User login
Failure of the Stem-Condyle Junction of a Modular Femoral Stem in Revision Total Knee Arthroplasty
Revision total knee arthroplasty (TKA) is frequently complicated by bone loss and ligament instability, necessitating specialized implants to increase constraint and transmit forces away from the joint surface. Femoral stems are commonly used to enhance fixation and distribute force from the condyles to the metaphysis or diaphysis, to higher-quality bone capable of sustaining the forces at the knee joint.
Modular implants are now commonplace in revision surgery, because they allow intraoperative customization of the implant to the patient’s anatomy, degree of bone loss, and need for metaphyseal or diaphyseal fixation. However, these advantages are not without a downside. The modular junction introduces potential weaknesses in the implant, which may lead to early failure.
We report a case of loosening of a Triathlon TS (Stryker) femoral component that was not evident on preoperative radiographs. To our knowledge, this complication has not been reported with this particular revision knee system. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman underwent 2-stage revision left TKA secondary to infection at an outside institution. She had undergone 17 prior knee surgeries with multiple revisions prior to this most recent revision surgery. A constrained implant was used at her last reimplantation secondary to ligamentous laxity after extensive débridement for infection. A Triathlon TS revision knee system with cemented stemmed tibial and femoral components was implanted; stems designed for uncemented fixation were cemented. She had a history of a quadriceps tendon tear, which was repaired prior to her revision, and quadricepsplasty was performed at the time of revision.
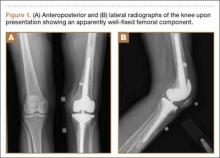
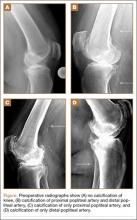
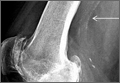
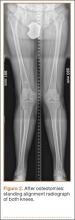
Seven years after this revision surgery, the patient presented to our clinic with progressive global instability, occasional effusions, and 2 documented episodes of frank dislocation. On examination, she was unstable in flexion and extension. Her extensor mechanism was intact, although with 7º active lag. She had a palpable quadriceps tendon defect. Her passive range of motion was 0º to 130º. Her active range of motion was 7º to 130º. Her erythrocyte sedimentation rate and C-reactive protein levels were within normal limits, and aspiration was negative for infection. Radiographs showed apparently well-fixed components with cemented femoral and tibial stems (Figures 1A, 1B).
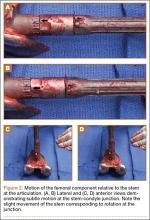
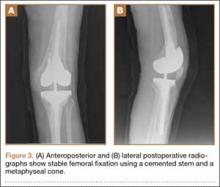
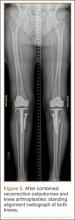
The patient underwent revision surgery for global instability with the surgical goal to upsize the polyethylene insert and advance the quadriceps to improve stability. In the operating room, a defect in the quadriceps mechanism was seen between the vastus medialis obliquus (VMO) and the patella, as well as a large effusion. Upon removal of the polyethylene insert, the tibial and patellar components were examined and found to be well fixed. The femoral component was grossly loose. On closer inspection, the condylar portion was found to be rotating in the axial plane freely on the well-fixed cemented stem in the femoral canal (Figures 2A-2D). The entire femoral component was removed with some difficulty because the well-fixed uncemented stem design was cemented in place. This required a small, anterior episiotomy of the femur. Reconstruction of the femur was performed using a trabecular metal cone, a cemented stem, and condylar component with distal and posterior augments (Figures 3A, 3B). A shorter, thinner stem was implanted and cemented into the previous cement mantle. A 19-mm constrained polyethylene liner was selected (the prior liner was 13 mm), which gave adequate stability with range of motion 0º to 130º. The VMO was advanced approximately 1.5 cm at the time of closure of the arthrotomy. The patient was implanted with the same Triathlon TS system, because the tibial component was well fixed, well positioned, and did not require revision.
Discussion
The need and use of stemmed, modular femoral components for revision TKA is neither questioned nor a novel concept in arthroplasty.1 Femoral bone defects encountered in revision arthroplasty generally lack sufficient cortical integrity to support an unstemmed component. Biomechanical analyses have reliably demonstrated improved initial stability and reduced relative motion provided by femoral stem extension.2,3 Correspondingly, significant translational and rotational movements of the femoral component when disconnected from the stem presumably correspond with clinical observations of instability.3 We report a unique case of failure of the modular junction of a stemmed femoral component in revision TKA that was not readily apparent on plain radiographs.
Dissociation of a cemented stem from the condylar portion of the component has been described at our institution with a different implant design.4 To our knowledge, we describe the first report of failure at the modular junction of the Triathlon TS femoral component.
Interestingly, relative motion has been shown to increase with increasing flexion in a biomechanical study2 using the same Triathlon TS system. The authors of that study found they were unable to complete testing at flexion greater than 30º because, absent the stabilizing influence of surrounding ligament and muscle, the sample deformation was so significant that it caused fracture.2 In the case of our patient, the incompetence of her extensor mechanism likely resulted in increased forces transmitted through the implant than might be expected in more physiologic circumstances. This higher stress may account in part for the failure of the implant at the known weakest point, the stem-condyle modular junction.
Modular implants are routinely used, given the variability of scenarios encountered in revision surgery and the need for customization to provide the best approximation of physiologic functioning of the joint. However, modular components introduce junctional points, which are potential points of failure. Stresses on the femoral component occur in multiple dimensions besides the axial loading and medial-lateral, anterior-posterior rocking seen with the tibial component. The maximum stress is observed at the distal-most aspect of the stiffest or most well-fixed components, in this case, the articulation between the cemented stem and the cemented condylar component. Poor distal femoral fixation compounds the problem.
Numerous case reports have documented such failures in other knee systems. Issack and colleagues5 described 2 cases of fracture through the taper lock between the femoral component and the stem extension in the Optetrak stemmed-constrained condylar knee prosthesis (Exactech). Westrich and colleagues6 reported disengagement of the locking bolt of the Insall-Burstein II Constrained Condylar Knee (Zimmer) leading to failure. Lim and colleagues4 reported stem-condyle junctional failure of the Total Condylar III (DePuy, Johnson & Johnson) due to locking-screw failure. Butt and colleagues7 reported a case of failure at the femoral component–stem junction caused by screw breakage. All of these cases involved failure at the condylar-stem junction that was readily apparent on routine preoperative imaging.
Our case is noteworthy because there was no preoperative radiographic evidence that the components were loose or the junction had failed. As with many revision systems observed by Fehring and colleagues,8 determination of fixation is often based on the appearance of the stem because the distal femoral interfaces may be obscured by the intercondylar box. This suggests that a loose component at the stem-condylar junction could easily be overlooked and not appropriately revised based on imaging alone. A solution for achieving stability at the time of revision surgery is to obtain good distal bone apposition and fixation. In this case, a cemented stem with a metaphyseal cone was used for femoral fixation (Figures 3A, 3B).
While long-term, abnormally high stress transmitted through the modular junction may account for the implant’s failure, to our knowledge, this is the first report of its kind related to this particular implant. If quadriceps weakness contributed to this failure, it is worth considering that quadriceps weakness is common after TKA and may persist without appropriate rehabilitation and activity. Furthermore, the lack of evidence on plain radiographs makes this particular form of failure very difficult to screen. A high degree of suspicion for loosening should be maintained in patients with pain and instability after revision TKA with this implant as well as with other modular revision knee systems.
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
2. Conlisk N, Gray H, Pankaj P, Howie CR. The influence of stem length and fixation on initial femoral component stability in revision total knee replacement. Bone Joint Res. 2012;1(11):281-288.
3. van Loon CJ, Kyriazopoulos A, Verdonschot N, de Waal Malefijt MC, Huiskes R, Buma P. The role of femoral stem extension in total knee arthroplasty. Clin Orthop Relat Res. 2000;(378):282-289.
4. Lim LA, Trousdale RT, Berry DJ, Hanssen AD. Failure of the stem-condyle junction of a modular femoral stem in revision total knee arthroplasty: a report of five cases. J Arthroplasty. 2001;16(1):128-132.
5. Issack PS, Cottrell JM, Delgado S, Wright TM, Sculco TP, Su EP. Failure at the taper lock of a modular stemmed femoral implant in revision knee arthroplasty. A report of two cases and a retrieval analysis. J Bone Joint Surg Am. 2007;89(10):2271-2274.
6. Westrich GH, Hidaka C, Windsor RE. Disengagement of a locking screw from a modular stem in revision total knee arthroplasty. A report of three cases. J Bone Joint Surg Am. 1997;79(2):254-258.
7. Butt AJ, Shaikh AH, Cameron HU. Coupling failure between stem and femoral component in a constrained revision total knee arthroplasty. J Coll Physicians Surg Pak. 2013;23(2):162-163.
8. Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res. 2003;(416):217-224.
Revision total knee arthroplasty (TKA) is frequently complicated by bone loss and ligament instability, necessitating specialized implants to increase constraint and transmit forces away from the joint surface. Femoral stems are commonly used to enhance fixation and distribute force from the condyles to the metaphysis or diaphysis, to higher-quality bone capable of sustaining the forces at the knee joint.
Modular implants are now commonplace in revision surgery, because they allow intraoperative customization of the implant to the patient’s anatomy, degree of bone loss, and need for metaphyseal or diaphyseal fixation. However, these advantages are not without a downside. The modular junction introduces potential weaknesses in the implant, which may lead to early failure.
We report a case of loosening of a Triathlon TS (Stryker) femoral component that was not evident on preoperative radiographs. To our knowledge, this complication has not been reported with this particular revision knee system. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman underwent 2-stage revision left TKA secondary to infection at an outside institution. She had undergone 17 prior knee surgeries with multiple revisions prior to this most recent revision surgery. A constrained implant was used at her last reimplantation secondary to ligamentous laxity after extensive débridement for infection. A Triathlon TS revision knee system with cemented stemmed tibial and femoral components was implanted; stems designed for uncemented fixation were cemented. She had a history of a quadriceps tendon tear, which was repaired prior to her revision, and quadricepsplasty was performed at the time of revision.
Seven years after this revision surgery, the patient presented to our clinic with progressive global instability, occasional effusions, and 2 documented episodes of frank dislocation. On examination, she was unstable in flexion and extension. Her extensor mechanism was intact, although with 7º active lag. She had a palpable quadriceps tendon defect. Her passive range of motion was 0º to 130º. Her active range of motion was 7º to 130º. Her erythrocyte sedimentation rate and C-reactive protein levels were within normal limits, and aspiration was negative for infection. Radiographs showed apparently well-fixed components with cemented femoral and tibial stems (Figures 1A, 1B).
The patient underwent revision surgery for global instability with the surgical goal to upsize the polyethylene insert and advance the quadriceps to improve stability. In the operating room, a defect in the quadriceps mechanism was seen between the vastus medialis obliquus (VMO) and the patella, as well as a large effusion. Upon removal of the polyethylene insert, the tibial and patellar components were examined and found to be well fixed. The femoral component was grossly loose. On closer inspection, the condylar portion was found to be rotating in the axial plane freely on the well-fixed cemented stem in the femoral canal (Figures 2A-2D). The entire femoral component was removed with some difficulty because the well-fixed uncemented stem design was cemented in place. This required a small, anterior episiotomy of the femur. Reconstruction of the femur was performed using a trabecular metal cone, a cemented stem, and condylar component with distal and posterior augments (Figures 3A, 3B). A shorter, thinner stem was implanted and cemented into the previous cement mantle. A 19-mm constrained polyethylene liner was selected (the prior liner was 13 mm), which gave adequate stability with range of motion 0º to 130º. The VMO was advanced approximately 1.5 cm at the time of closure of the arthrotomy. The patient was implanted with the same Triathlon TS system, because the tibial component was well fixed, well positioned, and did not require revision.
Discussion
The need and use of stemmed, modular femoral components for revision TKA is neither questioned nor a novel concept in arthroplasty.1 Femoral bone defects encountered in revision arthroplasty generally lack sufficient cortical integrity to support an unstemmed component. Biomechanical analyses have reliably demonstrated improved initial stability and reduced relative motion provided by femoral stem extension.2,3 Correspondingly, significant translational and rotational movements of the femoral component when disconnected from the stem presumably correspond with clinical observations of instability.3 We report a unique case of failure of the modular junction of a stemmed femoral component in revision TKA that was not readily apparent on plain radiographs.
Dissociation of a cemented stem from the condylar portion of the component has been described at our institution with a different implant design.4 To our knowledge, we describe the first report of failure at the modular junction of the Triathlon TS femoral component.
Interestingly, relative motion has been shown to increase with increasing flexion in a biomechanical study2 using the same Triathlon TS system. The authors of that study found they were unable to complete testing at flexion greater than 30º because, absent the stabilizing influence of surrounding ligament and muscle, the sample deformation was so significant that it caused fracture.2 In the case of our patient, the incompetence of her extensor mechanism likely resulted in increased forces transmitted through the implant than might be expected in more physiologic circumstances. This higher stress may account in part for the failure of the implant at the known weakest point, the stem-condyle modular junction.
Modular implants are routinely used, given the variability of scenarios encountered in revision surgery and the need for customization to provide the best approximation of physiologic functioning of the joint. However, modular components introduce junctional points, which are potential points of failure. Stresses on the femoral component occur in multiple dimensions besides the axial loading and medial-lateral, anterior-posterior rocking seen with the tibial component. The maximum stress is observed at the distal-most aspect of the stiffest or most well-fixed components, in this case, the articulation between the cemented stem and the cemented condylar component. Poor distal femoral fixation compounds the problem.
Numerous case reports have documented such failures in other knee systems. Issack and colleagues5 described 2 cases of fracture through the taper lock between the femoral component and the stem extension in the Optetrak stemmed-constrained condylar knee prosthesis (Exactech). Westrich and colleagues6 reported disengagement of the locking bolt of the Insall-Burstein II Constrained Condylar Knee (Zimmer) leading to failure. Lim and colleagues4 reported stem-condyle junctional failure of the Total Condylar III (DePuy, Johnson & Johnson) due to locking-screw failure. Butt and colleagues7 reported a case of failure at the femoral component–stem junction caused by screw breakage. All of these cases involved failure at the condylar-stem junction that was readily apparent on routine preoperative imaging.
Our case is noteworthy because there was no preoperative radiographic evidence that the components were loose or the junction had failed. As with many revision systems observed by Fehring and colleagues,8 determination of fixation is often based on the appearance of the stem because the distal femoral interfaces may be obscured by the intercondylar box. This suggests that a loose component at the stem-condylar junction could easily be overlooked and not appropriately revised based on imaging alone. A solution for achieving stability at the time of revision surgery is to obtain good distal bone apposition and fixation. In this case, a cemented stem with a metaphyseal cone was used for femoral fixation (Figures 3A, 3B).
While long-term, abnormally high stress transmitted through the modular junction may account for the implant’s failure, to our knowledge, this is the first report of its kind related to this particular implant. If quadriceps weakness contributed to this failure, it is worth considering that quadriceps weakness is common after TKA and may persist without appropriate rehabilitation and activity. Furthermore, the lack of evidence on plain radiographs makes this particular form of failure very difficult to screen. A high degree of suspicion for loosening should be maintained in patients with pain and instability after revision TKA with this implant as well as with other modular revision knee systems.
Revision total knee arthroplasty (TKA) is frequently complicated by bone loss and ligament instability, necessitating specialized implants to increase constraint and transmit forces away from the joint surface. Femoral stems are commonly used to enhance fixation and distribute force from the condyles to the metaphysis or diaphysis, to higher-quality bone capable of sustaining the forces at the knee joint.
Modular implants are now commonplace in revision surgery, because they allow intraoperative customization of the implant to the patient’s anatomy, degree of bone loss, and need for metaphyseal or diaphyseal fixation. However, these advantages are not without a downside. The modular junction introduces potential weaknesses in the implant, which may lead to early failure.
We report a case of loosening of a Triathlon TS (Stryker) femoral component that was not evident on preoperative radiographs. To our knowledge, this complication has not been reported with this particular revision knee system. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman underwent 2-stage revision left TKA secondary to infection at an outside institution. She had undergone 17 prior knee surgeries with multiple revisions prior to this most recent revision surgery. A constrained implant was used at her last reimplantation secondary to ligamentous laxity after extensive débridement for infection. A Triathlon TS revision knee system with cemented stemmed tibial and femoral components was implanted; stems designed for uncemented fixation were cemented. She had a history of a quadriceps tendon tear, which was repaired prior to her revision, and quadricepsplasty was performed at the time of revision.
Seven years after this revision surgery, the patient presented to our clinic with progressive global instability, occasional effusions, and 2 documented episodes of frank dislocation. On examination, she was unstable in flexion and extension. Her extensor mechanism was intact, although with 7º active lag. She had a palpable quadriceps tendon defect. Her passive range of motion was 0º to 130º. Her active range of motion was 7º to 130º. Her erythrocyte sedimentation rate and C-reactive protein levels were within normal limits, and aspiration was negative for infection. Radiographs showed apparently well-fixed components with cemented femoral and tibial stems (Figures 1A, 1B).
The patient underwent revision surgery for global instability with the surgical goal to upsize the polyethylene insert and advance the quadriceps to improve stability. In the operating room, a defect in the quadriceps mechanism was seen between the vastus medialis obliquus (VMO) and the patella, as well as a large effusion. Upon removal of the polyethylene insert, the tibial and patellar components were examined and found to be well fixed. The femoral component was grossly loose. On closer inspection, the condylar portion was found to be rotating in the axial plane freely on the well-fixed cemented stem in the femoral canal (Figures 2A-2D). The entire femoral component was removed with some difficulty because the well-fixed uncemented stem design was cemented in place. This required a small, anterior episiotomy of the femur. Reconstruction of the femur was performed using a trabecular metal cone, a cemented stem, and condylar component with distal and posterior augments (Figures 3A, 3B). A shorter, thinner stem was implanted and cemented into the previous cement mantle. A 19-mm constrained polyethylene liner was selected (the prior liner was 13 mm), which gave adequate stability with range of motion 0º to 130º. The VMO was advanced approximately 1.5 cm at the time of closure of the arthrotomy. The patient was implanted with the same Triathlon TS system, because the tibial component was well fixed, well positioned, and did not require revision.
Discussion
The need and use of stemmed, modular femoral components for revision TKA is neither questioned nor a novel concept in arthroplasty.1 Femoral bone defects encountered in revision arthroplasty generally lack sufficient cortical integrity to support an unstemmed component. Biomechanical analyses have reliably demonstrated improved initial stability and reduced relative motion provided by femoral stem extension.2,3 Correspondingly, significant translational and rotational movements of the femoral component when disconnected from the stem presumably correspond with clinical observations of instability.3 We report a unique case of failure of the modular junction of a stemmed femoral component in revision TKA that was not readily apparent on plain radiographs.
Dissociation of a cemented stem from the condylar portion of the component has been described at our institution with a different implant design.4 To our knowledge, we describe the first report of failure at the modular junction of the Triathlon TS femoral component.
Interestingly, relative motion has been shown to increase with increasing flexion in a biomechanical study2 using the same Triathlon TS system. The authors of that study found they were unable to complete testing at flexion greater than 30º because, absent the stabilizing influence of surrounding ligament and muscle, the sample deformation was so significant that it caused fracture.2 In the case of our patient, the incompetence of her extensor mechanism likely resulted in increased forces transmitted through the implant than might be expected in more physiologic circumstances. This higher stress may account in part for the failure of the implant at the known weakest point, the stem-condyle modular junction.
Modular implants are routinely used, given the variability of scenarios encountered in revision surgery and the need for customization to provide the best approximation of physiologic functioning of the joint. However, modular components introduce junctional points, which are potential points of failure. Stresses on the femoral component occur in multiple dimensions besides the axial loading and medial-lateral, anterior-posterior rocking seen with the tibial component. The maximum stress is observed at the distal-most aspect of the stiffest or most well-fixed components, in this case, the articulation between the cemented stem and the cemented condylar component. Poor distal femoral fixation compounds the problem.
Numerous case reports have documented such failures in other knee systems. Issack and colleagues5 described 2 cases of fracture through the taper lock between the femoral component and the stem extension in the Optetrak stemmed-constrained condylar knee prosthesis (Exactech). Westrich and colleagues6 reported disengagement of the locking bolt of the Insall-Burstein II Constrained Condylar Knee (Zimmer) leading to failure. Lim and colleagues4 reported stem-condyle junctional failure of the Total Condylar III (DePuy, Johnson & Johnson) due to locking-screw failure. Butt and colleagues7 reported a case of failure at the femoral component–stem junction caused by screw breakage. All of these cases involved failure at the condylar-stem junction that was readily apparent on routine preoperative imaging.
Our case is noteworthy because there was no preoperative radiographic evidence that the components were loose or the junction had failed. As with many revision systems observed by Fehring and colleagues,8 determination of fixation is often based on the appearance of the stem because the distal femoral interfaces may be obscured by the intercondylar box. This suggests that a loose component at the stem-condylar junction could easily be overlooked and not appropriately revised based on imaging alone. A solution for achieving stability at the time of revision surgery is to obtain good distal bone apposition and fixation. In this case, a cemented stem with a metaphyseal cone was used for femoral fixation (Figures 3A, 3B).
While long-term, abnormally high stress transmitted through the modular junction may account for the implant’s failure, to our knowledge, this is the first report of its kind related to this particular implant. If quadriceps weakness contributed to this failure, it is worth considering that quadriceps weakness is common after TKA and may persist without appropriate rehabilitation and activity. Furthermore, the lack of evidence on plain radiographs makes this particular form of failure very difficult to screen. A high degree of suspicion for loosening should be maintained in patients with pain and instability after revision TKA with this implant as well as with other modular revision knee systems.
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
2. Conlisk N, Gray H, Pankaj P, Howie CR. The influence of stem length and fixation on initial femoral component stability in revision total knee replacement. Bone Joint Res. 2012;1(11):281-288.
3. van Loon CJ, Kyriazopoulos A, Verdonschot N, de Waal Malefijt MC, Huiskes R, Buma P. The role of femoral stem extension in total knee arthroplasty. Clin Orthop Relat Res. 2000;(378):282-289.
4. Lim LA, Trousdale RT, Berry DJ, Hanssen AD. Failure of the stem-condyle junction of a modular femoral stem in revision total knee arthroplasty: a report of five cases. J Arthroplasty. 2001;16(1):128-132.
5. Issack PS, Cottrell JM, Delgado S, Wright TM, Sculco TP, Su EP. Failure at the taper lock of a modular stemmed femoral implant in revision knee arthroplasty. A report of two cases and a retrieval analysis. J Bone Joint Surg Am. 2007;89(10):2271-2274.
6. Westrich GH, Hidaka C, Windsor RE. Disengagement of a locking screw from a modular stem in revision total knee arthroplasty. A report of three cases. J Bone Joint Surg Am. 1997;79(2):254-258.
7. Butt AJ, Shaikh AH, Cameron HU. Coupling failure between stem and femoral component in a constrained revision total knee arthroplasty. J Coll Physicians Surg Pak. 2013;23(2):162-163.
8. Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res. 2003;(416):217-224.
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87(7):1487-1497.
2. Conlisk N, Gray H, Pankaj P, Howie CR. The influence of stem length and fixation on initial femoral component stability in revision total knee replacement. Bone Joint Res. 2012;1(11):281-288.
3. van Loon CJ, Kyriazopoulos A, Verdonschot N, de Waal Malefijt MC, Huiskes R, Buma P. The role of femoral stem extension in total knee arthroplasty. Clin Orthop Relat Res. 2000;(378):282-289.
4. Lim LA, Trousdale RT, Berry DJ, Hanssen AD. Failure of the stem-condyle junction of a modular femoral stem in revision total knee arthroplasty: a report of five cases. J Arthroplasty. 2001;16(1):128-132.
5. Issack PS, Cottrell JM, Delgado S, Wright TM, Sculco TP, Su EP. Failure at the taper lock of a modular stemmed femoral implant in revision knee arthroplasty. A report of two cases and a retrieval analysis. J Bone Joint Surg Am. 2007;89(10):2271-2274.
