruptures may involve transpatellar traction4 or tendon reinforcement with fascia lata, a semitendinosus band, or synthetic materials.3-5 Alternatively, tendon autograft and allografts have also been recommended, especially in extreme situations.1,6 Furthermore, animal experiments have shown that a compact platelet-rich fibrin scaffold (CPFS) has the potential to accelerate healing of patellar tendon defects and to act as a bioscaffold for graft augmentation.7
We describe the case of a 30-year-old man who underwent extensor mechanism reconstruction with cadaveric tendon–patellar tendon–bone allograft for failure of an infected primary end-to-end repair. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 30-year-old healthy man landed on an empty glass fish tank, resulting in a traumatic right-knee arthrotomy. On initial evaluation, the patient had a negative straight-leg-raise test and impaired knee extension. The patient was taken urgently to the operating room for irrigation and débridement and concurrent repair of the patellar tendon laceration. Antibiotic prophylaxis with 2 g of intravenous (IV) cefazolin was given in the emergency room.
Intraoperatively, after visualizing the patellar tendon laceration and excluding any associated chondral lesions, we proceeded with extensive débridement and irrigation using 9 L of normal saline pulse lavage. After we achieved a clean site, we proceeded to repair the patellar tendon using No. 2 FiberWire sutures (Arthrex, Naples, Florida) with a classic Krackow repair8 consisting of 2 sutures run in a 4-row fashion through the patella and the patellar tendon. The suture was securely tightened and then tested for stability to at least 90° of knee flexion. The retinaculum was repaired using No. 0 Vicryl sutures (Ethicon, Somerville, New Jersey). After wound closure and dressing, the patient was placed in a hinged knee brace locked in extension at all times after surgery. Antibiotic treatment with IV cefazolin was administered for 48 hours.
Postoperative management consisted of weight-bearing as tolerated on the operative limb and appropriate deep venous thrombosis prophylaxis. The patient followed up in clinic 2 weeks and 4 weeks after surgery. At 4 weeks, the patient was noted to have a secondary wound infection with superficial dehiscence and serosanguineous drainage. No wound opening was noticed, and local wound care was performed with a 1-week course of oral cephalexin. The patient was scheduled to follow up a few weeks later but did not follow up for a year.
At 1-year follow-up, the patient reported that he had had a steady progression of his knee range of motion (ROM) with decreased pain. However, over time, the patient noted subjective instability of the knee, with frequent falls occurring close to his 1-year follow-up. Examination of his knee showed that his active ROM ranged from 20° in extension to 120° in flexion, with a weak extensor mechanism. Passively, his knee could be brought to full extension. His incision was well healed, but it had an area of bogginess in the middle. Radiographs showed patella alta on the affected knee, with a lengthening of the patellar tendon of 7.70 cm on the right compared with 5.18 cm on the left. Magnetic resonance imaging (MRI) showed moderate-to-severe patellar tendinosis with small fluid pockets around the surgical material and evidence of acute patellar enthesopathy. The laboratory values showed a white blood cell count of 7580/μL (normal, 4500-11,000/μL), an erythrocyte sedimentation rate of 2 mm/h (normal, 1-15 mm/h), and a C-reactive protein level of 1.93 mg/dL (normal, 0.00-0.29 mg/dL). Based on the clinical examination and imaging findings, there was a concern for a possible chronic deep-tissue infection, in addition to failure of the primary patellar tendon repair. Operative versus nonoperative management options were discussed with the patient, and he elected to undergo surgery.
During surgery, the patellar laxity was confirmed, and the patellar tendon was noticed to be chronically thickened and surrounded by unhealthy tissue. Initially, an extensive soft-tissue débridement was performed, and all patellar tendon loculations visualized on the preoperative MRI were drained; a solid purulent-like fluid was expressed. Unfortunately, the extensive and required débridement did not allow the preservation of the patellar tendon. Appropriate cultures were taken and sent for immediate Gram-stain analysis, which returned negative. Tissue samples from the patellar tendon were also sent to the pathology department for analysis. Intraoperatively, the infrapatellar defect was filled temporarily with a tobramycin cement spacer mixed with 2 g of vancomycin in a manner similar to that of the Masquelet technique used for infected long-bone nonunions with bone loss.9,10 This technique is a 2-stage procedure that promotes the formation of a biologic membrane that allows bone healing in the reconstruction of long-bone defects. The first stage consists of a radical débridement with soft-tissue repair by flaps when needed, with the insertion of a polymethylmethacrylate cement spacer into the bone defect. The second stage is usually performed 6 to 8 weeks later, with removal of the spacer and preservation of the induced membrane, which is filled with iliac crest bone autograft augmented (if necessary) with demineralized allograft.
The incision was closed primarily, and after surgery, the patient was allowed to bear weight as tolerated in a hinged knee brace locked in extension. Final laboratory analysis from cultures and tissue samples revealed acute and chronic inflammation with more than 20 neutrophils per high-powered field. No organisms grew from aerobic, anaerobic, fungal, or mycobacterial cultures. The infectious disease service was consulted and recommended oral cephalexin.
Because all cultures were negative, all laboratory examinations did not indicate any residual infections, and no bony involvement was noticed intraoperatively or in the preoperative knee MRI, we decided to proceed with the second stage of the Masquelet technique after 2 weeks. The patient returned to the operating room for final reconstruction of his patellar tendon using a custom-ordered cadaveric tendon–patellar tendon–bone allograft, the length of which was determined by measuring the contralateral patellar tendon, ie, 5.18 cm (Figure 1A). The previous anterior knee incision was reopened and extended distally past the tibial tuberosity and proximally toward the quadriceps tendon. The antibiotic spacer was removed. We proceeded with a repeat irrigation and débridement and the allograft transfer. The selected allograft was customized by reducing the tibial bone component to an approximately 1×2-cm bone block and by reducing the allograft patellar thickness with an oscillating saw, leaving an approximately 2-mm thick patellar bone graft attached to the patellar tendon. In a similar technique using an oscillating saw, we shaved off the anterior cortex of the patient’s patella to accommodate, in a sandwich fashion, the patellar allograft. Proximally, the quadriceps tendon insertion was split longitudinally and partially separated from the superior pole of the patellar tendon to allow seating and fixation of the modified quadriceps allograft tendon component.