6. Westrich GH, Hidaka C, Windsor RE. Disengagement of a locking screw from a modular stem in revision total knee arthroplasty. A report of three cases. J Bone Joint Surg Am. 1997;79(2):254-258.
7. Butt AJ, Shaikh AH, Cameron HU. Coupling failure between stem and femoral component in a constrained revision total knee arthroplasty. J Coll Physicians Surg Pak. 2013;23(2):162-163.
8. Fehring TK, Odum S, Olekson C, Griffin WL, Mason JB, McCoy TH. Stem fixation in revision total knee arthroplasty: a comparative analysis. Clin Orthop Relat Res. 2003;(416):217-224.
Current Evidence Does Not Support Medicare’s 3-Day Rule in Primary Total Joint Arthroplasty
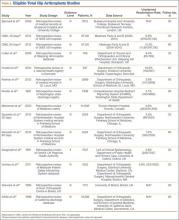
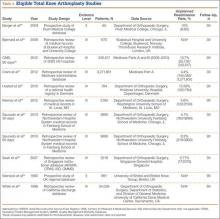
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
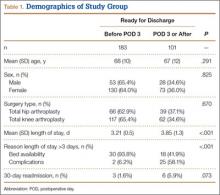
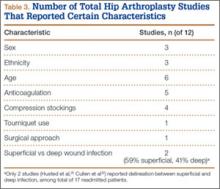
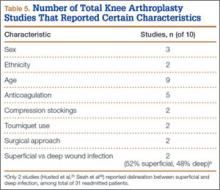
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
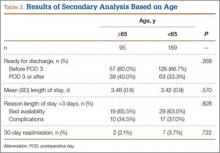
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
Medicare beneficiaries’ demand for total hip arthroplasty (THA) and total knee arthroplasty (TKA) has increased significantly over the past several years, with recent studies reporting 209,945 primary THAs and 243,802 primary TKAs performed annually.1,2 With this demand has come an increase in the percentage of patients discharged to an extended-care facility (ECF) for skilled nursing care or acute rehabilitation—an estimated 49.3% for THA and 41.5% for TKA.1,2 To qualify for discharge to an ECF, Medicare beneficiaries are required to have an inpatient stay of at least 3 consecutive days.3 Although the basis of this rule is unclear, it is thought to prevent hasty discharge of unstable patients.
We conducted a study to explore the effect of this policy on length of stay (LOS) in a population of patients who underwent primary total joint arthroplasty (TJA). Based on a pilot study by our group, we hypothesized that such a statuary requirement would be associated with increased LOS and would not prevent discharge of potentially unstable patients. Specifically, we explored whether patients who could have been discharged earlier experienced any later inpatient complications or 30-day readmission to justify staying past their discharge readiness.
Materials and Methods
Institutional review board approval was obtained for this study. Between 2011 and 2012, the senior authors (Dr. Wellman, Dr. Attarian, Dr. Bolognesi) treated 985 patients with Current Procedural Terminology (CPT) codes 27130 (THA) and 27447 (TKA). Of the 985 patients, 287 (29.13%) were discharged to an ECF and were included in the study. Three of the 287 were excluded: 2 for requiring preadmission for medical optimization and 1 for having another procedure with plastic surgery. All patients were admitted from home on day of surgery and had a standardized clinical pathway with respect to pain control, mobilization, and anticoagulation. Physical therapy and occupational therapy (PT/OT) were initiated on day of surgery and were continued daily until discharge.
The primary outcome was discharge readiness, defined as meeting the criteria of stable blood pressure, pulse, and breathing; no fever over 101.5°F for 24 hours before discharge; wound healing with no concerns; pain controlled with oral medications; and ambulation or the potential for rehabilitation at the receiving facility. Secondary outcomes were changes in PT/OT progress, medical interventions, and 30-day readmission rate. PT/OT progress was categorized as either slow or steady by the therapist assigned to each patient at time of hospitalization. Steady progress indicated overall improvement on several measures, including transfers, ambulation distance, and ability to adhere to postoperative precautions; slow progress indicated no improvement on these measures.
Results for continuous variables were summarized with means, standard deviations, and ranges, and results for categorical variables were summarized with counts and percentages. Student t test was used to evaluate increase in LOS, and the McNemar test for paired data was used to analyze rehabilitation gains from readiness-for-discharge day to the next postoperative day (POD). SAS Version 9.2 software (SAS Institute) was used for all analyses.
Results
Of the 284 patients included in the study, 203 were female (71.5%), 81 male (28.5%). Mean (SD) age was 68 (11) years (range, 21-92 years). One hundred seventy-nine patients (63.0%) underwent TKA, and 105 (37.0%) underwent THA. Two hundred twenty-seven patients (80.0%) were discharged to skilled nursing care, and 57 (20.1%) to inpatient rehabilitation. Mean (SD) LOS was 3.44 (0.92) days (range, 3-9 days). One hundred eighty-three patients (64.4%) were ready for discharge on POD 2, 76 (26.8%) on POD 3, and 25 (8.8%) after POD 3. Delaying discharge until POD 3 increased LOS by 1.08 days (P < .001). Two hundred nine patients (73.6%) were discharged on POD 3, and 75 (26.4%) after POD 3. Reasons for being discharged after POD 3 were lack of ECF bed availability (48 patients, 64.0%) and postoperative complications (27 patients, 36.0%). Patients ready for discharge on POD 2 had fewer complications than patients ready after POD 2 (P < .001).
Analysis of the 183 patients who were ready for discharge on POD 2 demonstrated a statistically significant (P = .038) change in rehabilitation progress by staying an additional hospital day. However, this difference was not clinically significant: Only 17.5% of patients improved, while 82.5% remained unchanged or declined in progress. Most important, among patients who demonstrated rehabilitation gains, the improvement was not sufficient to change the decision regarding discharge destination. Three patients (1.6%) ready for discharge on POD 2 were readmitted within 30 days of discharge (2 for wound infection, 1 for syncope). Risk for 30-day readmission or development of an inpatient complication in patients ready for discharge on POD 2 was not significant (P = .073). Table 1 summarizes the statistical results.
As age 65 years or older is one of the major criteria for Medicare eligibility, a secondary analysis was performed to explore whether there were age-related differences in the study outcomes. We found no significant differences between patients 65 years or older and patients younger than 65 years with respect to discharge readiness, LOS, postoperative complications, or 30-day readmission. Table 2 summarizes the statistical results based on age.
Discussion
Consistent with our pilot study,4 the majority of patients discharged to an ECF were ready for discharge on POD 2. Delaying discharge until POD 3 increased LOS by 1.08 days with no significant risk in 30-day readmission if patients were allowed to be discharged 1 day earlier. Different from our pilot study results, however, 17.5% of patients who stayed past their discharge readiness showed improvement in PT/OT progress, though this was not clinically sufficient to alter the decision regarding discharge destination. This difference can be attributed to the fact that the current study (vs the pilot study) was adequately powered for this outcome.
Our study was specifically designed to evaluate the effect of Medicare’s 3-day rule—the requirement of an inpatient hospital stay of at least 3 consecutive days to qualify for coverage for treatment at an ECF. This policy creates tremendous unnecessary hospitalization and resource utilization and denies patients earlier access to specialized postacute care. To put the economic implications of this policy in perspective, almost half of the 1 million TJAs performed annually are performed for Medicare beneficiaries, and almost half of those patients are discharged to an ECF.1,2,5 This equates to about 161,000 days of unnecessary hospitalization per year (64.4% of 250,000 patients), which translates into $310,730,000 in expenditures based on an average cost of $1930 per inpatient day for state/local government, nonprofit, and for-profit hospitals.6 Furthermore, with a growing trend toward outpatient TJA, the Medicare statute may leave substantial bills for patients who happen to require unplanned discharge to an ECF.
This study had its weaknesses. First, it was a retrospective review of charts at a single tertiary-care hospital. However, observer bias may have been eliminated, as the data were collected before a study was planned. An outcome such as discharge readiness, if prospectively assessed, could easily have been influenced by study personnel. Second, our patient sample was too small to definitively resolve this issue and be able to effect public policy change. However, there was sufficient power for the primary outcome. We also analyzed a consecutive group of patients who underwent a standardized postoperative clinical pathway with clear discharge-readiness criteria.
The effect of this study in the era of the Patient Protection and Affordable Care Act and its Bundled Payments for Care Improvement (BPCI) initiative deserves special attention. The BPCI initiative is divided into 4 models that reconcile payments associated with an episode of care (eg, TKA) against a predetermined payment amount.7 Relevant to our study, BPCI model 2 covers inpatient hospitalization up to 30, 60, or 90 days after discharge and includes a waiver of the 3-day rule for inpatient hospitalization. There are only 60 BPCI model 2–participating health care organizations. On the basis of our study results, we think the waiver is a step in the right direction, as no demonstrable benefits were realized from having patients stay hospitalized longer. However, the waiver should not be limited to select entities, and we hope that, with further research, the statutory requirement of 3-day inpatient hospitalization will be repealed.
Conclusion
Our study results call into question the validity of Medicare’s 3-day rule, and we hope they stimulate further research to definitively resolve this question. The majority of our study patients destined for discharge to an ECF could have been safely discharged on POD 2. The implications of reducing LOS cannot be overstated. From a hospital perspective, reducing LOS eliminates unnecessary hospitalization and resource utilization. From a patient perspective, it allows earlier access to specialized care and eliminates billing confusion. From a payer perspective, it may reduce costs significantly.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Cram P, Lu X, Callaghan JJ, Vaughan-Sarrazin MS, Cai X, Li Y. Long-term trends in hip arthroplasty use and volume. J Arthroplasty. 2012;27(2):278-285.e2.
3. Centers for Medicare & Medicaid Services. Medicare Coverage of Skilled Nursing Facility Care. Baltimore, MD: US Dept of Health and Human Services, Centers for Medicare & Medicaid Services. CMS Product No. 10153. http://www.medicare.gov/pubs/pdf/10153.pdf. Revised January 2015. Accessed August 24, 2015.
4. Halawi MJ, Vovos TJ, Green CL, Wellman SS, Attarian DE, Bolognesi MP. Medicare’s 3-day rule: time for a rethink. J Arthroplasty. 2015;30(9):1483-1484.
5. Inpatient surgery. Centers for Disease Control and Prevention, National Center for Health Statistics website. http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm. Updated April 29, 2015. Accessed August 24, 2015.
6 Hospital adjusted expenses per inpatient day by ownership. 2013. Kaiser Family Foundation website. http://kff.org/other/state-indicator/expenses-per-inpatient-day-by-ownership. Accessed August 24, 2015.
7. BPCI [Bundled Payments for Care Improvement] model 2: retrospective acute & post acute care episode. Centers for Medicare & Medicare Services website. http://innovation.cms.gov/initiatives/BPCI-Model-2. Updated August 20, 2015. Accessed August 24, 2015.
Are Knee and Hip Replacements Bad For the Heart?
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Researchers found that patients with osteoarthritis who had total knee or hip joint arthroplasty were at increased risk of myocardial infarction in the early post-operative period, according to a study published online ahead of print August 31 in Arthritis & Rheumatology. However, findings indicate that long-term risk of heart attack did not persist, while the risk for venous thromboembolism remained years after the procedure was performed.
The cohort study included 13,849 patients who underwent total knee replacement surgery and 13,849 matched controls that did not have surgery. Patients were ages 50 or older and were diagnosed with knee or hip osteoarthritis between January 2000 and December 2012.
Findings indicate that 306 patients in the arthroplasty group and 286 in the non-surgical group developed myocardial infarction during the follow-up period.
Risk of heart attack was significantly higher during the first postoperative month in those who had knee replacement surgery compared with those in the non-surgical group (hazard ratio 8.75), and gradually declined over time. Venous thromboembolism was a significant risk during the first month and over time for those who had total knee or total hip arthroplasty.
“Our findings provide the first general population-based evidence that osteoarthritis patients who have total knee or total hip replacement surgery are at increased risk of heart attack in the immediate postoperative period,” said Yuqing Zhang, DSc, Professor of Medicine and Epidemiology at Boston University School of Medicine. “The long-term risk of heart attack was insignificant, but risk of blood clots in the lung remained for years after surgery to replace a hip or knee damaged by osteoarthritis,” said Dr. Zhang.
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Suggested Reading
Lu N, Misra D, Neogi T, et al. Total joint arthroplasty and the risk of myocardial infarction - a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015 Aug 31 [Epub ahead of print].
Commentary to "CDC Will Soon Issue Guidelines for the Prevention of Surgical Site Infection"
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Analyzing the Guidelines: It Can't All Be Level I
The demand for total joint arthroplasty continues to rise, resulting in a steady increase in the number of primary total hip and knee replacements every year. Unfortunately, as these numbers rise, so will the number of periprosthetic joint infections (PJIs). The economic burden and patient morbidity associated with PJI has resulted in the creation of multiple orthopedic societies and committees focused on formulating “best practice” guidelines in order to reduce the rates of PJI as much as possible.
The new guidelines for surgical site infection (SSI) prevention by the Centers for Disease Control and Prevention (CDC) recently forced the orthopedic community to critically analyze the current literature. Dr. Javad Parvizi’s editorial elegantly notes that many areas of infection prevention and treatment are not well evaluated, and many of our day-to-day practices are based on low levels of evidence. Level I studies continue to be a costly and time-consuming challenge due to the already very low SSI rate, and, in order to show an improvement in this rate, thousands of patients are required for study. This makes a multicenter approach necessary to ensure adequate power, and a multicenter study often requires significant resources and funding outlets. These requirements have resulted in many of our practice recommendations being based on retrospective reviews, which have inherent methodological limitations. The retrospective nature of these studies lacks the experimental design necessary to confidently make treatment recommendations; however, they do allow us to look at what strategies have been tried, and in essence, how well they worked. Although level III and IV studies do not allow us to compare treatments head to head, they do give us some insights into viable treatment strategies and should not be completely disregarded. The results of retrospective studies allow us to design prospective experiments based on what we have observed as successful treatment modalities in particular patient cohorts.
An alternative approach for evaluating new and existing treatment strategies is through basic science translational research. Future advancements in PJI diagnosis and treatment will likely be founded upon translational research efforts from clinician scientists testing treatment protocols both on the benchtop and in animal models. The most glaring knowledge gaps in PJI should be identified through the combined efforts of the CDC, the Musculoskeletal Infection Society, the American Academy of Orthopaedic Surgeons, and the Orthopaedic Research Society. Coordinated efforts should be made and strategies executed to systematically fund translational projects that answer these questions. Translational studies will be able to safely and methodically evaluate new and even established treatment protocols for PJI in a cost-effective manner.
We have made great strides in the prevention and treatment of PJI over the past 2 decades. When working together as a cohesive profession, we will undoubtedly continue to advance our knowledge base and improve treatment recommendations for our patients.
Total Knee Replacement Is Effective in Patients With Rheumatoid Arthritis
According to a new study, total knee arthroplasty is highly effective in reducing clinically relevant knee pain to a greater extent than other subjective health-related quality-of-life indicies in patients with rheumatoid arthritis, although this improvement is less marked as compared to outcomes in patients with osteoarthritis. These study findings were published online ahead of print July 20 in Arthritis & Rheumatology.
The study included patients with rheumatologist-diagnosed arthritis undergoing primary total knee arthroplasty during 1999 to 2012. Indices of pain knee, and health-related quality of life were obtained in 3 consecutive 6-month intervals: preoperative, perioperative, and postoperative. Descriptive statistics and 1-way analysis of variance were used to compare total knee arthroplasty outcomes by diagnosis. Effect sizes and standardized response means were calculated between baseline and recovery.
Of the participating 18,897 patients, 834 people with rheumatoid arthritis, and 315 people with osteoarthritis had undergone index total knee arthroplasty at mean ages 65 and 68. Post total knee arthroplasty, significant improvements were observed for most domains of pain, function, and health-related quality of life within both disease groups, with greater impact in osteoarthritis. Based on the standardized response means, the maximum improvement was shown in index knee pain.
The Health Assessment Questionnaire II and the Short Form 36 physical component summary were the most responsive health-related quality of life indices in detecting post-total knee arthroplasty improvement in rheumatoid arthritis. A diagnosis of rheumatoid arthritis, lower income, and preoperative anxiety were independently associated with a lower degree of improvement in index knee pain following total knee arthroplasty.
Senior author Kaleb D. Michaud, PhD, Associate Professor in the Division of Rheumatology and Immunology at the University of Nebraska Medical Center in Omaha, and colleagues said that total knee replacement can serve as a “time machine” by which patients can return to a less disabled lifestyle, before the arthritic process catches up.
“A new knee can give osteoarthritis patients 10 to 20 years of painless use, whereas rheumatoid arthritis continues to affect the joint soon afterward,” the researchers said. “It’s an important and effective treatment, but patients with rheumatoid arthritis shouldn’t expect the same, often dramatic results experienced by their osteoarthritis counterparts,” Dr. Michaud said.
Suggested Reading
Dusad A, Pedro S, Mikuls TR, et al. Impact of total knee arthroplasty as assessed using patient-reported pain and health-related quality of life indices: rheumatoid arthritis versus osteoarthritis. Arthritis Rheumatol. 2015 July 20 [Epub ahead of print].
According to a new study, total knee arthroplasty is highly effective in reducing clinically relevant knee pain to a greater extent than other subjective health-related quality-of-life indicies in patients with rheumatoid arthritis, although this improvement is less marked as compared to outcomes in patients with osteoarthritis. These study findings were published online ahead of print July 20 in Arthritis & Rheumatology.
The study included patients with rheumatologist-diagnosed arthritis undergoing primary total knee arthroplasty during 1999 to 2012. Indices of pain knee, and health-related quality of life were obtained in 3 consecutive 6-month intervals: preoperative, perioperative, and postoperative. Descriptive statistics and 1-way analysis of variance were used to compare total knee arthroplasty outcomes by diagnosis. Effect sizes and standardized response means were calculated between baseline and recovery.
Of the participating 18,897 patients, 834 people with rheumatoid arthritis, and 315 people with osteoarthritis had undergone index total knee arthroplasty at mean ages 65 and 68. Post total knee arthroplasty, significant improvements were observed for most domains of pain, function, and health-related quality of life within both disease groups, with greater impact in osteoarthritis. Based on the standardized response means, the maximum improvement was shown in index knee pain.
The Health Assessment Questionnaire II and the Short Form 36 physical component summary were the most responsive health-related quality of life indices in detecting post-total knee arthroplasty improvement in rheumatoid arthritis. A diagnosis of rheumatoid arthritis, lower income, and preoperative anxiety were independently associated with a lower degree of improvement in index knee pain following total knee arthroplasty.
Senior author Kaleb D. Michaud, PhD, Associate Professor in the Division of Rheumatology and Immunology at the University of Nebraska Medical Center in Omaha, and colleagues said that total knee replacement can serve as a “time machine” by which patients can return to a less disabled lifestyle, before the arthritic process catches up.
“A new knee can give osteoarthritis patients 10 to 20 years of painless use, whereas rheumatoid arthritis continues to affect the joint soon afterward,” the researchers said. “It’s an important and effective treatment, but patients with rheumatoid arthritis shouldn’t expect the same, often dramatic results experienced by their osteoarthritis counterparts,” Dr. Michaud said.
According to a new study, total knee arthroplasty is highly effective in reducing clinically relevant knee pain to a greater extent than other subjective health-related quality-of-life indicies in patients with rheumatoid arthritis, although this improvement is less marked as compared to outcomes in patients with osteoarthritis. These study findings were published online ahead of print July 20 in Arthritis & Rheumatology.
The study included patients with rheumatologist-diagnosed arthritis undergoing primary total knee arthroplasty during 1999 to 2012. Indices of pain knee, and health-related quality of life were obtained in 3 consecutive 6-month intervals: preoperative, perioperative, and postoperative. Descriptive statistics and 1-way analysis of variance were used to compare total knee arthroplasty outcomes by diagnosis. Effect sizes and standardized response means were calculated between baseline and recovery.
Of the participating 18,897 patients, 834 people with rheumatoid arthritis, and 315 people with osteoarthritis had undergone index total knee arthroplasty at mean ages 65 and 68. Post total knee arthroplasty, significant improvements were observed for most domains of pain, function, and health-related quality of life within both disease groups, with greater impact in osteoarthritis. Based on the standardized response means, the maximum improvement was shown in index knee pain.
The Health Assessment Questionnaire II and the Short Form 36 physical component summary were the most responsive health-related quality of life indices in detecting post-total knee arthroplasty improvement in rheumatoid arthritis. A diagnosis of rheumatoid arthritis, lower income, and preoperative anxiety were independently associated with a lower degree of improvement in index knee pain following total knee arthroplasty.
Senior author Kaleb D. Michaud, PhD, Associate Professor in the Division of Rheumatology and Immunology at the University of Nebraska Medical Center in Omaha, and colleagues said that total knee replacement can serve as a “time machine” by which patients can return to a less disabled lifestyle, before the arthritic process catches up.
“A new knee can give osteoarthritis patients 10 to 20 years of painless use, whereas rheumatoid arthritis continues to affect the joint soon afterward,” the researchers said. “It’s an important and effective treatment, but patients with rheumatoid arthritis shouldn’t expect the same, often dramatic results experienced by their osteoarthritis counterparts,” Dr. Michaud said.
Suggested Reading
Dusad A, Pedro S, Mikuls TR, et al. Impact of total knee arthroplasty as assessed using patient-reported pain and health-related quality of life indices: rheumatoid arthritis versus osteoarthritis. Arthritis Rheumatol. 2015 July 20 [Epub ahead of print].
Suggested Reading
Dusad A, Pedro S, Mikuls TR, et al. Impact of total knee arthroplasty as assessed using patient-reported pain and health-related quality of life indices: rheumatoid arthritis versus osteoarthritis. Arthritis Rheumatol. 2015 July 20 [Epub ahead of print].
Knee Replacement Proves Effective for Degeneration Caused by Blount Disease
Total knee replacements can effectively treat degeneration caused by Blount disease, according to a study published online ahead of print July 11 in the Journal of Arthroplasty.
Middle-aged patients with Blount disease who underwent joint replacements on 1 or both knees were found to have stable knees, excellent range of motion, and no need for pain medications, according to the study conducted at Loyola University Medical Center in Illinois.
“With proper attention paid to technical details, patients with Blount or Blount-like deformity can undergo successful total knee arthroplasty,” said Harold Rees, MD, Assistant Professor of Reconstructive Surgery and Joint Replacement at Loyola University Chicago Stritch School of Medicine in Maywood, Illinois, and colleagues.
For the study, Dr. Rees and colleagues reviewed the records of 5 patients with Blount disease. Three patients had replacements on both knees and 2 patients had replacements on 1 knee. Four patients were African American and 4 were male. All were obese. The average age at the time of the knee replacements was 49.9. Patients were followed-up an average of 75.2 months after their knee replacements.
Mean proximal tibial metaphyseal-diaphyseal angle was 20.75 degrees. Each patient had substantial posteromedial tibial bony defects and 6 knees required extensive medial releases. Two knees required increased constraint at index procedure. One patient underwent bilateral revision surgery with rotating hinge prostheses.
The researchers used a scoring system, devised by the Knee Society, that combines clinical, functional, and satisfaction scores. The mean Knee Society score was 212.5, out of a maximum possible score of 255. Patients also were rated on the Western Ontario and McMaster Universities Osteoarthritis Index.
“The main purpose was to highlight surgical considerations in performing total knee arthroplasty in patients with Blount disease or Blount-like deformity. Despite a challenging patient population in which to perform total knee arthroplasty, we show that it can be done with a low risk of complication and reasonable medium-term results,” said the study authors. “Surgeons should be prepared to address posteromedial tibial bony defects and consider constrained arthroplasty at the index procedure,” they said.
Suggested Reading
Natoli RM, Nypaver CM, Schiff AP, et al. Total knee arthroplasty in patients with blount disease or blount-like deformity. J Arthroplasty. 2015 Jul 11 [Epub ahead of print].
Total knee replacements can effectively treat degeneration caused by Blount disease, according to a study published online ahead of print July 11 in the Journal of Arthroplasty.
Middle-aged patients with Blount disease who underwent joint replacements on 1 or both knees were found to have stable knees, excellent range of motion, and no need for pain medications, according to the study conducted at Loyola University Medical Center in Illinois.
“With proper attention paid to technical details, patients with Blount or Blount-like deformity can undergo successful total knee arthroplasty,” said Harold Rees, MD, Assistant Professor of Reconstructive Surgery and Joint Replacement at Loyola University Chicago Stritch School of Medicine in Maywood, Illinois, and colleagues.
For the study, Dr. Rees and colleagues reviewed the records of 5 patients with Blount disease. Three patients had replacements on both knees and 2 patients had replacements on 1 knee. Four patients were African American and 4 were male. All were obese. The average age at the time of the knee replacements was 49.9. Patients were followed-up an average of 75.2 months after their knee replacements.
Mean proximal tibial metaphyseal-diaphyseal angle was 20.75 degrees. Each patient had substantial posteromedial tibial bony defects and 6 knees required extensive medial releases. Two knees required increased constraint at index procedure. One patient underwent bilateral revision surgery with rotating hinge prostheses.
The researchers used a scoring system, devised by the Knee Society, that combines clinical, functional, and satisfaction scores. The mean Knee Society score was 212.5, out of a maximum possible score of 255. Patients also were rated on the Western Ontario and McMaster Universities Osteoarthritis Index.
“The main purpose was to highlight surgical considerations in performing total knee arthroplasty in patients with Blount disease or Blount-like deformity. Despite a challenging patient population in which to perform total knee arthroplasty, we show that it can be done with a low risk of complication and reasonable medium-term results,” said the study authors. “Surgeons should be prepared to address posteromedial tibial bony defects and consider constrained arthroplasty at the index procedure,” they said.
Total knee replacements can effectively treat degeneration caused by Blount disease, according to a study published online ahead of print July 11 in the Journal of Arthroplasty.
Middle-aged patients with Blount disease who underwent joint replacements on 1 or both knees were found to have stable knees, excellent range of motion, and no need for pain medications, according to the study conducted at Loyola University Medical Center in Illinois.
“With proper attention paid to technical details, patients with Blount or Blount-like deformity can undergo successful total knee arthroplasty,” said Harold Rees, MD, Assistant Professor of Reconstructive Surgery and Joint Replacement at Loyola University Chicago Stritch School of Medicine in Maywood, Illinois, and colleagues.
For the study, Dr. Rees and colleagues reviewed the records of 5 patients with Blount disease. Three patients had replacements on both knees and 2 patients had replacements on 1 knee. Four patients were African American and 4 were male. All were obese. The average age at the time of the knee replacements was 49.9. Patients were followed-up an average of 75.2 months after their knee replacements.
Mean proximal tibial metaphyseal-diaphyseal angle was 20.75 degrees. Each patient had substantial posteromedial tibial bony defects and 6 knees required extensive medial releases. Two knees required increased constraint at index procedure. One patient underwent bilateral revision surgery with rotating hinge prostheses.
The researchers used a scoring system, devised by the Knee Society, that combines clinical, functional, and satisfaction scores. The mean Knee Society score was 212.5, out of a maximum possible score of 255. Patients also were rated on the Western Ontario and McMaster Universities Osteoarthritis Index.
“The main purpose was to highlight surgical considerations in performing total knee arthroplasty in patients with Blount disease or Blount-like deformity. Despite a challenging patient population in which to perform total knee arthroplasty, we show that it can be done with a low risk of complication and reasonable medium-term results,” said the study authors. “Surgeons should be prepared to address posteromedial tibial bony defects and consider constrained arthroplasty at the index procedure,” they said.
Suggested Reading
Natoli RM, Nypaver CM, Schiff AP, et al. Total knee arthroplasty in patients with blount disease or blount-like deformity. J Arthroplasty. 2015 Jul 11 [Epub ahead of print].
Suggested Reading
Natoli RM, Nypaver CM, Schiff AP, et al. Total knee arthroplasty in patients with blount disease or blount-like deformity. J Arthroplasty. 2015 Jul 11 [Epub ahead of print].
Safety of Tourniquet Use in Total Knee Arthroplasty in Patients With Radiographic Evidence of Vascular Calcifications
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
Tourniquets are often used in total knee arthroplasty (TKA) to improve visualization of structures, shorten operative time, reduce intraoperative bleeding, and improve cementing technique. Despite these advantages, controversy remains regarding the safety of tourniquet use. Tourniquets have been associated with nerve palsies, vascular injury, and muscle damage.1-5 Some have hypothesized they may cause venous stasis or direct endothelial damage that may develop into deep vein thrombosis (DVT). Abdel-Salam and Eyres6 found an increased incidence of postoperative wound complications and DVTs associated with tourniquet use.
Moreover, investigators have analyzed the role of tourniquets in populations at high risk for wound complications. DeLaurentis and colleagues7 performed a prospective and retrospective analysis of 1182 TKA patients, 24 (2%) of whom had preexisting peripheral vascular disease (PVD), defined as a history of arterial insufficiency, absent dorsalis pedis and/or absent posterior tibial pulsations, and arterial calcifications. A tourniquet was used in each case. Arterial complications occurred in 6 of the 24 patients with PVD. As expected, the authors found that a history of intermittent claudication, pain at rest, and arterial ulcers resulted in a high risk for vascular complications. Further studies have supported this finding and expanded the list of predisposing factors to include previous vascular surgery and absent and asymmetric pedal pulsations.7-11 Of particular concern to total joint arthroplasty surgeons was the finding by DeLaurentis and colleagues7 that patients with radiographic evidence of calcification of the distal superficial femoral artery and/or popliteal artery were at risk for arterial complications. This finding is also supported by other studies.8,11 In TKA, damage to arterial structures proximal to the surgical field could manifest as impaired postoperative wound healing or an ischemic limb. Wound healing depends on adequate blood flow to the healing tissue, and any damage to arterial or venous structures can theoretically compromise this process.
Added to vascular/wound complications as concerning complications in orthopedic surgery is venous thromboembolism (VTE). The role of tourniquets in the formation of VTEs is controversial. A tourniquet has the potential to increase the risk for DVT because of the stasis of venous blood in the lower limb or possible damage to calcified blood vessels. Callam and colleagues12 studied the connection between artery disease and chronic leg ulcers and found that half the patients diagnosed with peripheral artery disease also had stigmata of chronic venous insufficiency. Therefore, the entities can occur in tandem, and surgeons should keep this in mind.
Here we report on a study we conducted to determine whether tourniquet use in TKA in patients with preexisting radiographic evidence of vascular disease increases the risk for wound complications or VTE.
Patients and Methods
We retrospectively reviewed 461 consecutive primary TKAs (373 patients) performed between January 2007 and June 2012 by 2 attending orthopedic surgeons specializing in adult reconstruction. Medical records and operative reports of 583 patients were examined after receiving institutional review board approval. Of these patients, 373 (64%) had a minimum of 12-month follow-up data available. Twelve months was deemed long enough to discover wound complications or DVTs secondary to the index procedure. Most of these outcomes manifest within the first 3 months after surgery and certainly by 12 months. Follow-up longer than 12 months may become a confounder, as wound complications outside the acute to subacute postoperative window could be related to patients’ underlying PVD and not directly to tourniquet use during surgery. Patient demographics and comorbidities were recorded. Comorbidities were obtained from preoperative medical evaluations and surgeons’ preoperative evaluations. All patients had preoperative palpable dorsalis pedis and posterior tibialis arterial pulses. No patient required preoperative vascular studies based on preoperative examination or comorbidities. No patient had prior vascular bypass surgery or stenting.
TKA was performed in a nonlaminar flow, positive-pressure, high-efficiency particulate air-filtered room with sterile toga/surgical helmet systems. For all patients, a pneumatic thigh tourniquet was applied, and the patient was prepared and draped. After limb exsanguination using a rubber bandage, the limb was elevated and the tourniquet inflated to a pressure of 250 to 300 mm Hg. The tourniquet was released either just before closure or immediately after closure in all cases; it was always let down before placement of final bandages.
Prophylactic chemical anticoagulation consisting of warfarin, aspirin, or enoxaparin was used in all patients and continued for 4 to 6 weeks after surgery. All patients received mechanical DVT prophylaxis with sequential compression devices, and all were mobilized out of bed beginning either the day of surgery or the next day. All patients received perioperative intravenous antibiotics, with the preoperative dose given before tourniquet inflation and the last postoperative dose stopped within 24 hours of surgery.
All patients who had primary TKA underwent preoperative medical evaluation and optimization. The patient’s hospital course was monitored closely, and complications noted by the orthopedic team were documented. Follow-up documentation was retrospectively reviewed for evidence of wound complications or VTE. Wound complications were defined as cellulitis, delayed wound healing, wound dehiscence, and/or periprosthetic joint infection. In the case of VTE, physical examination findings were not sufficient for inclusion. Venous duplex ultrasonography demonstrating the clot was reviewed before inclusion.
Preoperative radiographs were examined for arterial calcification (Figure). We refer to calcification seen above the knee joint as proximal calcification and to calcification observed below the joint as distal calcification. Patients exhibited calcification proximally only, distally only, or both proximally and distally. The 373 patients were placed into 2 groups based on whether they had preoperative arterial calcification on plain radiography of the knee. One group (285 patients with no radiographic evidence of preoperative knee arterial calcification) underwent 365 TKAs, and the other group (88 patients with radiographic evidence of preoperative knee arterial calcification) underwent 96 TKAs.
A sample size calculation was performed to determine how many patients were needed in each group with 80% power and an α of 0.05. With an estimated difference in VTE/wound complication rate between the calcification and no-calcification groups of 12%, we needed to review 316 TKAs total. This 12% difference was based on study findings of a 25% complication rate in PVD patients who underwent tourniquet-assisted TKA, and the rate of VTE/wound complication after TKA in patients overall, which can be up to 12%.7,13,14 We exceeded minimal enrollment and had 461 TKAs. Descriptive statistics were reported, with means and ranges provided where appropriate. Independent t test was used to evaluate the differences in continuous data (age) between the groups. Univariate analysis (using Pearson χ2 and Fisher exact tests) and multivariate logistic regression analysis were used to evaluate the effects of categorical variables (sex, comorbidity, calcification [presence, absence], and location of calcification [proximal only, distal only, both]) on wound complication and VTE rates. All tests were 2-tailed and performed with a type I error rate of 0.05. Data analysis was performed with SPSS Version 19.0 (SPSS).
Results
Patient characteristics are summarized in Table 1. Of the 373 patients, 285 lacked calcification, and 88 had calcification. Mean age was 67.73 years (range, 24-92 years) for all patients, 65.99 years (range, 24-89 years) for the no-calcification group, and 74.32 years (range, 54-92 years) for the calcification group; the calcification group demonstrated a trend toward older age, but the difference was not significantly different (P = .07). Of the 373 patients, 156 (41.82%) were male: 110 in the no-calcification group (38.60%) and 46 in the calcification group (52.27%); sex was significantly (P = .002) different between groups, with more males in the calcification group.
Data on total preoperative comorbidities are summarized in Table 2. Hypertension, hyperlipidemia, diabetes, and coronary artery disease (CAD) were the most common comorbidities, and they were all significantly (P ≤ .05) increased in the calcification group.
No patients had reported arterial complications, such as arterial bleeding, aneurysm, intimal tears, or loss of distal pulses. Wound complication after TKA was detected in 3.04% of all cases (Table 3). Rate of DVT after TKA was 2.60% of all cases, and rate of pulmonary embolism after TKA was 2.17% of all cases. Of the 96 TKAs with preoperative radiographic evidence of calcification, 47 (48.96%) had proximal calcification only, 11 (11.46%) had distal calcification only, and 38 (39.58%) had both proximal and distal calcification (Table 4). There was no significant difference between the rate of wound complication or VTE based on location of vascular calcification.
Univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative wound complication (odds ratio [OR], 1.04; 95% confidence interval [CI], 0.28-3.80; P > .05) (Table 5). Location of arterial knee calcification also did not increase the risk for postoperative wound complication. In addition, univariate analysis demonstrated that presence of arterial knee calcification did not increase the risk for postoperative VTE (OR, 1.20; 95% CI, 0.43-3.36; P > .05 (Table 6).
Of the 14 wound complications, the most common infections were cellulitis (5/14 cases; 35.71%) and infected hardware that required component revision (5/14 cases; 35.71%). Mean time from TKA to infection was 137.93 days (range, 5-783 days). The most common organism grown in culture from the wound was Staphylococcus (5/14 cases; 35.71%).
Additional univariate statistical analysis revealed that presence of diabetes, hypertension, prior VTE, CAD, and male sex was linked to higher incidence of wound complication (P < .05) (Table 5). When multivariate analysis was performed, hypertension, prior VTE, and male sex remained significant (P < .05) (Table 5).
Discussion
TKA is a safe and effective procedure used to treat osteoarthritis of the knee and improve patients’ quality of life.15 About 700,000 TKAs are performed annually in the United States.16 Because of improvements in preventive medicine and medical technology, life expectancy is increasing, and TKAs are now being performed in higher numbers and in an older patient population. Over the next few decades, these developments will lead to more postoperative complications. It is projected that, by 2030, the need for TKAs in the United States will increase by 673% to 3.48 million.17 Postoperative complications are rare but unfortunately often lead to poor outcomes or even mortality.18 To help minimize the number of postoperative complications, we must understand the safety of tourniquet use in TKA. Other investigators have concluded that tourniquet use is unsafe in patients with preoperative vascular calcifications on plain radiographs.7,8,11 The present study, designed to elucidate whether preoperative evidence of knee arterial calcification may predispose TKA patients to postoperative wound complication or VTE, had some important findings.
In our study, wound complication and VTE occurred in a considerable number of patients after TKA. Despite exceeding the number of patients calculated by the power analysis, our population may have been inadequate to fully detect statistical significance. Thus, our conclusion of failing to reject the null hypothesis may have been because of sample size, a type II error. We found that, after primary TKA, 3.04% of patients developed wound complications and 4.77% VTE. According to the literature, the incidence of infection after primary TKA is between 0.5% and 12%, and that of VTE reported within 3 months after TKA is 1.3% to 10%.13,14 Although we had 100% VTE prophylaxis, meeting the standard of care, VTE after TKA remains a postoperative complication.19 This study also found that a considerable percentage of primary TKA patients (23.59%) had preoperative calcification of the knee arteries. To our knowledge, this study was the first to quantify the incidence of knee arterial calcification in patients who underwent TKA.
Preoperative calcification of the knee arteries in patients who underwent TKA did not increase the risk for wound complication, VTE, or arterial damage. These calcifications, however, do pose an increased systemic vascular risk.20 Calcification of the vascular wall predicts increased cardiovascular risk, independent of classical cardiovascular risk factors.3,18,21-24 Clinically, patients who have both diabetes and calcifications are at significant excess risk for total mortality, stroke mortality, and cardiovascular mortality, compared with patients with diabetes but without such calcifications. They also had a significantly higher incidence of coronary heart disease events, stroke events, and lower extremity amputations.25,26
All our patients underwent tourniquet-assisted TKA. Although previous studies have indicated that tourniquet use may increase arterial complications and wound complications or even limb loss in patients with calcified arteries, we did not find this link.7,27 Our population had no reported arterial complications related to tourniquet use. Other, smaller studies have had similar findings. Vandenbussche and colleagues28 prospectively studied 80 TKA cases randomized to tourniquet use or no tourniquet use and found no postoperative nerve palsies, wound infections, wound healing problems, or hematomas. Our study is also in accord with studies that have reported tourniquet use did not increase risk for DVT.29 Therefore, unlike earlier data, our data demonstrated that tourniquet use in patients with knee arterial calcification was safe.7,27,30,31
Patients with calcification were more likely to have the medical comorbidities of hypertension, diabetes, hyperlipidemia, and CAD. All these comorbidities are linked to the development of arterial calcification, or atherosclerotic occlusive disease.32,33 As life expectancy and the need for TKA increase, it is likely that a larger percentage of TKA patients will have preoperative radiographic evidence of knee arterial calcification. Although current dogma is that tourniquet-assisted TKA is contraindicated for patients with preoperative radiographic evidence of femoral-popliteal calcification, our study results showed that this calcification should not affect preoperative TKA planning for these patients.
We divided our patients into 3 categories: those with proximal calcification (above the joint line), those with distal calcification (below the joint line), and those with both proximal and distal calcification. Location of arterial calcification did not have an effect on their rates of postoperative wound complication or VTE. We hypothesized that patients with proximal calcification would be at increased risk for direct arterial injury and subsequent wound complication because the tourniquet is placed proximally. Previous research has indicated that arterial occlusion and subsequent wound complication can occur because of low blood flow stemming from tourniquet use.7 Further, intraoperative manipulation (flexing) of a knee with calcified vessels causes arterial complications after TKA because these vessels are less elastic than nonatheromatous vessels.31 However, we found no such effect. At the same time, having arterial calcification might also be an indication of venous disease in this location,12 which may be especially important for proximal calcifications. Proximal DVT more likely is a precursor to pulmonary embolic events than distal DVT is.31,34 However, we found no difference in VTE rates among the 3 arterial location groups, which is supported by studies that have found that tourniquet use does not increase DVT incidence.29,35-40
Risk for wound complications was higher in male patients and in patients with diabetes, prior VTE, hypertension, or CAD. This finding is important because, with the increasing age of patients who undergo TKA, those with serious medical comorbidities will continue to need and have this surgery.17 Diabetes may increase the rate of wound complication because patients with diabetes have poor microcirculation, poor collagen synthesis, and reduced wound strength.41 Malinzak and colleagues42 demonstrated that, compared with patients without diabetes, those with diabetes had a significantly higher risk for infection after TKA. Prior VTE, specifically DVT, may increase the rate of wound complication because after DVT the deep veins may be damaged and exhibit valvular dysfunction. Labropoulos and colleagues43 showed that DVT history was strongly associated with ulcer nonhealing. Perhaps hypertension has been overlooked as a risk factor for wound complication in TKA. No previous studies have assessed the link between hypertension and wound complications after TKA. However, a study of wound healing after total hip arthroplasty found that, compared with normotensive patients, hypertensive patients had delayed wound healing, putting them at higher risk for infection.44 In addition, we found that patients with CAD were at increased risk for wound complications—an unexpected finding, as CAD traditionally is not a risk factor for infection or poor wound healing. Recently, however, CAD was identified as an independent risk factor for surgical site infections in posterior lumbar–instrumented arthrodesis.45 The etiology of this association is unknown. Also, male patients were at increased risk for wound complication. Male sex has been implicated as an independent risk factor for development of surgical site infections and has been established as an important predisposing factor for periprosthetic joint infections.46
It is possible that patients who present with diabetes, VTE, hypertension, or CAD before TKA should have a consultation with a vascular surgeon or should have TKA performed without a tourniquet, but this conclusion cannot be considered definitive without a large prospective randomized trial or possibly registry data. Our data indicate that patients with these comorbidities have higher rates of wound complications irrespective of preoperative radiographic calcifications. On the basis of our study results, however, we certainly recommend that patients with these risk factors have preoperative medical optimization. Orthopedic surgeons should take a thorough history and perform a meticulous physical examination on these patients to look for evidence of PVD. We recommend that, if vascular claudication is elicited in the history, or if there is evidence of peripheral arterial disease—such as hair loss, skin discoloration, dystrophic nail changes, or absent or unequal peripheral pulses—the ankle-brachial index test should be performed. If the index value is less than 0.9, then a preoperative vascular surgery consultation should be obtained.
This study had some weaknesses. First, it was retrospective, so it is possible that some wound or VTE complications were not reported and thus not found in the paper charts or electronic medical records. Some patients may have had VTE diagnostic scans at other hospitals, and their results may not have been recorded across databases. Moreover, some patients may have seen wound specialists for wound infections or wound healing problems, and these may not have been reported to the orthopedic surgeons. Second, though our patient population was not small, it may not have been of adequate size to fully detect statistical significance. We met our enrollment numbers based on our sample size calculations from an a priori power analysis; however, we still draw conclusions with the possibility of committing a type II error in mind by failing to reject the null hypothesis when in reality a statistically significant difference does exist. Third, none of our consecutive patients carried the preoperative diagnosis of PVD, and none had preoperative vascular surgery. Therefore, though calcifications were noted on radiographs, clinically our patients were asymptomatic with respect to vascular health. Last, the 2 groups were not randomized. All patients underwent tourniquet-assisted TKA.
Conclusion
To our knowledge, this is the largest study to examine the effect of preoperative knee arterial calcification on wound complication and VTE after tourniquet-assisted TKA. Contrary to previously published recommendations, we conclude that TKA can be safely performed with a tourniquet in the presence of preoperative radiographic evidence of such calcification. However, we recommend that patients with diabetes, hypertension, CAD, or prior VTE undergo an appropriate physical examination to elicit any signs or symptoms of vascular disease. If before surgery there is any question of vascular competence, a vascular surgeon should be consulted.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
1. Guanche CA. Tourniquet-induced tibial nerve palsy complicating anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(5):620-622.
2. Irvine GB, Chan RN. Arterial calcification and tourniquets. Lancet. 1986;2(8517):1217.
3. Patterson S, Klenerman L. The effect of pneumatic tourniquets on the ultrastructure of skeletal muscle. J Bone Joint Surg Br. 1979;61(2):178-183.
4. Rorabeck CH, Kennedy JC. Tourniquet-induced nerve ischemia complicating knee ligament surgery. Am J Sports Med. 1980;8(2):98-102.
5. Shenton DW, Spitzer SA, Mulrennan BM. Tourniquet-induced rhabdomyolysis. A case report. J Bone Joint Surg Am. 1990;72(9):1405-1406.
6. Abdel-Salam A, Eyres KS. Effects of tourniquet during total knee arthroplasty. A prospective randomised study. J Bone Joint Surg Br. 1995;77(2):250-253.
7. DeLaurentis DA, Levitsky KA, Booth RE, et al. Arterial and ischemic aspects of total knee arthroplasty. Am J Surg. 1992;164(3):237-240.
8. Holmberg A, Milbrink J, Bergqvist D. Arterial complications and knee arthroplasty. Acta Orthop Scand. 1996;67(1):75-8.
9. Hozack WJ, Cole PA, Gardner R, Corces A. Popliteal aneurysm after total knee arthroplasty. Case reports and review of the literature. J Arthroplasty. 1990;5(4):301-305.
10. Kumar SN, Chapman JA, Rawlins I. Vascular injuries after total knee arthroplasty: a review of the problem with special reference to the possible effects of the tourniquet. J Arthroplasty. 1998;13(2):211-216.
11. Rush JH, Vidovich JD, Johanson MA. Arterial complications and total knee arthroplasty. The Australian experience. J Bone Joint Surg Br. 1987;69(3):400-402.
12. Callam MJ, Harper DR, Dale JJ, Ruckley CV. Arterial disease in chronic leg ulceration: an underestimated hazard? Lothian and Forth Valley Leg Ulcer Study. Br Med J (Clin Res Ed). 1987;294(6577):929-931.
13. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86(5):688-691.
14. Geerts WH, Bergqvist D, Pinco G, et al. Prevention of venous thromboembolism. Chest. 2008;133(6 suppl):381S-453S.
15. Pulido L, Parvizi J, Macgibeny M, et al. In hospital complications after total joint arthroplasty. J Arthroplasty. 2008;23(6 Suppl 1):139-145.
16. Arthritis: data and statistics. Centers for Disease Control and Prevention website. http://www.cdc.gov/arthritis/data_statistics.htm. Updated March 11, 2015. Accessed July 27, 2015.
17. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
18. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710-1715.
19. Warwick D. Prevention of venous thromboembolism in total knee and hip replacement. Circulation. 2012;125(17):2151-2155.
20. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5(1):185-197.
21. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46(1):158-165.
22. Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283(21):2810-2815.
23. Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826-833.
24. Taylor AJ, Bindeman J, Feuerstein I, Cao F, Brazaitis M, O’Malley PG. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46(5):807-814.
25. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978-983.
26. Niskanen L, Siitonen O, Suhonen M, Uusitupa MI. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17(11):1252-1256.
27. Smith DE, McGraw RW, Taylor DC, et al. Arterial complications and total knee arthroplasty. J Am Acad Orthop Surg. 2001;9(4):253-257.
28. Vandenbussche E, Duranthon L, Couturier M, Pidhorz L, Augereau B. The effect of tourniquet use in total knee arthroplasty. Int Orthop. 2002;26(5):306-309.
29. Fukunda A, Hasegawa M, Kato K, Shi D, Sudo A, Uchida A. Effect of tourniquet application on deep vein thrombosis after total knee thrombosis. Arch Orthop Trauma Surg. 2007;127(8):671-675.
30. Butt U, Samuel R, Sahu A, Butt IS, Johnson DS, Turner PG. Arterial injury in total knee arthroplasty. J Arthroplasty. 2010;25(8):1311-1318.
31. Langkamer VG. Local vascular complications after knee replacement: a review with illustrative case reports. Knee. 2001;8(4):259-264.
32. Hussein A, Uno K, Wolski K, et al. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220-1225.
33. Ouriel K. Peripheral arterial disease. Lancet. 2001;358(9289):1257-1264.
34. Monreal M, Rufz J, Olazabal A, Arias A, Roca J. Deep venous thrombosis and the risk of pulmonary embolism. Chest. 1992;102(3):677-681.
35. Angus PD, Nakielny R, Goodrum DT. The pneumatic tourniquet and deep venous thrombosis. J Bone Joint Surg Br. 1983;65(3):336-339.
36. Fahmy NR, Patel DG. Hemostatic changes and postoperative deep-vein thrombosis associated with use of a pneumatic tourniquet. J Bone Joint Surg Am. 1981;63(3):461-465.
37. Harvey EJ, Leclerc J, Brooks CE, Burke DL. Effect of tourniquet use on blood loss and incidence of deep vein thrombosis in total knee arthroplasty. J Arthroplasty. 1997;12(3):291-296.
38. Simon MA, Mass DP, Zarins CK, Bidani N, Gudas CJ, Metz CE. The effect of a thigh tourniquet on the incidence of deep venous thrombosis after operations on the fore part of the foot. J Bone Joint Surg Am. 1982;64(2):188-191.
39. Stulberg BN, Insall JN, Williams GW, Ghelman B. Deep-vein thrombosis following total knee replacement. An analysis of six hundred and thirty-eight arthroplasties. J Bone Joint Surg Am. 1984;66(2):194-201.
40. Wakankar HM, Nicholl JE, Koka R, D’Arcy JC. The tourniquet in total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Br. 1999;81(1):30-33.
41. Vince K, Chivas D, Droll K. Wound complications after total knee arthroplasty. J Arthroplasty. 2007;22(4 Suppl 1):39-44.
42. Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84-88.
43. Labropoulos N, Wang E, Lanier S, Khan SU. Factors associated with poor healing and recurrence of venous ulceration. Plast Reconstr Surg. 2011;129(1):179-186.
44. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
45. Koutsoumbelis S, Hughes AP, Girardi FP, et al. Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am. 2001;93(17):1627-1633.
46. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
Recorrection Osteotomies and Total Knee Arthroplasties After Failed Bilateral High Tibial Osteotomies
High tibial osteotomy has proved successful in treating unicompartmental arthritis in young, active patients.1-3 However, this procedure fails over time because the other compartments deteriorate.4 The next step is conversion of the osteotomy to total knee arthroplasty (TKA). Conversion results vary, with several authors reporting poor outcomes5-9 and others reporting outcomes equal to those of primary TKA.10-14
The long-term success of TKA depends on proper restoration of the mechanical axis and soft-tissue balancing.15 Preexisting extra-articular deformity may adversely affect outcomes. A deformity of more than 15° may make it difficult to obtain intra-articular correction of an extra-articular deformity through soft-tissue balancing alone.16
In this article, we report the unique case of a patient whose bilateral high tibial osteotomies failed because of excessive extra-articular deformity. TKAs were performed consecutively, in 2 separate settings. Each TKA was combined with a recorrection tibial osteotomy in a single operation, allowing for re-creation of normal knee alignment with ligament balance. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man (weight, 250 pounds; body mass index, 30) underwent staged bilateral medial opening wedge osteotomies using distraction osteogenesis. A uniplanar external fixator was used for fixation on each knee. Before surgery, anatomical axis was 2° (right knee) and –1° (left knee) (Figure 1A), and tibial slope was 9° (right) and 8° (left) (Figures 1B, 1C). The procedures were performed 10 months apart. After surgery, anatomical alignment was 17° valgus (right knee) and 12° valgus (left knee) (Figure 2), and tibial slope was 20° (right) and 13° (left).
The patient received mild relief of his arthritis symptoms. Fifty-six months after the index operation, he decided to undergo conversion of the right high tibial osteotomy to TKA because of progressive painful arthritis of the knee. Excessive valgus alignment caused by the initial osteotomy raised concerns about being able to correct the extra-articular deformity intra-articularly while maintaining kinematic ligament balance. For this reason, a recorrection osteotomy was performed concurrently with the TKA. A posterior cruciate ligament–retaining (PCL-retaining) knee design (NexGen, Zimmer) was selected.
The procedure began with bone cuts for the TKA. Initial cuts were made on the femur. The tibial cut was made in valgus corresponding to the preoperative valgus deformity. The tibial recorrection osteotomy was made at the level of the original osteotomy site. A stemmed tibial component was used to cross the osteotomy site, correcting the valgus deformity and providing stability at the osteotomy site. A 3.5-mm locking compression T-plate (Synthes) was medially placed to prevent loss of correction and control rotation of the osteotomy during healing. The patient began range of motion on postoperative day 1. Continuous passive motion was not used. Protective weight-bearing continued for 6 weeks. After 6 weeks, and once there was radiograph evidence of healing at the osteotomy site, full weight-bearing was allowed.
After 4 months, the patient decided to undergo a similar procedure on the left knee. Postoperative rehabilitation was the same. A year after the bilateral TKAs, the patient maintained a Knee Society Score of 95 and a functional score of 90. After surgery, anatomical alignment was 6° (right knee) and 3° (left knee) (Figure 3), and tibial slope was 6° (right) and 7° (left) (Figures 4A, 4B). In each knee, the PCL was preserved with ligament balance.
Discussion
Clinical outcomes of TKA after high tibial osteotomy vary. Windsor and colleagues9 reported that knee arthroplasties after tibial osteotomy were less successful than primary TKAs. In small studies, both Staeheli and colleagues17 and Katz and colleagues5 found that TKA outcomes after osteotomy were satisfactory compared with outcomes of primary TKA without previous osteotomy. A meta-analysis by Ramappa and colleagues18 showed no difference in outcomes between TKAs with and without previous osteotomy. In addition, there were no differences in outcomes between TKAs performed after opening wedge versus closing wedge osteotomies.19
An arthritic knee compartment is unloaded when a high tibial osteotomy produces an extra-articular deformity. Neyret and colleagues7 reported difficulties in correcting angulations of 9° or more through soft-tissue release. Cameron and Welsh16 suggested pre–knee arthroplasty correction of the extra-articular deformity for malalignments of more than 15°. In cases of severe malalignment produced by an osteotomy, Katz and colleagues5 also suggested that a second osteotomy be performed to correct alignment before TKA.
For TKA after high tibial osteotomy, a neutral plateau resection removes more bone medially than laterally, creating medial laxity. Without correction of the tibial deformity, lateral release (or, as Krackow and Holtgrewe20 advocated, medial advancement) is required for ligament stability. Both technically demanding options may not provide complete stability throughout the arc of motion. In addition, neither corrects for rotational or sagittal deformities (the concern with correcting an extra-articular deformity with intra-articular ligament balancing).
Another option is valgus tibial resection, which maintains native ligament balance at the cost of excessive valgus alignment. In the low-demand patient, a condylar constrained implant provides a means of correcting the malalignment with knee stability.8,13,17 The increased restraint produces greater forces at the implant–bone interface and may risk early loosening.
The case presented here represents a unique situation of failed bilateral high tibial osteotomies with excessive valgus malalignment. In a similar situation, Papagelopoulos and colleagues21 suggested correcting fracture deformities before or at time of knee arthroplasty. Yoshina and colleagues22 reported using a stemmed tibial component with TKA in treating nonunion of a high tibial osteotomy. As mentioned, Katz and colleagues5 and Neyret and colleagues7 suggested preoperative correction of the osteotomy in cases of severe malalignment. Others have suggested combining recorrection osteotomy and knee arthroplasty in either consecutive operations or a single operation.23-26 Wolff and colleagues27 and Uchinou and colleagues28 described recorrection osteotomy performed concurrent with TKA. The present article is the first to report a case involving concurrent bilateral recorrection osteotomy and TKA.
In one setting, the recorrection osteotomy is performed after the bony cuts are made for the TKA. The initial tibial plateau resection is performed in valgus at the same degree of malalignment as the osteotomy. This allows the plane of the tibial resection to parallel the floor once the recorrection is finished. With use of a tibial stem crossing the osteotomy site and a derotation plate, adequate fixation of the osteotomy is obtained. The recorrection osteotomy prevents the ligaments from overlengthening, allows the native ligament balance of the knee, and preserves the PCL—which lets the surgeon obtain ligament balance for the TKA throughout the arc of motion, avoiding midstance instabilities and achieving knee alignment rotationally and in the coronal and sagittal planes.
The TKA used in the present case was a PCL-retaining design. Both posterior-stabilized and PCL-retaining designs are reasonable options for use in combination with recorrection osteotomy. A stemmed tibial component is needed to cross the osteotomy site. In our patient’s case, use of a PCL-retaining design was based on surgeon preference and experience.
Patella infera has been noted as a problem in studies on converting high tibial osteotomy to TKA.9,12,29 A postulated cause is scarring of the infrapatellar tendon after high tibial osteotomy. In addition, a higher incidence of lateral retinacular release has been identified.9-11 Patella infera did not occur in either knee in the present case, and lateral release was not required.
Our patient’s lateral radiographs (Figures 4A, 4B) showed persistence of the osteotomy plane anterior to the tibia. The osteotomy healed posteriorly but not completely anteriorly. This raises the issue of risk for nonunion when recorrection osteotomy is performed with TKA. Use of a stemmed tibial implant with a derotation plate provides the benefit of intramedullary fixation for the recorrection osteotomy. If the recorrection osteotomy were performed in a separate setting before TKA, plate fixation would be the primary fixation option. Should nonunion occur at the recorrection osteotomy site, revision of the tibial plateau with a new stemmed implant would be required in combination with plate fixation. Madelaine and colleagues30 reported on a series of 15 severe varus knees treated with both osteotomy and TKA. Two nonunions occurred. Fixation was a staple in one case and a cement wedge in the other. Risk for nonunion may be reduced with the combination of stemmed tibial implant and internal fixation with a derotation plate. Protective weight-bearing is recommended for the first 6 postoperative weeks.
Conclusion
Ligament imbalances produced by high tibial osteotomy and exacerbated by conversion to TKA are difficult to address. In this report, we have described successful single-stage high tibial osteotomy recorrection and TKA performed bilaterally in separate settings. With use of a stemmed tibial component and a derotation plate, solid fixation was obtained with an excellent clinical outcome. The malalignment was corrected while ligament balance was maintained for a PCL-retaining TKA design.
1. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
2. Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75(2):196-201.
3. Rinonapoli E, Mancini GB, Corvaglia A, Musiello S. Tibial osteotomy for varus gonarthrosis. A 10- to 21-year followup study. Clin Orthop Relat Res. 1998;(353):185-193.
4. Ritter MA, Fechtman RA. Proximal tibial osteotomy. A survivorship analysis. J Arthroplasty. 1988;3(4):309-311.
5. Katz MM, Hungerford DS, Krackow KA, Lennox DW. Results of total knee arthroplasty after failed proximal tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1987;69(2):225-233.
6. Mont MA, Antonaides S, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. A comparison with a matched group. Clin Orthop Relat Res. 1994;299:125-130.
7. Neyret P, Deroche P, Deschamps G, Dejour H. Total knee replacement after valgus tibial osteotomy. Technical problems [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1992;78(7):438-448.
8. Parvizi J, Hanssen AD, Spangehl MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
9. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
10. Amendola A, Rorabeck CH, Bourne RB, Apyan PM. Total knee arthroplasty following high tibial osteotomy for osteoarthritis. J Arthroplasty. 1989;(4 suppl):S11-S17.
11. Kazakos KJ, Chatzipapas C, Verettas D, Galanis V, Xarchas KC, Psillakis I. Mid-term results of total knee arthroplasty after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128(2):167-173.
12. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
13. Niinimaki T, Eskelinen A, Ohtonen P, Puhto AP, Mann BS, Leppilahti J. Total knee arthroplasty after high tibial osteotomy: a registry-based case–control study of 1,036 knees. Arch Orthop Trauma Surg. 2014;134(1):73-77.
14. van Raaij TM, Reijman M, Furlan AD, Verhaar JA. Total knee arthroplasty after high tibial osteotomy. A systematic review. BMC Musculoskelet Disord. 2009;10:88-98.
15. Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59(1):77-79.
16. Cameron HU, Welsh RP. Potential complications of total knee replacement following tibial osteotomy. Orthop Rev. 1988;17(1):39-43.
17. Staeheli JW, Cass JR, Morrey BF. Condylar total knee arthroplasty after failed proximal tibial osteotomy. J Bone Joint Surg Am. 1987;69(1):28-31.
18. Ramappa M, Anand S, Jennings A. Total knee replacement following high tibial osteotomy versus total knee replacement without high tibial osteotomy: a systematic review and meta analysis. Arch Orthop Trauma Surg. 2013;133(11):1587-1593.
19. Preston S, Howard J, Naudie D, Somerville L, McAuley J. Total knee arthroplasty after high tibial osteotomy: no differences between medial and lateral osteotomy approaches. Clin Orthop Relat Res. 2014;472(1):105-110.
20. Krackow KA, Holtgrewe JL. Experience with a new technique for managing severely overcorrected valgus high tibial osteotomy at total knee arthroplasty. Clin Orthop Relat Res. 1990;(258):213-224.
21. Papagelopoulos PJ, Karachalios T, Themistocleous GS, Papadopoulos ECh, Savvidou OD, Rand JA. Total knee arthroplasty in patients with pre-existing fracture deformity. Orthopaedics. 2007;30(5):373-378.
22. Yoshina N, Takai S, Watanabe Y, Nakamura S, Kubo T. Total knee arthroplasty with long stem for treatment of nonunion after high tibial osteotomy. J Arthroplasty. 2004;19(4):528-531.
23. Mont MA, Alexander N, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. Orthop Clin North Am. 1994;25(3):515-525.
24. Scott WN. Insall & Scott’s Surgery of the Knee. Vol 1. 4th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2006.
25. Gill T, Schemitsch EH, Brick GW, Thornhill TS. Revision total knee arthroplasty after failed unicompartmental knee arthroplasty or high tibial osteotomy. Clin Orthop Relat Res. 1995;(321):10-18.
26. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68(7):1035-1040.
27. Wolff AM, Hungerford DS, Pepe CL. The effect of extraarticular varus and valgus deformity on total knee arthroplasty. Clin Orthop Relat Res. 1994;(271):35-51.
28. Uchinou S, Yano H, Shimizu K, Masumi S. A severely overcorrected high tibial osteotomy: revision by osteotomy and a long stem component. Acta Orthop Scand. 1996;67(2):193-194.
29. Noda T, Yasuda S, Nagano K, Takahara Y, Namba Y, Inoue H. Clinico-radiological study of total knee arthroplasty after high tibial osteotomy. J Orthop Sci. 2000;5(1):25-36.
30. Madelaine A, Villa V, Yela C, et al. Results and complications of single-stage total knee arthroplasty and high tibial osteotomy. Int Orthop. 2014;38(10):2091-2098.
High tibial osteotomy has proved successful in treating unicompartmental arthritis in young, active patients.1-3 However, this procedure fails over time because the other compartments deteriorate.4 The next step is conversion of the osteotomy to total knee arthroplasty (TKA). Conversion results vary, with several authors reporting poor outcomes5-9 and others reporting outcomes equal to those of primary TKA.10-14
The long-term success of TKA depends on proper restoration of the mechanical axis and soft-tissue balancing.15 Preexisting extra-articular deformity may adversely affect outcomes. A deformity of more than 15° may make it difficult to obtain intra-articular correction of an extra-articular deformity through soft-tissue balancing alone.16
In this article, we report the unique case of a patient whose bilateral high tibial osteotomies failed because of excessive extra-articular deformity. TKAs were performed consecutively, in 2 separate settings. Each TKA was combined with a recorrection tibial osteotomy in a single operation, allowing for re-creation of normal knee alignment with ligament balance. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man (weight, 250 pounds; body mass index, 30) underwent staged bilateral medial opening wedge osteotomies using distraction osteogenesis. A uniplanar external fixator was used for fixation on each knee. Before surgery, anatomical axis was 2° (right knee) and –1° (left knee) (Figure 1A), and tibial slope was 9° (right) and 8° (left) (Figures 1B, 1C). The procedures were performed 10 months apart. After surgery, anatomical alignment was 17° valgus (right knee) and 12° valgus (left knee) (Figure 2), and tibial slope was 20° (right) and 13° (left).
The patient received mild relief of his arthritis symptoms. Fifty-six months after the index operation, he decided to undergo conversion of the right high tibial osteotomy to TKA because of progressive painful arthritis of the knee. Excessive valgus alignment caused by the initial osteotomy raised concerns about being able to correct the extra-articular deformity intra-articularly while maintaining kinematic ligament balance. For this reason, a recorrection osteotomy was performed concurrently with the TKA. A posterior cruciate ligament–retaining (PCL-retaining) knee design (NexGen, Zimmer) was selected.
The procedure began with bone cuts for the TKA. Initial cuts were made on the femur. The tibial cut was made in valgus corresponding to the preoperative valgus deformity. The tibial recorrection osteotomy was made at the level of the original osteotomy site. A stemmed tibial component was used to cross the osteotomy site, correcting the valgus deformity and providing stability at the osteotomy site. A 3.5-mm locking compression T-plate (Synthes) was medially placed to prevent loss of correction and control rotation of the osteotomy during healing. The patient began range of motion on postoperative day 1. Continuous passive motion was not used. Protective weight-bearing continued for 6 weeks. After 6 weeks, and once there was radiograph evidence of healing at the osteotomy site, full weight-bearing was allowed.
After 4 months, the patient decided to undergo a similar procedure on the left knee. Postoperative rehabilitation was the same. A year after the bilateral TKAs, the patient maintained a Knee Society Score of 95 and a functional score of 90. After surgery, anatomical alignment was 6° (right knee) and 3° (left knee) (Figure 3), and tibial slope was 6° (right) and 7° (left) (Figures 4A, 4B). In each knee, the PCL was preserved with ligament balance.
Discussion
Clinical outcomes of TKA after high tibial osteotomy vary. Windsor and colleagues9 reported that knee arthroplasties after tibial osteotomy were less successful than primary TKAs. In small studies, both Staeheli and colleagues17 and Katz and colleagues5 found that TKA outcomes after osteotomy were satisfactory compared with outcomes of primary TKA without previous osteotomy. A meta-analysis by Ramappa and colleagues18 showed no difference in outcomes between TKAs with and without previous osteotomy. In addition, there were no differences in outcomes between TKAs performed after opening wedge versus closing wedge osteotomies.19
An arthritic knee compartment is unloaded when a high tibial osteotomy produces an extra-articular deformity. Neyret and colleagues7 reported difficulties in correcting angulations of 9° or more through soft-tissue release. Cameron and Welsh16 suggested pre–knee arthroplasty correction of the extra-articular deformity for malalignments of more than 15°. In cases of severe malalignment produced by an osteotomy, Katz and colleagues5 also suggested that a second osteotomy be performed to correct alignment before TKA.
For TKA after high tibial osteotomy, a neutral plateau resection removes more bone medially than laterally, creating medial laxity. Without correction of the tibial deformity, lateral release (or, as Krackow and Holtgrewe20 advocated, medial advancement) is required for ligament stability. Both technically demanding options may not provide complete stability throughout the arc of motion. In addition, neither corrects for rotational or sagittal deformities (the concern with correcting an extra-articular deformity with intra-articular ligament balancing).
Another option is valgus tibial resection, which maintains native ligament balance at the cost of excessive valgus alignment. In the low-demand patient, a condylar constrained implant provides a means of correcting the malalignment with knee stability.8,13,17 The increased restraint produces greater forces at the implant–bone interface and may risk early loosening.
The case presented here represents a unique situation of failed bilateral high tibial osteotomies with excessive valgus malalignment. In a similar situation, Papagelopoulos and colleagues21 suggested correcting fracture deformities before or at time of knee arthroplasty. Yoshina and colleagues22 reported using a stemmed tibial component with TKA in treating nonunion of a high tibial osteotomy. As mentioned, Katz and colleagues5 and Neyret and colleagues7 suggested preoperative correction of the osteotomy in cases of severe malalignment. Others have suggested combining recorrection osteotomy and knee arthroplasty in either consecutive operations or a single operation.23-26 Wolff and colleagues27 and Uchinou and colleagues28 described recorrection osteotomy performed concurrent with TKA. The present article is the first to report a case involving concurrent bilateral recorrection osteotomy and TKA.
In one setting, the recorrection osteotomy is performed after the bony cuts are made for the TKA. The initial tibial plateau resection is performed in valgus at the same degree of malalignment as the osteotomy. This allows the plane of the tibial resection to parallel the floor once the recorrection is finished. With use of a tibial stem crossing the osteotomy site and a derotation plate, adequate fixation of the osteotomy is obtained. The recorrection osteotomy prevents the ligaments from overlengthening, allows the native ligament balance of the knee, and preserves the PCL—which lets the surgeon obtain ligament balance for the TKA throughout the arc of motion, avoiding midstance instabilities and achieving knee alignment rotationally and in the coronal and sagittal planes.
The TKA used in the present case was a PCL-retaining design. Both posterior-stabilized and PCL-retaining designs are reasonable options for use in combination with recorrection osteotomy. A stemmed tibial component is needed to cross the osteotomy site. In our patient’s case, use of a PCL-retaining design was based on surgeon preference and experience.
Patella infera has been noted as a problem in studies on converting high tibial osteotomy to TKA.9,12,29 A postulated cause is scarring of the infrapatellar tendon after high tibial osteotomy. In addition, a higher incidence of lateral retinacular release has been identified.9-11 Patella infera did not occur in either knee in the present case, and lateral release was not required.
Our patient’s lateral radiographs (Figures 4A, 4B) showed persistence of the osteotomy plane anterior to the tibia. The osteotomy healed posteriorly but not completely anteriorly. This raises the issue of risk for nonunion when recorrection osteotomy is performed with TKA. Use of a stemmed tibial implant with a derotation plate provides the benefit of intramedullary fixation for the recorrection osteotomy. If the recorrection osteotomy were performed in a separate setting before TKA, plate fixation would be the primary fixation option. Should nonunion occur at the recorrection osteotomy site, revision of the tibial plateau with a new stemmed implant would be required in combination with plate fixation. Madelaine and colleagues30 reported on a series of 15 severe varus knees treated with both osteotomy and TKA. Two nonunions occurred. Fixation was a staple in one case and a cement wedge in the other. Risk for nonunion may be reduced with the combination of stemmed tibial implant and internal fixation with a derotation plate. Protective weight-bearing is recommended for the first 6 postoperative weeks.
Conclusion
Ligament imbalances produced by high tibial osteotomy and exacerbated by conversion to TKA are difficult to address. In this report, we have described successful single-stage high tibial osteotomy recorrection and TKA performed bilaterally in separate settings. With use of a stemmed tibial component and a derotation plate, solid fixation was obtained with an excellent clinical outcome. The malalignment was corrected while ligament balance was maintained for a PCL-retaining TKA design.