We proceeded with the fixation of the allograft first distally on the patella. The anterior cortex of the tibial tuberosity was resected to allow the perfect seating of the bone block allograft. The graft was secured with a 4.0-mm fully threaded cancellous lag screw and reinforced with a 2.4-mm, 3-hole T-volar buttress plate (Synthes, Paoli, Pennsylvania). The plate was contoured to better fit the patient’s tibia. We sutured the patellar allograft tendon to the patella using two No. 2-0 FiberWire sutures in Krackow suture technique8 (Figures 1B, 1C). We obtained good fixation of the patellar tendon, and the distance between the patellar insertion and the inferior patellar pole was the same as before surgery: 5.57 cm and comparable to the contralateral side (Figures 2A-2C). The patellar allograft and autograft sandwich were secured with additional No. 2-0 FiberWire sutures, and the quadriceps allograft and autograft were secured with the cross-stitch technique with the same material. Fine suturing of the quadriceps tendon was done with No. 0 Vicryl sutures. After the fixation was completed, we tested the stability of the reconstruction and found good flexion up to 120°.
The postoperative protocol consisted of weight-bearing as tolerated in full extension and passive knee ROM, using a continuous passive ROM machine from 0° to 45° for the first 4 weeks, followed by active ROM, increased as tolerated, during the next 8 weeks.
The patient was seen in clinic 3 and 9 months after surgery. At the 3-month follow-up appointment, the patient’s examination showed knee ROM from 0° extension to 130° of flexion, no secondary infection signs, and radiographic evidence of a well-healing patellar allograft with symmetric patellar tendon length to the contralateral side. At 9-month follow-up, the patient’s active ROM was from 0° extension to 140° flexion (Figures 3A, 3B), and he had returned to his preinjury level of functioning.
Discussion
This case report describes the successful reconstruction of a patellar tendon defect with cadaveric tendon–patellar tendon–bone allograft. Extensor mechanism injuries are uncommon in general, and the incidence of patellar tendon injury is higher in men than in women.2 Patellar tendon tears occur frequently in active patients younger than 40 years, usually as a result of sudden quadriceps contraction with the knee slightly flexed.1 Treatment of patellar tendon injury is surgical, and functional outcomes for patients with this injury are equivalent to those of patients with quadriceps tendon injuries or patellar fractures.2 Acute patellar tendon tears can be repaired by end-to-end suturing or transosseous tunnel insertion in the tibia or patella.1 Reinforcement is often added between the patella and tibial tuberosity, using a semitendinosus band or wire.1 End-to-end suture is performed using a thick resorbable suture. It is important to avoid patella alta during suturing, comparing the position of the patella with the contralateral patella with the knee in 45° of flexion. In proximal avulsion, the tendon is anchored to the bone by 2 thick nonresorbable sutures through 2 parallel bone tunnels to the proximal pole of the patella. Distal avulsion is rare in adults, but it can be managed by using staples or suture anchors.1
End-to-end suturing of chronic patellar tendon defects is difficult more than 45 days after injury primarily because of difficulties in correcting patella alta secondary to the upward force exerted by the quadriceps tendon.1,3 Extreme situations similar to the case we present warrant Achilles or patellar tendon allograft for reconstruction of the extensor mechanism.1,3,6,9
Extensor mechanism allograft also provides an effective remedy for severe quadriceps deficiency caused by loss of the patella, patellar tendon, and quadriceps tendon in total knee arthroplasty.10 However, in such cases, late failure is common, and major quadriceps deficiency occurs after removal of the allograft material.10 To improve outcome, a novel technique using the medial gastrocnemius muscle transferred to the muscular portion of the vastus medialis and lateralis flaps provides a secure and strong closure of the anterior knee, thereby restoring the extensor mechanism of the knee.10
Patellar tendon reconstruction with allograft tissue has been successfully used, especially in cases related to chronic patellar tendon ruptures11 and total knee arthroplasty.6,12-14 Crossett and colleagues12 showed that, at 2-year follow-up, the average knee score for pain, ROM, and stability had improved from 26 points (range, 6-39 points) before surgery to 81 points (range, 40-92 points). The average knee score for function had also improved: 14 points (range, 0-35 points) before surgery to 53 points (range, 30-90 points).12 Primary repair may succeed in early intervention, but in an established rupture, allograft reconstruction is often necessary. Achilles tendon is the preferred allograft, with the calcaneus fragment embedded into the proximal tibia as a new tubercle and the tendon sutured into the remaining extensor mechanism.1,11 The repair is further protected using a cable loop from the superior pole of the patella to a drill hole in the upper tibia.9 Techniques have also been described involving passage of the proximal aspect of the allograft tendon through patellar bone tunnels and suture fixation to the native quadriceps tendon.11,15 However, in our technique, we shaved off the anterior cortex of the patient’s patella to allow a sandwich-type over-position of the allograft to secure fixation to the patella.