High tibial osteotomy has proved successful in treating unicompartmental arthritis in young, active patients.1-3 However, this procedure fails over time because the other compartments deteriorate.4 The next step is conversion of the osteotomy to total knee arthroplasty (TKA). Conversion results vary, with several authors reporting poor outcomes5-9 and others reporting outcomes equal to those of primary TKA.10-14
The long-term success of TKA depends on proper restoration of the mechanical axis and soft-tissue balancing.15 Preexisting extra-articular deformity may adversely affect outcomes. A deformity of more than 15° may make it difficult to obtain intra-articular correction of an extra-articular deformity through soft-tissue balancing alone.16
In this article, we report the unique case of a patient whose bilateral high tibial osteotomies failed because of excessive extra-articular deformity. TKAs were performed consecutively, in 2 separate settings. Each TKA was combined with a recorrection tibial osteotomy in a single operation, allowing for re-creation of normal knee alignment with ligament balance. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man (weight, 250 pounds; body mass index, 30) underwent staged bilateral medial opening wedge osteotomies using distraction osteogenesis. A uniplanar external fixator was used for fixation on each knee. Before surgery, anatomical axis was 2° (right knee) and –1° (left knee) (Figure 1A), and tibial slope was 9° (right) and 8° (left) (Figures 1B, 1C). The procedures were performed 10 months apart. After surgery, anatomical alignment was 17° valgus (right knee) and 12° valgus (left knee) (Figure 2), and tibial slope was 20° (right) and 13° (left).
The patient received mild relief of his arthritis symptoms. Fifty-six months after the index operation, he decided to undergo conversion of the right high tibial osteotomy to TKA because of progressive painful arthritis of the knee. Excessive valgus alignment caused by the initial osteotomy raised concerns about being able to correct the extra-articular deformity intra-articularly while maintaining kinematic ligament balance. For this reason, a recorrection osteotomy was performed concurrently with the TKA. A posterior cruciate ligament–retaining (PCL-retaining) knee design (NexGen, Zimmer) was selected.
The procedure began with bone cuts for the TKA. Initial cuts were made on the femur. The tibial cut was made in valgus corresponding to the preoperative valgus deformity. The tibial recorrection osteotomy was made at the level of the original osteotomy site. A stemmed tibial component was used to cross the osteotomy site, correcting the valgus deformity and providing stability at the osteotomy site. A 3.5-mm locking compression T-plate (Synthes) was medially placed to prevent loss of correction and control rotation of the osteotomy during healing. The patient began range of motion on postoperative day 1. Continuous passive motion was not used. Protective weight-bearing continued for 6 weeks. After 6 weeks, and once there was radiograph evidence of healing at the osteotomy site, full weight-bearing was allowed.
After 4 months, the patient decided to undergo a similar procedure on the left knee. Postoperative rehabilitation was the same. A year after the bilateral TKAs, the patient maintained a Knee Society Score of 95 and a functional score of 90. After surgery, anatomical alignment was 6° (right knee) and 3° (left knee) (Figure 3), and tibial slope was 6° (right) and 7° (left) (Figures 4A, 4B). In each knee, the PCL was preserved with ligament balance.
Discussion
Clinical outcomes of TKA after high tibial osteotomy vary. Windsor and colleagues9 reported that knee arthroplasties after tibial osteotomy were less successful than primary TKAs. In small studies, both Staeheli and colleagues17 and Katz and colleagues5 found that TKA outcomes after osteotomy were satisfactory compared with outcomes of primary TKA without previous osteotomy. A meta-analysis by Ramappa and colleagues18 showed no difference in outcomes between TKAs with and without previous osteotomy. In addition, there were no differences in outcomes between TKAs performed after opening wedge versus closing wedge osteotomies.19
An arthritic knee compartment is unloaded when a high tibial osteotomy produces an extra-articular deformity. Neyret and colleagues7 reported difficulties in correcting angulations of 9° or more through soft-tissue release. Cameron and Welsh16 suggested pre–knee arthroplasty correction of the extra-articular deformity for malalignments of more than 15°. In cases of severe malalignment produced by an osteotomy, Katz and colleagues5 also suggested that a second osteotomy be performed to correct alignment before TKA.
For TKA after high tibial osteotomy, a neutral plateau resection removes more bone medially than laterally, creating medial laxity. Without correction of the tibial deformity, lateral release (or, as Krackow and Holtgrewe20 advocated, medial advancement) is required for ligament stability. Both technically demanding options may not provide complete stability throughout the arc of motion. In addition, neither corrects for rotational or sagittal deformities (the concern with correcting an extra-articular deformity with intra-articular ligament balancing).
Another option is valgus tibial resection, which maintains native ligament balance at the cost of excessive valgus alignment. In the low-demand patient, a condylar constrained implant provides a means of correcting the malalignment with knee stability.8,13,17 The increased restraint produces greater forces at the implant–bone interface and may risk early loosening.
The case presented here represents a unique situation of failed bilateral high tibial osteotomies with excessive valgus malalignment. In a similar situation, Papagelopoulos and colleagues21 suggested correcting fracture deformities before or at time of knee arthroplasty. Yoshina and colleagues22 reported using a stemmed tibial component with TKA in treating nonunion of a high tibial osteotomy. As mentioned, Katz and colleagues5 and Neyret and colleagues7 suggested preoperative correction of the osteotomy in cases of severe malalignment. Others have suggested combining recorrection osteotomy and knee arthroplasty in either consecutive operations or a single operation.23-26 Wolff and colleagues27 and Uchinou and colleagues28 described recorrection osteotomy performed concurrent with TKA. The present article is the first to report a case involving concurrent bilateral recorrection osteotomy and TKA.
In one setting, the recorrection osteotomy is performed after the bony cuts are made for the TKA. The initial tibial plateau resection is performed in valgus at the same degree of malalignment as the osteotomy. This allows the plane of the tibial resection to parallel the floor once the recorrection is finished. With use of a tibial stem crossing the osteotomy site and a derotation plate, adequate fixation of the osteotomy is obtained. The recorrection osteotomy prevents the ligaments from overlengthening, allows the native ligament balance of the knee, and preserves the PCL—which lets the surgeon obtain ligament balance for the TKA throughout the arc of motion, avoiding midstance instabilities and achieving knee alignment rotationally and in the coronal and sagittal planes.
The TKA used in the present case was a PCL-retaining design. Both posterior-stabilized and PCL-retaining designs are reasonable options for use in combination with recorrection osteotomy. A stemmed tibial component is needed to cross the osteotomy site. In our patient’s case, use of a PCL-retaining design was based on surgeon preference and experience.
Patella infera has been noted as a problem in studies on converting high tibial osteotomy to TKA.9,12,29 A postulated cause is scarring of the infrapatellar tendon after high tibial osteotomy. In addition, a higher incidence of lateral retinacular release has been identified.9-11 Patella infera did not occur in either knee in the present case, and lateral release was not required.
Our patient’s lateral radiographs (Figures 4A, 4B) showed persistence of the osteotomy plane anterior to the tibia. The osteotomy healed posteriorly but not completely anteriorly. This raises the issue of risk for nonunion when recorrection osteotomy is performed with TKA. Use of a stemmed tibial implant with a derotation plate provides the benefit of intramedullary fixation for the recorrection osteotomy. If the recorrection osteotomy were performed in a separate setting before TKA, plate fixation would be the primary fixation option. Should nonunion occur at the recorrection osteotomy site, revision of the tibial plateau with a new stemmed implant would be required in combination with plate fixation. Madelaine and colleagues30 reported on a series of 15 severe varus knees treated with both osteotomy and TKA. Two nonunions occurred. Fixation was a staple in one case and a cement wedge in the other. Risk for nonunion may be reduced with the combination of stemmed tibial implant and internal fixation with a derotation plate. Protective weight-bearing is recommended for the first 6 postoperative weeks.
Conclusion
Ligament imbalances produced by high tibial osteotomy and exacerbated by conversion to TKA are difficult to address. In this report, we have described successful single-stage high tibial osteotomy recorrection and TKA performed bilaterally in separate settings. With use of a stemmed tibial component and a derotation plate, solid fixation was obtained with an excellent clinical outcome. The malalignment was corrected while ligament balance was maintained for a PCL-retaining TKA design.
1. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
2. Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75(2):196-201.
3. Rinonapoli E, Mancini GB, Corvaglia A, Musiello S. Tibial osteotomy for varus gonarthrosis. A 10- to 21-year followup study. Clin Orthop Relat Res. 1998;(353):185-193.
4. Ritter MA, Fechtman RA. Proximal tibial osteotomy. A survivorship analysis. J Arthroplasty. 1988;3(4):309-311.
5. Katz MM, Hungerford DS, Krackow KA, Lennox DW. Results of total knee arthroplasty after failed proximal tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1987;69(2):225-233.
6. Mont MA, Antonaides S, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. A comparison with a matched group. Clin Orthop Relat Res. 1994;299:125-130.
7. Neyret P, Deroche P, Deschamps G, Dejour H. Total knee replacement after valgus tibial osteotomy. Technical problems [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1992;78(7):438-448.
8. Parvizi J, Hanssen AD, Spangehl MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
9. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
10. Amendola A, Rorabeck CH, Bourne RB, Apyan PM. Total knee arthroplasty following high tibial osteotomy for osteoarthritis. J Arthroplasty. 1989;(4 suppl):S11-S17.
11. Kazakos KJ, Chatzipapas C, Verettas D, Galanis V, Xarchas KC, Psillakis I. Mid-term results of total knee arthroplasty after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128(2):167-173.
12. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
13. Niinimaki T, Eskelinen A, Ohtonen P, Puhto AP, Mann BS, Leppilahti J. Total knee arthroplasty after high tibial osteotomy: a registry-based case–control study of 1,036 knees. Arch Orthop Trauma Surg. 2014;134(1):73-77.
14. van Raaij TM, Reijman M, Furlan AD, Verhaar JA. Total knee arthroplasty after high tibial osteotomy. A systematic review. BMC Musculoskelet Disord. 2009;10:88-98.
15. Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59(1):77-79.
16. Cameron HU, Welsh RP. Potential complications of total knee replacement following tibial osteotomy. Orthop Rev. 1988;17(1):39-43.
17. Staeheli JW, Cass JR, Morrey BF. Condylar total knee arthroplasty after failed proximal tibial osteotomy. J Bone Joint Surg Am. 1987;69(1):28-31.
18. Ramappa M, Anand S, Jennings A. Total knee replacement following high tibial osteotomy versus total knee replacement without high tibial osteotomy: a systematic review and meta analysis. Arch Orthop Trauma Surg. 2013;133(11):1587-1593.
19. Preston S, Howard J, Naudie D, Somerville L, McAuley J. Total knee arthroplasty after high tibial osteotomy: no differences between medial and lateral osteotomy approaches. Clin Orthop Relat Res. 2014;472(1):105-110.
20. Krackow KA, Holtgrewe JL. Experience with a new technique for managing severely overcorrected valgus high tibial osteotomy at total knee arthroplasty. Clin Orthop Relat Res. 1990;(258):213-224.
21. Papagelopoulos PJ, Karachalios T, Themistocleous GS, Papadopoulos ECh, Savvidou OD, Rand JA. Total knee arthroplasty in patients with pre-existing fracture deformity. Orthopaedics. 2007;30(5):373-378.
22. Yoshina N, Takai S, Watanabe Y, Nakamura S, Kubo T. Total knee arthroplasty with long stem for treatment of nonunion after high tibial osteotomy. J Arthroplasty. 2004;19(4):528-531.
23. Mont MA, Alexander N, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. Orthop Clin North Am. 1994;25(3):515-525.
24. Scott WN. Insall & Scott’s Surgery of the Knee. Vol 1. 4th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2006.
25. Gill T, Schemitsch EH, Brick GW, Thornhill TS. Revision total knee arthroplasty after failed unicompartmental knee arthroplasty or high tibial osteotomy. Clin Orthop Relat Res. 1995;(321):10-18.
26. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68(7):1035-1040.
27. Wolff AM, Hungerford DS, Pepe CL. The effect of extraarticular varus and valgus deformity on total knee arthroplasty. Clin Orthop Relat Res. 1994;(271):35-51.
28. Uchinou S, Yano H, Shimizu K, Masumi S. A severely overcorrected high tibial osteotomy: revision by osteotomy and a long stem component. Acta Orthop Scand. 1996;67(2):193-194.
29. Noda T, Yasuda S, Nagano K, Takahara Y, Namba Y, Inoue H. Clinico-radiological study of total knee arthroplasty after high tibial osteotomy. J Orthop Sci. 2000;5(1):25-36.
30. Madelaine A, Villa V, Yela C, et al. Results and complications of single-stage total knee arthroplasty and high tibial osteotomy. Int Orthop. 2014;38(10):2091-2098.
1. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
2. Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75(2):196-201.
3. Rinonapoli E, Mancini GB, Corvaglia A, Musiello S. Tibial osteotomy for varus gonarthrosis. A 10- to 21-year followup study. Clin Orthop Relat Res. 1998;(353):185-193.
4. Ritter MA, Fechtman RA. Proximal tibial osteotomy. A survivorship analysis. J Arthroplasty. 1988;3(4):309-311.
5. Katz MM, Hungerford DS, Krackow KA, Lennox DW. Results of total knee arthroplasty after failed proximal tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1987;69(2):225-233.
6. Mont MA, Antonaides S, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. A comparison with a matched group. Clin Orthop Relat Res. 1994;299:125-130.
7. Neyret P, Deroche P, Deschamps G, Dejour H. Total knee replacement after valgus tibial osteotomy. Technical problems [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1992;78(7):438-448.
8. Parvizi J, Hanssen AD, Spangehl MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
9. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
10. Amendola A, Rorabeck CH, Bourne RB, Apyan PM. Total knee arthroplasty following high tibial osteotomy for osteoarthritis. J Arthroplasty. 1989;(4 suppl):S11-S17.
11. Kazakos KJ, Chatzipapas C, Verettas D, Galanis V, Xarchas KC, Psillakis I. Mid-term results of total knee arthroplasty after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128(2):167-173.
12. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
13. Niinimaki T, Eskelinen A, Ohtonen P, Puhto AP, Mann BS, Leppilahti J. Total knee arthroplasty after high tibial osteotomy: a registry-based case–control study of 1,036 knees. Arch Orthop Trauma Surg. 2014;134(1):73-77.
14. van Raaij TM, Reijman M, Furlan AD, Verhaar JA. Total knee arthroplasty after high tibial osteotomy. A systematic review. BMC Musculoskelet Disord. 2009;10:88-98.
15. Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59(1):77-79.
16. Cameron HU, Welsh RP. Potential complications of total knee replacement following tibial osteotomy. Orthop Rev. 1988;17(1):39-43.
17. Staeheli JW, Cass JR, Morrey BF. Condylar total knee arthroplasty after failed proximal tibial osteotomy. J Bone Joint Surg Am. 1987;69(1):28-31.
18. Ramappa M, Anand S, Jennings A. Total knee replacement following high tibial osteotomy versus total knee replacement without high tibial osteotomy: a systematic review and meta analysis. Arch Orthop Trauma Surg. 2013;133(11):1587-1593.
19. Preston S, Howard J, Naudie D, Somerville L, McAuley J. Total knee arthroplasty after high tibial osteotomy: no differences between medial and lateral osteotomy approaches. Clin Orthop Relat Res. 2014;472(1):105-110.
20. Krackow KA, Holtgrewe JL. Experience with a new technique for managing severely overcorrected valgus high tibial osteotomy at total knee arthroplasty. Clin Orthop Relat Res. 1990;(258):213-224.
21. Papagelopoulos PJ, Karachalios T, Themistocleous GS, Papadopoulos ECh, Savvidou OD, Rand JA. Total knee arthroplasty in patients with pre-existing fracture deformity. Orthopaedics. 2007;30(5):373-378.
22. Yoshina N, Takai S, Watanabe Y, Nakamura S, Kubo T. Total knee arthroplasty with long stem for treatment of nonunion after high tibial osteotomy. J Arthroplasty. 2004;19(4):528-531.
23. Mont MA, Alexander N, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. Orthop Clin North Am. 1994;25(3):515-525.
24. Scott WN. Insall & Scott’s Surgery of the Knee. Vol 1. 4th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2006.
25. Gill T, Schemitsch EH, Brick GW, Thornhill TS. Revision total knee arthroplasty after failed unicompartmental knee arthroplasty or high tibial osteotomy. Clin Orthop Relat Res. 1995;(321):10-18.
26. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68(7):1035-1040.
27. Wolff AM, Hungerford DS, Pepe CL. The effect of extraarticular varus and valgus deformity on total knee arthroplasty. Clin Orthop Relat Res. 1994;(271):35-51.
28. Uchinou S, Yano H, Shimizu K, Masumi S. A severely overcorrected high tibial osteotomy: revision by osteotomy and a long stem component. Acta Orthop Scand. 1996;67(2):193-194.
29. Noda T, Yasuda S, Nagano K, Takahara Y, Namba Y, Inoue H. Clinico-radiological study of total knee arthroplasty after high tibial osteotomy. J Orthop Sci. 2000;5(1):25-36.
30. Madelaine A, Villa V, Yela C, et al. Results and complications of single-stage total knee arthroplasty and high tibial osteotomy. Int Orthop. 2014;38(10):2091-2098.
Role of Surgical Dressings in Total Joint Arthroplasty: A Randomized Controlled Trial
Wound complications (eg, delayed wound healing, blisters, prolonged drainage) have been reported in up to 30% of patients who undergo elective total joint arthroplasty (TJA).1-6 Wound complications increase resource utilization, lengthen hospital stays, and increase costs.7-9 Prolonged wound healing and persistent wound drainage are also harbingers of both superficial and deep surgical site infections.5-11
In several studies, wound complications after TJA were the primary reason for hospital readmissions.12-15 As part of the Patient Protection and Affordable Care Act, hospitals will be penalized by the Centers for Medicaid & Medicare Services for unplanned hospital readmissions within 30 days after TJA. It is imperative, then, to reduce the risk factors and complications associated with surgical site infections to decrease unplanned readmissions.
Historically, little attention has been given to the role of surgical dressings and the effect of dressings on wound healing. Although many subspecialties (eg, cardiothoracic surgery, general surgery) have reported benefits in using occlusive dressings, adoption in TJA has been slow.16-18 At our institution about 5 years ago, we began using an occlusive silver-impregnated barrier dressing based on preliminary data from studies showing benefits of occlusive dressings in TJA.19,20
We conducted a study to determine if use of occlusive antimicrobial barrier dressings decreases rates of wound complications in TJA. We had 3 research questions: Compared with standard surgical dressings, are occlusive dressings associated with decreased rates of wound complications after TJA? Is there a difference in number of dressing changes required between the 2 dressing types? Is satisfaction higher for patients with occlusive dressings than for patients with standard dressings?
Patients and Methods
This randomized controlled trial (RCT) was reviewed and approved by the Institutional Review Board at Carolinas Healthcare. Patients were randomized by the research staff using a parallel, 1:1 allocation method. The randomization table was generated using a random number generator.
An a priori sample size estimate was made using a 2-tailed Fisher exact test with a .05 level of significance. Based on a study by Clarke and colleagues,21 we estimated the incidence of wound problems at 3% in the occlusive dressing (study) group and 13% in the standard dressing (control) group. We determined that 260 participants (130 per group) would be needed to achieve 80% power. We considered a 15% attrition rate for a total enrollment goal of 300 study participants (150 per group).
Between December 2010 and January 2013, patients presenting for either primary total hip arthroplasty (THA) or primary total knee arthroplasty (TKA) were recruited to participate in the study. Eligibility criteria (Table 1) were reviewed, and patients were enrolled by the senior surgeons, Dr. Springer, Dr. Beaver, Dr. Griffin, and Dr. Mason. All eligible participants who provided informed consent were randomized to receive either an occlusive antimicrobial barrier dressing (Aquacel Ag, ConvaTec) or standard surgical dressing (Primapore, Smith & Nephew). The occlusive dressing (Figure 1) consists of an outer barrier layer of hydrocolloid and a central island of hydrofiber, which absorbs and locks in any wound exudate within the fibers and prevents the creation of an overly moist wound environment that can lead to skin maceration and wound breakdown. In addition, the hydrofibers are embedded with ionic silver, which is released only at the site of wound exudate, or drainage; thus, there is no continuous exposure of the entire wound to silver. The standard dressing (Figure 2) consists of a central island of gauze enclosed in low-allergy acrylic adhesive tape.
All surgical dressings were placed over a closed incision in a sterile environment in the operating room after the procedure. The groups’ wound closures were identical.
A posterior approach was used for all THAs. The deep fascia was closed with a running barbed suture (Quill, Angiotech), the deep subcutaneous tissue with No. 1 Vicryl suture (Ethicon), and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch (Ethicon) was placed in the subcuticular layer and was followed with a skin adhesive (Dermabond, Ethicon). A closed suction drain, removed on postoperative day (POD) 1, was used for all THAs.
A standard medial parapatellar arthrotomy was used for all TKAs. The arthrotomy was closed with a running barbed suture, the deep subcutaneous tissue with No. 1 Vicryl suture, and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch was placed in the subcuticular layer and was followed with a skin adhesive. A closed suction drain was also used. In addition, a compressive wrap was placed over the dressing in the operating room and was removed the next morning. During the hospital stay, the surgical site was evaluated daily with a standard wound evaluation form.
In the standard dressing group, the bandage was removed for wound evaluation on POD 2, and the dressing was changed every other day during the hospital stay. The dressing was also changed as needed for wound drainage (Figure 3) or other minor wound-healing concerns.
In the occlusive dressing group, the dressing design allowed the dressing to remain in place for about 7 days. It was removed by a home health nurse during a visit closest to but not before the 7-day mark. In addition, it was changed at surgeon discretion if there were concerns about wound drainage or wound healing. For the occlusive barrier, wound drainage was evaluated by strike-through of drainage on the back side of the dressing (Figure 4). If more than 50% of the dressing was saturated, the bandage was changed and the wound evaluated. If there were no immediate concerns about wound complications (eg, infection, blistering), a new occlusive dressing was placed. Because the occlusive dressing was waterproof, patients in the study group were able to shower immediately after surgery. In the control group, patients were allowed to shower if the surgical dressing was kept dry, as the bandage was not waterproof.
Per the study protocol, all patients were discharged home and followed by a single home health agency. Mean hospital stay was 3 days (range, 0-8 days), which did not differ significantly between groups (P = .133). All home health nurses were trained in evaluation of postsurgical wounds and were aware of the study requirements. The nurses visited all patients 3 days a week until the scheduled 4-week postoperative follow-up with the treating physician or physician assistant. At each visit, the nurse evaluated the wound and surrounding skin using a standard wound document. Dressings were changed based on the criteria we have described. Concerns about wound status (eg, drainage, blistering, erythema) prompted removal of the dressing for further evaluation. The physician was notified of concerns about wound healing, which prompted an office visit for evaluation. The dressing remained in place for a minimum of 7 days but in all cases was removed as close to 7 days as possible, depending on the scheduled nursing visits. Once uneventful wound healing was complete, no further dressing was required. A final wound evaluation was conducted by the surgeon at the 4-week postoperative evaluation.
The primary outcome measure was wound complication (dichotomous variable). Wounds were assessed by describing the amount, type, and color of exudate (Figure 5). The appearance of the wound margins and the surrounding skin was also assessed. Because wounds could not be directly visualized in the occlusive dressing group, drainage (indicated by strike-through) was used as a measure of possible wound complications, prompting removal and full evaluation.
Secondary endpoints included additional wound treatment or surgical procedures for wound complications, number of dressing changes, and patient satisfaction. Patients completed a satisfaction questionnaire at each wound assessment (Figure 6). Using a visual analog scale (VAS), they rated their satisfaction with their ability to perform activities of daily living (personal hygiene, change clothes, sit comfortably, sleep comfortably), drawing a line on the VAS at a point between 0 (totally unsatisfied) and 100 (totally satisfied) for each satisfaction measure. This line was measured and recorded by the study coordinator. The 4 satisfaction measures were averaged for a composite satisfaction measure.