Another alternative to allograft reconstruction involves biocompatible scaffolds. Such scaffolds incorporate the use of platelets in a fibrin framework. A CPFS, produced from blood and calcium gluconate to improve healing of patellar tendon defects, has been described in animal studies.7 In the rabbit model, CPFS acts as a provisional bioscaffold that can accelerate healing of an injured patellar tendon repair, potentially secondary to several growth factors derived from platelets.7 Platelets are biocompatible sources of growth factors, and CPFS can act as a scaffold to restore the mechanical integrity of injured soft tissue.7,16 In addition, CPFS can act to lower donor-site morbidity associated with harvesting tissue autograft.7 However, to our knowledge, such scaffolds have not been used in human trials. The LARS biocompatible ligament (Corin Group PLC, Cirencester, United Kingdom), currently not approved by the US Food and Drug Administration, is used for reconstructions of isolated or multiple knee ligament injuries.17 This graft requires the presence of healthy tissue with good blood supply from which new tendon or ligament can grow in. Sometimes it is also used for extensor mechanism reconstruction after radical tumor resection around the knee; however, good results are achieved in only 59% of cases,18 and to our knowledge, only 1 case of primary repair of a patellar tendon rupture has been published.19
Techniques involving the use of tendon–patellar tendon–bone graft with fixation via the sandwich-type over-position of the allograft for chronic patellar tendon rupture have not been described in the literature. In our patient, given the extensive patellar tendon lesion and inflammation with chronic tissue degeneration, there was no option but to use allograft. To improve the patient’s outcome, we chose the strongest possible allograft, tendon–patellar tendon–bone graft.
Conclusion
Revision patellar tendon reconstruction is a challenging, but necessary, procedure to restore the extensor mechanism of the knee, especially in young, active individuals. Various options to reconstruct the tissue defects are available. Our patient was successfully treated with a tendon–patellar tendon–bone allograft reconstruction.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
1. Saragaglia D, Pison A, Rubens-Duval B. Acute and old ruptures of the extensor apparatus of the knee in adults (excluding knee replacement). Orthop Traumatol Surg Res. 2013;99(1 suppl):S67-S76.
2. Tejwani NC, Lekic N, Bechtel C, Montero N, Egol KA. Outcomes after knee joint extensor mechanism disruptions: is it better to fracture the patella or rupture the tendon? J Orthop Trauma. 2012;26(11):648-651.
3. Ecker ML, Lotke PA, Glazer RM. Late reconstruction of the patellar tendon. J Bone Joint Surg Am. 1979;61(6):884-886.
4. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63(6):932-937.
5. Levy M, Goldstein J, Rosner M. A method of repair for quadriceps tendon or patellar ligament (tendon) ruptures without cast immobilization. Preliminary report. Clin Orthop Relat Res. 1987;218:297-301.
6. Burks RT, Edelson RH. Allograft reconstruction of the patellar ligament. A case report. J Bone Joint Surg Am. 1994;76(7):1077-1079.
7. Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H. Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee. 2013;20(6):545-550.
8. Krackow KA, Thomas SC, Jones LC. Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics. 1988;11(6):909-917.
9. Brooks P. Extensor mechanism ruptures. Orthopedics. 2009;32(9):683-684.
10. Whiteside LA. Surgical technique: muscle transfer restores extensor function after failed patella-patellar tendon allograft. Clin Orthop Relat Res. 2014;472(1):218-226.
11. Farmer K, Cosgarea AJ. Procedure 25. Acute and chronic patellar tendon ruptures. In: Miller MD, Cole BJ, Cosgarea AJ, Sekiya JK, eds. Operative Techniques: Sports Knee Surgery. Philadelphia, PA: Saunders (Elsevier); 2008:397-417.
12. Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84(8):1354-1361.
13. Lahav A, Burks RT, Scholl MD. Allograft reconstruction of the patellar tendon: 12-year follow-up. Am J Orthop. 2004;33(12):623-624.
14. Yoo JH, Chang JD, Seo YJ, Baek SW. Reconstruction of a patellar tendon with Achilles tendon allograft for severe patellar infera--a case report. Knee. 2011;18(5):350-353.
15. Saldua NS, Mazurek MT. Procedure 37. Quadriceps and patellar tendon repair. In: Reider B, Terry MA, Provencher MT, eds. Operative Techniques: Sports Medicine Surgery. Philadelphia, PA: Saunders (Elsevier); 2010:623-640.
16. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91(1):4-15.
17. Ibrahim SAR, Ahmad FHF, Salah M, Al Misfer ARK, Ghaffer SA, Khirat S. Surgical management of traumatic knee dislocation. Arthroscopy. 2008;24(2):178-187.
18. Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328-334.
19. Naim S, Gougoulias N, Griffiths D. Patellar tendon reconstruction using LARS ligament: surgical technique and case report. Strategies Trauma Limb Reconstr. 2011;6(1):39-41.
Intra-Articular Dislocation of the Patella With Associated Hoffa Fracture in a Skeletally Immature Patient
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
In 1887, Midelfart1 first reported on an intra-articular dislocation of the patella, and since then approximately 50 cases have been reported in the worldwide literature.2 Also known as an inferior patellar dislocation, these rare traumatic events occur when the patella dislocates intra-articularly. Because the patella commonly rotates about its horizontal axis, the articular surface is facing proximally or distally. The patella becomes lodged within the trochlea and locks the knee joint. Most cases described in the literature involved adolescent boys, with the patella difficult to reduce. Most patients required open reduction, while those who underwent successful closed reduction often needed general anesthesia.3
Similarly, coronal shear fractures of the femoral condyle (ie, Hoffa fractures) are an uncommon fracture pattern typically seen in adults. These fractures are even more infrequent in skeletally immature patients, with fewer than 5 cases documented in the literature.4-7 In our case report, we present a 14-year-old boy with a coronal shear fracture of the femoral condyle associated with an intra-articular patellar dislocation. To our knowledge, this constellation of injuries has not been reported. Additionally, closed reduction of the patella was successful after intra-articular lidocaine injection, without the need for sedation or general anesthesia. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 14-year-old boy presented to our institution after sustaining a direct blow to his left knee. The injury occurred as he jumped and landed on a flexed knee while playing with friends. The patient was unable to ambulate after the injury, and his left knee was locked in a slightly flexed position. Examination in the emergency department showed the knee to be held in approximately 60º of flexion, with an obvious bony prominence noted anteriorly over the femoral condyles. The patient was unable to perform a straight leg raise or any active range of motion (ROM) at the knee. Radiographs performed with the knee maintained in flexion confirmed that the patella was displaced into the knee joint and was rotated with the articular surface facing distally. Also noted was a coronal shear fracture of the lateral femoral condyle (Figures 1A, 1B).