All statistical analyses were conducted using SAS Version 9.2 (SAS Institute). Standard univariate descriptive statistics (means, standard deviations, frequencies, proportions) were calculated and reported. Differences in mean values for continuous data were assessed with independent t test or Wilcoxon rank sum test. Chi-square test and Fisher exact test were used to determine differences between groups for categorical or dichotomous variables. A significance level of .05 was used for all statistical tests.
Results
The 300 patients who consented to participate in the study were randomized to receive either occlusive dressing or standard dressing. After randomization, 38 patients (15 occlusive, 23 standard) were withdrawn from the study (Table 2), leaving a final dataset of 262 patients, 141 in the occlusive group (67 THAs, 74 TKAs) and 121 in the standard group (49 THAs, 72 TKAs). There were no differences in proportion of THAs or TKAs, age, sex, or body mass index between the occlusive and standard groups (Table 3).
There were statistically significantly (P = .015) fewer wound complications in the occlusive dressing group (10%) than in the standard dressing group (22%). Blisters at or around the wound site were reported in significantly (P = .026) fewer patients with occlusive dressing (1/141, 0.7%) than standard dressing (7/121, 6%). Additional wound care was required in 9 patients (7%) in the standard group and 6 patients (4%) in the occlusive group (P = .27). Two patients (1.7%) in the standard group were readmitted for treatment of wound dehiscence; no one in the occlusive group was readmitted to the hospital or had to return to the operating room for treatment of a wound complication. The difference was not statistically significant (P = .13). There were also no significant (P = .81) differences in rate of wound complications between THA and TKA patients.
There were statistically significantly (P < .0001) fewer dressing changes in the occlusive dressing group. Mean number of dressing changes was 0.14 (median, 0; interquartile range, 0-0) in the occlusive group and 2.8 (median, 2; interquartile range, 1-3) in the standard group.
Compared with patients in the standard dressing group, patients in the occlusive dressing group reported significantly higher satisfaction scores. Mean overall patient satisfaction score was 92 in the occlusive group and 81 in the standard group (P < .0001). Patients in the occlusive group were more satisfied with their ability to take care of their personal hygiene, to change clothes, and to sit and sleep comfortably (Table 4).
Discussion
Wound complications after TJA are common, occurring in up to 30% of patients,1-6 and are associated with development of superficial and deep surgical site infections, increased resource utilization, and longer hospital stays.5-11 Although the role of surgical dressings has received little attention in TJA practice, other subspecialties have found that occlusive barrier dressings can reduce wound complications and promote wound healing.16,17 Mitotic cell division and leukocyte activity, which are critical in wound healing, increase under occlusive dressings. This cellular activity is disrupted with every dressing change, delaying wound healing (biological activity takes 3-4 hours to resume).22 In addition, occlusive dressings increase hypoxia, which promotes angiogenesis and accelerates wound healing.23
Despite being a prospective RCT, this study had several limitations. Because of the need to evaluate wounds and obvious differences between the 2 dressings (eg, color, ability to shower), it was not possible to blind the patient or surgeon to the dressing used. When rating satisfaction, patients were not able to directly compare the 2 dressings. The primary endpoint of the study was the complication rate; however, the deep periprosthetic infection rate may be a superior endpoint and would require a much larger study. Although we assumed that wound complications may be harbingers for periprosthetic infections, no patient in either group developed periprosthetic infection. Therefore, we cannot conclude that surgical dressings play a role in reducing infections. In addition, as the standard dressing was changed on POD 2 (per standard protocol) and the occlusive dressing could remain in place for up to 7 days, there was a selection bias in the evaluation of the number of dressing changes. However, given the characteristics of the standard dressing (eg, tape, gauze, nonocclusive), leaving it in place after POD 2 is not optimal. Therefore, we would expect to see a difference in the number of dressing changes. We think this comparison remains valid, as occlusive dressings were changed when there were indications of wound problems (eg, excessive drainage [strike-through], surrounding erythema, blistering). With an average of less than 1 dressing change in the occlusive group, we think this is a surrogate for uneventful wound healing and decreased wound complication, and our data support this. It is also important to test both dressing durability and patient tolerance for wearing a single dressing for 7 days.
Our RCT results showed that, compared with a standard dressing, an occlusive antimicrobial dressing was associated with a significant decrease in overall wound complications and blisters. These findings are similar to those of other studies of occlusive dressings in a number of surgical subspecialties.16,18 In an RCT of 200 patients who underwent elective and nonelective hip and knee surgery and were randomized to either absorbent perforated dressing with adhesive border (Cutiplast, Smith & Nephew) or Aquacel (ConvaTec) covered with vapor-permeable dressing (Tegaderm, 3M), Ravenscroft and colleagues20 found that Aquacel-plus-Tegaderm was 5.8 times more likely than Cutiplast to produce an uncompromised wound. Similarly, in an RCT of hydrofiber (Aquacel) and central pad (Mepore, Mölnlycke) dressings after primary THA and TKA, Abuzakuk and colleagues19 found significantly fewer dressing changes (43% vs 77%) and blisters (13% vs 26%) in the hydrofiber group than in the pad group.
Hopper and colleagues24 compared 50 consecutive patients treated with modern dressings (Aquacel) with 50 historical control patients treated with traditional surgical dressings (Mepore). Blisters developed in 20% of the patients in the traditional group and 4% of patients in the modern group (P = .028). The authors concluded that adverse outcomes of wound healing can be minimized with modern dressings.
A recent retrospective study by Cai and colleagues25 evaluated the incidence of acute periprosthetic infection (≤3 months after surgery) with use of occlusive (Aquacel) and standard dressings. Incidence of acute periprosthetic infection was 0.44% in the occlusive group and 1.7% in the standard group (P = .005). Incidence of wound-healing problems was not evaluated.
Our second aim in the present study was to evaluate the number of dressing changes required. There were significantly fewer dressing changes in the occlusive dressing group than in the standard dressing group. Therefore, wear time (amount of time a single dressing remains in place) was substantially longer for the occlusive group. In the study by Hopper and colleagues,24 wear time was significantly shorter for the traditional dressing than for the modern dressing (2 vs 7 days; P < .001), and the traditional dressing required more changes (3 vs 0; P < .001).
These findings are important for several reasons. Standard surgical dressings often require frequent changes. If left in place, they create an excessively moist wound environment that promotes blistering and delays wound healing. However, frequent dressing changes expose the wound and increase the risk for surgical site infection.26 A barrier dressing left in place from time of surgery prevents bacteria from entering and contaminating a healing wound. A study by Clarke and colleagues21 demonstrated higher skin colonization rates for patients who had dressings changed on POD 1 than for patients who had their first dressing change on POD 6.
Our third study aim was to evaluate patient satisfaction with surgical dressings. The orthopedic literature has little on this topic.23 Blisters and other wound complications can negatively affect satisfaction.2,3 Our data showed significant improvement in satisfaction, particularly regarding sterility and hygiene.
Other surgical subspecialties have found similar improvement in patient satisfaction with occlusive barrier dressings. In an RCT of 88 pediatric patients, Rasmussen and colleagues27 found that patients reported significantly less pain during changes of an occlusive adhesive dressing (Duoderm, ConvaTec) than during changes of a conventional Steristrip (3M) plus Cutiplast. According to the authors, the occlusive wound dressing seemed to minimize the physical and psychological trauma to the infant or child and lessen disruption of the child’s and the parents’ daily routines, because the children could be bathed immediately after surgery.
Our study did not specifically address cost. Cai and colleagues25 estimated that, if the Aquacel dressing were routinely used in every hip and knee arthroplasty, it would add about $27 million in cost. However, this must be balanced by the cost of managing infection after TJA. In the United States, at an estimated $50,000 to $100,000 per case and an annual incidence of 1% to 2%, the low-end cost for the treatment of periprosthetic infection would be $500 million.28 Cai and colleagues25 found a 4-fold reduction in periprosthetic infection when use of occlusive dressings was implemented. In addition, wound complications remain the number one reason for hospital readmission after TJA.12,13 Cost of hospital readmission, as well as financial penalties to institutions for unplanned readmission for wound complications, must be considered.
Conclusion
Our RCT results demonstrated that use of occlusive antimicrobial barrier dressings (vs standard surgical dressings) significantly reduced wound complications and dressing changes and improved overall patient satisfaction. These findings are similar to those in the literature on TJA and other surgical subspecialties. We conclude that occlusive surgical dressings reduce wound complications after TJA.
1. Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care. 2005;14(1):27-29.
2. Koval KJ, Egol KA, Hiebert R, Spratt KF. Tape blisters after hip surgery: can they be eliminated completely? Am J Orthop. 2007;36(5):261-265.
3. Lawrentschuk N, Falkenberg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. ANZ J Surg. 2002;72(10):716-719.
4. Mihalko WM, Manaswi A, Brown TE, Parvizi J, Schmalzried TP, Saleh KJ. Infection in primary total knee arthroplasty: contributing factors. Instr Course Lect. 2008;57:317-325.
5. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
6. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
7. Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48-54.
8. Gordon SM, Culver DH, Simmons BP, Jarvis WR. Risk factors for wound infections after total knee arthroplasty. Am J Epidemiol. 1990;131(5):905-916.
9. Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466(6):1368-1371.
10. Schmalzried TP. The infected hip: telltale signs and treatment options. J Arthroplasty. 2006;21(4 suppl 1):97-100.
11. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289.
12. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468.
13. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
14. Jordan CJ, Goldstein RY, Michels RF, Hutzler L, Slover JD, Bosco JA 3rd. Comprehensive program reduces hospital readmission rates after total joint arthroplasty. Am J Orthop. 2012;41(11):E147-E151.
15. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
16. Shinohara T, Yamashita Y, Satoh K, et al. Prospective evaluation of occlusive hydrocolloid dressing versus conventional gauze dressing regarding the healing effect after abdominal operations: randomized controlled trial. Asian J Surg. 2008;31(1):1-5.
17. Siah CJ, Yatim J. Efficacy of a total occlusive ionic silver-containing dressing combination in decreasing risk of surgical site infection: an RCT. J Wound Care. 2011;20(12):561-568.
18. Teshima H, Kawano H, Kashikie H, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today. 2009;39(10):848-854.
19. Abuzakuk TM, Coward P, Shenava Y, Kumar VS, Skinner JA. The management of wounds following primary lower limb arthroplasty: a prospective, randomised study comparing hydrofibre and central pad dressings. Int Wound J. 2006;3(2):133-137.
20. Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Ann R Coll Surg Engl. 2006;88(1):18-22.
21. Clarke JV, Deakin AH, Dillon JM, Emmerson S, Kinninmonth AW. A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care. 2009;18(1):5-8, 10-11.
22. Kloeters O. The use of a semi-occlusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair Regen. 2008;16(4):568-575.
23. Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective, randomized, controlled study. Ann Plast Surg. 1994;32(1):57-64.
24. Hopper GP, Deakin AH, Crane EO, Clarke JV. Enhancing patient recovery following lower limb arthroplasty with a modern wound dressing: a prospective, comparative audit. J Wound Care. 2012;21(4):200-203.
25. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
26. Berg A, Fleischer S, Kuss O, Unverzagt S, Langer G. Timing of dressing removal in the healing of surgical wounds by primary intention: quantitative systematic review protocol. J Adv Nurs. 2012;68(2):264-270.
27. Rasmussen H, Larsen MJ, Skeie E. Surgical wound dressing in outpatient paediatric surgery. A randomised study. Dan Med Bull. 1993;40(2):252-254.
28. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
Wound complications (eg, delayed wound healing, blisters, prolonged drainage) have been reported in up to 30% of patients who undergo elective total joint arthroplasty (TJA).1-6 Wound complications increase resource utilization, lengthen hospital stays, and increase costs.7-9 Prolonged wound healing and persistent wound drainage are also harbingers of both superficial and deep surgical site infections.5-11
In several studies, wound complications after TJA were the primary reason for hospital readmissions.12-15 As part of the Patient Protection and Affordable Care Act, hospitals will be penalized by the Centers for Medicaid & Medicare Services for unplanned hospital readmissions within 30 days after TJA. It is imperative, then, to reduce the risk factors and complications associated with surgical site infections to decrease unplanned readmissions.
Historically, little attention has been given to the role of surgical dressings and the effect of dressings on wound healing. Although many subspecialties (eg, cardiothoracic surgery, general surgery) have reported benefits in using occlusive dressings, adoption in TJA has been slow.16-18 At our institution about 5 years ago, we began using an occlusive silver-impregnated barrier dressing based on preliminary data from studies showing benefits of occlusive dressings in TJA.19,20
We conducted a study to determine if use of occlusive antimicrobial barrier dressings decreases rates of wound complications in TJA. We had 3 research questions: Compared with standard surgical dressings, are occlusive dressings associated with decreased rates of wound complications after TJA? Is there a difference in number of dressing changes required between the 2 dressing types? Is satisfaction higher for patients with occlusive dressings than for patients with standard dressings?
Patients and Methods
This randomized controlled trial (RCT) was reviewed and approved by the Institutional Review Board at Carolinas Healthcare. Patients were randomized by the research staff using a parallel, 1:1 allocation method. The randomization table was generated using a random number generator.
An a priori sample size estimate was made using a 2-tailed Fisher exact test with a .05 level of significance. Based on a study by Clarke and colleagues,21 we estimated the incidence of wound problems at 3% in the occlusive dressing (study) group and 13% in the standard dressing (control) group. We determined that 260 participants (130 per group) would be needed to achieve 80% power. We considered a 15% attrition rate for a total enrollment goal of 300 study participants (150 per group).
Between December 2010 and January 2013, patients presenting for either primary total hip arthroplasty (THA) or primary total knee arthroplasty (TKA) were recruited to participate in the study. Eligibility criteria (Table 1) were reviewed, and patients were enrolled by the senior surgeons, Dr. Springer, Dr. Beaver, Dr. Griffin, and Dr. Mason. All eligible participants who provided informed consent were randomized to receive either an occlusive antimicrobial barrier dressing (Aquacel Ag, ConvaTec) or standard surgical dressing (Primapore, Smith & Nephew). The occlusive dressing (Figure 1) consists of an outer barrier layer of hydrocolloid and a central island of hydrofiber, which absorbs and locks in any wound exudate within the fibers and prevents the creation of an overly moist wound environment that can lead to skin maceration and wound breakdown. In addition, the hydrofibers are embedded with ionic silver, which is released only at the site of wound exudate, or drainage; thus, there is no continuous exposure of the entire wound to silver. The standard dressing (Figure 2) consists of a central island of gauze enclosed in low-allergy acrylic adhesive tape.
All surgical dressings were placed over a closed incision in a sterile environment in the operating room after the procedure. The groups’ wound closures were identical.
A posterior approach was used for all THAs. The deep fascia was closed with a running barbed suture (Quill, Angiotech), the deep subcutaneous tissue with No. 1 Vicryl suture (Ethicon), and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch (Ethicon) was placed in the subcuticular layer and was followed with a skin adhesive (Dermabond, Ethicon). A closed suction drain, removed on postoperative day (POD) 1, was used for all THAs.
A standard medial parapatellar arthrotomy was used for all TKAs. The arthrotomy was closed with a running barbed suture, the deep subcutaneous tissue with No. 1 Vicryl suture, and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch was placed in the subcuticular layer and was followed with a skin adhesive. A closed suction drain was also used. In addition, a compressive wrap was placed over the dressing in the operating room and was removed the next morning. During the hospital stay, the surgical site was evaluated daily with a standard wound evaluation form.
In the standard dressing group, the bandage was removed for wound evaluation on POD 2, and the dressing was changed every other day during the hospital stay. The dressing was also changed as needed for wound drainage (Figure 3) or other minor wound-healing concerns.
In the occlusive dressing group, the dressing design allowed the dressing to remain in place for about 7 days. It was removed by a home health nurse during a visit closest to but not before the 7-day mark. In addition, it was changed at surgeon discretion if there were concerns about wound drainage or wound healing. For the occlusive barrier, wound drainage was evaluated by strike-through of drainage on the back side of the dressing (Figure 4). If more than 50% of the dressing was saturated, the bandage was changed and the wound evaluated. If there were no immediate concerns about wound complications (eg, infection, blistering), a new occlusive dressing was placed. Because the occlusive dressing was waterproof, patients in the study group were able to shower immediately after surgery. In the control group, patients were allowed to shower if the surgical dressing was kept dry, as the bandage was not waterproof.
Per the study protocol, all patients were discharged home and followed by a single home health agency. Mean hospital stay was 3 days (range, 0-8 days), which did not differ significantly between groups (P = .133). All home health nurses were trained in evaluation of postsurgical wounds and were aware of the study requirements. The nurses visited all patients 3 days a week until the scheduled 4-week postoperative follow-up with the treating physician or physician assistant. At each visit, the nurse evaluated the wound and surrounding skin using a standard wound document. Dressings were changed based on the criteria we have described. Concerns about wound status (eg, drainage, blistering, erythema) prompted removal of the dressing for further evaluation. The physician was notified of concerns about wound healing, which prompted an office visit for evaluation. The dressing remained in place for a minimum of 7 days but in all cases was removed as close to 7 days as possible, depending on the scheduled nursing visits. Once uneventful wound healing was complete, no further dressing was required. A final wound evaluation was conducted by the surgeon at the 4-week postoperative evaluation.
The primary outcome measure was wound complication (dichotomous variable). Wounds were assessed by describing the amount, type, and color of exudate (Figure 5). The appearance of the wound margins and the surrounding skin was also assessed. Because wounds could not be directly visualized in the occlusive dressing group, drainage (indicated by strike-through) was used as a measure of possible wound complications, prompting removal and full evaluation.
Secondary endpoints included additional wound treatment or surgical procedures for wound complications, number of dressing changes, and patient satisfaction. Patients completed a satisfaction questionnaire at each wound assessment (Figure 6). Using a visual analog scale (VAS), they rated their satisfaction with their ability to perform activities of daily living (personal hygiene, change clothes, sit comfortably, sleep comfortably), drawing a line on the VAS at a point between 0 (totally unsatisfied) and 100 (totally satisfied) for each satisfaction measure. This line was measured and recorded by the study coordinator. The 4 satisfaction measures were averaged for a composite satisfaction measure.
All statistical analyses were conducted using SAS Version 9.2 (SAS Institute). Standard univariate descriptive statistics (means, standard deviations, frequencies, proportions) were calculated and reported. Differences in mean values for continuous data were assessed with independent t test or Wilcoxon rank sum test. Chi-square test and Fisher exact test were used to determine differences between groups for categorical or dichotomous variables. A significance level of .05 was used for all statistical tests.
Results
The 300 patients who consented to participate in the study were randomized to receive either occlusive dressing or standard dressing. After randomization, 38 patients (15 occlusive, 23 standard) were withdrawn from the study (Table 2), leaving a final dataset of 262 patients, 141 in the occlusive group (67 THAs, 74 TKAs) and 121 in the standard group (49 THAs, 72 TKAs). There were no differences in proportion of THAs or TKAs, age, sex, or body mass index between the occlusive and standard groups (Table 3).
There were statistically significantly (P = .015) fewer wound complications in the occlusive dressing group (10%) than in the standard dressing group (22%). Blisters at or around the wound site were reported in significantly (P = .026) fewer patients with occlusive dressing (1/141, 0.7%) than standard dressing (7/121, 6%). Additional wound care was required in 9 patients (7%) in the standard group and 6 patients (4%) in the occlusive group (P = .27). Two patients (1.7%) in the standard group were readmitted for treatment of wound dehiscence; no one in the occlusive group was readmitted to the hospital or had to return to the operating room for treatment of a wound complication. The difference was not statistically significant (P = .13). There were also no significant (P = .81) differences in rate of wound complications between THA and TKA patients.
There were statistically significantly (P < .0001) fewer dressing changes in the occlusive dressing group. Mean number of dressing changes was 0.14 (median, 0; interquartile range, 0-0) in the occlusive group and 2.8 (median, 2; interquartile range, 1-3) in the standard group.
Compared with patients in the standard dressing group, patients in the occlusive dressing group reported significantly higher satisfaction scores. Mean overall patient satisfaction score was 92 in the occlusive group and 81 in the standard group (P < .0001). Patients in the occlusive group were more satisfied with their ability to take care of their personal hygiene, to change clothes, and to sit and sleep comfortably (Table 4).
Discussion
Wound complications after TJA are common, occurring in up to 30% of patients,1-6 and are associated with development of superficial and deep surgical site infections, increased resource utilization, and longer hospital stays.5-11 Although the role of surgical dressings has received little attention in TJA practice, other subspecialties have found that occlusive barrier dressings can reduce wound complications and promote wound healing.16,17 Mitotic cell division and leukocyte activity, which are critical in wound healing, increase under occlusive dressings. This cellular activity is disrupted with every dressing change, delaying wound healing (biological activity takes 3-4 hours to resume).22 In addition, occlusive dressings increase hypoxia, which promotes angiogenesis and accelerates wound healing.23
Despite being a prospective RCT, this study had several limitations. Because of the need to evaluate wounds and obvious differences between the 2 dressings (eg, color, ability to shower), it was not possible to blind the patient or surgeon to the dressing used. When rating satisfaction, patients were not able to directly compare the 2 dressings. The primary endpoint of the study was the complication rate; however, the deep periprosthetic infection rate may be a superior endpoint and would require a much larger study. Although we assumed that wound complications may be harbingers for periprosthetic infections, no patient in either group developed periprosthetic infection. Therefore, we cannot conclude that surgical dressings play a role in reducing infections. In addition, as the standard dressing was changed on POD 2 (per standard protocol) and the occlusive dressing could remain in place for up to 7 days, there was a selection bias in the evaluation of the number of dressing changes. However, given the characteristics of the standard dressing (eg, tape, gauze, nonocclusive), leaving it in place after POD 2 is not optimal. Therefore, we would expect to see a difference in the number of dressing changes. We think this comparison remains valid, as occlusive dressings were changed when there were indications of wound problems (eg, excessive drainage [strike-through], surrounding erythema, blistering). With an average of less than 1 dressing change in the occlusive group, we think this is a surrogate for uneventful wound healing and decreased wound complication, and our data support this. It is also important to test both dressing durability and patient tolerance for wearing a single dressing for 7 days.
Our RCT results showed that, compared with a standard dressing, an occlusive antimicrobial dressing was associated with a significant decrease in overall wound complications and blisters. These findings are similar to those of other studies of occlusive dressings in a number of surgical subspecialties.16,18 In an RCT of 200 patients who underwent elective and nonelective hip and knee surgery and were randomized to either absorbent perforated dressing with adhesive border (Cutiplast, Smith & Nephew) or Aquacel (ConvaTec) covered with vapor-permeable dressing (Tegaderm, 3M), Ravenscroft and colleagues20 found that Aquacel-plus-Tegaderm was 5.8 times more likely than Cutiplast to produce an uncompromised wound. Similarly, in an RCT of hydrofiber (Aquacel) and central pad (Mepore, Mölnlycke) dressings after primary THA and TKA, Abuzakuk and colleagues19 found significantly fewer dressing changes (43% vs 77%) and blisters (13% vs 26%) in the hydrofiber group than in the pad group.
Hopper and colleagues24 compared 50 consecutive patients treated with modern dressings (Aquacel) with 50 historical control patients treated with traditional surgical dressings (Mepore). Blisters developed in 20% of the patients in the traditional group and 4% of patients in the modern group (P = .028). The authors concluded that adverse outcomes of wound healing can be minimized with modern dressings.
A recent retrospective study by Cai and colleagues25 evaluated the incidence of acute periprosthetic infection (≤3 months after surgery) with use of occlusive (Aquacel) and standard dressings. Incidence of acute periprosthetic infection was 0.44% in the occlusive group and 1.7% in the standard group (P = .005). Incidence of wound-healing problems was not evaluated.
Our second aim in the present study was to evaluate the number of dressing changes required. There were significantly fewer dressing changes in the occlusive dressing group than in the standard dressing group. Therefore, wear time (amount of time a single dressing remains in place) was substantially longer for the occlusive group. In the study by Hopper and colleagues,24 wear time was significantly shorter for the traditional dressing than for the modern dressing (2 vs 7 days; P < .001), and the traditional dressing required more changes (3 vs 0; P < .001).