The patient received pain medication and an intra-articular lidocaine injection prior to a reduction attempt by the orthopedic resident. With the patient supine, the hip was gently flexed to relax the quadriceps muscle. As the knee was flexed up to 110º, the prominent patella was gripped between the thumb and fingers to gently free and elevate the patella out of the intercondylar notch.
After reduction, an immediate return of normal patellar contour and patellofemoral tracking was observed as the knee was gently extended. There was no obvious defect to the patellar or quadriceps tendons, and the patient was able to perform a straight-leg raise, confirming the integrity of the extensor mechanism. Radiographs performed after the reduction confirmed relocation of the patella in correct anatomic position, as well as a lateral femoral condyle fracture (Figures 2A, 2B). Magnetic resonance imaging (MRI) of the knee confirmed no full-thickness quadriceps or patellar tendon tear. A computed tomography (CT) scan of the knee showed a comminuted fracture of the lateral femoral condyle in the coronal plane, as well as multiple bone fragments within the joint (Figures 3A, 3B). The patient was placed in a bulky soft dressing and underwent open reduction and internal fixation of the fracture.
A 10-cm incision was made over the anterior aspect of the knee, and after dissection to the level of the retinaculum, a lateral parapatellar arthrotomy was performed. The patella was retracted medially to identify and free the fracture fragments. The fracture fragments were provisionally reduced and stabilized with three 0.065-in Kirschner wires. An area of osteochondral impaction proximal to the fracture was elevated and allograft bone was incorporated below the articular surface (Figures 4A, 4B). Rigid fixation of the fracture was achieved using 3 screws (2 Bio-Compression Screws [Arthrex Inc., Naples, Florida] and 1 Synthes cannulated screw [Synthes, West Chester, Pennsylvania]). The screws were placed in posteroanterior (PA) direction and inserted into the weight-bearing articular surface of the femoral condyle (Figures 4C, 4D). The screws were countersunk, and stable fixation with compression of the fracture was achieved. Reduction and screw position were verified with fluoroscopic views. The wound was closed in layers, and the patient was discharged home the next day.
Postoperatively, the patient was non-weight-bearing on the affected limb with a hinged-knee brace to allow for knee ROM exercises immediately. He was also given a continuous passive motion device to maintain knee motion. At the 6-week mark, the patient’s fracture alignment appeared to be well-maintained and showed interval healing. Clinically, the patient was noted to have limited knee ROM. The decision was made to take the patient to the operating room primarily for a manipulation under anesthesia and resection of scar tissue from postoperative arthrofibrosis. Arthroscopic screw removal was also planned as a secondary procedure at the same time in order to prevent the possibility of chondral injury from screw migration. During the procedure, the patient was noted to have improved ROM from 5º to 85º premanipulation to 5º to 110º postoperatively. At 3 months after the initial injury, the patient was allowed to begin progressive weight-bearing on the left knee. At most recent follow-up, after 12 months, the patient was able to ambulate and bear weight on the left leg without pain. Plain radiographs show a well-healed fracture with no evidence of collapse of the femoral condyle (Figures 5A, 5B). His active ROM of the left knee was 5º to 110º without pain (Figures 5C, 5D).
Discussion
In the vast majority of patellar dislocations, the patella dislocates laterally over the trochlear groove. Inferior, or intra-articular, dislocations of the patella are rare. The mechanism of injury is usually a blow onto the patella with a flexed knee. The 2 groups commonly involved are adolescent boys and the elderly.8,9 In young men, it is thought that lax patellar attachments place adolescents at higher risk for this type of injury.10-12 While patella fractures and frank extensor mechanism ruptures are uncommon in this age group, the same mechanism of injury can lead to stripping of the deep fibers of the patellar tendon from the superior pole of the patella.3,13 The intact superficial fibers of the tendon allow the patella to hinge and displace into the joint.14
Inferior dislocations of the patella are classified into 2 types based on the orientation of the articular surface and the presence of osteophytes.15 Type I inferior dislocations occur after a direct blow to a flexed knee forces the superior pole of the patella into the intercondylar notch. Type II dislocations are caused by osteophytes on the superior pole of the patella that become wedged in the intercondylar notch and dislocate the patella inferiorly. In type I dislocations, the patella is rotated in the horizontal plane and the articular surface often faces inferiorly, but type II dislocations do not involve rotation of the articular surface. Type II injuries are seen more commonly in the elderly.