These findings are important for several reasons. Standard surgical dressings often require frequent changes. If left in place, they create an excessively moist wound environment that promotes blistering and delays wound healing. However, frequent dressing changes expose the wound and increase the risk for surgical site infection.26 A barrier dressing left in place from time of surgery prevents bacteria from entering and contaminating a healing wound. A study by Clarke and colleagues21 demonstrated higher skin colonization rates for patients who had dressings changed on POD 1 than for patients who had their first dressing change on POD 6.
Our third study aim was to evaluate patient satisfaction with surgical dressings. The orthopedic literature has little on this topic.23 Blisters and other wound complications can negatively affect satisfaction.2,3 Our data showed significant improvement in satisfaction, particularly regarding sterility and hygiene.
Other surgical subspecialties have found similar improvement in patient satisfaction with occlusive barrier dressings. In an RCT of 88 pediatric patients, Rasmussen and colleagues27 found that patients reported significantly less pain during changes of an occlusive adhesive dressing (Duoderm, ConvaTec) than during changes of a conventional Steristrip (3M) plus Cutiplast. According to the authors, the occlusive wound dressing seemed to minimize the physical and psychological trauma to the infant or child and lessen disruption of the child’s and the parents’ daily routines, because the children could be bathed immediately after surgery.
Our study did not specifically address cost. Cai and colleagues25 estimated that, if the Aquacel dressing were routinely used in every hip and knee arthroplasty, it would add about $27 million in cost. However, this must be balanced by the cost of managing infection after TJA. In the United States, at an estimated $50,000 to $100,000 per case and an annual incidence of 1% to 2%, the low-end cost for the treatment of periprosthetic infection would be $500 million.28 Cai and colleagues25 found a 4-fold reduction in periprosthetic infection when use of occlusive dressings was implemented. In addition, wound complications remain the number one reason for hospital readmission after TJA.12,13 Cost of hospital readmission, as well as financial penalties to institutions for unplanned readmission for wound complications, must be considered.
Conclusion
Our RCT results demonstrated that use of occlusive antimicrobial barrier dressings (vs standard surgical dressings) significantly reduced wound complications and dressing changes and improved overall patient satisfaction. These findings are similar to those in the literature on TJA and other surgical subspecialties. We conclude that occlusive surgical dressings reduce wound complications after TJA.
Wound complications (eg, delayed wound healing, blisters, prolonged drainage) have been reported in up to 30% of patients who undergo elective total joint arthroplasty (TJA).1-6 Wound complications increase resource utilization, lengthen hospital stays, and increase costs.7-9 Prolonged wound healing and persistent wound drainage are also harbingers of both superficial and deep surgical site infections.5-11
In several studies, wound complications after TJA were the primary reason for hospital readmissions.12-15 As part of the Patient Protection and Affordable Care Act, hospitals will be penalized by the Centers for Medicaid & Medicare Services for unplanned hospital readmissions within 30 days after TJA. It is imperative, then, to reduce the risk factors and complications associated with surgical site infections to decrease unplanned readmissions.
Historically, little attention has been given to the role of surgical dressings and the effect of dressings on wound healing. Although many subspecialties (eg, cardiothoracic surgery, general surgery) have reported benefits in using occlusive dressings, adoption in TJA has been slow.16-18 At our institution about 5 years ago, we began using an occlusive silver-impregnated barrier dressing based on preliminary data from studies showing benefits of occlusive dressings in TJA.19,20
We conducted a study to determine if use of occlusive antimicrobial barrier dressings decreases rates of wound complications in TJA. We had 3 research questions: Compared with standard surgical dressings, are occlusive dressings associated with decreased rates of wound complications after TJA? Is there a difference in number of dressing changes required between the 2 dressing types? Is satisfaction higher for patients with occlusive dressings than for patients with standard dressings?
Patients and Methods
This randomized controlled trial (RCT) was reviewed and approved by the Institutional Review Board at Carolinas Healthcare. Patients were randomized by the research staff using a parallel, 1:1 allocation method. The randomization table was generated using a random number generator.
An a priori sample size estimate was made using a 2-tailed Fisher exact test with a .05 level of significance. Based on a study by Clarke and colleagues,21 we estimated the incidence of wound problems at 3% in the occlusive dressing (study) group and 13% in the standard dressing (control) group. We determined that 260 participants (130 per group) would be needed to achieve 80% power. We considered a 15% attrition rate for a total enrollment goal of 300 study participants (150 per group).
Between December 2010 and January 2013, patients presenting for either primary total hip arthroplasty (THA) or primary total knee arthroplasty (TKA) were recruited to participate in the study. Eligibility criteria (Table 1) were reviewed, and patients were enrolled by the senior surgeons, Dr. Springer, Dr. Beaver, Dr. Griffin, and Dr. Mason. All eligible participants who provided informed consent were randomized to receive either an occlusive antimicrobial barrier dressing (Aquacel Ag, ConvaTec) or standard surgical dressing (Primapore, Smith & Nephew). The occlusive dressing (Figure 1) consists of an outer barrier layer of hydrocolloid and a central island of hydrofiber, which absorbs and locks in any wound exudate within the fibers and prevents the creation of an overly moist wound environment that can lead to skin maceration and wound breakdown. In addition, the hydrofibers are embedded with ionic silver, which is released only at the site of wound exudate, or drainage; thus, there is no continuous exposure of the entire wound to silver. The standard dressing (Figure 2) consists of a central island of gauze enclosed in low-allergy acrylic adhesive tape.
All surgical dressings were placed over a closed incision in a sterile environment in the operating room after the procedure. The groups’ wound closures were identical.
A posterior approach was used for all THAs. The deep fascia was closed with a running barbed suture (Quill, Angiotech), the deep subcutaneous tissue with No. 1 Vicryl suture (Ethicon), and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch (Ethicon) was placed in the subcuticular layer and was followed with a skin adhesive (Dermabond, Ethicon). A closed suction drain, removed on postoperative day (POD) 1, was used for all THAs.
A standard medial parapatellar arthrotomy was used for all TKAs. The arthrotomy was closed with a running barbed suture, the deep subcutaneous tissue with No. 1 Vicryl suture, and the superficial subcutaneous layer with 2-0 Vicryl suture. A running 3-0 Monocryl stitch was placed in the subcuticular layer and was followed with a skin adhesive. A closed suction drain was also used. In addition, a compressive wrap was placed over the dressing in the operating room and was removed the next morning. During the hospital stay, the surgical site was evaluated daily with a standard wound evaluation form.
In the standard dressing group, the bandage was removed for wound evaluation on POD 2, and the dressing was changed every other day during the hospital stay. The dressing was also changed as needed for wound drainage (Figure 3) or other minor wound-healing concerns.
In the occlusive dressing group, the dressing design allowed the dressing to remain in place for about 7 days. It was removed by a home health nurse during a visit closest to but not before the 7-day mark. In addition, it was changed at surgeon discretion if there were concerns about wound drainage or wound healing. For the occlusive barrier, wound drainage was evaluated by strike-through of drainage on the back side of the dressing (Figure 4). If more than 50% of the dressing was saturated, the bandage was changed and the wound evaluated. If there were no immediate concerns about wound complications (eg, infection, blistering), a new occlusive dressing was placed. Because the occlusive dressing was waterproof, patients in the study group were able to shower immediately after surgery. In the control group, patients were allowed to shower if the surgical dressing was kept dry, as the bandage was not waterproof.
Per the study protocol, all patients were discharged home and followed by a single home health agency. Mean hospital stay was 3 days (range, 0-8 days), which did not differ significantly between groups (P = .133). All home health nurses were trained in evaluation of postsurgical wounds and were aware of the study requirements. The nurses visited all patients 3 days a week until the scheduled 4-week postoperative follow-up with the treating physician or physician assistant. At each visit, the nurse evaluated the wound and surrounding skin using a standard wound document. Dressings were changed based on the criteria we have described. Concerns about wound status (eg, drainage, blistering, erythema) prompted removal of the dressing for further evaluation. The physician was notified of concerns about wound healing, which prompted an office visit for evaluation. The dressing remained in place for a minimum of 7 days but in all cases was removed as close to 7 days as possible, depending on the scheduled nursing visits. Once uneventful wound healing was complete, no further dressing was required. A final wound evaluation was conducted by the surgeon at the 4-week postoperative evaluation.
The primary outcome measure was wound complication (dichotomous variable). Wounds were assessed by describing the amount, type, and color of exudate (Figure 5). The appearance of the wound margins and the surrounding skin was also assessed. Because wounds could not be directly visualized in the occlusive dressing group, drainage (indicated by strike-through) was used as a measure of possible wound complications, prompting removal and full evaluation.
Secondary endpoints included additional wound treatment or surgical procedures for wound complications, number of dressing changes, and patient satisfaction. Patients completed a satisfaction questionnaire at each wound assessment (Figure 6). Using a visual analog scale (VAS), they rated their satisfaction with their ability to perform activities of daily living (personal hygiene, change clothes, sit comfortably, sleep comfortably), drawing a line on the VAS at a point between 0 (totally unsatisfied) and 100 (totally satisfied) for each satisfaction measure. This line was measured and recorded by the study coordinator. The 4 satisfaction measures were averaged for a composite satisfaction measure.
All statistical analyses were conducted using SAS Version 9.2 (SAS Institute). Standard univariate descriptive statistics (means, standard deviations, frequencies, proportions) were calculated and reported. Differences in mean values for continuous data were assessed with independent t test or Wilcoxon rank sum test. Chi-square test and Fisher exact test were used to determine differences between groups for categorical or dichotomous variables. A significance level of .05 was used for all statistical tests.
Results
The 300 patients who consented to participate in the study were randomized to receive either occlusive dressing or standard dressing. After randomization, 38 patients (15 occlusive, 23 standard) were withdrawn from the study (Table 2), leaving a final dataset of 262 patients, 141 in the occlusive group (67 THAs, 74 TKAs) and 121 in the standard group (49 THAs, 72 TKAs). There were no differences in proportion of THAs or TKAs, age, sex, or body mass index between the occlusive and standard groups (Table 3).
There were statistically significantly (P = .015) fewer wound complications in the occlusive dressing group (10%) than in the standard dressing group (22%). Blisters at or around the wound site were reported in significantly (P = .026) fewer patients with occlusive dressing (1/141, 0.7%) than standard dressing (7/121, 6%). Additional wound care was required in 9 patients (7%) in the standard group and 6 patients (4%) in the occlusive group (P = .27). Two patients (1.7%) in the standard group were readmitted for treatment of wound dehiscence; no one in the occlusive group was readmitted to the hospital or had to return to the operating room for treatment of a wound complication. The difference was not statistically significant (P = .13). There were also no significant (P = .81) differences in rate of wound complications between THA and TKA patients.
There were statistically significantly (P < .0001) fewer dressing changes in the occlusive dressing group. Mean number of dressing changes was 0.14 (median, 0; interquartile range, 0-0) in the occlusive group and 2.8 (median, 2; interquartile range, 1-3) in the standard group.
Compared with patients in the standard dressing group, patients in the occlusive dressing group reported significantly higher satisfaction scores. Mean overall patient satisfaction score was 92 in the occlusive group and 81 in the standard group (P < .0001). Patients in the occlusive group were more satisfied with their ability to take care of their personal hygiene, to change clothes, and to sit and sleep comfortably (Table 4).
Discussion
Wound complications after TJA are common, occurring in up to 30% of patients,1-6 and are associated with development of superficial and deep surgical site infections, increased resource utilization, and longer hospital stays.5-11 Although the role of surgical dressings has received little attention in TJA practice, other subspecialties have found that occlusive barrier dressings can reduce wound complications and promote wound healing.16,17 Mitotic cell division and leukocyte activity, which are critical in wound healing, increase under occlusive dressings. This cellular activity is disrupted with every dressing change, delaying wound healing (biological activity takes 3-4 hours to resume).22 In addition, occlusive dressings increase hypoxia, which promotes angiogenesis and accelerates wound healing.23
Despite being a prospective RCT, this study had several limitations. Because of the need to evaluate wounds and obvious differences between the 2 dressings (eg, color, ability to shower), it was not possible to blind the patient or surgeon to the dressing used. When rating satisfaction, patients were not able to directly compare the 2 dressings. The primary endpoint of the study was the complication rate; however, the deep periprosthetic infection rate may be a superior endpoint and would require a much larger study. Although we assumed that wound complications may be harbingers for periprosthetic infections, no patient in either group developed periprosthetic infection. Therefore, we cannot conclude that surgical dressings play a role in reducing infections. In addition, as the standard dressing was changed on POD 2 (per standard protocol) and the occlusive dressing could remain in place for up to 7 days, there was a selection bias in the evaluation of the number of dressing changes. However, given the characteristics of the standard dressing (eg, tape, gauze, nonocclusive), leaving it in place after POD 2 is not optimal. Therefore, we would expect to see a difference in the number of dressing changes. We think this comparison remains valid, as occlusive dressings were changed when there were indications of wound problems (eg, excessive drainage [strike-through], surrounding erythema, blistering). With an average of less than 1 dressing change in the occlusive group, we think this is a surrogate for uneventful wound healing and decreased wound complication, and our data support this. It is also important to test both dressing durability and patient tolerance for wearing a single dressing for 7 days.
Our RCT results showed that, compared with a standard dressing, an occlusive antimicrobial dressing was associated with a significant decrease in overall wound complications and blisters. These findings are similar to those of other studies of occlusive dressings in a number of surgical subspecialties.16,18 In an RCT of 200 patients who underwent elective and nonelective hip and knee surgery and were randomized to either absorbent perforated dressing with adhesive border (Cutiplast, Smith & Nephew) or Aquacel (ConvaTec) covered with vapor-permeable dressing (Tegaderm, 3M), Ravenscroft and colleagues20 found that Aquacel-plus-Tegaderm was 5.8 times more likely than Cutiplast to produce an uncompromised wound. Similarly, in an RCT of hydrofiber (Aquacel) and central pad (Mepore, Mölnlycke) dressings after primary THA and TKA, Abuzakuk and colleagues19 found significantly fewer dressing changes (43% vs 77%) and blisters (13% vs 26%) in the hydrofiber group than in the pad group.
Hopper and colleagues24 compared 50 consecutive patients treated with modern dressings (Aquacel) with 50 historical control patients treated with traditional surgical dressings (Mepore). Blisters developed in 20% of the patients in the traditional group and 4% of patients in the modern group (P = .028). The authors concluded that adverse outcomes of wound healing can be minimized with modern dressings.
A recent retrospective study by Cai and colleagues25 evaluated the incidence of acute periprosthetic infection (≤3 months after surgery) with use of occlusive (Aquacel) and standard dressings. Incidence of acute periprosthetic infection was 0.44% in the occlusive group and 1.7% in the standard group (P = .005). Incidence of wound-healing problems was not evaluated.
Our second aim in the present study was to evaluate the number of dressing changes required. There were significantly fewer dressing changes in the occlusive dressing group than in the standard dressing group. Therefore, wear time (amount of time a single dressing remains in place) was substantially longer for the occlusive group. In the study by Hopper and colleagues,24 wear time was significantly shorter for the traditional dressing than for the modern dressing (2 vs 7 days; P < .001), and the traditional dressing required more changes (3 vs 0; P < .001).
These findings are important for several reasons. Standard surgical dressings often require frequent changes. If left in place, they create an excessively moist wound environment that promotes blistering and delays wound healing. However, frequent dressing changes expose the wound and increase the risk for surgical site infection.26 A barrier dressing left in place from time of surgery prevents bacteria from entering and contaminating a healing wound. A study by Clarke and colleagues21 demonstrated higher skin colonization rates for patients who had dressings changed on POD 1 than for patients who had their first dressing change on POD 6.
Our third study aim was to evaluate patient satisfaction with surgical dressings. The orthopedic literature has little on this topic.23 Blisters and other wound complications can negatively affect satisfaction.2,3 Our data showed significant improvement in satisfaction, particularly regarding sterility and hygiene.
Other surgical subspecialties have found similar improvement in patient satisfaction with occlusive barrier dressings. In an RCT of 88 pediatric patients, Rasmussen and colleagues27 found that patients reported significantly less pain during changes of an occlusive adhesive dressing (Duoderm, ConvaTec) than during changes of a conventional Steristrip (3M) plus Cutiplast. According to the authors, the occlusive wound dressing seemed to minimize the physical and psychological trauma to the infant or child and lessen disruption of the child’s and the parents’ daily routines, because the children could be bathed immediately after surgery.
Our study did not specifically address cost. Cai and colleagues25 estimated that, if the Aquacel dressing were routinely used in every hip and knee arthroplasty, it would add about $27 million in cost. However, this must be balanced by the cost of managing infection after TJA. In the United States, at an estimated $50,000 to $100,000 per case and an annual incidence of 1% to 2%, the low-end cost for the treatment of periprosthetic infection would be $500 million.28 Cai and colleagues25 found a 4-fold reduction in periprosthetic infection when use of occlusive dressings was implemented. In addition, wound complications remain the number one reason for hospital readmission after TJA.12,13 Cost of hospital readmission, as well as financial penalties to institutions for unplanned readmission for wound complications, must be considered.
Conclusion
Our RCT results demonstrated that use of occlusive antimicrobial barrier dressings (vs standard surgical dressings) significantly reduced wound complications and dressing changes and improved overall patient satisfaction. These findings are similar to those in the literature on TJA and other surgical subspecialties. We conclude that occlusive surgical dressings reduce wound complications after TJA.
1. Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care. 2005;14(1):27-29.
2. Koval KJ, Egol KA, Hiebert R, Spratt KF. Tape blisters after hip surgery: can they be eliminated completely? Am J Orthop. 2007;36(5):261-265.
3. Lawrentschuk N, Falkenberg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. ANZ J Surg. 2002;72(10):716-719.
4. Mihalko WM, Manaswi A, Brown TE, Parvizi J, Schmalzried TP, Saleh KJ. Infection in primary total knee arthroplasty: contributing factors. Instr Course Lect. 2008;57:317-325.
5. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
6. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
7. Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48-54.
8. Gordon SM, Culver DH, Simmons BP, Jarvis WR. Risk factors for wound infections after total knee arthroplasty. Am J Epidemiol. 1990;131(5):905-916.
9. Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466(6):1368-1371.
10. Schmalzried TP. The infected hip: telltale signs and treatment options. J Arthroplasty. 2006;21(4 suppl 1):97-100.
11. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289.
12. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468.
13. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
14. Jordan CJ, Goldstein RY, Michels RF, Hutzler L, Slover JD, Bosco JA 3rd. Comprehensive program reduces hospital readmission rates after total joint arthroplasty. Am J Orthop. 2012;41(11):E147-E151.
15. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
16. Shinohara T, Yamashita Y, Satoh K, et al. Prospective evaluation of occlusive hydrocolloid dressing versus conventional gauze dressing regarding the healing effect after abdominal operations: randomized controlled trial. Asian J Surg. 2008;31(1):1-5.
17. Siah CJ, Yatim J. Efficacy of a total occlusive ionic silver-containing dressing combination in decreasing risk of surgical site infection: an RCT. J Wound Care. 2011;20(12):561-568.
18. Teshima H, Kawano H, Kashikie H, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today. 2009;39(10):848-854.
19. Abuzakuk TM, Coward P, Shenava Y, Kumar VS, Skinner JA. The management of wounds following primary lower limb arthroplasty: a prospective, randomised study comparing hydrofibre and central pad dressings. Int Wound J. 2006;3(2):133-137.
20. Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Ann R Coll Surg Engl. 2006;88(1):18-22.
21. Clarke JV, Deakin AH, Dillon JM, Emmerson S, Kinninmonth AW. A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care. 2009;18(1):5-8, 10-11.
22. Kloeters O. The use of a semi-occlusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair Regen. 2008;16(4):568-575.
23. Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective, randomized, controlled study. Ann Plast Surg. 1994;32(1):57-64.
24. Hopper GP, Deakin AH, Crane EO, Clarke JV. Enhancing patient recovery following lower limb arthroplasty with a modern wound dressing: a prospective, comparative audit. J Wound Care. 2012;21(4):200-203.
25. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
26. Berg A, Fleischer S, Kuss O, Unverzagt S, Langer G. Timing of dressing removal in the healing of surgical wounds by primary intention: quantitative systematic review protocol. J Adv Nurs. 2012;68(2):264-270.
27. Rasmussen H, Larsen MJ, Skeie E. Surgical wound dressing in outpatient paediatric surgery. A randomised study. Dan Med Bull. 1993;40(2):252-254.
28. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
1. Cosker T, Elsayed S, Gupta S, Mendonca AD, Tayton KJ. Choice of dressing has a major impact on blistering and healing outcomes in orthopaedic patients. J Wound Care. 2005;14(1):27-29.
2. Koval KJ, Egol KA, Hiebert R, Spratt KF. Tape blisters after hip surgery: can they be eliminated completely? Am J Orthop. 2007;36(5):261-265.
3. Lawrentschuk N, Falkenberg MP, Pirpiris M. Wound blisters post hip surgery: a prospective trial comparing dressings. ANZ J Surg. 2002;72(10):716-719.
4. Mihalko WM, Manaswi A, Brown TE, Parvizi J, Schmalzried TP, Saleh KJ. Infection in primary total knee arthroplasty: contributing factors. Instr Course Lect. 2008;57:317-325.
5. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
6. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
7. Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am. 2009;91(1):48-54.
8. Gordon SM, Culver DH, Simmons BP, Jarvis WR. Risk factors for wound infections after total knee arthroplasty. Am J Epidemiol. 1990;131(5):905-916.
9. Jaberi FM, Parvizi J, Haytmanek CT, Joshi A, Purtill J. Procrastination of wound drainage and malnutrition affect the outcome of joint arthroplasty. Clin Orthop Relat Res. 2008;466(6):1368-1371.
10. Schmalzried TP. The infected hip: telltale signs and treatment options. J Arthroplasty. 2006;21(4 suppl 1):97-100.
11. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285-289.
12. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468.
13. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
14. Jordan CJ, Goldstein RY, Michels RF, Hutzler L, Slover JD, Bosco JA 3rd. Comprehensive program reduces hospital readmission rates after total joint arthroplasty. Am J Orthop. 2012;41(11):E147-E151.
15. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
16. Shinohara T, Yamashita Y, Satoh K, et al. Prospective evaluation of occlusive hydrocolloid dressing versus conventional gauze dressing regarding the healing effect after abdominal operations: randomized controlled trial. Asian J Surg. 2008;31(1):1-5.
17. Siah CJ, Yatim J. Efficacy of a total occlusive ionic silver-containing dressing combination in decreasing risk of surgical site infection: an RCT. J Wound Care. 2011;20(12):561-568.
18. Teshima H, Kawano H, Kashikie H, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today. 2009;39(10):848-854.
19. Abuzakuk TM, Coward P, Shenava Y, Kumar VS, Skinner JA. The management of wounds following primary lower limb arthroplasty: a prospective, randomised study comparing hydrofibre and central pad dressings. Int Wound J. 2006;3(2):133-137.
20. Ravenscroft MJ, Harker J, Buch KA. A prospective, randomised, controlled trial comparing wound dressings used in hip and knee surgery: Aquacel and Tegaderm versus Cutiplast. Ann R Coll Surg Engl. 2006;88(1):18-22.
21. Clarke JV, Deakin AH, Dillon JM, Emmerson S, Kinninmonth AW. A prospective clinical audit of a new dressing design for lower limb arthroplasty wounds. J Wound Care. 2009;18(1):5-8, 10-11.
22. Kloeters O. The use of a semi-occlusive dressing reduces epidermal inflammatory cytokine expression and mitigates dermal proliferation and inflammation in a rat incisional model. Wound Repair Regen. 2008;16(4):568-575.
23. Michie DD, Hugill JV. Influence of occlusive and impregnated gauze dressings on incisional healing: a prospective, randomized, controlled study. Ann Plast Surg. 1994;32(1):57-64.