Our patient was able to tolerate a closed reduction of the patella after an intra-articular lidocaine injection, and a successful reduction was achieved without great difficulty. However, the majority of reports describe the need for an open reduction of inferior patellar dislocations.3,8 When closed reductions were a success, they were performed under general anesthesia or conscious sedation.3 It is thought that the difficulty of reduction results from the tension of the quadriceps muscle pulling the patella superiorly into intercondylar notch.11,16 However, successful closed reduction may be more likely in patients with less patellar rotation and entrapment within the intercondylar notch, as well as in patients whose knee is near full extension at presentation.17-19 Successful closed reduction is also seen in elderly patients, where dislocation is generally caused by less forceful impact and held by osteophytes. In these patients, the knee is commonly held in extension.12,15,20-22
The fracture pattern seen in this case also shows a rare fracture in skeletally immature patients, with only a few case reports in the literature. Isolated coronal plane femur fractures account for 0.65% of all femur fractures and are usually seen in adults after high-energy trauma.23 In the skeletally immature, the fracture can occur with lower-energy mechanisms. The typical mechanism is thought to be a shearing force to the femur caused by an axial load to the knee in 90° or more of flexion.4,24 A CT scan is recommended for better identification of the fracture and to plan treatment.25,26 Because of their intra-articular nature and tenuous blood supply, Hoffa fractures tend to do poorly with nonoperative treatment and are prone to displacement and nonunion.27,28 The goal of operative treatment is to obtain anatomic reduction and rigid fixation. While operative fixation techniques are varied, screw fixation with multiple smaller diameter screws has equal pullout strength compared to larger screws and may minimize damage to the articular cartilage.29-31 By preserving blood supply to the fracture, and allowing for early active mobilization, operative treatment generally provides good long-term functional outcomes in these fracture patterns.24
Conclusion
We describe a case in which the patella of an adolescent boy dislocated inferiorly into the knee joint, with an associated coronal shear fracture of the lateral femoral condyle. To our knowledge, this constellation of injuries has not been reported. For this uncommon injury pattern, we recommend a sequential treatment algorithm to minimize morbidity. We recommend first attempting a closed reduction of the patella with adequate pain control to avoid the morbidity associated with general anesthesia. After a successful reduction, an advanced imaging study (eg, MRI) is advisable to assess for concomitant soft-tissue injuries and preoperative planning, if necessary. The mechanism of injury and force required to cause a patellar dislocation of this nature leaves a high likelihood of other injuries. When a fracture is noted on plain radiographs after reduction, a CT scan can provide important information for planning surgical fixation of the fracture. Even in a skeletally immature patient, the principle of direct reduction and stable interfragmentary fixation of an articular fracture is critical for long-term function, even after a significant trauma to the knee.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
1. Midelfart V. En sjelden luxation of patella. Norsk Magazin for Laegevidenskaben. 1887;4:588.
2. Kramer DE, Simoni MK. Horizontal intra-articular patellar dislocation resulting in quadriceps avulsion and medial patellofemoral ligament tear: a case report. J Pediatr Orthop B. 2013;22(4):329-332.
3. van den Broek TA, Moll PJ. Horizontal rotation of the patella. A case report with review of the literature. Acta Orthop Scand. 1985;56(5):436-438.
4. Flanagin BA, Cruz AI, Medvecky MJ. Hoffa fracture in a 14-year-old. Orthopedics. 2011;34(2):138.
5. Strauss E, Nelson JM, Abdelwahab IF. Fracture of the lateral femoral condyle. A case report. Bull Hosp Jt Dis Orthop Inst. 1984;44(1):86-90.
6. Biau DJ, Schranz PJ. Transverse Hoffa’s or deep osteochondral fracture? An unusual fracture of the lateral femoral condyle in a child. Injury. 2005;36(7):862-865.
7. McDonough PW, Bernstein RM. Nonunion of a Hoffa fracture in a child. J Orthop Trauma. 2000;14(7):519-521.
8. Brady TA, Russell D. Interarticular horizontal dislocation of the patella. A case report. J Bone Joint Surg Am. 1965;47(7):1393-1396.
9. Yuguero M, Gonzalez JA, Carma A, Huguet J. Intra-articular patellar dislocation. Orthopedics. 2003;26(5):517-518.
10. Frangakis EK. Intra-articular dislocation of the patella. A case report. J Bone Joint Surg Am. 1974;56(2):423-424.
11. Nanda R, Yadav RS, Thakur M. Intra-articular dislocation of the patella. J Trauma. 2000;48(1):159-160.
12. Choudhary RK, Tice JW. Intra-articular dislocation of the patella with incomplete rotation--two case reports and a review of the literature. Knee. 2004;11(2):125-127.
13. Chatziantoniou I, Diakos G, Pantelelli M. Horizontal dislocation of the patella. Case report. EEXOT. 2008;59(2):112-114.
14. McHugh G, Ryan E, Cleary M, Kenny P, O’Flanagan S, Keogh P. Intra-articular dislocation of the patella. Case Rep Orthop. 2013;2013:535803.
15. Bankes MJ, Eastwood DM. Inferior dislocation of the patella in the degenerate knee. Injury. 2002;33(6):528-529.
16. Theodorides A, Guo S, Case R. Intra-articular dislocation of the patella: A case report and review of the literature. Injury Extra. 2010;41(10):103-105.
17. Dimentberg RA. Intra-articular dislocation of the patella: case report and literature review. Clin J Sport Med. 1997;7(2):126-128.
18. Morin WD, Steadman JR. Case report of a successful closed reduction without anesthesia. Clin Orthop. 1993(297):179-181.
19. Murakami Y. Intra-articular dislocation of the patella. A case report. Clin Orthop. 1982;171:137-139.
20. Joshi RP. Inferior dislocation of the patella. Injury. 1997;28(5-6):389-390.
21. Garner JP, Pike JM, George CD. Intra-articular dislocation of the patella: two cases and literature review. J Trauma. 1999;47(4):780-783.
22. McCarthy TA, Quinn B, Pegum JM. Inferior dislocation of the patella: an unusual cause of a locked knee. Ir J Med Sci. 2001;170(3):209-210.
23. Manfredini M, Gildone A, Ferrante R, Bernasconi S, Massari L. Unicondylar femoral fractures: therapeutic strategy and long-term results. A review of 23 patients. Acta Orthop Belg. 2001;67(2):132-138.
24. Holmes SM, Bomback D, Baumgaertner MR. Coronal fractures of the femoral condyle: a brief report of five cases. J Orthop Trauma. 2004;18(5):316-319.
25. Nork SE, Segina DN, Aflatoon K, et al. The association between supracondylar-intercondylar distal femoral fractures and coronal plane fractures. J Bone Joint Surg Am. 2005;87(3):564-569.