24. Hopper GP, Deakin AH, Crane EO, Clarke JV. Enhancing patient recovery following lower limb arthroplasty with a modern wound dressing: a prospective, comparative audit. J Wound Care. 2012;21(4):200-203.
25. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case–control study. J Arthroplasty. 2014;29(6):1098-1100.
26. Berg A, Fleischer S, Kuss O, Unverzagt S, Langer G. Timing of dressing removal in the healing of surgical wounds by primary intention: quantitative systematic review protocol. J Adv Nurs. 2012;68(2):264-270.
27. Rasmussen H, Larsen MJ, Skeie E. Surgical wound dressing in outpatient paediatric surgery. A randomised study. Dan Med Bull. 1993;40(2):252-254.
28. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
Causes and Rates of Unplanned Readmissions After Elective Primary Total Joint Arthroplasty: A Systematic Review and Meta-Analysis
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
Total joint arthroplasty (TJA) is a clinically effective, cost-effective treatment for symptomatic arthritis.1,2 After TJA, patients report reduced pain, restored range of motion, high satisfaction, and ability to return to a more active lifestyle.3-7 The number of total hip arthroplasties (THAs) performed in the United States is expected to reach 572,000 by 2030, a 174% increase, and the number of total knee arthroplasties (TKAs) 3.5 million, nearly a 7-fold increase.8,9 Since 2005, the cost of THA has risen more than 4 times, to $13.43 billion, and the cost of TKA has risen more than 5 times, to $40.8 billion.8,9 Given the demand and price tag, TJA is the single largest cost in the Medicare budget.10
Given its potential to improve care and reduce costs, reducing readmission rates in the surgical setting is a priority for physicians and policymakers.11 Readmissions for TJA are highly scrutinized as a performance indicator—the Centers for Medicare & Medicaid Services (CMS) started including them in its readmissions penalty program in 2013—and were recently validated as a measure of surgical quality.12-14 Accurate assessments of readmissions after TJA are unclear, with rates ranging from 1% to 8.5% between 7 and 90 days after surgery.2,15-17 The early success of TJA as an elective (and more frequently outpatient) procedure has paradoxically translated to less tolerance for readmissions. Post-TJA complications resulting in readmission are subject to financial penalties, and there is an implicit judgment of inadequate surgical management.12
Not only is the readmission rate poorly characterized, but there is no consensus on the leading reasons for readmissions after primary elective unilateral TJAs. The range of rates, reasons, and follow-up periods reported in the literature is wide.18,19 CMS plans to monitor readmissions over 7 to 90 days after surgery (the period depends on the complication), whereas a significant portion of the orthopedic literature documents 90-day rates.19 In 2012, the Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation prepared for CMS a comprehensive report identifying rates of post-TJA complications and readmissions.20 The report, however, is limited to US hospitals and Medicare patients and therefore may overstate the rates, given this population’s documented comorbidities and the reimbursement variations between Medicare and commercial insurance.21 Lack of consensus on readmissions after primary elective unilateral TJAs requires that we synthesize available data to answer several questions: What is the overall readmission rate 30 and 90 days after TJA? What are the primary reasons for readmission 30 and 90 days after TJA? What are the cause-specific readmission rates? We performed a systematic review and a meta-analysis to answer these questions and to add clarity to the literature in order to help guide policy.
Materials and Methods
We performed a systematic review in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.22 Two reviewers independently completed structured searches of the Medline and Cochrane Central Register of Controlled Trials databases. Search terms were: (total hip replacement OR hip arthroplasty OR total hip arthroplasty OR total knee replacement OR knee arthroplasty OR total knee arthroplasty) AND (readmission OR complication OR discharge). They updated the search June 1, 2013. Four limits were applied: publication between January 1, 1982 and December 12, 2012; human subjects only; age 19+ years; and English-language articles. Study eligibility was determined by using standardized criteria as defined by the inclusion and exclusion criteria described in 3 stages: title review, abstract review, and full-article review. The reviewers also performed ancestry searches, including searches for major review articles and bibliographies of all retrieved studies, to identify additional studies not identified in the keyword searches. Discrepancies were resolved by author consensus.
Inclusion criteria were original studies that presented level I to III evidence and that were identified in structured online searches; published in English between January 1, 1982 and December 31, 2012; involved patients older than 19 years; and reported both readmission rates and reasons at follow-up 30 or 90 days after elective primary unilateral TJA, regardless of indication. Exclusion criteria were studies that reported data from hip fracture, knee fracture, and pelvis fracture cases; those that reported data from hemiarthroplasty, Birmingham hip resurfacing procedures, other resurfacing procedures, simultaneous bilateral hip or knee arthroplasties, unicompartmental knee arthroplasty, patellofemoral arthroplasty, metastatic or bone cancer, or revision hip or knee arthroplasty; those that did not report extractable reasons for readmission; those that reported complications but did not specify readmission rates; and those that reported readmission data only from after the 90-day follow-up window. In cases in which multiple studies reported data from the same patient population, only the largest or most recent report was used.
Two reviewers extracted the quantitative data from eligible studies. The 2 primary outcomes of interests were all-cause readmission rates, and reasons for readmission 30 and 90 days after TJA. Other extracted data were evidence level; publication journal, year, and country; data source (academic institution, Medicare); study design; number of patients; patient characteristics; surgical approach; follow-up period; overall readmission rate; anticoagulant use; tourniquet use; and compression stocking use. In addition, all post-TJA readmissions were assumed to be unplanned, except for staged sequential bilateral arthroplasty for osteoarthritis (excluded from analysis).
Readmission reasons were divided into 4 major categories as defined by the literature and the authors: thromboembolic disease, joint-specific reasons, surgical site infection, and surgical sequelae. The diagnoses in these categories are listed in Table 1. Other extracted reasons were cardiac dysrhythmia and pneumonia.
In cases in which there were at least 2 comparable studies, a meta-analysis was performed to obtain pooled estimates of the proportion of patients readmitted at 30 or 90 days. We calculated a Higgins I2 measure for between-study heterogeneity and random-effects analysis, using the method of DerSimonian and Laird23 if I2 was greater than 0.5. Pooled estimates were obtained for both overall and cause-specific reasons for readmission for all reasons reported in at least 3 studies. Small-study or publication bias was assessed using funnel plot asymmetry when at least 5 studies were analyzed as recommended.24 The meta-analytic findings for both overall and cause-specific readmission are presented as pooled proportions with 95% confidence intervals (CIs). All meta-analyses were performed using Stata 10.0.
Results
Fifteen unique TJA studies (12 THA, 10 TKA) met the criteria for the meta-analysis.20,25-38Figure 1 depicts the PRISMA flowchart for study identification.22
Of the 12 studies eligible for the THA analysis (Table 2), 6 were conducted in the United States,20,26,27,30,33,34 5 in Europe,25,28,29,32,35 and 1 in Canada.31 Seven of the 12 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (2 reported rates at both follow-ups). We analyzed a total of 113,396 patients at the 30-day window and 192,380 patients at the 90-day window. Mean age was 74.2 years. The included studies were variable and sparse in their reporting of specific characteristics (Table 3).
Of the 10 studies (2 prospective, 8 retrospective) eligible for the TKA analysis (Table 4), 6 were conducted in the United States,20,26,27,34,36,37 3 in Europe,25,29,35 and 1 in Asia.38 Four of the 10 studies reported readmission rates at 30 days, and 7 reported rates at 90 days (1 reported rates at both follow-ups).27 We analyzed a total of 3,278,635 patients at the 30-day window and 272,419 patients at the 90-day window. Mean age was 74.3 years. The included studies were quite variable and sparse in their reporting of specific characteristics (Table 5).
We performed random-effects meta-analyses of all unplanned readmissions at both 30 and 90 days (all I2s > 0.5). Among 5 THA studies that reported overall rates at 30 days,20,27,28,32,33 the estimated overall unplanned rate among the 120,272 index surgeries was 5.6% (95% CI, 3.2%-8.0%). Among 5 THA studies that reported overall rates at 90 days,20,25-27,31 the estimated overall unplanned rate among the 192,380 index surgeries was 7.7% (95% CI, 3.2%-12.2%) (I2 = 1.00). Among 3 TKA studies that reported overall rates at 30 days,27,37,38 the estimated overall unplanned rate among the 3,278,635 index surgeries was 3.3% (95% CI, 0.7%-5.9%). Among 5 TKA studies that reported overall rates at 90 days,20,25-27,36 the estimated overall unplanned rate among the 272,419 index surgeries was 9.7% (95% CI, 7.1%-12.4%) (I2 = 0.97).
30-Day Readmission Rates
The most common reason for readmission 30 days after THA discharge was joint-specific. This reason accounted for 39.3% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.2% (95% CI, 0.0%-4.6%; P < .001; I2 = 1.00) among 4 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 0.8%-2.5%; P < .001; I2 = 0.95) and thromboembolic disease (1.5%; 95% CI, 1.0%-1.9%; P < .001; I2 = 0.95). See Figure 2 for 30-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.1%; P < .001; I2 = 0.94). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported in only 1 study each.
The most common reason for readmission 30 days after TKA discharge was surgical site infection. This reason accounted for 12.1% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.4% (95% CI, 0.3%-0.6%; P < .001; I2 = 0.61) among 3 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.3% of the time. Joint-specific reasons were reported in 2 studies (95% CI, 0.0%-0.8%; P = .259; I2 = 0.94). Thromboembolic disease was reported in 4 studies (95% CI, 0.0%-0.7%; P = .067; I2 = 0.98) (Figure 3). Only these 3 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and “sequelae” were reported in only 1 study each.
90-Day Readmission Rates
Consistent with the 30-day THA results, the most common reason for readmission 90 days after THA discharge was joint-specific. This reason accounted for 31.2% of all unplanned readmissions among studies that reported joint-specific causes, with an estimated pooled rate of 2.4% (95% CI, 0.0%-4.9%; P < .001; I2 = 1.00) among 5 studies. The second and third most common reasons were surgical sequelae (1.6%; 95% CI, 1.0%-2.2%; P < .003; I2 = 0.83) and thromboembolic disease (1.0%; 95% CI, 0.7%-1.4%; P < .001; I2 = 0.97). See Figure 4 for 90-day THA readmission rates. The fourth most common readmission reason was surgical site infection (0.6%; 95% CI, 0.2%-1.0%; P < .001; I2 = 0.99). Only these 4 reasons could be pooled, as cardiac dysrhythmia, pneumonia, and bleeding were reported by only 1 study each.
Consistent with the 30-day TKA results, the most common reason for readmission 90 days after TKA discharge was surgical site infection. This reason accounted for 9.3% of all unplanned readmissions among studies that reported surgical site infections, with an estimated pooled rate of 0.9% (95% CI, 0.4%-1.4%; P < .001; I2 = 0.93) among 5 studies. The second and third most common reasons were joint-specific and thromboembolic disease, both occurring 0.7% of the time. Joint-specific reasons were reported in 5 studies (95% CI, 0.2%-1.1%; P =.003; I2 = 0.94). Thromboembolic disease was reported in 7 studies (95% CI, 0.3%-1.1%; P < .001; I2 = 0.97) (Figure 5). Bleeding was reported in 3 studies, with a pooled rate of 0.4% (95% CI, 0.0%-0.9%; P = .128; I2 = 0.83). Cardiac dysrhythmia was reported in 2 studies, with an estimated pooled rate of 0.3% (95% CI, 0.2%-0.5%; P < .001). Only these 5 reasons could be pooled, as pneumonia and “sequelae” were reported in only 1 study each.
Discussion
This study is the first systematic review and meta-analysis of the literature to identify overall and cause-specific readmission rates after TJA.
For THA, 30- and 90-day readmission rates were 5.6% and 7.7%, respectively. Joint-specific causes were the most common reason for readmission at both 30 and 90 days after THA. For TKA, 30- and 90-day rates were 3.3% and 9.7%, respectively. Surgical site infection was the most common reason for readmission at both 30 and 90 days after TKA.
Hospital readmissions are an important area of scrutiny for Medicare and the health care systems broadly. Readmissions after surgery are deemed quality indicators potentially suggesting incomplete management of active issues and inadequate preparation for discharge.39 Unplanned readmissions also place a significant economic burden on Medicare: $17.5 billion in 2010.40 Given their association with quality of overall surgical care, improved readmission rates have the potential to improve the standard of care and reduce costs.
Higher readmission rates will significantly affect hospitals as CMS shifts to bundling payments for acute-care episodes, such as TJA.41-43 Further, private and public health care payers are increasingly using unplanned 30- and 90-day readmission rates as a marker of quality of care. However, there is little agreement about readmission rates and reasons, let alone what follow-up window should be used to define orthopedic readmissions. One study involving the MEDPAR (Medicare Provider Analysis and Review) database found that a common reason for readmission after major hip or knee surgery was “aftercare” for surgical sequelae (10.3%)15; another study found a 15% increase in post-THA hospitalizations, most commonly for a mechanical complication (joint-related).44 There are no prior complete systematic reviews or meta-analyses of overall rates of readmissions after primary unilateral TJAs, or of the reasons for these readmissions. The closest such report, the Yale report to CMS, was skewed to a proportion of US hospitals treating a population prone to significant comorbidities.20
Although the strength of this study lies in its rigorous identification and extraction of data, notable clarifications must be made when synthesizing the information. First, the definitions of various thromboembolic events varied greatly. Some studies reported deep vein thrombosis (DVT) and pulmonary embolism (PE) separately, whereas others reported only DVT or only PE. Some studies reported rates of readmission for “thromboembolic disorder,” and one25 reported rates for DVT, PE, and thromboembolic disorder. To pool these related events, we created a composite definition that included DVT, PE, and thromboembolic disorders, which we termed thromboembolic disease. We also created a composite measure for joint-specific reasons for readmission. This category included joint infection that definitely required reentry into the joint, but using this category may have led to underestimation of surgical site infection rates, which were defined separately. Third, there was significant variation in documentation of surgical site infection among the studies included in this review. Some studies specified superficial wounds, whereas others did not categorize complications as superficial, deep, or intracapsular, which would qualify as a “joint-specific” cause. Despite this variation, surgical site infection after TJA was found to be the most common reason for readmission.
Our systematic review and meta-analysis were limited, as any others are, by the quality of studies investigated. Few studies reported cause-specific rates and reasons for readmission. Given the small sample, formal tests for small-study or publication bias could not be performed. Some studies included tremendous amounts of data, and International Classification of Diseases, Ninth Revision (ICD-9) codes were used without physician review of readmission diagnoses. In the absence of oversight, many readmissions could have been misinterpreted and incorrectly logged, or simply miscoded. Saucedo and colleagues27,45 found that readmission diagnostic codes were often unverified. Numerous other studies corroborated this lack of correlation with physician-derived readmission diagnoses in just 25% of cases.46-54 Another study limitation is the unknown number of patients who had TJA but presented and were subsequently readmitted to a different hospital. Last, as this review included patients who had surgery performed within a 30-year period, it could not address the shifts in postoperative management that occurred in that time, particularly with respect to anticoagulation. This limitation was partially addressed in THA by dividing final studies into 3 decades. Of these studies, only 1 was from the first decade, 3 were from the second, and the rest were from the third. Of the 3 from the second decade, only the study by Warwick and colleagues29 (1995) explicitly did not use anticoagulation, but compression stockings were used, and consequently there was a 4.0% rate of readmission for thromboembolic disease alone, compared with the study by White and colleagues34 (1998), which explicitly used anticoagulation and boasted a 1.7% rate of readmission for thromboembolic disease. This isolated comparison illustrates the effect of routine anticoagulation and the changes in surgical standards over the 3 decades.
The numbers from this systematic review and meta-analysis represent an international benchmark for TJA as a procedure. Knowing the top reasons for readmission will lead to more focus on joint-related and medical issues (surgical site infection, thromboembolic disease) before discharge to avoid readmission after elective unilateral primary TJA. Although readmission rates have received attention in the United States as a primary means of combating soaring health care costs, knowing the rates for a common procedure applies broadly as an indicator for standard of care worldwide, according to the World Health Organization.55 This study is the first systematic review and meta-analysis of documented readmission rates and reasons for readmission to identify overall and cause-specific rates after TJA. The hope is that our findings will add clarity to the literature and help guide the decisions of physicians and policymakers.
Conclusion
Readmission rates are an increasingly important metric in the United States and around the world, yet there is no consensus regarding overall readmission rates and reasons for readmission after primary unilateral TJAs. Our systematic review and meta-analysis of the literature found overall unplanned readmission rates of 5.6% (30 days) and 7.7% (90 days) for THA and 3.3% (30 days) and 9.7% (90 days) for TKA. At both 30 and 90 days, the most common readmission reasons were joint-specific (THA) and surgical site infection (TKA). New investigations should be directed toward developing countermeasures to lower the rates of readmission.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.
1. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
2. Cram P, Lu X, Kaboli PJ, et al. Clinical characteristics and outcomes of Medicare patients undergoing total hip arthroplasty, 1991–2001. JAMA. 2011;305(15):1560-1567.
3. de Vries LM, Sturkenboom MC, Verhaar JA, Kingma JH, Stricker BH. Complications after hip arthroplasty and the association with hospital procedure volume. Acta Orthop. 2011;82(5):545-552.
4. Mariconda M, Galasso O, Costa GG, Recano P, Cerbasi S. Quality of life and functionality after total hip arthroplasty: a long-term follow-up study. BMC Musculoskelet Disord. 2011;12:222.
5. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013;95(20):1869-1876.
6. Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89(3):526-533.
7. Mancuso CA, Salvati EA, Johanson NA, Peterson MG, Charlson ME. Patients’ expectations and satisfaction with total hip arthroplasty. J Arthroplasty. 1997;12(4):387-396.
8. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(suppl 3):144-151.
9. Kurtz SM, Ong KL, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
10. Bozic KJ, Rubash HE, Sculco TP, Berry DJ. An analysis of Medicare payment policy for total joint arthroplasty. J Arthroplasty. 2008;23(6 suppl 1):133-138.
11. Li LT, Mills WL, White DL, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175-1181.
12. Readmissions Reduction Program. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed July 27, 2015.
13. Tsai TC, Joynt KE, Orav J, Gawande AA, Jha AK. Variation in surgical readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134-1142.
14. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program [published correction appears in N Engl J Med. 2011;364(16):1582]. N Engl J Med. 2009;360(14):1418-1428.
15. Zmistowski B, Hozack WJ, Parvizi J. Readmission rates after total hip arthroplasty. JAMA. 2011;306(8):825.
16. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
17. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
18. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369.
19. Atkinson JG. Flaws in the Medicare readmission penalty. N Engl J Med. 2012;367(21):2056-2057.
20. Grosso LM, Curtis JP, Lin Z, et al. Hospital-level Risk-Standardized Complication Rate Following Elective Primary Total Hip Arthroplasty (THA) And/Or Total Knee Arthroplasty (TKA): Measure Methodology Report. Report prepared for Centers for Medicare & Medicaid Services. QualityNet website. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1228772504368. Submitted June 25, 2012. Accessed August 4, 2015.
21. Robinson JC. Analysis of Medicare and commercial insurer–paid total knee replacement reveals opportunities for cost reduction. Health Care Incentives Improvement Institute website. http://www.hci3.org/sites/default/files/files/HCI-2012-IssueBrief-L6-2.pdf. Published 2012. Accessed July 27, 2015.
22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188.
24. Higgins JP, Thompson SG. Quantifying heterogeniety in a meta-analysis. Stat Med. 2002;21(11):1539-1558.
25. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
26. Keeney JA, Adelani MA, Nunley RM, Clohisy JC, Barrack RL. Assessing readmission databases: how reliable is the information? J Arthroplasty. 2012;27(8 suppl):72-76.e1-e2.
27. Saucedo JM, Marecek GS, Wanke TR, Lee J, Stulberg SD, Puri L. Understanding readmissions after primary total hip and knee arthroplasty: who’s at risk? J Arthroplasty. 2014;29(2):256-260.
28. Seagroatt V, Tan HS, Goldacre M, Bulstrode C, Nugent I, Gill L. Elective total hip replacement: incidence, emergency readmission rate, and postoperative mortality. BMJ. 1991;303(6815):1431-1435.
29. Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77(1):6-10.
30. Kreder HJ, Deyo RA, Koepsell T, Swiontkowski MF, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am. 1997;79(4):485-494.
31. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
32. Cullen C, Johnson DS, Cook G. Re-admission rates within 28 days of total hip replacement. Ann R Coll Surg Engl. 2006;88(5):475-478.
33. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
34. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158(14):1525-1531.
35. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88(3):386-391.
36. Berger RA, Kusuma SK, Sanders SA, Thill ES, Sporer SM. The feasibility and perioperative complications of outpatient knee arthroplasty. Clin Orthop Relat Res. 2009;467(6):1443-1449.
37. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
38. Seah VW, Singh G, Yang KY, Yeo SJ, Lo NN, Seow KH. Thirty-day mortality and morbidity after total knee arthroplasty. Ann Acad Med Singapore. 2007;36(12):1010-1012.
39. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
40. The Revolving Door: A Report on U.S. Hospital Readmissions. An Analysis of Medicare Data by the Dartmouth Atlas Project. Stories From Patients and Health Care Providers by PerryUndem Research & Communication. Robert Wood Johnson Foundation. http://www.rwjf.org/content/dam/farm/reports/reports/2013/rwjf404178. Published February 2013. Accessed July 27, 2015.
41. Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM R. 2010;2(9):806-810.
42. Bosco JA 3rd, Karkenny AJ, Hutzler LH, Slover JD, Iorio R. Cost burden of 30-day readmissions following Medicare total hip and knee arthroplasty. J Arthroplasty. 2014;29(5):903-905.
43. McCormack R, Michels R, Ramos N, Hutzler L, Slover JD, Bosco JA. Thirty-day readmission rates as a measure of quality: causes of readmission after orthopedic surgeries and accuracy of administrative data. J Healthc Manag. 2013;58(1):64-76.
44. Bohm ER, Dunbar MJ, Frood JJ, Johnson TM, Morris KA. Rehospitalizations, early revisions, infections, and hospital resource use in the first year after hip and knee arthroplasties. J Arthroplasty. 2012;27(2)232-237.
45. Saucedo J, Marecek GS, Lee J, Huminiak L, Stulberg SD, Puri L. How accurately are we coding readmission diagnoses after total joint arthroplasty? J Arthroplasty. 2013;28(7):1076-1079.
46. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464-470.
47. Bozic KJ, Chiu VW, Takemoto SK, et al. The validity of using administrative claims data in total joint arthroplasty outcomes research. J Arthroplasty. 2010;25(6 suppl):58-61.
48. Cram P, Ibrahim SA, Lu X, Wolf BR. Impact of alternative coding schemes on incidence rates of key complications after total hip arthroplasty: a risk-adjusted analysis of a national data set. Geriatr Orthop Surg Rehabil. 2012;3(1):17-26.
49. Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973-981.
50. Cima RR, Lackore KA, Nehring SA, et al. How best to measure surgical quality? Comparison of the Agency for Healthcare Research and Quality Patient Safety Indicators (AHRQ-PSI) and the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) postoperative adverse events at a single institution. Surgery. 2011;150(5):943-949.
51. Steinberg SM, Popa MR, Michalek JA, Bethel MJ, Ellison EC. Comparison of risk adjustment methodologies in surgical quality improvement. Surgery. 2008;144(4):662-667.
52. Baron JA, Barrett J, Katz JN, Liang MH. Total hip arthroplasty: use and select complications in the US Medicare population. Am J Public Health. 1996;86(1):70-72.
53. HCUPnet. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality website. http://hcupnet.ahrq.gov. Accessed July 27, 2015.
54. Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J. 2011;5:80-85.
55. Parker SG. Do Current Discharge Arrangements From Inpatient Hospital Care for the Elderly Reduce Readmission Rates, the Length of Inpatient Stay or Mortality, or Improve Health Status? Health Evidence Network report. Copenhagen, Denmark: World Health Organization Regional Office for Europe; 2005. http://www.euro.who.int/__data/assets/pdf_file/0006/74670/E87542.pdf. Accessed July 27, 2015.