26. Allmann KH, Altehoefer C, Wildanger G, et al. Hoffa fracture--a radiologic diagnostic approach. J Belge Radiol. 1996;79(5):201-202.
27. Oztürk A, Ozkan Y, Ozdemir RM. Nonunion of a Hoffa fracture in an adult. Chir Organi Mov. 2009;93(3):183-185.
28. Lewis SL, Pozo JL, Muirhead-Allwood WF. Coronal fractures of the lateral femoral condyle. J Bone Joint Surg Br. 1989;71(1):118-120.
29. Arastu MH, Kokke MC, Duffy PJ, Korley RE, Buckley RE. Coronal plane partial articular fractures of the distal femoral condyle: current concepts in management. Bone Joint J. 2013;95-B(9):1165-1171.
30. Westmoreland GL, McLaurin TM, Hutton WC. Screw pullout strength: a biomechanical comparison of large-fragment and small-fragment fixation in the tibial plateau. J Orthop Trauma. 2002;16(3):178-181.
31. Jarit GJ, Kummer FJ, Gibber MJ, Egol KA. A mechanical evaluation of two fixation methods using cancellous screws for coronal fractures of the lateral condyle of the distal femur (OTA type 33B). J Orthop Trauma. 2006;20(4):273-276.
Spontaneous Osteonecrosis of Knee After Arthroscopy Is Not Necessarily Related to the Procedure
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
The term spontaneous osteonecrosis of the knee was first used by Ahlbäck1 in 1968. This term, and the acronym SONK (sometimes SPONK2), has subsequently been used by other authors to refer to an apparent osteonecrosis of the knee, most commonly occurring within the medial femoral condyle. SONK typically occurs in older women who usually do not have the typical osteonecrosis risk factors, such as steroid use, sickle-cell anemia, and excessive alcohol intake. Furthermore, the radiologic appearance of SONK differs from the typical avascular necrosis findings seen with radiography and magnetic resonance imaging (MRI). In particular, on MRI, the abnormality of SONK does not have the typical serpiginous margin of bone infarction, or the double-line sign indicating both sclerosis and granulation tissue.3 SONK is normally seen as a line of signal intensity on T1- and T2-weighted sequences; this line is adjacent to or parallels the subchondral bone with an adjacent area of extensive edema.
There is dispute over the cause of SONK. Yamamoto and Bullough4 proposed the lesion is in part a subchondral insufficiency fracture and staged it into 4 parts. Histologic findings suggest at least some SONK lesions are subchondral insufficiency fractures.5 Brahme and colleagues6 were the first to describe SONK occurring after arthroscopy, and others have documented this finding. The condition has also been referred to as osteonecrosis in the postoperative knee.7-13 An association of postoperative SONK with cartilage loss and meniscal tear has been proposed.7-13
We reviewed the clinical, radiologic, and MRI findings in 11 patients with evidence of postarthroscopy SONK to try to identify any risk factors that might predispose them to poor outcomes. Our study population consisted of 11 patients (12 knees) with SONK; 6 of the knees had the lesion before knee arthroscopy, and the other 6 developed the lesion after arthroscopy. We also considered MRI findings in a group of 11 age- and sex-matched patients who underwent knee arthroscopy and did not have or develop SONK. We reviewed the preoperative MRI findings of both groups for meniscal tear, meniscal extrusion, and cartilage loss. We had 2 hypotheses. First, patients with preoperative MRI findings of SONK would have articular cartilage changes, posterior root degeneration, and meniscal extrusion similar to those of patients who developed SONK after arthroscopy. Second, an age- and sex-matched group of patients who underwent arthroscopy and did not develop SONK would be similar in articular cartilage changes, posterior root degeneration or tear, and meniscal extrusion.
Materials and Methods
With institutional review board approval and waived informed consent, we reviewed all imaging studies, particularly the radiographs and MRI studies, of 11 patients (12 knees) who either had SONK before arthroscopy or developed it after arthroscopy. In all these cases, arthroscopy was performed to alleviate mechanical symptoms associated with meniscal tear.
On subsequent review by a musculoskeletal radiologist, 6 patients with SONK had an identifiable lesion before surgery. All patients’ symptoms had not improved with an earlier trial of conservative management. All preoperative and postoperative radiologic and MRI findings were reviewed. The patient group was assembled by writing to all the orthopedic surgeons who performed arthroscopy at our institution and asking for SONK cases seen in their practices. All but 2 cases were performed by a surgeon who treated a predominantly older, less active population. Clinical notes were reviewed for outcomes, and the musculoskeletal radiologist reviewed all radiologic studies. The 4 men and 7 women in the SONK group (1 woman had bilateral knee lesions) ranged in age from 43 to 74 years (mean, 63.8 years), and the 4 men and 7 women in the control group were age-matched to 43 to 75 years (mean, 63.6 years). The controls were chosen from a pool of patients who underwent knee arthroscopy at our institution.
MRI was performed using General Electric 1-T, 1.5-T, or 3-T magnets (GE Healthcare, Milwaukee, Wisconsin) or using Philips 1.5-T or open 0.7-T magnets (Philips Healthcare, Andover, Massachusetts). Imaging included sagittal and coronal proton density–weighted sequences and coronal and axial fat-suppressed T2-weighted sequences. SONK was diagnosed when a low signal line adjacent to the subchondral bone plate on the femoral or tibial condyles was present with an adjacent area of bone marrow edema in the respective condyle or when there was depression of the subchondral bone plate with adjacent edema. The MRI studies were reviewed for lesion location, and medial meniscus and lateral meniscus were reviewed for tear. Type of meniscal tear (horizontal cleavage, radial, complex degenerative) was documented, as was meniscal extrusion. The meniscus was regarded as extruded if the body extended more than 3 mm from the joint margin. Cartilage in the medial and lateral compartment was reviewed according to a modified Noyes scale listing 0 as normal, 1 as internal changes only, 2A as 1% to 49% cartilage loss, 2B as 50% to 90% loss of articular cartilage, 3A as 100% articular cartilage loss with subchondral bone plate intact, and 3B as 100% articular cartilage loss with ulcerated subchondral bone plate.14 Osteoarthritic severity was similarly classified using the Kellgren-Lawrence scale,15 where grade 0 is normal; grade 1 is unlikely to have narrowing of the joint space but potentially has osteophytic lipping; grade 2 has both definite narrowing of the joint space and osteophytes; grade 3 has narrowing of the joint space and multiple osteophytes, some sclerosis, and possible deformity of bone contour; and grade 4 has marked narrowing of the joint space, large osteophytes, severe sclerosis, and definite deformity of bone contour. Follow-up clinical notes and radiologic studies were reviewed in the assessment of patient outcomes.
All statistical analyses were performed with SAS 9.2 software (SAS Institute, Cary, North Carolina). Age data were evaluated with the Shapiro-Wilk test and graphical displays and were found to violate normality assumptions, so they are presented as medians and ranges; other variables are presented as count and column percentages. The Wilcoxon rank sum test was used to compare the 2 groups’ age distributions. Fisher exact tests were used to compare proportions between the 2 groups for the other variables. Statistical significance was set at P < .05.
Results
Table 1 lists the demographics and imaging characteristics of the 11 patients—6 had SONK before arthroscopy and 6 developed it after arthroscopy. Comparison of the 11 patients with SONK and the 11 controls is summarized with P values in Table 2. Representative cases that either presented before surgery or developed after surgery are shown in Figures 1 to 4. There were 6 prearthroscopy lesions and 6 postarthroscopy lesions—all 12 in the medial femoral condyle. Eleven of the 12 knees had a medial meniscal tear, and 1 knee had both medial and lateral meniscal tears. In 8 of the 12 knees, the lateral meniscus was normal; in 2 knees, it had mild degeneration; and, in 1 knee, it had a complex tear. Assessment of hyaline cartilage revealed medial cartilage loss ranging from 2A to 3B (median, 2B) in the patients with SONK, and lateral cartilage loss ranging from 0 to 2A (median, 0). At surgery, all knees had a partial medial meniscectomy, and 6 had a partial lateral meniscectomy. Ten of the 12 knees had chondroplasty, 9 patellar and 5 of the medial femoral condyle. Only 4 of the 11 patients with follow-up of more than 1 year went on to joint replacement. Six of the 12 had follow-up of more than 2 years. Of the 6 patients without an identifiable SONK lesion on MRI before arthroscopy, 4 had mild to moderate knee pain 0.5, 2.4, 3.5, and 4 years after surgery. For the other 2 patients, knee replacement was performed 1.5 and 1.8 years after surgery. Of the 6 patients with prearthroscopy SONK, 4 had mild to moderate knee pain 1.5, 3.7, 6.5, and 6.8 years after surgery; the other 2 had knee replacement 0.5 and 1.8 years after surgery. Articular cartilage degeneration and meniscal extrusion were similar (Table 1). In the control group, there was only 1 knee replacement, at 3 years, and the other 11 were functioning 2.6 to 5 years later. The longer follow-up resulted from selection of appropriate controls from the same year. Of the 6 SONK lesions found on preoperative MRI, 3 were read by the interpreting radiologist before surgery as possible SONK lesions, 2 were read as insufficiency fractures, and 1 was read as a possible insufficiency fracture.
Discussion
SONK is well described as a complication of arthroscopic knee surgery. However, this condition more commonly appears spontaneously in a population that has not had surgery. It has become clear that the term SONK may be misleading.16 In a recent series of postoperative subchondral fractures reported by MacDessi and colleagues,5 the average age of patients included in their study was 64 years. Pathologic analysis revealed subchondral fracture with callus formation in all cases. Only 2 knees had evidence of osteonecrosis, which appeared to be secondary to the fracture. Based on these findings, the authors concluded that “further investigation into the etiology of this condition is warranted.” A prominent association with medial meniscal tear has been noted, with the medial femoral condyle predominantly affected. As already mentioned, SONK differs from classical avascular necrosis on several points, including lack of the typical avascular osteonecrosis risk factors and absence of the serpiginous margin and double-line sign seen with typical bone infarction. In addition, the SONK lesions seen on radiographs and MRIs of the knee typically are in the medial femoral condyle and are very different from the typical area of infarction seen in patients with known risk factors for secondary osteonecrosis.
The cause of SONK is not known. Of more importance from a medicolegal standpoint is that these lesions are not necessarily related to arthroscopy.17 Interestingly, Pape and colleagues17 noted that some of the lesions they studied may have been present before surgery, which is what we found in 6 (50%) of the SONK knees in our study. Our data thus support the proposition that some SONK lesions are present before arthroscopy, and some cases of so-called postarthroscopy SONK may in fact have been progressing before surgery.
Our data also reinforce the importance of radiologist–orthopedic surgeon communication regarding the presence of SONK. We emphasize the importance of communicating the MRI findings clearly, whether the lesion is called SONK, SPONK, or insufficiency fracture. The orthopedic surgeons in our series may have been unaware of the presence of these lesions before arthroscopic meniscectomy, given the wide variety of terms being used in radiologic reports.
The natural history of spontaneous osteonecrosis of the medial tibial plateau has also been studied.18 There were 3 outcome patterns—acute extensive collapse of the medial tibial plateau, rapid progression to varying degrees of osteoarthritis, and complete resolution. It has been shown that resolution of SONK can occur in the early stages of the disease, within several months, but often the changes progress to bone destruction and articular cartilage collapse.19
In our series of patients, there was a female predominance, and mean age was 64 years. We investigated cartilage loss, meniscal tear, and meniscal extrusion to see if we could predict outcomes in patients who had the lesion before arthroscopy and if we could predict who might be at risk for developing the lesion after arthroscopy. Type of surgical procedure was also reviewed. For the sake of simplicity, we divided the follow-up patients into 2 groups: those managed with conservative treatment, which we deemed a reasonable outcome, and those who subsequently required knee joint replacement, which we deemed a poor outcome. As seen from our representative cases, both groups had patients with cartilage loss, meniscal tear, and meniscal extrusion to varying degrees. There were no risk factors pointing to a reasonable or poor outcome. In the group of patients with prearthroscopy lesions, we found the same problem. We were unable to identify a risk factor that might suggest a poor rather than a reasonable outcome. We must also emphasize that, in our review of patient charts, we could find no other causes for osteonecrosis. In particular, arthroscopic causes of acute chondral loss (eg, thermal wash, laser, bupivacaine pain pumps, epinephrine in irrigant) were not identified.
This study consisted of a series of cases managed at our institution over the past 8 years. Our data and this study had several limitations:
We may have been unable to identify other SONK cases that belonged in the group from our institution. In addition, we had only 11 patients for comparison with patients without SONK. Likewise, there were only 6 knees each in the prearthroscopy and postarthroscopy SONK groups. We also used images obtained from 1-T, 1.5-T, and 3-T closed MRI devices and one 0.7-T open device. These were, however, at the same institution.
Timing of our imaging was not uniform. In particular, in 3 of the patients who developed SONK after arthroscopy, preoperative MRI studies were performed quite some time before surgery. However, in these patients, more recent preoperative radiographs did not show any evidence of lesions. It can also be seen that postarthroscopy follow-up of patients varied. It is possible that, on longer follow-up, some of the cases we classified as having a reasonable outcome may have gone on to require total knee arthroplasty. One could argue that, in the patient who developed SONK within 1 year after surgery (Figure 4), the lesion was not related to the surgery. However, this patient’s radiographs 3 months after surgery did not show the SONK lesion but clearly showed prominent medial joint space narrowing—a new finding.
Only 1 musculoskeletal radiologist evaluated the radiographs, MRIs, and tomosynthesis (similar to computed tomography) studies for this investigation.
This lesion is not common, thus giving us a small group to analyze.
Despite our data limitations and the retrospective nature of this study, we compiled a reasonably representative sample of surgical SONK patients that matches other samples reported in the literature. Unfortunately, we could not identify any risk factors pointing to the likelihood of developing SONK or any risk factors pointing to either a reasonable or a poor prognosis in these patients. The etiology of the lesion remains an enigma. Our finding 6 cases of prearthroscopy lesions that did not necessarily result in a poor outcome, combined with our inability to identify any risk factors for SONK, points to the lack of a causal relationship with arthroscopy.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.
1. Ahlbäck S. Osteoarthritis of the knee. A radiographic investigation. Acta Radiol Diagn. 1968;(suppl 277):7-72.
2. Juréus J, Lindstrand A, Geijer M, Robertsson O, Tägil M. The natural course of spontaneous osteonecrosis of the knee (SPONK): a 1- to 27-year follow-up of 40 patients. Acta Orthop. 2013;84(4):410-414.
3. Zurlo JV. The double-line sign. Radiology. 1999;212(2):541-542.
4. Yamamoto T, Bullough PG. Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Joint Surg Am. 2000;82(6):858-866.
5. MacDessi SJ, Brophy RH, Bullough PG, Windsor RE, Sculco TP. Subchondral fracture following arthroscopic knee surgery. A series of eight cases. J Bone Joint Surg Am. 2008;90(5):1007-1012.
6. Brahme SK, Fox JM, Ferkel RD, Friedman MJ, Flannigan BD, Resnick DL. Osteonecrosis of the knee after arthroscopic surgery: diagnosis with MR imaging. Radiology. 1991;178(3):851-853.
7. Faletti C, Robba T, de Petro P. Postmeniscectomy osteonecrosis. Arthroscopy. 2002;18(1):91-94.
8. Johnson TC, Evans JA, Gilley JA, DeLee JC. Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy. 2000;16(3):254-261.
9. al-Kaar M, Garcia J, Fritschy D, Bonvin JC. Aseptic osteonecrosis of the femoral condyle after meniscectomy by the arthroscopic approach. J Radiol. 1997;78(4):283-288.
10. DeFalco RA, Ricci AR, Balduini FC. Osteonecrosis of the knee after arthroscopic meniscectomy and chondroplasty: a case report and literature review. Am J Sports Med. 2003;31(6):1013-1016.
11. Kusayama T. Idiopathic osteonecrosis of the femoral condyle after meniscectomy. Tokai J Exp Clin Med. 2003;28(4):145-150.
12. Prues-Latour V, Bonvin JC, Fritschy D. Nine cases of osteonecrosis in elderly patients following arthroscopic meniscectomy. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):142-147.
13. Santori N, Condello V, Adriani E, Mariani PP. Osteonecrosis after arthroscopic medial meniscectomy. Arthroscopy. 1995;11(2):220-224.
14. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-513.
15. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494-502.
16. Kidwai AS, Hemphill SD, Griffiths HJ. Radiologic case study. Spontaneous osteonecrosis of the knee reclassified as insufficiency fracture. Orthopedics. 2005;28(3):236, 333-236.
17. Pape D, Lorbach O, Anagnostakos K, Kohn D. Osteonecrosis in the postarthroscopic knee. Orthopade. 2008;37(11):1099-1107.
18. Satku K, Kumar VP, Chacha PB. Stress fractures around the knee in elderly patients. A cause of acute pain in the knee. J Bone Joint Surg Am. 1990;72(6):918-922.
19. Soucacos PN, Xenakis TH, Beris AE, Soucacos PK, Georgoulis A. Idiopathic osteonecrosis of the medial femoral condyle. Classification and treatment. Clin Orthop. 1997;(341):82-89.